Abstract
BACKGROUND
Recurrent pregnancy loss (RPL) occurs in 1–3% of all couples trying to conceive. No consensus exists regarding when to perform testing for risk factors in couples with RPL. Some guidelines recommend testing if a patient has had two pregnancy losses whereas others advise to test after three losses.
OBJECTIVE AND RATIONALE
The aim of this systematic review was to evaluate the current evidence on the prevalence of abnormal test results for RPL amongst patients with two versus three or more pregnancy losses. We also aimed to contribute to the debate regarding whether the investigations for RPL should take place after two or three or more pregnancy losses.
SEARCH METHODS
Relevant studies were identified by a systematic search in OVID Medline and EMBASE from inception to March 2019. A search for RPL was combined with a broad search for terms indicative of number of pregnancy losses, screening/testing for pregnancy loss or the prevalence of known risk factors. Meta-analyses were performed in case of adequate clinical and statistical homogeneity. The quality of the studies was assessed using the Newcastle-Ottawa scale.
OUTCOMES
From a total of 1985 identified publications, 21 were included in this systematic review and 19 were suitable for meta-analyses. For uterine abnormalities (seven studies, odds ratio (OR) 1.00, 95% CI 0.79–1.27, I2 = 0%) and for antiphospholipid syndrome (three studies, OR 1.04, 95% CI 0.86–1.25, I2 = 0%) we found low quality evidence for a lack of a difference in prevalence of abnormal test results between couples with two versus three or more pregnancy losses. We found insufficient evidence of a difference in prevalence of abnormal test results between couples with two versus three or more pregnancy losses for chromosomal abnormalities (10 studies, OR 0.78, 95% CI 0.55–1.10), inherited thrombophilia (five studies) and thyroid disorders (two studies, OR 0.52, 95% CI: 0.06–4.56).
WIDER IMPLICATIONS
A difference in prevalence in uterine abnormalities and antiphospholipid syndrome is unlikely in women with two versus three pregnancy losses. We cannot exclude a difference in prevalence of chromosomal abnormalities, inherited thrombophilia and thyroid disorders following testing after two versus three pregnancy losses. The results of this systematic review may support investigations after two pregnancy losses in couples with RPL, but it should be stressed that additional studies of the prognostic value of test results used in the RPL population are urgently needed. An evidenced-based treatment is not currently available in the majority of cases when abnormal test results are present.
Keywords: recurrent pregnancy loss, investigations, screening tests, diagnostic strategy
Introduction
Recurrent pregnancy loss (RPL), defined as two pregnancy losses prior to 20 weeks from the last menstrual period, occurs in 1–3% of all couples trying to conceive (ESHRE, 2017). Based on available data, there is consensus that women should not undergo extensive evaluation after a single first trimester or early second trimester pregnancy loss, given that these are relatively common and sporadic events with only a modestly increased risk of recurrence (Knudsen et al., 1991; Nybo Andersen et al., 2000; Cohain et al., 2017). In prospective studies, the risk of pregnancy loss increases with each loss from approximately 11% amongst nulligravidae to approximately 40% after three or more losses (Magnus et al., 2019).
Known risk factors for RPL are female age, previous pregnancy losses, parental structural chromosomal abnormalities, uterine anomalies, endocrine disturbances, antiphospholipid syndrome (APS) and inherited thrombophilia (Jauniaux et al., 2006). Even after comprehensive investigations, a cause for RPL is identified in fewer than 50% of couples (Alijotas-Reig and Garrido-Gimenez, 2013). Consequently, the majority of cases remain without a modifiable risk factor (Jaslow et al., 2010). Only female age and number of prior pregnancy losses have been consistently found to be prognostic factors for the majority of patients (ESHRE, 2017). The tests currently performed are often expensive, time-consuming and of uncertain prognostic value (Christiansen et al., 2005). Furthermore, there is no consensus about how many pregnancy losses couples should have experienced before evaluation is warranted, leading to a variety of RPL definitions.
The Royal College of Obstetricians and Gynaecologists defines RPL as three or more consecutive pregnancy losses (RCOG, 2011). The American Society for Reproductive Medicine Practice Committee defines RPL as two or more pregnancy losses confirmed by ultrasound or histology, not necessarily consecutive (ASRM Practice Committee, 2012). The most recent RPL guideline from ESHRE set the definition after a significant debate. It states that RPL could be considered after the loss of two or more pregnancies and stresses the importance of the need for further scientific research, including epidemiological studies on the effect of various RPL definitions on diagnosis, prognosis and treatment (ESHRE, 2017).
Although an evidence-based treatment is lacking for RPL, couples value a plan for the next pregnancy (Musters et al., 2013). Before trying to conceive, couples and clinicians attempt to find an explanation for their pregnancy losses and a treatment that will prevent a recurrence, especially in cases with modifiable risk factors, such as thyroid disorders and APS. This is why most guidelines advise investigations in RPL. However, there is no consensus on when to perform investigations for risk factors in couples with RPL.
There is a clear need for an evidence-based recommendation for when to initiate investigations in RPL. As such, the goal of this study was two-fold: first, to determine whether abnormal test results for factors that are definite or probable risk factors for RPL occur with equal frequency in women with two pregnancy losses versus those who have had three or more pregnancy losses; second, to recommend if investigations for RPL should take place after two or three or more pregnancy losses.
Methods
Search strategy
This review followed the PRISMA guidelines for reporting systematic reviews and meta-analyses (Fig. 1). A medical information specialist (JL) performed a systematic search in OVID MEDLINE and OVID EMBASE from inception to March 11th 2019, using both free text and controlled terms (i.e. MeSH-terms in MEDLINE). A search for RPL was combined with search filters for primary or secondary studies and a broad search for terms indicative of screening, obstetric history, two versus three or more pregnancy losses and the relevance/prevalence of known risk factors (Supplementary Table SI). We cross-checked reference lists and citing articles of identified relevant papers (in Web of Science) and adapted the search in case of additional relevant studies. The bibliographic records retrieved were imported and de-duplicated in ENDNOTE X7 © (Clarivate Analytics, Boston, MA, USA). Authors were contacted for additional details when required.
Figure 1.
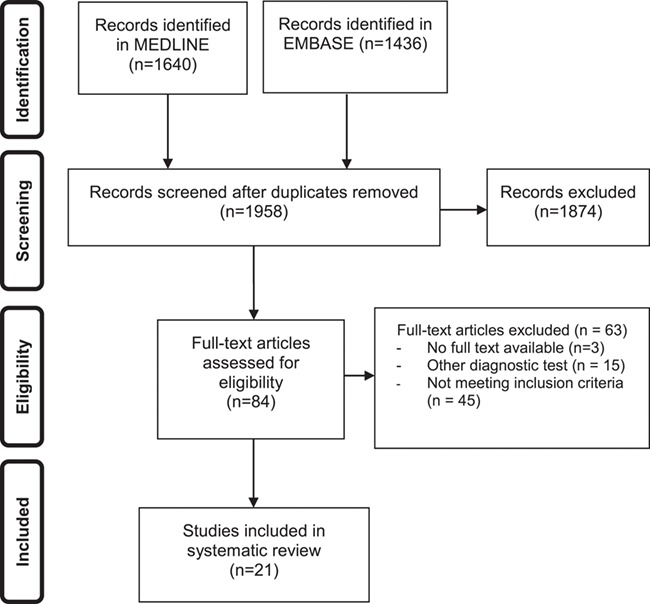
Study selection process for systematic review on the prevalence of abnormal evidence-based test result in women with recurrent pregnancy loss.
Selection criteria
Studies were selected if the prevalence of the abnormal test results for RPL was reported. Only studies which compared women with two pregnancy losses to women with three or more losses were included. Based on current reviews of the literature, the following evidence-based risk-factors for RPL were considered in this review: parental structural chromosomal abnormalities, uterine anomalies, APS, inherited thrombophilia and thyroid disorders. Results of parental chromosomal analysis were considered abnormal if significant rearrangements (e.g. balanced translocations and mosaics) were present. Studies were selected when chromosome analyses were performed with parental peripheral blood lymphocyte cultures. Studies for uterine anomalies were selected if diagnostic testing was performed by hysterosalpingography, hysteroscopy or sonohysterography. Congenital abnormalities (e.g. arcuate uterus, septate uterus, bicornuate uterus and unicornuate uterus) were considered as uterine anomalies.
APS was defined as the presence of thrombosis, pregnancy loss or female morbidity and persistent circulating antiphospholipid antibodies (aPL). aPLs (lupus anticoagulant, IgM anticardiolipin antibodies, IgG anticardiolipin antibodies and beta-2 glycoprotein 1 antibodies) were considered to be present if a test was positive on two occasions >12 weeks apart (Miyakis et al., 2006).
Inherited thrombophilia was defined in four different sub-categories: Factor V Leiden mutation, prothrombin gene mutation, protein S deficiency and protein C deficiency. Factor V Leiden mutation was considered abnormal if there was a heterozygous or homozygous factor V Leiden G1691A mutation found. Prothrombin gene mutation was defined as heterozygous or homozygous mutations for the G20210A prothrombin (factor II) gene. Functional protein C activity less than 70% and functional protein S activity less than 70% were considered abnormal.
Thyroid disorders were defined as serum levels of thyroid-stimulating hormone (TSH) <0.45 mU/L or TSH >4.5 mU/L with an abnormal free thyroxine level with or without the presence of thyroid peroxidase antibodies.
Studies were excluded when the population examined or the diagnostic methods used were not accurately defined. Only publications in English were considered in our selection.
Study selection
Studies were selected in a two-stage process using Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). First, the titles and abstracts from the electronic searches were examined independently by two reviewers (M.D. and A.M.K.), and full manuscripts of all citations that met the predefined selection criteria were obtained. Secondly, examinations of the full manuscripts were carried out to decide on inclusion or exclusion (M.D. and M.W.). In cases of duplicates, the most recent or the most complete publication was used. Any disagreements about inclusion were resolved by consensus or arbitration by a third reviewer (M.G.).
All selected papers were assessed for the following: study design, adequate sampling, adequate description of population characteristics, completeness of information in the data sets, and use of a validated diagnostic method.
Data collection and extraction
Data collection was performed by two reviewers (M.D. and M.W.) independently. Data were extracted based on patients’ characteristics, study quality, inclusion and exclusion criteria, diagnostic tools used and abnormal diagnostic test occurrence rates. Articles were judged on scientific quality according to the Strengthening the Reporting of Observational Studies in Epidemiology statement (White et al., 2015). Levels of evidence were attributed according to the Oxford Centre for evidence-based medicine (Oxford Centre for Evidence-based Medicine, 2009). The quality of each study was assessed with the Newcastle-Ottawa Scale.
Statistical analysis
In order to reach a consistent presentation of the data, all individual study results were translated into an odds ratio (OR) and 95% CI. In case of adequate clinical and statistical homogeneity with the same outcome measure, we performed meta-analyses using a random effect model. Heterogeneity was assessed using the I2 statistic. We took an I2 measurement greater than 50% to indicate substantial heterogeneity. To evaluate the possible presence of publication bias, a funnel plot was made for outcomes with data of at least 10 studies (Cochrane handbook). Review Manager 5 (RevMan version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to perform the meta-analyses.
Results
Of the 1958 publications identified, 21 publications met the inclusion criteria, entailing 8301 couples with RPL. Reference checking of the cited and citing articles of the included articles yielded no additional relevant articles (Fig. 1 shows the PRISMA flowchart of the selection process). Of the 21 articles included in this systematic review, 10 studies reported on chromosomal abnormalities (Michels et al., 1982; Diedrich et al., 1983; FitzSimmons et al., 1983; Schwartz and Palmer, 1983; Sachs et al., 1985; Sider et al., 1988; Goddijn et al., 2004; Jaslow et al., 2010; Bashiri et al., 2012; Asgari et al., 2013), 7 studies reported on testing for uterine anomalies (Weiss et al., 2005; Bohlmann et al., 2010; Jaslow et al., 2010; Souza et al., 2011; Bashiri et al., 2012; Seckin et al., 2012; Jaslow and Kutteh, 2013), 4 studies reported on testing for antiphospholipid syndrome (Jaslow et al., 2010; Bashiri et al., 2012; van den Boogaard et al., 2013; Guzel et al., 2015), 7 studies reported on testing for inherited thrombophilia (Sotiriadis et al., 2007; Jaslow et al., 2010; Bashiri et al., 2012; Karadeniz et al., 2012; Baumann et al., 2013; Ali et al., 2014; Guzel et al., 2015) and 2 studies reported on testing for thyroid disorders (Jaslow et al., 2010; Bashiri et al., 2012).
Quality of the studies
The characteristics of the included articles and quality assessment are reported in Table I and Supplementary Table SII. The studies were evidence-level IIb studies, i.e. cohort studies. Nineteen studies presented appropriate data and could be included in meta-analyses.
Table I.
Characteristics of the studies for chromosomal abnormalities, uterine anomalies, antiphospholipid syndrome, thrombophilia and thyroid disorders identified in a systematic review of RPL.
| Author | Year | Study type | Study population | Prevalence 2 pregnancy losses | Prevalence ≥ 3 pregnancy losses | Outcome measures |
|---|---|---|---|---|---|---|
| Chromosomal abnormalities | ||||||
| Michels et al. | 1982 | Cohort | 122 couples 2 PL n = 48 ≥ 3 PL n = 74 | Balanced translocations 8.4% (4/48) | Balanced translocations 5.4% (4/74) | Cytogenetic analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Diedrich et al. | 1983 | Cohort | 136 couples 2 PL n = 59 ≥ 3 PL n = 77 | Abnormal karyotype 10.2% (6/59) | Abnormal karyotype 11.9% (9/77) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| FitzSimmons et al. | 1983 | Cohort | 645 couples 2 PL n = 340 ≥ 3PL n = 305 | Abnormal karyotype 1.8% (6/340) | Abnormal karyotype 2.3% (7/305) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Schwartz et al. | 1983 | Cohort | 164 couples 2 PL n = 71 ≥ 3 PL n = 93 | Abnormal karyotype 5.6% (4/71) | Abnormal karyotype 5.4% (5/93) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Sachs et al. | 1985 | Cohort | 371 couples 2 PL n = 182 ≥ 3PL n = 189 | Abnormal karyotype 9.3% (17/182) | Abnormal karyotype 9.5% (18/189) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Sider et al. | 1988 | Cohort | 187 couples 2 PL n = 99 ≥ 3PL n = 88 | Abnormal karyotype 3.0% (3/99) | Abnormal karyotype 6.8% (6/88) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Goddijn et al. | 2004 | Cohort | 95 couples 2 PL n = 55 ≥ 3PL n = 40 | Abnormal karyotype 32.7% (18/55) | Abnormal karyotype 37.5% (15/40) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Jaslow et al. | 2010 | Cohort | 561 women 2 PL n = 281 ≥ 3PL n = 280 | Abnormal karyotype 2.8% (8/281) | Abnormal karyotype 5.4% (15/280) | Parental karyotypes showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Bashiri et al. | 2012 | Cohort | 114 couples 2 PL n = 34 ≥ 3 PL n = 80 | Abnormal karyotype (0/34) | Abnormal karyotype 4.0% (4/80) | Parental genetics (significant rearrangements (balanced translocations) showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Asgari et al. | 2013 | Cohort | 140 couples 2 PL n = 65 ≥ 3PL n = 75 | Abnormal karyotype 3.1% (2/65) | Abnormal karyotype 5.3% (4/75) | Chromosomal analysis from peripheral blood lymphocyte cultures showed no significant difference between 2 versus 3 or more pregnancy losses. |
| Uterine anomalies | ||||||
| Weiss et al. | 2005 | Cohort | 165 women 2 PL n = 67 ≥ 3 PL n = 98 | 22.4% (15/67) | 17.3% (17/98) | Identified by hysteroscopy. Considered abnormal were congenital anomalies. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Bohlmann et al. | 2010 | Cohort | 206 women 2 PL n = 78 ≥ 3 PL n = 119 | 9.2% (8/78) | 16.8% (20/119) | Identified by hysteroscopy. Considered abnormal were congenital abnormalities. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Jaslow et al. | 2010 | Cohort | 875 women 2 PL n = 401 ≥ 3PL n = 303 | 18.7% (75/401) | 18.2% (55/303) | Identified by hysterosalpingogram, hysteroscopy, sonohysterography. Considered abnormal were congenital anomalies, fibroids, polyps and septa, Asherman’s syndrome adhesions. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| De Souza et al. | 2011 | Cohort | 66 women 2 PL n = 23 ≥ 3 PL n = 43 | 17.3% (4/23) | 11.6% (5/43) | Identified by hysteroscopy. Considered abnormal was congenital anomalies. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Seckin et al. | 2012 | Cohort | 220 women 2 PL n = 151 ≥ 3 PL n = 69 | 26.5% (40/151) | 30.4% (21/69) | Diagnostic hysteroscopy. Considered abnormal was congenital anomaly. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Bashiri et al. | 2012 | Cohort | 114 women 2 PL n = 38 ≥ 3 PL n = 78 | 31.6% (12/38) | 23.1% (18/78) | Hysteroscopy or 3D ultrasound. Considered abnormal were septate uterus, unicornuate, bicornuate, fibroids, polyps and Asherman’s syndrome. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Jaslow et al. | 2013 | Cohort | 875 women 2 PL n = 389 ≥ 3 PL n = 486 | 6.7% (26/389) | 7.2% (35/486) | Three dimensional sonohysterography. Considered abnormal were congenital and acquired abnormalities. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Antiphospholipid syndrome | ||||||
| Jaslow et al. | 2010 | Cohort | 729 women 2 PL n = 409 ≥ 3 PL n = 320 | 15.6% (64/409) | 13.1% (42/320) | Lupus anticoagulant levels, Anticardiolipin IgG and IgM were measured. No difference was found between 2 versus 3 or more pregnancy losses. |
| Bashiri et al. | 2012 | Cohort | 120 women 2 PL n = 39 ≥ 3 PL n = 81 | 10.3% (4/39) | 13.6 (11/81) | Lupus anticoagulant. No difference in prevalence was found between 2 versus 3 or more pregnancy losses. |
| Van den Boogaard et al. | 2013 | Cohort | 2444 women 2 PL n = 1526 ≥ 3 PL n = 918 | 17.4% (265/1526) | 17.3% (159/918) | Lupus anticoagulant levels, Anticardiolipin IgG and IgM were measured. No difference was found between 2 versus 3 or more pregnancy losses. |
| Thrombophilia | ||||||
| Sotiriadis et al. | 2007 | Cohort | 99 women 2 PL n = 56 ≥ 3 PL n = 43 | 2 PL = 56 | 3 PL = 43 | There was no difference in the distribution of Factor V Leiden, FII G20210A and MTHFR between patients with 2 and 3 or more PLs. |
| Jaslow et al. | 2010 | Cohort | 243 women | Factor V Leiden 4.2% (6/144) Prothrombin gene mutation 2.6% (3/115) Protein S 3.5% (4/115) Protein S 0.9% (1/115) | Factor V Leiden 8.1% (8/99) Prothrombin gene mutation (0/85) Protein S 2.4% (2/85) Protein C (0/85) | Factor V Leiden mutation, prothrombin gene mutation, protein C activity, protein S activity. No difference was found between 2 versus 3 or more pregnancy losses. |
| Bashiri et al. | 2012 | Cohort | 120 women | Factor V Leiden 4.8% (1/21) Prothrombin gene mutation 13.6% (3/22) Protein S 3.8% (1/26) Protein C 7.7% (2/26) | Factor V Leiden 17.0% (8/47) Prothrombin gene mutation 4.5% (2/44) Protein S 13.6% (8/59) Protein C 8.2% (5/61) | Factor V Leiden mutation, prothrombin gene mutation, Protein S activity, Protein C activity. No difference was found between 2 versus 3 or more pregnancy losses. |
| Karadeniz et al. | 2012 | Cohort | 108 women 2 PL n = 42 ≥ 3 PL n = 66 | Factor V Leiden 9.5% (4/42) Prothrombin gene mutation (0/42) Protein S 16.6% (7/42) Protein C 16.6% (7/42) | Factor V Leiden 7.5% (5/66) Prothrombin gene mutation 1.5% (1/66) Protein S 12.2% (8/66) Protein C 18.2% (12/66) | Factor V Leiden mutation, prothrombin gene mutation, Protein S activity, Protein C activity. No difference was found between 2 versus 3 or more pregnancy losses. |
| Baumann et al. | 2013 | Cohort | 641 women 2 PL n = 240 ≥ 3 PL n = 401 | Factor V Leiden 8.3% (20/240) Prothrombin gene mutation 2.9% (7/240) | Factor V Leiden 7.2% (29/401) Prothrombin gene mutation 3.5% (14/401) | Factor V Leiden, prothrombin gene mutation. No difference was found between 2 versus 3 or more pregnancy losses. |
| Ali et al. | 2014 | Cohort | 250 patients 2 PL n = 125 ≥ 3 PL n = 125 | Factor V Leiden (0/23) Prothrombin gene mutation (0/175) Protein S 1.1% (2/175) Protein C 1.1% (2/175) | Factor V Leiden 11.5% (3/26) Prothrombin gene mutation 1.4% (2/140) Protein S 4.3% (6/140) Protein C 4.3% (6/140) | Factor V Leiden mutation, prothrombin gene mutation, protein C activity, protein S activity. No difference was found between 2 versus 3 or more pregnancy losses. |
| Guzel et al. | 2015 | Cohort | 252 women 2 PL n = 72 ≥ 3 PL n = 180 | Protein S deficiency (84.18 ± 11.69) Protein C deficiency (90.91 ± 23.35) | Protein S deficiency (89.02 ± 22.47) Protein C deficiency (106.57 ± 68.79) | Protein S deficiency and protein C deficiency. No difference was found between 2 versus 3 or more pregnancy losses. |
| Thyroid disorders | ||||||
| Jaslow et al. | 2010 | Cohort | 687 women 2 PL n = 396 ≥ 3 PL n = 291 | Abnormal TSH 8.0% (32/396) | Abnormal TSH 6.5% (19/291) | Serum levels of TSH < 0.45 mU/ml or > 4.5 mU/ml |
| Bashiri et al. | 2012 | Cohort | 118 women 2 PL n = 38 ≥ 3 PL n = 80 | Abnormal TSH 2.6% (1/38) | Abnormal TSH 16.3%(13/80) | Serum levels of TSH < 0.45 mU/ml or > 4.5 mU/ml. |
PL, pregnancy loss; TSH: thyroid-stimulating hormone, MTHFR: methylenetetrahydrofolate reductase.
Chromosomal abnormalities
A total of 10 studies (n = 2498) reported on the difference in prevalence of parental structural chromosomal abnormalities in women with two versus three or more pregnancy losses (Table I). When pooling the studies, we found insufficient evidence for a difference in the frequency of abnormal test results for parental structural chromosomal abnormalities between women with two pregnancy losses and three or more pregnancy losses (10 studies, OR 0.78, 95% CI: 0.55–1.10) (Fig. 2).
Figure 2.
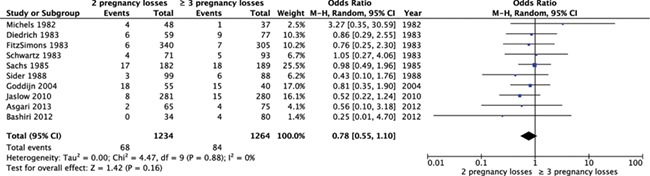
Forest plot of odds ratios of abnormal test results for parental chromosomal abnormalities in women with two pregnancy losses or three or more pregnancy losses.
When summarizing the individual proportions in the studies using meta-analysis, we found a chromosomal abnormality prevalence of 5.3% (95% CI 2.8–7.8) after two pregnancy losses and 6.6% (95% CI 3.8–9.3) after three pregnancy losses. These results indicate that differences in prevalence of chromosomal abnormalities after two or three pregnancy losses might be small, but that larger differences cannot be fully excluded.
The funnel plot did not show an indication of publication bias (Supplementary Fig. SI).
Uterine anomalies
Seven studies described the prevalence of uterine anomalies in women with two pregnancy losses compared to three or more pregnancy losses. Seven cohort studies (n = 2343) were eligible for meta-analysis and no significant difference in frequency of abnormal test results for uterine anomalies could be detected between women with two pregnancy losses and three or more pregnancy losses (seven studies, OR 1.00, 95% CI 0.79–1.27) (Fig. 3). When summarizing the individual proportions in the studies using meta-analysis, we found a prevalence of 18% (95% CI 11–25) after two pregnancies and 17% (95% CI 11–23) after three pregnancies. These results suggest that a clinically relevant difference in prevalence is unlikely.
Figure 3.
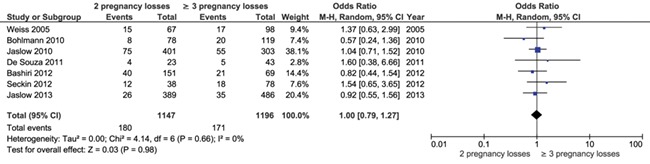
Forest plot of odds ratios of abnormal test results for uterine anomalies in women with two pregnancy losses or three or more pregnancy losses.
Antiphospholipid syndrome
Four included studies described the prevalence of APS in women with two pregnancy losses compared to three or more pregnancy losses. In a retrospective cohort study of 252 women with RPL, the levels of anticardiolipin antibodies IgG and IgM were compared between women with two versus three or more pregnancy losses. The test results of women with two pregnancy losses (n = 72) and three or more (n = 180) were not statistically significant different (anticardiolipin IgG 7.62 ± 2.45 versus 10.01 ± 4.16 GPLU/ml and IgM 4.76 ± 0.69 versus 4.22 ± 0.29 MPLU/ml) (Guzel et al., 2015).
Three studies were appropriate to be included for meta-analysis. No significant difference in frequency of abnormal results for APS was found between women with two pregnancy losses and three or more pregnancy losses (three studies, OR 1.04, 95% CI 0.86–1.25) (Fig. 4).
Figure 4.
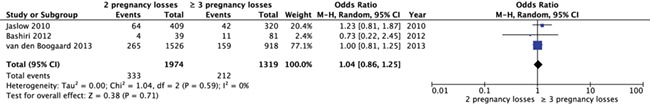
Forest plot of odds ratios of abnormal test results for antiphospholipid syndrome in women with two pregnancy losses or three or more pregnancy losses.
When summarizing the individual proportions in the studies using meta-analysis, we found a prevalence of 16% (95% CI 14–18) after two pregnancy losses and 15% (95% CI 12–18) after three pregnancy losses. These results suggest that a clinically relevant difference in prevalence is unlikely.
Inherited thrombophilia
Seven studies were identified which described the prevalence of inherited thrombophilia in women with two pregnancy losses compared to three or more pregnancy losses. A cohort study compared the prevalence of prothrombin gene mutation and Factor V Leiden mutation in 99 women with two or more pregnancy losses with 102 healthy controls. There was no difference in the distribution of Factor V Leiden and prothrombin gene mutation between patients with two and three or more pregnancy losses (Sotiriadis et al., 2007). In a retrospective cohort study of 252 women with RPL, different diagnostic tests were investigated. The results of cases with two pregnancy losses (n = 72) and more than two pregnancy losses(n = 180) were not significantly different for Protein S deficiency (84.18 ± 11.69 versus 89.02 ± 22.47) and Protein C deficiency (90.91 ± 23.35 versus 106.57 ± 68.79) (Guzel et al., 2015).
Five studies eligible for meta-analysis described the difference in prevalence of factor V Leiden mutation (n = 1109). Meta-analysis showed no significant difference in the prevalence of factor V Leiden mutation between women with two pregnancy losses and three or more pregnancy losses (five studies, OR 0.79, 95% CI 0.43–1.47) (Fig. 5a). Five studies described the difference in prevalence of prothrombin gene mutation (n = 1330). A meta-analysis showed no significant difference in frequency of prothrombin gene mutation between women with two pregnancy losses and three or more pregnancy losses (five studies, OR 1.08, 95% CI 0.44–2.62) (Fig. 5b). Four studies described the difference in prevalence of protein S deficiency (n = 708). A meta-analysis showed no significant difference in frequency between women with two pregnancy losses and three or more pregnancy losses (four studies, OR 0.72, 95% CI 0.27–1.94) (Fig. 5c). Four studies described the difference in prevalence of protein C deficiency (n = 710). A meta-analysis showed no significant difference in frequency between women with two pregnancy losses and three or more pregnancy losses (four studies, OR 0.73, 95% CI 0.34–1.54) (Fig. 5d).
Figure 5.
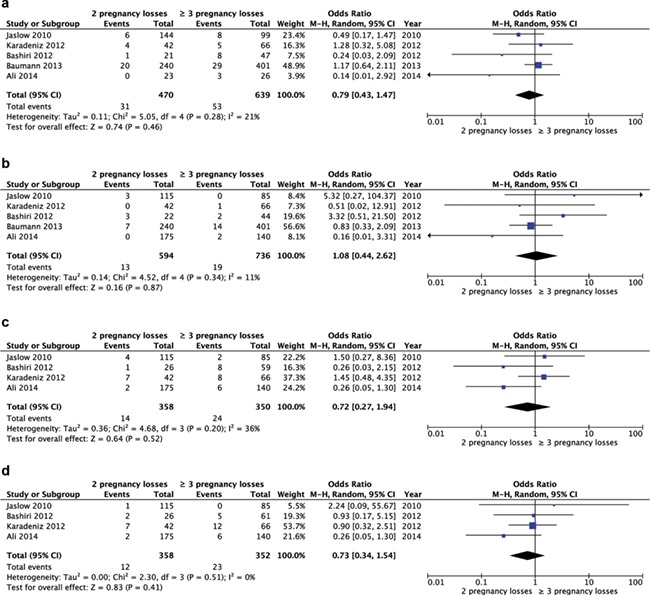
Forest plot of odds ratio of abnormal test results for thrombophilia in women with two pregnancy losses or three or more pregnancy losses. (a) Factor V Leiden mutation. (b) Prothrombin gene mutation. (c) Protein S deficiency. (d) Protein C deficiency.
Thyroid disorders
Two studies (n = 805) described the prevalence of thyroid disorders in women with two pregnancy losses versus three or more. We found insufficient evidence of a difference in frequency of abnormal results for thyroid disorders (two studies, OR 0.52, 95% CI 0.06–4.56, very low quality of evidence) (Fig. 6). There was substantial statistical heterogeneity (I2 of 76%) between the studies; therefore, this finding should be considered with care.
Figure 6.
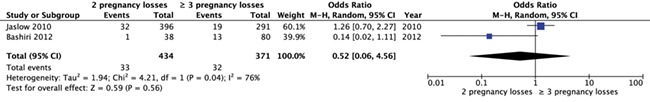
Forest plot of odds ratios of abnormal test results for thyroid disorders in women with two pregnancy losses or three or more pregnancy losses.
Discussion
This systematic review investigated the available literature on the prevalence of abnormal test results in women with RPL with different numbers of previous pregnancy losses. Overall, we found no difference in prevalence of abnormal test results for parental structural chromosome abnormalities, uterine anomalies, APS, inherited thrombophilia and thyroid disorders in women with two pregnancy losses compared with three or more pregnancy losses.
The most recent RPL guideline (ESHRE, 2017) recommends screening for antiphospholipid antibodies after two pregnancy losses. Thyroid screening and assessment of uterine anatomy is recommended for RPL, but no recommendation is given after how many pregnancy losses. Parental karyotyping is not routinely recommended. As the chance of finding an abnormality is very low, it should only be considered after an individual risk assessment (Franssen et al., 2005). As there is a weak association between RPL and hereditary thrombophilia and no available evidenced-based treatment, screening for hereditary thrombophilia is not routinely recommended in couples with RPL (ESHRE, 2017).
The results of this systematic review may support investigations after two pregnancy losses in couples with RPL, but it should be stressed that additional studies of the prognostic value of test results used in the RPL population are urgently needed. There is a paucity of effective evidenced-based treatments for the majority of abnormal tests for possible contributing factors for RPL. This is because many factors have been associated with RPL but few meet accepted criteria for causation. Therefore, testing should not be overvalued and the focus should be on tailor-made supportive care in women with RPL. Couples suffering from RPL need individualized management plans that include appropriate support and, in this context, testing for associated factors may help to reduce anxiety and manage expectations (Musters et al., 2013).
In this systematic review, the quantity and quality of the evidence on the comparison between the prevalence of abnormal test results between groups were low. It follows that any conclusions and recommendations should be drawn with care.
A methodological limitation of this study is the definition of the study groups. As, on average, 15–20% of women with two losses will experience a loss in the next pregnancy, some of the women in the group with two pregnancy losses would be in the other group if evaluated at a different time point. Comparing these groups at a certain moment in time is a fictitious reality, and large studies of the prognostic importance of test results would provide significant new insights into the clinical relevance of diverse clinical tests.
There was no statistical heterogeneity across studies; this suggests that the relative chance of pregnancy loss might be similar in different countries, which could imply that our results are highly generalizable. Two large cohort studies were present in all the meta-analyses and had an important weight factor in the analysis (Jaslow et al., 2010; Bashiri et al., 2012). The results of this systematic review were in line with these two studies. A large systematic review on uterine anomalies in women with RPL reported a prevalence of 10.9% (95% CI 3.6–33.3) uterine anomalies in women with two and 15.4% (95% CI 10.3–23) in women with three or more pregnancy losses, which was not significantly different (P = 0.572) (Chan et al., 2011).
In this systematic review, parental karyotyping was included, although in the last few years, less karyotyping is performed in some countries. In the work-up for couples with RPL, parental karyotyping of both parents is expensive, and there is a very low chance of a live born handicapped child with unbalanced chromosome abnormalities in the unselected RPL population (Franssen et al., 2006; Barber et al., 2010). These considerations have resulted in the recommendation not to perform routine karyotyping of all couples with RPL, but rather after an individual risk assessment (ESHRE, 2017). The treatment option for chromosome abnormalities in couples with RPL consists of PGD. However, limited evidence for PGD in couples with RPL shows no clear benefit of treatment. Couples should be offered genetic counselling and information on the treatment options (ESHRE, 2017).
We did not address genetic analysis of miscarriage tissue in this systematic review. Since genetic analysis is not routinely recommended, finding a fetal chromosomal abnormality does not necessarily rule out an underlying condition. However, it could be performed for explanatory purposes (ESHRE, 2017).
It is important to note that the presence of a particular abnormal test result in women with RPL does not prove causality for the pregnancy losses. Female age and number of prior pregnancy losses have been consistently found to be negative prognostic factors in numerous cohort studies (Parazzini et al., 1988; Knudsen et al., 1991; Quenby and Farquharson, 1993; Brigham et al., 1999; Bhattacharya et al., 2010; Lund et al., 2012; Kolte et al., 2014; Greenberg et al., 2015; Kling et al., 2016).
Female age at first live birth is almost 30 years in European populations, and with an increasing female age, the risk of embryonic aneuploidy increases. Therefore, embryonic aneuploidy will often be the etiology behind RPL, especially in women older than 36 years (Stephenson et al., 2002; Marquard et al., 2010). The decision on when to start investigations should depend on female age and previous pregnancy losses as well as other maternal conditions such as manifest autoimmune or coagulative disease, family history and the results from miscarriage tissue karyotyping, if performed (Bernardi et al., 2012). It should also be the result of shared decision-making by the doctor and couple whilst being compliant with available resources (ESHRE, 2017). Customized diagnostic testing should be considered, where some test can be performed and others omitted.
It should be noted that performing diagnostic testing after two pregnancy losses means that a higher number of couples will have to be investigated. Further studies are needed to assess the economic implications of such a change in policy.
We propose that future research should focus on the design of a dynamic prediction model for couples experiencing RPL. A dynamic model has the advantage that it allows for adaptations to changes in the underlying data over time (van Eekelen et al., 2017). In this model, age, previous pregnancy losses and other risk factors for RPL, such as APS, can be incorporated. If treatment possibilities are present for risk factors (i.e. APS), correction should be applied. With this prediction model, the chance of a live birth could be estimated more precisely. A prediction model can also be used to give positive message to couples suffering anxiety and depression following their pregnancy losses.
Conclusion
The prevalence of abnormal test results for RPL is low after two and three or more pregnancy losses. A difference in prevalence in uterine abnormalities and APS is unlikely in women with two versus three pregnancy losses. We cannot exclude a lower prevalence of chromosomal abnormalities, inherited thrombophilia and thyroid disorders following testing after two versus three pregnancy losses. The results of this systematic review may support testing after two pregnancy losses in couples with RPL, but it should be stressed that additional studies of the prognostic value of test results used in the RPL population are urgently needed. An evidenced-based treatment is not currently available in the majority of cases when abnormal test results are present.
Authors’ roles
M.D., A.M.K., J.L., S.Q., E.K., M.W. and M.G. all contributed substantially to the design of this review. The literature search was performed by J.L. M.D. A.M.K. and M.W. participated in the selection of studies and data-extraction. M.G. was reviewer in case consensus could not be reached directly. M.D. drafted the article; all other authors critically revised multiple versions of the manuscript. All authors gave their final approval of the version to be published.
Funding
No external funding was either sought or obtained for this study.
Conflict of interest
None declared.
Supplementary Material
References
- Ali N, Bhatti FA, Khan SA. Frequency of hereditary thrombophilia in women with recurrent pregnancy loss in northern Pakistan. J Obstet Gynaecol Res 2014;40:1561–1566. [DOI] [PubMed] [Google Scholar]
- Alijotas-Reig J, Garrido-Gimenez C. Current concepts and new trends in the diagnosis and management of recurrent miscarriage. Obstet Gynecol Surv 2013;68:445–466. [DOI] [PubMed] [Google Scholar]
- Asgari A, Ghahremani S, Saeedi S, Kamrani E. The study of chromosomal abnormalities and heteromorphism in couples with 2 or 3 recurrent abortions in Shahid Beheshti Hospital of Hamedan. Iran J Reprod Med 2013;11:201–208. [PMC free article] [PubMed] [Google Scholar]
- Barber JC, Cockwell AE, Grant E, Williams S, Dunn R, Ogilvie CM. Is karyotyping couples experiencing recurrent miscarriage worth the cost? BJOG 2010;117:885–888. [DOI] [PubMed] [Google Scholar]
- Bashiri A, Ratzon R, Amar S, Serjienko R, Mazor M, Shoham-Vardi I. Two versus three or more primary recurrent pregnancy losses are there any differences in epidemiologic characteristics and index pregnancy outcome? J Perinat Med 2012;40:365–371. [DOI] [PubMed] [Google Scholar]
- Baumann K, Beuter-Winkler P, Hackethal A, Strowitzki T, Toth B, Bohlmann MK. Maternal factor V Leiden and prothrombin mutations do not seem to contribute to the occurrence of two or more than two consecutive miscarriages in Caucasian patients. Am J Reprod Immunol 2013;70:518–521. [DOI] [PubMed] [Google Scholar]
- Bernardi LA, Plunkett BA, Stephenson MD. Is chromosome testing of the second miscarriage cost saving? A decision analysis of selective versus universal recurrent pregnancy loss evaluation. Is chromosome testing of the second miscarriage cost saving? A decision analysis of selective versus universal recurrent pregnancy loss evaluation 2012;98:156–61. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: are three miscarriages one too many? Analysis of a Scottish population-based database of 151,021 pregnancies. Eur J Obstet Gynecol Reprod Biol 2010;150:24–27. [DOI] [PubMed] [Google Scholar]
- Bohlmann MK, von Wolff D, Luedders W, Beuter-Winkler P, Diedrich K, Hornemann A, Strowitzki T. Hysteroscopic findings in women with two and with more than two first-trimester miscarriages are not significantly different. Reprod Biomed Online 2010;21:230–236. [DOI] [PubMed] [Google Scholar]
- Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod 1999;14:2868–2871. [DOI] [PubMed] [Google Scholar]
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, Coomarasamy A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update 2011;17:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen OB, Nybo Andersen AM, Bosch E, Daya S, Delves PJ, Hviid TV, Kutteh WH, Laird SM, Li TC, van der Ven K. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril 2005;83:821–839. [DOI] [PubMed] [Google Scholar]
- Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth 2017;17:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CA, Schmitz C, Genro VK, Martins A, Scheffel C, Oppermann ML, Cunha Filho JS. Office hysteroscopy study in consecutive miscarriage patients. Rev Assoc Med Bras 2011;57:397–401. [PubMed] [Google Scholar]
- Diedrich U, Hansmann I, Janke D, Opitz O, Probeck HD. Chromosome anomalies in 136 couples with a history of recurrent abortions. Hum Genet 1983;65:48–52. [DOI] [PubMed] [Google Scholar]
- van Eekelen R, van Geloven N, Wely M, McLernon DJ, Eijkemans MJ, Repping S, Steyerberg EW, Mol BW, Bhattacharya S, van der Veen F. Constructing the crystal ball: how to get reliable prognostic information for the management of subfertile couples. Hum Reprod 2017;32:2153–2158. [DOI] [PubMed] [Google Scholar]
- ESHRE guideline 'Recurrent Pregnancy Loss' ESHRE Early Pregnancy Guideline Development Group Version 2.0. November, 2017
- FitzSimmons J, Wapner RJ, Jackson LG. Repeated pregnancy loss. Am J Med Genet 1983;16:7–13. [DOI] [PubMed] [Google Scholar]
- Franssen MT, Korevaar JC, Leschot NJ, Bossuyt PM, Knegt AC, Gerssen-Schoorl KB, Wouters CH, Hansson KB, Hochstenbach R, Madan K et al. Selective chromosome analysis in couples with two or more miscarriages: case-control study. BMJ 2005;331:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index-control study. BMJ 2006;332:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddijn M, Joosten JH, Knegt AC, van derVeen F, Franssen MT, Bonsel GJ, Leschot NJ. Clinical relevance of diagnosing structural chromosome abnormalities in couples with repeated miscarriage. Hum Reprod 2004;19:1013–1017. [DOI] [PubMed] [Google Scholar]
- Greenberg T1, Tzivian L, Harlev A, Serjienko R, Mazor M, Bashiri A. Index pregnancy versus post-index pregnancy in patients with recurrent pregnancy loss. J Matern Fetal Neonatal Med 2015;28:63–67. [DOI] [PubMed] [Google Scholar]
- Guzel AI, Erkilinc S, Ozer I, Celik Y, Yilmaz N, Doganay M. Diagnostic value of screening tests in subgroups of women with recurrent pregnancy loss. J Matern-Fetal Neonat Med 2015;28:443–447. [DOI] [PubMed] [Google Scholar]
- Jaslow CR, Kutteh WH. Effect of prior birth and miscarriage frequency on the prevalence of acquired and congenital uterine anomalies in women with recurrent miscarriage: a cross-sectional study. Fertil Steril 2013;99:1916–1922. [DOI] [PubMed] [Google Scholar]
- Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril 2010;93:1234–1243. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Farquharson RG, Christiansen OB, Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod 2006;21:2216–2222. [DOI] [PubMed] [Google Scholar]
- Karadeniz RS, Altay MM, Ensari TA, Okyar A, Erol AO, Ozdokan S, Haberal A. There is no relationship between the number of subsequent pregnancy losses and thrombophilic factors. Turkiye Klinikleri J Med Sci 2012;32:376–381. [Google Scholar]
- Kling C, Magez J, Hedderich J, von Otte S, Kabelitz D. Two-year outcome after recurrent first trimester miscarriages: prognostic value of the past obstetric history. Arch Gynecol Obstet 2016;293:1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen UB, Hansen V, Juul S, Secher NJ. Prognosis of a new pregnancy following previous spontaneous abortions. Eur J Obstet Gynecol Reprod Biol 1991;39:31–36. [DOI] [PubMed] [Google Scholar]
- Kolte AM, van Oppenraaij RH, Quenby S, Farquharson RG, Stephenson M, Goddijn M, Christiansen OB. ESHRE special interest group early pregnancy. Non-visualized pregnancy losses are prognostically important for unexplained recurrent miscarriage. Hum Reprod 2014;29:931–937. [DOI] [PubMed] [Google Scholar]
- Lund M, Kamper-Jorgensen M, Nielsen HS, Lidegaard O, Andersen AM, Christiansen OB. Prognosis for live birth in women with recurrent miscarriage: what is the best measure of success? Obstet Gynecol 2012;119:37–43. [DOI] [PubMed] [Google Scholar]
- Magnus MC, Wilcox AJ, Morken N, Clarice R, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 2019;364:l869–l1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquard K, Westphal LM, Milki AA, Lathi RB. Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril 2010;94:1473–1477. [DOI] [PubMed] [Google Scholar]
- Michels VV, Medrano C, Venne VL, Riccardi VM. Chromosome translocations in couples with multiple spontaneous abortions. Am J Hum Genet 1982;34:507–513. [PMC free article] [PubMed] [Google Scholar]
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, Koike T, Meroni PL, Reber G et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006, 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- Musters AM, Koot YE, van den Boogaard NM, Kaaijk E, Macklon NS, van der Veen F, Nieuwkerk PT, Goddijn M. Supportive care for women with recurrent miscarriage: a survey to quantify women's preferences. Hum Reprod 2013;28:398–405. [DOI] [PubMed] [Google Scholar]
- Nybo Andersen AM1, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009) https://www.cebm.net/.
- Parazzini F, Acaia B, Ricciardiello O, Fedele L, Liati P, Candiani GB. Short-term reproductive prognosis when no cause can be found for recurrent miscarriage. Br J Obstet Gynaecol 1988;95:654–658. [DOI] [PubMed] [Google Scholar]
- Quenby SM, Farquharson RG. Predicting recurring miscarriage: what is important? Obstet Gynecol 1993;82:132–138. [PubMed] [Google Scholar]
- RCOG. Green-top Guideline No. 17 'The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage'. April 2011.
- Sachs ES, Jahoda MGJ, van Hemel JO. Chromosome studies of 500 couples with two or more abortions. Obstet Gynecol 1985, 1985;65:375–378. [PubMed] [Google Scholar]
- Schwartz S, Palmer CG. Chromosomal findings in 164 couples with repeated spontaneous abortions: with special consideration to prior reporductive history. Hum Genet 1983;63:28–34. [DOI] [PubMed] [Google Scholar]
- Seckin B, Sarikaya E, Oruc AS, Celen S, Cicek N. Office hysteroscopic findings in patients with two, three, and four or more, consecutive miscarriages. Eur J Contracept Reprod Health Care 2012;17:393–398. [DOI] [PubMed] [Google Scholar]
- Sider D, Wilson WG, Sudduth K, Atkin JF, Kelly TE. Cytogenetic studies in couples with recurrent pregnancy loss. South Med J 1988;81:1521–1524. [DOI] [PubMed] [Google Scholar]
- Sotiriadis A, Vartholomatos G, Pavlou M, Kolaitis N, Dova L, Stefos T, Paraskevaidis E, Kalantaridou SN. Combined thrombophilic mutations in women with unexplained recurrent miscarriage. Am J Reprod Immunol 2007;57:133–141. [DOI] [PubMed] [Google Scholar]
- Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod 2002;17:446–451. [DOI] [PubMed] [Google Scholar]
- The Practice Committee of the American Society for Reproductive Medicine (ASRM) Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103–1111. [DOI] [PubMed] [Google Scholar]
- Van den Boogaard E, Cohn DM, Korevaar JC, Dawood F, Vissenberg R, Middeldorp S, Goddijn M, Farquharson RG. Number and sequence of preceding miscarriages and maternal age for the prediction of antiphospholipid syndrome in women with recurrent miscarriage. Fertil Steril 2013;99:188–192. [DOI] [PubMed] [Google Scholar]
- Weiss A, Shalev E, Romano S. Hysteroscopy may be justified after two miscarriages. Hum Reprod 2005;20:2628–2631. [DOI] [PubMed] [Google Scholar]
- White RG, Hakim AJ, Salganik MJ, Spiller MW, Johnston LG, Kerr L, Kendall C, Drake A, Wilson D, Orroth K et al. Strengthening the reporting of observational studies in epidemiology for respondent-driven sampling studies: "STROBE-RDS" statement. J Clin Epidemiol 2015;68:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


