Abstract
Food-medicine plants play an important role in providing nutrition and treating chronic diseases, especially in many minority communities and developing regions. The coastal region of South China has abundant resources of medicinal plants. A long history of cross-cultural medicinal practices among different minority groups has facilitated the development of a remarkable dietary culture by using food-medicine plants. However, integrative ethnobotanical research on both nutritional and functional properties of the food-medicine plants in this region is still limited. In this study, 27 commonly used wild food-medicine plants were recorded and analyzed from the coastal region of South China. Most of them are good sources for calcium (47.83-1099.89 mg/100 g fresh weight), dietary fiber (3.00-31.87 mg/100 g fresh weight), iron (1.17-24.73 mg/100 g fresh weight), and vitamin C (0.44-68.32 mg/100 g fresh weight). Solanum americanum has the highest average nutritive value and is also considered to be good sources for proteins (7.90 g/100 g fresh weight). Medicinal properties of the studied species can be classified into 8 categories: treatment of the damp-heat syndrome, digestive diseases, urologic diseases, arthropathy, respiratory diseases, gynecological diseases, snake or insect bites, and uses as a tonic. Treating the damp-heat syndrome or expelling warm pathogenic factors is the most commonly used ethnomedicinal practice in the study area. The present study highlights that the local ethnomedicinal practices are deeply influenced by local natural conditions and customs. Food-medicine plants with superior key nutrients have been used regularly in the diet as medicinal food to alleviate common endemic diseases.
Keywords: food-medicine plants, ethnic food plants, nutritional properties, functional properties, coastal region of South China
In many cases, the use of plants as food or medicine can be difficult to differentiate,1–4 and the overlap between these 2 categories of uses is multidimensional.5 Some plants may be used only as a tonic or functional food to improve health instead of to treat a specific disease, but some others may be used as both food and medicine, even with the same organ parts and application methods.3,6,7 The food-medicine continuum is considered to play an important role in the treatment of chronic diseases and the improvement of physical fitness, especially in some developing countries and minority tribal areas.6–11 Therefore, integrative research on both food and medicine has become the trends of ethnopharmacological studies,2,3,6.8,9,12-15 and has been suggested by the World Health Organization.16 However, most previous studies have focused on the bioculture relationship between food and medicinal plants and (or) the decisive factors about the use and perceptions of food-medicines.1,3,13,17
South China is one of the most well-known regions for traditional Chinese medicine (TCM), and the region is home to the South China traditional medicine known as nan-yao (literally south-medicine in TCM). According to recent investigations, more than 600 medicinal plants in this region have been documented to be used by the different minority groups (eg, Li nationality, Yao nationality, and Miao nationality).11,18–20 Moreover, the efficacy, medicinal parts and methods can vary drastically among the minority groups and even in different Han groups, such as Cantonese, Hakka, and Hoklos.21,22 With the abundant resources of medicinal plants as well as the cross-cultural medication practices, edible medicinal plants are widely accepted and used in the diet of the local people. This combinational food-medicine dietary habit has facilitated the development of a distinct dietary culture in South China, for example, the medicinal soup and “liáng chá” (herbal tea) culture,20,21 and the highly localized health practices. For example, the Cantonese prefer to drink “liáng chá” to resist some ailments caused by the usually warm-wet climate in the Canton (Guangdong) region.20,23 Ethnopharmacological studies concerning nutritional properties of the food-medicine continuum and the documentation of the diverse medication practices can contribute to improve our public health knowledge and assist in informing the health policy.
Most previous ethnobotanical studies reported the medicinal properties of ethnomedicinal plants from the inland regions of South China.11,18,19,21–23 Yet species of local food-medicine plants and their nutritional properties are still poorly documented. With the calling on better understanding plants and people in harmony in plant sciences,24 it is important to document and study the plants and their utilizations in food and medicine in areas with rich cultural diversity.
The present study focuses on documenting the use by the minority groups and the nutritional properties of wild food-medicine plants in the coastal regions of South China to answer the following questions: (1) What are the common plant species consumed as the food-medicine continuum in daily life? (2) What are the nutritional and functional properties of these food-medicine plants? (3) Is there any relationship between the frequently used food-medicine plants and the common endemic diseases? (4) What are the diverse methods that the different minority groups apply while using the edible and medicinal plants?
Materials and Methods
Sampling and Ethnobotanical Data Collection
The field study covered 54 villages of south Fujian, Guangdong, Guangxi, and Hainan provinces of the coastal regions of South China (Figure 1). Yao nationality, Zhuang nationality, Li nationality, Miao nationality, and She nationality as well as Cantonese, Hakka, and Hoklos of the Han groups are the major minority groups in this region. The local traditional medication practice may be also influenced by the adjacent Dong nationality and Mulao nationality. Therefore, our study interviewed local people from all these groups. During the interview, 11 questions were asked concerning plant species, local name, harvest period, edible parts, edible methods, medicinal parts, medicinal use methods, efficacy, preparation methods, cultivation, and commercialization. Local healers were considered as the key informants for the ethnobotanical information. We also investigated the local traditional markets and restaurants to further document the information of edible plants. We further consulted the monograph of Chinese ethnopharmacology.25
Figure 1.
Location of the study areas (shaded area).
All the samples for the nutritional analyses and voucher specimens were collected in the field investigation during 2016. Each sample comprised 500 g of the edible portion. The edible part was randomly collected from a minimum of 25 healthy individual plants of one population at the harvest time. For widespread species, 2 to 3 populations (geographic interval at least 100 km apart) were collected. Voucher specimens were identified according to Flora of Hainan,26 Flora of Guangdong,27 and Flora Reipublicae Popularis Sinicae.28 Sources of samples and voucher information are included in Supplementary Table S1.
Sample Preparation for Nutritional Analyses
The edible part was collected in the field and immediately washed first with tap water and then with distilled water to remove soil and other debris, then air-dried at room temperature. Cleaned samples were cut into smaller pieces and dried at 60°C to constant weight, then ground into fine powder (2-mm-mesh pore size) and stored at room temperature.
Nutritional Evaluation
Total protein, dietary fiber, calcium, iron, carotene, and vitamin C were analyzed from the dried samples and treated as the key nutrients, following Grubben29 to calculate the average nutritive value (ANV) and to evaluate the nutritive value of vegetables. Each composition was measured twice from the same homogenized sample. We used the moisture value of each species from the published literature (Supplementary Table S2) to estimate the proximate composition of 100 g fresh edible part and calculate ANV and percent daily value.
Total Protein
Total proteins were determined from the nitrogen content by using the Kjeldahl method in conforming with AOAC 978.04.30 An amount of 0.5 g of dried sample was digested in sulfuric acid to measure the total protein with the percent nitrogen converted to protein using a factor of 6.25.
Total Dietary Fiber Assay
Because the samples consisted of leaves, stems, or fruits of the plant parts and the starch contents are less than 2%, the total dietary fiber was assayed employing the AOAC nonenzymatic-gravimetric method 993.21.30 The dried sample (0.5 g) was suspended in 25 mL H2O and incubated for 90 minutes at 37°C to solubilize water-soluble components and then the water-soluble fiber was precipitated by 95% ethanol. Total dietary fiber was determined by the weight of residue corrected for protein and ash content.
Calcium and Iron
The AOAC Atomic Absorption Spectrophotometric method 975.03 was used to estimate calcium and iron contents.30 Test solutions were prepared by the wet ash method. Accurately weighed 0.5 g dry sample was soaked with 20 mL HNO3: HClO4 (4:1, v/v) solution at room temperature overnight. Then it was heated on a hot plate to digest, slowly at first, until frothing ceased. If charring occurred, more HNO3:HClO4 (4:1, V/V) solution was added. The sample was heated until white fumes appeared and HNO3 was almost completely evaporated. The residual liquid (2-3 mL) was cooled and transferred into a 50 mL volumetric flask and diluted with deionized water to the 50 mL volume. Concentrations were determined by atomic absorption spectrophotometer using the following wavelengths: Ca (422.7 nm) and Fe (248.3 nm).
Carotene
The carotene content was measured by β-carotene and determined with the high-performance liquid chromatography (HPLC) method.31,32 The β-carotene was extracted from 10 g homogenized portions with petroleum ether:acetone (80:20, v/v). Extracting solution was then evaporated to dryness at 30°C. The residue was dissolved with a little petroleum ether and then purified by alumina column chromatography with petroleum ether:acetone (95:5, v/v) as the eluent. The final solution was diluted with eluent into 10 mL and then filtered through 0.45 µm membrane filters for HPLC analysis. Chromatography was performed with a monomeric C18 column (4.6 mm × 150 mm). The mobile phase consisted of methanol:acetonitrile (90:10, v/v), with a flow rate of 1.2 mL/min. The detector wavelength was set at 448 nm for the detection of β-carotene.
Vitamin C
The vitamin C concentration was determined by the titrimetric method (AOAC method 967.21) using 2,6-dichloroindophenol as a titrant.30 A sample of 5 g was extracted with the metaphosphoric acid (2%, w/v)–oxalic acid (2%, w/v) solution. The extracting solution was triturated until the test portion was in suspension. It was then diluted with the metaphosphoric acid (2%, w/v)–oxalic acid (2%, w/v) solution to 100 mL, mixed, and filtered. Then 10 mL filtrate was transferred into a 50 mL conical flask and titrated against 2,6-dichloroindophenol until the solution changed to pink color and persisted at least 15 seconds.
Data Analysis
The nutritional data of the edible parts and the use methods per species, medicinal parts and efficacies of the plant species by each minority group are summarized in Table 3. The differences between edible and medicinal parts as well as the medicinal uses by the different minority groups were compared. The categories of medicinal uses were summarized according to the functional properties of the plants corresponding to the endemic diseases.
Table 3.
Edible and Medicinal Use of the Studied Species.
| Taxa | Edible Parts and Uses | Medicinal Parts and Efficacy |
|---|---|---|
| Alternanthera sessilis | Young shoots: blanching and then stir-fry. | Whole plant: cure dysentery, epistaxis, hemoptysis, hematochezia, urethritis, mastitis, dysuria; external use to treatment of sore and furuncle, eczema, dermatitis, or snakebite. |
| Amaranthus blitum | Seedlings, young shoots and leaves: stir-fry, used in soups or egg pancakes, boiled and consumed in sauces. | Whole plant: cure dysentery, hot eyes, acute mastitis, hemorrhoids; seed: used for diuresis or improving eyesight. |
| A. spinosus | Seedlings, young shoots and leaves: stir fry, used in soups or egg pancakes, boiled and consumed in sauces. | Zhuang nationality Roots: cure dysentery and hemorrhoids; whole plant: cure dysentery, hemorrhoids, or metroptosis. |
| A. viridis | Seedlings, young shoots and leaves: stir fry, used in soups or egg pancakes, boiled and consumed in sauces. | Whole plant: used for heat-clearing and detoxifying or diuresis |
| Anredera cordifolia | Tender leaves and stems: stir fry, cooked in soups or with
noodles Tubercles: boiled like potatoes. |
Mulao nationality Vines and tubercles: used for replenishing blood |
| Artemisia argyi | Tender leaves: used in soups or egg pancakes; juice used for pastry. |
Yao nationality Whole plant: treatment of emmeniopathy,
stomachache, sore and furuncle, fetal irritability, or scrofula. Dong nationality Whole plant: treatment of emmeniopathy, stomachache, or scrofula. Mulao nationality Whole plant: treatment of dysmenorrhea or abnormal menstruation. Miao nationality Leaves: used for dysmenorrhea, metrorrhagia and metrostaxis, fetal irritability; external use to cure joint pain or cutaneous pruritus. |
| Asystasia gangetica | Young shoots and leaves: blanching then stir-fry. | Zhuang nationality Aerial parts: subside swelling when bruise or traumatic injury, cure metroptosis or rectocele. |
| Celosia argentea | Young shoots and leaves: stir-fry or boiled and consumed in
sauces Young inflorescences: stew with meat. |
Li nationality Whole plant: used for heat-clearing and damp-drying,
cooling blood and hemostasis or anti-pruritus; Seed: used for sore red swollen
eyes; inflorescence: used for cooling blood and hemostasis, removing liver-fire
for improving eyesight. She nationality Whole plant and seed: used for swelling and pain of eye, cataract, cutaneous pruritus. Mulao nationality Aerial parts: cure sore red swollen eyes or epistaxis. Miao nationality Seeds: cure sore red swollen eyes or keratitis. |
| Cheilocostus speciosus | Tender stems: peel off the older leaf sheath then stir fry, stews or braise with meat. |
Zhuang nationality Rhizomes: cure stomachache, fracture or impotence. Li nationality Rhizomes: used to diuresis detumescence, expelling wind-damp. |
| Commelina benghalensis | Young shoots: stir fry, boiled in soups, dried and stew with meat. | Whole plant: cure scanty dark urine, dysentery characterized by blood in the stool or furunculosis |
| C. communis | Young shoots: stir-fry, boiled in soups, dried and stew with meat. |
Dong nationality Whole plant: cure lumbago and edema. Miao nationality Inflorescences: cure keratitis, sore red swollen eyes, xerophthalmia, nyctalopia. |
| Eleutherococcus trifoliatus | Young shoots: blanching in water to remove the bitter principle and then stir fry or consumed in sauces, dried and boiled in soups. |
Yao nationality Roots and leaves: used in abnormal menstruation,
rheumatalgia, arthralgia, or pertussis. Zhuang nationality: Root and leaf: used in abnormal menstruation, rheumatalgia, fracture or bee sting. Mulao nationality Root and leaf: used for irregular menstruation or cold and cough. Miao nationality: Roots: cure cough, enteritidis, lithangiuria, diarrhea, rheumatoid arthritis, fracture, mastitis, or sore and ulcer. |
| Emilia sonchifolia | Seedlings, young shoots and leaves: stir fry, used in soups. |
Yao nationality Whole plant: ingested to cure cold, pharyngalgia,
pneumonia, urinary tract infection or diarrhea; external use to treatment of
scabies, traumatic injury, or snakebite. Zhuang nationality: Whole plant: ingested to cure dysentery, urocystitis, hemafecia, or bleeding from hemorrhoids; external use to treat scabies, traumatic injury, or snakebite. Li nationality Whole plant: used for heat-clearing and detoxifying, sore swollen poison, or hemafecia. Dong nationality Whole plant: used for treating flu, fever, swelling and pain in throat, nephritis, colitis, hepatitis, dysentery, cervicitis. Mulao nationality Whole plant: cure cold and fever. Miao nationality Whole plant: cure acute mastitis, sore and furuncle, tympanitis, acute tonsillitis, chronic gastroenteritis, urinary tract infection, or sprain. |
| Eryngium foetidum | Seedlings, tender leaves: stir fry, boiled and consumed in sauces, used as spice or condiment (raw or cooked), mixed with meat stuffing. | Yao nationality Whole plant: cure cold, bronchitis, diarrhea, oxyhepatitis, or odontalgia. |
| Gynura divaricata | Seedlings, young shoots and leaves: stir fry, used in soups, boiled and consumed in sauces, cooked with noodles. |
Yao nationality Leaves: used as ascaricide. Zhuang nationality Whole plant: cure traumatic injuries, carbuncle, or injury infection prevention clinically. Mulao nationality Whole plant: cure protracted dysentery. |
| Melastoma dodecandrum | Mature fruits: eaten raw, natural pigment. |
Yao nationality Whole plant: cure dysentery and enteritis. Zhuang nationality Roots: cure diarrhea or abnormal leukorrhea; whole plant: cure dysentery and enteritis; young leaf: used as hemostatics when incised wound. She nationality Whole plant or roots: cure rheumatalgia, hernia, dysmenorrhea, metrorrhagia and metrostaxis, dysentery with bloody stool, hemorrhoids and fistula, rubella. Dong nationality Whole plant: cure enteritis, dysentery or leukorrhagia. Miao nationality: Whole plant: cure habitual abortion. |
| Pandanus tectorius | Tender stems: peel off the rind and then stir fry Mature fruits: stew with meat. |
Li nationality Roots: cure cold, fever, hepatitis, cirrhotic ascites, nephritic edema, stranguria, rheumatic arthralgia or hernia; young leaves: cure cold and fever, heatstroke, gingival bleeding or crushed with soybean and then apply to affected areas for treatment of boils; cure cold and fever, stranguria with turbid urine, heat diarrhea, hernia, aphtha; fruits: used for dysentery, cough, stomachache, orchitis, hemorrhoids, dysuria, or cataract |
| Patrinia villosa | Young shoot and leaf: dried and used for soups. |
Zhuang nationality Roots: cure icteric hepatitis or treatment of
fester cause by snakebite. She nationality Whole plant: cure appendicitis or constipation. Mulao nationality Whole plant: cure swelling and pain of eye, swollen welling-abscess or dysentery. |
| Perilla frutescens var purpurascens | Tender leaves: spice (eg, cooked with fish or seafood). | Leaves: used for treating diuresis, analgesia or cold; seeds: used for eliminating phlegm, antitussive or relieving asthma. |
| Plantago asiatica | Seedlings and tender leaves: fresh stir fry or used in soups, dried and then used as tea. |
Li nationality Seeds and whole plant: urinary tract infection,
urinary calculus, nephritis or diarrhea. Mulao nationality Seeds: cure nephritis or urinary tract infection. |
| Polygonum perfoliatum | Young shoots: blanching and then stir fry. |
Yao nationality: Whole plant: treatment of eczema, children’s
diarrhea, snakebite, or used as ascaricide. Zhuang nationality Whole plant: treatment of cutaneous pruritus. Dong nationality Whole plant: stomachache, abdominal distention and pain, eczema or snakebite. Miao nationality Whole plant: cure cold, enteritis, diarrhea, snakebite or centipede bite. |
| Portulaca oleracea | Tender leaves and stems: stir fry, used in soups or egg pancakes, boiled and consumed in sauces, mixed with meat stuffing. |
Li nationality Whole plant: used for heat-clearing and detoxifying, dysentery. Mulao nationality Whole plant: cure tuberculosis, hemoptysis, hemafecia, or hematuria. |
| Rosa laevigata | Mature fruits: eaten raw or made into wine. |
Yao nationality Roots: treatment of used as ascaricide. She nationality Fruits: cure spermatorrhea, frequent micturition, metrorrhagia and metrostaxis, prolonged diarrhea and dysentery. Dong nationality Roots and fruits: cure tuberculosis, furuncle, metrorrhagia and metrostaxis. Mulao nationality Roots, leaves, flowers and fruits: cure spermatorrhea, enuresis, lingering dysentery, leukorrhea. Miao nationality Roots and fruits: cure spermatorrhea, impotence, children diarrhea or rectocele. |
| Smilax china | Young shoots: blanching in boiling water and then stir fry or consumed in sauces. |
Yao nationality Rhizomes: treatment of bone fracture. Zhuang nationality Rhizomes: cure gynecopathy or arthritis. Li nationality Rhizomes: diminish inflammation and relieve coughing. She nationality Whole plant: cure arthralgia, traumatic injury, enterogastritis, dysentery. Mulao nationality Rhizomes: used for reinforcing kidney to strengthen yang. Miao nationality Rhizome: cure rheumatalgia, numbness of sinews and bones, enteritis, diarrhea, diabetes, or treatment of cancer. |
| Solanum americanum | Young shoots and leaves: stir fry, used in soups, boiled and consumed in sauces, added in conjee. |
Dong nationality Whole plant: cure urodynia or sore and furuncle. Miao nationality Aerial parts: treatment for sore and furuncle, urinary tract infection or dysuria; root: cure hemoptysis or irregular menstruation. |
| S. torvum | Young fruits: stir fry with beef. | Roots: cure cold, chronic cough, stomachache, toothache, amenorrhea, swollen welling-abscess, traumatic injury; leaves: used for innominate inflammatory; fruit: improving eyesight. |
| Talinum paniculatum | Tender leaves and stems: stir fry, used in soups, boiled and consumed in
sauces Older root: stew with meat. |
Zhuang nationality Root: strengthen immunity and invigorate health
effectively, cure metroptosis, rectocele, lung vacuity cough. Dong nationality Roots: used as a tonic. Mulao nationality Roots: cure cough and hemoptysis. Miao nationality Roots: used as a tonic. |
Nutrient concentrations are reported on a per 100 g dry-weight basis and expressed as the mean value ± standard deviation (mean ± SD). The results were statistically analyzed using the JMP 13.1.0 software (SAS Institute Inc, Cary, NC). Analysis of variance (ANOVA) was conducted to analyze data at the 95% confidence level followed by the Duncan’s test for multiple comparisons.
Results and Discussion
Food-Medicine Plant Species in the Study Areas
A total of 27 wild food-medicine plant species belonging to 23 genera and 17 families were recorded in this study (Supplementary Table S1). All these 27 species have been commonly used by the local people either as seasonal wild fruits (ie, Melastoma dodecandrum Lour. and Rosa laevigata Michx.) or as vegetables (the remaining species). Twenty-two of them have been reported as folk medicines of South China.11,18,19,21,22 Nevertheless, 5 of the species have been used as traditional medicines in other regions beyond South China. They are Amaranthus blitum L. in Malaysia,33 A. viridis L. in India and Nepal,34 Commelina benghalensis L. in India,35 and Eleutherococcus trifoliatus (L.) S. Y. Hu and Patrinia villosa Juss. in southwest China.36,37
Some of the food-medicine plants are endemic and even commercialized in the coastal regions of South China (Figure 2). The most distinctive endemic food plant is Patrinia villosa, which is a traditional medicine well-known for its anti-inflammatory, antitumor, and antibacterial properties.37–40 This species tastes extremely bitter and it has the unpleasant foot odor, but its young shoot (Figure 2a) is a popular vegetable in Meizhou City, Guangdong Province. Patrinia villosa is under large-scale cultivation with a relatively high price (¥5/kg for fresh weight [FW]; ¥60/kg for dry weight [DW]). The young scape and young stems of Cheilocostus speciosus (J. Koenig) C. D. Specht (Figure 2g) have been used as an endemic wild vegetable in Hainan Province and can be sold at a relatively high price at ¥10 to ¥18/kg (FW). Because harvesting the tender upper part of the stem of Pandanus tectorius Parkinson ex Du Roi (Figure 2h) needs to cut down the whole tree, P. tectorius has been used as a vegetable mostly during the times of food scarcity. The fruits of Melastoma dodecandrum (Figure 2f) are popular as wild fruits in the study areas and can also be used to make wine and jelly candy.
Figure 2.
Edible parts of some endemic and commercialized food-medicine plant from the study area. a, Patrinia villosa. b, Gynura divaricate. c, Asystasia gangetica. d, Solanum torvum. e, S. torvum, show stir-fry with beef. f, Melastoma dodecandrum. g, Cheilocostus speciosus. h, Pandanus tectorius. i, Smilax china. Photographed by Yuan Xu.
Our study reports Asystasia gangetica (L.) T. Anderson (Figure 2c) as a wild vegetable in China for the first time. It is only used and cultivated in Chenmai County, Haikou City and Wenchang City of Hainan Province. Asystasia gangetica has also been used as a traditional leaf vegetable in South Africa,41 western Kenya,42 Vietnam,43 and India.44
Other commercialized food-medicine plants include Artemisia argyi Lévl. et Van., Eleutherococcus trifoliatus, Eryngium foetidum L., Gynura divaricata (L.) DC., Plantago asiatica L., Portulaca oleracea L., Rosa laevigata, Smilax china L. (Figure 2i), Solanum americanum Mill., Solanum torvum Swartz (Figure 2d and e) and Talinum paniculatum (Jacq) Gaertn, of which A. argyi, Eleutherococcus trifoliatus, Eryngium foetidum, G. divaricata, Plantago asiatica, Portulaca oleracea, Smilax china and T. paniculatum are also cultivated.
Of the 27 species, Amaranthus blitum, A. spinosus L., A. viridis, Anredera cordifolia, Eryngium foetidum, Solanum americanum, S. torvum and Talinum paniculatum are naturalized species originally from other continents.45–48 The coastal area of South China opened its door early to the west for trading. With the open culture and the frequent economic exchanges with Europe and America, the coastal region of South China has been viewed as a key area for the initial introduction of many naturalized plants into China.49–52
Nutritional Properties
The proximate composition of dry and fresh edible parts of the studied food-medicine plants is presented in Table 1 and Supplementary Table S2, respectively. The ANV calculated by the FW and the results are shown in Supplementary Table S2. Suggested serving size for the studied species were obtained from the National Nutrient Database for Standard Reference, Release 28 of US Department of Agriculture.53 For the leafy vegetables, the standard serving size followed the standard of Chinese cabbage (1 cup, 70 g; NDB No. 11116). For the stem vegetable Cheilocostus speciosus, the standard of asparagus (1 cup, 134 g; NDB No. 11011) was followed; and for Pandanus tectorius, we followed the standard of radishes (1 cup, 134 g; NDB No. 11011). For the fruits, we followed the standards of eggplant (1 cup, 82 g; NDB No. 11209) for Solanum torvum, blueberries (50 berries, 68 g; NDB No. 09050) for Melastoma dodecandrum, and apples (½ cup, 62.50 g; NDB No. 09003) for Rosa laevigata. Furthermore, daily value data of each nutrient category (relative to a 2000-cal diet) were obtained from the US Food and Drug Administration.54 Then, the percent daily value was calculated for a serving size for each studied species. Serving size, daily value, and percent daily value are shown in Table 2.
Table 1.
Proximate Composition of the Edible Parts (100 g, Dry Weight) of the Studied Species.
| Taxa* | Proteins (g) | Vitamin C (mg) | Carotene (mg) | Fiber (g) | Fe (mg) | Ca (mg) |
|---|---|---|---|---|---|---|
| Alternanthera sessilis | 18.19 ± 0.06defghi | 32.28 ± 1.48ijkl | 82.53 ± 0.40hi | 52.35 ± 1.20abcdefg | 95.74 ± 1.68bc | 2448.53 ± 11.51def |
| Amaranthus blitum | 29.12 ± 0.06a | 56.49 ± 1.78efghi | 159.65 ± 10.39fghi | 53.04 ± 1.06abcdefg | 52.77 ± 0.75cde | 1527.38 ± 30.00defgh |
| A. spinosus-1 | 17.85 ± 0.06 | 7.83 ± 0.13 | 184.00 ± 0.00 | 65.81 ± 0.82 | 77.49 ± 2.10 | 1128.92 ± 24.41 |
| A. spinosus-2 | 19.09 ± 0.04 | 24.40 ± 1.27 | 305.50 ± 0.71 | 47.75 ± 4.03 | 37.57 ± 0.23 | 2657.54 ± 32.18 |
| A. spinosus-3 | 21.58 ± 0.03 | 9.72 ± 0.22 | 449.10 ± 8.12 | 35.00 ± 0.28 | 39.08 ± 1.94 | 4267.43 ± 37.07 |
| A. spinosus (average) | 19.51cdefg | 13.98lm | 312.87cdef | 49.52abcdefg | 51.38de | 2684.63cde |
| A. viridis-1 | 28.70 ± 0.01 | 57.37 ± 0.47 | 182.45 ± 6.29 | 59.49 ± 0.97 | 45.52 ± 1.56 | 2141.44 ± 43.31 |
| A. viridis-2 | 19.20 ± 0.07 | 45.84 ± 0.91 | 103.00 ± 1.41 | 58.28 ± 0.78 | 25.02 ± 0.14 | 3765.08 ± 2.53 |
| A. viridis-3 | 25.48 ± 0.06 | 64.01 ± 1.01 | 271.50 ± 0.71 | 38.45 ± 0.49 | 28.97 ± 0.25 | 2452.47 ± 8.44 |
| A. viridis (average) | 24.46abcd | 55.74efghi | 185.65efgh | 52.07abcdefg | 33.17defg | 2786.33bcd |
| Anredera cordifolia | 12.30 ± 0.01ijkl | 65.61 ± 0.35cdefg | 567.50 ± 23.33a | 46.95 ± 0.78cdefg | 18.32 ± 0.57defg | 1685.61 ± 42.74defgh |
| Artemisia argyi | 21.94 ± 0.07bcde | 12.32 ± 0.20lm | 324.00 ± 5.66bcdef | 47.85 ± 1.20bcdefg | 21.43 ± 0.41defg | 1608.68 ± 69.22defgh |
| Asystasia gangetica | 21.19 ± 0.08bcde | 29.38 ± 1.21ijklm | 494.00 ± 0.00ab | 47.90 ± 1.13bcdefg | 43.59 ± 2.95defg | 2420.88 ± 34.11def |
| Celosia argentea-1 | 20.80 ± 0.02 | 46.14 ± 0.07 | 324.50 ± 3.54 | 41.61 ± 1.11 | 66.78 ± 0.39 | 3499.36 ± 44.02 |
| C. argentea-2 | 20.87 ± 0.01 | 15.04 ± 0.36 | 191.00 ± 8.49 | 58.45 ± 0.69 | 92.91 ± 2.50 | 1918.43 ± 56.02 |
| C. argentea-3 | 21.22 ± 0.08 | 23.44 ± 0.17 | 289.00 ± 8.91 | 43.60 ± 0.85 | 33.39 ± 0.81 | 2714.12 ± 38.32 |
| C. argentea-4 | 17.48 ± 0.03 | 34.31 ± 0.27 | 122.00 ± 4.24 | 43.75 ± 1.63 | 35.45 ± 0.14 | 2310.30 ± 11.19 |
| C. argentea (average) | 20.09cdef | 29.73jkl | 231.63defgh | 46.85cdefg | 57.13cd | 2610.55de |
| Cheilocostus speciosus | 10.55 ± 0.08jkl | 44.88 ± 0.95fghijk | ND | 38.30 ± 0.42efg | 15.21 ± 0.54efg | 2088.03 ± 37.84defg |
| Commelina benghalensis | 16.91 ± 0.01efghij | 72.46 ± 1.11bcdef | 335.41 ± 5.86bcde | 33.95 ± 0.92g | 59.06 ± 1.66cd | 1612.32 ± 39.96defgh |
| C. communis | 12.54 ± 0.09ijkl | 17.37 ± 0.49klm | 80.65 ± 2.33hi | 64.44 ± 0.70abcd | 105.14 ± 0.34ab | 891.78 ± 0.33ghi |
| Eleutherococcus trifoliatus | 20.67 ± 0.04cdef | 82.88 ± 0.60bcde | 134.00 ± 4.24ghi | 56.94 ± 0.46abcdefg | 11.60 ± 0.71efg | 356.91 ± 4.45hi |
| Emilia sonchifolia-1 | 24.37 ± 0.08 | 17.25 ± 0.76 | 251.70 ± 6.65 | 78.29 ± 1.02 | 51.79 ± 0.97 | 1432.52 ± 2.58 |
| E. sonchifolia-2 | 19.15 ± 0.08 | 20.01 ± 0.63 | 437.00 ± 5.66 | 46.05 ± 0.64 | 42.37 ± 0.34 | 555.59 ± 6.30 |
| E. sonchifolia-3 | 18.94 ± 0.01 | 38.79 ± 0.63 | 396.40 ± 1.98 | 48.05 ± 0.92 | 47.67 ± 0.06 | 1750.60 ± 0.99 |
| E. sonchifolia (average) | 20.82cde | 25.35jklm | 361.70bcd | 57.46abcdefg | 47.28def | 1246.24fghi |
| Eryngium foetidum | 14.11 ± 0.04fghijk | 86.03 ± 3.13bcd | 196.10 ± 4.10defgh | 41.55 ± 0.35defg | 53.29 ± 0.23cde | 356.11 ± 0.32hi |
| Gynura divaricata | 19.22 ± 0.02cdefgh | 11.00 ± 1.20lm | 313.50 ± 2.12cdef | 59.20 ± 1.27abcdef | 13.59 ± 0.92efg | 1350.17 ± 14.92efgh |
| Melastoma dodecandrum | 7.90 ± 0.07kl | 21.05 ± 0.04klm | 0.59 ± 0.02i | 67.33 ± 0.74abc | 141.08 ± 3.70a | 420.84 ± 1.47hi |
| Pandanus tectorius | 8.76 ± 0.04kl | 2.20 ± 0.00m | ND | 35.25 ± 0.49fg | 11.77 ± 0.13efg | 5499.46 ± 24.65a |
| Patrinia villosa-1 | 12.30 ± 0.08 | 89.97 ± 0.83 | 340.00 ± 7.07 | 67.61 ± 0.38 | 19.87 ± 0.47 | 584.31 ± 8.11 |
| P. villosa-2 | 14.22 ± 0.10 | 33.47 ± 0.18 | 430.00 ± 5.66 | 53.24 ± 0.44 | 14.70 ± 0.51 | 312.73 ± 11.44 |
| P. villosa (average) | 13.26hijkl | 61.72defgh | 385.00bc | 60.42abcde | 17.28efg | 448.52hi |
| Perilla frutescens var. purpurascens | 13.13 ± 0.07ghijkl | 23.49 ± 0.17jklm | 228.88 ± 0.13cdefgh | 71.65 ± 0.44ab | 140.06 ± 0.64a | 859.30 ± 10.12ghi |
| Plantago asiatica | 17.67 ± 0.08efghi | 61.19 ± 1.99cdefgh | 234.00 ± 1.41cdefgh | 45.65 ± 1.06cdefg | 41.18 ± 0.16defg | 3924.78 ± 26.53bc |
| Polygonum perfoliatum | 12.19 ± 0.08ijkl | 11.03 ± 0.09lm | 73.45 ± 0.92hi | 73.40 ± 0.87a | 9.94 ± 0.19efg | 4084.15 ± 106.82b |
| Portulaca oleracea-1 | 21.90 ± 0.11 | 26.37 ± 0.96 | 153.50 ± 2.12 | 59.70 ± 0.59 | 90.89 ± 3.57 | 2149.25 ± 43.20 |
| P. oleracea-2 | 10.45 ± 0.16 | 37.24 ± 0.14 | 175.50 ± 0.71 | 34.10 ± 0.42 | 187.15 ± 1.98 | 983.51 ± 5.00 |
| P. oleracea-3 | 17.28 ± 0.09 | 43.67 ± 1.34 | 313.00 ± 2.83 | 37.70 ± 0.28 | 68.44 ± 3.39 | 2116.71 ± 10.61 |
| P. oleracea (average) | 16.54efghij | 35.76hijkl | 214.00defgh | 43.83cdefg | 115.50ab | 1749.83defgh |
| Rosa laevigata | 7.19 ± 0.08l | 127.18 ± 0.35a | 6.24 ± 0.43i | 59.32 ± 0.73abcdef | 3.31 ± 0.09fg | 439.45 ± 2.88hi |
| Smilax china | 25.05 ± 0.04abc | 88.06 ± 2.04bc | 89.14 ± 0.58hi | 57.05 ± 0.49abcdefg | 10.48 ± 0.07efg | 520.68 ± 0.72hi |
| Solanum americanum-1 | 29.35 ± 0.05 | 49.25 ± 1.26 | 523.35 ± 2.33 | 63.03 ± 0.54 | 31.53 ± 1.23 | 818.14 ± 4.94 |
| S. americanum-2 | 22.06 ± 0.05 | 41.22 ± 0.84 | 462.00 ± 8.91 | 48.75 ± 0.21 | 45.46 ± 0.06 | 2368.92 ± 7.65 |
| S. americanum-3 | 28.88 ± 0.13 | 46.86 ± 0.23 | 704.86 ± 4.14 | 35.30 ± 0.71 | 41.42 ± 2.82 | 2031.77 ± 40.03 |
| S. americanum (average) | 26.76ab | 45.78ghij | 563.40a | 49.03cdefg | 39.47def | 1739.61defg |
| S. torvum-1 | 13.56 ± 0.22 | 84.52 ± 0.33 | 14.05 ± 0.21 | 44.99 ± 0.79 | 7.14 ± 0.12 | 182.60 ± 1.90 |
| S. torvum-2 | 15.12 ± 0.06 | 93.97 ± 1.53 | 19.25 ± 1.34 | 48.77 ± 0.88 | 5.62 ± 0.03 | 255.85 ± 2.54 |
| S. torvum-3 | 20.76 ± 0.28 | 98.26 ± 0.29 | 7.19 ± 0.09 | 72.36 ± 0.28 | 7.83 ± 0.32 | 428.25 ± 4.71 |
| S. torvum (average) | 16.48efghi | 92.25b | 13.50i | 55.37abcdef | 6.87g | 288.90i |
| Talinum paniculatum-1 | 20.09 ± 0.05 | 36.77 ± 0.09 | 252.50 ± 2.12 | 40.00 ± 0.14 | 31.56 ± 1.53 | 874.40 ± 15.98 |
| T. paniculatum-2 | 21.78 ± 0.04 | 28.25 ± 0.07 | 308.95 ± 8.56 | 38.25 ± 0.49 | 51.10 ± 0.06 | 1071.77 ± 11.94 |
| T. paniculatum (average) | 20.94cde | 32.51ijkl | 280.73cdefg | 39.13efg | 41.33defg | 973.09ghi |
* Numerical labels used for differentiating the samples from different localities (location information provided in Supplementary Table S1). ND indicates not detected. Values are given as average ± standard deviation (n = 2). For each column, different letters mean significant differences (P < 0.05) among species.
Table 2.
Serving Size, Daily Value, and Percent Daily Value of the Studied Species.
| Taxa | Serving Size (g) | Percent Daily Value | ||||
|---|---|---|---|---|---|---|
| Proteins | Vitamin C | Fiber | Fe | Ca | ||
| Alternanthera sessilis | 70.00 | 4.58 | 6.78 | 26.38 | 67.02 | 30.85 |
| Amaranthus blitum | 70.00 | 8.15 | 13.18 | 29.70 | 41.04 | 21.38 |
| A. spinosus | 70.00 | 4.92 | 2.94 | 24.96 | 35.97 | 33.83 |
| A. viridis | 70.00 | 6.85 | 13.01 | 29.16 | 25.80 | 39.01 |
| Anredera cordifolia | 70.00 | 1.10 | 4.90 | 8.41 | 4.56 | 7.55 |
| Artemisia argyi | 70.00 | 4.64 | 2.17 | 20.26 | 12.60 | 17.03 |
| Asystasia gangetica | 70.00 | 4.45 | 5.14 | 20.12 | 25.43 | 25.42 |
| Celosia argentea | 70.00 | 3.49 | 4.30 | 16.27 | 27.55 | 22.66 |
| Commelina benghalensis | 70.00 | 2.58 | 9.21 | 10.36 | 25.03 | 12.30 |
| C. communis | 70.00 | 1.44 | 1.66 | 14.80 | 33.53 | 5.12 |
| Cheilocostus speciosus | 134.00 | 2.43 | 8.62 | 17.65 | 9.74 | 24.06 |
| Eleutherococcus trifoliatus | 70.00 | 9.03 | 30.17 | 49.74 | 14.07 | 7.79 |
| Emilia sonchifolia | 70.00 | 2.19 | 2.22 | 12.08 | 13.81 | 6.55 |
| Eryngium foetidum | 70.00 | 2.65 | 13.48 | 15.62 | 27.83 | 3.35 |
| Gynura divaricata | 70.00 | 9.79 | 4.67 | 60.32 | 19.23 | 34.39 |
| Melastoma dodecandrum | 68.00 | 1.38 | 3.06 | 23.48 | 68.33 | 3.67 |
| Pandanus tectorius | 58.00 | 2.03 | 0.43 | 16.36 | 7.59 | 63.79 |
| Patrinia villosa | 70.00 | 3.36 | 13.03 | 30.62 | 12.16 | 5.68 |
| Perilla frutescens var purpurascens | 70.00 | 3.25 | 4.84 | 35.43 | 96.19 | 10.62 |
| Plantago asiatica | 70.00 | 3.78 | 10.92 | 19.56 | 24.50 | 42.03 |
| Polygonum perfoliatum | 70.00 | 2.52 | 1.90 | 30.36 | 5.71 | 42.23 |
| Portulaca oleracea | 70.00 | 1.62 | 2.92 | 8.59 | 31.44 | 8.57 |
| Rosa laevigata | 62.50 | 4.83 | 71.17 | 79.67 | 6.17 | 14.75 |
| Solanum americanum | 70.00 | 11.06 | 15.77 | 40.53 | 45.31 | 35.95 |
| S. torvum | 82.00 | 5.44 | 25.35 | 36.52 | 6.29 | 4.76 |
| Talinum paniculatum | 70.00 | 2.49 | 3.22 | 9.31 | 13.66 | 5.79 |
| Daily valuea | 50 g | 60 mg | 25 g | 18 mg | 1000 mg | |
a Daily value based on a caloric intake of 2000 calories as stablished by the US Food and Drug Administration.54
The range of protein content is from 7.19 ± 0.08 g (Rosa laevigata) to 29.12 ± 0.06 g (Alternanthera sessilis (L.) R. Br. ex DC.) of 100 g DW and from 0.79 g (Anredera cordifolia) to 7.90 g (Solanum americanum) of 100 g FW. The dietary fiber content varied from 33.95 ± 0.92 g (Commelina benghalensis) to 73.40 ± 0.87 g (Polygonum perfoliatum L.) of 100 g DW and from 3.00 g (Anredera cordifolia) to 31.87 g (Rosa laevigata) of 100 g FW. The calcium content fluctuated between 288.90 mg (Solanum torvum) to 5499.46 ± 24.65 mg (Pandanus tectorius) of 100 g DW, while 47.83 mg (Eryngium foetidum) and 1099.89 mg (Pandanus tectorius) of 100 g FW. The iron content ranged from 3.31 ± 0.09 mg (Rosa laevigata) to 141.08 ± 3.70 mg (Melastoma dodecandrum) of 100 g DW and 1.17 mg (Anredera cordifolia) to 24.73 mg (Perilla frutescens var. purpurascens (Hayata) H. W. Li) of 100 g FW. The carotene content was not detected (<0.04 mg/kg) for Pandanus tectorius and Cheilocostus speciosus. The lack of carotene was probably caused by the special edible parts of the 2 species. For Pandanus tectorius, its edible part is the “pith” of the young stem and almost no pigment can be found there. Among the remaining species, the highest carotene content was detected from Anredera cordifolia (567.50 ± 23.33 mg) of 100 g DW and Solanum americanum (166.32) of 100 g FW. The range of vitamin C content was from 2.20 mg (Pandanus tectorius) to 127.18 ± 0.35 mg (Rosa laevigata) of 100 g DW and 0.44 mg (Pandanus tectorius) to 68.32 mg (Rosa laevigata) of 100 g FW.
Solanum americanum has the highest ANV, as well as higher concentrations of several nutrients from each serving (11.06%, 15.77%, 40.53%, 45.31%, and 35.95% of the daily value for proteins, vitamin C, fiber, Fe and Ca, respectively). All the studied species can be considered good sources of dietary fiber. Their contribution of the daily fiber value per serving is between 8.41% (Anredera cordifolia) and 79.67% (Rosa laevigata). The contribution of proteins is limited. Only Gynura divaricata (9.79%) and Solanum americanum (11.06%) have higher percent daily value of proteins that is near or higher than 10%. Wild fruit, Rosa laevigata with 71.17% percent daily value, provides the maximum contribution for vitamin C per serving. Plantago asiatica (10.92%), Amaranthus viridis (13.01%), Patrinia villosa (13.03%), Amaranthus blitum (13.18%), Eryngium foetidum (13.48%) and Solanum americanum (15.77%) were considered good sources of vitamin C and Solanum torvum (25.35%) and Eleutherococcus trifoliatus (30.17%) are considered excellent sources with the percent daily value higher than 20%. The studied species also have a prominent iron content. Twenty species have percent daily value more than 10% and they range from 12.60% (Patrinia villosa) to 96.19% (Perilla frutescens var. purpurascens) per serving. For the contribution of calcium per serving, Perilla frutescens var. purpurascens (10.62%), Commelina benghalensis (12.30%), Rosa laevigata (14.75%) and Artemisia argyi (17.03%) are good sources and Amaranthus blitum (21.38%), Celosia argentea L. (22.66%), Cheilocostus speciosus (24.06%), Asystasia gangetica (25.42%), Alternanthera sessilis (30.85%), Amaranthus spinosus (33.83%), Gynura divaricata (34.39%), Solanum americanum (35.95%), Amaranthus viridis (39.01%), Plantago asiatica (42.03%), Polygonum perfoliatum (42.23%) and Pandanus tectorius (63.79%) are excellent sources.
Functional Properties
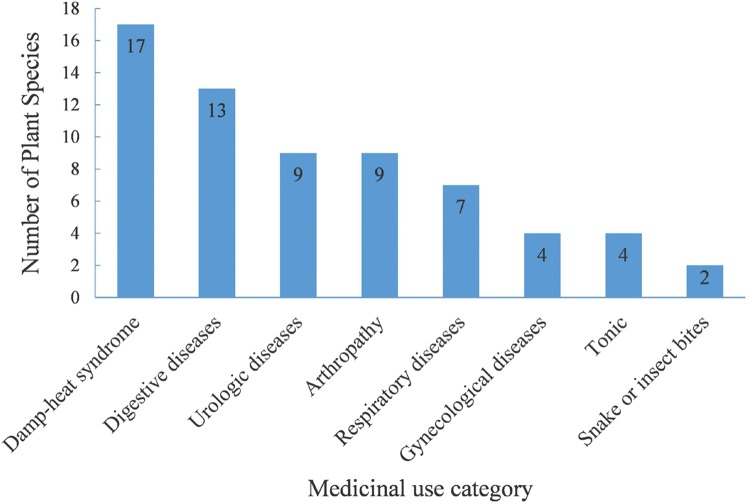
The medicinal efficacies of the investigated food-medicine plants are shown in Table 3 and can be classified into 8 categories (Figure 3): treatment of damp-heat syndrome (eg, aphtha, cutaneous pruritus, eczema, epistaxis, fever, pharyngalgia and swelling and pain of eye; 17 species), digestive diseases (eg, chronic gastroenteritis, constipation, dysentery, enteritidis and stomachache; 13 species), urologic diseases (eg, dysuria, hematuria, lithangiuria, nephritis, stranguria, urethritis and urocystitis; 9 species), arthropathy (eg, fracture, joint pain, lumbago, rheumatalgia and traumatic injury; 9 species), respiratory diseases (eg, asthma, bronchitis, cough, pertussis, pneumonia and tuberculosis; 7 species), gynecological diseases (eg, abnormal leukorrhea, abnormal menstruation, dysmenorrhea; mastitis, metroptosis and metrorrhagia; 4 species), snake or insect bites (2 species) and uses as a tonic (eg, to cure anemia, asthenia, and hypoimmunity; 4 species).
Figure 3.
Species number used for different diseases.
Usually, tonic is the most frequently used category in traditional Chinese food-medicine plants (eg, Panax ginseng C. A. Mey. and Dioscorea polystachya Turcz.), but the majority of food-medicine plants in the coastal region of South China have been used to treat the damp-heat syndrome (nearly 61%; Figure 3). Only 4 studied species, Anredera cordifolia, Rosa laevigata, Talinum paniculatum and Pandanus tectorius (fruit) are used as a tonic (Table 3; Figure 3). This dominant use of plants to treat damp-heat syndrome as well as the similar “liáng chá” (cool herb tea) culture in the region were likely developed to adapt to the local warm-wet climate in the coastal region of South China.20,23
Other common medicinal uses of food-medicine plants are to treat digestive diseases, urologic diseases, and arthropathy. These uses are likely closely related to the dietary habit and lifestyle of the local people. Because of their habit of eating raw seafood, anti-inflammatory and antibacterial food-plants are important supplement to the diet of the local people to prevent digestive diseases (eg, enteritis and diarrhea). Regular high-salt and high-purine diet intake increases the occurrences of hypertension, edema, dysuria and urarthritis. Food-medicine plants (like Plantago asiatica) that are used to treat urologic diseases can promote sodium and trioxypurine excretion to alleviate the above diseases. Besides, many local people frequently work on the ship, and rheumatism and bruises are hence common problems in the study area. Thus, the food-medicine plants that can treat arthropathy are always regarded as a household medicine or functional food in the coastal regions of South China.
Diversity and Differences in the Application Methods
The diversity of the application methods between food and medicine is mostly reflected in the different used parts of edible and medicinal plants (Table 3). Based on the edible parts and medicinal parts of the plant species, 3 different categories can be recognized.
First, the edible and medicinal parts are the same, but the harvest time is different. Usually, the harvest time for the edible parts is during the young stages, while that for medicinal parts is at the full-blooming stage. This category includes almost all the leafy vegetables (Alternanthera sessilis, Amaranthus blitum, A spinosus, A viridis, Anredera cordifolia, Artemisia argyi, Asystasia gangetica, Commelina benghalensis, Commelina communis L, Emilia sonchifolia (L.) DC. ex DC., Eryngium foetidum, Gynura divaricata, Patrinia villosa, Perilla frutescens var. purpurascens, Polygonum perfoliatum, Portulaca oleracea and Solanum americanum). All these species are most frequently used as “everyday” food, and most of them (except Artemisia argyi and Anredera cordifolia) are used to treat the damp-heat syndrome, likely to relieve ailments caused by the hot and humid climate in the coastal region of South China.
Second, edible and medicinal parts overlap. Most species included in this category have multiple medicinal parts and they are partly used as food, such as Pandanus tectorius (roots and fruits used as medicine, and fruits used as food), Plantago asiatica (the whole plant and seeds used as medicine, and the leaves used as food), Solanum torvum (roots, leaves and fruits used as medicine, and young fruits used as food), and Rosa laevigata (roots, leaves, flowers and fruits used as medicine, and fruits used as food). Only Talinum paniculatum has 2 edible parts (young stems, including some young leaves, and roots) and 1 medicinal part (roots). These species are often treated as functional foods with strong therapeutic effects and cannot be used as the “everyday” food.
The last category includes species with strictly different parts used for food and medicinal purposes, such as Cheilocostus speciosus (rhizomes used as medicine, and tender stems and tender scapes used as food), Eleutherococcus trifoliatus (roots and leaves used as medicine, and young shoots used as food), Melastoma dodecandrum (the whole plant, roots and young leaves used as medicine, and the mature fruits used as food), and Smilax china (the whole plant and rhizomes used as medicine, and the young shoots used as food). All these species are popular TCM and have been cultivated on a large scale.
The medicinal utilization methods are diverse in the coastal regions of South China. Medicinal parts for the same species can be different when used by different ethnic groups, even with different efficacies. Efficacies also can be various with different preparation methods (eg, external or internal use). For example, the efficacies of Smilax china are antiphlogosis and antitussive by the Li nationality, while the species is used to treat rheumatalgia and traumatic injury by other minority groups. The common efficacy of Gynura divaricata is to treat dysentery, but it may also be used to treat traumatic injuries and carbuncle for external use. The root of Solanum torvum is used for curing cold, chronic cough, stomachache, toothache, amenorrhea, swollen welling-abscess and traumatic injury, while the leaves are used for innominate inflammatory, and the fruits are used for improving eyesight.
Local Preferences of Food-Medicine Plants
Local preferences and ethnic use of food-medicine plants were normally affected by the local natural conditions, customs, as well as the long-term tradition of using medicinal plants.55–57 As a result of permanently living in the warm-wet zone in the coastal regions of South China, the local people particularly prefer the bitter foods. They regard food plants with bitter taste to be effective as heat-clearing agents. This perception is widespread and has influenced the consumption of both medicinal and food plants in the coastal region of South China.20 The concept of bitterness also conforms to the TCM knowledge that bitter medicinal plants usually have the cold property. Other studies have also reported that bitterness may have some additional medicinal properties.3,58,59 For example, the Sylheti people believe bitter food can treat diabetes.3
Conclusions
The coastal region of South China harbors abundant species diversity of food-medicine plants with at least 27 commonly used species documented by the present study. Most of them are good sources for dietary fiber, calcium, iron and vitamin C, with which the percent daily value is more than 10% per serving. Our study confirmed that the unique warm-wet climate in coastal South China has deeply influenced the consumption of the medicinal and food plants of the local ethnic groups. The most common medicinal use of the studied species is for heat-clearing, which is to treat some ailments caused by the specific hot-wet climatic conditions. Furthermore, the local people hence prefer to eat bitter food. The medicinal and edible parts are similar for most of the heat-clearing plants, and the medicinal parts may be used for “everyday” food at the young stage. Medicinal and edible parts of the functional food plants often overlap, and these species usually have strong therapeutic effects and cannot be used as “everyday” food. Almost all the heat-clearing plants are also cultivated and commercialized.
Supplemental Material
Supplemental Material, S_China_Food_Medicine_Plant-Supplementary_materials-revised for Nutritional and Functional Properties of Wild Food-Medicine Plants From the Coastal Region of South China by Yuan Xu, Dan Liang, Gang-Tao Wang, Jun Wen and Rui-Jiang Wang in Journal of Evidence-Based Integrative Medicine
Acknowledgments
The authors are thankful to the local people in the study areas, who shared the traditional knowledge related to the food-medicine plants.
Footnotes
Author Contributions: R-JW and YX designed the study; R-JW, YX, DL, and G-TW carried out the field study and compiled the database; R-JW, G-TW, and DL identified the plant species; YX and JW drafted the manuscript; YX and DL conducted experiments; YX, DL, and JW analyzed the data; R-JW, JW, and YX revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDA13020602).
ORCID iD: Yuan Xu, PhD  https://orcid.org/0000-0002-7922-6787
https://orcid.org/0000-0002-7922-6787
Ethical Approval: Not applicable.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Júnior WSF, de Oliveira Campos LZ, Pieroni A, Albuquerque UP. Biological and cultural bases of the use of medicinal and food plants. In: Albuquerque U, De Medeiros P, Casas A, eds. Evolutionary Ethnobiology. Cham, Switzerland: Springer; 2015:175–184. [Google Scholar]
- 2. Ferreira ICFR, Morales P, Barros L. Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications. Chichester, England: John Wiley & Sons; 2016. [Google Scholar]
- 3. Jennings HM, Merrell J, Thompson JL, Heinrich M. Food or medicine? The food-medicine interface in households in Sylhet. J Ethnopharmacol. 2015;167:97–104. [DOI] [PubMed] [Google Scholar]
- 4. Murray MT, Pizzorno J. The Encyclopedia of Natural Medicine. 3rd ed New York, NY: Simon and Schuster; 2012. [Google Scholar]
- 5. Leonti M. Herbal teas and the continuum of the food-medicine complex: field methods, contextualisation and cultural consensus. J Ethnopharmacol. 2014;151:1028–1030. [DOI] [PubMed] [Google Scholar]
- 6. Jiang S, Quave CL. A comparison of traditional food and health strategies among Taiwanese and Chinese immigrants in Atlanta, Georgia, USA. J Ethnobiol Ethnomed. 2013;9:61 doi:10.1186/1746-4269-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pieroni A, Quave CL. Functional foods or food medicines? On the consumption of wild plants among Albanians and southern Italians in Lucania. In: Pieroni A, Price LL, eds. Eating and Healing Traditional Food as Medicine. New York, NY: Haworth Press; 2006:101–129. [Google Scholar]
- 8. Towns AM, van Andel T. Wild plants, pregnancy, and the food-medicine continuum in the southern regions of Ghana and Benin. J Ethnopharmacol. 2016;179:375–382. [DOI] [PubMed] [Google Scholar]
- 9. Alarcon R, Pardo-de-Santayana M, Priestley C, Morales R, Heinrich M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J Ethnopharmacol. 2015;176:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivera D, Obon C, Inocencio C, et al. The ethnobotanical study of local Mediterranean food plants as medicinal resources in Southern Spain. J Physiol Pharmacol. 2005;56(suppl 1):97–114. [PubMed] [Google Scholar]
- 11. Hong LY, Guo ZY, Huang KH, et al. Ethnobotanical study on medicinal plants used by Maonan people in China. J Ethnobiol Ethnomed. 2015;11:32 doi:10.1186/s13002-015-0019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Etkin NL, Ross PJ. Should we set a place for diet in ethnopharmacology. J Ethnopharmacol. 1991;32:25–36. [DOI] [PubMed] [Google Scholar]
- 13. Leonti M. The co-evolutionary perspective of the food-medicine continuum and wild gathered and cultivated vegetables. Genet Resour Crop Evol. 2012;59:1295–1302. [Google Scholar]
- 14. Etkin NL. Edible Medicines: An Ethnopharmacology of Food. Tucson, AZ: University of Arizona Press; 2008. [Google Scholar]
- 15. Baker CB, Hacisalihoglu G. Excursions in teaching plant science through the local ethnobotany of the food-medicine continuum: field trips to traditional specialty food markets. In: Quave C, ed. Innovative Strategies for Teaching in the Plant Sciences. New York, NY: Springer; 2014:245–259. [Google Scholar]
- 16. World Health Organization. WHO Traditional Medicine Strategy: 2014-2023. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 17. Grivetti LE. Edible wild plants as food and as medicine: reflections on thirty years of fieldwork. In: Pieroni A, Price LL, eds. Eating and Healing: Traditional Food as Medicine. New York, NY: Haworth Press; 2006:11–38. [Google Scholar]
- 18. Zheng XL, Xing FW. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol. 2009;124:197–210. [DOI] [PubMed] [Google Scholar]
- 19. Zheng XL, Wei JH, Sun W, Li RT, Liu SB, Dai HF. Ethnobotanical study on medicinal plants around Limu Mountains of Hainan Island, China. J Ethnopharmacol. 2013;148:964–974. [DOI] [PubMed] [Google Scholar]
- 20. Li DL, Zheng XL, Duan L, et al. Ethnobotanical survey of herbal tea plants from the traditional markets in Chaoshan, China. J Ethnopharmacol. 2017;205:195–206. [DOI] [PubMed] [Google Scholar]
- 21. Au DT, Wu JL, Jiang ZH, Chen HB, Lu GH, Zhao ZZ. Ethnobotanical study of medicinal plants used by Hakka in Guangdong, China. J Ethnopharmacol. 2008;117:41–50. [DOI] [PubMed] [Google Scholar]
- 22. Li DL, Xing FW. Ethnobotanical study on medicinal plants used by local Hoklos people on Hainan Island, China. J Ethnopharmacol. 2016;194:358–368. [DOI] [PubMed] [Google Scholar]
- 23. Liu YJ, Ahmed S, Long CL. Ethnobotanical survey of cooling herbal drinks from southern China. J Ethnobiol Ethnomed. 2013;9:82 doi:10.1186/1746-4269-9-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crane PR, Ge S, Hong DY, et al. The Shenzhen declaration on plant sciences—uniting plant sciences and society to build a green, sustainable Earth. J Syst Evol. 2017;55:415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jia MR, Li XW. Zhong guo min zu yao zhi yao. Beijing, China: China Medical Science and Technology Press; 2005. [Google Scholar]
- 26. South China Institute of Botany Academia Sinica. Flora of Hainan. Vols 1-4 Beijing, China: Science Press; 1964-1977. [Google Scholar]
- 27. South China Institute of Botany Academia Sinica. Flora of Guangdong. Vols 1-6 Guangzhou, China: Guangdong Science and Technology Press; 1987-2009. [Google Scholar]
- 28. Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae. Flora Reipublicae Popularis Sinicae. Vols 1-80 Beijing, China: Science Press; 1959-2004. [Google Scholar]
- 29. Grubben GJH. Tropical Vegetables and Their Genetic Resources. Rome, Italy: International Board for Plant Genetic Resources; 1977. [Google Scholar]
- 30. Latimer GW. Official Methods of Analysis of AOAC International. 20th ed Rockville, MD: AOAC International; 2016. [Google Scholar]
- 31. Barba AIO, Hurtado MC, Mata MS, Ruiz VF, De Tejada MLS. Application of a UV-vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006;95:328–336. [Google Scholar]
- 32. Tee ES, Lim CL. Carotenoid composition and content of Malaysian vegetables and fruits by the AOAC and HPLC methods. Food Chem. 1991;41:309–339. [Google Scholar]
- 33. Amin I, Norazaidah Y, Hainida KE. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006;94:47–52. [Google Scholar]
- 34. Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Vivek C. Antinociceptive and antipyretic activities of Amaranthus viridis Linn. in different experimental models. Arch Biol Sci. 2010;62:397–402. [PMC free article] [PubMed] [Google Scholar]
- 35. Hasan SMR, Hossain MM, Akter R, Jamila M, Mazumder M, Hoque E, Rahman S. Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug Discov Ther 2009;3:211–227. [PubMed] [Google Scholar]
- 36. Ghorbani A, Langenberger G, Feng L, Sauerborn J. Ethnobotanical study of medicinal plants utilised by Hani ethnicity in Naban River Watershed National Nature Reserve, Yunnan, China. J Ethnopharmacol. 2011;134:651–667. [DOI] [PubMed] [Google Scholar]
- 37. He XR, Luan F, Zhao ZF, et al. The genus Patrinia: a review of traditional uses, phytochemical and pharmacological studies. Am J Chin Med. 2017;45:637–666. [DOI] [PubMed] [Google Scholar]
- 38. Lei JC, Yang CX, Yang Y, Zhang W, Yu JQ. Antioxidant and antitumour activities of extracts from Patrinia villosa and its active constituents. J Funct Foods. 2015;16:289–294. [Google Scholar]
- 39. Xiang Z, Chen N, Xu Y, et al. New flavonoid from Patrinia villosa. 2016;54:1219–1222. [DOI] [PubMed] [Google Scholar]
- 40. Xin-Jia Y, Wei L, Ying Z, et al. A new biphenyl neolignan from leaves of Patrinia villosa (Thunb.) Juss. Pharmacogn Mag. 2016;12:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Odhav B, Beekrum S, Akula U, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compos Anal. 2007;20:430–435. [Google Scholar]
- 42. Orech FO, Aagaard-Hansen J, Friis H. Ethnoecology of traditional leafy vegetables of the Luo people of Bondo district, western Kenya. Int J Food Sci Nutr. 2007;58:522–530. [DOI] [PubMed] [Google Scholar]
- 43. Sreeramulu N, Ndossi GD, Mtotomwema K. Effect of cooking on the nutritive value of common food plants of Tanzania: Part 1—Vitamin C in some of the wild green leafy vegetables. Food Chem. 1983;10:205–210. [Google Scholar]
- 44. Gopal T, Megha G, Chamundeeswari D, Reddy CU. Phytochemical and pharmacological studies on whole plant of Asystasia gangetica. Indian J Res Pharm Biotechnol. 2013;1:365–370. [Google Scholar]
- 45. Weber E, Sun SG, Li B. Invasive alien plants in China: diversity and ecological insights. Biol Invasions. 2008;10:1411–1429. [Google Scholar]
- 46. Wu SH, Sun HT, Teng YC, et al. Patterns of plant invasions in China: taxonomic, biogeographic, climatic approaches and anthropogenic effects. Biol Invasions. 2010;12:2179–2206. [Google Scholar]
- 47. Chen C, Wang QH, Wu JY, et al. Historical introduction, geographical distribution, and biological characteristics of alien plants in China. Biodivers Conserv. 2017;26:353–381. [Google Scholar]
- 48. Ma JS, Li HR. The Checklist of the Alien Invasive Plants in China. Beijing, China: Higher Education Press; 2018. [Google Scholar]
- 49. Axmacher JC, Sang W. Plant invasions in China—challenges and chances. Plos One. 2013;8:e64173. [DOI] [PMC free article] [PubMed]
- 50. Lu J, Li S, Wu Y, Jiang L. Are Hong Kong and Taiwan stepping-stones for invasive species to the mainland of China? Ecol Evol. 2018;8:1966–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang D, Zhang R, Kim KC, Suarez AV. Spatial pattern and determinants of the first detection locations of invasive alien species in mainland China. Plos One. 2012;7:e31734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mack RN. Global plant dispersal, naturalization, and invasion: pathways, modes, and circumstances. In: Ruiz GM, Carlton JT, eds. Invasive Species: Vectors and Management Strategies. Washington, DC: Island Press; 2003:3–30. [Google Scholar]
- 53. US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA national nutrient database for standard reference, release 28. 2016. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/sr17-sr28/. Accessed July 10, 2017.
- 54. US Food and Drug Administration. Guidance for industry: a food labeling guide. https://www.fda.gov/FoodLabelingGuide. Accessed July 25, 2017.
- 55. Yang L, Ahmed S, Stepp JR, et al. Comparative homegarden medical ethnobotany of Naxi healers and farmers in Northwestern Yunnan, China. J Ethnobiol Ethnomed. 2016;10:6 doi:10.1186/1746-4269-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang J, Pei SJ, Long CL. An ethnobotanical study of medicinal plants used by the Lisu people in Nujiang, northwest Yunnan, China. Econ Bot. 2004;58:S253–S264. [Google Scholar]
- 57. Liu Y, Dao Z, Yang C, Liu Y, Long C. Medicinal plants used by Tibetans in Shangri-la, Yunnan, China. J Ethnobiol Ethnomed. 2009;5:15 doi:10.1186/1746-4269-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pieroni A, Torry B. Does the taste matter? Taste and medicinal perceptions associated with five selected herbal drugs among three ethnic groups in West Yorkshire, Northern England. J Ethnobiol Ethnomed. 2007;3:21 doi:10.1186/1746-4269-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pieroni A, Sheikh QZ, Ali W, Torry B. Traditional medicines used by Pakistani migrants from Mirpur living in Bradford, Northern England. Complement Ther Med. 2008;16:81–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, S_China_Food_Medicine_Plant-Supplementary_materials-revised for Nutritional and Functional Properties of Wild Food-Medicine Plants From the Coastal Region of South China by Yuan Xu, Dan Liang, Gang-Tao Wang, Jun Wen and Rui-Jiang Wang in Journal of Evidence-Based Integrative Medicine