Abstract
Programmed cell death ligands (PD‐Ls) are expressed in tumor cells where they bind to programmed cell death‐1, an immunocyte co–receptor, resulting in tumor cell evasion from the immune system. Chemotherapeutic drugs have been recently reported to induce the expression of PD‐L, such as PD‐L1, in some cancer cells. However, little is known regarding PD‐L2 expression and its role in oral squamous cell carcinoma (OSCC). In this study, we examined the effect of cisplatin on the expression and regulation of PD‐L2 in OSCC cell lines and analyzed malignant behavior in PD‐L2‐expressing cells using colony, transwell and transformation assays. In addition, we examined PD‐L2 expression in the tumor tissues of OSCC patients using cytology and tissue microarray methods. In OSCC cell lines, cisplatin treatment upregulated PD‐L2 expression, along with that of the drug efflux transporter ABCG2, via signal transducers and activator of transcription (STAT) 1/3 activation. Moreover, PD‐L2‐positive or PD‐L2‐overexpressing cells demonstrated upregulation in both invasion and transformation ability but not in proliferation compared with PD‐L2‐negative or PD‐L2‐silencing cells. PD‐L2 expression was also observed in OSCC cells of cytology samples and tissue from OSCC patients. The intensity of PD‐L2 expression was correlated with more malignant morphological features in the histological appearance and an invasive pattern. Our findings indicate that cisplatin‐upregulated PD‐L2 expression in OSCC via STAT1/3 activation and the expression of PD‐L2 are likely to be associated with malignancy in OSCC. The PD‐L2 expression in cisplatin‐resistant OSCC cells may be a critical factor in prognosis of advanced OSCC patients.
Keywords: cisplatin, metastasis, oral squamous cell carcinoma, programmed cell death ligand 2, signal transducers and activator of transcription 1/3
Our findings indicate that cisplatin‐upregulated PD‐L2 expression in oral squamous cell carcinoma (OSCC) cells via STAT1/3 activation and the expression of PD‐L2 are likely to be associated with malignancy in OSCC. The PD‐L2 expression in cisplatin‐resistant OSCC cells may be a critical factor in the prognosis of advanced OSCC patients.
1. INTRODUCTION
Programmed cell death (PD)‐1 is an immune co–receptor that has been well characterized as a negative regulatory molecule of immunocytes at immune checkpoints. PD‐1 ligands belong to the B7 family of molecules and include PD‐L1 (B7‐H1) and PD‐L2 (B7‐DC).1, 2 PD‐L1 is widely expressed in a variety of immunocytes, including T cells, B cells, regulatory T cells, natural killer T cells, dendritic cells (DC) and tumor cells.3, 4 In tumor cells, PD‐L1 binds to PD‐1 on T cells and suppresses their immune function, which leads to the promotion of tumor growth, metastasis and cachexia.5 Indeed, immunotherapy using anti–PD‐1 antibodies has been reported to prevent immune tumor evasion from the immune system in patients with various solid malignancies, allowing immunocytes to appropriately attack the tumor cells.6
Programmed cell death‐2 is expressed in antigen‐presenting cells, including DC, macrophages, B cells and tumor cells.7, 8 Like PD‐L1, PD‐L2 has been reported to bind to PD‐1, resulting in the evasion of tumor cells from the immune system.9, 10
It has recently been revealed that the distribution of programmed cell death ligands (PD‐L) differs depending on the type of head and neck squamous cell carcinoma (HNSCC).11, 12 In HNSCC patients, PD‐L2 expression has been reported to be greater than that of PD‐L1.13 In addition, PD‐1/PD‐L2 interactions have been reported to have different association/dissociation kinetics, exhibiting a 2‐6‐fold higher affinity compared with PD‐1/PD‐L1 interactions.14 However, it remains unclear whether PD‐L2 expression and the ratio of PD‐L2/PD‐L1 expression play an important role in HNSCC.
Squamous cell carcinomas (SCC) comprise over 95% of malignant tumors in the head and neck region, including the oral cavity. Oral squamous cell carcinoma (OSCC) is the most common malignancy of the maxillofacial region, accounting for nearly 3% of all cancer cases worldwide.15 OSCC severely impairs patient quality of life because of the associated damage to speech, swallowing and mastication. However, the survival of patients with metastatic OSCC has significantly improved with treatments involving platinum‐based salt drugs and, more recently, immune checkpoint targeting therapies. Indeed, the inhibition of PD‐1/PD‐L1 binding using anti–PD‐1 antibodies has been reported to result in increased disease control and survival rates as compared with conventional chemotherapy alone.16, 17 Despite these therapeutic advances, the mortality of OSCC patient remains high due to the development of distant metastases and local and systemic recurrences, which are resistant to both chemotherapy and radiotherapy.18
Several studies have evaluated the ability of chemotherapy, including anti–tumor drugs such as cisplatin, to modulate tumor immunogenicity and to influence adaptive immunity.19 From these studies, it has recently been reported that cisplatin induces the expression of PD‐L1 in bladder cancer and non–small cell lung cancer.20, 21 However, the expression and regulatory mechanisms of PD‐L2 have not been evaluated in OSCC.
Therefore, in the present study, we evaluated the effect of cisplatin on the expression of PD‐L2 in OSCC cell lines. In addition, we evaluated whether PD‐L2 expression was involved in the malignancies observed in cisplatin‐resistant OSCC cell lines. Furthermore, PD‐L2 expression in the tumor tissues from OSCC patients was determined using cytology and tissue microarray methods.
2. MATERIALS AND METHODS
2.1. Ethics statement
This study was approved by the Ethics Review Board of Fukuoka Dental College (approval number: 432). All studies involving human participants were conducted in full compliance with the Declaration of Helsinki. All participants completed an informed consent form.
2.2. Cell culture
Human oral squamous carcinoma cells HSC‐2 and HSC‐3 were obtained from the Cell Resource Center, Institute of Developmental, Aging and Cancer, Tohoku University (Sendai, Japan). Cells were seeded at a density of 1 × 105 cells/mL and cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 in DMEM (Wako) supplemented with 10% FBS (Sigma‐Aldrich). Upon reaching sub‐confluence, cells were incubated in culture medium with or without cisplatin [cis‐Diammineplatinum(II)] dichloride (1‐5 μmol/L); Sigma‐Aldrich) for 0‐72 hours. In some experiments, the cells were incubated in culture medium containing cisplatin and fludarabine (10 μmol/L; STAT1 inhibitor; Sigma‐Aldrich) or cryptotanshinone (10 μmol/L; STAT3 inhibitor; Sigma‐Aldrich).
2.3. Cellular proliferation assay
Cellular proliferation was measured by using a Cell Counting Kit‐8 solution (CCK‐8; Dojindo Laboratories). Briefly, the cells were seeded in 96‐well plates at a density of 1000 cells/well in 100 μL D‐MEM. At 0, 24, 48 or 72 hour after cisplatin treatment, 10 μL of CCK8 reagent was added to each well and the plates were incubated at 37°C for 1 hour. Absorbance was read at 450 nm using a microplate reader (Mode 680; Bio‐Rad). Culture solution containing CCK‐8 reagent but without cells was used as a blank control.
2.4. RNA isolation and quantitative RT‐PCR
Total RNA was extracted from the cells using TRIzol Reagent. First‐strand cDNA was synthesized from 3 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen, Thermo Fisher Scientific). To detect mRNA expression, we selected gene‐specific primers based on the nucleotide sequence of the cDNA. QRT‐PCR analyses of the targeted mRNA were performed using the SYBR Prime Script RT‐PCR Kit with an ABI 7500 Real‐Time PCR System (Applied Biosystems). Reaction conditions were as follows: an initial step at 95°C for 30 seconds, followed by 40 cycles of denaturation at 95°C for 5 seconds, annealing at 60°C for 10 seconds, and extension at 72°C for 35 seconds. β‐actin was used as an internal standard to control for amplification variability due to differences in starting mRNA concentrations. The threshold cycle (Ct) was defined as the fractional cycle number. The gene expression level was expressed relative to that of β‐actin and the delta (delta Ct) method was used to calculate the fold change for each sample.
2.5. Western blot analysis
Cells were lysed in TNT buffer (Roche). Protein content was measured with a protein assay kit (Thermo Scientific). Briefly, 20 µg of each protein was subjected to 10.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Separated proteins were then electrophoretically transferred to PVDF membranes at 75 V for 1.5 hour at 4°C. The membranes were then incubated overnight with antibodies against the target molecules in a 5% skimmed milk solution at 4°C (Table S1). Blots were then washed in TBST (10 mmol/L tris–HCl, 50 mmol/L, NaCl 0.25% Tween‐20), incubated for 1 hour with HRP‐conjugated anti–rabbit or mouse IgG secondary antibodies, and developed using an enhanced chemiluminescence system (GE Healthcare). In some experiments, cellular total proteins were isolated as the membrane and cytoplasm fractions using the Mem‐PER Plus Membrane Protein Extraction Kit (Thermo Scientific). Briefly, cells were washed with wash buffer, and 0.75 mL of permeabilization buffer containing protease inhibitors was added. Cell suspensions were centrifuged at 16 000 g for 15 minutes at 4°C; the collected supernatant contained the cytosolic proteins. Membrane‐enriched pellets were incubated for 30 minutes with solubilization buffer and centrifuged at the same condition; the collected supernatant contained the membrane fraction.
2.6. Flow cytometry analysis and cell sorting
Cells were washed twice with PBS after treatment with Fc Receptor Blocking Solution (Human TruStain FcX; BioLegend) and incubated with the cell surface antigen of PD‐L2 (CD273) conjugated with phycoerythrin (PE, BioLegend) or ABCG2 (CD338) conjugated with PE‐Cy5 (BioLegend). The labeled cells were analyzed by flow cytometry analysis using the On‐chip system (On‐chip Biotechnologies). The ratio of each antibody‐positive cell to the total cells was quantified using the associated analysis software. In some experiments, PD‐L2‐positive or negative cells were sorted and collected using fluorescence‐activated cell sorting.
2.7. Colony assay
Cells were seeded at a low density of 1 × 103 cells/mL and cultured at 37°C in 100‐mm culture dishes. After 10 and 13 days, the colonies that were forming were stained with crystal biored and stained colonies were counted.
2.8. Transwell invasion assays
Cells were seeded onto 24‐well plates (6.5‐mm diameter; 8‐μm pore size chamber inserts; Corning, USA) for cell invasion assays. Briefly, cells were added to the upper collagen‐coated chamber of the transwell insert (1 × 103 cells/well). After 24 and 48 hours of incubation, the cells that remained at the top of the inserts were removed. Invasive cells that were present on the lower surface of the inserts were fixed with methanol and stained with calcein‐AM (Dojindo) for 15 minutes. The number of invasive cells was counted under a fluorescent microscope. Data were expressed as the average number of cells/transwell ± SD.
2.9. Transformation assay
Transforming assays were performed using Cytoselect 96‐well transforming plates in conjunction with a Soft Agar Colony Formation Kit (Cell Biolabs). Briefly, cell suspensions at a density of 1 × 104 cells/mL were mixed with an agar solution. The culture medium containing the mixed cell suspension was then incubated in 96‐well plates (100 μL/well) for 10 days at 37°C and 5% CO2. The formation of cell colonies was examined using a light microscope. After removal of the culture medium, lysis buffer was added to the wells, which were incubated for 15 minutes. The fluorescence at 520 nm excited at 480 nm was measued for colony formation in the agar floating culture using a microplate reader (Mode 680; Bio‐Rad).
2.10. Immunochemistry
Immunohistochemistry was performed for tissue microarray sections (Cat. No. OR208 US Biomax) using the Histofine Simple Stain MAX‐PO(R) kit (Nichirei). Briefly, antigen retrieval was performed by autoclave treatment and endogenous peroxidase activity was blocked by treatment with H2O2. Following incubation with anti–human PD‐L2 antibody (Cell Signaling Technology) then a secondary antibody (Nichirei), the tissue microarray sections were visualized using a DAB substrate kit (Nichirei), before counterstaining with hematoxylin. As a negative control, staining was performed without any primary antibody. The tissue microarray sections were independently examined by two researchers, including a pathologist. The PD‐L2 staining intensity of each tumor cell was classified into four levels relative to that of infiltrating macrophages as internal control in the same section (Figure S1): negative, no specific staining; low, weakly stained tumor cell; intermediate, moderately stained tumor cell; and high, strongly stained tumor cells. The histological grading (differentiation degree) and Yamamoto‐Kohama (YK)‐classification (invasive pattern) were also determined and the invasive pattern was categorized into three types: expansive type (YK‐1 and 2), intermediate type (YK‐3) and invasive type (YK‐4C and ‐4D). As for differentiation degree, we evaluated the morphological features in the observed tissue microarray spots rather than the information from the data sheet because the expression of PD‐L2 was heterogeneous in the same tumor nest.
For immunocytochemistry, cells cultured on coverslips were fixed with 4% paraformaldehyde and permeabilized by incubation with 0.2% Triton X‐100 in PBS. Following incubation with the primary antibodies, the antibody bindings were visualized using anti–mouse immunoglobulin G conjugated with Alexa Fluor 488 (1:200; Molecular Probes). Immunostained cells were then counterstained with DAPI (Vector Laboratories).
2.11. Collection of exfoliative cells and papanicolaou staining
Oral smears were taken from buccal sites in patients with OSCC using the EndoCervix‐Brush (Rover Medical Devices, KV Oss). Intraoperative cytology was performed on patients diagnosed with OSCC before surgery. Only specimens that were diagnosed as OSCC from postoperative pathology were used. In addition, the Japanese Society of Oral Surgery Oral Cancer Practice Guidelines, 2019 edition (draft), was used to provide guidelines for the diagnosis of OSCC. The brushes were immersed and washed in CytoRich Red Fluid to scatter the cells in the solution. After centrifugation, cell pellets were suspended in distilled water. Cell suspensions were refixed in 95% ethanol. Papanicolaou staining was used for cytopathology. The collected cells were stained using the same method as described for immunocytochemistry.
2.12. Silencing and overexpressing of programmed cell death ligands in HSC‐2
Silencer siRNA for PDCD1LG2 (B7DC) gene, 5′‐CCUAAGGAACUGUACAUAAtt‐3′, was purchased from Thermo Fisher Scientific. After culturing with 1 × 105 cells/mL in six‐well plates for 1 day, the cells were transfected with 40 nmol/L siRNA using the Lipofectamine RNAi MAX Reagent (Thermo Fisher Scientific) in accordance with the manufacturer's protocol. For the control transfection, Silencer Select Negative Control No. 1 siRNA (Thermo Fisher Scientific) was used.
The nucleotide coding sequences of human PD‐L2 were amplified by PCR using the human HSC‐2 cDNA as a template and the following PD‐L2 specific primers: sense 5′ primer AAC ATG ATC TTC CTC CTG CTA ATG T and antisense 3′ primer TCA GAT AGC ACT GTT CAC TTC CC. The plasmid was linearized by digestion with KpnI and XbaI, and the amplified cDNA (822bp) was inserted into p3xFlag‐CMV‐7.1 (Sigma‐Aldrich) using DNA ligase. The nucleotide sequences of the PCR‐amplified cDNA were confirmed by DNA sequencing. The plasmids were transfected into the cells using Lipofectamine 3000 (Thermo Fisher Scientific). We used western blot analysis to validate the expression level of PD‐L2 in transfected HSC‐2 cells with expressing plasmid or siRNA.
2.13. Statistical analyses
Data are expressed as the mean ± standard error of the mean (SEM). Differences were analyzed by one‐way analysis of variance, χ2 test and Scheffe's multiple comparison test. P‐values < 0.05 were considered significant.
3. RESULTS
3.1. Upregulation of programmed cell death ligand 2 and ABCG2 in cisplatin‐resistant HSC‐2 cells
To clarify the resistance of HSC‐2 cells to cisplatin, we first examined the effect of cisplatin on cell viability using the CCK assay (Figure 1A). HSC‐2 cells without cisplatin dramatically proliferated in a time‐dependent manner. However, after cisplatin treatment, the proliferation of HSC‐2 cells was suppressed in a dose‐dependent manner. The number of viable cells with cisplatin remained constant or was slightly decreased at cisplatin concentrations of 2 to 5 μmol/L. These HSC‐2 cells that survived with cisplatin treatment were considered a subset of cisplatin‐resistant HSC‐2 cells.
Figure 1.
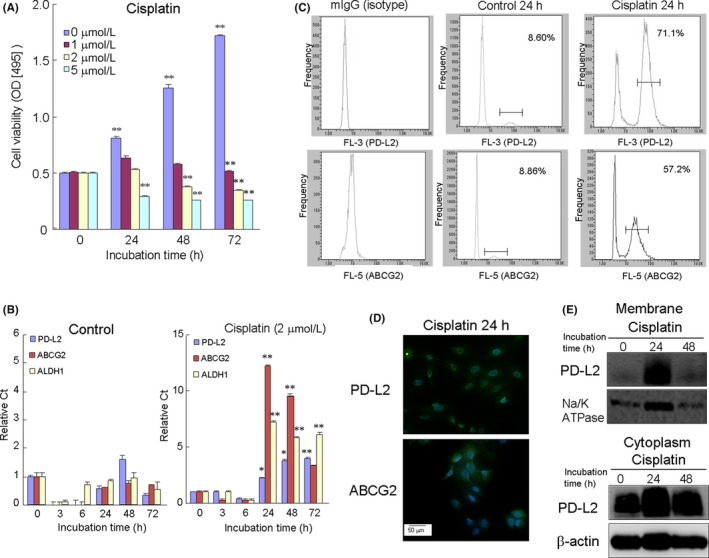
Cisplatin‐induced programmed death‐ligand 2 (PD‐L2) expression in anti–tumor drug‐resistant HSC‐2 cells. A, HSC‐2 cells were incubated with various concentrations of cisplatin for 0‐72 h. After cisplatin treatment, the number of viable cells was determined by the CCK8 assay. B, Expression of PD‐L2, ABCG2 and ALDH1 genes in HSC‐2 cells with or without cisplatin treatment (2 μmol/L). Samples were analyzed by RT‐PCR. Data shown are the means from seven culture wells (mean ± SEM). * and ** indicate P < 0.05 and P < 0.01, respectively, vs control (0 h). C, Cells were treated without (control) or with cisplatin (2 μmol/L). Flow cytometry analysis used 20 000 cells/each sample. Mouse IgG‐PE (isotype) was used as a negative control. D, The immunocytochemistry expression of PD‐L2 and ABCG2 in cisplatin‐treated HSC‐2 cells was also confirmed. Scale bar = 50 μm. E, Western blotting was conducted using targeted and β‐actin antibodies in membrane and cytoplasm fractions separated from HSC‐2 cells treated with cisplatin for 24 h. Similar results were obtained from three independent experiments
To clarify whether cisplatin‐induced PD‐L2 expression in OSCC, we examined the effect of cisplatin (2 μmol/L) on PD‐L2 gene expression using quantitative RT‐PCR (Figure 1B). Cisplatin treatment upregulated the gene expression of PD‐L2, ALDH1, a stem cell marker, and the ABCG2 gene, which is a drug efflux ATP‐binding cassette (ABC) transporter, in a time‐dependent manner. This upregulated expression reached a peak level at 24 hours after cisplatin treatment.
Subsets of anti–tumor drug‐resistant cells have been reported to depend on drug efflux ABC transporters.22, 23 ABCG2 is an ABC transporter that is a major contributor in cancer stem cells.24, 25 In addition, the activity of aldehyde dehydrogenases (ALDH), such as ALDH1, has been reported to define cancer stem cell populations in many cancer types and may act as a common marker for both normal and malignant stem cell populations.26, 27
The increase in the cell surface expression of PD‐L2 in cells treated with cisplatin was further confirmed using flow cytometry analysis, indicating the upregulation of PD‐L2 in cisplatin‐resistant HSC‐2 cells compared with the control (without cisplatin treatment; Figure 1C). Similar results were obtained using immunofluorescence staining, which showed that cisplatin treatment for 24 hours upregulated the expression of PD‐L2, as well as ABCG2, in HSC‐2 cells (Figure 1D). The cisplatin‐induced PD‐L2 expression was localized in both the cytoplasm and the membrane after separation of the cytoplasm and membrane fractions using western blot analysis (Figure 1E). Cisplatin treatment also upregulated the expression of PD‐L2 and ABCG2 in HSC‐3 cells, the other OSCC cell line (Figure 2S). Treatment with hydrogen peroxide (500 μmol/L), a reactive oxidant species, also upregulated PD‐L2 expression, together with ABCG2, and peaked 24 hours after treatment. In contrast, pretreatment of N‐acetylcysteine (10 μmol/L), an antioxidant drug, partially suppressed the cisplatin‐upregulated PD‐L2 expression.
Figure 2.
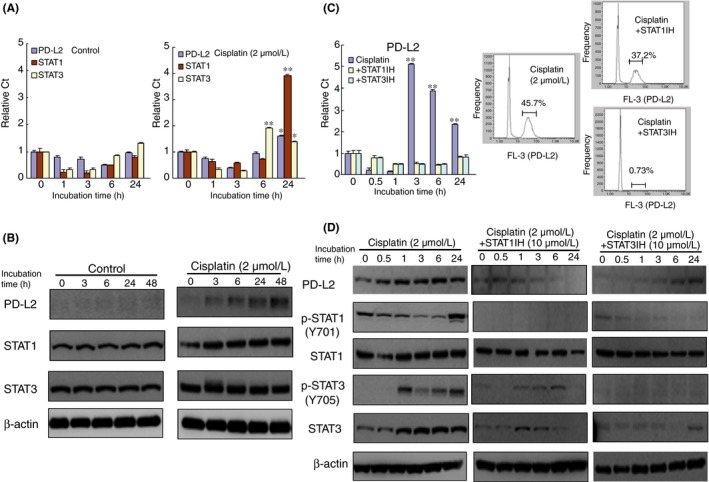
Cisplatin‐induced programmed death‐ligand 2 (PD‐L2) expression is mediated by signal transducers and activator of transcription 1/3 (STAT1/3) activation in HSC‐2 cells. Expression of PD‐L2, STAT1 and STAT3 in HSC‐2 cells with or without cisplatin treatment (2 μmol/L) for 0‐48 h using quantitative RT‐PCR analysis (A) and western blot analysis (B). Bar graphs show the mean from seven culture wells (mean ± SEM). * and ** indicate P < 0.05 and P < 0.01, respectively, vs control (0 h). The effect of a STAT1 or STAT3 inhibitor on PD‐L2 expression (C) and the activation of STAT1/3 phosphorylation (D). HSC‐2 cells were incubated with cisplatin in the presence or absence of a STAT1 or STAT3 inhibitor for 24 h. Similar results were obtained from three independent experiments
3.2. Signal transducers and activator of transcription 1/3 involvement in programmed cell death ligand 2 upregulation
A number of cytokines, including INF‐γ, GM‐CSF, IL‐4 and TNF‐α, have been reported to induce PD‐L expression.28 The reactions of these cytokines have been reported to be partially mediated by the transcription factors STAT and IRF. It has also been reported that INF‐γ induced PD‐L1 expression is mediated by JAK/STAT pathways.29, 30 To elucidate which transcription factors mediate cisplatin‐induced PD‐L2 expression, we next examined the effect of cisplatin on the activation of various kinases and transcriptional factors. Cisplatin treatment gradually induced STAT1 and STAT3 gene expression in HSC‐2 cells in a time‐dependent manner compared with the control (Figure 2A). Accordingly, cisplatin treatment upregulated the expression of the STAT1 and STAT3 proteins for 24 hours (Figure 2B). The cisplatin treatment also dominantly upregulated STAT3 expression and this protein remained phosphorylated more than 1 hour after treatment compared with STAT1 (Figure 2C). However, cisplatin treatment had little effect on the activation of other kinases, including AMPK, PTEN and PI3K (Figure S2).
Cisplatin treatment in the presence of fludarabine (10 μmol/L), a STAT1 inhibitor, or cryptotanshinone (10 μmol/L), a STAT3 inhibitor, decreased the relative number of PD‐L2‐positive cells compared with cisplatin alone (Figure 2D). The combination of cisplatin with either the STAT1 or STAT3 inhibitor also suppressed the phosphorylation of STAT1/3, particularly that of p‐STAT1.
3.3. The relationship between programmed cell death ligand 2 expression and malignancy in oral squamous cell carcinoma
Some studies have reported that PD‐L expression is associated with malignant potential, resulting in a decrease in overall survival in various cancers.31, 32 To further examine this issue, HSC‐2 cells were separated into PD‐L2‐positive and PD‐L2‐negative cells using fluorescence‐activated cell sorting, in which PD‐L2 expression was confirmed by western blot analysis (Figure 3A). To clarify whether PD‐L2 expression in HSC‐2 cells was associated with malignant behavior, we examined the effect of PD‐L2 expression on cellular proliferation using the colony assay (Figure 3B). There was no difference in proliferation activity between PD‐L2‐positive and PD‐L2‐negative cells on days 10 and 13. Next, we examined the tumor invasion ability, using transwell assays, and cellular transforming ability, using agar floating culture assays, in PD‐L2‐overexpressing and PD‐L2‐silencing cells. The number of cells that passed from the upper to the lower chamber through the collagen gel‐coated transwell increased in a time‐dependent manner (Figure 3C). In particular, a greater number of PD‐L2‐overexpressing or PD‐L2‐positive cells passed through the collagen gel compared with PD‐L2‐negative cells over a 48‐hour period. In contrast, the number of invading cells decreased with the silencing of PD‐L2 in collected PD‐L2 positive cells. Furthermore, the number of HSC‐2 cell colonies in the agar floating culture of PD‐L2‐overexpressing or PD‐L2‐positive cells was significantly greater than that observed for PD‐L2‐negative cells (Figure 3D).
Figure 3.
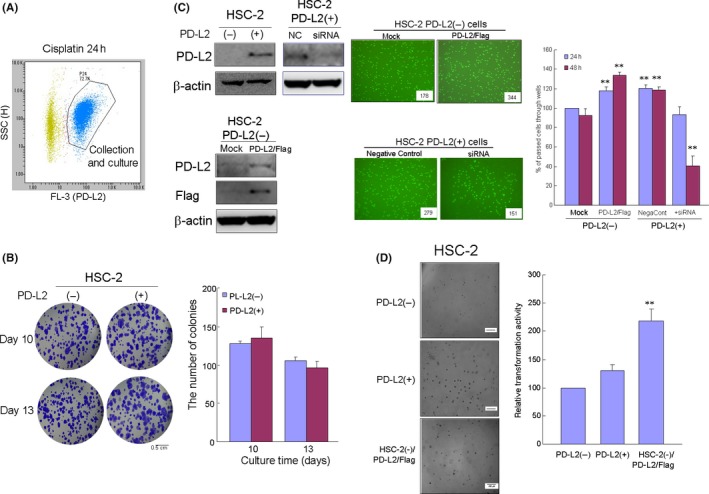
The invasion and transformation ability of cisplatin‐induced programmed death‐ligand 2 (PD‐L2)‐positive cells. A, Cells were treated with cisplatin (2 μmol/L), followed by separation into PD‐L2‐positive and PD‐L2‐negative cell populations using fluorescence‐activated cell sorting. B, Differences between the PD‐L2‐positive or expressing and PD‐L2‐negative or silencing cells in terms of proliferation (B) as determined by the colony formation assay, invasion (C) by the transwell assay and transformation (D) by the floating agar culture assay. Bar graphs show the mean from seven culture wells (mean ± SEM). * and ** indicate P < 0.05 and P < 0.01 vs mock cells at 48 h (C) or PD‐L2 (+) cells (D)
3.4. Oral squamous cell carcinoma patients expressing programmed cell death ligand 2
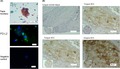
To clarify whether PD‐L2 is expressed in the tumor tissues of OSCC patients, we next examined PD‐L2 expression in OSCC cells from patients using cytological methods and commercial paraffin tissue microarrays in human oral cancers (Figure 4A,B) in conjunction with immunochemical staining. Using exfoliative cytological methods, we separated the cells derived from OSCC patients. These cells were severely atypical squamous cells with high nuclear‐cytoplasmic ratio, hyperchromatic nuclei and nuclear pleomorphism, suggesting OSCC as determined by papanicolaou staining. In addition, PD‐L2 expression in these cells was localized around the cellular membrane as determined by immunocytofluorescence. PD‐L2 expression was also observed in OSCC tissues derived from the tongue and gingiva of SCC patients, but not in normal tissues, in oral tissue microarray preparation. The rate of PD‐L2 positivity was 67.7% (113/ 167 in total tissues) and was dominant in males (P < 0.03) in the relationship between clinicopathological characteristics and PD‐L2 expression (Table S1). Furthermore, the intensity of PD‐L2 expression was significantly and positively correlated to the differentiation degree and invasive pattern (Table 1).
Figure 4.
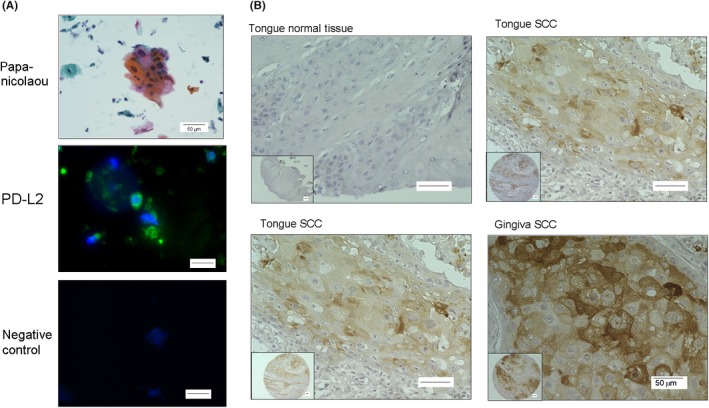
Programmed death‐ligand 2 (PD‐L2) expression in squamous cells isolated from oral squamous cell carcinoma (OSCC) patients and oral tissue microarray. A, Representative images of the exfoliative epithelium isolated from an OSCC patient and stained with papanicolaou and immunofluorescence. Scale bar = 50 μm. B, Immunohistochemical staining for PD‐L2 in various oral tissue samples by microarray. Scale bar = 50 μm
Table 1.
Correlation between programmed death‐ligand 2 (PD‐L2) expression and histological grading and invasive pattern
| Factors | PD‐L2 negative | PD‐L2 positive | |||
|---|---|---|---|---|---|
| n (%) (exp., st. resid.) | Low | Intermediate | High | P‐value | |
| (n = 46) | (n = 80) | (n = 22) | (n = 5) | ||
| Differentiation degree | |||||
| Grade I | 36 (78.3) | 38 (47.5) | 1 (4.5) | 0 (0) | 0.0001 |
| (22.549, 4.744)** | (39.216, −0.394) | (10.784, −4.510)** | (2.451, −2.229)* | ||
| Grade II | 10 (21.7) | 37 (46.5) | 13 (54.1) | 3 (60.0) | |
| (18.941, −3.203)** | (32.941, 1.335) | (9.059, 1.845) | (2.059, 0.870) | ||
| Grade III | 0 (0) | 5 (6.2) | 8 (36.4) | 2 (40.0) | |
| (4.510, −2.674)** | (7.843, −1.548) | (2.157, 4.527)** | (0.490, 2.309)* | ||
| Invasive pattern | |||||
| Expansive type (YK‐1, −2) | 30 (65.2) | 10 (12.5) | 0 (0) | 0 (0) | 0.000001 |
| (12.026, 7.212)** | (20.915, −4.021)** | (5.752, −3.016)** | (1.307, −1.353) | ||
| Intermediate type (YK‐3) | 16 (34.8) | 63 (78.8) | 15 (68.2) | 3 (60.0) | |
| (29.163, −4.818)** | (50.719, 4.127)** | (13.948, 0.503) | (3.170, −0.160) | ||
| Invasive type (YK‐4C, −4D) | 0 (0) | 7 (8.7) | 7 (31.8) | 2 (40.0) | |
| (4.801, −2.772)** | (8.366, −0.723) | (2.301, 3.538)** | (0.523, 2.195)* | ||
exp., expected value; st.resid., standardized residual.
P < 0.05, **P < 0.01.
4. DISCUSSION
Cisplatin‐based neoadjuvant chemotherapy in cancer patients is known to induce PD‐L1‐positive cells in tumors and immunocytes. In this study, we found that cisplatin treatment upregulated PD‐L2 and ABCG2 expression. ABCG2 is a drug efflux transporter in oral squamous tumors. Our findings indicate that the anti–tumor drug resistance in OSCC may be associated with PD‐L2 expression during chemotherapy.
It has recently been reported that cisplatin induces PD‐L1 expression in bladder cancer and non–small cell lung cancer cell lines.20, 21 In bladder cancer, cisplatin treatment serves to upregulate PD‐L1 expression via the ERK signaling pathway, followed by AP‐1. In addition, PD‐L1 is variably expressed in the cytoplasm and cell membrane in ovarian cancer cells.33 However, the mechanism of cisplatin‐induced PD‐L1 expression in these tumor cells remains largely unclear. In the current study, our results indicated that cisplatin treatment upregulated PD‐L2 expression in both the membrane and cytoplasm fractions of OSCC via STAT1/3 activation. PD‐L1 expression has been reported to be associated with the epithelial‐mesenchymal transition (EMT) observed during metastasis in lung and breast cancer, and esophageal SCC via ZEB1 activation.34, 35, 36 Moreover, cisplatin treatment has been reported to increase intracellular ROS levels37, 38 and treatment with antioxidants has been shown to ameliorate the toxic effects of cisplatin.39, 40 ROS themselves are known to induce the EMT in normal and SCC cells.41, 42, 43 Indeed, we found that hydrogen peroxide also upregulated PD‐L2 expression in HSC‐2 cells, whereas treatment of the antioxidant drug partially suppressed the cisplatin‐upregulated PD‐L2 expression in the present experiments. These findings, along with the present data, suggest that cisplatin‐induced PD‐L2 expression in OSCC is caused by the EMT via ROS induction.
Numerous reports have indicated that PD‐L expression in carcinogenesis is regulated by various intracellular signaling pathways. Indeed, the upregulation of PD‐L1 is believed to be driven by the activation of constitutive oncogenic signaling pathways, such as MAPK and PI3K‐AKT, as well as transcriptional factors, including HIF‐1, STAT3 and NF‐κB.44, 45 Furthermore, upregulation of PD‐L1 depends on IRF1 and STAT1 binding to their promoter, while PD‐L2 upregulation appears to depend on the IRF1 and STAT1/3 binding promoter.28, 29
In the present study, the presence of a STAT1 or STAT3 inhibitor partially suppressed the cisplatin‐induced PD‐L2 expression, suggesting that cisplatin may mediate STAT1/3 binding at the promoter sites of PDCD1LG2.
In general, cancers are regarded as heterogeneous tissues involving a variety of cells that originate from a unique and rare subset of cells that possess the ability to self‐renew and have the potential to differentiate into multiple cell lineages.46 Cancer stem cells, a subset of cancer cells with stem‐like properties, are responsible for tumor initiation, progression, recurrence and metastasis, as well as the cellular resistance to chemotherapy and radiotherapy.47, 48, 49 Cancer cells that are resistant to anti–tumor drugs have been reported to depend on ABC transporter activity.22, 23 ABCG2 is a member of the ABC transporter family and is a major factor in the drug resistance observed in cancer stem cells. Indeed, ABCG2 contribute to multidrug resistance to chemotherapy in several types of cancer.24, 25 In contrast, Abcg2 transporter gene knockout mice have been shown to be particularly sensitive to chemotherapeutic agents, indicating that ABCG2 is critical for maintaining drug‐resistant stem cells.50
In this study, we found that cisplatin treatment increased the percentage of ABCG2‐positive cells, so that >50% of the HSC‐2 cells were positive for ABCG2. This increase likely contributes to the stem cell properties of cells expressing ABCG2 and ALDH1 in OSCC, suggesting a possible mechanism for the high metastasis observed in anti–tumor drug‐resistant cells. Indeed, PD‐L2‐positive or PD‐L2‐overexpressing HSC‐2 cells in the current study had increased invasion and transformation ability. Thus, the subset of cancer stem cells with anti–tumor drug resistance among OSCC may become more malignant with increased invasion and transformation associated with upregulation of PD‐L2 expression.
Previous studies have shown that PD‐L1 and PD‐L2 have different prognostic roles in various tumors, suggesting that different tumor subsets exist, including in cancer stem cells.51, 52 Although PD‐L1 is known to be expressed in oral tissues derived from the tongue and salivary glands of cancer patients,53, 54, 55 the significance of PD‐L2 has not yet been systematically evaluated in OSCC. Thus, we analyzed cisplatin‐induced PD‐L2 expression in an OSCC cell line and in OSCC cytology and tissue samples derived from patients with OSCC. We found that PD‐L2 expression was observed in OSCC cells derived from the tongue and gingiva of patients but not in normal tissues.
In line with experimental findings, the intensity of PD‐L2 expression was positively correlated with more malignant morphological features, suggesting that poorly differentiated and invasive OSCC cells take advantage of expressing PD‐L2 to evade immune surveillance. In addition, at least one of two PD‐L was detected in the OSCC cells of four available tissue microarray spots from lymph node metastasis, implying that the two molecules may compensate each other to metastasize to lymph nodes (Figure S3).
In conclusion, cisplatin treatment upregulated PD‐L2 and ABCG2 expression in OSCC cell lines via STAT1/3 activation . In addition, upregulated PD‐L2‐positive cells had greater invasion and transformation ability and PD‐L2 expression was observed in epithelial cells derived from OSCC patients. Collectively, our novel findings suggest that cisplatin‐induced PD‐L2 expression in OSCC cells may be a critical factor in the prognosis of advanced OSCC patients.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K11062 to HK) and the Private University Research Branding Project at Fukuoka Dental College. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Sudo S, Kajiya H, Okano S, et al. Cisplatin‐induced programmed cell death ligand‐2 expression is associated with metastasis ability in oral squamous cell carcinoma. Cancer Sci. 2020;111:1113–1123. 10.1111/cas.14336
REFERENCES
- 1. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Latchman Y, Wood CR, Chernova T, et al. PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol. 2001;3:261‐268. [DOI] [PubMed] [Google Scholar]
- 3. Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD‐1/PD‐L pathway in mice. J Clin Invest. 2007;117:2974‐2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riella LV, Watanabe T, Sage PT, et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplant. 2011;11:832‐840. [DOI] [PubMed] [Google Scholar]
- 5. Dong H, Strome SE, Salomao DR, et al. Tumor‐associated B7–H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793‐800. [DOI] [PubMed] [Google Scholar]
- 6. Ichikawa M, Chen L. Role of B7–H1 and B7–H4 molecules in down‐regulating effector phase of T‐cell immunity: novel cancer escaping mechanisms. Front Biosci. 2005;10:2856‐2860. [DOI] [PubMed] [Google Scholar]
- 7. Brown JA, Dorfman DM, Ma F‐R, et al. Blockade of programmed death‐1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257‐1266. [DOI] [PubMed] [Google Scholar]
- 8. Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD‐L2 and its engagement with PD‐L2 promotes respiratory tolerance. J Exp Med. 2014;211:943‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jie H‐B, Gildener‐Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629‐2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565‐1570. [DOI] [PubMed] [Google Scholar]
- 11. Kim M‐Y, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD‐L1 and PD‐L2 expression in pulmonary squamous cell carcinoma: comparison with tumor‐infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;1:24‐33. [DOI] [PubMed] [Google Scholar]
- 12. Kano M, Hayano K, Hayashi H, et al. Survival benefit of neoadjuvant chemotherapy with S‐1 plus docetaxel for locally advanced gastric cancer: a propensity score‐matched analysis. Ann Surg Oncol. 2019;6:1805‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yearley JH, Gibson C, Yu NI, et al. PD‐L2 expression in human tumors: relevance to anti–PD‐1 therapy in cancer. Clin Cancer Res. 2017;23:3158‐3167. [DOI] [PubMed] [Google Scholar]
- 14. Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7–H1 and B7‐DC to programmed death‐1. Biochem Biophys Res Commun. 2003;307:672‐677. [DOI] [PubMed] [Google Scholar]
- 15. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 16. Herbst RS, Sznol M. Diminished but not dead: chemotherapy for the treatment of NSCLC. Lancet Oncol. 2016;17:1464‐1465. [DOI] [PubMed] [Google Scholar]
- 17. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non–small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Portugal LG. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22:34‐41. [DOI] [PubMed] [Google Scholar]
- 19. Shin J, Kim G, Lee JW, et al. Identification of ganglioside GM2 activator playing a role in cancer cell migration through proteomic analysis of breast cancer secretomes. Cancer Sci. 2016;1107:828‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fournel L, Wu Z, Stadler N, et al. Cisplatin increases PD‐L1 expression and optimizes immune check‐point blockade in non–small cell lung cancer. Cancer Lett. 2019;464:5‐14. [DOI] [PubMed] [Google Scholar]
- 21. Tsai TF, Lin JF, Lin YC, et al. Cisplatin contributes to programmed death‐ligand 1 (PD‐L1) expression in bladder cancer through ERK1/2-AP‐1 signaling pathway. Biosci Rep. 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scharenberg CW, Harkey MA, Torok‐Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507‐512. [DOI] [PubMed] [Google Scholar]
- 23. Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat Med. 2001;7:1028‐1034. [DOI] [PubMed] [Google Scholar]
- 24. Ho MM, Ng AV, Lam S, et al. Side population in human lung cancer cell lines and tumors is enriched with stem‐like cancer cells. Cancer Res. 2007;67:4827‐4833. [DOI] [PubMed] [Google Scholar]
- 25. Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3:1‐27. [PMC free article] [PubMed] [Google Scholar]
- 26. Ginestier C, Hur MH, Charafe‐Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237‐246. [DOI] [PubMed] [Google Scholar]
- 28. Garcia‐Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD‐L1 and PD‐L2 expression. Cell Rep. 2017;19:1189‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horlad H, Ma C, Yano H, et al. An IL‐27/Stat3 axis induces expression of programmed cell death 1 ligands (PD‐L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016;107:1696‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin C, Cao W, Ren Z, et al. GDNF secreted by nerves enhances PD‐L1 expression via JAK2‐STAT1 signaling activation in HNSCC. Oncoimmunology. 2017;6:e1353860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng M, Xiong G, Cao Z, et al. PD‐1/PD‐L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57‐65. [DOI] [PubMed] [Google Scholar]
- 32. Prat A, Navarro A, Paré L, et al. Immune‐related gene expression profiling after PD‐1 blockade in non–small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77:3540‐3550. [DOI] [PubMed] [Google Scholar]
- 33. Qu QX, Xie F, Huang Q, et al. Membranous and cytoplasmic expression of PD‐L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43:1893‐1906. [DOI] [PubMed] [Google Scholar]
- 34. Noman MZ, Janji B, Abdou A, et al. The immune checkpoint ligand PD‐L1 is upregulated in EMT‐activated human breast cancer cells by a mechanism involving ZEB‐1 and miR‐200. Oncoimmunology. 2017;6:e1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsutsumi S, Saeki H, Nakashima Y, et al. Programmed death‐ligand 1 expression at tumor invasive front is associated with epithelial‐mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017;108:1119‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raimondi C, Carpino G, Nicolazzo C, et al. PD‐L1 and epithelial‐mesenchymal transition in circulating tumor cells from non–small cell lung cancer patients: a molecular shield to evade immune system? Oncoimmunology. 2017;6:e1315488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008;68:6425‐6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santos NA, Catão CS, Martins NM, Curti C, Bianchi MLP, Santos AC. Cisplatin‐induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495‐504. [DOI] [PubMed] [Google Scholar]
- 39. El‐Beshbishy HA, Bahashwan SA, Aly HAA, Fakher HA. Abrogation of cisplatin‐induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur J Pharmacol. 2011;668:278‐284. [DOI] [PubMed] [Google Scholar]
- 40. Santos NA, Bezerra CS, Martins NM, et al. Hydroxyl radical scavenger ameliorates cisplatin‐induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother Pharmacol. 2008;61:145‐155. [DOI] [PubMed] [Google Scholar]
- 41. Fukawa T, Kajiya H, Ozeki S, Ikebe T, Okabe K. Reactive oxygen species stimulates epithelial mesenchymal transition in normal human epidermal keratinocytes via TGF‐beta secretion. Exp Cell Res. 2012;318:1926‐1932. [DOI] [PubMed] [Google Scholar]
- 42. Hirai M, Kitahara H, Kobayashi Y, et al. Regulation of PD‐L1 expression in a high‐grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol. 2017;50:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF‐beta1‐induced mitogen‐activated protein kinase activation and epithelial‐mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667‐675. [DOI] [PubMed] [Google Scholar]
- 44. Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD‐L1 expression in melanoma. Clin Cancer Res. 2014;20:3446‐3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD‐L1: a novel role of pro‐survival signalling in cancer. Ann Oncol. 2016;27:409‐416. [DOI] [PubMed] [Google Scholar]
- 46. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755‐768. [DOI] [PubMed] [Google Scholar]
- 47. Patrawala L, Calhoun T, Schneider‐Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696‐1708. [DOI] [PubMed] [Google Scholar]
- 48. Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396‐401. [DOI] [PubMed] [Google Scholar]
- 49. Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13‐24. [DOI] [PubMed] [Google Scholar]
- 50. Zhou G, Chen J, Lee S, Clark T, Rowley JD, Wang SM. The pattern of gene expression in human CD34(+) stem/progenitor cells. Proc Natl Acad Sci USA. 2001;98:13966‐13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death‐1 ligand‐1 and programmed death‐1 ligand‐2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947‐2953. [DOI] [PubMed] [Google Scholar]
- 52. Shin S‐J, Jeon YK, Kim P‐J, et al. Clinicopathologic analysis of PD‐L1 and PD‐L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23:694‐702. [DOI] [PubMed] [Google Scholar]
- 53. Nakano T, Takizawa K, Uezato A, Taguchi K, Toh S, Masuda M. Prognostic value of programed death ligand‐1 and ligand‐2 co–expression in salivary gland carcinomas. Oral Oncol. 2019;90:30‐37. [DOI] [PubMed] [Google Scholar]
- 54. Straub M, Drecoll E, Pfarr N, et al. CD274/PD‐L1 gene amplification and PD‐L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7:12024‐12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshida S, Nagatsuka H, Nakano K, et al. Significance of PD‐L1 expression in tongue cancer development. Int J Med Sci. 2018;15:1723‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


