Abstract
Background
Substantial research is dedicated to understanding the aging-related dynamics among individual differences in level, change, and variation across physical and cognitive abilities. Evaluating replicability and synthesizing findings has been limited by differences in measurements, samples, study design, and statistical analyses that confound between-person differences with within-person changes. Here, we systematically reviewed longitudinal results on the aging-related dynamics linking pulmonary function and cognitive performance.
Methods
Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines were used to systematically review longitudinal studies of pulmonary function and cognition.
Results
Only four studies thoroughly investigating cognitive and pulmonary longitudinal associations (three or more measurement occasions) were identified. Expanded review criteria identified three studies reporting two measurement occasions, and seven studies reporting one measurement of pulmonary function or cognition and two or more measurements of the other. We identified numerous methodological quality and risk for bias issues across studies.
Conclusions
Despite documented correlational associations between pulmonary function and cognition, these results show there is very limited research thoroughly investigating their longitudinal associations. This highlights the need for longitudinal data, rigorous methodological design including key covariates, and clear communication of methods and analyses to facilitate replication across an array of samples. We recommend systematic study of outcome measures and covariates, inclusion of multiple measures (e.g., peak expiratory flow, forced expiratory volume in 1 s, and forced vital capacity), as well as application of the same analytic approach across multiple datasets.
Keywords: Cognition, Longitudinal change, Pulmonary, Research methods and issues, Successful aging
Global aging research data are critical in helping us to better understand normal and abnormal or disease-related aging processes. Research has emphasized the dynamics of age-related changes in functional outcomes, including the influence of shared risk factors (Clouston et al., 2013; Spiro & Brady, 2008). For example, aging-related changes in functioning have been observed in a wide variety of biomarkers, including pulmonary function and cognitive abilities (Lara et al., 2015) and accumulating research has indicated pulmonary function may be a cross-sectional predictor of cognitive performance in young, middle-aged, and older adults (Albert et al., 1995; Anstey, Windsor, Jorm, Christensen, & Rodgers, 2004; Cerhan et al., 1998; Chyou et al., 1996; Cook et al., 1989; Deary, Whalley, Batty, & Starr, 2006; Emery, Huppert, & Schein, 1997; Emery, Pedersen, Svartengren, & McClearn, 1998; Min, Min, Paek, Sakong, & Cho, 2007; Pathan et al., 2011; Richards, Strachan, Hardy, Kuh, & Wadsworth, 2005; Russ, Starr, Stamatakis, Kivimäki, & Batty, 2015; Sachdev et al., 2006; Singh-Manoux et al., 2011). Yet, recent longitudinal findings regarding the association between decline in pulmonary function and decline in fluid cognitive abilities (Emery, Finkel, & Pedersen, 2012; Weuve et al., 2011) are inconsistent.
The utility of the extant research is limited by differences in study design and analytic models. Associations between biomarkers of aging including physiological or cognitive processes are notably clear when comparing individuals differing in chronological age (Hofer, Berg, & Era, 2003), but appear less consistent when evaluating associations among rates of changes as observed within individuals over time (Spiro & Brady, 2008). A limitation of cross-sectional analysis and, by extension between-person differences in time-dependent variables, is that the associations can arise due to age-related mean differences alone, in addition to individual differences in rates of change and time-specific variation (Hofer & Sliwinski, 2001). Further, pulmonary and cognitive measures are known to differ in their rates of change due to physiological differences (e.g., differential aging of aspects of the lungs and the brain) as well as broader factors including age, education, race/ethnicity, occupational attainment, activity level, anthropometric measures, and the presence of genetic or clinical pathology (Brewster et al., 2014; Dyer, 2012; Hofer, Flaherty, & Hoffman, 2006; Johnson et al., 2012; Karlamangla et al., 2009; Kraemer, Yesavage, Taylor, & Kupfer, 2000; Mungas et al., 2010; Salthouse, 2014; Vaz Fragoso & Gill, 2012). There are also non–aging related explanations for associations between baseline pulmonary function and cognitive functioning including differences in environment and childhood development (socioeconomic status, health, and nutrition within and across birth cohorts). To date, most studies examining associations between pulmonary and cognitive functioning have relied on either cross-sectional designs or use analytical models evaluating the effects of baseline function in one domain on change in another domain of functioning (Albert et al., 1995; Anstey et al., 2004; Cerhan et al., 1998; Chyou et al., 1996; Cook et al., 1989; Deary et al., 2006; Emery et al., 1997, 1998; Min et al., 2007; Pathan et al., 2011; Richards et al., 2005; Russ et al., 2015; Sachdev et al., 2006; Singh-Manoux et al., 2011). This is a particular problem for cross-sectional studies evaluating multivariate associations across a broad range of ages (Hofer et al., 2006; Kraemer et al., 2000) because associations among rates of change in these studies could arise from both “directional decline” (e.g., decrease in pulmonary functioning causing cognitive decline or the reverse), common causal processes (e.g., pollutant exposures damaging lungs and inciting neuroinflammation), or a normative aging process (e.g., resulting in average demographically corrected scores across all domains of functioning).
Because of these gaps, we do not know if a common aging process influences both pulmonary functioning and cognition. This problem is well exemplified by the often-studied areas of cognition and lung function. Despite an abundance of investigations, it appears numerous inconsistencies in methods and results remain. Here, we performed a systematic review of published longitudinal research investigating the association between changes in pulmonary function and cognitive performance in adults using three or more repeated measurements. To the best of our knowledge, this is the first systematic review of the longitudinal association between changes in pulmonary and cognitive functioning.
Method
Literature Search, Study Selection, and Data Extraction
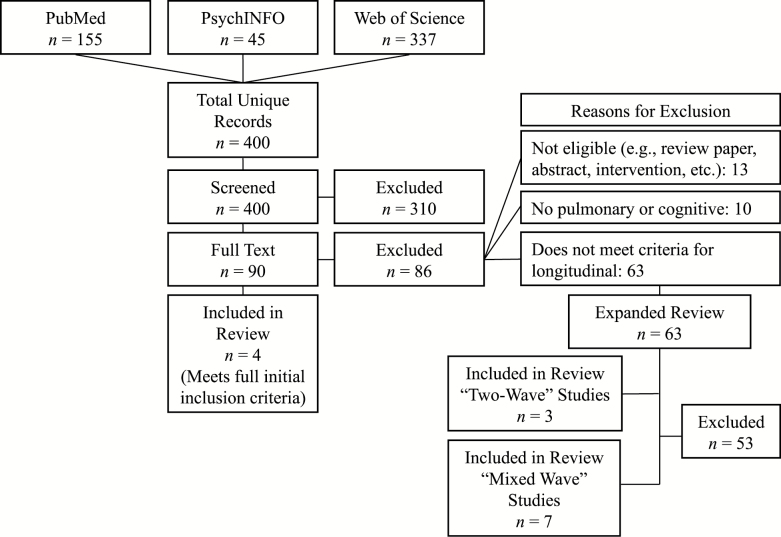
We followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement (Moher, Liberati, Tetzlaff, Altman, & Prisma Group, 2009). We also used the Critical Appraisal Skills Programme (CASP, 2018) to assess methodological quality (“Yes,” “No,” or “Can’t Tell” with overall rating of “Low,” “Medium,” or “High”) and the National Heart, Lung, and Blood Institute Quality Assessment Tool (NHLBI, 2014) with the PRISMA guidelines to assess risk of bias (overall rating at study level and outcome level of “Low,” “Medium,” or “High”). In November 2016, we performed a comprehensive literature search for longitudinal studies examining the association between pulmonary function and cognition in adults using PsychINFO, PubMed, and Web of Science (see Supplementary Table 1 for a comprehensive list of search terms). This search was updated on May 2017 and April 2018 to identify new articles published during the review period (see Figure 1 for total search details). Initial inclusion criteria included studies that (a) used individual-level data from adult (age ≥18) community-dwelling samples, (b) included objective measurements of both pulmonary and cognitive functioning and analysis of association (i.e., not merely covariates in a model of another outcome variable), (c) analyzed longitudinal data (i.e., three or more measurement occasions) on both pulmonary function and cognition, and (d) reported original data in English. We excluded studies not meeting these criteria as well as those reporting data from intervention studies (e.g., rehabilitation or drug trials). Because of a low number of studies meeting full inclusion criteria (n = 4), we expanded the inclusion criteria in two ways. First, we reviewed studies reporting two measurement occasions of both cognition and pulmonary function (“two-wave” studies). Second, we reviewed studies with a single measurement of one variable (pulmonary function or cognition) and two or more measurements of the other (“mixed-wave” studies).
Figure 1.
Total systematic review as of April 2018
Three authors (E. C. Duggan, R. B. Graham, and N D. Jenkins) followed a common data collection instrument to independently review and extract information from each study. All studies were reviewed independently at least twice, and the authors conferred at each review stage, reconciling disagreements through discussion.
Results
Of the 400 unique references identified by the search, four met the full original inclusion criteria. Although many references passed initial screening (n = 90), full-text review revealed most (n = 63) did not meet the longitudinal data criteria (i.e., pulmonary and cognitive data at three or more measurement occasions). On second expanded review, 10 of these 63 articles met partial inclusion criteria by assessing both pulmonary function and cognition on two occasions (“two-wave” studies; n = 3), or reporting baseline measures of one variable and two or more measurement occasions of the other variable (“mixed-wave” studies; n = 7). Figure 1 summarizes the study selection process and Supplementary Table 2 lists each reference with its primary selection decision. All articles are reviewed later, with longitudinal articles more thoroughly discussed and briefer summaries on “two-wave” and “mixed-wave” articles. Tables 1 and 2 summarize articles meeting full and partial inclusion criteria (respectively), whereas Table 3 and Supplementary Table 3 provide evaluation of methodological quality and risk of bias.
Table 1.
Summary of the Citations Meeting Full Longitudinal Inclusion Criteria for the Systematic Review: Sample and Study Characteristics, and Main Findings
| First author, year | Study | Sample characteristics | Geographic region | No. of participants | Waves of data collection | % Male | Baseline age range (years) | Pulmonary measures | Statistical method | |
|---|---|---|---|---|---|---|---|---|---|---|
| Emery, 2012 | Swedish Adoption/Twin Study of Aging (SATSA) | Population-twin | Sweden | 832 | 6 | 41 | 50 to 85 | FEV1 FVC |
Dual change score models | |
| Cognitive battery | (Latent) Domain: | Processing speed (Fluid intelligence) | Spatial ability (Fluid intelligence) | Memory (Fluid intelligence) | Verbal ability (Crystallized intelligence) | |||||
| Tests: | Digit symbol Figure identification |
Block design Card rotations |
Digit span Picture memory |
Information Synonyms Analogies |
||||||
| Main result | Decline in FEV1 and FVC led, directionally, to decline on the spatial ability and processing speed factors. | |||||||||
| Finkel, 2013 | Swedish Adoption/Twin Study of Aging (SATSA) | Population-twin | Sweden | 808 | 6 | 41 | 50 to 85 | FEV1 | Dual change score models | |
| Cognitive battery | (Latent) Domain: | Processing speed (Fluid intelligence) | Spatial ability (Fluid intelligence) | |||||||
| Tests: | Digit symbol Figure identification |
Block design Card rotations |
||||||||
| Main result | Genetic influences (as determined by studying monozygotic and dizygotive twin pairs) accounted for a significant proportion of variance in FEV1-related changes on the spatial ability and processing speed factors. | |||||||||
| MacDonald, 2011 | Victoria Longitudinal Study (VLS) | Population | Canada | 1,043 | 2 to 3 | 34 | 55 to 85 | PEF | Linear mixed-effects models | |
| Cognitive battery | (Latent) Domain: | Working memory | Fluid reasoning | Episodic memory | Semantic memory | Crystallized ability | ||||
| Test: | Computation span task | Letter series task | Word recall test | Fact recall test | Recognition vocabulary | |||||
| Main result | Decline in PEF associated with decline on computation span, fact recall, and vocabulary. | |||||||||
| Weuve, 2011 | Veteran’s Administration Normative Aging Study (VA-NAS) | Community sample | United States | 864 | Pulmonary: 2–5 cognitive: 3 |
100 | 49 to 97 | FEV1 | Linear mixed effects models and generalized estimating equation models | |
| Cognitive battery | (Latent) Domain: | Attention/working memory/ executive function | Visuospatial ability | Short-term memory | Verbal ability | |||||
| Tests: | Continuous performance | Constructional praxis | Immediate word list recall | Vocabulary | ||||||
| Digit span backward | Pattern comparison | Delayed word list recall | ||||||||
| Verbal fluency | Pattern memory | |||||||||
| Main result | FEV1 (baseline and change) was associated with change in constructional praxis and pattern comparison. | |||||||||
Note: FEV1 = forced expiratory flow (in 1 s); FVC = forced vital capacity; PEF = peak expiratory flow
Table 2.
Summary of Two-Wave Citations (n = 3) and Mixed-Wave Citations (n = 7) Partially Meeting Inclusion Criteria for the Systematic Review: Sample and Study Characteristics, and Main Findings
| First author, year | Study | Sample characteristics | Geographic region | No. of participants | Waves of data collection | % Male | Baseline age range (years) | Pulmonary measures | Statistical method | |
|---|---|---|---|---|---|---|---|---|---|---|
| Emery, 1998 | Swedish Adoption/ Twin Study of Aging (SATSA) | Population-Twin | Sweden | 444 | 2 | 40 | 40–84 | FEV1 | Hierarchical multiple regression | |
| Cognitive battery | (Latent) Domain: | Processing speed (Fluid intelligence) | Spatial ability (Fluid intelligence) | Memory (Fluid intelligence) | Verbal ability (Crystallized intelligence) | |||||
| Tests: | Digit symbol | Block design | Digit span | Information | ||||||
| Main result | Baseline FEV1 predicted performance in processing speed and spatial ability measures at baseline and 6-year follow-up, but did not predict change in any of the cognitive outcomes. | |||||||||
| Richards, 2005 | Medical Research Council National Survey of Health and Development (MRC NSHD) | Population | United Kingdom | 3,035 | 2 | 49.8 | 43 | FEV1 | Conditional change score models | |
| Cognitive battery | (Latent) Domain: | Processing speed/ concentration | Memory | Verbal ability | ||||||
| Tests: | Timed Peg Placement Test | Word List Recall | National Adult Reading Test | |||||||
| Letter Search | ||||||||||
| Main result | Baseline FEV1 was associated with processing speed at baseline and slower decline in processing speed over time. No association between FEV1 and memory or verbal ability. | |||||||||
| Starr, 2007 | 1947 Scottish Mental Survey | Population | Scotland | 298 | 2 | 47 | 64 | FEV1 PEF FVC |
Linear mixed effects models | |
| Cognitive battery | (Latent) Domain: | Non-verbal reasoning/ spatial ability | Verbal memory | Executive function | Processing speed | |||||
| Test(s): | Raven’s Progressive Matrices Block design |
Auditory Verbal Learning test | Use of common objects test | Digit symbol | ||||||
| Main result | Higher PEF was associated with better performance on all cognitive tests except Auditory Verbal Learning at age 64 and 66 years. | |||||||||
| Aiken-Morgan, 2018 | Baltimore Study of Black Aging - Patterns of Cognitive Aging | Population | United States | 407 | Pulmonary: 1 cognitive: 2 |
— | 48–95 | PEF | Multivariate and Univariate Analysis of Variance | |
| Cognitive battery | (Latent) Domain: | Inductive reasoning | Declarative memory | Processing speed | Working memory | Verbal ability | Executive functioning | |||
| Tests: | Letter Series Test | Hopkins Verbal Learning Task | Number Comparison Test | Alpha Span | Verbal Ability Test | Clock Drawing Test | ||||
| Shipley Institute of Living Scale Abstraction Test | Rey Auditory Verbal Learning Task | Identical Pictures Test Digit Symbol |
Operation Span Digit Span Backwards |
Shipley Institute of Living Verbal Meaning Test | ||||||
| Main result | Better lung function at baseline was significantly associated with cognitive stability over 3 years. | |||||||||
| Infurna, 2013 | Health and Retirement Study (HRS) | Population | United States | 4,177 | Pulmonary: 1 cognitive: 2 |
41 | 66.75* | FEV1 | Latent change score models | |
| Cognitive battery | (Latent) Domain: | Episodic memory | ||||||||
| Tests: | Word List Immediate Recall | |||||||||
| Word List Delayed Recall | ||||||||||
| Main result | FEV1 at baseline was significantly associated with 4-year change in memory; better pulmonary function predicted less memory decline. | |||||||||
| Koster, 2005 | Health, Aging and Body Composition (Health ABC) study | Population | United States | 2,574 | Pulmonary: 1 cognitive: 2 |
— | 70 –79 | FEV1 FVC |
Chi-square tests | |
| Cognitive battery | (Latent) Domain: | General cognitive status | ||||||||
| Tests: | Modified MMSE (3MS) | |||||||||
| Main result | Low FEV1 at baseline was significantly associated with 4 year decline in cognitive function. | |||||||||
| Pathan, 2011 | Atherosclerosis Risk in Communities Study | Population | United States | 10,975 | Pulmonary: 1 cognitive: 2–3 |
— | 45–64 | FEV1 FVC |
Multivariable linear regression | |
| Cognitive battery | (Latent) Domain: | Verbal memory | Executive function/ processing speed | Processing speed | ||||||
| Tests: | Delayed Word Recall | Digit Symbol | Word Fluency Test | |||||||
| Main result | Reduced FEV1 or FVC at baseline was significantly associated with worse cognitive function at baseline, and higher risk of dementia at follow-up. No association was observed between reduced FEV1 or FVC and cognitive decline. | |||||||||
| Swan, 1992 | National Heart, Lung, and Blood Institute (Bethesda, MD) Twin Study | Population | United States | 792 | Pulmonary: 1 cognitive: 2 |
– | MZ–57.1* DZ 56.3* |
FEV1 FVC |
Heritability analysis, t and F tests | |
| Cognitive battery | (Latent) Domain: | Processing speed | ||||||||
| Tests: | Digit symbol | |||||||||
| Main result | Cognitive decline in twin pairs measured by the digit symbol substitution test had significantly poorer lung function than twin pairs with no cognitive decline. | |||||||||
| Vidal, 2013 | AGES-Reykjavik study (2002–2006) Cohort | Population | Iceland | 5764 | Pulmonary: 2–3 cognitive: 1 |
33 | 52* | FEV1/height2 | ANOVA and logistic regression | |
| Cognitive battery | (Latent) Domain: | Memory | Processing speed | Executive function | General cognitive status | |||||
| Tests: | California Verbal Learning Test | Figure Comparison Test | CANTAB Spatial Working Memory Test | MMSE | ||||||
| Digit symbol | Digit Span Backwards | |||||||||
| Stroop | Stroop | |||||||||
| Main result | Lower FEV1/height2 at mid-life were more likely to have lower cognitive test scores or to develop MCI or dementia 23 years later. | |||||||||
| Whitfield, 1997 | MacArthur Research Network on Successful Aging Community Study | Population | United States | 224 | Pulmonary: 1 cognitive: 2 |
40 | 70 to 79 | PEF | Linear regression analyses and logistic regression | |
| Cognitive battery | (Latent) Domain: | Memory | Language | Conceptualization | Visuospatial ability | |||||
| Tests: | Delayed Verbal Memory | Boston Naming Test | Similarities | Delayed Recognition Span | ||||||
| Figure Copy | ||||||||||
| Main result | Baseline PEF was significantly associated with cognitive performance. PEF significantly predicted cognitive decline between intervals. | |||||||||
Note: FEV1 = forced expiratory flow (in 1 s); PEF = peak expiratory flow; MMSE = Mini-Mental Status Examination; MZ = Monozygotic twin pairs; DZ = Dizygotic twin pairs; *Age displayed as mean.
Table 3.
Summary of Methodological Quality using the CASP and Risk of Bias using the NHLBI Quality Assessment Tool
| First author (year) | Methodological quality | Risk of bias at study level? | Risk of bias at outcome level? | Notes on methodological limitations and risk of bias | |
|---|---|---|---|---|---|
| Longitudinal studies | Emery (2012) | Medium | High | Medium | Important confounds not included (e.g., height, smoking, and other health status). Implications clear but over stated. Effect sizes and other key statistics not reported. High attrition but consistent with study type. |
| Finkel (2013) | Medium | High | Medium | Important confounds not included (e.g., height, smoking, and other health status). Implications clear but over stated. Effect sizes and other key statistics not reported (including genetic and environmental contributions). High attrition but consistent with study type. | |
| MacDonald (2011) | High | Low | Low | No effect sizes, not all outcome data consistently reported (but can be calculated). High attrition but consistent with study type. Single cognitive measures per domain. | |
| Weuve (2011) | Medium | Low | High | Methods not clear and obscure potentially important information in the data, insufficient reporting, and over interpretation on small and/or no effects. Effect sizes and other key statistics not reported. High attrition but consistent with study type. | |
| “Two-Wave” studies | Emery (1998) | Medium | Medium | Medium | Controls for main confounds except smoking. FEV1 associated with fluid but not crystallized abilities (believable) but utility of pulmonary function as a predictor of cognitive performance over 6 years not believable (study not adequately longitudinal, only one association “approaching significance”). Attrition not sufficiently reported. Effect sizes and other key statistics not reported. |
| Richards (2005) | Low | Medium | High | Attrition inadequately reported. Inadequate follow-up (two measurement occasions pulmonary, one–two measurement occasions cognitive). Unusual and inconsistent application of analyses with high potential for bias (baseline pulmonary with change in cognition in adulthood, change in pulmonary with adolescent cognitive baseline). Effect sizes and other key statistics not reported. | |
| Starr (2007) | Medium | Medium | Medium | Study aims and structure not adequate to detect change over time (looking at association of smoking, but not particularly cognitive/pulmonary change; two measurements, 2 years apart). Choice of recruitment and details of high attrition not clearly detailed. Important confounds not included (e.g., height, and other health status). | |
| “Mixed Wave” studies | Aiken- Morgan (2018) | Low | High | Medium | Important confounds not included (e.g., height, smoking, and other health status). Inadequate follow-up (baseline pulmonary, two measurements of cognition 3 years apart). High attrition but consistent with study type. Effect sizes and other key statistics not reported. |
| Infurna (2013) | Low | High | High | Important confounds not included (e.g., height, smoking, and other health status). High levels of missing data, but well discussed. Inadequate follow-up and uses non validated memory measure as single cognitive outcome (baseline pulmonary, two cognitive measurements, 4 years apart). | |
| Koster (2005) | Low | Medium | High | Includes main confounds except smoking. Inadequate follow-up and uses two versions of single cognitive outcome with insufficient psychometrics (baseline pulmonary, two cognitive measurements, 4 years apart, abbreviated 3MS in 11% of sample). Effect sizes and other key statistics not reported. | |
| Pathan (2011) | Medium | Low | Medium | Inadequate follow-up (baseline pulmonary, two waves of cognitive data for 80% of the sample, 3–4 waves cognitive for est. <10%–20% sample [not clearly reported]). Effect sizes and other key statistics not reported. | |
| Swan (1992) | Low | High | High | Highly restricted sample increasing potential for bias. Inadequate design and follow-up (baseline pulmonary, two measurements of a single cognitive task [digit symbol] over 5 years in midlife). No analyses to directly look at pulmonary– cognitive association. Effect sizes and other key statistics not reported. | |
| Vidal (2013) | Medium | Medium | High | High attrition (27% of total sample has two pulmonary measurement occasions, 11% three pulmonary measurement occasions) Inadequate follow-up (one cognitive measurement at 23 years follow-up with no baseline). Effect sizes and other key statistics not reported. | |
| Whitfield (1997) | Medium | Low | Medium | Attrition just over 20% but consistent with study type. Inadequate follow-up (baseline pulmonary, two occasions of cognitive data 2 years apart). Effect sizes and other key statistics not reported. |
Note: CASP = Critical Appraisal Skills Programme (CASP, 2018); NHLBI = National Heart, Lung, and Blood Institute (NHLBI, 2014). See Supplementary Table 3.
Studies Meeting Full Inclusion Criteria: Longitudinal Measurement of Pulmonary Function and Cognition
Characteristics
Four publications met the full systematic review inclusion criteria, having at least three measurement occasions each of pulmonary function and cognition (Emery et al., 2012; Finkel, Reynolds, Emery, & Pedersen, 2013; MacDonald, DeCarlo, & Dixon, 2011; Weuve et al., 2011). These four articles were derived from three different longitudinal studies of aging: the Normative Aging Study (NAS; Weuve et al., 2011), the Swedish Adoption/Twin Study of Aging (SATSA; Emery et al., 2012; Finkel et al., 2013), and the Victoria Longitudinal Study (VLS; MacDonald et al., 2011).
Longitudinal study characteristics are summarized in Table 1. Overall, studies were either population based (VLS), twin population based (SATSA), or community sample based (NAS), all with moderately large numbers of participants (range = 808–1,035). Studies were heterogeneous in terms of sex, age, and geography/culture. One sample (NAS) included only men with a baseline age range of 49–97 years when cognitive testing began, whereas the rest were majority women (59%–66%; SATSA and VLS), with a baseline age range of mid-life (50 or 55 years) to 85 years. The samples differed in number of observational waves (ranging from 3 to 6), with intervals between waves ranging from 3 to 7 years. In terms of exclusion criteria, one study (SATSA) appeared to retain all participants except those with dementia diagnosis (with data before diagnosis being retained when applicable). Two studies (NAS and VLS) report exclusion of participants with chronic medical conditions at study enrollment, but no exclusion of participants based on medical or neurocognitive status following recruitment.
At least one type of pulmonary function measure was used in all studies and all methods appeared consistent with the American Thoracic Society recommendations for pulmonary testing (Miller et al., 2005). Three references (one for NAS and two for SATSA) used forced expiratory volume in 1 s (FEV1), one used forced vital capacity (FVC; SATSA; Emery et al., 2012), and one used peak expiratory flow (PEF; VLS). Data corrections and covariates also differed across studies. SATSA pulmonary raw data were corrected for height and sex before standard score transformation. NAS adjusted their overall models for age, height, education, previous computer experience, smoking, and baseline Mini-Mental State Examination. Similarly, VLS models adjusted for the influence of sex and age (but not height).
Each study included multiple cognitive tests (range = 5–9), treated as indicators of various (latent) cognitive constructs. Although there was some overlap across studies in terms of cognitive domains described and tests used, there were also numerous differences and inconsistencies in terminology (Table 1). For example, tasks considered as representing the working memory construct (e.g., digit span, computation span, and pattern memory; Strauss, Sherman, & Spreen, 2006) were described across studies as: memory, working memory, attention/working memory, executive function, and short-term memory. Furthermore, the fact recall test used in VLS was described as “semantic memory”; however, this test is in identical to the information test used in SATSA, which was described as “verbal ability” (both falling under the umbrella of crystallized abilities). All studies used cognitive tests as indicators of constructs, with one to three indicators per construct.
Methodological quality, risk of bias, and results
Methodological quality was medium to high. VLS (MacDonald et al., 2011) was the only study with high quality, and the other three medium quality articles were most affected by exclusion of important confounds (Emery and colleagues, 2012, and Finkel and colleagues, 2013, excluded height, smoking, and other health status) and incomplete or unclear data reporting (e.g., Weuve and colleagues, 2011, report pulmonary data in quartiles and cognitive data in percent change in standardized score; Emery and colleagues, 2012, Finkel and colleagues, 2013, and Weuve and colleagues, 2011, do not report effect sizes). Risk of bias at the study level ranged from low (VLS; MacDonald et al., 2011, and NAS; Weuve et al., 2011) to high (SATSA; Emery et al., 2012, Finkel, 2013) for similar reasons. Risk of bias at the outcome level was low for VLS, medium for SATSA (e.g., only two indicators are used per latent cognitive domain; methods are non-replicable based on information provided in the publications; poor operationalization of “genetic contributions” by Finkel and colleagues (2013), and high for NAS that used unusual treatment of cognitive and pulmonary data that increases risk for bias (as stated earlier, in addition to interpretation of nonsignificant and marginally significant associations; Weuve et al., 2011). Attrition was high across all four studies, but deemed fairly consistent with what is to be expected for longitudinal studies of this type. Study results described later should be considered in the context of these quality and risk for bias ratings.
Using data from the VLS, MacDonald and colleagues (2011) used Generalized Linear Models (GLM) to determine how changes in cognitive function and select physical health biomarkers (i.e., pulmonary function, grip strength, body mass index, and blood pressure) decline or improve in a related fashion over time. Using this approach, they reported four key findings relating to longitudinal cognitive and pulmonary functioning. First, pulmonary function (PEF) and all measures of cognition (five cognitive tasks) declined over time. Second pulmonary function decline was not associated with decline in performance on letter series and word recall. Third, pulmonary function decline was significantly associated with decline in performance on computation span, fact recall, and vocabulary. Finally, pulmonary function accounted for between-person age-differences on all three of these tasks.
Using data from NAS, Weuve and colleagues (2011) examined associations of change in pulmonary functioning (FEV1) over an approximate 12-year period (starting in 1984) with cognitive performance and change (using 11 cognitive measures) assessed over an approximate 9-year period (starting in 1993). Overall, FEV1 declined over time, with more decline in older participants and smokers. Change in cognition over time was not reported independently. The authors used generalized estimating equation regression models to examine the long-term mean FEV1 and mean annual change in FEV1 in relation to cognitive task performance first for all participants, and second for never-smokers only. Among all participants, higher long-term mean FEV1 (better lung function) was reportedly associated with better baseline visual spatial performance, better global cognition, and slower decline on measures of recall and pattern comparison (although not statistically significant, p = .10). Annual change in FEV1 was not associated with any of the cognitive measures or change in these measures over time. In never-smokers, long-term FEV1 associations were reported as more evident but generally weak (only the two visuospatial measures reached significance), and annual change in FEV1 was again not significantly associated with cognitive change.
Two publications present results obtained from analysis of the SATSA. Both used bivariate dual change score modeling, which is a form of structural equation modeling that combines difference scores with cross-lagged models. The authors used this approach in the first article to test the potential directionality of variable relationships (e.g., can change in pulmonary function statistically predict change in cognition or vice versa?), without making any a priori hypotheses (Emery et al., 2012). In the second article (Finkel et al., 2013), they further used dual change score model to estimate the predictive statistical relationships between three variable domains: (a) change in pulmonary function, (b) change in cognition, and (c) genetic and environmental factors (by fitting structural models to the covariance matrices for monozygotic and dizygotic twin pairs). Of note, both articles used 13 age bins when modeling longitudinal (within-person) age.
Emery and colleagues (2012) first modeled the longitudinal trajectories of their pulmonary measures (FEV1 and FVC) and four cognitive factors (verbal ability, spatial ability, memory, and processing speed, derived through principal components analysis of 10 cognitive tasks). The authors found pulmonary function (FEV1 and FVC) declined linearly and all cognitive factors declined curvilinearly (accelerating decline steepest for spatial ability and processing speed, moderate for memory, and modest for verbal factors). Next, they evaluated the extent to which pulmonary function predicted cognitive performance and vice versa using SEM models. Emery and colleagues reported decline in pulmonary function predicted decline on spatial ability, processing speed, and (to a lesser extent) verbal factors, but decline on each of the four cognitive factors did not predict decline in pulmonary function. The memory factor was not associated with change in pulmonary function.
On the basis of their findings that changes in pulmonary function most predicted changes in latent spatial ability and processing factors (Emery et al., 2012), SATSA researchers did a follow-up study to investigate the extent to which genetic or environmental factors might play a role (Finkel et al., 2013). We opted to retain this study in the systematic review due to the inclusion of a potentially influential covariate (genetic variation). Unlike their earlier article, FEV1 was the only pulmonary outcome. First, they examined genetic influence on cognition and pulmonary function independently. They found genetic factors influenced baseline and longitudinal change in cognitive performance. Genetic factors and pulmonary function were associated at baseline, but not longitudinally. Interestingly, data on genetic versus environmental contributions are not directly reported. Next, the authors tested to see whether (a) genetic influences on pulmonary function predicted aging changes in cognition, (b) genetic influences on cognition predicted aging changes in pulmonary function, or (c) genetic influences bidirectionally predicted cognition and pulmonary function. Results reportedly supported the first association, indicating genetic influences on pulmonary function accounted for a significant proportion of variance in aging changes in cognition. The authors interpreted this to mean that innate influences on pulmonary function (e.g., genetic characteristics of an individual’s pulmonary system) appeared to have more impact on cognitive decline than do lifestyle and environmental factors known to affect pulmonary function. However, the effects of genes on physiological and cognitive functioning may be variable over time and may be influenced by environmental factors, and this is an issue that cannot be adequately addressed with the author’s study design.
Overall, all four studies reported support for baseline associations between pulmonary function and cognition. Further, these studies also contribute to the widely cited literature demonstrating that pulmonary function and cognition each decline with age. Associations between change in pulmonary function and change in cognitive function, however, differed widely across these four studies. MacDonald and colleagues (2011) found decline in PEF associated with decline on computation span, fact recall, and vocabulary. Weuve and colleague (2011) found FEV1 (higher baseline and slower decline) was associated with better subsequent performance on measures of constructional praxis and pattern comparison. Emery and colleague (2012) reported decline in FEV1 and FVC were directionally related to decline on spatial and speed factors. Finally, Finkel and colleagues (2013) expanded on this finding a year later, reporting genetic influences (as determined by studying monozygotic and dizygotic twin pairs) accounted for a significant proportion of variance in aging-related changes on the spatial and speed factors.
Looking across studies, there was some consistency between longitudinal change in pulmonary functioning and visual spatial-like tasks; however, this conclusion is tenuous given the differences in sample, procedures to standardize measures, inclusion and treatment of key covariates, and analytic methods. Specifically, results from SATSA (Emery et al., 2012, Finkel et al., 2013) are hard to interpret because height, smoking, and presence of other health conditions (all know to affect pulmonary function data) were not included in the analyses, the lack of clarity in the methods makes it difficult to understand the validity and reliability of the results (and impedes the ability to replicate these findings), and the way “genetic contributions” were measured and treated in analyses by Finkel and colleagues (2013) is not clearly explained (and thus unknown). Results from Weuve and colleagues (2011) report pulmonary data using quartiles and cognitive data using percent change in standardized scores; this impedes the reader from understanding the range and clinical significance of the data, as well as understanding the potential meaning of findings that are marginally significant or trends. Finally, generalization across these four studies is limited by use of different pulmonary (FEV1, FVC, and PEF) and (more importantly) cognitive measures. Although all studies used psychometrically supported measures, not a single cognitive measure was consistent across studies, and cognitive domains were poorly defined and inadequately measured (i.e., only one or two indicators per domain for nearly all across studies). Ultimately, the main consistency across these four articles was the finding that pulmonary function change appeared to relate to cognitive change in their samples; however, results from these articles should be interpreted with particular caution due to these limitations in methodological quality and risk for bias.
Studies Meeting Partial Inclusion Criteria: Two Measurement Occasions Each of Pulmonary Function and Cognition (“Two-Wave” Studies)
Three publications assessed pulmonary function and cognition each at two measurement occasions (Emery, Pedersen, Svartengren, & McClearn, 1998; Richards, Strachan, Hardy, Kuh, & Wadsworth, 2005; Starr, Deary, Fox, & Whalley, 2007; see Table 2). First, Emery and colleagues (1998) analyzed pulmonary function (FEV1) and multiple cognitive measures at baseline and 6-year follow-up in 222 twin-pairs (n = 444) from the SATSA sample. Second, Starr and colleagues (2007) analyzed pulmonary function (FVC and PEF) and multiple cognitive measures at baseline and 2-year follow-up in 298 individuals from the 1947 Scottish Mental Health Survey. Finally, Richards and colleagues (2005) used data from 3,035 individuals in the Medical Research Council National Survey of Health and Development. They analyzed associations between pulmonary function (FEV1) and multiple cognitive measures from two mid-life occasions 10 years apart, as well as with adolescent cognitive ability, with some analyses inconsistently including one or two waves of data.
Methodological quality for the Emery and colleagues (1998) and Starr and colleagues (2007) articles was medium, primarily due to inadequate study design to detect longitudinal effects (two measurements 2 years apart for Starr and colleagues, 2007 and 6 years apart for Emery and colleagues, 1998). Risk of bias at the study and outcome level for these two studies was also medium, primarily due to issues with control of important confounds, insufficient reporting of attrition, study design flaws, and/or insufficient or absent reporting of effect sizes and other key statistics (e.g., Emery and colleagues, 1998, do not include information on smoking status, they are trying to fluid and/or crystallized cognitive change in short period of time [6 years] in a sample with a wide age range [baseline mean = 62.3 ± 7.7, range = 40–84]; Starr and colleagues, 2007, examine the contribution of smoking status to cognition performance after accounting for pulmonary function testing, and height and other health status not included in analyses). The Richards and colleagues (2005) article was rated with low methodological quality primarily due to inadequate follow-up (with two measurement occasions of pulmonary function and only one measurement of cognitive performance), poor data reporting that does not include details of attrition, effect sizes and other key statistics, and unusual application of statistical analyses (essentially with baseline pulmonary function at age 43 was analyzed in association cognitive performance at age 43 and 53, but change in pulmonary function from age 43 to 53 was associated with cognitive ability at age 15). Similarly, this article was rated with medium risk for bias at the study level and high risk for bias at the outcome level (due to the already stated study design and statistical methodological limitations).
Keeping methodological quality and risk for bias in mind, these three studies present support for associations between baseline pulmonary and cognitive abilities that are more fluid than crystallized, with Starr and colleagues (2007) noting higher PEF was significantly correlated with better fluid cognitive performance. Evidence was limited to support associations between pulmonary function baseline or change with cognitive change.
Studies Meeting Partial Inclusion Criteria: Mixed Single and Multiple Measurements of Pulmonary Function and Cognition (“Mixed-Wave” Studies)
Seven publications reported baseline measures of one variable (either pulmonary function or cognition) and serial (two or more) measures of the other (Aiken-Morgan, Gamaldo, Wright, Allaire, & Whitfield, 2018; Infurna & Gerstorf, 2013; Koster et al., 2005; Pathan et al., 2011; Swan, LaRue, Carmelli, Reed, & Fabsitz, 1992; Vidal et al., 2013; Whitfield et al., 1997; see Table 2). All data came from studies of aging not previously reported on in our review of longitudinal studies and two-wave studies and sample sized ranged from 224 to 10,975. Overall, five publications reported baseline pulmonary function with two measurement occasions of cognition (Aiken-Morgan et al., 2018; Infurna & Gerstorf, 2013; Koster et al., 2005; Swan et al., 1992; Whitfield et al., 1997) and one reported baseline pulmonary function with 2–4 measurement occasions of cognition (Pathan et al., 2011). Finally, one publication longitudinally measured pulmonary function (three occasions) and with a single cognition measurement on the final occasion (Vidal et al., 2013). Cognition was measured by one or more psychometric tests and/or verified diagnosis of mild cognitive impairment or dementia. Measures of pulmonary function included FEV1, FVC, and PEF, with seven publications using one pulmonary function measure and two publications using two or more.
Methodological quality (Table 3 and Supplementary Table 3) was rated low in four studies and medium in three studies. Further, risk of bias at the study level ranged from low to high, whereas risk of bias at the outcome level was medium to high. One of the most significant problems resulting in poorer ratings was exclusion of important confounds (height, smoking, and/or other health status in Aiken-Morgan et al., 2018; Infurna & Gerstorf, 2013; and Koster et al., 2005), inadequate follow-up (Whitfield et al., 1997 with one pulmonary and two cognitive waves, 2 years apart; Aiken-Morgan et al., 2018 with one pulmonary and two cognitive waves, 3 years apart; Pathan et al., 2011 with one pulmonary and three-to-four cognitive waves, over 14 years; Infurna & Gerstorf, 2013 and Koster et al., 2005 with one pulmonary and two cognitive waves, 4 years apart; Swan et al., 1992 with one pulmonary and two cognitive waves, 5 years apart; and Vidal et al., 2013 with one cognitive and two-to-three pulmonary waves). Further, contributes to poorer ratings were also affected by issues with data reporting (all of these studies except Infurna and Gerstorf, 2013, do not adequately attrition, effect sizes, and other key statistics) and data analysis (e.g., particularly Pathan et al., 2011 and Swan et al., 1992 with very unconventional approaches that analyze variations of the same data in multiple ways). The two mixed-wave studies with the best study quality (medium) and risk for bias (low study bias and medium outcome bias) were those conducted by Pathan and colleagues (2011) and Whitfield and colleagues (1997) that were most limited by inadequate follow-up and attrition (but did control for important confounds and had adequate analytical methods).
Considering poorer methodological quality and higher risk for bias, these mixed-wave studies best supported associations between baseline pulmonary function and cognitive performance. Five studies reported results broadly suggestive of low pulmonary function associations with cognitive decline or development of dementia (Aiken-Morgan et al., 2018; Infurna & Gerstorf, 2013; Koster et al., 2005; Swan et al., 1992; Whitfield, et al., 1997). Pathan and colleagues (2011) reported baseline concordant pulmonary and cognitive associations, but no associations between baseline pulmonary function and cognitive decline. In the only publication measuring pulmonary function over time (Vidal et al., 2013), there were baseline but no longitudinal associations between pulmonary function and cognitive performance, diagnosis of mild cognitive impairment, or diagnosis of dementia.
Discussion
In examining the current literature on longitudinal associations between pulmonary function and cognition, only four studies used adequately longitudinal methods with three or more measurement occasions. Expansion of review criteria allowed us to identify three additional studies using two pulmonary and cognitive measurement occasions, as well as seven studies using mixes of one pulmonary or cognitive measurement occasion with two or more measurement occasions of the other. Of these 14 studies combined, only one had high methodological quality and low risk for bias at both the study and outcome levels (MacDonald et al., 2011). Broadly, the most prevalent problems in the relatively higher quality studies with relatively less bias were issues with inadequate follow-up, inadequate inclusion of some important confounds, incomplete data reporting, and problems with attrition. Problems in relatively lower quality studies with relatively more bias tended to include study design and analysis problems, inadequate inclusion of many confounds, inadequate follow-up, and data reporting issues.
The most reliable finding across all studies is the support for a concordant cross-sectional association between pulmonary function and cognition (i.e., higher pulmonary function is associated with better cognitive performance and lower pulmonary function is associated with poorer cognitive performance) in mid-life and older adults. Despite substantial apparent support for correlational associations between pulmonary function and cognition documented throughout the literature, this systematic review shows there is very limited research thoroughly investigating their longitudinal associations, and there is currently little evidence to substantiate claims of longitudinal associations between pulmonary function and cognition.
Although the strength of this study lies in our rigorous evaluation of the longitudinal studies of pulmonary function and cognition, numerous limitations were also encountered. First, only four studies met the original inclusion criteria. This is perhaps the most important finding of our systematic review. Many studies (such as those reported in the two-wave and mixed-wave portions of this review) are often cited in the literature as providing support for a longitudinal association between pulmonary function and cognition. However, expanding the review criteria demonstrated that few studies (n = 3) even meet the lenient revised criteria of using two waves of cognitive pulmonary data. Similarly, only a handful (n = 7) of studies meet the even more lenient review criteria of using one cognitive or pulmonary wave of data with two or more waves of the other (five of the seven using only two data waves). Overall, studies with only two time points may not have sufficient within-person information to estimate rate of change reliably. In addition, examination of baseline level of one variable in relation to longitudinal change in another cannot adequately inform us of dynamic relationships over time. These two issues have been identified elsewhere as a feature of the broader literature examining longitudinal associations between cognition and other physical functioning biomarkers (Spiro & Brady, 2008).
Because this systematic review was designed to identify studies containing longitudinal data of both pulmonary function and cognition, it is likely that we did not capture all of the published studies that could potentially fit within the two-wave or the mixed-wave categories of studies. We also acknowledge the possibility that our procedures may not have identified some other relevant articles. For example, Marioni and colleagues (2015) and Harris and colleagues (2016) included both longitudinal measures of cognition and pulmonary function in their studies of telomere length and aging. Although we excluded these studies because they did not examine cognition and pulmonary function in relation to one another (and thus associations between the two cannot be ascertained with the data provided in the article), other studies may passively report such associations but were missed by our search terms due to emphasis on different outcome measures. Despite these limitations, however, the current review is still able to demonstrate key strengths and weaknesses within this body of literature (i.e., evidence for cross-sectional associations and limited evidence for longitudinal associations).
Even though cognition and pulmonary function are common outcomes in longitudinal studies of aging around the world, this systematic review incidentally highlights how their operationalization and analysis vary greatly across studies. Covariates known to play important roles in cognitive and/or pulmonary functioning such as sex, education, height, and health (e.g., body mass index, cardiovascular disease, diabetes, and smoking) were not consistently considered across studies and often frankly omitted. In addition, continuous variables such as age and FEV1 were sometimes modeled in a categorical manner (e.g., quartiles or multi-year age groups) and assessment and analysis of cognitive data was often quite restricted. Finally, even in our small sample of reviewed articles, longitudinal pulmonary outcomes differed somewhat and cognitive outcomes differed immensely, limiting our ability to make informative comparisons. This issue is hardly limited to our review, as inconsistency between outcome measures across studies of cognitive and physical aging remains a barrier to harmonization of research. Thus, future efforts to address these issues should include more systematic study of outcome measures and covariates, as well as application of the same analytic approach across multiple datasets. Similarly, studies would also benefit from the inclusion of multiple measures within the same domain (e.g., PEF, FEV1, and FVC to measure pulmonary function) as there is evidence to indicate that these measures have different sensitivity and rates of decline at different points in the lifespan, and this may or may not be differentially associated with various cognitive outcomes (Karlamangla et al., 2009; Vaz Fragoso & Gill, 2012).
Finally, because cognitive and pulmonary functions may share common vulnerabilities to a variety of environmental factors during early child development (and thus later in life), another potential limitation was our narrowing to studies of adults only. Although our cursory literature searches did not uncover any pediatric longitudinal studies of pulmonary function and cognition, other research reports positive association between greater lung function and better cognitive performance in children and adolescents (Suglia, Wright, Schwartz, & Wright, 2008).
Although research remains limited regarding the association between cognition and pulmonary function, as well as which measures of these abilities carry the most importance, there is much future research can do. This study highlights the need for longitudinal data, rigorous methodological design including key covariates, and clear communication of methods and analyses which facilitate replication across samples. Incorporating considerations of these factors into future research on aging-related changes in pulmonary function and cognition will benefit the increasingly important study of healthy and pathological aging.
Funding
This project is part of the Integrative Analysis of Longitudinal Studies of Aging and Dementia (IALSA) network, which is supported by the National Institute on Aging (P01-AG043362). E. C. Duggan was supported by the Vanier Canada Graduate Scholarship through the Natural Sciences and Engineering Research Council of Canada.
Conflict of Interest
The authors have no conflicts of interest to report.
Supplementary Material
References
- Aiken-Morgan A. T., Gamaldo A. A., Wright R. S., Allaire J. C., & Whitfield K. E (2018). Stability and change in cognitive status classification of black older adults. Journal of the American Geriatrics Society, 66, 179–183. doi:10.1111/jgs.15225 [DOI] [PubMed] [Google Scholar]
- Albert M. S., Jones K., Savage C. R., Berkman L., Seeman T., Blazer D., & Rowe J. W (1995). Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging, 10, 578–589. doi:10.1037/0882-7974.10.4.578 [DOI] [PubMed] [Google Scholar]
- Anstey K. J., Windsor T. D., Jorm A. F., Christensen H., & Rodgers B (2004). Association of pulmonary function with cognitive performance in early, middle and late adulthood. Gerontology, 50, 230–234. doi:10.1159/000078352 [DOI] [PubMed] [Google Scholar]
- Brewster P. W., Melrose R. J., Marquine M. J., Johnson J. K., Napoles A., MacKay-Brandt A., … Mungas D (2014). Life experience and demographic influences on cognitive function in older adults. Neuropsychology, 28, 846–858. doi:10.1037/neu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerhan J. R., Folsom A. R., Mortimer J. A., Shahar E., Knopman D. S., McGovern P. G., … Heiss G (1998). Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology, 44, 95–105. doi:10.1159/000021991 [DOI] [PubMed] [Google Scholar]
- Chyou P. H., White L. R., Yano K., Sharp D. S., Burchfiel C. M., Chen R., … Curb J. D (1996). Pulmonary function measures as predictors and correlates of cognitive functioning in later life. American Journal of Epidemiology, 143, 750–756. doi:10.1093/oxfordjournals.aje.a008812 [DOI] [PubMed] [Google Scholar]
- Clouston S. A., Brewster P., Kuh D., Richards M., Cooper R., Hardy R., … Hofer S. M (2013). The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiologic Reviews, 35, 33–50. doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. R., Evans D. A., Scherr P. A., Speizer F. E., Vedal S., Branch L. G., … Taylor J. O (1989). Peak expiratory flow rate in an elderly population. American Journal of Epidemiology, 130, 66–78. doi:10.1093/oxfordjournals.aje.a115324 [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme (CASP) (2018). CASP cohort study checklist [online] Retrieved from www.casp-uk.net Accessed August, 2018.
- Deary I. J., Whalley L. J., Batty G. D., & Starr J. M (2006). Physical fitness and lifetime cognitive change. Neurology, 67, 1195–1200. doi:10.1212/01.wnl.0000238520.06958.6a [DOI] [PubMed] [Google Scholar]
- Dyer C. (2012). The interaction of ageing and lung disease. Chronic Respiratory Disease, 9, 63–67. doi:10.1177/1479972311433766 [DOI] [PubMed] [Google Scholar]
- Emery C. F., Finkel D., & Pedersen N. L (2012). Pulmonary function as a cause of cognitive aging. Psychological Science, 23, 1024–1032. doi:10.1177/0956797612439422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery C. F., Huppert F. A., & Schein R. L (1997). Do pulmonary function and smoking behavior predict cognitive function? Findings from a British sample. Psychology and Health, 12, 265–275. doi:10.1080/08870449708407404 [Google Scholar]
- Emery C. F., Pedersen N. L., Svartengren M., & McClearn G. E (1998). Longitudinal and genetic effects in the relationship between pulmonary function and cognitive performance. The Journals of Gerontology,. Series B: Psychological Sciences and Social Sciences, 53, P311–P317. doi:10.1093/geronb/53B.P311 [DOI] [PubMed] [Google Scholar]
- Finkel D., Reynolds C. A., Emery C. F., & Pedersen N. L (2013). Genetic and environmental variation in lung function drives subsequent variation in aging of fluid intelligence. Behavior Genetics, 43, 274–285. doi:10.1007/s10519-013-9600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Marioni R. E., Martin-Ruiz C., Pattie A., Gow A. J., Cox S. R., … Deary I. J (2016). Longitudinal telomere length shortening and cognitive and physical decline in later life: The Lothian Birth Cohorts 1936 and 1921. Mechanisms of Ageing and Development, 154, 43–48. doi:10.1016/j.mad.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S. M., Berg S., & Era P (2003). Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychology and Aging, 18, 285–305. doi:10.1037/0882-7974.18.2.285 [DOI] [PubMed] [Google Scholar]
- Hofer S. M., Flaherty B. P., & Hoffman L (2006). Cross-sectional analysis of time-dependent data: Mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohort samples. Multivariate Behavioral Research, 41, 165–187. doi:10.1207/s15327906mbr4102_4 [DOI] [PubMed] [Google Scholar]
- Hofer S. M., & Sliwinski M. J (2001). Understanding ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology, 47, 341–352. doi:10.1159/000052825 [DOI] [PubMed] [Google Scholar]
- Infurna F. J., & Gerstorf D (2013). Linking perceived control, physical activity, and biological health to memory change. Psychology and Aging, 28, 1147–1163. doi:10.1037/a0033327 [DOI] [PubMed] [Google Scholar]
- Johnson J. K., Gross A. L., Pa J., McLaren D. G., Park L. Q., & Manly J. J; Alzheimer’s Disease Neuroimaging Initiative (2012). Longitudinal change in neuropsychological performance using latent growth models: A study of mild cognitive impairment. Brain Imaging and Behavior, 6, 540–550. doi:10.1007/s11682-012-9161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla A. S., Miller-Martinez D., Aneshensel C. S., Seeman T. E., Wight R. G., & Chodosh J (2009). Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology, 170, 331–342. doi:10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A., Penninx B. W., Bosma H., Kempen G. I., Newman A. B., Rubin S. M., … Kritchevsky S. B (2005). Socioeconomic differences in cognitive decline and the role of biomedical factors. Annals of Epidemiology, 15, 564–571. doi:10.1016/j.annepidem.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Kraemer H. C., Yesavage J. A., Taylor J. L., & Kupfer D (2000). How can we learn about developmental processes from cross-sectional studies, or can we?The American Journal of Psychiatry, 157, 163–171. doi:10.1176/appi.ajp.157.2.163 [DOI] [PubMed] [Google Scholar]
- Lara J., Cooper R., Nissan J., Ginty A. T., Khaw K. T., Deary I. J., … Mathers J. C (2015). A proposed panel of biomarkers of healthy ageing. BMC Medicine, 13, 222. doi:10.1186/s12916-015-0470-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald S. W., DeCarlo C. A., & Dixon R. A (2011). Linking biological and cognitive aging: Toward improving characterizations of developmental time. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66 (Suppl 1), i59–i70. doi:10.1093/geronb/gbr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni R. E., Shah S., McRae A. F., Ritchie S. J., Muniz-Terrera G., Harris S. E., … Deary I. J (2015). The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. International Journal of Epidemiology, 44, 1388–1396. doi:10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Crapo R., Hankinson J., Brusasco V., Burgos F., Casaburi R., … Wanger J; ATS/ERS Task Force (2005). General considerations for lung function testing. The European Respiratory Journal, 26, 153–161. doi:10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- Min J. Y., Min K. B., Paek D., Sakong J., & Cho S. I (2007). The association between neurobehavioral performance and lung function. Neurotoxicology, 28, 441–444. doi:10.1016/j.neuro.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., & Altman D. G; PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6, e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D., Beckett L., Harvey D., Farias S. T., Reed B., Carmichael O., … DeCarli C (2010). Heterogeneity of cognitive trajectories in diverse older persons. Psychology and Aging, 25, 606–619. doi:10.1037/a0019502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLIB) (2014). Quality assessment tool for observational cohort and cross-sectional studies. Bethesda, MD: National Institutes of Health, Department of Health and Human Services; Retrieved from https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort [Google Scholar]
- Pathan S. S., Gottesman R. F., Mosley T. H., Knopman D. S., Sharrett A. R., & Alonso A (2011). Association of lung function with cognitive decline and dementia: The Atherosclerosis Risk in Communities (ARIC) Study. European Journal of Neurology, 18, 888–898. doi:10.1111/j.1468-1331.2010.03340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., Strachan D., Hardy R., Kuh D., & Wadsworth M (2005). Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine, 67, 602–608. doi:10.1097/01.psy.0000170337.51848.68 [DOI] [PubMed] [Google Scholar]
- Russ T. C., Starr J. M., Stamatakis E., Kivimäki M., & Batty G. D (2015). Pulmonary function as a risk factor for dementia death: An individual participant meta-analysis of six UK general population cohort studies. Journal of Epidemiology and Community Health, 69, 550–556. doi:10.1136/jech-2014-204959 [DOI] [PubMed] [Google Scholar]
- Sachdev P. S., Anstey K. J., Parslow R. A., Wen W., Maller J., Kumar R., … Jorm A. F (2006). Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dementia and Geriatric Cognitive Disorders, 21, 300–308. doi:10.1159/000091438 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2014). Correlates of cognitive change. Journal of Experimental Psychology. General, 143, 1026–1048. doi:10.1037/a0034847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A., Dugravot A., Kauffmann F., Elbaz A., Ankri J., Nabi H., … Sabia S (2011). Association of lung function with physical, mental and cognitive function in early old age. Age (Dordrecht, Netherlands), 33, 385–392. doi:10.1007/s11357-010-9189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro A. III, & Brady C. B (2008)Integrating health into cognitive aging research and theory: Quo vadis? In Hofer S. M. & Alwin D. F. (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives (pp. 260–283). Thousand Oaks, CA: Sage Publications, Inc. doi:10.4135/9781412976589.n16 [Google Scholar]
- Starr J. M., Deary I. J., Fox H. C., & Whalley L. J (2007). Smoking and cognitive change from age 11 to 66 years: A confirmatory investigation. Addictive Behaviors, 32, 63–68. doi:10.1016/j.addbeh.2006.03.020 [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M. S., & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed). New York: Oxford University Press. [Google Scholar]
- Suglia S. F., Wright R. O., Schwartz J., & Wright R. J (2008). Association between lung function and cognition among children in a prospective birth cohort study. Psychosomatic Medicine, 70, 356–362. doi:10.1097/PSY.0b013e3181656a5a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan G. E., LaRue A., Carmelli D., Reed T. E., & Fabsitz R. R (1992). Decline in cognitive performance in aging twins. Heritability and biobehavioral predictors from the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology, 49, 476–481. doi:10.1001/archneur.1992.00530290058012 [DOI] [PubMed] [Google Scholar]
- Vaz Fragoso C. A., & Gill T. M (2012). Respiratory impairment and the aging lung: A novel paradigm for assessing pulmonary function. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 67, 264–275. doi:10.1093/gerona/glr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J. S., Aspelund T., Jonsdottir M. K., Jonsson P. V., Harris T. B., Lopez O. L., … Launer L. J (2013). Pulmonary function impairment may be an early risk factor for late-life cognitive impairment. Journal of the American Geriatrics Society, 61, 79–83. doi:10.1111/jgs.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Glymour M. M., Hu H., Sparrow D., Spiro A. III, Vokonas P. S., & Litonjua A. A (2011). Forced expiratory volume in 1 second and cognitive aging in men. Journal of the American Geriatrics Society, 59, 1283–1292. doi:10.1111/j.1532-5415.2011.03487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield K. E., Seeman T. E., Miles T. P., Albert M. S., Berkman L. F., Blazer D. G., & Rowe J. W (1997). Health indices as predictors of cognition among older African Americans: MacArthur studies of successful aging. Ethnicity and Disease, 7, 127–136. PMID: 9386953. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.