Abstract
Background
Human infection with a novel coronavirus named Middle East Respiratory Syndrome coronavirus (MERS-CoV) was first identified in Saudi Arabia and the Middle East in September, 2012, with 44 laboratory-confirmed cases as of May 23, 2013. We report detailed clinical and virological data for two related cases of MERS-CoV disease, after nosocomial transmission of the virus from one patient to another in a French hospital.
Methods
Patient 1 visited Dubai in April, 2013; patient 2 lives in France and did not travel abroad. Both patients had underlying immunosuppressive disorders. We tested specimens from the upper (nasopharyngeal swabs) or the lower (bronchoalveolar lavage, sputum) respiratory tract and whole blood, plasma, and serum specimens for MERS-CoV by real-time RT-PCR targeting the upE and Orf1A genes of MERS-CoV.
Findings
Initial clinical presentation included fever, chills, and myalgia in both patients, and for patient 1, diarrhoea. Respiratory symptoms rapidly became predominant with acute respiratory failure leading to mechanical ventilation and extracorporeal membrane oxygenation (ECMO). Both patients developed acute renal failure. MERS-CoV was detected in lower respiratory tract specimens with high viral load (eg, cycle threshold [Ct] values of 22·9 for upE and 24 for Orf1a for a bronchoalveolar lavage sample from patient 1; Ct values of 22·5 for upE and 23·9 for Orf1a for an induced sputum sample from patient 2), whereas nasopharyngeal specimens were weakly positive or inconclusive. The two patients shared the same room for 3 days. The incubation period was estimated at 9–12 days for the second case. No secondary transmission was documented in hospital staff despite the absence of specific protective measures before the diagnosis of MERS-CoV was suspected. Patient 1 died on May 28, due to refractory multiple organ failure.
Interpretation
Patients with respiratory symptoms returning from the Middle East or exposed to a confirmed case should be isolated and investigated for MERS-CoV with lower respiratory tract sample analysis and an assumed incubation period of 12 days. Immunosuppression should also be taken into account as a risk factor.
Funding
French Institute for Public Health Surveillance, ANR grant Labex Integrative Biology of Emerging Infectious Diseases, and the European Community's Seventh Framework Programme projects EMPERIE and PREDEMICS.
Introduction
Coronaviruses are large enveloped single-stranded RNA viruses that can infect and cause disease in many animal species, including bats, mice, birds, dogs, pigs, and cattle.1 In human beings, five respiratory coronaviruses have been described, causing common cold (229E and OC43), upper respiratory tract infections (NL63), or pneumonia (HKU1 and SARS).2 In September, 2012, a novel human coronavirus, named HCoV-EMC, was identified in two patients with severe respiratory disease.3, 4 This new coronavirus belongs to lineage C of the genus Betacoronavirus, and is genetically closely related to coronaviruses from various bat species in Africa and Eurasia.1, 5, 6 As of May 23, 2013, 44 laboratory-confirmed cases had been diagnosed in several countries (France, Germany, Jordan, Qatar, Saudi Arabia, Tunisia, and the UK).7, 8 Most patients with reported symptoms had severe respiratory disease, some with acute renal failure, and the case fatality rate is estimated at 50%.9 All cases originated from, or had a history of travel to, the Middle East, except for two secondary cases in the UK,4, 10 two in Tunisia,8 and one in France.11 A large cluster (>20) of cases has been documented in a hospital in Saudi Arabia, and another is suspected on the basis of a retrospective analysis of samples kept after an outbreak of respiratory diseases in a Jordanian hospital in April, 2012.12 On the basis of outbreak dynamics, HCoV-EMC was renamed Middle East Respiratory Syndrome coronavirus (MERS-CoV) by the International Committee on Taxonomy of Viruses.13
Whereas an as yet unidentified animal reservoir might have caused the initial outbreaks by introducing the virus into the human population, the occurrence of clusters, whether in the community or in hospitals, is a worrying development, because it might result from adaptation of the virus to inter-human transmission. This process of adaptation might have been pivotal in the switch from aborted outbreaks to the international pandemic of SARS-CoV in 2003–04.14 We report detailed clinical and virological information for two related cases of MERS-CoV disease, after nosocomial transmission of the virus from one patient to another in a French hospital in April, 2013.
Methods
Patients and genetic analysis
We report data for two patients who were admitted to hospital in April and May, 2013, in northern France. Their medical records were compiled and reviewed by their attending physicians. Spouses provided written informed consent for data and samples to be used for research and reporting purposes.
We extracted RNA from specimens from the upper (nasopharyngeal swabs) or the lower (bronchoalveolar lavage, sputum) respiratory tract and from whole blood, plasma, and serum specimens using the NucleoSpin Dx Virus or NucleoSpin RNA Blood Mini kits (Macherey-Nagel GmbH & Co KG, Düren, France) according to the manufacturer's instructions. For sputum, we applied a pretreatment with proteinase K (5 mg/mL for 10 min at 70°C) or Digest-EUR (Eurobio, Coutaboeuf, France) to reduce viscosity. We included sigma virus RNA (10 ng per assay) as a control for the extraction procedure and the absence of inhibitors.
We tested extracted nucleic acids by real-time RT-PCR assays targeting the upE, Orf1a, or Orf1b regions of the MERS-CoV genome as previously described15, 16 on a LightCycler 480 real-time PCR system (Roche, Coutaboeuf, France). The quality of the specimens was assessed by real-time RT-PCR targeting the GAPDH house-keeping gene. Positive control for Orf1a and upE real-time RT-PCR was an in-vitro transcribed RNA, combining the sequences of the Orf1a gene (from nucleotide 11172 to nucleotide 11414) and the upE gene (from nucleotide 27357 to nucleotide 27670) as the positive strand, designed based on the first published sequence of MERS-CoV.5 We did confirmatory sequence analysis on the RdRp and N gene regions as described.15 We assembled sequences using CLC Main WorkBench 6.8.3 software and aligned them with available MERS-CoV sequences: EMC/2012 (GenBank accession number JX869059), Jordan-N3/2012 (KC776174), England-1/2012 (KC164505), England/Qatar/2012 (KC667074), England 2/2013, and Munich/Abu_Dhabi/2013.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. As corresponding authors BG and SvdW had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Patient 1, a 64-year-old man, visited Dubai from April 9, to April 17, 2013. Fever and chills with diarrhoea (three to four bowel movements per day) started on April 22. Symptoms were much the same as those of a previous episode of sigmoiditis treated 6 months before. He also had a history of hypertension and diabetes, and had undergone renal transplantation in 1998, for renal failure secondary to diabetes. His existing treatments were mycophenolate mofetil, ciclosporin, and prednisone. He was admitted to the Valenciennes hospital on April 23, presenting with diarrhoea and fever reaching 39°C, arterial pressure at 137/66 mm Hg, and pulseoxymetric oxygen saturation of 96% on ambient air. At that time, he did not have any respiratory symptoms (cough or dyspnoea). Chest radiograph was normal (not shown).
Table 1 summarises biological data for patient 1. Blood cultures, stool analysis, urine antigen assays for Legionella spp and Pneumococcus spp, and plasma PCR for cytomegalovirus were negative. Treatment with ceftriaxone was initiated on April 24.
Table 1.
Haematological and blood chemical values for patient 1
| April 23 | April 26 | April 29 | May 8 | May 15 | Normal range | |
|---|---|---|---|---|---|---|
| Blood cells | ||||||
| Leucocyte count (cells per μL) | 8740 | 8980 | 11 700 | 11 800 | 9180 | 5500–15 500 |
| Neutrophils (%) | ND | ND | 87 | ND | ND | 23–45 |
| Lymphocytes (%) | ND | ND | 7 | ND | ND | 35–65 |
| Platelet count (cells per μL) | ND | 192 000 | 309 000 | 184 000 | 55 000 | 250 000–550 000 |
| Serum | ||||||
| Creatinine (μmol/L) | 318·6 | 300·8 | 274 | 220 | 177 | 40–130 |
| Blood urea nitrogen (mmol/L) | 29·9 | 27·7 | 23·7 | 41·6 | 31·5 | 1·2–3·3 |
| C-reactive protein (mg/L) | 152·30 | 206·40 | 163·60 | ND | 53 | <5 |
| Arterial blood | ||||||
| O2 (L/min) or FiO2 (%) | 0 | 5 L/min | 10 L/min | 80% | 70% FiO2 ECMO 50% |
.. |
| pH | ND | ND | 7·30 | 7·45 | 7·36 | 7·38–7·42 |
| Partial pressure of carbon dioxide (mm Hg) | ND | ND | 23·50 | 34 | 37·6 | 35–45 |
| Partial pressure of oxygen (mm Hg) | ND | ND | 59·40 | 72 | 79 | 70–100 |
| Bicarbonate (mEq/L) | ND | ND | 11·30 | 23·3 | 20·9 | 22–26 |
ND=not determined. FiO2=fraction of inspired oxygen. ECMO=extracorporeal membrane oxygenation.
An abdominal CT scan done on April 24 did not show any evidence of colitis, but lower thoracic images showed major pulmonary infiltrates (figure 1A ). On April 26, the patient developed dyspnoea and cough. Levofloxacin was added to ceftriaxone therapy. A CT scan of the lung was done on April 26, and showed a mostly peripheral interstitial infiltrate associated with right lower-lobe consolidation and left lower-lobe consolidation in the anterior basal, lateral basal, and posterior basal areas. A bronchoalveolar lavage was done and cytology showed a high number of neutrophils and macrophages. No specific pathogen was identified on direct microbiological examination of bronchoalveolar lavage fluid, and co-trimoxazole was added to the antimicrobial treatment regimen. Oxygen requirement increased daily to reach 10 L/min on April 29.
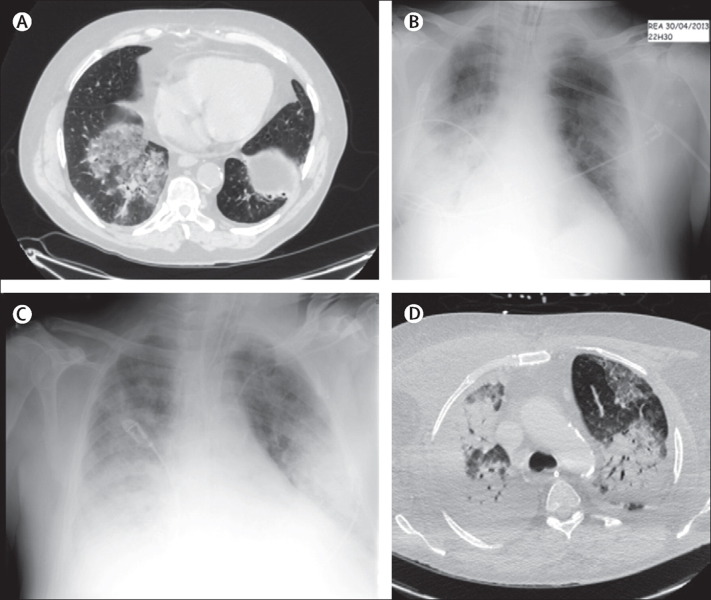
Figure 1.
Radiographs and CT scans of the chest of patient 1
(A) CT scans of the chest obtained on April 24. Substantial bilateral ground-glass opacity and consolidation can be seen. (B, C) Chest radiographs. Groundglass opacity and condensation, mainly on the lower right lobe, were noted on April 30 (B). Bilateral ground-glass opacity and consolidation were noted on May 8 (C). (D) On May 17, the CT scan of the chest showed a bilateral consolidation of the lung.
Patient 1 was transferred to the Douai hospital intensive-care unit (ICU) on April 29, where non-invasive mechanical ventilation was started, and antimicrobial therapy was modified to piperacilline plus tazobactam and linezolid. He had rapid respiratory deterioration, and was intubated on April 30 (figure 1B). Respiratory failure was followed by shock and renal failure, leading to noradrenaline administration and continuous venovenous haemofiltration.
On May 1, on the basis of the clinical presentation and the history of recent travel to Dubai, the hypothesis of an infection with MRES-CoV was raised, prompting the transfer of nasopharyngeal and stored bronchoalveolar lavage samples to the National Reference Center in Paris.
Further worsening of the respiratory status with a partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio of 100 despite low tidal volume and high-positive end expiratory pressure (PEEP) ventilation, inhaled nitric oxide, prone positioning, sedation, and neuromuscular blockade, prompted the implementation of extracorporeal membrane oxygenation (ECMO). Persisting haemodynamic support with norepinephrine at 0·5 mg/h was necessary. The patient was transferred to the ECMO referral centre ICU of Lille University Teaching Hospital on May 8 (figure 1C). As of May 27, the patient remained under ECMO therapy because of persisting severe acute respiratory distress syndrome (figure 1D). At 100% FiO2 on both ECMO and ventilator settings and with mean ECMO pump flow of 5·2 L/min, resulting PaO2 values did not exceed 80 mm Hg. Oxygen sweep flow was 12 L/min, resulting in mean partial pressure of carbon dioxide values of about 38 mm Hg. The patient had several haemorrhagic complications, contributing to renal failure and requirement for extrarenal epuration. No evidence of bacterial superinfection was noted before ECMO. Endotracheal aspirates remained sterile. After the implantation of ECMO, the patient had candidemia with Candida albicans and Candida glabrata. Empirical antifungal therapy with caspofungin had been started before ECMO. Patient 1 died on May 28, due to refractory multiple organ failure.
Patient 2, a 51-year-old man, was admitted to the Valenciennes hospital on April 26, for left arm deep venous thrombosis. He lived in the north of France and had not recently travelled abroad. His medical history included myocardial infarction in 2005, arterial hypertension, dyslipidaemia, and histamine-induced angioedema, for which he had needed systemic corticosteroid therapy (prednisone 40 mg per day) since June, 2012. He also had several episodes of deep venous thrombosis associated with the presence of low concentrations of an anticardiolipin antibody, IgM isotype, for which he was treated with a vitamin K antagonist. Patient 2 shared patient 1's room from April 26, to April 29. The room was 20 m2, and 1·5 m separated the two patients' beds. Both patients shared the same bathroom. During this 3-day period, patient 1 remained mostly confined to his bed, whereas patient 2 was able to move around. No nebulisers or known aerosol-generating procedures were used for patient 1. Because the diagnosis of MERS-CoV infection was not suspected, patient 1 wore no mask, and neither patient 2, nor staff nor visitors used protective equipment. Patient 2 was discharged from the hospital to his home on April 30.
On May 8, patient 2 presented with asthenia, myalgia, and cough. On May 9, he was rapidly directed to the Infectious Diseases Department of Lille University Teaching Hospital, since by that time he was a known contact of a confirmed case (positive results for MERS-CoV were available for patient 1 on May 7). On admission, he presented with fever (38°C), pulseoxymetric saturation of 95% on ambient air, and a respiratory rate of 15 breaths per min.
Table 2 summarises biological data for patient 2. Chest radiograph showed upper right lobe consolidation (figure 2A ). Oxygen therapy at 3 L/min and piperacillin plus tazobactam were started on April 30. The initial nasopharyngeal sample was inconclusive. The patient remained stable but with a high fever (39–40°C) between May 9 and May 12. Linezolid was added to the antimicrobial regimen on May 11. Diagnosis of MERS-CoV infection was confirmed on the basis of results from induced sputum analysis on May 11. Personal protective equipment—an FFP2 mask, gloves, gown, and goggles—was used to prevent secondary transmission during sputum induction.
Table 2.
Haematological and blood chemical values for patient 2
| May 9 | May 12 | May 15 | Normal range | |
|---|---|---|---|---|
| Blood cells | ||||
| Leucocyte count (cells per μL) | 7780 | 2250 | 3790 | 5500–15 500 |
| Neutrophils (%) | ND | 86 | ND | 23–45 |
| Lymphocytes (%) | ND | 14 | ND | 35–65 |
| Platelet count (cells per μL) | 154 000 | 100 000 | 64 000 | 250 000–550 000 |
| Serum | ||||
| Creatinine (μmol/L) | 53 | 53 | 398 | 40–130 |
| Blood urea nitrogen (mmol/L) | 5 | 6 | 19·9 | 1·2–3·3 |
| C-reactive protein (mg/L) | 217 | 319 | 237 | <5 |
| Arterial blood | ||||
| O2 (L/min) or FiO2 (%) | 3 L/min | 5 L/min | 100% FiO2 ECMO 100% | .. |
| pH | 7·48 | 7·49 | 7·29 | 7·38–7·42 |
| Partial pressure of carbon dioxide (mm Hg) | 31 | 30 | 42·5 | 35–45 |
| Partial pressure of oxygen (mm Hg) | 56 | 58 | 123 | 70–100 |
| Bicarbonate (mEq/L) | 23 | 22·4 | 19·6 | 22–26 |
ND=not determined. FiO2=fraction of inspired oxygen. ECMO=extracorporeal membrane oxygenation.
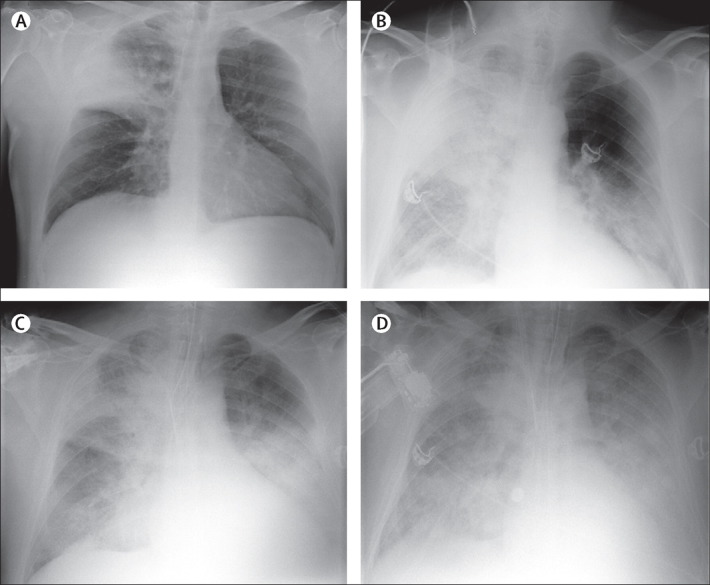
Figure 2.
Radiographs of the chest of patient 2
(A) Chest radiograph obtained on May 9 showed a systematic consolidation of the upper right lung lobe. (B) On May 12, ground-glass opacity and consolidation could also be seen in the lower left lobe. Bilateral ground-glass opacities and consolidation were noted afterwards on May 15 (C) and May 17 (D).
On May 12, the patient had acute respiratory failure, and oxygen therapy was increased to 5 L/min. Chest radiograph showed complete consolidation of the right lung and involvement of the lower left lobe (figure 2B). The patient was transferred to the ICU where oxygen therapy was progressively increased. Appearance of confusion, disorientation, and increasing hypoxaemia led to intubation and mechanical ventilation on May 13. Bronchoalveolar lavage was done, and no specific pathogen was identified, either on direct microbiological examination or culture. This sample was not tested for MERS-CoV, because the diagnosis had already been confirmed. After intubation, on the same day, FiO2 was increased to 1; inhaled nitric oxide and prone positioning became necessary. After intubation, dobutamine was introduced for biventricular dysfunction—confirmed by transthoracic echocardiogram—and progressively weaned over the next 48 h. Acute renal failure and anuria, despite volume expansion, resulted in haemodialysis on May 14. On May 14, PaO2 to FiO2 ratio was 90, despite inhaled nitric oxide, prone positioning, and low tidal volume and high PEEP ventilation, leading to implementation of ECMO. Neither norepinephrine nor dobutamine treatment were underway on the day of ECMO implantation. Chest radiograph showed bilateral diffuse alveolar-interstitial infiltrates on May 15 (figure 2C). The chest radiograph also showed bilateral ground-glass opacities with major consolidation (figure 2D). No bacterial superinfection was identified before ECMO. Patient 2's respiratory status has improved since May 26, with FiO2 at 70% on both ventilator and ECMO settings, ECMO flow at 5·2 L/min, and ECMO oxygen sweep flow at 5 L/min. Sedation is being progressively weaned.
On May 2, the National Influenza Center at the Institut Pasteur was notified by the French Institute for Public Health Surveillance of a possible case of infection with MERS-CoV. A nasopharyngeal swab obtained from patient 1 on April 30, analysed by real-time RT-PCR, was deemed negative (table 3 ). In view of the deterioration of his respiratory status, the patient remained classified as a possible case, awaiting the analysis of a bronchoalveolar lavage specimen obtained on April 26. Because of sample transportation issues, this specimen was received on May 7, and was shown to be positive by RT-PCR for both the upE and Orf1a targets with cycle threshold (Ct) values of 22·9 for upE and 24 for Orf1a (table 3), thus confirming the case. Parallel retesting of the April 30 nasopharyngeal swab resulted in Ct values of 37·2 for upE and 40 for Orf1a. A sputum sample obtained on May 7 was strongly positive (Ct <29) by RT-PCR for both targets, which further confirmed the diagnosis (table 3).
Table 3.
Genetic analysis of specimens
| Specimen type | Gene |
||
|---|---|---|---|
| upE (Ct) | Orf1a (Ct) | ||
| Patient 1 | |||
| April 26 | BAL* | 22·9 | 24 |
| April 30 | NP | 40 | 40 |
| April 30 | NP† | 37·2 | 40 |
| May 7 | SP‡ | 28·8 | 27·2 |
| May 7 | WB | 35·9 | 35·2 |
| May 9 | P | 40 | 38·8 |
| Patient 2 | |||
| May 9 | NPa | 37 | 40 |
| May 9 | NPb | 37·4 | Negative |
| May 11 | SP* | 22·5 | 23·9 |
| May 11 | NP | Inconclusive | Inconclusive |
Ct=cycle threshold. BAL=bronchoalveolar lavage. NP=nasopharyngeal swab. SP=sputum. NPS=nasopharyngeal swab. WB=whole blood. P=plasma.
Specimens on which sequencing was done (appendix).
Retest.
Specimen from which an isolate was obtained.
For patient 2, two nasal swabs obtained on May 9 both showed Ct values of about 37 at the detection limit of the RT-PCR for the upE target but were negative for the Orf1a target, casting serious doubts on the possibility of infection by the MERS-CoV. The diagnosis was subsequently confirmed on the basis of the analysis by the Cellule d'Intervention Biologique d'Urgence of an induced sputum sample from May 11: RT-PCR analysis showed Ct values of 22·5 for upE, and 23·9 for Orf1a (table 3), and of 25·6 for Orf1b. A nasopharyngeal swab from the same day gave inconclusive results.
Preliminary results of RT-PCR done on blood specimens from patient 1 on May 9 were positive for MERS-CoV in whole blood; weak but inconsistent detection was noted in plasma samples (table 3). No other types of specimens were analysed for either patient in this timeframe.
Virus isolation on Vero E6 (African green monkey kidney) cells was attempted from all respiratory specimens from both patients and an isolate was obtained from the sputum of patient 1. Preliminary analysis of serum samples from patient 1 from May 7 and May 9 shows reactivity in western blot with a recombinant N protein (data not shown).
Confirmatory sequence analysis of the RdRp and N gene segments was done directly on RNA extracted from the bronchoalveolar lavage specimen from patient 1 and sputum specimen from patient 2. The sequences from both patients were identical. The RdRp sequences showed the C→T polymorphism at position 15196, which distinguishes all available sequences from that of the EMC/2012 isolate.5 For the N gene, sequences were identical to that of the EMC isolate and did not show the short deletion (nucleotides 29736–29741) nor any of the polymorphisms recorded at positions 29714 (A→T) and 29723 (G→T) in the England/Qatar/2012 sequence, nor that found at position 29811 (C→T) in the Jordan-N3/2012 sequence (appendix).5, 15, 17 These results definitively establish that both patients were infected with MERS-CoV.
Discussion
This report describes the first two French cases of MERS-CoV infection with a case of patient-to-patient nosocomial transmission (panel ). Our findings suggest that the virus's incubation period could reach 9–12 days, a longer period than what was previously recorded, with clinical implications for the duration of quarantine. Our results also suggest that the best samples to detect the virus are those from the lower respiratory tract, rather than nasopharyngeal samples.
Panel. Research in context.
Systematic review
We searched PubMed on May 25, 2013, with the terms “HCoV-EMC” and “MERS-CoV” for articles published in English. Our search identified 24 reports linked to HCoV-EMC, starting with the initial report from Zaki and colleagues3 describing a previously unknown coronavirus isolated from the sputum of a 60-year-old man. Articles relevant to our paper are cited in the text. The search with “MERS-CoV” identified four articles, none of which were related to human transmission or clinical findings.
Interpretation
Our report provides evidence that patients with respiratory symptoms and a history of travel to the Middle East or contact with a known MERS-CoV case in the past 12 days should be isolated and investigated using lower respiratory tract samples. Our findings also suggest that immunosuppression is an aggravating factor, and could be associated with atypical clinical presentation.
The two cases we report show very similar clinical features compared with the only two other cases for which detailed clinical descriptions are available;3, 4 other reports do not have complete clinical data. Initial presentation included fever, chills, and myalgia. Respiratory symptoms with cough and dyspnoea soon became the predominant clinical symptoms, with a rapid deterioration of oxygenation and increasing oxygen requirements, leading to mechanical ventilation and ECMO. Later in the course of disease, and soon after ICU admission, severe renal failure with anuria needing renal replacement therapy developed. Lymphopenia was another common feature in our two patients, also noted in the report by Zaki and colleagues.3 Such a clinical presentation is reminiscent of that of patients with severe SARS, except that acute renal failure seemed less common in SARS cases. In fact, SARS-CoV was associated with a wide spectrum of clinical features—infected people presented initially with fever, myalgia, chills, and rigor and subsequently developed pneumonia.14, 18 Notably, patient 1 had diarrhoea among initial symptoms, a feature not yet reported in patients with MERS-CoV, but present upon admission in a quarter of patients with SARS.19 Patient 1 died on May 28, and patient 2 remains in the ICU under ECMO. The case-fatality rate in patients infected with MERS-CoV is high—it is estimated to be 50% in the 44 patients reported so far by WHO.8 This rate is higher than that of SARS, estimated at 15%, and strongly age-dependant.14 Whether this rate is higher for patients with MERS-CoV because of underreporting of milder forms of disease, a higher proportion of patients with underlying immunosuppressive disorders, as in our patients, or higher virulence of MERS-CoV compared with SARS-CoV has yet to be established.
For the two patients described here, MERS-CoV was detected at the time of the diagnosis of pneumonia. The data we have obtained so far show identical sequences for the viruses infecting both patients, and this sequence is also identical to most of the available reported MERS-CoV sequences. Whole genome sequencing and analysis of viral population diversities will be needed to further establish the degree of variability of the viruses of the French cases relative to previous cases and to work out the relatedness of the viruses of the two patients upon transmission.
Viral load was high in samples obtained from the lower respiratory tract, whereas the virus was almost undetectable in upper respiratory tract samples. Besides our two cases, available data for confirmed patients have shown positive detection by RT-PCR in upper respiratory tract specimens on days 4, 12, 13, and 144, 10, 20 after symptom onset, and in lower respiratory tract specimens on days 8, 17, and 194, 20 after symptom onset. A sputum sample taken 7 days after symptom onset has also been reported as having led to virus isolation.3 Notably, for patient 2, as for the London case reported in September, 2012, nasal and throat swabs were negative at the time at which sputum samples were positive.4
The little information available for virus shedding up to now is reminiscent of SARS-CoV, for which overall the virus was detected by RT-PCR in 76% of lower respiratory tract clinical samples, versus 37% of upper respiratory tract samples,21 and viral load was significantly higher in the lower respiratory tract compared with the upper respiratory tract. In the upper respiratory tract, the rate of viral shedding was initially low and increased to peak at around day 15.22 Whether MERS-CoV is present outside the respiratory tract will require additional studies. So far, there have been no reports of virus detection in stool or urine, and our preliminary data suggest that the virus might be present in blood. For SARS, the virus was readily detected in stool samples and more frequently after 10 days from onset of symptoms, whereas in blood samples detection was more frequent during the early course of illness.21 Although additional data for the kinetics and routes of viral shedding are needed for MERS-CoV, initial data suggest that for possible or suspect cases, especially when presenting early after the onset of symptoms, clinical samples from the lower respiratory tract should be obtained for confirmation of infection. Confirmation of an initially negative result on another sample taken a few days later should be recommended before exclusion of possible cases.
Patient 2 was admitted on April 26, at a time when pneumonia was already present in patient 1 and MERS-CoV was present in the bronchoalveolar lavage sample taken on the same day (although this result was not available at the time). For the next 3 days, the two patients shared the same room with no specific isolation measures other than standard precautions. 9–12 days after this exposure, patient 2 developed symptoms suggestive of lower respiratory tract infection (figure 3 ). In patient 2, infection with MERS-CoV was confirmed 2 days later on an induced sputum sample. This timeframe is at the high end of the 1–9 day incubation period reported in the two secondary cases from the UK10 and of the 2–10 day incubation period of SARS.23 In view of the very small number of patients for whom a well-defined window of exposure for MERS-CoV is available, this finding of human-to-human transmission through exposure during the upper range of reported incubation periods suggests that incubation periods beyond 10 days might be possible with MERS-CoV, with important implications for the duration of the quarantine needed to rule out infection in contacts. Furthermore, patient 2 had not recently travelled abroad, excluding any other source of contamination. However, the exact route of transmission from patient 1 to patient 2 remains unclear. Nosocomial transmission of MERS-CoV has been suspected in patients and health-care workers with respiratory symptoms in Jordan in April, 2012, and in an ongoing hospital outbreak with more than 20 cases in Saudi Arabia.24 Person-to-person transmission was further documented from one patient to two family members in the UK10 and for two cases reported in Tunisia.8 As for SARS-CoV, transmission through large respiratory droplets is currently thought to be the most likely route of MERS-CoV transmission between the two patients we describe. No aerosol-generating procedures were used with patient 1. The presence of diarrhoea in patient 1, possibly linked to MERS-CoV infection as previously described with SARS-CoV,25 might also have been a potential source of contamination of the environment. Systematic detection of MERS-CoV in stools of future patients might be important to document shedding of the virus through faeces and its potential contribution to viral transmission. During the hospital stay in Valenciennes, no personal protective equipment was used, and we did not record any secondary transmission in more than 100 health-care workers assessed for the development of symptoms or tested by RT-PCR for the presence of MERS-CoV (unpublished). Similarly, patient 2 was discharged from the hospital to his home and, up to now, no secondary cases have been detected in some 40 contacts. Low virus shedding in the upper respiratory tract might contribute to reduced transmissibility, although serological investigations will be needed to better assess the extent of transmission. The underlying disorder and immunosuppressive treatment of the two patients probably contributed to their increased susceptibility to the infection, and such background should be added to the list of criteria associated with increased suspicion of MERS-CoV infection. The risk that on acquisition of mutations MERS-CoV might become increasingly transmissible between human beings must also be kept in mind and continuously assessed as suggested.26
Figure 3.
Timeline of pertinent exposure, dates of illness, and virological findings in patients 1 and 2
Exposure (bold red line) shows the period during which the two patients shared the same room.
BAL=bronchoalveolar lavage. NP=nasopharyngeal swab. SP=sputum. Inc=Inconclusive. ECMO=extracorporeal membrane oxygenation. ICU=intensive-care unit.
For the Munich/Abu_Dhabi/2012 sequence see http://www.virology-bonn.de/
This online publication has been corrected. The corrected version first appeared at thelancet.com on June 28, 2013
Acknowledgments
Acknowledgments
The National Reference Center for Influenza Viruses and the CIBU are supported in part by funds from the French Institute for Public Health Surveillance (Institut de veille sanitaire, InVS). Work related to collection of data, implementation of the assays, and sequencing was done with funds from ANR grant Labex Integrative Biology of Emerging Infectious Diseases (IBEID, grant number ANR-10-LABX-62-IBEID from the French Government's Investissement d'Avenir programme) and from the European Community's Seventh Framework Programme (FP7/2007–2013) under the project EMPERIE (European Community grant agreement number 223498) and under the project PREDEMICS (grant agreement number 278433). We thank the nurses and all health-care workers from the hospitals in Valenciennes, Douai, and Lille who, in this crisis situation, took care of the patients and controlled the spread of this disease. We thank P Despres, S Paulous, and M P Frenkiel (Unit of Flavivirus-Host Molecular Interactions, Institut Pasteur) for preliminary serological analyses. We thank Eric Kipnis for editing of the report.
Contributors
BG, AF, and SvdW jointly wrote the report. BG, JP, LeM, CS, NE, XL, FV, and DM were involved in the treatment of patients 1 and 2 and the clinical management of their contacts. AG, SB, VE, VC, JCM, and SvdW were involved in the laboratory testing and interpretation of results from specimens. VE and SvdW did the analysis of the viral sequences. AM and DC were involved in the epidemiological investigation. All other contributors of the MERS-CoV study team participated in the treatment of patients 1 and 2, the clinical management of their contacts, the epidemiological investigation, and virological analyses. All coauthors provided comments and approved the final version of the report.
MERS-CoV study group
France: D Caparros (Pneumologie, Clinique Tessier, Valenciennes); L Vrigneaud, D Labatut, T Quemeneur (Néphrologie, Valenciennes); A-A Cracco (Hygiene, Valenciennes); A Guaguere, C Rousselin, E Lefebvre, P Morel, B Kowalski (Réanimation, Douai); T Coppin (Chirurgie vasculaire, Douai); K Faure, E Senneville, H Melliez (Maladies Infectieuses, Lille, Tourcoing); R Joly, P Goldstein (SAMU, Lille); A Vincentelli, N Rousse (Chirurgie cardio-vasculaire, Lille); R Favory, A Palud, E Parmentier-Decrucq, M Kauv (Réanimation, Lille); M Benassaya, D Briand, M Lazzerini, C Socratous (National Reference Center, Institut Pasteur, Paris); F Fichenick, (CIBU, Institut Pasteur, Paris); J Riou (Institut Pasteur, Paris); K Blanckaert (Antenne Régionale CCLIN, Lille); P Chaud, M-C Paty (InVS, Lille), J-P Legendre, S Segovia-Kueny (Agence Régionale de Santé Nord Pas de Calais, Lille).
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoek L. Human coronaviruses: what do they cause? Antivir Ther (Lond) 2007;12:651–658. [PubMed] [Google Scholar]
- 3.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Bermingham A, Chand MA, Brown CS. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 5.van Boheemen S, de Graaf M, Lauber C. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3:e00473. doi: 10.1128/mBio.00473-12. e00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annan A, Baldwin HJ, Corman VM. Human Betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19:456–459. doi: 10.3201/eid1903.121503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Novel coronavirus infection—update. May 15, 2013. http://www.who.int/csr/don/2013_05_15_ncov/en/index.html (accessed May 17, 2013).
- 8.WHO Novel coronavirus infection—update. May 22, 2013. http://www.who.int/csr/don/2013_05_22_ncov/en/index.html (accessed May 27, 2013).
- 9.WHO Novel coronavirus infection—update. May 23, 2013. http://www.who.int/csr/don/2013_05_23_ncov/en/index.html (accessed May 28, 2013).
- 10.Health Protection Agency (HPA) UK Novel Coronavirus Investigation team Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18:20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 11.Gulland A. Two cases of novel coronavirus are confirmed in France. BMJ. 2013;346:f3114. doi: 10.1136/bmj.f3114. [DOI] [PubMed] [Google Scholar]
- 12.WHO Novel coronavirus summary and literature—update. April 25, 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130425/en/index.html (accessed May 19, 2013).
- 13.de Groot RJ, Baker SC, Baric RS. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013 doi: 10.1128/JVI.01244-13. published online May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman VM, Müller MA, Costabel U. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:20334. doi: 10.2807/ese.17.49.20334-en. [DOI] [PubMed] [Google Scholar]
- 16.Corman VM, Eckerle I, Bleicker T. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:20285. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 17.Cotten M, Lam TT, Watson SJ. Full-genome deep sequencing and phylogenetic analysis of novel human Betacoronavirus. Emerg Infect Dis. 2013;19:736–742. doi: 10.3201/eid1905.130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomersall CD, Joynt GM, Lam P. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Med. 2004;30:381–387. doi: 10.1007/s00134-003-2143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 20.Buchholz U, Müller MA, Nitsche A. Contact investigation of a case of human novel coronavirus infection treated in a German hospital, October-November 2012. Euro Surveill. 2013;18:20406. [PubMed] [Google Scholar]
- 21.Cheng PKC, Wong DA, Tong LKL. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris JSM, Chu CM, Cheng VCC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DAT. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Novel coronavirus summary and literature—update. May 17, 2013. http://www.who.int/csr/disease/coronavirus_infections/update_20130517/en/index.html (accessed May 17, 2013).
- 25.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 26.McCloskey B, Zumla A, Stephens G, Heymann DL, Memish ZA. Applying lessons from SARS to a newly identified coronavirus. Lancet Infect Dis. 2013;13:384–385. doi: 10.1016/S1473-3099(13)70082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.