RA Afferents
Definition
Rapidly adapting (RA) mechanoreceptive afferents (also called fast adapting type I afferents) found in the skin. They are thought to be associated with Meissner corpuscles in glabrous skin and hair follicle and field receptors in hairy skin. Their receptive fields are generally small, and they have a low threshold to mechanical stimulation, particularly low frequency sinusoids (flutter, <60 Hz).
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_3
Functional Behavior
10.1007/978-3-540-29678-2_16
Rabies
Definition
Rabies is an acute, usually fatal encephalomyelitis caused by Rhabdoviridae. Highly endemic in parts of Africa, Asia, and Central and South America, rabies is almost always transmitted by an infected animal bites.
Infected people first develop fever, headache and skin sensation abnormalities (paresthesias) followed by paralysis (“dumb” form), hydrophobia, delirium or psychosis (“furious” form), then coma and death.
Confirmatory diagnosis is made by PCR assay of skin or saliva, but a negative result does not exclude the diagnosis. Pre-exposure vaccination is recommended for people who work with wild animals, travelers who anticipate prolonged stays in rural areas with high levels of endemic rabies as well as for cave explorers (spelunkers).
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_16
Radial-Arm Maze
Definition
An elevated maze with a central platform and, typically, eight radially-arranged alleys. The goal of a rat or mouse is to retrieve food hidden at the end of each alley without repeating an alley choice.
10.1007/978-3-540-29678-2_19
Radial Glia
Definition
The radial glia is morphologically defined as a type of cell that possesses an elongated fiber spanning the developing cerebral cortex from the ventricular surface to the pial surface with an ovoid cell body located within the ventricular zone. The radial glia retains a neurogenic capacity and also its processes serve as a scaffold for migrating neurons.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_14
Radial Histogenetic Division
Definition
A radially arranged region or territory of the brain, whose neurons primarily derive from a specific morphogenetic field (i.e. from a restricted ventricular sector of the neural plate/tube). The radial feature of brain histogenetic divisions is based on the predominant glial fiber-guided migration of immature neurons in their way from the ventricular (proliferative) zone to the mantle during development. Nevertheless, radial histogenetic divisions can contain immigrant cells coming from other fields by tangential migration.
10.1007/978-3-540-29678-2_5
Radial Migration
Definition
Projection neurons are produced locally in the telencephalic wall and migrate to the overlying cortical plate perpendicular to the pial surface.
Radiation Term
Definition
The volumetric source or sink of non-mechanical power in the balance of energy.
10.1007/978-3-540-29678-2_13
Radiculopathy
Definition
Radiculopathy refers to disease of the spinal nerve roots (from the Latin radix for root). Damage to the spinal nerve roots can lead e.g. to pain, numbness, weakness, and paresthesia (abnormal sensations in the absence of stimuli) in the limbs or trunk. Pain may be felt in a region corresponding to a dermatome, an area of skin innervated by the sensory fibers of a given spinal nerve.
10.1007/978-3-540-29678-2_14
Radioisotope
Definition
A radioactive isotope of an element.
Radioligand
Definition
A radiolabeled molecular probe for the visualization of a particular receptor sub-type; see Positron Emission Tomography (PET).
10.1007/978-3-540-29678-2_16
Radiopharmaceutical (Radiotracer)
Definition
A specific pharmaceutical, labeled with radioactive isotope.
Radiotracer Imaging
Definition
Radiotracer imaging techniques involve intravenously injecting various short-lived radiolabelled molecules and then using positron emission tomography (PET) or single photon emission computed tomography (SPECT) to measure one or more biological functions of dopaminergic neurons in a resting state.
10.1007/978-3-540-29678-2_4
Raf
Definition
A protein kinase and member of the MAPKK Kinase family. As a result of neurotrophic factor binding, MAPKKK is activated and phosphorylates MAPKK on its serine and threonine residues. The MAPKK then activates a MAPK through phosphorylation on its serine and tyrosine residues.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_14
RAGs (Regeneration-Associated Genes)
Definition
A series of changes in gene expression that occur in cell bodies (perikarya) of neurons with axon damage.
10.1007/978-3-540-29678-2_1
Random Process
Definition
The term “random process” denotes a series of uncorrelated events that are distributed either exponentially or in a Gaussian fashion.
10.1007/978-3-540-29678-2_3
Raphé Interpositus
Definition
A collection of neurons lined up on either side of the midline ventral to the abducens nucleus. The neurons in raphé interpositus are the saccade-related omnipause neurons.
10.1007/978-3-540-29678-2_15
10.1007/978-3-540-29678-2_19
Raphé Nuclei
Definition
The raphé nuclei are traditionally considered to be the medial portion of the reticular formation, and they appear as a ridge of cells in the center and most medial portion of the brain stem. The raphé nuclei have a vast impact upon the central nervous system. The raphé nuclei can be of particular interest to neurologists and psychologists since many of the neurons in the nuclei (but not the majority) are serotonergic, i.e. contain serotonin – a type of monoamine neurotransmitter.
Serotonin, also called 5-HT, seems to be the culprit in many of our modern psycho-pharmaceutical problems, such as anorexia, depression, and sleep disorders. It is not the sole culprit in the aforementioned disorders, but it is the area that the pharmacologists know how to affect in the best manner. It is important to note that pharmacology traditionally affects global serotonin levels, while the actions of the raphe nuclei are dependent on the complex interplay between nuclei.
10.1007/978-3-540-29678-2_19
Raphé Nuclei and Circadian Rhythm
Definition
The midbrain dorsal and median raphé nuclei known for their widespread, extensively overlapping, ascending serotonergic projections. The projections of each nucleus, serotonergic or not, contribute to a great many different brain functions. In the context of the circadian rhythm system, the innervation by the dorsal and median raphé is somewhat unique because the raphé efferent projections of those two nuclei do not overlap in the two primary components of the system, the suprachiasmatic nucleus (SCN) and the intergeniculate leaflet (IGL). The SCN is very heavily innervated by neurons with cell bodies in the median raphé nucleus.
The majority of these contain the neurotransmitter, serotonin, but many median raphé neurons projecting to the SCN contain a different, currently unknown, neurotransmitter. Neurons of the median raphé do not project to the IGL. In contrast, both serotonergic and non-serotonergic neurons in the dorsal raphé nucleus project to IGL, but not to the SCN. In addition, the median and dorsal raphé nuclei reciprocally connect to one another via serotonergic and non-serotonergic connections. The direct serotonergic median raphé-SCN projection has been implicated as an inhibitor of retinohypothalamic tract transmission of photic input to the SCN, while the dorsal raphé serotonergic projection to the IGL has been implicated in the non-photic regulation of circadian rhythm phase.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_9
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_19
Raphespinal Tract
Synonyms
Tractus raphespinalis
Definition
Projections of the magnocellular raphe nuclei (median zone of the reticular formation) to the gray matter of the spinal cord.
Pathways
Rapid Eye Movement (REM) Sleep
Definition
REM sleep (also called paradoxical sleep (PS) and activated sleep) is a distinctive sleep stage in mammals. Normally this stage of sleep appears after a period of non-REM (NREM) sleep and then alternates with episodes of NREM sleep throughout the sleep period. REM sleep is characterized by a constellation of events including the following: (i) low-amplitude synchronization of fast oscillations in the cortical electroencephalogram (EEG) (also called activated EEG); (ii) very low or absent muscle tone (atonia) in the electromyogram (EMG). The atonia is observed to be particularly strong on antigravity muscles, whereas the diaphragm and extra-ocular muscles retain substantial tone; (iii) singlets and clusters of rapid eye movements (REMs) in the electrooculogram (EOG); (iv) theta rhythm in the hippocampal EEG; and (v) spiky field potentials in the pons (P-wave), lateral geniculate nucleus, and occipital cortex (called ponto-geniculo-occipital (PGO) spikes). Supplemental to these polysomnographic signs, other REM sleep-specific physiological signs are: myoclonic twitches, most apparent in the facial and distal limb musculature; pronounced fluctuations in cardio-respiratory rhythms and core body temperature; penile erection in males and clitoral engorgement in females (tumescence). In humans, awakening from REM sleep typically yields detailed reports of hallucinoid dreaming, even in subjects who rarely or never recall dreams spontaneously.
REM sleep is critical for memory processing and improvement of learning. REM sleep is not identifiable in the fish, amphibian, or reptile classes. In birds REM sleep is seen only for brief periods of time, especially following hatching. Generally, REM sleep is considered to be a highly evolved behavioral stage of terrestrial mammals.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_14
10.1007/978-3-540-29678-2_19
Rapid Eye Movement (REM) Sleep Disorder
Definition
Rapidly Adapting Pulmonary Receptors
Rapidly Adapting Type I Mechanoreceptors
Definition
A mechanically sensitive sensory ending in the skin that adapts rapidly to a sustained indentation and therefore is sensitive to dynamic events such as vibration. It has small, well-defined receptive fields and the sensory terminal is believed to innervate the Meissner corpuscle.
Also known as FAI (fast-adapting type I) afferents in humans, RA (rapidly-adapting) receptors in the cat and QA (quickly-adapting) receptors in the primate.
10.1007/978-3-540-29678-2_3
Functional Behavior
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_5
Rapidly Adapting Type II Mechanoreceptors
Definition
A mechanically sensitive sensory ending in the skin that adapts rapidly to a sustained indentation and therefore is sensitive to dynamic events such as vibration. It has large, poorly-defined receptive fields and the sensory terminal is believed to innervate the Pacinian corpuscle.
Also known as FAII (fast-adapting type II) afferents in humans and PC (Pacinian Corpuscle) receptors in the cat and primate.
10.1007/978-3-540-29678-2_3
Functional Behavior
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_22
10.1007/978-3-540-29678-2_5
Rapsyn
Definition
Rapsyn (Receptor associated protein of the synapse) is important for initiating postsynaptic differentiation (pre-patterning) and is tightly associated with acetylcholine receptors suggesting that this complex becomes aggregated and stabilized at postsynaptic membranes.
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_3
Rarefaction
Definition
Areas of a propagating sound pressure wave of maximal decreased pressure (decrease below the static pressure).
10.1007/978-3-540-29678-2_1
Ras GTPases
Definition
A family of molecules that include RhoA, Rac and CDC42, signals within growth cones.
10.1007/978-3-540-29678-2_1
Rate Coding in Motor Units
Definition
Control of force output from an individual motor unit by regulation of motoneuron firing frequency.
10.1007/978-3-540-29678-2_13
Rate of Cross-Bridge Detachment
Definition
In the cross-bridge theory, cross-bridge attachment and detachment to the actin filament are quantitatively described by position-dependent rate functions. The detachment rate describes the first order kinetics of cross-bridge detachment from actin, while the attachment rate describes the first order kinetics of cross-bridge attachment to actin. In order for force production and contraction to always be in the same direction (i.e. a muscle always tends to shorten upon contraction and to produce tensile forces), these rate functions have to be asymmetric relative to the equilibrium point of the cross-bridge.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_6
Rathke's Pouch
Definition
The pituitary anlage from which a craniopharyngioma may arise.
10.1007/978-3-540-29678-2_14
Rating Task
Definition
A psychophysical task in which a subject is asked to state the magnitude of a stimulus either in absolute terms or relative to a reference.
Ratiometric Dye
Definition
Some dyes respond to a metabolic change with both increase and decrease of fluorescence, depending on how they are measured. For example, the fluorescence of the calcium sensitive dye fura increases with increasing calcium when excited at 340 nm, and decreases when excited at 380 nm. FRET-dyes (FRET means Fluorescence Resonance Energy Transfer) shift their emission spectrum, with the result that fluorescence decrease in one band, and increases in another.
These dyes can be evaluated by creating the ratio (hence the name ratiometric dye) of the two signals, creating a number that is independent of the absolute fluorescence strength.
10.1007/978-3-540-29678-2_6
Ray-finned Fishes
Definition
Also known as actinopterygian fishes. So named because of the flexible rays that provide the structural support of their fins. They make up approximately 95% of all living fishes and about half of all living vertebrate species.
10.1007/978-3-540-29678-2_5
RC Circuit
Definition
Electrical circuit consisting of a resistor and a capacitor.
10.1007/978-3-540-29678-2_3
RCS Rat
Definition
Royal College of Surgeons rat model of Retinitis Pigmentosa has a mutation affecting retinal pigment epithelium. The mutation leads to an inability to phagocytose the photoreceptor outer segment. The same gene mutation is found in human patients with Retinitis Pigmentosa.
10.1007/978-3-540-29678-2_9
rd/rd or rd1 Mouse
Definition
A mouse model of Retinitis Pigmentosa with a naturally occurring mutation of the beta-subunit of phosphodiesterase (an enzyme important in the visual transduction cascade). The same gene mutation is found in human patients with Retinitis Pigmentosa (see Inherited Retinal Degenerations).
10.1007/978-3-540-29678-2_9
Reach to Grasp Postural Strategy
Definition
A change in support reaction to postural perturbation in which a rapid reaching movement of the arm permits a stable object to be touched or grasped for support, in order to restore equilibrium.
10.1007/978-3-540-29678-2_16
Reaching Behavior
Definition
Goal-directed behavior of humans and animals that requires visual information for movement of arms and hands in reach for objects.
Reaching Movements
Definition
The act of reaching, bringing a part of the body in contact with an object, is a crucial component of many animal behaviors. Several vertebrate species use the distal portions of their forelimbs to explore and feed. Reaching movements are particularly important for primates, whose hands are capable of grasping and manipulating objects, and consequently these movements have been extensively investigated in humans and monkeys.
Characteristics
To reach for an object with the hand, the central nervous system (CNS) must map sensory input, which provides information about the object and hand locations in space, into motor output, comprising activations of shoulder and arm muscles that move the hand towards the target. Considering visually guided reaching, the location of a visual target is specified in retinal coordinates, proprioception gives information on the initial hand location in terms of arm muscle lengths, and muscle activations generate forces between arm segments. Thus, the CNS must transform sensory information into motor commands that are encoded in different 10.1007/978-3-540-29678-2_6. It is usually assumed that the CNS performs these sensorimotor transformations in two stages. First, sensory information is used to define a kinematic plan. Target location and hand location are mapped into a common reference frame and a difference vector or motor error is computed. Second, the movement is executed by mapping the plan into muscle activations. This transformation may be performed using sensory signals for correcting the motor commands while they are generated (10.1007/978-3-540-29678-2_6) or by pre-computing the appropriate commands (10.1007/978-3-540-29678-2_6). Since the delays involved in the conduction and processing of sensory signals may create instabilities in a feedback controller, the control of fast reaching movements requires feedforward control. Knowledge of the dynamical behavior of the musculoskeletal system necessary for pre-computing the appropriate motor commands is thought to be incorporated into the controller either explicitly as an 10.1007/978-3-540-29678-2_9 of the motor apparatus or implicitly as a collection of motor programs. The kinematic and dynamic characteristics as well as the muscle activation patterns observed during reaching movements have provided the experimental bases for the elaboration of these and other models of the computations involved in controlling reaching movements (10.1007/978-3-540-29678-2_13).
Kinematics
The motion of the arm during reaching, arm kinematics, is fully specified by the rotational motion of all the joints in the arm. Considering the wrist as the arm end-point to be positioned in space, three rotations at the shoulder (flexion-extension, adduction-abduction, internal-external rotation), and one at the elbow (flexion-extension) are required to characterize reaching kinematics. Since there are four joint angles, or degrees-of-freedom, for three spatial coordinates of the wrist, the system is redundant, i.e. the same spatial location can be reached with the arm in many different configurations. For example, it is possible to raise the elbow without moving the wrist. Moreover, there are infinite paths along which the wrist can be moved to reach a target location from a given start location. Thus, to plan a reaching movement the CNS has to select one out of infinite possible kinematics.
Simple invariant features have been observed in the kinematics of reaching movements and they have provided an indication of the strategy used by the CNS for the planning stage. When performing a point-to-point reaching movement between two points on a horizontal plane, the wrist paths are straight and the wrist tangential velocity has a “bell-shaped” profile with a single peak [1]. For unrestrained movements in a vertical plane, the hand path is not always straight but it is independent of the speed of the movement [2] and of the hand-held load [3] (Fig. 1b–c).
Reaching Movements. Figure 1.

Invariant wrist path and tangential velocity for point-to-point movements across speeds and loads. (a) The position in space of markers placed on the arm of subjects performing unrestrained reaching movements between two points in the sagittal plane is recorded. (b–c) The path, on the sagittal plane, of the wrist for upward and downward movements (between points 3 and 7) does not change with the speed (b, where 6 slow, 6 medium, and 6 fast movement paths are overlapped) and the hand-held load (c, where 6 unloaded, 6 with 2 lb load, and 6 with 4 lb load movement paths are overlapped). (d–e) Similarly, the tangential velocity profile for upward and downward (with inverted ordinates) movements between the same two points, once normalized for speed, does not change with speed (d) and load (e). Adapted from [3] copyright © 1985 by the Society of Neuroscience, with permission.
Moreover, the tangential velocity profiles for movement at different speeds have the same shape when normalized for speed (Fig. 1d). The existence of invariant kinematic features has been interpreted as evidence for kinematic planning of reaching movements. Moreover, the straightness of the wrist path has been interpreted as evidence for planning end-point trajectories or displacements. However, since movements are executed by changing joint angles, end-point planning also requires mapping desired end-point positions into joint angles (inverse kinematics).
Dynamics
Arm movements are generated by the forces applied on the arm segments by the contraction of the muscles interconnecting them as well as by gravity. Since the arm is a chain of articulated segments, the motion of one joint depends not only on the forces directly applied to it but also on the motion of the other joints and the forces applied to them. For example, during a sagittal-plane reach to a target at shoulder level, from a starting posture with the forearm at waist level and the upper arm vertical along the trunk, the shoulder flexes and the elbow extends. However, because of the intersegmental dynamics, the muscles generate a flexor torque at both shoulder and elbow joints. Thus, the transformation between kinematics and dynamics (inverse dynamics) is not trivial and how the CNS implements this transformation is still an open question.
The characteristics of the torque profiles generated by the muscle contractions suggest that the CNS uses simple rules to find approximate yet adequate solutions to the inverse dynamic problem. The net torque generated at each joint by all muscles acting on it can be estimated from the arm kinematics using a simplified dynamic model of the arm based on the Newtonian equations of motion. For point-to-point movements in the sagittal plane, from one central location to several peripheral locations arranged on a circle, the dynamic muscle torque (expressed as the net muscle torque minus the torque required to counteract gravity) at the shoulder and at the elbow are related almost linearly to each other [4] (Fig. 2).
Reaching Movements. Figure 2.

Scaling of dynamic muscle torques as a function of movement direction. (a) The elbow and shoulder muscle torques necessary for performing center-out reaching movements to 12 targets in the sagittal plane are estimated from the movement kinematics. (b) The average dynamic torque at the elbow and at the shoulder, obtained removing the torque required for resisting gravity from the total muscle torque at each joint, are plotted against each other, during the initial accelerating phase, for the 12 different directions (solid and dashed lines; open symbols represent the integrated torque, or impulse, at elbow and shoulder). The dynamic elbow and shoulder torque are approximately linearly related for all movements with a slope depending on the movement direction. Adapted from [4] copyright © 1997 by the American Physiological Society, with permission.
Both shoulder and elbow dynamic torque profiles have similar biphasic and synchronous shapes. Moreover, the relative amplitude of the two torque profiles changes with 10.1007/978-3-540-29678-2_13, with the same biphasic torque profile scaled at each joint by a coefficient that varies as a linear function of the angular displacement at both joints. Simple torque scaling rules have also been proposed as a mechanism to generate movements with invariant paths and tangential velocity with different speeds and loads [3]. These rules derive from the observation that scaling in time the anti-gravity torque profiles and both in amplitude and in time the dynamic torque profiles generates invariant kinematics.
Muscle Patterns
The patterns of muscle activation observed during reaching movements have a complex dependence on the movement direction and speed. For reaching in vertical planes, the electromyographic (EMG) waveforms are constructed by combining components related to both dynamic and gravitational torques [5]. The waveform components responsible for the dynamic torques (phasic activations) have an intensity and a timing that changes with the movement direction in a complex manner [6]. Each muscle has a distinct spatial and temporal pattern, with a recruitment intensity maximal in multiple directions and a recruitment timing changing gradually across directions. Moreover, the phasic activations scale in time with movement speed differently for different muscles.
Despite their complex dependence on the movement parameters, the muscle patterns for reaching are generated according to relatively simple rules. The changes in the muscle patterns for fast reaching movements in different directions on vertical planes are well captured by the combinations of a few time-varying 10.1007/978-3-540-29678-2_13 [7] (Fig. 3).
Reaching Movements. Figure 3.

Muscle synergies for reaching. (a) A set of five time-varying synergies, identified from the muscle patterns recorded during point-to-point movements between one central location and eight peripheral locations in the frontal and sagittal planes. (b) The activation waveforms of 17 shoulder and arm muscles are reconstructed (top, where the gray area represents the averaged EMG activity and the solid black line the synergy reconstruction) by scaling in amplitude and shifting in time (bottom, where the amplitude scaling coefficient is represented by the height of a rectangle and the onset latency by its horizontal position) and combining, muscle by muscle, each one of the five synergies. Different movements are reconstructed with different synergy combination coefficients. (c) The amplitude scaling coefficients are directionally tuned (10.1007/978-3-540-29678-2_4), with a tuning in most cases well captured by a cosine function. Adapted from [7] copyright © 2006 by the Society of Neuroscience, with permission.
A muscle synergy represents the coordinated activation of a group of muscle with specific activation profiles. Each synergy is modulated in intensity and delayed in time differently across movement directions and multiple synergies are combined to generate the observed muscle patterns. Such a combination mechanism may simplify the sensorimotor transformations for reaching by allowing a direct, low-dimensional mapping between kinematic plans and muscle patterns, and, thus, an implicit implementation of approximate inverse kinematics and inverse dynamic computations.
Neural Control
A distributed network of cortical areas in the parietal and frontal cortex and subcortical structures (spinal cord, cerebellum, basal ganglia) is involved in the neural control of reaching movements. This network functions in an integrated manner and it has not been possible to associate specific stages of the sensorimotor transformations to specific areas or neuronal populations. However, each area has a different degree of involvement into the different aspects of the control process. Spatial representation of limb position, target locations, and potential motor actions are highly expressed in the parietal cortex which is thought to be mainly involved in the early sensorimotor transformations. Selection and execution of motor actions are strongly expressed in the motor areas of the frontal cortex, from which most of the descending axons to the brain stem and the spinal cord originate, and which are believed to play a major role in transforming kinematic plans into descending commands closely related to the muscle patterns.
To understand the neural mechanisms underlying the sensorimotor transformations involved in reaching, the characteristics of the activity of individual neurons in many of the cortical areas involved have been investigated in monkeys. Recordings in the motor areas of the frontal cortex, composed by the primary motor cortex and six distinct premotor areas, have shown that the activity of most neurons is broadly tuned to the direction of movement [8]. The activity of each cell depends on the movement direction approximately as a cosine function, with a maximum in a 10.1007/978-3-540-29678-2_16 that varies from cell to cell. Thus, each cell is active for a broad range of movement directions. Conversely, each movement direction is associated by a pattern of graded activation of the entire neural population. In fact, the direction of movement, either during movement preparation or movement execution, can be approximately estimated using a “10.1007/978-3-540-29678-2_16,” the sum of the preferred direction vector of each recorded cell weighted by its firing rate change from baseline. These observations have been interpreted as an indication that the motor cortex in mainly involved in high-level movement representation in terms of spatial location of the hand. However, the activity of most of the cells in the motor cortex is also modulated by the posture of the arm [9] and by the movement dynamics [10]. Thus, the representation of both kinematic and dynamic features are likely to coexist in the motor cortex, as expected in a neural network implementing a coordinate transformation from a kinematic motor plan to dynamic motor commands.
Reaction
10.1007/978-3-540-29678-2_6
Reaction Time
Definition
The time from the presentation of a stimulus to the onset of the movement. Movement onset is usually defined either as the time a threshold in speed is exceeded or as the beginning of a burst of electromyographic activity, the latter criterion yielding smaller values.
10.1007/978-3-540-29678-2_5
Reaction Time Task
Definition
A class of experimental paradigms in which a response (a movement) occurs reflexively in response to the appearance of a sensory stimulus. Movement onset is usually defined either as the time a threshold in speed is exceeded or as the beginning of a burst of electromyographic activity, the latter criterion yielding smaller values. The reaction time is shorter in contrast to voluntary tasks in which the response requires the selection of a response goal that is dependent on other cognitive factors.
Reactive Astrocyte
Definition
When the cebtral nervous system (CNS) is damaged, inflamed or infected the astrocytes undergo a characteristic set of changes known as reactive gliosis. The cells may proliferate.
Morphologically they hypertrophy and generally put out more and longer processes. There are characteristic changes in the cytoskeleton with upregulation of GFAP, vimentin and nestin. The cells may secrete a range of cytokines and may express class II major histocompatibility complex (MHC) receptors.
After injury the cells may be neuroprotective, play a part in controlling inflammation and in resealing the blood-brain barrier.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_7
Reactive Gliosis
10.1007/978-3-540-29678-2_7
Reactive Oxygen Species: Superoxide Anions
10.1007/978-3-540-29678-2_14
Readily Releasable Secretory Vesicles
10.1007/978-3-540-29678-2_14
Reafference
Definition
Sensory input resulting from an animal’s own motor output.
Reafferent Control in Electric Communication
Synonyms
Electrocommunication; Electrical communication
Definition
Every motor act that an animal produces will elicit sensory input from its own receptors [1]. Termed reafference, this self-generated sensory input can be quite useful. For example, bats listen to the echoes of their own ultrasonic calls to navigate through the night, and sensory feedback from skeletal muscles can be used to improve motor control. On the other hand, reafferent input is often not informative, and it can even interfere with the detection of external sensory input. A major problem faced by all animals is distinguishing reafferent sensory input from external sensory input. This issue is particularly relevant to the subject of animal communication. A communicating animal must produce its own signal as well as detect the signals produced by other individuals. A central question in the neurobiology of communication behavior is how sensory systems are able to discriminate self-generated from externally produced signals.
Consider the problem of reafference for visual perception. Any movement of the eyes, either directly or indirectly, due to movements of the head or body, causes the visual input to the retina to shift dramatically. How does the visual system compensate for this shift and maintain sensitivity to external visual stimuli? Early experiments suggested that every time a motor command that induces eye movement is issued, a copy of that command is also sent to the visual system, which generates a negative image of the visual input expected to result from that movement [1,2]. Combining this negative image with actual visual input eliminates any self-induced changes. As a result, the perceived visual world maintains its stability and only externally generated visual inputs are detected.
This basic mechanism relies on two distinct features. First, the timing of motor output must be relayed to the sensory system through what is referred to as a 10.1007/978-3-540-29678-2_3 [2]. Second, the corollary discharge must activate a negative image of the reafferent input, a so-called 10.1007/978-3-540-29678-2_5 [1]. Research on weakly electric fish has provided insight into the neuronal implementation of these two features [3,4].
Characteristics
Quantitative Description
African mormyrid fish possess an electromotor system that generates weak electric signals from a specialized 10.1007/978-3-540-29678-2_5, as well as an electrosensory system for detecting these signals (Fig. 1a). This unique sensorimotor system serves two functions. Through 10.1007/978-3-540-29678-2_1, mormyrids are able to detect distortions in their own electric field caused by nearby objects and thereby locate and identify various features of those objects, as well as navigate through their environment. By sensing the electric signals generated by other individuals, mormyrids are also able to communicate within the electric modality.
Reafferent Control in Electric Communication. Figure 1.

(a) Schematic of the electric communication system in the mormyrid Brienomyrus brachyistius. The electric organ, shown in blue, is controlled by a command center in the hindbrain. Each descending command drives the production of a single electric organ discharge (EOD). External electric fields are detected by electroreceptors, whose distribution on the body surface is indicated by turquoise shading. Input from the electroreceptors converges onto an electrosensory region in the hindbrain, which also receives input from the electric organ command center. (b) Structure of electric signals in mormyrids. Head positive voltage is plotted upward. The electric organ discharge (EOD) has a fixed, characteristic waveform, while the pattern of EOD production, indicated by the 10.1007/978-3-540-29678-2_19 (SPI), is variable.
Electric signals in mormyrids consist of a fixed 10.1007/978-3-540-29678-2_5 separated by a variable 10.1007/978-3-540-29678-2_19 (SPI) (Fig. 1b). The EOD waveform conveys several aspects of the sender’s identity, such as its species, sex, dominance, and possibly even its individual identity [5]. The total duration of the EOD is a particularly salient variable across species, ranging from as little as 100 μs to over 10 ms, and it may also exhibit sex- and status-related differences, with dominant males having a two- to three-fold longer EOD than females. By contrast, the SPI is involved in communicating contextual information about the sender’s behavioral state and motivation. A variety of different patterns in the SPI have been linked with behaviors such as courtship and aggression [5].
In order for mormyrids to utilize the information available to them in these electric signals, however, they must first be able to distinguish their own EODs from those of other individuals. This distinction is made possible by a corollary discharge pathway that relays the timing of EOD production to central electrosensory regions (Figs. 1a and 2). By comparing incoming electrosensory information with an internal copy of their electromotor commands, they are able to distinguish their own electric signals from those of other nearby fish [4].
Reafferent Control in Electric Communication. Figure 2.

Electric communication pathways in mormyrids. The electromotor pathway is shown in blue, the electrosensory pathway in green, and the corollary discharge pathway in red. Excitatory connections are indicated by flat lines, inhibitory connections by solid circles. Abbreviations: BCA, bulbar command-associated nucleus; CN, command nucleus; MCA, mesencephalic command-associated nucleus; nELL, nucleus of the electrosensory lateral line lobe; PCN, precommand nuclear complex; MRN, medial relay nucleus; slem, sublemniscal nucleus; VP, ventroposterior nucleus.
Higher Level Structures
Electromotor Pathway
Each EOD is initiated by a group of neurons in the ventral hindbrain that together constitute the electric organ 10.1007/978-3-540-29678-2_3 (CN) [5]. The neurons in the CN project both directly and indirectly to an adjacent group of neurons that make up the medial relay nucleus (MRN). The neurons in the MRN receive the command from the CN and relay it down the spinal cord to electromotor neurons that drive the electric organ (Fig. 2). The activity in the CN, and therefore the SPI, is determined by a number of descending inputs, foremost of which is a precommand nuclear complex (PCN) consisting of two adjacent, but physiologically and anatomically distinct neuronal populations [5].
Electrosensory Pathway
The electrosensory system of mormyrids consists of three distinct pathways, one of which is relevant for electric communication (Fig. 2). The primary sensory afferents in this pathway receive input from so-called 10.1007/978-3-540-29678-2_11, and project to a region of the dorsal hindbrain termed the nucleus of the electrosensory lateral line lobe (nELL) [6]. The neurons in the nELL relay this electrosensory input to a large midbrain structure termed the 10.1007/978-3-540-29678-2_20, a sensory processing region considered homologous to the inferior colliculus of mammals.
Electric Organ Corollary Discharge Pathway
The EOD command issued by the CN is relayed not just down the spinal cord to the electric organ, but also to higher brain centers that provide a precise timing reference of EOD production (Fig. 2) [3,5]. This electric organ corollary discharge (EOCD) pathway plays an important role in electric communication. For electrosensory processing in the knollenorgan pathway, it gives rise to an inhibitory input to the nELL that serves to block responses to reafferent electrosensory input (Fig. 2) [4]. In addition, the EOCD pathway helps regulate EOD production, as it projects to an electromotor region that provides inhibitory input to the PCN (Fig. 2). As a result, the region that drives the CN to fire is inhibited each time an EOD is generated. This negative feedback, referred to as recurrent inhibition, plays a critical role in controlling the SPI [5].
Lower Level Components
Electric Organ
The electric organ of mormyrids is located at the base of the tail and consists of a homogenous population of disc-shaped, modified muscle cells called 10.1007/978-3-540-29678-2_5 (Fig. 1a) [7]. When they are activated in synchrony by input from spinal electromotor neurons, their individual electrical potentials summate and give rise to the EOD, the amplitude of which is typically a few volts. Differences in the EOD waveform across species and between the sexes are directly related to variations in electrocyte morphology [7].
Electroreceptors
The knollenorgans involved in electric communication typically contain a few receptor cells that are housed together within a single large capsule [8]. Knollenorgan receptors are broadly tuned to the spectrum of the species-specific EOD and are extremely sensitive, with thresholds as low as 0.1 mV. In response to outside positive-going voltage steps, they fire a single spike at a short fixed latency. This phase-locked activity is relayed by primary sensory afferents to the nELL.
Specialized Features of Time-Coding Circuitry
The electromotor and electrosensory pathways of mormyrids are characterized by several unique anatomical specializations. Both pathways contain high levels of calcium-binding protein and consist of large, spherical, adendritic cell bodies that give rise to thick, heavily myelinated axons. Synapses in both pathways are typically mixed chemical-electrical, and often form large terminals that envelope a significant portion of the postsynaptic soma. Unlike most brainstem nuclei that occur in bilateral pairs, the CN and MRN form unpaired, midline nuclei. All of these features have been associated with neural circuits in which spike timing precision is of the utmost importance [9]. For the electromotor system, this precision is critical for activating the electrocytes in synchrony and thereby maintaining a constant EOD waveform. For the electrosensory system, it is involved in accurate temporal coding of the EOD waveform.
Higher Level Processes
Distinguishing Self-Generated EODs from External EODs
Knollenorgan receptors respond equally to any EOD that is above threshold, whether it is generated by the fish’s own electric organ or that of another fish. In both cases, primary knollenorgan afferents generate a single spike that gives rise to an excitatory input to nELL [4]. However, the neurons in nELL also receive inhibitory input from the EOCD pathway [4], which causes the nELL neurons to respond quite differently to self-generated and external EODs (Fig. 3).
Reafferent Control in Electric Communication. Figure 3.
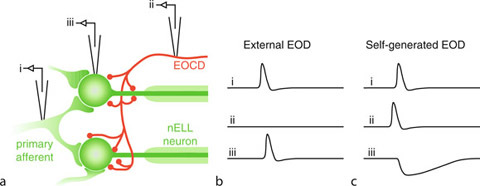
Corollary discharge-mediated inhibition of reafferent electrosensory input in the nucleus of the electrosensory lateral line lobe (nELL). (a) Primary knollenorgan afferents form large, excitatory, mixed chemical-electrical synapses onto the soma of large, adendritic spherical nELL neurons. The electric organ corollary discharge (EOCD) pathway also provides inhibitory input onto the soma and initial segment of nELL neurons. (b) Patterns of activity recorded from the electrode locations shown in (a) in response to an external EOD. (c) Patterns of activity recorded from the electrode locations shown in (a) in response to a self-generated EOD.
When knollenorgan afferents respond to an external EOD, the EOCD pathway is not active. As a result, the nELL neurons only receive the excitatory afferent input, which they relay to the midbrain (Fig. 3b). By contrast, when the fish generates its own EOD, the EOCD pathway also becomes active, providing inhibitory input to nELL neurons. This inhibition blocks the response of nELL neurons to afferent electrosensory input (Fig. 3c), and the signal therefore does not get relayed to the midbrain [4]. As the reafferent input for this system is simply a brief excitation, the corollary discharge-driven efference copy is simply a brief inhibition.
Temporal Coding of the EOD Waveform
The EOD of a neighboring fish will cause current to flow into one half of the body surface and out the other, meaning that knollenorgans on these two surfaces will be exposed to opposite stimulus polarities. As knollenorgans only respond to positive-going voltage steps, those located where current is entering the skin respond to the rising edge of the stimulus, while those located where current is exiting the skin respond to the falling edge. Thus, by comparing spike times from opposite sides of the body, a mormyrid can, in principle, determine the duration of the EOD waveform [6].
A primary projection site of nELL axons is the anterior exterolateral nucleus (ELa) in the torus semicircularis (Fig. 4a). Within the ELa, there are two distinct types of neurons, large cells and small cells, both of which receive excitatory input from nELL axons. Upon entering the ELa, the nELL axons immediately terminate onto 1 or 2 large cells, and then wind their way throughout the nucleus over distances of 3 to 4 mm before branching and terminating onto a large number of small cells [6]. The large cells project exclusively within the ELa, terminating on small cells with large inhibitory synapses [6]. Thus, the small cells receive phase-locked input from two different sources: excitatory input from nELL axons and inhibitory input from ELa large cells (Fig. 4b). However, the excitatory input is significantly delayed by the time it takes an action potential to propagate down the long, winding path of the nELL axon, suggesting an “anti-coincidence detection” model for comparing spike times from knollenorgans on opposite sides of the body [6].
Reafferent Control in Electric Communication. Figure 4.

Model of EOD waveform discrimination in mormyrids. (a) Neuroanatomy of the knollenorgan pathway. Excitatory connections are indicated by flat lines, inhibitory connections by solid circles. Primary afferents from knollenorgans project ipsilaterally onto the nucleus of the electrosensory lateral line lobe (nELL), which also receives inhibitory input from the electric organ corollary discharge pathway (EOCD). Axons from nELL ascend through the lateral lemniscus to project bilaterally to the anterior exterolateral nucleus (ELa) of the torus semicircularis, first onto large cells, then after winding throughout the nucleus, onto small cells. The large cells provide inhibitory input to the small cells. The small cells project ipsilaterally to the posterior exterolateral nucleus (ELp). (b) Schematic diagram showing the inputs to the small cell shown in (a) in response to a transverse square pulse. The ipsilateral side responds to the pulse onset, providing delayed excitatory input to the small cell, while the contralateral side responds to the pulse offset, providing inhibitory input to the small cell. c, Responses of the small cell shown in (b) to square pulses of varying duration. The green traces show the excitation provided by the nELL axon, while the red traces show the inhibition provided by the large cell. The blue traces show the resulting output of the small cell.
As an example, the small cell shown in Fig. 4b receives delayed excitatory input in response to stimulus onset and inhibitory input in response to stimulus offset. For short duration stimuli, this delayed excitatory input will arrive during the inhibition, and the small cell will not fire (Fig. 4c). As stimulus duration increases, however, there will be a greater delay before the inhibitory input reaches the small cell. If the duration is long enough such that the delayed excitatory input arrives before the inhibitory input, then the small cell will fire (Fig. 4c). Thus, a given small cell will only respond to EODs that are longer than some threshold duration. Assuming that different small cells receive input from nELL axons of varying delays, each small cell will have a different threshold value, and EOD duration will be reflected in the total number of active small cells [6].
Function
The Reafference Principle
Dealing with reafferent sensory input is a problem faced by all animals [1]. In the communication system of mormyrid electric fish, this problem is solved by a very simple, yet effective solution: incoming sensory input is blocked by inhibition every time the fish produces a signal. Thus, the fish only senses the electric signals produced by other individuals. Recent studies have shown that this same strategy is used by singing crickets to block auditory responses to their own song [10]. Thus, corollary discharge-driven inhibition may be a widespread solution to dealing with the problem of reafference.
However, reafferent stimuli may often be much more complex, and the temporary blanking of responses afforded by simple inhibition may not be an effective solution. The earlier description of the effects of eye movement on visual processing is an illustrative example. Rather than brief excitation, the reafferent input in this case is a complex pattern of excitation and inhibition across many neurons over time, which is dependent on the specific eye movement undertaken. It is not sufficient to simply block incoming visual input during any movement, because this would result in complete blindness. In this case, rather than simple inhibition, the corollary discharge activates a spatiotemporally complex efference copy that cancels out the sensory input arriving from each portion of the visual field in response to the movement [1].
For active electrolocation in mormyrids, the fish’s own EOD is the signal of interest, while those of other individuals constitute noise. Not surprisingly, then, the EOCD pathway provides excitatory, rather than inhibitory, input to the electrosensory pathway involved in active electrolocation and thereby facilitates reafferent sensory input [3]. However, much of this input is not informative, as it signals the presence of unchanging, or predictable, environmental features. In contrast to the hard-wired inhibition provided to the knollenorgan pathway, this corollary discharge-driven excitatory input can be altered through experience so that expected sensory input is nullified and only novel, informative input gets through [3]. This system provides an example of a modifiable efference copy, one that may be adjusted to compensate for changes in the sensory consequences of motor production.
Temporal Coding
Early research on electric communication in mormyrids focused on the SPI, because it was assumed that the EOD acted simply as a carrier signal for information encoded in a temporal pattern. The reasoning behind this was that EODs must be too brief to transmit any information. However, field recordings from mormyrids in the field revealed incredible species-specific diversity in the EOD waveform, as well as sex differences in many species [5]. Playback experiments in the field later demonstrated that these differences were behaviorally significant. In particular, EOD duration, or the relative timing of positive and negative voltage deflections were especially important [6]. These experiments therefore demonstrated that EOD recognition was mediated by a temporal code. In this chapter, we have seen a remarkable, yet simple, example of how the information contained within such a temporal code may be extracted through dedicated neuronal circuitry.
Realism (Metaphysical, Internal, Common Sense, Naïve, Scientific)
Definition
Realism is a metaphysical position concerning the status of objects, facts and properties which can be of the most different kinds. One may be a realist concerning objects in space and time like trees, rocks, and molecules, concerning abstract objects like numbers or values, properties like being red or facts like the fact that the earth is round. What does realism with respect to one or more of these types of items amount to? Unfortunately there is no shared view among the experts in the field as to how realism is best defined. The question is especially disputed among adherents of the various brands of realism and their critics, the so-called anti-realists. According to the definition shared by most (but not all) philosophers considering themselves realists, realism with respect to a certain item implies the following two claims: First, the existence claim (EC): The items in question exist. Secondly, the independence claim (IC): The items in question are neither themselves something mental (mere ideas or representations) nor is their existence in any way dependent on whether we represent them (that is, perceive them or think of them) in a particular way or not. If you believe, for example, that the earth exists independently of whether there is a being with mental states able to represent it then you are a realist about the earth. Realism is often restricted to certain types of items: one may be a realist concerning physical objects in space and time without being a realist concerning moral values. According to the two defining claims one might dispute realism concerning a certain item in two ways: by denying either (EC) or (IC). For example, realism about moral values can be denied either by denying that there are any such values in the first place or by admitting their existence but taking it to be completely dependent on our ability to devise such values.
According to the alternative definition put forward by anti-realists realism is not so much a theory about the nature of objects, facts or properties but a doctrine concerning the question of how the truth of sentences is best understood. The relevant conception of truth implies that truth is verification-transcendent, that is, a sentence might be true although we don’t have the slightest possibility to find out that it is true. Anti-realists use this definition to criticize realism, because they take the verification-transcendent conception of truth to be at odds with their preferred accounts concerning the question of what is implied when a speaker understands a proposition [1]. Realists have objected to this characterization of their position that they see no need to commit themselves to any substantial notion of truth whatsoever by endorsing (EC) and (IC) [2]. This essay will therefore follow the first definition.
Description of the Theory
Realism cannot only be held with respect to different items, it can also be formulated with varying strength. These variations in strength are mainly due to the fact that (IC) can be interpreted in various ways. According to the strongest reading, (IC1), the items in question exist independently whether any mind (not only human minds but also more powerful minds) has even the ability to represent them. It is then not only possible that there are items with nobody represented at a certain time but with could have been represented in principle, as was probably the case with the earth one billion years ago, but that there might even be items which lie completely outside of any representational power. A somewhat weaker reading, (IC2), would restrict this claim to human minds. Still, the world might contain many items we will not even have the possibility to form a conception of just as a chimpanzee is unable to form a conception of an electron [3]. In this sense our conception and a fortiori our knowledge of the world might always be limited and partial even if we lack the slightest evidence to suppose that they are limited and partial in that way. A considerably weaker reading, (IC3), would allow that there are many items we have never represented and we will never be able to discover but could at least form a conception of, so that we could at least speculate about their existence. A still weaker reading, (IC4), would allow that there are many items we have never represented but could have represented and would have been able to discover. The weakest reading, (IC5), only allows that the items in question can exist independently of whether someone actually represents them, but not independently of whether we can discover them or not.
The last three readings all make items in the world dependent in a certain way on our abiliy to represent them. Therefore, one might argue that they are too weak to convey the idea of independence which is inherent in realism. Realism is generally contrasted with idealism which holds that everything is in some sense dependent on our minds. True enough, there are forms of idealism which even contradict the weakest reading as it is the case with the idealism of Bishop Berkeley (1685–1753), who identified the existence of things with their actually being perceived. But there are many less radical forms of idealism (laying their emphasis on different kinds of dependence of the world of our mind or our representational capacities) which are compatible with these two readings. Note also, that the last two readings atleast don’t allow for a verification-transcendent notion of truth, because they imply that truths about the world have to be discoverable by us. Therefore, they would also fail to count as reconstructions of realism according to the second, anti-realist definition of realism. This explains why the term “realism” is generally associated with the stronger readings, but as will become clear below, Hilary Putnam’s 10.1007/978-3-540-29678-2_18 forms a notable exception.
The first two readings allow for insurmountable ignorance about parts of the world and the first three allow for certain kinds of radical error concerning parts of the world we have a conception of. It is not only possible that we err simply in mistaking something green for something blue or something spherical for a flat disc, we might even err in ascribing whole classes of properties to things that don’t possess. In this case, the concepts we make use of in our characterizations of the world (our “conceptual schemes”) don’t correspond to the internal structure of the world: we take the world to be coloured in the way it appears to our eyes, but it might be that in fact nothing is coloured in that sense. In fact, science tells us that the surfaces of tomatoes aren’t red in the way they appear red to our eyes, but that this appearance is largely due to the structure of our perceptual apparatus [3]. If science is right about these matters that we can say that it gives a more adequate picture of the world as it is than our everyday view. Considerations like these lead to interesting consequences concerning the question of how to deal with competing conceptions of reality which are not compatible with each other: According to realism there is a fact of the matter, how the world is. Therefore, either one of them will get closer to the true story about the world or both will fail in this attempt. Consequently, realism is opposed to various forms of relativism according to which truth and knowledge have to be relativized to culture, historical epoch, conceptual schemes and the like. Competing claims concerning the shape of the earth might then be correct relative to their specific cultural, historical and conceptual context and there might be no fact of the matter beyond these contexts allowing us to ask whether a claim, a theory or a conceptual scheme is correct or not. In contrast with these claims realism allows us to hold that the replacement of one theory or conceptual scheme by another scheme may be interpreted as progress in our endeavour to gain a picture of the world as it is independently of any of our representations of it.
Furthermore, there might be possibilities of large-scale error which open the door for certain notorious sceptical scenarios: the stronger versions of (IC) seem to allow that we could even be wrong about reality as a whole. Accordingly, it might be the case that we are always dreaming or, to cite another famous example, we might be all brains in vat filled with a nutritient and supplied not with real information about the world but only with hallucinations induced by a super-computer connected to us by nefarious neuroscientists [2,3] And considering that our revisions of our former world views also have to be couched in our conceptions of the world we can ask again whether these tensions will tell us the true story about the world as it really is [3]. In this sense realism leads to the consequence that all our epistemic accomplishments are in different respects fallible. Therefore a sceptical position which puts into doubt whether we will ever be able to gain knowledge about the world could possibly be true. A strong enough realism seems even to be one of the central presuppositions needed in order to make these kinds of sceptical hypotheses intelligible in the first place. Most philosophers supporting such a strong kind of realism don’t embrace scepticism, however. The fact that we have to admit the possible truth of scepticism should not be confounded with the fact that we have to take it seriously [2]. To the contrary, realists typically hold that they have the best explanation of how knowledge and scientific progress are possible inthe first place.
“10.1007/978-3-540-29678-2_18” is often used as a name for the kind of realism based on stronger readings of (IC) like (IC1) and (IC2). The term was originally coined by Hilary Putnam who refuses this kind of realism, because he takes the idea that a conception or theory of the world might be wrong, although it fulfilling all our predictions and following all our methodological constraints (coherence, elegance, simplicity etc.) to be incoherent. Additionally he has argued that metaphysical realism has to give up a commitment not only metaphysical realists would like to subscribe to: the claim that our representations of items in the world are connected with these items in a way which gives them a definite reference (e.g., that the concept “cat” refers to cats and not to rats) [4].
Internal realism is Putnam’s alternative to metaphysical realism and can be characterized roughly by following two claims: (IR1) A description of the world is true if it can be justified under epistemically ideal conditions. A description is justified if it is internally coherent and can be in principle verified, so that it is at least in principle possible for us to detect its truth. This implies that its truth does not consist of a kind of correspondence to facts in the world which are completely independent of our way of conceiving them and which might be completely inaccessible to us. (IR2) We have to acknowledge a certain kind of conceptual relativity according to which questions as to what kinds of objects there are or how many there are can’t be answered independently of the choice of a certain conceptual framework. If someone asks for example “How many objects are in this room?” the right answer depends on certain decisions concerning our concept of “object.” If we admit as objects only things which are not attached to other things my nose or a lampshade will not count as objects, if we do without this restriction, they will. In this sense there is no fact of the matter of how many objects are in the room which is independent of our concept of an object [4,5].
Conceptual relativity puts internal realism close to relativism. Putnam has emphasized, however, that internal realism is to be distinguished from relativism which he takes as holding a wrong conception of truth and considers even to be self-refuting. In his eyes, relativists typically their truth to mere rational acceptability. Therefore, according to relativism, the claim of the ancients that the earth was a flat disc was true at their time (although false today) because it was rationally acceptable in light of the available methods of investigation and evaluation at that time (but not in the light of the methods available today). However, the claim was not ideally rationally acceptable even at that time, because the conditions of verification were not ideal. A claim may lose its rational acceptability over time, but it can not lose its ideal rational acceptability. Relativism is self-refuting because in claiming its own absolute truth it exempts itself from the claim that all truths have to be relativized to certain historical conditions, conceptual schemes and so on [4].
Internal realism obviously only allows for weak readings of (IC) such as (IC4) and (IC5) because it takes the existence of the relevant items to be dependent on our conceptual resources and decisions and our ability in principle to verify what is the case. It can allow the existence of a certain rock in the desert even if it isn’t represented by anybody at any time. But the existence of rocks remains relative to the fact that we have the concept of a rock. It can also admit that there might be facts (e.g., in the past) we are not able to verify. But it can’t allow the possibility that reality might be a certain way if we can’t verify this under ideal conditions. Therefore, one might ask whether internal realism should be seen as a form of realism at all. It is no wonder that many have seen internal realism as a form of anti-realism [2].
Critics of internal realism have questioned among other things (i) whether it can be successfully distinguished from relativism [6], (ii) whether the specific examples Putnam gives of conceptual relativity cannot be accommodated within metaphysical realism, so that they don’t conflict with the claim that there are facts which are completely independent of our conceptual schemes [6], and (iii) whether ideal rational acceptability makes truth really accessible to us Putnam himself admitting that we can never tell whether we have reached ideal conditions and comparing this kind of idealization in question with unattainable idealizations such as frictionless surface. More recently Putnam himself has given up the claim that truth can be explained as idealized rational acceptability [7].
It is often assumed that realism with respect to spatio-temporal objects like rocks, chairs, etc., is a view dictated by common sense and held independently of any sophisticated knowledge about philosophical matters by “the plain man or woman on the street.” Realism of this kind is therefore often called “10.1007/978-3-540-29678-2_3.” Since common sense isn’t a developed philosophical doctrine it is not easy to decide to which reading of (IC) common sense realism is committed to. Arguably, common sense is not sophisticated enough to make the necessary distinctions required for any decision on these matters. Note, however, that philosophers with wildly diverging views also use this label for their own account of realism [2,7].
10.1007/978-3-540-29678-2_18 is often taken to be a position quite similar to common sense realism. In philosophical debates on perception Naïve realism is often taken to be a view according to which perception presents us the world by and large as it really is. For example, things not only appear to us as coloured (because of the specific nature of our perceptual apparatus) they really are coloured.
Scientific realism is a theory concerning the correct understanding of theoretical terms in scientific theories. Scientific theories make intensive use of theoretical terms like “molecule” “atom,” “electron” and the like which don’t refer to observable phenomena but play an indispensable role in the scientific explanation of such phenomena. We may say that with the help of these terms respective theoretical entities have been introduced into the scientific theory in question: molecules, atoms, electrons and so on. The behaviour of the observable phenomena is explained with the help of certain claims about the behaviour or state of these theoretical entities. The fact that water begins to boil at sea-level at 100°C is for example explained with the help of claims concerning the properties and the behaviour of H2O-Molecules. Because theoretical entities like molecules or atoms are not among the things which can be observed, the question arises as to whether we ever have any good reason to believe in their existence and to accept the respective claims about their properties and their behaviour as true. Scientific realism gives an affirmative answer to these questions. According to one of its classical formulations [8] we have to interpret theoretical terms as putatively referring expressions and we often have enough reason to accept claims containing such terms as at least approximately true. Furthermore, we can see scientific progress as a steady approximation toward the truth of the observable and the unobservable. The reality described by scientific theories is largely independent of our thoughts and theoretical commitments. Therefore, we can say that we not only introduce theoretical terms in order to facilitate empirical predictions and the organization of our observation-knowledge, we also discover that there are molecules and electrons etc. In this sense scientific realism clearly endorses (EC) and a strong version of (IC), although the precise strength is often left open because the discussion concentrates more on whether theoretical entities are claimed to exist at all. One of the main arguments put forward in favour of this position is based on the claim that we can only plausibly explain why scientific theories have the predictive success they have if we suppose that the theoretical claims referring to theoretical entities are approximately true [8,2]. A classical objection to this claim is the historical observation that theories can be predictively successful although they are largely wrong [9]. A further general objection to scientific realism is that it cannot deal with the fact that two successful theories with commitments to different theoretical entities might lead to the same empirical predictions. It is argued that in such cases there is no evidential basis allowing a decision between these theories. If theoretical statements can be literally true, however, as Scientific Realism would have it, such a decision must be possible in principle. Against this, Scientific Realists have argued that we should allow for a conception of evidential support that is not restricted to positive outcomes concerning prediction [8]. Sometimes scientific realism is and to imply a further claim which puts it into strong opposition to naïve realism or common sense realism. If there is, e.g., an irreconcilable collision between the common sense view of physical objects as continuous solids and the scientific view that they are swarms of molecules, the commitment to the existence of the theoretical entities of science demands that we give up our naïve and common sense views concerning the nature of reality [10].
Reality Monitoring
Definition
Reality monitoring is defined as the ability of distinguishing between external memories (e.g., those of events directly perceived or actions actually performed) and internal memories (e.g., those of events imagined or actions planned or intended to perform).
10.1007/978-3-540-29678-2_13
Realization
Definition
Mental properties, although not identical to physical properties, are still said to be physical properties in a broad sense in virtue of being realized by physical properties, just as a machine table, for instance, is implemented by but not identical to the states of its physical implementation. A central idea is that if property F realizes property G, then G is not something distinct from or something over and above F. Unlike identity, realization is asymmetric: F realizes G only if the instantiation of F in o necessitates or determines the instantiation of G in o but not vice versa, where the necessity in question is at least nomological necessity.
10.1007/978-3-540-29678-2_5
Reasoning
Definition
Reasoning is a process of drawing inferences from information that is taken for granted. Formal reasoning is within the scope of mathematics and philosophy. It is the study of inferences whose validity only derives from its formal structure. Mental reasoning is a function of the human brain. It comes into play whenever people go beyond what is explicitly given. It is the cognitive activity to infer that something must be true, or is likely to be true, given that the known information is true. The problem information is given by a number of statements which are called premises, and the task is to find a conclusion that follows from these premises. The following inference is a typical reasoning problem:
If a patient’s left hemisphere is damaged, then he has impaired reasoning abilities.
Alan’s left hemisphere is damaged.
––––––
Therefore, Alan has impaired reasoning abilities.
Although the premises (above the line) do not say anything about Alan’s reasoning abilities, most people immediately agree with what is stated in the conclusion (below the line). The conclusion necessarily – logically – follows from the premises. Another inference is given in the following example.
Mammals have a nervous system.
Birds have a nervous system.
Fishes have a nervous system.
––––––
All animals have a nervous system.
Although a reasoner might form the belief that the conclusion could be true, the premises do not warrant the truth of the conclusion. The reasoner is generating the hypothesis that the conclusion is true. The former inference is an example of deductive reasoning, while the latter is an instance of 10.1007/978-3-540-29678-2_18.
Characteristics
Deductive and Inductive Reasoning
Mental deductive reasoning is strongly related to formal logic. The latter serves as the normative model for the former (a critical assessment of this account from a neuroscience perspective can be found in [1]). To explore deductive reasoning in the psychological laboratory, people are typically asked to draw conclusions from given premises and later their responses are evaluated for logical validity. This evaluation is based on logical correctness only and does not account for the content of the statements (the deductive inference above is logically valid, although the content concerning the role of the left hemisphere is probably wrong; see below). In 10.1007/978-3-540-29678-2_18, the premises of the problem consist of an “if A then B” construct that posits B to be true if A is true. The two logically valid inferences are the Modus Ponens (if p then q; p; q, MP) and the Modus Tollens (if p then q; not-q; not-p, MT). Humans are pretty good in making inferences of the form MP, but they make many mistakes in the form MT [2], In syllogistic reasoning, the premises of the problem consist of quantified statements such as “All A are B,” “Some A are B,” “No A are B,” and “Some A are not B.” People often make many mistakes in syllogistic reasoning, in part because of the existence of a variety of biases [2]. The most frequently used sort of inferences in daily life (and in the psychological lab) are based on relations. In relational reasoning, at least two relational terms A r1 B and B r2 C are given as premises and the goal is to find a conclusion A r3 C that is consistent with the premises. The relations represent spatial (e.g., left of), temporal (e.g., earlier than), or more abstract information (e.g., is akin to). People are pretty good in making such inferences, but the difficulty depends on the number of premises, the order of terms and premises, the content, and the ease to envisage the content of the problem [3,4]. Moreover, in cases where a reasoning problem has multiple solutions, reasoners consistently prefer the same subset of possible answers – and often just a single solution [5].
Inductive reasoning has not as much to do with logic because the conclusion goes beyond the information given in the premises. The premises only provide good reasons for accepting the conclusion. Thus, inductive reasoning is not truth-preserving but it is the most important basis of our ability to create new knowledge. This new knowledge is often based on a limited number of observations from which we formulate a law recurring to a set of phenomenal experiences. Cognitive theories of induction typically describe it as a process in which hypotheses are generated, selected, and evaluated [6,7]. Although there is no generally accepted definition of the term “induction,” the majority of psychologists adopt the very broad definition that mental inductions are “all inferential processes that expand knowledge in the face of uncertainty” [6, p. 1]. Given that almost nothing is known about the neural basis of inductive reasoning this review is restricted to deductive reasoning. An easily accessible summary of behavioral findings on inductive reasoning can be found in Manktelow [8]. The main problems of research on inductive reasoning are summarized in Sloman and Lagnado [9].
Cognitive Theories of Reasoning
There are two main theories of deductive reasoning. They differ in the postulated underlying mental representations and the computational process that work on these representations. In one theory, it is believed that people think deductively by applying mental rules which are similar to rules in computer programs. In the other theory, deductive reasoning is conceived as a process in which the reasoner constructs, inspects, and manipulates 10.1007/978-3-540-29678-2_13. The rule-based theory is a syntactic theory of reasoning, as it is based on the form of the argument only, whereas, the 10.1007/978-3-540-29678-2_13 is a semantic theory, because it is based on the meaning (the interpretation) of the premises.
The rule-based theories are primarily represented by the work of Rips [10] and Braine and O’Brian [11]. These theories claim that reasoners rely on formal rules of inference akin to those of formal logic, and that inference is a process of proof in which the rules are applied to mental sentences (but cf. Stenning and Oberlander [12]). The formal rules govern sentential connectives such as “if” and quantifiers such as “any,” and they can account for relational inferences when they are supplemented with axioms governing transitivity, such as: For any a, b, and c, if a is taller than b and b is taller than c, then a is taller than c. The rules are represented in the human brain and the sequence of applied rules results in a mental proof, or derivation, which is seen as analogous to the proofs of formal logic [10].
The 10.1007/978-3-540-29678-2_13 has been developed by Johnson-Laird and colleagues [13–15]. According to the model theory, human reasoning relies on the construction of integrated mental representations of the information that is given in the reasoning problem’s premises. These integrated representations are models in the strict logical sense. They capture what is common to all the different ways in which the premises can be interpreted. They represent in “small scale” how “reality” could be – according to what is stated in the premises of a reasoning problem. The model theory distinguishes between three different mental operations. In the construction phase, reasoners construct the mental model that reflects the information from the premises. In the inspection phase, this model is inspected to find new information that is not explicitly given in the premises. In the variation phase, reasoners try to construct alternative models from the premises that refute the putative conclusion. If no such model is found, the putative conclusion is considered true [14].
Reasoning and the Brain
The two reasoning theories are related to different brain areas. The rule theory implies that reasoning is a linguistic and syntactic process, and so reasoning should depend on regions located in the left hemisphere. The model theory, in contrast, postulates that a major component of reasoning is not verbal, and so the theory predicts that the right cerebral hemisphere should play a significant role in reasoning [16]. More detailed predictions are related to specific brain areas. Here the rule theory assumes that the neural computations during reasoning are implemented in the language processing regions and here specifically in the temporal cortex, while the model theory predicts that the parietal and occipital cortical areas involved in spatial working memory, perception, and movement control are evoked by reasoning [17]. The lateralization of the reasoning process has been primarily investigated in patient studies, while brain imaging techniques allow for a more detailed localization of reasoning processes.
Patient Studies
Early studies of patients with brain-damages seemed to support the rule-based theories of reasoning. Conditional reasoning has been studied by Golding [18]. The author used the Wason-Selection-Task, which is probably the most important paradigm in behavioral research on human reasoning [19]. In the task, four cards are presented to the participants (see Fig. 1) and they are instructed to verify the rule “If there is a vowel on one side of the card, then there is an even number on the other side.” The participants are allowed to turn over the cards in order to verify the rule. The visible letters and numbers on the card correspond to the four possible propositions p, not-p, q, and not-q. According to the propositional calculus of formal logic the only correct choices are p (according to the MP a q must be on the other side) and not-q (according to the MT a not-p must be on the other side). However, only one of the left-hemisphere-damaged patients but half of the right-hemisphere-damaged patients selected the two correct cards. Deglin and Kinsbourne [20] studied syllogistic reasoning with psychiatric patients while recovering from transitory ictal suppression of one hemisphere by electroconvulsive therapy (ECT; that simulates a short-term lesion). The premises were familiar or unfamiliar and true or false. When the right hemisphere was suppressed, the participants tended to perform deductive inferences even when the factual answer was obviously false. While their left hemisphere was suppressed, the same participants used their prior knowledge and if the content was unfamiliar they completely refused to answer. Patient studies on relational reasoning have been reported by Caramazza et al. [21] and Read [22]. Caramazza et al. [21] presented relational premises such as “Mike is taller than George” to brain-damaged patients. After reading the statements they had to answer either a congruent (“Who is taller?”) or incongruent (“Who is shorter?”) question. The left-hemispheric patients showed impaired performance in all problems no matter they were congruent or incongruent. Right-hemispheric patients, in contrast, showed impaired performance only in the incongruent problems. Read [22] used two relational premises and asked patients who suffered from temporal-lobectomy to generate a conclusion from these statements. Overall, the left-hemispheric patients again performed weaker than the patients with right-temporal-lobectomy, but the right-hemispheric patients were more impaired with the incongruent conclusions.
Reasoning. Figure 1.

The Wason selection task.
The reported findings have been frequently used by neuroscientists to support the idea that reasoning is mainly a linguistic and syntactic process, but this interpretation seems awkward to many cognitive oriented reasoning researchers. Although lesions to the left hemisphere might result in a deficit in the processing of the linguistic elements of the problem and, thus, impair overall performance, it does not necessarily follow that the damage will also affect the reasoning process. It is likely that left-hemisphere lesions lead to an inability to process the linguistic aspects of a reasoning problem, but that for the pure reasoning process the right hemisphere is important. This interpretation would also explain most of the findings. For instance, in the studies by Caramazza et al. [21] and Read [22] the patients had problems in logically deducing the converse of relations. Moreover, Whitaker et al. [23] examined conditional reasoning in patients that had undergone a unilateral anterior temporal lobectomy, one group to the right hemisphere and the other group to the left hemisphere. The content of the problems was related to the participants’ prior knowledge of the world. Given the premises
If it rained, the streets will be dry.
It rained.
The right-hemisphere-damaged patients had a strong tendency to conclude “The streets will be wet” while the left-hemisphere-damaged patients concluded “The street will be dry.” In other words, these right-hemispheric patients were unable to perform the deduction in isolation from their prior knowledge, while the left-hemisphere patients relied on the linguistic content of the problem.
Brain Imaging Studies
Brain imaging studies have been conducted on all the main types of deductive inferences. As with the patient studies the early findings have been frequently interpreted in favor of the rule-based theories of reasoning, as they have shown that reasoning activates a fronto-temporal neural network often just in the left hemisphere [24,25]. However, more sophisticated experimental paradigms suggest this might be due to the confounding of linguistic processing and deductive reasoning. Knauff et al. [17] studied conditional reasoning problems by presenting premises such as “If the teacher is in love, then he likes pizza” to the participants. In half of the problems the second premise was “The teacher is in love” and the participants had to conclude (by MP) “The teacher likes pizza.” In the other half of problems the second premises was “The teacher does not like pizza” and the participants had to conclude (by MT) “The teacher is not in love.” Both types of problems activated a bilateral occipito-parietal-frontal network, including parts of the prefrontal cortex and the cingulate gyrus, the superior and inferior parietal cortex, the precuneus, and the visual association cortex. These finding are difficult to explain based on purely linguistic processes, as the activated brain areas are implicated in the processing of visual and spatial information and visuo-spatial working memory (→) (cf. [26–28]). Similar findings have been reported from a study on syllogistic reasoning. Goel et al. [29] used problems with semantic content (e.g., “All apples are red; all red fruit are sweet; therefore all apples are sweet”) and without semantic content (e.g., “All A are B; all B are C; therefore all A are C”). They found evidence for the engagement of both linguistic and spatial systems. The role of linguistic and spatial systems has been largely investigated by means of relational reasoning problems. In the study by Knauff et al. [30] such problems activated similar brain areas as the conditional problems did. However, the activity in visual association areas was even higher than during conditional reasoning. Goel and Dolan [31] addressed the question by using sentences with a spatial content. They again were either concrete (e.g., “The apples are in the barrel; the barrel is in the barn; therefore the apples are in the barn”) or abstract (e.g., “A are in B; B is in C; therefore A is in C”). They reported that all problems activated a similar bilateral occipito-parietal network no matter if they were concert or abstract.
Reasoning and Visual Mental Imagery
Many of the reported experiments seem to support the model theory of reasoning. However, it is essential not to confuse mental models with visual images (→) [32,33]. Visual images are structurally similar to real visual perceptions, and can represent objects, their colors and shapes, and the metrical distances between them. They have a limited resolution, but they can be scanned and mentally manipulated [34]. They are often accompanied by neural activity in visual association areas (→) and under certain conditions also activate the primary visual cortex (→) (e.g., [30,35,36]). In contrast, mental models are likely to exclude visual detail, to represent only the information relevant to inference and to take the form of multi-dimensional arrays that maintain ordinal and topological properties [33]. Visual images represent information in a modality-specific format, whereas spatial models are abstract and not restricted to a specific modality. To clarify the role of visual images in reasoning Knauff, et al. [37] conducted a combined behavioral and brain imaging study with four sorts of relations: (i) visuo-spatial relations that are easy to envisage visually and spatially, (ii) visual relations that are easy to envisage visually but hard to envisage spatially, (iii) spatial relations that are hard to envisage visually but easy to envisage spatially, and (iv) control relations that are hard to envisage either visually or spatially. This study highlighted two important findings: First, reasoners were significantly slower with the visual relations than with the other sorts of relations. This is called the visual-impedance effect [38]. And second: On the brain level, all types of reasoning problems evoked activity in the parietal cortices and this activity seems to be a “default mode” of brain functioning during reasoning. However, only the problems based on visual relations also activated areas of the visual cortices. Obviously, in the case of visual relations, reasoners cannot suppress a spontaneous visual image but its construction calls for additional activity in visual cortices and retards the construction of a mental model that is essential for the inferential process. Interestingly, congenitally totally blind people are immune to the visual-impedance effect, since they do not tend to construct disrupting visual images from the premises [39]. For a more detailed explanation on how visual images and mental models interact in reasoning the interested reader is directed to Knauff [4].
Content Effects and Belief Biases
How easy it is to visualize is only one aspect of the content of a reasoning problem. Another aspect is how well the content agrees with the reasoners previous experiences and prior knowledge. Many behavioral studies have shown that prior knowledge can significantly influence how efficiently a reasoning problem is solved. Technically speaking, the abstract (logical) truth value of an inference can be the same as the truth value of our prior knowledge – in this case the inference is supported. Or, the formal truth value conflicts with the truth value of the prior knowledge – then the inference is more difficult, which means it results in more errors or takes significantly longer. If an inference generated by a person is biased towards the truth value of the prior knowledge or even overwritten by it, this is called belief bias [40]. Some patient studies, as described, have therefore explored the effects of brain injuries on reasoning with concrete and abstract materials. Their findings agree with the brain imaging study by Goel et al. [29] in which evidence for the engagement of both linguistic and spatial systems have been found. Reasoning with a semantic content activated a left-hemispheric temporal system, whereas problems without semantic content activated an occipito-parietal network distributed over both hemispheres. Goel and Dolan [41] brought logic and belief into conflict and found evidence for the engagement of a left temporal lobe system during belief-based reasoning and a bilateral parietal lobe system during belief-neutral reasoning. Activation of right prefrontal cortex was found when the participants inhibited a response associated with belief-bias and correctly completed a logical task. When logical reasoning, in contrast, was overwritten by a belief-bias, there was engagement of ventral medial prefrontal cortex, a region implicated in affective processing. In the dual-processing theory, Goel, et al. therefore suggests that deductive reasoning is implemented in two separate systems whose engagement is primarily a function of the presence or absence of semantic content. Content-free reasoning seems to be stronger related to visuo-spatial cortical areas in the right hemisphere, whereas content-based reasoning recruits language-related areas in the left temporal cortex. If the content of the reasoning problem results in a conflict between belief and logic, this conflict recruits additional areas in the right prefrontal cortex.
Evaluation of Reasoning Theories
For a long time the psychology of reasoning was strongly committed to the assumption that reasoning should be studied in term of computational processes. How these computations are biologically implemented in the human brain has been conceived to be not sufficient, because each computational function can be computed on each hardware (and, thus, also the brain) that is equivalent to a Turing machine (e.g., [42]). However, reasoning research is a good example of where the assumption of implementation-independency fails. As there are many mappings possible between cortical regions and cognitive functions, neuroscientific data alone are certainly too weak to test cognitive theories. But, if such data are consistent with behavioral findings this can provide strong support for a cognitive theory of human reasoning. An outstanding example is the field of relational reasoning, where hardly any researchers defend an approach based on inference rules (e.g., [3]). The behavioral and neuroscientific evidence showing that people use their visuospatial system to preserve the structural properties of the world are too overwhelming. In other fields of reasoning the situation is more complicated (cf. [43]). Many researchers will agree that mental models play a key role if humans perform inferences based on conditionals and quantifiers [8]. On the other hand, there is also evidence that verbal, linguistic, and syntactic processes are also involved. The most reasonable corollary from the field of research is that human think deductively by applying different mental algorithms and that these algorithms are implemented in different brain areas. Content-free inferences are “real logical” inferences and they seem to rely on neural computations in the right parietal cortices the precuneus, and the extrastriate and (sometimes) striate cortex. They are accompanied by executive functions and control processes in the prefrontal cortex. When the logical problem is embedded into a semantic content or related to the reasoners’ beliefs additional linguistic and semantic processes in the left temporal cortex come into play. Another corollary from the neuro-cognitive research is that reasoning is a multi-component process and that the diverse components strongly overlap with the components of other cognitive functions. There is no single “cheater detection module” as proposed for reasoning about social contracts [44,45] much as there are no “pragmatic schemas” [46] that completely spare human to reason.
Rebound Bursting
Definition
Discharge of a burst of action potentials after the end of a hyperpolarizing influence, such as an inhibitory postsynaptic potential.
10.1007/978-3-540-29678-2_1
Recall
Definition
Recall is the ability to not only recognize something as having been experienced in the past, but also to retrieve, on demand, spatiotemporal details of the context in which the stimulus or event was originally encountered.
Receiver
Definition
In general an instrument that is able to register a signal. In communication theory, the receiver registers a signal, decodes it and reacts accordingly.
Recency
Definition
With respect to recognition, recency refers to the capacity to remember more accurately information which has just been experienced, as compared to events or items encountered further in time from retrieval.
Recent and Remote Memory
10.1007/978-3-540-29678-2_12
Receptive Field
Definition
The aspect (for example, a location or a temporal frequency) of the outer world that is represented by a given neuron in the brain is referred to as its receptive field.
Receptive Field, Visual
Definition
The receptive field of a “visual” neuron is the region of the visual field in which the presentation of a stimulus exerts a response of the neuron.
10.1007/978-3-540-29678-2_22
Receptive Field of Retinal Ganglion Cell
Definition
In physiological studies the visual field area over which a cell responds to light. The receptive field area corresponds roughly to the dendritic field area.
Receptive Field Selectivity
10.1007/978-3-540-29678-2_3
Receptor
Definition
The term receptor is an ambiguous term because, on the one hand, it is used as shorthand for sensory receptor cell. A sensory receptor (in physiology) is any structure which, on receiving environmental stimuli, produces an informative nerve impulse. The receptor recognizes a stimulus in the external or internal environment, initiates a transduction process by producing graded potentials (receptor potentials), from which all-or-none action potentials are elicited, that are conducted along afferent fibers originating in the same or adjacent cells. On the other hand, a membrane receptor, neurotransmitter receptor, etc. (in biochemistry/pharmacology) is a transmembrane glycoprotein, which is activated by ligands. Receptors to neurotransmitters are located at the plasma membrane. Upon binding by the specific transmitter, receptors can allow the passage of ions or activate enzymes, which ultimately modify the membrane potential. Ionotropic receptors are fast neurotransmitter-gated receptors formed by homomeric or heteromeric subunits outlining a channel, which allows influx or outflux of monovalent or divalent ions. Instead, metabotropic receptors are slow neurotransmitter-gated receptors, which are coupled to G proteins activating diverse effector mechanisms. Excitotoxicity is caused by ionotropic glutamate receptors of the AMPA, kainate and NMDA classes.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_7
10.1007/978-3-540-29678-2_19
Receptor Agonist
Definition
A chemical substance that binds to a cell membrane receptor and mimics the regulatory effects of endogenous signaling compounds such as neurotransmitters, neuromodulators and hormones.
Membrane Components
Receptor Cell
Definition
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_19
Receptor Channel
10.1007/978-3-540-29678-2_9
Receptor Current
Definition
Receptor current denotes the transmembrane current evoked, at the receptor membrane of a sensory receptor cell, by an impinging sensory stimulus through opening or closing of specific ion channels.
10.1007/978-3-540-29678-2_19
Receptor Desensitization
Definition
Receptor desensitization is a reduced response to a neurotransmitter or agonist drug due to a decrease in number of receptors available, or decreased activity of intracellular signaling pathways and ion channels, after prolonged exposure to the neurotransmitter or drug.
Desensitization also results from receptor internalization, the removal of receptors from a plasma membrane by endocytosis. Agonist-binding receptors are pinched off and drawn into the cytoplasm with membrane vesicles and either recycled to the cell surface or degraded.
10.1007/978-3-540-29678-2_9
Receptor Membrane
Definition
Receptor membrane denotes that region of a sensory receptor cell, where the transformed physico-chemical stimulus is converted, by a specific process called sensory transduction, into receptor current and receptor potential.
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_19
Receptor Potential
Definition
Receptor potential denotes the membrane potential change evoked, at the receptor membrane of a sensory receptor cell, by an impinging sensory stimulus through opening or closing of specific ion channels.
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_19
Receptor Regulation, Editing
Definition
A novel channel regulation of the ionotropic glutamate receptors. It occurs at a specific CAG codon for glutamine which changes to a CGG codon for arginine in the pre-mRNAs for a specific subtype of glutamate receptor subunits. The edited codon determines the Ca2+ permeability of the receptors containing the edited subunit.
10.1007/978-3-540-29678-2_9
Receptor Regulation, Phosphorylation
Definition
Certain types of neurotransmitter receptors, such as G protein-coupled receptors and ionotropic receptors, may be regulated by phosphorylation. These regulations are controlled by a combination of kinase and phosphatase, both of which are receptor-selective.
10.1007/978-3-540-29678-2_9
Receptor Regulation, Splicing
Definition
Alternative exon selection changes the receptor structure, modifying the property of the receptor. For example, the “flip/flop structures” of splice isoformes in the AMPA receptors determine the desensitization rate, and the C-terminal splice isoformes of many glutamate receptor subunits control their distribution in the subsynaptic membrane.
10.1007/978-3-540-29678-2_9
Receptor Regulation, Subunit Change
Definition
Almost all ionotropic receptors consist of different kinds of subunits. Their composition determines the functional properties and diversity of each receptor.
10.1007/978-3-540-29678-2_9
Receptor Trafficking
Synonyms
Post-synaptic receptor trafficking; Neurotransmitter receptor trafficking
Definition
Receptor trafficking is a term used to describe the movement of receptors within a neuron. It is used broadly to describe several distinct stages of receptor movement in neurons; the movement of newly synthesized receptors through the secretory pathway (Fig. 1), the movement of receptors into 10.1007/978-3-540-29678-2_1 or 10.1007/978-3-540-29678-2_4 and their targeting to the pre and postsynaptic domains of 10.1007/978-3-540-29678-2_19 respectively. In addition it is also used to describe the internalization of receptors from the plasma membrane as well as their subsequent intracellular trafficking. In all these incidents it should be noted receptor trafficking literally means the movement of receptors between distinct membrane and vesicular compartments of a neuron (Fig. 1).
Receptor Trafficking. Figure 1.

Schematic of the cellular steps of post-synaptic receptor trafficking in neurons. (1) Post-synaptic receptors are generally synthesized and processed within the endoplasmic reticulum (ER) and Golgi of the neuronal cell body. (2) Receptors are then inserted locally into the plasma membrane or trafficked along dendritic microtubules to distal synapses. (3) In both cases receptors are either inserted directly at synapses or in the extra-synaptic membrane followed by later diffusion to the synapse. (4) Anchoring proteins within the post-synaptic density (PSD) then limit trafficking from the synapse. (5) Following release from the PSD anchoring proteins, receptors are internalized via clathrin-mediated endocytosis directly at the synapse or by lateral diffusion to designated endocytic zones. Internalized receptors then traffic within the endosome system and are either recycled back to the plasma membrane (6) or targeted for degradation within lysosomes (7). At each of these steps a specific protein-protein interaction between a trafficking adaptor(s) and a trafficking motif(s) within post-synaptic receptors dictate their trafficking itineraries.
Characteristics
Description of the Process
In neurons, receptor trafficking is a process by which numerous neural functions, such as neuronal migration and 10.1007/978-3-540-29678-2_19 can be regulated. Receptor trafficking controls these functions by setting the capacity of a neuron to respond to an external cue. At the post-synaptic density for example, receptor trafficking can regulate the number of receptors available at any one time to respond to the pre-synaptic release of 10.1007/978-3-540-29678-2_14 molecules. As the amount of released neurotransmitter molecules often outweighs the number of available 10.1007/978-3-540-29678-2_16, the process of receptor trafficking can control the efficiency and amplitude of the post-synaptic response. Over the past decade our understanding of the cellular mechanisms utilized by neurons to control postsynaptic receptor trafficking has increased significantly. We have discovered amongst other things that the basic mechanisms that control postsynaptic receptor trafficking are largely conserved with those controlling receptor trafficking in general. Therefore we have chosen to use examples of postsynaptic receptor trafficking below to illustrate the process and regulation of general Receptor trafficking in neurons.
As Receptor trafficking involves the movement of receptors between distinct membrane and vesicular compartments, it is intuitive that receptors must be recognized by components of these distinct compartments or by the trafficking machinery at each step. Simply put, receptor trafficking involves the recognition of discrete motifs within receptors by components of the different compartments. The motifs within receptors are defined as 10.1007/978-3-540-29678-2_20 and the components of the distinct compartments as 10.1007/978-3-540-29678-2_20. Hence if a receptor contains a specific trafficking motif, it has the potential to be recognized by a specific adaptor molecule of the compartment/ trafficking pathway defined by that motif and to be moved there. The physical movement in most cases is mediated by an indirect interaction with the cytoskeleton.
Postsynaptic receptors are multi subunit receptors frequently composed of different sub-classes of subunits. Because of this heteromeric nature, it is common that different subunits within a single receptor may contain distinct trafficking motifs dictating specific movement to discrete compartments of the neuron. In the sections below I will describe examples that illustrate our present understanding of the motifs and adaptor molecules controling postsynaptic receptor trafficking.
Newly Synthesized Receptor Trafficking
In the synthesis of heteromeric receptors, active retention in the 10.1007/978-3-540-29678-2_18 (ER) is a commonly used process, whereby individual subunits are retained within the ER until they are correctly assembled into the mature receptor. This process acts primarily as a quality control measure ensuring the release of only functional molecules from the ER. The precise motifs and ER-proteins directing this retention are largely unknown for the vast majority of receptors. However it is thought and indeed the case in certain proteins that highly charged residues, which may be exposed in individual subunits but masked in the assembled receptor, play an important role in this retention process. In the case of the postsynaptic 10.1007/978-3-540-29678-2_14, an additional layer of regulation in ER receptor trafficking exists [1].
The predominant NMDA-type glutamate receptor in the brain is composed of 2 NR1 and 2 NR2 type subunits and the trafficking of this NMDA receptor from the ER into the secretory pathway is controlled by differential splicing of the NR1 subunit. The NR1 subunit gene contains the splicing cassettes C1, C2, C2′ that produce different cytoplasmic C-termini of the NR1 subunit. The NR1–1 splice variant, which is the main splice variant in the brain, encodes a C2 cassette and a C1 cassette, which contains a triple arginine (RRR) ER retention motif. If this NR1–1 splice variant assembles with NR2 subunits in the ER, mature assembled receptors will not exit the ER efficiently, due to the presence of this ER retention motif. However if the C1 cassette is expressed in combination with a C2′ cassette (NR1–3 splice variant) the assembled receptor successfully exits the ER. This is due to the presence of a specific trafficking motif within the C2′ cassette (Serine-Threonine-Valine-Valine, STVV) that overrides the RRR retention motif of the C1 cassette. This STVV trafficking motif is able to override the RRR retention motif, as it belongs to the family of 10.1007/978-3-540-29678-2_16 interacting motifs and specifically binds to the PDZ domain containing protein Sec23. Sec23 is a component of the COPII coat complex that promotes ER exit via COPII vesicle formation. Differential expression of these different splice variants is in addition controlled via neuronal activity. For example, increased neuronal activity promotes the insertion of the C2 cassette. This favors the C1 retention mechanism; reducing NMDA receptor exit from the ER and thus lowering overall surface expression of NMDA receptors and neuronal activity. In contrast blocking neuronal activity promotes C2′ insertion over C2, which promotes ER exit, the secretory trafficking of NMDA receptors and increases neuronal activity. In essence, the above example neatly illustrates how a commonly used mechanism (ER retention) can be used to control receptor trafficking from the ER and how an additional process can be employed to override this retention in order to respond to the needs of the system.
Trafficking Along Dendrites and Targeting to Synapses
Most postsynaptic receptors are synthesized in the 10.1007/978-3-540-29678-2_19 and once they have passed through the ER and Golgi are either inserted locally within the plasma membrane or travel large distances within 10.1007/978-3-540-29678-2_4 to reach the distal dendritic synapses. The latter is achieved predominantly via vesicular transport along 10.1007/978-3-540-29678-2_13 within the dendrite. To traffic receptors in this manner, specific linker proteins that bind to trafficking motifs within the cytoplasmic domain of receptors and to 10.1007/978-3-540-29678-2_13 move the vesicle along the microtubule. The microtubule motor proteins controlling this distal direction of vesicle movement (anterograde trafficking) belong to the kinesin superfamily proteins. It is estimated that a large anterograde transport vesicle may be associated on average with between 100–200 kinesin molecules. Once the target of this vesicle is reached (the dendritic synapse) exocytosis of these receptors occurs and the kinesin molecules are either degraded or recycled back to the soma for future use.
With respect to the linker proteins involved, an obvious pre-requisite is their ability to interact with kinesins. Of the linker proteins identified thus far, many have the ability to bind only a single kinesin protein but to broadly bind a variety of receptor types. This occurs as these linker proteins generally contain multiple interaction domains that can bind distinct trafficking motifs within different proteins. Furthermore some receptors, such as the 10.1007/978-3-540-29678-2_1, can bind multiple kinesins via different linker proteins. AMPA-receptors can bind convention kinesin via the linker protein the glutamate-receptor-interacting protein (GRIP1) and the kinesin family member, KIF1 via liprin-α [2]. Both interactions are mediated via carboxyl-terminal PDZ motifs in the cytoplasmic domains of the GluR2 and GluR3 AMPA receptor subunits. Thus a single postsynaptic receptor may encode multiple dendritic vesicular trafficking motifs, which can bind different linker proteins, which all participate in the anterograde movement of the receptor.
Under certain circumstances, postsynaptic receptors can also be trafficked in a retrograde fashion back to the soma. This involves a different set of linker proteins binding alternate trafficking motifs that have an affinity for retrograde microtubule motor proteins, such as the 10.1007/978-3-540-29678-2_4 superfamily of proteins. Finally exocytosis and insertion of receptors into the neuronal surface can occur at the synapse or at extra-synaptic sites followed by lateral diffusion of the receptors within the neuronal plasma membrane to the synaptic site. Once at the synapse, the binding of postsynaptic anchoring proteins subsequently retard further post-synaptic receptor trafficking and movement.
Endocytosis and Intracellular Trafficking
The next and penultimate step in the journey of neurotransmitter receptor trafficking is 10.1007/978-3-540-29678-2_5, where the receptor is targeted for internalization from the plasma membrane. The vast majority of postsynaptic receptors are internalized via clathrin-mediated endocytosis (CME). Endocytosis of postsynaptic receptors can occur at the synapse, but preferentially occurs at designated internalization sites lateral to the postsynaptic density. In which case, postsynaptic receptors need to disengage from their postsynaptic anchoring proteins prior to internalization. This occurs constitutively or in a signal-regulated manner, involving a post-translational modification that alters the association of the receptor with the postsynaptic density anchoring protein.
In general CME is mediated by endocytic trafficking motifs located in the cytoplasmic domains of postsynaptic receptors, which are recognized by the tetrameric clathrin-binding endocytic adaptor protein 2 complex (AP-2). Two classical endocytic motifs exist, a tyrosine (Y) based motif, YxxΦ, where –x- represents any amino acid and Φ is a hydrophobic amino acid and an acidic di-leucine motif, D/ExxxLL, (D = aspartic acid, E = glutamic acid and L = Leucine). These motifs, which have been found in the cytosolic domains of many neurotransmitter receptors, are recognized by different subunits of the cytosolic AP-2 complex. AP-2 binding in both cases however promotes membrane invagination and the recruitment of the clathrin lattice, leading to the formation of endocytic vesicles and receptor internalization. Some postsynaptic receptors encode a single endocytic motif, which is recognized by the AP-2 complex whilst others contain multiple. The inhibitory 10.1007/978-3-540-29678-2_7 heteropentamer for example, is composed of subunits that contain both tyrosine and di-leucine endocytic trafficking motifs, which all may be relevant in mediating GABAA receptor 10.1007/978-3-540-29678-2_5 [3].
Once internalized these postsynaptic receptor containing endocytic vesicles quickly mature, losing their clathrin lattice to first form early endosomes, which then subsequently mature into late/sorting endosomes. It is in late/sorting endosomes that internalized receptors are targeted either to be recycled back to the plasma membrane or alternatively for degradation in lysosomes. Again, the interaction between trafficking motifs within the receptor and compartment specific adaptors plays an important role. In the vast majority of cases the recycling of receptors is the default pathway, while trafficking to the lysosomal pathway involves an additional sorting step. The di-leucine motif mentioned above has been implicated in this step, through the binding of a related lysosomal specific adaptor complex to AP-2, the AP-3 complex. In addition to these peptide specific endocytic sorting motifs, the modification of receptor subunits by the addition of a 7 kilodalton(kDa) protein called 10.1007/978-3-540-29678-2_21 has been identified as a targeting signal for endosomal-lysosomal sorting, which is discussed in more detail below.
Regulation of the Process
Post-translation modifications, the addition of a molecule to a protein after it has been synthesized, provides an additional layer of regulation in receptor trafficking. Receptors can be modified by a number of different post-translational additions, such as lipids, inorganic ions or by the covalent attachment of small proteins. Listed below are some examples of how these different modifications can alter receptor trafficking itineraries.
Phosphorylation
10.1007/978-3-540-29678-2_16, the addition, or de-phosphorylation, the removal of an inorganic phosphate, are mediated by kinase or phosphatase enzymes respectively. This type of post-translational modification is used widely in neurons to regulate receptor trafficking, by altering the binding specificity of a trafficking adaptor to its linear trafficking motif. For example, the rate of GABAAR endocytosis is regulated by phosphorylation. All GABAAR heteropentamers contain a beta-type subunit, which encodes an atypical endocytic AP-2 binding motif that encompasses an established regulatory phosphorylation site [4]. Phosphorylation at this site results in the reduced affinity of the AP-2 complex for the endocytic motif, which interferes with the rate of GABAAR endocytosis. This example neatly demonstrates a phospho-dependent regulation of endocytosis via regulation of the endocytic adaptor protein AP-2 binding affinity.
Palmitoylation
10.1007/978-3-540-29678-2_16 is the reversible post-translational attachment of a saturated fatty acid, palmitic acid, to cysteine residues in a membrane protein via a thiol-ester bond. Palmitoyl acyl transferase enzymes mediate this reaction and in neurons the action of one such enzyme, the Golgi-specific DHHC zinc finger protein GODZ directs the palmitoylation of two classes of postsynaptic neurotransmitter receptors. These are the AMPA-type glutamate receptor and the GABAAR [5,6]. Palmitoylation of these receptors can modulate their membrane trafficking, by enhancing their hydrophobicity, which leads to an enhanced rate of surface expression. Palmitoylation of post-synaptic receptors therefore facilitates enhanced secretion of newly synthesized receptors.
10.1007/978-3-540-29678-2_21 and Sumoylation
10.1007/978-3-540-29678-2_21 (Ub) is a highly conserved 76 amino acid polypeptide that is covalently conjugated to lysine residues on target proteins or to itself by a reaction involving three classes of enzymes: an E1 activating enzyme, an E2 conjugating enzyme and an E3 ligase that also determines substrate specificity. Protein 10.1007/978-3-540-29678-2_21 is reversible and this is controlled by the action of de-ubiquitinating enzymes that cleave the Ub-protein bond. Modification of proteins by Ub chains (Ubn > 4) primarily targets them for degradation by the multi-subunit proteolytic complex called the 10.1007/978-3-540-29678-2_16. In contrast, a single ubiquitin (mono-ubiquitination) as well as multi-site mono-ubiquitination, functions as a signal in the endocytic pathway controlling proteins internalization and lysosomal degradation. Mono-ubiquitination functions as an efficient endocytic-lysosomal trafficking signal, as several endocytic adaptor proteins encode ubiquitin-binding domains, which specifically recognize, bind to and traffic ubiquitinated membrane proteins.
There is now compelling evidence demonstrating a role for ubiquitination in regulating the abundance of glutamate receptors and synapse-associated proteins. Ubiquitin dependent proteasomal degradation of PSD95 for example, a glutamate receptor synaptic anchoring protein, enhances the endocytosis rate of AMPA receptors. It does so by releasing AMPA receptors from the post-synaptic density and thereby enabling their interaction with the endocytic machinery [7]. This leads to a reduction in excitatory synaptic responses. With respect to the direct ubiquitination of glutamate receptors, a strong role for an E3 ligase complex of proteins called the Cullin E3 ligase (cul) complex has been discovered. The Cul3 adaptor protein actinfilin has been found to bind and mediate a Cul3 dependent ubiquitination of the GluR6 subunit of 10.1007/978-3-540-29678-2_11, controlling GluR6 levels and receptor accumulation at excitatory synapses [8]. The NR1 subunit of the NMDA-type of glutamate receptor is also targeted for ubiquitination by a cullin complex protein, the F-box protein Fbx2 [9]. Ubiquitination of NR1 by Fbx2 alternatively controls its retro translocation from the ER and ubiquitin-proteasome mediated degradation in an activity dependent manner, leading to a reduction in NMDA-dependent currents. Of the third class of mammalian glutamate receptors, the AMPA receptor, subunit ubiquitination has yet to be demonstrated, but is highly probable as the signal sequences targeting ubiquitination of the related C. elegans GLR-1 subunit, are conserved in all mammalian AMPA type glutamate receptors.
10.1007/978-3-540-29678-2_19 (small ubiquitin-related modifier), also named “sentrin,” is a 101-amino acid protein that can also be covalently attached to cytosolic lysine residues, in a process that is analogous to ubiquitination. 10.1007/978-3-540-29678-2_19 was originally associated only with the functions of nuclear proteins, however more recently sumoylation of neurotransmitter receptors has been demonstrated and this modification on these proteins, like ubiquitination can regulate receptor trafficking. Sumoylation of the GluR6 kainate-type of glutamate receptor for example, regulates the rate of kainate receptor endocytosis and modifies the efficiency of synaptic transmission [10]. How precisely sumoylation facilitates this process is at present unknown, but it is likely it either release kainate receptors from an anchoring protein or alter the binding affinity of the receptor to endocytic adaptors. Sumoylation of the GluR6 subunit in neurons is rapidly enhanced in response to kainate treatment, which implies this modification is employed in an autoregulatory type of response to agonist application.
Closing Comments
In this essay, I described the main cellular steps of neurotransmitter receptor trafficking that occur in a neuron (Fig. 1). Using specific examples of receptor trafficking for certain post-synaptic receptors, I have described how the different processes of receptor trafficking function at each step. In addition, I have outlined several post-translational modifications, which can regulate these processes of receptor trafficking and have explained how this regulation occurs and controls receptor trafficking.
Receptor Tyrosine Kinase
Definition
Receptor tyrosine kinase is a transmembrane receptor whose intracellular domain contains a kinase that is capable of transferring a phosphate group from ATP to a tyrosine of a protein. Many growth factors and extrinsic signaling molecules act through receptor tyrosine kinases.
10.1007/978-3-540-29678-2_7
Reciprocal Activation
Definition
Simultaneous activation of muscles with a mechanical action on a joint (agonist) and inhibition of muscles with the opposite mechanical action (antagonists).
Reciprocal activation may be of both central and peripheral origin and it is mediated by excitation of agonist motoneurons and reciprocal inhibition of antagonist motoneurons, via inhibitory spinal interneurons, by descending fibers (in voluntary movements) and by sensory afferents (in reflexive responses).
Reciprocal Dendrodendritic Synapse
Definition
Principal synapse of the olfactory bulb. This is a synapse between mitral cell lateral dendrites and granule cell dendrites. Depolarization of a mitral cell releases glutamate, which excites the postsynaptic granule cell. This cell releases GABA at the same synapse and inhibits the mitral cell in a reciprocal fashion.
10.1007/978-3-540-29678-2_15
Reciprocal Inhibition
Definition
A pattern of synaptic connection between neurons or groups of neurons where they each make an inhibitory synapse on the other neuron or group of neurons in a mutual or reciprocal fashion. This pattern of connectivity assures reciprocity/alternation in the activity of the two neurons/groups. The term also refers to inhibition of antagonist motoneurones when the agonist motoneurones are activated either as part of a stretch reflex or as part of a voluntary movement. Ia inhibitory interneurones play an important role in ensuring reciprocal inhibition, but other mechanisms also contribute.
10.1007/978-3-540-29678-2_9
10.1007/978-3-540-29678-2_9
Recognition
Definition
Recognition is the act during which a specific item or event is identified as having been experienced or encountered on a previous occasion. Variants of items or events that are not exactly like those previously experience can also be recognized by generalization or inference.
Recognition Memory
Synonyms
Familiarity; Recollection; Recency; Declarative memory; Explicit memory; Old/new recognition
Definition
Recognition memory refers to the ability of a system to classify a specific item or event as having been experienced or encountered on a previous occasion. Behavioral, neuropsychological, electrophysiological, and neuroimaging evidence indicate that recognition memory may be dissociable into the distinct processes of 10.1007/978-3-540-29678-2_6 and recollection.
Characteristics
The ability to form, retain and manipulate memories is necessary for an organism to adapt to its environment. The broad concept of memory is generally divided into procedural and declarative branches. Whereas procedural, or implicit, memory underlies the unconscious learning of motor, perceptual and habitual tasks, declarative, or explicit, memory refers to the conscious memory for facts and events. Declarative memory has been regarded as critical for providing temporal contiguity to conscious experience. Subsumed under the umbrella of declarative memory is recognition memory – the psychological ability to judge a particular item or event as having been previously encountered in the past. A hallmark of recognition memory is that it can both lead to a distinct feeling of oldness, or familiarity, as well as evoke vivid spatiotemporal details associated with the prior experience of the item or event. For example, imagine having a conversation with a stranger on a subway and then seeing that same stranger in a different setting a month later. Not only will you most likely have a strong sense of recognizing this person as familiar, but you also might be able to recall the specific train your were on during your previous encounter. Most current theoretical models maintain that there is qualitative distinction between recognition memory that is or is not complemented by contextual recall [1].
Such dual-process models label recognition unembellished with episodic content as “familiarity” and recognition with accompanying contextual particulars as “recollection.” This view can be contrasted with the less common single-process models which posit that the difference between familiarity and recollection is purely quantitative [2]. In this latter framework, familiarity and recollection vary along a continuum that captures how much background information is recalled at the time of recognition, with recollection obviously summoning more details than familiarity. Dual-process models also posit that memory traces can vary in strength, especially within the domain of familiarity, but there is a qualitative, as opposed to a quantitative, leap when one transitions from familiarity to recollection. Although dual-process models dominate the literature, and as we will show are supported by a wealth of converging evidence, the parsimony of single-process models makes them a viable alternative.
Several different experimental paradigms have been employed to independently assess the contribution of familiarity and recollection to recognition memory [3]. The remember/know procedure asks subjects to first study a list of words or pictures and then identify, within a list including both old and new items, those items which have been previously studied. If an item is judged as old, the subject further indicates whether he or she simply “knows” (K) this or can specifically “remember” (R) studying it (Fig. 1). Although the K judgment presumably reflects familiarity and the R judgment is analogous to recollection, it is difficult to tease these components apart because it is often the case that R judgments are preceded by implicit K judgments. Adding to the confound is the observation that the proportion of K and R judgments can be biased by manipulating the subjects' decision criteria. A similar procedure aimed at differentiating familiarity from recollection asks subjects to rate the confidence of their old judgments, with higher confidence ratings indicating recollection. Another common paradigm used to explore familiarity and recollection is a source judgment task. In this setup, subjects again encode items that the experimenter has specifically embedded in a specific context (e.g., a particular quadrant of the screen). In the test phase, subjects may be required to not only signal whether an item is new, but to also report some aspect of the context in which the item originally appeared. In this paradigm, the proportion of trials with incorrect or correct source judgments can provide indices of familiarity or recollection, respectively.
Recognition Memory. Figure 1.
A subject first encodes a set of words or pictures. Following a delay interval that is usually on the order of several minutes, subjects are presented with a list of words or pictures that include the initially studied items as well as new items. The subject’s task is to indicate which items are old and which items are new. If the subject responds old, he or she is asked to elaborate if the old judgment is simply due to some intuitive sense of “knowing” or if it is due to “remembering” specific details associated with the original encoding of the item in question. A “know” response presumably reflects a familiarity-based judgment whereas a “remember” response reveals a recollection-based judgment.
Through the application of the above-mentioned and other experimental manipulations, an emerging consensus is that familiarity and recollection are, to a certain degree, functionally distinct. So, for example, it has been shown that recollection benefits more than does familiarity from undivided attention at the time of encoding. Similarily recollection improves when subjects are given the opportunity to encode the initial list of items in more depth, which happens, for example, when subjects generate words instead of passively reading them. Conversely, familiarity judgements are more common when duration of encoding is limited. In addition, priming has been shown to have an effect on familiarity but not on recollection. While a full discourse on the relevant findings dissociating the two types of recognition memory is beyond the scope of this article, it is generally assumed that familiarity operates automatically and quickly whereas recollection requires voluntary control and time.
Given that these distinct cognitive phenomena must have neural correlates, a logical question is whether differences between familiarity and recollection are evident at the electrophysiological level. Event-related potentials (ERP) are one means to non-invasively measure the stimulus-locked neural activity of human subjects. Indeed, early ERP studies found that recognized items, at test, generated more positive ERPs than to unrecognized items. A series of studies then demonstrated a double dissociation between familiarity- and recollection-driven ERPs [1,4,5]. Specifically, recognition judgments based on familiarity elicit ERP effects that are more localized to frontal electrode sites and have a relatively short latency, starting around 300 ms and continuing for another 100–200 ms. Not surprisingly, frontally localized ERPs corresponding to familiar items have more positive amplitudes than the ERPs of correctly rejected new items. The hypothesized electrophysiological correlates of recollection, on the other hand, appears more localized to parietal sites. It, like the early frontal effect, is more positive for correctly recognized items than for correctly rejected items but is slower to develop, usually beginning at 400 ms post-stimulus onset and persisting for another 300–400 ms. The differential latencies of the ERPs parallel the behavioral observations that familiarity is more instinctive and thus quicker than the more effortful and slower recollection. Compellingly, old items receiving incorrect “new” classifications do not elicit the early frontal effect whereas false alarms do. This suggests that familiarity allows as to perceive oldness.
ERPs have relatively poor spatial resolution, which does not allow for a finer localization of the neural substrates of recognition. To overcome this particular limitation, investigators have turned to functional magnetic resonance imaging (fMRI). Making use of behavioral paradigms similar to the ones described above, several groups have started to delineate circumscribed areas of the brain responsible for familiarity and recollection [3,6]. These results have revealed distinctions between familiarity and recollection that parallel those obtained with ERPs. Familiarity has been shown to cause activity in a network of areas, including various frontal/prefrontal regions, medial temporal lobe (MTL) structures excluding the hippocampus, and the superior parietal lobule. The most convincing evidence centers on the MTL structures, which include the perirhinal, parahippocampal and entorhinal cortices. These structures not only exhibit reduced responses to old items, but this reduction correlates with the strength of the familiarity judgment, suggesting a neural substrate for the continuously scaled familiarity signal. Recollection-based judgments also tend also to activate several brain regions, most notably the hippocampus and a left lateral parietal cortex. These observations are consistent with the widely held belief that the hippocampus is critical for the recall of associations, which presumably constitutes a large component of the episodic retrieval inherent to recollection. Crucially for dual-process models, activity in the hippocampus does not increase for familiarity judgments. Intriguingly, the activity in the left lateral parietal region has been shown to increase even when items are mistakenly classified as old, again lending support to the existence of a brain network responsible for the subjective experience of oldness.
Another localization approach, complementing the fMRI studies, has been to examine patient groups with focal lesions. Because the hippocampus is particularly susceptible to hypoxia, patients suffering from lesions of the hippocampus are relatively easy to find. Investigations of these patients have revealed mixed results in that whereas some reveal significant impairment in tasks requiring recollection, others show equivalent deficits for both familiarity and recollection [3,7]. This has led some to postulate that while there may be important distinctions between the two kinds of recognition memory, all the structures within the MTL act as one integrated unit, which in turn contributes to both familiarity and recollection.
Notwithstanding the inconclusiveness of the human results, more controlled lesion studies in nonhuman primates have revealed that some division of labor is present within the classically defined MTL structures. Because we cannot ask monkeys to introspect about their recognition processes, it is difficult to tap into their different types of recognition memory; nevertheless, 10.1007/978-3-540-29678-2_4 and 10.1007/978-3-540-29678-2_4 paradigms have been extensively used to explore familiarity judgments. The basic format for this task requires the animal to encode an object (generally, but not always, by looking at it), to retain its identity throughout a delay interval, which can range from seconds to minutes or longer, and to then recall its identity by selecting the matching (or non-matching) object from a choice array containing two or more possible alternatives.
A meta-analysis of primate lesion studies, with the lesions restricted either to the hippocampus or to the surrounding perirhinal cortex, showed ablation of the perirhinal cortex impairs DNMS performance more than lesions of the hippocampus. Additionally, a correlation analysis showed that the magnitude of the DNMS performance deficit scaled positively with the extent of the perirhinal damage. While hippocampal damage also had a detrimental effect on the DNMS task, the size of the hippocampal lesion was negatively correlated with performance deficit, with the deficit becoming essentially negligible for monkeys totally lacking the hippocampus. This supports the view that the surrounding MTL structures play a more vital role in familiarity judgments than does the hippocampus itself.
The lesion studies described above are consistent with single cell recordings performed in the perirhinal cortex of nonhuman primates [8]. The monkeys in these experiments performed a serial recognition task in which the goal was to press one button if the stimulus was familiar (having been seen repeatedly on a daily basis or just recently presented) and to press another button if the stimulus was novel. Three distinct classes of neurons have been reported. Recency neurons decrease their response when a displayed stimulus is one that was seen a few trials back, regardless of whether it is highly familiar or novel for that session. Familiarity neurons exhibit a reduced response to stimuli which are familiar to the monkey. Finally, novelty neurons show a marked enhanced response to the first presentation of a novel stimulus, with subsequent presentations of it or other familiar stimuli evoking much lower or shorter responses. Remarkably, some of these neurons appear to have memory spans of up to 24 h, providing persuasive evidence that they are involved in the extraordinary capacity of primates to remember stimuli for long periods of time, even following single exposures. Interestingly, the general tendency of these neurons to decrease their response to known stimuli mirrors results of fMRI studies in which the strength of the familiarity signal determines the amount of activity decrease observed in MTL structures. Additional studies have shown that the response of PFC neurons to familiar items is less affected by noise manipulations, suggesting that these items are more accurately and efficiently represented.
Recently, neurophysiologists have begun describing in more detail the properties of the neural activity underlying familiarity in primates. ERP studies have shown results similar to those obtained in humans, namely that the magnitude of the ERP differentiates between novel and familiar stimuli, with familiar stimuli eliciting a more positive ERP. These differences are present even in simple fixation conditions, which speaks to the fundamental contribution of familiarity to everyday behavior. Furthermore, as the monkey becomes more familiar with the novel set, the ERPs to the two sets of images become systematically more similar. Familiar and novel items also produce differences in the temporal dynamics of single cell responses. Specifically, both elicit a similar initial peak in the firing rate (∼100 ms), but whereas the familiar response then quickly declines, the response to the novel images persists for an extended period of time. This suggests, again, that familiar items may be encoded more efficiently than novel ones, and that the prolonged response observed for novel images might contribute to the creation of new memories.
Although recollection is difficult to study in nonverbal animals, preliminary evidence does indicate that the hippocampus is essential [9,10]. Numerous studies have shown that removing the hippocampus in rats impairs their ability to use relational knowledge, particularly with regard to spatial relationships, which has been interpreted as reflecting deficits in associative recollection. Single cell recordings in monkeys have also demonstrated that the firing pattern of hippocampal neurons correlates with the simultaneous acquisition and immediate expression of arbitrary stimuli associations. It should be pointed out, however, that the long-term storage of associations is thought to rely on the surrounding MTL structures, particularly the perirhinal cortex. Hence, a precise and accurate account of the specific neural substrates of recollection will require more time and research.
In sum, there is strong evidence suggesting a dichotomy between familiarity- and recollection-based recognition memory. The precise nature of these differences is difficult to characterize given that the two processes interact extensively at the cognitive level; however, neurophysiological data obtained from both humans and primates has begun to elucidate possible neural substrates and processes that contribute to this distinction.
Recognition Neurons
Definition
Neurons that are able to signal by their response that a specific, generally complex stimulus has been recognized. Face neurons in the inferior temporal cortex are an example for recognition neurons (see also Grandmother Neuron).
Recollection
Recruitment
Definition
Abnormal increase in perceived loudness.
10.1007/978-3-540-29678-2_8
Recruitment in Acoustics
Definition
Abnormal increase in perceived loudness.
10.1007/978-3-540-29678-2_8
Recruitment of Motor Units
Definition
Central nervous system control of force output from a muscle by regulating the numbers and identities of motor units that are activated or de-activated (de-recruitment) during a movement.
10.1007/978-3-540-29678-2_13
Rectifying Gap Junctions
Definition
Rectifying gap junctions conduct ionic current better in one direction than in the other. In contrast to electrical synapses comprised of non-rectifying gap junctions, electrical synapses comprised of rectifying gap junctions transmit electrical signals in a unidirectional fashion.
10.1007/978-3-540-29678-2_5
Recurrent Brief Depression
Definition
Depressive episodes lasting at least 2 days but less than 2 weeks occurring at least once a month for 12 consecutive months. They often occur unpredictably but frequently. An average of two attacks a month is typical. Although the episodes are brief, symptoms are severe. Intense suicidal ideation is common.
10.1007/978-3-540-29678-2_13
Recurrent Facilitation
Definition
Recurrent Hypersomnia
Definition
Also known as Kleine-Levin syndrome, this disorder is characterized by periods of excessive sleepiness and long sleep times lasting days to weeks. It is most commonly found in adolescent males. During symptomatic periods, there is often increased food intake as well as cognitive and emotional dysfunction.
10.1007/978-3-540-29678-2_19
Recurrent Inhibition
Definition
Recurrent inhibition is a basic type of neuronal circuit throughout the central nervous system. In the spinal cord, motoneurons give off axon collaterals to excite GABAergic/glycinergic Renshaw cells, which mediate recurrent inhibition of motoneurons, Ia inhibitory interneurons, Renshaw cells and some cells of origin of the ventral spinocerebellar tract. Since the Renshaw cells also inhibit Ia inhibitory interneurons, which inhibit antagonistic motoneurons, activation of the Renshaw cells leads to removal of the inhibition of the antagonist motoneurons, and this phenomenon is termed recurrent facilitation.
10.1007/978-3-540-29678-2_9
10.1007/978-3-540-29678-2_9
Recurrent Network
Definition
A neural network architecture in which both feedforward and feed-back connections between neurons are present. In a fully recurrent architecture, the neurons are fully interconnected.
10.1007/978-3-540-29678-2_14
Recurrent Processing
Definition
Information is processed in a set of stages in which activation can propagate in feedback loops.
Red Nucleus
Definition
The red nucleus consists of two functionally entire separate divisions, the caudal magnocellular red nucleus and the more rostral parvocellular red nucleus. The magnocellular red nucleus is a large spherical nucleus in the midbrain containing very large neurons. It is the level of the oculomotor nucleus and the superior colliculus. It receives a very large input from the cerebellum by way of the superior cerebellar peduncle and also a small descending input from motor areas of the cerebral cortex. It projects to the contralateral facial and trigeminal nuclei in the brainstem and to the contralateral spinal cord, primarily to the cervical and lumbar enlargements that control the limbs. In fresh sections the magnocellular division of the red nucleus appears red or pinkish-yellow because of its marked vascularization, hence its name.
The parvocellular red nucleus, just rostral to the magnocellular division, consists of smaller neurons. In lower animals it is smaller than the magnocellular division but in primates, it is much larger. The parvocellular red nucleus receives a large input from the motor regions of the cerebral cortex and projects to the principle division of the ipsilateral inferior olive.
In summary, the magnocellular red nucleus is a premotor nucleus for muscles in the contralateral head and limb while the parvocellular red nucleus is a relay between the cerebral cortex and the inferior olive.
Reductionism (Anti-Reductionism, Reductive Explanation)
Definition
In philosophy of science and in the sciences, reductionism is sometimes a methodological stance; sometimes it is a substantive position. As a methodological stance, it is committed to understanding a system’s behavior analytically, i.e., in terms of the system parts and their interactions. As a substantive position, it anticipates the success of the reductionist methodology. Reduction sometimes is thought of in ontological terms; that is, as a commitment to the idea that, e.g., living systems consist of nothing other than physical constituents. This minimal commitment may be coupled with a claim that reduction holds in principle, even if not in practice, but does not require even this much. Reduction sometimes is an explanatory relation, either between theories or between domains. The former is theory reduction while the latter is reductive or mechanistic explanation.
Description of the Theory
Reductionism has been a persistent attractor for scientific thought. In physics, it led to some of the most striking successes of classical physics, such as the particle theory of light in the eighteenth century or 10.1007/978-3-540-29678-2_19 in the nineteenth century. In the biological sciences, the rapid growth of molecular genetics is a reductionistic triumph. In the 10.1007/978-3-540-29678-2_3, reductionism has been no less attractive, as is evidenced by the rise of what is called “cognitive neuroscience” in the twentieth century, or the dramatic successes of the neurosciences more generally. Opposition to reductionist approaches also has been prominent, especially in cognitive psychology. The successes of cognitive psychology and of 10.1007/978-3-540-29678-2_1 in the middle decades of the twentieth century encouraged the idea that reductionist methods were not necessary in order to explain human psychological capacities. In opposition to rather brute reductionistic claims such as that there is nothing but elementary particles, or that mental states are nothing but brain processes, champions of the special sciences feared they would lose their particular subject matter. Vitalistic or dualistic alternatives are not fashionable any more, but there are a number of antireductionist positions that have been recently defended.
Varieties of Reduction: Theory reduction
Theory reduction claims to deduce or “explain” one theory (the secondary or reduced theory) from another (the primary or reducing theory), perhaps as a limiting case. Theory reduction may involve theories at the same level of organization or at different levels of organization. A classical example for same level reduction, as discussed by Ernest Nagel [1], is the reduction of Newton’s mechanics to Einstein’s relativity theory; for reduction of theories at different levels Nagel refers to the relation of classical thermodynamics to statistical mechanics. The reducing theory is typically thought to be more general or more exact, or both. The reduced theory correspondingly is thought of as more restricted in its domain of application, or as an approximation, by comparison with the reducing theory. Thus, Newton’s mechanics applies only to velocities far from the velocity of light. The classical gas laws in thermodynamics apply only to gases at intermediate temperatures and pressures. When reducing and reduced theories are on the same level, or cover the same domains, we have “homogeneous” reductions. If reduction is possible at all, it can be achieved relatively easily, because the theories at least appear to have the same concepts. For example, both Newton and Einstein appeal to mass and velocity, although they in fact may not be the same concepts (insofar as the theory defines its concepts). When reducing and reduced theories refer to different levels, or cover different domains, we have “heterogeneous” reductions. If reduction is possible at all, it is more difficult in these cases, since the theories do not share the same vocabulary. For example, statistical mechanics has neither the notion of temperature nor that of pressure, though these are the key concepts of classical thermodynamics. So, in these cases, connections between the two domains or levels have to be forged, in the form of “10.1007/978-3-540-29678-2_2.” These connections are usually thought to be identifications between levels; e.g., mean kinetic energy is identified with temperature, at least within the domain of classical thermodynamics.
These identifications often lead to so-called “nothing buttery,” i.e., to the claim that the phenomena the upper level concepts originally referred to in fact are nothing but the entities the lower level concepts refer to. So, it is often said, temperature is nothing but mean molecular motion, and genes are nothing but nucleotide sequences. This sometimes leads to eliminativist claims concerning the reduced theory – that is, to denying the existence of things reduced [2]. In case there are suitable identifications available, reduction guarantees that the explanatory work done by the higher level theory can, at least in principle, be done by the lower level theory. The higher level theory can then, in principle, be eliminated without explanatory loss. In case the concepts of upper level theory are vaguer or more inexact, they cannot be identified with the more exact concepts of a lower level theory. Once again the original theory can be eliminated though in this case because it could not be reduced [3] (“phlogistaded”).
To take an example closer to the neurosciences, the trichromatic theory was an important case historically. Newton’s experiments with prisms revealed a visible spectrum ordered by wavelength. It was subsequently shown that any specific spectral hue could be matched by combining three different primary colors in different intensities. This led Thomas Young to propose, in 1801, that the retina contained exactly three different color-sensitive elements. This was subsequently confirmed and consolidated by the great physiologist, Hermann Helmholtz, with three classes of cones differing in their central sensitivities, though with substantial overlap of responses. This looked like a reduction of color theory to a trichromatic theory, which had broad acceptance into the middle of the last century. Unfortunately, not all the colors perceived by human subjects are represented in the physical spectrum. Brown is the most salient example of a color that is outside the physical spectrum. Hering eventually posited an “opponent process” theory which was aimed at more exactly describing the subjective phenomena of color perception. The existence of opponent processes at the neural level was eventually confirmed in the macaque. We still lack a decisive direct test, but this sort of case also is suggestive of theory reduction.
To consider a more contemporary example, John Bickle [2] argues that contemporary neuroscience captures the phenomena on offer from psychology concerning memory. In particular, he observes that Eric Kandel’s landmark work on 10.1007/978-3-540-29678-2_1 has forged a link between the mechanisms of long term potentiation (10.1007/978-3-540-29678-2_12) and 10.1007/978-3-540-29678-2_13. Memory consolidation is particularly important as the link between short-term and long-term memory. The key psychological phenomena include the importance of stimulus repetition, the time course relevant to fixation, and retrograde interference. The mechanisms, typically molecular, which characterize LTP capture these phenomena, at least to a first approximation. Bickle concludes that the “intended empirical applications of the two theories are virtually identical,” even if the theories differ in substantial ways.
Varieties of Reduction: Reductive or Mechanical Explanation
Reductive or mechanical explanations tell us why a certain entity instantiates a certain property, typically a property that is only attributed to the system as a whole [4,5]. So we might want to explain, e.g., how humans recognize faces, or how we acquire language. In trivial cases it suffices to simply add the corresponding properties of the system’s components. So we get the weight of brains from the weight of its parts. However, the behavior of the brain in vision or perception – both definitely more interesting properties – cannot be deduced in an equally simple manner. Here we need to know the arrangement of the components, particularly those of the visual areas, what properties they have, and how they interact among each other and with the visual stimuli. The capacity to see would be reductively explained if it followed from the above-mentioned factors and the natural laws that hold generally. Of course, at the moment, we do not yet have such a reduction in place. That need not trouble reductionists, insofar as they are committed to what we will or (perhaps) could achieve.
So, the aim of each reductive explanation is to explain (or predict) a system’s dispositions and properties solely by reference to its components, their properties, arrangement, and interactions. To be successful as a reductive or mechanistic explanation, several conditions must be met:
-
i.
There must be a systemic property to be explained
-
ii.
The property to be reduced must be functionally construable or reconstruable
-
iii.
The specified functional role must be filled by the system’s parts and their mutual interactions
-
iv.
The behavior of the system’s parts must follow from the behavior they show in isolation or in simpler systems than the system in question
These are demanding constraints, but without them no reductive explanation will be complete. What we are looking for, then, are functional characterizations of the properties to be reduced, and explanations of those functional properties in more fundamental terms. Usually we refer to these properties by concepts that classify properties at the system level, where specific patterns such as, e.g., learning a new person’s face brings the instantiation of a capacity to our notice. The time course of memory consolidation is one such functional property. The functional analysis is a precondition for constructing appropriate explanatory connections between the components and systemic behavior. If, however, this conceptual “priming procedure” fails, as it may in the case of 10.1007/978-3-540-29678-2_17, the corresponding reductive explanation fails, too [6].
Reductive or mechanistic explanations can be forged in two opposite directions. Given that we already know that some system S has a systemic property P one task is to provide a reductive explanation for P. For that we refer to the microstructure MS(S) of S, to the behavior of the components, and to the interaction among the components C i of S. With these resources, we try to show that S must have P given the analysis of its structure. So, in the case described above, it was important to the trichromatic theory that humans had exactly three color sensitive cones, and that these had appropriate spectral sensitivities. We also knew that humans could, typically, discriminate among spectral hues. So there was a systemic property (human discriminatory abilities) and there were microstructural features (three types of cones) that were appropriate. All this depended on adequate conceptual preparations. That is, we needed to know the range of discriminable colors. When it turned out that the systemic properties were different from those predicted by the trichromatic theory, that required a change in the understanding of the physiology.
In converse cases, we might at first only know the microstructure MS(S) of a system S and be uncertain concerning its exact capacities. The task, then, is to verify theoretically, or to forecast whether or not S has (a desired) systemic property P. To do so, we again must make use of adequate conceptual preparations and refer to the microstructure MS(S) of S, to the capacities and interactions of the components C i of S. This is a difficult procedure, since the combinatorial possibilities are daunting. Experimental procedures that follow from imposing deficits, whether experimental or accidental, fall into this category. Ablation studies in nineteenth century physiology follow this simple approach, though there are more sophisticated methods (e.g., knock-out genes) available to recent physiologists. So when Broca discovered a patient (Tan) that lacked the ability to produce coherent speech but who had normal comprehension, and who had damage to the left temporal lobe, he concluded that the temporal lobe was the location of articulate speech. Many fMRI experiments still follow this research strategy. They do not provide reductive explanations.
Of course, a system can be looked at from a “top down” perspective, a “bottom up” perspective, or from both simultaneously. In that case, we can stitch together the perspectives we garner from the bottom and the top.
Anti-Reductionistic Positions
If it turns out that some purported phenomenon is not real, the property to be reduced is not accepted. In that case, condition (i) fails, and reductive explanation fails right at the outset. If telepathy is not real, there is nothing to explain or reduce. At the extreme, this becomes 10.1007/978-3-540-29678-2_5, in which all psychological phenomena are rejected as real. Paul and Patricia Churchland [3], followed by John Bickle [2], defend largely eliminative positions, supported by an emphasis on theory reduction. If it turns out that one cannot functionalize a psychological property accepted for reductive explanation, even in principle, then condition (i) is satisfied, while (ii) is rejected. In this case, the systemic property is real but irreducible, and thereby it is synchronically (i.e., in a strong sense) emergent. Strong emergentism (10.1007/978-3-540-29678-2_5) is at least a type of property dualism [7]. The case in point is qualia. Commonly, it is held that, e.g., our sensation of red has an intrinsic character that cannot be captured in terms of function; and if this is so, then (ii) fails in this case [6]. If it turns out that the system components with their particular arrangement and interactions are not sufficient to account for the system’s behavior, then there is a failure of condition (iii). Again, a mechanistic explanation fails. If this is a consequence of limitations on our knowledge, then this is not a principled limitation. If it is a principled limitation, as the great neurophysiologists Sherrington and Eccles [8] maintained, then that is a failure of the reductionist/mechanist program. We would be driven to substantive dualism, 10.1007/978-3-540-29678-2_22 or Cartesianism (10.1007/978-3-540-29678-2_3). If the behavior of components, embedded in the appropriate context, is sufficient to explain the system behavior, but the behavior of the components in simpler constellations cannot account for their interactions within the more complex system, then condition (iv) fails, In that case, we are forced to a kind of 10.1007/978-3-540-29678-2_8; which establishes one type of emergentism. Irreducibility can coexist with mechanistic explanations [9].
Theory reduction also has its detractors, who usually contend that there will be a failure to provide appropriate bridge laws to connect the theories in heterogeneous reductions. Functionalists such as Jerry Fodor [10] and hold that psychological states are functional states (10.1007/978-3-540-29678-2_6), which can be realized in multiple ways, and conclude that therefore there will not be appropriate identities available. They conclude that this supports a kind of autonomy for psychological explanations relative to physiological explanations. A more radical failure would be the absence of sufficient physical conditions to explain psychological capacities. In this case, we would be driven again to either dualism or eliminativism.
Redundancy of Degrees of Freedom
Definition
An excess of elemental variables (degrees –of freedom) within a system as compared to a number of constraints imposed on the system in typical tasks; this term assumes that redundant elemental variables need to be eliminated to make the system controllable.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_4
Redundant Set, Redundancy Problem
Definition
Also called Bernstein’s problem, named after the scientist who has considered it as a major problem in motor control research; ambiguity in transforming a set of variables into a set of more numerous variables so that a unique solution of many possible solutions of the motor task must be chosen, for example, when it is necessary to distribute total torque acting on a joint into individual torques of muscles spanning this joint, or to choose one of many possible ways of combining different degrees of freedom of the body to reach the motor goal. See also the principle of neurological minimization.
10.1007/978-3-540-29678-2_5
Reference
Definition
Reference is the relation between an expression and what it refers to. A typical unambiguous concrete singular term like “the highest mountain” or “Gottlob Frege” refers to a certain concrete particular like a thing or a person. A [→] predicate like “is red” distributively refers to all those particulars to which it applies, i.e. to the red things. Sometimes the relation between a predicate and the set of things it applies to is also called “reference.”
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_12
Reference Frame
Definition
10.1007/978-3-540-29678-2_19
Reference Model
Definition
The Reference Model is used for a certain type of control design. It is a mathematical model that represents the desired behavior of the controlled physical system.
10.1007/978-3-540-29678-2_1
Referent Configuration
Definition
A position of the body or its segments at which muscles are silent in the absence of co-activation or, otherwise, produce net zero joint torques but generate activity and resistive forces in response to deviations from it; modified by control levels to produce motor actions.
10.1007/978-3-540-29678-2_5
Referent Trajectory of an Effector
Definition
Comprised of the positions of the effector’s associated with threshold configurations of the body at each instant of movement (see Threshold control).
10.1007/978-3-540-29678-2_5
Referential
Definition
Pointing to the meaning of an utterance.
10.1007/978-3-540-29678-2_3
Referred Pain
Definition
Referred pain is the phenomenon wherein nociceptive stimulation in one location results in the perception of pain in another location. In clinical practice, this phenomenon is most often thought of as involving the projection of pain from a visceral structure to the body surface. However, nociceptive stimulation of muscle, and possibly other somatic tissues, can also lead to referred pain. A number of mechanisms have been proposed to explain referred pain. These include, most importantly, the convergence of afferent neurons from the site of insult and the site of perceived pain. This may occur through the dichotomization of afferent fibers such that one branch of an axon terminates on, for example, a visceral structure, while another branch of the same axon terminates in the skin. Alternatively, two distinct peripheral sensory neurons may both terminate on the same dorsal horn neuron. In either instance, it is proposed that the brain would have difficulty in determining the true source of nociceptive input, and would preferentially attribute pain to the more familiar source of sensory input – hence the body surface.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_19
Reflex
Definition
Involuntary modification of activity in motoneurons in response to activation of sensory receptors.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_6
Reflex Adaptation
Definition
One of the simplest forms of motor learning. Inborn reflexes, such as an eyeblink reflex, are adaptable to new environmental conditions. For example, the force required for eyelid closure self-adjusts when the eyelid movement is impeded by an external load.
10.1007/978-3-540-29678-2_13
Reflex Chain
Definition
A sequence of reflexes where the action of the first reflex activates a set of receptors (e.g. proprioreceptors) that trigger a second reflex, and so on. The peripheral control hypothesis proposed that complex behaviours consist of simple reflexes that are linked together in a reflex chain. This hypothesis has been superseded by the central control hypothesis.
10.1007/978-3-540-29678-2_3
Reflex Sympathetic Dystrophy (RSD)
Definition
10.1007/978-3-540-29678-2_3
Reflexes
Definition
(Taken from Dr. Wilfrid Jänig’s essay on 10.1007/978-3-540-29678-2_1) Reflexes are functionally defined by an efferent (motor) output system that generates a distinct effector response when activated and by the population of afferent neurons stimulated. Reflexes are fragments of more complex somatomotor behaviors and are used in the laboratory as tools to study experimentally the central organization of neural regulation of movement.
Background
There is no universally accepted definition of reflexes that distinguishes them from voluntary responses to stimuli [1]. The Roman poet Ovid used the word reflex to describe “turning or bringing back.” Substitute “feed” for “bring” and we have the word “feedback.” In engineering, feedback refers to information about a process monitored by sensors and supplied to the controller of that process (see 10.1007/978-3-540-29678-2_6).
In biology, the first attempt at a definition of reflex is attributable to Georgiy Prochaska [2]: a behavior in response to an excitation, mediated by separate motor and sensory nerves. Prochaska saw the function of reflexes to be conservation of the individual, later called homeostasis by Claude Bernard [3]. The psychologist Herbert Spencer posited that reflexes were the atoms of the psyche; that the psyche was an assemblage of reflexes, and that instincts were reflex assemblies consolidated by repetition and transmitted in an hereditary manner [4]. The Russian clinical physiologist Ivan Sechenov went one step further, proposing that all motor acts in humans as well as animals were simply chains of elemental reflexes [5]. He argued that the appearance of spontaneity and volition was illusory and that all movements were in principle predicted by the history of prior events, sensory inputs and associated thoughts. His conception of reflexes included complex responses that involved choice, as well as learned responses that his successor Pavlov would later call conditioned reflexes. The ideas of Spencer and Sechenov were taken to their literal conclusion in the behaviorist theories of Watson and Skinner. These theories rejected all non-measurable explanations of behavior and replaced voluntary movement with operants: conscious arbitrary acts that have become associated with arbitrary stimuli through learning and arbitrary reinforcement.
Hughlings Jackson argued from clinical observations that movements ranged in a continuum from the most automatic or evolutionarily primitive to the least automatic or most evolutionarily advanced [6]. Primitive reflexes in humans were unmasked or released when the higher centers were damaged. The Jacksonian continuum from automatic to voluntary probably best encapsulates the current view of most neuroscientists. In this view, reflexes are brief, automatic and invariant responses to stimuli. But even this definition is problematic: Goldstein (1939) reviewed various responses called reflexes and found them all to be variable, state-dependent and mutable [1].
Jonathan Wolpaw recently argued that a single comprehensive hypothesis related to this question of distinguishing reflexes from voluntary actions developed in the nineteenth century, namely that the whole function of the nervous system is to convert sensory input into appropriate motor output [1]. Neuroscientists who say they are studying reflex behaviors are studying behaviors in which the connections from stimulus to response, from experience to behavior, are known to be, or at least believed to be, short and simple enough to be accessible to description with presently available methods, and they are excluding by one means or another voluntary behaviors, or behaviors involving connections so long and complex as to defy present-day analysis. Implicit in these definitions is the expectation that, as methodology and understanding advance, the class of reflex behaviors will grow larger and larger and the class of voluntary behaviors smaller and smaller.
When reading the essays on specific reflexes in this Encyclopedia it is useful to bear in mind the following comments of Francois Clarac [1]:
In normal behavior, reflexes are simple, fast reactions to the environment. The term should be confined to the simplest input-output reactions mediated by monosynaptic (or oligosynaptic) pathways at the lowest level, i.e. at the motoneuronal level. Reflexes should be viewed as elements of feedback control that each species possesses to react automatically to the environment.
The experimenter can induce a reflex artificially…. Reflexes then reduce to informative tests of CNS state. A reflex might be seen as a physiological “scalpel” permitting entrance into simple workings of the CNS, while not being a distinct and separable element when normal movements are considered. Thus although the understanding of motor behavior has benefited from reflex experiments, the normal functioning of the CNS, in which many afferent messages are integrated, should never be viewed as reflexive behavior even in the case of the “automatic” movements of invertebrates and lower vertebrates.
Overview of the Essays Grouped within the Topic “Reflexes”
In the following synopsis, key points are extracted or paraphrased from each of the essays in this volume related to reflexes.
10.1007/978-3-540-29678-2_1. Reflexes related to autonomic regulation of pelvic organs, cardiovascular system, functions of skin, gastrointestinal tract, airways, eye and pineal gland are mediated by spinal cord, brain stem or hypothalamus and are functionally defined by their afferent input and efferent output. They are di- or polysynaptic, organized at the segmental propriospinal or propriobulbar level and form the building blocks of autonomic regulations. Interneurons are important for the integration and coordination of different autonomic and somatomotor systems. Command signals from higher centers act primarily via these interneurons rather than directly with the final autonomic pathways.
10.1007/978-3-540-29678-2_3. The fact that reflexes are affected by activity-dependent plasticity throughout life (and even in utero) implies that the traditional distinction between unconditioned and conditioned reflexes is merely an artificial distinction imposed by an experimenter. In reality, most and probably all reflexes are conditioned in the sense that they have been shaped by activity. Those traditionally designated as “unconditioned,” such as the normal flexion withdrawal reflex that withdraws a limb from a painful stimulus, are reflexes that have undergone standard conditioning in the course of earlier life, and thus are similar in most normal individuals. In essence, “unconditioned reflexes” are simply reflexes that were conditioned before the experimenter began to observe them.
10.1007/978-3-540-29678-2_6. The word feedback is used extensively in engineering. In neurophysiology, it is used to describe the sensory signals used by the CNS to control a large number of bodily functions to maintain constancy of the internal environment (homeostasis). Signals from mechanoreceptors in muscles, joints and skin are involved in the control of movement, as well those from the eyes, ears and vestibular apparatus. All levels of the CNS from the spinal cord to the cerebellum and cerebral cortex receive feedback from mechanoreceptors and all these levels are involved in controlling even the simplest movements.
10.1007/978-3-540-29678-2_9. Despite more than a hundred years of research, the integration of spinal reflex circuitries and descending motor commands remains a challenge. For example, there is still no consensus about the mechanism by which the much-studied monosynaptic stretch reflex contributes to the activation of muscles during walking, if it makes a meaningful contribution at all. An understanding of spinal reflex networks is a requirement for developing useful therapeutic strategies in the rehabilitation of neurological disorders.
10.1007/978-3-540-29678-2_12. Locomotor reflexes play an essential role in the patterning of motor activity for walking. These reflexes have three major functions: (i) to regulate the timing of motor commands according to the mechanical state of the limbs and body, (ii) to control the magnitude of ongoing muscle activity, and (iii) to initiate corrective responses when the limbs or body are unexpectedly perturbed by events in the environment.
10.1007/978-3-540-29678-2_12. Long loop reflexes are automatic motor responses to somatosensory stimuli that are believed to be mediated via the cerebral cortex, hence the term transcortical reflex. By definition, long loop reflexes occur at latencies longer than the simplest reflexes mediated by segmental circuits within the spinal cord yet the latencies are too short to be mediated volitionally. For muscles in the hand, the fastest (spinal) reflexes to muscle stretch occur at latencies ~35 ms; long loop reflexes occur at latencies ~60 ms, whereas volitional responses occur at ~140 ms. However, automatic motor responses of comparable latencies can also be generated by tactile (cutaneous) stimuli that do not involve muscle stretch, so the term “long loop reflex” should not be restricted to those generated by muscle stretch.
10.1007/978-3-540-29678-2_16. Presynaptic inhibition (PSI) refers to a decrease of transmitter release at central synapses. For example, activation of afferent fibers originating in flexor muscles attenuates monosynaptic 10.1007/978-3-540-29678-2_5 in extensor motoneurons without detectable changes in the time course of the EPSPs, membrane potential or motoneurone excitability. PSI occurs widely within the CNS of both vertebrates and invertebrates. Synaptic efficacy at axon terminals from sensory afferents, descending systems or interneurons can be subject to PSI control by a number of neurotransmitters and presynaptic receptors.
10.1007/978-3-540-29678-2_18. Generating an optimal breathing pattern for O2 and CO2 homeostasis requires the integration of sensory information from a variety of receptors including central and peripheral chemoreceptors for adjusting the magnitude of alveolar ventilation and stretch receptors for regulating the depth and rate of breathing. Sensory input is also important in the coordination of breathing with other systems, such those required for speaking, eating, walking, running, and vomiting. Finally, sensory information is necessary for protection of the airways and lungs. Receptors in the nose, pharynx, larynx and lower airways elicit a variety of reflexes including coughs, sneezes and apnea that protect the airways from inhalation of noxious substances and increase mucous secretion that aids in their removal.
10.1007/978-3-540-29678-2_19. Spinal reflexes consisting of afferent and efferent components instigate the genital vasocongestion and neuromuscular tension responsible for sexual arousal (erection in men and vaginal lubrication and elongation in women), the triggering of ejaculation in men, and possibly orgasm in both sexes. While the spinal cord contains all the neural circuitry involved in the generation of genital arousal, many other body senses, emotions and cognitive processes mediating social awareness determine whether an individual person will orient towards sexual activity or lose interest.
Concluding thoughts. The difficulty in classifying motor acts as either voluntary, involuntary or reflexive is the inevitable consequence of the overlap in the attributes that describe these categories as well as the brain mechanisms that control them. The various essays summarized above all demonstrate this overlap in one way or another. The Jacksonian continuum, “most automatic” to “most voluntary,” should always be borne in mind when considering reflexive control of bodily functions.
Reflexive Saccades
Definition
These are also called reactive saccades, pro-saccades, or visual grasp reflex. A saccade elicited by visual, auditory and even tactile events, and direct gaze at the perceived location of these events. In freely behaving subjects they tend to be accompanied by head rotation when the eccentricity of this location exceeds 20° or so.
Depending on the modality and intensity of the stimuli, they occur at latencies of 150 to –350 ms. Considerable attentional effort is required if reflexive saccades are to be voluntarily suppressed.
10.1007/978-3-540-29678-2_15
10.1007/978-3-540-29678-2_19
Refractory Period
Definition
When, during an action potential, the membrane has undergone a full-blown depolarization (up to several tens of millivolts positive), the Na+ system is subsequently in a state of reduced responsiveness, from which it recovers slowly over several milliseconds. This period is called refractory period. There is often an initial short period during which the Na+ system cannot be activated at all, however strong the depolarization.
This is called the absolute refractory period. During the subsequent relative refractory period, the Na+ system responds in part.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_19
Regeneration
Regenerating axons grow well in the peripheral nervous system (PNS), but, in contrast, effective nerve regeneration rarely occurs in the central nervous system (CNS). Following 10.1007/978-3-540-29678-2_1, the distal segment of the injured axon degenerates (10.1007/978-3-540-29678-2_23), whereas the proximal segment usually remains intact. The most prominent change of the neural cell body following axotomy is the disintegration of 10.1007/978-3-540-29678-2_14 [1]. This phenomenon is called 10.1007/978-3-540-29678-2_3 (10.1007/978-3-540-29678-2_3). Chromatolysis that can occur in neural cell bodies in both the PNS and CNS involves not degenerating, but regenerating reactions of neurons to axotomy. Though morphological changes of neurons in relation to chromatolysis have been extensively studied, the molecular basis behind this phenomenon has not yet been clarified. Chromatolysis is a sign for neurons, when their axons are injured, to shift from the normal condition to the regenerating phase, leading to axonal growth. In the normal condition, neurons are mainly involved in the synthesis and release of transmitters. However, following injury, neurons should change their machinery to produce molecules that contribute to regeneration. Molecular changes in association with axonal degeneration and regeneration have been studied, and gene expression involving in axonal elongation is crucial in understanding the molecular mechanism of nerve regeneration (10.1007/978-3-540-29678-2_14). The molecular changes of neurons responding to axonal injury have been studied [2].
In the PNS, 10.1007/978-3-540-29678-2_19 and their basal laminae act as efficient scaffolds and sources of 10.1007/978-3-540-29678-2_14 required for the growth of regenerating axons, and adhesion molecules present on the surface of Schwann cells contribute to nerve regeneration. On the other hand, glial cells including 10.1007/978-3-540-29678-2_1 and 10.1007/978-3-540-29678-2_15 in the CNS play no supporting role in the growth of regenerating axons. In addition, there exist no extracellular matrices such as basal laminae in the CNS. Although axons in the CNS have the ability to regenerate after injury, the microenvironment appropriate for the growth of regenerating axons is not provided in the CNS. Cell transplantation using Schwann cells, 10.1007/978-3-540-29678-2_15, 10.1007/978-3-540-29678-2_14, 10.1007/978-3-540-29678-2_16, and 10.1007/978-3-540-29678-2_13 has been extensively studied to overcome this difficulty, as it could provide an efficient environment to enable regenerating axons to grow. Other studies have also been conducted on how to facilitate nerve regeneration via suppressing inhibitory factors using specific antibodies or via supplying trophic factors to the lesion using genetically altered cells to produce specific trophic factors.
Nerve Regeneration in the PNS
Axons are ensheathed by Schwann cells in the PNS, where each cell forms a 10.1007/978-3-540-29678-2_13 sheath segment around the axon with 10.1007/978-3-540-29678-2_14 intervening between the neighboring myelin sheath segments. Schwann cells of myelinated and unmyelinated fibers possess basal laminae on the surface facing the connective tissue, which are continuous even at the nodes of Ranvier. Therefore, the axon and associated Schwann cells reside within a basal lamina tube along its entire length.
Peripheral nerve fibers are located in the connective tissue compartment, an essential difference from the central nerve fibers, which are tightly packed within the brain and spinal cord without any structural extracellular component present between the nerve fibers. During Wallerian degeneration following axonal injury, remaining Schwann cells temporally proliferate and form cell strands called “10.1007/978-3-540-29678-2_19” within the basal lamina tube. Axonal sprouts that emerge at the nodes of Ranvier adjacent to lesions of the proximal stump extend as regenerating axons through the connective tissue compartment to the distal stump, in which they further elongate along Schwann cell columns.
The tip of the regenerating axon is specialized as a 10.1007/978-3-540-29678-2_7 [3]. Growth cones are formed at the growing tip of axons during development and regeneration. Growth cones emit filopodia and lamellipodia on the surface, which actively move in various directions to survey the surrounding environment. The structure of living growth cones and their impressive movement were first observed in the culture of single-dissociated neurons under cine-microscopy in Nakai’s pioneering work [4]. He proposed that filopodia and lamellipodia might represent sensors searching for appropriate targets during their extension [5]. Numerous studies have been performed regarding the structure and function of growth cones 10.1007/978-3-540-29678-2_7.
The growth of axonal sprouts is facilitated or suppressed depending on the conditions of myelin sheath degradation that occurs during Wallerian degeneration in the myelin sheath of the distal stump [6]. In the distal stump, regenerating axons come into contact with Schwann cell columns. Schwann cells play a critical role in nerve regeneration in the PNS; they provide cellular scaffolds for regenerating axons to grow through, and express trophic factors as well as adhesion molecules for promoting the extension and maintenance of regenerating axons. In Wallerian degeneration, Schwann cells disrupt myelin sheaths that have lost contact with axons into fragments called myelin balls that are subsequently transferred to and phagocytosed by macrophages. Thus, macrophages contribute to successful nerve regeneration. Schwann cells cooperate with macrophages not only in myelin sheath removal, but also in trophic factor production for promoting axon growth during nerve regeneration (10.1007/978-3-540-29678-2_19). Schwann cells that remain “quiescent” in Schwann cell columns during Wallerian degeneration become “active” by coming into contact with regenerating axons, following which they gradually ensheath axons and finally form myelin sheaths in myelinated fibers.
Schwann cells are primary sources of trophic factors for nerve regeneration. A large number of neurotrophic factors have been identified. A well-known neurotrophic factor is 10.1007/978-3-540-29678-2_14, a member of the neurotrophins that include 10.1007/978-3-540-29678-2_2, 10.1007/978-3-540-29678-2_14, and 10.1007/978-3-540-29678-2_14. 10.1007/978-3-540-29678-2_7 belong to another family that promotes the survival and neurite extension of neurons. 10.1007/978-3-540-29678-2_3 also have neurotrophic activity, which include 10.1007/978-3-540-29678-2_3 and 10.1007/978-3-540-29678-2_9. Neurotrophic molecules have been studied in relation to their corresponding receptors, effects on axonal cytoskeletons, and gene expression to explore the regeneration mechanisms of injured axons in the PNS and CNS (10.1007/978-3-540-29678-2_14).
On the other hand, in one experiment in which Schwann cells were killed by freeze-treatment, the damaged cells were removed by macrophages. Regenerating axons were then observed to vigorously grow through such basal lamina tubes in contact with the inner surface of the basal lamina [7]. This result indicates that peripheral nerve axons can grow through the acellular matrices (10.1007/978-3-540-29678-2_2). This is the theoretical basis for the use of artificial materials for nerve regeneration in the PNS. Basal laminae serve not only as the scaffold for growing axons, but also as a supply of trophic/nutritional factors that are adsorbed by heparan sulfate present on the outer surface of the basal laminae [8]. Thus, peripheral nerves are provided with dual structural insurance, Schwann cells and basal laminae, for successful nerve regeneration.
In a regular nerve suture, regenerating axons randomly enter distal Schwann cell columns. Therefore, it is not definite that regenerating axons can reach their original targets. The clinical estimation of functional recovery is important [9]. Several different methods of treatment have been developed and used clinically. In the case of crush injury, in which basal lamina tubes (endoneurial tubes) remain undisrupted, axonal sprouts can extend through the original Schwann cell tubes to the original targets. Therefore, nerve regeneration occurs readily, and the high correspondence in axon-target reinnervation is secured for accurate functional recovery. On the other hand, in the case of transection, the proximal and distal stump should be sutured by apposing directly, or using grafts including autologous nerve grafts and artificial tubes. In stump suturing, fascicular repair to connect the corresponding nerve fasciculi has been recommended to ensure that regenerating axons can reinnervate the original targets as accurately as possible (Regeneration: clinical aspects).
Neural connections in the 10.1007/978-3-540-29678-2_19 of the 10.1007/978-3-540-29678-2_3 can be reorganized depending on the input from the peripheral nervous system. The cortical neural organization including Sensory Cortex and 10.1007/978-3-540-29678-2_13 is not fixed, but can be modified subject to the functional demand. When the sensory inputs are lost due to peripheral nerve damage including the amputation of limbs or fingers, the corresponding areas of the somatosensory cortex are reorganized to receive inputs from the neighboring skin areas including the stump. A similar reorganization occurs in the motor cortex. Thus, motor and sensory representations of the cerebral cortex become remodeled following nerve injury and regeneration in the PNS. This means that patients should relearn how to appropriately perform an action through the remodeled cerebral cortex in rehabilitation. Reorganization of the cerebral cortex is a basis for the rehabilitation of limb activity (10.1007/978-3-540-29678-2_19; Regeneration: clinical aspects).
Artificial materials have been studied as guides for the growth of regenerating axons in the PNS. Collagen gel has been most commonly used. Other biodegradable polymers that have been utilized for nerve regeneration are polyglycolic acid, polylactic acid, poly-ε-caprolactone, alginate, and chitosan. Alginate is derived from brown seaweed, and chitosan is from the crustacian exoskeleton. These materials have been used as substrates for nerve regeneration in the PNS and CNS (10.1007/978-3-540-29678-2_20). A polyglycolic acid-collagen tube has been developed with good results [10].
Blood supply is a critical point for successful nerve regeneration. Blood capillaries that readily regenerate in the connective tissue compartment greatly contribute to peripheral nerve regeneration. Blood vessels, once damaged, rarely regenerate in the CNS. This is probably because, unlike in the PNS, there is no extracellular matrix which can act as a scaffold for developing vessels in the CNS. The loss of blood supply results in severe ischemia, which in turn leads to the suppression of tissue repair and the promotion of cavity formation in the CNS.
Motor Nerves: The Neuromuscular Junction
Motor nerves terminate at 10.1007/978-3-540-29678-2_14, which consist of presynaptic axon terminals and postsynaptic folds of the muscle fiber plasma membrane. In addition, terminal Schwann cells cover the presynaptic axon terminals, and the basal lamina, a continuation of the ordinary basal lamina of muscle fibers, is present on the folds of the muscle fibers. Following Wallerian degeneration, the presynaptic terminal disappears, and the postsynaptic folds at the endplate become gradually less distinct, but remain as a remnant of small folds. At the same time, terminal Schwann cells persist in the preterminal region. Regenerating axons enter the empty endplate, and become presynaptic terminals, thus forming new neuromuscular junctions. The presynaptic terminal can be formed in the absence of terminal Schwann cells, such as in the acellular scaffold [11]. In addition, regenerating axons can develop a presynaptic structure when they come into contact with the basal lamina at the site of the original postsynaptic folds. This indicates that the basal lamina at the endplate contains molecules that induce the regeneration of axons and cause postsynaptic specialization.
When muscle is partially denervated, 10.1007/978-3-540-29678-2_1 occurs from the intact neuromuscular junction. Following motor neuron injury, axon terminals are lost from the endplates of muscle fibers belonging to injured motor neurons. Responding to the denervation of endplates, axonal sprouts emanate from axon terminals of neighboring intact endplates, reinnervating denervated endplates. Thus, the 10.1007/978-3-540-29678-2_13 is enlarged, and the muscle activity can be kept almost unchanged. Terminal Schwann cells that have lost contact with axon terminals by denervation extend cell processes toward the neighboring intact endplates. Such Schwann cell processes act as guide tubes for axonal sprouts to elongate from the intact endplate to the denervated one (Role of sprouting in sustaining neuromuscular function in health and disease).
Sensory Nerves: Sensory Corpuscles in the Skin
Sensory nerve terminals occur as various types of organized corpuscles present in the skin. Representative sensory terminals include 10.1007/978-3-540-29678-2_16 and 10.1007/978-3-540-29678-2_13, which are composed of axon terminals and modified Schwann cells called lamellar cells. Following Wallerian degeneration, lamellar cells in these corpuscles become atrophic owing to the loss of contact with axons, but continue to exist for a long period of time. Upon the arrival of regenerating axons, lamellar cells begin to proliferate and take on the same structures as those found in the original corpuscles. For the acellular corpuscle, corpuscular basal laminae deprived of lamellar cells can also serve as a scaffold that promotes the regeneration of the original corpuscle following reinnervation. However, no new corpuscle regeneration in regions other than at the original corpuscle in the skin occurs (10.1007/978-3-540-29678-2_13; 10.1007/978-3-540-29678-2_16).
10.1007/978-3-540-29678-2_13-neurite complexes are different from Pacinian and Meissner corpuscles in that their axons make direct contact with Merkel cells. Merkel cells deprived of axon terminals tend to disappear, probably due to degeneration and/or movement to the surface of the epidermis. Upon reinnervation, Merkel cells reappear, partly due to the differentiation of keratinocytes, and then form Merkel-neurite complexes similar to the original ones present at the base of the epidermis (10.1007/978-3-540-29678-2_13).
Nerve Regeneration in the CNS
Nerve regeneration in the CNS, especially in the spinal cord, has been extensively studied for more than 100 years. No effective nerve regeneration or tissue repair occurs in lesions of the spinal cord, which usually results in cavity formation without distinct tissue repair. Following injury to the spinal cord, strong regenerative responses occur including the formation of growth cones and associated glial cell migration. However, such neural reactions do not develop as efficiently as they do in the PNS, and result in cavity formation without axonal extension into the lesion. As described above, the essential difference between the PNS and CNS is the presence of an extracellular matrix in the PNS, which is composed of basal laminae and collagen fibers. This means that there is no effective scaffold available for the growth of regenerating axons in the CNS. The same can be said for blood vessel regeneration, which requires an extracellular matrix scaffold to regenerate.
The 10.1007/978-3-540-29678-2_7 is usually produced around the cystic cavity at a chronic stage after injury. If the 10.1007/978-3-540-29678-2_16 is cut open, the fibroblast-like cells of the meningeal layer invade the lesion, contributing to the formation of dense glial scar of connective tissues composed of extracellular matrices including collagen fibers. In such cases, astrocytes form a barrier between the connective tissue and adjacent CNS tissue. Basal laminae are formed on the surface of astrocyte processes facing the connective tissues, and thus, the CNS tissue tends to segregate itself from the invading connective tissues using astrocytic scar tissue [12]. This kind of glial scar is regarded as the main obstacle preventing the growth of regenerating axons in the CNS. Connective tissue invasion followed by glial scarring is therefore an undesirable phenomenon for nerve regeneration in the CNS. On the other hand, in lesions in which the pia mater is not cut open, the tissue reaction is different. When the spinal cord is crush-injured, the pia mater is usually not damaged, with the pial basal lamina kept intact. Cavities of various sizes are usually formed in the lesion at chronic stages. In such cases, there is no cell invasion from the outside. Astrocytic proliferation is found along the margin of the cavity, and oligodendrocytes line the inner surface of the cavity margin.
Usually, astrocytes, oligodendrocytes, macrophages, and microglia all contribute to the formation of glial scars. The mechanisms for scar formation, roles of contributing cells, and expression of specific molecules including proteoglycans are complicated, and yet to be understood (10.1007/978-3-540-29678-2_7). Glial scars are thought to be the main impediments to the growth of regenerating axons. Regenerating axons from the proximal stump have to surpass the glial scar at the distal stump to invade the host spinal cord tissue. The digestion of proteoglycans has been proposed to promote axonal growth through glial scar in the spinal cord.
Cavities resulting from the degeneration of impaired tissues hamper axonal extension through the lesion. The margin of the cavity is not as thick as the astrocytic scar tissue as previously thought in the crush-injured spinal cord. Using appropriate techniques including cell transplantation, it may be possible to induce regenerating axons to grow through a region with cavities.
Functional assessment is important for nerve regeneration in the CNS, for which BBB scoring and other types of estimation of behavioral recovery have been employed [13].
At present, cell transplantation is being extensively studied to facilitate nerve regeneration in the injured spinal cord. Several varieties of cells have been used for transplantation, among which the major cell types include: Schwann cells, bone marrow stromal cells, olfactory ensheathing cells, choroid plexus ependymal cells, neural stem cells, macrophages, and those found in embryonic spinal cord tissue. Other studies have also been carried out which focused on the suppression of inhibitory factors such as 10.1007/978-3-540-29678-2_14 by the specific antibody.
Schwann Cells
Aguayo and colleagues showed that neurons within the CNS can induce the elongation of regenerating axons into peripheral nerves which were inserted at one end into the spinal cord and at the other end into the medulla oblongata [14]. This shows that regenerating axons from neurons in the CNS can extend if they are provided with an appropriate environment. Since this report, cell implantation aimed at CNS nerve regeneration has been extensively studied.
Schwann cells play a role in axonal elongation in grafted peripheral nerves, where regenerating axons come into contact with Schwann cells, which support the growth and maturation of regenerating axons, as in the case of the PNS. The utility of peripheral nerve grafts has prompted the use of cultured Schwann cells for transplantation in the CNS. Here, cultured Schwann cells mingle in matrigel, and are then placed into an artificial tube, after which the tube is subsequently grafted into the lesion of the spinal cord. Many regenerating axons extend through the tube, and some enter the distal stump. A few axons then form synapses with neurons present in the distal segment of the spinal cord, and behavioral improvement subsequently takes place [15].
Although Schwann cells also serve as effective conduits for regenerating axons in the CNS, extracellular matrices including basal laminae are inefficient as scaffolds for the growth of regenerating axons in the CNS, unlike in the PNS. Since Schwann cells possess basal laminae and the ability to produce collagen matrices, extracellular matrices can be brought into the lesion following Schwann cell transplantation. Therefore, astrocytes at the border of the lesion are apt to form barriers composed of cell processes with basal laminae on the surface facing the Schwann cell compartment, and regenerating axons cannot penetrate such astrocyte scar tissue. Studies have been concentrated to overcome this difficulty in Schwann cell transplantation (10.1007/978-3-540-29678-2_20).
The 10.1007/978-3-540-29678-2_15 is frequently used as an experimental model of nerve regeneration in the CNS. Since it is an isolated bundle composed of central nerve fibers, nerve regeneration can be more precisely evaluated than by using the spinal cord. Transplantation of peripheral nerves and Schwann cells into the optic nerve has been well studied [16]. A long peripheral nerve transplanted into the optic nerve can serve as a conduit for regenerating axons traveling from the optic nerve to the 10.1007/978-3-540-29678-2_19, in which some synaptic connections are formed. Some functional recovery of light sensation has also been reported using this technique.
Many retinal ganglion cells undergo retrograde degeneration after optic nerve injury, and this poses another major problem for optic nerve regeneration. The administration of trophic factors into the optic cup has been studied with the aim of promoting the survival of ganglion cells (Regeneration of optic nerve).
Bone Marrow Stromal Cells
Although they do not belong to the CNS, bone marrow stromal cells (BMSCs) have been used for transplantation into the spinal cord [17]. BMSCs are grafted by directly injecting them into the lesion or by infusing them through the cerebrospinal fluid (CSF) [18,19]. Some BMSCs then gather in the lesion and survive there for 2–3 weeks after grafting. BMSCs do not differentiate into neural cells after grafting into the spinal cord. In the rat, they tend to disappear from the spinal cord more than 4 weeks after grafting. Although BMSCs do not become integrated into lesions of the spinal cord, tissue repair including the suppression of cavity formation is facilitated. In addition, behavioral improvement is obvious in the spinal cord-injured rat. These findings imply that BMSCs can be used in transplants to treat spinal cord injury.
Since BMSCs can be obtained from the patients themselves and are not stem cells but ordinary functioning cells present in the bone marrow, they show promise for the clinical treatment of spinal cord injuries. Transplantation by infusing BMSCs into the CSF is more effective than direct injection into the lesion. Also, since BMSCs disappear several weeks after transplantation, they might release trophic factors that reverse the degeneration of damaged neural tissue. The clinical application of BMSCs by injecting them into the cerebrospinal fluid via lumbar puncture has progressed (10.1007/978-3-540-29678-2_20).
Bone marrow stromal cells can be trans-differentiated into Schwann cells and neurons, and the transplantation of trans-differentiated Schwann cells and neurons may be a promising technique for CNS as well as PNS regeneration [20].
Neural Stem Cells
Neural stem cells (NSCs) have been regarded as appropriate for cell transplantation to treat spinal cord injuries. After transplantation, these cells survive, migrate, and become integrated into the host tissue. They also have the ability to differentiate into neurons, astrocytes, and oligodendrocytes after transplantation.
There is hope that NSCs can be used after differentiation into neurons, astrocytes, and oligodendrocytes in vitro. Although NSCs are attractive for use in clinical therapy, the source of these cells is the most critical problem. There are strict limitations regarding the use of human embryos as sources of NSCs. It is also difficult to obtain NSCs from adult tissues. However, it is possible that NSCs can be acquired from the brains of deceased human bodies within a short period after death [21].
Another difficulty in using NSCs is the potential for unlimited cell proliferation, and, thus, the formation of cancer. Even in cases for which NSCs are used after differentiation in vitro, it is possible that a few undifferentiated cells may remain, and cause undesirable cell proliferation. Neural stem cells have been transplanted for spinal cord regeneration (10.1007/978-3-540-29678-2_20).
The Choroid Plexus
The choroid plexus is the main region where the cerebro-spinal fluid (CSF) is produced. It consists of epithelial cells and associated connective tissue containing plenty of sinusoidal blood vessels, and few fibroblasts and macrophages in a scanty collagen matrix. Grafting of the choroid plexus into the injured spinal cord promotes tissue repair including the growth and regeneration of axons in lesions [22]. Other in vitro and in vivo studies have indicated that choroid plexus epithelial cells (modified ependymal cells) might have the ability to promote nerve regeneration by rescuing neural tissues from degeneration and facilitating axonal growth. Considering that the CSF plays an important role in maintaining normal brain function, it should contain a variety of factors that promote the survival and proper activity of neural cells including neurons and glial cells.
Olfactory Ensheathing Cells
Recently, olfactory ensheathing cells (OECs) have been extensively studied for use in transplantation to promote nerve regeneration in the spinal cord. These cells possess the properties of Schwann cells and astrocytes, and grafted OECs become Schwann cells associated with axons and perineurial cells surrounding nerve fibers, as seen in the PNS [23]. Furthermore, functional recovery can be observed in accordance with histological improvement. It is obvious that OECs provide the guide for the extension of regenerating axons. OECs have had a great impact on the study of spinal cord regeneration; however, their effects have varied among such studies. In spite of the many studies performed so far, how OECs exert their effects in spinal cord regeneration has not yet been fully elucidated. Identification of OECs in in vivo experiments might be a crucial requisite for accurately understanding their roles in spinal cord regeneration. Genetically labeled OECs might be useful for long-term observation after transplantation (10.1007/978-3-540-29678-2_20).
Embryonic Spinal Cord Tissue
Satisfactory regeneration can be induced in the spinal cord in which a segment of the spinal cord obtained from an embryo has been transplanted in rats during the early postnatal period [24]. It has been proposed that embryonic tissue can overcome glial scaring of the cystic cavity in chronic injury of the spinal cord. Unfortunately, the use of embryonic tissues is greatly limited due to ethical problems.
Genetically Modified Cells
Experiments in which cells that were genetically transformed to secrete trophic factors have been transplanted into the injured CNS tissue have been conducted. Fibroblasts and other kinds of cells including OECs have been used for such experiments [25]. However, at present, genetically modified cells involve problems of ethics and safety that should be overcome before clinical application.
Immune System
Following injury to the CNS, microglia and macrophages invade the lesion. Microglia, macrophages, and T-cells have been demonstrated to play important roles in CNS protection and repair. Regulatory and autoimmune T-cells are contradictory regarding CNS protection. Autoimmune T-cells activate the microglia to secrete trophic factors for CNS repair (10.1007/978-3-540-29678-2_16). Macrophages preincubated with sciatic nerve fragments or autologous skin promoted repair of the optic nerve and spinal cord. Microglia and bone marrow-derived macrophages with the property of antigen-presenting cells secrete trophic factors that contribute to neuroprotection in the CNS (10.1007/978-3-540-29678-2_1). Studies on the functions of immune cells such as microglia, macrophages, and T-cells provide insights into the CNS mechanisms of protection against injury, and contribute to achieve CNS regeneration.
Inhibitory Molecules
It is believed that CNS regeneration cannot occur partly due to the presence of inhibitory molecules in the CNS. The main inhibitory molecules are associated with myelin sheaths and oligodendrocytes, including myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), and Nogo-A. Growth cones collapse when they encounter these inhibitory molecules. The administration of anti-Nogo A antibody promotes the growth of regenerating axons in the injured spinal cord and improves behavioral function in rats [26].
Another major inhibitor is chondroitin sulfate proteoglycan (CSPG) produced in the glial scar. CSPGs serve as barriers to growing axons. Regenerating axons are thought to be blocked at the boundary of the glial scar in the spinal cord injury (10.1007/978-3-540-29678-2_9).
Regeneration Associated Genes (RAGs)
Definition
Genes that are upregulated within the neuron following axotomy. The protein product of these genes such as tublin, GAP43 and others are anterogradely transported to and are critical to the elongation of the growth cone and regenerating axon.
10.1007/978-3-540-29678-2_14
10.1007/978-3-540-29678-2_16
Regeneration: Clinical Aspects
Definition
Outgrowth of 10.1007/978-3-540-29678-2_1 following clinical nerve injury and repair, resulting in functional restoration in denervated body parts.
Characteristics
Background
Injuries to peripheral nerve trunks constitute a major clinical problem [1,2]. Such injuries are most frequently seen in the upper extremity. The consequences are severe and the result is often permanent disturbances in sensory and motor functions of the hand. Normally, there is an interaction between the hand and the brain so that the hand is very well represented in the somatosensory cortex as well as the motor cortex [1,3,4]. A nerve injury implies a sudden de-afferentiation with arrest in inflow of sensory impulses to the brain. This results in a rapid cortical remodelling process where the “vacant” cortical area, previously representing the innervated area of the hand, is invaded by expanding adjacent cortical areas [1,3,4]. An analogous phenomenon occurs in the motor cortex.
If the nerve injury is surgically repaired, there is a regeneration of axons downstream of the distal nerve segment aiming at reinnervation of the denervated body part. To regain normal function, axons have to reinnervate their “correct” peripheral targets. However, there is always, in spite of meticulous surgical techniques, a large extent of misorientation of regenerating axons at the repair site and consequently an incorrect peripheral reinnervation [1]. With the reinnervation process, the cortical hand representation is again restored, however in a new and distorted pattern due to the peripheral mal-orientation. A relearning process is required that can be easily managed by the child’s brain but usually not by the adult brain [5]. Therefore, fine tactile discriminative functions are seldom or never fully restored in an adult patient. The process of clinical regeneration is influenced by a number of intrinsic and extrinsic factors, some of them reviewed below.
The Nerve Trunk and the Regeneration Process
The nerve trunk represents a composite tissue structure constructed to maintain continuity, nutrition and protection of its basic elements – the axons (Fig. 1). An axon is a long tubular process of the nerve cell body, which may be situated in a dorsal root ganglion (sensory axons), or the anterior horn of the spinal cord (motor axons). The nerve cell and its processes is called a neuron. The axons are ensheathed by Schwann cells that may produce a myelin sheath. The Schwann cell basal lamina contributes to constitute an “endoneurial tube.” The axons are closely packed within the endoneurial connective tissue inside 10.1007/978-3-540-29678-2_6 [1]. Each fascicle is surrounded by a perineurium, which is a multicellular laminated sheath of considerable mechanical strength, providing a diffusion barrier. The fascicles are embedded within an 10.1007/978-3-540-29678-2_5, which is a supporting and protective connective tissue sheath carrying a longitudinal network of epineurial blood vessels.
Regeneration: Clinical Aspects. Figure 1.

Microanatomy of peripheral nerve trunk and its component. (a) Fascicles surrounded by a multi-laminated perineurium (p) are embedded in loose connective tissue, the epineurium (epi). The outer layers of the epineurium are condensed into a sheath. (b and c) The appearance of unmyelinated and myelinated fibres, respectively, is shown. Schw Schwann cell; my myelin sheath; ax axons; nR node of Ranvier; end endoneurium. Reproduced with permission from Lundborg 2004.
Nerve injuries may be of several types and magnitudes. A severe compression or a crush lesion may disrupt axons while the ensheathing chain of Schwann cells and their basal lamina may be preserved. Disruption of axons results in degeneration of their distal segments, implying disintegration of the axonal elements and the myelin sheath. A regeneration process is then required where axons grow distally, following their original pathways, hereby reinnervating correct peripheral targets. The normal cortical representation of the body part is hereby re-established [3,4]. With transection of a nerve trunk, however, the situation is quite different: the “sprouts” that are formed by the transected proximal part of the axons may orient themselves into incorrect distal “Schwann cell tubes” that result in reinnervation of incorrect peripheral targets. Before the regeneration process is initiated, an “initial delay” may last for days or weeks. As a result of the injury a large number of nerve cell bodies in dorsal root ganglia may die, which excludes possibilities for regeneration of their corresponding axons [1]. Several physical, biochemical and other factors influence the course and functional outcome of the regeneration process [1].
Clinical Nerve Repair and Reconstruction
Repair and reconstruction of injured peripheral nerves span over a variety of techniques such as direct repair, 10.1007/978-3-540-29678-2_14, use of conduits, 10.1007/978-3-540-29678-2_14 and 10.1007/978-3-540-29678-2_5 anastomosis [1,6,7,8].
With 10.1007/978-3-540-29678-2_5, the nerve stumps are approximated by suturing the epineurial sheath, using external landmarks like longitudinal blood vessels to ensure a correct rotational adaptation of the nerve ends. Although the external aspect of the repair site may look perfect, this technique does not, however, ensure an absolutely correct matching of the fascicular structures inside the nerve trunk. A more correct mechanical adaptation may be achieved by fascicular repair or 10.1007/978-3-540-29678-2_7, which requires an internal dissection of the nerve so that separate bundles of fascicles are adapted towards each other. This technique may be justified in cases where sensory and motor fibres are running in separate, well-defined fascicles or fascicular groups, but otherwise the technique has no advantages over the epineurial technique. The various suture techniques may be combined and supplemented by fibrin glue. In 10.1007/978-3-540-29678-2_20, a small distance is left between the nerve ends that are enclosed in a tubular structure of biological or synthetic type. Such tubes, which may be biodegradable or non-degradable, may give equally good results as direct sutures of the nerve ends (Figs. 2, 3).
Regeneration: Clinical Aspects. Figure 2.

Epineurial suture. Adaptation of the nerve ends is achieved by single stitches in the superficial part of the epineurium along the circumference of the nerve. Reproduced with permission from Lundborg 2004.
Regeneration: Clinical Aspects. Figure 3.

Group fascicular suture. After resection of the epineurial tissue fascicular groups are approximated with single sutures in the connective tissue between separate fascicles or in the outer layer of the perineurium. Reproduced with permission from Lundborg 2004.
With more severe injury, there may be a defect in continuity of the nerve trunk. Such a situation may be seen in severe lacerations in the extremities or as a result of severe traction in the brachial plexus. Well-known examples are traction injuries occurring in difficult obstetrical situations or in adults involved in motorcycle or other types of traffic accidents. In such cases, the defect has to be bridged with a conduit to allow overgrowth of nerve fibres from the proximal to the distal nerve end. The most commonly used technique is to insert a nerve graft, usually harvested from the lower limb. Several cables of thin nerve grafts such as these are inserted between both of the nerve ends, using microsurgical techniques [7].
Nerve Transfers
In severe nerve injuries, a proximal nerve segment may not always be available. An alternate “donor nerve” may then be required to provide the distal segment of the injured nerve with axonal input from a proximal nerve segment, a so-called nerve transfer. The situation requires sacrifice of the donor nerve, which can then be transferred to the distal segment of the injured nerve [6]. Such nerve transfers are widely used in brachial plexus surgery for restoring function in paralysed muscle by using adjacent intact nerves as donors, but can also be applied to more distal nerve injuries, for instance, to achieve motor or sensory functions in the hand by transferring an intact, nearby non-injured nerve.
End-to Side (ETS) Nerve Repair
For more than 10 years it has been known that a distal nerve segment, when sutured in an end-to-side fashion to an adjacent intact nerve, can be reinnervated by sprouts from axons in the healthy donor nerve [8]. It was assumed that the intact axons in such cases may send out lateral sprouts that may reinnervate the sutured distal nerve segment. It was soon realised that this might be a new and promising possibility in clinical cases when routine nerve-grafting procedures where not possible, such as in root avulsions in brachial plexus injuries. The clinical results from these operations, as reported in the literature, are very variable.
Functional Remodelling of Brain Cortex
A nerve transection represents an acute deafferentiation with immediate and longstanding influence on the cortical representation of the innervated body part. For instance, deafferentiation due to median nerve transection results in rapid expansion of adjacent cortical areas, which then occupy the former median nerve cortical territories. If no regeneration occurs, as after an amputation, the extensive cortical reorganisation persists so that the cortical area, previously receiving input from the median nerve, remains occupied by expanding adjacent cortical areas. In amputation, severe cortical reorganisations in such cases may result in persistent 10.1007/978-3-540-29678-2_16 10.1007/978-3-540-29678-2_16. After a crush injury, regenerating axons are guided by their original Schwann cell tubes so that they reach their original skin locations, and the corresponding cortical hand representation is normalised. However, after transection and repair, this scenario is quite different due to peripheral axonal misorientation. The previously well-organised cortical representation is changed to a mosaic-like pattern [1,3,4] and the nerve does not recapture all of its original cortical territory.
Sensory Relearning and Sensory Re-Education
The outcome from nerve repair in adults is far from satisfactory and often disappointing, especially with respect to recovery of tactile discrimination [5]. One major factor is the new and distorted cortical hand representation – “the hand speaks a new language to the brain.” A relearning process is required, and it can be a difficult task for adults to require their lost functional sensibility. In hand rehabilitation, sensory relearning is based on the use of 10.1007/978-3-540-29678-2_19 protocols [9,10]. According to these strategies, the brain is reprogrammed based on a relearning process. First, the perception of different touch modalities and the capacity to localise touch is trained, followed by touching and exploration of items, presenting shapes and textures of varying and increasing difficulty to the patient with eyes open or closed. In this way, an alternate sense (vision) trains and improves the deficient sense “sensation.”
Factors Influencing the Outcome from Nerve Repair
The functional outcome of nerve repair may vary considerably between patients although identical techniques may be used. There are several factors that are known to influence the outcome of nerve repair.
Age
Although the functional recovery in adults is disappointing, especially with reference to recovering sensory functions, the situation is quite different among children who consistently show superior functional results after nerve repair [5]. This has usually been attributed to superior plasticity of the brain in children, with a better ability in central adaptation to the new pattern of afferent impulses presented by misdirected axons. A critical age period for recovery of functional sensibility in hands after nerve repair can be defined, the best results being seen in those younger than 10 years, followed by a rapid decline levelling out after late adolescence [5].
Cognitive Brain Capacities
In adults, specific cognitive capacities of the brain such as verbal learning capacity and visuo-spatial logic capacity may help to explain variations in the recovery of functional sensibility after nerve repair [1].
Timing of Repair
Nerve injuries should be repaired as soon as possible – if the condition allows. The posttraumatic nerve cell death, which usually occurs following nerve injury, can be reduced in this way. With early repair, the surgery is easier to perform since tissues may not yet be swollen, and the natural landmarks such as blood vessels can still be used to ensure a correct matching of the nerve ends. With increasing preoperative delay, there is a fibrosis of the distal nerve segment, atrophy of Schwann cells and there may be a progressive loss of neurons. After nerve transection the corresponding muscle atrophy rapidly, and after two years the muscle fibres may fragment and disintegrate.
Type of Nerve
The type of nerve that is injured considerably influences the functional outcome. If a pure motor nerve is injured, there is no risk of mismatch between motor and sensory cutaneous nerve fibres, thus optimising the accuracy in reinnervation. For pure sensory nerves, the situation is analogous. With mixed nerves, however, the situation is quite different with obvious risks for motor/sensory mismatch.
Level of Injury
After nerve transection, there is an initial delay of days or weeks followed by sprouting and axonal outgrowth. The regeneration in humans has been reported to be non-linear, with a gradually decreased regeneration rate in distal parts. In humans the average outgrowth rate is at most 1–2 mm/day. When digital nerves in fingers are injured, there is only a short distance separating the regenerating axons from their distal target, while more proximal lesions may have a very substantial distance to grow. Lesions to the median nerve at wrist level may require 3–4 months before the first signs of reinnervation of the hand occur. In brachial plexus lesions reinnervation of the hand seldom or never occurs because of the long regeneration distance.
Regeneration of Optic Nerve
Definition
Regeneration of retinal ganglion cell axons.
Characteristics
Higher Level Structure of Optic Nerve
The optic nerve is part of the central nervous system (CNS) and has a structure similar to other CNS tracts. The axons that form the optic nerve originate in the 10.1007/978-3-540-29678-2_7 and extend through the 10.1007/978-3-540-29678-2_15. As a tissue, the optic nerve has the same organization as the white matter of the brain in regard to its glia. There are three types of glial cells: oligodendrocytes, astrocytes and microglia.
Structural Regulation
Little structural and functional regeneration of the CNS takes place spontaneously following injury in adult mammals. In contrast, the ability of the mammalian peripheral nervous system (PNS) to regenerate axons after injury is well documented. A number of factors are involved in the lack of CNS regeneration, including: (i) the response of neuronal cell bodies against the damage, (ii) myelin-mediated inhibition by oligodendrocytes, (iii) glial scarring, by astrocytes, (iv) macrophage infiltration, and (v) insufficient trophic factor support.
Higher Level Process
The fundamental difference in the regenerative capacity between CNS and PNS neuronal cell bodies has been the subject of intensive research. In the CNS, the target normally conveys a retrograde trophic signal to the cell body. CNS neurons die because of trophic deprivation. Damage to the optic nerve disconnects the neuronal cell body from its target-derived trophic peptides, leading to the death of retinal ganglion cells (RGCs). Furthermore, the axotomized neurons become less responsive to the peptide trophic signals they do receive. The survival of certain types of CNS neurons depends on physiological activation of electrical activity or elevation of intracellular 10.1007/978-3-540-29678-2_3. On the other hand, adult PNS neurons are intrinsically responsive to neurotrophic factors and do not lose trophic responsiveness after axotomy [1].
Oligodendrocytes, which represent the myelinating glia in the CNS, carry on their surface axon growth-inhibiting molecules [2]. The hypothesis states that neurons in the CNS begin to lose their axonal regenerative capacity at roughly the period with the onset of myelination. Specific components of the myelin produced by the oligodendrocytes, such as 10.1007/978-3-540-29678-2_14 and 10.1007/978-3-540-29678-2_13, have been shown to inhibit axonal growth, and antibodies against these proteins resulted in axonal regrowth in the CNS.
The glial scar at the injury site is a biochemical and physical barrier to successful regeneration. It contains large numbers of reactive astrocytes, oligodendrocyte precursor cells, and CNS meningeal cells. A recent study suggests that injury-upregulated 10.1007/978-3-540-29678-2_2 synthesized within the CNS induces differentiation of astrocytes from neural progenitors, which may also contribute to glial scar formation after CNS injury [3]. The expression of repulsive molecules such as 10.1007/978-3-540-29678-2_19, 10.1007/978-3-540-29678-2_20, 10.1007/978-3-540-29678-2_14, 10.1007/978-3-540-29678-2_14, 10.1007/978-3-540-29678-2_16, 10.1007/978-3-540-29678-2_3 are related to the repulsive nature of glial scars [4]. The reactive glial extracellular matrix is directly associated with the failure of axonal regeneration, whereas the myelinated white matter beyond the glial scar is rather permissive for regeneration. Nevertheless, Moon and Fawcett [5] have shown that despite the reduction of scar formation by treatment with antibodies to 10.1007/978-3-540-29678-2_20, sufficient enhancement of spontaneous CNS regeneration was not obtained. There is no doubt that glial scars have a negative impact on CNS regeneration, although their precise contribution to the inhibitory nature of the CNS environment needs to be ascertained.
The injury is very slowly and poorly infiltrated by macrophages. The importance of macrophage infiltration is illustrated by the observation that it stimulates regenerative responses in the transected rat optic nerve axons [6]. However, microglial activation is considered to be a double-edged response. The first stage of activation includes a non-phagocytic state, where microglia become hypertrophic and produce molecules that are cytotoxic to neuronal cells, such as 10.1007/978-3-540-29678-2_20. However, microglia also release cytokines to promote regeneration, for example 10.1007/978-3-540-29678-2_20, to promote tissue repair by reducing astrocytic scar formation. In addition, trophic factors including 10.1007/978-3-540-29678-2_2 and 10.1007/978-3-540-29678-2_7, secreted by microglia, may also support regeneration.
Process Regulation
The ability of the mammalian PNS to regenerate axons after injury is well documented. Studies in the past decade have shown that the Schwann cell, one of the most important myelin components of the peripheral glia, plays a key role in regeneration. The proliferation and activation of Schwann cells leads to the production of various kinds of factors and other related molecules, to enhance the axons of the proximal nerve stump to grow through the distal stump. Activated Schwann cells express a variety of cell adhesion molecules including 10.1007/978-3-540-29678-2_14, 10.1007/978-3-540-29678-2_12 and their close homologues 10.1007/978-3-540-29678-2_3, N-cadherin and integrins, represented by 10.1007/978-3-540-29678-2_1 (α1β1 and α6β1-integrin), which mediate interactions between Schwann cells and axons, including growth cones. Besides these trophic factors and cell adhesion molecules, the Schwann cell supplies molecules to the extracellular matrix, such as 10.1007/978-3-540-29678-2_6, 10.1007/978-3-540-29678-2_12, 10.1007/978-3-540-29678-2_10 and 10.1007/978-3-540-29678-2_13, to the injured axons, which then extend their processes. Among these extracellular molecules, 10.1007/978-3-540-29678-2_12 is known to play an important role in establishing remyelination, since its absence in mice led to reduced compactness and delay of myelination [7].
Therapy
One strategy to elicit optic nerve regeneration is to provide a favorable environment by supplying neurotrophic factors and the transplantation of cells known to support axonal regeneration. Schwann cell is a strong candidate for transplantation, because optic nerve axons are known to regenerate, when the usual glial milieu is experimentally replaced by Schwann cells and/or peripheral nerve segments. Indeed, several experiments, involving CNS, have shown that exogenous supply of Schwann cells can improve axonal growth across the injured site [7].
Some cells such as gene-transfected astrocytes, 10.1007/978-3-540-29678-2_15, 10.1007/978-3-540-29678-2_5, differentiated embryonic stem (ES) cells, and neuronal stem cells, can induce elongation of CNS nerve fibers, however, it has not been established that the elongated nerve fibers are remyelinated. Many CNS axons are myelinated by oligodendrocytes. The optic nerve tract is a typical example. Myelinating cells, either of Schwann cell or oligodendrocyte origin, mediate the spacing of sodium channel clusters at the nodes of Ranvier to enable saltatory conduction, which is a prerequisite for normal neuronal activity and function. Therefore, even if the CNS can elongate its axons, remyelination of regenerated axons is indispensable for the re-establishment of CNS function.
Schwann cells myelinate in peripheral axons, they also remyelinate CNS axons when transplanted. They are “cells with a purpose” and amongst the best candidates for implantation to support CNS regeneration. Thus, it is expected that transplantation of Schwann cells could become a feasible clinical treatment in the future if the technical and surgical issues can be overcome.
In addition to Schwann cell implantation, various other approaches have been attempted, as mentioned above, but a single approach alone does not appear to provide an optimal condition for optic nerve regeneration. Instead, recent studies using combined approaches, for example, 10.1007/978-3-540-29678-2_3 with 10.1007/978-3-540-29678-2_14 [8], and CNTF with cAMP [9] have shown a synergistic effect on RGC axon regeneration. It is therefore suggested that combining various experimental approaches including neutralizing inhibitory molecules (e.g. 10.1007/978-3-540-29678-2_14 or 10.1007/978-3-540-29678-2_14), blocking inhibitory signaling pathways (e.g. Rho pathway inhibitor), supplementing appropriate neurotrophic factors (e.g. BDNF, 10.1007/978-3-540-29678-2_14 or CNTF), providing a favorable environment for axon regeneration (e.g. peripheral nerve graft or Schwann cells/ olfactory ensheathing glia transplantation), preventing scar tissue formation (e.g. 10.1007/978-3-540-29678-2_3), and elevating intrinsic regrowth capability (e.g. cAMP elevation), will help to provide the most favorable condition for optic nerve regeneration.
Regeneration of the Central Nervous System
Definition
Regeneration in general represents the replacement of lost body parts. Regeneration of the central nervous system (CNS) classically referred mainly to the re-growth of damaged neuronal axons. However, it has been realized that the replenishment of lost neural cells, and furthermore, the recovery of lost neural function, can be included in the concept of CNS regeneration. In fact, the attempt to recapitulate normal neural development has become a vital strategy for CNS regeneration.
Regionalization of the Vertebrate Central Nervous System
Definition
The vertebrate central nervous system first arises as a simple neural plate, which then forms a neural tube. The neural tube is divided into functionally and morphologically distinct regions. The first sign of regionalization in the central nervous system is the appearance of primary brain vesicles such as the prosencephalon, mesencephalon and rhombencephalon (Fig. 1).
Regionalization of the Vertebrate Central Nervous System. Figure 1.

Brain vesicles The fundamental brain plan is in the brain vesicles. Primary brain vesicles (prosencephalon, mesencephalon and rhombencephalon) are transformed into secondary brain vesicles. The fate of the brain vesicles is determined by a combination of expression of transcription factors. The anterior neural ridge and mes-metencephalic boundary function as signaling centers. Otx2 is expressed down to the mes-metencephalic boundary. Foxg1 is expressed in the prospective telencephalon. The di-mesencephalic boundary is determined by repressive interaction between Pax6 and En1/Pax2 and the mes-metencephalic boundary is determined by repressive interaction between Otx2 and Gbx2. The region where Otx2, En1 and Pax2 are expressed is the mesencephalon. Additional expression of Pax3/7 in the mesencephalic alar plate confers differentiation into the optic tectum. di diencephalons; mes mesencephalon; met metencephalon; pros prosencephalon; rhomb rhombencephalon; tel telencephalon.
As a result of the subdivision of the prosencephalon into the telencephalon and diencephalon and the rhombencephalon into the metencephalon and myelencephalon, five secondary brain vesicles are formed, which are the fundamental brain plan. The telencephalon differentiates into the cerebral cortex and nuclei. The diencephalon differentiates into the thalamus and hypothalamus. The retina, neurohypophysis and pineal body are also derivatives of the diencephalon. The mesencephalon differentiates into the optic tectum and tegmentum. The metencephalon differentiates into the cerebellum and the pons. The myelencephalon differentiates into the medulla oblongata.
The sulcus limitans divides the neural tube into the dorsal alar plate and the ventral basal plate (Fig. 2).
Regionalization of the Vertebrate Central Nervous System. Figure 2.

Neuromeres In the rhombencephalon, rhombomeres are formed, which are characterized by bulges and constrictions. Rhombomeres are compartments whose boundaries cells do not cross. In the prosencephalon, prosomere models are proposed. Zli is formed between p2 and p3 and functions as a signaling center. p1-p6 prosomere 1–6; r1-r6 rhombomere 1–6; zli zona limitans interthalamica.
Characteristics
Description of the Process
The fate of the region is determined by a combination of transcription factors expressed in the region. For the antero-posterior (AP) axis, boundaries function as organizing centers. Signals from the organizing center regulate expression of the transcription factors, thus regulating the fate of the adjacent region. For the dorso-ventral axis (DV), signaling centers are in the outside of the neural tube, the notochord and the dorsal midline ectoderm. Transcription factors that are homologous to those of Drosophila melanogaster are expressed in the vertebrate brain anlage and define the fate of the brain region.
Regionalization of the Prosencephalon
Otx1 and Otx2, homologs of orthodenticle (otd) of Drosophila, are expressed in the prosencephalon and mesencephalon. Expression of these genes differs with time; Otx2 is expressed from a very early stage whereas the Otx1 expression window is later. Otx2 plays a more important role in defining the region; Otx2 null mutant mice lack prosencephalon, mesencephalon and anterior rhombencephalon, although Otx1 null mutant mice show abnormality in the dorsal telencephalic cortex. Since Otx1 could be replaced by Otx2, it was suggested that the difference in the phenotypes of Otx1 and Otx2 null mutant mice stems from differences in expression patterns [1–3].
Emx1 and Emx2, homologues of empty spiracle (ems) are expressed in the telencephalon. These molecules may play a crucial role in arearization in the telencephalon, rather than defining the telencephalic region. Emx2 is expressed in a gradient, posterior high and anterior low. Fgf8 signal from the anterior and Wnt signal from the posterior (cortical hem) determine the pattern of Emx2 [1–3]. Wnt genes are homologs of Drosophila wingless (wg).
Foxg1 (BF1) is expressed in the telencephalon and defines the telencephalic region (Fig. 1). Six3 is expressed anterior to the zona limitans interthalamica (zli) and confers competence to express Foxg1 in response to Fgf8. Irx3 is expressed posterior to the zli and confers competence to express En1 and En2 in response to Fgf8. Six3, Irx and En are homologs of sine oculis, iroqois and engrailed respectively [4].
Puelles and Rubenstein had proposed that the prosencephalon consisted of six prosomeres (p1-p6), but then reduced it to four prosomeres [5] (Fig. 2).
P1 corresponds to the synencephalon, which is a prospective pretectum. P2 and P3 correspond to the parencephalon and are prospectively thalamus and prethalamus respectively. P4-P6 are the secondary prosencephalon, which gives rise to the telencephalon and hypothalamus. The zli is formed between p2 and p3 and functions as a signaling center [4–6]. P1-P3 are the epichordal part and P4-P6 are the prechordal part.
Regionalization of the Mesencephalon
The mesencephalon is characterized by a combinatorial expression of Otx2, En1 and Pax2 [7]. Otx2 is expressed down to the mes-metencephalic boundary (Fig. 1). The posterior limit of the mesencephalon corresponds to that of the Otx2 expression domain. Misexpression of Otx2 in the metencephalon changes the fate of the metencephalon to that of mesencephalon, i.e. the metencephalon differentiates into the tectum instead of the cerebellum after misexpression of Otx2. Otx2 knockout mice lack prosencephalon and mesencephalon. Misexpression of Gbx2, which is expressed in the metencephalon, causes an anterior shift in the posterior limit of the tectum. Fgf8, Pax2/5, En1/2 are in a positive feedback loop for their expression, so that misexpression of one of these molecules in the diencephalon activates the loop. Since Otx2 is intrinsically expressed in the diencephalon, misexpression of one of these genes changes the fate of the diencephalon to that of the mesencephalon [3,7,8]. Gbx2 is a vertebrate homolog of Drosophila unplugged and Pax genes contain a paired box, which was originally identified in the Drosophila paired gene.
Regionalization of the Rhombencephalon
The rhombencephalon is characterized by seven or eight swellings called rhombomeres (r) (Fig. 2). It was shown that the 10.1007/978-3-540-29678-2_14 in the hindbrain are 10.1007/978-3-540-29678-2_3 [2,6]. The spinal cord also shows a metameric pattern, which is characterized by motor nerves and dorsal root ganglia. Metamerism in the spinal cord is not however intrinsically formed, but is a reflection of the 10.1007/978-3-540-29678-2_19 of the somite [6]. It was shown that rhombomeres are true segments and form compartments whose boundaries are cell lineage restricting ones. Eph receptor tyrosine kinases and their ligands may be involved in lineage restriction. Receptors (EphA4, EphB2, EphB3) are expressed in odd-numbered rhombomeres (r3, r5) and their ephrin B ligands are expressed in the even numbered rhombomeres (r2, r4 and r6). The ephrin-Eph system is shown to produce repulsion, since the cells in the odd-numbered rhombomeres and those in the even-numbered rhombomeres do not intermingle [2]. Each rhombomere is characterized by a set of motor neurons. Orthologues of Drosophila Hox genes are expressed in an ordered and nested manner [2,6].
The identity of the rhombomere is determined by the combination of the expression of Hox genes [6]. Regulation of rhombomere identity by Hox genes has been shown by gain- and loss-of-function studies. Hoxb1 is uniquely expressed in r4. Some of the facial motor neurons that are produced in r4 migrate to r6 and vestibuloacoustic neurons migrate to the contralateral side in wild type mice. In Hoxb1-knock out mice, neurons produced in r4 do not migrate either to r4 or to the contralateral side, which suggests that the r4 is transformed to r2 in the mutant mice. On the other hand, misexpression of Hoxb1 in r2 changed its fate to that of r4.
Regulation of the Process by Signaling Centers
The fate of the brain vesicles is determined by a combination of transcription factors. Signals from the boundary regulate expression of the transcriptionm factors. The mes-metencephalic boundary (isthmus) was first recognized to function as a secondary organizer for the tectum and cerebellum [2,7,8]. This was first shown by ectopic transplantation of the brain vesicles. The alar plate of the diencephalon changed its fate and differentiated into the tectum when it was transplanted to the posterior part of the mesencephalon. The fate change did not occur in the anterior part of the mesencephalon. Transplantation of the isthmus to the diencephalon induced the tectum around the transplant, showing that the isthmus functions as the organizer.
Implantation of an Fgf8-soaked bead into the diencephalon mimicked transplantation of the isthmus, i.e. tectum was induced ectopically in the diencephalon by Fgf8 [7,8]. Furthermore, Fgf8 mutants in zebra fish and mice showed a disruption of the mes and r1. Later work all supported the idea that Fgf8 is a major organizing molecule in the isthmus (Fig. 1). Another secreted factor Wnt1 is first expressed widely in the mesencephalon and restricted to the posterior margin of the mesencephalon. Wnt1 null mutant mice show a severe deficit in midbrain and hindbrain. But later studies indicated that Wnt1 functions as a growth-accelerating factor. Fgf17 and Fgf18 have also been shown to function as growth promoting factors. Wnt1 is a homolog of Drosophila wingless (wg).
Among eight splicing isoforms of Fgf8, Fgf8a and Fgf8b are expressed in the isthmus. Misexpression by in ovo electroporation in chick embryos showed that Fgf8a changed the fate of the diencephalon to that of the mesencephalon and that Fgf8b changed the fate of the mesencephalon to that of the metencephalon. Since electroporation with a 1/100 dilution of Fgf8b expression vector exerted Fgf8a type effects, the difference in the effects of Fgf8a and Fgf8b may be due to difference in the intensity of the signal. A strong Fgf8 signal may activate the genes for cerebellar differentiation [7].
Signaling via FGF receptors, tyrosine kinase receptors (RTK), can activate the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K). Blocking of the Ras-ERK (MAPK) signaling pathway by the dominant negative form of Ras changed the fate of the metencephalon to that of the mesencephalon, i.e. the tectum developed instead of the cerebellum in the metencephalic region after misexpression of the dominant negative form of Ras. These results indicate that the strong Fgf8 signal activates the Ras-ERK pathway to cause differentiation into cerebellum [7]. Ras-ERK signaling is so strong that it may need negative regulators. Sprouty2, Sef (similar expression of Fgf8) and Mkp3 are induced by Fgf8, but regulate the pathway negatively. Sprouty2 is expressed overlappingly with Fgf8 and can be induced very rapidly by Fgf8 [7]. Repression of the Ras-ERK signaling pathway by misexpression of Sprouty2 changes the fate of the alar metencephalon to become the tectum. On the contrary, excess Ras-ERK signaling by application of dominant negative form of Sprouty2 results in an anterior shift in the mid-hindbrain boundary. Application of a specific inhibitor of the PI3K pathway indicated that this pathway is also activated by Fgf8 to induce Mkp3 and En2.
The anterior neural ridge also expresses Fgf8 and functions as a secondary organizer for the telencephalon. Fgf8 induces Foxg1 in the telencephalon (anterior to the zli), but induces En in the region posterior to the zli. It was shown that the difference in competence is dependent on the transcription factors expressed in the region. Six3 confers ability to express Foxg1 in response to Fgf8, whereas Irx3 confers ability to express En2 in response to Fgf8 [4]. Six3 is a homolog of Drosophila sine oculis.
The zli is another signaling center. There Shh is expressed and regulates the differentiation of the thalamic nuclei. Sox14 and Gbx2 are expressed in the young neurons of specific nuclei in the dorsal thalamus (Sox14: interstitial nucleus of the optic tract, perirotundic area; Gbx2: nucleus rotundus, posterior nucleus). High doses of Shh induce GliI, which in turn mediates expression of Sox14. On the other hand, low doses of Shh induce GliII, which in turn mediates expression of Gbx2 [4]. Shh (sonic hedgehog) is one of vertebrate homologs of Drosophila hedgehog, and Gli is the homolog of Drosophila Cubitus interuptus.
Regionalization Along Dorsoventral (DV) Axis
The floor plate and roof plate, which are situated at the ventral and dorsal midline respectively, segregate the bilateral halves of the neural tube. On each side, motor neurons differentiate in the ventral third, relay neurons in the middle third and smaller interneurons in the dorsal third. Pax3/7 and Pax6 are expressed in the dorsal and middle thirds respectively and Nkx2.2 is expressed in the most ventral part. Class II homeodomain proteins such as Nkx2.2, Nkx6.1, Nkx6.2 and bHLH transcription factor Olig2 are expressed in the most ventral part of the neural tube and Class I homeodomain (HD) proteins such as Pax6, Dbx2, Irx3 and Dbx1 are expressed dorsal to the Class II HD protein. Combination of these transcription factors defines the cell types along the DV axis.
Notochord was shown to have ventralizing activity. Implantation of the notochord lateral to the neural tube could induce floor plate and motor neurons near the implant. On the other hand, removal of the notochord results in extension of the dorsal markers to the ventral and motor neurons and the floor plate disappear. When notochord formation is genetically perturbed in mouse and zebra fish, ventral cell types are absent. For the ventralizing signal, Shh signaling was shown to play a crucial role. Shh is first expressed in the notochord, then the floor plate expresses Shh. Shh could elicit floor plate and motor neuron development ectopically. Centrally, Shh null mutant mice lack floor plate and motor neurons. Shh induces expression of ventral markers and the motor neuron marker islet1, but represses the ventral markers.
BMP4 and BMP 7 emanate from the roof plate and the dorsal ectoderm and antagonize the Shh signal. Thus, cell fate along the DV axis is determined by these signals.
For the DV axis in the mesencephalon, Pax3/7 are expressed in the alar plate of the mesencephalon and force it to differentiate as a tectum [7]. Shh is expressed in the floor plate of the mesencephalon. Misexpression of Shh in the mesencephalon represses Pax3/7 expression and changes the fate of the alar plate of the mesencephalon to the tegmentum (Fig. 1). After misexpression of Shh, motor neurons and dopaminergic neurons differentiate in the dorsal part of the mesencephalon.
Regulation of Neurotransmitter Release by Protein Phosphorylation
Definition
The release of neurotransmitters at 10.1007/978-3-540-29678-2_19 is brought about by the process of regulated 10.1007/978-3-540-29678-2_5, whereby a rise in the concentration of cytosolic Ca2+ triggers the fusion of a 10.1007/978-3-540-29678-2_19 with the plasma membrane and the release of the vesicle’s neurotransmitter content into the extracellular space. The proteins responsible for the sensing of the Ca2+ signal (synaptotagmin) and for vesicle docking and fusion (the SNARE proteins, SNAP-25, syntaxin and VAMP), have been identified and well characterized. In addition, several other proteins characterized through genetic approaches in flies, worms or mice are known to be either essential for neurotransmitter release (such as Munc13, Munc18-1 and NSF) or have important regulatory roles (such as cysteine string protein (CSP), Rabs and Rab effector proteins). In many neuronal cell types and various other kinds of secretory cells, exocytosis can be modulated through signalling pathways that result in phosphorylation of one or more of the key proteins involved in the exocytotic machinery [1]. The extent or kinetics of neurotransmitter release has been found to be modified by the action of several different protein kinases including PKA, PKC, Cdk5 and calmodulin-dependent protein kinase II (CaMKII). It has also been shown that protein phosphorylation by these, and other kinases, is required for various forms of 10.1007/978-3-540-29678-2_19. The changes in 10.1007/978-3-540-29678-2_14 that underlie synaptic modification are in part post- and in part pre-synaptic. Regulation of protein phosphorylation is important for presynaptic changes in neurotransmitter release which is, therefore, likely to be involved in learning and memory formation. Neurotransmitter release could be modified via phosphorylation of channels or receptors, but it is clear that components of the release machinery are direct targets for protein phosphorylation and these will be the focus of this review. The protein targets for the various protein kinases, and the particular amino acids within these substrates that are phosphorylated, are increasingly being identified, and this is allowing the physiological roles of specific phosphorylation events to be established. The strategy that is being used and is most informative is the expression of mutated forms of the proteins, in which the identified phosphorylated amino acid is rendered non-phosphorylatable (e.g. by changing serine to alanine) or phosphomimetic, by mutation of serine to the acidic amino acid glutamate or aspartate. We have concentrated here on proteins known to be important, based on genetic manipulation, as part of the exocytotic machinery for neurotransmitter release, for which the mutation strategy has defined a significant functional role for protein phosphorylation on a defined amino acid. Other presynaptic proteins that are substrates for protein phosphorylation are known [1], but their importance for neurotransmission has not yet been validated genetically.
Characteristics
Many aspects of neurotransmitter release can be modified following activation of protein kinases. These include an increase in release probability of vesicles, an increase in the size of the ready-releasable pool of synaptic vesicles, changes in Ca2+-sensitivity of the release mechanism or changes in the kinetics of individual fusion and release events [1]. This has led to the search for the protein substrates involved. Several key exocytotic proteins have been shown to be substrates for protein kinases in vitro, and some of these have been confirmed to be phosphorylated in intact cells in response to physiological stimuli. In even fewer cases has the phosphorylation of a specific protein been convincingly linked to one of the known effects on neurotransmitter release of activation of a specific kinase. Nevertheless, a number of examples of well defined regulation by protein phosphorylation are now known. The key presynaptic proteins involved in neurotransmitter release, which have been shown to be protein kinase substrates, are shown in Fig. 1, and the identified phosphorylation sites that are known are listed in Table 1.
Regulation of Neurotransmitter Release by Protein Phosphorylation. Figure 1.

Protein kinase substrates with established roles in the machinery for neurotransmitter release. Key synaptic proteins present on synaptic vesicles, the presynaptic membrane or the cytosol are shown, along with the protein kinases known to phosphorylate them in vitro. Only those proteins that have been confirmed through genetic approaches to be required for, or to regulate neurotransmitter release, are included. Proteins that are known to be phosphorylated in intact cells are indicated by asterisks.
Regulation of Neurotransmitter Release by Protein Phosphorylation. Table 1.
Identified protein kinase substrates involved in exocytosis and the kinases that phosphorylate them
| Protein | In vitro phosphorylation sites | In vivo phosphorylation sites | Functional significance tested? |
|---|---|---|---|
| CSP | PKA: S10 | S10 | Yes |
| Munc18-1 | PKC: S306, S313 Cdk5: T574 | S313 | Yes |
| Rabphilin 3A | PKA and PKC: S234, S274 | S234, S274 | No |
| Rim 1 | PKA: S413, S1548 CaMKII: S241, S287 (indirect) | S413 | Yes |
| SNAP-25 | PKA: T138 PKC: S187 | T138 S187 | Yes |
| Synapsin | PKA and CaMKI: S9 | As for in vitro | Yes |
| CaMKII: S566, S603 | |||
| ERK1: S62 | |||
| ERK2: S67 | |||
| MAPK and Cdk5: S549 | |||
| Cdk5: S551 | |||
| Synaptotagmin I | PKC and CaMKII: T112 | T112 | No |
| Syntaxin 1A | CK2 and ROCK: S14 DAPK: S188 | S14 | Yes |
Key synaptic proteins are listed that have been shown to be phosphorylated in vitro and whose phosphorylation sites have been identified. Only those proteins that have been confirmed through genetic approaches to be required for, or to regulate neurotransmitter release, are included.
We will concentrate on those proteins that have been confirmed to be important for neurotransmission through genetic approaches, and whose phosphorylation has been shown to be physiologically significant for exocytosis. Phosphorylation of several proteins has been linked to modification at various stages in the exocytotic process including vesicle mobilization (synapsins), vesicle recruitment into a releasable pool (RIM1), the maintenance of the ready-releasable pool size (SNAP-25) and late events during membrane fusion (CSP and Munc18-1).
The first presynaptic proteins whose phosphorylation was found to regulate neurotransmitter release were the synapsins, which have been extensively characterized both biochemically and functionally [1]. These proteins cross-link the reserve pool of synaptic vesicles to each other and to the cytoskeleton. Their phosphorylation by CaMKII following nerve terminal depolarization allows the release of the vesicles and, thereby, increases their availability for exocytosis. The functional significance of synapsins in the control of vesicle availability and recycling has been well established through the study of synapses from synapsin I and synapsin II knock-out mice.
A study using neurons from knock-out mice has shown that the Rab effector Rim1, which is localized on the presynaptic plasma membrane, is required to maintain the normal level of release probability in synapses and for 10.1007/978-3-540-29678-2_12 (LTP) at parallel fibre/Purkinje cell synapses of the cerebellum [2]. LTP at these synapses is dependent on presynaptic PKA. RIM1 is phosphorylated both in vitro and in vivo by PKA on Ser-413. The ability of this residue to be phosphorylated is necessary for the recovery of the wild-type phenotype when expressed in neurons from null mutant mice, as expression of non-phosphorylatable mutants was ineffective. In contrast, mutation of another putative PKA phosphorylation site, Ser- 1548 was without effect on the recovery in knock-out mice. This study suggests that phosphorylation of Ser-413 of RIM1 is a significant mechanism for the PKA-dependent plasticity that exists in certain types of synapses and that involves changes in neurotransmitter release. The mechanistic basis for the effect of RIM1 phosphorylation is, however, unknown.
SNAP-25 is one of the key SNARE proteins that associates with syntaxin and VAMP and mediates vesicle docking/fusion at the plasma membrane. Phosphorylation of SNAP-25 has been suggested to regulate the size of the ready-releasable pool of vesicles, based on data from studies on adrenal chromaffin cells [3,4]. SNAP-25 [5] can be phosphorylated both in vitro and in vivo by PKA and PKC on identified sites (Table 1), and this has been implicated in the functional effects of PKA and PKC activation on exocytosis. Activation of PKC has multiple effects on exocytosis, one of which is an increase in the rate of refilling of the ready releasable pool of vesicles. SNAP-25 is phosphorylated by PKC on Ser-187 and this reduces its association with other SNARE proteins. Expression of SNAP-25 in adrenal chromaffin cells with mutations in this residue either increased (phosphomimetic mutant) or impaired (non-phosphorylatable mutant) the rate of refilling of the vesicle pools [3]. This suggests that this effect of PKC activation could be through phosphorylation of Ser-187 of SNAP-25. In contrast, a study on hippocampal pyramidal neurons did not find any effect of mutating Ser-187 of SNAP-25 on neurotransmitter release, suggesting the existence of other functionally important PKC substrates that increase neurotransmitter release in hippocampal synapses. Neurotransmitter release can also be increased by activation of PKA. In addition, the tonic activity of PKA is linked to the maintenance of the pool of ready releasable vesicles in adrenal chromaffin cells, and this was revealed by the use of PKA inhibitors [4]. Expression of SNAP-25 mutated at Thr138, the identified PKA phosphorylation site, to produce a non-phosphorylatable mutant reduced the size of the initial fast burst of exocytosis in chromaffin cells, suggesting that this might be the target for PKA’s action on the ready releasable pool size. Phosphorylation of Thr138 has no effect on SNAP-25 binding to other SNAREs, although the effect on other protein interactions made by SNAP-25 is unknown.
CSP is a synaptic and secretory vesicle protein that has a chaperone role in the synapse. The phosphorylation status of CSP has been shown to affect late events in exocytosis that lead to changes in vesicle release kinetics and quantal size [6]. Overexpression of CSP in adrenal chromaffin cells was found to reduce the number of exocytotic events and also slowed vesicle release kinetics. CSP is phosphorylated in vitro on Ser-10 and this site was found to be phosphorylated in vivo. CSP phosphorylated on Ser-10 shows a reduced affinity for the syntaxin 1A and synaptotagmin I [6]. Expression of CSP with a mutation in Ser-10 (a non-phosphorylatable mutant) still reduced exocytosis but no longer modified the release kinetics. This suggests that Ser-10 is a target for protein phosphorylation, and that its phosphorylation can regulate neurotransmitter release through an effect on the time course of release from individual vesicles. It is currently unclear, however, whether the regulation of CSP is a consequence of PKA-mediated phosphorylation or phosphorylation by some other kinase that recognizes the motif at Ser-10.
Munc18-1 is a member of the Munc18/Sec1 family of proteins that function in essentially all intracellular membrane fusion events. It is essential for neurotransmission in mice and knock-out animals are paralysed and die in utero. Munc18-1 and its orthologues in other species appears to have both negative and positive functions exerted in part through its interaction with syntaxin. Munc18-1 is phosphorylated in vitro by PKC on Ser-306 and Ser-313, and by Cdk5 on Thr-574. Only phosphorylation on Ser-313 has so far been confirmed to occur in vivo [7]. Phosphorylation of Munc18-1 by PKC or mutation of Ser-306 and Ser-313 to glutamates reduces the affinity of Munc18-1 for binding syntaxin 1A. Significantly, expression of Munc18-1 with the phosphomimetic mutations in Ser-306 and Ser-313 mimics the effects of PKC in increasing the speed of single vesicle release events in chromaffin cells [8]. Another effect of PKC, to increase the number of exocytotic events was not observed as a consequence of expressing phosphomimetic Munc18-1, suggesting that another PKC substrate (SNAP-25?) must be involved in this effect of PKC. In contrast, expression of a phosphomimetic mutation of Munc18-1 at Thr574 only partially reproduced the effect of Cdk5 activation. The evidence suggests that Munc18-1 may be a physiological substrate for PKC, but the significance of the putative phosphorylation by Cdk5 is still unclear.
Phosphorylation of syntaxin 1A in vivo has been demonstrated and recently implicated in the exocytotic events that are involved in the insertion of new membrane in growing neuronal processes [9]. Syntaxin 1A was found to be the substrate for both casein kinase II [10] and the Rho-associated serine/threonine kinase (ROCK) [9], and was phosphorylated on Ser-14 by both kinases. This phosphorylation site was demonstrated to be functionally important as its phosphorylation increased the affinity of binding of tomosyn to syntaxin, and thereby inhibited its ability to form productive SNARE complexes. It was suggested that this would provide a mechanism for the spatial regulation of exocytosis. Other work has shown, however, that phosphorylation of Ser-14 of syntaxin increases its binding to synaptotagmin and its recovery in complexes with SNAP-25, which would be more consistent with a stimulatory effect on exocytosis. It is not known whether phosphorylation of syntaxin 1 does regulate neurotransmitter release.
The changes in neurotransmitter release that occur following phosphorylation of synapsin, RIM1, SNAP-25, CSP and Munc18-1 are also believed to be due to modifications in specific protein-protein interactions between these proteins and others in the exocytotic machinery. The molecular basis for the effects of phosphorylation, are in general still to be resolved. In particular, the effect of PKA phosphorylation of RIM1 and SNAP-25 on their protein-protein interactions is unknown. As noted above, more information is available on the molecular effects of other phosphorylation events. It is also possible that other substrate proteins could be crucial for mediating the protein kinase effects. We still have only limited knowledge of the significance of the phosphorylation of other exocytotic proteins, and the physiological conditions under which specific protein phosphorylation events occur and become relevant for changes in neurotransmission and synaptic plasticity. It is clear, however, that these are important mechanisms that contribute to learning and memory.
Regulatory Region
Definition
Regulatory region is a promoter, enhancer or other DNA sequence of a gene that is bound by transactivating factors that control gene expression. The best-studied regulatory regions are in DNA, but they also exist in RNA where they can be bound for example by micro RNA.
Regulatory Route
Definition
Regulatory route refers to the pathway whereby after synthesis in the rough endoplasmic reticulum, proteins are transported via the Golgi apparatus to be stored in granules, and exocytosed from granular storage.
Regulatory T Cells
Definition
A sub-population of T cells, which in their resting state bear markers, e.g. CD25, characteristic of activated T lymphocytes. These cells, which originate in the thymus, ensure peripheral tolerance of autoimmune T cells by a mechanism known to be characterized by cell-cell contact and cytokine secretion, but are not yet fully understood.
10.1007/978-3-540-29678-2_16
Reinforcement
Definition
This term was first used in classical conditioning by Pavlov to describe the process by which a conditioned stimulus (CS) came to substitute for the unconditioned stimulus (US) and thus elicit a conditioned response. Its current use in the classical conditioning literature is rather casual and denotes trials on which the CS is followed by the US in contrast to the term non-reinforcement which denotes trials where the CS is presented without the US.
Reinforcement is a core concept in operant conditioning. It has traditionally been used to refer to the process of strengthening a response or behavior. Positive reinforcement is often used to describe situations in which the occurrence of an instrumental response leads to a desirable outcome (or reward). Its counterpart, negative reinforcement, is used to refer to situations in which the occurrence of an instrumental response leads to either the removal or the postponement of an undesirable event, although, depending on the particular theoretical orientation, the terms escape and avoidance learning, respectively, are more likely to be employed.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_15
10.1007/978-3-540-29678-2_20
Reinforcement Learning in Neural Networks
Definition
An approach for training noisy networks based on increasing the likelihood of outputs that yield greater reward on average.
10.1007/978-3-540-29678-2_14
Reinforcement Learning in Animals
Definition
Reinforcement learning is a learning rule to search optimal value based on a reward signal, signifying to the organism which conditions are desirable and which are the undesirable ones.
Reinforcer
Definition
In associative conditioning theory, a reinforcer is an event that modifies the frequency of the behavior that preceded it. The term refers to operant learning (also called Skinnerian or instrumental learning), a form of associative learning in which animals learn to associate a behavioral action (for instance pressing a lever) to an outcome, either positive (a food reward) or negative (a punishment with an electric shock). Typically, the probability of the bar pressing response would increase if it is associated to the reward, but would decrease if it is associated to the punishment, a principle termed “the Law of Effect” by Edward Thorndike at the end of the 19th century. Note however, that the removal of a punishment can also act as a reinforcer: for instance, if bar pressing induces the end of a very loud noise, this behavior can increase because the end of the loud noise acts as a positive reinforcer.
Reinnervation
Definition
Return of lost nerve fibers (innervation) to a cell, tissue, organ.
10.1007/978-3-540-29678-2_14
Reinnervation of Muscle
Definition
The nerve supply to denervated muscle fibers can be restored by reinnervation; injured nerve fibers regrow their axons to reach and resupply or reinnervate the denervated muscle fibers.
10.1007/978-3-540-29678-2_1
Reinstatement
Definition
The return of a conditioned response following re-exposure to the unconditioned stimulus after extinction training.
10.1007/978-3-540-29678-2_12
Relation
Definition
In the basic binary case, a relation R is a rule specifying when an object a is related by R to b. In an abstract mathematical sense, a binary relation is simply the set of ordered pairs (a,b) upon which it holds. The domain of the relation is the set of a which are related to some b.
The range of the relation is the set of b which are related to by some a. The relation is reflexive if every object a is related to itself. The relation is symmetric if whenever a is related to b, then b is also related to a. The relation is transitive if whenever a is related to b, and b is related to c, then a is related to c. An equivalence relation is a relation that is reflexive, symmetric and transitive.
A relation f is a function if to each a in its domain, there is exactly one b to which a is related, and this b is said to be the value of the function at a, written b = f(a).
A function is one-to-one if whenever a1 and a2 are distinct, then so also are f(a1) and f(a2). The function is onto B if every object in B is in the range of the function.
A one-to-one correspondence between A and B is a oneto- one onto function with domain A and range B. That is, a one-to-one correspondence provides a way of matching objects in A to objects in B in such a way that every object in A corresponds to a unique object in B and every object in B is corresponded to by a unique object in A.
The concept of binary relation can be generalized to trinary relations, which holds of triples (a,b,c), and so on to any dimension, even to infinite dimensions.
10.1007/978-3-540-29678-2_16
Relational or Configural Navigation Strategy
Definition
Behavior relying on an allocentric reference frame and oriented by an internal spatial representation.
10.1007/978-3-540-29678-2_19
Relative Pain Unpleasantness
Definition
The amount of pain unpleasantness associated with a specific intensity of a pain sensation. It is a measure of how much a specific pain sensation bothers an individual. Equivalent intensities of pain may vary in unpleasantness, such as laboratory pain versus the pain of childbirth, or the pain of childbirth versus late stage cancer pain.
10.1007/978-3-540-29678-2_5
Releasing Values
Definition
Each stimulus has a certain attractiveness and may elicit a certain behavior. The attractiveness is measured by the releasing value. The natural, adequate stimulus has a releasing value of 100. Many stimulus have a lower releasing value, but some may have higher or supernormal releasing values.
Releasing-Hormone and Release-Inhibiting Hormone
Definition
These chemicals (mostly peptides) are produced by specific cells (neurosecretory cells) situated mainly in the hypothalamus and transported to the anterior pituitary gland. There they stimulate or inhibit a release of various anterior pituitary hormones. They include thyrotropin (TSH)-releasing hormone (TRH), which also acts as prolactin-releasing factor (PRF); adrenocorticotropin (ACTH) – releasing hormone (CRH); growth hormone (GH) – releasing hormone, (GHRH); growth hormone release-inhibiting hormone (somatostatin); gonadotropin (GnH) – releasing hormone (GnRH), sometimes called luteinizing-hormone (LH)- releasing hormone (LHRH), and prolactin release-inhibiting factor, now considered to be dopamine.
10.1007/978-3-540-29678-2_8
10.1007/978-3-540-29678-2_8
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_8
10.1007/978-3-540-29678-2_8
10.1007/978-3-540-29678-2_16
Reliabilism
Definition
Reliabilists claim that knowledge is true belief arrived at in a reliable manner, i.e. in a manner that makes it likely that the resulting belief is true.
10.1007/978-3-540-29678-2_11
REM
Definition
Rapid Eye Movement Sleep.
10.1007/978-3-540-29678-2_5
REM-off Cells
Definition
Extracellular single-unit-recording studies show that many neurons discharge at their highest rates (<5 spikes/sec) during waking, diminish their activity during non-REM (NREM) sleep, and become silent during REM sleep. Since these cells stop firing during REM sleep, they are called REM-off cells (also called PS-off cells). The majority of these REM-off cells are located in the locus coeruleus (LC) and raphé nuclei (RN). REM-off cells located in the LC contain the neurotransmitter noradrenaline and REM-off cells located in the RN contain the neurotransmitter serotonin. Although few in number, this type of cell is also present in the caudal part of the pedunculopontine tegmentum (PPT) and laterodorsal tegmentum (LDT).
10.1007/978-3-540-29678-2_12
10.1007/978-3-540-29678-2_14
10.1007/978-3-540-29678-2_14
Rapid Eye Movement (REM) Sleep
10.1007/978-3-540-29678-2_19
REM-on Cells
Definition
Neurons that exhibit increases in extracellularly recorded discharge rate during rapid eye movement (REM) sleep, rather than during waking and non-REM (NREM) sleep. This population of neurons is characterized by a progressively increasing mean tonic discharge rate as the animal moves from wake to NREM sleep and finally to REM sleep. This type of cell is mostly located in the pontine reticular formation, pedunculopontine tegmentum (PPT) and laterodorsal tegmentum (LDT). REM-on cells located within the pontine reticular formation contain the neurotransmitter glutamate and REM-on cells located in the PPT and LDT contain the neurotransmitter acetylcholine.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_7
10.1007/978-3-540-29678-2_14
REM Sleep
Definition
REM Sleep Behavior Disorder (RBD)
Definition
A parasomnia (a disorder involving abnormal behavior during sleep) characterized by the acting out of vivid, sometimes violent, confrontational or belligerent dreams during REM sleep. This happens because the REM-related atonia of voluntary muscles is lacking, allowing the muscles to move during dreaming. REM behavior disorder causes sleep disruption and potential injury to self or to others (e.g., a bed partner).
10.1007/978-3-540-29678-2_1
Remapping in Hippocampus
Definition
Remapping is the process of replacing one map representation with another. A representation is “remapped” when the map elements are scrambled. In the hippocampus, changing environments, or contexts, is associated with remapping.
10.1007/978-3-540-29678-2_19
REMO
Definition
Episodic retrieval mode; a component process of episodic retrieval that is required for remembering earlier experiences.
10.1007/978-3-540-29678-2_8
Remote Memory
Definition
The long-term representation of information that was learned months to years earlier.
10.1007/978-3-540-29678-2_13
Remyelination
Definition
Myelin sheaths are formed by Schwann cells in the peripheral nervous system, and by oligodendrocytes in the central nervous system. If myelin sheaths are degraded due to injury or pathological changes, new myelination develops on the surviving axons. In the central nervous system, remyelination is usually incomplete with a reduced number and irregular configuration of myelin lamellae over a long period of time.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_15
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_2
10.1007/978-3-540-29678-2_1
Renshaw Cell
Definition
Renshaw cells are inhibitory interneurons (using glycine and GABA as transmitters), which are located in the ventral horn of the spinal cord, receive their main excitatory inputs from collaterals of motoneurons and mediate recurrent inhibition of motoneurons, Ia inhibitory interneurons, Renshaw cells and cells of origin of the ventral spinocerebellar tract. Other inputs to Renshaw cells arise from sensory afferent fibers and tracts descending from supraspinal structures.
10.1007/978-3-540-29678-2_9
Repetition Maximum (RM)
Definition
Repetition Maximum represents the load used in resistance training. Performing sets of ten repetition maximum (RM) loads or less are typically used for resistance training, with one RM being the maximum weight an individual can lift once, and ten RM being the weight an individual can lift exactly ten times. These values represent 100% and ~70% of maximum capability for one RM and ten RM, respectively.
10.1007/978-3-540-29678-2_13
Replacement Neuromast
Definition
A superficial neuromast (hair cell of the lateral line system) having phylogenetic continuity with a neuromast found inside a canal in other taxa. This superficial configuration is most likely due to retarded development of canals in that taxon.
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_14
Repolarization
Definition
Repolarization is the return of membrane potential to its resting value. The term refers mostly to repolarization of the action potential, although more generally it also means the return to a more negative value after (forced) depolarization. Repolarization of the action potential is often carried by the outward flux of potassium ions mainly through delayed rectifying, voltage-gated potassium channels.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_14
Report
10.1007/978-3-540-29678-2_6
Representation (Mental)
Definition
The term “mental representation” is sometimes used to cover any mental item which is semantically evaluable, i.e. which has content, 10.1007/978-3-540-29678-2_20, refers to something, possesses 10.1007/978-3-540-29678-2_20, or is about something. Under this broad construal its extension includes beliefs, thoughts, memories, desires, perceptions and all other mental phenomena exhibiting the feature of intentionality.
But there also is a narrower construal of “mental representation” closely associated with the agenda of cognitive science. Under this narrower construal, mental representations are certain theoretical entities, i.e., semantically evaluable particulars which are postulated by classical or other types of cognitive architectures in order to explain processes and states which count as mental representations only in the broad sense.
Description of the Theory
Mental representations as theoretical entities postulated by cognitive scientists come in very different shapes. Think of a cognitive scientist working in the paradigm of classical architectures. Insofar as she tries to understand the mind as a complex system that receives, transforms and stores information, that is, as a complex symbol-manipulating system, her approach is based on the idea that mental phenomena should be explained by postulating mental representations (symbols). Or think of a cognitive scientist working in the paradigm of 10.1007/978-3-540-29678-2_3. Insofar as he tries to understand human behavior and mentality as based on the activity of neural networks, his approach, too, is based on the idea that mental phenomena should be explained by postulating mental representations, although these are notably different from those of classical architectures.
Mental representations as theoretical entities postulated by cognitive scientists come in great variety including, e.g., activity vectors in connectionist networks, Marr’s 2½ -D sketches in his theory of vision, Kosslyn’s mental images, the mental models of Johnson-Laird, or Fodor’s “sentences in the language of thought” [1]. Ironically, most mental representations are misleadingly labeled “mental” representations, for they are explicitly understood as certain neuronal or other physical structures. But certainly, they all qualify as mental in the weak sense that they are postulated in order to explain mental features. The mental features to be explained range from pattern recognition to intentional behavior, but in the following we will concentrate on the so called propositional attitudes like believing or desiring that something is the case. Generally speaking, propositional attitudes are mental states which can be ascribed with the help of “that”-clauses.
Representation
Representations are, of course, ubiquitous: There are words, photographs, paintings, maps, traffic signs, diagrams, graphs, music notes, X-ray photographs, digital images, and much more. They are not bound to a specific medium or syntax, and there are virtually no limits as to which things can represent which. A representation, as a self-representation, can represent itself, and two different things can represent each other. Representation should be distinguished from mere information, at least if the latter is taken to include things like the universe containing information about the big bang or the smoky sky containing the information that there is a fire. The idea behind distinguishing representation from information is that information cannot be false (otherwise it would not be information at all), whereas misrepresentation is possible. If this is correct, smoke does not represent fire, although it “indicates” or “means” fire [2]. It is notoriously hard to spell out precisely the necessary and together sufficient conditions which make something a representation, but there is a kind of consensus that every representation purports to stand for, denote, refer to, or be about something. Another important aim of philosophical thinking about representation is to build a useful classification of the many different forms of representation (see [3] and [4] for two very influential classificatory schemes).
Representational Theory of Mind
The best-known representational theory of propositional attitudes is developed by Fodor [1]. It is a paradigm instance of a 10.1007/978-3-540-29678-2_3 in cognitive science, and is best seen as an attempt to explain how propositional attitudes and reasoning processes can be physically realized. Strictly speaking, this is a two-step enterprise: The first step is concerned with the question how propositional attitudes and cognitive processes can be realized by computational relations and processes in which symbols are manipulated. The second step consists in explaining how these computational relations and processes can in principle be physically implemented. Fodor thinks that the second step is already established by the theoretical work of Turing and others and, of course, by the actual development of computers. Therefore, he sees his main task in making intelligible how propositional attitudes can be realized by computational relations. The central features of propositional attitudes which are to be explained include the following: They are (i) semantically evaluable, (ii) causally efficacious, and (iii) opaque.
In order to account for these features, Fodor does several things. First, he assumes that there are mental representations. These are held to be sentence-like physical structures in a “language of thought.” This means four things: Like sentences mental representations have propositional content; like sentences they are structured entities which have parts that themselves possess meaning; like sentences they have a compositional semantics, i.e., their meaning is a function of the meanings of their parts and the order of these parts; and these parts are “transportable” which means that the same parts can appear in many mental formulas (Fodor [1]: 137). Fodor calls the conjunction of these claims the Language of Thought Hypothesis. Second, Fodor then uses these views to formulate the representational thesis according to which propositional attitudes are relations between organisms and mental representations. For example, to believe that grass is green means, according to the representational thesis, to stand in a certain relation (the belief-relation) to a mental representation which means that grass is green. More generally, for any organism O, and any attitude A toward the proposition P, there is a computational relation R and a mental representation M such that (i) M means that P, and (ii) O has A if and only if O bears R to M (Fodor [1]: 17). That an organism bears a certain computational relation to a mental representation M is spelled out in the following way: The representation M occupies a certain causal or functional role in the organism; i.e. it is tokened in a special functionally defined area (e.g. the “belief-box” or the “desire-box”) and will be manipulated in a specific way. Third, Fodor claims that mental processes are causal sequences of tokenings of mental representations. This is best conceived as the view that causal relations between propositional states rely on computational processes that are sensitive to the structure of the involved mental representations.
This theory neatly explains the central features of propositional attitudes mentioned above as follows. (i) That propositional attitudes are semantically evaluable is accounted for by the fact that they are realized with the help of mental representations which are semantically evaluable. (ii) The deeper point in connection with second feature (causal efficacy) is this: Often causal relations between propositional attitudes contrive to respect their relations of content. For example, my thoughts that p and that (if p, then q) often cause me to think that q. This is explained by the fact that cognitive processes are computational processes which are structure-sensitive. This sensitivity to the syntax of mental representations is enough to explain the possibility of the parallel structure of logical and causal relations, because, as is well-known from logic, logical relations can be characterized syntactically. (iii) The belief that p and the belief that p* can be different beliefs, such that it is possible to believe that p without at the same time believing that p* (and vice versa), even when p and p* are both true or both false (or even possess the same truth-conditions). This feature of 10.1007/978-3-540-29678-2_15 can be explained by the representational theory under the assumption that the mental representations of p and p* are syntactically different. Because syntactically different representations are typically manipulated in different ways, it is no mystery how at a certain time, there can be the mental representation p, but not the mental representation p* in someone’s belief-box.
Main objections to Fodor’s representational theory concern two issues. The first is the issue whether there is empirical evidence against its implication that causally efficacious attitudes require actual tokenings of mental representations. The second is the issue whether the assumption that there are physical structures which have semantic content can be made plausible at all. This is the topic of the next section.
Physical Structures as Mental Representations
The representational theory as outlined above simply assumes that mental representations have a semantic content. Therefore, it remains another task for its proponents to explain how these representations being neural or physical entities can be semantically evaluable at all: How can physical or neuronal structures actually represent some state of affairs? This matter is not only of interest to the proponents of the representational theory, but is also of crucial interest for anyone else who takes a realistic stance on mental representations. Over the last two and a half decades, philosophers have developed several approaches to answer this question [1,5,6].
Dretske’s information-theoretic approach is rendered in terms of information, and analyzes the property of having semantic content as a form of carrying information: A certain structure S (e.g. a representation) has the semantic content that p if and only if S carries the information that p and the information that p is the most specific piece of information which S carries, i.e. S carries no other piece of information in which the information that p is nested (see Dretske [5]: 177). That S carries information about something X at all basically means that there is a certain causal correlation between S and X. The attractiveness of this approach lies in the fact that in principle it is no mystery how physical structures (rocks, clouds, or brains) can carry information. In order to explain how misrepresentation is possible, Dretske appeals to the learning period in which a representation is acquired. In a nutshell, the explanation is that only causal correlations during the learning period determine what S represents, whereas after the learning period S can be caused by different things which, then, are misrepresented by S. This information-theoretic account faces two major problems: (i) it only works for representations which are acquired through an individual learning history, and (ii) it cannot deal with the 10.1007/978-3-540-29678-2_4 (Fodor [1]: 101f). The latter problem is a fundamental problem for every broadly causal account of meaning or content: If the contents of A-representations are determined by their being caused by C-states, but sometimes A’s are brought about by some other cause C*, then how can the causal theory account for the difference between the case in which A’s represent C, but sometimes are caused by C*-states, and the case in which A’s represent the disjunction C-or-C*? Dretske’s appeal to a learning period would only be of help here if it were guaranteed that during this period all and only those factors cause A’s which should enter into the semantic content of A’s. But this seems to be false for empirical reasons.
Teleological accounts try to solve the disjunction problem by appeal to the biological function or purpose of representational states [6]. According to them, the mental representation R, although sometimes caused by dogs, represents wolfs and not wolfs-or-dogs, because it is the biological function of R-type representations to indicate wolfs and not wolfs-or-dogs. A major difficulty for this type of accounts is to explicate the notion of biological function in a non-semantic way. This is typically tried to be accomplished by an appeal to the (evolutionary) history of the organism. But it has turned out over the years that this is by far no easy task. Another problem lies in the fact that teleological accounts which appeal to evolution have counterintuitive consequences. For example, most people have the intuition that a perfect physical and behavioral duplicate of a human being would also have beliefs and desires. But if this duplicate has the wrong kind of history (or literally no history at all), it is thereby precluded from having mental representations. This arguably leads to an epistemological difficulty as well: If it is the evolutionary history of an organism which determines whether and which things are represented by its inner states, we can only know whether someone believes something if we know enough about its evolution.
Fodor [1] developed a third type of account which is based on the notion of asymmetric dependence. It runs along the following lines: A-states (of an organism O) represent C if and only if (i) under optimal conditions all C’s cause an A-token, and (ii) all A-tokens which are caused by a state C* are asymmetrically dependent on the causation of A-tokens by C’s. The idea behind Fodor’s notion of dependence can be caught by a question: Would the C*-state also have caused A-tokens if C-states did not cause A-tokens? If not, the causal relation between C*-states and A-tokens is dependent on the relation between C-states and A-tokens. This dependence is asymmetrical if it is not the other way round, i.e. if it is not true that the relation between C-states and A-tokens is dependent on the relation between C*-states and A-tokens. Although this is an ingenious proposal, as some philosophers have pointed out, it might be entirely misguided. Let us assume that tokens of A in an organism O mean “bird,” but sometimes are caused by big insects. According to Fodor’s proposal, this is the case because big insects would not cause A’s if birds did not cause A’s in O. But, now, what is it that precludes big insects to cause A’s in O even if there were no birds around? Why, in other words, should there be an asymmetric dependence relation at all? Certainly, if A were to mean “bird” in the first place, it would be quite plausible that some big insects cause A’s in O only because normally birds cause A’s in O. But that A means “bird” is exactly what Fodor’s theory tries to explain and, therefore, cannot be assumed by it.
Intentional Realism Versus Eliminativism
The representational theory of propositional attitudes and the project of naturalizing mental representations as sketched in the last section are committed to 10.1007/978-3-540-29678-2_9. Intentional Realism is the view that humans have intentional states which (i) more or less obey the laws of folk psychology and which (ii) have a semantic content that is (iii) causally efficacious. But these assumptions can, of course, be denied. Most prominently some philosophers favor an eliminative stance towards propositional attitudes. Churchland [7] argues that folk psychology is a rather unsuccessful theory which in the long run will be substituted by a much better explanation of human behavior developed by scientific psychology or neuroscience which is incompatible with the folk assumptions. But because intentional states like beliefs and desires are only theoretical entities postulated by folk psychology, Churchland argues, they will be eliminated when folk psychology is abandoned.
Intentional Realism and Eliminative Materialism are opposing views on the ends of a spectrum and there are positions in-between. A very prominent one is Dennett’s [8]. On the one hand, Dennett agrees with the Eliminative Materialists that there are in principle neuroscientific explanations of human behavior which are superior and incompatible with 10.1007/978-3-540-29678-2_9. On the other hand, he stresses that we cannot abandon intentional explanations altogether, because there are certain patterns in human behavior which can only be discovered from the intentional stance. Whether this or other “in-between” positions (as [9,10]) can be coherently defended is difficult to evaluate. What they all try to show is that the semantic contents of intentional states are real enough to underpin the autonomy of intentional explanations, but at the same time are not real enough to require their physical implementation. This can be put it in another way which perhaps is an exaggeration: These approaches aim to preserve mental representations in the broad sense (intentional states) without committing themselves to the existence of mental representations in the narrow sense. Faced with the empirical and conceptual problems of Intentional Realism and the smell of absurdity of eliminativism, this may be an attractive direction of inquiry for further theories of mental representation.
Repressed Memories
Definition
Memories for traumatic experiences that the mind supposedly banishes from conscious awareness due to their threatening nature. According to this theory, once repressed, these memories may return to consciousness.
The resulting memories are thought to be accurate in detail, and involve processes that are different from ordinary forgetting and remembering. Credible scientific support is lacking for these notions.
10.1007/978-3-540-29678-2_13
Repressor
Definition
A transcription factor that negatively regulates the expression of a gene.
Reproduction
Definition
Production of offspring.
Reproductive Organs
10.1007/978-3-540-29678-2_22
Reptilia
Definition
The amniote clade incorporating the last common ancestor of turtles, lizards, crocodiles and birds, and all descendents of that common ancestor.
10.1007/978-3-540-29678-2_16
Repulsive Guidance Molecule
Definition
The molecule by which growth cone movement is repelled.
10.1007/978-3-540-29678-2_1
RER
Definition
Res Cogitans
Definition
Latin phrase meaning “thinking thing,” introduced by Descartes to refer to the mind as opposed to the body (the res extensa or “extended thing”).
10.1007/978-3-540-29678-2_5
Rescorla-Wagner Model
Definition
This model of classical conditioning attributes variations in the effectiveness of conditioned stimulus-unconditioned stimulus (CS-US) pairings to variations in US processing. The model asserts that an US must be surprising for learning to occur. An US is defined as surprising if the discrepancy term (λ − VT) is different from zero. This discrepancy reflects the difference between the maximum conditioning the US can support (λ) and the associative strength of all the stimuli on the trial (VT). The equation for calculating a change in the associative strength of a CS is:
 |
where VT represents the total or sum of the individual associative strengths of all CSs present on that trial; α and β are fixed rate parameters (values from 0 to1) determined by the salience (physical properties) of the CS and US, respectively; λ is the maximum conditioning that the US can support.
10.1007/978-3-540-29678-2_20
Resetting
Definition
Alteration of a circadian rhythm such that it occurs earlier (advance) or later (delay) than predicted in subsequent cycles.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_3
Residual Brain Cells
Definition
Astrocytes, microglia and neurons are the residual brain cells. Along with cells of the immune system which migrate towards the brain in CNS disorders, these also play an important role in the etiology of these disorders.
10.1007/978-3-540-29678-2_3
Residual Hearing
Definition
The amount of hearing left after hearing loss.
10.1007/978-3-540-29678-2_8
Residual Schizophrenia
Definition
Constellation of symptoms which often occur after many years of a chronic course. Beside psychotic symptoms, patients suffer from symptoms of general cognitive impairment and affective flattening.
10.1007/978-3-540-29678-2_19
Resistance (Electrical)
Definition
Resistance (electrical) is the reciprocal of conductance and a measure of the resistance of an object to electric current flow.
10.1007/978-3-540-29678-2_15
Resistance Training
Definition
Resistance training or strength training can be defined as progressively overloading the neuromuscular system using near maximal muscle contractions against high resistance. Its purpose is to increase the ability to perform maximal contractions and increase muscle size.
10.1007/978-3-540-29678-2_13
Resonance
Definition
The frequency at which maximum output occurs.
10.1007/978-3-540-29678-2_8
Respiration — Neural Control
Synonyms
Respiratory system neurophysiology; Neuroanatomy and neurotransmitter/neuromodulator control
Definition
The neural control of respiration refers to functional interactions between networks of neurons that regulate movements of the lungs, airways and chest wall and abdomen, in order to accomplish (i) effective organismal uptake of oxygen and expulsion of carbon dioxide, airway liquids and irritants, (ii) regulation of blood pH.
Introduction
The neural control of respiration is still not completely understood, although remarkable progress has been made as instrumentation, technology and analytical procedures continue to improve at an accelerated pace. (Many excellent reviews are available that have followed progress in the field, and the interested reader is encouraged to consult them for particular areas of interest [1–14,16–18,20,21,23–26,28–31,33].)
It is axiomatic that biological cells are dependent on respiration for survival, proper function and 10.1007/978-3-540-29678-2_8. They require an efficient transport system that provides oxygen (O2) for aerobic metabolism and energy production and for extrusion of its end products, carbon dioxide (CO2) and water.
In mammals respiration takes on an additional, organismal meaning and significance, synonymous with ventilation; i.e., the act of breathing ambient air in and out to deliver O2 from the mouth and nasal passages to the lungs, and to transport CO2 from lungs to atmosphere.
The Respiratory Apparatus in Mammals
Effective organismal exchange of O2 and CO2 in mammals requires finely coordinated interactions between the organs of breathing and the 10.1007/978-3-540-29678-2_3. The latter consists of the heart acting as the pump for blood in which O2 and CO2 are dissolved and the vascular network, from capillaries to major arteries and veins, which serves as the transport line between the lungs and other O2-consuming, CO2-producing organs. A system of valves in the heart and in the sphincters surrounding arterioles regulates the flow of blood.
The organs of breathing (Fig. 1) can also be subdivided into pump and flow components. The pump machinery consists of the diaphragm, chest wall and abdominal muscles, while the transport system involves the mouth, nasal passages, bronchi, bronchioles and lungs. In the lungs, the alveoli, a vast network of air-filled sacs, are in intimate contact with blood capillaries where exchange of O2 and CO2 takes place. Airway resistance and airflow in and out of the lungs is affected by altering the tone of bronchiole smooth muscle, pharyngeal skeletal muscle, nasal musculature, as well as tongue position and the tone of the laryngeal (vocal fold) abductor and adductor muscles.
Respiration — Neural Control. Figure 1.

The respiratory apparatus. Left side, muscles that expand the chest for lung inflation during inspiration are illustrated. Arrows show the upward direction of rib movement. The middle upper segment illustrates the airways, from nasal passages to lungs. A cross section through the larynx shows the laryngeal folds in an open (abducted) state during inspiration. The middle lower segment shows the diaphragm, which contracts downward to inflate the lungs during inspiration. Phrenic nerve branches that innervate the diaphragm and cause the musculature to contract are also seen. Right, muscles of the chest wall and abdomen that contract to aid lung deflation during active expiration. Arrows show the direction of rib and abdominal muscle movements. Modified from [19].
Performance of the Respiratory Apparatus During Inspiration and Expiration
During inspiration, airflow into the lungs and alveoli is produced as the diaphragm contracts during periodic discharges of the phrenic and inspiratory intercostal nerves (Fig. 2); the discharges are activated by excitatory synaptic drive within the 10.1007/978-3-540-29678-2_2-spinal cord respiratory network. Contraction of the diaphragm changes its configuration from dome shaped to relatively flat. This increases the intrathoracic volume, resulting in an increase of negative intrapleural pressure that promotes lung inflation and inward airflow from the mouth to the lungs. Discharges in intercostal nerves contract the inspiratory muscles of the rib cage, moving the ribs upward and outward to further increase intrathoracic volume and inward airflow. (10.1007/978-3-540-29678-2_19) Movement of air into the lungs is further supported by cranial 10.1007/978-3-540-29678-2_13 discharges (10.1007/978-3-540-29678-2_1) that reduce upper airway resistance by contracting the muscles of the nasal passages and pharynx, move the tongue forward in the mouth (Respiratory control of hypoglossal motoneurons during sleep and wakefulness) and dilate the vocal folds by contracting abductor laryngeal muscles.
Respiration — Neural Control. Figure 2.

Discharges of the phrenic and laryngeal nerves (A) and changes in lung volume and airflow (B) during one respiratory cycle. In part B, I = inspiration, PI = post-inspiration, E = expiration. Part A adapted from Bianchi et al. 1995 [2]. Part B adapted from [24].
Until the end of the inspiratory phase, phrenic and inspiratory intercostal nerve discharges progressively increase, causing a gradual flattening of the diaphragm and expansion of the chest wall.
At the end of the inspiratory phase, phrenic, inspiratory intercostal and abductor laryngeal motoneurons stop discharging. A very brief silent period is followed by resumption of discharges that is less intense and declining as it progresses. During this transitional stage, referred to as either the postinspiratory or early expiratory phase, adductor motoneurons of the superior laryngeal nerve also discharge with declining intensity. The decrementing phrenic and laryngeal nerve discharge patterns result in a more gradual relaxation of the diaphragm, a reduced rate of outward airflow and thus a slowing in the rate of lung deflation. Alveolar collapse is opposed and functional residual capacity (FRC) is maintained, which has beneficial pulmonary consequences. Normally, only about 15% of air in the lungs is replaced by new air during each normal inspiration, and about the same amount of old air is expired. The slow replacement of air prevents sudden changes in blood gases that would destabilize respiratory control. Excessive increases and decreases of blood pO2, pCO2 and pH are also prevented when respiration is temporarily interrupted, for example during swallowing or phonation. Partial inflation during a normal FRC also maintains surfactant release and thus lung compliance, because the principal stimulus for liberation of surfactant appears to be direct mechanical distortion of type II alveolar cells [35].
Expiration during quiet breathing is mainly passive. Discharges of inspiratory cranial, phrenic and inspiratory intercostal nerves are silenced by synaptic inhibition in the brainstem and spinal cord. The chest wall and diaphragm return to their resting configurations and airway patency is maintained to allow passive outward airflow from the lungs to the atmosphere.
During active expiration, for example during exercise or coughing, discharges of the internal (expiratory) intercostal nerves move the lower ribs downward and inward. In addition, lumbar motoneuron discharges contract the abdominal muscles and compress the abdominal contents, pushing up the diaphragm and actively expelling air from the lungs. Upper airway patency is maintained by discharges of laryngeal abductor nerves and pharyngeal constrictor nerves.
The cycle of inspiration, postinspiration and expiration in the adult human is repeated, on average, about 15 times during quiet breathing.
Respiratory Muscles Contract in an Ordered Sequence that Optimizes Mechanical Advantage
The inspiratory pump muscles in both humans and quadrupeds discharge with a set temporal order that optimizes the mechanical advantage, or leverage of the muscles, and reduces the work of breathing [8]. Electromyographic studies in humans show that, relative to the onset of airflow into the airways, the diaphragm and the third dorsal external intercostal muscles are the first to contract, followed by the second parasternal intercostal and scalene muscles and lastly by the fifth dorsal external intercostal muscles. The order of recruitment is consistent with the relative inspiratory mechanical advantage that each of the muscles has, and the degree of inspiratory opening pressure exerted on the airways by each. The intensity and duration of unit discharges are greater for the diaphragm and third dorsal external intercostal 10.1007/978-3-540-29678-2_13 than for the other pump muscles. The pattern of recruitment of intercostal muscles not only expands the rib cage, but also applies stretch to the diaphragm to increase contractile strength.
Several factors have been posited for the recruitment and discharge patterns of the different pump muscles, including: (i) recruitment order of bulbospinal and spinal interneurons, (ii) different degrees of persistent and rhythmic inward currents and (iii) their spatial distribution over the soma and dendrites of motoneurons and (iv) graded distribution of inhibitory central respiratory drive potentials.
Central Nervous Control of the Respiratory Apparatus
Aggregates of neurons that discharge periodically during inspiration, post-inspiration or expiration are distributed bilaterally in the bulbar brainstem, from the rostral 10.1007/978-3-540-29678-2_16 (10.1007/978-3-540-29678-2_16) to the caudal border of the 10.1007/978-3-540-29678-2_13 (10.1007/978-3-540-29678-2_1). Synaptic interactions among the neurons establish the network respiratory rhythm, and their connections with cranial and spinal motoneurons and interneurons set up the timing and patterns of contraction in the muscles of respiration. Three regions of the medulla in particular have been studied for their roles in respiratory rhythmogenesis: the Pre10.1007/978-3-540-29678-2_16 Complex (10.1007/978-3-540-29678-2_16) and the para-facial and retrotrapezoid regions (Respiratory network analysis and isolated respiratory centre functions; [12]). Their functional integrity is essential for a normal respiratory rhythm, and in the PreBötzinger and para-facial areas neurons with autorhythmic pacemaker properties have been identified (Respiratory network analysis, isolated respiratory centre functions; 10.1007/978-3-540-29678-2_16).
Respiratory neurons of the brainstem receive modulatory synaptic input from non-respiratory regions such as the 10.1007/978-3-540-29678-2_13, pontine and medullary reticular formation, 10.1007/978-3-540-29678-2_3, 10.1007/978-3-540-29678-2_8, other 10.1007/978-3-540-29678-2_12 and cardiovascular regions of the brainstem as well as from extrapyramidal motor areas (10.1007/978-3-540-29678-2_1). These non-respiratory modulatory inputs adapt breathing rhythm and pattern to accommodate activities such as phonation, swallowing, coughing, physical exertion, defecation and postural change.
Rhythm Formation in Bulbar Respiratory Neurons
The 10.1007/978-3-540-29678-2_13 of medullary respiratory neurons normally oscillates between cycles of depolarization and hyperpolarization. The pattern of depolarization or hyperpolarization may be augmenting (increasing in intensity from onset to termination), decrementing (declining in intensity) or plateau (constant from onset to termination). In association with the patterns of depolarization, periodic discharges can be augmenting, decrementing or of constant intensity [9,25].
The rhythm and pattern of discharge in bulbar respiratory neurons result from a combination of intrinsic membrane ion 10.1007/978-3-540-29678-2_3, synaptic interactions among the neurons, and input from other CNS neurons and peripheral sensory afferents.
Intrinsic membrane ion conductances initiate membrane depolarization that triggers action potential discharge, control the rate of depolarization and hyperpolarization, and terminate action potential discharge [25,26] (PreBötzinger Complex Inspiratory Neurons and Rhythm Generation). Tonic excitatory drive comes from at least two sources. One is from CO2-sensitive neurons in the medulla (10.1007/978-3-540-29678-2_3; 10.1007/978-3-540-29678-2_13). A second is from non-respiratory reticular activating neurons. These excitatory inputs can be suppressed or reinforced by feedback synaptic input from medullary and pontine respiratory neurons. 10.1007/978-3-540-29678-2_3 and 10.1007/978-3-540-29678-2_13 afferents from the 10.1007/978-3-540-29678-2_3 (10.1007/978-3-540-29678-2_3), heart, lungs, chest wall and upper airways also influence discharge properties of bulbar respiratory neurons.
All afferent inputs and synaptic interactions among the respiratory and non-respiratory neurons are regulated chemically by 10.1007/978-3-540-29678-2_14, including excitatory and inhibitory amino acids, acetylcholine, peptides, monoamines and adenosine (Respiratory neurotransmitters and neuromodulators).
Rhythmic Properties, Connections and Functions of Respiratory Neurons in the Pons and Medulla
The roles that various types of bulbar neurons play in respiratory control have been investigated by: (i) measuring membrane potential and discharge properties during various phases of the respiratory cycle, (ii) identifying synaptic connections among them and (iii) observing their responses to changes in respiratory rhythm and ventilation. From such studies, theories of how the neurons interact as a network to generate rhythm have been proposed [2,16,25] (10.1007/978-3-540-29678-2_1). Elegant computer modeling studies based on the experimental findings have tested and support many of the proposed connections and predict additional ones (10.1007/978-3-540-29678-2_3).
Neurons in the respiratory-related regions of the rostral pons discharge with waves of excitatory and inhibitory postsynaptic potentials and intermittent bursts of action potentials and that are coincident with one of the three phases of the respiratory cycle, and some discharge more intensely at the transitions between phases (10.1007/978-3-540-29678-2_16). In unanesthetized cats prepared for chronic studies, respiratory rhythm is not obvious during sleep [2], and rhythmicity is diminished when nervous input from the lungs is intact. The pontine network of neurons modulates amplitude and timing of the respiratory muscles, and seems to promote inspiratory termination if pulmonary afferent feedback is impaired.
In respiratory regions of the medulla, six different types of medullary respiratory neurons differentiated by membrane potential and discharge properties have been identified: (i) Early-Inspiratory, (ii) Ramp-Inspiratory, (iii) Late-Inspiratory, (iv) Post-Inspiratory, (v) Late-Expiratory, (vi) Pre-Inspiratory (Fig. 3).
Respiration — Neural Control. Figure 3.

Medullary respiratory neurons thought to be involved in respiratory rhythmogenesis. The six types of neurons were recorded intracellulary in adult cats in vivo in studies performed by A. Haji and coworkers. Each pair of tracings shows neuron membrane potential (MP) and phrenic nerve activity (PNA). Figure courtesy of Prof. Dr. Akira Haji, Laboratory of Neuropharmacology, School of Pharmacy, Aichi Gakuin University, Nagoya.
Early-Inspiratory neurons are propriobulbar, that is, their cell bodies, dendrites and axons are restricted to bulbar regions. They depolarize suddenly to threshold shortly before phrenic nerve discharge begins at the onset of inspiration. One type of Early-Inspiratory neuron exhibits a discharge that is intense but decrementing, in association with a declining pattern of depolarization, until it terminates late in inspiration, before the phrenic nerve inspiratory discharge ceases [25]. The other type exhibits a constant, or plateau pattern of depolarization and discharge that begins and ends with the phrenic nerve inspiratory discharge [9]. In either case, two processes seem to be responsible for discharge termination. One is weak synaptic inhibition, possibly from Late-Inspiratory neurons, but a more prominent source is the development of a 10.1007/978-3-540-29678-2_3 that builds up as Ca2+ enters during cell discharge through high voltage-regulated channels. Thereafter, membrane potential is hyperpolarized by synaptic inhibition produced by the discharge of Post-Inspiratory and Late-Expiratory neurons. The proposed role of Early-Inspiratory neurons of the decrementing type is to impose synaptic inhibition on Late-Inspiratory and Post-Inspiratory neurons, which prevents premature termination of phrenic motoneuron discharges [25]. As for the plateau type, they seem to augment inspiratory phase excitatory synaptic drive, because input to Ramp-Inspiratory neurons has been demonstrated electrophysiologically [9].
Ramp-Inspiratory neurons are either propriobulbar and/or bulbospinal and provide excitatory synaptic drive to phrenic and intercostal inspiratory motoneurons and interneurons. They depolarize and discharge with an augmenting pattern at the beginning of inspiration, in parallel with phrenic nerve discharges. The pattern of depolarization and discharge in Ramp-I neurons is attributed to a combination of mutual recurrent excitation among the neurons and declining inhibitory synaptic input from Early-I neurons. Discharge is terminated by inhibitory synaptic input from Late-Inspiratory and Post-Inspiratory neurons.
Late-Inspiratory neurons are propriobulbar. During most of the inspiratory phase, their discharges are prevented by declining synaptic inhibition, probably set up by Early-Inspiratory neurons. They discharge coincident with the termination of Ramp-Inspiratory and phrenic nerve discharges and cease firing due to synaptic inhibition that seems to derive from discharges of Post-Inspiratory and Late-Expiratory neurons. Their proposed function is to initiate inspiratory phase termination [2,25] and mediate reflex inspiratory inhibition by slowly adapting lung stretch afferents (Respiratory Neurotransmitters and Neuromodulators).
Post-Inspiratory neurons are of two types. Some are cranial motoneurons that contract the pharyngeal constrictor and laryngeal adductor muscles. Others are propriobulbar and are thought to contribute to rhythm formation by securing termination of the inspiratory phase, and imposing a delay to the onset of expiratory neuron discharges. According to the network theory of rhythmogenesis, reciprocal inhibitory interactions between propriobulbar Post-I and Early-I neurons constitute the primary rhythm generator, and contribute to shaping the discharge patterns of inspiratory and expiratory neurons [25]. Post-Inspiratory neurons depolarize abruptly and discharge action potentials coincident with the termination of firing in Ramp-I neurons and with the arrest of phrenic nerve inspiratory phase discharges. Membrane depolarization and discharges in Post-I neurons exhibit declining patterns, which seem to be activity-dependent and mediated by a Ca2+-activated K+ conductance. Throughout the inspiratory phase, the neurons receive a declining wave of inhibitory postsynaptic potentials, evidently set up by Early-Inspiratory neurons. The rapid onset of depolarization and discharge in Post-I neurons is attributed to release from Early-I neuron inhibition and reactivation of low 10.1007/978-3-540-29678-2_22 and non-selective cationic currents that bring the membrane to threshold for action potential discharge. Throughout the expiratory phase, Post-I neurons receive synaptic inhibition set up by the discharge of Late-Expiratory neurons.
Late-Expiratory neurons are of two types, according to location and function. One group is located in the Bötzinger region of the rostral ventrolateral respiratory column (VRC) of the medulla, the other in the caudal region of the VRC (Alheid and McCrimmon: anatomy and function in the respiratory network). Late-E neurons in both regions have similar membrane potential and discharge properties but play different roles in respiratory control. Late-E neurons located in the caudal VRC are bulbospinal and provide excitatory synaptic drive to expiratory intercostal and lumbar motoneurons and interneurons. During inspiration and postinspiration, synaptic inhibition set up by Early-Inspiratory and Post-Inspiratory neurons prevents Late-E neuron discharge. The expiratory neurons depolarize gradually to threshold during the post-inspiratory phase and then discharge steadily during the expiratory phase. The Late-E neurons in the Bötzinger region of the VRC are both propriobulbar and bulbospinal. Their discharges result in inhibition of medullary, phrenic and intercostal inspiratory neurons during the expiratory phase.
Pre-Inspiratory neurons are propriobulbar. They discharge in short bursts at the end of expiration and sometimes at the end of inspiration. They are subject to augmenting synaptic inhibition during the inspiratory phase and declining inhibition during the postinspiratory phase. Based on these time-intensity profiles, they are thought to receive inhibitory synaptic input from Ramp-Inspiratory and Post-Inspiratory neurons. One function they might have is to initiate termination of discharge in Late-Expiratory neurons [15].
Network Hypothesis: How Bulbar Respiratory Neurons Express a Three-phase Rhythm that Controls Respiration in the Mature Respiratory Network [2,9,25] (Computational Modeling of the Respiratory Network)
According to the network model, bulbar respiratory neurons receive tonic excitatory drive from pH/CO2-sensitive chemoreceptor neurons and from neurons of the brainstem reticular activating system. Inspiration begins when Ramp-I neurons are released from inhibition and low-voltage activated (LVA) Ca2+ currents and non-selective cationic currents are activated. Ramp-like inspiratory discharges begin, driven by mutual recurrent excitation and by declining inhibition from Early-I neurons. Early-I discharges are terminated by Ca2+-activated K+ conductances, allowing disinhibition of Late-I and Post-I neurons that terminate discharge of Ramp-I neurons and arrest inspiration. Buildup of Ca2+-activated K+ conductances ends Post-I neuron inhibitory discharges, allowing discharge of Late-E neurons. Arrest of Late-E neuron discharge is initiated by Pre-I neurons and sustained by Early-I and Post-I neurons.
A key element in the cyclic regeneration of the inspiratory and expiratory phases is the termination of Early-I and Post-I discharges by activity-dependent Ca2+-activated K+ conductances.
Post-Inspiratory Discharges in Phrenic and Intercostal Inspiratory Motoneurons
The origin of postinspiratory discharge activity in inspiratory spinal motoneurons is not firmly established, but it does not arise from intrinsic membrane currents. Hypoxia increases its duration and hypercapnea shortens it. 10.1007/978-3-540-29678-2_9 and stretch receptors inhibit it, whereas withholding of lung inflation prolongs it.
Bulbospinal neurons have been identified in the medulla of the cat that discharge with two bursts; one beginning simultaneously with the onset of phrenic nerve inspiratory discharges and, a second that begins after a very brief pause and declines in synchrony with the postinspiratory discharge of phrenic nerve activity The discharge of these Inspiratory-Post-I (IPI) neurons and that of the phrenic nerve respond identically to activation of tracheal and pulmonary afferents. Thus, the postinspiratory discharge component in spinal inspiratory motoneurons may be linked to excitatory synaptic input from the medullary IPI neurons. The slow decline of the postinspiratory discharge in IPI neurons is attributed to integration of excitatory and inhibitory synaptic inputs coming from two populations of postinspiratory neurons within the medulla, one excitatory and the other inhibitory [27].
Cranial Motoneurons and Control of Flow in the Upper Airways
Cranial motoneurons with respiratory periodicity in the ventrolateral medulla have axons in the trigeminal (5th), facial (7th), glossopharnygeal (9th), vagal (10th) and hypoglossal (12th) cranial nerves. They innervate the muscles of the nostrils, pharynx, tongue and larynx, coordinating their positions and movements with those of the diaphragm, chest wall and abdominal muscles during breathing. Trigeminal motoneuron discharges open the mouth during breathing, whereas facial motoneurons flare the nostrils. Glossopharyngeal motoneurons discharge with an augmenting pattern during inspiration and contract the muscles of the pharynx and palate. Laryngeal motoneurons with an augmenting inspiratory discharge pattern contract the abductor muscles, those with a decrementing postinspiratory discharge contract and narrow the opening of the glottis and slow outward airflow as the lungs gradually deflate. Vagal motoneurons with an augmenting expiratory discharge contract the pharyngeal constrictor muscles during expiration to lower upper airway resistance. Hypoglossal motoneurons (Respiratory control of hypoglossal neurons during sleep and wakefulness) also regulate airway resistance and flow patterns by controlling tongue position.
Respiratory rhythm and pattern is derived from periodic excitatory and inhibitory synaptic input from the propriobulbar respiratory neurons described above. For laryngeal and hypoglossal motoneurons, at least, important sources of synaptic excitation and inhibition are respiratory neurons of the preBötzinger Complex and surrounding ventrolateral medulla [20,32].
The motoneurons are assigned other duties related to sneezing, coughing, movements of the mouth, swallowing, vomiting, etc., during which their discharge properties are appropriate for the movements they promote.
Spinal Motoneurons and Contraction of the Pump Muscles
The location, synaptic connections and electrophysiological properties of spinal respiratory neurons are described in detail elsewhere, with special emphasis on α-motoneurons that innervate the extrafusal muscles of respiration (10.1007/978-3-540-29678-2_19). Interested readers can also consult an earlier review [17].
Phrenic motoneurons innervating the diaphragm are located in the ventral horns of the lower cervical spinal segments, and receive bilateral excitatory synaptic drive from medullary bulbospinal Ramp-I neurons and inhibitory synaptic input from bulbospinal Late-E neurons of the Bötzinger Complex. The neurons depolarize and fire with an augmenting discharge pattern during inspiration and hyperpolarize with an augmenting pattern during expiration. Not all phrenic motoneurons reach threshold simultaneously, rather, there is a scatter in the discharge latencies (recruitment times) with respect to the onset of the population discharge in the phrenic nerve. It appears that the order of recruitment derives, at least in part, from similar temporal properties of the bulbospinal neurons that provide excitatory synaptic input. Some of the excitatory drive also comes from interneurons located in the phrenic motoneuron pool and at higher cervical levels, which are driven by excitatory input from bulbospinal inspiratory neurons [34].
Intercostal motoneurons that contract the scalene, sternocleidomastoid and intercostal muscles are located in the ventral horn at all levels of the thoracic spinal cord. Medullary bulbospinal neurons that control phrenic motoneurons are also responsible for the periodic depolarization, discharge and hyperpolarization of intercostal motoneurons. Some of the synaptic connections are direct and others are made through interneurons located in the same segment as the motoneuron pool and in other cervical and thoracic segments.
Lumbar spinal motoneurons innervate the rectus abdominis, external and internal oblique and transverse abdominal muscles of the abdomen, and produce contraction during active expiration.
Recurrent Inhibitory Interneurons with a very high frequency of action potential firing are found near to phrenic and intercostal motoneurons. They are activated to discharge by motor axon collaterals and in turn suppress motoneuron discharges [17]. The high-frequency discharge and the resulting feedback suppression of motoneuron discharges are reminiscent of the Renshaw inhibition that modulates discharge properties of limb motoneurons.
The Effects of Hypoxia on the Respiratory Apparatus: A Multiphase Reaction Involving the Carotid Bodies and the CNS Respiratory Network
Hypoxia produces dramatic disturbances of respiration. The initial response to acute hypoxia is increased breathing in an attempt to replenish O2. The immediate source of respiratory augmentation is stimulation of type 1 (glomus) cells of the carotid bodies (CB) located bilaterally in the bifurcation of the common carotid arteries, which leads to activation of CB afferents and reflex stimulation of the CNS respiratory network. (10.1007/978-3-540-29678-2_3).
If hypoxia is maintained, disturbances of synaptic function within the CNS convert breathing rhythm to gasping and apnea. (10.1007/978-3-540-29678-2_14). The CNS-derived hypoxic ventilatory response is a 5-component process, consisting of augmentation, apneusis or breath holding, protective apnea, gasping and terminal apnea. For each component, there are related changes in endogenous neurotransmitter and neuromodulator release and alterations in ion channel permeabilities.
Here, metabolic, enzymatic, ion channel and chemical neuromodulatory mechanisms that control the carotid body oxygen sensor and trigger the respiratory response to acute hypoxia are presented. The role of the CB in CO2/pH sensing and its importance in health and disease are also discussed (Carotid Body Chemoreceptors and Respiratory Drive). Sites and mechanisms within the CNS respiratory network that engender ventilatory disturbances and ultimate apnea are described. In one essay (Respiratory network responses to hypoxia) the hypoxic response is defined in terms of two phases and a comprehensive description of neural pathways, neurotransmitters and neuromodulators that mediate respiratory depression during the late hypoxic ventilatory response (HVR) is presented. In another essay (10.1007/978-3-540-29678-2_14), the energy cost of hypoxia to cells and its effects on ionic homeostasis are discussed. In addition, the HVR is presented as a 5-component process: augmentation, apneusis or breath holding, protective apnea, gasping and terminal apnea. For each component, accompanying changes in endogenous neurotransmitter and neuromodulator release and ion channel changes are described.
Concluding Comments
This overview has focused on respiratory control mechanisms in the adult mammal. Other contributions will show how the respiratory network develops before and shortly after birth (10.1007/978-3-540-29678-2_4) and how autorhythmic pacemaker neurons in the rodent medulla regulate respiration in the postnatal period (Respiratory pacemakers). Disturbances of respiratory control that are gene-related are also reviewed elsewhere (10.1007/978-3-540-29678-2_8).
Other important aspects of respiratory control not considered in this overview include: (i) neuroplasticity in the respiratory network, which allows readjustments of network responsiveness to injury and other environmental challenges (Respiratory neuroplasticity), (ii) 10.1007/978-3-540-29678-2_12 responding to liquid and chemical stimulation of laryngeal receptors, and (iii) respiratory control during sleep (10.1007/978-3-540-29678-2_15), (Respiratory control of hypoglossal neurons during sleep and wakefulness).
Hopefully, the reader will appreciate the valuable insights of the contributing authors into how respiration is controlled, and the innovative methods they utilize, including imaging techniques, (Respiratory network analysis, functional imaging) computer modeling (10.1007/978-3-540-29678-2_3) and the use of novel preparations to study integrated cardio-respiratory regulation (10.1007/978-3-540-29678-2_3).
Respiratory Bursting Neurons
Respiratory Central Pattern Generator
10.1007/978-3-540-29678-2_3
Respiratory Control of Hypoglossal Neurons During Sleep and Wakefulness
Synonyms
Respiratory control of the tongue and airway
Definition
Hypoglossal 10.1007/978-3-540-29678-2_13 control the tongue muscles; genioglossus muscle (tongue protrudor), hyoglossus and styloglossus (tongue retractors) and intrinsic muscles. Because the genioglossus as well as other tongue muscles participate in a range a motor acts (e.g., drinking, licking, swallowing and breathing), the hypoglossal motoneurons that innervate them receive numerous inputs from a variety of relevant brain areas. Since the tongue position affects airway patency and resistance, respiratory control is essential to coordinate respiratory muscle effort and airway resistance, minimizing resistance during inspiration and using the control of resistance during expiration to modify expiratory flow patterns. The hypoglossal control of the tongue assumes a greater importance during sleep when relaxation of the tongue can occlude the airway; a condition of 10.1007/978-3-540-29678-2_15.
Characteristics
Hypoglossal Motoneurons Anatomical Location
Hypoglossal 10.1007/978-3-540-29678-2_13 are 10.1007/978-3-540-29678-2_19 bilaterally in compact columns extending along the midline of the brainstem above and below the obex. For example a recent study in dogs [1] showed that the nuclei extend from 0.75 mm caudal to 3.45 mm rostral to the obex, with cells 0.37–2.12 mm below the dorsal surface, and symmetrically centered between 0.66 and 1.33 mm from the midline. There are a number of detailed locations studies including those in rats, cats, rabbits, frogs and monkeys as well as dogs. The hypoglossal motoneurons have varied and extensive dendritic arborizations that provide the potential for a wide range of afferent contacts. (Fig. 1) shows a schematic of the medullary respiratory neurons illustrating their general location and pattern of respiratory activity.
Respiratory Control of Hypoglossal Neurons During Sleep and Wakefulness. Figure 1.

A schematic showing the location of hypoglossal motoneurons relative to medullary respiratory neuron groups with representative recordings from the hypoglossal nerve, a ventral respiratory group inspiratory neuron and the phrenic nerve in an adult decerebrate rat. Abbreviations: PRG = pontine respiratory group, nVII = Facial nucleus, pFRG = parafacial respiratory group, XII = hypoglossal motor nucleus, RVLM = rostro ventrolateral medulla, Böt = Bötzinger complex, Pre-Böt = preBötzinger complex, VRG = ventral respiratory group, DRG = dorsal respiratory group, NA = nucleus ambiguus, UCIN = upper cervical inspiratory neurons.
Projections of Hypoglossal Motoneurons
The axons of these motoneurons course ventrally and slightly laterally to emerge from the medulla in the preolivary sulcus separating the olive and the pyramid, and form the twelfth cranial nerve (XII). The hypoglossal nerve innervates all the muscles of the tongue except for the palatoglossus muscle which is innervated by the vagus nerve (X) and the accessory nerve (XI). The tongue is a complex muscle, and the hypoglossal nerve bifurcates to form medial and lateral branches, with the medial branch innervating the protrusor tongue muscles, and the lateral branch innervating the retractor tongue muscles [2].
The ventrolateral and ventromedial subnuclei contain motoneurons that innervate the geniohyoid and genioglossus muscles respectively, and the dorsal subnucleus motoneurons innervate the hyoglossus and styloglossus muscles. The genioglossus muscle is of particular importance clinically because it is considered the main protruder and depressor muscle of the tongue. It is co-activated during inspiration with tongue retractor muscles [3] so that airway compliance is reduced, and upper airway patency increased during inspiration.
Respiratory Inputs to Hypoglossal Motoneurons
Hypoglossal motoneurons participate in rhythmic oro-facial motor acts such as mastication, licking, swallowing and breathing; receiving neural inputs from the brainstem rhythm-generating networks that generate these behaviors. They play a major role in respiratory airway control and the following focuses on the neural mechanisms by which respiratory drive is communicated to them.
A major function of the genioglossus muscle is to stiffen the pharyngeal airspace during inspiration so that it does not collapse during diaphragmatic contraction. Therefore, hypoglossal motoneurons are synaptically excited during inspiration (i.e., discharge action potential during inhalation); however, they are activated 200–300 ms before the phrenic motoneurons that innervate the 10.1007/978-3-540-29678-2_4 [3]. This pre-activation of hypoglossal motoneurons ensures that the airway is stiffened before the diaphragm contracts.
Although both the hypoglossal and phrenic motoneurons receive inspiratory signals from the medullary network that generates breathing, they receive their respective inspiratory commands from separate premotor populations. Inspiratory drive is transmitted to phrenic motoneurons by premotor neurons located in the ventral respiratory group (parallel to the nucleus ambiguus), while hypoglossal motoneurons receive respiratory drive from premotor neurons located in the medullary lateral tegmental field, lateral to the hypoglossal motor nucleus (Fig. 2) [4].
Respiratory Control of Hypoglossal Neurons During Sleep and Wakefulness. Figure 2.

A schematic representation of the neural mechanisms responsible for suppression of genioglossus muscle activity in sleep. (a) It is hypothesized that active inhibition and passive disfacilitation reduce hypoglossal (airway) motoneuron activity and hence genioglossus muscle tone in sleep. Several lines of evidence indicate that inhibitory processes play a predominant role in controlling airway motoneuron and muscle activity in sleep, particularly in REM sleep. There is also evidence indicating that withdrawal of excitatory sleep-related inputs (e.g., serotonin, orexin, noradrenaline) in sleep may reduce airway motoneuron excitability. (b) A typical example from a naturally behaving rat demonstrating that genioglossus (and neck) muscle activity is depressed in sleep, and particularly REM sleep. This figure was modified (with permission) from Morrison et al. Journal of Physiology, 2003, 552.3, pp. 975–991.
Although separate premotor populations relay inspiratory drive to phrenic and hypoglossal motoneurons, both release glutamate to activate post-synaptic non-NMDA receptors to induce inspiratory activation [5]. Unlike phrenic motoneurons, which are silenced during expiration by GABAergic and glycinergic inhibition, hypoglossal motoneurons receive no such inhibitory drive; instead, they are passively disfacilitated (withdrawal of excitation) during expiration [4]. Because the genioglossus is not only involved in the control of breathing, but is also in the control of speech, the lack of inhibition during expiration enables more effective modulation of their activity and thereby tongue muscles because motoneurons are more easily excited by other non-respiratory inputs.
Hypoglossal motoneurons are not only controlled by premotor neurons in the lateral tegmental field, they also receive respiratory signals from a population of interneurons located directly within the hypoglossal motor nuclei [4]. Hypoglossal interneurons are significantly smaller than motoneurons, they are active only during inspiration and they make inhibitory (e.g., GABA) connections with hypoglossal motoneurons. The precise role that interneurons play in transmitting inspiratory drive to hypoglossal motoneurons is unclear; however, it is hypothesized that they gate or filter pre-synaptic inputs.
Impact of Sleep on Hypoglossal Motoneuron Activity
10.1007/978-3-540-29678-2_19 suppresses the excitability of hypoglossal motoneurons. Although this review focuses on hypoglossal motoneurons, it should be made clear that the excitability of all somatic motoneurons including other airway motoneurons (e.g., trigeminal and facial) are also affected by sleep [6]. Understanding how hypoglossal motoneuron activity is regulated in sleep is clinically important because sleep-related reductions in their activity lead to reduced airway motor tone, airway narrowing and collapse in predisposed individuals (e.g., small airway). The suppression of airway motoneuron activity in sleep, and particularly during rapid-eye-movement (REM) sleep, is the primary cause of OSA.
The root cause of hypoglossal motoneuron suppression in sleep is unknown; however, recent work from our laboratory and others has begun to shed light on potential mechanisms. It is hypothesized that the neurocircuitry generating sleep (e.g., REM and 10.1007/978-3-540-29678-2_14) also innervates and regulates hypoglossal motoneuron activity. Although multiple neural circuits are involved in sleep generation, they can be subdivided into two categories – those that are excitatory and promote wakefulness and those that are inhibitory and promote sleep. Excitatory circuits are active during waking and project to both the cortex and motoneurons to promote behavioural arousal and high levels of motor tone [7]. Inhibitory circuits are active in sleep and project to and switch-off both the wake-promoting circuits as well as inhibiting airway motoneurons. Therefore, it is hypothesized that hypoglossal motoneurons are both actively inhibited and passively disfacilitated (withdrawal of excitatory inputs) during sleep (Fig. 3).
Respiratory Control of Hypoglossal Neurons During Sleep and Wakefulness. Figure 3.
Location of the premotor neurons that relay inspiratory drive to hypoglossal motoneurons. (a) Photograph of the premotor neurons in lateral tegmental field that are hypothesized to relay inspiratory drive to hypoglossal motoneurons. Premotor neurons were identified by visualizing the location of pseudorabies virus that was retrogradely transported from the genioglossus muscle where it had been injected. (b) Higher magnification of the black box in (a); brown cells represent hypoglossal premotor neurons. C and D, represent the anatomical distribution of hypoglossal premotor neurons (small dots) and hypoglossal motoneurons (small dots) plotted on schematic cross-sections of rat brainstem at 0–500 μm rostral to obex (c) and −100 to −600 μm caudal to obex (d). This figure was modified (with permission) from Chamberlin et al., J. Physiology, 579.2, 2007, pp 515–526.
The primary wake-promoting circuits consist of excitatory neurons located in the noradrenergic locus coeruleus, the serotonergic dorsal raphe, the orexinergic (also called hypocretin) lateral hypothalamus, the histaminergic tuberomammillary nucleus and the 10.1007/978-3-540-29678-2_4 10.1007/978-3-540-29678-2_16 and ventral tegmental area. The activity of these neural populations is highest in waking and minimal or silent in sleep. Because they project to motoneurons, it is hypothesized that withdrawal of noradrenergic, orexinergic, and serotonergic inputs may be a contributing factor to the reduction of motoneuron excitatory and hence muscle activity in sleep [6]. The major sleep-promoting system consists of inhibitory neurons located in the GABAergic ventrolateral and median preoptic areas of the anterior hypothalamus. The activity of these neurons is lowest in waking and highest in sleep (particularly non-REM sleep). Because they project to motoneurons, it is hypothesized that they actively inhibit (via GABA) hypoglossal motoneuron activity and thereby suppress airway motor tone in non-REM sleep.
Another source of motoneuron inhibition comes from the medial medullary reticular formation. Unlike neurons in the ventrolateral and median preoptic areas, these neurons contain both GABA and glycine, and are maximally active in REM sleep when genioglossus muscle tone is minimal or absent [7]. It therefore appears that together GABA and glycine play a role in regulating hypoglossal motoneuron activity in both non-REM and REM sleep. Although GABAergic and glycinergic neurons in the medial medullary reticular formation project to hypoglossal motoneurons and are active in REM sleep, they are not responsible for the 10.1007/978-3-540-29678-2_13 that typifies this state. Rather GABAergic and glycinergic inhibition are responsible for suppressing the phasic muscle twitches that characterize REM sleep [8]. The muscle atonia of REM sleep can not be explained by disfacilitation of excitatory because application of glutamate or glutamatergic receptor agonists (e.g., AMPA or NMDA) directly into airway (e.g., trigeminal) motoneurons can not reverse REM sleep atonia. Therefore, the cause of airway motoneuron inactivity and hence airway muscle atonia in REM sleep has yet to be identified. Identification of the neurochemical responsible for REM atonia requires attention because OSA is most common and severe during REM sleep.
Plasticity of Hypoglossal Motoneurons
Somatic motoneurons are generally considered to be passive neural relays that monotonically respond to pre-synaptic inputs; however, they are able to undergo remarkable degrees of plasticity. One type of motoneuron plasticity (neural plasticity) that is particularly relevant to hypoglossal control is respiratory 10.1007/978-3-540-29678-2_12 (LTF). LTF is characterized by a progressive increase in the inspiratory amplitude of the hypoglossal nerve (or genioglossus muscle) activity following exposure to 10.1007/978-3-540-29678-2_9 (an example is shown in Fig. 4) [9].
Respiratory Control of Hypoglossal Neurons During Sleep and Wakefulness. Figure 4.

A typical example of apnea-induced long-term facilitation (LTF) of genioglossus motor outflow in an anaesthetized rat. Basal levels of inspiratory genioglossus muscle activity were recorded 5-min before (baseline) and for 60-min after obstructive apneas (ten 15-s apneas each separated by 45 s). Each obstructive apnea caused a reflexive increase in the inspiratory activity of the genioglossus muscle (see under apnea-induced hypoxia). The cluster of ten apneas induced a persistent and progressive increase in peak inspiratory genioglossus muscle activity that lasted for over 60-min (i.e., LTF). The dotted line represents the average magnitude of genioglossus inspiratory efforts before apnea-induced LTF; note that genioglossus activity returned to baseline levels after apneas and then progressively increased to reach maximal levels at 60-min post-apnea.
LTF can only be evoked by intermittent hypoxia; exposure to continuous hypoxia does not evoke LTF. The central serotonergic system is required for LTF because blocking either serotonin release or serotonin receptors nullifies LTF. It is hypothesized that LTF is induced because intermittent hypoxia activates the serotonergic medullary raphe nuclei to release serotonin onto hypoglossal motoneurons. Intermittent serotonin receptor activation subsequently causes plastic changes in the excitability of hypoglossal motoneurons perhaps via group-I 10.1007/978-3-540-29678-2_13 glutamate receptors [10].
Since intermittent hypoxia causes persistent increases in hypoglossal motoneuron excitability and hence genioglossus motor output, and since hypoglossal motoneuron activity is lost in sleep and contributes to airway obstructions, then inducing LTF of during sleep may be an effective method for minimizing or reversing the root cause of obstructive sleep apnea. Therefore understanding the cellular mechanisms of LTF may provide the basis for rationale development of therapeutics for treating this prevalent (it affects about 5% of adults) sleep disorder.
Respiratory Control of the Tongue and Airway
Respiratory control of hypoglossal neurons during sleep and wakefulness
Respiratory CPG
10.1007/978-3-540-29678-2_3
Respiratory Cycle (Phase)
Definition
The neuronal cycle of respiration consists of three phases: inspiration in which inspiratory muscles contract, post-inspiration or passive expiration (stage 1 expiration) in which inspiratory muscles cease progressively to contract while activity of the adductor muscles of the upper airway reduces exhalation, and active expiration (stage 2 expiration) in which expiratory muscles contract.
Respiratory Kernel
Definition
An aggregate of neurons that is essential for a respiratory function.
10.1007/978-3-540-29678-2_4
Respiratory Memory
Respiratory Network
Definition
The central respiratory network consists of the respiratory neurons and generates respiratory rhythm and pattern.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_3
Respiratory Network Analysis, Functional Imaging
Synonyms
Visualization of respiratory centers; Microscopy of structure-function relationships in mammalian respiratory networks
Definition
Functional imaging of respiratory networks means optical analysis, with (Confocal microscopy), multiphoton microscopy (two-photon microscopy) or cameras (e.g. CCD- or CMOS-type) of structure-function relationships in rhythmically active neuronal brainstem networks involved in the control of breathing in mammals. Such imaging has so far only been carried out in vitro, specifically in (i) respiratory active slices, (ii) en bloc brainstem preparations from perinatal rodents and (iii) arterially-perfused “working-heart-brainstem” preparations of both newborn and adult rodents. Voltage-sensitive dye imaging of the activity of neuronal populations is feasible in the latter three in situ models. In contrast, simultaneous Ca2+ imaging of both the activity and basic morphology of single respiratory neurons, or clusters of such cells, has not been reported so far in the perfused preparation, and only in one case for en bloc medullas. In rhythmic slices, Ca2+ imaging is primarily done in inspiratory active interneurons and hypoglossal (XII) motoneurons. The latter neuron populations are also being used for optical analyses of subcellular processes such as activity- or metabolism-related changes of mitochondrial membrane potential, Ca2+ or redox state. Finally, expression of fluorescent proteins in transgenic mice and 10.1007/978-3-540-29678-2_6 labeling of neurotransmitter receptors are currently used for targeted electrophysiological recording from respiratory neurons in the slices. As outlined below, these approaches have provided important insights into both the structural organization and functional properties of respiratory centers. The following (yet mostly hypothetical scenario) would likely answer, with imaging techniques, most relevant questions regarding the neural control of breathing: Subpopulations of respiratory interneurons or output cells will be identified via a specific pattern of intrinsically-expressed fluorescent proteins and acutely fluorescence-labeled ion channels, receptors and/or transporters. Populations of these identified cells will then be loaded with cell-permeant fluorescent dyes for, e.g., simultaneous imaging of dynamic changes of signaling factors such as Ca2+ or pH in the cytosol and cellular organelles. At the same time, one or few of these cells will be whole-cell recorded (with further functional or morphological dyes in the patch electrode solution) for a correlation of dynamic changes of (sub)cellular activities with respiratory-related membrane potential oscillations or underlying membrane currents. Further improvement of computerized data processing and of the spatiotemporal resolution of fluorescent microscopy will enable simultaneous 4D-imaging of all these events for a thorough structure-function relationship of interactions between respiratory centers and (pre) motor circuits.
Characteristics
Medullary Respiratory Networks
Three major bilaterally-organized respiratory groups have been identified in the lower brainstem of mammals [1]. The dorsal respiratory group is primarily involved in the transmission of (chemosensory and mechanosensory) inputs to the medullary respiratory control system. The pontine respiratory group plays a major role in orchestrating the highly complex (pre/post)inspiratory-expiratory synaptic neuronal activity pattern for the innervation of diverse groups of respiratory muscles that are active during one or several of these phases. The ventral respiratory column contains various aggregations of respiratory neurons, among which some are capable of generating primary respiratory rhythms. Specifically, the 10.1007/978-3-540-29678-2_16 (preBötC) appears to be pivotal for the generation of inspiratory-related interneuronal and motor activities. Conversely, the parafacial respiratory group (pFRG), located between the pons and the more caudal preBötC, generates preinspiratory (and postinspiratory) activities that drive, e.g., expiratory abdominal muscles [1,2]. Both, the preBötC and pFRG remain active in distinct transverse brainstem slices from newborn rodents (see isolated respiratory centers). In more intact preparations, the pFRG and preBötC constitute presumably a dual respiratory center that may interact with the pontine and dorsal respiratory groups for generation of the full spectrum of respiratory activities [1]. As described below, voltage-sensitive dye imaging has been used for characterization of all three respiratory groups, whereas other imaging approaches were so far primarily used for studying the ventral respiratory column, in fact mostly presumptive or histologically-identified preBötC interneurons and inspiratory XII motoneurons.
Voltage-Sensitive Dye Imaging of Spatiotemporal Respiratory Patterns
Voltage-sensitive dye imaging is principally suitable to measure the activity of single cells. Though, this technique has been applied yet only to monitor, at low optical magnification, spatiotemporal activity patterns in large populations of respiratory neurons. In most studies, fluorescence signals were collected from the intact ventral brainstem surface. Two dyes are preferentially used for this approach. The agent Di-4-ANEPPS stains primarily superficial tissue layers, whereas the less lipophilic Di-2-ANEPEQ stains deeper brainstem structures in addition. Bath-application of these agents for time periods of 0.5 h to sometimes >1 h is necessary for effective staining of respiratory active regions in newborn rodent brainstems [2,3]. Decreases and increases in fluorescence intensity of the above voltage-sensitive dyes correlate with enhanced and attenuated neuronal activity, respectively, due to the fact that the fluorescence of Di-2-ANEPEQ and Di-4-ANEPPS is proportional to (neuronal) membrane potential [2]. Accordingly, in an area corresponding to the locus coeruleus, which is located between the pontine respiratory group and the pFRG, initial inspiratory-related voltage-sensitive dye imaged activity is followed by a pronounced period of decreased activity [3]. The time course of these optical signals is similar to that of the inspiratory depolarization and the subsequent postinspiratory hyperpolarization of single neurons in this area. In contrast, optical activity in the main area of the pFRG is primarily preinspiratory-related, with less pronounced postinspiratory activity compared to that observed with whole-cell recording from single pFRG neurons [2]. In the latter study, inspiratory-related activity was in particular pronounced in the region of cervical spinal motoneurons, and in an area located ~0.4–0.6 mm caudal to the caudal end of the facial motor nucleus [2]. The latter region corresponds well with the presumptive rostrocaudal extension of the preBötC [4]. Most areas of the ventral respiratory column, including the pFRG and preBötC, contain different classes of respiratory neurons. It is not clear at present, why no prominent respiratory-related optical activity is revealed in regions of the ventral respiratory column other than the pFRG and preBötC.
Despite these caveats, respiratory voltage-sensitive dye imaging provided important information regarding the structural organization and function of respiratory networks. In en bloc medullas from newborn rodents, voltage-sensitive dye imaging revealed that both the dorsal and pontine respiratory groups are active in regions similar to those previously identified in adult mammals with combined electrophysiological and histological techniques [3]. The seminal imaging study on the discovery of the pFRG [2] suggested that the area of the location of these rhythmogenic preinspiratory neurons may extend further rostrally than previously assumed. Regarding respiratory functions, voltage-sensitive dye imaging showed that anoxia-induced slowing of respiratory rhythm in newborn rat brainstems is accompanied by depressed preinspiratory and augmented postinspiratory medullary optical activities that coincide with the occurrence of inspiratory-related cervical nerve burst doublets (Fig. 1).
Respiratory Network Analysis, Functional Imaging. Figure 1.

Anoxic respiratory pattern shifts in newborn rat brainstem-spinal cords. (a) traces in box (50 averaged optical signals) show that pFRG activity (red dot) preceded preBötC activity (blue dot) in control. Images below box show fluorescence signals of the voltage-sensitive dye Di-2-ANEPEQ during time periods indicated by vertical red bars. (b) Hypoxic anoxia due to N2-gassed solution decreased inspiratory-related cervical nerve (C4) burst rate and induced double bursts (compare integrated C4 activities in (a) and (b)). Anoxia also suppressed preinspiratory optical pFRG activity (50 averages) and elicited a second optical peak in the pFRG, preBötC and intermittent regions. The latter activity appeared in the postinspiratory phase after the augmented C4 peak and coincided with secondary C4 activity. In original C4 traces, the amplitude of the second C4 peak was similar to that of initial activity, but was attenuated by averaging due to variation in the time of burst onset. Scale bar indicates percentage change in fluorescence. (From K. Ballanyi & H. Onimaru, unpublished).
The latter findings suggest that anoxia synchronizes the dual respiratory rhythm generators. This may result in enhanced postinspiratory medullary activity that triggers repetitive inspiratory motor bursts. All this shows that voltage-sensitive dye imaging is a potent tool for the analysis of normal and pathologically disturbed spatiotemporal patterns of respiratory activities, in particular when this technique is combined with electrophysiological recording of cellular respiratory bursting.
Imaging of Respiratory Ca2+ Oscillations and (Sub)Cellular Signaling Factors
In excitable cells, a notable rise of the free cytosolic [Ca2+] is associated with the Ca2+ influx caused by activity-related depolarization. Accordingly, Ca2+ imaging is a widely used tool for monitoring neuronal activity (see 10.1007/978-3-540-29678-2_14). The vast majority of Ca2+ imaging studies of the respiratory system has focused on preBötC neurons or preBötC-driven interneurons and XII motoneurons in rhythmic slices from perinatal rodents. In most of these reports, clusters of preBötC neurons were loaded with the membrane-permeant acetoxy-methyl (AM) form of Ca2±-sensitive dyes, in particular Fluo-4-AM, Calcium-Green-1-AM and Fura-2-AM [4–8].
In the first respiratory Ca2+ imaging study, cytosolic Ca2+ oscillations in preBötC neurons occurred synchronously with inspiratory-related XII activity [7]. Following pharmacological blockade of glutamatergic synaptic transmission, asynchronous Ca2+ oscillations persisted in a subpopulation of these cells, in line with the hypothesis that preBötC neurons have intrinsic bursting (“pacemaker”) properties [1,7]. For this CCD camera imaging study, Calcium-Green-1-AM was microinjected near the midline contralateral to the preBötC side to be imaged. This ensured that only cells were imaged that project their axons to the contralateral preBötC. As a caveat, such loading of cells via diffusion of dye from their axon to the cell bodies required “overnight,” i.e. >10 h, incubation times. This substantial delay between the generation of the acute slice and the start of recording may affect functional properties of rhythmogenic preBötC networks although basic inspiratory activity was preserved, at least in solution of artificially-elevated (7–8 mM) [K+] [7]. Alternatively, inspiratory Ca2+ oscillations can be monitored within <20 min after the injection of AM Ca2+ dye into the “online histologically-identified” preBötC [4] (Fig. 1). As a major advantage of this approach, preBötC rhythms can be studied for several hours in physiological (3 mM) K+ which ensures a higher sensitivity of preBötC rhythms to clinically-relevant agents such as opioids [4]. Although multiphoton microscopy was used in that report, neither the activity nor the gross morphology of preBötC neurons could be imaged at high spatial resolution at depths >80 μm into the tissue for yet unknown reasons. Similarly, CCD camera imaging and confocal laser scan microscopy are restricted to recording depths <70 μm [4–8]. Focal injection of Ca2+ dye limits the monitored area of active respiratory neurons to a circular spot with a diameter of 150–300 μm [4]. This limitation is overcome by loading cells unspecifically in the entire slice via bath-application of the AM Ca2+ dye which, however, penetrates <50 μm into the tissue. As further caveats, Ca2+ imaging may induce phototoxic effects (phototoxicity) on (respiratory) neurons and is subjected to bleaching of dye (10.1007/978-3-540-29678-2_6). The extent of these effects increases at both higher sampling rates and enhanced optical magnification for visualizations of (sub)cellular structures. Though, at scan rates of 1.5–3 scans/s most of the peak of respiratory-related Ca2+ oscillations is captured and continuous multiphoton or confocal imaging is possible for time periods >30 min [4] (Fig. 2), similar to CCD camera imaging (Fig. 3) (B.U. Keller, unpublished observations).
Respiratory Network Analysis, Functional Imaging. Figure 2.

Multiphoton/confocal imaging of the activity and morphology of inspiratory preBötC neurons. (a) cells located 0.59 mm caudal to the caudal end of facial nucleus in the preBötC of a 600 μm thick newborn rat brainstem slice were stained by pressure-injection (0.7–1.0 psi, 10 min) with Ca2+ sensitive dye, Fluo-4-AM. Movie (supplemental material) shows 90 s recording in 3 mM [K+] of Ca2+ oscillations in these neurons that were in phase with inspiratory population activity recorded from the contralateral PBC; bottom left trace in (b). Fluo-4-AM fluorescence intensity is plotted in arbitrary units (a.u.) against time. After washout of rhythm in 3 mM [K+], preBötC bursting and Ca2+ oscillations were restored by low concentrations of the metabotropic glutamate receptor agonist dihydroxyphenylglycine (DHPG) and the clinically used respiratory stimulant theophylline. (c) 3D animation (supplemental material) showing gross morphology of preBötC neurons and neighboring non-rhythmic cells obtained from z-stack (0.5 μm single step) image series encompassing areas starting 7.5 μm above to 7.5 μm below image plane of (a) (reproduced from [4] with permission).
Respiratory Network Analysis, Functional Imaging. Figure 3.

Whole-cell patch-clamp recording and simultaneous Ca2+ imaging of a rhythmically active hypoglossal motoneuron. (a) Fluorescence image of the soma and proximal dendrite of a patch-clamped hypoglossal motoneuron in a 700 μm thick mouse brainstem slice. (b) Whole-cell recording in current-clamp mode and simultaneous ratiometric CCD camera imaging of cytosolic Ca2+ concentrations. Rhythmic electrical discharges are paralleled by notable Ca2+ transients in the soma and six dendritic compartments, selected for analysis at distances of 24–76 μm from the soma (compartment positions are represented by boxes in (a)). Spontaneous bursts of action potentials, shown in the bottom trace as changes in membrane voltage (Vm) are accompanied by cytosolic Ca2+ rises up to 200 nM (reproduced from [8] with permission).
The correlation between inspiratory-related membrane potential oscillations (or underlying membrane currents) and intracellular Ca2+ can be analyzed during whole-cell recording of respiratory neurons that are loaded with Ca2+ dye via the recording patch-electrode (Fig. 3). The first study in that regard showed with photomultiplier-based “point” imaging in presumptive preBötC neurons that somatic Ca2+ rises with a magnitude of maximally 300 nM occur during the inspiratory drive potential, and that a major portion of this signal is due to Ca2+ influx via high voltage-gated Ca2+ channels [5]. The relation of membrane excitability with cytosolic Ca2+ transients and their buffering, or with other cellular signaling factors, has been studied thoroughly in inspiratory active XII cells [8] (Fig. 3). These cells are not only a potent model to study the inspiratory drive from the preBötC to motor networks, but also for analysis of the selective vulnerability of particular motoneurons in amyotrophic lateral sclerosis [8]. Disturbances of (sub)cellular signaling processes such as (respiratory-related oscillations of) mitochondrial Ca2+ and membrane potential or redox processes are studied in XII motoneurons with regard to the latter disease [8]. Rhythmic changes of (sub)cellular signaling factors have also been optically monitored in presumptive inspiratory preBötC neurons, e.g., in the context of anoxic respiratory depression [9].
Targeted Recording from Fluorescence-Tagged Respiratory Neurons
It is not clear, whether yet unidentified rhythmogenic respiratory neurons, in particular of the preBötC and pFRG, have specific morphological features such as a particular size and shape of the soma or the number and array of (primary) dendrites. If this were the case, such cells could be selectively targeted after fluorescence labeling for intracellular electrophysiological recording in the rhythmic slices. The above mentioned Ca2+-sensitive fluorescent dyes, and also other functional dyes such as the marker for mitochondrial potential Rhodamine-123, can principally be used as morphological markers (Fig. 2–4) (see neuron-glia imaging).
Respiratory Network Analysis, Functional Imaging. Figure 4.

Targeted recording from acutely fluorescence-tagged preBötC neurons. (a, b) tetramethylrhodamine conjugated to substance P (TMR-SP) labeling in preBötC neurons with different phenotypic properties. Infrared differential interference contrast (IR-DIC) and epifluorescence images are shown in left columns, with corresponding intracellular traces to the right. (a) TMR-SP− early inspiratory neuron with silent interburst intervals. (b) TMR-SP+ early inspiratory neuron with tonic low-frequency spiking properties. Scale bar (25 µm) applies to all images in (a, b). (c, e) simultaneous measurements of inspiratory activity and TMR-SP labeling in preBötC neurons. (a) fluo-4 image shows a peak acquisition of Ca2+ labeling; TMR-SP image shows TMR-SP+ cells in the same region. Scale bar: 50 µm. (d) changes in fluorescence intensity from regions of interest (ROIs) indicated by numerals in (c), plotted with synchronized XII activity (reproduced from [6] with permission).
Regarding Ca2+ dyes, Fura-2 provides a robust fluorescence signal at low (resting) cytosolic [Ca2+], whereas both Fluo-4 and Calcium-Green-1 fluoresce during rises of cytosolic Ca2+ (Fig. 2–4) [4–7]. Although recording from inspiratory (preBötC) interneurons in the rhythmic slices is routinely done under visual control, these superficial cells are surprisingly not routinely labeled during recording with high resolution morphological dyes, e.g., of the “Alexa” family.
Alternatively, subpopulations of respiratory neurons in acute medullary slices can be labeled with fluorescent markers for proteins in the plasma membrane or cytosol. In that context, it was assumed that rhythmogenic preBötC neurons are characterized by postsynaptic neurokinin-1 receptors that are normally activated by substance P [1,6]. Accordingly, fluorescence-tagging of substance P uptake via these receptors revealed that preBötC neurons can indeed be targeted [6] (Fig. 4). However, as shown in the latter study, the labeling was not specific and included various other types of (respiratory) brainstem neurons. In addition, respiratory neurons can be targeted in acute slices from transgenic mice that express a fluorescent protein coupled to a promoter such as glutamic acid decarboxylase [10]. Also this approach is yet not specific enough for identifying one particular population of candidate rhythmogenic respiratory neurons. Currently, fluorescence-tagged transcription factors that may be specific for rhythmogenic respiratory neurons, are being constructed in transgenic mice. However, it may turn out that such cells are not characterized by a single characteristic feature that can be visualized in the in vitro models, but rather by a unique pattern of transcription factors, (pacemaker) ion channels plus neurotransmitter receptors and/or transporters.
Acknowledgments
Work contributing to this study was supported by the Canadian Institutes of Health Research (CIHR), the Alberta Heritage Foundation for Medical Research (AHFMR), the Canada Foundation for Innovation (CFI-ASRIP), the Bundesministerium für Bildung und Forschung (BMBF) and the Berstein Center for Computational Neuroscience (BCCN).
Respiratory Network Analysis, Isolated Respiratory Center Functions
Synonyms
Dual respiratory center organization; Mammalian respiratory rhythm generators
Definition
Analysis of isolated respiratory centers means a reductionistic approach for studying the neural control of breathing using in vitro brainstem preparations (mostly from perinatal rodents) in which rhythmogenic respiratory networks remain active. The 10.1007/978-3-540-29678-2_16 has been identified as a brainstem region that generates respiratory rhythm in mammals and remains active in a transverse medullary slice preparation. In a more rostral transverse medullary slice without the preBötC, the pre/postinspiratory active parafacial respiratory group (pFRG) continues to drive facial (VII) motoneurons rhythmically. Although both groups of rhythmogenic neurons operate autonomously in the distinct brainstem slices, they appear to constitute a 10.1007/978-3-540-29678-2_4, at least in less reduced “ 10.1007/978-3-540-29678-2_5” brainstem-spinal cord preparations from perinatal rodents and juvenile rats in vivo. This hypothesis is based on a differential action of opioids on functionally inspiratory (preBötC-driven) or expiratory (pFRG-driven) motor activities in vivo and in vitro suggesting that the pFRG provides a pivotal excitatory drive to the preBötC. Conversely, anoxia appears to synchronize and enhance the activities of both rhythm generators, resulting in pronounced postinspiratory medullary activities and lumbar/facial motor bursting that are accompanied by inspiratory-related nerve burst doublets. The latter findings suggest that the preBötC and pFRG are capable of adjusting their interactions to cope in particular with pathological disturbances of breathing.
Characteristics
Respiratory Network Organization in vitro and in vivo
Three major anatomically defined respiratory groups have been identified in the lower brainstem of mature mammals in vivo by microelectrode analysis of respiratory-related extra- or intracellular activities and subsequent histological identification of the recording sites [1] (Fig. 1). The 10.1007/978-3-540-29678-2_4 is a relay site of peripheral mechano- and chemoreceptor inputs to primary respiratory networks, whereas the pontine respiratory group is important for the generation of the multiphase neuronal activity pattern, which is projected to the respiratory muscles [1]. The 10.1007/978-3-540-29678-2_22 (or rather column) contains arrays of interneurons, which are involved in the generation of the basic rhythm [1,3,4] (Fig. 1). In 1984, Suzue reported that respiratory activity in mammals is retained in vitro, specifically in isolated brainstem-spinal cord preparations from newborn rats [3] (Fig. 1). Extra- and intracellular electrophysiological recording of rhythmic drive potentials and/or action potential discharge in histologically-identified brainstem sites established that different classes of respiratory neurons are active in this en bloc model in areas corresponding to those in adult mammals in vivo [1,3,4] (Fig. 1). Neonatal rat ventral respiratory column neurons have been classified according to the phase relation of their cellular bursting with inspiratory-related cervical nerve bursting. This revealed that such neurons are active during one or several phases of the in vitro respiratory rhythm, which is comprised of a preinspiratory, inspiratory, postinspiratory and an active expiratory (“E-2”) component in brainstem-spinal cord preparations [3,4–7] (Fig. 1).
Respiratory Network Analysis, Isolated Respiratory Center Functions. Figure 1.

Respiratory groups, centers and neuron classes in mammals. Sharp microelectrode membrane potential recordings in the left part of the figure revealed rhythmic depolarizations in six classes of adult cat medullary respiratory neurons. These cells discharge action potentials (black bars) in a specific phase relation with the inspiratory (I) plus postinspiratory (post-I) activities of the phrenic nerve which activates the diaphragm, i.e. the main inspiratory muscle. Grey areas indicate periods of inhibition via GABAA and glycine receptors. Modified with permission from D.W. Richter (in Comprehensive Human Physiology, eds. R. Greger, U. Windhorst; Springer-Verlag, Berlin Heidelberg, 1996). The dorsal schematic view on an adult cat brainstem shows the simplified distribution of I neurons (black areas) and expiratory (E, dotted areas) neurons in the pontine, dorsal and ventral respiratory groups (PRG, DRG, VRG). Note that the rostral portion of the VRG is named ventral respiratory column (VRC). Modified with permission from [1]. The attached ventral view on a newborn rat brainstem shows the locations and rostrocaudal extensions of the parafacial respiratory group (pFRG) and pre-Bötzinger Complex (preBötC) rhythmogenic respiratory centers with reference to the caudal end of the facial (VII) motonucleus, VIIc. The constancy of the rostrocaudal extensions of respiratory marker nuclei such as the VII nucleus and the inferior olive allowed the generation of “calibrated” newborn rat brainstem-spinal cord (“en bloc”) preparations with a defined content of (respiratory) brainstem tissue [2]. The ventral brainstem view also shows the location of cranial nerves and blood vessels, which are used as landmarks for insertion of “patch-clamp” electrodes in the en bloc model for “whole-cell” recordings of membrane potentials from different types of newborn rat VRC neurons. In the right part of the figure, the activity patterns of such neurons are aligned with reference to inspiratory-related activity of ventral cervical nerve roots (C3–6) forming the phrenic nerve. Specifically, these neurons are I neurons (sublabeled Insp-I, Insp-II, Insp-III after [3]), two types of E neurons, and preinspiratory (plus postinspiratory) active “Pre-I”-type pFRG neurons. Modified with permission from H. Onimaru, A. Arata, I. Homma (Respiration & Circulation 46, 773–782, 1998). Abbreviations: E-2, active expiratory phase; BötC, Bötzinger Complex; V-XII, cranial nerves, specifically V, trigeminus; VI, abducens; VIII, vestibulocochleat; IX, glossopharyngeus; X, vagus; XI, accessorius; XII, hypoglossus; C 1, 1st ventral cervical nerve.
Isolation of Respiratory Centers
The findings from a large number of studies using the en bloc brainstem model greatly advanced the understanding of cellular mechanisms involved in the neural control of breathing [3,4]. In that regard, findings from one seminal study [8] supported in 1991 the long-standing hypothesis that breathing movements originate from a limited area, a “noeud vitale,” in the “upper neck” [1]. Specifically, microsection of the newborn rat brainstem-spinal cord preparation was combined with suction electrode recording of cranial and spinal nerve activities to consolidate first the conclusion from previous findings on that model [3] that neither the pontine nor the dorsal respiratory group are necessary for fictive inspiratory-related rhythm [8]. Instead, inspiratory rhythm was irreversibly blocked when microsection affected a medullary area, named the preBötC, which extended ~200 μm in rostrocaudal direction (Fig. 2). Finally, this study demonstrated that the preBötC remains inspiratory active after isolation in a transverse brainstem slice (Fig. 2).
Respiratory Network Analysis, Isolated Respiratory Center Functions. Figure 2.

Isolation of the inspiratory center. (a) the upper panel shows a schematic lateral sagittal section through the ventral aspect of the newborn rat brainstem. The numbers correspond to consecutive 75 μm transverse sections through the brainstem-spinal cord model (Fig. 1). The sections were carried out in rostral to caudal direction, while recording with suction electrodes inspiratory-related cervical nerve bursts which are displayed, after integration, in the lower panel. Such experiments revealed an irreversible block of rhythmic discharge following section 8. These findings were complemented by results from corresponding recordings from inspiratory active cranial nerves during sectioning in caudorostral direction. This identified the preBötC as a brainstem region with a rostrocaudal extension by ~200 μm (see box) which is important for generation of respiratory rhythm. Abbreviations others than those in Fig. 1: LRN, lateral reticular nucleus; RFN, retrofacial nucleus; SO, superior olive; rVRG, rostral ventral respiratory group; cNA, caudal nucleus ambiguus. (b) The preBötC remains inspiratory active in a newborn rat brainstem slice with a thickness >175 μm. Rhythmically active neurons in the area of the ventrolateral medulla rhythmically drive XII motoneurons which innervate via the XII nerve genioglossal tongue muscles for patency of the upper airways during inspiration. (a and b modified with permission from [8].) (c) The constancy of respiratory marker nuclei such as the inferior olive enables the generation of preBötC slices with rostrocaudal boundaries which are “calibrated” by comparison with a newborn rat brainstem atlas [9]. The latter study introduced a terminology for such slices. In this example the “m-preBötC[500/-0.70]W-P2.5” slice contains the preBötC in the middle (“m-preBötC”), is 500 μm thick with the caudal boundary 0.70 mm caudal to VIIc, and was produced from a 2.5 days-old (P2.5) Wistar (W) rat. (d) calibrated preBötC slices generate robust inspiratory-related rhythm in the area of the VRC in superfusate with physiological (3 mM) instead of routinely used 7–11 mM [K+]. This rhythm is very sensitive to low concentrations of the μ-opioid receptor agonist DAMGO. Note that the 3 mM K+ rhythm is effectively restored within few minutes of application of the opioid receptor antagonist naloxone (1 μM). c and d modified with permission from [9]. (e) in a different preBötC slice, 3 mM K+ rhythm was abolished shortly after raising superfusate Ca2+ from 1 mM (the lower limit of the proposed physiological range) to 1.5 mM (the upper limit). Note the incomplete recovery of inspiratory rhythm following washout of raised Ca2+. Modified with permission from [2].
The view that the preBötC constitutes an essential respiratory center is supported since its discovery by numerous in vivo and in vitro studies [3,4]. However, the preBötC is not the only autonomous respiratory center in mammals. Already more than 20 years ago, the hypothesis has been proposed that pre/postinspiratory active “Pre-I” neurons are important for maintenance of the rhythmic activity of inspiratory medullary networks in the newborn rat en bloc preparation [3]. More recent findings from voltage-sensitive dye imaging of spatiotemporal respiratory patterns and concomitant electrophysiological recording of membrane potential indicated that Pre-I neurons form the pFRG, a functionally and anatomically defined respiratory group [6] (Fig. 1). The pFRG remains rhythmically active and drives VII motoneurons in a transverse slice of brainstem tissue that rostrally neighbors the preBötC [7] (Fig. 3). Most pFRG neurons are active during both the preinspiratory and postinspiratory phase, but are subject to pronounced inhibition via hyperpolarizing GABAA and glycine receptor-mediated inhibitory postsynaptic potentials during the inspiratory phase [3,5–7] (Fig. 1). In contrast, pFRG neurons are continuously active for a time period of several seconds in the slices with rhythmic VII nerve activity [7] (Fig. 3). This supports earlier assumptions that the preBötC is responsible for inspiratory inhibition of Pre-I cells in the en bloc medullas [3]. Although “reference” inspiratory motor activity is missing in the rhythmic pFRG slices, it is likely that the sustained neuronal and VII nerve activities (Fig. 3) span the preinspiratory, inspiratory plus postinspiratory phases.
Respiratory Network Analysis, Isolated Respiratory Center Functions. Figure 3.

Rhythmic pFRG activity in a transverse slice of brainstem tissue rostral to the preBötC. (a) the newborn rat en bloc model was transected slightly rostral to the most rostral XII root to obtain a transverse slice for simultaneous suction electrode recording from the VII nerve and whole-cell recording of membrane potential (MP) from neurons within the ventrolateral medulla. (b) membrane potential oscillations of putative Pre-I neuron in the rostral block were synchronous with VII nerve activity in “Suzue-type” solution with 6.2 mM K+ and 2.4 mM Ca2+. Shortly before the recording, the preparation was treated for 10–15 min with DAMGO (1 μM) for enhancement of such bursting. (c) histological reconstruction revealed that the transection was between VIIc and the rostral boundary of the preBötC, thus in the region corresponding to the BötC in adult mammals (compare Fig. 1). (d) in a preparation transected at a level similar to that in the experiment of a–c, but without removing the rostral block, DAMGO in 6.2 mM K+ and 2.4 mM Ca2+ abolished inspiratory-related C4 activity in the caudal aspect of the transected preparation, but restored VII nerve rhythm, which was transiently depressed due to the transection procedure. These findings suggest that pFRG neurons in rostral medullary slices, not including the preBötC, produce rhythmic bursting which is facilitated by opioids, in contrast to a strong depressing action of such drugs on more caudal preBötC-driven rhythms. Modified with permission from [7,8].
Bursting of pFRG neurons during these phases is in accordance with the finding that branches of the VII nerve innervate muscles of the alae nasi that decrease the nasal airway resistance in cats and dogs before, during and after inspiration [7]. Conversely, the finding that interneurons in the ventrolateral aspect of preBötC slices induce rhythmic activity of XII motoneurons (Fig. 2) strongly suggests that the rhythm in that model is inspiratory-related [8], because subgroups of XII motoneurons innervate the tongue during inspiration for patency of the upper airways [1,4].
Determinants of Isolated Respiratory Center Activities
The rhythmic activities of the isolated respiratory centers are not identical with those in intact animals or less reduced in vitro preparations such as the “working heart brainstem preparation” of rodents [see corresponding chapters]. Though, activities in the rhythmic slices share several features with respiratory behaviors in vivo. For example, in juvenile rats preBötC-driven inspiratory activity is blocked by opioids, whereas rhythmic contractions of pFRG-driven expiratory abdominal muscles are not inhibited [4,5]. Similar to these in vivo findings, opioids depress preBötC-driven (motor) rhythms in vitro, whereas pFRG-driven cellular and nerve activities are not inhibited [4,5,7]. The effects of various neuromodulators on the in vitro respiratory-related rhythms are influenced by the experimental conditions, which differ notably between laboratories. In particular the superfusate concentrations of K+ and Ca2+ vary between 3–11 mM and 0.8–2.4 mM, respectively, despite the notion that these cations strongly modulate neuronal excitability [2]. Regarding the action of opioids, preBötC slice rhythms in physiological K+ (3 mM) and 1 mM Ca2+ are blocked by low nanomolar concentrations of opioids (Fig. 2), whereas close to micromolar concentrations are needed to depress rhythms in preBötC slices or en bloc medullas in superfusate with elevated K+ (Fig. 3) [7,9]. Furthermore, preBötC slices generate long-term and robust rhythm in 3 mM K+ and 1 mM Ca2+ (corresponding to the lower range of the physiological spectrum), whereas rhythm is depressed by elevation of Ca2+ to 1.5 mM (the proposed upper limit of the physiological range) [2,9] (Fig. 2). In 1.5 mM Ca2+, preBötC slice rhythm is reactivated by raised K+ leading to the view that isolated inspiratory center activity is determined by an extracellular “Ca2+/K+ antagonism” [2].
Respiratory center rhythms depend also critically on the physical dimensions of the in vitro models. For example, findings in the newborn rat en bloc model indicated that the pFRG drives pre/postinspiratory bursting of lumbar motoneurons in spinal L1–2 segments via premotoneurons which are located caudal to the preBötC [5]. This view was substantiated by the observation in juvenile rats in vivo that brainstem transection at the caudal end of the VII nucleus, which partially overlaps with the pFRG [6] (Figs. 1 and 3), abolished pre/postinspiratory bursting of expiratory abdominal muscles innervated by L1–2 lumbar motoneurons [4]. Similar transection experiments in the newborn rat en bloc model revealed that the transection level critical for blocking pre/postinspiratory lumbar bursting is quite close to the rostral instead of the caudal end of the VII nucleus [2]. The absence of respiratory lumbar bursting in such transected preparations suggests that pFRG neurons responsible for this motor behavior are located in the most rostral aspect of the pFRG [2] (Fig. 1). However, it is for example also possible that axons from more caudal pFRG neurons inducing lumbar respiratory bursting project first rostrally and may thus have been transected [2,10]. This indicates that results from transection experiments need to be considered with caution. Furthermore, Pre-I neurons are not only found in the main area of the pFRG, but also within the preBötC, and even in regions caudal to the preBötC. In addition to Pre-I and expiratory cells, the preBötC in both perinatal rodent en bloc medullas and in vivo contains various subclasses of inspiratory neurons [3] (Fig. 1). Conversely, inspiratory (and expiratory) neurons are also active in the main area of the pFRG. While the rostral portion of the pFRG co-locates with the VII nucleus, the caudal part of the pFRG partially covers an area corresponding to the Bötzinger Complex in mature mammals. Finally, the pFRG also more or less overlaps medially with the Retrotrapezoid nucleus, which is one presumptive site of central respiratory chemosensitivity [4].
Due to the overlap of primary (rhythmogenic) areas and secondary (chemosensitive) respiratory drive regions, in concert with a rostrocaudally dispersed distribution of distinct classes of respiratory neurons, the respiratory centers can not be isolated without portions of functionally and/or anatomically different structures that may interact with these centers. Despite these caveats, the reductionistic approach has already, and will further, provide important information on the neural control of breathing. For example, multiphoton/confocal Ca2+ imaging has been adapted to study the activity and gross morphological features such as soma size or shape of preBötC neurons, located in a histologically defined rostrocaudal area of “calibrated” preBötC slices that operate in physiological cation solution [9] (Fig. 2) [see Respiratory network analysis, functional imaging]. A structure-function relationship of the isolated respiratory centers may be feasible by using the calibrated in vitro models in combination with nerve and intracellular electrophysiological recording plus Ca2+ and voltage-sensitive dye imaging, which was crucial for identification of the pFRG [6] [see Respiratory network analysis, functional imaging].
A Dual pFRG-preBötC Respiratory Center
Findings in the en bloc brainstem model and juvenile rats in vivo suggest that the pFRG and the preBötC constitute a dual respiratory center. This hypothesis has been first proposed according to the above described distinct effects of opioids on inspiratory and pre/postinspiratory motor behaviors. These findings led to the conclusion that opioids inhibit breathing, at least partly, due to depression of synaptic excitatory transmission between the pFRG and the preBötC [4,5]. This view that excitatory drive from the pFRG to the preBötC is necessary for robust activity of the preBötC has already been proposed much earlier based on the finding in the en bloc model that focal lesion of the area including the pFRG impairs inspiratory cervical motor output [3]. However, as stated above the preBötC slices are capable of generating robust rhythm in physiological ion solution for several hours in the absence of structures corresponding to the main location of the pFRG [2,9]. The finding that rhythmic VII nerve activity in the pFRG slices is not depressed, but rather stimulated, by μ-opioid receptor agonists [7] (Fig. 3) supports the view that this respiratory center may be in particular important for breathing during the perinatal period, when the brainstem is presumably subject to a surge by endogenous opioids [4].
Anoxia represents a further approach for analyzing the cooperativity of the pFRG and the preBötC. In the en bloc model, both hypoxic and chemical anoxia synchronize and enhance the activities of the pFRG and preBötC rhythm generators. This results in pronounced and persistent (>20 min) postinspiratory medullary activities and lumbar/facial motor nerve bursting during anoxia which is accompanied by inspiratory-related nerve burst doublets [10]. A causal relation between the latter anoxia-related phenomena is suggested by the finding that control pre/postinspiratory lumbar bursting is absent (similar to anoxia-induced enhancement of postinspiratory lumbar bursting and inspiratory-related nerve burst doublets) upon transection of the newborn rat en bloc preparation between the preBötC and the caudal end of the VII nucleus [10]. These results suggest that the anoxic postinspiratory augmentation of medullary interneuronal and lumbar/facial nerve bursting as well as inspiratory motor burst doublets require an interaction between the preBötC and the pFRG [10].
In summary, the rhythmogenic preBötC and pFRG appear to constitute a dual respiratory center, which adjusts its activity to cope with pathological disturbances of breathing. Under the influence of opioids, boosted pFRG activity may partly compensate for the depressed intrinsic preBötC interneuronal activity and provide enhanced drive for breathing efforts. During oxygen depletion, a functional reorganization of the preBötC and pFRG for synchronized and augmented bursting may optimize uptake of oxygen by enhancing single breaths. However, the extent of cooperativity between these respiratory centers during normal breathing is not clear yet. In particular, it remains to be shown that the pFRG has a similarly important role for breathing in mature mammals compared to newborns. That the pFRG is active in vivo after birth is indicated by the above finding of pre/postinspiratory abdominal muscle activity in juvenile rats [4,5]. It may be important to study whether the pFRG in mature mammals is closely related to the chemosensitive Retrotrapezoid nucleus [4]. That this may be the case is suggested by the anatomical overlap of these brainstem regions and by the findings that neurons in both regions are excited by raised levels of CO2 and H+ and project to other respiratory areas including the preBötC [3,4].
Respiratory Network Responses to Hypoxia
Definition
Respiratory network responses to 10.1007/978-3-540-29678-2_8 refer to the complex interactions between groups of neurons located mainly in the medulla, pons and midbrain that are responsible for control of ventilation during hypoxia. The physiologic mechanisms underlying these processes involve intricate interplay of 10.1007/978-3-540-29678-2_14 released from respiratory neurons and glial cells. The ventilatory response to hypoxia is precisely controlled and distinctive patterns emerge during postnatal development. The functional role of this process is not only to adapt the respiration during hypoxic conditions, but also to ensure cell survival, especially during the vulnerable period of development.
Characteristics
The mammalian ventilatory response to hypoxia is biphasic. It consists of an initial increase in minute ventilation, followed by a later decline in ventilation which is termed hypoxic ventilatory depression (Fig. 1).
Respiratory Network Responses to Hypoxia. Figure 1.

Representative recording of minute ventilation during a 20-min hypoxic challenge followed by 10 min recovery in normoxia in a 14-day old rat pup. Please note initial increase in minute ventilation (HVR) followed by progressive time-dependent reduction in ventilatory output (HVD).
In developing mammals, the magnitude of hypoxic ventilatory depression is particularly prominent such that minute ventilation decreases below normoxic level [1]. The early component of hypoxic ventilatory response is mediated mainly through the 10.1007/978-3-540-29678-2_16 in the 10.1007/978-3-540-29678-2_3. The type I glomus cells in the carotid body are the primary site of oxygen sensing signal. The afferent signal is then transmitted to sensory terminals of the carotid sinus nerve and is subsequently projected to the nucleus of solitary tract (nTS). The nTS is located in the brainstem region and provides the first central synaptic relay to peripheral chemoreceptor afferent inputs. Other nuclei at this level of brainstem that play a role in respiratory control and the HVR include the nucleus ambiguus, the area postrema, the dorsal motor nucleus of vagus, the hypoglossal nucleus and the pre-Botzinger complex. A variety of neuromodulators in these areas play a crucial role in the central mediated hypoxic ventilatory response. Several studies have shown that the early response to hypoxia is mediated through platelet activating factor receptor pre-synaptically [2,3] and post-synaptically by N-methyl-D-aspartate (NMDA) glutamate receptors [4,5], which then activate downstream signaling pathways such as protein kinase C, tyrosine kinases, and calcium calmodulin kinase [6]. The hypoxic ventilatory depression is mediated through several complex mechanisms. In addition to hypoxia-induced reductions in metabolism, several neuromodulators have been thus far identified as playing a role in the hypoxic ventilatory depression, namely adenosine, γ-aminobutyric acid (GABA), serotonin (5-HT), opioids, and platelet derived growth factor (PDGF-β) receptors.
In this section, we will discuss the respiratory neuronal network with particular emphasis in the caudal brainstem, and will delineate specific neuromodulators mediating each component of hypoxic ventilatory response from a developmental perspective.
Respiratory Neuronal Network
The peripheral chemoreceptors are located in carotid bodies and aortic bodies. These areas contain glomus cells of 2 types. The type I glomus cells in the carotid bodies is oxygen sensing cells of the peripheral chemoreceptor. The hypoxic stimulus is then transmitted to the sensory terminal of the carotid sinus nerve (CSN). From CSN, the signal projects to the several regions of the nucleus of solitary tract (nTS), the first synapses for primary afferents originating from peripheral chemoreceptors. Retrograde tracer studies reveal that the medial, dorsomedial, lateral and commissural regions of the nTS receive dense innervations from peripheral chemoreceptor afferent fibers. The nTS, the main neuonal nucelus of the 10.1007/978-3-540-29678-2_4, has interconnections to other respiratory neurons including the pontine respiratory and the 10.1007/978-3-540-29678-2_22. The pontine respiratory group is composed of the lateral and medial parabrachial and Kollicker-Fuse nucleus, which play a role in diaphragmatic motor control and respiratory rhythm modulation. The ventral respiratory group is divided into the rostral and the caudal group. The rostral part of ventral respiratory group includes the Botzinger complex, the pre-Botzinger complex, and the parafacial respiratory group. The pre-Botzinger complex and the parafacial respiratory group are believed to encompass the kernel for respiratory rhythmogenesis. In addition, there are influences from many rostral brain areas to the respiratory neurons including the suprapontine nuclei, midbrain, diencephalons, hypothalamus, cerebellum, and regions of the cerebral cortex.
Neuromodulators
Early HVR
Platelet Activating Factor Receptors
Platelet activating factor and its cognate receptor (PAFR) are proposed to modulate glutamatergic signaling presynaptically, thereby influencing the release of glutamate into the synaptic cleft. PAFR activity has now been conclusively implicated in the acute ventilatory response to hypoxia [2,3].
NMDA Glutamate Receptors
In the cardiorespiratory control regions, N-methyl-D-aspartate glutamate (NMDA) receptors mediate critical components of the respiratory pattern generation, cardiovascular regulation and HVR. The early response to hypoxia is mediated through NMDA glutamate receptors [4,5]. NMDA receptors are widely expressed throughout the brain, including the respiratory control areas such as nTS. Previous studies have demonstrated that systemic and targeted brainstem administration of NMDA glutamate receptor antagonists is associated with attenuation of HVR in adult and developing animals. In addition, hypoxia induces an increase in glutamate concentration within the nTS of conscious rats, which is associated with an increase in minute ventilation. However, the hypercapneic (hypercapnia) ventilatory response is not affected by NMDA glutamate receptors [5]. The structure of NMDA glutamate receptors consists of heterodimeric, mandatory subunits that include one or more of the splice variants of the NMDA NR1 subunit and additional NR2 and NR3 subunits. Activation of NMDA receptors in the caudal brainstem of conscious rats involves tyrosine phosphorylation of both NR1 and NR2A/B subunits. In addition, the role of NMDA receptor in HVR is developmentally regulated such that an increasing dependency on NMDA glutamate receptor emerges over time and transition from an immature to a more mature hypoxic response requires NMDA receptor-bearing neurons within the nTS.
Non-NMDA Receptors
Previous studies have indicated the potential role of AMPA glutamate receptors in the ventilatory control and the HVR. Administration of NBQX (a selective non-NMDA receptor antagonist) did not affect ventilatory output in adult conscious mice and cats, but led to marked respiratory depression in neonatal animals. Microinjection of the AMPA glutamate receptor blocker NBQX within the nTS of anesthetized adult rats resulted in attenuation of ventilatory responses following carotid body stimulation. However, NBQX failed to modulate hypoxia-induced c-Fos activation in the adult rat. AMPA receptors appear to influence the respiratory pattern in the immature animal. The respiratory rhythm generation in neurons within the pre-Botzinger complex of neonatal rats is dependent on AMPA receptor activity. Notwithstanding, the role of AMPA glutamate receptors in the developmental of respiratory control may be limited to the regulation of timing mechanisms during normoxia, but not in mediating the hypoxic ventilatory responses [7].
Intracellular Downstream Signaling Pathways Underlying the early HVR
During the early HVR, NMDA receptors (NMDA-R) activation elicits calcium influx, and subsequent activation of phospholipase C (PLC), mitogen-activated protein kinase kinase (SEK) and calcium calmodulin kinase 2 (CaCmII). Our previously proposed model suggests that activation of PLC leads to translocation of protein kinase C, and phosphorylation of serine/threonine residues in the intracellular domain of NMDA receptors. SEK phosphorylates stress activated protein kinase 2 (JNK-2) leading to activation of the activator protein-1 complex (AP-1). CaCmII activates neuronal nitric oxide (NO) synthase resulting in NO formation. NMDA receptor activation will also lead to phosphorylation of IκB with subsequent activation of nuclear kappa B (NF-κB) and activation of tyrosine kinase (TK) by tyrosine phosphorylation (PY) (Fig. 2).
Respiratory Network Responses to Hypoxia. Figure 2.

Schematic diagram of signaling pathways that are operational in respiratory neurons within the nucleus of the solitary tract during hypoxia. Signal transduction proteins for which there is definitive evidence are shown in red. (See text for more details).
Of all the downstream signaling pathways, the functional role of PKC on respiratory control neurons has been studied extensively. PKC activation within the respiratory neurons of the ventral medullary group is associated with increased respiratory drive potentials. Endogenous PKC activity modulates tonic activity and excitability of the expiratory neurons in the cat. PKC within the caudal brainstem underlies critical components of both tonic respiratory drive and the HVR [6]. Most of the known PKC isoforms are expressed with in the dorsocaudal brainstem, and activation of both Ca2+- dependent and Ca2+- independent PKC isoforms occurs in the nTS during hypoxia [6]. PKC exerts a significant influence on respiratory timing during normoxia in the early postnatal period, and the effect decreases with advancing age. In contrast, hypoxia-induced PKC activation is absent in the immature animal and emerges concomitantly with the appearance of NMDA dependency. Nitric oxide (NO) is another important neuromodulator with a dual role in hypoxic chemotransduction. While NO derived from endothelial nitric oxide synthase (eNOS) exerts an inhibitory effect at the carotid body level, NO derived from neuronal nitric oxide synthase (nNOS) in the caudal brainstem plays a significant role in sustaining ventilation during the second phase of the HVR. Activation of NMDA receptors will lead to opening of a voltage-dependent calcium channel, calcium calmodulin binding and subsequent nNOS activation. The intracellular NO will in turn modulate glutamate release, either through activation of cGMP-dependent protein phosphorylation cascades, or by retrograde activation of the pre-synaptic neuron. Therefore, nNOS acts an excitatory neurotransmitter during the HVR and may prevent the early onset of hypoxia-induced ventilatory depression. In addition, we have recently identified a mechanism whereby deoxyhemoglobin elicited by the presence of environmental hypoxia activates the formation of S-nitrosothiols through a very tightly regulated process, and that these compounds lead to excitation of respiratory-related neurons within the nTS, and thus contribute to the early phase of HVR [8].
Late HVR
As the duration of hypoxia is extended, some degree of ventilatory depression will develop. This component of HVR is extremely prominent in developing animals. Several neuromodulators including γ-amino-butyric acid (GABA), serotonin (5-HT), adenosine, opioid receptors, and platelet-derived growth factor (PDGF)-β receptors have all been shown to play contributory roles to the emergence of the hypoxic ventilatory depression associated with prolonged hypoxia. We will briefly delineate the role of each neuromodulator in the late phase of HVR. GABA acts through two GABA receptor subtypes, GABA-A and GABA-B. GABA-A receptors modulate tidal volume, whereas GABA-B receptors modulate respiratory frequency and pattern of breathing. It is postulated that the hypoxic ventilatory depression is the result of imbalance between the excitatory glutamate and the inhibitory GABA [4]. In addition, hypoxic ventilatory depression of developing animals is partly mediated through the neuro-depressant effect of GABA. Another neuromodulator, adenosine plays an important role in hypoxic ventilatory depression during the early postnatal period, and the effect decreases with maturation. Among the major 4 adenosine receptors (A1, A2A, A2B and A3), adenosine A1 and A2A receptors are postulated to play a role in the hypoxic ventilatory depression. The inhibitory effect of adenosine A1 receptors may involve postsynaptic hyperpolarization, presynaptic depression of synaptic transmission, modulation of cAMP mediated pathway and activation of potassium channels. While adenosine A1 receptors are involved in cardiorespiratory control during normoxia, adenosine A2A receptors play a critical role in the hypoxic ventilatory depression. Serotonin (5-HT) has been shown to play a role in hypoxic ventilatory depression in both adult and developing animals. This neurotransmitter exerts multiple effects on respiratory control, and modulates both the respiratory rhythm generator and the respiratory motoneurons. Among the myriad of 5-HT receptor subtypes, 5-HT1A and 5-HT2 receptors have been shown to play a role in respiratory control. While 5-HT exerts an excitatory effect on the central respiratory rhythm generator within the rostral medulla area, it inhibits the hypoglossal inspiratory output in developing animals, possibly through activation of 5-HT2 receptors. The release of endogenous 5-HT may signal the termination of the early hypoxic augmentation, possibly through activation of 5-HT1A receptors. 5-HT1A receptors are present in the hypoglossal nucleus of developing rats. Their density is high in the newborn and decreases with increasing postnatal age. Since the use of morphine led to occasional onset of respiratory depression, it became apparent that opioids are involved in respiratory functions within the CNS. Interestingly, endogenous opioids have been shown to play a role in the late phase of hypoxic ventilatory response, whereby ventilatory depression may be partly mediated through opioid-mediated neuronal inhibition. Opioids modulate the respiratory frequency and tidal volume through activation of μ- and δ-opioid receptors respectively. The caudal brainstem, especially the nTS and the nucleus ambiguus, seem to be important sites for opioid-induced inhibition of respiration. Opioid receptors display a distinct maturation pattern during the early postnatal period. The μ-opioid receptor binding sites are present during the mid-fetal period and are located in the cardiorespiratory-related brainstem nuclei, whereas the δ-opioid receptors primarily appear during the postnatal period. Both μ(1) and μ(2) opioid receptors are involved in opioid-induced respiratory depression in early postnatal period.
Finally, we have shown that hypoxia specifically triggers the release of the PDGF polypeptide isoform called PDGF-BB from glial cells, which in turn leads to subsequent activation of PDGF-β receptor in the nTS, where it reduces ventilatory output [9]. Both PDGF-B chains and PDGF-β receptors are abundantly expressed in nTS neurons of adult rats [9], and activation of the receptors leads to down-regulation of ligand-gated ion channels, such as NMDA glutamate receptors. PDGF-β receptor activation is an important contributor to the hypoxic ventilatory depression at all postnatal ages, but is more critical in the immature animals. The increased expression of PDGF-β receptors in the caudal brainstem of immature animals may provide additional protection against hypoxia-induced 10.1007/978-3-540-29678-2_1. In fact, PDGF-β receptors exert their role in promoting neuronal cells survival via two major signaling pathways, namely the phosphoinositide 3 kinase (PI3K)/Akt and the MEK/MAPK pathways. Activation of PDGF-β receptors leads to tyrosine phosphorylation of sites that will activate Ras kinase. Ras can activate PI3K, which in turn may phosphorylate PI3K, which in turn may phosphorylate the serine-threonine protein kinase called Akt, the latter phosphorylating BAD at serine 136. Phosphorylated BAD binds to cytosolic 14-3-3 protein, whereas dephosphorylated BAD binds elements of the Bcl-2 complex such as Bcl-x to promote apoptosis (Fig. 2). In fact, hypoxia-induced phosphorylation of PDGF-β receptors in the caudal brainstem of adult rats is temporally associated with activation of an anti-apoptotic mechanism via the PI3 kinase-dependent phosphorylation of both Akt and BAD pathways [10]. This mechanism may prevent induction of apoptosis in the respiratory neurons during hypoxia, and may contribute to the well known increased hypoxic tolerance of the brainstem neurons.
Respiratory Neuroplasticity
Synonyms
Respiratory memory; Respiratory recovery from injury; Lasting alteration of breathing reflexes
Definition
Respiratory Neuroplasticity has been defined as “a persistent change in the neural control system (morphology and/or function) based on prior experience” [1]. Plasticity exists in various forms in all of the segments of the respiratory network; the afferent, central control and efferent segments. The plasticity may be classed as recovery from injury, respiratory memory, and lasting alterations of protective reflexes such as cough.
Characteristics
The basic respiratory rhythm and pattern is generated by neural networks in what is called the ventral respiratory column (VRC) of the medulla. The column spans several groupings of cells, called nuclei, in the ventral lateral medulla from nearly the beginning of the cervical spinal cord almost to the pons. However, breathing is modulated by many other regions of the medulla, such as the midline raphe, the main source of the neurotransmitter serotonin. Neural networks in the pons, cerebellum and cerebrum can also affect breathing and play roles in breathing plasticity.
The central respiratory neural networks use information, or afferent input, from various sensors. Important examples include the carotid and aortic bodies that sense carbon dioxide concentrations, as well as acidity (pH) but are the predominant sensors of oxygen in the blood. There are also sensors in the lung that transmit information about lung distension, irritation and excess fluid. Sensors within the medulla itself feedback pH of the brain tissue. Since carbon dioxide and pH are in chemical balance in the body, and brain tissue only functions well in a narrow range of pH, it is important that the respiratory control system tightly control elimination of carbon dioxide produced by metabolism as well as provide oxygen.
Neurons that connect with muscles are called motor neurons. Two important groups of such neurons that reside in each side of the spinal cord are known as phrenic motor neurons. Phrenic motor neurons receive drive, or efferent input, from the VRC directly and in many species indirectly from another group of medullary cells called the dorsal respiratory group (Fig. 1a) Phrenic motor neurons send axons through the phrenic nerves to provide drive to the diaphragm, the major muscle that expands the lungs and produces inspiration of air.
Respiratory Neuroplasticity. Figure 1.

Firing rate histograms from multi-site recordings during induction of long-term facilitation (LTF). a, schematic dorsal view of the cat brainstem showing midline raphe, Ventral Respiratory Column (VRC), and the Dorsal Respiratory Group (DRG) b, Data segments show firing rate histograms of 17 neurones and integrated phrenic nerve activity, recorded simultaneously at the indicated sites during the first (I) and fifth (II) period of carotid chemoreceptor stimulation and 6 min following the fifth and final stimulus and induction of LTF (III). Numbers on the right are the firing rates that correspond to the highest “bin.” This demonstrates that phrenic amplitude has nearly doubled, while the rate of cycling has increase. Note that some cells have persistent greater peak activity with concomitant shorter durations of activity corresponding to the shorter respiratory phases. Other cells that may be inhibitory to inspiratory activity have persistent decreases in activity (adapted from Fig. 2 of [4] with permission).
Recovery from Injury
In response to loss of partial function, such as removal of important afferent input, the respiratory neural networks can recover significant normal function. This plasticity varies among species and with age. After surgical removal of 10.1007/978-3-540-29678-2_3, normal response to low oxygen, hypoxia, is temporarily lost, but can be recovered in some species, such as cats or rats. Human asthmatics who have had carotid bodies removed show little or no recovery of function. Dogs, ponies and goats recover much less of this function while one-day-old goats that have carotid bodies removed completely recover normal responses to hypoxia. Mechanisms of recovery include up-regulation of alternative input, in this case that from the aortic bodies and perhaps other tissues as well as up-regulation of the efferent limb so that phrenic motor neurons become more responsive to drive.
In response to spinal cord injury, pathways that have little or no activity normally can become active. If one half of the spinal cord is cut, the diaphragm on that side becomes paralyzed because the efferent output of the VRC for phrenic motor neurons is interrupted. However, under increased respiratory drive, alternate, usually inactive pathways from the uninjured side can be activated resulting in partial recovery of function. This is known as the “crossed phrenic phenomenon” [1,2].
Memory
Long-term facilitation (LTF, Fig. 1b), an increase in respiratory motor output that persists more than 1 h, is another type of plasticity and a robust type of memory. Induction and expression of this memory can be blocked by serotonin and brain derived neurotrophic factor antagonists [3]. LTF is induced by repeated brief, intermittent, but not extended, hypoxia, chemical stimulation of carotid chemoreceptors, or electrical stimulation of the carotid sinus nerve or brain stem midline but not by hypercapnia. In some experiments with cats, rats, dogs and goats, LTF can increase measures of phrenic nerve activity to approximately twice that of baseline.
Altered activity and connectivity among neurons in the VRC and raphe neurons have been identified in spike train data sets in which; (i) the constituent neurons had respiratory-modulated firing patterns, (ii) significant changes in firing rate during carotid chemoreceptor stimulation were correlated with altered respiratory efferent activity, (iii) persistent firing rate changes were expressed during LTF, (iv) there was evidence of effective connectivity between the recorded neurons appropriate to contribute to LTF, and (v) changes in measures of effective connectivity between these neurons after induction of LTF were greater than those during different control periods [2,4].
LTF has been demonstrated in rats in both the activity of the phrenic nerve and in sympathetic nerve activity that is involved in control of blood pressure [9]. Human beings who have sleep apnea are exposed to brief periods of hypoxia each night, similar to many protocols that produce LTF in animal experiments. These people have increased sympathetic nerve activity during the day when they are not hypoxic, and that activity has increased modulation with their breathing. The increased sympathetic nerve activity probably contributes to their increased incidence of high blood pressure, cardiovascular disease and stroke. Normal awake human subjects experimentally exposed to brief, intermittent hypoxia show persistent changes in breathing pattern, they breathe more shallowly and faster as well as with less variability [5]. However, they do not have an over-all increase in breathing similar to LTF in some animals. In contrast, subjects with sleep apnea have a persistent increase in breathing in response experimental intermittent hypoxia during sleep [3].
The plasticity of the neural network expressed as LTF can therefore be both adaptive and maladaptive. It may act to stabilize upper airways and prevent further hypoxia. However, if the intermittent hypoxia persists it may contribute to hypertension and attendant illness.
Sudden infant death syndrome (SIDS) is the most common cause of death in infants between 2 weeks and 1 year of age. Some SIDS cases appear to result from fetal neural damage that later compromises responses to breathing or blood pressure challenges during sleep. A major risk factor is pre- or post-natal tobacco smoke exposure. The deficits appear to involve alterations in neural network function within regions involved in oxygen-sensing and cardiovascular control. A developmental abnormality in serotonergic neurons in the caudal raphe, i.e., a major part of the network implicated in LTF, may result in a failure of protective responses to life-threatening stressors during sleep [6].
Finally, the respiratory networks demonstrate a “meta-plasticity” in that early exposure to hypoxia or hyperoxia can produce life long changes in respiratory behaviors, responses and plasticity [1]. Neonatal hyperoxia produces plastic changes that lead to blunted responses to hypoxia in later life, whereas hypoxia in infancy produces greater adult hypoxic ventilatory response and increased expression of LTF [1].
Airway Defensive Reflexes
Cough is an essential component of pulmonary defense and is the most common manifestation of pulmonary disease. Cough is the single most common reason why sick patients visit physicians in the United States. The function of cough is to remove fluids, mucus, and/or foreign bodies from the respiratory tract by the generation of high velocity airflows. These airflows during cough are generated by a complex motor pattern involving three phases: inspiration, compression, and expulsion. The inspiratory phase of cough is generated by a large burst of activity in inspiratory muscles, such as the diaphragm. The compressive phase of cough is produced by laryngeal closure caused by a burst in expiratory laryngeal muscles during rapidly increasing expiratory thoracic and abdominal muscle activity. The resulting large increase in lung air pressure produces very high airflows (up to 12 L s−1 in humans) when the larynx opens and the expulsive phase begins. The expulsive phase is characterized by extremely large bursts of activity in expiratory thoracic and abdominal muscles.
In the lower airways, slowly adapting receptors (SARs), rapidly adapting receptors (RARs), and pulmonary C-fibers all can influence the production of cough. There is little doubt that RARs can elicit cough. SARs have a permissive role in the production of cough. The exact role of C-fibers in the production of cough is more controversial, with some groups supporting an excitatory role and others supporting an inhibitory role. Sensory information is processed in the 10.1007/978-3-540-29678-2_2, where the basic elements responsible for the production of cough are located. Pulmonary afferent information is processed by second order interneurons located near to and in various subnuclei of the nucleus of the tractus solitarius.
It was once thought that neural networks separate from those controlling normal breathing, eupnea, controlled other reflexes that defend the upper airways and lungs, such as cough. Recent research has revealed that the brainstem neural networks that produce eupnea also are involved in coughing and sneezing as well as other less well known reflexes. The process by which the brainstem neural network for breathing can be involved in the production of other behaviors is known as reconfiguration. That is, the breathing network changes its “circuit diagram” to allow for the generation of a non-breathing behavior [2]. This process involves alteration of the discharge patterns and effective connectivity of neurons in the respiratory network. The reconfiguration process may also involve “conscription” of neurons that have little to do with breathing but have activity patterns that are selective for certain behaviors, such as cough. This conscription may include recruitment of previously silent neurons and significant modification of the activities of neurons during cough that were not modulated during breathing. There is good evidence that these processes take place and that, in addition to the network that controls breathing, coughing is also controlled by another brainstem control mechanism known as a gate. In essence, the system can be functionally subdivided into a controller (the gate) and an effector (the brainstem respiratory network). The controller regulates the excitability of the behavior and the effector is responsible for the coordination of motor drive to respiratory muscles for cough. During breathing the gate, or controller, is functionally quiescent and the respiratory network is primarily involved in the production of breathing. When RARs are stimulated, the gate becomes active and the brainstem respiratory network reconfigures to produce coughing [7].
Plasticity of the Cough Reflex
There is considerable evidence that cough can undergo significant plasticity in both humans and animals, especially during induced or naturally occurring airway disease. This plasticity is usually manifest in the form of an increased number of coughs in response to a given stimulus and/or increased sensitivity to inhaled irritants. The relative role of central and peripheral mechanisms in this plasticity is less well understood.
In humans, chronic spontaneous cough lasting for years is well documented and can occur in a variety of conditions, such as smoking, asthma, chronic obstructive pulmonary disease, upper airway disorders, and gastro-esophageal reflux. In many of these conditions, the sensitivity of humans to inhaled irritants is elevated but this enhanced cough sensitivity resolves with successful treatment of the underlying disorder. However, tobacco smoke exposure during childhood is associated with cough in adulthood, suggesting that there is a permanent alteration of some important part of the cough reflex [8]. The increased sensitivity of the cough reflex in these patients is consistent with plasticity. It is presumed that hyperexcitable peripheral afferents are responsible for the enhanced coughing in these patients, but the potential contribution of central mechanisms has been difficult to address in humans.
Very similar observations have been made in animal models of airway disease and it is well established that airway sensory afferents responsible for cough undergo significant plasticity in many of these conditions. It also has been shown in an animal model that inflammation of one region of the airway will elicit an enhanced cough response to stimulation of a non-inflamed region of the airway. Presumably the sensory afferent responsiveness in the non-inflamed region of the airway was normal. This suggests that plasticity can occur in the central cough neural networks.
Cough can undergo hypoexcitability during neurological diseases. Stroke, Parkinson’s Disease and Multiple Sclerosis are all associated with cough weakness or an inability to cough at all. This cough impairment can contribute to an increased susceptibility to aspiration in these patient groups. In stroke, cough impairment can occur even if the lesion does not include the brainstem. This fact, in combination with the knowledge that cough can be produced voluntarily suggest that suprapontine mechanisms can be important in the regulation of cough excitability in awake humans. The extent to which these mechanisms can be subject to plasticity is unknown. More research must be performed to gain a greater understanding of these mechanisms.
Respiratory Neurotransmitters and Neuromodulators
Synonyms
Neurochemicals; Endogenous receptor agonists
Definition
Chemicals synthesized by neurons involved in generating rhythm and pattern in the respiratory network, and in transmitting input signals from central and peripheral chemoreceptors and mechanoreceptors to respiratory interneurons and motoneurons (Fig. 1).
Respiratory Neurotransmitters and Neuromodulators. Figure 1.

A schematic diagram of the neuronal interactions between the respiratory center and other modulatory structures, and of putative neurotransmitters and neuromodulators involved in the neuronal control of respiratory rhythm and pattern generation. The main neurotransmitters of respiratory neurons are glutamate, GABA and glycine. Glutamate mediates excitatory transmissions through AMPA and NMDA receptors and GABA mediates inhibitory transmissions through GABAA receptors. They are also utilized in the phase-switching process which is regulated by the pneumotaxic descending inputs and pulmonary SAR afferents. Transduction of hypoxic signals within the peripheral chemoreceptors depends primarily on O2-sensitive K+ channels associated with neuroactive substances such as ACh and dopamine. These signals are transmitted to relay neurons in the NTS by glutamate through AMPA receptors. Hypercapnia stimulates preferentially the central chemosensitive area near the ventral surface of the medulla, where ACh acts as a mediator of such signals. Many neuroactive substances other than amino acids have been implicated in modulating the respiratory rhythm. Serotonergic, noradrenergic and dopaminergic inputs modulate the respiratory neuronal discharge and respiratory rhythm. Muscarinic and nicotinic cholinergic modulations are also apparent. Several neuroactive peptides including SP, SS, CCK and opioids affect respiration. They act either presynaptically or postsynaptically to modulate synaptic transmission within the primary neuronal network as well as at the input and output relay nuclei. These substances are not involved in generation of a basic eupnea. Interactions among various neuroactive substances are essential for precise control of the normal functioning and adaptive processes in the central organization of respiratory rhythm and pattern.
Active Phase
The period of the respiratory cycle (phase) in neurons characterized by membrane depolarization to threshold for the generation of action potentials [2,9].
Silent Phase
The period of the respiratory cycle characterized by membrane hyperpolarization that prevents action potential generation [2,9].
Characteristics
Neurotransmitter Functions in the Respiratory Network
Rhythmic fluctuations of membrane potential in respiratory neurons evolve from neurotransmitter- and membrane conductance-dependent, periodic barrages of 10.1007/978-3-540-29678-2_9s and 10.1007/978-3-540-29678-2_5s that occur with precise timing during the respiratory cycle. Neurotransmitter-dependent excitatory synaptic connections between synchronously active neurons evoke bursts of action potential discharge, while discharges of reciprocally activated neurons periodically release inhibitory 10.1007/978-3-540-29678-2_14 that hyperpolarize membrane potential away from firing threshold. Tonic neurotransmitter release provides a continual excitatory bias on what appears to be all types of respiratory neurons, whereas tonic release of inhibitory neurotransmitter can have a stabilizing effect on membrane potential.
Inhibitory Amino Acids (GABA and glycine)
There are three types of phasic inhibition in respiratory neuron activities; reciprocal inhibition, recurrent inhibition and phase-transition inhibition, as well as tonic inhibition. All four types are characterized by membrane hyperpolarization and lowered input resistance [1,3,4,7].
Phasic inhibition during the inactive phase
GABA initiates IPSPs during the inactive phase by binding to a GABAA-type of receptor in respiratory neurons. During the inactive phase, temporal summation of IPSPs hyperpolarizes membrane potential near to the equilibrium potential for chloride ions (Cl−).
Inhibition during Phase Transitions
The GABAA receptor-mediated mechanism plays an essential role during transition from one respiratory phase to another. During transition from the inspiratory to the expiratory phase, postinspiratory (early expiratory) IPSPs occur in augmenting inspiratory (aug -I) neurons. During transition from late expiration to inspiration, inspiratory IPSPs are observed in augmenting expiratory (aug-E) neurons. In the latter case, activation of GABAB receptors is partially involved, leading to increased potassium (K+) conductances.
Inhibition during the Active Phase
Glycine mediates IPSPs during the later part of stage 2 expiration in aug-E neurons, and IPSPs during late inspiration in aug-I neurons. Aug-I neurons also show IPSPs during early part of inspiration, but whether GABA, glycine or both are involved is unclear.
Tonic Inhibition
GABAA, GABAB and glycine receptor-mediated postsynaptic inhibitions are active in respiratory neurons to help stabilize membrane potential level.
Inhibition of Spinal Motoneurons
GABA mediates the inhibition of phrenic motoneurons during expiration through GABAA receptors [6,8]. This inhibition comes from aug-E neurons of the Bötzinger complex. GABAA mechanisms are also involved in the raphe-stimulated inhibition of the respiratory neuronal activity. The GABAB mechanism decreases transmitter release by acting at presynaptic site and hyperpolarizing phrenic motoneurons through activation of K+ conductances at postsynaptic sites [6].
Excitatory Amino Acid (Glutamate)
All types of respiratory neurons exhibit glutamate-activated EPSPs, in association with lowered input resistance and induction of action potential discharge. They summate temporally to bring membrane potential to discharge threshold [1,3,4,7].
Phasic Excitation during the Active Phase
Glutamate initiates EPSPs during the active phase in most types of respiratory neurons by activating both AMPA- and NMDA-type glutamate receptors. The sequential activation of the two types of postsynaptic receptor is required for production of respiratory-related bursts of discharge. In aug-E neurons, depolarization and discharge are due to AMPA receptor activation, as is phasic recurrent excitation in aug-I neurons. Metabotropic glutamate receptor-mediated mechanisms have no significant effect on membrane potential of 10.1007/978-3-540-29678-2_2.
Inspiratory Off-Switch (10.1007/978-3-540-29678-2_9) Mechanism
The NMDA mechanism plays an important role in IOS of pontine as well as medullary respiratory neurons. IOS is accomplished by a sequential activation of late inspiratory (late-I) and postinspiratory (post-I) neurons to produce barrages of IPSPs in aug-I neurons. During 10.1007/978-3-540-29678-2_1 caused by NMDA blockade, active phase depolarization of late-I and post-I neurons and their firing activity are decreased. The discharge activity of aug-I neurons is also decreased during apneusis.
Tonic Excitation
Respiratory neurons receive glutamatergic tonic inputs that activate both NMDA and AMPA receptors.
Pneumotaxic Descending Inputs
Termination of the IOS can be produced by afferents originating from the 10.1007/978-3-540-29678-2_16. Glutamate through the NMDA mechanism responsible for IOS is present in the pontine structure. However, the pontine descending inputs generating fast EPSPs in bulbar respiratory neurons are not mediated by NMDA receptors, but by AMPA receptors.
Pulmonary Mechanoreceptor Afferent Inputs
Glutamate mediates the primary afferent excitation of the NTS neurons in the 10.1007/978-3-540-29678-2_8 pathways, primarily through the activation of AMPA receptors. The Hering-Breuer inspiratory promotion reflex induced by application of lung deflation during expiration is mediated by NMDA receptors.
Excitatory Drive to Bulbospinal Motoneurons
Glutamate mediates the bulbospinal transmission of respiratory drive acting on both AMPA and NMDA receptors [6,8]. Contribution of the former is greater than that of the latter to motor outputs. AP4 receptors are located at the presynaptic sites of the inspiratory bulbospinal terminals. Short term potentiation is mediated by NMDA receptors, which augment EPSPs and prolong depolarization of phrenic motoneurons. Activation of metabotropic glutamate receptors affects the inspiratory-modulated activity of phrenic motoneurons via distinct mechanisms at pre- and postsynaptic sites.
Excitatory Drive to Spinal Motoneurons
The major glutamatergic excitatory drives to phrenic, intercostal and abdominal motoneurons come from bulbospinal neurons that activate AMPA/Kainate- and NMDA-types of receptors
Neurotransmitters, Neuromodulators and Responsiveness to Hypercapnea and Hypoxia
Carbon dioxide and its acid byproduct, H+ ion, constitute the primary respiratory stimulus within the central nervous system. Sensitivity to CO2/pH is up-regulated or down-regulated by several different neurotransmitters and 10.1007/978-3-540-29678-2_14 [5,7]. Acetylcholine (ACh) activation of muscarinic receptors on neurons close to the ventrolateral surface of the medulla increases respiratory responsiveness to CO2/pH+. Adrenergic cell groups have also been reported to increase respiratory responsiveness to hypercapnea/acidosis in the rostral ventrolateral medulla. Glutamate is also a neurotransmitter candidate for tonic excitation of respiratory neurons mediated by central CO2/pH+ sensitive neurons. A GABAergic mechanism in the caudal hypothalamus dampens respiratory responsiveness to hypercapnea/acidosis.
Respiratory responsiveness to hypoxia is modulated peripherally by neuromodulators in the carotid bodies and within the central respiratory network. Generally, excitatory transmission is cholinergic, whereas dopamine (DA) plays an inhibitory role in carotid body chemoreceptors. Hypoxic release of ACh activates nicotinic receptors, leading to augmentation of hypoxia-induced depolarization and further release of ACh and other neurotransmitters. DA release, on the other hand, suppresses carotid body discharges by activating D2-type receptors. Glomus cells contain other compounds that are released during hypoxia, including serotonin (5-HT), enkephalins, prostaglandins, ATP, adenosine, substance P (SP), cholecystokinins (CCK), nitric oxide and atrial natriuretic peptide.
SP localized within vagal afferent fibers appears to act as a neurotransmitter or modulator of chemo- and baroreceptor fibers. Central dopaminergic mechanisms are also involved in modulating the chemoreflex respiratory control.
In the central respiratory network, glutamate release from the afferent glossopharyngeal terminals in the nucleus of the solitary tract (NTS) activates AMPA receptors on relay neurons in response to hypoxia. Release and local accumulation of GABA and/or neuromodulators, including catecholamines and opioids, are important factors leading to late hypoxic depression. GABA mediates inhibition in the NTS neurons that respond to stimulation of the carotid sinus nerve. Accumulation of metabolic byproducts such as adenosine may increase KATP channel currents in postsynaptic neurons. Hypoxia also increases endogenous 5-HT levels and increases K+ currents via 5-HT1A receptors in respiratory neurons, resulting in depression of respiratory neurons.
Respiratory Neuromodulation by Monoamines and Peptides
It has been more difficult to assess the neuromodulatory roles of serotonin, catecholamines and peptides in the central respiratory network. Their actions are generally slow in onset, discreet, state-variable, and dependent on a vast array of receptor subtypes. Assessment of function is often assumed from the effects of exogenous receptor agonists and antagonists, not all of which are suitably selective [1,4,7].
Serotonergic Agents
5-HT has diverse effects on respiratory neurons. Respiratory neuron excitability is increased postsynaptically by 5-HT2, 5-HT1C and 5HT4 receptor agonists, and decreased postsynaptically via 5-HT1A and 5HT7 agonists. Activation of 5-HT1A and 5HT7 receptors depresses the cAMP-protein kinase A pathway, 5HT4 receptors activate it, and 5-HT2 receptor activation stimulates the PLC/PLA- protein kinase C pathway.
Catecholaminergic Inputs
The effects of catecholamines (noradrenaline, adrenaline, DA) are also diverse and dependent on which subtypes of receptor are affected. Noradrenaline and adrenaline have a predominantly depressant effect on bulbar respiratory neurons. Dopaminergic mechanisms exert a tonic inhibitory influence in the central pathways involving in the hypoxic ventilatory responses through D2 receptors, and increase central respiratory responsiveness to CO2 via D1 receptors.
Peptides and Hormones
SP and thyrotropin-releasing hormone (TRH) have excitatory, and somatostatin (SS) and 10.1007/978-3-540-29678-2_15 have depressant effects on respiration. CCK produces either excitatory or inhibitory effects, depending on its receptor types activated. Individual neuropeptides often coexist and interact with classical neurotransmitters in respiratory neurons. They play some roles in the central control of respiratory activity including the chemoreflex [1,4,7].
Substance P
SP mediates excitatory neurotransmission and integration of the peripheral chemoreflex in the NTS through NK1 receptors. SP reverses the respiratory neuronal depression induced by SS or opioids. Hypoxia induces desensitization of NK1 receptors to SP in the NTS neurons, leading to a decline of hyperventilation during hypoxia. SP is a transmitter of the pulmonary C fiber-mediated reflex which induces a rapid shallow breathing and/or apnea.
Thyrotropin-Releasing Hormone
TRH had postsynaptic excitatory effects on neurons in the NTS, nucleus ambiguus and pre-Bötzinger complex. TRH enhances glutamatergic transmissions and counteracts the inhibitory effects of opioids. TRH also seems to be involved in central (CO2/pH+) chemoreception (10.1007/978-3-540-29678-2_3).
Somatostatin
SS has an inhibitory effect on respiratory neurons. Anesthesia or sleep enhances the effects of SS. SS metabolites potentiate the voltage-dependent, non-inactivating outward K+ currents (IM current) in the NTS neurons.
Opioid Peptides
Opioid peptides may be the most important peptides endogenously involved in respiratory modulation in the brainstem. Opioid peptides depress respiratory neuron activity by increasing K+ conductances through µ receptors. Endogenous opioids negatively interact with the CO2-sensitive cholinergic transmission, and with the glutamatergic transmission of respiratory neuronal activity.
Cholecystokinin
CCK octapeptide (CCK8) causes various effects on respiration; It stimulates respiration by stimulating either the forebrain or the medullary region and depresses ventilation by stimulating vagal afferents. The activation of CCKA receptors causes inhibition of respiratory neurons due to an increase of K+ conductances, while that of CCKB receptors produces excitation due to a decrease in K+ conductances. Further, activation of CCKB receptors by endogenous CCK reduces the GABA-mediated fast inhibitory responses in the NTS neurons.
Progesterone
Endogenous progesterone is a respiratory stimulant [10]. It increases ventilatory responsiveness to CO2. In pregnancy and during the luteal phase of the menstrual cycle, it accounts for hyperventilation and low CO2. Endogenous progesterone also has a beneficial effect on the upper airways. It increases tonic and phasic geneoglossus activities.
Respiratory Pacemaker Neuron
Definition
Neuron with an intrinsic ability to generate rhythmic bursts.
Respiratory Pacemakers
Synonyms
Respiratory bursting neurons; Respiratory pacemaker neurons
Definition
A respiratory pacemaker is a neuron with the intrinsic ability to generate rhythmic 10.1007/978-3-540-29678-2_2 that emerge through voltage-, time- and calcium- dependent ion fluxes [1]. These ion fluxes give rise to rhythmic membrane fluctuations that are defined as “10.1007/978-3-540-29678-2_4” or “pacemaker potentials.” The ion fluxes leading to drive potentials are carried by sodium, calcium, and/or non-specific cations, but many ionic conductances contribute to shape and frequency of these potentials. The drive potentials can, but do not always give rise to a series of action potentials. A drive potential that gives rise to action potentials is called a “burst”. Bursts that are generated by ionic conductances intrinsic to the neuron are often referred to as “10.1007/978-3-540-29678-2_9.” Two types of pacemaker neurons have been described in the respiratory network: (i) Pacemakers that depend on CAN current and are blocked by cadmium are referred to as “10.1007/978-3-540-29678-2_3” [2]. (ii) Pacemakers dependent on the persistent sodium current burst even in the presence of cadmium. These neurons are referred to as “10.1007/978-3-540-29678-2_3” [2].
A neuron that generates pacemaker activity only in the presence of a neuromodulator is generally called a “10.1007/978-3-540-29678-2_3.” As described in the next paragraph, the dynamic regulation of pacemaker neurons is the rule, and not the exception. Hence, it is conceivable that all respiratory pacemakers are “conditional”.
Characteristics
Quantitative Description
The identity of a respiratory pacemaker is not fixed, but dynamically regulated. Non-pacemakers can be turned into respiratory pacemakers, and conversely respiratory pacemakers can become non-pacemakers [1]. This dynamic process is not all-or-none. A non-pacemaker can turn into a weakly bursting or strongly bursting pacemaker, and weak pacemakers can turn into strong pacemakers. Weak pacemakers are characterized by small amplitude, intrinsically generated 10.1007/978-3-540-29678-2_4 that give rise to one or two action potentials. Bursts can occur irregularly in some, while regularly in other neurons. Thus, the discharge properties of respiratory neurons cover a wide range from non-bursting, to weak-irregular bursting to strong-regular bursting [1,3]. Transformation of pacemakers and non-pacemakers and their dynamic regulation are not unique for the respiratory system. For example the discharge patterns of neocortical and thalamic neurons change dramatically during the transition from wake to sleep [1,4,5] and there are numerous other examples in which neurons can loose or attain pacemaker properties.
Mechanistically, this is not surprising as the pacemaker property emerges through a complex, and modifiable ratio of different ionic currents in which inward currents (typically carried by Na+ or Ca++) are larger than outward currents (typically carried by K+ or Cl- currents).
In the functional network the ratio of these ion channels is continuously modulated by endogenously released neuromodulators, such as amines and peptides. In the respiratory system, induction of pacemaker properties has been demonstrated for serotonin, acetylcholine, norepinephrine, TRH (10.1007/978-3-540-29678-2_20) and substance P [1,3,6]. It is likely that many still unexamined neuromodulators can induce and suppress pacemaker properties in respiratory neurons.
In the functional network intrinsically generated drive potentials are also dynamically regulated by synaptic transmission: Intrinsically generated bursts can be activated by excitatory synaptic inputs, and thus function as a mechanism to boost or amplify synaptic inputs. But the boosting mechanism must also be considered dynamically, since concurrently occurring synaptic inhibitory mechanisms can also suppress these intrinsically generated drive potentials. Thus, in the functional network concurrent inhibitory synaptic mechanisms can regulate the bursting mechanism to the extent that excitatory synaptic drive is necessary to activate bursting in a pacemaker neuron. Synaptic mechanisms play also critical role in timing the onset of a burst, even in pacemakers that have strong intrinsic bursting properties. Thus, the bursting property must be considered as a dynamic property that is highly influenced by fine balance of synaptic as well as neuromodulatory mechanisms.
Due to the tight interaction between synaptic and intrinsic membrane properties, demonstrating pacemaker properties is challenging. It must be shown that the rhythmicity recorded in a neuron is generated intrinsically and is not the result of rhythmic synaptic input that emerges through network interactions. In the respiratory network pharmacological approaches are typically used to isolate pacemaker neurons [6,7]. Exogenously applied neurotransmitter antagonists can block inhibitory and excitatory neurotransmission which eliminates rhythmic synaptic population inputs. The pharmacological approaches are usually combined with electrophysiological approaches that take advantage of the voltage-dependency of ion channels [7]. Brief de- or hyperpolarizing current injections can reset ongoing pacemaker activity by advancing or delaying the generation of a pacemaker burst. Long-lasting de- or hyperpolarizing current injections can accelerate or slow the frequency of pacemaker activity. Brief depolarizing current pulses can prematurely trigger, while hyperpolarizing current pulses can prematurely terminate ongoing pacemaker bursts. It is important to be aware that pharmacological approaches can be misleading. For example low concentrations of extracellular calcium can block synaptic transmission, but at the same time, this manipulation can induce pacemaker properties in non-pacemakers by blocking 10.1007/978-3-540-29678-2_7 [2]. Conversely, low calcium concentrations could block the activation of the CAN current, which plays a major role in evoking bursts in some respiratory neurons [2]. Bicucculline, is a substance that blocks 10.1007/978-3-540-29678-2_6, but at higher concentration it can also block potassium channels which could induce pacemaker properties.
An even greater challenge is the interpretation of lesion experiments in a functional network. Pacemaker, synaptic and modulatory properties are highly integrated elements of the functional network and provide the respiratory network with the necessary adaptability and flexibility for survival. The removal of any of these elements will change the overall network property, and whether its removal abolishes rhythmicity is neither an indicator for its importance nor its specific role in respiratory rhythm generation [1].
Higher Level Structures
The majority of neuronal networks in the brain generate rhythmic activity, and pacemakers are found in the majority of these networks including networks within the spinal cord, medulla, 10.1007/978-3-540-29678-2_14, 10.1007/978-3-540-29678-2_2, thalamus, 10.1007/978-3-540-29678-2_12, ventral tegmentum area (VTA), 10.1007/978-3-540-29678-2_8 and 10.1007/978-3-540-29678-2_1 [1,8]. In many cases it is unclear how the rhythmicity in general and how pacemakers in particular contribute to these network functions, and the respiratory network is no exception. Pacemaker neurons have been identified in various areas that belong to the respiratory network, including the NTS [6], the pre-Bötzinger complex [1] and the parafacial nucleus [9]. Pacemakers are also found in areas that are driven by the respiratory network including the locus coeruleus [10]. While most pacemakers were identified in vitro slices, it is likely that recordings in more intact networks reveal that pacemaker neurons are more abundant than generally expected. Intact networks have more active modulatory systems that can promote pacemaker properties, such as norepinephrine and serotonin.
Lower Level Components
In general terms, the ionic mechanisms that give rise to pacemaker activity are very heterogeneous. These mechanisms typically involve a complex interaction between voltage-dependent and voltage-independent components of ion channels within their intra- and extracellular environment [1]. In general, a neuron depolarizes and ultimately bursts either in response to the activation of inward currents that are carried by sodium and/or calcium ions, or in response to the cessation of outward currents that are carried by potassium ions. The inward currents include the hyperpolarization-activated current (Ih current), the persistent sodium current, various low- and high-voltage activated calcium currents and the calcium-activated non-specific cation (CAN) current [1]. The ongoing burst is commonly terminated by either of two principal ionic mechanisms. (i) The channels responsible for the inward current inactivate. Such properties may play a major role in determining bursting in neurons dependent on persistent sodium current. (ii) The calcium or sodium influx during the ongoing burst can activate calcium- or sodium-dependent potassium currents that hyperpolarize the membrane and thereby terminate the burst. Possible mechanisms that cause a repolarization can include voltage-independent intracellular signals, and slow activation or inactivation properties of inward or outward currents.
Structural Regulation
There is currently no characteristic anatomical structure that defines a respiratory pacemaker neuron. Similarly there are many different discharge patterns that characterize a respiratory pacemaker neuron. “Irregular-” and “regular-bursting” neurons are differentiated by the regularity of the burst periodicity. Given that rhythmic drive potentials can arise through a variety of ionic mechanisms, it is not surprising that the same anatomical region may contain different types of pacemaker neurons. The fact that the same anatomical region contains more than one type of pacemaker neuron is not the exception, but presumably the rule [1]. It is assumed that different types of pacemaker neurons play different roles in the generation of network activity, an issue of much ongoing research [1,2,3,8,9]. This complexity is not unique to the nervous system: cardiac pacemakers for example are also very diverse.
Higher Level Processes
Pacemaker neurons are embedded in complex neuronal networks. Hence there are many synaptic and modulatory processes that govern the activity of a pacemaker neuron [1,3]. Many principle insights into the interactions between pacemaker neurons, synaptic transmission and 10.1007/978-3-540-29678-2_14 were gained from studying small neuronal networks of invertebrates. It can be expected that medullary pacemakers are modulated by various inputs from networks outside the 10.1007/978-3-540-29678-2_13, and vice versa that pacemakers influence networks outside the medulla. Unfortunately, recordings from pacemakers in more intact networks are sparse. Thus very little is known about these potential network interactions.
Lower Level Processes
Neuromodulators play a critical role in modulating the cellular events that govern the discharge pattern of a pacemaker neuron. Endogenously released neuromodulators can phosphorylate voltage-dependent ion channels, or alter second messenger pathways and intracellular calcium thereby changing ion channel properties. This complex interplay between neuromodulators, the intracellular milieu and voltage-dependent ion fluxes will significantly alter pacemaker activity. In doing so, neuromodulators can determine the burst frequency, the amplitude and shape of the drive potential [3]. Neuromodulators are also responsible for the fact that the pacemaker property itself is not a fixed property as described above.
Function
As described above, pacemakers are embedded in synaptically organized networks and therefore pacemaker activity itself is influenced by synaptic inputs [1]. Thus, in general pacemaker activity can not be regarded as a “driver” of network activity. Thus assigning a specific function to a pacemaker neuron becomes difficult if not impossible, since this property can not be separated from the other properties that determine its discharge. Tonic excitatory or inhibitory synaptic inputs can determine the frequency of pacemaker activity. Excitatory synaptic input can prematurely trigger pacemaker activity, which means that synaptic inputs can determine the timing of pacemaker activity. A pacemaker burst can act as a non-linear amplifier of synaptic excitatory inputs, while synaptic inhibitory inputs can act as leak currents that will greatly suppress pacemaker activity.
Various functions have been ascribed to pacemaker neurons. It is thought that pacemakers can influence regularity, burst amplitude and frequency of respiratory activity. Due to differences in their voltage-dependence cadmium-sensitive and insensitive pacemakers may assume different roles in regulating frequency versus amplitude of respiratory bursts [3].
Process Regulation
The number, the types of pacemakers, and the degree of their bursting properties in a functional neuronal network will be continuously regulated by neuromodulators and synaptic interactions. Consequently, the contribution of pacemaker properties to the overall network output will not be fixed [1,2]. By altering for example the number of active pacemaker neurons a network can assume different configurations that can lead to different network outputs. These complex modulatory interactions imbue neuronal networks with a high degree of plasticity. This is an essential prerequisite for generating a rhythmic behavior that has to continuously adapt to changes in behavioral, environmental and metabolic conditions.
Pathology
It has been hypothesized that the suppression of pacemaker properties may lead to the failure of gasping and possibly Sudden Infant Death Syndrome [1,2].
Therapy
In vitro studies suggest that pacemaker neurons are important in regulating regularity of respiratory rhythmic activity. Hence, a better understanding of the ionic basis of pacemaker activity and their modulation may be an important step towards developing rational therapies or strategies to prevent neurological disorders associated with erratic breathing.
Respiratory Plasticity
Respiratory Recovery from Injury
Respiratory Reflexes
Synonyms
Pulmonary reflexes; Breuer-Hering reflexes; Deflation reflex; Central chemoreceptors; Peripheral chemoreceptors; Carotid chemoreflex; Slowly adapting pulmonary stretch receptors; Rapidly adapting pulmonary receptors; Irritant receptors; Cough; Respiratory plasticity
Definition
Respiratory reflexes encompass a significant repertoire of responses to a variety of sensory receptors regulating the depth and frequency of individual breaths and participating in the protection of airways from potentially damaging inhaled substances. Specifically, receptors in the airways and lungs sense the relative inflation or deflation of the lungs as well as the presence of inhaled irritants, and elicit appropriate responses via brainstem respiratory circuits to maintain the integrity and efficient function of the lungs and airways. Central (brain) chemoreceptors and peripheral chemoreceptors in contact with arterial blood, evoke changes in breathing to maintain appropriate levels of oxygen and carbon dioxide as well as pH.
Characteristics
Continuous, rhythmic breathing movements are essential for the homeostatic regulation of arterial blood gases, acid-base balance and, ultimately, for the maintenance of life itself. Neurons responsible for generating respiratory rhythm and shaping it into the detailed pattern of activity evident on respiratory motor output are located predominantly in two brainstem regions (see Alheid & McCrimmon this volume). One group forms a long column of cells in the ventrolateral medulla in close proximity to the nucleus ambiguus. Termed the ventral respiratory column (VRC), this group extends rostrally from the spinal-medullary junction to a region ventral to facial nucleus. A second group, termed the dorsal respiratory group, is localized in the dorsomedial medulla, mainly within the ventrolateral nucleus of the solitary tract (NTS). These neurons fire in bursts phase locked to the breathing rhythm. Most fire either during inspiration (inspiratory neurons) or expiration (expiratory neurons) although some fire in bursts that span phase transitions between inspiration and expiration.
The magnitude and rate of respiratory efforts generated by brainstem respiratory neurons are regulated to maintain brain and arterial tensions of oxygen (O2), carbon dioxide (CO2), and pH within narrow limits despite large variations in metabolic requirements. A variety of chemical and mechanical receptors located in the airways, lungs, and chest-wall provide the sensory feedback essential to optimization of the breathing pattern. Sensory feedback from arterial and central (brain) chemoreceptors as well as lung mechanoreceptors modulates the breathing pattern, e.g., tidal volume and breathing frequency.
Receptors distributed throughout the airways also help defend the respiratory system. Afferent-evoked protective reflexes include apnea (a transient cessation of breathing), shallow rapid breathing, coughing, sneezing, mucus secretion, and airway constriction. These reflexes protect the airways from irritants and facilitate the removal of inhaled substances potentially harmful to the lungs and airways.
Chemoreceptors
Regulating the level of the metabolic product CO2 and maintenance of tissue oxygenation are principal roles of the respiratory system. In performing these tasks, the central circuitry generating respiratory pattern receives sensory input from chemoreceptors located in the brain (central chemoreceptors) and the arterial system (peripheral chemoreceptors). Through the regulation of CO2, the respiratory system also adjusts pH and hence contributes importantly to acid-base balance.
Central chemoreceptors have a relatively greater role than peripheral receptors in regulating CO2 and pH and they have been identified in several regions of the brain [1]. Most are in the medulla and pons including: (i) regions at the ventral surface of the medulla, particularly in the retrotrapezoid nucleus, (ii) midline raphe serotonergic neurons, (iii) the NTS, (iv) the preBötzinger complex [a subregion of the VRC] and (v) noradrenergic neurons in the locus coeruleus. Additional chemosensitive sites have been identified in: (vi) the fastigial nucleus of the cerebellum, and (vii) the posterior hypothalamus. The relative importance of several sites including the NTS, retrotrapezoid nucleus and caudal raphe may vary with physiological conditions such as the sleep–wake state. Central O2 chemoreceptors appear to exist but little ventilatory response to hypoxia is observed after peripheral deafferentation when CO2 levels are held constant.
Peripheral chemoreceptors have a dominant role in eliciting the ventilatory increases in response to hypoxia [2]. Peripheral chemoreceptors are located in both the carotid and aortic bodies but the carotid bodies are quantitatively much more important in regulating breathing. The aortic bodies contribute relatively more to cardiovascular adjustments. Afferent fibers emanating from the carotid bodies have a low discharge rate at normal resting levels of arterial O2 and CO2 but increase their discharge in response to a decrease in the partial pressure of arterial O2 (PO2) or to an increase in the partial pressure of arterial CO2 (PCO2) or to decreases in pH.
Overall, chemoreceptors are remarkably sensitive to PCO2. Increasing arterial PCO2 by 1–3 mm Hg from a normal resting value of about 40 mm Hg can cause a doubling of ventilation. About 60% of this response is contributed by central chemoreceptors. In contrast, there is little ventilatory response to hypoxia until arterial PO2 decreases below 60 mm Hg from a normal resting value of 80–100 mm Hg.
Chemoafferent fibers from the carotid body are contained in the carotid sinus nerve (CSN), a branch of the glossopharyngeal nerve, with the cell bodies of (first order) CSN chemoafferent neurons found mainly within the petrosal ganglia. CSN chemoreceptor afferent fibers are a mixture of unmyelinated C-fiber axons and myelinated A-fibers. The principal fast transmitter in these afferents appears to be glutamate, however, dopamine appears to be present in about 40% of the C-fibers. Other potential chemoafferent transmitters include substance P and ATP.
Carotid body afferents target 2nd-order caudal NTS neurons [3], specifically within its commissural division (SolC; Fig. 1).
Respiratory Reflexes. Figure 1.

Carotid body chemoreceptor afferent terminations within the nucleus of the solitary tract (NTS). (a & b) NTS subnuclei at two rostrocaudal levels. (c & d) Central pathways of carotid chemoreceptors projected onto Nissl-stained coronal sections of the medulla at the levels approximating those diagramed in A & B. The axons of these sensory fibers are carried by the carotid sinus nerve (CSN), a branch of the glossopharyngeal nerve [9]. They enter the medulla near the level of the facial nucleus and run caudally within the solitary tract (sol) terminating predominantly within the commissural subregion (SolC) in the caudal aspect of the NTS. NTS 2nd-order neurons relay this afferent input directly (or indirectly via NTS higher-order neurons) to respiratory regions in the ventrolateral medulla and pons. Compare with the distribution of lung mechanoreceptor afferents in Fig. 3. Abbreviations: 10N dorsal motor nucleus of the vagus; 12 hypoglossal nerve; 12N hypoglossal nucleus; AP area postrema; CC central canal; IO inferior olive; sol solitary tract; SolDL dorsolateral subnucleus; SolIM intermediate subnucleus; SolM medial subnucleus; SolVL ventrolateral subnucleus; VRC ventral respiratory column of the ventrolateral medulla.
Within the NTS, intrinsic polysynaptic pathways provide recurrent excitatory feedback that can initially increase the excitability of 2nd- and higher-order NTS chemoafferent interneurons. Inhibitory GABAergic neurons in SolC are also activated during hypoxic stimulation of breathing and the initial activation of NTS neurons is followed by local inhibition that ultimately limits the excitatory responses of the NTS 2nd and higher order neurons.
Multiple pathways emanating from the NTS are involved in processing carotid chemoreceptor afferent activity. Both second and higher order neurons in SolC are the source of extrinsic projections to the brainstem and forebrain. Among brainstem targets are VRC respiratory neurons. Additionally, SolC neurons relay chemoafferent input to the rostral dorsolateral pons in the region of the parabrachial and Kölliker-Fuse nuclei. These nuclei contain respiratory neurons and are collectively referred to as the pontine respiratory group (PRG). Some neurons in the PRG are likely relays for chemoafferent (and other visceral) inputs to higher brain structures (in addition to the direct forebrain projections from the NTS). PRG neurons also provide descending inputs that coordinate respiratory activity with other systems such as cardiovascular control as well as with nociceptive afferent input.
Plasticity in Chemoreceptor Breathing Responses
Short and long-term modifications (plasticity) occur in the breathing response to chemoreceptor activation. Respiratory plasticity accommodates the changing demands of development as well as environmental demands such as changing PO2 levels at varying altitudes, and physiological demands created by pathological changes in the efficiency of the airways and lungs.
Plasticity of the hypoxia reflex is evident in changes in respiratory pattern occurring in multiple stages. The acute response to hypoxia is characterized by increases in both respiratory frequency and the volume of each breath (tidal volume). There is a progressive increase in tidal volume (termed short-termed potentiation) over a period of seconds to minutes. Upon returning to normal oxygen levels there is a slow return to the normal tidal volume. There is also a post-hypoxic decline in breathing frequency lasting several minutes in which respiratory frequency declines below pre-hypoxic levels. While the mechanisms underlying these changes are not well understood, short term potentiation may involve recurrent excitation within the chemoreceptor pathway in the NTS as well as pre-synaptic changes (e.g. calcium accumulation) in NTS or downstream neurons. Post hypoxic frequency decline, on the other hand, may require participation of neurons in the ventrolateral pons.
As illustrated in Fig. 2, repeated episodes of hypoxia lasting from seconds to minutes result in an additional form of plasticity consisting of a long-term facilitation (LTF) of respiratory motor output that can persist for several hours [1].
Respiratory Reflexes. Figure 2.

Respiration-related neural activity in an anesthetized and artificially ventilated rat demonstrates a form of respiratory plasticity known as respiratory long-term facilitation (LTF). On the left in each trace, electrical activity in the phrenic nerve (the nerve innervating the diaphragm, the principal inspiratory muscle) is integrated such that each peak represents a “fictive breath” that the artificially ventilated rat had intended to make. Under conditions of normal arterial oxygen and carbon dioxide (pre-hypoxia baseline; blue traces), the phrenic nerve bursts are rhythmic and relatively constant in frequency and amplitude. Ventilating the rats with lowered oxygen levels (red traces) causes differential effects on phrenic nerve activity depending upon the pattern of hypoxia exposure (intermittent, upper trace; continuous, lower trace). In both conditions, phrenic bursts increase in amplitude, primarily reflecting the increased activation of carotid body (peripheral) chemoreceptors. Following either pattern of hypoxia exposure, phrenic nerve activity also asymptotes toward baseline levels over several minutes following the return to normoxia (green traces). However, after intermittent, but not after continuous hypoxia, phrenic nerve burst amplitude again increases slowly and progressively over the next hour, even though arterial oxygen and carbon dioxide are at pre-hypoxia levels. This slow increase reflects LTF that is elicited in response to the repetitive exposure to hypoxia. This facilitation requires release of serotonin in the region of the motor neurons (Data provided by T.L. Baker-Hermann and G.S. Mitchell, reproduced with permission from Fundamental Neuroscience, 2nd Edition, edited by LR Squire, FE Bloom, SK McConnell, JL Roberts, NC Spitzer, MJ Zigmond, Academic Press, San Diego, 2003).
LTF is observed at motoneurons innervating respiratory pump muscles (e.g. phrenic and external intercostal motoneurons in the cervical and thoracic spinal cord) and upper airway muscles (e.g. hypoglossal motoneurons) and has been related to brainstem serotonergic afferents to these cells (via 5HT-2A receptors) as well as to up-regulation of the peptide, brain derived neurotrophic factor (BDNF), and the molecular signalling proteins activated by BDNF receptors (e.g. TrkB).
In humans, an etiologic role for central chemoreceptors in the medullary arcuate nucleus (located at the medial ventral surface of the brain) has been postulated in sudden infant death syndrome (SIDS) and this has been supported by observations of abnormal development of arcuate serotonin receptors [4]. Disruption of normal chemoreceptor function is also suggested in congenital central hypoventilation syndrome (CCHS). CCHS patients with a polyalanine expansion mutation in the PHOX2B gene have negligible sensitivity to elevated PCO2 or hypoxemia [5].
Airway Receptors
Respiratory reflexes arising from the airways serve both in protecting the airways as well as in regulating the depth and frequency of breathing. Protective reflexes include apnea, cough, sneeze, mucus secretion, and airway constriction that both protect the airways from irritants and facilitate the removal of potentially harmful substances. Sensory feedback also helps coordinate breathing with other behaviours such as locomotion or vocalization.
Receptors in the Nasal Passages and Pharynx
Receptors in the mucous membranes of the nasal cavities are sensitive to cold and pressure changes associated with breathing as well as to inhaled irritants such as cigarette smoke and ammonia. Branches of the trigeminal nerve, including the anterior ethmoidal and maxillary nerves convey the sensory information to the central nervous system. Respiratory motor responses to activation of these receptors include sneezing and apnea. Additional reflex components occur secondarily to alterations in the activity of the autonomic nervous system and include mucus secretion, bradycardia, and increased blood pressure.
The diving reflex is also elicited by receptors with afferent fibers in the trigeminal nerve. This reflex is elicited by water on the face or in nasal passages, and consists of apnea and peripheral vasoconstriction, along with marked increases in arterial pressure and bradycardia. Stimulation of the anterior ethmoidal nerve, which innervates the nasal passages, mimics the diving reflex. Its central terminations are found mainly in ventral aspects of the spinal trigeminal nucleus at levels caudal to the facial nucleus with additional terminations in the NTS and paratrigeminal nucleus.
Laryngeal Receptors
The larynx is richly innervated by several subgroups of sensory receptors [6,7]. Their afferent fibers are mainly in the recurrent and superior laryngeal branches of the vagus nerve with terminations in the NTS. The receptors are located at the entrance to the trachea and lower airways and elicit strong protective reflexes including cough and apnea. The pronounced apneas elicited from laryngeal receptors has also suggested that abnormal development of their reflex pathways could contribute to SIDS. In contrast, a subset of laryngeal receptors promotes airway patency via activation of airway dilating muscles such as the genioglossus and posterior cricoarytenoid.
Receptors in the Lower Airways
Receptors within the lungs and lower airways, i.e., those below the larynx, are classified into two main types based on whether the sensory afferent fibers are myelinated or unmyelinated [6,8,9]. The afferent axons arising from both groups travel in the vagus nerves and terminate in middle and caudal aspects of the NTS. Receptors with myelinated axons constitute airway mechanoreceptors and are activated by distension of the airway during lung inflation or by a reduction in airway dimensions during lung deflation, especially deflations below the normal end-expiratory volume. Two groups of receptors, slowly (SAR) and rapidly (RAR) adapting receptors, are identified based on their sensitivity to distension of the airways during lung inflation and the rate of accommodation in their response. An additional group of receptors, termed deflation activated receptors (DARs), are more prominent in small animals (e.g. rats). DARs share several common properties with RARs, which are more readily observed in larger animals and activation of either RARs or DARs elicits augmented inspiratory efforts.
SARs are located in airway smooth muscle. Their activation by lung inflation inhibits inspiratory motor activity, thereby shortening inspiratory duration and reducing tidal volume (termed the Breuer-Hering inspiration-inhibiting reflex). Maintaining inflation into the expiratory period prolongs expiratory duration (Breuer-Hering expiratory facilitatory reflex). The Breuer-Hering reflexes are activated during normal resting breathing in most mammals while in humans they appear to only significantly influence breathing pattern when tidal volumes increase to 2–3 times their resting values as may occur during muscular exercise. Activation of SARs has several additional effects, including reductions in airway smooth muscle tone resulting in bronchodilation, and reductions in heart rate and systemic vascular resistance.
RARs are located in airway epithelium and submucosa. While they are activated by lung inflation they are less responsive than SARs and tend to have little activity under normal breathing conditions. Their discharge adapts rapidly to lung inflation and they typically fire irregularly, giving rise to one or a few action potentials during lung inflation. They respond with a significantly more sustained discharge to inhaled irritants. RARs are implicated in a number of potent airway protective reflexes, including augmented breaths (sighs), airway constriction, mucus secretion and laryngeal closure. While they are generally believed to trigger coughing, this function has recently been related to a specific subset of polymodal A∂-fibers (termed cough receptors; [10]).
DARs. Some researchers group DARs with RARs and the degree to which these represent the same or separate populations requires further examination. Nevertheless, lung deflation triggers reflexes that shorten expiratory duration and increase inspiratory effort. There is also a reflex narrowing (adduction) of the glottis and stimulation of low intensity diaphragm activity during expiration that slows expiration. Together these responses help prevent alveolar collapse, and are particularly important in human infants as well as in other small mammals that have highly compliant chest walls.
C-Fibers constitute the largest class of pulmonary vagal afferent fibers (~75%). They are polymodal and nociceptive, responding to a variety of inflammatory mediators and inhaled irritants. Reflex changes in breathing in response to C-fiber activation involve rapid shallow breathing interspersed with apneas; additional reflex effects include bronchoconstriction, mucus secretion, hypotension, bradycardia and airway mucosal vasodilation. Beyond identification of the regions of C-fiber termination within the NTS, little is known concerning central pathways mediating these responses.
Second and Higher-Order Neurons in Reflexes From Airway Mechanoreceptors
Central Pathways of SARs
SAR primary afferent fibers terminate within mid to caudal portions of the NTS (Fig. 3) [6]. Only two functional classes of NTS neurons receive monosynaptic input from SARs (Fig. 3).
Respiratory Reflexes. Figure 3.

The topographical distribution within the NTS of three classes of pulmonary afferents. The terminal distribution slowly and rapidly adapting stretch receptors (SARs and RARs, respectively) and bronchopulmonary C-fibers is shown at two rostrocaudal levels of the NTS. The three afferent systems in general project to topographically separate NTS targets. The principal projections of their 2nd order neurons are also indicated. Among the known projections is an inhibitory projection of pump (P-) cells to RAR relay neurons in the NTS commissural subnucleus. See Fig. 1 for abbreviations (used with permission from [6]).
One type, termed Iβ neurons, exhibits inspiratory discharge patterns, and activation of SARs increases the discharge rate of these neurons. Iβ neurons are bulbospinal premotor neurons that monosynaptically excite phrenic motoneurons. These motoneurons innervate the diaphragm and this reflex accordingly provides positive feedback excitation of diaphragm inspiratory activity.
A second group of NTS neurons, termed pump-neurons, mediate Breuer-Hering reflex changes in respiratory rhythm. Pump neurons receive monosynaptic SAR input but are readily distinguished from Iβ neurons by the general absence of an inspiratory discharge when SAR input is removed. Consistent with the broad effects they elicit on respiratory pattern, their axons arborize extensively within pontomedullary regions (ventrolateral medulla and rostral dorsolateral pons) containing neurons responsible for generating the respiratory pattern. Many pump neurons are inhibitory, containing GABA with only a small percentage also containing glycine. Experimental evidence also suggests that there may be excitatory pump neurons, however, these cells have not yet been directly identified.
Central Pathways of RARs
RAR primary afferent fibers terminate in caudal aspects of the NTS where they monosynaptically excite neurons termed RAR interneurons (Fig. 3) [6]. Like pump neurons, these interneurons provide extensive axonal arborizations to pontomedullary regions involved in respiratory pattern generation. RAR interneurons are believed to be excitatory and presumably facilitate the discharge of inspiratory neurons. RAR activation accordingly elicits augmented inspirations such as sighs, and the large inspiration preceding a cough. RARs are also stimulated by decreases in lung compliance that result from alveolar collapse. The resulting RAR-triggered large inspiration stretches the lung, reopening collapsed alveoli.
Summary
Although seemingly effortless in the healthy individual, generating an optimal breathing pattern for O2 and CO2 homeostasis requires the integration of sensory information arising from a variety of receptors. These include multiple central and peripheral chemoreceptors for adjusting the magnitude of alveolar ventilation as well as multiple mechanoreceptors that respond to stretch of the airways and regulate the relative depth and rate of breathing to reduce energy expenditure. Sensory input is also important in the coordination of breathing with other systems, such those required for speaking, eating, walking, running, vomiting. Finally, sensory information is necessary for protection of the airways and lungs. Receptors in the nose, pharynx, larynx and lower airways elicit a variety of reflexes including coughs, sneezes and apnea that protect the airways from inhalation of noxious substances and increase mucous secretion that aids in their removal. Failure of any of these systems can be catastrophic, severely compromising the quality of life for an individual or ultimately leading to their death.
Respiratory Sinus Arrhythmia
10.1007/978-3-540-29678-2_3
Responding Conditioning
10.1007/978-3-540-29678-2_3
Response, Instrumental
Definition
A voluntary, conditioned response to a cue performed for reinforcement.
Response Acquisition
10.1007/978-3-540-29678-2_12
Response Extinction
Definition
The result of extinction, observed as a decrease in conditioned responses to a conditioned stimulus following non-reinforced presentations of the conditioned stimulus.
10.1007/978-3-540-29678-2_12
Response Inhibition
Definition
Inhibitory control is the ability to suppress behaviors that are inappropriate under the circumstances. Neuropsychological studies of the prefrontal cortex indicate that this function arises from the orbitomedial divisions, most probably via descending projections to structures such as the amygdala, basal ganglia and hypothalamus.
10.1007/978-3-540-29678-2_16
Rest-Activity Cycle
Definition
The fundamental alternation between extended periods of activity and rest that define a complete circadian cycle. Can also define ultradian (much less than 24 h) cycles.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_9
10.1007/978-3-540-29678-2_19
Resting Membrane Potential
Definition
The resting potential is a stable membrane potential in non-excitable cells or, in excitable cells, the most stable membrane potential between action potentials without excitatory or inhibitory inputs. In some excitable tissues, a resting potential cannot be defined because of continuous changes in membrane potential.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_1
Resting Tremor
Definition
Approximately 70% of patients notice tremor as the first symptom of Parkinson disease. Onset of tremor is usually in one hand and it may later involve the contralateral upper limb or ipsilateral lower limb.
Typically, the tremor is 3–5 Hz rhythmic “pill-rolling” movements of the thumb and index finger while the hand is at rest. There may be abduction and adduction of the thumb, or flexion and extension of the wrist, or of the metacarpophalyngeal joints. The tremor may also extend to the forearm with pronation–supination or even to the elbow and upper arm. During early disease, tremor is often intermittent and is evident only under stress. Tremor is worsened by anxiety, fatigue, and sleep deprivation. It diminishes with voluntary activity but may reappear with static posture (e.g. outstretched hands) and is absent during sleep. Resting tremor is enhanced by mental task performance, such as serial seven subtractions, and by motor task performance in a different body part. The hand tremor may also be enhanced during ambulation. Compared to essential tremor, the resting tremor of Parkinson disease is generally less prone to exacerbation by caffeine or improvement with alcohol.
10.1007/978-3-540-29678-2_16
Restless Legs Syndrome
Definition
Restless limbs syndrome is a common disorder with a prevalence of 5–15% in western countries. It is characterized by a distressing desire to move the legs, motor restlessness brought on by rest, worsening symptoms in the evenings and at night, and periodic limb movements during sleep. Although it can be seen with peripheral neuropathy, most cases of restless legs are not accompanied by neuropathy. There is an association between restless limbs and brain dopamine and iron deficiency. Therefore, checking iron and ferritin levels is part of the workup for restless legs syndrome. If iron deficiency is detected, it should be evaluated (anemia workup, etc.) and treated with iron supplementation. If a sleep study reveals sleep apnea together with periodic limb movements during sleep, the apnea component should be treated. Symptomatic treatment of restless legs and limb movements during sleep usually begins with a dopamine agonist (pramipexole or ropinirole). Dopamine agonists have longer durations of action compared to levodopa and one or two evening/bedtime dose(s) may suffice. If dopamine agonists are not well tolerated, a controlled release formulation of carbidopa/levodopa should be tried next, and the dose titrated as tolerated. Other adjunct medications include gabapentin, benzodiazepines, and low potency opioids as a last resort.
10.1007/978-3-540-29678-2_19
Ret
Definition
The signaling component of the glial cell line-derived neurotrophic factor (GDNF) family receptor complex. Ligand-binding to GPI-linked GFRα receptors (1–4) and subsequent induction of Ret dimerization results in Ret phosphorylation and activation of several signaling cascades, including those involving MAPK, PI3K and PLCγ.
10.1007/978-3-540-29678-2_7
10.1007/978-3-540-29678-2_14
Retention
Definition
Retention is the ability to maintain in mind information about a stimulus when that stimulus is no longer physically present. Retention span can vary from on the order of seconds or minutes to months or even years.
Reticular Core
Reticular Formation
Synonyms
Formatio reticularis; Substantia reticularis; Isodendritic core; Reticular core
Definition
The reticular formation is a netlike structure of cells and fibers that extends throughout the core of the brainstem. It maintains vegetative functions, plays an essential role in coordinated motor behaviors and exerts control on cortical and thalamocortical activation. Extensive damage to the reticular formation is incompatible with survival.
Characteristics
Anatomical Organization and Concepts
The term reticular formation was coined by anatomists in the last century to describe a region in the core of the 10.1007/978-3-540-29678-2_2 characterized by scattered neurons of various sizes and shapes with long, sparsely branching dendrites lying in and among multiple, differently oriented fiber systems. The dendrites of neighboring neurons overlap extensively with each other, giving the structure a netlike (“reticular”) appearance. The reticular formation extends continuously from the caudal medulla oblongata to the 10.1007/978-3-540-29678-2_13 (and possibly beyond, see below).
Based on differences in cytoarchitecture, cytochemistry and connections, the reticular formation has been divided into three longitudinal zones, a median zone, which contains the serotoninergic raphe nuclei (Greek raphe = seam), a medial zone, characterized by big cells intermingling with many small ones and a lateral zone, which consists predominantly of (Fig. 1) small neurons and has fewer fibers than the medial zone (Fig. 1a–c).
Reticular Formation. Figure 1.

(a) Photomicrographs showing some reticular formation nuclei in the median, medial, intermediate and lateral zones. Prominent, big neurons of the gigantocellular nucleus can be easily recognized. The gigantocellular nucleus is enlarged in (b), showing large and small neurons (arrows) side-by-side, one of the characteristics of the reticular formation. The very small cells (arrowheads) are not neurons, but glial cells. (c) shows an enlargement of the parvocellular reticular nucleus, which has neurons that are obviously smaller than those in the gigantocellular nucleus. Note also the different sizes and shapes of neurons. The facial nerve nucleus, which is not included in the reticular formation, is shown in (d). Its neurons are all of similar size and appearance and are more densely packed than those in the gigantocellular and parvocellular reticular nuclei (compare b, c, d). Abbreviations: 7n facial nucleus, g7 genu of facial nerve; Gi gigantocellular nucleus; IRt intermediate reticular nucleus; PCRt parvocellular reticular nucleus. Arrows point to neurons; arrowheads point to glial cells. The scale bar in (a) represents 0.5 mm in (a), 0.125 mm in (b–d).
More recently, an intermediate zone has been delineated between the medial and lateral ones (Fig. 1a, [1]). The intermediate zone comprises large, medium and small cells and exhibits slightly stronger 10.1007/978-3-540-29678-2_1 staining as compared to the adjacent medial and lateral zones. 10.1007/978-3-540-29678-2_3 nuclei (e.g. facial and cochlear) and relay nuclei (e.g. cuneate and red nuclei), which largely consist of densely packed neurons of similar appearance (Fig. 1d) are not included in the reticular formation.
Nuclei of the Recticular Formation
By analyzing Nissl stained (10.1007/978-3-540-29678-2_14) sections of the human and rabbit brainstem Olszewski and colleagues noted cytoarchitectural heterogeneities, which led them (and subsequently others in other species) to suggest that the reticular formation consists of different nuclei [2,10]. An overview of reticular formation nuclei is given in Table 1.
Reticular Formation. Table 1.
Nuclei of the brainstem reticular formation
Nuclei are ordered from caudal to rostral, colored boxes indicate where the nuclei are situated in the brainstem: blue – Medulla oblongata; orange – Pons; green – Mesencephalon. At medullary levels an intermediate zone has been delineated. Whereas the zones are easily recognizable at medullary levels (caudal), it is more difficult to distinguish them at rostral pontine and mesencephalic levels. Thus, at mesencephalic levels the allocation of nuclei to distinct zones is fairly tentative. Abbreviation: n nucleus.
At the medullary level of the medial reticular formation, the 10.1007/978-3-540-29678-2_14 has been delineated, which is composed of prominent multipolar giant cells, as well as large, medium sized and some small neurons (Fig. 1a, b). It is surrounded by the gigantocellular nucleus pars alpha and the lateral and dorsal paragigantocellular nuclei, all of which also contain large neurons. The gigantocellular nucleus extends from the obex to the rostral medulla oblongata and is continuous caudally with the ventral reticular nucleus and rostrally with the caudal pontine reticular nucleus. The caudal pontine reticular nucleus extends to the rostral pons where it merges with the oral pontine reticular nucleus, which in turn extends rostralward to the level of the decussation of the superior cerebellar peduncle. Dorsal and ventral to the caudal pontine reticular nucleus are the dorsomedial tegmental and ventral pontine reticular nuclei respectively. The lateral zone consists, in order from 10.1007/978-3-540-29678-2_3 to rostral, of the dorsal reticular (caudal medulla), parvocellular (medulla; Fig. 1c), subceruleus (pons) and cuneiform nucleus (pons, 10.1007/978-3-540-29678-2_13). The mesencephalic reticular formation is dominated by the deep mesencephalic nucleus, which some authors regard as part of the medial and others as part of the lateral zone of the reticular formation. Some of the above mentioned nuclei have been further subdivided (see [2,3]).
In addition to the above-mentioned nuclei are some that are regarded by some, but not all, authors to be part of the reticular formation, e.g. the lateral reticular nucleus, 10.1007/978-3-540-29678-2_16, locus ceruleus, pedunculopontine and laterodorsal tegmental nuclei, retrorubral field, and ventral tegmental area. It sometimes seems to be a matter of personal taste whether a nucleus is included in the reticular formation or not.
The Veticular Formation and the Ascending Reticular Activity System
In 1949, Moruzzi and Magoun published a seminal paper describing studies in which they demonstrated that stimulation of the reticular formation in lightly anaesthetized cats evokes a desynchronization of the cortical 10.1007/978-3-540-29678-2_5, closely resembling the changes observed in the human EEG upon transition from sleep to wakefulness or relaxation and drowsiness to alertness and attention [4]. Such EEG changes could be elicited by stimulation of the medial medullary, pontine and mesencephalic reticular formation and dorsal hypothalamus and subthalamus. They proposed a series of relays in the reticular formation ascending to the basal diencephalon and exerting influence on widespread areas of the cortex via a “diffuse thalamic projection system.” These and other observations led to the concept of an ascending reticular activating system (often referred to in the literature as ARAS), which spurred extensive research and had an enormous influence on subsequent views concerning the neural basis of consciousness. Because potentials could be recorded from large cortical territories following stimulation of afferents from a particular source and impulses from several sources were found to reach the same region, it was assumed that the system would be entirely diffusely organized. Unfortunately, the term “ascending reticular activating system” describing a physiological concept was soon frequently used synonymously with the morphological term “reticular formation,” which led Olszewski to comment [5]: “There are presently two reticular formations – an anatomic one and a physiologic one – and they do not correspond to each other.”
The Concept of the Isodendritic Core
Due to the confusion surrounding the term reticular formation and the lack of a generally accepted definition, some scientists suggested that use of the construct be discontinued altogether (e.g. [5,6]), whereas others attempted to define it more precisely [1 and refs. therein]. Based on analyses of Golgi stained (10.1007/978-3-540-29678-2_7) brain sections of different species Ramon-Moliner and Nauta described not one defining characteristic of reticular formation, but rather an aggregation of several morphological features that they concluded are found together only in the reticular formation: i) cytological polymorphism, large and small neurons are side by side (Fig. 1b); ii) generalized dendrites, long, radiating, relatively rectilinear and sparsely branching processes, iii) considerable dendritic overlap and iv) free intermingling of dendrites and passing myelinated and unmyelinated fiber bundles. Ramon-Moliner and Nauta called neurons (Fig. 2) with the generalized dendritic patterns found in the reticular formation “isodendritic” (from the Greek isos: similar, uniform, Fig. 2a) and distinguished them from allodendritic (allos: different) and idiodendritic (idios: peculiar) neurons (Fig. 2b).
Reticular Formation. Figure 2.

(a) Isodendritic neurons have long, sparsely branching dendrites (arrows) and overlapping dendritic fields (asterisks). (b) Allodendritic neurons have short dendrites, which ramify close to the cell body. Dendrites of neighbouring neurons largely do not overlap with each other. These Golgi preparations are adapted from figs. 6 and 7 of [1], with permission from Wiley Interscience.
Idio- and allo-dendritic neurons, also sometimes referred to as “hodophob” (hodos: path, pathway; phob: fearing), are characterized by short dendrites that ramify close to the cell body and do not extend into passing fiber bundles (Fig. 2b). These neuron types are found, e.g. in sensory nuclei, like the cuneate nucleus. In contrast, the “reticular” isodendritic neurons are regarded as “hodophil” (phil: friendly).
Ramon-Moliner and Nauta’s concept of reticular formation does not necessarily preclude the existence of regional differences and is not incompatible with parcellations suggested by Olszewski and others. Thus, although the extensively overlapping dendritic fields of reticular neurons make it difficult, if not impossible, to draw definite lines around nuclei, the division of the reticular formation into nuclei is nevertheless helpful and necessary (and widely employed today) to describe the locations of nerve cells, electrode placements and lesions within the reticular formation. Applying the criteria of Ramon-Moliner and Nauta, the lateral reticular nucleus is not part of the reticular formation because it consists of allodendritic neurons and has a restricted set of connections with the 10.1007/978-3-540-29678-2_19 and 10.1007/978-3-540-29678-2_3. In contrast, e.g. the 10.1007/978-3-540-29678-2_16, locus ceruleus, pedunculopontine and laterodorsal tegmental nuclei, retrorubral field and ventral tegmental area have sufficient reticular characteristics to be included. Furthermore, if these criteria are applied, the reticular formation is not confined to the brainstem, but also includes structures in the forebrain, such as the 10.1007/978-3-540-29678-2_12 and preoptic areas and the magnocellular 10.1007/978-3-540-29678-2_2 [7]. Even if these areas are conservatively excluded from the reticular formation, their neuroanatomical organization suggests that they process information in a manner similar to that which occurs in the reticular formation of the brainstem.
Connections and Functions of the Reticular Formation of the Brainstem
The reticular formation maintains pivotal vegetative functions (e.g. respiration, heart beat, blood pressure) and plays an essential role in coordinated motor behaviors (e.g. swallowing, chewing and 10.1007/978-3-540-29678-2_12). In addition, it functions as an intermediary through which amygdala, septum, and basal ganglia gain access to the autonomic and motor systems [8]. Via its ascending projections, the reticular formation exerts control on cortical and thalamocortical activation and functions.
Medial Reticular Formation
Reticular neurons give rise to long ascending (e.g. to the cerebral 10.1007/978-3-540-29678-2_3) and descending (e.g. to the sacral level of the spinal cord) axons that give off several collaterals along their course, thereby interconnecting different parts of the reticular formation [3]. For example the oral and caudal pontine reticular nuclei receive half of their afferents from other parts of the brainstem reticular formation. Other main afferents to the medial reticular formation arise in the 10.1007/978-3-540-29678-2_16 and sensory cortices, zona incerta, fields of Forel, lateral hypothalamus, preoptic area, substantia nigra pars reticulata, superior colliculus, central gray, cerebellum and spinal cord. The medial reticular formation projects mainly to the spinal cord and motor cranial nerve nuclei and to the laterodorsal and pedunculopontine tegmental nuclei, intralaminar thalamic nuclei, fields of Forel, parafascicular thalamic nucleus, zona incerta and lateral hypothalamus. The projections of the individual nuclei of the medial reticular formation differ in degree rather than kind. Thus, all parts of the medial reticular formation project to the telencephalon, but the largest number of neurons projecting there is located at the mesencephalic level, whereas only a few are situated at caudal pontine and medullary levels. Similarly, whereas many neurons in the gigantocellular nucleus project to the spinal cord, only a few in the mesencephalic reticular formation do so [3].
Neurons in the dorsal two thirds of the caudal pontine and medullary medial reticular formation project to the intermediate and ventral horn of the spinal cord, where the premotor interneurons and motorneurons for the axial and proximal musculature (i.e. trunk, hip, back, shoulder and neck) are situated. Thus, they play an important role in the control of posture, integration of the movements of body and limbs and the orientation of body and head. Because orienting movements of the head and eye are tightly linked, it might not be surprising that parts of the medial reticular formation that project to the spinal cord also possess neurons that innervate eye muscle motor neurons (oculomotor and 10.1007/978-3-540-29678-2_1 nuclei), which are necessary for horizontal 10.1007/978-3-540-29678-2_7 control. Neurons controlling the phylogenetically later developed vertical gaze control (trochlear and oculomotor nuclei) are situated further rostrally in the medial reticular formation.
Neurons in the medial reticular formation also influence wide areas of the cerebral cortex via their strong ascending projections to the laterodorsal and pedunculopontine tegmental nuclei and, to a lesser extent, to intralaminar thalamic nuclei (see Essay on Mesopontine Tegmentum).
Lateral Reticular Formation
The lateral reticular formation receives afferents from the primary motor and 10.1007/978-3-540-29678-2_19, 10.1007/978-3-540-29678-2_9, 10.1007/978-3-540-29678-2_3, bed nucleus of stria terminalis, central gray, trigeminal nuclei, rostral part of the 10.1007/978-3-540-29678-2_14, red nucleus, other parts of the reticular formation and cerebellum. Neurons in the lateral reticular formation project predominantly to motor cranial nuclei (e.g. trigeminus, facial and hypoglossal) and the medial reticular formation.
Sensory information from jaw muscle spindle afferents and oral cavity (via trigeminal nuclei), taste (via the rostral part of the nucleus of the solitary tract), visceral information (via the insular cortex) and oral motor information (from the motor cortex) are relayed to and integrated within the lateral reticular formation, which in turn projects to motor cranial nuclei containing neurons for muscles involved in swallowing, chewing and salivation. In addition, inputs from structures commonly regarded as being involved in emotional processing, such as 10.1007/978-3-540-29678-2_1, bed nucleus of stria terminalis and 10.1007/978-3-540-29678-2_16 are integrated in the lateral reticular formation and relayed to, e.g. facial motor neurons and autonomic centers.
The connections conceptualized
These anatomical data provide a general idea about the connections, but are insufficient to explain the precise circuits that underlie the functions of the reticular formation. Against the common perception that the reticular formation is organized in a diffuse way, the complex behaviors it is involved in require very specific and precisely tuned connections. Breathing, for example, requires the coordinated movements of jaws, lips, tongue, pharynx and larynx, can be controlled voluntarily, but usually happens automatically and has to adapt for eating, speaking, fighting or fleeing. Thus, a high level of specificity must exist in the reticular formation, but it is the very structure of the reticular formation that makes such a specificity very difficult to detect.
So, what might such an apparently disorganized structure be good for? To appreciate the functional anatomical organization of the reticular formation, it might be useful to compare it to a system that is quite differently organized, such as the primary motor or sensory system. The so-called lemniscal system carries sensory information via two synapses to the primary sensory cortex. Such a sensory pathway of minimal interruption rigorously maintains the topography of the sensory periphery and thus permits the exact localization of the source of the sensory stimulus. Hence, this system is very well suited for discriminative functions. The reticular formation, in contrast, consisting of neurons with long dendrites receiving multiple heterogeneous inputs and emitting axons that collateralize a lot, is especially well suited for integrative functions. As Nauta and Feirtag [9] state “Life depends on the innervation of the viscera: in a way all the rest is biological luxury. And vital systems ought to be organized on the principle that no single excitation should greatly affect their workings.” It remains an exciting challenge to untangle the precise underlying neuronal networks.
Reticulospinal Cells (Neurons)
Definition
Neurons located within the reticular formation with an axonal projection to the spinal cord. Reticulospinal cells project to different levels of the spinal cord, some activating exclusively the most rostral segments whereas others activate the caudal ones.
Reticulospinal Long-Lead Burst Neurons
Definition
Reticulospinal neurons, in general, are neurons that have their somata in the mesencephalic, pontine, or medullary reticular formation and have axons that project at least as far as the upper-most spinal cord. Reticulospinal 10.1007/978-3-540-29678-2_12 (RS-LLBNs) (10.1007/978-3-540-29678-2_2) are reticulospinal neurons that discharge before and during gaze saccades in a preferred direction that depends on the particular cell. Like other reticulospinal neurons, RS-LLBNs integrate input from multiple sources and project to multiple targets. In the case of RS-LLBNs, the purpose is to generate coordinated eye, head, and sometimes trunk movements.
Characteristics
Higher-Order Processes
RS-LLBNs are part of a system of descending pathways that mediate orienting toward areas or objects of interest. The stereotypical 10.1007/978-3-540-29678-2_15 depends on the species. Macaque monkeys move only their eyes if the target of interest is near the current direction of gaze, but will make a combined eye and head gaze movement for targets further away. Cats preferentially move the eyes and head together, and typically also redirect their pinnae and maybe their trunk. Rodents do all of the above with greater movement of the trunk. Human orienting is more flexible, but usually includes combined eye and head movements. The descending system that mediates this behavior includes the reticulospinal pathway, the tectospinal pathway, the corticospinal pathway, as well as less direct pathways.
The command to execute an orienting movement originates in cerebral cortex and involves many of the same structures as saccade generation. The 10.1007/978-3-540-29678-2_12, the 10.1007/978-3-540-29678-2_19, and the 10.1007/978-3-540-29678-2_6, which are themselves interconnected, project to the deep and intermediate layers of the 10.1007/978-3-540-29678-2_19 as well as to other brainstem locations. Signals for these cortical areas, as well as those from subcortical areas, are integrated in the superior colliculus, and the colliculus issues the principal command to move the eye, head, etc. This command is conveyed to the brainstem, including the 10.1007/978-3-540-29678-2_19 and reticulospinal neurons, and directly to the spinal cord. In cats, this “tectospinal” pathway is strong and consists of well studied 10.1007/978-3-540-29678-2_20. In primates, the tectospinal pathway is weak and probably includes neurons that differ from the cat TRSNs. This means that primates rely heavily on reticulospinal pathways to orient.
The superior collicular projection to the brainstem provides the principal input to the RS-LLBNs and a smaller input to other reticulospinal neurons. Efferents from cerebral cortex provide additional input to RS-LLBNs, but not as much as to other reticulospinal neurons [1]. Cortical inputs arise from motor cortex and possibly premotor cortex. Another input to reticulospinal neurons, including RS-LLBNs, is from the 10.1007/978-3-540-29678-2_6 of the midline cerebellum. The fastigial nucleus, in turn, receives indirect input from the superior colliculus, the FEF, the SEF, and motor cortex, and represents another pathway by which these cortical structures can influence RS-LLBNs. In fact, orienting movements in cats and monkeys are severely disrupted by chemical inactivation of the fastigial nucleus. Many reticulospinal neurons that receive input from the superior colliculus also receive input from the vestibular system [2], although the relevance for RS-LLBNs is controversial. Caudal reticulospinal neurons receive input from collaterals of more rostral reticulospinal neurons [1,3–5].
Parts of the RS-LLBN System
RS-LLBNs as well as other reticulospinal neurons are found in the medullary 10.1007/978-3-540-29678-2_14, pontomedullary 10.1007/978-3-540-29678-2_14, and in the mesencephalic reticular formation in and near the 10.1007/978-3-540-29678-2_9 and the H-fields of Forel (called the riMLF in the monkey). Neurons have different properties in different areas, and in different species according to current incomplete data. In general, neurons in NRPc and dorsal NRG have horizontal preferred directions and appear to innervate horizontal eye and head movers, while those in ventral NRG and in the midbrain have vertical preferred directions and appear to innervate vertical eye and head movers. Behaviorally identified RS-LLBNs have mainly been studied using intra-axonal recording and subsequent injection with horseradish peroxidase.
Cats
The best studied RS-LLBNs are “eye-neck neurons,” which have their somata in the NRPc rostral and/or ventral to the abducens nucleus [3]. Collaterals of the descending axon arborize in the abducens nucleus, the prepositus nucleus, the medial vestibular nucleus (all related to horizontal eye movements), the facial nucleus (mediating pinna movement), the dorsal NRG and the nucleus reticularis ventralis (containing reticulospinal neurons), and paramedian cell groups projecting to the cerebellum (Fig. 1) [3].
Reticulospinal Long-Lead Burst Neurons. Figure 1.

Camera lucida drawing of part of a cat reticulospinal neuron drawn in a parasagittal plane. The large soma is located in the PPRF just rostral to the abducens nucleus (VI), and the descending axon gives off branches that terminate in the abducens and facial motor nuclei (VI and VII), nucleus prepositus hypoglossi (PH), nucleus intercalatus (IC), the medial and lateral vestibular nuclei (VM and VL), the nucleus reticularis gigantocellularis (NRG), and the nucleus reticularis ventralis (NRV). Symbols show where the branches left the plane of section, and are coded according to where the branches terminated (see Key). Other abbreviations; G VII = genu of the VIIth nerve, NRPc = nucleus reticularis pontis caudalis, PRN = paramedian reticular nucleus, XII = hypoglossal nucleus, IO = inferior olive, CT = corticospinal tract. Adapted from Grantyn et al. [3].
During attempted gaze shifts in head-fixed cats having electromyographic (EMG) electrodes implanted in the neck muscles, a typical eye-neck neuron exhibits a burst of spikes that begins 66–132 ms before the saccade (hence the long-lead designation), peaks during the saccade, and slowly decays to an end after saccade end but roughly coinciding with the end of the phasic component of neck EMG activity [6]. Eye-neck neurons also have a moderate firing rate during sustained EMG activity when the head is held eccentrically. Phasic and sustained activity increases with increasing eye movement towards the 10.1007/978-3-540-29678-2_9 side and with increasing EMG activity in the ipsilateral neck muscles, but is not perfectly correlated with either alone. These discharges and the axonal arborization of these neurons both suggest a role in the coordinated activation of eye and neck muscles.
A second group of more caudally located RS-LLBNs has similar discharges but with very little sustained activity when the head is stationary [4]. Somata are mostly located caudal to the abducens nucleus, but one was located rostral to the abducens. The axons do not collateralize to innervate any cranial motor nuclei, but rather innervate the paramedian reticular nucleus, interstitial cell groups in the MLF (which both project to the cerebellum), and nucleus reticularis ventralis. Preferred directions could have large vertical components, and one neuron had a contralateral preferred direction and an axon that descended in and innervated the contralateral medulla and spinal cord. The lack of projections to oculomotor structures, the projection to the spinal cord, and the phasic burst all imply these neurons function as head burst-neurons.
A third group of RS-LLBNs have their somata in the H-fields of Forel – the cat counterpart of the monkey rostral interstitial nucleus of the MLF. Called “augmenting neurons” by some, these neurons have a long build up beginning about 200 ms before upward gaze movements. The burst lasts about as long as the head movement in head-free alert cats and has a peak rate that is proportional to head velocity [7]. They also have a low-rate spontaneous discharge that is independent of eye position, and thus are only borderline LLBNs. Projections of augmenting neurons were studied electrophysiologically. Besides projecting to the spinal cord, augmenting neurons project to the midbrain cuneiform nucleus (containing saccade-related neurons) [8] and strongly to reticulospinal neurons both rostral and caudal to the abducens nucleus (NRPc and NRG) [5]. There are more typical LLBNs located at the caudal border of the H fields, but they do not have projections to the spinal cord.
Monkey
Very little research has been devoted to reticulospinal neurons in monkeys. LLBNs have been recorded in NRPc that fire throughout the duration of the head movement in gaze saccades, but their potential spinal projections were not explored. Two groups of confirmed RS-LLBNs have been found in head-stabilized monkeys, but there are surely more than this.
Two RS-LLBNs with their somata in NRPc anterior and ventral to the abducens nucleus had preferred directions that were ipsiversive for one and down for the other [9]. Burst leads averaged 17 and 42 ms, and the number of spikes in the burst increased weakly with saccade size. As the monkeys had their heads stabilized, burst parameters could not be analyzed in relation to head movements. Axons traveled outside the paramedian tracts on their way to the spinal cord, and issued collaterals that innervated NRG, the middle of the prepositus nucleus, the paramedian reticular nuclei (both of which project to the cerebellum), and to the nucleus reticularis ventralis (Fig. 2). With the exception of the soma locations, these RS-LLBNs are similar to the head burst-neurons in the cat (group 2, above).
Reticulospinal Long-Lead Burst Neurons. Figure 2.

Camera lucida drawing of a monkey reticulospinal neuron drawn in a parasagittal plane. The large soma is located in the PPRF just rostral to the abducens nucleus (ABD), and the descending axon gives off dorsally-coursing branches that terminate in the medullary reticular formation, the nucleus prepositus hypoglossi (NPH), and the dorsal and ventral paramedian reticular nucleus (PRNd and PRNv). Staining faded as the main axon entered the spinal cord (dotted line). Inset shows the firing rate (FR) of the neuron during saccades, shown as a horizontal component (H) and vertical component (V). Other abbreviations; G = genu of the VIIth nerve, NRG = nucleus reticularis gigantocellularis, XII = hypoglossal nucleus, IO = inferior olive, PT = pyramidal tract. Adapted from Scudder et al. [9].
Three RS-LLBNs had their somata just lateral to the interstitial nucleus of Cajal [10]. All discharged preferentially for upward saccades beginning 25–105 before saccade onset, and the number of spikes increased with increasing saccade amplitude. The axons descended in the MLF and issued no collaterals until the caudal pons. They subsequently innervated raphe pontis, raphe obscurus, and the paramedian reticular nucleus, all of which project to the cerebellum, including oculomotor-related areas in the first two cases. Axons additionally innervated raphe interpositus and the dorsal NRG, both containing neurons that are part of the saccadic burst generator, and more ventral parts of NRG containing reticulospinal neurons.
Both types of RS-LLBNs have heavy projections to the cerebellum via relay nuclei. These projections are perhaps especially appropriate in the monkey relative to the cat because primates are credited with more flexible eye-head strategies, and could rely on the cerebellum to insure the different combinations of eye and head movements end with gaze directed at the target.
Lower level Processes
The innervation of the spinal cord has been studied in detail for cat reticulospinal neurons, but not for RS-LLBNs specifically. This is because staining of the intra-axonally filled RS-LLBNs described above always faded before reaching their terminations in the cord. Doubtlessly, RS-LLBNs share some features of the more general innervation. Reticulospinal neurons with somata in NRPc and NRG and receiving input from the superior colliculus project to upper cervical segments, including interneuron and motoneuron pools for the neck (Rexed laminae VII, VIII, IX). Motoneurons that are monosynaptically contacted by these reticulospinal neurons include those innervating the multisegmental dorsal muscles, splenius (a horizontal neck rotator) and biventer cervicis and complexus (a vertical neck rotator). Individual neurons in NRG innervate either splenius or biventer/complexus, but poorly innervate the other. Some NRPc and NRG reticulospinal neurons project to interneurons in the lower cervical segments (innervating forelimbs) and possibly lumbar segments (innervating hindlimbs). Activity in fore- and hind-limb muscles has been observed during orienting movements, but these could be a postural reaction as much as a direct product of orienting behavior. Reticulospinal neurons with somata in the H fields and receiving input from the superior colliculus make monosynaptic connections with biventer cervicis and complexus motoneurons, but few with splenius, confirming the specificity of the H fields for vertical movements [1,5].
Pathology
Excitotoxic destruction of the somata of neurons in the cat NRPc and NRG on one side produced severe to moderate deficits in producing ipsiversive gaze saccades, depending on the extent of the lesion. Vertical gaze saccades were also impaired when the head was oriented to the ipsilesional side. The loss of eye-saccades is presumably due to the destruction of the cells of the 10.1007/978-3-540-29678-2_2, and the loss of head-saccades is presumably due to destruction of the RS-LLBNs. 10.1007/978-3-540-29678-2_5 of the H-fields in cats produced debilitating asymmetries in neck muscle tone when done unilaterally, and major deficits in vertical head saccades when done bilaterally. Lesions in humans produced by tumors or infarcts would likely result in more severe deficits due to the destruction of the many fibers of passage as well as destruction of reticulospinal neurons.
Reticulospinal Neurons in Eye Movements
Definition
Neurons of the brainstem reticular formation that project to the spinal cord. Anatomical studies have demonstrated that, on their way to the spinal premotor centers, axons of resticulospinal neurons (RSNs) emit numerous collaterals to various oculomotor and vestibular centers.
Together with some superior colliculus (SC) output neurons showing a similar pattern of projections, those RSNs that receive input from the SC are thus ideally positioned to decompose the collicular “desired gaze displacement” signal into motor commands for the eye and head platforms.
10.1007/978-3-540-29678-2_5
10.1007/978-3-540-29678-2_19
Reticulospinal Tract
Synonyms
Tractus reticulospinal ant; Anterior reticulospinal tract
Definition
The medial reticulospinal tract begins in the caudal pontine reticular nucleus and in the caudal portion of the oral pontine reticular nucleus. It descends in the medial longitudinal fasciculus in the spinal cord. Its fibers terminate mostly in lamina VII and VIII of the spinal gray matter; but they also run in lamina IX in which the motoneurons for the trunk musculature lie.
Pathways
Reticulotectal Long-Lead Burst Neurons
Synonyms
RTLLBs
Definition
Saccade related long-lead burst neurons of the primate mesencephalic reticular formation (cMRF) that project to the SC.
Characteristics
Higher Order Structure
Although they belong to the reticular formation, due to their location and projections RTLLBs can be thought to belong to a satellite system of the SC.
Parts of This Structure
Figure 1 illustrates the salient morphological and physiological features of RTLLBs following the intracellular study of their discharge in alert squirrel monkeys, their subsequent injection with a tracer and the camera lucida reconstruction of their axons in the frontal plane [1]. RTLLB somata (Fig. 1b, arrow) are located in an area receiving strong input from saccade related neurons of the SC (see TLLBs). The territory occupied by RTLLB somata probably receive input from additional classes of SC neurons, as indicated by axonal reconstructions of single X neurons of the cat [2,3] and the monkey [4,1]. The axonal terminations of RTLLBs are contained within the intermediate and deeper layers of the SC, in both sides of the brain (Fig. 1b).
Reticulotectal Long-Lead Burst Neurons. Figure 1.
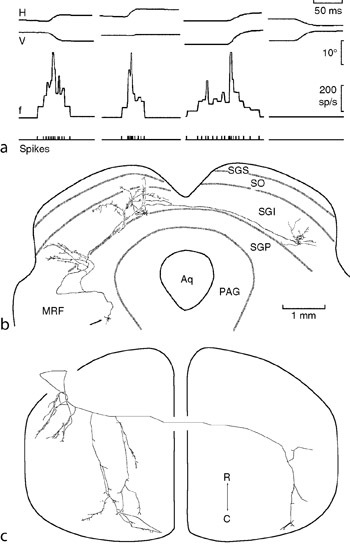
Salient morphological and physiological features of RTLLBs (reprinted from [1], with permission). (a) Saccade related discharge pattern for one RTLLB. (b) Frontal reconstruction of the axonal system of the same neuron from serial sections. (c) The axonal system of the same neuron as it appears when looking down upon the surface of the SC. Scale in (b) applies to both (b) and (c). Abbreviations: Aq, aqueduct; C, caudal; MRF, mesencephalic reticular formation; PAG, periaqueductal grey; R, rostral; SGI, stratum griseum intermediale; SGP, stratum griseum profundum; SGS, stratum griseum superficiale; SO, stratum opticum.
Functions of the Structure
RTLLBs emit bursts of discharge which precede contraversive saccades, whether upward, downward or horizontal (Fig. 1a) by about 20 ms on average. They often do not discharge for saccades in the opposite direction (Fig. 1a, right-most example). Although the on-direction of many RTLLBs is roughly horizontal, the existence of RTLLBs with vertical and oblique on-directions has been documented [1]. The activity of RTLLBs provides a good estimate of the metrics of impending saccades; the correlation coefficient between the number of spikes in the burst, and the amplitude of saccades in their on-direction can be as high as 0.85.
Higher Order Function
RTLLBs could belong to a highly conserved satellite system of the SC as cells with quite similar morphology have been encountered in lower phyla. Neurons with bilateral projections largely confined to the optic tectum have been found in the nucleus lateralis profundus mesencephali of the snake [5] and the dorsolateral tegmental nucleus in fish [6]. Also, saccade related signals were discovered in the intertectal commissure of fish [7] before the existence of saccade related signals in the superior colliculus of mammals became known. Given their discharge pattern and projections, RTLLBs are eminently qualified to supply the SC with an efference copy signal indicative of the metrics of ongoing saccades. Together with the visual input carried by L neurons (see 10.1007/978-3-540-29678-2_19) RTLLBs endow the SC with the machinery needed to implement the Vector Subtraction hypothesis (see 10.1007/978-3-540-29678-2_6).
Quantitative Measure for This Structure
Besides detailed quantitative descriptions of the pattern of their discharge, the 3D spatial distribution of the terminals deployed in the SC by single functionally identified RTLLBs has been described in squirrel monkeys [8]. When overlaid on a horizontal map of the SC, they can occupy a considerable portion of the rostrocaudal and mediolateral extent of its deeper layers (Fig. 1c).
Retina
Definition
The light-sensitive layered neural tissue that lines the posterior hemisphere of the eye and contains the photoreceptors (rods and cones) and the initial processing machinery for the primary visual pathways. It is a highly organized structure whose function is to capture, process, and transmit visual images to the brain. The signals generated by photoreceptors are then processed by other neurons in the retina before being transmitted to the brain as trains of action potentials by the axons of the retinal ganglion cells. Additionally, the retina serves to detect changes in ambient levels of light to regulate a multitude of non-visual photoresponses such as the pupillary light reflex.
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_22
Retinal Bipolar Cells
Synonyms
Retinal bipolar neurons
Definition
Bipolar cells are interneurons in the retina (10.1007/978-3-540-29678-2_22), which transfer visual information from photoreceptors (rods and cones; 10.1007/978-3-540-29678-2_16) to amacrine (Retinal direction selectivity: Role of starburst amacrine cells) and ganglion cells (retinal ganglion cells). Bipolar cells consist of multiple (9–12) subtypes that differ in their morphology, synaptic connectivity, and response properties. Different types of bipolar cells process different visual modalities in parallel pathways. The following article describes the structure, distribution, synaptic connectivity, and function of bipolar cells in the mammalian retina.
Characteristics
Quantitative Description
Morphology of Bipolar Cells
The name “bipolar cell” is derived from its morphology. Bipolar cells have a cell body in the 10.1007/978-3-540-29678-2_9 from which a primary dendrite extends into the 10.1007/978-3-540-29678-2_15 and an axon extends into the 10.1007/978-3-540-29678-2_9 (10.1007/978-3-540-29678-2_22). Morphologically two major types, cone bipolar and rod bipolar cells, can be distinguished with respect to their connections with photoreceptors (10.1007/978-3-540-29678-2_16. The dendrites of cone bipolar cells contact cone photoreceptors almost exclusively and are thus involved in high-acuity daytime vision and colour vision (10.1007/978-3-540-29678-2_3. The dendrites of rod bipolar cells contact rod photoreceptors and are involved in night or 10.1007/978-3-540-29678-2_19 vision.
Bipolar cells make up about 40% of all retinal neurons (apart from photoreceptors) and are the most numerous interneurons in the retina [1]. Each bipolar type is found across the retina and forms a regular mosaic.
Diffuse Bipolar Cells
Cone bipolar cells are subdivided into OFF and ON bipolar cells [2]. The OFF bipolar cells hyperpolarise in response to light, whereas the ON bipolar cells depolarise. The axons of OFF bipolar cells stratify in the outer half of the inner plexiform layer. The axons of ON bipolar cells stratify in the inner half of the inner plexiform layer.
The OFF and ON cone bipolar types are further subdivided into at least nine subtypes with respect to their stratification in the inner plexiform layer and their connections with cones (Fig. 1) [1–6].
Retinal Bipolar Cells. Figure 1.

Bipolar cell types in primate retina as analysed from Golgi-impregnated macaque retina. The axon terminals stratify at different levels of the inner plexiform layer (IPL). Diffuse bipolar cells (DB1 – DB6) non-selectively contact multiple cones in the outer plexiform layer (OPL). There are two types of midget bipolars, flat midget bipolar (FMB or OFF midget) and invaginating bipolar cells (IMB or ON midget). Both types contact single M- or L-cones and carry a chromatic signal. Blue cone bipolar (BB) cells contact S-cones selectively and carry an S-cone ON signal. Rod bipolar cells contact rod spherules and transfer scotopic signals.
Most cone bipolar types contact five to ten cones, and have thus been named diffuse bipolar (DB) cells [3]. Some of these subtypes can be selectively labelled with immunohistochemical methods. An example of an immunohistochemically labelled ON bipolar type (DB6) from macaque retina is shown in Fig. 2.
Retinal Bipolar Cells. Figure 2.

Diffuse bipolar cells in primate retina. DB6 cells (Fig. 1) are immunohistochemically labelled. Scale bar: 25 μm.
Figure 3 shows the mosaic formed by DB6 cells in whole mount view. The fine dendrites form a dense meshwork across the outer plexiform layer. The somata are located in the outer half of the inner nuclear layer, and the axons tile the retina in a regular mosaic.
Retinal Bipolar Cells. Figure 3.

Diffuse bipolar cells in primate retina. Whole mount view of immunohistochemically labelled DB6 cells. The cells are shown at different focal levels. Scale bar: 20 μm.
In recent years, a number of studies have estimated the density of bipolar cell types using a variety of methods including electron microscopy, Golgi-impregnation, immunohistochemistry, intracellular injection and photofilling [3–5]. The major conclusions from these quantitative studies are that all types have comparable densities ranging between 1,000 and 3,000 cells/mm2, and thus no type of bipolar cell usually predominates. The only exception to this rule is the midget bipolar cell in primate fovea (see below). The density of cone bipolar cells is usually lower than the cone density. However, since most bipolar cell types contact multiple cones, it is assumed that all cones provide input to all types of cone bipolar cells [1,3,6].
Bipolar Cell Types Involved with Colour Vision
Most mammals possess two types of cones, one that is maximally sensitive to short wavelengths of the visible spectrum (S or blue cone), and one that is maximally sensitive to long wavelengths [7]. Mammals with two cone types have dichromatic colour vision. Trichromatic colour vision is based on the presence of a third cone type that is maximally sensitive to medium wavelengths. Among placental mammals, trichromatic colour vision has only been described in primates (10.1007/978-3-540-29678-2_3) [7].
Three types of cone bipolar cells are involved with colour vision: blue cone bipolar cells, and two types of midget bipolar cells. The blue cone bipolar cells (BB, Fig. 1) are ON cells and receive input exclusively from S-cones. The axons of blue cone bipolar cells stratify close to the ganglion cell layer where they provide output to a special type of ganglion cell, the blue ON/yellow OFF ganglion cell [1,3,4,6,8].
Each blue cone bipolar cell receives input from between one and five S-cones (convergence), and each S cone contacts between one and five blue cone bipolar cells (divergence). The maximal density of blue cone bipolar cells is approximately 800 cells/mm2, thus they are among the least numerous bipolar types in the retina.
In mammals, midget bipolar cells [1,3,4,6,9] are probably unique to primate retina where they are thought to play a role in trichromatic colour vision. Midget bipolar cells can be subdivided into ON and OFF types. The ON midget bipolar dendrites make 10.1007/978-3-540-29678-2_9 with cones, and have thus been named invaginating midget bipolar (IMB, Figure 1) cells. The OFF midget bipolar dendrites make flat synapses with cones, and are thus called flat midget bipolar (FMB, Fig. 1) cells. In the central retina, each midget bipolar cell receives input from a single cone and in turn contacts one ON or one OFF midget (parvocellular projecting) ganglion cell. Thus, each midget bipolar cell carries the chromatic signal of the cone type it contacts to the inner plexiform layer.
In central retina, midget bipolar cells are the most numerous cone bipolar type. The density of FMB cells was estimated in macaque retina. In central retina, their density follows the cone density (> 10,000 cells/mm2). In peripheral retina, FMB cells contact more than one cone, and thus their density (< 5000 cells/mm2) drops below the cone density [9].
Rod Bipolar Cells
In all mammalian retinae only one type of rod bipolar cell studied to date has been described (RB, Fig. 1). Each rod bipolar cell contacts between ∼20 and 100 rod spherules (Retinal ribbon synapses) in the outer plexiform layer. Rod bipolar cells are depolarised by light, and thus are ON cells [2]. The axon terminals of rod bipolar cells are located at the border of the inner plexiform layer with the ganglion cell layer. The axon terminals of rod bipolar cells tile the retina in a non-overlapping mosaic (Fig. 4).
Retinal Bipolar Cells. Figure 4.

Whole mount view of the axonal terminals of two types of ON bipolar cells in primate retina (courtesy of Patricia Jusuf, NVRI, Melbourne). The two types of cell (rod bipolar and DB6) form separate mosaics but might share some postsynaptic cell types. Scale bar: 10 μm.
Rod bipolar axons provide output to AII amacrine cells, which then feed the rod signal into cone pathways [2,5,6].
The density of rod bipolar cells varies depending on the retinal location and between species. For example, in cat retina the density ranges between ∼20,000 and 46,000 cells/mm2, whereas in rabbit the density ranges between ∼2,000 and 5,000 cells/mm2. In macaque retina, rod bipolar cells are absent from the fovea, and the maximal density is ∼15,000 cells/mm2 at about 1–3 mm distance from the fovea. Thus, the density of rod bipolar cells is higher than that of individual cone bipolar cell types, but cone bipolar cells outnumber rod bipolar cells in total [1].
The ratio between rods and rod bipolar cells (numerical convergence) varies between species. It is 10:1 in central macaque retina, 15:1 in the area centralis of cat retina, and 50:1 in rabbit retina. However, the divergence between rods and rod bipolar cells is relatively constant. For all species studied, and at all eccentricities, between one and four rod bipolar cells are postsynaptic to each rod.
Description of the Structure
Synaptic Connectivity in the Outer Plexiform Layer
The synaptic terminal of a rod photoreceptor (rod spherule) is a relatively simple structure. It contains one or two synaptic ribbons (Retinal ribbon synapses), which are presynaptic to usually two rod bipolar and two horizontal cell processes at a single synaptic invagination. The rod bipolar cell dendrites form the central elements at this synapse [5].
The synaptic terminal of a cone photoreceptor (cone 10.1007/978-3-540-29678-2_3; Retinal ribbon synapses) is a much more complex synapse (Fig. 5a).
Retinal Bipolar Cells. Figure 5.

Schematic drawing of a cone pedicle in macaque monkey retina. (a) Four presynaptic ribbons and four triads are shown. Invaginating dendrites of horizontal cells (red) form the lateral elements, invaginating dendrites of ON bipolar cells (green) form the central elements of the triads. Flat contacts (blue) are mainly made by OFF bipolar cells. Desmosome-like junctions (black bars) are located at a distance of about 1.5 μm underneath the pedicle. (b) The same pedicle with the laminated expression of postsynaptic glutamate (red and blue) and GABA receptors (blue and green) is shown.
It comprises of between 20 and 50 invaginating and several hundred flat contacts. The invaginating contacts consist of a presynaptic ribbon, one or two central elements deriving from ON cone bipolar cells, and two lateral processes deriving from horizontal cells (triad). The 10.1007/978-3-540-29678-2_6 are located at the base of the cone pedicle and derive mostly from OFF bipolar cells. In total, each cone pedicle makes about 500 contacts. The number of postsynaptic cells is lower as some cells receive multiple contacts. About 10–15 individual cone bipolar cells, comprising of a variety of different types, are postsynaptic to a cone pedicle [3]. Thus, at the cone pedicle, the first synapse in the retina, the light signal is distributed into multiple pathways [1,6,10].
Until recently, all invaginating bipolar processes were thought to belong to ON bipolar cells, whereas all flat contacts were thought to belong to OFF bipolar cells. However, in primate this rule applies strictly only to ON bipolar and OFF midget bipolar cells. Most OFF bipolar types make a mixture of both types ofcontact [3]. It is now known that it is the type of 10.1007/978-3-540-29678-2_7 receptor expressed at bipolar dendrites that determines whether they are ON or OFF types.
In addition to the layers of invaginating and flat contacts, a third postsynaptic layer has been detected at a distance of about 1.5 μm from the cone pedicle base. This layer consists of junctions that have the appearance of desmosomes (“desmosome-like junctions”) but do not contain any known protein normally present at junctions. Instead, these structures are postsynaptic densities that are located on horizontal cell processes and express ionotropic glutamate receptor subunits [10].
Glutamate and GABA Receptors in the Outer Plexiform Layer
The neurons postsynaptic to photoreceptors express different types of glutamate receptors [6]. The ON bipolar cells express sign inverting metabotropic glutamate receptors (mGluR6) that act via G-proteins. Horizontal cells and OFF bipolar cells express sign conserving ionotropic (10.1007/978-3-540-29678-2_1, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and 10.1007/978-3-540-29678-2_11) glutamate receptors.
The 10.1007/978-3-540-29678-2_14 of horizontal cells is 10.1007/978-3-540-29678-2_7 (gamma amino butyric acid). GABAA and GABAc receptors are consistently found on the dendrites of bipolar cells. The presence of different types of postsynaptic receptors on different processes at the cone pedicle base results in a laminated arrangement (Fig. 5b) [10].
Connectivity of Bipolar Cells in the Inner Plexiform Layer
In the inner plexiform layer, bipolar axon terminals form ribbon synapses (Retinal ribbon synapses) onto two postsynaptic processes (10.1007/978-3-540-29678-2_4). Cone bipolar axons usually contact one amacrine and one ganglion cell process, whereas rod bipolar axons do not contact ganglion cells but form dyads onto two amacrine processes, one of which belongs to the 10.1007/978-3-540-29678-2_1 [2]. Different types of diffuse bipolar cells form different numbers of output synapses [5], but relatively little is known about the identity of their postsynaptic targets.
Bipolar axons make excitatory (sign-conserving) glutamatergic synapses. The two postsynaptic processes at a bipolar dyad usually express two different types of ionotropic glutamate receptors (AMPA, kainate and 10.1007/978-3-540-29678-2_14) [6].
The synaptic input to bipolar cells in the inner plexiform layer derives from amacrine cells containing the inhibitory neurotransmitters GABA or 10.1007/978-3-540-29678-2_7. The axon terminals of different bipolar types vary with respect to the expression, subunit composition and frequency of GABAA, GABAC and glycine receptor clusters [1,6].
Higher Level Structures
OFF bipolar cells transfer their signals to OFF ganglion cells, whereas ON bipolar cells contact ON ganglion cells (retinal ganglion cells). The ganglion cells then transfer the bipolar signals to distinct regions in the brain. As bipolar axons stratify in distinct strata of the inner plexiform layer, they can only contact ganglion cells stratifying in the same stratum [1]. Some bipolar types provide input to only one type of ganglion cell (e.g., midget bipolar to midget ganglion cells in primate; CD15-OFF bipolar cells to ON-OFF direction-selective ganglion cells in rabbit (retinal ganglion cells; Retinal direction selectivity: Role of starburst amacrine cells) [4]. Some ganglion cell types receive input from only one bipolar type (e.g., midget ganglion cells). Other bipolar cell types contact more than one type of ganglion cell, e.g., DB3 cells in primate retina presumably contact OFF parasol (10.1007/978-3-540-29678-2_13) and the outer (OFF) tier of small bistratified cells (10.1007/978-3-540-29678-2_11) [3]. Finally, several types of bipolar cells can provide input to the same type of ganglion cell, e.g., cat alpha and beta cells, primate parasol cells and direction-selective cells in rabbit retina (retinal ganglion cells; Retinal direction selectivity: Role of starburst amacrine cells) [1].
Function
Different morphological types of diffuse bipolar cells play different functional roles [6]. Physiological differences could be based on the presence of different types of glutamate receptors on bipolar dendrites, as well as distinct patterns of inhibitory input from horizontal and amacrine cells. For example, the type b2 and b3 OFF bipolar cells in the ground squirrel stratify at different levels of the inner plexiform layer and express distinct types of ionotropic glutamate receptors in the outer plexiform layer. The b2 cells express AMPA receptors and are involved in the transmission of transient responses to light. The b3 cells express kainate receptors and are involved with sustained light response. Studies in tiger salamander showed that different types of bipolar cells, with axon terminals at different levels of the inner plexiform layer, differ with respect to their light response characteristics. The idea that parallel streams in the visual system first diverge at the level of the outer flexiform layer is thus supported in all retinas studied so far (10.1007/978-3-540-29678-2_22). The question why the brain requires multiple afferent signals streams, and how these distinct streams are processed in the brain, remains a major challenge for visual neuroscience.
Retinal Color Vision in Primates
Synonyms
Color vision; Color processing
Definition
Color Vision in the Retina
The eyes of nearly all animals contain multiple classes of cone photoreceptor (10.1007/978-3-540-29678-2_16), and the ability to discriminate objects by their 10.1007/978-3-540-29678-2_19 (10.1007/978-3-540-29678-2_3) is an almost universal feature of animal visual systems studied so far. The neural processes that give rise to color sensations begin in the retina, where certain neurons show selectivity for distinct regions of the visible spectrum. Color is not a property of objects or of retinal processes, but is a result of the brain's ability to interpret these neural signals. Humans and other primates normally show tri-variant (10.1007/978-3-540-29678-2_20) color vision whereas most other mammals show more rudimentary (10.1007/978-3-540-29678-2_4 or “red-green color blind”) color vision.
Characteristics
Description of the Process
Encoding Spectral Signals
Most humans show trichromatic color vision. This means that the eye contains three distinct classes of cone photoreceptors that are sensitive to different wavelength ranges in the visible spectrum, and that differential activation of these photoreceptors can be analyzed by the brain to yield color sensations. The cone photoreceptors are active under 10.1007/978-3-540-29678-2_16 (daylight) conditions whereas 10.1007/978-3-540-29678-2_19 (night) vision is served by a single class of rod photoreceptor and thus is color blind (10.1007/978-3-540-29678-2_3).
Figure 1 gives an overview of spectral signal processing in the primate retina. Objects in the environment (in this example, a fruit-bearing tree) reflect more or less strongly the incident photons in the visible spectrum (the visible range is approximately 400–700 nm in the electromagnetic spectrum). For example (Fig. 1), a ripe red fruit on the tree reflects more long-wavelength photons than a green leaf on the tree, but the leaf will reflect more medium-wavelength photons than the fruit does. The shapes of these reflectance curves are determined by the physical-chemical properties of the objects, and the spectrum and intensity of the light that illuminates the objects. Thus, color is not a property of the objects per se but is a result of the brain's ability to interpret the spectral reflectance of an object, relative to the reflectance of other objects in the 10.1007/978-3-540-29678-2_22. This is the most important fact to learn about color vision and is an essential prerequisite for understanding spectral processing in the retina.
Retinal Color Vision in Primates. Figure 1.

First stages of color vision. From left to right: objects in the environment show different reflectance spectra (in this example, the upper row shows fruit and the lower row shows foliage). These spectra are integrated into three separate wavelength bands by the short (S), medium (M) and long-wavelength (L) sensitive cones. The cone excitations are transformed into two cone-opponent signals (M − L) and (S − [M + L]) for transmission to the brain. The brain interprets these signals to yield color sensations.
The first stage of vision is the transduction of light by photoreceptors (10.1007/978-3-540-29678-2_16). Each cone photoreceptor expresses one of three types of proteins called cone 10.1007/978-3-540-29678-2_15, which together with the vitamin A derivative 11-cis-retinal form the only light-dependent stage of vision. The amino acid sequence of the opsin “tunes” its spectral sensitivity towards the short (peak ∼430 nm), medium (∼530 nm) or long (∼560 nm) wavelength regions of the spectrum [1]. In other words, the probability of photon absorption is maximal at one of three constant positions in the spectrum, yet each receptor absorbs photons across a wide band of the visible spectrum (Fig. 1). Because the absorption spectra are broad, wavelength is confounded with intensity in each receptor's response. For example, a bright light at 500 nm or a dim light at 430 nm could produce identical photon absorptions by the short-wavelength sensitive (S) cones. It is only by comparing the output of different receptor classes that specific information about wavelength can be recovered. The photoreceptor responses or “cone excitations” are thus shown as the relative heights of the bars in Fig. 1, because each receptor effectively ignores the wavelength of absorbed photons across its sensitivity range. In summary, the ripe fruit on the tree yields a red sensation because it activates long wavelength sensitive (L) cones more than it activates the medium– (M) and short wavelength-sensitive (S) cones, whereas a leaf on the tree appears green because it activates the M cones more than the L or S cones. The reflectance spectra of these objects are collapsed into this trivariant, or trichromatic, signal at the first stage of color vision.
Neural Signals Underlying Color Sensations
How are the signals that enable color sensations transmitted to the brain? The retinal structures described in the following sections yield two main signals called “10.1007/978-3-540-29678-2_3” signals (Fig. 1). This term conveys the idea that activity of one cone type is subtracted from or “opposes” the activity of another cone type. One opponent channel pits the activity of M against L cones, the other pits the activity of S cones against a combination of M and L cones. Distinct anatomical pathways in the retina form the substrate of these opponent channels. For simplicity these are often referred to as “red-green” and “blue-yellow” pathways in the retina, although the perceptual color axes do not correspond exactly to the cone-opponent axes. It is also important to note that most retinal neurons respond to changes in either brightness (intensity) or color (relative spectral reflectance) of a stimulus, so do not form exclusive color-detecting channels [2] (10.1007/978-3-540-29678-2_3).
Red-Green Pathways in the Retina
There is general agreement that the so-called “midget-parvocellular projecting” retinal pathway carries the M-L opponent signals which serve the red-green axis of color vision. This pathway forms the dominant output of the primate retina (it comprises ∼80% of all 10.1007/978-3-540-29678-2_15 fibers), and carries signals serving high-acuity spatial vision in addition to signals for red-green color vision. The genes encoding M and L cone opsins diverged relatively recently in the evolutionary history of primates, yielding trichromatic color vision from the primordial dichromatic system thought to be common to most mammals [1,3]. The evolution of high-acuity spatial vision in primates may have enabled the more recent evolution of red-green color vision [4,5]. The idea is illustrated in Fig. 2. Figure 2a shows a schematic drawing of the primate eye, with the eye's output neurons (retinal ganglion cells) concentrated near the point of highest visual acuity (the 10.1007/978-3-540-29678-2_6). The M and L cones are very tightly packed near the fovea to enable this high acuity, and each cone makes contact with two bipolar cells (Retinal ribbon synapses) of the midget-parvocellular pathway in addition to contacting other bipolar cells [6]. One of the midget bipolar cells responds to brightness decrements (off-type response) and the other responds to brightness increments (on-type response). These connections show one-to-one specificity (Fig. 2b), so the spatial acuity of the cone array can be preserved at subsequent processing stages. The spectral (M or L) signature of each cone in the array will likewise be preserved by this chain of excitatory synaptic connections, and yields four response signatures in midget-parvocellular cells: red-on, red-off, green-on and green-off. The red-green color signals thus are “piggybacked” on the system for high-acuity spatial signals.
Retinal Color Vision in Primates. Figure 2.

Red-green chromatic pathways in the eye. (a) schematic cross section of the eye showing concentration of ganglion cells near the fovea (F) on the visual axis (dashed red line). Ganglion cell axons form the optic nerve. (b) connections of midget-parvocellular pathway neurons near the fovea. Each cone is contacted in a one-to-one fashion by both on-type and off-type midget bipolar cells. The bipolar cells in turn contact midget ganglion cells. (c) connections of midget-parvocellular pathway neurons in mid-peripheral retina. For simplicity, only on-type connections are shown. Some midget bipolar cells receive convergent input from multiple cones, and most ganglion cells receive convergent input from multiple bipolar cells. Both these convergent steps will degrade the spectral purity of the ganglion cell response. (d) organization of inhibitory inputs to midget-parvocellular pathway ganglion cells. Horizontal cells and amacrine cells make widespread connections and feed mixed spectral signals to ganglion cells.
The anatomical organization of the midget-parvocellular pathway in the peripheral retina is shown in Fig. 2c. Here, many of the midget bipolar cells make contact with two or more cones and will receive a mixed spectral signal if they contact cones of different type. The bipolar cells likewise make convergent connections with ganglion cells (Retinal ganglion cells) [2,7]. The spatial acuity and spectral purity of ganglion cell signals should thus decrease in the peripheral retina, and both spatial acuity and red-green color vision in the peripheral visual field are correspondingly poor [8].
How do the cone opponent properties of midget-parvocellular ganglion cells arise? Inhibitory interneurones (10.1007/978-3-540-29678-2_8 and 10.1007/978-3-540-29678-2_1) are the most likely source of the opponent cone signals (Fig. 2d). One subtype of horizontal cell in primate retina (the H1 subtype) collects signals from M and L cones and provides feedback inhibition to these cones as well as to bipolar cells [2,7]. Anatomical connections of amacrine cells with midget-parvocellular ganglion cells are less well-understood, but as is the case with horizontal cells, amacrine cells should pool spatial signals originating in large numbers of M and L cones. Both these cell types thus provide a spectrally mixed inhibitory input to antagonize or “oppose” the spectrally pure (in the fovea) or biased (in the peripheral retina) excitatory inputs from midget bipolar cells.
There is functional (physiological) evidence that both excitatory and inhibitory inputs to parvocellular ganglion cells show greater spectral selectivity than predicted by the pure “random wiring” scheme outlined above, but to date there is no direct anatomical evidence for cone-selective connections in the midget-parvocellular pathway [2,7]. This functional selectivity likely arises from subtle changes in the strength of synaptic connections rather than specific wiring between specialized cell classes, of the type described below for the blue-yellow retinal pathway.
Blue-Yellow Pathways in the Retina
The short wavelength sensitive (S) cones form less than 10% of all cones in the primate retina (Fig. 3a). Molecular studies show that a distinct S cone pigment emerged before the mammalian radiation, and thus could form the basis for a primordial color vision system in mammals [1,5]. The main synaptic output of S cones in primates is to a single class of bipolar cell called the blue cone bipolar cell. These bipolar cells form a specific network contacting S cones (Fig. 3b) and transmit on-type signals (corresponding to increases in the 10.1007/978-3-540-29678-2_16 of S cones) from the S cone array. The blue cone bipolar cells contact a specific class of ganglion cell called the small bistratified (blue-on) cell. The small bistratified cells get excitatory input from S cones and inhibitory input from M and L cones, to yield a “blue-on, yellow off” cone opponent response [9]. There are two sources of inhibition from M and L cones. First, the small bistratified cell receives synaptic input from off-type diffuse bipolar cells, which contact predominantly M and L cones. Second, the S cones and the blue-cone bipolar cells receive inhibitory input from the H2 subtype of horizontal cell, which gets synaptic input from all cone types. This spectrally mixed inhibitory input opposes the spectrally pure S cone input to blue cone bipolar cells [7]. The selective connections of S cones with blue cone bipolar cells and H2 horizontal cells are preserved across the primate retina, so the deterioration in blue-yellow chromatic sensitivity with increasing visual field eccentricity is not as marked as the deterioration in red-green sensitivity [8].
Retinal Color Vision in Primates. Figure 3.

Short-wavelength sensitive (S) cone pathway. (a) fragment of the cone photoreceptor mosaic in macaque retina [modified from ref. 15]. (b) connections of blue-cone bipolar cells (grey) with S cones (blue plaques) in marmoset retina [modified from ref. 16]. The dendrites of blue cone bipolar cells make dominant contact with S cones. (c) schematic vertical section through primate retina showing connections of a small bistratified (blue-on) ganglion cell with blue cone bipolar and diffuse bipolar cells. (d) a second source of cone opponent inputs to blue-on ganglion cells arises from inhibitory feedback by the H2 subclass of horizontal cell.
The question how off-type signals from S cones are transmitted to the brain has not been fully resolved. At least two types of sparsely-branched or “wide field” ganglion cells show blue-off type responses, and presumed targets of these cells in the brain show large receptive fields [7,10]. One of these cell types also shows intrinsic, melanopsin-based photosensitivity and may contribute to 10.1007/978-3-540-29678-2_3 entraining as well as to color vision [7,11]. Connections from S cones to off-type midget bipolar cells have been reported in macaque fovea, but S cones in marmoset retina make negligible connections to midget cells and only sparse connections with diffuse, off-type bipolar cells [6,12]. Whether these differences reflect true species differences or methodological differences is not yet clear.
In summary, there is clear evidence for a selective network transmitting on-type signals from S cones to the brain to yield a “primordial” dichromatic color vision channel. A specific network of S cone connections to bipolar cells has also been shown in mouse retina [13], and the question whether this or other elements of S-cone circuits are common to other diurnal mammals has become an interesting topic in comparative neurology.
Higher Level Processes
Central Targets for Chromatic Signals
The main target for all ganglion cell axons in primates is the dorsal 10.1007/978-3-540-29678-2_12 of the 10.1007/978-3-540-29678-2_20. The dominant input to the parvocellular layers of the LGN is from midget ganglion cells, and accordingly in trichromatic primates most parvocellular cells show red-green chromatic opponent properties. Geniculocortical relay cells (10.1007/978-3-540-29678-2_7) in the parvocellular layers project to granular layer 4Cβ in the 10.1007/978-3-540-29678-2_16. By contrast, blue-on and blue-off type responses are segregated to the intercalated or koniocellular division of the LGN [10]. Koniocellular relay cells show relatively diffuse cortical projections including supragranular layers 3 and 4A in the primary visual cortex: consistently, blue-on and blue-off type responses of presumed geniculate afferents can be recorded from these layers [14]. The two chromatic streams thus remain segregated at least to the early stages of cortical processing, and the mechanism by which these channels combine to enable color perception remains an outstanding and fascinating topic in neuroscience.
Retinal Direction Selectivity: Role of Starburst Amacrine Cells
Definition
Direction-Selectivity in the Retina
Computing the direction of image motion is an essential task for the visual system. The retina’s ability to detect the direction of image motion (10.1007/978-3-540-29678-2_4 or DS) was first described more than 40 years ago [1]. 10.1007/978-3-540-29678-2_4 (DSGCs) (retinal ganglion cells) fire vigorously when a stimulus is moving in a certain direction (“preferred”), while remaining silent when the same stimulus moves in the opposite (“null”) direction (Fig. 1). 10.1007/978-3-540-29678-2_19 (SACs) [2,3] are retinal interneurons closely intertwined with the direction-selective circuitry in the retina. While there is common consensus that SACs are crucial for the computation of motion direction, the exact nature of their involvement is still controversial. For review and further reading see [4,5].
Retinal Direction Selectivity: Role of Starburst Amacrine Cells. Figure 1.

(a) Fluorescent dye-injected ON/OFF direction-selective ganglion cell in a flat mounted rabbit retina (pseudo-color codes retinal depth). The two dendritic arbors stratify in the ON (red) and OFF (green) sublaminae of the IPL. (b) Electrical responses of a DSGC to a bar moving in 12 directions across its receptive field (see inset): both the leading (ON) and the trailing edge (OFF) of the bar elicit responses (p.d.: preferred, n.d.: null direction).
Characteristics
(Quantitative) Description
Direction-Selective Ganglion Cells (DSGCs)
Direction-selective ganglion cells (retinal ganglion cells) have been primarily studied in rabbit retina, where they account for 10% of the ganglion cells. They have functionally and morphologically equivalent counterparts in other mammals and in non-mammalian vertebrates. Most research has focused on the ON/OFF DSGC (Fig. 1a), which has a bistratified dendritic tree with one arborization in the outer half (the OFF sublamina) of the 10.1007/978-3-540-29678-2_9, and another arborization in the inner half (the ON sublamina) of the IPL.
This arrangement allows responses to the direction of image motion of dark objects on a light background – mediated by the OFF arbor – as well as to objects brighter than the background – mediated by the ON arbor. The cell comes in four functional subtypes, each preferring one particular direction of motion (as an example see Fig. 1b). A second type of retinal direction-selective cell is the monostratified ON DS ganglion cell, which prefers one of three particular directions (see Function). Each DSGC subtype tiles the retina with little dendritic overlap making directional information for any of the preferred directions available at every retinal location.
Starburst Amacrine Cells (SACs)
The dendrites of SACs closely co-fasciculate with the dendrites of DSGCs, and therefore have long been implicated in the computation of direction selectivity. (Strictly speaking, SAC neurites are not dendrites as they not only receive input, but also make output synapses – this is the case for most amacrine cells.) Unlike most other retinal neurons, SACs display a tremendous dendritic overlap (30–70 fold coverage) and, hence, offer plenty of “substrate” to provide the different DSGC subtypes with adequate neural circuitry. When SACs are removed from the circuitry, e.g., by gene-targeted cell ablation [6], direction-selective responses in DSGCs are abolished, confirming that SACs play a crucial role for direction-selectivity.
Starburst cells have been found in non-mammalian and mammalian species including primates. SAC morphology is well conserved among species: several primary dendrites radiate symmetrically from the soma before dividing into smaller branches (Fig. 2). The distal third of the branches is decorated with bead-like swellings (varicosities) [3]. SACs contain two transmitters, 10.1007/978-3-540-29678-2_7 and 10.1007/978-3-540-29678-2_1. Due to the presence of ACh, they are also called “cholinergic amacrine cells.” Two subtypes of SACs exist: OFF SACs co-stratify with the OFF dendritic arbor of the ON/OFF DSGCs, whereas ON SACs co-stratify with the ON dendritic arbor of the ON/OFF DSGC and the ON DSGC dendritic arbor. Despite the opposite polarity of their light responses, ON- and OFF-SACs are considered functionally equivalent.
Retinal Direction Selectivity: Role of Starburst Amacrine Cells. Figure 2.

Starburst amacrine cell in a flat mounted rabbit retina injected with a fluorescent dye. The cell’s morphology is reminiscent of a “starburst” fireworks display.
Description of the Process
As recognized in the first studies on DSGCs in rabbit retina [1], the generation of direction-selectivity could be attributed to spatially offset inhibition biased towards the “null” direction. This proposal assumes asymmetrical wiring, such that the DSGC receives inhibition preferentially from interneurons displaced to one side of its receptive field (Fig. 3a). It was proposed that motion in the “null” direction triggers inhibition (via the spatially displaced interneurons) before the stimulus reaches and excites the DSGC directly. If the inhibition is sufficiently delayed or, alternatively, long-lasting, it will coincide with the direct excitation and prevent the DSGC from responding. Motion in the opposite (“preferred”) direction, on the other hand, will also trigger inhibition, but too late to prevent the DSGC from responding.
Retinal Direction Selectivity: Role of Starburst Amacrine Cells. Figure 3.

(a) Schematic retinal cross section illustrating the direction-selectivity circuitry. The central SAC (*) serves (here: inhibits) two DSGCs with opposite preferred directions (indicated by arrow). For simplicity, cholinergic input, other amacrine cells involved and connections in the ON sublamina of the IPL are omitted. Proposed direction-selectivity mechanisms: (b) Direction-selective inhibition: preferred motion (i) elicits a response in the DSGC, because the left SAC’s dendrite, which is connected to the DSGC, is not activated by this motion direction, while the right SAC’s dendrite is activated but not connected. Null direction motion (ii) elicits no response in the DSGC, because the left SAC’s dendrite is activated and inhibits the DSGC. (c) Direction-selective excitation: the left SAC (or, alternatively, another amacrine) inhibits the excitatory input from bipolar cells presynaptically to the DSGC for null direction motion [2], but not for preferred direction motion [1]. A similar mechanism could also be implemented via excitatory (cholinergic) connections from SACs.
Pre- or Postsynaptic
It was originally proposed that direction-selectivity is computed by such a delay-based “veto”-mechanism from non-directional inputs in the DSGC itself. Detailed electrical recordings from DSGCs (reviewed in [5]), however, revealed that the inputs that DSGCs receive are already directionally tuned, with inhibitory input being larger for “null” direction motion, and excitatory input being larger for “preferred” direction motion. While is consistent with spatially offset inhibition in general, this finding suggests that the DSGC’s response is substantially determined by the ratio of excitation and inhibition, and less by their temporal sequence in the DSGC, as originally proposed. More importantly, this indicates that direction-selectivity is already computed in interneurons presynaptic to the DSGC. Postsynaptic processing in the dendrites of DSGCs essentially supplements the presynaptic direction-selective mechanisms by sharpening the directional tuning.
Multiple Mechanisms
Retinal direction-selectivity is largely independent of contrast, mean brightness and velocity of the stimulus. This robustness alone suggests that different mechanisms at multiple levels participating in the generation and amplification of direction-selectivity [reviewed in 5]. This is also reflected by the fact that both inhibitory and excitatory inputs to DSGCs are by themselves direction-selective.
Direction-Selective Inhibition
Pharmacologically blocking 10.1007/978-3-540-29678-2_7 has consistently been shown to abolish direction-selectivity in DSGCs, indicating that the direction-selective inhibition is mediated by GABAergic input [8]. This GABAergic input is at least partially provided by SACs (Fig. 3b). More importantly, light-evoked Ca2+ signals optically recorded in the distal SAC dendrites are direction-selective (Fig. 3a) [9]. Thus, it is highly likely that GABA release from SACs is also direction-selective, because the SACs’ output synapses are located in the distal dendrites [3], and their GABA release has been shown to be Ca2+-dependent [10]. Furthermore, SACs appear to make direct GABAergic synapses with DSGCs, which seem to be highly asymmetrical and fit the requirements for spatially offset inhibition. Paired recordings have shown that DSGCs receive inhibitory synaptic input from SACs located on the “null side” of the DSGC’s receptive field, but do not receive synaptic input from SACs on the “preferred side” [11]. Alternatively, (additional) directionally tuned inhibitory input may come from other, yet to be identified amacrine cells; however, direct evidence for this is lacking so far.
Direction-Selective Excitation
The excitatory input to DSGCs is 10.1007/978-3-540-29678-2_7 as well as cholinergic. The glutamatergic input comes from bipolar cells (Retinal ribbon synapses) and appears to be directionally tuned, but it is not yet clear by which synaptic circuitry. Such tuning could result from suppression of bipolar cell activity for “null” direction motion (Fig. 4c); however, it is not known which amacrine cell could supply the required spatially offset inhibition to the bipolar cell terminals. The SAC is a potential candidate, and ultrastructural evidence for SAC output onto bipolar cell terminals does exist, but such contacts seem to be too sparse [3]. Alternatively, bipolar cell activity could be enhanced by motion in the “preferred” direction (facilitation), but again an appropriate pathway has not yet been unequivocally identified.
Retinal Direction Selectivity: Role of Starburst Amacrine Cells. Figure 4.

(a) Dendritic Ca2+ responses (as relative change in fluorescence ΔF/F) optically recorded from a distal SAC dendrite to a bar grating moving in four different directions. Only motion from the soma roughly towards the tip of the imaged dendrite (indicated by a blue cross) elicits a response (from [9], Fig. 4a, modified). (b) SAC dendritic branches signal centrifugal motion.
It is likely that DSGCs receive excitatory input from SACs via cholinergic synapses, because DSGCs express 10.1007/978-3-540-29678-2_1. ACh receptor blockers reduce the firing of DSGCs, and SACs are considered the only source of ACh in the retina [2]. Since ACh release from SACs is Ca2+ dependent and SAC dendritic Ca2+ signals are direction-selective, one would expect that ACh release is also direction-selective. Nonetheless, the cholinergic pathway is enigmatic in several ways (reviewed in [4]): Laser ablation of SACs on the preferred side of a DSGC reduced its excitatory input. However, paired recordings of SACs and DSGCs failed to show direct cholinergic connections [11]. Blocking ACh receptors appears to reduce direction-selectivity in DSGCs for certain stimuli (like bar gratings). On the other hand, in the presence of GABA receptor blockers, ACh antagonists reduce DSGC responses independent of motion direction, suggesting that cholinergic excitation is symmetrical [12].
Higher Level Processes
Direction Selectivity from Network Interactions
Various models of retinal direction-selectivity are based on network interactions to explain the generation of the direction-selective signals observed in the DSGCs (reviewed in [4,5,13]. The models differ in the complexity of interactions, and by the number and types of neurons recruited, but the basic principles are similar: spatially offset inhibition and asymmetrical wiring. Depending on the model, the spatially offset inhibition is provided by SACs or by not yet identified amacrine cells. In either case, most, if not all, network models assign some central role to SACs, ranging from simply relaying signals to DSGCs to providing essential direction-selective output. As a general mechanism for rendering signals direction-selective, both facilitation and suppression have been suggested.
SAC Networks
Starburst cells form a network with attractive properties. Reciprocal cholinergic excitation among SACs is prominent in the developing retina, but seems to be strongly reduced during retinal maturation. GABAergic interactions between neighboring SACs are prominent in the adult retina, and have long been suspected to be crucial for the generation of direction-selectivity (e.g., [13]). An excited SAC inhibits its neighbors, which in turn reduces their inhibition onto the first SAC, and effectively amplifies excitation. Thus, SAC dendrites pointing in the same direction could stabilize each other’s responses. Such network interaction may well serve to enhance DS [10]. Nonetheless, GABAergic inhibition appears not to be pivotal to render SAC output direction-selective, because blocking GABA receptors does neither abolish direction selectivity in the SACs’ dendritic Ca2+ not in their somatic volatage response [14].
Lower Level Processes
Direction Selectivity from Intrinsic SAC Properties
While in the network models direction selectivity arises primarily from neuronal interactions, a second group of models suggests that direction selectivity is initially generated in SAC dendrites as a result of intrinsic properties. Note that network models and intrinsic models are not necessarily mutually exclusive, but rather complementary.
At first glance SACs appear to be very symmetrical neurons (Fig. 2), which seems to disagree with the role of a detector of motion direction. In fact, SACs are better viewed as a collection of “wedge-shaped” direction detectors represented by the primary dendrites (Fig. 4b). The dendritic branches are largely electrically isolated and respond independently to local light stimulation [8]. Thus, they can be considered as largely “autonomous” computational units [14]. In contrast to the whole cell, the dendrites are indeed highly polarized structures. Synaptic inputs and outputs are differentially distributed along the dendrites: input synapses are located along the whole length, whereas output synapses are associated with the varicosities on the distal third of the branches [3]. Each principal branch responds more strongly to centrifugal motion (towards the dendritic tips) than to centripetal motion (towards the soma) [8], thus displaying dendritic direction selectivity. The mechanism underlying dendritic direction selectivity in SACs is not yet fully understood. Several “cell-autonomous” models of SAC dendritic direction selectivity have been proposed, each of them employing different (but not necessarily mutually exclusive) intrinsic mechanisms and properties.
Morphology and Amplification. Computational studies have suggested that the starburst dendrite morphology, with its steady increase of input synapses towards the dendritic tips, would generate a weak direction-selective dendritic signal by itself (e.g., [13]). This small direction-dependent difference in membrane potential (10.1007/978-3-540-29678-2_13) could be amplified by other mechanisms, such as differential activation of voltage-gated channels. Starburst cells express several types of voltage-gated Ca2+ channels (10.1007/978-3-540-29678-2_3) that would be suitable. Support for this comes from the fact that blocking Ca2+ channels that are predominant on SACs abolishes direction selectivity in DSGCs, while leaving the DSGC’s responsiveness to light intact. It is also possible that voltage-gated Na+ channels (10.1007/978-3-540-29678-2_19) play a role; however, it is uncertain whether SACs generate spikes carried by Na+.
Chloride Gradient along the Dendritic Branches. Immunocytochemical and physiological experiments suggest that a differential expression of the chloride transporters NKCC1 and KCC2 (10.1007/978-3-540-29678-2_3 leads to a chloride concentration ([Cl−]) gradient within the SAC dendrite (with high [Cl−] in and near the soma) [15]. Such a gradient would render the proximal GABAergic inputs excitatory, whereas the distal GABAergic inputs would remain inhibitory. In this model, the temporal sequence in which proximal and distal GABAergic inputs occur (centrifugal vs. centripetal) lead to the generation of direction-selective transmitter release from the SAC distal dendrites.
Intracellular “Calcium Wave”. An important question is how spatially offset inhibition can be delayed long enough so that it coincides with excitation in the DSGC. A similar question arises for the signal propagation within the SAC dendrite: If constructive and/or destructive interactions of inputs create dendritic direction selectivity in SACs, what causes the required delay? As electrical propagation seems too fast, it has been proposed that an intracellular Ca2+ “wave” supported by Ca2+-induced Ca2+ release (within the SAC) may provide suitable delays.
Other, Yet Unidentified Interneurons in the DS Circuitry
A general problem with proposing that interneurons other than the SAC perform essential direction-selectivity computations is lack of neuronal “substrate.” Retinal neurons usually tile the retina; such that their density appears too low to build local circuitries dedicated to each of the seven DSGC subtypes at every retinal location (see Function). Local dendritic processing – as implemented in SACs – may be a solution. In the case of bipolar cells, as has been proposed, this would be demanding. To tune the output of single branches of a bipolar cell axon to different preferred directions would require a tremendous locality of processing.
Involved Structures
Ultrastructural Basis of Direction Selectivity
While it is commonly agreed that retinal direction selectivity requires spatial asymmetries in the wiring of the circuitry, a directly corresponding anatomical correlate has not been unequivocally identified. It has so far not been possible to predict a DSGC’s preferred direction from its anatomy. Although at the ultrastructural level complex arrangements of amacrine cell synapses onto DSGC dendrites have been described [16], systematic asymmetries in the wiring of the direction-selectivity circuitry have not been found. The only available evidence for asymmetrical wiring comes from paired recordings [10]. Another puzzle is the location of GABA release sites on SACs. Synapses with ACh-containing vesicles could be located in the varicosities, whereas, due to the lack of conventional ultrastructural features, GABA release sites have not yet been localized on SAC dendrites.
Function
For visually oriented animals, it is a matter of survival to swiftly detect moving objects (10.1007/978-3-540-29678-2_12) and reliably discriminate their direction of motion. In addition, motion of the whole visual field (10.1007/978-3-540-29678-2_7) provides important information about head/body movements (10.1007/978-3-540-29678-2_22; 10.1007/978-3-540-29678-2_15). Hence, coding the direction of image motion is an important task for the visual system. The three preferred motion directions of the ON DSGCs correspond to rotation around the axes defined by the three 10.1007/978-3-540-29678-2_19 in the inner ear (reviewed in [4]). The cells respond preferentially to global motion and project to the 10.1007/978-3-540-29678-2_1, thus, ON DSGCs are thought to provide a correctional signal for 10.1007/978-3-540-29678-2_5 and gaze-stabilization. This is supported by the finding that ablating SACs leads to a loss of the 10.1007/978-3-540-29678-2_15 [6]. The four preferred directions of the ON/OFF DSGCs are roughly aligned with the extraocular rectus muscles and, therefore, with the cardinal ocular rotation directions. ON/OFF DSGCs seem to contribute some input to the optokinetic system, however, they send projections to the 10.1007/978-3-540-29678-2_19 and the 10.1007/978-3-540-29678-2_12, indicating that their signals also serve other functions, possibly including control of spatial attention (10.1007/978-3-540-29678-2_22). The responses of ON/OFF DSGCs to moving objects are attenuated by synchronous motion of the background, indicating that they preferentially signal local motion. Nonetheless, there is no functional evidence so far that signals from retinal DSGCs take part in higher visual processing of motion direction.
Development
Up to now there is no satisfactory explanation of how the direction selectivity circuitry is wired during retinal development. That direction selectivity is established at the time of eye-opening suggests that the wiring process does not require visual stimulation.
Retinal Flow
10.1007/978-3-540-29678-2_15
Retinal Ganglion Cells
Definition
The retinal ganglion cells (RGCs) are the output stage of retinal information processing. They are the only cells in the retina with axons that leave the eye. The ganglion cell axons form the 10.1007/978-3-540-29678-2_15 and transmit retinal information – in the form of spike trains – to the visual target areas in the brain. The name “ganglion cell” derives from the anatomical notion that these cells constitute the “ganglion nervi optici,” i.e. the cluster of somata that give rise to the fibers of the optic nerve. The ganglion cell somata are located in the 10.1007/978-3-540-29678-2_7, the innermost layer of the retina. The dendrites of the ganglion cells ramify in the 10.1007/978-3-540-29678-2_9 where they are postsynaptic to bipolar cell axons and amacrine cell processes (Retinal ribbon synapses). There are more than a dozen different types of ganglion cell in all mammalian retinae studied so far. They differ in dendritic field size and dendritic branching pattern, and they receive input from different bipolar and amacrine cell types, and hence have different functional properties. The various types are specialized to encode different aspects of a visual scene, e.g. fine spatial detail (visual resolution), brightness, color, or movement. These ganglion cell types are the basis of distinct parallel visual pathways relaying a decomposed representation of the visual scene to distinct target areas in the brain [1–5].
Characteristics
Description
Among mammals, ganglion cells have been morphologically and functionally most thoroughly studied in the cat, the rabbit, and some primates [1,2,4,6,7]. More recently, detailed morphological classifications of mouse and rat ganglion cells have been added [8]. These comparative studies suggest that there is a basically similar set of about 15–20 ganglion cell types in all mammals, but formal proofs of homology are still lacking (Figs. 1–3). The accounting of types in any one species differs between authors depending on the classification criteria applied. For historical reasons, the nomenclature of types differs between species. The present article focuses on the most thoroughly studied ganglion cell types in the cat and primates, which continue to serve as benchmarks for ganglion cell classifications.
Retinal Ganglion Cells. Figure 1.

A selection of ganglion cell types in the cat retina. The cells have been dye-injected and are seen in flat view, all drawn at the same scale. Cat ganglion cell types are named by Greek letters. Each type is characterized by a specific morphology, e.g. alpha cells have a large soma and a large dendritic tree formed by stout dendrites, whereas kappa cells have a small soma and a large dendritic tree formed by fine dendrites. The axons exiting from the soma have been omitted. Cell drawings kindly provided by David M. Berson.
Retinal Ganglion Cells. Figure 3.

Midget (upper row) and parasol (lower row) ganglion cells in the retina of the marmoset, a diurnal primate. The cells have been dye-injected and are seen in flat view, all drawn at the same scale. Both cell types increase in size with increasing distance from the fovea (distance given for each cell), but at each location the midget cells are smaller than the parasol cells. Axons are marked by arrows. Note that these marmoset cells are smaller than their presumed counterparts in cat, the beta and alpha cells (Fig. 4); 6 mm is peripheral in the smaller marmoset retina. Modified from [5], courtesy Paul R. Martin.
Alpha Ganglion Cells/Primate Parasol Cells
Alpha ganglion cells have been identified morphologically in all mammalian species studied to date [6]. At every retinal location, they are the type with the largest soma, the largest-caliber, fastest-conducting axon, and a large dendritic field. The dendritic tree is circular-to-oval with stout radial, relatively densely branched dendrites that rarely overlap, and it is monostratified in the IPL (Figs. 1, 2, and 4).
Retinal Ganglion Cells. Figure 2.

Examples of ganglion cells that have been individually injected with the fluorescent dye lucifer yellow. (a) Flat view of a neighboring alpha (right) and beta (left) ganglion cell in peripheral dog retina. (b) Flat view of a group of neighboring alpha cells in central rat retina. The scale bar applies to A & B. A, modified from Peichl (1992) J. Comp. Neurol. 324:590–602; B, modified from Peichl (1989) J. Comp. Neurol. 286:120–139.
Retinal Ganglion Cells. Figure 4.

Variation of ganglion cell population density and ganglion cell size across the cat retina. Left: Schematic map of the ganglion cell density in a whole retina; radial cuts were made to flatten the retina. From the region of highest ganglion cell density, the 10.1007/978-3-540-29678-2_1 in temporal retina, cell density decreases monotonically towards the retinal periphery. The open circle signifies the optic nerve head. Right: Dye-injected alpha and beta ganglion cells at three distances from the area centralis, showing that dendritic field size increases as cell density decreases, while the size difference between alpha and beta cells is maintained. D, dorsal; V, ventral; N, nasal; T, temporal. The left scale bar applies to the map, the right scale bar to the individual cells. Modified from Peichl (1990) Optometrie 3/1990:3–12.
Alpha cells have been identified as the brisk-transient (Y) cells of physiology [1,6,9]. They comprise two functional subtypes, ON alpha cells and OFF alpha cells, as defined by their response to light (see dendritic stratification below). The receptive fields of alpha/Y cells are large and have a concentric organization with an excitatory centre and a larger antagonistic, inhibitory surround [9,10]. Alpha/Y cells show a vigorous transient (phasic) response whenever there is a stimulus change; their response to stationary, constant stimuli decays within the first few tenths of a second following stimulus onset. Alpha/Y cells respond best to rapid spatial or temporal changes of coarse patterns, hence they can be considered as “novelty detectors.” They are very sensitive to low luminance contrasts, but not to chromatic stimuli.
The corresponding cell type in primate retinae is thought to be the parasol (= umbrella-shaped) cell [4,5]. Due to its axonal projection to the magnocellular (M) layers (of the lateral geniculate nucleus), it is also termed M cell. The parasol cell is morphologically very similar to the alpha cells in other species (Fig. 3).
Like the alpha cells of non-primates, the parasol cells comprise two functional subtypes, ON and OFF, and their relatively large receptive fields have an antagonistic centre-surround organization [10]. The response to visual stimuli is transient. Parasol cells respond well to achromatic “luminance” stimuli and poorly to chromatic stimuli. There are also, however, functional differences between primate parasol and cat alpha cells. For example, alpha cells show non-linear spatial summation whereas parasol cells show linear summation. Thus, the question of correspondence between parasol and alpha cells remains contentious [6].
Beta Ganglion Cells/Primate Midget Cells
The beta ganglion cells have medium-sized somata and medium-caliber axons. Their dendritic trees are very small and circular-to-oval, with radial, relatively densely branched dendrites that rarely overlap (Figs. 1, 2, and 4). Like those of alpha cells, they are monostratified in the IPL. In fact, beta cells look like miniature versions of alpha cells. Accordingly, they collect input from only a few bipolar cells and are the high-resolution (visual acuity) system of the retina. Beta cells have been identified as the brisk-sustained (X) cells of physiology [1,9]. Like the alpha/Y cells, the beta/X cells comprise two functional subtypes, ON and OFF, and their small receptive fields show an antagonistic centre-surround organization [9,10]. However, their response to visual stimuli is sustained (tonic). Beta/X cells respond well to small, high-contrast, stationary stimuli.
In primate retinae, the ganglion cell type with the smallest dendritic and thus receptive field is termed midget ganglion cell [4,5]. Due to its axonal projection to the 10.1007/978-3-540-29678-2_16, it is also termed P cell. The midget cells may be homologous to the beta cells of cat and other mammals. Midget ganglion cells have medium-sized somata and very small dendritic trees that are relatively densely branched and monostratified in the IPL (Fig. 2). They comprise two functional subtypes, ON and OFF, with concentric antagonistic receptive fields [10]. The dendritic trees of the central-most midget cells near the 10.1007/978-3-540-29678-2_6 are so small that each contacts just one midget bipolar cell (Retinal ribbon synapses), which likewise contacts just one cone. This foveal 1:1:1 connectivity, termed the midget system, is the anatomical basis for the high visual acuity of diurnal primates and man. As a corollary, the single cone input conveys to a midget cell the spectral tuning of that cone. The midget cells are widely considered to be the retinal keystone of the red-green chromatic channel of trichromatic primates, doing “double duty” in spatial resolution and color vision. The blue-yellow chromatic channel is implemented by other ganglion cell types [4] (10.1007/978-3-540-29678-2_3).
Interestingly, rabbit, mouse and rat do not possess a ganglion cell type that easily fits the morphological characteristics of beta cells [6–8]. It is possible that the beta cell is not as ubiquitous as the alpha cell, and that in some species another type of ganglion cell subserves spatial resolution.
Intrinsically Photosensitive, Melanopsin-Containing Ganglion Cells
A recently discovered ganglion cell type that appears to subserve “non-image-forming” functions is the “intrinsically photosensitive retinal ganglion cell” (ipRGC) [4,11]. The most intriguing feature of ipRGCs is that they contain the putative photopigment melanopsin and are directly sensitive to light. In addition, they receive conventional photoreceptor input via bipolar cells. The ipRGCs seem to play a major role in the entrainment of 10.1007/978-3-540-29678-2_3, as evidenced by their projection to the circadian pacemaker, the 10.1007/978-3-540-29678-2_19, and in regulating pupil constriction, as evidenced by their projection to the 10.1007/978-3-540-29678-2_16. The ipRGCs constitute a small fraction of the ganglion cells (1–3% in rodent retinae, ~0.2% in primate retina). Their dendritic trees in the IPL are relatively large but sparsely branched. The cells show a sluggish, tonic ON response that is monotonically increasing with the light intensity.
Other Ganglion Cell Types
Many of the non-alpha and non-beta ganglion cell types occur at low densities, each constituting a small fraction of the total ganglion cell population. In primates, these other types mostly have larger dendritic fields than the parasol cells; some are sparsely and some rather densely branched [4]. In the cat, there is a wider range from rather small to very large types (Fig. 1). Some of these types have a concentric antagonistic receptive field organization, others do not, and for some morphologically recognized types, functional data are lacking.
Examples of further well-studied ganglion cell types are two kinds of direction-selective (DS) ganglion cells, the monostratified ON-DS cell and the bistratified ON/OFF-DS cell (Retinal direction selectivity: Role of starburst amacrine cells). They are specialized to detect retinal image movement. Another example is the primate “small bistratified” cell, which shows a blue-yellow antagonism and serves in color processing [4] (10.1007/978-3-540-29678-2_3).
Dendritic Stratification and Light Response
ON ganglion cells have a receptive field centre that is activated by a light increase, OFF ganglion cells are activated by a light decrease in the receptive field center. These response characteristics are determined by their input neurons. The inner plexiform layer (IPL) is morphologically and functionally clearly stratified to keep these different processing circuits segregated (Fig. 5).
Retinal Ganglion Cells. Figure 5.

Schematic drawing of the functional stratification of the inner plexiform layer (IPL) in the mammalian retina as seen in a transverse section. The dendritic trees of OFF ganglion cells stratify in the outer (distal) part of the IPL, those of ON ganglion cells in the inner (proximal) part. The ON/OFF direction selective (DS) ganglion cell has a bistratified dendritic tree with dendrites in the ON and the OFF stratum. Ganglion cell types are rendered in different colors. Abbreviations: INL, inner nuclear layer; GCL, ganglion cell layer.
Cells stratifying in the outer part of the IPL are OFF cells, whilst those stratifying in the inner part are ON cells. The stratification level of the ganglion cell’s dendritic field ensures appropriate (ON or OFF) bipolar cell input. The actual thickness of the two strata differs from species to species, in some they are nearly equal, in others the ON stratum may be twice as thick as the OFF stratum (because it also contains the axon terminals of the rod bipolar cells, which are ON cells; Retinal ribbon synapses).
Both the alpha/parasol ganglion cells and the beta/midget ganglion cells are dichotomous, comprising equally numerous subpopulations of ON and OFF cells. Their monostratified dendritic trees ramify in the inner sublamina and the outer sublamina of the IPL, respectively (Fig. 5). The ON-DS ganglion cells also monostratify in the inner sublamina, while the ON/OFF-DS ganglion cells have bistratified dendritic trees.
Retinal Topography and Population Properties
A basic requirement of retinal organization is that the complete computing machinery has to be present at each retinal location in order to process a visual stimulus wherever it hits the retina. To achieve this, ganglion cells (and most other retinal neurons) are distributed economically across the retina. The members of each functional type (e.g. ON alpha cells, OFF alpha cells, ON beta cells, and OFF beta cells) tile the retina, such that their dendritic trees cover the retinal surface without gaps and without too much overlap [1]. The grain of this tessellation is finest in the central retina (the area centralis of most mammals, and the fovea of primates), where highest visual acuity is achieved. It becomes coarser toward the retinal periphery, where complete coverage is obtained by a less dense spacing and larger individual dendritic trees for each ganglion cell type (Figs. 3 and 4). Relative size differences between the ganglion cell types are preserved across the retina.
The alpha/parasol ganglion cells with their large dendritic trees require a low packing density. Depending on species, they only constitute between 1 and 10% of the total ganglion cell population [6]. Nevertheless, they exhibit uniform coverage and contribute to processing at each retinal point. The beta/midget ganglion cells have much smaller dendritic trees and correspondingly higher packing densities. In the cat retina, about 50% of the ganglion cells are beta cells, in the primate retina, about 80% of the ganglion cells are midget cells.
Lower Level Processes
The response characteristics of the different ganglion cell types are thought to be set mainly by the specific mix of synaptic inputs from different bipolar and amacrine cell types [1–3,5]. The sustained (tonic) beta/midget cells are probably driven by tonic bipolar cells, and the transient (phasic) alpha/parasol cells by phasic bipolar cells (Retinal bipolar cells). A ganglion cell’s specific dendritic geometry, and hence electrotonic properties (10.1007/978-3-540-29678-2_5), also contribute to its response characteristics. The antagonistic surround of a ganglion cell’s receptive field is mediated by lateral inhibitory input, partly directly from amacrine cells to the ganglion cell, and partly from horizontal cells to the bipolar cells (10.1007/978-3-540-29678-2_18 in the retina). The ganglion cells operate at high (10.1007/978-3-540-29678-2_16) as well as low (10.1007/978-3-540-29678-2_19) light levels, i.e. the cone pathway and the rod pathway converge onto the same ganglion cells. The luminance-dependent functional switch from the cone pathway to the rod pathway is regulated by dopaminergic amacrine cells [2].
Higher Level Processes
There is homologous electrical coupling by 10.1007/978-3-540-29678-2_7 between the dendrites of neighboring alpha/parasol cells of the same centre sign (ON with ON, OFF with OFF), and heterologous 10.1007/978-3-540-29678-2_7 between alpha/parasol cells and certain amacrine cell types. ON/OFF-DS cells also show homologous gap junctional coupling, likely to be restricted to partners with the same direction tuning. Modulated electrical coupling allows changing associations between neighboring ganglion cells. It probably is the basis for the high incidence of synchronous firing among homotypic neighbors. Synchronous firing, i.e. concerted activity among multiple ganglion cells, which has been observed in retinal multineuron recordings, may represent a “population coding” or “multiplexing” that is more powerful in encoding visual stimuli than the independent activity of individual cells [12]. Beta/midget cells do not show such electrical coupling, probably because signal spread across neighbors would decrease spatial resolution.
Each ganglion cell type projects to specific thalamic and/or midbrain target structures and specific subdivisions within these target structures [4,5]. This indicates segregation of the various retinal processing channels also at higher processing levels. The 10.1007/978-3-540-29678-2_12 and 10.1007/978-3-540-29678-2_19 are targets for several ganglion cell types. Other nuclei are more selectively targeted, and some ganglion cell types innervate several targets by axon collaterals.
Function
Visual acuity, dominantly mediated by the beta/midget system, is of more importance to some species than to others. In the diurnal primates, the midget system provides the anatomically possible maximum of acuity by fully exploiting the tight foveal cone packing. In the crepuscular-to-nocturnal cat, there is a considerable convergence from cones to beta cells even in the area centralis, “giving away” some of the acuity theoretically possible with the cone packing density. Here perhaps, evolutionary pressure was less on high acuity and more on a good signal-to-noise ratio at dim light. The latter requires signal summation over several photoreceptors, in addition to the presence of rods in the area centralis.
Alpha/parasol cells, with their large receptive fields, are thought to contribute little to acuity or to color vision. Their particular responsiveness to changing stimuli (novelty detectors), and their fast conduction velocity, would make them an “alarm” or “warning” system to direct visual attention (10.1007/978-3-540-29678-2_22) to objects entering the visual field. Alpha/parasol cells also play an important role in global form perception (10.1007/978-3-540-29678-2_6) and depth perception (10.1007/978-3-540-29678-2_2). Both alpha/parasol and beta/midget cells project to the lateral geniculate nucleus and hence provide major inputs for cortical, “higher” visual processing.
The ON direction-selective ganglion cells project to the 10.1007/978-3-540-29678-2_1, the ON/OFF direction-selective ganglion cells project to the optokinetic system, the superior colliculus and the lateral geniculate nucleus. Both types are thought to contribute to the discrimination between self-movement and object movement (Retinal direction selectivity: Role of amacrine cells). The intrinsically photosensitive ganglion cells are specialized to encode ambient light intensities, they are involved in synchronizing circadian rhythms with the solar day and in regulating the pupillary light reflex. These three ganglion cell types are examples for retinal channels feeding into subcortical, “lower” visual processing systems.
For many of the less well-characterized ganglion cell types, we lack a clear idea of their role in image analysis. On the one hand, it is clear that there are basic parallel processing channels feeding specific parts of visual information into ganglion cell types with different response properties. On the other hand there is increasing awareness that retinal image processing works by a finely tuned and stimulus-dependent interplay of these many different channels, rather than by a strict dedication of “one type for this task, one type for that task.” For example, primate parasol cells, despite their large receptive fields, play a significant role in hyperacuity (10.1007/978-3-540-29678-2_22). Actually, there seem to be more types of ganglion cell (and of other retinal neurons) than we need to account for the functions we currently attribute to the retina [2]. We still have to learn a lot about this intriguing piece of neural tissue.
Lateral Interactions
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_4
10.1007/978-3-540-29678-2_7
Retinal Implant
Definition
Device intended to restore useful vision in pathologies that selectively affect the photo-detectors of the retina while leaving relatively intact the other retina neurons and the fibers of the optic nerve (such as retinitis pigmentosa and macular degeneration). These devices share with cochlear implants the basic design principles and requirements, but are at a much earlier stage of development than cochlear implants (there is at least a 20–30 years gap), because of the greater information density (thousands of hair cells on the basilar membrane vs. millions of photodetectors on the retina) and the more complex structure of the sensor.
10.1007/978-3-540-29678-2_3
Retinal Lateral Interactions
Synonyms
Lateral interactions in the retina
Definition
The influence of signals generated by retinal neurons on the activity of other neurons laterally distant in the retina.
Many processes are covered by this term, some acting over short distances between immediately adjacent neurons, and others acting over virtually the entire extent of the retina [1]. Lateral interactions occur at all levels in the retina, from the 10.1007/978-3-540-29678-2_16, the input neurons of the retina, through to ganglion cells (retinal ganglion cells), the output neurons of the retina. Lateral interactions may be positive or negative. Light falling on a patch of retina may augment the signal generated in neurons in an adjacent patch or it may diminish this signal. Lateral interactions are often time-dependent and may also depend on special features of the stimulus such as stimulus velocity or the wavelength of the illuminating light.
There are several different mechanisms known to mediate lateral interactions. Some forms of lateral interaction, particularly those occurring in the inner retina, are known only from indirect and incomplete evidence and are not well understood.
Characteristics
The best known form of lateral interaction in the retina is lateral inhibition. Lateral inhibition was inferred by Ernst Mach in the 1860s on the basis of psychophysical experiments. Much later it was shown that lateral inhibition was one of the first neural operations performed in the compound eye of the horseshoe crab, Limulus, where eccentric cells, roughly the equivalent of ganglion cells in the vertebrate retina, are thought to make inhibitory 10.1007/978-3-540-29678-2_19 with their neighbors (Fig. 1). Hartline and his colleagues quantified the effect of lateral inhibition on the steady-state firing rate of eccentric cells by writing simultaneous equations [2]. The firing of every eccentric cell is influenced by the firing of every other eccentric cell, so that, considering only a pair of illuminated eccentric cells, A and B, the responses of A and B, rA, and rB are given by:
 |
 |
where eA is the response of A in the absence of stimulation to B, KA,B is a term representing the strength of inhibitory connection from B to A, rB is the response of B, and r0 B is a threshold firing rate for B below which it exerts no inhibition on A. Terms for the response of B have analogous meanings. These simple piecewise linear equations give a good approximation of the responses of eccentric cells for different patterns of light falling on the Limulus eye and capture the essential features of lateral inhibition.
Retinal Lateral Interactions. Figure 1.

The idea underling the quantitative description of inhibitory interactions in the Limulus retina [2]. A pair of adjacent eccentric cells, A and B, each capable of independent excitation by light, are shown as cell bodies with axons sending information to the brain and collaterals that make local inhibitory connections with each other. The excitation of one cell tends to reduce the response of its neighbor. A subtle but important feature of the inhibition, reflected in this diagram and in the descriptive equations, is that it is recurrent. By this is meant that for any cell, the inhibitory effects of neighbors are exerted upstream of any inhibitory output from that cell.
The organization of the vertebrate retina is radically different from that of Limulus and yet lateral inhibition is similarly one of its first processing steps. To understand how lateral inhibition operates in the outer vertebrate retina it is necessary to consider all three general classes of neuron found there, their lateral interactions, and their receptive fields.
Photoreceptors have very small receptive fields, though in many instances these are at least slightly broader than expected from the anatomical dimensions of the photoreceptor. The explanation for this enlargement is that photoreceptors are weakly coupled together via 10.1007/978-3-540-29678-2_7) so that signals leak from one photoreceptor to its neighbors. Quantitative models of signal spread through the photoreceptor network have been based on square or hexagonal networks in which each photoreceptor is represented by a resistor and a capacitor, coupled to its neighbors through resistors representing gap junctions (Fig. 2). This kind of model gives a reasonable approximation for the spread of small signals through the network but misses some of the network’s time-dependent properties. More sophisticated models, incorporating the voltage-dependent conductances of photoreceptors, show that, at least for the amphibian and turtle retina, the network has strongly time-dependent properties [4,5].
Retinal Lateral Interactions. Figure 2.

A schematic representation of part of a network of coupled photoreceptors based on [3]. In this model, photoreceptors are arranged in a square grid separated by a distance, D. The resistors, rs, represent the coupling resistance of the gap junctions between adjacent photoreceptors while the photoreceptors themselves, positioned at every node of the grid, are represented by a capacitance and a membrane resistance.
Photoreceptors pass signals to both horizontal cells and bipolar cells (Retinal ribbon synapses) (Fig. 3). Horizontal cells are strongly coupled homotypically via gap junctions, with the result that signals spread widely throughout the network of horizontal cells, giving these neurons very broad receptive fields. Because the coupling between horizontal cells is so strong, individual cells can be ignored and the network approximated to a continuous and homogeneous sheet. The distribution of voltage in this model is given by a second-order partial differential equation for which useful analytical solutions have been found [6].
Retinal Lateral Interactions. Figure 3.

The receptive fields of the neurons in the outer retina and an explanatory schematic showing how these receptive fields are generated. For simplicity only one spatial dimension is shown, equivalent to mapping receptive fields using a long thin bar of light moved orthogonal to its long axis. Photoreceptors, shown in green, have narrow receptive fields, only slightly wider than the width of an individual receptor. Horizontal cells (blue), in contrast, have wide receptive fields because they collect signals from all their overlying photoreceptors and are strongly coupled to each other, thereby allowing signals to spread laterally. Bipolar cells (orange) collect signals from a relatively small group of overlying photoreceptors but also receive signals of opposite sign from nearby horizontal cells. This arrangement gives rise to a receptive field in which light falling in the center of the field, dominated by direct input from overlying photoreceptors, produces a response whose sign is opposite to that produced by more lateral stimuli in which horizontal cell input dominates.
Bipolar cells, in contrast to horizontal cells, are not strongly coupled and also differ from horizontal cells in having a receptive field comprising two distinct regions. A relatively narrow central region receives input from overlying photoreceptors but outside this lies an annulus of inhibitory, or antagonistic surround. If a spot of light falling in the central region depolarizes the bipolar cell, a spot falling in the surround would hyperpolarize the cell, and a large patch of light covering both center and surround would produce no sustained response (although it would produce transient responses when the light comes on and when it goes off). Loosely speaking, the bipolar cell receptive field is generated from the sum of the inputs from overlying photoreceptors and a sign-reversed input from horizontal cells of much larger spatial extent, as summarized in Fig. 3. How horizontal cell inputs “sum” is not entirely clear and there is evidence supporting both inhibitory synaptic feedback to photoreceptor terminals and inhibitory synaptic feedforward to bipolar cells as well. Neither of these proposed mechanisms is entirely satisfactory and an alternative idea based on extracellular current flow has recently been revived [7].
The lateral spread of signal between neurons coupled together via gap junctions can be inferred from intracellular recording from a pair of cells. Current injected into one cell produces a voltage in the other, and by examining pairs of neurons at known separations, it is possible to measure signal spread directly and estimate the electrical parameters of the network. This direct approach has been applied to photoreceptors and horizontal cells but a less direct method has proved useful between other cell types. The injection of small tracer molecules, often fluorescent molecules, into a single cell, implies the presence of coupling gap junctions if fluorescence appears in nearby cells. This method is not easily amenable to quantitative analysis, moreover gap junctions differ in the size of tracer molecule they allow to pass, nevertheless it has the huge advantages that intracellular injection into only a single neuron is required, and even very sparse connections can be revealed this way. The tracer molecules Biocytin and Neurobiotin have revealed a previously unsuspected wealth of coupling, both homo- and heterotypic, between neurons in the inner retina. Although this coupling implies the lateral spread of signal, electrical coupling between neurons is known to serve other functions, such as the synchronization of spiking, and it may transpire that the spatial aspects of this coupling are inconsequential.
Higher Level Processes
Perceptual illusions (10.1007/978-3-540-29678-2_22), such as Mach bands and the Hermann grid illusion, originate from lateral interactions in the retina [8].
Lower Level Components
Lateral interactions are mediated through gap junctions and synapses. In some instances it is possible to say which specific gap junction subunits are present in which retinal neurons but in many instances this is not yet known. The synapses and their transmitters mediating lateral interactions are known in outline but, as always, the details of the inner retina are much less clear than those of the outer retina.
Process Regulation
A large body of evidence shows that many, but not all, of the gap junctions that mediate lateral signal spread in the retina can be functionally closed by transmitters and 10.1007/978-3-540-29678-2_14. Best known is the example of coupling between horizontal cells in the retinas of fish and turtle [9,10]. 10.1007/978-3-540-29678-2_4, which is released by neurons in the inner retina, and plays a role in switching the retina from 10.1007/978-3-540-29678-2_19 to 10.1007/978-3-540-29678-2_16 conditions, clearly uncouples horizontal cells by elevating intracellular 10.1007/978-3-540-29678-2_3, thereby decreasing the receptive field size of these neurons and greatly restricting the ability of injected dye molecules to move between cells. A similar effect is seen in AII 10.1007/978-3-540-29678-2_1 [11] that, in low-light conditions when rod photoreceptors dominate, are coupled together and are a crucial link in the transmission of rod signals.
In addition to dopamine, 10.1007/978-3-540-29678-2_14 also closes gap junctions, in this case acting through 10.1007/978-3-540-29678-2_3, and probably also plays a role in light/dark adaptation, though how these two agents, dopamine and nitric oxide and perhaps others, act together and what their different functional roles might be, is not known. In general though, there are good theoretical arguments for modulating the spatial dimensions of lateral inhibition to optimize it for different ambient light intensities. In flies, the measured spatial extent of lateral inhibition is reasonably well matched to theory [12] but in vertebrate retina no similar comparison has been attempted.
Function
Lateral inhibition clearly enhances edges, which, very likely, is a necessary step in the task of parsing the visual world into separate, identifiable objects. Unfortunately, this idea does not lead easily to a quantitative theory.
Another way of looking at the function of lateral inhibition, initiated by H. B. Barlow and subsequently built on by others, has its roots in 10.1007/978-3-540-29678-2_9 and is known as predictive coding (10.1007/978-3-540-29678-2_19). The starting point for this approach is the postulate that the visual system is interested in all spatial information, i.e. edges are accorded no special a priori value. Key ideas are (1) that the retina has been optimized for efficiency of information transfer to the brain. This is a plausible conjecture, if only because there are obvious anatomical constraints on the thickness of the 10.1007/978-3-540-29678-2_15 and therefore the number of output channels from the retina. A more subtle constraint is exercised by intrinsic noise within a neuron that limits its information carrying capacity. (2) There exist statistical regularities in the spatial and temporal structure of the images falling on the retina. For example, the intensity of light falling on any point in the retina is likely to be similar to the intensities found adjacent to that point, first, because of blurring caused by the eye’s optical limitations, and second, because the visual world is not a random pattern of dots. (3) From point 2, we can say that some information is redundant and, on the basis of point 1, ought not to be transmitted to the brain.
Lateral inhibition performs this task of redundancy removal and permits the efficient use of the limited bandwidth provided by retinal neurons. Simply put, this view of lateral inhibition is that it is a way of computing a best guess for the signal expected at any point in the retina, based on a weighted sum of the signals from points around it. This best guess is then subtracted from the actual signal, thereby allowing any deviations from the guess to fill the bandwidth of that neuron.
On the basis of these arguments we would predict that the strength and extent of lateral inhibition should be matched to the statistics of different visual environments. An important contributor to these statistics is the variance in the number of photons arriving, which is a function of light intensity. At low light intensities a statistically reliable best guess requires that a large number of points be included, in other words lateral inhibition should become broader [12]. At higher light levels, where signal-to-noise ratios are higher, the best guess would include only those points in the immediate neighborhood.
The spatial characteristics of different scenes, a beach versus a forest for example, ought, from the principles of predictive coding, to elicit different lateral interactions within the retina. Some evidence from ganglion cell recordings suggests that this may be occurring in the inner retina, probably mediated by amacrine cells, though the mechanism is presently unknown [13].
While lateral inhibition has provided a path into some of the deepest questions concerning the design of the nervous system, positive lateral interactions have also stimulated a careful consideration of function.
The function of coupling between horizontal cells is fairly apparent, but what about photoreceptor coupling? At first sight this is puzzling, not only because photoreceptor coupling is apparently the opposite of lateral inhibition, for which there is a good understanding, but also because it must degrade spatial resolution.
A crucial concept in understanding photoreceptor coupling is that photoreceptors are very high-gain detectors for which noise is an inescapable problem. Some of this noise is produced by the thermal activation of the molecules involved in transduction (10.1007/978-3-540-29678-2_16) but a large contribution comes from chance fluctuations in the number of photons caught. Even for cones operating in the photopic range, few enough photons are caught per integration time that these fluctuations compromise the reliability of the signal. Coupling between photoreceptors is a form of signal averaging that improves the signal-to-noise ratio by canceling out some of the uncorrelated noise found in every cell. This engineering trick necessarily involves trading a gain in reliability (improved signal-to-noise ratio) for spatial resolution. But the trade is definitely worthwhile. In the case of mammalian cones, coupling increases the signal-to-noise ratio by about 70% by allowing some signal to leak into neighboring photoreceptors. The spatial blurring this causes actually turns out to be less than the blurring caused by the imperfect optics of the eye, so in fact no resolution is sacrificed by coupling [14].
While mammalian cones have coupling that is, in effect, strong enough to spread signals only to immediate neighbors but no further, poikilothermic vertebrates have somewhat stronger coupling between their photoreceptors. Undoubtedly the same arguments about signal-to-noise ratio apply, but as first noticed by Hodgkin and his collaborators, signal spreads a long way through the rod network shortly after a light comes on, but then contracts down to a much smaller area. A plausible, though unproven idea stemming from this finding is that the retina, at the level of bipolar cells, might employ two sampling strategies to read the rod network: one with good temporal resolution but poor spatial resolution, and the other with the converse properties [5].
Retinal Photoreceptors
Synonyms
Visual cells; Rods; Cones
Definition
Photoreceptor cells are light-sensitive neurons that respond with a graded change in the release of 10.1007/978-3-540-29678-2_14 (10.1007/978-3-540-29678-2_7) from their synaptic terminal. Photoreceptors are found in most classes of metazoan organism and vary widely in structure and function. Photoreceptors are predominantly located in the retina of both invertebrate and vertebrate eyes, where they are involved in vision, but they may also be located extraocularly on the integument or in the brain.
Characteristics
Quantitative Description
Photoreceptors are neurons that respond with a graded change in transmembrane potential (10.1007/978-3-540-29678-2_13), to the absorption of photons by light-sensitive proteins (10.1007/978-3-540-29678-2_16) embedded in specialized regions of their plasma membrane. The size, shape, ultrastructure, biochemistry, electrical properties and developmental origins of photoreceptors vary considerably across the animal kingdom. For the sake of brevity, the following descriptions are related to vertebrate visual photoreceptors (rather than non-visual retinal or extraocular photoreceptors), and in most cases, draw upon mammalian examples.
Photoreceptor Types
The vertebrate retina is inverted by virtue of its developmental origins and, consequently, the photoreceptors are situated close to the sclera at the back of the eye. They point away from the incident light, which must traverse the other retinal layers before reaching the photoreceptors [1]. There are two distinct types of retinal photoreceptor: rods that operate under low light (10.1007/978-3-540-29678-2_19) levels of illumination and 10.1007/978-3-540-29678-2_3 that operate under bright light (10.1007/978-3-540-29678-2_16 conditions). The rod-cone nomenclature reflects differences in aspects of photoreceptor morphology in the mammalian retina: the outer segments of rods are rod-like whereas cone outer segments are typically conical (Fig. 1).
Retinal Photoreceptors. Figure 1.

Schematic representations of vertebrate rod and single cone retinal photoreceptors. Oil droplets – found in the photoreceptor ellipsoid in some species – are not shown. See text for details.
However, it should be remembered that this is not always the case – even within the same retina – and it can be misleading to classify photoreceptors using morphology alone; rods and cones also differ in their biochemistry, physiology and function.
The human retina contains a single type of rod photoreceptor but three spectrally distinct cone types, which each contain a different cone photopigment maximally sensitive to blue, green and red light (see 10.1007/978-3-540-29678-2_16). The cones of humans and other placental mammals, lungfish and elasmobranchs are all of the “single” type. Amphibians, marsupials, monotremes, birds, reptiles and teleost fish possess an additional photoreceptor type that consists of a pair of closely opposed cone cells. They are usually referred to as double cones when the two members are unequal in size – with a larger “principal” and a smaller “accessory” member – and twin cones when the two members are of similar size and shape. The closely opposed outer segments of the two members are separated from those of other photoreceptors – but not each other – by the processes of retinal pigmented epithelium (RPE) cells; the two members are thought to be both optically and electrically coupled.
Double cones are more widely distributed among the vertebrate classes, and both members usually contain the same spectral type of photopigment. Often, but not always, the principal member (and occasionally the accessory member) contains an oil droplet (see below). Twin cones occur predominantly in teleost fishes, and may have identical or distinct photopigments in each member, even within the same retina.
Retinal Topography
The absolute density and relative proportion of rods and cones varies as a function of visual ecology. Strongly diurnal species (e.g. most birds, turtles) have a lower rod:cone ratio than nocturnal species (e.g. many rodents) and, in some cases, lack rods altogether (e.g. lizards). The average human retina contains about 4.6 million cones and 92 million rods, giving a rod to cone ratio of about 20:1 (excluding the 10.1007/978-3-540-29678-2_6). In contrast, nocturnal rodents have rod to cone ratios of around 100:1 [2].
Moreover, the topographic distribution of photoreceptors across the retina is usually non-uniform. Most animals have a retina with one or more areas of increased photoreceptor density, which permit increased spatial sampling of the retinal image and provide high acuity vision over a defined region of visual space. The areas are often circular and usually occur in the central (10.1007/978-3-540-29678-2_1) or temporal (10.1007/978-3-540-29678-2_1) retina, but may also form horizontal streaks (10.1007/978-3-540-29678-2_1).
In some cases, the vitreal surface of the retina is indented above the area of highest photoreceptor density. This type of area is called a fovea, and the foveal indentation or pit is a result of the lateral displacement of secondary retinal neurons from the optical path, to provide the incident light with unimpeded access to the photoreceptor layer. The shape of the foveal pit varies in curvature, and may serve to magnify the image projected onto the retina by virtue of the difference in refractive index between the vitreous and retina. The human fovea contains approximately 200,000 cones, with reported peak densities ranging from 100,000 to 320,000 cells mm−2. Cone density falls rapidly with increasing eccentricity: at 10° eccentricity – or 3 mm from the centre of the fovea – it averages 7,000 cones mm−2. Rods are excluded from the human fovea but attain their highest density of around 160,000 cells mm−2 in a perifoveal ring located at 18° eccentricity or about 5 mm from the centre of the fovea [3].
Description of the Structure
Vertebrate photoreceptors are comprised of three morphologically and functionally distinct regions: the outer segment, inner segment and synaptic terminal.
Outer Segment
Human rod outer segments are 2 μm in diameter and vary in length with retinal eccentricity from 24 μm at the periphery to 40 μm in the parafoveal region. Human foveal cone outer segments are 30–40 μm in length, are less tapered/conical than cones located in the periphery and have a diameter of about 0.8 μm [1]. The outer segments of cones in the peripheral retina are more conical but only about half as long. Photoreceptor outer segment dimensions also vary widely with habitat and life history. For example, nocturnal species, or those inhabiting light-limited environments such as the deep sea, tend to have longer and wider outer segments than diurnal species in order to capture more of the available light.
Rod outer segments consist of stacks of isolated membranous discs or “saccules” bounded by the plasma membrane. Each saccule is around 19 nm in thickness. The periodicity of saccule spacing is 28 nm and, in humans, there are around 1,000 saccules in each rod outer segment. In contrast, cone outer segments are formed by multiple infolding of the plasma membrane. Both the saccule and infolded plasma membranes are packed with photopigment molecules that absorb the incident light. The high lipid content of the outer segment endows it with a high refractive index relative to the surrounding extracellular space. Consequently, the outer segment acts as a waveguide, and light entering at the base is contained and propagated along its length by total internal reflection.
The base of the outer segment is connected to the inner segment by a modified non-motile cilium, which projects through a narrow cytoplasmic bridge (“ciliary stalk”) that is 1 μm in length. In some species, such as rodents, a second contiguous cytoplasmic bridge is observed. A number of fine processes originating from the inner segment, called calycal processes, extend distally along the outer segment for approximately one third of its length and probably provide mechanical support. In rod outer segments, the calycal processes are contiguous, with indentations in the plasma membrane that overlie – but do not invaginate – scalloped incisures in the radial edge of the saccule membrane.
Inner Segment
The inner segment contains all the structures necessary for cellular metabolism and protein synthesis. It may also contain organelles that help to capture and/or spectrally filter the incident light and to focus it onto the outer segment. The most important structures are described below:
Ellipsoid
Photoreceptors contain numerous 10.1007/978-3-540-29678-2_13 in the distal portion of their inner segment. These are packed together in a highly refractile body called the ellipsoid, and may be oriented with their long axis parallel to that of the photoreceptor, as in mammals, or clumped randomly. Photoreceptors are energetically demanding cells and the mitochondria must generate sufficient adenosine triphosphate (ATP) to support cellular function, in particular the maintenance of sodium/potassium (Na±/K±) pumps (Ion transport) in the plasma membrane, the production of photopigment molecules and the turnover of guanosine 3′,5′ cyclic monophosphate (cGMP; see 10.1007/978-3-540-29678-2_16).
In the mammalian retina, cones contain many more mitochondria than rods, but it is not readily apparent why they should do so on a metabolic basis [4]. It is possible that they also have an optical function by increasing the refractive index of the inner segment. Both the inner and outer segments have a higher refractive index than the surrounding medium and, at least in cones, the inner segment is significantly wider than the outer segment. Light striking the inner segment is funnelled by total internal reflection into the outer segment, a physical phenomenon known as waveguiding. Consequently, the cross-sectional area of the inner segment, rather than that of the outer segment, defines the photon capture area of the photoreceptor. Human foveal cones are 2.3 μm in diameter whereas those in the peripheral retina are 7.9 μm in diameter, a difference that confers a 12-fold increase in photon capture area and, therefore, optical sensitivity [1].
The ellipsoids of some species show further specializations. The proximal region of the cone ellipsoid in some lizards contains aggregations of extended endoplasmic reticula, known as refractile bodies, which may well have a light-gathering function in addition to any putative metabolic storage role. Similarly, tree shrews (Tupaia sp.) have large distended mitochondria (“megamitochondria”) with visible but irregular cristae, which may confer an enhanced light-gathering ability to the ellipsoid. This structure could replace the colorless oil droplets present in the cones of their ancestors and retained by marsupials but lost by other placental mammals [5]. Other modified mitochondria (ellipsosomes) are present in the ellipsoids of teleost fish and contain filtering pigments that resemble reduced cytochrome C.
Oil Droplets
The photoreceptors of a number of species contain an inclusion located either within or just distal to the ellipsoid, known as an oil droplet. As their name suggests, oil droplets are composed predominantly of lipids, but they may also contain light-absorbing carotenoid pigments. Pigmented oil droplets are found in the cones of birds, turtles, lizards and lungfish; colorless oil droplets occur in some geckos, anuran amphibians, chondrostean fishes, marsupials and some monotremes. Oil droplets are absent from lampreys, teleosts, elasmobranchs, snakes, crocodilians and placental mammals [6].
Pigmented oil droplets act as filters that tune the spectral sensitivity of the photoreceptor, in most cases narrowing the spectral sensitivity function and shifting the wavelength of peak sensitivity to a wavelength longer than the wavelength of maximum absorbance (λmax) of the photopigment in the outer segment. Non-pigmented oil droplets probably serve a similar function to ellipsosomes in capturing and focusing light into the outer segment.
Paraboloid and Hyperboloid
The rod and cone inner segments of some holosteans, amphibians, birds and reptiles contain a granular structure proximal to the ellipsoid known as the paraboloid (cones) or hyperboloid (rods). It is thought to act as a store of glycogen, presumably supporting the high metabolic activity of the cell.
Myoid
The myoid region lies proximal to the ellipsoid (and paraboloid/hyperboloid if present) but distal to the nucleus. It contains free ribosomes, rough endoplasmic reticulum (RER) and the Golgi apparatus, and it is the site of protein synthesis in the photoreceptor. Photopigment 10.1007/978-3-540-29678-2_13 from the nucleus migrate to the RER and are translated into opsin proteins (see 10.1007/978-3-540-29678-2_16) that are eventually packaged into small vesicles by the Golgi apparatus. These vesicles migrate to the ciliary stalk, pass through the cytoplasmic bridge into the outer segment and become incorporated in the saccule and plasma membranes. In some lamprey and lizard species, the entire myoid contains a diffuse yellow-orange pigment that, like the pigmented oil droplets, spectrally filters the incident light before it reaches the outer segment.
In some “lower” vertebrates (e.g. fish, amphibians) the myoid is motile. Cone myoids contract in the light and elongate in the dark; the opposite is true for the rod myoid. These so called “retinomotor movements” are substantial (50–70 μm) and represent a form of light/dark adaptation, the function of which is to shield the rods in the retinal pigmented epithelium during the day but fully expose them at night. Myoid contraction and elongation is controlled by both light-dependent and endogenous (circadian) mechanisms [7].
Synaptic Terminal
Beneath the myoid lies the nucleus and, proximal to this, a thin fiber (axon) that connects the inner segment to the synaptic terminal. The synaptic terminal of a photoreceptor cell ramifies in the outer plexiform layer of the retina and is the site of communication with other retinal neurons. The graded changes in outer segment transmembrane potential that occur as a result of the phototransduction process propagate electrotonically (10.1007/978-3-540-29678-2_5) to the synaptic terminal and are communicated to other retinal neurons in two ways. Firstly, the electrical potential may be transferred passively to adjacent photoreceptors via low-resistance intercellular junctions, called 10.1007/978-3-540-29678-2_7. Secondly, changes in membrane potential at the synaptic terminal alter the internal Ca2+concentration and modulate the rate at which 10.1007/978-3-540-29678-2_19 fuse with the plasma membrane and release neurotransmitter (glutamate) into the synapse.
The morphology of rod and cone synaptic terminals differs markedly. The rod synaptic terminal (“spherule”) is roughly spherical and contains a single invagination (“synaptic cleft”) within which lie the processes of two to five rod bipolar cells and two horizontal cells (Retinal ribbon synapses; 10.1007/978-3-540-29678-2_9; 10.1007/978-3-540-29678-2_22). The cone synaptic terminal (“10.1007/978-3-540-29678-2_3”) is much larger and almost pyramidal in shape. In the mammalian retina, peripheral cones have much larger pedicles than foveal cones and display as many as 50 synaptic clefts. Like the rod spherule, the synaptic clefts of cone pedicles contain the processes of two or more cone bipolar cells and two horizontal cells [8].
In both rod and cone synaptic terminals, the active zone immediately above the synaptic clefts contain one (cones) or two (rods) synaptic ribbons that modulate the release of neurotransmitter (see Ribbon synapses). Rod and cone bipolar cell processes entering the synaptic cleft are termed “central” or “invaginating” processes. Other bipolar cell processes contact the cone pedicle on either side of the invaginating processes (“semi-invaginating” processes) or diffusely across the base of the pedicle (“flat” processes). Consequently, each cone pedicle may make several hundred synapses with 10 or more postsynaptic neurons.
Higher Level Structures
In the mammalian retina, rods contact only one type of bipolar cell, the rod ON bipolar (Retinal ribbon synapses). Cone photoreceptors synapse with up to 11 different types of ON and OFF cone bipolar cells. In other vertebrates, both rods and cones may contact the same bipolar cell, which may be either ON or OFF and either rod- or cone-dominated. Photoreceptors are presynaptic to horizontal cells in a sign-conserving manner, i.e. 10.1007/978-3-540-29678-2_8 of the photoreceptor transmembrane potential results in a hyperpolarization of the horizontal cell. However, horizontal cells feed back onto photoreceptors in a sign-inverting manner that antagonizes the effects of transmembrane hyperpolarization, reduces glutamate release and allows the outer retina to adapt to steady illumination [1].
Regulation of the Structure
In the dark, a balanced flow of cations into the outer segment and out of the inner segment (known as the dark current) maintains a moderate depolarization of the transmembrane potential. The magnitude of the dark current in the rods (−34 pA) and cones (−30 pA) is similar in the macaque monkey. Stimulation of photoreceptors with light causes a reduction in the dark current due to phototransduction. This change in dark current is called the photocurrent and, although both rods and cones are capable of signaling the absorption of a single photon of light, the photocurrent produced by a single photoisomerization event (see 10.1007/978-3-540-29678-2_16) is much smaller in cones (33 fA) than it is in rods (700 fA) [1]. Consequently, more photons per unit time are required by cones than rods to provide a large enough change in glutamate release at the synaptic terminal to be reliably detected by the bipolar cells and, therefore, rods are better for vision under low levels of illumination.
There are also qualitative differences in the time course of the photocurrent between rods and cones. The magnitude of the photocurrent peaks about 50 ms after the onset of light in primate cones but takes up to four times longer in the rods. Moreover, the rod photocurrent decays more slowly, taking up to one second to return to zero, whereas cones recover up to five times faster. These differences in response and recovery kinetics enable cone pathways to respond to higher temporal frequencies than the rods, although at the expense of absolute sensitivity.
Photoreceptor outer segments are renewed continuously. Rod saccules are shed from the distal tip of the outer segment in packets of 8–30, where they are taken up in phagocytotic vesicles (“phagosomes”) by RPE cells. Shed saccules are replaced by the synthesis of additional membrane at the base of the outer segment, where new photopigment molecules generated in the inner segment are incorporated. Cones shed and regenerate membrane in a similar fashion to rods, although newly synthesized photopigment molecules can diffuse to any location in the membrane, as the outer segment is not compartmentalized. Membrane shedding follows a circadian rhythm; cone membranes are shed after dusk when the visual system begins to rely on rods, and rod saccules are shed at dawn when cones become dominant [9].
Function
The retina is a two-dimensional sensor array that extracts salient features from the image of a three-dimensional world projected onto the back of the eye by the lens and cornea (10.1007/978-3-540-29678-2_22). The size, packing density, spectral sensitivity and electrical response characteristics of the photoreceptors limit the spatial, temporal and chromatic information that can be extracted from that image.
Rods are more sensitive than cones and are used in dim light (scotopic) conditions. Most vertebrates, including humans, have only one spectral class of rod and, consequently, are essentially color blind at night. Cones, on the other hand, function at higher (photopic) light levels. The possession of both scotopic and photopic photoreceptor types – otherwise known as a duplex retina – has the primary function of extending the range of light intensities over which the visual system is operational. For most animals, this range varies over 10 log units from starlight to bright sunlight [1].
Where multiple spectral types of cone are present, their outputs can be compared by secondary neurons to extract chromatic information from the retinal image. The ability to distinguish objects based on their color (spectral reflectance) independently of brightness is called color vision (10.1007/978-3-540-29678-2_3). Most mammals have only two spectral types of cone and have a dichromatic color vision system. Humans and some primates have three spectral cone types and are trichromats. Birds have a tetrachromatic color vision system based on four spectral types of single cone photoreceptor [10]. The function of double/twin cones is unclear, despite being the most numerous photoreceptor types in many species. Limited evidence suggests that, at least in the avian retina, double cones mediate purely achromatic (brightness discrimination) tasks, including motion detection, and are not involved in color vision.
Retinal Pigment Epithelium
Definition
These cells form the outer part of the vertebrate retina, forming the outer blood-retinal barrier and providing key roles in chromophore recycling and transport of metabolites and metabolic byproducts.
10.1007/978-3-540-29678-2_9
10.1007/978-3-540-29678-2_16
Retinal Ribbon Synapses
Definition
Ribbon synapses are specialized synapses of the primary sensory neurons of the eye (retinal 10.1007/978-3-540-29678-2_16) and ear (10.1007/978-3-540-29678-2_8 and vestibular hair cells). They are also formed by Retinal ribbon synapses, vestibular receptor cells, and fish 10.1007/978-3-540-29678-2_5. Morphologically, these synapses are characterized by a presynaptic electron-dense bar, the 10.1007/978-3-540-29678-2_18, at the site of 10.1007/978-3-540-29678-2_14 release (Fig. 1). Ribbon synapses support continuous release of the neurotransmitter 10.1007/978-3-540-29678-2_7, and modulate the rate of release in response to graded changes in membrane potential (10.1007/978-3-540-29678-2_13).
Retinal Ribbon Synapses. Figure 1.

Schematic diagrams of ribbon synapses in the retina and inner ear, drawn approximately to scale (as indicated). (a). Rod photoreceptor terminal (spherule) containing a vesicle-covered synaptic ribbon with an arciform density at its base. Postsynaptic horizontal cell (white) and rod bipolar cell (orange) processes protrude into the invagination, opposed to the site of transmitter release. (b) Cone photoreceptor terminal (pedicle) containing four separate ribbons with their associated arciform densities, and postsynaptic invaginations of horizontal cell (white) and ON-cone bipolar cell (orange) processes. OFF-cone bipolar cells (purple) make synapses at flat contacts, outside the invaginations. (c) Cone bipolar cell terminal (orange) containing multiple small ribbons. Postsynaptic amacrine cell (yellow) and ganglion cell (green) processes are opposed to the sites of neurotransmitter release. (d) Type I (I.) and type II (II.) inner ear hair cells contain spherical synaptic bodies, covered with tethered vesicles. Type I hair cells form a single calyceal terminal, whereas type II hair cells make synapses with individual afferent boutons, each bouton receiving input from a single ribbon.
Characteristics
Description of the Structure
Anatomically, ribbon synapses are characterized by the presence of a structural specialization of the 10.1007/978-3-540-29678-2_1, the synaptic ribbon. The synaptic ribbon is a planar structure in retinal photoreceptors and bipolar cells, and a spheroid structure in inner ear hair cells where it is also referred to as a “synaptic body” (Fig. 1). The ribbons are attached to the plasma membrane and typically extend perpendicularly into the cytoplasm up to several hundred nanometers. The ribbon is surrounded by a monolayer of 10.1007/978-3-540-29678-2_19 that are tethered to the ribbon by fine filaments. Vesicles at the base of the ribbon are docked on the plasma membrane and represent the readily releasable pool of synaptic vesicles. Those tethered further up the ribbon provide a reserve pool of synaptic vesicles. Synaptic ribbons vary in size and shape depending on the cell, but for a particular cell type, such as the mammalian rod photoreceptor, the total ribbon area and geometry vary comparatively little, even between species.
Photoreceptor ribbon synapses are the most structurally complex of ribbon synapses. Photoreceptor ribbon synapses are formed by an invagination into the photoreceptor terminal, over the apex of which lays the synaptic ribbon, and into which postsynaptic processes from 10.1007/978-3-540-29678-2_8 (10.1007/978-3-540-29678-2_18, 10.1007/978-3-540-29678-2_9, 10.1007/978-3-540-29678-2_22) and bipolar cells protrude (Fig. 1). Rod photoreceptor terminals (referred to as spherules) contain a single invagination that gives rise to one or two ribbon synapses [1], whereas cone photoreceptor terminals (referred to as 10.1007/978-3-540-29678-2_3) contain 10–30 invaginations, depending on whether they are in the central or peripheral retina, each invagination corresponding to a single ribbon synapse (Fig. 1). The large synaptic ribbons of rod photoreceptors curve around the invagination in a characteristic horseshoe shape, which can be clearly discerned at the light microscopic level by immunofluorescent staining of ribbon proteins (such as bassoon or RIBEYE, see Fig. 2).
Retinal Ribbon Synapses. Figure 2.

(a) Individual arc-shaped synaptic ribbons, labeled with an antibody to RIBEYE (red), are seen within rod and cone photoreceptor terminals (green) in a confocal micrograph through the 10.1007/978-3-540-29678-2_15 of the rat retina. (b) Rod ribbon synapse ultrastructure is observed in more detail by transmission electron microscopy. The synaptic ribbon, in transverse section, is perpendicular to the cell membrane, and overlies an arciform density (red arrowhead). Vesicles of neurotransmitter are evident throughout the terminal, with a subset of these tethered to the ribbon. Postsynaptic horizontal and rod bipolar cell processes invade the terminal, opposed to the site of neurotransmitter release.
The photoreceptor synaptic ribbon is attached to the plasma membrane via a linear, trough-shaped structure, the arciform density. The arciform density defines the site of neurotransmitter release, or active zone, of the photoreceptor ribbon synapse (Fig. 2b). The plasma membrane is pinched where it contacts the arciform density to form a ridge along the underside of the invagination. Synaptic vesicles at the base of the ribbon are docked on the plasma membrane, fusing on either side of the arciform density. Two postsynaptic horizontal cell processes, known as “lateral elements,” extend their tips close to the synaptic ribbon. One or more bipolar cell processes, known as “central elements”, occupy the central region of the invagination, with their tips located at a further distance from the ribbon. An electron micrograph of a photoreceptor ribbon synapse will often exhibit a “triad” arrangement, with two horizontal cell processes flanking a single bipolar cell process within the invagination postsynaptic to the ribbon. Rods make synaptic contacts with only one type of bipolar cell, the rod bipolar cell. Cones contact both ON-bipolar cells (depolarize in light) and OFF-bipolar cells (hyperpolarize in light) (Retinal ribbon synapses). At cone ribbon synapses it is only the ON-bipolar cells that extend their dendrites into the invagination; OFF-bipolar cells contact cone pedicles outside the invaginations at basal contacts.
Bipolar cell and hair cell ribbon synapses are simpler in structure (Fig. 1). They both lack invaginations and arciform densities. It is unclear how the ribbons are anchored to the plasma membrane in these cells. As in photoreceptors, bipolar cell synaptic ribbons are planar structures sitting perpendicular to the plasma membrane above a linear active zone, although they are smaller than photoreceptor ribbons. Two postsynaptic processes from amacrine cells (Retinal direction selectivity: Role of starburst amacrine cells) and ganglion cells (retinal ganglion cells) abut the bipolar cell membrane, one on either side of the active zone. Each bipolar cell terminal forms multiple ribbon synapses.
Hair cells also contain multiple ribbons, averaging ~20/cell, each covered with a layer of one hundred to several hundred tethered synaptic vesicles. Each hair cell ribbon synapse releases glutamate onto a single postsynaptic ending/contact. Type I vestibular hair cells are enveloped by a single calyceal terminal, which receives the output from all of the hair cell’s ribbon synapses; whereas each ribbon synapse of a type II vestibular hair cell has a separate post-synaptic dendrite [2]. Hair cells in the cochlea also have only one post-synaptic afferent/dendrite per ribbon [3]. Hair cell synaptic ribbons are often spherical, but elongated and planar ribbons are also found, especially in type II vestibular hair cells.
Quantitative Description of Ribbon Synapses in the Retina
Reconstruction of rod spherules in the cat retina reveal a planar synaptic ribbon ~2 μm in length (or two half-sized ribbons), extending from the plasma membrane into the cytoplasm by ~0.4 μm. Each face of the ribbon binds approximately 385 synaptic vesicles, 65 of which lie along the bottom of the ribbon docked at the plasma membrane on each side of the arciform density. Cone synaptic ribbons are smaller but more numerous than in rods. Cone ribbons are ~1 μm long and extend from the plasma membrane into the cytoplasm by ~0.2 μm. Although different ribbons within a cone terminal vary in length, the total ribbon length and number of tethered vesicles per cone is quite uniform within a retinal locus. The 20 or so ribbons in a primate foveal cone tether a total of ~3,600 synaptic vesicles, ~720 of which are docked at the plasma membrane [4]. Bipolar cells, depending on their type, contain from 30–100 synaptic ribbons, but they are small compared to photoreceptor ribbons, tethering several dozen vesicles each. The total number of ribbons and associated docked vesicles per synaptic terminal may be related to the information content that has to be transmitted by the neuron [4]. For example, rod photoreceptors transmit the detection of single photons in very dim light, like starlight, whereas cones must transmit finely graded changes over orders of magnitude of light intensity (10.1007/978-3-540-29678-2_16, 10.1007/978-3-540-29678-2_16).
The most detailed and quantitative description of a ribbon synapse has been obtained for the mammalian rod photoreceptor. Digital reconstruction of primate and cat rod spherules from serial electron micrographs has revealed the following invariant features of rod ribbon synapses [1]: Each spherule contains a single invagination approximately 1μm in diameter, and two synaptic units. Post-synaptically, each unit is made up of two lateral elements (horizontal cell dendrites) and at least one central element (rod bipolar cell dendrite). Although there are always two sets of postsynaptic processes, presynaptically a spherule may contain either one or two synaptic ribbons. However, even in cases where a spherule contains a single ribbon, the ribbon contacts two discrete arciform densities, each ~1.0 μm in length. Typically, the two arciform densities are in different planes, so that a single ribbon will twist to contact both. The volume of extracellular space within the invagination is small, roughly 0.1μm3, and the most distant bipolar cell glutamate receptors from the active zone (as defined by the arciform density) are within 1.5 μm. Thus, diffusion of glutamate to receptors on all postsynaptic processes should be rapid.
Higher Level Structures
The principal component of synaptic ribbons is the ribbon-specific protein, RIBEYE, composed of a unique N-terminal domain possessing a self-aggregating activity that may be important for the polymerization of the ribbons, and a C-terminal domain that is identical (minus the first 20 amino acids) to the transcriptional co-repressor, C-terminal binding protein 2 (CtBP2) [5]. Other proteins associated with the ribbons are components of the presynaptic matrix at conventional synapses including RIM (rab3-interacting protein), and the large (>400 kD) scaffolding proteins bassoon and piccolo, suggesting that at least some aspects of ribbon function are equivalent to that served by the presynaptic matrix at the active zone of conventional synapses. Bassoon has been localized by post-embedding immuno-electron microscopy to the base of photoreceptor ribbons close to the plasma membrane, whereas piccolo and RIM are localized towards the distal portion of the ribbon [6]. In mice deficient in bassoon, photoreceptor and hair cell synaptic ribbons are either absent or free-floating in the cytoplasm [7,8] suggesting that bassoon is required for anchoring the ribbons to the plasma membrane.
The presynaptic Ca2+ channels at ribbon synapses are localized to the plasma membrane at the base of the ribbons [6]. Ribbon synapse Ca2+ channels belong to the L-type Ca2+ channel family (10.1007/978-3-540-29678-2_3), and are of the subtypes Cav1.3 (α1D) in cone photoreceptors and hair cells, and Cav1.4 (α1F) in rod and cone photoreceptors and some bipolar cells. A distinctive characteristic of the ribbon synapse Ca2+ channels is that they are non-inactivating, an essential property for maintaining transmitter release during prolonged depolarization, as occurs in darkness.
The majority of synaptic vesicle-associated proteins are identical between ribbon and conventional synapses; however, there are a few significant differences [9]. For example, ribbon synapses are the only synapses known not to contain 10.1007/978-3-540-29678-2_19, peripheral membrane proteins of synaptic vesicles at conventional synapses. Synapsins immobilize synaptic vesicles in the absence of action potentials by linking them to the actin-based cytoskeleton, and then release them upon Ca2+ influx to replenish the readily releasable pool. At ribbon synapses, which are tonically active, synaptic vesicles are in constant flux and synapsins are not needed. Retinal ribbon synapses also differ in which syntaxin gene they express. At conventional synapses, syntaxin 1 is one of the key proteins catalyzing synaptic vesicle fusion. Retinal ribbon synapses are the only synapses known not to contain syntaxin 1 but to contain syntaxin 3 instead. This substitution may reflect differences in the regulation of synaptic vesicle fusion between ribbon and conventional synapses.
Function
Ribbon synapses are formed by sensory neurons of the visual, auditory, and vestibular systems. These neurons are electrotonically compact (10.1007/978-3-540-29678-2_5), and track changes in external stimuli with graded changes in their membrane potential (in contrast to axon-bearing neurons that fire action potentials). The graded changes in membrane potential in turn modulate the rate of tonic release of the neurotransmitter, glutamate. Synaptic ribbons are not found in neurons that undergo action potential-driven transmitter release. As at other chemical synapses, neurotransmitter is released at ribbon synapses by the Ca2+-dependent fusion of neurotransmitter-filled synaptic vesicles with the plasma membrane. Calcium enters the nerve terminal through voltage-sensitive Ca2+ channels that open in response to membrane depolarization and close upon hyperpolarization. Photoreceptors are depolarized in darkness and undergo a continuous stream of synaptic vesicle fusion in this state. Absorption of photons hyperpolarizes the photoreceptor, closing Ca2+ channels and reducing glutamate release (10.1007/978-3-540-29678-2_16). The synaptic ribbons most likely serve to maintain a pool of synaptic vesicles in close proximity to the active zone, and ensure the continual replenishment of synaptic vesicles during prolonged depolarizations. They have also been proposed to facilitate compound fusion of vesicles, or simultaneous fusion of multiple vesicles [10].
The critical role of the synaptic ribbons in 10.1007/978-3-540-29678-2_19 at ribbon synapses is illustrated by the phenotype of the bassoon knockout mouse. Elimination of bassoon prevents synaptic ribbons from anchoring at the plasma membrane. The physiological consequence is a drastic impairment of synaptic transmission between photoreceptors and depolarizing bipolar cells [7]. Likewise, in hair cells of the bassoon knockout mouse, patch-clamp recordings (10.1007/978-3-540-29678-2_9) indicate a 50% reduction in fast 10.1007/978-3-540-29678-2_5. The hair cell recordings also reveal substantially smaller Ca2+ currents in the bassoon knockout mouse compared to wild-type, suggesting that recruitment and stabilization of Ca2+ channels at hair cell active zones may be dependent on association with synaptic ribbons [8].
Retinal Essays: (photoreceptor outer segments), (Retinal ribbon synapses) and (lateral interactions in the retina).
Retinal Slip
Definition
Motion of the visual image on the surface of the retina. Slip of the visual image across large portions of the retina is the stimulus that stimulates optokinetic eye movements, and also the stimulus that produces the adaptation (improvement) of the optokinetic system.
10.1007/978-3-540-29678-2_15
Retinitis Pigmentosa
Definition
A group of hereditary retinal degenerations characterized by loss of peripheral vision (constricted visual fields) and night vision (nyctalopia). Although a vast variety of genetic mutations have been identified, patients with Retinitis Pigmentosa display similar symptoms and ocular fundus appearance in the end stage of the disease: bone spicule pigment deposits, pale atrophic optic nerve head and attenuated blood vessels.
10.1007/978-3-540-29678-2_9
Retino-geniculo-cortical Pathway
Definition
A large fraction (at least 90%) of retinal ganglion cells project to visual cortex through the lateral geniculate nucleus. This pathway is thought to be most important for visual perception. Non-geniculo-cortical pathways, such as the pathways through the suprachiasmatic nucleus or pre-tectum involve smaller numbers of ganglion cells and in some cases have specialized functions (e.g. suprachiasmatic nucleus is involved in diurnal rythyms).
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_22
10.1007/978-3-540-29678-2_22
Retinohypothalamic Tract
Synonyms
RHT
Definition
A monosynaptic neural projection that extends from the retina to the hypothalamus.
Characteristics
Origin and Projections
The retinohypothalamic tract (RHT) originates from a subset of retinal ganglion cells (RGCs). In rodents, this small cohort of cells represents only about 1–2% of all RGCs of which 80% or more express the 10.1007/978-3-540-29678-2_16, thereby making them intrinsically photosensitive. However, compared to rod and cone photoreceptors, these photoreceptive cells are relatively insensitive to light. The sparse, varicose dendritic arbors of murine 10.1007/978-3-540-29678-2_9 arise from 2 or 3 primary dendrites emanating from perikarya about 20 μm in diameter. ipRGC dendritic fields have a mean diameter of 450 μm and tile the retina with substantial overlap. The arbors themselves contain melanopsin and are capable of 10.1007/978-3-540-29678-2_16, giving each of these cells a capture radius of approximately 15° of visual space. These overlapping dendrites coupled with the relative photic insensitivity of ipRGCs are consistent with the sensory characteristics observed in many non-visual responses to light such as the synchronization (10.1007/978-3-540-29678-2_5) of circadian rhythms to the astronomical day. Relative to the visual system, the circadian axis requires higher levels of light to elicit a response and can integrate photic stimuli over longer temporal intervals and broader spatial domains. In essence, while the visual system functions like a camera, the “photoreceptive net” arising from the widely distributed ipRGCs functions like a photographer’s light meter [1,2].
In most animals examined to date, the ipRGCs are distributed evenly throughout the retina. In rats and primates, however, there is a shallow density cline peaking in the superiotemporal and parafoveal retinal domains, respectively. The principal target of the RHT is the 10.1007/978-3-540-29678-2_19, the site of a primary 10.1007/978-3-540-29678-2_3. The RHT is the anatomical route by which information about environmental light levels is conveyed from the eye to the SCN, where it is processed and used to entrain a multitude of circadian rhythms to the prevailing 10.1007/978-3-540-29678-2_12. Among these are circadian rhythms of activity, daily variations in core body temperature, and 24-h rhythms in levels of hormones such as 10.1007/978-3-540-29678-2_13 and cortisol [2,3].
The topography of the retinal innervation to the SCN is highly variable across mammalian species and even varies significantly among the rodents. In general, the ventrolateral aspect of the SCN receives the most dense innervation from the retina and is coextensive with the distribution of vasoactive intestinal peptide (VIP)-positive cells. However, it should be noted that the entire SCN receives some retinal afferents [3,4].
The degree to which the retina projects to each SCN also varies significantly among mammals. Contralaterality of retinal projections to central visual structures is highly correlated with the lateral placement of the eyes on the head. For example, mammals such as rodents with very limited binocular vision due to laterally positioned eyes show a high degree of contralaterality of the retinal projections to the dorsal lateral geniculate nucleus (LGN). By contrast, the retinal projections of primates and cats that have frontally positioned eyes are not contralaterally dominant, but rather extend an equal number of contralateral and ipsilateral retinal projections to the geniculate body.
The degree of bilaterality of the RHT demonstrates no such correlation to the lateralization or frontalization of the eyes. Perhaps the most striking example is that of the scaly anteater whose laterally placed eyes results in a total binocular field of only 15°, a feature that is reflected in the optic chiasm where greater than 99% of retinal fibers cross the midline and project to the contralateral lateral geniculate. However, both eyes project an equal number of axons to each SCN. As previously mentioned, primates, due to very frontalized eyes, have an extensive binocular visual field and accordingly, have equally weighted bilateral projections to central visual sites. This is in stark contrast to the projections to the SCN which are heavily ipsilaterally weighted. For example, in the gibbon, almost 90% of fibers emerging from one retina project to the ipsilateral SCN, whereas visual projections are balanced [5].
In the hamster, about 5% of the RGCs of the RHT bifurcate and send axonal collaterals to both SCN. In addition, some RGCs that project to the SCN via the RHT also send axonal collaterals to other non-visual retinorecipient sites in the brain such as the 10.1007/978-3-540-29678-2_9 and the 10.1007/978-3-540-29678-2_15. Whether this is a common feature across all mammals remains to be determined [4]. It should be noted that while the SCN is the primary and best-studied target of the RHT, other hypothalamic sites receive RHT innervation. These include the retrochiasmatic area, the subparaventricular zone, the perisupraoptic nucleus and the lateral hypothalamus [2,3]. Understanding how these regions mediate responses to light is a subject of ongoing investigation.
Neurotransmitters
There is abundant evidence that 10.1007/978-3-540-29678-2_7 is the primary neurotransmitter of the RHT [6,7]. Among this evidence is the presence of anti-glutamate immunoreactivity in presynaptic terminals within the SCN of rat and mouse. Furthermore, glutamate release can be induced in the SCN by electrically stimulating the optic nerve and glutamate application mimics that effect of light on the SCN. However, some investigators have observed that application of glutamate onto the SCN does not accurately mimic the circadian phase shifting effect of light, although administration of the glutamate agonist N-methyl-D-aspartate (NMDA) does indeed mimic light and these effects can be blocked by NMDA antagonists. Intraperitoneal injection of MK801, a competitive NMDA receptor antagonist, blocks the phase-shifting effect of light on mouse circadian locomotor rhythms [4].
A hallmark of the lateral geniculate, a well-characterized target of glutamatergic neurotransmission, is an abundance of the 10.1007/978-3-540-29678-2_7 vesicular transporters VGluT1 and VGluT2. The SCN, however, shows low expression of both of these proteins. The presence of glutamate receptors in the SCN, however, reinforces a prominent role for glutamate as the primary neurotransmitter of the RHT. In addition to NMDA receptors, ionotropic receptors sensitive for the glutamate agonist α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) are found throughout the SCN. In particular, receptors composed of the GluR2 subunits are localized to the ventrolateral aspect of the SCN where most axonal terminals of RGCs are found. It must be considered that glutamate receptors are frequently expressed in astrocytes, thus raising the possibility that glutamate functions indirectly as a neuromodulator. Alternatively, astrocytes themselves may serve as a source of glutamate, released in response to some other signal originating from the RHT. Finally, derivatives of glutamate, such as N-acetylaspartylglutamate (NAAG) may play a role in RHT neurotransmission. NAAG is released from RGC terminals in a calcium-dependent manner and can be converted to its constituent amino acids within the synaptic cleft. The prospect of NAAG as a primary RHT neurotransmitter is diminished by the fact that it is only found in a fraction of the retinorecipient aspect of the SCN [4].
Nitric oxide (NO) also plays a critical role in transferring information about environmental light levels from the retina to the SCN. The phase shifting effect of light (or glutamate) on SCN slice preparations can be faithfully mimicked by NO generators such as sodium nitroprusside. Moreover, these effects are blocked by the application of the competitive nitric oxide synthase (NOS) inhibitor N G-nitro-L-arginine methyl ester (L-NAME). This inhibition, however, can be reversed by increasing the availability of L-arginine, the natural substrate of NOS. The emerging evidence suggests that glutamate released from the presynaptic terminals of retinal afferents binds to NMDA receptors in postsynaptic SCN cells. This, in turn, results in a transient increase in intracellular calcium concentrations, thereby leading to activation of nNOS and the subsequent production of NO [4,8]. Because of the inherent instability of NO, such a mechanism could provide very fine spatial and temporal resolution to signaling occurring at the synapses of retinal afferents.
10.1007/978-3-540-29678-2_16 is one of a handful of peptide transmitters that may modulate the glutamatergic-based signaling of the RHT. PACAP is colocalized with glutamate and it has been suggested that all melanopsin-containing RGCs express PACAP [9]. Evidence from other systems where a small-molecule and a peptide neurotransmitter coexist in presynaptic terminals has shown that the small molecule transmitter is released upon weak or transient presynaptic stimulation. Stronger tonic stimulation results in release of both classes of transmitter, thereby encoding a broad dynamic range of stimulus strength. Similarly, weak retinal illumination may result in the release of glutamate from RHT terminals, while higher levels of light induce the release of PACAP.
Several labs have conducted experiments where PACAP has been applied in vitro to SCN slices or infused in vivo to hamsters. These experiments have not produced a consensus regarding PACAP’s role in RHT neurotransmission. Additionally, mice null for PACAP show modest circadian effects. They show no re-entrainment deficits when exposed to a shifted light:dark cycle, no diminution in the lengthening of circadian period in response to constant light, and no loss of 10.1007/978-3-540-29678-2_13 behavior, i.e., acute light-induced suppression of nocturnal locomotor activity. However, these animals did exhibit attenuated circadian responses to 10.1007/978-3-540-29678-2_16 or 10.1007/978-3-540-29678-2_16 pulses of light and they displayed a modestly shortened 10.1007/978-3-540-29678-2_6 circadian 10.1007/978-3-540-29678-2_16. The PACAP-specific receptor PAC1 has been knocked out in mice. The behavioral phenotype of these mice is rather subtle; they exhibit light-induced phase delays of circadian activity rhythms that are about 30% longer than wild-type mice [4]. By contrast, mice null for the VPAC2 receptor, which binds PACAP and VIP, are 10.1007/978-3-540-29678-2_1. This dramatic phenotype, however, is difficult to attribute to PACAP signaling because of the non-specificity of the receptor [10].
Finally, the tachykinin, substance P (SP), has been implicated as a neuromodulator of the RHT. This implication has been challenged because localization of SP to any RGCs, much less RGCs that comprise the RHT, has been equivocal. It has been reported that SP does not colocalize with PACAP in the retina and that bilateral enucleation does not abolish SP immunoreactivity. SP’s proposed neuromodulatory role in RHT transmission remains to be shown conclusively [4].
Concluding Remarks
The identification and characterization of ipRGCs has provided new insight into a major constituent of the RHT. The specifics of how information is transferred from the retinal afferents to SCN neurons, and how this information is subsequently processed by these neurons remains a critical gap in our body of knowledge. However, it also represents a fertile field for future investigations.
Retinotopic
Definition
Topographic arrangement of visual pathways and visual centers that reflects the spatial organization of the neurons responding to visual stimuli in the retina.
10.1007/978-3-540-29678-2_5
Retinotopic Frame of Reference
Definition
Also, “Oculocentric frame of reference.” A reference frame specifying the location of a visual target with respect to the position of the eyes in space.
Retinotopy
Definition
The visual field is represented in orderly maps in occipital cortex in retinal centered coordinates. The left half of the visual field is represented in the right hemisphere and vice versa, whereas the upper visual field is mapped to the inferior occipital cortex (below the calcarine sulcus) and vice versa. Therefore, when subjects hold their eye position constant (e.g. when they maintain central fixation), the region of occipital cortex that responds to a particular stimulus in the visual field can be determined within these maps. This is known as the retinotopic representation of the visual stimulus.
10.1007/978-3-540-29678-2_19
10.1007/978-3-540-29678-2_22
10.1007/978-3-540-29678-2_22
Retroambiguus Nucleus
Synonyms
Nucl. Retroambiguus; Retro-ambiguus nucleus
Definition
Nuclear region of the myelencephalon continuing to the upper cervical cord and integrated in cardiorespiratory functions.
10.1007/978-3-540-29678-2_13
Prosencephalon
Retrograde Amnesia
Definition
There is memory loss for events prior to the incident (e.g., trauma), but memories from the distant past and the period following the incident are intact.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_13
Retrograde Degeneration
10.1007/978-3-540-29678-2_3
Retrograde Interference
Definition
The disruption of transfer from short- to long-term memory by distractions introduced after the initial items are acquired.
Retrograde Messenger
Definition
Retrograde messenger is a chemical substance that is released from postsynaptic neurons and acts on presynaptic neurons. In the nervous system, information coded by action potentials is transferred from neuron to neuron at a specialized site called “synapse” (Fig. 1). The transmission at 10.1007/978-3-540-29678-2_3 is generally one-directional. Neurotransmitters are released from presynaptic terminals on the arrival of action potentials, and transmit a signal to postsynaptic neurons by activating the corresponding receptors (Fig. 1a). In contrast to this fundamental anterograde information transfer, the signaling from postsynaptic to presynaptic neurons is called retrograde signaling (Fig. 1b) [1,2]. The retrograde signaling can be mediated by either a diffusible factor that is called “retrograde messenger,” or a direct interaction of presynaptic and postsynaptic membrane-bound elements. In typical cases, a retrograde messenger is released from the postsynaptic site lacking morphologically specialized structures for release (e.g. 10.1007/978-3-540-29678-2_1), activates the receptors located on presynaptic terminals, and influences the function of presynaptic terminals (i.e. transmitter release) (Fig. 1b).
Retrograde Messenger. Figure 1.

Synaptic transmission and retrograde signaling. In synaptic transmission (a), neurotransmitters are released from presynaptic terminals, and bind to postsynaptic receptors. In retrograde signaling (b), retrograde messengers are released from postsynaptic neurons, and activate presynaptic receptors.
Characteristics
Quantitative Description
The substances so far proposed as retrograde messengers are 10.1007/978-3-540-29678-2_3 (endocannabinoids) [3], 10.1007/978-3-540-29678-2_14 (NO), 10.1007/978-3-540-29678-2_3 (CO), arachidonic acid, platelet-activating factor, neurotrophic factors, and some classical neurotransmitters or neuropeptides [2]. Among them, endocannabinoids are the most widely accepted substances as retrograde messengers in the brain. Major endocannabinoids are arachidonoylethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG) (Fig. 2).
Retrograde Messenger. Figure 2.

Chemical structures of two major endocannabinoids.
Anandamide is the amide of arachidonic acid with ethanolamine, and the molecular weight is 347.54. 2-AG is the glycerol derivative in which the second hydroxyl group is linked to arachidonic acid residue by an ester bond, and the molecular weight is 378.55. The structural features of endocannabinoids are quite different from classical neurotransmitters, and shared by lipid messengers such as eicosanoids, which mediate signals of inflammation and pain.
Lower Level Components
Arachidonoylethanolamide
Arachidonoylethanolamide, known as “anandamide,” was the first endocannabinoid to be identified. The name of anandamide is based on the Sanskrit word for bliss and tranquility, ananda. Anandamide binds to both CB1 and CB2 10.1007/978-3-540-29678-2_3, but displays lower affinity for CB2 compared to CB1 receptors.
2-Arachidonylglycerol
2-Arachidonoylglycerol (2-AG) is another major endocannabinoid, and binds to both CB1 and CB2 receptors. 2-AG is widely distributed in the brain and periphery. The level of 2-AG is reported to be much higher (ca. 170 times) than anandamide in brain tissues. This molecule is the most likely candidate for the endocannabinoid that mediates retrograde synaptic modulation at hippocampal and cerebellar synapses.
Other Putative Endocannabinoids
Other putative endocannabinoids include noladin ether and virodhamine. Noladin ether is an ether-linked analogue of 2-AG. Virodhamine is the ester of arachidonic acid with ethanolamine. It is not determined whether these molecules actually mediate retrograde signals.
Higher Level Processes
Interneuronal Communication
In the anterograde signaling, classical neurotransmitters, neuropeptides, neurotrophic factors, and some other substances are released from presynaptic terminals, and produce rapid changes in membrane potential (i.e. generation of postsynaptic potentials) as well as long-term structural and metabolic changes in the postsynaptic cells. In the retrograde signaling, retrograde messengers including some of the substances used for the anterograde signaling are released from postsynaptic cells, and influence the function or morphology of presynaptic neurons. In addition to these diffusible factors, direct interactions of presynaptic and postsynaptic membrane-bound elements (e.g. cadherins) are also involved in interneuronal communication between presynaptic and postsynaptic neurons.
Lower Level Processes
Synthesis of Endocannabinoids (Anandamide and 2-AG)
Anandamide formation in neurons is a two-step process. The first step is the transfer of an arachidonic acid group from the sn-1 position of phosphatidylcholine to the head group of phosphatidylethanolamine (PE) by the enzyme N-acyltransferase, resulting in the formation of N-arachidonyl-PE. The second step is the cleavage of N-arachidonyl-PE by phospholipase D (PLD), which produces anandamide and phosphatidic acid (Fig. 3a) 2-AG is formed through two distinct pathways. The first step of the main pathway is the cleavage of phosphatidylinositol (PI) by the enzyme PI-specific phospholipase C (PI-PLC), producing 1,2-diacylglycerol (DAG). The second step is the further cleavage of DAG by DAG lipase, yielding 2-AG (Fig. 3b). The alternative pathway involves phospholipase A1 and lyso-PLC. The PLD and DAG lipases responsible for endocannabinoid formation have been identified. Among several types of PI-PLC, β type PLC (PLCβ) is the most important for 2-AG formation.
Retrograde Messenger. Figure 3.

Pathways of formation and degradation of two major endocannabinoids. PLD phospholipase D; FAAH fatty acid amide hydrolase; PLC phospholipase C; PLA 1 Phospholipase A1; DAG diacylglycerol; MGL monoacylglycerol lipase.
Breakdown of Endocannabinoids (Anandamide and 2-AG)
Anandamide is broken down into arachidonic acid and ethanolamine by the enzyme fatty acid amide hydrolase (FAAH) (Fig. 3a). 2-AG is broken down into arachidonic acid and glycerol by the enzyme monoacylglycerol lipase (MGL) (Fig. 3b). These enzymes have been identified. They exhibit unique distributions in the brain. In general, FAAH is a postsynaptic enzyme, whereas MGL is presynaptic.
CB1 Receptor Signaling at Presynaptic Terminals
The CB1 receptor is densely distributed on presynaptic axons and terminals in various regions of the brain. They include excitatory synapses on cerebellar Purkinje cells (both climbing fiber and parallel fiber synapses), inhibitory synapses on cerebellar Purkinje cells, part of hippocampal inhibitory synapses including CCK-positive basket cell to pyramidal cell synapses, and inhibitory synapses from the striatum to globus pallidus. Activation of presynaptic CB1 receptors suppresses the release of the transmitters (glutamate or GABA). This CB1-mediated suppression can be caused by inhibition of voltage-gated Ca2+ channels, activation of K+ channels, direct effect on release machinery, or some other unknown mechanisms.
Process Regulation
The release of retrograde messengers from postsynaptic neurons is generally controlled by neural activity. For example, endocannabinoids are produced on demand in response to depolarization-induced elevation of intracellular Ca2+ concentration [4,5], or activation of Gq-coupled receptors such as group I metabotropic glutamate receptors [6] and M1/M3 muscarinic receptors [7] (Fig. 4).As endocannabinoids are membrane-permeable, they are considered to diffuse across the plasma membrane to the extracellular space immediately after production (Fig. 4).
Retrograde Messenger. Figure 4.

Endocannabinoid-mediated retrograde signaling. Endocannabinoid production is induced by postsynaptic depolarization-triggered Ca2+ influx, or activation of Gq-coupled receptors such as group I metabotropic glutamate receptors (I-mGluRs) and M1/M3 muscarinic receptors. The endocannabinoids that are produced are then released from postsynaptic neurons, and activate presynaptic CB1 receptors to suppress the transmitter release.
Endocannabinoids are produced much more effectively when Ca2+ elevation and receptor activation coincide [8]. The released endocannabinoids then work as retrograde messengers (Fig. 4), and are removed from the extracellular space through uptake and enzymatic degradation. Other membrane-permeable factors such as NO and CO can be produced in response to certain conditions, and released to the extracellular space. In contrast, membrane-impermeable factors including classical neurotransmitters and neuropeptides are supposed to be stored in vesicles, and secreted through exocytotic mechanisms in response to certain triggering stimuli such as an elevation of intracellular Ca2+ concentration.
Function
Retrograde messengers play important roles in formation, maturation, and plasticity of synaptic connections. Among them, most attention has been focused on their crucial roles in activity-dependent modulation of synaptic transmission, including both short-term and long-term forms of synaptic plasticity.
Endocannabinoids mediate retrograde signals involved in several forms of short-term synaptic plasticity including DSI, DSE, and receptor-driven retrograde suppression. The CB1 receptor is widely distributed in the brain, and located densely on many, but not all, types of presynaptic terminals. At CB1-expressing synapses, endocannabinoids released from the postsynaptic neurons activate presynaptic CB1 receptors, and thereby suppress the transmitter release. Endocannabinoid release is triggered by elevation of intracellular Ca2+ concentration, activation of Gq-coupled receptors, or combination of the two [8] (Fig. 4). Under physiological conditions, endocannabinoid-mediated retrograde suppression is triggered by synaptic activity that can produce postsynaptic Ca2+ elevation and Gq-coupled receptor activation. Thus, the endocannabinoid-mediated retrograde suppression provides a feedback mechanism, by which the postsynaptic neurons receiving synaptic inputs can retrogradely influence the function of CB1-expressing presynaptic terminals. This endocannabinoid-mediated retrograde suppression is reversible and thus classified as a form of short-term synaptic plasticity. However, endocannabinoids are also involved in long-term synaptic plasticity, especially long-term depression (LTD), in some brain regions including the striatum and amygdala [9,10].
It has been reported that dopamine can be released from dendrites of dopaminergic neurons. Some other classical neurotransmitters or neuropeptides are also proposed to be released from dendrites [2]. They include glutamate, GABA, and dynorphin. Although the actual release mechanisms of these substances are not fully elucidated, it is likely that these substances also contribute to short-term plasticity.
Synaptic activity can induce long-term potentiation (LTP) or depression (LTD) depending on the pattern of activity. Membrane-permeable factors such as NO, CO, arachidonic acid and platelet-activating factor have been proposed to contribute to long-term synaptic plasticity, especially LTP. However, there are many controversial results, and general consensus has not yet been reached as to the possible roles of these factors as retrograde messengers in long-term synaptic plasticity.
Retrograde messengers also play important roles in formation, maturation and refinement of synaptic connections, especially at early developmental stages. These processes require information exchange between presynaptic and postsynaptic cells through anterograde and retrograde signals. Although molecular mechanisms of these signals are not clearly elucidated, it has been proposed that neurotrophic factors, including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), could play important roles in these processes.
Therapy
Cannabinoids for Therapies
Marijuana, which contains the natural cannabinoid Δ9-tetrahydrocannabinol, as well as several commercially-available synthetic cannabinoids (nabilone and dronabinol) are clinically effective for several disorders such as nausea from cancer chemotherapy, chronic pain, and exhaustion in AIDS patients. However, these drugs also have psychoactive side effects such as dizziness and thinking abnormalities. They are inherent problems, because the CB1 receptor is widely distributed in the brain. To avoid these problems, it has been attempted to develop drugs that enhance the endocannabinoid signaling in a target-specific manner.
Retrograde Neuron Reaction
10.1007/978-3-540-29678-2_14
Retrograde Tracing
Definition
A method for visualizing the neurons of origin of an axonal pathway. A dye is injected into the region of the nervous system where axons are thought to terminate. The dye is absorbed by the axons and transported back to the cell bodies allowing them to be visualized.
Retrograde Tracing Techniques
Definition
Neuron tracing techniques take advantage of the fact that axoplasmic transport of materials goes in both directions within the axon. Retrograde flow of materials is toward the cell body. Early studies of retrograde transport used the enzyme horseradish peroxidase (HRP), which after injection into nerve tissue is picked up at axon terminals by micropinocytosis. The HRP is transported back to the cell body of the neuron where it can be made visible with a variety of chemical reactions. More recent studies use retrograde immunocytochemical and fluorescent labeling techniques to visualize neuron morphology.
Retrograde Transport
Definition
Retrograde transport is a process, mediated by the microtubule motor dynein, by which chemical messages are sent from the axon back to the cell. Fast retrograde transport can cover over 100 mm/day.
Retronasal/Orthonasal Olfaction
Definition
Orthonasal smell perception occurs when volatile molecules are pumped in through the external nares of the nose and activate the sensory cells in the olfactory epithelium. This is the route used to sense odors in the environment.
Retronasal stimulation occurs during food ingestion, when volatile molecules released from the food in the mouth are pumped, by movements of the mouth, from the back of the oral cavity up through the nasopharynx to the olfactory epithelium. It is activated only when breathing out through the nose between mastications or swallowings. This is the route used to sense aromas of food.
10.1007/978-3-540-29678-2_6
10.1007/978-3-540-29678-2_15
Retrospective Monitoring
Definition
Retrospective monitoring refers to metamemory experiences when one searches for and retrieves the origin and content of information stored in long-term memory.
It has been studied by examining the experiences of “tip-of-the-tongue” (TOT), which one has when the information one tries to remember feels like right on the edge of the tongue), and of “feeling-of-knowing” (FOK).
10.1007/978-3-540-29678-2_13
Retroviral Vectors
Definition
These are retroviruses used to insert novel genes into neurons or other cells by infecting them.
Rev-erbα
Definition
Member of the nuclear hormone receptors with hemin as a potential ligand. Identified as the main circadian repressor of the Clock and Bmal1 genes. Nuclear orphan receptor that binds to the consensus sequence ([A/T]A[A/T]NT[A/G]GGTCAtermedRORE, in the promoter of target genes. The gene is transcribed from the opposite strand of the erba gene, which is a cellular homolog of the viral oncogene v-erbA.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_3
Reverberation
Definition
The persistence of sound in an enclosed space, as a result of multiple reflections, after the sound from the source has stopped.
10.1007/978-3-540-29678-2_1
Reverberation Time
Definition
The time between the offset of the originating sound and when the reverberant sound remaining in the enclosed space is 60 dB (a factor of 1,000 in pressure) less than the level of the originating sound.
10.1007/978-3-540-29678-2_1
Reversal Learning
Definition
In reversal learning, a particular discrimination task is first learned and then the reinforcement contingencies are reversed. In other words, once the subject has learned to discriminate a reinforced from a non-reinforced stimulus, it has to learn to reverse its response to such stimuli. Such reversals tend to be difficult since there are negative transfer effects; e.g. the individual tends to persist in responding to the stimulus that was originally reinforced. Eventually, however, this tendency becomes weaker, and the response to the alternative stimulus becomes more frequent until it is consistently evoked.
Reversal Potential
Definition
Reversal potential (also called Nernst potential) is the membrane voltage at which there is no net flow of a particular ion from one side of the membrane to the other.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_19
Reverse Real-Time quantitative PCR (RT-qPCR)
Definition
Complementary DNA (cDNA) is first made from an RNA template, using a reverse transcriptase enzyme. A specific sequence of cDNA is then amplified and the amount of product produced at the end of each PCR cycle is evaluated by measuring signal strength of fluorescent markers. Since PCR generates products at an exponential rate, the relative abundance of template can be compared between samples. Alternatively the absolute abundance of template can be determined if reference dilutions of template are used. RT-qPCR can also refer to Reverse Transcriptase quantitative PCR, Real-time quantitative PCR (PCR).
10.1007/978-3-540-29678-2_19
Reverse Signaling: Nervous System Development
Definition
Reverse signaling refers to the signaling mechanism by which a known membrane-bound ligand also functions as a receptor to trigger intracellular signaling events in the ligand-bearing cell, thereby modifying its behavior. Such dual function of a membrane protein as both ligand and receptor allows the ligand-receptor system to mediate bi-directional signal exchange between two neighboring cells, thus greatly increasing the plasticity of intercellular communications. Two major reverse signaling pathways mediated by ephrin and semaphorin have been implicated in regulating the development of the nervous system.
Characteristics
Description of Reverse Signaling Mechanism
Ephrin Reverse Signaling
Ephrins were originally identified as ligands for the Eph-family receptor tyrosine kinases. Ephrins are divided into A and B subclasses. A subclass ephrins are tethered to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor, while each B subclass ephrin contains a hydrophobic transmembrane segment spanning the plasma membrane followed by a short cytoplasmic domain [1–3]. Based on sequence homology and their preference for binding ephrins, Eph receptor tyrosine kinases are also classified into two subgroups, including ephrin-A-binding Eph-As and ephrin-B-binding EphBs [1–3].
Early studies of the ephrin-Eph interaction focused on understanding the signaling mechanism by which activation of Eph by ephrin modulates downstream signaling proteins to regulate cellular behaviors. This type of Eph-mediated signaling in response to ephrin binding is called 10.1007/978-3-540-29678-2_6 (Fig. 1). Each Eph family member has a highly conserved domain architecture in the cytoplasmic region that is comprised of a juxtamembrane region, a kinase domain, a SAM (sterile α domain) domain and a PDZ-domain binding motif [1–3]. Binding of ephrin to the extracellular region of Eph induces conformational changes, allowing the Eph cytoplasmic domain to modulate the activity and/or subcellular localization of downstream signaling proteins, which then modulate cytoskeletal reorganization leading to an attractive or repulsive response.
Reverse Signaling: Nervous System Development. Figure 1.

Bi-directional signaling mediated by two ligand-receptor systems. Binding of ephrin and semaphorin activates Eph and Plexin forward signaling, respectively. Binding of Eph and Plexin can also activate ephrin and transmembrane semaphorin to trigger reverse signaling, respectively. The identity of cell surface protein (colored in blue) that activates semaphorin reverse signaling during neural development is unknown.
Later studies show that the binding between ephrin and Eph is capable of triggering downstream signaling events in both ephrin-expressing- (i.e., reverse signaling) and Eph-expressing (i.e., forward signaling) cells (Fig. 1), and thus mediates bi-directional signaling [1–3]. The action of ephrins as receptors to mediate reverse signaling relies on their ability to modulate the activity and/or subcellular localization of downstream signaling proteins in response to Ephs. EphrinBs can utilize both phospho-tyrosine residues and the PDZ domain-binding motif in its cytoplasmic domain to recruit downstream signaling proteins. For instance, Eph-induced ephrin-B1 phosphorylation of the conserved tyrosine residues provides a docking site for the 10.1007/978-3-540-29678-2_19 of the adaptor protein Grb4 [4]. Grb4 in turn links ephrin-B1 to multiple signaling pathways through the binding of its multiple 10.1007/978-3-540-29678-2_19 to proline-rich proteins (e.g., Abl-interaction protein-1 (Abi-1), c-Cbl-associated protein (CAP) and scaffolding protein axin), thus modulating 10.1007/978-3-540-29678-2_6 and cytoskeletal reorganization [1,3]. Ephrin-Bs can also recruit downstream signaling proteins (e.g., glutamate-receptor-interacting-protein-1 (GRIP1), GRIP2, systenin, protein kinase C-interacting protein-1 (PICK1), tyrosine phophatase PTP-BL, and PDZ-RGS3) in a phosphorylation-independent way via the PDZ domain-binding motif in their C-terminus [1,3]. Ephrin-B-mediated reverse signaling can also directly stimulate the enzymatic activity of Fak (focal adhesion kinase) to regulate focal adhesion [5].
Although ephrin-As do not have a cytoplasmic domain, they are also capable of mediating reverse signaling in response to Eph binding [1–3]. Ephrin-As are localized to 10.1007/978-3-540-29678-2_12, specific plasma membrane microdomains consisting of glycosphingo-lipids and cholesterol, where ephrin-As presumably associate with other membrane protein complexes. The activation of ephrin-As can lead to the recruitment of intracellular signaling proteins such as the 10.1007/978-3-540-29678-2_19 family kinase Fyn to lipid rafts, which in turn regulates downstream proteins to modulate cytoskeletal reorganization and cell adhesion [6].
Semaphorin Reverse Signaling
Semaphorins are a large family of secreted and transmembrane proteins that share a conserved ~500 amino-acid Semaphorin (Sema) domain at the amino terminus [7]. Semaphorins can also mediate bi-directional signaling. Semaphorins mediate forward signaling by functioning as ligands to bind and activate their receptors Plexin and 10.1007/978-3-540-29678-2_14, which in turn initiate a cascade of signaling events in Plexin and/or neuropilin-expressing cells to regulate cytoskeletal changes for directed axonal growth and cell movement. Like ephrin-Bs, some transmembrane semaphorins can also mediate reverse signaling by utilizing their cytoplasmic domains to recruit intracellular signaling proteins (Fig. 1). For instance, binding of Plexin-A1 to the extracellular region of Semaphorin-6D increases the association of its cytoplasmic domain with the Abl tyrosine kinase, but decreases its association with Mena, a member of the enabled (Ena)/vasodilator-stimulated phosphoprotein (Vasp) family proteins [7]. In Drosophila, it also appears likely that direct association of the transmembrane semaphorin-1a via its cytoplasmic domain with the fruitfly Enabled protein mediates semaphorin-1a-dependent reverse signaling [8]. The changes in the activity and/or localization of these semaphorin-associating intracellular signaling proteins contribute to the cytoskeletal reorganization necessary for cell migration and axonal projections.
Function of Reverse Signaling in Neural Development
The function of reverse signaling in a ligand-receptor system mediating bi-directional signaling in neural development can be specifically assessed in several ways [2]. For instance, wild-type ligand or receptor in mice would be replaced with a mutant version incapable of interacting with intracellular signaling proteins by gene targeting, thus selectively inactivating reverse- or forward signaling, respectively. The contribution of reverse or forward signaling would then be assessed by comparing the phenotype displayed in the above mutants to that caused by the loss of both forward- and reverse signaling that occurs in receptor or ligand null mutants. Reverse signaling can also be selectively inactivated in zebrafish and Xenopus by expressing a dominant-negative version of a transmembrane ligand in which the cytoplasmic domain mediating reverse signaling is deleted. The dominant-negative mutant is still able to mediate forward signaling through binding to its receptor, but interferes with reverse signaling through competing with wild-type counterpart for receptor binding. In Drosophila, the contribution of reverse signaling can be determined by assessing whether null ligand mutants are rescued by expression of a mutant ligand that is capable of activating its receptor but defective in reverse signaling. The phenotype that is not rescued by the reverse-signaling-defective mutant ligand likely reflects the function of reverse signaling mediated by this ligand.
Ephrin Reverse Signaling in the Vertebrate Neural Development
The Formation of Anterior Commissure Tract
The projection of both acP axon tract and acA axon tract of 10.1007/978-3-540-29678-2_1 in mice requires ephrin reverse signaling [1,2]. Both ephrin-B2 and Eph-B2 are required for the guidance of acP axons. However, ephrin-B2 is expressed in acP axons and functions in a cell-autonomous manner, whereas Eph-B2 is expressed in cells underlying acP axons and is required non-cell-autonomously for the projection of acP axons. That the guidance of acP axons requires an intact cytoplasmic domain of ephrin-B but not the kinase domain of Eph-B2 supports the involvement of ephrin-B2 reverse signaling in the guidance of acP axons. The activation of ephrin-B2 reverse signaling by Eph-B2 appears to initiate a repulsive response that guides acP axons toward the midline. The guidance of both acP and acA axons also requires the activation of ephrin-A reverse signaling by Eph-A4, which functions non-cell-autonomously as a ligand to attract acP and acA axons. The identity of the ephrin activated by Eph-B4 in acP and acA axons remains unclear.
Retinotectal Mapping Along Dorsal-ventral Axis
In the vertebrate visual system, retinal ganglion cells in the eye project axons into the optic tectum in a topographic fashion along both anterior-posterior and dorsal-ventral axes. While topographic projections of retinal ganglion axons along the anterior-posterior axis are directed by the ephrin-As-Eph-As forward signaling [1,3], dorsoventral topographic projections appear to require the ephrin-B-mediated reverse signaling [2]. In Xenopus, retinal ganglion cell axons display a decreasing dorsal-to-ventral expression gradient of ephrin-B2 and B3 in the retina, while Eph-B1 shows a complementary expression pattern (i.e., decreasing ventral-to-dorsal gradient) on cells in the optic tectum. In vivo and in vitro studies suggest strongly that Eph-B1 in the ventral tectum activates ephrin-B2 and B3 expressed on dorsal retinal ganglion axons to initiate reverse signaling leading to an attractive response, which targets dorsal ganglion axons toward the ventral tectum.
Establishment of Vomeronasal Map
The vomeronasal organ (VNO) is involved in detecting pheromones. VNO axons projected from the vomeronasal epithelium are targeted topographically to specific glomeruli comprised of sensory projections in the accessory olfactory bulb (AOB) during embryonic development. Topographic projections of VNO axons appear to involve an attractive response mediated by ephrin-A5 reverse signaling when activated by Eph-A6 [2]. Ephrin-A5 is expressed differentially in VNO axons and required for their topographic projections. The difference in the expression level of ephrin-A5 appears to dictate onto which regions of the accessory olfactory bulb VNO axons are targeted: i.e., axons with higher level of ephrin-A5 are targeted to regions with higher level of Eph-A6 and vice versa.
Semaphorin Reverse Signaling in Neural Development in Drosophila
Several recent studies, including our own, suggest strongly that transmembrane semaphorin-1a-mediated reverse signaing plays an important role in regulating neural development in Drosophila [8–10]. While it has been shown that reverse signaling mediated by transmembrane semaphorin-6D is required for the guidance of myocardial patterning in vertebrates [7], future studies are needed to determine if semaphorin reverse signaling is also involved in regulating the vertebrate neural development.
The Formation of Giant-fiber-motor Neuron Synapse in Drosophila
The giant fiber system of Drosophila is involved in controlling the jump-and-flight response. During development, a giant interneuron in the giant fiber system of the brain projects an axon into the second thoracic segment where the axon forms synapses with a motor neuron, which in turn controls the activity of the jump muscle. Semaphorin-1a is required both pre- and postsynaptically for the formation of giant-fiber-motor neuron synapes, suggesting a role for semaphorin to mediate bi-directional signaling between pre- and postsynaptic partners [8]. Overexpression of wild-type semaphorin-1a, but not a truncated semaphorin-1a mutant protein lacking the cytoplasmic domain, causes a gain-of-function phenotype. These data suggest that the participation of semaphorin-1a in synapse formation involves the action of semaphorin-1a reverse signaling. It remains to be determined whether Plexin or other semaphorin-interacting proteins function as a ligand to activate semaphorin-1a in synapse formation.
Photoreceptor Axon Guidance in Drosophila
In the Drosophila adult visual system, R1-R6 photoreceptors project their axons from the retina to the superficial layer of the optic lobe, the lamina. During development, R1-R6 axons temporally stop at their intermediate target region in between two layers of glial cells prior to establishing synaptic connections with lamina neurons. We found recently that the transmembrane semaphorin-1a functions cell-autonomously in photoreceptor axons for the proper arrangement at the intermediate target region [9]. The function of semaphorin-1a in photoreceptor axons requires its cytoplasmic domain, consistent with a role for semaphorin-1a as a receptor to mediate reverse signaling. The identity of cell surface proteins that activate semaphorin-1a reverse signaling in photoreceptor axons remains to be determined.
Dendritic Targeting of Olfactory Projection Neurons in Drosophila
In the development of the Drosophila olfactory system, projection neurons project their dendrites onto discrete units called glomeruli in the 10.1007/978-3-540-29678-2_1, the first olfactory information relay center equivalent to olfactory bulb in vertebrates. Different types of odorant receptor axons form one-to-one precise connections to dendrites projected from different types of projection neurons at glomeruli. Semaphorin-1a displays a differential expression pattern on dendrites of projection neurons. Genetic analysis showed that semaphorin-1a is required differentially in projection neurons for the targeting of their dendrites onto discrete glomeruli in a cell-autonomous manner, indicating a role for semaphorin-1a as a receptor for dendritic targeting [10]. Consistently, the cytoplasmic domain of semaphorin-1a is shown to be indispensable for its function, which likely reflects its role in recruiting downstream signaling proteins within the dendrites of projection neurons. The guidance cue that activates semaphorin-1a reverse signaling in dendrites is unknown.
Reward
Definition
A reward or positive reinforcer is any stimulus an animal will work to obtain. Often these stimuli have biological significance to the animal such as food, shelter or sex, a class of stimuli sometimes referred to as primary or unlearned reinforcers. Other types of reward are initially affectively neutral but acquire value through being associated with a primary reinforcer.
An example of such a stimulus for humans is money which acquires value by virtue of its capacity to be exchanged for other kinds of primary reinforcers such as food or shelter.
10.1007/978-3-540-29678-2_22
Reward Signal in Neural Networks
Definition
A scalar performance measure used for reinforcement learning of networks.
10.1007/978-3-540-29678-2_14
RFLP
Definition
Restriction fragment length polymorphism. These are polymorphisms that change restriction sites. RFLPs with known chromosomal locations were used in linkage analysis, with Southern blotting, to map disease genes until the advent of microsatellite markers.
10.1007/978-3-540-29678-2_2
Rheobase
Definition
Strength of a rectangular depolarizing direct current (DC) current necessary to elicit an action potential.
10.1007/978-3-540-29678-2_1
Rheological Models
Definition
A rheological model consists of an assembly of one-dimensional rheological elements, each of which can be seen as a black box with two protruding terminals.
Description of the Theory
Each rheological element is characterized by a deterministic relationship between the (history of the) relative displacement (or elongation) u(t) between the terminals and the (history of the) applied force f(t). In other words, knowing the displacement function u(t) for all past times t up to the present, the present value of the force can be uniquely determined by some mathematical operation or vice versa. The merit of this one-dimensional oversimplification of the complexity of a true continuum constitutive theory (q.v.) lies in the fact that, by combining a small number of rheological elements in series and in parallel, a wealth of mathematically tractable, surprisingly varied and suggestive force-elongation responses is obtained. Two rheological elements are said to be connected in series if one of the terminals of the first is connected to one of the terminals of the second, in such a way that the remaining two unconnected terminals are considered as the terminals of the new combined-element black box. Let [f 1(t), u 1(t)] and [f 2(t), u 2(t)] denote, respectively, the force-elongation pairs of the first and second elements connected in series. The main feature of a series combination is that both elements experience the same force, while the elongation of the combined black box is the sum of the elongations of the original elements. Denoting by [f(t), u(t)] the force-elongation pair of the combined series black box:
 |
(1) |
Two elements are said to be connected in parallel if each terminal of one element is connected to a counterpart in the other element. The two common terminals thus obtained are considered as the terminals of the combined black box. As a result, both elements experience necessarily the same elongation, while the forces are added to produce the response of the combined element. Using the same notation as before, for the 10.1007/978-3-540-29678-2_16:
 |
(2) |
Some of the most common elements in use are: the 10.1007/978-3-540-29678-2_12, the 10.1007/978-3-540-29678-2_12 (or linear dashpot) and the 10.1007/978-3-540-29678-2_3. The linear spring is characterized by two material constants: the rest length L 0 and the stiffness constant k. By convention, in the linear spring the elongation u is measured with respect to the rest length (in other words, when the elongation vanishes, the distance between the terminals is equal to the rest length). The force at time t is then proportional to the elongation at that time, namely:
 |
(3) |
Thus, the linear spring provides a purely elastic response, whereby the past history of the 10.1007/978-3-540-29678-2_4 plays no role, except for the fact that the material always remembers its “original” rest length. The linear damper, on the other hand, is completely characterized by a single material constant c called the viscous constant. There is no rest length. The force-elongation relation is given by the equation:
 |
(4) |
In a linear damper the only fact that counts in determining the force between the terminals at a given time is the speed of elongation  at that time. In other words, the past history plays a role, albeit limited to the very immediate past. More sophisticated history elements can be defined. Finally, the contractile element is a useful device with important applications to muscle 10.1007/978-3-540-29678-2_13. It can be thought of as a frictionless slider that produces no force, whatever the value of the elongation may be. In muscle mechanics applications, however, it is usually assumed that this behavior is characteristic of the inactive state only and that the contractile element may be activated, so that in the active state the force-elongation response abides by an ad-hoc law (for example, a so-called force-length relation).
at that time. In other words, the past history plays a role, albeit limited to the very immediate past. More sophisticated history elements can be defined. Finally, the contractile element is a useful device with important applications to muscle 10.1007/978-3-540-29678-2_13. It can be thought of as a frictionless slider that produces no force, whatever the value of the elongation may be. In muscle mechanics applications, however, it is usually assumed that this behavior is characteristic of the inactive state only and that the contractile element may be activated, so that in the active state the force-elongation response abides by an ad-hoc law (for example, a so-called force-length relation).
To illustrate the variety of material responses that can be obtained by means of rheological models, the Maxwell model, obtained by placing a linear spring and a linear dashpot in series is considered. It is not difficult to show that the response of the Maxwell model is completely contained in the following first-order ordinary differential equation:
 |
(5) |
If a force f
0 is suddenly applied, an instantaneous elongation of value 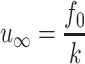 develops completely at the expense of the spring, while the dashpot does not have time to react. As time goes on, however, if the force is kept at a constant value, the elongation will keep growing steadily at the expense of the deformation of the damper. If the force is suddenly removed, the spring goes instantaneously back to its original length, while the damper immediately stops deforming. At the end of the process, therefore, the Maxwell model retains a residual deformation. The response of a system to a suddenly applied load that remains constant in time is known as 10.1007/978-3-540-29678-2_3.
develops completely at the expense of the spring, while the dashpot does not have time to react. As time goes on, however, if the force is kept at a constant value, the elongation will keep growing steadily at the expense of the deformation of the damper. If the force is suddenly removed, the spring goes instantaneously back to its original length, while the damper immediately stops deforming. At the end of the process, therefore, the Maxwell model retains a residual deformation. The response of a system to a suddenly applied load that remains constant in time is known as 10.1007/978-3-540-29678-2_3.
If a linear spring and a damper are combined in parallel, the result is the Voigt model. It is governed by the differential equation:
 |
(6) |
A sudden application of a force is met with no instantaneous response, since the damper cannot react immediately. As time goes on, however, an exponential growth of the elongation is observed which approaches asymptotically the value  . If the force is suddenly removed, the spring will slowly bring back the system to its original rest length. This is the type of behavior observed when sitting on a feather- or down-filled cushion. This effect is sometimes described as “delayed 10.1007/978-3-540-29678-2_5,” although the response is anything but elastic.
. If the force is suddenly removed, the spring will slowly bring back the system to its original rest length. This is the type of behavior observed when sitting on a feather- or down-filled cushion. This effect is sometimes described as “delayed 10.1007/978-3-540-29678-2_5,” although the response is anything but elastic.
A more realistic description of the behavior of many materials is obtained by combining in parallel a Maxwell model with a linear spring. The result is known as the Kelvin model or the standard linear solid. Its creep response is similar to that of a Voigt model, except that, just like a Maxwell model, it also has an instantaneous elastic response, governed by the spring added in parallel.
If instead of subjecting the various models to a sudden force they are subjected to a sudden elongation, the response obtained in terms of the decay of the resulting force as time goes on is known as relaxation.
Rheostasis
Definition
Regulation of the internal environment of an animal to a stable condition with a changing reference setpoint.
10.1007/978-3-540-29678-2_8
Rheumatoid Arthritis (RA)
Definition
RA is traditionally considered a chronic, inflammatory autoimmune disorder that causes the immune system to attack the joints. It is a disabling and painful inflammatory condition, which can lead to substantial loss of mobility due to pain and joint destruction. RA is a systemic disease, often affecting extra-articular tissues throughout the body including the skin, blood vessels, heart, lungs, and muscles.
Rhinencephalon
The olfactory bulb and those structures that receive afferents form the olfactory bulb are classified as being part of the rhinencephalon. They include primarily the olfactory tract and the basal olfactory area, parts of the amygdaloid body, septum verum and prepiriform cortex.
10.1007/978-3-540-29678-2_3
Rhizotomy
Definition
A surgical procedure in which spinal nerve roots are cut.
Rho
Definition
The Greek letter ρ, used to denote the rest phase of the circadian rest-activity cycle. Occurs at night in diurnal animals, and in the day in nocturnal animals.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_3
Rho Family of Small Guanosine Triphosphatases (Rho GTPases)
Definition
Rho family of small guanosine triphosphatases (Rho GTPases) are important intracellular signaling proteins involved in various aspects of neuronal morphogenesis including migration, polarity, axon growth and guidance, dendrite arborization, spine plasticity, and synapse formation. Acting as intramolecular switches, the Rho GTPases transduce signals from various extracellular ligands to the cytoskeleton. They exist in two states: a GTP-bound active state, and a GDP-bound inactive state. Guanine nucleotide exchange factors turn on Rho GTPases by facilitating the exchange of GDP for GTP, and GTPase activating proteins increase their GTPase activity, helping to turn them off.
10.1007/978-3-540-29678-2_3
10.1007/978-3-540-29678-2_7
10.1007/978-3-540-29678-2_14
Rho GTPases
Synonyms
Rho Family of Small GTP-Binding Proteins
Definition
The Rho family of small GTP-binding proteins consists of 22 mammalian proteins related to each other based on the similarity of their amino acid sequence to the first family member to be identified, RhoA. These proteins are relatively small (less than 25 kDa) and all possess an intrinsic GTPase activity, which hydrolyzes the guanosine triphosphate (GTP) into guanosine diphosphate (GDP). The bound nucleotide regulates the activity of the GTPase, rendering it inactive or active in the case of GDP or GTP, respectively.
Characteristics
The Rho GTPases are expressed in all cells from fertilization through adulthood and their activity is critical to many aspects of cell biology that are required for normal functioning of the organism [1]. When active, the Rho GTPases can activate a diverse array of intracellular signaling pathways. RhoA, Rac1, and Cdc42, the best characterized members of the Rho family of small GTPases, regulate the assembly of the 10.1007/978-3-540-29678-2_1 and 10.1007/978-3-540-29678-2_13 cytoskeleton, a filamentous network of proteins within a cell that control cell shape, cell adhesion, cell polarity and cell migration. Specificity of Rho GTPase signaling is achieved by the coordinated regulation of the nucleotide bound state of the GTPase and the region of the cell to which the active form of the protein is targeted.
The Cycling of GTP and GDP Regulates Rho GTPase Activity
Each of these monomeric GTPases acts as a molecular switch to control the downstream signaling pathways. The cycling of GDP-GTP binding to Rho GTPases is tightly regulated by three families of proteins. The GTPase is unable to exchange the bound GDP for free GTP in the cytosol without the help of proteins termed 10.1007/978-3-540-29678-2_3. In response to external stimuli, GEFs are activated and bind the GDP-bound conformation of Rho GTPases leading to the release of GDP. Then, the Rho GTPases in a free-nucleotide transition state are able to bind GTP in the cytosol and activate downstream effectors in their active GTP-bound conformation. The activity is terminated by 10.1007/978-3-540-29678-2_7, which enhance the intrinsic GTPase activity, leading to the inactive state of the GTPase. Additionally, guanine nucleotide dissociation inhibitors (GDIs) can sequester Rho GTPases in their GDP-bound, inactive state (Fig. 1).
Rho GTPases. Figure 1.

Cycling of Rho GTPases. Rho GTPases exist in either an inactive, GDP-bound state or an active, GTP-bound state. Three families of proteins tightly regulate this GDP/GTP cycle. GEFs stimulate the exchange of GDP for GTP, thereby activating the GTPases. GAPs enhance the intrinsic GTPase activity, leading to the inactive GDP-bound form. GDIs bind to the GDP-bound GTPase and sequester the protein in the cytosol.
Subcellular Localization of Rho GTPases
While inactive GTPases are usually restricted to the cytoplasm, the active forms are localized to the surface of membranes within the cell, such as plasma, golgi, or endosomal membranes. The correct intracellular localization of active GTPases is critical for their regulation and coupling to downstream signaling pathways. The targeting information is contained in the CAAX box present at the C-terminal end of the GTPase amino acid sequence [2]. This sequence is modified by the addition of an isoprenyl group on the side chain of the cysteine, which is then able to insert into membrane lipid bilayers. Additionally, there is a short stretch of basic residues upstream of the CAAX box that confers specificity to each Rho GTPase and influences into which membrane the isoprenyl chain inserts. The specificity in action is also achieved by the restricted subcellular distribution of GAPs and GEFs, which results in the inactivation/activation of the Rho GTPases being tightly coupled to specific regions of the cell.
Functions of Rho GTPases During Development of the Nervous System
Neuron Polarization and Axon Specification
During the initial stages of the development of the nervous system when neural precursors are generated by cell division, the cells lack features such as dendrites or axons and are non-polarized [3]. To begin the process of forming axons and dendrites the cells must specify which region of the plasma membrane will begin to extend away from the cell body in order to form elongated structures known as 10.1007/978-3-540-29678-2_14. These structures will ultimately become the single axon and several dendrites of the nascent 10.1007/978-3-540-29678-2_14, a process known as neuronal polarization. In vitro studies have revealed key roles for the Rho GTPases Cdc42 and Rac1 in the initial polarization of a neuron [4]. Cdc42 is targeted to a sub-region of the plasma membrane and recruits the polarity complex of Par3, Par6, and the atypical protein kinase C, a conserved multiprotein complex used throughout evolution to polarize different cell types. The polarity complex in turn recruits the GEFs Tiam1 and STEF, which catalyze the exchange of GDP for GTP on Rac1. The active Rac1 mediates the formation of filamentous actin and neurite formation ultimately polarizing the cell.
Axon Guidance
As the nervous system develops, newborn neurons extend axons towards their cognate targets. The neuronal growth cone, located at the tip of the growing axon, is a highly motile structure acting as a sophisticated signal transduction device, capable of recognizing extracellular guidance cues and translating them into directed neurite outgrowth [5]. Over the past 15 years, a combination of cellular and genetic studies has led to the identification of highly conserved families of guidance molecules that can be either membrane-bound factors or secreted molecules, acting over short or long distances, respectively, to guide the growth of axons. They include the classical molecular cues: netrins, slits, ephrins, and semaphorins [6].
Cytoskeletal rearrangements are crucial during growth cone guidance. The growth cone is enriched in the cytoskeletal elements F-actin and microtubules that are rapidly remodeled in response to environmental cues and direct the migration of the growth cone [5]. There is now compelling evidence demonstrating a role for RhoA, Rac1, and Cdc42 as important signaling elements downstream of most, if not all, guidance cue receptors [7]. Indeed, Rho GTPases mediate a cascade of responses from receptors to actin remodeling within the neuronal growth cone. For instance, Rac1 interacts directly with the semaphorin receptor plexin-B1, suggesting that Rac1 plays a role in mediating the repulsive activities of semaphorins. On the other hand, the Rho-specific GEF, PDZ-RhoGEF LARG, interacts with plexin B to activate RhoA signaling, provoking growth cone repulsion and collapse. Ephexin, a GEF for RhoA and Cdc42, binds to Eph receptors to modulate Ephrin-induced growth cone collapse, whereas the slit receptors Robo mediate axon repulsion by interacting with srGAP that inhibits Cdc42. Finally, Rac1 and Cdc42 are important mediators of the signaling response of axons to the netrin-1 receptor DCC. Overall, it is clear that Rho GTPases are important regulators in axon pathfinding and guidance, and it is the correct balance of localized Rho GTPase activities through GEFs and GAPs that will determine the appropriate attractive and repulsive response of an axon to extracellular cues.
Dendritogenesis and Structural Plasticity of the Dendritic Arbor
During the maturation of connectivity within the central nervous system (CNS), dendrites are highly dynamic. The branched structure of the dendrites is highly enriched in the cytoskeletal elements and it is the controlled remodeling of these structures that is responsible for the shape and complexity of the dendritic arbor. In many cases, dendritic spines are the location of the synaptic connections and their size and number correlate with their ability to transmit the correct information from axon to dendrite. The ability of Rho GTPases to convert the upstream signals into cytoskeletal changes leads to remodeling of the dendrites [8]. For example, N-methyl D-aspartate (NMDA) receptors transmit excitatory transmissions mediated by L-glutamate. Activation of NMDA receptors increases the activity of Cdc42 and Rac1 while decreasing the activity of RhoA, leading to the stabilization of the dendritic spines [7]. The regulation of the growth and elaboration of the whole dendritic architecture represent an important mechanism of plasticity in the central nervous system.
Axon Regeneration in the CNS
The activity of the Rho GTPase family member RhoA is known to induce acto-myosin contractility within the cell and to produce mechanical forces that can retract actin-dependent structures such as neurites [9]. Inhibition of RhoA in neuronal cells leads to neurite extension over substrates that would not normally be permissive for neurite outgrowth. Many reports are now suggesting that RhoA is highly activated following lesions in the CNS and mediates many of the signals associated with the growth suppressive environment of the CNS following injury. For example, myelin associated glycoprotein (MAG) is released from the damaged myelin sheath and binds to the NOGO receptor to activate RhoA, preventing regrowth of the damaged axons. This is accomplished because RhoA activates in turn Rho-kinase (ROCK), which is able to induce intracellular acto-myosin contractility that prevents axon outgrowth following injury. Therefore, RhoA is now a promising pharmacological target for therapy in an aim to promote 10.1007/978-3-540-29678-2_1 in the CNS following injury.
Implication of Rho GTPase Dysfunction as Being Causative for Mental Retardation
The importance of Rho GTPases in the nervous system development is further highlighted by research studies linking dysfunction in Rho GTPase signaling pathways and mental retardation in the adult. In this situation mutations in specific genes affect the developmental program such that the organism develops abnormally [7]. Malformed dendrites and dendritic spines are common in these conditions and are hypothesized to be important indicators of the mechanisms of impaired cognition in mental retardation. For example, in non-syndromic x-linked mental retardation, three genes out of a total of 13 have been pinpointed to be mutated and are encoding either regulators of Rho GTPases or downstream mediators of the pathways activated by Rho GTPases. In particular, the OPHN-1 gene encodes the protein oligophrenin-1, which contains a RhoGAP domain shown to negatively regulate RhoA, Rac1, and Cdc42. It is specifically expressed at high levels in both axons and dendrites throughout the brain and a mutation causing decreased levels of the oligophrenin-1 mRNA is associated with mental retardation. In addition, the ARHGEF6 gene encodes a GEF that activates Rac1 and Cdc42 and a truncation mutant protein missing the first 28 amino acids is associated with mental retardation. Finally, the serine/threonine kinase activity of PAK3, a member of the p21-activated protein kinase family (PAK) family of proteins, acting downstream of Rac1 and Cdc42, has been found to be compromised in individuals with this neurological disorder.
Concluding Remarks
RhoA, Rac1, and Cdc42 regulate a wide variety of intracellular signaling pathways to mediate many aspects of the development of the nervous system. The Rho family of GTPases includes 19 other members, the roles of which are relatively unknown. Future research will undoubtedly reveal many novel functions of this diverse family of proteins in the development of the nervous system.
Rhodopsins
10.1007/978-3-540-29678-2_16
Rhombencephalon
Definition
The rhombencephalon (Greek for rhombus-shaped brain) is the caudal part of the developing neural tube, the hindbrain, which is composed of the metencephalon (pons and cerebellum) and myelencephalon (medulla).
Rhombomere
10.1007/978-3-540-29678-2_5
RHT
Rhynchocephalia
Definition
Sister taxon to the Squamata (lizards, snakes, amphisbaenians) and incorporating the living Tuatara of New Zealand, Sphenodon. The term Sphenodontia refers to a subset of Rhynchocephalia that excludes the most basal forms.
10.1007/978-3-540-29678-2_16
Rhythm
Definition
Periodic change of an entity. In behavior, several rhythms are distinguished by their periods: circadian (about 24 h), circalunar (about 28 days), circannual (about 1 year).
10.1007/978-3-540-29678-2_8
Rhythmic Jaw Movements
10.1007/978-3-540-29678-2_13
Rhythmic Movements
The Behavior
Rhythmic movements are motor acts that are characterized by the activation of groups of muscles in a recurring or cyclic pattern. Rhythmic movements are found in all animals ranging from invertebrates to man and include various behaviors that are continuously ongoing, like respiration, are episodic, like swimming, 10.1007/978-3-540-29678-2_13 and 10.1007/978-3-540-29678-2_23, or brief like 10.1007/978-3-540-29678-2_19 and the 10.1007/978-3-540-29678-2_19. The rhythmic movements are generated by localized neuronal networks, called 10.1007/978-3-540-29678-2_3, or CPGs. Activity in the CPGs directly controls the timing (rhythm) and phasing (pattern) of 10.1007/978-3-540-29678-2_13, whose activity in turn activates the 10.1007/978-3-540-29678-2_13 needed to generate the rhythmic movements; e.g., the limb muscles acting on the leg during walking or intercostals muscles and the diaphragm acting on the lungs during respiration. Thus, the term CPG alludes to the fact that these neuronal networks are restricted to specific regions of the central nervous system and, when appropriately activated, are capable of generating both the timing and phasing of rhythmic movements without receiving patterned sensory information. The CPG for respiration or mastication in vertebrates is, for example, localized in the 10.1007/978-3-540-29678-2_2 while the CPGs for swimming, scratching and walking are localized in the 10.1007/978-3-540-29678-2_19. The CPGs controlling rhythmic movements in invertebrates are typically localized to ganglionic structures, like to the 10.1007/978-3-540-29678-2_19 that controls the foregut movements in Crustaceans or to the chain of midbody ganglia that control leech swimming. Traditionally, rhythmic movements were studied in intact or semi-intact animals. Because of the distinct network localization, numerous preparations have been developed where the part of the central nervous system that contains the CPG network can be studied in isolation in vitro.
External Control
Although CPGs are able to intrinsically generate the timing and phasing of rhythmic movements, they do not function in isolation. Rather, in most cases their activity is often turned on and off by an external command signal. For example, when a cat starts to walk or a fish starts to swim, the spinal CPGs are activated by activity in descending fiber tracts originating in the brainstem [1,2]. Initiating signals originating in the forebrain are funneled through the 10.1007/978-3-540-29678-2_2 and conveyed to nuclei in the diencephalon (diencephalic locomotor region) and mesencephalon (10.1007/978-3-540-29678-2_13) and then to excitatory reticulospinal neurons in brainstem 10.1007/978-3-540-29678-2_12. The reticulospinal neurons then project to the spinal locomotor network and provide the external 10.1007/978-3-540-29678-2_5 drive needed to initiate and maintain the rhythmic activity. Similarly, descending inputs from “head ganglia” to CPGs in the stomatogastric ganglion or the swimming CPG in leech can turn specific rhythmic motor behaviors on and off [3]. Sensory inputs, like loud sound or sudden changes in light, might be the direct trigger for the descending signal leading to escape or startle responses [4], which is followed by more persistent rhythmic movements. Sensory inputs are also triggers for the scratching movements where the motor behavior is initiated by tactile stimulation applied to the skin [5].
Sensory Information
Although rhythmic motor outputs can be generated in the absence of sensory information CPGs receive sensory feedback. Some of these sensory signals cause corrections of the rhythmic movements, as when a person is stumbling over an object. In this case 10.1007/978-3-540-29678-2_19 mediate the corrective signal to the CPG circuit [6]. Other sensory signals are involved in phase transitions and amplitude modulation of the rhythmic movements and are caused by 10.1007/978-3-540-29678-2_16 feedback from the moving appendages. Examples of sensory inputs that are involved in phase transitions are feedback from 10.1007/978-3-540-29678-2_19 in the lungs that regulate the transition from inspiration to expiration, joint receptors in the hip in mammals that regulate the transition from stance to swing and sensory receptors in the wing of the locust that influence the transition from wing depression to wing elevation [6,7]. Load receptors, like 10.1007/978-3-540-29678-2_20, or stretch receptors in muscles, like 10.1007/978-3-540-29678-2_13, also provide proprioceptive cues for phase transitions and are actively involved in modulating the amplitude variation of rhythmic movements [7–9].
Basic Network Features
The intrinsic function of rhythmic motor networks is defined by the synaptic interconnections between the CPG neurons in the network and the membrane properties of the neurons. A minimal characterization of the network function therefore requires that CPG component neurons are identified, that the connectivity between individual neurons is established, and that the salient membrane properties are described. An analysis at this level of detail has only been obtained in a limited number of rhythmic motor systems both in invertebrates and in vertebrates.
Notable examples in invertebrates of CPGs that have been characterized in detail are the swim CPGs in the mollusks Clione [10], and Tritonia [11], the heart-beat network in leech [3,12], and the CPG circuits in the stomatogastric ganglion controlling foregut movements in Crustacea [13]. Because of the small number of cells (less than 30 neurons), the complete network connectivity has been worked out and the cellular properties of individual CPG neurons have been determined in great detail. From the analysis of these small CPG networks, it is clear that each CPG network has its specific characteristics and that none of them are alike. However, several basic network and cellular building blocks can be extracted from the analysis [11]. Network elements include extensive reciprocal inhibition in a 10.1007/978-3-540-29678-2_8 fashion, delayed feed-forward excitation, 10.1007/978-3-540-29678-2_5 and 10.1007/978-3-540-29678-2_19. These network elements alone do not determine the timing and phasing that the CPG network produces but they interact with cellular properties that actively interpret the synaptic activity and contribute to timing and phasing. Such cellular elements are 10.1007/978-3-540-29678-2_2 that can provide sustained rhythmic drive, post-inhibitory rebound firing that helps escape inhibition and is generated by h-channels (10.1007/978-3-540-29678-2_8) and T-type calcium channels, 10.1007/978-3-540-29678-2_16 generated by 10.1007/978-3-540-29678-2_16, 10.1007/978-3-540-29678-2_3 (10.1007/978-3-540-29678-2_3 channels) that amplify and prolong synaptic inputs, delayed activation generated by activation of potassium channels with slow kinetics (10.1007/978-3-540-29678-2_1), and spike-frequency adaptation generated by 10.1007/978-3-540-29678-2_7 [14].
In vertebrates, the CPG organizations for swimming in lamprey and in Xenopus tadpole have been revealed in great detail [15,16]. The core of these networks consists of 10.1007/978-3-540-29678-2_5 10.1007/978-3-540-29678-2_5 and inhibitory glycinergic 10.1007/978-3-540-29678-2_9. The glutamatergic interneurons project ipsilaterally and provide the excitatory drive to other CPG interneurons and motoneurons necessary to produce sustained rhythmic drive on one side of the cord. The glycinergic interneurons are commissural interneurons projecting to the contralateral side where they connect to all CPG neurons and motor neurons and mediate reciprocal inhibition segmentally so that when one side is active the other side is inactive. This half center organization is the basis for the side-to-side undulatory swimming. The rhythm itself is not dependent on inhibitory connections, but can be generated in a network of mutually coupled excitatory neurons. Each one of the about 100 spinal segments that makes up the lamprey spinal cord appear to contain such a basic CPG unit [17]. These units are coupled both in the ascending and descending directions. These connections provide the basis for the 10.1007/978-3-540-29678-2_9 of muscular activity along the length of the body that is required for the animal to swim. Similarly to invertebrate CPGs, a large number of intrinsic membrane properties influence the rhythmogenic capability of the swim CPG neurons and participate in patterning of the motor output [18,19].
The large number of neurons controlling any given behavior in mammals has made it difficult to reveal the detailed network organization of, for example, the CPGs controlling mammalian 10.1007/978-3-540-29678-2_22 [20], 10.1007/978-3-540-29678-2_13 [21] or respiration [22–24]. Knowledge about the functionally of these CPGs is, however, advancing rapidly. For the walking CPG, the key network functions are the rhythm generation, ipsilateral coordination of flexors and extensors across the same or different joints in a limb, and 10.1007/978-3-540-29678-2_12 [20]. The rhythm is generated by glutamatergic ipsilateral projecting interneurons [25]. The exact identity of these neurons has not been determined. The circuits underlying coordination of flexors and extensors segmentally and intersegmentally include inhibitory 10.1007/978-3-540-29678-2_9 and Renshaw cells, as well as a number of unidentified interneurons. Functional analysis of left-right circuitries in the mammals suggests that 10.1007/978-3-540-29678-2_9 provided by commissural interneurons is involved in binding motor synergies along the cord, while inhibitory intrasegmental commissural connections control segmental alternation and excitatory commissural connections control synchronous activity [20]. It thus appears that some basic characteristics of swimming CPG and walking CPG network structure are preserved. However, the commissural circuitries seem more complex in the walking CPG than what has been described for the swimming CPG. Additionally, while network elements in the swimming CPG appear to be composed of homogenous populations of neurons, similar network elements, such as the rhythm generation network in the walking CPG, appear to be composed of more heterogeneous populations of neurons. Thus, additional network layers are added when moving from a non-limbed to a limbed CPG. Similar to what is seen in invertebrate and lower vertebrate neurons, mammalian CPG neurons express to a variable degree rhythmogenic/pacemaker-like membrane properties or phase-regulating membrane properties [14,22–24,26].
A new addition to the CPG network analysis is methods for genetically dissecting the neuronal circuits. Such methods include genetic silencing or activation of molecularly defined populations of neurons and have been applied to both invertebrate and vertebrate CPGs. In networks with many neurons, such manipulations can more directly link a population of CPG neurons to a specific network function than traditional electrophysiological methods are able to do [27].
Neuromodulation of Rhythmic Movements
A lesson that has been learned from studies of CPGs in both invertebrates and vertebrates is that the overall network function is flexible and can be changed by 10.1007/978-3-540-29678-2_14 acts on individual CPG neurons and connectivity [28,29]. The neuromodulation may be the result of neurotransmitters and hormones released from sources outside the CPG circuits (10.1007/978-3-540-29678-2_14) or the neuromodulation may be the result of neurotransmitters and signals released from active CPG neurons (10.1007/978-3-540-29678-2_14). Examples of extrinsic 10.1007/978-3-540-29678-2_14 are the numerous amine and peptide containing systems that project to stomatogastric ganglion [28,30] and the descending 5-HT or dopamine systems in vertebrates [31]. Examples of intrinsic 10.1007/978-3-540-29678-2_14 are neurotransmitters released from swim CPG neurons in Tritonia [32], adenosine released from CPG neurons tadpole [18] and endocannabinoids from CPG neurons in the lamprey [33]. In all cases, the targets for neuromodulators are ligand-gated ion channels and/or chemical or electrical synaptic transmission. Because neuromodualtors have these ubiquitous network targets, they can change timing, phasing or amplitude of the rhythmic movements separately or all of these parameters at ones. Thus in some cases, the neuromodulation is a fine tuning of the rhythmic motor behavior, while in others the neuromodulation causes dramatic switching in the motor coordination.
Disorders of Rhythmic Movements
Primary disorders of rhythmic movements are not common, although a number of respiratory dysfunctions involve defects in network function and/or its modulation [22]. Secondary disorders of rhythmic movements are due to injury of the external control systems, for example, damage to the spinal cord that leads to loss of ambulatory ability below the lesion. The ultimate way of restoring the rhythmic motor behavior after spinal cord injury is to promote re-growth or regeneration of the severed fibers across the injury. An alternative approach is neuro-rehabilitation. Experiments in animals with spinal cord injury have shown that sustained 10.1007/978-3-540-29678-2_12 on a treadmill in combination with drugs that activate the spinal locomotor CPG can lead to substantial recovery of the lost locomotor capability [34,35]. Clinical trials have shown that humans with partial spinal cord injury also can benefit from such 10.1007/978-3-540-29678-2_12 [34,35].
Ribonuclease (RNase)
Definition
An enzyme that catalyses the hydrolysis of an RNA resulting in either cleavage to smaller RNA units or by degradation to constituent nucleotides.
Ribosome
Definition
A ribosome is a non-membranous organelle that translates of a mRNA molecule into a polypeptide chain. It consists of 65% ribosomal RNA and 35% ribosomal proteins.
Riddoch Phenomenon or Syndrome
Definition
When visual cerebro-cortical area V5 is disconnected from area V1 (with which it is reciprocally connected and from which it normally receives its visual input) but has a secondary visual input that reaches it without passing through area V1, the subject can still experience visual motion consciously though crudely.
10.1007/978-3-540-29678-2_2
10.1007/978-3-540-29678-2_22
Rigidity
Definition
An increased resistance to passive stretch that is nearly equal in both agonist and antagonist muscles and generally uniform throughout the range of motion of the joint being tested. It may be sustained (plastic or lead pipe) or intermittent and rachetty (cogwheel). Although cogwheel rigidity is usually thought to be Parkinsonian rigidity complicated by Parkinsonian tremor, it may occur in the absence of tremor and the frequency felt by the examiner tends to be higher than that of the visible resting tremor.
10.1007/978-3-540-29678-2_16
Rigor Configuration
Definition
The rigor configuration in the cross-bridge cycle is associated with the end-state of the power stroke with the nucleotide products (ADP and P) having been released. In order to advance from the rigor configuration, ATP is required to release the cross-bridge from actin.
10.1007/978-3-540-29678-2_13
10.1007/978-3-540-29678-2_16
10.1007/978-3-540-29678-2_19
RNA Interference
Definition
RNA interference – this procedure is abbreviated RNAi. It consists of the down-regulation of gene expression by using specific double-stranded ribonucleic acids. The specific or chosen RNA base pairs with its complementary strand of mRNA resulting in the degradation of the latter.
10.1007/978-3-540-29678-2_7
RNA Localization
10.1007/978-3-540-29678-2_13
RNA Synthesis
10.1007/978-3-540-29678-2_4
RNA Translation
Synonyms
Protein synthesis; Polypeptide synthesis
Definition
RNA translation is the process whereby the genetic information encoded in messenger RNA (10.1007/978-3-540-29678-2_13) is translated by specialized cytoplasmic complexes (ribosomes) into a polypeptide. This process is essential for the function of all cell types, and each step of translation represents a highly regulated event.
Characteristics
Eukaryotic mRNAs are initially transcribed from genetic information encoded in the nuclear DNA. After processing by various nuclear proteins (e.g., splicing factors), mRNAs are exported to, and subsequently translated within, the cytoplasm. The basic process translates the sequence of bases organized in three letter codons within the mRNA into a specific polypeptide string of linked amino acids. As more is learned about mRNA translation, it has become apparent that this process is a highly regulated event with several levels of control including storage of mRNAs in a non-translating state and restriction of translation to specific sub-domains within the cytoplasm. Many of these regulatory events are mediated by sequences encoded within the mRNA itself. Each mRNA can be functionally divided into several regions including the 5′ cap, the 5′ untranslated region (UTR), the 10.1007/978-3-540-29678-2_15 (ORF), the 3′ UTR and a 3′ domain consisting of repeated adenosine bases that are added post-transcriptionally (Fig. 1).
RNA Translation. Figure 1.

RNA translation: Initiation – RNA binding proteins that specifically interact with the 5′ m7G cap and 3′ UTR regions of mRNAs form the translation initiation complex. A complex of poly A binding protein (pABP, red) interacts with the 3′ poly A tail, while eukaryotic Initiation Factors (eIFs) form a complex at the 5′ end of the mRNA. The major proteins in this complex include the cap-binding protein eIF4E (yellow), and the eIF4G (orange), eIF3 (green) and eIF4A (pale blue). They serve to position the 40S (small) ribosomal subunit on the mRNA. In most cases of mRNA translation, the 40S subunit is thought to scan along the mRNA in a 5′-3′ direction until it reaches an AUG codon where a specialized tRNA (fMET) brings in the first amino acid and the 60S (large) ribosomal subunit joins the complex to form a complete ribosome.
In a generalized model, the ORF is the most important region, with the information within encoding a specific protein, while the UTRs are thought to play roles in regulating localization, degradation and restrictions in the translation of the mRNA.
The core component of the cellular complex that reads the genetic information in the ORF and translates it into a specific protein is the ribosome. Several ribosomes can bind to a single mRNA to form a larger translation complex called a polysome. The genetic information in the ORF of the mRNA is read in three base segments by a collection of transfer RNA (tRNA) molecules that contain a complementary RNA code (anti-codon) that recognizes and binds a particular nucleotide triplet (codon) in the mRNA. A parallel cellular process specifically links each tRNA to the amino acid that corresponds to the codon (Fig. 2). The ribosomal complex processes along the mRNA and through the progressive recruitment of specific amino-acid linked tRNAs. The amino acids delivered by the tRNAs to the ribosome are covalently joined to produce the polypeptide encoded by the mRNA. This process is regulated at several levels. For example, proteins that will remain inside the cell are translated on free ribosomes in the cytoplasm while the ribosomes-mRNA-nascent polypeptide complexes translating exported or membrane bound proteins are trafficked to the endoplasmic reticulum. However, there are several other regulatory events during mRNA translation including: editing of the mRNA after it is transcribed (RNA editing), sub-cellular localization of mRNAs, sub-cellular restriction of mRNA translation, sequestering multiple non-translating mRNAs in protein complexes and selective mRNA degradation.
RNA Translation. Figure 2.

Codon usage table: Codons are grouped by the first (left) and second (top) position. Note that in many cases the third position is redundant and that codons with a common first and second position base base often encode encode the same amino acid.
Details of mRNA Translation
The translation process is often divided into three stages (Fig. 1): Initiation –where a complex of proteins (eukaryotic initiation factors, eIFs) recruits ribosome(s) to the mRNA where they move to the beginning of the ORF, Elongation – which encompasses the processing of the ribosome along the coding region of the mRNA linking amino acids brought by specific transfer RNAs (tRNAs) into a growing polypeptide chain and Termination – which occurs at the end of the ORF characterized by the disassembly of the ribosomal complex and release of the full-length polypeptide.
Initiation
Eukaryotic ribosomes contain a large and small subunit, each formed from a specific collection of ribosomal proteins and non-coding ribosomal RNAs (rRNAs). When an mRNA is to be translated, the small subunit of the ribosome first binds to a site “upstream” (on the 5′ side) of the ORF. This activity is mediated by an initiation-complex consisting of eukaryotic initiation factor (eIF) proteins that bind to the 7 methylguanosine cap (m7G) [RNA binding proteins Structures/Processes/Conditions] in the 5′ and UTR. This also requires interaction with proteins that bind the polyA+ sequence of the 3′ UTR (Fig. 1). The small ribosome subunit then proceeds along the 5′ UTR region of mRNA in a 5′ -> 3′ direction until it encounters an AUG-10.1007/978-3-540-29678-2_19 (Fig. 1). At this point it joins with a large ribosomal subunit to form a functional ribosome. Ribosomes contain two sites that contain tRNA molecules, the P-site which usually contains a peptidyl-tRNA molecule (i.e., a tRNA with the growing peptide attached) and an A-site which normally recruits aminoacytled –tRNAs which bring in additional amino acids for incorporation into the polypeptide (Fig. 1). Translation is initiated by an “initiator” tRNA, the only tRNA that can bind directly to an empty P-site of the ribosome. Most often in eukaryotes, this initiator tRNA encodes a methionine (Met) amino acid. At this point the ribosome is ready to recruit additional aminoacytled –tRNAs and proceed to synthesise a full length polypeptide. In special cases, other mechanisms can also initiate mRNA translation. This includes termination re-initiation, where ribosomes are re-directed to translate the same mRNA multiple times. Also, initiation can occur at an AUG codon other than the one nearest the m7G cap (leaky scanning), and ribosome shunting. Finally, there is an alternative to m7G cap-dependent translation initiation where internal ribosome entry sites (IRES) along the mRNA direct translation.
Elongation
In eukaryotic cells, there are 20 amino acids commonly used for protein synthesis. Each tRNA contains a region with three unpaired nucleotides (anti-codon) which binds the corresponding codon in the mRNA. The use of three bases to encode each amino acid means that there are actually (43) different possibilities that can be used to uniquely encode them providing 64 unique identities. Thus, there are more codons than amino acids and therefore considerable redundancy in the code, with some amino acids encoded by four or more tRNAs with different anticodons. A separate process couples each specific amino acid to their representative encoding tRNA(s) via an activating enzyme (aminoacyl-tRNA synthetase). Also, some tRNAs can recognize more than one codon. This modified base pairing at the third nucleotide of a codon is called “wobble-pairing.” For example, the phenylalanine tRNA with the anticodon 3′ AAG 5′ recognizes UUC and UUU. Also, some codons are reserved for specialized functions. AUG signals for the beginning of each ORF and for the amino acid methionine (Fig. 2). As a result, there is usually a methionine at the amino terminal of the polypeptide synthesized from the mRNA. Any AUG codons after this point are interpreted for the insertion of an internal methionine.
Elongation of a nascent ribosome associated polypeptide is an iterative process in which a series of specific aminoacytled –tRNAs, are recruited to their respective three-base codons within the A-site adjacent to the P-site (Fig. 1). Once this occurs, elongation factors hydrolyze GTP (an energy source) to covalently link to the incoming amino acid to the existing polypeptide (Fig. 1). At this point the tRNA at the P site is released and the ribosome moves one codon (3 bases) downstream. The newly-arrived tRNA at the A-site, while still attached to the nascent polypeptide, shifts to the P site and opens the A site for recruitment of an aminoacyl-tRNA that carries an amino acid corresponding to the next codon. This occurs via another protein elongation factor and requires the energy of hydrolysis of another molecule of GTP. Once the ribosome complex clears the recruitment site, it is possible to recruit additional ribosome complexes to a single mRNA. Often a single mRNA molecule is translated simultaneously by many ribosomes. This multi-ribosome complex is called a polysome.
Termination
Translation of a protein is normally finished when the ribosome reaches one or more 10.1007/978-3-540-29678-2_19 (UAA, UAG, UGA) (Fig. 2). Normally, there are no corresponding tRNA molecules with anti-codons for STOP codons. Instead, specific proteins (release factors) recognize these codons when they arrive at the A site of the ribosome and release the completed polypeptide (Fig. 1). During this process the ribosome complex is dissociated back into its corresponding subunits, which are then available for translation of additional mRNAs (Fig. 1).
Regulation of mRNA Translation
Regulation of mRNA translation appears to be a much more common mechanism for regulating protein expression that was previously thought. There are several mechanisms that limit mRNA translation to specific cellular sub-domains. In addition, cytoplasmic mRNAs have a finite life and like mRNA transcription, degradation of mRNAs is also a regulated process. This includes the surveillance and destruction of aberrant mRNAs as the proteins translated from these mRNAs would potentially be detrimental to cell function. Also, there is an entire RNA interference [Structures/Processes/Conditions] regulatory system that employs small non-coding RNA molecules that also regulate translation and/or mRNA degradation (i.e., RNAi).These events are regulated through specific RNA binding proteins [Structures/Processes/Conditions] that interact with the RNA either before or after it is exported into the cytoplasm for translation. It is now thought that there is a dynamic balance between translation by ribosomes and cytoplasmic RNA regulatory complexes for access to mRNAs.
RNA Transport Particles
In many cell types, mRNAs are transported to specific sub-domains to regulate protein expression. This process is important for establishing cellular asymmetry during cell division or directed cell migration. This process also appears critical for aspects of neuronal function, including axon guidance and nerve regeneration [1]. The RNA particles that contain these transported mRNAs also contain several of the proteins involved in translation including ribosomal subunits (Fig. 1). It is thought that translation is suppressed during mRNA transport.
Processing-bodies
Processing bodies (P-bodies) are thought to be primarily sites of mRNA degradation although there are cases where they also sequester mRNAs away from the translational protein complexes to be released later. Degradation-specific P-bodies contain a complex that break down the mRNA by removing the 7mG cap and a 5′-3′ exonuclease that subsequently breaks down the mRNA [2]. These structures also appear to be a destination for mRNAs that are being regulated by the RNAi pathway.
Stress Granules
In mammalian cells, stress granules appear during events that seem to require a rapid shift in the translational events within a cell [3]. They are proposed to be sites where pools of translationally arrested mRNAs are stored as they contain several proteins also involved in mRNA translation such as the small ribosomal subunit. Functionally, these structures seem to have a role in sorting, remodeling and exporting mRNAs either for subsequent translation or storage in complexes such as P-bodies.
Preventing Translation of Mutated mRNAs
Eukaryotic cells possess several surveillance mechanisms to ensure the fidelity and appropriate translation of cytoplasmic mRNAs. Translation of aberrant mRNAs would obviously present a disadvantage to a cell. Defects in mRNA molecules can arise via mutations in the DNA of the encoding gene, or by errors that occur during transcription. Some of these errors would have no effect on translation, especially if they occur in the third (wobble) position of a codon due to codon redundancy (Fig. 2). However, mRNAs with incorrect amino acid codons or premature STOP codons would produce truncated proteins that would be ineffective or even harmful.
Nonsense Mediated mRNA Decay
One process that removes non-sense mutations (pre-mature stop codons) is the nonsense mediated decay (NMD) pathway [4]. Briefly, the complex of the RNA binding proteins that mediate joining of the last exons during nuclear RNA splicing remain associated with the mRNA as it is exported to the cytoplasm. During the first round of mRNA translation, these complexes are removed. If the ribosome complex dissociates from an mRNA via a premature termination codon, one or more of these complexes are not removed, and their retention marks the aberrant mRNA for selective destruction.
Nonstop mRNA Decay and the Exosome
Nonstop transcripts contain no functional STOP codon. These mutations often occur during mRNA processing either by abnormal splicing or premature addition of the polyA+ tail. During translation of these mRNAs, termination does not occur and the ribosome complex reaches the end of the poly(A) tail. If this occurs, the Ski7 protein binds to the empty A site overhanging the 3′ end of the mRNA and recruits it to the exosome [5]. This exosome complex is the primary mediator of 3′-5′ mRNA degradation that targets old mRNAs for degradation due to shortening of their 3′ poly A+ tail.
RNase H
Definition
Ribonuclease is a general term for enzymes which catalyse the hydrolysis of RNA into smaller fragments. Ribonucleases are grouped into several sub-classes within Enzyme Class 2.7. (phosphorolytic enzymes) and Enzyme Class 3.1 (hydrolytic enzymes). RNase H cleaves the 3′-O-P-bond of RNA in a DNA/RNA duplex to produce 3′-hydroxyl and 5′-phosphate terminated products. Since RNase H hydrolyses RNA in a DNA:RNA duplex without degrading the DNA, it is commonly used to remove the RNA template after first strand cDNA synthesis.
10.1007/978-3-540-29678-2_19
Rod and Frame, Rod and Disc
Definition
Protocols to assess the impact of a static frame or a rotating disk on the visual perception of verticality (svv).
10.1007/978-3-540-29678-2_22
Rod Photoreceptor
Definition
Rod-shaped photoreceptors in the vertebrate retina that mediate vision at low intensity light levels. Rods are far more numerous than cones in humans and are of only one type with peak spectral sensitivity ~500 nm.
10.1007/978-3-540-29678-2_16
Rod Spherule
Definition
Synaptic terminal of a rod photoreceptor that provides output to rod bipolar and horizontal cells.
10.1007/978-3-540-29678-2_8
10.1007/978-3-540-29678-2_16
Roll Off
Definition
The amount of attenuation of sound magnitude provided by a filter for waves outside of the filter’s passband, usually expressed in dB/octave.
10.1007/978-3-540-29678-2_1
Romberg Sign
Definition
Simply put, while standing and with the feet together, a positive Romberg sign is when the subject sways or steps out with the eyes closed but not with the eyes open. The amount of sway should make the examiner worried that the subject will fall since most people will sway a little with this test.
The interpretation of an abnormal Romberg test is not simple. A great deal of the nervous system is involved when performing the Romberg test and multiple small deficits in many systems could be present. In other words, the nervous system functions together. For instance, adequate strength in the legs is required. Proximal or distal weakness will prevent the subject from making corrections in stance. Significant cerebellar dysfunction will cause the subject to be unsteady with feet together and eyes open, while mild cerebellar dysfunction might only reveal itself with the eyes closed.
With intact cerebellar and motor function, one maintains balance using the visual, vestibular and proprioceptive systems. Since the Romberg test is performed with the eyes closed, abnormalities in either of the other two systems can cause an abnormal Romberg test. Classically, the Romberg test assumes that the vestibular system is intact. That may not be a good assumption if dizziness is the primary complaint. In the classic situation, if the subject closes their eyes and significantly sways or steps out, the implication is that proprioception is impaired. If proprioceptive and vestibular functions were both intact, closing the eyes would not be a problem.
Proprioceptive function is transmitted through large diameter myelinated peripheral nerves and the dorsal columns of the spinal cord to nuclei in the brain stem. Peripheral neuropathy, vitamin B12 deficiency, multiple sclerosis and neurosyphilis are examples of diseases to consider when the Romberg sign is positive.
10.1007/978-3-540-29678-2_22
Root Neurons
Definition
Special neurons unique to the rodent cochlear nucleus located in the auditory nerve root.
10.1007/978-3-540-29678-2_3
Ror
Definition
Nuclear orphan receptor related to the retinoic acid receptors. Binds to the consensus sequence ([A/T]A[A/T]NT[A/G]GGTCA termed RORE, in the promoter of target genes.
10.1007/978-3-540-29678-2_3
Rostral Interstitial Nucleus of the MLF (riMLF)
Definition
Located at the mesodiencephalic junction, this is the most rostral of the interstitial nuclei of the MLF.
10.1007/978-3-540-29678-2_5
Rostral Ventrolateral Medulla (RVLM)
Definition
The RVLM is part of the ventrolateral medulla and located just caudal to the facial nucleus. It is a vasomotor nucleus and contains (in addition to interneurons) sympathetic premotor neurons related to the sympathetic cardiomotor, cutaneous vasoconstrictor, muscle vasoconstrictor, renal vasoconstrictor, visceral (non-renal) vasoconstrictor pathways and to the adrenal medulla (probably cells secreting noradrenaline). These populations of sympathetic premotor neurons are viscerotopically organized.
10.1007/978-3-540-29678-2_1
10.1007/978-3-540-29678-2_2
Rotation Vector
Definition
Three dimensional eye positions can be expressed in rotation vector form, where the direction of the vector specifies the axis of rotation from primary position and its length specifies the rotation angle.
10.1007/978-3-540-29678-2_22
Rough Endoplasmic Reticulum (RER)
Definition
The endoplasmic reticulum (ER) is an extensive membrane network of tubes and sac-like structures held together by the cytoskeleton. Some parts of the ER are covered with ribosomes on the surface, which give them a rough appearance at the level of the electron microscope. Ribosomes assemble amino acids into proteins based on instructions from the nucleus. and insert the freshly produced proteins directly into the ER, which processes them and then passes them on to the Golgi apparatus.
Route Navigation (or Taxon Navigation)
Definition
In route navigation the traveler is guided by a memorized set of turns and straight paths to reach a goal. Rule sets such as “at the big rock turn left; next, at the fallen tree bear right and proceed…” are examples of route learning. In animals route learning is based on operant conditioning. In humans it is usually accomplished by “following directions” generated by another (cultural transmission). Route learning can be effective but is inflexible.
10.1007/978-3-540-29678-2_15
10.1007/978-3-540-29678-2_19
Route Navigation Strategy
Definition
Behavior relying on an egocentric reference frame and directed by chaining sequences of taxon and praxis strategies.
10.1007/978-3-540-29678-2_19
RTLLBs
Rubrobulbar Tract
Synonyms
Tractus rubrobulbaris
Definition
Descending fibers of the rubrobulbar tract and rubrospinal tract terminate on the interneurons in the lateral reticular formation and the dorsolateral intermediate zone of the spinal cord and directly on motoneurons of the nucleus of the facial nerve. The rubrobulbar tract and rubrospinal tract have a somatotopic arrangement.
10.1007/978-3-540-29678-2_13
Rubrospinal Tract
Synonyms
Tractus rubrospinalis
Definition
Somatotopically arranged fiber bundles between the red nucleus and spinal cord. Runs in the lateral column of the spinal cord, originating in the magnocellular portion of the red nucleus, going to the spinal cord segments as far as the thoracic cord. Regulates the tone of important flexors.
10.1007/978-3-540-29678-2_13
RVOR (Rotational VOR)
10.1007/978-3-540-29678-2_22
Footnotes
*Corresponding author.
Contributor Information
Marc D. Binder, Email: mdbinder@u.washington.edu
Nobutaka Hirokawa, Email: hirokawa@m.u-tokyo.ac.jp.
Uwe Windhorst, Email: siggi.uwe@t-online.de.
References
- 1.Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42(2):223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- 2.Soechting JF, Lacquaniti F. Invariant characteristics of a pointing movement in man. J Neurosci. 1981;1(7):710–720. doi: 10.1523/JNEUROSCI.01-07-00710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci. 1985;5(9):2318–2330. doi: 10.1523/JNEUROSCI.05-09-02318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb GL, Song Q, Almeida GL, Hong DA, Corcos D. Directional control of planar human arm movement. J Neurophysiol. 1997;78(6):2985–2998. doi: 10.1152/jn.1997.78.6.2985. [DOI] [PubMed] [Google Scholar]
- 5.Flanders M, Herrmann U. Two components of muscle activation: scaling with the speed of arm movement. J Neurophysiol. 1992;67:931–943. doi: 10.1152/jn.1992.67.4.931. [DOI] [PubMed] [Google Scholar]
- 6.Flanders M, Pellegrini JJ, Geisler SD. Basic features of phasic activation for reaching in vertical planes. Exp Brain Res. 1996;110(1):67–79. doi: 10.1007/BF00241376. [DOI] [PubMed] [Google Scholar]
- 7.d’Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci. 2006;26(30):7791–7810. doi: 10.1523/JNEUROSCI.0830-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2(11):1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol. 1997;77(2):826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- 10.Kalaska JF, Cohen DA, Hyde ML, Prud’homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci. 1989;9(6):2080–2102. doi: 10.1523/JNEUROSCI.09-06-02080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.von Holst E, Mittelstaedt H. Das reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- 2.Sperry R. Neural basis of spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 3.Bell C, Bodznick D, Montgomery J, Bastian J. The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol. 1997;50:17–31. doi: 10.1159/000113352. [DOI] [PubMed] [Google Scholar]
- 4.Bell CC, Grant K. Corollary discharge inhibition and preservation of temporal information in a sensory nucleus of mormyrid electric fish. J Neurosci. 1989;9:1029–1044. doi: 10.1523/JNEUROSCI.09-03-01029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson BA. Electric signaling behavior and the mechanisms of electric organ discharge production in mormyrid fish. J Physiol (Paris) 2002;96:403–417. doi: 10.1016/S0928-4257(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 6.Xu-Friedman MA, Hopkins CD. Central mechanisms of temporal analysis in the knollenorgan pathway of mormyrid electric fish. J Exp Biol. 1999;202:1311–1318. doi: 10.1242/jeb.202.10.1311. [DOI] [PubMed] [Google Scholar]
- 7.Bass AH. Electric organs revisited: Evolution of a vertebrate communication and orientation organ. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley; 1986. pp. 13–70. [Google Scholar]
- 8.Zakon HH. The electroreceptive periphery. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley; 1986. pp. 103–156. [Google Scholar]
- 9.Carr CE, Friedman MA. Evolution of time coding systems. Neural Comput. 1999;11:1–20. doi: 10.1162/089976699300016773. [DOI] [PubMed] [Google Scholar]
- 10.Poulet JFA, Hedwig B. A corollary discharge maintains auditory sensitivity during sound production. Nature. 2002;418:872–876. doi: 10.1038/nature00919. [DOI] [PubMed] [Google Scholar]
- 1.Dummett M. The seas of language. Oxford: Oxford University Press; 1993. [Google Scholar]
- 2.Devitt M. Realism and truth, 2nd edn with an new afterword by the author. Princeton: Princeton University Press; 1997. [Google Scholar]
- 3.Nagel T. The view from nowhere. Oxford: Oxford University Press; 1986. [Google Scholar]
- 4.Putnam H. Reason, truth, and history. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- 5.Putnam H. The many faces of realism. La Salle, Ill: Open Court; 1987. [Google Scholar]
- 6.Field H. Realism and relativism. J Philos. 1982;79:553–567. [Google Scholar]
- 7.Putnam H. The threefold cord mind, body, and world. New York: Columbia University Press; 1999. [Google Scholar]
- 8.Boyd R. The current status of scientific realism. In: Leplin J, editor. Scientific realism. Berkeley and Los Angeles: University of California Press; 1984. pp. 41–82. [Google Scholar]
- 9.Laudan L. A confutation of convergent realism. In: Leplin J, editor. Scientific realism. Berkeley and Los Angeles: University of California Press; 1984. pp. 218–249. [Google Scholar]
- 10.Churchland PM. Scientific realism and the plasticity of mind. Cambridge: Cambridge University Press; 1979. [Google Scholar]
- 1.Cosmides L, Tooby J. Neurocognitive adaptations designed for social exchange. In: Buss DM, editor. Evolutionary psychology handbook. New York: Wiley; 2005. pp. 584–627. [Google Scholar]
- 2.Evans JStBT, Newstead SE, Byrne RMJ. Human reasoning. The psychology of deduction. Hove: Lawrence Erlbaum Associates; 1993. [Google Scholar]
- 3.Goodwin GP, Johnson-Laird PN. Reasoning about relations. Psychol Rev. 2005;112:468–493. doi: 10.1037/0033-295X.112.2.468. [DOI] [PubMed] [Google Scholar]
- 4.Knauff M. A neuro-cognitive theory of relational reasoning with visual images and mental models. In: Held C, Knauff M, Vosgerau G, editors. Mental models and the mind: a concept at the intersection of cognitive psychology, neuroscience, and philosophy of mind. North-Holland: Elsevier; 2006. [Google Scholar]
- 5.Rauh R, Hagen C, Knauff M, Kuß T, Schlieder C, Strube G. From preferred To alternative mental models in spatial reasoning. Spatial Cognition and Computation. 2005;5:239–269. [Google Scholar]
- 6.Holland JH, Holyoak KJ, Nisbett RE, Thagard PR. Induction: processes of inference, learning, and discovery. Cambridge, MA: MIT; 1986. [Google Scholar]
- 7.Holyoak KJ, Nisbett RE. Induction. In: Sternberg RJ, Smith EE, editors. The psychology of human thought. New York: Cambridge University Press; 1988. pp. 50–91. [Google Scholar]
- 8.Manktelow KI. Reasoning and Thinking. Hove: Psychology; 1999. [Google Scholar]
- 9.Sloman SA, Lagnado D. The problem of induction. In: Morrison R, Holyoak K, editors. Cambridge handbook of thinking and reasoning. New York: Cambridge University Press; 2005. pp. 95–116. [Google Scholar]
- 10.Rips LJ. The psychology of proof. Cambridge, MA: MIT; 1994. [Google Scholar]
- 11.Braine MDS, O’Brian DP, editors. Mental logic. Mahwah, NJ: Lawrence Erlbaum; 1998. [Google Scholar]
- 12.Stenning K, Oberländer K. A cognitive theory of graphical and linguistic reasoning: logic and implementation. Cogn Sci. 1995;19:97–140. [Google Scholar]
- 13.Johnson-Laird PN. Mental models. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 14.Johnson-Laird PN, Byrne RMJ. Deduction. Hove: Erlbaum; 1991. [Google Scholar]
- 15.Johnson-Laird PN. Mental models and deduction. Trend Cogn Sci. 2001;5:434–442. doi: 10.1016/s1364-6613(00)01751-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Laird PN. Mental models, deductive reasoning, and the brain. In: Gazzaniga S, editor. The cognitive neurosciences. Cambridge, MA: MIT; 1994. pp. 999–1008. [Google Scholar]
- 17.Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: a functional MRI study. Cognitive Brain Research. 2002;13:203–212. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- 18.Golding E. The effect of unilateral brain lesion on reasoning. Cortex. 1981;17:31–40. doi: 10.1016/s0010-9452(81)80004-4. [DOI] [PubMed] [Google Scholar]
- 19.Wason PC. Reasoning. In: Foss B, editor. New horizons in psychology. Harmondsworth, UK: Penguin; 1966. pp. 135–151. [Google Scholar]
- 20.Deglin VL, Kinsbourne M. Divergent thinking styles of the hemispheres: how syllogisms are solved during transitory hemisphere suppression. Brain Cogn. 1996;31:285–307. doi: 10.1006/brcg.1996.0048. [DOI] [PubMed] [Google Scholar]
- 21.Caramazza A, Gordon J, Zurif EB, DeLuca D. Right-hemispheric damage and verbal problem-solving behavior. Brain Lang. 1976;3:41–46. doi: 10.1016/0093-934x(76)90005-5. [DOI] [PubMed] [Google Scholar]
- 22.Read DE. Solving deductive-reasoning problems after unilateral temporal lobectomy. Brain Lang. 1981;12:116–127. doi: 10.1016/0093-934x(81)90008-0. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker HA, Markovits H, Savary F, Grou C, Braun C. Inference deficits after brain damage. J Clin Exp Neuropsychol. 1991;13:38. [Google Scholar]
- 24.Goel V, Gold B, Kapur S, Houle S. The seats of reason: a localization study of deductive and inductive reasoning using PET (O15) blood flow technique. Neuroreport. 1997;8:1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- 25.Goel V, Gold B, Kapur S, Houle S. Neuroanatomical correlates of human reasoning. J Cogn Neurosci. 1998;10:293–302. doi: 10.1162/089892998562744. [DOI] [PubMed] [Google Scholar]
- 26.Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc London B Biol Sci. 1997;352:1421–1428. doi: 10.1098/rstb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 28.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 29.Goel V, Büchel C, Frith C, Dolan RJ. Dissociation of mechanisms underlying syllogistic reasoning. Neuroimage. 2000;12:504–514. doi: 10.1006/nimg.2000.0636. [DOI] [PubMed] [Google Scholar]
- 30.Knauff M, Kassubek J, Mulack T, Greenlee MW. Cortical activation evoked by visual mental imagery as measured by functional MRI. Neuroreport. 2000;11:3957–3962. doi: 10.1097/00001756-200012180-00011. [DOI] [PubMed] [Google Scholar]
- 31.Goel V, Dolan RJ. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia. 2001;39:901–909. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- 32.Johnson-Laird PN. Imagery, visualization, and thinking. In: Hochberg J, editor. Perception and cognition at century’s end. San Diego, CA: Academic; 1998. pp. 441–467. [Google Scholar]
- 33.Knauff M, Schlieder C. Spatial inference: no difference between mental images and models. Behav Brain Sci. 2004;27:589–590. [Google Scholar]
- 34.Kosslyn SM. Image and brain: the resolution of the imagery debate. Cambridge, MA: MIT; 1994. [Google Scholar]
- 35.Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- 36.Kosslyn SM, Thompson WL. When is early visual cortex activated during visual mental imagery? Psychol Bull. 2003;129:723–746. doi: 10.1037/0033-2909.129.5.723. [DOI] [PubMed] [Google Scholar]
- 37.Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: behavioral measures and cortical activity. J Cogn Neurosci. 2003;4:559–573. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- 38.Knauff M, Johnson-Laird PN. Visual imagery can impede reasoning. Mem Cogn. 2002;30:363–371. doi: 10.3758/bf03194937. [DOI] [PubMed] [Google Scholar]
- 39.Knauff M, May E. Mental imagery, reasoning, and blindness. Quart J Exp Psychol. 2006;59:161–177. doi: 10.1080/17470210500149992. [DOI] [PubMed] [Google Scholar]
- 40.Evans JStBT. Bias in human reasoning. Hove: Lawrence Erlbaum; 1989. [Google Scholar]
- 41.Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87(1):B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- 42.Pylyshyn ZW. Computation and cognition: toward a foundation for cognitive science. Cambridge, MA: MIT; 1984. [Google Scholar]
- 43.Held C, Knauff M, Vosgerau G, editors. Mental models and the mind. A conception in the intersection of cognitive psychology, neuroscience, and philosophy of mind. North-Holland: Elsevier; 2006. [Google Scholar]
- 44.Cosmides L. The logic of social exchange: has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition. 1989;31:187–276. doi: 10.1016/0010-0277(89)90023-1. [DOI] [PubMed] [Google Scholar]
- 45.Stone V, Cosmides L, Tooby J, Kroll N, Knight R. Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proc Natl Acad Sci. 2002;99(17):11531–11536. doi: 10.1073/pnas.122352699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng PW, Holyoak KJ. Pragmatic reasoning schemas. Cogn Psychol. 1985;17:391–416. doi: 10.1016/0010-0285(85)90014-3. [DOI] [PubMed] [Google Scholar]
- 47.Fangmeier T, Knauff M, Ruff CC, Sloutsky V (2006) The neural correlates of logical thinking: an event-related fMRI study. J Cogn Neurosci (in press)
- 1.Mu Y, Otsuka T, Horton A-C, Scott D-B, Ehlers M-D. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;240(3):581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 2.Shin H, Wyszynski M, Huh K-H, Valtschanoff J-G, Lee J-R, Ko J, Streuli M, Weinberg R-J, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- 3.Kittler J-T, Delmas P, Jovanovic J-N, Brown D-A, Smart T-G, Moss S-J. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kittler J-T, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo I-L, Jovanovic J-N, Pangalos M-N, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T, Rumbaugh G, Huganir R-L. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Keller C-A, Yuan X, Panzanelli P, Martin M-L, Alldred M, Sassoè-Pognetto M, Lüscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colledge M, Snyder E-M, Crozier R-A, Soderling J-A, Jin Y, Langeberg L-K, Lu H, Bear M-F, Scott J-D. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salinas G-D, Blair L-A, Needleman L-A, Gonzales J-D, Chen Y, Li M, Singer J-D, Marshall J. Actinfilin is a Cul3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J Biol Chem. 2006;281:40164–40173. doi: 10.1074/jbc.M608194200. [DOI] [PubMed] [Google Scholar]
- 9.Kato A, Rouach N, Nicoll R-A, Bredt D-S. Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination. Proc Natl Acad Sci USA. 2005;102:5600–5605. doi: 10.1073/pnas.0501769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin S, Nishimune A, Mellor J-R, Henley J-M. Sumoylation regulates kainate-receptor-mediated synaptic transmission. Nature. 2007;447:321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson W. The role of decision processes in remembering and knowing. Mem Cognit. 1996;24(4):523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- 3.Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci. 2003;7(7):313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 4.Jager T, Mecklinger A, Kipp KH. Intra- and inter-item associations doubly dissociate the electrophysiological correlates of familiarity and recollection. Neuron. 2006;52(3):535–545. doi: 10.1016/j.neuron.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23(9):3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonsalves BD, et al. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47(5):751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 9.Wirth S, et al. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300(5625):1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 10.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 1.Nagel E. The structure of science: problems in the logic of scientific explanation. London: Routledge & Kegan Paul; 1961. [Google Scholar]
- 2.Bickle J. Philosophy and neuroscience: a ruthlessly reductive account. Dordrecht: Kluwer; 2003. [Google Scholar]
- 3.Churchland PS. Neurophilosophy: towards a unified understanding of the mind-brain. Cambridge: MIT; 1986. [Google Scholar]
- 4.Bechtel W, Richardson RC. Discovering complexity: decomposition and localization as strategies in scientific research. Princeton: Princeton University Press; 1993. [Google Scholar]
- 5.Craver C, Darden L. Discovering mechanisms in neurobiology: the case of spatial memory. In: Machamer PK, Grush R, McLaughlin P, editors. Theory and method in neuroscience. Pittsburgh: University of Pittsburgh Press; 2001. [Google Scholar]
- 6.Stephan A (2001) Phänomenaler Pessimismus. In: Pauen M, Stephan A (eds) Phänomenales Bewusstsein – Rückkehr zur Identitätstheorie? Paderborn, mentis, pp 342–363
- 7.Stephan A (1999) Emergenz. Von der Unvorhersagbarkeit zur Selbstorganisation. 2nd printing, mentis, Paderborn 2005
- 8.Popper K, Eccles J. The self and its brain: an argument for interactionism. Berlin Heidelberg New York: Springer; 1977. [Google Scholar]
- 9.Boogerd FC, Bruggeman FJ, Richardson RC, Stephan A, Westerhoff HV. Emergence and its place in nature: a case study of biochemical networks. Synthese. 2005;145:131–164. [Google Scholar]
- 10.Fodor J. The language of thought. New York: Thomas Crowell; 1975. [Google Scholar]
- 1.Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res. 2000;130:417–432. doi: 10.1007/s002219900250. [DOI] [PubMed] [Google Scholar]
- 2.Prochaska G. The principles of physiology, Prochaska on the nervous system. London: The Sydenham Society; 1784. p. 463. [Google Scholar]
- 3.Bernard C. Leçons sur les phénomènes de la vie communes aux animaux et aux des végéraux. Paris: Baillière; 1878. pp. 114–121. [Google Scholar]
- 4.Spencer H. Principles of psychology. 2. New York: Appleton; 1855. p. 648. [Google Scholar]
- 5.Sechenov IM. Reflexes of the brain (Refleksy Golovnogo Mozga) In: Subkov AA, editor. Im sechenov, selected works. Moscow: State Publishing House; 1863. [Google Scholar]
- 6.Hughlings Jackson J. On the evolution and dissolution of the nervous system. Croonian lectures 3, 4 and 5 to the Royal Society of London. Lancet. 1884;1:555–739. [Google Scholar]
- 1.Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- 2.Kiryu-Seo S, Gamo K, Tachibana T, Tanaka K, Kiyama H. Unique anti-apoptotic activity of EAAC1 in injured motor neurons. EMBO J. 2006;25:3411–3421. doi: 10.1038/sj.emboj.7601225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dontchev VD, Letourneau PC. Growth cones integrate signaling from multiple guidance cues. J Histochem Cytochem. 2003;51:435–444. doi: 10.1177/002215540305100405. [DOI] [PubMed] [Google Scholar]
- 4.Nakai J. Dissociated dorsal root ganglia in tissue culture. Am J Anat. 1956;99:81–129. doi: 10.1002/aja.1000990105. [DOI] [PubMed] [Google Scholar]
- 5.Nakai J, Kawasaki Y. Studies on the mechanism determining the course of nerve fibers in tissue culture. I. The reaction of the growth cone to various obstructions. Z Zellforsch Mikrosk Anat. 1959;51:108–122. doi: 10.1007/BF00345083. [DOI] [PubMed] [Google Scholar]
- 6.Torigoe K, Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Exp Neurol. 1998;150:254–262. doi: 10.1006/exnr.1997.6775. [DOI] [PubMed] [Google Scholar]
- 7.Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto E, Mizoguchi A, Hanada K, Ide C. Basic fibroblast growth factor promotes extension of regenerating axons of peripheral nerve. In vitro experiment using a Schwann cell basal lamina tube model. J Neurocytol. 1997;26:511–528. doi: 10.1023/a:1015410023132. [DOI] [PubMed] [Google Scholar]
- 9.Lundborg G, Rosen B, Dahlin L, Holmberg J, Rosen I. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg [Br] 2004;29:100–107. doi: 10.1016/j.jhsb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Inada Y, Fukuda S, Yoshitani M, Nakada A, Itoi S, Kanemaru S, Endo K, Shimizu Y. Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube. Brain Res. 2004;1027:18–29. doi: 10.1016/j.brainres.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278:12599–12600. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 12.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 13.Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 15.Xu XM, Zhang SX, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Hosokawa M, Morigiwa K, Ohashi Y, Fukuda Y. Bcl-2 overexpression does not enhance in vivo axonal regeneration of retinal ganglion cells after peripheral nerve transplantation in adult mice. J Neurosci. 2002;22:4468–4477. doi: 10.1523/JNEUROSCI.22-11-04468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Suzuki Y, Noda T, Bai H, Kitada M, Kataoka K, Chou H, Ide C. Bone marrow stromal cells enhance differentiation of co-cultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res. 2003;72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- 19.Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, Mizuta, Kuno S, Ide C. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp.Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Kimura K, Matsumoto N, Ide C. Isolation of neural stem cells from the forebrain of deceased early postnatal and adult rats with protracted post-mortem intervals. J Neurosci Res. 2003;74:533–540. doi: 10.1002/jnr.10769. [DOI] [PubMed] [Google Scholar]
- 22.Ide C, Kitada M, Chakrabortty S, Taketomi M, Matumoto N, Kikukawa S, Mizoguchi A, Kawaguchi S, Endo K, Suzuki Y. Grafting of choroid plexus ependymal cells promotes the growth of regenerating axons in the dorsal funiculus of rat spinal cord – A preliminary report. Exp Neurol. 2001;167:242–251. doi: 10.1006/exnr.2000.7566. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi S, Iseda T, Nishio T. Effects of an embryonic repair graft on recovery from spinal cord injury. Prog Brain Res. 2004;143:155–162. doi: 10.1016/S0079-6123(03)43015-X. [DOI] [PubMed] [Google Scholar]
- 25.Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- 26.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 1.Lundborg G. Nerve injury and repair. Regeneration, reconstruction and cortical re-modelling. Philadelphia: Elsevier; 2004. [Google Scholar]
- 2.McAllister RM, et al. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J Hand Surg Br. 1996;21:4–13. doi: 10.1016/s0266-7681(96)80004-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaas JH, Florence SL. Mechanisms of reorganization in sensory systems of primates after peripheral nerve injury. Adv Neurol. 1997;73:147–158. [PubMed] [Google Scholar]
- 4.Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 5.Lundborg G, Rosen B. Sensory relearning after nerve repair. Lancet. 2001;358:809–810. doi: 10.1016/S0140-6736(01)06001-9. [DOI] [PubMed] [Google Scholar]
- 6.Dvali L, Mackinnon S. Nerve repair, grafting, and nerve transfers. Clin Plast Surg. 2003;30:203–221. doi: 10.1016/s0094-1298(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 7.Millesi H. Nerve grafting. Clin Plast Surg. 1984;11:105–113. [PubMed] [Google Scholar]
- 8.Viterbo F. A new method for treatment of facial palsy: the cross-face nerve transplantation with end-to-side neurorraphy. Rev Soc Bras Cir Plast Estet Reconstr. 1993;8:29. [Google Scholar]
- 9.Dellon AL. Evaluation of sensibility and re-education of sensation in the hand. Baltimore: Williams and Wilkins; 1981. [Google Scholar]
- 10.Rosén B, Balkenius C, Lundborg G. Sensory re-education today and tomorrow. Review of evolving concepts. Br J Hand Ther. 2003;8:48–56. [Google Scholar]
- 1.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration (Review) Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 2.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 3.Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- 4.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 5.Moon LD, Fawcett JW. Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGFbeta1 and beta2. Eur J Neurosci. 2001;14:1667–1677. doi: 10.1046/j.0953-816x.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 7.Dezawa M, Adachi-Usami E. Role of Schwann cells in retinal ganglion cell axon regeneration. Prog Retin Eye Res. 2000;19:171–204. doi: 10.1016/s1350-9462(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 8.Cui Q, Cho K-S, So K-F, Yip H. Synergistic effect of Nogo-neutralizing antibody IN-1 and ciliary neurotrophic factor on axonal regeneration in adult rodent visual systems. J Neurotrauma. 2004;21:617–625. doi: 10.1089/089771504774129946. [DOI] [PubMed] [Google Scholar]
- 9.Cui Q, Yip HK, Zhao RC, et al. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 1.Boncinelli E. Otx and Emx homeobox genes in brain development. Neuroscientist. 1999;5:164–172. [PubMed] [Google Scholar]
- 2.Brown M, Keynes R, Lumsden A. The developing brain. New York: Oxford University Press; 2001. [Google Scholar]
- 3.Simeone A. Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet. 2000;16:237–240. doi: 10.1016/s0168-9525(00)02000-x. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 6.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura H, Katahira T, Matsunaga E, Sato T. Isthmus organizer for midbrain and hindbrain development. Brain Res Brain Res Rev. 2005;49:120–126. doi: 10.1016/j.brainresrev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Alvarado-Mallart RM. The chick/quail transplantation model: discovery of the isthmic organizer center. Brain Res Brain Res Rev. 2005;49:109–113. doi: 10.1016/j.brainresrev.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 1.Leenders AGM, Sheng Z-H. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1α by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 3.Nagy G, Matti U, Nehring RB, Binz T, Rettig J, Neher E, Sorensen JB. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci. 2002;22:9278–9286. doi: 10.1523/JNEUROSCI.22-21-09278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sorensen JB. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki Y, Nishiki T-I, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- 6.Evans GJO, Wilkinson MC, Graham ME, Turner KM, Chamberlain LH, Burgoyne RD, Morgan A. Phosphorylation of cysteine string protein by PKA: implications for the modulation of exocytosis. J Biol Chem. 2001;276:47877–47885. doi: 10.1074/jbc.M108186200. [DOI] [PubMed] [Google Scholar]
- 7.Craig TJ, Evans GJO, Morgan A. Physiological regulation of Munc18/nSec1 phosphorylation on serine-313. J Neurochem. 2003;86:1450–1457. doi: 10.1046/j.1471-4159.2003.01955.x. [DOI] [PubMed] [Google Scholar]
- 8.Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJO, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- 9.Sakisawa T, Baba T, Tanaka S, Izumi G, Yasumi M, Takai Y. Regulation of SNARES by tomosyn and ROCK: implication in extension and retraction of neurites. J Cell Biol. 2004;166:17–25. doi: 10.1083/jcb.200405002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foletti DL, Lin R, Finley MA, Scheller RH. Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J Neurosci. 2000;20:4535–4544. doi: 10.1523/JNEUROSCI.20-12-04535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Fodor J. Psychosemantics. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- 2.Dretske F. Naturalizing the mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 3.Goodman N. Languages of art. Indianapolis: Hackett; 1976. [Google Scholar]
- 4.Peirce CS. Collected papers. Cambridge, MA: Harvard University Press; 1935. [Google Scholar]
- 5.Dretske F. Knowledge and the flow of information. Cambridge, MA: MIT Press; 1981. [Google Scholar]
- 6.Millikan R. Language, thought, and other biological categories. Cambridge, MA: MIT Press; 1984. [Google Scholar]
- 7.Churchland P. Eliminative materialism and the propositional attitudes. J Philos. 1981;78:67–90. [Google Scholar]
- 8.Dennett D. The intentional stance. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- 9.Beckermann A. Is there a problem about intentionality? Erkenntnis. 1996;45:1–23. [Google Scholar]
- 10.Cummins R. Meaning and mental representation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 1.Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- 4.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- 5.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. II. Populations Of coupled pacemaker neurons. J Neurophysiol. 1999b;82:398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MI. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979;59:1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- 7.Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- 8.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostals muscles. Physiol Rev. 2004;86:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of the respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 10.Feldman JL (1986) Neurophysiology of breathing in mammals. In: Handbook of physiology. The nervous system. intrinsic regulatory system in the brain. Am Physiol Soc, Sect. 1, vol. IV, Chap. 9. Washington, DC, pp 463–524
- 11.Feldman JL, McCrimmon DR. Neural control of breathing. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental neuroscience. 2. Amsterdam: Academic; 2003. [Google Scholar]
- 12.Feldman JL, Mitchell GG, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath; prenatal maturation of respiratory neural control. J Physiol. 2005;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 15.Klages S, Bellingham MC, Richter DW. Late expiratory inhibition of stage 2 expiratory neurons in the cat – a correlate of expiratory termination. J Neurophysiol. 1993;70:1307–1315. doi: 10.1152/jn.1993.70.4.1307. [DOI] [PubMed] [Google Scholar]
- 16.Long S, Duffin J. The neuronal determinants of respiratory rhythm. Prog Neurobiol. 1986;27:101–182. doi: 10.1016/0301-0082(86)90007-9. [DOI] [PubMed] [Google Scholar]
- 17.Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:144–191. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- 18.Nattie EE. Central chemosensitivity, sleep and wakefulness. Respiration Physiology and Neurobiology. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- 19.Netter FH (1997) Atlas of Human Anatomy, 2nd Edition. In: Dalley AE (ed) Novartis, Hanover, NJ. p. 523 pp. 967–990
- 20.Ono K, Shiba K, Nakazawa K, Shimoyama I. Synaptic origin of the respiratory-modulated activity of laryngeal motoneurons. Neuroscience. 2006;140:1079–1088. doi: 10.1016/j.neuroscience.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 21.Pierrefiche O, Shevtsova NA, St-John WM, Paton JF, Rybak IA. Ionic currents and endogenous rhythm generation in the pre-Botzinger complex: modelling and in vitro studies. Advances in Experimental Medicine and Biology. 2004;551:121–6. doi: 10.1007/0-387-27023-x_19. [DOI] [PubMed] [Google Scholar]
- 22.Purvis LK, Smith JC, Koizumi H, Butera RJ. Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. Journal of Neurophysiology. 2007;97:1515–26. doi: 10.1152/jn.00908.2006. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respiration Physiology and Neurobiology. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Richerson GB, Boron WF. Control of ventilation. In: Boron WF, Boulpaep EL, editors. Medical Physiology. Philadelphia: Elsevier Saunders; 2005. pp. 712–734. [Google Scholar]
- 25.Ritchter DW. Neural regulation of respiration: Rhythmogenesis and afferent control. In: Gregor R., Windhorst U., editors. Comprehensive Human Physiology. Berlin: Springer; 1996. pp. 2079–2095. [Google Scholar]
- 26.Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Current Opinions in Neurobiology. 1992;26:788–93. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 27.Richter DW, Ballantyne D. On the significance of post-inspiration. Funtonanalyse biologischer Systeme. 1988;18:149–156. [Google Scholar]
- 28.Richter DW, Pierrefiche O, Lalley PM, Polder HR. Voltage-clamp analysis of neurons within deep layers of the brain. Journal of Neuroscience Methods. 1996;67:121–131. [PubMed] [Google Scholar]
- 29.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends in Neuroscience. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 30.Rybak IA, Shevtsova NA, Paton JF, Dick TE, St.-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Resp. Physiol. Neurobiol. 2004a;143:307–319. doi: 10.1016/j.resp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Rybak IA, Shevtsova NA, Paton JF, Pierrefiche O, St-John WM, Haji A. Modelling respiratory rhythmogenesis: focus on phase switching mechanisms. Advances in Experimental Medicine and Biology. 2004b;551:189–94. doi: 10.1007/0-387-27023-x_29. [DOI] [PubMed] [Google Scholar]
- 32.Shao XM, Feldman JL. Cholinergic neurotransmission in PreBötzinger Complex modulates excitability of respiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respiration Physiology. 2000;122:131–47. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- 34.Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studies with cross-correlation in the decerebrate rat. Experimental Brain Research. 1996;110:196–204. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- 35.Wood PG, Daniels CB, Orgeig S. Functional significance and control of release of pulmonary surfactant in the lizard lung. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 1995;269:R838–R847. doi: 10.1152/ajpregu.1995.269.4.R838. [DOI] [PubMed] [Google Scholar]
- 1.Brandes IF, Zuperku EJ, Dean C, Hopp FA, Jakovcevic D, Stuth EA. Retrograde labeling reveals extensive distribution of genioglossal motoneurons possessing 5-HT2A receptors throughout the hypoglossal nucleus of adult dogs. Brain Res. 2007;1132:110–119. doi: 10.1016/j.brainres.2006.10.099. [DOI] [PubMed] [Google Scholar]
- 2.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- 3.Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflugers Archiv. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- 4.Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 5.Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in vivo. Neuroscience. 2006;138:1407–1424. doi: 10.1016/j.neuroscience.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 6.Peever JH, McGinty D (2007) Why do we sleep? In: Lavigne G, Choinière M, Sessle Band oja P (eds) Sleep and Pain. International Association for the Study of Pain (IASP) Press, pp. 10–15
- 7.Siegel JM. REM sleep. In: Kryger MH, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: W.B. Saunders; 2005. pp. 120–135. [Google Scholar]
- 8.Brooks P, Peever JH (in press) Role of chloride-mediated inhibition in the neurochemical control of airway motoneurons during natural sleep. Adv Exp Biol Med
- 9.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 10.Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- 2.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onimaru H, Homma I. Optical imaging of respiratory neuron activity from the dorsal view of the lower brainstem. Clin Exp Pharmacol Physiol. 2005;32:297–301. doi: 10.1111/j.1440-1681.2005.04187.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruangkittisakul A, Schwarzacher SW, Ma Y, Poon B, Secchia L, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally-imaged respiratory center neurons in online-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frermann D, Keller BU, Richter DW. Calcium oscillations in rhythmically active respiratory neurones in the brainstem of the mouse. J Physiol. 1999;515:119–131. doi: 10.1111/j.1469-7793.1999.119ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes JA, Del Negro CA. Neurokinin receptor-expressing pre-botzinger complex neurons in neonatal mice studied in vitro. J Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- 7.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 8.von Lewinski F, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol. 2001;533:227–236. doi: 10.1111/j.1469-7793.2001.0227b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Botzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 1.Feldman JL (1986) Neurophysiology of breathing in mammals. In: Handbook of physiology. The nervous system. Intrinsic regulatory systems in the brain, vol 4, chapter 9. Am Physiol Soc,Washington, DC, Sect 1, pp 463–524
- 2.Ruangkittisakul A, Secchia L, Bornes TD, Palathinkal DM, Ballanyi K. Dependence on extracellular Ca2+/K+antagonism of inspiratory centre rhythms in slices and en bloc preparations of newborn rat brainstem. J Physiol. 2007;584:489–508. doi: 10.1113/jphysiol.2007.142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 4.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- 8.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taccola G, Secchia L, Ballanyi K. Anoxic persistence of lumbar respiratory bursts and block of lumbar locomotion in newborn rat brainstem-spinal cords. J Physiol. 2007;585:507–524. doi: 10.1113/jphysiol.2007.143594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simakajornboon N, Graff GR, Torres JE, Gozal D. Modulation of hypoxic ventilatory response by systemic platelet-activating factor receptor antagonist in the rat. Respir Physiol. 1998;114(3):213–225. doi: 10.1016/s0034-5687(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Holt GA, Graff GR, Torres JE. Platelet-activating factor modulates cardiorespiratory responses in the conscious rat. Am J Physiol. 1998;275(2):R604–R611. doi: 10.1152/ajpregu.1998.275.2.R604. [DOI] [PubMed] [Google Scholar]
- 4.Kazemi H, Hoop B. Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol. 1991;70:1–7. doi: 10.1152/jappl.1991.70.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Graff GR, Gozal E, Torres JE. Modulation of the hypoxic ventilatory response by Ca2+-dependent and Ca2+-independent protein kinase C in the dorsocaudal brainstem of conscious rats. Respir Physiol. 1998;112(3):283–290. doi: 10.1016/s0034-5687(98)00038-3. [DOI] [PubMed] [Google Scholar]
- 7.Whitney GM, Ohtake PJ, Simakajornboon N, Xue YD, Gozal D. AMPA glutamate receptors and respiratory control in the developing rat: anatomic and pharmacological aspects. Am J Physiol Regul Integr Comp Physiol. 2000;278:R520–R528. doi: 10.1152/ajpregu.2000.278.2.R520. [DOI] [PubMed] [Google Scholar]
- 8.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413(6852):171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-beta receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- 10.Simakajornboon N, Szerlip NJ, Gozal E, Anonetapipat JW, Gozal D. In vivo PDGF beta receptor activation in the dorsocaudal brainstem of the rat prevents hypoxia-induced apoptosis via activation of Akt and BAD. Brain Res. 2001;895:111–118. doi: 10.1016/s0006-8993(01)02054-6. [DOI] [PubMed] [Google Scholar]
- 1.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94(1):358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 2.Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Invited review: Neural network plasticity in respiratory control. J Appl Physiol. 2003;94(3):1242–1252. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- 3.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92(1):27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 4.Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol (Lond) 2001;532:483–497. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol. 2004;140(1):1–8. doi: 10.1016/j.resp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Sahni R, Fifer WP, Myers MM. Identifying infants at risk for sudden infant death syndrome. Curr Opin Pediatr. 2007;19(2):145–149. doi: 10.1097/MOP.0b013e32808373b6. [DOI] [PubMed] [Google Scholar]
- 7.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir Physiol Neurobiol. 2006;152(3):255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joad JP, Sekizawa S, Chen CY, Bonham AC. Air pollutants and cough. Pulm Pharmacol Ther. 2007;20(4):347–354. doi: 10.1016/j.pupt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92(1):87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 1.Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- 4.Denavit-Saubié M, Foutz AS. Neuropharmacology of respiration. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural control of the respiratory muscles. New York: CRC press; 1997. pp. 143–157. [Google Scholar]
- 5.Eyzaguirre C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol Res. 2005;38:341–345. doi: 10.4067/s0716-97602005000400005. [DOI] [PubMed] [Google Scholar]
- 6.Feldman JL, McCrimmon DR. Neuronal control of breathing. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. New York: Academic press; 1999. pp. 1063–1090. [Google Scholar]
- 7.Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther. 2000;86:277–403. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 8.Hilaire G, Montau R. Brainstem and spinal control of respiratory muscles during breathing. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural control of the respiratory muscles. New York: CRC press; 1997. pp. 91–105. [Google Scholar]
- 9.Richter DW. Neural regulation of respiration: rhythmogenesis and afferent control. In: Greger R, Windhorst W, editors. Comprehensive Human Physiology. Berlin: Springer; 1996. pp. 2079–2095. [Google Scholar]
- 10.Saaresranta T, Polo O. Hormones and breathing. Chest. 2002;122:2165–2182. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- 1.Ramirez JM, Tryba AK, Peña FP. Pacemaker neurons: an integrative view. Curr Opin Neurobiol. 2004;14(6):665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and non-pacemaker neurons. J Neurophysiol. 2006;95(4):2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- 4.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 5.Steriade M. Neocortical cell classes are flexible entities. Nat Rev Neurosci. 2004;5:121–134. doi: 10.1038/nrn1325. [DOI] [PubMed] [Google Scholar]
- 6.Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229(4708):67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- 7.Tryba AK, Pena F, Ramirez JM. Stabilization of bursting in respiratory pacemaker neurons. J Neurosci. 2003;23(8):3538–3546. doi: 10.1523/JNEUROSCI.23-08-03538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshavsky YI. Cellular and network properties in the functioning of the nervous system: from central pattern generators to cognition. Brain Res Brain Res Rev. 2003;41:229–267. doi: 10.1016/s0165-0173(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 9.Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res. 1989;76(3):530–536. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- 10.Ballantyne D, Andrzejewski M, Mückenhoff K, Scheid P. Rhythms, synchrony and electrical coupling in the Locus coeruleus. Respir Physiol Neurobiol. 2004;143(2–3):199–214. doi: 10.1016/j.resp.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 1.Lee L-Y, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 2.Yu J. Airway mechanosensors. Respir Physiol Neurobiol. 2005;148:217–243. doi: 10.1016/j.resp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Berry-Kravis EM, Zhou L, Rand CM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med. 2006;174(10):1139–1144. doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296(17):2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 5.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112(2):123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 6.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol (Lond) 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paton JFR, Deuchars J, Li Y-W, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience. 2001;105(1):231–248. doi: 10.1016/s0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- 10.Sant’Ambrogio G, Tsubone H, Sant’Ambrogio FB. Sensory information from the upper airway: role in the control of breathing. Respir Physiol. 1995;102:1–16. doi: 10.1016/0034-5687(95)00048-i. [DOI] [PubMed] [Google Scholar]
- 1.Ramon-Moliner E, Nauta WJH. The isodendritic core of the brain stem. J Comp Neurol. 1966;126:311–336. doi: 10.1002/cne.901260301. [DOI] [PubMed] [Google Scholar]
- 2.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 3.Jones BE. Reticular formation: cytoarchitecture, transmitters, and projections. In: Paxinos, editor. The rat nervous system. 2. Sydney: Academic; 1995. pp. 155–171. [Google Scholar]
- 4.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 5.Olszewski (1957) Reticular formation of the brain. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT (eds) Little, Brown, Boston, MA, p 56
- 6.Blessing WW. The lower brainstem and bodily homeostasis. Oxford, NY: Oxford University Press; 1997. [Google Scholar]
- 7.Leontovich TA, Zhukova GP. The specificity of the neuronal structure and cography of the reticular formation in the brain and spinal cord of carnivore. J Comp Neurol. 1963;121:347–379. doi: 10.1002/cne.901210305. [DOI] [PubMed] [Google Scholar]
- 8.Zahm DS. The evolving theory of basal forebrain functional-anatomical ‘macrosystems’. Neurosci Biobehav Rev. 2006;30:148–72. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Nauta WJH, Feirtag M. The organization of the brain. Sci Am. 1979;241:88–111. doi: 10.1038/scientificamerican0979-88. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski J, Baxter D. Cytoarchitecture of the human brain stem. Basel: Karger; 1954. [Google Scholar]
- 1.Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66:205–241. doi: 10.1016/s0301-0082(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 2.Peterson BW, Fukushima K. The reticulospinal mechanism and its role in generating vestibular and visuomotor reflexes. In: Sjolund B, Bjorklund A, editors. Brain stem control of spinal mechanisms. New York: Elsevier; 1982. pp. 225–251. [Google Scholar]
- 3.Grantyn AA, Ong-Meang Jacques V, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat II. Morphological properties as revealed by intro-axonal injections of horseradish peroxidase. Exp Brain Res. 1987;66:355–377. doi: 10.1007/BF00243310. [DOI] [PubMed] [Google Scholar]
- 4.Grantyn AA, Hardy O, Olivier E, Gourdon A. Relationships between task-related discharge patterns and axon morphology of brainstem projection neurons involved in orienting eye and head movements. In: Shimazu H, Shinoda Y, editors. Vestibular and brain stem control of eye, head, and body movements. New York: Karger; 1992. pp. 255–273. [Google Scholar]
- 5.Isa T, Sasaki S. Mono- and disynaptic pathways from Forel’s field H to dorsal neck motoneurones in the cat. Exp Brain Res. 1992;88:580–593. doi: 10.1007/BF00228187. [DOI] [PubMed] [Google Scholar]
- 6.Grantyn AA, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat I. Behavioral properties. Exp Brain Res. 1987;66:339–354. doi: 10.1007/BF00243309. [DOI] [PubMed] [Google Scholar]
- 7.Isa T, Naito K. Activity of neurons in Forel’s field H during orienting head movements in alert head-free cats. Exp Brain Res. 1994;100:187–199. doi: 10.1007/BF00227190. [DOI] [PubMed] [Google Scholar]
- 8.Isa T, Itouji T. Axonal trajectories of single Forel’s field H neurones in the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1992;89:484–495. doi: 10.1007/BF00229872. [DOI] [PubMed] [Google Scholar]
- 9.Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. II. Pontine neurons. J Neurophysiol. 1996;76:353–370. doi: 10.1152/jn.1996.76.1.353. [DOI] [PubMed] [Google Scholar]
- 10.Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. I. Descending projections from the mesencephalon. J Neurophysiol. 1996;76:332–352. doi: 10.1152/jn.1996.76.1.332. [DOI] [PubMed] [Google Scholar]
- 1.Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol. 1988;60:263. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- 2.Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting to the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46:243. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- 3.Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol. 1985;239:276. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- 4.Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. J Neurophysiol. 1988;60:232. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- 5.Dacey DM, Ulinski PS. Optic tectum of the eastern garter snake, Thamnophis sirtalis. V. Morphology of brainstem afferents and general discussion. J Comp Neurol. 1986;245:423. doi: 10.1002/cne.902450402. [DOI] [PubMed] [Google Scholar]
- 6.Niida A, Ohono T. An extensive projection of fish dorsolateral tegmental cells to the optic tectum revealed by intra-axonal dye marking. Neurosci Lett. 1984;48:261. doi: 10.1016/0304-3940(84)90048-x. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone JR, Mark RF. Evidence for efference copy for eye movements in fish. Comp Biochem Physiol. 1969;30:931. doi: 10.1016/0010-406x(69)90048-6. [DOI] [PubMed] [Google Scholar]
- 8.Bozis A, Moschovakis AK. Neural network simulations of the primate oculomotor system. III. A one-dimensional one-directional model of the superior colliculus. Biol Cybern. 1998;79:215. doi: 10.1007/s004220050472. [DOI] [PubMed] [Google Scholar]
- 1.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 2.Nelson R, Kolb H. ON and OFF pathways in the vertebrate retina and visual system. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge: The MIT Press; 2003. pp. 260–278. [Google Scholar]
- 3.Boycott B, Wässle H. Parallel processing in the mammalian retina. The Proctor lecture. Investig Ophthalmol Vis Sci. 1999;40:1313–1327. [PubMed] [Google Scholar]
- 4.Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 5.Sterling P. How retinal circuits optimize the transfer of visual information. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge: M.I.T. Press; 2003. pp. 243–268. [Google Scholar]
- 6.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 8.Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 9.Wässle H, Grünert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vis Res. 1994;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 10.Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 1.Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- 2.Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 4.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 5.Mollon JD. “Tho' she kneel'd in the place where they grew.” The uses and origins of primate colour vision. J Exp Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. J Neurosci. 2003;23:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr Opin Neurobiol. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai M, Mullen KT. Cone weights for the two cone-opponent systems in peripheral vision and asymmetries of cone contrast sensitivity. Vision Res. 2006;46:4346–4354. doi: 10.1016/j.visres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Dacey DM, Lee BB. The ‘blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 10.Szmajda BA, Buzás P, FitzGibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proc Natl Acad Sci USA. 2006;103:19512–19517. doi: 10.1073/pnas.0606970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 12.Lee SCS, Grünert U. Connections of diffuse bipolar cells in primate retina are biased against S-cones. J Comp Neurol. 2007;502:126–140. doi: 10.1002/cne.21284. [DOI] [PubMed] [Google Scholar]
- 13.Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426:668–671. doi: 10.1038/nature02167. [DOI] [PubMed] [Google Scholar]
- 15.Roorda A, Metha AB, Lennie P, Williams DR. Packing arrangement of the three cone classes in primate retina. Vision Res. 2001;41:1291–1306. doi: 10.1016/s0042-6989(01)00043-8. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Ghosh KK, Martin PR, Grünert U. Analysis of two types of cone bipolar cells in the retina of a New World monkey, the marmoset, Callithrix jacchus. Visual Neurosci. 1999;16:707–719. doi: 10.1017/s0952523899164101. [DOI] [PubMed] [Google Scholar]
- 1.Barlow HB, Hill RM, Levick WR. Rabbit retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masland RH, Mills JW. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Famiglietti EV., Jr “Starburst” amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- 4.Vaney DI, He S, Taylor WR, Levick WR. Direction-selective ganglion cells in the retina. In: Zanker JM, Zeil J, editors. Motion Vision – Computational, Neural, and Ecological Constraints. Berlin: Springer; 2001. pp. 13–56. [Google Scholar]
- 5.Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 7.Ariel M, Daw NW. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982;324:161–185. doi: 10.1113/jphysiol.1982.sp014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- 9.Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Fried SI, Münch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- 11.He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- 12.Chiao CC, Masland RH. Starburst cells nondirectionally facilitate the responses of direction-selective retinal ganglion cells. J Neurosci. 2002;22:10509–10513. doi: 10.1523/JNEUROSCI.22-24-10509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg-Graham LJ, Grzywacz NM. A model of the directional selectivity circuit in retina: transformations by neurons singly and in concert. In: McKenna T, Zornetzer SF, Davis JL, editors. Single Neuron Computation. San Diego, CA: Academic Press; 1992. pp. 347–375. [Google Scholar]
- 14.Miller RF, Bloomfield SA. Electroanatomy of a unique amacrine cell in the rabbit retina. Proc Natl Acad Sci USA. 1983;80:3069–3073. doi: 10.1073/pnas.80.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrikov KE, Dmitriev AV, Keyser KT, Mangel SC. Cation–chloride cotransporters mediate neural computation in the retina. Proc Natl Acad Sci USA. 2003;100:16047–16052. doi: 10.1073/pnas.2637041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dacheux RF, Chimento MF, Amthor FR. Synaptic input to the ON-OFF directionally selective ganglion cell in the rabbit retina. J Comp Neurol. 2003;456:267–278. doi: 10.1002/cne.10521. [DOI] [PubMed] [Google Scholar]
- 1.Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 2.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 3.Wässle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 4.Dacey D. Origins of perception: retinal ganglion cell diversity and the creation of parallel pathways. In: Gazzaniga MS, editor. The cognitive neurosciences III. Cambridge Mass: MIT Press; 2004. pp. 281–301. [Google Scholar]
- 5.Martin PR, Grünert U. Ganglion cells in mammalian Retinae. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge Mass: MIT Press; 2004. pp. 410–421. [Google Scholar]
- 6.Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7:155–169. doi: 10.1017/s0952523800011020. [DOI] [PubMed] [Google Scholar]
- 7.Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- 9.Levick WR. Receptive fields of cat retinal ganglion cells with special reference to the alpha cells. Progr Ret Eye Res. 1996;15:457–500. [Google Scholar]
- 10.Rodieck RW. The first steps in seeing. Sunderland Mass: Sinauer Associates; 1998. [Google Scholar]
- 11.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 12.Meister M, Berry MJ. The neural code of the retina. Neuron. 1999;22:435–450. doi: 10.1016/s0896-6273(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 1.Passaglia CL, Enroth-Cugell C, Troy JB. Effects of remote stimulation on the mean firing rate of cat retinal ganglion cells. J Neurosci. 2001;21:5794–5803. doi: 10.1523/JNEUROSCI.21-15-05794.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartline HK, Ratliff F. Inhibitory interaction of receptor units in the eye of Limulus. J Gen Physiol. 1957;40:357–376. doi: 10.1085/jgp.40.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb TD, Simon EJ. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976;263:257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detwiler PB, Hodgkin AL, McNaughton PA. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb TD. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976;263:239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 8.Spillmann L. The Hermann grid illusion: a tool for studying human perspective field organization. Perception. 1994;23:691–708. doi: 10.1068/p230691. [DOI] [PubMed] [Google Scholar]
- 9.Teranishi T, Negishi K, Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983;301:243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- 10.Dowling JE. The Charles F. Prentice Medal Award Lecture 1991: dopamine; a retinal neuromodulator. Optom Vis Sci. 1992;69:507–514. doi: 10.1097/00006324-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci. 2004;21:791–805. doi: 10.1017/S0952523804045122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan MV, Laughlin SB, Dubs A. Predictive coding: a fresh view of inhibition in the retina. Proc R Soc Lond B Biol Sci. 1982;216:427–459. doi: 10.1098/rspb.1982.0085. [DOI] [PubMed] [Google Scholar]
- 13.Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- 14.DeVries SH, Qi X, Smith R, Makous W, Sterling P. Electrical coupling between mammalian cones. Curr Biol. 2002;12:1900–1907. doi: 10.1016/s0960-9822(02)01261-7. [DOI] [PubMed] [Google Scholar]
- 1.Rodieck RW. The first steps in seeing. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 2.Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic – adaptive design. Prog Retin Eye Res. 2000;19:711–777. doi: 10.1016/s1350-9462(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 3.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 4.Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- 5.Knabe W, Skatchkov S, Kuhn HJ. “Lens mitochondria” in the retinal cones of the tree-shrew Tupaia belangeri. Vis Res. 1997;37:267–271. doi: 10.1016/s0042-6989(96)00199-x. [DOI] [PubMed] [Google Scholar]
- 6.Walls GL. The vertebrate eye and its adaptive radiation. New York: Hafner; 1942. [Google Scholar]
- 7.Burnside B, Wang E, Pagh-Roehl K, Rey H. Retinomotor movements in isolated teleost retinal cone inner-outer segment preparations (CIS-COS): effects of light, dark and dopamine. Exp Eye Res. 1993;57:709–722. doi: 10.1006/exer.1993.1179. [DOI] [PubMed] [Google Scholar]
- 8.Haverkamp S, Grünert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000;196:245–313. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- 10.Hart NS. The visual ecology of avian photoreceptors. Prog Retin Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- 1.Migdale K, Herr S, Klug K, Ahmad K, Linberg K, Sterling P, Schein S. Two ribbon synaptic units in rod photoreceptors of macaque, human, and cat. J Comp Neurol. 2003;455:100–112. doi: 10.1002/cne.10501. [DOI] [PubMed] [Google Scholar]
- 2.Lysakowski A, Goldberg J. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol. 1997;389:419–433. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. doi: 10.1016/s0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 4.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz F, Konigstorfer A, Sudhof T. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 6.tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick O, tom Dieck S, Altrock W, Ammermuller J, Weiler R, Garner C, Gundelfinger E, Brandstatter J. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 8.Khimich D, Nouvian R, Pujol R, tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- 9.Morgans CW. Presynaptic proteins of ribbon synapses in the retina. Microsc Res Tech. 2000;50:141–150. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Prescott ED, Zenisek D. Recent progress towards understanding the synaptic ribbon. Curr Opin Neurobiol. 2005;15:431–436. doi: 10.1016/j.conb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 1.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 2.Provencio I. Melanopsin Cells. 1.20:423–431. In: Basbaum AI, Bushnell M, Smith D, Beauchamp G, Firestein S, Dallos P, Oertel D, Masland R, Albright T, Kaas J, Gardner E, editors. The senses: a comprehensive reference. Amsterdam: Elsevier; 2007. p. 4640. [Google Scholar]
- 3.Moore RY, Leak RK. Suprachiasmatic Nucleus. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian clocks: handbook of behavioral neurobiology. New York: Kluwer Publishers; 2001. p. xxiii. [Google Scholar]
- 4.Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Magnin M, Cooper HM, Mick G. Retinohypothalamic pathway: a breach in the law of Newton-Muller-Gudden? Brain Res. 1989;488:390–397. doi: 10.1016/0006-8993(89)90737-3. [DOI] [PubMed] [Google Scholar]
- 6.Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 7.Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- 8.Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- 9.Hannibal J. Roles of PACAP-containing retinal ganglion cells in circadian timing. Int Rev Cytol. 2006;251:1–39. doi: 10.1016/S0074-7696(06)51001-0. [DOI] [PubMed] [Google Scholar]
- 10.Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:335–338. doi: 10.1046/j.1365-2826.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- 1.Tao HW, Poo M. Retrograde signaling at central synapses. Proc Natl Acad Sci USA. 2001;98:11009–11015. doi: 10.1073/pnas.191351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 2.Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 5.Maejima T, Ohno-Shosaku T, Kano M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res. 2001;40:205–210. doi: 10.1016/s0168-0102(01)00241-3. [DOI] [PubMed] [Google Scholar]
- 6.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 7.Fukudome Y, et al. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signaling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- 8.Hashimotodani Y, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 10.Gerdeman GL, Ronesi J, Lovinger DM. Potsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 1.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 2.Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- 3.Murai KK, Pasquale EB. “Eph” ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 4.Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- 5.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 6.Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 8.Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin-1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- 9.Cafferty P, Yu L, Long H, Rao Y. Semaphorin-1a functions as a guidance receptor in the Drosophila visual system. J Neurosci. 2006;26:3999–4003. doi: 10.1523/JNEUROSCI.3845-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 1.Pipkin AC. Applied mathematics series. Berlin: Springer; 1972. Lectures on viscoelasticity theory. [Google Scholar]
- 2.Fung YC. Biomechanics. New York: Spinger; 1981. [Google Scholar]
- 3.Epstein M, Herzog W. Theoretical models of skeletal muscle. Chichester: Wiley; 1998. [Google Scholar]
- 1.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson D, Silletti J, Murphy G, D’Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Watabe-Uchida M, Govek EE, Van Aelst L. Regulators of Rho GTPases in neuronal development. J Neurosci. 2006;26:10633–10635. doi: 10.1523/JNEUROSCI.4084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 6.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 7.Govek EE, Newey SE, Van Aelst L. The role of Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 8.Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–212. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- 9.Ellezam B, Dubreuil C, Winton M, Loy L, Dergham P, Selles-Navarro I, McKerracher L. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog Brain Res. 2002;137:371–380. doi: 10.1016/s0079-6123(02)37028-6. [DOI] [PubMed] [Google Scholar]
- 1.Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J Neurophysiol. 2003;90:1714–27. doi: 10.1152/jn.00202.2003. [DOI] [PubMed] [Google Scholar]
- 2.Jordan LM. Initiation of locomotion in mammals. Ann N Y Acad Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 3.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 4.Fetcho JR. Spinal network of the Mauthner cell. Brain Behav Evol. 1991;37:298–316. doi: 10.1159/000114367. [DOI] [PubMed] [Google Scholar]
- 5.Stein PS. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:213–29. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- 6.Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In Brooks V (ed) Handbook of Physiology, Bethesda, pp 1176–236
- 7.Pearson KG. Proprioceptive regulation of locomotion. Curr Opin Neurobiol. 1995;5:786–91. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 8.Hultborn H. State-dependent modulation of sensory feedback. J Physiol. 2001;533:5–13. doi: 10.1111/j.1469-7793.2001.0005b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlovsky GN, Deliagina TG, Grillner S. Neuronal control of locomotion. From mollusc to man. Oxford: Oxford University Press; 1999. [Google Scholar]
- 11.Getting P. Comparative analysis of invertebrate spinal pattern generators. In: Cohen A, Rossignol S, Grillner S, editors. Neural control of rhythmic movements in vertebrates. New York: Wiley; 1988. pp. 101–28. [Google Scholar]
- 12.Calabrese RL, Peterson E. Neral control of heartbeat in the leech, Hirido medicinalis. In: Roberts A, Roberts B, editors. Neural origin of rhythmic movements. Cambridge: Cambridge University Press; 1983. pp. 195–221. [Google Scholar]
- 13.Selverston A, Moulin M. The crustacean stomatogastric system. Berlin: Springer; 1987. [Google Scholar]
- 14.Harris-Warrick RM. Voltage-sensitive ion channels in rhythmic motor systems. Curr Opin Neurobiol. 2002;12:646–51. doi: 10.1016/s0959-4388(02)00377-x. [DOI] [PubMed] [Google Scholar]
- 15.Grillner S, Wallen P, Brodin L, Lansner A. Neuronal network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties, and simulation. Annu Rev Neurosci. 1991;14:169–99. doi: 10.1146/annurev.ne.14.030191.001125. [DOI] [PubMed] [Google Scholar]
- 16.Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao FY. Central circuits controlling locomotion in young frog tadpoles. Ann N Y Acad Sci. 1998;860:19–34. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- 17.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–86. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 18.Dale N, Kuenzi FM. Ion channels and the control of swimming in the Xenopus embryo. Prog Neurobiol. 1997;53:729–56. doi: 10.1016/s0301-0082(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 19.Grillner S. Ion channels and locomotion. Science. 1997;278:1087–8. doi: 10.1126/science.278.5340.1087. [DOI] [PubMed] [Google Scholar]
- 20.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 21.Lund J, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006;21(3):167–74. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- 22.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–74. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–72. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 25.Kiehn O, Quinlan KA, Restrepo CE, Lundfald L, Borgius L, et al. Excitatory components of the walking mammalian locomotor CPG. Brain Res Rev. 2008;57(1):56–63. doi: 10.1016/j.brainresrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–59. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 27.Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–21. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- 28.Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- 29.Kiehn O, Katz P. Making circuits dance: modulation of motor pattern generation. In: Katz P, editor. Beyond neurotransmission. neuromodulation and its importance for information processing. Oxford: Oxford University Press; 1999. pp. 275–317. [Google Scholar]
- 30.Nusbaum M, Beenhakker M. A small-systems approach to motor pattern generation. Nature. 2002;417(6886):343–250. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 32.Katz P, Getting P, Frost W. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature. 1994;367:729–31. doi: 10.1038/367729a0. [DOI] [PubMed] [Google Scholar]
- 33.El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev. 2008;57(1):29–36. doi: 10.1016/j.brainresrev.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23:560–70. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- 35.Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res. 1999;123:349–65. doi: 10.1016/s0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- 1.Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng J, Maquat LE. Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol Cell Biol. 1993;13:1892–1902. doi: 10.1128/mcb.13.3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frischmeyer PA, van Hoof A, O’Donnell K, Guerrerio AL, Parker R, Dietz HC (2002) An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. 95:2258–2261 [DOI] [PubMed]


