Abstract
Many species of the genus Bipolaris are important plant pathogens and often cause leaf spot, root rot, and seedling blight in an extremely wide range of hosts around the world. In recent years, maize leaf spot caused by Bipolaris species has frequently occurred with complex symptoms and is becoming increasingly serious in Sichuan Province of China. To investigate the population diversity of Bipolaris spp. and their corresponding symptoms in maize, 747 samples of maize leaf spot were collected from 132 sampling sites in 19 administrative districts of Sichuan Province from 2011 to 2018. Based on morphological characteristics, pathogenicity testing, and phylogenetic analysis of the rDNA internal transcribed spacer (ITS) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes, a total of 1186 Bipolaris isolates were identified as B. maydis, B. zeicola, B. cynodontis, B. oryzae, B. setariae, and B. saccharicola, among which B. maydis and B. zeicola were the dominant pathogenic species, accounting for 57.34% and 42.07% of the isolates, respectively. We found that B. zeicola isolates were mainly distributed in high altitude and cool mountainous areas, while B. maydis was more widely distributed in Sichuan Province. The typical symptoms caused by the Bipolaris species were clearly distinct in maize. The typical symptoms caused by B. maydis were elongated strip lesions, or fusiform, elliptical lesions, and those caused by B. zeicola were narrow linear lesions. Herein, B. saccharicola was first reported on maize and caused subrotund lesions. This study provides useful information for disease diagnosis and management for Bipolaris leaf spot in maize.
Keywords: maize leaf spot, Bipolaris, identification, symptom, diagnosis
1. Introduction
Maize (Zea mays L.) is one of the most important cereal crops around the world. In China, maize is planted on a total of 42.399 million hectares annually, and the annual production reached 259.071 million tons in 2017 [1]. In southwest China, the cultivation area and production of maize are the third largest in China, accounting for approximately 30% and 30%, respectively [2]. However, maize plantings in this region endure complex ecological conditions with altitudes ranging from 200 to 2500 m. In addition, diverse cultivation practices, including straw return and no- or reduced-tillage, are widely performed, resulting in the gradual increase of maize disease over the year [3]. Recently, leaf spot disease characterized by symptoms of elongated, elliptical lesions or small, narrow linear lesions has occurred and increased in incidence yearly, especially among summer-sown maize plants. However, the complexity and diversity of the symptoms make determining the pathogen species based only on the leaf spot symptoms in the field very difficult. Our preliminary diagnosis suggested that species of the Bipolaris genus may be the major causal organisms of this kind of maize leaf spot in Sichuan Province. Therefore, in this study, we focused on Bipolaris species, and the maize leaf spot caused by Bipolaris species was referred to as Bipolaris leaf spot of maize.
Bipolaris (anamorph of the ascomycetous genus Cochliobolus), which has more than 100 species, is an important genus of plant pathogens [4,5,6,7,8]. Most Bipolaris species are associated with leaf spot or blight, root rot, ear rot, seedling blight, and other diseases of cultivated and wild gramineous plants [6,7]. Many species of the Bipolaris genus are of considerable economic importance, such as B. oryzae, B. maydis and B. sorokiniana, causing devastating diseases in cereal crops [6,7,8]. Southern leaf blight caused by B. maydis is an important maize disease worldwide [5,6,7,9,10]. In China, a more than 30% production reduction in maize is likely caused by southern leaf blight in some regions with serious disease occurrence [2]. In addition, B. zeicola also causes northern leaf blight, which resulted in a heavy economic loss in the maize belt of the USA in the 1940s [5]. In China, northern leaf blight has become an important factor in maize production in northeastern China and northern China and has also spread towards Southwest China [11,12,13]. In Sichuan, with the changes in cultivation practices and replacement of the main maize varieties, Bipolaris leaf spot of maize has tended to increase in recent years [14].
Bipolaris species were formerly described as Helminthosporium. In several taxonomic refinements, the Helminthosporium species were segregated into four genera: Bipolaris, Curvularia, Drechslera, and Exserohilum [4,5]. These genera were morphologically similar and known as helminthosporioid fungi. Some species of these sister genera were also reported to cause maize leaf spot and often mixed infections formed under certain circumstances, especially for the genera Curvularia and Exserohilum [5,6]. Multiple species of Curvularia, including C. lunata, C. pallescens, C. eragrostidis, C. clavata, C. intermedia, C. inaequalis, C. spicifera, and C. papendorfii, were reported to infect leaves, sheaths, and bracts of maize [15,16,17,18]. Exserohilum turcicum and E. rostratum were also reported to cause serious maize leaf spot [19,20]. Under field conditions, the symptoms of maize leaf spot caused by these species were very similar and diverse. Accurately distinguishing these species based on symptoms alone is problematic. The identification of Bipolaris species is typically based on morphological characteristics; however, many species have similar characteristics, and conidial features are sometimes variable depending on isolates and culture conditions [6,8,21,22,23]. Recently, molecular phylogenetic analyses based on rDNA internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), translation elongation factor 1α (TEF1α), and large subunit of nuclear ribosomal DNA (LSU) made it possible to determine clear phylogenetic positions in the genus Bipolaris and its sister genera [6,22,24,25]. Thus, accurate identification of Bipolaris species based on morphological characteristics combined with molecular data has become a trend.
Sichuan Province, located in southwest China, is characterized by a mild and cool climate, with abundant rainfall and less sunshine. The maize planting region in Sichuan has an average annual temperature of 16–18 °C, and an average annual rainfall of 1000–1200 mm. However, this kind of climate is relatively favorable for the infection, colonization, reproduction, and dispersal of Bipolaris species [12,14]. Moreover, symptoms of these diseases are especially complicated by host variety differences and variations in pathogen virulence. To our knowledge, the occurrence and population structure of Bipolaris species associated with maize leaf spot has not been investigated in Sichuan, China. Thus, the objectives of this study were as follows: (i) to identify the Bipolaris diversity associated with maize leaf spot in Sichuan based on morphological and phylogenetic analyses; (ii) to ascertain the corresponding symptoms on maize leaves caused by different Bipolaris species; and (iii) to verify the pathogenicity and population distribution of the dominant species to provide guidance for disease diagnosis and control.
2. Results
2.1. Symptom Types of Maize Leaf Spot Caused by Bipolaris Species
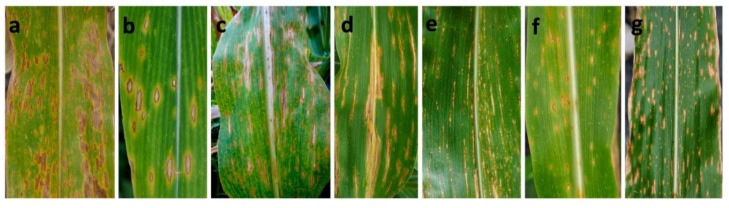
A total of 747 symptomatic samples were collected from 132 sampling sites from 2011 to 2018 in 19 administrative districts of Sichuan Province of China. Based on the shape and size of the lesions, the following five typical symptom types were noted in the infected maize leaves collected from the field. Type I (341 samples): fusiform or elliptical lesions, 6–20 mm × 4–11 mm (Figure 1a,b); Type II (135 samples): longitudinally elongated lesions, restricted by veins, developing into long strips of lesions, 5–40 mm × 3–10 mm (Figure 1c,d); Type III (126 samples): long, narrow linear lesions, 3–20 mm × 0.5–2 mm (Figure 1e); Type IV (100 samples): subrotund lesions, which were smaller than the lesions of Type I (Figure 1f); Type V (45 samples): punctiform or minute necrotic lesions (Figure 1g). Type I was the dominant symptom type, accounting for 45.6% of symptoms. In general, more than one symptom type was observed in one sample site or even in the same field.
Figure 1.
Typical symptoms of Bipolaris leaf spot of maize in the field. (a,b): Type I symptoms included fusiform or elliptical lesions; (c,d): Type II symptoms were characterized by elongated, nearly long strip lesions restricted by veins; (e): Type III symptoms were long, narrow linear lesions; (f): Type IV symptoms were nearly circular lesions that were smaller than the Type I lesions; (g): Type V symptoms were punctiform or minute necrotic lesions.
2.2. Morphological Identification of Bipolaris Species Associated with Maize Leaf Spot
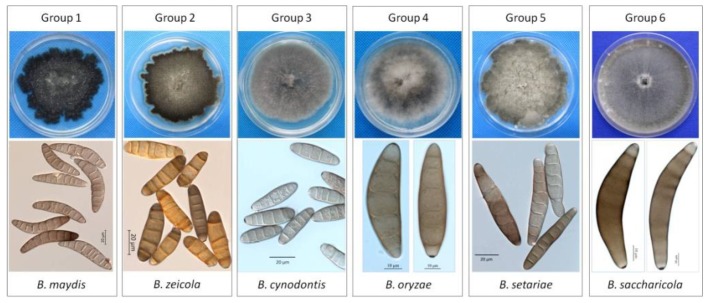
A total of 1186 Bipolaris single-spore isolates were obtained from all the collected maize leaf spot samples (Table 1 and Figure S1). The 1186 isolates were classified into 6 groups according to their cultural and morphological characteristics. Group 1 included 680 isolates, accounting for 57.34%, fitting the description of B. maydis, and Group 2 included 499 isolates, accounting for 42.07%, fitting the description of B. zeicola. In addition, Group 3 consisted of 3 isolates matching the description of B. cynodontis, and Group 4 had one isolate fitting the description of B. oryzae. Two isolates in Group 5 and one isolate in Group 6 fit the description of B. setariae and B. saccharicola, respectively. Group 1 and Group 2 were the predominant groups. A summary of the morphological data for these Bipolaris species in Groups 1-6 is presented in Table 2 and Figure 2.
Table 1.
Isolate number of Bipolaris species from maize in Sichuan Province, China.
| Sampling Regions | No. of Sampling Sites | B. maydis | B. zeicola | B. cynodontis | B. oryzae | B. setariae | B. saccharicola |
|---|---|---|---|---|---|---|---|
| Chengdu | 23 | 214 | 97 | 0 | 0 | 2 | 0 |
| Deyang | 12 | 82 | 144 | 3 | 0 | 0 | 0 |
| Meishan | 11 | 94 | 52 | 0 | 0 | 0 | 1 |
| Ya’an | 13 | 35 | 83 | 0 | 0 | 0 | 0 |
| Mianyang | 15 | 68 | 23 | 0 | 0 | 0 | 0 |
| Neijiang | 6 | 36 | 40 | 0 | 1 | 0 | 0 |
| Nanchong | 6 | 36 | 21 | 0 | 0 | 0 | 0 |
| Leshan | 12 | 32 | 6 | 0 | 0 | 0 | 0 |
| Yibin | 3 | 21 | 3 | 0 | 0 | 0 | 0 |
| Guang’an | 5 | 17 | 5 | 0 | 0 | 0 | 0 |
| Luzhou | 3 | 11 | 9 | 0 | 0 | 0 | 0 |
| Guangyuan | 7 | 9 | 7 | 0 | 0 | 0 | 0 |
| Suining | 4 | 7 | 0 | 0 | 0 | 0 | 0 |
| Ziyang | 2 | 5 | 1 | 0 | 0 | 0 | 0 |
| Bazhong | 2 | 6 | 0 | 0 | 0 | 0 | 0 |
| Liangshan | 2 | 0 | 5 | 0 | 0 | 0 | 0 |
| Ganzi | 1 | 2 | 3 | 0 | 0 | 0 | 0 |
| Zigong | 2 | 3 | 0 | 0 | 0 | 0 | 0 |
| Dazhou | 3 | 2 | 0 | 0 | 0 | 0 | 0 |
| Total | 132 | 680 | 499 | 3 | 1 | 2 | 1 |
Table 2.
Summary of morphological data for Bipolaris isolates.
| Group | Species | Colony Characterization | Growth Rate (mm/day) | Conidia | |||
|---|---|---|---|---|---|---|---|
| Average Length (μm) | Average Width (μm) | Length-Width Ratio | Shape | ||||
| 1(680) † | B. maydis | dark grey to black appearance | 5.7 ± 0.20a * | 93.5 ± 4.0a (52–126) | 13.9 ± 0.35b (10–17) | 6.6 ± 0.14a (5.6–7.6) | fusiform, slightly curved, light fawn to dark brown, 7–11 distoseptate, hilum distinct, 3–5 μm |
| 2(499) | B. zeicola | blackish grey, entire or irrengular margin | 4.5 ± 0.19d | 64.9 ± 3.1c (42–92) | 14.4 ± 0.57b (9–21) | 4.5 ± 0.07c (3.8–5.0) | slightly curved or straight, narrow elliptic to obclavate, helvus, isabelline to dark brown, 6–11 distoseptate, hilum inconspicuous |
| 3(3) | B. cynodontis | grey to greyish black | 5.1 ± 0.11bc | 38.2 ± 0.7d (30–45) | 11.3 ± 0.15c (10–13) | 3.4 ± 0.06d (2.8–4.3) | slightly curved or straight, cylindrical to elliptic, light olivaceous green to brown or golden brown, 3–9 distoseptate, hilum inconspicuous |
| 4(1) | B. oryzae | white to slight grey, fluffy cottony | 4.8 ± 0.09cd | 93.0 ± 4.6a (54–129) | 15.9 ± 0.54a (10–21) | 5.8 ± 0.12b (4.8–6.8) | curved, rarely straight, fusiform, obclavate, slightly brown to brown, 6–12 distoseptate, hilum slightly protruding |
| 5(2) | B. setariae | slight grey to dark grey, with abundant aerial mycelia | 5.5 ± 0.13ab | 80.3 ± 2.7b (41–120) | 13.4 ± 0.33b (9–18) | 6.0 ± 0.19b (3.8–9.4) | straight or slightly curved, fusiform or obclavate, pale brown to dark brown, 5–10 distoseptate, hilum inconspicuous or slightly protruding |
| 6(1) | B. saccharicola | olivaceous grey to olivaceous black, moderate aerial mycelium giving a cottony appearance | 5.8 ± 0.30a | 102.4 ± 3.4a (72–138) | 17.2 ± 0.69a (10–24) | 6.2 ± 0.29ab (4.4–10.8) | curved, rarely straight, fusiform, subhyaline to pale brown or brown, 5–11 distoseptate, hila inconspicuous, brown, slightly protuberant |
Note: † The numbers shown in parentheses represent the number of isolates in each group. * The mean difference is significant at the 0.05 level; a–d: the values with the same letter in a column do not significantly differ according to Duncan’s multiple range test.
Figure 2.
Morphology and cultural characteristics of six Bipolaris groups.
Colony characteristics (Table 2 and Figure 2): Distinct colony morphology on potato dextrose agar (PDA) was observed for each group after 7 days. The isolates in Group 1 produced dark gray to black colonies with abundant sporulation. The colonies produced by Group 2 isolates were blackish gray with marginally pigmented zones, abundant sporulation and irregular margins. The isolates in Group 3 produced gray to grayish dark colonies with sparse gray aerial mycelia, and the colonies showed a cottony appearance. The colonies from Group 4 exhibited white to slightly gray mycelia with a fluffy cottony appearance. The colonies produced by Group 5 isolates were slightly gray to dark gray, with abundant aerial mycelia. The isolate from Group 6 produced olivaceous gray to olivaceous black, moderate aerial mycelia with a cottony appearance.
Growth rate (Table 2): Isolates from different groups exhibited different growth rates. The maximum mycelial growth was observed in Group 6 (5.8 ± 0.3 mm/day), followed by those from Group 1 (5.7 ± 0.2 mm/day), Group 5 (5.5 ± 0.13 mm/day), Group 3 (5.1 ± 0.11 mm/day), Group 4 (4.8 ± 0.09 mm/day), and Group 2 (4.5 ± 0.19 mm/day).
Conidial morphology (Table 2 and Figure 2): Two conidial types were observed among the six groups based on the following conidia shapes: curved or slightly straight (observed in Groups 1, 4, and 6) and straight or slightly curved (observed in Groups 2, 3, and 5). The conidia sizes of Groups 1, 4, and 6 were similar, but the conidia color produced by the Group 1 isolates was light fawn to dark brown, which was different from those of Groups 4 and 6. The length-width ratio of Group 1 was larger than those of Group 4 and Group 6. Group 4 isolates produced fusiform, obclavate, slightly brown to brown conidia. The conidia produced by Group 6 isolates were fusiform, subhyaline to pale brown or brown, and the conidia of Group 6 were relatively longer and prominently curved. Isolates of Groups 2, 3, and 5 had significant differences in conidia color. The conidia produced by the Group 2 isolates were narrowly elliptical to obclavate, helvus, and isabelline to dark brown. Group 3 produced cylindrical to ellipsoidal, light olivaceous green to brown or golden brown conidia, and the conidia of Group 5 were fusiform or obclavate, and pale brown to dark brown.
2.3. Phylogenetic Analysis
A total of 173 isolates from the six morphological groups were subsequently selected for further molecular analyses based on their ITS and GAPDH gene sequences. The amplification success rates of ITS and GAPDH were 92.5% and 86.7%, respectively. The GenBank accession numbers of these Bipolaris isolates are listed in Table S1. The referred sequences of helminthosporioid fungi (35 Bipolaris, 11 Curvularia, 6 Drechslera, and 13 Exserohilum) and one other species, Alternaria alternata, originating from GenBank and used for phylogenetic analysis are listed in Table 3.
Table 3.
Details of the isolates used in this study, including the hosts, locations, and GenBank accession numbers of the generated sequences.
| Species | Strain No.1 | Host | Location Country | GenBank Accession Numbers2 | References | |
|---|---|---|---|---|---|---|
| ITS | GAPDH | |||||
| Alternaria alternata | EGS 34.0160 | Arachis hypogaea | India | AF071346 | AF081400 | [24] |
| Bipolaris axonopicola | BRIP 11740 | Axonopus fissifolius | Australia | KX452443 | KX452409 | [23,26] |
| B. bamagaensis | BRIP 13577 | Brachiaria subquadripara | Australia | KX452445 | KX452411 | [23,26] |
| B. chloridis | CBS 242.77 | Chloris gayana | Australia | JN192372 | JN600961 | [5,6] |
| B. cynodontis | ICMP 6128 | Cynodon dactylon | New Zealand | JX256412 | JX276427 | [25] |
| B. cynodontis | CBS 109894 | Cynodon dactylon | Hungary | KJ909767 | KM034838 | [6] |
| B. drechsleri | CBS 136207 | Microstegium vimineum | USA | KF500530 | KF500533 | [6] |
| B. drechsleri | CBS 136208 | Microstegium vimineum | USA | KF500532 | KF500535 | [6] |
| B. heveae | CBS 241.92 | Hevea sp. | Nigeria | KJ909763 | KM034843 | [6,23] |
| B. luttrellii | 14643-1 | Dactyloctenium aegyptium | Australia | AF071350 | AF081402 | [6,24] |
| B. maydis | CBS 137271/C5 | Zea mays | USA | AF071325 | KM034846 | [6,24] |
| B. maydis | AR 5182 | Sorghum bicolor | Japan | KM230388 | KM034844 | [6] |
| B. maydis | AR 5183 | Sorghum bicolor | Japan | KM230390 | KM034848 | [6] |
| B. maydis | M 1122/C4 | Zea mays | USA | KM230389 | KM034847 | [6] |
| B. maydis | CBS 136.29 | Zea mays | Japan | KJ909769 | KM034845 | [6] |
| B. oryzae | MFLUCC 100715 | Oryza sativa | Thailand | JX256416 | JX276430 | [25] |
| B. oryzae | MFLUCC 100733 | Oryza sativa | Thailand | JX256417 | KM042898 | [25] |
| B. oryzae | MAFF 235499 | Oryza sativa | Japan | KJ922383 | KM042897 | [6] |
| B. oryzae | AR3797 | Panicum virgatum | USA | KM230392 | KM042894 | [6] |
| B. oryzae | AR5204 | Panicum virgatum | USA | KM230393 | KM042895 | [6] |
| B. saccharicola | CBS 155.26 | Saccharum officinarum | —3 | KY905674 | KY905686 | [22] |
| B. saccharicola | CBS 325.64 | Saccharum officinarum | — | KY905675 | KY905687 | [22] |
| B. salviniae | BRIP 16571 | Salvinia auriculata | Brazil | KJ415535 | KJ415411 | [23,26] |
| B. setariae | CBS141.31 | — | — | EF452444 | EF513206 | [22,27] |
| B. setariae | CBSHN01 | Cassava | China | GU290228 | — | [28] |
| B. setariae | CPC 28802 | Imperata cylindrica | Thailand | MF490811 | MF490833 | [23,29] |
| B. setariae | UTHSC 05-3211 | Human | USA | HE792921 | — | [21] |
| B. sorokiniana | MAFF 236448 | Zea mays | Japan | KJ909792 | KM034826 | [6] |
| B. sorokiniana | MAFF 235501 | Zea mays | Japan | KJ909791 | KM034825 | [6] |
| B. sorokiniana | MAFF 238877 | Hordeum vulgare | Japan | KJ909790 | KM034824 | [6] |
| B. sorokiniana | CBS 110.14 | Hordeum sp. | USA | KJ922381 | KM034822 | [6,23] |
| B. sorokiniana | CPC 28832 | Triticum aestivum | Thailand | MF490812 | MF490834 | [23,29] |
| B. zeae | BRIP 11512 | Zea mays | Australia | KJ415538 | KJ415408 | [23,30] |
| B. zeicola | AR5166 | Sorghum sp. | USA | KJ909788 | KM034813 | [6] |
| B. zeicola | AR 5168 | Sorghum sp. | USA | KM230397 | KM034814 | [6] |
| B. zeicola | FIP 532 | Zea mays | USA | KM230398 | KM034815 | [6] |
| Curvularia ellisii | CBS193.62 | Air | Pakistan | JN192375 | JN600963 | [5] |
| C. hawaiiensis | CBS 173.57 | Oryza sativa | Hawaii | JN601029 | JN600966 | [5] |
| C. hawaiiensis | BRIP 10971 | Chloris gayana | Australia | JN601030 | JN600967 | [5] |
| C. hawaiiensis | BRIP 15933 | Chloris gayana | Australia | JN601028 | JN600965 | [5,6,8] |
| C. inaequalis | CBS 102.42 | Sand dune soil | France | KJ922375 | KM061787 | [6] |
| C. lunata | CBS730.96 | Human lung biopsy | USA | JX256429 | JX276441 | [25] |
| C. lunata | CBS157.34 | Unknown | Indonesia | JX256430 | JX276442 | [25] |
| C. pallescens | CBS 156.35 | Air | Java | KJ922380 | KM083606 | [6] |
| C. richardiae | BRIP 4371 | Richardia brasiliensis | Australia | KJ415555 | KJ415391 | [30] |
| C. spicifera | CBS 274.52 | Soil | Spain | JN192387 | JN600979 | [5,6] |
| C. trifolii | ICMP6149 | Setaria glauca | New Zealand | JX256434 | JX276457 | [25] |
| Drechslera avenae | CBS 189.29 | — | — | AY004795 | AY004827 | [8,31] |
| D. avenae | CBS 279.31 | — | — | AY004796 | AY004828 | [8,31] |
| D. avenae (Pyrenophora chaetomioides) | DAOM 208989 | — | — | AF081445 | AF081371 | [8] |
| D. dactylidis | DAOM 92161 | — | — | AY004781 | AY004812 | [8,31] |
| D. erythrospila | CBS 108941 | — | — | AY004782 | AY004813 | [8,31] |
| D. erythrospila | DAOM 55122 | — | — | AY004783 | AY004814 | [8,31] |
| Exserohilum holmii | BRIP 12679 | Dactyloctenium radulnas | Australia | LT837846 | LT882542 | [32] |
| E. minor | BRIP 14614 | Dactyloctenium aegyptium | Australia | LT837468 | LT715885 | [32] |
| E. pedicellatum | CBS 375.76 | Oryza sativa | Turkey | KT265259 | LT715879 | [32] |
| E. rostratum | PZ-3-4 | Zea mays | China | MG780267 | MK558815 | In this study |
| E. rostratum | PZ-3-40 | Zea mays | China | MG780268 | MK558816 | In this study |
| E. rostratum | ER9-8-1 | Zea mays | China | MG780269 | MK558817 | In this study |
| E. rostratum | BRIP 10995 | Zea mays | Australia | LT837823 | LT882566 | [32] |
| E. rostratum | BRIP 11416 | Zea mays | Australia | LT837466 | LT883543 | [32] |
| E. rostratum | BRIP 11417 | Zea mays | Australia | LT837836 | LT882553 | [32] |
| E. turcicum | BRIP 12267 | Sorghum bicolor | Australia | LT837482 | LT883553 | [32] |
| E. turcicum | BRIP 13326 | Sorghum sudanense | Australia | LT837480 | LT883551 | [32] |
| E. turcicum | CBS 195.26 | Zea mays | Indonesia | LT837485 | LT882583 | [32] |
| E. turcicum | CBS 330.64 | Zea mays | USA | LT837484 | LT715874 | [32] |
Note: 1 AR, FIP: Isolates housed in Systematic Mycology and Microbiology Laboratory, United States Department of Agriculture, Agricultural Research Service, Beltsville, Maryland; BRIP: Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; CPC: working collection of P.W. Crous, housed at the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; EGS: Collection of E. G. Simmons; ICMP: International Collection of Microorganisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; MAFF: Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Ibaraki, Japan; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand. 2 ITS: internal transcribed spacers and intervening 5.8S nrDNA; GAPDH: partial glyceraldehyde-3-phosphate dehydrogenase gene. 3—, not available.
2.3.1. Phylogeny Based on the ITS Region
A maximum-parsimony tree was constructed based on the ITS gene region of 160 isolates obtained in this study, 20 reference taxa and an outgroup (Alternaria alternata), as shown in Figure S2 (TL = 62 steps, CI = 0.935, RI = 0.994, and RCI = 0.930). There were 465 characteristics in this analysis, including 398 conserved characteristics and 23 parsimony informative characteristics. A total of 160 isolates were clustered into six separate clades corresponding to B. maydis, B. zeicola, B. setariae, B. saccharicola, B. cynodontis and B. oryzae, which was consistent with the morphological analyses.
2.3.2. Phylogeny Based on the GAPDH Region
A maximum-parsimony tree was constructed based on the GAPDH gene region containing 150 isolates obtained in this study, 19 reference taxa and an outgroup (Alternaria alternata), as shown in Figure S3 (TL = 129 steps, CI = 0.907, RI = 0.994, and RCI = 0.902). For the 498 characteristics used in the phylogenetic analysis, 385 were conserved, and 57 were parsimony informative. The tree contained six primary clades, including B. maydis, B. zeicola, B. setariae, B. saccharicola, B. cynodontis, and B. oryzae, with their corresponding Bipolaris species from GenBank.
2.3.3. Phylogeny Based on the ITS + GAPDH Regions
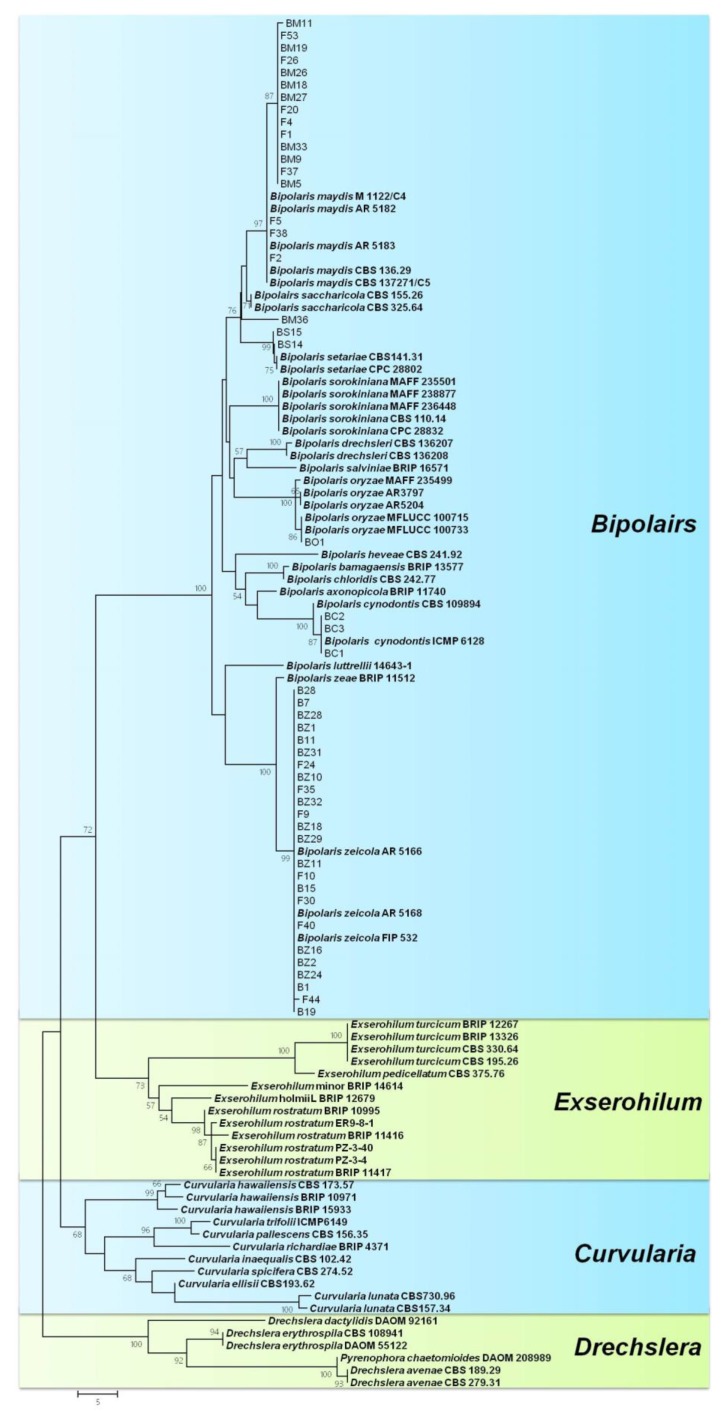
To evaluate the phylogenetic relationship among species of Bipolaris, multilocus phylogenetic analysis of the combined sequences of ITS and GAPDH gene regions was performed among 81 isolates of Bipolaris (48 isolates from this study), 13 isolates of Exserohilum, 11 isolates of Curvularia, and 6 isolates of Drechslera (used as outgroup). The dataset contained 780 characteristics, of which 537 were consistent and 203 were parsimony informative (TL = 298 steps, CI = 0.557, RI = 0.910, and RCI = 0.507). The phylogram was divided into four clades, as shown in Figure 3. Species of Bipolaris formed a well-supported clade (100% bootstrap values), clearly separated from other graminicolous helminthosporioid genera, such as Exserohilum, Curvularia, and Drechslera. Similar to the ITS and GAPDH trees, six Bipolaris species in this study were clearly separated in this tree and clustered into six distinct clades with their corresponding species from GenBank.
Figure 3.
Phylogram generated from parsimony analysis based on combined internal transcribed spacer (ITS) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequenced data of the accepted species of Bipolaris, Curvularia, and Exserohilum. Numbers on the branching points are ≥50% bootstrap values from a bootstrap test of 1000 replicates. Isolates obtained from NCBI are indicated in bold. The tree was rooted with Drechslera species.
2.4. Fungal Species and Corresponding Field Symptom Types
The isolation frequencies of fungal species isolated from the maize leaf spot samples with different symptom types are recorded in Table 4. B. maydis and B. zeicola could be isolated from all symptom types, and these two species were simultaneously isolated from 111 samples. B. maydis were mainly isolated from fusiform, elliptical (Type I) and elongated (Type II) lesions, with 54.4% and 77.8% isolation frequencies, respectively. A total of 432 isolates of B. maydis were obtained from Type I and Type II samples. Type I is the most typical symptom caused by B. maydis in the field and is highly concerned by growers. It has been learned from investigation that a lower isolation frequency of B. maydis from Type I should be attributed to the use of fungicides during the growing season. However, most B. zeicola were isolated from long narrow linear lesions (Type III), with 100% isolation frequency, and 235 isolates of B. zeicola were obtained. Except for these main types, both B. maydis and B. zeicola could be obtained from subrotund (Type IV) and punctiform (Type V) samples. B. saccharicola and B. setariae were isolated from subrotund lesions (Type IV). B. cynodontis and B. oryzae were isolated from punctiform lesions (Type V). Moreover, a fair number of isolates of the genera Curvularia, Colletotrichum and Exserohilum were isolated along with the Bipolaris species in the present study. One hundred fifty-one Curvularia isolates were frequently obtained from samples of Type IV and Type V symptoms, but the subrotund lesions caused by Curvularia spp. were smaller and more densely distributed than lesions caused by Bipolaris species. Ninety-nine isolates of Colletotrichum spp. were mainly isolated accompanying Bipolaris species from Type I and Type II samples. Eleven isolates of E. rostratum were isolated from 4 Type I samples.
Table 4.
The isolation frequencies of fungal species isolated from the maize leaf spot samples with different field symptom types.
| Symptom Types | Number of Samples | Bipolaris maydis | B. zeicola | B. cynodontis | B. oryzae | B. setariae | B. saccharicola | Curvularia spp. | Colletotrichum spp. | Exserohilum rostratum |
|---|---|---|---|---|---|---|---|---|---|---|
| Type I: Fusiform or elliptic | 341 | 54.3% | 9.97% | 0 | 0 | 0 | 0 | 0 | 13.2% | 1.17% |
| Type II: Elongate | 135 | 77.8% | 18.5% | 0 | 0 | 0 | 0 | 8.89% | 22.2% | 0 |
| Type III: Narrow linear | 126 | 27.0% | 100% | 0 | 0 | 0 | 0 | 15.1% | 0 | 0 |
| Type IV: Subrotund | 100 | 50.0% | 24.0% | 0 | 0 | 1.00% | 1.00% | 28.0% | 3.00% | 0 |
| Type V: Punctiform or necrotic | 45 | 26.7% | 22.2% | 2.22% | 2.22% | 0 | 0 | 46.7% | 24.4% | 0 |
| Total | 747 | 51.7% | 29.3% | 0.13% | 0.13% | 0.13% | 0.13% | 10.7% | 11.9% | 0.54% |
Note: The isolation frequency is equal to the number of samples with fungal species isolated/total number of samples.
2.5. Pathogenicity Tests
Seventy-four representative isolates from six identified Bipolaris species were used for pathogenicity testing on maize (Table S2). After one week of inoculation, all inoculated leaves developed characteristic lesions, whereas untreated controls had no symptoms. Five kinds of symptoms were observed in the pathogenicity test: elliptical, fusiform, subrotund, narrow linear, and punctiform. Leaves inoculated with B. maydis showed small, light brown watery spots at 48 h after inoculation. Subsequently, these small spots developed into subrotund, or elliptical, fusiform lesions in 5–7 days. Some lesions joined together and caused a large area of leaf death. The main symptoms caused by B. zeicola were small spots in the first three days, and then, for some isolates, narrow linear lesions appeared and extended to 5–13 mm in length at 7 days after inoculation. The isolate BM36 of B. saccharicola caused subrotund spots of approximately 5 mm in diameter on maize leaves at 7 days after inoculation. Leaves inoculated with B. setariae isolates showed symptoms of small subrotund spots after 7 days of inoculation. Only small chlorotic punctiform spots appeared on maize leaves after 3 days when inoculated with B. cynodontis and B. oryzae, but the spots did not extend further over time. The diseased leaves were collected at 10 days after inoculation, and the recovered fungi were consistent with the inoculated species.
Virulence differentiation was observed among the predominant species of B. maydis and B. zeicola with different symptoms (Figure 4). According to the lesion length, isolate virulence was divided into three categories (Table 5). Moderately and weakly virulent isolates of B. maydis accounted for 30.6% and 22.2%, respectively, causing elliptical to subrotund lesions of approximately 2–5 mm length (Figure 4a,b). B. maydis isolates with high virulence caused larger fusiform, elliptical or subrotund lesions 6–8 mm in length (Figure 4c) and accounted for 47.2% of the total isolates. B. zeicola isolates of weak virulence accounted for 45.2% of the total isolates, only showing small punctiform or white pinhole-like lesions (Figure 4d). B. zeicola isolates with moderate virulence causing small subrotund spots (Figure 4e) accounted for approximately 32.2% of isolates, while highly virulent B. zeicola isolates accounted for 22.6% of isolates and caused conspicuous long narrow linear lesions, which was the main symptom type observed under field conditions (Figure 4f).
Figure 4.
Symptoms in maize leaves after inoculation with the two predominant Bipolaris species: B. maydis and B. zeicola. (a–c): different symptoms at 7 days after inoculation with B. maydis, each representing weak (a), moderate (b), and high (c) virulence; (d–f): different symptoms at 7 days after inoculation with B. zeicola, each representing weak (d), moderate (e), and high (f) virulence.
Table 5.
Virulence differentiation of the two preponderant Bipolaris species.
| Species | Virulence Level | No. of Isolate | Incubation Period (h) | Average Lesion Length (mm) |
|---|---|---|---|---|
| B. maydis | High virulence | 17 | 24 | 6.5 ± 0.36b (6–8) |
| Moderate virulence | 11 | 24 | 4.8 ± 0.34c (4.3–5.2) | |
| Weak virulence | 8 | 48 | 3.1 ± 0.38e (2.7–3.8) | |
| B. zeicola | High virulence | 7 | 24 | 7.6 ± 0.99a * (6.2–8.9) |
| Moderate virulence | 10 | 48 | 4.3 ± 0.30d (4–5.1) | |
| Weak virulence | 14 | 72 | 2.5 ± 0.36f (1.8–2.9) |
Note: * The mean difference is significant at the 0.05 level; a–f: the values with the same letter in a column do not significantly differ according to Duncan’s multiple range test.
3. Discussion
Maize leaf spot is a serious fungal disease worldwide [33]. Accurate diagnosis of Bipolaris leaf spot of maize and the associated causal agents plays a key role in the effective management of this disease [7]. According to morphological, phylogenetic, and pathogenicity analysis, our study demonstrated that six Bipolaris species were associated with maize leaf spot in Sichuan Province, China. Among them, B. maydis and B. zeicola were the dominant pathogenic species, accounting for 57.42% and 42.07% of the isolates, respectively. B. cynodontis, B. oryzae, B. setariae, and B. saccharicola could also cause maize leaf spot but were extremely rare, implying that they might not occupy a major niche for infection on maize.
Previously, some research results on the isolation and identification of pathogens from diseased maize leaves in China have indicated the predominance of Bipolaris species [34,35]. Due to the widespread use of susceptible hybrid maize varieties, Bipolaris leaf spot has become the major disease in almost all maize production regions, especially in summer-sown maize fields [14]. In addition to the widely distributed B. maydis and B. zeicola, some other Bipolaris species, such as B. sorokiniana, have also been reported as harmful pathogens of maize [6,34,36]. B. sorokiniana, the causal agent of root rot and leaf spot in wheat, was also recently reported to cause leaf spot in summer-sown maize in North China [36,37]. However, in this study, B. sorokiniana was not isolated from the maize samples. We found serious B. sorokiniana infection on volunteer wheat seedlings in wheat-maize rotation fields in Sichuan, while isolates of B. sorokiniana showed only very weak virulence on maize plants [38]. It was possible that the infection of maize by B. sorokiniana required certain strict conditions or that the maize plants used for the pathogenicity test in our study were resistant varieties. The wheat-maize rotation is a traditional planting system in Sichuan, therefore the infection of maize by B. sorokiniana is still of great importance. In this study, B. saccharicola, B. setariae, B. cynodontis, and B. oryzae were also pathogenic species on maize. B. saccharicola is a novel species isolated from Saccharum officinarum [22]. This work is the first report of maize leaf spot caused by B. saccharicola. Both B. setariae and B. cynodontis have been reported on maize; however, the former is mainly distributed in Canada, India, and Pakistan [4], and the latter is distributed in Australia and the United States [4,6]. To our knowledge, this is also the first report of B. setariae and B. cynodontis as causal agents of maize leaf spot in China. Studies by Amorio and Cumagun suggested that in the rice-maize cropping system, B. oryzae was not a potential source of inoculum for leaf spot of maize, and likewise, B. maydis was not a potential source of inoculum for brown spot of rice [39]. However, B. oryzae shows considerable genetic variation within the species; thus, several biotypes and pathotypes may exist within the species [40,41]. The potential threat posed by B. oryzae to maize production cannot be underestimated. Since Bipolaris species can persist on crop debris for a long time after harvest, this might increase the survival and spread of these pathogens to some extent. Thus, certain environmental conditions may be especially conducive to the interaction of Bipolaris species among different hosts. These findings indicate that long-term monitoring of the composition of Bipolaris species causing maize leaf spot is still needed.
Comparing the field symptoms with the symptoms shown in the pathogenicity test, the results showed that different Bipolaris species caused unique symptom types. Isolates of B. zeicola were mainly isolated from type III field symptoms. In the pathogenicity tests, the narrow linear lesions were only observed after inoculation with the isolates of B. zeicola obtained in this study. In Yunnan and Shaanxi Provinces of China, B. zeicola isolates were also reported to produce long, narrow linear lesions [12,42,43]. Therefore, based on these results, long narrow linear lesions are the typical symptom caused by B. zeicola. Isolates of B. maydis were mainly isolated from Type I and Type II symptoms. In the pathogenicity tests, the symptoms caused by B. maydis isolates were mostly fusiform and elliptical (Type I), and in the late stages, the lesions joined together, causing similar elongated lesions. Therefore, elongated, fusiform and elliptical lesions were the typical symptoms caused by B. maydis, which was consistent with the results of previous studies [34,35,36]. Moreover, some subrotund or punctiform lesions were also found to be caused by B. maydis or B. zeicola isolates, which increased the difficulty of disease diagnosis. B. saccharicola and B. setariae were isolated from maize leaves with subrotund lesions (Type IV) and caused subrotund lesions in the pathogenicity tests. B. cynodontis and B. oryzae were isolated from punctiform lesions (Type V) and caused punctiform lesions in the pathogenicity tests. In addition, we also observed a regional difference in the geographic distribution of these two dominant species in Sichuan Province. B. zeicola was mainly isolated from Ya’an City, Luding County of Ganzi Prefecture, Xichang City of Liangshan Prefecture, and Beichuan County of Mianyang City, where maize plants were usually planted at high altitude and in cool climates. The samples collected in these regions were almost all Type III. Compared to B. zeicola, B. maydis had a more extensive distribution. Except for Liangshan Prefecture, B. maydis was isolated from each sampling region. Although there were some situations in which the infection of Curvularia spp., Colletotrichum spp. and Exserohilum spp. would further increase the difficulty of disease diagnosis, we can distinguish these species by their particular symptom and distribution characteristics.
Previous studies have shown that the virulence of B. maydis and B. zeicola isolates is diversified and that these species have different pathotypes [13,42,44,45]. It was reported that race 3 of B. zeicola might be a mountain ecotype, favoring high humidity and cool temperature in mountain areas at high elevations [42,46,47,48,49]. B. zeicola isolates from Yunnan Province of China, collected from approximately 550 m to 2535 m, were mostly identified as race 3, producing long, narrow linear lesions [42]. Zhang et al. reported that narrow linear lesions on the leaves of mature maize plants were caused by race 3 of B. zeicola in Shaanxi Province of China [43]. Therefore, considering these results and the lesion types of our maize samples, most isolates of B. zeicola in Sichuan Province may belong to race 3. B. maydis is generally subdivided into four races, namely, O, T, C, and S, and among them, race O is the predominant pathogen of maize in China and the disease characterized by the appearance of elongated or fusiform lesions on the leaves of susceptible plants [44,45,50,51]. We inferred that isolates of B. maydis in Sichuan may mostly belong to race O. Our pathogenicity tests demonstrated that virulence differentiation existed among isolates of B. maydis and B. zeicola, which was basically consistent with the findings of previous studies. It is necessary to focus on high virulence groups and establish early-warning lines to avoid huge maize losses. Although most isolates of B. zeicola were moderately or weakly virulent in the pathogenicity tests in this study, the maize leaf spot diseases caused by B. zeicola were relatively serious in some mountainous locations. Perhaps the virulence testing conditions were somewhat different from the real environmental conditions, and were not suitable for B. zeicola infection. Therefore, in future work, we suggest simulating the pathogenicity of B. zeicola in cold areas at high altitudes. As a result of the race diversity of B. maydis and B. zeicola, the disease symptoms on maize are very complicated, and thus, further studies should be devoted to race detection and virulence determination of Bipolaris spp. and to the elucidation of the mechanisms of pathogen differentiation.
In conclusion, to explore the species diversity of Bipolaris and the corresponding symptoms, a continuous investigation of maize leaf spot was carried out in Sichuan, China. We verified that B. maydis, B. zeicola, B. cynodontis, B. oryzae, B. setariae, and B. saccharicola were able to cause maize leaf spot, while B. maydis and B. zeicola were the main causal agents of Bipolaris leaf spot of maize. These two dominant species were able to cause mixed infections but were distinguished by different symptom characteristics. B. maydis was distributed throughout Sichuan Province and mainly caused elongated, fusiform, and elliptical lesions on maize leaves. B. zeicola was mainly distributed in cool mountain areas at a high elevation and caused long, narrow linear lesions. These findings contribute greatly to the understanding of the pathogens causing Bipolaris maize leaf spot disease.
4. Materials and Methods
4.1. Sample Collection and Isolation
From 2011 to 2018, more than 700 symptomatic samples of maize leaves with visible punctiform, linear, fusiform, elliptical, or irregular lesions (at least five maize leaves for each sample) were collected from 132 sampling sites in 19 administrative districts of Sichuan Province during the maize growing season. All sampling sites were distributed in different villages or fields of different towns. Some sites yielded more than one sample, but the samples always originated from different fields.
Isolations were made from one to three lesions per leaf. Tissues were removed from the margins of single lesions, surface-sterilized in 75% ethanol for 30 s and 1% NaClO for 30 s, and rinsed thrice in sterile distilled water. The pieces were placed on sterile potato dextrose agar (PDA) plates containing streptomycin at a concentration of 30 μg·mL−1. All PDA plates were incubated at 25 °C in the dark for 5 days. Single-spore cultures were prepared from all fungal colonies that displayed the characteristics of helminthosporioid fungi following the method described by Gong et al. [52]. Conidia were first removed directly from cultured colonies using a sterilized acupuncture needle and dispersed on a plug of 2% water agar on a glass slide. A single spore was picked and placed on another water agar plug under a microscope. Finally, a water agar plug containing only one single spore was transferred to a new PDA plate and cultured at 25 °C until a clear colony was obtained. One isolate per lesion was obtained. Pure cultures were maintained on PDA slants at 4 °C for short-term storage and in 25% glycerol at −80 °C for long-term storage.
4.2. Morphological Analysis
All isolates were cultured on PDA media at 25 °C in the dark for 5–7 days. Colony diameter was measured each day from two perpendicular cross-sections until the plates were fully covered. The daily growth rate was calculated based on the values from three replicates. For observation of conidia and conidiophores, sterile cellophane (approximately 2 cm × 2 cm sizes) was placed on a water agar plate, and then mycelial plugs (5 mm diameter) were placed on one end of the cellophane and incubated at 25 °C. After 5–7 days, the cellophane was gently removed with a tweezer and placed on a glass slide for observation under a compound microscope (Axio Imager Z2, Carl Zeiss, Germany). The sizes and shapes of 50 conidia were measured for each isolate. The isolates were tentatively identified based on a comparison with morphological characteristics of colonies and conidia reported in previous taxonomic studies [5,6,22,53].
4.3. DNA Extraction and Sequence Amplification
Cultures were grown for 5–7 days on PDA at 25 °C. Mycelia were scraped from the colony surface using a sterile medicine spoon. Genomic DNA of each isolate was extracted using the Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. DNA concentration and quality were estimated using a Thermo Scientific NanoDrop™ 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware, USA). Then, DNA was diluted to a final concentration of 30 ng·μL−1.
The ITS and GAPDH gene sequences of the representative isolates were amplified and sequenced. PCR amplification was conducted with the primer pair ITS1/ITS4 (5’-TCCGTAGGTGAACCTGCGG-3′ and ITS4: 5’-GCTGCGTTCTTCATCGATGC-3′) for the ITS region [54] and GPD1/GPD2: (5′-CAACGGCTTCGGTCGCATTG-3′ and GPD2: 5′-GCCAAGCAGTTGGTTGTGC-3′) for the GAPDH gene [24]. PCR reactions were performed in a final volume of 30 μL containing 1 μL of genomic DNA (30 ng·μL−1), 1 μL of each primer (10 μM), 12 μL of ddH2O, and 15 μL of 2 × Taq PCR MasterMix (Tiangen Biotech, Beijing, China). The ITS region was amplified at 94 °C for 5 min, followed by 30 cycles of 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 2 min with a final extension of 72 °C for 10 min. For the GAPDH region, the amplification program included an initial denaturation step at 96 °C for 2 min, followed by 30 cycles of 1 min at 96 °C, 1 min at 52 °C and 45 s at 72 °C with a final extension of 10 min at 72 °C. The PCR products were analyzed by electrophoresis in 1% agarose gels. DNA sequencing was performed by Sangon Biotech Co. Ltd. (Chengdu, China).
4.4. Phylogenetic Analysis
The obtained sequences were compared by BLASTn on the NCBI database, and then reference nucleotide sequences were downloaded from NCBI. All sequences were aligned with Clustal X v2.0, and their characteristics were weighted equally. Maximum-parsimony trees were constructed with MEGA 6.0 based on the Kimura 2-parameter model [55]. The bootstrap values provided on the phylogenetic dendrogram were generated with 1000 replicates, and alignment gaps were excluded. All sequence data generated in this study were deposited in GenBank, and their accession numbers were obtained. The tree length (TL), consistency index (CI), retention index (RI), and rescaled consistency index (RCI) were also calculated.
4.5. Pathogenicity Tests
To identify whether all isolated Bipolaris species were pathogenic to maize, a total of 74 representative isolates, including 36 isolates of B. maydis, 31 isolates of B. zeicola and all isolates of the other four less common species, were selected for pathogenicity tests. Seeds of the susceptible maize variety Zhenghong 505 were grown in plastic pots (12 cm in height, 13 cm in diameter of the top) at 23 °C in a greenhouse. Before inoculation, isolates were grown on PDA plates at 27 °C for 5 to 7 days until adequately sporulating. Then, conidia were suspended in sterile water with 0.2% Tween 20 and adjusted to a concentration of 1 × 105 conidia per mL. For each maize plant, 5 mL of conidial suspension was evenly sprayed using a handheld sprayer when plants were at the five- to six-leaf stage [56]. The leaves of control plants were sprayed with sterile distilled water. Six replicates for each isolate and an equal number of inoculated control plants were included. After inoculation, maize plants were covered with plastic film for moisture retention for 24 h at 25 °C in a greenhouse, and then the plastic film was removed. The incubation period was recorded. Lesion length and type were recorded on the third and fourth leaves at 7 days after inoculation. The experiment was repeated three times.
Data from three repetitions of the experiment were combined and subjected to analysis of variance (ANOVA). The presence of lesions and isolate virulence were evaluated by lesion length at 7 days post inoculation as previously described by Kong et al. [50], where high virulence corresponded to lesion length greater than or equal to 6 mm; moderate virulence to lesion length greater than or equal to 4 mm and less than 6 mm; and weak virulence to lesion length less than 4 mm. The organisms were reisolated from the diseased inoculation sites at 10 days after inoculation and identified as the inoculated isolates based on the method mentioned above for the identification of Bipolaris species.
Acknowledgments
We are grateful to Ying Li, Khalilullah Soomro, Tingmin Pan and Min Liu for their help during pathogenicity tests. We are also grateful to all of the other Plant Protection Stations for supporting our research.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/3/229/s1, Figure S1: Map of the Bipolaris isolates distribution in the nineteen administrative districts of Sichuan Province, Figure S2: Consensus maximum-parsimony tree based on the ITS gene sequences from 161 Bipolaris isolates, Figure S3: Consensus maximum-parsimony tree based on the partial GAPDH gene sequences from 149 Bipolaris isolates, Table S1: GenBank accession numbers of Bipolaris isolates obtained in this study, Table S2: Symptoms and the virulence levels of Bipolaris isolates on maize.
Author Contributions
Conceptualization, G.G., X.S., and X.Q.; methodology, X.S., X.Q., and G.G.; software, X.S. and W.W.; validation, X.S. and G.G.; formal analysis, X.L., W.W., and H.Z.; investigation, X.S. and X.Q.; resources, C.W.; data curation, H.Z.; writing—original draft preparation, X.S.; writing—review and editing, X.C. and G.G.; visualization, X.S. and X.L.; supervision, M.Z. and G.G.; project administration, H.C. and G.G.; funding acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Maize Innovational Team of Industry Technology System of Modern Agriculture (Grant Number sccxtd-2020-02).
Conflicts of Interest
The authors declare no conflict of interest in this work. All forms of financial support are acknowledged in the contribution. This work does not involve any human participants or animals. All authors have offered the consent to submission.
References
- 1.National Bureau of Statistics of China . China Statistical Yearbook. China Statistics Press; Beijing, China: 2018. [Google Scholar]
- 2.Li G.K., Pan G.T. The utilization present situation and study advances of the germplasm in southwest maize zone. J. Maize Sci. 2005;13:3–7. [Google Scholar]
- 3.Chen Z.H. Ecological regions and variety requirements of the southwest corn. J. Mt. Agric. Biol. 2016;35:1–9. [Google Scholar]
- 4.Sivanesan A. Graminicolous Species of Bipolaris, Curvularia, Drechslera, Exserohilum and Their Teleomorphs. C.A.B. International; Wallingford, UK: 1987. pp. 1–261. Mycological Papers No. 158. [Google Scholar]
- 5.Manamgoda D.S., Cai L., Bahkali A.H., Chukeatirote E., Hyde K.D. Cochliobolus: An overview and current status of species. Fungal Divers. 2011;51:3–42. doi: 10.1007/s13225-011-0139-4. [DOI] [Google Scholar]
- 6.Manamgoda D.S., Rossman A.Y., Castlebury L.A., Crous P.W., Madrid H., Chukeatirote E., Hyde K.D. The genus Bipolaris. Stud. Mycol. 2014;79:221–288. doi: 10.1016/j.simyco.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengyella L., Yekwa E.L., Nawaz K., Iftikhar S., Tambo E., Alisoltani A., Feto N.A., Roy P. Global invasive Cochliobolus species: Cohort of destroyers with implications in food losses and insecurity in the twenty-first century. Arch. Microbiol. 2018;200:119–135. doi: 10.1007/s00203-017-1426-6. [DOI] [PubMed] [Google Scholar]
- 8.Hyde K.D., Nilsson R.H., Alias S.A., Ariyawansa H.A., Blair J.E., Cai L., de Cock A.W.A.M., Dissanayake A.J., Glockling S.L., Goonasekara I.D., et al. One stop shop: Backbones trees for important phytopathogenic genera: I. Fungal Divers. 2014;67:21–125. doi: 10.1007/s13225-014-0298-1. [DOI] [Google Scholar]
- 9.Tatum L.A. The southern corn leaf blight epidemic. Science. 1971;171:1113–1116. doi: 10.1126/science.171.3976.1113. [DOI] [PubMed] [Google Scholar]
- 10.Ullstrup A.J. The impacts of the southern corn leaf blight epidemics of 1970–1971. Annu. Rev. Phytopathol. 1972;10:37–50. doi: 10.1146/annurev.py.10.090172.000345. [DOI] [Google Scholar]
- 11.Sun G.Y., Wang Q., Zhang R., Mao Q. Identification and biological characteristics analysis of corn leaf spot caused by Bipolaris zeicola in Shaanxi Province. Acta Phytopathol. Sin. 2006;36:494–500. [Google Scholar]
- 12.Zhang X.F., Cui L.N., Li X., Zou C.J., Yang X.R. Biological characters and the resistance study of corn leaf spot caused by Bipolaris zeicola in southwest China. J. Maize Sci. 2013;21:128–133. [Google Scholar]
- 13.Zhang J.X., Wu Y.X., Ho H.H., Lu C.H., He Y.Q. Cultural characteristics and pathogenic variations among Cochliobolus carbonum isolates in Yunnan Province of China. J. Plant Pathol. Microbiol. 2013;4:210. doi: 10.4172/2157-7471.1000210. [DOI] [Google Scholar]
- 14.Ye K.H., Gong G.S., Qi X.B., Yuan J.C., Jiang C.X., Sun X.F., Wang Y.Y., Yang J.Z. Effect of cultivation measures on sheath blight and southern leaf blight of corn. Plant Prot. 2015;41:154–159. [Google Scholar]
- 15.Li P.P., Cao Z.Y., Dong J.G., Zhang L.H., Jia H., Liu N., Li S.H., Hao Z.M., Gu S.Q., Wang X.Y. First report of Bipolaris papendorfii causing corn leaf spot in China. Plant Dis. 2013;97:1506–1507. doi: 10.1094/PDIS-02-13-0203-PDN. [DOI] [PubMed] [Google Scholar]
- 16.Manamgoda D.S., Rossman A.Y., Castlebury L.A., Chukeatirote E., Hyde K.D. A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): Human and plant pathogens. Phytotaxa. 2015;212:175–198. doi: 10.11646/phytotaxa.212.3.1. [DOI] [Google Scholar]
- 17.Li G.F., Liu K.X., Xiao S.Q., Lu Y.Y., Xue C.S., Wang G.Q. First report of leaf spot of maize (Zea mays) caused by Bipolaris spicifera in China. Plant Dis. 2016;100:855. doi: 10.1094/PDIS-07-15-0750-PDN. [DOI] [Google Scholar]
- 18.Gan L., Dai Y., Yang X., Du Y., Ruan H., Shi N., Chen F. First report of leaf spot caused by Cochliobolus eragrostidis on corn (Zea mays L.) in Fujian Province, China. Plant Dis. 2018;102:439–440. doi: 10.1094/PDIS-03-17-0372-PDN. [DOI] [Google Scholar]
- 19.Tsai J.N., Tsai W.H., Chen J.L. Pathogenicity of Exserohilum rostratum on corn and weeds in the corn fields. Plant Pathol. Bull. 2001;10:181–186. [Google Scholar]
- 20.Weems J.D., Bradley C.A. Exserohilum turcicum race population distribution in the North Central United States. Plant Dis. 2018;102:292–299. doi: 10.1094/PDIS-01-17-0128-RE. [DOI] [PubMed] [Google Scholar]
- 21.da Cunha K.C., Sutton D.A., Fothergill A.W., Cano J., Gené J., Madrid H., De Hoog S., Crous P.W., Guarro J. Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J. Clin. Microbiol. 2012;50:4061–4066. doi: 10.1128/JCM.01965-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin-Felix Y., Groenewald J.Z., Cai L., Chen Q., Marincowitz S., Barnes I., Bensch K., Braun U., Camporesi E., Damm U., et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayawardena R.S., Hyde K.D., Jeewon R., Ghobad-Nejhad M., Wanasinghe D.N., Liu N.G., Phillips A.J.L., Oliveira-Filho J.R.C., da Silva G.A., Gibertoni T.B., et al. One stop shop II: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50. Fungal Divers. 2019;94:41–129. doi: 10.1007/s13225-019-00418-5. [DOI] [Google Scholar]
- 24.Berbee M.L., Mona P., Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91:964–977. doi: 10.1080/00275514.1999.12061106. [DOI] [Google Scholar]
- 25.Manamgoda D.S., Cai L., McKenzie E.H.C., Crous P.W., Madrid H. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers. 2012;56:131–144. doi: 10.1007/s13225-012-0189-2. [DOI] [Google Scholar]
- 26.Tan Y.P., Crous P.W., Shivas R.G. Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP) Mycol. Prog. 2016;15:1203–1214. doi: 10.1007/s11557-016-1240-6. [DOI] [Google Scholar]
- 27.Andrie R.M., Schoch C.L., Hedges R., Spatafora J.W., Ciuffetti L.M. Homologs of ToxB, a host-selective toxin gene from Pyrenophora tritici-repentis, are present in the genome of sister-species Pyrenophora bromi and other members of the Ascomycota. Fungal Genet. Biol. 2008;45:363–377. doi: 10.1016/j.fgb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Shi T., Li C.P., Li J.F., Cai J.M., Huang G.X. First report of leaf spot caused by Bipolaris setariae on cassava in China. Plant Dis. 2010;94:919. doi: 10.1094/PDIS-94-7-0919A. [DOI] [PubMed] [Google Scholar]
- 29.Marin-Felix Y., Senwanna C., Cheewangkoon R., Crous P.W. New species and records of Bipolaris and Curvularia from Thailand. Mycosphere. 2017;8:1555–1573. doi: 10.5943/mycosphere/8/9/11. [DOI] [Google Scholar]
- 30.Tan Y.P., Madrid H.R., Crous P.W., Shivas R.G. Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas. Plant Pathol. 2014;43:589–603. doi: 10.1007/s13313-014-0315-6. [DOI] [Google Scholar]
- 31.Zhang G.J., Berbee M.L. Pyrenophora phylogenetics inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 2001;93:1048–1063. doi: 10.1080/00275514.2001.12063240. [DOI] [Google Scholar]
- 32.Hernández-Restrepo M., Madrid H., Tan Y.P., da Cunha K.C., Gené J., Guarro J., Crous P.W. Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia. 2018;41:71–108. doi: 10.3767/persoonia.2018.41.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X.M., Jin Q.M., Shi J., Wang Z.Y., Li X. The status of maize diseases and the possible effect of variety resistance on disease occurrence in the future. Acta Phytopathol. Sin. 2006;36:1–11. [Google Scholar]
- 34.Guo Y.J., Niu Y.C., Deng H. Bipolaris and Curvularia species associated with corn leaf spot in northern China. Plant Prot. 2016;42:39–46. [Google Scholar]
- 35.Dai Y.L., Gan L., Ruan H.C., Shi N.N., Du Y.X., Chen F.R., Yang X.J. Pathogen identification of small leaf spot on sweet corn plants in Fujian. Fujian J. Agric. Sci. 2017;32:1341–1349. [Google Scholar]
- 36.Guo N., Ni X., Shi J., Ma J.Y., Xue C.S., Chen J. The occurrence and pathogen identification of leaf spot on maize. Acta Phytopathol. Sin. 2017;47:1–8. [Google Scholar]
- 37.Acharya K., Dutta A.K., Pradhan P. Bipolaris sorokiniana (Sacc.) Shoem.: The most destructive wheat fungal pathogen in the warmer areas. Aust. J. Crop. Sci. 2011;5:1064–1071. [Google Scholar]
- 38.Sun X.F., Zhang D.X., Gong G.S., Qi X.B., Ye K.H., Zhou Y., Liu N., Chang X.L. Spot blotch on volunteer wheat plants in Sichuan, China. J. Plant Pathol. 2015;97:173–176. [Google Scholar]
- 39.Amorio D.J.H., Cumagun C.J.R. Pathogenicity and cytological examination of adapted and non-adapted Bipolaris species on resistant and susceptible cultivars of rice and corn. Mycosphere. 2017;8:377–391. doi: 10.5943/mycosphere/8/3/3. [DOI] [Google Scholar]
- 40.Cholil A., de Hoog G.S. Variability in Drechslera oryzae. Trans. Br. Mycol. Soc. 1982;79:491–496. doi: 10.1016/S0007-1536(82)80041-7. [DOI] [Google Scholar]
- 41.Ahmadpour A., Castell-Miller C., Javan-Nikkhah M., Naghavi M.R., Dehkaei F., Leng P.Y., Puri K.D., Zhong S. Population structure, genetic diversity, and sexual state of the rice brown spot pathogen Bipolaris oryzae from three Asian countries. Plant Pathol. 2018;67:181–192. doi: 10.1111/ppa.12714. [DOI] [Google Scholar]
- 42.Lu C.H., Wu Y.X., Ho H.H., Mao Z.C., He Y.Q. Identification of races and mating types of Cochliobolus carbonum from corn in the Yunnan Province in China. J. Phytopathol. 2014;162:313–321. doi: 10.1111/jph.12194. [DOI] [Google Scholar]
- 43.Zhang R., Wang Q., Sun G.Y., Mao Q., Gleason M.L. First report of race 3 of Bipolaris zeicola on corn in China. Plant Dis. 2007;91:1360. doi: 10.1094/PDIS-91-10-1360A. [DOI] [PubMed] [Google Scholar]
- 44.Wang M., Wang S.Q., Ma J., Yu C.J., Gao J.X., Chen J. Detection of Cochliobolus heterostrophus races in South China. J. Phytopathol. 2017;165:681–691. doi: 10.1111/jph.12607. [DOI] [Google Scholar]
- 45.Gan L., Dai Y.L., Ruan H.C., Shi N.N., Du Y.X., Chen F.R., Yang X.J. Pathotype and its population structure of Cochliobolus heterostrophus in Fujian Province. Chin. Agric. Sci. Bull. 2018;34:147–151. [Google Scholar]
- 46.Lodge D.J., Leonard K.J. A cline and other patterns of genetic variation in Cochliobolus carbonum isolates pathogenic to corn in North Carolina. Can. J. Bot. 1984;62:995–1005. doi: 10.1139/b84-138. [DOI] [Google Scholar]
- 47.Welz H.G., Leonard K.J. Phenotypic variation and parasitic fitness of races of Cochliobolus carbonum on corn in North Carolina. Phytopathology. 1993;83:593–601. doi: 10.1094/Phyto-83-593. [DOI] [Google Scholar]
- 48.Tsukiboshi T., Kimigafukuro T., Sato T. Identification of races of Bipolaris zeicola, the causal fungus of Helminthosporium leaf spot on corn in Japan. Ann. Phytopathol. Soc. Jpn. 1987;53:647–649. doi: 10.3186/jjphytopath.53.647. [DOI] [Google Scholar]
- 49.Jones M.J., Dunkle L.D. Analysis of Cochliobolus carbonum races by PCR amplification with arbitrary and gene-specific primers. Phytopathology. 1993;83:366–370. doi: 10.1094/Phyto-83-366. [DOI] [Google Scholar]
- 50.Kong L.X., Zhao J.Y., Li Q.S., Wang L.S., Luo P.C. Identification and population dynamics of physiological races of Bipolaris maydis in Hebei. Acta Agric. Boreali Sin. 2005;20:90–93. [Google Scholar]
- 51.Zhang L.X., Dong M., Yang L.M., Wang J.H., Tan G.J. Identification of physiological races of Bipolaris maydis and their sensitivities to diniconazole in Anhui Province. Acta Phytopathol. Sin. 2011;41:441–444. [Google Scholar]
- 52.Gong G.S., Xu Q., Zhang M., Yang J.Z., Chen H.B., Shen S.A., Tang T.F. A simple method for single fungal spore isolation. J. Maize Sci. 2010;18:126–127. [Google Scholar]
- 53.Zhang T.Y., Sun G.Y. Flora Fungorum Sinicorum. Volume 31: Helminthosporioid Hyphomycetes. Science Press; Beijing, China: 2010. pp. 1–109. [Google Scholar]
- 54.White T.F., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky F.S., White T.T., editors. PCR Protocol: A Guide to Methods and Applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 55.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balint-Kurti P.J., Carson M.L. Analysis of quantitative trait loci for resistance to southern leaf blight in juvenile maize. Phytopathology. 2006;96:221–225. doi: 10.1094/PHYTO-96-0221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.