Summary
The small intestinal (SI) epithelium harbors a heterogeneous population of lymphocytes that mediate mucosal damage and repair in celiac disease (CD). The composition and roles of human proximal SI intra‐epithelial innate lymphoid cells (ILCs), and their alterations in CD, are not well understood. We report that duodenal intra‐epithelial ILCs predominantly consist of natural killer (NK)p44+CD127− cytotoxic ILC1s and NKp44−CD127+ helper ILC1s, while ILC3s only represent a minor population. In patients with newly diagnosed or active CD (ACD) and refractory CD type 1 (RCD I), the frequency of SI NKp44+ ILCs is decreased, with restoration of NKp44+ ILC frequency observed in patients adhering to a gluten‐free diet who show evidence of mucosal healing. Moreover, the frequency of SI NKp44− ILCs is increased in ACD and RCD I patients and correlates with the severity of villous atrophy and epithelial damage, as assessed by serum levels of fatty acid binding protein 2 (FABP2). We show that the ILC alterations in CD represent a phenotypic shift of cytotoxic ILC1s rather than an increase in helper ILC1s or transdifferentiation of ILC1s to ILC3s, and activation‐induced loss of NKp44 by cytotoxic ILC1s is associated with increased interferon (IFN)‐γ expression and release of lytic granules. These findings suggest that intra‐epithelial NKp44−CD127− cytotoxic ILC1s may contribute to mucosal damage in CD.
Keywords: autoimmunity, cell surface molecules, human, inflammation, innate lymphoid cells
In this study, we show that duodenal intraepithelial ILCs predominantly consist of NKp44+ CD127− cytotoxic ILC1s (ieILC1s) and NKp44− CD127+ helper ILC1s, while CD127+ ILC3s only represent a minor population. In celiac disease (CD), cytotoxic ILC1s undergo a phenotypic transition (downregulate NKp44 expression) and upregulate IFN‐γ. This phenomenon is mirrored in vitro upon activation of cytotoxic ILC1s and is associated with increased intracellular IFN‐γ and granzyme‐B expression and lytic granule release. These findings suggest that intraepithelial NKp44− CD127− cytotoxic ILC1s may foster intestinal inflammation and contribute to enterocyte damage in CD.
Introduction
Celiac disease (CD) is a common autoimmune disorder with intestinal and systemic manifestations triggered by consumption of wheat or related proteins in genetically predisposed individuals 1, 2. The activation of adaptive and innate immune responses by gliadin peptides results in small intestinal mucosal damage, and adherence to a gluten‐free diet (GFD), the only effective available treatment, leads to a decrease in inflammation, recovery of mucosal architecture and relief of symptoms 3. A minority of patients (1–5%), however, have persistence or recurrence of gastrointestinal symptoms and villous atrophy despite adherence to a GFD for >1 year 4, 5, and are deemed to have refractory celiac disease (RCD) 6.
The mechanisms underlying gliadin‐mediated activation of the adaptive immune response are relatively well defined in CD, but the nature and regulation of the innate immune response within the epithelial compartment is not fully understood 7. Intraepithelial lymphocytes (IELs) constitute a diverse population, including thymus‐derived T cells of the αβ and γδ lineage and subsets of bone marrow‐derived innate lymphoid cells (ILCs) 8, 9. It has been shown that IELs, specifically cytotoxic T cell receptor (TCR)‐αβ+ T cells, which undergo natural killer (NK)‐like reprogramming on interacting with epithelial stress‐induced ligands, and IL‐15, up‐regulated upon exposure to toxic gliadin peptides, promote epithelial destruction 10, 11. Contributions of other types of lymphoid cells to the innate immune response in CD are suspected, but these are currently unclear 12, 13, 14, 15, 16, 17, 18, 19, 20. Recent studies have implicated ILCs in the pathogenesis of a variety of intestinal inflammatory disorders 21; however, data regarding the repertoire of human proximal small intestinal (SI) ILCs and their alterations in CD are limited 9, 12, 13, 14, 15, 22.
To gain insight into the composition and dynamics of intra‐epithelial ILCs in CD we analyzed the phenotypes of duodenal intra‐epithelial ILCs in healthy adults, patients with active CD (ACD) and RCD type 1 (RCD I) and CD patients on GFD and performed correlative studies and in‐vitro assays to assess potential cell functions.
Materials and methods
Biopsy collection and patient characteristics
Duodenal biopsies (four to five specimens) were obtained from adult CD patients, including 10 with biopsy‐proven, newly diagnosed or active CD (ACD), 15 patients adherent to a GFD for greater than 6 months (0·73–28·81 years, median = 7·83 years), six patients with RCD I and 15 control subjects. ACD patients were on a gluten‐containing diet, were seropositive and had villous atrophy (Marsh score >2) on histopathological examination. Celiac patients with persistent villous atrophy and with persistent or recurrent symptoms despite strict adherence to a GFD for >1 year were considered to have RCD. After excluding other possible causes of villous atrophy and symptoms, patients on a strict GFD and without a clonal population of immunophenotypically aberrant IELs were considered to have RCD type I for this study. Control subjects underwent upper endoscopy for gastrointestinal symptoms, but had normal duodenal mucosa without inflammation. Clinical data were obtained from the treating physicians. The biopsy specimens were collected after obtaining written informed consent from patients and the study was performed in accordance with the Declaration of Helsinki using a protocol approved by the institutional review board of Columbia University Irving Medical Center, New York.
Histopathological analysis
Formalin‐fixed paraffin‐embedded (FFPE) duodenal biopsies from patients and controls were stained with hematoxylin and eosin for morphological analysis. Severity of villous atrophy was assessed using the modified Marsh–Oberhuber scoring system (0 = normal histology, <40 IEL/100 epithelial cells; 1 = normal histology, >40 IEL/100 epithelial cells; 2 = hyperplastic crypts, normal villi, >40 IEL/100 epithelial cells; 3a = hyperplastic crypts, partial villous atrophy, >40 IEL/100 epithelial cells; 3b = hyperplastic crypts, subtotal villous atrophy, >40 IEL/100 epithelial cells; and 3c = hyperplastic crypts, total villous atrophy, > 40 IEL/100 epithelial cells) 23.
IEL isolation
IELs were extracted from biopsies as described previously, with minor modifications 24. Freshly obtained duodenal biopsies were incubated in Hanks’s balanced salt solution (HBSS) (gibco, Gaithersburg, MD, USA) supplemented with 1 mM dithiothreitol (Sigma‐Aldrich, St Louis, MO, USA), 1 mM ethylenediamine tetraacetic acid (EDTA) (Sigma‐Aldrich) and 1% (vol/vol) fetal calf serum (FBS) (gibco), while shaking for 30 min at 37°C. The cell suspension was filtered through a 100 µm cell strainer (Corning, Tewksbury, MA, USA) to separate tissue pieces. After centrifugation and resuspension of IELs in FBS containing 10% (vol/vol) dimethyl sulphoxide (DMSO) (Sigma‐Aldrich), aliquots of 1 × 106 cells/mL were stored in liquid nitrogen.
Antibodies and flow cytometry
IELs were incubated on ice with conjugated antibodies in phosphate‐buffered saline (PBS) containing 3% FBS for 30 min. Antibodies specific for human CD19 (HIB19), CD34 (581), CD45 (HI30), TCR‐α/β (IP26), TCR‐γ/δ (B1), CD107α (1D4B), CD117 (104D2), CD122 (TU27), CD127 (A019D5), chemoattractant receptor‐homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) (BM16) and NKp44 (P44‐8) were from Biolegend (San Diego, CA, USA); anti‐CD3 (OKT3 and UCHT1), CD14 (TUK4) and granzyme B (GB11) were from Invitrogen (Carlsbad, CA, USA); anti‐CD56 (B159) and CD123 (9F5) were from BD Biosciences (Franklin Lakes NJ, USA); anti‐CD8α (RPA‐T8), CD103 (Ber‐ACT8), human leukocyte antigen D‐related (HLA‐DR) (LN3), NKp44 (44.189), interleukin (IL)‐17A (eBio64DEC17), interferon (IFN)‐γ (4S.B3), GATA binding protein 3 (GATA 3) (TWAJ), T‐bet (eBio4B10), Eomesodermin (Eomes) (WD1928) and retinoic acid‐related orphan receptor gamma t (RORγt) (AFKJS‐9) were from eBioscience (San Diego, CA, USA); anti‐CD16 (3G8) was from BD Pharmingen (San Jose, CA, USA); and anti‐NKG2D (REA797) was from Miltenyi Biotech (Bergisch Gladbach, Germany).
Fluorochrome‐labeled antibodies directed against human CD3, CD14, CD16, CD19, CD34, CD45, TCR‐α/β, TCR/δ, NKp44, CD123 and CD103 were used to identify CD45+ lineage‐negative (Lin−) intraepithelial ILCs. Fluorescence minus one (FMO) controls were used for all experiments. Cells were acquired on a BD LSR Fortessa cell analyzer (BD Bioscience), and results were analyzed with FlowJo software, version 9.9 (Treestar, Ashland, OR, USA).
Analysis of ILC cytokine and transcription factor expression
IELs were stimulated with phorbol myristate acetate and ionomycin (PMA/IO) (1:500; Biolegend) for 3 h at 37°C in RPMI complete medium (CM), with the following composition: RPMI‐1640 medium supplemented with 10% (vol/vol) FBS, 1% (vol/vol) penicillin/streptomycin, 25 mM HEPES, 1% (vol/vol) non‐essential amino acids (NEAA), 1% (vol/vol) L‐glutamine and 100 μM 2‐mercaptoethanol (all from gibco) in the presence of brefeldin A (eBioscience). The cells were fixed and permeabilized with the forkhead box protein 3 (FoxP3)/transcription factor staining buffer kit (eBioscience), according to the manufacturer’s protocol. After gating on ILCs, the intracellular expression of IFN‐γ and IL‐17A and transcription factors GATA 3, T‐bet, Eomes and RORγT was analyzed.
In‐vitro assessment of ILC NKp44 expression stability and cell viability
IELs were stimulated with PMA/IO for 0·5, 1, 2 and 3 h at 37°C in CM. After gating on ILC subpopulations, percentages of NKp44+CD103+ ILC1s were recorded at all time‐points. The percentage of NKp44+ and NKp44−CD103+ apoptotic cells was assessed by staining with annexin V (Biolegend), according to the manufacturer’s protocol. The rate of apoptosis was calculated as the ratio of annexin V+CD103+ ILCs at the point of isolation (time 0) to time‐points 1, 2, 3 and 4, respectively. Additionally, CD127 expression was assessed at all time‐points.
Serum fatty acid‐binding protein 2 (FABP2) analysis
Serum was obtained on the same day as the duodenal biopsy specimens, and levels of FABP2 were determined by enzyme‐linked immunosorbent assay (ELISA) (R&D systems, Minneapolis, MN, USA), as previously described 25.
Statistical analysis
Difference in mean patient age in the different disease categories was evaluated by the Kruskal–Wallis one‐way analysis of variance. Distribution of the modified Marsh scores was assessed by the χ2 test. Difference in the intracellular expression of IFN‐γ and IL‐17A and cell surface expression of CD107a before and after in‐vitro PMA/IO stimulation in control ILCs, as well as differences in the frequencies of NKp44+ and NKp44− ILC1s in controls, ACD, GFD and RCD I samples were assessed by the Mann–Whitney U‐test. Correlation between serum levels of FABP2 and the frequency of NKp44+ and NKp44− ILC1s was analyzed using Spearman’s rho. Difference in the frequency and mean fluorescence intensity (MFI) of IFN‐γ‐producing NKp44− ILC1s in control and ACD samples after PMA/IO stimulation was evaluated by the Wilcoxon matched‐pairs signed‐rank test. All P‐values were two‐sided, and differences were considered statistically significant when P < 0·05. All data were analyzed with GraphPad Prism version 6 software (GraphPad, San Diego, CA, USA).
Results
Patient and biopsy characteristics
Clinical characteristics and biopsy histopathology of all subjects included in this study are described in Table 1. All celiac patients were confirmed to have celiac disease based on prior characteristic histopathological features and positive serology [anti‐tissue transglutaminase (anti‐tTG) and/or anti‐endomysial antibody]. Controls lacked villous atrophy and had negative celiac serology. The mean ages of patients in different CD groups varied, in line with previous observations. RCD I was diagnosed more frequently in older individuals 26, 27 and 17% were seropositive for anti‐tTG immunoglobulin (Ig)A antibodies. Biopsies from CD patients showed differences in the distribution of Marsh scores or severity of villous atrophy in the different disease groups. All ACD and RCD I patients had villous atrophy and the majority of GFD patients had normalized villous architecture 28.
Table 1.
Patient and biopsy characteristics
| Controls (n = 15) % | Newly diagnosed celiac disease (ACD) n = 10 | Celiac disease patients on a gluten‐free diet (GFD) n = 15 | Refractory celiac disease I patients (RCD I) n = 6 | P‐values | ||
|---|---|---|---|---|---|---|
| Age (years) | Age, mean | 57 | 34 | 53 | 60 | 0·0326* |
| Age, standard error | 20 | 14 | 15 | 23 | ||
| Age range | 20–80 | 20–68 | 23–78 | 33–88 | ||
| Sex | Female | 7 | 6 | 10 | 3 | n.s.† |
| Male | 8 | 4 | 5 | 3 | ||
| Seropositive | Seropositive | 0 | 10 | 2 | 1 | |
| Marsh score | 0 | 14 | 0 | 7 | 0 | < 0·0001† |
| 1 | 1 | 0 | 4 | 0 | ||
| 2 | 0 | 0 | 1 | 0 | ||
| 3a–c | 0 | 10 | 3 | 6 |
Kruskal–Wallis test.
χ2 test; n.s. = not significant.
Phenotypic characterization of normal duodenal intraepithelial ILCs
After exclusion of T cells, B cells, monocytes and classical NK cells, Lin−CD45+ cells were analyzed for the expression of NKp44 and CD103 in duodenal IEL fractions. In healthy controls, more than 95% of the Lin−CD45+ cells were CD103+ and comprised two subsets, NKp44+ and NKp44− (Fig. 1a). We analyzed the expression of transcription factors (T‐bet, RORγt, GATA3 and Eomes) and a panel of markers (CD127, CD117, HLA‐DR, CD56, NKG2D, CD8α, CD122 and CRTH2) to further delineate the nature of the NKp44+ and NKp44− cells. The majority of NKp44+ cells expressed T‐bet (Fig. 1b, upper panel) and lacked CD127 expression, with a subset expressing CD56, a profile consistent with cytotoxic intra‐epithelial ILC1s (ieILC1s) (76·84%), also known as NK‐like ILCs 29, 30, 31. The NKp44+ population included a minor component of RORγt+ cells that expressed CD127, CD117 and HLA‐DR, a profile indicative of ILC3s (4·29%) 21, 32. The majority of NKp44− cells also expressed T‐bet (Fig. 1b, lower panel), with major subsets expressing CD127 and CD8α, consistent with helper ILC1s (11·6%) 33, 34. No distinct population of CRTH2+ cells was detected, confirming an absence of ILC2s within the epithelium.
Figure 1.
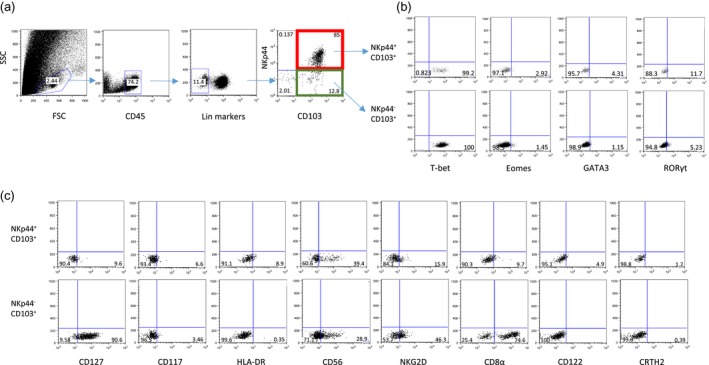
Phenotypic analysis of intra‐epithelial innate lymphoid cells (ILCs) isolated from normal small intestine biopsies. (a) Gating strategy for CD45+ lineage− [CD3−CD19−CD14−CD16−CD123−CD34−T cell receptor (TCR)γ/δ−TCRα/β−] CD103+NKp44+ and CD103+NKp44− intra‐epithelial ILCs. (b) Intracellular expression of indicated transcription factors in CD103+ NKp44+ (upper panel) and CD103+NKp44− (lower panel) ILCs. (c) Expression of indicated cell surface markers in CD103+NKp44+ cells (upper panel) and CD103+NKp44− cells (lower panel) ILCs. Dot‐plots represent one of three independent experiments.
These results demonstrate that duodenal intra‐epithelial CD103+ ILCs can be divided into NKp44+ and NKp44− ILCs, with NKp44+ cells mainly constituting cytotoxic ILC1s and NKp44− cells largely comprising helper ILC1s.
Cytokine profiles and functional attributes of duodenal intraepithelial ILCs
Since ILC1s and ILC3s were identified as the intra‐epithelial ILC subsets, we evaluated expression of intracellular IFN‐γ and IL‐17A, the main effector cytokines of ILC1s and ILC3s 29, 35, 36, and assessed granzyme B expression as well as the degranulation of cytotoxic granules upon stimulation. As expected, we observed a significant increase in IFN‐γ expressing ILCs compared with unstimulated controls (Fig. 2a). Based on CD127 expression and in line with previous studies, we confirmed IFN‐γ production by both CD127− (cytotoxic ILC1) and CD127+ (non‐cytotoxic ILC1 and ILC3) subsets (Fig. 2b) 29. Consistent with the identification of ILC3s among intra‐epithelial ILCs, we detected IL‐17A expression that was significantly elevated following stimulation compared with unstimulated controls (Fig. 2a). The median percentage of ILCs expressing IFN‐γ was threefold higher than cells expressing IL‐17A, underlining the predominance of T‐bet+ group 1 ILCs. We observed significantly increased expression of CD107a (Fig. 2a,b), a marker of release of lytic granules, and up‐regulation of granzyme B (Fig. 2b) in only CD127− ILCs, confirming the cytotoxic potential of this subset.
Figure 2.
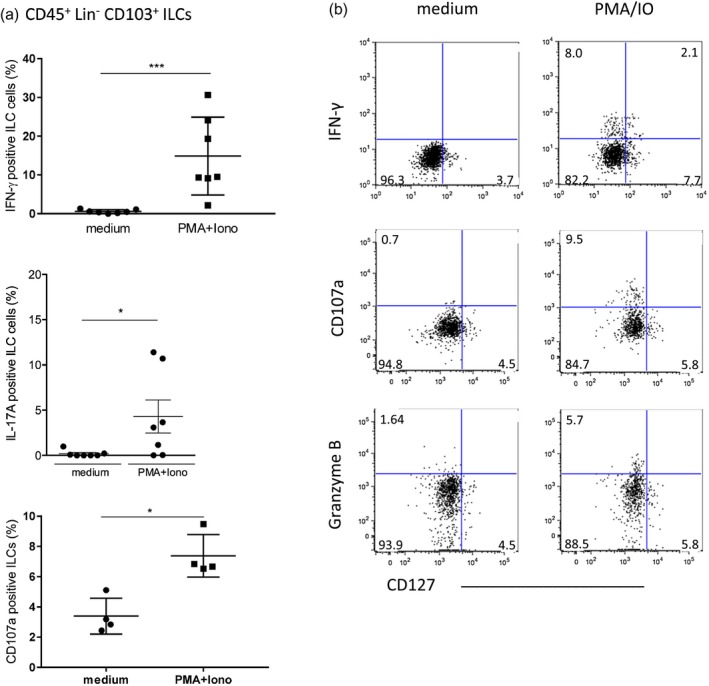
Interferon (IFN)‐γ, interleukin (IL)‐17A, CD107a and granzyme B expression of total intraepithelial innate lymphoid cells (ILCs) after stimulation in vitro. (a) IFN‐γ, IL‐17A and CD107a‐expressing ILC1s. IFN‐γ: medium = 0.57 ± 0.18 (n = 7), PMA/IO = 14.87 ± 3.80 (n = 7); IL‐17A: medium = 0.18 ± 0.13 (n = 7), PMA/IO = 4.29 ± 1.82 (n = 7); CD107a: medium = 3.39 ± 0.59 (n = 4), PMA/IO = 7.38 ± 0.70 (n = 4) [mean percentage ± standard error of the mean (SEM.)]. (b) Representative dot‐plot of stimulated CD127+ and CD127− ILC1s. Dot‐plots represent one of three independent experiments ***P < 0·0001; *P < 0·05.
Activation‐induced NKp44 loss in cytotoxic ILC1s
We observed a loss of NKp44 expression after PMA/IO stimulation in CD103+ ILCs (Fig. 3a). To investigate if the increase in NKp44− ILCs was due to an expansion of helper ILC1s, transdifferentiation of NKp44+ ILC1s to ILC3s or the consequence of a phenotypic transition, i.e. loss of NKp44 expression by cytotoxic ILC1s, we stimulated IELs isolated from healthy adults with PMA/IO, and assessed the percentage of CD103+NKp44+ ILCs as well as their rate of apoptosis over time. The frequency of NKp44+ ILCs decreased upon stimulation, without a significant change in the apoptotic rate (Fig. 3b,c). Activation of ILCs also led to increased CD107a and granzyme B expression, as shown previously (Fig. 2b). The percentage of CD127+ ILCs did not change upon stimulation (Fig. 3d), arguing against transdifferentiation of NKp44+CD127− cytotoxic ILC1s to other CD127+ ILC subsets.
Figure 3.
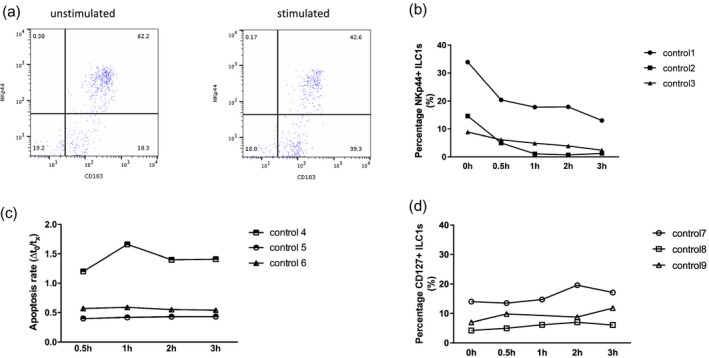
Stimulation of control innate lymphoid cells (ILCs) with phorbol myristate acetate and ionomycin (PMA/IO) in vitro. (a) Down‐regulation of NKp44 expression after PMA/IO stimulation in control ILCs. (b) Percentage of NKp44+ ILCs after 0·5, 1, 2 and 3 h stimulation. (c) Apoptotic rate is shown as the ratio of annexin V+ cells at time‐point 0 (isolation) and at time‐points 1–4. (d) Percentage of CD127+ ILCs after 0·5, 1, 2 and 3 h stimulation. Graphs represent three independent experiments.
Composition of duodenal intraepithelial ILCs is altered in CD
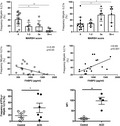
While TCR+ IELs accumulate during the active state of CD, the percentage of CD7(bright)+ intracellular CD3ε+/surfaceCD3− lymphocytes, which are now known to represent ILCs, has been reported to decrease in pediatric and adult patients 37, 38. Hence, we determined if there was a change in the relative abundance of NKp44− and NKp44+ ILCs in patients with ACD, RCD I and GFD. The frequency of NKp44+ ILCs was significantly decreased in ACD, RCD I and GFD patients, while the frequency of NKp44− ILCs in all disease categories showed a significant increase compared to controls (Fig. 4a,b).
Figure 4.
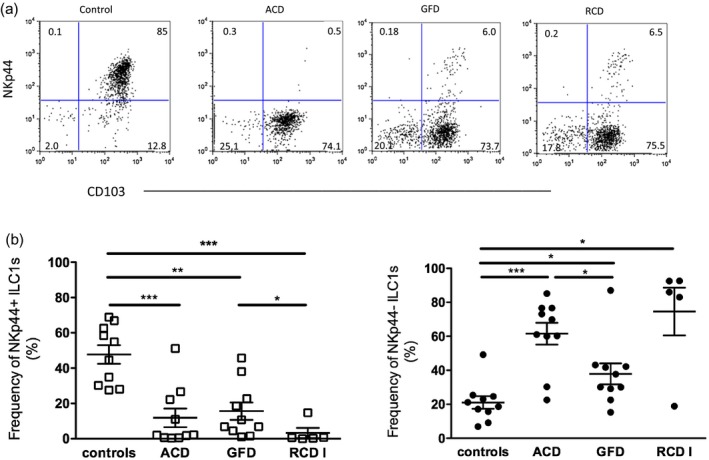
Innate lymphoid cell (ILC) composition in controls and patients with celiac disease (CD). (a) Dot‐plots represent one of five to 10 independent experiments. (b) The frequencies of NKp44+ and NKp44− ILC1s in controls, patients with active CD (ACD), patients on a gluten‐free diet (GFD) and patients with refractory CD type 1 (RCD I). Left: controls = 47·68 ± 5·25 (n = 10), ACD = 11·85 ± 5·31 (n = 10), GFD = 15·56 ± 4·92 (n = 10), RCD = I 3·27 ± 2·86 (n = 5); right: controls = 21·07 ± 3·68 (n = 10), ACD = 61·55 ± 6·43 (n = 10), GFD = 37·87 ± 6·16 (n = 10), RCD I = 74·6 ± 14·05 (n = 5) [mean percentage ± standard error of the mean (SEM.)]. ***P < 0·0001; **P < 0·01; *P < 0·05.
Of note, the NKp44− ILC1 frequency was significantly lower in GFD patients compared to ACD patients. RCD I patients, despite being on GFD, had significantly fewer NKp44+ ILC1s than patients with uncomplicated CD on GFD (Fig. 4b). Although the frequency of NKp44+ ILC1s in GFD patients as a group did not differ significantly from ACD patients (Fig. 4b), an inverse correlative trend of NKp44+ ILC1 frequency was noted when GFD patients were stratified by the Marsh score (see section below and Supporting information, Fig. S1).
ILC alterations in CD correlate with the severity of mucosal damage and are associated with enhanced IFN‐γ expression
Next, we sought to analyze the relationship between the frequencies of NKp44+ and NKp44− ILCs and severity of mucosal damage in CD patients. For this analysis, CD biopsies were stratified according to the modified Marsh score into three groups (Marsh scores 0, 1–2 and 3) 23. The frequency of NKp44+ ILCs correlated inversely with the severity of villous atrophy being significantly higher in CD patients with Marsh 0 than Marsh 3 scores; conversely, the frequency of NKp44− cells was significantly higher in patients with Marsh 3 than Marsh 0 scores (Fig. 5a). As the levels of FABP2, a marker of epithelial cell damage and turnover, have been shown to correlate with the degree of villous atrophy in CD 39, we analyzed serum FABP2 levels in 15 CD patients (ACD, n = 7; GFD, n = 8) and three controls. Increasing FABP2 levels correlated with decreasing frequencies of NKp44+ and increasing frequencies of NKp44− ILCs (Fig. 5b).
Figure 5.
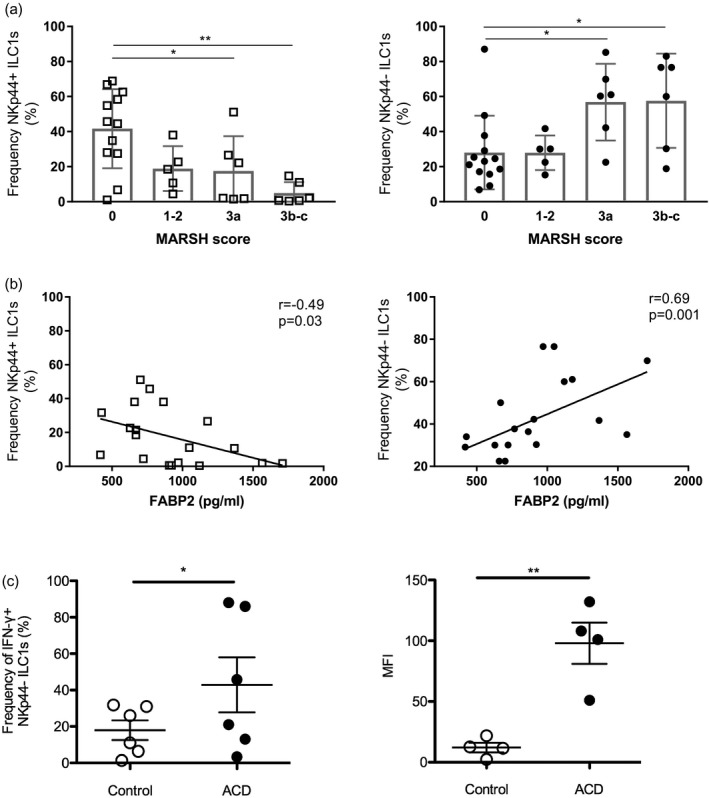
Correlation between natural killer (NK)p44+ and NKp44− innate lymphoid cell (ILC) frequencies and intestinal epithelial cell damage in celiac disease (CD) patients. (a) Frequency of NKp44+ and NKp44− intraepithelial ILCs in CD patients, including patients with active CD (ACD) and patients on a gluten‐free diet (GFD), and controls stratified for the degree of villous atrophy based on Marsh scores 0–3. Left: Marsh 0 = 41·68 ± 6·51 (n = 13), 1–2 = 18·88 ± 5·72 (n = 5), 3a = 17·55 ± 8·11 (n = 6), 3b–c = 4·95 ± 2·56 (n = 6); right: Marsh 0 = 28·04 ± 5·82 (n = 13), 1–2 = 27·92 ± 4·40 (n = 5); 3a = 56·85 ± 8·94 (n = 6), 3b–c = 57·57 ± 10·98 (n = 6) [mean percentage ± standard error of the mean (SEM.)]. (b) Correlation between serum fatty acid binding protein 2 (FABP2) levels and frequencies of NKp44+ and NKp44− ILCs in CD patients (n = 15) and controls (n = 3). (c) Stimulation of control and active CD (ACD) NKp44− CD103+ ILC1s with phorbol myristate acetate and ionomycin (PMA/IO) in vitro. Left: percentage of interferon (IFN)‐γ producing NKp44− CD103+ ILC1s. Control = 24·95 ± 4·82 (n = 6), ACD = 60·18 ± 16·29 (n = 6) (mean percentage ± s.e.m.); right: MFI of IFN‐γ expression in NKp44− CD103+ ILC1s. Control = 12·03 ± 3·98 (n = 4), ACD = 98 ± 17·01 (n = 4) (mean percentage ± SEM.). **P < 0·01.
Lastly, to assess for any difference in the cytokine response of ILCs in CD, we compared intracellular IFN‐γ expression in NKp44− ILC1s from control and ACD patients. The frequency of IFN‐γ‐producing NKp44− ILC1s was significantly higher in ACD patients. Moreover, the mean fluorescence intensity (MFI) of IFN‐γ expression was significantly higher in NKp44− ILC1s of ACD patients indicative of increased IFN‐γ production (Fig. 5c).
Discussion
Investigations during recent years have led to the recognition of diverse types of ILCs with distinct functions 40. The frequencies and phenotypes of ILC subsets differ between various anatomical sites, and the localization of specific ILCs in different compartments relates to their roles in immune and inflammatory responses 30, 41. Schmitz et al. showed that the composition of proximal intestinal intra‐epithelial ILCs changes with age, CD56+ conventional natural killer (cNK) cells being the dominant population at the fetal stage, with CD127− cytotoxic ILCs and CD127+ helper ILCs predominating in children and adults, respectively 13. In our analysis of healthy adult duodenal intraepithelial ILCs, CD103+ NKp44+ CD127− IFN‐γ‐expressing cytotoxic ILC1s were the predominant subset, followed by NKp44− CD127+ helper ILC1s and IL‐17A‐expressing RORγt+ ILC3s, while ILC2s were not identified. These findings are in line with those of Kramer et al., who reported ILC1s to represent the dominant subset and a low frequency of ILC3s in the human duodenum 41.
A novel finding of our study was the loss of NKp44 expression by intra‐epithelial cytotoxic ILC1s in CD. NKp44, a member of the natural cytotoxicity receptors (NCRs), was first identified in 1998 to be an activating receptor mediating non‐major histocompatibility complex (MHC)‐restricted cytotoxicity of NK cells 42. It is a type I transmembrane glycoprotein with a single extracellular V‐type Ig‐like domain and a cytoplasmic tail containing an immunoreceptor tyrosine‐based inhibitory motif (ITIM) 43. Upon ligand binding, NKp44 interacts with a dimer of immunoreceptor tyrosine‐based activation motif (ITAM) of the adaptor DNAX‐activating protein 12 (DAP12/KARAP/Tyrobp), via the positively charged lysine in the transmembrane region, to transmit an activating signal 42, 44. NKp44 signaling is important for potentiating NK cell cytotoxicity against tumor cells 45, 46 and the recognition and lysis of virus‐infected cells 47. NKp44 expression has also been documented in other immune cells, including plasmacytoid dendritic cells 48, cytotoxic ILC1s 29 and a subset of ILC3s 49, potentially conferring NK‐like signaling programs to these cells 50. In the context of ILC1s, NKp44 expression has been used as a marker to distinguish cytotoxic intraepithelial ILC1s (ieILC1s) from classic helper ILC1s 29.
Lower frequencies of NKp44+ ILCs have previously been reported in other gastrointestinal diseases, including ileal lamina propria ILC3s in inflammatory bowel disease (IBD) and rectal mucosal ILC1s in non‐celiac wheat sensitivity (NCWS) 33, 51, but the reason for attrition of NKp44+ ILCs has not been explored. We observed a marked decrease in NKp44+ ILCs in ACD (and RCD I). Additionally, in‐vitro studies showed activation‐induced loss of NKp44 expression on cytotoxic ILC1s. These findings suggest that the decrease in NKp44 expression is the consequence of an inflammation/activation‐induced phenotypic shift of cytotoxic ILC1s to NKp44− CD127− ILCs rather than loss due to cell death, migration to another site, transdifferentiation into ILC3s or a relative expansion of helper NKp44− CD127+ ILC1s. This observation is congruent with prior reports that did not identify any difference in the fraction of intraepithelial (or lamina propria) CD127+ ILCs between adult CD patients and healthy individuals 12, 14. The long‐term fate of NKp44−CD127− ILCs is unknown. It is unclear whether CD127− ILCs up‐regulate NKp44 expression upon resolution of inflammation or if they are replaced by newly generated NKp44+ ILCs.
The precise mechanisms underlying the loss of NKp44 are not known. Up‐regulation of indoleamine 2,3‐dioxygenase (IDO) and prostaglandin E2 (PGE2) in CD 52, 53, 54 could potentially contribute, similarly to what has been observed in NK cells 55, 56. Of interest, Marafini et al. reported an absence of NKp44/NKp46 double‐positive NK cells and NK T cells in the intestinal epithelia in ACD 57. Functional exhaustion of NK cells during chronic infections and anti‐tumor immune responses 58, 59 is associated with downregulation of NKp44 among other antigens 60. However, in contrast to NKp44−CD127− cytotoxic ILCs in CD, exhausted NK cells show diminished cytolytic activity and decreased expression of effector cytokines.
The correlation of intraepithelial NKp44− ILC frequency with increased severity of villous atrophy and increased serum levels of FABP2, a marker for enterocyte damage, as well as increased IFN‐γ expression and enhanced cytotoxic potential, suggests roles of cytotoxic ILC1s in fostering inflammation and mediating epithelial destruction in CD. These findings support the superior cytocidal activity of CD3− IELs previously reported by Leon et al. 61. A few studies have explored mechanisms of intraepithelial ILC activation in CD and suggested roles for factors derived from both innate and adaptive immune cells. While immunogenic gliadin peptides activate HLA‐DQ2 or ‐DQ8 restricted T cells in CD, some gliadin peptides can induce an epithelial stress response resulting in the up‐regulation of IL‐15 62, 63, 64, which has been shown to stimulate ILC1s in CD 12, 13, 15, 29. Other cytokines, e.g. TNF, IL‐2 and IL‐21, released by gliadin responsive lamina propria CD4+ T cells have also been reported to stimulate the proliferation of Lin− innate IELs, in addition to enhancing their survival 65. Additionally, non‐gluten proteins such as amylase‐trypsininhibitors (ATIs) have been demonstrated to initiate an innate immune response by binding to Toll‐like receptor (TLR)‐4 on monocytes, macrophages and dendritic cells 66, but it is not known if they can activate ILCs. We found no evidence of ILC activation by gluten and non‐gluten proteins (data not shown). Further studies are awaited to elucidate other relevant contact dependent and independent mechanisms of intraepithelial ILC activation in CD and other inflammatory disorders.
RCD, a rare complication of CD, is currently subdivided into two types, RCD I and RCD II, based on the absence or presence of a clonal population of immunophenotypically aberrant IELs 67. RCD II is a neoplastic disorder, also referred to as cryptic enteropathy‐associated T cell lymphoma or ‘in‐situ’ lymphoma 68 and the aberrant IELs have recently been shown to derive from CD127−CD122+ (IL‐15Rβ) ILCs 12, 15. Acquisition of gain‐of‐function mutations in Janus kinase 1 (JAK1) and signal transducer and activator of transcription 3 (STAT3), which enhance response to IL‐15, are thought to contribute to the clonal expansion of these ILCs 15. The etiology of RCD I is unresolved and is probably multi‐factorial. Up to a third of patients are seropositive for tissue transglutaminase antibodies 69, 70, 71, 72, suggesting either inadvertent gluten intake or a protracted inflammatory or immune response to ingested gluten. An increased frequency of infections has also been documented in RCD I patients 73, 74. Although polyclonal expansions of intraepithelial CD3+ CD8+TCR+ T cells are well recognized, ILC alterations have not been previously examined in RCD I. We observed a similar ILC profile and phenotypic shift of cytotoxic ILC1s (NKp44 loss) in RCD I as in ACD, suggesting an analogous mucosal inflammatory milieu. Although we recommend further exploration of the biological consequences of NKp44 loss by ILCs in CD and RCD, this marker does not appear to be suitable for the evaluation of aberrant IELs in RCD II, due to its infrequent expression 12.
In summary, this study is the first, to our knowledge, to characterize duodenal intraepithelial ILC alteration in CD. We identified a phenotypic shift of cytotoxic ILCs associated with enhanced cytotoxic potential and increased IFN‐γ expression, which correlated with the severity of mucosal damage. It remains to be determined whether therapeutic targeting of specific ILC subsets can dampen mucosal inflammation and hasten the healing of SI mucosa in CD.
Disclosures
The authors declare no conflicts of interest.
Author contributions
G. B. conceived the study idea; M. U., A. B. and G. B. designed the experiments. G. B., M. U., A. B., C. B., X. Y., P. G. and G. B. analyzed data. P. G., S. K., S. L. and B. L. provided control and celiac disease biopsy samples; A. A. provided digested wheat proteins and rice proteins for the stimulation studies. S. G., B. R., P. G. and G. B. supervised the study. X. Y. and G. B. drafted and wrote the manuscript; all authors provided critical comments and reviewed the manuscript.
Supporting information
Fig. S1. Frequency of NKp44+ and NKp44− ILC1s in patients on a gluten‐free diet. NKp44+ and NKp44− ILC1s in CD patients on a gluten‐free diet (GFD) for at least 6 months stratified by degree of persistent villous atrophy compared with controls and ACD patients. Left: ACD = 11·85 ± 5·31 (n = 10), MARSH 3 = 15·02 ± 10·95 (n = 3), 1‐2 = 18·88 ± 5·72 (n = 5), 0 = 28·96 ± 12·9 (n = 5), Controls = 47·68 ± 5·25 (n = 10); right: ACD = 61·55 ± 6·43 (n = 10), MARSH 3 = 41·97 ± 0·73 (n = 3), 1‐2 = 27·92 ± 4·40 (n = 5), 0 = 36·52 ± 13·44 (n = 5), Controls = 21·07 ± 3·68 (n = 10) (Mean percentage ± SEM) ** = P < 0·01.
Acknowledgements
We would like to thank Cyrus Garcia for help with sample processing and obtaining and organizing the clinical data. This study was made possible with a research grant from the Celiac Disease Center at Columbia University and a Bouncer Foundation grant.
Contributor Information
X. Yu, Email: xy2314@cumc.columbia.edu.
G. Bhagat, Email: gb96@cumc.columbia.edu.
References
- 1. Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–43. [DOI] [PubMed] [Google Scholar]
- 2. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet 2018; 391:70–81. [DOI] [PubMed] [Google Scholar]
- 3. Abadie V, Sollid LM, Barreiro LB, Jabri B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Ann Rev Immunol 2011; 29:493–525. [DOI] [PubMed] [Google Scholar]
- 4. Roshan B, Leffler DA, Jamma S et al The incidence and clinical spectrum of refractory celiac disease in a North American referral center. Am J Gastroenterol 2011;106:923–8. [DOI] [PubMed] [Google Scholar]
- 5. van Wanrooij RLJ, Bouma G, Bontkes HJ et al Outcome of referrals for non‐responsive celiac disease in a tertiary center: low incidence of refractory celiac disease in the Netherlands. Clin Transl Gastroen 2017; 8:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludvigsson JF, Bai JC, Biagi F et al Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 2014; 63:1210–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jabri B, Sollid LM. Tissue‐specific immune responses. Tissue‐mediated control of immunopathology in coeliac disease. Nat Rev Immunol 2009; 9:858–70. [DOI] [PubMed] [Google Scholar]
- 8. McDonald BD, Jabri B, Bendelac A. Diverse developmental pathways of intestinal intra‐epithelial lymphocytes. Nat Rev Immunol 2018; 18:514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Kaer L, Olivares‐Villagomez D. Development, homeostasis, and functions of intestinal intra‐epithelial lymphocytes. J Immunol 2018; 200:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hue S, Mention JJ, Monteiro RC et al A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004; 21:367–77. [DOI] [PubMed] [Google Scholar]
- 11. Meresse B, Curran SA, Ciszewski C et al Reprogramming of CTLs into natural killer‐like cells in celiac disease. J Exp Med 2006; 203:1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitz F, Tjon JM, Lai Y et al Identification of a potential physiological precursor of aberrant cells in refractory coeliac disease type II. Gut 2013; 62:509–19. [DOI] [PubMed] [Google Scholar]
- 13. Schmitz F, Kooy‐Winkelaar Y, Wiekmeijer AS et al The composition and differentiation potential of the duodenal intra‐epithelial innate lymphocyte compartment is altered in coeliac disease. Gut 2016; 65:1269–78. [DOI] [PubMed] [Google Scholar]
- 14. Marafini I, Monteleone I, Di Fusco D et al TNF‐alpha producing innate lymphoid cells (ILCs) are increased in active celiac disease and contribute to promote intestinal atrophy in mice. PLOS ONE 2015; 10:e0126291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ettersperger J, Montcuquet N, Malamut G et al Interleukin‐15‐dependent T‐cell‐like innate intra‐epithelial lymphocytes develop in the intestine and transform into lymphomas in celiac disease. Immunity 2016; 45:610–25. [DOI] [PubMed] [Google Scholar]
- 16. Montalvillo E, Bernardo D, Martinez‐Abad B et al Increased intra‐epithelial valpha24 invariant NKT cells in the celiac duodenum. Nutrients 2015; 7:8960–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grose RH, Cummins AG, Thompson FM. Deficiency of invariant natural killer T cells in coeliac disease. Gut 2007; 56:790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marafini I, Monteleone I, Di Fusco D et al Celiac disease‐related inflammation is marked by reduction of Nkp44/Nkp46‐double positive natural killer cells. PLoS ONE 2016; 11:e0155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jabri B, de Serre NP, Cellier C et al Selective expansion of intra‐epithelial lymphocytes expressing the HLA‐E‐specific natural killer receptor CD94 in celiac disease. Gastroenterology 2000; 118:867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunne MR, Elliott L, Hussey S et al Persistent changes in circulating and intestinal gamma delta T cell subsets, invariant natural killer T cells and mucosal‐associated invariant T cells in children and adults with coeliac disease. PLOS ONE 2013; 8;e76008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geremia A, Arancibia‐Carcamo CV. Innate lymphoid cells in intestinal inflammation. Front Immunol 2017; 8:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Unen V, Li N, Molendijk I et al Mass cytometry of the human mucosal immune system identifies tissue‐ and disease‐associated immune subsets. Immunity 2016; 44:1227–39. [DOI] [PubMed] [Google Scholar]
- 23. Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11:1185–94. [DOI] [PubMed] [Google Scholar]
- 24. Mayassi T, Ladell K, Gudjonson H et al Chronic inflammation permanently reshapes tissue‐resident immunity in celiac disease. Cell 2019; 176:967–81.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uhde M, Indart AC, Yu XCB et al Markers of non‐coeliac wheat sensitivity in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Gut 2019; 68:377–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chander U, Leeman‐Neill RJ, Bhagat G. Pathogenesis of enteropathy‐associated T cell lymphoma. Curr Hematol Malig Rep 2018; 13:308–17. [DOI] [PubMed] [Google Scholar]
- 27. Malamut G, Afchain P, Verkarre V et al Presentation and long‐term follow‐up of refractory celiac disease: comparison of type I with type II. Gastroenterology 2009; 136:81–90. [DOI] [PubMed] [Google Scholar]
- 28. Wahab PJ, Meijer JW, Mulder CJ. Histologic follow‐up of people with celiac disease on a gluten‐free diet: slow and incomplete recovery. Am J Clin Pathol 2002; 118:459–63. [DOI] [PubMed] [Google Scholar]
- 29. Fuchs A, Vermi W, Lee JS et al Intra‐epithelial type 1 innate lymphoid cells are a unique subset of IL‐12‐ and IL‐15‐responsive IFN‐gamma‐producing cells. Immunity 2013; 38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simoni Y, Fehlings M, Kloverpris HN et al Human innate lymphoid cell subsets possess tissue‐type based heterogeneity in phenotype and frequency. Immunity 2017; 46:148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim AI, Li Y, Lopez‐Lastra S et al Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017; 168:1086–100.e10. [DOI] [PubMed] [Google Scholar]
- 32. Hepworth MR, Fung TC, Masur SH et al Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria‐specific CD4(+) T cells. Science 2015; 348:1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernink JH, Peters CP, Munneke M et al Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013; 14:221–9. [DOI] [PubMed] [Google Scholar]
- 34. Van Kaer L, Algood HM, Singh K et al CD8alphaalpha(+) innate‐type lymphocytes in the intestinal epithelium mediate mucosal immunity. Immunity 2014; 41:451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517:293–301. [DOI] [PubMed] [Google Scholar]
- 36. Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 2012; 37:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spencer J, MacDonald TT, Diss TC, Walker‐Smith JA, Ciclitira PJ, Isaacson PG. Changes in intra‐epithelial lymphocyte subpopulations in coeliac disease and enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine). Gut 1989; 30:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Camarero C, Eiras P, Asensio A et al Intra‐epithelial lymphocytes and coeliac disease: permanent changes in CD3−/CD7+ and T cell receptor gammadelta subsets studied by flow cytometry. Acta Paediatr 2000; 89:285–90. [PubMed] [Google Scholar]
- 39. Adriaanse MPM, Tack GJ, Passos VL et al Serum I‐FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharm Ther 2013; 37:482–90. [DOI] [PubMed] [Google Scholar]
- 40. Vivier E, Artis D, Colonna M et al Innate lymphoid cells: 10 years on. Cell 2018; 174:1054–66. [DOI] [PubMed] [Google Scholar]
- 41. Kramer B, Goeser F, Lutz P et al Compartment‐specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLOS Pathog 2017; 13:e1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vitale M, Bottino C, Sivori S et al NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non‐major histocompatibility complex‐restricted tumor cell lysis. J Exp Med 1998; 187:2065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cantoni C, Ponassi M, Biassoni R et al The three‐dimensional structure of the human NK cell receptor NKp44, a triggering partner in natural cytotoxicity. Structure 2003; 11:725–34. [DOI] [PubMed] [Google Scholar]
- 44. Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol 2014; 92:221–9. [DOI] [PubMed] [Google Scholar]
- 45. Parodi M, Favoreel H, Candiano G et al NKp44‐NKp44 ligand interactions in the regulation of natural killer cells and other innate lymphoid cells in humans. Front Immunol 2019;10;719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debre P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013; 122:2935–42. [DOI] [PubMed] [Google Scholar]
- 47. Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol 2001; 31:2680–9. [DOI] [PubMed] [Google Scholar]
- 48. Bonaccorsi I, Cantoni C, Carrega P et al The immune inhibitory receptor LAIR‐1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFN alpha production. PLOS ONE 2010: 5;e15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoorweg K, Peters CP, Cornelissen F et al Functional differences between human NKp44(–) and NKp44(+) RORC+ innate lymphoid cells. Front Immunol 2012; 3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Villanova F, Flutter B, Tosi I et al Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ILC3 in psoriasis. J Invest Dermatol 2014; 134:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Di Liberto D, Mansueto P, D'Alcamo A et al Predominance of type 1 innate lymphoid cells in the rectal mucosa of patients with non‐celiac wheat sensitivity: reversal after a wheat‐free diet. Clin Transl Gastroenterol 2016; 7:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Branski D, Karmeli F, Gross‐Kieselstein E, Abrahamov A, Rachmilewitz D. Prostaglandins in small intestinal mucosa of children with celiac disease. J Pediatr Gastroenterol Nutr 1984; 3:672–5. [DOI] [PubMed] [Google Scholar]
- 53. Lavo B, Knutson L, Loof L, Hallgren R. Gliadin challenge‐induced jejunal prostaglandin E2 secretion in celiac disease. Gastroenterology 1990; 99:703–7. [DOI] [PubMed] [Google Scholar]
- 54. Torres MI, Lopez‐Casado MA, Lorite P, Rios A. Tryptophan metabolism and indoleamine 2,3‐dioxygenase expression in coeliac disease. Clin Exp Immunol 2007; 148:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pietra G, Manzini C, Rivara S et al Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72:1407–15. [DOI] [PubMed] [Google Scholar]
- 56. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer‐cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3‐dioxygenase and prostaglandin E2. Blood 2008; 111:1327–33. [DOI] [PubMed] [Google Scholar]
- 57. Marafini I, Monteleone I, Di Fusco D et al Celiac disease‐related inflammation is marked by reduction of Nkp44/Nkp46‐double positive natural killer cells. PLOS ONE 2016; 11:e0155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wherry EJ, Teichgraber V, Becker TC et al Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225–34. [DOI] [PubMed] [Google Scholar]
- 59. Bi J, Tian Z. NK cell exhaustion. Front Immunol 2017; 8:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaufmann DE, Walker BD. Programmed death‐1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS 2008; 3:362–7. [DOI] [PubMed] [Google Scholar]
- 61. Leon F, Roldan E, Sanchez L, Camarero C, Bootello A, Roy G. Human small‐intestinal epithelium contains functional natural killer lymphocytes. Gastroenterology 2003; 125:345–56. [DOI] [PubMed] [Google Scholar]
- 62. Setty M, Discepolo V, Abadie V et al Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intra‐epithelial killer cells and active celiac disease. Gastroenterology 2015; 149:681–91.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meresse B, Chen ZG, Ciszewski C et al Coordinated induction by IL15 of a TCR‐independent NKG2D signaling pathway converts CTL into lymphokine‐activated killer cells in celiac disease. Immunity 2004; 21:357–66. [DOI] [PubMed] [Google Scholar]
- 64. Hue S, Mention JJ, Monteiro RC et al A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004; 21:367–77. [DOI] [PubMed] [Google Scholar]
- 65. Kooy‐Winkelaar YMC, Bouwer D, Janssen GMC et al CD4 T‐cell cytokines synergize to induce proliferation of malignant and nonmalignant innate intra‐epithelial lymphocytes. Proc Natl Acad Sci USA 2017; 114:E980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Junker Y, Zeissig S, Kim SJ et al Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll‐like receptor 4. J Exp Med 2012; 209:2395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol 2005; 19:413–24. [DOI] [PubMed] [Google Scholar]
- 68. Cellier C, Delabesse E, Helmer C et al Refractory sprue, coeliac disease, and enteropathy‐associated T‐cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356:203–8. [DOI] [PubMed] [Google Scholar]
- 69. Malamut G, Meresse B, Cellier C, Cerf‐Bensussan N. Refractory celiac disease: from bench to bedside. Semin Immunopathol 2012; 34:601–13. [DOI] [PubMed] [Google Scholar]
- 70. Arguelles‐Grande C, Brar P, Green PHR, Bhagat G. Immunohistochemical and T‐cell receptor gene rearrangement analyses as predictors of morbidity and mortality in refractory celiac disease. J Clin Gastroenterol 2013; 47:593–601. [DOI] [PubMed] [Google Scholar]
- 71. Ilus T, Kaukinen K, Virta LJ et al Refractory coeliac disease in a country with a high prevalence of clinically‐diagnosed coeliac disease. Aliment Pharm Ther 2014; 39:418–25. [DOI] [PubMed] [Google Scholar]
- 72. Hollon JR, Cureton PA, Martin ML, Puppa ELL, Fasano A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet‐adherent non‐responsive celiac disease patients. BMC Gastroenterol 2013; 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meresse B, Malamut G, Cerf‐Bensussan N. Celiac disease: an immunological Jigsaw. Immunity 2012; 36:907–19. [DOI] [PubMed] [Google Scholar]
- 74. Perfetti V, Baldanti F, Lenti MV et al Detection of active Epstein–Barr virus infection in duodenal mucosa of patients with refractory celiac disease. Clin Gastroenterol Hepatol 2016; 14:1216–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Frequency of NKp44+ and NKp44− ILC1s in patients on a gluten‐free diet. NKp44+ and NKp44− ILC1s in CD patients on a gluten‐free diet (GFD) for at least 6 months stratified by degree of persistent villous atrophy compared with controls and ACD patients. Left: ACD = 11·85 ± 5·31 (n = 10), MARSH 3 = 15·02 ± 10·95 (n = 3), 1‐2 = 18·88 ± 5·72 (n = 5), 0 = 28·96 ± 12·9 (n = 5), Controls = 47·68 ± 5·25 (n = 10); right: ACD = 61·55 ± 6·43 (n = 10), MARSH 3 = 41·97 ± 0·73 (n = 3), 1‐2 = 27·92 ± 4·40 (n = 5), 0 = 36·52 ± 13·44 (n = 5), Controls = 21·07 ± 3·68 (n = 10) (Mean percentage ± SEM) ** = P < 0·01.


