Abstract
Background
Acute low back pain (LBP) is a common health problem. Non‐steroidal anti‐inflammatory drugs (NSAIDs) are often used in the treatment of LBP, particularly in people with acute LBP. In 2008, a Cochrane Review was published about the efficacy of NSAIDs for LBP (acute, chronic, and sciatica), identifying a small but significant effect in favour of NSAIDs compared to placebo for short‐term pain reduction and global improvement in participants with acute LBP. This is an update of the previous review, focusing on acute LBP.
Objectives
To assess the effects of NSAIDs compared to placebo and other comparison treatments for acute LBP.
Search methods
We searched CENTRAL, MEDLINE, Embase, PubMed, and two trials registers for randomised controlled trials (RCT) to 7 January 2020. We also screened the reference lists from relevant reviews and included studies.
Selection criteria
We included RCTs that assessed the use of one or more types of NSAIDs compared to placebo (the main comparison) or alternative treatments for acute LBP in adults (≥ 18 years); conducted in both primary and secondary care settings. We assessed the effects of treatment on pain reduction, disability, global improvement, adverse events, and return to work.
Data collection and analysis
Two review authors independently selected trials to be included in this review, evaluated the risk of bias, and extracted the data. If appropriate, we performed a meta‐analysis, using a random‐effects model throughout, due to expected variability between studies. We assessed the quality of the evidence using the GRADE approach. We used standard methodological procedures recommended by Cochrane.
Main results
We included 32 trials, with a total of 5356 participants (age range 16 to 78 years). Follow‐up ranged from one day to six months. Studies were conducted across the globe, the majority taking place in Europe and North‐America. Africa and the Eastern Mediterranean region were not represented. We considered seven studies at low risk of bias. Performance and attrition were the most common biases. There was often a lack of information on randomisation procedures and allocation concealment (selection bias); studies were prone to selective reporting bias, since most studies did not register their trials. Almost half of the studies were industry‐funded.
There is moderate quality evidence that NSAIDs are slightly more effective in short‐term (≤ 3 weeks) reduction of pain intensity (visual analogue scale (VAS), 0 to 100) than placebo (mean difference (MD) ‐7.29 (95% confidence interval (CI) ‐10.98 to ‐3.61; 4 RCTs, N = 815). There is high quality evidence that NSAIDs are slightly more effective for short‐term improvement in disability (Roland Morris Disability Questionnaire (RMDQ), 0 to 24) than placebo (MD ‐2.02, 95% CI ‐2.89 to ‐1.15; 2 RCTs, N = 471). The magnitude of these effects is small and probably not clinically relevant. There is low quality evidence that NSAIDs are slightly more effective for short‐term global improvement than placebo (risk ratio (RR) 1.40, 95% CI 1.12 to 1.75; 5 RCTs, N = 1201), but there was substantial heterogeneity (I² 52%) between studies. There is very low quality evidence of no clear difference in the proportion of participants experiencing adverse events when using NSAIDs compared to placebo (RR 0.86, 95% CI 0.63 to 1.18; 6 RCTs, N = 1394). There is very low quality evidence of no clear difference between the proportion of participants who could return to work after seven days between those who used NSAIDs and those who used placebo (RR 1.48, 95% CI 0.98 to 2.23; 1 RCT, N = 266).
There is low quality evidence of no clear difference in short‐term reduction of pain intensity between those who took selective COX‐2 inhibitor NSAIDs compared to non‐selective NSAIDs (mean change from baseline ‐2.60, 95% CI ‐9.23 to 4.03; 2 RCTs, N = 437). There is moderate quality evidence of conflicting results for short‐term disability improvement between groups (2 RCTs, N = 437). Low quality evidence from one trial (N = 333) reported no clear difference between groups in the proportion of participants experiencing global improvement. There is very low quality evidence of no clear difference in the proportion of participants experiencing adverse events between those who took COX‐2 inhibitors and non‐selective NSAIDs (RR 0.97, 95% CI 0.63 to 1.50; 2 RCTs, N = 444). No data were reported for return to work.
Authors' conclusions
This updated Cochrane Review included 32 trials to evaluate the efficacy of NSAIDs in people with acute LBP. The quality of the evidence ranged from high to very low, thus further research is (very) likely to have an important impact on our confidence in the estimates of effect, and may change the estimates.
NSAIDs seemed slightly more effective than placebo for short‐term pain reduction (moderate certainty), disability (high certainty), and global improvement (low certainty), but the magnitude of the effects is small and probably not clinically relevant.
There was no clear difference in short‐term pain reduction (low certainty) when comparing selective COX‐2 inhibitors to non‐selective NSAIDs.
We found very low evidence of no clear difference in the proportion of participants experiencing adverse events in both the comparison of NSAIDs versus placebo and selective COX‐2 inhibitors versus non‐selective NSAIDs.
We were unable to draw conclusions about adverse events and the safety of NSAIDs for longer‐term use, since we only included RCTs with a primary focus on short‐term use of NSAIDs and a short follow‐up. These are not optimal for answering questions about longer‐term or rare adverse events.
Plain language summary
Anti‐inflammatory drugs for acute low back pain
Review question
We examined the effect of non‐steroidal anti‐inflammatory drugs (NSAIDs), such as diclofenac, ibuprofen, and naproxen, for people with acute low back pain. Acute low back pain is defined as the presence of pain in the back, below the ribs and above the buttocks, for under 12 weeks. We compared NSAIDs to placebo, paracetamol, other NSAIDs, other drugs, and non‐drug treatments.
Background
Acute low back pain is common, and causes pain and disability. Physicians often prescribe NSAIDs to treat acute low back pain. Different types of NSAIDs are available, both over‐the‐counter and as prescription drugs.
Study characteristics
We searched for randomised controlled trials that were published or registered before 7 January 2020. We included 32 trials with 5356 participants. Trial participants were 16 to 78 years old and had acute low back pain. Study length varied from one day to six months. The studies took place in many different countries. More than half of the studies was done in Europe and North‐America.
Key results
NSAIDs were slightly more effective than placebo for pain reduction in the first three weeks. On average, the pain intensity decreased by 7.3 points on a 100‐point scale. This means there was a small difference between the two treatments, but it was not clinically relevant. People receiving NSAIDs also scored 2.0 points better on a 24‐point disability scale than those receiving placebo. This is unlikely to be of real‐world benefit. There was a similar number of side effects between people receiving NSAIDs and people receiving placebo. However, the type of studies that we investigated are not designed to find side effects. Therefore, we should be careful about drawing conclusions based upon these findings.
We also compared two different types of NSAIDs; non‐selective NSAIDs versus COX‐2 inhibitors. We found no clear differences in effect. There was also a similar number of reported side effects of the digestive system, such as abdominal pain, nausea, diarrhoea, or stomach symptoms.
Quality of the evidence
There is moderate quality evidence that NSAIDs are slightly more effective than placebo for reducing short‐term pain, and high quality evidence that they are slightly more effective than placebo for reducing disability in acute low back pain. The magnitude of the effect is very small.
Summary of findings
Background
Description of the condition
Low back pain (LBP) is one of the most prevalent health problems worldwide, and still one of the leading causes of years lived with disability, according to the most recently published global burden of disease study (GBD 2016). It is usually defined as pain, muscle tension, or stiffness localised below the costal margin and above the inferior gluteal folds (Koes 2006). The lifetime prevalence of non‐specific LBP is estimated at 60% to 70% in industrialised countries (Hoy 2010). This affects personal lives, causing activity limitations and work absence, but also brings with it an economic burden, with high socioeconomic costs (Hoy 2010; Lidgren 2003); especially when a chronic state of LBP develops (Steenstra 2005). In the first six weeks, recovery occurs in a substantial number of participants. However, there is increasing debate about the numbers of recurrent pain episodes and chronicity, since participants continue to report pain after one year (Costa 2012; Itz 2013; Manchikanti 2014; Pengel 2003). Current guidelines on the treatment of non‐specific LBP are consistent in their focus on early and gradual activation, patient education, avoiding bedrest, and addressing psychosocial factors to prevent chronicity; and on prescribing analgesic medication for short periods, where necessary, in the case of acute LBP (Oliveira 2018).
Description of the intervention
As stated above, most guidelines recommend staying active. Better pain control may ease this process, therefore, the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) can be of value. If pain medication is considered, NSAIDs are recommended (Oliveira 2018). Previously, guidelines recommended various types of analgesics (Koes 2010). Recently, an updated review on paracetamol for LBP found high‐quality evidence that paracetamol (4 g per day) is no better than placebo for relieving acute LBP (Saragiotto 2016). In some adapted guidelines, this new evidence is already incorporated, for instance, the National Institute for Health and Care Excellence (NICE) guidelines from the UK (Bernstein 2017). The most recent guidelines from the USA recommend non‐pharmacological treatments first, given that most people with (sub)acute LBP improve over time, regardless of treatment. But if pharmacological treatment is considered, then NSAIDs or muscle relaxants are recommended as first line options (Qaseem 2017). In such circumstances, NSAIDs are often recommended because of their known analgesic and anti‐inflammatory effects. However, the drawback is that they are also associated with a variety of potential adverse events, particularly gastrointestinal and cardiovascular effects (Brune 2015).
How the intervention might work
The main therapeutic effects of NSAIDs derive from their ability to inhibit the production of prostaglandins. The first enzyme in the pathway of prostaglandin synthesis is cyclooxygenase (COX). Both COX‐1 and COX‐2 contribute to the production of prostaglandins when inflammation and pain are present, and for autoregulation and homeostasis of the human body. COX‐1 is the dominant source for the production of prostaglandins that are responsible for gastric epithelial protection and haemostasis. COX‐2 is important for prostaglandin synthesis induced by cytokines and stress. NSAIDs inhibit the COX enzyme, and thus block the synthesis of prostaglandins, reducing inflammation, pain, and fever (Grosser 2011). Two types of NSAIDs are available: non‐selective NSAIDs that inhibit both COX‐1 and COX‐2 enzymes (e.g. ibuprofen, diclofenac, naproxen); and selective COX‐2 inhibitors that only inhibit the COX‐2 enzyme (e.g. nimesulide, celecoxib). The latter was developed because non‐selective NSAIDs were often associated with gastrointestinal adverse events. Blocking COX‐1 also reduced gastric protection, leading to an increased risk for gastrointestinal complications (e.g. gastric ulcer, perforation, stomach bleeding (Sostres 2013)). Selective COX‐2 inhibitors decrease this risk, however they increase the risk for cardiovascular adverse events. For instance, rofecoxib, a selective COX‐2 inhibitor, was withdrawn from the market for this reason. Similar concerns arose around the cardiovascular safety of traditional NSAIDs (CNT Collaboration 2013; Trelle 2011; Walker 2018). There is evidence that this risk is duration‐ and dose‐dependent (Pepine 2017). Therefore, whenever NSAIDs are prescribed, one should always take into account the risk for gastrointestinal or cardiovascular adverse events (Brune 2015; Walker 2018), and if possible, choose the shortest duration and lowest effective doses (Pepine 2017). NSAIDs vary in their degree of COX‐2 selectivity. The choice of the best fitting NSAID further depends on patient characteristics, their medical history, and the type of complaint.
Why it is important to do this review
This Cochrane Review is part of a series on the effect of NSAIDs for LBP, and is an update of a Cochrane Review first published in 2000 (van Tulder 2000). The previous update included 65 randomised controlled trials on acute LBP, chronic LBP, and sciatica (Roelofs 2008). Due to the high number of trials, we split the review into three separate Cochrane Reviews on the use of NSAIDs for different types of LBP. The response to NSAIDs may differ for acute LBP, compared to chronic LBP or sciatica. The reviews on chronic LBP (Enthoven 2016), and sciatica (Rasmussen‐Barr 2016), have been published. This review focuses on the efficacy of NSAIDs for acute LBP.
Objectives
To assess the effects of non‐steroidal anti‐inflammatory drugs compared to placebo and other comparison treatments for acute low back pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials, conducted in both primary and secondary care settings. The original protocol of this review included only English, German, and Dutch studies. The present update had no language restrictions.
Types of participants
We included subjects aged 18 years or older, treated for acute non‐specific low back pain (LBP). We defined LBP as pain below the costal margin and above the inferior gluteal folds. Acute LBP was defined as having LBP symptoms for less than 12 weeks. Within acute LBP, we included both acute (less than six weeks) and subacute LBP (6 to 12 weeks). If the study authors did not describe the duration of LBP, but LBP was labelled as acute, we also included the study. If a study included mixed populations (like acute or subacute and chronic LBP), we only included the study if they presented data for acute LBP separately. If a minor part of the study population (< 10%) experienced pain radiating to one or both legs to the knee, or a flare‐up (acute exacerbations of chronic LBP), we included the study and performed a sensitivity analysis at a later stage, if applicable. We excluded studies on subjects with chronic LBP, flare‐ups, or sciatica, as well as studies that included participants with LBP caused by specific pathological entities, such as infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis, or fractures.
Types of interventions
We included trials that assessed one or more types of non‐steroidal anti‐inflammatory drugs (NSAID). Additional interventions were allowed if there was a contrast for NSAIDs in the trial. For example, studies comparing NSAIDs plus muscle relaxants versus muscle relaxants alone would be included, while studies comparing NSAIDs plus muscle relaxants versus NSAIDs alone were not. We also excluded studies if they combined NSAIDs with other drugs, making it difficult to distinguish the actual effect of the NSAID, e.g. a study comparing NSAIDs plus muscle relaxants versus paracetamol would be excluded. We included studies that compared NSAIDs to another type of NSAID. We excluded a study if it compared an NSAID to the same NSAID with the mode of delivery as the only difference, or if different NSAIDs were used in the same group, and no distinction was made in the analysis.
We clustered comparisons of NSAIDs versus reference treatments into the following categories:
NSAIDs versus placebo (the main comparison)
Selective COX‐2 inhibitors versus non‐selective NSAIDs
NSAIDs versus paracetamol
NSAIDs versus other drug treatment
NSAIDs versus non‐drug treatment
The NSAID arm could include both selective and non‐selective NSAIDs, except for the comparison of selective COX‐2 inhibitors versus non‐selective NSAIDs, where this was specified.
Types of outcome measures
As outcome measures, we used the four primary outcomes that were already defined in the protocol and previous version of the Cochrane review (Roelofs 2008). We added adverse events as a fifth primary outcome. These outcomes are described below. We set the minimal duration of follow‐up at one day, with at least one outcome measured in the first three weeks. We divided the timing of outcome assessment into two main categories:
1) Short‐term follow‐up: ranging from one day to three weeks. If there were more outcome assessments around this time point, we used the strategy of including outcomes closest to three weeks.
2) Long‐term follow‐up: ranging from longer than three and up to 12 weeks. If there were more outcome assessments around this time point, we used the strategy of including outcomes closest to twelve weeks.
Primary outcomes
Primary outcome measures were:
1) pain intensity (e.g. Visual Analogue Scale (VAS) or Numerical Rating Scale (NRS))
2) back pain‐specific functional status (e.g. Roland Morris Disability Questionnaire (RMDQ), Oswestry Disability Index (ODI))
3) global measure (e.g. overall improvement, proportion of participants recovered)
4) adverse events (proportion of participants experiencing adverse events)
5) return to work (e.g. return to work status, number of days off work)
We evaluated pain intensity and disability as continuous outcomes. We considered a between‐group difference of more than 10% of the scale (e.g. 10 points on a 0 to 100 scale) to be clinically relevant. A mean difference smaller than this was considered not clinically relevant. For the global measure of improvement, we used dichotomous outcomes; if there were categories in range of improvement, we counted categories such as 'almost recovered' and 'completely recovered', 'good' and 'very good or excellent', and 'a lot' to 'complete recovery' responses as recovered. For adverse events and return to work, we used dichotomous outcomes, usually proportion of participants.
Secondary outcomes
There were no secondary outcomes.
Search methods for identification of studies
Electronic searches
We identified RCTs that met our inclusion criteria by searching the following databases, with no language restrictions, to 7 January 2020:
Cochrane Central Register of Controlled Trials (CENTRAL, 2020, Issue 1) in the Cochrane Library; includes the Back and Neck Group Trials Register; CRS Web (searched 7 January 2020);
MEDLINE Ovid Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) (1946 to 7 January 2020);
Embase (1980 to 2020 Week 01);
PubMed (1946 to January 2016);
ClinicalTrials.gov (searched 7 January 2020);
ICTRP (searched 7 January 2020).
We conducted searches in May 2012 (for publications between June 2007 and May 2012), and repeated them annually until January 2020. Search strategies are presented in Appendix 1, Appendix 2, Appendix 3, Appendix 4. We searched PubMed in 2015 and 2016 for studies not in MEDLINE, using the strategy recommended by Duffy 2014. We began searching MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Daily, and MEDLINE(R)) in 2017 because it allowed us to search several MEDLINE databases in one search. In 2017, we began searching CENTRAL and the Cochrane Back and Neck (CBN) Group Trials Register in CRS Web; previously they were searched in CRS standalone. An experienced information specialist developed the strategies following the updated methods guideline of the CBN and the Cochrane Handbook for Systematic Reviews of Interventions (the Handbook (Furlan 2015; Higgins 2011)).
Searching other resources
We screened the reference lists of all included trials, as well as (systematic) reviews on NSAIDs for acute LBP. We also reassessed the studies on acute low back pain included in the previous version of this review (Roelofs 2008).
Data collection and analysis
Selection of studies
Two review authors (BK and PR, PR and WG, or WG and WE) independently screened all search results. We excluded clearly ineligible studies based on title and abstract. We retrieved full‐text articles for all remaining studies, and two review authors independently conducted the screening for inclusion. We resolved disagreements via consensus, and consulted a third review author (WG, WE or PR) in case of uncertainty, or if disagreements persisted.
Data extraction and management
Two review authors (WG and PR) independently extracted the data using a standardized data extraction form provided by the CBN. We extracted data on:
study characteristics: type of study and randomisation, population, setting, description of interventions, and reference treatments, follow‐up time, trial registration, funding
characteristics of participants: number of participants, gender, mean age, duration of current symptoms, inclusion and exclusion criteria
primary outcomes and any relevant additional information
We extracted follow‐up data at several time points, and defined an outcome assessment as relevant if it was measured between one day and 12 weeks of follow‐up. We contacted corresponding authors for further information if potentially relevant information was missing or not available, for data extraction due to a different format. We resolved any disagreement through consensus.
Assessment of risk of bias in included studies
Two review authors (WG and WE, or WG and PR) independently assessed the risk of bias of the included studies using a stepwise approach. First, we used the thirteen criteria recommended by Furlan 2015, described in Table 3 and Table 4. Since this review evaluated the efficacy of specific medication for acute LBP, namely NSAIDs, we added an additional criterion concerning funding or sponsorship. We separately scored these fourteen criteria as yes, no, or unsure, and reported these in the 'Risk of bias' tables, including the rationale for our decision. In cases of unsure judgement of the risk of bias, we attempted to contact corresponding authors of newly included trials for extra information. We did not contact authors of previously included trials, since earlier attempts to contact these authors for the previous version of this review were unsuccessful.
1. Sources of risk of bias.
| Bias domain | Source of Bias | Possible answers |
| Selection | (1) Was the method of randomisation adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the participant blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the careprovider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the dropout rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/No/Unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/No/Unsure |
| Performance | (10) Were co‐interventions avoided or similar? | Yes/No/Unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/No/Unsure |
2. Criteria for a judgement of 'yes' for the sources of risk of bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, pre‐ordered sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance or social security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the participants. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the participant. |
| 3 | Index and control groups are indistinguishable for the participants, or if the success of blinding was tested among the participants and it was successful. |
| 4 | Index and control groups are indistinguishable for the careproviders, or if the success of blinding was tested among the careproviders and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored 'yes' if the success of blinding was tested among the outcome assessors and it was successful or:
• for participant‐reported outcomes in which the participant is the outcome assessor (e.g. pain, disability): the blinding procedure is adequate for outcome assessors if participant blinding is scored 'yes' • for outcome criteria assessed during scheduled visit and that supposes a contact between participants and outcome assessors (e.g. clinical examination): the blinding procedure is adequate if participants are blinded, and the treatment or adverse effects of the treatment cannot be noticed during clinical examination • for outcome criteria that do not suppose a contact with participants (e.g. radiography, magnetic resonance imaging): the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed when assessing the main outcome • for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and careproviders (e.g. co‐interventions, hospitalisation length, treatment failure), in which the careprovider is the outcome assessor: the blinding procedure is adequate for outcome assessors if item '4' (caregivers) is scored 'yes' • for outcome criteria that are assessed from data from the medical forms: the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed on the extracted data |
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up, and does not lead to substantial bias, a 'yes' is scored. (N.B. these percentages are arbitrary, not supported by literature). |
| 7 | All randomised participants are reported and analysed in the group to which they were allocated by randomisation for the most important moments of effect measurement (minus missing values) irrespective of non‐compliance and co‐interventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of a protocol, by assessing that the published report includes enough information to make this judgement. |
| 9 | Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of participants with neurological symptoms, and value of main outcome measure(s). |
| 10 | If there were no co‐interventions, or if they were similar between the index and control groups. |
| 11 | The review author determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number, and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore, it is necessary to assess how many sessions each participant attended. For single‐session interventions (e.g. surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. For example:
• When the outcome measures were not valid. There should be evidence from a previous or present scientific study that the primary outcome can be considered valid in the context of the present. • Industry‐sponsored trials. The conflict of interest (COI) statement should explicitly state that the researchers have had full possession of the trial process from planning to reporting without funders with potential COI having any possibility to interfere in the process. If, for example, the statistical analyses have been done by a funder with a potential COI, usually 'unsure' is scored. |
Published by Furlan 2015; these instructions were adapted from van Tulder 2003, Boutron 2005, and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Each of these fourteen criteria corresponds to a specific type of bias at the domain level. Therefore, as a second step, we assessed the risk of bias at domain level: selection bias (criteria 1, 2, 9), performance bias (criteria 3, 4, 10, 11), detection (or measurement) bias (criteria 5, 12), attrition bias (criteria 6, 7), reporting bias (criteria 8), and other (potential) biases (criteria 13, 14). We resolved disagreements by consensus, and consulted a third review author (PR or WE) if disagreements persisted.
Measures of treatment effect
Following the recommendations in the Handbook (Higgins 2011), we analysed dichotomous outcomes by calculating the risk ratio (RR). We analysed continuous outcomes by calculating the mean difference (MD) when the same instrument was used to measure outcomes, or the standardized mean difference (SMD) when different instruments were used. We expressed the uncertainty with 95% confidence intervals (CI).
Unit of analysis issues
We included cross‐over trials if the data from the first phase (until the cross‐over) were available, to aid comparability. Cluster‐randomized trials were analysed based on the level of allocation, for example, a cluster of participants.
Some of the included studies had more than two study arms. To avoid unit of analysis error, we split the control group to prevent overestimation of the number of participants for the same intervention. For example, in the Dreiser 2003 and Babej‐Dolle 1994 studies, we divided the placebo group into two subgroups, by dividing the number of events and number of cases by two for dichotomous outcomes, or by dividing the sample size by two, and assuming similar mean and standard deviations reported for continuous outcomes in both subgroups.
Dealing with missing data
For trials that were included in the previous review: data that were not reported in the study, nor added in the previous review after consultation of the study authors, were considered missing for this update. In case of unclear or mixed duration or location of back pain, with no subgroup analyses presented, we moved the study to 'Studies awaiting classification', while we tried to contact the authors. In a future update, these studies could either be moved to 'Included' or 'Excluded' studies, depending on the trial author response.
For new trials: in case of missing data, we e‐mailed the corresponding author. Additional data provided by authors were used in the analyses. If data were not described in the text, but were shown in graphs, two review authors (PR and WG) collected the data from the graphs by estimation. If needed, we recalculated data to provide standard deviations. We performed the calculations according to the Handbook (Higgins 2011).
Assessment of heterogeneity
Statistical heterogeneity was assessed with the I² statistic. Values of I² below 40% suggested no important heterogeneity, above 75% suggested considerable heterogeneity. If values of I² were between 40% and 75%, moderate to substantial heterogeneity could be present (Furlan 2015; Higgins 2011). We performed visual inspection of the forest plot and the overlap of confidence intervals. If the I² value was below 40%, we pooled the results; if it was above 75%, we did not pool. For values between 40% and 75%, we based the decision for pooling on the evaluation of the heterogeneity. Clinical heterogeneity was assessed for all included studies that reported similar outcomes. We judged the studies on the setting, population source of the participants, and the intervention. For the latter, we took variations in the type of NSAIDs that were used, dosage, mode of delivery, and duration of treatment into account. If studies were clinically too heterogeneous, we did not pool them. If pooling was feasible, we used a random‐effects model throughout this review, due to expected variability between studies (e.g. differences in populations and interventions as described above).
Assessment of reporting biases
According to CBN, publication bias should be examined when at least ten studies are included in the meta‐analysis (Furlan 2015). No comparisons included more than six trials, therefore, it was not possible to construct a funnel plot or to draw conclusions concerning publication bias. There were no language restrictions to prevent reporting bias due to language.
Data synthesis
We assessed the overall quality of the evidence for each outcome using the GRADE approach, as recommended in the Handbook, and adapted in the CBN's updated method guidelines (Furlan 2015; GRADE Working Group 2004; Guyatt 2008; Higgins 2011; Appendix 5). We determined the quality of evidence for each outcome based on five domains: limitations in study design and implementation (risk of bias), inconsistency (heterogeneity), indirectness (inability to generalise), imprecision (insufficient or imprecise data), and publication bias. We judged these five domains for all studies that measured a particular outcome and could be included in a meta‐analysis. The quality of the evidence for a specific outcome could be reduced by one or more levels, depending on the performance of the studies against these five factors. For the considerations used to define the level of evidence, refer to Appendix 5. The overall quality of the evidence for each outcome is the result of the combination of this assessment of all domains.
The GRADEpro GDT enabled us to import data from Review Manager 5.3 to create the 'Summary of findings' tables for the main comparison (GRADEpro GDT; Review Manager 2014).
'Summary of findings' tables
We considered 'NSAIDs versus placebo' our main comparison. We added a second 'Summary of findings' table for 'Selective COX‐2 inhibitors versus non‐selective NSAIDs', since we considered this a clinically important comparison. We considered all five primary outcome measures important, and as such, we presented all of them in the 'Summary of findings' tables. We presented the outcomes in the short‐term (follow‐up ≤ 3 weeks), which was deemed most relevant in the case of acute LBP, except for the reporting of adverse events, which had no limitations in follow‐up time.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses on the analysis of NSAIDs versus non‐drug treatment (one subgroup for physiotherapy and spinal manipulation, and one subgroup for bedrest), but these were not applicable, due to lack of available data. A subgroup analysis of selective versus non‐selective NSAIDs was planned but not completed, due to lack of data.
Sensitivity analysis
We planned two sensitivity analyses for each comparison. In the first sensitivity analysis, we excluded studies with a final judgement of a high risk of bias from the analysis. The second sensitivity analysis included the studies with solely acute LBP participants. We left studies with a case‐mix of participants with acute LBP and a small subgroup of the study population (< 10%) with additional sciatic or flare‐up complaints out of this analysis. We could only perform both sensitivity analyses in a few comparisons, most often for NSAIDs versus placebo. In some comparisons this was not possible because all studies were judged as having a high risk of bias, or available data were lacking.
Results
Description of studies
Results of the search
For this update, we identified 5786 references through database and trial registry searches (Figure 1). After removing duplicates, we screened 4680 titles and abstracts, and subsequently assessed 96 full‐text publications. In total, we included 32 publications in the present update: 26 studies that focused on acute low back pain (LBP) from the previous review (Aghababian 1986; Agrifoglio 1994; Amlie 1987; Babej‐Dolle 1994; Bakshi 1994; Brown 1986; Colberg 1996; Dreiser 2003; Hosie 1993; Innes 1998; Jaffe 1974; Lacey 1984; Metscher 2001; Nadler 2002; Orava 1986; Pohjolainen 2000; Postacchini 1988; Schattenkirchner 2003; Stratz 1990; Szpalski 1990; Szpalski 1994; Videman 1984; Waterworth 1985; Wiesel 1980; Ximenes 2007; Yakhno 2006); and six new publications (Hancock 2007; Miki 2018; Plapler 2016; Shin 2013; von Heymann 2013; Zippel 2007).
1.
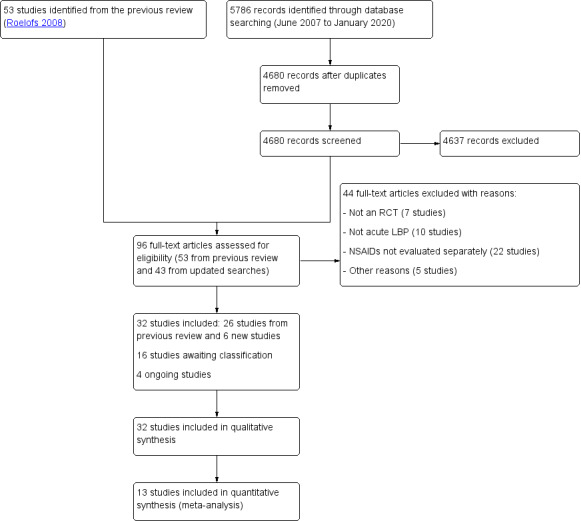
Study flow diagram
There are 16 studies still awaiting classification. We contacted the authors of these studies to ask for clarification, unless we were unable to find any currently active contact information. The reasons for not being classified were: an unclear or mixed duration of pain (Aoki 1983; Borghi 2018; Davoli 1989; Famaey 1998; Hingorani 1970; Hingorani 1975; Jacobs 1968; Sweetman 1987; Waikakul 1995; Waikakul 1996; Zolotovskaya 2015); an unclear or mixed location of pain (Basmajian 1989; Milgrom 1993; Predel 2019); the use of different non‐steroidal anti‐inflammatory drugs (NSAID) in one treatment arm that was not specified (NCT01374269); or a completed pilot study for which we are awaiting the results (TCTR20141027001). Usually, there was no subgroup analysis presented, or the reported data were insufficient to be used in our analyses. By retrieving more information from the authors, we aimed to clarify if subgroup analyses would be possible in a future update. Further details of the studies can be found in the 'Characteristics of studies awaiting classification' table.
There were four ongoing studies recruiting participants, thus no results were available for this review (CTRI/2018/11/016371; NCT03861611; NCT04111315; TCTR20151118003). Further details can be found in the 'Characteristics of ongoing studies' table.
Included studies
We included 32 trials with a total of 5356 participants (sample size ranged from 30 to 372). Ages of included participants ranged from 16 to 78 years; one trial had no age limits, but did not specify the age range of the back pain subgroup (Lacey 1984). All of the included studies were published in English, except for one which was in German (Metscher 2001). The studies were conducted in Germany (six studies), USA (four studies), UK (three studies), Finland (three studies), Belgium (two studies), Italy (two studies), Brazil, Norway, Austria, France, Australia, Canada, Japan, South‐Korea, New Zealand and Russia (one study each). One study was conducted in three European countries (Belgium, Germany, Poland), while one study took place in nine Latin‐American countries (Brazil, Venezuela, Ecuador, Argentina, Chile, Mexico, Colombia, Costa Rica, and Peru). Settings were most often general practice or outpatient clinics. In some cases, the setting was the emergency department or an occupational health centre. For further details of the studies refer to the 'Characteristics of included studies' table.
Comparisons were as follows:
nine trials compared one or more types of NSAIDs with a placebo (Amlie 1987; Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Lacey 1984; Nadler 2002; Postacchini 1988; Szpalski 1994; von Heymann 2013);
three trials compared one or more types of NSAIDs with paracetamol (Miki 2018; Nadler 2002; Wiesel 1980);
seventeen trials compared different types of NSAIDs (Aghababian 1986; Agrifoglio 1994; Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hosie 1993; Jaffe 1974; Orava 1986; Plapler 2016; Pohjolainen 2000; Schattenkirchner 2003; Stratz 1990; Wiesel 1980; Ximenes 2007; Yakhno 2006; Zippel 2007); two of these trials compared selective COX‐2 inhibitors with non‐selective NSAIDs (Pohjolainen 2000; Ximenes 2007);
four trials compared one or more types of NSAIDs with other drugs (Brown 1986; Innes 1998; Metscher 2001; Videman 1984);
seven trials compared one or more types of NSAIDs with non‐drug treatment (Hancock 2007; Nadler 2002; Postacchini 1988; Shin 2013; Szpalski 1990; von Heymann 2013; Waterworth 1985).
Three studies had multiple groups of NSAID treatment, comparing two different types of NSAIDs to other treatment (Babej‐Dolle 1994; Dreiser 2003; Wiesel 1980). Comparators in the group of other drugs were acetaminophen with codeine, tramadol hydrochloride, and meptazinol. Comparators in the group of non‐drug treatment were spinal manipulation, physiotherapy, bedrest, heat wrap, and motion style acupuncture treatment.
The duration of follow‐up ranged from one day to six months. Most studies only reported short‐term results (follow‐up one day to three weeks). We defined long‐term follow‐up as longer than three weeks and up to 12 weeks. Only five trials had a follow‐up duration longer than three weeks (Hancock 2007; Miki 2018; Postacchini 1988; Shin 2013; von Heymann 2013).Of these five trials, von Heymann 2013 reported that they completed assessments at 12 weeks, but they did not report the data; Postacchini 1988 only reported a combination score of improvement in pain, disability, and spinal mobility (all three combined in one 'global measure') at two months; Miki 2018 only presented a mean pain difference score between groups at four weeks. Shin 2013 presented long‐term outcomes at four weeks, but in both study arms, they allowed participants to pursue other treatments of their choice once they completed the intervention treatment session (reportedly due to ethical reasons). This implies the results after the first follow‐up at 30 minutes may be difficult to interpret or generalize. Hancock 2007 presented long‐term follow‐up outcomes (84 days) while maintaining the intervention groups throughout the follow‐up period.
In four studies, a part of the study population had sciatic complaints or a flare‐up of chronic back complaints (Babej‐Dolle 1994; Bakshi 1994; Jaffe 1974; Orava 1986); in one study, none of the screened people were excluded, despite the strict exclusion criteria (Plapler 2016); one study used a very selected population of young male army trainees (Wiesel 1980). One study used an inadequate dosage of the reference medication (Yakhno 2006).
Fourteen studies were industry‐sponsored. The pharmaceutical companies that funded the studies were most often the developer of the study drug (Aghababian 1986; Amlie 1987; Babej‐Dolle 1994; Bakshi 1994; Brown 1986; Dreiser 2003; Hosie 1993; Innes 1998; Nadler 2002; Plapler 2016; Pohjolainen 2000; Schattenkirchner 2003; Ximenes 2007; Zippel 2007). One study, with spinal manipulation as a comparison was funded by two organisations for manual therapy (von Heymann 2013). A few studies clearly stated they were funded by sponsors that did not have any influence on data collection, management, analysis, and reports (Hancock 2007); by a hospital foundation (Waterworth 1985); a national association for musculoskeletal pain studies (Miki 2018); or that they received an unrestricted grant (Yakhno 2006). The remaining fourteen studies did not mention their funding sources.
For declarations of (and potential conflicts of) interest: one author of a study with motion‐style acupuncture treatment as a comparison was supported by an Asian medicine institute (Shin 2013); one study was written by a paid consultant and co‐authored by employees of the health sciences institute of a pharmaceutical company (Nadler 2002); one study received statistical help from an employee of a pharmaceutical company (Lacey 1984); one study received editorial support (Ximenes 2007). One study was funded by a local pharmaceutical company that was involved in study design, protocol development, obtaining and evaluating the data, and writing the manuscript together with the authors, and all authors received grants and consulting fees for this (Plapler 2016).
Excluded studies
We described the reasons for excluding studies in the 'Characteristics of excluded studies' table. There were four main categories:
Not a randomised controlled trial (RCT; e.g. no intervention, a clinical series, case report, review or commentary (Anaya 2014; Arul Prakasam 2011; Buchbinder 2010; Day 2013; Shikhkerimov 2016; von Uberall 2013; Yastrebov 2012));
Not acute LBP (i.e. LBP was of longer duration, or it did not concern LBP in general (Blazek 1986; Chrubasik 2003; Coats 2004; Driessens 1994; Evans 1980; Ingpen 1969; Matsumo 1981; Muckle 1986; Shell 2012; Siegmeth 1978));
NSAIDs were not evaluated separately (e.g. NSAIDs were added for blinding purposes only, or the comparison group only involved the mode of administration (Allegrini 2009; Altan 2019; Berry 1988; Borenstein 1990; Bruggemann 1990; Cohen 2017; Costantino 2011; Dehghan 2014; Dehghan 2015; Friedman 2015; Friedman 2016; Friedman 2018; Geller 2016; Górska 2005; Ilic 2009; IRCT2013052213146N2; Kuhlwein 1990; Listrat 1990; Ostojic 2017; Stark 2014; Vetter 1988; Voicu 2019));
Other reasons (e.g. no (or insufficient) study results were available because we did not find the original study results (Pena 1990), the study terminated early (Schreijenberg 2017); or the duration of follow‐up was less than one day (Eken 2014; Lee 2008; Serinken 2016)).
Risk of bias in included studies
We presented the assessment of the risk of bias of included studies at the domain level in Figure 2 and Figure 3. Domains included selection, performance, detection, attrition, reporting, and other biases. After final assessment at the domain level, we determined there were seven studies with an overall judgement of low risk of bias (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Innes 1998; Pohjolainen 2000; Szpalski 1994; Yakhno 2006).
2.
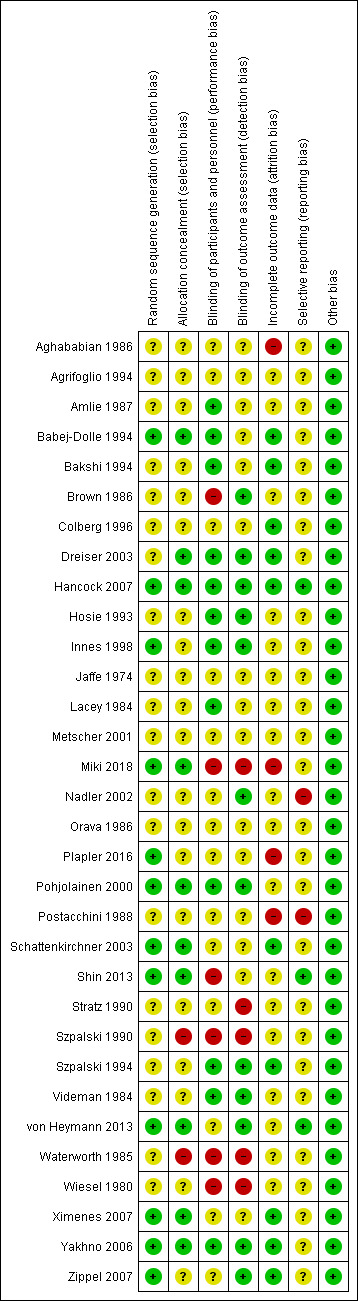
'Risk of bias' summary per domain: review authors' judgements about each 'Risk of bias' item for each included study
3.
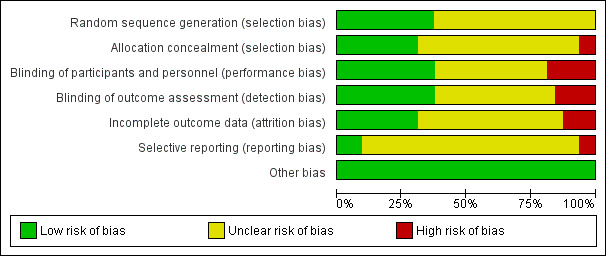
'Risk of bias' graph per domain: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
Of the 32 included studies, 12 reported an adequate randomisation procedure (Babej‐Dolle 1994; Hancock 2007; Innes 1998; Miki 2018; Plapler 2016; Pohjolainen 2000; Schattenkirchner 2003; Shin 2013; von Heymann 2013; Ximenes 2007; Yakhno 2006; Zippel 2007). Ten studies adequately concealed treatment allocation (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Miki 2018; Pohjolainen 2000; Schattenkirchner 2003; Shin 2013; von Heymann 2013; Ximenes 2007; Yakhno 2006). The majority of studies did not report the method of randomisation or allocation concealment, thus, we scored these studies as unclear risk on both items.
Two‐thirds of the studies showed similar characteristics at baseline (Agrifoglio 1994; Amlie 1987; Bakshi 1994; Colberg 1996; Dreiser 2003; Hancock 2007; Hosie 1993; Innes 1998; Miki 2018; Nadler 2002; Orava 1986; Plapler 2016; Pohjolainen 2000; Shin 2013; Szpalski 1994; Videman 1984; von Heymann 2013; Waterworth 1985; Ximenes 2007; Yakhno 2006; Zippel 2007). The other studies either did not report baseline characteristics, or did not provide enough details to compare them. Several studies reported that baseline characteristics were similar, however, they did not provide details of the actual baseline characteristics for the acute LBP subgroup. We scored these as unclear risk of bias.
Overall, we determined 12 studies to have a low risk of selection bias (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Innes 1998; Miki 2018; Plapler 2016; Pohjolainen 2000; Schattenkirchner 2003; Shin 2013; von Heymann 2013; Ximenes 2007; Yakhno 2006).
Blinding
Performance bias
Fifteen studies adequately blinded participants (Amlie 1987; Bakshi 1994; Dreiser 2003; Hancock 2007; Hosie 1993; Innes 1998; Lacey 1984; Orava 1986; Pohjolainen 2000; Stratz 1990; Szpalski 1994; Videman 1984; von Heymann 2013; Yakhno 2006; Zippel 2007). Twelve trials adequately blinded careproviders (Amlie 1987; Babej‐Dolle 1994; Bakshi 1994; Dreiser 2003; Hosie 1993; Innes 1998; Lacey 1984; Pohjolainen 2000; Schattenkirchner 2003; Szpalski 1994; Videman 1984; Yakhno 2006). Thirteen studies adequately blinded outcome assessors (Babej‐Dolle 1994; Brown 1986; Dreiser 2003; Hancock 2007; Hosie 1993; Innes 1998; Nadler 2002; Pohjolainen 2000; Szpalski 1994; Videman 1984; von Heymann 2013; Yakhno 2006; Zippel 2007). The remaining studies either (i) had inadequate blinding of participants, careproviders, and outcome assessors (scored at high risk); or (ii) provided insufficient details to determine adequacy of blinding (scored as unclear risk). In two studies, blinding of careproviders was not possible, since they had to perform either real or sham spinal manipulation; however, in both studies, the outcome assessors were blinded (Hancock 2007; von Heymann 2013).
Regarding co‐interventions: we allowed paracetamol as rescue medication. No other analgesics or anti‐inflammatory drugs were allowed. Five studies allowed the use of paracetamol as rescue medication (Amlie 1987; Metscher 2001; von Heymann 2013; Yakhno 2006; Zippel 2007), whereas in one study, the use of rescue medication terminated trial participation (Dreiser 2003). In one study, all participants received 1g of paracetamol four times a day (Hancock 2007). Three studies prescribed bedrest to all participants (Szpalski 1990; Szpalski 1994; Wiesel 1980). We scored 14 studies that avoided co‐interventions at low risk of bias (Aghababian 1986; Amlie 1987; Babej‐Dolle 1994; Bakshi 1994; Brown 1986; Hancock 2007; Hosie 1993; Metscher 2001; Pohjolainen 2000; Schattenkirchner 2003; Stratz 1990; von Heymann 2013; Waterworth 1985; Zippel 2007). We scored one study at high risk of bias because they did not restrict co‐interventions after the first follow‐up at 30 minutes, and participants could choose between inpatient and outpatient treatment, which influenced the amount of additional treatment for each participant (Shin 2013). We scored the remaining studies as unclear risk of bias.
Most studies provided insufficient information on compliance, therefore, we scored them as unclear risk of bias. Four studies either reported that compliance was acceptable or provided details regarding compliance (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Stratz 1990).
Overall, we determined twelve studies to be at low risk of performance bias (Amlie 1987; Babej‐Dolle 1994; Bakshi 1994; Dreiser 2003; Hancock 2007; Hosie 1993; Innes 1998; Lacey 1984; Pohjolainen 2000; Szpalski 1994; Videman 1984; Yakhno 2006).
Detection bias
The majority of studies adequately reported the timing of outcome assessments, and this timing was similar in most cases; therefore, we scored 28 studies at low risk of bias. Four studies either did not report clearly on the timing of the outcome assessment (and we scored them as unclear risk of bias (Brown 1986; Shin 2013)); or they had different timing of outcome assessment between participants (and we scored them at high risk of bias (Babej‐Dolle 1994; Stratz 1990)). This domain also concerned the adequate blinding of the outcome assessor, which we listed above (see Performance bias; we scored 13 trials at low risk).
Overall, we determined twelve studies to have a low risk of detection bias (Brown 1986; Dreiser 2003; Hancock 2007; Hosie 1993; Innes 1998; Nadler 2002; Pohjolainen 2000; Szpalski 1994; Videman 1984; von Heymann 2013; Yakhno 2006; Zippel 2007).
Incomplete outcome data
We scored most studies at low risk of bias concerning dropout rates. However, three studies did not adequately report dropouts and we scored them as unclear risk of bias (Lacey 1984; Shin 2013; Wiesel 1980); five studies reported substantial drop‐out rates or clear differences between groups, and we scored them at high risk of bias (Aghababian 1986; Miki 2018; Plapler 2016; Postacchini 1988; von Heymann 2013). Twelve studies performed an intention‐to‐treat (ITT) analysis and we scored them at low risk of bias (Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hancock 2007; Schattenkirchner 2003; Shin 2013; Szpalski 1994; von Heymann 2013; Ximenes 2007; Yakhno 2006; Zippel 2007). The remaining trials either did not report (unclear risk of bias), or did not perform an ITT analysis (high risk of bias).
Overall, we determined ten studies to have a low risk of attrition bias (Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hancock 2007; Schattenkirchner 2003; Szpalski 1994; Ximenes 2007; Yakhno 2006; Zippel 2007).
Selective reporting
Three RCTs registered their study protocol in an accessible clinical trial registry, published it, or made their study protocol available on request (Hancock 2007; Shin 2013; von Heymann 2013). We scored registered trials at low risk for reporting bias. If there was no study protocol, we scored the risk of bias as unclear. We scored two studies at high risk of bias for selective reporting: Nadler 2002 had a study protocol available, but several outcomes of the primary treatment groups were not compared, and they did not report the results of two comparison groups; and Postacchini 1988 reported they intended to recruit a control group of untreated participants, but did not include this group in the final analyses because not enough participants agreed to enrol in this group, and most of them were lost to follow‐up. They reported this in the discussion section, but not in the methods section.
Therefore, we determined only three studies at low risk for the domain of reporting bias (Hancock 2007; Shin 2013; von Heymann 2013).
Other potential sources of bias
We did not find any other potential sources of bias. Therefore, we determined all studies to be at low risk for the domain of other potential sources of bias.
Summary
To summarise, the most common sources of bias were due to insufficient information, for instance, on the method of randomisation, allocation concealment (selection bias), and blinding (performance and detection bias). Often, there was no information available as to whether they performed an ITT analysis (attrition bias). Most did not register their study protocols, which increases the risk for selective reporting (reporting bias). Lastly, it remains unclear if there was publication bias.
Effects of interventions
Summary of findings for the main comparison. NSAIDs compared to placebo in people with acute low back pain.
| NSAIDs compared to placebo in people with acute low back pain | ||||||
| Patient or population: adults (≥ 18 years of age) with acute low back pain Setting: primary and secondary care settings, mainly general practice and outpatient clinics Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with NSAIDs | |||||
|
Pain intensity VAS (0 to 100; lower = better) Follow‐up: ≤ 3 weeks (range 7 to 15 days) |
The mean pain intensity in the placebo group ranged from 7.9 to 33.9 | The mean pain intensity in the NSAID group was 7.29 lower (10.98 lower to 3.61 lower) |
‐ | 815 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | MID is 10 points on a 0 to 100 scale |
|
Disability RMDQ (0 to 24; lower = better) Follow‐up: ≤ 3 weeks (range 7 to 14 days) |
The mean disability in the placebo group ranged from 6.0 to 7.3 | The mean disability in the NSAID group was 2.02 lower (2.89 lower to 1.15 lower) |
‐ | 471 (2 RCTs) | ⊕⊕⊕⊕ High | MID is 2.4 points on a 0 to 24 scale |
|
Proportion of participants experiencing global improvement Various dichotomised Likert scales; lower = better Follow‐up ≤ 3 weeks (range 1 to 15 days) |
Study population | RR 1.40 (1.12 to 1.75) | 1201 (5 RCTs) | ⊕⊕⊝⊝ Low b,c | ||
| 367 per 1000 | 514 per 1000 (412 to 643) | |||||
|
Proportion of participants experiencing adverse events Follow‐up range 1 day to 12 weeks |
Study population | RR 0.86 (0.63 to 1.18) | 1394 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,d,e,f | ||
| 111 per 1000 | 95 per 1000 (70 to 130) | |||||
|
Return to work (%) Follow‐up: 7 days |
Study population | RR 1.48 (0.98 to 2.23) |
266 (1 RCT) |
⊕⊝⊝⊝ Very lowa,g | ||
| 212 per 1000 | 314 per 1000 (208 to 473) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; VAS: visual analogue scale; RMDQ: Roland Morris Disability Questionnaire; RR: risk ratio; RCT: randomised controlled trial; MID: minimal important difference | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. More than 25% of the included participants were from studies with a high risk of bias. bDowngraded one level due to inconsistency. There is moderate to substantial heterogeneity with an I² of 52% and a wide variance in point estimates across studies. cDowngraded one level due to indirectness. Two studies (three treatment arms) included a small percentage of participants with additional sciatic complaints (different population). NSAID tablets, capsules or intramuscular injections were used (different intervention). Treatment time and timing of outcome assessments ranged (differences in outcome), which could make the results less generalisable. dDowngraded one level due to inconsistency. On visual inspection, there is a wide variance in point estimates across studies. Follow‐up duration to measure and report adverse events varied greatly, and was probably too short in a few studies to adequately detect all adverse events. eDowngraded one level due to indirectness. Two studies (three treatment arms) included a small percentage of participants with additional sciatic complaints (different population). Most studies had a relatively short follow‐up time (ranging from 1 day to 2 to 3 weeks), except for one study (follow‐up time 12 weeks). Therefore, it is unclear if the follow‐up time frame was sufficient to measure and report all relevant outcomes regarding adverse events. fDowngraded one level due to imprecision. The total number of events was less than 300. gDowngraded two levels due to imprecision. Only one study was included in the comparison, with a total number of events far less than 300. The 95% CI includes both no effect and the threshold of appreciable benefit.
Summary of findings 2. Selective COX‐2 inhibitors compared to non‐selective NSAIDs for acute low back pain.
| Selective COX‐2 inhibitors compared to non‐selective NSAIDs for acute low back pain | ||||||
| Patient or population: adults (≥ 18 years of age) with acute low back pain (LBP) Setting: primary and secondary care settings, mainly general practice and outpatient clinics Intervention: selective COX‐2 inhibitors Comparison: non‐selective NSAIDs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐selective NSAIDs | Risk with COX‐2 inhibitors | |||||
|
Change in pain intensity from baseline VAS (0 to 100; lower = better) Follow‐up ≤ 3 weeks (range 7 to 10 days) |
The mean change in pain intensity from baseline in the non‐selective NSAID group ranged from 38 to 41 | The mean change in pain intensity from baseline in the COX‐2 inhibitors group was 2.60 lower (9.23 lower to 4.03 higher) | ‐ | 437 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | MID is 10 points on a 0 to 100 scale |
|
Disability ODI (0 to 50; lower = better) Follow‐up ≤ 3 weeks (range 7 to 10 days) |
One trial reported a mean difference in disability score of ‐7.00 (95% CI ‐13.15 to ‐0.85) after 10 days, showing a statistically significant and clinically relevant difference in favour of the nimesulide arm. One trial reported a mean decrease in baseline disability of 32% in both the valdecoxib and diclofenac arm at 1 week follow‐up, showing no clear difference between study arms. |
‐ | 437 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | MID is 5 points on a 0 to 50 scale | |
|
Proportion of participants experiencing global improvement % of dichotomized Likert scale Follow‐up ≤ 3 weeks (7 days) |
One trial reported the percentage of participants who reported their pain relief as 'a lot better' or 'completely better' at 1 week follow‐up, which was similar in both the valdecoxib (80%) and diclofenac (81%) arm, showing no clear difference between study arms. | 333 (1 RCT) |
⊕⊕⊝⊝ Lowa,c | |||
|
Proportion of participants experiencing adverse events Follow‐up range 10 to 37 days |
Study population | RR 0.97 (0.63 to 1.50) | 444 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d | ||
| 248 per 1000 | 240 per 1000 (156 to 372) | |||||
|
Return to work (%) Follow‐up: N/A |
Not reported | ‐ | ‐ | N/A | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; VAS: visual analogue scale; ODI: Oswestry Disability Index; RR: risk ratio; RCT: randomised controlled trial; N/A: not available; MID: minimal important difference | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. More than 25% of the included participants were from a study with high risk of bias. bDowngraded one level due to inconsistency. There is moderate to substantial heterogeneity with an I² of 57%, and a wide variance in point estimates. cDowngraded one level due to imprecision. The total number of events was far less than 300, leading to a wide confidence interval. dDowngraded two levels due to imprecision. The total number of events was far less than 300, leading to a wide confidence interval. The 95% confidence interval includes both no effect and the threshold of appreciable benefit or harm.
We used a random‐effects model to pool results throughout this review, assuming some between‐study variation, when taking into account the differences in population, types and frequency of NSAIDs used, follow‐up duration, and the country where the trial was performed. This approach provided us with a slightly more conservative estimate of the 95% confidence interval (CI). We planned subgroup analyses, but we could not conduct these due to lack of available data.
1. NSAIDs compared to placebo
See Table 1 for this comparison.
Nine studies compared NSAIDs with placebo (Amlie 1987; Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Lacey 1984; Nadler 2002; Postacchini 1988; Szpalski 1994; von Heymann 2013), two of which compared two different NSAIDs with placebo (Babej‐Dolle 1994; Dreiser 2003). Diclofenac was the most common NSAID evaluated (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Postacchini 1988; von Heymann 2013). Other studies evaluated ibuprofen (two studies), piroxicam, dipyrone, and tenoxicam. We considered four studies at low risk of bias (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Szpalski 1994). Follow‐up ranged from one day to two months. Treatment duration ranged from one day to four weeks. Most often, tablets or capsules were used as the mode of delivery, except for Babej‐Dolle 1994, which used intramuscular injections, and Szpalski 1994, which started with an intramuscular injection followed by capsules. Postacchini 1988 compared diclofenac tablets to an anti‐edema gel that functioned as a placebo.
One study added a placebo for blinding purposes only, and did not report results for the comparison of NSAID versus placebo (Nadler 2002). One study only reported a combination score of improvement in pain, disability, and spinal mobility, which showed no significant differences between NSAID and placebo after three weeks and two months (Postacchini 1988). One study closed the placebo arm early, after an interim analysis showing superiority of both treatment arms (diclofenac or spinal manipulation) compared to placebo (von Heymann 2013). They presented data of the combined group of both active treatment arms compared to placebo, without providing a subgroup analysis of diclofenac versus placebo, hence, these data could not be used in this comparison.
Primary outcomes
Pain intensity
Four studies (five treatment arms, N = 815) reported on short‐term pain reduction from baseline (visual analogue scale (VAS) 0 to 100) and provided data at a time point relevant for our review that could be pooled (Amlie 1987; Dreiser 2003; Hancock 2007; Szpalski 1994). One study reported on pain intensity at a maximum of six hours follow‐up only, and was excluded from the comparison (Babej‐Dolle 1994). NSAIDs reduced pain intensity more than placebo (mean difference (MD) ‐7.29, 95% CI ‐10.98 to ‐3.61; I² 35%; Analysis 1.1; Figure 4). The magnitude of the effect is small and probably not clinically relevant. The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
1.1. Analysis.
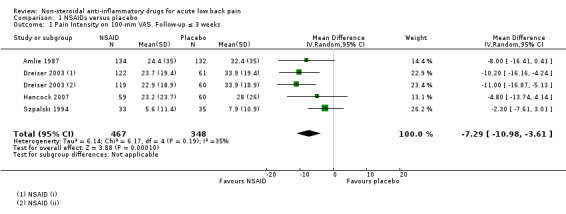
Comparison 1 NSAIDs versus placebo, Outcome 1 Pain Intensity on 100‐mm VAS. Follow‐up ≤ 3 weeks.
4.
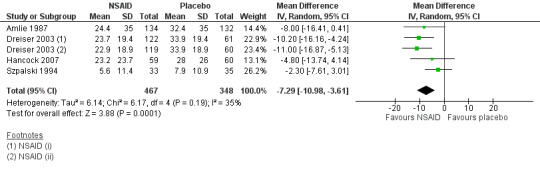
Forest plot of comparison: 1 NSAIDs versus placebo, outcome: 1.1 Pain intensity on 100‐mm VAS. Follow‐up ≤ 3 weeks.
Disability
Two studies (three treatment arms, N = 471) reported on short‐term change in disability from baseline (Roland Morris Disability Questionnaire (RMDQ) 0 to 24 scale) during three weeks follow‐up (Dreiser 2003; Hancock 2007). NSAIDs reduced disability more than placebo (MD ‐2.02, 95% CI ‐2.89 to ‐1.15; I² 0%; Analysis 1.2; Figure 5). The difference is small and probably not clinically relevant. The quality of this evidence is high; we did not downgrade the evidence.
1.2. Analysis.
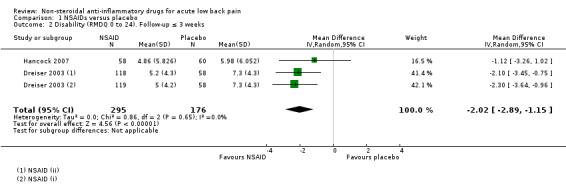
Comparison 1 NSAIDs versus placebo, Outcome 2 Disability (RMDQ 0 to 24). Follow‐up ≤ 3 weeks.
5.
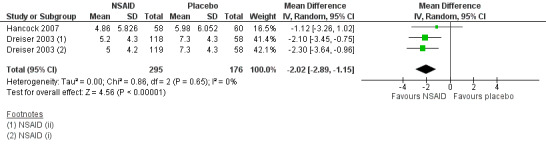
Forest plot of comparison: 1 NSAIDs versus placebo, outcome: 1.2 Disability (RMDQ 0 to 24). Follow‐up ≤ 3 weeks.
Global improvement
Five studies (seven treatment arms, N = 1201) reported on the proportion of participants experiencing global improvement (Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Lacey 1984; Szpalski 1994). There was a greater likelihood that those who received NSAIDs experienced global improvement over those who took placebo (risk ratio (RR) 1.40, 95% CI 1.12 to 1.75; I² 52%; Analysis 1.3; Figure 6). The effect size is probably not clinically relevant. The heterogeneity could not be explained, but some differences existed between the studies: different cutoff points to define improvement; different scales and types of outcome measures; varied timing of outcome assessments (ranged from two days to two weeks); and different modes of delivery. The quality of this evidence is low; we downgraded the evidence two levels due to inconsistency and indirectness.
1.3. Analysis.
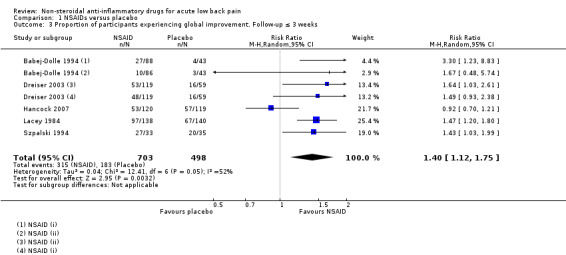
Comparison 1 NSAIDs versus placebo, Outcome 3 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks.
6.
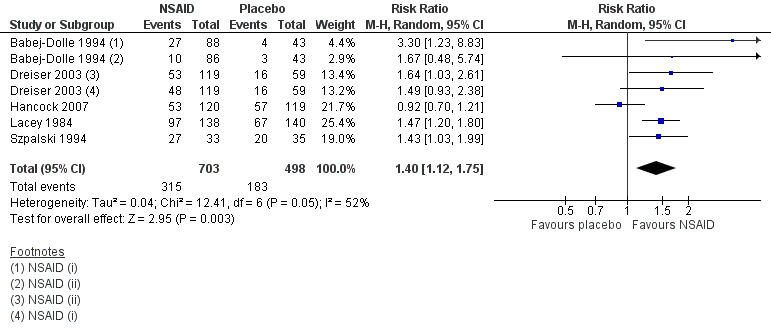
Forest plot of comparison: 1 NSAIDs versus placebo, outcome: 1.3 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks.
Adverse events
Six studies (eight treatment arms, N = 1394) reported on the proportion of participants experiencing adverse events (Amlie 1987; Babej‐Dolle 1994; Dreiser 2003; Hancock 2007; Lacey 1984; Szpalski 1994). The results between the NSAID and placebo groups were inconclusive for experiencing adverse events (RR 0.86, 95% CI 0.63 to 1.18; I² 0%; Analysis 1.4). The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, inconsistency, indirectness, and imprecision.
1.4. Analysis.
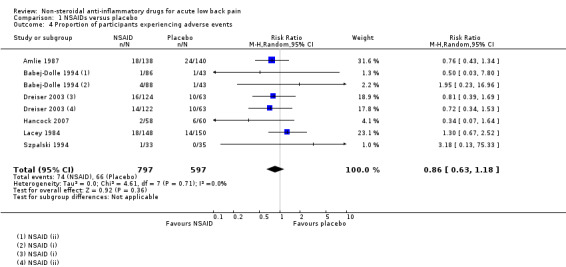
Comparison 1 NSAIDs versus placebo, Outcome 4 Proportion of participants experiencing adverse events.
Return to work
One study (N = 266) reported data on return to work (Amlie 1987). The results for return to work were inconclusive between the NSAID and placebo groups (RR 1.48; 95% CI 0.98 to 2.23); the difference was not clinically relevant. The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias (one level), and imprecision (two levels).
Long‐term follow‐up & sensitivity analyses
Two studies provided long‐term follow‐up data. Hancock 2007 showed no significant differences between NSAIDs and placebo on the mean pain score, mean disability score, and mean global perceived effect score after 12 weeks. Postacchini 1988 used a combination score of improvement in pain, disability, and spinal mobility, which showed no significant differences between NSAIDs and placebo after two and six months.
We performed one sensitivity analysis for pain intensity; removing the studies at high risk of bias did not change the results (Amlie 1987; Lacey 1984).
We performed two sensitivity analyses for global improvement; removing the study at high risk of bias did not change the result (Lacey 1984). Removing studies with mixed participant population increased the heterogeneity, and the result was no longer statistically significant (RR 1.29, 95% CI 0.97 to 1.72; I² 58%; Babej‐Dolle 1994; Lacey 1984).
We performed two sensitivity analyses for adverse events; (i) removing the studies at high risk of bias (Amlie 1987; Lacey 1984), and (ii) removing studies with a mixed participant population (Babej‐Dolle 1994; Lacey 1984). This did not change the results in either analyses.
We did not conduct sensitivity analyses for the other outcomes.
2. Selective COX‐2 inhibitors compared to non‐selective NSAIDs
See Table 2 for this comparison.
Seventeen studies compared NSAIDs to other NSAIDs (Aghababian 1986; Agrifoglio 1994; Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hosie 1993; Jaffe 1974; Orava 1986; Plapler 2016; Pohjolainen 2000; Schattenkirchner 2003; Stratz 1990; Wiesel 1980; Ximenes 2007; Yakhno 2006; Zippel 2007), two of which compared a selective COX‐2 inhibitor to a non‐selective NSAID (Pohjolainen 2000; Ximenes 2007). We considered four studies at low risk of bias (Babej‐Dolle 1994; Dreiser 2003; Pohjolainen 2000; Yakhno 2006). Diclofenac was the most common NSAID used for comparison (Agrifoglio 1994; Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Schattenkirchner 2003; Stratz 1990; Yakhno 2006; Zippel 2007). The other types of NSAIDs used were naproxen, ibuprofen, indomethacin, diflunisal, meloxicam, lornoxicam, aceclofenac, felbinac, ketorolac‐trometamol, etofenamat, dexketoprofen, and phenylbutazone. The latter NSAID (phenylbutazone) was withdrawn from the market for safety reasons. Follow‐up time ranged from one day to two weeks; this was similar for treatment duration. Most often, NSAIDs were used in the form of tablets or capsules, except for Babej‐Dolle 1994, Stratz 1990, and Zippel 2007, which used intramuscular injections; Colberg 1996, which started with either an intramuscular diclofenac injection or an intravascular meloxicam injection followed by tablets; and Hosie 1993, which compared ibuprofen capsules with a felbinac foam.
For further information on the results of the fifteen studies comparing non‐selective NSAIDs to each other, refer to the subheading 'Non‐selective versus non‐selective NSAIDs' below. When focusing on the two studies that compared selective COX‐2 inhibitors versus non‐selective NSAIDs, Pohjolainen 2000 compared nimesulide to ibuprofen (10 days) in a double‐blind, double‐dummy concept, and we considered it at low risk of bias. Ximenes 2007 compared valdecoxib versus diclofenac (7 days) in a double‐blind but not double‐dummy concept, and we considered it at high risk of bias.
Primary outcomes
Pain intensity
Two studies (N = 437) reported on short‐term pain reduction from baseline (Pohjolainen 2000; Ximenes 2007). The I² statistic was 57%, indicating moderate to substantial statistical heterogeneity. On a clinical level, these studies were sufficiently comparable to pool results. The pooled mean change in pain intensity score from baseline was ‐2.60 (95% CI ‐9.23 to 4.03), indicating no clear difference in pain reduction (Analysis 2.1). The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and inconsistency.
2.1. Analysis.
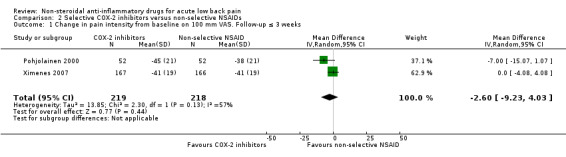
Comparison 2 Selective COX‐2 inhibitors versus non‐selective NSAIDs, Outcome 1 Change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 3 weeks.
Disability
Both studies (N = 437) used the Oswestry Disability Index (0 to 50 scale). Pohjolainen 2000 reported a substantial improvement in mean disability score within both groups at 10‐day follow‐up. Nimesulide reduced disability more than ibuprofen (MD ‐7.00, 95% CI ‐13.15 to ‐0.85); this amount was clinically relevant. Ximenes 2007 reported similar improvement in both groups at one‐week follow‐up, with a mean decrease in baseline disability of 32% in both the valdecoxib and diclofenac arm, and no clear difference between study arms. The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
Global improvement
Ximenes 2007 (N = 333) reported that at day 7, the percentage of participants reporting pain relief as 'a lot' or 'complete' was 80% in the valdecoxib arm versus 81% in the diclofenac arm, showing no clear difference between study arms. The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision. Pohjolainen 2000 did not report data on global improvement.
Adverse events
Both studies (N = 444) reported on the proportion of participants experiencing adverse events. There was no clear difference between the treatment arms (RR 0.97, 95% CI 0.63 to 1.50; I² 22%; Analysis 2.2). The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias (one level), and imprecision (two levels). Both studies reported on the proportion of participants experiencing gastrointestinal adverse events. The results were inconclusive (RR 0.60, 95% CI 0.33 to 1.09; I² 14%; Analysis 2.3).
2.2. Analysis.
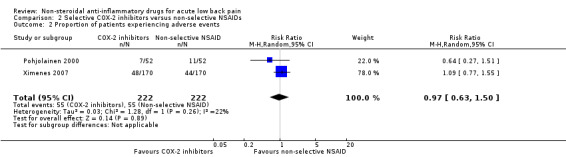
Comparison 2 Selective COX‐2 inhibitors versus non‐selective NSAIDs, Outcome 2 Proportion of patients experiencing adverse events.
2.3. Analysis.
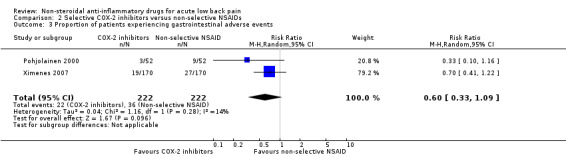
Comparison 2 Selective COX‐2 inhibitors versus non‐selective NSAIDs, Outcome 3 Proportion of patients experiencing gastrointestinal adverse events.
Return to work
There were no data reported for this outcome.
Long‐term follow‐up & sensitivity analyses
There was no long‐term follow‐up for either study.
We performed a sensitivity analysis removing the study with high risk of bias (Ximenes 2007) from the results for pain intensity, adverse events, and gastrointestinal adverse events (Analysis 2.1; Analysis 2.2; Analysis 2.3). This did not change the overall results and conclusion.
We did not perform the second sensitivity analysis since the studies did not include mixed participant populations.
3. Non‐selective versus non‐selective NSAIDs
As previously mentioned, fifteen studies compared a non‐selective NSAID to another non‐selective NSAID (Aghababian 1986; Agrifoglio 1994; Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hosie 1993; Jaffe 1974; Orava 1986; Plapler 2016; Schattenkirchner 2003; Stratz 1990; Wiesel 1980; Yakhno 2006; Zippel 2007), three of which we considered at low risk of bias (Babej‐Dolle 1994; Dreiser 2003; Yakhno 2006). Diclofenac was used as a comparison drug in nine studies (Agrifoglio 1994; Babej‐Dolle 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Schattenkirchner 2003; Stratz 1990; Yakhno 2006; Zippel 2007), and was compared to many different NSAIDs: aceclofenac (twice), dipyrone, piroxicam, meloxicam, ibuprofen, etofenamat, lornoxicam, and dexketoprofen. Other NSAIDs used in comparisons were ibuprofen (twice), diflunisal (twice), indomethacin (twice), naproxen, aspirin, ketorolac‐trometamol, phenylbutazone (off the market), and felbinac foam. We will descriptively summarise the results of these studies below.
Primary outcomes
Pain intensity
Two studies reported on pain intensity at a maximum of six hours follow‐up only; we excluded them from this comparison (Babej‐Dolle 1994; Zippel 2007).
Thirteen studies (N = 1823) reported data on pain intensity at a relevant time point. Ten studies showed no clear difference in pain relief on different scales (either 4‐ or 5‐point ordinal scales, 100‐mm VAS, 11‐point numeric rating scale (NRS), or pain point calculations) after three to eight days (Agrifoglio 1994; Bakshi 1994; Colberg 1996; Dreiser 2003; Hosie 1993; Jaffe 1974; Orava 1986; Plapler 2016; Stratz 1990; Wiesel 1980).
Two studies explored acelofenac and diclofenac. Schattenkirchner 2003 (N = 227) reported that aceclofenac reduced pain intensity more than diclofenac; the results were statistically significant but not clinically relevant (between‐group difference of 5.5 on 100‐mm VAS). Agrifoglio 1994 (N = 100) found no clear difference between the two NSAIDs.
Aghababian 1986 (N = 56) reported that 100% of those who took diflunisal reported none or mild pain after two weeks compared to 88% of those who took naproxen, but no significance tests were reported, and the difference was not clinically relevant.
Yakhno 2006 (N = 220) reported a sum of pain intensity differences from baseline to day six of 4.2 for those who took lornoxicam versus 3.8 for those who took diclofenac, a statistically significant but not clinically relevant difference.
The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
Disability
Five studies (N = 1006) reported data on disability at a relevant time point, four of which showed no clear difference in disability or functional status between groups, measured with either the RMDQ (Dreiser 2003; Zippel 2007), or a 4‐point ordinal scale (Jaffe 1974; Orava 1986).
One study showed that aceclofenac reduced pain more than diclofenac by a between‐group difference of 4.5% on the 100‐point Quebec Back Pain Disability Score; the trail authors stated the results were statistically significant but not clinically relevant (Schattenkirchner 2003).
The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
Global improvement
Seven studies (N = 987) reported data on the proportion of participants who experienced global improvement, five of which showed similar improvement between groups, with no statistically significant or clinically relevant differences (Aghababian 1986; Bakshi 1994; Colberg 1996; Dreiser 2003; Stratz 1990).
Agrifoglio 1994 (N = 100) reported that 87% of those who took aceclofenac reported global improvement versus 79% of participants who took diclofenac; a statistically significant but not clinically relevant difference.
Babej‐Dolle 1994 (N = 174) reported that 32% of those who received dipyrone intramuscular injections were completely recovered after two days versus 12% of the participants who received diclofenac intramuscular injections; a statistically and clinically relevant difference.
The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
Adverse events
Fourteen studies (N = 2337) reported data on adverse events, 11 of which showed no clear difference between treatments in the proportion of participants experiencing adverse events (Aghababian 1986; Bakshi 1994; Colberg 1996; Dreiser 2003; Hosie 1993; Jaffe 1974; Plapler 2016; Schattenkirchner 2003; Stratz 1990; Yakhno 2006; Zippel 2007).
Aghababian 1986 (N = 56) reported no adverse events.
Three studies reported a difference in the proportion of participants experiencing adverse events: Agrifoglio 1994 (N = 100) reported 2% in the aceclofenac arm versus 12% in the diclofenac arm; Babej‐Dolle 1994 (N = 174) reported 5% in the dipyrone versus 1% in the diclofenac arm; and Orava 1986 (N = 133) reported 18% in the diflunisal arm versus 31% in the indomethacin arm; this difference was considered both statistically significant and clinically relevant.
Schattenkirchner 2003 (N = 227) also evaluated aceclofenac and diclofenac, but found no clear difference.
The quality of this evidence is moderate; we downgraded the evidence one level due to risk of bias.
Return to work
Wiesel 1980 (N = 30) found no clear difference for return to work between those who took aspirin or phenylbutazone. The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, indirectness, and imprecision.
4. NSAIDs compared to paracetamol
Three studies, with four treatment arms, compared NSAIDs to paracetamol or acetaminophen (Miki 2018; Nadler 2002; Wiesel 1980). They respectively compared loxoprofen, ibuprofen, and aspirin or phenylbutazone (two study arms) to paracetamol, all in the form of capsules or tablets. We considered none of the studies at low risk of bias. Follow‐up ranged from four days to four weeks. Treatment duration ranged from two days to four weeks.
Primary outcomes
Pain intensity
All studies reported on pain intensity, but not all in comparable formats. Nadler 2002 used a mean change score (NRS scale 0 to 5), and did not directly compare ibuprofen versus acetaminophen; similar to Miki 2018, which reported a pain difference score between the two study arms. We pooled the results from these two studies (N = 289) using the standardised mean difference (SMD), which showed no clear difference between NSAID and paracetamol on short‐term pain relief (SMD ‐0.12, 95% CI ‐0.35 to 0.12; I² 0%; Analysis 3.1). This is comparable with an MD of ‐0.07 (95% CI ‐0.25 to 0.11; I² 0%).
3.1. Analysis.
Comparison 3 NSAIDs versus paracetamol, Outcome 1 Mean difference in pain intensity on various scales. Follow‐up ≤ 3 weeks.
The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
Wiesel 1980 used pain‐point calculations, a sum score that added ‘points’ each day, depending on the severity of back pain. Therefore, we could not compare their results to the other studies, although results were similar (i.e. no significant differences in pain reduction at follow‐up among two NSAID arms and one paracetamol arm).
Disability
Nadler 2002 (N = 219) reported no clear difference in the mean change scores in disability for NSAIDs compared to paracetamol after four days. No other studies reported on disability outcomes. The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
Global improvement
There were no data reported on this outcome.
Adverse events
Two studies (N = 289) reported on the proportion of participants who experienced adverse events. Nadler 2002 (N = 219) reported 10% in the NSAID arm versus 4% in the paracetamol arm, and no serious side effects. Miki 2018 (N = 70) reported 14% in the NSAID arm versus 3% in the paracetamol arm (Analysis 3.2). We did not pool these results because of considerable clinical heterogeneity. Specifically, (i) Nadler 2002 had a short follow‐up duration (four days with two days of treatment), while Miki 2018 had a longer follow‐up (four weeks of treatment and follow‐up); and (ii) Miki 2018 had high rates of loss to follow‐up for reasons not reported. The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
3.2. Analysis.
Comparison 3 NSAIDs versus paracetamol, Outcome 2 Proportion of participants experiencing adverse events.
Return to work
Wiesel 1980 (N = 45) reported data on return to work, showing no clear differences among the three arms (three NSAID arms and one paracetamol arm). The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, indirectness, and imprecision.
Long‐term follow‐up & sensitivity analyses
One study reported data on the pain difference score after four weeks, showing no clear difference between the two study arms (Miki 2018).
We did not perform sensitivity analyses for this comparison, since there were no studies at low risk of bias, and none with mixed participant populations.
5. NSAIDs compared to other drug treatment
Four studies compared NSAIDs to other drugs (Brown 1986; Innes 1998; Metscher 2001; Videman 1984). Two of these compared NSAIDs to acetaminophen with codeine (Brown 1986; Innes 1998); Metscher 2001 compared NSAIDs to tramadol hydrochloride, and Videman 1984 compared NSAIDs to meptazinol. We considered one study at low risk of bias (Innes 1998). Follow‐up ranged from seven days to three weeks. Treatment duration ranged from seven days (or until pain free) to a maximum of three weeks. The studies used either tablets or capsules.
Primary outcomes
Pain intensity
All four studies (N = 391) reported on pain intensity, but data were inadequately reported, and therefore, we were unable to conduct a meta‐analysis. Innes 1998 only reported a mean change score after six hours, and we excluded it from this comparison. Brown 1986 used pain assessments by the participant on a 3‐point ordinal scale, showing a curve of slow improvement over 15 days. Metscher 2001 found that those who took NSAIDs reported a mean pain change score (VAS 0 to 100 scale) of ‐6 (SD 4) after seven days compared to those who took tramadol hydrochloride, which was statistically significant but not clinically relevant. Videman 1984 reported a mean pain reduction (VAS 0 to 100 scale) of 45 in the NSAID arm versus 40 in the meptazinol arm (data extracted from graphs), showing no clear difference between study arms. The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
Disability
There were no data reported on this outcome.
Global improvement
We pooled the results of two studies (N = 162) that used the same reference drug as a comparison (acetaminophen with codeine (Brown 1986; Innes 1998)). Both studies reported on the proportion of participants who experienced global improvement within a three‐week follow‐up time frame. The pooled RR was 1.01 (95% CI 0.81 to 1.25; I² 0%; Analysis 4.1). The quality of this evidence is moderate; we downgraded the evidence one level due to imprecision.
4.1. Analysis.
Comparison 4 NSAIDs versus other drug treatment, Outcome 1 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks.
Adverse events
All four studies (N = 391) comparing NSAIDs to other drug treatments reported on the proportion of participants experiencing adverse events. Clinical heterogeneity was considerable, and therefore, we decided not to pool these data. Those who took NSAIDs were more likely to report adverse events than those who took other drugs (RR ranged from 0.53 to 0.83, 95 % CI ranged from 0.18 to 2.41; Analysis 4.2). The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
4.2. Analysis.
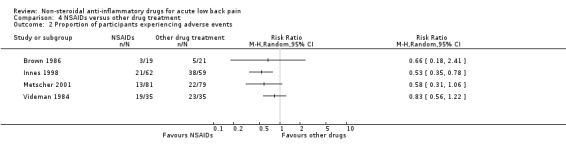
Comparison 4 NSAIDs versus other drug treatment, Outcome 2 Proportion of participants experiencing adverse events.
Return to work
There were no data reported on this outcome.
Long‐term follow‐up & sensitivity analyses
There was no long‐term follow‐up available for this comparison.
For global improvement, we performed a sensitivity analysis by removing the study at high risk of bias (Brown 1986). This did not change the results. We were unable to perform sensitivity analyses for the other outcomes.
6. NSAIDs compared to non‐drug treatment
Seven studies compared NSAIDs to non‐drug treatment (Hancock 2007; Nadler 2002; Postacchini 1988; Shin 2013; Szpalski 1990; von Heymann 2013; Waterworth 1985). Specifically, four studies compared NSAIDs to spinal manipulation (Hancock 2007; Postacchini 1988; von Heymann 2013; Waterworth 1985); two studies compared NSAIDs to physiotherapy (Postacchini 1988; Waterworth 1985); two studies compared NSAIDs to bedrest (Postacchini 1988; Szpalski 1990); one to heat wrap (Nadler 2002); and one to motion style acupuncture treatment (MSAT (Shin 2013)). We considered one study at low risk of bias (Hancock 2007). Follow‐up ranged from four days to six months, and treatment duration from one day to four weeks. NSAIDs were generally administered in tablet or capsule form, with the exception of Shin 2013, who administered the NSAID as a single intramuscular injection. Due to considerable clinical heterogeneity, we decided not to pool these studies. Analyses are presented below, grouped according to the non‐drug comparison treatment. We were unable to perform the planned sensitivity analyses for this comparison.
6a. NSAIDs compared to spinal manipulation or physiotherapy
Pain intensity
Four studies (six treatment arms, N = 353) compared NSAIDs to spinal manipulation, physiotherapy, or both (Hancock 2007; Postacchini 1988; von Heymann 2013; Waterworth 1985). We considered one study at low risk of bias (Hancock 2007). All four studies reported on pain reduction from baseline (VAS 0 to 100 scale) at a relevant time point. The I² statistic was 94%, representing substantial statistical heterogeneity, and consequently, we did not pool the data.
Hancock 2007 showed no clear difference in pain reduction when NSAIDs were compared with spinal manipulation (MD 0.80, 95% CI ‐7.11 to 8.71; Analysis 5.1). In contrast, von Heymann 2013 showed that spinal manipulation reduced pain more than NSAIDs (MD 18.31, 95% CI 15.62 to 21.00; Analysis 5.1); this was clinically relevant.
5.1. Analysis.
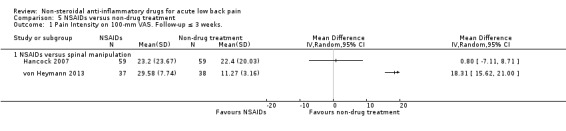
Comparison 5 NSAIDs versus non‐drug treatment, Outcome 1 Pain Intensity on 100‐mm VAS. Follow‐up ≤ 3 weeks..
Waterworth 1985 presented pain intensity scores on a 4‐point scale, and showed no clear difference after 12 days. Postacchini 1988 only presented a combination score of improvement in pain, disability, and spinal mobility, and showed no clear difference between NSAIDs and either spinal manipulation or physiotherapy, after three weeks and two months. The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, inconsistency, and imprecision.
Disability
Two studies (N = 193) reported on short‐term disability (Hancock 2007; von Heymann 2013). von Heymann 2013 did not report mean baseline data on disability, only median baseline values, and the mean change in disability from baseline. von Heymann 2013 showed that spinal manipulation reduced disability more than NSAIDs by a statistically significant and clinically relevant difference; Hancock 2007 showed no clear difference in disability reduction. The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, inconsistency, and imprecision.
Global improvement
Two studies (three treatment arms; N = 180) reported on global improvement (von Heymann 2013; Waterworth 1985). von Heymann 2013 showed that those who received spinal manipulation reported global improvement over NSAIDs by a statistically significant and clinically relevant difference; Waterworth 1985 showed no clear difference between study arms (Analysis 5.2). The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, inconsistency, and imprecision.
5.2. Analysis.
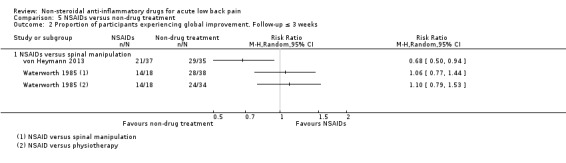
Comparison 5 NSAIDs versus non‐drug treatment, Outcome 2 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks.
Hancock 2007 reported global perceived effect, and found no clear difference between study arms (NSAID, spinal manipulation, and placebo spinal manipulation).
Adverse events
Two studies (N = 189) reported data on adverse events, showing no clear difference between treatments (Hancock 2007; von Heymann 2013). von Heymann 2013 reported no adverse events, therefore the risk ratio was not estimable and the data could not be pooled (Analysis 5.3). The quality of this evidence is very low; we downgraded the evidence three levels due to risk of bias, and imprecision (two levels).
5.3. Analysis.
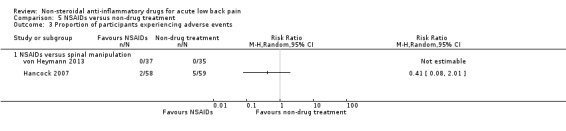
Comparison 5 NSAIDs versus non‐drug treatment, Outcome 3 Proportion of participants experiencing adverse events.
Waterworth 1985 reported two adverse events in the NSAID group (indigestion and nausea), but did not measure or evaluate adverse events in the comparison groups.
Return to work
One study reported the number of days off‐work, showing no clear difference between NSAIDs and spinal manipulation (von Heymann 2013). The quality of this evidence is low; we downgraded the evidence two levels due to risk of bias and imprecision.
Long‐term follow‐up
One study reported long‐term follow‐up data (Hancock 2007). After 12 weeks, there were no clear differences between NSAIDs and spinal manipulation in mean pain score, mean disability score, and mean global perceived effect.
6b. NSAIDs compared to bedrest
Two studies (N = 130) compared NSAIDs to bedrest. Postacchini 1988 presented a combination score of improvement in pain, disability, and spinal mobility, and showed no clear difference between NSAIDs and bedrest after three weeks and two months. Szpalski 1990 did not present any relevant outcomes, except for adverse events; they reported that two participants in the NSAID group withdrew because of adverse events (1 participant with gastric symptoms,1 participant with gastric symptoms and a skin rash).
6c. NSAIDs compared to heat wrap
One study compared NSAIDs to a heat wrap (Nadler 2002; N = 371). It showed that a heat wrap relieved pain better than NSAIDs (mean pain relief 2.61 versus 1.68 after four days; NRS (0 to 5); statistically significant and clinically relevant difference), and reduced disability more than NSAIDs (mean disability reduction 4.9 versus 2.7 after four days; RMDQ (0 to 24), but this difference was not large enough to be considered clinically relevant. Global improvement was not mentioned. The number of adverse events was similar among study arms (heat wrap 6%, 7/113; and NSAID 10%, 11/106). There was no long‐term follow‐up.
6d. NSAIDs compared to motion style acupuncture treatment (MSAT)
One study compared one intramuscular NSAID injection to one MSAT session (Shin 2013; N = 58). It showed that the MSAT session reduced pain (mean (SD) pain intensity reduction from baseline 4.17 (3.05) versus 5.83 (2.61); NRS (0 to 10); and disability (mean (SD) improvement in functional status from baseline 36.34 (29.1) versus 56.41 (24.86); ODI (0 to 50)) more than NSAIDs after two weeks; these differences were statistically significant and clinically relevant.
After the first 30 minutes, the selection of treatment was no longer restricted, thus participants were allowed to use any other treatment (medication or otherwise), if they wished. Therefore, results reported after the 30‐minute follow‐up should be interpreted with caution. There were no adverse events.
Discussion
Summary of main results
In this updated Cochrane Review, aimed at assessing the efficacy of non‐steroidal anti‐inflammatory drugs (NSAID) for acute low back pain (LBP), we included 32 trials with a total of 5356 participants, ranging in age from 16 to 78 years old. We included 26 trials from the previous review (Roelofs 2008), and added six trials from the searches conducted between 2007 and 2020 (Hancock 2007; Miki 2018; Plapler 2016; Shin 2013; von Heymann 2013; Zippel 2007). Follow‐up was usually short (≤ 3 weeks). Almost half of the studies were industry‐funded. The most common biases in the 'Risk of bias' assessment were performance and attrition bias. Often, there was a lack of information on randomisation procedures and allocation concealment, which is a risk for selection bias, and studies were prone to selective reporting bias, since most studies did not register their trials. However, for the latter it is important to realise most studies were published long before trial registries existed (around 2004). Therefore, most of the studies included in this review did not have the possibility yet to register their trials.
There is moderate quality evidence that NSAIDs are slightly more effective than placebo for short‐term pain reduction (0 to 100 visual analogue scale (VAS)), with a pooled mean difference (MD) in pain intensity of ‐7.29 (95% confidence interval (CI) ‐10.98 to ‐3.61; I² 35%; 4 RCTs, 5 treatment arms, N = 815), a small difference, which is likely not clinically relevant. There is high quality evidence that NSAIDs are slightly more effective than placebo for short‐term reduction of disability (0 to 24 Roland Morris Disability Questionnaire (RMDQ)), with a pooled MD of ‐2.02 (95% CI ‐2.89 to ‐1.15; I² 0%; 2 RCTs, 3 treatment arms, N = 471), a small difference, which is likely not clinically relevant. There is low quality evidence that NSAIDs may be slightly more effective for short‐term global improvement than placebo, with a pooled risk ratio (RR) for experiencing global improvement of 1.40 (95% CI 1.12 to 1.75; I² = 52%; 5 RCTs, 7 treatment arms, N = 1201). However, there was moderate to substantial heterogeneity between studies, and in a sensitivity analysis in which we removed two studies with a mixed participant population, the result was no longer significant (RR 1.29, 95% CI 0.97 to 1.72; I² = 58%). There is very low quality evidence of no clear difference in the proportion of participants who experienced adverse events between those who used NSAIDs and those who used placebo (RR 0.86, 95% CI 0.63 to 1.18; I² = 52%; 6 RCTs, 8 treatment arms, N = 1394). Data on adverse events are usually better documented in observational studies. Most of the trials in this review studied participants for a relatively short‐term treatment and follow‐up, which is expected given the subject of acute LBP. Consequently, these may not be optimal for answering questions about adverse events. There is very low quality evidence that there is no clear difference between the proportion of participants who could return to work after seven days between those who used NSAIDs and those who used placebo (RR 1.48, 95% CI 0.98 to 2.23; 1 RCT, N = 266).
There is low quality evidence that there is no clear difference between selective COX‐2 inhibitor NSAIDs and non‐selective NSAIDs in short‐term pain reduction, with a pooled mean change in pain intensity from baseline of ‐2.60 (95% CI ‐9.23 to 4.03; I² = 57%; 2 RCTs, N = 437). There is moderate quality evidence of conflicting results for short‐term improvement of disability (2 RCTs, N = 437). Low quality evidence from one trial (N = 333) found no clear difference between groups in the proportion of participants experiencing global improvement. Very low quality evidence found no clear difference between groups for adverse events (RR 0.97, 95% CI 0.63 to 1.50; I² = 22%; 2 RCTs, N = 444); or for specific gastrointestinal adverse events (RR 0.60, 95% CI 0.33 to 1.09; I² = 14%; 2 RCTs, N = 444). A sensitivity analysis in which we removed the study with a high risk of bias did not change these results. No data were reported on return to work.
Overall completeness and applicability of evidence
This was an update for the Cochrane Review on NSAIDs for LBP (Roelofs 2008). In the previous review, we included trials on LBP of all types and duration. This review focused on acute LBP, therefore, we had strict criteria on duration and type of pain. We excluded studies with a mixed population of acute with chronic or recurrent LBP, or with a large percentage of participants with additional sciatic complaints, in order to keep the results clear and specific. Thus, fewer trials met the inclusion criteria for this update, but the results may be more applicable.
Study populations were rather diverse and often heterogeneous, including a broad range of participants who varied in age and amount of complaints. Both general practitioner (GP) practices and outpatient clinics were used for the source population. In older studies, the study population was sometimes even admitted to the hospital for inpatient treatment. This makes it complex to draw conclusions that fit all people with acute LBP. Furthermore, various types of NSAIDs were used, with different ways of administration, frequencies, doses, and duration of use; adding to the complexity of our attempt to compare a wide range of NSAIDs. The comparison became too heterogeneous to pool, especially when NSAIDs were compared to other drugs, or a combination of other drugs. These different sources of heterogeneity also limited the feasibility of sensitivity or stratified analyses, for instance, regarding the duration of LBP (acute versus subacute) or the dose, duration of treatment, and the mode of delivery of the NSAID. Lastly, not all outcome measures were equally reported. Work‐related outcomes (e.g. return to work status, number of days off work) and long‐term follow‐up (up to three months) were often unavailable, although these could be relevant outcomes for people and their GPs, to help predict the course and prognosis of an episode. Although, when considering the longer‐term outcomes, it is important to keep in mind that the effect of the (usually) short‐term drug treatment may be less relevant at those time points. Despite this variation in (and sometimes lack of useful) outcome reporting, the results of our review appear to be similar to the previous review, which showed a small effect in the quantitative analysis in favour of NSAIDs compared to placebo on pain intensity and global improvement (Roelofs 2008).
One could argue whether our main findings of a mean difference in pain intensity of ‐7.29 on a 0 to 100 VAS scale and a reduction of 2.02 points on the 0 to 24 RMDQ in favour of NSAIDs over placebo are clinically meaningful differences for an individual. A review of the literature shows this is questionable. International consensus was reached on minimal important change (MIC) values of frequently used participant‐reported outcome measures in the field of LBP to aid practical guidance. Reasonable MIC values are ‐15.00 on a 0 to 100 VAS scale and ‐5.00 for the RMDQ. When measuring change from baseline, a 30% improvement was considered a useful threshold for identifying clinically meaningful improvement for each of these measures (Ostelo 2008). However, these are considered to be individual‐level changes. What we consider a minimally important between‐group difference has not yet been established, and is context‐dependent. Other reviews in the field of back pain consider differences in treatment effect of less than 10% of the scale, or 9 points on a 100‐point scale a small effect, and not clinically relevant. Thus, the effect sizes shown in this review do not pass the threshold of clinical relevance, or a clinical meaningful change in an individual.
Adverse events rates for NSAIDs are better documented in observational studies. Our review considered NSAIDs for acute LBP, and most studies focused on short‐term use of NSAIDs with a short follow‐up time. Most sample sizes were too small for evaluating adverse events, or not sufficiently powered to detect rare adverse events. It is possible that the proportion of adverse events was underestimated, or that rare or uncommon adverse events were missed. We should refrain from conclusions concerning adverse events and the safety of NSAIDs for longer‐term use.
Quality of the evidence
Sample sizes of the included RCTs differed widely (ranging from 30 to 372) and follow‐up time was usually short. Relevant information was not always mentioned, for instance, the method of randomisation was not reported and allocation concealment was not adequately described. About half of the studies did not report sufficiently on blinding, so we judged them at high risk of bias because of lack of blinding. Most studies did not register their trials in a publicly accessible clinical trial registry, mostly because they were published before these existed. Therefore, reporting bias is often unclear and cannot be excluded. About half of the trials avoided co‐interventions, the others we considered unclear or at high risk. One trial had no treatment restrictions at all after the first 30 minutes. Compliance was often not mentioned or clearly described, and was only reported by a few studies. A main problem for many of the studies was funding. We scored half of the studies at high risk of bias because of funding by a pharmaceutical company, and often there were conflicts of interest for one or more of the authors, or the sponsor was involved in data collection and analysis, sometimes even writing the report. Two studies clearly stated that the sponsor was not involved in data collection and analysis, and had no influence on the report; these we scored at low risk of bias.
One of the problems we encountered during data‐extraction was that similar outcomes were presented in very different ways across studies. For example, there was substantial variation in the definition and cutoff points for 'global improvement'. Over the years, trials moved from reporting mainly physiological outcomes that combined Likert scales of pain, disability, and global improvement, to reporting separate outcomes, and pain‐ and disability‐specific measurement tools. However, even more recent studies do not always report their outcome measures in a standard way, which impedes the ability to easily compare studies and pool data. Greater consistency in reporting would facilitate higher quality comparisons of studies on acute LBP. A task force of the NIH (National Institutes of Health) Pain Consortium developed research standards for chronic LBP regarding definitions, a minimal data set, reporting outcomes, and future research (Deyo 2014). Recently, an international steering committee developed a preliminary core outcome measurement set that specifies instruments to be included in every clinical trial involving people with non‐specific LBP, which will be useful for studies reporting on acute LBP (Chiarotto 2018). Hopefully, this will further improve the comparability of outcomes in future research in acute LBP, and increase the quality of the body of evidence according to the GRADE criteria.
To conclude, there is moderate to high quality evidence that NSAIDs are slightly more effective than placebo in reducing pain and disability, respectively, in the short‐term. We downgraded the evidence for pain intensity because of high risk of bias. The magnitude of the difference was small, and often there were methodological shortcomings. However, when we only included the studies with a low risk of bias in a sensitivity analysis, the results were consistent, and did not change. The present findings regarding the efficacy of NSAIDs for acute LBP seem to be in line with the results of the previous Cochrane Review (Roelofs 2008).
Potential biases in the review process
We aimed to present a complete overview of the efficacy of NSAIDs for acute LBP, and therefore, the review process was thorough, and we tried to be transparent in all steps taken. We used the current guidelines recommended by the Cochrane Back and Neck Group (CBN) and the Cochrane Handbook for Systematic Reviews of Interventions, and graded the quality of the evidence using the GRADE approach (Furlan 2015; GRADE Working Group 2004; Guyatt 2008; Higgins 2011). The information specialist from the CBN conducted a comprehensive literature search. All abstracts were carefully and independently screened by two review authors. Moreover, we checked the references of all included and possibly relevant studies, and of other reviews published from 2008 onwards. It is still possible that we did not identify all studies (due to publication bias or non‐registered trials), but with extensive searches and checks, we tried to reduce this risk to a minimum. The original review protocol stated that only trials published in English, German or Dutch would be included. To be sure of overall completeness, there were no restrictions on language for this review update. However, we did not re‐screen all foreign language abstracts from prior to 2008, so there is a probability that we missed a foreign language study from before 2008 that could have been included. We also included studies with NSAIDs that were no longer available on the market (e.g. phenylbutazone), aiming to provide a complete overview. A potential drawback could be that the results are less applicable to NSAIDs currently on the market. After analysis, this concerned one study, which could not be included in a meta‐analysis, and did not influence the results or conclusions of this review.
We tried to be strict on the inclusion criteria for this review to retrieve results that were as 'clean' and reliable as possible. For instance, we excluded recurrent LBP to prevent possible bias. Our underlying assumption was that people with recurrent LBP are most probably people with chronic LBP, who may have already tried several therapies, unsuccessfully. They may be non‐responsive to therapy, and thus, belong to a different trial population than people with acute LBP. Still, in many of the (mostly) older studies, the inclusion criteria concerning the type and location of LBP were not mentioned in a very specific manner, or not stated clearly. In reality, the eligibility criteria for the study population might not have been too strict at that time. Sometimes, this was not completely clear, and it was up to us, as review authors, to make a decision whether to include or exclude the study. Main considerations to include were a clearly stated majority of people in the study population who suffered from acute LBP, with only a small minority that may have had additional leg pain, for instance. In a sensitivity analysis, we checked if excluding these from the analysis would change the results. We intended to be transparent in this process, and to make understandable and reproducible decisions. However, it may be that someone else would have decided differently. In case of a clearly mixed study population (either type of back pain, e.g. acute, chronic or recurrent, sciatica; or location of back pain, e.g. also upper back or neck), or if it was unclear what type of (low) back pain the study population had, we included the study as 'awaiting classification'. Usually, there was no subgroup analysis, and the reported data were insufficient to use. We contacted trial authors for clarification, with plans to include these data in a future update. The sixteen studies currently awaiting classification could have had an impact on the results of this review.
Publication bias was difficult to assess due to the limited number of trials included in the meta‐analysis. Almost half of the included trials were supported by pharmaceutical companies, as stated before. Another 43% did not provide (sufficient) information regarding funding. A recent systematic review showed that sponsorship of drug and device studies by the manufacturing company leads to more favourable efficacy results and conclusions, when compared to non‐industry sponsored studies (e.g. any other source of sponsorship). The review authors suggest the existence of an 'industry bias' that cannot be explained by standard 'Risk of bias' assessments (Lundh 2017). In other words, a form of publication bias could be likely.
Agreements and disagreements with other studies or reviews
A review on NSAIDs for spinal pain had similar findings (Machado 2017). They found moderate quality evidence that NSAIDs were effective in reducing pain and disability in the immediate term (less than 2 weeks) compared to placebo, in participants with acute LBP, but the magnitude of the difference was small, and did not exceed the threshold for a clinically relevant treatment effect. Their findings are consistent with our review. A recently published overview of clinical practice guidelines for non‐specific LBP showed that all eight included guidelines currently recommend NSAIDs for acute LBP, either as a first or second choice medication (Schreijenberg 2019). Usually, they recommend to take into consideration potential adverse events or contra‐indications, and to give the lowest effective dose for the shortest possible period of time. Newer guidelines tend to move towards non‐pharmacological treatments, with short‐term use of NSAIDs in addition to usual care, only after careful consideration.
If we compare our results with the other two recently published Cochrane Reviews on NSAIDs for different subgroups of LBP, we observe that the quality of most of the evidence is comparable (low to very low (Enthoven 2016; Rasmussen‐Barr 2016)). However, two of our outcomes only contained studies at low risk of bias, with sufficient numbers of participants to be assessed as moderate to high quality evidence. In agreement with the review on chronic LBP, there is moderate to high (respectively low in Enthoven 2016) quality evidence that NSAIDs are slightly more effective than placebo regarding pain intensity and disability, but it is questionable whether this effect is clinically meaningful. There were no differences in efficacy between different types of NSAIDs. This seems in line with our results. According to the review on NSAIDs for sciatica, there was low quality evidence that global improvement was slightly better in participants with NSAIDs, compared to placebo (Rasmussen‐Barr 2016). This is in agreement with our review, although in a sensitivity analysis in which we removed the studies with a mixed participant population, the result was no longer significant. Furthermore, there was very low quality evidence that the efficacy of NSAIDs for pain reduction was not significant, which is in disagreement with our review.
Authors' conclusions
Implications for practice.
For people with acute low back pain (LBP), non‐steroidal anti‐inflammatory drugs (NSAIDs) were found to be slightly better in reducing pain (moderate quality evidence) and disability (high quality evidence) than placebo in the short‐term. However, the magnitude of the effect is small and probably not clinically relevant. There is low quality evidence that there is no clear difference between selective COX‐2 inhibitor NSAIDs and non‐selective NSAIDs in reducing pain in the short‐term. In all cases, potential (gastrointestinal) adverse events should be taken into account.
Implications for research.
Almost half of the studies in this review were industry‐sponsored, and often they were relatively old. The quality of the evidence ranged from high to very low. Since acute LBP is a frequent condition and morbidity is high, future research is needed to establish strong, and high‐quality evidence regarding the use of NSAIDs in acute LBP. We encourage researchers to use the previously mentioned preliminary core outcome sets for clinical trials on non‐specific LBP recently developed by an international steering committee, and to report all outcomes that are deemed clinically relevant to acute LBP to improve the comparability of results (Chiarotto 2018).
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2020 | New citation required but conclusions have not changed | This is an update of a previously published review on NSAIDS for low back pain (LBP) (Roelofs 2008). The reviews on chronic LBP (Enthoven 2016), and sciatica (Rasmussen‐Barr 2016), have been published. This review focuses on acute LBP. We included 32 trials. |
| 7 January 2020 | New search has been performed | We performed a new search and added six new trials to the review. We used the updated Cochrane Back and Neck method guideline (Furlan 2015). We had changes in authorship: WH van der Gaag and WTM Enthoven were added to the author team; RJPM Scholten and RA Deyo resigned. |
Acknowledgements
The authors thank Shireen Harbin and Maggie Tiong of the Cochrane Back and Neck Group for updating the literature searches. We are grateful to the authors of the studies who provided additional data or information. We also thank Richard Deyo and Rob Scholten for their contributions to the previous review (Roelofs 2008), and Andrew Lingard and Nicolas Henschke for their peer review and valuable feedback.
Appendices
Appendix 1. CENTRAL search strategy
Last searched 7 January 2020
1 MESH DESCRIPTOR Back Pain EXPLODE ALL AND CENTRAL:TARGET
2 dorsalgia AND CENTRAL:TARGET
3 backache AND CENTRAL:TARGET
4 (lumb* or back) next pain AND CENTRAL:TARGET
5 coccyx or coccydynia or spondylosis AND CENTRAL:TARGET
6 MESH DESCRIPTOR Spine EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Spinal Diseases EXPLODE ALL AND CENTRAL:TARGET
8 lumbago or discitis AND CENTRAL:TARGET
9 disc near herniat* AND CENTRAL:TARGET
10 disk NEAR herniat* AND CENTRAL:TARGET
11 spinal fusion AND CENTRAL:TARGET
12 facet near joint* AND CENTRAL:TARGET
13 MESH DESCRIPTOR Intervertebral Disc EXPLODE ALL AND CENTRAL:TARGET
14 postlaminectomy AND CENTRAL:TARGET
15 arachnoiditis AND CENTRAL:TARGET
16 failed near back AND CENTRAL:TARGET
17 MESH DESCRIPTOR Cauda Equina EXPLODE ALL AND CENTRAL:TARGET
18 lumb* near vertebra* AND CENTRAL:TARGET
19 spinal near stenosis AND CENTRAL:TARGET
20 slipped near disc* AND CENTRAL:TARGET
21 slipped NEAR disk* AND CENTRAL:TARGET
22 degenerat* near disc* AND CENTRAL:TARGET
23 degenerat* near disk* AND CENTRAL:TARGET
24 stenosis near spine AND CENTRAL:TARGET
25 stenosis near root AND CENTRAL:TARGET
26 stenosis near spinal AND CENTRAL:TARGET
27 displace* near disc* AND CENTRAL:TARGET
28 displace* near disk* AND CENTRAL:TARGET
29 prolap* near disc* AND CENTRAL:TARGET
30 prolap* near disk* AND CENTRAL:TARGET
31 MESH DESCRIPTOR Sciatic Neuropathy EXPLODE ALL AND CENTRAL:TARGET
32 sciatic* AND CENTRAL:TARGET
33 back disorder* AND CENTRAL:TARGET
34 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 AND CENTRAL:TARGET
35 nsaid* AND CENTRAL:TARGET
36 MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL AND CENTRAL:TARGET
37 MESH DESCRIPTOR Cyclooxygenase Inhibitors EXPLODE ALL AND CENTRAL:TARGET
38 MESH DESCRIPTOR Cyclooxygenase 2 Inhibitors EXPLODE ALL AND CENTRAL:TARGET
39 non‐steroidal anti inflammat* AND CENTRAL:TARGET
40 non‐steroidal antiinflammat* AND CENTRAL:TARGET
41 non‐steroidal anti‐inflammat* AND CENTRAL:TARGET
42 cyclooxygenase NEAR3 inhibitor* AND CENTRAL:TARGET
43 cyclo‐oxygenase NEAR3 inhibitor* AND CENTRAL:TARGET
44 aspirin AND CENTRAL:TARGET
45 acetylsalicyl* AND CENTRAL:TARGET
46 carbasalate calcium AND CENTRAL:TARGET
47 diflunisal AND CENTRAL:TARGET
48 aceclofenac AND CENTRAL:TARGET
49 alclofenac AND CENTRAL:TARGET
50 diclofenac AND CENTRAL:TARGET
51 indometacin or indomethacin AND CENTRAL:TARGET
52 sulindac AND CENTRAL:TARGET
53 meloxicam AND CENTRAL:TARGET
54 piroxicam AND CENTRAL:TARGET
55 dexibuprofen AND CENTRAL:TARGET
56 dexketoprofen AND CENTRAL:TARGET
57 fenoprofen AND CENTRAL:TARGET
58 flurbiprofen AND CENTRAL:TARGET
59 ibuprofen AND CENTRAL:TARGET
60 ketoprofen AND CENTRAL:TARGET
61 naproxen AND CENTRAL:TARGET
62 tiapro* AND CENTRAL:TARGET
63 metamizol AND CENTRAL:TARGET
64 phenylbutazone AND CENTRAL:TARGET
65 phenazone AND CENTRAL:TARGET
66 propyphenazone AND CENTRAL:TARGET
67 celecoxib AND CENTRAL:TARGET
68 etoricoxib AND CENTRAL:TARGET
69 nabumeton AND CENTRAL:TARGET
70 parecoxib AND CENTRAL:TARGET
71 rofecoxib AND CENTRAL:TARGET
72 celecoxib AND CENTRAL:TARGET
73 valdecoxib AND CENTRAL:TARGET
74 lumiracoxib AND CENTRAL:TARGET
75 parecoxib AND CENTRAL:TARGET
76 vioxx AND CENTRAL:TARGET
77 celebrex AND CENTRAL:TARGET
78 bextra AND CENTRAL:TARGET
79 prexige AND CENTRAL:TARGET
80 arcoxia AND CENTRAL:TARGET
81 etodolac AND CENTRAL:TARGET
82 floctafenine AND CENTRAL:TARGET
83 meclofenam* AND CENTRAL:TARGET
84 meloxicam AND CENTRAL:TARGET
85 oxaprozin AND CENTRAL:TARGET
86 piroxicam AND CENTRAL:TARGET
87 tenoxicam AND CENTRAL:TARGET
88 tolmetin AND CENTRAL:TARGET
89 #88 OR #87 OR #86 OR #85 OR #84 OR #83 OR #82 OR #81 OR #80 OR #79 OR #78 OR #77 OR #76 OR #75 OR #74 OR #73 OR #72 OR #71 OR #70 OR #69 OR #68 OR #67 OR #66 OR #65 OR #64 OR #63 OR #62 OR #60 OR #61 OR #59 OR #58 OR #57 OR #56 OR #55 OR #54 OR #53 OR #52 OR #51 OR #50 OR #49 OR #48 OR #47 OR #46 OR #45 OR #44 OR #43 OR #42 OR #41 OR #40 OR #39 OR #38 OR #37 OR #36 OR #35 AND CENTRAL:TARGET
90 #89 AND #34 AND CENTRAL:TARGET
91 (2018 OR 2019 OR 2020):YR AND CENTRAL:TARGET
92 #91 AND #90
2015 search in CRS standalone database. The terms ketoprofen and cycooxygenase were added, an alternative spelling for indometacin was included, and some proximity search operators were revised. There was no date limit because the yield was small.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 lumbar next pain or coccyx or coccydynia or spondylosis
#5 MeSH descriptor: [Spine] explode all trees
#6 MeSH descriptor: [Spinal Diseases] explode all trees
#7 lumbago and discitis and disc near herniation
#8 spinal fusion
#9 spinal neoplasms
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 nsaid*
#29 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees
#30 MeSH descriptor: [Cyclooxygenase Inhibitors] explode all trees
#31 MeSH descriptor: [Cyclooxygenase 2 Inhibitors] explode all trees
#32 non‐steroidal anti inflammat*
#33 non‐steroidal anti‐inflammat*
#34 (cyclooxygenase or cyclo‐oxygenase) next/3 inhibitor*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin or indomethacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 parecoxib
#67 vioxx
#68 celebrex
#69 bextra
#70 prexige
#71 arcoxia
#72 etodolac
#73 floctafenine
#74 meclofenam*
#75 meloxicam
#76 oxaprozin
#77 piroxicam
#78 tenoxicam
#79 tolmetin
#80 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79
#81 #27 and #80
#82 #81 in Trials
2014 search. Some duplicate terms were removed.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 lumbar next pain or coccyx or coccydynia or spondylosis
#5 MeSH descriptor: [Spine] explode all trees
#6 MeSH descriptor: [Spinal Diseases] explode all trees
#7 lumbago OR discitis OR disc near degeneration OR disc near prolapse OR disc near herniation
#8 spinal fusion
#9 spinal neoplasms
#10 facet near joints
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 nsaid*
#29 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees
#30 MeSH descriptor: [Cyclooxygenase Inhibitors] explode all trees
#31 MeSH descriptor: [Cyclooxygenase 2 Inhibitors] explode all trees
#32 non‐steroidal anti inflammat*
#33 non‐steroidal anti‐inflammat*
#34 aspirin
#35 acetylsalicyl*
#36 carbasalate calcium
#37 diflunisal
#38 aceclofenac
#39 alclofenac
#40 diclofenac
#41 indometacin
#42 sulindac
#43 meloxicam
#44 piroxicam
#45 dexibuprofen
#46 dexketoprofen
#47 fenoprofen
#48 flurbiprofen
#49 ibuprofen
#50 ketoprofen
#51 naproxen
#52 tiapro*
#53 metamizol
#54 phenylbutazone
#55 phenazone
#56 propyphenazone
#57 celecoxib
#58 etoricoxib
#59 nabumeton
#60 parecoxib
#61 rofecoxib
#62 celecoxib
#63 valdecoxib
#64 lumiracoxib
#65 parecoxib
#66 vioxx
#67 celebrex
#68 bextra
#69 prexige
#70 arcoxia
#71 etodolac
#72 floctafenine
#73 meclofenam*
#74 meloxicam
#75 oxaprozin
#76 piroxicam
#77 tenoxicam
#78 tolmetin
#79 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78
#80 #27 and #79
#81 #80 Publication Date from 2013 to 2014, in Trials
2012 search
#1 MeSH descriptor Back Pain explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor Low Back Pain explode all trees
#5 (lumbar next pain) or (coccyx) or (coccydynia) or (sciatica) or (spondylosis)
#6 MeSH descriptor Spine explode all trees
#7 MeSH descriptor Spinal Diseases explode all trees
#8 (lumbago) or (discitis) or (disc near degeneration) or (disc near prolapse) or (disc near herniation)
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor Intervertebral Disk explode all trees
#13 postlaminectomy
#14 arachnoiditis
#15 failed near back
#16 MeSH descriptor Cauda Equina explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor Sciatic Neuropathy explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
#29 nsaid*
#30 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees
#31 MeSH descriptor Cyclooxygenase Inhibitors explode all trees
#32 MeSH descriptor Cyclooxygenase 2 Inhibitors explode all trees
#33 non‐steroidal anti inflammat*
#34 non‐steroidal anti‐inflammat*
#35 aspirin
#36 acetylsalicyl*
#37 carbasalate calcium
#38 diflunisal
#39 aceclofenac
#40 alclofenac
#41 diclofenac
#42 indometacin
#43 sulindac
#44 meloxicam
#45 piroxicam
#46 dexibuprofen
#47 dexketoprofen
#48 fenoprofen
#49 flurbiprofen
#50 ibuprofen
#51 ketoprofen
#52 naproxen
#53 tiapro*
#54 metamizol
#55 phenylbutazone
#56 phenazone
#57 propyphenazone
#58 celecoxib
#59 etoricoxib
#60 nabumeton
#61 parecoxib
#62 rofecoxib
#63 celecoxib
#64 valdecoxib
#65 lumiracoxib
#66 etoricoxib
#67 parecoxib
#68 vioxx
#69 celebrex
#70 bextra
#71 prexige
#72 arcoxia
#73 etodolac
#74 floctafenine
#75 meclofenam*
#76 meloxicam
#77 naproxen
#78 oxaprozin
#79 piroxicam
#80 tenoxicam
#81 tolmetin
#82 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81)
#83 (#28 AND #82), from 2007 to 2012
Appendix 2. MEDLINE search strategies
Last searched 7 January 2020
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random*.ti,ab.
6 placebo.ab,ti.
7 drug therapy.fs.
8 trial.ab,ti.
9 groups.ab,ti.
10 or/1‐9
11 (animals not (humans and animals)).sh.
12 10 not 11
13 dorsalgia.tw,kf.
14 exp Back Pain/
15 backache.tw,kf.
16 ((lumb* adj pain) or (back adj pain)).tw,kf.
17 coccyx.tw,kf.
18 coccydynia.tw,kf.
19 sciatica.tw,kf.
20 exp sciatic neuropathy/
21 spondylosis.tw,kf.
22 lumbago.tw,kf.
23 back disorder*.tw,kf.
24 or/13‐23
25 exp Anti‐Inflammatory Agents, Non‐Steroidal/
26 nsaid*.tw,kf.
27 non‐steroidal antiinflammat*.tw,kf.
28 non‐steroidal anti‐inflammat*.tw,kf.
29 aspirin.tw,kf. or exp Aspirin/
30 acetylsalicyl*.tw,kf.
31 exp Salicylic Acid/
32 carbasalate calcium.tw,kf.
33 diflunisal.tw,kf. or exp Diflunisal/
34 aceclofenac.tw,kf.
35 alclofenac.tw,kf. (
36 diclofenac.tw,kf. or exp Diclofenac/
37 (indometacin or indomethacin).tw,kf. or exp Indomethacin/
38 sulindac.tw,kf. or exp Sulindac/
39 meloxicam.tw,kf.
40 piroxicam.tw,kf. or exp Piroxicam/
41 dexibuprofen.tw,kf.
42 dexketoprofen.tw,kf.
43 fenoprofen.tw,kf. or exp Fenoprofen/
44 flurbiprofen.tw,kf. or exp Flurbiprofen/
45 ibuprofen.tw,kf. or exp Ibuprofen/
46 ketoprofen.tw,kf. or exp Ketoprofen/
47 naproxen.tw,kf. or exp Naproxen/
48 tiapro*.tw,kf.
49 metamizol.tw,kf. or exp Dipyrone/
50 phenylbutazone.tw,kf. or exp Phenylbutazone/
51 phenazone.tw,kf. or exp Antipyrine/
52 propyphenazone.tw,kf.
53 celecoxib.tw,kf.
54 etoricoxib.tw,kf.
55 nabumeton.tw,kf.
56 parecoxib.tw,kf.
57 or/25‐56
58 exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
59 ((cyclooxygenase adj3 inhibitor*) or (cyclo‐oxygenase adj3 inhibitor*)).tw,kf.
60 rofecoxib.tw,kf.
61 celecoxib.tw,kf.
62 valdecoxib.tw,kf.
63 lumiracoxib.tw,kf.
64 etoricoxib.tw,kf.
65 parecoxib.tw,kf.
66 vioxx.tw,kf.
67 celebrex.tw,kf.
68 bextra.tw,kf.
69 prexige.tw,kf.
70 arcoxia.tw,kf.
71 etodolac.tw,kf. or exp Etodolac/
72 floctafenine.tw,kf.
73 exp Meclofenamic Acid/
74 meclofenam*.tw,kf.
75 meloxicam.tw,kf.
76 oxaprozin.tw,kf.
77 piroxicam.tw,kf. or exp Piroxicam/
78 tenoxicam.tw,kf.
79 tolmetin.tw,kf. or exp Tolmetin/
80 or/58‐79
81 57 or 80
82 12 and 24 and 81
83 limit 82 to yr=2018‐2020
84 limit 82 to ed=20181112‐20200107
85 83 or 84
2017 search. The .mp. field was changed to .tw,kf. and the study design filter was revised.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 comparative study.pt.
5 random*.ti,ab.
6 placebo.ab,ti.
7 drug therapy.fs.
8 trial.ab,ti.
9 groups.ab,ti.
10 or/1‐9
11 (animals not (humans and animals)).sh.
12 10 not 11
13 dorsalgia.tw,kf.
14 exp Back Pain/
15 backache.tw,kf.
16 ((lumb* or back) adj pain).tw,kf.
17 coccyx.tw,kf.
18 coccydynia.tw,kf.
19 sciatica.tw,kf.
20 exp sciatic neuropathy/
21 spondylosis.tw,kf.
22 lumbago.tw,kf.
23 back disorder*.tw,kf.
24 or/13‐23
25 exp Anti‐Inflammatory Agents, Non‐Steroidal/
26 nsaid*.tw,kf.
27 non‐steroidal antiinflammat*.tw,kf.
28 non‐steroidal anti‐inflammat*.tw,kf.
29 aspirin.tw,kf. or exp Aspirin/
30 acetylsalicyl*.tw,kf.
31 exp Salicylic Acid/
32 carbasalate calcium.tw,kf.
33 diflunisal.tw,kf. or exp Diflunisal/
34 aceclofenac.tw,kf.
35 alclofenac.tw,kf.
36 diclofenac.tw,kf. or exp Diclofenac/
37 (indometacin or indomethacin).tw,kf. or exp Indomethacin/
38 sulindac.tw,kf. or exp Sulindac/
39 meloxicam.tw,kf.
40 piroxicam.tw,kf. or exp Piroxicam/
41 dexibuprofen.tw,kf.
42 dexketoprofen.tw,kf.
43 fenoprofen.tw,kf. or exp Fenoprofen/
44 flurbiprofen.tw,kf. or exp Flurbiprofen/
45 ibuprofen.tw,kf. or exp Ibuprofen/
46 ketoprofen.tw,kf. or exp Ketoprofen/
47 naproxen.tw,kf. or exp Naproxen/
48 tiapro*.tw,kf.
49 metamizol.tw,kf. or exp Dipyrone/
50 phenylbutazone.tw,kf. or exp Phenylbutazone/
51 phenazone.tw,kf. or exp Antipyrine/
52 propyphenazone.tw,kf.
53 celecoxib.tw,kf.
54 etoricoxib.tw,kf.
55 nabumeton.tw,kf.
56 parecoxib.tw,kf.
57 or/25‐56
58 exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
59 ((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).tw,kf.
60 rofecoxib.tw,kf.
61 celecoxib.tw,kf.
62 valdecoxib.tw,kf.
63 lumiracoxib.tw,kf.
64 etoricoxib.tw,kf.
65 parecoxib.tw,kf.
66 vioxx.tw,kf.
67 celebrex.tw,kf.
68 bextra.tw,kf.
69 prexige.tw,kf.
70 arcoxia.tw,kf.
71 etodolac.tw,kf. or exp Etodolac/
72 floctafenine.tw,kf.
73 exp Meclofenamic Acid/
74 meclofenam*.tw,kf.
75 meloxicam.tw,kf.
76 oxaprozin.tw,kf.
77 piroxicam.tw,kf. or exp Piroxicam/
78 tenoxicam.tw,kf.
79 tolmetin.tw,kf. or exp Tolmetin/
80 or/58‐79
81 57 or 80
82 12 and 24 and 81
83 limit 82 to yr=2016‐2017
84 limit 82 to ed=20160114‐20170719
85 83 or 84
Search strategy for 2015 and 2016 in MEDLINE In‐Process & Other Non‐Indexed Citations. Last searched 14 January 2016. In 2015, the study design filter was edited, some truncated terms were revised, the term "cyclooxygenase adj3 inhibitor* was added, and an alternative spelling for indometacin was included.
1 randomized controlled trial.ti,ab.
2 controlled clinical trial.ti,ab.
3 pragmatic.ti,ab.
4 comparative study.ti,ab.
5 clinical trial.ti,ab.
6 randomi#ed.ab.
7 placebo.ab,ti.
8 drug therapy.fs.
9 randomly.ab,ti.
10 trial.ab,ti.
11 groups.ab,ti.
12 or/1‐11
13 dorsalgia.ti,ab.
14 Back Pain.ti,ab.
15 backache.ti,ab.
16 (lumbar adj pain).ti,ab.
17 coccyx.ti,ab.
18 coccydynia.ti,ab.
19 sciatica.ti,ab.
20 sciatic neuropathy.ti,ab.
21 spondylosis.ti,ab.
22 lumbago.ti,ab.
23 back disorder$.ti,ab.
24 or/13‐23
25 nsaids.mp.
26 non‐steroidal antiinflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
27 non‐steroidal anti‐inflammat$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
28 aspirin.mp.
29 acetylsalicyl$.mp.
30 Salicylic Acid.mp.
31 carbasalate calcium.mp.
32 diflunisal.mp.
33 aceclofenac.mp.
34 alclofenac.mp.
35 diclofenac.mp.
36 (indomethacin or indometacin).mp.
37 sulindac.mp.
38 meloxicam.mp.
39 piroxicam.mp.
40 dexibuprofen.mp.
41 dexketoprofen.mp.
42 fenoprofen.mp.
43 flurbiprofen.mp.
44 ibuprofen.mp.
45 ketoprofen.mp.
46 naproxen.mp.
47 tiapro$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
48 metamizol.mp.
49 phenylbutazone.mp.
50 phenazone.mp.
51 propyphenazone.mp.
52 celecoxib.mp.
53 etoricoxib.mp.
54 nabumeton.mp.
55 parecoxib.mp.
56 or/25‐55
57 ((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
58 rofecoxib.mp.
59 celecoxib.mp.
60 valdecoxib.mp.
61 lumiracoxib.mp.
62 etoricoxib.mp.
63 parecoxib.mp.
64 vioxx.mp.
65 celebrex.mp.
66 bextra.mp.
67 prexige.mp.
68 arcoxia.mp.
69 etodolac.mp.
70 floctafenine.mp.
71 Meclofenamic Acid.mp.
72 meclofenamate.mp.
73 meloxicam.mp.
74 oxaprozin.mp.
75 piroxicam.mp.
76 tenoxicam.mp.
77 tolmetin.mp.
78 or/57‐77
79 56 or 78
80 12 and 24 and 79
81 limit 80 to yr=2015‐2016
82 limit 80 to ed=20150624‐20160114
83 81 or 82
2014 search strategy
1 randomized controlled trial.ti,ab.
2 controlled clinical trial.ti,ab.
3 comparative study.ti,ab.
4 clinical trial.ti,ab.
5 randomized.ab.
6 placebo.ab,ti.
7 drug therapy.fs.
8 randomly.ab,ti.
9 trial.ab,ti.
10 groups.ab,ti.
11 or/1‐10
12 dorsalgia.ti,ab.
13 Back Pain.ti,ab.
14 backache.ti,ab.
15 (lumbar adj pain).ti,ab.
16 coccyx.ti,ab.
17 coccydynia.ti,ab.
18 sciatica.ti,ab.
19 sciatic neuropathy.ti,ab.
20 spondylosis.ti,ab.
21 lumbago.ti,ab.
22 back disorder$.ti,ab.
23 or/12‐22
24 Anti‐Inflammatory Agents, Non‐Steroidal.mp.
25 nsaids.mp.
26 non‐steroidal anti inflammat$.mp.
27 non‐steroidal anti‐inflammat$.mp.
28 aspirin.mp.
29 acetylsalicyl$.mp.
30 Salicylic Acid.mp.
31 carbasalate calcium.mp.
32 diflunisal.mp.
33 aceclofenac.mp.
34 alclofenac.mp.
35 diclofenac.mp.
36 indomethacin.mp.
37 sulindac.mp.
38 meloxicam.mp.
39 piroxicam.mp.
40 dexibuprofen.mp.
41 dexketoprofen.mp.
42 fenoprofen.mp.
43 flurbiprofen.mp.
44 ibuprofen.mp.
45 ketoprofen.mp.
46 naproxen.mp.
47 tiapro$.mp.
48 metamizol.mp.
49 phenylbutazone.mp.
50 phenazone.mp.
51 propyphenazone.mp.
52 celecoxib.mp.
53 etoricoxib.mp.
54 nabumeton.mp.
55 parecoxib.mp.
56 or/24‐55
57 (cyclooxygenase inhibitors or cyclooxygenase 2 inhibitors).mp.
58 rofecoxib.mp.
59 celecoxib.mp.
60 valdecoxib.mp.
61 lumiracoxib.mp.
62 etoricoxib.mp.
63 parecoxib.mp.
64 vioxx.mp.
65 celebrex.mp.
66 bextra.mp.
67 prexige.mp.
68 arcoxia.mp.
69 etodolac.mp.
70 floctafenine.mp.
71 Meclofenamic Acid.mp.
72 meclofenamate.mp.
73 meloxicam.mp.
74 oxaprozin.mp.
75 piroxicam.mp.
76 tenoxicam.mp.
77 tolmetin.mp.
78 or/57‐77
79 56 or 78
80 11 and 23 and 79
2012 search strategy
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 comparative study.pt.
4 clinical trial.pt.
5 randomized.ab.
6 placebo.ab,ti.
7 drug therapy.fs.
8 randomly.ab,ti.
9 trial.ab,ti.
10 groups.ab,ti.
11 or/1‐10
12 (animals not (humans and animals)).sh.
13 11 not 12
14 dorsalgia.ti,ab.
15 exp Back Pain/
16 backache.ti,ab.
17 exp Low Back Pain/
18 (lumbar adj pain).ti,ab.
19 coccyx.ti,ab.
20 coccydynia.ti,ab.
21 sciatica.ti,ab.
22 sciatic neuropathy/
23 spondylosis.ti,ab.
24 lumbago.ti,ab.
25 back disorder$.ti,ab.
26 or/14‐25
27 exp Anti‐Inflammatory Agents, Non‐Steroidal/
28 nsaids.mp.
29 non‐steroidal anti inflammat$.mp.
30 non‐steroidal anti‐inflammat$.mp.
31 aspirin.mp. or exp Aspirin/
32 acetylsalicyl$.mp.
33 exp Salicylic Acid/
34 carbasalate calcium.mp.
35 diflunisal.mp. or exp Diflunisal/
36 aceclofenac.mp.
37 alclofenac.mp.
38 diclofenac.mp. or exp Diclofenac/
39 indometacin.mp. or exp Indomethacin/
40 sulindac.mp. or exp Sulindac/
41 meloxicam.mp.
42 piroxicam.mp. or exp Piroxicam/
43 dexibuprofen.mp.
44 dexketoprofen.mp.
45 fenoprofen.mp. or exp Fenoprofen/
46 flurbiprofen.mp. or exp Flurbiprofen/
47 ibuprofen.mp. or exp Ibuprofen/
48 ketoprofen.mp. or exp Ketoprofen/
49 naproxen.mp. or exp Naproxen/
50 tiapro$.mp.
51 metamizol.mp. or exp Dipyrone/
52 phenylbutazone.mp. or exp Phenylbutazone/
53 phenazone.mp. or exp Antipyrine/
54 propyphenazone.mp.
55 celecoxib.mp.
56 etoricoxib.mp.
57 nabumeton.mp.
58 parecoxib.mp.
59 or/27‐58
60 exp cyclooxygenase inhibitors/ or exp cyclooxygenase 2 inhibitors/
61 rofecoxib.mp.
62 celecoxib.mp.
63 valdecoxib.mp.
64 lumiracoxib.mp.
65 etoricoxib.mp.
66 parecoxib.mp.
67 vioxx.mp.
68 celebrex.mp.
69 bextra.mp.
70 prexige.mp.
71 arcoxia.mp.
72 etodolac.mp. or exp Etodolac/
73 floctafenine.mp.
74 exp Meclofenamic Acid/
75 meclofenamate.mp.
76 meloxicam.mp.
77 naproxen.mp. or exp Naproxen/
78 oxaprozin.mp.
79 piroxicam.mp. or exp Piroxicam/
80 tenoxicam.mp.
81 tolmetin.mp. or exp Tolmetin/
82 or/60‐81
83 59 or 82
84 13 and 26 and 83
85 limit 84 to yr="2007 ‐ 2012"
86 limit 84 to ed=20070601‐20120524
87 85 or 86
Appendix 3. Embase search strategy
Last searched 7 January 2020
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 allocat*.ti,ab.
9 assign*.ti,ab.
10 blind*.ti,ab.
11 ((controlled adj7 study) or (controlled adj7 design)).ti,ab.
12 (crossover or cross‐over).ti,ab.
13 (compare or compared or comparing or comparison or comparative).ti,ab.
14 ((singl* adj7 mask*) or (doubl* adj7 mask*) or (trebl* adj7 mask*) or (tripl* adj7 mask*)).ti,ab.
15 placebo?.ti,ab.
16 random*.ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 dorsalgia.tw,kw.
25 back pain.tw,kw.
26 exp BACKACHE/
27 (lumb* adj pain).tw,kw.
28 coccyx.tw,kw.
29 coccydynia.tw,kw.
30 sciatica.tw,kw.
31 exp ISCHIALGIA/
32 spondylosis.tw,kw.
33 lumbago.tw,kw.
34 or/24‐33
35 exp Nonsteroid Antiinflammatory Agent/
36 nsaid*.tw,kw.
37 non‐steroidal anti‐inflammator*.tw,kw.
38 non‐steroidal antiinflammat*.tw,kw.
39 exp Acetylsalicylic Acid/
40 acetylsalicyl*.tw,kw.
41 carbasalate calcium.tw,kw. or exp Carbasalate Calcium/
42 diflunisal.tw,kw. or exp DIFLUNISAL/
43 aceclofenac.tw,kw. or exp ACECLOFENAC/
44 alclofenac.tw,kw. or exp ALCLOFENAC/
45 diclofenac.tw,kw. or exp DICLOFENAC/
46 exp INDOMETACIN/ or (indometacin or indomethacin).tw,kw.
47 sulindac.tw,kw. or exp SULINDAC/
48 meloxicam.tw,kw. or exp MELOXICAM/
49 exp PIROXICAM/ or piroxicam.tw,kw.
50 dexibuprofen.tw,kw. or exp DEXIBUPROFEN/
51 dexketoprofen.tw,kw. or exp DEXKETOPROFEN/
52 exp FENOPROFEN/ or fenoprofen.tw,kw.
53 flurbiprofen.tw,kw. or exp FLURBIPROFEN/
54 ibuprofen.tw,kw. or exp IBUPROFEN/
55 ketoprofen.tw,kw. or exp KETOPROFEN/
56 naproxen.tw,kw. or exp NAPROXEN/
57 tiapro*.tw,kw.
58 metamizol.tw,kw. or exp Dipyrone/
59 phenylbutazone.tw,kw. or exp PHENYLBUTAZONE/
60 phenazone.tw,kw. or exp PHENAZONE/
61 exp PROPYPHENAZONE/ or propyphenazone.tw,kw.
62 celecoxib.tw,kw. or exp CELECOXIB/
63 etoricoxib.tw,kw. or exp ETORICOXIB/
64 exp Nabumetone/ or nabumeton.tw,kw.
65 parecoxib.tw,kw. or exp PARECOXIB/
66 or/35‐65
67 exp Cyclooxygenase 2 Inhibitor/
68 ((cyclooxygenase adj3 inhibitor*) or (cyclo‐oxygenase adj3 inhibitor*)).tw,kw.
69 rofecoxib.tw,kw. or exp ROFECOXIB/
70 valdecoxib.tw,kw. or exp VALDECOXIB/
71 lumiracoxib.tw,kw. or exp LUMIRACOXIB/
72 etoricoxib.tw,kw. or exp ETORICOXIB/
73 parecoxib.tw,kw. or exp PARECOXIB/
74 vioxx.tw,kw.
75 celebrex.tw,kw.
76 bextra.tw,kw.
77 prexige.tw,kw.
78 arcoxia.tw,kw.
79 etodolac.tw,kw. or exp ETODOLAC/
80 floctafenine.tw,kw. or exp FLOCTAFENINE/
81 exp Meclofenamic Acid/
82 meclofenam*.tw,kw.
83 oxaprozin.tw,kw. or exp OXAPROZIN/
84 exp PIROXICAM/ or piroxicam.tw,kw.
85 tenoxicam.tw,kw. or exp TENOXICAM/
86 tolmetin.tw,kw. or exp TOLMETIN/
87 or/67‐86
88 66 or 87
89 23 and 34 and 88
90 limit 89 to yr=2018‐2020
91 limit 89 to em=201846‐202001
92 90 or 91
2017 search. The study design filter and some truncated terms were revised and the .tw,kw. field was searched instead of .mp.
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 allocat*.ti,ab.
9 assign*.ti,ab.
10 blind*.ti,ab.
11 (controlled adj7 (study or design or trial)).ti,ab.
12 (crossover or cross‐over).ti,ab.
13 (compare or compared or comparing or comparison or comparative).ti,ab.
14 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
15 placebo?.ti,ab.
16 random*.ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 dorsalgia.tw,kw.
25 back pain.tw,kw.
26 exp BACKACHE/
27 (lumb* adj pain).tw,kw.
28 coccyx.tw,kw.
29 coccydynia.tw,kw.
30 sciatica.tw,kw.
31 exp ISCHIALGIA/
32 spondylosis.tw,kw.
33 lumbago.tw,kw.
34 or/24‐33
35 exp Nonsteroid Antiinflammatory Agent/
36 nsaid*.tw,kw.
37 non‐steroidal anti‐inflammator*.tw,kw.
38 non‐steroidal antiinflammat*.tw,kw.
39 exp Acetylsalicylic Acid/
40 acetylsalicyl*.tw,kw.
41 carbasalate calcium.tw,kw. or exp Carbasalate Calcium/
42 diflunisal.tw,kw. or exp DIFLUNISAL/
43 aceclofenac.tw,kw. or exp ACECLOFENAC/
44 alclofenac.tw,kw. or exp ALCLOFENAC/
45 diclofenac.tw,kw. or exp DICLOFENAC/
46 exp INDOMETACIN/ or (indometacin or indomethacin).tw,kw.
47 sulindac.tw,kw. or exp SULINDAC/
48 meloxicam.tw,kw. or exp MELOXICAM/
49 exp PIROXICAM/ or piroxicam.tw,kw.
50 dexibuprofen.tw,kw. or exp DEXIBUPROFEN/
51 dexketoprofen.tw,kw. or exp DEXKETOPROFEN/
52 exp FENOPROFEN/ or fenoprofen.tw,kw.
53 flurbiprofen.tw,kw. or exp FLURBIPROFEN/
54 ibuprofen.tw,kw. or exp IBUPROFEN/
55 ketoprofen.tw,kw. or exp KETOPROFEN/
56 naproxen.tw,kw. or exp NAPROXEN/
57 tiapro*.tw,kw.
58 metamizol.tw,kw. or exp Dipyrone/
59 phenylbutazone.tw,kw. or exp PHENYLBUTAZONE/
60 phenazone.tw,kw. or exp PHENAZONE/
61 exp PROPYPHENAZONE/ or propyphenazone.tw,kw.
62 celecoxib.tw,kw. or exp CELECOXIB/
63 etoricoxib.tw,kw. or exp ETORICOXIB/
64 exp Nabumetone/ or nabumeton.tw,kw.
65 parecoxib.tw,kw. or exp PARECOXIB/
66 or/35‐64 (519934)
67 exp Cyclooxygenase 2 Inhibitor/
68 ((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).tw,kw.
69 rofecoxib.tw,kw. or exp ROFECOXIB/
70 valdecoxib.tw,kw. or exp VALDECOXIB/
71 lumiracoxib.tw,kw. or exp LUMIRACOXIB/
72 etoricoxib.tw,kw. or exp ETORICOXIB/
73 parecoxib.tw,kw. or exp PARECOXIB/
74 vioxx.tw,kw.
75 celebrex.tw,kw.
76 bextra.tw,kw.
77 prexige.tw,kw.
78 arcoxia.tw,kw.
79 etodolac.tw,kw. or exp ETODOLAC/
80 floctafenine.tw,kw. or exp FLOCTAFENINE/
81 exp Meclofenamic Acid/
82 meclofenam*.tw,kw.
83 oxaprozin.tw,kw. or exp OXAPROZIN/
84 exp PIROXICAM/ or piroxicam.tw,kw.
85 tenoxicam.tw,kw. or exp TENOXICAM/
86 tolmetin.tw,kw. or exp TOLMETIN/
87 or/67‐86
88 66 or 87
89 23 and 34 and 88
90 limit 89 to yr="2016‐2017"
91 limit 89 to em=201602‐201729
92 90 or 91
2015 search. The study design filter was revised, some truncated terms were revised, the term "cyclooxygenase adj3 inhibitor* was added, and an alternative spelling for indometacin was included.
1 Randomized Controlled Trial/
2 exp Controlled Clinical Trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 allocat$.mp.
9 assign$.mp.
10 blind$.mp.
11 ((control$ or compar$ or prospectiv$ or clinical) adj25 (trial or study)).mp.
12 (crossover or cross‐over).mp.
13 factorial$.mp.
14 (followup or follow‐up).mp.
15 placebo$.mp.
16 random$.mp.
17 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
18 volunteer$.mp.
19 or/1‐18
20 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
21 human/ or normal human/ or human cell/
22 20 and 21
23 20 not 22
24 19 not 23
25 dorsalgia.mp.
26 back pain.mp.
27 exp BACKACHE/
28 (lumbar adj pain).mp.
29 coccyx.mp.
30 coccydynia.mp.
31 sciatica.mp.
32 exp ISCHIALGIA/
33 spondylosis.mp.
34 lumbago.mp.
35 or/25‐34
36 exp Nonsteroid Antiinflammatory Agent/
37 nsaids.mp.
38 non‐steroidal anti‐inflammator$.mp.
39 exp Acetylsalicylic Acid/
40 acetylsalicyl$.mp.
41 carbasalate calcium.mp. or exp Carbasalate Calcium/
42 diflunisal.mp. or exp DIFLUNISAL/
43 aceclofenac.mp. or exp ACECLOFENAC/
44 alclofenac.mp. or exp ALCLOFENAC/
45 diclofenac.mp. or exp DICLOFENAC/
46 exp INDOMETACIN/ or (indometacin or indomethacin).mp.
47 sulindac.mp. or exp SULINDAC/
48 meloxicam.mp. or exp MELOXICAM/
49 exp PIROXICAM/ or piroxicam.mp.
50 dexibuprofen.mp. or exp DEXIBUPROFEN/
51 dexketoprofen.mp. or exp DEXKETOPROFEN/
52 exp FENOPROFEN/ or fenoprofen.mp.
53 flurbiprofen.mp. or exp FLURBIPROFEN/
54 ibuprofen.mp. or exp IBUPROFEN/
55 ketoprofen.mp. or exp KETOPROFEN/
56 naproxen.mp. or exp NAPROXEN/
57 tiapro$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
58 metamizol.mp. or exp Dipyrone/
59 phenylbutazone.mp. or exp PHENYLBUTAZONE/
60 phenazone.mp. or exp PHENAZONE/
61 exp PROPYPHENAZONE/ or propyphenazone.mp.
62 celecoxib.mp. or exp CELECOXIB/
63 etoricoxib.mp. or exp ETORICOXIB/
64 exp Nabumetone/ or nabumeton.mp.
65 parecoxib.mp. or exp PARECOXIB/
66 or/36‐65
67 exp Cyclooxygenase 2 Inhibitor/
68 ((cyclooxygenase or cyclo‐oxygenase) adj3 inhibitor*).mp.
69 rofecoxib.mp. or exp ROFECOXIB/
70 valdecoxib.mp. or exp VALDECOXIB/
71 lumiracoxib.mp. or exp LUMIRACOXIB/
72 etoricoxib.mp. or exp ETORICOXIB/
73 parecoxib.mp. or exp PARECOXIB/
74 vioxx.mp.
75 celebrex.mp.
76 bextra.mp.
77 prexige.mp.
78 arcoxia.mp.
79 etodolac.mp. or exp ETODOLAC/
80 floctafenine.mp. or exp FLOCTAFENINE/
81 exp Meclofenamic Acid/
82 meclofenam$.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
83 oxaprozin.mp. or exp OXAPROZIN/
84 exp PIROXICAM/ or piroxicam.mp.
85 tenoxicam.mp. or exp TENOXICAM/
86 tolmetin.mp. or exp TOLMETIN/
87 or/67‐86
88 66 or 87
89 24 and 35 and 88
90 limit 89 to yr="2014‐2015"
91 limit 89 to em=201414‐201525
92 90 or 91
2014 search strategy. The study design filter was adjusted.
1 Clinical Article/
2 exp Clinical Study/)
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 or 30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or normal human/ or human cell/
34 32 and 33
35 32 not 34
36 31 not 35
37 dorsalgia.mp.
38 back pain.mp.
39 exp BACKACHE/
40 (lumbar adj pain).mp.
41 coccyx.mp.
42 coccydynia.mp.
43 sciatica.mp.
44 exp ISCHIALGIA/
45 spondylosis.mp.
46 lumbago.mp.
47 or/37‐46
48 exp Nonsteroid Antiinflammatory Agent/
49 nsaids.mp.
50 non‐steroidal anti‐inflammatory.mp.
51 exp Acetylsalicylic Acid/
52 acetylsalicyl$.mp.
53 carbasalate calcium.mp. or exp Carbasalate Calcium/
54 diflunisal.mp. or exp DIFLUNISAL/
55 aceclofenac.mp. or exp ACECLOFENAC/
56 alclofenac.mp. or exp ALCLOFENAC/
57 diclofenac.mp. or exp DICLOFENAC/
58 exp INDOMETACIN/ or indometacin.mp.
59 sulindac.mp. or exp SULINDAC/
60 meloxicam.mp. or exp MELOXICAM/
61 exp PIROXICAM/ or piroxicam.mp.
62 dexibuprofen.mp. or exp DEXIBUPROFEN/
63 dexketoprofen.mp. or exp DEXKETOPROFEN/
64 exp FENOPROFEN/ or fenoprofen.mp.
65 flurbiprofen.mp. or exp FLURBIPROFEN/
66 ibuprofen.mp. or exp IBUPROFEN/
67 ketoprofen.mp. or exp KETOPROFEN/
68 naproxen.mp. or exp NAPROXEN/
69 tiapro$.mp.
70 metamizol.mp. or exp Dipyrone/
71 phenylbutazone.mp. or exp PHENYLBUTAZONE/
72 phenazone.mp. or exp PHENAZONE/
73 exp PROPYPHENAZONE/ or propyphenazone.mp.
74 celecoxib.mp. or exp CELECOXIB/
75 etoricoxib.mp. or exp ETORICOXIB/
76 exp Nabumetone/ or nabumeton.mp.
77 parecoxib.mp. or exp PARECOXIB/
78 or/48‐77
79 exp Cyclooxygenase 2 Inhibitor/
80 rofecoxib.mp. or exp ROFECOXIB/
81 valdecoxib.mp. or exp VALDECOXIB/
82 lumiracoxib.mp. or exp LUMIRACOXIB/
83 etoricoxib.mp. or exp ETORICOXIB/
84 parecoxib.mp. or exp PARECOXIB/
85 vioxx.mp.
86 celebrex.mp.
87 bextra.mp.
88 prexige.mp.
89 arcoxia.mp.
90 etodolac.mp. or exp ETODOLAC/
91 floctafenine.mp. or exp FLOCTAFENINE/
92 exp Meclofenamic Acid/
93 meclofenam$.mp.
94 oxaprozin.mp. or exp OXAPROZIN/
95 exp PIROXICAM/ or piroxicam.mp.
96 tenoxicam.mp. or exp TENOXICAM/
97 tolmetin.mp. or exp TOLMETIN/
98 or/79‐97
99 78 or 98
100 36 and 47 and 99
101 limit 100 to yr="2013 ‐ 2014"
102 limit 100 to em=201314‐201414
103 101 or 102
2013 search strategy. The study design filter was revised.
1 Clinical Article/
2 exp Clinical Study/
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 and 30
32 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
33 human/ or normal human/ or human cell/
34 32 and 33
35 32 not 34
36 31 not 35
37 dorsalgia.mp.
38 back pain.mp.
39 exp BACKACHE/
40 (lumbar adj pain).mp.
41 coccyx.mp.
42 coccydynia.mp.
43 sciatica.mp.
44 exp ISCHIALGIA/
45 spondylosis.mp.
46 lumbago.mp.
47 exp Low Back Pain/
48 or/37‐47
49 exp Nonsteroid Antiinflammatory Agent/
50 nsaids.mp.
51 non‐steroidal anti‐inflammatory.mp.
52 exp Acetylsalicylic Acid/
53 acetylsalicyl$.mp.
54 carbasalate calcium.mp. or exp Carbasalate Calcium/
55 diflunisal.mp. or exp DIFLUNISAL/
56 aceclofenac.mp. or exp ACECLOFENAC/
57 alclofenac.mp. or exp ALCLOFENAC/
58 diclofenac.mp. or exp DICLOFENAC/
59 exp INDOMETACIN/ or indometacin.mp.
60 sulindac.mp. or exp SULINDAC/
61 meloxicam.mp. or exp MELOXICAM/
62 exp PIROXICAM/ or piroxicam.mp.
63 dexibuprofen.mp. or exp DEXIBUPROFEN/
64 dexketoprofen.mp. or exp DEXKETOPROFEN/
65 exp FENOPROFEN/ or fenoprofen.mp.
66 flurbiprofen.mp. or exp FLURBIPROFEN/
67 ibuprofen.mp. or exp IBUPROFEN/
68 ketoprofen.mp. or exp KETOPROFEN/
69 naproxen.mp. or exp NAPROXEN/
70 tiapro$.mp.
71 metamizol.mp. or exp Dipyrone/
72 phenylbutazone.mp. or exp PHENYLBUTAZONE/
73 phenazone.mp. or exp PHENAZONE/
74 exp PROPYPHENAZONE/ or propyphenazone.mp.
75 celecoxib.mp. or exp CELECOXIB/
76 etoricoxib.mp. or exp ETORICOXIB/
77 exp Nabumetone/ or nabumeton.mp.
78 parecoxib.mp. or exp PARECOXIB/
79 or/49‐78
80 exp Cyclooxygenase 2 Inhibitor/
81 rofecoxib.mp. or exp ROFECOXIB/
82 valdecoxib.mp. or exp VALDECOXIB/
83 lumiracoxib.mp. or exp LUMIRACOXIB/
84 etoricoxib.mp. or exp ETORICOXIB/
85 parecoxib.mp. or exp PARECOXIB/
86 vioxx.mp.
87 celebrex.mp.
88 bextra.mp.
89 prexige.mp.
90 arcoxia.mp.
91 etodolac.mp. or exp ETODOLAC/
92 floctafenine.mp. or exp FLOCTAFENINE/
93 exp Meclofenamic Acid/
94 meclofenam$.mp.
95 oxaprozin.mp. or exp OXAPROZIN/
96 exp PIROXICAM/ or piroxicam.mp.
97 tenoxicam.mp. or exp TENOXICAM/
98 tolmetin.mp. or exp TOLMETIN/
99 or/80‐98
100 79 or 99
101 36 and 48 and 100
102 limit 101 to yr="2012 ‐ 2013"
103 limit 101 to em=201218‐201314
104 102 or 103
2012 search strategy
1 Clinical Article/
2 exp Clinical Study/
3 Clinical Trial/
4 Controlled Study/
5 Randomized Controlled Trial/
6 Major Clinical Study/
7 Double Blind Procedure/
8 Multicenter Study/
9 Single Blind Procedure/
10 Phase 3 Clinical Trial/
11 Phase 4 Clinical Trial/
12 crossover procedure/
13 placebo/
14 or/1‐13
15 allocat$.mp.
16 assign$.mp.
17 blind$.mp.
18 (clinic$ adj25 (study or trial)).mp.
19 compar$.mp.
20 control$.mp.
21 cross?over.mp.
22 factorial$.mp.
23 follow?up.mp.
24 placebo$.mp.
25 prospectiv$.mp.
26 random$.mp.
27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
28 trial.mp.
29 (versus or vs).mp.
30 or/15‐29
31 14 and 30
32 human/
33 Nonhuman/
34 exp ANIMAL/
35 Animal Experiment/
36 33 or 34 or 35
37 32 not 36
38 31 not 36
39 37 and 38
40 38 or 39
41 dorsalgia.mp.
42 back pain.mp.
43 exp BACKACHE/
44 (lumbar adj pain).mp.
45 coccyx.mp.
46 coccydynia.mp.
47 sciatica.mp.
48 exp ISCHIALGIA/
49 spondylosis.mp.
50 lumbago.mp.
51 exp Low Back Pain/
52 or/41‐51
53 exp Nonsteroid Antiinflammatory Agent/
54 nsaids.mp.
55 non‐steroidal anti‐inflammatory.mp.
56 exp Acetylsalicylic Acid/
57 acetylsalicyl$.mp.
58 carbasalate calcium.mp. or exp Carbasalate Calcium/
59 diflunisal.mp. or exp DIFLUNISAL/
60 aceclofenac.mp. or exp ACECLOFENAC/
61 alclofenac.mp. or exp ALCLOFENAC/
62 diclofenac.mp. or exp DICLOFENAC/
63 exp INDOMETACIN/ or indometacin.mp.
64 sulindac.mp. or exp SULINDAC/
65 meloxicam.mp. or exp MELOXICAM/
66 exp PIROXICAM/ or piroxicam.mp.
67 dexibuprofen.mp. or exp DEXIBUPROFEN/
68 dexketoprofen.mp. or exp DEXKETOPROFEN/
69 exp FENOPROFEN/ or fenoprofen.mp.
70 flurbiprofen.mp. or exp FLURBIPROFEN/
71 ibuprofen.mp. or exp IBUPROFEN/
72 ketoprofen.mp. or exp KETOPROFEN/
73 naproxen.mp. or exp NAPROXEN/
74 tiapro$.mp.
75 metamizol.mp. or exp Dipyrone/
76 phenylbutazone.mp. or exp PHENYLBUTAZONE/
77 phenazone.mp. or exp PHENAZONE/
78 exp PROPYPHENAZONE/ or propyphenazone.mp.
79 celecoxib.mp. or exp CELECOXIB/
80 etoricoxib.mp. or exp ETORICOXIB/
81 exp Nabumetone/ or nabumeton.mp.
82 parecoxib.mp. or exp PARECOXIB/
83 or/53‐82
84 exp Cyclooxygenase 2 Inhibitor/
85 rofecoxib.mp. or exp ROFECOXIB/
86 valdecoxib.mp. or exp VALDECOXIB/
87 lumiracoxib.mp. or exp LUMIRACOXIB/
88 etoricoxib.mp. or exp ETORICOXIB/
89 parecoxib.mp. or exp PARECOXIB/
90 vioxx.mp.
91 celebrex.mp.
92 bextra.mp.
93 prexige.mp.
94 arcoxia.mp.
95 etodolac.mp. or exp ETODOLAC/
96 floctafenine.mp. or exp FLOCTAFENINE/
97 exp Meclofenamic Acid/
98 meclofenam$.mp.
99 oxaprozin.mp. or exp OXAPROZIN/
100 exp PIROXICAM/ or piroxicam.mp.
101 tenoxicam.mp. or exp TENOXICAM/
102 tolmetin.mp. or exp TOLMETIN/
103 or/84‐102
104 83 or 103
105 40 and 52 and 104
106 limit 105 to yr="2007 ‐ 2012"
107 limit 105 to em=200712‐201220
108 106 or 107
Appendix 4. Search strategies for clinical trials registries and PubMed
Clinical Trials.gov
Last searched 7 January 2020
Search terms field: back pain and NSAIDS
First posted from 11/12/2018 to 01/07/2020
2014 search
Basic search: “back pain” and NSAIDS
2012 search
Condition: back pain
AND
Intervention: NSAID
WHO ICTRP
Last searched 7 January 2020
Basic search: back pain and NSAIDS
2012 search
Condition: back pain
AND
Intervention: NSAID
PubMed
Last searched 14 January 2016
((nsaids OR non‐steroidal anti‐inflammator* OR non‐steroidal antiinflammator* OR aspirin OR acetylsalicyl* OR salicylic acid OR carbasalate calcium OR diflunisal OR aceclofenac OR alclofenac OR diclofenac OR indomethacin OR indometacin OR sulindac OR meloxicam OR piroxicam OR dexibuprofen OR dexketoprofen OR fenoprofen OR flurbiprofen OR ibuprofen OR ketoprofen OR naproxen OR tiapro* OR metamizol OR phenylbutazone OR phenazone OR propyphenazone OR celecoxib OR etoricoxib OR nabumeton OR parecoxib OR cyclooxygenase inhibitor* OR cyclo‐oxygenase inhibitor* OR rofecoxib OR celecoxib OR valdecoxib OR lumiracoxib OR etoricoxib OR parecoxib OR vioxx OR celebrex OR bextra OR prexige OR arcoxia OR etodolac OR floctafenine OR Meclofenamic Acid OR meclofenamate OR meloxicam OR oxaprozin OR piroxicam OR tenoxicam OR tolmetin) AND (back pain OR sciatica OR lumbar pain OR lumbago OR dorsalgia OR backache OR back disorder*) AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb])) limit to 2015/06/24‐2016/01/14
Appendix 5. The GRADE approach to evidence synthesis
The quality of evidence will be categorized as follows, using the GRADE approach (GRADE Working Group 2004; Guyatt 2008; Furlan 2015):
High (⊙⊙⊙⊙): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊙⊙⊙○): further research is likely to have an important impact on the confidence in the estimate of effect, and may change the estimate.
Low (⊙⊙○○): further research is very likely to have an important impact on the confidence in the estimate of effect and is likely to change the estimate.
Very low (⊙○○○): very little confidence in the effect estimate.
No evidence: no RCTs were identified that addressed this outcome.
The quality of the evidence for a specific outcome can be reduced by one or more levels, depending on the performance of the studies (that measured that particular outcome) on five domains. The evidence available to answer each sub‐question will be graded on the domains in the following manner:
1. Study design and risk of bias
Only randomized controlled trials were included. Special attention was drawn to adequacy of allocation concealment, blinding and follow‐up. The risk of bias of included trials was assessed independently by two review authors, based on criteria described in in the updated method guidelines of CBN (Furlan 2015). First, each of the criteria was scored as 'yes', 'no', or 'unsure' (Table 3; Table 4). Second, a judgement was made on the domain level. Finally, an overall judgement of the risk of bias of each study was reached by consensus. We downgraded the evidence by one level if > 25% of the participants were included from studies with an overall judgement of a high risk of bias.
2. Inconsistency
The evidence was downgraded by one level when heterogeneity or variability in results was substantial (I² > 50%), or if there were inconsistent findings, e.g. large differences in treatment effect estimates, or in the direction of effect across studies.
3. Indirectness
Indirectness refers to the generalizability of the study population, the chosen intervention, and the outcomes, e.g. the extent to which the study population in the trials is representative of those defined in the inclusion criteria of the review. The evidence was downgraded one level when there was an uncertainty about generalizability of the results.
4. Imprecision
Studies with small sample sizes may lead to imprecision of results, with few events and wide confidence intervals (CIs) around the effect estimate. The evidence was downgraded by one level if the results were considered imprecise due to either (1) or (2); or by two levels if both were applicable. 1) When the sample size was small, with few events and a wide CI; if there was only one study that could be included in the comparison; or if there was more than one trial but the total number of events was less than 300 for dichotomous data, or if the number of participants was less than 400 for continuous data. No precise cutoff exists for 'insufficient' data, but these numbers were used as a general rule of thumb (Furlan 2015; Mueller 2007). 2) If the 95% CI around the pooled estimate of effect includes both a) no effect and b) appreciable benefit or appreciable harm in dichotomous outcomes (threshold is a relative risk reduction or relative risk increase of > 25%), or if the 95% CI includes both a) no effect and b) the upper or lower confidence limit crosses an effect size (standardized mean difference) of 0.5 in either direction in continuous outcomes.
5. Publication bias
The quality of evidence would be downgraded by one level if a funnel plot suggested publication bias. However, in this review, the amount of studies in the meta‐analysis of each specific comparison was not large enough to develop a funnel plot or draw conclusions concerning potential publication bias.
Data and analyses
Comparison 1. NSAIDs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity on 100‐mm VAS. Follow‐up ≤ 3 weeks | 4 | 815 | Mean Difference (IV, Random, 95% CI) | ‐7.29 [‐10.98, ‐3.61] |
| 2 Disability (RMDQ 0 to 24). Follow‐up ≤ 3 weeks | 2 | 471 | Mean Difference (IV, Random, 95% CI) | ‐2.02 [‐2.89, ‐1.15] |
| 3 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks | 5 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.12, 1.75] |
| 4 Proportion of participants experiencing adverse events | 6 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.18] |
Comparison 2. Selective COX‐2 inhibitors versus non‐selective NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in pain intensity from baseline on 100 mm VAS. Follow‐up ≤ 3 weeks | 2 | 437 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐9.23, 4.03] |
| 2 Proportion of patients experiencing adverse events | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.63, 1.50] |
| 3 Proportion of patients experiencing gastrointestinal adverse events | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.09] |
Comparison 3. NSAIDs versus paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean difference in pain intensity on various scales. Follow‐up ≤ 3 weeks | 2 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.35, 0.12] |
| 2 Proportion of participants experiencing adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. NSAIDs versus other drug treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.81, 1.25] |
| 2 Proportion of participants experiencing adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. NSAIDs versus non‐drug treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity on 100‐mm VAS. Follow‐up ≤ 3 weeks. | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 NSAIDs versus spinal manipulation | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants experiencing global improvement. Follow‐up ≤ 3 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 NSAIDs versus spinal manipulation | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Proportion of participants experiencing adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 NSAIDs versus spinal manipulation | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aghababian 1986.
| Methods | RCT, open‐label. Randomisation procedure not described Follow‐up time: 14 days |
|
| Participants |
Population: 56 participants, 11 women, 22 men Setting: people presenting at the emergency department of the University of Massachusetts Medical Center (1 site) Inclusion criteria: mild to moderate acute LBP, age 18 to 60 years Exclusion criteria: taking analgesics, chronic back pain, pain longer than 72 hours, history of bleeding disorders, high blood pressure, heart, kidney, liver, or ulcer disease, pregnant or breast feeding, allergic reactions to analgesics or NSAIDs |
|
| Interventions | NSAID (i): diflunisal capsules, 1000 mg initially, 500 mg every 8 to 12 hrs, 2 wks (N = 16) NSAID (ii): naproxen capsules, 500 mg initially, 250 mg every 6 to 8 hrs, 2 wks (N = 17) | |
| Outcomes | No. of participants (%) reporting none or mild pain (on a ordinal 4‐point scale) after 2 weeks (i) 16/16 (100%) (ii) 15/17 (88%). No. of participants (%) reporting global improvement (on a ordinal 4‐point scale) after 2 weeks (i) 16/16 (100%) (ii) 15/17 (88%) No significance tests reported No adverse experiences were reported by the participants |
|
| Funding | Funding by Merck Sharp & Dohme, developer of diflunisal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | High risk | High drop‐out rate: 32/65 participants (49%). 14 lost to FU; 2 withdrew after initial evaluation; 7 were withdrawn (5 took other medication in addition to the study medication; 2 suffered from chronic LBP, not acute) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | No ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | No table with baseline characteristics |
| Co‐interventions avoided or similar | Low risk | Additional treatment of both groups included bedrest, local application of heat, rehabilitative exercise, and other measures as appropriate |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Agrifoglio 1994.
| Methods | RCT; multicentre, single‐blind (observer blind), randomised, parallel group study Randomization procedure not described Follow‐up time: 8 days |
|
| Participants |
Population: 100 participants, 40 women, 60 men. Mean age 42.2 years (range 19 to 68) Setting: enrolled from 5 centres in Italy Inclusion criteria: acute lumbago, onset less than 48 hours ago, age 18 to 70 years, pain intensity at least 50 mm on VAS Exclusion criteria: any disorder which might interfere with the study drug, usage of anticoagulants or other drugs which may interfere with the treatment assessment, pregnancy, breastfeeding, or hormonal contraception |
|
| Interventions | NSAID (i): aceclofenac 150 mg IM b.i.d. for 2 days + 100 mg tablets b.i.d. for 5 days + 1 placebo tablet for 5 days (N = 50) NSAID (ii): diclofenac 75 mg IM b.i.d. for 2 days + 50 mg tablets t.i.d. for 5 days (N = 50) | |
| Outcomes | Mean improvement in pain intensity (VAS; 0 to 100 mm) after 8 days: (i) 65 (ii) 62 Global improvement good/very good in (i) 87% and (ii) 79% of participants Adverse events: (i) 1 (ii) 6 participants |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | (i) 9/50 withdrew = 18% (ii) 8/50 withdrew = 16% |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | No ITT analysis mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Amlie 1987.
| Methods | RCT; double‐blind, parallel placebo‐controlled multicentre trial. Randomisation procedure not described Follow‐up time: 7 days |
|
| Participants |
Population: 282 participants, 116 women, 166 men Setting: 27 GPs, company doctors, and rheumatologists participated in the trial, conducted in Norway Inclusion criteria: acute LBP, onset within the previous 48 hours, free from LBP for the last 3 months, age 18 years to 60 years Exclusion criteria: radicular symptoms, ankylosing spondylitis, rheumatoid arthritis, history of peptic ulcer or severe dyspepsia, hypersensitivity to aspirin or other NSAIDs, pregnancy or lactation; and any other hematologic, hepatic, renal, pulmonary, cardiac, or systemic disease |
|
| Interventions | NSAID (i): piroxicam 20 mg capsules, single daily dose of 40 mg (2 capsules) for the first two days, then single daily dose of 20 mg (1 capsule) for the next 5 days; 7 days (N = 140). Reference treatment (ii): placebo capsules, single daily dose of 40 mg (2 capsules) for the first two days, then single daily dose of 20 mg (1 capsule) for the next 5 days; 7 days (N = 142). | |
| Outcomes | (i) More pain relief than (ii) measured with visual analogue scale after 3 days. After 7 days no significant differences Consumption of additional analgesics: (i) 49 (N = 134) versus (ii) 62 (N = 132), with a mean consumption of 3.2 (i) versus 5.9 (ii) tablets after 7 days. Return to work after 7 days: (i) 42 (N = 134) versus (ii) 28 participants. Adverse effects: similar (i) 18 (13%) (ii) 24 (17%) |
|
| Funding | Study supported by Pfizer, developer of Piroxicam | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | To maintain double‐blind conditions, placebo capsules of identical appearance were administered in the same way as the study drug. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 5,6%. (i) 6/140 withdrew. (ii) 10/142 withdrew. (16/242) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | No ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | Paracetamol 500 mg, maximum 6 to 8 tablets a day, was used as rescue medication. In a very few cases, a combination of paracetamol and codeine was permitted for more severe pain. The consumption of rescue analgesics was recorded for each participant during the trial. |
| Compliance acceptable | Unclear risk | Compliance not mentioned; they do mention that 12 withdrawals were due to protocol violations. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Babej‐Dolle 1994.
| Methods | RCT; observer‐blind, multicentrer study Follow‐up time: 1 to 2 days (depending on amount of injections) |
|
| Participants |
Population: 260 participants, 126 women, 134 men Setting: 16 medical offices of GPs throughout Germany, May 1990 to December 1990 Inclusion criteria: age 18 years or older; lumbago or sciatic pain Exclusion criteria: hypersensitivity to drugs or drug‐related malfunction of liver or kidney, polyneuropathy, previous disk surgery or vertebral fractures, psychiatric disease, alcohol and drug abuse, pregnancy or lactation. No use of other analgesics, spasmolytics, or NSAIDs besides study medication. |
|
| Interventions | NSAIDs (i): dipyrone IM, 5 mL (= 2.5 g), once daily, 1 to 2 injections, 1 to 2 days (N = 88) NSAIDs (ii): diclofenac IM, 3 mL (= 75 mg), once daily, 1 to 2 injections, 1 to 2 days (N = 86) Reference treatment (iii): placebo, 5 mL isotonic saline, once daily, 1 to 2 injections, 1 to 2 days (N = 86) | |
| Outcomes | Mean (SD) pain intensity (VAS) at baseline and after 6 hours: (i) 80.2 (15.4), 33.4 (25.5); (ii) 79.2 (14.5), 41.7 (25.9); (iii) 78.2 (14.8), 54.8 (25.3). (i) significantly better than (ii) and (iii) No. (%) of participants recovered after 2 days (characterising their general well‐being as 'very well'): (i) 27 (32%); (ii) 10 (12%); (iii) 7 (9%) Adverse events: (i) 4 participants (1 withdrew), (ii) 1 participant, (iii) 2 participants |
|
| Funding | The study was funded by Hoechst AG, developer of Dipyrone | |
| Notes | Some participants with sciatic pain, not clear how many, although 22 were pretreated because of this; no subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a random number generator |
| Allocation concealment (selection bias) | Low risk | Drugs were randomised, pre‐packed and provided by pharmaceutical company in individualised participant's kits, which were assigned by the investigator according to the participant's order of entry to the study. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | There was no possibility of using matched preparations, therefore, treatments were given observer‐blind to three parallel groups of outpatients. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | There was no possibility of using matched preparations, but the preparation of the injection and the injection itself were carried out by two different persons. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Treatments were given observer‐blind to three parallel groups of outpatients; and assessments were performed by someone from the investigational staff who was unaware of the drug administered. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate (14/260 = 5.4%) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar; 22 participants with sciatic complaints were pre‐treated with NSAIDs, they had an even distribution among the 3 groups: (i) 7 participants, (ii) 9 participants, (iii) 6 participants |
| Co‐interventions avoided or similar | Low risk | Sciatic pain was pre‐treated in 22 participants (mostly with diclofenac, piroxicam, or ibuprofen). Besides the study medication, no other analgesics, spasmolytics, or anti‐inflammatory drugs were allowed. |
| Compliance acceptable | Low risk | Compliance acceptable |
| Timing outcome assessments similar | High risk | Similar timing of outcome assessments, but if a participant was pain‐free, there was no second injection. Maximum follow‐up was set on 24 hours after the last injection. This implies some participants had 24 hours of follow‐up and others 48 hours. |
| Other bias | Low risk | None |
Bakshi 1994.
| Methods | RCT; double‐blind, multicentre study. Randomisation procedure not described. Follow‐up time: 14 days |
|
| Participants |
Population source: 132 participants, 62 men, 70 women Setting: 7 outpatient clinics in Austria Inclusion criteria: acute back pain less than 1 week, moderate to severe pain (> 50 mm on VAS), at least 2 objective signs of lumbosacral pathology (tenderness, limited ROM, or SLR < 75 degrees), LBP due to mechanical cause Exclusion criteria: hypersensitivity to aspirin or NSAIDs, gastroduodenal ulcer, history of gastrointestinal bleeding, severe cardiac, hepatic, or renal insufficiency, severe hypertension, history of haemopoietic or bleeding disorders, pregnancy, sensory or motor deficits in lower extremities, and infective, inflammatory, neoplastic, metabolic, or structural cause |
|
| Interventions | NSAIDs (i): diclofenac 75 mg b.i.d., 14 days (N = 66) NSAIDs (ii): piroxicam 20 mg b.i.d. 2 days and after that, once a day plus a placebo capsule once a day for 12 days (N = 66) | |
| Outcomes | Mean pain intensity at rest (VAS) at baseline and after 4, 8, and 15 days: (i) 70.0, 43.3, 30.6, 22.7; (ii) 67.1, 44.5, 27.8, 21.0 No. (%) of participants improved after 14 days: (i) 54 (82%), 58 (88%). Not significant Adverse events (i) 13 participants (1 withdrawal), (ii) 12 participants (4 withdrawals) |
|
| Funding | Funding by Ciba‐Geigy (now Novartis), developer of diclofenac resinate | |
| Notes | Some participants with additional radicular symptoms, not clear how many; no subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Matching capsules were provided to maintain double‐blind conditions. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Matching capsules were provided to maintain double‐blind conditions. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Dropout rate 18% (24/132) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | Administration of analgesic/anti‐inflammatory drugs other than the study medication was not permitted during the trial. In the 3 days preceding the trial, only paracetamol was allowed. |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Brown 1986.
| Methods | RCT; open‐label. Randomisation procedure not described Follow‐up time: 15 days |
|
| Participants |
Population: 47 participants, 22 women, 18 men. Mean age 29 years (range 18 to 59) Setting: University of Maryland Health Center Inclusion criteria: initial or recurrent mild to moderate acute LBP, age 18 to 59 years Exclusion criteria: pregnant or nursing women, allergy to aspirin or other NSAIDs, history of peptic ulcer, gastrointestinal bleeding or bleeding disorders, significant hypertension; cardiovascular, renal, or hepatic disease, recurrent chronic pain, neurologic signs or symptoms, fracture of the lumbosacral spine |
|
| Interventions | NSAID (i): diflunisal (500 mg tablets), initial dose 2 tablets (1000 mg), followed by 1 tablet (500 mg) every 12 hrs, 15 days (N = 19) Reference treatment (ii): acetaminophen with codeine (300 mg + 30 mg tablets), initial dose 2 tablets (660 mg), followed by 1 tablet (330 mg) every 4 hours, 15 days (N = 21) | |
| Outcomes | Pain assessments by participant and investigator on 3‐point ordinal scale showed similar improvement curves (data in graphs) No. of participants rating drugs as excellent or very good (i) 9 (ii) 9. No significant differences Side effects: more side effects in (ii) 10 (in 5 participants) than in (i) 3 (in 3 participants). No participants withdrew because of side effects. |
|
| Funding | Study supported by a grant from Merck Sharp & Dohme (developer of diflunisal) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Study co‐ordinator dispensed all medications and was not involved in participant evaluation. None of the investigators were aware of the medication taken by the participant. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Dropout rate 14.8% (7/47) 7 participants were withdrawn because they failed to return for follow‐up evaluation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | No ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | No table with baseline characteristics |
| Co‐interventions avoided or similar | Low risk | No other analgesic/anti‐inflammatory treatment was allowed. Each participant did the same postural exercises from day 3 on. |
| Compliance acceptable | Unclear risk | Compliance not mentioned |
| Timing outcome assessments similar | Unclear risk | Timing similar |
| Other bias | Low risk | None |
Colberg 1996.
| Methods | RCT; randomised, controlled, parallel, open multicentre study. Randomisation procedure not described. Follow‐up time: 8 days |
|
| Participants | Population: 183 participants, 103 men, 80 women Setting: 12 centres of orthopaedic surgeons, internal specialists, and general practitioners in Germany Inclusion criteria: age 18 years or older, acute lumbago, onset within 48 hours prior to treatment Exclusion criteria: chronic or chronically recurrent LBP, disc prolapse, whiplash injury or direct trauma, history of, or active gastrointestinal ulcer, coagulation or bleeding disorders, hypersensitivity to analgesics or NSAIDs, use of oral anticoagulants or lithium therapy, pregnant or breastfeeding women, or women without adequate contraception | |
| Interventions | NSAIDs (i): meloxicam IV 1.5 mL (= 15 mg) IV on day 1 (1 injection), followed by 1 tablet (15 mg) daily for 7 days; 8 days (N = 92) NSAIDs (ii): diclofenac IM 3ml (= 75 mg) IM on day 1 (1 injection), followed by 1 tablet (100 mg, slow‐release) daily for 7 days; 8 days (N = 91) | |
| Outcomes | Percentage of participants with no or mild pain during movement after 8 days: (i) 91%; (ii) 88% Percentage of participants recovered after 8 days, on overall improvement, functional status, and tolerance: (i) 89%, 67%, 96%; (ii) 91%, 54%, 95% Adverse events: (i) 6 participants (1 severe), (2 withdrew), (ii) 8 participants (1 severe), (2 withdrew) |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 5,5% (10/183) In both groups, 3 withdrawals because of insufficient efficacy, and 2 because of an adverse event; total withdrawals: 10 participants |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Compliance was assessed by the dispensing record and the number of trial medication tablets taken, but not mentioned in results. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Dreiser 2003.
| Methods | RCT; double‐blind; double‐dummy randomised, placebo‐controlled, parallel group; multicentre Follow‐up time: 8 days Drugs were randomised according to a random scheme in blocks of 3, provided by pharmaceutical company; the lowest available randomisation number was assigned to the participants at each site when entering the study. |
|
| Participants |
Population: 372 participants, 187 women, 182 men. Mean age approximately 40 years Setting: 54 clinics of general practitioners in France Inclusion criteria: age 18 to 60 years, with untreated acute low back pain, onset within 2 days, pain ≥ 50 mm on 100‐mm VAS, not due to an associated radiculalgia; not radiating below gluteal fold, pain intermittent or constant, and aggravated by mechanical factors Exclusion criteria: hypersensitivity to diclofenac, ibuprofen, paracetamol, aspirin; current disease status that could interfere with safety or efficacy of study medication; drug or alcohol abuse; anticoagulant therapy or other concomitant treatments; pregnant or nursing; sensory or motor deficits in lower extremities; previous episode of LBP within 3 months; chronic LBP, or an infective, inflammatory, neoplastic, metabolic or structural cause for back pain |
|
| Interventions | NSAID (i): diclofenac‐K 12.5 mg, initial dose 2 tablets (25 mg), 7 days flexible dose 1 to 2 tablets every 4 to 6 hours, maximum 6 tablets a day; 8 days (N = 124) NSAID (ii): ibuprofen 200 mg, initial dose 2 tablets (400 mg), 7 days flexible dose 1 to 2 tablets every 4 to 6 hours, maximum 6 tablets a day; 8 days (N = 122) Reference treatment (iii): placebo, initial dose 2 tablets, 7 days flexible dose 1 to 2 tablets every 4 to 6 hours, maximum 6 tablets a day; 8 days (N = 126) | |
| Outcomes | Pain intensity, mean changed score (SD), 100 mm VAS, after 7 days: (i, N = 122) ‐48.4 (26.1); (ii, N = 119) ‐48.8 (24.0); (iii, N = 121) ‐37.5 (26.9); (i) vs (iii) and (ii) vs (iii) significantly lower (P < 0.001)
Roland‐Morris Back Pain Questionnaire (0 to 24, no to maximal disability) mean changed score (SD) after 7 days: (i, N = 119) ‐8.6 (5.7); (ii, N = 118) ‐8.1 (5.2); (iii, N = 116) ‐5.7 (5.3); (i) vs (iii), and (ii) vs (iii) significantly lower (P < 0.001) Global improvement: a lot/complete in (i) 53/119 (45%) and (ii) 48/119 (40%) Adverse events: (i) 16 participants (4 withdrew); (ii) 14 participants (4 withdrew); (iii) 20 participants (8 withdrew) |
|
| Funding | Study supported by Novartis Consumer Health (developer of diclofenac‐K (Voltaren K)) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the study sponsor arranged the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Adequate: drugs were randomised according to a random scheme in blocks of 3, provided by the study sponsor; the lowest available randomisation number was assigned to the participants at each site when entering the study. Each package of study medication looked equal and was labelled with a randomisation number. Sealed decoding envelopes were supplied to the sites for un‐blinding in case of emergency; all were returned unopened to the study sponsor. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Blinding was achieved by double‐dummy approach. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 7% (26/369 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol registered |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Rescue medication consisted of 1 or 2 tablets of paracetamol (500 mg); the use of rescue medication terminated the participation of the participant in the trial. No even distribution among groups. |
| Compliance acceptable | Low risk | Daily use of study medication was recorded in participant diary, and study medication left at the end of the trial was counted. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Hancock 2007.
| Methods | RCT; randomised, double‐blind, double‐dummy, parallel trial with 4 arms Follow‐up time: 12 weeks (outcomes recorded at baseline, 1, 2, 4, and 12 weeks) |
|
| Participants |
Population: 240 participants, 105 women and 135 men. Mean age (SD) 40.7 years (15.6); mean duration of current symptoms (SD) 9.13 days (9.31) Setting: 19 GPs from 14 general practices in an urban population in Australia, recruitment between June 2005 and October 2006 Inclusion criteria: all participants with low back pain (with or without leg pain) of less than 6 weeks duration, causing moderate pain and moderate disability (measured by adaptations of items 7 and 8 of SF‐36) Exclusion criteria: present episode of pain not preceded by a pain‐free period of at least 1 month, in which care was not provided; known or suspected serious spinal pathology; nerve root compromise (with at least two of these signs: myotomal weakness, dermatomal sensory loss, or hyporeflexia of the lower limb reflexes); presently taking NSAIDs or undergoing spinal manipulation; any spinal surgery within the preceding 6 months; and contraindication to paracetamol, diclofenac, or spinal manipulative therapy |
|
| Interventions | All participants: paracetamol 1g four times daily (until recovery or for a maximum of 4 weeks) and advice given by the GP NSAID (i): diclofenac 50 mg twice daily and placebo manipulative therapy (N = 60) NSAID (ii): diclofenac 50 mg twice daily and spinal manipulative therapy (N = 60) Reference treatment (iii): spinal manipulative therapy and placebo drug twice daily (N = 59; 1 excluded after randomisation) Reference treatment (iv): double placebo (N = 60) |
|
| Outcomes | Mean (SD) number of days to recovery, counted as the first pain‐free day (pain score 0 or 1 on range 0 to 10) Hazard ratio of (i) and (ii) vs (iii) and (iv): 1.09 (95% CI 0.84 to 1.42). No significant difference Hazard ratio of (ii) vs (iv): 1.10 (95% CI 0.76 to 1.60, P = 0.609). No significant difference Median days to recovery: (i) and (ii): 13 (95% CI 10 to 16); (iii) and (iv): 16 (95% CI 14 to 18). No significant difference The effects of NSAIDs and spinal manipulative therapy did not interact significantly. Secondary outcomes (pain, disability, function, global perceived effect): no significant differences at any time point Adverse events: total 22 participants (22/239; 9%) (i, ii) 11 participants (1 withdrew); (iii, iv) 11 participants (none withdrew) Additional data retrieved after contacting the author: PI after 14 days, mean NRS (SD): (i, N = 59) 2.32 (2.367); (iii, N = 59) 2.24 (2.003); (iv, N = 60) 2.80 (2.602). No significant differences Disability after 14 days, mean RMDQ (SD): (i, N = 58) 4.86 (5.826); (iii, N = 59) 4.29 (5.408); (iv, N = 60) 5.98 (6.052). No significant differences Global perceived effect after 14 days, mean global perceived effect (SD): (i, N = 59) 3.47 (1.716); (iii, N = 59) 3.73 (1.215); (iv, N = 60) 3.12 (2.059). No significant differences PI after 84 days, mean NRS (SD): (i, N = 57) 1.05 (2.108); (iii, N = 58) 0.98 (2.098); (iv, N = 60) 0.93 (1.903). No significant differences Disability after 84 days, mean RMDQ (SD): (i, N = 57) 2.28 (5.095); (iii, N = 58) 2.03 (4.433); (iv, N = 60) 2.42 (5.093). No significant differences Global perceived effect after 84 days, mean global perceived effect (SD): (i, N = 57) 4.32 (1.638); (iii, N = 58) 4.52 (1.525); (iv, N = 60) 4.35 (1.696). No significant differences |
|
| Funding | Funding source (Australia's National Health and Medical Research Council) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The active diclofenac was donated by Alphapharm (a generic drug manufacturing company based in Australia). | |
| Notes | Declarations of interest: one of the authors was a member of an advisory board about paracetamol for GlaxoSmithKline. Payments went to an audited hospital account for teaching and research purposes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule developed by statistician not involved in data collection or analysis. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Participants were blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | GPs were blinded, but the physiotherapist who would give either active or placebo spinal manipulative therapy could obviously not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate (< 20% dropouts) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis was performed; the authors mentioned five participants who did not get the correct spinal manipulative therapy intervention as allocated. |
| Selective reporting (reporting bias) | Low risk | Trial was registered and study protocol was published. |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | Co‐interventions similar in the active and placebo arms. Participants were asked not to seek other treatments for their acute LBP during intervention and follow‐up, and a record of additional treatments was kept for any participants who took other treatments within this time. |
| Compliance acceptable | Low risk | Compliance was acceptable. Unused medications were collected and compliance was assessed using various methods. |
| Timing outcome assessments similar | Low risk | Timing of outcome assessments similar |
| Other bias | Low risk | None |
Hosie 1993.
| Methods | RCT; multicentre, double‐blind, double‐dummy. Randomisation procedure not described Follow‐up time: 2 weeks |
|
| Participants |
Population: 287 participants, 136 women, 151 men. Aged 18 to 63 years Setting: 28 general practices in the UK Inclusion criteria: acute low back injury/LBP, onset less than 1 month ago Exclusion criteria: sciatica, 2 or more episodes of LBP in the previous 6 months, nerve root pressure, previous vertebral fractures, spinal stenosis, spondylolisthesis, marked scoliosis, ankylosing spondylitis, osteoarthritis, Paget's disease, metabolic bone disease, systemic connective tissue disorders, malignancy, infection, referred pain from intra‐abdominal or intra‐pelvic disease, pregnancy, lactation, allergy to NSAIDs, history of bronchial asthma or peptic ulcer, gastrointestinal, renal, hepatic, cardiovascular, metabolic, haematological, or dermatological disease |
|
| Interventions | NSAID (i): ibuprofen (capsules, 400 mg) 3 times daily + placebo foam 3 times daily, 14 days (N = 147) NSAID (ii): felbinac (foam, 3%) 3 times daily + placebo capsules 3 times daily, 14 days (N = 140) | |
| Outcomes | Participants (%) reporting none or mild severity after 1 and 2 weeks (i) 84, 92 (ii) 76, 88. No significant differences between the groups Adverse events (EA): No. of side effects (i) 22 AEs reported by 21 participants (8 withdrew), (ii) 26 AEs reported by 22 participants (3 withdrew) |
|
| Funding | Not mentioned. However, the address for correspondence is the Medical Department, Lederle Laboratories, and Traxam (felbinac) was a registered trademark of Lederle Laboratories, UK. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Blinding was achieved by double‐dummy approach. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Dropout rate 18% (52/287 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | ITT analysis performed, based on available data, but for ITT‐analysis dropout too high (analysis based on 172 participants). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | participants were instructed not to use any other oral, injectable, or topical analgesic or antiinflammatory medication, and to continue any ongoing physiotherapy without change. |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Innes 1998.
| Methods | RCT; double‐blind; multicentre. Randomised according to computer‐generated random allocation table, exact procedure not described. Follow‐up time: 7 to 9 days |
|
| Participants |
Population: 122 emergency department participants with acute LBP, 26 women, 96 men. Mean age (SD), 34.5 (10), range 19 to 62 years Setting: 6 university and community hospital emergency departments in Canada Inclusion criteria: acute musculoskeletal low back pain (moderate/severe), onset within the previous 72 hours, age 18 to 60 years, weight > 50 kg, well enough for discharge within 4 hours, requirement of oral analgesics Exclusion criteria: treatment with investigational drug in previous 4 weeks; adverse events due to NSAIDs; hypersensitivity to analgesics, antipyretics, or NSAIDs; anti‐coagulants use within 4 weeks, concurrent treatment with other medications influencing pain intensity evaluations; active peptic ulcer within 6 months; anticoagulant use; conditions requiring treatment beyond analgesics; pregnancy or breastfeeding; alcohol or drug abuse; chronic or recurrent LBP or neurologic cause; interfering co‐existing injury or illness |
|
| Interventions | NSAID (i): Dose 1 to 4 per day: ketorolac tromethamine 10 mg (1 capsule) + placebo (1 capsule), every 4 to 6 hours as needed, up to 4 doses per 24 hours. Dose 5 and 6 per day, if required: acetaminophen 650 mg per dose (2 capsules); up to 7 days (N = 62). Reference treatment (ii): Dose 1 to 4 per day: acetaminophen 300 mg + codeine 30 mg per capsule (2 capsules), every 4 to 6 hours as needed. Dose 5 and 6 per day, if required: acetaminophen 650 mg per dose (2 capsules); up to 7 days (N = 60) | |
| Outcomes | Pain mean changed score (SD), 100‐mm VAS, after 6 hours: (i, N = 55) ‐6.4 (17); (ii, N = 58) ‐5.4 (16), no significant differences % participants reporting 'a lot/complete' pain relief after 4 days: (i) 53%; (ii) 55%, no significant differences % participants reporting 'no/mild' impairment after 4 days: (i) 67%; (ii) 62%, no significant differences Adverse events: (i) 21 participants (0 withdrew); (ii) 38 participants (7 withdrew) | |
| Funding | Study funded by Hoffmann‐La Roche of Canada (developer of Toradol = ketorolac tromethamine). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Exact procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | All drugs were prepared in identical capsules to preserve double‐blinding. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | All drugs were prepared in identical capsules to preserve double‐blinding. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | A blinded consultant entered all data and performed statistical analyses. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Dropout rate: 18.9% (23/122 withdrew) 16 (10 (i) and 6 (ii)) withdrew prematurely because of analgesic inefficacy, 7 (ii) withdrew because of side effects |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unclear if pain relief and impairment was also measured after 7 days; it was not mentioned. Study protocol locally approved, not registered. |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Drug were packaged in blister cards that separated each day's drug supply into six doses labelled 1 to 6. Each 'dose' consisted of two capsules. |
| Compliance acceptable | Unclear risk | Not mentioned if blister cards with used/unused medication were collected. Participants were asked to record all medication intake in their study diary. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Jaffe 1974.
| Methods | RCT; double‐blind, between‐participant study with matched pairs. Randomisation procedure not described Follow‐up time: 7 days Information extracted from Group A (acute low back pain with or without sciatica) |
|
| Participants |
Population: subgroup of 30 participants (consecutive attenders at the surgery) with acute lumbar pain, with or without sciatica, 20 women, 10 men. Mean age (SD), 40.5 (13.5) (i), 38.1 (10.6) (ii); range not mentioned Setting: surgery department in England Inclusion criteria: acute LBP, with or without sciatica, of mechanical and/or degenerative origin Exclusion criteria: peptic ulcer, renal or hepatic impairment, history of intolerance to study drugs, children (no upper age limit) |
|
| Interventions | NSAID (i): alclofenac capsules 1 g t.i.d.; 7 days (N = 15) NSAID (ii): indomethacin capsules 50 mg t.i.d.; 7 days (N = 15) | |
| Outcomes | Mean (SEM) change score in pain intensity (5‐point scale, 0 to 4) and functional status (4‐point scale, 0 to 3) after 7 days for acute LBP: (i) 1.46 (0.28), 1.80 (0.03); (ii) 1.45 (0.27), 1.53 (0.23). Not significant Adverse events: no data presented on adverse events for subgroup with acute LBP. These are the AEs in the whole group (60 participants), consisting of both acute and chronic LBP: (i) 5 participants; (ii) 3 participants (none withdrew). |
|
| Funding | Not mentioned. Berk Pharmaceuticals Limited is the manufacturer of alclofenac (Prinalgin) and provided the capsules. | |
| Notes | Part of the population had additional sciatica complaints. Not mentioned how many, no subgroup analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | 'Members of a matched pair were randomly allocated', randomisation procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Double‐blind, details not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Double‐blind, details not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 3,3% (1/30 lost to follow‐up) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | 'Patients were instructed to take no other pain‐killing medication unless they informed the clinician'. Follow‐up of this advice not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Lacey 1984.
| Methods | RCT; double‐blind, placebo‐controlled. Randomisation procedure not described. Follow‐up time: 14 days Information extracted from subgroup with acute back sprain |
|
| Participants |
Population: subgroup of 337 participants with acute back or sacroiliac pain (< 72 hours). Age and sex ratio unknown Setting: 215 participating general practitioners, conducted in the United Kingdom Inclusion criteria: acute back or sacroiliac pain, diagnosed within the last 72 hours or less Exclusion criteria: not mentioned |
|
| Interventions | NSAID (i): piroxicam 10 mg capsules, four times per day first two days, two times per day next 12 days; 14 days (N = 168) Reference treatment (ii): identical placebo capsules, four times per day first two days, two times per day next 12 days; 14 days (N = 169) | |
| Outcomes | Participant (%) improved after 1 week only in subgroups with initial moderate/severe pain (i) 82%/49% (ii) 53%/38%. No differences for subgroup with mild initial pain. Results after 2 weeks not reported. No data presented on adverse events for subgroup with back pain. The overall numbers, including other musculoskeletal disorders, were similar: (i) 12% (3% withdrew) (ii) 9% (2,5% withdrew). |
|
| Funding | Not mentioned. Statistical help was given by an employee of Pfizer Central Research. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐blind, identical placebo capsules |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, identical placebo capsules |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Unclear risk | Not mentioned for subgroup |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | Baseline characteristics not mentioned for subgroup |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Metscher 2001.
| Methods | RCT; double‐blind, randomised, controlled, multicentre. Article in German. Randomisation procedure not described. Follow‐up time: 7 days (outcome measurements at baseline, after 3 and 7 days) |
|
| Participants |
Population: 192 participants, 87 women, 105 men. Median age 47 years (range 20 to 70) Setting: 24 centres in Germany, from November 1998 until March 1999 Inclusion criteria: age 18 to 70 years; acute LBP; pain 100‐mm VAS ≥ 50 mm; onset within 48 hours; not indicated for other than analgesic treatment Exclusion criteria: not mentioned |
|
| Interventions | NSAID (i): dexketoprofen‐trometamol 25 mg t.i.d.; 7 days (N = 97) Reference treatment (ii): tramadol hydrochloride 50 mg t.i.d.; 7 days (N = 95) | |
| Outcomes | Pain difference, mean changed score, 100‐mm VAS, after 7 days: (i, N = 81) vs (ii, N = 79) ‐6 (4) (P < 0.05) Adverse events: (i) 13 participants; (ii) 22 participants | |
| Funding | Not mentioned | |
| Notes | Unclear presentation of results (data on pain scores extracted from graph) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Dropout rate 17.1% (33/193 withdrew: 1 only baseline measurements; 10 stopped therapy prematurely or failed therapy; 22 protocol violations) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | Not mentioned, no table 1 |
| Co‐interventions avoided or similar | Low risk | Paracetamol was allowed as rescue medication, maximum 4 tablets of 500 mg |
| Compliance acceptable | Unclear risk | Compliance was assessed by the recordings in the participant diaries. Unused medication was not collected. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Miki 2018.
| Methods | RCT; open‐label, non‐inferiority trial. Computer‐generated randomisation Follow‐up time: 4 weeks |
|
| Participants |
Population: 127 participants, 84 women, 43 men. Mean age (SD) (i) 66.73 (2.29) (ii) 63.5 (19.4) Setting: 1 outpatient hospital in Japan, recruitment from July 2014 until September 2017 Inclusion criteria: age older than 20 years old and initiation of LBP in the 4 weeks prior to study entry Exclusion criteria: seeking a second opinion for a prior consultation, cancer‐related pain, presence of neurological symptoms (e.g. pain radiating down the leg), traumatic cases, such as falls, evidence of bone fractures, surgery within the prior 6 months, current use of full, regularly recommended doses of an analgesic, pregnancy, autoimmune diseases, inflammatory rheumatic disordered, cardiopulmonary restrictions, severe kidney or liver function disorders, acute duodenal or ventricular ulcer, psychiatric disorders, or the presence of laboratory data outside the normal limits |
|
| Interventions |
NSAID (i): loxoprofen 60 mg, 3 times daily; 4 weeks (N = 63) Reference treatment (ii): acetaminophen 600 mg, 4 times daily; 4 weeks (N = 64) |
|
| Outcomes | Pain difference mean changed score, NRS, after 2 weeks: (i) vs (ii) ‐0.51 (95% CI ‐1.70 to 0.67), not significant. After 4 weeks: (i, N = 35) vs (ii, N = 35) ‐0.80 (95% CI ‐2.08 to 0.48), not significant Adverse events: (i) 1 participant (GI complaints); (ii) 5 participants (of which 3 GI complaints) | |
| Funding | The study was funded by a Japanese association for the study of musculoskeletal pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure was performed by a person not involved in the treatment of participants. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Not blinded; open‐label trial |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Not blinded; open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | High risk | Additional information by authors: treatment was performed by a single orthopaedist, and outcomes were assessed by the same physician. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | High risk | High dropout rate 44.9% (57/127 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | No ITT analysis, only a PP analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered at local ethics committee; (inter)national trial registry not mentioned. The primary outcome pain intensity (NRS) after 2 and 4 weeks was not presented clearly in the results section of the article. |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | External medication for pain was not allowed, with the exception of topical anaesthetics. No other supplementary analgesic medication was given during the treatment period. |
| Compliance acceptable | High risk | Additional information by authors: compliance was not measured. Participants were told that they could stop the medication if their symptoms improved, or if any adverse events occurred. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Nadler 2002.
| Methods | RCT; randomised, single (investigator) blind, multicentre study. Stratified randomisation, allocation procedure not described Follow‐up time: 4 days (2 days treatment time) |
|
| Participants |
Population: 371 participants, 216 women, 155 men. Mean age (SD): 36 (10.59) years Setting: 11 sites, conducted in the USA Inclusion criteria: age 18 to 55 years, acute nonspecific LBP; pain intensity ≥ 2 on 6‐point scale (at least moderate intensity); no low back trauma within the preceding 48 hours; an answer of "yes" to the question "Do the muscles in your low back hurt?" Exclusion criteria: radiculopathy or other neurologic deficits; history of back surgery; fibromyalgia; diabetes mellitus; hypersensitivity for NSAIDs, acetaminophen, or heat; peptic ulcer or gastrointestinal bleedings; renal or hepatic disorders; anticoagulant treatment; pregnancy; daily back pain for more than three consecutive months |
|
| Interventions | NSAID (i): ibuprofen 200 mg, 2 tablets t.i.d. + 2 placebo tablets once a day; 2 days (N = 106) Reference treatment (ii): acetaminophen 500 mg, 2 tablets q.i.d.; 2 days (N = 113) Reference treatment (iii): heat wrap, 40 °C, approximately 8 hours of wear per day; 2 days (N = 113) Reference treatment (iv): unheated wrap, 8 hours/day; 2 days (N = 19), no outcome measures presented Reference treatment (v): placebo, 2 tablets q.i.d.; 2 days (N = 20), no outcome measures presented | |
| Outcomes | Pain mean changed score (NRS scale 0 to 5), after 4 days: (i) ‐1.7; (ii) ‐2.0; (iii) ‐2.6; (i) vs (ii) not presented; (i) vs (iii) significantly less effective (P < 0.001) RMDQ (scale 0 to 24, none to maximal disability), mean changed score after 4 days: (i, N = 101) ‐2.7, (ii, N = 104) ‐2.9, (iii, N = 110) ‐4.9; (i) vs (ii) not presented; (i) vs (iii) significantly less effective (P < 0.001) Adverse events: no serious side effects occurred. Systemic adverse events: (i) 10.4% (11/106; 1 withdrew), (ii) 4.4% (5/113), (iii) 6.2% (7/113) |
|
| Funding | Funding by the Procter and Gamble company (developer of the used ThermaCare Heat Wrap). | |
| Notes | Declarations of interest: Dr. Nadler is a paid consultant for Procter & Gamble and most of the co‐authors are employees of the Procter and Gamble Health Sciences Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation, procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | The participants using tablets (i, ii, v) were blinded for which tablet group they were in; the participants using wraps (iii, iv) were blinded for which wrap they received; but none of them were blinded for both tablets and heat wraps. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Investigator blinded, data sets determined before the database was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 2.2% (8/371 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | ITT analysis performed, but data not presented. Results of ITT population matched the results of the PP populations, so only the evaluable subject population was reported. |
| Selective reporting (reporting bias) | High risk | Study protocol available, but several data from primary treatment groups were not compared, and results of reference treatment (iv) and (v) were not presented. |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Subjects were asked not to use other treatments. |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Orava 1986.
| Methods | RCT; double‐blind, multicentre. Randomization procedure not described. Follow‐up time: 1 week. |
|
| Participants |
Population: 133 participants, 41 women, 92 men. Age range 18 to 71 Setting: multicentre investigation at 8 cities by 8 physicians, conducted in Finland Inclusion criteria: acute lumbago, onset less than 2 weeks ago, bothersome LBP and considerable functional disability, no previous use of analgesics, antiinflammatory, or muscle relaxing agents for this episode Exclusion criteria: stable, chronic LBP; LBP due to disorders of the pelvic region or spinal disorders (prolapse/hernia of an intervertebral disc); pregnancy or nursing; hypersensitivity to salicylates or indomethacin; current treatment with systemic corticosteroids or anticoagulants; active peptic ulcer or gastrointestinal haemorrhage; significant liver of kidney disease; haemopoietic disorders |
|
| Interventions | NSAID (i): diflunisal 500 mg (capsules) b.i.d.; 7 days (N = 66) NSAID (ii): indomethacin 50 mg (capsules) t.i.d.; 7 days (N = 67) | |
| Outcomes | Data shown in graphs, extracted from graphs. Mean score difference in pain (4‐point Likert scale, range 0 to 3, no pain to severe pain) 0 to 7 days: (i) ‐1.0, (ii) ‐1.1 (at rest); (i) ‐1.6, (ii) ‐1.5 (on active movement). No significant differences Mean score difference in functional disability (4‐point Likert scale, range 0 to 3, no disability to severe disability) 0 to 7 days: (i) ‐1.5, (ii) ‐1.55. No significant differences Adverse events: (i) 12 participants = 18.2% (2 withdrew), (ii) 21 participants = 31.3% (6 withdrew) |
|
| Funding | Not mentioned | |
| Notes | Partially flare participants: 126 participants with acute lumbago, 7 participants with acute exacerbation of chronic lumbago; no subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | The number and dosing of both medicines were made similar in both groups. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 6% (8/133 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Plapler 2016.
| Methods | RCT; double‐blind, randomised, double‐dummy clinical trial (non‐inferiority) Computer‐generated randomisation Follow‐up time: 10 days (maximum treatment duration 5 days). Outcome measurements at baseline, 60 minutes, 2 days, 4 days, 10 days |
|
| Participants |
Population: 83 participants, 216 women, 155 men. Mean age (SD): 36 (10.59) years Setting: outpatient clinics of 2 research centres in Brazil Inclusion criteria: diagnosed with moderate or severe acute LBP (VAS > 40 mm), aged 18 to 65 years, able to give written informed consent; women of childbearing age had to agree to use contraceptive methods throughout the study Exclusion criteria: weight < 50 kg; severe congestive heart failure; current alcoholism or illegal drug use; presence of fever or signs of infection; kidney disease; fracture; fibromyalgia; cancer; neuropsychiatric disease; rheumatologic disease; history of peptic ulcer disease; gastrointestinal bleeding or hemorrhagic diathesis; cerebrovascular disease; haemostatic disorders or use of anticoagulants; pregnancy; lactation; postoperative people at high risk of bleeding; history of hypersensitivity to NSAIDs; nasal polyps and asthma. No participation in another experimental study 6 months prior to study entry. |
|
| Interventions |
NSAID (i): ketorolac‐trometamol sublingual 10 mg t.i.d.; 5 days (N = 66)
NSAID (ii): naproxen oral 250 mg, t.i.d.; 5 days (N = 67) From 2nd to 5th day, if participant had VAS > 40 mm, an increased dose of four times per day was allowed. |
|
| Outcomes | VAS‐scores are not mentioned separately, only combined scores are mentioned and focus on pain relief by comparing VAS scores 1 hour before and after medication. Relevant outcomes (VAS‐scores) at day 4 are not mentioned. No significant differences mentioned. Adverse events: (i) 42 adverse events (1 withdrew); (ii) 35 adverse events (0 withdrew) |
|
| Funding | EMS Sigma Pharma is a domestic pharmaceutical company in Brazil. This study was funded by EMS and they were involved in study design, protocol development, obtaining the data, and evaluating the data together with the authors. The manuscript was written by the authors, together with EMS medical writing services. All authors received grants and consulting fees from EMS Industry. | |
| Notes | 83 people were screened, none of them were excluded, despite the strict exclusion criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated lottery, randomly assigned., |
| Allocation concealment (selection bias) | Unclear risk | Each participant received a numbered kit in order of arrival. The trial design says double‐dummy and double‐blind assignments; but NSAID (i) was administered sublingually and NSAID (ii) was administered orally, therefore, this seems not possible. It was not mentioned if both tested and reference medication looked identical. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | It was written that both medical staff and participants were blinded to treatment assignments; but different way of administering the medication and no details were mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | It was written that both medical staff and participants were blinded to treatment assignments; but different way of administering the medication and no details were mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | High risk | High dropout rate: 24% (20/83 lost to follow‐up/withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | High risk | ITT analysis performed for adverse events only; PP analysis for effect |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered at local ethical committee. (inter)national trial registry not mentioned. Not mentioned if pain outcome measure (RPR) was registered like this. |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar, except for weight |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Pohjolainen 2000.
| Methods | RCT; double‐blind, double‐dummy, multicentre. Randomised according to a list in permutation blocks, concealed allocation Follow‐up time: 10 days |
|
| Participants |
Population: 104 participants, sex unknown. Mean age 42 years (range 19 to 63) Setting: 1 hospital outpatient clinic and 1 occupational health care centre, both in Helsinki Inclusion criteria: age 18 to 65 years; acute LBP, onset within 30 days Exclusion criteria: chronic LBP for more than 4 weeks; sciatica syndrome (LBP with radiation to an extremity below the knee); secondary cause of LBP; osteoporotic fracture or a history of lumbar spine surgery; pregnancy or lactation; significant systematic disease; history of peptic ulceration; allergy to NSAIDs |
|
| Interventions | NSAID (i): nimesulide 100 mg b.i.d. + placebo once a day; 10 days (N = 52) NSAID (ii): ibuprofen 600 mg t.i.d.; 10 days (N = 52) | |
| Outcomes | Oswestry LBP disability questionnaire, pre‐mean (SD), post‐mean day 10 (SD): (i, N = 52) 35.8 (15.0), 10.0 (10.8); (ii, N = 52) 35.1 (19.1), 16.5 (19.0), (i) vs (ii) significantly lower (P < 0.05)
Average pain intensity and relief scores (100‐mm VAS), pre‐mean (SD), post‐mean day 10 (SD): (i, N = 52) 57.9 (20.6), 12.8 (15.4); (ii, N = 52) 55.2 (21.4), 18.5 (19.9). No significant differences Adverse events: (i) 7 participants = 13% (2 withdrew); (ii) 11 participants = 21% (1 withdrew) |
|
| Funding | Partially supported by Rhône‐Poulenc, a pharmaceutical company. Drugs supplied by Helsinn Healthcare SA, patent holder of nimesulide. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to a list prepared in permutation blocks, random allocation |
| Allocation concealment (selection bias) | Low risk | Concealed allocation, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐blind, double‐dummy |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 4.8% (5/104 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not mentioned |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | No therapy other than the study medication was permitted during the trial. No analgesics, muscle relaxants, topical preparations, local injections or non‐drug therapies (e.g. physiotherapy, massage, or bedrest) were permitted. |
| Compliance acceptable | Unclear risk | Participants were asked to return any unused medication. The monitor counted the numbers of unused pills and decided whether the participant had been compliant. Compliance criteria were assessed by the following: instances of not appearing for visits, incomplete diaries, or medication not taken according to the protocol. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Postacchini 1988.
| Methods | RCT. Randomisation procedure not described Follow‐up time: 6 months (3 weeks, 2 and 6 months) Information extracted from Group I, Subgroup A (acute low back pain) and Group II, Subgroup A (acute back pain radiating to the buttocks and/or thighs and no neurological deficit) |
|
| Participants |
Population: Group I, Subgroup A = 76 participants and Group II, Subgroup A = 83 participants; in total 159 participants with acute LBP (Group I = 271 participants; Group II = 188 participants. Respectively 235 and 163 after retracting the participants lost to follow‐up or who interrupted or changed their assigned treatment), 34 women, 42 men. Mean age 36.3 years (I A) and 37.7 (II A) (range 17 to 58) Setting: two Low Back Clinics in Rome, Italy, between January 1985 and October 1986 Inclusion criteria: age 17 to 59 years; acute LBP of less than 4 weeks duration, no LBP in preceding six months Exclusion criteria: neoplastic or infectious diseases of the spine, pregnant or nursing women, serious general diseases, psychiatric disturbances or medico‐legal litigation |
|
| Interventions | NSAID (i): diclofenac 'full dosage', 10 to 14 days (acute participants) (N = 16 and 18 (I A and II A)) Reference treatment (ii): chiropractic manipulation (N = 17 and 18 (I A and II A)) Reference treatment (iii): physiotherapy (N = 15 and 16 (I A and II A)) Reference treatment (iv): bedrest (N = 15 and 14 (I A and II A)) Reference treatment (v): placebo (anti‐oedema gel; (N = 13 and 17 (I A and II A)) | |
| Outcomes | Mean improvement on combined pain, disability, and spinal mobility score (range 5 to 32 from poor to excellent clinical status) after 3 wks, 2 and 6 months Group I subgroup A: (i) 3.0, 10.7, 14.0 (ii) 7.5, 9.7, 12.3 (iii) 5.0, 8.4, 10.2 (iv) 5.4, 7.5, 7.3 (v) 1.8, 7.3, 11.0. (ii) significantly better than others after 3 wks; no other differences. After 2 and 6 months no significant differences Group II subgroup A: (i) 4.7, 8.7, 10.3 (ii) 6.3, 9.2, 12.1 (iii) 3.7, 6.0, 10.1 (iv) 4.1, 5.7, 10.3 (v) 2.2, 5.1, 9.8. (ii) significantly better than others after 3 wks (P < 0.05). No other differences. After 2 and 6 months no significant differences No data on side‐effects reported |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | An approximately equal number of participants was assigned to each type of treatment. Unclear how this was done |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | High risk | Dropout rate: 13% in Group I (36/271 withdrew), 13% in Group II (25/188 withdrew). Unclear how many in Subgroup A. "The present study did not include a control group of untreated patients, because only a few patients agreed to undergo no treatment and most of them were lost to follow‐up"; this additional placebo group not mentioned in results, not clear how many people in this group originally |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | High risk | In the discussion, they mentioned that a control group of untreated participants was not included, because only a few participants agreed to undergo no treatment and most of them were lost to follow‐up. In the methods, they do not mention this group. |
| Similarity at baseline characteristics | Unclear risk | Not mentioned in detail |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Schattenkirchner 2003.
| Methods | RCT; double‐blind; multicentre. Randomisation procedure not described Follow‐up time: 8 days |
|
| Participants |
Population: 227 participants, mainly from general practices, 85 women, 142 men. Mean age 45.3 (SD ± 10.1) years Setting: 15 centres in Germany, (mainly) general practices, March 2000 to October 2000 Inclusion criteria: age 20 to 65 years; localised, uncomplicated, acute LBP associated with degenerative spinal disorders; participants having pain without analgesic therapy during previous 24 hours; and with a pain intensity score at rest of at least 60 on a 100‐mm VAS Exclusion criteria: suspicion of serious underlying spinal condition (e.g. sciatica), non‐specific back symptoms related to abdominal, pelvic, or thoracic pathology; prior neurological deficits in lower extremities; surgery for LBP; lumbosacral facet syndrome; history of haematological or bleeding disorders; severe cardiac, hepatic, or renal insufficiency; severe hypertension; connective tissue diseases; history of GI ulcer or bleeding; hypersensitivity to aspirin or NSAIDs; alcohol/drug abuse; pregnant/lactating |
|
| Interventions | NSAID (i): aceclofenac 100 mg b.i.d., 10 days or until asymptomatic (N = 114) NSAID (ii): diclofenac 75 mg b.i.d., 10 days or until asymptomatic (N = 113) | |
| Outcomes | Mean change in pain score (100‐mm VAS), from baseline, after 8 to 10 days (SD): (i, N = 114) ‐62.4 (24.5); (ii, N = 113) ‐56.8 (22.6); (ITT analysis, N = 227). QBPDS (functional disability score) change (%), from baseline, after 4 days (SD): (i, N = 100) 25.5 (14.8); (ii, N = 105) 20.6 (12.1); (PP analysis, N = 205) Adverse events: (i) 17 participants (14.9%); (ii) 18 participants (15.9%), none withdrew because of AEs |
|
| Funding | Funding not mentioned. Sponsor UCB Pharma (developer of aceclofenac) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by the generation of a randomisation list using permutable blocks. |
| Allocation concealment (selection bias) | Low risk | Each drug package was identified by a unique code number and was delivered to the participant according to the ascending order of their number. Sealed emergency envelopes with identity of the drugs kept in a secure place, to be opened only in case of a medical emergency, to be returned to the sponsor at the end of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Double‐blind, no double‐dummy |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind. No person conducting the trial had access to the randomisation list before the study was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 4.4% (10/227 withdrew). (i) 6 early cure; (ii) 1 early cure, 2 lack of efficacy, 1 personal reasons |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol mentioned in manuscript, but not registered |
| Similarity at baseline characteristics | Unclear risk | According to text, similar baseline characteristics in both treatment groups, but data were not shown, no table 1. |
| Co‐interventions avoided or similar | Low risk | During the study, participants were not allowed to take any other NSAIDs (except for the study medication), muscle relaxants, benzodiazepines, analgesics, corticosteroids, or coumarinics, or to receive physical or chirotherapy |
| Compliance acceptable | Unclear risk | Compliance was assessed by the recordings in the participant diaries. (Unused) study medication was not collected. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Shin 2013.
| Methods | RCT, assessor‐blinded Follow‐up time: 24 weeks Outcomes measured at baseline, 30 minutes, after 2, 4, and 24 weeks |
|
| Participants |
Population: 58 participants, 24 women and 34 men. Mean age (SD): 38.31 (7.97) years Setting: 2 hospitals in South Korea Inclusion: participants 20 to 60 years with acute LBP of < 4 weeks duration, with or without radiating pain to the limb with an Oswestry Disability Index (ODI) value ≥ 60% as an indicator of severe disability Exclusion: serious disease that could cause LBP (e.g. cancer, vertebral fracture, spinal infection); chronic disease that could interfere with the effect of the treatment or the interpretation of treatment results (e.g. cardiovascular disease, diabetic neuropathy, fibromyalgia); progressive neurological deficit or severe neurological symptoms; conditions inappropriate or unsafe for acupuncture (e.g. haemorrhagic disease, blood coagulation disorders); current intake of corticosteroids, immunosuppressant drugs, psychiatric medicine; experience of gastrointestinal side effects after taking NSAIDs, or current treatment for gastrointestinal disease; pregnancy; and reluctance to accept the treatment regimens or examinations (e.g. X‐ray, MRI) of this study |
|
| Interventions |
NSAID (i): NSAID injection group, received 1 IM injection of conventional diclofenac (N = 29); FU at 30 minutes, 2, 4, and 24 weeks Reference treatment (ii): Motion Style Acupuncture Treatment (MSAT) group, received 1 session of MSAT (N = 29); FU at 30 minutes, 2, 4, and 24 weeks |
|
| Outcomes | Mean (SD) pain intensity change from baseline (NRS 0 to 10): (i) 4.17 (3.05), (ii) 5.83 (2.61), P = 0.0305 (2 weeks); (i) 6.84 (1.9), (ii) 6.64 (2.47), P = 0.7221 (24 weeks) Mean improvement in functional status (Oswestry Disability Index) from baseline: (i) 36.34 (29.1), (ii) 56.41 (24.86), P = 0.0066 (2 weeks); (i) 80.83 (13.58), (ii) 73.23 (20.24), P = 0.0995 (24 weeks) Patient global impression of change (PGIC; subjective assessment of improvement): no outcomes at relevant time points Adverse events: there were no adverse events reported |
|
| Funding | Not mentioned | |
| Notes | In both groups, the selection of treatment after the initial treatment session was not restricted because of ethical reasons. This implies the results after the first follow‐up at 30 minutes are not clean, and are difficult to generalise. Declaration of interest: one of the authors was supported by the Korea Institute of Oriental Medicine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence generated by statistician not involved in data collection or analysis. |
| Allocation concealment (selection bias) | Low risk | Randomised numbers were kept in sealed envelopes by a researcher who had no direct contact with study participants. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Blinding of participants and practitioners was not possible because of the nature of the treatment. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Blinding of participants and practitioners was not possible because of the nature of the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Assessor‐blinding was achieved by blinding the assessor performing outcome assessment and case report form (CRF) data entry to the random allocation. Statistical analysis was performed by an independent statistician who was blinded to the identification of each treatment group, but blinding of participants not possible; therefore unclear risk. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Unclear risk | Not clearly mentioned, but outcomes presented after 2, 4, 24 weeks for the following number of participants: (i) N = 23, 20, 27; (ii) N = 25, 21, 24. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | Trial was registered |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | High risk | Choice for participants for inpatient or outpatient treatment. Inpatients received an integrative package (herbal medicine, Chuna manipulation, bee venom pharmaco‐acupuncture, acupuncture) for 5 sessions a week, outpatients received 1 to 2 sessions a week. In both groups, the selection of treatment after the initial treatment session was not restricted because of ethical reasons. This implies the results after the first follow‐up at 30 minutes are not clean and difficult to generalise. |
| Compliance acceptable | Unclear risk | Not applicable |
| Timing outcome assessments similar | Unclear risk | Timing similar |
| Other bias | Low risk | None |
Stratz 1990.
| Methods | RCT; single‐blind, multicentre study. Randomization procedure not described. Article in German. Follow‐up time: 3‐5 days (baseline and final measurements; maximum follow‐up time 2 days after the last IM injection). |
|
| Participants |
Population: 96 participants with acute LBP, 49 women, 47 men; mean age 50.8 years, aged between 16 and 78 years Setting: participants visiting four participating physicians, conducted in Germany Inclusion criteria: first episode of acute lumbago, or acute onset after a long symptom‐free period Exclusion criteria: age < 14 years, pregnancy or lactation, allergy, current use of corticosteroid or anti‐rheumatic treatment with half‐life > 24 hours, other NSAIDs or anticoagulant treatment |
|
| Interventions | NSAID (i): diclofenac‐natrium IM 3 mL (= 75mg), 1 to 3 injections (with a minimum of 16 hours between injections), 1 to 3 days (N = 47) NSAID (ii): etofenamat (Rheumon®) IM 2 mL (= 1000 mg), 1 to 3 injections (with a minimum of 16 hours between injections), 1 to 3 days (N = 49) | |
| Outcomes | Similar amount of IM injections was needed in both groups, no significant differences. Mean pain score (11‐point NRS), at baseline and after 3 to 5 days: (i) 5.3, 2.7, (ii) 5.3, 2.4 No. of participants recovered after 3 to 5 days (therapeutic success: 'good' or 'very good'): (i) 27/47, (ii) 34/49. Not significant Adverse events: (i) 2 (none withdrew), (ii) 0 (none withdrew) |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | participants were blinded; procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Careproviders were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 1/96 = 1% |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | Baseline characteristics similar according to text, but no Table 1 available. |
| Co‐interventions avoided or similar | Low risk | No use of anti‐rheumatica, anti‐flogisti or analgesic medication other than study drug. No use of corticosteroids. If further treatment with oral or topical NSAIDs was necessary after injections, this was recorded. This was the case in (i) 28/47 (60%) and (ii) 23/49 (47%) of the participants. |
| Compliance acceptable | Low risk | 1 person in group (i) (diclofenac IM) withdrew after the first injection, because of no benefit. All the others seemed to have complied to the treatment and follow‐up program. |
| Timing outcome assessments similar | High risk | Timing not similar; final measurements 2 days after last injection, this could be either after the first, second, or third injection. |
| Other bias | Low risk | None |
Szpalski 1990.
| Methods | RCT; use of randomisation table Follow‐up time: 14 days (clinical assessment at days 0 and 14) |
|
| Participants |
Population: 110 participants, 51 women, 59 men. Mean age (SD): 40.2 (13.7) years Setting: an outpatient department (October 1988 to March 1999), conducted in Belgium Inclusion criteria: acute LBP, pain present < 2 weeks, asymptomatic period of at least 4 months Exclusion criteria: LBP related to industrial accident covered by insurance, specific (spinal) pathology, such as disc protrusion or spinal trauma |
|
| Interventions | NSAID (i): tenoxicam 20 mg, 1 tablet daily (14 days), and bedrest (7 days strict, 7 days intermittent; (N = 49) Reference treatment (ii): bedrest, (7 days strict, 7 days intermittent; (N = 50) | |
| Outcomes | Mean % improvement (SD) between baseline and 14 days in ROM (i) 123 (24), (ii) 114 (23); significant (P < 0.05) After 14 days of treatment: 86% (i) versus 70% (ii) no need for further treatment Adverse events: (i) 2 participants (2 withdrew) |
|
| Funding | Not mentioned | |
| Notes | Physical examination only outcome. Outcome presented as percentages, baseline values not presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A randomisation table was used |
| Allocation concealment (selection bias) | High risk | Not possible by study design; no placebo control |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 10% (11/110) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | Baseline characteristics similar, but only age and sex mentioned, no other baseline values presented. |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Szpalski 1994.
| Methods | RCT; double‐blind, placebo‐controlled. Randomisation procedure not described Follow‐up time: 15 days (clinical evaluation at days 1, 8, and 15) |
|
| Participants |
Population source: 73 participants, 26 women, 47 men. Mean age approximately 38 years Setting: not mentioned Inclusion criteria: acute LBP, pain present for less than 2 weeks, first presentation or first reappearance after an asymptomatic period of at least 6 months Exclusion criteria: work accident covered by worker's compensation, spinal pathology (e.g. herniated disc or spinal trauma), pregnancy or lactation, hypersensitivity to NSAIDs, history of gastrointestinal ulceration, current use of NSAIDs, anticoagulants, oral antidiabetics, or lithium |
|
| Interventions | NSAID (i): tenoxicam 20 mg IM injection on day 1 + 20 mg capsules, 1 per day, for day 2 to 14 (+ 7 days bedrest; (N = 37)) Reference treatment (ii): placebo IM injection on day 1 + placebo capsules, 1 per day, for day 2 to 14 (+ 7 days bedrest; (N = 36)) | |
| Outcomes | Mean pain intensity on VAS on day 1, 8, and 15 (SD): (i) 7.4 (1.5), 1.9 (2.0), 0.6 (1.1); (ii) 7.1 (2.0), 2.8 (2.0), 0.8 (1.1). (i) significantly better on day 8 (P = 0.043). Overall clinical assessment by the investigator after 1 week: (i) 27/33 (82%), (ii) 20/35 (57%) either markedly improved or cured, no significant differences Adverse events: (i) 1 participant (none withdrew) |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐blind, double‐dummy: active and placebo tablets were matched in appearance (shape, size and colour) |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 6.8% (5/73) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed. 5 participants were not evaluated as they did not return for follow‐up after the baseline visit (4 from (i) and 1 from (ii)). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Videman 1984.
| Methods | RCT; double‐blind. Randomisation procedure not described. Follow‐up time: 3 weeks |
|
| Participants |
Population: 70 participants, 29 women, 41 men. Mean age 37 years (age range 20 to 64) Setting: outpatient clinic, conducted in Finland Inclusion criteria: acute LBP, duration from 1 day to 30 days Exclusion criteria: pregnant or nursing women; haematological, renal, hepatic, respiratory, or circulatory disorders; history of peptic ulceration or gastro‐intestinal upset; sensitivity or dependence to narcotic analgesics or benzomorphan derivatives, weight < 45 kg or > 95 kg |
|
| Interventions | NSAID (i): diflunisal 250 mg, 1 capsule q.i.d., and 1 placebo tablet q.i.d., 3 weeks (N = 35) Reference treatment (ii): meptazinol 200 mg, 1 tablet q.i.d., and 1 placebo capsule q.i.d., 3 weeks (N = 35) | |
| Outcomes | Mean change in degree of pain on 100‐mm visual analogue scale at three weeks (i) 45 (ii) 40. Similar improvement regarding capacity for daily tasks (data in graphs). No significant differences Adverse effects: (i) 19 participants (1 withdrew), (ii) 23 participants (none withdrew) |
|
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐blind, double‐dummy |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 2.9% (2/70) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
von Heymann 2013.
| Methods | RCT, double‐blind, double‐dummy design Follow‐up time: 12 weeks. Outcome measurements: before treatment, after 3 days, between 7 and 9 days after treatment |
|
| Participants |
Population: 101 participants randomised; 69 during initial 3‐arm phase, 32 during 2‐arm phase. N = 1: no treatment registered; N = 100 received treatment Setting: 5 outpatient orthopaedic or general practices in 4 different cities in Germany, between February 2003 and September 2008 (interim analysis June 2006) Inclusion criteria: people aged 18 to 55 years, presenting with acute (for < 48 hr before randomisation) LBP, and written informed consent Exclusion criteria: known intolerance to NSAID or paracetamol, occurrence of LBP or spinal manipulation for any cause within the last 3 months, known or suspected abuse of alcohol or drugs, metabolic, or malignant, or any serious organic or neurological disease, atopic diathesis, any structural disturbances of the spine (osteoporosis, scoliosis, disc herniation, spondylolisthesis, hip dysplasia, and others). Women with childbearing potential had to undertake effective contraception. For real sham manipulation, people with dysfunction of the sacroiliac joint (SIJ) were excluded (by functional and pain‐provocation tests). |
|
| Interventions |
NSAID (i): Sham manipulation and Diclofenac 50 mg, t.i.d.; 7 to 9 days (N = 37) Reference treatment (ii): Spinal manipulation and diclofenac‐like placebo, t.i.d.; 7 to 9 days (N = 38) Reference treatment (iii): Sham manipulation and diclofenac‐like placebo t.i.d.; 7 to 9 days (N = 25); placebo‐arm was closed after an interim‐analysis with 69 subjects who completed the study |
|
| Outcomes | Mean change in disability (RMDQ, 0 to 24) from baseline to 7 to 9 days (SD): (i) 4.75 (4.93), (ii) 7.71 (4.88) Pain intensity 100‐mm VAS: data shown in graph. Comparison between both active treatments and placebo only, and comparison of diclofenac vs spinal manipulation Quality of life (SF‐12): data shown in graph Cumulative dose of and number of days of rescue medication taken: (i) 6.41 (10.67) and 1.92 (2.61); (ii) 2.22 (3.73) and 1.19 (1.77); no significant differences Off‐work time (days): (i) 1.80 (2.10), (ii) 1.24 (1.69); no significant differences Overall clinical impression by blinded investigator after 3 and 7 to 9 days (complete relief or improved): (i) 20/37 (54%) and 21/37 (57%); (ii) 30/35 (86%), 29/35 (83%) Adverse events: none were registered |
|
| Funding | Funding by the German Organization for Manual Medicin (Deutsche Gesellschaft fúr Manuelle Medizin, DGMM), and doctors seminar for manual spine‐ and extremities therapy (Aerzteseminar fúr Manuelle Wirbelsaeulen‐ und Extremitaetentherapie, MWE). The first author is a member of the board of DGMM. No other conflicts of interest regarding any medical measures tested. | |
| Notes | An interim analysis was performed when 69 participants completed the study. Due to statistically significant superiority of active compared with placebo treatment, the placebo arm was closed. The trial continued with 2 active treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | participants were randomised using a phone call to the involved and responsible Institute of Biometrics. |
| Allocation concealment (selection bias) | Low risk | Numbered folders and numbered boxes with the trial medication; both diclofenac and placebo prepared in an identical way. After a phone call to the biometric institute, the physician received the randomised number of the folder, allocating the subject to one of the trial arms. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐dummy design, participants were blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Sham manipulation could only be performed in single‐blind manner. Outcome assessment took place by another physician (blinded investigator). |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Outcome assessment by a physician other than the one performing the spinal/sham manipulation. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | High risk | > 20% dropouts in placebo group |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed: "93 subjects were evaluable and formed the collective intention‐to‐treat (22 placebo, 36 diclofenac, 35 spinal manipulation)" |
| Selective reporting (reporting bias) | Low risk | Protocol is available on request in German |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | Paracetamol 500 mg, maximum 6 tablets a day, was used as rescue medication. No other concomitant analgesic medication, acupuncture, or homeopathy was allowed. |
| Compliance acceptable | Unclear risk | Protocol violation in 8/101 participants; and a lot of participants in the placebo group withdrew. |
| Timing outcome assessments similar | Low risk | Timing of outcome assessments similar |
| Other bias | Low risk | None |
Waterworth 1985.
| Methods | RCT. Randomisation procedure not described Follow‐up time: 12 days (clinical assessment day 0; after 3 to 4 days and 10 to 12 days) |
|
| Participants |
Population: 112 participants, 38 women, 70 men. Aged 18 to 50 years Setting: participants with LBP presenting to their general practitioner, conducted in New Zealand Inclusion criteria: sudden onset of moderate to severe LBP, with or without radiation to the back of the thighs, aggravated by sitting or physical activity, relieved by rest, present for less than 1 month; pain at least bothersome with a considerable degree of functional incapacity Exclusion criteria: established spinal disorders; pregnant women; aspirin hypersensitivity; long‐term use of NSAIDs, steroids or anticoagulants; haematological, renal ,or hepatic disease; history of peptic ulcer |
|
| Interventions | NSAID (i): diflunisal 500 mg capsules: 1000 mg immediately, then 500 mg b.i.d., 10 days (N = 36) Reference treatment (ii): physiotherapy: local heat, ultrasound, and exercises (5 weekly sessions of 45 minutes; (N = 34)) Reference treatment (iii): spinal manipulation and/or McKenzie therapy (5 sessions of 45 minutes weekly; (N = 38)) | |
| Outcomes | Mean change in pain intensity on 4‐point scale after 4 and 12 days: (i) ‐0.9, ‐1.7 (ii) ‐0.9, ‐1.6 (iii) ‐1.1, ‐1.7. No significant differences in pain and mobility. Global improvement measured with a good or excellent overall response as rated by the participants: (i) 77%, (ii) 70%, (iii) 73% Adverse effects: (i) 2 participants; not measured in other groups |
|
| Funding | Funding by the hospital foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 3.6% (4/112) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | During the study period, daytime bedrest, muscle relaxant drugs, local anaesthetic or steroid infiltration into the back, acupuncture, etc. were not permitted. |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Wiesel 1980.
| Methods | RCT; randomisation procedure not described; prospective study Follow‐up time: 2 weeks |
|
| Participants |
Population: 45 participants admitted to a military hospital for bedrest, all men, aged 17 to 34 years (mean age 23 years) Setting: army hospital in the USA Inclusion criteria: non‐radiating LBP, no previous back pain, normal neurologic and straight leg raising results, normal lumbar roentgenograms Exclusion criteria: discovering entities, such as spina bifida, on the roentgenogram |
|
| Interventions | All participants were admitted to the army hospital for bedrest. NSAID (i): aspirin 625 mg capsules, 4 times per day, 2 weeks (N = 15) NSAID (ii): phenylbutazone 100 mg capsules, 4 times per day (first 5 days), no further information (N = 15) Reference treatment (iii): acetaminophen (dosage not given), twice daily, 2 weeks (N = 15) |
|
| Outcomes | Mean no. of days before return to full activity (i) 5.7 (ii) 6.5 (iii) 5.7. No significant differences. No data on side‐effects given. |
|
| Funding | Not mentioned | |
| Notes | Study population consisted of young, US army men (combat trainees), which is a different population than usual. Phenylbutazone was taken off the market due to severe adverse effects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Similarity at baseline characteristics | Unclear risk | Baseline characteristics not mentioned for study section on NSAIDs |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Ximenes 2007.
| Methods | RCT; double‐blind, double‐dummy; multicentre Follow‐up time: 7 days. Adverse events were monitored until 30 days after the final administration of the study drug. |
|
| Participants |
Population: 340 participants, 173 women, 167 men. Mean age (SD): (i) 41.6 (11.7), (ii) 40.1 (12.7) Setting: 31 centres in 9 Latin American countries (Brazil, Venezuela, Ecuador, Argentina, Chile, Mexico, Colombia, Costa Rica, and Peru), November 2002 to May 2003 Inclusion criteria: age 18 to 65 years, acute LBP, duration of ≤ 72 hours, no previous episodes of acute LBP in the previous 6 weeks, VAS pain score ≥ 50 mm (100‐mm scale) and moderate to severe pain (categorical scale) Exclusion criteria: back pain of neurologic etiology, back pain did not qualify as a Quebec Task Force class 1a or 2a; presence of inflammatory conditions (e.g. arthritis), conditions of chronic pain, malignancy or IBD; uncontrolled hypertensive, hepatic, or renal disorders; participants subject of active workers compensation or litigation cases; history of allergic reactions to NSAIDs or sulfonamides; pregnant or lactating women |
|
| Interventions | NSAIDs (i): valdecoxib 40 mg daily, with a second dose on day 1; 7 days (N = 170) NSAIDs (ii): diclofenac 75 mg b.i.d.; 7 days (N = 170) | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100‐mm VAS) at 7 days; (i, N = 167 vs ii, N = 166) 0.26 (‐3.76 to 4.28)
Mean difference (95% CI) Oswestry LBPDQ (0 to 24‐point scale) at 7 days; (i vs ii) 0.02% (‐3.21 to 3.16) Adverse events (no of participants, % (95% CI)): (i, N = 170) 48 participants, 28.2% (21.7 to 35.7%), (0 withdrew); (ii, N = 170) 44 participants, 25.9% (19.6% to 33.3%), (4 withdrew) |
|
| Funding | Funded by Pfizer Inc., manufacturer of Valdecoxib. Editorial support provided by C. Scott, MD at Parexel, (biopharmaceutical R&D company). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule generated by the study sponsor before the start of the study; stratified by categorical baseline pain intensity (moderate or severe), 1:1 |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Unclear risk | Double‐blind concept, it was written that it was double‐dummy, but the frequency of administration differs between both types of NSAIDs (one or two times a day). It is unclear how "all patients and study personnel were blinded to the identity of the study medication." |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Double‐blind concept, but unclear if it was double‐dummy. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Unclear risk | Double‐blind concept, but unclear if it was double‐dummy. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 7% (24/340 withdrew) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | MITT (modified intention‐to‐treat) analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not mentioned |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Not mentioned |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Yakhno 2006.
| Methods | RCT; double‐blind, double‐dummy; multicentre Follow‐up time: 7 days |
|
| Participants |
Population: 220 participants, sex not mentioned. Mean age (SD): (i) 39.3 (9.1); (ii) 40.3 (9.7) Setting: 6 centres in Russia Inclusion criteria: age 18 to 55 years, nonspecific acute LBP, duration ≤ 5 days, pain score ≥ 5 on 11‐point NRS, requiring medical treatment Exclusion criteria: pregnant or breastfeeding women; previous episode of LBP within the last 6 months; specific spinal pathology or symptoms related to other pathologies; intake of analgesics ≤ 3 hrs preceding inclusion; intake of antiepileptics, antidepressants, barbiturates, anxiolytics, or muscle relaxants ≤ 24 hrs preceding inclusion; scheduled daily intake of one of the above medications; concomitant treatment with anticoagulants or platelet‐aggregation inhibitors; contraindication or allergy to study drugs; alcohol/drug abuse/dependency; episodes of GI disorders, oedema, dizziness, headache; history of aspirin‐induced asthma |
|
| Interventions | NSAIDs (i): lornoxicam on day 1: 16 mg once a day and 8 mg once a day; day 2 to 7: 8 mg b.i.d.; 7 days (N = 110) + matching (diclofenac‐like) placebo NSAIDs (ii): diclofenac‐k on day 1: 100 mg once a day and 50 mg once a day; day 2 to 7: 50 mg b.i.d.; 7 days (N = 110) + matching (lornoxicam‐like) placebo | |
| Outcomes | Sum of pain intensity differences from baseline on day 1 to 6 (SE); (i, N = 109) 4.2 (0.17); (ii, N = 110) 3.8 (0.17); (i) significantly lower than (ii) P < 0.05 Adverse events (no of participants, %): (i) 27 participants (24.5%), 1 withdrew; (ii) 28 participants (25.5%), 1 withdrew |
|
| Funding | Study was supported by an unrestricted grant from Nycomed, manufacturer of lornoxicam. | |
| Notes | Inadequate dosage of diclofenac‐k (50 mg b.i.d. instead of t.i.d.) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | A computer‐generated randomisation schedule assigned treatments in equal ratio to sequential participants. Participants were assigned to the next consecutive participant number in a sequential ascending order. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Double‐blind, double‐dummy |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | Low dropout rate: 1.4% (3/220) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol registration not mentioned |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Unclear risk | Paracetamol (acetaminophen) was allowed as rescue medication. |
| Compliance acceptable | Unclear risk | Not mentioned |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
Zippel 2007.
| Methods | RCT, double‐blind (non‐inferiority study) Follow‐up time: 2 days |
|
| Participants |
Population: 370 participants, 202 women and 168 men; mean age ± SD (range): dexketoprofen group: 48.5 ± 13.31 (21 to 75), diclofenac group: 50.0 ± 13.26 (20 to 74) Setting: multicentre trial conducted in 21 centres in Belgium, Germany, and Poland Inclusion: male/female outpatients aged 18 to 75 years with acute low back pain of moderate to severe intensity (≥ 50 mm on a visual analogue scale (VAS)), and of no more than 1 week's duration Exclusion: low back pain secondary to systemic or degenerative diseases, vertebral fractures or compressions; intervertebral disc hernia; neoplastic, infectious or metabolic diseases; and neurological pain. General contraindications to the use of NSAIDs. Concomitant treatment with steroidal drugs, alternative therapies, and use of any analgesic within 6 hours prior to inclusion in the study |
|
| Interventions |
NSAID (i): dexketoprofen 50 mg IM (Menarini, Florence, Italy), twice daily; 2 days (N = 183) NSAID (ii): diclofenac 75 mg IM (Voltarol®, Novartis Pharmaceuticals Ltd, Horsham, West Sussex, UK), twice daily; 2 days (N = 187) |
|
| Outcomes | Outcomes in ITT population Mean (± SD) changes in sum of analogue pain intensity difference scores (SAPID0‐6) from baseline to 6 hours after the first dose 111.8 ± 116.54 (i) versus 112.7 ± 105.71 (ii). Adjusted mean 111.9 (i) and 112.7 (ii); Adjusted ratio of means 0.993. 95% lower CI (two‐sided) 0.79 Adjusted mean SAPID0‐last score was 296.0 mm/h in (i) and 283.8 mm/h in (ii), with no statistical differences between treatments (P = 0.567). The median change in RDQ scores was ‐6.0 for both treatment groups (P = 0.695), showing an improvement on the disability scale Rescue medication: taken by 39% of participants in (i) and 33% of participants in (ii), no statistical differences between treatments (P = 0.235) Adverse events: (i) 50/183 = 27% (4 participants withdrew), (ii) 58/187 = 31% (2 participants withdrew) |
|
| Funding | Financially supported by a grant from Menarini Ricerche SpA, Florence, Italy (manufacturer of dexketoprofen). Not mentioned if they had a role in study design, data collection, or analysis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | participants were randomly assigned according to a computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes ‐ participants | Low risk | Study drugs were administered by a person from outside the investigational team. It was not mentioned if they were similar in size, shape, and colour. |
| Blinding of participants and personnel (performance bias) All outcomes ‐ careproviders | Unclear risk | Study drugs were administered by a person from outside the investigational team. It was unclear if this was their own careprovider. |
| Blinding of outcome assessment (detection bias) All outcomes ‐ outcome assessors | Low risk | Study drugs were administered by a person from outside the investigational team in order to maintain the double‐blind nature of the study. |
| Incomplete outcome data (attrition bias) All outcomes ‐ dropouts | Low risk | (i) 10/173 participants withdrew, (ii) 10/177 participants withdrew |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis | Low risk | ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not mentioned |
| Similarity at baseline characteristics | Low risk | Baseline characteristics similar |
| Co‐interventions avoided or similar | Low risk | Paracetamol 500 mg, maximum 3g a day, was used as rescue medication. |
| Compliance acceptable | Unclear risk | Compliance was not mentioned, (unused) study medication was not collected. |
| Timing outcome assessments similar | Low risk | Timing similar |
| Other bias | Low risk | None |
b.i.d. = twice a day; CI = confidence interval; CRF = case report form; FU = follow‐up; GI = gastrointestinal; GP = general practitioner; IM = intramuscular; ITT = intention‐to‐treat; IV = intravenous; LBP = low back pain; MRI = magnetic resonance imaging; NRS = numeric rating scale; NSAID = non‐steroidal anti‐inflammatory drug; PI = pain intensity; PP = per protocol; QBPDS = Quebec Back Pain Disability Scale; RCT = randomised controlled trial; RMDQ = Roland‐Morris Disability Questionnaire; q.i.d.= four times a day; ROM = range of motion; SD = standard deviation; SLR = straight‐leg raise; SF‐12/36 = 12 or 36‐item Short Form Health Survey; t.i.d. = three times a day; VAS = visual analogue scale; wks = weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allegrini 2009 | NSAIDs used in both groups, comparison concerned only the mode of administration of the same NSAID (piroxicam patch versus cream) |
| Altan 2019 | NSAIDs were not evaluated separately: a combination of NSAIDs and myorelaxants was evaluated |
| Anaya 2014 | Not a RCT; clinical series |
| Arul Prakasam 2011 | Not a RCT; prospective study, no randomisation |
| Berry 1988 | NSAIDs used in both groups; only addition of muscle relaxant evaluated |
| Blazek 1986 | No acute LBP; sciatica |
| Borenstein 1990 | NSAIDs used in both groups; only addition of muscle relaxant evaluated |
| Bruggemann 1990 | NSAIDs used in both groups; only addition of vitamins B1/B6/B12 evaluated |
| Buchbinder 2010 | No RCT; commentary on Cochrane Review |
| Chrubasik 2003 | No acute LBP; chronic LBP in flare |
| Coats 2004 | No acute LBP; chronic LBP in flare |
| Cohen 2017 | The pharmacotherapy group could use different types of medication; no subgroup analysis of NSAID users |
| Costantino 2011 | NSAIDs used in both groups, comparison concerned only the way of administration (mesotherapeutic versus systemic administration) |
| Day 2013 | No RCT; case report and review |
| Dehghan 2014 | NSAIDs were used in all three groups, no comparison group without NSAIDs. Only addition of thermotherapy or cryotherapy evaluated |
| Dehghan 2015 | NSAIDs were used in both groups, no comparison group without NSAIDs. Only addition of gabapentin evaluated |
| Driessens 1994 | No acute LBP; chronic LBP |
| Eken 2014 | No relevant timing of outcome assessments, follow‐up time 30 minutes (minimum follow‐up time for this systematic review is 1 day) |
| Evans 1980 | No acute LBP; chronic LBP in flare |
| Friedman 2015 | NSAIDs were used in all three groups, no comparison group without NSAIDs. Only addition of either cyclobenzaprine or oxycodone/acetaminophen evaluated |
| Friedman 2016 | NSAIDs were used in both groups, no comparison group without NSAIDs. Only addition of diazepam versus placebo evaluated |
| Friedman 2018 | NSAIDs were used in all three groups, no comparison group without NSAIDs. Only addition of either orphenadrine or methocarbamol versus placebo evaluated. |
| Geller 2016 | NSAIDs were used in both groups, no comparison group without NSAIDs. Only addition of B vitamins evaluated. |
| Górska 2005 | NSAIDs used in both groups; no comparison group without NSAIDs. Only addition of muscle relaxant evaluated. |
| Ilic 2009 | NSAIDs used in both groups; comparison concerned two generic formulations of the same NSAID (nimesulide). |
| Ingpen 1969 | No acute LBP; chronic LBP |
| IRCT2013052213146N2 | NSAIDs were used in all groups, no comparison group without NSAIDs. |
| Kuhlwein 1990 | NSAIDs used in both groups; only addition of vitamins B1/B6/B12 evaluated. |
| Lee 2008 | No relevant timing of outcome assessments, follow‐up time 1 hour (minimum follow‐up time for this systematic review was 1 day). |
| Listrat 1990 | NSAIDs used in both groups, comparison concerned only the mode of administration of the same NSAID (tenoxicam tablets versus intramuscular injections). |
| Matsumo 1981 | No acute LBP; chronic LBP |
| Muckle 1986 | No acute LBP; acute lumbar disc syndrome with an affected nerve root |
| Ostojic 2017 | NSAIDs were used both groups, no comparison group without NSAIDs. Only addition of paracetamol evaluated. |
| Pena 1990 | Information derived from a review. The original trial of Bontoux was not found. The reported data were not sufficient to be part of the current review. |
| Schreijenberg 2017 | No study results available. Study was terminated early because of low recruitment rates. |
| Serinken 2016 | No relevant timing of outcome assessments, follow‐up time 30 minutes (minimum follow‐up time for this systematic review was 1 day). NSAIDs used in both groups, only additional topical NSAID gel vs placebo tested. |
| Shell 2012 | No acute LBP; chronic LBP lasting > 6 weeks, confirmed by corresponding author. |
| Shikhkerimov 2016 | Not a RCT |
| Siegmeth 1978 | No LBP; radiologically confirmed lumbar osteoarthritis. Presence and duration of LBP was not mentioned. |
| Stark 2014 | Oral NSAIDs were included for blinding purposes only (N = 5). |
| Vetter 1988 | NSAIDs were used in both groups, no comparison group without NSAIDs. |
| Voicu 2019 | A combination of medication was used; NSAIDs were not evaluated separately. |
| von Uberall 2013 | Not a RCT; secondary analysis on 4 non‐interventional studies on NSAIDs. |
| Yastrebov 2012 | Not a RCT; no randomisation, intense lumbar pain syndrome, LBP not specifically mentioned. |
NSAID = non‐steroidal anti‐inflammatory drug; LBP = low back pain; RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Aoki 1983.
| Methods | RCT; double‐blind. Randomisation procedure not described Follow‐up: 14 days. Outcome measurements after 1 and 2 weeks |
| Participants |
Population: 237 participants Inclusion criteria: adults presenting at clinical centres with principal complaint LBP Exclusion criteria: history of gastrointestinal, hepatic, or renal disease, those with complications or requiring surgery, history of drug allergy, anticoagulants, abnormal baseline laboratory values, pregnancy, nursing mothers and women with childbearing potential. |
| Interventions |
NSAID (i): piroxicam 20 mg capsules, once per day, 14 days (N = 116) NSAID (ii): indomethacin 25 mg capsules, three times per day, 14 days (N = 114) |
| Outcomes | Global improvement, adverse events |
| Notes | 1. Duration of LBP is not clear (unspecified) 2. We were unable to find contact details to ask for clarification (21 October 2019). |
Basmajian 1989.
| Methods | RCT; double‐blind, multicentre. Randomisation procedure not described Follow‐up: 7 days. Outcome measurements after 2, 4, and 7 days |
| Participants |
Population and setting: 175 participants from 18 clinics Inclusion criteria: acute incidence of trauma or musculoskeletal strain, rigid criteria of clinical pain and spasm for the previous 7 days were required |
| Interventions |
NSAID (i): diflunisal capsules 500 mg twice per day (N = 44) NSAID plus muscle relaxant (ii): diflunisal capsules 500 mg + 5 mg cyclobenzaprine twice per day (N = 43) Muscle relaxant (iii): cyclobenzaprine (flexeril) capsules 5 mg twice per day (N = 43) Reference treatment (iv): placebo capsules twice per day (N = 45) |
| Outcomes | Marked improvement, adverse events |
| Notes | 1. Both LBP and neck pain included, no distinction was made; no subgroup analysis 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Borghi 2018.
| Methods | RCT; double‐blind, placebo‐controlled, cross‐over study; computer‐generated randomisation Follow‐up: 90 days. Outcome measurements at baseline, 5 minutes, 1, 6, 12, and 24 hours after each injection; and after 5, 15, 30, and 90 days |
| Participants |
Population: 80 participants, May 2012 to April 2014 Inclusion criteria: people suffering from LBP of < 6 months duration, older than 18 years, written informed consent; non‐surgical lumbago including disc syndrome, spinal stenosis, postural back pain Exclusion criteria: paediatric patients, people with allergies to meloxicam (or other non‐steroidal drugs), people with contraindications to the local puncture (such as infection of the skin at the puncture level), and people unable to express an informed consent to the treatment or available for the follow‐up |
| Interventions |
NSAID (i): periradicular meloxicam injections (N = 40)
Reference treatment (ii): periradicular saline injections (N = 20) The amount of injections differed per participant depending on NRS score change (1 to 3 injections). |
| Outcomes | Pain reduction, need for analgesic medication, level of physical activity, quality of sleep |
| Notes | 1. LBP < 6 months included; no subgroup analysis of only (sub)acute LBP 2. We contacted the corresponding author to ask for clarification (21 September 2019) |
Davoli 1989.
| Methods | RCT; randomisation procedure not described Follow‐up: 7 days. Outcome measurements at baseline and after 7 days |
| Participants |
Population: 30 participants, 26 women and 4 men, mean age 62 years, range 45 to 80 years Inclusion criteria: acute or recurrent low back pain, with or without radiation, degree of pain at least 2 on a 5‐point scale Exclusion criteria: pregnant or lactating women, gastroduodenal ulcer, depression, severe hepatic, renal or cardiovascular insufficiency (or both), severe alterations of blood chemistry, hypersensitivity or intolerance to piroxicam, aspirin, or other NSAIDS, use of NSAIDs or corticosteroids in previous 30 days |
| Interventions |
NSAID (i): etodolac 200 mg bid, 7 days (N = 15) NSAID (ii): piroxicam‐beta‐cyclodextrin one 20 mg tablet, 7 days (N = 15) |
| Outcomes | Pain, adverse events |
| Notes | 1. Both acute and recurrent LBP included, no distinction made 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Famaey 1998.
| Methods | Open label randomised trial, multicentre; randomisation procedure not described Follow‐up: 4 weeks |
| Participants |
Population and setting: 196 outpatients, 123 female, 73 male Inclusion criteria: people with subacute or chronic low back pain without a specific diagnosis, such as root entrapment syndromes, with at least severe pain at either rest, motion, standing or at night Exclusion criteria: acute LBP; active or previous history of gastric or duodenal ulcers, renal, or hepatic diseases, history of asprin or NSAID intolerance; treatment with corticosteroids. |
| Interventions | NSAIDs (i): nimesulide 100 mg, b.i.d., 4 weeks (N = 95) Reference treatment (ii): diclofenac sodium 50 mg b.i.d., 4 weeks (N = 101) |
| Outcomes | Sum of participants with none, mild, moderate / sum of participants with severe, very severe pain at: rest, motion, night, standing. Adverse events. |
| Notes | 1. Only subacute and chronic LBP included; no distinction made; no subgroup analysis of subacute LBP 2. We retrieved an e‐mail address and contacted the author (21 October 2019) to ask for clarification, but we received a message that the address no longer existed. We were unable to find other currently active contact details. |
Hingorani 1970.
| Methods | CCT; double‐blind; not randomised Follow‐up: 7 days. Outcome measurements at baseline, and after 3 and 7 days |
| Participants | Population and setting: 83 participants with acute low back pain warranting inpatient treatment, 59 men, 24 women Inclusion criteria: pain in the lower back of acute or chronic onset Exclusion criteria: neoplasms, myeloma, Paget's disease, collagen disease, rheumatoid arthritis, ankylosing spondylitis, and people with contraindications to either drug |
| Interventions | NSAID (i): indomethacin 25 mg, q.i.d., 7 days (N = 40) NSAID (ii): oxyphenbutazone 100 mg, q.i.d., 7 days (N = 43) |
| Outcomes | Total pain score (4‐point scale), total physical examination score (fingertip‐floor distance, inch), total functional status score (movements; 4‐point scale), paracetamol use, adverse events |
| Notes | 1. LBP of both acute and chronic onset included, no distinction made; no subgroup analysis 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Hingorani 1975.
| Methods | RCT; double‐blind, cross‐over; randomisation procedure not described |
| Participants |
Population: 50 participants, 32 men, 18 women, mean age 48.4 years Inclusion criteria: age 20 to 70 years, acute backache necessitating a stay in hospital of at least 3 weeks Exclusion criteria: concomitant treatment with steroids, gold, or mono‐amine oxidase inhibitors, systemic cause of backache, history of peptic ulcer, possibility of pregnancy |
| Interventions | NSAIDs (i): azapropazone 300 mg q.i.d., 1 week (N = ?) NSAIDs (ii): ketoprofen 50 mg q.i.d., 1 week (N = ?) |
| Outcomes | No data presented except for adverse events |
| Notes | 1. Both acute and recurrent LBP included, no distinction made. 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Jacobs 1968.
| Methods | RCT; double‐blind; participants were allotted the next serial number in their diagnostic category and given the appropriate numbered bottle. |
| Participants | Population and setting: 110 participants with clinically diagnosed prolapsed intervertebral disc with or without radicular pain attending an outpatient clinic, maximum 60 years of age Inclusion criteria: acute or chronic LBP, age < 60 years Exclusion criteria: neoplastic, metabolic, or other bone disease, pregnancy, diabetes, epilepsy, peptic ulcer |
| Interventions |
NSAID (i): indomethacin 25 mg capsules, 3 capsules per day for 2 days, 4 capsules per day next 5 days (N = 25 with nerve root pain, and N = 30 without nerve root pain) Reference treatment (ii): placebo capsules (N = 25 with nerve root pain, and N = 30 without nerve root pain) |
| Outcomes | Data on effectiveness in graphs; adverse events |
| Notes | 1. Both acute and acute flare of chronic LBP included, no distinction made; no subgroup analysis 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Milgrom 1993.
| Methods | RCT; randomisation according to their military identification numbers Follow‐up time: 10 weeks |
| Participants |
Population and setting: 70 male infantry recruits with over exertion back pain, 32 with thoracic and 40 with lumbar pain, mean age 18 years Inclusion criteria: no history of back pain, no back trauma, back pain exertionally related to carrying loads on the back, not present or markedly improved on non‐exertional activities, no sciatica, no sudden onset |
| Interventions | NSAID (i): ibuprofen 800 mg t.i.d., 7 days (N = 24) Reference treatment (ii): paracetamol 1000 mg t.i.d., 7 days (N = 24) Reference treatment (iii): no drug treatment (N = 22) |
| Outcomes | % participants cured after 10 weeks, adverse events |
| Notes | 1. Not LBP, but both lumbar and thoracic pain, no distinction made 2. We contacted the corresponding author to ask for clarification (21 September 2019) |
NCT01374269.
| Methods | RCT Follow‐up time: 6 months. Outcome measurements at baseline, 4, 12, and 24 weeks |
| Participants |
Population and setting: participants who were assigned to the social security system and living in the metropolitan area, consulting the ambulatory health centres in Medellin, Colombia, 2009 to 2012 Inclusion criteria: participants (aged 18 to 60 years) with subacute LBP (lasting 4 to 12 weeks) with or without radiculopathy Exclusion criteria: a specific cause for the pain (infection, tumour, ankylosing spondylitis, inflammatory conditions, or cauda equine syndrome), the presence of red flags, scoliosis > 15 °, depression or mental illness, history of gastrointestinal bleeding, renal failure, intake of anticoagulants or antiplatelet drugs, NSAID allergy |
| Interventions |
NSAID (I): naproxen capsules, 500 mg per day, 10 days OR celecoxib 200 mg per day, 10 days (N = 45) Reference treatment (ii): protocolised back pain exercise by a physiotherapist, 3 times a week, 4 weeks, 12 sessions in total (N = 45) |
| Outcomes | VAS, ODI, RMDQ, SF‐36 (quality of life), missed workdays, relapses of lumbar pain, additional treatments, medical consultations |
| Notes | 1. Either naproxen or celecoxib was used, no distinction was made, no subgroup analysis presented 2. We contacted the corresponding author to ask for clarification (21 September 2019) |
Predel 2019.
| Methods | RCT; randomised, double‐blind, multicentre, multicountry, parallel‐group design (3 groups). Randomisation using an interactive‐response technology, with a randomisation list in blocks (5 per block; 2:2:1 ratio). Follow‐up: 8 to 10 days. Outcome assessments at day 2, 4, 6, and 8 to 10 days |
| Participants |
Population and setting: 635 participants with acute back or neck pain presenting to 19 sites in Germany or Russia Inclusion criteria: aged 18 or above, acute back or neck pain (duration > 24 hours but < 21 days) resulting in pain on movement (POM) score of 5 or higher (0 to 10 NRS) for at least one of five standardized POM procedures Exclusion criteria: history of three or more episodes of back or neck pain in the last 6 months, or if they had chronic back or neck pain (defined as pain for three weeks or longer), if they had pain due to an identifiable cause, back or neck surgery, or rehabilitation in the last 12 months, use of prohibited medication (including any anti‐inflammatory drugs, hepinaroids, or muscle relaxants) within 3 days prior to study entry |
| Interventions |
NSAID (i): ibuprofen (400 mg) and caffeine (100 mg; oral), 3 times daily for 5 days NSAID (ii): ibuprofen (400 mg; oral), 3 times daily for 5 days Reference treatment (iii): placebo (oral), 3 times daily for 5 days |
| Outcomes | Pain on movement (NRS) between baseline and the morning of day 2. Safety and tolerability measures. Global assessment of efficacy. Pain at rest (NRS). Disability (ODI) |
| Notes | 1. Mixed population of participants with neck and back pain. The group of back pain participants for the relevant comparison of NSAID (NSAID (ii); N = 140) versus placebo (reference treatment (iii); N = 67) was N = 207. The results of these two groups were not compared separately for the subgroup of back pain (only NSAID (i) vs NSAID (ii), and NSAID (i) vs reference treatment (iii) 2. We contacted the corresponding author to ask for detailed results of the subgroup analysis of back pain participants in this group (22 January 2020) |
Sweetman 1987.
| Methods | RCT; parallel group design (3 groups); randomisation procedure not described Follow‐up time: 7 days. Outcome measurements at baseline, 1, and 7 days |
| Participants |
Population and setting: 122 participants, 57 women, 65 men, aged 15 to 72 years Ambulant outpatients from 12 general practitioners Inclusion criteria: acute LBP, current episode longer than 24 hours and less than 28 days Exclusion criteria: signs of nerve root compression, arthritis, ankylosing spondylitis, spinal infections, malignancy, renal or hepatic disease, peptic ulcers, pregnant or lactating women, sensitivity to test medications |
| Interventions | NSAID (i): mefenamic acid 500 mg, one tablet t.i.d. + placebo chlormezazone and paracetamol, two capsules t.i.d. (N = 40) Reference treatment (ii): chlormezanone 100 mg and paracetamol 450 mg, two capsules t.i.d. + placebo mefenamic acid, one tablet t.i.d. (N = 42) Reference treatment (iii): ethoheptazine 75 mg, meprobamate 150 mg and aspirin 250 mg two capsules t.i.d. + placebo (either mefenamic acid placebo or chlormezazone + paracetamol placebo) t.i.d. (N = 40) |
| Outcomes | Pain, adverse events |
| Notes | 1. Mixed population of acute and recurrent LBP (about half of the participants had chronic LBP with an acute flare), no distinction made 2. We were unable to find contact details to ask for clarification (21 October 2019) |
TCTR20141027001.
| Methods | RCT, open‐label; pilot study (safety/efficacy study); follow‐up time: 7 days |
| Participants |
Population and setting: 29 participants with acute low back pain in Thailand Inclusion criteria: age 18 to 65 years, nonspecific acute low back pain (≥ 4/10 NRS) for under 72 hours Exclusion criteria: history of lumbar and spinal accidents in the previous year or lumbosacral surgery, history of osteoporosis, immunodeficiency, diabetes mellitus, hypertension, cardiovascular disease, thyroid/endocrine gland disease and asthma, active ulcer, epilepsy, seizures, liver disease, renal disease, hypertension, known allergies for ibuprofen or herbal/pollen grain, pregnancy or lactation, certain co‐medication, or recent treatment with test medication |
| Interventions |
NSAID (i): NSAIDs; oral Ibuprofen 400 mg, 3 times daily (7 days) Reference treatment (ii): Thai herbal medicine; oral Ayurved Siriraj Sahatsatara recipe (AVS023) 1350 mg, 3 times daily (7 days) |
| Outcomes | Pain intensity (NRS); disability (ODI); safety (adverse events and blood test) |
| Notes | 1. Study title. A single‐blind randomised controlled trial of AVS023 poly‐herbal formula for acute low back pain: a pilot study 2. We contacted the corresponding author to ask for clarification (21 September 2019) |
Waikakul 1995.
| Methods | RCT; participants were randomly allocated to 2 groups according to the last 2 digits of their hospital numbers Follow‐up time: 6 weeks. Outcome measurements at baseline and after 1 and 6 weeks |
| Participants | Population and setting: 72 hospital patients with non‐surgical low back pain, 20 men, 52 women Inclusion criteria: non‐surgical low back pain including disc syndrome, spondylosis, mild spondylolisthesis, spinal stenosis, and postural back pain, aged 15 years and older Exclusion criteria: presence of digestive, haematological, hepatic, and renal disorders, hypersensitivity to proprionic acid derivatives, long‐term administration of steroids, indication for surgery |
| Interventions | NSAID (i): Loxoprofen 60 mg t.i.d., 6 weeks (N = 37) NSAID (ii): naproxen 250 mg t.i.d., 6 weeks (N = 35) |
| Outcomes | Therapeutic results according to criteria of the Japanese orthopaedic Academic Society, adverse events |
| Notes | 1. Duration of LBP not clear 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Waikakul 1996.
| Methods | RCT; single‐blind; randomisation procedure not described Follow‐up time: 3 weeks. Outcome measurements at baseline, and after 1, 2, and 3 weeks |
| Participants |
Population and setting: 64 ambulant people, 16 men, 48 women
Inclusion criteria: non‐surgical lumbago including disc syndrome, spinal stenosis, postural back pain Exclusion criteria: need for surgery |
| Interventions | NSAID (i): indomethacin plaster twice a day, 4 weeks plus 1 mg oral vitamin B b.i.d. (N = 30) NSAID (ii): diclofenac emulgel, 2 cm 4 times a day, 4 weeks plus 1 mg oral vitamin B bid (N = 34) |
| Outcomes | 30‐point total rating scale, % of participants with good improvement, safety and usefulness, adverse events |
| Notes | 1. Duration of LBP not clear 2. We were unable to find contact details to ask for clarification (21 October 2019) |
Zolotovskaya 2015.
| Methods | Open, prospective, follow‐up by simple randomisation Follow‐up time: 3 months |
| Participants | Population: 80 participants, 49 women, 31 men |
| Interventions | Randomization in four groups of 20: NSAID (i): etoricoxib 90 mg/day (N = 20) NSAID (ii): nimesulide 100 mg/day (N = 20) NSAID (iii): diclofenac 100 mg/day (N = 20) NSAID (iv): meloxicam 15 mg/day (N = 20) |
| Outcomes | Pain, blood pressure, lab tests |
| Notes | 1. Mixed population of back pain, no distinction made in location or in duration 2. We contacted the corresponding author to ask for clarification (21 Septemer 2019) |
b.i.d. = twice a day; LBP = low back pain; ODI = Oswestry Disability Index; NSAID = non‐steroidal anti‐inflammatory drug; RCT = randomised controlled trial; q.i.d. = four times a day; RMDQ = Roland‐Morris Disability Questionnaire; SF‐12/36 = 12 or 36‐item Short Form Health Survey; t.i.d. = three times a day; VAS = visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
CTRI/2018/11/016371.
| Trial name or title | A comparative study of the efficacy and tolerability of fixed dose combination of etoricoxib and thiocolchicoside and thiocolchicoside alone in patients with painful muscle spasm |
| Methods | RCT, randomised, open‐label. Follow‐up time: 7 days |
| Participants |
Population and setting: 100 participants with painful muscle spasms (such as torticollis, lumbago, backache) attending the orthopaedic outpatient department of a hospital in Bangalore, India Inclusion criteria: age 18 to 65 years, with painful muscle spasms, attending the orthopaedic outpatient department Exclusion criteria: history of liver and kidney damage, cardiovascular disease, asthma or acid peptic diseases, severe concurrent systemic diseases or anticoagulant therapy, malignancy, osteoporosis or a history of spine surgery, pregnancy or lactation, allergies or intolerances to NSAIDs and skeletal muscle relaxants, treatment with study medication 1 week prior to study enrolment |
| Interventions |
NSAID (i): Etoricoxib (60 mg) + thiocolchicoside (4 mg; oral), b.i.d. for 7 days Reference treatment (ii): Thiocolchicoside (4 mg; oral), b.i.d. for 7 days |
| Outcomes | Pain (VAS). Patients' global assessment in response to treatment |
| Starting date | Not yet recruiting |
| Contact information | S. Priyanka; drpriyanka110@gmail.com |
| Notes |
NCT03861611.
| Trial name or title | A comparison of NSAIDs for acute, non‐radicular low back pain. A randomised trial |
| Methods | RCT, double‐blind, randomised, parallel assignment. Follow‐up time: 5 days |
| Participants |
Population and setting: 198 participants with acute, new onset low back pain presenting to the emergency department of a hospital in New York, USA Inclusion criteria: age 18 to 64 years, with functionally impairing (score > 5 on the 0 to 24 RMDQ) low back pain of musculoskeletal etiology, non‐radicular, non‐traumatic, pain duration > 2 weeks Exclusion criteria: flank pain (originating from tissues lateral to the paraspinal muscles), not available for follow‐up, pregnancy, having a chronic pain syndrome; allergies or intolerances of, or contra‐indications to investigational medications |
| Interventions |
NSAID (i): Ketorolac 10 mg (oral), 3 times daily (5 days as needed) NSAID (ii): Ibuprofen 600 mg (oral), 3 times daily (5 days as needed) NSAID (iii): Diclofenac 50 mg (oral), 3 times daily (5 days as needed) Participants in all three study arms received an additional 15‐minute educational intervention |
| Outcomes | Functional impairment (RMDQ); LBP worsening; LBP frequency; analgesic or NSAID usage |
| Starting date | July 2019; currently recruiting |
| Contact information | Eddie Irizarry, MD; eddiriza@montefiore.org |
| Notes |
NCT04111315.
| Trial name or title | Efficacy of metamizole versus ibuprofen and a short educational intervention versus standard care in acute and subacute low back pain: a randomised, factorial trial |
| Methods | RCT, randomised, factorial, double‐blind, controlled; follow‐up time: 14 days for pain, 42 days for disability |
| Participants |
Population and setting: 120 participants with a new low back pain episode presenting to GPs in the region of Bern, Switzerland Inclusion criteria: age 18 years or older, seeking care for a new onset of non‐specific or specific LBP (pain duration of less than 12 weeks LBP prior to the baseline visit), the GP plans to prescribe a non‐opioid pain medication for pain control Exclusion criteria: presence of red flags, active malignancy or history of haematologic disorder, known intolerance or contraindications against the study medications, immune deficiency or under immunosuppressant treatment, current opioid use, pregnancy |
| Interventions |
NSAID (i): Metamizole 0.5 mg (oral), 3 times daily 2 capsules for 4 days, followed by an as needed regimen (days 4 to 42) + educational intervention NSAID (ii): Metamizole 0.5 mg (oral), 3 times daily 2 capsules for 4 days, followed by an as needed regimen (days 4 to 42) + standard care NSAID (iii): Ibuprofen 500 mg (oral), 3 times daily 2 capsules for 4 days, followed by an as needed regimen (days 4 to 42) + educational intervention NSAID (iv): Ibuprofen 500 mg (oral), 3 times daily 2 capsules for 4 days, followed by an as needed regimen (days 4 to 42) + standard care |
| Outcomes | Pain (NRS). Disability (Core Outcome Measures Index) |
| Starting date | December 2019; currently recruiting |
| Contact information | Maria Wertli, MD PhD; Maria.Wertli@insel.ch |
| Notes |
TCTR20151118003.
| Trial name or title | Efficacy of Ayurved SIRIRAJ SAHATTHARA recipe, in patient with acute low back pain, an open‐label randomised controlled trial |
| Methods | RCT, open‐label, non‐inferiority; follow‐up time: 7 days |
| Participants |
Population and setting: 90 participants with acute low back pain in Thailand Inclusion criteria: age 18 to 65 years, nonspecific acute low back pain (≥ 4/10 NRS) for under 72 hours Exclusion criteria: history of lumbar and spinal accidents in the previous year or lumbosacral surgery, history of osteoporosis, immunodeficiency, diabetes mellitus, hypertension, cardiovascular disease, thyroid or endocrine gland disease and asthma, active ulcer, epilepsy, seizures, liver disease, renal disease, hypertension, known allergies for ibuprofen or herbal/pollen grain, pregnancy or lactation, certain co‐medication or recent treatment with test medication |
| Interventions |
NSAID (i): NSAIDs; oral Ibuprofen 400 mg, 3 times daily (7 days) Reference treatment (ii): Thai herbal medicine; oral Ayurved Siriraj Sahatsatara recipe (AVS023) 1350 mg, 3 times daily (7 days) |
| Outcomes | Pain intensity (NRS). Disability (ODI). Safety (adverse events and blood test) |
| Starting date | March 2016; currently recruiting |
| Contact information | Somruedee Chatsiricharoenkul, MD; somruedee.cha@mahidol.ac.th |
| Notes | 1. TCTR20141027001 seems to be related to an (already completed) pilot study: A single‐blind randomised controlled trial of AVS023 poly‐herbal formula for acute low back pain: a pilot study. This study was followed by the current prospective study (TCTR20151118003) that is currently recruiting, no results are presented yet. 2. We contacted the corresponding author to ask for the current status (21 September 2019) |
b.i.d. = twice a day; GP = general practitioner; LBP = low back pain; ODI = Oswestry Disability Index; NSAID = non‐steroidal anti‐inflammatory drug; NRS = numeric rating scale; RCT = randomised controlled trial; q.i.d. = four times a day; RMDQ = Roland‐Morris Disability Questionnaire; SF‐12/36 = 12 or 36‐item Short Form Health Survey; t.i.d. = three times a day; VAS = visual analogue scale
Differences between protocol and review
This review is an update of a previously published review on non‐steroidal anti‐inflammatory drugs (NSAIDs) for non‐specific low back pain (van Tulder 2000). A protocol was published in 1997 for the original review, but not for this review, which focused on NSAIDS for acute low back pain (LBP). There were some changes to the 1997 protocol that we specified in the methods and will describe here. In general, we followed the guidance of Furlan 2015 along with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the protocol, only randomised and double‐blind controlled trials were included, in the current review we included all types of RCTs. The protocol identified five comparisons, the first two of which remained (NSAIDs versus placebo and NSAIDs versus paracetamol). We considered NSAIDs versus placebo the main comparison. The protocol included two additional comparisons, NSAIDs versus narcotic analgesics, and NSAIDs versus muscle relaxants, which we combined into one broader comparison of NSAIDs versus other drug treatment. We added a comparison of selective COX‐2 inhibitor NSAIDs versus non‐selective NSAIDs, and a comparison of NSAIDs versus non‐drug treatment. We removed the comparison of NSAIDs versus NSAIDs plus muscle relaxants, since there was no contrast for NSAIDs in this comparison.
We added adverse events as a fifth primary outcome measure. We did not include the secondary outcome measures that were mentioned in the protocol (physiological outcomes, generic functional status, and medical consumption), and we specified the timing of follow‐up (minimal follow‐up duration of one day, and at least one outcome assessment in the first three weeks), since this review concerned people with acute LBP.
The protocol did not state any age limits. The current methods included studies with subjects aged 18 years or older, because we aimed to identify all studies focusing on adults. However, in practice, we still included a study if age limits were not mentioned, if there were no age limits, or if subjects aged 16 or above (instead of 18) were included. Usually, only a small minority of the study population were younger than our 18‐year limit in these cases.
Electronic databases for the searches were added or removed as described under Electronic searches.
Contributions of authors
BW Koes, PDDM Roelofs, WH van der Gaag, and WTM Enthoven screened titles and abstracts. WH van der Gaag, PDDM Roelofs and WTM Enthoven performed methodological quality assessment. A third review author was consulted in case of disagreement between two review authors. Data extraction and analysis were performed by WH van der Gaag and PDDM Roelofs. WH van der Gaag wrote the initial draft of the manuscript, and all authors critically reviewed the manuscript.
Sources of support
Internal sources
-
Department of General Practice, Erasmus Medical Center, Rotterdam, Netherlands.
In‐kind support
-
Research Centre Innovations in Care, Rotterdam University of Applied Sciences, Rotterdam, Netherlands.
In‐kind support
-
Department of Health Sciences, Community and Occupational Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
In‐kind support
External sources
No sources of support supplied
Declarations of interest
Wendelien H van der Gaag has no known conflicts of interest.
Pepijn DDM Roelofs has no known conflicts of interest.
Wendy TM Enthoven has no known conflicts of interest.
Maurits W van Tulder was one of the Co‐ordinating Editors of the Cochrane Back and Neck Group and is currently on the Editorial Board. Editorial Board members are required to author reviews to remain current in methods. During the last three years, he has received research funding from the Netherlands Organisation for Health Research and Development, and the Dutch Health Insurance Council to his institution; and his institution received travel, accommodation, and meeting expenses from the European Pain Federation EFIC, and the Danish Occupational Therapy Association.
Bart W Koes has no known conflicts of interest.
New
References
References to studies included in this review
Aghababian 1986 {published data only}
- Aghababian RV, Volturo GA, Heifetz IN. Comparison of diflunisal and naproxen in the management of acute low back pain. Clinical Therapeutics 1986;9(Suppl C):47‐51. [PubMed] [Google Scholar]
Agrifoglio 1994 {published data only}
- Agrifoglio E, Benvenutti M, Gatto P, Albanese L, Chrubino P, Marinoni EC, et al. Aceclofenac: a new NSAID in the treatment of acute lumbago. Multicentre single blind study vs. diclofenac. Acta Therapeutica 1994;20:33‐43. [Google Scholar]
Amlie 1987 {published data only}
- Amlie E, Weber H, Holme I. Treatment of acute low back pain with piroxicam: results of a double‐blind placebo‐controlled trial. Spine 1987;12:473‐6. [DOI] [PubMed] [Google Scholar]
Babej‐Dolle 1994 {published data only}
- Babej‐Dolle R, Freytag S, Eckmeyer J, Zerle G, Schinzel S, Schmeider G, et al. Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: randomized observer‐blind multicenter study. International Journal of Clinical Pharmacology and Therapeutics 1994;32:204‐9. [PubMed] [Google Scholar]
Bakshi 1994 {published data only}
- Bakshi R, Thumb N, Bröll H, Klein G, Mayrhofer F, Rainer F, et al. Treatment of acute lumbosacral back pain with diclofenac resinate: results of a double‐blind comparative trial versus piroxicam. Drug Investigation 1994;8:288‐93. [Google Scholar]
Brown 1986 {published data only}
- Brown FL, Bodison S, Dixon J, Davis W, Nowoslawski J. Comparison of diflunisal and acetaminophen with codeine in the treatment of initial or recurrent acute low back pain. Clinical Therapeutics 1986;9(Suppl C):52‐8. [PubMed] [Google Scholar]
Colberg 1996 {published data only}
- Colberg K, Hettich M, Sigmund R, Degner FL. The efficacy and tolerability of an 8‐day administration of intravenous and oral meloxicam: a comparison with intramuscular and oral diclofenac in patients with acute lumbago. German Meloxicam Ampoule Study Group. Current Medical Research and Opinion 1996;13:363‐77. [DOI] [PubMed] [Google Scholar]
Dreiser 2003 {published data only}
- Dreiser RL, Marty M, Ionescu E, Gold M, Liu JH. Relief of acute low back pain with diclofenac‐K 12.5 mg tablets: a flexible dose, ibuprofen 200 mg and placebo‐controlled clinical trial. International Journal of Clinical Pharmacy and Therapeutics 2003;41(9):375‐85. [DOI] [PubMed] [Google Scholar]
Hancock 2007 {published and unpublished data}
- Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, Day RO, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first‐line treatment for acute low back pain: a randomised controlled trial. Lancet 2007;370(9599):1638‐43. [DOI] [PubMed] [Google Scholar]
- Hancock MJ, Maher CG, Latimer J, et al. Manipulative therapy and/or NSAIDs for acute low back pain: design of a randomized controlled trial [ACTRN012605000036617]. BMC Musculoskel Dis 2005; 6: 57. 2005;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosie 1993 {published data only}
- Hosie GAC. The topical NSAID, felbinac, versus oral ibuprofen: a comparison of efficacy in the treatment of acute lower back injury. British Journal of Clinical Research 1993;4:5‐17. [Google Scholar]
Innes 1998 {published data only}
- Innes GD, Croskerry P, Worthington J, Beveridge R, Jones D. Ketorolac versus acetaminophen‐codeine in the emergency department treatment of acute low back pain. Journal of Emergency Medicine 1998;16(4):549‐56. [DOI] [PubMed] [Google Scholar]
Jaffe 1974 {published data only}
- Jaffe G. A double‐blind, between‐patient comparison of alclofenac ('Prinalgin' ) and indomethacin in the treatment of low back pain and sciatica. Current Medical Research and Opinion 1974;2:424‐9. [DOI] [PubMed] [Google Scholar]
Lacey 1984 {published data only}
- Lacey PH, Dodd GD, Shannon DJ. A double‐blind placebo controlled study of piroxicam in the management of acute musculoskeletal disorders. European Journal of Rheumatology and Inflammation 1984;7:95‐104. [PubMed] [Google Scholar]
Metscher 2001 {published data only}
- Metscher B. Kubler U. Jahnel‐Kracht H. Dexketoprofen‐trometamol and tramadol in acute lumbago [Dexketoprofen‐Trometamol und Tramadol bei akuter Lumbago]. Fortschritte der Medizin. Originalien 2001;118(4):147‐51. [PubMed] [Google Scholar]
Miki 2018 {published and unpublished data}
- Miki K, Ikemoto T, Hayashi K, Arai Y, Sekiguchi M, Shi K, et al. Randomized open‐label non‐inferiority trial of acetaminophen or loxoprofen for patients with acute low back pain. Journal of Orthopaedic Science 2018 Oct 3;23(3):483‐7. [PUBMED: PMID: 30292383] [DOI] [PubMed] [Google Scholar]
Nadler 2002 {published data only}
- Nadler SF, Steiner DJ, Erasala GN, Hengehold DA, Hinkle RT, Beth Goodale M, et al. Continuous low‐level heat wrap therapy provides more efficacy than Ibuprofen and acetaminophen for acute low back pain. Spine 2002;27(10):1012‐7. [DOI] [PubMed] [Google Scholar]
Orava 1986 {published data only}
- Orava S. Medical treatment of acute low back pain. Diflunisal compared with indomethacin in acute lumbago. International Journal of Clinical Research 1986;6:45‐51. [PubMed] [Google Scholar]
Plapler 2016 {published data only}
- Plapler PG, Scheinberg MA, Ecclissato CC, Bocchi de Oliveira MF, Amazonas RB. Double‐blind, randomized, double‐dummy clinical trial comparing the efficacy of ketorolac trometamol and naproxen for acute low back pain. Drug Design, Development and Therapy 2016;10:1987‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pohjolainen 2000 {published data only}
- Pohjolainen T, Jekunen A, Autio L, Vuorela H. Treatment of acute low back pain with the COX‐2‐selective anti‐inflammatory drug nimesulide: results of a randomized, double‐blind comparative trial versus ibuprofen. Spine 2000;25(12):1579‐85. [DOI] [PubMed] [Google Scholar]
Postacchini 1988 {published data only}
- Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low back pain: a comparative study. Neuro‐Orthopedics 1988;6:28‐35. [Google Scholar]
Schattenkirchner 2003 {published data only}
- Schattenkirchner M, Milachowski KA. A double‐blind, multicentre, randomised clinical trial comparing the efficacy and tolerability of aceclofenac with diclofenac resinate in patients with acute low back pain. Clinical Rheumatology 2003;22(2):127‐35. [DOI] [PubMed] [Google Scholar]
Shin 2013 {published data only}
- Shin JS, Ha IH, Lee J, Choi Y, Kim MR, Park BY, et al. Effects of motion style acupuncture treatment in acute low back pain patients with severe disability: a multicenter, randomized, controlled, comparative effectiveness trial. Pain 2013;154(7):1030‐7. [DOI] [PubMed] [Google Scholar]
- Shin JS, Ha IH, Lee TG, Choi Y, Park BY, Kim MR, Lee MS. Motion style acupuncture treatment (MSAT) for acute low back pain with severe disability: a multicenter, randomized, controlled trial protocol. BMC Complement Altern Med 2011;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratz 1990 {published data only}
- Stratz T. Intramuscular etofenamate in the treatment of acute lumbago. Effectiveness and tolerance in comparison with intramuscular diclofenac‐Na. Fortschritte der Medizin 1990;108:264‐6. [PubMed] [Google Scholar]
Szpalski 1990 {published data only}
- Szpalski M, Poty S, Hayez JP, Debaize JP. Objective assessment of trunk function in patients with acute low back pain treated with Tenoxicam. A prospective controlled study. Neuro‐orthopedics 1990;10:41‐7. [Google Scholar]
Szpalski 1994 {published data only}
- Szpalski M, Hayez JP. Objective functional assessment of the efficacy of tenoxicam in the treatment of acute low back pain: a double blind placebo‐controlled study. British Journal of Rheumatology 1994;33:74‐8. [DOI] [PubMed] [Google Scholar]
Videman 1984 {published data only}
- Videman T, Heikkila J, Partanen T. Double‐blind parallel study of meptazinol versus diflunisal in the treatment of lumbago. Current Medical Research and Opinion 1984;9:246‐52. [DOI] [PubMed] [Google Scholar]
von Heymann 2013 {published data only}
- Heymann WJ, Schloemer P, Timm J, Muehlbauer B. Spinal high‐velocity low amplitude manipulation in acute nonspecific low back pain: a double‐blinded randomized controlled trial in comparison with diclofenac and placebo. Spine 2013;38(7):540‐8. [DOI: 10.1097/BRS.0b013e318275d09c] [DOI] [PubMed] [Google Scholar]
Waterworth 1985 {published data only}
- Waterworth RF, Hunter IA. An open study of diflunisal, conservative and manipulative therapy in the management of acute mechanical low back pain. New Zealand Medical Journal 1985;May:372‐5. [PubMed] [Google Scholar]
Wiesel 1980 {published data only}
- Wiesel SW, Cuckler JM, Deluca F, Jones F, Zeide MS, Rothman RH. Acute low back pain: an objective analysis of conservative therapy. Spine 1980;5:324‐30. [DOI] [PubMed] [Google Scholar]
Ximenes 2007 {published data only}
- Ximenes A, Robles M, Sands G, Vinueza R. Valdecoxib is as efficacious as diclofenac in the treatment of acute low back pain. Clinical Journal of Pain 2007;23(3):244‐50. [DOI] [PubMed] [Google Scholar]
Yakhno 2006 {published data only}
- Yakhno N, Guekht A, Skoromets A, Spirin N, Strachunskaya E, Ternavsky A, et al. Analgesic efficacy and safety of lornoxicam quick‐release formulation compared with diclofenac potassium: Randomised, double‐blind trial in acute low back pain. Clinical Drug Investigation 2006;26(5):267‐77. [DOI] [PubMed] [Google Scholar]
Zippel 2007 {published data only}
- Zippel H, Wagenitz A. A multicentre, randomised, double‐blind study comparing the efficacy and tolerability of intramuscular dexketoprofen versus diclofenac in the symptomatic treatment of acute low back pain.. Clinical Drug Investigation 2007;27(8):533‐43. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allegrini 2009 {published data only}
- Allegrini A, Nuzzo L, Pavone D, Tavella‐Scaringi A, Giangreco D, Bucci M, et al. Efficacy and safety of piroxicam patch versus piroxicam cream in patients with lumbar osteoarthritis: A randomized, placebo‐controlled study. Arzneimittel‐Forschung/Drug Research 2009;59(8):403‐9. [DOI] [PubMed] [Google Scholar]
Altan 2019 {published data only}
- Altan L, Kasapoglu Aksoy M, Kösegil Öztürk E. Efficacy of diclofenac & thiocolchioside gel phonophoresis comparison with ultrasound therapy on acute low back pain; a prospective, double‐blind, randomized clinical study. Ultrasonics 2019 Jan;91:201‐5. [DOI] [PubMed] [Google Scholar]
Anaya 2014 {published data only}
- Anaya A, Plantmason L, Dhaliwal G. Back attack. Journal of General Internal Medicine 2014;29(1):255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arul Prakasam 2011 {published data only}
- Arul Prakasam KC, Salman P, Senthilkumar N. Comparative assessment of analgesic effect of different NSAID's in the management of low back pain. International Journal of PharmTech Research 2011;3(3):1260‐4. [Google Scholar]
Berry 1988 {published data only}
- Berry H, Hutchinson DR. Tizanidine and ibuprofen in acute low‐back pain: results of a double‐blind multicentre study in general practice. Journal of International Medical Research 1988;16:83‐91. [DOI] [PubMed] [Google Scholar]
Blazek 1986 {published data only}
- Blazek M, Keszthelyi B, Varhelyi M, Korosi O. Comparative study of biarison and voltaren in acute lumbar pain and lumbo‐ischialgia. Therapia Hungarica (English Edition) 1986;34:163‐6. [PubMed] [Google Scholar]
Borenstein 1990 {published data only}
- Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clinical Therapeutics 1990;12:125‐31. [PubMed] [Google Scholar]
Bruggemann 1990 {published data only}
- Bruggemann G, Koehler CO, Koch EM. Results of a double‐blind study of diclofenac + vitamin B1, B6, B12 versus diclofenac in patients with acute pain of the lumbar vertebrae. A multicenter study. Klinische Wochenschrift 1990;68:116‐20. [DOI] [PubMed] [Google Scholar]
Buchbinder 2010 {published data only}
- Buchbinder R. Topical NSAIDs provide effective relief of acute musculoskeletal pain compared to placebo, with no increase in risk of adverse effects. Evidence‐Based Medicine 2010;15(6):177‐8. [DOI] [PubMed] [Google Scholar]
Chrubasik 2003 {published data only}
- Chrubasik S, Model A, Black A, Pollak S. A randomized double‐blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology (Oxford) 2003;42(1):141‐8. [DOI] [PubMed] [Google Scholar]
Coats 2004 {published data only}
- Coats TL, Borenstein DG, Nangia NK, Brown MT. Effects of valdecoxib in the treatment of chronic low back pain: results of a randomized, placebo‐controlled trial. Clinical Therapeutics 2004;26(8):1249‐60. [DOI] [PubMed] [Google Scholar]
Cohen 2017 {published data only}
- Cohen MM, Smit V, Andrianopoulos N, Ben‐Meir M, Taylor DM, Parker SJ, et al. Acupuncture for analgesia in the emergency department: a multicentre, randomised, equivalence and non‐inferiority trial. Medical Journal of Australia 2017;206(11):494‐9. [DOI] [PubMed] [Google Scholar]
Costantino 2011 {published data only}
- Costantino C, Marangio E, Coruzzi G. Mesotherapy versus systemic therapy in the treatment of acute low back pain: a randomized trial. Evidence‐Based Complementary and Alternative Medicine: eCAM 2011;2011:317183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2013 {published data only}
- Day RO, Graham GG. Therapeutics: non‐steroidal anti‐inflammatory drugs (NSAIDs). BMJ (Online) 2013;346:f3195. [DOI: 10.1136/bmj.f3195] [DOI] [PubMed] [Google Scholar]
Dehghan 2014 {published data only}
- Dehghan M, Farahbod F. The efficacy of thermotherapy and cryotherapy on pain relief in patients with acute low back pain, a clinical trial study. Journal of Clinical and Diagnostic Research 2014;8(9):LC01‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dehghan 2015 {published data only}
- Dehghan M, Farahbod F. Evaluation of the therapeutic effect of oral gabapentin on the severity of acute low back pain [Persian]. Scientific Journal of Kurdistan University of Medical Sciences 2015;20(1):97‐104. [Google Scholar]
Driessens 1994 {published data only}
- Driessens M, Famaey JP, Orloff S, Chochrad I, Cleppe D, Brabanter G, et al. Efficacy and tolerability of sustained‐release ibuprofen in the treatment of patients with chronic back pain. Current Therapeutic Research, Clinical and Experimental 1994;55:1283‐92. [Google Scholar]
Eken 2014 {published data only}
- Eken C, Serinken M, Elicabuk H, Uyanik E, Erdal M. Intravenous paracetamol versus dexketoprofen versus morphine in acute mechanical low back pain in the emergency department: a randomised double‐blind controlled trial. Emergency Medicine Journal 2014;31(3):177‐81. [DOI] [PubMed] [Google Scholar]
Evans 1980 {published data only}
- Evans DP, Burke MS, Newcombe RG. Medicines of choice in low back pain. Current Medical Research and Opinion 1980;6:540‐7. [DOI] [PubMed] [Google Scholar]
Friedman 2015 {published data only}
- Friedman BW, Dym AA, Davitt M, Holden L, Solorzano C, Esses D, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA 2015;314(15):1572‐80. [DOI: 10.1001/jama.2015.13043] [DOI] [PubMed] [Google Scholar]
Friedman 2016 {published data only}
- Friedman BW, Irizarry E, Solorzano C, Khankel N, Zapata J, Zias E, et al. Diazepam is no better than placebo when added to naproxen for acute low back pain. Annals of Emergency Medicine 2017 Aug;70(2):169‐76. [DOI: 10.1016/j.annemergmed.2016.10.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2018 {published data only}
- Friedman BW, Cisewski D, Irizarry E, Davitt M, Solorzano C, Nassery A, et al. A randomized, double‐blind, placebo‐controlled trial of Naproxen with or without Orphenadrine or Methocarbamol for acute low back pain. Annals of Emergency Medicine 2018;71(3):348‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geller 2016 {published data only}
- Geller M, Mibielli MA, Nunes CP, Fonseca AS, Goldberg SW, Oliveira L. Comparison of the action of diclofenac alone versus diclofenac plus B vitamins on mobility in patients with low back pain. Journal of Drug Assessment 2016;5(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Górska 2005 {published data only}
- Górska J. Effects of back pain treatment with tizanidine [Polish]. Ortopedia, Traumatologia, Rehabilitacja 2005;7(3):306‐9. [PubMed] [Google Scholar]
Ilic 2009 {published data only}
- Ilic KV, Sefik‐Bukilica M, Jankovic S, Vujasinovic‐Stupar N. Efficacy and safety of two generic copies of nimesulide in patients with low back pain or knee osteoarthritis. Reumatismo 2009;61(1):27‐33. [DOI] [PubMed] [Google Scholar]
Ingpen 1969 {published data only}
- Ingpen ML. A controlled clinical trial of sustained‐action dextropropoxyphene hydrochloride. British Journal of Clinical Practice 1969;23:113‐5. [PubMed] [Google Scholar]
IRCT2013052213146N2 {published data only}
- IRCT2013052213146N2. Compare between non‐steroidal anti‐inflammatory drugs and different frequencies of pulse low level laser therapy in acute low back pain. en.irct.ir/trial/13065 (first registered 15 December 2013).
Kuhlwein 1990 {published data only}
- Kuhlwein A, Meyer HJ, Koehler CO. Reduced diclofenac administration by B vitamins: results of a randomized double‐blind study with reduced daily doses of diclofenac (75 mg diclofenac versus 75 mg diclofenac plus B vitamins) in acute lumbar vertebral syndromes. Klinische Wochenschrift 1990;68:107‐15. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee HKH, Ting SM, Lau FL. A randomised control trial comparing the efficacy of tramadol and paracetamol against ketorolac and paracetamol in the management of musculoskeletal pain in the emergency department. Hong Kong Journal of Emergency Medicine. January 2008;15(1):5‐11. [Google Scholar]
Listrat 1990 {published data only}
- Listrat V, Dougados M, Chevalier X, Kramer F, Amor B. Comparison of the analgesic effect of tenoxicam after oral or intramuscular administration. Drug Investigation 1990;2(Suppl 3):51‐2. [DOI: 10.1007/BF03258217] [DOI] [Google Scholar]
Matsumo 1981 {published data only}
- Matsumo S, Kaneda K, Nohara Y. Clinical evaluation of Ketoprofen (Orudis) in lumbago: a double‐blind comparison with diclofenac sodium. British Journal of Clinical Practice 1981;35(7‐8):266. [PUBMED: PMID 6459111] [PubMed] [Google Scholar]
Muckle 1986 {published data only}
- Muckle DS. Flurbiprofen for the treatment of soft tissue trauma. American Journal of Medicine 1986;80:76‐80. [DOI] [PubMed] [Google Scholar]
Ostojic 2017 {published data only}
- Ostojic P, Radunovic G, Lazovic M, Tomanovic‐Vujadinovic S. Ibuprofen plus paracetamol versus ibuprofen in acute low back pain: a randomized open label multicenter clinical study. Acta Reumatologica Portuguesa 2017;42(1):18‐25. [PubMed] [Google Scholar]
Pena 1990 {published data only}
- Pena M. Etodolac: analgesic effects in musculoskeletal and postoperative pain. Rheumatology International 1990;10(Suppl):9‐16. [DOI] [PubMed] [Google Scholar]
Schreijenberg 2017 {published data only}
- Schreijenberg M, Luijsterburg PA, Trier YD, Rizopoulos D, Koopmanschap MA, Voogt L, et al. Efficacy of paracetamol, diclofenac and advice for acute low back pain in general practice: design of a randomized controlled trial (PACE Plus). BMC Musculoskeletal Disorders 2017;18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serinken 2016 {published data only}
- Serinken M, Eken C, Tunay K, Golcuk Y. Ketoprofen gel improves low back pain in addition to IV dexketoprofen: a randomized placebo‐controlled trial. American Journal of Emergency Medicine 2016;34(8):1458‐61. [DOI] [PubMed] [Google Scholar]
Shell 2012 {published data only}
- Shell WE, Charuvastra EH, Dewood MA, May LA, Bullias DH, Silver DS. A double‐blind controlled trial of a single dose naproxen and an amino acid medical food theramine for the treatment of low back pain. American Journal of Therapeutics 2012;19(2):108‐14. [DOI] [PubMed] [Google Scholar]
Shikhkerimov 2016 {published data only}
- Shikherimov RK. The use of nimesulide in the treatment of acute low back pain [Russian] [Применение нимесулида в терапии острой боли в нижней частиспины]. Zhurnal Nevrologii i Psikhiatrii Imeni S S 2016;116(5):28‐32. [DOI] [PubMed] [Google Scholar]
Siegmeth 1978 {published data only}
- Siegmeth W, Sieberer W. A comparison of the short‐term effects of ibuprofen and diclofenac in spondylosis. Journal of International Medical Research 1978;6:369‐74. [DOI] [PubMed] [Google Scholar]
Stark 2014 {published data only}
- Stark J, Petrofsky J, Berk L, Bains G, Chen S, Doyle G. Continuous low‐level heat wrap therapy relieves low back pain and reduces muscle stiffness.. Physician & Sportsmedicine 2014;42(4):39‐48. [DOI] [PubMed] [Google Scholar]
Vetter 1988 {published data only}
- Vetter G, Bruggemann G, Lettko M, Schwieger G, Asbach H, Biermann W, et al. Shortening diclofenac therapy by B vitamins. Results of a randomized double‐blind study, diclofenac 50 mg versus diclofenac 50 mg plus B vitamins, in painful spinal diseases with degenerative changes. Zeitschrift fur Rheumatologie 1988;47:351‐62. [PubMed] [Google Scholar]
Voicu 2019 {published data only}
- Voicu VA, Mircioiu C, Plesa C, Jinga M, Balaban V, Sandulovici R, et al. Effect of a new synergistic combination of low doses of acetylsalicylic acid, caffeine, acetaminophen, and chlorpheniramine in acute low back pain. Frontiers in Pharmacology 2019;10:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Uberall 2013 {published data only}
- Uberall MA, Essner U, Muller‐Schwefe GH. 2‐week efficacy and tolerability of flupirtine MR and diclofenac in patients with acute low/back pain – results of a post‐hoc subgroup analysis of patient‐level data from four non‐interventional studies [German] [2‐Wochen‐Wirksamkeit und ‐Verträglichkeit von Flupirtin MR und Diclofenac bei akuten Kreuz‐/Rückenschmerzen]. MMW Fortschritte der Medizin 2013 Dec;155 Suppl 4:115‐23. [DOI] [PubMed] [Google Scholar]
Yastrebov 2012 {published data only}
- Yastrebov DN, Shpagin MV, Artifexo SB. Pathophysiological substantiation of epidural administration of Tenoxicam in dorsalgia treatment. Sovremennye Tehnologii v Medicine 2012;1:133‐6. [Google Scholar]
References to studies awaiting assessment
Aoki 1983 {published data only}
- Aoki T, Kuroki Y, Kageyama T, Irimajiri S, Mizushima Y, Yamamoto K. Multicentre double‐blind comparison of piroxicam and indomethacin in the treatment of lumbar diseases. European Journal of Rheumatology and Inflammation 1983;6:247‐52. [PubMed] [Google Scholar]
Basmajian 1989 {published data only}
- Basmajian JV. Acute back pain and spasm: a controlled multicenter trial of combined analgesic and antispasm agents. Spine 1989;14:438‐9. [PubMed] [Google Scholar]
Borghi 2018 {published data only}
- Borghi B, Aurini L, White PF, Tognù A, Rossi B, Fini G, et al. Treatment of recent onset low back pain with periradicular injections of meloxicam: a randomized, double blind, placebo controlled cross‐over study. Minerva Anestesiologica 2018 May;84(5):590‐8. [DOI] [PubMed] [Google Scholar]
Davoli 1989 {published data only}
- Davoli L, Ciotti G, Biondi M, Passeri M. Piroxicam‐beta‐cyclodextrin in the treatment of low‐back pain. Controlled study vs etodolac. Current Therapeutic Research, Clinical and Experimental 1989;46:940‐7. [Google Scholar]
Famaey 1998 {published data only}
- Famaey JP, Bruhwyler J, Geczy J, Vandekerckhove K, Appelboom T. Open controlled randomized multicenter comparison of nimesulide and diclofenac in the treatment of subacute and chronic low back pain. Journal of Clinical Research 1998;1:219‐38. [Google Scholar]
Hingorani 1970 {published data only}
- Hingorani K, Biswas AK. Double‐blind controlled trial comparing oxyphenbutazone and indomethacin in the treatment of acute low back pain. British Journal of Clinical Practice 1970;24:120‐3. [PubMed] [Google Scholar]
Hingorani 1975 {published data only}
- Hingorani K, Templeton JS. A comparative trial of azapropazone and ketoprofen in the treatment of acute backache. Current Medical Research and Opinion 1975;3:407‐12. [DOI] [PubMed] [Google Scholar]
Jacobs 1968 {published data only}
- Jacobs JH, Grayson MF. Trial of anti‐inflammatory agent (indomethacin) in low back pain with and without radicular involvement. British Medical Journal 1968;3:158‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milgrom 1993 {published data only}
- Milgrom C, Finestone A, Lev B, Wiener M, Floman Y. Overexertional lumbar and thoracic back pain among recruits: a prospective study of risk factors and treatment regimens. Journal of Speech Disorders 1993;6:187‐93. [DOI] [PubMed] [Google Scholar]
NCT01374269 {published data only}
- NCT01374269. Improvement in pain, function and HRQoL (Health Related Quality of Life) in subacute low back pain: a controlled clinical trial of exercise versus NSAIDs. clinicaltrials.gov/ct2/show/study/NCT01374269 (first posted 15 June 2011).
Predel 2019 {published data only}
- Predel HG, Ebel‐Bitoun C, Lange R, Weiser T. A randomized, placebo‐ and active‐controlled, multi‐country, multi‐center parallel group trial to evaluate the efficacy and safety of a fixed‐dose combination of 400 mg ibuprofen and 100 mg caffeine compared with ibuprofen 400 mg and placebo in patients with acute lower back or neck pain. Journal of Pain Research 2019;12:2771‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweetman 1987 {published data only}
- Sweetman BJ, Baig A, Parsons DL. Mefenamic acid, chlormezanone‐paracetamol, ethoheptazine‐aaspirin‐meprobamate: a comparative study in acute low back pain. British Journal of Clinical Practice 1987;41:619‐24. [PubMed] [Google Scholar]
TCTR20141027001 {published data only}
- TCTR20141027001. A single‐blind randomized controlled trial of AVS023 poly‐herbal formula for Acute low back pain: a pilot study. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20141027001 (first registered 27 October 2014).
Waikakul 1995 {published data only}
- Waikakul S, Soparat K. Effectiveness and safety of loxoprofen compared with naproxen in nonsurgical low back pain: a parallel study. Clinical Drug Investigation 1995;10:59‐63. [Google Scholar]
Waikakul 1996 {published data only}
- Waikakul S, Danputipong P, Soparat K. Topical analgesics, indomethacin plaster and diclofenac emulgel for low back pain: a parallel study. Journal of the Medical Association of Thailand 1996;79:486‐90. [PubMed] [Google Scholar]
Zolotovskaya 2015 {published data only}
- Zolotovskaya IA, Davydkin IL. Effect of nonsteroidal anti‐inflammatory drugs on the indicators of cardiovascular risk in patients with acute nonspecific back pain [Russian]. Terapevticheskii Arkhiv 2015;87(12):18‐25. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2018/11/016371 {published data only}
- CTRI/2018/11/016371. A comparative study of the efficacy and tolerability of fixed dose combination of etoricoxib and thiocolchicoside and thiocolchicoside alone in patients with painful muscle spasm. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/11/016371 (first registered 16 November 2018 ).
NCT03861611 {published data only}
- NCT03861611. A comparison of NSAIDs for acute, non‐radicular low back pain. A randomized trial. clinicaltrials.gov/ct2/show/NCT03861611 (first posted 4 March 2019).
NCT04111315 {published data only}
- NCT04111315. Efficacy of metamizole versus ibuprofen and a short educational intervention versus standard care in acute and subacute low back pain: a randomized, factorial trial. clinicaltrials.gov/ct2/show/NCT04111315 (first posted 1 October 2019).
TCTR20151118003 {published data only}
- TCTR20151118003. Efficacy of Ayurved SIRIRAJ SAHATTHARA recipe, in patient with acute low back pain, the open‐label randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20151118003 (first registered 17 November 2015).
Additional references
Bernstein 2017
- Bernstein IA, Malik Q, Carville S, Ward S. Low back pain and sciatica: summary of NICE guidance. BMJ 2017;356:i6748. [DOI: 10.1136/bmj.i6748] [DOI] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58:1233‐40. [DOI] [PubMed] [Google Scholar]
Brune 2015
- Brune K, Patrignani P. New insights into the use of currently available non‐steroidal anti‐inflammatory drugs. Journal of Pain Research 2015;8:105‐18. [DOI: 10.2147/JPR.S75160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiarotto 2018
- Chiarotto A, Boers M, Deyo RA, Buchbinder R, Corbin TP, Costa LOP, et al. Core outcome measurement instruments for clinical trials in non‐specific low back pain. Pain 2018;159(3):481–95. [DOI: 10.1097/j.pain.0000000000001117; PUBMED: 29194127] [DOI] [PMC free article] [PubMed] [Google Scholar]
CNT Collaboration 2013
- Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Coxib and traditional NSAID Trialists' (CNT) Collaboration. Vascular and upper gastrointestinal effects of non‐steroidal anti‐inflammatory drugs: meta‐analyses of individual participant data from randomised trials. Lancet 2013 Aug;382(9894):769‐79. [DOI: 10.1016/S0140-6736(13)60900-9; PUBMED: 23726390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2012
- Costa da LCM, Maher CG, Hancock, MJ, McAuley JH, Herbert RD, Costa LOP. The prognosis of acute and persistent low‐back pain: a meta‐analysis. Canadian Medical Association Journal 2012;184(11):E613‐2. [DOI: 10.1503/cmaj.111271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyo 2014
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH Task Force on research standards for chronic low back pain. Journal of Pain 2014 Jun;15(6):569‐85. [DOI: 10.1016/j.jpain.2014.03.005; PUBMED: 24787228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enthoven 2016
- Enthoven WTM, Roelofs PDDM, Deyo RA, Tulder MW, Koes BW. Non‐steroidal anti‐inflammatory drugs for chronic low back pain. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. Editorial Board of the Cochrane Back and Neck Group. 2015 Updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660‐73. [DOI: 10.1097/BRS.0000000000001061; PUBMED: 26208232] [DOI] [PubMed] [Google Scholar]
GBD 2016
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211‐59. [DOI: 10.1016/S0140-6736(17)32154-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Working Group 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ June 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 31 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Grosser 2011
- Grosser T, Smyth E, FitzGerald GA. Anti‐inflammatory, antipyretic, and analgesic agents; pharmacology of gout. In: Brunton LL, Chabner BA, Knollman BC editor(s). Goodman & Gilman's Pharmacological Basis of Therapeutics. 12th Edition. New York: McGraw‐Hill, 2011:962‐77. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoy 2010
- Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Practice & Research Clinical Rheumatology 2010;24:769‐81. [DOI: 10.1016/j.berh.2010.10.002] [DOI] [PubMed] [Google Scholar]
Itz 2013
- Itz CJ, Geurts JW, Kleef M, Nelemans P. Clinical course of non‐specific low back pain: a systematic review of prospective cohort studies set in primary care.. European Journal of Pain (London, England) 2013 Jan;17(1):5‐15. [PUBMED: 22641374] [DOI] [PubMed] [Google Scholar]
Koes 2006
- Koes BW, Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ 2006 June 17;332(7555):1430‐4. [DOI: 10.1136/bmj.332.7555.1430; PUBMED: 16777886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koes 2010
- Koes BW, Tulder MW, Lin CW, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. European Spine Journal 2010 Dec;19(12):2075‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lidgren 2003
- Lidgren L. The bone and joint decade 2000‐2010. Bulletin of the World Health Organization 2003;81(9):629. [PUBMED: PMC2572548] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2017
- Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non‐steroidal anti‐inflammatory drugs for spinal pain: a systematic review and meta‐analysis. Annals of the Rheumatic Diseases 2017;76:1269‐78. [DOI: 10.1136/annrheumdis-2016-210597] [DOI] [PubMed] [Google Scholar]
Manchikanti 2014
- Manchikanti L, Singh V, Falco FJE, Ramsin M, Benyamin MD, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation: Technology at the Neural Interface 2014;17(52):3‐10. [DOI: 10.1111/ner.12018] [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of Internal Medicine June 2007;146(12):878–81. [DOI: 10.7326/0003-4819-146-12-200706190-00009] [DOI] [PubMed] [Google Scholar]
Oliveira 2018
- Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non‐specific low back pain in primary care: an updated overview. European Spine Journal 2018 Nov;27(11):2791‐2803. [DOI: 10.1007/s00586-018-5673-2] [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008 Jan 1;33(1):90‐4. [DOI: 10.1097/BRS.0b013e31815e3a10; PUBMED: 18165753] [DOI] [PubMed] [Google Scholar]
Pengel 2003
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ 2003 Aug 9;327(7410):323. [DOI: 10.1136/bmj.327.7410.323; PUBMED: 12907487] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pepine 2017
- Pepine CJ, Gurbel PA. Cardiovascular safety of NSAIDs: additional insights after PRECISION and point of view. Clinical Cardiology 2017 Dec;40(12):1352‐6. [DOI: 10.1002/clc.22814; PUBMED: 29247518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2017
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2017;166:514‐30. [DOI: 10.7326/M16-2367] [DOI] [PubMed] [Google Scholar]
Rasmussen‐Barr 2016
- Rasmussen‐Barr E, Held U, Grooten WJA, Roelofs PDDM, Koes BW, Tulder MW, et al. Non‐steroidal anti‐inflammatory drugs for sciatica. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012382] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saragiotto 2016
- Saragiotto BT, Machado GC, Ferreira ML. Pinheiro MB, Abdel Shaheed C, Maher CG. Paracetamol for low back pain. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schreijenberg 2019
- Schreijenberg M, Koes BW, Lin CWC. Guideline recommendations on the pharmacological management of non‐specific low back pain in primary care – is there a need to change?. Expert Review of Clinical Pharmacology 2019;12(2):145‐57. [DOI: 10.1080/17512433.2019.1565992] [DOI] [PubMed] [Google Scholar]
Sostres 2013
- Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti‐inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Research & Therapy 2013 Jul 24 [Epub ahead of print];15(Suppl 3):S3. [DOI: 10.1186/ar4175; PUBMED: 24267289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steenstra 2005
- Steenstra IA, Verbeek JH, Heymans WM, Bongers PM. Prognostic factors for duration of sick leave in patients sick listed with acute low back pain: a systematic review of the literature. Occupational and Environmental Medicine 2005;62:851‐60. [DOI: 10.1136/oem.2004.015842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trelle 2011
- Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non‐steroidal anti‐inflammatory drugs: network meta‐analysis. BMJ 2011 Jan;342:c7086. [DOI: 10.1136/bmj.c7086; PUBMED: 21224324] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Walker 2018
- Walker C, Biasucci LM. Cardiovascular safety of non‐steroidal anti‐inflammatory drugs revisited. Postgraduate Medicine 2018;130(1):55‐71. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Roelofs 2008
- Roelofs PDDM, Deyo RA, Koes BW, Scholten RJPM, Tulder MW. Non‐steroidal anti‐inflammatory drugs for low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000396.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Tulder 2000
- Tulder MW, Scholten RJ, Koes BW, Deyo RA. Non‐steroidal anti‐inflammatory drugs for low back pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000396; CD000396; PUBMED: PMID: 10796356] [DOI] [PubMed] [Google Scholar]


