Abstract
A set of genetically engineered isogenic cell lines is developed to express either folate receptor alpha or mesothelin, and a control cell line negative for both antigens. These cell lines also express fluorescent and bioluminescent reporter transgenes. The cell lines are used to authenticate specificity and function of a T-cell biofactory, a living vector that is developed to express proportionate amounts of engineered proteins upon engaging with disease cells through their specific antigenic biomarkers. The engineered cell lines are also used to assess the cytolytic function and specificity of primary T cells engineered with chimeric antigen receptors; and the specificity of monoclonal antibodies. The strategy described can be used to generate other cell lines to present different disease-specific biomarkers for use as quality control tools.
Keywords: artificial antigen presenting cell, authentication of biologics, CAR T cells, cell engineering, quality control
With recent advances in cell-based therapeutics,[1–4] a compelling need has emerged for quality control tools for validating cell-based products for their efficacy and safety.[5] More sophisticated molecular and cell-based tools are needed[6] to address reproducibility issues in the research environment, which has been reported at a staggering $28 billion per year,[7] and to ensure consistency when considered for human use. We have recently reported[8]’ on transforming a T cell into a living vector that synthesizes proteins with desired properties in situ upon interacting with the antigen-presenting target cell (i.e., antigen-specific T-cell biofactory). We report here on the design and use of a set of genetically engineered isogenic cell lines for validating antigen-specificity of the T-cell biofactories. We show that these cell lines can additionally be used to confirm the antigen specificity and function of other biologics, such as primary T cells engineered to express chimeric antigen receptors (CAR T cells) and monoclonal antibodies. These studies present an approach that can be used for quality control and validation of engineered biological agents; a need that is now well documented.[9–12]
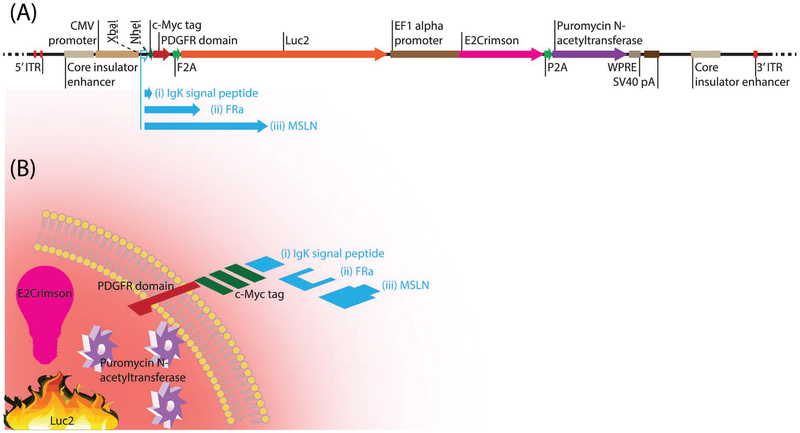
Figure 1 shows a schematic of the plasmids used to generate the isogenic cell lines with or without the surface expression of folate-receptor alpha (FRa) and mesothelin (MSLN) on cells. These constructs were introduced into a human ovarian cancer cell line, A2780cis, which does not express endogenous FRa or MSLN and thus also served as the negative control. These constructs were designed to translate the following under a constitutive promoter: i) Ig kappa signal peptide at the N-terminus of the antigen to be displayed on the surface of the cell; ii) c-Myc sequence at the C-terminus of the antigen to confirm the antigen display using an anti-c-Myc antibody; and iii) platelet derived growth factor receptor (PDGFR) transmembrane domain fused at the C-terminus of the c-Myc tag peptide to anchor the antigen-c-Myc complex to the plasma membrane. The design allowed for Ig kappa signal peptide in the empty vector (control) to be used for display of c-Myc on the cell surface, while it could be exchanged with the nucleotide sequences for the antigens (i.e., FRa or MSLN) that have their endogenous signal peptides. These vectors were used to engineer the parental FRanegMSLNneg A2780cis cell line to generate three different cell lines: i) FRanegMSLNnegLuc2-2A-E2Crimson+A2780cis; ii) FRa+MSLNnegLuc2-2A-E2Crimson+A2780cis; and iii) FRanegMSLN+Luc2-2A-E2Crimson+A2780cis. We used a PiggyBac system for stable integration of these vectors, following DNA transfection into our cell line of interest. Low transfection efficiency can be a challenge when it comes to the generation of stably transformed cell lines. In order to overcome this barrier, we utilized multiple strategies. These included 1) a PiggyBac transposon, capable of carrying a large genetic payload,[13] along with PiggyBac transposase for stable integration of the desired transgenes into the genome of A2780cis cells; 2) an antibiotic-resistant gene (puromycin N-acetyltransferase) and drug selection for ablation of non-transfected A2780cis cells and to establish a stable pool of genetically transformed cells; and 3) a far-red fluorescent protein E2Crimson (a noncytotoxic tetrameric variant of DsRed fluorescent protein; Ex = 611 nm, Em = 646 nm) for fluorescence-activated cell sorting (FACS)-based enrichment to establish a more homogenous population of A2780cis cells with enhanced expression of the desired transgenes. Therefore, by utilizing a combination of these three strategies, we were able to reproduce this process and generate three isogenic cell lines.
Figure 1.
Schematic of the plasmids and isogenic cell lines. A) PiggyBac vector plasmid modified with insert for engineering A2780cis cell line for expression of i) Luc2 and E2Crimson; ii) FRa, Luc2, and E2Crimson; iii) MSLN, Luc2, and E2Crimson. A common PiggyBac transposon vector plasmid backbone was prepared with a) puromycin N-acetyltransferase selection marker, b) Luc2 bioluminescent, and c) E2Crimson fluorescent reporter transgenes. An XbaI restriction endonuclease site was introduced at the 5’ end of the Ig kappa signal peptide nucleotide sequence and NheI site at its 3’ end. The rationale for the placement of these restriction endonuclease sites was that while Ig kappa signal peptide in the empty vector (control) could be used to display c-Myc on the cell surface, it could be exchanged with the nucleotide sequences for the antigens (i.e., FRa or MSLN). B) A2780cis cell engineered to express i) Luc2 and E2Crimson; ii) FRa, Luc2, and E2Crimson; iii) MSLN, Luc2, and E2Crimson.
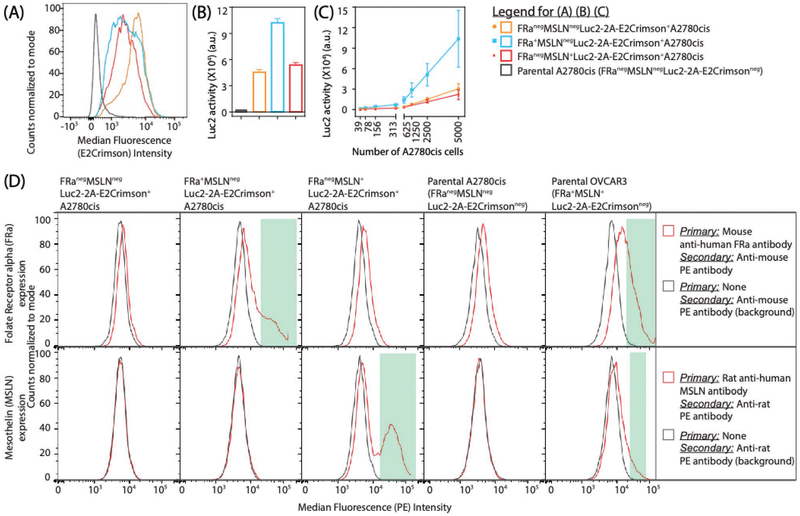
The reporter (E2Crimson and Luc2) activities and the relative expression of the target antigen (FRa and MSLN) in the engineered A2780cis cell lines were confirmed (Figure 2). Following the puromycin-based stable selection and FACS-based enrichment to generate a more homogenous cell population, the cells were further expanded and E2Crimson was analyzed using flow cytometry (Figure 2A). A bioluminescence signal (Figure 2B) corresponding to firefly luciferase activity (Luc2, Promega) (broad emission spectrum that peaks at 560 nm in vitro and 610 nm in vivo) was also used to detect the selected cells after expansion. As expected, the signal due to Luc2 activity was linearly correlated with the number of engineered A2780cis cells (Figure 2C). Both reporters, Luc2 and E2Crimson, have been used by others for improved in vivo imaging of tumor cells engineered to express them.[14] Additionally, Luc2 can also be used to indicate the live–dead status of the transformed A2780cis cells. This is because ATP, which is an indicator of the metabolic activity of live cells, is essential for Luc2 activity and rapidly depletes when cells lose viability. The surface expression level of the two antigens (FRa or MSLN) on the respective engineered A2780cis variants, compared to the variant that is negative for both antigens, was determined by flow cytometry (Figure 2D). Confirmation of binding of these reference antibodies (anti-FRa or anti-MSLN) is also an indication that the engineered A2780cis cell lines can be used as a resource to authenticate the antibodies against these antigens prior to their use and to assess the specificity and affinity of new antibodies being developed.
Figure 2.
Characterization of engineered A2780cis cell lines. The engineered A2780cis cell lines were generated using plasmids encoding desired transgenes sub-cloned in a PiggyBac transposon vector and PiggyBac transposase. The transformed cells were selected using puromycin treatment (negative selection) and selected by FACS-based enrichment (positive selection). The selected cells were expanded and assessed for A) E2Crimson fluorescent protein expression using flow cytometry, B,C) Luc2 (firefly luciferase) activity using bioluminescence analysis. B) The vertical histogram bars show the average Luc2 activity from a total of 5000 differently engineered A2780cis variants and non-engineered parental A2780cis. C) The Luc2 activity in the engineered A2780cis cell lines was fit using a linear regression model, Luc2 = m* (no. of engineered A2780cis cells) + c, where m is the slope (indicating the relationship between Luc2 activity and the number of engineered A2780cis cells) and c is the intercept (indicating background activity) (B,C) The error bars extend 1 standard deviation (SD) above the mean (n = 4) and can also be considered as one half-width of an 68% confidence interval for that mean. D) FRa and MSLN expression on the cell surface of respective A2780cis variants was analyzed using flow cytometry. The expression was compared to i) the A2780cis variant that is negative for both antigens (FRanegMSLNnegLuc2-2A-E2Crimson+A2780cis), ii) parental A2780cis (endogenous FRanegMSLNneg), and iii) parental OVCAR3 (endogenous FRa+MSLN+).
The use of the engineered A2780cis cell lines as a quality control tool-set to assess the target-specific capabilities of two Effector T-cell models (T-cell biofactory or CAR T cell) was demonstrated (Figure 3). The reporter activity in three different T-cell biofactories (Figure 3A) and in the engineered A2780cis cell lines in response to CAR T cells (Figure 3B) was measured when incubated with target and nontarget cells. The plasmids used to engineer T cells, as described previously,[8] also included c-Myc sequence. When expressed in the engineered Effector T cells, the resulting c-Myc tag forms a part of the extracellular portion of the CAR. The detection of c-Myc tag with Alexa647-labeled anti-c-Myc monoclonal antibody (mAb) confirmed the successful expression of antigen-specific CAR in the T cells (Figure S1, Supporting Information).
Figure 3.
Application of engineered A2780cis cell lines as a quality control tool-set. T-cell biofactories (panel A) and primary CAR+ T cells (panel B) were assessed for their specificity against cell surface FRa and MSLN. A) The T-cell biofactory (see our recent work, ref. [8] for more details) were assessed for their engineered function to synthesize desired proteins/reporter) upon engaging their target antigen, that is, when incubated with the A2780cis variants that are i) negative for FRa and MSLN (FRanegMSLNnegLuc2-2A-E2Crimson+A2780cis), ii) express FRa but not MSLN (FRa+MSLNnegLuc2-2A-E2Crimson+A2780cis); and iii) express MSLN but not FRa (FRanegMSLN+Luc2-2A-E2Crimson+A2780cis). Nluc reporter activity from the T-cell biofactories is shown in the graphs. B) Primary CAR+ T cells—i) FRa-specific or ii) MSLN-specific—were assessed for their target-specific cytolytic function by assessing the Luc2 activity in the target/nontarget engineered A2780cis cell lines. The T-cell biofactory Nluc reporter activity (panel A) or Luc2 activity in (panel B) target/nontarget engineered A2780cis cell lines were fit using a four-parameter logistic model, Activity = Activitymin + (Activitymax - Activitymin)/(1 + 10(b × (X - log10EC50))) where X is the log10 of the number of Effector T cells, Activitymax is an estimated parameter defining an upper asymptote for the reporter activity, Activitymin is an estimated parameter defining a lower asymptote for the reporter activity, b is a “Hill” parameter defining the slope at the inflection point of the fitted curve, and EC50 is an estimated parameter representing the X value corresponding to (Activitymax - Activitymin)/2, the error bars are 1 SD above and below the mean (n = 4) and can also be considered as one half-width of an 68% confidence interval for that mean.
Figure 3A reports on the engineered function of the T-cell biofactory,[8] that is, autonomous synthesis of a protein with desired properties upon stimulation by the target antigen-presenting cells. The results indicate that this function in the two T-cell biofactories, that is, regulated expression of Nluc (Promega) upon engaging their target (FRa+ or MSLN+) A2780cis cells is distinct and is statistically greater (p <0.0001 at all effector-to-target ratios) when compared to nontarget stimulation. (Note: the feasibility of T-cell biofactory[8] was determined in Jurkat Clone E6–1 as a parental cell line). A certain level of nonspecific Nluc expression was observed when FRa-specific T-cell biofactories were incubated with the nontarget FRanegMSLN+Luc2-2A-E2Crimson+A2780cis cells (Figure 3A(i) and A(iii)). This could potentially be due to the FRa-specific single-chain variable fragment (scFv) that forms the extracellular portion of CAR and may nonspecifically recognize the parental A2780cis cells. This was also observed in the flow cytometry data (Figure 2D) where anti-FRa-antibody exhibited slight nonspecific affinity toward FRanegA2780cis cell line variants.
Figure 3B shows the target cell-specific cytolytic function of the primary T cells engineered to express FRa-specific or MSLN-specific CAR, compared to the nonspecific cytolytic function toward nontarget cells. The engineered A2780cis cell lines are useful for this application as they include a Luc2 transgene that, as discussed earlier, can be used to determine the live-dead status of the target or nontarget cells. The comparative analysis of the Luc2 signal in the engineered A2780cis cell lines indicates that the cytolytic activity of the engineered primary T cells toward their target antigen-presenting cells (FRa or MSLN) is specific and statistically significant (p <0.002; at the effector-to-target ratio 8:1 or less). The difference between the means of two co-incubations (effector and target vs effector and nontarget) at two ratios (i.e., 8:1; 4:1) was found to be larger at 48 h compared to 72 h (Figure S2, Supporting Information). Based on this, the duration of 48 h was deemed to be more sensitive for assessing the cytolytic function of the primary CAR T cells and their off-target reactivity. A similar comparison was also made at 24 h vs 48 h demonstrating higher sensitivity for a 48-h duration of the assay (data not shown). Higher cytolytic activity of MSLN-CAR+ T cells was observed toward their target MSLN+ A2780cis cells compared to that of FRa-CAR+ T cells toward FRa+ A2780cis cells (Figure 3B). One explanation for this is reflected in the relatively reduced surface expression of FRa over FRa+MSLNnegLuc2-2A-E2Crimson+A2780cis compared to MSLN over FRanegMSLN+Luc2-2A-E2Crimson+A2780cis and can be observed in the flow cytometry data shown in Figure 2D as reduced binding of anti-FRa-antibody toward FRa+A2780cis cell line variants.
Cell engineering is an emerging strategy for targeting many diseases that evade detection by the immune system or involve its malfunction.[15–18] As with any new therapeutic approach, a standardized tool-set, quality control methodology, and availability of authentication resources are critical for the scale-up and subsequent adoption in clinical applications.[5] As CAR specificity relies on heavy and light chain of the target-specific antibodies fused into an scFv, it shares the challenges that antibodies face, that is, target binding, cross-reactivity, and reproducibility.[12] To address these challenges, strategies for enhanced validation of antibodies are typically based on genetic validation, recombinant expression, independent antibodies, orthogonal validation, and capture mass spectrometry validation.[19]
In this work, we draw a parallel with the approach of recombinant expression suggested for authenticating antibodies[19]; but with the difference that the recombinant antigen is expressed in the context of the whole cell. The resulting set of isogenic A2780cis cell lines can thus be used as a quality control tool-set to not only determine the specificity of cell-based biologics but also their functional activity. Our engineered cells offer an advantage in terms of safety and ease of use over the radioactive chromium-51 (51Cr) release assay, an industry-standard for assessing the cytolytic ability of engineered T cells. The engineered A2780cis cell lines can serve as a resource,[20] using bioluminescence as an alternative to radioactivity for authenticating the specificity and function of FRa-specific or MSLN-specific T-cell biofactories.
Additionally, we used the same resource to validate engineered primary CAR T cells and monoclonal antibodies against FRa and MSLN antigens both of which have been found to be overexpressed in multiple solid tumors (e.g., FRa has been found to be overexpressed in ovarian, cervical, endometrial, breast, lung, kidney, colorectal, bladder, pleura, brain;[21,22] and MSLN is prevalent in malignant mesothelioma, pancreatic, ovarian, and lung adenocarcinoma[23–26]). Furthermore, no lethal toxicities have been observed with FRa-targeting[22] or MSLN-targeting[27] agents. The impact of this work is furthermore in the general strategy that can be used to present other disease-specific cell-surface biomarkers that are either already known (e.g., prostate-specific membrane antigen for prostate cancer, disialoganglioside GD2 for neuroblastoma, human epidermal growth factor receptor 2 for breast cancer) or that remain to be discovered. The resulting cell-based tool-set can be used as a standardized quality control methodology to authenticate emerging biologic technologies and validate therapeutics that will target these biomarkers.[28]
Supplementary Material
Acknowledgements
Research reported in this publication was supported in part by the National Cancer Institute (R21CA193064; R21CA236640) and the National Institute of Biomedical Imaging and Bioengineering (DP2EB024245) of the National Institutes of Health (NIH); and the Defense Advanced Research Projects Agency (DARPA) (D19AP00024). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or DARPA. Part of this work was funded by through the NIH Director’s New Innovator Award Program (https://commonfund.nih.gov/newinnovator), Grant Number DP2EB024245; and DARPA Young Faculty Award (https://www.darpa.mil/work-with-us/for-universities/young-faculty-award), Grant Number D19AP00024. P.B. thanks Maksim Mamonkin and Malcolm Brenner (Baylor College of Medicine); Michael Kershaw (Peter MacCallum Cancer Centre, Melbourne, Australia); Dario Campana (National University of Singapore, Singapore); and Sean Yu (Epoch Life Sciences, Missouri City, TX, USA) for helpful discussions.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma, https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm (accessed: July 2019).
- [2].FDA approval brings first gene therapy to the United States, https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm (accessed: July 2019).
- [3].Levine BL, Miskin J, Wonnacott K, Keir C, Mol. Ther.–Methods Clin. Dev 2017, 4, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang X, Rivière I, Mol. Ther.–Oncolytics 2016, 3, 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lipsitz YY, Timmins NE, Zandstra PW, Nat. Biotechnol 2016,34, 393. [DOI] [PubMed] [Google Scholar]
- [6].Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR,Cell Stem Cell 2014, 14, 141. [DOI] [PubMed] [Google Scholar]
- [7].Freedman LP, Cockburn IM, Simcoe TS, PLoS Biol. 2015, 13, e1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Repellin CE, Patel P, Beviglia L, Javitz H, Sambucetti L,Bhatnagar P, Adv. Biosyst 2018, 2, 1800210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Howat WJ, Lewis A, Jones P, Kampf C, Pontén F, vander Loos CM, Gray N, Womack C, Warford A, Methods 2014, 70, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Voskuil JLA, F1000Research 2017, 6, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reiss PD, Min D, Leung MY, F1000Research 2014, 3, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bordeaux J, Welsh AW, Agarwal S, Killiam E, Baquero MT,Hanna JA, Anagnostou VK, Rimm DL, BioTechniques 2010, 48, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li MA, Turner DJ, Ning ZM, Yusa K, Liang Q, Eckert S,Rad L, Fitzgerald TW, Craig NL, Bradley A, Nucleic Acids Res.2011, 39, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luker KE, Pata P, Shemiakina II, Pereverzeva A, Stacer AC, Shcherbo DS, Pletnev VZ, Skolnaja M, Lukyanov KA, Luker GD, Pata I, Chudakov DM, Sci. Rep 2015, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fischbach MA, Bluestone JA, Lim WA, Sci. Transl. Med 2013,5, 179ps7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kershaw MH, Westwood JA, Darcy PK, Nat. Rev. Cancer 2013,13, 525. [DOI] [PubMed] [Google Scholar]
- [17].Zhen AJ, Kamata M, Rezek V, Rick J, Levin B, Kasparian S,Chen ISY, Yang OO, Zack JA, Kitchen SG, Mol. Ther 2015,23, 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sautto GA, Wisskirchen K, Clementi N, Castelli M, Diotti RA,Graf J, Clementi M, Burioni R, Protzer U, Mancini N, Gut. 2015,65, 512 10.1136/gutjnl-2014-308316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J,Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M,Yamamoto T, Nat. Methods 2016, 13, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bandrowski A, Brush M, Grethe JS, Haendel MA,Kennedy DN, Hill S, Hof PR, Martone ME, Pols M, Tan SC,Washington N, Zudilova-Seinstra E, Vasilevsky N, J. Comp. Neurol 2016, 524, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheung A, Bax HJ, Josephs DH, Ilieva KM, Pellizzari G, Opzoomer J, Bloomfield J, Fittall M, Grigoriadis A, Figini M, Canevari S, Spicer JF, Tutt AN, Karagiannis SN, Oncotarget 2016, 7, 52553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Low PS, Kularatne SA, Curr. Opin. Chem. Biol 2009, 13, 256. [DOI] [PubMed] [Google Scholar]
- [23].Kelly RJ, Sharon E, Pastan I, Hassan R, Mol. Cancer Ther 2012, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hassan R, Ho M, Eur. J. Cancer 2008, 44, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, Pastan I, Clin. Cancer Res 2007, 13, 1571. [DOI] [PubMed] [Google Scholar]
- [26].Hassan R, Bera T, Pastan I, Clin. Cancer Res 2004, 10, 3937. [DOI] [PubMed] [Google Scholar]
- [27].Chang K, Pastan I, Willingham MC, Int. J. Cancer 1992, 50, 373. [DOI] [PubMed] [Google Scholar]
- [28].O’Hurley G, Sjosted E, Rahman A, Li B, Kampf C, Ponten F, Gallagher WM, Lindskog C, Mol. Oncol 2014, 8, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.