Abstract
DNA vaccines expressing codon-optimized Venezuelan equine encephalitis virus (VEEV) and Ebola virus (EBOV) glycoprotein genes provide protective immunity to mice and nonhuman primates when delivered by intramuscular (IM) electroporation (EP). To achieve equivalent protective efficacy in the absence of EP, we evaluated VEEV and EBOV DNA vaccines constructed using minimalized Nanoplasmid expression vectors that are smaller than conventional plasmids used for DNA vaccination. These vectors may also be designed to co-express type I interferon inducing innate immune agonist genes that have an adjuvant effect. Nanoplasmid vaccinated mice had increased antibody responses as compared to those receiving our conventional pWRG7077-based vaccines when delivered by IM injection, and these responses were further enhanced by the inclusion of the innate immune agonist genes. The Nanoplasmid VEEV DNA vaccines also significantly increased protection against aerosol VEEV challenge as compared to the pWRG7077 VEEV DNA vaccine. Although all mice receiving the pWRG7077 and Nanoplasmid EBOV DNA vaccines at the dose tested survived EBOV challenge, only mice receiving the Nanoplasmid EBOV DNA vaccine that co-expresses the innate immune agonist genes failed to lose weight after challenge. Our results suggest that Nanoplasmid vectors can improve the immunogenicity and protective efficacy of alphavirus and filovirus DNA vaccines.
Keywords: DNA Vaccine, Nanoplasmid, Genetic Adjuvant, Innate Immune Agonist, RIG-I, CpG, Immunostimulatory RNA, Ebola virus, Venezuelan equine encephalitis virus
Graphical Abstract
Nanoplasmid DNA vaccine vectors can improve the protective efficacy of DNA vaccines in the absence of electroporation. Here we show that intramuscular injection of Nanoplasmid vectors expressing the glycoprotein genes of Venezuelan equine encephalitis virus (VEEV) and Ebola virus (EBOV) protect against lethal viral challenge in mice.
Introduction
Despite their ability to trigger potent innate and adaptive immune responses, the widespread use of DNA vaccines has been hampered logistically by the requirement of specialized methods such as electroporation (EP) for effective delivery.1, 2, 3, 4, 5 Delivery methods that require specialized training, advanced technology, or access to a sustainable power source pose significant challenges in remote areas or when enacting a ring vaccination strategy in an outbreak setting. A simpler approach for DNA vaccine delivery that can provide protective efficacy comparable to EP could be advantageous for the broader applicability of this platform. One possible approach for improving DNA vaccine immunogenicity is to modify the DNA vaccine backbone itself. This can include modification of the promoter to enhance antigen expression, removal of antibiotic selection markers, and elimination of nonfunctional sequences that can limit expression. Nature Technology Corporation (NTC) has tested this approach by developing minimalized Nanoplasmid expression vectors.6,7 These vectors are smaller than traditional DNA vaccine plasmids, allowing for improved uptake and persistence in transfected cells. Large extragenic regions of bacterial DNA can also mediate transgene silencing in certain tissues, a phenomenon that may be avoided by the use of shorter DNA sequences.8 This subsequently leads to increased transgene expression that can yield enhanced immune responses and sustained improvements in immunological memory.9 NTC has also designed Nanoplasmid vectors that co-express innate immune immunostimulatory RNA (isRNA) agonists to function as type I interferon-αβ (IFN-αβ)-inducing genetic adjuvants. These vectors co-express a retinoic acid-inducible gene I (RIG-I) double-stranded RNA (dsRNA) agonist (NTC-eRNA) or the dsRNA RIG-I agonist as well as a Toll-like receptor 9 (TLR9) stimulating CpG motif (NTC-eRNA-CpG). IFN-αβ is a powerful genetic adjuvant, enhancing both cellular and humoral responses.10, 11, 12, 13 NTC previously tested an influenza H5N1 hemagglutinin (HA) vaccine expressed from the NTC-eRNA vector.14 Vaccination with the NTC-eRNA/HA vaccine significantly improved antibody titers and antibody binding avidity compared to the standard NTC/HA vector. Additionally, as IFN-αβ is required for optimal DNA vaccine immunogenicity,1,15,16 inclusion of an IFN-αβ stimulating agonist may compensate for any reduction in the immunogenicity of a DNA vaccine delivered in the absence of EP.
Our laboratory has developed and evaluated DNA vaccines for several highly pathogenic biodefense-related targets, including the alphavirus Venezuelan equine encephalitis virus (VEEV). Natural mosquito-borne transmission of VEEV to humans can result in severe neurological disease, but aerosol VEEV infection may yield increased morbidity and mortality compared to that observed with natural infection.17, 18, 19, 20 We previously demonstrated that a candidate VEEV DNA vaccine (pWRG/VEEV) expressing codon-optimized E2 and E1 envelope glycoprotein (GP) genes delivered by intramuscular (IM)-EP elicits robust VEEV-specific immune responses and protects mice and nonhuman primates (NHPs) against aerosol VEEV challenge.21,22 Subsequently, the pWRG/VEEV vaccine candidate proved to be safe and highly immunogenic in a Phase 1 clinical study, with all subjects developing VEEV-neutralizing antibodies following IM-EP delivery.23
We have also previously developed and tested a candidate Zaire ebolavirus (EBOV) GP DNA vaccine (pWRG/EBOV). When delivered by IM-EP, this DNA vaccine elicited protective immunity against IM EBOV challenge in mice and NHPs.24,25 pWRG/EBOV-vaccinated NHPs developed pre-challenge EBOV-neutralizing antibodies, as well as high numbers of EBOV-specific T cells.25 Our data suggest that DNA vaccination may be an effective means of eliciting protective immunity against filovirus infection, as both cell-mediated and humoral immune responses are likely required for protection against EBOV challenge.26, 27, 28, 29, 30, 31, 32, 33
Here, we explored the potential benefit of Nanoplasmid vectors engineered to express the codon-optimized VEEV and EBOV GP genes without and with co-expression of the innate immune agonists. Specifically, we evaluated the immune responses and protective efficacy elicited by each of these vaccine candidates following IM injection in a murine model. Our results suggest a potential path forward for VEEV and EBOV Nanoplasmid DNA vaccines delivered by IM injection in the absence of EP.
Results
Nanoplasmid Vectors Exhibit Increased In Vitro Antigen Production Compared to pWRG7077 Vectors
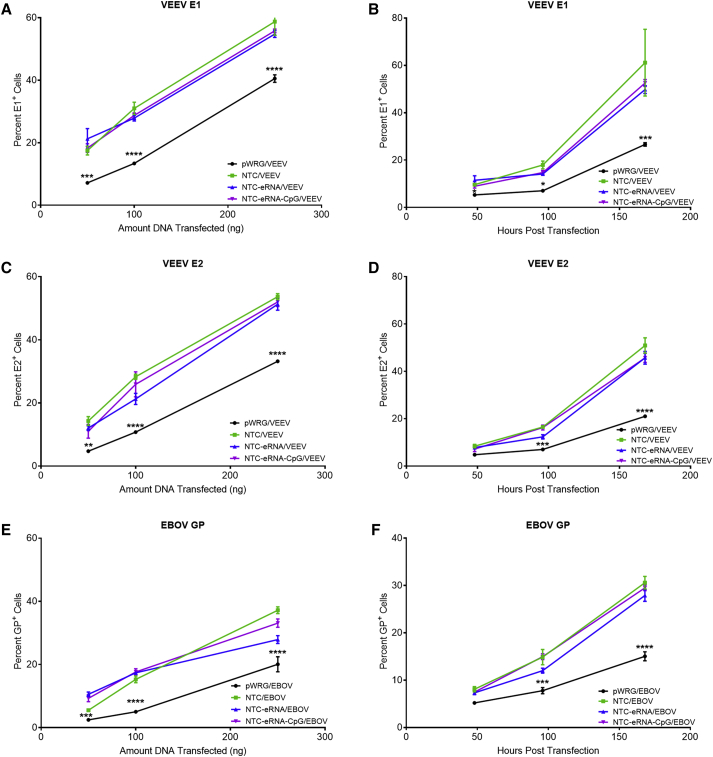
Previous reports suggest that Nanoplasmid vectors improve expression levels and duration of expression compared to conventional plasmids used for DNA vaccination.7 To examine this in the context of the VEEV and EBOV Nanoplasmid constructs, we compared transient in vitro antigen expression from the various Nanoplasmid vectors to that of our standard pWRG7077 vector. For this, COS-7 cells were transfected with 50, 100, or 250 ng of the individual Nanoplasmid constructs or our standard pWRG7077 vaccine plasmids, and the cells were harvested 48 h after transfection for analysis of antigen expression levels by flow cytometry. At all DNA concentrations tested, transfection with the various Nanoplasmid constructs resulted in a significantly increased percentage of VEEV E1+, VEEV E2+, and EBOV GP+ cells compared to pWRG7077 transfected cells (Figures 1A, 1C, and 1E). To determine whether the increases in antigen expression observed for the Nanoplasmid constructs persist over a longer period of time, we harvested COS-7 cells transfected with 50 ng of the individual Nanoplasmid constructs or our standard pWRG7077 vaccine plasmids at various time points for a period of 7 days after transfection for analysis of antigen expression levels by flow cytometry. In these experiments, significantly increased percentages of VEEV E1+, VEEV E2+, and EBOV GP+ cells were observed for the Nanoplasmid constructs as compared to the pWRG7077-based constructs up to 7 days post-transfection (Figures 1B, 1D, and 1E). Representative histogram plots of VEEV E1 expression are shown in Figure S1.
Figure 1.
Transfection with Nanoplasmid Vectors Improves Antigen Expression
COS-7 cells transfected with 50, 100, or 250 ng of the pWRG7077 or various Nanoplasmid DNA vaccine plasmids were harvested 48 h post transfection, and the number of cells positive for surface expression of (A) VEEV E1, (C) VEEV E2 , or (E) EBOV GP were quantitated by flow cytometric analysis. Additional cell cultures were transfected with 50 ng of the pWRG7077 or various Nanoplasmid DNA vaccine plasmids and harvested at the indicated time points. The cells positive for surface expression of (B) VEEV E1, (D) VEEV E2, or (F) EBOV GP were quantitated by flow cytometric analysis. Data are presented as mean averages ± SEM from two independent experiments with samples from each time point performed in triplicate. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. p values were determined by two-way ANOVA with Dunnett’s multiple comparison test.
Nanoplasmid VEEV Vectors Improve Humoral, but Not Cellular, Immune Responses in Mice
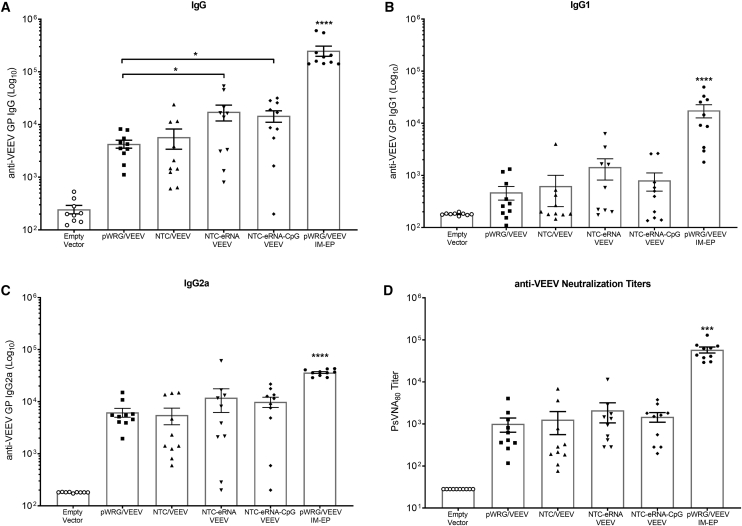
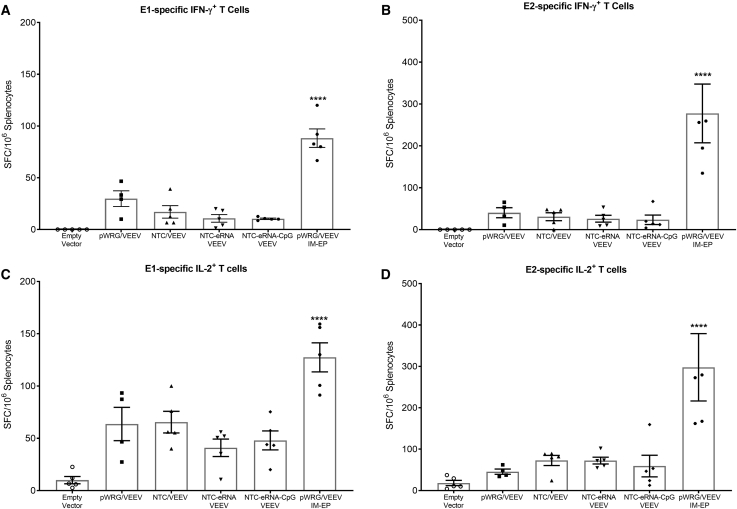
To evaluate the potential immunological benefit of the Nanoplasmid VEEV constructs, we vaccinated groups of 15 BALB/c mice two times, 3 weeks apart, by IM injection of either the pWRG/VEEV DNA vaccine or the various Nanoplasmid VEEV DNA vaccines as described in the Materials and Methods. Vaccination with the NTC/VEEV construct resulted in slightly improved, but statistically similar, total immunoglobulin G (IgG) anti-VEEV antibody levels compared to those observed in mice receiving the pWRG/VEEV vaccine (Figure 2A). In contrast, the NTC/VEEV construct with the added isRNA RIG-I agonist yielded a significant increase in total IgG titers. However, additional inclusion of the CpG motif within this construct did not further boost the total IgG response compared to the NTC-eRNA/VEEV vaccine. Although not statistically significant, vaccination with the NTC-eRNA/VEEV or NTC-eRNA-CpG/VEEV constructs also resulted in IgG1 and IgG2a subtype antibody titers that trended slightly higher than those for the pWRG/VEEV or standard NTC/VEEV constructs (Figures 2B and 2C). Moreover, vaccination with both isRNA-expressing Nanoplasmids trended toward slightly enhanced VEEV-neutralizing antibody generation, suggesting a broad improvement in the humoral response (Figure 2D). Unlike for the antibody response, we observed little discernable difference in the cell-mediated immunity elicited in vaccinated mice, as those receiving either the pWRG/VEEV or various Nanoplasmid VEEV DNA vaccines displayed similar numbers of VEEV E1- or E2-specific IFN-γ+ (Figures 3A and 3B) and interleukin-2+ (IL-2+) T cells (Figures 3C and 3D) as quantified by ELISPOT. Of note, no groups vaccinated with any of the VEEV DNA vaccines delivered by IM injection developed humoral or cell-mediated immune responses equivalent to those observed for the pWRG/VEEV IM-EP control group.
Figure 2.
isRNA Expressing Nanoplasmids Significantly Improve the Humoral Response Elicited by VEEV GP DNA Vaccination
Groups of female BALB/c mice were vaccinated with 5 μg of pWRG/VEEV or the various VEEV Nanoplasmids on days 0 and 21 by IM injection. VEEV GP-specific (A) total IgG antibody titers and (B) IgG1 and (C) IgG2a subtype titers were quantified by ELISA in sera collected 21 days after the second vaccination. (D) PsVNA80 neutralization titers of vaccinated mice were also determined using these sera samples. Data represent the group mean averages ± SEM. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. p values were determined by one-way ANOVA with Tukey’s post hoc test.
Figure 3.
VEEV Nanoplasmid Vaccination Does Not Increase T Cell Responses following IM Delivery
Groups of female BALB/c mice were vaccinated with 5 μg of pWRG/VEEV or the various VEEV Nanoplasmids on days 0 and 21 by IM injection and then euthanized on day 28 for isolation of splenocytes. Splenocytes were stimulated with pools of 15-mer, overlapping peptides spanning the VEEV E1 or E2 proteins, and VEEV E1- or E2-specific (A and B) IFN-γ+ T cells and (C and D) IL-2+ T cells were quantified via ELISPOT. Data represent the group mean averages ± SEM. ∗∗∗∗p < 0.0001. p values were determined by one-way ANOVA with Tukey’s post hoc test.
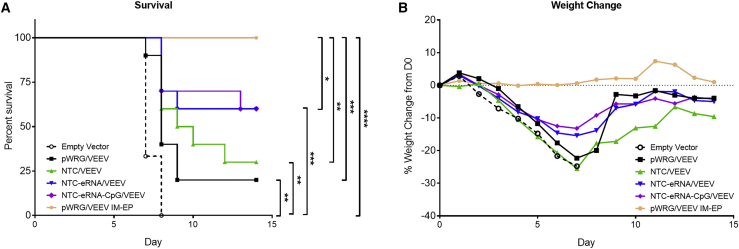
IM Vaccination with Nanoplasmid VEEV Vectors Partially Protects Mice against Aerosol VEEV Challenge
We next evaluated the protective efficacy of the pWRG/VEEV and Nanoplasmid VEEV DNA vaccines administered by IM delivery. 4 weeks after the second and final vaccination, the vaccinated mice were challenged with 104 plaque-forming units (PFU; ∼10,000 median lethal doses [LD50]) of VEEV via the aerosol route. As expected, all mice receiving the empty vector control exhibited clinical signs of disease to include weight loss, ruffled fur, and inactivity, and all succumbed to disease or were euthanized in accordance with early endpoint criteria by day 8 post-infection (Figures 4A and 4B). Mice vaccinated with the pWRG/VEEV and NTC/VEEV constructs exhibited 20% and 30% survival, respectively, following viral challenge, which represented a significant improvement relative to that of the empty vector control mice. However, both the pWRG/VEEV and NTC/VEEV groups exhibited similar levels of weight loss to that of the negative control mice following challenge. Protection was further enhanced in mice receiving either the NTC-eRNA/VEEV or NTC-eRNA-CpG/VEEV DNA vaccine, with a 60% survival rate observed for these groups. In addition, mice in these groups exhibited less post-challenge weight loss compared to the other IM vaccination groups. As observed previously, all mice in the pWRG7077/VEEV IM-EP control displayed no clinical signs of disease post-challenge and all survived. The protection observed for this group was also significantly higher than that of the IM vaccination groups.
Figure 4.
Vaccination with VEEV Nanoplasmids Expressing isRNA Improves DNA Vaccine Protective Efficacy against Aerosol VEEV Challenge
Vaccinated mice were challenged with 104 PFU of VEEV by the aerosol route. (A) Survival and (B) weight loss of mice receiving pWRG/VEEV or the various VEEV Nanoplasmids following VEEV challenge are shown. Weight data represent the percent change in the collective weight of all mice per group relative to the day 0 pre-challenge weight as measured daily. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. p values were determined by log rank test.
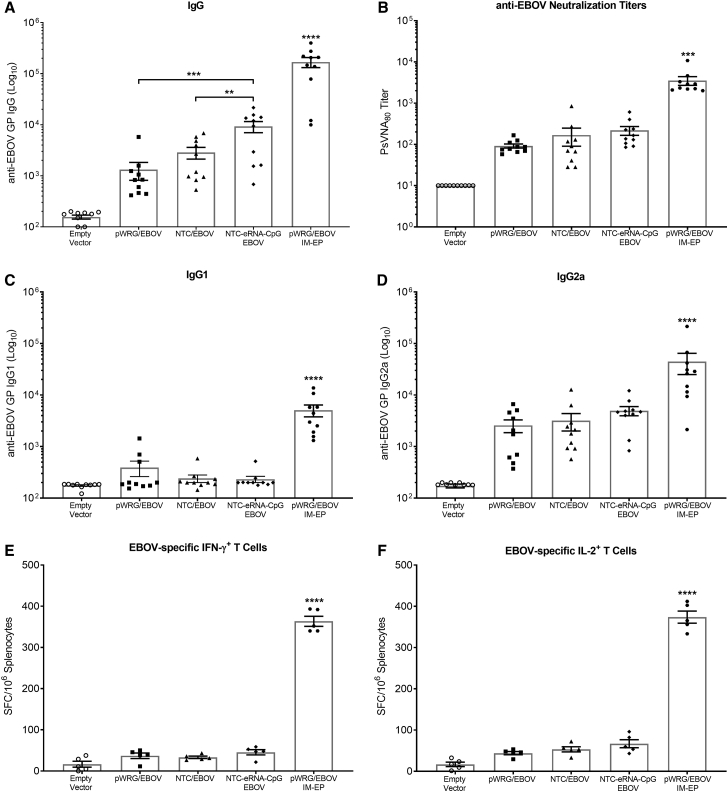
Nanoplasmid Vectors Improve EBOV DNA Vaccine Immunogenicity and Provide Protection from EBOV Challenge in Mice
Since vaccination with the Nanoplasmid vectors improved the protective efficacy of our VEEV vaccine in the context of IM delivery in the absence of EP, we expanded our studies to evaluate the benefit of the Nanoplasmid vectors for vaccination against EBOV. Because we did not observe a significant difference in immunogenicity or protective efficacy between the NTC-eRNA/VEEV and NTC-eRNA-CpG/VEEV vaccines, we chose to only include the NTC-eRNA-CpG/EBOV vaccine in this study. We vaccinated groups of 15 BALB/c mice two times, 3 weeks apart, by IM injection of the pWRG/EBOV, NTC/EBOV, or NTC-eRNA-CpG/EBOV vaccines as described in the Materials and Methods. As observed in the VEEV study, vaccination with NTC/EBOV resulted in slightly improved but statistically similar total IgG anti-EBOV antibody levels compared to those of mice receiving the pWRG/EBOV vaccine (Figure 5A). Also mirroring the VEEV results, the addition of the innate immune stimulating eRNA-CpG motifs to the NTC/EBOV DNA vaccine significantly improved the total IgG response. We also observed slight increases in EBOV-neutralizing antibody titers for both Nanoplasmid vaccines and IgG2a antibody production for the NTC-eRNA-CpG/EBOV group, although not to statistically significant levels (Figures 5B–5D). Vaccination with the EBOV Nanoplasmids also did not improve EBOV-specific IFN-γ+ and IL-2+ T cell responses as quantified by ELISPOT (Figures 5E and 5F). As for VEEV, no groups vaccinated by IM injection approached the immunogenicity levels measured for the pWRG/EBOV IM-EP control group.
Figure 5.
Vaccination with EBOV Nanoplasmids Enhances DNA Vaccine Immunogenicity
Female BALB/c mice were vaccinated with 25 μg of pWRG/EBOV or the various EBOV Nanoplasmids on days 0 and 21 by IM injection. EBOV GP-specific antibody responses were analyzed 21 days after the second vaccination. (A) ELISA end-point and (B) PsVNA80 neutralization titers are shown. Additionally, EBOV GP-specific (C) IgG1 and (D) IgG2a subtype titers were quantified by ELISA. Cohorts of 5 mice/group were euthanized on day 28 for isolation of splenocytes. Splenocytes were stimulated with pools of 15-mer, overlapping peptides spanning EBOV GP, and EBOV-specific (E) IFN-γ+ T cells and (F) IL-2+ T cells were quantified via ELISPOT. Data represent the group mean averages ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. p values were determined by one-way ANOVA with Tukey’s post hoc test.
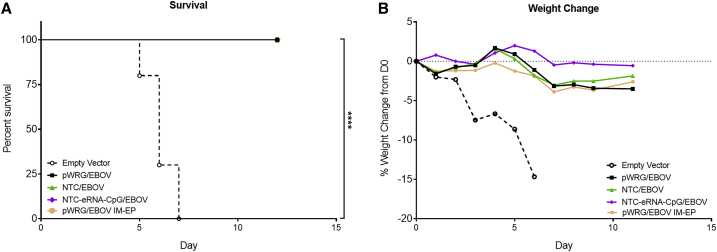
29 days after the second vaccination, the remaining mice were challenged with 2,000 PFU of ma-EBOV by intraperitoneal (IP) injection. All mice in the empty vector control group exhibited clinical signs of disease to include severe weight loss and ruffled fur, and all succumbed to disease or were euthanized in accordance with early endpoint criteria by day 7 post-infection. In contrast, all mice receiving either the pWRG/EBOV, NTC/EBOV, or NTC-eRNA-CpG/EBOV vaccine survived viral challenge (Figure 6A). Interestingly, only the mice receiving the NTC-eRNA-CpG/EBOV failed to lose any weight following viral challenge (Figure 6B). Because all EBOV vaccinated mice survived viral challenge, we next quantified the anti-nucleoprotein (NP) serum antibody response following EBOV challenge in an attempt to indirectly determine if there were any potential differences in the ability of the immune responses elicited after vaccination for the different groups to control viral replication. However, all mice that survived EBOV challenge exhibited similar levels of anti-NP IgG (data not shown).
Figure 6.
DNA Vaccination with EBOV Nanoplasmids Is Protective against EBOV Challenge
Vaccinated mice were challenged with 2,000 PFU of ma-EBOV by the IP route. (A) Survival and (B) weight loss following ma-EBOV challenge. Weight data represent the percent change in the collective weight of all mice per group relative to the day 0 pre-challenge weight as measured daily. ∗∗∗∗p < 0.0001. p values were determined by log-rank test.
Discussion
A major advantage of DNA vaccination is the ability to elicit high levels of both humoral and cellular immune responses, making DNA vaccines an ideal platform for eliciting protective immunity against various pathogens. We have previously demonstrated that vaccination with codon-optimized VEEV GP and EBOV GP DNA vaccines could protect both mice and NHP from viral challenge when delivered by IM-EP.12,13 The goal of the studies presented here was to determine whether next-generation, minimalized DNA vaccine plasmids expressing IFN-αβ stimulating innate immune agonists could enhance the humoral and/or cell-mediated immune responses elicited following IM delivery in the absence of EP in mice. To our knowledge, this is the first report to examine this approach for biodefense-related pathogens.
We initially found that the various Nanoplasmid vectors improved in vitro VEEV and EBOV antigen expression as compared to the pWRG7077 vector when using equal DNA concentrations. However, this improved antigen production did not seem to have a significant impact on short-term vaccine immunogenicity, as both the NTC/VEEV and NTC/EBOV vaccines elicited comparable immune responses to the respective pWRG7077-based vaccines. Instead, it appears that the inclusion of the isRNA innate immune agonist had the greatest influence on vaccine immunogenicity. Despite the importance of RIG-I signaling in generating antigen-specific T cells,34 we did not measure a significant increase in the numbers of IFN-γ+ or IL-2+ T cells for either antigen. This is in agreement with previous findings that the isRNA expression did not enrich T cell immunity, possibly due to the lack of a hairpin structure in the dsRNA transcribed from the Nanoplasmids.14,35 Conversely, inclusion of the isRNA adjuvant yielded improved viral GP-specific IgG titers and trended toward improved virus neutralizing antibody titers. This is most likely due to IFN-αβ’s ability to upregulate co-stimulatory molecules, priming CD4+ T cells, and driving the subsequent B cell response.36,37 IFN-αβ also amplifies the sensitivity of the B cell receptor, boosting the ability of naive B cells to produce high-affinity antibodies.38 Somewhat surprisingly, the addition of CpG motifs had a limited effect on VEEV DNA vaccine immunogenicity. One possible explanation for this result is the limited effect of TLR9 signaling on DNA vaccine immunogenicity.1,39 Alternatively, our results may suggest that a peak response was achieved by inclusion of the RIG-I agonist, and more subtle immune differences may be better discerned at a lower vaccine dose.
The results from our challenge studies also suggest that the increases in DNA vaccine immunogenicity afforded by the Nanoplasmids may subsequently enhance protective efficacy. Because neutralizing antibodies directed against the envelope glycoproteins is the most widely accepted correlate of protection against VEEV,40, 41, 42 the trend toward increased neutralization measured for the NTC-eRNA/VEEV and NTC-eRNA-CpG/VEEV vaccinated groups represents one possible explanation for the improved protection from aerosol VEEV challenge. However, neutralizing antibody titers are not always significantly associated with protection against VEEV challenge by the aerosol route. Non-neutralizing antibodies have also proven effective at preventing lethal alphavirus infection, and it has been reported that vaccine-elicited IgG2a (i.e., Th1 phenotype) correlates strongly with protection from aerosol VEEV challenge.43 This effect may be due to IgG2a’s broad range of effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement fixation.40 The co-expression of isRNA from the Nanoplasmid vector resulted in increased IgG2a production, albeit not to a statistically significant degree. However, IM vaccinated mice that had a higher ratio of IgG2a to IgG1 appeared to be better protected from VEEV challenge (Figure S2), suggesting that the improvement in IgG2a levels may have been substantial enough to afford greater protection from challenge. Taken together, the IgG2a data combined with the improved neutralizing capability suggests that the isRNA expressing Nanoplasmid vectors can shape multiple facets of the adaptive immune response required for protection against VEEV challenge.
EBOV Nanoplasmid vaccination resulted in a similar immune profile as was observed in our VEEV studies. Vaccination with NTC/EBOV resulted in antibody responses that trended higher than those quantified in the pWRG/EBOV group. The humoral response was further boosted by the addition of the dsRNA/CpG adjuvant. Notably, both Nanoplasmid vaccinated groups exhibited higher levels of IgG2a than did the pWRG/EBOV group. While the correlates of protection for EBOV remain unclear, several reports have detailed the role of IgG2a in conferring protection to EBOV challenge in mice.28,44 Moreover, Warfield et al.45 previously reported that an EBOV VLP vaccine elicited strong ADCC activity in vaccinated NHPs. Follow-on studies suggested that this vaccine elicited weakly neutralizing, but highly protective Th1 antibodies.46 Our study suggests a similar protective effect of IgG2a, as IM vaccinated mice with a higher ratio of IgG2a to IgG1 lost less weight following challenge (Figure S3). Although we were not able to quantify significant changes in protection to EBOV challenge at the DNA dose tested here, it is noteworthy that Nanoplasmid vaccination did not negatively impact protective efficacy. In fact, the sustained weight seen in the NTC-eRNA-CpG/EBOV group suggests that the isRNA may have the ability to augment protective efficacy beyond what was measured here. Follow-on EBOV challenge studies performed using a range of DNA vaccine doses will be required to definitively determine the impact of the Nanoplasmid vector platform.
Overall, our results demonstrate that vaccination with next-generation Nanoplasmid vectors can improve DNA vaccine immunogenicity when delivered by simple IM injection in the absence of EP. Furthermore, the inclusion of innate immune agonists, such as dsRNA and/or CpG motifs, can significantly boost protective efficacy. However, several questions remain to be addressed in future studies. Of interest is the ability of the Nanoplasmids to improve immunological memory, as well as their ability to spur affinity maturation. Previous reports demonstrating sustained antigen production from Nanoplasmid transfected cells suggest that these effects are possible, but we have yet to characterize them as they were outside the scope of these studies. Finally, as no groups vaccinated by IM injection generated immune responses equal to those elicited by IM-EP, further studies are required to determine whether the Nanoplasmid vectors sufficiently enhance DNA vaccine immunogenicity such that they could offer protective immunity to NHPs, which are a more relevant model of human disease.
Materials and Methods
DNA Vaccines
Construction of the pWRG/VEEV DNA vaccine candidate expressing the E3-E2-6K-E1 genes of VEEV subtype IAB was previously described.21 Briefly, codon-optimization of the structural genes, minus the capsid protein coding region, of VEEV IAB strain Trinidad donkey (GenBank: L01442) was accomplished using the Gene Optimizer bioinformatic algorithm followed by synthesis of the codon-optimized genes (Geneart, Regensburg, Germany). pWRG/VEEV was constructed by cloning the synthesized codon-optimized genes into the NotI and BglII restriction sites of pWRG7077, downstream of the cytomegalovirus immediate-early promoter. The pWRG/EBOV DNA vaccine plasmid was constructed in a similar manner by inserting the codon-optimized GP genes (Geneart, Regensburg, Germany) of EBOV-Kikwit 1995 (GenBank: U28077) into the pWRG7077 eukaryotic expression vector as described previously.24 Codon-optimization of the EBOV GP gene sequence resulted in the ablation of the 7U/8U genomic editing motif of EBOV, preventing production of soluble GP (sGP). The Nanoplasmid vaccines were produced by standard restriction digestion-mediated transfer of the pWRG7077 VEEV and EBOV transgenes into the Nanoplasmid NTC9385R, NTC9385R-eRNA41H, or NTC9385R-eRNA41H-CpG vector. Plasmid NTC9385R is a Nanoplasmid expression vector that contains a bacterial backbone comprising a 140 bp RNA-based sucrose selectable antibiotic free marker (RNA-OUT) and a 300 bp R6K origin.6 NTC9385R derivatives NTC9385R-eRNA41H and NTC9385R-eRNA41H-CpG RNA co-express isRNA with antigen. The RNA is transcribed by either RNA Pol II (isRNA encoded downstream of transgene in the 3′ UTR; CpG RNA) or RNA Pol III (isRNA transcribed independently from transgene; eRNA41H, a 114 bp RNA Pol III convergently transcribed non-palindromic dsRNA based isRNA that activates RIG-I).14,47 In these vectors, EBOV or VEEV transgenes are expressed from the CMV promoter upstream of the HTLV-IR expression enhancer.48 Research-grade pWRG7077 vaccine plasmids were manufactured by Aldevron (Fargo, ND), while research-grade Nanoplasmid vaccine plasmids were manufactured by Nature Technology Corporation (Lincoln, NE).
Gene Expression
COS-7 cells were transfected with multiple concentrations of the respective VEEV or EBOV GP expressing plasmids using Fugene HD (Promega, Madison, WI) according to the manufacturer’s instructions. Cultures were incubated at 37°C for the indicated time, after which transfected cells were harvested and surface glycoprotein expression was quantified by flow cytometry. For quantification of surface VEEV E1 protein, monoclonal antibody 3B2A-9 was used as the primary antibody at a concentration of 1.5 μg/mL. VEEV E2 surface expression was quantified by monoclonal antibody 1A3A-9 at a concentration of 1.5 μg/mL. For EBOV GP quantification, monoclonal antibody 12B5-1-1 was used as the primary antibody at a concentration of 5 μg/mL. The secondary antibody for both assays was an Alexa Fluor 488-labeled goat anti-mouse antibody (Becton Dickinson, Franklin Lakes, NJ) diluted to 1:200 in FACS buffer. Samples were measured on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) and analyzed using FlowJo software (FlowJo, Ashland, OR).
VEEV Mouse Vaccinations and Challenge
Groups of 15 female BALB/c mice, aged 6- to 8-weeks old (The Jackson Laboratory, Bar Harbor, ME) were anesthetized and vaccinated two times with a 3-week interval by IM injection into the anterior tibialis with 5 μg of codon-optimized pWRG/VEEV, NTC/VEEV, NTC-eRNA/VEEV, or NTC-eRNA-CpG/VEEV DNA plasmid diluted in calcium-and magnesium-free PBS. A negative control group of mice received 5 μg of the pWRG7077 empty vector delivered by IM injection on the same schedule. A positive control group of mice received 5 μg of the codon-optimized pWRG/VEEV DNA plasmid delivered by IM-EP. For IM-EP, mice were anesthetized and then vaccinated in the anterior tibialis muscle with 20 μL of DNA solution using a 3/10 cm3 U-100 insulin syringe inserted into the center of an Ichor Medical Systems TriGrid electrode array (San Diego, CA) with 2.5 mm electrode spacing. Injection of DNA was followed immediately by electrical stimulation at an amplitude of 250 V/cm, and the total duration was 40 ms over a 400 ms interval. Sera were collected prior to vaccination on days 0 and 21 by submandibular bleed. 5 mice/group were euthanized on day 28 and splenocytes were isolated for T cell analysis. Sera were isolated from blood samples collected from the remaining mice on day 42. Mice were subsequently challenged on day 50 with a target dose of 104 PFU of VEEV IAB strain Trinidad Donkey via the aerosol route. For challenge, mice were placed into a class III biological safety cabinet located inside a biosafety level 3 containment suite and exposed in a whole-body aerosol chamber to a VEEV aerosol created by a Collison nebulizer for 10 min as previously described.49 VEEV IAB was diluted to an appropriate starting concentration in Hanks’ balanced salt solution containing 1% fetal bovine serum for use in aerosol generation. Samples collected from the all-glass impinger (AGI) attached to the aerosol chamber were analyzed by plaque assay on Vero cells using standard methods as previously described to determine the inhaled dose of VEEV.50 The mice were monitored daily for clinical score and survival, and any animals found to meet early endpoint criteria were euthanized. The collective weights of all mice per group were measured daily. 25 days after challenge, all surviving mice were euthanized by exsanguination under deep anesthesia.
Research was conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
EBOV Mouse Vaccinations and Challenge
Groups of 15 female BALB/c mice, aged 6- to 8-weeks old (The Jackson Laboratory, Bar Harbor, ME) were vaccinated two times at 3-week intervals. Mice vaccinated by IM injection received 25 μg of codon-optimized pWRG/EBOV, NTC/EBOV, or NTC-eRNA-CpG/EBOV DNA plasmid diluted in calcium-and magnesium-free PBS. A negative control group of mice received 25 μg of the pWRG7077 empty vector delivered by IM injection on the same schedule. A positive control group of mice received 5 μg of the codon-optimized pWRG/EBOV DNA plasmid by IM-EP as described above.24,25 Sera were collected prior to vaccination on days 0 and 21 by submandibular bleed. 5 mice per group were euthanized on day 28 for T cell analysis. The remaining mice were observed until day 42, when sera were harvested for antibody analysis. Mice were subsequently challenged on day 50 with 2,000 PFU of mouse-adapted EBOV Mayinga-76 (ma-EBOV) via the IP route. The mice were monitored daily for clinical score and survival. The collective weights of all mice per group were measured daily. 15 days following challenge, the surviving mice were euthanized by exsanguination under deep anesthesia.
ELISA
High bind ELISA plates (Corning, Corning, NY) were coated with virus-like particles (VLPs) expressing either the VEEV E1/E2 antigen or the EBOV GP antigen encoded within the DNA vaccines. VLPs were produced by co-transfection of plasmids expressing the codon-optimized glycoprotein genes (E3-E2-6K-E1) of VEEV IAB (strain Trinidad donkey) or EBOV GP (strain Kikwit 1995) and a scaffolding murine leukemia virus (MLV) Gag protein as previously described.12,13 Briefly, HEK293T cells were transfected with pWRG/MLVGag and either pWRG/VEEV or pWRG/EBOV. Cell supernatants were collected at 24 and 48 h post-transfection, pooled, clarified by centrifugation, and filtered through a 0.45 μm filter. VLPs were concentrated through a Centricon filter unit with a 100-kDa cutoff (EMD Millipore, Burlington, MA) according to manufacturer’s instructions. VLPs were then pelleted through a 20% sucrose cushion in virus resuspension buffer (VRB; 130 mM NaCl, 20 mM HEPES, pH 7.4) by centrifugation for 2 h at 106,750 × g in an SW32 rotor at 4°C. VLP pellets were re-suspended overnight in VRB at 4°C, pooled, and diluted 10-fold with VRB. The diluted VLPs were re-pelleted without a sucrose cushion as described above. VLPs were re-suspended in 1/1,000 volume of VRB relative to starting supernatant and then stored at −80°C. ELISA plates were coated at a concentration of 150 ng/well, and then incubated overnight at 4°C. The following day, plates were washed with PBS containing 0.05% Tween-20 and then blocked with Neptune Block (ImmunoChemistry Technologies, Bloomington, MN) for 2 h at 37°C. Plates were washed again prior to being loaded with 2-fold serial dilutions of mouse sera in duplicate (dilution range 1:200 to 1:25,600). Serum dilutions were carried out in Neptune Block. Plates were incubated at ambient temperature for 1 h prior to being washed, and then incubated with a 1:1,000 dilution of horseradish peroxidase (HRP) conjugated goat anti-mouse (SeraCare Life Sciences, Gaithersburg, MD) in Neptune Block for 1 h at ambient temperature. Plates were washed again and then developed with SureBlue TMB substrate (SeraCare Life Sciences, Gaithersburg, MD). Absorbance at the 450 nm wavelength was detected with a Tecan M1000 microplate reader (Tecan Group, Switzerland). Pooled naive sera collected prior to vaccination were used as an internal control for each assay group. A plate cutoff value was determined based on 2 × the average absorbance of the pooled pre-bleed sera from each respective group. End-point titers were determined using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). For antibody subtyping ELISA, HRP-conjugated goat anti-mouse IgG1 and anti-mouse IgG2a secondary antibodies (Bethyl Laboratories, Montgomery, TX) were used at a 1:10,000 dilution. EBOV nucleoprotein ELISA plates were coated with recombinant EBOV nucleoprotein (PanThera Biopharma, Aiea, HI) at a concentration of 10 μg/mL and assayed as described above.
Pseudovirion Neutralization Assay
The pseudovirion neutralization assay (PsVNA) used to detect neutralizing antibodies in sera was described previously.12,13,51 This uses a replication-restricted, recombinant vesicular stomatitis virus (rVSV∗ΔG) expressing luciferase, which is pseudotyped with either the VEEV IAB E1/E2 glycoproteins (Trinidad donkey) or Ebola GP (Kikwit 1995).51 Briefly, heat-inactivated mouse sera (56°C for 30 min) was first diluted 1:20, followed by 5-fold serial dilutions that were mixed with an equal volume of Eagle’s minimum essential medium with Earle’s salts and 10% fetal bovine sera containing 4,000 fluorescent focus units of VEEV E1/E2 or EBOV GP pseudovirions and 10% guinea pig complement (Cedarlane, Burlington, NC). The sera and pseudovirion mixture was incubated overnight at 4°C. Following this incubation, 50 μL was inoculated onto Vero-76 cell monolayers in clear bottom, black-walled 96-well plates in duplicate. Plates were incubated at 37°C for 18–24 h. The media were discarded and cells were lysed according to the luciferase kit protocol (Promega, Madison, WI). A Tecan M200 Pro (Tecan Group, Switzerland) was used to acquire luciferase data. The values were graphed using GraphPad Prism software and used to calculate the percent neutralization normalized to cells alone and pseudovirions alone as the minimum and maximum signals, respectively. The percent neutralization values for duplicate serial dilutions were plotted. 80% PsVNA (PsVNA80) titers were interpolated from 4-parameter curves, and geometric mean titers were calculated in GraphPad Prism 8.
ELISPOT
Mouse T cell ELISPOT reagents were obtained from Mabtech (Cincinnati, OH). Antigen specific IFN-γ+ and IL-2+ T cells were quantified per manufacturer’s instructions. Positive control wells were stimulated with 10 ng/mL phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO) and 500 ng/mL ionomycin (Sigma-Aldrich, St. Louis, MO). Test splenocyte wells were stimulated with the appropriate peptides at a concentration of 10 μg/mL. Cells were incubated for 20 h at 37°C in 5% CO2. Positive spots were visualized on a CTL Imager and counting was performed with Immunospot software (Cellular Technology, Shaker Heights, OH). Splenocytes from VEEV vaccinated mice were stimulated with pooled 15-mer peptides containing an 11-base overlap spanning either the VEEV IAB E1 or E2 envelope glycoprotein (Pepscan, Lelystad, Netherlands). Splenocytes from EBOV vaccinated mice were stimulated with pooled 15-mer peptides containing a 10-base overlap spanning the envelope glycoprotein of EBOV (Mimotopes, Victoria, Australia).
Statistical Analysis
All data are presented as the mean of individual mice ± the standard error of the mean (SEM). Statistical analysis was performed using a Student’s t test, a one-way ANOVA followed by a Tukey post-test, or a two-way ANOVA with Dunnett’s multiple comparison test. Kaplan Meier survival curve analysis using a log rank test was performed to determine p value significance of vaccinated groups surviving lethal challenge compared to the control group using GraphPad Prism 8 for Windows. Further, log10 transformations were applied to VLP end-point ELISA titers using GraphPad Software as described above.
Author Contributions
Conceptualization: J.J.S., L.C.D., and C.J.S. Methodology: J.J.S., L.C.D., J.A.W., and C.S.S. Validation: J.J.S. and C.J.S. Investigation: J.J.S., L.C.D., C.J.S., C.S., K.W.S., and S.A.K. Resources: J.J.S. and C.J.S. Writing – Original Draft: J.J.S. and L.C.D. Writing – Review & Editing: J.J.S. and L.C.D. Visualization: J.J.S. Supervision: J.J.S., L.C.D., and C.S.S. Project Administration: J.J.S., L.C.D., and C.S.S. Funding Acquisition: J.J.S., L.C.D., and C.S.S.
Conflicts of Interest
J.A.W. is an employee of, and has an equity interest in, Nature Technology Corporation. The author acknowledges that there is a potential conflict of interest inherent in the publication of this manuscript and asserts that an effort to reduce or eliminate that conflict has been made where possible. The remaining authors declare no competing interests.
Acknowledgments
The authors would like to thank Colonel Olivier Flusin, MD/PhD for his contributions to this manuscript. This research was supported by project numbers CB10204 and CB3947 from The Joint Science & Technology Office for Chemical & Biological Defense (JSTO-CBD) to the U.S. Army Medical Research Institute for Infectious Disease (USAMRIID). Pseudovirion neutralization assay development was supported in part by the Postgraduate Research Participation Program at USAMRIID administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. DOE and U.S. Army Medical Research and Material Command (USAMRMC). The content is solely the responsibility of the authors and does not necessarily represent the official views of The Joint Science & Technology Office for Chemical & Biological Defense office. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.04.009.
Contributor Information
John J. Suschak, Email: john.j.suschak.ctr@mail.mil.
Lesley C. Dupuy, Email: lesley.dupuy@nih.gov.
Supplemental Information
References
- 1.Ishii K.J., Kawagoe T., Koyama S., Matsui K., Kumar H., Kawai T., Uematsu S., Takeuchi O., Takeshita F., Coban C., Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 2.Suschak J.J., Wang S., Fitzgerald K.A., Lu S. Identification of Aim2 as a sensor for DNA vaccines. J. Immunol. 2015;194:630–636. doi: 10.4049/jimmunol.1402530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R., Epstein J., Baraceros F.M., Gorak E.J., Charoenvit Y., Carucci D.J., Hedstrom R.C., Rahardjo N., Gay T., Hobart P. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA. 2001;98:10817–10822. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupuy L.C., Richards M.J., Reed D.S., Schmaljohn C.S. Immunogenicity and protective efficacy of a DNA vaccine against Venezuelan equine encephalitis virus aerosol challenge in nonhuman primates. Vaccine. 2010;28:7345–7350. doi: 10.1016/j.vaccine.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Luke J., Carnes A.E., Hodgson C.P., Williams J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine. 2009;27:6454–6459. doi: 10.1016/j.vaccine.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams J.A., Luke J., Johnson L., Hodgson C. pDNAVACCultra vector family: high throughput intracellular targeting DNA vaccine plasmids. Vaccine. 2006;24:4671–4676. doi: 10.1016/j.vaccine.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Lu J., Zhang F., Xu S., Fire A.Z., Kay M.A. The extragenic spacer length between the 5′ and 3′ ends of the transgene expression cassette affects transgene silencing from plasmid-based vectors. Mol. Ther. 2012;20:2111–2119. doi: 10.1038/mt.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutzler M.A., Weiner D.B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramson J.L., Dayball K., Hall J.R., Millar J.B., Miller M., Wan Y.H., Lin R., Hiscott J. Super-activated interferon-regulatory factors can enhance plasmid immunization. Vaccine. 2003;21:1363–1370. doi: 10.1016/s0264-410x(02)00694-1. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki S., Amara R.R., Yeow W.S., Pitha P.M., Robinson H.L. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 2002;76:6652–6659. doi: 10.1128/JVI.76.13.6652-6659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suschak J.J., Bagley K., Shoemaker C.J., Six C., Kwilas S., Dupuy L.C., Schmaljohn C.S. The Genetic Adjuvants Interleukin-12 and Granulocyte-Macrophage Colony Stimulating Factor Enhance the Immunogenicity of an Ebola Virus Deoxyribonucleic Acid Vaccine in Mice. J. Infect. Dis. 2018;218(suppl_5):S519–S527. doi: 10.1093/infdis/jiy378. [DOI] [PubMed] [Google Scholar]
- 13.Suschak J.J., Bagley K., Six C., Shoemaker C.J., Kwilas S., Spik K.W., Dupuy L.C., Schmaljohn C.S. The genetic adjuvant IL-12 enhances the protective efficacy of a DNA vaccine for Venezuelan equine encephalitis virus delivered by intramuscular injection in mice. Antiviral Res. 2018;159:113–121. doi: 10.1016/j.antiviral.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Luke J.M., Simon G.G., Söderholm J., Errett J.S., August J.T., Gale M., Jr., Hodgson C.P., Williams J.A. Coexpressed RIG-I agonist enhances humoral immune response to influenza virus DNA vaccine. J. Virol. 2011;85:1370–1383. doi: 10.1128/JVI.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suschak J.J., Wang S., Fitzgerald K.A., Lu S. A cGAS-Independent STING/IRF7 Pathway Mediates the Immunogenicity of DNA Vaccines. J. Immunol. 2016;196:310–316. doi: 10.4049/jimmunol.1501836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tudor D., Riffault S., Carrat C., Lefèvre F., Bernoin M., Charley B. Type I IFN modulates the immune response induced by DNA vaccination to pseudorabies virus glycoprotein C. Virology. 2001;286:197–205. doi: 10.1006/viro.2001.0957. [DOI] [PubMed] [Google Scholar]
- 17.Steele K., Reed D.S., Glass P.J., Hart M.K., Ludwig G.V., Pratt W.D., Parker M.D., Smith J.F. Plague. In Medical Aspects of Biological Warfare. In: Dembek Z.F., editor. Office of the Surgeon General, Department of the Army and US Army Medical Department Center and School; 2007. pp. 241–270. [Google Scholar]
- 18.Tsai T.F. Arboviral infections in the United States. Infect. Dis. Clin. North Am. 1991;5:73–102. [PubMed] [Google Scholar]
- 19.Franz D.R., Jahrling P.B., McClain D.J., Hoover D.L., Byrne W.R., Pavlin J.A., Christopher G.W., Cieslak T.J., Friedlander A.M., Eitzen E.M., Jr. Clinical recognition and management of patients exposed to biological warfare agents. Clin. Lab. Med. 2001;21:435–473. [PubMed] [Google Scholar]
- 20.Hanson R.P., Sulkin S.E., Beuscher E.L., Hammon W.M., McKinney R.W., Work T.H. Arbovirus infections of laboratory workers. Extent of problem emphasizes the need for more effective measures to reduce hazards. Science. 1967;158:1283–1286. doi: 10.1126/science.158.3806.1283. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy L.C., Richards M.J., Ellefsen B., Chau L., Luxembourg A., Hannaman D., Livingston B.D., Schmaljohn C.S. A DNA vaccine for venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin. Vaccine Immunol. 2011;18:707–716. doi: 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuy L.C., Richards M.J., Livingston B.D., Hannaman D., Schmaljohn C.S. A Multiagent Alphavirus DNA Vaccine Delivered by Intramuscular Electroporation Elicits Robust and Durable Virus-Specific Immune Responses in Mice and Rabbits and Completely Protects Mice against Lethal Venezuelan, Western, and Eastern Equine Encephalitis Virus Aerosol Challenges. J. Immunol. Res. 2018;2018:8521060. doi: 10.1155/2018/8521060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannaman D., Dupuy L.C., Ellefsen B., Schmaljohn C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. 2016;34:3607–3612. doi: 10.1016/j.vaccine.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 24.Grant-Klein R.J., Van Deusen N.M., Badger C.V., Hannaman D., Dupuy L.C., Schmaljohn C.S. A multiagent filovirus DNA vaccine delivered by intramuscular electroporation completely protects mice from ebola and Marburg virus challenge. Hum. Vaccin. Immunother. 2012;8:1703–1706. doi: 10.4161/hv.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant-Klein R.J., Altamura L.A., Badger C.V., Bounds C.E., Van Deusen N.M., Kwilas S.A., Vu H.A., Warfield K.L., Hooper J.W., Hannaman D. Codon-optimized filovirus DNA vaccines delivered by intramuscular electroporation protect cynomolgus macaques from lethal Ebola and Marburg virus challenges. Hum. Vaccin. Immunother. 2015;11:1991–2004. doi: 10.1080/21645515.2015.1039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart M.K. Vaccine research efforts for filoviruses. Int. J. Parasitol. 2003;33:583–595. doi: 10.1016/s0020-7519(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 27.Hevey M., Negley D., VanderZanden L., Tammariello R.F., Geisbert J., Schmaljohn C., Smith J.F., Jahrling P.B., Schmaljohn A.L. Marburg virus vaccines: comparing classical and new approaches. Vaccine. 2001;20:586–593. doi: 10.1016/s0264-410x(01)00353-x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson J.A., Hevey M., Bakken R., Guest S., Bray M., Schmaljohn A.L., Hart M.K. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 29.Dye J.M., Herbert A.S., Kuehne A.I., Barth J.F., Muhammad M.A., Zak S.E., Ortiz R.A., Prugar L.I., Pratt W.D. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl. Acad. Sci. USA. 2012;109:5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzi A., Yoshida R., Miyamoto H., Ishijima M., Suzuki Y., Higuchi M., Matsuyama Y., Igarashi M., Nakayama E., Kuroda M. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS ONE. 2012;7:e36192. doi: 10.1371/journal.pone.0036192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu X., Audet J., Wong G., Pillet S., Bello A., Cabral T., Strong J.E., Plummer F., Corbett C.R., Alimonti J.B., Kobinger G.P. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 2012;4:138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 32.Raymond J., Bradfute S., Bray M. Filovirus infection of STAT-1 knockout mice. J. Infect. Dis. 2011;204(Suppl 3):S986–S990. doi: 10.1093/infdis/jir335. [DOI] [PubMed] [Google Scholar]
- 33.Warfield K.L., Olinger G., Deal E.M., Swenson D.L., Bailey M., Negley D.L., Hart M.K., Bavari S. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J. Immunol. 2005;175:1184–1191. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- 34.Kandasamy M., Suryawanshi A., Tundup S., Perez J.T., Schmolke M., Manicassamy S., Manicassamy B. RIG-I Signaling Is Critical for Efficient Polyfunctional T Cell Responses during Influenza Virus Infection. PLoS Pathog. 2016;12:e1005754. doi: 10.1371/journal.ppat.1005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D., Liu Y., Zhang Y., Xu J., Hong K., Sun M., Shao Y. Adjuvant effects of plasmid-generated hairpin RNA molecules on DNA vaccination. Vaccine. 2007;25:6992–7000. doi: 10.1016/j.vaccine.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 36.Luft T., Pang K.C., Thomas E., Hertzog P., Hart D.N., Trapani J., Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 37.Gallucci S., Lolkema M., Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 38.Braun D., Caramalho I., Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int. Immunol. 2002;14:411–419. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 39.Spies B., Hochrein H., Vabulas M., Huster K., Busch D.H., Schmitz F., Heit A., Wagner H. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 2003;171:5908–5912. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 40.Bennett A.M., Elvin S.J., Wright A.J., Jones S.M., Phillpotts R.J. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine. 2000;19:337–347. doi: 10.1016/s0264-410x(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 41.Phillpotts R.J., Jones L.D., Howard S.C. Monoclonal antibody protects mice against infection and disease when given either before or up to 24 h after airborne challenge with virulent Venezuelan equine encephalitis virus. Vaccine. 2002;20:1497–1504. doi: 10.1016/s0264-410x(01)00505-9. [DOI] [PubMed] [Google Scholar]
- 42.Hart M.K., Lind C., Bakken R., Robertson M., Tammariello R., Ludwig G.V. Onset and duration of protective immunity to IA/IB and IE strains of Venezuelan equine encephalitis virus in vaccinated mice. Vaccine. 2001;20:616–622. doi: 10.1016/s0264-410x(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 43.Schmaljohn A.L., Johnson E.D., Dalrymple J.M., Cole G.A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297:70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 44.Duehr J., Wohlbold T.J., Oestereich L., Chromikova V., Amanat F., Rajendran M., Gomez-Medina S., Mena I., tenOever B.R., García-Sastre A. Novel Cross-Reactive Monoclonal Antibodies against Ebolavirus Glycoproteins Show Protection in a Murine Challenge Model. J. Virol. 2017;91:e00652-17. doi: 10.1128/JVI.00652-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warfield K.L., Swenson D.L., Olinger G.G., Kalina W.V., Aman M.J., Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J. Infect. Dis. 2007;196(Suppl 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 46.Blaney J.E., Marzi A., Willet M., Papaneri A.B., Wirblich C., Feldmann F., Holbrook M., Jahrling P., Feldmann H., Schnell M.J. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog. 2013;9:e1003389. doi: 10.1371/journal.ppat.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiyama T., Gursel M., Takeshita F., Coban C., Conover J., Kaisho T., Akira S., Klinman D.M., Ishii K.J. CpG RNA: identification of novel single-stranded RNA that stimulates human CD14+CD11c+ monocytes. J. Immunol. 2005;174:2273–2279. doi: 10.4049/jimmunol.174.4.2273. [DOI] [PubMed] [Google Scholar]
- 48.Luke J.M., Vincent J.M., Du S.X., Gerdemann U., Leen A.M., Whalen R.G., Hodgson C.P., Williams J.A. Improved antibiotic-free plasmid vector design by incorporation of transient expression enhancers. Gene Ther. 2011;18:334–343. doi: 10.1038/gt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart M.K., Pratt W., Panelo F., Tammariello R., Dertzbaugh M. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine. 1997;15:363–369. doi: 10.1016/s0264-410x(96)00204-6. [DOI] [PubMed] [Google Scholar]
- 50.Pratt W.D., Gibbs P., Pitt M.L., Schmaljohn A.L. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine. 1998;16:1056–1064. doi: 10.1016/s0264-410x(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 51.Martins K., Carra J.H., Cooper C.L., Kwilas S.A., Robinson C.G., Shurtleff A.C., Schokman R.D., Kuehl K.A., Wells J.B., Steffens J.T. Cross-protection conferred by filovirus virus-like particles containing trimeric hybrid glycoprotein. Viral Immunol. 2015;28:62–70. doi: 10.1089/vim.2014.0071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.