Abstract
Non-cariogenic sweet substances, like sugar alcohols, are used to decrease the risk of caries by reducing the growth of dental plaque. The aim of our study was to reveal the impact of xylitol and erythritol on the growth and biofilm formation of cariogenic bacteria including as a novelty, set of clinical mutans streptococci and Scardovia wiggsiae and to assess the possible synergistic influence of these polyols. We found both xylitol and erythritol to express high growth inhibition effect on cariogenic bacteria. In synergistic effect experiments, 10% polyol combination with excess of erythritol was found to be more effective against growth of Streptococcus mutans and the combination with excess of xylitol more effective against growth of Streptococcus sobrinus and S. wiggsiae. In biofilm inhibition experiments, solutions of 10% polyols in different combinations and 15% single polyols were equally effective against mutans streptococci. At the same time, higher biofilm formation of S. wiggsiae compared to experiments without polyols was detected in different polyol concentrations for up to 34%. In conclusion, both erythritol and xylitol as well as their combinations inhibit the growth of different cariogenic bacteria. Biofilm formation of mutans streptococci is also strongly inhibited. When applying polyols in caries prophylaxis, it is relevant to consider that the profile of pathogens in a particular patient may influence the effect of polyols used.
Subject terms: Pathogens, Biofilms, Oral diseases, Infection
Introduction
Contemporary theories support the role of non-cariogenic sweet substances, like sugar alcohols, as the one way to reduce the risk of caries. Clinical investigations have shown that xylitol (XYL), a pentitol type sugar alcohol, can be used as a safe and effective caries-limiting sweetener1. Regular use of oral hygiene adjuvants and xylitol-containing products has shown to inhibit the growth of caries-associated bacteria, to cut the growth of dental plaque and to decrease the incidence of dental caries1. There are numerous science-based regulatory propositions for the use of xylitol as a caries-limiting agent2.
Recently, a tetritol-type alditol, erythritol (ERY), has shown potential as a non-cariogenic sugar substitute. Erythritol is well tolerated in the gastrointestinal tract and well absorbed in small intestine but not metabolized in the body and thereby it is a non-caloric polyol. Erythritol has been shown to significantly and in larger extent than xylitol to reduce the dental plaque weight3,4. Erythritol’s mechanism of action is generally similar to xylitol and it is to reduce growth of the plaque-related biofilm, and streptococci do not produce neither lactic nor other acids from erythritol2,4–7.
Etiology of caries in humans is mostly associated with mutans streptococci, namely Streptococcus mutans and Streptococcus sobrinus8. In vitro experiments have shown direct inhibitory effect of erythritol and xylitol on the growth of mutans streptococci3,9. It has been supposed that combinations of xylitol and erythritol may reduce the incidence of caries more effectively than either alditol alone2, however, synergistic inhibitory effect of xylitol and erythritol on mutans streptococci needs to be confirmed first.
Additionally, the influence of polyols on etiological factors of caries has mainly been focused on mutans streptococci and no information about polyol effect on newly discovered oral pathogen associated with childhood caries, Scardovia wiggsiae10 is available so far. As mostly only type strains are used in experiments studying polyol efficacy the information about number of clinical strains are needed.
The aim of the present study was to reveal the effect of xylitol and erythritol on the growth and biofilm formation of cariogenic bacteria S. mutans, S. sobrinus and S. wiggsiae, also to assess the possible synergistic influence of these polyols.
Results
Cariogenic bacterial growth inhibition by different erythritol and xylitol concentrations
Inhibitory effect of different concentrations (15%, 7.5%, 3.75%, 1.9% and 0.9%) of polyols was tested on two clinical strains of S. sobrinus (S. sobrinus HUMB 13000, S. sobrinus HUMB 13104), two clinical strains of S. mutans (S. mutans HUMB 13076, S. mutans HUMB 13034) and S. wiggsiae DSM 22547 strain. Clinical strains were chosen on the basis of their origin - one adult and one child oral strain (Table 1). Individual strain data is provided in the supplementary materials (Supplementary Fig. 1a,b). Though the optical density was measured in different time points during the experiments the detectable growth was achieved only by 24 h (data not shown) and consequently 24 h data were analysed.
Table 1.
The list of bacterial strains used in the study.
| No | Microbe | Collection number | Origin of the strain |
|---|---|---|---|
| 1 | Streptococcus sobrinus | HUMB 13000 | oral cavity, child |
| 2 | Streptococcus sobrinus | HUMB 13087 | oral cavity, child |
| 3 | Streptococcus sobrinus | HUMB 13104 | oral cavity, adult |
| 4 | Streptococcus sobrinus | HUMB 13038 | oral cavity, adult |
| 5 | Streptococcus sobrinus | HUMB 13105 | oral cavity, adult |
| 6 | Streptococcus mutans | HUMB 13005 | oral cavity, child |
| 7 | Streptococcus mutans | HUMB 13076 | oral cavity, child |
| 8 | Streptococcus mutans | HUMB 13034 | oral cavity, adult |
| 9 | Streptococcus mutans | HUMB 13033 | oral cavity, adult |
| 10 | Streptococcus mutans | HUMB 13102 | oral cavity, adult |
| 11 | Scardovia wiggsiae | DSM 22547 | oral cavity, type strain |
Bacterial growth inhibition by different erythritol and xylitol concentrations was strain specific, but in average similarly high inhibitory effect (inhibition more than 40%) of both polyols at 15% concentration to all studied cariogenic bacteria (Fig. 1a,b) was found. The strains of S. mutans were more sensitive to polyols than two other cariogenic bacteria. The growth of S. mutans was inhibited up to 85% by 15% erythritol, more than 50% by 7.5% and also by 3.75% of erythritol and approx. 1/3 by 7.5% of xylitol (Supplementary Fig. 2a,b).
Figure 1.
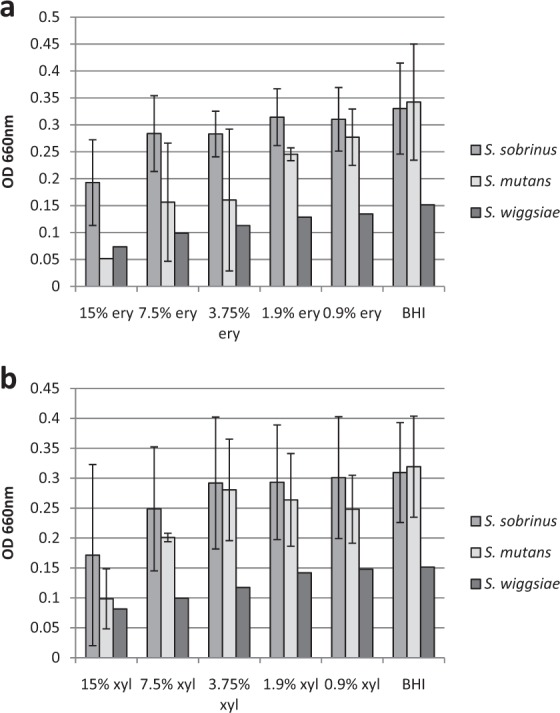
Inhibitory effect of erythritol (a) and xylitol (b) to the growth of studied two S. sobrinus, (S. sobrinus HUMB 13000, S. sobrinus HUMB 13104) two S. mutans (S. mutans HUMB 13076, S. mutans HUMB 13034) and S. wiggsiae DSM 22547 strain after 24 h incubation. The growth was measured spectrophotometrically by determining the optical density at 660 nm. Average of two median results from triplicate experiments and standard deviations of the studied strains in are presented.
Synergistic effect of polyols on growth inhibition of cariogenic bacteria
According to the bacterial growth inhibition test results, 10% of final polyol concentration was chosen for studying synergistic effect of polyols. The polyols were used in different combinations and two concentrations of triclosan were used as controls.
In these experiments, five clinical strains of S. sobrinus (S. sobrinus HUMB 13000, HUMB 13087, HUMB 13104, HUMB 13038, HUMB 13105) and five clinical strains of S. mutans (S. mutans HUMB 13005, HUMB 13076, HUMB 13034, HUMB 13033, HUMB 13102) and additionally S. wiggsiae DSM 22547 strain (Table 1) were used in order to get more generalized view. Inter-strain variability (shown as SD) in the growth inhibition by polyols is presented in Fig. 2. Erythritol and xylitol, in all studied combinations and individually, inhibited the growth of cariogenic bacteria compared to BHI. Both polyols in 15% of concentrations were the most effective.
Figure 2.
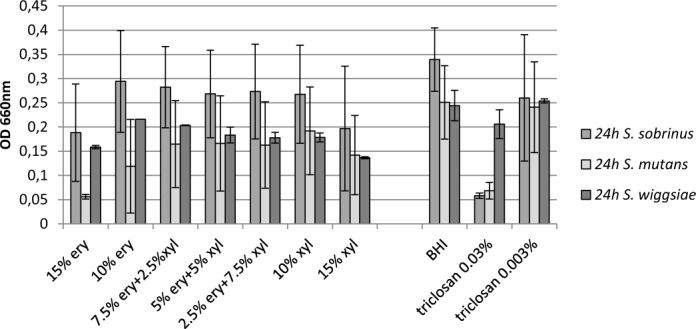
Synergistic effect of polyols and triclosan on the growth of 5 S. sobrinus (HUMB 13000, HUMB 13087, HUMB 13104, HUMB 13038, HUMB 13105), 5 S. mutans (HUMB 13005, HUMB 13076, HUMB 13034, HUMB 13033, HUMB 13102) and S. wiggsiae DSM 22547 strains after 24 h incubation. The growth was measured spectrophotometrically by determining the optical density at 660 nm. Average of two median results from triplicate experiments and standard deviations of the studied strains in are presented.
Within the three combinations tested (7.5% ERY + 2.5% XYL, 5% ERY + 5% XYL and 2.5% ERY + 7.5% XYL), the effect on S. mutans, S. sobrinus and S. wiggsiae remained relatively consistent (Fig. 2). We found 10% polyol combination with excess of erythritol to be more effective against S. mutans and the combination with excess of xylitol more effective against S. sobrinus and S. wiggsiae (Fig. 2). Still, 15% of polyol concentration showed higher inhibitory effect on the cariogenic bacteria than any of polyol 10% combinations had). The solution of 0.03% of triclosan, served as a positive control, had high effect (growth inhibition more than 70%) against mutans streptococci but not against S. wiggsiae (Supplementary Fig. 3). At the same time, the lower triclosan concentration (0.003%) did not have growth inhibitory effect against cariogenic bacteria.
Inhibition of biofilm formation
The biofilm inhibition of cariogenic bacteria by polyols was strain specific. The results of the biofilm inhibition tests of the cariogenic bacteria are presented in Fig. 3. Solutions of 10% polyols in different combinations and 15% single polyols were equally effective against mutans streptococci (biofilm inhibition of S. sobrinus 57–69% and for S. mutans 63–85%; Supplementary Fig. 4). On the contrary, we found higher biofilm formation of S. wiggsiae compared to BHI in different polyol concentrations for 1–34%. Biofilm formation of S. wiggsiae was increased in the 10% polyol combinations for 2–15%, in 10% single polyol solutions for 1–24% and in 15% single polyol solution for 8–34%. (Fig. 3, Supplementary Fig. 4).
Figure 3.
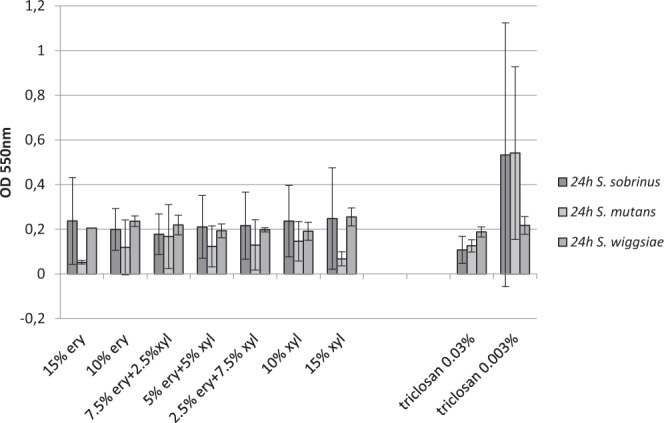
Synergistic effect of polyols and triclosan on the inhibition of biofilm of 5 S. sobrinus (HUMB 13000, HUMB 13087, HUMB 13104, HUMB 13038, HUMB 13105), 5 S. mutans (HUMB 13005, HUMB 13076, HUMB 13034, HUMB 13033, HUMB 13102) and S. wiggsiae DSM 22547 strains after 24 h incubation. The biofilm was measured spectrophotometrically by determining the optical density at 550 nm. Average of two median results from triplicate experiments and standard deviations of the studied strains in are presented.
The control solution, 0.03% triclosan, inhibited biofilm formation of mutans streptococci (85% for S. sobrinus and 66% for S. mutans) while had no effect on S. wiggsiae. The 0.003% triclosan solution had moderate inhibitory effect (36%) on S. sobrinus, but biofilm-supporting effect on S. mutans (−5%) and S. wiggsiae (−14%).
Altogether, 10% polyol concentrations like also 15%, were effective against mutans streptococci, inhibiting their growth and biofilm formation. The growth of S. wiggsiae was also inhibited by polyols, but at the same time biofilm formation was increased.
Discussion
Our experiments showed consistently growth inhibitory effect of polyols, both singularly and in combinations, on the set of clinical strains of mutans streptococci and newly detected cariogenic species S. wiggsiae. At the same time, the inhibition of biofilm formation by polyols was dependent on the type of organism and the polyol(s) studied. For example, inhibition of biofilm formation was clearly evident in case of mutans streptococci and mostly missing or even biofilm enhancing in case of S. wiggsiae.
Xylitol was one of the first sugar alcohol acknowledged by its effect against cariogenic bacteria in 1970ies. In recent years another polyol, erythritol, has been found to possess similar effect4. In concordance with previous studies11, we found that S. mutans was more sensitive to polyols than other studied cariogenic bacteria (S.sobrinus and S. wiggsiae). Though even very low (0.01%) polyol concentrations are able to inhibit the growth of mutans streptococci12, we found high polyol concentrations (15%) to be the most effective in growth inhibition.
Singularly, both erythritol and xylitol were found to be effective in the growth inhibition of the cariogenic bacteria in our study although strain-specific differences existed. Differences between individual strains of cariogenic bacteria have also been found in previous studies4,11. Accordingly, several clinical isolates of different hosts were used for our experiments to get more reliable results. Inhibition of biofilm formation in case of mutans streptococci was high and even more expressed than growth inhibition. No association between magnitudes of inhibition of growth and adherence was found previously11. At the same time, we found that although the growth inhibition of S. wiggsiae by polyols was significant, the biofilm formation was even increased. Söderling & Hietala-Lenkkeri11 presented similar results where erythritol decreased glass surface adherence of mutans streptococci, with the exception of S. salivarius in which case the adherence was increased.
Several compounds have been found to be synergistic with xylitol in inhibiting biofilm formation of mutans streptococci13,14. In our study, we explored the potential synergistic effect of xylitol and erythritol. In that regard, we used xylitol and erythritol combinations in 10% final concentration as we found it to be optimal against mutans streptococci, inhibiting their growth and biofilm formation. At the same time, all 10% polyol combinations had lower growth inhibition effect on the cariogenic bacteria than high (15%) individual polyol concentrations, but probably more difficult to achieve in oral cavity. The polyol combinations with higher proportion of erythritol were more active in growth inhibition of S. mutans. Similar implication about erythritol being more effective than xylitol against S. mutans induced dental problems has been made by de Cock et al.15,16. At the same time the combination with higher proportion of xylitol were more active in growth inhibition of S. sobrinus and S. wiggsiae. Given the emerging role of S. wiggsiae in advanced and initial caries17, this observed trade off might be relevant to take into account when choosing the use of different individual or combinations of polyols for applications. No clear synergistic effects were found for inhibition of growth and biofilm formation concerning the studied cariogenic bacteria. Overall, based on the various effects on distinct species of cariogenic bacteria by erythritol, xylitol and their combinations, personalized use of polyols may be suggested according to patient’s existing pathogens.
Triclosan is a broad-spectrum antibacterial agent widely used in hygiene especially oral care products. The solution of 0.03% of triclosan had high inhibitory effect on the growth and biofilm formation of mutans streptococci. The inhibitory effect was comparable to high polyol concentrations. At the same time, no significant effect on the growth or biofilm formation of 0.03% triclosan on S. wiggsiae was found. The lower, 0.003% triclosan concentration showed positive inhibition effect on S. sobrinus biofilm formation, but slight biofilm enhancing effect on S. mutans and S. wiggsiae. Although antibacterial effect of triclosan on S. sobrinus and S. mutans is widely acknowledged18,19 its effect on biofilm formation is not fully investigated. Sub-inhibitory triclosan concentrations have shown to enhance the biofilm formation of S. mutans18 but to our best knowledge no respective results on S. sobrinus and S. wiggsiae have been published before.
Limitation of our study include moderate number of tested strains and lack of type strains in case of mutans streptococci. At the same time, our study included two different polyols and three different species of cariogenic bacteria, including also novel species S. wiggsiae.
Conclusions
Both erythritol and xylitol as well as their combinations inhibit the growth of clinical strains of mutans streptococci and S. wiggsiae, newly recognized cariogenic bacterium. Biofilm formation of mutans streptococci is also strongly inhibited. When applying polyols (singularly or in combinations) in caries prophylaxis, it is relevant to consider the profile of pathogens in a particular patient and thus optimize the choice of polyols based on this profile.
Materials and Methods
Chemicals and strains
Erythritol (Cargill R&D Centre Europe, Vilvoorde, Belgium) and xylitol (≥99%, Sigma-Aldrich Co, St. Louis, USA) were tested.
Altogether 11 cariogenic bacteria (5 Streptococcus sobrinus, 5 Streptococcus mutans clinical isolates and 1 Scardovia wiggsiae DSM 22547 type strain; Table 1) were included into the study. The clinical isolates were acquired from the Human Microbiota Biobank of Tartu (HUMB; http://www.eemb.ut.ee/eng/).
Bacterial growth inhibition by different polyol concentrations
The effect of polyols was studied using slightly modified method previously described by Mäkinen, et al.2,4. Briefly, the tested substances were sterilized by filtration at desired concentration and added to the brain-heart infusion (BHI, Oxoid Limited, Basingstoke, Hampshire, UK) medium (sterilization by autoclaving at 121 °C for 15 min). The bacteria were incubated in aerobic conditions at 37 °C for 24 hours on blood agar (Oxoid Limited, Basingstoke, Hampshire, UK) (S. mutans, S. sobrinus) or in anaerobic conditions with gases: 90%N, 5%CO2, 5% H2 (Whitley Anaerobic Workstations A35, Don Whitley Scientific Limited, Bingley, West Yorkshire, UK) at 37 °C for 24 hours on fastidious anaerobe agar (S. wiggsiae) (LAB090, Lab M Limited, Heywood, Lancashire, UK). The microtiter plate wells (CELLSTAR, 96 well polystyrene suspension culture microplates, F-bottom, Greiner Bio-One GmbH, Kremsmünster, Austria) were inoculated with equal amounts (200 µl) of bacterial (the final test-concentration of 105 CFU/ml) and polyol solution. The bacterial cells were grown in aerobic or anaerobic conditions at 37 °C on the microtiter plate, monitoring the growth up to the late exponential growth phase. The density of bacterial growth was detected spectrophotometrically in absorbance microplate reader (Sunrise, Tecan Group Ltd., Männedorf, Switzerland) at 660 nm. The time points for measuring were 0, 1, 2, 4, 6 and 24 hours.
The cultivations were carried out with all organisms, using the polyol concentrations as indicated in Table 2 as well as negative (BHI) and positive (triclosane) controls. All bacterial strains were tested in 3 repeats for 2 times.
Table 2.
Polyol and triclosan concentrations used in the study.
| Inhibition study | Synergistic study | Biofilm study | ||||
|---|---|---|---|---|---|---|
| Polyol/triclosan concentration (%) | ||||||
| Cariogenic pathogens | ERY | XYL | Polyol | Triclosan | Polyol | Triclosan |
| S. sobrinus, S mutans, S. wiggsiae | 15 | 15 | 15% ERY | 0.03 | 15% ERY | 0.03 |
| 7.5 | 7.5 | 10% ERY | 0.003 | 10% ERY | 0.003 | |
| 3.75 | 3.75 | 7.5% ERY + 2.5%XYL | 7.5% ERY + 2.5%XYL | |||
| 1.9 | 1.9 | 5% ERY + 5% XYL | 5% ERY + 5% XYL | |||
| 0.9 | 0.9 | 2.5% ERY + 7.5% XYL | 2.5% ERY + 7.5% XYL | |||
| 10% XYL | 10% XYL | |||||
| 15% XYL | 15% XYL | |||||
ERY erythritol, XYL xylitol.
The tested polyol concentrations (weight/volume) included 15%, 7.5%, 3.75%, 1.9%, and 0.9% (Table 2). The growth inhibition percentages were calculated as ratios between average tests and BHI values.
Synergistic effect of polyols on growth inhibition of cariogenic bacteria in vitro
After defining the inhibitory effect of erythritol and xylitol in total of 5 concentrations for S. wiggsiae and two strains of S. sobrinus and S. mutans, the most optimal summary polyol concentration was defined and used in further studies in different proportions (Table 2).
In addition, growth inhibition capacity of triclosan as positive control was tested in two concentrations (0.03% and 0.003%)18. The growth inhibition percentages were calculated as ratios between average tests and BHI values.
Inhibition of biofilm formation testing
Inhibition of bacterial biofilm production by polyols in BHI solution was measured using a semi-quantitative adherence assay on 96-well microtiter plates (CELLSTAR, 96 well polystyrene suspension culture microplates, F-bottom)20,21. The bacteria were cultivated at 37 °C for 24 h on blood agar (S. mutans, S. sobrinus) or fastidious anaerobe agar (S. wiggsiae). The microtiter plate wells were inoculated with equal amounts (200 µl) of bacterial (the final test-concentration of 105 CFU/ml) and polyol solution. The plates were covered and incubated aerobically or anaerobically (90%N, 5%CO2, 5% H2) at 37 °C for 24 h. At the end of incubation, the liquid in the wells was poured out and each well was washed three times with 0.25 ml of sterile phosphate buffered saline (PBS).
Plates were stained for 10 min with 0.1 ml of 0.04% crystal violet per well. The wells were washed four times with distilled water to remove the unbound crystal violet dye and dried for 2 h at 37 °C. After adding 0.1 mL of 95% (v/v) ethanol to each well, optical density (OD) in absorbance microplate reader at 550 nm was measured with the preceding shaking of 5 min with the amplitude of 5 mm to release the stain from the biofilms.
Inhibition of biofilm of cariogenic bacteria was tested using different polyol concentrations alone and in combinations (XYL 15%, XYL 10%, XYL 7.5% + ERY 2.5%, XYL 5% + ERY 5%, XYL 2.5% + ERY 7.5%, ERY 10%, ERY 15%) (Table 2). Biofilm inhibition capacity of triclosan as positive control was tested in two concentrations (0.03% and 0.003%)18. All bacterial strains were tested in 3 repeats for 2 times.
The biofilm inhibition percentages were calculated as ratios between tests averages and BHI values.
Statistical analysis
All experiments were performed in triplicate and repeated twice. Each value represents the average of two median results from triplicate experiments.
Supplementary information
Acknowledgements
This study was supported by Estonian Research Council (grant No. IUT34–19) and Estonian Ministry of Education and Research (grant No. KOGU-HUMB). The authors are thankful to Joan Vermeiren and Peter de Cock for fruitful scientific discussion and to Dagmar Hoidmets for technical assistance.
Author contributions
S.K. contributed in study design, data analysis, writing the paper. I.S. contributed in study design, data analysis, statistical analysis. A.C. contributed in data analysis, writing the paper. D.B. contributed in data analysis, writing the paper. R.M. contributed in study design, data analysis, writing the paper. All authors have read and approved the final version of this manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
Anirikh Chakrabarti and Douwina Bosscher were employed by Cargill during the preparation of this manuscript, and Cargill produced erythritol. No potential conflict of interest was reported by other authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63153-x.
References
- 1.Janakiram C, Deepan Kumar CV, Joseph J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J Nat Sci Biol Med. 2017;8:16–21. doi: 10.4103/0976-9668.198344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäkinen KK. Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med Princ Pract. 2011;20:303–320. doi: 10.1159/000324534. [DOI] [PubMed] [Google Scholar]
- 3.Runnel R, et al. Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J Dent. 2013;41:1236–1244. doi: 10.1016/j.jdent.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Mäkinen KK, et al. Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res. 2005;39:207–215. doi: 10.1159/000084800. [DOI] [PubMed] [Google Scholar]
- 5.Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T. Noncariogenicity of erythritol as a substrate. Caries Res. 1992;26:358–362. doi: 10.1159/000261468. [DOI] [PubMed] [Google Scholar]
- 6.Hashino E, et al. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:435–451. doi: 10.1111/omi.12037. [DOI] [PubMed] [Google Scholar]
- 7.Janus MM, et al. Effect of erythritol on microbial ecology of in vitro gingivitis biofilms. J Oral Microbiol. 2017;9:1337477. doi: 10.1080/20002297.2017.1337477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banas JA, Drake DR. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health. 2018;18:129. doi: 10.1186/s12903-018-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganter J, Hellwig E, Doerken S, Al-Ahmad A. In vitro evaluation of the cariogenic potential of rebaudioside A compared to sucrose and xylitol. Clin Oral Investig. 2020;24:113–122. doi: 10.1007/s00784-019-02908-x. [DOI] [PubMed] [Google Scholar]
- 10.Tanner AC, et al. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söderling EM, Hietala-Lenkkeri AM. Xylitol and erythritol decrease adherence of polysaccharide-producing oral streptococci. Curr Microbiol. 2010;60:25–29. doi: 10.1007/s00284-009-9496-6. [DOI] [PubMed] [Google Scholar]
- 12.Söderling EM, Ekman TC, Taipale TJ. Growth inhibition of Streptococcus mutans with low xylitol concentrations. Curr Microbiol. 2008;56:382–385. doi: 10.1007/s00284-007-9076-6. [DOI] [PubMed] [Google Scholar]
- 13.Zou Y, Lee Y, Huh J, Park JW. Synergistic effect of xylitol and ursolic acid combination on oral biofilms. Restor Dent Endod. 2014;39:288–295. doi: 10.5395/rde.2014.39.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ, et al. Synergistic inhibition of Streptococcal biofilm by ribose and xylitol. Arch Oral Biol. 2015;60:304–312. doi: 10.1016/j.archoralbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.de Cock P. Erythritol Functional Roles in Oral-Systemic Health. Adv Dent Res. 2018;29:104–109. doi: 10.1177/0022034517736499. [DOI] [PubMed] [Google Scholar]
- 16.de Cock P, et al. Erythritol Is More Effective Than Xylitol and Sorbitol in Managing Oral Health Endpoints. Int J Dent. 2016;2016:9868421. doi: 10.1155/2016/9868421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kressirer CA, et al. Scardovia wiggsiae and its potential role as a caries pathogen. J Oral Biosci. 2017;59:135–141. doi: 10.1016/j.job.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedran TB, Grignon L, Spolidorio DP, Grenier D. Subinhibitory concentrations of triclosan promote Streptococcus mutans biofilm formation and adherence to oral epithelial cells. PLoS One. 2014;9:e89059. doi: 10.1371/journal.pone.0089059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobos O, Padilla A, Padilla C. In vitro antimicrobial effect of bacteriocin PsVP-10 in combination with chlorhexidine and triclosan against Streptococcus mutans and Streptococcus sobrinus strains. Arch Oral Biol. 2009;54:230–234. doi: 10.1016/j.archoralbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68:2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakeri S, Kermanshahi RK, Moghaddam MM, Emtiazi G. Assessment of biofilm cell removal and killing and biocide efficacy using the microtiter plate test. Biofouling. 2007;23:79–86. doi: 10.1080/08927010701190011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


