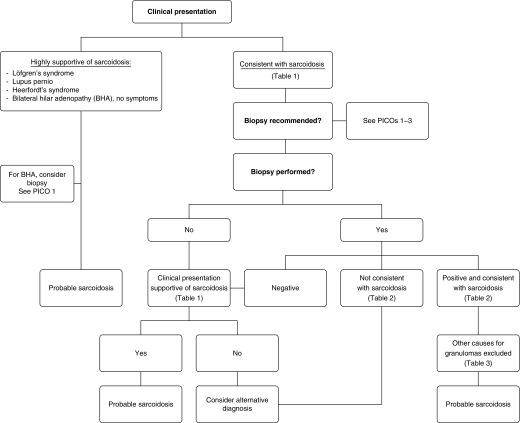
Abstract
Background: The diagnosis of sarcoidosis is not standardized but is based on three major criteria: a compatible clinical presentation, finding nonnecrotizing granulomatous inflammation in one or more tissue samples, and the exclusion of alternative causes of granulomatous disease. There are no universally accepted measures to determine if each diagnostic criterion has been satisfied; therefore, the diagnosis of sarcoidosis is never fully secure.
Methods: Systematic reviews and, when appropriate, meta-analyses were performed to summarize the best available evidence. The evidence was appraised using the Grading of Recommendations, Assessment, Development, and Evaluation approach and then discussed by a multidisciplinary panel. Recommendations for or against various diagnostic tests were formulated and graded after the expert panel weighed desirable and undesirable consequences, certainty of estimates, feasibility, and acceptability.
Results: The clinical presentation, histopathology, and exclusion of alternative diagnoses were summarized. On the basis of the available evidence, the expert committee made 1 strong recommendation for baseline serum calcium testing, 13 conditional recommendations, and 1 best practice statement. All evidence was very low quality.
Conclusions: The panel used systematic reviews of the evidence to inform clinical recommendations in favor of or against various diagnostic tests in patients with suspected or known sarcoidosis. The evidence and recommendations should be revisited as new evidence becomes available.
Keywords: cardiac sarcoidosis, endobronchial ultrasound biopsy, pulmonary hypertension, rare lung disease, granuloma
Contents
Summary of Recommendations
Lymph Node Sampling
Screening for Extrapulmonary Disease
Diagnostic Evaluation of Suspected Extrapulmonary Disease
Introduction
Methods
Diagnosis
Clinical Presentation
Histopathology
Diagnostic Testing
Question 1: Should Lymph Node Sampling Be Performed in a Patient Presenting with Asymptomatic Bilateral Hilar Lymphadenopathy?
Question 2: Should Patients with Suspected Sarcoidosis and Mediastinal and/or Hilar Lymphadenopathy, for Whom It Has Been Determined That Tissue Sampling Is Necessary, Undergo EBUS-guided Lymph Node Sampling or Mediastinoscopy as the Initial Mediastinal and/or Hilar Lymph Node Sampling Procedure?
Question 3: Should Patients with Sarcoidosis Who Do Not Have Ocular Symptoms Undergo Screening for Ocular Sarcoidosis by Routine Eye Examination?
Question 4: Should Patients with Sarcoidosis Who Do Not Have Renal Symptoms Undergo Screening for Renal Sarcoidosis by Routine Serum Creatinine Testing?
Question 5: Should Patients with Sarcoidosis Who Do Not Have Hepatic Symptoms Undergo Screening for Hepatic Sarcoidosis by Routine Transaminase and Alkaline Phosphatase Testing?
Question 6: Should Patients with Sarcoidosis Who Do Not Have Symptoms or Signs of Hypercalcemia Undergo Screening for Abnormal Calcium Metabolism by Routine Serum Calcium and Vitamin D Testing?
Question 7: Should Patients with Sarcoidosis Undergo Screening for Hematological Abnormalities by Routine Complete Blood Cell Count Testing?
Question 8: Should Patients with Sarcoidosis Who Do Not Have Cardiac Symptoms or Signs Undergo Routine Screening for Cardiac Sarcoidosis using ECG, TTE, or 24-Hour Ambulatory ECG Monitoring?
Question 9: Should Patients Who Are Suspected of Having Cardiac Sarcoidosis Undergo Cardiac MRI, TTE, or PET as an Initial Imaging Test?
Question 10: Should Patients with Sarcoidosis Who Are Suspected of Having PH Undergo TTE?
Conclusions
Summary of Recommendations
Lymph Node Sampling
-
1.
In patients for whom there is a high clinical suspicion for sarcoidosis (e.g., Löfgren’s syndrome, lupus pernio, or Heerfordt’s syndrome), we suggest NOT sampling lymph nodes (conditional recommendation, very low-quality evidence). Remarks: Patients who do not undergo lymph node sampling require close clinical follow-up.
-
2.
For patients presenting with asymptomatic, bilateral hilar lymphadenopathy, we make no recommendations for or against obtaining a lymph node sample. Remarks: If lymph node sampling is not obtained, close clinical follow-up is a reasonable alternative approach.
-
3.
For patients with suspected sarcoidosis and mediastinal and/or hilar lymphadenopathy for whom it has been determined that tissue sampling is necessary, we suggest endobronchial ultrasound (EBUS)-guided lymph node sampling, rather than mediastinoscopy, as the initial mediastinal and/or hilar lymph node sampling procedure (conditional recommendation, very low-quality evidence).
Screening for Extrapulmonary Disease
-
1.
For patients with sarcoidosis who do not have ocular symptoms, we suggest a baseline eye examination to screen for ocular sarcoidosis (conditional recommendation, very low-quality evidence).
-
2.
For patients with sarcoidosis who have neither renal symptoms nor established renal sarcoidosis, we suggest baseline serum creatinine testing to screen for renal sarcoidosis (conditional recommendation, very low-quality evidence).
-
3.
For patients with sarcoidosis who have neither hepatic symptoms nor established hepatic sarcoidosis, we suggest baseline serum alkaline phosphatase testing to screen for hepatic sarcoidosis (conditional recommendation, very low-quality evidence).
-
4.
For patients with sarcoidosis who have neither hepatic symptoms nor established hepatic sarcoidosis, we make no recommendation for or against baseline serum transaminase testing.
-
5.
For patients with sarcoidosis who do not have symptoms or signs of hypercalcemia, we recommend baseline serum calcium testing to screen for abnormal calcium metabolism (strong recommendation, very low-quality evidence).
-
6.
If assessment of vitamin D metabolism is deemed necessary in a patient with sarcoidosis, such as to determine if vitamin D replacement is indicated, we suggest measuring both 25- and 1,25-OH vitamin D levels before vitamin D replacement (conditional recommendation, very low-quality evidence).
-
7.
We suggest that patients with sarcoidosis undergo baseline complete blood cell count testing to screen for hematological abnormalities (conditional recommendation, very low-quality evidence).
-
8.
For patients with extracardiac sarcoidosis who do not have cardiac symptoms or signs, we suggest performing baseline ECG to screen for possible cardiac involvement (conditional recommendation, very low-quality evidence).
-
9.
For patients with extracardiac sarcoidosis who do not have cardiac symptoms or signs, we suggest NOT performing routine baseline transthoracic echocardiography (TTE) or 24-hour ambulatory ECG (Holter) monitoring to screen for possible cardiac involvement (conditional recommendation, very low-quality evidence). Remarks: The panel recognizes the low risks attendant to the use of TTE or Holter to screen for cardiac sarcoidosis. Thus, these tests should be considered on a case-by-case basis.
Diagnostic Evaluation of Suspected Extrapulmonary Disease
-
1.
For patients with extracardiac sarcoidosis and suspected cardiac involvement, we suggest cardiac magnetic resonance imaging (MRI), rather than positron emission tomography (PET) or TTE, to obtain both diagnostic and prognostic information (conditional recommendation, very low-quality evidence).
-
2.
For patients with extracardiac sarcoidosis and suspected cardiac involvement who are being managed in a setting in which cardiac MRI is not available, we suggest dedicated PET, rather than TTE, to obtain diagnostic and prognostic information (conditional recommendation, very low-quality evidence).
-
3.
For patients with sarcoidosis in whom pulmonary hypertension (PH) is suspected, we suggest initial testing with TTE (conditional recommendation, very low-quality evidence). Remarks: “PH is suspected” refers to clinical manifestations, including exertional chest pain and/or syncope, exam findings of a prominent P2 or S4, reduced 6-minute walk distance, desaturation with exercise, reduced DlCO, increased pulmonary artery diameter relative to ascending aorta diameter (e.g., by computed tomography [CT] scan), elevated brain natriuretic factor, and/or fibrotic lung disease.
-
4.
For patients with sarcoidosis in whom PH is suspected and a transthoracic echocardiogram is suggestive of PH, we suggest right heart catheterization to definitively confirm or exclude PH (conditional recommendation, very low-quality evidence).
-
5.
For patients with sarcoidosis in whom PH is suspected and a transthoracic echocardiogram is NOT suggestive of PH, the need for right heart catheterization should be determined on a case-by-case basis (best practice statement).
Introduction
The purpose of this clinical practice guideline is to make recommendations that address uncertainties that are commonly confronted by clinicians relating to the diagnosis and detection of sarcoidosis. The target audience is pulmonary, rheumatology, or other clinicians who manage patients with suspected or confirmed pulmonary sarcoidosis. This guideline was developed by an ad hoc committee of experts from the American Thoracic Society with guidance from experienced methodologists to objectively identify and summarize the best available evidence. The quality of the evidence was poor in most cases, reflecting the need for additional high-quality research to guide clinical practice. As such, clinicians, patients, payers, and other stakeholders should not consider these recommendations as mandates. Moreover, no guideline or recommendation can consider all potential clinical circumstances. Thus, clinicians are encouraged to apply the recommendations within the clinical context of each individual patient, including the patient’s values and preferences, and on the basis of regional factors, such as the prevalence of alternative diagnoses or consideration of alternative diagnostic approaches when the preferred diagnostic modality is unavailable.
Methods
A multidisciplinary panel of experts in sarcoidosis was composed to construct clinically important questions related to diagnostic testing for sarcoidosis. Systematic reviews were then performed to inform recommendations that answered each question. The panel used the Grading of Recommendations, Assessment, Development, and Evaluation approach to formulate and grade the strength of the recommendations. The guideline included three patients who participated on the guideline panel and provided perspective on patient values and preferences. A detailed description of the methods, including the implications of the strengths of the recommendation (i.e., strong vs. conditional) and the meaning of best practice statements, are described in the online supplement. The guideline underwent anonymous peer review by four content experts and one methodologist. After multiple cycles of review and revision, the guideline was reviewed and approved by a multidisciplinary board of directors. The guideline will be reviewed by the American Thoracic Society 3 years after publication, and it will be determined if updating is necessary.
Diagnosis
The diagnosis of sarcoidosis is not standardized, but is based on three major criteria: a compatible clinical presentation, the finding of nonnecrotizing granulomatous inflammation in one or more tissue samples (not always required, as discussed subsequently here), and the exclusion of alternative causes of granulomatous disease. Presently, there are no established objective measures to determine if each of these diagnostic criteria has been satisfied, and, therefore, the diagnosis of sarcoidosis is never fully secure. In this section of the article, these three diagnostic criteria will be discussed separately.
Clinical Presentation
The clinical presentation of sarcoidosis exhibits a spectrum of manifestations ranging from the asymptomatic state to that of progressive and relapsing disease. Disease progression often leads to pulmonary impairment or, in some cases, death due to complications of progressive pulmonary fibrosis or from cardiac involvement, including sudden cardiac death (arrhythmias) or congestive heart failure (myocarditis). The global health implications of sarcoidosis remain unknown, but new evidence indicates that the disease is much more prevalent than previously estimated (1), and mortality among patients with sarcoidosis is much higher than previously reported in some patient populations (e.g., 2.4-times higher mortality in African American women with sarcoidosis compared with a matching cohort without sarcoidosis [2]). There is great variability in the number of organs clinically involved with sarcoidosis, which adds to diagnostic uncertainty based on highly variable clinical presentations. Whereas many sarcoidosis cases are a diagnostic dilemma, certain clinical features of sarcoidosis are considered so highly specific for the disease that they have been deemed diagnostic (3). These include Löfgren’s syndrome (4), lupus pernio (5), and Heerfordt’s syndrome (6). Other features have been strongly associated with sarcoidosis, such as bilateral hilar adenopathy in patients without B symptoms (fevers, night sweats, and weight loss) (7).
To standardize organ involvement in sarcoidosis, consensus criteria were originally established in 1999 (8) and updated in 2014 (9). These instruments assumed that the patient had known sarcoidosis, and assessed the probability of specific organ involvement. The 2014 document was sponsored by the World Association of Sarcoidosis and Other Granulomatous Disorders. In that document, the criteria for sarcoidosis involvement of each organ were established on the basis of consensus among sarcoidosis experts using a structured Delphi methodology, and the confidence of organ involvement was further qualified on a scale of highly probable, probable, or possible. Consensus for a specific criterion was considered achieved if more than 70% of the experts agreed. Some clinical features failed to reach a consensus. Two recent reports using similar methodology from groups of sarcoidosis experts have developed clinical criteria for the diagnosis of cardiac (10) and neurologic sarcoidosis (11).
Table 1 provides a summary of clinical features and related relative probabilities supporting a diagnosis of sarcoidosis based on history, physical examination, imaging, and laboratory testing. This table is not an exhaustive list of the clinical manifestations of sarcoidosis, but encompasses clinical features of sarcoidosis that are relatively common and specific enough to inform the clinical suspicion of the disease. In recognition of the central role of imaging during the initial assessment of interstitial lung diseases, as briefly summarized in Table 1, we refer the interested reader to a more comprehensive review of this topic (12). This table has also simplified certain criteria from the more detailed World Association of Sarcoidosis and Other Granulomatous Disorders document (8), such as by combining various forms of uveitis under one heading.
Table 1.
Clinical Features Supportive of a Diagnosis of Sarcoidosis
| Highly Probable | Probable | |
|---|---|---|
| History | Löfgren’s syndrome* | Seventh cranial nerve paralysis |
| Treatment-responsive renal failure | ||
| Treatment-responsive CM or AVNB | ||
| Spontaneous/inducible VT with no risk factors | ||
| Physical | Lupus pernio | Maculopapular, erythematous, or violaceous skin lesions |
| Uveitis | Subcutaneous nodules | |
| Optic neuritis | Scleritis | |
| Erythema nodosum | Retinitis | |
| Lacrimal gland swelling | ||
| Granulomatous lesions on direct laryngoscopy | ||
| Symmetrical parotid enlargement | ||
| Hepato-/splenomegaly | ||
| Imaging | Bilateral hilar adenopathy (CXR, CT, and PET) | Upper lobe or diffuse infiltrates (CXR, CT, and PET) |
| Perilymphatic nodules (chest CT) | Peribronchial thickening (CT) | |
| Gadolinium enhancement on MRI (CNS) | Two or more enlarged extra thoracic nodes (CT, MRI, and PET) | |
| Osteolysis, cysts/punched-out lesion, trabecular pattern bone (X-ray, CT, and MRI) | Increased inflammatory activity in heart (MRI, PET, and gallium) | |
| Parotid uptake (gallium and PET) | Imaging showing enlargement or nodules in liver or spleen (CT, PET, and MRI) | |
| Inflammatory lesions in bone (gallium, PET, and MRI) | ||
| Other testing | Hypercalcemia or hypercalciuria with abnormal vitamin D metabolism† | Reduced LVEF with no risk factors (echo and MRI) |
| Elevated ACE level test‡ | ||
| Nephrolithiasis with calcium stone, no vitamin D testing | ||
| BAL lymphocytosis or elevated CD4:CD8 ratio | ||
| Alkaline phosphatase greater than three times the upper limit of normal | ||
| New-onset, third-degree AV block in young or middle-aged adults |
Definition of abbreviations: ACE = angiotensin-converting enzyme; AV = atrioventricular; AVNB = atrioventricular node block; CM = cardiomyopathy; CNS = central nervous system; CT = computed tomography; CXR = chest X-ray; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; PET = positron emission tomography; VT = ventricular tachycardia.
Löfgren’s syndrome is defined as bilateral hilar adenopathy with erythema nodosum and/or periarticular arthritis.
Abnormal vitamin D metabolism is defined as normal to low parathyroid hormone, normal to elevated 1,25-dihydroxyvitamin D, and normal to low 25-hydroxyvitamin D.
ACE elevated above 50% of the upper limit of normal was considered abnormal.
Table 1 does not account for the presence of multiple clinical features for the diagnosis of sarcoidosis that may reinforce the diagnosis of sarcoidosis beyond that when only one feature is present. Nevertheless, we endorse the proposed list of clinical features to aid clinicians during the initial diagnostic evaluation of patients with suspected sarcoidosis.
Histopathology
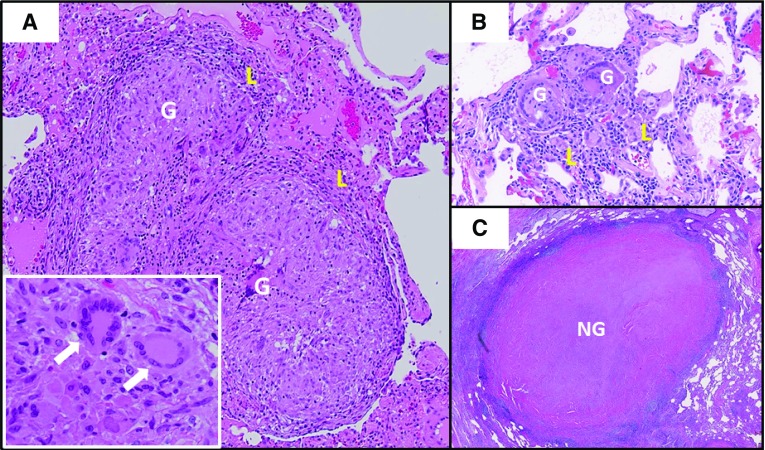
Given that the clinical manifestations of sarcoidosis are often nonspecific, histological evaluation of tissue granulomas is often required to establish the diagnosis. Histological features that are typical of a sarcoidosis granuloma include the presence of well-formed, concentrically arranged layers of immune cells, most prominent being the central core of macrophage aggregates and multinucleated giant cells. An outer layer of loosely organized lymphocytes, mostly T cells, is often observed with a few interposed dendritic cells. In some cases, the granulomas are surrounded by isolated collections of B lymphocytes. Sarcoidosis granulomas are most often nonnecrotic; however, variants of sarcoidosis, particularly the nodular pulmonary sarcoidosis phenotype, can present with a mixture of necrotic and nonnecrotic granulomas (13).
The differential diagnosis of granulomatous diseases is broad, as noted in the next section. Table 2 and Figure 1 provide histopathological features that are useful for discriminating sarcoidosis from other causes, although histopathologic features alone cannot distinguish sarcoidosis from other granulomatous diseases. Certain granulomatous diseases may have similar histological features, such as berylliosis (chronic beryllium disease).
Table 2.
Key Pathological Features of Sarcoidosis
| Favors Sarcoidosis | Against Sarcoidosis |
|---|---|
| Granuloma presence | |
| Numerous | Few |
| Absent but with nodular hyalinized fibrosis representing healed granulomas (scattered multinucleated giant cells may be detectable) | Absent |
| Granuloma morphology | |
| Compact, tightly formed collections of large “epithelioid” histiocytes and multinucleated giant cells. Granulomas tend to stay discrete | Loosely organized collections of mononuclear phagocytes/multinucleated giant cells |
| Nonnecrotic or focal and usually minimal ischemic necrosis | • Extensive necrosis |
| • Dirty necrosis (containing nuclear debris) | |
| • Palisading granulomas | |
| Fibrosis beginning at the granuloma periphery with extension centrally into the granuloma, with or without calcification | |
| Lesion location | |
| Perilymphatic; around bronchovascular bundles and fibrous septa containing pulmonary veins, and near visceral pleura | • Lack of lymphangitic distribution |
| • Intraalveolar granulomas | |
| In necrotizing sarcoid angiitis and granulomatosis: granulomatous angiitis with invasion of vascular walls | |
| | |
| Accompanying histology | |
| Sparse surrounding lymphocytic infiltrate | • Robust surrounding inflammatory infiltrate (including lymphocytes, neutrophils, eosinophils, and plasma cells) |
| • Secondary lymphoid follicles | |
| Microorganism stains and cultures | |
| Negative | Positive |
| Multidisciplinary clinical features | |
| Intra- and extrathoracic involvement | Extrathoracic involvement only |
Figure 1.
Comparison of pulmonary sarcoidosis granuloma histology to other granulomatous lung diseases. (A) Typical sarcoidosis histology with well-formed granulomas comprised of macrophage aggregates (G) and featuring multinucleated giant cells (white arrows, inset), with minimal surrounding lymphocytic inflammation (L). (B) Hypersensitivity pneumonitis featuring smaller granulomas (G) with more extensive surrounding lymphocytic alveolitis (L). (C) A large acellular necrotizing granuloma (NG) caused by pulmonary Histoplasma capsulatum infection.
Exclusion of alternative causes
To ensure diagnostic accuracy of sarcoidosis, the differential diagnosis must be considered and alternative diagnoses reliably excluded during the initial diagnostic evaluation or in presumed established sarcoidosis cases with atypical clinical features, such as those refractory to immune suppression treatment. Although tissue histopathology may reveal an alternative diagnosis (Figure 1), granulomas found in patients with sarcoidosis have no unique histologic features to differentiate them from all other granulomatous diseases. The diagnosis of sarcoidosis, therefore, requires a complete history and physical examination and, when indicated, additional testing to exclude other disorders, especially those that also produce granulomas (14). The differential diagnosis of sarcoidosis is typically categorized into granulomatous disorders of infectious and noninfectious causes (Table 3 provides representative examples of each). An alternative schema classifies these diagnoses according to the affected organ system(s) (Table 4).
Table 3.
Key Infectious and Noninfectious Differential Diagnoses for Granulomatous Lesions within Commonly Biopsied Sites
| Granulomatous Lesion within These Sites: |
Testing and Clinical Pearls | |||||
|---|---|---|---|---|---|---|
| Lung | Lymph Node | Skin | Liver | Bone Marrow | ||
| Infectious etiologies | ||||||
| Bacteria | ||||||
| Tuberculosis* | X | X | X | X | X | Culture is diagnostic gold standard; IFN-γ release assay used for screening, and preferable to tuberculin skin testing due to anergy |
| Nontuberculous mycobacteria (MAC and M. kansasii)* | X | X | X | X | X | Culture is the gold standard |
| Aspiration pneumonia* | X | Culture | ||||
| Brucella | X | X | X | X | Serum agglutination and ELISA; livestock exposure history | |
| Tropheryma whippelii | X | X | Periodic acid–Schiff stain; immunohistochemistry testing; diarrhea, weight loss, and joint pains | |||
| Mycobacterium leprae | X | Culture is the gold standard, but can be difficult; histology; PCR | ||||
| Francisella tularensis | X | X | Serologic assay, then repeat in 2 wk; rabbit exposure | |||
| Bartonella henselae | X | X | Titers >1:256; cat exposure | |||
| Coxiella burnetii | X | X | Serology; PCR; livestock exposure | |||
| Fungi | ||||||
| Aspergillus* | X | X | X | Culture; Aspergillus IgG; histology | ||
| Histoplasma* | X | X | X | X | Culture; urine histoplasma antigen | |
| Blastomyces* | X | X | Culture; histology; blasto Ag is nonspecific | |||
| Coccidioides* | X | X | Serologic tests using EIA for IgM and IgG; then confirmatory immunodiffusion | |||
| Cryptococcus | X | X | X | X | Cryptococcal serum antigen | |
| Pneumocystis | X | Histology; screen with β-d-glucan assay | ||||
| Viruses | ||||||
| Herpes zoster | X | X | Granulomas may occasionally be found | |||
| Parasitic | ||||||
| Toxoplasma gondii | X | X | X | Toxoplasma serologic assay IgM and IgG | ||
| Schistosomiasis | X | X | X | Serology and microscopic visualization of eggs in stool or urine | ||
| Leishmaniasis | X | X | Histology and PCR for Leishmania | |||
| Echinococcosis | X | X | EIA; ultrasound imaging | |||
| Enterobius | X | X | Pinworm paddle test, then microscopy | |||
| Dirofilaria | X | Histology; eosinophilia | ||||
| Noninfectious etiologies | ||||||
| Malignancy | ||||||
| Lymphoma* | X | X | X | X | X | Clonal cell population; rarely can have elevated serum ACE |
| Sarcoid-like reaction to tumor* | X | X | X | X | X | PET useful for selecting biopsy site but not diagnostic; biopsy must be performed to diagnose |
| Lymphomatoid granulomatosis | X | Atypical clonal EBV-positive B cells; multiple pulmonary nodules with lymphocytic transmural angiitis and granulomas noted sometimes in skin | ||||
| Germ cell tumor | X | Serum α fetoprotein, human chorionic gonadotropin, lactate dehydrogenase | ||||
| Autoimmune or immune dysfunction | ||||||
| ANCA-associated vasculitides (GPA, MPA, and EGPA) | X | X | MPO or PR3 ANCA+, renal disease, necrotizing vasculitis; eosinophilic infiltration if EGPA | |||
| GLILD associated with CVID | X | X | Nonnecrotizing granulomas, LIP, and follicular bronchiolitis on lung biopsy; hypogammaglobulinemia and recurrent infections | |||
| Rheumatoid nodules | X | Multiple subpleural nodules in patient with anti-CCP antibodies, arthralgias; necrotizing granulomas | ||||
| Langerhans cell histiocytosis | X | X | X | X | X | Young smoker; multiple bizarre-shaped upper lung zone cysts and/or nodules; Langerhans cell stain CD1a and S100 positive; eosinophilic granulomas most common |
| IgG4-related disease | X | X | X | X | X | Elevated serum IgG4; elevated tissue IgG4+ plasma cell count and IgG4:IgG ratio; granulomas rare; differential diagnosis with multicentric Castleman disease |
| Inflammatory bowel disease | X | X | X | GI symptoms; granulomatous bronchiolitis | ||
| Primary biliary cholangitis | X | Cholestasis; antimitochondrial antibodies; portal based, poorly formed granulomas with bile duct destruction | ||||
| Primary sclerosing cholangitis | X | Cholestasis; P-ANCA+; ulcerative colitis associated; biliary strictures present, granulomas rare and not associated with bile duct destruction | ||||
| Autoimmune hepatitis | Abnormal liver function tests and autoantibodies (e.g., anti–smooth muscle); syncytial multinucleated giant cells are rare in adults but may be observed in children or adolescents | |||||
| Exposures | ||||||
| Hypersensitivity pneumonitis* | X | X | Organic exposure, small poorly formed interstitial granulomas in interstitium, prominent lymphocytic infiltrates, chronic inflammatory infiltrates accentuated around bronchioles | |||
| Hot tub lung syndrome (MAC exposure with hypersensitivity features) | X | X | Aerosolized water exposure, MAC cultured from sputum, lung or hot tub, large well-formed granulomas in bronchiole lumens | |||
| Pneumoconiosis (such as beryllium, titanium, aluminum, zirconium, cobalt, and others) | X | X | X | Inorganic exposure history | ||
| Drug-induced granulomatous disease (including but not limited to IFN, checkpoint inhibitor, anti-TNF, and/or biologic therapies)* | X | X | X | X | X | Usually nonnecrotizing granulomas. Drug exposure history essential. Seewww.pneumotox.com for full list |
| Foreign body granulomatosis (such as talc aspirated or injected, tattoo ink)* | X | X | X | Serum ACE elevated in many patients; particles found on biopsy; perivascular granulomas | ||
| Steatosis (lipogranulomas) | X | Central lipid vacuole; ingestion of mineral oil or hepatic steatosis | ||||
| Idiopathic | ||||||
| Sarcoidosis | X | X | X | X | X | Multisystemic; well formed, usually nonnecrotic granulomas |
| Necrotizing sarcoid granulomatosis | X | X | Granulomatous pneumonitis with necrosis and vasculitis; multiple necrotic lung nodules | |||
| Histiocytic necrotizing lymphadenitis (Kikuchi’s disease) | X | Cervical lymphadenopathy and low-grade fever. Granulomas are not found, although necrotic areas with histiocytes are present | ||||
| GLUS | X | X | X | X | Lacks progressive lung parenchymal disease, elevated serum calcium, 1,25-dihydroxyvitamin D, and ACE | |
| Bronchocentric granulomatosis | X | Associated with asthma and Aspergillus infection in 50%. Necrotizing granulomas exclusively in bronchi and bronchioles | ||||
Definition of abbreviations: ACE = angiotensin-converting enzyme; Ag = antigen; EIA = enzyme-linked immunoassays; ANCA = antineutrophil cytoplasmic antibody; CCP = cyclic citrullinated peptide; CVID = common variable immune deficiency; EBV = Epstein-Barr virus; EGPA = eosinophilic GPA; GI = gastrointestinal; GLILD = granulomatous–lymphocytic interstitial lung disease; GLUS = granulomatous lesions of unknown significance syndrome; GPA = granulomatosis with polyangiitis; LIP = lymphocytic interstitial pneumonia; MAC = Mycobacterium avium complex; M. kansasii = Mycobacterium kansasii; MPA = microscopic polyangiitis; MPO = myeloperoxidase; p-ANCA = perinuclear ANCA; PR3 = PR3-ANCA; PET = positron emission tomography; TNF = tumor necrosis factor.
More commonly found alternative diagnoses for granulomatous disease in U.S. populations. The differential diagnosis should be prioritized on the basis of the individual’s clinical history and presentation.
Table 4.
Key Differential Diagnoses for Sarcoidosis within Individual Organ Systems
| Organ System | Noninfectious Differential Diagnoses | Infectious Differential Diagnoses |
|---|---|---|
| Central nervous system | IgG4-related disease | Bacteria |
| Chronic variable immunodeficiency | • Tuberculosis | |
| Rosai-Dorfman disease | • Brucella | |
| Histiocytoses | Fungi | |
| • Histiocytosis X | • Aspergillus | |
| • Erdheim-Chester | • Coccidioidomycosis | |
| Lymphomatoid granulomatosis | • Cryptococcosis | |
| Granulomatosis with polyangiitis | Parasites | |
| Rheumatoid nodules | • Amoeba | |
| Amyloidosis | • Toxoplasmosis | |
| Cholesterol granuloma | • Schistosomiasis | |
| Foreign body | • Taenia solium | |
| Drugs/toxins/heavy metals | • Echinococcus | |
| Sarcoid-like reaction to tumor | • Paragonimiasis | |
| CNS malignancies ranging from glioblastoma to lymphoma | Viruses | |
| • Varicella zoster | ||
| • Herpes simplex | ||
| Eyes | Inflammatory bowel disease | Parinaud oculoglandular syndrome |
| ANCA vasculitides | • Bartonella | |
| Vogt-Koyanagi-Harada disease | • Francisella | |
| Blau syndrome | Bacteria | |
| • Tuberculosis | ||
| • Syphilis | ||
| Viruses | ||
| • Cytomegalovirus | ||
| • Varicella zoster | ||
| Toxoplasmosis | ||
| Sinonasal | Granulomatosis polyangiitis | Bacteria |
| Eosinophilic granulomatosis with polyangiitis | • Tuberculosis | |
| Cholesterol granuloma | • Atypical | |
| NK/T-cell lymphoma | Mycobacteria | |
| Foreign body | • Klebsiella | |
| Drugs/toxins | Rhinoscleromatis | |
| • Cocaine | • Syphilis | |
| • Narcotics | Fungi | |
| • Aspergillus flavus | ||
| • Histoplasmosis | ||
| Parasites | ||
| • Leishmaniasis | ||
| • Rhinosporidiosis | ||
| Parotid/salivary/lacrimal glands | Granulomatosis polyangiitis | Bacteria |
| Ductal obstruction (calculus, tumor) | • Tuberculosis | |
| Crohn’s disease | • Atypical mycobacteria | |
| Heart | Giant cell myocarditis | Bacteria |
| Acute rheumatic heart disease | • Tuberculosis | |
| Granulomatosis with polyangiitis | • Syphilis | |
| Erdheim-Chester | • Tropheryma whippelii | |
| Arrhythmogenic right ventricular dysplasia | Fungi | |
| Foreign body | • Aspergillus | |
| Drugs/toxins | ||
| Granulomatous lesions of unknown significance | ||
| Spleen | Chronic variable immunodeficiency | Bacteria |
| Sarcoid-like reaction to tumor | • Tuberculosis | |
| Fungi | ||
| • Histoplasmosis | ||
| Parasites | ||
| • Leishmaniasis | ||
| Kidney | Granulomatosis polyangiitis | Bacteria |
| Chronic lymphocytic leukemia | • Tuberculosis | |
| Drugs | Fungi | |
| • Allopurinol | • Histoplasmosis | |
| • Antivirals | • Coccidioidomycosis | |
| • Anticonvulsants | Viral | |
| • β-Lactams | • Adenovirus | |
| • Diuretics | ||
| • Erythromycin | ||
| • Fluoroquinolones | ||
| • NSAIDs | ||
| • Proton pump inhibitors | ||
| • Rifampin | ||
| • Sulfonamides | ||
| • Vancomycin | ||
| Muscle | Non-Hodgkin lymphoma | Bacteria |
| Crohn’s disease | • Tuberculosis | |
| Thymoma-myasthenia gravis | • Syphilis | |
| Foreign body | • Brucella | |
| Primary biliary cirrhosis (primary biliary cholangitis) | Fungi | |
| Cryofibrinogenemia | • Pneumocystis jirovecii | |
| • Cryptococcosis | ||
| Virus | ||
| • Human T-lymphotrophic virus 1 |
Definition of abbreviations: ANCA = antineutrophil cytoplasmic antibody; CNS = central nervous system; NK = natural killer; NSAIDs = nonsteroidal antiinflammatory drugs.
Tuberculosis (TB) and atypical mycobacterial infections can mimic sarcoidosis. These infections can be screened for by staining biopsies for acid-fast bacilli and latent TB infection can be detected by performing IFN-γ release assay testing or delayed-type hypersensitivity skin testing to TB antigens, as was previously recommended as a standard approach in patients with suspected sarcoidosis (14). It should be noted that false-negative IFN-γ release or skin test results can occur in those with acutely active forms of TB or sarcoidosis due to concurrent T-cell anergy; thus, negative test results should be interpreted with caution (15). When possible, sputum smear and culture for acid-fast bacilli (16) and molecular testing (17) for mycobacterial species is encouraged for patients residing in areas endemic for TB. Fungal infections (e.g., histoplasmosis) should also be considered in those with suspected sarcoidosis, including staining biopsies for fungal infections. Tissue culture, culture of BAL fluid, antigen detection in urine and/or blood, and serologic tests for fungal-specific antibodies may be used to confirm the diagnosis (18).
Additional testing for other infectious and noninfectious etiologies is guided by clinical and/or radiologic findings (Tables 3 and 4). Hypersensitivity pneumonitis and chronic beryllium disease should be considered in patients with a history of occupational and/or environmental exposures associated with these disorders. The blood lymphocyte proliferation test is diagnostic for chronic beryllium disease (19). Although BAL fluid analysis is insufficient to establish a specific diagnosis of any interstitial lung disease, BAL can be useful for excluding infections or malignancy or to identify cellular patterns suggestive of eosinophilic or hypersensitivity pneumonitis (20).
Sarcoidosis-like granulomatous reactions have been described in numerous clinical conditions and in association with several medications, including immunotherapeutics, such as immune checkpoint inhibitors, anti–TNF-α (tumor necrosis factor-α), and other immune modulating drugs (21). Sarcoid-like reactions to tumor should be considered in patients with granulomatous adenopathy who are suspected of having malignancy or in those with a recent or concomitant history of neoplasm (22). Erdheim-Chester, a histiocytic disorder with clinical and radiologic features similar to sarcoidosis, may be differentiated from sarcoidosis on the basis of histopathologic staining for the CD68 marker (23). The small-vessel antineutrophil cytoplasmic antibody–associated vasculitides (especially granulomatosis with polyangiitis) may affect the upper and lower airways, similar to sarcoidosis. Most patients with these vascular inflammatory diseases, however, exhibit MPO and/or PR3 antineutrophil cytoplasmic antibodies.
Patients with common variable immune deficiency may develop noncaseating granulomas in lymphoid or solid organs, referred to as granulomatous–lymphocytic interstitial lung disease, which mimics multiorgan sarcoidosis. Granulomatous–lymphocytic interstitial lung disease due to common variable immune deficiency should be suspected in patients with apparent sarcoidosis who have hypogammaglobulinemia, a history of recurrent sinopulmonary infections, autoimmune disease, or splenomegaly. IgG4-related disease may resemble pulmonary sarcoidosis (bilateral hilar adenopathy and/or lung nodules on CT of the chest) and extrapulmonary, multiorgan sarcoidosis (24); pathology can usually differentiate IgG4-related disease from sarcoidosis (25). Elevated serum IgG4 levels (high IgG4:IgG ratio) is present in approximately 66% of patients with IgG4 disease (26), and elevated tissue plasma cell IgG4 may further differentiate this disorder from sarcoidosis.
Diagnostic Testing
Question 1: Should Lymph Node Sampling Be Performed in a Patient Presenting with Asymptomatic Bilateral Hilar Lymphadenopathy?
Rationale for question
Isolated involvement of mediastinal and hilar lymph nodes is a common presentation of sarcoidosis, and is readily detected by a routine chest X-ray. Many such patients are asymptomatic, and sarcoidosis is only suspected on the basis of radiographic testing for an unrelated reason. Patients with sarcoidosis with asymptomatic lymph node involvement generally have self-limited disease, and do not require treatment. However, the finding of enlarged hilar and mediastinal lymph nodes during radiographic testing is often alarming to healthcare providers and patients alike, primarily out of concern for an alternative diagnosis, such as occult malignancy or latent infection. Previous studies have reported that asymptomatic bilateral hilar lymphadenopathy is almost always caused by sarcoidosis (27), and, given the benign nature of this phenotype, there is clinical equipoise for pursuing diagnostic sampling in such patients.
Summary of evidence
Our systematic review identified 2,106 potentially relevant articles; the full text of 75 was reviewed. One study reported enrolling patients with bilateral hilar lymphadenopathy, but included both symptomatic and asymptomatic patients (28), so the panel decided to separately consider 16 studies that enrolled patients with suspected radiographic stage 1 sarcoidosis (29–44). The study that enrolled patients with symptomatic and asymptomatic bilateral hilar lymphadenopathy confirmed sarcoidosis in 72% (95% confidence interval [CI], 61–81%), but found lymphoma in 10% (95% CI, 5.3–19%) and other diagnoses (i.e., nonlymphomatous malignancy, silicosis, fibrosis, and amyloidosis) in 7.7% (95% CI, 3.6–15.8%). The 16 studies that enrolled patients with suspected radiographic stage 1 sarcoidosis collectively included 556 patients who underwent at least 1 sampling procedure. Sarcoidosis was confirmed in 85% (95% CI, 82–88%) of patients, an alternative diagnosis was made in 1.9% (95% CI, 1–3.7%) of patients, and sampling was nondiagnostic in 11% (95% CI, 8–14%) of patients. Among the alternative diagnoses, 38% (95% CI, 14–69%) were TB and 25% (95% CI, 7.1–59%) were lymphoma. The only complication reported was a case of mediastinitis that occurred after an esophageal endoscopic ultrasound procedure.
Committee conclusions
The committee acknowledged that most patients with bilateral hilar lymphadenopathy will be confirmed to have sarcoidosis, especially among those presenting with Löfgren’s syndrome, lupus pernio, or Heerfordt’s syndrome. In asymptomatic bilateral hilar lymphadenopathy cases, sampling will be nondiagnostic in a substantial number of patients, and an alternative diagnosis (e.g., malignancy or infections) will be identified in a few cases, but those few cases may have important treatment implications. Factors to consider when weighing the risks and benefits of biopsy may include: regional prevalence of alternative infectious etiologies; patient-specific risk factors for malignancy, infection, or enhanced procedural risk; enlarging lymph nodes; likelihood of obtaining close follow-up; and patient preference. Finally, the availability of a maximally safe, efficacious, and cost-effective means of biopsy procedure is considered. Thus, the committee concluded that the decision to biopsy asymptomatic patients with bilateral hilar adenopathy should be made on a case-by-case basis.
Recommendations
-
1.
In patients for whom there is a high clinical suspicion for sarcoidosis (e.g., Löfgren’s syndrome, lupus pernio, or Heerfordt’s syndrome), we suggest NOT sampling lymph nodes (conditional recommendation, very low-quality evidence). Remarks: Patients who do not undergo lymph node sampling require close clinical follow-up.
-
2.
For patients presenting with asymptomatic bilateral hilar lymphadenopathy, we make no recommendations for or against obtaining a lymph node sample. Remarks: If lymph node sampling is not obtained, close clinical follow-up was considered a reasonable alternative approach.
Research needs
All but one study selected for the systematic review enrolled patients with suspected sarcoidosis; therefore, the probability of finding sarcoidosis and alternative diagnoses in unselected patients with asymptomatic bilateral hilar adenopathy is uncertain and requires further investigation. Studies are needed to determine which clinical factors, including CT or chest radiographic manifestations, and/or biomarkers are the best determinants of pretest probability of sarcoidosis and which radiographic features are predictive of disease progression (45). Such predictive modeling might enable certain patients to be spared from biopsy, while heightening the need for early diagnosis in others. Studies are also needed to determine the true incidence of both minor and major complications of biopsy. Finally, studies that determine long-term outcomes, including treatment and disease course, are needed to determine the utility of early biopsy in asymptomatic patients.
Question 2: Should Patients with Suspected Sarcoidosis and Mediastinal and/or Hilar Lymphadenopathy, for Whom It Has Been Determined That Tissue Sampling Is Necessary, Undergo EBUS-guided Lymph Node Sampling or Mediastinoscopy as the Initial Mediastinal and/or Hilar Lymph Node Sampling Procedure?
Rationale for question
Tissue sampling is often helpful in the diagnostic evaluation of suspected sarcoidosis. It begins with the least-risky and least-invasive method, such as sampling skin or peripheral lymph node (e.g., axillary lymph node) abnormalities suggestive of sarcoidosis. However, most new sarcoidosis cases lack skin and peripheral lymph node findings, and invasive testing is pursued from the start. Superiority of EBUS-guided lymph node sampling over transbronchial lung biopsy in pulmonary sarcoidosis has already been established (46–48). The committee asked whether EBUS-guided lymph node sampling or mediastinoscopy is preferable.
Summary of evidence
Our systematic review identified 703 potentially relevant articles; the full text of 64 was reviewed and 29 were selected. There were no studies comparing EBUS-guided sampling to mediastinoscopy; all studies were nonrandomized studies that reported the diagnostic yield and other outcomes of either EBUS-guided lymph node sampling (30, 31, 36, 39, 41, 43, 44, 49–60) or mediastinoscopy (37, 61–70) in patients with suspected sarcoidosis.
EBUS-guided lymph node sampling had a diagnostic yield of 87% (95% CI, 94–91%). Among the diagnostic samples, 98% confirmed sarcoidosis and 2% found an alternative diagnosis, including lymphoma, TB, and lung cancer. There were no reported major complications of EBUS, and only one case of post-procedure stridor, yielding a reported complication rate of <0.1%. Only one mediastinoscopy study (published in 2006) reported outcomes in the same fashion as the EBUS studies (37); it found that mediastinoscopy had a diagnostic yield of 98% (95% CI, 90–100%). Among the diagnostic samples, 91% confirmed sarcoidosis and 9% found reactive lymphadenopathy. The other mediastinoscopy studies (all published before 1970) reported that 96% (95% CI, 94–97%) of procedures confirmed sarcoidosis, and the remaining 4% (95% CI, 3–6%) found an alternative diagnosis or were nondiagnostic.
Committee conclusions
The committee weighed the observations that mediastinoscopy has a higher diagnostic yield than EBUS-guided lymph node biopsy (98% and 87%, respectively), but EBUS-guided lymph node biopsy is less invasive than mediastinoscopy. The committee recognized probable underestimation of the risk of mediastinoscopy given the bias toward reporting only severe complications, implying that less severe complications, like vocal cord damage and their associated costs and inconveniences, were not counted (71, 72). In lieu of rigorous data directly comparing complication rates of mediastinoscopy to EBUS specifically among patients with suspected sarcoidosis, the committee considered a recent systemic review of 9 studies (960 cases) comparing complications of mediastinoscopy and EBUS among patients undergoing mediastinal staging of lung cancer. The systemic review found a statistically higher complication rate for mediastinoscopy (73). The committee also agreed that costs are typically lower for procedures such as EBUS that are performed in an endoscopy room compared with an operating room for mediastinoscopy, and that EBUS is better tolerated than mediastinoscopy because general anesthesia may be avoided (74, 75), although the committee acknowledged that dissenting opinions exist (76, 77). Finally, the committee noted the ease of adding transbronchial biopsy when lymphadenopathy is accompanied by radiographic findings of parenchymal disease, or endobronchial biopsy when mucosal abnormalities are noted during endoscopy, which further increase the yield of bronchoscopy with EBUS (78). The committee concluded that the advantages of EBUS-guided lymph node sampling for the 87% of patients in whom it is diagnostic outweigh the additional risks and burdens to the 13% of patients who require an additional sampling procedure.
The role of conventional blind transbronchial needle aspiration (TBNA) was not specifically investigated. Prior studies directly comparing blind TBNA to EBUS-guided TBNA show the performance of the latter to be significantly better (79, 80) with much higher negative predictive value (80). Nonetheless, the committee believed that conventional TBNA is a low-risk procedure that is widely available and, therefore, is a reasonable alternative to EBUS when the latter is unavailable.
Recommendation
-
1.
For patients with suspected sarcoidosis and mediastinal and/or hilar lymphadenopathy for whom it has been determined that tissue sampling is necessary, we suggest EBUS-guided lymph node sampling, rather than mediastinoscopy, as the initial mediastinal and/or hilar lymph node sampling procedure (conditional recommendation, very low-quality evidence).
Research needs
The committee concluded that there is a need for research to determine whether the addition of CT or chest radiographic manifestations, or genomic or biochemical profiling, increases diagnostic yield beyond histologic assessment for tissue samples obtained via EBUS-guided sampling.
Question 3: Should Patients with Sarcoidosis Who Do Not Have Ocular Symptoms Undergo Screening for Ocular Sarcoidosis by Routine Eye Examination?
Rationale for question
Sarcoidosis can involve almost every portion of the eye, including the orbit, anterior and posterior chambers, lacrimal gland, sclera, and conjunctiva. Uveitis and retinal involvement are the most concerning manifestations, because they can result in blindness and are not typically apparent on a routine physical examination. Some patients present with uveitis as their initial clinical manifestation. Prevalence of ocular involvement varies by sex and race, with higher rates noted in women and Japanese and persons of African descent. The diagnosis of ocular sarcoidosis is based on a combination of signs and tests (81), which are often not sought until symptoms develop. The committee asked whether patients with sarcoidosis without ocular symptoms should undergo ophthalmologic screening for ocular sarcoidosis.
Summary of evidence
Our systematic review identified 582 potentially relevant articles; the full text of 25 was reviewed and 18 were selected. None of the studies compared eye exams to no eye exams; all were nonrandomized studies that enrolled patients with extraocular sarcoidosis and reported the frequency of abnormal eye exams and other outcomes (82–99). Although the question is intended for patients with sarcoidosis without ocular symptoms, all studies included patients with and without ocular symptoms.
Eye exams identified abnormalities consistent with ocular sarcoidosis in 26% (95% CI, 23–29%) of patients with sarcoidosis. The most common abnormality was anterior uveitis, which was detected in 53% (95% CI, 41–64%) of patients. Ocular symptoms were present in 78% (95% CI, 64–91%) of patients with an eye exam abnormality. Ocular disease was judged severe enough to warrant topical or systemic corticosteroid treatment in 83% (95% CI, 74–93%) of patients.
Committee conclusions
The systematic review estimated the prevalence of ocular sarcoidosis to be 26%. The committee was concerned that this estimate is higher than the actual prevalence among the population of interest (patients with sarcoidosis without ocular symptoms), as most patients with ocular abnormalities had ocular symptoms. However, other studies have similarly estimated the prevalence of ocular sarcoidosis to be 20–40% (100), and one study indicated that some patients with uveitis are asymptomatic (101). Among Japanese patients, the prevalence may be >50% (102, 103).
Regardless of the exact prevalence, the committee concluded that: 1) eye exams are required for detection of ocular involvement and are neither harmful nor burdensome; 2) vision is very precious to patients; and 3) treatment may be beneficial in reducing harm (i.e., reduction of loss of vision). The impact of treatment is based on the committee’s clinical experience and the systematic review, which suggested that most treated patients had improvement or stabilization of their visual acuity. However, the systematic review did not target treatment, and, therefore, better evidence about treatment of ocular sarcoidosis may exist. The committee’s recommendations also factored in the ancillary benefits of routine eye exams, such as identification of abnormalities that may guide nonimmunosuppressive therapy (e.g., for glaucoma) or detection of sarcoidosis treatment–related toxicity (e.g., hydroxychloroquine-induced retinopathy) that could impair vision if not identified.
Recommendation
-
1.
For patients with sarcoidosis who do not have ocular symptoms, we suggest a baseline eye examination to screen for ocular sarcoidosis (conditional recommendation, very low-quality evidence).
Research needs
The identification of risk factors for ocular sarcoidosis, particularly the more devastating forms of the disease, could obviate the need for screening all patients with sarcoidosis with eye exams. A better understanding of the natural history of ocular sarcoidosis, the identification of predictors of which patients will develop new onset of eye involvement after baseline negative screening and among those with active disease at baseline who will experience persistent or recurrent disease, and new diagnostic modalities are all needed (104). In those who initially have no evidence of eye involvement, the committee recommends ophthalmology evaluation based on development of new symptoms (Table 5). However, additional research is needed to determine if there is a clinical benefit, such as earlier detection and more effective treatment, to routine eye exams compared with exams based on the development of symptoms. Finally, treatment trials that enroll patients with ocular sarcoidosis are needed, because most treatment trials have enrolled patients with uveitis of many causes, including sarcoidosis.
Table 5.
Best Practice Recommendations for Detection of Delayed Onset of Extrapulmonary Sarcoidosis Manifestations after Negative Baseline Screening
| Test Parameter | Routine Testing for New Sarcoidosis Involvement | New Conditions Triggering a Specific Testing for Extrapulmonary Sarcoidosis Involvement |
|---|---|---|
| Calcium | Annually | Kidney stones |
| Acute or acute on chronic renal failure | ||
| Creatinine | Annually | — |
| Alkaline phosphatase | Annually | — |
| Eye exam | None | Change in vision |
| • Floaters | ||
| • Blurry | ||
| • Visual field loss | ||
| Eye pain, photophobia, or redness (sustained) | ||
| Cardiac testing (see Questions 9) | None | Chest pains |
| Palpitations | ||
| Near syncope/syncope | ||
| Sustained bradycardia or tachycardia | ||
| Dyspnea out of proportion to lung disease | ||
| New ECG findings | ||
| Pulmonary hypertension testing (see Question 10) | None | Clinical signs of pulmonary hypertension (see main text) |
Approximately 23% of patients with sarcoidosis will develop a new disease manifestation within 3 years of baseline evaluation. Annual testing is recommended for calcium, creatinine, and alkaline phosphatase, because these manifestations are often asymptomatic. In contrast, routine testing is not recommended for ocular or heart sarcoidosis, unless the patient presents with related symptoms, as above.
Question 4: Should Patients with Sarcoidosis Who Do Not Have Renal Symptoms Undergo Screening for Renal Sarcoidosis by Routine Serum Creatinine Testing?
Rationale for question
Sarcoidosis can cause compromised kidney function in a subgroup of patients through two mechanisms: 1) parenchymal granulomatous inflammation or 2) consequent to altered calcium metabolism (e.g., nephrocalcinosis, nephrolithiasis). Timely treatment (e.g., immune suppression) can attenuate sarcoidosis-induced renal complications. The committee asked if routine creatinine screening is indicated in patients with sarcoidosis with no known kidney disease.
Summary of evidence
Our systematic review identified 469 potentially relevant articles; the full text of 12 was reviewed and 8 were selected. None of the studies compared renal function testing to no testing; all were nonrandomized studies that reported the frequency of abnormal renal function and other outcomes (88, 99, 105–110). Only two studies included serum creatinine testing as a renal function test; the other studies used 24-hour urine collection alone or in combination with other tests. Meta-analysis of the selected studies found that abnormal renal function was detected in 7% (95% CI, 3–11%) of patients. Six out of eight studies reported kidney biopsy among those with abnormal renal function. Granulomas and nephrocalcinosis were the findings reported in most studies, although the frequency of occurrence varied broadly, ranging from 1% to 63% for granulomas and 0–50% for nephrocalcinosis. The committee’s confidence in the estimated frequency of the pathological abnormalities was limited by the wide ranges and concern about selection bias due to nonconsecutive selection of patients for kidney biopsy.
Committee conclusions
The guideline committee recognized that the prevalence of renal dysfunction identified among patients with sarcoidosis was modest. However, the committee’s discussion led to the following conclusions: 1) renal sarcoidosis is often asymptomatic; 2) progressive or persistent renal dysfunction is associated with poor clinical outcomes; 3) renal function testing is not harmful; and 4) most patients respond to therapy. The last conclusion is based on both the committee’s clinical experience and the systematic review, which suggested that roughly 90% of patients treated with immune suppression to suppress granulomatous inflammation and related vitamin D–mediated hypercalcemia, together with intravenous fluids, and/or other therapies to further correct hypercalcemia (a cause of renal dysfunction in sarcoidosis) had improvement or correction of coexisting renal dysfunction (105, 108). However, the systematic review was not designed to assess treatment effects, and, therefore, better evidence about treatment of renal sarcoidosis may exist.
The combination of serum creatinine testing being safe and potentially detecting a condition with a poor prognosis that responds well to treatment if detected early prompted the guideline committee to conclude that the desirable consequences of renal function testing exceed the undesirable consequences. The committee acknowledged its uncertainty about whether serum creatinine is the best test to screen for renal sarcoidosis, because many studies used 24-hour urine collection. It decided that creatinine testing is easy and less costly and, therefore, favored its use.
Failure to respond to treatment within 1 month indicates either irreversible sarcoidosis-related renal manifestations, such as nephrocalcinosis or glomerular sclerosis, or an alternative diagnosis. Alternative renal disorders are common among patients with sarcoidosis according to studies of patients with sarcoidosis who presented with renal insufficiency and underwent a diagnostic renal biopsy. These include membranous glomerulonephritis, IgA nephropathy, and focal glomerular sclerosis (111, 112). Failure of renal dysfunction in a patient with sarcoidosis to respond to immune suppression treatment should prompt further diagnostic testing.
In patients with an established diagnosis of sarcoidosis-induced renal dysfunction caused by granulomatous interstitial nephritis or hypercalcemia, relapses are common after withdrawal of immune suppression (113). As such, the panel considers it standard practice to monitor the serum creatinine in all patients with established renal sarcoidosis, especially after de-escalation of immune suppression, and annually in those who have no prior history of renal involvement (Table 5).
Recommendation
-
1.
For patients with sarcoidosis who have neither renal symptoms nor established renal sarcoidosis, we suggest baseline serum creatinine testing to screen for renal sarcoidosis (conditional recommendation, very low-quality evidence).
Research needs
The clinical presentation of renal sarcoidosis is often insidious, and renal damage is progressive without treatment. Elevated creatinine or available imaging technologies are not specific for renal sarcoidosis. Renal biopsy is often necessary to reliably establish the diagnosis of renal sarcoidosis, but carries risks of bleeding, pain, and, rarely, arteriovenous fistula formation. Future studies should consider convenient, noninvasive biomarkers of renal sarcoidosis; examples include evaluation of 24-hour urine parameters and research focused on prognosis, response to therapy, and mechanisms of fibrosis (114).
Question 5: Should Patients with Sarcoidosis Who Do Not Have Hepatic Symptoms Undergo Screening for Hepatic Sarcoidosis by Routine Transaminase and Alkaline Phosphatase Testing?
Rationale for question
The liver is commonly involved in sarcoidosis and is more common in African Americans than white individuals (88). Although liver fibrosis, cirrhosis, and portal hypertension can result and require transplantation, the prevalence of these outcomes and the long-term consequences of hepatic sarcoidosis are not established, and the indications for treating hepatic sarcoidosis are unclear. Thus, the benefits of routine screening for hepatic sarcoidosis, based on liver function tests, is unknown. The committee asked if patients with sarcoidosis presenting with no liver manifestations should undergo routine baseline screening with liver function tests.
Summary of evidence
Our systematic review identified 575 potentially relevant articles; the full text of 15 was reviewed and 8 were selected to inform the guideline committee. None of the studies compared liver function testing to no testing; all were nonrandomized studies that reported the frequency of abnormal liver function and other outcomes (83, 88, 99, 115–119). Only one study explicitly stated that the patients had no hepatic symptoms; the others only implied that most patients had minimal to no hepatic symptoms. Meta-analysis of the selected studies found that liver function testing was abnormal in 12% (95% CI, 6–19%) of patients. Among those who underwent subsequent liver biopsy, granulomas were identified in 96% (95% CI, 88–99%). The committee’s confidence in the generalizability of this result was limited by concern about selection bias due to nonconsecutive selection of patients for liver biopsy. The proportion of patients with abnormal liver function tests in whom systemic corticosteroids were initiated varied widely across studies, ranging from 25% to 95%.
Committee conclusions
The evidence suggests that liver laboratory abnormalities will be found in 12% of patients with sarcoidosis who undergo routine liver function testing, most of whom have hepatic granulomas. We did not identify a specific pattern of liver function abnormalities indicative of hepatic sarcoidosis, but some studies suggest that liver granulomas are more often associated with increases of alkaline phosphatase and less frequently with rises in transaminases (120).
The committee discussed reports that immune suppression and ursodeoxycholic acid reduce transaminitis and cholestasis in patients with related symptoms (pruritus) (121), suggesting that treatment is effective in reducing disease activity in patients with symptomatic hepatic sarcoidosis. However, according to both the committee’s clinical experience and the systematic review, it is uncertain whether these effects can be extrapolated to asymptomatic patients with hepatic sarcoidosis. The systematic review found no difference in resolution or improvement of abnormal liver function tests with treatment; however, it was not designed to estimate the effects of treatment of hepatic sarcoidosis, and, therefore, better evidence about treatment may exist. Treatment decisions should not be undertaken carelessly, because death due to cirrhosis or liver failure is uncommon (122), yet the risks of chronic treatment, especially immune suppression, are significant. The committee also noted that prednisone treatment may confound the assessment, as it can cause transaminitis. The committee agreed that the evidence is too scarce to conclude whether treatment affects progression to cirrhosis or reduces the need for liver transplantation.
Taken together, the evidence suggests that more than 1 out of every 10 patients with sarcoidosis who undergo liver function testing will be identified as having liver involvement, but the implication on treatment is unclear. The committee concluded that there is value in identifying patients with liver involvement at the time of initial diagnosis, and to screen for liver involvement annually even if the initial screen is negative (Table 5), if for no other reason than to avoid hepatotoxic treatments, and to follow such patients more carefully for the development of symptoms that warrant treatment.
Recommendations
-
1.
For patients with sarcoidosis who have neither hepatic symptoms nor established hepatic sarcoidosis, we suggest baseline serum alkaline phosphatase testing to screen for hepatic sarcoidosis (conditional recommendation, very low-quality evidence).
-
2.
For patients with sarcoidosis who have neither hepatic symptoms nor established hepatic sarcoidosis, we make no recommendation for or against baseline serum transaminase testing.
Research needs
Studies are needed to delineate the best screening test for hepatic sarcoidosis, such as alkaline phosphatase, serum transaminases, or others. In addition, little is known about the natural history of hepatic sarcoidosis. Case series demonstrate a broad range of disease outcomes, from spontaneous resolution to rapid progression to cirrhosis (123). Future studies should use long-term longitudinal follow-up to determine the natural history of hepatic sarcoidosis and consider the influence of genetic and environmental factors. Better tools are needed to: 1) detect progression from hepatitis to early fibrosis and cirrhosis, such as liver elastography ultrasound; 2) more accurately characterize disease phenotypes; and 3) identify patients who might need and respond best to therapy.
Question 6: Should Patients with Sarcoidosis Who Do Not Have Symptoms or Signs of Hypercalcemia Undergo Screening for Abnormal Calcium Metabolism by Routine Serum Calcium and Vitamin D Testing?
Rationale for question
Abnormal calcium metabolism in sarcoidosis can lead to hypercalcemia, hypercalciuria, and their manifestations, including kidney stones and renal failure; it is the most common cause of sarcoidosis-related renal insufficiency. Dysfunctional calcium metabolism can also result in elevated bone resorption and increased renal and intestinal absorption of calcium. The mechanisms of abnormal calcium metabolism are likely multifactorial, including increased 1α‐hydroxylase production by granulomatous macrophages, which converts 25-(OH) vitamin D to 1,25-(OH)2 vitamin D, increased expression of parathyroid hormone–related protein in sarcoidosis macrophages (124), and cytokine and other growth factor production (125, 126). Although hypercalcemia- or hypercalciuria-related symptoms may bring the problem to attention, the committee asked whether patients with sarcoidosis without symptoms should undergo screening for abnormal calcium metabolism by baseline serum calcium and vitamin D testing.
Summary of evidence
Our systematic review identified 1,531 potentially relevant articles; the full text of 14 was reviewed and 11 were selected to inform the guideline committee. None of the studies compared serum calcium or vitamin D testing to no testing; rather, all were nonrandomized studies that reported the frequency of abnormal calcium testing (88, 99, 105, 109, 127–133). One of the studies further reported the consequences of untreated and treated hypercalcemia, as well as the frequency of abnormal vitamin D testing among patients with sarcoidosis (127).
Hypercalcemia was detected in 6% (95% CI, 4–8%) of patients, with renal failure developing in 42% (95% CI, 33–52%) of untreated patients. In the one study that reported vitamin D testing, no patient had an elevated 25-(OH) vitamin D level, 84% (95% CI, 79–88%) had a low 25-(OH) vitamin D level, 11% (95% CI, 8–15%) had a high 1,25-(OH)2 vitamin D level, and 0.4% (95% CI, 0.07–2%) had a low 1,25-(OH)2 vitamin D level. There were no differences in the 25-(OH) vitamin D and 1,25-(OH)2 vitamin D levels among patients with hypercalcemia and normal serum calcium levels. However, 1,25-(OH)2 vitamin D levels were relatively higher than 25-(OH) vitamin D levels among patients with a history of hypercalcemia compared with patients who did not have a history of hypercalcemia.
Committee conclusions
Our systematic review suggests that serum hypercalcemia will be detected in 6% of patients with sarcoidosis who undergo routine serum calcium testing; nearly half will develop renal failure. Our systematic review did not target treatment of sarcoidosis-related hypercalcemia, and, therefore, better evidence about treatment may exist. However, according to the committee’s clinical experience and our systematic review, hypercalcemia improves or resolves in more than 90% of patients who are treated with immune suppression. In contrast, once hypercalcemia-induced renal failure is established, chronic renal impairment is common, despite immune suppression. The committee concluded that, despite the low prevalence of hypercalcemia, the accessibility of a nonburdensome test, coupled with the opportunity to intervene before the development of irreversible consequences, warrants baseline testing, and annual screening thereafter (Table 5). In addition, the committee determined that a strong recommendation for initial screening was indicated, despite the very low quality of evidence, because the importance of the potential benefits of early detection of hypercalcemia (i.e., the prevention of irreversible kidney disease) substantially outweighs the harms, burdens, and costs of testing.
The committee noted that data to support 1,25-(OH)2 vitamin D testing for abnormal calcium metabolism was neither extensively studied nor supported by the systematic review, although it was recognized that there may be other reasons for 1,25-(OH)2 vitamin D testing in patients with sarcoidosis. For example, it has been hypothesized that 1,25-(OH)2 vitamin D may be a biomarker of granulomatous burden, with one study reporting an association between disease chronicity and 1,25-(OH)2 vitamin D levels (134). A comprehensive discussion of alternative reasons for 1,25-(OH)2 vitamin D testing is beyond the scope of this guideline, but is provided elsewhere (135). Testing of 25-(OH) vitamin D levels is often undertaken in the primary care setting, and may be useful in conjunction with 1,25-(OH)2 vitamin D levels in a subset of patients with sarcoidosis, such as those with severe fatigue or exposed to chronic corticosteroids, for whom vitamin D repletion may be beneficial (136).
Patients who require calcium repletion are at risk for hypercalciuria and/or hypercalcemia, and need to be monitored closely, because the effects of calcium and vitamin D supplementation are complex. Studies have reported a small, but significant, increase in hypercalcemia with calcium and vitamin D supplementation (136–138), with another finding that withdrawal of calcium and vitamin D supplementation improved the hypercalcemia (127). Whereas hypercalcemia caused by vitamin D supplementation did not cause sustained renal damage, vitamin D supplementation was shown to be ineffective in terms of improved bone health or other benefits (123). The committee had no basis to recommend routine vitamin D screening but did recommend that both types of vitamin D testing (1,25-[OH]2 and 25-[OH] vitamin D) be undertaken in patients who are being evaluated for possible vitamin D replacement.
Recommendations
-
1.
For patients with sarcoidosis who do not have symptoms or signs of hypercalcemia, we recommend baseline serum calcium testing to screen for abnormal calcium metabolism (strong recommendation, very low-quality evidence).
-
2.
If assessment of vitamin D metabolism is deemed necessary in a patient with sarcoidosis, such as to determine if vitamin D replacement is indicated, we suggest measuring both 25- and 1,25-OH vitamin D levels before vitamin D replacement (conditional recommendation, very low-quality evidence).
Research needs
Although the cause of abnormal calcium metabolism, hypercalcemia, and hypercalciuria appears to be multifactorial, better delineation of the mechanisms, such as genetic variation, may help define better screening tests and/or ways to treat this manifestation of sarcoidosis. The question of whether 24-hour urine calcium or other biomarkers are better assessments of abnormal calcium metabolism than serum calcium or vitamin D testing needs to be evaluated. The potential benefits of calcium and vitamin D supplementation on bone health and/or the potential antiinflammatory effects of vitamin D supplementation need to be weighed against the risk of hypercalcemia and hypercalciuria, and the role of monitoring of vitamin D metabolism after vitamin D supplementation also needs to be further established. Finally, whether low 25-(OH) vitamin D or elevated 1,25-(OH)2 vitamin D levels are biomarkers for disease severity and/or granulomatous burden needs to be determined.
Question 7: Should Patients with Sarcoidosis Undergo Screening for Hematological Abnormalities by Routine Complete Blood Cell Count Testing?
Rationale for question
Hematologic abnormalities (i.e., leukopenia, lymphopenia, anemia, thrombocytopenia, and/or pancytopenia) are manifestations of sarcoidosis that raise the possibility of bone marrow involvement, although this is not the most common cause of such abnormalities. Splenomegaly with sequestration may be a more common cause of hematologic abnormalities. In the absence of splenomegaly, compartmentalization of white blood cells to the site of organ involvement is a common cause of leukopenia and lymphopenia (3, 130, 139–141). Additional causes of hematologic abnormalities are hepatic sarcoidosis, nonsarcoidosis medical problems, and treatment with immunosuppressive therapies, like methotrexate. Less-frequent hematologic abnormalities attributed to sarcoidosis include eosinophilia, hemolytic anemia, and idiopathic thrombocytopenia. Pancytopenia may be a presenting manifestation of sarcoidosis, but the cytopenia more often presents as a secondary finding in those with active disease. As a result, the committee asked whether patients with sarcoidosis should undergo a baseline complete blood cell count testing to screen for hematological abnormalities.
Summary of evidence
Our systematic review identified 2,767 potentially relevant articles; the full text of 13 was reviewed and 10 were selected. None of the studies compared complete blood cell testing to no testing; rather, all were nonrandomized studies that reported the frequency of anemia, leukopenia, or lymphopenia (88, 130–133, 141–145). Only four of the studies were published within the past 20 years; six were published more than 50 years ago.
Complete blood cell counts identified anemia in 22% (95% CI, 14–30%) of patients; the frequency of bone marrow granulomas among patients with anemia was 38% (95% CI, 13–64%). Complete blood cell counts also identified leukopenia in 4% (95% CI, 1–7%) of sarcoidosis when <4,000 cells/mm3 defined leukopenia, and in 30% (95% CI, 26–34%) when <5,000 cells/mm3 defined leukopenia. The frequency of lymphopenia varied from 27% in one study to 55% in another study. In one study, there was no difference in the frequency of anemia or lymphopenia when comparing healthy persons and patients with sarcoidosis. In two studies, most abnormalities occurred once and did not persist. No study reported the frequency with which treatment was changed on the basis of the results of the complete blood counts, or the response to treatment. The committee’s confidence in the estimated frequency of bone marrow granulomas was diminished by the wide CI due to small sample size and the risk of selection bias due to nonconsecutive selection of patients for bone marrow biopsy.
Committee conclusions
The evidence suggests that hematological abnormalities are commonly detected in patients with sarcoidosis by a peripheral blood complete blood cell count and differential cell count. However, the abnormalities are often transient and, according to our systematic review, might be just as common among healthy control subjects. The committee noted that leukopenia and lymphopenia are common complications of sarcoidosis, and are related in almost all cases to inflammatory mechanisms, such as granulomatous bone marrow infiltration (142) and the systemic effects of inflammatory mediators, such as TNF-α (146). Thus, there is no compelling reason to sample the bone marrow for alternative diagnoses in cases of sarcoidosis accompanied by leukopenia. Anemia, however, was common and an important finding, because it can contribute to sarcoidosis symptoms and signs, such as fatigue and shortness of breath, which may impact a patient’s care. It can also be an indicator of other medical problems, and a complete blood cell count is usually needed when initiating cytotoxic immunosuppressive therapy. Taken together, the committee concluded that anemia, whether attributable to sarcoidosis or not, is clinically relevant, important to patients, and requires further evaluation.
Recommendation
-
1.
We suggest that patients with sarcoidosis undergo baseline complete blood cell count testing to screen for hematological abnormalities (conditional recommendation, very low-quality evidence).
Research needs
The literature addressing hematologic abnormalities in sarcoidosis is decades old, and it is unclear whether it is applicable today, given the different laboratory tests and thresholds that are currently used. Splenectomy was previously used to treat cytopenia due to hypersplenism; even though splenectomy is less common today, there are no definitive alternative approaches that have been evaluated as treatments for hematologic abnormalities due to sarcoidosis. Lymphopenia and CD4, CD8, and CD19 lymphocyte subsets have been proposed as markers of more severe sarcoidosis (147), and patients with lymphopenia with sarcoidosis may be particularly responsive to anti–TNF-α treatment (146), although additional study is needed.
Question 8: Should Patients with Sarcoidosis Who Do Not Have Cardiac Symptoms or Signs Undergo Routine Screening for Cardiac Sarcoidosis Using ECG, TTE, or 24-Hour Ambulatory ECG Monitoring?
Rationale for question
Cardiac sarcoidosis is an underrecognized manifestation of sarcoidosis that can present with sudden cardiac death. These features make early detection and management of cardiac sarcoidosis desirable. Providers should ask specifically about symptoms of palpitations, lightheadedness, chest pain, and syncope, because these presenting signs and symptoms greatly increase the likelihood of cardiac sarcoidosis. ECG, TTE, and 24-hour ambulatory ECG (Holter) monitoring are noninvasive methods to potentially screen asymptomatic patients for cardiac sarcoidosis. However, the tests provide fundamentally different information. Although the ECG and Holter monitoring appraise conductivity within the heart, TTE assesses the mechanical function of the heart. The committee asked if any of these tests should be employed in screening asymptomatic patients with sarcoidosis for cardiac involvement.
Summary of evidence
Our systematic review identified 1,212 potentially relevant articles. For ECG, we reviewed the full text of 13 articles and selected 4 (148–151). For TTE, we reviewed the full text of 30 articles and selected 2 (148, 149). For Holter monitoring, we reviewed the full text of six articles and selected two (148, 152). None of the studies compared the diagnostic test(s) to no testing; all were nonrandomized studies that reported the results of one or more diagnostic tests. Most enrolled consecutive patients who were being followed in a sarcoidosis or pulmonary clinic. No study specifically enrolled patients with sarcoidosis without cardiac symptoms; the studies either enrolled both symptomatic and asymptomatic patients or did not report whether the patients had symptoms.
ECGs were abnormal in 7% (95% CI, 4–11%) of patients with sarcoidosis. The definition of abnormal varied among studies, but all included conduction abnormalities and ventricular ectopy. Results of the two ECG studies were too different to aggregate: one study reported a sensitivity and specificity of 9% (95% CI, 1–27%) and 97% (95% CI, 86–100%), respectively, whereas the other reported a sensitivity and specificity of 92% (95% CI, 65–99%) and 73% (95% CI, 54–86%), respectively, potentially reflecting the use of different reference standards. An abnormal ECG was associated with an increased risk of cardiac events, including atrioventricular block, ventricular tachycardia, and systolic dysfunction (hazard ratio [HR], 11.27; 95% CI, 3.29–38.64) and a trend toward increased all-cause mortality (44% vs. 36%; relative risk [RR], 1.40; 95% CI, 0.80–2.42) assessed over a median follow-up of 27 years.
TTEs were abnormal in 11% (95% CI, 5–17%) of patients with sarcoidosis, typically defined as a diminished ejection fraction and/or wall motion abnormalities inconsistent with coronary artery disease. An abnormal TTE identified cardiac sarcoidosis with a sensitivity and specificity of 25% (95% CI, 10–47%) and 97% (95% CI, 86–99%), respectively. It also predicted conduction system abnormalities (58% vs. 22%; RR, 2.6; 95% CI, 1.38–4.92).
Holter monitoring was abnormal in 5% (95% CI, 1–9%) of patients with sarcoidosis. An abnormal ambulatory ECG monitor was defined as frequent premature ventricular contractions in one study, and ventricular ectopy or conduction abnormalities in the other study. Abnormal ambulatory ECG monitoring identified cardiac sarcoidosis with a sensitivity and specificity of 56% (95% CI, 40–70%) and 95% (95% CI, 87–98%), respectively.
Only one of the selected studies evaluated all three modalities in a single population, finding that ECG, TTE, and ambulatory ECG monitoring have sensitivities of 9% (95% CI, 1–27%), 25% (95% CI, 10–47%), and 50% (95% CI, 29–71%), respectively, and specificities of 97% (95% CI, 86–100%), 95% (95% CI, 83–99%), and 97% (95% CI, 86–100%), respectively (148).
Committee conclusions
The committee recognized the hazards of comparing diagnostic tests across studies (i.e., it is uncertain whether differences are due to the test or the population) and, therefore, heavily weighted the only study in the systematic review that evaluated all three tests within a single population (148). Because the study found that each test alone is insensitive, the committee considered the possibility of using combinations of tests to screen for cardiac sarcoidosis with the plan to pursue advanced testing (cardiac magnetic resonance [CMR] and/or cardiac PET [cPET]) if any test is positive and to forego advanced testing if all three tests are negative. The committee began with ECG plus echocardiography, because both tests have attributes that are favorable in screening tests (i.e., they are inexpensive, noninvasive, reliable, and easily administered). If both tests are performed, their combined sensitivity (i.e., the likelihood that one of the tests will be abnormal in patients with cardiac sarcoidosis) is only 32%, providing minimal clinical utility. The committee next assessed TTE plus ambulatory ECG monitoring, because they are the two tests with the highest sensitivity. Their combined sensitivity is 63%, which was deemed insufficient for screening, particularly given that ambulatory ECG monitoring often requires more than one office visit. Finally, the committee considered ECG plus ambulatory ECG monitoring, but, again, the combined sensitivity was judged too low for routine screening.
These conclusions were supported by a more recent study that similarly assessed all three tests within a single population (153); this study was not included in the systematic review, because it couldn’t be determined if the study defined the tests as abnormal using the same criteria that were established a priori for the systematic review. Notably, in the first study that compared the three tests in a single population (148), all three diagnostic tests were specific. However, the more recent study (153) did not confirm the high specificity, and, therefore, the potential role was not pursued further.
Regardless of the tests’ inability to detect cardiac sarcoidosis, ECG identified patients at increased risk of cardiac events. Given that ECG is readily available in many institutions, noninvasive, harmless, and relatively inexpensive, the committee concluded that baseline ECG was recommended to identify patients who may warrant additional evaluation.
Recommendations
-
1.
For patients with extracardiac sarcoidosis who do not have cardiac symptoms or signs, we suggest performing baseline ECG to screen for possible cardiac involvement (conditional recommendation, very low-quality evidence).
-
2.
For patients with extracardiac sarcoidosis who do not have cardiac symptoms or signs, we suggest NOT performing routine baseline TTE or 24-hour continuous ambulatory ECG (Holter monitor) to screen for possible cardiac involvement (conditional recommendation, very low-quality evidence). Remarks: The panel recognizes the low risks attendant to the use of TTE or 24-hour continuous ambulatory ECG (Holter monitor) to screen for cardiac sarcoidosis. Thus, these tests should be considered on a case-by-case basis.
Research needs
The committee concluded that large research studies that compare diagnostic tests within a single population are needed to identify the optimal screening test(s) for cardiac sarcoidosis in asymptomatic individuals with sarcoidosis. Research studies are also needed to determine whether screening with CMR is warranted. Alternative screening tests for cardiac sarcoidosis should be developed and investigated, to detect cardiac sarcoidosis before it causes symptoms, and significant morbidity and mortality.
Question 9: Should Patients Who Are Suspected of Having Cardiac Sarcoidosis Undergo Cardiac MRI, TTE, or PET as an Initial Imaging Test?
Rationale for question
Given the risks and treatment implications for accurate detection of cardiac sarcoidosis, as previously discussed, definitive detection is a high clinical priority. Endomyocardial biopsy, even when aided with electroanatomic mapping guidance, is unreliable for the detection of cardiac sarcoidosis (e.g., ∼13% diagnostic yield), and requires specialized equipment and skills, is invasive, and has potentially serious complications (10, 154). Thus, imaging techniques are more commonly used for cardiac sarcoidosis detection. TTE is the most widely available imaging procedure, and is shown in small, isolated studies to detect clinically symptomatic cardiac sarcoidosis (148). CMR and fludeoxyglucose F 18 (18F-FDG) cPET are both reported to be effective for the detection of cardiac sarcoidosis (155); however, uncertainty exists on the basis of inconclusive and conflicting reports as to which imaging modality is preferred, particularly among patients with established extracardiac sarcoidosis with suspected cardiac involvement. Figure 2 demonstrates typical ECG, CMR, and cPET manifestations of cardiac sarcoidosis.
Figure 2.
Typical ECG and radiographic features of cardiac sarcoidosis. (A) ECG demonstrates first-degree A-V block (P-R interval, 200 ms) and right bundle branch block. (B) Cardiac magnetic resonance showing multifocal abnormal late gadolinium enhancement involving the mid- to epicardial lateral ventricular wall (arrowheads). (C) Cardiac positron emission tomography demonstrates intense hypermetabolic uptake of 18F-fluorodeoxyglucose in the lateral left ventricular wall (arrow).
Summary of evidence
Our systematic review identified 2,152 potentially relevant articles. No study included all three modalities; therefore, we sought studies that separately evaluated CMR, cPET, and TTE. For CMR, we reviewed the full text of 45 articles and selected 11 (156–166). For cPET, we reviewed the full text of 34 articles and selected 6 (164–169). For TTE, we reviewed the full text of 30 articles and selected 2 (148, 149). Most studies specified that the patients had “suspected cardiac sarcoidosis,” defined as an abnormal ECG or cardiac symptoms, although asymptomatic patients frequently outnumbered symptomatic patients.
Cardiac MRIs were abnormal in 27% (95% CI, 23–31%). In all cases, CMR was considered abnormal if there was late gadolinium enhancement (LGE). An abnormal CMR was associated with increased overall mortality (9.9% vs. 4.7%; RR, 2.54; 95% CI, 0.38–17.16), cardiac mortality (13% vs. 1.5%; RR, 9.00; 95% CI, 1.93–41.97), aborted sudden cardiac death (28% vs. 0%), ventricular arrhythmias (38% vs. 3.6%; RR, 11.71; 95% CI, 2.59–52.92), diastolic heart failure (67% vs. 33%; RR, 2.0; 95% CI, 1.39–2.88), other heart failure (47% vs. 4%; RR, 11.88; 95% CI, 3.69–38.21), atrial arrhythmias (36% vs. 12%; RR, 3.01; 95% CI, 1.53–5.93), complete heart block (12% vs. 1.4%; RR, 9.5; 95% CI, 1.10–81.72), and PH (25% vs. 8%; RR, 3.17; 95% CI, 1.19–8.39). In addition, three studies reported a markedly increased risk of major cardiac events (i.e., ventricular arrhythmias, sudden cardiac death, implantable cardioverter–defibrillator shocks, etc.), but the estimates could not be pooled due to differential reporting across studies. Sensitivity and specificity were deemed biased estimates, because CMR is a component of the reference standard.
cPET scans were abnormal in 52% (95% CI, 43–60%) of patients with sarcoidosis, using a variety of definitions of “abnormal.” An abnormal cPET scan was associated with major adverse cardiac events, although the estimate could not be pooled due to differential reporting across studies and was associated with a trend toward increased overall mortality (HR, 1.33; 95% CI, 0.68–2.26). Sensitivity and specificity were deemed biased estimates, because cPET scanning is a component of the reference standard.
TTEs were abnormal in 11% (95% CI, 5–17%) of patients with sarcoidosis, typically defined as a diminished ejection fraction and wall motion abnormalities inconsistent with coronary artery disease. An abnormal TTE identified cardiac sarcoidosis with a sensitivity and specificity of 25% (95% CI, 10–47%) and 97% (95% CI, 86–99%), respectively. It also predicted conduction system abnormalities (58% vs. 22%; RR, 2.6; 95% CI, 1.38–4.92).
Three studies evaluated both CMR and cPET, thereby enabling comparison of the tests within a population (164–166). In one study, adverse cardiac events were better predicted by CMR (HR, 10.63; 95% CI, 1.4–80.78) than cPET (HR, 2.29; 95% CI, 0.72–7.33) (166). This was supported by another study, although the outcome measures were different for CMR (RR, 8.33; 95% CI, 1.18–58.51) and cPET (HR, 3.3; 95% CI, 1.1–10.0) (164). The third study compared sensitivity and specificity, which the panel deemed too biased to report (165).
Committee conclusions
The guideline panel recognized that comparing the diagnostic accuracy of CMR with LGE to cPET was difficult due to: 1) the lack of a reference standard that is independent of both tests; and 2) lack of studies that directly compared the tests in large cohorts of patients with suspected cardiac sarcoidosis. Regarding the former, the tests themselves are included in the reference standards used by most of the studies we reviewed (2006 Japanese Ministry of Health and Welfare diagnostic criteria include CMR as a minor diagnostic criterion [170] and the Heart Rhythm Society diagnostic criteria rely on either CMR or cPET for diagnosis of cardiac sarcoidosis [10]), which means that sensitivity and specificity are biased estimates. Nonetheless, the selected studies clearly demonstrate that TTE has a lower sensitivity for cardiac sarcoidosis detection.
The clinical importance of CMR-LGE abnormalities is supported by several observations: 1) CMR-LGE has direct histopathological correlation with CS (157); 2) the prevalence of CMR-LGE abnormalities among patients with sarcoidosis approaches the prevalence of CS reported by serial autopsies (157, 163, 171); 3) the absence of CMR-LGE findings predicts no serious cardiac events for at least 3 years to follow (172); and 4) the extent of LGE correlates with the risk of future adverse events (173). Likewise, 18F-FDG PET uptake has been shown to predict future adverse cardiac events (174).
The body of evidence supporting the prognostic capability of CMR is larger than that supporting cPET. Moreover, CMR is less expensive, more widely available, and less prone to the false-positive results, such as those that can result from failure to convert cardiac myocyte metabolism fully away from carbohydrates. cPET may be less reproducible, despite identical patient preparation (175). On the basis of these considerations, CMR emerged as the preferred modality for imaging patients with suspected cardiac sarcoidosis, with the caveats that CMR with LGE is contraindicated in the setting of advanced kidney disease, and considering emerging evidence indicating that CMR and cPET are complementary for the detection of myocardial fibrosis and inflammation, respectively (176). It should be emphasized that these imaging technologies are continually advancing; thus, the capacity to detect clinically important cardiac sarcoidosis manifestations are expected to further improve in coming years.
The committee recognizes that subclinical cardiac involvement may develop over time in some patients with sarcoidosis based on advanced imaging modalities, such as CMR or cPET, but the committee does not recommend routine screening (e.g., ECG, TTE, CMR, and cPET) for cardiac sarcoidosis in those who are asymptomatic (Table 5). This recommendation is based on the current consensus that clinically silent cardiac sarcoidosis is associated with a benign prognosis (177).
Recommendations
-
1.
For patients with extracardiac sarcoidosis and suspected cardiac involvement, we suggest cardiac MRI, rather than cPET or TTE, to obtain both diagnostic and prognostic information (conditional recommendation, very low-quality evidence).
-
2.
For patients with extracardiac sarcoidosis and suspected cardiac involvement who are being managed in a setting in which cardiac MRI is not available, or when CMR results are inconclusive, we suggest dedicated cPET, rather than a TTE, to obtain diagnostic and prognostic information (conditional recommendation, very low-quality evidence).
Research needs
Future studies are encouraged to develop disease-specific biomarkers of granulomatous inflammation that could further enhance the performance of existing imaging techniques. For example, combined use of CMR and cPET, or integration of CMR-LGE with T1 or T2 parameters, may improve the detection of acute and chronic disease manifestations (177). Newer applications of PET are being developed, such as imaging based on deoxy-3′-[18F]-fluorothymidine (178) or 4′[methyl-11C]-thiothymidine uptake (179), which have the advantage of not requiring dietary restriction, as per 18F-FDG PET. Larger studies are needed to further validate these newer imaging modalities and combinations before strong recommendations can be offered on the basis of the rigorous Grading of Recommendations, Assessment, Development, and Evaluation approach upon which clinical practice guidelines are based. Research is also needed to develop readily available biomarkers (e.g., blood derived) to serve as convenient and less-expensive surrogates for advanced imaging modalities and to guide therapy.
Question 10: Should Patients with Sarcoidosis Who Are Suspected of Having PH Undergo TTE?
Rationale for recommendation
Sarcoidosis-associated PH (SAPH) occurs in 5–20% of patients seen in sarcoidosis clinics (180–183). Nearly half of patients with sarcoidosis with persistent dyspnea have been found to have SAPH (184, 185), and SAPH is an independent risk factor for increased mortality in sarcoidosis (181, 186). Other clinical manifestations, including exertional chest pain and/or syncope, exam findings of a prominent P2 or S4, reduced 6-minute walk distance, desaturation with exercise, reduced DlCO, increased pulmonary artery diameter relative to ascending aorta diameter (e.g., by CT scan), elevated brain natriuretic factor, and fibrotic lung disease, are proposed as methods to identify patients at risk for SAPH (182, 184, 187–189), but these clinical parameters are unreliable. TTE is the most commonly recommended method to initially screen for the presence of PH (189, 190). Thus, we investigated the utility of TTE in the evaluation of possible SAPH.
Summary of evidence
Our systematic review identified 137 potentially relevant articles; the full text of 13 was reviewed and 9 were selected. None of the studies compared TTE to no testing; rather, all were nonrandomized studies that selected patients with sarcoidosis (most enrolled patients who had persistent respiratory symptoms despite treatment of their sarcoidosis) and reported the frequency of abnormal TTE results (180, 184, 191–197). Some studies also reported the rate of confirmation by right heart catheterization. Of note, the definition of PH has evolved through the years, which may bias the populations included in our literature review.
TTE identified abnormalities suggestive of PH (i.e., high estimated systolic pulmonary arterial pressure) in 29% (95% CI, 20–39%) of patients. Among those with an abnormal TTE, 78% (95% CI, 67–86%) were subsequently confirmed to have PH by right heart catheterization, indicating that 78% of the abnormal TTEs were true positives and 22% were false positives. The proportion of patients with confirmed PH for whom treatment was initiated or changed was not reported in any of the studies.
An association between PH and lung disease severity was noted in the systemic review. Patients with PH had more severe lung disease than patients without PH, with a mean difference of the percent predicted FVC of −16.5% (95% CI, −22.4% to −10.6%). Although not selected during the systematic review, another series known to committee members found that impaired diffusing capacity is also correlated with PH, perhaps even more than diminished FVC, but the correlation between TTE and right heart catheterization pressures was most reliable (198).
The systematic review had several limitations. One was the criteria for identifying PH by TTE. The studies that we selected estimated right ventricular end systolic pressure (or pulmonary artery systolic pressure) based on tricuspid regurgitation (TR). However, up to one-third of patients do not have a sufficient TR jet to estimate pressure. TTE may identify right ventricular strain due to PH, but this alternative measure was not accounted for in the selected studies. Guidance on how to use evidence of right ventricular strain to detect pulmonary artery hypertension has been published elsewhere (190).
Another limitation was the inability of elevated pulmonary artery pressure identified by TTE to distinguish between precapillary PH and elevated pressure due to left ventricular dysfunction. These different types of PH have different causes and treatment (190), and different prognoses. In one study, patients with sarcoidosis with precapillary PH had worse survival than those with left ventricular dysfunction (185).
Committee conclusions
The evidence suggests that if TTE is performed on patients with sarcoidosis with suspected PH (i.e., persistent dyspnea despite treatment of their sarcoidosis), 29% will be abnormal, among which PH will be confirmed by right heart catheterization in roughly three-fourths and excluded in one-fourth. The committee concluded that TTE is a worthwhile screening test for suspected SAPH, because TTE abnormalities are prevalent and correlate directly with PH severity (185), but falsely abnormal TTE is too common to justify decision-making based on TTE alone. Right heart catheterization is necessary to confirm PH and to distinguish postcapillary PH (World Health Organization group II PH) from pre-capillary PH (World Health Organization group V PH), which are managed differently. Appropriate therapy has been shown to improve outcomes (183, 199–201), although the finding has not been universal (202).
Recommendations
-
1.
For patients with sarcoidosis in whom PH is suspected, we suggest initial testing with TTE (conditional recommendation, very low-quality evidence). Remarks: “PH is suspected” refers to clinical manifestations, including exertional chest pain and/or syncope, exam findings of a prominent P2 or S4, reduced 6-minute walk distance, desaturation with exercise, reduced DlCO, increased pulmonary artery diameter relative to ascending aorta diameter (e.g., by CT scan), elevated brain natriuretic factor, and fibrotic lung disease.
-
2.
For patients with sarcoidosis in whom PH is suspected and a transthoracic echocardiogram is suggestive of PH, we suggest right heart catheterization to definitively confirm or exclude SAPH (conditional recommendation, very low-quality evidence).
-
3.
For patients with sarcoidosis in whom PH is suspected and a transthoracic echocardiogram is NOT suggestive of PH, the need for right heart catheterization should be determined on a case-by-case basis.
-
4.
Finally, routine screening for SAPH is unnecessary in those who are asymptomatic (Table 5).
Research needs
The impact of underlying sarcoidosis on echocardiogram needs to be better defined. Sarcoidosis can directly affect the heart, including the right and left ventricle. In addition, parenchymal lung disease, especially pulmonary fibrosis, and hilar adenopathy impact the pulmonary artery architecture. Future studies are needed to better understand how such changes affect the accuracy of the echocardiogram. The estimated pulmonary artery pressure is based on TR, the severity of which may be influenced by right heart failure. The value of other radiographic features for predicting right ventricular failure is demonstrated in other forms of PH (190), and this needs to be considered in sarcoidosis; for example, CMR can visualize the right ventricle, and may provide a more reliable evaluation of right ventricular performance. Future studies are needed to evaluate the diagnostic and prognostic value of biomarkers, such as pro-BNP, to augment the detection and management of PH among patients with sarcoidosis (181, 203)
Conclusions
Committee members concur that there is a pressing need for higher-quality evidence to guide clinical practice relating to the diagnosis and detection of sarcoidosis, and to better define the natural history of disease progression in each organ system. A large body of related data has been published, but very few of these studies were properly designed to guide clinical practice. Nonetheless, this document establishes the current standards of care based on a rigorous and unbiased analysis of the available data, and provides direction for future investigations to further refine clinical practice to improve diagnosis and detection of sarcoidosis. Figure 3 provides an algorithm that incorporates the recommendations of these guidelines within the scope of clinical practice for the diagnosis and detection of sarcoidosis.
Figure 3.
Schematic of recommended diagnostic algorithm. The figure outlines a general approach to the diagnosis of sarcoidosis and refers to tables presented with this article. PICO = problem, intervention, comparison, outcome question format.
Supplementary Material
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems.
Members of the subcommittee are as follows:
Elliott D. Crouser, M.D. (Co-Chair)1
Lisa A. Maier, M.D. (Co-Chair)2
Robert P. Baughman, M.D. (Co-Chair)3
Eric Abston, M.D.4
Richard C. Bernstein, M.D.5
Ron Blankstein, M.D.6
Catherine A. Bonham, M.D.7
Edward S. Chen, M.D.8
Daniel A. Culver, D.O.9
Wonder Drake, M.D.10
Marjolein Drent, M.D.11,12
Alicia K. Gerke, M.D.13
Michael Ghobrial, M.D.9
Praveen Govender, M.D.4
Nabeel Hamzeh, M.D.13
W. Ennis James, M.D.14
Marc A. Judson, M.D.15
Liz Kellermeyer, M.S.L.S.16
Shandra Knight, M.S.L.S.16
Laura L. Koth, M.D.17
Adam S. Morgenthau, M.D.18
Karen C. Patterson, M.D.19,20
Venerino Poletti, M.D.21,22
Subha V. Raman, M.D.23
Melissa H. Tukey, M.D.24
Gloria E. Westney, M.D.25
Kevin C. Wilson, M.D.4
1Division of Pulmonary, Critical Care, and Sleep Medicine and 23Division of Cardiovascular Medicine, the Ohio State University, Columbus, Ohio; 2Division of Environmental and Occupational Health and 16Library and Knowledge Services, National Jewish Health, Denver, Colorado; 3Department of Medicine, University of Cincinnati Medical Center, Cincinnati, Ohio; 4Division of Pulmonary, Allergy, Sleep, and Critical Care Medicine, Boston University School of Medicine, Boston, Massachusetts; 5Division of Pulmonary, Allergy, and Critical Care Medicine, Penn State University, Hershey, Pennsylvania; 6Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts; 7Division of Pulmonary and Critical Care Medicine, University of Virginia, Charlottesville, Virginia; 8Division of Pulmonary and Critical Care Medicine, Johns Hopkins Medical School, Baltimore, Maryland; 9Department of Medicine, Respiratory Institute, Cleveland Clinic, Cleveland, Ohio; 10Division of Infectious Diseases, Vanderbilt University, Nashville, Tennessee; 11Interstitial Lung Disease Center of Excellence, Department of Pulmonology, St. Antonius Hospital, Nieuwegein, the Netherlands; 12Department of Toxicology and Pharmacology, Faculty of Health, Medicine and Life Sciences, University Maastricht, Maastricht, the Netherlands; 13Division of Pulmonary, Critical Care, and Occupational Medicine, University of Iowa, Iowa City, Iowa; 14Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine, Medical University of South Carolina, Charleston, South Carolina; 15Division of Pulmonary Medicine, Albany Medical College, Albany, New York; 17Division of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco, California; 18Division of Pulmonary, Critical Care, and Sleep Medicine, Icahn School of Medicine at Mount Sinai, New York, New York; 19Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 20Brighton and Sussex Medical School, Brighton, England; 21Department of Diseases of the Thorax, Morgagni Hospital, Forlì, Italy; 22Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Aarhus, Denmark; 24Kaiser Permanente Northern California, Oakland, California; and 25Division of Pulmonary and Critical Care Medicine, Morehouse School of Medicine, Atlanta, Georgia
Acknowledgment
The committee acknowledges the American Thoracic Society (ATS) for supporting this project, including members of the ATS Documents Committee, and ATS staff, Kimberly Lawrence, John Harmon, and Judy Corn, for administrative assistance. It also appreciates the many peer reviewers and representatives of patients with sarcoidosis who took part in the face-to-face meetings. Special thanks also to Dr. Konstantin Shiloh for providing histopathology images for Figure 1.
Footnotes
Supported by the American Thoracic Society.
This official clinical practice guideline was approved by the American Thoracic Society February 2020
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202002-0251ST.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: E.D.C. served as a consultant for Beckman Coulter Inc. and aTyr Pharma; served as a speaker for Beckman Coulter Inc.; and received research support from Beckman Coulter Inc. and Bristol-Myers Squibb. L.A.M. received research support from aTyr Pharma and Mallinckrodt. R.P.B. received research support from Actelion, AstraZeneca, aTyr Pharma, Bayer, Celgene, Genentech, Gilead, Mallinckrodt, and Novartis; served as a speaker for Genentech and Mallinckrodt; served as a consultant for Actelion, KinBio, Mallinckrodt, and Novartis; and served on an advisory committee for Actelion and Mallinckrodt. R.C.B. served on an advisory committee for Mallinckrodt. D.A.C. received personal fees from Janssen, aTyr Pharma, Mallinckrodt, and Boehringer Ingelheim; and received research support from Mallinckrodt and Boehringer Ingelheim. A.K.G. received research support from Mallinckrodt. N.H. received research support from aTyr Pharma. W.E.J. served on an advisory committee for Mallinckrodt. M.A.J. received research support from Mallinckrodt and Novartis Pharma AG. L.L.K.’s spouse/life partner served as a consultant for Medlogix, TASC (PharmaHealthLabs), Glenmark, NGM Pharma, GlaxoSmithKline, and Regeneron; and served on an advisory committee for Regeneron. A.S.M. served as a consultant and received royalties from Auven Therapeutics; and had ownership investments from Philip Morris International Inc. that are now divested. K.C.W. is employed by American Thoracic Society as Chief of Documents and Documents Editor with an interest in the success of ATS guidelines. E.A., R.B., C.A.B., E.S.C., W.D., M.D., M.G., P.G., L.K., S.K., K.C.P., V.P., S.V.R., M.H.T., and G.E.W. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Clinical Problems
References
- 1.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139:144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tukey MH, Berman JS, Boggs DA, White LF, Rosenberg L, Cozier YC. Mortality among African American women with sarcoidosis: data from the Black Women’s Health Study. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:128–133. [PMC free article] [PubMed] [Google Scholar]
- 3.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis: American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 4.Grunewald J, Eklund A. Sex-specific manifestations of Löfgren’s syndrome. Am J Respir Crit Care Med. 2007;175:40–44. doi: 10.1164/rccm.200608-1197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiteri MA, Matthey F, Gordon T, Carstairs LS, James DG. Lupus pernio: a clinico-radiological study of thirty-five cases. Br J Dermatol. 1985;112:315–322. doi: 10.1111/j.1365-2133.1985.tb04859.x. [DOI] [PubMed] [Google Scholar]
- 6.Poate TW, Sharma R, Moutasim KA, Escudier MP, Warnakulasuriya S. Orofacial presentations of sarcoidosis: a case series and review of the literature. Br Dent J. 2008;205:437–442. doi: 10.1038/sj.bdj.2008.892. [DOI] [PubMed] [Google Scholar]
- 7.Winterbauer RH, Belic N, Moores KD. Clinical interpretation of bilateral hilar adenopathy. Ann Intern Med. 1973;78:65–71. doi: 10.7326/0003-4819-78-1-65. [DOI] [PubMed] [Google Scholar]
- 8.Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H., Jr Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group: a case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86. [PubMed] [Google Scholar]
- 9.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 10.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Stern BJ, Royal W, III, Gelfand JM, Clifford DB, Tavee J, Pawate S, et al. Definition and consensus diagnostic criteria for neurosarcoidosis: from the Neurosarcoidosis Consortium Consensus Group. JAMA Neurol. 2018;75:1546–1553. doi: 10.1001/jamaneurol.2018.2295. [DOI] [PubMed] [Google Scholar]
- 12.Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603–619. doi: 10.1016/j.ccm.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosen Y. Four decades of necrotizing sarcoid granulomatosis: what do we know now? Arch Pathol Lab Med. 2015;139:252–262. doi: 10.5858/arpa.2014-0051-RA. [DOI] [PubMed] [Google Scholar]
- 14.Costabel U, Hunninghake GW Sarcoidosis Statement Committee; American Thoracic Society; European Respiratory Society; World Association for Sarcoidosis and Other Granulomatous Disorders. ATS/ERS/WASOG statement on sarcoidosis. Eur Respir J. 1999;14:735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 15.Amicosante M. IGRAs for tuberculosis in sarcoidosis patients: is the immune response to mycobacteria helpful in the differential diagnosis or still a confounding factor? Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:85–86. [PubMed] [Google Scholar]
- 16.Pai M, Nicol MP, Boehme CC. Tuberculosis diagnostics: state of the art and future directions. Microbiol Spectr. 2016;4:TBTB2-0019-2016. doi: 10.1128/microbiolspec.TBTB2-0019-2016. [DOI] [PubMed] [Google Scholar]
- 17.Shinnick TM, Good RC. Diagnostic mycobacteriology laboratory practices. Clin Infect Dis. 1995;21:291–299. doi: 10.1093/clinids/21.2.291. [DOI] [PubMed] [Google Scholar]
- 18.Nin CS, de Souza VV, do Amaral RH, Schuhmacher Neto R, Alves GR, Marchiori E, et al. Thoracic lymphadenopathy in benign diseases: a state of the art review. Respir Med. 2016;112:10–17. doi: 10.1016/j.rmed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Balmes JR, Abraham JL, Dweik RA, Fireman E, Fontenot AP, Maier LA, et al. ATS Ad Hoc Committee on Beryllium Sensitivity and Chronic Beryllium Disease. An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. Am J Respir Crit Care Med. 2014;190:e34–e59. doi: 10.1164/rccm.201409-1722ST. [DOI] [PubMed] [Google Scholar]
- 20.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 21.Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-induced sarcoidosis-like reactions. Chest. 2018;154:664–677. doi: 10.1016/j.chest.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Payá-Llorente C, Cremades-Mira A, Estors-Guerrero M, Martínez-Hernández N, Alberola-Soler A, Galbis-Carvajal JM. Mediastinal sarcoid-like reaction in cancer patients. Pulmonology. 2018;24:61–63. doi: 10.1016/j.pulmoe.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Gong L, He XL, Li YH, Ren KX, Zhang L, Liu XY, et al. Clonal status and clinicopathological feature of Erdheim-Chester disease. Pathol Res Pract. 2009;205:601–607. doi: 10.1016/j.prp.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277–288. doi: 10.1007/s00535-011-0386-x. [DOI] [PubMed] [Google Scholar]
- 25.Carmona EM, Kalra S, Ryu JH. Pulmonary sarcoidosis: diagnosis and treatment. Mayo Clin Proc. 2016;91:946–954. doi: 10.1016/j.mayocp.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 27.Reich JM, Brouns MC, O’Connor EA, Edwards MJ. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest. 1998;113:147–153. doi: 10.1378/chest.113.1.147. [DOI] [PubMed] [Google Scholar]
- 28.Boujaoude Z, Dahdel M, Pratter M, Kass J. Endobronchial ultrasound with transbronchial needle aspiration in the diagnosis of bilateral hilar and mediastinal lymphadenopathy. J Bronchology Interv Pulmonol. 2012;19:19–23. doi: 10.1097/LBR.0b013e3182442b89. [DOI] [PubMed] [Google Scholar]
- 29.Fritscher-Ravens A, Sriram PV, Topalidis T, Hauber HP, Meyer A, Soehendra N, et al. Diagnosing sarcoidosis using endosonography-guided fine-needle aspiration. Chest. 2000;118:928–935. doi: 10.1378/chest.118.4.928. [DOI] [PubMed] [Google Scholar]
- 30.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest. 2007;132:1298–1304. doi: 10.1378/chest.07-0998. [DOI] [PubMed] [Google Scholar]
- 31.Hong G, Lee KJ, Jeon K, Koh WJ, Suh GY, Chung MP, et al. Usefulness of endobronchial ultrasound guided transbronchial needle aspiration for diagnosis of sarcoidosis. Yonsei Med J. 2013;5:1416–1421. doi: 10.3349/ymj.2013.54.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwashita T, Yasuda I, Doi S, Kato T, Sano K, Yasuda S, et al. The yield of endoscopic ultrasound-guided fine needle aspiration for histological diagnosis in patients suspected of stage I sarcoidosis. Endoscopy. 2008;40:400–405. doi: 10.1055/s-2007-995593. [DOI] [PubMed] [Google Scholar]
- 33.Koerner SK, Sakowitz AJ, Appelman RI, Becker NH, Schoenbaum SW. Transbronchinal lung biopsy for the diagnosis of sarcoidosis. N Engl J Med. 1975;293:268–270. doi: 10.1056/NEJM197508072930603. [DOI] [PubMed] [Google Scholar]
- 34.Koonitz CH, Joyner LR, Nelson RA. Transbronchial lung biopsy via the fiberoptic bronchoscope in sarcoidosis. Ann Intern Med. 1976;85:64–66. doi: 10.7326/0003-4819-85-1-64. [DOI] [PubMed] [Google Scholar]
- 35.Leonard C, Tormey VJ, O’Keane C, Burke CM. Bronchoscopic diagnosis of sarcoidosis. Eur Respir J. 1997;10:2722–2724. doi: 10.1183/09031936.97.10122722. [DOI] [PubMed] [Google Scholar]
- 36.Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Kawata Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863–868. doi: 10.1111/j.1440-1843.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 37.Pakhale SS, Unruh H, Tan L, Sharma S. Has mediastinoscopy still a role in suspected stage I sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:66–69. [PubMed] [Google Scholar]
- 38.Pauli G, Pelletier A, Bohner C, Roeslin N, Warter A, Roegel E. Transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 1984;85:482–484. doi: 10.1378/chest.85.4.482. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro C, Oliveira A, Neves S, Campainha S, Nogueira C, Torres S, et al. Diagnosis of sarcoidosis in the endobronchial ultrasound-guided transbronchial needle aspiration era. Rev Port Pneumol. 2014;20:237–241. doi: 10.1016/j.rppneu.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Trisolini R, Lazzari Agli L, Cancellieri A, Poletti V, Candoli P, Paioli D, et al. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:147–151. [PubMed] [Google Scholar]
- 41.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Ichihara S, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg. 2012;143:1324–1329. doi: 10.1016/j.jtcvs.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 42.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, et al. Transesophageal bronchoscopic ultrasound-guided fine needle aspiration for diagnosis of sarcoidosis. Respiration. 2013;85:137–143. doi: 10.1159/000345207. [DOI] [PubMed] [Google Scholar]
- 43.Oki M, Saka H, Ando M, Nakashima H, Shiraki A, Murakami Y, et al. How many passes are needed for endobronchial ultrasound-guided transbronchial needle aspiration for sarcoidosis? A prospective multicenter study. Respiration. 2018;95:251–257. doi: 10.1159/000485661. [DOI] [PubMed] [Google Scholar]
- 44.Yanardağ H, Caner M, Kaynak K, Uygun S, Demirci S, Karayel T. Clinical value of mediastinoscopy in the diagnosis of sarcoidosis: an analysis of 68 cases. Thorac Cardiovasc Surg. 2006;54:198–201. doi: 10.1055/s-2005-872996. [DOI] [PubMed] [Google Scholar]
- 45.Drent M, De Vries J, Lenters M, Lamers RJS, Rothkranz-Kos S, Wouters EF, et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol. 2003;13:2462–2471. doi: 10.1007/s00330-003-1965-x. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima T, Yasufuku K, Kurosu K, Takiguchi Y, Fujiwara T, Chiyo M, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis: comparisons with other bronchoscopic diagnostic modalities. Respir Med. 2009;103:1796–1800. doi: 10.1016/j.rmed.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–556. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 48.Madan K, Dhungana A, Mohan A, Hadda V, Jain D, Arava S, et al. Conventional transbronchial needle aspiration versus endobronchial ultrasound-guided transbronchial needle aspiration, with or without rapid on-site evaluation, for the diagnosis of sarcoidosis: a randomized controlled trial. J Bronchology Interv Pulmonol. 2017;24:48–58. doi: 10.1097/LBR.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 49.von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in ’t Veen JC, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013;309:2457–2464. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 50.Friedel H, Dorscheid HO, Kirsch M, Mucke T. The significance of bronchoscopy and mediastinoscopy for the diagnosis of sarcoidosis [in German] Z Tuberk Erkrank Thoraxorg. 1964;121:152–159. [PubMed] [Google Scholar]
- 51.Aragaki-Nakahodo AA, Baughman RP, Shipley RT, Benzaquen S. The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir Med. 2017;131:65–69. doi: 10.1016/j.rmed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Balwan A. Endobronchial ultrasound-guided transbronchial needle aspiration using 19-G needle for sarcoidosis. J Bronchology Interv Pulmonol. 2018;25:260–263. doi: 10.1097/LBR.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 53.Szlubowski A, Kuzdzał J, Pankowski J, Obrochta A, Soja J, Hauer J, et al. Ultrasound guided transbronchial needle aspiration as a diagnostic tool for lung cancer and sarcoidosis [in Polish] Pneumonol Alergol Pol. 2008;76:229–236. [PubMed] [Google Scholar]
- 54.Li K, Jiang S. A randomized controlled study of conventional TBNA versus EBUS-TBNA for diagnosis of suspected stage I and II sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:211–218. [PubMed] [Google Scholar]
- 55.Low SY, Koh MS, Ong TH, Phua GC, Anantham D. Use of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in the diagnosis of granulomatous mediastinal lymphadenopathy. Ann Acad Med Singapore. 2014;43:250–254. [PubMed] [Google Scholar]
- 56.Navasakulpong A, Auger M, Gonzalez AV. Yield of EBUS-TBNA for the diagnosis of sarcoidosis: impact of operator and cytopathologist experience. BMJ Open Respir Res. 2016;3:e000144. doi: 10.1136/bmjresp-2016-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raddaoui E, Alhamad EH, Zaidi SN, Arafah M, AlHabeeb FF. Utility of endoscopic ultrasound-guided transbronchial fine-needle cytology in the diagnosis of sarcoidosis: a Saudi experience. Cytojournal. 2014;11:31. doi: 10.4103/1742-6413.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay A, Stather DR, MacEachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest. 2009;136:340–346. doi: 10.1378/chest.08-2768. [DOI] [PubMed] [Google Scholar]
- 59.Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, et al. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29:1182–1186. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 60.Dziedzic DA, Peryt A, Orlowski T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin Respir J. 2017;11:58–63. doi: 10.1111/crj.12304. [DOI] [PubMed] [Google Scholar]
- 61.Tucker JA, Gartner W. Mediastinoscopy with special reference to the diagnosis of sarcoid. Ann Otol Rhinol Laryngol. 1970;79:937–943. doi: 10.1177/000348947007900510. [DOI] [PubMed] [Google Scholar]
- 62.Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest. 1959;36:343–352. doi: 10.1378/chest.36.4.343. [DOI] [PubMed] [Google Scholar]
- 63.Nielsen EG, Olsen H. Mediastinoscopy: an analysis of 223 cases. Dan Med Bull. 1966;13:193–197. [PubMed] [Google Scholar]
- 64.Maassen W. Results and significance of mediastinoscopy and other procedures of thoracic biopsy [in German] Tuberk Grenzgeb Einzeldarst. 1967;19:1–160. [PubMed] [Google Scholar]
- 65.Jepsen O. Mediastinoscopy. Copenhagen: Einar Munkegaard Forlag; 1966. [Google Scholar]
- 66.Löfgren S, Snellman B. Principles and procedures for obtaining biopsies in sarcoidosis. Acta Med Scand Suppl. 1964;425:225–227. doi: 10.1111/j.0954-6820.1964.tb05756.x. [DOI] [PubMed] [Google Scholar]
- 67.Palva T. Mediastinal sarcoidosis. Acta Otolaryngol Suppl. 1964;188(Suppl 188) doi: 10.3109/00016486409134571. [DOI] [PubMed] [Google Scholar]
- 68.Pätiälä J. Observations on the significance of mediastinoscopy in the diagnosis of sarcoidosis. Acta Med Scand Suppl. 1964;425:239–240. doi: 10.1111/j.0954-6820.1964.tb05761.x. [DOI] [PubMed] [Google Scholar]
- 69.Mikhail JR, Mitchell DN. Mediastinoscopy: a diagnostic procedure in hilar and paratracheal lymphadenopathy. Postgrad Med J. 1971;47:698–704. doi: 10.1136/pgmj.47.553.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergh NP, Rydberg B, Schersten T. Mediastinal exploration by the technique of Carlens. Dis Chest. 1964;46:399–410. doi: 10.1378/chest.46.4.399. [DOI] [PubMed] [Google Scholar]
- 71.Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH, Jr, et al. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg. 2006;82:1185–1189. doi: 10.1016/j.athoracsur.2006.05.023. [Discussion, pp. 1189–1190]. [DOI] [PubMed] [Google Scholar]
- 72.Cybulsky IJ, Bennett WF. Mediastinoscopy as a routine outpatient procedure. Ann Thorac Surg. 1994;58:176–178. doi: 10.1016/0003-4975(94)91095-2. [DOI] [PubMed] [Google Scholar]
- 73.Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Endosonography versus mediastinoscopy in mediastinal staging of lung cancer: systematic review and meta-analysis. Ann Thorac Surg. 2016;102:1747–1755. doi: 10.1016/j.athoracsur.2016.05.110. [DOI] [PubMed] [Google Scholar]
- 74.Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, et al. REMEDY Trial Investigators. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186:255–260. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinfort DP, Liew D, Conron M, Hutchinson AF, Irving LB. Cost-benefit of minimally invasive staging of non–small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol. 2010;5:1564–1570. doi: 10.1097/JTO.0b013e3181e8b2e6. [DOI] [PubMed] [Google Scholar]
- 76.Li WWL, van der Heijden EFM, Verhagen AFTM. Mediastinoscopy after negative endoscopic mediastinal nodal staging: can it be omitted? Eur Respir J. 2015;46:1846–1848. doi: 10.1183/13993003.01166-2015. [DOI] [PubMed] [Google Scholar]
- 77.Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg. 2010;89:S2084–S2089. doi: 10.1016/j.athoracsur.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 78.Hu LX, Chen RX, Huang H, Shao C, Wang P, Liu YZ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration versus standard bronchoscopic modalities for diagnosis of sarcoidosis: a meta-analysis. Chin Med J (Engl) 2016;129:1607–1615. doi: 10.4103/0366-6999.184458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gnass M, Szlubowski A, Soja J, Kocoń P, Rudnicka L, Ćmiel A, et al. Comparison of conventional and ultrasound-guided needle biopsy techniques in the diagnosis of sarcoidosis: a randomized trial. Pol Arch Med Wewn. 2015;125:321–328. doi: 10.20452/pamw.2828. [DOI] [PubMed] [Google Scholar]
- 80.Liran L, Rottem K, Gregorio FZ, Avi A, Neville B. A novel, stepwise approach combining conventional and endobronchial ultrasound needle aspiration for mediastinal lymph node sampling. Endosc Ultrasound. 2019;8:31–35. doi: 10.4103/eus.eus_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Acharya NR, Browne EN, Rao N, Mochizuki M International Ocular Sarcoidosis Working Group. Distinguishing features of ocular sarcoidosis in an international cohort of uveitis patients. Ophthalmology. 2018;125:119–126. doi: 10.1016/j.ophtha.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Birnbaum AD, French DD, Mirsaeidi M, Wehrli S. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122:934–938. doi: 10.1016/j.ophtha.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ungprasert P, Tooley AA, Crowson CS, Matteson EL, Smith WM. Clinical characteristics of ocular sarcoidosis: a population-based study 1976–2013. Ocul Immunol Inflamm. 2019;27:389–395. doi: 10.1080/09273948.2017.1386791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SY, Lee HG, Kim DS, Kim JG, Chung H, Yoon YH. Ocular sarcoidosis in a Korean population. J Korean Med Sci. 2009;24:413–419. doi: 10.3346/jkms.2009.24.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans M, Sharma O, LaBree L, Smith RE, Rao NA. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. 2007;114:325–333. doi: 10.1016/j.ophtha.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 86.Dróbecka E, Swituła M, Godowska J, Skórska I, Płodziszewska M, Szopiński J. Ocular manifestations in sarcoidosis [in Polish] Klin Oczna. 1999;101:201–204. [PubMed] [Google Scholar]
- 87.Atmaca LS, Atmaca-Sönmez P, Idil A, Kumbasar OO, Celik G. Ocular involvement in sarcoidosis. Ocul Immunol Inflamm. 2009;17:91–94. doi: 10.1080/09273940802596526. [DOI] [PubMed] [Google Scholar]
- 88.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 89.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–127. [PubMed] [Google Scholar]
- 90.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102:297–301. doi: 10.1016/0002-9394(86)90001-2. [DOI] [PubMed] [Google Scholar]
- 91.James DG, Anderson R, Langley D, Ainslie D. Ocular sarcoidosis. Br J Ophthalmol. 1964;48:461–470. doi: 10.1136/bjo.48.9.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siltzbach LE, James DG, Neville E, Turiaf J, Battesti JP, Sharma OP, et al. Course and prognosis of sarcoidosis around the world. Am J Med. 1974;57:847–852. doi: 10.1016/0002-9343(74)90160-0. [DOI] [PubMed] [Google Scholar]
- 93.Jackson H. Ocular sarcoidosis. Postgrad Med J. 1970;46:501–504. doi: 10.1136/pgmj.46.538.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–655. doi: 10.1016/0002-9394(78)90184-8. [DOI] [PubMed] [Google Scholar]
- 95.Khanna A, Sidhu U, Bajwa G, Malhotra V. Pattern of ocular manifestations in patients with sarcoidosis in developing countries. Acta Ophthalmol Scand. 2007;85:609–612. doi: 10.1111/j.1600-0420.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 96.Sungur G, Hazirolan D, Bilgin G. Pattern of ocular findings in patients with biopsy-proven sarcoidosis in Turkey. Ocul Immunol Inflamm. 2013;21:455–461. doi: 10.3109/09273948.2013.775311. [DOI] [PubMed] [Google Scholar]
- 97.Sheu SJ, Chang FP, Wu TT, Chuang CT. Ocular sarcoidosis in southern taiwan. Ocul Immunol Inflamm. 2010;18:152–157. doi: 10.3109/09273941003637502. [DOI] [PubMed] [Google Scholar]
- 98.Baughman RP, Lower EE, Ingledue R, Kaufman AH. Management of ocular sarcoidosis. Sarc Vasc Diff Lung Dis. 2012;29:26–33. [PubMed] [Google Scholar]
- 99.Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–379. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 100.Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13:840–849. doi: 10.1016/j.autrev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Pefkianaki M, Androudi S, Praidou A, Sourlas V, Zakynthinos E, Brazitikos P, et al. Ocular disease awareness and pattern of ocular manifestation in patients with biopsy-proven lung sarcoidosis. J Ophthalmic Inflamm Infect. 2011;1:141–145. doi: 10.1007/s12348-011-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA International Workshop on Ocular Sarcoidosis Study Group. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103:1418–1422. doi: 10.1136/bjophthalmol-2018-313356. [DOI] [PubMed] [Google Scholar]
- 103.Ohara K, Okubo A, Sasaki H, Kamata K. Intraocular manifestations of systemic sarcoidosis. Jpn J Ophthalmol. 1992;36:452–457. [PubMed] [Google Scholar]
- 104.Yang SJ, Salek S, Rosenbaum JT. Ocular sarcoidosis: new diagnostic modalities and treatment. Curr Opin Pulm Med. 2017;23:458–467. doi: 10.1097/MCP.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bergner R, Hoffmann M, Waldherr R, Uppenkamp M. Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:126–132. [PubMed] [Google Scholar]
- 106.Richmond JM, Chambers B, D’Apice AJ, Whitworth JA, Kincaid-Smith P. Renal disease and sarcoidosis. Med J Aust. 1981;2:36–37. [PubMed] [Google Scholar]
- 107.Ricker W, Clark M. Sarcoidosis; a clinicopathologic review of 300 cases, including 22 autopsies. Am J Clin Pathol. 1949;19:725–749. doi: 10.1093/ajcp/19.8.725. [DOI] [PubMed] [Google Scholar]
- 108.MacSearraigh ET, Doyle CT, Twomey M, O’Sullivan DJ. Sarcoidosis with renal involvement. Postgrad Med J. 1978;54:528–532. doi: 10.1136/pgmj.54.634.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lebacq E, Desmet V, Verhaegen H. Renal involvement in sarcoidosis. Postgrad Med J. 1970;46:526–529. doi: 10.1136/pgmj.46.538.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Löfgren S, Snellman B, Lindgren AG. Renal complications in sarcoidosis; functional and biopsy studies. Acta Med Scand. 1957;159:295–305. [PubMed] [Google Scholar]
- 111.Löffler C, Löffler U, Tuleweit A, Waldherr R, Uppenkamp M, Bergner R. Renal sarcoidosis: epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31:306–315. [PubMed] [Google Scholar]
- 112.Stehlé T, Joly D, Vanhille P, Boffa JJ, Rémy P, Mesnard L, et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: a multicenter study. Orphanet J Rare Dis. 2013;8:65. doi: 10.1186/1750-1172-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hilderson I, Van Laecke S, Wauters A, Donck J. Treatment of renal sarcoidosis: is there a guideline? Overview of the different treatment options. Nephrol Dial Transplant. 2014;29:1841–1847. doi: 10.1093/ndt/gft442. [DOI] [PubMed] [Google Scholar]
- 114.Mahévas M, Lescure FX, Boffa JJ, Delastour V, Belenfant X, Chapelon C, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009;88:98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
- 115.Cremers J, Drent M, Driessen A, Nieman F, Wijnen P, Baughman R, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 2012;24:17–24. doi: 10.1097/MEG.0b013e32834c7b71. [DOI] [PubMed] [Google Scholar]
- 116.Kahi CJ, Saxena R, Temkit M, Canlas K, Roberts S, Knox K, et al. Hepatobiliary disease in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:117–123. [PubMed] [Google Scholar]
- 117.Vatti R, Sharma OP. Course of asymptomatic liver involvement in sarcoidosis: role of therapy in selected cases. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:73–76. [PubMed] [Google Scholar]
- 118.Kennedy PT, Zakaria N, Modawi SB, Papadopoulou AM, Murray-Lyon I, du Bois RM, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006;18:721–726. doi: 10.1097/01.meg.0000223911.85739.38. [DOI] [PubMed] [Google Scholar]
- 119.Cowdell RH. Sarcoidosis: with special reference to diagnosis and prognosis. Q J Med. 1954;23:29–55. [PubMed] [Google Scholar]
- 120.Baughman RP, Koehler A, Bejarano PA, Lower EE, Weber FL., Jr Role of liver function tests in detecting methotrexate-induced liver damage in sarcoidosis. Arch Intern Med. 2003;163:615–620. doi: 10.1001/archinte.163.5.615. [DOI] [PubMed] [Google Scholar]
- 121.Bakker GJ, Haan YC, Maillette de Buy Wenniger LJ, Beuers U. Sarcoidosis of the liver: to treat or not to treat? Neth J Med. 2012;70:349–356. [PubMed] [Google Scholar]
- 122.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Brusselen D, Janssen CE, Scott C, Bevers N, Roskams T, Wouters C, et al. Budd-Chiari syndrome as presenting symptom of hepatic sarcoidosis in a child, with recurrence after liver transplantation. Pediatr Transplant. 2012;16:E58–E62. doi: 10.1111/j.1399-3046.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 124.Zeimer HJ, Greenaway TM, Slavin J, Hards DK, Zhou H, Doery JC, et al. Parathyroid-hormone-related protein in sarcoidosis. Am J Pathol. 1998;152:17–21. [PMC free article] [PubMed] [Google Scholar]
- 125.Koeffler HP, Reichel H, Bishop JE, Norman AW. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 126.Sexton DJI, O’Reilly MW, Geoghegan P, Kinsella SM, Moran PJ, O’Regan AW. Serum fibroblastic growth factor 23 in acute sarcoidosis and normal kidney function. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:139–142. [PubMed] [Google Scholar]
- 127.Baughman RP, Janovcik J, Ray M, Sweiss N, Lower EE. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:113–120. [PubMed] [Google Scholar]
- 128.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from the Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 129.James DG. Diagnosis and treatment of sarcoidosis. BMJ. 1956;2:900–904. doi: 10.1136/bmj.2.4998.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayock RL, Bertrand P, Morrison CE, Scott JH. Manifestations of sarcoidosis: analysis of 145 patients with a review of nine series selected from the literature. Am J Med. 1963;35:67–89. doi: 10.1016/0002-9343(63)90165-7. [DOI] [PubMed] [Google Scholar]
- 131.McCort JJ, Wood RH. Sarcoidosis a clinical and roentgenologic study of twenty-eight proved cases. Arch Intern Med (Chic) 1947;80:293. doi: 10.1001/archinte.1947.00220150003001. [DOI] [PubMed] [Google Scholar]
- 132.Ferguson RH, Paris J. Sarcoidosis; study of twenty-nine cases, with a review of splenic, hepatic, mucous-membrane; retinal, and joint manifestations. AMA Arch Intern Med. 1958;101:1065–1084. doi: 10.1001/archinte.1958.00260180055007. [DOI] [PubMed] [Google Scholar]
- 133.Cummings MM, Dunner E, Williams JH., Jr Epidemiologic and clinical observations in sarcoidosis. Ann Intern Med. 1959;50:879–890. doi: 10.7326/0003-4819-50-4-879. [DOI] [PubMed] [Google Scholar]
- 134.Kavathia D, Buckley JD, Rao D, Rybicki B, Burke R. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med. 2010;104:564–570. doi: 10.1016/j.rmed.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tebben PJ, Singh RJ, Kumar R. Vitamin D–mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521–547. doi: 10.1210/er.2016-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kamphuis LS, Bonte-Mineur F, van Laar JA, van Hagen PM, van Daele PL. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res. 2014;29:2498–2503. doi: 10.1002/jbmr.2262. [DOI] [PubMed] [Google Scholar]
- 137.Bolland MJ, Wilsher ML, Grey A, Horne AM, Fenwick S, Gamble GD, et al. Randomised controlled trial of vitamin D supplementation in sarcoidosis. BMJ Open. 2013;3:e003562. doi: 10.1136/bmjopen-2013-003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Capolongo G, Xu LH, Accardo M, Sanduzzi A, Stanziola AA, Colao A, et al. Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis. J Investig Med. 2016;64:1025–1034. doi: 10.1136/jim-2016-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomas PD, Hunninghake GW. Current concepts of the pathogenesis of sarcoidosis. Am Rev Respir Dis. 1987;135:747–760. doi: 10.1164/arrd.1987.135.3.747. [DOI] [PubMed] [Google Scholar]
- 140.Browne PM, Sharma OP, Salkin D. Bone marrow sarcoidosis. JAMA. 1978;240:2654–2655. [PubMed] [Google Scholar]
- 141.Lower EE, Smith JT, Martelo OJ, Baughman RP. The anemia of sarcoidosis. Sarcoidosis. 1988;5:51–55. [PubMed] [Google Scholar]
- 142.Yanardağ H, Pamuk GE, Karayel T, Demirci S. Bone marrow involvement in sarcoidosis: an analysis of 50 bone marrow samples. Haematologia (Budap) 2002;32:419–425. [PubMed] [Google Scholar]
- 143.Gupta D, Rao VM, Aggarwal AN, Garewal G, Jindal SK. Haematological abnormalities in patients of sarcoidosis. Indian J Chest Dis Allied Sci. 2002;44:233–236. [PubMed] [Google Scholar]
- 144.Sharma SK, Mohan A, Guleria JS. Clinical characteristics, pulmonary function abnormalities and outcome of prednisolone treatment in 106 patients with sarcoidosis. J Assoc Physicians India. 2001;49:697–704. [PubMed] [Google Scholar]
- 145.Israel HL, Sones M. Sarcoidosis; clinical observation on one hundred sixty cases. AMA Arch Intern Med. 1958;102:766–776. doi: 10.1001/archinte.1958.00260220082008. [DOI] [PubMed] [Google Scholar]
- 146.Crouser ED, Lozanski G, Fox CC, Hauswirth DW, Raveendran R, Julian MW. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti–tumor necrosis factor-alpha therapy. Chest. 2010;137:1432–1435. doi: 10.1378/chest.09-2576. [DOI] [PubMed] [Google Scholar]
- 147.Sweiss NJ, Salloum R, Gandhi S, Alegre ML, Sawaqed R, Badaracco M, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS One. 2010;5:e9088. doi: 10.1371/journal.pone.0009088. [Published erratum appears in PLoS One 5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 149.Burstow DJ, Tajik AJ, Bailey KR, DeRemee RA, Taliercio CP. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63:478–482. doi: 10.1016/0002-9149(89)90323-8. [DOI] [PubMed] [Google Scholar]
- 150.Nagao S, Watanabe H, Sobue Y, Kodama M, Tanaka J, Tanabe N, et al. Electrocardiographic abnormalities and risk of developing cardiac events in extracardiac sarcoidosis. Int J Cardiol. 2015;189:1–5. doi: 10.1016/j.ijcard.2015.03.175. [DOI] [PubMed] [Google Scholar]
- 151.Langer SW, Iversen E, Vestbo J, Viskum K. Electrocardiographic changes in patients with intrathoracic sarcoidosis: influence on prognosis. Sarcoidosis. 1995;12:42–45. [PubMed] [Google Scholar]
- 152.Suzuki T, Kanda T, Kubota S, Imai S, Murata K. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106:1021–1024. doi: 10.1378/chest.106.4.1021. [DOI] [PubMed] [Google Scholar]
- 153.Hena KM, Yip J, Jaber N, Goldfarb D, Fullam K, Cleven K, et al. FDNY Sarcoidosis Clinical Research Group. Clinical course of sarcoidosis in world trade center-exposed firefighters. Chest. 2018;153:114–123. doi: 10.1016/j.chest.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vaidya VR, Abudan AA, Vasudevan K, Shantha G, Cooper LT, Kapa S, et al. The efficacy and safety of electroanatomic mapping-guided endomyocardial biopsy: a systematic review. J Interv Card Electrophysiol. 2018;53:63–71. doi: 10.1007/s10840-018-0410-7. [DOI] [PubMed] [Google Scholar]
- 155.Ohira H, Birnie DH, Pena E, Bernick J, Mc Ardle B, Leung E, et al. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2016;43:259–269. doi: 10.1007/s00259-015-3181-8. [DOI] [PubMed] [Google Scholar]
- 156.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest. 2005;128:30–35. doi: 10.1378/chest.128.1.30. [DOI] [PubMed] [Google Scholar]
- 157.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patel AR, Klein MR, Chandra S, Spencer KT, Decara JM, Lang RM, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail. 2011;13:1231–1237. doi: 10.1093/eurjhf/hfr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 160.Cain MA, Metzl MD, Patel AR, Addetia K, Spencer KT, Sweiss NJ, et al. Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am J Cardiol. 2014;113:1556–1560. doi: 10.1016/j.amjcard.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 161.Kouranos V, Tzelepis GE, Rapti A, Mavrogeni S, Aggeli K, Douskou M, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:1437–1447. doi: 10.1016/j.jcmg.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 162.Stanton KM, Ganigara M, Corte P, Celermajer DS, McGuire MA, Torzillo PJ, et al. The utility of cardiac magnetic resonance imaging in the diagnosis of cardiac sarcoidosis. Heart Lung Circ. 2017;26:1191–1199. doi: 10.1016/j.hlc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 163.Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging. 2015;16:634–641. doi: 10.1093/ehjci/jeu294. [DOI] [PubMed] [Google Scholar]
- 164.Bravo PE, Raghu G, Rosenthal DG, Elman S, Petek BJ, Soine LA, et al. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol. 2017;241:457–462. doi: 10.1016/j.ijcard.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 166.Wicks EC, Menezes LJ, Barnes A, Mohiddin SA, Sekhri N, Porter JC, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018;19:757–767. doi: 10.1093/ehjci/jex340. [DOI] [PubMed] [Google Scholar]
- 167.Yokoyama R, Miyagawa M, Okayama H, Inoue T, Miki H, Ogimoto A, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195:180–187. doi: 10.1016/j.ijcard.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 168.Sperry BW, Tamarappoo BK, Oldan JD, Javed O, Culver DA, Brunken R, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11:336–345. doi: 10.1016/j.jcmg.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 169.Sgard B, Brillet PY, Bouvry D, Djelbani S, Nunes H, Meune C, et al. Evaluation of FDG PET combined with cardiac MRI for the diagnosis and therapeutic monitoring of cardiac sarcoidosis. Clin Radiol. 2018;74:81, e9–81,e18. doi: 10.1016/j.crad.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 170.Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 171.Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9:e005001. doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shafee MA, Fukuda K, Wakayama Y, Nakano M, Kondo M, Hasebe Y, et al. Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol. 2012;60:448–453. doi: 10.1016/j.jjcc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 173.Agoston-Coldea L, Kouaho S, Sacre K, Dossier A, Escoubet B, Chillon S, et al. High mass (>18g) of late gadolinium enhancement on CMR imaging is associated with major cardiac events on long-term outcome in patients with biopsy-proven extracardiac sarcoidosis. Int J Cardiol. 2016;222:950–956. doi: 10.1016/j.ijcard.2016.07.233. [DOI] [PubMed] [Google Scholar]
- 174.Flores RJ, Flaherty KR, Jin Z, Bokhari S. The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis. J Nucl Cardiol. doi: 10.1007/s12350-018-01504-y. [online ahead of print] 12 Nov 2018. [DOI] [PubMed] [Google Scholar]
- 175.Alvi RM, Young BD, Shahab Z, Pan H, Winkler J, Herzog E, et al. Repeatability and optimization of FDG positron emission tomography for evaluation of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2019;12:1284–1287. doi: 10.1016/j.jcmg.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 176.Vita T, Okada DR, Veillet-Chowdhury M, Bravo PE, Mullins E, Hulten E, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11:e007030. doi: 10.1161/CIRCIMAGING.117.007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ribiero ML, Jellis CL, Joyce E, Callahan TD, Hachamovitch R, Culver DA. Update on cardiac sarcoidosis. Ann Am Thorac Soc. 16:1341–1350. doi: 10.1513/AnnalsATS.201902-119CME. [DOI] [PubMed] [Google Scholar]
- 178.Martineau P, Pelletier-Galarneau M, Juneau D, Leung E, Nery PB, de Kemp R, et al. Imaging cardiac sarcoidosis with FLT-PET compared with FDG/perfusion-PET: a prospective pilot study. JACC Cardiovasc Imaging. 2019;12:2280–2281. doi: 10.1016/j.jcmg.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 179.Hotta M, Minamimoto R, Kubota S, Awaya T, Hiroi Y. 11C-4DST PET/CT imaging of cardiac sarcoidosis: comparison with 18-F-FDG and cardiac MRI. Clin Nucl Med. 2018;43:458–459. doi: 10.1097/RLU.0000000000002059. [DOI] [PubMed] [Google Scholar]
- 180.Alhamad EH, Idrees MM, Alanezi MO, Alboukai AA, Shaik SA. Sarcoidosis-associated pulmonary hypertension: clinical features and outcomes in Arab patients. Ann Thorac Med. 2010;5:86–91. doi: 10.4103/1817-1737.62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Parikh KS, Dahhan T, Nicholl L, Ruopp N, Pomann GM, Fortin T, et al. Clinical features and outcomes of patients with sarcoidosis-associated pulmonary hypertension. Sci Rep. 2019;9:4061–40030. doi: 10.1038/s41598-019-40030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J. 2008;32:296–302. doi: 10.1183/09031936.00175907. [DOI] [PubMed] [Google Scholar]
- 183.Keir GJ, Walsh SL, Gatzoulis MA, Marino PS, Dimopoulos K, Alonso R, et al. Treatment of sarcoidosis-associated pulmonary hypertension: a single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:82–90. [PubMed] [Google Scholar]
- 184.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128:1483–1489. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 185.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138:1078–1085. doi: 10.1378/chest.09-2002. [DOI] [PubMed] [Google Scholar]
- 186.Kirkil G, Lower EE, Baughman RP. Predictors of mortality in pulmonary sarcoidosis. Chest. 2018;153:105–113. doi: 10.1016/j.chest.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 187.Baughman RP, Shlobin OA, Wells AU, Alhamad EH, Culver DA, Barney J, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med. 2018;139:72–78. doi: 10.1016/j.rmed.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 188.Huitema MP, Spee M, Vorselaars VM, Boerman S, Snijder RJ, van Es HW, et al. Pulmonary artery diameter to predict pulmonary hypertension in pulmonary sarcoidosis. Eur Respir J. 2016;47:673–676. doi: 10.1183/13993003.01319-2015. [DOI] [PubMed] [Google Scholar]
- 189.Duong H, Bonham CA. Sarcoidosis-associated pulmonary hypertension: pathophysiology, diagnosis, and treatment. Clin Pulm Med. 2018;25:52–60. doi: 10.1097/CPM.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 191.Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129:1246–1252. doi: 10.1378/chest.129.5.1246. [DOI] [PubMed] [Google Scholar]
- 192.Joyce E, Kamperidis V, Ninaber MK, Katsanos S, Debonnaire P, Schalij MJ, et al. Prevalence and correlates of early right ventricular dysfunction in sarcoidosis and its association with outcome. J Am Soc Echocardiogr. 2016;29:871–878. doi: 10.1016/j.echo.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 193.Liu L, Xu J, Zhang Y, Fang L, Chai Y, Niu M, et al. Interventional therapy in sarcoidosis-associated pulmonary arterial stenosis and pulmonary hypertension. Clin Respir J. 2017;11:906–914. doi: 10.1111/crj.12435. [DOI] [PubMed] [Google Scholar]
- 194.Maimon N, Salz L, Shershevsky Y, Matveychuk A, Guber A, Shitrit D. Sarcoidosis-associated pulmonary hypertension in patients with near-normal lung function. Int J Tuberc Lung Dis. 2013;17:406–411. doi: 10.5588/ijtld.12.0428. [DOI] [PubMed] [Google Scholar]
- 195.Pabst S, Hammerstingl C, Grau N, Kreuz J, Grohe C, Juergens UR, et al. Pulmonary arterial hypertension in patients with sarcoidosis: the Pulsar single center experience. Adv Exp Med Biol. 2013;755:299–305. doi: 10.1007/978-94-007-4546-9_38. [DOI] [PubMed] [Google Scholar]
- 196.Patel MB, Mor-Avi V, Murtagh G, Bonham CA, Laffin LJ, Hogarth DK, et al. Right heart involvement in patients with sarcoidosis. Echocardiography. 2016;33:734–741. doi: 10.1111/echo.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rapti A, Kouranos V, Gialafos E, Aggeli K, Moyssakis J, Kallianos A, et al. Elevated pulmonary arterial systolic pressure in patients with sarcoidosis: prevalence and risk factors. Hai. 2013;191:61–67. doi: 10.1007/s00408-012-9442-4. [DOI] [PubMed] [Google Scholar]
- 198.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:108–116. [PubMed] [Google Scholar]
- 199.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130:1481–1488. doi: 10.1378/chest.130.5.1481. [DOI] [PubMed] [Google Scholar]
- 200.Bonham CA, Oldham JM, Gomberg-Maitland M, Vij R. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest. 2015;148:1055–1062. doi: 10.1378/chest.14-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barnett CF, Bonura EJ, Nathan SD, Ahmad S, Shlobin OA, Osei K, et al. Treatment of sarcoidosis-associated pulmonary hypertension: a two-center experience. Chest. 2009;135:1455–1461. doi: 10.1378/chest.08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ford HJ, Baughman RP, Aris R, Engel P, Donohue JF. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ. 2016;6:557–562. doi: 10.1086/688775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thakkar V, Stevens W, Prior D, Youssef P, Liew D, Gabbay E, et al. The inclusion of N-terminal pro-brain natriuretic peptide in a sensitive screening strategy for systemic sclerosis-related pulmonary arterial hypertension: a cohort study. Arthritis Res Ther. 2013;15:R193. doi: 10.1186/ar4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.