Abstract
A 47-year-old man with metastatic melanoma presented with refractory hyperlactaemic acidosis following the first dose of the mono-carboxylase transporter 1 inhibitor AZD3965 within a “first time in man” clinical trial. The mechanism of the agent and the temporal relationship suggested that this event was potentially drug related and recruitment was suspended. However, urinary metabolomics showed extensive abnormalities even prior to drug administration, leading to investigations for an underlying metabolic disorder. The lack of clinical symptoms from the elevated lactate and low blood glucose suggested a diagnosis of “hyper-Warburgism”, where the high tumour burden was associated with extensive glucose uptake and lactate efflux from malignant cells, and the subsequent impact on blood biochemistry. This was supported by an FDG-PET scan showing extensive glucose uptake in numerous metastases and lack of uptake in the brain. A review of the literature showed 16 case reports of “hyper-Warburgism” in non-haematological malignancies, none of them with melanoma, with most associated with a poor outcome. The patient was treated symptomatically, but died 2 months later. The development of AZD3965 continues with the exclusion of patients with elevated plasma lactate at screening added to the protocol as a safety measure.
Trial identification number ClinicalTrials.Gov. NCT01791595.
Subject terms: Cancer metabolism, Drug development
Background
We present the case of a patient with metastatic melanoma who presented with malignant lactic acidosis, this biochemical abnormality being made clinically apparent by treatment with a novel antitumour agent-targeting metabolism, AZD3965.
Malignant lactic acidosis is a rare complication of solid malignancies, being more common in haematological cancers. An increase in lactate production as a result of the Warburg effect in cancer cells is considered to play an important role in its pathogenesis.1–4 The Warburg effect is considered one of the hallmarks of cancer,5 and describes the tendency of malignant cells to favour glucose metabolism via glycolysis over oxidative phosphorylation, even in the presence of oxygen. Whilst this appears significantly less efficient for energy production, yielding only two molecules of adenosine triphosphate (ATP) per glucose molecule, compared with 36 produced within oxidative phosphorylation, the process may confer benefit to cancer cells through enhanced availability of metabolic substrates that are essential for cell proliferation, e.g., lipids, amino acids and nucleotides.2,4,6,7 Through this metabolic reprogramming, driven by oncogenic genes, such as mTOR, c-MYC and hypoxia-inducible factor 1 (HIF-1), the cancer cells are able to maintain their high proliferation rate.2,3,6 Tumour cells can upregulate lactate transporters, in particular the monocarboxylate transporters (MCT) 1 and MCT-4 to remove the excess lactate produced. AZD3965 is an inhibitor of MCT-1 currently in Phase 1/2 clinical development (ClinicalTrials.Gov. NCT01791595) and will inhibit the transport of lactate out of or into cells that do not express MCT-4, the alternative route of excretion. Therefore, it can potentially exploit the dependency of cancer cells on aerobic glycolysis, leading to an accumulation of intracellular lactate, feedback inhibition of glycolysis and pH imbalance.
In this case, a patient with metastatic melanoma presented with severe hyperlactaemic acidosis, following a single dose of the MCT-1 inhibitor, AZD3965, within a clinical trial. Given the temporal relationship with the agent, it was important to determine the cause of this syndrome, both to guide the patient’s immediate management, and also to ensure that further development of this agent could be performed safely.
Methods
A 47-year-old man with no significant past medical history was diagnosed with BRAF wild-type metastatic melanoma from an unknown primary following needle biopsy of enlarged inguinal lymph nodes. Over the following 18 months, his treatments included inguinal dissection with adjuvant radiotherapy, combination immunotherapy on diagnosis of metastatic disease with ipilimumab and nivolumab, discontinued after three cycles due to grade 4 autoimmune hepatitis, and subsequently three cycles of dacarbazine chemotherapy with progressive disease. He then entered a trial for the first-in-human dose escalation of an oral MCT-1 inhibitor, AZD3965. His baseline trial CT scan demonstrated extensive lymphadenopathy, bone and liver metastases.
Twelve hours after the first dose, he developed severe vomiting unresponsive to anti-emetics. On admission, he was apyrexial but dehydrated with tachycardia (107 bpm) and reduced skin turgor, but normal blood pressure (153/44 mmHg). Arterial blood gas revealed metabolic acidosis (pH: 7.06, standard HCO3−: 9 mmol/L, base excess: −23 and CO2: 2.8 mmHg). Venous lactate was elevated at 7.7 mmol/L (normal range 0.5–2.2 mmol/L). Capillary glucose was 4.1 mmol/L (normal range 4–11 mmol/L). Urine was positive for protein (++), blood (+) and ketones (+++). Imaging revealed no acute changes compared with his baseline scan.
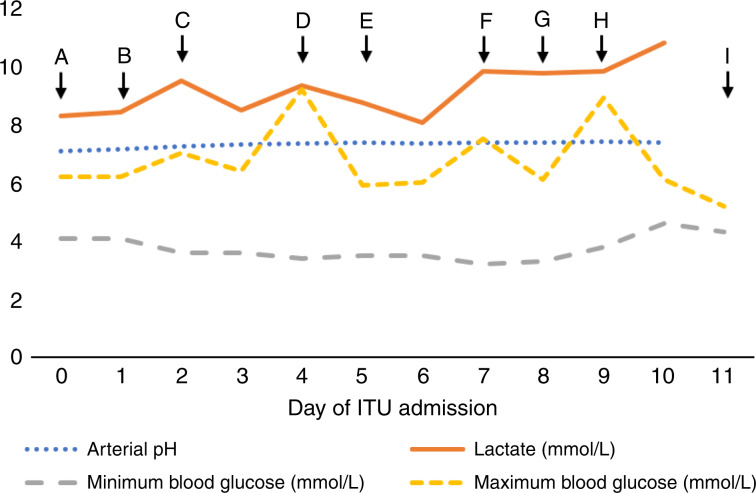
Following rehydration, he was transferred to the intensive care unit (ICU) due to persistent hyperlactaemic acidosis (pH: 7.09, lactate 8.29 mmol/L and base excess −21), where he received continuous veno-venous haemodiafiltration (CVVHDF via the Baxter Prismaflex system) and oral sodium bicarbonate (2 g of BD). Throughout admission, he had asymptomatic intermittent hypoglycaemia that was resistant to 20% dextrose infusion, administered from day 2 until day 4, with 10% dextrose infusion given from day 8 onwards, and high calorie intake (see Fig. 1). Serum insulin and C-peptide concentrations were not increased. He remained in the ICU for 10 days for CVVHDF, and IV electrolyte and thiamine supplementation, but despite the severe biochemical abnormalities, he remained clinically well, mobile and ate a normal diet. He was discharged from the ICU to the ward for a further 7 days, and then to home, by taking oral bicarbonate (2 g of TDS) and monitoring his blood sugar via a continuous glucose monitor. Lactate was still elevated at 9.2 mmol/L on discharge.
Fig. 1. Lactate, arterial pH and blood glucose measured during initial admission to the intensive care unit.
Blood glucose was measured on arterial blood gas except on days 9 and 10, where it was recorded as capillary blood glucose. Lactate and pH were not measured on day 11. The highest recorded lactate values and the lowest recorded pH values are shown for each day. A, admission to the ITU. B, started continuous veno-venous haemodiafiltration (CVVHDF). C, started 20% intravenous dextrose. D, stopped intravenous dextrose. E, started intravenous vitamin supplementation (pabrinex). F, stopped CVVHDF. G, started 10% intravenous dextrose. Started oral bicarbonate therapy (2 g of BD). H, oral bicarbonate increased (2 g of TDS). I, transferred to the ward.
The seriousness of this event led to suspension of trial recruitment, whilst the study team investigated the cause. No similar toxicity had been observed in any other patient in the trial.
Results
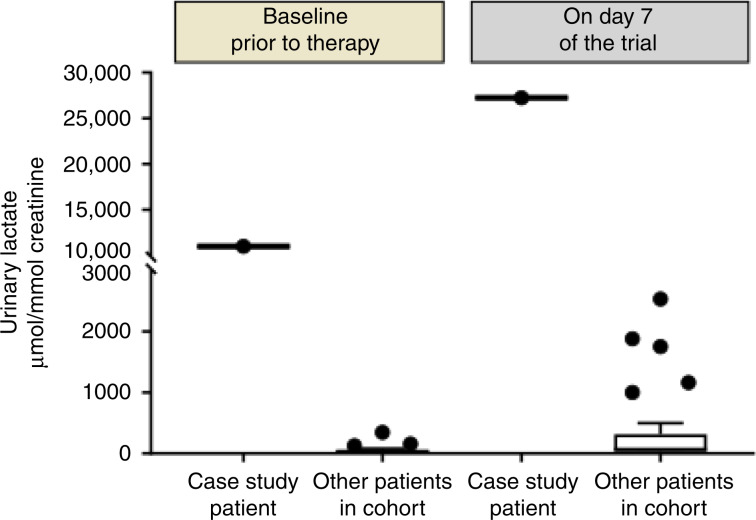
1H Nuclear Magnetic Resonance spectroscopy analysis revealed high urine ketone and lactate levels in samples taken before the initial dose, with a significant increase following the start of treatment (Fig. 2). The possibility of an underlying inborn error of metabolism unveiled by treatment with AZD3965, such as a disorder of gluconeogenesis or oxidative phosphorylation, was considered. Metabolic investigations were performed, and no abnormalities were identified apart from persistently increased lactic acid in blood and urine (see Table 1). These results, the lack of any previous clinical history suggesting a metabolic disorder and the very mild clinical response to significant acidosis suggested that an inherited metabolic disorder as a cause of lactic acidaemia was unlikely. Given the mechanism of the drug, we also considered congenital MCT-4 deficiency, but strong MCT-4 expression was detected in his original tumour resection samples suggesting that this was unlikely (Supplementary Fig. 1). Pharmacokinetic analysis did not show excessive exposure to AZD3965 compared with previous patients; the maximum concentration of the drug in the patient (Cmax) was 71% of the mean values in this cohort, while the estimated exposure over 24 h (AUC0–24) was 103% of the mean values in this cohort. The most likely explanation for the persistent lactic acidaemia in this patient was therefore considered to be a result of the abnormal glucose metabolism found in the cancer, and not due to the patient’s underlying physiology.
Fig. 2. Urinary lactate in the patient before and following treatment with one dose of AZD3965 and stay in the intensive care unit with administration of IV 20% dextrose.
Compared with other patients in the clinical trial treated at the same dose of AZD3965. Urinary lactate was measured with 1H nuclear magnetic resonance spectroscopy metabolomics analysis.
Table 1.
The results of key metabolic investigations in the patient.
| Investigation | Result | Reference range | Interpretation |
|---|---|---|---|
| Serum insulin (when glucose was 2.2 mmol/L) | <6 pmol/L | Appropriate insulin for hypoglycaemia | |
| Blood lactate | 13.4 mmol/L | <1.8 | Elevated ratio, not consistent with pyruvate dehydrogenase deficiency |
| Blood pyruvate | 0.28 mmol/L | 0.04–0.15 | |
| Blood alanine | 1.04 mmol/L | 0.2–0.5 | |
| Blood 3-OH butyrate | 0.26 mmol/L | Normal ratio | |
| Plasma non-esterified fatty acids | 0.65 mmol/L | ||
| Plasma ammonia | 33 µmol/L | <50 | |
| Blood spot acylcarnitines | Normal | ||
| Urine organic acids | Elevated lactate and pyruvate, and no increase in Kreb’s cycle intermediates. Elevated ketones with no increase in dicarboxylic acids | Consistent with lactic acidosis and ketosis |
The patient was re-admitted 1 week later due to recurrence of hyperlactaemic acidosis (venous lactate 13.8 mmol/L, arterial pH: 7.29), with nausea, upper abdominal discomfort and bloating. His acidosis worsened despite intravenous bicarbonate, and he returned to the ICU for CVVHDF. Symptomatology appeared to be driven by the acidosis rather than lactate or glucose levels; we hypothesised that medical treatment of his hypoglycaemia with glucose (see Fig. 1) was driving increased lactate production in the tumour with the resultant impact on his blood biochemistry; therefore, we started the patient on a ketogenic diet with some improvement in symptoms. A review of the literature at this time found reported cases of “hyper-Warburgism” where in the context of high tumour burden, high glucose uptake in the malignant cells impacts on blood biochemistry, with blood sampling demonstrating low glucose and high lactate as we had observed in this case (see Table 2).
Table 2.
Reported cases of lactic acidosis in patients with solid malignancies.
| Malignancy | Age | Lactate range (mmol/L) | Arterial pH | Liver metastases present | Intervention | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Breast adenocarcinoma | 86 | 7.5–12 | 7.35 | Yes |
Thiamine Sodium bicarbonate Chemotherapy |
Died (weeks) | 3 |
| Breast adenocarcinoma | 31 | 16 | NS | Yes |
Thiamine Sodium bicarbonate Supportive care |
Died (8 h) | 19 |
| Colorectal adenocarcinoma | 64 | 7.2–20.1 | 6.99 | Yes |
Sodium bicarbonate Multivitamins Supportive care |
Died (6 days) | 11 |
| Colorectal adenocarcinoma | 44 | >11 | 7.24 | Yes |
Sodium bicarbonate Chemotherapy Starch loading Thiamine Hydrochlor-thiazide |
Resolved | 20 |
| Prostate adenocarcinoma | 81 | 9.5–13.6 | 7.23 | Yes |
Chemotherapy Prednisolone Thiamine Sodium bicarbonate |
Died (days) | 6 |
| Gastric adenocarcinoma | 81 | 4.0–6.6 | 7.43 | Yes | Supportive care | Died (days) | 21 |
| Squamous cell lung cancer | 84 | 13.5–14 | 7.13 | No | Sodium bicarbonate | Died (15 days) | 22 |
| SCLC | 55 | 26 | 7.17 | Yes |
Radiotherapy Chemotherapy |
Died (5 days) | 23 |
| SCLC | 57 | 25.5 | 7.18 | Yes | NS | Died | 23 |
| SCLC | 79 | 4.5 | 7.33 | Yes | NS | NS | 24 |
| SCLC | 70 | 15 | 7.29 | No |
Chemotherapy Sodium bicarbonate |
Resolved | 25 |
| SCLC | 73 | 4.9–25 | 6.8 | Yes |
Sodium bicarbonate Supportive care |
Died (days) | 26 |
| Small-cell carcinoma of the liver | 77 | 13 | 7.14 | Primary in the liver | Supportive care | Died (days) | 27 |
| CUP | 25 | 171.5 | 7.08 | Yes |
Haemodialysis Sodium bicarbonate |
Died (8 days) | 28 |
| CUP | 76 | 7.7 | NS | Yes |
Sodium bicarbonate CRRT |
Died (15 days) | 29 |
| CUP | 14 | NS | NS | Yes |
Chemotherapy Supportive care |
Died (2 months) | 30 |
| Melanoma | 49 | 6.8–16 | 7.05 | Yes |
Haemofiltration Sodium bicarbonate Multivitamins Supportive care |
Died (2 months) |
CRRT continuous renal replacement therapy, CUP carcinoma of unknown primary, SCLC small-cell lung cancer.
Previous case reports of lactic acidosis with solid malignancies compared with this case (in bold). Supportive care includes treatments not directly related to relieving acidosis or treating an underlying malignancy, e.g., antibiotics, pantoprazole (for haematemesis), vasopressors, transfusion and standard palliative care. NS: not stated, or for arterial pH only “metabolic acidosis” was stated without giving a value.
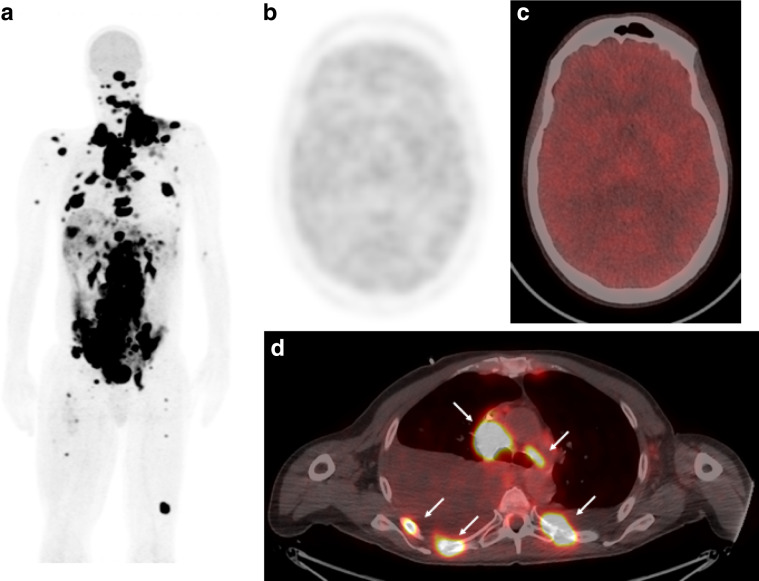
Unfortunately, he was re-admitted 1 month later with recurrent acidosis and a venous lactate of 11.6 mmol/L, which was treated with sodium bicarbonate infusions. A re-staging CT showed disease progression, and a PET scan showed significant volume of FDG-avid disease throughout the body (Fig. 3a, d). We also noted reduced uptake in normal tissues most obvious in the brain, as the organ that has the highest physiological uptake (this is consistent with a change in brain metabolism from glucose to lactate or fatty acid metabolism, Fig. 3b, c). This scan, with his resistant hyperlactaemia and asymptomatic hypoglycaemia, supported a theory of “hyper-Warburgism” as the cause for his hyperlactaemic acidosis. Unfortunately, the patient died 2 weeks later from progressive disease. Baseline lactate-level assessment was added to the protocol as a safety measure for subsequent development of AZD3965.
Fig. 3.
FDG PET showing extensive uptake in tumour metastases throughout the body on maximum intensity projections (a). Reduced uptake in the brain (b, c) is shown on the maximum intensity projection and fused axial images (hot iron scale) and compared with a cross section of FDG-avid nodal and bone metastases in the thorax (d, indicated by white arrows). The PET scan was performed on a GE 710 PET-CT scanner with a dose of 3.5 MBq/kg 18F fludeoxyglucose in 3-min bed positions.
Discussion
Lactic acidosis is associated with high mortality,1,8 and treatment consists of correcting the underlying cause where possible, and attempting to enhance clearance of lactate and reduce acidosis. Symptomatic treatment with NaHCO3 is often used; however, its utilisation is controversial due to lack of an effective response, and a potential association with increased mortality.8,9 Other treatments include thiamine supplementation, to encourage oxidation of lactate via the action of pyruvate dehydrogenase,1 or haemodialysis to remove excess lactate.1,10 In the context of malignancy-related lactic acidosis, case reports suggest that the mainstay of treatment is systemic therapy for the underlying cancer such as chemotherapy; however, the feasibility will depend on the patient’s physiological reserve, cancer type and previous treatment history.1,11
In this case, the PET scan showed high glucose uptake by the tumour with extensive disease burden. Melanoma is known to express high levels of GLUT-1,12 and in vitro studies show high levels of glycolysis and lactate production.13–15 The elevated urinary metabolites prior to therapy, the lack of symptoms with high lactate and low glucose (the patient only became symptomatic when acidotic) and the low glucose uptake in the brain on PET scan all suggest that this was a chronic state. The temporal relationship to treatment and the increase in urinary metabolites following therapy suggest that the AZD3965 precipitated the admission. MCT is responsible for both influx and efflux of lactate, and will reduce import by key tissues such as the liver and kidney. This impact on normal tissues can be most readily observed by the rise in urinary lactate seen in all subjects (Fig. 2). We presume that the single dose temporarily interfered with plasma clearance by the liver and other organs, by precipitating the symptomatic deterioration, and inadvertently the medical team worsened the condition by seeking to reverse the asymptomatic hypoglycaemia. However, in light of the eventual diagnosis and the subsequent continued deterioration, we suspect that this event would have occurred shortly with further disease progression, even in the absence of this treatment.
Case reports of “Hyper-Warburgism” show that in the majority of cases, this was associated with a rapidly fatal outcome (see Table 2); many present themselves unwell with advanced cancer and receive symptomatic measures only. To the authors' knowledge, this is the first report in association with malignant melanoma, with cases more commonly associated with haematological malignancies.1,3,16–18 The treatments received in case reports and outcomes are listed in Table 2; only two cases were resolved, both with disease that responded to chemotherapy. Interestingly, a high proportion of cases had primary or metastatic liver involvement (14 out of 16 cases), and the majority of the cases unfortunately resulted in a fatal outcome (13 out of 16). In the presented case, NaHCO3 improved the patient symptoms as it helped to correct blood acidosis. However, there has been a link to mortality in cancer-related acidosis,8 and NaHCO3 can increase acidosis if excess CO2 is not cleared effectively. Its use may help protect the heart from the impact of acidosis, until effective systemic therapy can be used, if this is an option.
Overall, this case highlights the potential complexity of clinical development of agents targeting tumour metabolism, the requirement for baseline metabolic assessment before treatment commences and the need for a multidisciplinary team to investigate and manage patients who develop complications on therapy.
Supplementary information
Acknowledgements
We acknowledge the support of the research nurses and clinical teams in caring for the patients on this trial.
Author contributions
R.M. and A.G. performed the literature review and compiled the case report. J.W., A.B., N.L. and F.J. aided in the care and investigation of the patient. C.B. performed and interpreted the immunohistochemical analysis of MCT-1 and MCT-4. G.P. performed and interpreted the radiological analysis. H.K. and A.S. performed and interpreted the urinary lactate analysis. S.H. and R.P. led the clinical development of AZD3965 and the investigation of this event. All authors contributed to and reviewed the final paper.
Ethics approval and consent to participate
The clinical trial of AZD3965 is ethically approved by the Newcastle and North Tyneside Regional Ethics Committee 1 (REC number 12/NE/0345) and run according to the standards of Good Clinical Practice as set out in the Declaration of Helsinki.
Consent to publish
The patient gave separate informed consent for publication of his case and the associated images.
Data availability
There are no raw data published in this paper.
Competing interests
The authors declare no competing interests.
Funding information
The AZD3965 clinical trial is sponsored and funded by the Cancer Research UK Centre for Drug Development. The patient was cared for at the Newcastle Experimental Cancer Medicine Centre, which is jointly funded by the Cancer Research UK and the Department of Health, UK (grant number RES/0190/7915). The urine lactate studies were performed at the Imperial College London Experimental Cancer Medicine Centre under a statement of work funded by CRUK (grant number CRUKD/12/004). Immunohistochemistry was performed in the MRC/EPSRC Newcastle Molecular Pathology Node under a Specialist Unit Agreement with CRUK.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rosie McNeillis, Alastair Greystoke
Change history
3/12/2020
A Correction to this paper has been published: 10.1038/s41416-020-0801-2
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-0727-8.
References
- 1.Ruiz JP, Singh AK, Hart P. Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. ScientificWorldJournal. 2011;11:1316–1324. doi: 10.1100/tsw.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groot R, Sprenger RA, Imholz AL, Gerding MN. Type B lactic acidosis in solid malignancies. Neth. J. Med. 2011;69:120–123. [PubMed] [Google Scholar]
- 4.DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.van der Mijn JC, Kuiper MJ, Siegert CEH, Wassenaar AE, van Noesel CJM, Ogilvie AC. Lactic acidosis in prostate cancer: consider the Warburg effect. Case Rep. Oncol. 2017;10:1085–1091. doi: 10.1159/000485242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Son YK, An WS. Effect of sodium bicarbonate administration on mortality in patients with lactic acidosis: a retrospective analysis. PLoS ONE. 2013;8:e65283. doi: 10.1371/journal.pone.0065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adeva-Andany MM, Fernandez-Fernandez C, Mourino-Bayolo D, Castro-Quintela E, Dominguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi: 10.1155/2014/627673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luft FC. Lactic acidosis update for critical care clinicians. J. Am. Soc. Nephrol. 2001;12(Suppl 17):S15–S19. doi: 10.1681/ASN.V12suppl_1s15. [DOI] [PubMed] [Google Scholar]
- 11.Gharia B, Seegobin K, Mahida H, Shaikh M, Matthews Hew T, Pham D. Fatal type B lactic acidosis associated with metastatic colorectal cancer: a case report with review of literature, pathogenesis, and treatment. J. Investig. Med. High. Impact Case Rep. 2018;6:2324709618788101. doi: 10.1177/2324709618788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch A, Lang SA, Wild PJ, Gantner S, Mahli A, Spanier G, et al. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget. 2015;6:32748–32760. doi: 10.18632/oncotarget.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, et al. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J. Biol. Chem. 2011;286:42626–42634. doi: 10.1074/jbc.M111.282046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratnikov BI, Scott DA, Osterman AL, Smith JW, Ronai ZA. Metabolic rewiring in melanoma. Oncogene. 2017;36:147–157. doi: 10.1038/onc.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer GM, Vashisht Gopal YN, McQuade JL, Peng W, DeBerardinis RJ, Davies MA. Metabolic strategies of melanoma cells: Mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma Res. 2018;31:11–30. doi: 10.1111/pcmr.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Kim HJ, Choi S, Kim CK, Lee NS, Lee KT, et al. A case of type B lactic acidosis in acute leukemia. Yonsei Med. J. 2010;51:460–462. doi: 10.3349/ymj.2010.51.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brault C, Zerbib Y, Delette C, Marc J, Gruson B, Marolleau JP, et al. The Warburg effect as a type B lactic acidosis in a patient with acute myeloid leukemia: a diagnostic challenge for clinicians. Front. Oncol. 2018;8:232. doi: 10.3389/fonc.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia P, Plumb TJ, Fillaus JA. Type B lactic acidosis associated with multiple myeloma. Am. J. Kidney Dis. 2013;62:633–637. doi: 10.1053/j.ajkd.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 19.McConnell AA, Parfitt VL, Walker PR. An unusual case of shock in a young woman. Postgrad. Med. J. 1989;65:120. doi: 10.1136/pgmj.65.760.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinoza AM, Venook AP. Lactic acidosis and colon cancer: oncologic emergency? Clin. Colorectal Cancer. 2011;10:194–197. doi: 10.1016/j.clcc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Krimmel JD, Packer CD. Type B lactic acidosis in a patient with gastric adenocarcinoma and extensive hepatic metastases. Med. Princ. Pract. 2015;24:391–393. doi: 10.1159/000430445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao KS, Mehta R, Ferlinz J. Unusual presentation of cancer-induced lactic acidosis. Postgrad. Med. J. 1988;64:475. doi: 10.1136/pgmj.64.752.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheriff DS. Lactic acidosis and small cell carcinoma of the lung. Postgrad. Med. J. 1986;62:297–298. doi: 10.1136/pgmj.62.726.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesbey G. Lactic acidosis in oat cell carcinoma with extensive hepatic metastases. Arch. Intern Med. 1981;141:816–817. doi: 10.1001/archinte.1981.00340060124034. [DOI] [PubMed] [Google Scholar]
- 25.Fujimura M, Shirasaki H, Kasahara K, Matsuda T. Small cell lung cancer accompanied by lactic acidosis and syndrome of inappropriate secretion of antidiuretic hormone. Lung Cancer. 1998;22:251–254. doi: 10.1016/S0169-5002(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 26.Oh DJ, Dinerman E, Matthews AH, Aron AW, Berg KM. Refractory lactic acidosis in small cell carcinoma of the lung. Case Rep. Crit. Care. 2017;2017:6148350. doi: 10.1155/2017/6148350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otten, M., Sepehrkhouy, S., van Everdingen, K. & Haas, L. Primary small cell carcinoma of the liver, a rare entity. BMJ Case Rep.2013, bcr2013201990 (2013). [DOI] [PMC free article] [PubMed]
- 28.Chau WK, Yang CF, Chou YH, Ho CH. Aggressive undifferentiated carcinoma of unknown primary site complicated by lactic acidosis after bleeding: a case report. Jpn. J. Clin. Oncol. 2002;32:210–214. doi: 10.1093/jjco/hyf050. [DOI] [PubMed] [Google Scholar]
- 29.El Imad T, El Khoury L, Geara AS. Warburg’s effect on solid tumors. Saudi J. Kidney Dis. Transpl. 2014;25:1270–1277. doi: 10.4103/1319-2442.144266. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JC, Esparza SD, Knez VM, Sakamoto KM, Moore TB. Severe lactic acidosis in a 14-year-old female with metastatic undifferentiated carcinoma of unknown primary. J. Pediatr. Hematol. Oncol. 2004;26:780–782. doi: 10.1097/00043426-200411000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no raw data published in this paper.