Abstract
Background
The role of postoperative radiotherapy in pathological T2–3N0M0 esophageal squamous cell carcinoma is unknown. We aimed to evaluate the efficacy and safety of postoperative radiotherapy in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma.
Materials and Methods
Patients aged 18–72 years with pathological stage T2–3N0M0 esophageal squamous cell carcinoma after radical surgery and without neoadjuvant therapy were eligible. Patients were randomly assigned to surgery alone or to receive postoperative radiotherapy of 50.4 Gy in supraclavicular field and 56 Gy in mediastinal field in 28 fractions over 6 weeks. The primary endpoint was disease‐free survival. The secondary endpoints were local‐regional recurrence rate, overall survival, and radiation‐related toxicities.
Results
From October 2012 to February 2018, 167 patients were enrolled in this study. We analyzed 157 patients whose follow‐up time was more than 1 year or who had died. The median follow‐up time was 45.6 months. The 3‐year disease‐free survival rates were 75.1% (95% confidence interval [CI] 65.9–85.5) in the postoperative radiotherapy group and 58.7% (95% CI 48.2–71.5) in the surgery group (hazard ratio 0.53, 95% CI 0.30–0.94, p = .030). Local‐regional recurrence rate decreased significantly in the radiotherapy group (10.0% vs. 32.5% in the surgery group, p = .001). The overall survival and distant metastasis rates were not significantly different between two groups. Grade 3 toxicity rate related to radiotherapy was 12.5%.
Conclusion
Postoperative radiotherapy significantly increased disease‐free survival and decreased local regional recurrence rate in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma with acceptable toxicities in this interim analysis. Further enrollment and follow‐up are warranted to validate these findings in this ongoing trial.
Implications for Practice
The value of adjuvant radiotherapy for patients with node‐negative esophageal cancer is not clear. The interim results of this phase III study indicated that postoperative radiotherapy significantly improved disease‐free survival and decreased local‐regional recurrence rate in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma compared with surgery alone with acceptable toxicities. The distant metastasis rates and overall survival rates were not different between the two groups. Adjuvant radiotherapy should be considered for pathologic T2–3N0M0 thoracic esophageal squamous cell carcinoma. Prospective trials to identify high‐risk subgroups are needed.
Keywords: Randomized controlled trial, Esophageal cancer, Treatment modality
Short abstract
This phase III randomized controlled study compared surgery alone with surgery followed by postoperative radiotherapy in patients with pathological T2‐3N0M0 esophageal squamous cell carcinoma. Interim results are reported.
Introduction
Esophageal cancer is a worldwide malignancy that yields 455,784 new cases and 400,169 deaths as estimated in 2012 1. Although the National Comprehensive Cancer Network (NCCN) guideline recommends observation for postoperative patients, a number of studies reported high recurrence rates, and the median time to recurrence was within 2 years after surgery 2, 3. However, recent data still indicate local‐regional recurrence rates of 35.7%–41.8%, which accounted for the major failure pattern rather than systemic metastases 4, 5, 6.
Evidence of the efficacy of postoperative radiotherapy for patients with positive lymph nodes was identified in squamous cell carcinoma and adenocarcinoma 7, 8, 9. However, for node‐negative patients, local‐regional recurrence rates are estimated to be as high as 26.4%–45.1% 10, 11, 12, which suggested that greater attention should be paid to this subgroup. To the best of our knowledge, there has not been a single prospective, randomized study focusing on the efficacy and safety of postoperative radiotherapy for these early‐stage patients. Our phase II study indicated that postoperative radiotherapy significantly improved 5‐year disease‐free survival and the overall survival rate in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma. Evaluation using propensity score–matched analysis further demonstrated survival benefits 13. Based on this knowledge and these findings, we conducted this prospective, phase III, randomized controlled study comparing surgery alone and postoperative radiotherapy in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma. Currently, the study has met the criteria for interim analysis, and we report the interim results here.
Materials and Methods
Patient Eligibility
Patients who received R0 esophagectomy and at least two‐field lymphadenectomy (resection of mediastinal and abdominal lymph nodes) as their first treatment and who were pathologically confirmed as having T2–3N0 thoracic esophageal squamous cell carcinoma, according to the Union for International Cancer Control (UICC) 7th tumor‐node‐metastasis (TNM) classification, were included. Eligible patients were 18–72 years of age, with a Karnofsky performance status ≥70, and had adequate hematological, pulmonary, cardiac, renal, and hepatic functions. Patients with residual diseases, recurrences, or distant metastases before randomization; severe postoperative complications or comorbidities that ruled them out for receiving radiotherapy; or a history of other secondary malignancies were excluded. All patients provided written informed consent. Ethical committee approval was received on September 27, 2012, and the trial was registered at the http://clinicaltrials.gov (NCT01745107).
Randomization
Random assignment was performed after esophagectomy, using a computer‐generated random number code at a 1:1 ratio. Treatment allocations were not masked. After informed consent was obtained, clinicians uncovered the number and assigned patients to the given group.
Treatment
Postoperative workup included history taking and physical examination, routine hematological and biochemical profiles, pulmonary function test, and an electrocardiogram. Mandatory staging procedures comprised neck/chest/abdomen computed tomography (CT) scans, cervical/abdominal lymph node ultrasonography, a barium meal, brain magnetic resonance imaging, and a radionucleotide bone scan. Positron emission tomography (PET)‐CT scan and endoscopic biopsy were performed when suspicious lesions were detected.
Radiotherapy began after surgical wound healing but did not exceeded 3 months after surgery. Photon beams from a linear accelerator with energy of 6 MV and intensity‐modulated radiation therapy (IMRT) was administrated. Pre‐ and postoperative imaging were reviewed to determine the location of the tumor bed. Clinical target volume (CTV) was defined from cricothyroid membrane to 3 cm below carina for proximal diseases and from T1 vertebra to 3 cm below tumor bed for middle and lower disease, which involved 1R/L, 2R/L, 3p, 4R/L, 7, part 8, and part 10L mediastinal lymph nodes. Anastomosis was included when proximal tumor margin was less than 3 cm or proximal disease was observed. Planning target volume included CTV and an additional 5 mm in three‐dimensional directions. A total dose of 50.4 Gy for supraclavicular field and 56 Gy for mediastinal field (divided by the upper edge of clavicular head) was delivered in 28 fractions.
Patients were followed up every 3 months during the first 2 years after randomization, for 6 months during the 3rd and 4th year, and every year after 5 years. Recurrence was confirmed by diagnostic imaging for lymph nodes and distant metastases. For superficial recurrence sites such as supraclavicular lymph nodes, fine‐needle aspiration was required; for reconstructed‐esophagus recurrence, endoscopic biopsy was required.
Outcomes
The primary endpoint was disease‐free survival (DFS), which was calculated from the day of R0 surgery to the day of first recurrence or death from any cause or censor. Secondary endpoints included local‐regional recurrence rate, overall survival (OS), and radiation‐related toxicities. Regional lymph nodes were defined from supraclavicular to celiac area for thoracic esophageal cancer according to the UICC 7th staging manual. OS was calculated from the day of R0 surgery to death or censor. Toxicities were defined according to Common Terminology Criteria for Adverse Events 4.0. The outcomes were assessed by clinicians who were independent of the trial.
Statistical Analysis
This study aimed to detect a 5‐year DFS difference of 21.4% from 50.3% in the surgery‐alone group to 71.7% in the postoperative radiotherapy group, with 80% power and 5% type I error, 6 years of recruitment, and 5 years of follow‐up. Using a two‐sided log‐rank test, we anticipated that 105 events were required in 216 patients, assuming 10% dropout or loss to follow‐up; therefore, we needed 240 patients, with 120 patients in each group. The interim analysis was conducted when 50 events out of 105 events were observed. Using O'Brien and Fleming's test boundaries, the Z score test cutoff at the interim analysis for stopping and rejecting the null hypothesis was 2.54, and the Z score test cutoff at the interim analysis for stopping and rejecting the alternative was 0.34.
All the data were analyzed using the intention‐to‐treat principle. Median follow‐up was calculated according to reverse Kaplan‐Meier estimates; OS and DFS were calculated from the date of surgery using the Kaplan‐Meier method and compared using the log‐rank test. Equality of the censoring distributions between groups was assumed. The Cox proportional hazards model, with the assumptions of proportional hazards confirmed based on both Schoenfeld's residuals test and the parallel log[‐log(S(t))] curves between groups, were used to estimate the hazard ratio between the exposure and the control group. We compared the failure pattern of the two groups according to the status of local‐regional recurrence and distant metastasis using the Kaplan‐Meier estimate, and the cumulative incidence could be obtained using 1 ‐ Kaplan‐Meier survival rate. All analyses were done using R software, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
From October 2012 to February 2018, 167 patients were enrolled in the study: 82 patients were assigned to surgery alone and 85 patients to surgery plus postoperative radiotherapy. To calculate survival time, we included 157 patients who were followed up for more than 1 year or who had died. Finally, 77 patients in the surgery group and 80 patients in the radiotherapy group were included in the analysis; patients who declined to receive radiation after randomization were not excluded (Fig. 1). The baseline demographic and clinical characteristics were well balanced between the two groups (Table 1). Most patients (84.7%) were men, and the median age was 59 years. About 22.9% of patients experienced ≥5% weight loss before surgery, and 62.4% of patients had a tumor of more than 5 cm length. Most tumors were located in the middle (65.6%) and lower (31.2%) esophagus. The proximal margin length was more than 5 cm in 43.9% of patients. T3 (78.3%) accounted for the major stage in both groups. G1–G2 tumors (72.0%) were predominant in all patients. In the surgery‐alone group, 87.0% of patients had more than 16 lymph nodes removed compared with 72.5% in the postoperative radiotherapy group. Lymphovascular invasion was presented in 10.4% of patients in the surgery‐alone group and in 17.5% in the postoperative radiotherapy group.
Figure 1.
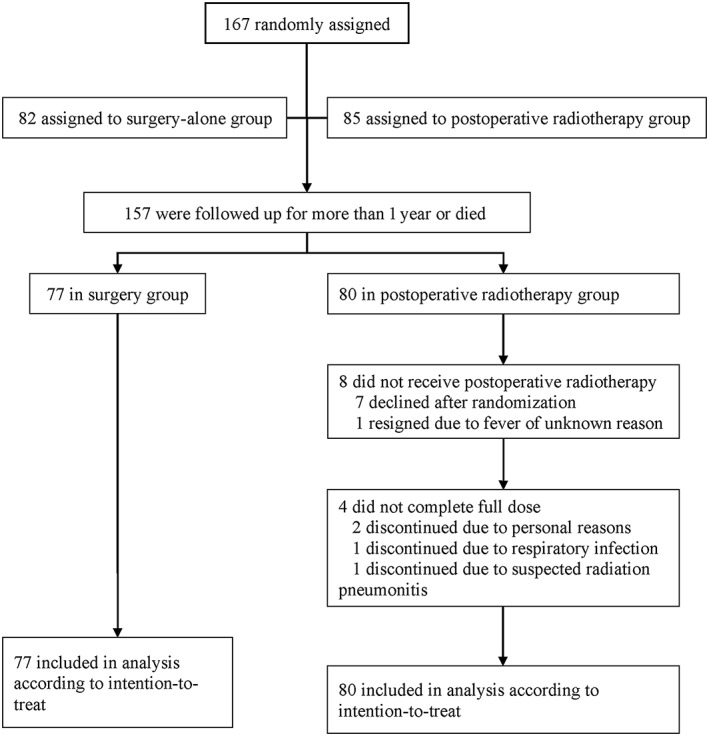
Study flow diagram.
Table 1.
Baseline characteristics of patients with thoracic esophageal squamous cell carcinoma followed up for more than 1 year
| Characteristic | S alone group (n = 77) | S + R group (n = 80) | All patients (n = 157) |
|---|---|---|---|
| Sex | |||
| Male | 62 (80.5) | 71 (88.8) | 133 (84.7) |
| Female | 15 (19.5) | 9 (11.2) | 24 (15.3) |
| Age, years | |||
| ≤65 | 62 (80.5) | 71 (88.8) | 133 (84.7) |
| >65 | 15 (19.5) | 9 (11.2) | 24 (15.3) |
| Median (IQR) | 61 (56.5–64.5) | 58 (52–62.8) | 59 (55–64) |
| Weight loss | |||
| <5% | 59 (76.6) | 62 (77.5) | 121 (77.1) |
| ≥5% | 18 (23.4) | 18 (22.5) | 36 (22.9) |
| Tumor length, cm | |||
| <5 | 27 (35.1) | 32 (40.0) | 59 (37.6) |
| ≥5 | 50 (64.9) | 48 (60.0) | 98 (62.4) |
| Median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–6) |
| Tumor location | |||
| Upper | 4 (5.2) | 1 (1.2) | 5 (3.2) |
| Middle | 49 (63.6) | 54 (67.5) | 103 (65.6) |
| Lower | 24 (31.2) | 25 (31.2) | 49 (31.2) |
| Proximal margin length, cm | |||
| ≤5 | 43 (55.8) | 45 (56.2) | 88 (56.1) |
| >5 | 34 (44.2) | 35 (43.8) | 69 (43.9) |
| Median (IQR) | 5 (5–6) | 5 (5–6) | 5 (5–6) |
| T | |||
| T2 | 20 (26.0) | 14 (17.5) | 34 (21.7) |
| T3 | 57 (74.0) | 66 (82.5) | 123 (78.3) |
| Removed lymph nodes | |||
| ≤16 | 10 (13.0) | 22 (27.5) | 32 (20.4) |
| >16 | 67 (87.0) | 58 (72.5) | 125 (79.6) |
| Median (IQR) | 24 (19.5–32.5) | 22 (16–30) | 23 (17.5–30.5) |
| Grade | |||
| G1–G2 | 55 (71.4) | 58 (72.5) | 113 (72.0) |
| G3–G4 | 22 (28.6) | 22 (27.5) | 44 (28.0) |
| LVSI | 8 (10.4) | 14 (17.5) | 22 (14.0) |
| Complete RT | 68 (85.0) |
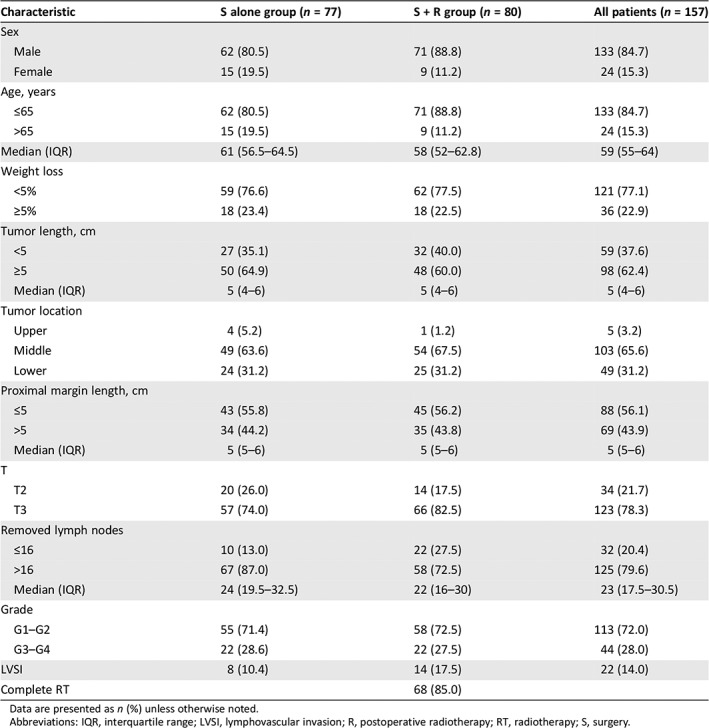
Data are presented as n (%) unless otherwise noted.
Abbreviations: IQR, interquartile range; LVSI, lymphovascular invasion; R, postoperative radiotherapy; RT, radiotherapy; S, surgery.
Protocol Compliance and Toxicity
The median time interval from surgery to radiation was 57 days (interquartile range [IQR] 49–64). A total of 72 out of 80 patients (90.0%) received radiotherapy, and 68 (85.0%) completed the full dose without interruptions. Among the eight patients who did not receive radiotherapy, seven declined after informed consent and randomization, and one resigned because of persistent fever of unknown reason after surgery. In addition, four patients did not reach the planned full dose because of personal issues (two patients), respiratory infection (one patients), and suspected radiation pneumonitis (one patient). Patients who discontinued treatment received more than 20 fractions of dose.
In the 68 patients who received radiation in the radiotherapy group, no grade 4 or 5 toxicities were observed. Grade 3 toxicities were also rare: leukopenia in three patients (4.2%), neutropenia in one patient (1.4%), thrombocytopenia in one patient (1.4%), cough in two patients (2.8%), esophagitis in one patient (1.4%), and dermatitis in one patient (1.4%). The most frequent toxicities were esophagitis and leukopenia. During the follow‐up period, seven patients (8.8%) in the radiotherapy group experienced grade 3 anastomosis stricture that required endoscopic dilation, compared with five (6.5%) in the surgery group. All available adverse events that occurred during radiotherapy are summarized in Table 2.
Table 2.
Acute toxicities related to radiotherapy
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4/5 |
|---|---|---|---|---|
| Leukopenia | 30 (41.7) | 27 (37.5) | 3 (4.2) | 0 |
| Neutropenia | 23 (31.9) | 9 (12.5) | 1 (1.4) | 0 |
| Anemia | 3 (4.2) | 0 | 0 | 0 |
| Thrombopenia | 4 (5.6) | 3 (4.2) | 1 (1.4) | 0 |
| Cough | 20 (27.8) | 7 (9.7) | 2 (2.8) | 0 |
| Esophagitis | 35 (48.6) | 27 (37.5) | 1 (1.4) | 0 |
| Pneumonitis | 0 | 1 (1.4) | 0 | 0 |
| Dermatitis | 46 (63.9) | 10 (13.9) | 1 (1.4) | 0 |
| Fever | 0 | 1 (1.4) | 0 | 0 |
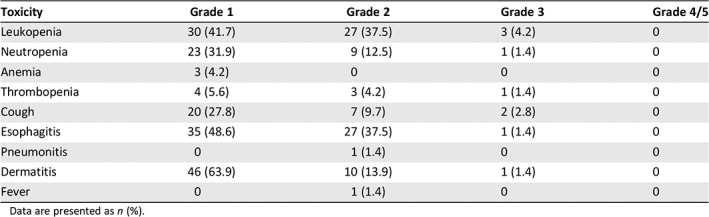
Data are presented as n (%).
Survival
At the last follow‐up on February 2018, patients were followed up for a median time of 45.6 months (IQR 32.2–57.5). Overall, 50 (31.8%) of the 157 patients experienced treatment failure or death, including 31 patients (40.3%) in the surgery group and 19 (23.8%) in the radiotherapy group. The 1‐, 2‐, and 3‐year DFS rates in the radiotherapy group were 87.5% (95% confidence interval [CI] 80.5%–95.1%), 78.1% (95% CI 69.4%–87.9%), and 75.1% (95% CI 65.9%–85.5%), which were significantly higher than 77.9% (95% CI 69.2%–87.8%), 65.5% (95% CI 55.5%–77.2%), and 58.7% (95% CI 48.2%–71.5%) in the surgery group (hazard ratio [HR] 0.53, 95% CI 0.30–0.94, p = .030; Fig. 2A). Twenty‐nine patients had died at last follow‐up, including 16 (20.8%) patients in the surgery group and 13 patients (16.2%) in the radiotherapy group (p = .465). Among the 16 deaths in the surgery group, 12 (75.0%) were cancer‐specific deaths, and 4 patients died for other reasons: 1 died from infection secondary to ileus, 2 from complications of salvage chemotherapy, and 1 from suicide. Of 13 deaths in the radiotherapy group, 10 (76.9%) died from disease progression, 1 from pneumonia, 1 from heart failure, and 1 from secondary malignancy. The 1‐, 2‐, and 3‐ year OS rates in the radiotherapy group and the surgery group were 96.3% (95% CI 92.2%–100%), 90.8% (95% CI 84.5%–97.6%), and 89.3% (95% CI 82.6%–96.6%) and 96.1% (95% CI 91.9%–100%), 92.0% (95% CI 86.1%–98.4%), and 81.1% (95% CI 72.3%–91.0%), respectively (HR 0.79, 95% CI 0.38–1.64, p = .527; Fig. 2B). Neither group reached median DFS or OS.
Figure 2.
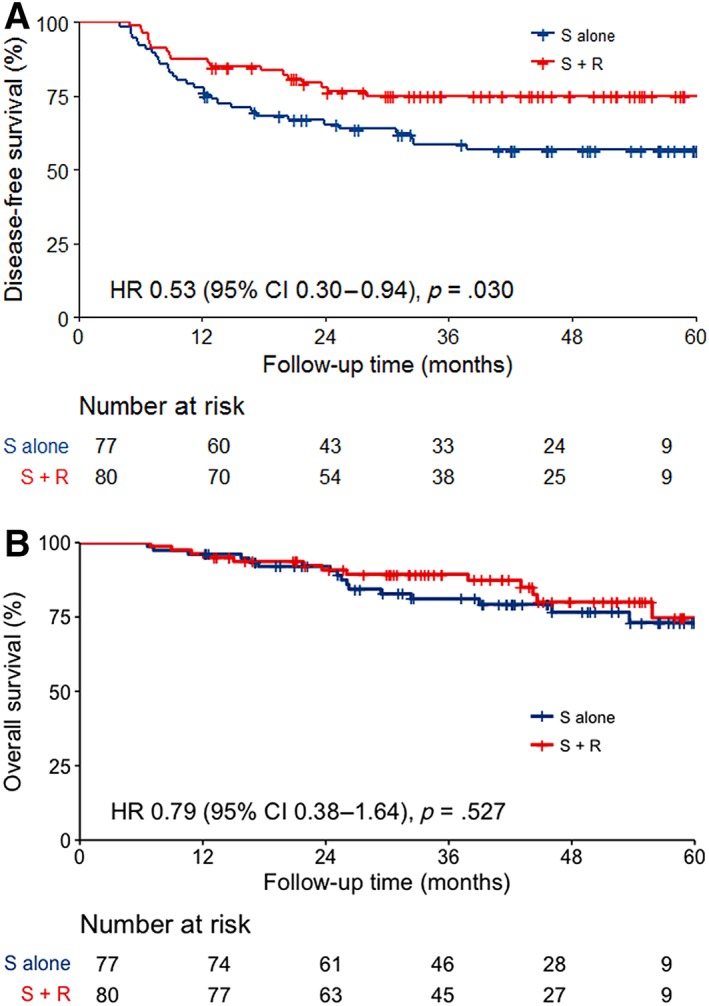
Survival curves. (A): Disease‐free survival curves for surgery‐alone and postoperative radiotherapy groups. (B): Overall survival curves in surgery‐alone and postoperative radiotherapy groups.Abbreviations: CI, confidence interval; HR, hazard ratio; R, postoperative radiotherapy; S, surgery.
Recurrence Pattern
The overall recurrence rate was 29.9%. The median time to recurrence was 11.3 months from surgery. Twenty‐nine patients (37.7%) in the surgery group and 18 patients (22.5%) in the radiotherapy group underwent recurrence (p = .038). Local‐regional recurrences were observed in 33 patients (21.0%), with significantly more in the surgery group than in the radiotherapy group (25 patients [32.5%] vs. 8 patients [10.0%], p < .001; Fig. 3A). Mediastinal and supraclavicular lymph nodes were the most common local‐regional failure sites. Twenty‐three patients (14.6%) had distant metastases, including 12 (15.6%) in the surgery group and 11 (13.8%) in the radiotherapy group (p = .708; Fig. 3B). The lungs were the most common distant metastasis sites. The salvage therapies usually started within 1 months of recurrence. The approaches of salvage therapies are summarized in supplemental online Table 1.
Figure 3.
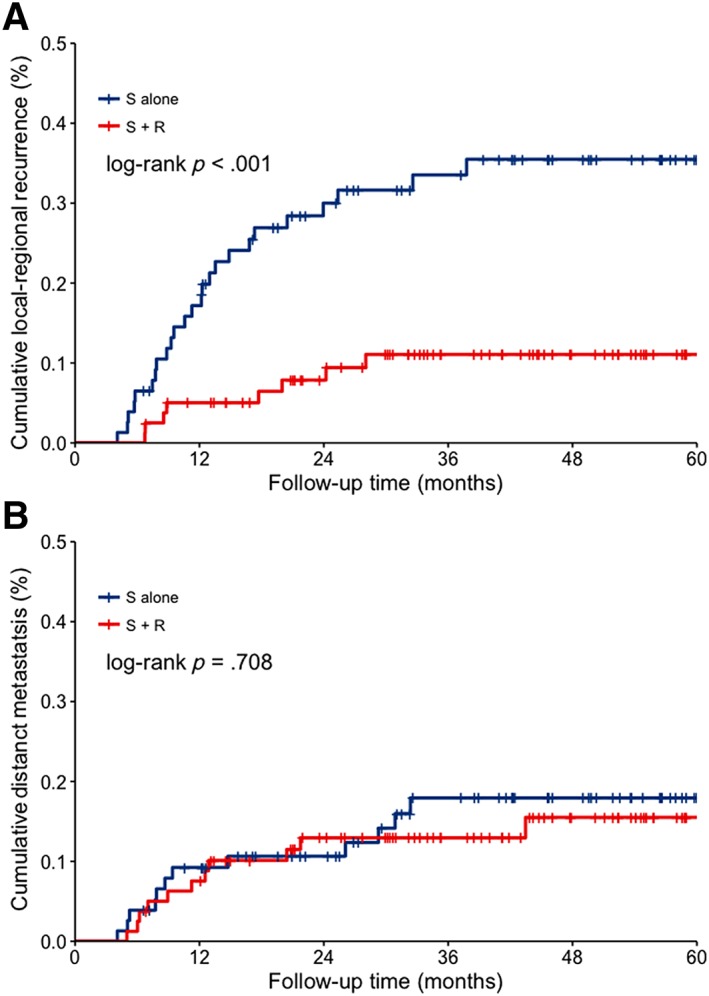
Cumulated recurrence curves. (A): Local‐regional recurrence in surgery‐alone and postoperative radiotherapy groups. (B): Distant metastasis in surgery‐alone and postoperative radiotherapy groups.Abbreviations: R, postoperative radiotherapy; S, surgery.
Discussion
The interim results of our study indicated that postoperative radiotherapy significantly improved DFS and decreased local‐regional recurrence rate, with acceptable adverse events, in patients with pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma compared with surgery alone. The distant metastasis rates were not different between the two groups. Potential benefits of postoperative radiotherapy were seen in terms of OS but require longer follow‐up time.
Evidence of adjuvant radiotherapy for patients with esophageal carcinoma is limited, especially for stage IIA or T2–3N0M0 disease. Schreiber et al. analyzed the Surveillance, Epidemiology, and End Results database and note that the median OS increased from 27 months to 46 months and the 3‐year OS increased from 46.4% to 51.9% for stage IIA patients but without a significant difference 7. Another study also indicated that in stage IIA disease, although 5‐year OS increased from 28.5% to 35% and the median survival increased from 21 to 35 months, no significant benefits between two groups were observed 14. In a Chinese study, Xiao et al. reported the results of a prospective trial. Although the effect did not reach statistical significance, potential 3‐year absolute OS benefits of 8% were observed in stage IIA disease (64.0% vs. 56.0%, p = .634) 15. The potential survival benefits of adjuvant radiotherapy for stage IIA patients need further evaluation (Table 3).
Table 3.
Summary of outcomes for postoperative radiotherapy in IIA/N0 esophageal cancer
| Study | Pathology | Stage | Treatment | Sample size | Median OS, months | 3‐year OS, % | 5‐year OS, % | p value |
|---|---|---|---|---|---|---|---|---|
| Schreiber et al. 7 | AC and SCC | IIA (T3N0) | PORT | 69 | 46 | 51.9 | — | .245 |
| Surgery | 190 | 27 | 46.4 | — | ||||
| Worni et al. 14 | SCC | IIA | PORT | 44 | 35 | — | 35.0 | >.05 |
| Surgery | 216 | 21 | — | 28.5 | ||||
| Wong et al. 9 | AC and SCC | T3–4Nx/0 | PORT | 217 | — | 41.3 | — | .24 |
| Surgery | 1148 | — | 46.9 | — |
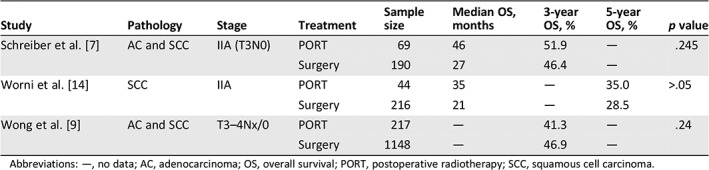
Abbreviations: —, no data; AC, adenocarcinoma; OS, overall survival; PORT, postoperative radiotherapy; SCC, squamous cell carcinoma.
We conducted a phase II study of postoperative IMRT in 95 patients with pT3N0M0 thoracic esophageal cancer. Compared with surgical patients during the same period, significant improvement in 5‐year OS (74.3% vs. 59.9%, p = .010) and DFS (71.0 vs. 51.7%, p = .002) were observed. The overall recurrence rate was significantly lower in the postoperative IMRT group (22.9% vs. 42.6%, p < .001), as was the local‐regional recurrence rate (18.8% vs. 34.7%, p = .002). Moreover, when analyzing patients who underwent surgery alone, overall recurrence (41.6% vs. 43.0%, p = .72) and local‐regional recurrence (37.2% vs. 33.6%, p = .35) were not different between pT2 and pT3 disease 16. Based on these data, Yang et al. further used propensity score–matched analysis to compare patients who underwent surgery alone with postoperative IMRT. As a result, the 5‐year DFS improved from 50.3% to 71.7% (p = .009) and OS improved from 58.8% to 75.7% (p = .017) in the postoperative IMRT group. Similarly, the overall recurrence and local‐regional recurrence rates were significantly lower in the postoperative radiotherapy group (p = .001) 13. Our interim results for the prospective trial were similar to the propensity score–matched analysis, which further validated the importance of postoperative radiotherapy for pT2–3N0M0 patients.
According to the recurrence pattern after R0 esophagectomy for squamous cell carcinoma, the most frequent failure sites are mediastinal and bilateral supraclavicular lymph nodes. Mariette et al. followed up 439 patients after two‐field lymphadenectomy and found that cervical recurrences were more frequently detected in squamous cell carcinoma, especially in upper and middle third disease 3. Cai et al. also indicated that local‐regional recurrence was the most common failure pattern. The anastomosis, supraclavicular area, mediastinal 1‐5, and 7 station lymph nodes were the most frequent recurrence sites for upper and middle thoracic esophageal carcinoma 17. According to a study, a T‐shaped clinical target volume area including bilateral supraclavicular and entire mediastinum could cover 87.2% of the recurrent sites 6. As indicated, the upper abdominal lymph node metastasis rate was only 2.1%–10.4%, with a higher incidence in lower thoracic esophagus and node‐positive patients. In addition, intra‐abdominal failure was low in squamous cell carcinoma and was not decreased by postoperative radiotherapy 15, 18. Therefore, we considered the bilateral supraclavicular region, upper mediastinal, and subcarina to be high‐risk regions in our study.
Adjuvant chemotherapy failed to prove its efficacy in node‐negative esophageal squamous cell carcinoma. The JCOG 9204 trial showed that adjuvant chemotherapy only benefited node‐positive, rather than ‐negative, patients in terms of DFS 19. Several retrospective studies also failed to demonstrate the superiority of adjuvant chemotherapy in pN0 patients 20, 21. There are no randomized clinical trials or large‐sample‐size retrospective analyses comparing adjuvant radiotherapy and chemoradiation in patients with esophageal cancer. Peyre et al. reported that the number of involved lymph nodes could be used to predict systemic disease. The frequency of systemic disease after esophagectomy was 16% for those without nodal involvement 22. Because local‐regional recurrence is the major failure pattern for N0 patients, postoperative radiotherapy could be the optimal therapy for N0 patients. In addition, this study investigated postoperative radiotherapy in patients with low tumor burden (T2–3). Postoperative chemoradiation was recommended to patients with node‐positive or deep tumor invasion (T3–T4a) in esophageal adenocarcinoma 23. Similar evidence for chemoradiation was found in squamous cell carcinoma for patients with high tumor burden, which may not be suitable for T2–3N0 patients. In consideration of comparable efficacy but fewer toxicities, we use postoperative radiotherapy instead of chemoradiation in this trial.
We used intention‐to‐treat analysis in this study and did not exclude the eight patients who failed to accomplish scheduled radiotherapy. Under these circumstances, the DFS, local‐regional recurrence, and overall recurrence rates still favored the postoperative radiotherapy group, which strengthened the conclusion that radiation could lower the risk of recurrence and improve the survival of these patients.
The timely salvage therapy could be one of the reasons that OS did not show a statistically positive result in the current study. It usually started within 1 month after recurrence. For patients in the surgery‐alone group, salvage therapies generally included concurrent chemoradiation for local‐regional recurrence and chemotherapy for distant metastasis, whereas for patients who received prophylactic radiotherapy, salvage chemotherapy was the most common option. Studies indicated that salvage radiation or chemoradiation in local‐regionally recurrent esophageal cancer after esophagectomy produced effective survival improvements 24. However, the survival rates of patients who received salvage radiation were not as high as those for patients who received prophylactic postoperative radiation 25. Although the OS did not reach a significant difference, the postoperative radiotherapy group showed an absolute 3‐year OS increase of 8.2%. The final result of the current study still calls for long‐term follow‐up.
This study also has some limitations. First, most of our patients came from the same institute; therefore, this population may not be representative of all patients with thoracic esophageal squamous cell carcinoma. Second, we used extended two field lymph node dissection, and PET‐CT was not a required examination after surgery, which means there might be some overlooked upper mediastinal lymph nodes. However, the median number of removed lymph nodes was 23, and the postoperative images of every patient were clearly reviewed before enrollment. For suspicious lesions, PET‐CT was required to exclude residual, recurrent, or metastatic disease. Furthermore, the relatively high 3‐year DFS rate of 58.7% and OS rate of 81.1% in the surgery group implied a high‐quality surgery. Finally, our study investigated postoperative radiotherapy for early‐stage esophageal carcinoma, which is not a routine practice according to current guidelines. However, we provided a possible suggestion for those patients who are understaged before surgery or who received esophagectomy as their first treatment. In this group of patients, adjuvant therapy should be considered.
Conclusion
This study suggested that postoperative radiotherapy in pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma could potentially increase DFS and reduce local‐regional recurrence with low‐grade toxicities. However, further enrollment and long‐term follow‐up are needed to validate the efficacy and safety of this treatment strategy.
Author Contributions
Conception/design: Zefen Xiao
Provision of study material or patients: Zongmei Zhou, Dongfu Chen, Qinfu Feng, Jun Liang, Jima Lv, Xiaozhen Wang, Xin Wang, Lei Deng, Wenqing Wang, Nan Bi, Tao Zhang, Yexiong Li, Shugeng Gao, Qi Xue, Yousheng Mao, Kelin Sun, Xiangyang Liu, Dekang Fang, Dali Wang, Jian Li, Jun Zhao, Kang Shao, Zhishan Li, Xinjie Chen, Lei Han, Lifang Wang, Jie He, Zefen Xiao
Collection and/or assembly of data: Wei Deng, Jinsong Yang, Wenjie Ni, Chen Li, Xiao Chang, Weiming Han
Data analysis and interpretation: Wei Deng, Jinsong Yang, Zefen Xiao
Manuscript writing: Wei Deng, Jinsong Yang, Zefen Xiao
Final approval of manuscript: Wei Deng, Jinsong Yang, Wenjie Ni, Chen Li, Xiao Chang, Weiming Han, Zongmei Zhou, Dongfu Chen, Qinfu Feng, Jun Liang, Jima Lv, Xiaozhen Wang, Xin Wang, Lei Deng, Wenqing Wang, Nan Bi, Tao Zhang, Yexiong Li, Shugeng Gao, Qi Xue, Yousheng Mao, Kelin Sun, Xiangyang Liu, Dekang Fang, Dali Wang, Jian Li, Jun Zhao, Kang Shao, Zhishan Li, Xinjie Chen, Lei Han, Lifang Wang, Jie He, Zefen Xiao
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendix
Supplemental Tables
Supplemental Figures
Acknowledgments
This work was supported by the Capital Clinical Characteristic Application Research of China (Z121107001012004), the Capital Health Research and Development of Special of China (2016‐2‐4021), and the National Key Projects of Research and Development of China (2016YFC0904600).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two‐field lymphadenectomy. Br J Surg 2000;87:1426–1433. [DOI] [PubMed] [Google Scholar]
- 3. Mariette C, Balon JM, Piessen G et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616–1623. [DOI] [PubMed] [Google Scholar]
- 4. Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two‐field and three‐field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol 2010;5:707–712. [DOI] [PubMed] [Google Scholar]
- 5. Hsu PK, Wang BY, Huang CS et al. Prognostic factors for post‐recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg 2011;15:558–565. [DOI] [PubMed] [Google Scholar]
- 6. Liu Q, Cai XW, Wu B et al. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: Implications for the clinical target volume design of postoperative radiotherapy. PLoS One 2014;9:e97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schreiber D, Rineer J, Vongtama D et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol 2010;5:244–250. [DOI] [PubMed] [Google Scholar]
- 8. Shridhar R, Weber J, Hoffe SE et al. Adjuvant radiation therapy and lymphadenectomy in esophageal cancer: A SEER database analysis. J Gastrointest Surg 2013;17:1339–1345. [DOI] [PubMed] [Google Scholar]
- 9. Wong AT, Shao M, Rineer J et al. The impact of adjuvant postoperative radiation therapy and chemotherapy on survival after esophagectomy for esophageal carcinoma. Ann Surg 2017;265:1146–1151. [DOI] [PubMed] [Google Scholar]
- 10. Chen G, Wang Z, Liu XY et al. Clinical study of modified Ivor‐Lewis esophagectomy plus adjuvant radiotherapy for local control of stage IIA squamous cell carcinoma in the mid‐thoracic esophagus. Eur J Cardiothorac Surg 2009;35:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Sun ZG, Wang Z. Clinical study on lymph node metastatic recurrence in patients with N0 esophageal squamous cell cancer. Dis Esophagus 2011;24:182–188. [DOI] [PubMed] [Google Scholar]
- 12. Guo XF, Mao T, Gu ZT et al. Clinical study on postoperative recurrence in patients with pN0 esophageal squamous cell carcinoma. J Cardiothorac Surg 2014;9:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Zhang W, Xiao Z et al. The impact of postoperative conformal radiotherapy after radical surgery on survival and recurrence in pathologic T3N0M0 esophageal carcinoma: A propensity score‐matched analysis. J Thorac Oncol 2017;12:1143–1151. [DOI] [PubMed] [Google Scholar]
- 14. Worni M, Martin J, Gloor B et al. Does surgery improve outcomes for esophageal squamous cell carcinoma? An analysis using the surveillance epidemiology and end results registry from 1998 to 2008. J Am Coll Surg 2012;215:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao ZF, Yang ZY, Liang J et al. Value of radiotherapy after radical surgery for esophageal carcinoma: A report of 495 patients. Ann Thorac Surg 2003;75:331–336. [DOI] [PubMed] [Google Scholar]
- 16. Yang JS, Liu X, Xiao ZF, Liang J et al. Prospective phase II study of three‐dimensional radiotherapy after radical surgery for pT2‐3N0M0 esophageal cancer [in Chinese]. Chin J Radiat Oncol 2015;24(1):29–32. [Google Scholar]
- 17. Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol 2010;96:104–107. [DOI] [PubMed] [Google Scholar]
- 18. Lu JC, Tao H, Zhang YQ et al. Extent of prophylactic postoperative radiotherapy after radical surgery of thoracic esophageal squamous cell carcinoma. Dis Esophagus 2008;21:502–507. [DOI] [PubMed] [Google Scholar]
- 19. Ando N, Iizuka T, Ide H et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol 2003;21:4592–4596. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Zhao L, Lin B et al. Adjuvant therapeutic modalities following three‐field lymph node dissection for stage II/III esophageal squamous cell carcinoma. J Cancer 2017;8:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Li W, Lyu X et al. Adjuvant chemotherapy with paclitaxel and cisplatin in lymph node‐positive thoracic esophageal squamous cell carcinoma. Chin J Cancer Res 2017;29:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peyre CG, Hagen JA, DeMeester SR et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: A multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979–985. [DOI] [PubMed] [Google Scholar]
- 23. National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers, Version 2. 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed October 30, 2019. [DOI] [PubMed]
- 24. Kobayashi R, Yamashita H, Okuma K et al. Salvage radiation therapy and chemoradiation therapy for postoperative locoregional recurrence of esophageal cancer. Dis Esophagus 2014;27:72–78. [DOI] [PubMed] [Google Scholar]
- 25. Yamashita H, Nakagawa K, Tago M et al. Salvage radiotherapy for postoperative loco‐regional recurrence of esophageal cancer. Dis Esophagus 2005;18:215–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Appendix
Supplemental Tables
Supplemental Figures


