Abstract
Diabetic kidney disease remains the most common cause of end-stage kidney disease in the world. Despite reductions in incidence rates of myocardial infarction and stroke in people with diabetes over the past 3 decades, the risk of diabetic kidney disease has remained unchanged, and may even be increasing in younger individuals afflicted with this disease. Accordingly, changes in public health policy have to be implemented to address the root causes of diabetic kidney disease, including the rise of obesity and diabetes, in addition to the use of safe and effective pharmacological agents to prevent cardiorenal complications in people with diabetes. The aim of this article is to review the mechanisms of pathogenesis and therapies that are either in clinical practice or that are emerging in clinical development programs for potential use to treat diabetic kidney disease.
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
SGLT2 inhibitors significantly improve renal outcomes across a wide range of DKD stages and albuminuria.
GLP-1 receptor agonists decrease albuminuria, however very modest eGFR preservation effects are observed long term.
DPP4-inhibitors did not show renal protection to date.
Newer generation of more selective, non-steroidal mineralocorticoid receptor antagonists (MRAs) are in clinical trials and will reveal whether these agents can improve renal outcomes without side effects associated with the less specific MRAs.
Agents targeting inflammatory and fibrotic pathways are of significant therapeutic interest in studies of DKD.
Clinical trials with endothelin receptor antagonists and uric acid lowering agents are ongoing to examine renal protection in DKD.
Beyond potential use of these therapies, future studies are needed to detail the safety and efficacy of combination therapies.
Diabetic kidney disease (DKD) accounts for 44.5% of end-stage kidney disease (ESKD) in developed countries, which requires dialysis or kidney transplantation and increases the risk of cardiovascular disease (CVD) (1). Therefore, identification of safe new agents that can prevent or delay the onset of DKD could help alleviate a significant public health burden. Most renal protection trials have either failed, demonstrated harm, or reported effects that are below expectations based on data from experimental models (Table 1). For example, within the past 10 years, trials with early or with dual renin-angiotensin-aldosterone system (RAAS) blockade, inhibition of protein kinase C-β, endothelin receptor antagonists, and the antioxidant bardoxolone have reported disappointing results (2). Most recently, trials with sodium glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated consistent and robust renal protection effects. Accordingly, this review will summarize novel therapies targeting mechanisms involved in DKD pathogenesis, such as neurohormonal activation, tubuloglomerular feedback, and renal inflammation and fibrosis.
Table 1.
Summary of Failed Clinical Trials with Therapeutic Interventions for DKD
| Name of Study | Agent(s) | Patient Cohort | Status | Reason for Study Failure |
|---|---|---|---|---|
| VA NEPHRON-D | ACEi + ARB | 1448 participants with proteinuric DKD, mean SBP ≥ 140 mm Hg and mean DBP ≥ 80 mm Hg | Prematurely stopped | Safety concerns; worse renal outcomes |
| HALT-PKD | ACEi + ARB | 1044 participants with autosomal dominant polycystic kidney disease | Completed | No renal benefit |
| ALTITUDE | ACEi/ARB + DRI | 8561 participants with T2D and CKD or CV disease | Prematurely stopped | Renal/CV safety signals |
| ONTARGET | ACEi + ARB vs. ACEi or ARB monotherapies | 25,620 participants aged 55 years or older with established atherosclerotic vascular disease or with diabetes with end-organ damage | Completed | Worse renal outcomes |
| MDRD | Low-protein diet | 1785 participants with CKD | Completed | Did not significantly slow the progression of renal disease |
| TREAT | Erythropoietin | 4038 participants with anemia, CKD, and T2D | Completed | Did not affect the risk of death and major CV and renal events |
| SUN-Overt | Sulodexide and GAG pathways | 1248 patients with T2D, renal impairment, and significant proteinuria | Prematurely stopped | Did not affect the risk of composite of a doubling of baseline serum creatinine, development of ESRD, or serum creatinine ≥6.0 mg/dL |
| SUN-Micro | Sulodexide and GAG pathways | 1056 participants with T2D and ACRs of 35–200 mg/g in men and 45–200 mg/g in women | Completed | Did not decrease ACR, no effect renal |
| ASCEND | ET-A antagonist | 1392 participants with T2D and nephropathy | Prematurely stopped | Renal safety issues |
| BEACON | Nrf2 (bardoxolone) | 2185 participants with T2D and Stage 4 CKD | Prematurely stopped | Renal/CV safety concerns |
| CAP-KD | Carbon absorption (AST-120) | 460 participants with progressive CKD | Completed | No renal benefit. Did not significantly affect the composite of doubling of serum creatine, increase in serum creatinine level to ≥6.0 mg/dL, need for dialysis or transplantation or death |
| HARP-3 | AngII/NEPi (LCZ696) | 414 participants (40% with a history of diabetes at baseline) with eGFR 20–60 mL/min/1.73 m2 and without heart failure | Completed | No renal benefit |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACR, ; ARB, angiotensin-receptor blocker; CKD, chronic kidney disease; CV: cardiovascular; DBP, diastolic blood pressure; DKD, diabetic kidney disease; DRI, ; ESRD, end-stage renal disease; ET-A, endothelin-A; SBP, systolic blood pressure; T2D, type 2 diabetes.
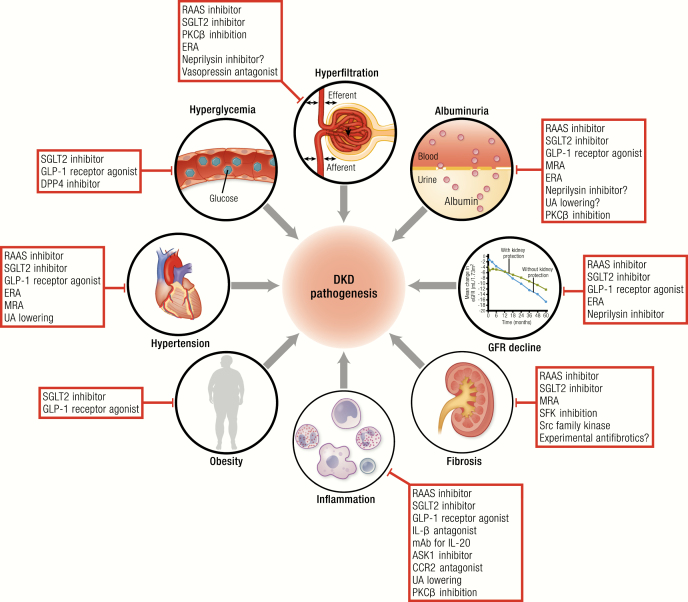
Pathophysiology of DKD
The pathogenesis of DKD is complex and involves both hemodynamic and nonhemodynamic mechanisms of renal injury. According to the classical paradigm of DKD, early in the disease process, intraglomerular hypertension and single-nephron hyperfiltration are a common consequence of hyperglycemia and hyperinsulinemia associated with diabetes. The majority of patients with DKD develop elevated albumin excretion before exhibiting kidney function decline and ultimately ESKD (3). One of the earliest abnormalities that can be detected in animals and humans leading to DKD is therefore single-nephron hyperfiltration. Interestingly, reported rates of hyperfiltration at the whole kidney level vary significantly, between 10% and 67% in people with type 1 diabetes (T1D) and 6% to 73% in people with type 2 diabetes (T2D) (3). Nevertheless, these hemodynamic changes have been associated with increased intraglomerular wall tension and shear stress, leading to activation of pro-inflammatory cytokines, albuminuria, and kidney disease progression (4, 5).
Although the pathogenesis of hyperfiltration is not yet completely understood, changes in both preglomerular (afferent) and postglomerular (efferent) arteriolar tone have been strongly implicated (3). A variety of vasoactive mediators that regulate efferent and afferent arteriolar tone have been implicated in the pathogenesis of DKD. These factors are therefore either being used or investigated as DKD therapies, as discussed below. The RAAS, which is activated in the setting of diabetes, plays a central role in the development and progression of DKD, in part via inducing efferent constriction and intraglomerular hypertension, and by activating a variety of downstream pro-inflammatory and pro-fibrotic pathways described in further detail in this review and elsewhere (6). RAAS inhibition decreases efferent arteriolar resistance and consequently decreases intraglomerular pressure and suppresses other nonhemodynamic injury pathways (6, 7). Therefore, RAAS inhibition has been the cornerstone of primary and secondary renal protection in patients with diabetes over the past 20 years. Unfortunately, RAAS blockade reduces but does not completely normalize hyperfiltration (8) and does not fully prevent renal injury (9). Moreover, large randomized controlled trials examining renal and cardiovascular outcomes showed that dual RAAS blockade results in significant side effects such as increased risk of hyperkalemia and acute kidney injury (10–12), suggesting that additive effects of therapies acting at the efferent arteriole may cause more harm than benefit. A potential caveat to this cautionary note is that novel, nonsteroidal mineralocorticoid receptor antagonists (MRAs) such as finerenone are being studied as a DKD therapy on top of standard of care, as discussed in detail in the subsequent section (13). This avenue of investigation is predicated based on the idea that this type of MRA may attenuate both hemodynamic and pro-fibrotic injury caused by aldosterone, with a lower risk of hyperkalemia and sex steroid–based side effects. Beyond the RAAS, further supportive evidence for the role of pathogenic mediators associated with efferent constriction has emerged from trials examining endothelin receptor antagonists, which also likely act in part by dilating the efferent arteriole leading to antiproteinuric effects and prevention of renal function loss, as discussed in the endothelin receptor antagonism section that follows (14).
Over the past decade, new components of the RAAS have been identified that may elucidate mechanisms of kidney injury in diabetes. For example, angiotensin-converting enzyme 2 (ACE2), which has a 40% homology to ACE, was identified in the kidney (15, 16), and acts by cleaving the C-terminal amino acid of angiotensin (Ang) II, thereby generating Ang (1–7). Ang (1–7) counteracts the adverse effects of Ang II by reducing inflammation, oxidative stress, and lipotoxicity (16). Diabetic animal models with ACE2 downregulation are associated with increased oxidative stress (17), albuminuria, glomerulosclerosis (18), and tubular injury (19), whereas increased ACE2 activity slows DKD progression (20). Consistent with animal models, patients with DKD were shown to have decreased expression of glomerular and tubular ACE2 (21, 22). Because of such strong mechanistic rationale for renal protection, ACE2 has been investigated as a potential therapeutic target in murine models. Recombinant ACE2 lowers blood pressure; and attenuates oxidative stress, fibrosis, and mesangial cell proliferation; and diminishes DKD progression (16, 23). Despite strong data from animal studies, little is known about potential protective effects of agents that modulate ACE2/Ang (1–7) mechanisms in humans (24).
For the preglomerular circulation, a host of factors have been linked with afferent vasodilatation, resulting in renal hyperperfusion and hyperfiltration. Historically, these have included upregulation of nitric oxide synthase, atrial natriuretic peptide, and cyclooxygenase-2 under the influence of ambient hyperglycemia (25–27). Unfortunately, blockade of nitric oxide synthase and cyclooxygenase-2 can have significant adverse effects such as hypertension and vasoconstriction, and no current therapies specifically target atrial natriuretic peptide. More recently, the afferent vasodilatory roles of SGLT2 and vasopressin have been more widely appreciated, as discussed later, and are important emerging targets for pharmacological therapies in patients with diabetes.
In addition to hemodynamic factors, there are a number of nonhemodynamic mediators that contribute to DKD progression that are primarily activated on the basis of ambient hyperglycemia, including protein kinase C-β, mediators of oxidative stress, advanced-glycosylation end-product (AGE) activation and receptor for AGE (RAGE) as well as inflammatory cytokines and chemokines. Although many of these nonhemodynamic factors have been recognized for their kidney injury potential for many years, it is only recently that some of them have started to take on clinical significance as possible therapeutic targets. For example, therapy-directed oxidative stress has been completed and more are under way with agents targeting Kelch-like ECH-associated protein 1 and nuclear factor erythroid 2-related factor 2 (NRF2) mechanisms, and other efforts related to protein kinase C β (PKCβ) and AGE-RAGE are at various stages of clinical trial development, as reviewed in the sections that follow. For inflammatory pathways, monocyte chemoattractant protein-1, tumor necrosis factor-α, IL-1, IL-6, IL-8, and apoptosis-signaling kinase 1 (ASK1) pathways are augmented under the influence of hyperglycemia in experimental studies, and activate intracellular signalling cascades including JAK-STAT and natural killer-κB that have been linked with mesangial expansion, glomerulosclerosis, tubulointerstitial fibrosis, and DKD progression (28). In this review, we will describe the pathogenic importance of some of these mediators, with a focus on those being tested in preclinical or clinical studies. Similarly, factors linked with renal parenchymal fibrosis have been a major focus clinically in recent years because of increasing recognition that approximately 20% of patients with T2D have a form of nonalbuminuric kidney disease characterized by impaired kidney function with tubulointerstitial fibrosis. In patients with this nonalbuminuric phenotype (29), it is not known if traditional therapies such as RAAS inhibitors are renal protective because dedicated trial have not been undertaken in this population. TGFβ and its downstream effector, connective tissue growth factor (CTGF), are pivotal mediators of kidney fibrosis in preclinical models. Preclinical and clinical studies have therefore been planned or undertaken to antagonize these mediators. Unfortunately, a dedicated trial using a monoclonal antibody against TGFβ was terminated early because of a lack of efficacy (30). A small phase 1 trial with an anti-CTGF antibody did show modest clinical efficacy in patients with T2D, but no studies with this agent appear to be under way in publicly available databases. Agents specifically targeting TGFβ and CTGF are therefore not addressed further in this review (31). Similarly, other historical agents targeting fibrosis in DKD such as pirfenidone are not being used in ongoing trials and are therefore not discussed further in this review (32). Accordingly, mechanisms linked with renal fibrosis, as well as their respective therapies, such as uric acid, Src kinases, and autophagy, are described in further detail in the sections that follow (33).
Brief overview of current therapeutic strategies for DKD
Based on available clinical trial data, current therapeutic approaches for kidney protection in people with diabetes include management of hyperglycemia, blood pressure control, and use of RAAS inhibitors, which reduce blood pressure and intraglomerular hypertension. In people with T2D, intensive glycemic control reduced the risk of microvascular complications including ESKD in the Action in Diabetes and Vascular Disease Preterax and Diamicron MR Controlled Evaluation Post-Trial Observational Study (ADVANCE-ON) (34). Despite increasing diabetes prevalence (35), improved glycemic and blood pressure control has kept DKD incidence rates stable over the past 20 years, and about 40% of people still develop kidney disease, highlighting the need for novel therapeutic strategies. Further, more recent data have suggested that rates of DKD are increasing over time, especially in younger individuals, underscoring the need to identify and use new therapies (36, 37). Novel agents targeting mechanisms, such as glomerular hyperfiltration, inflammation, and fibrosis, have been a major focus for development of new treatments. Accordingly, the aim of this review is to present state-of-the-art knowledge on recent developments in pharmacotherapies for DKD.
Glucose-Lowering Therapies
SGLT2 inhibition
Effects of SGLT2 inhibition on metabolic and cardiovascular parameters.
Medications that block SGLT have been used in clinical practice for approximately 6 years. By blocking the reabsorption of glucose, SGLT2i leads to weight loss of ∼2 to 3 kg and a decrease in HbA1c by ∼0.7% (38, 39). The effects on metabolic parameters, such as HbA1c, are most pronounced in people with preserved kidney function, and attenuated in those with chronic kidney disease (CKD) resulting from a reduction in glucosuria (38–40). Beyond these glucosuria-mediated effects, SGLT2i also induces acute natriuresis, which is maximal for the initial 3 days, and then returns back to baseline (38, 39, 41–43). As a consequence of this initial natriuretic effect, however, plasma volume decreases modestly by approximately 7% (44), and then remains below baseline over the longer term (45–47). For context, in the same study, in the group treated with a thiazide, there was durable effect on plasma volume at 12 weeks, which distinguishes these 2 types of natriuresis agents. Head–head plasma volume studies with SGLT2i vs. loop diuretics have not been published and so the relative effect of these 2 drug classes on cardiovascular hemodynamics in patients with T2D is not yet known. Direct measures of plasma volume with radiolabeled albumin in people with T2D have demonstrated these changes over 12 weeks (45). Moreover, in longer term clinical trials, including cardiovascular safety trials (CVOTs), hematocrit increases modestly over both the short and long term (45, 48). Although it is not yet known if the rise in hematocrit is solely related to circulating volume depletion and/or increased erythropoietin production, changes in plasma volume are important for a variety of reasons. First, blood pressure lowering by 3 to 5 mmHg systolic and 1 to 2 mmHg diastolic may be in part secondary to plasma volume contraction, which may in turn reduce the risk of DKD, CVD, and hospitalization for heart failure (HHF) (49–51). Blood pressure–lowering effects of SGLT2i occur across the range of estimated glomerular filtration rate (eGFR) down to CKD stage 4 (40). In addition to the importance of plasma volume contraction in relation to blood pressure lowering, plasma volume contraction may also be an important mediator of the decline in HHF risk, as suggested by analyses demonstrating that hemoconcentration is responsible for a large component of the cardiovascular (CV) benefit reported in CVOTs (48). Magnetic resonance imaging studies showing a decline in sodium content in the skin suggest an alteration of the nonosmotic sodium and lower total body sodium content with SGLT2i (52). Plasma volume contraction, markers of hemoconcentration, and a decline in body sodium content may therefore ultimately have important implications for HHF risk.
SGLT2i has a variety of other physiological effects in the CV system that have been demonstrated in humans, and that may also contribute to beneficial cardiorenal outcomes in clinical trials. In people with T1D and T2D, empagliflozin reduces arterial stiffness, and in individuals with T2D, dapagliflozin reduces endothelial dysfunction (53–56). Beyond possible benefits on blood pressure control, by lowering arterial stiffness, cardiac afterload may be attenuated, which may protect against subendocardial ischemia and left ventricular remodeling (57). Improving endothelial function may also lower blood pressure and serve to maintain adequate myocardial perfusion by dilating coronary arteries leading to CV protection.
Effects of SGLT2i on the kidney.
In addition to these systemic effects, SGLT2i exerts important hemodynamic effects in the kidney mediated through tubuloglomerular feedback pathways at the afferent arteriole, effects that pertain to hyperfiltering patients with T1D and could theoretically be relevant to patients with T2D and to those with nondiabetic CKD (35, 58–61). In brief, under conditions of ambient hyperglycemia in the setting of diabetes, proximal tubular reabsorption of sodium and glucose is enhanced because of augmented SGLT2 bioactivity, resulting in less sodium delivery to the macula densa (62). The decrease in distal sodium delivery to the macula densa leads to less sodium reabsorption via the Na+/K+/2Cl– transporter on the luminal membrane surface, which is an energy-dependent process, resulting in less ATP breakdown to adenosine. In this part of the nephron, adenosine acts as a vasoconstrictor via the adenosine 1 receptor. Under normal physiological circumstances, a decline in sodium delivery to the macula densa occurs as a result of effective circulating volume depletion. Based on a decrease in vasoconstrictor activity, and to preserve renal function under conditions of volume depletion, the afferent arteriole dilates, leading to increases in renal blood flow and glomerular pressure, thereby preserving kidney function. However, in the setting of diabetes, in which renal function, blood pressure, and circulating volume start out at a normal baseline, afferent vasodilatation has been linked with hyperfiltration, thereby predisposing to glomerular injury over time. By blocking SGLT2 pharmacologically, the delivery of sodium to the macula densa is restored back to physiological levels, leading to increased afferent tone and decreased renal perfusion back to normal levels that do not overcompensate or predispose to glomerular hypoperfusion and kidney injury.
The impact of SGLT2i on hyperfiltration is well established, based on seminal studies by Vallon et al. and others (63). However, critical observations around the role of the afferent arteriole in response to these therapies were only made recently with the availability of multiphoton in vivo microscopy. In a streptozotocin-induced model of T1D, Kidokoro et al. reported that hyperfiltration is ameliorated with SGLT2i and made the additional crucial measurements of afferent arteriolar diameter in the same nephrons before and after drug administration, which decreased in response to empagliflozin (64). Moreover, they reported that urine adenosine increased significantly, in line with the tubuloglomerular feedback hypothesis. Finally, they demonstrated that by blocking adenosine signalling pharmacologically, the hemodynamic impact of empagliflozin was entirely lost, indicating that the natriuresis-ATP breakdown-adenosine-A1-receptor binding cascade is required for SGLT2i to mediate changes in kidney function associated with renal protection. Interestingly, blockade of other preglomerular vasodilators associated with hyperfiltration – nitric oxide and prostanoids – did not affect SGLT2i-related changes in kidney function. In human translational physiology studies, to define whether alterations in kidney function and hyperfiltration reported in animals also occur in humans, we examined the impact of empagliflozin on GFR and renal blood flow in young adults with T1D and reported that, similar to observations in animals, hyperfiltration and renal hyperperfusion are significantly attenuated with SGLT2i, in conjunction with increased urinary excretion of adenosine (65–68). Although the previously described mechanisms are well described in people with T1D and hyperfiltration as well as in rodent models of T1D (e.g., streptozotocin-induced diabetes), the changes induced in renal physiology by SGLT2i in people with T2D may differ. As compared with young adults with T1D and hyperfiltration, older adults with T2D have lower whole-kidney GFR, but higher single-nephron filtration resulting from age- and disease-associated declines in nephron numbers (69). Although albuminuria is a clinically important marker of DKD and CV health, at lower levels it may not imply progressive nephropathy and is also affected by factors such as nephron loss and renal function decline. These factors may, in part, explain discordance between albuminuria and other renal endpoints (70–72). In addition, they use concurrent RAAS blockers that likely modulate renal responses to other drugs. As such, recently presented data showed that in people with T2D, dapagliflozin reduced GFR without increasing renal vascular resistance, suggesting postglomerular vasodilatory effects. Although adenosine levels were increased, they were not related to the decline in GFR. On the other hand, various urinary prostaglandins were increased, which could have driven the overall vasodilatory response (73). Thus, although the dip in GFR is consistently demonstrated, responsible mechanisms may be different in various populations across different age groups and disease durations, depending on renal hemodynamic status at baseline as well as the use of concomitant medications such as RAAS blockers and baseline macronutrient and salt intake.
SGLT2i in human clinical trials.
Beyond experimental work and smaller human mechanistic studies, the physiological effects of SGLT2i can also be appreciated in larger human clinical trials. In studies designed to determine the effect of SGLT2i on glycemic and metabolic parameters, in response to this class of glucose-lowering therapy, eGFR decreases acutely – even after a single dose, by 3 to 5 mL/min/1.73 m2, and then tends to return back toward baseline over the subsequent 8 to 12 weeks while on therapy (41). This initial “dip” in eGFR likely reflects acute hemodynamic effects and is reversible after cessation of therapy, as reflected by the observation that eGFR rapidly returns toward baseline after a washout of several weeks (74). Thus, the initial, temporary eGFR dip does not appear to have significant safety implications as long as the dip is not excessive, and the eGFR level stays within a safe range according to clinical judgement. After approximately 70 to 90 weeks, people with T2D treated with SGLT2i consistently have better preservation of kidney function compared with patients treated with either placebo or active glucose-lowering agents such as sulfonylureas (50, 75, 76). Along with better preservation of eGFR compared with placebo, SGLT2i consistently reduce albuminuria by 30% to 50% in adults with T2D (77, 78), including those with and without renal function impairment down to eGFR values of approximately 30 mL/min/1.73 m2 at study baseline. Along with antialbuminuric effects across CKD stages, SGLT2i reduces blood pressure independent of eGFR or glycemic lowering in people with T2D, highlighting the natriuresis-related basis for many of the renal effects of these therapies.
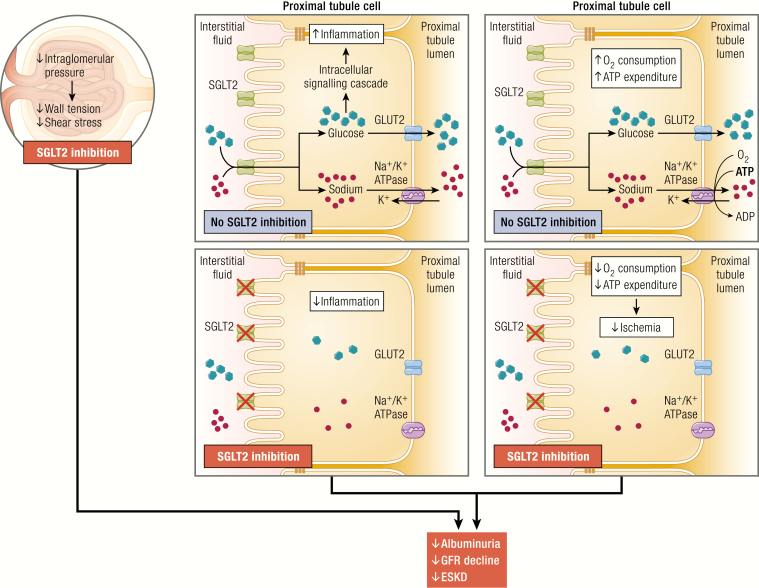
Of the various protective mechanisms associated with the class, it is perhaps the hemodynamic effects of SGLT2i that are the most widely appreciated and studied. Yet, other nonglycemic, nonhemodynamic factors may be involved and equally contribute to beneficial cardiorenal outcomes in clinical trials. For example, SGLT2i reduces markers of oxidative stress in experimental models and, in humans, suppresses mediators of inflammation and fibrosis, and lowers uric acid concentrations by inducing uricosuria (79). In experimental models, SGLT2i reduces levels of nuclear factor-κB, IL-6, monocyte activator protein-1, and other factors implicated in target end-organ injury in diabetes, as reviewed previously (61). In humans, SGLT2i also reduces IL-6, and suppresses levels of kidney injury molecule-1 (80). Although it is not yet known why SGLT2i suppresses inflammation, several mechanisms may be involved (81) (Fig. 1). First, in the kidney, by reducing intraglomerular pressure, wall tension and shear stress in the glomerulus are reduced, which has been linked with reduced risk of renal inflammation/fibrosis (25). Second, by blocking the transit of glucose across tubular cells, studies with cultured human proximal tubular cells have suggested that the intracellular production of pro-inflammatory and pro-fibrotic pathways is more directly inhibited (82). Finally, SGLT2i leads to less sodium being reabsorbed on the luminal side of the renal tubule, leading to less sodium reaching the Na+/K+ ATPase located on the basolateral side. Therefore, SGLT inhibition may attenuate the ATP consumption needed to reabsorb sodium, thereby reducing renal oxygen consumption and protecting against renal hypoxia. As a consequence of this decline in ischemia-related factors, pro-inflammatory and pro-fibrotic pathways may also be suppressed. Importantly, protective influences on hemodynamic and nonhemodynamic pathways are not mutually exclusive and may both play central roles in renal protection reported in CVOTs, in Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) and possibly in novel clinical setting including people with T1D and those with nondiabetic CKD who are being recruited and assessed in ongoing primary renal endpoint studies such as Effects of Dapagliflozin in Non-diabetic Patients With Proteinuria (NCT03190694), A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA-CKD; NCT03036150), and The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY; NCT03594110).
Figure 1.
Proposed anti-inflammatory mechanisms in the kidney mediated by SGLT2 inhibition in diabetes. SGLT2, sodium glucose co-transporter 2.
SGLT2i in large cardiovascular outcome trials.
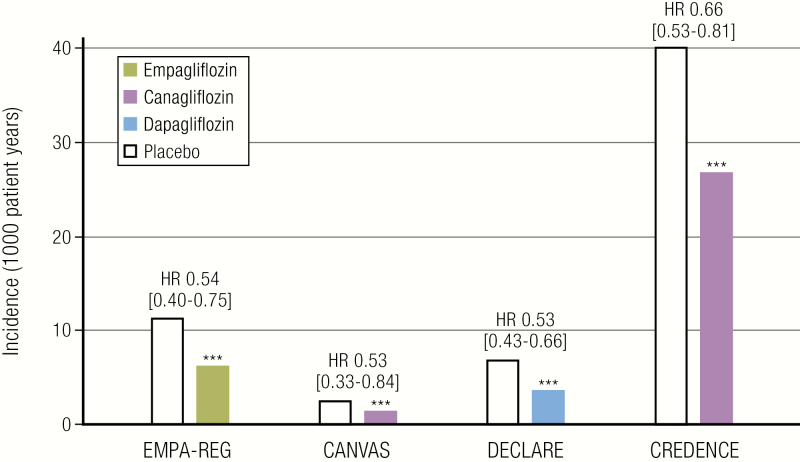
To determine if the compelling mechanistic rationale for the use of SGLT2i translated into both safety and efficacy in patients with T2D, large cardiovascular outcome trials were performed or are still under way with agents in this class (Table 2) (49–51). In 2015, the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) trial was reported. In this trial involving 7020 participants with established CV disease and T2D, empagliflozin reduced the primary 3-point nonfatal myocardial infarction, nonfatal stroke, and CV death (MACE) outcome significantly, and also reduced CV death, overall mortality, and HHF (51). From a renal perspective, the composite of progression of albuminuria, doubling of creatinine, renal replacement therapy, or renal death was significantly reduced by 39%, driven by declines in the risk of reaching the first 3 components of the composite endpoint (Fig. 2) (75). The safety profile of empagliflozin was reassuring, and there was a significant decrease in the risk of acute kidney injury. In 2017, the results of the second SGLT2i CVOT – the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program – was reported (50). In brief, in this trial involving participants with (∼2/3) and without (∼1/3) established CVD, 3-point MACE and HHF were also significantly reduced, but the effect on mortality did not reach significance. For renal outcomes, progression of albuminuria was reduced by 40%, and the composite of 40% decline in eGFR or renal replacement therapy or renal death reduced by 40%. In contrast with EMPA-REG OUTCOME, however, the risks of amputation and fracture were significantly increased in the canagliflozin treatment group, effects that have not yet been reported in CVOTs with other SGLT2i agents to date. As in EMPA-REG OUTCOME, the risk of acute kidney injury tended to be lower with canagliflozin. The third SGLT2i CVOT, Dapagliflozin Effect on Cardiovascular Events (DECLARE TIMI-58), with dapagliflozin was performed in the lowest CV risk cohort – with established CVD in only 40% of the cohort (49). Despite this low baseline risk, dapagliflozin significantly reduced the co-primary endpoint of CV death or HHF. In DECLARE TIMI-58, the risks of mortality or MACE were not, however, reduced and the positive trial result was primarily on the basis of a decline in HHF. For renal endpoints, the authors reported ≥40% decrease in eGFR to <60 mL/min/1.73 m2, end-stage renal disease (ESRD), or death from renal or CV disease was reduced by 24%, whereas the composite ≥40% eGFR to <60 mL/min/1.73 m2, ESRD, or death from renal disease was reduced by 47%. The ≥40% eGFR decline to <60 mL/min/1.73 m2 outcome alone was reduced by 46% and the combined risk of ESRD or renal death, despite low numbers of events, was reduced by 59% (83). Cardiorenal composite outcomes were improved with dapagliflozin consistently across prespecified subgroups by eGFR and the presence or absence of atherosclerotic CV disease, suggesting SGLT2i mediated early prevention and reduction in DKD progression across a broad range of baseline risk in patients with T2D – a view supported by recent meta-analyses (84). What makes the observations from DECLARE TIMI-58 distinct from the results of EMPA-REG OUTCOME and the CANVAS Program was the lower baseline risk profile of the cohort. Only 7.4% of the participant cohort had GFR <60 mL/min/1.73 m2 and the mean eGFR was 85 mL/min/1.73 m2 in DECLARE – 10 mL/min/1.73 m2 higher than the other trials, and yet participants still derived significant renal protection by dapagliflozin. The renal benefit of dapagliflozin on eGFR was observed by 3 years of treatment in DECLARE-TIMI 58, which is longer than in previously reported trials, likely because of the low prevalence of increased albuminuria and slow rate of renal function decline in people with preserved renal function (83). Subsequent meta-analyses have further reported that renal benefits are statistically even greater in people with better preservation of kidney function at baseline (49). As in EMPA-REG OUTCOME and CANVAS, the risk of acute kidney injury was lower in dapagliflozin treated patients in the DECLARE trial, intriguing observations that merit further investigation to better understand the physiological basis for this effect.
Table 2.
Summary of Selected Large Clinical Trials with SGLT2i, DPP-4 Inhibitors, PKCβ Inhibitors, Anti-inflammatory Agents, Mineralocorticoid Receptor Antagonists, and Endothelin Receptor Antagonists
| Study | Treatment Arms | Duration | Patient Cohort | Outcome |
|---|---|---|---|---|
| SGLT2i | ||||
| Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) NCT01131676 | Empagliflozin 10 mg or 25 mg daily vs. placebo | Up to 4.6 years | 7020 T2D patients with established CV complications (≥18 years) | Primary: 14% reduction in 3-point MACE pooled from 10 mg and 25 mg empagliflozin doses Secondary: 35% reduction in hospitalization for HF, 39% reduction in the composite renal endpoint (new macroalbuminuria, doubling of serum creatinine and GFR ≤45, renal replacement therapy, renal death) |
| Canagliflozin Cardiovascular Assessment Study (CANVAS Program) NCT01032629 | Canagliflozin 100 mg or 300 mg daily vs. placebo | 3.6 years | 10,142 T2D patients with established vascular complications or ≥2 CV risk factors (>30 years) | Primary: 14% reduction in 3-point MACE Secondary: 27% reduction in progression of albuminuria, 70% increase in regression of albuminuria, 40% reduction in the composite renal endpoint (40% reduction in eGFR, renal replacement therapy, renal death) |
| Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) NCT02065791 | Canagliflozin 100 mg daily vs. placebo | 2.6 years | 4401 T2D patients with Stage 2 or 3 CKD and macroalbuminuria and on ACEi/ARB | Primary: 30% reduction in ESKD, S-creatinine doubling, renal/CV death Secondary: 20% reduction in MACE; 39% reduction in hospitalization for CHF; 34% reduction in composite renal endpoint (ESKD, doubling of serum Cr, renal death) |
| Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI 58) NCT01730534 | Dapagliflozin 10 mg vs. placebo | 4.2 years | 17,160 T2D patients with high risk for CV events | Primary: No reduction in MACE; 17% reduction in CV death, hospitalization resulting from HF Secondary: 24% reduction in renal composite endpoint (≥40% decrease in eGFR to <60 and/or ESRD and/or renal or CV death |
| DAPA-CKD NCT02065791 | Dapagliflozin vs. placebo | Ongoing | T2D with DKD or nondiabetic kidney disease with eGFR ≥25 to ≤75 and mL/min/1.73 m2 UACR ≥200 to ≤5000 mg/g | Ongoing: Kidney composite endpoint (≥50% sustained decline in eGFR, ESKD, or kidney or CVD death) |
| EMPA-KIDNEY (NCT03594110) | Empagliflozin vs. placebo | Ongoing | DKD (T2D or T1D) or nondiabetic kidney disease with eGFR ≥20 to <45 mL/min/1.73 m2OR eGFR ≥45 to <90 mL/min/1.73 m2 with UACR ≥200 mg/g | Ongoing: Composite outcome of time to first occurrence of kidney disease progression (ESKD, sustained decline in eGFR to <10 mL/min/1.73 m2, ESKD, kidney death, or a sustained decline of ≥40% in eGFR from randomization), or cardiovascular death |
| DPP-4 Inhibitors | ||||
| The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) NCT00790205 | Sitagliptin 100 mg vs. placebo | 3 years | 14,671 T2D patients with established CV disease (≥50 years) | Primary: No reduction in 4-point MACE, hospitalization for unstable angina |
| The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus Thrombolysis in Myocardial Infarction (SAVOR-TIMI 53) NCT01107886 | Saxagliptin 5 mg vs. placebo | 2.1 years | 16,492 T2D patients with established CV disease or multiple risk factors for CV disease | Primary: No reduction in 3-point MACE |
| Cardiovascular Outcomes Study of Alogliptin in Patients with Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) NCT00968708 | Alogliptin 25 mg vs. placebo | 18 months | 5380 T2D patients with recent acute coronary syndrome event | Primary: No reduction in 3-point MACE |
| The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) NCT01897532 | Linagliptin 5 mg vs. placebo | 1.9 years | 6991 T2D patients with high risk for CV events, BMI ≤45 | Primary: No reduction in 3-point MACE. Secondary: No reduction in the composite of adjudication-confirmed ESRD, death due to renal failure, or a sustained decrease of at least 50% in eGFR from baseline |
| Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes (CAROLINA) NCT01243424 | Linagliptin 5 mg vs. Glimepiride 1–4 mg | >6 years | 6 033 T2D patients at increased CV risk or established CV disease | Primary: No reduction in the composite endpoint of MACE or hospitalization for unstable angina pectoris |
| PKCβ Inhibitors | ||||
| Treatment of Peripheral Neuropathy in Patients With Diabetes NCT00044421 | Ruboxistaurin mesylate 32 mg vs. placebo | 2.7 years | 707 T2D participants with diabetic neuropathy | Patients treated with ruboxistaurin had lower urinary albumin-to-creatinine ratio and higher estimated GFR |
| Anti-inflammatory Agents | ||||
| The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) NCT01327846 | Canakinumab 300 mg vs canakinumab 150 mg vs placebo | 3.7 years | 10,061 adults with a history of myocardial infarction and systemic inflammation (elevated high sensitivity CRP >2 mg/mL) -40% had T2D and 46% of patient with CKD in the trial had T2D | 15% reduction in 3-point MACE. Subsequent post hoc analyses demonstrated that the risk of MACE was reduced in people with CKD and in those with albuminuria or diabetes. |
| A Study to Evaluate the Safety and Efficacy of CCX140-B in Subjects With Diabetic Nephropathy NCT01447147 | CCX140-B 10 mg (CCR2 inhibitor) vs CCX140-B 5 mg vs. placebo | 52 weeks | 332 T2D patients with proteinuria, GFR ≥25 mL/min/1.73 m2 | Albuminuria lowering |
| Effects of Selonsertib in Patients with Diabetic Kidney Disease NCT02177786 | 1:1:1:1 allocation to selonsertib (oral daily doses of 2, 6, or 18 mg) or placebo | 48 weeks | 333 adults moderate-to-advanced DKD (eGFR of 15–60 mL/min/1.73 m2 at screening) and albuminuria, defined as a urine albumin-to-creatinine ratio (UACR) ≥600 mg/g if stage 3a CKD, UACR) ≥300 mg/g if stage 3b CKD, and UACR) ≥150 mg/g if stage 4 CKD | Primary endpoint: no difference in eGFR at 48 weeks. B In post hoc analyses, from 4 and 48 weeks, eGFR decline was reduced by 71% for the 18-mg group vs. placebo (difference 3.11 mL/min/1.73 m2 per year, P = 0.043). Effects on urine albumin-to-creatinine ratio did not differ between selonsertib and placebo. |
| A Multicenter Clinical Trial of Allopurinol to Prevent Kidney Function Loss in Type 1 Diabetes NCT02017171 | Allopurinol vs. placebo | Ongoing Start date: February 2014 Completion date: June 30, 2019 | 530 patients with T1D and microalbuminuria or moderate macroalbuminuria or evidence of kidney function decline regardless of albuminuria | Primary endpoint: iohexol GFR at the end of the 2-month washout period |
| Mineralocorticoid Receptor Antagonists | ||||
| MinerAlocorticoid Receptor Antagonist Tolerability Study–Heart Failure ARTS-HF NCT01807221 | Finerenone (multiple doses) vs. eplerenone | 90 days | 1066 patients with worsening HF and reduced ejection fraction and CKD and/or T2D | Finerenone reduced a composite endpoint of death from any cause, cardiovascular hospitalizations, or emergency presentation for worsening HF; reduced albuminuria |
| ARTS–Diabetic Nephropathy ARTS-DN NCT1874431 | Finerenone (multiple doses) vs. placebo | 90 days | 823 T2D patients with high or very high albuminuria who are on ACEs or ARBs | Reduced albuminuria |
| Efficacy and Safety of Finerenone in Subjects with Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD) NCT02540993 | Finerenone (10 mg vs. 20 mg vs. placebo) | Ongoing Start date September 17, 2015 Estimated end date May 25 2020 | 5734 T2D patients with DKD (persistent high albuminuria or very high albuminuria) | Ongoing Primary endpoint – time to first occurrence of the composite of onset of kidney failure, a sustained decrease of eGFR ≥40% from baseline over at least 4 weeks and renal death |
| Efficacy and Safety of Finerenone in Subjects with Type 2 Diabetes Mellitus and a Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD) NCT02540993 | Finerenone (10 mg vs. 20 mg vs. placebo) | Ongoing Start date September 17, 2015 Estimated end date June 21, 2021 | 7437 T2D patients with DKD (persistent high albuminuria or very high albuminuria) | Ongoing Primary endpoint – time to first occurrence of the composite endpoint of cardiovascular death and nonfatal cardiovascular events (myocardial infarction, stroke, or hospitalization for heart failure) |
| Endothelin Receptor Antagonists | ||||
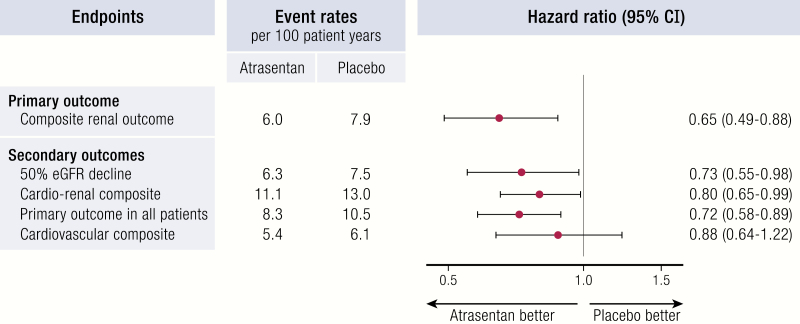
| SONAR NCT01858532 | Atrasentan 0.75 mg vs. placebo | 2.2 years | 2648 patients with T2D, eGFR 25–75 mL/min/1.73 m2 and ACR 300-500 mg/g on RAAS blockers | Early termination (lower number of events), 35% relative risk reduction of doubling of serum creatinine or ESKD |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CHF, congestive heart failure; CKD, chronic kidney disease; Cr, creatinine; CV, cardiovascular; DPP, dipeptidyl peptidase; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; HF, heart failure; MACE, myocardial infarction, nonfatal stroke, and cardiovascular death; MI, myocardial infarction; PKCβ, protein kinase C β; SGLT2i, sodium glucose co-transporter 2 inhibitors; T2D, type 2 diabetes; UACR, urine albumin-to-creatinine ratio.
Figure 2.
Summary of renal composite outcomes with SGLT2 inhibition in EMPA-REG OUTCOME, CANVAS, DECLARE, and CREDENCE Trials. The renal composite outcome shown for EMPA-REG, CANVAS, and CREDENCE is incident or worsening nephropathy, defined as progression to macroalbuminuria; a doubling of the serum creatinine level; the initiation of renal-replacement therapy; or death from renal disease. For DECLARE, the renal composite outcome shown is sustained decline of at least 40% in estimated glomerular filtration rate to <60 mL/min/1.73 m2, end-stage renal disease, or death from renal causes. CANVAS, Canagliflozin Cardiovascular Assessment Study; CREDENCE, Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy; DECLARE, Dapagliflozin Effect on Cardiovascular Events; EMPA-REG, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes; HR, hazard ratio.
SGLT2i in primary renal outcome trials.
Beyond secondary renal outcome results from CVOTs, in 2019, the results of the CREDENCE trial were reported (85). In this DKD cohort with eGFR between 30 and 90 mL/min/1.73 m2 and macroalbuminuria, the primary endpoint, which was the composite of ESKD, doubling of serum creatinine, or renal or CV death was reduced by 30% after canagliflozin treatment. Albuminuria was reduced by 31% in the canagliflozin treatment group and eGFR decline attenuated by 2.7 mL/min/year/1.73 m2. The results observed were similar across patients with different albuminuria levels and CKD status. Similar to previously reported CVOTs, the risk of HHF decreased by 39%; however, cardiovascular and renal death outcomes decreased, but did not reach statistical significance. Perhaps as expected, the participant cohort in the CREDENCE study was at a much higher risk of renal disease progression compared with risks reported in previous CVOTs, since the CREDENCE cohort had significantly lower mean eGFR (56 mL/min/1.73 m2) and higher albuminuria and blood pressure at baseline. In addition to being the first primary renal outcome trial reported, the results of this trial were critical to show the safety profile for the use of these medications in patients with DKD. Unlike in the CANVAS study with canagliflozin, the CREDENCE study did not detect any signal of amputation or fracture risk. On the basis of physiological data suggesting nonglycemic pathways that underlie renal protection with SGLT2i, 2 additional renal outcome trials, EMPA-KIDNEY and DAPA-CKD, are under way and are also enrolling people with CKD but without diabetes. These trials will therefore be critical to extend observations from small mechanistic trials in participants without diabetes, and will ultimately demonstrate whether the results from CREDENCE will alter general practice pattern in normoglycemic individuals (86, 87).
Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide (GLP)-1 is an intestinal hormone secreted following food ingestion. It contributes to reducing postprandial glucose levels by stimulating insulin secretion, reducing glucagon release, slowing down gastric emptying, reducing hepatic glucose production, as well as satiety induction, reducing meal size (88). Because of its short half (˜2–3 minutes) following degradation by the enzyme dipeptidyl peptidase (DPP)-4, GLP-1 is not suitable for clinical application. However, DPP-4-resistant GLP-1 receptor agonists have been developed with half-lives of hours to days (89). These drugs are frequently used for glucose-lowering in patients with T2D. Beyond reductions in HbA1c levels of 0.3% to 1.9%, GLP-1 receptor agonists also lower blood pressure (˜2–3 mm Hg) body weight (3 kg), while improving lipid profiles (90, 91). Because all these factors are associated with the development of CVD and DKD, there is a rationale for cardiorenal protective effects with GLP-1 receptor agonists. Accordingly, to establish both the safety and efficacy of GLP-1 receptor agonists, these agents were extensively examined in CVOTs across a range of study cohorts, as summarized in Table 3. In a meta-analysis including CVOTs for lixisenatide, exenatide, liraglutide, and semaglutide, all-cause mortality was reduced by 12% (hazard ratio [HR], 0.88, 95% confidence interval [CI], 0.81-0.95, P = 0.002), cardiovascular mortality by 13% (HR, 0.87, 95% CI, 0.79-0.96, P = 0.007), and 3-point MACE by 10% (HR, 0.90, 95% CI, 0.82-0.99, P = 0.033) compared with placebo (92). However, treatment effects for a shorter acting agent, lixisenatide, was smaller in magnitude compared with the longer acting agents, such as liraglutide (93–96), suggesting clinically important heterogeneity within this class of medications. GLP-1 receptor agonists appear to mainly affect atherosclerosis-related endpoints, in contrast with SGLT2i agents that reduce the risk of HHF but not myocardial infarction or stroke (92). CVOTs with GLP-1 receptor agonists have also included secondary renal analyses, which have yielded important results. In the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) (93), Liraglutide Effects and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) (96, 97), Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes, (95) and Exenatide Study of Cardiovascular Event Lowering (92) trials, decrements in absolute urinary albumin excretions as well as reductions in the endpoint of new-onset or persistent macroalbuminuria were observed. Remarkably, these renal effects do not appear to depend on changes in traditional renal risk factors (98, 99), including body weight (100). Although dedicated clinical renal endpoint data with GLP-1 receptor agonists are currently lacking, the Study Comparing Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes (T2D) and Moderate or Severe Chronic Kidney Disease (CKD) (AWARD-7) trial with dulaglutide in patients with CKD stages 3 and 4 showed significant antialbuminuric effects despite similar glycemic control vs. the active comparator, insulin glargine, further suggesting that antialbuminuric effects are independent of glycemic lowering (101). In 1 of the most recently published CVOTs, Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes, a cohort with the lowest overall CVD risk profile and lowest renal risk also reported a significant 12% reduction in the primary outcome, 3-point MACE, which was driven by the 24% reduction in the risk of stroke. This trial did not necessarily yield new insight into potential cardiorenal protective mechanisms because HbA1c declined by 0.61% and body weight by 1.5 kg (102). In this relatively low-risk group for DKD progression, the majority of study participants had preserved kidney function and were normoalbuminuric (103). The secondary nephropathy endpoint (new macroalbuminuria, 30% fall in eGFR, or renal replacement therapy) was reduced by 15%; only the progression to macroalbuminuria component was significant. In line with effects in previous work, urine albumin-to-creatinine ratio (UACR) was reduced by 18%, in the absence of significant renal function preservation over time. The modest effect on albuminuria is consistent with previous GLP1-RA studies and tends to occur early during the course of treatment. The absence of an effect on renal function preservation may have been anticipated based on the study cohort’s very low risk for DKD progression at baseline (preserved baseline eGFR, low prevalence, and degree of albuminuria). The trial duration would therefore likely have had to be much longer to capture more definitive renal endpoints such as eGFR decline. Interestingly, in sensitivity analyses, the risk of a 40% decline in eGFR was significantly lower in dulaglutide-treated participants, albeit with a small number of events. Finally, in the first completed CVOT with an oral GLP-1 receptor agonist, A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes with oral semaglutide, 3-point MACE (and each of the MACE components) was not significantly reduced, whereas the HR for all-cause mortality was 0.51 (95% CI, 0.31-0.84) despite a relatively small number of events (104). No renal data have been published to date.
Table 3.
Cardiovascular Outcome Trials With Glucagon Like Peptide-1 Receptor Agonists
| ELIXA | LEADER | SUSTAIN 6 | EXSCEL | HARMONY | REWIND | PIONEER-6 | |
|---|---|---|---|---|---|---|---|
| No. of patients | 6068 | 9340 | 3297 | 14,752 | 9463 | 9901 | 3183 |
| GLP-1 RA agent | Lixisenatide | Liraglutide | Semaglutide | Exenatide | Albiglutide | Dulaglutide | Semaglutide (oral) |
| Dosing | Daily | Daily | Weekly | Weekly | Weekly | Weekly | Daily |
| History of prior CVD | 100% | 81% | 83% | 73% | 100% | 31% | 85% (CVD or CKD) |
| Mean age (years) | 60 | 64 | 54 | 62 | 64 | 66 | 66 |
| Women | 30% | 36% | 39% | 38% | 31% | 46% | 32% |
| Median follow-up | 2.1 years | 3.8 years | 2.1 years | 3.2 years | 1.6 years | 5.4 years | 15.9 months |
| Diabetes duration (years) | 9.3 | 12.8 | 13.9 | 13.1y | 14.2y | 10.5 | 14.7 |
| Baseline A1c | 7.7% | 8.7% | 8.7% | 8.1% | 8.8% | 7.3% | 8.2% |
| Baseline eGFR (mL/min/1.73 m2) | 76 | ~75 | ~75 | 76 | 79 | 77 | 74 |
| UACR | Median 10.4 mg/g | Median ∼50 mg/g (eGFR<60 mL/min/1.73 m2) and ∼16 mg/g (eGFR ≥60 mL/min/1.73 m2) | N/A | N/A | N/A | 1.82 mg/g | N/A |
| SBP | 130 | 136 | 136 | 135 | 135 | 137 | 135 |
| DBP | N/A | 77 | 77 | 80 | 77 | 78 | 76 |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); N/A, not available; RA, ; SBP, systolic blood pressure; UACR, albumin to creatinine ratio (mg/g).
Despite consistent effects showing albuminuria lowering with GLP-1 receptor agonists, the mechanisms responsible for the decline in albuminuria remain poorly understood. Outside of modifying traditional renal risk factors, it has been suggested that decreased urine albumin excretion rates may be due to direct GLP-1 receptor effects in the human kidney (3). This may be of clinical and/or physiological importance because the GLP-1 receptor has been localized to the afferent arteriole in some human studies (105). To determine if this afferent localization translates into effects on renal function, carefully designed studies in people with T2D using inulin and p-aminohippuric acid infusion techniques measured changes in GFR and renal plasma flow failed to demonstrate that GLP-1 receptor agonists reduce glomerular pressure or have other beneficial renal hemodynamic actions (3, 106). This is in line with observed eGFR trajectories in larger clinical trials where no clinically relevant effects on eGFR deterioration or slopes were observed over the long term (97, 99), with the exception of a modest eGFR preservation effect in the 30 to 60 mL/min/1.73 m2 subgroup in LEADER (97), and a 1 and 2 mL/min/y preservation of eGFR in AWARD-7 at 52 weeks (100).
Regarding renal tubular effects, a number of studies indicate that GLP-1 receptor agonists acutely induce sodium excretion, likely because of inhibition of the sodium hydrogen antiporter NHE3 in the proximal tubule (3, 107, 108), possibly through effects on protein kinase A–serine phosphorylation pathways (109, 110). NHE3, which is located at the brush border of the renal proximal tubule, is bound to a complex that also contains DPP4. Pharmacological doses of GLP-1 or GLP-1R agonists increase intrarenal cAMP generation, protein kinase A activation, and phosphorylation of NHE3 at the PKA consensus sites Ser552 and Ser605, which reduces its activity. Conversely, GLP-1R blockade with exendin 9 reduces renal cortical phosphorylation at Ser552 of NHE3 (111). The GLP-1 receptor agonist-induced increment in fractional lithium clearance and urinary pH further support the concept that these agents therapies act via NHE3-related effects (112). However, to what extent the temporary increase in natriuresis, which does not seem to alter intra- or extracellular volumes, contributes to renal outcomes, is uncertain. In addition, the role of circulating neurohormones such as atrial natriuretic peptide as a cause of the natriuresis in humans remains uncertain. Animal models have supported the concept that GLP-1 receptor agonism mediates natriuresis via atrial natriuretic peptide, an observation not replicated in humans with T2D (113). Other proposed mechanisms underlying the potential renoprotective effects of GLP-1 receptor agonists include: 1) reduction of inflammation/oxidative stress; 2) improvement of insulin sensitivity and mitochondrial function, possibly via improved glycemic control and alterations in pathways related to adaptive thermogenesis such as AMPK-SIRT-1-PGC1-alpha cell signaling (114) and effects on mTOR-dependent HIF-1alpha activation (115); and 3) direct effects of GLP-1 on the tubular cell, independent of receptor-mediated mechanisms, possibly via GLP-1 breakdown products (116–118). Regardless of the principal mechanisms, existing renal data support the concept that clinical benefits are largely on the basis of preventing albuminuria progression, a surrogate measure of renal and cardiovascular risk with all of its limitations, rather than preventing harder renal outcomes (70–72, 119). Specifically, although albuminuria is strongly associated with renal function decline and end-stage kidney disease, this is certainly not always the case, and many patients with albuminuric DKD progress. Nevertheless, data from AWARD-7 and LEADER have suggested a modest effect on preserving eGFR in patients with eGFR <60 mL/min/1.73 m2 at baseline (120). Whether GLP-1 receptor agonists are renal protective will ultimately be known after the completion of the Semaglutide on the Progression of Renal Impairment in Subjects With Type 2 Diabetes and Chronic Kidney Disease (NCT03819153) trial with semaglutide, which is being tested in patients with DKD at baseline. Based on effects on urinary albumin to creatinine ratio and intriguing but modest protection against renal function decline in cohort from CVOTs with eGFR impairment, these dedicated, long-term studies in people with diabetes and renal function impairment are necessary to assess whether or not GLP-1 receptor agonists alter hard renal outcomes.
Renal effects and therapeutic implication of DPP-4 inhibitors
The other incretin-based therapy is the DPP-4 inhibitor class, which prevents degradation of GLP-1, thereby lowering postprandial glucose levels. As with GLP-1 receptor agonists, DPP-4 inhibitors are also natriuretic, an effect that is incompletely understood but has been attributed to inhibition of the sodium hydrogen antiporter NHE3 in the proximal tubule, and to more distally acting pathways via the stromal cell–derived factor 1-alpha. A proximally acting natriuresis would be expected to increase sodium excretion and to trigger tubuloglomerular feedback mechanisms, although these agents do not affect eGFR in mechanistic studies or eGFR in clinical trials over time (110). As a consequence, it has been suggested that distal natriuretic mechanisms resulting from DPP-4 inhibitor mediated increases in levels of the cytokine stromal cell–derived factor-1 alpha are the predominant cause of natriuresis with these agents (121). Because of distal location of this natriuretic effect, tubuloglomerular feedback is not activated and renal function is not affected. Beyond this lack of hemodynamic effect, DPP-4 inhibitors decrease oxidative stress, inflammation, albuminuria, and glomerular sclerosis in animal models (122). Existing clinical data related to DPP-4 inhibitors suggests that in contrast with GLP-1 receptor agonists, they are neutral in terms of renal and vascular protection. Aside from a 28% greater reduction in albuminuria after a 24-week treatment with linagliptin compared with placebo in a pooled analysis with 217 patients with T2D and albuminuria (123), renal protective effects have not been observed in dedicated prospective clinical trials. For example, in the Efficacy, Safety & Modification of Albuminuria in Type 2 Diabetes Subjects With Renal Disease With Linagliptin trial involving 360 individuals with T2D and albuminuria, treatment with linagliptin for 24 weeks significantly improved glycemic control but failed to lower albuminuria or improve eGFR when compared with placebo (124). An even longer treatment duration CVOT study, Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus, showed that in 6979 participants with T2D and high CV and renal risk, linagliptin treatment for 2.2 years was noninferior for CV or renal outcomes compared with placebo across the spectrum of kidney disease (125, 126).
SGLT2i, incretins, and the potential for combination therapy
Given that the mechanisms involved in renoprotection with SGLT2i appear to differ from the potential renoprotective effects observed with GLP-1 receptor agonists and DPP-4 inhibitors, it is important to determine if a combination of these agents have additive renal benefits. Additionally, improved glycemic control with various glucose lowering therapies, especially GLP1-RA, may play a role in improving renal function and therefore should be addressed in future studies. To date, only a single study has examined whether the SGLT2 and the DPP-4 inhibitor combination can lower albuminuria in people with T2D and moderate to severe CKD. In the Albuminuria-lowering Effect of Dapagliflozin Alone and in Combination With Saxagliptin and Effect of Dapagliflozin and Saxagliptin on Glycaemic Control in Patients With Type 2 Diabetes and Chronic Kidney Disease trial, people with micro- or macroalbuminuria and eGFR 25 to 75 mL/min/1.73 m2 randomized to dapagliflozin alone or in combination with saxagliptin had a significant decrease in albuminuria by 21% and 38% compared with placebo, respectively. Between-group albuminuria differences were not significant even though dual therapy produced additional glycemic lowering (85). Whether adding a DPP-4 inhibitor to SGLT2i-based therapy provides additive long-term renal protection requires a large dedicated outcome trial.
Despite the strong rationale for both SGLT2i and GLP-1 receptor agonists reviewed previously, no studies in humans have assessed the role of combining SGLT2i and GLP-1 receptor agonists as a cardiorenal protective strategy. To date, evidence supporting the use of combination SGLT2i plus GLP-1 receptor agonists has been limited to the DURATION-8 trial examining exenatide, dapagliflozin, and their combination. Although DURATION-8 suggested improved glycemic measures and cardiovascular risk with dual treatment in patients with T2D, the effects on renal function are not yet reported (127). However, with respect to body weight and blood pressure lowering, effects were greater with combination therapy vs. either drug alone (128). Whether these metabolic and blood pressure effects translate into renal or CV benefits is not yet known, but warrants further investigation.
Therapeutic implications
On the basis of observations from CVOTs, clinical practice guidelines have started to change over the past 3 years, resulting principally in modified recommendations around when to use SGLT2i and GLP-1 receptor agonists in people with established CVD and suboptimal glycemic control (129, 130). Subsequent changes to guidelines have included recommendations around when to consider the use of SGLT2i in people with HF and diabetes and have also lowered the lower eGFR limit for these agents as a recognition of the preserved cardiorenal effects in patients down to an eGFR of 30 mL/min/1.73 m2. With the results of CREDENCE, with a background of supportive secondary renal data from CVOTs involving >40,000 participants, it seems reasonable to expect a broad-based shift in renal guidelines for patients with proteinuric DKD in the near future. Within 6 weeks of the CREDENCE trial results being released, the American Diabetes Association Living standards of Medical Care in Diabetes issued an update to its guidelines stating that clinicians should consider the use of an SGLT2i in patients with T2D and DKD (eGFR ≥30 and particularly in those with >300 mg/g albuminuria) to reduce risk of kidney disease progression, cardiovascular events, or both (Grade A recommendation). The recommendation also states that for patients with T2D with CKD who are at increased risk for CV events, use of a GLP-1 receptor agonist may reduce risk of progression of albuminuria, cardiovascular events, or both (Grade C).
From a safety perspective, SGLT2i therapies are generally well tolerated. The most common side effect is genital mycotic infections, which may occur in 5% to 10% of users, fortunately leads to few discontinuations in clinical trials (51). Other extremely rare side effects such as diabetic ketoacidosis have been reported in some but not all studies at a frequency of <0.5% (49). Patients prescribed SGLT2i should be provided with “sick day” advice to avoid becoming volume depleted around episodes of intercurrent illness or hospitalization (131). For GLP-1 receptor agonists, the most common side effect is nausea and gastrointestinal upset (significant side effects leading to discontinuation, 0.5%-2.0%), which can be managed with slow up-titration of therapy and starting at lower doses (96).
Insights that have been obtained from recent CVOTs and DKD trials therefore have direct implications for the treatment of patients in general practice, and in endocrine, cardiology, and nephrology specialty clinics (132–135). SGLT2i can, based on currently available data, be used safely with other agents that influence blood pressure and renal function. Nevertheless, there are specific clinical circumstances that require more caution, including hospitalized patients, the perioperative setting, patients with history of urinary and genital tract infections, or dynamic circulating volume status, as described elsewhere (136).
Novel Therapeutic Areas Targeting Hyperglycemia-Related Pathways
Protein kinase Cβ inhibition and DKD
PKCβ has been proposed as an important regulator of the pro-oxidant and pro-apoptotic function, and is thought to be activated in the setting of diabetes by hyperglycemia and activation of the RAAS (137). PKCβ activation is in turn linked with vasoconstriction and the development of diabetic vascular dysfunction (137). In both experimental and clinical studies, PKCβ inhibition with LY333531 (ruboxistaurin) has salutary cardiorenal effects (138–140). In a murine model, for example, LY333531 reduced apoptosis of glomerular endothelial cells in animals with evidence of DKD (141). In small human mechanistic studies, ruboxistaurin reduced renal hyperfiltration, albuminuria and the urinary excretion of pro-inflammatory and pro-fibrotic biomarkers including TGF-β (142–144). In the systemic circulation in patients with T2D, ruboxistaurin attenuates hyperglycemia-mediated endothelial dysfunction (145, 146). In long-term studies of ruboxistaurin, the effect on albuminuria has not been consistent. For example, in studies focused on diabetic retinopathy progression, kidney outcome rates did not differ between ruboxistaurin vs. placebo (147). In contrast, in a phase 3, randomized, double-blind, placebo-controlled, multicenter clinical trial (NCT00044421) in people with T2D and diabetic neuropathy, ruboxistaurin therapy for 36 months was associated with lower urinary albumin-to-creatinine ratio and higher estimated GFR compared with placebo (148). Although adequately powered clinical trials are warranted to determine whether PKC-β inhibition impedes progression of DKD in T1D and/or T2D, no trials are currently under way to elucidate these potential benefits. Nevertheless, PKC-β-related pathways may still have relevance for several novel therapeutic agents, discussed in the following section.
Selected antioxidant therapies in DKD
In addition to activation of PKCβ, hyperglycemia has been linked with increased levels of oxidative stress, resulting in renal injury. As a consequence, therapies to suppress oxidative stress are a promising target in DKD and have shown benefit in animal models. For example, nicotinamide adenine dinucleotide phosphate oxidases (NOX) isoform 1 and 4 are upregulated in the setting of ambient hyperglycemia and promote oxidative stress pathways leading to DKD progression. Conversely, inhibition of NOX1 and 4 with GKT137831 attenuates histological evidence of diabetic nephropathy in murine models (149, 150). Other experimental models using pharmacological inhibitors of NOX4 have reported kidney protective effects, although molecules used in studies in this field may have multiple targets, including PKCβ, making it difficult to be certain which physiological effect is dominant (151). In terms of translation of this work to humans, a safety and efficacy trial of oral GKT137831 failed to achieve the primary outcome of albumin excretion reduction in people with T2D (NCT02010242), suggesting more work is required to understand the clinical relevance of suppressing oxidative stress with this modality in humans (152). As a final comment, the NOX5 isoform (expressed in humans but not in rodents) has also been linked with target organ injury in diabetes including DKD, but has not yet been examined in clinical trials (153, 154).
A second major class of antioxidants that are being studied in the setting of DKD are related to the Kelch-like ECH-associated protein 1 and NRF2 system, which regulates genes involved in cellular redox homeostasis (155). In a previous earlier phase clinical trial with bardoxolone called BEAM (156), the NRF2 activator bardoxolone was associated with an increase in eGFR at 3 weeks, in conjunction with a rise in UACR, for reasons that are not yet well understood. The subsequent BEACON trial with bardoxolone was terminated early because of concerns around cardiovascular risk and increases in UACR and B-type natriuretic peptide, both markers of cardiorenal risk (157). It is not yet clear whether the risks observed with bardoxolone are unique to this agent or it is a class effect. Ongoing trials in DKD and Alport syndrome will therefore exclude patients with elevated baseline B-type natriuretic peptide (BNP) levels in an effort to exclude people at high risk of volume overload, as reviewed elsewhere (155).
Inhibitors of AGE and extracellular AGEs that act on the RAGE in DKD
The altered intracellular metabolism characteristic of diabetes also includes accumulation of intracellular AGEs and extracellular AGEs that act on RAGE (158). Renal accumulation of AGEs has been implicated in the pathogenesis of DKD by increasing oxidative stress and by activating PKCβ (158–161). In a murine model, continuous infusion of DNA aptamers (chemical equivalent of an antibody capable of binding to proteins or other cellular targets) directed against RAGE attenuated the development and progression of experimental diabetic nephropathy by blocking the AGE-RAGE axis (162). The results from the phase 2 clinical trial evaluating the 6-month effects of the RAGE inhibitor, TTP488, on urinary ACR in people with T2D and persistent elevated albumin excretion has not been published (NCT00287183).
Nonglucose-Lowering Agents
Anti-inflammatory agents
Hyperglycemia, intraglomerular hypertension, neurohormonal activation, and a variety of other factors activate pro-inflammatory and pro-fibrotic pathways, thereby leading to renal injury in the setting of diabetes. Therefore, agents targeting inflammatory and fibrotic pathways have been of significant therapeutic interest in renal protection studies in preclinical and clinical studies of DKD. Agents that more selectively suppress inflammation and fibrosis, without altering renal or systemic hemodynamics, have the potential to be used safely in combination with traditional renal protective strategies (RAAS inhibition, blood pressure control) and with newer glucose-lowering agents because of a lower likelihood of causing hemodynamic compromise. We have focused the review of these medications to those that have been studied in clinical trials in humans, including 1L-β antagonists and agents that antagonize monocyte chemoattractant protein (MCP-1). Other molecules targeting inflammation have been investigated in people with DKD but are not under active development, including Pyridorin, pentoxifylline, and therapies that target Janus kinase–signal transducer and activator of transcription signaling (baricitinib) (163). Synthetic analogues of medium chain-length fatty acids such as PBI-4050 (3-pentylbenzenacetic acid sodium salt) have also been designed as synthetic analogues of medium chain-length fatty acids including free fatty acid receptor 1 and GPR84 to suppress renal inflammation. In an experimental model of T2D, PBI-4050 reduced glomerular injury, albuminuria, GFR decline, and proinflammatory/pro-fibrotic pathways. Although of potential significant future interest, human data with PBI-4050 are not available (164).
1L-β is a pro-inflammatory factor secreted largely in circulating monocytes, but can also be induced in epithelial cells in kidneys and podocytes (165). Genetic variants in the IL-1-related genes are associated with an increased risk of ESKD (165). Of particular interest for cardiorenal protection are agents that block IL-1β such as canakinumab. Canakinumab is a human monoclonal antibody that leads to sustained reductions in fibrinogen, IL-6, and C-reactive protein (CRP) without affecting lipid levels in people with T2D (166). The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) was conducted in 10,061 adults with a history of myocardial infarction and systemic inflammation (elevated high-sensitivity CRP >2 mg/mL) to specifically target inflammation without confounding lipid-lowering effects in people with and without CKD. In the overall cohort, 40% had a history of T2D, whereas 46% of patients with CKD who enrolled in the trial had a background of T2D. Canakinumab treatment at 150 mg daily for 3.7 years reduced the risk of the primary 3-point MACE endpoint compared with placebo (HR, 0.85, P = 0.021), and also reduced the secondary 4-point MACE (addition of hospitalization for unstable angina requiring urgent revascularization, HR 0.83, P = 0.0005) (167). The main effect of canakinumab was driven by a lower incidence of myocardial infarction. Subsequent post hoc analyses demonstrated that the risk of MACE was reduced in people with CKD (eGFR <60 mL/min/1.73 m2) and in those with albuminuria or diabetes (168). Although this observation is important to show that canakinumab is well tolerated in people with CKD or diabetes, canakinumab did not impact CKD progression, with the caveat that the cohort in CANTOS was not enriched for CKD risk factors such as albuminuria or impaired eGFR. Therefore, to determine if this agent is protective against CKD progression, future trials with canakinumab should to enroll much higher risk individuals with DKD, possibly for an extended period. Dedicated studies will also be needed to determine whether blockade of IL-1β could result in safe, complementary effects when combined with more contemporary therapies that mitigate the risk of CKD progression such as SGLT2i and GLP-1RAs.
Recent studies in both diabetic mice and humans suggest upregulation of a pro-inflammatory cytokine, IL-20, which is further increased in those with renal dysfunction (169). Reactive oxygen species and hyperglycemia were shown to upregulate IL-20 stimulating podocyte production of TGF-β1, VEGF, MCP-1, MMP-9, and podocyte apoptosis through the caspase-8 pathway, thereby promoting renal fibrosis. Given the contribution of IL-20 to inflammation and diabetic glomerulopathy, it may be an important therapeutic target to mitigate renal injury and slow down DKD progression. A human recombinant monoclonal antibody for IL-20 has already shown a reassuring safety profile in initial human studies (170). Given promising findings of renal protection with anti-IL-20 monoclonal antibodies in murine models, future studies are needed to translate findings in animal models to humans and determine how such therapies can be combined with other anti-inflammatory agents to enhance protective effects.
ASK1 is another important signaling pathway through which oxidative stress promotes inflammation, apoptosis, and fibrosis. During conditions of increased oxidative stress and/or reactive oxygen species, ASK1 is activated through autophosphorylation, leading to activation of the MAPKs p38 and c-Jun N-terminal Kinase, which drive CKD progression through inflammation, apoptosis, and fibrosis (171–173). Specifically, in people with DKD, ASK1 may play a central role in maintaining the pathological interaction between oxidative stress and inflammation resulting in progressive nephron loss. Selonsertib is a potent inhibitor of ASK1 and is expected to slow or halt the progression of renal disease (174). The long-term efficacy and safety of selonsertib was tested in a phase 2 clinical trial in participants with DKD over 48 months. Although the primary endpoint (change from baseline eGFR at 48 weeks) was not different vs. placebo, in post hoc analyses examining eGFR changes from week 4 to week 48, the rate of eGFR decline was significantly reduced by 3.33 mL/min/1.73 m2 per year at the highest 18-mg dose of selonsertib vs. placebo, suggesting a renal protective effect (175).
An increasing body of literature also suggests an important role of chemokines and chemokine receptors in the pathogenesis of DKD (176). MCP-1, also known as C-C motif chemokine ligand 2 (CCL2) is linked with renal parenchymal inflammation in experimental models (177). Urinary excretion of CCL2 correlates with DKD severity in T2D patients (178). In animal models of DKD, inhibition of CCL2 is associated with renal protection, including decreases in glomerulosclerosis, podocyte loss, and albuminuria (179, 180), whereas in people with DKD and albuminuria, agents that target CCL2 significantly decrease albuminuria (181, 182). Pilot studies with DMX-200, an antagonist of C-C motif chemokine receptor 2, have also shown a significant additive albuminuria lowering effects when added for 12 weeks to RAAS blockade with irbesartan in people with T2D. Clinical studies with a larger sample size using DMX-200 are ongoing. A 52-week treatment with another selective C-C motif chemokine receptor 2 antagonist (CCX140-B) on top of standard of care also resulted in albuminuria-lowering effects in people with T2D and DKD (181). Data regarding long-term preservation of kidney function with these agents are, however, not yet available (181).
In summary, promising observations, especially around surrogate markers including albuminuria, suggest a possible role for anti-inflammatory agents as renal protective agents in people with DKD. Enriched longer term clinical endpoint trials are required to ultimately determine whether there is a role for these therapies in the clinical management of people with DKD, both in terms of safety and efficacy.
Mineralocorticoid receptor antagonists
The mineralocorticoid hormone aldosterone is produced in the adrenal cortex and is stimulated by factors such as hypovolemia, sodium deficiency, and particularly angiotensin II. Aldosterone, through intracellular mineralocorticoid receptor activation, maintains blood pressure by promoting reabsorption of sodium and water in the distal tubule (13). However, in people with diabetes, increased aldosterone activity in the kidney is linked with the development of hypertension, glomerular injury, renal vasoconstriction, and proteinuria (183). Aldosterone synthesis is only partly prevented by ACE inhibition or angiotensin II receptor blocker therapies (184). Targeting aldosterone with MRAs, such as spironolactone and eplerenone, reduces albuminuria on top of RAAS blockade in people with DKD; however, because of undesired side effects such as hyperkalemia and gynecomastia, their use is limited (185–187). Newer generations of more selective, nonsteroidal MRAs, most notably finerenone, are in development and could potentially have a more favorable side effect profile compared with older MRAs. In earlier studies such as the Mineralocorticoid Receptor Antagonist Tolerability Study–Heart Failure and ARTS–Diabetic Nephropathy, finerenone reduced albuminuria, and the risk of hyperkalemia leading to discontinuation was low (188, 189). Two ongoing, separate phase 3 trials are examining the effects of finerenone on DKD and cardiovascular outcomes, including Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (NCT02545049) and Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (NCT02540993) (190, 191).
Endothelin receptor antagonists
The endothelin family comprises 3 endothelins (ET-1, ET-2, and ET-3), which were discovered in the late 1980s and may bind to either the ETA or ETB receptor. The ET system plays an important role in sodium and water regulation. ETB activation predominantly accounts for the natriuresis via effects at the proximal tubule, and also leads to vasodilatation, whereas ETA activation is linked with sodium retention and vasoconstriction (192, 193). Accordingly, for pharmacological blockade of ET receptors to be effective and lead to blood pressure lowering, natriuresis, and efferent relaxation, it is critical to take advantage primarily of ETA antagonism and to avoid ETB blockade to reduce the risk of volume retention and heart failure, a significant risk that often occurs in people with T2D and CKD (194, 195). The Achilles’ heel of many ERAs has, unfortunately, been a relative lack of drug selectivity for ETA (with some exceptions such as zibotentan), leading to concomitant ETA and ETB blockade and clinically apparent edema and/or heart failure, especially at higher ETA/ETB receptor antagonist (ERA) drug doses where deleterious ETB blocking effects are more apparent.
Several factors, all of them activated in people with T2D and CKD, stimulate the release of ET-1, such as hyperglycemia, dyslipidemia, endothelial dysfunction, and oxidative stress. ET-1 can in turn cause kidney injury through potent efferent arteriolar vasoconstrictive effects, causing glomerular hypertension. Other deleterious effects caused by ET-1 release include activation of pro-inflammatory and pro-fibrotic pathways (196).
Experimental and clinical studies have shown that ETA and ERAs reduce proteinuria and preserve kidney function in the longer term. The salutary effects of ERAs on kidney protection may in part be explained by hemodynamic effects. In hypertensive patients with CKD, ERA strategies increased effective renal plasma flow and reduced filtration fraction, suggesting that ET-1 activation is mediated by vasoconstriction of the efferent arteriole and glomerular hyperfiltration (197, 198). However, other effects of ERAs contributing to long-term kidney preservation may also be involved. For example, ERAs preserve endothelial glycocalyx function, which can be injured in the setting of diabetes, and is essential for preventing albumin leakage and a key regulator of vascular homeostasis. Improvements in glycocalyx function are accompanied by reductions in albuminuria, highlighting a potential link between improvements in glycocalyx function, glomerular permselectivity, and albuminuria (199). Finally, ERAs preserve podocyte function and improve glomerular selectivity as demonstrated in cultured podocytes derived from animal and human cell lines (200). It is important to note in this context that increased protein exposure to podocytes may release ET-1 and thereby cause kidney damage (200). Whether ERAs exert direct beneficial effects on podocytes or whether these beneficial effects are secondary to improvement in glycocalyx function and decreased albumin leakage, thereby reducing podocyte exposure to filtered albumin and other proteins, remains to be elucidated. ERAs therefore exert protective effects in the kidney that may be ubiquitous and beneficial across different CKD etiologies. The best evidence for kidney protection has, however, been derived from trials involving patients with T2D.
Because these beneficial effects are observed only in relatively small studies, ERAs are currently not available for clinical use. In previous work, a large phase 3 outcome trial with the ERA avosentan was completed but the trial was stopped early because of an increased risk of congestive HF associated with this ERA (194). In hindsight, the relatively low selectivity of avosentan for the ETA receptor as well as the high doses (25 and 50 mg/d) tested in this trial likely increased the rate of fluid retention and congestive HF in a population with diabetes and stage 3 and 4 CKD who are particularly vulnerable to fluid retention. This trial reinforced the importance of careful dose selection when clinical trials are performed in people with CKD, especially for drugs with a narrow therapeutic window (201).
Compared with avosentan, the ERA atrasentan exhibits a higher selectivity for the ETA receptor. Atrasentan was originally developed for prostate cancer and has been tested in clinical trials at doses of 10 mg/d. Low doses of atrasentan (0.75 and 1.25 mg/d) resulted in significant reductions in albuminuria with modest fluid retention in a phase 2 study (202). These beneficial effects led to the design of the Study of Diabetic Nephropathy with Atrasentan (SONAR) trial, which used a design that is novel to clinical trials in people with diabetes and CKD. In contrast to other trials, the SONAR trial used an active open-label run-in period, enrichment period, during which all participants received atrasentan for 6 weeks. The purpose of the enrichment period was to identify atrasentan responders, defined by a reduction in albuminuria of more than 30%, who also did not have a body weight increase more than 3 kg and BNP >300 pg/mL (203). The intent was to enrich the study population with individuals who are likely to exhibit benefit on long-term kidney outcomes and who are at the same time unlikely to develop heart failure in clinical practice. Unfortunately, the trial was stopped early because of a lower than anticipated event rate for the primary kidney outcome over the trial period. Despite the early termination, lower number of events, and hence lower statistical power, the trial demonstrated a statistically significant 35% relative risk reduction for the primary outcome of doubling of serum creatinine or ESKD (Fig. 3) (14). Nevertheless, the incidence of hospital admissions for heart failure with atrasentan (3.5%) was higher vs. placebo (2.6%), indicating that despite the precautionary approaches implemented in the trial careful monitoring of fluid retention (body weight and BNP monitoring) is necessary if ERAs were to be used in clinical practice.
Figure 3.
Forest plot of hazard ratios for the primary and secondary outcomes of the SONAR Trial. Composite renal outcome: composite of doubling of serum creatinine (sustained for ≥30 days) or end-stage kidney disease (eGFR <15 mL/min/1.73 m2 sustained for ≥90 days, chronic dialysis for ≥90 days, kidney transplantation, or death from kidney failure. Cardiorenal composite endpoint: doubling serum creatinine, end-stage kidney disease, cardiovascular death, nonfatal myocardial infarction, or non-fatal stroke. Primary outcome in all patients (responders and nonresponders combined). Cardiovascular composite endpoint: cardiovascular death, nonfatal myocardial infarction, or non-fatal stroke. Abbreviations: CI, confidence interval; eGFR, glomerular filtration rate; SONAR, Study of Diabetic Nephropathy with Atrasentan.
Clinical studies with 2 other ERAs are currently ongoing. Aprocitentan is under investigation for treatment of resistant hypertension (NCT03541174). Sparsentan, a dual-acting angiotensin II receptor blocker (irbesartan) and highly selective ETA is being tested for its renoprotective effect in a phase 3 trial of patients with primary focal segmental glomerulosclerosis (NCT03493685) and in another trial in patients with IgA nephropathy (NCT03762850). In summary, these trials, together with the results of the SONAR, will ultimately define whether ERAs are used for the treatment of CKD, both in people with and without diabetes.
Neprilysin inhibitors
The neutral endopeptidase neprilysin contributes to the breakdown of several natriuretic peptides, which regulate sodium and fluid balance by promoting natriuresis and diuresis, and also promotes vasodilatation. A neprilysin inhibitor combined with valsartan reduced mortality and heart failure hospitalization in people with heart failure with reduced ejection fraction in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (204). Interestingly, in this overall cohort composed of 8399 participants, compared with enalapril, sacubitril/valsartan led to a slower rate of eGFR decline (–1.3 vs. –1.8 mL/min/1.73 m2 per year; P < 0.0001), even though urine protein excretion increased (205). In the overall cohort, 45% of participants had diabetes at baseline. In the subgroup with diabetes, the impact of sacubitril/valsartan on kidney protection was greater compared with participants without diabetes, an effect that was accompanied by a statistically significant, but clinically small improvement in glycemic control in post hoc analyses of the trial (between-group reduction in HbA1c, 0.13%, 95% CI, 0.05-0.22, P = 0.0023) (206). For the enhanced kidney protection in the subgroup with T2D, it is possible that normalization of glomerular pressure with these therapies contributes both to eGFR preservation and changes in albuminuria, a hypothesis that merits further testing (207). In patients with preserved ejection fraction who participated in the Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction trial (NCT01920711), sacubitril/valsartan also had renal beneficial effects because this agent reduced the risk of worsening renal function, which occurred in 1.4% of patients in the sacubitril–valsartan group and in 2.7% in the valsartan group (HR, 0.50; 95% CI, 0.33-0.77) (208).
Because renal dysfunction in HF is often due to kidney hypoperfusion, a vasodilatory effect may be particularly beneficial in this population to mitigate progressive kidney blood flow decline and hypoxic injuries in the setting of HF (209). Beneficial physiological effects in the kidney with sacubitril/valsartan were also suggested in the United Kingdom Heart and Renal Protection-III involving 414 participants (40% with a history of diabetes at baseline) with eGFR 20 to 60 mL/min/1.73 m2 and without HF. In this shorter term, 12-month trial, sacubitril/valsartan reduced blood pressure, NT-pro-BNP, and troponin I significantly more compared with irbesartan, but without affecting eGFR or UACR (210). Thus, based on available evidence, neprilysin inhibitor-based therapy has a salutary effect on kidney function, only in the setting of HF, and effects may be greatest in those with DKD vs. nondiabetic kidney disease.
Uric acid-lowering agents
The relationship between uric acid, hypertension, and kidney disease was first noted in the 1870s. Uric acid has been shown to be an independent risk factor for progression of CKD in general, and especially in the setting of diabetes, as well as a risk factor for cardiovascular mortality in people with diabetes through activation of oxidative stress and neurohormones including the RAAS (211–213). In human trials, therapies to lower serum uric acid, including allopurinol, reduce albumin excretion and slow eGFR decline in adults with and without T2D (214–217). In adults with T1D, plasma uric acid lowering with febuxostat modestly lowered blood pressure without affecting the RAAS, suggesting that lowering uric acid may influence other hemodynamic or inflammatory mechanisms (218). A randomized, placebo controlled, double blinded, crossover trial in 26 older adults with T1D found no treatment effect of allopurinol on albumin excretion or measured GFR by 51Cr-EDTA over 2 months. The negative findings may relate to the short duration of therapy because other studies have shown that it takes at least 4 months to observe an effect of uric acid lowering on albumin excretion (216). As a caveat, because of the sample size, it would not have been feasible to determine the effect of baseline uric acid levels on trial outcomes. The Preventing Early Renal function Loss (PERL) trial is under way and will help determine whether uric acid lowering with allopurinol vs. placebo will protect against progression of DKD in adults with T1D (219, 220). In contrast with other kidney endpoint trials, in this review, which used indirect creatinine-based estimates of GFR, the PERL trial used direct iohexol-derived measures of GFR to assess kidney function change over time, which are more precise and accurate that conventional clinical measurements.
Vasopressin-lowering agents
Vasopressin concentrations are elevated in the setting of T1D and T2D (221–223). The leading stimuli for vasopressin secretion are increased plasma osmolality and/or decreased arterial circulating volume. Indeed, a relative fluid deficit secondary to glycosuria is thought to explain the higher vasopressin concentrations observed in people with diabetes. In addition to stimulating water reabsorption, vasopressin has antinatriuretic properties (222, 224–228) and increases the mRNA expression and subsequent protein abundance of the Na+/K+ ATPase (229, 230). Vasopressin also has important intrarenal hemodynamic effects that could raise intraglomerular pressure and promote kidney injury, irrespective of the underlying cause of CKD. Vasopressin receptors are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. Although further research is needed, the majority of renal effects are likely mediated by actions on the V2 receptors that are abundantly found in the vascular endothelium, smooth muscle, and tubular cells of the kidney. V2 receptors are expressed on the afferent arterioles, and short-term infusion of vasopressin results in afferent arteriolar vasodilation through direct V2 agonism, in addition to efferent arteriolar vasoconstriction via RAAS activation, which collectively increases GFR in experimental models (221, 231, 232) and in postcardiac surgery patients (228). Further, vasopressin infusion increases renal oxygen consumption, likely by virtue of increased sodium reabsorption and Na+/K+-ATPase with resultant renal hypoxia (228). Consistent with these observations, the selective vasopressin receptor 1a antagonist, relcovaptan, improves renal oxygenation in a murine model (233). Further, dual V1 and V2 antagonism potentiates the renoprotection of RAAS inhibition in rats with renal mass reduction (234).
Outside the setting of DKD, the selective V2 antagonist, tolvaptan, is renal protective in adults with autosomal-dominant polycystic kidney disease (235, 236). Tolvaptan also resulted in a slower eGFR decline than placebo in people with later stage autosomal-dominant polycystic kidney disease over 12 months (237). A significant advantage of vasopressin blockers is their dual site of action (glomerular and tubular), which is mechanistically attractive in DKD because these patients are at risk for both glomerular and tubulointerstitial injury. Although thirst and dry mouth are the most commonly reported adverse effects of selective vasopressin V2 antagonists, it is important to note that tolvaptan and satavaptan have been associated with risk for increased of changes in liver enzymes, thereby requiring people taking these therapies to undergo liver function monitoring (237). Further, satavaptan has been associated with increased risk of gastrointestinal bleeding (238, 239). Lixivaptan, another selective V2 antagonist, does not appear to be associated with gastrointestinal bleeding or hepatotoxicity (240, 241). V2 antagonism has been shown to reduce albuminuria and prevent hyperfiltration in an animal model of DKD (232). However, there are no published human data available on the effects of vasopressin receptor antagonism on mechanisms of disease or clinical DKD progression in the setting of diabetes.
Other Emerging Therapeutic Targets
Autophagy
Autophagy, which is a cellular recycling process, is crucial in maintaining kidney homeostasis, and is modulated by several mechanisms implicated in the pathogenesis of DKD, including RAAS activation, insulin resistance, oxidative stress, AGEs, and hypoxia (242, 243). Autophagy defects, which impair self-degradation and repair of damaged organelles and proteins, occur at different stages of kidney disease (242). In fact, in DKD, autophagy is important to protect against podocyte loss, albuminuria (244), and profibrotic pathways associated with hyperglycemia (245). Accordingly, a murine model of diabetes using a knockout of a critical autophagy pathway (autophagy-related gene 5) was associated with progression of DKD (245–247). Conversely, stimulating autophagy by decreasing activation of the mTORC1 in diabetic mice was shown to slow down DKD progression (245, 248, 249). Similarly, autophagy may protect mesangial cells from apoptosis stimulated by TGF-β1 via TAK1 and PI3K-AKT related pathways in DKD (250). Because DKD is associated with decreased autophagy and increased apoptosis, upregulation of autophagy mediated pathways may be a useful therapeutic target.
Although several drugs have demonstrated inhibition or activation of autophagy experimentally, these efforts are still in early development. For example, metformin is known to upregulates AMPK signaling, which may stimulate autophagy function (245). More work, however, is required to determine the clinical relevance of autophagy to human disease and how we can use therapeutic agents to modify such mechanisms for renal protection.
SRC family kinases
Fibrotic diseases account for up to 45% of deaths in the developed world, and kidney fibrosis is a recognized complication of DKD (251). Despite the gravity of fibrosis and importance in diabetes, approved antifibrotic therapies for DKD do not yet exist. Src family kinases is a group of proto-oncogenic nonreceptor tyrosine kinases that are ubiquitously expressed in all cell types and implicated in fibrosis through activation of profibrotic processes including epidermal growth factor receptor and signal transducer and activator of transcription 3. Inhibition of Src family kinases has been shown to attenuate tissue fibrosis (251). For example, inhibition of Src kinase by PP1 has been shown to block both epidermal growth factor receptor and signal transducer and activator of transcription 33 (252). Src kinase is activated by hyperglycemia, which leads to activation of epidermal growth factor receptor and MAPK and collagen IV synthesis (253). Src inhibitors (PP2 and SU-6656) and small interfering RNA-mediated Src knockdown has been shown to inhibit the activation of TNF-α-converting enzyme, epidermal growth factor receptor, MAPK (ERK1/2 and p38), and collagen IV accumulation (253). In a T1D murine model, administration of PP2 was associated with attenuation of albuminuria, glomerular matrix protein accumulation, glomerular basement membrane thickening, and podocyte loss (253, 254). Accordingly, studies examining the relationship of these pathways with human disease is warranted.
Metabolic peptides
Experimental models suggest that diabetes is associated with an environment that initially upregulates ATP consumption because of: 1) increased Na+ reabsorption mediated by glucosuria, prolonged exogenous supraphysiological insulin exposure (255–260), and elevated vasopressinergic and RAAS activity (222, 225, 227, 228); and 2) increased filtration of Na+ resulting from elevated GFR (i.e., hyperfiltration) (261–263). Further, emerging animal data suggest that in diabetes, the kidneys are unable to sufficiently compensate for the increased ATP consumption because of the effects of insulin resistance and mitochondrial dysfunction on substrate utilization (impaired ATP generation) (256–259). The net effect of the mismatch between increased ATP consumption and decreased ATP generation is increased renal O2 consumption because the majority of ATP produced in the kidneys is through aerobic metabolism (264, 265). The high O2 demand is also followed by increased perfusion to deliver more O2. However, in the kidneys, increased perfusion results in increased GFR and, thus, higher filtered sodium and ATP consumption. This vicious cycle contributes to a supraphysiological increase in renal O2 consumption and ultimately renal hypoxia. In fact, animal diabetes models suggest that renal O2 consumption is increased by 40% in all cortical segments and by 160% each in the S3 segment and medullary collecting duct (266–270). Further, experimental data have established that increased renal O2 consumption results in decreased renal oxygenation and hypoxia in diabetes (270–273).
The metabolic imbalance between renal energy expenditure and substrate utilization underlying DKD is an attractive therapeutic target. There are also small peptides found to selectively target mitochondria to promote oxidative phosphorylation and improve ATP synthesis in preclinical studies (274–277). For example, the Szeto-Schiller (SS) peptide SS-31, which is mitochondria-targeted tetrapeptide that has been shown to scavenge mitochondrial reactive oxygen species, thereby accelerate ATP recovery and reduce ischemic kidney injury (277). SS-31 has also shown to selectively bind to cardiolipin, inhibit cytochrome c peroxidase activity, promote electron transport, and optimize mitochondrial ATP generation (275, 276). More recently, SS31 administration in participants with T2D was found to ameliorate markers of inflammation and oxidative stress (278). In addition to identifying novel therapies, there are several existing interventions which could be repurposed in combination to decrease renal ATP consumption and/or improve ATP generation. A promising example includes combining agents which may lower renal ATP consumption (e.g., vasopressin receptor blockers (227, 228), SGLT2i, dual SGLT1 and 2 inhibitors (271, 279)) with interventions to improve ATP generation (e.g., metabolic peptides, insulin sensitizers, artificial pancreas systems, glucose-responsive insulin). Artificial pancreas technology and glucose-responsive insulin provides intermittent periods without active insulin and are thought to augment insulin sensitivity in animal diabetes models (255, 280) while not jeopardizing glycemic control.
Future Directions
The cardiovascular, kidney, and endocrine communities have witnessed a metamorphosis in the understanding of the pathogenesis and management of diabetes related complications over the past 5 years. During that time, the focus of antihyperglycemic agents has shifted away from hyperglycemia toward the use of other agents, such as SGLT2i, for their glucose-independent and natriuretic effects. As a result, nephrologists and cardiologists have had to get more accustomed to using medications that have traditionally been used by general practitioners, internists, and endocrinologists. Beyond these currently approved glucose-lowering medications, MRAs and endothelin receptor antagonists appear to be the most likely to move into the clinic in the future for use as kidney protective therapies, although the mechanisms of hyperkalemia with MRAs and volume retention with endothelin receptor antagonists will need to be clearly understood to mitigate the risks of such side effects.
As part of the potential use of these therapies, future studies are needed to detail the safety and efficacy of combination therapies. For example, can combination SGLT2i and endothelin receptor antagonists lead to an additive reduction in UACR while at the same time avoid fluid retention? Alternatively, it will be important to determine if putative beneficial hemodynamic effects of SGLT2i lead to additional kidney or cardiovascular protection when combined with agents that target inflammation, fibrosis, and oxygen metabolism. While elucidating such clinically important mechanistic questions, major efforts are also needed to optimize the use of therapies with proven efficacy, including SGLT2i and GLP1-receptor agonists, in patients with T2D. Furthermore, appropriate research strategies will have to be completed, including ongoing clinical trials, to identify novel nondiabetic groups of patients who may benefit from therapies targeting pathways that are ubiquitously linked with cardiorenal disease.
Glossary
Abbreviations
- ACE
angiotensin-converting enzyme
- AGE
advanced-glycosylation end-product
- Ang
angiotensin
- ASK1
apoptosis-signaling kinase 1
- BNP
- CACE
- CCL2
- CI
confidence interval
- CKD
chronic kidney disease
- CRP
C-reactive protein
- CTGF
connective tissue growth factor
- CV
cardiovascular
- CVD
cardiovascular disease
- CVOT
cardiovascular safety trial
- DKD
diabetic kidney disease
- DPP
dipeptidyl peptidase
- eGFR
estimated glomerular filtration rate
- ERA
ETA/ETB receptor antagonist
- ESKD
end-stage kidney disease
- ET
endothelin
- GLP-1
glucagon-like peptide
- HHF
hospitalization for heart failure
- HR
hazard ratio
- MACE
myocardial infarction, nonfatal stroke, and cardiovascular death
- MCP
monocyte chemoattractant protein
- MRA
mineralocorticoid receptor antagonist
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- NRF2
nuclear factor erythroid 2-related factor 2
- PKCβ
protein kinase C β
- RAAS
renin-angiotensin-aldosterone system
- RAGE
receptor for advanced-glycosylation end-product
- SGLT2i
sodium glucose co-transporter 2 inhibitor
- SS
Szeto-Schiller
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- UACR
urine albumin-to-creatinine ratio
Additional Information
Disclosure Summary: All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Y.L., D.H.R., and H.L.H. have nothing to declare. P.B. receives salary and research support by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIDDK; K23 DK116720-01), in addition to research support by Thrasher Research Fund, Juvenile Diabetes Research Foundation (JDRF), NIDDK/DiaComp, International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical & Translational Sciences Institute (CCTSI), and Center for Women’s Health Research at University of Colorado; has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, and Horizon Pharma; and served on the advisory board of XORTX. D.Z.I.C. has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck, and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, and Merck.
Reference and Notes
- 1. Collins AJ, Foley RN, Herzog C, et al. US renal data system 2010 annual data report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8, e1-526. [DOI] [PubMed] [Google Scholar]
- 2. Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, et al. Horizon 2020 in diabetic kidney disease: the clinical trial pipeline for add-on therapies on top of renin angiotensin system blockade. J Clin Med. 2015;4(6):1325–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tonneijck L, Muskiet MH, Smits MM, van et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zatz R, Meyer TW, Rennke HG, Brenner BM. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc Natl Acad Sci U S A. 1985;82(17):5963–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15 Suppl 1:S55–S57. [DOI] [PubMed] [Google Scholar]
- 6. Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23(7):579–591. [DOI] [PubMed] [Google Scholar]
- 7. Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol. 2006;17(6):1703–1709. [DOI] [PubMed] [Google Scholar]
- 8. de Azevedo MJ, Ramos OL, Gross JL. Lack of effect of captopril on glomerular hyperfiltration in normoalbuminuric normotensive insulin-dependent diabetic patients. Horm Metab Res. 1997;29(10):516–519. [DOI] [PubMed] [Google Scholar]
- 9. Ficociello LH, Perkins BA, Silva KH, et al. Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol. 2007;2(3):461–469. [DOI] [PubMed] [Google Scholar]
- 10. Fried LF, Emanuele N, Zhang JH, et al. ; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903. [DOI] [PubMed] [Google Scholar]
- 11. Vidt DG. Telmisartan, ramipril, or both in patients at high risk for vascular events. Curr Hypertens Rep. 2008;10(5):343–344. [DOI] [PubMed] [Google Scholar]
- 12. Parving HH, Brenner BM, McMurray JJ, et al. ; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. [DOI] [PubMed] [Google Scholar]
- 13. Lytvyn Y, Godoy LC, Scholtes RA, van Raalte DH, Cherney DZ. Mineralocorticoid antagonism and diabetic kidney disease. Curr Diab Rep. 2019;19(1):4. [DOI] [PubMed] [Google Scholar]
- 14. Heerspink HJL, Parving HH, Andress DL, et al. ; SONAR Committees and Investigators. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. [DOI] [PubMed] [Google Scholar]
- 15. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. [DOI] [PubMed] [Google Scholar]
- 16. Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong J, Guo D, Chen CB, et al. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57(2):314–322. [DOI] [PubMed] [Google Scholar]
- 18. Oudit GY, Herzenberg AM, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168(6):1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72(5):614–623. [DOI] [PubMed] [Google Scholar]
- 20. Nadarajah R, Milagres R, Dilauro M, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82(3):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74(12):1610–1616. [DOI] [PubMed] [Google Scholar]
- 22. Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51(4):613–623. [DOI] [PubMed] [Google Scholar]
- 23. Liu CX, Hu Q, Wang Y, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17(1-2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burns WC, Velkoska E, Dean R, Burrell LM, Thomas MC. Angiotensin II mediates epithelial-to-mesenchymal transformation in tubular cells by ANG 1-7/MAS-1-dependent pathways. Am J Physiol Renal Physiol. 2010;299(3):F585–F593. [DOI] [PubMed] [Google Scholar]
- 25. Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes. 2012;3(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cherney DZ, Reich HN, Jiang S, et al. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;303(7):R710–R718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cherney DZ, Miller JA, Scholey JW, et al. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes. 2008;57(3):688–695. [DOI] [PubMed] [Google Scholar]
- 28. VR ALBVR, Tan SH, Candasamy M, Bhattamisra SK. Diabetic nephropathy: An update on pathogenesis and drug development. Diabetes Metab Syndr. 2019;13(1):754–762. [DOI] [PubMed] [Google Scholar]
- 29. Klimontov VV, Korbut AI. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab Syndr. 2019;13(1):474–479. [DOI] [PubMed] [Google Scholar]
- 30. Voelker J, Berg PH, Sheetz M, et al. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28(3):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adler SG, Schwartz S, Williams ME, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol. 2010;5(8):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma K, Ix JH, Mathew AV, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22(6):1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jun M, Ohkuma T, Zoungas S, et al. ; ADVANCE Collaborative Group. Changes in albuminuria and the risk of major clinical outcomes in diabetes: results from ADVANCE-ON. Diabetes Care. 2018;41(1):163–170. [DOI] [PubMed] [Google Scholar]
- 35. Bjornstad P, Cherney DZ, Maahs DM, Nadeau KJ. Diabetic kidney disease in adolescents with type 2 diabetes: new insights and potential therapies. Curr Diab Rep. 2016;16(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA. [DOI] [PubMed] [Google Scholar]
- 37. Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. [DOI] [PubMed] [Google Scholar]
- 38. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. [DOI] [PubMed] [Google Scholar]
- 39. Cherney D, Perkins BA, Lytvyn Y, Heerspink H, Rodríguez-Ortiz ME, Mischak H. The effect of sodium/glucose cotransporter 2 (SGLT2) inhibition on the urinary proteome. Plos One. 2017;12(10):e0186910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93(1):231–244. [DOI] [PubMed] [Google Scholar]
- 41. Bjornstad P, Laffel L, Tamborlane WV, et al. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care. 2018;41(8):e129–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. León Jiménez D, Cherney DZI, Bjornstad P, Guerra LC, Miramontes González JP. Antihyperglycemic agents as novel natriuretic therapies in diabetic kidney disease. Am J Physiol Renal Physiol. 2018;315(5):F1406–F1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int. 2016;89(3):524–526. [DOI] [PubMed] [Google Scholar]
- 44. Eickhoff MK, Dekkers CCJ, Kramers BJ, et al. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med. 2019;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verma S, McMurray JJV, Cherney DZI. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2(9):939–940. [DOI] [PubMed] [Google Scholar]
- 47. Lovshin JA, Cherney DZ. Sodium transport in diabetes: two sides to the coin. Nat Rev Nephrol. 2019;15(3):125–126. [DOI] [PubMed] [Google Scholar]
- 48. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–363. [DOI] [PubMed] [Google Scholar]
- 49. Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 50. Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 51. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 52. Karg MV, Bosch A, Kannenkeril D, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sugiyama S, Jinnouchi H, Kurinami N, et al. The SGLT2 inhibitor dapagliflozin significantly improves the peripheral microvascular endothelial function in patients with uncontrolled type 2 diabetes mellitus. Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin attenuates cardiac remodeling in mice model of cardiac pressure overload. Am J Hypertens. 2019;32(5):452–459. [DOI] [PubMed] [Google Scholar]
- 58. Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38(5):356–363. [DOI] [PubMed] [Google Scholar]
- 60. Goldenberg RM, Berall M, Chan CTM, et al. Managing the course of kidney disease in adults with type 2 diabetes: from the old to the new. Can J Diabetes. 2018;42(3):325–334. [DOI] [PubMed] [Google Scholar]
- 61. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94(1):26–39. [DOI] [PubMed] [Google Scholar]
- 62. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098–2107. [DOI] [PubMed] [Google Scholar]
- 63. Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140(4):303–315. [DOI] [PubMed] [Google Scholar]
- 65. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. [DOI] [PubMed] [Google Scholar]
- 66. Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int. 2014;86(5):1057–1058. [DOI] [PubMed] [Google Scholar]
- 67. Rajasekeran H, Lytvyn Y, Bozovic A, et al. Urinary adenosine excretion in type 1 diabetes. Am J Physiol Renal Physiol. 2017;ajprenal 00043 02017. [DOI] [PubMed] [Google Scholar]
- 68. Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57(12):2599–2602. [DOI] [PubMed] [Google Scholar]
- 69. Denic A, Mathew J, Lerman LO, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376(24):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heerspink HJL, Coresh J, Gansevoort RT, Inker LA. Change in albuminuria as a surrogate endpoint in chronic kidney disease - Authors’ reply. Lancet Diabetes Endocrinol. 2019;7(5):336–337. [DOI] [PubMed] [Google Scholar]
- 71. Heerspink HJL, Greene T, Tighiouart H, et al. ; Chronic Kidney Disease Epidemiology Collaboration. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2):128–139. [DOI] [PubMed] [Google Scholar]
- 72. Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium . Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26(8):2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Van Bommel EJ, Muskiet MH, van Baar MJB, et al. Dapagliflozin Reduces Measured GFR by Reducing Renal Efferent Arteriolar Resistance in Type 2 Diabetes. San Francisco, CA: American Diabetes Association 79th Scientific Sessions; 2019:Abstract S-157. [Google Scholar]
- 74. Barnett AH, Mithal A, Manassie J, et al. ; EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–384. [DOI] [PubMed] [Google Scholar]
- 75. Wanner C, Inzucchi SE, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 76. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. [DOI] [PubMed] [Google Scholar]
- 77. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860–1870. [DOI] [PubMed] [Google Scholar]
- 78. Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–621. [DOI] [PubMed] [Google Scholar]
- 79. Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308(2):F77–F83. [DOI] [PubMed] [Google Scholar]
- 80. Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20(8):1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van Raalte DH, Cherney DZI. Sodium glucose cotransporter 2 inhibition and renal ischemia: implications for future clinical trials. Kidney Int. 2018;94(3):459–462. [DOI] [PubMed] [Google Scholar]
- 82. Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. [DOI] [PubMed] [Google Scholar]
- 84. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. [DOI] [PubMed] [Google Scholar]
- 85. Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 86. Rajasekeran H, Reich HN, Hladunewich MA, et al. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol. 2018;314(3):F412–F422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Škrtić M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24(1):96–103. [DOI] [PubMed] [Google Scholar]
- 88. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 89. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. [DOI] [PubMed] [Google Scholar]
- 90. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dieter BP, Alicic RZ, Tuttle KR. GLP-1 receptor agonists in diabetic kidney disease: from the patient-side to the bench-side. Am J Physiol Renal Physiol. 2018;315(6):F1519–F1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bethel MA, Patel RA, Merrill P, et al. ; EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–113. [DOI] [PubMed] [Google Scholar]
- 93. Pfeffer MA, Claggett B, Diaz R, et al. ; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. [DOI] [PubMed] [Google Scholar]
- 94. Holman RR, Bethel MA, Mentz RJ, et al. ; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 96. Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mann JFE, Ørsted DD, Brown-Frandsen K, et al. ; LEADER Steering Committee and Investigators. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. [DOI] [PubMed] [Google Scholar]
- 98. Mann JFE, Ørsted DD, Buse JB. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(22):2197–2198. [DOI] [PubMed] [Google Scholar]
- 99. Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):859–869. [DOI] [PubMed] [Google Scholar]
- 100. Tuttle KR, Lakshmanan MC, Rayner B, Zimmermann AG, Woodward B, Botros FT. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Diabetes Obes Metab. 2019;21(6):1493–1497. [DOI] [PubMed] [Google Scholar]
- 101. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. [DOI] [PubMed] [Google Scholar]
- 102. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019. [DOI] [PubMed] [Google Scholar]
- 103. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet (London, England). 2019. [DOI] [PubMed] [Google Scholar]
- 104. Husain M, Birkenfeld AL, Donsmark M, et al. ; PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. [DOI] [PubMed] [Google Scholar]
- 105. Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 106. Tonneijck L, Smits MM, Muskiet MH, et al. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2016;39(11):2042–2050. [DOI] [PubMed] [Google Scholar]
- 107. Muskiet MH, Tonneijck L, Smits MM, et al. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab. 2016;18(2):178–185. [DOI] [PubMed] [Google Scholar]
- 108. Tonneijck L, Smits MM, Muskiet MHA, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;297(6):F1647–F1655. [DOI] [PubMed] [Google Scholar]
- 110. Crajoinas RO, Oricchio FT, Pessoa TD, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301(2):F355–F363. [DOI] [PubMed] [Google Scholar]
- 111. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605–628. [DOI] [PubMed] [Google Scholar]
- 112. Skov J, Pedersen M, Holst JJ, et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18(6):581–589. [DOI] [PubMed] [Google Scholar]
- 113. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38(1):132–139. [DOI] [PubMed] [Google Scholar]
- 114. Zhou J, Poudel A, Chandramani-Shivalingappa P, Xu B, Welchko R, Li L. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-α cell signaling pathway. Endocrine. 2019;64(2):271–283. [DOI] [PubMed] [Google Scholar]
- 115. Carlessi R, Chen Y, Rowlands J, et al. GLP-1 receptor signalling promotes β-cell glucose metabolism via mTOR-dependent HIF-1α activation. Sci Rep. 2017;7(1):2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bangshaab M, Gutierrez A, Huynh KD, et al. Different mechanisms involved in liraglutide and glucagon-like peptide-1 vasodilatation in rat mesenteric small arteries. Br J Pharmacol. 2019;176(3):386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moellmann J, Klinkhammer BM, Onstein J, et al. Glucagon-like peptide 1 and its cleavage products are renoprotective in murine diabetic nephropathy. Diabetes. 2018;67(11):2410–2419. [DOI] [PubMed] [Google Scholar]
- 118. Guglielmi V, Sbraccia P. GLP-1 receptor independent pathways: emerging beneficial effects of GLP-1 breakdown products. Eat Weight Disord. 2017;22(2):231–240. [DOI] [PubMed] [Google Scholar]
- 119. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 120. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–2031. [DOI] [PubMed] [Google Scholar]
- 121. Lovshin JA, Rajasekeran H, Lytvyn Y, et al. Dipeptidyl peptidase 4 inhibition stimulates distal tubular natriuresis and increases in circulating SDF-1α1-67 in patients with type 2 diabetes. Diabetes Care. 2017;40(8):1073–1081. [DOI] [PubMed] [Google Scholar]
- 122. Muskiet MH, van Raalte DH, van Bommel EJ, van Bommel E, Smits MM, Tonneijck L. Understanding EMPA-REG OUTCOME. Lancet Diabetes Endocrinol. 2015;3(12):928–929. [DOI] [PubMed] [Google Scholar]
- 123. Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36(11):3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017;19(11):1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. McGuire DK, Alexander JH, Johansen OE, et al. ; CARMELINA Investigators. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139(3):351–361. [DOI] [PubMed] [Google Scholar]
- 126. Rosenstock J, Perkovic V, Johansen OE, et al. ; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–1016. [DOI] [PubMed] [Google Scholar]
- 128. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543–1556. [DOI] [PubMed] [Google Scholar]
- 129. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McFarlane P, Cherney D, Gilbert RE, Senior P. Chronic kidney disease in diabetes. Can J Diabetes. 2018;42(Suppl 1):S201–S209. [DOI] [PubMed] [Google Scholar]
- 131. Peacock SC, Lovshin JA, Cherney DZI. Perioperative considerations for the use of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Anesth Analg. 2018;126(2):699–704. [DOI] [PubMed] [Google Scholar]
- 132. Cherney DZ, Udell JA. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation. 2016;134(24):1915–1917. [DOI] [PubMed] [Google Scholar]
- 133. Perkins BA, Udell JA, Cherney DZ. No need to sugarcoat the message: is cardiovascular risk reduction from SGLT2 inhibition related to natriuresis? Am J Kidney Dis. 2016;68(3):349–352. [DOI] [PubMed] [Google Scholar]
- 134. Rajasekeran H, Cherney DZ, Lovshin JA. Do effects of sodium-glucose cotransporter-2 inhibitors in patients with diabetes give insight into potential use in non-diabetic kidney disease? Curr Opin Nephrol Hypertens. 2017;26(5):358–367. [DOI] [PubMed] [Google Scholar]
- 135. Rajasekeran H, Kim SJ, Cardella CJ, et al. Use of canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: a case series. Diabetes Care. 2017;40(7):e75–e76. [DOI] [PubMed] [Google Scholar]
- 136. Peacock SC, Lovshin JA, Cherney DZI. Perioperative considerations for the use of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Anesth Anal. 2017. [DOI] [PubMed] [Google Scholar]
- 137. Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87(5):1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Noh H, King GL. The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl. 2007;(106):S49–S53. [DOI] [PubMed] [Google Scholar]
- 139. Budhiraja S, Singh J. Protein kinase C beta inhibitors: a new therapeutic target for diabetic nephropathy and vascular complications. Fundam Clin Pharmacol. 2008;22(3):231–240. [DOI] [PubMed] [Google Scholar]
- 140. Thallas-Bonke V, Cooper ME. Tandem inhibition of PKC in Diαβetic nephropathy: it takes two to tango? Diabetes. 2013;62(4):1010–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang ZB, Zhang S, Li Y, et al. LY333531, a PKCβ inhibitor, attenuates glomerular endothelial cell apoptosis in the early stage of mouse diabetic nephropathy via down-regulating swiprosin-1. Acta Pharmacol Sin. 2017;38(7):1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cherney DZ, Konvalinka A, Zinman B, et al. Effect of protein kinase Cbeta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care. 2009;32(1):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gilbert RE, Kim SA, Tuttle KR, et al. Effect of ruboxistaurin on urinary transforming growth factor-beta in patients with diabetic nephropathy and type 2 diabetes. Diabetes Care. 2007;30(4):995–996. [DOI] [PubMed] [Google Scholar]
- 144. Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28(11):2686–2690. [DOI] [PubMed] [Google Scholar]
- 145. Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90(1):107–111. [DOI] [PubMed] [Google Scholar]
- 146. Mehta NN, Sheetz M, Price K, et al. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther. 2009;23(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW; PKC-DRS, PKC-DMES, and PKC-DRS 2 Study Groups . Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol. 2007;2(4):631–636. [DOI] [PubMed] [Google Scholar]
- 148. Tuttle KR, McGill JB, Bastyr EJ 3rd, Poi KK, Shahri N, Anderson PW. Effect of ruboxistaurin on albuminuria and estimated GFR in people with diabetic peripheral neuropathy: results from a randomized trial. Am J Kidney Dis. 2015;65(4):634–636. [DOI] [PubMed] [Google Scholar]
- 149. Gorin Y, Cavaglieri RC, Khazim K, et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol. 2015;308(11):F1276–F1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gray SP, Jha JC, Kennedy K, et al. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia. 2017;60(5):927–937. [DOI] [PubMed] [Google Scholar]
- 151. Qin J, Peng Z, Yuan Q, et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX pathways. Sci Rep. 2019;9(1):4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Genkyotex. Safety and efficacy of oral GKT1377831 in patient with type 2 diabetes and albuminuria. NCT02010242. 2015. [Google Scholar]
- 153. Touyz RM, Anagnostopoulou A, Rios F, Montezano AC, Camargo LL. NOX5: molecular biology and pathophysiology. Exp Physiol. 2019;104(5):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jha JC, Banal C, Okabe J, et al. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66(10):2691–2703. [DOI] [PubMed] [Google Scholar]
- 155. Nezu M, Suzuki N, Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. 2017;45(6):473–483. [DOI] [PubMed] [Google Scholar]
- 156. Pergola PE, Raskin P, Toto RD, et al. ; BEAM Study Investigators. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. [DOI] [PubMed] [Google Scholar]
- 157. de Zeeuw D, Akizawa T, Audhya P, et al. ; BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369(26):2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions. Eur J Pharmacol. 2018;833:158–164. [DOI] [PubMed] [Google Scholar]
- 159. Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Peng KY, Horng LY, Sung HC, Huang HC, Wu RT. Hepatocyte growth factor has a role in the amelioration of diabetic vascular complications via autophagic clearance of advanced glycation end products: Dispo85E, an HGF inducer, as a potential botanical drug. Metabolism. 2011;60(6):888–892. [DOI] [PubMed] [Google Scholar]
- 161. Penfold SA, Coughlan MT, Patel SK, et al. Circulating high-molecular-weight RAGE ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010;78(3):287–295. [DOI] [PubMed] [Google Scholar]
- 162. Matsui T, Higashimoto Y, Nishino Y, Nakamura N, Fukami K, Yamagishi SI. RAGE-Aptamer blocks the development and progression of experimental diabetic nephropathy. Diabetes. 2017;66(6):1683–1695. [DOI] [PubMed] [Google Scholar]
- 163. Cherney DZI, Bakris GL. Novel therapies for diabetic kidney disease. Kidney Int Suppl (2011). 2018;8(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Li Y, Chung S, Li Z, et al. Fatty acid receptor modulator PBI-4050 inhibits kidney fibrosis and improves glycemic control. JCI Insight. 2018;3(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Anders HJ. Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol. 2016;27(9):2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ridker PM, Howard CP, Walter V, et al. ; CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–2748. [DOI] [PubMed] [Google Scholar]
- 167. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 168. Ridker PM, MacFadyen JG, Glynn RJ, et al. Inhibition of Interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405–2414. [DOI] [PubMed] [Google Scholar]
- 169. Chang MS, Hsu YH. The role of IL-20 in chronic kidney disease and diabetic nephropathy: pathogenic and therapeutic implications. J Leukoc Biol. 2018;104(5):919–923. [DOI] [PubMed] [Google Scholar]
- 170. Šenolt L, Leszczynski P, Dokoupilová E, et al. Efficacy and safety of anti-interleukin-20 monoclonal antibody in patients with rheumatoid arthritis: a randomized phase IIa trial. Arthritis Rheumatol. 2015;67(6):1438–1448. [DOI] [PubMed] [Google Scholar]
- 171. Verzola D, Gandolfo MT, Ferrario F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72(10):1262–1272. [DOI] [PubMed] [Google Scholar]
- 172. Liles JT, Corkey BK, Notte GT, et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J Clin Invest. 2018;128(10):4485–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fujisawa T, Takeda K, Ichijo H. ASK family proteins in stress response and disease. Mol Biotechnol. 2007;37(1):13–18. [DOI] [PubMed] [Google Scholar]
- 174. Lin JH, Zhang JJ, Lin SL, Chertow GM. Design of a phase 2 clinical trial of an ASK1 inhibitor, GS-4997, in patients with diabetic kidney disease. Nephron. 2015;129(1):29–33. [DOI] [PubMed] [Google Scholar]
- 175. Chertow GM, Pergola PE, Chen F, Kirby BJ, Sundy JS, Patel UD; GS-US-223-1015 Investigators . Effects of selonsertib in patients with diabetic kidney disease. J Am Soc Nephrol. 2019;30(10):1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs. 2016;25(9):1045–1058. [DOI] [PubMed] [Google Scholar]
- 177. Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17(2):368–377. [DOI] [PubMed] [Google Scholar]
- 178. Tashiro K, Koyanagi I, Saitoh A, et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Darisipudi MN, Kulkarni OP, Sayyed SG, et al. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am J Pathol. 2011;179(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sayyed SG, Hägele H, Kulkarni OP, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52(11):2445–2454. [DOI] [PubMed] [Google Scholar]
- 181. de Zeeuw D, Bekker P, Henkel E, et al. ; CCX140-B Diabetic Nephropathy Study Group. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–696. [DOI] [PubMed] [Google Scholar]
- 182. Menne J, Eulberg D, Beyer D, et al. ; Emapticap Study Group. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant. 2017;32(2):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Albaghdadi M, Gheorghiade M, Pitt B. Mineralocorticoid receptor antagonism: therapeutic potential in acute heart failure syndromes. Eur Heart J. 2011;32(21):2626–2633. [DOI] [PubMed] [Google Scholar]
- 184. Maeda K, Yasunari K, Sato EF, Yoshikawa J, Inoue M. Activation of protein kinase C and nicotinamide adenine dinucleotide phosphate oxidase in leukocytes of spontaneously hypertensive rats. Hypertens Res. 2003;26(12):999–1006. [DOI] [PubMed] [Google Scholar]
- 185. Morales E, Millet VG, Rojas-Rivera J, et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant. 2013;28(2):405–412. [DOI] [PubMed] [Google Scholar]
- 186. Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–951. [DOI] [PubMed] [Google Scholar]
- 187. Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 28Suppl 4:iv82–iv98. [DOI] [PubMed] [Google Scholar]
- 188. Sato N, Ajioka M, Yamada T, et al. ; ARTS-HF Japan study group. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circ J. 2016;80(5):1113–1122. [DOI] [PubMed] [Google Scholar]
- 189. Katayama S, Yamada D, Nakayama M, et al. ; ARTS-DN Japan study group. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J Diabetes Complications. 2017;31(4):758–765. [DOI] [PubMed] [Google Scholar]
- 190. Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bakris GL, Agarwal R, Chan JC, et al. ; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. Jama. 2015;314(9):884–894. [DOI] [PubMed] [Google Scholar]
- 192. Stuart D, Chapman M, Rees S, Woodward S, Kohan DE. Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346(2):182–189. [DOI] [PubMed] [Google Scholar]
- 193. Stuart D, Rees S, Woodward SK, Koesters R, Strait KA, Kohan DE. Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion. BMC Nephrol. 2012;13:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mann JF, Green D, Jamerson K, et al. ; ASCEND Study Group. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wenzel RR, Littke T, Kuranoff S, et al. ; SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86(5):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Dhaun N, Macintyre IM, Melville V, et al. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54(1):113–119. [DOI] [PubMed] [Google Scholar]
- 198. Dhaun N, Ferro CJ, Davenport AP, Haynes WG, Goddard J, Webb DJ. Haemodynamic and renal effects of endothelin receptor antagonism in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22(11):3228–3234. [DOI] [PubMed] [Google Scholar]
- 199. Boels MG, Avramut MC, Koudijs A, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65(8):2429–2439. [DOI] [PubMed] [Google Scholar]
- 200. Barton M, Tharaux PL. Endothelin and the podocyte. Clin Kidney J. 2012;5(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Heerspink HL, de Zeeuw D. Pharmacology: defining the optimal dose of a new drug: a crucial decision. Nat Rev Nephrol. 2009;5(9):498–500. [DOI] [PubMed] [Google Scholar]
- 202. de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25(5):1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Heerspink HJL, Andress DL, Bakris G, et al. Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20(6):1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 205. Waanders F, Vaidya VS, van Goor H, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ruggenenti P, Remuzzi G. Combined neprilysin and RAS inhibition for the failing heart: straining the kidney to help the heart? Eur J Heart Fail. 2015;17(5):468–471. [DOI] [PubMed] [Google Scholar]
- 208. Solomon SD, McMurray JJV, Anand IS, et al. ; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 209. Gottlieb SS. Renal effects of adenosine A1-receptor antagonists in congestive heart failure. Drugs. 2001;61(10):1387–1393. [DOI] [PubMed] [Google Scholar]
- 210. Haynes R, Judge PK, Staplin N, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138(15):1505–1514. [DOI] [PubMed] [Google Scholar]
- 211. Pilemann-Lyberg S, Hansen TW, Tofte N, et al. Uric acid is an independent risk factor for decline in kidney function, cardiovascular event and mortality in patients with type 1 diabetes. Diabetes Care. 2019. [DOI] [PubMed] [Google Scholar]
- 212. Lytvyn Y, Bjornstad P, Lovshin JA, et al. Association between uric acid, renal haemodynamics and arterial stiffness over the natural history of type 1 diabetes. Diabetes Obes Metab. 2019;21(6):1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bjornstad P, Maahs DM, Rivard CJ, et al. Serum uric acid predicts vascular complications in adults with type 1 diabetes: the coronary artery calcification in type 1 diabetes study. Acta Diabetol. 2014;51(5):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65(4):543–549. [DOI] [PubMed] [Google Scholar]
- 216. Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132. [PubMed] [Google Scholar]
- 217. Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2–7. [DOI] [PubMed] [Google Scholar]
- 218. Stronski A, Achimova E, Paiuk O, et al. Direct magnetic relief recording using As40S60: Mn-Se nanocomposite multilayer structures. Nanoscale Res Lett. 2017;12(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Maahs DM, Caramori L, Cherney DZ, et al. ; PERL Consortium. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Afkarian M, Polsky S, Parsa A, et al. ; PERL Study Group. Preventing early renal loss in diabetes (PERL) study: a randomized double-blinded trial of allopurinol-rationale, design, and baseline data. Diabetes Care. 2019;42(8):1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bardoux P, Martin H, Ahloulay M, et al. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci U S A. 1999;96(18):10397–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bjornstad P, Maahs DM, Jensen T, et al. Elevated copeptin is associated with atherosclerosis and diabetic kidney disease in adults with type 1 diabetes. J Diabetes Complications. 2016;30(6):1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Velho G, Ragot S, El Boustany R, et al. Plasma copeptin, kidney disease, and risk for cardiovascular morbidity and mortality in two cohorts of type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Jensen T, Bjornstad P, Johnson RJ, Sippl R, Rewers M, Snell-Bergeon JK. Copeptin and estimated insulin sensitivity in adults with and without type 1 diabetes: The CACTI Study. Can J Diabetes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bjornstad P, Johnson RJ, Snell-Bergeon JK, et al. Albuminuria is associated with greater copeptin concentrations in men with type 1 diabetes: a brief report from the T1D exchange Biobank. J Diabetes Complications. 2017;31(2):387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Boone M, Kortenoeven M, Robben JH, Deen PM. Effect of the cGMP pathway on AQP2 expression and translocation: potential implications for nephrogenic diabetes insipidus. Nephrol Dial Transplant. 2010;25(1):48–54. [DOI] [PubMed] [Google Scholar]
- 227. Ricksten SE, Bragadottir G, Redfors B. Renal oxygenation in clinical acute kidney injury. Crit Care. 2013;17(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand. 2009;53(8):1052–1059. [DOI] [PubMed] [Google Scholar]
- 229. Bertuccio CA, Ibarra FR, Toledo JE, Arrizurieta EE, Martin RS. Endogenous vasopressin regulates Na-K-ATPase and Na(+)-K(+)-Cl(-) cotransporter rbsc-1 in rat outer medulla. Am J Physiol Renal Physiol. 2002;282(2):F265–F270. [DOI] [PubMed] [Google Scholar]
- 230. Blot-Chabaud M, Djelidi S, Courtois-Coutry N, et al. Coordinate control of Na,K-atpase mRNA expression by aldosterone, vasopressin and cell sodium delivery in the cortical collecting duct. Cell Mol Biol (Noisy-Le-Grand). 2001;47(2):247–253. [PubMed] [Google Scholar]
- 231. Tamaki T, Kiyomoto K, He H, et al. Vasodilation induced by vasopressin V2 receptor stimulation in afferent arterioles. Kidney Int. 1996;49(3):722–729. [DOI] [PubMed] [Google Scholar]
- 232. El Boustany R, Taveau C, Chollet C, et al. Antagonism of vasopressin V2 receptor improves albuminuria at the early stage of diabetic nephropathy in a mouse model of type 2 diabetes. J Diabetes Complications. 2017;31(6):929–932. [DOI] [PubMed] [Google Scholar]
- 233. Cernecka H, Droebner K, Modritzki T, Collin M-P, Eitner F, Kolkhof P. Selective vasopressin V1a receptor antagonism improves renal oxygenation and perfusion in acute kidney injury. J Am Soc Nephrol. 2018;FR-PO101. [Google Scholar]
- 234. Perico N, Zoja C, Corna D, et al. V1/V2 Vasopressin receptor antagonism potentiates the renoprotection of renin-angiotensin system inhibition in rats with renal mass reduction. Kidney Int. 2009;76(9):960–967. [DOI] [PubMed] [Google Scholar]
- 235. Devuyst O, Chapman AB, Gansevoort RT, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28(5):1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Gansevoort RT, Meijer E, Chapman AB, et al. ; TEMPO 3:4 Investigators. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol Dial Transplant. 2016;31(11):1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Torres VE, Chapman AB, Devuyst O, et al. ; REPRISE Trial Investigators. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930–1942. [DOI] [PubMed] [Google Scholar]
- 238. Wong F, Watson H, Gerbes A, et al. ; Satavaptan Investigators Group. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut. 2012;61(1):108–116. [DOI] [PubMed] [Google Scholar]
- 239. Ginès P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D; HypoCAT Study Investigators . Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48(1):204–213. [DOI] [PubMed] [Google Scholar]
- 240. Ghali JK, Orlandi C, Abraham WT; CK-LX2401 Study Investigators . The efficacy and safety of lixivaptan in outpatients with heart failure and volume overload: results of a multicentre, randomized, double-blind, placebo-controlled, parallel-group study. Eur J Heart Fail. 2012;14(6):642–651. [DOI] [PubMed] [Google Scholar]
- 241. Pellegrini L, Woodhead J, Shoda L, Siler SQ, Howell BA, Orlandi C. Lixivaptan, a novel vasopressin V2 receptor antagonist in development for the treatment of autosomal dominant polycystic kidney disease. Kidney Week 2017: ABSTRACT: FR-PO326. 2019. [Google Scholar]
- 242. Woods TC, Satou R, Miyata K, et al. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol. 2019;49(4):331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Yang D, Livingston MJ, Liu Z, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75(4):669–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lenoir O, Jasiek M, Hénique C, et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015;11(7):1130–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Liu N, Xu L, Shi Y, Zhuang S. Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res. 2017;2017:3560238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Inoki K, Mori H, Wang J, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Gödel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121(6):2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Lin TA, Wu VC, Wang CY. Autophagy in chronic kidney diseases. Cells. 2019;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Zhou D, Liu Y. Therapy for kidney fibrosis: is the Src kinase a potential target? Kidney Int. 2016;89(1):12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yan Y, Ma L, Zhou X, et al. Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 2016;89(1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Taniguchi K, Xia L, Goldberg HJ, et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes. 2013;62(11):3874–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Wang J, Zhuang S. Src family kinases in chronic kidney disease. Am J Physiol Renal Physiol. 2017;313(3):F721–F728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Lay AC, Hurcombe JA, Betin VMS, et al. Prolonged exposure of mouse and human podocytes to insulin induces insulin resistance through lysosomal and proteasomal degradation of the insulin receptor. Diabetologia. 2017;60(11):2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. [DOI] [PubMed] [Google Scholar]
- 257. Haase VH. The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease. Kidney Int. 2006;69(8):1302–1307. [DOI] [PubMed] [Google Scholar]
- 258. Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4(4):216–226. [DOI] [PubMed] [Google Scholar]
- 259. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–1122. [DOI] [PubMed] [Google Scholar]
- 260. Nizar JM, Shepard BD, Vo VT, Bhalla V. Renal tubule insulin receptor modestly promotes elevated blood pressure and markedly stimulates glucose reabsorption. JCI Insight. 2018;3(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Cherney DZ, Miller JA, Scholey JW, et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care. 2010;33(6):1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Lovshin JA, Škrtić M, Bjornstad P, et al. Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2018;314(4):F667–F674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Bjornstad P, Cherney DZ, Snell-Bergeon JK, et al. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with type 1 diabetes. Nephrol Dial Transplant. 2015;30(10):1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Cohen JJ. Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am J Physiol. 1979;236(5):F423–F433. [DOI] [PubMed] [Google Scholar]
- 265. Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31. [DOI] [PubMed] [Google Scholar]
- 266. Körner A, Eklöf AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43(5):629–633. [DOI] [PubMed] [Google Scholar]
- 267. Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol. 2016;311(6):F1217–F1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol. 2016;311(6):F1378–F1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol. 2016;310(11):F1269–F1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46(8):1153–1160. [DOI] [PubMed] [Google Scholar]
- 271. Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40(2):123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Palmer BF. Disturbances in renal autoregulation and the susceptibility to hypertension-induced chronic kidney disease. Am J Med Sci. 2004;328(6):330–343. [DOI] [PubMed] [Google Scholar]
- 274. Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171(8):2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Birk AV, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22(6):1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Escribano-Lopez I, Diaz-Morales N, Iannantuoni F, et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Rep. 2018;8(1):15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981;240(5): F357–F371. [DOI] [PubMed] [Google Scholar]
- 280. Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev. 2008;4(1):10–17. [DOI] [PubMed] [Google Scholar]