The latent-to-lytic switch in KSHV infection is one of the critical events regulated by the major replication and transcription activator KSHV protein called RTA. Chromatin modification of the viral genome determines the phase of the viral life cycle in the host. Here, we report that LXA4 interacts with a host chromatin modulator, especially SMARCB1, which upregulates the KSHV ORF50 promoter. SMARCB1 has also been recognized to be a tumor suppressor protein which controls many tumorigenic events associated with the hedgehog (hh) signaling pathway. We also observed that LXA4 treatment reduces PD-L1 expression and that PD-L1 expression is an important immune evasion strategy used by KSHV for its survival and maintenance in the host. Our study underscores the role of LXA4 in KSHV biology and emphasizes that KSHV is strategic in downregulating LXA4 secretion in the host to establish latency. This study also uncovers the therapeutic potential of LXA4 and its targetable receptor, AhR, in KSHV’s pathogenesis.
KEYWORDS: KSHV, LXA4, hedgehog, SMARCB1, RTA, PD-L1, aspirin, Kaposi's sarcoma-associated herpesvirus, latency, lipoxin, cyclooxygenase, hedgehog signaling, lytic switch
ABSTRACT
Lipoxin A4 (LXA4) is an endogenous lipid mediator with compelling anti-inflammatory and proresolution properties. Studies done to assess the role of arachidonic acid pathways of the host in Kaposi's sarcoma-associated herpesvirus (KSHV) biology helped discover that KSHV infection hijacks the proinflammatory cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) pathways and concurrently reduces anti-inflammatory LXA4 secretion to maintain KSHV latency in infected cells. Treatment of KSHV-infected cells with LXA4 minimizes the activation of inflammatory and proliferative signaling pathways, including the NF-κB, AKT, and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways, but the exact mechanism of action of LXA4 remains unexplored. Here, using mass spectrometry analysis, we identified components from the minichromosome maintenance (MCM) protein and chromatin-remodeling complex SMARCB1 and SMARCC2 to be LXA4-interacting host proteins in KSHV-infected cells. We identified a higher level of nuclear aryl hydrocarbon receptor (AhR) in LXA4-treated KSHV-infected cells than in untreated KSHV-infected cells, which probably facilitates the affinity interaction of the nucleosome complex protein with LXA4. We demonstrate that SMARCB1 regulates both replication and transcription activator (RTA) activity and host hedgehog (hh) signaling in LXA4-treated KSHV-infected cells. Host hedgehog signaling was modulated in an AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR)-S6 kinase-dependent manner in LXA4-treated KSHV-infected cells. Since anti-inflammatory drugs are beneficial as adjuvants to conventional and immune-based therapies, we evaluated the potential of LXA4 treatment in regulating programmed death-ligand 1 (PD-L1) on KSHV-carrying tumor cells. Overall, our study identified LXA4-interacting host factors in KSHV-infected cells, which could help provide an understanding of the mode of action of LXA4 and its therapeutic potential against KSHV.
IMPORTANCE The latent-to-lytic switch in KSHV infection is one of the critical events regulated by the major replication and transcription activator KSHV protein called RTA. Chromatin modification of the viral genome determines the phase of the viral life cycle in the host. Here, we report that LXA4 interacts with a host chromatin modulator, especially SMARCB1, which upregulates the KSHV ORF50 promoter. SMARCB1 has also been recognized to be a tumor suppressor protein which controls many tumorigenic events associated with the hedgehog (hh) signaling pathway. We also observed that LXA4 treatment reduces PD-L1 expression and that PD-L1 expression is an important immune evasion strategy used by KSHV for its survival and maintenance in the host. Our study underscores the role of LXA4 in KSHV biology and emphasizes that KSHV is strategic in downregulating LXA4 secretion in the host to establish latency. This study also uncovers the therapeutic potential of LXA4 and its targetable receptor, AhR, in KSHV’s pathogenesis.
INTRODUCTION
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a causative agent of Kaposi’s sarcoma (KS) and two other lymphoproliferative disorders of B-cell origin, primary effusion lymphoma (PEL) (1, 2) and a plasmablastic variant of multicentric Castleman’s disease (MCD) (2, 3). KSHV is associated with a biphasic life cycle, comprising a latent phase, regulated by latency-associated nuclear antigen 1 (LANA-1 or ORF73), and an active lytic phase, essential for viral progression and dissemination (4, 5). The maintenance of latency is a default state for the virus and is marked by the expression of limited latent viral proteins LANA-1, viral cyclin, viral FLIP, and kaposins (kaposins A, B, and C), which aid KSHV with survival in the host and which give KSHV an opportunity to revert to an active state, allowing viral replication and progression as needed.
The double-stranded KSHV genome of 165 to 170 kb exists as a closed circular episome tethered to the host chromosomes. The circular episome is associated with cellular histones and gets chromatinized, which facilitates maintenance and replication of the viral genome during latent infection (6). Among the critical steps in the herpesvirus life cycle is the switch from latent to lytic replication, mediated by the KSHV replication and transcription activator (RTA) (7, 8). This step is mediated by alteration in the histone modification (9). Genetic studies have revealed a close resemblance between the N-terminal DNA-binding domain of the RTA complex of KSHV and that of Epstein-Barr virus (EBV) (10). A relatively less conserved carboxyl acidic activation domain exhibits vigorous transactivation activity (11). Studies have shown that promoters of several lytic cycle genes, such as ORF57, viral G protein-coupled receptor (vGPCR), and viral interferon regulatory factor 1 (vIRF1), possess high-affinity RTA binding sites. Also, RTA binding sites present near the origins of lytic cycle DNA replication (12) are responsible for an open chromatin conformation, which favors viral replication. Few other genes that lack RTA binding sites get activated via recruitment of RTA at the promoter with the help of host transcription factors (13). Studies have also shown that the abrupt expression of RTA is sufficient to activate lytic replication by putting a halt to KSHV latency (14, 15). Most available antiviral therapies to date target lytic cycle replication and are known not only to have low efficacy but also to cause severe side effects. Our lab explored the therapeutic potential of lipoxin A4 (LXA4), which is an anti-inflammatory metabolite of the arachidonic acid pathway and has fewer side effects than other antiviral therapies (16, 17).
LXA4 binds to the host ALXR receptor in KSHV-infected cells and creates an anti-inflammatory environment by mitigating the level of secreted inflammatory cytokines (tumor necrosis factor alpha, interleukin-1β [IL-1β], and IL-6) and signaling (NF-κB, AKT, extracellular signal-regulated kinase 1/2 [ERK1/2]) pathways essential for inflammation and tumorigenesis (16). Since we observed promising implications of LXA4 as a therapeutic agent for KS, we attempted to elucidate the mechanism through which LXA4 may alter host and viral factors important to lytic reactivation and viral progression in KSHV latently infected cells. Our findings showed the efficacy of LXA4 in changing viral genome chromatinization in a way that would facilitate lytic reactivation. Our study suggests the potential usage of LXA4 as one of the safe therapeutic molecules to cure KSHV-associated diseases. Using mass spectrometry (MS), we identified several LXA4-interacting nuclear proteins, such as nucleosome assembly protein 1 (NAP1), high-mobility group protein B1 (HMGB1), minichromosome maintenance (MCM) protein, and SWI/SNF complex components SMARCC2 and SMARCB1, which are important to viral replication and chromatinization processes (13, 18, 19). The SWI/SNF complex, along with other cofactors, such as TRAP/mediators, participates in chromatin remodeling and disruption of nucleosomes to aid ATP-dependent transcription factor binding. Histone modification facilitated by histone acetyltransferase (HAT) and histone deacetylase (HDAC) help in the process of chromatin modulation (13). Several types of activators, including nuclear receptors, c-myc proto-oncoprotein, CCAAT-enhancer-binding protein (C/EBP), and Gli-1 (the effector of the hedgehog [hh] signaling pathway), physically or functionally interact with the SWI/SNF complex component SMARCB1 (20).
In the present study, we identified the manipulation of the host hh pathway during KSHV infection upon LXA4 treatment through (i) chromatin-remodeling complex component SMARCB1 and (ii) AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling. Evaluating the effect of LXA4 on the signaling pathways associated with tumorigenesis and immune escape, both of which are important for KSHV infection, would give us a new insight into the ongoing efforts to find a cure to KSHV-associated malignancies.
RESULTS
LXA4 affinity purification of cellular proteins in KSHV-infected cells.
To identify LXA4-interacting host cellular proteins, LXA4 was covalently coupled to a solid Sepharose support using CarboxyLink resin (Pierce) and the cross-linking agent 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) (Fig. 1A). Nuclear extracts from BCBL-1 cells were incubated with either LXA4-coupled resin or untreated (UT) resin, washed extensively, and then eluted with 0.1 M glycine HCl, pH 2.5. The cellular proteins from BCBL-1 cells, which were unique for their LXA4 binding capacity, were bound to the column. These host proteins were isolated, run in an SDS gel, stained with colloidal blue (Fig. 1B, lanes 3 and 4), and subsequently identified using mass spectrometry (Table 1). Several polypeptides were identified from LXA4-positive eluted fractions and validated by Western blotting, including minichromosomal maintenance (MCM) proteins (MCM2 and MCM3), SMARCB1, and SMARCC2 (Fig. 1C). Interestingly, mass spectrometry analysis also identified these polypeptides from cellular transcription cofactors, such as the SWI/SNF complex (SMARCC2 and SMARCB1, indicated in boldface in Table 1) and MCM complexes. Findings from previous study by Gwack et al. (21) showed that SMARCC2 is one of the KSHV RTA-interacting proteins. In light of these findings, the identification of SMARCC2 as one of the LXA4-interacting cellular proteins in our experiment suggests a probable role of LXA4 treatment on RTA induction in KSHV-infected cells.
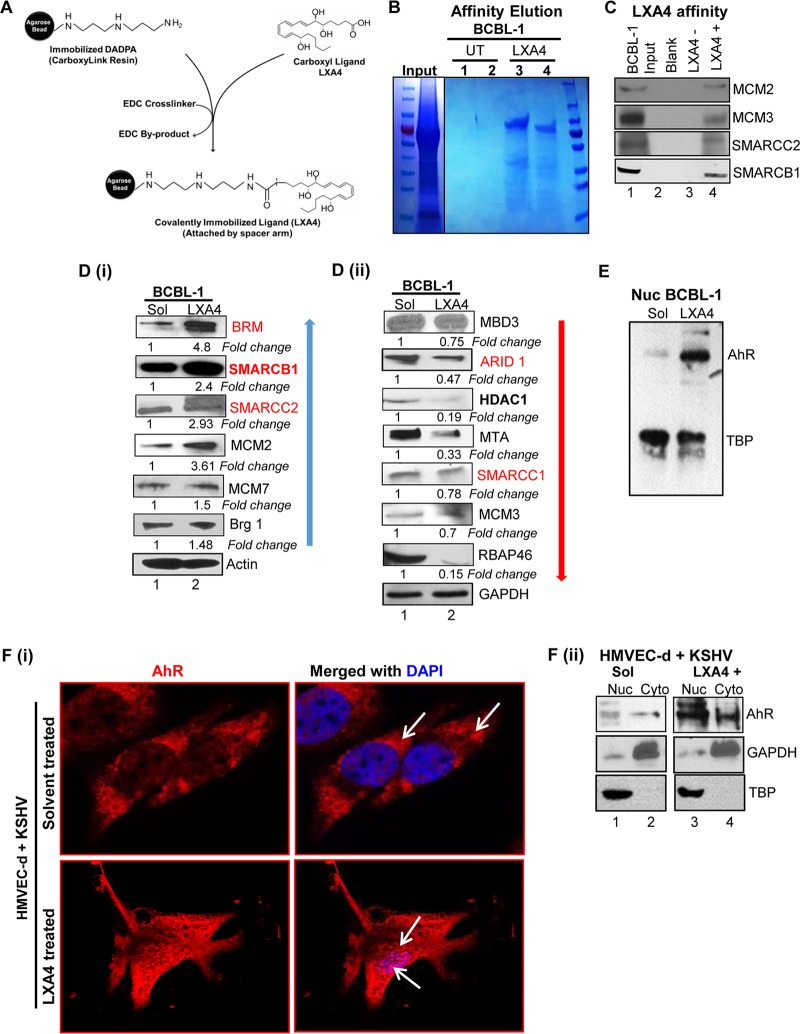
FIG 1.
Identification of LXA4 affinity-purified cellular proteins. (A) Strategy for immobilization of LXA4 on CarboxyLink resin using the cross-linker EDC. DADPA, diaminodipropylamine. (B) Coomassie staining, after SDS-PAGE analysis, of protein bound to and eluted from LXA4 affinity resin or mock affinity resin after elution with 1 mM LXA4. (C) Western blot analysis for candidate LXA4 affinity-purified proteins identified by LC-MS/MS. Input proteins (BCBL-1 cell crude extract), blank (reaction buffer; lane Input), mock affinity (BCBL-1 cell nuclear lysate and LXA4-negative) resin-purified proteins (lane LXA4−), and LXA4 affinity (BCBL-1 cell nuclear lysate and LXA4-positive) resin-purified proteins (lane LXA4+) were assayed with antibodies for MCM2, MCM3, SMARCC2, and SMARCB1, as indicated to the right of each blot. (D) Western blot analysis for understanding the effect of LXA4 treatment on the expression of the SWI/SNF and MCM proteins. β-Actin and GAPDH were used as the loading control. The fold change in expression was calculated by considering the level of protein in the solvent (Sol)-treated sample as 1. Representative data from three experiments are shown here. (E) Western blot for AhR in nuclear lysates of solvent (ethanol)-treated and 100 nM LXA4 (48 h)-treated BCBL-1 cells. Representative data from three experiments are shown here. (F) (i) Immunofluorescence analysis of AhR in latently infected HMVEC-d cells treated with solvent (ethanol) or 50 nM LXA4. Latently infected HMVEC-d cells (infected with KSHV at 30 DNA copies/cell for 72 h) were treated with either solvent (ethanol) or LXA4 (50 nM) for 20 min. The cells were fixed, permeabilized, and incubated overnight with the primary AhR rabbit antibody. The cells were then washed and incubated in secondary anti-rabbit immunoglobulin conjugated to Alexa Fluor 594 for 2 h and mounted with mounting medium containing the nuclear stain DAPI (blue). Stained cells were viewed under an Olympus confocal laser scanning microscope (FluoView FV10i). White arrows indicate AhR nuclear translocation. (ii) Western blot of AhR in cytoplasmic (Cyto) and nuclear (Nuc) lysates of solvent (ethanol)- and LXA4 (48 h)-treated latently infected HMVEC-d cells (infected with KSHV at 30 DNA copies/cell for 72 h). These membranes were stripped and immunoblotted with anti-GAPDH and anti-TATA-binding protein (anti-TBP) antibodies to check the purity of cytoplasmic and nuclear lysates, respectively.
TABLE 1.
Mass spectrometric analysis to identify LXA4 binding proteins in BCBL-1 cells
| GenBank accession no.|locus | −10 lgPb | Coverage % | No. of peptides | Avg mass (Da) | Descriptiona |
|---|---|---|---|---|---|
| P55209|NP1L1_HUMAN | 89.29 | 14 | 3 | 45,374 | Nucleosome assembly protein 1-like 1, OS = Homo sapiens, GN = NAP1L1, PE = 1, SV = 1 |
| Q99733|NP1L4_HUMAN | 72.73 | 5 | 1 | 42,823 | Nucleosome assembly protein 1-like 4, OS = Homo sapiens, GN = NAP1L4, PE = 1, SV = 1 |
| P25205|MCM3_HUMAN | 128.79 | 15 | 7 | 90,981 | DNA replication licensing factor MCM3, OS = Homo sapiens, GN = MCM3, PE = 1, SV = 3 |
| Q14566|MCM6_HUMAN | 103.34 | 6 | 4 | 92,889 | DNA replication licensing factor MCM6, OS = Homo sapiens, GN = MCM6, PE = 1, SV = 1 |
| P49736|MCM2_HUMAN | 101.25 | 6 | 4 | 101,896 | DNA replication licensing factor MCM2, OS = Homo sapiens, GN = MCM2, PE = 1, SV = 4 |
| Q8TAQ2|SMRC2_HUMAN | 81.25 | 3 | 2 | 132,879 | SWI/SNF complex subunit SMARCC2, OS = Homo sapiens, GN = SMARCC2, PE = 1, SV = 1 |
| Q9UNS2|CSN3_HUMAN | 126.08 | 11 | 4 | 47,873 | COP9 signalosome complex subunit 3, OS = Homo sapiens, GN = COPS3, PE = 1, SV = 3 |
| Q12824|SNF5_HUMAN | 218.33 | 26 | 2 | 44,141 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member, OS = Homo sapiens, GN = SMARCB1, PE = 1, SV = 2 |
| P35250|RFC2_HUMAN | 73.13 | 7 | 2 | 39,157 | Replication factor C subunit 2, OS = Homo sapiens, GN = RFC2, PE = 1, SV = 3 |
| P55209|NP1L1_HUMAN | 72.79 | 7 | 2 | 45,374 | Nucleosome assembly protein 1-like 1, OS = Homo sapiens, GN = NAP1L1, PE = 1, SV = 1 |
| P09429|HMGB1_HUMAN | 166.36 | 22 | 8 | 24,894 | High-mobility group protein B1, OS = Homo sapiens, GN = HMGB1, PE = 1, SV = 3 |
| P24534|EF1B_HUMAN | 153.92 | 24 | 3 | 24,764 | Elongation factor 1-beta, OS = Homo sapiens, GN = EEF1B2, PE = 1, SV = 3 |
| Q14232|EI2BA_HUMAN | 89.85 | 10 | 2 | 33,712 | Translation initiation factor alpha subunit of eukaryotic initiation factor 2B, OS = Homo sapiens, GN = EIF2B1, PE = 1, SV = 1 |
| P68104|EF1A1_HUMAN | 81.08 | 9 | 3 | 50,141 | Elongation factor 1-alpha 1, OS = Homo sapiens, GN = EEF1A1, PE = 1, SV = 1 |
| Q92905|CSN5_HUMAN | 80.73 | 7 | 2 | 37,579 | COP9 signalosome complex subunit 5, OS = Homo sapiens, GN = COPS5, PE = 1, SV = 4 |
| Q9H9Q2|CSN7B_HUMAN | 80.68 | 11 | 2 | 29,622 | COP9 signalosome complex subunit 7b, OS = Homo sapiens, GN = COPS7B, PE = 1, SV = 1 |
| P19338|NUCL_HUMAN | 207.06 | 16 | 10 | 76,615 | Nucleolin, OS = Homo sapiens, GN = NCL, PE = 1, SV = 3 |
Text in bold indicates the specific LXA4 binding components of the SWI/SNF complex protein, as identified by mass spectrometric analysis. OS, organism name; GN, gene name; PE, protein existence; SV, sequence version.
IgP, protein confidence score.
We then compared the expression of proteins belonging to the SWI/SNF, MCM, and nucleosome remodeling deacetylase (NuRD) complex in BCBL-1 cells treated with solvent or LXA4 for 48 h. The Western blotting results showed significant changes in proteins of the SWI/SNF complex, as indicated by an increase (blue arrows) in Fig. 1Di or a decrease (red arrows) in Fig. 1Dii. We also compared the expression of SMARCB1 (an SWI/SNF complex component identified by mass spectrometry; Table 1) in BJAB cells treated with solvent or LXA4 for 48 h. The Western blotting result for BJAB cells treated with solvent or LXA4 for 48 h showed no significant change in the expression of the SMARCB1 protein (data not shown), suggesting a specific effect of LXA4 treatment on KSHV-infected cells.
AhR expression in KSHV-infected cells treated with LXA4.
LXA4 binds to two types of receptors: lipoxin A4 receptor/formyl peptidyl receptor (ALX/FPR) and the nuclear ligand-activated transcription factor called aryl hydrocarbon receptor (AhR) (22). A nuclear fraction of BCBL-1 cells treated with solvent or LXA4 was immunoblotted for AhR protein to evaluate the impact of LXA4 treatment on the localization of the AhR protein. We observed an increased nuclear expression of the AhR protein in the LXA4-treated fraction compared to that in solvent-treated BCBL-1 cells (Fig. 1E). We also validated the immunostaining pattern of the AhR protein in human dermal microvascular endothelial (HMVEC-d) cells latently infected (72 h) with KSHV (30 DNA copies/cell) and treated with solvent or LXA4 for 20 min. A rapid nuclear translocation of AhR in cells stimulated with LXA4 was observed (Fig. 1Fi, bottom). On the other hand, HMVEC-d cells infected with KSHV and treated with solvent showed a dispersed cytoplasmic and nuclear distribution of AhR (Fig. 1Fi, top). Similarly, we observed an increased AhR protein level, especially in the nuclear fraction of HMVEC-d cells infected with KSHV and then treated with LXA4 (Fig. 1Fii, lane 3), compared to that in cells treated with solvent (Fig. 1Fii, lane 1). This observation suggests that at higher concentrations (>30 nmol/liter) of LXA4, AhR, which is otherwise located mostly in the cytosol, gets released from the cytosol and is translocated to the nucleus. We thus hypothesized that AhR might be responsible for the LXA4 interaction with nuclear proteins, as identified by mass spectrometry (Table 1).
This observation concurs with that of previous studies showing that at higher concentrations (>30 nmol/liter), LXA4 binds to AhR and that following this binding AhR joins with the AhR nuclear translocator (ARNT) to mediate the cellular response in carcinogenic cancer cells (23). Observations from the previous study indicated that the ATPase subunit of SWI/SNF complexes is a bona fide coactivator for AHR/ARNT-dependent transcription of the genes associated with the regulation of lipid metabolism (24).
Interaction between SMARCB1 and LANA-1 in KSHV-infected cells.
We also observed an increase in SMARCB1 expression of 2.5-fold with a subsequent decrease in KSHV latency protein (LANA-1) when BCBL-1 cells were treated with LXA4 (100 nM for 48 h) (Fig. 2A).
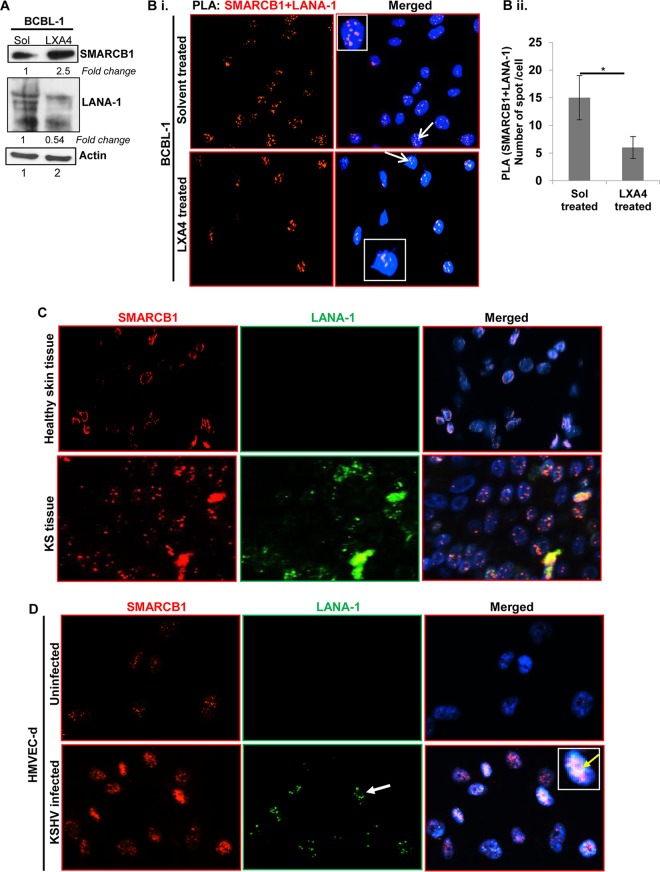
FIG 2.
Interaction between SMARCB1 and LANA-1 in KSHV-infected cells. (A) Western blot for SMARCB1 and LANA-1 in BCBL-1 cells treated with solvent (ethanol) or LXA4 (100 nM for 48 h). β-Actin was used as the loading control. The fold change in expression was calculated by considering the level of protein in the solvent-treated sample as 1. (B) Proximity ligation assay (PLA) to visualize the interaction between SMARCB1 and LANA-1. (i) BCBL-1 cells treated with solvent or LXA4 (100 nM) for 48 h were processed as described in Materials and Methods and reacted with the indicated pairs of primary antibodies, followed by PLA to assess the interactions between SMARCB1 and LANA-1. Nuclei were stained with DAPI and viewed at a ×40 magnification. The PLA reaction was detected using the Duolink red detection agent. Red PLA spots in the nucleus indicate a positive PLA signal, suggesting interactions between the two proteins. (ii) Quantitative analysis of the average number of SMARCB1 plus LANA-1 interaction PLA spots per cell is presented in the histogram. *, P < 0.05. (C and D) Healthy skin tissue (C) and KS skin tissue (D) and HMVEC-d cells uninfected or infected with KSHV (30 DNA copies/cell for 48 h) were washed, fixed, permeabilized, blocked using 5% milk at room temperature for 30 min, and incubated with target-specific primary antibodies (SMARCB1 and LANA-1), followed by staining with secondary antibodies conjugated to Alexa Fluor 488 (green) and Alexa Fluor 594 (red) for immunofluorescence assay. Nuclei were counterstained using DAPI (blue) and viewed at a ×40 magnification. Insets show enlarged cells.
We performed a proximity ligation assay (PLA) to assess protein-protein interactions to study the interactions between viral latency protein (LANA-1) and host (SMARCB1) proteins under solvent treatment (Fig. 2Bi, top) or LXA4 treatment (Fig. 2Bi, bottom). Red dots in the nuclei indicate a positive PLA signal, suggesting interactions among the host SMARCB1 and viral LANA-1 proteins (Fig. 2Bi). Interestingly, during KSHV infection, we observed significant nuclear interactions (red dots) between LANA-1 and SMARCB1 (Fig. 2Bi, top). BCBL-1 cells treated with LXA4 showed only a limited association between SMARCB1 and LANA-1 (Fig. 2Bi, bottom). Quantitative signal analysis for the average number of SMARCB1–LANA-1 interaction PLA spots per cell was done. We observed significant association between SMARCB1 and LANA-1 in the solvent-treated cells compared to that in LXA4-treated BCBL-1 cells. (Fig. 2Bii).
To understand the in vivo physiological relevance of SMARCB1 induction, KS skin tissue sections were immunostained for SMARCB1. KS skin tissue sections showed an increase in SMARCB1 expression compared to that for healthy skin tissue, suggesting the importance of SMARCB1 during KSHV infection (Fig. 2C). Similarly, we observed more abundant immunostaining for SMARCB1 in the primary endothelial cells (HMVEC-d cells) infected with KSHV than in uninfected cells (Fig. 2D, red). KSHV infectivity was confirmed by LANA-1 staining (Fig. 2C and D). LANA-1 staining was negative in the healthy skin tissue (Fig. 2C) and uninfected cells (Fig. 2D). Forty percent of KSHV-infected HMVEC-d cells were positive for LANA-1-specific nuclear punctate staining (Fig. 2D, green, white arrow). We observed LANA-1 and SMARCB1 colocalization in KSHV-infected HMVEC-d cells (Fig. 2D, yellow). HMVEC-d cells were infected with KSHV (multiplicity of infection [MOI], 30), treated with LXA4 (100 nM, 48 h), and stained for the KSHV genome and the host protein SMARCB1 (Fig. 3A). The KSHV genome staining colocalized with SMARCB1 (Fig. 3A, top, white arrows), suggesting an interaction between the KSHV genome (green) and SMARCB1 (red) in the nucleus during primary infection. Using a whole-viral-genome fluorescence in situ hybridization (FISH) probe, we explored whether SMARCB1 interacts with the KSHV genome in KSHV-infected HMVEC-d cell nuclei. We observed that almost all the KSHV genome in the infected HMVEC-d cells treated with LXA4 colocalized with nuclear SMARCB1 (Fig. 3A, bottom). This finding indicated the specificity of the FISH analyses as well as the absence of a free viral genome in the nucleus. The multiple SMARCB1 colocalization spots may have been due to the SMARCB1 interaction with all of the viral genome in LXA4-treated de novo-infected HMVEC-d cells. In cells containing one copy of KSHV DNA, as shown by a single FISH spot, only one SMARCB1 immunofluorescent colocalization spot was observed (Fig. 3A, white arrow). In contrast, in cells with multiple KSHV genomes, numerous SMARCB1-colocalizing spots, representing the interaction of individual genomes and SMARCB1, were observed (Fig. 3A, bottom). These findings indicate that SMARCB1 may continuously interact with the KSHV genome in de novo infected cells and thereby contribute to viral replication.
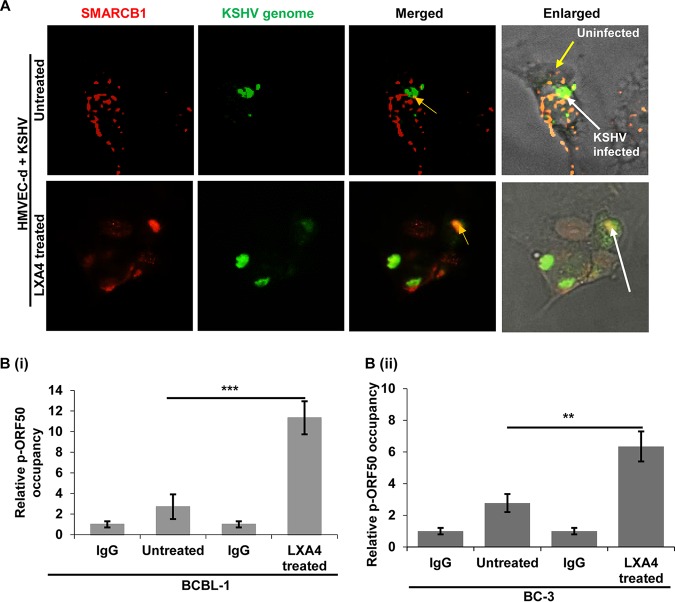
FIG 3.
SMARCB1 and KSHV genome interaction. (A) HMVEC-d cells were infected with KSHV, washed, and incubated with solvent or LXA4 for 48 h. The cells were fixed, permeabilized, and immunostained with an anti-rabbit SMARCB1 antibody followed by donkey anti-rabbit immunoglobulin conjugated to Alexa Fluor 594 secondary antibodies. The cells were then subjected to in situ hybridization with a spectrum green-labeled whole-KSHV-genome probe and viewed under a microscope at a ×40 magnification. (B) ChIP analysis of SMARCB1 in BCBL-1 and BC-3 cells treated with solvent or LXA4 for 48 h. SMARCB1 was immunoprecipitated from nuclei isolated from BCBL-1 and BC-3 cells that had been treated with solvent or LXA4 for 48 h, and bound DNA was analyzed by real-time RT-PCR with primers specific to regions (100 to 200 bp) flanking the transcription start sites of the indicated genes (ORF73 promoter and ORF50 promoter). Promoter occupancy relative to that obtained by IgG immunoprecipitation is presented for pORF50. The results are presented as the mean ± SD of data from three independent experiments. **, P < 0.01; ***, P < 0.001.
We then asked whether SMARCB1 binds to the latent or lytic KSHV gene promoters to regulate their transcription. To determine this, we carried out a chromatin immunoprecipitation (ChIP) assay of KSHV-infected BCBL-1 and BC-3 cells with and without LXA4 treatment by pulling down the DNA associated with SMARCB1 and performed quantitative PCR (qPCR) using primers corresponding to the promoter regions of the KSHV ORF73 (latent) and ORF50 (lytic) genes (Fig. 3B).
To validate the specificity of the SMARCB1 antibody (Ab) used, the results are presented as the fold increase in promoter occupancy over that for the IgG control. These results demonstrated that the SMARCB1 antibody coprecipitated with the KSHV ORF50 promoter (pORF50) with different efficiencies in the BCBL-1 (Fig. 3Bi) and BC-3 (Fig. 3Bii) cell lines treated with solvent or LXA4 (100 nM for 48 h). These results demonstrate that SMARCB1 interacts with KSHV episomal genome DNA and occupies the ORF50 promoter during KSHV infection. Our results also suggest significantly increased ORF50 promoter activity in LXA4-treated PEL cells compared to that in solvent-treated PEL cells. These results demonstrate that SMARCB1 interacts with KSHV episomal genome DNA and occupies its ORF50 promoter but not its ORF73 promoter.
SMARCB1 upregulates the KSHV ORF50 promoter in PEL cells.
We observed that treating the BCBL-1 cells with LXA4 showed an 80% decrease in the HDAC1 protein level compared to that in KSHV latently infected cells (P < 0.001) (Fig. 1Dii, lane 2). This decrease in the HDAC1 level could favor the reactivation of latent KSHV and could dramatically remodel the viral genome topology and chromatin architecture to support the RTA (ORF50)-induced lytic switch. We further showed that SMARCB1 interacts with the KSHV whole genome and occupies ORF50 promoter activity during infection (Fig. 3A and B), which is an indication that SMARCB1 might play a crucial role in the viral chromatinization that promotes lytic induction. Thus, we further investigated the effect of SMARCB1 expression on the KSHV ORF50 promoter in the absence of other viral proteins. HEK293T cells were cotransfected with an increasing quantity (0 to 2 μg) of an SMARCB1 expression plasmid along with a plasmid (p2500Luc) expressing a KSHV ORF50 promoter-luciferase (Luc) reporter gene, encoding the region corresponding to 2,500 bases upstream of ORF50. At 48 h after transfection, lysates were assayed for the mean relative luciferase activity of KSHV ORF50-Luc, normalized to the cotransfected Renilla activity. Increasing quantities of SMARCB1 induced the KSHV ORF50 promoter in a dose-dependent manner (Fig. 4A). The ORF50 promoter was activated by 2.38-, 3.08-, and 5.74-fold after transfection with 0.5, 1, and 2 μg of the SMARCB1 expression plasmid, respectively (Fig. 4A).
FIG 4.
Effect of SMARCB1 on KSHV lytic cycle induction and ATPase activity of the SWI/SNF complex. (A) Effect of SMARCB1 on the KSHV ORF50 promoter. HEK293T cells were transfected, using the Lipofectamine 2000 reagent, with an increasing quantity of SMARCB1 expression plasmids (0, 0.5, 1, or 2 μg), 1 μg of the ORF50 promoter-luciferase constructs, and 0.2 μg of the Renilla reporter construct (as a transfection control). Cells were harvested at 36 h after transfection. Alteration in ORF50 promoter activity was determined after normalization to the Renilla activity as a transfection efficiency control. The data represent the mean fold induction from at least three independent experiments ± SD. *, P < 0.05; ***, P < 0.001. (B) SMARCB1 silencing. TREx-BCBL-1-RTA cells were transduced using 5 μg/ml Polybrene at an MOI of 0.1 with the SMART vector-inducible lentiviral shRNAs directed against control shRNA (sh-Control) or SMARCB1 (sh-SMARCB1). Cells were selected with 3 μg/ml puromycin for 72 h. shRNA expression was then induced with 1.0 μg/ml doxycycline. (i) Total protein was extracted 72 h after doxycycline induction and subjected to Western blotting using antibody to cellular proteins (SMARCB1, Brg1, GAPDH) and the viral protein RTA (K8). Membranes exposed to LI-COR secondary antibodies were scanned using a LI-COR Odyssey infrared scanner. Representative data from three experiments are shown here. (ii) cDNA was isolated, and real-time qPCR for SMARCB1 was done. Expression of the gene for SMARCB1 was normalized to that of the gene for GAPDH. Results are presented as the mean ± SD from three independent experiments. Statistical analysis was done by using Student's t test. ***, P < 0.001. (C) HMVEC-d cells were transduced with either control shRNA lentiviral particles (sh-Control; catalog no. sc-108080; Santa Cruz Biotechnology, Inc.) or an shRNA lentiviral particle against SMARCB1 (sh-SMARCB1; catalog no. sc-35668-V; Santa Cruz Biotechnology, Inc.) and selected with 3 μg/ml puromycin for 5 to 7 days. These transduced cells were either left uninfected or infected with KSHV (30 DNA copies/cell). Total proteins were extracted in RIPA lysis buffer and subjected to Western blotting using antibody for viral protein (K8, ORF50) and cellular proteins Brg1, SMARCB1, and GAPDH. Membranes exposed to LI-COR secondary antibodies were scanned using a LI-COR Odyssey infrared scanner. A representative blot for the experiments is presented. GAPDH was used as the loading control. (D) ATPase activity. The ATPase activity of isolated chromatin-remodeling complex from TREx-BCBL-1-RTA cells (untreated), TREx-BCBL-1-RTA SMARCB1 KD cells, and TREx-BCBL-1-RTA cells with an expression plasmid for SMARCB1 overexpression was measured in terms of ATP hydrolysis on immunoprecipitation Dynabeads. This ATPase activity was further quantified by a luciferase assay, performed using the ADP-Glo Max assay (catalog number V7001; Promega), as discussed in Materials and Methods. Results are presented as the mean ± SD from three independent experiments, and statistical analysis was done by using Student's t test. *, P <0.05; ***, P < 0.001.
SMARCB1 downregulation by sh-SMARCB1 reduces KSHV lytic protein expression.
Since we observed that SMARCB1 interacts with the KSHV genome and occupies the ORF50 promoter during infection (Fig. 3A and B), we sought to determine the probable role of SMARCB1 in KSHV lytic cycle induction. For this experiment, a pool of three SMART vector-inducible lentiviral short hairpin RNAs (shRNAs) provided by Dharmacon was used to silence SMARCB1 in TREx-BCBL-1-RTA cells. The TREx-BCBL-1-RTA cells used for this experiment get reactivated while shRNA is induced using doxycycline (Dox) at a concentration of 1 μg/ml. The percentage of live cells was calculated using the trypan blue exclusion method, followed by the determination of SMARCB1 protein levels. Western blotting showed a significant 80% decrease in the expression of SMARCB1 in SMARCB1-specific shRNA (sh-SMARCB1)-treated TREx-BCBL-1-RTA cells compared to that in control shRNA (sh-Control)-treated cells (Fig. 4Bi, lane 2). We also observed a significant decrease of 72% in the level of protein expression of Brg1 when SMARCB1 was silenced (Fig. 4Bi, lane 2). We also observed significant decreases of 82% and 72% in the levels of RTA and K8 viral protein expression, respectively, in sh-SMARCB1-treated TREx-BCBL-1-RTA cells compared to the level in sh-Control-treated cells (Fig. 4Bi, lane 2).
SMARCB1 gene silencing was confirmed by qPCR using primers specific for the SMARCB1 gene. A substantial SMARCB1 gene silencing of 60% (Fig. 4Bii) relative to the level of expression of the control (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) gene was observed.
To confirm the effect of SMARCB1 silencing on other components of the SWI/SNF complex, HMVEC-d cells were transduced using SMARCB1-specific shRNA lentiviral particles. One set of these transduced cells was infected with KSHV to show the effect of SMARCB1 on viral protein expression (Fig. 4C). We observed a decrease of 61% in the RTA (ORF50) protein level in sh-SMARCB1-treated cells (Fig. 4C, lane 4) compared to that in sh-Control-treated cells (Fig. 4C, lane 3). When sh-SMARCB1-treated cells were infected with KSHV, they showed a 65% reduction in K8 expression compared to that in sh-Control-treated KSHV-infected HMVEC-d cells (Fig. 4C, lane 4).
The loss of SMARCB1 affects the ATPase activity of the SWI/SNF complex.
RTA interacts with the chromatin-remodeling SWI/SNF complex, which contains DNA-dependent ATPase activity (21). Therefore, we compared the ATPase activity of the chromatin-remodeling complex isolated from sh-SMARCB1-treated TREx-BCBL-1-RTA cells and TREx-BCBL-1-RTA SMARCB1-overexpressing cells with that of untreated TREx-BCBL-1-RTA cells (Fig. 4D). SMARCB1 knockdown using sh-SMARCB1 significantly downregulated the ATPase activity of the chromatin-remodeling complex compared to that for the untreated TREx-BCBL-1-RTA cells (ATP hydrolysis, 16.2% versus 25.3%; P < 0.05), as represented in Fig. 4D. On the contrary, the ATPase activity of the chromatin-remodeling complex isolated from SMARCB1-overexpressing PEL cells was approximately 1.5-fold higher than that isolated from untreated TREx-BCBL-1-RTA cells (ATP hydrolysis, 39.7% versus 25.3%; P < 0.001) (Fig. 4D). These results demonstrate that the DNA-dependent ATPase activity is enhanced by SMARCB1 expression, as it is a core subunit of the SWI/SNF complex.
LXA4 treatment downregulated hedgehog signaling mediator Gli-1.
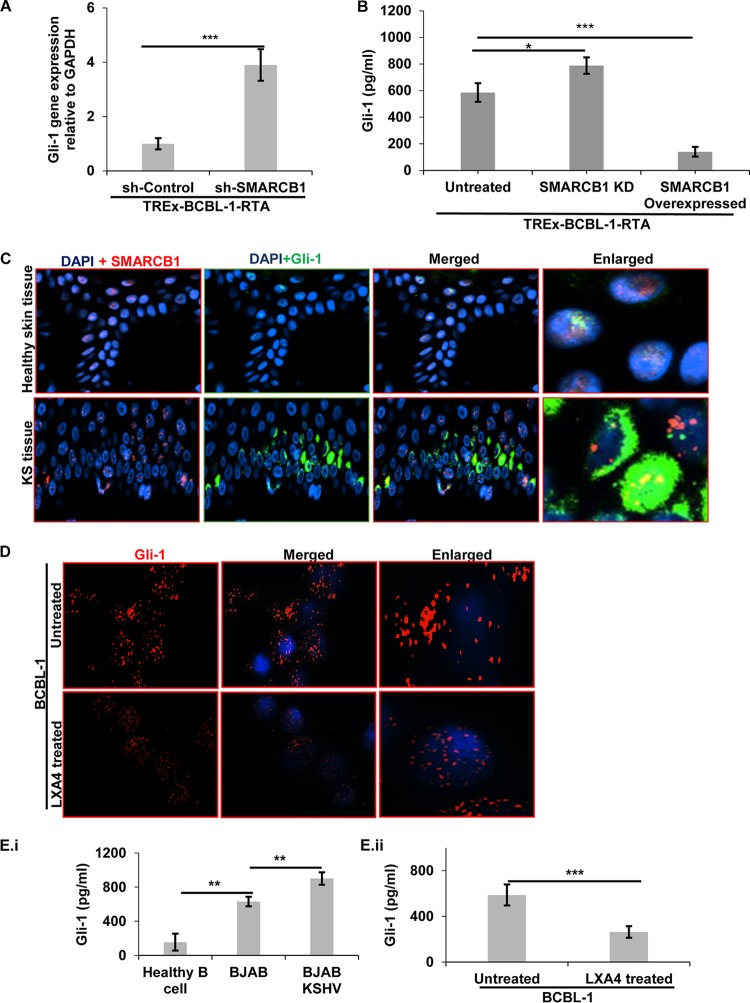
In our previous study, we observed that LXA4 modulated cell signaling pathways associated with cell proliferation and survival (25). In the present study, we found host SMARCB1 to be crucial for KSHV lytic induction. Since SMARCB1 has been shown to control tumorigenesis (26), crippling SMARCB1 would allow Gli-1 to activate the hh transcriptional program (27). Gli-1 is a member of the Kruppel family of zinc finger-containing transcription factors overexpressed in various cancers, such as human malignant glioma, medulloblastoma, and basal cell carcinoma (28, 29). The Gli family of transcription factors, though very important in tumorigenesis, cell proliferation, and metastasis, has not been studied in KSHV-related cancers. We sought to determine the level of Gli-1 expression in SMARCB1-silenced KSHV-infected cells (Fig. 5A). We observed a 3.95-fold increase in Gli-1 expression in SMARCB1-silenced cells compared to that in sh-Control-treated cells, as shown by qPCR analysis (Fig. 5A). In order to evaluate the consequence of SMARCB1 silencing on Gli-1 at the protein level, we measured the Gli-1 levels in KSHV-infected cells using an enzyme-linked immunosorbent assay (ELISA). A significant increase in the level of Gli-1 expression in TREx-BCBL-1-RTA cells with SMARCB1 knockdown (KD) compared to that in SMARCB1-overexpressing TREx-BCBL-1-RTA cells was observed (P < 0.05) (Fig. 5B). We also observed a significant decrease in Gli-1 expression in TREx-BCBL-1-RTA cells with a SMARCB1 expression plasmid compared to that in TREx-BCBL-1-RTA cells (P < 0.001) (Fig. 5B).
FIG 5.
Expression of Gli-1. (A) Gli-1 gene expression relative to GAPDH gene expression for TREx-BCBL-1-RTA cells treated with sh-Control and sh-SMARCB1 was determined using real-time PCR. Results are presented as the mean ± SD from three independent experiments, and statistical analysis was done by using Student's t test. ***, P < 0.001. (B) The amount of Gli-1 in lysates obtained from TREx-BCBL-1-RTA cells (untreated), TREx-BCBL-1-RTA SMARCB1 KD cells, and TREx-BCBL-RTA cells with an expression plasmid for SMARCB1 overexpression was estimated using ELISA. Results are presented as the mean ± SD from three independent experiments, and statistical analysis was done by using Student's t test. *, P < 0.05; ***, P < 0.001. (C) Healthy skin and KS skin tissue specimens were deparaffinized, hydrated, permeabilized, blocked with signal enhancer, reacted with anti-SMARCB1 and anti-Gli-1 antibodies, washed, and incubated with Alexa Fluor 594 (red) and Alexa Fluor 488 (green) secondary antibodies, respectively. The images were merged with those for DAPI-stained nuclei. (D) BCBL-1 cells and LXA4-treated BCBL-1 cells were fixed, permeabilized, washed, incubated with anti-Gli-1, washed, and incubated with Alexa Fluor 594 (red). Nuclei were stained with DAPI. Immunofluorescence assay staining for Gli-1 in LXA4-treated BCBL-1 cells showed the nuclear translocation of Gli-1. (E) Gli-1 expression in lysates obtained from healthy B cells, KSHV-negative cells (BJAB), and BJAB-KSHV cells (i) and from KSHV-positive cells (BCBL-1 cells and LXA4-treated BCBL-1 cells) (ii) was estimated using the ELISA method. The experiments were repeated three times. The results are presented as the mean ± SD, and statistical analysis was done by using Student's t test. **, P < 0.01; ***, P < 0.001.
Healthy skin tissue shows specific nuclear staining for both SMARCB1 (red) and Gli-1 (green) protein (Fig. 5C, top). We observed both a cytoplasmic and a nuclear distribution of Gli-1, along with the nuclear localization of SMARCB1, in KS skin tissue (Fig. 5C). Comparing KS skin tissue to healthy skin, we observed a significant increase in Gli-1 (green) expression in KS skin tissue, suggesting its substantial role during KS progression (Fig. 5C, Enlarged, bottom). These observations prompted us to further study the modulation of the hedgehog pathway by LXA4 treatment. LXA4-treated BCBL-1 cells showed Gli-1 expression (red) exclusively in the nuclei (Fig. 5D, bottom). In contrast, untreated BCBL-1 cells showed a diffused pattern of staining, which was predominantly concentrated within the nuclei, along with specks of staining in the cytoplasm, as analyzed by immunofluorescence microscopy (Fig. 5D, top). We also performed an ELISA and observed a decrease in the Gli-1 protein level in LXA4-treated cells compared to that in BCBL-1 cells mock treated with solvent (263.30 ± 50.3 versus 587.14 ± 91.4 pg/ml) (Fig. 5Eii), suggesting the downregulation of Gli-1 by LXA4 treatment of BCBL-1 cells. A significantly (P < 0.01) higher level of Gli-1 in BJAB-KSHV cells than in KSHV- and EBV-negative BJAB-KSHV cells (Fig. 5Ei) is portentous of the role played by Gli-1 in KSHV infection. Importantly, we observed a significantly (P < 0.01) higher level of Gli-1 levels in BJAB and BJAB-KSHV cells than in healthy B cells (Fig. 5Ei), suggesting the involvement and role of Gli-1 in oncogenesis in tumor cells.
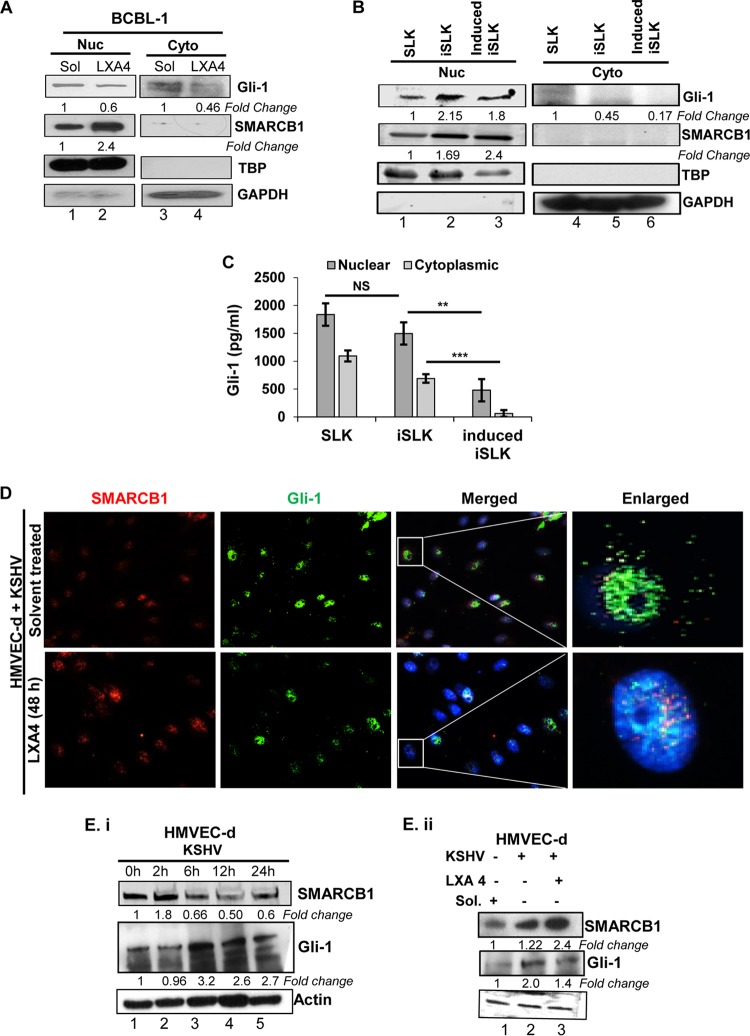
To further confirm the observations from the immunofluorescence results of Gli-1 nuclear translocation upon LXA4 treatment, we prepared nuclear and cytoplasmic fractions from LXA4-treated and solvent-treated BCBL-1 cells and analyzed them for SMARCB1 and Gli-1 protein levels by Western blotting (Fig. 6A). SMARCB1 and Gli-1 were confined to the nucleus under both conditions (Fig. 6A, lanes 1 and 2), with some diffused expression of Gli-1 being seen in the cytoplasm of solvent-treated BCBL-1 cells (Fig. 6A, lanes 3 and 4).
FIG 6.
LXA4 treatment regulates the nuclear translocation of Gli-1 and the SMARCB1–Gli-1 interaction in the nuclei of KSHV-infected cells. (A) The nuclear (Nuc) and cytoplasmic (Cyto) fractions were isolated from BCBL-1 cells and LXA4-treated BCBL-1 using a nuclear isolation kit and immunoblotted with anti-SMARCB1 and anti-Gli-1 antibodies. These membranes were stripped and immunoblotted with anti-GAPDH and anti-TATA-binding protein (anti-TBP) antibodies to check the purity of the cytoplasmic and nuclear lysates, respectively, and to confirm equal loading. (B) Shuttling of Gli-1. Immunoblotting showed the total nuclear translocation of the Gli-1 protein in induced iSLK cells compared with that in iSLK cells. (C) Gli-1 expression in lysates obtained from the nuclear and cytoplasmic fractions of SLK cells, latently infected SLK cells (iSLK), and induced iSLK cells was estimated using an ELISA kit. The experiments were repeated three times, and the results are expressed as the mean ± SD. **, P < 0.01; ***, P < 0.001; NS, not significant. (D) Nuclear translocation of Gli-1 during the treatment of de novo KSHV-infected HMVEC-d cells with LXA4. (E) (i) HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 0, 2, 6, 12, and 24 h. The Western blot shows the fold change in protein expression for SMARCB1 and Gli-1 in KSHV-infected HMVEC-d cells when expression for uninfected cells was considered 1. (ii) HMVEC-d cells were infected with KSHV (30 DNA copies/cell), and lysates were prepared from LXA4-treated cells or cells mock treated with solvent and were immunoblotted for SMARCB1 and Gli-1. The expression for uninfected HMVEC-d cells was considered 1. The values presented are the averages of results from three independent experiments, and statistical analysis was done by using Student's t test.
Similar, comparable results were also obtained for SMARCB1 and Gli-1 cytonuclear distribution patterns in the iSLK and induced iSLK cell lines (Fig. 6B and C). In concurrence with our previous findings in BCBL-1 cells treated with LXA4 (Fig. 5D), we observed translocation of Gli-1 into the nuclei of KSHV-infected HMVEC-d cells treated with LXA4 (Fig. 6D), whereas Gli-1 was distributed both in the cytoplasm and in the nucleus of the infected HMVEC-d cells treated with the solvent (Fig. 6D). HMVEC-d cells treated with KSHV for 0, 2, 6, 12, and 24 h showed a significant initial increase in SMARCB1 expression, suggesting requisition of SMARCB1 for primary infection of virus (Fig. 6Ei, lane 2). We also observed a subsequent rise in Gli-1 expression post-KSHV infection, when the level of protein expression in cells treated with the solvent was considered to be a value of 1 (Fig. 6Ei).
LXA4 regulates the hedgehog pathway through SMARCB1.
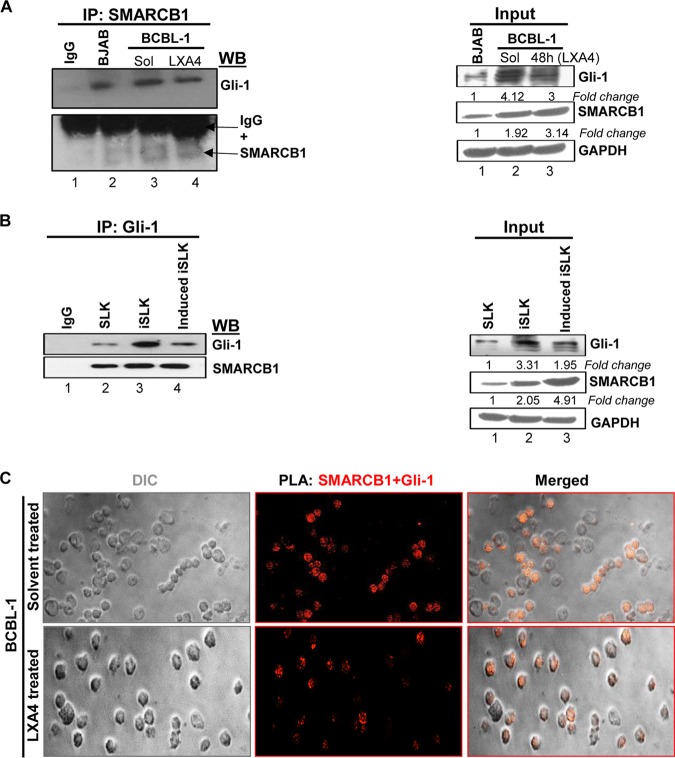
RTA regulates many cellular transcription factors to favor KSHV replication inside the host cell. Interestingly, SMARCB1 is a tumor suppressor and a transcription factor that controls many host cell cycle and cell proliferation genes. Therefore, we investigated the possible interactions between SMARCB1 and the tumorigenic gene (Gli-1) that might play a significant role in modulating the tumor microenvironment of KSHV-infected cells. Immunoprecipitation assays were performed using BJAB cells (KSHV negative/EBV negative) and BCBL-1 cells treated with solvent or LXA4 (48 h) (Fig. 7A). We found that SMARCB1 interacted with Gli-1 in both LXA4-treated and solvent-treated PEL cells (Fig. 7A). Compared to the amount of SMARCB1 that could be pulled down from the solvent-treated BCBL-1 cell lysate, more SMARCB1 could be pulled down from the LXA4-treated BCBL-1 cell lysate, which could have possibly interacted with Gli-1 to directly inactivate it, to show a less significant interacting band (Fig. 7A, top, lane 4 versus lane 3). To validate this finding, we performed a similar experiment using iSLK cells (latent infection model) and the induced iSLK cells (lytic infection model) and obtained a result comparable to the earlier result (Fig. 7B). In these experiments, the evident increase in Gli-1 expression in latently infected BCBL-1 and iSLK cells could be explained by the lower level of expression of SMARCB1 during latency, as SMARCB1 directly inhibits Gli-1 expression (Fig. 7A and B, right). Similar findings have been shown by others (27). Results from the proximity ligation assay (PLA) also verified the interaction between SMARCB1 and Gli-1 in PEL cells treated with solvent or LXA4 (Fig. 7C).
FIG 7.
SMARCB1 interacts with Gli-1 in KSHV-infected cells (A) (Left) Immunoprecipitation (IP) assay. BCBL-1 cells treated with solvent or LXA4 (48 h) and BJAB cells (KSHV and EBV negative) were harvested, and the lysates were precipitated with SMARCB1 and analyzed by immunoblotting with Gli-1 and SMARCB1. (Right) The input for the left panel. Representative data from three experiments are shown here. (B) (Left) Immunoprecipitation assay using SLK, iSLK, and induced iSLK cells. The cells were harvested, and the lysates were precipitated with Gli-1 and analyzed by immunoblotting with Gli-1 and SMARCB1. (Right) The input for the left panel. Representative data from three experiments are shown here. (C) Proximity ligation assay (PLA) to detect the interaction between SMARCB1 and Gli-1. BCBL-1 cells treated with solvent or LXA4 were washed, fixed, permeabilized, and reacted with SMARCB1 and Gli-1 primary antibodies, followed by PLA to assess the interactions between SMARCB1 and Gli-1. Nuclei were counterstained with DAPI. The differential interference contrast (DIC) image shown in the merged panels corresponds to the fluorescence image. The PLA reaction was detected using the Duolink red detection agent. Red puncta in the nucleus indicate a positive PLA signal, suggesting interactions between the two proteins. Images were captured at a ×40 magnification.
In LXA4-treated PEL cells, this interaction may lead to a subsequent decrease in Gli-1 expression. Since we observed the nuclear translocation of Gli-1 when PEL cells were treated with LXA4 (Fig. 5D), we hypothesize that the increased SMARCB1 expression in LXA4-treated cells (Fig. 8Ai) may directly prevent the transcription of Gli-1 (Fig. 8Aii). We had observed the same when we stained and probed LXA4-treated KSHV-infected HMVEC-d cells for SMARCB1 and Gli-1 (Fig. 6D).
FIG 8.
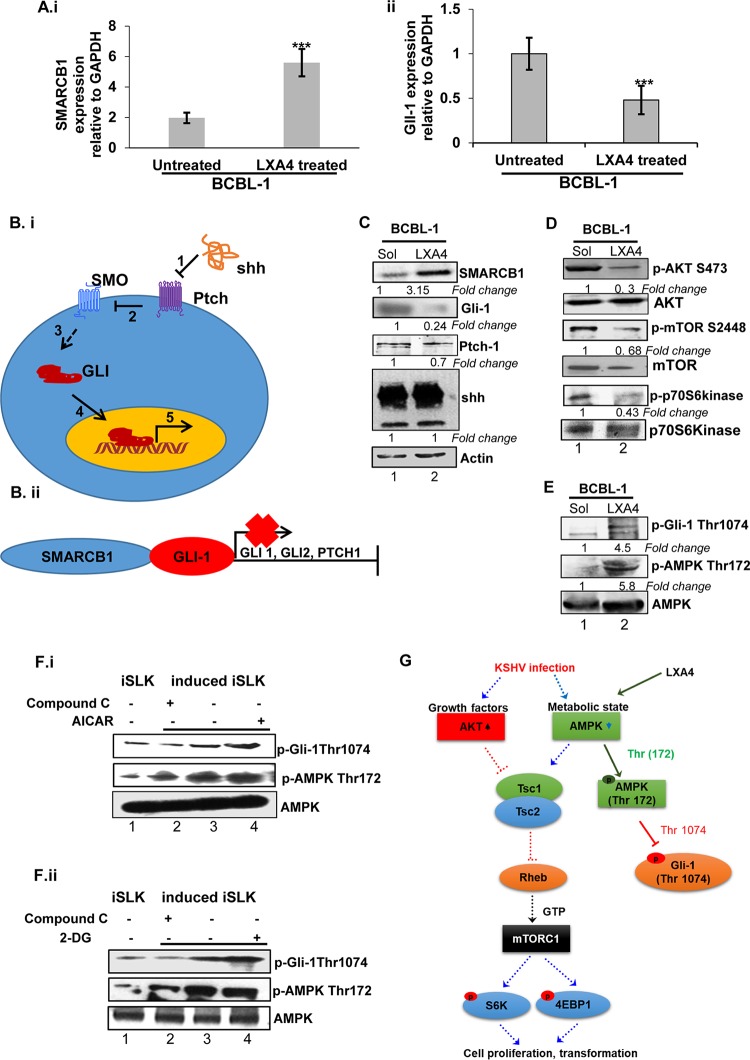
Noncanonical hedgehog signaling in LXA4-treated BCBL-1 cells. (A) cDNA from LXA4-treated and untreated BCBL-1 cells was harvested, and gene expression for SMARCB1 (i) and Gli-1 (ii) was examined. Each bar represents the fold change in gene expression ± standard deviation (SD) from three independent experiments. These fold changes were calculated after normalization to the expression of the GAPDH gene. (B) (i) Schematic showing the steps of canonical hedgehog signaling. In step 1, the shh ligand protein binds to the Ptch receptor. In step 2, in the absence of the ligand, Ptch inhibits SMO, a downstream protein in the pathway. In step 3, the binding of shh relieves SMO inhibition, leading to activation of the GLI transcription factors: the activators GLI1 and GLI2 and the repressor GLI3. In step 4, activated GLI accumulates in the nucleus, and in step 5, activated GLI controls the transcription of hh target genes. (ii) SMARCB1 directly prevents the transcription of the glioma-associated oncogene homologue (GLI), thus resulting in reduced downstream hedgehog (hh) pathway target genes. (C) Lysates were extracted from LXA4-treated and solvent-treated BCBL-1 cells, and the blots were probed for Gli-1, Ptch-1, and shh. There was no change in the expression of the shh ligand (both the precursor and the 19-kDa active forms), but a significant decrease in the expression of Gli-1 and Ptch-1 was observed. (D) Downregulation of the LXA4-mediated Akt/mTOR pathway. Lysates obtained from latently infected and LXA4-treated BCBL-1 cells were immunoblotted with primary antibody for AKT, phosphorylated AKT (p-AKT), mTOR, phosphorylated mTOR (p-mTOR), S6 kinase, and phosphorylated S6 kinase (pS6kinase). A representative image of the blots with the fold change in the phosphorylation of AKT, mTOR, and S6 kinase is illustrated. (E) Phosphorylation of Gli-1 (p-Gil-1 Thr 1074) inactivates Gli-1, possibly in an AMPK-dependent manner, as represented by Western blotting. (F) iSLK and induced iSLK cells were mock treated or treated with AICAR and/or compound C (i) and 2-DG and/or compound C (ii) and analyzed by Western blotting for AMPK, phospho-AMPK, and phospho-Gli (Thr 1074). (G) Schematic showing the effect of LXA4 treatment on hedgehog signaling in an AMPK-mTOR-S6 kinase-dependent manner. KSHV infection leads to activation of AKT/mTOR signaling in B cells and endothelial cells, and this pathway is essential for both the lytic and the latent phases of the KSHV life cycle. LXA4 binds to the ALX receptor to reduce the levels of KSHV infection-induced AKT and the ERK pathway. LXA4 activates AMPK (Thr 172) to phosphorylate Gli-1 at Thr 1074, causing Gli-1 degradation, which affects cell proliferation and tumorigenesis. Dashed arrows show the pathway followed in KSHV-infected cells. Solid arrows show the pathway followed by LXA4.
LXA4 treatment regulates hedgehog signaling in an AMPK-mTOR-S6 kinase-dependent manner.
The mechanism of action of LXA4 in KSHV-infected cells was explored as a result of two findings: (i) the interaction of LXA4 with nucleosome complex components (Fig. 1D) and (ii) the overall decreased expression of Gli-1 in HMVEC-d cells infected with KSHV and treated with LXA4 compared to that in solvent-treated HMVEC-d cells (Fig. 6Eii). We observed a similar decrease in the expression of Gli-1 in PEL cells treated with LXA4 compared to solvent-treated cells (Fig. 8C). Surprisingly, this decrease in Gli-1 and Ptch-1 expression was not dependent on sonic hedgehog (shh) ligand activity, as we did not observe any significant change in shh expression (for either the 45-kDa precursor or the 19-kDa amino-terminal peptide, responsible for all known hedgehog signaling) (Fig. 8C) in the solvent- or LXA4-treated (48 h) PEL cells. Changes in the levels of various host cell signaling proteins specific to KS cell proliferation/survival (AKT and mTOR pathway) (Fig. 8D) and oncogenesis/immune suppression (programmed death-ligand 1 [PD-L1]) (Fig. 9) were evaluated. In KSHV latently infected cells, AKT, mTOR, and its downstream p70S6 showed increased expression, signifying proliferation and increased survival of the infected cells (Fig. 8D, lane 1). On treating BCBL-1 cells with LXA4, we observed decreased phosphorylation of AKT/mTOR proteins (Fig. 8D, lane 2). Interestingly, we found increased phosphorylation of Gli-1 at Thr 1074 in LXA4-treated PEL cells (P < 0.001) (Fig. 8E). We performed Western blotting (Fig. 8F) to confirm the effect of activators (2-deoxy-d-glucose [2-DG] and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate [AICAR]) and an inhibitor (compound C) of the AMPK pathway on the hedgehog pathway and Gli-1 stability. We cultured iSLK cells (latent infection model) and induced iSLK cells (lytic infection model) and treated those with AICAR or 2-DG (1 mM) or compound C (4 μM). We observed that the chemical activation of AMPK using AICAR or 2-DG led to the increased phosphorylation of AMPK, causing the phosphorylation of Gli-1 at Thr 1074, leading to the degradation of Gli-1 (Fig. 8F, lane 4). The effect of compound C was an antagonist to treatment with AICAR and 2-DG (Fig. 8F, lane 2). These observations suggest that the LXA4 treatment of PEL cells resembled the chemical effect of the AMPK activator (Fig. 8E and F). Gli-1 phosphorylation at Thr 1074 led to the degradation of Gli-1 through AMPK activation (through the phosphorylation of AMPK at Thr 172) and reduced the oncogenic potency of Gli-1 by preventing the transcription of target genes Gli-1 and Ptch-1. The destabilized Gli-1 may decrease the angiogenic processes associated with vascular endothelial growth factor C (VEGF-C)/vascular endothelial growth factor receptor 2 (VEGFR2)/VEGF-A expression (30–32).
FIG 9.
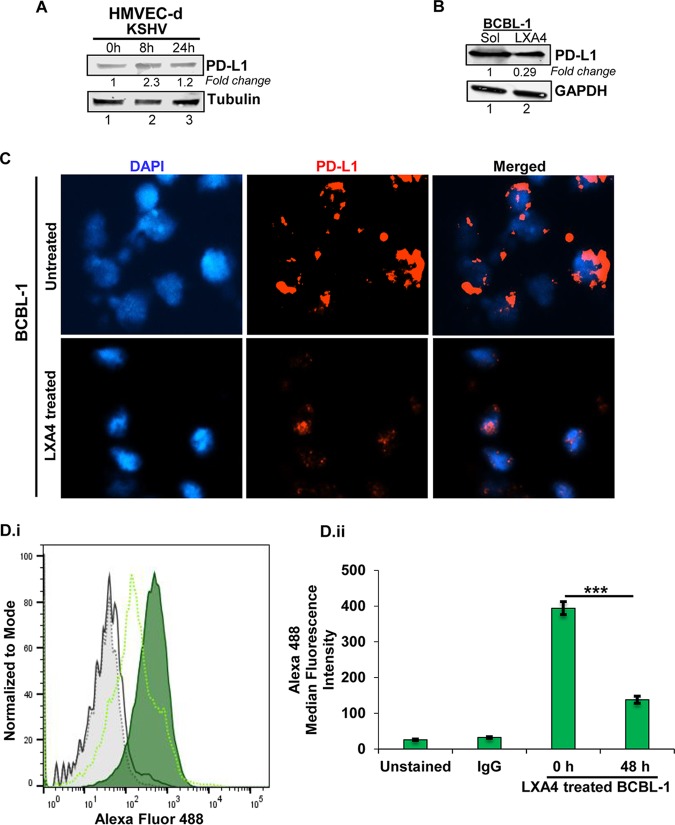
(A and B) Lysates from HMVEC-d cells infected with KSHV (30 DNA copies/cell) for the indicated times (0, 8, and 24 h) (A) and BCBL-1 cells (B) were Western blotted for PD-L1, stripped, and immunoblotted for tubulin and GAPDH, respectively. Representative data from three experiments are shown here, with the fold change being indicated. (C) In vitro PEL cell models. BCBL-1 cells treated with LXA4 were fixed and stained for PD-L1 using an Alexa Fluor 594-labeled antibody. (D) (i) BCBL-1 cells and LXA4-treated BCBL-1 cells were fixed, probed for surface PD-L1, stained with Alexa Fluor 488-labeled antibody, and analyzed by FACS using a Becton, Dickinson LSR II flow cytometer and FlowJo software. (ii) The hatched black peak represents unstained cells, the black peak represents the IgG control, and the solid green peak and hatched green peak represented stained solvent-treated and LXA4-treated BCBL-1 cells, respectively. ***, P < 0.001.
LXA4 may work as an immune checkpoint to reduce inflammation and validation of LXA4 as an anti-inflammatory drug for KSHV-infected cells.
Programmed death-ligand 1 (PD-L1) usually leads to diminished antitumor T-cell responses, which mediates the immune escape of tumor cells (33–35). We observed that de novo KSHV infection of primary HMVEC-d cells for 8 h increased PD-L1 expression by more than 2-fold (Fig. 9A, lane 2). PEL cells were treated with LXA4, and PD-L1 expression was measured via immunofluorescence staining and flow cytometry (Fig. 9C and D, respectively). We observed a one-third (P < 0.001) decrease in the level of expression of the immunomodulatory protein PD-L1 in PEL cells treated with LXA4 compared to that in BCBL-1 cells mock treated with solvent (Fig. 9D, lane 2). Since mTOR and AKT activate PD-L1 in the tumor microenvironment (36), that could probably explain the increased PD-L1 expression in latently infected cells compared to that in LXA4-treated BCBL-1 cells (Fig. 9B to D).
DISCUSSION
The KSHV latent-to-lytic switch in latently infected PEL cells primarily depends on the activation of RTA (ORF50), which can initiate lytic cycle gene expression and complete productive viral replication. Our study demonstrates that LXA4 treatment of KSHV-infected cells modulates the chromatinization of the KSHV genome to facilitate the lytic switch and replication, and it also alters host cell signal transduction.
Histone-DNA complex formation makes DNA inaccessible for transcription, replication, or DNA repair (37). The histone acetyltransferases (HATs) and the chromatin-remodeling complexes are cellular machinery allowing the access of target promoters to transcription factors (38, 39). HATs facilitate the process by adding acetyl groups to the amino-terminal tails of histones. In contrast, chromatin-remodeling complexes use the energy of ATP hydrolysis to weaken the interactions between histone core particles and DNA (40). In this report, we show that LXA4 treatment of KSHV-infected cells reduces the expression of the HDAC protein (Fig. 1Dii, lane 2). HDAC is a negative regulator of primary lytic cycle switch ORF50 (11) and acts as a sensor for lytic replication in infected cells, thereby controlling the latent and lytic phases of the KSHV life cycle (41). Using carboxyl affinity purification and mass spectrometry analysis, we identified that LXA4 has an affinity to the components required for KSHV latency establishment, chromatin maintenance, and genome replication. Previous studies have shown that at higher concentrations (>30 nmol/liter), LXA4 binds to AhR, the aryl hydrocarbon receptor; following this binding, AhR enters the nucleus and joins with the AhR nuclear translocator (ARNT) to activate the transcription of genes (22). Observations from the previous study indicated that the ATPase subunit of SWI/SNF complexes is a bona fide coactivator for AHR/ARNT-dependent transcription of the genes associated with the regulation of lipid metabolism. Infection of HMVEC-d cells with KSHV followed by LXA4 treatment showed the nuclear translocation of the AhR protein (Fig. 1Fi and ii), further supporting the overexpression and probable nuclear translocation of AhR in LXA4-treated BCBL-1 cells (Fig. 1E). The nuclear translocation of AhR might be responsible for the affinity interaction of cellular nucleosome complex proteins to LXA4 (Table 1). Among these LXA4-interacting proteins are MCM proteins, nucleosome assembly protein 1 (NAP1), high-mobility group protein B1 (HMGB1), and SWI/SNF components (Table 1). Nucleosome assembly protein 1-like protein 1 (NAP1L1) has been reported to associate with KSHV latency protein LANA-1 (42). Our study underscores the role of the LXA4 binding components identified by mass spectrometry analysis in viral replication and transcription. Major subunits of SWI/SNF were identified from our mass spectrometry analysis (Table 1), and immunoblot assays explicitly demonstrated that SMARCB1 is one of the core components of the SWI/SNF complex, which was upregulated in BCBL-1 cells treated with LXA4 for 48 h (Fig. 1D). Our study demonstrates that SMARCB1 can activate the KSHV lytic cycle switch ORF50 promoter and sustains ORF50’s expression (Fig. 4A).
Previous studies have shown that during KSHV latency, the PRC complex and other repressive chromatin-associated factors, such as H3K27me3, are identified on lytic gene-coding regions of the KSHV genome (43). The role of CREB-binding protein (CBP) and SWI/SNF in the regulation of the RTA (ORF50) promoter was investigated by Gwack and coworkers (21), who demonstrated that the C-terminal activation domain of RTA forms a complex with TRAP mediators, SWI/SNF, and CBP. During KSHV reactivation, RTA recruits these cellular factors onto the RTA promoter and other RTA-responsive lytic promoters, resulting in their transcriptional activation (21). Collectively, these studies show that KSHV reactivation requires the recruitment of HATs by cellular and viral transcription factors to the viral promoters, resulting in KSHV chromatin remodeling. We demonstrate that RTA-mediated KSHV reactivation and the recruitment of SWI/SNF at the RTA promoter require SMARCB1 activity to reorganize the viral promoter chromatin structure and recruit basal transcriptional factors, such as Brg1, to initiate regulated viral gene expression (Fig. 3A and B and 4D). ChIP analysis (Fig. 3B) showed that SMARCB1 binds to the KSHV lytic promoter (ORF50) but not the latency promoter (ORF73). We show that LXA4 treatment leads to HDAC inhibition (Fig. 1Dii), which may lead to a robust increase in pORF50 association with SMARCB1. This finding is in agreement with previous reports showing NaB-induced histone acetylation to be responsible for the recruitment of the SMARCB1 chromatin-remodeling complex to ORF50 (44). Silencing of SMARCB1 decreased Brg1 expression (Fig. 4Bi and Fig. 4C) and reduced ATPase activity (Fig. 4D), which is required for RTA activation. These findings are supported by previous reports, which suggested that the residual activity of SWI/SNF and Brg1 depends on SMARCB1 expression (45). We observed a change in the expression of ARID1 when SMARCB1 was silenced (data not shown), and ARID1 is associated with drug resistance and tumor progression (46).
It has already been validated that SMARCB1 directly prevents the transcription of Gli-1, further preventing downstream target gene expression (27). In our present work, we found similar results in a lytic model of KSHV-infected cells in which the knocking down of SMARCB1 increased Gli-1 expression (Fig. 5A and B) both at the gene level and at the protein level. In particular, our findings from Fig. 5Ei shows a significant increase in Gli-1 levels in KSHV-infected BJAB cells. Also, treating PEL cells with LXA4 showed a significant dip in Gli-1 levels (Fig. 5Eii). These findings were further supported by the increased nuclear SMARCB1 expression in KSHV-infected cells compared to that in LXA4-treated cells, which could have directly inhibited nuclear Gli-1 activity (Fig. 6). We further verified the SMARCB1–Gli-1 interaction with a more confirmatory experiment (Fig. 7). We observed an interaction between SMARCB1 and Gli-1 in both BCBL-1 cells (Fig. 7A) treated with solvent and BCBL-1 cells treated with LXA4, while similar results were replicated and validated in iSLK cell (latent infection) and induced iSLK cell (lytic infection) models (Fig. 7B). Also, we observed an overall decrease in Gli-1 expression in induced iSLK cells and LXA4-treated BCBL-1 cells compared to that in iSLK and BCBL-1 cells.
Here, for the first time, we show that treating PEL cells with LXA4 reduced Gli-1 expression. Decreased Gli-1 expression in LXA4-treated BCBL-1 cells could not be explained by the canonical hedgehog pathway (Fig. 8Bi). We explored the cross talk between AMPK/AKT and hedgehog signaling pathways in KSHV-infected cells (Fig. 8G). The phosphatidylinositol 3-kinase/AKT/mTOR pathway is highly upregulated and is vital for cell survival in KSHV-infected cells (47). We observed a decrease in AKT/mTOR/S6 kinase 1 signaling along with an upregulated AMPK level in LXA4-treated BCBL-1 cells. Our results suggest that activated AMPK might be associated with the phosphorylation of Gli-1 at the Thr 1074 site (Fig. 8E and Fi and ii). This observation was in a previous report from Li et al., who showed that AMPK phosphorylated Gli-1 at three novel sites and induced Gli-1 protein degradation (48). Together, our study established cross talk between the host AMPK-dependent mTOR/S6 kinase 1 and hh pathways, which provides a mechanism for hh ligand-independent Gli-1 downregulation in LXA4-treated BCBL-1 cells (Fig. 8C).
We report that decreased Gli-1 expression upon LXA4 treatment is primarily regulated through noncanonical host hh signaling. HDAC inhibition upon LXA4 treatment may lead to the induction of ORF50. Reactivation of KSHV is an extremely complex process involving a combination of both viral and cellular factors, including recruitment of SMARCB1 and the SWI/SNF complex at the RTA promoter, unwinding the replication nucleosome to allow viral replication. As the SWI-SNF complex regulates nucleosome mobilization, the nucleosome density at Gli-1 target promoters may be increased in LXA4-treated PEL cells, causing an evident decrease in overall Gli-1 expression by direct transcriptional inhibition by SMARCB1, as shown by Jagani et al. (27).
KS tumor progression is dependent on various angiogenic (VEGF-A, VEGF-C, angiogenin, and angiopoietin 2) factors (49–51). Importantly, total Gli-1 can transcriptionally upregulate the expression of VEGF-C and thereby promote angiogenesis (32). We performed a tumor formation assay to understand the role of Gli-1 in tumorigenesis. We examined the effect of a Gli-1 inhibitor (GANT-61) on tumor formation in a human Kaposi’s sarcoma tumor-derived cell line (KS-IMM cells). KS-IMM cells are an immortalized KS cell line that have the general characteristics of KS spindle cells but that no longer carry KSHV and are an excellent model for testing the effectiveness of inhibitor angiogenesis, inflammation, and tumorigenesis (17, 52). We monitored the sphere-forming efficiency (SFE) and the average volume of the formed spheres, which were both significantly decreased compared to those in mock-treated (nontreated) control tumor spheres (data not shown). These observations suggest the potential role of Gli-1 inhibitors in regressing tumor formation during KS.
Recent studies emphasized that many inflammatory diseases and cancers overexpress programmed death-ligand 1 (PD-L1) to evade the host immune system and result in a poor prognosis in patients (53, 54). Likewise, aggressive B-cell lymphomas and virus- and immunodeficiency-associated malignancies, including KS, have been reported to overexpress PD-L1 (55, 56). A recent study reported that KSHV infection of human monocytes increased PD-L1 prosurvival cell surface protein and uncovered a novel mechanism of KSHV-guided immune regulation to allow virus progression and survival in the host (57). In concurrence with their findings, we observed that KSHV de novo infection upregulates PD-L1 expression in primary endothelial cells (Fig. 9A). Interestingly, when KSHV-positive PEL cells were treated with LXA4, a significant reduction in PD-L1 expression was observed (Fig. 9B to D). Along with our previous studies underscoring the antiangiogenic and anti-inflammatory potential of LXA4 (16, 17), there is considerable potential for LXA4 treatment to inhibit PD-L1 expression in the infected cells (Fig. 9B). Our findings also demonstrated why KSHV downregulates LXA4 secretion (17, 25), possibly to efficiently take over the host immune system and reduce PD-L1 expression (Fig. 9B to D).
Anti-inflammatory nonsteroidal anti-inflammatory drugs (aspirin) and COX-2 inhibitors, in combination with antitumor immunity-enhancing chimeric antigen receptor T-cell therapy, have shown promise in many cancers, and a lot more have gained attention recently (58). COX-2 inhibitors and aspirin have immune-suppressive properties and inhibit the expression of T-lymphocyte-coinhibitory molecules CTLA4, PD-1, and the PD-1 ligand, PD-L1 (59–62). Aspirin treatment was synergistic with an anti-PD-L1 blockade in eradicating tumors (63). As cancer immunotherapy with an immune-checkpoint blockade, anti-PD-1/PD-L1, and anti-CTLA-4 antibodies revolutionized cancer medicine (53, 54), an addition of safe therapeutics like LXA4 and aspirin-triggered lipoxin might be an attractive choice in combination therapies against KSHV-linked cancers (58).
Overall, our findings suggest that treatment of KSHV-infected cells with LXA4 not only destabilizes viral latency through chromatin modulation but also suppresses tumor growth by modulating the cellular hedgehog and PD-1/PD-L1-associated signaling pathways (Fig. 10). These significant findings warrant attention to venture into the unexplored hh signaling associated with the KSHV life cycle. Considering next-generation hh/Gli inhibitors with improved efficacy in various cancers, it would be important to test their therapeutic potential along with anti-inflammatory LXA4 in in vivo mouse models of PEL and KS. Here, we also reemphasize the prospective role of LXA4 as a safe adjuvant to available immunotherapies against KSHV infection, which can be tested in other viral malignancies, too.
FIG 10.
LXA4, a potential therapeutic agent against KSHV. (A) The overall anti-inflammatory action of LXA4 in vivo is attributed to its interactions with multiple receptors, including (i) direct activation of ALX as a receptor agonist and (ii) direct activation of the nuclear receptor AhR. (B) Lipoxin modulates hedgehog signaling and adaptive immunity in KSHV-infected cells. Previous work from Sharma-Walia’s lab (17, 25) has shown that the ALX/FPR receptor is vital for LXA4 signaling and that blocking this receptor elevates the levels of NF-κB, ERK, and COX-2. LXA4 downregulates the AKT pathway in KS-IMM cells and KSHV-infected primary endothelial cells. In the present study, we postulate that LXA4 may be an endogenous ligand that facilitates the translocation of AhR into the nucleus, where it heterodimerizes with the AhR nuclear translocator (ARNT). AhR-ARNT induces the transcription of target genes by the recruitment of various components of the transcriptional machinery, such as ATP-dependent chromatin-remodeling components, such as Brg-1 (the mechanism has been explored in other systems but has yet to be proved in KSHV-infected cells). The proposed mechanism of action of LXA4 is based on our two current findings: (i) the interaction of LXA4 with nucleosome complex components possibly mediated by AhR and (ii) the overall decreased expression of Gli-1 in LXA4-treated PEL cells compared to that in untreated PEL cells. LXA4 inhibits Gli-1 expression noncanonically at the cytoplasmic level through AMPK activation (indicated by “1 Cytoplasm”). The AKT pathway is regulated by AMPK and, in turn, regulates the mTOR/S6 kinase 1 pathway. The phosphorylation of Gli-1 at the Thr 1074 site in LXA4-treated PEL cells suggests the direct inhibition of hedgehog signaling mediated through AMPK. LXA4 treatment decreased PD-L1 expression, which is related to a decrease in AKT activation and the associated inflammation in LXA4-treated BCBL-1 cells. LXA4 also inhibits Gli-1 expression noncanonically at the nuclear level (indicated by “2 Nucleus”). LXA4 treatment of KSHV-infected BCBL-1 cell inhibited HDAC expression and increased SMARCB1 expression. SMARCB1 directly interacts with Gli-1 to repress its transcriptional activity. SMARCB1, importantly, regulates RTA epigenetically to initiate and carry viral lytic gene replication and viral progression.
MATERIALS AND METHODS
Cells.
PEL cells (KSHV positive [KSHV+]/Epstein-Barr virus negative [EBV−]; BCBL-1 and BC-3 cells), BJAB cells (KSHV- and EBV-negative B lymphoma cells), as well as BJAB-KSHV cells (64) (a generous gift from Blossom Damania, University of North Carolina) were cultured in RPMI 1640 medium, GlutaMAX supplement (Gibco Life Technologies, Grand Island, NY) supplemented with 10% (vol/vol) fetal bovine serum (FBS; HyClone, Logan, UT), and penicillin-streptomycin (Gibco). TREx-BCBL-1-RTA and TREx-BCBL-1 cells (a gift from J. Jung, University of Southern California) were cultured in the above-mentioned medium containing 200 μg/ml hygromycin B. The SLK cell line was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (vol/vol) FBS, penicillin-streptomycin, and 2 mM l-glutamine (Gibco). The iSLK.219 cell line was grown and maintained in DMEM supplemented with 10% FBS and penicillin-streptomycin containing G418 (250 μg/ml), hygromycin (400 μg/ml), and puromycin (10 μg/ml) (65). Doxycycline (0.2 μg/ml) was used for lytic cycle induction in iSLK cells. Primary human dermal microvascular endothelial cells (HMVEC-d cells; Lonza, Walkersville, MD) were cultured in EBM-2 supplemented with EGM-2 bullet kit medium (Lonza). All cells were tested for mycoplasma and confirmed to be negative using a MycoAlert mycoplasma detection kit (Lonza). Healthy B cells were prepared from whole blood from healthy donors by the standard protocol in our lab using CD19 Dynabeads (Thermo Fisher Scientific) (66).
Induction of KSHV lytic cycle.
These studies were approved by the RFUMS Institutional Review Board. After informed consent was obtained in accordance with the Declaration of Helsinki, peripheral blood was collected in sodium heparin Vacutainer tubes (Becton, Dickinson, Franklin Lakes, NJ). Induction of the KSHV lytic cycle was achieved by treatment with tetradecanoyl phorbol acetate (20 ng/ml) or 3 mM sodium butyrate for 48 h. TREx-BCBL-1-RTA cells were induced with doxycycline at a final concentration of 1 μg/ml. KSHV virions were produced and purified from BCBL-1 cells according to the methods described previously (67). For de novo KSHV infection, 60 to 80% confluent HMVEC-d cells were washed twice with phosphate-buffered saline (PBS) and infected with purified KSHV at 30 DNA copies/cell (multiplicity of infection [MOI]), and unless stated otherwise, were used for all experiments.
Plasmids.
The KSHV ORF50 promoter construct (p2500Luc) was obtained from George Miller (Yale University School of Medicine, New Haven, CT) (68). pDONR223_SMARCB1_WT was a gift from Jesse Boehm, William Hahn, and David Root (Addgene overexpression plasmid no. 81791). Cells were transfected using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The effect of the SMARCB1 expression plasmid on the KSHV ORF50 promoter (p2500Luc construct, a 2,500-bp ORF50 promoter-luciferase reporter cloned in the pcDNA3.1 vector from Invitrogen) was measured using a luciferase reporter assay (Promega, Madison, WI).
Reagents.
LXA4 (catalog no. 90410) was purchased from Cayman Chemical Company. For the ATPase assay, an ADP-Glo Max assay (catalog no. V7002), Dynabeads protein A (catalog no. 10001D), and single-stranded DNA (for use in the 1× ATPase reaction buffer) were purchased from Promega, Invitrogen, and Integrated DNA Technologies, respectively. Doxycycline (Dox; catalog no. D3072), spectinomycin (catalog no. S0692), Polybrene (catalog no. TR-1003), AMPK activators (2-deoxy-d-glucose [2-DG; catalog no. D8375] and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate [AICAR; catalog no. A9978), and inhibitor (compound C; CAS 866405-64-3) were bought from Sigma-Aldrich.
Antibodies.
Rabbit antibodies (Abs) against human SMARCB1 (clone D9C2, catalog no. 8745), Brg1 (clone D1Q7F, catalog no. 49360), Brm (clone D9E8B, catalog no. 11966), ARID1A (clone D2A8U, catalog no. 12354), ARID1B (clone E9J4T, catalog no. 92964), AMPKα (clone D5A2, catalog no. 5831), mTOR (catalog no. 2972), phospho-mTOR (Ser 2448; catalog no. 2971), AKT (catalog no. 9272), phospho-AKT (catalog no. 9271), and PD-L1 (clone PD-L1 [E1L3N] XP, catalog no. 13684) were obtained from Cell Signaling Technology. Rabbit anti-Ptch-1 (clone ab53715) and TATA-binding protein (TBP; clone ab63766) antibodies were obtained from Abcam, Inc. (Cambridge, MA). Rabbit anti-phospho-Gli-1 (clone 12935) and phospho-AMPK (Thr 172; clone 40H9, catalog no. 2535) antibodies were obtained from Signalway Antibody (MD, USA). Mouse antibodies (Abs) against human Gli-1 (catalog no. sc-515781) and Brg-1 (catalog no. sc-17796) were obtained from Santa Cruz Biotechnology, Inc. (Sana Cruz, CA). We used KSHV K8 (clone 8C12G10G1) antibody from Abnova. LANA-1 antibody was obtained from Bala Chandran, University of South Florida, and anti-KSHV RTA was obtained from Abbiotec, LLC (San Diego, CA).
Horseradish peroxidase-conjugated secondary antibodies were obtained from KPL, Inc. (Gaithersburg, MD). Secondary antibodies conjugated with Alexa Fluor 488 and Alexa Fluor 594 were obtained from Thermo Fisher Scientific. The secondary antibodies anti-mouse immunoglobulin tagged with IR Dye 680RD and anti-rabbit immunoglobulin tagged with IR Dye 800CW were from LI-COR Biotechnology, Lincoln, NE.
LXA4 affinity purification.
CarboxyLink agarose column (catalog no. 44899; Pierce/Thermo Fisher Scientific) was equilibrated, washed, and resuspended using coupling buffer (0.1 M morpholineethanesulfonic acid, pH 4.7) at 1:1 (vol/vol). Resuspended CarboxyLink resin was mixed 1:1 with a solution of 1 mM LXA4 (resuspended in reaction buffer) or with reaction buffer lacking LXA4 (control resin) for 5 min of mixing by end-over-end rotation. An equal volume (∼200 μl) of the freshly prepared coupling reagent 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC; 120 mg/ml) in reaction buffer was added to the CarboxyLink resin samples (with or without LXA4) and mixed end-over-end at 25°C for 3 h. The resins were then transferred to a 1-ml chromatography column and allowed to drain by gravity. The columns were then washed with 5 column volumes of binding buffer to equilibrate for 1 h at 4°C. The columns were washed extensively (10 column volumes) in the washing buffer. One milligram of BCBL-1 cell lysate was incubated with each sealed column with end-over-end mixing for 1 h. Proteins were then eluted sequentially with elution buffer (0.1 to 0.2 M glycine-HCl, pH 2.5 to 3.0). The eluted fractions were assayed by SDS-PAGE and stained with Coomassie blue. Protein bands deemed to be enriched in the LXA4 resin relative to the control resin were isolated and subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the Rosalind Franklin proteomics facility.
Immunoprecipitation.
Cells were lysed in lysis buffer (product no. 87788; Thermo Fisher Scientific). Two hundred micrograms of cell lysate was precleared with 30 μl protein A/G-plus agarose beads by rotation at 4°C for 1 h. The precleared supernatants were incubated with 2 μg of antibody by rotation at 4°C for 4 h and then incubated with 30 μl protein A/G-plus agarose beads by rotation at 4°C overnight. The samples were collected after centrifugation, washed, and then suspended in SDS-PAGE sample loading buffer. After boiling for 5 min, the samples were subjected to SDS-PAGE and analyzed by Western blotting with the specific antibodies indicated above.
ChIP.
To detect the direct association of SMARCB1 with viral DNA, a chromatin immunoprecipitation (ChIP) assay was performed using a truChIP chromatin shearing kit (Covaris, Woburn, MA). Briefly, untreated BCBL-1 and BC-3 cells (3 × 107) or cells treated with LXA4 for 48 h were fixed with 1% (vol/vol) methanol-free formaldehyde in fixing buffer for 5 min, cross-linking was quenched using quenching buffer for 5 min at room temperature, and the cells were washed twice with cold PBS and then incubated in lysis buffer to break the cell membrane. Intact nuclei were collected by centrifugation at 1,700 × g for 5 min at 4°C and resuspended in shearing buffer containing protease inhibitors, and chromatin shearing was performed on an adaptive focused acoustics (AFA) ultrasonicator (Covaris model M220). Following chromatin shearing, ChIP was performed. Briefly, cellular debris was cleared from the sheared chromatin by centrifugation, and the supernatant was incubated overnight at 4°C with 2 μg of SMARCB1 antibody. Samples were incubated in ChIP-grade protein G magnetic beads for 2 h at 4°C to collect immune complexes and then washed successively with low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris [pH 8.1], 150 mM NaCl), then high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris [pH 8.1], 500 mM NaCl), and then LiCl wash buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris [pH 8.1]). DNA-protein complexes were eluted in 1% SDS prepared in 0.1 M NaHCO3. Cross-linking was reversed by adding 1 μl RNase A and NaCl (0.3 M) and incubating at 65°C for 5 h. Protein was removed by incubating the lysate with proteinase K at 55°C for 1 h. Subsequently, DNA was purified and resuspended in nuclease-free water. Real-time PCR was performed with the following KSHV-specific primers: primers for pORF73, CAAGGTTAAAGTGGGTTTGCTG and GGTTATTGGCCGTTTCTGTTTC, and primers for pORF50, GACAGTCCGCCATACTCTTC and CTGGCTCTACCACATCTTCATAG. ChIP results are represented as the fold enrichment over that for the IgG control.
Isolation of nuclear and cytoplasmic fractions for Western blot analysis.
Cells were harvested and used for the preparation of nuclear and cytoplasmic extracts using a nuclear extraction kit (catalog no. ab113474; Abcam). Briefly, cells were trypsinized, washed with PBS supplied with phosphatase inhibitor, and lysed with 1× hypotonic buffer to disrupt the plasma membrane. The cytoplasmic fraction was collected after pelleting the nuclei by centrifugation at 500 × g for 2 min. The nuclear pellet was washed two additional times with 1× hypotonic buffer to remove any loosely bound cytoplasmic contaminants before resuspension in complete lysis buffer. After measurement of the protein concentrations with a bicinchoninic acid protein assay reagent (Pierce, Rockford, IL), nuclear and cytoplasmic extracts were subjected to Western blotting with different antibodies. The purity of the nuclear extracts and cytoplasmic extracts was assessed by immunoblotting with anti-TATA-binding protein (anti-TBP) and anti-GAPDH antibodies, respectively.
Western blot analysis.
The whole-cell protein lysates were prepared using radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with protease inhibitor cocktail. Cells were incubated on a rocker at 4°C for 15 min, sonicated, and clarified by centrifugation for 15 min at 4°C at 15,000 × g. Equal amounts of samples were resolved by SDS-PAGE and subjected to Western blotting. Immunoreactive bands were developed by an enhanced chemiluminescence (ECL; catalog no. 34096; Thermo Fisher Scientific, Rockford, IL, USA) reaction, and the bands were scanned and quantitated using an AlphaImager system (Alpha Innotech Corporation, San Leonardo, CA).
To detect the primary antibodies (SMARCB1, ORF50, K8, Brg1, GAPDH, actin, tubulin, PD-L1), secondary anti-mouse and anti-rabbit immunoglobulin (IRDye 680 and IRDye 800 labeled, respectively) antibodies were used, and immunoblots were visualized using a LI-COR Odyssey system. Each experiment was performed in triplicate.
Lentivirus-mediated silencing of SMARCB1 in TREx-BCBL-1-RTA cells.
Three inducible SMART lentiviral human SMARCB1 shRNA constructs (clone identifiers, V3SH7669 [catalog no. 224915395], V3SH7669 [catalog no. 226644793], and V3SH7669 [catalog no. 227554669]; Dharmacon, GE Healthcare, Lafayette, CO) were tested for their ability to silence SMARCB1 efficiently. After transduction, cells were distributed into a complete medium and placed at 37°C in a humidified 5% CO2 atmosphere. After 48 h of transduction, the cells were selected with 3 μg/ml puromycin for 72 h and shRNA expression was induced using 1 μg/ml doxycycline (Dox). The usage of Dox for the induction of shRNA also reactivates these KSHV-infected cells. The percentages of live cells were measured at different time point until 72 h, using the trypan blue dye exclusion method. We did not observe any significant difference in the number of viable cells after treatment of TREx-BCBL-1-RTA cells with sh-SMARCB1 and the number after treatment with sh-Control. Cell lysates were harvested at 72 h after Dox induction, and the SMARCB1 knockdown efficiency was analyzed using Western blotting.
SMARCB1 lentiviral particle-mediated silencing in HMVEC-d cells.
HMVEC-d cells were transduced with either control shRNA lentiviral particles (sh-Control; catalog no. sc-108080; Santa Cruz Biotechnology, Inc.) or shRNA lentiviral particles against SMARCB1 (sh-SMARCB1; catalog no. sc-35668-V; Santa Cruz Biotechnology, Inc.). After transduction, the cells were distributed into a complete medium and placed at 37°C in a humidified 5% CO2 atmosphere. After 24 h, the transduced cells were selected by 3-μg/ml puromycin for 5 to 7 days. These transduced cells were either left uninfected or infected with KSHV (30 DNA copies/cell). The percentage of live cells at different time points was calculated using the trypan blue dye exclusion method. We did not observe any significant difference in the number of viable cells after treatment of the cells with sh-SMARCB1 and the number after treatment with sh-Control. The cell lysates were harvested, and the SMARCB1 knockdown efficiency was analyzed by Western blotting.
Immunofluorescence assay.
PEL cells were fixed on slides, permeabilized with ice-cold acetone for 15 min, washed twice in 1× PBS, and then incubated with target-specific primary antibodies against the specific proteins, followed by fluorescent dye-conjugated secondary antibodies. All images were acquired at the magnifications indicated in the figure legends.
HMVEC-d cells grown on eight-chambered slides were either left uninfected or infected with KSHV (30 DNA copies/cell), washed with Hanks balanced salt solution, fixed with 4% paraformaldehyde solution for 20 min, and permeabilized using 0.2% Triton X-100 in PBS for 5 min, followed by washing with PBS. The cells were then blocked using 5% milk for 30 min at room temperature and incubated with target-specific primary antibodies, followed by staining with secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 594. The stained cells were mounted in mounting medium with DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen) and visualized with a Nikon 80i fluorescence microscope equipped with MetaMorph digital imaging software.
Healthy and KS skin tissue sections obtained from the AIDS and Cancer Specimen Resource (ACSR; San Francisco, CA) were deparaffinized with HistoChoice clearing reagent and hydrated with water before microwave treatment in antigen retrieval buffer (Sigma-Aldrich) for 15 min. Sections were blocked with a blocking solution (5% nonfat dry milk in PBS), followed by incubation with a specific primary antibody overnight at 4°C. These sections were incubated with secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 594 for 2 h, mounted in mounting medium with DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen), and visualized with a Nikon 80i fluorescence microscope equipped with MetaMorph digital imaging software.
Immunofluorescence and FISH.
Uninfected and KSHV-infected HMVEC-d cells were fixed as described above and incubated with rabbit anti-SMARCB1 antibodies, followed by donkey anti-rabbit immunoglobulin Alexa Fluor 594 secondary antibodies. These cells were fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.2% Triton X-100 for 5 min, and treated with 0.1 M Tris-HCl (pH 7.0) for 2 min and 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) twice for 2 min each time. The cells were treated with denaturing solution (70% formamide in 2× SSC) at 70°C for 2 min, washed, dried, and subjected to in situ hybridization. A KSHV fluorescence in situ hybridization (FISH) probe was developed and validated by the Anatomic Pathology FISH Laboratory, Medical College of Virginia, Richmond, VA. The entire spectrum green-labeled bacterial artificial chromosome (BAC36) KSHV genome was used as a probe. The KSHV probe was diluted (1:10) in DenHyb hybridization solution (Insitus Biotechnologies, Albuquerque, NM) and incubated with cells for hybridization in a humidified chamber at 37°C overnight. The slides were sequentially washed in 2× SSC (10 min) and deionized water (1 min), and the nuclei were counterstained with DAPI and examined.
Proximity ligation assay microscopy.
A Duolink PLA kit (catalog no. DUO92101; Sigma) was used to detect protein-protein interactions. BCBL-1 cells (treated with solvent or LXA4) were cultured, fixed, permeabilized with prechilled acetone, and blocked with Duolink blocking buffer for 30 min at 37°C. The cells were incubated with primary antibodies (to SMARCB1, LANA-1/SMARCB1, and Gli-1) diluted in Duolink antibody diluents for 1 h, washed, and then further incubated for another 1 h at 37°C with species-specific PLA probes (plus and minus probes) under hybridization conditions and in the presence of 2 additional oligonucleotides to facilitate the hybridization of PLA probes only if they were in proximity (<40 nm). A ligation mixture and ligase were then added to join the two hybridized oligonucleotides to form a closed circle. Several cycles of rolling-circle amplification using the ligated circle as a template were performed by adding an amplification solution to generate a concatemeric product extending from the oligonucleotide arm of the PLA probe. Lastly, a detection solution consisting of fluorescently labeled oligonucleotides was added, and the labeled oligonucleotides were hybridized to the concatemeric products. The signal was detected as a distinct fluorescent dot in the Texas Red channel and analyzed by fluorescence microscopy. PLA was quantified using Duolink software.
Measurement of host gene expression by real-time RT-PCR.
To assess changes in host gene expression due to LXA4 treatment, total RNA was isolated using the TRIzol reagent, and a cDNA library was created using a high-capacity cDNA reverse transcription kit (Life Technologies). The cycle threshold (CT) values for each gene were determined using gene-specific primers and SYBR green probe-based real-time reverse transcription-PCR (RT-PCR). ΔCT values relative to the values for GAPDH were assessed for each condition. The value for gene expression under the uninfected/untreated conditions was arbitrarily set to 1, and the fold induction was based on the ΔCT differential relative to this condition. The following primers were used for the reactions and were obtained from RealTimePrimers: for SMARCB1, forward primer 5′-AGA AGT TGA TGA CGC CTG AG-3′ and reverse primer 5′-AGG ACT CGA TCT GCT GTC TG-3′, and for Gli-1, forward primer 5′-TGTTCAACTCGATGACCC-3′ and reverse primer 5′-GTCATGGGGACCACAAGG-3′.
ATPase assay.
An ATPase assay was carried out for TREx-BCBL-1-RTA cells (nontransfected), TREx-BCBL-1-RTA cells with SMARCB1 KD, and the SMARCB1 expression plasmid using a previously described procedure (69). Briefly, the nuclear extract was prepared, further isolating the remodeling complex. The isolated chromatin-remodeling complexes were found to retain catalytic activity while bound to the beads used in the immunoprecipitations. The luciferase assay was performed in small volumes in 96-well opaque-bottom plates for the sensitive quantitation of ATPase catalytic activity using the ADP-Glo Max assay (catalog no. V7001; Promega).
Flow cytometry.
BCBL-1 cells and LXA4-treated BCBL-1 cells were stained for programmed death-ligand 1 (PD-L1; cell membrane protein) and anti-rabbit IgG (H+L); the F(ab′)2 fragment (Alexa Fluor 488) was used as a secondary antibody before analysis using FlowJo software.
ELISA for Gli-1 detection.
The levels of Gli-1 in the cell lysates were measured using a commercially available enzyme immunoassay (MyBioSource). Briefly, cells were collected and centrifuged for 5 min at 1.000 × g at 2 to 8°C. The supernatant was removed, and the cells were resuspended with 1× PBS (pH 7.2 to 7.4). The suspended cells were centrifuged for 5 min at 1,000 × g and 2 to 8°C, the cells were diluted with 1× PBS (pH 7.2 to 7.4), and the Gli-1 concentration was estimated. Each sample was run in triplicate, and the assay was repeated a minimum of 3 times. The quantities of Gli-1 among the various samples were normalized by the protein content.
Statistical analysis.
All results are expressed as mean ± standard deviation (SD) from at least three independent experiments (n ≥ 3) to ensure reproducibility.
ACKNOWLEDGMENTS
We thank Robert Dickinson for help with the fluorescence-activated cell sorter (FACS) analyses at the RFUMS FACS/cell sorter core facility. We thank Xinli Yang at the Midwest Proteome Centre, RFUMS, mass spectrometry facility for mass spectrometry analysis and HRSA. We thank Patricia Loomis for providing help with confocal microscopy. We also thank Li Wan, a summer research student, for technical support. We gratefully acknowledge Jae Jung, University of Southern California, for the TREx-BCBL-1-RTA cells. We are grateful to Rolf Renne (University of Florida) for providing iSLK producer cells. We are thankful to the NIH AIDS Reagent Program for healthy and KS skin tissue specimens. We thank Bala Chandran from the University of South Florida for the LANA-1 antibody. We thank George Miller, Yale University School of Medicine, for the kind gift of p2500Luc, an ORF50 promoter-luciferase reporter construct.
We gratefully acknowledge the support of National Institutes of Health grant R01CA192970 to N.S.-W. The RFUMS mass spectrometry facility is supported by NIH grant NCRR S100D010662, and HRSA is supported by grant 76HFU3610-01-00.
REFERENCES
- 1.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A 96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YB, Rahemtullah A, Hochberg E. 2007. Primary effusion lymphoma. Oncologist 12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 3.Schulz TF. 1999. Epidemiology of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. Adv Cancer Res 76:121–160. doi: 10.1016/S0065-230X(08)60775-7. [DOI] [PubMed] [Google Scholar]
- 4.Ye F, Lei X, Gao SJ. 2011. Mechanisms of Kaposi’s sarcoma-associated herpesvirus latency and reactivation. Adv Virol 2011:193860. doi: 10.1155/2011/193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. 1998. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol 72:8309–8315. doi: 10.1128/JVI.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth Z, Brulois K, Lee HR, Izumiya Y, Tepper C, Kung HJ, Jung JU. 2013. Biphasic euchromatin-to-heterochromatin transition on the KSHV genome following de novo infection. PLoS Pathog 9:e1003813. doi: 10.1371/journal.ppat.1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukac DM, Renne R, Kirshner JR, Ganem D. 1998. Reactivation of Kaposi’s sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 8.Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. 1998. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toth Z, Maglinte DT, Lee SH, Lee HR, Wong LY, Brulois KF, Lee S, Buckley JD, Laird PW, Marquez VE, Jung JU. 2010. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog 6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukac DM, Yuan Y. 2007. Reactivation and lytic replication of KSHV In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 11.Gwack Y, Byun H, Hwang S, Lim C, Choe J. 2001. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi’s sarcoma-associated herpesvirus open reading frame 50. J Virol 75:1909–1917. doi: 10.1128/JVI.75.4.1909-1917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song MJ, Deng H, Sun R. 2003. Comparative study of regulation of RTA-responsive genes in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol 77:9451–9462. doi: 10.1128/jvi.77.17.9451-9462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwack Y, Hwang S, Lim C, Won YS, Lee CH, Choe J. 2002. Kaposi’s sarcoma-associated herpesvirus open reading frame 50 stimulates the transcriptional activity of STAT3. J Biol Chem 277:6438–6442. doi: 10.1074/jbc.M108289200. [DOI] [PubMed] [Google Scholar]
- 14.Wu TT, Usherwood EJ, Stewart JP, Nash AA, Sun R. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol 74:3659–3667. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang PJ, Shedd D, Gradoville L, Cho MS, Chen LW, Chang J, Miller G. 2002. Open reading frame 50 protein of Kaposi’s sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J Virol 76:3168–3178. doi: 10.1128/jvi.76.7.3168-3178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekharan JA, Sharma-Walia N. 2015. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res 8:181–192. doi: 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marginean A, Sharma-Walia N. 2015. Lipoxins exert antiangiogenic and anti-inflammatory effects on Kaposi’s sarcoma cells. Transl Res 166:111–133. doi: 10.1016/j.trsl.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Blow JJ, Hodgson B. 2002. Replication licensing—defining the proliferative state? Trends Cell Biol 12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tye BK. 1999. MCM proteins in DNA replication. Annu Rev Biochem 68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Kalimuthu SN, Chetty R. 2016. Gene of the month: SMARCB1. J Clin Pathol 69:484–489. doi: 10.1136/jclinpath-2016-203650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwack Y, Baek HJ, Nakamura H, Lee SH, Meisterernst M, Roeder RG, Jung JU. 2003. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi’s sarcoma-associated herpesvirus RTA-mediated lytic reactivation. Mol Cell Biol 23:2055–2067. doi: 10.1128/mcb.23.6.2055-2067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaldach CM, Riby J, Bjeldanes LF. 1999. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry 38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 23.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. 2006. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Hankinson O. 2002. Functional involvement of the Brahma/SWI2-related gene 1 protein in cytochrome P4501A1 transcription mediated by the aryl hydrocarbon receptor complex. J Biol Chem 277:11821–11827. doi: 10.1074/jbc.M110122200. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekharan JA, Huang XM, Hwang AC, Sharma-Walia N. 2016. Altering the anti-inflammatory lipoxin microenvironment: a new insight into Kaposi’s sarcoma-associated herpesvirus pathogenesis. J Virol 90:11020–11031. doi: 10.1128/JVI.01491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter JF. 2010. Crippling SWI-SNF makes tumors GLI-ful. Nat Med 16:1374–1376. doi: 10.1038/nm1210-1374. [DOI] [PubMed] [Google Scholar]
- 27.Jagani Z, Mora-Blanco EL, Sansam CG, McKenna ES, Wilson B, Chen D, Klekota J, Tamayo P, Nguyen PT, Tolstorukov M, Park PJ, Cho YJ, Hsiao K, Buonamici S, Pomeroy SL, Mesirov JP, Ruffner H, Bouwmeester T, Luchansky SJ, Murtie J, Kelleher JF, Warmuth M, Sellers WR, Roberts CW, Dorsch M. 2010. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat Med 16:1429–1433. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghali L, Wong ST, Green J, Tidman N, Quinn AG. 1999. Gli1 protein is expressed in basal cell carcinomas, outer root sheath keratinocytes and a subpopulation of mesenchymal cells in normal human skin. J Invest Dermatol 113:595–599. doi: 10.1046/j.1523-1747.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Stephen D, Joyner A, Curran T. 2005. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene 24:4026–4036. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter RL, Paw I, Zhu H, Sirkisoon S, Xing F, Watabe K, Debinski W, Lo HW. 2015. The gain-of-function GLI1 transcription factor TGLI1 enhances expression of VEGF-C and TEM7 to promote glioblastoma angiogenesis. Oncotarget 6:22653–22665. doi: 10.18632/oncotarget.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Geradts J, Dewhirst MW, Lo HW. 2012. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene 31:104–115. doi: 10.1038/onc.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter RL, Lo HW. 2012. Hedgehog pathway and GLI1 isoforms in human cancer. Discov Med 13:105–113. [PMC free article] [PubMed] [Google Scholar]
- 33.Xie QK, Zhao YJ, Pan T, Lyu N, Mu LW, Li SL, Shi MD, Zhang ZF, Zhou PH, Zhao M. 2016. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology 5:e1181252. doi: 10.1080/2162402X.2016.1181252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang X, Yu PC, Long D, Liao XL, Zhang S, You XM, Zhong JH, Li LQ. 2018. Prognostic value of PD-L1 expression in patients with primary solid tumors. Oncotarget 9:5058–5072. doi: 10.18632/oncotarget.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Drake CG, Pardoll DM. 2015. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lastwika KJ, Wilson W III, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. 2016. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 37.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 38.Workman JL. 2006. Nucleosome displacement in transcription. Genes Dev 20:2009–2017. doi: 10.1101/gad.1435706. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez JL, Chandy M, Carrozza MJ, Workman JL. 2007. Activation domains drive nucleosome eviction by SWI/SNF. EMBO J 26:730–740. doi: 10.1038/sj.emboj.7601524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. 1997. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol 71:314–324. doi: 10.1128/JVI.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta N, Thakker S, Verma SC. 2016. KSHV encoded LANA recruits nucleosome assembly protein NAP1L1 for regulating viral DNA replication and transcription. Sci Rep 6:32633. doi: 10.1038/srep32633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunther T, Grundhoff A. 2010. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog 6:e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. 2003. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol 77:11425–11435. doi: 10.1128/jvi.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Sansam CG, Thom CS, Metzger D, Evans JA, Nguyen PT, Roberts CW. 2009. Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer Res 69:8094–8101. doi: 10.1158/0008-5472.CAN-09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe H, Maeda D, Hino R, Otake Y, Isogai M, Ushiku AS, Matsusaka K, Kunita A, Ushiku T, Uozaki H, Tateishi Y, Hishima T, Iwasaki Y, Ishikawa S, Fukayama M. 2012. ARID1A expression loss in gastric cancer: pathway-dependent roles with and without Epstein-Barr virus infection and microsatellite instability. Virchows Arch 461:367–377. doi: 10.1007/s00428-012-1303-2. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt AP, Damania B. 2012. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Front Immunol 3:401. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li YH, Luo J, Mosley YY, Hedrick VE, Paul LN, Chang J, Zhang G, Wang YK, Banko MR, Brunet A, Kuang S, Wu JL, Chang CJ, Scott MP, Yang JY. 2015. AMP-activated protein kinase directly phosphorylates and destabilizes hedgehog pathway transcription factor GLI1 in medulloblastoma. Cell Rep 12:599–609. doi: 10.1016/j.celrep.2015.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadagopan S, Sharma-Walia N, Veettil MV, Bottero V, Levine R, Vart RJ, Chandran B. 2009. Kaposi’s sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis. J Virol 83:3342–3364. doi: 10.1128/JVI.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, Greene W, Yeh IT, Graham D, Gao SJ. 2007. Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol 81:3980–3991. doi: 10.1128/JVI.02089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavallin LE, Ma Q, Naipauer J, Gupta S, Kurian M, Locatelli P, Romanelli P, Nadji M, Goldschmidt-Clermont PJ, Mesri EA. 2018. KSHV-induced ligand mediated activation of PDGF receptor-alpha drives Kaposi’s sarcomagenesis. PLoS Pathog 14:e1007175. doi: 10.1371/journal.ppat.1007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albini A, Fontanini G, Masiello L, Tacchetti C, Bigini D, Luzzi P, Noonan DM, Stetler-Stevenson WG. 1994. Angiogenic potential in vivo by Kaposi’s sarcoma cell-free supernatants and HIV-1 tat product: inhibition of KS-like lesions by tissue inhibitor of metalloproteinase-2. AIDS 8:1237–1244. doi: 10.1097/00002030-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M, Kroemer G, Zitvogel L. 2016. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44:1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Buchbinder E, Hodi FS. 2015. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest 125:3377–3383. doi: 10.1172/JCI80012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. 2013. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronaghy A, Wang HY, Thorson JA, Medeiros LJ, Xie Y, Zhang X, Sheikh-Fayyaz S. 2017. PD-L1 and Notch1 expression in KSHV/HHV-8 and EBV associated germinotropic lymphoproliferative disorder: case report and review of the literature. Pathology 49:430–435. doi: 10.1016/j.pathol.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Host KM, Jacobs SR, West JA, Zhang Z, Costantini LM, Stopford CM, Dittmer DP, Damania B. 2017. Kaposi’s sarcoma-associated herpesvirus increases PD-L1 and proinflammatory cytokine expression in human monocytes. mBio 8:e00917-17. doi: 10.1128/mBio.00917-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, Zhu B, Li Y. 2017. Resolution of cancer-promoting inflammation: a new approach for anticancer therapy. Front Immunol 8:71. doi: 10.3389/fimmu.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis e Sousa C. 2015. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markosyan N, Chen EP, Evans RA, Ndong V, Vonderheide RH, Smyth EM. 2013. Mammary carcinoma cell derived cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activity. Breast Cancer Res 15:R75. doi: 10.1186/bcr3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. 2011. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res 71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anani W, Shurin MR. 2017. Targeting myeloid-derived suppressor cells in cancer. Adv Exp Med Biol 1036:105–128. doi: 10.1007/978-3-319-67577-0_8. [DOI] [PubMed] [Google Scholar]
- 63.Ooki A, Del Carmen Rodriguez Pena M, Marchionni L, Dinalankara W, Begum A, Hahn NM, VandenBussche CJ, Rasheed ZA, Mao S, Netto GJ, Sidransky D, Hoque MO. 2018. YAP1 and COX2 coordinately regulate urothelial cancer stem-like cells. Cancer Res 78:168–181. doi: 10.1158/0008-5472.CAN-17-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nun TK, Kroll DJ, Oberlies NH, Soejarto DD, Case RJ, Piskaut P, Matainaho T, Hilscher C, Wang L, Dittmer DP, Gao SJ, Damania B. 2007. Development of a fluorescence-based assay to screen antiviral drugs against Kaposi’s sarcoma associated herpesvirus. Mol Cancer Ther 6:2360–2370. doi: 10.1158/1535-7163.MCT-07-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sturzl M, Gaus D, Dirks WG, Ganem D, Jochmann R. 2013. Kaposi’s sarcoma-derived cell line SLK is not of endothelial origin, but is a contaminant from a known renal carcinoma cell line. Int J Cancer 132:1954–1958. doi: 10.1002/ijc.27849. [DOI] [PubMed] [Google Scholar]
- 66.Sharma-Walia N, Chandran K, Patel K, Veettil MV, Marginean A. 2014. The Kaposi’s sarcoma-associated herpesvirus (KSHV)-induced 5-lipoxygenase-leukotriene B4 cascade plays key roles in KSHV latency, monocyte recruitment, and lipogenesis. J Virol 88:2131–2156. doi: 10.1128/JVI.02786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu FX, Chong JM, Wu L, Yuan Y. 2005. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol 79:800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye J, Shedd D, Miller G. 2005. An Sp1 response element in the Kaposi’s sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J Virol 79:1397–1408. doi: 10.1128/JVI.79.3.1397-1408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanton BZ, Hodges C, Crabtree GR, Zhao K. 2017. A general non-radioactive ATPase assay for chromatin remodeling complexes. Curr Protoc Chem Biol 9:1–10. doi: 10.1002/cpch.16. [DOI] [PMC free article] [PubMed] [Google Scholar]