1. Introduction
Despite its extensive history as a folk treatment for numerous health conditions [28], controlled clinical studies on the efficacy of cannabis have only recently begun to accumulate. The past half-century has witnessed several notable achievements in the science of cannabis, including the extraction and identification of delta-9-tetrahydrocannabinol (THC) as the primary psychoactive constituent of the cannabis plant [20] and the identification of an endocannabinoid system in the mammalian brain [26,51,71]. Further, interest in the utility of cannabinoids as potential analgesics has greatly increased over the past decade.
Strong and consistent preclinical evidence from rodent models has suggested that cannabinoids might be a promising class of analgesics [1,9,35,43,67]. However, efficacy data from human clinical trials in patients with chronic non-cancer pain (CNCP) outcomes are equivocal. Previous systematic reviews have generally found modest evidence for the efficacy of cannabinoids on self-reported clinical pain outcomes across CNCP disorders, with iatrogenic effects outpacing clinical gains [3,6,8,17,19,25,44,48,69].
Although reasons for this modest clinical translation are unclear, previous reviews have concluded that there is a lack of high-quality evidence and have called for more rigorous clinical trials. Quantitative sensory testing (QST) might be a valuable tool to improve study rigor and bolster mechanistic understanding of cannabinoid effects on clinical pain. QST broadly refers to psychophysical methods for systematically quantifying somatosensory function in individuals with and without chronic pain [73]. Previous work suggests that QST can help identify changes in pain-related neural processing, making it an important tool for analgesic development [7]. Because QST uses calibrated noxious stimuli to evaluate acute pain responses in a controlled setting, key sources of variability are minimized by standardizing sensory input parameters. Though QST responses are subject to substantial inter- and intraindividual variability [21], increased pain sensitivity on measures of QST predicts worse clinical pain outcomes in numerous clinical trials [24,66,85,86].
Recently, Lotsch, Weyer-Menkhoff, and Tegeder [43] conducted a review of six studies that examined cannabinoid-based analgesia in human experimental settings. They found mixed outcomes across studies for responses to QST stimuli following cannabinoid administration. De Vita and colleagues [74] expanded on this work and conducted the first systematic review and meta-analysis on cannabinoid administration during QST in healthy adults. In total, they synthesized findings from 18 placebo-controlled studies and concluded that cannabinoids exerted small effects on pain thresholds, but not overall pain intensity, during QST. Further, they suggested that cannabinoid-related analgesia might occur via indirect influences (i.e., changes in affective processes). Although this review provided valuable information about cannabinoid-related analgesia on measures of pain threshold, tolerance, and primary hyperalgesia in healthy individuals, it did not include studies assessing cannabinoid analgesia to QST in chronic pain populations. Given that chronic pain patients tend to show increased pain sensitivity compared to healthy controls [46], it is of interest to understand the effects of cannabinoids on experimentally-induced pain in controlled settings among chronic pain patients.
In the present review, we systematically synthesize the evidence for cannabinoid analgesic effects in both healthy adults and patients with CNCP focusing on QST outcomes. Specifically, we examine the existing evidence by grouping results based on study sample, cannabinoid compounds, and sensory domain.
2. Methods
A protocol for this systematic review was established and pre-registered on PROSPERO (CRD42018117367) on 12/19/2018. Data were extracted between 09/24/2018 and 01/08/2019 and the review was conducted in four stages: (1) compiling of a potential abstract pool based on search terms, (2) abstract review, (3) full-text review of eligible abstracts, and (4) data extraction from included full-texts.
2.1. Search Procedure
Reviewers used the Covidence web-based platform (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia; www.covidence.org) for systematic reviews to organize compiled abstracts. Searched databases included PsycINFO, Cochrane, Google Scholar, Embase, and Pubmed. Search terms were: (“cannabi*” OR “marijuana” OR “marihuana” OR “hashish” OR “THC”) AND [(“pain”) AND (“quantitative sensory testing” OR “pain testing” OR “calibrated noxious stimuli” OR “threshold” OR “tolerance” OR “cold pressor” OR “cold water bath” OR “cold” OR “mechanical” OR “pressure” OR “thermal” OR “heat” OR “electrical” OR “capsaicin” OR “hyperalgesia” OR “allodynia” OR “temporal summation” OR “windup” OR “diffuse noxious inhibitory control” OR “conditioned pain modulation” OR “nociceptive flexion reflex”)].
2. 2. Eligibility Criteria
2.2.a. Abstract Criteria for Full-Text Review
Peer-reviewed publications were eligible for full-text review contingent on the following criteria: (1) relevant search terms appeared in the abstract, (2) the publication was written in the English language, (3) the study included human subjects only, (4) at least one cannabinoid agent (i.e., plant-based or synthetic) was used, (5) at least one QST measure was used, (6) the manuscript was accepted for publication prior to August 2018, and (7) the full text was available. Four reviewers were involved in this stage (CJM, JEL, ENP, and PHF). Each was randomly assigned as a reviewer for half of the available abstracts, so that each abstract was reviewed independently by two individuals. Abstracts were considered for full-text review if both reviewing authors marked the abstract for inclusion. Any conflicts were resolved by PHF or ENP.
2.2.b. Full-text Criteria for Data Extraction
Full-texts associated with eligible abstracts were further reviewed using the following criteria: (1) the study included a placebo control, and (2) individuals were randomized to drug conditions. Two authors reviewed all full-texts (CJM and JEL). Full-texts were included in the systematic review if both reviewing authors marked the full-text for inclusion, and we decided a priori that PHF would resolve any conflicts. However, there were no conflicts between CJM and JEL in this process (i.e., perfect inter-rater reliability).
2.2.c. Additional Manual Literature Search
Reference sections and search engines were manually searched in addition to the procedure described above. Articles resulting from this manual search were compared to articles resulting from the above-described systematic search, yielding 5 new articles. Abstracts from these studies were added to Covidence manually. CJM and JEL reviewed these 5 abstracts to determine their eligibility for full-text review, and PHF resolved any conflicts.
2.3. Definitions of Predictor and Outcome Variables
We examined the overall effects reported in eligible full-texts using a Population, Intervention, Comparison, Outcome (PICO) framework. First, we broadly grouped studies based on population. Studies were further sub-grouped based on cannabinoid compound. Finally, studies were further sub-grouped by QST sensory modality. For each subgrouping, we summarize findings, examine potential dose-response effects, describe the consistency of effects across studies, and offer brief conclusions.
2.3.a. Population
Although no study has provided direct evidence to determine whether individuals with chronic pain have altered endocannabinoid system function, previous work robustly shows differences in pain responses across quantitative sensory tests between individuals with and without chronic pain. For this reason, studies were first broadly grouped based on population in order to identify meaningful patterns of results. Studies within the “Healthy Adult Samples Only” category included samples of healthy, pain-free adults aged 18 and older. Studies within the “Patient Samples Included” category included either a sample of patients with CNCP only or a sample composed of individuals both with and without CNCP.
2.3.b. Cannabinoid Compounds
Because the term cannabinoid includes a broad array of compounds with distinct structures and mechanisms, it is important to narrowly interpret study results to the specific compound of study rather than the class of cannabinoid compounds. Further confounding factors of results could include routes of administration (e.g., inhaled, oral), duration of use, concentration, food effects, and cannabinoid combinations. Furthermore, previous work suggests that cannabinoid compounds can have drastically different psychobehavioral effects. For instance, although THC is associated with dose-dependent intoxication, cannabidiol (CBD) has no such dose-dependent effect and does not have intoxicating effects [61,63]. Other studies have suggested that CBD may mitigate some of the negative effects of THC, either enzymatically or through negative allosteric modulation of CB1 receptors [68]. No set of studies has definitively determined whether one cannabinoid compound, form (synthetic cannabinoid or phytocannabinoid), or route of administration is preferable to pain patients [39,60]. For these reasons, we sub-grouped studies based on cannabinoid compound and form to draw more meaningful conclusions about the extant literature. Studies were classified as “Inhaled Cannabis” if the cannabis was smoked or vaporized cannabis flower. Studies were classified as “Synthetic Cannabinoids” if the study used either dronabinol or nabilone, both single-molecule synthetic pharmaceutical products. Studies were classified as “Combined THC and CBD Formulations” if the drug product tested contained both THC and CBD (e.g., the plant derived pharmaceutical Sativex, which contains THC and CBD in roughly equal concentrations). Finally, studies were classified as “Other Endocannabinoid Modulator” if the cannabinoid agent did not fit the previously described categories (e.g., novel compounds).
2.3.c. Sensory Domains
Eligible full-texts were further sub-grouped based on the QST sensory domain tested, which included heat, cold, mechanical, electrical, visceral, and chemical modalities. The “heat” domain refers to noxious stimuli delivered through a thermal stimulator. The “cold” domain refers to studies using tests involving exposure to noxious cold stimuli (e.g., cold pressor test). The “mechanical” domain refers to noxious stimuli delivered via pressure algometry or punctate probes. The “electrical” domain refers to noxious stimuli administered by an electrodermal stimulator. The “visceral” domain refers to noxious stimuli delivered via esophageal or rectal balloon distension. Finally, the “chemical” domain refers to stimuli delivered via topical or injectable irritants. Given that some studies interrogated pain processing via multiple modalities, we present results separately by domain. For this reason, some studies are reported in more than one sensory domain.
2.3.d. Outcome Measures
Within each sub-group, we describe outcomes based on pain threshold, pain tolerance, evoked allodynia, and secondary hyperalgesia. Pain threshold refers to the lowest stimulus intensity reported as painful. Pain tolerance refers to the maximum stimulus intensity or duration reported as bearable. Evoked allodynia is a reported painful sensation to a non-noxious stimulus. Finally, secondary hyperalgesia is a reported increase in pain sensitivity to a noxious stimulus applied over a region that surrounds, but does not include, the primary affected area.
2.4. Extraction of Study Information
Two authors (CJM and JEL) divided the final set of full-text articles to extract relevant study information, including study agents, dosing information, experimental design, sample characteristics, evoked-pain methods, and outcomes. For studies including non-cannabinoid agents, only contrasts related to cannabinoid vs. placebo outcomes were considered. All extractions were cross-checked for accuracy.
2.5. Risk of Bias Assessment
In reviewing included studies, two authors (CJM and JEL) blindly completed independent risk of bias assessments. Criteria and scoring were adapted from two prior systematic reviews involving QST measures as primary outcomes [41,54].
The following domains were assessed: 1) blinding of participant to drug assignment; 2) blinding of experimenter to drug assignment; 3) appropriateness and clarity of inclusion criteria and recruitment approach; 4) age and sex matching of cases and controls; 5) control for, or exclusion of, established confounders (e.g., medication and caffeine use prior to testing; clinical pain level; menstrual phase; medical comorbidity or other individual difference factors known to affect QST response; reported time frame in which QST was administered).
Risk was coded on a 0–2 scale, with “low risk” assigned 0, “moderate risk” assigned 1, and “high risk” assigned 2. Scores were then summed across categories to yield a total risk score that ranged from 0 to 10, with higher scores indicating higher risk of bias. Specific guidance for scoring within each category and risk level were adapted from Lewis et al. [41] and shown in Table 1. All discordant scores within each domain were reviewed by a third author (PHF) and discussed as a group (JEL, CJM, PHF) until consensus was reached.
Table 1.
Criteria used in the risk of bias assessment
| Category | Low Risk | Moderate Risk | High Risk |
|---|---|---|---|
| Participant Blind | Explicitly stated and described | Stated or implied, but not described | Not blinded or not stated |
| Experimenter Blind | Explicitly stated and described | Stated or implied, but not described | Not blinded or not stated |
| Inclusion Criteria | Adequately described and justified inclusion criteria and recruitment strategy | One of the following: ICa) inadequate inclusion criteria justification or ICb) inadequate recruitment strategy and/or description | Both (ICa) and (ICb) |
| Age and Sex Matching | Both of the following: ASMa) ≤10% difference between groups in mean age; ASMb) ≤10% difference between groups in sex distribution | >10% difference on either (ASMa) or (ASMb) | >10% difference on both (ASMa) and (ASMb) |
| Confounder Control | Explicitly stated control of at least 4 confounders listed above | Explicitly stated control of 3 confounders listed above | <3 confounders controlled or not explicitly stated |
3. Results
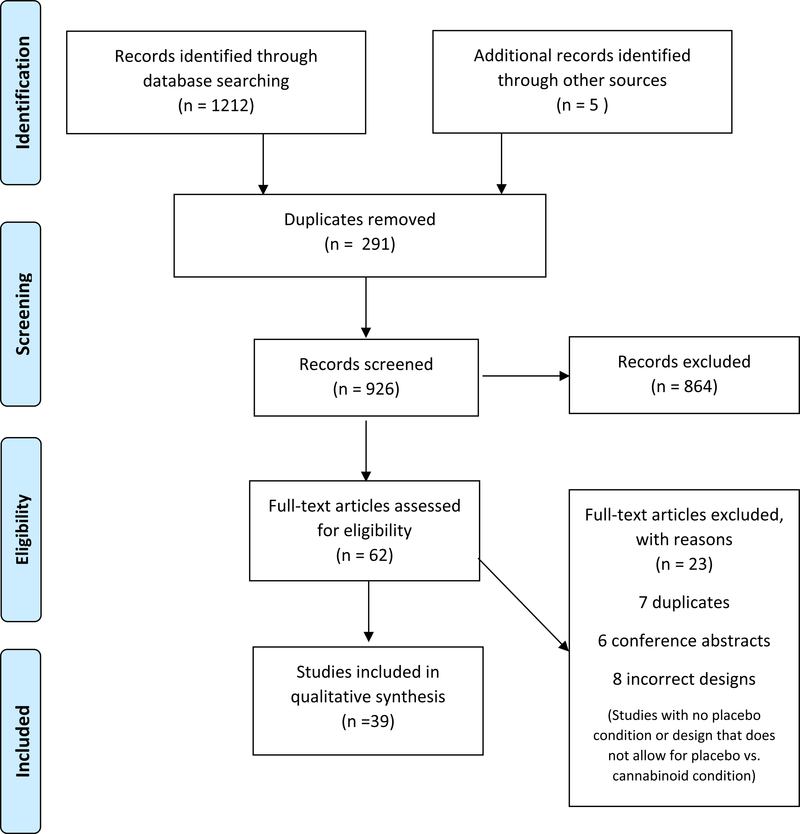
Figure 1 provides a flowchart of the number of studies included at each stage of the above-described procedures. A total of 1217 abstracts were screened. Among these abstracts, 291 were duplicates, and 864 abstracts were excluded based on search criteria. A total of 62 abstracts underwent full-text review, and 23 of them were excluded because some additional duplicates were uncovered and some studies had incorrect designs, conference abstracts, incorrect intervention, and incorrect study outcomes. A total of 39 articles were reviewed for the qualitative synthesis of our study. Characteristics of these included studies are summarized in Table 2.
Figure 1:
PRISMA Flow Chart
Table 2.
Characteristics of included studies
| Authors (Country) | Sample (N) | Age | Other sample characteristics | Cannabinoid Info | QST Sensory Domain | Risk of Bias | Effect |
|---|---|---|---|---|---|---|---|
| Studies Including Healthy Adults Only | |||||||
| Cooper et al., 2013 (USA) | Healthy Adults (30) | 27 (Mean) |
Male: 50% Ethnicity: Black (67%), White (20%), Other (13%) Cannabis Use History: Current use |
Type:
1. Inhaled Cannabis 2. Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 1. 1.98%, 3.56% THC in 800mg cigarettes; 3–7 puffs 2. 10mg, 20mg Dose-Response: Y |
Cold | 4 | Mixed (null and positive) |
| Cooper et al., 2016 (USA) | Healthy Adults (42) | 28 (Mean) |
Male: 50% Ethnicity: Black (62%), White (26%), Other (12%) Cannabis Use History: Current use of >3 cigarettes, >4 times weekly for >4 weeks prior to screening |
Type: Inhaled Cannabis Active Dose: 3.56%, 5.6% THC; puffed with 40-s intervals until cigarette 70% pyrolized Dose-Response: Y |
Cold | 4 | Mixed (null and positive) |
| Cooper et al., 2018 (USA) | Healthy Adults (18) | 29.9 (Mean) |
Male: 67% Ethnicity: Black (55%), White (28%), Other (17%) Cannabis Use History: Current user of >3 cigarettes, >4 times weekly for >4 weeks prior to screening |
Type: Inhaled Cannabis Active Dose: 5.6% THC; puffed until 800mg cigarette was 70% pyrolized Dose-Response: N |
Cold | 5 | No comparison of cannabis v. placebo; cannabis + opioid only |
| Esfandyari et al., 2007 (USA) | Healthy Adults (52) | 35.5 (Mean) |
Male: 42% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 7.5mg Dose-Response: N |
Visceral | 2 | Mixed (null and positive) |
| Greenwald & Stitzer, 2000 (USA) | Healthy Adults (13) | 18–45 (Range) |
Male: 69% Ethnicity: White (80%), Other (20%) Cannabis Use History: Current use between 3–20 cigarettes per week in the month prior to screening |
Type: Inhaled Cannabis Active Dose: 3.55% THC; 18 puffs of cigarette weighing 750–990mg Dose-Response: Y |
Heat | 3 | Mixed (null and positive) |
| Gregg et al., 1976 (USA) | Healthy Adults (15) | 24.5 (Mean) |
Male: 100% Ethnicity: White (100%) Cannabis Use History: Previous use, but not over moderate use |
Type: Synthetic Cannabinoid (IV) Active Dose: .022mg/kg, .044mg/kg Dose-Response: Y |
Mechanical, Electrical | 3 | Hyperalgesia |
| Hill et al., 1974 (USA) | Healthy Adults (31) | 21–30 (Range) |
Male: 100% Ethnicity: NR Cannabis Use History: NR |
Type: Inhaled Cannabis Active Dose: 1.4% THC, ~12mg Dose-Response: N |
Electrical | 8 | Hyperalgesia |
| Kalliomäki et al., 2012 (Sweden) | Healthy Adults (30) | 29.3 (Mean) |
Male: 100% Ethnicity: NR Cannabis Use History: Naïve |
Type: Synthetic Cannabinoid (Nabilone, capsule) Active Dose: 1, 2, or 3mg Dose-Response: Y |
Heat, Mechanical to Capsaicin | 4 | Completely null |
| Kalliomäki, et al., 2013 (Sweden) | Healthy Adults (43) | 28.1 years (Mean) |
Male: 100% Ethnicity: NR Cannabis Use History: NR |
Type: Other (AZD1940, oral) Active Dose: 400μg, 800μg Dose-Response: Y |
Heat, Mechanical to Capsaicin | 4 | Completely null |
| Karniol et al., 1975 (Brazil) | Presumably Healthy Adults (5) | 25–29 (Range) |
Male: 100% Ethnicity: NR Cannabis Use History: NR |
Type: THC:CBN (Cannabinol, liquid) Active Dose: 25mg THC, 50mg CBN, 25mg THC+12.5mg CBN, 25mg THC+25mg CNB, and 25mg THC+50mg CBN Dose-Response: Y |
Cold | 6 | Completely null |
| Kraft et al., 2008 (Germany) | Healthy Adults (18) | 23.5 (Mean) |
Male: 0% Ethnicity: NR Cannabis Use History: Naïve |
Type: THC:CBD (2:1, oral) Active Dose: 20mg total Dose-Response: N |
Heat, Electrical to Capsaicin | 2 | Mixed (null and hyperalgesia) |
| Lee et al., 2013 (UK) | Healthy Adults (12) | 24–34 (Range) |
Male: 100% Ethnicity: NR Cannabis Use History: Naïve |
Type: Synthetic Cannabinoid (NR, capsule) Active Dose: 15mg Dose-Response: N |
Mechanical to Capsaicin | 4 | Mixed (null and positive) |
| Libman & Stern, 1985 (Canada) | Healthy Adults (78) | 21 (Mean) |
Male: 0% Ethnicity: White (100%) Cannabis Use History: 50% twice monthly use for >2 years, 50% did not exceed lifetime use of 24 times |
Type: Synthetic Cannabinoid (NR, capsule) Active Dose: 10mg, 20mg Dose-Response: Y |
Mechanical | 2 | Mixed (null and positive) |
| Milstein et al., 1974 (Canada) | Healthy Adults (64) | 38.3 (Mean) |
Male: 50% Ethnicity: NR Cannabis Use History: 50% with regular use in the past year, 50% with no use in the past year |
Type: Inhaled Cannabis Active Dose: 1.5% THC; 600mg total Dose-Response: N |
Heat, Mechanical | 3 | Null |
| Milstein et al., 1975 (Canada) | Healthy Adults (31) | 32.8 (Mean) |
Male: 52% Ethnicity: NR Cannabis Use History: 50% use 2–365 times in the past year, 50% Naïve |
Type: Inhaled Cannabis Active Dose: 1.5% THC; 600mg total Dose-Response: N |
Mechanical | 4 | Positive |
| Naef et al., 2003 (Switzlerland) | Healthy Adults (12) | 26 (Mean) |
Male: 50% Ethnicity: NR Cannabis Use History: Naïve |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 20mg Dose-Response: N |
Mechanical, Heat, Electrical | 3 | Completely null |
| Naef et al., 2004 (Switzlerland) | Healthy Adults (8) | 26–50 (Range) |
Male: 50% Ethnicity: NR Cannabis Use History: Naïve |
Type: Synthetic Cannabinoid (Dronabinol, aerosol inhalation and IV) Active Dose: 0.053mg/kg Dose-Response: N |
Cold | 4 | Hyperalgesia |
| Raft et al., 1977 (USA) | Healthy Adults (10) | 18–28 (Range) |
Male: 100% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (NR, IV) Active Dose: 0.022mg/kg, and 0.044mg/kg Dose-Response: Y |
Mechanical, Electric | 6 | Completely null |
| Redmond et al., 2008 (Canada) | Healthy Adults (17) | 22.9 (Mean) |
Male: 41% Ethnicity: NR Cannabis Use History: No use >3 months prior to screening |
Type: Synthetic Cannabinoid (Nabilone, oral) Active Dose: .5mg, 1mg Dose-Response: Y |
Heat | 3 | Completely null |
| Roberts et al., 2006 (USA) | Healthy Adults (13) | 18–49 (Range) |
Male: 54% Ethnicity: NR Cannabis Use History: No recent use |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 5mg Dose-Response: N |
Heat | 3 | Completely null |
| Rukwied et al., 2003 (UK) | Healthy Adults (20) | 29 (Mean) |
Male: 50% Ethnicity: NR Cannabis Use History: NR |
Type: Other (HU210, skin patch) Active Dose: 50μl HU210 solution covering 4cm2 of skin for 24 hrs Dose-Response: N |
Heat, Mechanical | 8 | Mixed (null and positive) |
| van Amerongen et al., 2018 (Netherlands) | Healthy Adults (24) | 24 (Mean) |
Male: 52% Ethnicity: White (96%), Others (4%) Cannabis Use History: No history of illicit drug use or positive drug screen |
Type: Synthetic Cannabinoid (Namisol, capsule) Active Dose: 10mg Dose-Response: N |
Heat, Electrical, Mechanical | 2 | Mixed (null and hyperalgesia) |
| Wallace et al., 2007 (USA) | Healthy Adults (19) | 28.9 (Mean) |
Male: 58% Ethnicity: White (37%), Others (63%) Cannabis Use History: Some use in the past 6 months, but abstained 1 month prior to screening |
Type: Inhaled THC Active Dose: 2%, 4%, and 8% THC by body weight, 4 puffs Dose-Response: Y |
Cold, Heat, Mechanical | 1 | Mixed (null and hyperalgesia) |
| Walter et al., 2015 (Germany) | Healthy Adults (30) | 27.4 (Mean) |
Male: 50% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (NR, capsule) Active Dose: 20mg Dose-Response: N |
Electrical | 8 | Hyperalgesia |
| Walter et al., 2016 (Germany) | Healthy Adults (15) | 26.5 (Mean) |
Male: 53% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (NR, capsule) Active Dose: 20mg Dose-Response: N |
Chemical | 6 | Completely null |
| Zeidenberg et al., 1973 (USA) | Healthy Adults (4) | 25–29 (Range) |
Male: 100% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (NR, capsule) Active Dose: 15mg Dose-Response: N |
Heat | 3 | Significance testing results NR |
| Studies Including Patient Samples | |||||||
| Abrams et al., 2007 (USA) | Adults with symptomatic HIV-associated sensory neuropathy (50) | 48.5 (Mean) |
Male: 87% Race: White (45%), Black (38%), Hispanic (15%), Others (2%) Cannabis Use History: >5 times previous use |
Type: Inhaled Cannabis Active Dose: 3.56% THC; ~5 puffs of 0.9g cigarette Dose-Response: N |
Heat to Capsaicin, Mechanical | 3 | Mixed (null and positive) |
| Conte et al., 2009 (Italy) | Adults with secondary progressive MS (17) | 51.1 (Mean) |
Male: 29% Ethnicity: NR Cannabis Use History: Naïve |
Type: THC:CBD (Sativex, oromucosal spray) Active Dose: Each spray had 2.7mg THC and 2.5mg CBD; Max dose was 48 sprays over 24hrs Dose-Response: N |
Electrical | 4 | Mixed (null and positive) |
| Klooker et al., 2011 (Netherlands) | Adults with irritable bowel syndrome (22) | 32.5 (Mean) |
Male: 32% Ethnicity: NR Cannabis Use History: Free from use >2 months prior to screening |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 5mg, 10mg Dose-Response: Y |
Visceral | 6 | Completely null |
| Malik et al., 2017 (USA) | Adults with unexplained non-cardiac chest pain (13) | 40.8 (Mean) |
Male: 15% Ethnicity: NR Cannabis Use History: NR |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 10mg daily for 4 weeks Dose-Response: N |
Visceral | 4 | Mixed (null and positive) |
| Nurmikko et al., 2004 (UK & Belgium) | Adults with unilateral peripheral neuropathic pain and allodynia (125) | 53.4 (Mean) |
Male: 44% Ethnicity: White (97%), Others (3%) Cannabis Use History: No use 7 days prior to randomization |
Type: THC:CBD (Sativex, sublingual spray) Active Dose: Each spray had 2.7mg THC and 2.5mg CBD; Max dose was 48 sprays in 24 hours Dose-Response: N |
Mechanical | 2 | Completely positive |
| Salim et al., 2005 (Germany) | Adults with chronic neuropathic pain (21) | 50.9 (Mean) |
Male: 62% Ethnicity: NR Cannabis Use History: No current use |
Type: Other (Ajulemic acid, oral) Active Dose: 40mg first 4 days; 80mg last 3 days; 14 days total use Dose-Response: N |
Mechanical | 3 | Completely null |
| Serpell et al., 2014 (UK, Czech Republic, Romania, Belgium, and Canada) | Adults with peripheral neuropathic pain (246) | 57.3 (Mean) |
Male: 39% Ethnicity: White (99%), Others (1%) Cannabis Use History: None in the past year |
Type: THC:CBD (NR, oromucosal spray) Active Dose: Each spray had 2.7mg THC and 2.5mg CBD; Max dose was 48 sprays in 24hrs Dose-Response: N |
Mechanical | 3 | Completely null |
| Skrabek et al,. 2008 (Canada) | Adults with fibromyalgia (40) | 48.9 (Mean) |
Male: 8% Race: NR Cannabis Use History: No use of cannabis for pain management |
Type: Synthetic Cannabinoid (Nabilone, capsule) Active Dose: 0.5mg/day 1st week, 1mg/day 2nd week, 1.5mg/day 3rd week, 2mg/day 4th week Dose-Response: Y |
Mechanical | 3 | Completely null |
| Svendsen et al., 2004 (Denmark) | Adults with MS and central pain (24) | 50 (Median) |
Male: 42% Ethnicity: NR Cannabis Use History: No use within 3 months of screening |
Type: Synthetic Cannabinoid (Dronabinol, capsule) Active Dose: 2.5mg daily at start, increased by 2.5mg every other day; Max dose of 5mg BID (10mg daily total) Dose-Response: N |
Cold, Heat, Mechanical | 2 | Mixed (null and positive) |
| Wallace et al 2015 (USA) | Adults with painful diabetic peripheral neuropathy (16) | 56.9 (Mean) |
Male: 56% Race: White (44%), Black (50%), Hispanic (6%) Cannabis Use History: No use within 30 days of screening |
Type: Inhaled Cannabis Active Dose: 1%, 4%, 7% THC yielding 4mg, 16mg, 28mg THC per dosing session, respectively Dose-Response: Y |
Mechanical | 2 | Completely positive |
| Wilsey et al., 2008 (USA) | Adults with central and peripheral neuropathic pain (38) | 46 (Median) |
Male: 53% Ethnicity: White (87%), Black (3%), Hispanic (3%), Others (7%) Cannabis Use History: Previous use required, but required to abstain from use 30 days prior to participation |
Type: Inhaled Cannabis Active Dose: 3.5%, 7% THC; 9 puffs total Dose-Response: Y |
Heat, Mechanical | 1 | Completely null |
| Wilsey et al., 2013 (USA) | Adults with central and peripheral neuropathic pain (39) | 50 (Mean) |
Male: 72% Ethnicity: White (72%), Black (13%), Hispanic (8%), Others (8%) Cannabis Use History: Previous use required, but required to abstain from use 30 days prior to participation |
Type: Inhaled Cannabis Active Dose: 1.29%, 3.53% THC; 8–12 puffs total Dose-Response: Y |
Heat, Mechanical | 1 | Mixed (null and positive) |
| Wilsey et al., 2016 (USA) | Adults with injury and disease of the spinal cord (42) | 46.4 (Mean) |
Male: 69% Ethnicity: White (62%), Black (12%), Hispanic (17%), Others 9%) Cannabis Use History: Required to refrain 7 days prior to participation |
Type: Inhaled Cannabis Active Dose: 2.9%, 6.7% THC; 8–12 puffs total Dose-Response: Y |
Heat, Mechanical | 0 | Completely null |
3.1. Healthy Control Samples Only
3.1.a. Inhaled cannabis
Eight studies examined inhaled cannabis effects on QST outcomes in healthy controls. Five of the 8 studies [11,12,14,22,76] used samples of experienced cannabis users, and one study [27] did not report participants’ cannabis use status. Two studies used both experienced cannabis users and cannabis-naïve samples [49,50]. Sample sizes ranged from 13–64. All effects are in comparison to placebo (cannabis that contained 0% THC). THC concentration ranged from 0 to 8% and reported THC doses ranged from 0 to 31 mg. Detailed information on THC concentration, dose, and cannabis use history is presented in Table 2.
3.1.a.1. Heat
Three studies probed inhaled cannabis-related analgesia via heat stimuli [22,50,76]. One study found an analgesic effect on heat pain threshold [22]. The other studies showed null effects on heat pain threshold and heat hyperalgesia induced through intradermal capsaicin [50,76].
3.1.a.2. Cold
Four studies examined inhaled cannabis effects on cold pain responses [11,12,14,76]. Two of these studies reported analgesic effects on cold pain threshold and tolerance [12,14]. The remaining studies reported null results on cold pain threshold [11,76] and tolerance [11].
3.1.a.3. Mechanical
Three studies conducted QST under inhaled cannabis using mechanical stimuli [49,50,76]. Two studies reported analgesic effects on at least one mechanical pain stimulus [49,76]. The other study reported null effects on pressure pain threshold [50].
3.1.a.4. Electrical
Only one study examined the effects of inhaled cannabis on responses to electrical stimuli. Hill [27] reported hyperalgesic effects on tests of electrical pain threshold and tolerance.
3.1.a.5. Dose-Response
Three of the 8 studies evaluated dose-response effects of inhaled cannabis and found significant dose-response relationships [12,22,76]. Cooper et al [12] found analgesic effects on cold pain tolerance, but not threshold, comparing a low dose (1.98% THC; 3–7 puffs; 11 mg) to placebo. Greenwald and Stitzer [22] found an analgesic effect only at the high dose (3.55% THC; 9 puffs). Wallace et al. [76] found a null effect at a low dose (2% THC by weight; 4 puffs), analgesic effect at an intermediate dose (4% THC by weight; 4 puffs), and hyperalgesic effect at a high dose (8% THC by weight; 4 puffs).
3.1.a.6. Conclusion
Five out of 8 (62.5%) studies demonstrated an analgesic benefit of inhaled cannabis on at least one QST outcome measure. These positive findings should be interpreted against the backdrop of several null results and inconsistencies – both within and across studies – in the type of QST response affected and the dose at which analgesia was observed. Hyperalgesia was observed in two studies, and in one study this was observed at a high dose, when lower doses in the same study produced null and analgesic effects. This suggests an inverted-U dose-response relation between inhaled cannabis and QST outcomes. Also, the majority of studies were based upon experienced cannabis users. No study examined the analgesic effects of inhaled cannabis on chemical or visceral stimuli.
3.1.b. Synthetic Cannabinoids
A total of 15 studies examined the effects of synthetic cannabinoid pharmaceuticals (dronabinol; nabilone, a synthetic cannabinoid analog with greater potency and bioavailability than dronabinol), vaporized synthetic cannabinoids, or intravenous synthetic cannabinoids on QST responses in healthy adults. Among 15 studies, three used samples of experienced cannabis users [12,23,42], four used cannabis-naïve samples [33,40,52,53], three did not allow for recent use of cannabis [4,31,58], and five did not report participants’ cannabis use status [18,34,57,79,87]. Sample sizes ranged from 8–78. Of the 15 studies, one study administered the synthetic cannabinoids through aerosol inhalation, three studies used intravenous administration, and the rest of the studies used oral administration. In terms of dose, the range of dronabinol dose was 5 to 20mg, and the range of nabilone dose was 0.5 to 3mg. The dose range for intravenous administration was .022mg/kg body weight to .044mg/kg bodyweight, and dosing for aerosol administration was .052mg/kg body weight.
3.1.b.1. Heat
Five studies examined the effects of synthetic cannabinoids on responses to heat stimuli [4,31,33,52,58]. All five studies reported null results on all heat QST outcomes. One study [87] did not report statistical tests.
3.1.b.2. Cold
Six studies examined synthetic cannabinoid effects on responses to cold stimuli [4,12,31,34,52,53]. Only one study reported an analgesic effect [12]. Four studies reported null effects [4,31,34,52], and one study reported a hyperalgesic effect [53].
3.1.b.3. Mechanical
Seven studies examined synthetic cannabinoid effects on response to mechanical stimuli [4,23,33,40,42,52,57]. van Amerongen et al. [4] reported hyperalgesic effect and the rest of the studies reported null effects across outcome measures.
3.1.b.4. Electrical
Five studies examined responses to electrical stimuli under synthetic cannabinoids [4,23,52,57,79]. Two reported null effects [23,57], and three reported hyperalgesic effects [4,52,79].
3.1.b.5. Chemical
One study chemically induced trigeminal excitation and found a null effect on pain outcomes [78].
3.1.b.6. Visceral
One study used colonic distension following synthetic cannabinoids administration [18] and found no effect on visceral pain threshold, but a hyperalgesic response to increasing distention pressure was observed.
3.1.b.7. Dose-Response
Five out of the 15 (33.3%) studies assessed dose-response effects of synthetic cannabinoids [12,23,31,33,34]. Only one study reported a positive effect of high-dose dronabinol (20mg) on cold pain threshold, and positive effects of high and low (10mg) doses on cold pain tolerance [12].
3.1.b.8. Conclusion
Only one out of 15 studies (6.7%) supported an analgesic benefit of synthetic cannabinoids on QST outcomes, which was a dose-dependent response [12]. Importantly, all participants in this one positive study were daily cannabis smokers, a methodological feature that distinguishes the study from most other studies reviewed. Although five other studies failed to show an analgesic response to synthetic cannabinoids using the cold pressor task, none included daily cannabis users. From these data, then, we can conclude that synthetic cannabinoids, in general, are unlikely to alter acute pain sensitivity to QST in healthy adults who are not frequent cannabis users. Because most of the studies showing hyperalgesic effects did not report cannabis use status, it is unclear how use at the time of study participation contributed to results. Overall, however, the bulk of the evidence (60%; 9 out of 15) for synthetic cannabinoid formulations suggests an absence of any effect on QST outcomes and a considerable proportion of studies (33.3%; 5 out of 15) showed a hyperalgesic effect, especially in response to an electrical stimulus.
3.1.c. Combined THC and CBD formulations
Only one study used a combined THC/CBD agent to examine QST outcomes in 18 healthy adults [37]. This study reported on QST outcomes across several sensory domains. The sample was based upon 18 cannabis-naïve participants. In this study, the unspecified 20mg pharmaceutical quality cannabis plant extract that contained THC and CBD in the ratio of 2:1 was administered orally.
3.1.c.1. Heat
Kraft and colleagues [37] reported null effects on heat pain threshold and tolerance following THC/CBD formulation administration.
3.1.c.2. Mechanical
Kraft et al. [37] also reported null effects on pin prick and brush-induced pain in both non-sensitized and sensitized skin.
3.1.c.3. Electrical
Finally, Kraft et al. [37] found mixed effects on electrical stimuli; whereas there were null effects on electrical pain threshold and tolerance on both non-sensitized skin and the skin area with secondary hyperalgesia, there was a hyperalgesic effect on sensitized skin stimulated at 250 Hz.
3.1.c.4. Dose-Response
Kraft and colleagues [37] did not examine dose-response effects.
3.1.c.5. Conclusion
It is difficult to provide a meaningful conclusion for this domain, as only one study was available. The pattern of responses was not consistent across sensory domains tested in the study. No study examined the analgesic effects of combined THC/CBD formulations on a cold, chemical, or visceral stimulus.
3.1.d. Other Endocannabinoid Modulators
Two studies examined the effects of other endocannabinoid modulators on QST outcomes in healthy adults [32,59]. Neither study reported participants’ cannabis use history. Sample sizes ranged from 20–43. Kalliomaki and colleagues [32] used 400 and 800μg of oral solution of AZD1940– a novel peripherally restricted cannabinoid CB1/CB2 receptor agonist. Rukwied and colleagues [59] administered four 12 mm (in diameter) skin patches soaked with 50μl cannabinoid receptor ligand HU210.
3.1.d.1. Heat
Rukwied and colleagues [59] reported analgesic effects of HU210 (i.e., a cannabinoid CB1/CB2 receptor agonist) on responses to painful heat stimulation after capsaicin injection. Kalliomaki and colleagues [32], on the other hand, reported a null effect on heat pain threshold following administration of AZD1940–a novel peripherally restricted cannabinoid CB1/CB2 receptor agonist.
3.1.d.2. Mechanical
On tests of brush allodynia after intradermal capsaicin injection, the HU210 produced an analgesic effect on brush allodynia [59], whereas the AZD1940 was associated with null effects [32].
3.1.d.3. Dose-Response
Neither study examined dose-response relationships.
3.1.d.4. Conclusion
The two reviewed studies found mixed effects of other endocannabinoid modulators on QST outcomes, which is likely partly attributable to vast differences in mechanisms of action between the HU210 agent (i.e., a CB1 receptor agonist) and AZD1940 formulation (i.e., a peripherally acting CB1/CB2 receptor agonist). Notably, positive outcomes reported by Rukwied and colleagues [59] were only obtained at certain assessment time points in a repeated measures design with a small sample (N=20). The analgesic evidence of non-THC/CBD endocannabinoid modulators is unclear with these current data.
3.2. Patient Samples Included
3.2.a. Inhaled cannabis
Five studies used inhaled cannabis to measure changes in calibrated pain responses among patients with primary neuropathic pain [80,81] and neuropathy secondary to diabetes [77], HIV [16], and spinal cord injury or disease [82]. Inclusion criteria in these studies allowed for both cannabis-naïve participants and those with positive cannabis use history; however, all stipulated that participants needed to refrain from use at least 7–30 days before enrollment. Sample sizes ranged from 16–50. All effects are compared to placebo (0% THC). THC concentrations ranged from 0 to 7% for total THC doses of 0 to 34mg.
3.2.a.1. Heat
Four studies used painful heat stimulation in patients with neuropathic pain [16,80–82]. All studies reported null effects.
3.2.a.2. Mechanical
Five studies used painful mechanical stimulation in patients with neuropathic pain [16,77,80–82]. Only one of these studies reported a positive effect (i.e., brush and von Frey hair evoked pain; [77]), with null effects across remaining studies.
3.2.a.3. Dose-Response
Four out of the 5 studies (80%) evaluated dose-response effects of inhaled cannabis [77,80–82]. Only one study reported a significant dose-response effect of cannabis [77]. Specifically, the high dose (7% THC; 3 puffs; 28mg) showed the greatest reduction in brush and von Frey hair evoked pain, followed by the medium dose (4% THC; 3 puffs; 16mg), low dose (1% THC; 3 puffs; 4mg), and placebo (0% THC; 3 puffs; 0mg; [77]).
3.2.a.4. Conclusion
The bulk of the evidence (80%; 4 out of 5 studies) suggests null effects of inhaled cannabis on QST outcomes among patients with neuropathic pain. History of cannabis use was mixed among these samples. The lone analgesic finding from Wallace and colleagues [77] was qualified by a small sample (N=16) and dose-dependent effects that deviated from prior literature. Whereas a previous study had shown hyperalgesia at a high dose of inhaled cannabis in healthy subjects [76], Wallace et al. [77] found analgesia exclusively at the high dose. No study examined the analgesic effects of inhaled cannabis on cold, electrical, chemical, or visceral pain stimuli. Hyperalgesic effects were not reported among these studies.
3.2.b. Synthetic Cannabinoids
Four studies examined QST outcomes among patients with clinical pain after synthetic cannabinoid (i.e., dronabinol, nabilone) administration. Clinical conditions studied under these conditions include Multiple Sclerosis (MS; [72]), fibromyalgia [65], Irritable Bowel Syndrome (IBS; [36]), and functional chest pain disorders [47]. Participants in these studies were generally asked to abstain from cannabis use 7–60 days prior to study participation. Sample sizes ranged from 13–40 participants. The dose range for dronabinol was 2.5mg daily for one week to 10mg daily for four weeks, and the lone nabilone study used a 2mg daily dose for 7 days [65].
3.2.b.1. Heat
Only one study examined the effects of synthetic cannabinoids on heat pain and found null results in patients with MS and central pain [72].
3.2.b.2. Cold
Svendsen and colleagues [72] also found null effects using noxious cold stimuli in their MS sample.
3.2.b.3. Mechanical
Of the two studies that examined mechanical pain outcomes under synthetic cannabinoids [65,72], only one showed an analgesic effect [72]. Specifically, patients with MS and central pain had higher pressure pain thresholds after cannabinoid use; however, there were no differences in punctate temporal summation and brush allodynia. There were also null effects on the number of tender points and pressure pain threshold in fibromyalgia patients following synthetic cannabinoids administration [65].
3.2.b.4. Visceral
Two studies used visceral stimuli to examine calibrated pain responses [36,47]. Both studies found null results among patients with IBS or functional chest pain.
3.2.b.5. Dose-Response
Two out of the 4 studies assessed dose-response effects of synthetic cannabinoids [36,65]. None showed dose-response effects.
3.1.b.6. Conclusion
Only one out of four studies (25%) supported synthetic cannabinoid-related analgesia during systematized, painful stimulation. No study examined the effects of synthetic cannabinoids on electrical or chemical pain stimuli. Notably, the study providing evidence for synthetic cannabinoid-related analgesia reported one significant finding (i.e., increased pressure pain threshold in MS patients) across seven QST measures tested, and the authors did not correct for multiple testing. Furthermore, the QST assessment was conducted before and after an extended treatment period (approximately 3 weeks) rather than in an acute administration design. Hyperalgesic effects were not reported among these studies. While most studies in this category required individuals to abstain from cannabis use for a period of time before participation, previous cannabis use history was not well-documented.
3.2.c. Combined THC and CBD formulations
Three studies used a combined THC:CBD agent (i.e., Sativex) to examine QST responses in patients with clinical pain, including neuropathic pain [64] or MS [10]. One study used cannabis-naïve participants [10], and two studies required that participants refrained from cannabis use 7 days [55] or one year [64] prior to study participation. Sample sizes ranged from 17 to 246 participants. The range of Sativex doses was 2.7mg to 129.6mg THC and 2.5mg to 120mg CBD.
3.2.c.1. Mechanical
Two studies in patients with neuropathic pain examined responses during delivery of mechanical stimuli [55,64]. One study found analgesic effects on brush and punctate allodynia [55], but null effects were observed on these outcomes in the other study [64].
3.2.c.2. Electrical
Only one study used painful, electrical stimuli to examine pain outcomes in patients with MS. Specifically, the flexion reflex – which assesses spinal and supraspinal pain pathways involved in pain control – was used as an electrical QST outcome. The authors found positive effects on RIII threshold (i.e., pain threshold) and reflex area (i.e., level of pain perception; [10]).
3.2.c.3. Dose-Response
None of these studies examined dose-response effects.
3.2.c.4. Conclusion
Two out of three studies (66.7%) supported analgesic benefits of combined THC/CBD formulations during QST among individuals with clinical pain. However, these positive findings should be interpreted with caution because (1) only three studies were available for two different QST modalities; and (2) none of the studies tested dose-response relationships. Hyperalgesic effects were not reported among these studies. Prior experience with cannabis use and sample sizes greatly varied across studies.
3.2.d. Other Endocannabinoid Modulators
One study examined the effects of a non-THC/CBD endocannabinoid modulator in patients with neuropathic pain who did not report cannabis use at the time of the study.
3.2.d.1. Mechanical
Salim and colleagues [62] reported null effects on pain responses to mechanical stimuli (i.e., von Frey hair-evoked pain) following ajulemic acid administration among patients with neuropathic pain.
3.2.d.2. Dose-Response
This study did not examine dose-response relationships.
3.2.d.3. Conclusion
It is difficult to provide a meaningful conclusion for this domain based on one study that tested effects on 21 participants.
4. Discussion
Our systematic review of 39 placebo-controlled studies found a lack of consistent evidence for beneficial cannabinoid effects on QST responses in comparison to placebo among samples with and without CNCP. This finding is commensurate with results from a recent systematic review and meta-analysis of studies in healthy volunteers [74] and expands this work by examining studies with a broader battery of QST measures and those including CNCP samples. Further, our findings from studies of experimentally induced pain are consistent with conclusions of numerous systematic reviews of the clinical efficacy of cannabinoids for CNCP, which have generally shown weak and inconsistent effects of cannabinoids on measures of clinical pain [3,6,8,17,19,25,44,45,48,69].
As highlighted in previous reviews, many studies examining cannabinoid-related analgesia have had relatively small sample sizes and have weak to moderate methodological rigor [25,69]. These concerns make it difficult to conclude whether potential analgesic effects from cannabinoids have been masked by study limitations, only occur under specific circumstances for individuals with specific characteristics, or truly do not outperform placebo responses. The studies reviewed here generally were deemed to have low-to-moderate risk of bias. However, heterogeneity in research design and methods across studies has substantially limited the potential to establish a reliably reproducible cannabinoid analgesic effect using QST. Prospective studies should select key study design elements (e.g., see Table 3) that promote rigor and reproducibility. A unique contribution of the present review is its inclusive focus on multiple cannabinoids and routes of administration, dose-response effects, a wide range of QST stimuli, and a differentiation of results obtained from healthy participants versus patients with CNCP. We structured our review to identify patterns in the extant literature that may inform the development of future studies. Recommendations based on our observations are described below and summarized in Table 3.
Table 3.
Consistent study limitations and recommendations for future work examining cannabinoid-related analgesia
| Category | Limitations | Recommendations |
|---|---|---|
| Study Sample | 1. Few studies in clinical populations 2. Poor characterization of psychosocial mediators/moderators |
1. Focus on clinical pain conditions, rather than healthy controls 2. Characterize sex differences 3. Measure the impact of cannabis use history 4. Examine the influence of pain catastrophizing and negative affect |
| Cannabinoid Agents | 1. Limited reports on dose-response relationships 2. Limited work on non-THC agents 3. Poor characterization of pharmacodynamic profiles |
1. Examine dose-response relationships 2. Categorize “low”, “medium”, and “high” doses consistent with previous literature to avoid heterogeneity 3. Report constituent effects in plant-based cannabis or combined formulations |
| QST Approach | 1. Wide heterogeneity in QST battery 2. Limited tests of central sensitization 3. Poor control for multiple tests and outcome measures |
1. Conduct QST batteries restricted to empirically-selected and clinically-relevant tests and outcome measures 2. Include dynamic QST protocols (e.g., conditioned pain modulation, temporal summation) 3. Use multiple comparisons corrections 4. Complete QST in both an area of clinical pain and non-painful body site |
4.1. Differences in Healthy Subjects versus CNCP
The overall pattern of findings did not substantively vary between healthy subjects and patients with CNCP. Examination of the literature based on sample type revealed two main observations. First, CNCP studies—particularly those involving patients with neuropathic pain—more frequently employed mechanical tests of allodynia relative to studies in healthy controls. Cannabinoid administration, though, did not consistently produce analgesia for allodynia tests, and positive findings were observed under unique experimental contexts that have not yet been reproduced. In fact, the evidence is so variable that we can only conclude that any observable experimental effect of cannabinoid analgesia involving QST stimuli is likely to be nuanced and not broadly generalizable. Second, most studies testing cannabinoid-related analgesia via QST in clinical samples used patients with neuropathic pain or patients with MS and central pain. Evidence for efficacy of inhaled cannabis, synthetic cannabinoids (dronabinol), and other endocannabinoid modulators (HU210 and AZD1940) was limited for patients with neuropathic pain or MS; however, there was modest evidence for efficacy of combined THC/CBD formulations in a limited number of studies.
Recommendations:
The evidence gathered to date does not suggest that basic tests of cannabinoid analgesia to QST stimuli are stronger, more consistent, or more informative when conducted among healthy subjects compared to patients. In addition to dose-escalating studies in healthy volunteers, future research efforts may focus on rigorous, reproducible study designs in well-characterized clinical samples that help answer specific clinical research questions. Future research should also determine whether there are differences in the endogenous function of the endocannabinoid system or processing of exogenous cannabinoids as a function of chronic pain status.
4.2. Differences across Cannabinoid Compounds
The term “cannabis” is commonly used as an umbrella term that refers to a broad range of compounds from the cannabis plant and its synthetic derivatives. These compounds have different routes of administration and vary substantially in pharmacokinetic and pharmacodynamic properties [13,29,30,75]. Studies inconsistently controlled for factors (e.g., calorie intake, caffeine consumption) that might affect the pharmacodynamic properties of cannabinoids. Our literature search revealed studies that could be categorized across four broad classes of cannabinoids: cannabis plant (smoked or vaporized), synthetic cannabinoid formulations, combined THC/CBD formulations, and other endocannabinoid modulators.
For inhaled, whole plant, cannabis, the majority of studies with healthy adults observed significant analgesia on QST outcomes. However, only one of five studies in CNCP participants demonstrated analgesia. Dose-response evaluation in these studies suggest an inverted U-shape response to dose, but dose calculations for inhaled botanical cannabis products can be challenging and were not able to be calculated in all studies. Additional research, particularly using methods in which dose delivery can be more carefully controlled, is needed to reconcile the differences in outcomes between healthy and CNCP populations and to identify target doses most likely to be effective for analgesia.
The bulk of the evidence suggests that synthetic cannabinoids products, including dronabinol and nabilone, do not provide analgesia to QST stimuli in either healthy subjects or patients with CNCP. The two studies (out of a total of 19) that did reveal an analgesic effect of synthetic cannabinoids were rigorously conducted, but each was a small study (Ns ≤ 30) and had notable methodological or analytic caveats that will require direct replication, including use of a daily cannabis smoking sample [12] and an extended treatment design in MS patients [72].
THC/CBD hybrid compounds and other endocannabinoid modulators were sparsely studied among healthy subjects and revealed mixed findings. Kraft et al. [37] conducted the most comprehensive study of THC/CBD in healthy subjects and reported null findings across QST responses to heat, mechanical, and electrical stimuli. The absence of analgesic effects across these tests, however, is qualified by the fact that the study was potentially underpowered to detect an effect (N=18). Alternatively, there was somewhat consistent, positive evidence in support of THC/CBD’s analgesic effect on QST measures in CNCP patients (two of three studies), but inter-study heterogeneity precludes firm conclusions about the potential reproducibility of observed effects.
Recommendations:
Taken together, the available evidence does not support a consistent trend for QST-examined analgesic responses to synthetic cannabinoids in either healthy subjects or patients with chronic pain. Future studies are encouraged to (1) compare effects among cannabis-naïve and daily cannabis users, given the imbalance of studies reporting positive evidence for analgesia among daily users and adverse effects for individuals who were naïve to cannabis; (2) examine agents that combine THC and CBD, given that there was a trend for analgesia in two out of three studies among CNCP patient populations; (3) establish dose-response relationship and improve dose measurement for inhaled cannabis studies; and (4) use extended dosing paradigms as a comparison to acute dosing paradigms. It is possible that cannabinoid effects on the peripheral and/or central processing of noxious stimuli differ as a function of dose quantity and/or frequency. Further, future research should potentially prioritize THC/CBD formulations to examine cannabinoid-induced changes in pain processing among neuropathic pain patients, but it is unclear whether similar mechanisms are affected across other clinical pain conditions.
4.3. Differences across QST Measures
QST approaches used in the reviewed literature varied widely. Between and within studies, there were generally inconsistent outcomes across QST approaches, and many studies did not control for multiple comparisons. Only two notable patterns emerged. First, although most studies did not show synthetic cannabinoid-related analgesia, most positive findings were observed in tests of cold pain sensitivity. Second, several studies, primarily in healthy controls, reported hyperalgesic responses to electrical stimulation.
It is unclear why these two sensory domains showed these trends. Regarding observed hyperalgesia, QST responses are generally known to be influenced by a wide range of individual difference factors, such as ethnicity and affective disposition [15]. It is possible that such factors could have contributed to the hyperalgesic response to cannabis, but most studies were underpowered to explore these possibilities through moderation analysis. Additional factors, such as cannabis use history and participants’ sex, may have also played a role in promoting hyperalgesia; indeed, three of the hyperalgesic effects were observed in cannabis naïve samples [4,37,53], and one study examining sex differences found that men exhibited greater cannabis-induced analgesia than women [14]. Another mechanistic possibility might be binding of exogenous cannabinoid ligands to CB1 and CB2 receptors, especially in frontolimbic regions. Specifically, CB1 receptors are pervasively expressed along limbic, nociceptive, descending pain modulatory pathways [2]. Exogenous cannabinoids can impart both excitatory and inhibitory responses, resulting in complex biochemical reactions that yield pro- or anti-nociceptive outcomes [56,83].
Recommendations:
Researchers are encouraged to select QST approaches that are especially relevant to the clinical population in question (e.g., brush/von Frey tests in patients with neuropathic pain, rectal balloon distension in patients with IBS, pressure pain in patients with fibromyalgia) or focus on dynamic QST measures thought to measure central sensitization. Multiple comparisons corrections should be considered when administering a larger QST battery. Finally, future studies should critically evaluate the roles of sex, ethnicity, and affective-motivational processes to aid in interpretation of results. None of the studies with clinical samples reported analyses that linked QST changes with changes in clinical pain ratings. Future research should examine whether any specific QST approaches have specific clinical relevance for capturing cannabinoid-related analgesia.
4.4. Dose-Response Findings
Nearly half of the studies (44%) evaluated dose-response effects and findings varied both in magnitude and direction, with some studies showing the strongest analgesic effects at the highest dose [22] and others showing the stronger effects at lower doses [12,76]. More than half of the studies (53%) that tested dose-response effects failed to find any response variation across doses [31–34,36,57,65,81,82], and patterns of findings were not substantively different between cannabinoid compounds or QST modalities. Notably, to date, no study using THC/CBD formulations has examined dose-response effects.
Interpretation of these findings is challenged by several factors that can be addressed in future research. Importantly, dose has not been clearly and consistently operationalized across studies to permit reproducible research. For example, “low,” “moderate,” and “high” dose designations have typically been used to reference THC content, but these designations may not be comparable across compounds, or even within studies using the same compound due to methodological challenges in consistent/complete dose delivery (e.g. using a paced puffing procedure for inhaled cannabis is subject to titration via puff topography), as well as potential individual differences in drug absorption and metabolism [29]. Additionally, with respect to inhaled cannabis, the true “dose” of cannabis flower likely involves a complex interaction of a variety of constituent compounds with THC, and the pharmacodynamics and kinetic profile of these interactions is not well understood. Thus, although the extant literature provides a signal that analgesic responses to cannabinoid administration may be dose dependent, the strength of that evidence across studies is weak and limited by poor dose operationalization and experimental control.
Recommendations:
Future studies should critically examine dose-response relationships and match doses to previous work to reduce heterogeneity. It should be also noted that concentration and the pharmacodynamics and pharmacokinetic properties of drugs largely depends on metabolism, which substantially varies across individuals [70,84]. To better understand dose-response, studies should also consider examining individual differences in metabolism of cannabinoids, and how they are associated with the level of analgesic effects.
4.5. Strengths and Limitations
One strength of this review is its thorough assessment of the evidence for cannabinoid-related analgesia to systematized pain stimuli [38,73]. Another strength is the inclusion of studies that used clinical pain samples, thus providing a clinically-relevant expansion on De Vita and colleagues’ review. Despite these strengths, there were several notable limitations. First, the data gathered did not permit a meta-analysis. De Vita and colleagues [74] previously conducted a meta-analysis using studies on experimental pain in the context of cannabis in healthy adults. However, because our research questions were broad and inclusive of a wide range of studies, there was substantial heterogeneity of study designs, populations, cannabinoid compounds, and QST outcomes that precluded a valid meta-analysis. Second, we determined analgesic, null, or hyperalgesic evidence using a p-value cutoff because this was the most consistently available metric across studies. However, this may not accurately reflect actual clinical effects in an underpowered or methodologically flawed study [5]. Third, Covidence, a web-based systematic review platform that we used in the present study, does not retain information on voting history of abstract screening. Hence, we were not able to calculate inter-rater reliability (i.e., Cohen’s Kappa coefficient) for the agreement for abstract inclusion. However, we note that any conflicts were resolved by another author (either PHF or ENP) as a tie-breaking vote, and then discussed as a group to form consensus. Finally, we could not provide insights on dynamic QST measures, given limited evidence.
4.6. Conclusion
Consistent with previous reviews, the present systematic review found poor consistency of findings for the efficacy of cannabinoids as an analgesic agent. Limitations in reviewed studies hinder conclusions about the efficacy of cannabinoids as analgesic agents. Future work should focus on clinical populations, examine dose-response relationships, and better characterize mediating and moderating factors.
Acknowledgement
Funding for this research was supported by the NINDS T32 NS070201 (for CJM’s postdoctoral training), NIDA F32DA049393 (awarded to CJM), NIDA K23DA035915 (awarded to PHF), NHLBI F32HL143941 (awarded to JEL), and NIDA R01DA043075 (awarded RV). We would like to acknowledge Mr. Hunter Land for his insightful comments on portions of this manuscript.
Footnotes
Conflict of Interest
Authors CJM, JEL, CMC, JGN, DD, CCN, and PHF do not have a conflict of interest. ENP is currently employed by Canopy Growth Corporation, a cannabis company. However, Canopy Growth Corporation had no role on this manuscript besides supporting ENP’s time. Furthermore, Canopy Growth Corporation did not supply product for any of the studies included in the review. RV has received consulting fees or honoraria from Zynerba Pharmaceuticals, Canopy Health Innovations, Battelle Memorial Institute, and Brain Solutions Inc.
References
- [1].Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007;10:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alexander SPH, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol 2007;152:602–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, Lindblad AJ, Korownyk C, Kolber MR. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64:e78–e94. [PMC free article] [PubMed] [Google Scholar]
- [4].van Amerongen G, Siebenga P, de Kam ML, Hay JL, Groeneveld GJ. Effect profile of paracetamol, Δ9-THC and promethazine using an evoked pain test battery in healthy subjects. Eur J Pain 2018;22:1331–1342 [DOI] [PubMed] [Google Scholar]
- [5].Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567:305–307. [DOI] [PubMed] [Google Scholar]
- [6].Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, Abrams DI, Prasad H, Wilsey B, Indyk D. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015;16:1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].“Misha Backonja M, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede R-D, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013;154:1807–1819. [DOI] [PubMed] [Google Scholar]
- [8].Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache 2015;29:7–14. [DOI] [PubMed] [Google Scholar]
- [9].Chesher GB, Dahl CJ, Everingham M, Jackson DM, Marchant‐Williams H, Starmer GA. The effect of cannabinoids on intestinal motility and their antinociceptive effect in mice. Br J Pharmacol 1973;49:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, Giacomelli E, Gabriele M, Aragona M, Tomassini V, Pantano P, Pozzilli C, Inghilleri M. Cannabinoid-induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain 2009;13:472–477. [DOI] [PubMed] [Google Scholar]
- [11].Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 2018;43:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology 2013;38:1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend 2009;103:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cooper ZD, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend. 2016;167:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007;68:515–521. [DOI] [PubMed] [Google Scholar]
- [17].Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can Fam Physician 2015;61:e372–e381. [PMC free article] [PubMed] [Google Scholar]
- [18].Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Liver Physiol 2007;293:G137–G145. [DOI] [PubMed] [Google Scholar]
- [19].Fitzcharles M, Ste‐Marie PA, Häuser W, Clauw DJ, Jamal S, Karsh J, Landry T, Leclercq S, Mcdougall JJ, Shir Y. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Am J Physiol Liver Physiol 2007;293:G137–G145. [DOI] [PubMed] [Google Scholar]
- [20].Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964;86:1646–1647. [Google Scholar]
- [21].Geber C, Klein T, Azad S, Birklein F, Gierthmühlen J, Huge V, Lauchart M, Nitzsche D, Stengel M, Valet M. Test–retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain 2011;152:548–556. [DOI] [PubMed] [Google Scholar]
- [22].Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend 2000;59:261–275. [DOI] [PubMed] [Google Scholar]
- [23].Gregg JM, Small EW, Moore R, Raft D, Toomey TC. Emotional response to intravenous delta9tetrahydrocannabinol during oral surgery. J Oral Surg 1976;34:301–313. [PubMed] [Google Scholar]
- [24].Grosen K, Fischer IWD, Olesen AE, Drewes AM. Can quantitative sensory testing predict responses to analgesic treatment? Eur J Pain 2013;17:1267–1280. [DOI] [PubMed] [Google Scholar]
- [25].Häuser W, Fitzcharles M-A, Radbruch L, Petzke F. Cannabinoids in pain management and palliative medicine: An overview of systematic reviews and prospective observational studies. Dtsch Arztebl Int 2017;114:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci 1990;87:1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hill SY, Goodwin DW, Schwin R, Powell B. Marijuana: CNS depressant or excitant? Am J Psychiatry 1974;131:313–315. [DOI] [PubMed] [Google Scholar]
- [28].Hudson R, Puvanenthirarajah N. Cannabis for pain management: Pariah or panacea? Univ West Ont Med J 2018;87:58–61. [Google Scholar]
- [29].Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007;4:1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hunault CC, Böcker KBE, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology 2014;231:4723–4733. [DOI] [PubMed] [Google Scholar]
- [31].Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin 2008;24:1017–1024. [DOI] [PubMed] [Google Scholar]
- [32].Kalliomäki J, Annas P, Huizar K, Clarke C, Zettergren A, Karlsten R, Segerdahl M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 2013;40:212–218. [DOI] [PubMed] [Google Scholar]
- [33].Kalliomäki J, Philipp A, Baxendale J, Annas P, Karlsten R, Segerdahl M. Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 2012;39:336–342. [DOI] [PubMed] [Google Scholar]
- [34].Karniol IG, Shirakawa I, Takahashi RN, Knobel E, Musty RE. Effects of Δ9-tetrahydrocannabinol and cannabinol in man. Pharmacology 1975;13:502–512. [DOI] [PubMed] [Google Scholar]
- [35].Keedy NH, Keffala VJ, Altmaier EM, Chen JJ. Health locus of control and self-efficacy predict back pain rehabilitation outcomes. Iowa Orthop J 2014;34:158–65. [PMC free article] [PubMed] [Google Scholar]
- [36].Klooker TK, Leliefeld KEM, Van Den Wijngaard RM, Boeckxstaens GEE. The cannabinoid receptor agonist delta- 9- tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil 2011;23:30–e2. [DOI] [PubMed] [Google Scholar]
- [37].Kraft B, Frickey N, Kaufmann R, Reif M, Frey R, Gustorff B, Kress H. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008;109:101–110. [DOI] [PubMed] [Google Scholar]
- [38].kumar Reddy KS, Naidu MUR, Rani PU, Rao TRK. Human experimental pain models: a review of standardized methods in drug development. J Res Med Sci 2012;17:587–595. [PMC free article] [PubMed] [Google Scholar]
- [39].Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci 2017;19:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, Menon DK, Tracey I. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 2013;154:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J pain 2012;13:936–944. [DOI] [PubMed] [Google Scholar]
- [42].Libman E, Stern MH. The effects of Δ9 THC on cutaneous sensitivity and its relation to personality. Pers Individ Dif 1985;6:169–174. [Google Scholar]
- [43].Lötsch J, Weyer-Menkhoff I, Tegeder I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain 2018;22:471–484. [DOI] [PubMed] [Google Scholar]
- [44].Lynch ME, Campbell F. Cannabinoids for treatment of chronic non‐cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 2011;72:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol 2015;10:293–301. [DOI] [PubMed] [Google Scholar]
- [46].Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010;150:439–450. [DOI] [PubMed] [Google Scholar]
- [47].Malik Z, Bayman L, Valestin J, Rizvi-Toner A, Hashmi S, Schey R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- [48].Meng H, Johnston B, Englesakis M, Moulin DE, Bhatia A. Selective cannabinoids for chronic neuropathic pain: a systematic review and meta-analysis. Anesth Analg 2017;125:1638–1652. [DOI] [PubMed] [Google Scholar]
- [49].Milstein SL, MacCannell K, Karr G, Clark S. Marijuana-produced changes in pain tolerance. Experienced and non-experienced subjects. Int Pharmacopsychiatry 1975;10:177–182. [DOI] [PubMed] [Google Scholar]
- [50].Milstein SL, Maccannell KL, Karr GW, Clark S. Marijuana produced changes in cutaneous sensitivity and affect : Users and non-users. Pharmacol Biochem Behav 1974;2:367–374. [DOI] [PubMed] [Google Scholar]
- [51].Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–65. [DOI] [PubMed] [Google Scholar]
- [52].Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 2003;105:79–88. [DOI] [PubMed] [Google Scholar]
- [53].Naef M, Russmann S, Petersen-Felix S, Brenneisen R. Development and pharmacokinetic characterization of pulmonal and intravenous Delta-9-Tetrahydrocannabinol (THC) in humans. J Pharm Sci 2004;93:1176–1184. [DOI] [PubMed] [Google Scholar]
- [54].Nahman-Averbuch H, Shefi T, Schneider VJ, Li D, Ding L, King CD, Coghill RC. Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. Pain 2018;159:1202–1223. [DOI] [PubMed] [Google Scholar]
- [55].Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133:210–220. [DOI] [PubMed] [Google Scholar]
- [56].Pernía-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schüttler J. Spinal endocannabinoids and CB1 receptors mediate C-fiber–induced heterosynaptic pain sensitization. Science 2009;325:760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Raft D, Gregg J, Ghia J, Harris L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain; Psychological correlates of the analgesic response. Clin Pharmacol Ther 1977;21:26–33. [DOI] [PubMed] [Google Scholar]
- [58].Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9- tetrahydrocannabinol and morphine. Eur J Pharmacol 2006;530:54–58. [DOI] [PubMed] [Google Scholar]
- [59].Rukwied R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain 2003;102:283–288. [DOI] [PubMed] [Google Scholar]
- [60].Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use–basic prevalence and related health outcomes: A scoping review and synthesis. Int J Drug Policy 2018;52:87–96. [DOI] [PubMed] [Google Scholar]
- [61].Russo EB. Cannabidiol claims and misconceptions. Trends Pharmacol Sci 2017;38:198–201. [DOI] [PubMed] [Google Scholar]
- [62].Salim K, Schneider U, Burstein S, Hoy L, Karst M. Pain measurements and side effect profile of the novel cannabinoid ajulemic acid. Neuropharmacology 2005;48:1164–1171. [DOI] [PubMed] [Google Scholar]
- [63].Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, Etges T, Sommerville K. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy Behav 2018;88:162–171. [DOI] [PubMed] [Google Scholar]
- [64].Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, Ehler E. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014;18:999–1012. [DOI] [PubMed] [Google Scholar]
- [65].Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain 2008;9:164–173. [DOI] [PubMed] [Google Scholar]
- [66].Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, Borsook D, Edwards RR, Harris RE, Wager TD. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain 2017;18:757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sofia RD, Vassar HB, Knobloch LC. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 1975;40:285–295. [DOI] [PubMed] [Google Scholar]
- [68].Solowij N, Broyd S, Greenwood L, van Hell H, Martelozzo D, Rueb K, Todd J, Liu Z, Galettis P, Martin J. A randomised controlled trial of vaporised Δ 9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci 2019;269:17–35. [DOI] [PubMed] [Google Scholar]
- [69].Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018;159:1932–1954. [DOI] [PubMed] [Google Scholar]
- [70].Su MK, Seely KA, Moran JH, Hoffman RS. Metabolism of classical cannabinoids and the synthetic cannabinoid JWH‐018. Clin Pharmacol Ther 2015;97:562–564. [DOI] [PubMed] [Google Scholar]
- [71].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 1995;215:89–97. [DOI] [PubMed] [Google Scholar]
- [72].Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Uddin Z, MacDermid JC. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med 2016;17:1694–1703. [DOI] [PubMed] [Google Scholar]
- [74].De Vita MJ, Moskal D, Maisto SA, Ansell EB. Association of cannabinoid administration with experimental pain in healthy adults: A systematic review and meta-analysis. JAMA Psychiatry 2018;75:1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wachtel S, ElSohly M, Ross S, Ambre J, De Wit H. Comparison of the subjective effects of Δ 9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology 2002;161:331–339. [DOI] [PubMed] [Google Scholar]
- [76].Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, Abramson I. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiol 2007;107:785–796. [DOI] [PubMed] [Google Scholar]
- [77].Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015;16:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Walter C, Oertel BG, Felden L, Kell CA, Nöth U, Vermehren J, Kaiser J, Deichmann R, Lötsch J. Brain mapping-based model of Δ 9-tetrahydrocannabinol effects on connectivity in the pain matrix. Neuropsychopharmacology 2016;41:1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Walter C, Oertel BG, Lötsch J. THC may reproducibly induce electrical hyperalgesia in healthy volunteers. Eur J Pain 2015;19:516–518. [DOI] [PubMed] [Google Scholar]
- [80].Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013;14:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008;9:506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016;17:982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology 2017;124:105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yamada H, Ishii Y, Oguri K. Metabolism of drugs of abuse: its contribution to the toxicity and the inter-individual differences in drug sensitivity. J Heal Sci. 2005;51:1–7. [Google Scholar]
- [85].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–28. [DOI] [PubMed] [Google Scholar]
- [86].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–1198. [DOI] [PubMed] [Google Scholar]
- [87].Zeidenberg P, Clark WC, Jaffe J, Anderson SW, Chin S, Malitz S. Effect of oral administration of Δ9 tetrahydrocannabinol on memory, speech, and perception of thermal stimulation: Results with four normal human volunteer subjects. Preliminary report. Compr Psychiatry 1973;14:549–556. [DOI] [PubMed] [Google Scholar]