Abstract
The hamster species used as research models include the Syrian (golden), Mesocricetus auratus; the Chinese (striped-back), Cricetulus griseus; the Armenian (gray), C. migratorius; the European, Cricetus cricetus; and the Djungarian, Phodopus campbelli (Russian dwarf) and P. sungorus (Siberian dwarf). Hamsters are classified as members of the order Rodentia, suborder Myomorpha, superfamily Muroidea and in family Cricetidae. Animals in this family are characterized by large cheek pouches, thick bodies, short tails, and an excess of loose skin. They have incisors that erupt continuously and cuspidate molars that do not continue to grow ((I 1/1, C 0/0, PM 0/0, M 3/3) × 2 = 16). In 2010, it was reported that approximately 146,000 hamsters were used in research in the United States (United States Department of Agriculture, 2010).
Keywords: Mesocricetus auratus, Syrian hamster, golden hamster, Cricetulus griseus, Chinese hamster, Cricetulus migratorius, Armenian hamster, Cricetus cricetus, European hamster, Phodopus sungorus, Siberian dwarf Djungarian hamster, Phodopus campbelli, Russian dwarf Djungarian hamster, research, husbandry, diseases, proliferative enteritis, Lawsonia, Tyzzer's disease, Clostridium, Salmonellosis, Helicobacter, pneumonia, polyomavirus, parvovirus, adenovirus, papillomavirus, Sendai, PVM, LCMV, protozoa, nematodes, cestodes, mites, non-infectious diseases, amyloid, metabolic, diabetes, neoplasia
I. Introduction
The hamster species used as research models include the Syrian (golden), Mesocricetus auratus; the Chinese (striped-back), Cricetulus griseus; the Armenian (gray), C. migratorius; the European, Cricetus cricetus; and the Djungarian, Phodopus campbelli (Russian dwarf) and P. sungorus (Siberian dwarf). Hamsters are classified as members of the order Rodentia, suborder Myomorpha, superfamily Muroidea, and in family Cricetidae. Animals in this family are characterized by large cheek pouches, thick bodies, short tails, and an excess of loose skin. They have incisors that erupt continuously and cuspidate molars that do not continue to grow ((I 1/1, C 0/0, PM 0/0, M 3/3) × 2 = 16). In 2010, it was reported that approximately 146,000 hamsters were used in research in the United States (United States Department of Agriculture, 2010).
II. Syrian Hamster
A. Introduction
The reader is referred to the American College of Laboratory Animal Medicine Series reference entitled The Laboratory Rabbit, Guinea Pig, Hamster and Other Rodents (Suckow et al., 2012) for a comprehensive source of information on hamster biology and diseases, experimental techniques, and research models.
1. Description
The Syrian or golden hamster (Mesocricetus auratus) originated in Syria and naturally resides in the arid, temperate regions of southeast Europe and Asia Minor. In their native environment, hamsters live in deep tunnels that provide cooler temperatures and higher humidity than the general desert environment. They are nocturnal animals in the laboratory, but field research has shown diurnal activity in females in the wild (Gattermann et al., 2008). The adult Syrian hamster usually grows to a length of 6–8 inches (14–19 cm) and weighs between 110 and 140 g. The adult female of this breed tends to be larger than the male. The hamster has a small blunt tail and smooth, short hair. Normal coloration is reddish gold, with a grayish-white ventrum. Hair-coat colors also include cream, albino, piebald, and cinnamon; the length of hair can also vary (Harkness et al., 2010). Hamster ears are pointed, with dark pigmentation, and the eyes are small, dark, and bright. Male hamsters can be identified by prominent flank glands and by large testicles that protrude behind the body on each side of the tail. The normal gross anatomy for the golden hamster has been described in this section (Hoffman et al., 1968, Murray, 2012).
2. Use in Research
Practically all Syrian hamsters now in use as laboratory animals originated from one litter captured in Syria in 1930. The use of the golden hamster as a laboratory animal was initiated by Saul Adler, who sought a laboratory animal susceptible to infection with Leishmania (Adler, 1948). Only three littermates, one male and two females, were retained in captivity, and it is the progeny of these animals that were first imported to the United States in 1938. By 1973, the hamster had become the third most commonly used laboratory animal in the United States, behind mice and rats. Hamster use in research has steadily declined by approximately 67% since its peak in the early 1970s; currently hamsters are less frequently used than mice, rats, rabbits, and guinea pigs (United States Department of Agriculture, 2010).
Hamsters have several unique anatomical and physiological features that make them desirable research models. In addition, they are susceptible to a variety of carcinogens and develop certain tumors other animal models do not. Metabolic diseases can be induced in hamsters through dietary manipulation, and they develop a variety of inherited diseases that are similar to human syndromes. Furthermore, hamsters are relatively free of pathogens yet are susceptible to several experimental infectious diseases (Valentine et al., 2012).
Hamsters are used often for carcinogenesis studies; in fact, the hamster cheek-pouch carcinogenesis model is a popular model to study oral tumor formation (Vairaktaris et al., 2007, Vairaktaris et al., 2008a, Vairaktaris et al., 2008b). They are also used extensively to study pancreatic ductal adenocarcinoma through the administration of nitrosamines (Konishi et al., 1998, Uchida et al., 2008) or via the transplantable cell line, PGHAM-1, which models metastatic pancreatic cancer (Fukuhara et al., 2005, Uchida et al., 2008). The hamster is also susceptible to respiratory tract tumors and can be induced to develop nonsmall cell lung carcinoma through a course of injections of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), with or without the addition of hyperoxia (Oreffo et al., 1993, Sunday et al., 1995). The role of Simian virus 40 (SV40), a polyomavirus, in human cancers remains controversial (Rollison et al., 2004); however, the hamster remains a valuable model for investigation of this viral disease process. Hamsters injected with SV40 develop a variety of tumors depending on the route of inoculation and the age of hamster when inoculated (Allison et al., 1967, Cicala et al., 1993, Sroller et al., 2008). Additionally, the Syrian hamster is used to study the effects of exogenous estrogenic compounds on tumor development, with 100% of male hamsters developing renal tumors after the administration of estrogens (Li and Li, 1996; Liehr, 1997). Finally, the hamster is one of the few animal models that permit the replication of human adenoviruses, which holds promise as a potential cancer therapeutic (Hjorth et al., 1988, Thomas et al., 2006).
Hamsters, like humans, are highly susceptible to metabolic diseases and present with several related clinical signs and syndromes. The hamster is commonly used as a model for cholesterol cholelithiasis, which can be induced via excess dietary cholesterol or by feeding a sucrose-rich diet (Cohen et al., 1989, Khallou et al., 1991, Trautwein et al., 1999). Hamsters are also susceptible to diabetes mellitus induced by differing methods. Chemical agents such as streptozotocin (STZ) or alloxan can be used; however, STZ may be more effective and reliable than alloxan (Phares, 1980). The addition of nicotinamide (dosed intraperitoneally) at 15 min before STZ injection results in partial protection against the beta-cytotoxic effect of STZ, resulting in partial preservation of insulin stores (Fararh et al., 2002, Masiello et al., 1998). Diabetes mellitus can also be induced via dietary modifications. A high-fat (15%) diet containing modest cholesterol (0.12%) fed for three weeks will induce type 2 diabetes along with related comorbidities such as obesity, hyperinsulinemia, hyperleptinemia, hypercholesterolemia, and hypertriglyceridemia (Van Heek et al., 2001). Syrian hamsters of the albino-panda-albino (APA) strain develop diabetes with nephropathy following STZ injections and also develop coronary lesions (Horiuchi et al., 2005). Syrian hamsters possess similar lipid metabolism to humans and are useful models for atherosclerosis, induced via dietary manipulation (Mitchell and McLeod, 2008, Simionescu et al., 1993, Wissler, 1991).
Syrian hamsters have spontaneous genetic mutations that manifest with conditions resembling human cardiovascular disease. Cardiomyopathy in the Syrian hamster is a naturally occurring, inherited condition and, as such, is an established animal model for both dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). In the hamster, both DCM and HCM are caused by a defect in the sarcoglycan gene, a component of the dystrophin complex (Bajusz et al., 1969, Escobales and Crespo, 2006, Escobales and Crespo, 2008, Goineau et al., 2001, Ikeda and Ross, 2000, Lipskaia et al., 2007, Ryoke et al., 1999, Sakamoto et al., 1997). Cardiomyopathic hamster lines include the original polymyopathic line 1.50, as well as BIO 82.62, BIO TO-2, BIO 53, and UMX-7.1. Some strains are characterized by significant cardiac hypertrophy, some by ventricular dilation without hypertrophy, and still other strains show compensatory hypertrophy progressing to left ventricular dilation (Cruz et al., 2007, Goineau et al., 2001, Homburger, 1979, Ikeda and Ross, 2000, Sakamoto et al., 1997).
Syrian hamsters were originially introduced as laboratory animals that could be infected with Leishmania (Adler, 1948). Their susceptibility to experimentally induced infectious diseases continues to make them valuable infectious disease models. Hamsters serve as experimental models of Hantavirus pulmonary syndrome (McElroy et al., 2004, Milazzo et al., 2002, Wahl-Jensen et al., 2007). Hamsters are also susceptible to the coronovirus that leads to severe acute respiratory syndrome (SARS); therefore, they are also useful for efficacy studies for vaccinations and immunotherapy treatments against this virus (Roberts et al., 2005). Hamsters are susceptible to fungal infections, including Histoplasma spp., and are sensitive to small inocula. They then can be involved in refinements for disease diagnosis. Most of the fungi grow in the spleen, lymph nodes, and liver. Hamsters infected with Mycoplasma pneumoniae are used as models of localized infection in the respiratory tract (Brunner, 1997).
Other pathogens to which hamsters are susceptible include Mycobacteria spp., Clostridium difficile (Kokkotou et al., 2008), Treponema pallidum (Kajdacsy-Balla et al., 1993), Toxoplasma spp. (Pavesio et al., 1995), and Babesia spp. (Wozniak et al., 1996). In addition, hamsters can serve as models of leprosy, atypical tuberculosis, and leptospirosis, as well as other protozoal and helminthic infections. Leishmania infantum infection causes polymyositis and may be a new model for inflammatory myopathy (Paciello et al., 2010). Syrian hamsters have historically been valuable for the study of prion disease. Laboratory mice are now the animal model of choice for this research area, yet hamster strains are still occasionally used in the study of prions because of their susceptibility to scrapie, transmissible mink encephalopathy (TME), Creutzfeldt–Jakob disease, and Gerstmann–Staussler syndrome (GSS) (Lowenstein et al., 1990). These prions cause slow, progressive, degenerative diseases in the central nervous system (CNS). Hamsters develop amyloid-like deposits in their brains, which may be similar to extracellular deposits of amyloid found in human Alzheimer’s disease (Czub et al., 1986). Scrapie prions replicate to high titers in the brains of several species of hamsters, making it possible to compare the human and hamster forms of the disease in a single host (Lowenstein et al., 1990, Marsh and Hanson, 1978). Further information about the prion diseases can be obtained in reviews by Prusiner, 1991, Trevitt and Collinge, 2006.
In addition to the above models, hamsters are preferred for several other experimental uses. Chronic obstructive pulmonary disease (COPD) and emphysema can be induced via a single intratracheal dose of porcine pancreatic elastase (Borzone et al., 2007) or feeding a diet deficient in copper (Soskel et al., 1984). Hamsters are also used to study gastropathy related to administration of nonsteroidal anti-inflammatory drugs (NSAIDs) (Kolbasa et al., 1988, Fitzpatrick et al., 1999).
In 1976, the hamster oocyte was discovered to be penetrable by human spermatozoa (Yanagimachi et al., 1976). Since that time, one of the main uses of Syrian hamsters in the biomedical setting has been to aid in the assessment of human fertility using the zona-free hamster oocyte assay, which analyzes the ability of sperm to capacitate eggs, undergo the acrosome reaction, and fuse with the oocyte (Barros et al., 1978). Results obtained from the assay correlate well with human in vitro fertilization results, but the process is labor-intensive and difficult to standardize. As new techniques are developed to assess male fertility (such as intracytoplasmic sperm injection), the hamster oocyte assay has begun to wane in popularity (Aitken, 2006).
B. Biology
1. Anatomy and Physiology
a. Development
A newborn M. auratus pup weighs 2–3 g. It is hairless, with eyes and ears closed. A unique feature of this rodent pup is that incisor teeth are visible at birth. At approximately day 4–5 of age, the ears will open; at day 9, hair growth is first observed; and between days 14 and 16, the eyes will open (Mulder, 2012). By weaning at day 21 of age, the pup weighs 35–40 g. By maturity at 6–8 weeks, males weigh 85–110 g and females weigh 95–120 g. There may be additional increase in weight with increased age. Male and female hamsters can be identified by comparing the anogenital distances (longer in the male) and by observing mammae on the ventrum of the female or noting the prominence of the posterior scrotum of the male.
The reproductive life span begins around 6–8 weeks and continues until 14 months of age (Mulder, 2012). The total life span averages 2 years, with the potential for aging up to 3 years. It is of interest to note that the average life span of the female golden hamster may be markedly shorter than that of males, depending on strain and source of the animals (Bernfeld et al., 1986). The short life cycle of the Syrian hamster, ranging between 18 and 24 months, makes it an excellent animal for the study of development and the effect of teratogenic agents. The eighth day of pregnancy is the optimal time for teratogenic studies, when hourly development of the fetal pups can be observed (Ferm, 1967). Normative physiological data, such as heart rate and respiration, can be found in Table 5.1 . Serum blood chemistry values have been provided in Table 5.2 . It should be mentioned that serum chemistry parameters may differ between sexes and strains of hamsters (Maxwell et al., 1985).
Table 5.1.
Normative Data – Syrian (Golden) Hamstera
| Adult weight | |
| Male | 85–140 g |
| Female | 95–120 g |
| Life-span | |
| Average | 2 years |
| Maximum expected | 3 years |
| Chromosome number (diploid) | 44 |
| Water consumption | 30 ml/day |
| Food consumption | 10–15 g/day (adult) |
| Body temperature | 36.2–37.5°C |
| Puberty | |
| Male | 6–8 weeks (90 g) |
| Female | 8–12 weeks (90–100 g) |
| Gestation | 15–18 days |
| Litter size | 4–12 pups |
| Birth weight | 2–3 g |
| Eyes open | 15 days |
| Weaning | 21 days (35–40 g) |
| Heart rate | 280–412 |
| Respiratory frequency | 74 (33–127) |
| Leukocyte counts | |
| Total | 7.62 × 103/mm |
| Neutrophils | |
| Segmented | 21.9% |
| Nonsegmented | 8.0% |
| Lymphocytes | 73.5% |
| Monocytes | 2.5% |
| Eosinophils | 1.1% |
| Basophils | 1.1% |
| Erythrocyte sedimentation rate | 1.64 mm/h |
| Platelets | 670.0 × 103/mm (indirect) |
| Red blood cells | 7.50 × 106/mm |
| Hemoglobin | 16.8% |
From Aeromedical Review (1975).
Table 5.2.
Mean ± SD Serum Blood Chemistry Values for Adult Syrian Hamstersa
| Serum analyte | Units | Male | Female |
|---|---|---|---|
| Glucose | mg/dl | 84.0 ± 18.5 | 100.0 ± 16.6 |
| Urea nitrogen | mg/dl | 23.2 ± 4.1 | 27.5 ± 4.6 |
| Creatinine | mg/dl | 0.40 ± 0.89 | 0.50 ± 0.15 |
| Sodium | mEq/l | 148.0 ± 3.70 | 148.0 ± 3.70 |
| Potassium | mEq/l | 6.50 ± 0.75 | 6.40 ± 0.73 |
| Chloride | mEq/l | 104.0 ± 3.10 | 104.0 ± 3.60 |
| Bicarbonate | mEq/l | 29.9 ± 2.9b | |
| Calcium | mg/dl | 12.6 ± 0.59 | 13.2 ± 1.38 |
| Phosphorus | mg/dl | 5.40 ± 1.00 | 5.50 ± 1.09 |
| Magnesium | mg/dl | 2.50 ± 0.20 | 2.20 ± 0.10 |
| Alanine aminotransferase | IU/l | 44.7 ± 25.9 | 50.3 ± 18.3 |
| Aspartate aminotransferase | IU/l | 61.2 ± 39.1 | 53.3 ± 22.7 |
| Alkaline phosphatase | IU/l | 126 ± 6 | |
| Lactate dehydrogenase | IU/l | 257 ± 63.6 | 208 ± 54.7 |
| Creatinine kinase | IU/l | 469 ± 174 | 520 ± 184 |
| Protein, total | g/l | 63 ± 3.2 | 59 ± 3.4 |
| Albumin | g/l | 43 ± 2.2 | 41 ± 2.8 |
| Cholesterol | mg/dl | 143 ± 23.5 | 158 ± 35.3 |
| Triglycerides | mg/dl | 209 ± 53.3 | 212 ± 52.7 |
| Bilirubin, total | mg/dl | 0.3 ± 0.09 | 0.3 ± 0.13 |
| Bile acids | μmol/l | 0.9 ± 0.2 | |
| Uric acid | 4.6 ± 0.5 | 4.4 ± 0.5 | |
| Luteinizing hormone | ng/ml | 10–30 | 20–40 (basal) |
| 1500–2000 | |||
| (late proestrus) | |||
| Follicle stimulating hormone | ng/ml | 200–300 | 100–200 (basal) |
| 400–600 | |||
| (preovulatory, estrus) | |||
| Prolactin | ng/ml | 5–10 | 10–15 (basal) |
| 30 (late proestrus) | |||
| Thyroid stimulating hormone | ng/ml | 300b | |
| Thyroxine (T4) | μg/dl | 3–7b | |
| Triiodothyronine (T3) | ng/dl | 30–80b | |
| Cortisol | μg/dl | 2.75 ± 0.44 | 0.33 ± 0.04 |
| (start of light) | |||
| Progesterone | ng/ml | 1.0 (basal) | |
| 10–12 (proestrus) | |||
| 6–8 (estrus, diestrus) | |||
| Estradiol | pg/ml | 5–10 (basal) | |
| 300–400 (proestrus) | |||
| Testosterone | ng/ml | 1.5–2.0 |
Summarized from Loeb and Quimby (1999).
Gender not specified.
b. Oral Cavity
i. Cheek Pouches
The cheek pouches, bilateral invaginations of the oral mucosa, are found in the lateral buccal walls. Often these highly distensible pouches are used by the hamster for temporary storage of food and bedding materials. These pouches do not contain glands but are rich in mast cells, are highly vascular, and lined with stratified squamous epithelium (deArruda and Montenegro, 1995). Blood supply to the pouches is carried by branches from the external carotid artery (Davis et al., 1986). More specifically, the pouches are supplied by six small arteries in the neck and face that are potentially important in controlling cheek pouch blood flow (Davis et al., 1986). The pouches can easily be everted (Fig. 5.1 ), with their blood flow intact, and have been used extensively for microvascular studies of inflammation, tumor growth, vascular smooth muscle function, and ischemia reperfusion studies (Svensjo, 1990, Hedqvist et al., 1990, Bertuglia and Reiter, 2007). These pouches lack an intact lymphatic drainage pathway and are therefore described as ‘immunologically privileged.’ Studies have shown that the surface density of Langerhans cells in the cheek pouches is markedly decreased, which may contribute to the specialized immune status of the tissue (Bergstresser et al., 1980). The pouch tissue will support the long-term survival of transplanted foreign tissue without immunological rejection. As mentioned previously, the Syrian hamster model of carcinogenesis in the cheek pouch is one of the best animal systems for the evaluation of human oral cancer development (Gimenez-Conti and Slaga, 1993).
Figure 5.1.
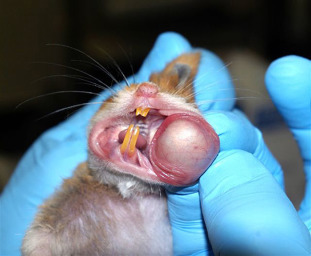
The cheek pouch has been manually everted for illustrative purposes. Note the vasculature supplying the pouch.
Credit: Jerald Silverman and Academic Press.
ii. Dentition
Due to the morphological makeup of their crown teeth, retention of fine food particles often occurs, and Syrian hamsters develop dental caries under defined conditions of diet and oral flora (Krasse, 1966). Studies show that the caries rate in hamsters is influenced not only by the amount of carbohydrate in the diet, but also by the form of carbohydrate. The presence or absence of vitamins in the diet is also thought to be a contributing factor (Shklar, 1972). Historical reports have suggested that caries may be caused by infectious bacteria and transmissible among rodents via oral routes (Jordan and van Houte, 1972). While hamsters were useful models at one time for studying caries-induced lesions, other rodent models are now more common (Bowen, 2013).
c. Gastrointestinal System
i. Stomach and Intestines
The hamster has a distinctly compartmentalized stomach consisting of two parts: the glandular stomach and the nonglandular forestomach. The forestomach and glandular stomach are separated from each other by the incisurae of the greater and lesser curvatures (Magalhaes, 1968). The nonglandular forestomach is functionally similar to that of ruminants and has an elevated pH level and microflora that contribute to digestion through a fermentation process.
The incidence of neoplasms in Syrian hamsters varies by study. These differences are likely due to age, strain differences, breeding environment, diet, and other unknown factors. Two studies showed high incidences of spontaneous neoplasms in the gastrointestinal tract (Fortner, 1957, Van Hoosier and Trentin, 1979), while other studies do not document such findings (Tanaka et al., 1991). The experimental induction of papillomas and adenocarcinoma in the forestomach and intestines, as well as adenomatous polyps in the colon, historically validated the hamster model of gastrointestinal carcinogenesis (Homburger, 1968).
Syrian hamsters respond predictably to intragastric administration of purified cholera enterotoxin, presenting with intraluminal accumulation of fluid in the small bowel, cecum, and proximal colon. Therefore, this animal was historically used to study pharmacological agents, such as indomethacin, polymyxin B sulfate, glucose electrolyte solutions, and colchicine that may inhibit intestinal fluid secretions (Lepot and Banwell, 1976).
ii. Pancreas/Gallbladder/Biliary Tract
In the hamster, the major pancreatic ducts join the common bile duct shortly before it enters the duodenum. This anatomical configuration is similar to that of mice and rats, but is distinct from other mammals, including humans. The pancreas of the Syrian hamster is similar in function to that of the mouse and rat.
The Syrian hamster can serve as a model for pancreatic carcinogenesis. Most commonly, pancreatic tumors are induced by the subcutaneous administration of nitrosamines, but the transplantable cell line (PGHAM-1), mentioned previously, can also reproduce metastatic pancreatic cancer (Uchida et al., 2008).
d. Pulmonary System
The conductive airways of the Syrian hamster contain a limited number of glandular structures, primarily in the proximal trachea, which facilitates modeling chronic bronchitis (Hayes et al., 1977). The pulmonary vascular bed is similar to that of humans and hamsters develop pulmonary lesions that resemble human centrilobular emphysema when exposed to intratracheal porcine pancreatic elastase (Borzone et al., 2007, Kleinerman, 1972). Spontaneous bronchiogenic and pulmonary cancers are rare; hence, the Syrian hamster is a good model to study chemical carcinogenesis of the respiratory tract (Homburger, 1968).
e. Genitourinary System
In the Syrian hamster, the reproductive and urogenital tracts develop from the same embryonic germinal ridge, rendering the kidneys highly responsive to estrogen. As a consequence, administration of estrogen to male hamsters leads to renal tumors and represents a critical model for studying the effects of exogenous estrogenic compounds on tumor development (Li et al., 1993). Hamsters are one of the most reliable models for studying the effect of chemical carcinogens on the urinary bladder (Van Hoosier and Ladgies, 1984).
f. Endocrine System
Hamsters are reported to be the first model in which the equivalent of Addisonian adrenal necrosis could be studied (Frenkel, 1956). The adrenal glands show a distinct difference in size by 4 weeks of age, depending on the sex of the animal. Male adrenal glands reportedly have a greater number of reticular cells within the adrenal cortex, accounting for a size double that of female adrenal glands (Militzer et al., 1990).
g. Immunological System
Hamsters have unique immune system characteristics. Hamsters do not reject skin allografts to the same extent as compared to rejection by other laboratory animals, and they have enhanced susceptibility to select infections (Streilein, 1978). Streilein et al. (1980) determined, based upon skin grafting experiments, that the original littermates identified in 1930 had very little alloantigenic variation. In addition, few mutational changes in this defined gene pool have occurred since the introduction of the hamster into biomedical use (Streilein et al., 1980). Many immunological studies have focused on the organization of major histocompatibility complex (MHC) class I genes in hamsters. While diversity exists at the MHC class II locus, the region is likely similar among the strains of Syrian hamsters that are available for research (Hixon et al., 1996).
Related to their short gestation period, the ontogeny of the thymic system and associated cellular immunity in Syrian hamsters is delayed compared to other rodents. In addition, only four of the five immunoglobulin (Ig) classes have been described in the hamster, i.e., IgM, IgG, IgA, and IgE, while IgD remains to be defined, and at least two strains of inbred hamsters are deficient in the sixth component of complement. Another IgG isotype, classified as IgG3, has been isolated from some strains of inbred Syrian hamsters. This immunoglobulin is differentiated from IgG1 and IgG2 by its affinity for protein A (Coe et al., 1995). Immunodeficiency has not been linked to deficiencies in IgG3.
Structural information for hamster immunoglobulins has been limited; however the first crystal structure of a hamster IgG Fab fragment and the complete cDNA sequence of the stimulatory antibody HL4E10 (which contains the first example of a hamster lambda light chain) has been identified. As the HL4E10 antibody is uniquely costimulatory for γδ T cells, humanized versions may be of clinical relevance in treating γδ T cell dysfunction-associated diseases, such as chronic non-healing wounds and cancer (Verdino et al., 2011).
h. Secretory and Sebaceous Glands
i. Harderian Glands
Harderian glands are pigmented lacrimal glands located posterior to the ocular globes. These secretory glands release a lipid- and porphyrin-rich material that lubricates the eyes and eyelids. Additionally, the harderian gland is a site of immune response, a source of thermoregulatory lipids and pheromones, a photoprotective organ and part of a retinal–pineal axis. Marked sexual dimorphism of these glands in Syrian hamsters was first reported in the 1950s and has not been shown to exist in the Chinese, Armenian, or Djungarian hamster. Female and male Syrian hamsters differ most significantly in the type of lipid droplets secreted by the Harderian glands and in the relative concentration of secreted porphyrin (females secrete up to 103-times more porphyrin than males) (Buzzell, 1996). This glandular dimorphism is androgen-dependent and exhibits seasonal variation. A complete histologic description of the gland has been published (Buzzell, 1996).
ii. Flank Glands
Coarse hair over darkly pigmented skin can be readily observed in the costovertebral area in males (Fig. 5.2 ). The flank glands of the Syrian hamster are dermal structures composed of sebaceous glands that produce secretions in response to androgens. When the male is sexually excited, hair over these glands becomes wet, and the male may appear pruritic. These glandular secretions are likely used for territorial marking. The female also has dorsal sebaceous glands, but they are not as easily identified and the secretions are associated with the estrous cycle (Hamilton and Montagna, 1950).
Figure 5.2.
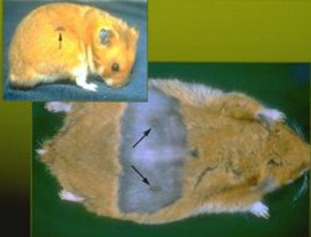
The flank glands in the male hamster (arrows) are used as sex glands and for olfactory marking. Females also have these glands, although they are less prominent.
i. Hibernation
Hibernation is a state of inactivity and metabolic depression in endotherms. This behavior, not exhibited in mice, rats, or guinea pigs, enables hamsters to be used for a variety of unique experimental objectives in behavioral and physiological research (Horwitz et al., 2013, Lyman, 1979, Storey, 2010). Hibernation ability varies among different hamster species and between individual animals; however, exposure to cold stimulates the hamster to gather food, and it will hibernate at a temperature of approximately 5°C (±2°). Unlike the European hamster, which is a true hibernator, the Syrian hamster is not used extensively for hibernation studies since it may not reliably enter hibernation when exposed to cold temperatures and bouts of hibernation may be short (Lyman, 1982). Because cold exposure and hibernation in the hamster are associated with desaturation of white adipose tissue, hamsters are useful for studies of factors controlling the saturation of fat.
2. Genetics
Syrian hamsters have a diploid chromosome number of 44. Numerous mutations have been introduced since the establishment of this animal model in the 1930s (Yoon and Peterson, 1979). Eighteen of the mutations involve coat and eye color; the earliest mutations produced brown, cream, piebald, and white hamsters. Six mutations involve the neuromuscular system, and six are identifiable by quantity or texture of hair. Breeders have also developed inbred strains of hamsters, some of which are of value to researchers because of genetically transmitted diseases or conditions, and unique susceptibility to teratogenic and carcinogenic agents (Homburger, 1972). In 2014, the first successful transgenic hamsters were created, promoting the future use of genetically engineered hamsters as disease models (Gao et al., 2014). Hamster embryonic stem cell lines have also been established (Doetschman et al., 1988).
3. Nutrition
Hamsters can be maintained on standard rodent diets, but relatively little research has been done on specific nutritional requirements of hamsters (Newberne and McConnell, 1979). Nonetheless, commercial rodent feed (intended for mice and rats) is generally used as the basic diet for hamsters, and hamsters placed on these formulations have normal growth and reproduction. Regardless of gender, Syrian hamsters consume approximately the same amount of food daily, between 5.5 and 8.9 g, during growth and development. Although once commonplace, additional supplementation of grains, fruits, and vegetables is unnecessary and, should be discouraged because of the associated risk of exposure to unwanted contaminants (Coates, 1991, Mulder, 2012, Slater, 1972).
Syrian hamsters may have nutritional requirements that differ from other rodents, potentially due to the presence of a nonglandular forestomach and initial digestion via fermentation. For hamsters, unlike other rodents, soybean meal offers better nutritional efficiency than fish meal. Carbohydrates in the diet can induce changes in both the glucose and lipid metabolism in hamsters (Kasim-Karakas et al., 1996). The mineral requirements for zinc, copper, and potassium are increased in the Syrian hamster, although the levels of other minerals are similar to those of the rat (Newberne and McConnell, 1979). Syrian hamsters require sources of many of the B vitamins and also need a source of non-nutritive bulk (Warner and Ehle, 1976). Vitamin E has been reported as essential for preventing myocytolysis in cardiomyopathic hamsters; deficiencies in this vitamin, combined with oxidative stress, may play a role in the pathogenesis of heart disease in hamsters (Sakanashi et al., 1991). In addition, vitamin E can reduce fatty streak accumulation in hypercholesterolemic hamsters (Xu et al., 1998).
For animals used in research, it is imperative that the diet be adequate to ensure that the biological responses obtained are, in fact, related to the experimental procedure (Newberne and Fox, 1980). Studies of hamster nutrition have shown that increased rates of survival for male and female hamsters are linked to long-term diets of 20 g lactalbumin/100 g of food (Birt et al., 1982). In addition, variations in dietary components can influence the outcome of spontaneous disease (Birt and Pour, 1985, Birt et al., 1985). Studies have shown that hamsters changed from a diet of rodent chow to semipurified feed are susceptible to colocolic intussusception within 7–10 days of the change to the nutritionally refined diet (Cunnane and Bloom, 1990).
Although it is generally recommended that laboratory animals be fed in a manner that minimizes food contamination with excreta, Syrian hamsters are an exception. If food hoppers are used for hamsters, the feed pellets must be able to fall through the slots to the floor of the cage (Harkness et al., 1977). In a hamster study that began with observations of failing health, decreased conception, and increased cannibalism, the problems were traced to a change in feeders. The feeders that contributed to these problems had 5/16-inch-wide slots that prevented the food from dropping to the cage floor. Because hamsters have a broad muzzle, the animals were forced to bite the food simultaneously from both sides of the individual metal strips of the feeder. The situation resulted in broken teeth and severe weight loss due to starvation.
Placement of the food directly on the floor of the cage, in addition to or in lieu of the use of a feeder, is preferred for adults and young hamsters (Fig. 5.3 ), who can begin to eat solid dry food at about 7–10 days of age. Like many other rodents, hamsters are naturally coprophagic. The placement of food on the floor of the enclosure is acceptable per federal regulations (Code of Federal Regulations (CFR), 2013). Fluid requirements are approximately 8.5 ml per 100 g body weight, but can vary significantly between genders (National Research Council, 1995), potentially linked to their natural adaptations for water conservation (Committee on Rodents, 1996). The use of a stainless steel sipper tube for drinking water or administration of other fluids is advised since hamsters can bite or chew through glass or plastic (Fig. 5.4 ). The location of the sipper tube must be sufficiently low for the smallest animal that is caged, as even nursing pups benefit from fluids, in addition to milk from the dam, to prevent gastrointestinal disturbances.
Figure 5.3.

Shoe-box caging for hamsters. Note the placement of food on the cage bottom.
Figure 5.4.

Syrian hamster drinking from elongated stainless steel sipper tube.
4. Pharmacology
Hamsters are apparently more sensitive to the metabolic effects of corticosteroids than some other laboratory animals, and are less responsive to histamine. Hamsters are very resistant to morphine; it generally has no sedative or hypnotic effects (Houchin, 1943, Tseng et al., 1979). Hamsters are also susceptible to Clostridum difficile overgrowth (discussed in greater detail under Bacterial Infections, Section I.C.1.a) following the administration of several commonly used antibiotics, including lincomycin, clindamycin, ampicillin, vancomycin, erythromycin, cephalosporins, gentamicin, and penicillin (Percy and Barthold, 2007).
5. Mating and Reproduction
The male hamster is sexually mature at approximately 90 g body weight. In the female, estrus begins within 6–8 weeks, yet it is recommended that breeding be withheld until the hamster reaches a weight of 90–100 g. Copulation activity may begin as early as 4 weeks of age, but it is unusual for pregnancy to occur before 8 weeks of age. For both genders, the ability to reproduce decreases at approximately 14 months of age. However, senescent females can often be successfully bred with younger males, even though there is a notable increase in defective ova and a decrease in number of offspring produced (Slater, 1972). The female has a 4-day estrous cycle that can be assessed by evaluation of the vaginal discharge. The end of ovulation (usually day 2 of the cycle) is marked by the appearance of a copious postovulatory discharge that fills the vagina and may extrude through the vaginal orifice. The discharge is creamy white, opaque, and very viscous, with a distinct odor. The female can be successfully mated in the evening of the third day after this postovulatory discharge.
Hamsters are usually test-mated by trial placement to determine if the female is receptive to the male. All animals should be caged individually for at least 1 week, allowing males to establish cage dominance and the females to cycle normally. On the third day following a post-ovulatory discharge, a female is introduced into a cage with a male approximately 1–2 h prior to the start of the dark cycle. It has been reported that the females are receptive to mating for approximately 16 h from early evening until mid-afternoon on the following day (Ciaccio and Lisk, 1971). If the female is ready for mating, she will quickly assume a position of lordosis with hindlegs spread and tail erect, and will maintain this position if the male exhibits interest. If mating does not occur within 5 min, or if the female is aggressive, she is removed and another female can be presented to the male. If copulation occurs, the pair can be left together until the following light cycle. With a normal dark cycle, ovulation and fertilization generally occur during the early morning hours, and this (the day of separation) is considered day 1 of gestation.
Gestation in the Syrian hamster is from 15 to 18 days in length. Disturbances should be minimized during pregnancy; after mating, the female can be moved to a separate nesting cage for at least 2 days prior to and 10 days after parturition to minimize maternal rejection or cannibalization of the litter. Despite early accounts of successful cross-fostering of pups with nursing mothers (Richards, 1966, Rowell, 1960), there are no recent peer-reviewed published accounts of successful cross-fostering of hamsters, although there are anecdotal comments online. Bottle feeding of newborn hamsters is very difficult and rarely (if ever) successful.
Another breeding mechanism is to trio-breed with one male and two females in the cage for 1–2 weeks, followed by the removal of the females to a separate cage for parturition. Since Syrian female hamsters tend to be aggressive, measures should be taken to reduce chances for injury as a result of fighting. It is recommended that breeding pairs/trios include a male hamster that is older than the female(s). For adequate veterinary care, breeding hamsters should be checked daily for fight wounds.
Female hamsters may undergo pseudopregnancy, usually as a result of an infertile mating. The female hamster can be examined for postovulatory discharge on days 5 and 9 after mating. If the discharge is present, she is exhibiting normal estrous cycles and is not pregnant. A hamster that is pregnant will have a distinct gain in weight, with abdominal distension, approximately 10 days after mating.
Studies have shown that the time of mating and the light–dark cycle under which the animals are housed have effects on the time of parturition (Viswanathan and Davis, 1992). Just prior to parturition, the female becomes restless and alternates between eating, grooming, and nest building. An increase in respiratory rate is also a sign that the litter can be expected to deliver within the next several hours. The most common time for parturition is on the 16th day of gestation, and parturition itself usually lasts for more than 3 h. A change toward maternal behavior occurs abruptly in late gestation for female Syrian hamsters; this differs from the gradual onset of maternal behavior observed throughout gestation in mice and rats (Buntin et al., 1984).
Litters range in size from four to 12 pups, with six to eight pups being the most common size. It is possible to sex the pups at birth by comparing the distance from the external urethral orifice to the anus (greater in males), but it is preferable to leave the litter undisturbed for the first 7–10 days after birth. During this time, fresh food pellets and water are provided for the mother, but no cage changes should be performed. Cannibalism may occur if the mothers are potentially stressed or threatened; alternatively, a mother may put pups into her cheek pouch due to transient stress, but then removes them when she becomes calm. If it is necessary to disturb the litter, the dam should be provided with food pellets on the cage floor with which she can stuff her cheek pouches. This may decrease the likelihood of cannibalism of newborn pups by the mother.
Hamster pups should remain with the dam until they are at least 19 days of age. Normal weaning time is 21–28 days, and the estrous cycle does not usually resume for the mother until 1–8 days following parturition (Battles, 1985). Young from different litters can usually be housed together until 40–50 days of age, when it becomes necessary to separate the females due to aggression. Males from the same litter may be kept together for a longer period of time.
6. Management and Husbandry
a. Caging and Environment
Hamsters can be maintained in colonies; however, mature animals are usually caged separately because of their tendency to fight. Females to be mated must be given some degree of isolation from adult males and other pregnant or lactating females.
A hamster weighing 60 g or less requires about 10 in2 of space. An animal over 60 g should have 13–19 in2 depending on body weight. A female with a litter should have approximately 121 in2. The height of the cage for hamsters must be 6 inches from the cage floor to the cage top (CFR, 2008; Institute of Laboratory Animal Research, 2011).
Caging used for other laboratory rodents is acceptable for hamsters provided it is escape-proof. Hamsters are capable of chewing through thick wood and aluminum. Doors and corners must be close-fitting, and latches must be secure. Plastic shoe-box cages with locking lids are recommended. It is essential to have a solid bottom for nesting females and for their young. Preference testing of hamsters found that solid-floored cages with bedding material were more readily inhabited than wire cages; however, age and/or prior experience may have affected the choice by the animals (Arnold and Estep, 1994).
Recommended bedding materials include processed hardwood chips, sawdust, shavings, corncobs, and certified paper products (Fig. 5.5). It has been shown that, without nesting material, hamsters have a preference for pine shavings over aspen shavings, and corn cob and aspen shavings are preferred over wood pellets. Interestingly these preferences were eliminated when nesting material (paper towel) was provided (Lanteigne and Reebs, 2006). Aromatic hydrocarbons in these materials may induce nonspecific hepatic enzymes in the hamster (Harkness, 1994). Normal urine output is slight, and hamsters tend to consistently use one corner of the cage for elimination. Replacement of bedding materials can be routinely done once or twice weekly, and can be left for up to 10–14 days, particularly when it is desirable to leave a litter undisturbed.
Figure 5.5.

Example of plastic tube that can be placed in the cage to provide enrichment.
Male and female group-housed hamsters typically fight, but stable groups have been reported when animals were housed together starting at a young age. Use of enrichment devices may reduce aggression between cage mates (Arnold and Westbrook, 1998). Environmental enrichment for hamsters should include some sort of burrow, pipe, tube, or shelter to mimic natural habitats of underground burrows (Fig. 5.5 ) (Arnold and Westbrook, 1998, Baumans, 2005). Additionally, nest material (or material that provides ability for nest building) is recommended, as hamsters of both genders make nests (Gattermann et al., 2001, Lanteigne and Reebs, 2006, Richards, 1969). Hamsters that had bedding material 40–80 cm in depth showed signifigantly less cage-bar chewing and increased burrow construction than hamsters housed in bedding that was only 10 cm deep (Hauzenberger et al., 2006). Hamsters also use running wheels; these devices can be added to the housing cage as a form of environmental enrichment (Beaulieu and Reebs, 2009).
Cages used for housing adult hamsters must be maintained in an environment of approximately 68–79°F with 30–70% humidity (Institute for Laboratory Animal Research, 2011). Hamsters are fairly adaptable to cooler temperatures, with one study showing that pre-hibernation hamsters prefer temperatures around 8°C (46°F), while post-hibernation hamsters show a preference for higher temperatures around 24°C (75°F) (Gumma et al., 1967).
A daily light period of 12–14 h is recommended. The longer 14-h period is required for breeding colonies. A light intensity of 323 lux (30 ft-candles) measured approximately 1 m above the floor has been recommended for rodents (Institute for Laboratory Animal Research, 2011).
b. Handling and Restraint
Hamsters are nocturnal animals, so they tend to be quite inactive during the light cycle in the animal facility. Males are more docile and easier to handle than females. Frequent handling can contribute to reduced aggressiveness, but a startled or awakened hamster is likely to roll on its back and threaten to bite.
To safely manipulate hamsters, place a small cup or container in the cage. The animal will usually enter the container, and the container with the hamster can be quickly moved to another cage. The easiest method of hand restraint is to grasp the hamster around the head and shoulders, approaching the animal carefully from the rear. Another method is to approach the animal in much the same way, but grasp only the skin. With the loose skin bunched securely in the hand, the skin is taut over the thorax and abdomen. As the animal is lifted, the hand holding the hamster is rotated so that the hamster’s body is supported (Fig. 5.6 ). An alternative to this method is to approach from the animal’s head, so that the thumb and forefinger are gripping the base of the tail; as before, the loose skin is secured between the fingers and the palmar surface before lifting. Still another method is to approach from the head and enclose the entire body with one hand. The thumb is placed at the base of the rear leg, with the first and second fingers on the opposite side at the base of the tail. The third and fourth fingers restrain the head and forelegs.
Figure 5.6.
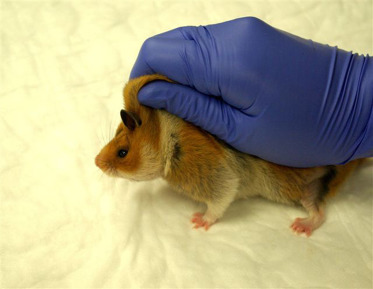
One-hand restraint of hamsters is demonstrated. The excessive loose skin is gathered tautly around the neck as the animal is lifted.
Credit: Jerald Silverman and Academic Press.
C. Diseases
1. Infectious Diseases
a. Bacterial Infections
i. Proliferative Enteritis (Transmissible Ileal Hyperplasia)
Proliferative enteritis is an infectious disease of hamsters that results in high morbidity and mortality. Proliferative enteritis is characterized by diarrhea in weanling hamsters with segmental proliferative lesions in the epithelium of the terminal ileum. This disease has also been refered to as regional enteritis, enzootic intestinal adenocarcinoma, transmissible ileal hyperplasia, and ‘wet tail.’ While the term ‘wet tail’ has been used extensively to describe this disease, this terminology can be confusing since it merely denotes a clinical description of diarrhea, and there are several other diseases that cause diarrhea in hamsters (Frisk, 2012).
Etiology
While the incidence of proliferative enteritis has decreased since it was first reported in the late 1950s (Cooper and Gebhart, 1998), this disease entity remains a concern in hamster colonies due to its extremely contagious nature and high rates of morbidity and mortality.
The causative organism isolated from hamsters with proliferative enteritis is Lawsonia intracellularis (Stills, 1991, Cooper et al., 1997a). L. intracellularis, related to Desulfovibrio desulfuricans, is a gram-negative, nonspore forming, slightly curved rod (1.5 × 0.35 μm) that is an obligate intracellular bacterium (Fox et al., 1994). In addition, this bacterium is challenging to manipulate or culture in cell lines (Cooper and Gebhart, 1998). It causes proliferative enteropathy in a number of other species including pigs, ferrets, horses, deer, and rabbits (Cooper et al., 1997a,b; Fox et al., 1994).
Clinical Signs
Watery diarrhea results in characteristic moist, matted fur on the tail, perineum, and ventral abdomen. Other clinical signs include dehydration, inactivity and a hunched appearance, inferred to be secondary to abdominal pain. Abdominal distention, hypothermia, and convulsions can occur just prior to death. Prolapse of the rectum or intussusception is often noted (Friedman, 1965, Frisk, 2012). Death occurs in 50–90% of cases associated with an outbreak, usually within 48 h after onset of clinical signs (Freidman, 1965). Chronic courses of proliferative enteritis have also been observed in hamsters with mild diarrhea and weight loss (Frisk et al., 1977, Jacoby et al., 1975, Lawson and Gebhart, 2000); however, it is important to recognize that the disease may be self-limiting without clinical signs. Jacoby et al. (1975) observed hamsters after experimental transmission of proliferative ileitis and divided clinical signs into acute, subacute, and chronic. Acute signs occurred in 10% of hamsters 7–10 days after inoculation, the primary sign being hemorrhagic diarrhea. Subacute signs of delayed growth and diarrhea appeared 21–30 days after transmission. The chronic disease did not produce clinical signs, with those animals showing normal growth rates.
Transmission and Epizootiology
Natural transmission most likely occurs by the fecal–oral route, following ingestion of contaminated fecal material. Increased severity and development of disease have been associated with factors such as overcrowding, transport, surgery, limited and purified diets (Decker and Henderson, 1959), transplantation of neoplasms (Lussier and Pavilanis, 1969), and experimental leishmaniasis (Frenkel, 1972). Cross-species transmission has been shown to occur experimentally between infected swine and hamsters (McOrist and Lawson, 1987). Vertical transmission has not been evaluated; however, it is not considered likely that L. intracellularis can cross the placenta to infect the fetus. In addition, it is unknown how long L. intracellularis can survive in the environment and if this is important in natural infections (Cooper and Gebhart, 1998).
Necropsy Findings
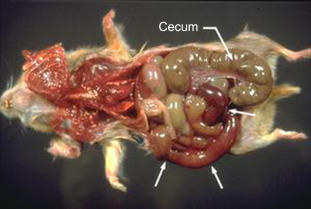
Gross lesions can include a segmental thickening and congestion of the ileum, enlargement of the mesenteric lymph nodes, peritonitis, and adhesions, although lesions are not always observed (Fig. 5.7 ). Histopathologic changes are characterized by hyperplasia of columnar mucosal epithelial cells in the terminal ileum, proliferation of glandular epithelium, and lymphadenitis with lymphoid hyperplasia, edema, and leukocytic infiltration of sinusoids (Frisk et al., 1977). Intestinal crypts may be lengthened, with increased mitosis, decreased numbers of goblet cells, and villar atrophy (Fig. 5.8 ). Finally, L. intracellularis can often be identified, using Warthin–Starry silver stain, in the apical cytoplasm of crypt enterocytes (Cooper and Gebhart, 1998).
Figure 5.7.
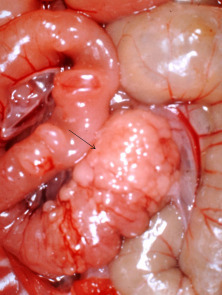
The abdominal viscera of a hamster with proliferative enteritis. The arrow denotes the thickening of the terminal jejunum and ileum.
Reprinted with permission from J.G. Fox and J.C. Murphy.
Figure 5.8.
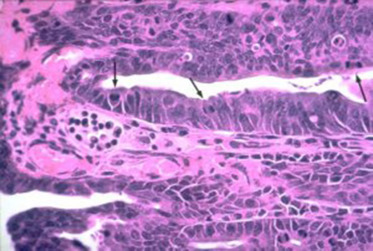
The crypt epithelium in an animal with proliferative enteritis. There are an increased number of mitotic figures (arrows) coupled with cellular immaturity in the epithelium.
Reprinted with permission from Harold F. Stills Jr.
Pathogenesis and Diagnosis
Weanling hamsters are very susceptible to this disease, but become less susceptible by 6 weeks of age, and resistant to infection by 10 weeks of age (Jacoby and Johnson, 1981). The lesions observed in the ileum develop in two phases following the experimental transmission of the disease (Jacoby, 1978). The initial phase is characterized by hyperplasia, which begins as a focal lengthening of villi. Approximately 3 weeks following transmission, an inflammatory phase begins, associated with focal or segmental necrosis of crypt epithelium. The evolution of the lesions is closely associated with a particulate bacterial antigen that can be detected by immunoperoxidase staining or in situ hybridization in the cytoplasm of mucosal epithelial cells. It is not clear what mechanism is utilized by L. intracellularis to localize to the gastrointestinal tract; however, cellular receptors or factors in the microenvironment may be important (Cooper and Gebhart, 1998). The proposed model for entry into the crypt epithelial cells involves attachment of the bacteria to the microvillus brush border, ingestion by endocytosis, and release from vacuoles into the cytoplasm of the cell. Released bacteria may then multiply within the epithelial cells prior to cell rupture. Additional bacteria may then attach to neighboring epithelial cells and spread the infection more rapidly (Jasni et al., 1994). Serum antibodies have been detected that are specific for the intracytoplasmic antigen, which may be of diagnostic value (Stills, 1991). Commercially available polymerase chain reaction (PCR) assays are readily available for detection of L. intracellularis in fecal samples (Cooper et al., 1997b, Jones et al., 1993).
Differential Diagnosis
Other infectious diseases that should be considered for hamsters with diarrhea are Tyzzer’s disease (Clostridium piliforme), Clostridium difficile enterotoxemia, and salmonellosis. Microbiologic and pathologic findings should distinguish between the various possibilities. When observed, the described proliferative changes involving the ileum are pathognomonic for the disease (Frisk, 2012).
Prevention, Control, and Treatment
Prior to obtaining hamsters for the biomedical facility, one should review the vendor/supplier history of the animal colonies with regard to enteritis. Animals should be purchased from a colony with minimal disease history, and they should not be mixed with animals from other sources. Hamsters with diarrhea should be separated and isolated from other animals. Treatment should be supportive and aggressive to correct nutritional and electrolyte imbalances. Antibiotic therapy indicated for L. intracellularis should be administered, although treatment has only been moderately successful. Tetracycline (10 mg/kg PO q12 h for 5–7 days), enrofloxacin (10 mg/kg PO or IM q12 h for 5–7 days), and trimethoprim-sulfa combinations (30 mg/kg PO q12 h for 5–7 days) have been recommended; these can be added to drinking water to control infections (Donnelly, 1997). Colony depopulation, facility sanitation, and repopulation with uninfected hamsters remain the best way to eliminate proliferative ileitis (Frisk, 2012).
Research Complications
Enteritis can be a major problem because of its prevalence, variable morbidity (20–60%), and high mortality (approximately 90%).
ii. Tyzzer’s Disease
This condition was first reported in Japanese Waltzing mice but has since been diagnosed in several other species including rats, rabbits, gerbils, cats, rhesus monkeys, dogs, horses, guinea pigs, and hamsters (Ganaway et al., 1971, Waggie et al., 1987). The disease is caused by Clostridium piliforme, a spore-forming intracellular bacterium. Transmission is believed to occur through the oral ingestion of C. piliforme spores from the feces of infected animals (Waggie et al., 1987). Although Tyzzer’s disease has only been sporadically reported in hamsters, transmission to hamsters is a possibility whenever hamsters are housed near susceptible species (Frisk, 2012). Clinical signs include roughened hair coats, diarrhea, and high mortality in animals that tend to be of weaning age or immunosuppressed (Donnelly, 1997). Reported necropsy lesions include enterocolitis, lymphadenitis, and multifocal necrotizing hepatitis (Fig. 5.9 ) (Nakayama et al., 1975). The diagnosis depends on the demonstration of the characteristic organism in the affected tissue, particularly in the epithelial and smooth muscle cells of the ileum, cecum, and colon, following special staining with Giemsa or silver techniques (Waggie et al., 1987). In experimental infections, inflammatory lesions may be present within 2 days of inoculation, while foci of liver necrosis occur within 4 days (Waggie et al., 1987). Infection with C. piliforme may not always manifest into clinical disease in the hamster. Outbreaks may have lesions localized in the intestines, the liver, or primarily in cardiac muscle, with or without intestinal involvement (Nakayama et al., 1976, Magaribuchi et al., 1977, Zook et al., 1977). The most important factors in the control and prevention of Tyzzer’s disease involve improved sanitation and isolation, since elimination of C. piliforme spores is critical for containing an outbreak. Treatment is not usually described in reported outbreaks. Oxytetracycline was added to the water of a pet store supplier of hamsters with an outbreak without success (Motzel and Gibson, 1990).
Figure 5.9.
Gross lesions in Tyzzer’s disease include hepatomegaly and multifocal hepatic necrosis (arrows) as seen on the left. Intestinal lesions, seen on the right, involve the ileum through the colon and include loss of tone and serosal edema. In some cases, hyperemia and hemorrhage may occur.
Reprinted with permission from Sherri L. Motzel.
iii. Clostridium Difficile
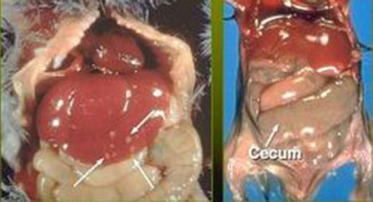
Enteritis associated with this bacterium has been linked to inappropriate antibiotic administration (i.e., antibiotic-associated enteritis), stress, experimental manipulation, and heavy environmental contamination with C. difficile (Ryden et al., 1991, Rehg and Lu, 1982, Blankenship-Paris et al., 1995b). Antibiotics associated with enterocolitis in hamsters include lincomycin, clindamycin, ampicillin, vancomycin, erythromycin, cephalosporins, gentamicin, and penicillin (Percy and Barthold, 2007); C. difficile overgrowth subsequently occurs and can cause enterocolitis due to alterations to intestinal microflora (Frisk, 2012). C. difficile can also cause disease in hamsters unrelated to antibiotic use. Hamsters may unexpectedly die with or without signs of diarrhea and have lesions of cecitis. Affected hamsters may vary in age from juveniles to adults (Hart et al., 2010). It is postulated that cecal dysbiosis results in cecal hyperplasia, overgrowth of the bacteria, and resultant necrotizing cecitis (Fig. 5.10 ) (Ryden et al., 1991). A reported outbreak with toxigenic, cytotoxin B-positive C. difficile resulted in profuse, watery, and hemorrhagic diarrhea that was highly associated with mortality (Chang and Rohwer, 1991). Histologic findings included typhlitis and colitis in these adult hamsters.
Figure 5.10.
Lesions of Clostridium difficile enterocolitis. Note the distended cecum and markedly hemorrhagic distal small intestine (arrows).
Reprinted with permission from Susan V. Gibson.
Additionally, hamsters used as models of atherosclerosis and placed on high-fat and -cholesterol diets may be prone to development of enteric disease associated with toxigenic C. difficile; necrohemorrhagic typhlitis and cecal mucosal hyperplasia were commonly noted in these hamsters (Blankenship-Paris et al., 1995b). Alterations in diet may be risk factors in disease development due to changes in intestinal microflora, pH, and ability to mount immune responses (Blankenship-Paris et al., 1995a). The development of antibodies against the virulence factors, toxins A and B, has proved useful in preventing disease relapse and subsequent reinfections in hamsters (Kink and Williams, 1998). Control of an outbreak of C. difficile-associated disease may be accomplished by depopulation, decontamination of animal holding rooms with chlorine dioxide, and repopulation (Hart et al., 2010).
Experimental infection with C. difficile serves as an important model for studying the human disease. This model has provided valuable information with regard to the role of toxins in the pathogenesis and potential treatments of the disease (Goulding et al., 2009).
iv. Salmonellosis
The rarity of salmonellosis in hamsters is likely attributable to well-managed facilities, improved quality of animals, regulated diets, and standards of animal care (Percy, 1987). This disease is rare in hamsters, although outbreaks had been reported historically (Innes et al., 1956). At necropsy, multifocal hepatic necrosis without enteritis has been described. Histologically, the disease is characterized by septic thrombi involving the veins and venules, an unusual feature of bacterial infection in hamsters. Preventive procedures, should salmonellosis be suspected, should include the isolation of hamsters from other rodents and quality control procedures to preclude the introduction of contaminated food or bedding. Antibiotic treatment typically is unrewarding (Frisk, 1987).
v. Helicobacter SPP.
Helicobacter spp. are motile gram-negative bacteria that are curved to spiral to fusiform in morphology. The hamster intestine is naturally colonized with several Helicobacter spp. that are not typically associated with clinical disease. Species identified in the hamster include H. cinaedi, H. mesocricetorum, H. cholecystus, H. aurati, and a novel Helicobacter species (in the H. bilis cluster) (Fox et al., 2009, Patterson et al., 2000a, Patterson et al., 2000b, Whary and Fox, 2004). H. cinaedi has been isolated from the intestinal tract of hamsters and has not been shown to cause pathological changes in this species (Gebhart et al., 1989). However, it has been shown to cause enteritis, proctocolitis, and rectal infection in humans (Gebhart et al., 1989, Whary and Fox, 2004). Hamsters should be considered a potential source of infection in humans, especially immonocompromised individuals (Fox, 2002). H. mesocricetorum has been isolated from the feces of hamsters and is considered a nonpathological commensal of the intestine (Simmons et al., 2000). Its causal association with pancreatic lesions has not been established. H. cholecystus has been isolated from hamsters with cholangiofibrosis and centrilobular pancreatitis in hamsters (Franklin et al., 1996). H. aurati has been associated with several experimentally induced lesions. Syrian hamsters infected with H. aurati had gastritis, chronic and progressive typhlocolitis, intestinal metaplasia, and dysplastic lesions in the large intestine (Patterson et al., 2000a, Patterson et al., 2000b). Hamsters showed either no clinical signs or chronic weight loss/poor body condition. Helicobacter spp. were also isolated from a hamster with gastric adenocarcinoma (Nambier et al., 2006; Patterson et al., 2000a, Patterson et al., 2000b). A novel Helicobacter species was identified, from the livers of aged hamsters, that appears closely related to H. bilis and may play a role in hepatobiliary disease: the livers from those hamsters had lesions of chronic hepatitis, hepatic dysplasia, and biliary hyperplasia (Fox et al., 2009).
vi. Pneumonia
Etiology and Prevalence
A survey originated in 1975 listed pneumonia as the second-most common hamster disease after diarrhea, and implicated Pasteurella pneumotropica, Streptococcus pneumoniae, and other Streptococcus spp. in the disease process (Renshaw et al., 1975); however, their importance in producing clinical disease of hamsters is unclear. Nonetheless, Pasteurella and Streptococcus are commonly listed in health reports for hamsters. Infection with Corynebacterium paulometabulum has been reported as a suspected cause of acute pneumonia in hamsters (Tansey et al., 1995); however, nasal infections with another strain, C. kutscheri, were subclinical in hamsters (Amao et al., 1991).
Clinical Signs
Overt manifestations of disease may include depression, anorexia, and nasal and ocular discharges, with ‘chattering’ and respiratory distress.
Pathogenesis
Various causes of stress, including significant variations from recommended environmental temperatures, may be contributing and predisposing factors to respiratory disease in the hamster.
Differential Diagnosis
A judicious assessment of clinical signs, lesions, and the results of microbiology laboratory reports is essential to definitively diagnose the etiologic agent (see above) of pneumonia in hamsters.
Prevention, Control, and Treatment
Stressful situations should be avoided, and affected animals should be isolated. If treatment is necessary, the use of antibiotics to which the etiologic organism is sensitive may be appropriate. A number of antibiotics are associated with fatal enterocolitis in this species; therefore, careful selection of antimicrobials is imperative.
b. Viral Infections
i. Prevalence
Current recommendations are that several viral infections should be monitored serologically in hamster breeding units. Most viral infections do not manifest any clinical disease in hamsters, with the exception of hamster polyomavirus (HAPyV) and Rodent protoparvovirus 1 (a species designation that also contains viral strains such as mouse parvovirus). Viruses for which one should routinely screen include, but are not limited to, lymphocytic choriomeningitis virus (LCMV), the Protoparvovirus genus, murine pneumonia virus (MPnV), Mammalian orthoreovirus (reovirus type 3; Reo 3), and Sendai virus (SV) (Mulder, 2012). Multiple groups have reported on the presence of antibodies to numerous viruses in Syrian hamsters.
ii. Lymphocytic Choriomeningitis Virus
The hamster is the most common animal species to transmit LCMV to humans (Cassano et al., 2012); however, the laboratory mouse, Mus musculus, is the primary reservoir for the virus.
Etiology
The infection is caused by an RNA virus of the arenavirus group.
Clinical Signs
Disease manifestation is ultimately dependent on a variety of factors including virus strain, dose of virus administered, route of infection, age of the host, strain of the host, and host immunocompetence (Barthold and Smith, 2007). Clinical signs may vary depending on whether the LCMV infection is natural or experimentally induced. In adult hamsters, natural infection generally causes an acute short-term infection that rarely causes illness. Infections in perinatally exposed animals remain subclinical, despite the fact that hamsters are shedding large amounts of virus during this period. Approximately half of hamsters infected congenitally or as newborns remain persistently infected and may develop chronic, progressive fatal disease characterized by inactivity, weight loss and wasting (Skinner and Knight, 1979). Impaired reproductive performance has been reported for chronically infected female hamsters (Parker et al., 1976).
Transmission
The implantation of tumors, unknowingly containing LCMV, has been the principal method of transmission to laboratory hamsters. Transmission in natural infections is primarily due to direct contact, although fomites and aerosols have been implicated in the spread of LCMV (Fox et al., 2002). High concentrations of virus have been found in the blood, organs, urine, and feces of Syrian hamsters. Viral shedding occurs primarily in the urine and saliva, but also in feces, milk, semen, and nasal secretions (Skinner and Knight, 1979). LCMV can be transmitted vertically or horizontally.
Necropsy Findings
Histopathology of tissues from animals that were perinatally infected and unable to clear the infection develop chronic disease characterized by lymphocytic infiltration of the liver, lung, pancreas, kidney, spleen, meninges, and brain (Genovesi and Peters, 1987, Oldstone and Dixon, 1969, Parker et al., 1976) as well as a chronic glomerulonephropathy and widespread vasculitis. The progressive glomerulonephritis can be attributed to antigen–antibody complex deposition in the arterioles and glomerular basement membranes of the infected kidney (Buchmeier and Oldstone, 1978, Oldstone and Dixon, 1969).
Pathogenesis
The experimental infection of young adult hamsters results in a viremia that decreases in titer over a period of 3 months. Virus excreted in the urine persists longer and is detectable in greater amounts than that found in blood. Complement-fixing antibodies appear by 10 days postinfection, reach peak levels by day 60, and decline slowly thereafter. Some hamsters infected neonatally remain healthy and follow a pattern of infection similar to that of young adults. However, other neonates develop disease with persistent viremia and lower levels of both complement-fixing and neutralizing antibodies. The presence of viral antigen and γ-globulin in the glomeruli of affected hamsters suggests an immune complex mechanism for the glomerulonephropathy, analogous to that reported for LCMV disease in mice (Buchmeier and Oldstone, 1978, Parker et al., 1976).
Differential Diagnosis
Other potential causes of wasting disease include graft versus host disease and any procedures resulting in suppression of normal immune responses. Possible renal lesions should be differentiated from glomerular amyloidosis.
Prevention, Control, and Treatment
A quality-assurance program that includes the regular testing of hamster colonies for antibodies and transplantable tumors for virus, with the elimination of infected animals or tumors, is the principal means of prevention. If dirty-bedding sentinels are used to screen hamster colonies for LCMV, care must be taken when interpreting negative results since LCMV is best transmitted via direct contact and viral transmission through dirty bedding is limited (Ike et al., 2007). Since feral mice may be reservoirs of infection, their direct or indirect contact with experimental animal colonies should be avoided.
Research Complications
LCMV is zoonotic and can be transmitted to humans through contact with rodents (see Chapter 28). The spectrum of disease manifested in humans varies from asymptomatic infection to rare cases of severe infection localized to the central nervous system. Studies that utilize LCMV-infected hamsters require Animal Biosafety Level 3 containment (CDCP-NIH, 2009). In a survey of biological contaminants, LCMV was isolated from 28% of hamster transplantable tumors (Nicklas et al., 1993). Humans can be infected either by direct contact or by inhalation of infectious aerosolized rodent excretions or secretions (Amman et al., 2007, Bowen et al., 1975, Skinner and Knight, 1979). In 2005, three human patients died and one became seriously ill after receiving organs from a common infected donor. It was revealed that the donor had recently acquired a pet hamster that was seropositive for LCMV. Furthermore, 3% of the pet hamsters from the rodent distributor implicated in the outbreak were seropositive for the virus (Jay et al., 2005).
iii. Sendai Virus
Etiology
Sendai virus is a single-stranded pleomorphic RNA virus and is the type species of the genus Respirovirus of the Paramyxoviridae family. Although mice are believed to be the natural host and most common laboratory animal affected, rats and hamsters are susceptible to natural infection (Percy and Palmer, 1997). Initial reports of the condition in hamsters were from Sendai, Japan (Matsumoto et al., 1954).
Clinical Signs
SV infection may lead to mortality in newborn pups; however, most infections are subclinical in hamsters.
Epizootiology and Transmission
An enzootic form of the infection was reported historically at a research facility in association with the periodic, but continuous, introduction of susceptible hamsters from a commercial vendor (Profeta et al., 1969). Transmission studies in mice have indicated that direct contact with infected rodents or contaminated fomites constitutes the primary route of infection. Aerosol inhalation has been successfully used to experimentally infect hamsters (Blandford and Charlton, 1977).
Necropsy Findings
Consolidation of the lungs has been described (Profeta et al., 1969). Experimental infections in hamsters have resulted in hyperplasia of the nasal mucosal epithelium, hyperplasia of bronchial epithelium, peribronchial edema, and peribronchial lymphocytic infiltration, which resolves within 2 weeks postinoculation (Percy and Palmer, 1997). These findings concurred with those seen in a SV vaccine study (Tagaya et al., 1995). Lesions and sites of viral replication within the respiratory tract are similar to those reported in strains of laboratory mice (Percy and Palmer, 1997).
Pathogenesis
Studies done in the mouse have shown that this agent causes a descending infection that is typically restricted to the mucociliary epithelium of conducting airways, but is capable of spreading to the alveolar epithelium (Brownstein, 2007). In male Syrian hamsters intranasally inoculated with Sendai, viral antigen was present postinoculation day 3 in the respiratory tract epithelium of the nasal passages and trachea. Antigen was present in the bronchioles by day 5, and antibodies were present by day 7, remaining at high levels throughout the 21-day study (Percy and Palmer, 1997).
Differential Diagnosis
Additional causes of pneumonia to exclude from the list of differentials include Corynebacterium spp. (Tansey et al., 1995), Streptococcus pneumoniae, Pasteurella pneumotropica, other Streptococcus spp., and MPnV (Renshaw et al., 1975).
Prevention, Control, and Treatment
Based on the likelihood that other laboratory animal species are the source of SV infections observed in hamsters, experimental hamsters should be housed in rooms separate from mice, rats, and guinea pigs. Hamsters from different sources should not be housed in the same room unless all sources are known to be free of the virus. In addition, analogous procedures described for mice should be applicable to hamsters (Parker and Richter, 1982). Dirty-bedding sentinels have been shown to be only variably efficacious in detecting SV outbreaks in mice (Compton et al., 2004), therefore, colony surveillance measures may need to be modified. Animal-derived biological products should be screened by PCR or hamster antibody production (HAP) test before use in colony hamsters (Cassano et al., 2012).
Research Complications
SV infection may be lethal to suckling hamsters (Profeta et al., 1969). In addition, reports of immunosuppressive effects of the virus in other species may be extrapolated to infection in hamsters (Garlinghouse and Van Hoosier, 1978). Due to effects on the nasal mucosal epithelium, and given the importance of olfactory cues to the hamster, SV infection may complicate studies of behavior and olfactory function in hamsters (Murphy and Schneider, 1970, Percy and Palmer, 1997).
iv. Murine Adenovirus (MADV)
Infections with Murine adenovirus have been reported in mice, rats, and other rodents, including hamsters, although no specific hamster adenovirus has been isolated. Hamsters can be experimentally infected with adenoviruses from a variety of other species. Infections are typically subclinical in hamsters unless the animal is stressed or immunocompromised (Richter, 1986). Mice can be naturally infected with two strains, MADV strain FL (now known as MAdV A) and K87 (now known as MAdV B). Hamsters can be serologically positive for antibodies to MAdV A, although reports of adenoviral infections are sporadic (Suzuki et al., 1982). Naturally occurring enteric adenovirus infection in hamsters, closely resembling infection with MAdVB in mice, is not associated with clinical disease and affects animals less than 24 days of age (Gibson et al., 1990). Adenoviral intranuclear inclusion bodies may be found in the intestinal epithelium in young hamsters.
v. Hamster Polyomavirus (HaPyV)
Hamster polyomavirus was first described in Germany in association with spontaneous skin epitheliomas from which viral particles were later identified (Graffi et al., 1967). The same virus also causes lymphoma, which is atypical of a polyomavirus (Delmas et al., 1985). HaPyV is a double-stranded, non enveloped DNA virus, and member of the Polyomaviridae family in the Polyomavirus genus (ICTV, 2013).
Epizootiology
The exact origin of HaPyV has not been elucidated. The virus has been isolated from the spleen and kidney of subclinically infected European hamsters, suggesting them as the natural host, with infection transferred to Syrian hamsters inadvertently after species co-mingling (Hannoun et al., 1974, Percy and Barthold, 2007). The virus is likely transmitted horizontally via ingestion of virions or through contaminated fomites (Ambrose and Coggin, 1975, Coggin et al., 1983). HaPyV is unusual in that it displays tropism for both undifferentiated keratinocytes and also lymphocytes. Due to this variable tissue tropism, it may cause two different disease syndromes. The syndrome observed in an individual hamster depends upon the immunological status of the animal and the age of the hamster when infected. One syndrome occurs in naive juvenile hamsters that manifests as an epizootic multicentric lymphoma involving the mesentery, intestines, liver, kidney, and thymus (Barthold et al., 1987, Graffi et al., 1969, Simmons et al., 2001). Lymphoma may also be induced by injection of virus or viral DNA (Barthold et al., 1987, Graffi et al., 1969, Graffi et al., 1970). With the second disease syndrome, hamsters develop trichoepitheliomas on the face, feet, neck, back, flanks, and abdomen. These tumors typically develop in hamsters aged 3 months to 1 year (Coggin et al., 1985, Graffi et al., 1970, Scherneck and Feunteun, 1990). Simultaneous formation of both epitheloimas and lymphomas in a single hamster rarely happens (Barthold et al., 1987). Virus likely persists in the renal tubular epithelium and is shed in urine. Additionally, virus-containing shed keratinocytes or enterocytes released in the feces can be a source of infection (Simmons et al., 2001).
Clinical Signs
Hamsters with lymphoma will likely have signs that are dependent on the organ system infiltrated with disease, but generally signs include weight loss, dyspnea, and dehydration. Palpable masses may be found upon examination. Hamsters with trichoepithelomas present with nodules within the cutis. The tumors may grow gradually and regress spontaneously (Barthold et al., 1987, Coggin et al., 1978).
Prevention and Control
Therapy is not recommended. Culling of the entire colony, and decontamination of the facility, has failed to prevent new outbreaks in some facilities. The virus is stable in the environment, but is susceptible to DNase, phenols, and KOH. It has shown resistance to UV light, formaldehyde vapor, RNase, proteinase K, and chlorinated and iodinated disinfectants (Ambrose and Coggin, 1975, Coggin et al., 1983, Manci et al., 1984).
vi. Hamster Parvovirus (Rodent Protoparvovirus 1 (RPV-1))
Hamster parvovirus (HaPV) was first described in 1982 in a colony of suckling hamsters that experienced high mortality (Gibson, 1983). Hamster parvovirus is a non enveloped single-stranded DNA virus in the family Parvoviridae. The virus shares homology with the mouse parvoviruses, including 94.6% homology with mouse parvovirus 1 (MPV-1) and over 98% homology with MPV-3 (Besselsen et al., 1996, Besselsen et al., 2006). Currently, the International Committee on Viral Taxonomy considers all the rodent parvoviruses (mouse parvovirus, H-1 parvovirus, Kilham rat virus, minute virus of mice) as one species, Rodent protoparvovirus 1, based on similarity of sequenced genomes, although multiple substrains can be identified.
Epizootiology and Pathogenesis
There is evidence that the hamster is not the natural host for any strain of RPV-1 because rodent parvoviruses are typically subclinical in their natural host and multiple rodent parvoviruses cause a similar course of disease when inoculated into young hamsters. The mouse is likely the natural host of this strain of RPV-1 due to the fact that MPV-3 is nearly identical to HaPV (Besselsen et al., 2006, Besselsen et al., 2008, Christie et al., 2010). Furthermore, experimental infection of mice with the hamster-derived strain of RPV-1 had similar pathogenesis to mice infected with the strain MPV-1 (Christie et al., 2010). However, infection of hamsters with MPV-3 has not been demonstrated. HaPV is likely transmitted via ingestion or inhalation of viral particles (Jacoby et al., 1996).
Clinical Disease
Infection in adult hamsters is typically subclinical. Young hamsters (2–4 weeks old) are most susceptible to HaPV. Affected hamsters are runted with incisior teeth abnormalites, domed craniums, small testicles, and a potbellied appearance (Gibson, 1983). Hemmorhagic disease has also been reported, causing diarrhea, ataxia, and mortality (Besselsen et al., 1999). Hamsters that survive typically seroconvert within 1–3 weeks, but animals remain persistently infected for several weeks. It has not been determined whether hamsters are shedding viral particles during that time. Humoral immunity prevents re-infection (Jacoby et al., 1996).
Diagnosis, Prevention, and Control
Diagnosis can be made with PCR using MPV-3/HaPV specific primers (Besselson et al., 1999, 2006; Christie et al., 2010). Monitoring cell lines, serum, and tumors prior to use in the colony is the primary way to prevent HaPV from being inadvertently introduced. Outbreak management should include quarantine, facility disinfection, and re-stocking with new hamsters (Jacoby et al., 1996, Cassano et al., 2012).
c. Parasitic Diseases
i. Protozoa
Fecal smears of Syrian hamsters may contain a large number and variety of organisms. Yet, their etiologic role in enteric disease remains a matter for speculation, as they have been found in comparable numbers in diverse species in both healthy and diseased animals. The presence of Spironucleus muris (previously Hexamita sp.) has been reported as an incidental finding (Wagner et al., 1974). Tritrichomonas muris has been successfully eradicated from the intestinal tract using a regimen of 80 mg of metronidazole administered intragastrically for 6 days (Taylor et al., 1993). This protocol is insufficient for the eradication of Giardia muris, which has a high prevalence in hamsters but is not associated with significant clinical signs or lesions. If removal of Giardia is deemed necessary, there have been successful reports of adding dimetridazole to the drinking water to eliminate Giardia (Moore, 1990, Sebesteny, 1969).
ii. Nematodes
The hamster has been reported to be susceptible to several species of pinworms, including Syphacia mesocriceti, Syphacia criceti, Syphacia stroma, Syphacia peromysci, Syphacia obvelata, Syphacia muris, Aspiculuris tetraptera (in Siberian dwarf hamsters, specifically), and Dentostomella translucida (Burr et al., 2012). Mice have been known to transmit Syphacia obvelata to Syrian hamsters (Watson, 1946), and Syrian hamsters can become infected with S. muris, the rat oxyurid, as a consequence of direct contact with infected rats (Ross et al., 1980). Eradication has been reported by two treatment courses with piperazine citrate (10 mg/ml of drinking water) for 7 days separated by 5 days without treatment (Unay and Davis, 1980). Treatment strategies for mice and rats are well documented, with avermectins and benzimidazoles being the most widely used anthelmintics for pinworm eradication (Pritchett, 2007, Pritchett and Johnston, 2002). Studies detailing treatment strategies in hamsters are limited, but treatment can most likely be adapted from the treatment of other rodents. The pinworm life cycle is direct, and transmission occurs through direct contact and fomites such as dirty cages and bedding material (Wightman et al., 1978).
iii. Cestodes
Etiology (Prevalence, Host Range)
Hamsters are susceptible to a number of cestode infections of the family Hymenolepididae, including Rodentolepis nana, Hymenolepis diminuta, and Rodentolepis microstoma. Historically, Rodentolepis nana, the dwarf tapeworm, was one of the most important internal parasites found in hamsters (Renshaw et al., 1975); infection has been found in animals from commercial colonies (Pinto et al., 2001). Rodentolepis nana ranges in size from 25 to 40 mm in length and is usually found in the small intestine. The host range includes mice, rats, nonhuman primates, and humans, but host-specificity is uncertain. It has been postulated that the human strain of R. nana may be non-infective to rodents (Macnish et al., 2002).
Clinical Signs
The consequences of infection are usually benign, but the effects depend on the number of parasites and degree of intestinal occlusion, as impactions have been reported with large worm burdens (Soave, 1963).
Epizootiology and Transmission
Rodentolepis nana is the only known tapeworm with either a direct or an indirect cycle; flour beetles or fleas serve as the intermediate host. The direct cycle predominates and is 14–16 days in length, while the indirect life cycle is variable. Autoinfection can also occur when eggs hatch within the host in which they were produced, rather than being excreted through the feces. Eggs then undergo development into mature worms within the intestinal tract of the original host. Evidence supports autoreinfection as a means for massive clinical infection in humans, as well as potentially causing clinical disease in rodents. Induced immunity through the direct life cycle can prevent autoreinfection (Heyneman, 1961).
Diagnosis
A diagnosis can be made by the demonstration of eggs in the feces or by isolation of the mature worm in the intestines at necropsy. R. nana can be distinguished from H. diminuta by the presence of hooks on the scolex in the former. Molecular techniques such as PCR-RFLP are also available for identification (Casanova et al., 2001, Macnish et al., 2003).
Prevention, Control, and Treatment
Preventive and control measures include isolation and quarantine of newly acquired animals, effective insect and wild-rodent control, and regular sanitation of cages and ancillary equipment. Praziquantel, thiabendazole, and niclosamide have been reported to be effective and safe (Ronald and Wagner, 1975). A study using H. diminuta as the cestode model showed several effective oral single-dose agents including praziquantel, bunamidine, niclosamide, cambendazole, and mebendazole (Ostlind et al., 2004).
Research Complications
Although any infection associated with morbidity and mortality may interfere with research, the primary significance of R. nana is its potential transmissibility to humans, despite growing evidence of host-adapted strains. Accordingly, personnel working with infected hamsters should be informed of potential transmission and receive instruction in appropriate hygienic procedures.
iv. Mites
Etiology and Prevalence
Ascariasis in hamsters is predominantly associated with two species of the genus Demodex (D. criceti and D. aurati); D. cricetuli infests Armenian hamsters. In addition, infections with ear mites (Notoedres sp.), the tropical rat mite (Ornithonyssus bacoti), and a nasal mite (Spleorodens clethrionomys) have also been reported. Despite high infection rates with Demodex spp., clinical signs of skin disease are uncommon.
Clinical Signs
Clinical signs are rare, even with high infestations (Nutting, 1961). Alopecia, predominantly of the rump and back, with dry, scaly skin has been noted in association with D. aurati and D. criceti (Estes et al., 1971). Notoedric mange in the female hamster usually affects only the ears; however, in males, lesions may also be observed on the nose, genitalia, tail, and feet.
Pathology
Reported cases of demodectic mange were characterized by dilated hair follicles that contained debris and mites, loss of hair shaft, an increase in thickness of the corneum, and little evidence of inflammation.
Pathogenesis
The direct life cycle takes approximately 24 days to complete. D. criceti and D. aurati complete their life cycles within different areas of the epidermis, permitting dual-species infection (Nutting, 1961). Males may be more susceptible to infection and disease than females. Clinical disease, when seen, has mostly been associated with immunosuppressive factors, such as underlying disease, advanced age, tumors, etc. (Estes et al., 1971, Tani et al., 2001). Demodectic mange has been observed in hamsters involved in a lymphosarcoma transmission study and a chemical carcinogenesis study, although lesions were apparently more related to increasing age than experimental procedures (Estes et al., 1971).
Diagnosis and Treatment
A diagnosis can be established by the demonstration of mites in skin scrapings, even though the presence of Demodex spp. in association with lesions does not necessarily establish a cause-and-effect relationship. Topical amitraz is the standard treatment for demodicosis. Regimes vary from weekly to every 14 days, with treatment length recommended for at least 4 weeks following a negative skin scrape (Hasegawa, 1995, Meredith, 2006). Other drugs reported to be effective are coumaphos and oral ivermectin (Hasegawa, 1995, Tani et al., 2001). While it may be an extreme approach, weekly cleansings with shampoos (selenium sulfide and benzoyl peroxide) can be used in combination with topical treatment (Hasegawa, 1995) for those critical research animals that are particularly affected.
2. Neoplastic Diseases
a. Introduction
The degree of background tumor incidence in Syrian hamsters has been a controversial topic in the literature. Although some reports state that hamsters have a low incidence of naturally occurring tumors, other groups have found the occurrence of spontaneous tumors to be quite high (Homburger, 1983, Pour et al., 1976a). For example, the overall incidence of spontaneous malignant neoplasms in Syrian hamsters has been estimated to be 3.7%, based on 435 tumors found in 11,972 animals (Van Hoosier and Trentin, 1979). Tanaka et al. (1991) reported tumor incidences in male hamsters of 10.6% and 11.5% in two cohorts, and Pour et al., 1976a, Pour et al., 1976b, Pour et al., 1976c noted an overall incidence of 32% in one colony versus 42% in another. Factors affecting the difference in numbers of tumors reported include the strain, age, and sex distribution of the animals observed; the environmental conditions and source of the colony (i.e., genetic differences between colonies); and the extent to which the animals are examined grossly and microscopically. There is general consensus that tumors occur more frequently in females than in males (Pour et al., 1976a, Van Hoosier and Trentin, 1979).
b. Benign Neoplasms
Reported benign neoplasms of the Syrian hamster include intestinal polyps, adrenal adenomas, splenic hemangiomas, islet cell pancreatic tumors, hepatic adenomas, squamous papillomas of the forestomach, and fibroadenomas of the mammary gland. One report found the incidence of benign tumors to be 10% in males and 13% in females when 200 control animals from the BIO 15.16 line were examined (Homburger, 1983).
c. Malignant Neoplasms
Lymphosarcoma is the most frequently reported malignant tumor of the Syrian hamster. Other malignancies include adrenocortical carcinoma, renal cell carcinoma, and subcutaneous sarcoma (Homburger, 1983).
Of special interest in this regard are the reported outbreaks of horizontally transmitted malignant lymphomas in a hamster colony, with an incidence of 50–90% in young inbred and random-bred animals (Ambrose and Coggin, 1975). The agent associated with the disease is the HaPyV (Coggin et al., 1983, Prokoph et al., 1996). These reports are historically important because the disease poses an epizootic threat to experimental colonies and because the disease condition in hamsters is a valuable model for understanding host–virus relationships in other species.
3. Miscellaneous Diseases
a. Amyloidosis and Associated Nephrotic Syndrome
Amyloidosis, a disease in which normally soluble proteins polymerize as insoluble fibrils, is a principal cause of death in hamsters on long-term experiments. The two components of amyloid are amyloid A (AA), which is derived from amyloid fibrils, and amyloid P (AP), also known as ‘female protein’ (FP), a member of the pantraxin family of plasma proteins (Tennent et al., 1993). Studies have shown that sex hormones regulate the expression of AP and that levels in females are normally 100- to 200-fold greater than levels in males (Coe et al., 1981). When compared to males, female Syrian hamsters have a distinct predisposition to acquire amyloidosis, with increased severity and earlier onset. Organs involved typically include the liver, kidney, stomach, adrenal, thyroid, and spleen (Coe and Ross, 1990). Testosterone has been linked to the inhibition of hepatic synthesis of AP, which is the homolog of primary importance in the deposition of amyloid (Coe and Ross, 1990). The incidence of nephrotic syndrome due to amyloidosis was reported to be 6% in a colony of Syrian hamsters, with ascites and anasarca observed (Murphy et al., 1984). Affected hamsters can present with extensive subcutaneous edema, ascites and hydrothorax (Fig. 5.11 ). The primary gross lesions are pale tan, enlarged, and misshapen kidneys (Fig. 5.12 ). Serum albumin is decreased and the total globulin component is increased in hamsters over 1 year of age. Proteinuria, hypercholesterolemia, triglyceridemia, and high levels of creatinine have been reported (Murphy et al., 1984). Clinical signs and laboratory test findings of low albumin, ascites, and proteinuria are classic for a diagnosis of nephrotic syndrome, as described in humans. Histologically, characteristic amyloid deposits are present initially in the glomeruli of the kidney and subsequently in a variety of tissues. Because amyloidosis in mice is strain-associated, genetic factors should be considered in the etiology and pathogenesis of the disease in hamsters. Amyloidosis in the Syrian hamster is used as a model to understand the pathogenesis of the same disease in humans, including the form linked to Alzheimer’s disease (Coe et al., 1997). Amylodosis can be induced experimentally using casein or lipopolysaccharide (LPS) subcutaneous injections (Hol et al., 1986, Niewold et al., 1987).
Figure 5.11.
(A) Male hamster exhibiting nephrotic syndrome: note the distended abdomen indicative of subcutaneous edema and ascites. (B) Skin reflected showing subcutaneous edema.
Reprinted with permission from J.G. Fox and J.C. Murphy.
Figure 5.12.
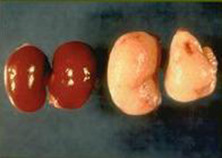
Classic appearance of amyloidosis in the kidneys on the right. Normal hamster kidneys are shown on the left for comparison.
Reprinted with permission from J. Derrell Clark.
b. Polycystic Disease
Polycystic disease is a common postmortem finding in Syrian hamsters, with cysts having been observed in up to 76% of hamsters over 1 year of age (Gleiser et al., 1970). The liver is a common site, but several organs may be affected, including the cecum, kidneys, ovaries, and spleen (Table 5.3 ). The liver lesions are related to developmental defects of normal ductal structures (i.e., bile ducts), whereas cysts in other organs likely develop from dilations of the lymphatic system. The condition may be associated with distension of the abdomen, but other clinical signs have not been recorded. Typical findings include cysts of variable size that can be unilocular to multilocular (Kaup et al., 1990). At necropsy, the cysts are thin-walled with clear watery fluid varying in color from amber to green. Findings in one study mentioned that the proteinaceous nature of the fluid resulted in white solidified collections within cysts in the cecal walls (Kaup et al., 1990). Reports have found that a higher incidence of intraperitoneal cysts occurs in European compared to Syrian hamsters (Kaup et al., 1990).
Table 5.3.
Frequency of Cysts at Various Sites
| Organ systems | Number (%) |
|
|---|---|---|
| Syrian hamstera | European hamsterb | |
| Gastrointestinal system | ||
| Esophagus | 1/40 (2.5%) | — |
| Liver | 17/40 (42.5%) | 54/150 (36.0%) |
| Cecum | — | 18/150 (12.0%) |
| Colon | — | 1/150 (0.7%) |
| Reproductive system | ||
| Seminal vesicle | 4/17 (23.5%) | — |
| Epididymis | 8/17 (47.0%) | — |
| Uterus | 1/23 (4.35%) | — |
| Ovary | 1/23 (4.35%) | 13/150 (8.7%) |
| Endocrine | ||
| Adrenal | 1/40 (2.5%) | — |
| Pancreas | 5/40(12.5%) | — |
| Other | ||
| Kidney | 2/40 (5.0%) | 7/150 (4.7%) |
| Spleen | — | 2/150(1.3%) |
c. Chronic Hepatitis
Chronic hepatitis and cirrhosis were first described as an incidental finding during various carcinogen studies (Chesterman and Pomerance, 1965). Disease has been linked to dietary contamination, infection with bacterial pathogens, and immune system abnormalities, yet a common etiology has not been identified (Brunnert and Altman, 1991, Fox et al., 2009). The primary means of diagnosis has been from necropsy, since there are usually no clinical signs of disease, even in cirrhotic animals (Hamilton and Reynolds, 1983). In most strains, females are more commonly affected than males (Homburger, 1972). Significant elevations of both alanine aminotransferase (ALT) and bile acids may be seen on serum clinical chemistries (Brunnert and Altman, 1991).
d. Atrial Thrombosis
Atrial thrombosis is a common finding in aged Syrian hamsters. The exact incidence varies greatly, from 16% reported in one colony to 73% incidence in another (McMartin, 1977; Pour, 1976a). Males and females are equally affected, but females develop the condition at an earlier age. Atrial thrombosis may be most commonly seen in the APA strain of hamster, accompanied by cardiac hypertrophy that develops with age (Doi et al., 1987). Clinical signs are suggestive of heart failure and include tachypnea, tachycardia, and cyanosis (Sichuk et al., 1965). Upon necropsy, thrombi are present, primarily in the left atrium. One study also noted that there was bilateral ventricular hypertrophy in the hearts with thrombosis and myxomatous thickening of the atrioventricular valves. However, the valvular lesions were also found in all aged hamsters, regardless of whether thrombi were present or not (McMartin and Dodds, 1982). Doi et al. (1987) propose that an increase in relative and absolute heart weights seen in older APA hamsters is suggestive of cardiac hypertrophy. It is postulated that thrombi occur as a result of local blood stasis, secondary to heart failure (McMartin and Dodds, 1982).
III. Chinese Hamster
A. Introduction
The Chinese hamster (Cricetulus griseus), also known as the striped-back hamster, was first used as a laboratory animal in 1919 (Fig. 5.13 ) (Yerganian, 1985). Benefits such as small size, polyestrous cycle, short gestation period, and low chromosome number encouraged the use of this specific hamster breed in biomedical research. Today, use of this animal in research is greatly overshadowed by the extensive use of cell lines derived from its ovarian cells. Chinese hamster ovary (CHO) cells are used almost exclusively for cell culturing experiments to obtain heterologous protein products (Oka and Rupp, 1990). Over the last two decades, CHO cells have been used to synthesize a wide array of recombinant therapeutic proteins that have been utilized clinically for the treatment of many human diseases (Jayapal et al., 2007).
Figure 5.13.

Appearance of the adult Chinese hamster, Cricetulus griseus. Note the dark stripe of fur along the dorsal midline.
The Chinese hamster has been shown to be susceptible to a number of infectious disease agents, such as bacteria, mycobacteria, protozoa, diphtheria, rabies, influenza, and equine encephalitis (Yerganian, 1958). Spontaneous hereditary diabetes mellitus, with similarities to the human disease, has been described (Meier and Yerganian, 1959) as well as susceptibility to experimental induction of stomach and esophageal cancer by oral diethylnitrosamine (Baker et al., 1974).
Chinese hamsters can be purchased commercially and fare well under standardized laboratory housing conditions. Because their size is comparable to that of the laboratory mouse, a similar style of caging is adequate.
B. Biology
The Chinese hamster, like the Syrian hamster, has a cheek pouch that can be utilized as an immunologically privileged site (Yerganian, 1958). Another unique biological feature of this animal is its low chromosome number of (2n = 22), which is beneficial for cytogenetic studies. The 10 large pairs of autosomes and two sex chromosomes can be readily differentiated. The constant diploidy maintained in cell culture provides a stable cell system for assessment of agents with known or suspected mutagenic and carcinogenic properties.
Adult animals weigh between 39 and 46 g, and are approximately 9 cm long. Newborns weigh 1.5–2.5 g. The average normal life span under laboratory conditions is 2.5–3.0 years. Adult males have exceptionally large testicles; also, the spleen and brain in both sexes are relatively larger, with respect to overall body size, than those of the Syrian hamster (Festing, 1972). The normal hemogram is shown in Table 5.4 (Moore, 1966).
Table 5.4.
| Parameter | Mean ± SD |
|---|---|
| Erythrocytes (106/μl) | 7.1 ± 0.01 |
| Packed cell volume (%) | 42.1 ± 5.6 |
| Hemoglobin (g/dl) | 12.4 |
| Leukocytes (103/μl) | 5.5 |
| Neutrophils (%) | 19.3 ± 2.2 |
| Bands (%) | 0.2 ± 0.1 |
| Lymphocytes (%) | 76.1 ± 7.8 |
| Monocytes (%) | 2.1 ± 0.3 |
| Eosinophils (%) | 1.7 ± 0.7 |
| Basophils | 0.1 ± 0.04 |
| Sedimentation rate (mm/h) | 3.5 ± 1.7 |
| Bleeding time (s)c | 55 |
Table from Feeney (2012) reprinted with permission from Academic Press.
From Moore (1966).
From Yerganian et al. (1955).
There appear to be no unique dietary needs for Chinese hamsters. They do very well on standard rodent chow, but wheat germ may be used as a supplement for breeders. The average daily water intake was shown to be 11.4 ml per 100 g body weight for males and 12.9 ml per 100 g body for females (Thompson, 1971).
Early attempts to breed Chinese hamsters under laboratory conditions were unsuccessful until a reversed illumination schedule was employed to establish a production colony (Yerganian, 1958). Sexual maturity is indicated by vaginal opening, with a mucus-like, creamy material frequently secreted at the beginning of estrus. The estrous cycle consists of four phases, with distinct behavioral characteristics associated with vaginal orifice changes (Yerganian, 1958). Routine examinations of the vulva can assist with determining estrus and optimal breeding time. Progesterone levels are significantly different during the estrus cycle and pregnancy. During the 4-day cycle, maximal synthesis of progesterone occurs on day 3, which differs from the low levels found on day 3 of the Syrian hamster cycle (Sato et al., 1984). Normal reproductive data are shown in Table 5.5 (Moore, 1965).
Table 5.5.
Reproductive Data for the Chinese Hamster
| Weaned | 21–25 daysa |
| Sexually mature | 8–12 weeksa |
| Type of estrous cycle | Polyestrusb |
| Duration of estrous cycle | 4 daysa |
| Length of estrus | 6–8 hra |
| Ovulation time | Immediately before estrusb |
| Copulation | 2–4 h after start of dark periodc |
| Implantation | 5–6 daysb |
| Gestation | 20.5 daysa |
| Average litter size | 4.5–5.2b |
| Number of mammae | 8b |
| Postpartum estrus | 4 daysa |
Because females can become very aggressive immediately following mating and even kill the male, hand mating was originally used for breeding purposes. However, selective monogamous breeding with docile females having high fecundity was found to be very successful for establishing breeding colonies (Calland et al., 1986). It has also been shown that Chinese hamsters can be mated in groups (Cisar et al., 1972). Infertility in young female hamsters may be due to an excess growth of hair around the vulva, preventing penile penetration during copulation attempts.
Pregnancy is indicated by a closed vagina with dry, pale, and scaly perineal tissues at day 4 following mating. Progesterone levels in peripheral blood increase through day 12 of pregnancy, stabilize through day 18, peak on day 19, and then dramatically drop prior to parturition (Sato et al., 1984). Dystocia may occur as a result of fetal wedging in the proximal portion of the vagina during parturition attempts. The fetuses can be saved by surgical removal.
Newborn animals have front incisors. Body hair appears at 3–4 days of age, with complete coverage in 7 days. Eyes and ears open within 10–14 days, and testicles descend in males at about 30 days of age. As animals approach sexual maturity, aggressive females may fight until dominance is established.
C. Diseases
1. Infectious Diseases
The Chinese hamster appears to be experimentally susceptible to a number of infectious disease agents; yet, little has been reported concerning spontaneous infections in this species. Tyzzer’s disease is similar to that seen in Syrian hamsters. The presence of antibodies against murine viruses has also been reported in Chinese hamsters. Parasitic infections may include persistent intestinal colonization with Trichomonas spp; however, few reports of other infecting endo- and ectoparasites exist in the literature. Despite the identification of Demodex sinocricetuli (Desch and Hurley, 1997) in Cricetulus barabensis (a species considered to be synonymous with C. griseus), there appears to be a very low susceptibility to demodectic mange in Chinese hamsters (Benjamin and Brooks, 1977).
2. Metabolic/Genetic Diseases
a. Diabetes Mellitus
Spontaneous diabetes mellitus was first recognized in 1957 during the course of inbreeding (Meier and Yerganian, 1959). The disease is similar in a number of aspects to insulin-dependent diabetes of humans (Yerganian, 1965).
Etiology
The disease is associated with a degranulation of the β-cells of the pancreatic islets of Langerhans, resulting in a primary defect in the biosynthesis of insulin.
Clinical Signs
Animals can show signs as early as 18 days of age, but the disease may occur at any age. Polydipsia and polyuria develop, with up to 50–70 ml of urine passed in 24 h. Urine staining and scald may develop on the abdomen. At the onset of disease, there may be an initial weight gain, but animals can become lethargic and require close clinical management. Occasionally, hamsters develop blindness and nonspecific conjunctivitis and alopecia may be seen. Animals are very susceptible to mild stress of any kind, and sudden death may be triggered by such procedures as cage transfer and changes in environmental parameters. Diabetic females may be infertile, but hamsters that do become pregnant tend to have increased numbers of abortions and fetal deaths at delivery.
Epizootiology
The disease appears to be transmitted as a recessive factor. When glucosuria was used to characterize diabetes, it was shown that four recessive genes were involved (Butler and Gerritsen, 1970). If any two of the four genes were homozygous, glucosuria could result. Apparently the duration, severity, and constancy of glucosuria is controlled by modifier genes. It has also been shown that 100% of the offspring become diabetic if the parents are ketotic (Gerritsen et al., 1970).
Necropsy Findings
Macroscopic lesions are confined mainly to the kidneys, which are slightly enlarged, spongy, and friable in diabetic animals. The renal pelvis may be dilated. When hydronephrosis is seen, retained urine is clear but odoriferous, and the urinary bladder is usually distended with urine. In some animals, the liver may be moderately enlarged with a yellow to gray color.
Microscopically, the pancreatic islets of Langerhans are decreased in number (Meier and Yerganian, 1959). There is a decrease in the number of β-cells; remaining cells stain lightly basophilic with cytoplasmic granulation and vacuolization. There is periodic acid-Schiff (PAS)-positive material within the cytoplasm that accumulates around pyknotic nuclei. Ultrastructural findings in the pancreas have been characterized (Boquist, 1969). Renal convoluted tubules contain much protein precipitate, and glomeruli are hypocellular with marked sclerosis. Intercapillary homogeneous material can be observed that is PAS positive. Bowman’s capsule may be slightly to moderately thickened, and adhesions may be present between the glomerulus and capsule. PAS-positive material is also found in the basement membrane, which may appear wrinkled and slightly thickened. The liver shows an intact lobular arrangement with extensive vacuolization of cells with perinuclear haloes. Intracytoplasmic material is PAS-positive, but negative when stained for fat. PAS-positive material is occasionally found in pericardial adipose tissue.
Pathogenesis
The basic defect is a degranulation of β-cells, which results in a decreased amount of insulin production with a reciprocal increase in glucagon. In highly inbred glucosuric strains with diabetes, there is a greatly reduced level of pancreatic insulin and a significantly elevated level of glucagon in both pancreas and stomach. A decrease in lactate dehydrogenase (LDH) isozymes appears to be associated with severity of the diabetic condition (Chang et al., 1977). A contributing factor to the observed renal pathology may be subnormal levels of specific glycosidases in the kidneys, with a resulting change in turnover of tissue glycoproteins (Chang, 1981).
Differential Diagnosis
Because diabetes mellitus is a spontaneous disease, it should be ruled out whenever a colony illness occurs. If the animals are being used as a model to study diabetes, the experimental protocol will dictate the diagnostic monitoring procedures. Certainly Tyzzer’s disease must be considered both in the initial differential phases and also as a secondary complication, since diabetic animals are very susceptible to stress and subsequent immunosuppression.
Treatment and Control
It has been previously suggested that treatment with hypoglycemic drugs may be indicated in breeding females in an attempt to maintain inbred lines (Meier and Yerganian, 1961).
Research Complications
The disease could potentially occur in animals being used in research protocols unrelated to diabetes. Because cellular metabolism is affected, cytogenetic studies could produce unreliable data.
3. Traumatic Diseases
Female littermates can become very aggressive as they reach maturity. Severe bite wounds, especially about the tail and head area, can be inflicted, and death is not an uncommon occurrence. Litters should be separated before fighting becomes a problem. Following attempts at breeding, the female can become quite aggressive to the male, so some means of removing the male must be anticipated.
4. Neoplastic Diseases
In general, Chinese hamsters have a low incidence of spontaneous tumors, with mainly the liver and reproductive organs involved. The rarity of spontaneous and induced leukemias may reflect the absence of innate tumor viruses.
Uterine adenocarcinomas were detected in 30 of 120 females (Ward and Moore, 1969). The growths were firm and white with implantation frequently seen on the visceral and parietal peritoneum. Approximately 10% of affected hamsters had lung metastases. Another report showed 11 of 77 affected with similar characteristics except that no pulmonary metastasis was seen (Benjamin and Brooks, 1977). Vaginal bleeding was the sign most often seen initially. The incidence of ovarian tumors was significantly increased with radiation exposure, but was rarely reported in control animals (Kohn and Gultman, 1964).
Hepatomas were found in 66 of 253 animals (Ward and Moore, 1969). These were benign and most often occurred as multiple nodules. Nodular hyperplasia, a nonneoplastic lesion, was seen in 111 of 157 animals in another survey (Benjamin and Brooks, 1977).
Pancreatic adenocarcinomas were reported in three 3-year-old females that were partially inbred for the development of spontaneous diabetes mellitus (Poel and Yerganian, 1961); however, it is believed that these tumors are rare in nondiabetic animals.
5. Miscellaneous Diseases
a. Cerebral Hemorrhage
Hemorrhage occurred in 20% of both control and experimental animals in a 131I chronic toxicity study, (Ward and Moore, 1969). Deaths occurred at 1–2 years of age. Grossly, the hemorrhage was most evident between the cerebral hemispheres, with blood often in the lateral ventricles. Microscopically, the hemorrhage was shown to be caused by inflammation and necrosis of the anterior cerebral artery. A homogeneous PAS-positive material could be seen within the media of the diseased artery. The vessel wall was greatly thickened in chronic cases. The cause of the cerebral hemorrhage has not been determined but it is postulated to involve an inflammatory or degenerative change in the anterior cerebral artery. The true incidence of cerebral hemorrhage is not known since other reports have not seen cerebral hemorrhage in necropsies of control or naive Chinese hamsters (Benjamin and Brooks, 1977).
b. Periodontitis
This condition was found in a strain of Chinese hamster with hereditary diabetes mellitus (Cohen et al., 1961). The lesion is characterized by absorption of alveolar bone, inflammation, and pocket formation due to splitting of the epithelial attachment. The disease corresponds to that seen in humans with diabetes mellitus.
c. Nephrosclerosis
In a study of 157 animals, 46 had evidence of nephrosclerosis (Benjamin and Brooks, 1977). The pathology was different from the intercapillary glomerulosclerosis associated with diabetes mellitus. Grossly, pitting and a decrease in size were noted in severely affected kidneys. Microscopically, tubular degeneration, mild interstitial fibrosis, and focal atrophy of the cortex were seen early in the disease process. In more advanced conditions, hyaline sclerosis of glomeruli, more severe interstitial fibrosis, and tubular degeneration were noted.
d. Spondylosis
The incidence and extent of spondylosis were increased in hamsters with spontaneous diabetes mellitus compared to nondiabetic control animals (Silberberg and Gerritsen, 1976).
e. Pulmonary Granulomas
Pulmonary granulomas were observed in 54 of 157 animals (Benjamin and Brooks, 1977). Grossly, the lesions appeared as subpleural, yellowish gray foci, measuring 1–3 mm in diameter with variable involvement of the lung parenchyma. Microscopically, lesions consisted of alveolar collections of lipid-filled macrophages, mixed inflammatory cells, septal fibrosis, and occasional cholesterol clefts. Affected animals were housed in both suspended wire cages and plastic shoe-box cages with different types of bedding; the etiology remains unknown.
IV. Armenian Hamster
A. Introduction
The Armenian hamster (Cricetulus migratorius), also known as the gray hamster, was first introduced as a laboratory research animal in the 1960s because of its susceptibility to mutagenic and carcinogenic agents (Fig. 5.14 ). Cytological features are comparable to those of the Chinese hamster, so this species is also used for cytogenetic studies. Like the Syrian hamster, the Armenian hamster is highly susceptible to oncogenic viruses but has a high tolerance to both homologous and heterologous transplantable tumors. Armenian hamsters are used to study infection with prion diseases.
Figure 5.14.

Armenian hamster (Cricetulus migratorius).
B. Biology
Care and management procedures are similar to those of the Chinese hamster. Body size and weight (33–80 g) are also similar. The diploid chromosome number is 22, with the X and Y chromosome of equal size. Captured animals are aggressive, but if reared in the laboratory, they can be bred successfully. Sexual maturity occurs around 50 days of age. The gestation period is 18–19 days, with an average litter size of five to seven pups.
C. Diseases
Reports concerning spontaneous infectious diseases in the Armenian hamster are rare. The expression of spontaneous amyloidosis differs in gender-specific AP expression and susceptibility to AA amyloidosis from that seen in the Syrian hamster (de Beer et al., 1993). Hepatocellular carcinomas have been reported in animals exposed to estrogen (Coe et al., 1990).
Skin lesions have been attributed to mite infestations, which have been identified as Demodex cricetuli (Hurley and Desch, 1994). This mite is similar to D. aurati of the Syrian hamster and occupies hair follicles, particularly along the face and back.
V. European Hamster
A. Introduction
The European hamster (Cricetus cricetus) developed some importance as a laboratory model when several wild-caught animals from a West German industrial area were found to have bronchogenic squamous cell carcinoma. It has since been found to be susceptible to N-diethylnitrosamine (DEN), with the subsequent development of respiratory tumors (Mohr et al., 1973). The European hamster is believed to be a more suitable model than the Syrian hamster for highly concentrated and prolonged smoke-inhalation studies (Reznik et al., 1975). The Bern Convention of 1979 established the European hamster as a strictly protected species in Appendix II, and later became listed in Appendix IV of the Habitats Directive, therefore being provided strict legal protection in all European countries (Ziomek and Banaszek, 2007). There are no breeding colonies of these animals presently housed in the United States; instead, sources are from European institutional research breeding colonies. They are not widely used research animals, and most of their use in the biomedical field involves studies of hibernation.
B. Biology
European hamsters are nocturnal, and they hibernate during the winter months in the wild. They are the largest hamster species, being minimally three-times the size of a Syrian hamster, with males larger than the females. Their outward appearance consists of white faces and feet, bodies with reddish brown dorsums, cranioventral black patches, and caudolateral white patches. These hamsters tend to be very aggressive, are easily frightened and will attack and bite. Those in captivity through laboratory breeding have become much easier to handle. Each litter develops a defined social order, with the heaviest male being dominant (Reznik-Schuller et al., 1974). The average life span of a research-bred European hamster is 34 months for females and 31 months for males, which may be related to the higher reported incidence of neoplasia in males (Ernst et al., 1989). Their life span is 4–10 years in the wild (Reznik-Schuller et al., 1974). Like the Chinese hamster, the European hamster has a chromosome diploid number of 2n = 22. A normal hemogram is presented in Table 5.6 .
Table 5.6.
Normal Hemogram of the European Hamster
| Hemogram |
Silverman and Chavannes (1977) |
Emminger et al. (1975) |
||
|---|---|---|---|---|
| Value | Percentage | Value | Percentage | |
| Leukocytes (103/ml) | 7.4 ± 2.6 | 8.3 ± 2.2 | ||
| Neutrophils | 1.71 ± 0.06 | 23.2 ± 2.5 | 2.87 ± 3.74 | 34.6 ± 17 |
| Lymphocytes | 5.47 ± 0.06 | 74.0 ± 2.3 | 5.02 ± 0.39 | 60.0 ± 17.6 |
| Monocytes | 0.192 ± 0.015 | 2.6 ± 0.6 | 0.083 ± 0.022 | 1.00 ± 1.00 |
| Eosinophils | 0.005 ± 0.002 | 0.07 ± 0.10 | 0.093 ± 0.018 | 1.13 ± 0.83 |
| Basophils | 0.001 ± 0.002 | 0.02 ± 0.07 | 0 | 0 |
| Thrombocytes (103/ml) | 210 ± 32 | |||
| RBC (106/ml) | 7.64 ± 0.42 | 7.45 ± 0.49 | ||
| PCV (%) | 49.2 ± 1.6 | |||
| Hemoglobin (g/dl) | 18.0 ± 0.7 | |||
Water consumption is 5 ml/100 g body weight, and average food consumption is 2.9 g/100 g body weight in summer (August) and 1.8 g/100 g body weight in winter (November) (Silverman and Chavannes, 1977). These animals are mainly seed eaters, but will readily consume a standard laboratory rodent diet.
Reproductive data are shown in Table 5.7 . Females have a regular four-stage estrus cycle of 4–6 days (Reznik et al., 1979). Proestrus lasts a few hours, estrus lasts 1–2 days, there is a metestrus of 6h, and diestrus lasting 2–4 days (Reznik-Schȕller et al., 1974). Estrus is determined by vaginal smears and by test-mating, using a steel mesh divider to keep the pair separated. The female is only receptive to the male for a short period during estrus, and markedly aggressive during the other three stages of the cycle. When no aggressiveness is observed, hamsters may be mated (Mohr et al., 1973). Females tend to bear one to two litters per year, each with six to nine pups (Reznik-Schuller et al., 1974). Newly weaned animals (25 days postpartum) have an average body weight of 75 g, with 6-month-old females and males approaching 300 and 400 g, respectively (Mohr et al., 1973). Sexual activity is not observed in winter months, during which time females and males are very aggressive toward each other. In the nonbreeding season, the female’s vagina is closed and the male’s scrotum is decreased in size with the testes situated in the abdominal cavity.
Table 5.7.
Reproductive Data for the European Hamstera
| Sexual maturity | Females, 80–90 days |
| Males, 60 days | |
| Estrus cycle | 4–6 days |
| Gestation | 18–21 days (captured) |
| 15–17 days (laboratory-born) | |
| Litter size | 7–9 |
| Weaning | 25–28 days |
Data from Mohr et al. (1973).
Anatomy of the European hamster has been studied extensively. The exocrine pancreas was described in an attempt to determine the suitability of this animal as a model for pancreatic cancer (Spikermann and Althoff, 1980). The nasal cavity has been fully described, as have comparative analyses of organ weights (Reznik and Jensen, 1979, Reznik et al., 1973).
Photoperiodic regulation of annual cycles has been described in European hamsters (Pevet, 1988). The critical photoperiod, at which time gonadal regression is induced, is between 15 and 15.5 h. Studies have also implicated a circannual rhythm in physiological variations in these animals, including changes in body weight and food intake even under conditions of constant photoperiods (Masson-Pevet et al., 1994, Wollnik and Schmidt, 1995). There is a slight reduction in activity in the winter months. As mentioned previously, these hamsters are true hibernators. Hibernation affects thrombocyte and leukocyte values, but no significant difference in these values has been noted in nonhibernating animals during winter or summer (Reznik et al., 1979).
C. Diseases
Spontaneous neoplasia (bengin or malignant) is common in older European hamsters, with up to a 70% incidence by 2 years of age. Neoplasia is also slightly more prevalent in males than in females. The most frequent tumors in descending order are leukemias and lymphomas, adrenal pheochromocytomas, and granulosa cell tumors in females (Ernst et al., 1989). Thymomas discovered in a small number of examined European hamsters resembled benign human thymomas (Ghadially and Illman, 1965). Thymic tumors were associated with large numbers of mast cells, which are not normally seen in the human form of disease (Ghadially and Illman, 1965). Similar to the Syrian and Chinese hamsters, the European hamster has a very low incidence of spontaneous pulmonary neoplasia (Ernst et al., 1989). In a small group of males (n=8), animals were found to be generally free of endoparasites, ectoparasites, and blood parasites (Silverman and Chavannes, 1977). One outbreak of Spironucleus muris associated with chronic enteritis was reported in seven newly captured European hamsters (Maatthiesen et al., 1976).
European hamsters are prone to developing cysts within the peritoneal cavity, particularly in the liver. Cysts tend to occur more often in females than in males and additional locations for cysts include the cecum, ovaries, spleen, kidney, and colon (Kaup et al., 1990).
Secondary bacterial infections with Corynebacteria, Staphylococcus, Pasteurella pneumotropica, and Pasteurella multicoda have been associated with pathological processes resembling fistulated abscesses in the head and jaws of the European hamster. The development of such disorders as malocclusion, osteomyelitis, and dysplasia appears to increase with age and suggest that the European hamster could be of use in dental research (Kunstyr et al., 1987). These disease processes in the mouth are often complicated by secondary bacterial infections, which may be fatal (Ernst et al., 1989).
VI. Djungarian Hamster
A. Introduction
The Djungarian hamsters are Phodopus campbelli (Russian dwarf) and P. sungorus (Siberian dwarf) (Fig. 5.15 ). Initially these animals were assumed to be subspecies; however, they are now believed to be separate species. The two may be confused because the common name ‘Djungarian’ is often used to denote either species. The most obvious factor to phenotypically differentiate P. campbelli from P. sungorus is the lack of dramatic coat color change in response to a short photoperiod: P. sungorus molts to a pure white haircoat, while P. campbelli retains its gray haircoat. Karyotype analysis using G-banding and chromosomal painting with probes from the Syrian and Chinese hamsters has confirmed close phylogenetic relationship between P. sungorus and P. campbelli (Romanenko et al., 2007). Unique C-banding patterns in P. sungorus chromosomes distinguish the karyotype from that of P. campbelli (Ross, 1998). The Russian dwarf hamster has been traced to Siberia, China, and Mongolia and is a distant relative of the Syrian hamster (Cooper et al., 1991). The Siberian dwarf hamster is native to the steppes of Kazakhstan, Manchuria, and northern China (Wynne-Edwards and Lisk, 1984). These hamsters range from 50 to 100 mm in body length, with an additional 10 mm of tail. Body weights range from 18 to 25 g, with mature males reaching 40–50 g. The dorsal fur is gray, and a dark stripe runs dorsally along the length of the body. The fur of the ventrum, limbs, and tail tends to be white. Djungarian hamsters generally live for 9–15 months, although survival up to 2 years has been reported (Lawrie and Megahy, 1991). The normal karyotype of the Djungarian species is 2n = 28 chromosomes. These hamsters have a high incidence of neoplasia and are susceptible to carcinogens, and can be infected with oncogenic viruses, particularly Rous sarcoma virus (RSV), Human mastadenovirus A (formerly known as human adenovirus 12), and SV40 (Pogosianz, 1975). The dwarf hamsters are extensively used in behavior and reproductive physiology studies.
Figure 5.15.

Dungarian hamsters. Males (left) are larger than females.
B. Biology
Dwarf hamsters have the most compressed reproductive cycle of any eutherian mammal. They can mate on the day of parturition (following an 18-day gestation) and deliver the second litter, while weaning the first, within a 36-day time period (Newkirk et al., 1997). Similar to the Syrian and Chinese hamsters, the two Djungarian hamster species have a 4-day estrus cycle with spontaneous ovulation (Erb et al., 1993). Pregnancy in Phodopus campbelli is dependent on continued secretion of progesterone by the corpus luteum through late gestation (Edwards et al., 1995). Prolactin levels in this species are absent during midgestation and resume in late gestation. Possible roles for this reappearance of activity include influences on lactogenesis, mammary gland development, and the regulation of maternal behavior toward newborns (Edwards et al., 1995). Successful reproduction in P. campbelli is dependent on monogamous parental care by both males and females (Wynne-Edwards and Lisk, 1984, Wynne-Edwards and Lisk, 1987). In contrast, male P. sungorus do not participate in the rearing of offspring. The nonaggressive behavior of females facilitates the maintenance of breeding pairs throughout life, with weaning of offspring occurring at 3 weeks of age. Females can bear between one and 18 litters, with each consisting of one to nine pups, in their reproductive years (Pogosianz, 1975). Reproductive development in females is accelerated when exposed to males of the same species (Reasner and Johnston, 1988). Increased food hoarding has been observed in P. sungorus as a behavioral adaptation to provide accessible energy during pregnancy (Bartness, 1997). Endocrinology in P. sungorus is similar to that of other rodent species, yet differs from that of P. campbelli. This implies that the two species of Djungarian hamsters have undergone evolutionary selection pressures with respect to reproductive endocrinology (Mcmillan and Wynne-Edwards, 1998). As adults, these hamsters are biologically dependent on the critical photoperiod, which is approximately 13 h (versus 12.5 h in the Syrian and 15.5 h in the European hamsters) (Pevet, 1988). Changes in photoperiod influence seasonal changes in breeding activities, thermoregulation, hair-coat growth, and fat metabolism (Pogosianz, 1975, Ebling, 1994). Djungarian hamsters have been widely used in studies of the pineal gland and melatonin secretion in mediating the effects of photoperiod. These hamsters are unusual in that they do not hibernate even when exposed to temperatures below − 40°C (Schlenker, 1985). Seasonal acclimation of blood-gas transport during periods of colder temperatures is facilitated by an increased relative heart weight, increased surface area of erythrocytes, and slightly altered hemoglobin content, all of which aid in oxygen transport (Puchalski and Heldmaier, 1986). These animals also decrease their resting metabolic rates and increase their capacity for nonshivering thermogenesis to adapt to inclement temperatures (Schlenker, 1985).
Djungarian hamsters are omnivorous (Sawrey et al., 1984). They can be maintained on the same diet as Syrian hamsters and are less costly to maintain because of their smaller size (Pogosianz, 1975).
C. Diseases
High incidence of neoplasia, particularly of the oral cavity, skin, and mammary glands has been reported in Russian dwarf hamsters, particularly in females (Lawrie and Megahy, 1991). In a study of 30 animals ranging from 6 to 12 months of age, 30% were found to have metastatic tumors that consisted of fibromas (n = 4), fibrosarcomas (n = 2), mammary adenocarcinomas (n = 2), and fibroma with liver cell carcinoma (n = 1) (Cooper et al., 1991). The incidence of the tumors has changed over time, with mammary tumors increasing and skin tumors decreasing in frequency; this may reflect genetic alterations linked to further inbreeding (Pogosianz and Sokova, 1982). Affected dwarf hamsters are reported to exhibit rapid weight loss, similar to that seen with disease conditions of bronchopneumonia and incisor malocclusion. Dermatologic conditions of trichophytosis have been reported (Pogosianz, 1975). Other skin problems include alopecia and ventral dermatitis caused by demodectic mites.
Female Russian dwarf hamsters prevented from breeding may develop cystic ovaries, with clinical presentations of swollen abdomens and bloody vaginal discharge (Lawrie and Megahy, 1991).
Hypersensitivity to bedding materials, particularly cedar chips, has been empirically reported. Affected dwarf hamsters may develop alopecia with dry skin and have secondary bacterial infections (McGuire, 1993).
Enteritis with rectal prolapse, although common in other varieties of hamsters, has not been noted in the Russian hamster (Lawrie and Megahy, 1991).
References
- Adler S. Origin of the golden hamster, Cricetus auratus, as a laboratory animal. Nature. 1948;162:256–257. doi: 10.1038/162256b0. [DOI] [PubMed] [Google Scholar]
- Aitken R.J. Sperm function tests and fertility. Int. J. Androl. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- Allison A.C., Chesterman F.C., Baron S. Induction of tumors in adult hamsters with simian virus 40. J. Natl. Cancer Inst. 1967;38:567–572. [PubMed] [Google Scholar]
- Amao H., Akimoto T., Takahashi K., Nakagawam M., Saito M. Isolation of Corynebactrium kutscheri from aged Syrian hamsters (Mesocricetus auratus) Lab. Anim. Sci. 1991;41:265–267. [PubMed] [Google Scholar]
- Ambrose K.R., Coggin J.H.J. An epizootic in hamsters of lymphomas of undetermined origin and mode of transmission. J. Natl. Cancer Inst. 1975;54:877–879. [PubMed] [Google Scholar]
- Amman B.R., Pavlin B.I., Albariño C.G., Comer J.A., Erickson B.R., Oliver J.B. Pet rodents and fatal lymphocytic choriomeningitis in transplant patients. Emerg. Infect. Dis. 2007;13:719. doi: 10.3201/eid1305.061269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C., Westbrook R.D. Enrichment in group-housed laboratory golden hamsters. Anim. Welf. Inf. Cent. Newsl. 1998;8:22–24. [Google Scholar]
- Arnold C.E., Estep D.Q. Laboratory caging preferences in golden hamsters (Mesocricetus auratus) Lab. Anim. 1994;28:232–238. doi: 10.1258/002367794780681598. [DOI] [PubMed] [Google Scholar]
- Bajusz E., Baker J.R., Nixon C.W., Homburger F. Spontaneous, hereditary myocardial degeneration and congestive heart failure in a strain of Syrian hamsters. Infect. Immun. 1969;56:105–129. doi: 10.1111/j.1749-6632.1969.tb16721.x. [DOI] [PubMed] [Google Scholar]
- Baker J.R., Mason M.M., Yerganian G., Weisburger E.K., Weisburger J.H. Induction of tumors of the stomach and esophagus in inbred Chinese hamsters by oral diethylnitrosamine. Proc. Soc. Exp. Biol. Med. 1974;146:291–293. doi: 10.3181/00379727-146-38090. [DOI] [PubMed] [Google Scholar]
- Barros C., Gonzalez J., Herrera E., Bustos-Obregon E. Fertilizing capacity of human spermatozoa evaluated by actual penetration of foreign eggs. Contraception. 1978;17:87–92. doi: 10.1016/0010-7824(78)90064-1. [DOI] [PubMed] [Google Scholar]
- Barthold S.W., Bhatt P.N., Johnson E.A. Further evidence for papovavirus as the probable etiology of transmissible lymphoma of Syrian hamsters. Lab. Anim. Sci. 1987;37:283. [PubMed] [Google Scholar]
- Barthold S.W., Smith A.L. Lymphocytic choriomeningitis virus. In: Fox J.G., Barthold S.W., Davisson M.T., Newcomer C.E., Quimby F.W., Smith A.L., editors. The Mouse in Biomedical Research. second ed. Elsevier; New York: 2007. pp. 179–213. [Google Scholar]
- Bartness T.J. Food hoarding is increased by pregnancy, lactation, and food deprivation in Siberian hamsters. Am. J. Physiol. 1997;272:R118–R125. doi: 10.1152/ajpregu.1997.272.1.R118. [DOI] [PubMed] [Google Scholar]
- Battles A.H. The biology, care, and diseases of the Syrian hamster. Compend. Contin. Educ. Pract. Vet. 1985;7:815–825. [Google Scholar]
- Baumans V. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J. 2005;46:162–170. doi: 10.1093/ilar.46.2.162. [DOI] [PubMed] [Google Scholar]
- Beaulieu A., Reebs S.G. Effects of bedding material and running wheel surface on paw wounds in male and female Syrian hamsters. Lab. Anim. 2009;43:85–90. doi: 10.1258/la.2008.007088. [DOI] [PubMed] [Google Scholar]
- Benjamin S.A., Brooks A.L. Spontaneous lesions in Chinese hamsters. Vet. Pathol. 1977;14:449–462. doi: 10.1177/030098587701400504. [DOI] [PubMed] [Google Scholar]
- Bergstresser P.R., Toews G.B., Gilliam J.N., Streilein J.W. Unusual numbers and distribution of Langerhans cells in skin with unique immunologic properties. J. Invest. Dermatol. 1980;74:312–314. doi: 10.1111/1523-1747.ep12543542. [DOI] [PubMed] [Google Scholar]
- Bernfeld P., Homburger F., Adams R.A., Soto E., Van Dongen C. Base-line data in a carcinogen-susceptible first generation hybrid strain of Syrian golden hamsters: FID Alexander. J. Natl. Cancer Inst. 1986;77:165–171. [PubMed] [Google Scholar]
- Bertuglia S., Reiter R.J. Melatonin reduces ventricular arrhythmias and preserves capillary perfusion during ischemia – reperfusion events in cardiomyopathic hamsters. J. Pineal Res. 2007;42:55–63. doi: 10.1111/j.1600-079X.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Besselsen D.G., Pintel D.J., Purdy G.A., Besch-Williford C.L., Franklin C.L., Hook R.R. Molecular characterization of newly recognized rodent parvoviruses. J. Gen. Virol. 1996;77:899–911. doi: 10.1099/0022-1317-77-5-899. [DOI] [PubMed] [Google Scholar]
- Besselsen D.G., Gibson S.V., Besch-Williford C.L., Purdy G.A., Knowles R.L., Wagner J.E. Natural and experimentally induced infection of Syrian hamsters with a newly recognized parvovirus. Comp. Med. 1999;49:308–312. [PubMed] [Google Scholar]
- Besselsen D.G., Romero M.J., Wagner A.M., Henderson K.S., Livingston R.S. Identification of novel murine parvovirus strains by epidemiological analysis of naturally infected mice. J. Gen. Virol. 2006;87:1543–1556. doi: 10.1099/vir.0.81547-0. [DOI] [PubMed] [Google Scholar]
- Besselsen D.G., Franklin C.L., Livingston R.S., Riley L.K. Lurking in the shadows: emerging rodent infectious diseases. ILAR J. 2008;49:277–290. doi: 10.1093/ilar.49.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D.F., Pour P.M. Interaction of dietary fat and protein in spontaneous diseases of Syrian golden hamsters. J. Natl. Cancer Inst. 1985;75:127–133. [PubMed] [Google Scholar]
- Birt D.F., Schuldt G.H., Salmasi S. Survival of hamsters fed graded levels of two protein sources. Lab. Anim. Sci. 1982;32:363–366. [PubMed] [Google Scholar]
- Birt D.F., Patil K., Pour P.M. Comparative studies on the effects of semipurified and commercial diet on longevity and spontaneous and induced lesions in the Syrian golden hamster. Nutr. Cancer. 1985;7:167–177. doi: 10.1080/01635588509513851. [DOI] [PubMed] [Google Scholar]
- Blandford G., Charlton D. Studies of pulmonary and renal immunopathology after nonlethal primary Sendai viral infection in normal and cyclophosphamide-treated hamsters. Am. Rev. Respir. Dis. 1977;115:305–314. doi: 10.1164/arrd.1977.115.2.305. [DOI] [PubMed] [Google Scholar]
- Blankenship-Paris T.L., Chang J., Dalldorf F.G., Gilligan P.H. In vivo and in vitro studies of Clostridium difficile-induced disease in hamsters fed an atherogenic, high fat diet. Lab. Anim. Sci. 1995;45:47–53. [PubMed] [Google Scholar]
- Blankenship-Paris T.L., Walton B.J., Hayes Y.O., Chang J. Clostridium difficile infection in hamsters fed an atherogenic diet. Vet. Pathol. 1995;32:269–273. doi: 10.1177/030098589503200308. [DOI] [PubMed] [Google Scholar]
- Boquist L. Pancreatic islet morphology in diabetic Chinese hamsters. Acta Pathol. Microbiol. Scand. 1969;75:399–414. [PubMed] [Google Scholar]
- Borzone G., Liberona L., Olmos P., Saez C., Meneses M., Reyes T. Rat and hamster species differences in susceptibility to elastase-induced pulmonary emphysema relate to differences in elastase inhibitory capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1342–R1349. doi: 10.1152/ajpregu.00343.2007. [DOI] [PubMed] [Google Scholar]
- Bowen G.S., Calisher C.H., Winkler W.G., Kraus A.L., Fowler E.H., Garman R.H. Laboratory studies of a lymphocytic choriomeningitis virus outbreak in man and laboratory animals. Am. J. Epidemiol. 1975;102:233–240. doi: 10.1093/oxfordjournals.aje.a112152. [DOI] [PubMed] [Google Scholar]
- Bowen W.H. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- Brownstein D.G. Sendai virus and pneumonia virus of mice (MPNV) In: Fox J.G., Barthold S.W., Davisson M.T., Newcomer C.E., Quimby F.W., Smith A.L., editors. second ed. vol. 2. Elsevier; New York: 2007. pp. 281–310. (The Mouse in Biomedical Research). [Google Scholar]
- Brunner H. Models of mycoplasma respiratory and genital tract infections. Wien Klin Wochenschr. 1997;109:569–573. [PubMed] [Google Scholar]
- Brunnert S.R., Altman N.H. Laboratory assessment of chronic hepatitis in Syrian hamsters. Lab. Anim. Sci. 1991;41:559–562. [PubMed] [Google Scholar]
- Buchmeier M.J., Oldstone M.B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J. Immunol. 1978;120:1297–1304. [PubMed] [Google Scholar]
- Buntin J.D., Jaffe S., Lisk R.D. Changes in responsiveness to newborn pups in pregnant, nulliparous golden hamsters. Physiol. Behav. 1984;32:437–439. doi: 10.1016/0031-9384(84)90259-2. [DOI] [PubMed] [Google Scholar]
- Burr H.N., Paluch L.R., Roble G.S., Lipman N.S. Parasitic diseases. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. pp. 839–866. [Google Scholar]
- Butler L., Gerritsen G.C. A comparison of the modes of inheritance of diabetes in the Chinese hamster and the KK mouse. Diabetologia. 1970;6:163–167. doi: 10.1007/BF01212224. [DOI] [PubMed] [Google Scholar]
- Buzzell G.R. Sexual dimorphism in the Harderian gland of the Syrian hamster is controlled and maintained by hormones, despite seasonal fluctuations in hormone levels: functional implications. Microsc. Res. Tech. 1996;34:133–138. doi: 10.1002/(SICI)1097-0029(19960601)34:2<133::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Calland C.J., Wightman S.R., Neal S.B. Establishment of a Chinese hamster breeding colony. Lab. Anim. Sci. 1986;36:183–185. [PubMed] [Google Scholar]
- Casanova J., Santalla F., Durand P., Vaucher C., Feliu C., Renaud F. Morphological and genetic differentiation of Rodentolepis straminea (Goeze, 1752) and Rodentolepis microstoma (Dujardin, 1845) (Hymenolepididae) Parasitol. Res. 2001;87:439–444. doi: 10.1007/s004360100379. [DOI] [PubMed] [Google Scholar]
- Cassano A., Rasmussen S., Wolf F.R. Viral diseases. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press; Waltham, MA: 2012. pp. 821–837. [Google Scholar]
- Centers for Disease Control and Prevention – National Institutes of Health (CDCP-NIH) Biosafety in Microbiological and Biomedical Laboratories. fifth ed. US Government Printing Office; Washington, DC: 2009. HHS Publ. http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf. [Google Scholar]
- Chang A.Y. Biochemical abnormalities in the Chinese hamster (Cricetulus griseus) with spontaneous diabetes. Int. J. Biochem. 1981;13:41–43. doi: 10.1016/0020-711x(81)90134-8. [DOI] [PubMed] [Google Scholar]
- Chang A.Y., Noble R.E., Wyse B.M. Comparison of highly inbred diabetic and nondiabetic lines in the Upjohn colony of Chinese hamsters. Diabetes. 1977;26:1963–1971. doi: 10.2337/diab.26.11.1063. [DOI] [PubMed] [Google Scholar]
- Chang J., Rohwer R.G. Clostridium difficile infection in adult hamsters. Lab. Anim. Sci. 1991;41:548–552. [PubMed] [Google Scholar]
- Chesterman F.C., Pomerance A. Cirrhosis and liver tumours in a closed colony of golden hamsters. Br. J. Cancer. 1965;19:802–811. doi: 10.1038/bjc.1965.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie R.D., Marcus E.C., Wagner A.M., Besselsen D.G. Experimental infection of mice with hamster parvovirus: evidence for interspecies transmission of mouse parvovirus 3. Comp. Med. 2010;60:123–129. [PMC free article] [PubMed] [Google Scholar]
- Ciaccio L.A., Lisk R.D. Hormonal control of cyclic estrus in the female hamster. Am. J. Physiol. 1971;221(3):936–942. doi: 10.1152/ajplegacy.1971.221.3.936. [DOI] [PubMed] [Google Scholar]
- Cicala C., Pompetti F., Carbone M. SV40 induces mesotheliomas in hamsters. Am. J. Pathol. 1993;142:1524–1533. [PMC free article] [PubMed] [Google Scholar]
- Cisar C.F., Gumperz E.P., Nicholson F.S., Moore W., Jr. A practical method for production of breeding of Chinese hamsters (Cricetulus griseus) Lab. Anim. Sci. 1972;22:725–727. [PubMed] [Google Scholar]
- Coates M.E. Nutrition and feeding. In: Poole T., editor. seventh ed. vol. 1. Blackwell Science; Oxford: 1991. pp. 45–60. (The UFAW Handbook on the Care and Management of Laboratory Animals). [Google Scholar]
- Code of Federal Regulations (CFR) Title 9; Animals and Service; Part 3: Standards; Subpart B: Specifications for the Humane Handling, Care, Treatment and Transportation of Guinea Pigs and Hamsters. Office of the Federal Register; Washington, DC: 2013. [Google Scholar]
- Coe J.E., Ross M.J. Amyloidosis and female protein in the Syrian hamster. Concurrent regulation by sex hormones. J. Exp. Med. 1990;171:1257–1267. doi: 10.1084/jem.171.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe J.E., Margossian S.S., Slayter H.S., Sogn J.A. Hamster female protein. A new pentraxin structurally and functionally similar to C-reactive protein and amyloid P component. J. Exp. Med. 1981;153:977–991. doi: 10.1084/jem.153.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe J.E., Ishak K.G., Ross M.J. Estrogen induction of hepatocellular carcinomas in Armenian hamsters. Hepatology. 1990;11:570–577. doi: 10.1002/hep.1840110408. [DOI] [PubMed] [Google Scholar]
- Coe J.E., Schell R.F., Ross M.J. Immune response in the hamster: definition of a novel IgG not expressed in all hamster strains. Immunology. 1995;86:141–148. [PMC free article] [PubMed] [Google Scholar]
- Coe J.E., Cieplak W., Hadlow W.J., Ross M.J. Female protein, amyloidosis, and hormonal carcinogenesis in Turkish hamster: differences from Syrian hamster. Am. J. Physiol. 1997;273:R934–R941. doi: 10.1152/ajpregu.1997.273.3.R934. [DOI] [PubMed] [Google Scholar]
- Coggin J.H., Jr., Thomas K.V., Huebner R. Horizontally transmitted lymphomas of Syrian hamsters. Fed. Proc. (United States) 1978;37:2086–2088. [PubMed] [Google Scholar]
- Coggin J.H.J., Bellomy B.B., Thomas K.V., Pollock W.J. B-cell and T-cell lymphomas and other associated diseases induced by an infectious DNA viroid-like agent in hamsters (Mesocricetus auratus) Am. J. Pathol. 1983;110:254–266. [PMC free article] [PubMed] [Google Scholar]
- Coggin J.H.J., Hyde B.M., Heath L.S., Leinbach S.S., Fowler E., Stadtmore L.S. Papovavirus in epitheliomas appearing on lymphoma-bearing hamsters: lack of association with horizontally transmitted lymphomas of Syrian hamsters. J. Natl. Cancer Inst. 1985;75:91–97. [PubMed] [Google Scholar]
- Cohen B.I., Matoba N., Mosbach E.H., McSherry C.K. Dietary induction of cholesterol gallstones in hamsters from three different sources. Lipids. 1989;24:151–156. doi: 10.1007/BF02535254. [DOI] [PubMed] [Google Scholar]
- Cohen M.M., Shklar G., Yerganian G. Periodontal pathology in a strain of Chinese hamster, Cricetulus griseus, with hereditary diabetes mellitus. Am. J. Med. 1961;31:864–867. doi: 10.1016/0002-9343(61)90027-4. [DOI] [PubMed] [Google Scholar]
- Committee on Rodents, Institute of Laboratory Animal Resources . Laboratory Animal Management: Rodents. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Compton S.R., Homberger F.R., Paturzo F.X., Clark J.M. Efficacy of three microbiological monitoring methods in a ventilated cage rack. Comp. Med. 2004;54:382–392. [PubMed] [Google Scholar]
- Cooper D.M., Gebhart C.J. Comparative aspects of proliferative enteritis. J. Am. Vet. Med. Assoc. 1998;212:1446–1451. [PubMed] [Google Scholar]
- Cooper D.M., Swanson D.L., Barns S.M., Gebhart C.J. Comparison of the 16S ribosomal DNA sequences from the intracellular agents of proliferative enteritis in a hamster, deer, and ostrich with the sequence of a porcine isolate of Lawsonia intracellularis. Int. J. Syst. Bacteriol. 1997;47:635–639. doi: 10.1099/00207713-47-3-635. [DOI] [PubMed] [Google Scholar]
- Cooper D.M., Swanson D.L., Gebhart C.J. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia intracellularis specific multiplex PCR assay. Vet. Microbiol. 1997;54:47–62. doi: 10.1016/s0378-1135(96)01264-3. [DOI] [PubMed] [Google Scholar]
- Cooper J.E., Knowler C., Pearson A.J. Tumours in Russian hamsters (Phodopus sungorus) Vet. Rec. 1991;128:335–336. doi: 10.1136/vr.128.14.335-a. [DOI] [PubMed] [Google Scholar]
- Cruz N., Arocho L., Rosario L., Crespo M.J. Chronic administration of carvedilol improves cardiac function in 6-month-old Syrian cardiomyopathic hamsters. Pharmacology. 2007;80:144–150. doi: 10.1159/000103254. [DOI] [PubMed] [Google Scholar]
- Cunnane S.C., Bloom S.R. Intussusception in the Syrian golden hamster. Br. J. Nutr. 1990;63:231–237. doi: 10.1079/bjn19900110. [DOI] [PubMed] [Google Scholar]
- Czub M., Braig H.R., Diringer H. Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titers and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J. Gen. Virol. 1986;67:2005–2009. doi: 10.1099/0022-1317-67-9-2005. [DOI] [PubMed] [Google Scholar]
- Davis M.J., Ferrer P.N., Gore R.W. Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am. J. Physiol. 1986;250:H291–H303. doi: 10.1152/ajpheart.1986.250.2.H291. [DOI] [PubMed] [Google Scholar]
- deArruda M.S.P., Montenegro M.R. The hamster cheek pouch: an immunologically privileged site suitable to the study of granulomatous infections. Rev. Inst. Med. Trop. Sao Paulo. 1995;37:303–309. doi: 10.1590/s0036-46651995000400004. [DOI] [PubMed] [Google Scholar]
- de Beer M.C., de Beer F.C., Beach C.M., Gonnerman W.A., Carreras I., Sipe J.D. Syrian and Armenian hamsters differ in serum amyloid A gene expression. Identification of novel Syrian hamster serum amyloid A subtypes. J. Immunol. 1993;150:5361–5370. [PubMed] [Google Scholar]
- Decker R.H., Henderson L.M. Hydroxyanthranilic acid as a source of niacin in the diets of the chick, guinea pig and hamster. J. Nutr. 1959;68:17–24. doi: 10.1093/jn/68.1.17. [DOI] [PubMed] [Google Scholar]
- Delmas V., Bastien C., Scherneck S., Feunteun J. A new member of the polyomavirus family: the hamster papovavirus. Complete nucleotide sequence and transformation properties. EMBO J. 1985;4:1279–1286. doi: 10.1002/j.1460-2075.1985.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desch C.E., Jr., Hurley R.J. Demodex sinocricetuli: new species of hair follicle mite (Acari: Demodicidae) from the Chinese form of the striped hamster, Cricetulus barabensis (Rodentia: Muridae) J. Med. Entomol. 1997;34:317–320. doi: 10.1093/jmedent/34.3.317. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Williams P., Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev. Biol. 1988;127:224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- Doi K., Yamamoto T., Isegawa N., Doi C., Mitsuoka T. Age-related non-neoplastic lesions in the heart and kidneys of Syrian hamsters of the APA strain. Lab. Anim. 1987;21:241–248. doi: 10.1258/002367787781268765. [DOI] [PubMed] [Google Scholar]
- Donnelly T.M. Disease problems of small rodents. In: Hillyer E.V., Quesenberry K.E., editors. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery. Saunders; Philadelphia, PA: 1997. pp. 307–327. [Google Scholar]
- Ebling F.J.P. Photoperiodic differences during development in the dwarf hamsters Phodopus sungorus and Phodopus campbelli. Gen. Comp. Endocrinol. 1994;95:475–482. doi: 10.1006/gcen.1994.1147. [DOI] [PubMed] [Google Scholar]
- Edwards H.E., Reburn C.J., Wynne-Edwards K.E. Daily patterns of pituitary prolactin secretion and their role in regulating maternal serum progesterone concentrations across pregnancy in the Djungarian hamster (Phodopus campbelli) Biol. Reprod. 1995;52:814–823. doi: 10.1095/biolreprod52.4.814. [DOI] [PubMed] [Google Scholar]
- Emminger A., Reznik G., Reznik-Schuller H., Mohr U. Differences in blood values depending on age in laboratory-bred European hamsters (Cricetus cricetus L.) Lab. Anim. 1975;9:33–42. doi: 10.1258/002367775780994899. [DOI] [PubMed] [Google Scholar]
- Erb G.E., Edwards H.E., Jenkins K.L., Mucklow L.C., Wynne-Edwards K.E. Induced components in the spontaneous ovulatory cycle of the Djungarian hamster (Phodopus campbelli) Physiol. Behav. 1993;54:955–959. doi: 10.1016/0031-9384(93)90308-3. [DOI] [PubMed] [Google Scholar]
- Ernst H., Kunstyr I., Rittinghausen S., Mohr U. Spontaneous tumours of the European hamster (Cricetus cricetus L.) Z. Versuchstierkd. 1989;32:87–96. [PubMed] [Google Scholar]
- Escobales N., Crespo M.J. Angiotensin II-dependent vascular alterations in young cardiomyopathic hamsters: role for oxidative stress. Vascul. Pharmacol. 2006;44:22–28. doi: 10.1016/j.vph.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Escobales N., Crespo M.J. Early pathophysiological alterations in experimental cardiomyopathy: the Syrian cardiomyopathic hamster. P.R. Health Sci. J. 2008;27:307–314. [PubMed] [Google Scholar]
- Estes P.C., Richter C.B., Franklin J.A. Demodectic mange in the golden hamster. Lab. Anim. Sci. 1971;21(6):825–828. [PubMed] [Google Scholar]
- Fararh K.M., Atoji Y., Shimizu Y., Takewaki T. Isulinotropic properties of nigella sativa oil in streptozotocin plus nicotinamide diabetic hamster. Res. Vet. Sci. 2002;73:279–282. doi: 10.1016/s0034-5288(02)00108-x. [DOI] [PubMed] [Google Scholar]
- Feeney W.P. The Chinese or Striped-Back Hamster. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press; Boston: 2012. pp. 907–922. [Google Scholar]
- Ferm V.H. The use of the golden hamster in experimental teratology. Lab. Anim. Care. 1967;17:452–462. [PubMed] [Google Scholar]
- Festing M. Hamsters. In: University Federation for Animal Welfare, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. Churchill-Livingstone; Edinburgh: 1972. pp. 242–256. [Google Scholar]
- Fitzpatrick L.R., Sakurai K., Le T. Effect of naproxen on the hamster gastric antrum: ulceration, adaptation and efficacy of anti-ulcer drugs. Aliment Pharmacol. Ther. 1999;13:1553–1562. doi: 10.1046/j.1365-2036.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- Fortner J.G. Spontaneous tumors, including gastrointestinal neoplasms and malignant melanomas, in the Syrian hamster. Cancer. 1957;10:1152–1156. doi: 10.1002/1097-0142(195711/12)10:6<1153::aid-cncr2820100610>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fox J.G. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50:273–283. doi: 10.1136/gut.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.G., Dewhirst F.E., Paster B.J., Shames B., Yan L.L., Murphy J.C. Intracellular Campylobacter-like organism from ferrets and hamsters with proliferative bowel disease is a Desulfovibrio sp. J. Clin. Microbiol. 1994;32:1229–1237. doi: 10.1128/jcm.32.5.1229-1237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.G., Newcomer C.E., Rozmiarek H. Selected zoonoses. In: Fox J.G., Cohen B.J., Loew F.M., editors. Laboratory Animal Medicine. second ed. Academic Press; New York: 2002. pp. 1060–1098. [Google Scholar]
- Fox J.G., Shen Z., Muthupalani S., Rogers A.R., Kirchain S.M., Dewhirst F.E. Chronic hepatitis, hepatic dysplasia, fibrosis, and biliary hyperplasia in hamsters naturally infected with a novel Helicobacter classified in the H. bilis cluster. J. Clin. Microbiol. 2009;47:3673–3681. doi: 10.1128/JCM.00879-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin C.L., Beckwith C.S., Livingston R.S., Riley L.K., Gibson S.V., Besch-Williford C.L. Isolation of a novel Helicobacter species, Helicobacter cholecystus sp. nov., from the gallbladders of Syrian hamsters with cholangiofibrosis and centrilobular pancreatitis. J. Clin. Microbiol. 1996;34:2952–2958. doi: 10.1128/jcm.34.12.2952-2958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J.K. Effects of hormones on the adrenal necrosis produced by Besnoitia jellisoni in golden hamsters. J. Exp. Med. 1956;103(3):375–398. doi: 10.1084/jem.103.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J.K. Infection and immunity in hamsters. Prog. Exp. Tumor Res. 1972;16:326–367. [PubMed] [Google Scholar]
- Friedman M.H. “Wet-tail disease” of hamsters. Lab. Anim. Dig. 1965;1:19. [Google Scholar]
- Frisk C.S. Bacterial and mycotic diseases. In: van Hoosier G.L., McPherson C.W., editors. Laboratory Hamsters. Academic Press; Orlando, FL: 1987. pp. 111–133. [Google Scholar]
- Frisk C.S. Bacterial and fungal diseases. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. pp. 797–820. [Google Scholar]
- Frisk C.S., Wagner J.E., Owens D.R. Hamster enteritis: a review. Lab. Anim. 1977;11:79–85. doi: 10.1258/002367777781005613. [DOI] [PubMed] [Google Scholar]
- Fukuhara M., Uchida E., Tajiri T., Aimoto T., Naito Z., Ishiwata T. Re-expression of reduced VEGF activity in liver metastases of experimental pancreatic cancer. J. Nippon Med. Sch. 2005;72:155–164. doi: 10.1272/jnms.72.155. [DOI] [PubMed] [Google Scholar]
- Ganaway J.R., Allen A.M., Moore T.D. Tyzzer’s disease. Am. J. Pathol. 1971;64:717–732. [PMC free article] [PubMed] [Google Scholar]
- Gao M., Zhang B., Liu J., Guo X., Li H., Wang T. Generation of transgenic golden Syrian hamsters. Cell Res. 2014;24:380–382. doi: 10.1038/cr.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlinghouse L.E., Jr., Van Hoosier G.L., Jr. Studies on adjuvant-induced arthritis, tumor transplantability, and serologic response to bovine serum albumin in Sendai virus infected rats. Am. J. Vet. Res. 1978;39:297–300. [PubMed] [Google Scholar]
- Gattermann R., Fritzsche P., Neumann K., Al‐Hussein I., Kayser A., Abiad M. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) J. Zool. 2001;254:359–365. [Google Scholar]
- Gattermann R., Johnston R.E., Yigit N., Fritzsche P., Larimer S., Özkurt S. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol. Lett. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart C.J., Fennell C.L., Murtaugh M.P., Stamm W.E. Campylobacter cinaedi is normal intestinal flora in hamsters. J. Clin. Microbiol. 1989;27:1692–1694. doi: 10.1128/jcm.27.7.1692-1694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesi E.V., Peters C.J. Susceptibility of inbred Syrian golden hamsters (Mesocricetus auratus) to lethal disease by lymphocytic choriomeningitis virus. Exp. Biol. Med. 1987;185:250–261. doi: 10.3181/00379727-185-42541. [DOI] [PubMed] [Google Scholar]
- Gerritsen G.C., Needham L.B., Schmidt F.L., Dulin W.E. Studies on the prediction and development of diabetes in offspring of diabetic Chinese hamsters. Diabetologia. 1970;6:158–162. doi: 10.1007/BF01212223. [DOI] [PubMed] [Google Scholar]
- Ghadially F.N., Illman O. Naturally occurring thymomas in the European hamster. J. Pathol. Bacteriol. 1965;90:465–469. doi: 10.1002/path.1700900214. [DOI] [PubMed] [Google Scholar]
- Gibson S.V. Mortality in weanling hamsters associated with tooth loss. Lab. Anim. Sci. 1983;33:497. [Google Scholar]
- Gibson S.V., Rottinghaus M.S., Wagner J.E., Stills H.F.J., Stogsdill P.S., Kinden D.A. Naturally acquired enteric adenovirus infection in Syrian hamsters (Mesocricetus auratus) Am. J. Vet. Res. 1990;51:143–147. [PubMed] [Google Scholar]
- Gimenez-Conti I.B., Slaga T.J. The hamster cheek pouch carcinogenesis model. J. Cell Biochem. Suppl. F. 1993;17:83–90. doi: 10.1002/jcb.240531012. [DOI] [PubMed] [Google Scholar]
- Gleiser C.A., Van Hoosier G.L., Jr., Sheldon W.G. A polycystic disease of hamsters in a closed colony. Lab. Anim. Care. 1970;20:923–929. [PubMed] [Google Scholar]
- Goineau S., Pape D., Guillo P., Ramee M.P., Bellissant E. Hemodynamic and histomorphometric characteristics of dilated cardiomyopathy of Syrian hamsters (bio TO-2 strain) Can. J. Physiol. Pharmacol. 2001;79:329–337. [PubMed] [Google Scholar]
- Goulding D., Thompson H., Emerson J., Fairweather N.F., Dougan G., Douce G.R. Distinctive profiles of infection and pathology in hamsters infected with Clostridium difficile strains 630 and B1. Infect. Immun. 2009;77:5478–5485. doi: 10.1128/IAI.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffi A., Schramm T., Graffi I., Bierwolf D., Bender E. Virus-associated skin tumors of the Syrian hamster. J. Natl. Cancer Inst. 1967;40:867–873. [PubMed] [Google Scholar]
- Graffi A., Bender E., Schramm T., Kuhn W., Schneiders F. Induction of transmissible lymphomas in Syrian hamsters by the application of DNA from viral hamster papovavirus-induced tumors and by cell-free filtrates from human tumors. Med. Sci. 1969;64:1172–1175. doi: 10.1073/pnas.64.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffi A., Bender E., Schramm T., Graffi I., Bierwolf D. Studies on the hamster papilloma and the hamster virus lymphoma. Bibl. Haematol. 1970;36:293. doi: 10.1159/000391720. [DOI] [PubMed] [Google Scholar]
- Gumma M.R., South F.E., Allen J.N. Temperature preference in golden hamsters. Anim. Behav. 1967;15:534–537. doi: 10.1016/0003-3472(67)90055-3. [DOI] [PubMed] [Google Scholar]
- Hamilton J.B., Montagna W. The sebaceous glands of the hamster. I. Morphological effects of androgens on integumentary structures. Am. J. Anat. 1950;86:191–233. doi: 10.1002/aja.1000860203. [DOI] [PubMed] [Google Scholar]
- Hamilton J.M., Reynolds T. Cholangiofibrosis in the Syrian golden hamster. Vet. Rec. 1983;112:359–360. doi: 10.1136/vr.112.15.359. [DOI] [PubMed] [Google Scholar]
- Hannoun C., Guillon J.C., Chatelain J. Natural latent infection of the european hamster (“cricetus cricetus,” linne) with a papovavirus. I. Isolation of virus in golden hamster and new-born mice (author’s transl)] Ann. Microbiol. (Paris) 1974;125:215–226. [PubMed] [Google Scholar]
- Harkness J.E. Small rodents. Vet. Clin. North Am. Small Anim. Pract. 1994;21:89–102. doi: 10.1016/s0195-5616(94)50004-4. [DOI] [PubMed] [Google Scholar]
- Harkness J.E., Wagner J.E., Kusewitt D.F., Frisk C.S. Weight loss and impaired reproduction in the hamster attributable to an unsuitable feeding apparatus. Lab. Anim. Sci. 1977;27:117–118. [PubMed] [Google Scholar]
- Harkness J.E., Turner P.V., VandeWoude S., Wagner J.E. Harkness and Wagner’s Biology and Medicine of Rabbits and Rodents. Williams and Wilkins, Media; Pennsylvania, PA: 2010. [Google Scholar]
- Hart M., O’Conner E., Davis M. Multiple peracute deaths in a colony of Syrian hamster. Lab. Anim. Sci. 2010;40:325–327. doi: 10.1038/laban0410-99. [DOI] [PubMed] [Google Scholar]
- Hasegawa T. A case report of the management of demodicosis in the golden hamster. J. Vet. Med. Sci. 1995;57:337–338. doi: 10.1292/jvms.57.337. [DOI] [PubMed] [Google Scholar]
- Hauzenberger A.R., Gebhardt-Henrich S.G., Steiger A. The influence of bedding depth on behaviour in golden hamsters (Mesocricetus auratus) Appl. Anim. Behav. Sci. 2006;100:280–294. [Google Scholar]
- Hayes J.A., Christensen T.G., Snider G.L. The hamster as a model of chronic bronchitis and emphysema in man. Lab. Anim. Sci. 1977;27:762–770. [PubMed] [Google Scholar]
- Hedqvist P., Raud J., Dahlen S.E. Microvascular actions of eicosanoids in the hamster cheek pouch. Adv. Prostaglandin Thromboxane Leukot. Res. 1990;20:153–160. [PubMed] [Google Scholar]
- Heyneman D. Studies on helminth immunity. III. Experimental verification of autoinfection from cysticercoids of Hymenolepis nana in the white mouse. J. Infect. Dis. 1961;109:10–18. doi: 10.1093/infdis/109.1.10. [DOI] [PubMed] [Google Scholar]
- Hixon M.L., Lewis A.M.J., Levine A.S., Chattopadhyay S.K. Limited diversity in the major histocompatibility complex class II loci of Syrian hamster DNA. Lab. Anim. Sci. 1996;46:679–681. [PubMed] [Google Scholar]
- Hjorth R.N., Bonde G.M., Pierzchala W.A., Vernon S.K., Wiener F.P., Levner M.H. A new hamster model for adenoviral vaccination. Arch. Virol. 1988;100:279–283. doi: 10.1007/BF01487691. [DOI] [PubMed] [Google Scholar]
- Hoffman R.A., Robinson P.F., Magalhaes H. The Golden Hamster: Its Biology and Use in Medical Research. Iowa State Univ. Press; Ames, IA: 1968. [Google Scholar]
- Hol P.R., Snel F.W., Niewold T.A., Gruys E. Amyloid-enhancing factor (AEF) in the pathogenesis of AA-amyloidosis in the hamster. Virchows Arch. B Cell. Pathol. Incl. Mol. Pathol. 1986;52:273–281. doi: 10.1007/BF02889968. [DOI] [PubMed] [Google Scholar]
- Homburger F. The Syrian golden hamster in chemical carcinogenesis research. Prog. Exp. Tumor Res. 1968;10:164–237. [Google Scholar]
- Homburger F. Disease models in Syrian hamsters. Prog. Exp. Tumor Res. 1972;16:69–86. doi: 10.1159/000393365. [DOI] [PubMed] [Google Scholar]
- Homburger F. Myopathy of hamster dystrophy: history and morphologic aspects. Ann. N Y Acad. Sci. 1979;317:2–17. [PubMed] [Google Scholar]
- Homburger F. Background data on tumor incidence in control animals (Syrian hamsters) Prog. Exp. Tumor Res. 1983;26:259–265. doi: 10.1159/000407264. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Takatori A., Inenaga T., Ohta E., Ishii Y., Kyuwa S. Histopathological studies of aortic dissection in streptozotocin-induced diabetic APA hamsters. Exp. Anim. 2005;54:363–367. doi: 10.1538/expanim.54.363. [DOI] [PubMed] [Google Scholar]
- Horwitz B.A., Chau S.M., Hamilton J.S., Song C., Gorgone J., Saenz M. Temporal relationships of blood pressure, heart rate, baroreflex function, and body temperature change over a hibernation bout in Syrian hamsters. Am. J. Physiol-Reg. I. 2013;305:R759–R768. doi: 10.1152/ajpregu.00450.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchin O.B. Toxic levels of morphine for the hamster. Exp. Biol. Med. 1943;54:339–340. [Google Scholar]
- Hurley R.J., Desch C.E., Jr. Demodex cricetuli: new species of hair follicle mite (Acari: Demodecidae) from the Armenian hamster, Cricetulus migratorius (Rodentia: Cricetidae) J. Med. Entomol. 1994;31:529–533. doi: 10.1093/jmedent/31.4.529. [DOI] [PubMed] [Google Scholar]
- ICTV, 2013. ICTV Master Species List 2013 – International Committee on Taxonomy of Viruses. <http://www.ictvonline.org/virusTaxonomy.asp>.
- Ike F., Bourgade F., Ohsawa K., Sato H., Morikawa S., Saijo M. Lymphocytic choriomeningitis infection undetected by dirty-bedding sentinel monitoring and revealed after embryo transfer of an inbred strain derived from wild mice. Comp. Med. 2007;57:272–281. [PubMed] [Google Scholar]
- Ikeda Y., Ross J., Jr. Models of dilated cardiomyopathy in the mouse and the hamster. Curr. Opin. Cardiol. 2000;15:197–201. doi: 10.1097/00001573-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Innes J.R.M., Wilson C., Ross M.A. Epizootic Salmonella enteritidis infection causing septic pulmonary phlebothrombosis in hamsters. J. Infect. Dis. 1956;98:133–141. doi: 10.1093/infdis/98.2.133. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 2011. [Google Scholar]
- Jacoby R.O. Transmissible ileal hyperplasia of hamsters. I. Histogenesis and immunocytochemistry. Am. J. Pathol. 1978;91:433–450. [PMC free article] [PubMed] [Google Scholar]
- Jacoby, R.O., Johnson, E.A., 1981. Transmissible ileal hyperplasia. In: Hamster Immune Responses in Infectious and Oncologic Diseases, pp. 267–289.
- Jacoby R.O., Osbaldiston G.W., Jonas A.M. Experimental transmission of atypical ileal hyperplasia of hamsters. Lab. Anim. Sci. 1975;25:465–473. [PubMed] [Google Scholar]
- Jacoby R.O., Ball-Goodrich L.J., Besselsen D.G., McKisic M.D., Riley L.K., Smith A.L. Rodent parvovirus infections. Lab. Anim. Sci. 1996;46:370–380. [PubMed] [Google Scholar]
- Jasni S., McOrist S., Lawson G.H.K. Experimentally induced proliferative enteritis in hamsters: an ultrastructural study. Res. Vet. Sci. 1994;56:186–192. doi: 10.1016/0034-5288(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Jay M.T., Glaser C., Fulhorst C.F. The arenaviruses. J. Am. Vet. Med. Assoc. 2005;227:904–915. doi: 10.2460/javma.2005.227.904. [DOI] [PubMed] [Google Scholar]
- Jayapal K.P., Wlaschin K.F., Hu W., Yap M.G. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem. Eng. Progress. 2007;103:40–47. [Google Scholar]
- Jones G.F., Ward G.E., Murtaugh M.P., Lin G., Gebhart C.J. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J. Clin. Microbiol. 1993;31:2611–2615. doi: 10.1128/jcm.31.10.2611-2615.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H.V., van Houte J. The hamster as an experimental model for odontopathic infections. Prog. Exp. Tumor Res. 1972;16:539. doi: 10.1159/000393388. [DOI] [PubMed] [Google Scholar]
- Kajdacsy-Balla A., Howeedy A., Bagasra O. Experimental model of congenital syphilis. Infect. Immun. 1993;61:3559–3561. doi: 10.1128/iai.61.8.3559-3561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasim-Karakas S.E., Vriend H., Almario R., Chow L.C., Goodman M.N. Effects of dietary carbohydrates on glucose and lipid metabolism in golden Syrian hamsters. J. Lab. Clin. Med. 1996;128:208–213. doi: 10.1016/s0022-2143(96)90013-x. [DOI] [PubMed] [Google Scholar]
- Kaup F.J., Konstyr I., Drommer W. Characteristics of spontaneous intraperitoneal cysts in golden hamsters and European hamsters. Exp. Pathol. 1990;40:205–212. doi: 10.1016/s0232-1513(11)80298-7. [DOI] [PubMed] [Google Scholar]
- Khallou J., Riottot M., Parquet M., Verneau C., Lutton C. Biodynamics of cholesterol and bile acids in the lithasic hamster. Br. J. Nutr. 1991;66:479–492. doi: 10.1079/bjn19910049. [DOI] [PubMed] [Google Scholar]
- Kink J.A., Williams J.A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman J. Some aspects of pulmonary pathology in the Syrian hamster. Prog. Exp. Tumor Res. 1972;16:287–299. doi: 10.1159/000393377. [DOI] [PubMed] [Google Scholar]
- Kohn H.I., Gultman P.H. Life span, tumor incidence, and intercapillary glomerulosclerosis in the Chinese hamster (Cricetulus griseus) after whole-body and partial-body exposure to X-rays. Radiat. Res. 1964;21:622–643. [PubMed] [Google Scholar]
- Kokkotou E., Moss A.C., Michos A., Espinoza D., Cloud J.W., Mustafa N. Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters. Antimicrob. Agents Chemother. 2008;52:1121–1126. doi: 10.1128/AAC.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbasa K.P., Lancaster C., Olafsson A.S., Gilbertson S.K., Robert A. Indomethacin-induced gastric antral ulcers in hamsters. Gastroenterology. 1988;95:932–944. doi: 10.1016/0016-5085(88)90166-7. [DOI] [PubMed] [Google Scholar]
- Konishi Y., Tsutsumi M., Tsujiuchi T. Mechanistic analysis of pancreatic ductal carcinogensis in hamsters. Pancreas. 1998;16:300–306. doi: 10.1097/00006676-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch. Oral Biol. 1966;11 doi: 10.1016/0003-9969(66)90107-5. 429-IN14. [DOI] [PubMed] [Google Scholar]
- Kunstyr I., Ernst H., Merkt M., Reichart P. Spontaneous pathology of the European hamster (Cricetus cricetus). Malocclusion, dysplastic, and inflammatory processes on the jaws. Z. Versuchstierkd. 1987;29:171–180. [PubMed] [Google Scholar]
- Lanteigne M., Reebs S.G. Preference for bedding material in Syrian hamsters. Lab. Anim. 2006;40:410–418. doi: 10.1258/002367706778476424. [DOI] [PubMed] [Google Scholar]
- Lawrie A.M., Megahy I.W. Tumours in Russian hamsters. Vet. Rec. 1991;128:411–412. doi: 10.1136/vr.128.17.411. [DOI] [PubMed] [Google Scholar]
- Lawson G.H.K., Gebhart C.J. Proliferative enteropathy. J. Comp. Path. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- Lepot A., Banwell J.G. The Syrian hamster: a reproducible model for studying changes in intestinal fluid secretion in response to enterotoxin challenge. Infect. Immun. 1976;14:1167–1171. doi: 10.1128/iai.14.5.1167-1171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.J., Gonzalez A., Banerjee S., Banerjee S.K., Li S.A. Estrogen carcinogenesis in the hamster kidney: role of cytotoxicity and cell proliferation. Environ. Health Perspect. 1993;101(Suppl. 5):259–264. doi: 10.1289/ehp.93101s5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.J., Li S.A. Estrogen carcinogenesis in the hamster kidney: a hormone-driven multistep process. Prog. Clin. Biol. Res. 1996;394:255–267. [PubMed] [Google Scholar]
- Liehr J.G. Hormone-associated cancer: mechanistic similarities between human breast cancer and estrogen-induced kidney carcinogenesis in hamsters. Environ. Health Perspect. 1997;105(Suppl 3):565–569. doi: 10.1289/ehp.97105s3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipskaia L., Pinet C., Fromes Y., Hatem S., Cantaloube I., Coulombe A. Mutation of the delta-sarcoglycan is associated with Ca(2+)-dependent vascular remodeling in the Syrian hamster. Am. J. Pathol. 2007;171:162–171. doi: 10.2353/ajpath.2007.070054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb W., Quimby F., editors. second ed. Taylor and Francis; Philadelphia, PA: 1999. The Clinical Chemistry of Laboratory Animals. [Google Scholar]
- Lowenstein D.H., Butler D.A., Westaway D., McKinley M.P., DeArmond S.J., Prusiner S.B. Three hamster species with different scrapie incubation times and neuropathological features encode distinct prion proteins. Mol. Cell Biol. 1990;10:1153–1163. doi: 10.1128/mcb.10.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier G., Pavilanis V. Presence of intranuclear inclusion bodies in proliferative ileitis of the hamster (Mesocricetus auratus). A preliminary report. Lab. Anim. Care. 1969;19:387. [PubMed] [Google Scholar]
- Lyman C.P. Usefulness of the hamster in the study of hibernation. In: Altman P.L., Dittman Katz D., editors. Vol 2. Fed. Am. Soc. Exp. Biol.; Bethesda, Maryland: 1979. p. 431. (Inbred and Genetically Defined Strains of Laboratory Animals). [Google Scholar]
- Lyman C.P. Sensitivity to arousal. In: Lyman C.P., editor. Hibernation and Torpor in Mammals and Birds. Academic Press; New York: 1982. pp. 77–91. [Google Scholar]
- Maatthiesen T., Kunstyr I., Tuch K. Hexamita-muris infection in mice and European hamsters in a laboratory animal colony. Z. Versuchstierkd. 1976;18:113–120. [PubMed] [Google Scholar]
- Macnish M.G., Morgan U.M., Behnke J.M., Thompson R.C.A. Failure to infect laboratory rodent hosts with human isolates of Rodentolepis (=Hymenolepis) nana. J. Helminthol. 2002;76:37–43. doi: 10.1079/joh200198. [DOI] [PubMed] [Google Scholar]
- Macnish M.G., Ryan U.M., Behnke J.M., Thompson R.C.A. Detection of the rodent tapeworm Rodentolepis (=Hymenolepis) microstoma in humans. A new zoonosis? Int. J. Parasitol. 2003;33:1079–1085. doi: 10.1016/s0020-7519(03)00137-1. [DOI] [PubMed] [Google Scholar]
- Magalhaes H. Gross anatomy. In: Hoffman R.A., Robinson P.F., Magalhaes H., editors. The Golden Hamster – Its Biology and Use in Medical Research. Iowa State University Press; Ames, IA: 1968. pp. 91–109. [Google Scholar]
- Magaribuchi T., Koshimizu K., Fujiwara K. An outbreak of “wet tail” in hamsters due to the Tyzzer’s organism. Exp. Anim. 1977;26:123–129. [PubMed] [Google Scholar]
- Manci E.A., Heath L.S., Leinbach S.S., Coggin J.H., Jr. Lymphoma-associated ulcerative bowel disease in the hamster (Mesocricetus auratus) induced by an unusual agent. Am. J. Pathol. 1984;116:1–8. [PMC free article] [PubMed] [Google Scholar]
- Marsh R.F., Hanson R.P. The Syrian hamster as a model for the study of slow virus diseases caused by unconventional agents. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1978;37:2076–2078. [PubMed] [Google Scholar]
- Masiello P., Broca C., Gross R., Roye M., Manteghetti M., Hillaire-Buys D. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- Masson-Pevet M., Naimi F., Canguilhem B., Saboureau M., Bonn D., Pevet P. Are the annual reproductive and body weight rhythms in the male European hamster (Cricetus cricetus) dependent upon a photoperiodically entrained circannual clock? J. Pineal Res. 1994;17:151–163. doi: 10.1111/j.1600-079x.1994.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Nagata I., Kariya Y., Ohaski K. Studies on a strain of pneumotropic virus of hamster. Nagoya J. Med. Sci. 1954;17:93–97. [Google Scholar]
- Maxwell K.O., Wish C., Murphy J.C., Fox J.G. Serum chemistry reference values in two strains of Syrian hamsters. Lab. Anim. Sci. 1985;35:67–70. [PubMed] [Google Scholar]
- McElroy A.K., Smith J.M., Hooper J.W., Schmaljohn C.S. Andes virus M genome segment is not sufficient to confer the virulence associated with andes virus in Syrian hamsters. Virology. 2004;326:130–139. doi: 10.1016/j.virol.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McGuire, J., 1993. Phodopus sungorus (Russian dwarf hamsters). <http://netvet.wustl.edu>.
- McMartin D.N. Spontaneous atrial thrombosis in aged Syrian hamsters. I. Incidence and pathology. Thromb. Haemost. 1977;38:447–456. [PubMed] [Google Scholar]
- McMartin D.N., Dodds W.J. Animal model of human disease: atrial thrombosis in aged Syrian hamsters. Am. J. Pathol. 1982;107:277. [PMC free article] [PubMed] [Google Scholar]
- Mcmillan H.J., Wynne-Edwards K.E. Evolutionary change in the endocrinology of behavioral receptivity: divergent roles for progesterone and prolactin within the genus Phodopus. Biol. Reprod. 1998;59:30–38. doi: 10.1095/biolreprod59.1.30. [DOI] [PubMed] [Google Scholar]
- McOrist S., Lawson G.H.K. Possible relationship of proliferative enteritis in pigs and hamsters. Vet. Microbiol. 1987;15:293–302. doi: 10.1016/0378-1135(87)90017-4. [DOI] [PubMed] [Google Scholar]
- Meier H., Yerganian G. Spontaneous hereditary diabetes mellitus in Chinese hamster (Cricetulus griseus). I. pathological findings. Proc. Soc. Exp. Biol. Med. 1959;100:810–815. doi: 10.3181/00379727-100-24786. [DOI] [PubMed] [Google Scholar]
- Meier H., Yerganian G. Spontaneous diabetes mellitus in the Chinese hamster (Cricetulus griseus). III. Maintenance of a diabetic hamster colony with the aid of hypoglycemic therapy. Diabetes. 1961;10:19–21. doi: 10.2337/diab.10.1.19. [DOI] [PubMed] [Google Scholar]
- Meredith A. Skin diseases and treatment of hamsters. In: Paterson S., editor. Skin Diseases of Exotic Pets. Blackwell Science Ltd; Oxford: 2006. pp. 251–263. [Google Scholar]
- Milazzo M.L., Eyzaguirre E.J., Molina C.P., Fulhorst C.F. Maporal viral infection in the Syrian golden hamster: a model of hantavirus pulmonary syndrome. J. Infect. Dis. 2002;186:1390–1395. doi: 10.1086/344735. [DOI] [PubMed] [Google Scholar]
- Militzer K., Herberg L., Buttner D. The ontogenesis of skin and organ characteristics in the Syrian golden hamster. II. Body and organ weights as well as blood glucose and plasma levels. Exp. Pathol. 1990;40:139–153. doi: 10.1016/s0232-1513(11)80337-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P.L., McLeod R.S. Conjugated linoleic acid and atherosclerosis: studies in animal models. Biochem. Cell Biol. 2008;86:293–301. doi: 10.1139/o08-070. [DOI] [PubMed] [Google Scholar]
- Mohr U., Schuller H., Reznik G., Althoff J., Page N. Breeding of European hamsters. Lab. Anim. Sci. 1973;23:799–802. [PubMed] [Google Scholar]
- Moore G.J. Giardia and Trichomonas infections in Syrian hamsters: efficacy of dimetridazole therapy and containment of infection. Anim. Tech. 1990;41:133–136. [Google Scholar]
- Moore W., Jr. Observations on the breeding and care of the Chinese hamster, Cricetulus griseus. Lab. Anim. Care. 1965;15:95–101. [PubMed] [Google Scholar]
- Moore W., Jr. Hemogram of the Chinese hamster. Am. J. Vet. Res. 1966;27:608–610. [PubMed] [Google Scholar]
- Motzel S.L., Gibson S.V. Tyzzer disease in hamsters and gerbils from a pet store supplier. J. Am. Vet. Med. Assoc. 1990;197:1176–1178. [PubMed] [Google Scholar]
- Mulder G. Anatomy, physiology, and behavior. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. pp. 765–777. (2012) [Google Scholar]
- Murphy J.C., Fox J.G., Niemi S.M. Nephrotic syndrome associated with renal amyloidosis in a colony of Syrian hamsters. J. Am. Vet. Med. Assoc. 1984;185:1359–1362. [PubMed] [Google Scholar]
- Murphy M.R., Schneider G.E. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Murray K. Anatomy, physiology, and behavior. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. pp. 753–765. [Google Scholar]
- Nakayama M., Saegusa J., Itoh K., Kiuchi Y., Tamura T., Ueda K. Transmissible enterocolitis in hamsters caused by Tyzzer’s organism. Jpn. J. Exp. Med. 1975;45:33–41. [PubMed] [Google Scholar]
- Nakayama M., Machii K., Goto Y., Fujiwara K. Typhlohepatitis in hamsters infected perorally with the Tyzzer’s organism. Jpn. J. Exp. Med. 1976;46:309–324. [PubMed] [Google Scholar]
- National Research Council . Nutritional Requirements of Laboratory Animals. fourth ed. National Academy Press; Washington, DC: 1995. [Google Scholar]
- Newberne P.M., Fox J.G. Nutritional adequacy and quality control of rodent diets. Lab. Anim. Sci. 1980;30:352–365. [PubMed] [Google Scholar]
- Newberne P.M., McConnell R.G. Nutrition of the Syrian golden hamster. Prog. Exp. Tumor Res. 1979;24:127–138. doi: 10.1159/000402090. [DOI] [PubMed] [Google Scholar]
- Newkirk K.D., Mcmillan H.J., Wynne-Edwards K.E. Length of delay to birth of a second litter in dwarf hamsters (Phodopus): evidence for post-implantation embryonic diapause. J. Exp. Zool. 1997;278:106–114. [PubMed] [Google Scholar]
- Nicklas W., Kraft V., Meyer B. Contamination of transplantable tumors, cell lines, and monoclonal antibodies with rodent viruses. Lab. Anim. Sci. 1993;43:296–300. [PubMed] [Google Scholar]
- Niewold T.A., Hol P.R., van Andel A.C., Lutz E.T., Gruys E. Enhancement of amyloid induction by amyloid fibril fragments in hamster. Lab. Invest. 1987;56:544–549. [PubMed] [Google Scholar]
- Nutting W.B. Demodex aurati sp. nov. and D. criceti, ectoparasites of the golden hamster (Mesocricetus auratus) Parasitology. 1961;51:515–522. doi: 10.1017/s0031182000070761. [DOI] [PubMed] [Google Scholar]
- Oka M.S., Rupp R.G. Large-scale animal cell culture: a biological perspective. Bioprocess Tech. 1990;10:71–92. [PubMed] [Google Scholar]
- Oldstone M.B., Dixon F.J. Pathogenesis of chronic disease associated with persistentlymphocytic choriomeningitis viral infection I. Relationship of antibody production to disease in neonatally infected mice. J. Exp. Med. 1969;131:1–19. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreffo V.I., Lin H.W., Padmanabhan R., Witschi H. K-ras and p53 point mutations in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced hamster lungg tumo. Carcinogenesis. 1993;14:451–455. doi: 10.1093/carcin/14.3.451. [DOI] [PubMed] [Google Scholar]
- Ostlind D.A., Mickle W.G., Smith S.K., Cifelli S., Ewanciw D.V. The Hymenolepis diminuta-golden hamster (Mesocricetus auratus) model for the evaluation of gastrointestinal anticestode activity. J. Parasitol. 2004;90:898–899. doi: 10.1645/GE-3356RN. [DOI] [PubMed] [Google Scholar]
- Paciello O., Wojcik S., Gradoni L., Oliva G., Trapani F., Lovane V. Syrian hamster infected with Leishmania infantum: a new experimental model for inflammatory myopathies. Muscle Nerve. 2010;41:355–361. doi: 10.1002/mus.21502. [DOI] [PubMed] [Google Scholar]
- Parker J.C., Richter C.B. Viral diseases of the respiratory system. In: Foster H.L., Small J.D., Fox J.G., editors. The Biology of the Laboratory Mouse. Academic Press; New York: 1982. pp. 107–155. [Google Scholar]
- Parker J.C., Igel H.J., Reynolds R.K., Lewis A.M.J., Rowe W.P. Lymphocytic choriomeningitis virus infection in fetal, newborn, and young adult Syrian hamsters. Infect. Immun. 1976;13:967–981. doi: 10.1128/iai.13.3.967-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M.M., Schrenzel M.D., Feng Y., Fox J.G. Gastritis and intestinal metaplasia in Syrian hamsters infected with Helicobacter aurati and two other microaerobes. Vet. Pathol. 2000;37:589–596. doi: 10.1354/vp.37-6-589. [DOI] [PubMed] [Google Scholar]
- Patterson M.M., Schrenzel M.D., Feng Y., Xu S., Dewhirst F.E., Paster B.J. Helicobacter aurati sp. nov., a urease-positive Helicobacter species cultured from gastrointestinal tissues of Syrian hamsters. J. Clin. Microbiol. 2000;38:3722–3728. doi: 10.1128/jcm.38.10.3722-3728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavesio C.E., Chiappino M.L., Gormley P., Setzer P.Y., Nichols B.A. Acquired retinochoroiditis in hamsters inoculated with ME 49 strain toxoplasma. Invest. Ophthalmol. Vis. Sci. 1995;36:2166–2175. [PubMed] [Google Scholar]
- Percy D.H. Zoonoses in laboratory animals. Can. Vet. J. 1987;28:268–269. [PMC free article] [PubMed] [Google Scholar]
- Percy D.H., Palmer D.J. Pathogenesis of Sendai virus infection in the Syrian hamster. Lab. Anim. Sci. 1997;47:132–137. [PubMed] [Google Scholar]
- Percy D.H., Barthold S.W. Hamster. In: Percy D.H., Barthold S.W., editors. Pathology of Laboratory Rodents and Rabbits. third ed. Blackwell; Ames, Iowa: 2007. pp. 179–204. [Google Scholar]
- Pevet P. The role of the pineal gland in the photoperiodic control of reproduction in different hamster species. Reprod. Nutr. Dev. 1988;28:443–458. doi: 10.1051/rnd:19880310. [DOI] [PubMed] [Google Scholar]
- Phares C.K. Streptozotocin-induced diabetes in Syrian hamsters: new model of diabetes mellitus. Experientia. 1980;36:681–682. doi: 10.1007/BF01970137. [DOI] [PubMed] [Google Scholar]
- Pinto R.M., Goncalves L., Gomes DC., Noronha D. Helminth fauna of the golden hamster Mesocricetus auratus in Brazil. Contemp. Top. Lab. Anim. Sci. 2001;40:21–26. [PubMed] [Google Scholar]
- Poel W.E., Yerganian G. Adenocarcinoma of the pancreas in diabetes-prone Chinese hamsters. Am. J. Med. 1961;31:861–863. doi: 10.1016/0002-9343(61)90026-2. [DOI] [PubMed] [Google Scholar]
- Pogosianz H.E. Djungarian hamster—a suitable tool for cancer research and cytogenetic studies. J. Natl. Cancer Inst. 1975;54:659–664. doi: 10.1093/jnci/54.3.659. [DOI] [PubMed] [Google Scholar]
- Pogosianz H.E., Sokova O.I. Tumours of the Dungarian hamster. IARC Sci. Publ. 1982;34:451–455. [PubMed] [Google Scholar]
- Pour P., Kmoch N., Greiser E., Mohr U., Althoff J., Cardesa A. Spontaneous tumors and common diseases in two colonies of Syrian hamsters. I. Incidence and sites. J. Natl. Cancer Inst. 1976;56:931–935. doi: 10.1093/jnci/56.5.931. [DOI] [PubMed] [Google Scholar]
- Pour P., Mohr U., Althoff J., Cardesa A., Kmoch N. Spontaneous tumors and common diseases in two colonies of Syrian hamsters. II. Respiratory tract and digestive system. J. Natl. Cancer Inst. 1976;56:937–948. doi: 10.1093/jnci/56.5.937. [DOI] [PubMed] [Google Scholar]
- Pour P., Mohr U., Althoff J., Cardesa A., Kmoch N. Spontaneous tumors and common diseases in two colonies of Syrian hamsters. III. Urogenital system and endocrine glands. J. Natl. Cancer Inst. 1976;56:949–961. doi: 10.1093/jnci/56.5.949. [DOI] [PubMed] [Google Scholar]
- Pritchett K.R. Helminth parasites of laboratory mice. In: Fox J.G., Barthold S.W., Davisson M.T., Newcomber C.E., Quimby F.W., Smith A.L., editors. The Mouse in Biomedical Research, vol. Diseases. second ed. Academic Press; New York: 2007. pp. 551–564. [Google Scholar]
- Pritchett K.R., Johnston N.A. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp. Top. Lab. Anim. Sci. 2002;41:36–46. [PubMed] [Google Scholar]
- Profeta M.L., Lief F.S., Plotkin S.A. Enzootic Sendai infection in laboratory hamsters. Am. J. Epidemiol. 1969;89:316–324. doi: 10.1093/oxfordjournals.aje.a120944. [DOI] [PubMed] [Google Scholar]
- Prokoph H., Arnold W., Schwartz A., Scherneck S. In vivo replication of hamster polyomavirus DNA displays lymphotropism in hamsters susceptible to lymphoma induction. J. Gen. Virol. 1996;77:2165–2172. doi: 10.1099/0022-1317-77-9-2165. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. Molecular biology and transgenetics of prion diseases. Crit. Rev. Biochem. Mol. Biol. 1991;26:397–438. doi: 10.3109/10409239109086789. [DOI] [PubMed] [Google Scholar]
- Puchalski W., Heldmaier G. Seasonal changes of heart weight and erythrocytes in the Djungarian hamster, Phodopus sungorus. Comp. Biochem. Physiol. A. 1986;84:259–263. doi: 10.1016/0300-9629(86)90610-9. [DOI] [PubMed] [Google Scholar]
- Reasner D.S., Johnston R.E. Acceleration of reproductive development in female Djungarian hamsters by adult males. Physiol. Behav. 1988;43:57–64. doi: 10.1016/0031-9384(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Rehg J.E., Lu Y.S. Clostridium difficile typhlitis in hamsters not associated with antibiotic therapy. J. Am. Vet. Med. Assoc. 1982;181:1422–1423. [PubMed] [Google Scholar]
- Renshaw H.W., Van Hoosier G.L., Jr., Amend N.K. A survey of naturally occurring diseases of the Syrian hamster. Lab. Anim. 1975;9:179–191. doi: 10.1258/002367775780994592. [DOI] [PubMed] [Google Scholar]
- Reznik G., Jensen K.A. Anatomy of the nasal cavity of the European hamster (Cricetus cricetus) Z. Versuchstierkd. 1979;21:321–340. [PubMed] [Google Scholar]
- Reznik G., Reznik-Schuller H., Mohr U. Comparative studies of organs in the European hamster (Cricetus cricetus L.), the Syrian golden hamster (Mesocricetus auratus W.), and the Chinese hamster (Cricetulus griseus M.) Z. Versuchstierkd. Bd. 1973;15:S272–S282. [PubMed] [Google Scholar]
- Reznik G., Reznik-Schuller H., Schostek H., Deppe K., Mohr U. Comparative studies concerning the suitability of European hamsters and Syrian golden hamsters for investigations on smoke exposure. Arzneimittelforschung. 1975;25:923–926. [PubMed] [Google Scholar]
- Reznik G., Reznik-Schuller H., Emminger A., Mohr U. Comparative studies of blood from hibernating and nonhibernating European hamsters (Cricetus cricetus L.) Lab. Anim. Sci. 1979;25:210–215. [PubMed] [Google Scholar]
- Reznik-Schuller H., Reznik G., Mohr U. The European hamster (Cricetus cricetus L.) as an experimental animal: breeding methods and observations of their behaviour in the laboratory. Z. Versuchstierkd. 1974;16:S48–S58. [PubMed] [Google Scholar]
- Richards M.P.M. Maternal behaviour in the golden hamster: responsiveness to young in virgin, pregnant, and lactating females. Anim. Behav. 1966;14:310–313. doi: 10.1016/s0003-3472(66)80088-x. [DOI] [PubMed] [Google Scholar]
- Richards M.P.M. Effects of oestrogen and progesterone on nest building in the golden hamster. Anim. Behav. 1969;17:356–361. doi: 10.1016/0003-3472(69)90022-0. [DOI] [PubMed] [Google Scholar]
- Richter C.B. Mouse adenovirus, K virus, pneumonia virus of mice. In: Bhatt P.N., Jacoby R.O., Morse I.H.C., editors. Viral and Mycoplasmal Infections of Laboratory Rodents. Academic Press; New York: 1986. pp. 137–192. [Google Scholar]
- Roberts A., Vogel L., Guarner J., Hayes N., Murphy B., Zaki S. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J. Virol. 2005;79:503–511. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollison D.E., Page W.F., Crawford H., Gridley G., Wacholder S., Martin J. Case control study of cancer among US army veterans exposed to simian virus 40-contaminated adenovirus vaccine. Am. J. Epidemiol. 2004;160:317–324. doi: 10.1093/aje/kwh212. [DOI] [PubMed] [Google Scholar]
- Romanenko S.A., Volobouev V.T., Perelman P.L., Lebedev V.S., Serdukova N.A., Trifonov V.A. Karyotype evolution and phylogenetic relationships of hamsters (Cricetidae, Muroidea, Rodentia) inferred from chromosomal painting and banding comparison. Chromosome Res. 2007;15:283–298. doi: 10.1007/s10577-007-1124-3. [DOI] [PubMed] [Google Scholar]
- Ronald N.C., Wagner J.E. Treatment of Hymenolepsis nana in hamsters with yomesan (niclosamide) Lab. Anim. Sci. 1975;25:219–220. [PubMed] [Google Scholar]
- Ross C.R., Wagner J.E., Wrightman S.R., Dill S.E. Experimental transmission of Syphacia muris among rats, mice, hamsters, and gerbils. Lab. Anim. Sci. 1980;30:35–37. [PubMed] [Google Scholar]
- Ross P.D. Phodopus sungorus. Am. Soc. Mammol. 1998:1–9. (Mammalian Species No. 595). Available at: https://kataloge.thulb.uni-jena.de/DB=1/LNG=EN/CLK?IKT=12&TRM=636891429. [Google Scholar]
- Rowell T.E. Retrieving and other behavior in lactating golden hamsters. Proc. Zool. Soc. 1960;135:265–282. [Google Scholar]
- Ryden E.B., Lipman N.S., Taylor N.S., Rose R., Fox J.G. Clostridium difficile typhlitis associated with cecal mucosal hyperplasia in Syrian hamsters. Lab. Anim. Sci. 1991;41:553–558. [PubMed] [Google Scholar]
- Ryoke T., Gu Y., Mao L., Hongo M., Clark R.G., Peterson K.L. Progressive cardiac dysfunction and fibrosis in the cardiomyopathic hamster and effects of growth hormone and angiotensin-converting enzyme inhibition. Circulation. 1999;100:1734–1743. doi: 10.1161/01.cir.100.16.1734. [DOI] [PubMed] [Google Scholar]
- Sakamoto A., Ono K., Abe M., Jasmin G., Eki T., Murakami Y. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc. Natl. Acad. Sci. USA. 1997;94:13873–13878. doi: 10.1073/pnas.94.25.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanashi T., Sako S., Nozuhara A., Adachi K., Okamoto T., Koga Y. Vitamin E deficiency has a pathological role in myocytolysis in cardiomyopathic Syrian hamster (BIO 14.6) Biochem. Biophys. Res. Commun. 1991;181:145–150. doi: 10.1016/s0006-291x(05)81393-2. [DOI] [PubMed] [Google Scholar]
- Sato T., Komeda K., Shirama K. Plasma progesterone concentrations during the estrous cycle, pregnancy, and lactation in the Chinese hamster, Cricetulus griseus. Jikken Dobutsu. 1984;33:501–508. doi: 10.1538/expanim1978.33.4_501. [DOI] [PubMed] [Google Scholar]
- Sawrey D.K., Baumgardner D.J., Campa M.J., Ferguson B., Hodges A.W., Dewsbury D.A. Behavioral patterns of Djungarian hamsters: an adaptive profile. Anim. Learn. Behav. 1984;12:297–306. [Google Scholar]
- Scherneck S., Feunteun J. The hamster polyomavirus—a brief review of recent knowledge. Arch. Geschwulstforsch. 1990;60:271–278. [PubMed] [Google Scholar]
- Schlenker E.H. Ventilation and metabolism of the Djungarian hamster (Phodopus sungorus) and the albino mouse. Comp. Biochem. Physiol. A. 1985;82:293–295. doi: 10.1016/0300-9629(85)90857-6. [DOI] [PubMed] [Google Scholar]
- Sebesteny A. Pathogenicity of intestinal flagellates in mice. Lab. Anim. 1969;3:71–77. doi: 10.1258/002367774780943805. [DOI] [PubMed] [Google Scholar]
- Shklar G. Experimental oral pathology in the Syrian hamster. Prog. Exp. Tumor Res. 1972;16:518. doi: 10.1159/000393387. [DOI] [PubMed] [Google Scholar]
- Sichuk G., Bettigole R.E., Der B.K., Fortner J.G. Influence of sex hormones on thrombosis of left atrium in Syrian (golden) hamsters. Am. J. Physiol. 1965;208:465–470. doi: 10.1152/ajplegacy.1965.208.3.465. [DOI] [PubMed] [Google Scholar]
- Silberberg R., Gerritsen G.C. Aging changes in intervertebral discs and spondylosis in Chinese hamsters. Diabetes. 1976;25:477–483. doi: 10.2337/diab.25.6.477. [DOI] [PubMed] [Google Scholar]
- Silverman J., Chavannes J.M. Biological values of the European hamster (Cricetus cricetus) Lab. Anim. Sci. 1977;27:641–645. [PubMed] [Google Scholar]
- Simionescu N., Sima A., Dobrian A., Tirziu D., Simionescu M. Pathobiochemical changes of the arterial wall at the inception of atherosclerosis. Curr. Top. Pathol. 1993;87:1–45. doi: 10.1007/978-3-642-76849-1_1. [DOI] [PubMed] [Google Scholar]
- Simmons J.H., Riley L.K., Besch-Williford C.L., Franklin C.L. Helicobacter mesocricetorum sp. nov., a novel Helicobacter isolated from the feces of Syrian hamsters. J. Clin. Microbiol. 2000;38:1811–1817. doi: 10.1128/jcm.38.5.1811-1817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.H., Riley L.K., Franklin C.L., Besch-Williford C.L. Hamster polyomavirus infection in a pet Syrian hamster (Mesocricetus auratus) Vet. Path. 2001;38:441–446. doi: 10.1354/vp.38-4-441. [DOI] [PubMed] [Google Scholar]
- Skinner H.H., Knight E.H. The potential role of Syrian hamsters and other small animals as reservoirs of lymphocytic choriomeningitis virus. J. Small Anim. Pract. 1979;20:145–161. doi: 10.1111/j.1748-5827.1979.tb07023.x. [DOI] [PubMed] [Google Scholar]
- Slater G.M. The care and feeding of the Syrian hamster. Prog. Exp. Tumor. Res. 1972;16:42–49. doi: 10.1159/000393363. [DOI] [PubMed] [Google Scholar]
- Soave O.A. Diagnosis and control of common diseases of hamsters, rabbits, and monkeys. J. Am. Vet. Med. Assoc. 1963;142:285–290. [PubMed] [Google Scholar]
- Soskel N.T., Watanabe S., Sandberg L.B. Mechanisms of lung injury in the copper-deficient hamster model of emphysema. CHEST. 1984;85(Suppl. 6):70S–73S. doi: 10.1378/chest.85.6_supplement.70s. [DOI] [PubMed] [Google Scholar]
- Spikermann V.A.R., Althoff J. The fine structure of the exocrine pancreas of the European hamster (Cricetus cricetus L.): electron microscopic investigations. Z. Versuchstierkd. 1980;22:36–42. [PubMed] [Google Scholar]
- Sroller V., Vilchez R.A., Stweart A.R., Wong C., Butel J.S. Influence of the viral regulatory region on tumor induction by simian virus 40 in hamsters. J. Virol. 2008;82:871–879. doi: 10.1128/JVI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stills H.F., Jr. Isolation of an intracellular bacterium from hamsters (Mesocricetus auratus) with proliferative ileitis and reproduction of the disease with a pure culture. Infect. Immun. 1991;59:3227–3236. doi: 10.1128/iai.59.9.3227-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey K.B. Out cold: biochemical regulation of mammalian hibernation – a mini-review. Gerontology. 2010;56:220–230. doi: 10.1159/000228829. [DOI] [PubMed] [Google Scholar]
- Streilein J.W. Hamster immune responses: experimental models linking immunogenetics, oncogensis, and viral immunity. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1978;37:2023–2108. [PubMed] [Google Scholar]
- Streilein J.W., Duncan W.R., Homburger F. Immunogenetic relationships among genetically defined, inbred domestic Syrian hamster strains. Transplantation. 1980;30:358–361. doi: 10.1097/00007890-198011000-00010. [DOI] [PubMed] [Google Scholar]
- Suckow M.A., Stevens K.A., Wilson R.P. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. [Google Scholar]
- Sunday M.E., Willett C.G., Graham S.A., Oreffo V.I., Linnoila R.I., Witschi H. Histochemical characterization of non-neuroendocrine lung tumors and neuroendocrine cell hyperplasia induced hamster lung by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone with or without hyperoxia. Am. J. Pathol. 1995;147:740–752. [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Matsubara J., Saito M., Muto T., Nakagawa M., Imaizumi K. Serological survey of laboratory rodents for infection with Sendai virus, mouse hepatitis virus, reovirus type 3, and mouse adenovirus. Jpn. J. Med. Sci. Biol. 1982;35:249–254. doi: 10.7883/yoken1952.35.249. [DOI] [PubMed] [Google Scholar]
- Svensjo E. The hamster cheek pouch as a model in microcirculation research. Eur. Respir. J. Suppl. 1990;12:595s–600s. [PubMed] [Google Scholar]
- Tagaya M., Morie M.I., Miyadai T. Efficacy of temperature-sensitive Sendai virus vaccine in hamsters. Lab. Anim. Sci. 1995;45:233–238. [PubMed] [Google Scholar]
- Tanaka A., Hisanaga A., Ishinishi N. The frequency of spontaneously-occurring neoplasms in the male Syrian golden hamster. Vet. Hum.Toxicol. 1991;33:318–321. [PubMed] [Google Scholar]
- Tani K., Iwanaga T., Sonoda K., Hayashiya S., Hayashiya M., Taura Y. Ivermectin treatment of demodicosis in 56 hamsters. J. Vet. Med. Sci. 2001;63:1245–1247. doi: 10.1292/jvms.63.1245. [DOI] [PubMed] [Google Scholar]
- Tansey G., Roy A.F., Bivin W.S. Acute pneumonia in a Syrian hamster: isolation of a Corynebacterium species. Lab. Anim. Sci. 1995;45:366–367. [PubMed] [Google Scholar]
- Taylor D.M., Farquhar C.F., Neal D.L. Studies on the eradication of intestinal protozoa of Syrian hamsters in quarantine and their transfaunation to mice. Lab. Anim. Sci. 1993;43:359–360. [PubMed] [Google Scholar]
- Tennent G.A., Baltz M.L., Osborn G.D., Butler P.J., Noble G.E., Hawkins P.N. Studies of the structure and binding properties of hamster female protein. Immunology. 1993;80:645–651. [PMC free article] [PubMed] [Google Scholar]
- Thomas M.A., Spencer J.F., La Regina M.C., Dhar D., Tollefson A.E., Toth K. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Thompson R. The water consumption and drinking habits of a few species and strains of laboratory animals. J. Inst. Anim. Tech. 1971;22:29–36. [Google Scholar]
- Trautwein E.A., Siddiqui A., Hayes K.C. Characterization of the bile acid profile in developing male and female hamsters in response to dietary cholesterol challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999;124:93–103. doi: 10.1016/s1095-6433(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Trevitt C.R., Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129:2241–2265. doi: 10.1093/brain/awl150. [DOI] [PubMed] [Google Scholar]
- Tseng L.F., Ostwald T.J., Loh H.H., Li C.H. Behavioral activities of opioid peptides and morphine sulfate in golden hamsters and rats. Psychopharmacology. 1979;64:215–218. doi: 10.1007/BF00496065. [DOI] [PubMed] [Google Scholar]
- Uchida E., Matsushita A., Yanagi K., Hiroi M., Aimoto T., Nakamura Y. Experimental pancreatic cancer model using PGHAM-1 cells: characteristics and experimental therapeutic trials. J. Nippon Med. Sch. 2008;75:325–331. doi: 10.1272/jnms.75.325. [DOI] [PubMed] [Google Scholar]
- Unay E.S., Davis B.J. Treatment of Syphacia obvelata in the Syrian hamster (Mesocricetus auratus) with piperazine citrate. Am. J. Vet. Res. 1980;41:1899–1900. [PubMed] [Google Scholar]
- United States Department of Agriculture, 2010. Animal Care Annual Report of Activities. United States Department of Agriculture, Animal and Plant Heath Inspection Service. Available at: <http://www.aphis.usda.gov/animal_welfare/efoia/downloads/2010_Animals_Used_In_Research.pdf>.
- Vairaktaris E., Papageorgiou G., Derka S., Moulavassili P., Nkenke E., Kessler P. Expression of ets-1 is not affected by N-ras or H-ras during ral oncogenesis. J. Cancer Res. Clin. Oncol. 2007;133:227–233. doi: 10.1007/s00432-006-0161-1. [DOI] [PubMed] [Google Scholar]
- Vairaktaris E., Papakosta V., Derka S., Vassiliou S., Nkenke E., Spyridonidou S. H-ras and c-fos exhibit similar expression patterns during most stages of oral oncogenesis. In Vivo. 2008;22:621–628. [PubMed] [Google Scholar]
- Vairaktaris E., Spyridonidou S., Papakosta V., Vyllliotis A., Lazaris A., Perrea D. The hamster model of sequential oral oncogenesis. Oral Oncol. 2008;44:315–324. doi: 10.1016/j.oraloncology.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Valentine H., Daugherity E.K., Singh B., Maurer K.J. Anatomy, physiology, and behavior. In: Suckow M.A., Stevens K.A., Wilson R.P., editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. first ed. Academic Press; Waltham, MA: 2012. pp. 875–906. 2012. [Google Scholar]
- Van Heek M., Austin T.M., Faarley C., Cook J.A., Tetzloff G.G., Davis H.R. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 2001;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- Van Hoosier G.L., Jr., Trentin J.J. Naturally occurring tumors of the Syrian hamster. Prog. Exp. Tumor Res. 1979;23:1–12. doi: 10.1159/000401419. [DOI] [PubMed] [Google Scholar]
- Verdino P., Witherden D.A., Podshivalova K., Rieder S.E., Havran W.L. cDNA Sequence and Fab Crystal Structure of HL4E10, a Hamster IgG Lambda Light Chain Antibody Stimulatory for γδ T Cells. PLoS One. 2011;6(5):e19828. doi: 10.1371/journal.pone.0019828. http://dx.doi.org/10.1371/journal.pone.0019828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan N., Davis F.C. Timing of birth in Syrian hamsters. Biol. Reprod. 1992;47:6–10. doi: 10.1095/biolreprod47.1.6. [DOI] [PubMed] [Google Scholar]
- Waggie K.S., Thornburg L.P., Grove K.J., Wagner J.E. Lesions of experimentally induced Tyzzer’s disease in Syrian hamsters, guinea pigs, mice, and rats. Lab. Anim. 1987;21:155–160. doi: 10.1177/002367728702100213. [DOI] [PubMed] [Google Scholar]
- Wagner J.E., Doyle R.E., Ronald N.C., Garrison R.G., Schmitz J.A. Hexamitiasis in laboratory mice, hamsters, and rats. Lab. Anim. Sci. 1974;24:349–354. [PubMed] [Google Scholar]
- Wahl-Jensen V., Chapman J., Asher L., Fisher R., Zimmerman M., Larsen T. Temporal analysis of andes virus and sin nombre virus infections of Syrian hamsters. J. Virol. 2007;81:7449–7462. doi: 10.1128/JVI.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.C., Moore W., Jr. Spontaneous lesions in a colony of Chinese hamsters. Lab. Anim. Care. 1969;19:516–521. [PubMed] [Google Scholar]
- Warner R.G., Ehle F.R. Nutritional idiosyncrasies of the golden hamster (Mesocricetus auratus) Lab. Anim. Sci. 1976;26:670–673. [PubMed] [Google Scholar]
- Watson J.M. Helminths infective to man in the Syrian hamster. Brit. Med. J. 1946;2:578. doi: 10.1136/bmj.2.4476.578-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whary M.T., Fox J.G. Natural and experimental Helicobacter infections. Comp. Med. 2004;54:128–158. [PubMed] [Google Scholar]
- Wightman S.R., Pilitt P.A., Wagner J.E. Dentostomella translucida in the Mongolian gerbil (Meriones unguiculatus) Lab. Anim. Sci. 1978;28:290–296. [PubMed] [Google Scholar]
- Wissler R.W. Update on the pathogenesis of atherosclerosis. Am. J. Med. 1991;91:3S–9S. doi: 10.1016/0002-9343(91)90050-8. [DOI] [PubMed] [Google Scholar]
- Wollnik F., Schmidt B. Seasonal and daily rhythms of body temperature in the European hamster (Cricetus cricetus) under semi-natural conditions. J. Comp. Physiol. B. 1995;165:171–182. doi: 10.1007/BF00260808. [DOI] [PubMed] [Google Scholar]
- Wozniak E.J., Lowenstine L.J., Hemmer R., Robinson T., Conrad P.A. Comparative pathogenesis of human WA1 and babesia microti isolates in a Syrian hamster model. Lab. Anim. Sci. 1996;46:507–515. [PubMed] [Google Scholar]
- Wynne-Edwards K.E., Lisk R.D. Djungarian hamsters fail to conceive in the presence of multiple males. Anim. Behav. 1984;32:626–628. [Google Scholar]
- Wynne-Edwards K.E., Lisk R.D. Male–female interactions across the female estrous cycle: a comparison of two species of dwarf hamster (Phodopus campbelli and Phodopus sungorus) J. Comp. Psychol. 1987;101:335–344. [PubMed] [Google Scholar]
- Xu R., Yokoyama W.H., Irving D., Rein D., Walzem R.L., German J.B. Effect of dietary catechin and vitamin E on aortic fatty streak accumulation in hypercholesterolemic hamsters. Atherosclerosis. 1998;137:29–36. doi: 10.1016/s0021-9150(97)00248-7. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Yanagimachi H., Rogers B.J. The use of the zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol. Reprod. 1976;15:471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]
- Yerganian G. The striped-back or Chinese hamster. J. Natl. Cancer Inst. 1958;20:705–727. [PubMed] [Google Scholar]
- Yerganian G. Spontaneous diabetes mellitus in the Chinese hamster, Cricetulus griseus. Int. Cong. Ser. Excerpta Med. 1965;84:612–627. [Google Scholar]
- Yerganian G. The biology and genetics of the Chinese hamster. In: Gottesman M., editor. Molecular Cell Genetics. Wiley; New York: 1985. pp. 3–36. [Google Scholar]
- Yerganian G., Klein E., Roy A. Physiological studies on Chinese hamster, Cricetulus griseus. II. A method for study of bleeding time in hamsters. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1955;14:424. [Google Scholar]
- Yoon C.H., Peterson J.S. Recent advances in hamster genetics. Prog. Exp. Tumor Res. 1979;24:157–161. doi: 10.1159/000402093. [DOI] [PubMed] [Google Scholar]
- Ziomek J., Banaszek A. The common hamster, Cricetus cricetus in Poland: status and current range. Folia Zool. 2007;56:235–242. [Google Scholar]
- Zook B.C., Huang K., Rhorer R.G. Tyzzer’s disease in Syrian hamsters. J. Am. Vet. Med. Assoc. 1977;171:833–836. [PubMed] [Google Scholar]


