Abstract
Geriatric assessment (GA) is used in oncology to identify deficits in older patients with cancer that may affect treatment choice. We examine GA in 550 patients with early breast cancer, including both younger (<65 years) and older women (aged 65 years or older), to assess the potential value of this tool in younger, presumed “healthier” patients. Although older women have more GA‐identified deficits overall, younger patients are more anxious. Suboptimal physical function was problematic across the age spectrum. GA domains can identify major deficits in younger patients beyond those likely to be uncovered in routine investigation.
Short abstract
Despite current definitions of geriatric, there is no age at which a patient is technically considered older. Geriatric assessment deficits may have implications for younger patients too. This article explores the frequency of geriatric assessment deficits identified in younger patients and examines the value of this tool in a sample that includes both younger (<65 years) and older (≥65 years) women with early breast cancer.
Introduction
Geriatric assessment (GA) is recognized as an important tool in oncology practice to identify deficits in older patients with cancer that can help estimate life expectancy 1, predict treatment‐related toxicity 2, 3, and assess the risk of hospitalization. In addition, the GA can identify problems such as falls, polypharmacy, and inadequate social support, for which evidence‐based interventions may improve outcomes. A brief GA can be readily administered in clinical practice 4, 5 and includes all major domains relevant to identifying deficits of importance in treatment decisions of older patients with cancer. The mental status exam and Timed Up and Go (TUG) test are administered by the clinician or research personnel, whereas the remainder of the brief GA relies on patient self‐report to identify key deficits. This reflects much of current health outcomes research, which focuses on patient‐reported outcomes 6. Our prior research has shown that in adults age 65 years and older who have a Karnofsky Performance Status (KPS) score of 80 or higher, the brief GA can identify major deficits that may affect cancer treatment tolerance and outcomes 7.
The cutoff age (65–70) used to define “geriatric” is arbitrary, based on factors such as Medicare and social security eligibility. In practice, there is no age at which a patient is considered “older”; therefore, in theory, “geriatric” deficits may have implications for younger patients as well. In this study, we explore the frequency of GA deficits identified in younger patients and examine the value of this tool in a sample that includes both younger (<65) and older (≥65) women with early breast cancer.
Materials and Methods
The sample consists of women with early breast cancer (stage I–III) enrolled in the Carolina Senior Registry (NCT01137825); some of the women were enrolled through an exercise intervention trial (NCT011789983, NCT02167932, NCT02328313, NCT037611706) for home‐based walking during chemotherapy. In the intervention trials, all study participants completed the brief GA prior to chemotherapy and exercise intervention. Measures included in the brief GA have been described previously in detail 4, 7. Table 1 includes cut points for the identification of significant deficits.
Table 1.
Study participant characteristics before chemotherapy (n = 550)
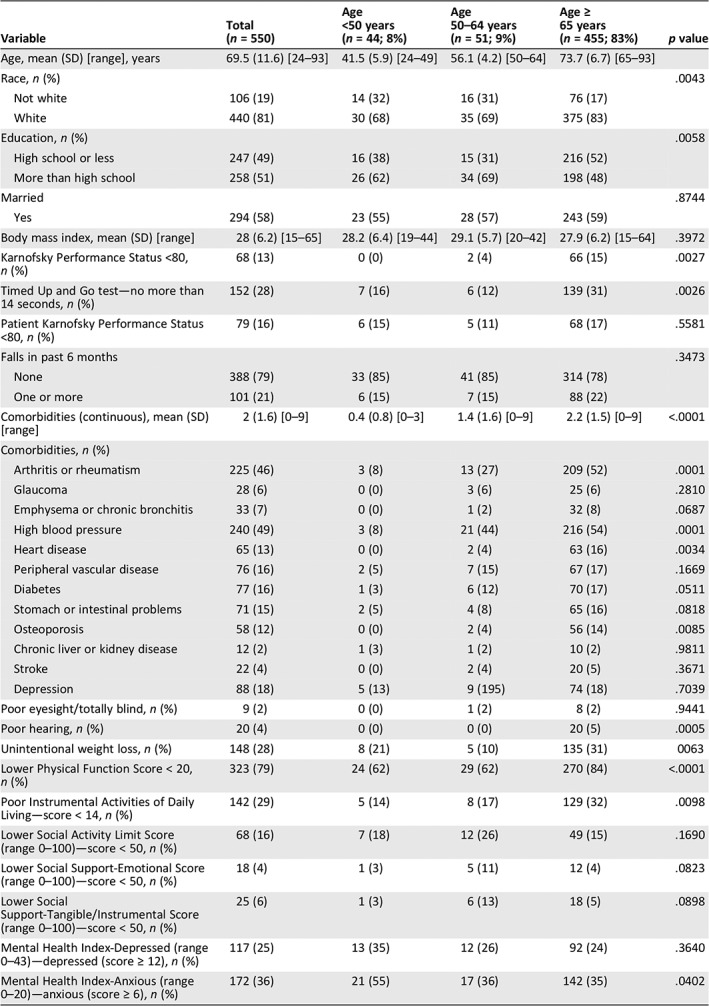
| Variable | Total (n = 550) | Age <50 years (n = 44; 8%) | Age 50–64 years (n = 51; 9%) | Age ≥ 65 years (n = 455; 83%) | p value |
|---|---|---|---|---|---|
| Age, mean (SD) [range], years | 69.5 (11.6) [24–93] | 41.5 (5.9) [24–49] | 56.1 (4.2) [50–64] | 73.7 (6.7) [65–93] | |
| Race, n (%) | .0043 | ||||
| Not white | 106 (19) | 14 (32) | 16 (31) | 76 (17) | |
| White | 440 (81) | 30 (68) | 35 (69) | 375 (83) | |
| Education, n (%) | .0058 | ||||
| High school or less | 247 (49) | 16 (38) | 15 (31) | 216 (52) | |
| More than high school | 258 (51) | 26 (62) | 34 (69) | 198 (48) | |
| Married | .8744 | ||||
| Yes | 294 (58) | 23 (55) | 28 (57) | 243 (59) | |
| Body mass index, mean (SD) [range] | 28 (6.2) [15–65] | 28.2 (6.4) [19–44] | 29.1 (5.7) [20–42] | 27.9 (6.2) [15–64] | .3972 |
| Karnofsky Performance Status <80, n (%) | 68 (13) | 0 (0) | 2 (4) | 66 (15) | .0027 |
| Timed Up and Go test—no more than 14 seconds, n (%) | 152 (28) | 7 (16) | 6 (12) | 139 (31) | .0026 |
| Patient Karnofsky Performance Status <80, n (%) | 79 (16) | 6 (15) | 5 (11) | 68 (17) | .5581 |
| Falls in past 6 months | .3473 | ||||
| None | 388 (79) | 33 (85) | 41 (85) | 314 (78) | |
| One or more | 101 (21) | 6 (15) | 7 (15) | 88 (22) | |
| Comorbidities (continuous), mean (SD) [range] | 2 (1.6) [0–9] | 0.4 (0.8) [0–3] | 1.4 (1.6) [0–9] | 2.2 (1.5) [0–9] | <.0001 |
| Comorbidities, n (%) | |||||
| Arthritis or rheumatism | 225 (46) | 3 (8) | 13 (27) | 209 (52) | .0001 |
| Glaucoma | 28 (6) | 0 (0) | 3 (6) | 25 (6) | .2810 |
| Emphysema or chronic bronchitis | 33 (7) | 0 (0) | 1 (2) | 32 (8) | .0687 |
| High blood pressure | 240 (49) | 3 (8) | 21 (44) | 216 (54) | .0001 |
| Heart disease | 65 (13) | 0 (0) | 2 (4) | 63 (16) | .0034 |
| Peripheral vascular disease | 76 (16) | 2 (5) | 7 (15) | 67 (17) | .1669 |
| Diabetes | 77 (16) | 1 (3) | 6 (12) | 70 (17) | .0511 |
| Stomach or intestinal problems | 71 (15) | 2 (5) | 4 (8) | 65 (16) | .0818 |
| Osteoporosis | 58 (12) | 0 (0) | 2 (4) | 56 (14) | .0085 |
| Chronic liver or kidney disease | 12 (2) | 1 (3) | 1 (2) | 10 (2) | .9811 |
| Stroke | 22 (4) | 0 (0) | 2 (4) | 20 (5) | .3671 |
| Depression | 88 (18) | 5 (13) | 9 (195) | 74 (18) | .7039 |
| Poor eyesight/totally blind, n (%) | 9 (2) | 0 (0) | 1 (2) | 8 (2) | .9441 |
| Poor hearing, n (%) | 20 (4) | 0 (0) | 0 (0) | 20 (5) | .0005 |
| Unintentional weight loss, n (%) | 148 (28) | 8 (21) | 5 (10) | 135 (31) | 0063 |
| Lower Physical Function Score < 20, n (%) | 323 (79) | 24 (62) | 29 (62) | 270 (84) | <.0001 |
| Poor Instrumental Activities of Daily Living—score < 14, n (%) | 142 (29) | 5 (14) | 8 (17) | 129 (32) | .0098 |
| Lower Social Activity Limit Score (range 0–100)—score < 50, n (%) | 68 (16) | 7 (18) | 12 (26) | 49 (15) | .1690 |
| Lower Social Support‐Emotional Score (range 0–100)—score < 50, n (%) | 18 (4) | 1 (3) | 5 (11) | 12 (4) | .0823 |
| Lower Social Support‐Tangible/Instrumental Score (range 0–100)—score < 50, n (%) | 25 (6) | 1 (3) | 6 (13) | 18 (5) | .0898 |
| Mental Health Index‐Depressed (range 0–43)—depressed (score ≥ 12), n (%) | 117 (25) | 13 (35) | 12 (26) | 92 (24) | .3640 |
| Mental Health Index‐Anxious (range 0–20)—anxious (score ≥ 6), n (%) | 172 (36) | 21 (55) | 17 (36) | 142 (35) | .0402 |
The data were analyzed in three age cohorts: age < 50, age 50–64, and age 65 and older. Percentages and means are compared between groups using chi‐squared tests for categorical variables and analysis of variance for continuous variables.
Results
Table 1 summarizes our findings. Among the 550 women, 455 (82%) were age 65 or older, 51 (9%) age 50–64, and 44 (8%) less than age 50. Women age 65 plus were more likely to be white (p = .004), have a level of education of high school or less (p = .006), and have lower KPS <80 (p = .003), slower gait speed (TUG >14 seconds; p = .003), more comorbidities (p < .0001), poorer hearing (p = .0005), higher rates of unintentional weight loss (p = .006), worse physical function (p < .0001), and more instrumental activities of daily living (IADL) deficits (p = .01). Women under age 50 had greater anxiety (Mental Health Index [MHI]; p = .04). There were no significant differences among the age cohorts for patient‐reported KPS, falls, depression, eyesight, social activity limitations, and social support—tangible (instrumental) or emotional. Figure 1 presents individual components of the physical function scale, with significant differences found in 8 of 10 components.
Figure 1.
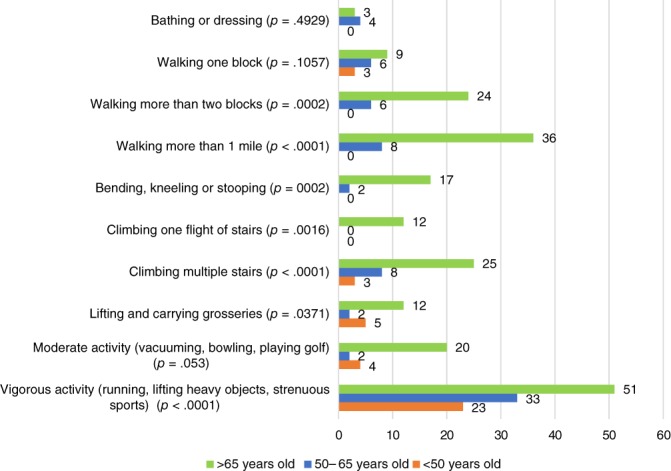
Physical function deficits (%).
Discussion
Our findings confirm the high proportion of older women with GA‐identified deficits. However, we also found that younger patients had substantial deficits detected by the brief GA. We note that women of all ages who participated in the intervention trials had been deemed “fit” for the chemotherapy by their oncology provider. Figure 1 shows that 23% of women age < 50 and 33% of women age 50–64 had difficulties with vigorous activity, such as running and lifting heavy objects. Being able to maintain levels of activity and exercise are important to quality of life and living independently in the community 8. Furthermore, 15% of patients <65 years experienced a fall in the past 6 months, and 16% of women <50 years and 12% of women age 50–65 years had TUG scores >14 seconds. Slower gait speeds and recurrent falls are considered indicators of poor outcomes in older patients 9 but would be a concern among younger patients as well.
Participants age 50–64 are generally thought to be “healthy”; however, their mean number of comorbidities was similar to that of women age 65 years and older (1.6 vs. 2.1). The age 50–64 cohort also had the highest proportion with social activity limitations (26%) and lowest social support (11% emotional, 13% tangible/instrumental). The MHI‐13 is an accurate method for detecting anxiety and depression in patients with cancer 10. In our study, women age < 50 years had the highest proportion defined as anxious (55%) or depressed (35%), reflecting the emotional toll of a cancer diagnosis.
The brief GA takes on average 10 minutes for the professionally assessed items and 20 minutes for patient‐reported items 7, and there is growing interest in making the brief GA an integral component of cancer care in older patients 5, especially for chemotherapy decisions 2. Our findings suggest that a brief assessment may also be valuable in treatment decisions for patients with breast cancer under age 65. Our findings pertaining to functional capacity among patients <50 and 50–64 years are alarming; 62% of both age groups had suboptimal physical function scores, and 14% and 17% had IADL limitations, respectively. This is noteworthy as this age group included patients who were scheduled to receive adjuvant chemotherapy. One would expect to find even higher rates of deficits in both younger and older patients with metastatic disease receiving palliative chemotherapy. Our study shows that domains evaluated in the GA are likely to identify major deficits in younger patients beyond those uncovered in the routine history and physical examination. Clinicians may wish to select a limited set of GA measures to incorporate into their routine medical evaluations, to reduce the amount of time that the full GA entails. The GA is likely to be helpful in evaluating all patients with cancer, and revising it to add assessment such as financial toxicity should be the subject for further research. Our data indicate that GA is not just for older patients.
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
Editor's Note: See the related article, “When It Comes to Geriatric Assessment, Rome Was Not Built in One Day” by Armin Shahrokni, Stuart Lichtman, and Beatriz Korc‐Grodzicki on https://doi.org/10.1634/theoncologist.2020-0015 of this issue.
References
- 1. Guerard EJ, Deal AM, Chang Y et al. Frailty index developed from a cancer‐specific geriatric assessment and association with mortality among older adults with cancer. J Natl Compr Canc Netw 2017;15:894–902. [DOI] [PubMed] [Google Scholar]
- 2. Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishijima TF, Deal AM, Williams GR et al. Chemotherapy toxicity risk score for treatment decisions in older adults with advanced solid tumors. The Oncologist 2018;23:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams GR, Deal AM, Jolly TA et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol 2014;5:245–251. [DOI] [PubMed] [Google Scholar]
- 5. Hurria A. Geriatric assessment in oncology practice. J Am Geriatr Soc 2009;57(suppl 2):S246–S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basch E, Dueck AC, Rogak LJ et al. Feasibility of implementing the patient‐reported outcomes version of the Common Terminology Criteria for Adverse Events in a multicenter trial: NCCTG N1048. J Clin Oncol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jolly TA, Deal AM, Nyrop KA et al. Geriatric assessment‐identified deficits in older cancer patients with normal performance status. The Oncologist 2015;20:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kushi LH, Doyle C, McCullough M et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 9. Guerard EJ, Deal AM, Williams GR et al. Falls in older adults with cancer: Evaluation by oncology providers. J Oncol Pract 2015;11:470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pergolotti M, Langer MM, Deal AM et al. Mental status evaluation in older adults with cancer: Development of the Mental Health Index‐13. J Geriatr Oncol 2019;10:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]


