Abstract
Lipocalin-2 (LCN2) is a secreted molecule, expressed in various cell types, that is involved in the progression of numerous diseases and disorders. The biological functions and expression levels of LCN2 in diseases including pancreatic cancer, pancreatitis (acute and chronic), and diabetes mellitus, suggest the potential role of LCN2 as a biomarker and/or therapeutic target. However, findings on the role of LCN2 in pancreatic diseases have been contradictory. In pancreatic cancer and pancreatitis, LCN2 has been identified as a potential biomarker; increased expression levels in various biological specimens correlate with the presence of the disease and may be able to differentiate cancer and chronic pancreatitis from healthy subjects. LCN2 is also known to be an adipokine; it is upregulated in obesity and is a common co-factor in the development of pancreatic diseases. Emerging research suggests LCN2 is elevated in type 2 diabetes mellitus, but the exact role of LCN2 in this disease is not clear. In this review, we summarize research on LCN2 as it relates to pancreatic diseases, highlighting the discrepancies in the literature. By explaining and clarifying the role of LCN2 in these disorders, we aim to promote research in developing novel diagnostic and treatment strategies to reduce the burden of pancreatic diseases.
Keywords: Lipocalin-2, neutrophil gelatinase-associated lipocalin, pancreatitis, pancreatic cancer, obesity
Introduction
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), lipocalin 24p3, migration stimulating factor inhibitor (MSFI), superinducible protein 24 (SIP24), Ch21, α1-microglobulin related protein, uterocalin and siderocalin, is secreted from multiple tissue and cell types. LCN2 was originally isolated from the gelatinase subcellular compartment of human neutrophils supporting the innate immune responses that help fight bacterial infections1. Increased LCN2 expression has been associated with the innate immune system for immune cell migration, localization, infiltration, and adhesion,2 as well as a part of the inflammatory responses related to inflammatory bowel disease,3 psoriasis,4, 5 metabolic syndrome,6, 7 neurodegenerative diseases,8-10 and cancer11, 12.
Diseases of the exocrine and endocrine pancreas, including pancreatic ductal adenocarcinoma (PDAC), acute pancreatitis (AP), chronic pancreatitis (CP), and certain types of diabetes, have all been linked to inflammation and increased LCN2 expression. Understanding the pathophysiology that associates inflammation and LCN2 expression to disease initiation and progression could assist in the development of new treatment strategies. Among pancreatic diseases, PDAC has the worst outcome, with a survival rate of less than 9%, indicating that new, more effective diagnostic and treatment strategies are needed13. Both, PDAC and pancreatitis, particularly CP, are strongly associated with fibrosis and chronic inflammation14, 15. In AP, a single bout of the disease can alter inflammatory pathways resulting in recurrent AP or CP16, 17. Moreover, CP-associated inflammation and fibrosis increases the risk for developing PDAC18, 19. Emerging studies are now linking the gut microbiome diversity with PDAC outcomes and resistance to common treatments like gemcitabine 20, 21. Therefore, as a regulator of immune cell migration and infiltration during inflammation, LCN2 may be one of the mediators driving increased risk and worse prognosis in patients with PDAC and other pancreatic disorders.
A comprehensive literature review was performed in order to organize and assess the state of LCN2 research and how it relates to pancreatic diseases. This review discusses current knowledge relating LCN2 function and expression with PDAC, AP, CP, and diabetes mellitus. Additionally, the potential of LCN2 to serve as a biomarker and therapeutic target for some of these pancreatic diseases is discussed.
Physiologic Functions of LCN2
LCN2 is a 25kDa protein22, 23 that is essential in modulating iron homeostasis1. It binds to three identified receptors, megalin, solute carrier family 22 member 17 (SLC22A17), and melanocortin-4 (MC4R). Megalin, also known as LDL receptor related protein 2 (LRP2), facilitates renal reabsorption of LCN224. LCN2 binds specifically to SLC22A17, also known as 24p3R, and is expressed in several tissues, including pancreatic stellate cells25, 26. Finally, MC4R binds to osteoblast-derived LCN2 in the neurons of the hypothalamus, activating the MC4R-dependent appetite-suppressing pathway27.
LCN2 binds ferric iron using a siderophore cofactor and serves a major role in the innate immune system1. Siderophore cofactors are produced by plants, fungi, bacteria,28 and mammals29. LCN2 binds bacterial or mammalian siderophores, such as enterobactin,29 which are secreted to chelate iron from host proteins during stress conditions, such as inflammation30. Iron scavenging by bacterial siderophores causes neutrophils in the host to secrete LCN2 to sequester the iron-siderophore complexes away from bacteria. As a result, LCN2 inhibits bacterial growth as part of the defense mechanism of the innate immune response system31. A variety of proinflammatory signals induce LCN2 expression, including lipopolysaccharide (LPS), and IL-1β32. Some of these responses are mediated through the activation of NF-KB32.
Another function of LCN2 is to bind and transport lipophilic molecules such as fatty acids and steroids. LCN2 has a binding affinity for cholesterol, retinol, and retinoic acid33. For this reason, LCN2 is suggested to aid in lipid-mediated signal transductions, such as the metabolic homeostasis of thermogenesis in adipose tissue via retinoid regulation34, 35. As an adipokine, LCN2 expression correlates positively with adiposity, hypertriglyceridemia, hyperglycemia, and insulin resistant36. The critical role of LCN2 during inflammation and association with adipose tissue make it a potential target molecule for diagnosis or treatment of many inflammatory diseases and obesity-associated cancers.
LCN2 Expression and Mechanisms in PDAC
LCN2 expression has been assessed as a possible diagnostic and/or a prognostic indicator of oncogenesis in a variety of tissues37, 38. However, there are contradictory findings regarding the contributions of LCN2 in the pathogenesis of these cancers. For example, some studies indicate that increased LCN2 expression results in reduced invasion, metastasis, and angiogenesis of tumor cells and suppresses the process of epithelial-to-mesenchymal transition in certain cancers39-45. Conversely, other studies indicate poor prognosis of cancer outcomes (including PDAC), with increased levels of LCN2 associated with increased growth and survival of oncogenic cells, drug resistance, metastasis, angiogenesis, and invasion46-51. It is possible this apparent controversy is a result of the body trying to produce more LCN2 to reduce invasion and metastasis of aggressive cancers, however this will need to be confirmed in in vitro and in vivo experiments.
In PDAC, LCN2 expression levels begin to increase in pancreatic intraepithelial neoplasia (PanIN) lesions as early as PanIN1, and increased expression correlates with malignant progression to PDAC52-61. LCN2 is highly upregulated in the blood and pancreatic fluid, so it could help discriminate PDAC from other diseases and healthy subjects60, 62. This increased expression of LCN2 is also observed in various preclinical mouse models of PDAC26. However, as with other cancers, findings from research attempting to elucidate the molecular mechanisms of LCN2 in PDAC have been inconclusive and at times contradictory. Both in vitro and in vivo studies, using orthotopic and subcutaneous preclinical mouse models, have reported mixed results regarding the role of LCN2 in PDAC growth, invasion, and metastasis43, 50, 63. Some of these studies suggest that LCN2 serves as a pancreatic tumor suppressor,43, 63 while others propose it is a pancreatic tumor promoter50. These heterogenous results could potentially be explained by the study design, as the studies did not allow for simultaneously assessing the contribution of the immune system in the progression of PDAC. To address this concern, our laboratory used a genetically engineered mouse model (GEMM) of PDAC and crossed it with an Lcn2 null mice to show that lack of Lcn2 decreased inflammation and fibrosis of the tumor microenvironment26. Moreover, whole body deletion of Lcn2 in this GEMM prolonged survival of mice predisposed to develop PDAC due to Kras expression and feeding of a high-fat diet26.
In addition to regulating inflammation in the tumor microenvironment, LCN2 expression may also modulate metastasis and angiogenesis. LCN2 depletion is observed in poorly differentiated PDAC tissue (mesenchymal-like), and is thought to be necessary for invasion and metastasis40, 41, 43, 44, 53. Relative to these observations, epidermal growth factor decreases LCN2 expression via NF-KB inhibition in PDAC63. This down regulation of LCN2 is brought about by the activation of the EGFR/MEK/ERK signaling pathway, which subsequently inhibits E-cadherin, a regulator of the epithelial-to-mesenchymal transition (EMT), along with a reduction of NF-KB activation, another regulator of EMT39, 63. Alternatively, the NF-KB p50 protein and the nuclear protein IKBζ form a transcription complex on the Lcn2 gene promoter and generate Lcn2 mRNA for increased expression64. Since LCN2 may be either pro- or anti- oncogenic depending on the type of cancer, it will be critical for future research to establish the mechanism that causes the transition between excess LCN2 secretion and an inhibition of LCN2 expression in PDAC.
In the tumor microenvironment, LCN2 inhibits angiogenesis by reducing VEGF expression, leading to a hypovascular tumor environment44, 65, 66. Typically, preventing angiogenesis inhibits tumor growth; however, in PDAC, the hypovascular environment is detrimental as it limits effective delivery of chemotherapy. Therefore, inhibiting LCN2-induced hypovascularity, possibly through antibody neutralization or directed small molecule inhibition treatment could enhance treatment effectiveness. Altering the expression of, or preventing LCN2 secretion may be key to future therapeutic approaches for PDAC.
Aside from therapies, clinical research on LCN2 focuses on whether it can be used as an early biomarker of PDAC or for staging of the cancer. LCN2 expression has been assessed as a diagnostic biomarker in urine, serum, bile, pancreatic fluid/juice and fluid from pancreatic cysts (Table 1).26, 43, 52-54, 56, 60, 61, 67, 68. One group showed that LCN2 in combination with miR-196b and TIMP1 is 100% specific and 100% sensitive in distinguishing patients with PanINs and stage I PDAC from healthy subjects amongst a cohort of high risk individuals; however, this combination did not perform as well in discriminating PDAC from CP (sensitivity 50%, specificity 80%)54. It has been suggested that effective use of LCN2 as a diagnostic biomarker for PDAC can be improved by comparing serial levels in urine samples, with doubling in concentration being indicative of PDAC69. Further studies are needed to assess temporal trends in other fluids, including serum and bile.
Table 1:
Summary of LCN2 Expression Levels in Human Subjects with Pancreatic Diseases.
| Pancreatic Disease |
Biospecimen | Detection Method | LCN2 Expression (Summary) |
Year + Ref. |
|---|---|---|---|---|
| PDAC | Tissue (core biopsy) | RT-PCR IHC | ↑ in tumor tissue vs. normal tissue | 2006 61 |
| Tissue | IHC | Predominantly expressed in PanIN-1 and PanIN-2 lesions | 2008 43 | |
| Tissue | IHC | ↑ with increasing PanIN score | 2008 53 | |
| Serum | ELISA | ↑ in PC with diabetes ↓ after surgical removal of PDAC |
2013 56 | |
| Plasma | ELISA | ↑ in PDAC compared to healthy controls | 2013 60 | |
| Cyst Fluid | ARCHITECHT Analyzer | ↑ in inflammatory cystic group over cystic neoplasm | 2018 68 | |
| Serum | ELISA | ↑ in familial pancreatic cancer | 2018 54 | |
| CP vs. PDAC | Plasma | ELISA | ↑ in CP and PDAC patients, but could not differentiate CP from PDAC | 2013 52 |
| Urine Bile | ELISA | Differentiated PDAC from CP | 201667 | |
| Serum + Plasma | ELISA | ↑ in PDAC compared to CP and normal | 2017 26 | |
| AP | Serum | ELISA | ↑ between 24 and 48 hours and differentiated mild vs. severe AP | 2010 71 |
| Serum | Fluorescent Immunoassay Triage® | ↑ compared to ventilated patients without pancreatitis | 2013 75 | |
| Urine Serum | ARCHITECHT Analyzer ELISA | Mild ↑ in urine between 24 and 72h No significant change in serum | 2016 72 | |
| Serum | ELISA | ↑ but no significant different between mild and severe | 201673 | |
| Urine | Urinalysis | ↑ correlates with severity | 2016 76 | |
| Serum | MILLIPLEX MAP human metabolic magnetic beads | ↑ after AP Mild ↑ associated with RAP, but not significant | 2017 77 | |
| Diabetes Mellitus | Serum | ↑ in type 2 diabetes mellitus | 201356 |
AP, acute pancreatitis; CP, chronic pancreatitis; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry
LCN2 Expression in Acute and Chronic Pancreatitis
AP is usually a self-limited disorder where duration and degree of organ failure determines the severity of the disease, with the most severe cases resulting in multi-systemic organ failure and death70. Expression of LCN2 has been associated with increased severity of AP; however these findings are debatable71-73. Some studies suggest urine LCN2 is an effective early marker for AP74-76. In particular, these studies show a strong correlation between LCN2 elevation and severity of AP. Other studies looking at the physiologic changes occurring after AP has resolved found a delayed increase of LCN2 expression in AP patients when alcohol was the etiology 77. Elevation in LCN2 was particularly noted in obese subjects with excess abdominal adiposity, but only after AP had resolved77. This delayed elevation in LCN2 may be associated with a lingering inflammatory response and may be involved in the progression to recurrent AP (RAP) or CP. A study examining the expression of LCN2 across various etiologies of AP and controlling for adiposity is needed to better understand the role of LCN2 in the progression of these diseases.
Acute kidney injury (AKI) is a somewhat common complication seen in moderately severe and severe AP, and associated with worse clinical outcomes including increased length of stay. Increased LCN2 expression has been associated with development and severity of acute kidney injury (AKI)73. LCN2 is currently being assessed for applicability as an biomarker of AKI since it may become elevated prior to changes in serum creatinine, the current clinical standard marker for AKI72,73,78,79. This could be particularly helpful in the subset of AP patients who develop delayed organ failure. Therefore, LCN2 may have a potential role for predicting both long term and acute progression of AP.
CP is a progressive and degenerative disease with symptomatic pain and inflammation, characterized by an irreversible and deleterious effect on the exocrine function. CP-associated inflammation is caused by an infiltration of immune cells like T-cells, particularly CD4+ and CD8+ T-cells,80 and macrophages and progressive fibrosis of the pancreas81. CP patients have an increased lifetime risk for developing PDAC with a standardized incidence ratio of 15-16 times higher than the general population, possibly due to the similarities in inflammatory infiltration and fibrosis between the two diseases82.
No biomarkers are clinically available to differentiate CP from other gastrointestinal disorders like AP and PDAC. Due to the nature of CP being associated with increased, chronic inflammation and immune cell filtration, several groups have looked into whether LCN2 can be used as a biomarker for CP. LCN2 is elevated in CP subjects compared to healthy individuals, as measured in pancreatic fluid, urine, and bile52, 67. Some studies indicate LCN2 is elevated in both CP and PDAC and proposed it is due to overlapping mechanisms of chronic inflammation52-54, 60. However, other studies assessing LCN2 in urine or bile collected intraoperatively suggest LCN2 concentrations could differentiate CP and PDAC67. Being able to differentiate CP from AP or patients with other gastrointestinal disorders by assessing concentrations of LCN2 in the aforementioned studies is a large step towards potentially using LCN2 as a biomarker in the clinic. However, there is still a need for several large, multi-center studies to determine whether LCN2 can be used as an effective biomarker for detecting early stages of CP where most diagnostic uncertainty exists. Additionally, although LCN2 is elevated in CP, there is a lack of research on the exact role of LCN2 in the development or persistence of CP and whether LCN2 blockade could be a therapeutic target for CP.
LCN2 Expression in Obesity-Related Pancreatic Diseases
There is a strong link between obesity and diabetes mellitus, AP and its severity, and PDAC83. Elevation of LCN2 has been implicated in the development of chronic low-grade inflammation associated with obesity84. Obesity is also correlated with increased immune system activation, with elevated serum and visceral adipose tissue concentrations of LCN284. Recent studies have demonstrated that LCN2 can predict early stages of type 2 diabetes mellitus in obese women85. In both children and adults, an increase in LCN2 expression has been positively correlated with increased weight, BMI, waist-to-hip ratio, waist circumference, percent body fat, and serum concentrations of insulin, glucose, and triglycerides, while being negatively correlated with high-density lipoproteins7, 86, 87. Several studies have linked increased LCN2 expression to insulin resistance and sensitivity, glucose uptake, and obesity88, 89. In mice, LCN2 expression in adipocytes and hepatocytes was regulated by obesity, and elevation in Lcn2 enhanced insulin resistance90.
Diet appears to play a critical role in LCN2 function and influences biological processes. A recently published analysis of Lcn2 in mice concluded that under normal diet the Lcn2 knockout mice phenotype and physiology were virtually identical to controls91. When provided a high fat diet, Lcn2 null mice exhibited several physiologic differences compared to controls including, generation of more brown adipose depots, increased oxygen consumption, increased diet consumption, and reduced weight gain compared to a normal diet control or wild type mice fed a high fat diet. These findings underscore the link between LCN2, obesity, and metabolism91
Medical professionals often recommend changes in diet and increased exercise to reduce obesity and risk of obesity-related diseases. However, in a study on the effects of obesity in Korean women, increased exercise was insufficient to reduce serum LCN2 levels92. This is unexpected since other studies indicated exercise can reduce inflammation, even in obesity, in both men and women93-95. Future studies should address whether exercise before, during, and/or after inflammatory pancreatic disease alters circulating LCN2 as well as other inflammatory cytokines and adipokines or whether the changes are related to ethnicity. This information will be essential to clinicians when developing patient-specific plans for reducing risk for obesity-related pancreatic diseases, and ultimately to improve patient outcomes in these diseases.
Conclusions
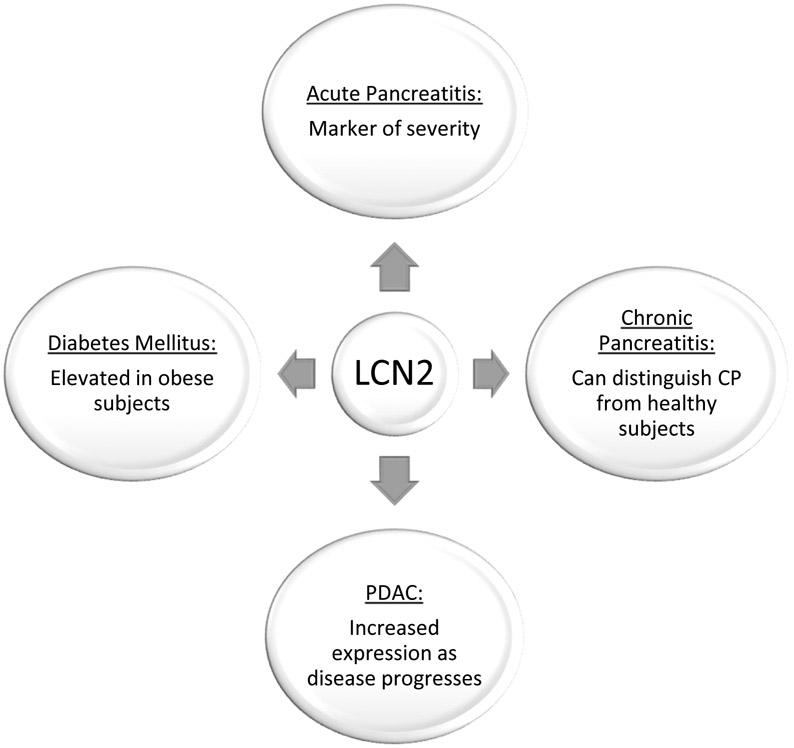
LCN2 expression is increased in AP, CP, pancreatic cancer, and diabetes mellitus (Figure 1); however, the underlying mechanisms and its function in initiation and progression of these diseases remains unclear. Changes in LCN2 expression may be a useful early diagnostic biomarker, but further validation using samples derived from larger multi-institutional cohorts is needed. Since LCN2 has a role in the innate response of bacterial infections, further investigations should consider the potential impact of subsets of gut or local pancreatic microbiota to initiate LCN2 secretion. Understanding the functions of LCN2 in AP and CP will be critical to better understanding disease progression, and targeting immune-associated proteins like LCN2 may potentially be a therapeutic option. Additional studies are needed understanding the diagnostic and therapeutic of LCN2 expression in PDAC. Experiments using GEMMs of PDAC will likely be necessary, because PDAC is typically advanced at the time of diagnosis making it difficult to understand the molecular mechanisms of tumor pathogenesis and progression. Further research into the function of LCN2 will further guide our understanding of its potential use as a diagnostic biomarker and potential therapeutic target, and hopefully create new opportunities to improve the care of patients with pancreatic diseases.
Figure 1:
Summary of the Role of LCN2 in Pancreatic Diseases
Acknowledgments
Grant support:
Research in this publication was supported by: The National Pancreas Foundation (ZC-M), The National Cancer Institute (NCI) R01CA223204 (ZC-M), and by the NCI and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award number U01DK108327 (DC, ZC-M, PH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest/disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK: The neutrophil lipocalin ngal is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Molecular cell 2002; 10: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 2.Schroll A, Eller K, Feistritzer C, Nairz M, Sonnweber T, Moser PA et al. : Lipocalin-2 ameliorates granulocyte functionality. European journal of immunology 2012; 42: 3346–3357. [DOI] [PubMed] [Google Scholar]
- 3.Oikonomou KA, Kapsoritakis AN, Theodoridou C, Karangelis D, Germenis A, Stefanidis I et al. : Neutrophil gelatinase-associated lipocalin (ngal) in inflammatory bowel disease: Association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol 2012; 47: 519–530. [DOI] [PubMed] [Google Scholar]
- 4.Ataseven A, Kesli R, Kurtipek GS, Ozturk P: Assessment of lipocalin 2, clusterin, soluble tumor necrosis factor receptor-1, interleukin-6, homocysteine, and uric acid levels in patients with psoriasis. Dis Markers 2014; 2014: 541709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Hadidi H, Samir N, Shaker OG, Otb S: Estimation of tissue and serum lipocalin-2 in psoriasis vulgaris and its relation to metabolic syndrome. Arch Dermatol Res 2014; 306: 239–245. [DOI] [PubMed] [Google Scholar]
- 6.Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H: Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends in endocrinology and metabolism: TEM 2017; 28: 388–397. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang JL, Tso AWK et al. : Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clinical chemistry 2007; 53: 34–41. [DOI] [PubMed] [Google Scholar]
- 8.Naude PJ, Nyakas C, Eiden LE, Ait-Ali D, van der Heide R, Engelborghs S et al. : Lipocalin 2: Novel component of proinflammatory signaling in alzheimer's disease. FASEB J 2012; 26: 2811–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang E, Kim JH, Lee S, Kim JH, Seo JW, Jin M et al. : Phenotypic polarization of activated astrocytes: The critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J Immunol 2013; 191: 5204–5219. [DOI] [PubMed] [Google Scholar]
- 10.Al Nimer F, Elliott C, Bergman J, Khademi M, Dring AM, Aeinehband S et al. : Lipocalin-2 is increased in progressive multiple sclerosis and inhibits remyelination. Neurol Neuroimmunol Neuroinflamm 2016; 3: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty S, Kaur S, Guha S, Batra SK: The multifaceted roles of neutrophil gelatinase associated lipocalin (ngal) in inflammation and cancer. Biochimica et biophysica acta 2012; 1826: 129–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Chan YR: Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine 2011; 56: 435–441. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 14.Komar HM, Hart PA, Cruz-Monserrate Z, Conwell DL, Lesinski GB: Local and systemic expression of immunomodulatory factors in chronic pancreatitis. Pancreas 2017; 46: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong X, Sun T, Kong F, Du Y, Li Z: Chronic pancreatitis and pancreatic cancer. Gastrointest Tumors 2014; 1: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB et al. : Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clinical Gastroenterology and Hepatology 2016; 14: 738–746. [DOI] [PubMed] [Google Scholar]
- 17.Zheng L, Xue J, Jaffee EM, Habtezion A: Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013; 144: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav D, Lowenfels AB: The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144: 1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcomb DC: Inflammation and cancer - v. Chronic pancreatitis and pancreatic cancer. Am J Physiol-Gastr L 2004; 287: G315–G319. [DOI] [PubMed] [Google Scholar]
- 20.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D et al. : Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017; 357:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W et al. : Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019; 178: 795–806 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triebel S, Bläser J, Reinke H, Tschesche H: A 25 kda α2 -microglobulin-related protein is a component of the 125 kda form of human gelatinase. FEBS Letters 1992; 314: 386–388. [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N: Isolation and primary structure of ngal, a novel protein associated with human neutrophil gelatinase. The Journal of biological chemistry 1993; 268: 10425–10432. [PubMed] [Google Scholar]
- 24.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N: The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS letters 2005; 579: 773–777. [DOI] [PubMed] [Google Scholar]
- 25.Devireddy LR, Gazin C, Zhu X, Green MR: A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 2005; 123: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, Chavez-Tomar M, Lesinski GB, Bekaii-Saab T et al. : Lipocalin-2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer research 2017; 77: 2647–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z et al. : Mc4r-dependent suppression of appetite by bone-derived lipocalin 2. Nature 2017; 543: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hider RC, Kong X: Chemistry and biology of siderophores. Nat Prod Rep 2010; 27: 637–657. [DOI] [PubMed] [Google Scholar]
- 29.Devireddy LR, Hart DO, Goetz DH, Green MR: A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010; 141: 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischbach MA, Lin H, Liu DR, Walsh CT: How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol 2006; 2: 132–138. [DOI] [PubMed] [Google Scholar]
- 31.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK et al. : Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004; 432: 917–921. [DOI] [PubMed] [Google Scholar]
- 32.Cowland JB, Sorensen OE, Sehested M, Borregaard N: Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by il-1 beta, but not by tnf-alpha. J Immunol 2003; 171: 6630–6639. [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Foncea R, O'Byrne SM, Jiang H, Zhang Y, Deis JA et al. : Lipocalin 2, a regulator of retinoid homeostasis and retinoid-mediated thermogenic activation in adipose tissue. The Journal of biological chemistry 2016; 291: 11216–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tso AW, Xu A, Chow WS, Lam KS: Adipose tissue and the metabolic syndrome: Focusing on adiponectin and several novel adipokines. Biomark Med 2008; 2: 239–252. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y: Small lipid-binding proteins in regulating endothelial and vascular functions: Focusing on adipocyte fatty acid binding protein and lipocalin-2. Br J Pharmacol 2012; 165: 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW et al. : Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007; 53: 34–41. [DOI] [PubMed] [Google Scholar]
- 37.Roli L, Pecoraro V, Trenti T: Can ngal be employed as prognostic and diagnostic biomarker in human cancers? A systematic review of current evidence. The International journal of biological markers 2017; 32: e53–e61. [DOI] [PubMed] [Google Scholar]
- 38.Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS et al. : Roles of neutrophil gelatinase-associated lipocalin (ngal) in human cancer. Oncotarget 2014; 5: 1576–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng M, Feng J, Chen W, Wang W, Wu X, Zhang J et al. : Lipocalin2 suppresses metastasis of colorectal cancer by attenuating nf-kappab-dependent activation of snail and epithelial mesenchymal transition. Molecular cancer 2016; 15: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanai J, Mammoto T, Seth P, Mori K, Karumanchi SA, Barasch J et al. : Lipocalin 2 diminishes invasiveness and metastasis of ras-transformed cells. The Journal of biological chemistry 2005; 280: 13641–13647. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y, Kim JS: Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. International journal of cancer 2006; 118: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 42.Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW et al. : Neutrophil gelatinase-associated lipocalin (ngal) an early-screening biomarker for ovarian cancer: Ngal is associated with epidermal growth factor-induced epithelio-mesenchymal transition. International journal of cancer 2007; 120: 2426–2434. [DOI] [PubMed] [Google Scholar]
- 43.Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB et al. : Neutrophil gelatinase-associated lipocalin: A novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer research 2008; 68: 6100–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatesha S, Hanai J, Seth P, Karumanchi SA, Sukhatme VP: Lipocalin 2 antagonizes the proangiogenic action of ras in transformed cells. Molecular cancer research : MCR 2006; 4: 821–829. [DOI] [PubMed] [Google Scholar]
- 45.Xu B, Jin DY, Lou WH, Wang DS: Lipocalin-2 is associated with a good prognosis and reversing epithelial-to-mesenchymal transition in pancreatic cancer. World journal of surgery 2013; 37: 1892–1900. [DOI] [PubMed] [Google Scholar]
- 46.Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A: Neutrophil gelatinase-associated lipocalin (ngal) is a predictor of poor prognosis in human primary breast cancer. Breast cancer research and treatment 2008; 108: 389–397. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA: The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2005; 11: 5390–5395. [DOI] [PubMed] [Google Scholar]
- 48.lannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C et al. : The neutrophil gelatinase-associated lipocalin (ngal), a nf-kappab-regulated gene, is a survival factor for thyroid neoplastic cells. Proceedings of the National Academy of Sciences of the United States of America 2008; 105: 14058–14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K et al. : Clinical evidence for a protective role of lipocalin-2 against mmp-9 autodegradation and the impact for gastric cancer. European journal of cancer 2007; 43:1869–1876. [DOI] [PubMed] [Google Scholar]
- 50.Leung L, Radulovich N, Zhu CQ, Organ S, Bandarchi B, Pintilie M et al. : Lipocalin2 promotes invasion, tumorigenicity and gemcitabine resistance in pancreatic ductal adenocarcinoma. PloS one 2012; 7: e46677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z et al. : Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: Significant correlation with cell differentiation and tumour invasion. Journal of clinical pathology 2007; 60: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaur S, Baine MJ, Guha S, Ochi N, Chakraborty S, Mallya K et al. : Neutrophil gelatinase-associated lipocalin, macrophage inhibitory cytokine 1, and carbohydrate antigen 19-9 in pancreatic juice: Pathobiologic implications in diagnosing benign and malignant disease of the pancreas. Pancreas 2013; 42: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J et al. : Early diagnosis of pancreatic cancer: Neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. British journal of cancer 2008; 98: 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartsch DK, Gercke N, Strauch K, Wieboldt R, Matthai E, Wagner V et al. : The combination of mirna-196b, lcn2, and timp1 is a potential set of circulating biomarkers for screening individuals at risk for familial pancreatic cancer. Journal of Clinical Medicine 2018; 7: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slater EP, Fendrich V, Strauch K, Rospleszcz S, Ramaswamy A, Matthai E et al. : Lcn2 and timp1 as potential serum markers for the early detection of familial pancreatic cancer. Translational oncology 2013; 6: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Mesallamy HO, Hamdy NM, Zaghloul AS, Sallam AM: Clinical value of circulating lipocalins and insulin-like growth factor axis in pancreatic cancer diagnosis. Pancreas 2013; 42: 149–154. [DOI] [PubMed] [Google Scholar]
- 57.Zabron AA, Horneffer-van der Sluis VM, Wadsworth CA, Laird F, Gierula M, Thillainayagam AV et al. : Elevated levels of neutrophil gelatinase-associated lipocalin in bile from patients with malignant pancreatobiliary disease. The American journal of gastroenterology 2011; 106: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 58.Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD et al. : Discovery of new markers of cancer through serial analysis of gene expression: Prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer research 2001; 61: 4320–4324. [PubMed] [Google Scholar]
- 59.Budzynska A, Nowakowska-Dulawa E, Marek T, Boldys H, Nowak A, Hartleb M: Differentiation of pancreatobiliary cancer from benign biliary strictures using neutrophil gelatinase-associated lipocalin. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2013; 64: 109–114. [PubMed] [Google Scholar]
- 60.Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A et al. : Potentials of plasma ngal and mic-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PloS one 2013; 8: e55171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurell H, Bouisson M, Berthelemy P, Rochaix P, Dejean S, Besse P et al. : Identification of biomarkers of human pancreatic adenocarcinomas by expression profiling and validation with gene expression analysis in endoscopic ultrasound-guided fine needle aspiration samples. World journal of gastroenterology 2006; 12: 3344–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaur S, Sharma N, Krishn SR, Lakshmanan I, Rachagani S, Baine MJ et al. : Muc4-mediated regulation of acute phase protein lipocalin 2 through her2/akt/nf-kappab signaling in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2014; 20: 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong Z, Chakraborty S, Sung B, Koolwal P, Kaur S, Aggarwal BB et al. : Epidermal growth factor down-regulates the expression of neutrophil gelatinase-associated lipocalin (ngal) through e-cadherin in pancreatic cancer cells. Cancer 2011; 117: 2408–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohda A, Yamazaki S, Sumimoto H: The nuclear protein ikappabzeta forms a transcriptionally active complex with nuclear factor-kappab (nf-kappab) p50 and the lcn2 promoter via the n- and c-terminal ankyrin repeat motifs. The Journal of biological chemistry 2016; 291: 20739–20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freeny PC, Traverso LW, Ryan JA: Diagnosis and staging of pancreatic adenocarcinoma with dynamic computed tomography. Am J Surg 1993; 165: 600–606. [DOI] [PubMed] [Google Scholar]
- 66.Sofuni A, lijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K et al. : Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol 2005; 40: 518–525. [DOI] [PubMed] [Google Scholar]
- 67.Hogendorf P, Durczynski A, Skulimowski A, Kumor A, Poznanska G, Strzelczyk J: Neutrophil gelatinase-associated lipocalin (ngal) concentration in urine is superior to ca19-9 and ca 125 in differentiation of pancreatic mass: Preliminary report. Cancer biomarkers : section A of Disease markers 2016; 16: 537–543. [DOI] [PubMed] [Google Scholar]
- 68.Lipinski M, Degowska M, Rydzewska G: Cystic fluid neutrophil gelatinase-associated lipocalin (ngal) concentration in differential diagnosis of pancreatic cystic lesions: A new factor enters the scene? Prz Gastroenterol 2018; 13: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delanaye P, Rozet E, Krzesinski JM, Cavalier E: Urinary ngal measurement: Biological variation and ratio to creatinine. Clin Chim Acta 2011; 412: 390. [DOI] [PubMed] [Google Scholar]
- 70.Beger HG, Rau B, Mayer J, Pralle U: Natural course of acute pancreatitis. World journal of surgery 1997; 21: 130–135. [DOI] [PubMed] [Google Scholar]
- 71.Chakraborty S, Kaur S, Muddana V, Sharma N, Wittel UA, Papachristou GI et al. : Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. The American journal of gastroenterology 2010; 105: 2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sporek M, Dumnicka P, Gala-Bladzinska A, Ceranowicz P, Warzecha Z, Dembinski A et al. : Angiopoietin-2 is an early indicator of acute pancreatic-renal syndrome in patients with acute pancreatitis. Mediators Inflamm 2016; 2016: 5780903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sporek M, Dumnicka P, Gala-Bladzinska A, Mazur-Laskowska M, Walocha J, Ceranowicz P et al. : Determination of serum neutrophil gelatinase-associated lipocalin at the early stage of acute pancreatitis. Folia medica Cracoviensia 2016; 56: 5–16. [PubMed] [Google Scholar]
- 74.Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, Rydzewska G: Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015; 44: 448–452. [DOI] [PubMed] [Google Scholar]
- 75.Linko R, Pettila V, Kuitunen A, Korhonen AM, Nisula S, Alila S et al. : Plasma neutrophil gelatinase-associated lipocalin and adverse outcome in critically ill patients with ventilatory support. Acta Anaesthesiol Scand 2013; 57: 855–862. [DOI] [PubMed] [Google Scholar]
- 76.Rybak K, Sporek M, Gala-Bladzinska A, Mazur-Laskowska M, Dumnicka P, Walocha J et al. : Urinalysis in patients at the early stage of acute pancreatits. Przegl Lek 2016; 73: 88–92. [PubMed] [Google Scholar]
- 77.Singh RG, Pendharkar SA, Plank LD, Petrov MS: Role of human lipocalin proteins in abdominal obesity after acute pancreatitis. Peptides 2017; 91: 1–7. [DOI] [PubMed] [Google Scholar]
- 78.Cho SY, Hur M: Neutrophil gelatinase-associated lipocalin as a promising novel biomarker for early detection of kidney injury. Ann Lab Med 2018; 38: 393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siddappa PK, Kochhar R, Sarotra P, Medhi B, Jha V, Gupta V: Neutrophil gelatinase-associated lipocalin: An early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open 2019; 3: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grundsten M, Liu GZ, Permert J, Hjelmstrom P, Tsai JA: Increased central memory t cells in patients with chronic pancreatitis. Pancreatology 2005; 5: 177–182. [DOI] [PubMed] [Google Scholar]
- 81.Habtezion A, Algul H: Immune modulation in acute and chronic pancreatitis. Pancreapedia Exocrine Pancreas Knowledge Base 2016. [Google Scholar]
- 82.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR et al. : Pancreatitis and the risk of pancreatic cancer. New England Journal of Medicine 1993; 328: 1433–1437. [DOI] [PubMed] [Google Scholar]
- 83.Malli A, Li F, Conwell DL, Cruz-Monserrate Z, Hussan H, Krishn SR: The burden of systemic adipoisity on pancreatic disease: Acute pancreatitis, non-alcoholic fatty pancreas disease, and pancreatic cancer. Journal of the Pancreas 2917; 18: 365–368. [PMC free article] [PubMed] [Google Scholar]
- 84.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Silva C, Rotellar F et al. : Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. Journal of molecular medicine 2009; 87: 803–813. [DOI] [PubMed] [Google Scholar]
- 85.Rashad NM, El-Shal AS, Etewa RL, Wadea FM: Lipocalin-2 expression and serum levels as early predictors of type 2 diabetes mellitus in obese women. IUBMB life 2017; 69: 88–97. [DOI] [PubMed] [Google Scholar]
- 86.Xiao Y, Xu A, Hui X, Zhou P, Li X, Zhong H et al. : Circulating lipocalin-2 and retinol-binding protein 4 are associated with intima-media thickness and subclinical atherosclerosis in patients with type 2 diabetes. PloS one 2013; 8: e66607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaki ME, El-Bassyouni H, Youness E, Mohamed N: Lipocalin-2 is an inflammatory biomarker associated with metabolic abnormalities in egyptian obese children Journal of Applied Pharmaceutical Science 2015; 5: 007–012. [Google Scholar]
- 88.Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA et al. : Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 2010; 59: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM et al. : Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010; 59: 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z et al. : The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007; 56: 2533–2540. [DOI] [PubMed] [Google Scholar]
- 91.Ishii A, Katsuura G, Imamaki H, Kimura H, Mori KP, Kuwabara T et al. : Obesity-promoting and anti-thermogenic effects of neutrophil gelatinase-associated lipocalin in mice. Sci Rep 2017; 7: 15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee M, Kwon DY, Kim MS, Choi CR, Park MY, Kim AJ: Genome-wide association study for the interaction between bmr and bmi in obese korean women including overweight. Nutr Res Pract 2016; 10: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh S, Tanaka K, Warabi E, Shoda J: Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc 2013; 45: 2214–2222. [DOI] [PubMed] [Google Scholar]
- 94.You T, Arsenis NC, Disanzo BL, Lamonte MJ: Effects of exercise training on chronic inflammation in obesity : Current evidence and potential mechanisms. Sports Med 2013; 43: 243–256. [DOI] [PubMed] [Google Scholar]
- 95.de Souza DC, Matos VAF, dos Santos VOA, Medeiros IF, Marinho CSR, Nascimento PRP et al. : Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, iga, and lipid peroxidation responses in obese males. Frontiers in Physiology 2018; 9: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]