Abstract
Background
The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) has recommended that isocitrate dehydrogenase 1 and 2 wildtype (IDH1/2wt) diffuse lower-grade gliomas (LGGs) World Health Organization (WHO) grade II or III that present with (i) a telomerase reverse transcriptase promoter mutation (pTERTmt), and/or (ii) gain of chromosome 7 combined with loss of chromosome 10, and/or (iii) epidermal growth factor receptor (EGFR) amplification should be reclassified as diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV (IDH1/2wt astrocytomas WHO IV). This paper describes the overall survival (OS) of IDH1/2wt astrocytoma WHO IV patients, and more in detail patients with tumors with pTERTmt only.
Methods
In this retrospective multicenter study, we compared the OS of 71 IDH1/2wt astrocytomas WHO IV patients, with radiological characteristics of LGGs, with the OS of 197 IDH1/2wt glioblastoma patients. Moreover, we compared the OS of 22 pTERTmt only astrocytoma patients with the OS of the IDH1/2wt glioblastoma patients.
Results
Median OS was similar for IDH1/2wt astrocytoma WHO IV patients (23.8 mo) and IDH1/2wt glioblastoma patients (19.2 mo) (Cox proportional hazards model: hazard ratio [HR] 1.27, 95% CI: 0.85–1.88, P = 0.242). OS was also similar in patients with IDH1/2wt astrocytomas WHO IV, pTERTmt only, and IDH1/2wt glioblastomas (HR 1.15, 95% CI: 0.64–2.10, P = 0.641).
Conclusions
The presented data confirm the cIMPACT-NOW recommendation and we propose that IDH1/2wt astrocytomas WHO IV in the absence of other qualifying mutations should be classified as IDH1/2wt glioblastomas.
Keywords: astrocytoma, glioblastoma, cIMPACT-NOW, IDH, TERT
Key Points.
IDH1/2wt astrocytomas WHO IV have a similar OS as IDH1/2wt glioblastomas.
pTERTmt only astrocytomas also have a similar OS as IDH1/2wt glioblastomas.
IDH1/2wt astrocytomas WHO IV should be classified as IDH1/2wt glioblastomas.
Importance of the Study.
The cIMPACT-NOW committee has recommended the classification of the new glioma subtype diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV. We show that these IDH1/2wt astrocytomas WHO IV, presenting with typical clinical, radiological, and histological characteristics of diffuse lower-grade gliomas, but that have either a TERT promoter mutation and/or EGFR amplification and/or gain of chromosome 7 and loss of chromosome 10 have a similar poor prognosis as glioblastomas. In the present report, all included cases had MRI scans that were fully consistent with a grade II or III tumor, and thus the histological findings do not simply represent a biopsy bias. Moreover, we identified a series of cases with only a TERT promoter mutation and confirmed their poor prognosis. Our data therefore support the reclassification of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV as IDH1/2wt glioblastomas in a revised WHO classification.
The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) aims to aid in the taxonomy of primary brain tumors in the period between official editions of the World Health Organization (WHO) classifications of brain tumors. In the third cIMPACT-NOW report, the committee recommended to reclassify isocitrate dehydrogenase 1 and 2 wildtype (IDH1/2wt) diffuse lower-grade gliomas (LGGs) of WHO grades II and III as diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV (IDH1/2wt astrocytomas WHO IV) if they present with (i) a telomerase reverse transcriptase promoter (pTERT) mutation (mt), and/or (ii) gain of chromosome 7 combined with loss of chromosome 10 (7+/10−), and/or (iii) epidermal growth factor receptor (EGFR) amplification (amp).1–5 Although this classification defines the diagnostic molecular criteria for IDH1/2wt astrocytomas WHO IV, the data on the clinical characteristics and survival of these tumors are still very limited. Firstly, it is not clear whether the prognosis of patients presenting with IDH1/2wt astrocytomas WHO IV with classical radiological characteristics of LGGs (ie, absence of ring-like contrast enhancement with central necrosis) is similar to the prognosis of IDH1/2wt glioblastoma patients. Secondly, pTERTmt IDH1/2wt astrocytomas without 7+/10− or EGFRamp (pTERTmt only) are now also assigned IDH1/2wt astrocytomas WHO IV, but it is unclear whether their prognosis is indeed similar to the other IDH1/2wt astrocytomas WHO IV. Rare cases have been described of more benign types of IDH1/2wt astrocytomas harboring a pTERT mutation.4,6 The main objective of this retrospective study is to evaluate the clinical presentation and survival outcome of a cohort of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV according to the cIMPACT-NOW criteria presenting with MRI characteristics of an LGG, and specifically the outcome of cases with pTERT mutations only.
Materials and Methods
Patient Population
In this retrospective multicenter cohort study, adult (≥18 y) patients with a newly diagnosed predominantly supratentorial IDH1/2wt LGG (WHO grade II or III) were identified from the Erasmus Medical Center Cancer Institute, the Haaglanden Medical Center, and the Leiden University Medical Center pathology databases as well as from the previously published dataset of Erasmus MC patients from Wijnenga et al.7 Histopathological diagnoses were determined by local dedicated neuropathologists. Patients were included if (i) IDH1/2 mutation status, the copy number status of chromosome 7 and chromosome 10, and the amplification status of EGFR had been assessed with a glioma tailored next-generation sequencing (NGS) panel, and (ii) MRI scans at the time of diagnostic surgery were available for review.8 Patients with a histological diagnosis of an LGG but presenting with lesions suggestive of glioblastoma (ring-like contrast enhancement with evidence of central necrosis on the MRI at the time of histological diagnosis) were excluded (Fig. 1A). An historical cohort from the Erasmus MC of IDH1/2wt glioblastoma patients diagnosed with the NGS panel in a routine diagnostic setting between 2013 and 2019 was used to compare overall survival (OS).8 The design of the study was approved by the institutional review boards of the participating centers and was conducted according to national and local regulations.
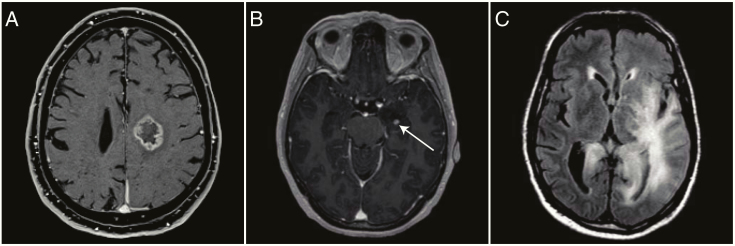
Fig. 1.
MRIs made at the time of histological diagnosis of 3 different IDH1/2wt LGG patients. (A) Ring-like contrast enhancement on cT1w imaging, suggestive of glioblastoma; the patient was excluded from further analysis. (B) Minor contrast enhancement on cT1w imaging, not suggestive of glioblastoma; the patient was included for further analysis. (C) Typical gliomatosis cerebri on FLAIR imaging; a confluent hyperintense abnormality in at least 3 separate brain lobes.
Baseline Tumor Characteristics and Additional Molecular Analysis
For glioma targeted NGS, patient tumor material was cut into 5 µm formalin-fixed paraffin-embedded slices and selected for regions with the highest tumor cell percentage as defined by the local neuropathologists. DNA was isolated using 5% Chelex 100 resin (Bio-Rad) and proteinase K digestion. NGS with a targeted neuro-oncology panel and single nucleotide polymorphism–based loss of heterozygosity analysis were performed as previously described.9 If pTERT status was not covered by the NGS panel, a SNaPshot assay was performed for the 2 hotspot mutations in gliomas (C228T and C250T) as previously described.10
Clinical Characteristics
The collected baseline clinical characteristics included sex, age at diagnosis, Karnofsky performance status (KPS) before and 3 months after surgery, date and symptom of onset, surgical procedure (biopsy or resection), histopathological diagnosis, and primary treatment after surgery. OS was measured from the date of the diagnostic MRI scan until death or was censored at the date of last follow-up.
Radiological Characteristics
Radiological data were taken from T2-weighted (T2w) images, T2-weighted fluid-attenuated inversion recovery (FLAIR) images, and T1-weighted images before and after intravenous contrast administration (cT1w). Baseline radiological characteristics were assessed using the MRI made before diagnostic surgery and included tumor location (hemisphere, lobe[s], basal ganglia, thalamus, brainstem, cerebellum), growth pattern (gliomatosis cerebri, multifocal), and presence and pattern of contrast enhancement (patchy, ring-like, nodular). The MRI scans were reviewed by the first author (C.M.S.T.), and scans with more than minor contrast enhancement (patchy, nodular) were also reviewed by the last author (M.J.v.d.B.). The radiological diagnosis gliomatosis cerebri was defined as a confluent hyperintense FLAIR or T2w abnormality in at least 3 separate brain lobes (Fig. 1C).
Statistical Analysis
OS was estimated using the Kaplan–Meier method and curves were compared using the log-rank test. Categorical variables were compared using Fisher’s exact test. Continuous numeric variables were compared using the Mann–Whitney U-test and the Kruskal–Wallis test. The Cox proportional hazards model was used for univariable and multivariable analysis. All factors for univariable analysis were included in the multivariable analysis based on known prognostic effect from previous literature.11–15 All P-values below 0.05 were considered to be statistically significant. Statistical analysis was performed using R (v3.6.0) and RStudio (v1.0.153).
Results
Cohort Distribution
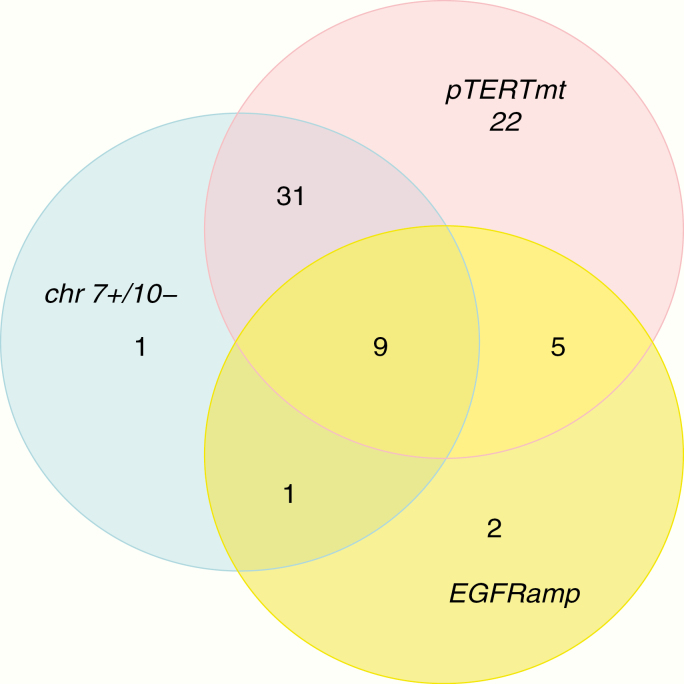
A set of 126 IDH1/2wt LGG patients as confirmed by NGS analysis was identified from the 3 participating centers and assessed for eligibility. The patients were in part identified from a previous study on LGGs and in part during routine diagnostics.5,7,8 Thirty-nine of these 126 patients were excluded: 3 patients with solely infratentorial lesions, 2 patients with insufficient NGS data, and 34 patients with ring-like contrast enhancement on MRI at the time of histological diagnosis. In 29 IDH1/2wt LGG patients, minor enhancement was present which was fully compatible with a grade II or III histology and these cases were included in this series. The included 87 patients were reclassified into 71 patients with molecular features of glioblastoma (IDH1/2wt astrocytomas WHO IV) and 16 patients without molecular features of glioblastoma (IDH1/2wt astrocytomas WHO II and III). Reclassification into IDH1/2wt astrocytomas WHO IV was based on the presence of a pTERT mutation in 67 patients, EGFR amplification in 17 patients, and the combined 7+/10− signature in 42 patients (Fig. 2). In 22 of 67 pTERTmt patients the diagnosis of IDH1/2wt astrocytomas WHO IV was based solely on the pTERT mutation. A reference set containing 197 IDH1/2wt glioblastomas was identified from the Erasmus MC. All IDH1/2wt glioma patients were operated upon between October 2002 and May 2019.
Fig. 2.
A Venn diagram of the number of patients with TERT promoter mutations, EGFR amplification, and the signature of 7+/10− within our cohort of IDH1/2wt LGGs.
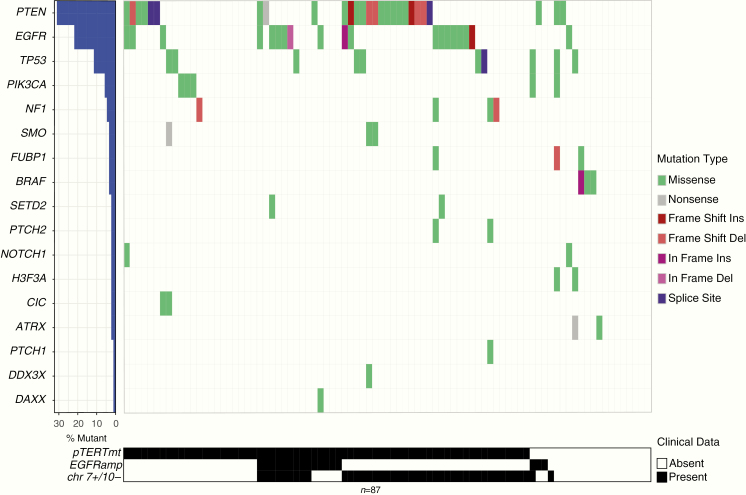
The molecular features of our cohort were consistent with those frequently found in astrocytic tumors (Fig. 3). Mutations in ATRX, BRAF, and H3F3A were only observed in IDH1/2wt astrocytomas WHO II and III (12.5%, 18.8%, and 12.5%, respectively). Mutations in EGFR and PTEN were frequently observed in IDH1/2wt astrocytomas WHO IV (25.4% and 35.2%, respectively), but mutations in these genes were also sporadically seen in IDH1/2wt astrocytomas WHO II and III (6.3% and 12.5%, respectively). Three patients with a BRAF mutation were identified. Of the 2 patients with an H3F3A mutation, 1 had the H3F3A K27M mutation and 1 had the H3F3A G34R mutation.
Fig. 3.
A waterfall plot of the mutations as picked up by the glioma-specific NGS panel within the IDH1/2wt LGGs.
Baseline Clinical and Radiological Characteristics
IDH1/2wt astrocytomas WHO IV patients had a higher age of onset than those with IDH1/2wt astrocytomas WHO II and III and IDH1/2wt glioblastomas (58 y vs 45 y and 55 y, respectively; P = 0.006). IDH1/2wt astrocytoma WHO IV patients presented more often with epilepsy than the IDH1/2wt glioblastoma patients (64.8% vs 27.4%, P < 0.001). IDH1/2wt glioblastoma patients were operated upon sooner after the presenting symptom than IDH1/2wt astrocytomas WHO IV patients (first symptom to first surgery: 1.1 mo vs 2.9 mo, P < 0.001; diagnostic scan to first surgery: 0.5 mo vs 1.3 mo, P < 0.001). A biopsy was performed more frequently in the IDH1/2wt astrocytomas WHO IV compared with the IDH1/2wt astrocytomas WHO II and II and the IDH1/2wt glioblastomas (83.1% vs 56.2% and 16.8%, respectively; P < 0.001). After the diagnostic surgery, IDH1/2wt glioblastomas were commonly treated with chemoradiation, while less than half of the IDH1/2wt astrocytomas WHO IV received both chemotherapy and radiotherapy (chemoradiation: 89.8% vs 42.3%, P < 0.001). Other clinical characteristics of the IDH1/2wt gliomas are summarized in Table 1.
Table 1.
The clinical information of all IDH1/2wt gliomas as confirmed by a glioma specific NGS panel for diagnostic purposes*
| Characteristics | IDH1/2wt Astrocytomas WHO IV | IDH1/2wt Astrocytomas WHO II and III | P a | IDH1/2wt Glioblastomas | P b | IDH1/2wt Astrocytomas WHO IV, pTERTmt only | P c | All Patients | P d |
|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 71 | 16 | 197 | 22 | 284 | ||||
| Sex, n (%) | 0.259 | 0.767 | 1.000 | 0.311 | |||||
| Female | 24 (33.8) | 8 (50) | 62 (31.5) | 7 (31.8) | 94 (33.1) | ||||
| Male | 47 (66.2) | 8 (50) | 135 (68.5) | 15 (68.2) | 190 (66.9) | ||||
| Age, y | 0.012 | 0.010 | 0.394 | 0.006 | |||||
| Median | 58 | 45 | 55 | 62 | 56 | ||||
| Range | 19–78 | 21–69 | 18–84 | 19–78 | 18–84 | ||||
| Age groups, n (%) | |||||||||
| <40 y | 6 (8.5) | 5 (31.3) | 19 (9.6) | 2 (9.1) | 30 (10.6) | ||||
| 40–60 y | 32 (45.1) | 6 (37.5) | 117 (59.4) | 8 (36.4) | 155 (54.6) | ||||
| >60 y | 33 (46.5) | 5 (31.3) | 61 (31.0) | 12 (54.5) | 99 (34.9) | ||||
| Follow-up, y | 0.185 | 0.857 | 0.252 | 0.331 | |||||
| Median | 1.4 | 2.9 | 1.5 | 1.2 | 1.5 | ||||
| Interquartile range (IQR) | 0.8–2.5 | 0.8–4.1 | 1–2.2 | 0.7–2.3 | 0.9–2.4 | ||||
| First symptom to surgery, mo | 0.081 | <0.001 | 0.476 | <0.001 | |||||
| Median | 2.9 | 8 | 1.1 | 2.2 | 1.5 | ||||
| IQR | 1.5–5.8 | 1.8–13.9 | 0.6–2.3 | 1.3–5.0 | 0.8–3.4 | ||||
| First scan to surgery, mo | 0.956 | <0.001 | 0.582 | <0.001 | |||||
| Median | 1.3 | 1.3 | 0.5 | 1.1 | 0.6 | ||||
| IQR | 0.7–3.2 | 0.6–4.9 | 0.1–0.8 | 0.7–2.3 | 0.2–1.3 | ||||
| Histopathology, n (%) | 0.678 | <0.001 | 1.000 | <0.001 | |||||
| Glioma WHO II | 45 (63.4) | 9 (56.3) | 0 (0) | 15 (68.2) | 54 (19.0) | ||||
| Glioma WHO III | 14 (19.7) | 3 (18.8) | 0 (0) | 2 (9.1) | 17 (6.0) | ||||
| Glioblastoma | 0 (0) | 0 (0) | 197 (100) | 0 (0) | 197 (69.4) | ||||
| Glioma NOS | 12 (16.9) | 4 (25) | 0 (0) | 5 (22.7) | 16 (5.6) | ||||
| Symptom of onset, n (%) | 0.102 | <0.001 | 0.359 | <0.001 | |||||
| Epilepsy | 46 (64.8) | 6 (37.5) | 54 (27.4) | 13 (59.1) | 106 (37.3) | ||||
| Incidental finding | 2 (2.8) | 1 (6.3) | 9 (4.6) | 0 (0) | 12 (4.2) | ||||
| Other | 23 (32.4) | 9 (56.3) | 134 (68.0) | 9 (40.9) | 166 (58.5) | ||||
| Surgery modality, n (%) | 0.039 | <0.001 | 0.686 | <0.001 | |||||
| Resection | 12 (16.9) | 7 (43.8) | 164 (83.2) | 2 (9.1) | 183 (64.4) | ||||
| Biopsy | 59 (83.1) | 9 (56.3) | 33 (16.8) | 20 (90.9) | 101 (35.6) | ||||
| Primary treatment, n (%) | 0.094 | <0.001 | 0.724 | <0.001 | |||||
| No further treatment | 9 (12.7) | 6 (37.5) | 9 (4.6) | 4 (18.2) | 24 (8.5) | ||||
| Chemotherapy | 14 (19.7) | 1 (6.3) | 4 (2.0) | 6 (27.3) | 19 (6.7) | ||||
| Radiotherapy | 18 (25.4) | 2 (12.5) | 7 (3.6) | 6 (27.3) | 27 (9.5) | ||||
| Chemoradiation | 30 (42.3) | 7 (43.8) | 177 (89.8) | 6 (27.3) | 214 (75.4) | ||||
| Preoperative KPS | 0.218 | 0.248 | 0.545 | 0.068 | |||||
| Median | 90 | 100 | 90 | 90 | 90 | ||||
| 90–100, n (%) | 50 (70.4) | 14 (87.5) | 122 (61.9) | 14 (63.6) | 186 (65.5) | ||||
| ≤80, n (%) | 21 (29.6) | 2 (12.5) | 75 (38.1) | 8 (36.4) | 98 (34.5) | ||||
| Postoperative KPS | 0.259 | 0.889 | 0.400 | 0.286 | |||||
| Median | 90 | 100 | 90 | 80 | 90 | ||||
| 90–100, n (%) | 39 (55.7) | 12 (75) | 106 (53.8) | 10 (47.6) | 157 (55.5) | ||||
| ≤80, n (%) | 31 (44.3) | 4 (25) | 91 (46.2) | 11 (52.4) | 126 (44.5) |
*The indicated P-values compared a) IDH1/2wt astrocytomas WHO IV vs IDH1/2wt astrocytomas WHO II and III, b) IDH1/2wt astrocytomas WHO IV vs IDH1/2wt glioblastomas, c) IDH1/2wt astrocytomas WHO IV, pTERTmt only vs other IDH1/2wt astrocytomas WHO IV, and d) IDH1/2wt astrocytomas WHO IV vs IDH1/2wt astrocytomas WHO II and III vs IDH1/2wt glioblastomas.
The MRI characteristics at the time of histological diagnosis of 83 IDH1/2wt LGGs are shown in Table 2. Minor nodular or patchy contrast enhancement was present in 29 of the 83 IDH1/2wt LGGs (Fig. 1B). No occipital lesions were identified in IDH1/2wt astrocytomas WHO II and III. Slight infiltration into the brainstem was more frequently observed in IDH1/2wt astrocytomas WHO II and III compared with IDH1/2wt astrocytomas WHO IV (37.5% and 9%, respectively; P = 0.009). No other differences were observed between the IDH1/2wt astrocytomas WHO IV and the IDH1/2wt astrocytomas WHO II and III in location distribution, growth pattern (including gliomatosis cerebri), or presence of subtle contrast enhancement. In addition, no radiological differences were identified between pTERTmt only and other IDH1/2wt astrocytomas WHO IV. Gliomatosis cerebri was present in 35.8% of the IDH1/2wt astrocytomas WHO IV and 18.8% of the IDH1/2wt astrocytomas WHO II and III. The majority of the IDH1/2wt astrocytomas WHO IV were located in the temporal lobe, the insular region, and the parietal lobe. In IDH1/2wt astrocytoma WHO II and III patients more than half of the lesions were observed in the temporal lobe.
Table 2.
Radiological characteristics of IDH1/2wt LGGs as determined on MRI at the time of histological diagnosis*
| Characteristics | IDH1/2wt Astrocytomas WHO IV | IDH1/2wt Astrocytomas WHO II and III | P a | IDH1/2wt Astrocytomas WHO IV, pTERTmt only | P b |
|---|---|---|---|---|---|
| Patients, n | 67 | 16 | 21 | ||
| Hemisphere, n (%) | 0.636 | 0.933 | |||
| Right | 23 (34.3) | 7 (43.8) | 8 (38.1) | ||
| Left | 30 (44.8) | 5 (31.3) | 8 (38.1) | ||
| Bilateral | 14 (20.9) | 4 (25) | 5 (23.8) | ||
| Tumor location, n (%) | |||||
| Frontal lobe | 33 (49.3) | 6 (37.5) | 0.421 | 11 (52.4) | 0.391 |
| Parietal lobe | 34 (50.7) | 5 (31.3) | 0.178 | 13 (61.9) | 0.569 |
| Temporal lobe | 50 (74.6) | 10 (62.5) | 0.360 | 18 (85.7) | 1.000 |
| Occipital lobe | 17 (25.4) | 0 (0) | 0.034 | 8 (38.1) | 0.763 |
| Insula | 39 (58.2) | 6 (37.5) | 0.168 | 15 (71.4) | 0.376 |
| Corpus callosum | 23 (34.3) | 3 (18.8) | 0.368 | 10 (47.6) | 0.568 |
| Basal ganglia | 32 (47.8) | 6 (37.5) | 0.580 | 12 (57.1) | 0.567 |
| Thalamus | 25 (37.3) | 7 (43.8) | 0.776 | 10 (47.6) | 0.775 |
| Brainstem | 6 (9) | 6 (37.5) | 0.009 | 4 (19.0) | 0.223 |
| Cerebellar | 1 (1.5) | 1 (6.3) | 0.350 | 1 (4.8) | 0.420 |
| Growth pattern, n (%) | |||||
| Gliomatosis cerebri | 24 (35.8) | 3 (18.8) | 0.241 | 11 (52.4) | 0.394 |
| Multifocal | 6 (9.0) | 1 (6.3) | 1.000 | 1 (4.8) | 0.636 |
| Minor contrast enhancement, n (%) | |||||
| Present | 21 (31.3) | 8 (50) | 0.146 | 5 (23.8) | 1.000 |
*Only patients with available cT1w imaging and either FLAIR or T2w imaging were incorporated in this table. The indicated P-values compared a) IDH1/2wt astrocytomas WHO IV vs IDH1/2wt astrocytomas WHO II and III, and b) IDH1/2wt astrocytomas WHO IV, pTERTmt only vs other IDH1/2wt astrocytomas WHO IV.
Survival Data of All IDH1/2wt Gliomas
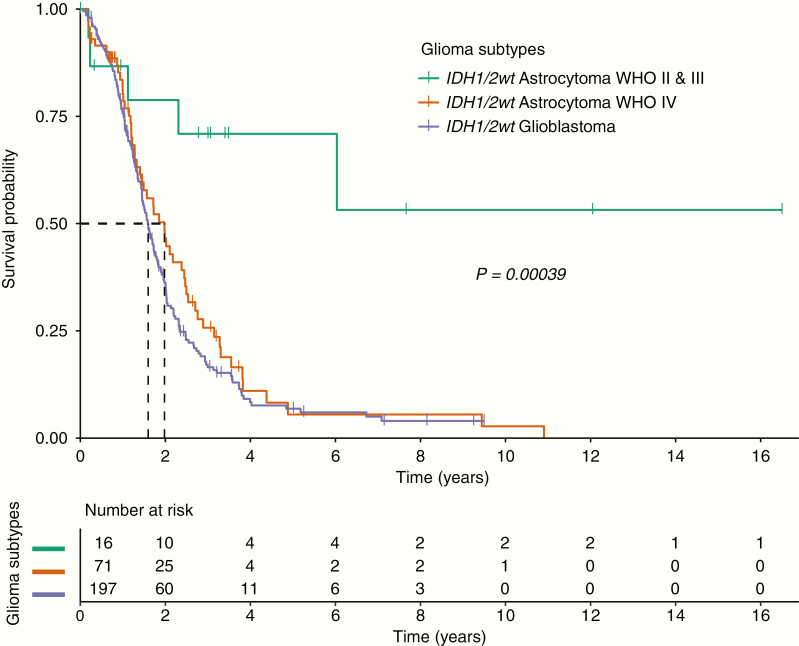
At the time of analysis, 223 of 284 IDH1/2wt glioma patients were deceased: 53 IDH1/2wt astrocytoma WHO IV patients (74.6%), 165 IDH1/2wt glioblastoma patients (83.8%), and 5 IDH1/2wt astrocytoma WHO II and III patients (31.3%). Median follow-up of all IDH1/2wt glioma patients was 1.5 years (IDH1/2wt astrocytomas WHO IV: 1.4 y, IDH1/2wt glioblastomas: 1.5 y, IDH1/2wt astrocytomas WHO II and III: 2.9 y; Table 1). Median OS for the IDH1/2wt astrocytomas WHO IV and the IDH1/2wt glioblastomas was similar (23.8 mo vs 19.2 mo, log-rank test: P = 0.25), but the median OS of the IDH1/2wt astrocytomas WHO II and III was significantly longer compared with the other 2 glioma subtypes (median OS not reached, log-rank test: P < 0.001; Fig. 4).
Fig. 4.
Kaplan–Meier curves of the OS of the IDH1/2wt astrocytomas WHO II and III, the IDH1/2wt astrocytomas WHO IV, and the IDH1/2wt glioblastomas. The dashed line represents the median OS.
Univariable analysis identified lower KPS before surgery as a significant unfavorable prognostic factor for survival (KPS ≤80 vs KPS 90–100: HR 1.54, 95% CI: 1.17–2.02, P = 0.002; Supplementary Table 1). Other prognostic factors from univariable analysis that showed a level of significance <0.10 included sex and age of onset, in which male patients were associated with a worse outcome (male vs female: HR 1.33, 95% CI: 1.00–1.76, P = 0.05) and younger patients were associated with a better prognosis (<40 y vs 40–60 y: HR 0.62, 95% CI: 0.39–0.97; >60 y vs 40–60 y: HR 1.02, 95% CI: 0.77–1.36; P = 0.07). Multivariable analysis taking into account age, sex, KPS before surgery, and the type of first surgery confirmed the similar survival of IDH1/2wt astrocytomas WHO IV and IDH1/2wt glioblastomas (HR 1.27, 95% CI: 0.85–1.88, P = 0.242) and the better survival in IDH1/2wt astrocytomas WHO II and III compared with IDH1/2wt astrocytomas WHO IV (HR 0.30, 95% CI: 0.12–0.78, P = 0.013; Supplementary Fig. 1). Adding primary treatment after surgery to the multivariable analysis led to similar results, although power of the analysis was reduced in comparison to the previously mentioned model (Supplementary Fig. 2).
The survival of the IDH1/2wt astrocytomas WHO IV, pTERTmt only was similar to the survival of the IDH1/2wt glioblastomas (median OS: 14.4 mo vs 19.2 mo, log-rank test: P = 0.89; Supplementary Fig. 3). At the time of analysis, 18 pTERTmt only patients (81.8%) were deceased, and median follow-up of the pTERTmt only patients was 1.2 years. Univariable analysis identified both sex and KPS before surgery as significant prognostic factors, in which male patients and patients with a KPS below 80 had a shorter OS (male vs female: HR 1.40, 95% CI: 1.02–1.92, P = 0.04; KPS ≤80 vs KPS 90–100: HR 1.40, 95% CI: 1.04–1.89, P = 0.03; Supplementary Table 2). The similar survival in IDH1/2wt astrocytomas WHO IV, pTERTmt only and IDH1/2wt glioblastomas remained after correction for confounding factors in multivariable analysis (IDH1/2wt glioblastomas vs IDH1/2wt astrocytomas WHO IV, pTERTmt only: HR 1.15, 95% CI: 0.64–2.10, P = 0.641; Supplementary Fig. 4).
In 3 pTERTmt only patients EGFR mutations were identified (A289D, A289V, and P596L). It could be argued that these 3 tumors should not be classified as pTERTmt only IDH1/2wt astrocytomas WHO IV. However, even without these 3 samples, survival of pTERTmt only patients was similar to IDH1/2wt glioblastomas (median OS: 14.4 mo vs 19.2 mo, log-rank test: P = 0.94; Supplementary Fig. 5).
Discussion
Diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma WHO grade IV represent a new glioma subtype proposed by the cIMPACT-NOW committee. The diagnostic criteria require testing for pTERT mutation status, for chromosome copy alterations of chromosome 7 and 10, and for EGFR amplification, all of which can be routinely performed. This study’s confirmation of poor survival in IDH1/2wt LGG patients (with clinical, radiological, and histological characteristics meeting the classical criteria for grade II or III glioma) in the presence of these molecular markers supports a reclassification in the next official WHO classification of IDH1/2wt astrocytomas WHO IV to IDH1/2wt glioblastomas. Moreover, patients with IDH1/2wt astrocytomas with only pTERT mutations had a similar poor outcome.
In this cohort, we did not find a BRAF mutation in the IDH1/2wt astrocytomas WHO IV group and therefore also no BRAF mutations in the pTERTmt only group. This is important because BRAF mutations are also found in pleomorphic xanthoastrocytomas and these tumors may have pTERT mutations and a markedly improved survival compared with other glioma subtypes.4,6
Of note, the OS of IDH1/2wt glioblastoma patients in our dataset may appear markedly longer than historical cohorts (19.2 mo vs 14–16 mo in large studies).13,16-18 However, the longer survival is largely explained by the measurement of OS from first diagnostic scan until death, whereas most cohorts measure from date of randomization, which typically adds 2–3 months. In addition, our dataset contained a relatively young population of IDH1/2wt glioblastoma patients and age is a well-known prognostic factor for poor survival in gliomas.11–15 Until recently routine NGS was only performed in younger glioblastoma patients. However, after correction for age in the multivariable analysis the OS of the IDH1/2wt astrocytomas WHO IV and the IDH1/2wt glioblastomas remained similar.
The most important limitation of our study is its retrospective design. This design made it impossible to control for the treatment regimens after surgery, which may have impacted on survival. Due to the non-glioblastoma radiological and histological diagnosis, most IDH1/2wt astrocytomas WHO IV were treated less intensively and this may have adversely affected outcome. Moreover, our study was restricted to patients in whom IDH1/2 status was assessed with a glioma dedicated NGS panel. This was reflected in a younger IDH1/2wt glioblastoma cohort as mentioned earlier. Finally, we used the original clinical diagnosis without review, as this was the way the patients were initially diagnosed.
In conclusion, this study has shown similar survival of patients with IDH1/2wt astrocytomas WHO IV and IDH1/2wt glioblastomas. Furthermore, this similar survival is also present in IDH1/2wt astrocytoma patients with pTERTmt only. Our data therefore support the classification of IDH1/2wt astrocytomas WHO IV with the IDH1/2wt glioblastomas, without further distinctions. Further prospective studies should try to understand why these tumors do not show the classical radiological and histopathological characteristics of glioblastoma.
Supplementary Material
Conflict of interest statement. H.J.D. is a member of the advisory board of Abbvie. M.S. has received a speaker honorarium from GE Healthcare and financial compensation for independent review for Parexel Ltd. P.J.F. is a member of the advisory board of Abbvie. M.J.v.d.B. has received speaker honoraria from Agios, Celgene, Boehringer, BMS, Carthera, Bayer, and Abbvie. There are no other conflicts of interest.
Authorship statement. Study conception and design: C.M.S.T., L.D., M.M.J.W., J.A.F.K., A.J.P.E.V., H.J.D., P.N.A., J.M.K., S.G.v.D., M.S., M.J.B.T., P.J.F., M.J.v.d.B. Material preparation, data collection, and analysis: C.M.S.T., L.D., M.M.J.W., J.A.F.K., H.J.D., P.N.A., J.M.K., S.G.v.D., P.J.F., M.J.v.d.B. Statistical analysis: C.M.S.T., P.J.F., M.J.v.d.B. Writing and revision of manuscript: C.M.S.T., L.D., M.M.J.W., J.A.F.K., A.J.P.E.V., H.J.D., P.N.A., J.M.K., S.G.v.D., M.S., M.J.B.T., P.J.F., M.J.v.d.B.
Funding
None.
References
- 1. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stichel D, Ebrahimi A, Reuss D, et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136(5):793–803. [DOI] [PubMed] [Google Scholar]
- 5. Wijnenga MMJ, Dubbink HJ, French PJ, et al. Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol. 2017;134(6):957–959. [DOI] [PubMed] [Google Scholar]
- 6. Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126(6):907–915. [DOI] [PubMed] [Google Scholar]
- 7. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Synhaeve NE, van den Bent MJ, French PJ, et al. Clinical evaluation of a dedicated next generation sequencing panel for routine glioma diagnostics. Acta Neuropathol Commun. 2018;6(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubbink HJ, Atmodimedjo PN, van Marion R, et al. Diagnostic detection of allelic losses and imbalances by next-generation sequencing: 1p/19q co-deletion analysis of gliomas. J Mol Diagn. 2016;18(5):775–786. [DOI] [PubMed] [Google Scholar]
- 10. Koopmans AE, Ober K, Dubbink HJ, et al. ; Rotterdam Ocular Melanoma Study Group Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2014;55(9):6024–6030. [DOI] [PubMed] [Google Scholar]
- 11. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 14. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 16. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.