Abstract
Background and aims
Balanced nutrition which can help in maintaining immunity is essential for prevention and management of viral infections. While data regarding nutrition in coronavirus infection (COVID-19) are not available, in this review, we aimed to evaluate evidence from previous clinical trials that studied nutrition-based interventions for viral diseases (with special emphasis on respiratory infections), and summarise our observations.
Methods
A systematic search strategy was employed using keywords to search the literature in 3 key medical databases: PubMed®, Web of Science® and SciVerse Scopus®. Studies were considered eligible if they were controlled trials in humans, measuring immunological parameters, on viral and respiratory infections. Clinical trials on vitamins, minerals, nutraceuticals and probiotics were included.
Results
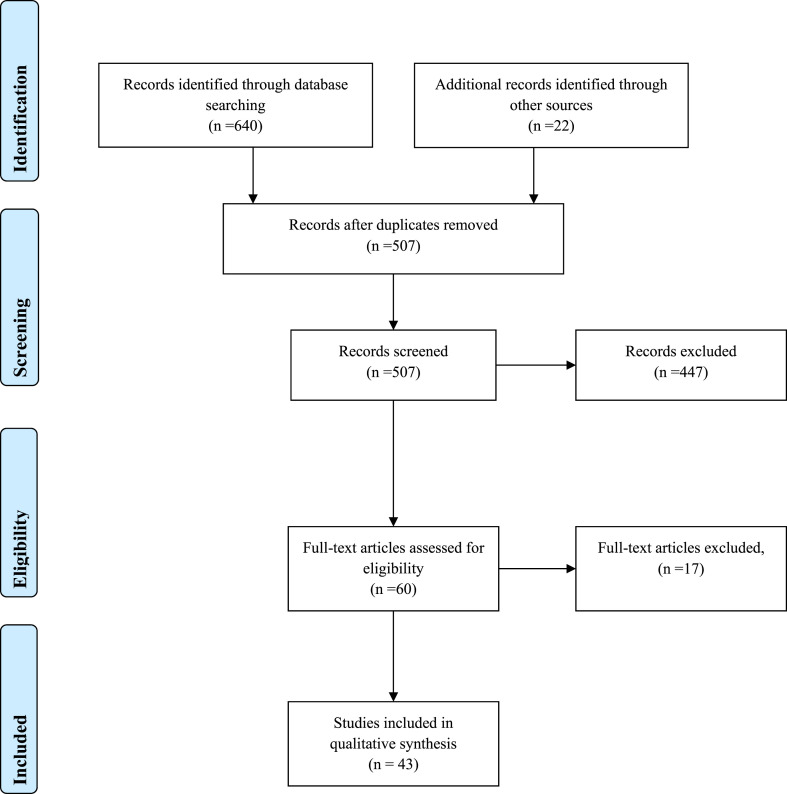
A total of 640 records were identified initially and 22 studies were included from other sources. After excluding duplicates and articles that did not meet the inclusion criteria, 43 studies were obtained (vitamins: 13; minerals: 8; nutraceuticals: 18 and probiotics: 4). Among vitamins, A and D showed a potential benefit, especially in deficient populations. Among trace elements, selenium and zinc have also shown favourable immune-modulatory effects in viral respiratory infections. Several nutraceuticals and probiotics may also have some role in enhancing immune functions. Micronutrients may be beneficial in nutritionally depleted elderly population.
Conclusions
We summaries possible benefits of some vitamins, trace elements, nutraceuticals and probiotics in viral infections. Nutrition principles based on these data could be useful in possible prevention and management of COVID-19
1. Introduction
Considering the current pandemic of COVID-19 where no effective preventive and curative medicine is available, a healthy immune system is one of the most important weapons . There are several vitamins and trace elements which are essential for the normal functioning of the immune system [1]. Furthermore, supplementation of these have shown positive impact on enhancing immunity in viral infections. Vitamin A and D supplementationhas increased the humeral immunity of paediatric patients following influenza vaccination [2]. High dose zinc supplementation has shown immune enhancement in patients with torquetenovirus (TTV) [3]. Similarly, selenium supplementation has shown a positive response after an influenza vaccination challenge [4]. In addition to micronutrients, several herbals and probiotics also have shown effectiveness for treatment and prevention of viral infections [5]. Moreover, several nutraceuticals and probiotics have also shown a supportive role in enhancing immune responses [6,7].
Malnutrition increases morbidity, mortality, and causes significant economic impact on the health care systems, while the economic situation of a country influences all aspects of optimal nutrition care [8]. The increased risk of morbidity and mortality caused by malnutrition is a result of the increased rate of infections, as well as by delayed recovery. Furthermore, infections increase the demand for several nutrients [9]. It is well-recognized that nutrition is a crucial factor in modulating immune homeostasis. Protein-energy malnutrition or even subclinical deficiencies of one micronutrient may impair one’s immune responses [10]. Recently, Calder et al. has highlighted the importance of optimal nutritional status to protect against viral infections [11] and Wu et al. has provided nutritional advices to reduce damages to the lungs from coronavirus and other lung infections [12]. Acknowledging both these valuable reviews, we used a systematic searching strategy and evaluated the highest quality evidence from clinical trials for both the prevention and treatment of viral diseases by means of nutritional interventions. Priority has been given for supplementation of vitamins, trace-elements, nutraceuticals and probiotics.
In the light of the current pandemic of COVID-19, we wanted to evaluate the evidence on enhancing immunity in viral infections. Hence, this review mainly focuses on, influenza-like viral infections; however, other studies on viral infections have also been included. Finally, practical recommendations have been drawn on both preventive and therapeutic nutritional interventions for COVID-19.
2. Methods
This was conducted using a systematic search strategy and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. PRISMA checklist is attached as a supplementary material.
2.1. Search strategy
A comprehensive search of the literature was conducted in the following databases; PubMed® (U.S. National Library of Medicine, USA), Web of Science® (Thomson Reuters, USA) and SciVerse Scopus® (Elsevier Properties S.A, USA) for studies published until 23rd March 2020. The search strategy is shown in Table A as a supplementary material. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible studies. All citations were imported into a bibliographic database (EndNote X8; Thomson Reuters) and duplicates were removed. This search process was conducted independently by two reviewers (RJ and PR) and the final group of articles to be included in the review was determined after an iterative consensus process.
2.2. Study selection, data extraction and quality assessment
Title, abstract and then full-text of all articles were screened for eligibility. Studies were considered eligible for data extraction if they met the following inclusion criteria: RCTs in humans, measuring immunological parameters, on viral infection and respiratory infections, and only articles in English language. We excluded interventional studies conducted on HIV patients, due to large body of evidence and not being directly related to respiratory viral infections and studies on infants. Data were extracted from the included articles by one author (PS) by using a standardized form. A second author checked the accuracy of the data extracted (RJ), and discrepancies were corrected by the involvement of a third author where necessary (PR). The following information was extracted from each study: a) details of the study (study setting, year of publication and study design), b) study population, sample size (male/female) and age of the subjects in years, c) primary intervention(s) and control group and d) details of the main antiviral/immunological outcomes reported. Outcomes evaluated were classified as a) Clinical response – incidence of disease, duration, severity and symptoms or b) Immunological response – cellular changes, serological response, and other relevant immunological phenomenon. These are reported in the relevant tables for vitamins, minerals, nutraceutical and probiotics, for comparison of similar outcomes between interventions. The Jadad scale (0–5, where <3 indicates poor quality) was used to assess the methodological quality of the trials included in the review [14]. The Jadad scale score of each included study is reported in respective tables.
3. Results
A diagram showing the details of studies included is shown in Fig. 1 . A total of 640 recorded were identified initially from PubMed, Scopus and Web of Science databases. In addition, 22 studies were included from other sources. After excluding duplicates and articles that did not meet the inclusion criteria, we obtained 60 articles with full-texts which were read for further evaluation, where another 17 were excluded as irrelevant. Overall, we included 43 articles of which 13 were on vitamins, 8 on minerals, 18 on nutraceuticals and 4 on probiotics.
Fig. 1.
Flow of information through the different phases of a systematic review.
3.1. Vitamins and multi-nutrient supplements
A summary of RCTs on vitamins and multi-nutrient supplements that are discussed below is presented in Table 1 .
Table 1.
Immunological effect of Vitamins and multi-nutrients.
| Author; Published Year; Country |
Nutrient | Study design; Duration; Jadad score |
Study population; Sample size (I/C); Male/Female; Age (years) |
Intervention; Control; Dose/Frequency |
Purpose | Significant anti-viral outcome |
|---|---|---|---|---|---|---|
| Siddiqui et al. [16] 2001; Pakistan |
Vitamin A | C; 30 days; 0 points |
Healthy participants; 20/20; 30/10; 10–35 |
IG: Vitamin A (100000 IU on 1st vaccine day and 100000 IU on the following day) CG: No placebo Both groups received anti-rabies vaccine |
Study the role of Vitamin A in enhancing humoral immunity produced by anti-rabies vaccine | Clinical: NA Immunological: IG group had significantly greater (2.1 times) serum anti-rabies titre than CG. |
| Patel et al. [2] 2019; USA |
Vitamin A and Vitamin D | R, DB, PC; 28 days; 2 points |
Healthy children; 39/40; 33/46; 2–8 |
IG: Oral gummy (Vitamin A 20,000 IU and Vitamin D 2000 IU), on days 0 and 28 CG: Oral gummy placebo, on days 0 and 28 Both groups received influenza vaccine |
Study benefit of vitamin A&D supplements on humoral immune responses following paediatric influenza vaccination | Clinical: NA Immunological: Higher antibody responses among children who entered the study with insufficient or deficient levels of RBP and 25-hydroxyvitamin D. |
| Abu-Mouch et al. 2011 [24]; Israel |
Vitamin D | R, C; 48 weeks; 1 point |
Chronic hepatitis C (HCV) patients; 36/36; 39/33; 18–65 |
IG: Vitamin D3 (2000 IU/day) with antiviral therapy CG: Antiviral therapy alone |
Determine whether adding Vitamin D improves HCV response to antiviral therapy | Clinical: Similar in both groups. Immunological: Significantly more IG patients were HCV-RNA negative (at week 4, 12 and 24). Vitamin D supplementation was strongly and independently associated with sustained virological response in multivariate analysis. |
| Aglipay et al. [21] 2017; Canada |
Vitamin D | R, DB, C; 4–8 months; 5 points |
Healthy children; 349/354; 404/296; 1–5 |
IG: Vitamin D3 high dose (2000 IU/day) CG: Vitamin D3 standard dose (400 IU/day) |
Compare effects of high-dose vs. standard-dose vitamin D supplementation on prevention of viral upper respiratory tract infections among children | Clinical: No significant difference in incidence of wintertime upper respiratory tract infections in IG compared to CG Immunological: NA |
| Ginde et al. [23] 2017; USA |
Vitamin D | R, DB, PC; 12 months; 5 points |
Elderly participants; 55/52; 45/62; ≥60 |
IG: High-dose group (Vitamin D3 100,000 IU/month) CG: Standard-dose group (A Placebo, for participants taking 400–1000 IU/day as part of usual care or 12000 IU of vitamin D3/month, for participants taking <400 IU/day as part of usual care) |
Evaluate efficacy of high dose monthly Vitamin D for prevention of acute respiratory infection in older long-term care residents | Clinical: IG had significantly lesser number of acute respiratory infections than CG. Immunological: NA |
| Goncalves-Mendes et al. [22] 2019; France |
Vitamin D | R, DB, PC; 3 months; 5 points |
Elderly participants (Vitamin D deficient); 19/19 Both genders ˃65 |
IG: Vitamin D (6 doses 100,000 IU, 1 vial/15 days) CG: Placebo (6 doses, 1 vial/15 days) Both groups received influenza vaccine |
Study whether Vitamin D supplementation in deficient elderly persons could improve influenza sero-protection and immune response. | Clinical: NA Immunological: IG had a higher TGFβ plasma level in response to influenza vaccination without improved antibody response. Vitamin D seems to direct lymphocyte polarization toward a tolerogenic immune response. |
| Nimer and Mouch [25] 2012; Israel |
Vitamin D | R, C; 24 weeks; 1 point |
Chronic HCV patients; 20/30; 31/19; 18–65 |
IG: Vitamin D3 (2000 IU/day) with antiviral therapy CG: Antiviral therapy alone |
Examine whether vitamin D improved viral response and predicted treatment outcome in patients with HCV genotype 2–3. | Clinical: NA Immunological: Ninety-five percent in IG were HCV RNA negative at week 4 and 12. At 24 weeks sustained virological response was significantly more in IG. Logistic regression analysis identified vitamin D supplement as an independent predictor of viral response. |
| Andreone et al. [29] 2001; Italy |
Vitamin E | R, C; 3 months; 2 points |
Chronic hepatitis B, (HBV) patients; 15/17; NM; I: 37 ± 15 C: 42 ± 14 |
IG: Vitamin E (300 mg twice daily) CG: No treatment |
Study the role of Vitamin E as a treatment for Chronic HBV | Clinical: NA Immunological: Significantly higher complete response, HBV-DNA negativization, alanine aminotransferase normalization observed in IG. |
| Fiorino et al. [30] 2017; Italy |
Vitamin E | R, C; 12 months; 3 points |
Children with chronic HBV; 23/23; 34/12; 2–17 |
IG: Vitamin E (15 mg/kg/day) CG: No treatment |
Evaluate the safety and efficacy of vitamin E for the treatment of paediatric HBeAg-positive chronic HBV | Clinical: NA Immunological: Significantly more patients in IG had anti-HBe seroconversion and a virological response. |
| Hemilä and Kaprio [27] 2008; Finland |
Vitamin E and β-carotene | R, DB, PC; 5–8 years; 3 points |
Male participants (smoked at least 5 cigarettes/day and initiated smoking at ≤ 20 years); 10,784/10,873; Males only; 50–69 |
IG: Three groups a) Vitamin E (α-tocopheryl acetate, 50 mg/day) b) β-carotene (20 mg/day) c) Both vitamin E and β-ca CG: Placebo |
Examine the effects of vita-min E and pneumonia risk in males who initiated smoking at an early age | Clinical: Vitamin E supplementation had no effect on the risk of pneumonia in participants with body weight in a range from 70 to 89 kg. Vitamin E increased the risk of pneumonia in participants with body weight <60 kg and in participants with body weight >100 kg. The harm of vitamin E supplementation was restricted to participants with dietary vitamin C intake above the median. Immunological: NA |
| Meydani et al. [28] 2004; USA |
Vitamin E | R, DB, PC; 12 months; 5 points |
Elderly participants; 231/220; 113/338; ≥65 |
IG: Vitamin E (α-tocopherol, 200 IU) in soybean oil, one capsule/day CG: Placebo (4 IU of vitamin E) in soybean oil, one capsule/day |
Investigate effect vitamin E supplementation on respiratory infections in elderly nursing home residents. | Clinical: IG did not have a statistically significant incidence of lower respiratory tract infections. However, a protective effect was noted on upper respiratory tract infections, particularly the common cold. Immunological: NA |
| Girodon et al. [33] 1999; France |
Multi-nutrient (Trace elements [zinc and selenium sulphide] or vitamins [beta carotene, ascorbic acid, and vitamin E]) | R, DB, PC; 2 years; 4 points |
Elderly participants; 182:180:181/182; 185/540; 5–103 |
IG: Three groups a) Trace element: zinc sulphate and selenium sulphide (Zinc 20 mg, Selenium 100 μg) b) Vitamins: ascorbic acid (120 mg), beta carotene (6 mg), αtocopherol (15 mg) c) Trace element and vitamin CG: Placebo group (calcium phosphate and microcrystalline cellulose) |
Effect of long-term daily supplementation with trace elements or vitamins in immunity and incidence of infections in institutionalized elderly. | Clinical: Correction of specific nutrient deficiencies was observed after 6 months and was maintained for the first year, during which there was no effect of any treatment on delayed-type hypersensitivity skin response. Number of patients without respiratory tract infections during the study was higher in groups that received trace elements. Immunological: Antibody titers after influenza vaccine were higher in groups that received trace elements alone or associated with vitamins, whereas the vitamin group had significantly lower antibody titers. |
| Graat et al. [34] 2002; Netherland |
Multi-nutrient (retinol, beta-carotene, ascorbic acid, vitamin E, cholecalciferol, vitamin K, thiamine, niacin riboflavin, pantothenic acid, pyridoxine, cyanocobalamin, zinc, selenium, iron, copper magnesium, iodine, calcium, phosphor, manganese, chromium, molybdenum and silicium) and Vitamin E | R, DB, PC; 15 months; 5 points |
Elderly participants; 163:164:172/153 Both genders ≥60 |
IG: Three groups a) Multivitamin-Mineral, 2 capsule/day b) Vitamin E (200 mg), 2 capsule/day c) Multivitamin-Mineral Plus vitamin E, 2 capsule/day CG: Placebo (soybean oil), 2 capsule/day |
Study the effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly. | Clinical: Neither daily multivitamin mineral supplementation at physiological dose nor 200 mg of vitamin E showed a favourable effect on incidence and severity of acute respiratory tract infections in well-nourished non- institutionalized elderly individuals. Immunological: NA |
C – Controlled; CG – Control group; DB – Double blind; DNA – deoxyribonucleic acid; HBV – Hepatitis B virus; HBeAg – Hepatitis B e-antigen; – Hepatitis B HCV – Hepatitis C virus; IG – Interventional group; IU – International units; NA – Not applicable; PC – Placebo controlled; R- Randomized; RNA – Ribonucleic acid; RBP – Retinol binding protein; TGF – Transforming growth factor.
3.1.1. Vitamin A
Vitamin A is a fat-soluble vitamin, which is crucial for maintaining vision, promoting growth and development, and protecting epithelium and mucosal integrity in the body [15]. It is known to play an important role in enhancing immune function, and having a regulatory function in both cellular and humoral immune responses [15]. Vitamin A supplementation to infants has shown the potential to improve antibody response after some vaccines, including measles [15] and anti-rabies vaccination (2.1 times) [16]. In addition an enhanced immune response to influenza virus vaccination has also been observed in children (2–8 years) who were vitamin A and D-insufficient at baseline, after supplementation with vitamin A and D [2].
3.1.2. Vitamin D
Vitamin D, another fat-soluble vitamin, that plays a vital role in modulating both innate and adaptive immune responses [17]. Epidemiological data has linked vitamin D deficiency to increased susceptibility to acute viral respiratory infections [18]. Recent reviews evaluating possible mechanisms suggest that vitamin D plays an important modulatory role of the innate immune responses to respiratory viral infections, such as Influenza A and B, parainfluenza 1 and 2, and Respiratory syncytial virus (RSV) [19]. A systematic review on the role of vitamin D in the prevention of acute respiratory infections, which included 39 studies (4 cross-sectional studies, 8 case-control studies, 13 cohort studies and 14 clinical trials), noted that observational studies predominantly report statistically significant associations between low vitamin D status and increased risk of both upper and lower respiratory tract infections [20]. However, results from RCTs included in the above systematic review were conflicting, possibly, reflecting heterogeneity in dosing regimens and baseline vitamin D status in study populations [20]. Few RCT have been conducted subsequent to the above systematic review. A study by Aglipay et al. on the effect of high-dose (2000 IU/day) vs. standard-dose (400 IU/day) vitamin D supplementation on viral upper respiratory tract infections did not show any significant difference between the two group [21]. However, only about 1/3 of the study population had vitamin D levels <30 ng/ml. A recent RCT on the impact of vitamin D supplementation on influenza vaccine response in deficient elderly person, showed that it promotes a higher TGFβ plasma level without improving antibody production, and suggested that supplementation seems to direct the lymphocyte polarization toward a tolerogenic immune response [22]. Similarly in another RCT, a monthly high-dose (100,000 IU/month) vitamin D supplementation reduced the incidence of acute respiratory infections in older long-term care residents, in comparison to a standard dose group (12,000 IU/month) [23]. It is evident that the role of vitamin D supplementation on antiviral immunity against respiratory infections is likely to depend on the vitamin D status of the individual. Furthermore, vitamin D has demonstrated a beneficial effect in other viral infections, for example adding vitamin D to conventional Peg-α-2b/ribavirin therapy for treatment-naïve patients with chronic HCV genotype 1 infection significantly improved the viral response [24], and a similar effect has also been observed in patients with HCV genotype 2–3 [25].
3.1.3. Vitamin E
Vitamin E, a fat-soluble vitamin, is a potent antioxidant and has the ability to modulate host immune functions [26]. Vitamin E deficiency is known to impairs both humoral and cellular immunity [26]. However, few studies have shown that vitamin E supplementation might cause harmful effects on the incidence of infectious disease. A study among 50–69 years old adult smokers showed that vitamin E supplementation increases the risk of pneumonia [27]. Similarly, supplementation of vitamin E (200 IU/day) did not have a statistically significant effect on lower respiratory tract infections in elderly nursing home residents [28]. However positive effects of vitamin E have been observed in the treatment of chronic hepatitis B in a small pilot RCT, where a significantly higher normalization of liver enzymes and HBV-DNA negativization, was observed in the vitamin E group [29]. Similar results have been observed in a RCT in the paediatric population, where vitamin E treatment resulted in a higher anti-HBe seroconversion and virological response [30].
3.1.4. Vitamin C
Vitamin C is known as an essential antioxidant and enzymatic co-factor for many physiological reactions in the body, such as hormone production, collagen synthesis and immune potentiation [31]. In-vivo animal studies in mice have shown that it is an essential factor for the antiviral immune responses against the influenza A virus (H3N2) through the increased production of interferon-α/β, especially at the early stages of the infection [31]. However, our literature search was unable to identify RCTs examining the use of vitamin C for the treatment for specific viral infections. Furthermore, a systematic review and meta-analysis on the role of vitamin C for preventing and treating the common cold, did not find any conclusive evidence to indicate that there is benefit of using vitamin C mega-dose prophylaxis in the community to reduce the incidence of common cold, which is most often caused by viral infections [32].
3.1.5. Multi-nutrients supplements
As evident from the studies described above, micronutrient deficiency suppresses immune functions by affecting the T-cell-mediated immune response and adaptive antibody response, and leads to dysregulation of the balanced host response [1]. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Supplementations with various combinations of trace-elements and vitamins have shown beneficial effects on the antiviral immune response. A RCT including 725 institutionalized elderly patients, studying delayed-type hypersensitivity skin response, humoral response to influenza vaccine, and infectious morbidity and mortality showed that low-dose supplementation of zinc together with selenium provides an increase to the humoral response after vaccination in comparison to the control group [33]. Antibody titers after influenza vaccine were higher in groups that received trace elements alone or with vitamins, whereas the vitamins only group had significantly lower antibody titers [33]. The number of patients without respiratory tract infections during the study was higher in groups that received trace elements (zinc sulphate and selenium sulfide) [33]. However in another RCT neither daily multivitamin-mineral supplementation nor vitamin E (200 mg/day) showed a favourable effect on incidence and severity of acute respiratory tract infections in well-nourished non-institutionalized elderly [34]. On the contrary, this study noted an increased severity, illness-duration, number of symptoms and restriction of activity in the group supplemented with vitamin E.
3.2. Trace elements
A summary of RCTs on trace element supplements that are discussed below is presented in Table 2 .
Table 2.
Immunological effect of Minerals.
| Author; Published Year; Country |
Nutrient | Study design; Duration; Jadad score |
Study population; Sample size (I/C); Male/Female; Age (years) |
Intervention; Control; Dose/Frequency |
Purpose | Significant anti-viral outcome |
|---|---|---|---|---|---|---|
| Iovino et al. [3] 2018; Italy |
Zinc | R, C; 100 days; 1.5 points |
Patients undergoing autologous stem cell transplantation for multiple myeloma; 9/9; 12/6; 47-72; |
IG: Zinc sulphate 600 mg/day (150 mg of elementary zinc/day) CG: No placebo Both groups received standard therapy |
Investigate a possible therapeutic effect of zinc in improving the immune reconstitution after stem cell transplantation. | Clinical: NA Immunological: CD4+ naïve lymphocytes and T-cell receptor excision circles showed a significant increase only in the IG. Moreover, the load of Torquetenovirus, increased at day +100 only in the CG. |
| Acevedo-Murillo et al. [37] 2019; Mexico |
Zinc | R, TB, PC; Throughout hospital stay; 5 points |
Children with Pneumonia; 50/53; 57/46; 1 (month) – 5 |
IG: Zinc sulphate (10 mg for <1-year-old or 20 mg otherwise) CG: Placebo (glucose, 20 mg) |
Evaluate immunomodulatory effect of zinc supplementation in children with pneumonia younger than 5 years old. | Clinical: Higher improvement in the clinical status, respiratory rate and oxygen saturation was seen IG compared to CG. Immunological: An increase in Interferon-γ (IFNγ) and Interleukin-2 (IL-2) after treatment in the IG was observed. |
| Provinciali et al. [38] 1998; Italy |
Zinc or Zinc plus arginine | R, C; 60 days; 1 point |
Elderly participants; 33:34/31; Both genders; 64–100 |
IG: Two groups a) Zinc sulphate (400 mg/day) b) Zinc sulphate (400 mg/day) with Arginine (4 g/day) CG: No placebo All groups received influenza vaccine |
Evaluate whether oral supplementation with zinc or zinc/arginine increases the antibody response to influenza vaccine or modulates lymphocyte phenotype in elderly subjects. | Clinical: Supplementation increased zinc plasma concentrations restoring the age-related impairment in zinc concentrations. Immunological: The antibody titre against influenza viral antigens was not increased in both IGs in comparison with subjects receiving vaccine alone. The number of CD3, CD4 or CD8 lymphocytes was also not affected in both IGs. |
| Ivory et al. [4] 2017; UK |
Selenium | R, DB, PC; 12 weeks; 5 points |
Healthy participants with suboptimal Selenium (plasma level <110 ng/ml); 84/35; 65/54; 50–64 |
IG: Four groups a) Selenium 50 μg/day b) Selenium 100 μg/day c) Selenium 200 μg/day (a-c as Selenium yeast tablet) d) Selenium 50 μg/day as enriched onions with meals CG: Two groups e) Yeast without added selenium f) Non-enriched onions with meals |
Measure both cellular and humoral immune responses to flu vaccine in healthy older individuals with marginal Selenium status after Selenium supplementation. | Clinical: NA Immunological: Selenium-yeast increased Tctx-Antibody-dependent cellular cytotoxicity cell counts in blood before flu vaccination and a dose-dependent increase in T cell proliferation, IL-8 and IL-10 secretion after in vivo flu challenge. Positive effects were contrasted by lower granzyme B content of CD8 cells. Selenium-onions also enhanced T cell proliferation after vaccination, IFNγ and IL-8 secretion, granzyme and perforin content of CD8 cells but inhibited TNF-a synthesis. Onion on its own reduced the number of NK cells in blood. Mucosal flu-specific antibody responses were unaffected by Selenium supplementation. |
| Broome et al. [40] 2004; UK |
Selenium | DB, PC; 15weeks; 2 points |
Healthy participants; (non-smoking); 44/22; 33/33; 20–47 |
IG: Two groups a) 50 μg of Selenium/day (as sodium selenite) b) 100 μg of Selenium/day (as sodium selenite) CG: Placebo (soybean oil with no selenium) |
Assess whether administration of small selenium supplements to healthy subjects leads to functional changes in immune status and the rates of clearance and mutation of a picornavirus | Clinical: Selenium supplementation increased plasma selenium concentrations and the body exchangeable selenium pool. Immunological: Selenium supplementation increased lymphocyte phospholipid and cytosolic glutathione peroxidase activity. Selenium supplements augmented cellular immune response through an increased production of interferon and other cytokines, an earlier peak T cell proliferation, an increase in T helper cells and more rapid clearance of poliovirus. Humoral immune responses were unaffected. |
| Goldson et al. [41] 2011; UK |
Selenium | R, DB, PC; 12 weeks; 4 points |
Healthy participants (non-smoking); 18:21:23:17:18/20; Both genders; 50–64 |
IG: Five groups a) Selenium 50 μg/day b) Selenium 100 μg/day c) Selenium 200 μg/day (a-c, as enriched yeast) d) Non-enriched onions e) Enriched onions (50 μg/day) (d-e with meals) CG: Placebo |
Determine effect of different doses and forms of Selenium on gene expression of selenoproteins (SEPW1, SEPS1, SEPR) and responses to an immune function challenge (influenza vaccine). | Clinical: NA Immunological: There was a significant increase in SEPW1 mRNA in the Se-enriched onion group compared with unenriched onion group. SEPR and SEPW1 did not change significantly over the duration of the supplementation period in the CG or Selenium-yeast groups, except at week 10 when SEPW1 mRNA levels were significantly lower in the 200 mg/day Selenium-yeast group compared to the CG. Levels of SEPS1 mRNA increased significantly 7 days after the influenza vaccine challenge, the magnitude of the increase in SEPS1 gene expression was dose-dependent, with a significantly greater response with higher Se supplementation. |
| Hawkes et al. [42] 2009; USA |
Selenium | R, DB, C; 48 weeks; 3 points |
Health participants; 42∗ NM; 18–45 |
IG: High selenium yeast tablet (Baker’s yeast with sodium selenite, 300 μg selenium/tablet) CG: Low selenium yeast tablet (Baker’s yeast without sodium selenite, <1.3 μg/tablet) |
Study whether an increased intake of dietary selenium affects immune function | Clinical: Supplementation increased selenium levels by 50%. Immunological: Consumption of the low-selenium yeast induced anergy in delayed type hypersensitivity (DTH) skin responses and increased counts of Natural killer (NK) cells and T lymphocytes expressing both subunits of the high affinity IL2R. DTH skin responses and IL2R + cells did not change in the high-selenium group, suggesting Se supplementation blocked induction of DTH anergy. No differences between groups in other leukocyte phenotypes, serum immunoglobulins, or complement factors. |
| Turnlund et al. [46] 2004; USA |
Copper | C; 148 days; 1 point |
Healthy participants; 9/10; 19:0; 38 ± 7 |
I: Copper with meals 0–18 days - 1.6 mg per day 19–129 days – 7 mg per day 130–148 days - 7.8 mg per day C: No placebo |
Determine the effect of long-term high copper intake on indexes of copper status, oxidant damage, and immune function | Clinical: NA Immunological: Ceruloplasmin activity, benzylamine oxidase, and super-oxide dismutase were significantly higher at the end of the second period than at the end of the first. Polymorphonuclear cell count, the percentage of white blood cells, lymphocyte count, and IL2R were affected by copper supplementation. Antibody titre for the Beijing strain of influenza virus was significantly lower in IG after immunization than in CG. |
∗ Group allocation not mentioned; C – Controlled; CD – Cluster of differentiation; CG – Control group; DTH – Delayed type hypersensitivity; IFN – Interferon; IG – Intervention group; IL – Interleukin; MRU - Metabolic research unit; NK – Natural killer cells; NA – Not applicable; NM – Not mentioned; PC – Placebo controlled; R – Randomized; TB – Triple blind; SEP – selenoproteins; TNF – Tumour necrosis factor.
3.2.1. Zinc
Zinc is an essential trace element which plays an important role in growth, development, and the maintenance of immune function [35,36]. Zinc deficiency has been associated with an increased susceptibility to infectious diseases, including viral infections. Studies have shown that the zinc status of an individual is a critical factor that can influence immunity against viral infections, with zinc-deficient populations being at increased risk of acquiring infections, such as HIV or HCV [35]. Few RCTs have evaluated the effect of zinc supplementation on the immune response. A study by Acevedo-Murillo et al. among 103 children (1 month–5 years) with pneumonia showed a statically significant clinical improvement (duration of illness, respiratory rate and oxygen saturation) in the zinc supplemented group compared to placebo [37]. They also demonstrated an increase in the cytokine response in Th1 pattern (IL-2 and INF-γ) only in the zinc group, with Th2 cytokines (IL-4 and IL-10) being elevated or remaining high in both groups. Another RCT on oral supplementation of high-dose zinc (150 mg/day) after stem cell transplantation, demonstrated that it enhances thymic function and the output of new CD4+ naïve T cells, helping to prevent the reactivation of TTV [3]. However, a study by Provincial et al. concluded that although prolonged supplementation with zinc (400 mg/day) or zinc + arginine (4 d/day) in the elderly (age 64–100 years) restores zinc plasma concentrations, it is ineffective in inducing or ameliorating the antibody response or number of CD3, CD4 or CD8 lymphocytes after influenza vaccination [38].
3.2.2. Selenium
Selenium is another trace element that has a wide range of pleiotropic effects, ranging from antioxidant effects to anti-inflammatory properties [39]. Low selenium status has been associated with an increased risk of mortality, poor immune function, and cognitive decline, while a higher selenium concentration or selenium supplementation has shown antiviral effects [39]. This has been demonstrated in a study by Broome et al., who evaluated whether an increase in selenium intake (50–100 μg/day) improves immune function in adults with marginal selenium concentration [40]. Selenium supplementation increased plasma selenium concentrations, and lymphocyte phospholipid and cytosolic glutathione peroxidase activities, the cellular immune response was increased (increased IFN-γ and other cytokines), with an earlier peak T-cell proliferation, and an increase in T-helper cells. However, humoral immune responses were unaffected [40]. Furthermore selenium supplemented subjects also showed a more rapid clearance of the poliovirus.
A 12-week lasting RCT on healthy adults, with sub-optimal selenium concentration (<110 ng/ml), supplemented with daily capsules of yeast enriched with selenium showed both beneficial and detrimental effects [4]. In this study the immune response to flu vaccine (immune challenge) was assessed in selenium supplemented and control groups. Selenium supplementation resulted in a dose-dependent increase in T-cell proliferation, IL-8 and IL-10. However, positive effects were contrasted by lower granzyme B content of CD8 cells. Furthermore, mucosal flu-specific antibody responses were unaffected by selenium supplementation [4]. A similar RCT lasting 12-weeks showed that selenium supplementation significantly improves selenoprotein W (SEPW1) mRNA, while after an influenza vaccination, a dose dependent increase in selenoprotein S (SEPS1) gene expression was observed [41]. Furthermore, selenium supplementation has also demonstrated effects on the delayed type hypersensitivity (DTH) skin response [42]. In this study low-selenium yeast (control group) induced anergy in DTH skin responses and increased counts of NK cells, while DTH skin responses in the high-selenium (treatment) group were normal, suggesting that selenium supplementation blocked the induction of DTH anergy [42].
3.2.3. Copper
Copper plays a crucial role in immunity by participating in the development and differentiation of immune cells [43]. In-vitro studies have shown that copper demonstrates antiviral properties. For example, thujaplicin-copper chelates inhibit replication of human influenza viruses [44], while intracellular copper has been shown to regulate the influenza virus life cycle [45]. Turnlund et al. conducted a study to determine the effect of long-term high copper intake on indices of copper status, oxidant damage, and immune function [46]. Their results showed that plasma ceruloplasmin activity, benzylamine oxidase, and superoxide dismutase were significantly higher when copper intake was 7.8 mg/day, in comparison to 1.6 mg/day, indicating an improvement in antioxidant status. However, the higher copper intake (7.8 mg/day) significantly reduced the percentage of circulating neutrophils, serum IL-2R and the antibody titer against the Beijing strain of influenza [46].
3.2.4. Magnesium
Magnesium plays an important role in controlling immune function by exerting a marked influence on immunoglobulin synthesis, immune cell adherence, antibody-dependent cytolysis, Immunoglobulin M (IgM) lymphocyte binding, macrophage response to lymphokines, and T helper-B cell adherence [47]. Although some in-vitro and in-vivo studies suggests that magnesium is likely to play a role in the immune response against viral infections [48], our literature search was not able to identify any RCTs that demonstrated a beneficial effect of magnesium supplementation on immunity against viral infections.
3.3. Nutraceuticals supplements
Nutraceuticals are products that claim physiological benefit or protection against a chronic disease. These products may range from isolated nutrients, herbal products, dietary supplements, genetically engineered designer foods, specific diets, and processed foods, such as cereals, soups, and beverages [49]. Some nutraceuticals have shown promising results in enhancing immune function. A very recent study by McCarty et al. reported that certain nutraceuticals may help provide relief to people infected with encapsulated RNA viruses, such as influenza and coronavirus by boosting immune responses [6]. Our study found 18 RCTs conducted on nutraceuticals as shown in Table 3 [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]]. All studies, except the prebiotic study, showed enhanced immune responses after treatment [52].
Table 3.
Immunological effect of Nutraceuticals.
| Author; Published Year; Country |
Nutrient | Study design; Duration; Jadad score |
Study population; Sample size (I/C); Male/Female; Age (years) |
Intervention; Control; Dose/Frequency |
Purpose | Significant anti-viral outcomes |
|---|---|---|---|---|---|---|
| Ahmed et al. [50] 2014; USA |
Polyphenol-enriched protein powder | R, DB, PC; 17 days; 2 points |
Healthy long-distance runners; 16/15; Both genders; 19–45 |
IG: Blueberry–green tea-polyphenol soy protein complex (PSPC) CG: Soy protein isolate, with non-polyphenolic food 40 g/day; 2 doses (20 g/morning, 20 g/lunch) |
Study the protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to viral infection | Clinical: NA Immunological: A significant difference in ability of serum from IG versus CG athletes to protect cells in culture from killing by vesicular stomatitis virus following strenuous exercise. Serum of subjects who ingested PSPC significantly delayed an exercise-induced increase in virus replication. |
| Brull et al. [51] 2016; Netherland |
Plant stanol ester | R, DB, PC; 8 weeks; 3 points |
Asthma patients; 29/29; 16/42; 18–70 |
IG: Plant stanol enriched soy-based yogurts, 4 g CG: soy-based yogurt without added plant stanol esters, 4 g 4 g plant stanols/day |
Evaluate in-vivo the plant stanol esters effect on the immune response in asthma patient | Clinical: NA Immunological: IG showed higher antibody titres against hepatitis A virus post-vaccination. Substantial reductions in plasma IgE, IL-1ß, and TNF-⍺ shown in IG. Increase in serum plant stanol concentrations correlated significantly with decrease in IL-13 and Th1 switch in Th1/Th2 balance. No absolute differences in cytokine production between groups. |
| Bunout et al. [52] 2002; Switzerland |
Prebiotic mixture | R, SB, PC; 28 weeks; 4.5 points |
Healthy elderly participants; 20/23; NM; ≥70 |
IG: Prebiotic mixture (70% raftilose and 30% raftiline), 3 g sachet CG: Maltodextrin powder, 3 g sachet 6 g/day (Two 3 g sachets/day) |
Study the effect of prebiotics on the immune response to vaccination in the elderly | Clinical: NA Immunological: No changes in serum proteins, albumin, immunoglobulins, and secretory IgA. Antibodies against influenza B increased significantly from weeks 0–8, with no significant differences between groups. Antibodies against influenza A did not increase. No effects of prebiotics on IL-4 and INF secretion by cultured monocytes were observed. |
| De Luca et al. [53] 2012; Italy |
Coenzyme Q 10, Vitamin E, Selenium aspartate, and l-methionine | R, DB, PC; 6 months; 2 points |
Patients with HPV skin warts; 36/32; 36/32; I: 31.4 ± 9.7 C: 30.5 ± 9.6 |
IG: Coenzyme Q 10 (12.5 mg), vitamin E (12.5 mg), selenium aspartate (12.5 mg), and l-methionine (50 mg), in soy phospholipids (147 mg) per capsule CG: Soy phospholipids (147 mg) per capsule 4 capsules/day |
Study the ability of a nutraceutical mixture to accelerate recovery and inhibit recurrences of a chronic muco-cutaneous DNA-virus infections | Clinical: The nutraceutical induced significantly faster healing with reduced incidence of relapses as compared to CG Immunological: IG had decreased viral load and increased antiviral cytokine and peroxynitrite plasma levels. Plasma antioxidant capacity was higher in IG versus CG. |
| Elsaid et al. [54] 2018; Egypt |
Arabinoxylan rice bran (Biobran/MGN-3) | R, DB, PC; 1 month; 3 points |
Healthy elderly participants; 6/6; 6/6; ≥56 |
IG: Sachets with Biobran/MGN-3 (500 mg), maltitol (1000 mg), dextrin (200 mg), hydroxypropyl distarch phosphate (280 mg), and tricalcium phosphate (20 mg). CG: Sachets with maltitol (1000 mg), dextrin (200 mg), hydroxypropyl distarch phosphate (780 mg), and tricalcium phosphate (20 mg) 1 sachet per day |
Study whether arabinoxylan rice bran (Biobran/MGN-3) could counteract this decline in NK/NKT cell activity in elderly | Clinical: NA Immunological: IG had no effect on the total percentage of NK cells, however IG had enhanced cytotoxic activity of induced NK cell expression of CD107a, when compared with baseline values and with the CG. |
| McElhaney et al. [55] 2006; Canada |
COLD-fx: Root extract of North American ginseng (Panax quinquefolium) | R, DB, PC; 4 months; 5 points |
Healthy elderly Participants; 22/21; 21/22; ≥65 |
IG: Extract from Panax quinquefolium, containing 90% polyfuranosyl-pyranosyl-saccharides, 200 mg/capsule CG: microcrystalline cellulose, 200 mg/capsule 2 capsules (400 mg/day) every morning |
Study efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults | Clinical: Frequency and duration of acute respiratory infections during the first two months was found similar in both groups. However, during the last 2 months significantly fewer subjects in the COLD-fX group had acute respiratory infections. The duration of symptoms during the last 2 months was significantly shorter in the COLD-fX group. Immunological: NA |
| Moyad et al. [56] 2008; USA |
EpiCor: a dried Saccharomyces cerevisiae fermentate (modified yeast) |
R, DB, PC; 12 weeks; 4 points |
Healthy participants recently vaccinated for influenza; 52/64; 50/66; 18–76 |
IG: EpiCor: A Saccharomyces cerevisiae fermentate, 500 mg/capsule CG: identical placebo, 500 mg/capsules 1 capsule (500 mg/day) every morning |
Determine if EpiCor taken daily reduces the incidence and duration of colds or flu-like symptomatic features in healthy individuals recently vaccinated against seasonal influenza. |
Clinical: Subjects receiving EpiCor experienced a statistically significant reduction in the incidence and duration of colds or flu. Immunological: NA |
| Muller et al. [57] 2016; USA |
Broccoli sprout homogenates (BSH) | R, DB, PC; 4 days; 5 points |
Healthy participants; 13/16; 10/19; I: 25.5 ± 1.5 C: 27.6 ± 1.5 |
IG: BSH – a shake was about 200 g (containing about 111 g of fresh broccoli sprouts) daily CG: Similar dose of alfalfa sprout homogenate (ASH) daily |
Study the effect of Broccoli sprouts and Live Attenuated Influenza Virus (LAIV) on Peripheral blood NK cells | Clinical: NA Immunological: LAIV significantly reduced NKT (day 2 and 21) and T cell (day 2) populations. LAIV Decreased NK cell CD56 and CD158b expression, while significantly increasing CD16 expression and cytotoxic potential (day2). BSH supplementation further increased LAIV-induced granzyme B production (day 2) in NK cells compared to ASH and in BSH group granzyme B levels appeared to be negatively associated with influenza RNA levels in nasal lavage fluid cells. |
| Nabeshima et al. [58] 2012; Japan |
Maoto (multicomponent formulation extracted from four plants: Ephedra Herb, Apricot Kernel, Cinnamon Bark, and Glycyrrhiza Root |
R, C; 5 days; 3 points |
Influenza patients; 10/18 (8:10); 14/14; 20–64 |
IG: Maoto granules 2.5 g three times/day CG: Oseltamivir 75 mg two times/day (n = 8) or Zanamivir 20 mg two times/day (n = 10) |
Compare the efficacy of Maoto with neuraminidase inhibitors in the treatment of seasonal influenza | Clinical: No significant between-group differences were found in total symptom score among three groups. Immunological: Viral persistent rates and serum cytokine levels (IFN-a, IL6, IL-8, IL-10, and TNF-a) during the study period showed no differences among 3 groups. Hence the clinical and virological efficacy of Maoto was similar to neuraminidase inhibitor |
| Nantz et al. [59] 2012; USA |
Aged Garlic Extract (AGE) powder | R, DB, PC; 90 days; 5 points |
Healthy participants; 56/56; 49/63; 21–50 |
IG: AGE capsule 2.56 g/day CG: Placebo capsule 4 capsules/day |
Study the effect of aged garlic extract on immune function and the severity of cold and flu symptoms | Clinical: After 90 days illness diary entries showed that the incidence of colds and flu, a secondary outcome, were not statistically different. However, IG appeared to have reduced severity and a reduction in the number of days and incidences where the subjects functioned sub-optimally and the number of work/school days missed due to illness. Immunological: After 45 days of consuming of AGE, γδ-T cells and NK cells were shown to proliferate better compared to CG. |
| Nantz et al. [60] 2013; USA |
Cranberry polyphenols | R, DB, PC; 10 weeks; 5 points |
Healthy participants; 22/23; 14/31; 21–50 |
IG: Cranberry beverage (cranberry components from juice, filtered water, sugar, natural flavors, citric acid, and sucralose), 450 ml/bottle CG: Placebo beverage (color-Red 40 and Blue 1), calorie-, and sweetener-matched beverage without cranberry components), 450 ml/bottle 1 bottle to be taken through the day |
Evaluate ability of cranberry phytochemicals to modify immunity, specifically γδ-T cell proliferation | Clinical: In the IG, the incidence of illness was not reduced, however significantly fewer symptoms of illness were reported. Immunological: The proliferation index of γδ-T cells in culture was almost five times higher after 10 weeks in IG compared to CG. |
| Negishi et al. [61] 2013; Japan |
Mekabu fucoidan (MF) (a sulphated polysaccharide extracted from seaweed) | R, DB, PC; 4 weeks; 5 points |
Healthy elderly participants; 35/35; 6/64; >60 |
IG: granules with 300 mg of MF and 300 mg of dextrin CG: granules with 600 mg dextrin only Granules mixed with lunch and taken daily |
Study immune response to seasonal influenza vaccination after supplementation of Fucoidan from seaweed | Clinical: NA Immunological: The IG had higher antibody titres against all 3 strains contained in the seasonal influenza virus vaccine than the placebo group. In the IG, natural killer cell activity tended to increase from baseline 9 weeks after MF intake, but not in CG. |
| Rauš et al. [62] 2015; Germany |
Echinaforce Hotdrink (Echinacea purpurea plant extract) | R, DB, C; 10 days; 5 points |
Influenza patients; 203/217; 210/210; 12–70 |
IG: Echinaforce Hotdrink 3 days (5 × 5 ml), followed by (3 × 5 ml) on the following 7 days CG: Oseltamivir 75 mg two times/day for 5 days followed by 5 days of placebo |
Compare a new echinacea formulation with oseltamivir, in the treatment of influenza | Clinical: Recovery from illness was comparable in both groups at days 1, 5 and 10. Non-inferiority was demonstrated for each day and overall. Echinaforce Hotdrink is as effective as oseltamivir in early treatment of clinically diagnosed and virologically confirmed influenza virus infections. Immunological: NA |
| Roman et al. [63] 2013; USA |
AHCC (Active hexose correlated compound), a Basidiomycetes mushroom extract | R, C; 3 weeks; 2 points |
Healthy participants; 14/15; 16/13; I: 60.8 ± 4.0 C: 57.8 ± 5.3 |
IG: AHCC capsule 3 g/day C:G No placebo used |
To study immune response to influenza vaccine with AHCC supplementation. | Clinical: NA Immunological: Flow cytometric analysis of lymphocyte subpopulations revealed that AHCC supplementation significantly increased NKT cells and CD8 T cells post-vaccination compared to CG. Analysis of antibody production 3 weeks post-vaccination revealed that AHCC supplementation significantly improved protective antibody titres to influenza B, while the improvement was not significant in the CG. |
| Thies et al. [64] 2001; UK |
Five types of capsulated oil blends (parallel intervention) Flaxseed oil (ALA), Evening primrose oil (GLA), Arachidonic Acid (AA), docosahexaenoic acid (DHA) and Fish oil (FO) |
R, DB, PC; 12 weeks; 4 points |
Healthy participants; 38(ALA:8; GLA:7; AA: 8; DHA:8; FO:7)/8 24/22; 55–75 |
IG: oils rich in ALA, GLA, AA, DHA, or EPA plus DHA) Each capsule contained 445 mg of the oil blend. 9 capsules/d CG: placebo oil (an 80:20 mix of palm and sunflower seed oils) |
Determine effect of dietary supplementation with oil blends rich in ALA, GLA, AA, DHA, or EPA plus DHA (fish oil) on the NK cell activity of human peripheral blood mononuclear cells | Clinical: NA Immunological: The fatty acid composition of plasma phospholipids changed significantly in the GLA, AA, DHA, and fish oil groups. NK cell activity was not significantly affected by the placebo, ALA, GLA, AA, or DHA treatment. Fish oil caused a significant reduction in NK cell activity that was fully reversed by 4 weeks after supplementation had ceased. |
| Tiralongo et al. [65] 2016; Australia |
Elderberries (Sambucus nigra) | R, DB, PC; 15–16 days; 5 points |
Healthy participants (economy class passengers travelling overseas); 158/154 106/206; ≥18 |
IG: Elderberry capsules (300 mg of elderberry extracts) CG: Placebo capsules priming phase 2 capsules/day (600 mg/day) while travelling and overseas 3 capsules/day (900 mg/day) |
Determine if a standardised membrane filtered elderberry extract has beneficial effects, respiratory, and mental health in air travellers | Clinical: Most cold episodes occurred in the CG; however, the difference was not significant. CG participants had a significantly longer duration of cold episode days and the average symptom score over these days was also significantly higher. A significant reduction of cold duration and severity in air travellers with elderberry capsule. Immunological: NA |
| Yakoot & Salem [66] 2012; Egypt |
Spirulina platensis (cynobacterium) | R, DB, C; 6 months; 5 points |
Chronic hepatitis C patients; 30/29; Both genders; 18–70 |
IG: Spirulina 500 mg dry powder extract capsule CG: Silymarin 140 mg capsule One capsule 3 times/day |
Study effects of Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. | Clinical: NA Immunological: In Spirulina group 4 patients had a complete end of treatment virological response and 2 patients had partial response. However, the difference was not statistically significant at the end of both 6 months. |
| Zunino et al. [67] 2014; USA |
Freeze-dried grape powder (Vitis vinifera) | R, DB, CO; 9 weeks; 4 points |
Obese adults; 24/24; 8/16; 20–50 |
IG: One packet with 46 g of grape powder CG: One packet with 46 g, similar flavour with food starch and tapioca maltodextrin, two potassium salts and silicon dioxide 2 packets/day (morning and night) |
Study influence of dietary grapes on Inflammation in obese adults | Clinical: NA Immunological: No difference was observed for the production of T-cell cytokines between groups. The production of TNF-⍺ was increased in the supernatants from lipopolysaccharide-activated peripheral blood mononuclear cells in IG. A modest increase in the proliferation of the CD8 T-lymphocyte population was observed at 24h post-activation. |
AA – Arachidonic acid; AGE – Aged garlic extract; AHCC – Active hexose correlated compound; ASH - Alfalfa sprout homogenate; BSH – Broccoli sprout homogenates; C – Controlled; CD – Cluster of differentiation; CO – Cross-over; CG – Control group; DB – Double blind; DHA – Docosahexaenoic acid; DNA - Deoxyribonucleic acid; FO – Fish oil; HPV – Human papilloma virus; IG – Interventional group; IgA – Immunoglobulin A; IgE – Immunoglobulin E; IL – Interleukin; INF – Interferon; LAIV – Live attenuated influenza virus; MF – Mekabu fucoidan; NA – Not applicable; NK – Natural killer cells; NM – Not mentioned; PC – Placebo controlled; PSPC - Polyphenol soy protein complex; R – Randomized; SB – Single blind; Th – T helper cells; TNF – Tumour necrosis factor.
3.4. Probiotic supplements
Probiotics are defined as live micro-organisms that confer a health benefit to the host, including on the gastrointestinal tract, when administered in adequate amounts [68]. They also stimulate immune response by increasing the antibody production [69]. The results of a meta-analysis by Kang et al. implied that probiotics have a modest effect in common cold reduction [7]. Our review found 4 studies on probiotics [[70], [71], [72], [73]], where Lactobacillus and Bifidobacterium strains have been used as treatments (Table 4 ). All these studies have found that probiotic supplementation either reduces the severity or shortens the duration of infection. Three of these studies showed the efficacy of Lactobacillus for treatment of respiratory tract infection of viral origin [[70], [71], [72]]. The remaining study highlighted a significant association between Bifidobacterium and increased immune function and intestinal microbiota in elderly [73].
Table 4.
Immunological effect of Probiotics.
| Author; Published Year; Country |
Nutrient | Study design; Duration; Jadad score |
Study population; Sample size (I/C); Male/Female; Age (years) |
Intervention; Control; Dose/Frequency |
Purpose | Significant anti-viral outcome |
|---|---|---|---|---|---|---|
| Akatsu et al. [73] 2013; Japan |
Probiotic Bifidobacterium longum (BB536) |
R, DB, PC; 12 weeks; 5 points |
Elderly fed by enteral tube; 23/22; 13; 32 >65 |
IG: BB536 powder 2 g/sachet CG: Placebo powder (an internal matrix, consisting mainly of dextrin), 2 g/sachet 1 sachet two times/day (4 g/day) |
Study effects of supplementation with Bifidobacterium longum on immune function and intestinal microbiota in elderly | Clinical: NA Immunological: BB536 intake significantly increased cell numbers of bifidobacteria in faecal microbiota. There was a tendency toward an increase of serum IgA in IG compared with CG. BB536 intake did not significantly affect hemagglutination inhibition titres in response to influenza vaccine. NK cell activity decreased significantly in CG but not in IG. |
| Berggren et al. [70] 2011; Sweden |
Probiotic lactobacilli Lactobacillus plantarum HEAL 9 (DSM 15312) and Lactobacillus paracasei 8700:2 (DSM 13434) |
R, DB, PC; 12 weeks; 4 points |
Healthy participants; 137/135; 92/180 18–65y |
IG: Probiotic sachet, Lyophilised lactobacilli and maltodextrin, 1g sachet CG: Placebo powder (maltodextrin), 1 g/sachet 1 sachet/day (1 g/day) |
Investigate whether consumption of probiotic lactobacilli could affect naturally acquired common cold infections in healthy subjects. | Clinical: Incidence of acquiring one or more common cold episode, number of days with common cold symptoms and total symptom score was reduced significantly in IG. Reduction in pharyngeal symptoms was significant. Immunological: Proliferation of B lymphocytes was significantly counteracted in IG in comparison with CG. |
| Boge et al. [71] 2009; France |
Probiotic Actimel® A fermented dairy drink with probiotic strain Lactobacillus casei DN-114001 (CNCMI-1518), combined with ferments in yoghurt, Streptococcus thermophilus and Lactobacillus bulgaricus |
R, DB, C; 13 weeks; 5 points |
Healthy elderly participants; 113/109; 74/148 ≥70y |
IG: Actimel® 100 g/bottle CG: a non-fermented control dairy product 100 g/bottle Two bottle/day (200 g/day) |
Investigate effect of regular consumption of probiotic drink Actimel® on specific antibody responses to influenza vaccination in healthy elderly. | Clinical: NA Immunological: Titres against the influenza B strain increased significantly more in the IG. Significant differences in seroconversion between the groups by intended to treat analysis were still found 5 months after vaccination. |
| de Vrese et al. [72] 2006; Germany |
Probiotic bacteria Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, Bifidobacterium bifidum MF 20/5 |
R, DB, PC; 3 and 5 months (2 winter/spring periods); 3 points |
Healthy participants; 158/153; Both genders; 18–67 |
IG: Tablet with spray dried probiotic 5 × 107 cfu plus vitamins and minerals CG: Tablet with vitamin minerals only 1 tablet/day |
Investigate the effect of long-term consumption of probiotic bacteria on viral respiratory tract infections | Clinical: Intake of probiotic had no effect on incidence of common cold infections, but significantly shortened duration of episodes by almost 2 days and reduced the severity of symptoms. Immunological: IG had a larger increase in cytotoxic T plus T suppressor cell counts and in T helper cell counts. |
BB536 – Bifidobacterium longum 536; CG – Control group; DB – Double blind; IG – Interventional group; IgA – Immunoglobulin A; NA – Not applicable; NK – Natural killer cells; PC – Placebo controlled; R – Randomized.
4. Discussion
To the best of our knowledge, this is the first systematic review reporting nutritional interventions to enhance immunity in viral infections taking into consideration the current epidemic of COVID-19. This comprehensive review reports evidence on several vitamins, particularly A, D and E, as well as few trace elements, such as zinc and selenium. Furthermore, a large number of nutraceuticals and several probiotics have also shown immune enhancing effects for either preventing or treating viral infections, especially influenza-like illnesses.
Several vitamins are essential for the proper functioning of the immune system [1]. A well balanced and varied diet is essential not only to minimize vitamin deficiencies, but also to avoid unnecessary excess consumption or supplementation [74]. According to our findings, vitamin supplementation, especially vitamin D may be beneficial in people who are either deficient or insufficient. Theoretically, vitamin E is a potent antioxidant and has an ability to modulate the host immune functions. However, most of studies in our review reported adverse effects of vitamin E supplementation on the immune response. Similarly, evidence does not support supplementation of vitamin E in cardiovascular disease and cancer prevention. In fact, high-dosage of vitamin E supplementation may increase all-cause mortality. Similar to vitamins, several trace elements are essential for proper immune functions. A disrupted zinc homeostasis affects immune cells by several mechanisms leading to abnormal lymphopiesis, disturbed intercellular communication via cytokines, and poor innate host defense via phagocytosis and oxidative burst [75]. Similarly selenium has a complex immunological mechanism but mainly through its incorporation into selenoproteins [76]. Currently nutraceuticals have received considerable interest for their properties in improve general health, prevent diseases and delay ageing and increase life expectancy [77]. Although cellular mechanism on immunomodulating effects of various nutraceuticals are not well understood, one of the possible mechanism is anti-oxidant and anti-inflammatory activities of nutraceuticals [77]. Our review has reported several beneficial nutraceuticals, however it is important to note that the efficacy and safety of nutraceuticals depend on their ingredients, as well as various other factors including, methods of extraction [78]. Probiotics regulate the functions of systemic and mucosal immune cells and intestinal epithelial cells of the host to regulate immune function [79], but not all probiotics demonstrate similar health benefits [80], therefore, probiotic products should be carefully selected depending on the clinical situation, in order to obtain the relevant beneficial effect.
In addition to micronutrients, obesity has long been associated with higher risks of chronic non-communicable diseases. However, recent evidence suggests that it may also be associated with infectious diseases [81]. Very recent clinical findings of patients with COVID-19 shows severity of the disease is independently associated with BMI ≥28 kg.m-2 (OR, 5.872; 95% CI, 1.595 to 21.621; P = 0.008) [82]. Translational data suggested that an alteration in the metabolic profile of T cells in obese individuals impairs the activation and function of these critical adaptive immune cells [83]. A RCT conducted by El-Kader and Al-Jiffri, in 100 obese patients with chronic HCV infection, identified that the mean values of white blood cells, total neutrophil count, monocytes, CD3, CD4 and CD8 lymphocytes were significantly decreased in the group that underwent a weight loss program in comparison to the control group [84].
A few limitations of this review shall be highlighted; first, a meta-analysis has not been performed due to heterogenicity of studies, especially in relation to reported outcomes. Secondly, we excluded a large quantum of research on supplementation of different nutrients for patients with HIV infection. However, we believe including clinical trials on HIV may dilute the well-timed message of this review, targeting respiratory infection, like COVID-19. Furthermore, quality assessment using the Jadad scale identified 13 studies (<30%) with a score <3 points, indicating poor methodological quality. However, these were not excluded, especially since a meta-analysis was not performed for pooled estimates. Furthermore, another 24 studies (>54%) had a score >3, indicating acceptable/good methodological quality. Finally, although exercise is one of the lifestyle changes that is known to increase immunity and reduces viral infection [85], we consider reviewing the effects of exercise on immune function beyond the scope of the present review. Same applies for other parameters that can alter the immune capacity, e.g. stress [86]. Furthermore, a large quantum of in-vitro and in-vivo animal studies have been conducted on antiviral effects of vitamins, trace elements and nutraceuticals against several viral diseases including influenza virus [87,88]. However, it is difficult to draw conclusions on efficiency and safety or derive recommendations for human use from these studies. Therefore, these require further exploration through well-designed human clinical trials, especially considering the current COVID-19 pandemic. In the absence of specific prophylaxis or vaccination for this viral infection, below recommendation will be helpful for prevention and treatment of patient with COVID-19.
4.1. Recommendations for prevention and treatment of viral infections
Recommendations are summarized in Table 5 . In addition to basic hygienic practices, proper dietary and lifestyle behaviors are essential for prevention and treatment of respiratory viral diseases, such as COVID-19. Everyone including self-quarantine patients are encouraged to follow food based dietary guidelines from their respective national governing bodies, in addition to recommendations given below [89]. For example, everyone should consume at least five portions of fruit and vegetables each day and all main meals should contain starchy carbohydrate preferably a wholegrain variety. Moreover, two to three portions of meat or equivalent (for vegetarians: pulses and other suitable protein rich foods) should be included on a daily basis [89]. However, taking multi-vitamin-mineral (MVM) supplement for a short period at least during this pandemic many be beneficial, since achieving a well-balanced and varied diet is difficult due to several logistics and financial difficulties during lockdowns or self-quarantine. Furthermore, those who are malnourished or at risk of malnutrition should take extra precautionary care to improve their energy, protein and micronutrient levels [90]. Ideally, a trained dietician or nutritionist should prescribe diet, after taking into considering socio-economic factors. In addition to protein and energy malnutrition, the presence of micronutrient deficiency should be identified early and corrected by therapeutic doses of the respective micronutrient. In the absence of the individual micronutrient deficiencies, every malnourished should take a MVM supplement [91]. On the other hand, a patient with excess body weight (BMI>25 kg m−2) should lose at least 5% body weight over a period of 12 weeks to improve their immunity [92]. Patients with diabetes mellitus require a varied and balanced diet to maintain blood glucose and enhance immune functions [93], they should give priority to foods with low glycemic index, limit consumption of high fat and starchy or sugary foods, and choose lean protein variety [93].
Table 5.
Recommendations for prevention or treatment of viral infections.
| Condition/nutrient | Prevention | Treatment | Dose | Food sourcesa |
|---|---|---|---|---|
| Healthy | Follow local food based dietary guideline [89] | Initial nutritional screening using validated nutritional assessment tool (e.g. NRS-2002) and treat accordingly [100] | NA | NA |
| Malnutrition | Those with protein-energy malnutrition require structured dietary advices focusing on increasing calories. Furthermore, they may require MVM [90] | Refer to dieticians/nutritionist. Personalized dietary advices are required with support of ONS and MVM. | NA | NA |
| Obesity | Follow caloric restricted dietary plan covering all major food groups in adequate portions, under health specialist supervision [81,82,92] | Weight loss not advisable [83] | NA | NA |
| Diabetes | Foods with low glycemic index, limit consumption of high fat and starchy or sugary foods, and choose lean protein variety [93] | Refer to dieticians/nutritionist. Personalized dietary advices are required [102,103] | NA | NA |
| Energy intake | No change | Increase by 10% [101] | NA | NA |
| Multi-nutrients | Supplementation may be effective for vulnerable population and those who have poor dietary practices [33,91] | Supplementation may be effective for those who have poor dietary intake before and during the illness [91] | P:1 x RDI T: 1x RDI |
NA |
| Vitamin A | Supplementation may be effective [2,16] | Supplementation may be effective [2,16] | P:5000IU/d T:20000IU/d |
AS: Liver, eggs, milk, cheese V:Dark green leafy vegetables, carrots, mangos, papayas, sweet potatoes |
| Vitamin D | Supplementation may be effective especially those who are deficient and those who are in self-quarantine [21,23,97,98] | Measure serum vitamin status and treat accordingly [22,24,25,98]. | P: 5000IU/d T:10000IU/d |
AS: Oily fish (salmon, sardines), egg yolk, liver V: Mushrooms |
| Vitamin E | Supplementation may be harmful [27,28] | Supplementation may be harmful [29,30,34] | P:NR T:NR |
AS: Eggs, tuna, salmon V: Wheat germ, sunflower seeds, sunflower oil, almonds, peanuts, |
| Vitamin C | Supplementation unlikely to be beneficial [32] | Supplementation may be effective [32] | P:NR T:1 g/d |
AS: liver, oyster V: citrus fruits, guava, strawberries, pineapple, broccoli, tomato, |
| Zinc | Supplementation may be effective [35] | Supplementation may be effective [3,37,38] | P:20 mg/d T:150 mg/d |
AS: Oysters, beef, pork, chicken V: Backed beans, cashews, pumpkin seeds, almonds, peas |
| Selenium | Supplementation may be effective [4,40,41] | Supplementation may be effective [4,42] | P:50 μg/d T:200 μg/d |
AS: Turkey, eggs, pork, chicken, milk V: Brazilnuts, sunflower seeds, Tofu, whole grain cereals |
| Cooper | Supplementation may be effective [46] | Supplementation unlikely to be beneficial | P:1.6 mg/d T:NR |
AS: Oysters, shellfish, organ meats V: wheat-bran cereals, whole-grain products, seeds and nuts |
| Magnesium | Supplementation unlikely to be beneficial | Supplementation unlikely to be beneficial | P:NR T:NR |
AS: salmon, chicken, beef V: Green leafy vegetables, legumes, nuts, seeds, and whole grains |
| Nutraceuticals | Supplementation could be beneficial depending on the ingredient [50,[54], [55], [56], [57],[59], [60], [61],[63], [64], [65],67] | Supplementation could be beneficial depending on the ingredient [51,53,58,62,66] | Depend on the product | Garlic, oily fish, cranberry juices, broccoli sprouts |
| Probiotics | Supplementation could be beneficial depending on the strain [[70], [71], [72]] | Supplementation could be beneficial depending on the strain [7,[70], [71], [72]] | Depend on the product | Yogurt, curd |
Food sources are from USDA database, MVM - Multivitamin/mineral Supplements; ONS - Oral nutritional supplements; RDI: Recommended Daily Intake; NA: Not applicable; NR: Not recommended; AS- Animal sources; V- Vegetarian sources.
Furthermore, it is important to remember that micronutrient deficiencies such as Vitamin D and B12 are well documented in the South Asian countries [94]. Micronutrient deficiencies are highly prevalent even in high-income countries, especially among vulnerable populations such as infants, children, adolescents, during pregnancy and lactation and the elderly [95]. Those who have restricted dietary habits such as food allergies, vegetarian of any subtype and those who have chronic diseases are also at a high risk for micronutrient deficiencies [96]. It is safe to consume MVM on a daily basis to optimize nutritional needs and maintain satisfactory immune function in such circumstances [91]. With regards to the global vitamin D deficiency especially in the population of the northern hemisphere during the winter, supplementation of vitamin D (5000IU/daily) may be effective for both high risk e.g. diabetes and obese individuals, and self-quarantined individuals [97]. Toxicity of vitamin D is rare and moderately high doses (2000–5000 IU/daily) can be taken for years [98]. The common practice of taking high dose of vitamin C and E found to be inefficient to enhance immunity except vitamin E for viral hepatitis [32,34]. Nearly 1/5 of the world’s population is at risk of inadequate zinc intake [99], hence we recommend the supplementation of zinc (20mg/daily) for optimal immune function [11]. Similarly, selenium supplementation (50μg/daily) has shown beneficial effect for enhancing immunity.
Regarding nutraceuticals, many single and combined products have shown effectiveness in enhancing immunity in viral infections including influenza. Depending on the availability; many nutraceuticals can be used to enhance immunity. Among over 20 different products; garlic, oily fish, cranberry juices and broccoli sprouts are relatively readily available options [57,58,60,64]. Probiotics have been effective for improving the immunity in general and Lactobacillus varieties can be recommended to prevent influenza like viral infections [70,71].
Every patient who has been diagnosed with COVID-19 must be screened for malnutrition on admission using a validated nutrition screening tool (e.g. NRS-2002) [100]. In addition to dietary assessment, they are all required to be tested for serum vitamin D levels, and if facilities are available, it is recommended to assess micronutrient deficiencies. According to serum vitamin D levels, deficient or insufficient patients must receive therapeutic doses of vitamin D according to local guidelines [98]. Other vitamin deficiencies also should be treated accordingly. MVM (1xRDI) can be recommended to most patients with viral infections especially those who have poor dietary intake during the illness [91]. Patients receiving intensive care facilities should be treated by a critical care dietician/nutritionist. Furthermore, some patients may need oral nutrition supplement (ONS) to achieve recommended calories and protein intake. Resting energy expenditure increases by 10% during viral infection, which should be considered and energy intake should be increased by 10% during the illness [101]. Among trace elements, zinc (150mg/daily) and selenium (200 μg/daily) supplementation could be beneficial to improve immunity during viral infections [4,37]. Along with proper energy and nutritional intake, several nutraceuticals and Lactobacillus containing probiotics can be supplemented to improve the immunity of the patients with viral infections [70,72]. Furthemore, it has been observed that patients with diabetes have severe disease progression and higher mortality [82] therefore it is recommended to provide a personalized diet with help of qualified nutritionist/dietician and hospital catering system [102]. Gupta et al. recently listed clinical consideration for patients with diabetes with regards to COVID-19 [103].
5. Conclusion
For a viral disease like COVID-19, where no pharmacological strategies for prevention or treatment are presently available and where the exact time of the ending of the alarming situation is unknown, nutritional strategies for enhancing immunity is something to be explored. In addition to treating malnutrition and weight reduction in obese healthy subjects, in this review we have highlighted the potential preventive and therapeutic application of few vitamins, trace elements, several nutraceuticals and probiotics. In the current global context with limited movements, it is difficult to obtain a balanced and varied diet. Therefore, achieving recommended amounts of calories and micronutrient will be a challenge and elective micronutrient supplementations may be beneficial especially for vulnerable populations such as the elderly.
Icmje form
Not relevant since this is a review.
Funding
The authors received no external or internal funding for this study.
Authors’ contributions
RJ devised the conceptual idea. RJ and PR searched databases. RJ, PS and PR were involved in retrieving data. RJ, PR and PS drafted the manuscript. CJ provided immunological inputs. RJ and MC made recommendation; MC revised the manuscript. All authors provided critical feedback on manuscript. All authors read and approved the final manuscript.
Ethics declarations
Not applicable.
Declaration of competing interest
Nothing to declare.
Acknowledgements
To all frontline health workers during this pandemic.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2020.04.015.
Abbreviation
- AA
Arachidonic acid
- AHCC
Active hexose correlated compound
- BB536
Bifidobacterium longum 536
- BSH
Broccoli sprout homogenates
- CD
Cluster of differentiation
- COVID-19
Coronavirus disease 2019
- DHA
Docosahexaenoic acid
- DTH
Delayed type hypersensitivity
- HBeAg
Hepatitis B e-antigen
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- IFN
Interferon
- IL
Interleukin
- IL-2R
Interleukin-2 receptor
- INF
Interferon
- LAIV
Live attenuated influenza virus
- MF
Mekabu fucoidan
- NK
Natural killer cells
- NRS 2002
Nutritional risk screening-2002
- PSPC
Polyphenol soy protein complex
- RBP
Retinol binding protein
- RCT
Randomized control trial
- SEP
Selenoproteins
- TGF
Transforming growth factor
- Th –
T helper cells
- TNF
Tumour necrosis factor
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 2.Patel N. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11(10) doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iovino L. High-dose zinc oral supplementation after stem cell transplantation causes an increase of TRECs and CD4+ naive lymphocytes and prevents TTV reactivation. Leuk Res. 2018;70:20–24. doi: 10.1016/j.leukres.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Ivory K. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36(2):407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mousa H.A. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J Evid Based Complementary Altern Med. 2017;22(1):166–174. doi: 10.1177/2156587216641831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang E.-J. The effect of probiotics on prevention of common cold: a meta-analysis of randomized controlled trial studies. Korean journal of family medicine. 2013;34(1):2–10. doi: 10.4082/kjfm.2013.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis L.J. Costs of hospital malnutrition. Clin Nutr. 2017;36(5):1391–1396. doi: 10.1016/j.clnu.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Rytter M.J. The immune system in children with malnutrition--a systematic review. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2001;85(Suppl 2):S75–S80. doi: 10.1079/bjn2000297. [DOI] [PubMed] [Google Scholar]
- 11.Calder P.C.C., A.C, Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Preprints. 2020:2020030199. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J.Z.,P. 2020. Treatment strategies for reducing damages to lungs in patients with coronavirus and other infections. . Preprints; p. 2020020116. [Google Scholar]
- 13.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad A.R. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z. Role of vitamin A in the immune system. J Clin Med. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui F.Q. The role of vitamin A in enhancing humoral immunity produced by antirabies vaccine. East Mediterr Health J. 2001;7(4–5):799–804. [PubMed] [Google Scholar]
- 17.Aranow C. Vitamin D and the immune system. J Invest Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monlezun D.J. Vitamin D status and acute respiratory infection: cross sectional results from the United States National Health and Nutrition Examination Survey, 2001-2006. Nutrients. 2015;7(3):1933–1944. doi: 10.3390/nu7031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zdrenghea M.T. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1) doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 20.Jolliffe D.A., Griffiths C.J., Martineau A.R. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–329. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Aglipay M. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves-Mendes N. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10(65) doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginde A.A. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Mouch S. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naive patients. World J Gastroenterol. 2011;17(47):5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimer A., Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18(8):800–805. doi: 10.3748/wjg.v18.i8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriguchi S., Muraga M. Vitamin E and immunity. Vitam Horm. 2000;59:305–336. doi: 10.1016/s0083-6729(00)59011-6. [DOI] [PubMed] [Google Scholar]
- 27.Hemila H., Kaprio J. Vitamin E supplementation and pneumonia risk in males who initiated smoking at an early age: effect modification by body weight and dietary vitamin C. Nutr J. 2008;7:33. doi: 10.1186/1475-2891-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meydani S.N. Vitamin E and respiratory tract infections in elderly nursing home ResidentsA randomized controlled trial. JAMA. 2004;292(7):828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreone P. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antivir Res. 2001;49(2):75–81. doi: 10.1016/s0166-3542(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 30.Fiorino S. Vitamin E for the treatment of children with hepatitis B e antigen-positive chronic hepatitis: a systematic review and meta-analysis. World J Hepatol. 2017;9(6):333–342. doi: 10.4254/wjh.v9.i6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune network. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemila H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girodon F. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. Arch Intern Med. 1999;159(7):748–754. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- 34.Graat J.M., Schouten E.G., Kok F.J. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly PersonsA randomized controlled trial. JAMA. 2002;288(6):715–721. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 35.Read S.A. The role of zinc in antiviral immunity. Advances in Nutrition. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Advances in nutrition. 2013;4:176–190. doi: 10.3945/an.112.003210. Bethesda, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acevedo-Murillo J.A. Zinc supplementation promotes a Th1 response and improves clinical symptoms in fewer hours in children with pneumonia younger than 5 Years old. A randomized controlled clinical trial. Frontiers in Pediatrics. 2019;7(431) doi: 10.3389/fped.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provinciali M. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998;27(6):715–722. doi: 10.1093/ageing/27.6.715. [DOI] [PubMed] [Google Scholar]
- 39.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 40.Broome C.S. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 41.Goldson A.J. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomised controlled trial. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0014771. e14771-e14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawkes W.C., Hwang A., Alkan Z. The effect of selenium supplementation on DTH skin responses in healthy North American men. J Trace Elem Med Biol : organ of the Society for Minerals and Trace Elements (GMS) 2009;23(4):272–280. doi: 10.1016/j.jtemb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Li C., Li Y., Ding C. The role of copper homeostasis at the host-pathogen Axis: from bacteria to fungi. Int J Mol Sci. 2019;20(1):175. doi: 10.3390/ijms20010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyamoto D. Thujaplicin-copper chelates inhibit replication of human influenza viruses. Antivir Res. 1998;39(2):89–100. doi: 10.1016/s0166-3542(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 45.Rupp J.C. Host cell copper transporters CTR1 and ATP7A are important for influenza A virus replication. Virol J. 2017;14(1):11. doi: 10.1186/s12985-016-0671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnlund J.R. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am J Clin Nutr. 2004;79(6):1037–1044. doi: 10.1093/ajcn/79.6.1037. [DOI] [PubMed] [Google Scholar]
- 47.Liang R.Y. Magnesium affects the cytokine secretion of CD4(+) T lymphocytes in acute asthma. J Asthma. 2012;49(10):1012–1015. doi: 10.3109/02770903.2012.739240. [DOI] [PubMed] [Google Scholar]
- 48.Chaigne-Delalande B. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science (New York, N.Y.) 2013;341(6142):186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalra E.K. Nutraceutical--definition and introduction. AAPS PharmSci. 2003;5(3) doi: 10.1208/ps050325. E25-E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed M. The protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to virus infection. Phytother Res. 2014;28(12):1829–1836. doi: 10.1002/ptr.5208. [DOI] [PubMed] [Google Scholar]
- 51.Brüll F. Dietary plant stanol ester consumption improves immune function in asthma patients: results of a randomized, double-blind clinical trial1. Am J Clin Nutr. 2016;103(2):444–453. doi: 10.3945/ajcn.115.117531. [DOI] [PubMed] [Google Scholar]
- 52.Bunout D. Effects of prebiotics on the immune response to vaccination in the elderly. J Parenter Enteral Nutr. 2002;26(6):372–376. doi: 10.1177/0148607102026006372. [DOI] [PubMed] [Google Scholar]
- 53.De Luca C. Coenzyme Q(10), vitamin E, selenium, and methionine in the treatment of chronic recurrent viral mucocutaneous infections. Nutrition (Burbank, Los Angeles County, Calif.) 2012;28(5):509–514. doi: 10.1016/j.nut.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Elsaid A.F., Shaheen M., Ghoneum M. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: a randomized, double-blind, placebo-controlled clinical trial. Experimental and therapeutic medicine. 2018;15(3):2313–2320. doi: 10.3892/etm.2018.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McElhaney J.E. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. J Alternative Compl Med. 2006;12(2):153–157. doi: 10.1089/acm.2006.12.153. [DOI] [PubMed] [Google Scholar]
- 56.Moyad M.A. Effects of a modified yeast supplement on cold/flu symptoms. Urol Nurs. 2008;28(1):50–55. [PubMed] [Google Scholar]
- 57.Müller L. Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: a randomized, double-blind study. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0147742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nabeshima S. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J Infect Chemother. 2012;18(4):534–543. doi: 10.1007/s10156-012-0378-7. [DOI] [PubMed] [Google Scholar]
- 59.Nantz M.P. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr. 2012;31(3):337–344. doi: 10.1016/j.clnu.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Nantz M.P. Consumption of cranberry polyphenols enhances human γδ-T cell proliferation and reduces the number of symptoms associated with colds and influenza: a randomized, placebo-controlled intervention study. Nutr J. 2013;12(1):161. doi: 10.1186/1475-2891-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Negishi H. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J Nutr. 2013;143(11):1794–1798. doi: 10.3945/jn.113.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rauš K. Effect of an echinacea-based hot drink versus oseltamivir in influenza treatment: a randomized, double-blind, double-dummy, multicenter, noninferiority clinical trial. Curr Ther Res Clin Exp. 2015;77:66–72. doi: 10.1016/j.curtheres.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roman B.E. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013;33(1):12–17. doi: 10.1016/j.nutres.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Thies F. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n−3 or n−6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73(3):539–548. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- 65.Tiralongo E., Wee S.S., Lea R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: a randomized, double-blind placebo-controlled clinical trial. Nutrients. 2016;8(4) doi: 10.3390/nu8040182. 182-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yakoot M., Salem A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterol. 2012;12(1):32. doi: 10.1186/1471-230X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zunino S.J. Dietary grape powder increases IL-1β and IL-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br J Nutr. 2014;112(3):369–380. doi: 10.1017/S0007114514000890. [DOI] [PubMed] [Google Scholar]
- 68.Sanders M.E. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46(Supplement_2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 69.Kanauchi O. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr Pharmaceut Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berggren A. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011;50(3):203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 71.Boge T. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27(41):5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 72.de Vrese M. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44):6670–6674. doi: 10.1016/j.vaccine.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 73.Akatsu H. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J Parenter Enteral Nutr. 2013;37(5):631–640. doi: 10.1177/0148607112467819. [DOI] [PubMed] [Google Scholar]
- 74.Miller E.R., III Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 75.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nasri H. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med. 2014;5(12):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y.-H. Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality. Sci Rep. 2016;6 doi: 10.1038/srep24253. 24253-24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan F., Polk D.B. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27(6):496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J.-S. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine. 2009;27(7):1073–1079. doi: 10.1016/j.vaccine.2008.11.114. [DOI] [PubMed] [Google Scholar]
- 81.Milner J.J., Beck M.A. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Annals of the American Thoracic Society. 2017;14(Supplement_5):S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abd El-Kader S.M., Al-Jiffri O.H. Impact of weight reduction on selected immune system response among Hepatitis C virus Saudi patients. Afr Health Sci. 2018;18(2):417–427. doi: 10.4314/ahs.v18i2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37(4):157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morey J.N. Current directions in stress and human immune function. Current opinion in psychology. 2015;5:13–17. doi: 10.1016/j.copsyc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bouvier N.M., Lowen A.C. Animal models for influenza virus pathogenesis and transmission. Viruses. 2010;2(8):1530–1563. doi: 10.3390/v20801530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bodewes R. In vitro assessment of the immunological significance of a human monoclonal antibody directed to the influenza a virus nucleoprotein. Clin Vaccine Immunol : CVI. 2013;20(8):1333–1337. doi: 10.1128/CVI.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Who . Regional office; Europe: 2020. Food and nutrition tips during self-quarantine. [Google Scholar]
- 90.Bda COVID-19/coronavirus - advice for the general public. 2020 27/03/2020 29/03/2020] https://www.bda.uk.com/resource/covid-19-corona-virus-advice-for-the-general-public.html AvailaCble from:
- 91.Ward E. Addressing nutritional gaps with multivitamin and mineral supplements. Nutr J. 2014;13 doi: 10.1186/1475-2891-13-72. 72-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santos M.S. Immunological effects of low-fat diets with and without weight loss. J Am Coll Nutr. 2003;22(2):174–182. doi: 10.1080/07315724.2003.10719291. [DOI] [PubMed] [Google Scholar]
- 93.Idf COVID-19 and diabetes. 2020 9/4/2020 11/4/2020] https://www.idf.org/aboutdiabetes/what-is-diabetes/covid-19-and-diabetes.html Available from:
- 94.Gopalan H.S., Misra A., Jayawardena R. Nutrition and diabetes in south Asia. Eur J Clin Nutr. 2018;72(9):1267–1273. doi: 10.1038/s41430-018-0219-6. [DOI] [PubMed] [Google Scholar]
- 95.Bruins M.J. Considerations for secondary prevention of nutritional deficiencies in high-risk groups in high-income countries. Nutrients. 2018;10(1):47. doi: 10.3390/nu10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirby M., Danner E. Nutritional deficiencies in children on restricted diets. Pediatr Clin. 2009;56(5):1085–1103. doi: 10.1016/j.pcl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 98.Marcinowska-Suchowierska E. Vitamin D toxicity-A clinical perspective. Front Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00550. 550-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0050568. e50568-e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reber E. Nutritional risk screening and assessment. J Clin Med. 2019;8(7):1065. doi: 10.3390/jcm8071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kosmiski L. Energy expenditure in HIV infection. Am J Clin Nutr. 2011;94(6):1677S–1682S. doi: 10.3945/ajcn.111.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou J., Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216. doi: 10.1016/j.metabol.2020.154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta R. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes & Metabolic Syndrome. 2020;14(3):211. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.