Summary
As with all viruses, the severe acute respiratory syndrome coronavirus (SARS‐CoV) utilizes specific host cell factors during its infection cycle. Some of these factors have been identified and are now increasingly scrutinized as targets to intervene with infection. In this brief review, we describe the current understanding of how the SARS‐CoV is able to use the cellular machinery for its replication.
Introduction
Viruses are infectious agents incapable of reproduction on their own. They replicate within cells by diverting the cellular machinery to their own advantage. Because these host–pathogen interactions are ultimately the basis of disease, knowledge about this interplay is of great interest, not the least for the development of rational strategies to combat infections. In general, much is known about the pathogens but little about the contributions of the host. In this short review we focus on the severe acute respiratory syndrome coronavirus (SARS‐CoV) describing what is currently known of the cell’s contributions during the successive phases of the infection cycle, i.e. entry, replication and assembly (Fig. 1). Where it is necessary and possible, reference is made to relevant information about these processes for other coronaviruses.
Figure 1.
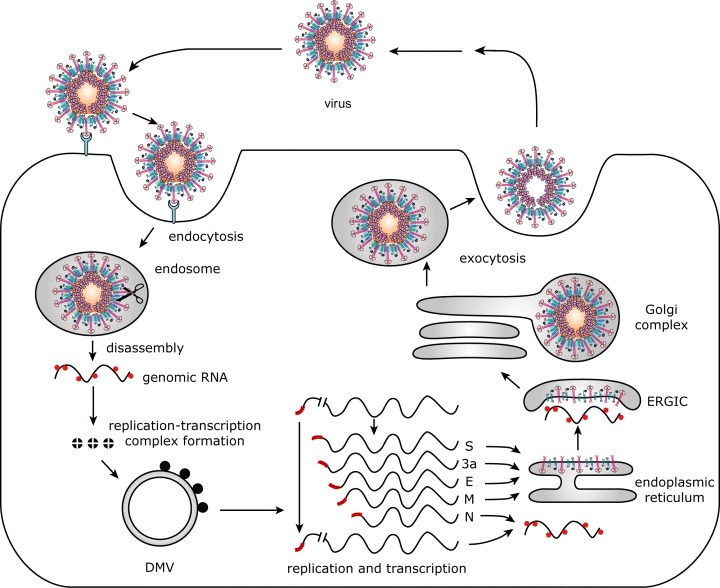
SARS‐CoV life cycle. The infection cycle of the SARS‐CoV starts with the binding of the virion to the receptor ACE2. After endocytosis, the S protein is cleaved by cathepsin L (represented by the scissors), after which the viral envelope fuses with the host cell membrane. Next, the virus disassembles, releasing its genomic RNA into the cytoplasm of the host cell. Translation of the replicase genes produces two large precursor proteins (pp1a and pp1ab) the many cleavage products of which (nsp1‐16) collectively constitute the functional replication–transcription complexes on double‐membraned vesicles (DMVs). Genes located downstream of the replicase genes are expressed from a 3′‐coterminal nested set of subgenomic mRNAs, each of which contains a short 5′ leader sequence derived from the 5′ end of the genome (shown in red). Many copies of N protein package the genomic RNA into a helical nucleocapsid. The envelope proteins (S, 3a, E and M) are inserted into the ER membrane, and accumulate in the ER‐to‐Golgi intermediate compartment (ERGIC) to meet the nucleocapsid and, subsequently, to assemble into particles by budding. Finally, the complete virions are transported out of the cell via the constitutive secretory pathway.
Coronaviruses constitute a family of enveloped, positive‐stranded RNA viruses that usually cause respiratory or intestinal infections in various species. Their relevance has increased considerably by the recent emergence of new human coronaviruses (HCoV) such as the SARS‐CoV (Drosten et al., 2003; Ksiazek et al., 2003; Peiris et al., 2003), HCoV‐NL63 (van der Hoek et al., 2004) and HCoV‐HKU1 (Woo et al., 2005), which all cause severe respiratory tract disease. Coronaviruses can be divided into three groups on the basis of serological and genetic criteria. The taxonomic position of the SARS‐CoV has not been formally assigned, but the virus was considered most closely related to the coronaviruses of group 2 (Snijder et al., 2003), which includes among others the mouse hepatitis virus (MHV). Coronavirus particles contain a helical nucleocapsid structure, consisting of one copy of the viral genomic RNA packaged by multiple copies of the nucleocapsid (N) protein. Their lipid envelope accommodates three to four membrane proteins, with the membrane (M), envelope (E) and spike (S) proteins being invariably present (reviewed by de Haan and Rottier, 2005). The SARS‐CoV contains a 29.7 kb genomic RNA (Marra et al., 2003; Rota et al., 2003) with the typical coronavirus genome organization, which is characterized by the occurrence of a distinctive set of genes positioned in a fixed order: 5′‐1a‐1b‐S‐E‐M‐N‐3′. The two partly overlapping open reading frames (ORFs) 1a and 1b comprise approximately two‐thirds of the genome and encode all viral components required for viral RNA synthesis. In addition, the SARS‐CoV contains a set of accessory genes of unclear function between the S and E and the M and N genes (Yount et al., 2005), which differ distinctly from the so‐called group‐specific genes found in other coronaviruses.
Entry
Coronavirus entry is driven by the S glycoprotein, which is a class I fusion protein (Bosch et al., 2003). Trimers of the S protein form the peplomers that radiate from the virion envelope, giving it its characteristic corona solis‐like appearance. While the S proteins of some coronaviruses – notably those of group 2 – are cleaved during their maturation by furin‐like enzymes (de Haan et al., 2004), this does not appear to be the case for the SARS‐CoV S protein (Xiao et al., 2003; Bisht et al., 2004; Song et al., 2004; Simmons et al., 2005). Nevertheless, an amino‐terminal S1 and a carboxy‐terminal S2 subunit can be distinguished, which are responsible for receptor binding and membrane fusion respectively. The receptor‐binding domain in the SARS‐CoV S protein has been mapped to residues 318–510 (Babcock et al., 2004; Wong et al., 2004). It appears that in different coronaviruses the receptor‐binding domain occurs in different regions of the S1 subunit (reviewed by de Haan and Rottier, 2005). The ectodomain of the S2 subunit contains two heptad repeat (HR) regions, a sequence motif characteristic of coiled coils, while the fusion peptide is predicted to be located immediately upstream of the first HR region (Bosch et al., 2004). Binding of the S1 subunit to the receptor is thought to trigger a series of conformational changes that ultimately results in the formation of an antiparallel heterotrimeric six‐helix bundle by the two HR regions – characteristic of class I fusion proteins – and, consequently, the close colocation of the putative fusion peptide and the transmembrane domain. These structural rearrangements in the S protein generate the energy that drives the fusion of the viral and cellular lipid membrane, a process in which cholesterol appears to be an essential cofactor (Thorp and Gallagher, 2004).
The first host component essential for SARS‐CoV infection is the angiotensin‐converting enzyme 2 (ACE2). It was identified as the main receptor soon after the identification of the virus as the cause of SARS (Li et al., 2003; Wang et al., 2004). ACE2 is a metalloprotease that is abundantly expressed in human heart, kidney, testis, gastrointestinal tract and lungs (Kuhn et al., 2004). Using a mouse model, ACE2 was also shown to be the crucial receptor in vivo (Kuba et al., 2005). While human ACE2 functions as an efficient receptor for the 2002–03 SARS‐CoV, this is much less so for the 2003–04 viruses and for the palm civet SARS‐CoV (Li et al., 2005a). These differences are explained by amino acid substitutions in the receptor‐binding domain of the SARS‐CoV S protein (2005a, 2005b; Qu et al., 2005). Conversely, rat ACE2, which does not support infection by SARS‐CoV, and mouse ACE2, which does so only very inefficiently (Li et al., 2004), contain amino acid differences relative to the human sequence that are also likely to interfere with ACE2–S interaction (Li et al., 2005b). Enzymatic activity of ACE2 was shown not to be required for the receptor to be functional (Moore et al., 2004), nor does binding of SARS‐CoV S protein to ACE2 alter its enzymatic activity (Kuhn et al., 2004). S protein binding does, however, result in downregulation of ACE2 cell surface expression (Kuba et al., 2005). Strikingly, ACE2 was shown to protect, in a mouse model, against lung damage caused by SARS‐CoV and other agents. Therefore, downregulation of ACE2 may contribute to the severity of lung pathology observed upon SARS‐CoV infection (Imai et al., 2005).
Unlike most other group 2 coronaviruses, SARS‐CoV does not have a heamagglutinin‐esterase‐like attachment factor. Yet, several lectins (DC‐SIGN, DC‐SIGNR/L‐SIGN/CD209L and LSECtin) have been demonstrated to function as such by binding to the S protein (Jeffers et al., 2004; Marzi et al., 2004; Yang et al., 2004; Gramberg et al., 2005; Chan et al., 2006). Expression of these lectins in the absence of ACE2 did not lead to infection driven by retroviral particles containing the SARS‐CoV S protein; in the presence of ACE2 infectious retroviral entry was augmented (Marzi et al., 2004; Gramberg et al., 2005). Furthermore, it was found that the presence of L‐SIGN allowed very inefficient entry of SARS‐CoV (Jeffers et al., 2004), although in another study this lectin did not appear to facilitate infection of SARS‐CoV in ACE2 expressing cells (Chan et al., 2006). Nevertheless, DC‐SIGN and L‐SIGN expressing cells, as well as dendritic cells, which could not be infected themselves, were able to promote cell‐mediated transfer of virus to susceptible target cells (Marzi et al., 2004; Yang et al., 2004; Chan et al., 2006). Interestingly, L‐SIGN also appears to play a protective role in SARS‐CoV infection. Compared with cells heterozygous for L‐SIGN, cells homozygous for L‐SIGN (with respect to a polymorphism in the extracellular neck region) show a higher binding capacity for SARS‐CoV, higher proteasome‐dependent viral degradation and a lower capacity for cell‐mediated transfer of virus (Chan et al., 2006). These results correlate with a genetic risk association study in which individuals homozygous for L‐SIGN appear less susceptible to SARS‐CoV infection (Chan et al., 2006).
The entry pathway of coronaviruses has not been clearly established. While some coronaviruses appear to use an endosomal route of infection (Nash and Buchmeier, 1997; Hansen et al., 1998; Nomura et al., 2004), others seem to enter cells at the plasma membrane (Nash and Buchmeier, 1997). Conceivably, the route of entry taken is determined both by the coronavirus (strain) and by the host cell. In the case of SARS‐CoV, lysomotropic agents were demonstrated to block the entry both of lentiviral particles pseudotyped with the SARS‐CoV S protein (Simmons et al., 2004; Yang et al., 2004; Huang et al., 2006) and of SARS‐CoV itself (Huang et al., 2006), indicating that acidification of endosomes is required for entry. However, activation of the S protein by proteases was sufficient for bypassing the entry inhibition caused by lysomotropic agents (Matsuyama et al., 2005; Simmons et al., 2005) and resulted in cell–cell fusion (Simmons et al., 2004; Matsuyama et al., 2005). Apparently, low pH per se is not required for fusion. Accordingly, infection mediated by the SARS‐CoV S protein could be inhibited by specific inhibitors of the pH‐sensitive endosomal protease cathepsin L (Simmons et al., 2005; Huang et al., 2006). Because the SARS‐CoV S protein is not cleaved during biogenesis and maturation of the virion, it is feasible that cathepsin L cleaves the protein closely upstream of the SARS‐CoV fusion peptide thereby locating this hydrophobic peptide near to the newly generated N terminus of the membrane‐anchored subunit, as is generally the case for class I fusion proteins. Whether cathepsin L (‐like) cleavage is essential for the activation of S proteins of coronaviruses in general or, for instance, just for those not processed by furin enzymes has yet to be established. Interestingly, the infection by HCoV‐NL63, which also uses ACE2 as its receptor (Hofmann et al., 2005) and whose S protein is uncleaved, is less low‐pH sensitive and is not blocked by cathepsin l‐inhibitors (Huang et al., 2006), indicating that two coronaviruses that utilize the same receptor can enter cells through distinct mechanisms.
RNA replication
Nothing is known about the initial fate of the viral nucleocapsid once it is delivered to the cell’s cytoplasm by the fusion of viral and cellular membranes. Whether it disengages from the viral envelope components, whether it is transported to a specific location and how it is disassembled to allow translation of the viral RNA remains elusive. SARS‐CoV genome expression starts with the translation of the very large ORF1a and ORF1b genes. The more downstream ORF1b is translated by translational readthrough using a ribosomal frameshift mechanism for which a ‘slippery’ sequence and a pseudoknot are required (Thiel et al., 2003). As a result, two very large polyproteins (pp1a and pp1ab; 4382 and 7073 amino acids, respectively) are produced, which are predicted to be cleaved by two viral proteinases into 16 subunits (nsp1‐16) (Snijder et al., 2003; Ziebuhr, 2004). These cleavage products collectively constitute the replication–transcription complex. The replication–transcription complex mediates both genome replication and the transcription of a 3′‐coterminal nested set of subgenomic mRNAs from which the genes located downstream of ORF1b are expressed. Each of the subgenomic mRNAs contains a short 5′‐ leader sequence derived from the 5′ end of the genome, which is acquired by a mechanism that involves discontinuous synthesis of subgenomic minus strands and which is dependent on transcription regulatory sequences located upstream of most genes (reviewed by Sawicki and Sawicki, 2005).
Several cleavage products of the SARS‐CoV pp1a and pp1ab (nsp3, 4 and 6) contain hydrophobic domains that presumably mediate their anchoring in cellular membranes where they may function in the recruitment of the viral replication complex (Harcourt et al., 2004; Ziebuhr, 2004; 2005). Although the precise mechanism that leads to the formation of these replication complexes has not yet been elucidated, increasing evidence indicates that coronaviruses attach their replication machinery to the limiting membrane of autophagosomes. In the cellular process of autophagy, compartments bound by double membranes sequester regions of the cytosol and then mature and degrade their cytosolic contents (Kirkegaard et al., 2004). Cells infected with either MHV or SARS‐CoV accumulate large double‐membraned vesicles (DMVs) to which the viral replication complexes are localized (Gosert et al., 2002; Goldsmith et al., 2004). Consistently, the coronavirus replication complexes were demonstrated to colocalize with markers of autophagosomes (2004a, 2004b,b). Furthermore, the cellular machinery of autophagy is essential for the formation of these double‐membraned compartments in cells infected with MHV and the replication of this virus is severely impaired in its absence (Prentice et al., 2004a). However, MHV replication complexes have also been localized to late endosomal membranes using electron microscopy (van der Meer et al., 1999). To what extent and how coronaviruses exploit these intracellular transport routes, remains to be elucidated.
Once the replication–transcription complexes are formed, coronaviral RNA replication and transcription not only involves viral proteins, but also several cellular proteins. In addition to the N protein, which is also required for efficient RNA replication (Almazan et al., 2004; Schelle et al., 2005), several heterologous nuclear ribonucleoprotein (hnRNP) family members (hnRNPA1, PTB and SYNCRYP), known to be involved in premRNA processing, were found to bind to different regions of the (+) and (–) strand genomic RNA and to affect coronavirus replication and transcription (reviewed by Shi and Lai, 2005). Other RNA‐binding proteins have also been suggested to play a role in coronavirus replication, such as m‐aconitase (Nanda and Leibowitz, 2001) and poly‐A‐binding protein (Spagnolo and Hogue, 2000). The N proteins from MHV and SARS‐CoV were shown to interact with hnRNAP A1 (Wang and Zhang, 1999; Luo et al., 2005). Interactions between viral RNA, N protein and cellular proteins such as hnRNPs may result in the formation of ribonucleoprotein structures that mediate replication and/or discontinous transcription by the replication–transcription complexes. Clearly, our understanding of this elementary process of the infection cycle, particularly of the host cell contributions, is rudimentary.
Assembly
Once sufficient levels of new genomic RNA and structural components start to accumulate, assembly of virions ensues. Coronaviruses assemble their particles by budding of the helical nucleocapsid through membranes early in the secretory pathway, in particular the endoplasmic reticulum to Golgi intermediate compartment (ERGIC). The involvement of host cellular factors in this process has hardly been explored. Work has so far been concentrated on the viral components and their interplay (reviewed by de Haan and Rottier, 2005). It is clear that the M protein, a triple spanning membrane protein, is the key player in coronavirus assembly. It is the most abundant protein in the virion, which, through its interactions with every known component of the virion, orchestrates the entire assembly process. The M protein exerts its central role in assembly by selecting and organizing the viral envelope components at the assembly sites and by mediating the interactions with the nucleocapsid to allow the budding of virions. Two types of interactions appear to effect the incorporation of the nucleocapsid into the virion: protein–protein interactions between the M protein and the N protein and protein–RNA interactions between the M protein and the viral genome. At the level of the membrane the M protein interacts with itself, to generate the basic molecular framework of the envelope, with the E protein, to induce the budding and release of the M protein‐modified membrane, and with the S protein, to assemble the spikes into the viral envelope. In addition, the SARS‐CoV M protein interacts with the 3a protein, resulting in its incorporation into particles (Ito et al., 2005). The 3a protein is another triple‐spanning membrane protein, hence similar to M, also in the fact that it is (O‐)glycosylated. Interestingly, also for some other coronaviruses such triple‐spanning proteins have been predicted (Oostra et al., 2006), though their occurrence in virions has not been observed. These latter proteins have been shown to be dispensable, which also appears to be the case for 3a (Yount et al., 2005).
The coronavirus envelope proteins have the capacity to assemble, just by themselves, uniform envelopes, which have the same appearance and dimensions as normal virions. Such virus‐like particles form independently of the N protein, E and M being the minimally required proteins (Vennema et al., 1996; reviewed by de Haan and Rottier, 2005). This has also been reported for the SARS‐CoV (Mortola and Roy, 2004; Hsieh et al., 2005), although others have claimed the M and N proteins to be necessary and sufficient for formation of virus‐like particles (Huang et al., 2004). In this respect, it is perhaps worth mentioning the varying importance of the E protein for the assembly of infectious coronaviruses. Thus, while the E protein is essential for the production of infectious porcine transmissible gastroenteritis virus (Ortego et al., 2002), this is not the case for MHV (Kuo and Masters, 2003) and SARS‐CoV (L. Enjuanes, pers. comm.). The small hydrophobic E protein is a viroporin; it forms ion channels (Wilson et al., 2004) and is able to alter the membrane permeability of cells (Madan et al., 2005). Viroporins of other enveloped viruses were also shown to enhance the membrane permeability and to promote virus budding and release (reviewed by Gonzalez and Carrasco, 2003).
In general, viral glycoproteins determine the site of virion budding. For coronaviruses this is, however, not so apparent because the most obvious candidate, the M protein localizes to the Golgi complex, beyond the site of budding. Also the E and S proteins do not localize to the budding compartment (Nal et al., 2005; reviewed by de Haan and Rottier, 2005). It might well be that the lateral association of the envelope proteins creates novel localization signals that direct the multimeric complexes to the budding site. Alternatively, interactions of the M protein with the nucleocapsid could determine the localization of budding. Whereas early in infection the N protein colocalizes with the replication complexes, which are almost entirely discrete from the sites of M protein accumulation, at later times the helicase and the N proteins appear to colocalize with the M protein (Bost et al., 2001). The relocation of the helicase–N complex may serve as a mechanism to deliver the newly synthesized RNA and nucleocapsids to the assembly sites and to facilitate the retention of the M protein in the intermediate compartment. Considering what is becoming known about the assembly processes of other viruses, particularly of HIV‐1 (Freed, 2004), it is safe to expect that also coronaviruses rely on an arsenal of host factors for their virion assembly and budding. Yet, none has so far been identified.
Besides the coronavirus proteins also the coronavirions themselves are subject to intracellular maturation processes while on their way through the constitutive secretory pathway out of the cell. Thus, while the n‐glycans of the SARS‐CoV M and S proteins mature (Nal et al., 2005) and the 3a protein becomes o‐glycosylated (Oostra et al., 2006), the coronavirus particles undergo structural maturation during their transport through the Golgi complex (reviewed by de Haan and Rottier, 2005). The significance of this maturation process, which is seen morphologically as a rearrangement of the inner ribonucleoprotein accompanied by shrinkage of the particle, remains unclear. It has so far not been reported for SARS‐CoV.
Perspective
Ever since its identification as the cause of SARS, knowledge about the SARS‐CoV has accumulated at breathtaking pace, obviously by profiting from the pre‐SARS insights in coronavirology. However, as for other coronaviruses, this knowledge is mostly limited to aspects of the molecular biology of the virus and of the disease process. Relatively little do we know about the structure of the virion and, as outlined in this review, about the contributions of the host cell to infection. It is only now that the latter issue is becoming amenable to research due in particular to the explosive technical developments in the fields of (functional) genomics and proteomics.
Acknowledgements
C.A.M.deH. is supported by the Netherlands Organization for Scientific Research (NWO‐VIDI‐700.54.421). The authors thank Marije Brouwer for making the drawing.
References
- Almazan, F. , Galan, C. , and Enjuanes, L. (2004) The nucleoprotein is required for efficient coronavirus genome replication. J Virol 78: 12683–12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock, G.J. , Esshaki, D.J. , Thomas, W.D. , Jr Ambrosino And, D.M. (2004) Amino acids 270–510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol 78: 4552–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht, H. , Roberts, A. , Vogel, L. , Bukreyev, A. , Collins, P.L. , Murphy, B.R. , et al. (2004) Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA 101: 6641–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, B.J. , Van Der Zee, R. , De Haan, C.A. , and Rottier, P.J. (2003) The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol 77: 8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, B.J. , Martina, B.E. , Van Der Zee, R. , Lepault, J. , Haijema, B.J. , Versluis, C. , et al. (2004) Severe acute respiratory syndrome coronavirus (SARS‐CoV) infection inhibition using spike protein heptad repeat‐derived peptides. Proc Natl Acad Sci USA 101: 8455–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost, A.G. , Prentice, E. , and Denison, M.R. (2001) Mouse hepatitis virus replicase protein complexes are translocated to sites of M protein accumulation in the ERGIC at late times of infection. Virology 285: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, V.S. , Chan, K.Y. , Chen, Y. , Poon, L.L. , Cheung, A.N. , Zheng, B. , et al. (2006) Homozygous L‐SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat Genet 38: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten, C. , Gunther, S. , Preiser, W. , Van Der Werf, S. , Brodt, H.R. , Becker, S. , et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- Freed, E.O. (2004) HIV‐1 and the host cell: an intimate association. Trends Microbiol 12: 170–177. [DOI] [PubMed] [Google Scholar]
- Goldsmith, C.S. , Tatti, K.M. , Ksiazek, T.G. , Rollin, P.E. , Comer, J.A. , Lee, W.W. , et al. (2004) Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis 10: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, M.E. , and Carrasco, L. (2003) Viroporins. FEBS Lett 552: 28–34. [DOI] [PubMed] [Google Scholar]
- Gosert, R. , Kanjanahaluethai, A. , Egger, D. , Bienz, K. , and Baker, S.C. (2002) RNA replication of mouse hepatitis virus takes place at double‐membrane vesicles. J Virol 76: 3697–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramberg, T. , Hofmann, H. , Moller, P. , Lalor, P.F. , Marzi, A. , Geier, M. , et al. (2005) LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 340: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan, C.A. , and Rottier, P.J. (2005) Molecular interactions in the assembly of coronaviruses. Adv Virus Res 64: 165–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan, C.A. , Stadler, K. , Godeke, G.J. , Bosch, B.J. , and Rottier, P.J. (2004) Cleavage inhibition of the murine coronavirus spike protein by a furin‐like enzyme affects cell–cell but not virus–cell fusion. J Virol 78: 6048–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, G.H. , Delmas, B. , Besnardeau, L. , Vogel, L.K. , Laude, H. , Sjostrom, H. , and Noren, O. (1998) The coronavirus transmissible gastroenteritis virus causes infection after receptor‐mediated endocytosis and acid‐dependent fusion with an intracellular compartment. J Virol 72: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt, B.H. , Jukneliene, D. , Kanjanahaluethai, A. , Bechill, J. , Severson, K.M. , Smith, C.M. , et al. (2004) Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain‐like protease activity. J Virol 78: 13600–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Hoek, L. , Pyrc, K. , Jebbink, M.F. , Vermeulen‐Oost, W. , Berkhout, R.J. , Wolthers, K.C. , et al. (2004) Identification of a new human coronavirus. Nat Med 10: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, H. , Pyrc, K. , Van Der Hoek, L. , Geier, M. , Berkhout, B. , and Pohlmann, S. (2005) Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA 102: 7988–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, P.K. , Chang, S.C. , Huang, C.C. , Lee, T.T. , Hsiao, C.W. , Kou, Y.H. , et al. (2005) Assembly of severe acute respiratory syndrome coronavirus RNA packaging signal into virus‐like particles is nucleocapsid dependent. J Virol 79: 13848–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Yang, Z.Y. , Kong, W.P. , and Nabel, G.J. (2004) Generation of synthetic severe acute respiratory syndrome coronavirus pseudoparticles: implications for assembly and vaccine production. J Virol 78: 12557–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, I.C. , Bosch, B.J. , Li, F. , Li, W. , Lee, K.H. , Ghiran, S. , et al. (2006) SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2‐expressing cells. J Biol Chem 281: 3198–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y. , Kuba, K. , Rao, S. , Huan, Y. , Guo, F. , Guan, B. , et al. (2005) Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, N. , Mossel, E.C. , Narayanan, K. , Popov, V.L. , Huang, C. , Inoue, T. , et al. (2005) Severe acute respiratory syndrome coronavirus 3a protein is a viral structural protein. J Virol 79: 3182–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers, S.A. , Tusell, S.M. , Gillim‐Ross, L. , Hemmila, E.M. , Achenbach, J.E. , Babcock, G.J. , et al. (2004) CD209L (L‐SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA 101: 15748–15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard, K. , Taylor, M.P. , and Jackson, W.T. (2004) Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek, T.G. , Erdman, D. , Goldsmith, C.S. , Zaki, S.R. , Peret, T. , Emery, S. , et al. (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953–1966. [DOI] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Gao, H. , Guo, F. , Guan, B. , et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 11: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, J.H. , Li, W. , Choe, H. , and Farzan, M. (2004) Angiotensin‐converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci 61: 2738–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, L. , and Masters, P.S. (2003) The small envelope protein E is not essential for murine coronavirus replication. J Virol 77: 4597–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Moore, M.J. , Vasilieva, N. , Sui, J. , Wong, S.K. , Berne, M.A. , et al. (2003) Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Greenough, T.C. , Moore, M.J. , Vasilieva, N. , Somasundaran, M. , Sullivan, J.L. , et al. (2004) Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin‐converting enzyme 2. J Virol 78: 11429–11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhang, C. , Sui, J. , Kuhn, J.H. , Moore, M.J. , Luo, S. , et al. (2005a) Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J 24: 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Li, W. , Farzan, M. , and Harrison, S.C. (2005b) Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science 309: 1864–1868. [DOI] [PubMed] [Google Scholar]
- Luo, H. , Chen, Q. , Chen, J. , Chen, K. , Shen, X. , and Jiang, H. (2005) The nucleocapsid protein of SARS coronavirus has a high binding affinity to the human cellular heterogeneous nuclear ribonucleoprotein A1. FEBS Lett 579: 2623–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan, V. , Garcia Mde, J. , Sanz, M.A. , and Carrasco, L. (2005) Viroporin activity of murine hepatitis virus E protein. FEBS Lett 579: 3607–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra, M.A. , Jones, S.J. , Astell, C.R. , Holt, R.A. , Brooks‐Wilson, A. , Butterfield, Y.S. , et al. (2003) The genome sequence of the SARS‐associated coronavirus. Science 300: 1399–1404. [DOI] [PubMed] [Google Scholar]
- Marzi, A. , Gramberg, T. , Simmons, G. , Moller, P. , Rennekamp, A.J. , Krumbiegel, M. , et al. (2004) DC‐SIGN and DC‐SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol 78: 12090–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama, S. , Ujike, M. , Morikawa, S. , Tashiro, M. , and Taguchi, F. (2005) Protease‐mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA 102: 12543–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Meer, Y. , Snijder, E.J. , Dobbe, J.C. , Schleich, S. , Denison, M.R. , Spaan, W.J. , and Locker, J.K. (1999) Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J Virol 73: 7641–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.J. , Dorfman, T. , Li, W. , Wong, S.K. , Li, Y. , Kuhn, J.H. , et al. (2004) Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin‐converting enzyme 2. J Virol 78: 10628–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola, E. , and Roy, P. (2004) Efficient assembly and release of SARS coronavirus‐like particles by a heterologous expression system. FEBS Lett 576: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nal, B. , Chan, C. , Kien, F. , Siu, L. , Tse, J. , Chu, K. , et al. (2005) Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J Gen Virol 86: 1423–1434. [DOI] [PubMed] [Google Scholar]
- Nanda, S.K. , and Leibowitz, J.L. (2001) Mitochondrial aconitase binds to the 3′ untranslated region of the mouse hepatitis virus genome. J Virol 75: 3352–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, T.C. , and Buchmeier, M.J. (1997) Entry of mouse hepatitis virus into cells by endosomal and nonendosomal pathways. Virology 233: 1–8. [DOI] [PubMed] [Google Scholar]
- Nomura, R. , Kiyota, A. , Suzaki, E. , Kataoka, K. , Ohe, Y. , Miyamoto, K. , et al. (2004) Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J Virol 78: 8701–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra, M. , De Haan, C.A. , De Groot, R.J. , and Rottier, P.J. (2006) Glycosylation of the severe acute respiratory syndrome coronavirus triple‐spanning membrane proteins 3a and M. J Virol 80: 2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego, J. , Escors, D. , Laude, H. , and Enjuanes, L. (2002) Generation of a replication‐competent, propagation‐deficient virus vector based on the transmissible gastroenteritis coronavirus genome. J Virol 76: 11518–11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J.S. , Lai, S.T. , Poon, L.L. , Guan, Y. , Yam, L.Y. , Lim, W. , et al. (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice, E. , Jerome, W.G. , Yoshimori, T. , Mizushima, N. , and Denison, M.R. (2004a) Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem 279: 10136–10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice, E. , McAuliffe, J. , Lu, X. , Subbarao, K. , and Denison, M.R. (2004b) Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J Virol 78: 9977–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, X.X. , Hao, P. , Song, X.J. , Jiang, S.M. , Liu, Y.X. , Wang, P.G. , et al. (2005) Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J Biol Chem 280: 29588–29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota, P.A. , Oberste, M.S. , Monroe, S.S. , Nix, W.A. , Campagnoli, R. , Icenogle, J.P. , et al. (2003) Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–1399. [DOI] [PubMed] [Google Scholar]
- Sawicki, S.G. , and Sawicki, D.L. (2005) Coronavirus transcription: a perspective. Curr Top Microbiol Immunol 287: 31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelle, B. , Karl, N. , Ludewig, B. , Siddell, S.G. , and Thiel, V. (2005) Selective replication of coronavirus genomes that express nucleocapsid protein. J Virol 79: 6620–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S.T. , and Lai, M.M. (2005) Viral and cellular proteins involved in coronavirus replication. Curr Top Microbiol Immunol 287: 95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, G. , Reeves, J.D. , Rennekamp, A.J. , Amberg, S.M. , Piefer, A.J. , and Bates, P. (2004) Characterization of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) spike glycoprotein‐mediated viral entry. Proc Natl Acad Sci USA 101: 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, G. , Gosalia, D.N. , Rennekamp, A.J. , Reeves, J.D. , Diamond, S.L. , and Bates, P. (2005) Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA 102: 11876–11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder, E.J. , Bredenbeek, P.J. , Dobbe, J.C. , Thiel, V. , Ziebuhr, J. , Poon, L.L. , et al. (2003) Unique and conserved features of genome and proteome of SARS‐coronavirus, an early split‐off from the coronavirus group 2 lineage. J Mol Biol 331: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H.C. , Seo, M.Y. , Stadler, K. , Yoo, B.J. , Choo, Q.L. , Coates, S.R. , et al. (2004) Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J Virol 78: 10328–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo, J.F. , and Hogue, B.G. (2000) Host protein interactions with the 3′ end of bovine coronavirus RNA and the requirement of the poly (A) tail for coronavirus defective genome replication. J Virol 74: 5053–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel, V. , Ivanov, K.A. , Putics, A. , Hertzig, T. , Schelle, B. , Bayer, S. , et al. (2003) Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol 84: 2305–2315. [DOI] [PubMed] [Google Scholar]
- Thorp, E.B. , and Gallagher, T.M. (2004) Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J Virol 78: 2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema, H. , Godeke, G.J. , Rossen, J.W. , Voorhout, W.F. , Horzinek, M.C. , Opstelten, D.J. , and Rottier, P.J. (1996) Nucleocapsid‐independent assembly of coronavirus‐like particles by co‐expression of viral envelope protein genes. EMBO J 15: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , and Zhang, X. (1999) The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo . Virology 265: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Chen, J. , Zheng, A. , Nie, Y. , Shi, X. , Wang, W. , et al. (2004) Expression cloning of functional receptor used by SARS coronavirus. Biochem Biophys Res Commun 315: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, L. , McKinlay, C. , Gage, P. , and Ewart, G. (2004) SARS coronavirus E protein forms cation‐selective ion channels. Virology 330: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S.K. , Li, W. , Moore, M.J. , Choe, H. , and Farzan, M. (2004) A 193‐amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin‐converting enzyme 2. J Biol Chem 279: 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P.C. , Lau, S.K. , Chu, C.M. , Chan, K.H. , Tsoi, H.W. , Huang, Y. , et al. (2005) Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79: 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Chakraborti, S. , Dimitrov, A.S. , Gramatikoff, K. , and Dimitrov, D.S. (2003) The SARS‐CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun 312: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z.Y. , Huang, Y. , Ganesh, L. , Leung, K. , Kong, W.P. , Schwartz, O. , et al. (2004) pH‐dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC‐SIGN. J Virol 78: 5642–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount, B. , Roberts, R.S. , Sims, A.C. , Deming, D. , Frieman, M.B. , Sparks, J. , et al. (2005) Severe acute respiratory syndrome coronavirus group‐specific open reading frames encode nonessential functions for replication in cell cultures and mice. J Virol 79: 14909–14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr, J. (2004) Molecular biology of severe acute respiratory syndrome coronavirus. Curr Opin Microbiol 7: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr, J. (2005) The coronavirus replicase. Curr Top Microbiol Immunol 287: 57–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


