Abstract
Event-related potentials (ERPs) reveal the temporal dynamics of emotional processing and are easily assessed in children. Yet, little longitudinal research has examined ERPs sensitive to emotion across development. We aimed to systematically identify timing and spatial distributions of ERPs sensitive to emotion in a longitudinal sample of youth (N = 62) using principal component analysis (PCA), and evaluate stability and change in emotional responses across development. Participants completed an emotional interrupt paradigm in childhood (Mage = 9.38, SD = 0.42), early adolescence (Mage = 13.03, SD = 0.24), and mid-adolescence (Mage = 15.16, SD = 0.17). ERPs were recorded to unpleasant, pleasant, and neutral images. Participants were instructed to respond to a target while viewing images. Two components sensitive to emotion emerged across development: P300/early late positive potential (LPP) and late LPP. The P300/early LPP component was characterized by an enhanced positivity for unpleasant compared to pleasant and neutral images. The late LPP was enhanced for both unpleasant and pleasant compared to neutral images, and more positive for unpleasant compared to pleasant images. The components showed moderate to strong stability. Overall LPP magnitude decreased from childhood into adolescence. There was a developmental shift in distributions from occipital sites in childhood to centroparietal sites in mid-adolescence. Results support use of PCA to inform scoring windows and electrode selection. The shift in distribution may reflect developmental focalization in underlying neural circuitry. Future work is needed using multimodal approaches to further understand the relationship between ERPs and changes in neural circuitry across development.
1. Introduction
Attending and responding to emotionally salient environmental stimuli is necessary and adaptive. Neural processing of emotion has been a major focus of research across areas from affective and cognitive neuroscience to development and clinical psychology and psychiatry. Indeed, dysregulation of emotion is a key process underlying the development of both internalizing and externalizing psychopathology (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Compas et al., 2017; Sheppes, Suri, & Gross, 2015). As such, there has been a growing interest in understanding and measuring emotion across multiple analytical levels and methods.
While functional magnetic resonance imaging (fMRI) is a common methodology for studying the neural circuits involved in emotion (Guyer et al., 2008; Hare et al., 2008; Ochsner et al., 2009), neurophysiological measures, particularly event-related potentials (ERPs), offer valid measures of the time course of emotional processing that are economical and relatively easy to administer across development (Codispoti, Ferrari, & Bradley, 2007; Foti, Hajcak, & Dien, 2009; Hajcak, MacNamara, & Olvet, 2010; MacNamara et al., 2016; Olofsson, Nordin, Sequeira, & Polich, 2008). Of particular relevance, the late positive potential (LPP) is an ERP component that indexes the elaborative processing of motivationally salient stimuli. The LPP is characterized by a sustained positive deflection traditionally beginning around 300 ms post stimulus onset and persisting across stimulus presentation time that is sensitive to emotional and salient, compared to neutral, stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). Visual processing of salient stimuli occurs across widely distributed circuitry, incorporating the amygdala, anterior insula, anterior cingulate cortex (ACC), and orbitofrontal cortex, with the amygdala in particular having projections throughout much of the prefrontal cortex (PFC) and providing feedback information via loops to the visual cortex (Pessoa & Adolphs, 2010). Activation of regions of the visual cortex appears to be particularly relevant in generating the scalp-recorded LPP, but LPP has also been linked to a distributed network of both cortical and subcortical regions, as well as bidirectional coupling between occipitoparietal/visual cortex and PFC (Moratti, Saugar, & Strange, 2011). Between-subjects analyses using separate recordings of ERP and fMRI signals suggest that the LPP correlates with activation in lateral occipital, parietal, and inferotemporal cortices, as well as amygdala, ACC, anterior insula, and ventral striatum/nucleus accumbens (Sabatinelli, Keil, Frank, & Lang, 2013; Sabatinelli, Lang, Keil, & Bradley, 2007). Similarly, trial by trial analysis of simultaneously recorded EEG-BOLD signals within-subjects associated the magnitude of the LPP with activation in the inferior, middle and superior temporal cortices, occipital cortex, insula, orbitofrontal cortex, and amygdala/hippocampus (Liu, Huang, McGinnis-Deweese, Keil, & Ding, 2012). Similarly, in children and adolescents, between-subjects correlations indicated that LPP to emotional faces corresponded with activation in inferior frontal gyrus (IFG)/orbitofrontal gyrus (OFG), left supplementary motor area, right superior parietal lobule, and bilateral amygdala (Bunford, Kujawa, Fitzgerald, Monk, & Phan, 2018).
The transition from childhood to adolescence is characterized by developmental change in the neural systems involved in emotional processing and regulation. Specifically, perceptual regions of the occipital and temporal lobes tend to be characterized by diffuse activation early in development, with focalization and refinement into adolescence. On the other hand, affective and motivational regions, such as the amygdala and striatum, tend to develop in a curvilinear pattern with an increase in activation earlier in adolescence followed by a decrease in late adolescence into early adulthood (for a review, see Nelson, Jarcho, & Guyer, 2016). In addition to developmental changes in activation of key regions involved in emotional processing, bidirectional interactions between subcortical and cortical regions continue to evolve and develop across childhood and adolescence. For example, there is evidence for a developmental shift from greater positive to negative coupling between amygdala and regulatory frontal regions including medial PFC and ACC (Casey, Jones, & Hare, 2008; Silvers et al., 2017; Wu, Kujawa et al., 2016), as well as a general decrease in amygdala activation from childhood to adulthood (Gee et al., 2013). While fMRI is useful for disentangling regions of activation and functional connectivity, ERPs are well-suited for examining the temporal course of emotional processing across development, yet there is little longitudinal research examining this.
There is some evidence from prior research that the LPP decreases in magnitude from childhood into adolescence and is more temporally protracted with age (Kessel et al., 2018; Kujawa, Weinberg, Hajcak, & Klein, 2013; MacNamara et al., 2016). This developmental decrease in the LPP has been associated with age-related decreases in activation in the bilateral IFG/OFG and amygdala (Bunford et al., 2018). Additionally, there appears to be a developmental shift in the scalp distribution of LPP from occipital regions in middle childhood to more adult-like patterns of activation over centroparietal sites in adolescence (Kujawa, Klein, & Hajcak, 2012). Preliminary evidence from a small sample spanning a broad age range indicates the LPP is relatively stable over two years in children and adolescents (Kujawa, Klein, & Proudfit, 2013). Consistent with this, adult research supports the stability of ERP amplitudes across time (Cassidy, Robertson, & O’Connell, 2012), and recent evidence suggests that the LPP has good test-retest reliability and internal consistency over a one-month span in adolescents and young adults (Bondy et al., 2018). The LPP appears to be a valid indicator of individual differences in emotion processing and can be measured beginning in early childhood (Hajcak & Dennis, 2009; Kujawa, Proudfit, & Klein, 2014).
Most of the literature on developmental changes in the neural bases of emotion examines associations with age in cross-sectional studies, and there is little longitudinal research aimed at methodically disentangling the development of ERP components sensitive to emotion. ERPs are well-suited for longitudinal studies of emotional processing in order to evaluate developmental change and stability of earlier and later stages of processing. To this end, we sought to systematically identify the timing and scalp distributions of ERPs sensitive to emotion in a longitudinal sample of youth assessed at three discrete time points spanning a period of six years from middle childhood to middle adolescence. The goals of the study were to leverage the strengths of ERPs to inform understanding of typical development of the time course of emotional processing and to optimize measurement of emotion-relevant ERPs across childhood and adolescence. Principal component analysis (PCA) was used to empirically identify temporally and spatially distinct ERP components in response to emotional stimuli at each time point (Dien, 2012). This approach is particularly useful when studying the LPP across development, as prior PCA research in adults indicates the LPP is actually composed of several overlapping, yet distinguishable positivities (Foti et al., 2009; Weinberg & Hajcak, 2011). The data-driven approach to identifying the timing and location of the peaks for each component makes PCA additionally useful for developmental research given evidence of shifts in the scalp distribution of the LPP across the transition from childhood to early adolescence (Kujawa et al., 2013). Subsequently, for those components that emerged consistently at each assessment, stability and developmental changes in the magnitude and topographical distribution of the ERPs was evaluated. Based on existing cross-sectional research on the development of the LPP and amygdala activation, we hypothesized that the LPP component will emerge across development, but a decrease in emotional reactivity will be observed from middle childhood to middle adolescence and reflected by a decrease in LPP magnitude across time (Bunford et al., 2018; Kessel et al., 2018; MacNamara et al., 2016). Furthermore, we expected the possibility of a developmental shift in the scalp distribution of the LPP from being maximal over occipital regions in childhood, to more centroparietal regions in adolescence (Kujawa, Klein, & Hajcak, 2012).
2. Method
2.1. Participants
Participants were a subset of a longitudinal community sample (N = 559), originally recruited at age 3 (with an additional 50 participants added to the sample at age 6). Participants were assessed again at approximately age 9, 12, and 15 (full sample description available in Kujawa et al. (2014)) as part of a larger study of early precursors to depression and other psychiatric disorders. The current study presents longitudinal EEG data collected during an emotional interrupt task administered at the age 9, 12, and 15 year assessments. A total of 451 participants completed the task at the age 9 assessment, and 434 completed the task at the age 12 assessment. The age 15 assessment is currently ongoing. Data from the first 75 participants to have completed the most recent follow-up were included in the present within-subjects analyses. Longitudinal change and stability of ERPs modulated by reward and loss feedback has been previously examined in this subsample (Kujawa et al., 2018), allowing results from these 2 tasks to be compared. Of these participants, 5 were excluded due to missing data from the emotional interrupt task at one of the assessments. An additional 8 participants were excluded for having fewer than 15 artifact-free, correct trials per condition at one or more of the assessments. The final sample included 62 participants with a mean age of 9.38 (SD = 0.42) at the middle childhood assessment, a mean age of 13.03 (SD = 0.24) at the early adolescence assessment, and a mean age of 15.16 (SD = 0.17) at the middle adolescence assessment. The sample was 43.1% female, 7.7% Hispanic/Latino, 96.9% Caucasian, 1.5% African American, and 1.5% Asian American. Most participants were right-handed (87.1%). Of this sample, one participant was missing behavioral data due to a technical error. Protocols for this study were approved by the Institutional Review Board at Stony Brook University. Informed consent was obtained from all parents and assent from minor participants.
2.2. Measures: Emotional Interrupt Task
At each EEG assessment, participants completed an emotional interrupt paradigm used successfully in previous research to elicit the LPP in children and adolescents (Kujawa et al., 2012; Nelson, Perlman, Hajcak, Klein, & Kotov, 2015). Sixty developmentally appropriate images selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) were presented randomly in each of the two blocks, resulting in 120 total trials. Twenty images were pleasant (e.g. children playing, happy people, cute animals), twenty images were unpleasant (e.g. weapons or mild violence, sad or angry peoples, aggressive animals), and twenty images were neutral (e.g. household objects, people with neutral expressions, neutral scenes) (Kujawa et al., 2016; 2018). A fixation cross (+) was presented at the beginning of each trial for 800 ms followed by an image for 1000 ms, a target arrow (< or >) for 150 ms, and the same image for another 400 ms. Participants were instructed to click either the right or left button on the mouse to indicate whether the presented target was pointing to the right or left. The intertrial interval varied randomly from 1500 ms to 2000 ms.
2.3. Materials
Stimuli were presented on a Dell Optiplex 760 with an Intel duo core processor using Presentation software (Version 17.1, Build 03.15.14) and a Dell 17” monitor. The computer operating system was Windows 7 for the age 9 and 12 assessments, and Windows 10 for the age 15 assessment.
2.4. EEG Data Collection and Processing
At each assessment, EEG was recorded continuously using a 34-channel system from BioSemi with electrode offsets generally below 20 μV (FCz and Iz added to a 32 electrode cap; BioSemi, Amsterdam, Netherlands). The common-sense mode electrode and driven right leg electrode formed the ground and reference electrodes, and electrodes were placed on the right and left mastoids. Facial electrodes were placed approximately 1 cm above and below one eye, and 1 cm from the outer corner of the right and left eye to record electrooculogram. Data were digitized at 24-bit resolution with a least significant bit value of 31.25 nV and a sampling rate of 1024 Hz.
Offline processing was completed using BrainVision Analyzer software (Brain Products, Munich, Germany). Data were referenced to the mean mastoid recordings, band-pass filtered from .1 to 30 Hz, and each trial was segmented from 200 ms prior to stimulus onset to 1000 ms after onset. Faulty recordings at single electrodes were resolved through interpolation using the signal from surrounding electrodes. Eye-blink correction and artifact rejection were conducted using semiautomated procedures identifying voltage steps of more than 50 μV between sampling points, voltage differences of 300 μV within a trial, and voltage differences less than .50 μV within 100 ms intervals. Additional artifacts were identified and removed through visual inspection. For the unpleasant condition, participants had an average of 30.97 (range: 15 – 39) correct, artifact-free trial segments at age 9, 33.00 (range: 16 – 40) at age 12, and 35.27 (range: 23 – 40) at age 15. For the pleasant condition, the averages were 31.19 (range: 15 – 40) at age 9, 33.44 (range: 16 – 40) at age 12, and 34.84 (range: 18 – 40) at age 15. Finally, for the neutral condition the average trial segments were 32.31 (range: 13 – 39) at age 9, 35.08 (range: 24 – 40) at age 12, and 35.66 (range: 22 – 40) at age 15. Results of a 3 (age: age 9, age 12, age 15) × 3 (condition: pleasant, unpleasant, neutral) repeated-measures ANOVA to test main effects of age and condition on number of artifact-free trials revealed that there was a significant main effect of both age (F(2, 122) = 11.96, p < .001, ) and condition (F(2, 122) = 10.08, p < .001, ). Across conditions, the number of correct, artifact-free trials increased with age, from age 9 to 12 (F(1, 61) = 7.99, p = .006, ), from age 9 to 15 (F(1, 61) = 21.47, p < .001, ), and from age 12 to 15 (F(1, 61) = 4.34, p = .042, ). Across assessments, the number of correct, artifact-free trials was greater for neutral trials compared to both unpleasant (F(1, 61) = 14.85, p < .001, ) and pleasant (F(1, 61) = 14.08, p < .001, ) trials. The comparison between unpleasant and pleasant conditions was not significant (p = .805). Data were baseline corrected to 200 ms pre-stimulus onset, and ERPs were averaged separately for unpleasant, pleasant, and neutral trials.
2.5. Data Analysis
2.5.1. Behavioral analyses.
For each picture type (unpleasant, pleasant, neutral), mean accuracy and reaction time (RT) for correct trials were calculated. Responses less than 150 ms or greater than 2150 ms after target onset were counted as errors. We computed 3 (age: age 9, age 12, age 15) × 3 (condition: pleasant, unpleasant, neutral) repeated-measures ANOVAs to test main effects of age and condition as well as age × condition interactions on accuracy and RT. When the assumption of sphericity was violated, Greenhouse-Geisser correction was used for all repeated-measures ANOVAs.
2.5.2. PCA.
ERP components were empirically identified through temporospatial PCA, a method which extracts linear combinations of data from all time points and recording sites to delineate patterned electrocortical activity (Dien & Frischkoff, 2005; Foti et al., 2009; Kujawa et al., 2018; Weinberg & Hajcak, 2011). The temporal PCA was conducted first using the ERP PCA Toolkit, version 2.54 in Matlab (Dien, 2010), with all time points from each participant’s averaged data specified as variables, and participants, trial types, and recording sites specified as observations. Promax rotation was used to rotate the simple structure in the temporal domain (Dien, 2010; Dien, Khoe, & Mangun, 2007). A parallel analysis (Horn, 1965) comparing the Scree of the present dataset to the Scree from a fully random dataset was conducted on the resulting Scree plot (Cattell, 1966), yielding 8 temporal factors (TF) at age 9 and 10 factors at ages 12 and 15 accounting for a greater proportion of variance than those generated by the random dataset. The retained temporal factors were then subjected to a spatial PCA (Dien, 2010; Dien et al., 2007). All recording sites were specified as variables, and observations were specified as participants, trial types, and temporal factor scores. Infomax was used to rotate the factors to independence in the spatial domain (Dien, 2010). The parallel test of the resulting Scree plot extracted 3 spatial factors (SF) at ages 9 and 12 and 2 SF at age 15 from each of the temporal factors. Overall, the temporospatial PCA resulted in 24 TF/SF combinations at age 9, 30 TF/SF combinations at age 12, and 20 TF/SF combinations at age 15 that in sum accounted for 29.6%, 28.4%, and 34.9% of the unique variance in the ERP waves at each assessment, respectively.
The components that accounted for at least 0.5% unique variance were subjected to a robust analysis of variance (ANOVA; Dien, 2017; Keselman, Wilcox, & Lix, 2003) to determine which components were significantly modulated by emotional condition. Specifically, PCA factors were converted to microvolt scaling, and the ANOVA procedures were conducted in ERP PCA Toolkit with 4999 bootstrapping simulations. Simulations were run 11 times, with median p-values reported to account for potential variability in the resulting p-values. Significance was defined as resulting values where the median p-value plus two standard deviations remained below 0.05 (Dien, 2017). In order to account for the possibility of Type I errors due to multiple comparisons, a False Discovery Rate (FDR) correction using the Benjamini-Hochberg method (Benjamini & Hochberg, 1995) was applied to robust ANOVAs identifying significant components (27 tests). This method adjusts the critical p-value based on the expected proportion of Type I errors among the significant effects. Components meeting the FDR correction are reported in Table 1. Microvolt-scaled PCA factor scores for significant components (peak electrode and time point) at each age were then exported from ERP PCA Toolkit for further statistical analysis. Following significant main effects of emotional condition based on 3 (condition: pleasant, unpleasant, neutral) × 3 (age: age 9, age 12, age 15) repeated-measures ANOVAs, we conducted simple effects contrasts to test for significant differences between each pair of conditions.
Table 1.
Temporospatial factor combinations sensitive to pleasant, unpleasant, and neutral images at each assessment
| Factor combination | Unique variance (%) | Temporal peak (ms) | Peak electrode | Main effect of condition TWJt/c (2.0, 48.9) (p)* | Description |
|---|---|---|---|---|---|
| Age 9 | |||||
| TF1/SF1 | 13.82 | 358 | PO4 | 12.66 (.001) | Occipital relative positivity for unpleasant compared to pleasant, and unpleasant compared to neutral |
| TF1/SF2 | 7.26 | 358 | O2 | 7.31 (.004) | Occipital relative positivity for pleasant compared to neutral, and pleasant compared to unpleasant |
| TF2/SF1 | 2.92 | 955 | O2 | 9.89 (<.001) | Occipital relative positivity for pleasant compared to neutral, unpleasant compared to pleasant, and unpleasant compared to neutral |
| Age 12 | |||||
| TF1/SF1 | 11.67 | 369 | Pz | 9.46 (.001) | Parietal relative positivity for unpleasant compared to pleasant, and unpleasant compared to neutral |
| TF2/SF2 | 1.51 | 972 | O1 | 12.40 (.033) | Occipital relative positivity for pleasant compared to neutral, unpleasant compared to pleasant, and unpleasant compared to neutral |
| TF2/SF3 | 0.51 | 972 | Fp2 | 4.78 (.020) | Frontal relative positivity for neutral compared to unpleasant |
| Age 15 | |||||
| TF1/SF1 | 19.83 | 388 | FCz | 25.71 (<.001) | Frontocentral relative positivity for unpleasant compared to pleasant, and unpleasant compared to neutral |
| TF2/SF1 | 1.75 | 977 | Cz | 9.14 (.001) | Central relative positivity for unpleasant compared to pleasant, and unpleasant compared to neutral |
Note:
p-values are FDR-corrected.
2.5.3. Stability and developmental change analyses.
Two temporospatial factors from the PCA consistently emerged across development with comparable timing in the PCA (see Table 1): one that peaked between 358 and 388 ms after stimuli presentation at occipitoparietal sites in childhood/early adolescence and central sites in mid-adolescence (i.e., P300 or early LPP; TF1/SF1 at each age), and one that peaked between 955 and 977 ms after emotional stimuli presentation at occipital sites in childhood/early adolescence and central sites in mid-adolescence (i.e., late LPP; TF2/SF1 at age 9 and 15, TF2/SF2 at age 12). Two scoring approaches were used to evaluate stability and developmental change in the P300/early LPP and late LPP components: PCA factor scores were converted to microvolt scaling at the peak electrode (Dien, 2012; 2017), and more traditional mean activity measures were extracted from averaged ERPs for each participant before the PCA across a time window around the peaks identified in the corresponding PCA components (i.e., 325–425 ms for the P300/early LPP and 900–1000 ms for the late LPP). Specifically, mean activity values in microvolts from windows surrounding the peaks identified by the PCA (i.e., P300 or early LPP: 325–425 ms; late LPP: 900–1000 ms) were extracted using BrainVision Analyzer. Consistent with prior evidence of developmental shifts in the scalp distribution of the LPP, the peak electrode for each component derived from the PCA changed across development from occipital to centroparietal electrodes (Kujawa et al., 2013). Thus, mean activity from the original data before the PCA was extracted from separate poolings of centroparietal electrodes (FCz, Cz, and Pz) and occipital electrodes (O1, O2, and Oz) corresponding to both the early and late LPP components. Analyses of stability at individual electrode sites are provided for comparison in Supplemental Results (Tables S1–S2).
All stability and developmental change analyses were conducted in SPSS. To assess the stability of the ERP response to unpleasant, pleasant, and neutral stimuli, both Pearson’s r bivariate correlations to measure rank-order stability between two assessments and intraclass correlations (ICC) to examine both rank-order and mean-level stability between two assessments and across all three assessments were calculated. These correlations were conducted at the same regions (i.e., centroparietal or occipital pooled electrode sites for mean activity measures at each assessment, and the same corresponding PCA factor at each assessment). ICCs were computed using two-way mixed single measures with absolute agreement. Cicchetti, Butcher, and Nelson’s (1994) guidelines for psychological tests were followed to interpret ICCs from a psychometrics perspective. Specifically, coefficients are considered to have poor stability below .40, indicating a lack of combined rank-order and mean-level stability (Cicchetti et al., 1994). Coefficients between .40 and .59 indicate fair stability, coefficients between .60 and .74 indicate good stability, and coefficients between .75 and 1.00 indicate excellent stability, according to the guidelines (Cicchetti et al., 1994). Given the emphasis on the magnitude, rather than significance, of these coefficients, stability analyses were not corrected for multiple comparisons While it is expected that there will be moderate rank-order stability across development, supporting reliability of the measures, lower mean-level stability might be expected, given evidence that the magnitude of emotional reactivity changes from childhood to adolescence. Specifically, mean-level stability may be affected by these developmental changes, and as such, ICCs may be lower compared to rank-order stability in the Pearson’s r bivariate correlations. Stability was also examined using subtraction-based difference scores and residual scores to isolate variance in the ERPs specific to emotional processing (Luck, 2005; Meyer, Lerner, de los Reyes, Laird, & Hajcak, 2017). Results from the subtraction-based difference scores were generally comparable to the residual scores, so only results from residual scores are reported in order to simplify the presentation of results.
Lastly, we conducted repeated-measures ANOVAs to examine developmental changes in the magnitude of the early and late LPPs. When the assumption of sphericity was violated, Greenhouse-Geisser correction was used. For the PCA factors as well as occipital and centroparietal ERP mean activity measures, we computed 3 (age: age 9, age 12, age 15) × 3 (condition: pleasant, unpleasant, neutral) repeated-measures ANOVAs. In order to account for the possibility of Type I errors, FDR correction was applied to all main and interaction effects in these analyses (18 analyses total).
3. Results
3.1. Behavioral Data
Table 2 presents the mean accuracy and RT at each assessment for each condition. In the unpleasant condition, participants made 6.72 (SD = 3.65) errors on average at age 9, 3.76 (SD = 2.77) at age 12, and 2.74 (SD = 1.97) at age 15. For the pleasant condition, participants made 6.46 (SD = 3.62) errors on average at age 9, 3.65 (SD = 2.93) at age 12, and 3.18 (SD = 1.97) at age 15. For the neutral condition, participants made 5.26 (SD = 3.54) errors on average at age 9, 3.07 (SD = 2.20) at age 12, and 2.39 (SD = 1.75) at age 15. Across all three conditions, participants made 17.85 (SD = 9.75) errors on average at age 9, 8.86 (SD = 7.04) at age 12, and 6.43 (SD = 5.00) at age 15. Results of a 3 (age) × 3 (condition) repeated-measures ANOVA for accuracy revealed that there was a significant effect of age, F(1.32, 79.34) = 44.98, p < .001, , such that accuracy increased over time across picture types from age 9 to 12 (F(1, 60) = 35.42, p < .001, ), from age 9 to 15 (F(1, 60) = 63.50, p < .001, ), and from age 12 to 15 (F(1, 60) = 11.58, p = .001, ). There was also a significant effect of condition, F(2, 120) = 18.77, p < .001, , such that accuracy was lower following unpleasant compared to neutral pictures (F(1, 60) = 28.82, p < .001, ) and pleasant compared to neutral pictures (F(1, 60) = 25.37, p < .001, ). The comparison between unpleasant and pleasant picture types did not reach significance (p = .357). The interaction between age and condition was not significant (p = .132).
Table 2.
Mean accuracy and reaction time (ms) at each assessment for each condition
| Unpleasant M (SD) |
Neutral M (SD) |
Pleasant M (SD) |
|
|---|---|---|---|
| Accuracy (%) | |||
| Age 9 | 79.34 (13.93) | 83.53 (14.88) | 80.00 (13.49) |
| Age 12 | 90.70 (8.05) | 93.57 (6.90) | 91.93 (8.16) |
| Age 15 | 94.63 (5.28) | 96.03 (4.99) | 94.10 (5.53) |
| Reaction Time (ms) | |||
| Age 9 | 556.95 (146.38) | 544.72 (147.63) | 559.83 (164.00) |
| Age 12 | 494.54 (137.07) | 491.27 (129.71) | 491.80 (126.60) |
| Age 15 | 411.60 (111.29) | 413.45 (116.10) | 413.48 (109.66) |
Results of a 3 (age) × 3 (condition) repeated-measures ANOVA for RT revealed that there was a significant effect of age, F(2, 120) = 20.49, p < .001, , such that RT decreased over time across picture types: from age 9 to 12 (F(1, 60) = 6.74, p = .012, ), from age 9 to 15 (F(1,60) = 40.09, p < .001, ), and from age 12 to 15 (F(1, 60) = 15.49, p < .001, ). There was no significant main effect of condition on RT (p = .112), nor was there a significant interaction between age and condition on RT (p = .116).
3.2. PCA of ERPs Sensitive to Emotion Condition at Each Assessment
ERP waves and scalp distributions for both the centroparietal and occipital poolings prior to the PCA are presented in Figure 1. Temporospatial factors from the PCA that accounted for at least 0.5% unique variance in the ERP at each age and were significantly modulated by emotion (based on robust ANOVAs conducted in ERP PCA Toolkit with FDR correction applied) are presented in Table 1. ERP waves and scalp distributions for the P300/early LPP and late LPP PCA factors are presented in Figures 2 and 3, respectively. The means for the PCA derived P300/early LPP and late LPP for each condition at each age are presented in Table 3.
Figure 1.
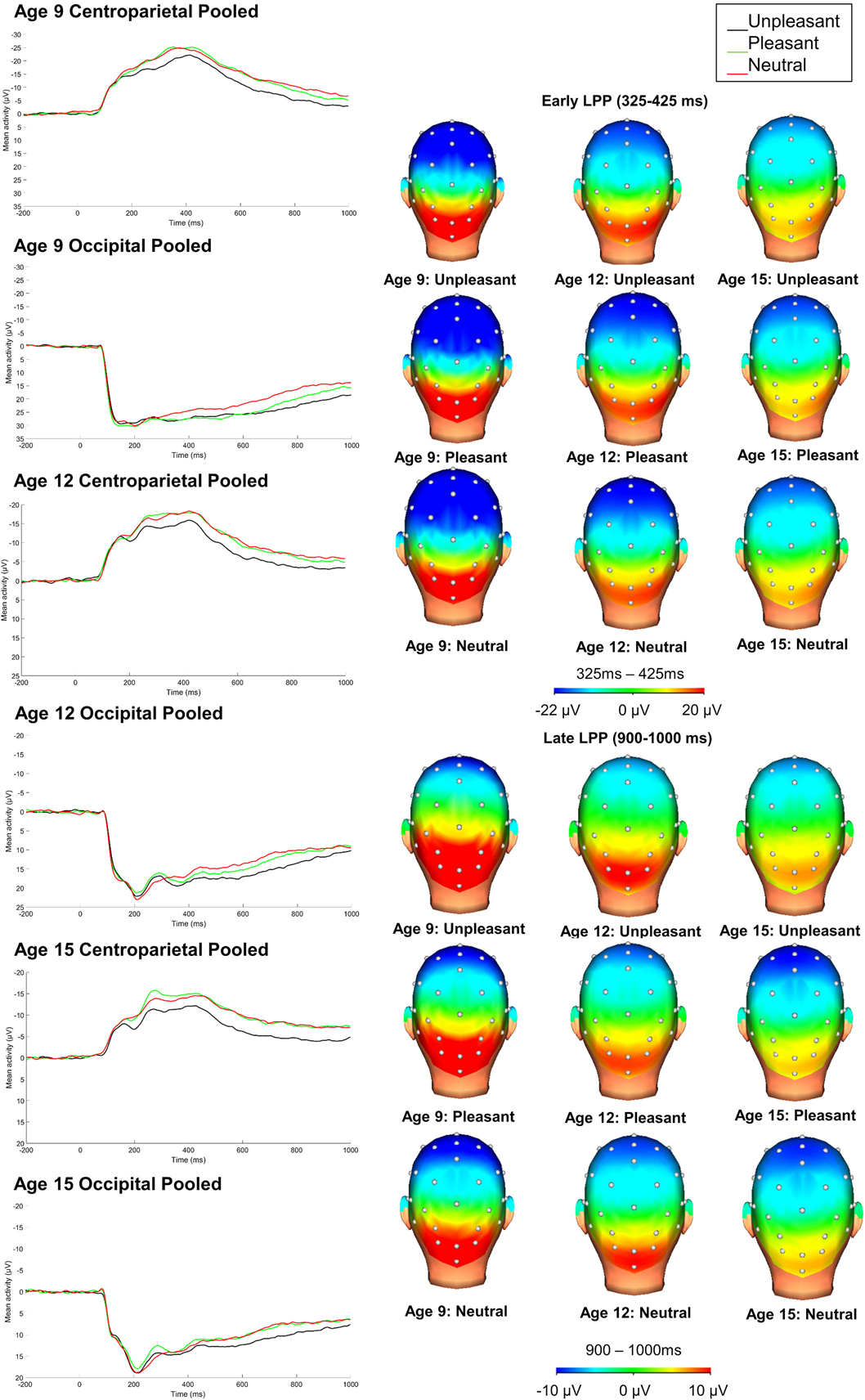
Average ERP responses (negative up) to unpleasant, pleasant, and neutral stimuli at the centroparietal and occipital prior to PCA (34-channel montage with a linked mastoid reference). Please note that in order to depict emotion modulation of ERPs across all ages, ERPs are presented using a wider scale at age 9 compared to age 12 and 15.
Figure 2.
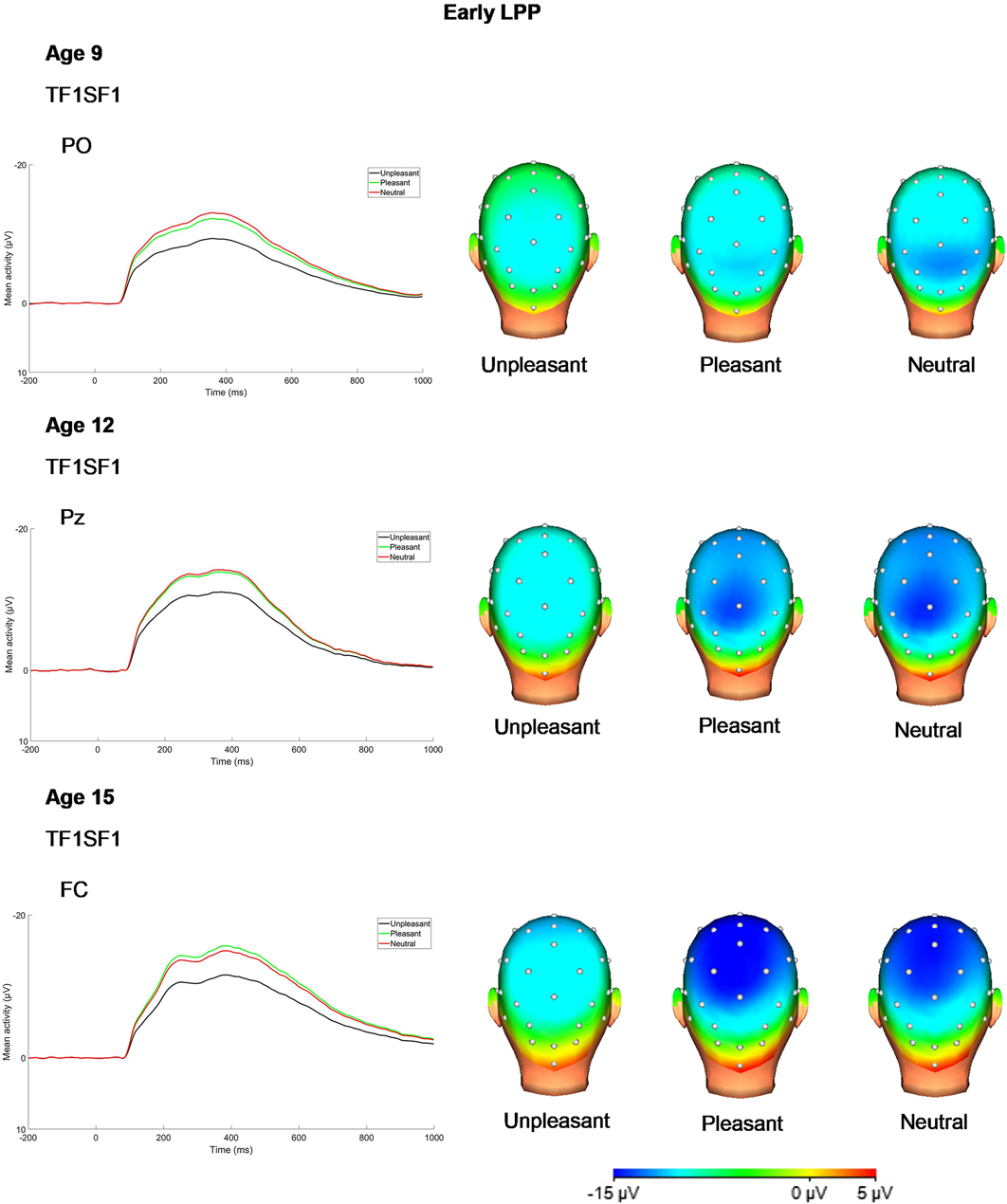
PCA temporospatial factor ERPs and scalp distributions at each age for P300/early LPP (34-channel montage with linked mastoid reference).
Figure 3.
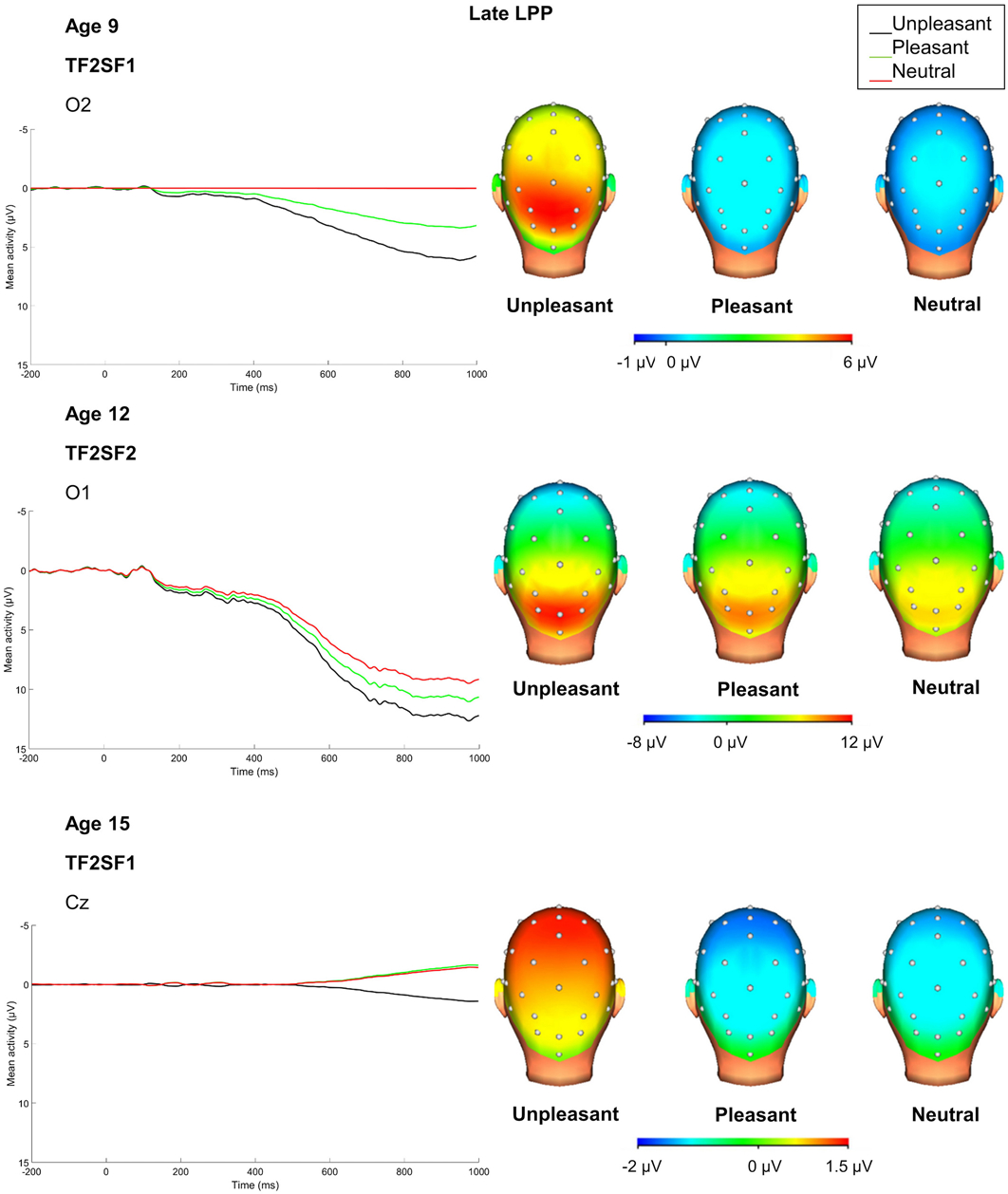
PCA temporospatial factor ERPs and scalp distributions at each age for late LPP. Please note that given differences in overall magnitude of ERPs at each age, the scale for scalp distributions differs at each assessment (34-channel montage with linked mastoid reference).
Table 3.
Descriptive statistics, bivariate correlations (Pearson’s r), and intraclass correlations (ICC) of the peak factors derived from the PCA for the two components corresponding to the early late positive potential (LPP) and late LPP at each assessment/age
| Early LPP | ||||||||||
| Unpleasant | −9.37 (10.69) | −11.05 (7.16) | −11.60 (6.39) | .30* | .52*** | .50*** | .27* | .52*** | .43*** | .39*** |
| Pleasant | −12.28 (11.46) | −13.88 (7.79) | −15.68 (6.43) | .46*** | .63*** | .40*** | .42*** | .61*** | .32** | .42*** |
| Neutral | −13.12 (9.77) | −14.21 (6.33) | −14.99 (5.67) | .35** | .67*** | .35** | .36** | .66*** | .30** | .40*** |
| Unpleasant residuals | 0.00 (6.27) | 0.00 (5.34) | 0.00 (4.24) | .10 | .26* | .29* | .10 | .25* | .27* | .20** |
| Pleasant residuals | 0.00 (6.61) | 0.00 (5.20) | 0.00 (3.95) | −.09 | .21 | −.001 | −.09 | .21 | −.001 | .01 |
| Late LPP | ||||||||||
| Unpleasant | 6.37 (9.62) | 12.64 (6.92) | 1.42 (6.45) | .24 | .20 | .22 | .18* | .08 | .17 | .14** |
| Pleasant | 3.61 (8.02) | 11.03 (6.62) | −1.64 (5.56) | .20 | −.11 | .17 | .13 | −.04 | .12 | .05 |
| Neutral | 0.27 (11.30) | 9.48 (5.97) | −1.45 (5.61) | .19 | .04 | .17 | .11 | .01 | .13 | .08* |
| Unpleasant residuals | 0.00 (8.97) | 0.00 (4.86) | 0.00 (5.68) | .27* | .24 | .14 | .23* | .24* | .13 | .19** |
| Pleasant residuals | 0.00 (7.68) | 0.00 (4.78) | 0.00 (5.22) | .04 | −.07 | .11 | .03 | −.07 | .10 | .04 |
Note: T1 = age 9; T2 = age 12; T3 = age 15;
p < .001;
p < .01;
p < .05
The component corresponding with P300/early LPP was significantly more positive to unpleasant images relative to both neutral (F(1, 61) = 53.74, p < .001, ) and pleasant images across assessments (F(1, 61) = 56.88, p < .001, ). Paired samples t-tests indicated that unpleasant was significantly different from neutral at each assessment (age 9: t(61) = 4.63, p < .001; age 12: t(61) = 4.47, p < .001; age 15: t(61) = 6.16, p < .001), and that unpleasant was significantly different from pleasant at each assessment (age 9: t(61) = 3.60, p = .001; age 12: t(61) = 3.86, p < .001; age 15: t(61) = 7.23, p < .001). The comparisons between pleasant and neutral images for early LPP did not reach significance across assessments (p = .692). Results of paired samples t-tests were consistent with this finding (ps > .175).
In contrast, the component corresponding with late LPP was more positive in response to both unpleasant (F(1, 61) = 38.31, p < .001, ) and pleasant (F(1, 61) = 6.66, p = .012, ) images compared to neutral images across ages, and in response to unpleasant compared to pleasant images (F(1, 61) = 22.60, p < .001, ). Paired samples t-tests indicated that unpleasant was significantly different from neutral at each assessment (age 9: t(61) = 4.05, p < .001; age 12: t(61) = 5.00, p < .001; age 15: t(61) = 3.63, p = .001), and that pleasant was significantly different from neutral at age 9 (t(61) = 2.25, p = .028) and age 12 (t(61) = 2.44, p = .017), but not age 15 (p = .817). Results of paired samples t-tests also showed that unpleasant was significantly different from pleasant at each assessment (age 9: t(61) = 2.42, p = .019; age 12: t(61) = 2.53, p = .014; age 15: t(61) = 3.69, p < .001). As these two components were the only two to emerge at every assessment with comparable timings, analyses of stability and developmental changes focused on the P300/early LPP and late LPP.
3.3. Stability of Emotion ERPs from Childhood through Adolescence
Pearson’s r bivariate correlations and ICCs for all LPP measures are presented in Tables 3 and 4.
Table 4.
Descriptive statistics, bivariate correlations (Pearson’s r), and intraclass correlations (ICC) of the mean activity measures of early and late LPP
| T1 M (SD) | T2 M (SD) | T3 M (SD) | T1 to T2 r | T2 to T3 r | T1 to T3 r | T1 to T2 ICC | T2 to T3 ICC | T1 to T3 ICC | T1, T2, and T3 ICC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Early LPP | ||||||||||
| Unpleasant centroparietal | −21.00 (10.21) | −14.88 (8.34) | −11.66 (6.43) | .47** | .65** | .62** | .38*** | .58*** | .35*** | .41*** |
| Pleasant centroparietal | −24.88 (11.63) | −17.72 (8.73) | −14.70 (6.39) | .58** | .70** | .59** | .45*** | .62*** | .31*** | .43*** |
| Neutral centroparietal | −24.32 (9.37) | −17.63 (7.09) | −13.83 (5.84) | .65** | .74** | .48** | .48*** | .62*** | .23*** | .39*** |
| Unpleasant centroparietal residual | 0.00 (6.43) | 0.00 (5.98) | 0.00 (3.86) | .11 | .34** | .39** | .11 | .32** | .35** | .24*** |
| Pleasant centroparietal residual | 0.00 (6.81) | 0.00 (5.85) | 0.00 (3.63) | −.08 | .28* | .005 | −.08 | .25* | .01 | .03 |
| Unpleasant occipital | 27.73 (14.36) | 18.75 (9.52) | 14.23 (7.19) | .71** | .75** | .66** | .51*** | .64*** | .31*** | .44*** |
| Pleasant occipital | 27.64 (14.24) | 17.77 (10.15) | 13.18 (7.27) | .68** | .80** | .56** | .49*** | .67*** | .25*** | .41*** |
| Neutral occipital | 24.93 (14.91) | 16.72 (9.37) | 13.54 (7.03) | .72** | .80** | .72** | .54*** | .72*** | .38*** | .50*** |
| Unpleasant occipital residual | 0.00 (6.56) | 0.00 (5.61) | 0.00 (3.47) | .37** | .33** | .21 | .37** | .30** | .18 | .29*** |
| Pleasant occipital residual | 0.00 (7.72) | 0.00 (5.19) | 0.00 (3.59) | .17 | .17 | .09 | .16 | .16 | .07 | .13* |
| Late LPP | ||||||||||
| Unpleasant centroparietal | −3.12 (7.51) | −3.23 (6.64) | −4.14 (5.56) | .19 | .26* | .32* | .19 | .26* | .31** | .25*** |
| Pleasant centroparietal | −5.65 (7.25) | −5.15 (7.00) | −7.41 (5.14) | .34** | .31* | .36** | .34** | .28** | .33** | .32*** |
| Neutral centroparietal | −7.37 (8.33) | −6.04 (5.33) | −7.15 (5.08) | .36** | .33** | .18 | .32** | .32** | .16 | .26*** |
| Unpleasant centroparietal residual | 0.00 (6.94) | 0.00 (6.12) | 0.00 (4.82) | .05 | .03 | .24 | .05 | .03 | .23* | .10 |
| Pleasant centroparietal residual | 0.00 (6.90) | 0.00 (5.70) | 0.00 (4.37) | .11 | −.04 | .16 | .11 | −.04 | .15 | .09 |
| Unpleasant occipital | 19.28 (12.63) | 10.94 (7.98) | 8.24 (6.45) | .62** | .70** | .59** | .43*** | .65*** | .30*** | .40*** |
| Pleasant occipital | 16.08 (11.92) | 9.20 (8.11) | 6.75 (5.58) | .57** | .59** | .42** | .43*** | .53*** | .22** | .36*** |
| Neutral occipital | 14.15 (13.85) | 9.31 (6.84) | 6.69 (5.77) | .60** | .60** | .59** | .44*** | .55*** | .34*** | .41*** |
| Unpleasant occipital residual | 0.00 (7.86) | 0.00 (5.32) | 0.00 (4.43) | .23 | .20 | .11 | .22* | .20 | .10 | .17* |
| Pleasant occipital residual | 0.00 (8.13) | 0.00 (5.36) | 0.00 (4.38) | .08 | −.05 | −.007 | .08 | −.05 | −.01 | .02 |
Note: T1 = age 9; T2 = age 12; T3 = age 15;
p < .001;
p < .01;
p < .05;
Early LPP time window: 325–425 ms; Late LPP time window: 900–1000 ms
3.3.1. P300/Early LPP: PCA factor score measures.
Regarding the P300/early LPP, PCA peak factor scores for each emotional condition across all three ages showed moderate to strong rank-order stability (Pearson’s r), and generally acceptable ICCs. Stability of unpleasant LPP from age 9 to age 12 and 15 was lower, and stability of residual scores was more modest (see Table 3).
3.3.2. P300/Early LPP: Mean activity measures.
Regarding the centroparietal mean activity measures within time windows around the peak of the corresponding P300/early LPP PCA factor, P300/early LPP for each emotional condition demonstrated strong rank-order stability (Pearson’s r) across all three ages. ICCs indicated a lack of stability from age 9 to age 15, with more stable measures from age 12 to age 15. Again, residual scores showed low stability. See Figure S1 for scatterplots depicting stability of the P300/early LPP component at centroparietal poolings from age 9 to age 12 and from age 12 to age 15 for each condition.
Similar patterns were observed for mean activity measures at occipital sites. P300/early LPP for each emotional condition showed strong rank-order stability (Pearson’s r) across all three ages and ICCs indicated that measures were generally stable. Stability from age 9 to age 15 was again lower, and residual scores showed only modest stability (see Table 4).
3.3.3. Late LPP: PCA factor score measures.
Regarding the late LPP, PCA peak factor scores for each emotional condition across all three ages showed weak to modest rank-order stability (Pearson’s r) and were generally not significant for LPP to pleasant, unpleasant, and neutral conditions, and residual scores. Similarly, ICCs indicated lack of stability of late LPP PCA peak factor scores across all ages (see Table 3).
3.3.4. Late LPP: Mean activity measures.
Regarding the mean activity measures within time windows around the peak of the corresponding late LPP PCA factor over centroparietal sites, rank-order stability (Pearson’s r) was generally modest but significant. However, ICCs indicated a lack of combined mean-level and rank-order stability across the assessments. Stability of residuals scores was low and generally not significant.
The late LPP scored as mean activity over occipital sites demonstrated stronger stability than at centroparietal sites. Rank-order stability (Pearson’s r) was mostly strong and significant across all three age and emotional conditions. ICCs were generally fair to good, with the exception of the comparison between age 9 and 15. On the other hand, stability (both Pearson’s r and ICCs) was more modest and often not significant for residual scores across the three age comparisons (see Table 4). See Figure S2 for scatterplots depicting stability of the late LPP component at centroparietal poolings from age 9 to age 12 and from age 12 to age 15 for each condition.
3.4. Developmental Change in ERPs Sensitive to Emotion from Childhood through Adolescence
Next, to examine developmental changes in ERP measures, 3 (age) × 3 (emotion condition) repeated-measures ANOVAs were computed for both early and late LPP PCA and mean activity measures. Figure 4 presents early and late LPP centroparietal and occipital mean activity scores to illustrate developmental changes across each age and emotion condition.
Figure 4.
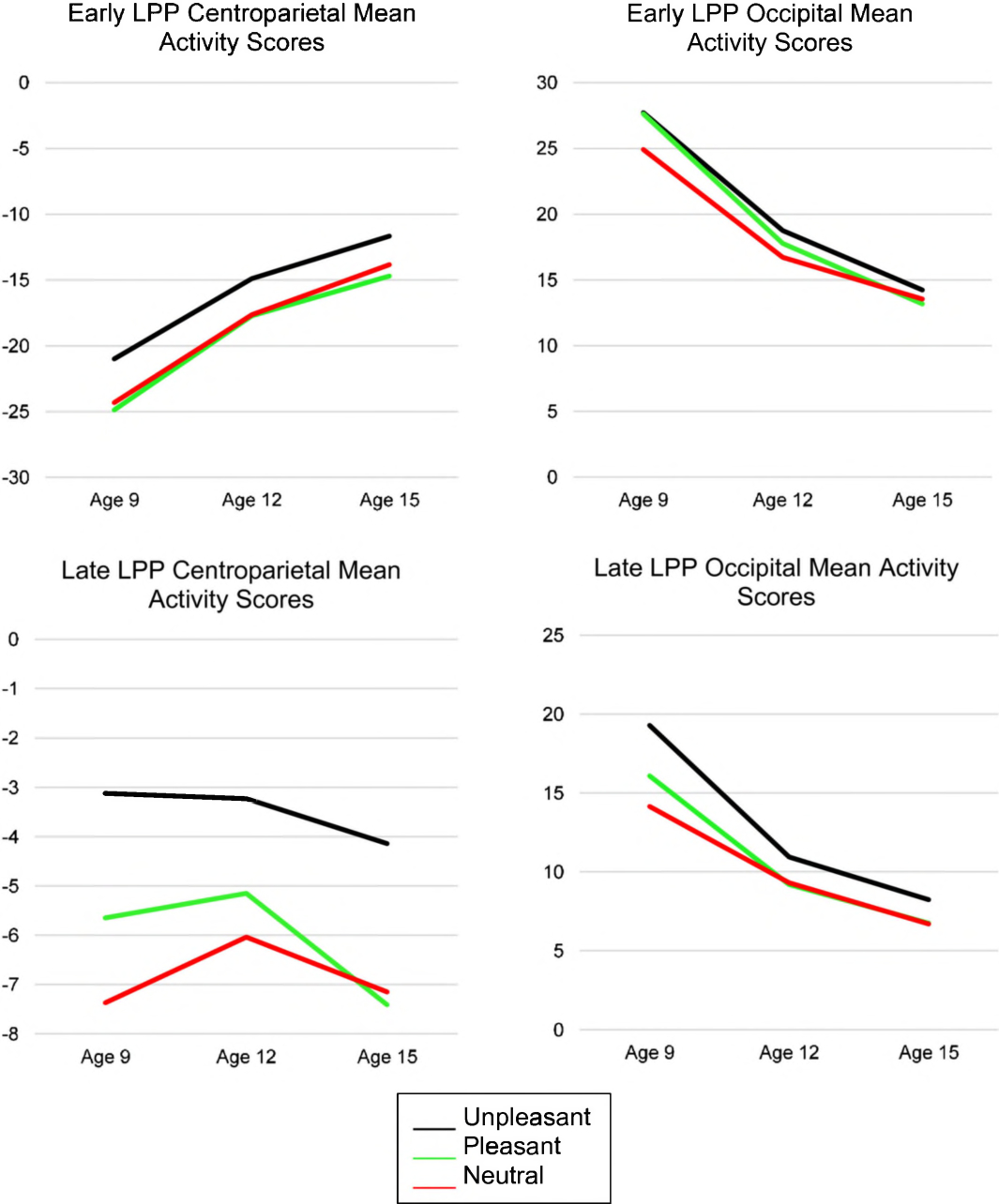
Developmental changes in the early and late LPP centroparietal and occipital mean activity scores across each assessment and emotion condition.
3.4.1 P300/Early LPP: PCA factor score measures.
As expected, for the P300/early LPP, there was a significant effect of condition on the early LPP PCA factor scores, F(2, 122) = 40.42, FDR-corrected p = .009, , such that, as reported in section 3.2, the component was more positive for unpleasant images compared to both neutral (F(1, 61) = 53.74, p < .001, ) and pleasant (F(1, 61) = 56.88, p < .001, ) images across ages. The comparison between pleasant and neutral images was not significant (p = .692). There was a trending significant main effect of age, F(1.44, 88.06) = 3.38, FDR-corrected p = .073, ., such that from age 9 to 15, the component became less positive across all conditions, F(1, 61) = 5.17, p = .026, . Comparisons between ages 9 to 12 and 12 to 15 did not reach significance (ps > .091). There was no significant interaction between condition and age (FDR-corrected p = .515). See Table 3 for the means for each condition at each age for the PCA-derived P300/early LPP components.
3.4.2. P300/Early LPP: Mean activity measures.
For the P300/early LPP centroparietal mean activity measures, there were main effects of condition (F(2, 122) = 29.94, FDR-corrected p = .002, ) and age (F(1.59, 96.94) = 74.85, FDR-corrected p = .002, ), but no significant interaction between condition and age (FDR-corrected p = .660). Similar to the early LPP PCA findings, the component was more positive for unpleasant compared to both neutral (F(1, 61) = 31.12, p < .001, ) and pleasant (F(1, 61) = 52.93, p < .001, ) images. The comparison between positive and neutral images was not significant (p = .227). LPP became more positive across conditions at centroparietal sites from age 9 to 12 (F(1, 61) = 52.93, p < .001, ), from age 9 to 15 (F(1, 61) = 111.31, p < .001, ), and from age 12 to 15 (F(1, 61) = 32.71, p < .001, ).
For the P300/early LPP occipital ERP mean activity scores, there were significant main effects of both condition (F(2, 122) = 6.94, FDR-corrected p = .002, ) and age (F(1.36, 83.13) = 77.91, FDR-corrected p = .003, ). Main effects were qualified by a trend for an interaction between condition and age (F(3.10, 188.84) = 2.82, uncorrected p = .038, FDR-corrected p = .057, ) on the P300/early occipital ERP mean activity scores. The effect of condition was significant at age 9, F(2, 122) = 5.62, p = .005, , such that the component was more positive in the unpleasant (F(1, 61) = 10.22, p = .002, ) and pleasant (F(1,61) = 6.70, p = .012, ) conditions compared to the neutral condition. The comparison between pleasant and unpleasant conditions did not reach significance (p = .926). The effect of condition was also significant at age 12, F(2, 122) = 3.92, p = .022, , such that the component was more positive in the unpleasant compared to neutral conditions (F(1, 61) = 7.48, p = .008, ). The comparisons between unpleasant and pleasant (p = .206) and neutral and pleasant (p = .119) did not reach significance. However, emotional modulation of P300/early LPP at occipital sites at age 15 did not reach significance (p = .071). See Table 4 for the means for each condition at each age for the mean activity scored P300/early LPP.
3.4.3. Late LPP: PCA factor score measures.
There were main effects of both condition (F(2, 122) = 23.35, FDR-corrected p = .004, ) and age (F(2, 122) = 76.11, FDR-corrected p = .005, ) on the late LPP PCA factor scores. As reported in section 3.2, the component was more positive for both pleasant (F(1, 61) = 6.66, p = .012, ) and unpleasant (F(1, 61) = 38.31, p < .001, ) images compared to neutral images across ages. Additionally, the component was more positive for unpleasant compared to pleasant images across ages (F(1, 61) = 22.60, p < .001, ). Across conditions, the component was more positive at age 12 compared to age 9, F(1, 61) = 59.56, p < .001, , but less positive at age 15 compared to 9 (F(1, 61) = 15.84, p < .001, ) and age 15 compared to 12 (F(1, 61) = 158.91, p < .001, ). The interaction between condition and age did not reach significance (FDR-corrected p = .090). See Table 3 for the means for each condition at each age for the PCA derived late LPP.
3.4.4. Late LPP: Mean activity measures.
For the late centroparietal ERP mean activity scores, there was only a significant main effect of condition (F(2, 122) = 26.60, FDR-corrected p = .006, ), such that the component was more positive for unpleasant compared to both neutral (F(1, 61) = 42.61, p < .001, ) and pleasant (F(1, 61) = 28.00, p < .001, ) images across ages. The comparison between neutral and pleasant did not reach significance (p = .081). There was no significant effect of age (FDR-corrected p = .138) nor an interaction between condition and age (FDR-corrected p = .502).
For the late LPP occipital ERP mean activity scores, there were main effects of both condition (F(2, 122) = 17.95, FDR-corrected p = .003, ) and age (F(1.36, 82.84) = 49.33, FDR-corrected p = .018, ), which were qualified by a significant interaction between condition and age (F(3.33, 202.97) = 2.94, FDR-corrected p = .047 ). The effect of condition was significant at age 9, F(2, 122) = 10.29, p < .001, , such that the component was more positive for the unpleasant condition compared to the neutral (F(1, 61) = 21.01, p < .001, ) and pleasant (F(1, 61) = 8.77, p = .004, ) images. The comparison between the pleasant and neutral conditions did not reach significance (p = .120). The component was also modulated by emotion at age 12, although a somewhat weaker effect was observed, F(2, 122) = 3.99, p = .021, , such that the component was more positive for the unpleasant condition compared to the neutral (F(1, 61) = 5.66, p = .021, ) and pleasant (F(1, 61) = 6.24, p = .015, ) conditions. The comparison between pleasant and neutral did not reach significance (p = .869). Finally, the effect of condition at age 15 was comparable in magnitude to age 12, F(2, 122) = 4.36, p = .015, , and the component was more positive for the unpleasant condition compared to the neutral (F(1, 61) = 7.17, p = .010, ) and pleasant (F(1, 61) = 6.73, p = .012, ) conditions.
To better understand this interaction, we compared each pair of assessments on the late LPP in the unpleasant and pleasant conditions using repeated-measures ANCOVAs, covarying late LPP in the neutral condition. For the unpleasant condition, there was a significant decrease from age 9 to age 12 (F(1, 59) = 7.46, p = .008, ), and from age 9 to age 15 (F(1, 59) = 12.42, p = .001, ). Comparisons for the unpleasant condition between ages 12 and 15 were not significant (p = .590). Similarly, for the pleasant condition, there was a significant decrease from age 9 to age 12 (F(1, 59) = 6.73, p = .012, ), and from age 9 to age 15 (F(1, 59) = 5.21, p = .026, ). Comparisons for the pleasant condition between ages 12 and 15 were not significant (p = .560). See Table 4 for the means for each condition at each age for the mean activity scored late LPP.).
4. Discussion
The aim of the present study was to use ERPs to assess the time course of emotional processing in a longitudinal sample from middle childhood through mid-adolescence. Several key findings emerged. First, temporospatial PCA at each assessment indicated comparable timing of both an earlier and later LPP modulated by emotional condition at each of the three waves of assessments. Next, emotional modulation of the earlier and later components were distinct in that P300/early LPP was characterized by a relative positivity for unpleasant compared to both pleasant and neutral images, while the late LPP presented as an enhanced positivity for both unpleasant and pleasant compared to neutral images. This possibly reflects greater sustained, rather than early attention, towards pleasant stimuli. Third, PCA factor scores for the P300/early LPP and mean activity scores for the early and late LPP evinced moderate to strong rank-order stability and generally acceptable mean-level stability from middle childhood to middle adolescence, but the late LPP PCA factor scores were not stable across development. Stability was stronger from early to mid-adolescence than middle childhood to adolescence and for LPP scores in response to a single condition rather than residual scores. Finally, we observed a developmental shift in the scalp distribution of P300/early LPP from occipital electrodes in middle childhood to centroparietal electrodes in mid-adolescence, with a pattern of decreasing magnitude over occipital sites in mid-adolescence compared to middle childhood and early adolescence that was also characterized by less emotional modulation.
Though several ERP components sensitive to emotion were empirically identified using PCA, only those components corresponding to an early and late LPP appeared consistently at all three assessments. Prior research also suggests that the traditionally observed sustained LPP may be produced by several overlapping and potentially functionally distinct relative positivities in the ERP wave (Foti et al., 2009; Weinberg & Hajcak, 2011). The lack of consistent emergence of an emotion-modulated ERP component reflecting early attentional processing, such as the early posterior negativity (EPN) or P1 is surprising. In a prior PCA of ERPs to emotional stimuli in a smaller sample of children, we found some evidence of an earlier EPN, followed by overlapping positivities consistent with the current results (Kujawa, Weinberg, et al., 2013). Current findings suggest that compared to P300 or LPP, earlier components may be less consistently modulated by emotion across development. This null finding could be influenced by developmental changes in neural circuity underlying earlier stages of processing (MacNamara et al., 2016) or variation in the perceptions of the complexity of task images, which has been found to modulate early processing components but not later elaborative processing components, like LPP (Bradley, Hamby, Löw, & Lang, 2007). These findings have important implications for understanding development of the neural bases of emotion from childhood to adolescence, as well as informing developmental psychophysiology methods.
By examining multiple scoring approaches including PCA factor scores, mean activity measures, and residual scores, our results provide insights into the stability of ERP measures of emotional reactivity across 6 years of development. Regarding the developmental stability of the P300/early LPP, both the PCA factor scores and the mean activity scores evinced moderate to strong stability from middle childhood to mid-adolescence. Stronger stability was observed during the transition from early to middle adolescence, rather than middle childhood to adolescence, a finding that is consistent with our prior research on the developmental stability of reward-related ERP components (Kujawa et al., 2018). For the late LPP, the PCA factor approach yielded generally lower stability than the mean activity approach, likely reflecting changes in the PCA factor solution at each assessment. Additionally, the late LPP indexed by mean activity scores demonstrated stronger stability over occipital regions rather than the centroparietal regions, consistent with the emergence of a positivity over centroparietal sites later in adolescence. Importantly, stability of residual scores for the P300/early LPP and the late LPP was generally low. This is not surprising, as both residual and subtraction-based approaches to difference scores have previously been associated with low reliability, likely due to noise and measurement error, individual differences in baseline levels of activity, and the tendency for ERP components to be highly correlated with one another (Burt & Obradovic, 2013; Furr, & Bacharach, 2013; Levinson, Speed, Infantolino, & Hajcak, 2017).
Although it is surprising that the early LPP/P300 component presented as an overall negativity at centroparietal sites., this is consistent with prior PCAs in adults. Weinberg and Hajcak (2011), for example, found a P300/early LPP component using PCA in an undergraduate sample that was also negative and maximal over centroparietal sites. Additionally, large negative deflections over central and frontal sites and more positive values over the posterior region, similar to the values observed in the present study, have been observed in children (e.g., Solomon, DeCicco, & Dennis, 2011). This may in part be driven by the positioning of dipoles contributing to the ERP signal; the LPP is often observed to be more occipitally maximal in children (e.g., Hajcak & Dennis, 2009), which may lead to a positivity occipitally and more of a negativity centrally and frontally.
In general, results suggest that PCA, rather than traditional scoring approaches, offers a number of benefits to developmental neuroscience research. First, PCA allows for latent components sensitive to cognitive and emotional processes to be isolated without requiring residual or difference scores to adjust for overlap in components and isolate the effects of the condition of interest. PCA is also particularly helpful when the timing and scalp distributions of ERPs change across development. At the same time, it is important to note that when there are changes in the underlying structure of ERP components, the stability of PCA factor scores across development is diminished. In such instances, a combined approach may be preferable, using PCA to inform selection of scoring windows and electrode sites and the mean activity levels to assess stability and reliability over time. Both developmental changes in childhood compared to adolescence and the tendency for EEG data collected from children to have a lower signal-to-noise ratio than data from older populations (Hӓmmerer, Li, Völkle, Müller, & Lindenberger, 2013) could contribute to changes in the magnitude and distribution of ERP components, potentially reducing the stability of PCA factor scores and residual scores compared to that of mean activity scores (also see Kujawa et al., 2018).
Although we found some evidence of a reduction in the magnitude of the LPP across development, our results revealed complexity in the development of emotional processing as measured by ERPs. Specifically, we found a developmental shift in the P300/early LPP from increased magnitude of ERPs over occipital sites in middle childhood to centroparietal sites in mid-adolescence. Importantly, emotional modulation of the P300/early LPP and late LPP over occipital site was also reduced from childhood into adolescence. That is, developmental changes in LPP magnitude from childhood to adolescence are characterized both by a shift in the scalp distribution, as well as relatively reduced emotional modulation, possibly reflecting reduced emotional reactivity and/or improvement in emotion regulation. The results from the behavioral data, specifically an increase in accuracy and decrease in RT with age, provide additional support for this notion. Neuroimaging research indicates that the development of perceptual regions of the brain is characterized by broadly distributed activation in childhood, followed by periods of refinement and localization (Nelson et al., 2016), a theme paralleled in another review that found developmental focalization in prefrontal cortical regions (Durston et al., 2006). The age-related changes in the magnitude and maximal distribution of the LPP components found in the present study may reflect the recruitment of narrower, specified networks during development, such as decreases in the activation of the amygdala and a shift in the recruitment of prefrontal regions from ventrolateral to more dorsomedial with age (Casey et al., 2008; Silvers et al., 2017). This is supported by source localization research on the LPP which demonstrate associations between the magnitude of the LPP and activation in subcortical regions and the visual cortices (Liu et al, 2012), as well as age-related decreases in activation in the ventrolateral prefrontal cortex and bilateral amygdala (Bunford et al., 2018). Furthermore, research on the neural underpinnings of the scalp-recorded LPP in adults suggests that the late LPP component could reflect a reverberation of activity from the prefrontal-parietal network (Moratti et al., 2011), with the amygdala initiating the reentry into the visual cortex (Sabatinelli, Lang, Bradley, Costa, & Keil, 2009). This could have contributed to the lack of stability for the late LPP component over the centroparietal sites.
A limitation to the present study is the repeated presentation of the pleasant, unpleasant, and neutral images at each assessment. While the assessments were three years apart, and the use of consistent images ensures developmental conclusions are not driven by alterations to the task, it is possible that the salience of the images was diminished with repeated administrations or that practice effects contributed to developmental decreases in accuracy and RT. Although habituation to the stimuli, rather than a decrease in emotional reactivity with age, may have contributed to the present results, prior cross-sectional evidence also supports developmental changes in LPP magnitude and distribution (Kujawa et al., 2012; MacNamara et al., 2016). Further, a study on the repetition of stimuli within a single assessment revealed that modulation of the LPP to emotional stimuli remained intact, despite a general decrease in magnitude across trials (Codispoti, Ferrari, & Bradley, 2006). Regarding the developmental changes in accuracy and RT, the potential that practice effects contributed to the findings cannot be ruled out. Nonetheless, evidence of improvements in processing speed from childhood to adolescence (Kail, 1991) supports the interpretation of these effects as reflecting true developmental changes, rather than the result of repeated administrations of the task. Because the developmental assessments began in middle childhood and concluded in mid-adolescence, our current understanding of the development of these processes is limited. Current research suggests that the development of these emotional reactivity and regulation process continues developing into early adulthood (Silvers et al., 2017).
Future research is needed to comprehensively capture the development of emotion-modulated ERPs starting in early childhood and into early adulthood. Additionally, further research needs to address the statistical and methodological challenges identified in the current study. Subsequent developmental research should focus on combining PCA and traditional mean activity scoring methods for ERPs, as changes in the structure and spatial distribution of ERPs is expected with age. Additionally, alternative approaches to quantifying difference scores, such as latent variable approaches, should be explored to parse out the effects of measurement error and state differences (Burt & Obradovic, 2013). Moreover, multimodal research utilizing both EEG and fMRI is needed to further understand the relationship between scalp recorded ERPs and activation of underlying neural circuitry. ERPs are generated through the summed activity of large populations of cortical neurons (Luck, 2005), thus combining EEG with fMRI would provide enhanced spatial resolution and the assessment of key subcortical regions of emotion networks, including amygdala. Multimodal imaging studies could additionally elucidate whether changes in the magnitude of the LPP are reflective of a developmental decrease in emotional reactivity with age, or of more complex developmental changes in the underlying neural systems that influence scalp recorded ERPs.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grant R01 MH069942 to DNK.
Footnotes
The authors have no conflicts of interest to declare.
References
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bondy E, Stewart JG, Hajcak G, Weinberg A, Tarlow N, Mittal VA, & Auerbach RP (2018). Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology, 55(2), e12977 10.1111/psyp.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, & Lang PJ (2007). Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology, 44(3), 364–373. 10.1111/j.1469-8986.2007.00520.x [DOI] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Fitzgerald KD, Monk CS, & Phan KL (2018). Convergence of BOLD and ERP measures of neural reactivity to emotional faces in children and adolescents with and without anxiety disorders. Biological Psychology, 134, 9–19. 10.1016/j.biopsycho.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Burt KB, & Obradovic J (2013). The construct of psychophysiological reactivity: Statistical and psychometric issues. Developmental Review, 33(1), 29–57. 10.1016/j.dr.2012.10.002 [DOI] [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–126. 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SM, Robertson IH, & O’Connell RG (2012). Retest reliability of event-related potentials: evidence from a variety of paradigms. Psychophysiology, 49(5), 659–664. 10.1111/j.1469-8986.2011.01349.x [DOI] [PubMed] [Google Scholar]
- Cattell RB (1966). The scree test for the number of factors. Multivariate Behavioral Research, 1(2), 245–276. 10.1207/s15327906mbr0102_10 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Butcher J, & Nelson L (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. 10.1037/1040-3590.6.4.284 [DOI] [Google Scholar]
- Codispoti M, Ferrari V, & Bradley M (2007). Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience, 19(4), 577–586. 10.1162/jocn.2007.19.4.577 [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, & Bradley MM (2006). Repetitive picture processing: autonomic and cortical correlates. Brain Research, 1068(1), 213–220. 10.1016/j.brainres.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Compas BE, Jaser SS, Bettis AH, Watson KH, Gruhn MA, Dunbar JP, … Thigpen JC (2017). Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychological Bulletin, 143(9), 939–991. 10.1037/bul0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Dien J (2010). The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods, 187(1), 138–145. 10.1016/j.jneumeth.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Dien J (2012). Applying principal components analysis to event-related potentials: A tutorial. Developmental Neuropsychology, 37(6), 497–517. 10.1080/87565641.2012.697503 [DOI] [PubMed] [Google Scholar]
- Dien J (2017). Best practices for repeated measures ANOVAs of ERP data: Reference, regional channels, and robust ANOVAs. International Journal of Psychophysiology, 111, 42–56. 10.1016/j.ijpsycho.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Dien J, & Frischkoff GA (2005). Principal components analysis of ERP data In Handy TC (Ed.), Event-Related potentials: A methods handbook (pp. 189–207). Cambridge, MA: MIT Press. [Google Scholar]
- Dien J, Khoe W, & Mangun GR (2007). Evaluation of PCA and ICA of simulated ERPs: Promax vs. infomax rotations. Human Brain Mapping, 28(8), 742–763. 10.1002/hbm.20304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, & Casey BJ (2006). A shift from diffuse to focal cortical activity with development. Developmental Science, 9(1), 1–8. 10.1111/j.1467-7687.2005.00454.x [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- Furr RM, & Bacharach VR (2013). Psychometrics: An introduction. Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala: Prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, … Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. 10.1162/jocn.2008.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, & Dennis TA (2009). Brain potentials during affective picture processing in children. Biological Psychology, 80(3), 333–338. 10.1016/j.biopsycho.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li SC, Völkle M, Müller V, & Lindenberger U (2013). A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology, 50(1), 111–123. 10.1111/j.1469-8986.2012.01476.x [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30(2), 179–185. 10.1007/bf02289447 [DOI] [PubMed] [Google Scholar]
- Kail R (1991). Developmental change in speed of processing during childhood and adolescence. Psychological Bulletin, 109(3), 490–501. 10.1037/0033-2909.109.3.490 [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Wilcox RR, & Lix LM (2003). A generally robust approach to hypothesis testing in independent and correlated group designs. Psychophysiology, 40(4), 586–596. 10.1111/1469-8986.00060 [DOI] [PubMed] [Google Scholar]
- Kessel EM, Nelson BD, Kujawa A, Hajcak G, Kotov R, Bromet EJ, … Klein DN (2018). Hurricane Sandy exposure alters the development of neural reactivity to negative stimuli in children. Child Development, 89(2), 339–348. 10.1111/cdev.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Carroll A, Mumper E, Mukherjee D, Kessel EM, Olino T, … Klein DN (2018). A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. International Journal of Psychophysiology, 132(Pt B), 323–330. 10.1016/j.ijpsycho.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Danzig AP, Black SR, Bromet EJ, Carlson GA, … Klein DN (2016). Neural reactivity to emotional stimuli prospectively predicts the impact of a natural disaster on psychiatric symptoms in children. Biological Psychiatry, 80(5), 381–389. 10.1016/j.biopsych.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Hajcak G (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2(4), 458–467. 10.1016/j.dcn.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Proudfit GH (2013). Two-year stability of the late positive potential across middle childhood and adolescence. Biological Psychology, 94(2), 290–296. 10.1016/j.biopsycho.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123(2), 287 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Hajcak G, & Klein DN (2013). Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal–spatial PCA. Developmental Psychobiology, 55(5), 539–550. 10.1002/dev.21058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Levinson AR, Speed BC, Infantolino ZP, & Hajcak G (2017). Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology, 54(4), 601–607. 10.1111/psyp.12813 [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, & Ding M (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2005). An introduction to the event-related potential technique. Cambridge, MA: The MIT Press. [Google Scholar]
- MacNamara A, Vergés A, Kujawa A, Fitzgerald KD, Monk CS, & Phan KL (2016). Age-related changes in emotional face processing across childhood and into young adulthood: Evidence from event-related potentials. Developmental Psychobiology, 58(1), 27–38. 10.1002/dev.21341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, de los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54, 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Moratti S, Saugar C, & Strange BA (2011). Prefrontal-occipitoparietal coupling underlies late latency human neuronal responses to emotion. Journal of Neuroscience, 31(47), 17278–17286. 10.1523/JNEUROSCI.2917-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Hajcak G, Klein DN, & Kotov R (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine, 45(12), 2545–2556. 10.1017/S0033291715000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, … Gross JJ (2009). Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science, 20(11), 1322–1331. 10.1111/j.1467-9280.2009.02459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, & Adolphs R (2010). Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience, 11(11), 773–783. 10.1038/nrn2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, & Lang PJ (2013). Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology, 92(3), 513–519. 10.1016/j.biopsycho.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Costa VD, & Keil A (2009). The timing of emotional discrimination in human amygdala and ventral visual cortex. Journal of Neuroscience, 29(47), 14864–14868. 10.1523/JNEUROSCI.3278-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, & Bradley MM (2007). Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex, 17(5), 1085–1091. 10.1093/cercor/bhl017 [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, & Gross JJ (2015). Emotion regulation and psychopathology. Annual Review of Clinical Psychology, 11, 379–405. 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, … Ochsner KN (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 25, 128–137. 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B DeCicco JM, & Dennis TA (2012). Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 110–119. 10.1016/j.dcn.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 23(10), 2994–3007. 10.1162/jocn.2011.21630 [DOI] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, … Phan KL (2016). Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Human Brain Mapping, 37(5), 1684–1695. 10.1002/hbm.23129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


