Abstract
Purpose
Substantial preclinical evidence and case reports suggest that MEK inhibition is an active approach in tumors with BRAF mutations outside the V600 locus, and in BRAF fusions. Thus, Subprotocol R of the NCI-MATCH study tested the MEK inhibitor trametinib in this population.
Methods
The NCI-MATCH study performed genomic profiling on tumor samples from patients with solid tumors and lymphomas progressing on standard therapies or with no standard treatments. Patients with pre-specified fusions and non-V600 mutations in BRAF were assigned to Subprotocol R using the NCI-MATCHBOX algorithm. The primary endpoint was objective response rate (ORR).
Results
Among 50 patients assigned, 32 were eligible and received therapy with trametinib. Of these, 1 had a BRAF fusion and 31 had BRAF mutations (13 and 19 with class 2 and 3 mutations, respectively). There were no complete responses; 1 patient (3%) had a confirmed partial response (patient with breast ductal adenocarcinoma with BRAF G469E mutation) and 10 patients had stable disease as best response (clinical benefit rate 34%). Median progression free survival was 1.8 months and median overall survival was 5.7 months. Exploratory subgroup analyses showed that patients with colorectal adenocarcinoma (n=8) had particularly poor PFS. No new toxicity signals were identified.
Conclusions
Trametinib did not show promising clinical activity in patients with tumors harboring non-V600 BRAF mutations, and the subprotocol did not meet its primary endpoint.
Keywords: Trametinib, BRAF, MATCH, MEK, genomics, fusions
Introduction
Molecularly-guided therapy has made a major impact in certain cancer types in tumors with particular genomic alterations (e.g. EGFR mutations, BRAF V600E mutations, ALK fusions). Most commonly identified mutations in cancer, by contrast, have no validated targeted therapy, despite extensive preclinical data suggesting effective therapeutic strategies in some cases. The National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) Trial is a platform trial with multiple phase II tumor agnostic arms designed to evaluate genomically-targeted treatment strategies across multiple identified genomic changes in cancer.
BRAF inhibitors with or without a MEK inhibitor have demonstrated substantial clinical efficacy in BRAF V600 mutated melanoma, lung cancer, thyroid cancer, hairy cell leukemia, and other cancers.1–5 However, non-V600 BRAF mutations are identified in a substantial portion of patients across cancers (up to 3% total in some publicly available databases), with no validated molecularly guided therapy for them.6,7 These non-V600 BRAF mutations activate mitogen-activated protein kinase (MAPK) pathway signaling similarly (albeit generally slightly less robustly) than the BRAF V600 mutations.8,9 Similarly, fusions in BRAF, which remove the inhibitory-RAS binding domain and hyperactivate MAPK signaling, are also present across cancers.6,7,10,11 Several pre-clinical studies, particularly in melanoma models harboring BRAF L597 mutations and BRAF fusions, suggested BRAF inhibitor monotherapy would not be effective. In contrast, MEK inhibitors demonstrated substantial pre-clinical efficacy in these studies.12,13 In addition, several case reports have demonstrated that MEK inhibitors could produce excellent clinical responses in patients with these molecular variants.12,14–16
MEK inhibitors have shown variable degrees of activity in several settings, including BRAF V600 mutant melanoma, NRAS mutant melanoma, low-grade serous ovarian cancer, plexiform neurofibromas, thyroid cancer, and low-grade gliomas, with more limited responses in KRAS mutant pancreatic cancer or lung cancer.17–22 Trametinib, a selective, allosteric inhibitor of MEK1/2, is approved in BRAF V600 mutant melanoma (alone or in combination with the BRAF inhibitor dabrafenib).17,23 This agent has also been extensively studied in pre-clinical and clinical scenarios, and is the only FDA approved MEK inhibitor monotherapy. Herein, we report the results for NCI-MATCH Subprotocol R: A Phase II study of trametinib in patients with BRAF fusions, or with non-V600 BRAF mutations.
Methods
Subprotocol overview
The NCI-MATCH trial, developed by ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) and the National Cancer Institute (NCI), aimed to find signals of efficacy for treatments targeted to actionable molecular alterations found in any tumor type. The R subprotocol, reported here, was a single arm, phase 2 trial to test the efficacy and safety of trametinib in patients with cancers harboring fusions or non-V600 mutations in BRAF. The study was reviewed by the NCI central IRB and all patients signed written informed consent. The study was conducted according to the Declaration of Helsinki.
Patient selection
Eligible patients were adults with any solid tumor, lymphoma or myeloma who progressed on standard treatment or for whom no standard treatment was available and whose tumor contained an eligible BRAF variant (either by profiling a fresh biopsy with the NCI-MATCH assay24 or after determination by an assay performed on tumor in a CLIA-approved NCI-MATCH accepted laboratory). Adequate hematopoietic, liver and kidney function, an Eastern Cooperative Oncology Group (ECOG) performance status ≤1, were required. Patients were excluded if they had prior treatment with a MEK inhibitor, prior significant cardiac disease (including arrhythmias, treatment-refractory hypertension, decreased cardiac ejection fraction), or prior interstitial lung disease.
Tumor sequencing and subprotocol assignment
Between August 2015 to May 11, 2017, a central network of laboratory reporting was used to determine eligibility. Biopsy specimens in buffered formalin (29 patients) and/or cytology specimens with smears and in CytolytR for cell block preparation (two patients) or archived formalin-fixed paraffin-embedded tissue blocks (2 patients) were shipped overnight to the CLIA-accredited central processing laboratory for the trial. Tumor profiling in these cytology or biopsy specimens was accomplished as previously described24, using an NGS panel of 143 genes that identified single nucleotide variants (SNV), indels, amplifications and selected fusions. Central immunohistochemistry (IHC) assays for expression of PTEN, MLH1, MSH2 as reported previously25 were done on 29 tumors and in a commercial laboratory for one tumor. Patients were assigned using a validated NCI designed informatics rules algorithm (MATCHBOX) (Manuscript under review). After May 11, 2017, patient’s eligibility was initially determined by a referral from a certified genomic laboratory (https://ecog-acrin.org/nci-match-eay131-designated-labs) and later confirmed by the NCI-MATCH central laboratory network. Two patients were enrolled through this referral method. Patients who had pre-specified fusions or mutations in BRAF (Supplemental Table 1) were assigned to Subprotocol R. Pre-specified mutations were identified based on levels of evidence which include the following: Level 1 – gene variant approved for selection of an approved drug, Level 2 – gene variant an eligibility criteria for ongoing clinical trial or has been identified in N of 1 responses, and/or Level 3 – preclinical inferential data that provide biological evidence sufficient to support the use of the variant in treatment.
Evaluation of Response and Toxicity
Patients were treated with trametinib 2 mg daily until disease progression, unacceptable toxicity, or patient/physician choice to discontinue therapy. Dose reductions were permitted to trametinib 1.5 mg daily, then to 1.0 mg daily for severe or persistent toxicities. Objective response was evaluated every 8 weeks using RECIST 1.1 criteria (for solid tumors) or Lugano criteria (lymphomas).26,27 Patients continued on trametinib until progressive disease, unacceptable toxicity, or self-discontinuation. Toxicity was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Statistical Considerations
The primary objective of NCI-MATCH study was to evaluate the objective response rate (ORR) for each subprotocol, defined as proportion of patients with best overall response of complete response or partial response based on applicable criteria (20,21). The ORR was compared against a null benchmark value of 5%. A response rate of 5/31 patients (16%) or more was predefined as a signal of promising activity. This design had approximately 92% power to conclude an agent’s activity is promising if its true ORR is 25%, with one-sided type I error rate of 1.8%. Allowing for 10% ineligibility rate, the accrual goal was 35 patients for this subprotocol. Secondary objectives included progression-free survival at 6 months (PFS6), PFS, overall survival (OS), toxicity assessment, and evaluation of predictive biomarkers (co-mutations or other factors that potentially predict response). PFS was defined as time from treatment start to disease progression or death from any cause; OS was defined as time from treatment start to death from any cause. Both PFS and OS were estimated using Kaplan-Meier method. In an exploratory, unplanned fashion, we assessed PFS and OS based on prior therapies, location of mutation (exon 11 vs. 15), histology, co-occurring mutations, BRAF allele frequency, and BRAF mutation class (as defined by Yao et al)9. Co-occurring mutations were classified as concurrent RAS vs. no RAS mutations (mutations in KRAS, NRAS, HRAS), or PI3K pathway vs. no PI3K pathway (mutations in PI3K, AKT, MTOR, TSC1).
Results
Patients
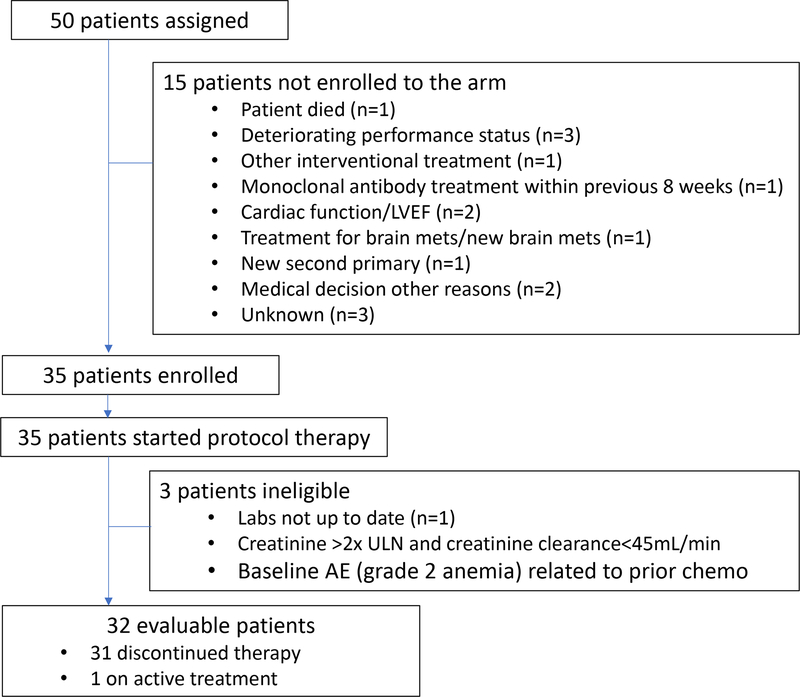
Subprotocol R was activated August 12, 2015. Between August 12, 2015 and August 17, 2017, 50 patients were assigned to Subprotocol R. Thirty-five patients were enrolled to the subprotocol and 15 patients were ineligible to enroll (Figure 1). Of these 35 patients, all received at least one dose of protocol therapy, 3 were found to be ineligible after treatment, and thus 32 patients were evaluable for efficacy endpoints. Table 1 lists patient characteristics; median age was 65.5 years (range 40–83), and 18 (58%) were female. Most patients had ECOG PS of 1 (n=26; 81%) and 69% (n=22) of patients had 3 or more prior therapies. Tumor histopathologic classification is listed in Table 1. GI cancers (n=8, 25%, of which 7 were colorectal adenocarcinoma), lung adenocarcinomas (n=9, 28%), and prostate adenocarcinoma (n=4, 12%, 3 with neuroendocrine differentiation) were the most common subtypes enrolled.
Figure 1:
CONSORT diagram showing numbers of patients assigned, enrolled, and evaluable, as well as reasons for lack of enrollment or evaluability. LVEF: left ventricular ejection fraction; ULN: upper limit of normal.
Table 1:
Patient Characteristics
| Characteristics | No. of patients (%) |
|---|---|
| Total no. of evaluable patients | 32 |
| Age (median, range) | 65.5(40,83) |
| Sex | |
| Male | 14 (44%) |
| Female | 18 (56%) |
| Race | |
| White | 30 (100%) |
| Unknown | 2 |
| Ethnicity | |
| Hispanic | 2 (7%) |
| Non-Hispanic | 28 (93%) |
| Unknown | 2 |
| ECOG PS | |
| 0 | 6 (19%) |
| 1 | 26 (81%) |
| No. of prior therapies | |
| 1 | 6 (19%) |
| 2 | 4 (12%) |
| 3 | 4 (12%) |
| >3 | 18 (56%) |
| Weight loss in previous 6 months | |
| < 5% | 26 (81%) |
| 5 to < 10% | 4 (12%) |
| 10 to < 20% | 3 (6%) |
| Tumor Histology | |
| Gastrointestinal | 8 (25%) |
| Adenocarcinoma of colon | 6 |
| Adenocarcinoma of rectum | 1 |
| Intrahepatic cholangiocarcinoma | 1 |
| Gynecologic | 4 (12%) |
| Serous adenocarcinoma of ovary | 1 |
| Malignant mixed Mullerian tumor of uterus | 1 |
| Endometrioid endometrial adenocarcinoma | 1 |
| Melanoma of vulva | 1 |
| Breast (ductal carcinoma) | 1 (3%) |
| Lung adenocarcinoma | 9 (28%) |
| Adenocarcinoma | 6 |
| Adenosquamous carcinoma | 1 |
| Hepatoid adenocarcinoma | 1 |
| Sarcomatoid adenocarcinoma | 1 |
| Genitourinary | 5 (16%) |
| Prostate adenocarcinoma | 1 |
| Prostate adenocarcinoma with neuroendocrine differentiation | 3 |
| Osteosarcoma of the renal pelvis | 1 |
| Lymphoma | 2 (6%) |
| Cutaneous T-cell anaplastic large cell lymphoma | 1 |
| Diffuse large B-cell lymphoma | 1 |
| Spindle cell component of parotid epithelial- myoepithelial carcinoma | 1 |
| Adenocarcinoma of unknown primary site | 2 (6%) |
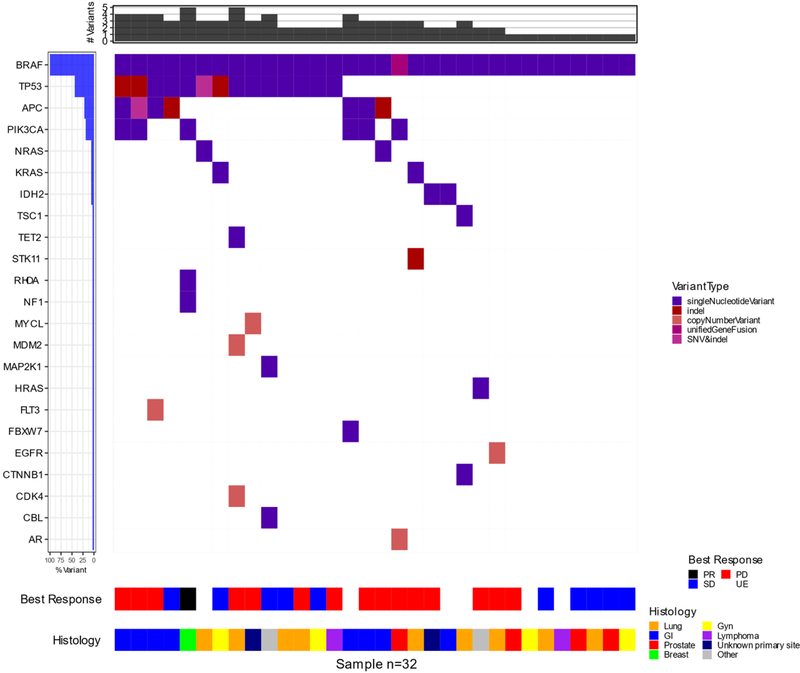
Various BRAF mutations were identified, as well as a single BRAF fusion (n=1) (Supplementary Table 2, Supplementary Figure 1). Exon 11 mutations were identified in 13 patients (42%), including in G464 (n=2), G466 (n=4), and G469 (n=7). Exon 15 mutations were present in 18 patients (58%), including N581 (n=3), D594 (n=11), L597 (n=2), and K601 (n=1). Co-occurring mutations were also diverse, including those in APC (n=10), HRAS (n=1), KRAS (n=2), NRAS (n=2), PIK3CA (n=6), and TP53 (n=16).
Efficacy
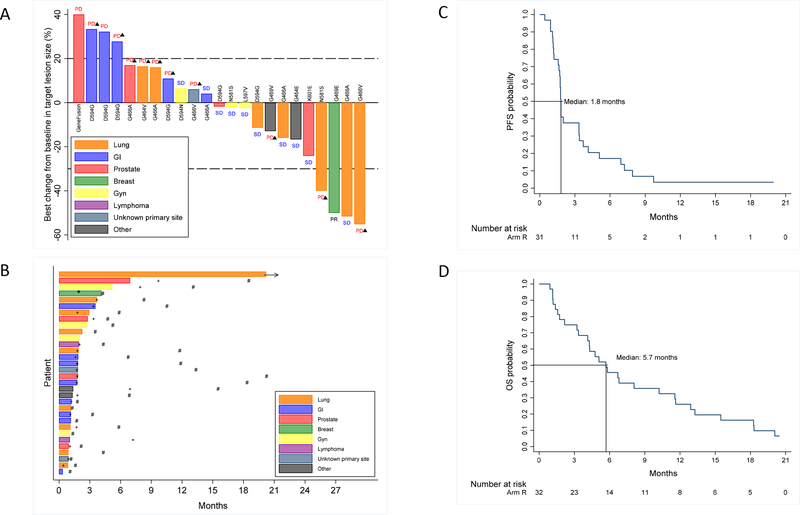
Of the 32 patients evaluable for efficacy endpoints, there were no complete responses, 1 patient had a partial response, 10 had stable disease, and 15 had progressive disease. Six patients did not have any imaging assessment prior to death (n=5), or withdrawal (n=1) (Figure 2A). Notably, the patient who withdrew after one cycle remained alive at 20.8 months post-registration. Thus, we observed a response rate of 3% (1/32, 90% CI 0.2%−14%), and a potential clinical benefit rate of 34% (90% CI: 21%−50%). The patient with a partial response had invasive breast cancer with a BRAF G469E mutation; at 4 months on treatment she had a maximal partial response (with 50% tumor shrinkage) but died suddenly at 4.3 months (potentially drug-related, and of unknown cause) without progression. Four additional patients had stable disease with PFS >6 months, including one patient with lung adenocarcinoma with BRAF G469A mutation who remains on therapy for 22 cycles (20.4 months) without progression, and a prostate cancer patient with a BRAF K601E mutation with a near partial response with progression at 9.7 months after starting therapy (Figure 2B, Table 2, Supplementary Figure 2).
Figure 2:
(A) Waterfall plot showing depth of response at best change from baseline in target lesion size; best confirmed response noted as PD (progressive disease), SD (stable disease), or PR (partial response) in 24 patients whose best change in target lesion size was evaluable (6 patients without any follow-up imaging assessment and 3 patients without confirmatory follow-up assessment of their target lesions were not included), triangle denotes new lesion as cause of PD; (B) “Swimmer’s” plot showing treatment duration for all 32 evaluable patients and their occurrence of response (*), disease progression (+) and death (#); (C) Progression-free survival (1 patient was censored at registration and did not contribute to the analysis); (D) Overall Survival
Table 2:
Patients with response or prolonged stable disease
| Histology | Mutation type | Concurrent mutated genes | Best conf. response | # Cycles | PD | Dead | PFS time (months) | OS time (months) |
|---|---|---|---|---|---|---|---|---|
| Ductal carcinoma of breast | Gly469Glu | NF1, PIK3CA, RHOA, TP53 | PR | 5 | No | Yes | 4.1 | 4.3 |
| Spindle cell neoplasm | Gly464Glu | CBL, MAP2K1, TP53 | SD | 2 | Yes | Yes | 6.9 | 15.5 |
| Endometrioid endometrial adenocarcinoma | Leu597Val | KRAS, TP53 | SD | 6 | Yes | Yes | 7.9 | 13.1 |
| Adenocarcinom of prostate with neuroendocrine differentiation | Lys601Glu | No | SD | 8 | Yes | Yes | 9.7 | 18.5 |
| Adenocarcinoma of lung | Gly469Ala | No | SD | 22 | No | No | 20.0 | 20.4 |
The median PFS was 1.8 months (90% CI: 1.7, 3.4), with an estimated 6-month PFS rate of 17% (90% CI 8%, 30%) (Figure 2C). The estimated 6-month OS rate was 46% (90% CI: 30%, 59%), and the median OS was 5.7 (90% CI: 4.1, 8.1) months (Figure 2D). At last follow up, 29 (of 32) patients had died (3 patients were alive at 2.2, 20.4, and 20.8 months).
In exploratory analyses, we assessed whether histology, co-occurring mutations, BRAF allele frequency, and type of BRAF mutation affected benefit from trametinib (Figure 3;). Given the small sample size and post-hoc analyses, we did not formally statistically compare subgroups, rather, we provided the hazard ratios (HR) and associated 95% confidence intervals (CI) from univariate Cox proportional hazard models. We did not observe obvious differences in clinical outcomes in patients based on prior therapies (Supplementary Figure 3). A trend toward improved OS was observed in patients with exon 11 BRAF mutations (HR=0.45, 95% CI: 0.20, 1.00) -(Supplementary Figure 4). Patients with colorectal adenocarcinomas had particularly poor PFS (HR=3.22, 95% CI: 1.29, 8.02) (Supplementary Figure 5). Some trends toward improved PFS (HR=0.36, 95% CI: 0.15, 0.90) and OS (HR=0.50, 95% CI: 0.21, 1.20) were observed in patients lacking concurrent PI3K pathway gene mutations, albeit with small numbers, with no differences observed in patients with or without concurrent RAS mutations (Supplementary Figures 6–7). Interestingly, lower than median BRAF allele frequency also seemed associated with slightly better PFS (HR=0.76, 95% CI: 0.36, 1.61) and OS (HR=0.67, 95% CI: 0.32, 1.43) (Supplementary Figure 8). Finally, we assessed mutation class (class 2 vs. class 3; see discussion); class 2 mutations appeared associated with improved PFS (HR=0.50, 95% CI: 0.22, 1.14) and OS (HR=0.62, 95% CI: 0.29, 1.31) (Supplementary Figure 9).
Figure 3:
Oncoprint showing spectrum and allele frequency of identified BRAF mutations, co-occurring mutations, histology, and best overall response).
Safety
Adverse events at least possibly related to treatment are listed in Supplementary Table 3. All 35 patients enrolled started protocol therapy, but one patient declined all intervention and symptom assessment shortly after starting treatment, and adverse event data was not assessed, so the analysis population for toxicity was the 34 patients who were treated and reported adverse event data. Nine deaths on study were noted; two were considered possibly related to treatment (one patient with sudden death several days after the development of extreme fatigue and was found to have decreased cardiac ejection fraction and one patient with a thromboembolic event approximately one month following an ankle fracture, both events judged possibly due to drug or disease). Worst grade toxicity otherwise was grade 1–2 (n=16, 47%) or grade 3 (n=12, 35%). Toxicities were consistent with other MEK inhibitor studies overall, and included anemia (n=13, 38%), nausea (n=12, 35%), peripheral edema (n=11, 32%), and acneiform rash (n=11, 32%). Of the 35 treated patients, the median number of cycles was 2 (range 1–22). Among 31 eligible patients who had discontinued therapy, six (19%) discontinued due to toxicity, fifteen patients (48%) discontinued treatment due to disease progression, 4 due to death on study (2 due to disease, 2 to possible drug-related toxicities), 3 due to other complicating disease, 1 due to other reason, and 2 patients withdrew (Supplemental Table 4).
Discussion
In this study of trametinib in patients with BRAF non-V600 mutations, we found that trametinib had relatively low activity and the primary endpoint was not met. Too few patients with BRAF fusions (n=1) were included to characterize the activity of trametinib in this population. A few patients did experience clinical benefit with responses (3%) or prolonged stable disease. The toxicities observed were consistent with other studies of trametinib, without obvious new safety signals.
The explanation for this lack of benefit is not entirely evident. Patients were heavily pre-treated and multiple histologies were enrolled. Exploratory subgroup analyses were assessed to potentially identify signals of benefit or particularly poorly performing populations, although it should be noted that these were post-hoc and underpowered for definitive conclusions. Patients with concurrent PI3K pathway mutations seemed to experience worse PFS and OS, possibly indicating that parallel signaling networks may have driven resistance in many patients. BRAF and/or MEK inhibition has had little success in many tumor types, for example in colorectal cancer, which comprised 20% of patients in this study (and had particularly poor outcomes).28,29 By contrast, only one patient (with melanoma of the vulva; who failed to respond) had a tumor type historically more sensitive to MEK inhibitors based on previously available data (e.g. melanoma, thyroid cancer). Thus, the available evidence suggests that histology (or molecular features that accompany histology) continues to play a role and provide context for mutations common to distinct cancer types. Further, 5 patients had concurrent RAS mutations, which typically do not respond to MEK inhibition; this may have contributed to the poor responses.
Another potential explanation lies in the types of BRAF mutations identified. One potentially useful framework and nomenclature is the Class 1–3 mutations recently described.8,9 Class 1 mutations (limited to BRAF V600 mutations) signal as constitutively active monomers, whereas class 2 (G469, L597, K601) signal as constitutively active dimers. By contrast, class 3 mutations (D594, G466, A581) have impaired kinase activity (or are kinase dead), bind more tightly to wild type RAF, and often exhibit RAS activation triggered by other mechanisms (e.g. RAS mutations, NF1 deletions, growth factor signaling). Thus, class 3 BRAF mutated tumors may signal through multiple pathways, similar to RAS mutated tumors. Historically, most responses to MEK inhibitors16,30 and newer agents (ERK inhibitors)31 have come in tumors with class 2 rather than class 3 mutations. Most patients in this series had class 3 mutations (n=19), potentially explaining the relative lack of activity; most patients that benefited in our study had, by contrast, class 2 mutations. Of note, the patient with the transient partial response had a class 3 BRAF mutation (G469E) as well as an NF1 inactivating mutation, but the patient with prolonged stable disease had a class 3 mutation (D594G) without a RAS or NF1 co-mutation (Supplemental Table 3), thus suggesting this is just one piece of the puzzle. One could suggest that assessing the degree of pERK expression might be a potential readout to determine MAPK signaling dependency and MEK inhibitor sensitivity (and lack of pERK as a possible marker of resistance), although this was not feasible for this study.
While trametinib did not show substantial activity in this population, newer agents to target MAPK signaling are in development. These include inhibitors of ERK, the final canonical member of the MAPK cascade, which has shown some modest clinical activity in early studies.31 In addition, next generation BRAF inhibitors (so-called paradox-breaker, or dimer-disrupting BRAF inhibitors) may also hold promise.32 These agents have shown promise in BRAF V600 mutant melanoma resistant to BRAF/MEK inhibitors, as well as those with non-V600 mutations and fusions in BRAF. However, no large scale studies in this population have been performed.
In conclusion, single agent trametinib had low rates of clinical activity in patients with heavily pre-treated, metastatic cancers harboring non-V600 mutations in BRAF. This contrasts with a number of case reports, largely in melanoma, showing responses in patients with these mutations. Further study might help distinguish subpopulations that benefit from trametinib. In the interim however, trametinib cannot be recommended as a single agent in patients harboring these mutations.
Supplementary Material
Statement of translational relevance.
Mutations in BRAF outside of the 600th codon (BRAF non-V600) or BRAF fusions activate MAPK pathway signaling, and may be targetable by MEK inhibitors. We conducted a phase II study of trametinib in patients with solid tumors and lymphomas harboring BRAF non-V600 mutations or fusions to characterize the activity of this population. Overall, trametinib had low activity (3% response rate) and is thus not a recommended treatment option in this population. Additional treatment options are needed for patients harboring these genomic alterations.
Acknowledgements
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA233270, CA233230, CA233290, CA233329, CA233180.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Conflicts of interest: DBJ serves on advisory boards for Array Biopharma, BMS, Incyte, Merck, and Novartis, and receives grant support from BMS and Incyte outside the scope of this study.
References
- 1.Long GV, Eroglu Z, Infante J, et al. Long-Term Outcomes in Patients With BRAF V600-Mutant Metastatic Melanoma Who Received Dabrafenib Combined With Trametinib. J Clin Oncol 2018;36:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016;17:1248–60. [DOI] [PubMed] [Google Scholar]
- 3.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307–16. [DOI] [PubMed] [Google Scholar]
- 4.Tiacci E, Park JH, De Carolis L, et al. Targeting Mutant BRAF in Relapsed or Refractory Hairy-Cell Leukemia. N Engl J Med 2015;373:1733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004;116:855–67. [DOI] [PubMed] [Google Scholar]
- 9.Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson KE, Lipson D, Stephens PJ, et al. BRAF Fusions Define a Distinct Molecular Subset of Melanomas with Potential Sensitivity to MEK Inhibition. Clin Cancer Res 2013;19:6696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botton T, Yeh I, Nelson T, et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell Melanoma Res 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlman KB, Xia J, Hutchinson K, et al. BRAF L597 mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dankner M, Lajoie M, Moldoveanu D, et al. Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanoma. Clin Cancer Res 2018. [DOI] [PubMed] [Google Scholar]
- 14.Menzies AM, Yeh I, Botton T, et al. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:773–81. [DOI] [PubMed] [Google Scholar]
- 18.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 2019;20:1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dombi E, Baldwin A, Marcus LJ, et al. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N Engl J Med 2016;375:2550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champer M, Miller D, Kuo DY. Response to trametinib in recurrent low-grade serous ovarian cancer with NRAS mutation: A case report. Gynecol Oncol Rep 2019;28:26–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–45. [DOI] [PubMed] [Google Scholar]
- 23.Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res 2011;17:989–1000. [DOI] [PubMed] [Google Scholar]
- 24.Lih CJ, Harrington RD, Sims DJ, et al. Analytical Validation of the Next-Generation Sequencing Assay for a Nationwide Signal-Finding Clinical Trial: Molecular Analysis for Therapy Choice Clinical Trial. J Mol Diagn 2017;19:313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury JD, Wang WL, Prieto VG, et al. Validation of Immunohistochemical Assays for Integral Biomarkers in the NCI-MATCH EAY131 Clinical Trial. Clin Cancer Res 2018;24:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopetz S, Desai J, Chan E, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol 2015;33:4032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlman KB, Xia J, Hutchinson K, et al. BRAFL597 Mutations in Melanoma Are Associated with Sensitivity to MEK Inhibitors. Cancer Discov 2012;2:791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan RJ, Infante JR, Janku F, et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov 2018;8:184–95. [DOI] [PubMed] [Google Scholar]
- 32.Yao Z, Gao Y, Su W, et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nat Med 2019;25:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.