SUMMARY
Aberrant hyperphosphorylation of the protein phosphatase 2A catalytic subunit (PP2Ac) at Tyr307 has been associated with aggressive disease and poor clinical outcome in multiple cancers. However, the study of reversible phosphorylation at this site has relied entirely upon the use of antibodies—most prominently, the clone E155. Here, we provide evidence that the E155 and F-8 phospho-Tyr307 antibodies cannot differentiate between phosphorylated and unphosphorylated forms of PP2Ac. The form of PP2Ac bound by these antibodies in H358 cells is unphosphorylated at the C-terminal tail. Furthermore, these antibodies are sensitive to additional protein modifications that occur near Tyr307, including Thr304 phosphorylation and Leu309 methylation, when these post-translational modifications are present. Thus, studies that used these antibodies to report PP2Ac hyperphosphorylation require reinterpretation, as these antibodies cannot be reliably used as readouts for a single PP2Ac post-translational modification (PTM) change.
In Brief
Inhibitory hyperphosphorylation of the PP2A catalytic subunit in cancer has been correlated with poor prognosis in numerous studies. Mazhar et al. show that the phospho-Tyr307-specific antibodies commonly used to detect this inhibitory mark are in fact agnostic to their intended target, binding unphosphorylated PP2A with equal affinity.
Graphical Abstract

INTRODUCTION
Protein phosphatase 2A (PP2A) is a ubiquitously expressed enzyme that negatively regulates numerous anti-apoptotic and mitogenic pathways (Narla et al., 2018). Often, cellular PP2A exists as a trimeric holoenzyme consisting of a catalytic subunit (C, also called PP2Ac), a scaffolding subunit (A), and a regulatory subunit (B). The role of PP2A as a tumor suppressor gene was first demonstrated in cellular transformation models in which PP2A inhibition contributed to oncogenesis (Hahn et al., 2002). Since then, multiple mechanisms of PP2A inactivation in cancer have been identified. PP2A is commonly inhibited via the overexpression of endogenous inhibitors such as cancerous inhibitor of PP2A (CIP2A) and SET nuclearproto-oncogene (SET). In addition, somatic mutations of the A subunit, decreased expression of A and B subunits, genomic loss of B subunits, and post-translational modifications of the PP2Ac carboxy-terminus have all been reported in cancer and are associated with diminished PP2A activity and cancer progression (O’Connor et al., 2018; Sangodkar et al., 2016).
The last six amino acids of the carboxyl tail of PP2Ac are conserved back to yeast and contain a number of post-translational modifications. The terminal amino acid Leu309 can undergo reversible carboxyl methylesterification, a process that is regulated by leucine carboxyl methyl transferase-1 (LCMT-1) and protein phosphatase methylesterase-1 (PME-1) (Lee et al., 1996; Lee and Stock, 1993). PP2Ac methylation at this site is associated with an active form of PP2A that promotes holoenzyme assembly with specific methyl-sensitive B subunits (Yu et al., 2001; Longin et al., 2007; Hwang et al., 2016). Furthermore, phosphorylation at Thr304 has been detected by multiple groups using mass spectrometry (Zhou et al., 2013; Mertinset al., 2014), and phospho-mimetic mutants at this site suggest that this phosphorylation event may disrupt certain B subunits from binding to the A-C dimer (Longin et al., 2007). Of particular interest to the oncology field, Tyr307 was identified to be phosphorylated by multiple receptor and non-receptor tyrosine kinases frequently activated in cancer, including the epidermal growth factor receptor (EGFR), insulin receptor (INSR), protooncogene tyrosine-protein kinase Src (SRC), and lymphocyte-specific protein tyrosine kinase (LCK). In vitro phosphorylation of Tyr307 on PP2Ac reduced catalytic activity by 90% through an unknown mechanism (Chen et al., 1992). Subsequent studies using Tyr307 phospho-mimetic mutants showed decreased B regulatory subunit binding, again suggesting that phosphorylation at this site may also disrupt holoenzyme assembly (Longin et al., 2007).
After these findings, aberrant hyperphosphorylation of PP2Ac at Tyr307 was reported in multiple diseases varying from cancer to neurodegenerative disease to asthma (Chen et al., 2017; Yang etal., 2013; Kobayashi et al., 2011). These studies primarily used a phospho-specific antibody clone E155, sold by Epitomics, that was developed against a synthetic peptide phosphorylated at Tyr307. Antibodies sold by Santa Cruz (clone F-8) and R&D Systems (polyclonal) have also been widely used. Despite appearing in multiple high-impact journals, the E155 clone used to specifically detect Tyr307 phosphorylation on PP2Ac has not been previously validated. The original datasheet provided by Epitomics displays a western blot showing an increase in signal when cells are stimulated with the epidermal growth factor (EGF). It also states in the text that the antibody “only detects PP2A phosphorylated on Tyrosine 307,” but data to show a lack of cross-reactivity with unphosphorylated PP2Ac are not provided. In this study, we demonstrate that this antibody, as well as those distributed by Santa Cruz and R&D Systems, are capable of detecting PP2Ac when it is un-phosphorylated at Tyr307 and that the form of PP2Ac detected by these antibodies is primarily unphosphorylated at this residue. In addition, we show that the antibodies are differentially sensitive to nearby post-translational modifications, including phosphorylation at Thr304 and methylation at Leu309, thus requiring a reinterpretation of previous data generated using these antibodies.
RESULTS
Multiple Commercially Available Antibodies Marketed as “Phospho-Tyr307 Specific” Detect the Phospho- Incompetent Form of PP2Ac
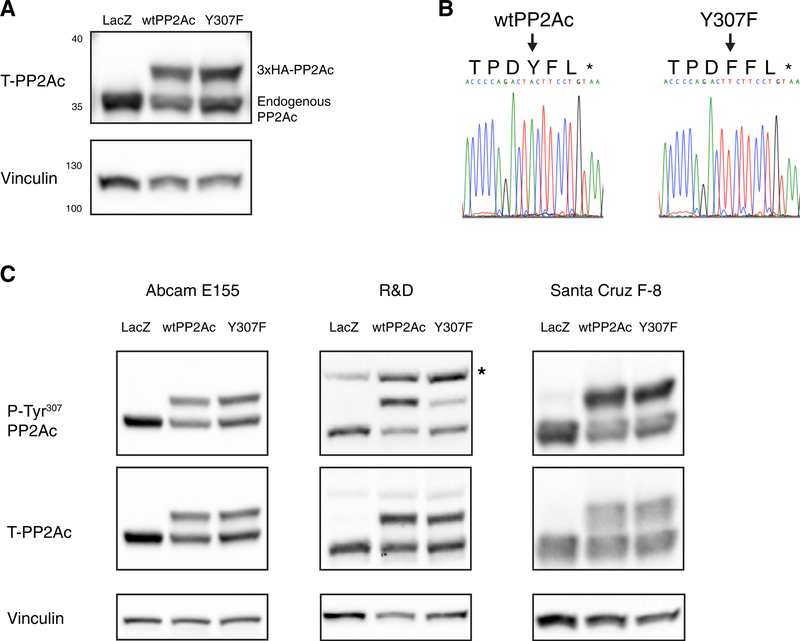
To investigate the specificity of PP2Ac phospho-Tyr307 anti-bodies, we stably overexpressed an N-terminal 3xHA-tagged wild-type and Y307F mutant PP2Ac in H358 lung adenocarcinoma cells (Figure 1A). Overexpression was confirmed using an N-terminal-specific total PP2Ac antibody, and PP2Ac sequence status was confirmed by Sanger sequencing (Figures 1Aand 1B). The mutation of tyrosine to phenylalanine results in a loss of the phosphorylatable hydroxyl of tyrosine, thus rendering the phenylalanine non-phosphorylatable (Tarrant and Cole, 2009). Western blot probing of these engineered H358 cell lines for phospho-Tyr307 PP2Ac was performed using three highly cited and commercially available phospho-Tyr307 PP2Ac antibodies. All three antibodies detected the non-phosphorylatable Y307F mutant form of PP2Ac (Figures 1C and S1). The monoclonal antibodies from Abcam and Santa Cruz detected wild-type and Y307F forms with equal intensity, while the polyclonal R&D antibody demonstrated a significant decrease in signal for the Y307F form of PP2Ac (Figure S1), suggesting that the hydroxyl group on tyrosine may be required for the binding of certain pools of immunoglobulin G (IgG) clones. Since all three antibodies are capable of binding the phospho-incompetent Y307F mutant, these results suggest that the antibodies bind to the unphosphorylated form of Tyr307 on PP2Ac.
Figure 1. Multiple Antibodies Directed against Phosho-Tyr307 PP2Ac Detect Both Wild-Type PP2Ac and the Phospho-Incompetent Y307F Mutant.
(A) Representative western blot showing total PP2Ac in the H358 cell line expressing either control LacZ, 3xHA-tagged wtPP2Ac, or 3xHA- tagged Y307F PP2Ac.
(B) Sequence confirmation of wild-type PP2Ac and Y307F mutant from mRNA.
(C) Western blots for LacZ, wtPP2Ac, and Y307F expressing lines probed with phosho-Tyr307 antibodies. The image is representative of 4 (E155 and F-8) or 6 (R&D) independent experiments. Asterisk indicates non-specific band See also Figure S1.
Inhibition of Cellular Tyrosine Phosphatases Increases Global Tyrosine Phosphorylation but Fails to Enhance Signal from Phospho-Tyr307-Directed PP2Ac Antibodies
One possible explanation for the detection of Y307F by these antibodies is that the level of Tyr307 phosphorylation in H358 cells is very low due to constitutive dephosphorylation by tyrosine phosphatases. In the absence of the preferred antigen, these antibodies may be binding non-specifically to PP2Ac even in the unphosphorylated form. To test this hypothesis, H358 cells expressing either a LacZ control or 3xHA-wtPP2Ac were treated with peroxyvanadate to inhibit cellular tyrosine phosphatases and enhance cellular phospho-tyrosine residues. Western blot analysis using a pan-phospho-Tyr antibody demonstrated that peroxyvanadate treatment resulted in a global increase in tyrosine phosphorylation (Figures 2A and S2). Using phospho-specific antibodies directed against specific residues such as Tyr1068 on the EGFR and Tyr419 and Tyr530 on the SRC, we further validated the effect of peroxyvanadate treatment (Figures 2B, 2C, and S2). With the exception of Tyr530 on SRC, peroxyvanadate treatment significantly enhanced phosphorylation of these tyrosine residues. Alternatively, western probing with the phos-pho-Tyr307 PP2Ac antibodies demonstrated no change in signal between untreated and peroxyvanadate-treated cells (Figures 2D–2F and S2). These results indicate that the antigen being detected by the phospho-Tyr307 antibodies is insensitive to tyrosine phosphatase activity and that the antibodies are likely binding non-specifically to the unphosphorylated form of PP2Ac.
Figure 2. Inhibition of Tyrosine Phosphatases via Peroxyvanadate Treatment Does Not Enhance Signal from Phospho-Tyr307 Antibodies.
(A-F) Western blot validation of peroxyvanadate-treated H358 cells expressing either LacZ or wtPP2Ac probed with (A) Pan phospho Tyrosine, (B) phospho- EGFR, and (C) phospho-SRC. Western blots for phospho-Tyr307 PP2Ac antibodies from (D) Abcam, (E) R&D Systems, and (F) Santa Cruz for peroxyvanadate- treated H358 cells expressing either LacZ or wtPP2Ac. Images are representative of three independent experiments.
Results for EGFR (B), SRC (C), and E155 (D) originate from the same blot that was cut at appropriate molecular weights and probed with the antibodies stated. Therefore, they share the same Vinculin loading control. Separate blots were run for pan-phospho-tyrosine, R&D, and Santa Cruz. See also Figure S2.
Alkaline Phosphatase Strips Global Tyrosine Phosphorylation but Does Not Abrogate Signal from Phospho-Tyr307 PP2Ac Antibodies
An alternate method to determine whether these antibodies are sensitive to a phosphorylated residue is to abrogate phospho-dependent tyrosine signals by treating the blots with a universal phosphatase. Lysates expressing either LacZ control or 3xHA-wtPP2Ac were run in duplicate on western blots and transferred to a nitrocellulose membrane. The membranes were cut and then subjected to either buffer alone or alkaline phosphatase (AP) treatment. AP treatment resulted in an overall decrease of tyrosine phosphorylation (Figures 3A and S3). To confirm the effects of AP treatment on specific proteins, control-treated and AP-treated membranes were probed with phospho-EGFR and phospho-SRC antibodies (Figures 3B, 3C, and S3). AP treatment resulted in an almost complete abrogation of signal from these phospho-Tyr-specific antibodies. However, AP treatment did not reduce the signal seen from the phospho-Tyr307 antibodies (Figures 3D–3F). This further suggests that the signal detected from these antibodies is insensitive to the phosphorylation state of PP2Ac.
Figure 3. Signal from Phospho-Tyr307 Antibodies Is Insensitive to Alkaline Phosphatase Treatment.
(A-F) Western blot validation of control or alkaline phosphatase (AP)-treated H358 cells expressing either LacZ or wtPP2Ac probed with (A) Pan phospho Tyrosine, (B) phospho-EGFR, and (C) phospho-SRC. Western blotsfor phospho-Tyr307 PP2Ac antibodiesfrom (D) Abcam, (E) R&D Systems, and (F) Santa Cruz for control or AP-treated H358 cells expressing either LacZ or wtPP2Ac. Images are representative of three independent experiments.
The pan-phospho-tyrosine blot was stripped and cut at appropriate molecularweights and reprobed with phospho-SRC and E155. These three share the same Vinculin loading control (A, C, and D). A second blot was run, treated with AP, and cut for phospho-EGFR and R&D. These two share the same vinculin loading control (B and E). A separate blot was run and treated for Santa Cruz.
See also Figures S3 and S4.
AP treatment is known to strip phosphorylation on serines and threonines as well. Since Thr304 on PP2Ac is a reported phosphorylation site near Tyr307 (Zhou et al., 2013; Mertins et al., 2014), we investigated whether these antibodies could be binding specifically to phosphorylated Thr304. We determined the ability of our AP treatment to dephosphorylate threonines that precede a proline (to reflect the TPDYFL motif on PP2Ac). AP treatment reduced global phosphorylation on threonines followed by prolines (Figure S4). AP treatment also reduced phosphorylation on PP1 and cMYC at Thr320 and Thr58, respectively (Figure S4), both of which are Thr-Pro motifs. Since AP treatment did not reduce the signal seen from the phospho-Tyr307 antibodies, it is unlikely that phosphorylation at this residue is high enough to contribute to the signal detected by phospho-Tyr307 antibodies
Antibodies Marketed as Specific to Phospho-Tyr307 Are Also Sensitive to Changes in Thr304 Phosphorylation and Leu309 Methylation When These Modifications Are Present
To investigate whether the phospho-Tyr307 antibodies are sensitive to a different post-translational modification near the target residue, we obtained synthetic peptides containing the last 20 carboxy (C) terminal amino acid residues of PP2Ac (Figure 4A). The C-terminal of PP2Ac can be phosphorylated at Thr304 and may also undergo carboxyl methylesterification at Leu309 (Lee and Stock, 1993). To determine if the binding of the phospho-Tyr307 antibodies is affected by the presence of these modifications, an indirect ELISA was performed using synthetic peptides that are either unmodified, phosphorylated at Thr304, phosphorylated at Tyr307, or methylated at Leu309. The Abcam E155 and Santa Cruz F-8 phospho-Tyr307 antibodies recognize the unmodified synthetic peptide and phospho-Tyr307 synthetic peptide with equal affinity, while the R&D polyclonal binds the phospho-Tyr307 peptide with 48% greater affinity (Figure 4B). Furthermore, the Abcam antibody is not influenced by phosphorylation at Thr304, whereas the Santa Cruz antibody has reduced binding upon Thr304 phosphorylation. However, R&D has an increased preference for this peptide over the unmodified peptide. Methylation at Leu309 has a dramatic effect on the binding of all phospho-Tyr307 antibodies, reducing binding by 80%−90%, compared to the unmodified peptide (Figure 4B). Based on these results, all three antibodies are able to bind the PP2Ac C-terminal tail when it is devoid of any post-translational modifications (PTMs). Furthermore, the signal from these antibodies is affected by phosphorylation at Thr304 as well as methylation at Leu309 when these modifications are present.
Figure 4. Phospho-Tyr307 Antibodies Bind Unmodified PP2Ac and Are Differentially Sensitive to Modifications at Thr304, Tyr307, and Leu309.
(A) Schematic of the last six amino acids of the 20-amino-acid-long synthetic peptides used for indirect ELISA.
(B) Relative affinity of phospho-Tyr307 antibodies for phospho-Thr304, phospho-Tyr307, and methyl-Leu309 PP2Ac synthetic peptides normalized against the unmodified peptide.
(C) Confirmation of modified PP2Ac peptides using pan-phospho-threonine, pan-phospho-tyrosine, and methyl-PP2Ac specific antibodies. Graphs represent the average of three independent experiments. Bars are mean ± SD. ***p<0.001, **p<0.01, ns, not significant.To confirm the presence of the respective PTMs on the synthetic peptides, ELISAs measuring antibody binding of pan-phospho-threonine (Proline), pan-phospho-tyrosine, and methyl PP2Ac antibodies were also performed (Figure 4C). The results indicate the presence of the respective PTMs on the synthetic peptides used.
Synthetic Peptides Phosphorylated at Tyr307 Are Detected with Decreased Efficiency after Tryptic Cleavage
Despite multiple studies in which global phosphorylated tyrosines in cells were enriched and their presence reported by mass spectrometry (Hornbeck et al., 2015), phosphorylation at Tyr307 on PP2Ac has never been detected by this method. To determine if there is a technical hurdle in detecting phospho-Tyr307 peptides by mass spectrometry, we used liquid chromatography-mass spectrometry (LC-MS) to analyze a synthetic peptide bearing the last 20 amino acids of PP2Ac in either its unmodified form or its Tyr307 phosphorylated form. Both the unmodified and Tyr307 peptides were subject to tryptic digestion, a mainstay step in the processing of cellular proteins for LC-MS. We detected the peptide RGEPHVTR and two peptide fragments containing Tyr307 for both the unmodified (Figure S5, top) and phosphorylated (Figure S5, bottom) peptides. The control fragment RGEPHVTR is detected with roughly equal intensity regardless of the phosphorylation status of Tyr307 (Table 1). However, detection of the phosphorylated peptides is reduced, compared to the non-phosphorylated controls. This difference in detection cannot be attributed to differences in tryptic digestion, as that would have resulted in a stronger peak for undigested fragments, which is not seen. Rather, it may be that the majority of the small, cleaved phosphorylated products are not retained on the column or may not ionize as well in the mass spectrometer. Since tryptic cleavage is the preferred method for peptide generation, this result may explain the lack of Tyr307 detection by MS in previous reports, especially if naturally occurring levels of phosphorylation at this site are very low.
Table 1.
Relative Intensity of Unmodified and Phospho-Tyr307 Peptides
| PP2Ac Synthetic Peptide | Intensity Ratio (Phospho/Unmodified) |
|---|---|
| Control fragment (RGEPHVTR) | 0.92 ± 0.05 (ns) |
| RTPDYFL | 0.49 ± 0.06* |
| TPDYFL | 0.21 ± 0.03*** |
Intensity of cleaved fragments after trypsin treatment of synthetic peptides that are either unmodified or Tyr307 phosphorylated was determined using mass spectrometry. Intensity ratio represents the mean ± SD of 3 experiments. See also Figure S5.
p < 0.001,
p < 0.05; ns, not significant
DISCUSSION
Insufficient validation of antibodies has been a major factor contributing to the 201Creproducibility crisis’’ in biomedical research (Baker, 2015). Forsstrom et al. (2014) used a high-throughput array of 2.1 million overlapping peptides and mapped the binding affinities of 14 antibodies. In addition to their intended targets, significant binding was observed by all 14 antibodies against un-related proteins that contained a portion of the epitope sequence (Forsstrom et al., 2014). Thus, all antibodies used for western blotting must be validated using knockdown or knockout strategies to ensure that any off-target proteins do not run at the same size as the target protein. Antibodies generated against PTMs require an additional level of scrutiny, as their usefulness depends on their ability to recognize the target proteins only if it bears the respective modification. We have provided evidence that multiple antibodies directed against phospho-Tyr307 on PP2Ac also recognize the protein when it is unmodified and are differentially sensitive to additional PTMs on neighboring residues. The antibody distributed by Abcam, clone E155, is the most well-known of the three, although it is frequently cited as having been purchased from Epitomics, referencing its previous manufacturer. After receiving unpublished data from our group, Abcam now sells this clone as a total-PP2Ac antibody. However, our data suggest that the ability of this antibody to detect PP2Ac is significantly reduced when it is methylated at Leu309. Considering that methylation levels in cells vary from 50% to 90% (Favre et al., 1994; Sontag et al., 2013), the signal from this antibody cannot be considered reflective of total PP2Ac either. As a result, the interpretation of data generated using these antibodies presents a challenge.
Multiple groups have used the E155 antibody to demonstrate a correlation between hyperphosphorylation at Tyr307 and reduced overall survival, as well as reduced progression-free survival in cancer patients (Cristobal et al., 2014; Chen et al., 2017). Numerous additional studies, published as recently as 2019 (Gao et al., 2019), have used these antibodies to report hyperphosphorylation of Tyr307 as a marker for PP2A inhibition. However, based on our data, both the E155 and F-8 clones detect the unmodified and Tyr307 phosphorylated forms of PP2Ac with equal affinity. Only the polyclonal R&D antibody shows a modest preference for the Tyr307 phosphorylated form, but this preference is also extended toward Thr304 phosphorylation, making it challenging to interpret results from this antibody as well. In contrast, the F-8 clone binds less efficiently to peptides phosphorylated at Thr304. Additionally, all three antibodies bind the methylated Leu309 form of PP2Ac with reduced efficiency. In this issue of Cell Reports, Frohner et al. (2020) further demonstrates that a combination of PTMs such as Tyr307 phosphorylation and Leu309 methylation also differentially influence binding. Given the multiple inputs influencing these antibodies, it is unlikely that any change in signal can be easily attributed to any single PTM without additional experiments to rule out all other possibilities.
Despite the preference for phosphorylated PP2Ac seen with the R&D polyclonal antibody, it appears that the form of PP2Ac in H358 cells is mostly unphosphorylated at Tyr307, as AP treatment does not remove the signal, despite successfully dephosphorylating other tyrosine residues. Furthermore, Tyr307 phosphorylation could not be induced at this site via chemical inhibition of tyrosine phosphatases (Figure 2). Oddly, Tyr307 phosphorylation in cells has never previously been reported by MS. While SRC can phosphorylate this site in vitro (Chen et al., 1992), phosphotyrosine enrichment and LC-MS of SRC-transformed mouse embryonic fibroblasts (MEFs) did not detect phosphorylation at Tyr307 (but did pick up phosphorylation at Tyr284 and Tyr127 on PP2Ac) (Luo et al., 2008). Tryptic cleavage of the synthetic PP2Ac C-terminal peptide yields the RTPDYFL and TPDYFL peptides, which are detected with less intensity when phosphorylated (Figure S5; Table 1). Future work attempting to detect phosphorylation at this site should employ an alternate digestion method, such as LysC, to see if this fragment is detected with improved efficiency. Finally, the future use of any phospho-Tyr307 antibody, and in general any antibodies binding to targets of extremely low abundance or antibodies that recognize epitopes bearing multiple PTMs, must be met with extreme caution.
STAR★METHODS
LEAD CONTACT
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Goutham Narla (gnarla@med.umich.edu).
MATERIALS AVAILABILITY
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Culture
H358 lung adenocarcinoma cells were obtained from American Type Culture Collection and maintained in RPMI-1640 supplemented with 10% FBS and 0.5% Penicillin/Streptomycin. Cell were kept at 37°C with 5% CO2 in a humidified incubator.
METHOD DETAILS
Generation of 3xHA-PPP2Ac overexpressing line
3xHA-tagged PPP2CA from pcDNA3 (a gift from Dr. David Brautigan) was cloned into pLX301 (Addgene plasmid # 25895) using Gateway Technology (ThermoFisher Scientific #12535–019) according to the manufacturers protocol. For viral transduction, 293T cells were transfected with pMD2.G, psPAX2 and pLX301–3xHA-PPP2CA in Opti-MEM (ThermoFisher Scientific #31985062) using Lipofectamine 3000 (ThermoFisher Scientific #L300015). Transfection media was removed after 6hrs, and media containing viral particles was collected 30hrs after transfection. H358 cells were exposed to viral media for 14hrs. Cells were allowed to grow for an additional 24–48hrs before confirming overexpressing of tagged protein.
Generation and Confirmation Y307F mutation
The Y307F mutation was introduced into the plx301–3xHA-PPP2CA plasmid using site-directed mutagenesis (Agilent #210519). To confirm the wild-type and Y307F mutant status in the exogenously expressed PP2Ac mRNA, RNA was isolated (Roche #11741985001) from both wtPP2Ac and Y307F expressing cells and reverse transcribed (ThermoFisher #18080051) using oligo dT primers. The cDNA was amplified (Promega #M7501) using primers specific to the exogenously expressed PP2Ac. The PCR product was purified (ThermoFisher #78201) and submitted for Sanger sequencing.
Immunoblotting
Lysis buffer: Thermo-Fisher 14321D supplemented with 100mM NaCl and protease and phosphatase inhibitors (Roche). Cell lysates were cleared and protein concentration was quantified (Pierce™ BCA Protein Assay Kit). 60 mg of protein was run on 12% TGX Stain-free gels (Bio-rad 4568044) and transferred to nitrocellulose membranes using the Trans-Blot Turbo Transfer system. Membranes were blocked in 3% non-fat milk and probed with primary and secondary antibodies in 0.5% non-fat milk. Proteins were detected using chemiluminescence (GE #RPN2232) and imaged using the Chemidoc™ XRS+. Quantification was done using the ImageLab™ software. For indirect ELISA, 25ng of a 20 amino acid synthetic peptide corresponding to the carboxyl terminus of PP2Ac (either unmodified or containing the stated post-translational modification) was used to coat immune assay plates (ThermoFisher Scientific #467120) overnight at 4°C. Plates were blocked with 2%BSA and probed with primary (1ug/mL) and secondary antibody in 1%BSA. TMB (3,3’,5,5’-Tetramethylbenzidine) was used as a substrate and absorbance read at 450nm.
Alkaline Phosphatase Assay
Identical proteins for both control and alkaline phosphatase treatment were run on the same gel. After transferring the proteins to a nitrocellulose membrane, the membrane was cut and half was incubated in 1x FastAP buffer alone while the other half was incubated in 300 units of Alkaline phosphatase (ThermoFisher Scientific #EF0651) for 1hr at 37°C with agitation. The membranes were rinsed in 1x PBS-Tween and blocked with 3% non-fat milk before being probed with the antibodies indicated. The control and alkaline phosphatase treated blots were imaged simultaneously to ensure identical exposure times.
Peroxyvanadate treatment
To prepare peroxyvanadate solution, hydrogen peroxide (30%) and 0.1M sodium orthovanadate were combined to a working concentration of 10mM for each. This was further diluted 1:100 in media for a final concentration of 100uM peroxyvanadate. Cells were treated for 45 minutes, after which the peroxyvanadate media was removed, cells were rinsed in cold PBS and scraped directly into lysis buffer.
Detection of unmodified and phospho-Tyr307 PP2Ac synthetic peptides by mass spectrometry
Synthetic peptides of 20 amino acids containing Tyr307 were generated for unmodified and phospho-Tyr307 (ThermoFisher Waltham, MA). Two micrograms of each synthetically generated peptide were digested with sequencing grade trypsin (Promega, Madison, WI) at an enzyme:substrate ratio of 1:20 overnight at 37°C in 50mM Tris. Two hundred and fifty picomoles of each digestion were analyzed in triplicate by LC-MS/MS using a LTQ-Orbitrap Elite mass spectrometer (Thermo Scientific, San Jose, CA) equipped with a nanoAcquity™ Ultra-high pressure liquid chromatography system (Waters, Taunton, MA). Mobile phases were aqueous phase A (0.1% formic acid in water) and organic phase B (0.1% formic acid in acetonitrile). Peptides were loaded onto a nanoACQUITY UPLC® 2G-V/M C18 desalting trap column (180 μm × 20mm nano column, 5 μm, 100 A°) at flow rate of 0.300μl/minute. Subsequently, peptides were resolved in a nanoACQUITY UPLC® BEH300 C18 reversed phase column (75 μm × 250mm nano column, 1.7 μm, 100 A°; Waters, Milford, MA) followed by a gradient elution of 1%−60% of phase B over 60 minutes. A nano ES ion source at a flow rate of 300 nL/min, 1.5 kV spray voltage, and 270°C capillary temperature was utilized to ionize peptides. Full scan MS spectra (m/z 380–1800) were acquired at a resolution of 60,000 followed by twenty data dependent MS/MS scans. MS/MS spectra were generated by collision induced dissociation of the peptide ions (normalized collision energy = 35%; activation Q = 0.250; activation time = 20 ms) to generate a series of b-and y-ions as major fragments. LC-MS/MS raw data were acquired using the Xcalibur software an (Thermo Fisher Scientific, version 2.2 SP1). Extraction of the m/z corresponding to each peptide was used to determine area under the curve (AUC) for each peptide. Ratios of unmodified to modified peptide were determined using AUC intensities.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance was determined using a Student’s t test calculated by Prism 8. Graphs represent the mean and standard deviation. P values are represented as: * < 0.05, ** < 0.01, *** < 0.001. All experiments were performed at least three times with the exact n indicated in the figure legends.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N-terminal T-PP2Ac | Abcam | Cat#ab106262; Lot#GR3234128–6; RRID:AB_10860464 |
| Vinculin | Cell Signaling | Cat#13901; Lot#5; RRID:AB_2728768 |
| P-EGFR Y1068 | Cell Signaling | Cat#3777; Lot#21; RRID:AB_2096270 |
| T-EGFR | Cell Signaling | Cat#2232; Lot#11; RRID:AB_331707 |
| P-SRC Y419 | Cell Signaling | Cat#2101; Lot#4; RRID:AB_331697 |
| P-SRC Y530 | Cell Signaling | Cat#2105; Lot#9; RRID:AB_331034 |
| T-SRC | Cell Signaling | Cat#2123; Lot#3; RRID:AB_2106047 |
| Pan P-Tyrosine | Cell Signaling | Cat#8954; RRID:AB_2687925 |
| Pan P-Threonine-Proline | Cell Signaling | Cat#9391; Lot#11; RRID:AB_331801 |
| P-PP1 T320 | Cell Signaling | Cat#2581; Lot#1; RRID:AB_330823 |
| T-PP1 | Cell Signaling | Cat#2582; Lot#1; RRID:AB_330822 |
| P-cmyc T58 | Abcam | Cat#ab28842; RRID:AB_731667 |
| T-cmyc | Cell Signaling | Cat#9402; RRID:AB_2151827 |
| PP2Ac (E155) formerly P-Tyr307 | Abcam | Cat#ab32104; Lot#GR96171 −13/ GR17965–24; RRID:AB_777385 |
| P-Tyr307 PP2Ac | R&D Systems | Cat#AF3989; Lot#YZW0316011; RRID:AB_2169636 |
| P-Tyr307 PP2Ac (F-8) | Santa Cruz | Cat#sc-271903; Lot#B1914; RRID:AB_10611810 |
| Methyl-PP2Ac (7C10) | Gift from Dr. Egon Ogris | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| PP2Ac synthetic peptides | UMICH Proteomics & Peptide Synthesis Core | N/A |
| Lipofectamine 3000 | ThermoFisher Scientific | Cat#L300015 |
| Opti-Mem | ThermoFisher Scientific | Cat#31985062 |
| cOmplete, EDTA-free Protease Inhibitor Cocktail | Roche | Cat#11873580001 |
| PhosSTOP | Roche | Cat#4906845001 |
| FastAP Thermosensitive Alkaline Phosphatase | ThermoFisher Scientific | Cat#EF0651 |
| Sodium Orthovanadate (Vanadate) | NEB | Cat#P0758S |
| 3,3’,5,5’-Tetramethylbenzidine | Sigma Aldrich | Cat#T2885–1G |
| Immuno 96-Well Plates (MediSorp) | Thermo Scientific | Cat#467320 |
| Critical Commercial Assays | ||
| Gateway Cloning Kit | ThermoFisher Scientific | Cat#12535–019 |
| QuikChange Lightning Site-Directed Mutagenesis Kit | Agilent | Cat#210519 |
| mRNA Isolation Kit | Roche | Cat#11741985001 |
| SuperScript III First-Strand Synthesis System | ThermoFisher Scientific | Cat#18080051 |
| PCR Master Mix | Promega | Cat#M7501 |
| ExoSAP-IT PCR Product Cleanup Reagent | ThermoFisher Scientific | Cat#78201 |
| Pierce BCA Protein Assay Kit | ThermoFisher Scientific | Cat#23225 |
| ECL Prime Western Blotting System | GE | Cat#RPN2232 |
| Experimental Models: Cell Lines | ||
| H358 | ATCC | Cat#CRL-5807 |
| Oligonucleotides | ||
| mutagenesis primer_Y307F_FW | ThermoFisher Scientific | gggtcttacaggaagaagtctggggtacgac |
| mutagenesis primer_Y307F_RV | ThermoFisher Scientific | gtcgtaccccagacttcttcctgtaagaccc |
| Recombinant DNA | ||
| plx301 | Addgene | Cat#25895 |
| pMD2.G | Addgene | Cat#12259 |
| psPAX2 | Addgene | Cat#12260 |
Highlights.
PP2Ac hyperphosphorylation studies rely upon the use of poorly validated antibodies
These antibodies readily bind the unphosphorylated form of the protein
Signal from the antibodies is unchanged by phosphatase treatment
The antibodies demonstrate a preference for the unmethylated form of PP2Ac
ACKNOWLEDGMENTS
The authors would like to thank Dr. Egon Ogris, Dr. Susann Brady-Kalnay, Dr. Bingcheng Wang, and Dr. Mark Jackson for critical input regarding the project. This work was supported by the NIH under award R01CA181654 granted to G.N.
Footnotes
DECLARATION OF INTERESTS
G.N. is a scientific founder and has an equity interest in RAPPTA Therapeutics, a company seeking to develop and commercialize novel small-molecule PP2A activators. G.N. and Dr. Egon Ogris have filed a patent on the methyl C-specific 7C10 antibody used in this manuscript on its prognostic and/or predictive utilization in cancer. They are seeking a commercial partner for this technology.
DATA AND CODE AVAILABILITY
This study did not generate any new codes or datasets.
The graphical abstract was created with Biorender.com
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://dol.org/10.1016/].celrep.2020.02.012.
REFERENCES
- Baker M. (2015). Reproducibility crisis: Blame it on the antibodies. Nature 521,274–276. [DOI] [PubMed] [Google Scholar]
- Chen J, Martin BL, and Brautigan DL (1992). Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257, 1261–1264. [DOI] [PubMed] [Google Scholar]
- Chen PM, Chu PY, Tung SL, Liu CY, Tsai YF, Lin YS, Wang WL, Wang YL, Lien PJ, Chao TC, and Tseng LM (2017). Overexpression of phosphoprotein phosphatase 2A predicts worse prognosis in patients with breast cancer: a 15-year follow-up. Hum. Pathol. 66, 93–100. [DOI] [PubMed] [Google Scholar]
- Cristobal I, Manso R, Rincon R, Carames C, Zazo S, Del Pulgar TG, Cebrian A, Madoz-Gurpide J, Rojo F, and Garcia-Foncillas J. (2014). Phosphorylated protein phosphatase 2A determines poor outcome in patients with metastatic colorectal cancer. Br. J. Cancer 111, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre B, Zolnierowicz S, Turowski P, and Hemmings BA (1994). The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J. Biol. Chem. 269, 16311–16317. [PubMed] [Google Scholar]
- Forsstrom B, Axnas BB, Stengele KP, Buhler J, Albert TJ, Richmond TA, Hu FJ, Nilsson P, Hudson EP, Rockberg J, and Uhlen M. (2014). Proteome-wide epitope mapping of antibodies using ultra-dense peptide arrays. Mol. Cell. Proteomics 13, 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohner IE, Mudrak I, Schϋchner S, Anrather D, Hartl M, Sontag J-M, Sontag E, Wadzinski BE, Preglej T, Ellmeier W, et al. (2020). PP2Ac phos-pho-Tyr307 antibodies used to assess PP2A activity are not specific for this modification but are sensitive to other PP2Ac modifications. Cell Rep. 30, this issue, 3183–3194. [DOI] [PubMed] [Google Scholar]
- Gao, Zhao X,L, Liu S, Li Y, Xia S, Chen D, Wang M, Wu S, Dai Q, Vu H,et al. (2019). gamma-6-Phosphogluconolactone, a Byproduct of the Oxidative Pentose Phosphate Pathway, Contributes to AMPK Activation through Inhibition of PP2A. Mol. Cell 76, 857–871.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, Decaprio JA, and Weinberg RA (2002). Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol 22,2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, and Skrzypek E. (2015). PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Lee JA, and Pallas DC (2016). Leucine Carboxyl Methyltransferase 1 (LCMT-1) Methylates Protein Phosphatase 4 (PP4) and Protein Phosphatase 6 (PP6) and Differentially Regulates the Stable Formation of Different PP4 Holoenzymes. J. Biol. Chem 291, 21008–21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Mercado N, Barnes PJ, and Ito K. (2011). Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS ONE 6, e27627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, and Stock J. (1993). Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem 268, 19192–19195. [PubMed] [Google Scholar]
- Lee J, Chen Y, Tolstykh T, and Stock J. (1996). A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc. Natl. Acad. Sci. USA 93, 6043–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, and Janssens V. (2007). Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J. Biol. Chem 282, 26971–26980. [DOI] [PubMed] [Google Scholar]
- Luo W, Slebos RJ, Hill S, Li M, Brabek J, Amanchy R, Chaerkady R, Pandey A, Ham AJ, and Hanks SK (2008). Global impact of oncogenic Src on a phosphotyrosine proteome. J. Proteome Res. 7, 3447–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins P, Yang F, Liu T, Mani DR, Petyuk VA, Gillette MA, Clauser KR, Qiao JW, Gritsenko MA, Moore RJ, et al. (2014). Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol. Cell. Proteomics 13, 1690–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla G, Sangodkar J, and Ryder CB (2018). The impact of phosphatases on proliferative and survival signaling in cancer. Cell. Mol. Life Sci 75, 2695–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Perl A, Leonard D, Sangodkar J, and Narla G. (2018). Therapeutic targeting of PP2A. Int. J. Biochem. Cell Biol 96, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangodkar J, Farrington CC, Mcclinch K, Galsky MD, Kastrinsky DB, and Narla G. (2016). All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase. FEBS J. 283, 1004–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag JM, Nunbhakdi-Craig V, and Sontag E. (2013). Leucine carboxyl methyltransferase 1 (LCMT1)-dependent methylation regulates the association of protein phosphatase 2A and Tau protein with plasma membrane microdomains in neuroblastoma cells. J. Biol. Chem 288, 27396–27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant MK, and Cole PA (2009). The chemical biology of protein phosphorylation. Annu. Rev. Biochem 78, 797–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang X, Duan C, Lu L, and Yang H. (2013). Alpha-synuclein overexpression increases phospho-protein phosphatase 2A levels via formation of calmodulin/Src complex. Neurochem. Int. 63, 180–194. [DOI] [PubMed] [Google Scholar]
- Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, Mcquoid MJ, and Pallas DC (2001). Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol. Biol. Cell 12, 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Di Palma S, Preisinger C, Peng M, Polat AN, Heck AJ, and Mohammed S. (2013). Toward a comprehensive characterization of a human cancer cell phosphoproteome. J. Proteome Res. 12, 260–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.