Key Points
Question
Is the effect of dapagliflozin in patients with heart failure and reduced ejection fraction consistent in patients with and without type 2 diabetes mellitus?
Findings
In this exploratory analysis of a randomized clinical trial that included 4744 patients, dapagliflozin, compared with placebo, added to recommended therapy significantly reduced the risk of the primary composite outcome of a first episode of worsening heart failure (hospitalization for heart failure or an urgent heart failure visit requiring intravenous therapy) or cardiovascular death in patients with diabetes (hazard ratio, 0.75) and in patients without diabetes (hazard ratio, 0.73). In patients without diabetes, the hazard ratio was 0.74 in individuals with a glycated hemoglobin of at least 5.7% and the hazard ratio was 0.67 in those with a glycated hemoglobin less than 5.7%.
Meaning
Dapagliflozin was effective at reducing cardiovascular morbidity and mortality in patients with heart failure and reduced ejection fraction independent of diabetes status.
Abstract
Importance
Additional treatments are needed for heart failure with reduced ejection fraction (HFrEF). Sodium-glucose cotransporter 2 (SGLT2) inhibitors may be an effective treatment for patients with HFrEF, even those without diabetes.
Objective
To evaluate the effects of dapagliflozin in patients with HFrEF with and without diabetes.
Design, Setting, and Participants
Exploratory analysis of a phase 3 randomized trial conducted at 410 sites in 20 countries. Patients with New York Heart Association classification II to IV with an ejection fraction less than or equal to 40% and elevated plasma N-terminal pro B-type natriuretic peptide were enrolled between February 15, 2017, and August 17, 2018, with final follow-up on June 6, 2019.
Interventions
Addition of once-daily 10 mg of dapagliflozin or placebo to recommended therapy.
Main Outcomes and Measures
The primary outcome was the composite of an episode of worsening heart failure or cardiovascular death. This outcome was analyzed by baseline diabetes status and, in patients without diabetes, by glycated hemoglobin level less than 5.7% vs greater than or equal to 5.7%.
Results
Among 4744 patients randomized (mean age, 66 years; 1109 [23%] women; 2605 [55%] without diabetes), 4742 completed the trial. Among participants without diabetes, the primary outcome occurred in 171 of 1298 (13.2%) in the dapagliflozin group and 231 of 1307 (17.7%) in the placebo group (hazard ratio, 0.73 [95% CI, 0.60-0.88]). In patients with diabetes, the primary outcome occurred in 215 of 1075 (20.0%) in the dapagliflozin group and 271 of 1064 (25.5%) in the placebo group (hazard ratio, 0.75 [95% CI, 0.63-0.90]) (P value for interaction = .80). Among patients without diabetes and a glycated hemoglobin level less than 5.7%, the primary outcome occurred in 53 of 438 patients (12.1%) in the dapagliflozin group and 71 of 419 (16.9%) in the placebo group (hazard ratio, 0.67 [95% CI, 0.47-0.96]). In patients with a glycated hemoglobin of at least 5.7%, the primary outcome occurred in 118 of 860 patients (13.7%) in the dapagliflozin group and 160 of 888 (18.0%) in the placebo group (hazard ratio, 0.74 [95% CI, 0.59-0.94]) (P value for interaction = .72). Volume depletion was reported as an adverse event in 7.3% of patients in the dapagliflozin group and 6.1% in the placebo group among patients without diabetes and in 7.8% of patients in the dapagliflozin group and 7.8% in the placebo group among patients with diabetes. A kidney adverse event was reported in 4.8% of patients in the dapagliflozin group and 6.0% in the placebo group among patients without diabetes and in 8.5% of patients in the dapagliflozin group and 8.7% in the placebo group among patients with diabetes.
Conclusions and Relevance
In this exploratory analysis of a randomized trial of patients with HFrEF, dapagliflozin compared with placebo, when added to recommended therapy, significantly reduced the risk of worsening heart failure or cardiovascular death independently of diabetes status.
Trial Registration
ClinicalTrials.gov Identifier: NCT03036124
This exploratory analysis of a randomized trial examined the effect of dapagliflozin on a composite of worsening heart failure or cardiovascular death on individuals with heart failure with reduced ejection fraction with and without diabetes.
Introduction
Inhibitors of the sodium-glucose cotransporter 2 (SGLT2), initially developed as a treatment for individuals with type 2 diabetes, prevent reabsorption of filtered glucose and lower blood glucose levels by increasing urinary excretion.1,2,3 In trials of participants with type 2 diabetes, these drugs led to a reduction in incident heart failure, which occurred early after randomization and appeared to be independent of baseline and time-dependent changes in glycated hemoglobin, suggesting this benefit may not necessarily be due to glucose lowering and might also be found in individuals without diabetes.4,5,6,7,8 In parallel, experimental evidence emerged suggesting that SGLT2 inhibitors may have cardioprotective actions independent of blood glucose level.9,10,11 The Dapagliflozin And Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial included patients with heart failure and reduced ejection fraction with and without type 2 diabetes. Dapagliflozin reduced the risk of worsening heart failure events and cardiovascular death and improved symptoms.12,13 The key question arising from the DAPA-HF trial is how the effects of dapagliflozin compared in patients with and without diabetes, and whether the findings of this trial support the hypothesis that SGLT2 inhibition might be an effective treatment for patients with heart failure, including those without diabetes. In this exploratory analysis, the efficacy of dapagliflozin, along with metabolic and hemodynamic changes and adverse events, were analyzed in patients with heart failure with reduced ejection fraction with and without diabetes and across the range of baseline glycated hemoglobin levels in DAPA-HF.
Methods
Trial Design and Oversight
The design and conduct of this double-blind, randomized, placebo-controlled trial are published and the protocol and statistical analysis plan (SAP) are available in Supplement 1 and Supplement 2.12,13 The trial was approved by the ethics committee at each study site and all patients provided written informed consent. The trial was reviewed by an independent data monitoring committee.12,13
Study Participants
Adults aged at least 18 years with New York Heart Association (NYHA) class II, III, or IV symptoms; an ejection fraction of 40% or less; and an elevated level of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) were eligible for inclusion.12,13 Patients were required to receive standard drug and device therapy for heart failure. The aim of the study was to enroll patients with heart failure and reduced ejection fraction. Individuals with type 2 diabetes were to continue to receive their glucose-lowering treatments, but these could be adjusted as required.
Exclusion criteria included recent treatment with or intolerance of an SGLT2 inhibitor, symptoms of hypotension or a systolic blood pressure of less than 95 mm Hg, type 1 diabetes mellitus, and an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73m2 of body surface area (or rapidly declining kidney function).12,13 Race and ethnicity were recorded in this study based on US Food and Drug Administration recommendations and investigators were asked to report participants’ race and ethnicity according to the categories in this guidance.14,15
Baseline Categorization of Diabetes Status
Investigators recorded whether patients had a history of diabetes at the enrollment visit (visit 1). Patients also underwent measurement of their glycated hemoglobin level in a central laboratory at visit 1 and again at the randomization visit (visit 2), which occurred 14 (±7) days later. For this prespecified subgroup analysis, patients were categorized as having diabetes if they had a history of diabetes or their glycated hemoglobin was at least 6.5% (≥48 mmol/mol) at visits 1 and 2. Patients with a glycated hemoglobin level less than 5.7% (<39 mmol/mL) at visits 1 and 2 were considered to have a normal glycated hemoglobin. For the purposes of this trial, patients with a glycated hemoglobin of at least 5.7% and less than 6.5% were considered to have prediabetes.12,13
Randomization
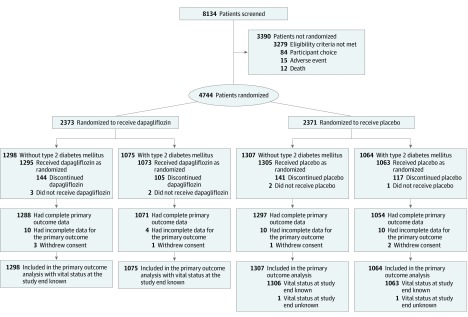
Patients were randomized to receive 10 mg of dapagliflozin once daily or matching placebo, in accordance with the sequestered fixed randomization schedule, using balanced blocks to ensure an approximate 1:1 ratio of the 2 treatments (Figure 1).12,13 The randomization codes were generated in blocks of 4 using a computer-generated random number generator. The blocks were not revealed to the investigators and randomization was performed via an interactive voice/web response system. Randomization was stratified based on a diagnosis of type 2 diabetes or a glycated hemoglobin level of at least 6.5% (≥48 mmol/mol) at the enrollment visit (visit 1).
Figure 1. Enrollment, Randomization, and Follow-up of Participants in a Study of the Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes.
The primary outcome was time to the first occurrence of cardiovascular death, hospitalization for heart failure, or an urgent heart failure hospital visit requiring intravenous therapy.
Study Intervention and Procedures
Patients were evaluated 14 and 60 days after randomization, with additional study visits at 4 months and at 4-month intervals thereafter. The full schedule of assessments is provided in the protocol (Supplement 1). Dose reduction (to 5 mg daily of dapagliflozin or matching placebo) or temporary discontinuation was permitted in the instance of any episode of an acute, unexpected decline in eGFR; volume depletion; or hypotension (or to avoid these), with a subsequent increase in dose (or restarting treatment) advised if possible.
End Points
The primary outcome for the trial was the composite of a first episode of worsening heart failure or cardiovascular death. An episode of worsening heart failure was defined as an unplanned hospital admission because of worsening heart failure or an urgent hospital visit requiring treatment for worsening heart failure with intravenous therapy.16 The first of the secondary outcomes was the composite of hospital admission for worsening heart failure or cardiovascular death. The additional secondary outcomes were the total number of hospital admissions for heart failure (including repeat admissions) and cardiovascular deaths; change from baseline to 8 months in the Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score (range, 0-100; higher scores indicate fewer symptoms; ≥5-point change considered clinically meaningful)17; the incidence of a composite worsening kidney function outcome, consisting of a 50% or greater sustained (ie, ≥28 days) decline in eGFR, kidney failure (defined as a sustained [ie, ≥28 days] eGFR <15 mL/min/1.73m2, sustained dialysis treatment, or kidney transplant) or kidney-related death; and death from any cause.12,13 The clinical outcomes described were adjudicated by a blinded end point committee.16
Diabetes status at baseline was one of the prespecified exploratory analysis subgroups. The SAP stated that the primary outcome, the cardiovascular death component of this composite, and the first secondary outcome would be analyzed in the prespecified subgroups (Supplement 2). The SAP also stated that outcomes would be analyzed among patients without diabetes according to whether their glycated hemoglobin level was less than 5.7% (normal) or greater than or equal to 5.7% (prediabetes). Analysis of the change from baseline to each visit for glycated hemoglobin, eGFR, body weight, and blood pressure were prespecified exploratory outcomes.
We conducted additional, post hoc analyses of outcomes according to glycated hemoglobin levels, examining this as a categorical variable (tertiles) in patients without diabetes and a continuous variable in all patients. We also conducted a post hoc analysis of change in hematocrit.
The prespecified analyses of adverse events included serious adverse events, adverse events associated with discontinuation of study treatment, adverse events of interest (ie, volume depletion, kidney events, major hypoglycemia, bone fractures, diabetic ketoacidosis, amputations), and laboratory findings of note. Other adverse events were not routinely collected in view of the extensive prior reporting of these adverse events for dapagliflozin. Doubling of serum creatinine was a prespecified exploratory end point.
Statistical Analysis
The statistical assumptions underlying DAPA-HF are published and the SAP is available in Supplement 2.12,13 Data from all randomized patients were included in this intention-to-treat analysis of the primary and secondary outcomes. Baseline characteristics were summarized as means and SDs, medians and interquartile ranges (IQRs), or percentages.
Patients without diabetes were divided into 2 categories based on their baseline glycated hemoglobin level: less than 5.7% (normal) or greater than or equal to 5.7% (prediabetes). In a post hoc analysis, patients without diabetes were also divided into 3 groups by tertile of glycated hemoglobin at baseline: less than or equal to 5.6%, 5.7% to 5.9%, and greater than or equal to 6.0%. The effect of treatment according to continuous glycated hemoglobin as a fractional polynomial was examined in an additional post hoc analysis.
Longitudinal measures, such as glycated hemoglobin level and body weight, were analyzed using a mixed model for repeated measurements (adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient). The least squares mean differences between treatment groups were estimated with 95% CIs and plotted for each group. The same approach was used in the post hoc analysis of hematocrit.
Time-to-event data were evaluated using Kaplan-Meier estimates and Cox proportional hazards models as prespecified in the SAP (Supplement 2). In a post hoc sensitivity analysis, we included trial site as an explanatory variable in the Cox models. The proportionality assumption was tested by Schoenfeld residuals and was met (P > .05 for all outcomes). Effect modification of treatment effect by baseline diabetes status was assessed by a likelihood ratio test. Total events (including repeat hospital admissions) were analyzed using a semiparametric proportional rates model to test the treatment effect and to quantify the treatment difference.18
The KCCQ total symptom score was analyzed as a composite rank-based end point, incorporating patient vital status (dead or alive) at 8 months and change in score from baseline to 8 months in patients who were alive, using the rank analysis of covariance method, with a corresponding win ratio used to estimate the magnitude of treatment effect (additional details are available in the SAP in Supplement 2).19 Adverse events were analyzed in patients who were randomized and received at least 1 dose of dapagliflozin or placebo and compared using a Fisher exact test.
These exploratory analyses were performed using Stata, version 15 (StataCorp), and SAS, version 9.4 (SAS Institute). All P values provided are 2-sided, and P <.05 was considered nominally statistically significant.
Results
Patients
Of the 4744 patients included, 2605 (55%) did not have diabetes. Of the remaining 2139 patients, 1983 (92.7%) had a history of diabetes at screening and an additional 156 (7.3%) were found to have previously undiagnosed diabetes (ie, a glycated hemoglobin ≥6.5% at visit 1 [enrollment] and visit 2 [randomization]). Of the 2605 patients without diabetes, 1748 (67.1%) had a glycated hemoglobin level of at least 5.7% at visit 1 or visit 2 and 839 (32.2%) had a glycated hemoglobin level less than 5.7% at both visit 1 and visit 2. In addition, 12 patients had only a single glycated hemoglobin measurement of less than 5.7% and 6 patients were missing both baseline glycated hemoglobin measurements (these 18 patients were included in the normal glycated hemoglobin group).
The baseline characteristics of patients with and without diabetes were well balanced between patients assigned to receive dapagliflozin or placebo within each patient group (Table 1). Patients without diabetes were significantly less likely to be black and to have an ischemic etiology than participants with diabetes (Table 1). Mean body mass index, heart rate, systolic blood pressure, and NT-proBNP level were significantly lower in participants without diabetes compared with those with diabetes. Mean eGFR was significantly higher in participants without diabetes compared with those with diabetes. The mean glycated hemoglobin level in patients without diabetes was 5.8%, compared with 7.4% in those with diabetes. The median (IQR) duration of diabetes was 7.41 (2.73-13.5) years.
Table 1. Characteristics of Patients at Baseline in a Study of the Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes.
| Characteristic | Mean (SD)a | |||
|---|---|---|---|---|
| No diabetes (n = 2605)b | Diabetes (n = 2139)b | |||
| Dapagliflozin (n = 1298) | Placebo (n = 1307) | Dapagliflozin (n = 1075) | Placebo (n = 1064) | |
| Age, y | 66.0 (11.8) | 66.4 (11.5) | 66.3 (9.9) | 66.7 (9.8) |
| Sex, No. (%) | ||||
| Women | 324 (25.0) | 308 (23.6) | 240 (22.3) | 237 (22.3) |
| Men | 974 (75.0) | 999 (76.4) | 835 (77.7) | 827 (77.7) |
| Race, No. (%)c | ||||
| White | 918 (70.7) | 926 (70.8) | 744 (69.2) | 745 (70.0) |
| Black | 50 (3.9) | 48 (3.7) | 72 (6.7) | 56 (5.3) |
| Asian | 311 (24.0) | 314 (24.0) | 241 (22.4) | 250 (23.5) |
| Other | 19 (1.5) | 19 (1.5) | 18 (1.7) | 13 (1.2) |
| Hispanic or Latino, No. (%) | 197 (15.2) | 209 (16.0) | 184 (17.1) | 178 (16.7) |
| Region, No. (%) | ||||
| Europe | 601 (46.3) | 602 (46.1) | 493 (45.9) | 458 (43.0) |
| Asia/Pacific | 306 (23.6) | 311 (23.8) | 237 (22.0) | 242 (22.7) |
| South America | 217 (16.7) | 226 (17.3) | 184 (17.1) | 190 (17.9) |
| North America | 174 (13.4) | 168 (12.9) | 161 (15.0) | 174 (16.4) |
| NYHA functional classification, No. (%)d | ||||
| II | 938 (72.3) | 903 (69.1) | 668 (62.1) | 694 (65.2) |
| III | 352 (27.1) | 391 (29.9) | 395 (36.7) | 360 (33.8) |
| IV | 8 (0.6) | 13 (1.0) | 12 (1.1) | 10 (0.9) |
| Heart rate/min | 70.8 (11.9) | 70.9 (12.0) | 72.3 (11.3) | 72.3 (11.4) |
| Systolic blood pressure, mm Hg | 121.1 (16.4) | 120.1 (15.7) | 123.2 (16.1) | 123.4 (16.9) |
| Left ventricular ejection fraction, % | 31.0 (6.8) | 30.8 (6.9) | 31.4 (6.6) | 31.0 (6.8) |
| Hemoglobin A1c, % | 5.7 (0.4) | 5.8 (0.4) | 7.4 (1.5) | 7.4 (1.6) |
| NT-proBNP, median (IQR), pg/mL | 1414 (821-2424) | 1412 (840-2551) | 1479 (903-2885) | 1487 (889-2759) |
| BMI | 27.3 (5.9) | 27.1 (5.6) | 29.3 (5.9) | 29.4 (6.1) |
| Principal cause of heart failure, No. (%) | ||||
| Ischemic | 660 (50.8) | 681 (52.1) | 656 (61.0) | 677 (63.6) |
| Nonischemic | 515 (39.7) | 518 (39.6) | 342 (31.8) | 312 (29.3) |
| Unknown | 123 (9.5) | 108 (8.3) | 77 (7.2) | 75 (7.0) |
| Medical history, No. (%) | ||||
| Hospitalization for heart failure | 608 (46.8) | 594 (45.4) | 516 (48.0) | 533 (50.1) |
| Atrial fibrillation | 507 (39.1) | 515 (39.4) | 409 (38.0) | 387 (36.4) |
| eGFR, mL/min/1.73 m2 | 67.8 (19.3) | 67.8 (19.1) | 63.9 (19.6) | 62.8 (19.1) |
| eGFR <60 mL/min/1.73 m2, No. (%) | 480 (37.0) | 464 (35.5) | 482 (44.8) | 500 (47.0) |
| Device therapy, No. (%) | ||||
| Implantable cardioverter defibrillatore | 347 (26.7) | 318 (24.3) | 275 (25.6) | 302 (28.4) |
| Cardiac resynchronization therapyf | 110 (8.5) | 93 (7.1) | 80 (7.4) | 71 (6.7) |
| Heart failure medication at randomization visit, No. (%) | ||||
| β-blocker | 1240 (95.5) | 1251 (95.7) | 1038 (96.6) | 1029 (96.7) |
| Diuretic | 1191 (91.8) | 1214 (92.9) | 1025 (95.3) | 1003 (94.3) |
| Mineralocorticoid receptor antagonist | 913 (70.3) | 928 (71.0) | 783 (72.8) | 746 (70.1) |
| ACE inhibitor | 737 (56.8) | 752 (57.5) | 595 (55.3) | 577 (54.2) |
| Angiotensin receptor blocker | 357 (27.5) | 335 (25.6) | 318 (29.6) | 297 (27.9) |
| Digitalis | 224 (17.3) | 234 (17.9) | 221 (20.6) | 208 (19.5) |
| Sacubitril-valsartan | 138 (10.6) | 141 (10.8) | 112 (10.4) | 117 (11.0) |
| Glucose-lowering medication at randomization visit, No. (%) | ||||
| Biguanide | 4 (0.3) | 6 (0.5) | 505 (47.0) | 515 (48.4) |
| Sulfonylurea | 0 | 0 | 229 (21.3) | 211 (19.8) |
| DPP-4 inhibitor | 0 | 0 | 161 (15.0) | 149 (14.0) |
| GLP-1 receptor agonist | 0 | 0 | 11 (1.0) | 10 (0.9) |
| Insulin | 0 | 0 | 274 (25.5) | 266 (25.0) |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Unless otherwise noted. Percentages may not total 100 because of rounding.
A total of 993 patients (41.8%) in the dapagliflozin group and 990 in the placebo group (41.8%) had a history of diabetes at baseline. An additional 82 patients in the dapagliflozin group and 74 in the placebo group had previously undiagnosed diabetes, defined as a glycated hemoglobin level of 6.5% or greater (≥48 mmol/mol) measured in a central laboratory at both screening and randomization.
Race was reported by the investigators. Of the 69 participants with race designated as “other,” 47 were described as “mixed race” and the remainder had miscellaneous designations (n = 16) or no additional information provided (n = 6).
New York Heart Association (NYHA) functional classification: I, no limitation of physical activity (ordinary physical activity does not cause undue fatigue, palpitation, dyspnea); II, slight limitation of physical activity (comfortable at rest; ordinary physical activity results in fatigue, palpitation, dyspnea); III, marked limitation of physical activity, comfortable at rest, less than ordinary activity causes fatigue, palpitation, or dyspnea; IV, unable to carry on any physical activity without discomfort, symptoms of heart failure at rest, discomfort increases if any physical activity is undertaken.
Either implantable cardioverter defibrillator or cardiac resynchronization therapy with a defibrillator.
Cardiac resynchronization therapy with or without a defibrillator.
At baseline, median (IQR) KCCQ total symptom scores were significantly higher (indicating better health) in patients without diabetes than in those with diabetes (79.2 [61.5-91.7] vs 75.0 [56.3-91.7]; P < .001; Table 2). A total of 1995 patients (93.3%) with diabetes and 2447 (93.9%) without diabetes took a renin-angiotensin system blocker and 1529 patients (71.5%) with diabetes and 1841 (70.7%) without diabetes took a mineralocorticoid receptor antagonist.
Table 2. Primary and Secondary Cardiovascular End Points in a Study of the Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes.
| Outcome | Dapagliflozin (n = 2373) | Placebo (n = 2371) | Absolute risk difference (95% CI) | Hazard ratio (95% CI) | P value | P value for interaction | ||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Participants per 100 patient-years | No. (%) | Participants per 100 patient-years | |||||
| No diabetes | 1298 | 1307 | ||||||
| Diabetes | 1075 | 1064 | ||||||
| Primary composite outcome and individual components | ||||||||
| Cardiovascular death, hospitalization for heart failure, or an urgent heart failure visita | ||||||||
| No diabetes | 171 (13.2) | 9.2 | 231 (17.7) | 12.7 | 4.5% (1.7 to 7.3) | 0.73 (0.60 to 0.88) | .002 | .80 |
| Diabetes | 215 (20.0) | 14.6 | 271 (25.5) | 19.4 | 5.5% (1.9 to 9.0) | 0.75 (0.63 to 0.90) | .002 | |
| Hospitalization for heart failure or an urgent heart failure visit | ||||||||
| No diabetes | 95 (7.3) | 5.1 | 150 (11.5) | 8.2 | 4.2% (1.9 to 6.4) | 0.62 (0.48 to 0.80) | <.001 | .22 |
| Diabetes | 142 (13.2) | 9.6 | 176 (16.5) | 12.6 | 3.3% (0.3 to 6.4) | 0.77 (0.61 to 0.95) | .018 | |
| Hospitalization for heart failure | ||||||||
| No diabetes | 93 (7.2) | 5.0 | 146 (11.2) | 8.0 | 4.0% (1.8 to 6.2) | 0.63 (0.48 to 0.81) | <.001 | .26 |
| Diabetes | 138 (12.8) | 9.3 | 172 (16.2) | 12.2 | 3.3% (0.3 to 6.3) | 0.76 (0.61 to 0.95) | .017 | |
| Urgent heart failure visit | ||||||||
| No diabetes | 3 (0.2) | 0.2 | 12 (0.9) | 0.6 | 0.7% (0.1 to 1.4) | 0.25 (0.07 to 0.89) | .032 | .25 |
| Diabetes | 7 (0.7) | 0.4 | 11 (1.0) | 0.7 | 0.4% (−0.4 to 1.3) | 0.62 (0.24 to 1.59) | .32 | |
| Cardiovascular death | ||||||||
| No diabetes | 106 (8.2) | 5.5 | 125 (9.6) | 6.5 | 1.4% (−0.8 to 3.6) | 0.85 (0.66 to 1.10) | .23 | .70 |
| Diabetes | 121 (11.3) | 7.7 | 148 (13.9) | 9.7 | 2.7% (−0.2 to 5.5) | 0.79 (0.63 to 1.01) | .06 | |
| Secondary outcomes | ||||||||
| Cardiovascular death or hospitalization for heart failure | ||||||||
| No diabetes | 169 (13.0) | 9.1 | 227 (17.4) | 12.4 | 4.3% (1.6 to 7.1) | 0.73 (0.60 to 0.89) | .002 | .83 |
| Diabetes | 213 (19.8) | 14.4 | 268 (25.2) | 19.1 | 5.4 (1.8 to 8.9) | 0.75 (0.63 to 0.90) | .002 | |
| No. of first and recurrent heart failure hospitalizations and cardiovascular deathb | ||||||||
| No diabetes | 239 | 12.5 | 327 | 17.0 | 0.73 (0.59 to 0.91) | .005 | .74 | |
| Diabetes | 328 | 21.0 | 415 | 27.3 | 0.77 (0.63 to 0.94) | .011 | ||
| Worsening kidney functionc | ||||||||
| No diabetes | 10 (0.8) | 0.5 | 15 (1.1) | 0.8 | 0.4% (−0.4 to 1.2) | 0.67 (0.30 to 1.49) | .33 | .86 |
| Diabetes | 18 (1.7) | 1.2 | 24 (2.3) | 1.6 | 0.6% (−0.6 to 1.8) | 0.73 (0.39 to 1.34) | .31 | |
| Death from any cause | ||||||||
| No diabetes | 133 (10.2) | 6.9 | 151 (11.6) | 7.8 | 1.3% (−1.1 to 3.7) | 0.88 (0.70 to 1.12) | .30 | .45 |
| Diabetes | 143 (13.3) | 9.1 | 178 (16.7) | 11.7 | 3.4% (0.4 to 6.5) | 0.78 (0.63 to 0.97) | .027 | |
| Change in KCCQ total symptom score at 8 mod | Baseline | Change | Baseline | Change | ||||
| No diabetes | 79.2 (60.4 to 92.7) | 5.4 (4.3 to 6.4) | 79.2 (62.5 to 91.7) | 3.1 (2.1 to 4.2) | 1.15 (1.05 to 1.26) | .004 | .18 | |
| Diabetes | 75.0 (55.2 to 91.7) | 7.0 (5.7 to 8.3) | 75.0 (57.3 to 91.7) | 3.5 (2.1 to 4.9) | 1.22 (1.11 to 1.35) | <.001 | ||
Abbreviations: IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Analyzed as time to first occurrence of any of these events; an urgent visit was defined as a hospital visit in which intravenous therapy for heart failure was administered.
Analyzed by the semiparametric proportional rates model16; the treatment effect is a rate ratio.
Composite outcome analyzed as time to first occurrence of 50% or greater reduction in eGFR sustained for at least 28 days, kidney failure, or death from kidney-related causes. Kidney failure was defined as eGFR less than 15 mL/min/1.73m2 sustained for at least 28 days, chronic dialysis treatment sustained for at least 28 days, or kidney transplant.
Scores range from 0 to 100, with higher scores indicating fewer symptoms and physical limitations associated with heart failure. Baseline scores are reported as median (IQR) and change as mean (95% CI). The treatment effect is shown as a win ratio. A value greater than 1 indicates superiority.
End Points
Patients without diabetes had a lower rate of the prespecified primary end point, the composite of a first episode of worsening heart failure or cardiovascular death, than patients with diabetes (eg, 17.7% vs 25.5% in the placebo groups) (Table 2, Figure 2, and Figure3A). Among participants without diabetes, the rate of the primary end point was lower in those with a baseline glycated hemoglobin less than 5.7% than in those with a level greater than or equal to 5.7% (eg, 16.9% vs 18.0% in the placebo groups) (Figure 3A). In contrast, the overall change in the KCCQ total symptom score did not differ significantly: increase of 3.1 (95% CI, 2.1-4.2) from baseline to 8 months in the placebo group in participants without diabetes compared with 3.5 (95% CI, 2.1-4.9) in those with diabetes (Table 2).
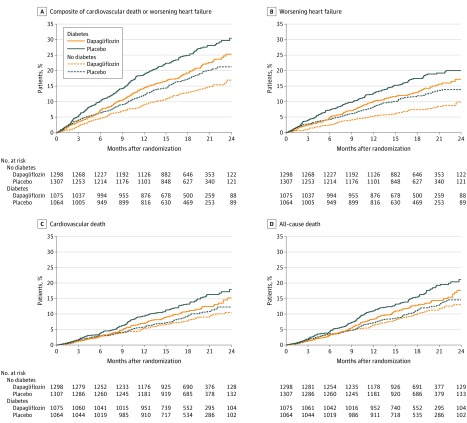
Figure 2. Exploratory Analyses of Worsening Heart Failure and Cardiovascular and All-Cause Death in a Study of the Effect of Dapagliflozin on Patients With Heart Failure With and Without Diabetes.
A, The median (interquartile range [IQR]) follow-up time for the primary end point was 18.0 (13.8-21.3) months in the dapagliflozin group and 17.7 (13.3-21.2) months in the placebo group in patients without diabetes and 17.2 (12.9-20.8) months in the dapagliflozin group and 16.6 (12.4-20.8) months in the placebo group for patients with diabetes. B, The median (IQR) follow-up times were the same in each group for the worsening heart failure event outcome as for the primary outcome. C, For cardiovascular death, median (IQR) follow-up times were 18.4 (14.4-21.5) months in the dapagliflozin group and 18.2 (14.3-21.5) months in the placebo group for patients without diabetes and 18.2 (14.1-21.3) months in the dapagliflozin group and 18.0 (13.8 -21.3) months in the placebo group among patients with diabetes. D, For all-cause death, median (IQR) follow-up times were 18.4 (14.4-21.5) months in the dapagliflozin group and 18.3 (14.3-21.5) months in the placebo group among participants without diabetes and 18.2 (14.1-21.3) months in the dapagliflozin group and 18.0 (13.8 -21.3) months in the placebo group among patients with diabetes.
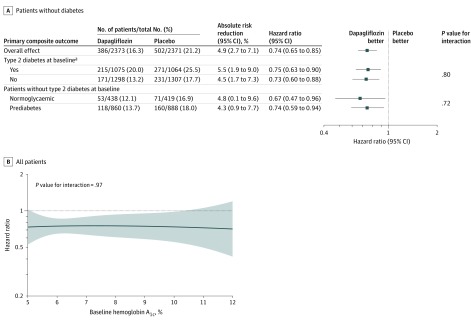
Figure 3. Prespecified Primary Composite End Point Based on Diabetes Status and Glycated Hemoglobin in a Study of the Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes.
A, In patients without diabetes, analysis of patients with a glycated hemoglobin greater than or equal to 5.7% (prediabetes) and less than <5.7% (normoglycemia) was prespecified. A total of 1983 patients had a history of diabetes at baseline and 156 had glycated hemoglobin greater than or equal to 6.5% at baseline. B, The solid black line represents a continuous hazard ratio and the dotted line represents a hazard ratio of 1 (ie, no difference between treatments). The shaded area represents the 95% CI around the hazard ratio.
The effects of dapagliflozin on the primary composite outcome, each of the individual mortality and hospital admission outcomes, and on urgent visits for worsening heart failure requiring intravenous treatment are shown in Table 2 and eFigure 1 in Supplement 3. The primary composite outcome occurred in 171 of 1298 patients (13.2%) in the dapagliflozin group and 231 of 1307 (17.7%) in the placebo group (hazard ratio, 0.73 [95% CI, 0.60-0.88]) among patients without diabetes and in 215 of 1075 patients (20.0%) in the dapagliflozin group and 271 of 1064 (25.5%) in the placebo group among patients with diabetes (hazard ratio, 0.75 [95% CI, 0.63-0.90]) (P value for interaction = .80; Table 2). The effect of dapagliflozin on all other outcomes did not differ significantly between patients with and without diabetes, as was the case for the kidney composite outcome (which was not improved significantly by dapagliflozin overall). In post hoc sensitivity analyses, adjusting for trial site gave similar results (eTable in Supplement 3).
Among patients without diabetes at baseline, the numbers of patients with the primary end point were 118 (13.7%) in the dapagliflozin group and 160 (18.0%) in the placebo group among those with a glycated hemoglobin of at least 5.7% (hazard ratio, 0.74 [95% CI, 0.59-0.94]) and 53 (12.1%) in the dapagliflozin group and 71 (16.9%) in the placebo group among patients with a glycated hemoglobin less than 5.7% (hazard ratio, 0.67 [95% CI, 0.47-0.96]) (P value for interaction = .72; Figure 3A). A post hoc analysis of patients without diabetes divided into 3 groups by baseline glycated hemoglobin is shown in eFigure 2 in Supplement 3. An additional post hoc analysis that used glycated hemoglobin as a continuous measure demonstrated benefit of dapagliflozin across the range of levels (P value for interaction for the primary end point = .97; Figure 3B). Cardiovascular death and all-cause death outcomes can be found in eFigure 3 in Supplement 3.
Between baseline and 8 months, the mean KCCQ total symptom score increased by 2.2 (95% CI, 0.7-3.7) points more in the dapagliflozin group than in the placebo group in patients without diabetes and by 3.5 (95% CI, 1.6-5.4) points more in patients with diabetes (P value for interaction = .18; Table 2).
In individuals without diabetes, significantly more patients in the dapagliflozin group than in the placebo group reported an increase of at least 5 points (the minimally important difference) in the KCCQ total symptom score (57.7% vs 51.7%; odds ratio, 1.12 [95% CI, 1.03-1.22]; P < .01) and significantly fewer reported a decrease of at least 5 points (26.0% vs 31.3%; odds ratio, 0.88 [95% CI, 0.81-0.97]; P < .01). In individuals with diabetes, significantly more patients in the dapagliflozin group than in the placebo group also reported an increase of at least 5 points (58.9% vs 49.9%; odds ratio, 1.20 [95% CI, 1.09-1.31]; P < .001) and significantly fewer reported a decrease of at least 5 points (24.5% vs 34.8%; odds ratio, 0.78 [95% CI, 0.71-0.87]; P < .001) (P value for interaction for improvement = .294; P value for interaction for deterioration = .075).
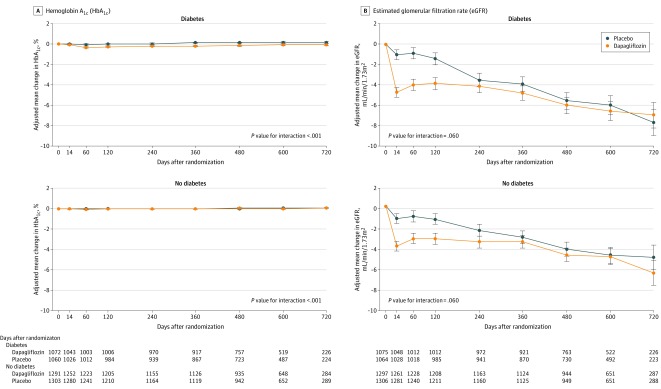
Changes in laboratory measures, weight and blood pressure, and hematocrit, adjusting for baseline value, are shown in Figure 4, Figure 5, and Figure 6 and eFigure 4 in Supplement 3. The placebo-corrected change in glycated hemoglobin between baseline and 4 months in patients without diabetes was 0.003% (95% CI, −0.022 to 0.028), compared with −0.261% (95% CI, −0.354 to −0.169) in patients with diabetes, with a significant interaction between diabetes status at baseline and the effect of dapagliflozin, compared with placebo, on glycated hemoglobin over the duration of the trial (P value for interaction <.001) (Figure 4A). There was an initial decrease in eGFR with dapagliflozin in both groups, although the between-treatment difference had attenuated by 4 months (placebo-corrected change in participants without diabetes, −1.9 mL/min/1.73m2 [95% CI, −2.6 to −1.2]; participants with diabetes, −2.4 mL/min/1.73m2 [95% CI, −3.3 to −1.6]), with no interaction between diabetes status and the effect of dapagliflozin (Figure 4B).
Figure 4. Effect of Dapagliflozin on Hemoglobin A1c and Estimated Glomerular Filtration Rate Among Patients With Heart Failure With and Without Diabetes.
Means and 95% CIs were derived from a mixed-effect model adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient. Least square mean changes along with 95% CIs are shown. The interaction between diabetes status at baseline and the effect of dapagliflozin, compared with placebo, on each variable of interest over the duration of the trial was tested.
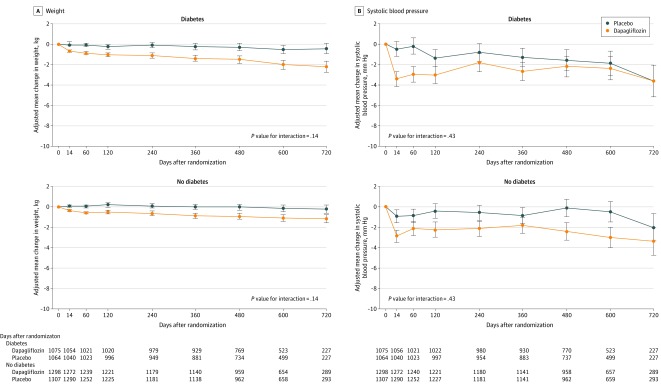
Figure 5. Effect of Dapagliflozin on Weight and Systolic Blood Pressure Among Patients With Heart Failure With and Without Diabetes.
Means and 95% CIs were derived from a mixed-model adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient. Least square mean changes along with 95% CIs are shown. The interaction between diabetes status at baseline and the effect of dapagliflozin, compared with placebo, on each variable of interest over the duration of the trial was tested.
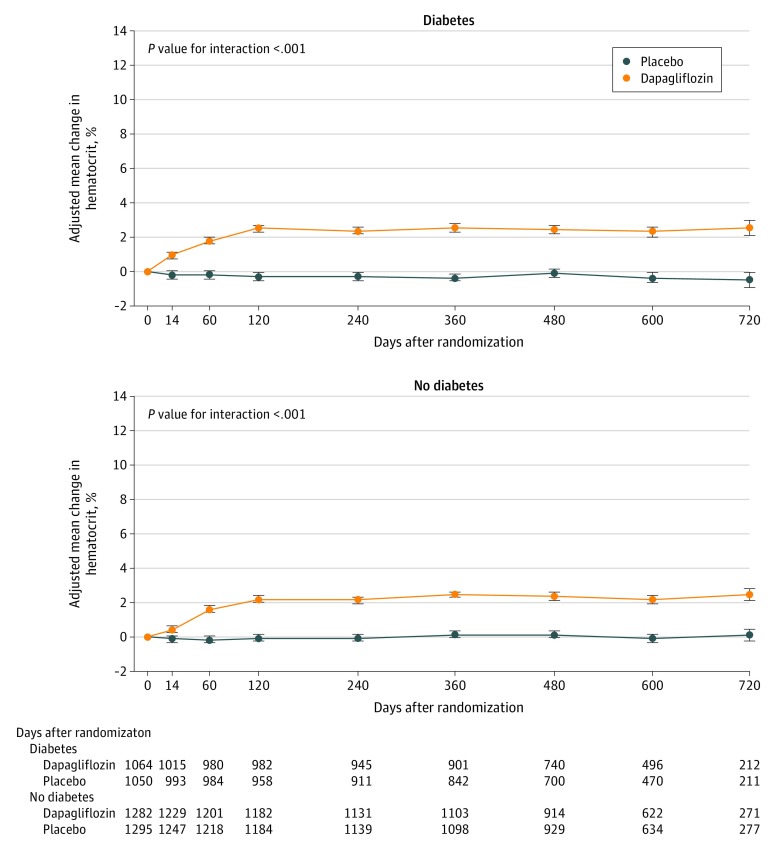
Figure 6. Post Hoc Analysis of the Effect of Dapagliflozin on Hematocrit Among Patients With Heart Failure With and Without Diabetes.
Means and 95% CIs were derived from a mixed-effect model adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient. Least square mean changes along with 95% CIs are shown. The interaction between diabetes status at baseline and the effect of dapagliflozin, compared with placebo, on each variable of interest over the duration of the trial was tested.
Weight declined in both patient groups (eg, placebo-corrected decrease in those without diabetes, −0.7 kg [95% CI, −0.9 to −0.4] at 4 months vs −0.8 kg [95% CI. −1.1 to −0.5] at 8 months), but there was no interaction between diabetes status and the effect of dapagliflozin (Figure 5A). Similarly, systolic blood pressure declined in both patient groups (placebo-corrected decrease at 4 months, −1.8 mm Hg [95% CI, −2.8 to −0.8] in those without diabetes vs −1.6 mm Hg [95% CI, −2.8 to −0.4] in those with diabetes), but there was no interaction between diabetes status and the effect of dapagliflozin (Figure 5B).
In a post hoc analysis, hematocrit increased with dapagliflozin in both patient groups, with plateau reached after approximately 4 months (placebo-corrected change, 2.3% [95% CI, 2.0-2.5] in participants without diabetes vs 2.8% [95% CI, 2.5-3.1] in patients with diabetes), with an interaction between diabetes status and the effect of dapagliflozin (P value for interaction <.001) (Figure 6).
In patients without diabetes, NT-proBNP decreased by 144 pg/mL (95% CI, 13-276) in the dapagliflozin group and increased by 84 pg/mL (95% CI, −89 to 257) in the placebo group between baseline and 8 months (between-treatment difference, −278 pg/mL [95% CI, −485 to −71]; P = .009). The corresponding changes in participants with diabetes were a decrease of 257 (95% CI, 100-415) pg/mL in the dapagliflozin group and an increase of 121 (95% CI, −64 to 306) pg/mL in the placebo group (between-treatment difference, −333 pg/mL [95% CI, −562 to −104]; P = .004) (P value for interaction = .73).
Tolerability and Adverse Events
The study medication was stopped in 144 patients (11.1%) in the dapagliflozin group and 141 (10.8%) in the placebo group among patients without diabetes and 105 (9.8%) in the dapagliflozin group and 117 (11.0%) in the placebo group among patients with diabetes. Doubling of serum creatinine occurred in 22 patients (1.7%) without diabetes in the dapagliflozin group and 36 patients (2.8%) in the placebo group (P = .08); among participants with diabetes, doubling of serum creatinine occurred in 21 patients (2.0%) in the dapagliflozin group and 41 (3.9%) in the placebo group (P = .01).
The most common adverse events of interest were those related to volume depletion and kidney impairment (Table 3). The incidence of these adverse events did not differ significantly between dapagliflozin and placebo in either patient group. Three patients (0.06%) experienced definite or probable diabetic ketoacidosis in the trial (all were patients with diabetes randomized to receive dapagliflozin). Eight patients (0.17%) experienced major hypoglycemia in the trial, and all 8 had diabetes (4 randomized to receive dapagliflozin and 4 randomized to receive placebo). Overall, 25 patients (0.5%) had an amputation (1 [0.1%] in the dapagliflozin group and 3 [0.2%] in the placebo group among patients without diabetes and 12 [1.1%] in the dapagliflozin group and 9 [0.8%] in the placebo group among patients with diabetes).
Table 3. Post Hoc Analysis of Adverse Events of Interest, Adverse Events Leading to Discontinuation of Study Treatment, and Serious Adverse Events.
| Outcome | No. (%) | Odds ratio (95% CI)b | P value for interaction | |
|---|---|---|---|---|
| Dapagliflozin (n = 2368)a | Placebo (n = 2368)a | |||
| No diabetes | 1295 | 1305 | ||
| Diabetes | 1073 | 1063 | ||
| Any serious adverse event (including death) | ||||
| No diabetes | 448 (34.6) | 481 (36.9) | 0.91 (0.77-1.06) | .16 |
| Diabetes | 447 (41.7) | 513 (48.3) | 0.77 (0.65-0.91) | |
| Discontinuation of study drug due to adverse event | ||||
| No diabetes | 68 (5.3) | 59 (4.5) | 1.17 (0.82-1.67) | .09 |
| Diabetes | 43 (4.0) | 57 (5.4) | 0.74 (0.49-1.11) | |
| Adverse Events of Interest | ||||
| Volume depletion | ||||
| No diabetes | 94 (7.3) | 79 (6.1) | 1.21 (0.89-1.66) | .40 |
| Diabetes | 84 (7.8) | 83 (7.8) | 1.00 (0.73-1.38) | |
| Kidney adverse event | ||||
| No diabetes | 62 (4.8) | 78 (6.0) | 0.79 (0.56-1.11) | .36 |
| Diabetes | 91 (8.5) | 92 (8.7) | 0.98 (0.72-1.32) | |
| Fracture | ||||
| No diabetes | 27 (2.1) | 25 (1.9) | 1.09 (0.63-1.89) | .58 |
| Diabetes | 22 (2.1) | 25 (2.4) | 0.87 (0.49-1.55) | |
| Amputation | ||||
| No diabetes | 1 (0.1) | 3 (0.2) | 0.34 (0.03-3.23) | .24 |
| Diabetes | 12 (1.1) | 9 (0.8) | 1.32 (0.56-3.16) | |
| Major hypoglycemiac | ||||
| No diabetes | 0 | 0 | ||
| Diabetes | 4 (0.4) | 4 (0.4) | 0.99 (0.25-3.97) | |
| Diabetic ketoacidosis | ||||
| No diabetes | 0 | 0 | ||
| Diabetes | 3 (0.3) | 0 | ||
The safety population included patients who received at least 1 dose of the trial medication.
Odds ratio are obtained from logistic regression and an odds ratio of less than 1 favors dapagliflozin.
Major hypoglycemia was defined as hypoglycemia requiring the assistance of another person to actively administer carbohydrates, glucagon, or take other corrective action.
Discussion
The key finding from these exploratory analyses of patients with heart failure with reduced ejection fraction was that the effect of the SGLT2 inhibitor dapagliflozin on the primary and principal secondary outcome did not differ in individuals with and without diabetes. Furthermore, among the individuals without diabetes, the reduction in the primary outcome with dapagliflozin was consistent in those with a glycated hemoglobin greater than or equal to 5.7% and less than 5.7% (ie, in those with prediabetes and those with a normal glycated hemoglobin).20,21 These data provide evidence that the benefits of SGLT2 inhibition are not limited to people with diabetes or prediabetes and are applicable to patients with heart failure with reduced ejection fraction, irrespective of glycemic status.
Dapagliflozin reduced glycated hemoglobin in patients with type 2 diabetes but had no effect on this measure in patients without diabetes. However, the effects of dapagliflozin on weight, blood pressure, hematocrit, eGFR, and NT-proBNP were directionally similar in those with and without diabetes, although significantly more pronounced in the former group for hematocrit.
These findings suggest the benefits of dapagliflozin were independent of plasma glucose lowering. Other mechanisms of action for SGLT2 inhibitors have been proposed, including a diuretic effect.22,23 While this mechanism was not measured directly in the present trial, the early decreases in systolic blood pressure, weight, and eGFR and the increase in hematocrit were consistent with a diuretic action. However, very little is known about the effects of SGLT2 inhibitors on urinary sodium and water excretion when added to conventional diuretic therapy, especially in patients with heart failure, and particularly in those without diabetes.22,24,25,26 There are other potential explanations for the decrease in eGFR and increase in hematocrit, including augmentation of tubulo-glomerular feedback and increase in kidney erythropoietin secretion.27,28,29 The time course of the changes in eGFR and hematocrit observed were quite different, with the initial decrease in eGFR reversing after 14 days, whereas hematocrit increased progressively over the first 4 months, plateauing thereafter.
Other diuresis-independent actions, including effects on ion transporters, fibrosis, adipokines, sympathetic nervous system activity, and vascular function, have also been proposed, although clinical evidence supporting these is sparse.3,10,30,31 SGLT2 inhibitors may reduce left ventricular mass and an effect on cardiac remodeling could explain the decrease in NT-proBNP observed with dapagliflozin.3,9,10,32 Prevention of decline in kidney function is also likely to be beneficial in heart failure.
The rate of adverse events related to volume depletion was low and not significantly different between participants with and without diabetes. Other prespecified adverse events were infrequent in both groups of patients, and discontinuation of study drug was also uncommon in the 2 groups. Neither major hypoglycemia nor diabetic ketoacidosis occurred in any patient without diabetes. Although there was no statistically significant effect on the prespecified kidney outcome, this occurred in few patients treated with dapagliflozin. However, doubling of serum creatinine (a prespecified exploratory outcome) was less common in patients who received dapagliflozin, both in patients with and without diabetes.
Limitations
This study has several limitations. First, although this analysis was prespecified, the results reported in this study are based on subgroup analysis. Second, some analyses were post hoc, including those that evaluated outcomes according to glycated hemoglobin levels by tertiles in patients without diabetes and as a continuous variable in all patients and those that evaluated changes in hematocrit levels. Third, additional information that might have helped explain the effects of SGLT2 inhibitors in heart failure was not collected. Fourth, the diagnosis of previously unknown diabetes was based on 2 consecutively elevated glycated hemoglobin levels (≥6.5%), and an alternative diagnostic approach (eg, an oral glucose tolerance test and fasting plasma glucose measurement) might have recategorized some patients.18,19 Fifth, to fit with the scheduled assessments in the trial, “prediabetes” was pragmatically defined as 1 glycated hemoglobin level measurement of at least 5.7% at visit 1 or visit 2 (ie, not having 2 consecutively normal levels [<5.7%] or having known or previously undiagnosed diabetes).
Conclusions
In this exploratory analysis of a randomized clinical trial of patients with heart failure with reduced ejection fraction, dapagliflozin compared with placebo, when added to recommended therapy, significantly reduced the risk of worsening heart failure or cardiovascular death independently of diabetes status.
Trial protocol
Statistical analysis plan
eResults
Data sharing statement
References
- 1.Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51(5):1145-1149. doi: 10.1021/jm701272q [DOI] [PubMed] [Google Scholar]
- 2.Pfister M, Whaley JM, Zhang L, List JF. Inhibition of SGLT2: a novel strategy for treatment of type 2 diabetes mellitus. Clin Pharmacol Ther. 2011;89(4):621-625. doi: 10.1038/clpt.2011.16 [DOI] [PubMed] [Google Scholar]
- 3.Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098-2107. doi: 10.1007/s00125-018-4669-0 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 7.Fitchett D, Zinman B, Wanner C, et al. ; EMPA-REG OUTCOME trial investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME trial. Eur Heart J. 2016;37(19):1526-1534. doi: 10.1093/eurheartj/ehv728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356-363. doi: 10.2337/dc17-1096 [DOI] [PubMed] [Google Scholar]
- 9.Yurista SR, Silljé HHW, Oberdorf-Maass SU, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21(7):862-873. doi: 10.1002/ejhf.1473 [DOI] [PubMed] [Google Scholar]
- 10.Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Prog Cardiovasc Dis 2019;62(4):349-357. doi: 10.1016/j.pcad.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 11.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931-1944. doi: 10.1016/j.jacc.2019.01.056 [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, DeMets DL, Inzucchi SE, et al. ; DAPA-HF Committees and Investigators . A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019;21(5):665-675. doi: 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to treat Type 2 Diabetes US Food and Drug Administration; 2008. Accessed December 3, 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diabetes-mellitus-evaluating-cardiovascular-risk-new-antidiabetic-therapies-treat-type-2-diabetes
- 15.Collection of Race and Ethnicity Data in Clinical Trials: Guidance for Industry and Food and Drug Administration Staff US Food and Drug Administration; 2016. Accessed December 3, 2019. https://www.fda.gov/media/75453/download
- 16.Hicks KA, Mahaffey KW, Mehran R, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71(9):1021-1034. doi: 10.1016/j.jacc.2017.12.048 [DOI] [PubMed] [Google Scholar]
- 17.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62:711-730. doi: 10.1111/1467-9868.00259 [DOI] [Google Scholar]
- 19.Wang D, Pocock S. A win ratio approach to comparing continuous non-normal outcomes in clinical trials. Pharm Stat. 2016;15(3):238-245. doi: 10.1002/pst.1743 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S13-S28. doi: 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 21.Chatterton H, Younger T, Fischer A, Khunti K; Programme Development Group . Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ. 2012;345:e4624. doi: 10.1136/bmj.e4624 [DOI] [PubMed] [Google Scholar]
- 22.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? a differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479-487. doi: 10.1111/dom.13126 [DOI] [PubMed] [Google Scholar]
- 23.McMurray J. EMPA-REG - the “diuretic hypothesis”. J Diabetes Complications. 2016;30(1):3-4. doi: 10.1016/j.jdiacomp.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 24.Devineni D, Vaccaro N, Polidori D, Rusch S, Wajs E. Effects of hydrochlorothiazide on the pharmacokinetics, pharmacodynamics, and tolerability of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Clin Ther. 2014;36(5):698-710. doi: 10.1016/j.clinthera.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 25.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140(18):1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
- 26.Kosiborod M, Gause-Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complications. 2017;31(7):1215-1221. doi: 10.1016/j.jdiacomp.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 27.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752-772. doi: 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 28.Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140(4):303-315. doi: 10.1161/CIRCULATIONAHA.118.037418 [DOI] [PubMed] [Google Scholar]
- 29.Yanai H, Katsuyayama H. A possible mechanism for renoprotective effect of sodium-glucose cotransporter 2 inhibitor: elevation of erythropoietin production. J Clin Med Res. 2017;9(2):178-179. doi: 10.14740/jocmr2857w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojcik C, Warden BA. Mechanisms and evidence for heart failure benefits from SGLT2 inhibitors. Curr Cardiol Rep. 2019;21(10):130. doi: 10.1007/s11886-019-1219-4 [DOI] [PubMed] [Google Scholar]
- 31.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108-2117. doi: 10.1007/s00125-018-4670-7 [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Mazer CD, Yan AT, et al. ; EMPA-HEART CardioLink-6 Investigators . Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693-1702. doi: 10.1161/CIRCULATIONAHA.119.042375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eResults
Data sharing statement