Abstract
Aim: Recently identified human bocavirus (HBoV) types 2 and 3 have been associated with acute gastroenteritis in children. We studied 878 stool specimens from children with acute gastroenteritis and 112 controls (43 children with unspecified fever, 33 with respiratory tract infection and 36 healthy children) for known HBoVs. The same specimens were previously studied for rotaviruses, noroviruses, sapoviruses, adenoviruses, coronaviruses and aichivirus.
Methods: HBoVs were detected by PCR and positive amplicons were sequenced to identify HBoV1, HBoV2, HBoV3 and HBoV4.
Results: HBoV of any type was found in 85 (9.7%) cases of acute gastroenteritis and in 6 (5.4%) controls. HBoV1 was detected in 49 (5.6%) cases and 2 (1.8%) controls, HBoV2 in 29 (3.3%) cases and 2 (1.8%) controls and HBoV3 in 8 (0.9%) cases and 2 (1.8%) controls. No HBoV4 was found. HBoV as a single infection was found in 16 (1.8%) cases and in 6 (5.4%) controls; in the remaining cases, a known gastroenteritis virus was also found. Among the single HBoV infections, HBoV2 was the most common type with 8 (50%) cases.
Conclusion: HBoVs are rarely found alone in children with acute gastroenteritis. Further studies are warranted to confirm a possible specific association of HBoV2 with gastroenteritis.
Keywords: Acute gastroenteritis, Children, Human bocavirus, Polymerase chain reaction
Abbreviations
- AGE
acute gastroenteritis
- HBoV
human bocavirus
- HBoV1
human bocavirus 1
- HBoV2
human bocavirus 2
- HBoV3
human bocavirus 3
- HBoV4
human bocavirus 4
- PCR
polymerase chain reaction
Key notes
-
•
We found human bocaviruses (HBoVs) 1, 2 and 3 at respective rates 5.6%, 3.3% and 0.9% in children with acute gastroenteritis but mostly in combination with known gastroenteritis viruses.
-
•
Of the cases with HBoV as a single agent (1.8% of all AGE cases), HBoV2 accounted for 50%.
-
•
Further studies are warranted to confirm the possible role of HBoV2 in acute gastroenteritis; otherwise, the role of human bocaviruses appears small.
Introduction
The first human bocavirus (HBoV1) was described in 2005 by Allander et al. (1) as a result of screening of nasopharyngeal aspirates of children with respiratory tract infection. Since then, HBoV1 has been connected to respiratory tract infections (2, 3, 4), and the evidence for this has increased with the use of serological studies (5). On the other hand, co‐infections with other respiratory pathogens are common and HBoV1 has also been found in asymptomatic subjects (6, 7, 8).
Furthermore, several studies have shown that HBoV1 may also be present in faecal samples of children with acute gastroenteritis (AGE) (9, 10, 11, 12, 13), and to complicate matters, patients with HBoV1 in respiratory tract samples have been reported to have diarrhoea (3). As in the case of the respiratory tract, simultaneous presence of HBoV1 with other, previously established gastroenteritis viruses is common in faecal specimens (10, 12, 13), and no clear connection between HBoV1 and AGE of children has been established (11, 13, 14).
Since 2009, three new human bocaviruses have been identified (14, 15, 16). HBoV2 was found in a stool specimen of a Pakistani child in 2009 (15), and since then, HBoV2 has been detected in several studies on children with AGE (14, 17, 18, 19, 20, 21). Again, co‐infections with known human gastroenteritis viruses have commonly been found (18, 20) as well as shedding in stools of asymptomatic children (20). Therefore, the role of HBoV2 as an enteric pathogen is still not confirmed. HBoV2 has also been detected in stools of children with respiratory tract infection (22) but not at all (23) or rarely (24) in respiratory tract samples.
HBoV3 was also originally detected in a stool sample in 2009 (14). Several other studies have confirmed the presence of HBoV3 in faecal samples of patients with gastroenteritis, but detection rates have been lower than those of HBoV2 (19, 20, 21).
Recently, HBoV4 was found in faecal samples of children and adults (16), but the significance of this virus may be regarded as unknown.
To elucidate the role of different human bocaviruses in AGE of children, we tested stool specimens from 878 children seen in hospital because of AGE. The samples were collected in a 2‐year prospective study from August 2006 to August 2008 (25). This material had been tested previously for known gastroenteritis viruses including rotaviruses, noroviruses, sapoviruses, adenoviruses, coronaviruses and aichivirus (25, 26, 27, 28, 29).
Material and Methods
Patients and specimens
The prospective study on the aetiology of AGE in children was approved by the Ethics Committee of Pirkanmaa Hospital District and conducted at Tampere University Hospital from August 2006 to August 2008 and at Kuopio University Hospital from September 2006 to August 2007 (25).
Children under 15 years of age with AGE admitted to the paediatric outpatient clinic or to the hospital ward, or those who came down with AGE during hospitalization were eligible for the study. Informed consent was obtained from the guardians of all study subjects diagnosed with AGE by a paediatrician or included in the study as controls. A total of 878 stool specimens were obtained from children with AGE (one per subject) and 112 stool specimens were collected as controls including three different groups of patients: 43 specimens from children with fever of unknown origin (some of these also had vomiting but not diarrhoea), 33 specimens from children with respiratory tract infection and 36 specimens from healthy children admitted for examinations. Because the study material was not originally collected for HBoV studies, the selection of the control material was not optimal for HBoVs. Also, the number of control cases remained small causing some limitations for statistical analyses, as discussed later.
Rotaviruses, caliciviruses including norovirus genogroups I and II and sapoviruses, and human coronaviruses (229E, OC43, HKU1 and NL63) had previously been studied using the same material (25, 26, 27). In addition, 516 stool specimens were previously tested for aichivirus and 724 for adenoviruses (28, 29). All enteric viruses were tested using polymerase chain reaction (PCR) method, except for adenoviruses; either a PCR or ProSpecT® enzyme‐linked immunosorbent assay‐kit (Oxoid, Basingstoke, UK) was used.
Laboratory methods
Suspensions 10% w/v were made by diluting stool specimens in phosphate‐buffered saline. Viral nucleic acid was extracted using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer′s protocol (this method was tested to be suitable for DNA extraction).
HBoV DNA was amplified by PCR, and a two‐step PCR method was used to increase sensitivity. In the first PCR, reaction volume was 50 μL containing 5 μL of the sample DNA, 1× Green GoTaq® Flexi Buffer (Promega, Madison, WI, USA), 1.5 mmol/L of GoTaq® MgCl2 (Promega), 200 μmol/L of each dNTP (Promega), 2.5 U of GoTaq DNA polymerase (Promega) and 0.5 μmol/L of HBoV NS1 primers (Sigma‐Aldrich, St Louis, Mo, USA). PCR programme was run as follows: denaturation at 94°C for 3 min, 35 cycles of amplification (40 sec at 94°C, 30 sec at 62°C, 65 sec at 72°C) and final extension at 72°C for 5 min. The first amplification produced a 960‐bp amplicon of gene NS1 encoding for a non‐structural protein (Table 1).
Table 1.
The oligonucleotide primers for HBoVs
| Name | Sequence* | Position** | Size |
|---|---|---|---|
| HBoV NS1 fwd | GGACGTGGTSCGTGGGAAC | 1089–1107(+) | 960 bp |
| HBoV NS1 rev | GTCCTGTGAATGWGTAGGACAAAGG | 2024–2048(−) | |
| HBoV NS1 2nd fwd | CCWGTAATTATWTCCACTAACCA | 1764–1786(+) | 200 bp |
| HBoV NS1 2nd rev | AGAGTACAKTCGTACTCATTRAA | 1941–1963(−) | |
| Boca NS‐1 fwd*** | TATGGCCAAGGCAATCGTCCAAG | 1545–1567(+) | 291 bp |
| Boca NS‐1 rev*** | GCCGCGTGAACATGAGAAACAGA | 1813–1835(−) |
In the second PCR, there were two pools of primers, pool Boca (30) and pool HBoV, producing amplicons of 291 and 200 bp in size, respectively (Table 1). Pool HBoV‐primers were designed to detect all HBoVs, especially the newer ones, and pool Boca primers already in use in our laboratory for HBoV1 were included in the PCR. Reaction volume was 50 μL containing 2 μL of the 1st PCR product, 150 μmol/L of each dNTP and 0.5 μmol/L of HBoV NS1 2nd primers or Boca NS‐1 primers, and the rest of the reaction conditions were as in the 1st PCR. PCR programme was run as follows: 3 min at 94°C, 30 cycles of amplification (30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C) and at 72°C for 5 min.
PCR products were visualized in gel electrophoresis, and positive results were confirmed by sequencing using ABI PRISM™ 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences were analysed using Sequencher™ 4.8 program (Gene Codes Corporation, Ann Arbor, MI, USA) and compared to reference strains by NCBI Blast®‐program to determine HBoV types.
Statistical methods
Statistical analyses were conducted using PASW Statistics 18 program (SPSS®, Chicago, IL, USA). Statistical significance was calculated using Fisher′s exact test, and p < 0.05 was considered significant.
Results
Altogether, 878 stool specimens were collected from children with AGE and 112 specimens from control groups including 43 specimens from children with fever of unknown origin, some of whom also had vomiting, 33 specimens from children with respiratory tract infection, and 36 specimens from healthy children admitted for examinations. In 719 (81.9%) of the 878 stool specimens of children with AGE, one or more viruses, including rotaviruses, noroviruses, sapoviruses, adenoviruses, coronaviruses, aichivirus and human bocaviruses, were found. In the combined control groups, 22 (19.6%) of 112 specimens were positive for at least one of these viruses, excluding sapoviruses and aichivirus that were not detected from controls.
Human bocaviruses were detected in 91 (9.2%) cases of all stool samples, 85 (9.7%) of the 878 cases with AGE and 6 (5.4%) of the 112 (non‐AGE) controls. There was not a statistically significant difference in the amount of HBoV‐positive cases between AGE group and combined controls (p = 0.165).
HBoV1 was detected in 49 (5.6%) cases of AGE. There was also one (2.3%) positive sample in the group of children with fever of unknown origin and one (3.0%) positive sample in the group of children with respiratory tract infection; in the healthy children, there were no positive samples. (Table 2.) The differences in detection rates of HBoV1 between AGE and the separate control groups were not statistically significant (p = 0.529). During the first season (August 2006–August 2007), there were 20 cases positive for HBoV1 and during the second season (September 2007–August 2008), 31. One of the samples in the AGE group contained both HBoV1 and HBoV2.
Table 2.
Number and per cent of human bocavirus (HBoV)‐positive cases among different study groups
| Virus | Number (%) of stool specimens in different study groups | All (N = 990) | |||
|---|---|---|---|---|---|
| AGE* (N = 878) | Fever** (N = 43) | RTI*** (N = 33) | Healthy children (N = 36) | ||
| HBoV1 | 49 (5.6%) | 1 (2.3%) | 1 (3.0%) | 0 | 51 (5.2%) |
| HBoV2 | 29 (3.3%) | 1 (2.3%) | 0 | 1 (2.8%) | 31 (3.1%) |
| HBoV3 | 8 (0.9%) | 1 (2.3%) | 1 (3.0%) | 0 | 10 (1.0%) |
| Total | 86 (9.8%)† | 3 (7.0%) | 2 (6.1%) | 1 (2.8%) | 92 (9.3%)† |
*Children with acute gastroenteritis.
**Children with unknown fever.
***Children with respiratory tract infection.
†One of the samples contained both HBoV1 and HBoV2, so this case is calculated here twice. The actual number of HBoV‐positive cases in AGE group is 85 (9.7)% and in all samples 91 (9.2)%.
Twenty‐nine (3.3%) specimens in the AGE group were positive for HBoV2. In the control groups, there was one (2.3%) positive sample in children with fever of unknown origin and one (2.8%) positive sample in the group of healthy children, but none in the children with respiratory tract infection. (Table 2.) The differences in detection rates of HBoV2 between AGE and the separate control groups were not statistically significant (p = 0.949). Numbers of HBoV2‐positive samples during the first and the second season were 18 and 13, respectively.
HBoV3 was detected in 8 (0.9%) cases of children with AGE, in one (2.3%) case of children with fever of unknown origin, and in one (3.0%) case of children with respiratory tract infection, and in none of the healthy children (Table 2.). The differences in detection rates of HBoV3 between AGE and the separate control groups were not statistically significant (p = 0.260). All HBoV3‐positive cases were found during the first season.
In this study, no HBoV4 was detected.
In 16 (1.8%) cases of AGE, human bocavirus was the only virus detected, whereas in the combined controls, all 6 (5.4% of all controls) cases were single infections with HBoV. Among the 16 cases of AGE in which HBoV was the only virus detected in stool, HBoV2 was the most common one with 8 (50.0% of all single infections) cases, followed by HBoV1 with 7 (43.8%) cases and HBoV3 with one (6.3%) case (Table 3). Conversely, among the 69 cases of mixed infections, 42 (60.9% of the HBoV‐positive mixed infections) contained HBoV1, 21 (30.4%) contained HBoV2, and 7 (10.1%) cases contained HBoV3. In one sample, both HBoV1 and HBoV2 were detected. The proportion of HBoV2 was greater in the single infections than in mixed infections and vice versa for HBoV1, but the differences in the proportions of different HBoVs between mixed and single infections were not statistically significant (p = 0.428).
Table 3.
Human bocavirus (HBoV)‐positive findings with other viruses in acute gastroenteritis of childhood
| HBoV | HBoV alone | Co‐infection with one virus | Co‐infection with more than one virus* | Total | |||
|---|---|---|---|---|---|---|---|
| Rota | Noro | Sapo | Adeno | ||||
| HBoV1 | 7 | 14 | 13 | 0 | 5 | 10 | 49 |
| HBoV2 | 8 | 11 | 5 | 1 | 1 | 3 | 29 |
| HBoV3 | 1 | 4 | 2 | 0 | 0 | 1 | 8 |
| Total | 16 | 29 | 20 | 1 | 6 | 14** | 86** |
*Including rotaviruses (8), noroviruses (4), sapoviruses (2), adenoviruses (1), aichivirus (4), coronaviruses (3).
**The case including both HBoV1 and HBoV2 calculated twice.
Forty‐two (85.7%) of 49 HBoV1‐positive cases, 21 (72.4%) of 29 HBoV2‐positive cases and 7 (87.5%) of 8 HBoV3‐positive cases were mixed infections with known gastroenteritis viruses. Proportion of mixed infections did not differ significantly between different HBoVs (data not shown).
In mixed infections, rotaviruses and noroviruses were the most common gastroenteritis viruses detected with bocaviruses (Table 3.). In six cases negative for rotaviruses and noroviruses, HBoVs were detected together with adenovirus. Fifty‐six of the mixed infections contained two different viruses, and in 13 cases, there were more than two viruses. The one case with both HBoV1 and HBoV2 in the stool also had rotavirus in the same specimen.
The seasonal distribution of HBoV findings is shown in Fig. 1. Most of the HBoV‐positive cases were detected from November to June. From November to March, over 10% of all stool samples collected per month were positive for some of the HBoVs, exception being January 2008 with low detection rate of 1.9%.
Figure 1.
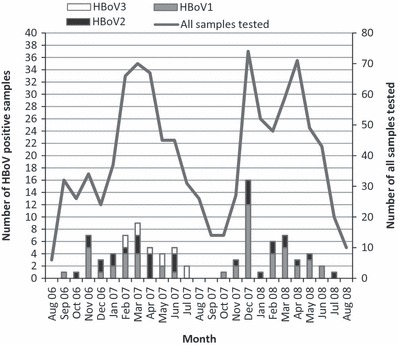
All human bocavirus (HBoV)‐positive findings and monthly numbers of all tested samples during the study period, August 2006–August 2008. (Notice two vertical scales).
Discussion
This study was set‐up to examine whether HBoVs in general and bocavirus types HBoV2 and HBoV3 in particular could be linked with AGE in childhood as aetiological agents. As a whole, we could not confirm such an aetiological role of any of the HBoVs in AGE in children, although HBoV2 appeared more common than the other types when HBoV was found in stool without other viruses.
In this study, human bocaviruses were detected in 9.7% of children with AGE. HBoV1 was detected in 5.6% of all AGE cases, which concurs with previous studies of HBoV1 in children with AGE (0.8–9.1%) (10, 12, 13, 14). However, the differences in the rate of HBoV1 in AGE and control groups were not statistically significant, and in very few cases, HBoV1 was detected alone without well‐established gastroenteritis viruses such as rotavirus or norovirus, which were likely to have a causative role in such cases. As a single infection, HBoV1 was found in only 0.8% of AGE cases.
As shown in Fig. 1, there is a peak in HBoV1 findings during December 2007. This peak is most likely due to the waterborne AGE outbreak that occurred in Nokia, a town near to Tampere, and was caused by contamination of drinking water by sewage water (29). During the outbreak, there was an unusual amount of mixed infections with severe gastrointestinal symptoms (29). Fifty stool samples from children connected to the Nokia outbreak were tested during our study, and we found eight HBoV1‐positive cases (all of these were mixed infections) and two HBoV2‐positive cases (one was a mixed infection). Even if all ‘Nokia cases’ were excluded from the calculations, the differences between HBoVs (separately for HBoV1, HBoV2 and HBoV3) in AGE and control groups did not change outstandingly.
HBoV2 was detected in 3.3% of the samples of the children with AGE. This is a lower detection rate than those reported from Australia (17.2%) (14) and China (20.4–24.6%) (17, 20), but close to the 3.6% reported from South Korea (18). HBoV2 was detected as a single virus in 0.9% of AGE cases. While we could not confirm a specific association of HBoV2 with AGE in children, there were nevertheless numerically more ‘pure’ cases (eight in all) of HBoV2 than other HBoVs, leaving a small possibility of a specific association of HBoV2 with gastroenteritis. Arthur et al. (14) in their case–control study found a statistically significant association between HBoV2 and AGE, but in other studies, despite the common findings in stool specimens, the causal association of HBoV2 and AGE has been weak (17, 20).
In this study, HBoV3 was found in 0.9% of the AGE samples over 2 years. HBoV3 was not detected during the second season at all, and there was only one case of HBoV3 as a single virus in specimen without other gastroenteritis viruses. In earlier studies, detection rates of HBoV3 in children with AGE have been 0.9–2.7% (14, 20), and in general, HBoV3 has been less common than HBoV2 in stool samples of children with AGE (19, 20).
We were unable to find any HBoV4 in our study. This was also the case in several other recent studies (19, 20, 21), and therefore, the role of this virus remains unclear.
We did a thorough work‐up of most of the established gastroenteritis viruses including rotaviruses, noroviruses, sapoviruses, enteric adenoviruses, coronaviruses and aichivirus (astroviruses or bacterial pathogens were not studied) and found co‐infections in 81.2% of all bocavirus‐positive AGE cases. Similar rates (approximately 74–80%) have been detected in earlier studies in which other gastroenteritis viruses have been investigated using adequate methods (20, 21). In such co‐infections, it is reasonable to assume that the known gastroenteritis viruses actually have a causative role and HBoV may be either shed from the respiratory tract or infecting the intestinal tract with no pathogenic role in AGE. In the future, simultaneous testing of respiratory and stool samples together with serologic testing should be carried out to clarify this assumption.
The number of the ‘pure’ HBoV‐positive cases was small and could not be positively associated with AGE. Nevertheless, it is noteworthy that in 50.0% of the single infections, HBoV2 was the bocavirus detected, whereas in the mixed infections, its proportion was only 30.4%.
In this study, most of the HBoV‐positive cases were detected from November to June. The highest proportional detections rates, comparing to number of collected samples per month, were in winter months (Fig. 1). There was no remarkable difference in seasonality between HBoV1 and HBoV2, but HBoV3 was detected only from February 2007 to July 2007. In some previous studies, HBoV1 was detected throughout the year, but some higher incidence during winter months was also seen (3, 11). HBoV2 was also detected throughout the year, and the highest incidences were detected from February to April (17).
The sizes of the control groups were a limitation in our study and also a limitation for a reliable statistical analysis of causative role of HBoVs in AGE. In principle, using all three groups, that is, children with respiratory tract infection, children with fever and vomiting, and healthy children as controls, might be justified, but the size of each group remained too small, and some statistical comparison was made with pooled controls, which is not optimal. Originally, this material was not collected for bocavirus studies. Because of these limitations and because other viruses were frequently found in cases of AGE, we focused more on infections with HBoV as a single pathogen indicating specific association with AGE.
Further studies are warranted, and a study with simultaneous collection of specimens from respiratory tract and stools is in progress to investigate the type‐specific association of HBoVs with respiratory or gastrointestinal tract, respectively. Collection of the serum samples is also being carried out in our next studies for serological testing and the detection of HBoV viraemia.
In conclusion, we investigated stool samples from a large number of children with AGE and found human bocaviruses 1, 2 and 3 at respective rates of 5.6%, 3.3% and 0.9%, but bocaviruses were seldom detected alone without other viruses like rotaviruses and noroviruses. Even those cases that appeared to be single HBoV infections may actually have been co‐infections if a more comprehensive work‐up on viruses such as astroviruses had been performed and bacterial pathogens had also been investigated. In the total material, HBoV2 and HBoV3 did not stand out as having any stronger association with AGE than HBoV1, but in the ‘pure’ HBoV cases of AGE, HBoV2 was slightly overrepresented.
Conflict of interest and funding
No conflict of interest. No specific funding.
Acknowledgements
We would like to thank study nurse Marjo Salonen, laboratory supervisor Marjo Salminen and our laboratory technicians, especially Emilia Halttunen, for excellent work in this project. We also thank Heini Huhtala for indispensable help with statistical analyses.
References
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 2005; 102: 12891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brieu N, Guyon G, Rodiere M, Segondy M, Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 2008; 27: 969–73. [DOI] [PubMed] [Google Scholar]
- 4. Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. Human bocavirus in children: mono‐detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 2010; 49: 158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantola K, Hedman L, Allander T, Jartti T, Lehtinen P, Ruuskanen O, et al. Serodiagnosis of human bocavirus infection. Clin Infect Dis 2008; 46: 540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longtin J, Bastien M, Gilca R, Leblanc E, de Serres G, Bergeron MG, et al. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis 2008; 14: 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Linstow ML, Hogh M, Hogh B. Clinical and epidemiologic characteristics of human bocavirus in danish infants: results from a prospective birth cohort study. Pediatr Infect Dis J 2008; 27: 897–902. [DOI] [PubMed] [Google Scholar]
- 8. Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 2010; 201: 1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pham NT, Trinh QD, Chan‐It W, Khamrin P, Nishimura S, Sugita K, et al. Human bocavirus infection in children with acute gastroenteritis in Japan and Thailand. J Med Virol 2011; 83: 286–90. [DOI] [PubMed] [Google Scholar]
- 10. Vicente D, Cilla G, Montes M, Perez‐Yarza EG, Perez‐Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis 2007; 13: 636–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu JM, Li DD, Xu ZQ, Cheng WX, Zhang Q, Li HY, et al. Human bocavirus infection in children hospitalized with acute gastroenteritis in China. J Clin Virol 2008; 42: 280–5. [DOI] [PubMed] [Google Scholar]
- 12. Lee JI, Chung JY, Han TH, Song MO, Hwang ES. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis 2007; 196: 994–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng WX, Jin Y, Duan ZJ, Xu ZQ, Qi HM, Zhang Q, et al. Human bocavirus in children hospitalized for acute gastroenteritis: a case‐control study. Clin Infect Dis 2008; 47: 161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog 2009; 5: e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, et al. A newly identified bocavirus species in human stool. J Infect Dis 2009; 199: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis 2010; 201: 1633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu ZQ, Cheng WX, Li BW, Li J, Lan B, Duan ZJ. Development of a real‐time PCR assay for detecting and quantifying human bocavirus 2. J Clin Microbiol 2011; 49: 1537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han TH, Kim CH, Park SH, Kim EJ, Chung JY, Hwang ES. Detection of human bocavirus‐2 in children with acute gastroenteritis in South Korea. Arch Virol 2009; 154: 1923–7. [DOI] [PubMed] [Google Scholar]
- 19. Kantola K, Sadeghi M, Antikainen J, Kirveskari J, Delwart E, Hedman K, et al. Real‐time quantitative PCR detection of four human bocaviruses. J Clin Microbiol 2010; 48: 4044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin Y, Cheng WX, Xu ZQ, Liu N, Yu JM, Li HY, et al. High prevalence of human bocavirus 2 and its role in childhood acute gastroenteritis in China. J Clin Virol 2011; 52: 251–3. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Gonzalez R, Zhou H, Li J, Li Y, Paranhos‐Baccala G, et al. Detection of human bocavirus 3 in China. Eur J Clin Microbiol Infect Dis 2011; 30: 799–805. [DOI] [PubMed] [Google Scholar]
- 22. Shan TL, Zhang W, Guo W, Cui L, Yuan CL, Dai XQ, et al. The first detection of human bocavirus 2 infections in China. J Clin Virol 2009; 46: 196–7. [DOI] [PubMed] [Google Scholar]
- 23. Chieochansin T, Kapoor A, Delwart E, Poovorawan Y, Simmonds P. Absence of detectable replication of human bocavirus species 2 in respiratory tract. Emerg Infect Dis 2009; 15: 1503–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han TH, Chung JY, Hwang ES. Human bocavirus 2 in children, South Korea. Emerg Infect Dis 2009; 15: 1698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasanen S, Lappalainen S, Halkosalo A, Salminen M, Vesikari T. Rotavirus gastroenteritis in Finnish children in 2006–2008, at the introduction of rotavirus vaccination. Scand J Infect Dis 2011; 43: 58–63. [DOI] [PubMed] [Google Scholar]
- 26. Rasanen S, Lappalainen S, Salminen M, Huhti L, Vesikari T. Noroviruses in children seen in a hospital for acute gastroenteritis in Finland. Eur J Pediatr 2011; 170: 1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Risku M, Lappalainen S, Rasanen S, Vesikari T. Detection of human coronaviruses in children with acute gastroenteritis. J Clin Virol 2010; 48: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaikkonen S, Rasanen S, Ramet M, Vesikari T. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol Infect 2010; 138: 1166–71. [DOI] [PubMed] [Google Scholar]
- 29. Rasanen S, Lappalainen S, Kaikkonen S, Hamalainen M, Salminen M, Vesikari T. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol Infect 2010; 138: 1227–34. [DOI] [PubMed] [Google Scholar]
- 30. Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol 2006; 35: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


