Abstract
Group-living animals vary in social behavior across multiple dimensions, including group size, extent of cooperation, and the specificity vs. gregariousness of social behavior. Use of standardized assessments of social behavior across different species serves a dual function of revealing specific behavioral components that may underlie collective behavior in natural habitats, and providing a basis for comparative methods to understand neural processes supporting social behaviors. Degus (Octodon degus) are South American caviomorph rodents that nest and forage in groups with relatively low genetic relatedness. Flexibility in group membership is likely supported by gregariousness toward strangers, but the relative preference for strangers compared with familiar individuals has not been systematically tested. We assessed the specificity of social preferences in female degus using same-sex partner preference tests. Degus huddled extensively with both familiar and unfamiliar peers, with no average preference for one over the other. Detailed analysis of social interactions demonstrated an effect of familiarity on social investigation and aggressive behaviors, indicating that degus recognized familiar conspecifics, even though it did not impact huddling. This behavioral profile is thus far unique to degus; in similar tests, meadow and prairie voles exhibit strong partner preferences for known peers, while mice exhibit low social huddling and spend relatively less time in social chambers. Understanding how group living species differ in specific aspects of social behavior such as familiarity/novelty preference and propensity for social contact will offer a foundation to interpret differences in neural systems supporting sociality.
Keywords: partner preference, partner preference test, familiarity, social behavior, affiliation, sociality, Octodon degus, degu
INTRODUCTION
All animals engage in some form of social interaction, but species differ widely in the nature and extent of these interactions. One distinction of particular relevance to group living species is the propensity to interact with familiar versus unfamiliar conspecifics. It might be expected that species with stable and/or family-based groups would exhibit intrinsic preferences for familiar individuals, while a tendency to quickly affiliate with or tolerate strangers may accommodate more dynamic social groupings, allowing groups to be relatively preserved even in the case of high turnover of group members. Laboratory tests of preference for familiar vs. unfamiliar peers (i.e. same-sex conspecifics) in group-living animals support this in the limited number of examples to date, but testing a greater diversity of species using a uniform experimental paradigm will aid in establishing the generality of this finding. By assessing familiarity preferences in degus—a highly social, relatively gregarious rodent now used in social neuroscience studies—we aim to gain an understanding of how their natural behavior relates to partner preference test behavior, as well increase our general understanding of interspecific variation in peer partner preferences.
Degus (Octodon degus) are a highly social species of rodent from Chile. As caviomorph rodents, degus are part of a distinct suborder of rodents from mice and voles, separated by over 66 million years of evolution (Fabre et al., 2012). The cohabitation patterns of degus suggest a high level of female peer affiliation, with individual burrows often including only 0–2 males but 1–8 females (Hayes et al., 2009; Ebensperger et al., 2012; Fulk, 1976; Ebensperger et al., 2002). Females often care for and nurse one-another’s offspring (Ebensperger et al., 2002), and stability of female relationships has been found to increase during lactation (Wey et al., 2013). Notably, degu social groups are characterized by relatively low levels of genetic relatedness (Davis et al., 2016a; Ebensperger et al., 2004; Quirici et al., 2011), indicating that kin selection is not a significant driver of social living in degus. This is also supported by the fact that degus are far more sensitive to whether or not they have encountered another individual, or that individual’s scent, than they are to genetic relatedness (Villavicencio et al., 2009). When group membership changes, this may have fitness consequences that differ by sex. The direct fitness of females increased with higher numbers of females in stable groups, while male fitness was high in large but less stable groups (Ebensperger et al., 2016). As many breeding females do not live long enough to give birth to more than one litter (Davis et al., 2016a; Ebensperger et al., 2009, but see Meserve et al. 1995), building and maintaining social groups may depend on rapid acceptance of initial strangers. This sociality may be promoted by a variety of environmental factors: both predation risk and burrowing costs have been associated with group size (Ebensperger et al., 2012), and fitness advantages of larger group sizes appear to be stronger during years of lower precipitation and low food availability (Ebensperger et al., 2014). The dynamic group structure and high sociality of degus make this species offers a unique test case for investigating peer affiliation, and provides a valuable point of contrast with other social rodents.
The partner preference test (PPT) is an extended test of familiarity vs. novelty preferences that was originally developed to assess preferences for mates in socially monogamous prairie voles (Microtus ochrogaster) (e.g. Williams et al., 1992; Winslow et al., 1993). In the PPT, a focal animal is allowed to move between a neutral empty chamber and chambers containing a tethered opposite-sex or same-sex familiar partner and stranger for a three-hour interval. This relatively long test duration allows animals to shift from exploration to resting social contact, with familiarity preferences manifesting by the second hour and well-established by the third hour (Williams et al., 1992). The test has been used to assess mate preferences in prairie voles and other monogamous species (Goodson et al., 2004; Smiley et al., 2012; Carp et al., 2016), but also offers insight into the selectivity of non-reproductive relationships between same-sex peers, such as those present in group living species. Previous studies have shown that prairie voles, which often reside in mate pairs and small family groups (Getz et al., 1993) exhibit highly selective preferences for huddling with familiar same-sex conspecifics (DeVries et al., 1997; Beery et al., 2018; Lee et al., 2019). Meadow voles (Microtus pennsylvanicus) are seasonally social rodents that form social groups that become exclusive over the course of winter (Madison et al., 1984; Madison and McShea, 1987); individuals of this species also exhibit strong same-sex partner preferences for familiar conspecifics (Parker and Lee, 2003; Beery et al., 2008, 2009). In contrast, mice and rats live in groups of widely ranging size and composition (Berdoy and Drickamer, 2007). These species exhibit a high level of social investigation, but do not prefer to interact with familiar individuals (Schweinfurth et al., 2017; Beery et al., 2018). The assessment of degus, for whom field data reveal variable social groups, should provide valuable insight into species-specific variation in this standardized assessment and how it relates to natural behavior.
The value of studying degu social behavior using standardized measures like the PPT also comes from the increasing use of degus for studying the physiological mechanisms of social behavior (Colonnello et al., 2011). A number of neuroscience tools have already been applied toward studying the degu brain, including neuroimaging (Bock et al., 2012), electrophysiology (Perryman, 2011), brain lesions (Uekita and Okanoya, 2011), morphometry (Sobrero et al., 2016), neuropeptide receptor localization (Beery et al., 2016), and the construction of a 3D, stereotaxic brain atlas (Kumazawa-Manita et al., 2013). Laboratory husbandry methods have become well established (Colby et al., 2012), and specific frequencies of different social behaviors have been described and quantified in animals of different life stages (e.g., juveniles: Sergio M. Pellis et al., 2010; Wilson, 1982; adults: Fulk, 1976). By comparing specific social behaviors in degus with other models used in social neuroscience (most notably mice, prairie voles, and meadow voles), it will be possible to better frame the similarities and differences of the degu brain with these other species.
Based on reports cited above indicating that kinship has little impact on the species’ social organization, it might be expected that females readily establish new relationships with initially unfamiliar conspecifics. Here we confirm that prediction, finding that, in contrast with mice, degus spent nearly all of their time in social chambers, but in contrast with voles, degus show no systematic preference for familiar conspecifics compared with strangers.
MATERIALS AND METHODS
Animal subjects
Degus from an in-house breeding colony were housed in same-sex groups of 2–4 individuals in plastic cages (50.8 × 40.6 × 21.6 cm or 61 × 43.2 × 20.3 cm—dependent on group size). Degus were fed a 1:1 mixture of chinchilla and guinea pig “Teklad” feeds (Envigo; Indianapolis, IN). The light cycle was 12:12 light:dark; degus are diurnal so all tests were run during their light cycle. Ten adult female degus (mean±SEM: 353±49 days of age, range: 157–623 days) were the focal subjects in partner preference tests, with ten cohoused female degus serving as “partners.” Unfamiliar females, age-matched within approximately one-month, served as “strangers.” There were no significant correlations between age and any outcome measure (e.g. age at testing vs. time huddling: R=.25, p=0.49), thus it was not used as a factor in analyses.
Although strangers shared the same vivarium, the animals designated as strangers had never encountered the focal animal directly. Females were the focus of the present study, as degu groups in the wild rarely contain more than 2 males, but may contain of up to 8 females. Animal care and use followed recommendations of the Guide for the Care and Use of Laboratory Animals published by the Natural Research Council, adhered to federal and institutional guidelines, and was approved by the Institutional Animal Care and Use Committee at the University of Montana, Missoula.
Partner Preference Tests
Peer PPTs were conducted in a linear 3-chambered apparatus scaled for degus (30×30×112cm) according to previously described methods (Beery et al., 2018). Familiar “partner” and novel “stranger” conspecifics were tethered at opposite ends of the apparatus. Tethered animals were acclimated to the chamber for 5 min before placement of the focal female degu in the center, neutral chamber. Tests lasted 180 minutes and were video recorded for analysis. Location of the partner and the stranger was alternated between successive tests. Partners were re-used as strangers 0–1 times, a minimum of one day after initial testing.
Data Analysis
Video recordings were scored for time in each chamber and duration of resting body-contact (huddling) using Intervole Timer v1.6 (A. Beery) without knowledge of partner and stranger positions. The number of aggressive interactions was tallied at the same time. Partner preference was defined as significantly more time adjacent to the cagemate partner than the stranger. Preference score was defined as relative preference for the partner (time adjacent to the partner/(partner+stranger). Secondary analysis of detailed social interactions was conducted using BORIS v7 (Friard and Gamba, 2016) to quantify time spent in anogenital investigation, face-to-body investigation, grooming, and agonistic behaviors (wrestling, boxing, or biting) toward the partner and stranger.
Statistical tests are listed with the results, and were conducted two-tailed. Analyses were conducted in R v3.3.0 (the R project for Statistical Computing) and GraphPad Prism 7.0.
RESULTS
Social Preference
Test (focal) degus spent an average of 97±0.9% of the PPT in one of the chambers containing a stimulus degu (the partner or the stranger), rather than the center, “neutral” chamber. On average, subjects did not exhibit a preference for the partner versus the stranger chamber, though degus clearly preferred social chambers over the unoccupied center chamber (p<0.0001, one-way RM-ANOVA; partner vs. center: p<0.0001, stranger vs. center: p<0.0001, partner vs. stranger: p=0.69, Tukey’s HSD, Fig 1A). Approximately half of the testing time (49.4±6.3%) was spent in resting contact with a stimulus degu. Degus huddled extensively with both familiar and unfamiliar stimulus individuals, showing no apparent systematic preference for one over the other (t(9)=0.47, p=0.65, paired t-test, Fig 1B). All individual degus crossed back and forth between social chambers, exploring both sides. This was illustrated by the fluctuations and zero crossings of the cumulative time each animal spent in the partner compared with the stranger chamber (Fig 1C). The same pattern was seen with huddling, where most degus appeared to split their time between huddling with partners versus strangers (Fig 1D).
Figure 1.
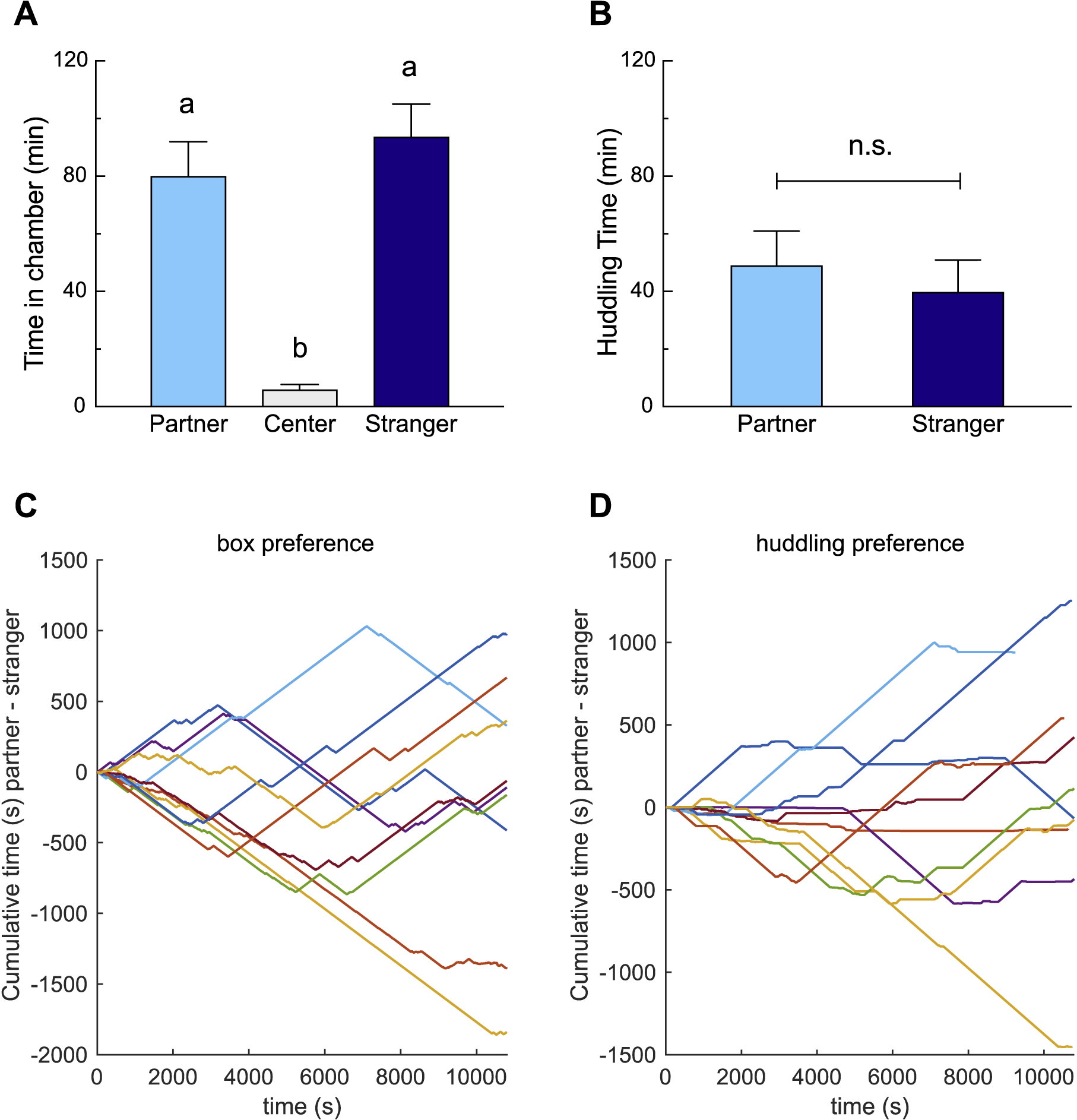
Partner preference test behavior. A. Degus spent significantly more time in the social chambers than in the center, unoccupied chamber (each p<0.0001). Time in the social chambers did not differ from each other. B. Degus huddled extensively with both partners and strangers. C. Cumulative distribution of chamber preference (partner chamber-stranger chamber) over the 3h test. D. Cumulative distribution of social preference (partner huddling-stranger huddling) over 3h. Individual preferences were heterogenous: some degus huddled principally with the partner or stranger, while others huddled with both individuals. Different letters denote groups that are significantly different (Tukey’s test).
Interaction behavior
Additional social interactions were quantified to assess whether the nature of social investigation and interaction differed between focals and their familiar cage-mates versus novel conspecifics beyond huddling and chamber time. Four interactive behaviors were quantified: sniffing of the head or body, anogenital investigation/sniffing of the rear, grooming, and agonistic interactions. Duration of interactions was found to differ depending on social familiarity (stranger vs. partner: F(1,36) = 6.261, p = 0.017), behavior type (sniffing head or body, sniffing rear, grooming, or agonistic interaction: F(3,36) = 4.033, p = 0.014), and the interaction between the two (F(3,36) = 3.18, p = 0.036; 2-way RM-ANOVA).
Post-hoc tests of the effect of familiarity on each behavior revealed that only agonistic interactions differed significantly between partners and strangers (p=0.0017; Sidak’s multiple comparisons test). Most degus exhibited few to no aggressive interactions, but a few (3 out of 10 females) were highly aggressive (range: 0–451 aggressive interactions, median: 4, mean: 106±55), and their aggression was biased towards strangers. Females directed 105.2±55.3 episodes of aggression toward novel degus, but only 0.8±0.49 episodes toward familiar partners. Low stranger huddling (<20 min) but not low partner huddling was associated with higher counts of total aggression (t(8)=−3.22, p=0.01) and stranger directed aggression (t(8)=−3.21, p=0.01).
DISCUSSION
Degus exhibited extensive social interaction during the partner preference test, spending nearly the entire test in occupied chambers, and half of the test in resting social contact with a same-sex peer. Degus exhibited no preference for huddling with the partner over a stranger. This finding is consistent with predictions based on the known social organization of degus, in which groups of typically unrelated females cohabitate, nurse one-another’s young, and maintain contact in the field (Beery et al., 2016; Davis et al., 2016b; Ebensperger et al., 2002, 2012, 2009; Fulk, 1976; Hayes et al., 2009) Importantly, as illustrated by Figure 1C and D, degus explored and huddled with both subjects throughout the test; many focal animals spent time in one social chamber during the first few minutes or hours, then shifted to the other over the course of the experiment. Most focal degus huddled for over 10 minutes with both the partner and stranger, suggesting that the interest in strangers was not only one of active investigation, but also of relatively inactive, affiliative physical contact. There were multiple exceptions, with three focal degus engaging in primarily agonistic interactions toward the stranger rather than huddling.
Degu behavior in the PPT is distinct from that of other rodents described to date. Like degus, mice (Beery et al., 2018) and rats (Beery and Shambaugh, unpublished data) do not display partner preferences for familiar same-sex peers in the 3h PPT, nor do they appear to form specific social bonds under typical conditions (rats: Schweinfurth et al., 2017; mice: Harrison et al., 2016). However, mice and rats appear less interested in sustained social interaction, spending little time in social contact in the peer PPT, and much less time in social chambers compared with degus. Vole species have been extensively tested in the PPT. Both meadow and prairie voles display high levels of social contact and time in the social chambers in peer PPTs, as did degus in the present study, but unlike degus, peer huddling in both species of voles is selectively biased toward the familiar partner (DeVries et al., 1997; Parker and Lee, 2003; Beery et al., 2008, 2009; Beery and Zucker, 2010; Anacker et al., 2016a, 2016b; Beery et al., 2018; Goodwin et al., 2019; Lee et al., 2019). Peer partner preference in voles is not limited to a single partner; seasonally social meadow voles housed in groups in winter-like photoperiods exhibit equivalently strong preferences for each of their cagemates relative to strangers (Beery et al., 2009). Thus familiarity preferences in this species are a characteristic of the social group.
Different patterns of social behavior in the peer PPT may be related to species-specific differences in group membership, and one pattern is not clearly “more social” than another. For example, in prairie voles, high selective affiliation for familiar peers is accompanied by high aggression towards unfamiliar ones (Lee et al., 2019). While meadow voles groups are selective, changes in group membership do occur subsequent to predation and immigration (reviewed in (Beery, 2019). Thus, despite preference for familiar voles, new individuals may be added to the group. This may be facilitated by reduced aggression towards strangers in winter-like day lengths (Lee et al., 2019). Group composition may thus be shaped by selective affiliation, selective aggression, and lack of selectivity. Among rodents tested to date, female degus appear uniquely motivated to spend time in close social contact with unfamiliar conspecifics. This gregarious social motivation may be functionally related to degus’ variable group structure in the field.
The lack of partner preference in degus seen in the present study does not reflect lack of social recognition. Detailed examination of investigation behavior revealed that degus interacted more with strangers, and that this was disproportionately higher for some types of behaviors (i.e., there was an interaction between social familiarity and behavior type). These results are also consistent with previous observations showing increased interaction between degus that have not been previously exposed to one-another, independent of kinship (Villavicencio et al., 2009). While these data cannot offer insight into the causes of motivation toward strangers, a seeming inevitable consequence would be that degus learn about and establish a relationship with the new conspecific. This is true both in the case of aggressive interactions, where a dominance relationship may be forming, and of the non-aggressive huddling, where the relationship is more affiliative. Both agonistic and affiliative social interactions have been shown to be rewarding in some contexts in other rodents (e.g. Panksepp and Lahvis, 2007; Dölen et al., 2013; Golden et al., 2017; Borland et al., 2017; Goodwin et al., 2019). Future work would be necessary to determine whether extended exposures to the stranger result in specific patterns of behavior, or neural changes related to learning and memory.
The present demonstration that female degus are highly social with both familiar and unfamiliar, same-sex conspecifics can be placed into an ecological context. Research in wild degus has revealed that groups experience relatively high turn-over both within and across seasons, reflecting the introduction and inclusion of new group members (Ebensperger et al. 2009). Field studies also demonstrate a number of benefits to group-living in females, and suggest that environmental factors that put strains on survival may also increase selective pressures for sociality (Ebensperger et al., 2016, 2014; Ebensperger and Wallem, 2002). While females may benefit from increasing number of females in the group, there appear to be fitness costs associated with increased numbers of males (Hayes et al., 2019). Males may still benefit from larger groups due to their apparently opportunistic mating patterns, and the increased likelihood of finding reproductive partners (Ebensperger et al., 2019). Thus, both males and females seem predisposed to establish affiliative interactions with novel (sex-specific) individuals.
Affiliative social behavior is often described as a unified construct, despite the occurrence of markedly variable combinations of constituent behaviors across species (Goodson, 2013). Such variability in individual behaviors applies even to species that share common descriptions of types of social behavior, such as “group living.” The present study reveals a pattern of behavior characterized by a high degree of social interaction and physical contact in the absence of preference for long-term social partners, or novel individuals. Species-specific variation in social preferences highlights the advantages of studying multiple and diverse social organisms. Characterization of the nature of behavioral differences between “social” species should therefore improve our understanding of the mechanisms that support sociality in its many forms.
Figure 2.
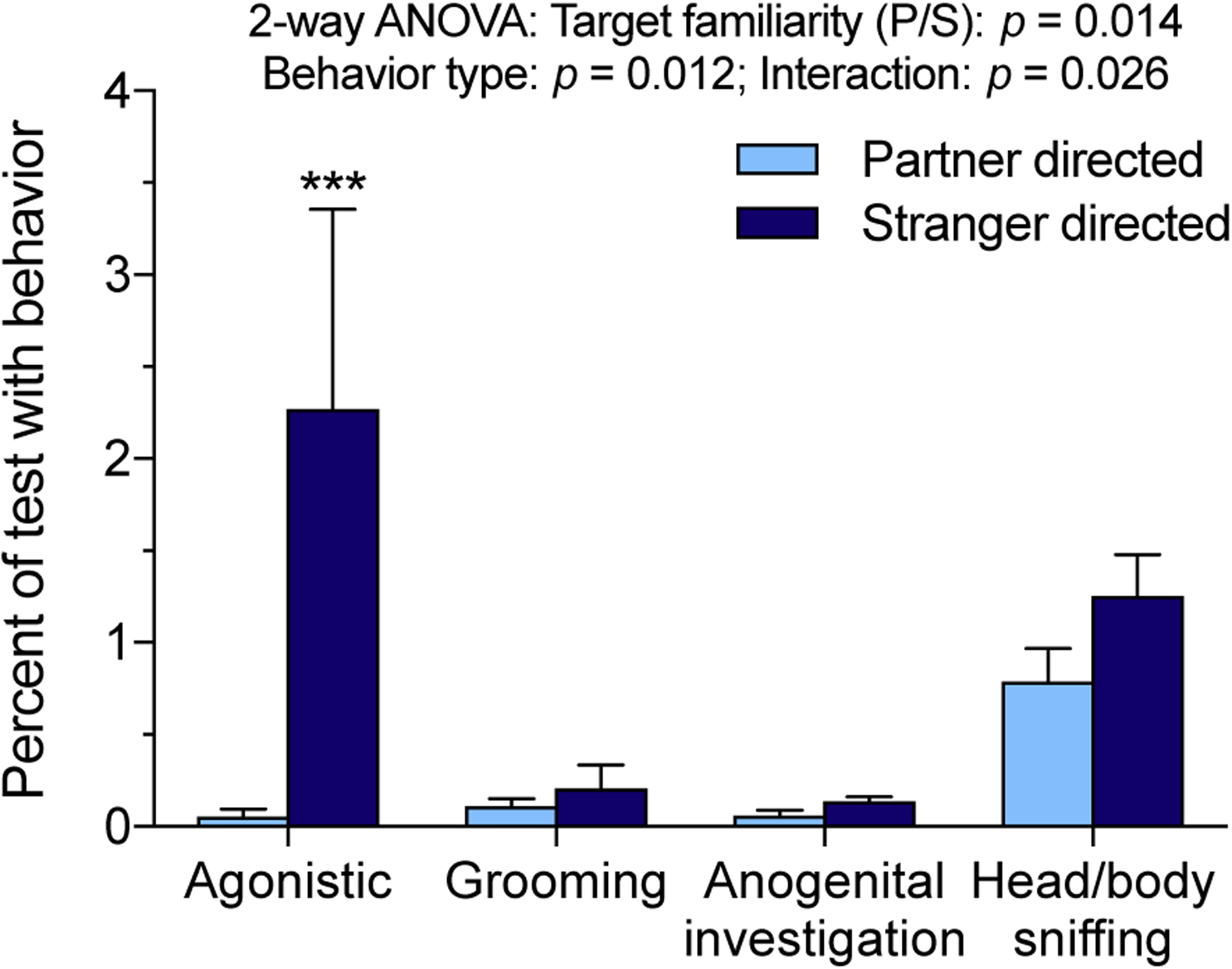
Time spent in additional social interaction behaviors. Across behaviors there was a significant main effect of tethered stimulus degu familiarity (partner vs. stranger) as well as behavior type, and an interaction of familiarity and behavior (2-way RM-ANOVA). Comparison of partner and stranger directed behavior within categories revealed significant variation in agonistic behavior with familiarity (Sidak’s multiple comparisons). *** p < 0.001.
Highlights.
Group-living animals exhibit diverse social structures
Comparative study of familiarity/novelty preference should aid understanding of different mechanisms underlying sociality
Degus are group-living South American rodents, studied in both the lab and field
We assessed the selectivity of social preferences in female degus using same-sex partner preference tests
Degus were highly social, choosing both familiar and unfamiliar companions over an empty chamber
Degus recognized but did not prefer contact with familiar group members over novel strangers
These patterns are related to those found in mice, rats, prairie voles and meadow voles
ACKNOWLEDGEMENTS
We are grateful to Sarah Lopez for assistance with apparatus design and planning. This work was supported by the National Institute Of Mental Health of the National Institutes of Health (award R15MH113085).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
None.
REFERENCES
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK, 2016a. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology 68, 156–162. 10.1016/j.psyneuen.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, Beery AK, 2016b. Stress impairs new but not established relationships in seasonally social voles. Horm. Behav 79, 52–57. 10.1016/j.yhbeh.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Beery A, Kamal Y, Sobrero R, Hayes LD, 2016. Comparative neurobiology and genetics of mammalian social behavior, in: Ebensperger LA, Hayes LD (Eds.), Sociobiology of Caviomorph Rodents. John Wiley & Sons, Ltd, Chichester, UK, pp. 59–90. 10.1002/9781118846506.ch3 [DOI] [Google Scholar]
- Beery AK, 2019. Frank Beach award winner: Neuroendocrinology of group living. Horm. Behav 107, 67–75. 10.1016/j.yhbeh.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, Blandino KL, 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Front. Behav. Neurosci 12 10.3389/fnbeh.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, Zucker I, 2008. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm. Behav 54, 153–159. 10.1016/j.yhbeh.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Routman DM, Zucker I, 2009. Same-sex social behavior in meadow voles: Multiple and rapid formation of attachments. Physiol. Behav 97, 52–57. 10.1016/j.physbeh.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–673. 10.1016/j.neuroscience.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Berdoy M, Drickamer LC, 2007. Chapter 32 Comparative Social Organization and Life History of Rattus and Mus 14. [Google Scholar]
- Bock J, Riedel A, Braun K, 2012. Differential changes of metabolic brain activity and interregional functional coupling in prefronto-limbic pathways during different stress conditions: functional imaging in freely behaving rodent pups. Front. Cell. Neurosci 6 10.3389/fncel.2012.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Frantz KJ, Aiani LM, Grantham KN, Song Z, Albers HE, 2017. A novel operant task to assess social reward and motivation in rodents. J. Neurosci. Methods 287, 80–88. 10.1016/j.jneumeth.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL, 2016. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus). Am. J. Primatol 78, 326–339. 10.1002/ajp.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby LA, Rush HG, Mahoney MM, Lee TM, 2012. Degu, in: The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Elsevier, pp. 1031–1053. 10.1016/B978-0-12-380920-9.00044-4 [DOI] [Google Scholar]
- Colonnello V, Iacobucci P, Fuchs T, Newberry RC, Panksepp J, 2011. Octodon degus. A useful animal model for social-affective neuroscience research: Basic description of separation distress, social attachments and play. Neurosci. Biobehav. Rev., Pioneering Research in Affective Neuroscience: Celebrating the Work of Dr. Jaak Panksepp 35, 1854–1863. 10.1016/j.neubiorev.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Davis GT, Vásquez RA, Poulin E, Oda E, Bazán-León EA, Ebensperger LA, Hayes LD, 2016a. Octodon degus kin and social structure. J. Mammal 97, 361–372. 10.1093/jmammal/gyv182 [DOI] [Google Scholar]
- Davis GT, Vásquez RA, Poulin E, Oda E, Bazán-León EA, Ebensperger LA, Hayes LD, 2016b. Octodon degus kin and social structure. J. Mammal 97, 361–372. 10.1093/jmammal/gyv182 [DOI] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS, 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75, 295–301. [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensperger L, Veloso C, Wallem P, 2002. Do female degus communally nest and nurse their pups? J. Ethol 20, 143–146. 10.1007/s10164-002-0063-x [DOI] [Google Scholar]
- Ebensperger LA, Chesh AS, Castro RA, Tolhuysen LO, Quirici V, Burger JR, Hayes LD, 2009. Instability Rules Social Groups in the Communal Breeder Rodent Octodon degus. Ethology 115, 540–554. 10.1111/j.1439-0310.2009.01635.x [DOI] [Google Scholar]
- Ebensperger LA, Correa LA, León C, Ramírez-Estrada J, Abades S, Villegas Á, Hayes LD, 2016. The modulating role of group stability on fitness effects of group size is different in females and males of a communally rearing rodent. J. Anim. Ecol 85, 1502–1515. 10.1111/1365-2656.12566 [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, Correa LA, Ly Prieto Á, Pérez de Arce F, Abades S, Hayes LD, 2019. Multiple mating is linked to social setting and benefits the males in a communally rearing mammal. Behav. Ecol 30, 675–687. 10.1093/beheco/arz003 [DOI] [Google Scholar]
- Ebensperger LA, Sobrero R, Quirici V, Castro RA, Tolhuysen LO, Vargas F, Burger JR, Quispe R, Villavicencio CP, Vásquez RA, Hayes LD, 2012. Ecological drivers of group living in two populations of the communally rearing rodent, Octodon degus. Behav. Ecol. Sociobiol 66, 261–274. 10.1007/s00265-011-1274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebensperger LA, Villegas Á, Abades S, Hayes LD, 2014. Mean ecological conditions modulate the effects of group living and communal rearing on offspring production and survival. Behav. Ecol 25, 862–870. 10.1093/beheco/aru061 [DOI] [Google Scholar]
- Ebensperger LA, Wallem PK, 2002. Grouping increases the ability of the social rodent, Octodon degus, to detect predators when using exposed microhabitats. Oikos 98, 491–497. 10.1034/j.1600-0706.2002.980313.x [DOI] [Google Scholar]
- Ebensperger LuisA., Hurtado M, Soto-Gamboa M, Lacey EileenA., Chang AnnT., 2004. Communal nesting and kinship in degus (Octodon degus). Naturwissenschaften 91 10.1007/s00114-004-0545-5 [DOI] [PubMed] [Google Scholar]
- Fabre P-H, Hautier L, Dimitrov D, P Douzery EJ, 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol. Biol 12, 88 10.1186/1471-2148-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M, 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol 7, 1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Fulk GW, 1976. Notes on the Activity, Reproduction, and Social Behavior of Octodon degus. J. Mammal 57, 495–505. 10.2307/1379298 [DOI] [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B, 1993. Social Organization of the Prairie Vole (Microtus ochrogaster). J. Mammal 74, 44–58. 10.2307/1381904 [DOI] [Google Scholar]
- Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, Shaham Y, 2017. Compulsive Addiction-like Aggressive Behavior in Mice. Biol. Psychiatry, Sociopathy, Impulsivity, Aggression 82, 239–248. 10.1016/j.biopsych.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, 2013. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology 38, 465–478. 10.1016/j.psyneuen.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P, 2004. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm. Behav 45, 136–143. 10.1016/j.yhbeh.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, Beery AK, 2019. Comparative role of reward in long-term peer and mate relationships in voles. Horm. Behav 111, 70–77. 10.1016/j.yhbeh.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N, Lopes PC, König B, 2016. Oxytocin and Social Preference in Female House Mice (Mus musculus domesticus). Ethology 122, 571–581. 10.1111/eth.12505 [DOI] [Google Scholar]
- Hayes LD, Chesh AS, Castro RA, Tolhuysen LO, Burger JR, Bhattacharjee J, Ebensperger LA, 2009. Fitness consequences of group living in the degu Octodon degus, a plural breeder rodent with communal care. Anim. Behav 78, 131–139. 10.1016/j.anbehav.2009.03.022 [DOI] [Google Scholar]
- Hayes LD, Correa LA, Abades S, Gao CL, Ebensperger LA, 2019. Male group members are costly to plurally breeding Octodon degus females. Behaviour 156, 1–36. 10.1163/1568539X-00003525 [DOI] [Google Scholar]
- Kumazawa-Manita N, Katayama M, Hashikawa T, Iriki A, 2013. Three-dimensional reconstruction of brain structures of the rodent Octodon degus: a brain atlas constructed by combining histological and magnetic resonance images. Exp. Brain Res 231, 65–74. 10.1007/s00221-013-3667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DM, FitzGerald RW, McShea WJ, 1984. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav. Ecol. Sociobiol 15, 9–17. [Google Scholar]
- Madison DM, McShea W, 1987. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Am. Zool 27, 899–908. [Google Scholar]
- Panksepp JB, Lahvis GP, 2007. Social reward among juvenile mice. Genes Brain Behav. 6, 661–671. 10.1111/j.1601-183X.2006.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Lee TM, 2003. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J. Comp. Psychol 117, 283–9. 10.1037/0735-7036.117.3.283 [DOI] [PubMed] [Google Scholar]
- Perryman JI, 2011. Circadian and homeostatic components of sleep across sex and development in the diurnal rodent, Octodon degus. The University of Michigan. [Google Scholar]
- Quirici V, Faugeron S, Hayes LD, Ebensperger LA, 2011. Absence of kin structure in a population of the group-living rodent Octodon degus. Behav. Ecol 22, 248–254. 10.1093/beheco/arq196 [DOI] [Google Scholar]
- Schweinfurth MK, Neuenschwander J, Engqvist L, Schneeberger K, Rentsch AK, Gygax M, Taborsky M, 2017. Do female Norway rats form social bonds? Behav. Ecol. Sociobiol 71, 98. [Google Scholar]
- Pellis Sergio M., Pellis Vivien C., Reinhart Christine J., 2010. The evolution of social play, in: Worthman Carol M. (Ed.), Formative Experiences: The Interaction of Caregiving, Culture, and Developmental Psychobiology. Cambridge University Press, Cambridge ; New York. [Google Scholar]
- Smiley KO, Vahaba DM, Tomaszycki ML, 2012. Behavioral effects of progesterone on pair bonding and partner preference in the female zebra finch (Taeniopygia guttata). Behav. Processes 90, 210–216. 10.1016/j.beproc.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Sobrero R, Fernández-Aburto P, Ly-Prieto Á, Delgado SE, Mpodozis J, Ebensperger LA, 2016. Effects of Habitat and Social Complexity on Brain Size, Brain Asymmetry and Dentate Gyrus Morphology in Two Octodontid Rodents. Brain. Behav. Evol 87, 51–64. 10.1159/000444741 [DOI] [PubMed] [Google Scholar]
- Uekita T, Okanoya K, 2011. Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav. Brain Res 219, 302–309. 10.1016/j.bbr.2011.01.042 [DOI] [PubMed] [Google Scholar]
- Villavicencio CP, Márquez IN, Quispe R, Vásquez RA, 2009. Familiarity and phenotypic similarity influence kin discrimination in the social rodent Octodon degus. Anim. Behav 78, 377–384. 10.1016/j.anbehav.2009.04.026 [DOI] [Google Scholar]
- Wey TW, Burger JR, Ebensperger LA, Hayes LD, 2013. Reproductive correlates of social network variation in plurally breeding degus (Octodon degus). Anim. Behav 85, 1407–1414. 10.1016/j.anbehav.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS, 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav 26, 339–49. [DOI] [PubMed] [Google Scholar]
- Wilson SC, 1982. Contact-promoting behavior, social development, and relationship with parents in sibling juvenile degus (Octodon Degus). Dev. Psychobiol 15, 257–268. 10.1002/dev.420150309 [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–8. 10.1038/365545a0 [DOI] [PubMed] [Google Scholar]


