Significance
Visually cued animals that inhabit the deep sea must signal to one another in order to facilitate group behaviors, yet the capacity and mechanisms for information transfer in such a dimly lit habitat are largely uninvestigated. By examining in situ behavioral footage of the Humboldt squid, Dosidicus gigas, we demonstrate the potential for a deep-living social animal to visually convey and receive large quantities of information by combining complex pigmentation patterning with whole-body luminescence. Our findings reveal a capability for information sharing comparable to advanced forms of animal communication known from well-lit habitats. This may have important implications for ecosystem processes, as information sharing between abundant predators is involved in energy and nutrient transfer throughout the world’s oceans.
Keywords: behavioral ecology, social evolution, deep-sea biology, visual signaling
Abstract
Visual signals rapidly relay information, facilitating behaviors and ecological interactions that shape ecosystems. However, most known signaling systems can be restricted by low light levels—a pervasive condition in the deep ocean, the largest inhabitable space on the planet. Resident visually cued animals have therefore been hypothesized to have simple signals with limited information-carrying capacity. We used cameras mounted on remotely operated vehicles to study the behavior of the Humboldt squid, Dosidicus gigas, in its natural deep-sea habitat. We show that specific pigmentation patterns from its diverse repertoire are selectively displayed during foraging and in social scenarios, and we investigate how these behaviors may be used syntactically for communication. We additionally identify the probable mechanism by which D. gigas, and related squids, illuminate these patterns to create visual signals that can be readily perceived in the deep, dark ocean. Numerous small subcutaneous (s.c.) photophores (bioluminescent organs) embedded throughout the muscle tissue make the entire body glow, thereby backlighting the pigmentation patterns. Equipped with a mechanism by which complex information can be rapidly relayed through a visual pathway under low-light conditions, our data suggest that the visual signals displayed by D. gigas could share design features with advanced forms of animal communication. Visual signaling by deep-living cephalopods will likely be critical in understanding how, and how much, information can be shared in one of the planet’s most challenging environments for visual communication.
In animal communication, complex signals develop to maximize information content and to enhance the efficacy with which information is relayed (1). Information can be rapidly shared through vision, and, thus, visual signals are commonly used by social animals with highly dynamic lifestyles (2). However, this sensory modality hinges on the presence of adequate light levels. The deep ocean is an all but totally dark environment and is thus not known to harbor animals that display complex visual signals beyond bioluminescent displays (3).
Most cephalopods are visually cued predators that spend the majority of their lives in the deep ocean (4–6). Many deep-living species are highly social (7), and some display dynamic, coordinated movements similar to animals that live in well-lit waters (8). Some of the deep-dwelling species are also highly cannibalistic (9, 10). Therefore, despite the light-restricted nature of their primary habitat, the lifestyles of many deep-living cephalopods probably incorporate the need to visually convey large quantities of information rapidly.
However, the visual behaviors of cephalopods have been studied almost exclusively in shallow-living species. In combination with postures and locomotion, they generate pigmentation patterns by controlling the size of chromatophores in their skin, which are widely recognized as visual signals that facilitate all manner of activities, including social behaviors (6). Evidence of semanticity (signals tied to specific meanings) and discreteness (signal combinations convey specific information) have been found in the visual signaling of some shallow-living cephalopods (11, 12), suggesting complexity comparable with advanced forms of animal communication (13).
Although some species of deep-living cephalopods have been recently shown to display diverse repertoires of pigmentation patterns comparable to their shallow-living relatives (14–16), the manner in which these visual displays and associated behaviors are utilized remains unknown. Moreover, it is unknown how these behaviors could be adequately perceived under the low-light conditions characterizing the primary habitat. The goals of this study were to 1) associate the visual behaviors of deep-living cephalopods with ecological context, such as foraging and social scenarios; 2) investigate their behavioral syntax; and 3) determine how such behaviors could be conveyed and received in the dark.
Study Species and Location
To investigate the role of visual signals in foraging and group behaviors in deep-living cephalopods, we used high-definition (HD) video cameras mounted on electrohydraulic and electric remotely operated vehicles (ROVs) to study the behavior of the Humboldt squid (Dosidicus gigas) in its natural daytime habitat in the California Current at depths between 266 and 848 m (Materials and Methods). This squid is a gregarious migratory species endemic to the pelagic zone of the eastern Pacific Ocean, where it is known to exert substantial ecological impacts on pelagic communities through its role as a high-level predator (7, 17). Three aspects of the species’ behavior make D. gigas well suited to our behavioral research focus using ROVs. Unlike many deep-living squids, it is attracted to and is seemingly unperturbed by the presence of ROVs (16); it reliably pursues and captures prey items in front of the vehicles (18); and it is social (i.e., it forms interacting shoals that exhibit foraging and antipredator behaviors) (19). Thus, interaction between individuals within groups can be readily observed (8, 20). In addition, tagging studies show that D. gigas migrates vertically and, in doing so, spends the majority of day and night hours at light levels darker than a moonless night at the sea surface (21–24). Daytime hours are typically spent at depths >200 m, where solar illumination is greatly diminished (3, 25), especially in productive regions like those of our study area. At night, D. gigas migrates closer to the surface, albeit not as shallow if the moon is full (21).
Despite the low light levels of its typical habitat, D. gigas must coordinate group activities, most likely through a visual pathway. Shipboard acoustic techniques have shown that this species is capable of exceptionally polarized schooling behavior during foraging activity at night, which involves horizontal group movements punctuated by bouts of coordinated feeding (8). Although not tested, relevant decisions, such as where to forage, when to initiate feeding, or how to establish feeding priority, presumably require signaling within squid groups. Like many studied shallow-living squid, D. gigas displays an impressive repertoire of pigmentation patterns (SI Appendix, Fig. S1A and Table S1) (16, 26) that would presumably be the signals by which such information is relayed. As hypothesized for shoaling squid, some of these behaviors are quite subtle (6)—how such behaviors are used in an ecologically relevant manner is an open question.
Standardized ROV observations were made of 30 squid, split evenly between different ecological contexts (Materials and Methods). The majority of the diverse postural, chromatic, and locomotor components that we recorded in D. gigas (SI Appendix, Fig. S1 A and B and Table S1) have been described from prior ROV studies (16) and from animal-borne video packages (20, 26), which suggests a conserved signaling repertoire in this species. These constituents of the overall physical appearance of a squid were frequently utilized in nonrandom combinations (probabilistic co-occurrence model, alpha = 0.05) (SI Appendix, Fig. S2A). Thus, the behaviors we recorded were unlikely to be artifacts of the artificial lighting and noise generated by the ROVs. Although behavior may be altered in squid under observation, we focused on the behavioral variation associated with other ecological factors while holding that of the ROV constant.
Results and Discussion
Chromatic Behaviors Associated with Foraging in Groups.
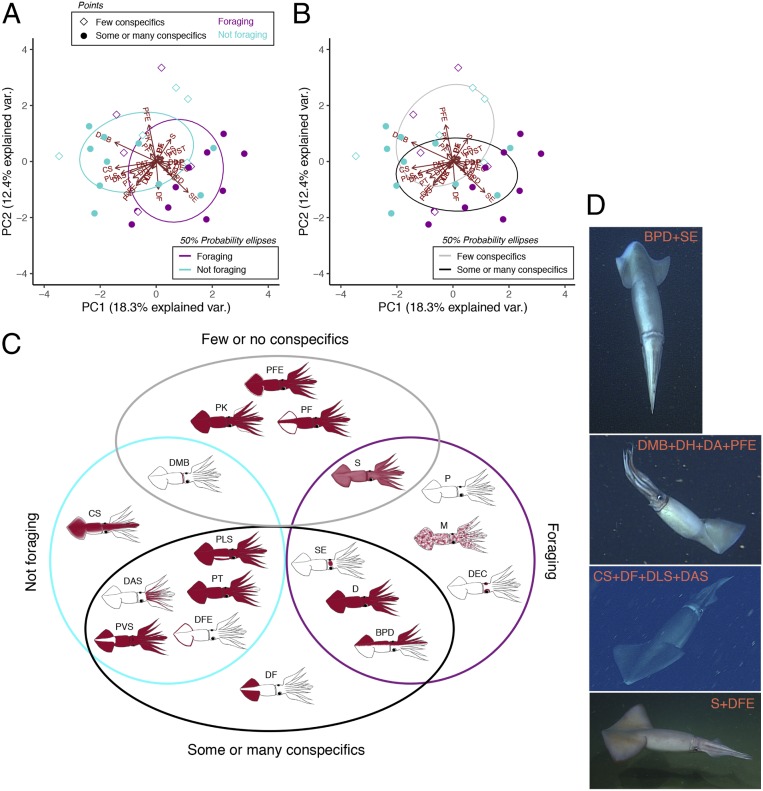
Many marine taxa inhabiting well-lit surface waters use specific visual signals to indicate intent when foraging (27) and in social scenarios (28). To our knowledge, however, visual behaviors have not been associated with similar ecological contexts in a deep-living animal. In its deep daytime habitat, we found that D. gigas more frequently utilized the variable chromatic displays flashing (rapid alteration between pale [P] and dark [D]; P = 0.24) and flickering (dynamic mosaic of scattered pigmentation; P = 0.019) when in the presence of many conspecifics (ANOVA with Tukey’s honest significant difference [HSD]; SI Appendix, Fig. S2B and Table S2), which is congruent with prior observations of their use during group spawning activities in shallower waters (20). Using principal component analysis (PCA), we found that variance in chromatic behavioral components by D. gigas at depth was aligned with foraging status (18.3%; Fig. 1A) and conspecific abundance (12.4%; Fig. 1B), whereas variance in posture and locomotion showed no alignment with conspecific abundance (SI Appendix, Fig. S2C) distinct from foraging status (Fig. 2 C and D). This suggests that chromatic behaviors, in particular, may have signaling functions related to group behaviors.
Fig. 1.
Pigmentation patterning associated with foraging and group behaviors in D. gigas. (A and B) PCA and vectors of chromatic components with 50% probability ellipses encircling foraging status clusters (A) and conspecific abundance clusters (B). In both, each of the 30 squid is represented by a point that is colored by foraging status and shaped by conspecific abundance category with proximity indicating behavioral similarity. Red arrows and abbreviations represent the vectors, or relative contribution of different components to the behavioral trends among squid. Var., variation. (C) Venn diagram illustrating the ecological context under which squid utilized specific chromatic components based on component vectors and other PCA results (Materials and Methods). (D) Examples of various chromatic components analyzed in this study. See Table 1 for component abbreviations. In this study, foraging and not foraging distinguish whether or not a squid attempted to capture prey.
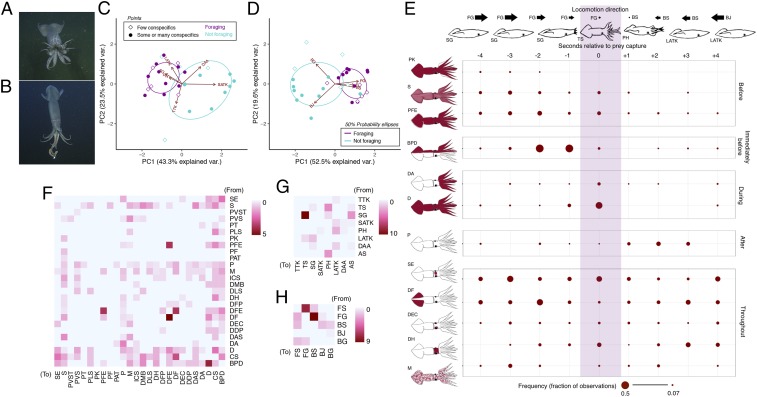
Fig. 2.
Consistencies in the arrangement of pigmentation patterns during foraging in D. gigas. (A and B) Representative images of arm strike (A) and tentacle strike (B), the two stereotyped strategies squid used to capture prey. (C and D) PCAs and vectors of postures (C) and locomotion (D) with 50% probability ellipses encircling foraging status clusters. In both, each D. gigas (n = 30) is represented by a point that is colored by foraging status and shaped by conspecific abundance category, with proximity indicating behavioral similarity. Red arrows and abbreviations represent the vectors, or relative contribution of different components to the behavioral trends among squid. Var., variation. (E) The frequency of chromatic components displayed by foraging squid, or those that attempted to capture prey (n = 15), with respect to prey-capture attempts (shaded in purple). The general suite of postures and locomotion is also illustrated. (F–H) Adjacency network heatmaps of chromatic (F), postural (G), and locomotor (H) components displayed by squid during the 4 s preceding and following prey-capture attempts, with loops (i.e., prolonged, single-component display) removed to highlight transitions between different components. Color denotes the number of occurrences that squid transitioned from components on the vertical axes to components on the horizontal axes (warmer, more adjacencies; paler, fewer adjacencies). See Table 1 for component abbreviations.
Humboldt squid relied on considerably more chromatic behavioral components (28) compared with postural (8) and locomotor components (5) (Table 1 and SI Appendix, Table S1). We used PCA to identify 18 chromatic components that distinguished the behavior of squid in different ecological contexts—13 of which were utilized by D. gigas when surrounded by large numbers of conspecifics, or squid that were foraging (Fig. 1C). Many of these components are known to serve as interspecific and intraspecific signals in shallow-water cephalopods. Notably, P and D along the longitudinal axis (bilaterally pale and dark [BPD]; Fig. 1 C and D) functions in antagonistic displays between rival males during courtship-associated competition in the Caribbean reef squid, Sepioteuthis sepioidea (6), and the mourning cuttlefish, Sepia plangon (29). Humboldt squid, which are among the most cannibalistic of all cephalopods (9), displayed this behavior when foraging in the presence of many conspecifics, suggesting its potential utility as a signal of intent to conspecifics during contentious foraging aggregations.
Table 1.
Abbreviations of behavioral components analyzed in this study
| Abbreviation | Component |
| Chromatic behaviors | |
| BPD | Bilaterally pale and dark |
| CS | Countershaded |
| D | Dark |
| DAS | Dark arm stripes |
| DA | Dark arms |
| DDP | Dark dorsal patch |
| DEC | Dark eye circle |
| DFE | Dark fin edges |
| DFP | Dark fin patch |
| DF | Dark fins |
| DH | Dark head |
| DK | Dark keels |
| DLS | Dark lateral stripes |
| DMB | Dark mantle base |
| ICS | Inverse countershaded |
| M | Mottle |
| P | Pale |
| PAT | Pale arm tips |
| PE | Pale eyes |
| PFE | Pale fin edges |
| PF | Pale fins |
| PK | Pale keels |
| PLS | Pale lateral stripes |
| PT | Pale tentacles |
| PVS | Pale ventral shield |
| PVST | Pale ventral stripe |
| S | Sandy |
| SE | Shaded eye |
| Postural behaviors | |
| AS | Arm strike |
| DAA | Dorsal arm arch |
| LATK | Loose arm tips keeled |
| PH | Prey handling |
| SATK | Stiff arm tips keeled |
| SG | Strike glide |
| TS | Tentacle strike |
| TTK | Trailing tentacles keeled |
| Locomotor behaviors | |
| BG | Back glide |
| BJ | Back jet |
| BS | Back swim |
| FG | Forward glide |
| FS | Forward swim |
It has been suspected, but not yet tested, that large, visually cued pelagic predators with potentially dangerous prey-capture structures and strategies use visual cues involving pigmentation to organize feeding order when foraging in groups (30). Consistent with findings of acoustic-based research (8), we observed that no matter how frenzied group foraging activity became, squid avoided direct contact and physical competition for individual prey with one another. This may indicate that the squid perceived and responded to visual displays of their counterparts that were demonstrating foraging intent. Intraspecific signals that aid in the prevention of unnecessary competition for prey and physical antagonistic interactions have fitness consequences (31, 32).
Arrangement of Chromatic Behaviors during Foraging.
Visual signals can interact in nonrandom ways, such that not only do the signals themselves convey information, but also their syntax—an attribute that many interpret as a building block of language (33, 34). Typical drivers of language development such as elaborate social structure are seemingly absent among cephalopods (6), yet two shallow-living species, S. sepioidea and Sepioteuthis lessoniana (oval squid), show evidence of syntax in their use of chromatic components (11, 12). To our knowledge, syntactic signal usage is largely unexplored in pelagic and deep-living cephalopods like D. gigas (6, 35), let alone any other deep-living animal.
Prey-capture attempts by D. gigas in our study (Fig. 2 A and B) were readily recognizable, and the squid utilized a stereotyped suite of postures (Fig. 2C) and locomotion (Fig. 2D) preceding and following strikes at prey (Fig. 2 E, G, and H). This accounted for the majority of positive component associations determined by a probabilistic co-occurrence model (SI Appendix, Fig. S2A). Foraging squid (n = 15) thus provided the unique opportunity to examine how deep-living squid arrange chromatic components with respect to a fixed event.
Although we were limited by sample size, adjacency network analysis suggested that foraging D. gigas may consistently arrange chromatic components in a hierarchical manner, similar to what has been recorded in sexual and hostile interactions of S. sepioidea (11). Squid pursuing prey were predominantly darkened (D, sandy [S], or countershaded [CS]) and then shifted to disruptive pigmentation (BPD) immediately before striking at prey. During the strike, D. gigas typically went D, followed by an abrupt transition to P (Fig. 2 E and F). These whole-body components are similar to “signifiers” (11), or signals that indicate strong and precise messages—perhaps in this case, an intent and attempt to capture prey. Signifiers associated with prey pursuit and capture were adjacent to more subtle patterning: pale stripes along the lateral mantle (pale lateral stripes [PLS]) preceded CS; dark arm stripes (DAS) occurred before and after S; a dark patch on top of the head (shaded eye [SE]) preceded BPD and followed CS; and a darkened anterior mantle margin (dark mantle base [DMB]) followed S, CS, and BPD (Fig. 2F). These more subtle components are similar to “modifiers” (11), or more widely distributed signals that may serve as accents to signifiers—possibly denoting the type and location of prey, and thus the impending prey-capture strategy undertaken by a foraging squid. During the entire foraging sequence, squid concurrently shifted between pale fin edges (PFE) and dark fin edges (DFE), and then between DFE and entirely dark fins (DF), and finally back to PFE from DFE (Fig. 2F). Both SE and DMB, as well as the fin components and a pale ventral mantle tip (pale ventral shield [PVS]), showed prolonged display, comparable to many whole-body components (S, CS, and BPD; SI Appendix, Fig. S2D). These components and their usage may resemble “positionals” (11), or auxiliary components that provide additional information. As such, they may not be directly related to the foraging sequence, but could serve as signals of social dominance (32), signaler quality (36), or deimatic behaviors (6) directed at nearby conspecifics. The equivalent of DFE and SE in S. lessoniana, when displayed together, signify the winner of fights among male squid (12).
Variation in the arrangement of chromatic behaviors in D. gigas could also be attributable to diematic displays directed at the ROVs. Chromatic behaviors displayed during prey capture are relatively consistent, regardless of ecological context in the anagolous bobtail squid, Sepiola affinis, and the common cuttlefish, Sepia officinalis (37, 38). This suggests that they may serve to startle or distract predators when an individual is temporarily vulnerable while hunting prey (37, 38). While the ROVs may have been viewed as a potential threat by D. gigas in our study, cannibalistic conspecifics (9) present at the time are also a likely threat that could have warranted similar signaling.
Bioluminescent Backlighting and the Illumination of Chromatic Behaviors.
The limited light regime of the deep ocean (3, 25) could preclude adequate visual perception of chromatic behaviors by D. gigas or their deep-living counterparts. Bioluminescence is a ubiquitous ecological trait in the ocean (39), where it can comprise visual signaling (40). Bioluminescent signals typically involve changes in light intensity—these can be generated by altering conditions within the photophores (41) or by manipulating the emitted light using other anatomical features. For instance, some nocturnal marine fishes flash large cutaneous photophores using muscular “shutters” (42, 43); similarly, the deep-living octopus squids Octopoteuthis deletron and Taningia danae use chromatophores to control light produced by large arm-tip photophores (14, 44).
As opposed to the common tactic of altering the intensity of light produced by individual photophores, D. gigas may use pigmentation patterning to selectively reveal and conceal different regions of an entirely luminescent body. While in most cases, photophores are external and designed to project light outward, those of D. gigas are instead internal and radiate light within the muscle tissue of the fins, mantle, head, and arms. Numerous (a single D. gigas may have hundreds) small subcutaneous (s.c.) photophores that consist of relatively rudimentary aggregations of photogenic tissue are embedded throughout the muscle tissue (Fig. 3 A–C) and cause the entire animal to glow (45–49). Pigmentation patterns are generated by the overlying chromatophore layer, which is cutaneous. Therefore, we postulate that D. gigas displays pigmentation patterns against a glowing backdrop created by bioluminescence, which would likely enhance pattern visibility under low-light conditions. The shallow-living Hawaiian bobtail squid, Euprymna scolopes, uses a different mode of bioluminescence to glow and does not combine this with complex chromatophore patterning (50).
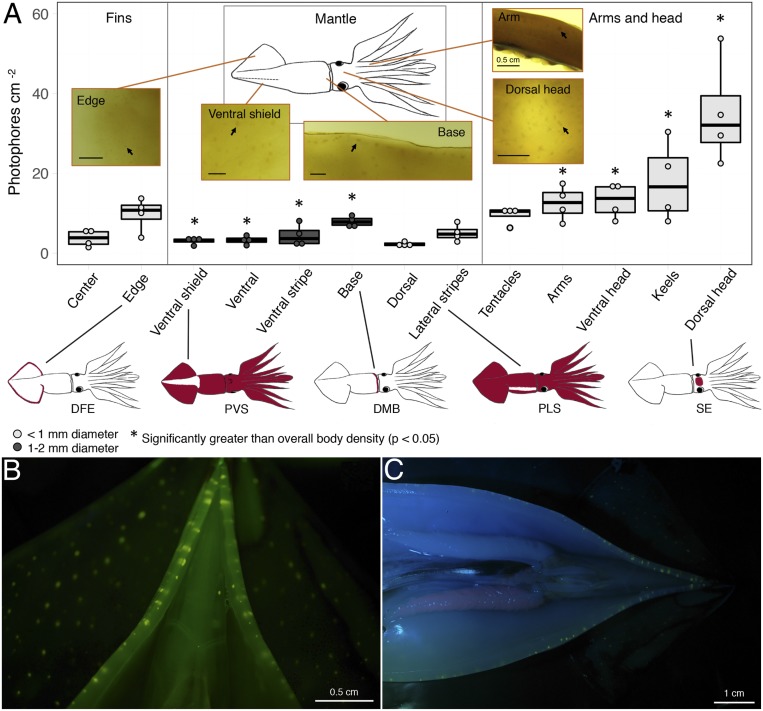
Fig. 3.
Proposed mechanism by which pigmentation patterns can function as visual signals under low-light conditions. (A) Spatial patterns of s.c. photophore density and size with respect to pigmentation-changing regions in D. gigas. A, Insets are images of photophores illuminated from below using white light. Arrows point to individual photophores. (Scale bars, 0.5 cm.) In the plot, light gray and dark gray boxes and points indicate regions dominated by small or large photophores, respectively. Dark horizontal lines show the mean value, with boxes and vertical lines, respectively, representing the two inner and outer quartiles of the data (shown as points) (n = 4). Examples of pigmentation patterns corresponding to regions with higher than average photophore density are included beneath the plot. (B and C) Fluorescence of s.c. photophores in the fins and mantle tip (B) and along the mantle (C) (photos: Steven Haddock, MBARI). Photophores were illuminated by blue (465 nm) light with a yellow long-pass filter used on the camera lens. See Table 1 for component abbreviations.
Photophore arrangement can contain information in marine taxa, including squid such as Watasenia scintillans (firefly squid), which have sex-specific cutaneous photophore distribution patterns (51). s.c. photophores in D. gigas are found in the muscle tissue throughout the body, but concentrations in their distribution align with ecologically important chromatic components. By examining captured specimens of D. gigas (n = 4; Materials and Methods), we found that regions of higher than average s.c. photophore density aligned with the chromatic components identified as important during prey capture, or when in the presence of many conspecifics—especially the more subtle “modifiers” and “positionals,” including DFE (P = 0.12), PVS (P = 0.01), DMB (P < 0.001), PLS (P = 0.8), DAS (P = 0.022), and SE (P < 0.001) (linear mixed-effects analyses; Fig. 3A and SI Appendix, Table S3). Squid may therefore glow more brightly in these regions of their body, thereby enhancing contrast and, thus, the visual perception of subtle chromatic displays.
Bioluminescent backlighting likely enhances the visibility of the pigmentation patterns of D. gigas under the limited light regime of the deep ocean. However, limitations of video cameras used in our study meant that we were unable to document the proposed signaling mechanism in the wild. Therefore, it is possible that photophores in D. gigas serve additional functions unrelated to visual signaling—which would indicate that pigmentation patterns are perceived by using remarkable low-light vision. The precise visual capabilities of D. gigas are unknown, but measurements of eye diameter (52) and visual acuity (53) suggest a potential ability to resolve objects in dim-light conditions at distances relevant to grouping (1 m between adjacent individuals on average; ref. 8). Recent camera developments have allowed researchers to film in situ bioluminescent behavior of deep-sea species using ROVs (54), and application of these techniques may further reveal how D. gigas uses s.c. bioluminescence at depth.
The Evolution of Visual Signal Complexity in Deep-Sea Squids.
Our data indicate that the chromatic behaviors displayed by a deep-living social squid could share design features with advanced forms of animal communication (2), as specific components seem to be tied to specific contexts (semanticity) and may be combinable in distinct ways (discreteness). Both features are indicative of signal complexity—the latter suggesting that multiple components may interact with one another to alter the message they convey, but not precluding the possibility that some components could elicit behavioral responses on their own (55). The limiting factor in addressing this uncertainty is adequately quantifying the behavioral responses of signal recipients in D. gigas. Pelagic and deep-living squids like D. gigas and other species in the family Ommastrephidae are notoriously difficult to maintain in captivity for experimental procedures, even for short durations.
Content-based selective pressures (1), or those that maximize information content, seem relevant as potential drivers for diverse pigmentation patterning in D. gigas. The ability for individuals to rapidly signal foraging intent and competitive quality (the quality plus hypothesis; ref. 55) could help avoid antagonistic interactions in dense foraging aggregations. Almost all chromatic components displayed by D. gigas have bilateral symmetry, suggesting that they may additionally be shaped by selection favoring receiver variability (55), or the need to convey information to multiple recipients—in this case, multiple conspecifics simultaneously in a three-dimensional habitat.
It is also likely that efficacy-based selective pressures (1), or those that facilitate information transfer, play a role in shaping the visual displays of D. gigas. Environmental variability—particularly ambient light conditions—would presumably be a key driver for the evolution of background bioluminescence. The efficacy trade-off hypothesis suggests that, even within a sensory modality, there is no single signaling component that is most effective for all communication scenarios (55). The Humboldt squid primarily inhabits low-light conditions under which s.c. background bioluminescence would presumably enhance the visibility of pigmentation patterns. But during brief daytime excursions into the epipelagic, where light levels are higher (20, 21), and under shallow nighttime conditions (21, 24) with reliably persistent ambient bioluminescence (40, 56), pigmentation patterns could be visible without background bioluminescence.
Humboldt squid constitute a monotypic genus in the family Ommastrephidae, where three subfamilies are currently recognized: Ommastrephinae, Illicinae, and Todarodinae (7, 57). Although thorough behavioral investigations have only been conducted on one species each in the Ommastrephinae (D. gigas; refs. 16, 20, and 26) and Illicinae (Illex illecebrosus; ref. 58), it is likely that most, if not all, members of the three subfamilies display diverse pigmentation patterns because they are highly social (7). However, only the Ommastrephinae possess the numerous s.c. photophores indicative of background bioluminescence (ref. 7 and SI Appendix, Fig. S3). Indeed, some members of this group have appropriately glowing common names—e.g., luminous flying squid, Eucleoteuthis luminosa, and neon flying squid, Ommastrephes bartramii. The proposed signaling mechanism may be common in the Ommastrephinae.
The most obvious ecological feature distinguishing the Ommastrephinae from the rest of the family Ommastrephidae is that they are primarily oceanic, whereas the Illicinae and Todarodinae, and also the ecologically similar but unrelated family Loliginidae, primarily inhabit productive current systems in slope and shelf habitats (ref. 7 and SI Appendix, Fig. S3). All three of the latter clades lack bioluminescent capability (ref. 7 and SI Appendix, Fig. S3). Perhaps aspects of slope and shelf systems, such as shallower waters or plentiful ambient bioluminescence, in conjunction with the visual ecology of resident squids do not necessitate backlighting for adequate perception of pigmentation patterns. On the other hand, background bioluminescence may give members of the Ommastrephinae a competitive advantage in oceanic habitats, and thereby restrict other ecologically similar clades of social squids to nearshore ecosystems.
Conclusions
The Ommastrephinae are the most abundant and ecologically active group of squids on the planet, where they primarily inhabit light-restricted environments. We have shown that the manner by which D. gigas, a species in this subfamily, displays pigmentation patterns in its deep-sea habitat is indicative of semanticity and discreteness. Such complexity is analogous to forms of advanced animal communication known from well-lit habitats and challenges assumptions of limited information content in the visual behaviors of deep-living animals. We hypothesize that the bioluminescence created using s.c. photophores backlights the pigmentation patterns and thereby enhances the visual perception of these signals under low-light conditions. The evolution of complex visual signals in these squids may therefore have been driven by content- and efficacy-based selection. Improved low-light filming capability from ROVs should further reveal how D. gigas and other members of the Ommastrephinae use s.c. bioluminescence at depth.
Visual signaling is probably crucial in allowing species in the Ommastrephinae to coordinate complex schooling behaviors, facilitate collective decisions, and maintain group cohesion during movement behaviors in the deep ocean. These activities are not only critical to their life history, but are likely to be involved in obtaining and distributing nutritional resources throughout the world’s oceans. Further investigation of cephalopod signals in the deep ocean will be important in understanding how, and how much, information can be shared in one of the planet’s most challenging environments for visual communication.
Materials and Methods
ROV Specifications and Observations.
The Monterey Bay Aquarium Research Institute (MBARI) electrohydraulic ROVs Ventana and Doc Ricketts and electric ROV Tiburon (https://www.mbari.org/at-sea/vehicles/) recorded footage of D. gigas from various deep pelagic and demersal habitats within Astoria Submarine Canyon, OR, and Monterey Submarine Canyon, CA, from 2005 to 2012. All vehicles were outfitted with Ikegami HDL45 HD cameras, white halogen lights, and conductivity, temperature, depth, dissolved oxygen, and navigation sensors. Footage was logged directly onto 1080i D5 HD videotape, and tapes were annotated for organisms by MBARI Video Lab scientists and synchronized with hydrographic parameters in MBARI’s Video Annotation Reference System (VARS) (59). Thirty dive tapes containing footage of D. gigas from 2005 to 2012 were selected for observation based on annotated foraging activity, population density, and comments from MBARI Video Lab scientists indicating high quality of footage. On each tape, we observed one squid: the first in a specified population that stayed in frame for at least 14 s at a distance permitting accurate behavioral observations. We hypothesized that squid in larger groups would have more opportunities to interact with conspecifics and, therefore, may display intraspecific communication behaviors more often than squid in smaller groups. We observed 10 squid from each of the following conspecific abundance levels: many (≥30 total other conspecifics), some (10 to 29 conspecifics), and few (<10 conspecifics). We calculated the total number of surrounding conspecifics by counting all of the D. gigas in the 1 min of footage prior to and following the 14-s focal observation and adding these totals to the number of surrounding conspecifics observed during the focal observation. Because squid entered and exited the field of view, it is possible that we slightly overestimated surrounding conspecific abundance (18). At a focal distance of 1.5 m, cameras recorded an area of 4.01 m2; in some cases, during observations, the telephoto lens was used to magnify the focal animal, thus temporarily reducing the field of view. Surrounding total conspecific abundance ranged from 30 to 245 (average ± SE; 88 ± 19) in the many group, 11 to 29 (18 ± 2) in the some group, and 0 to 8 (3 ± 1) in the few group. We also recorded the foraging status of each individual. A squid was determined to be foraging if it attempted to capture prey via arm strike, tentacle strike, or both (Fig. 2 A and B). Many squid are opportunist predators (7), and, therefore, it is likely that some of the D. gigas we defined as not foraging were searching for prey. Five foraging and five nonforaging individuals were selected for observation in each of the three conspecific abundance categories—thus, 15 foraging individuals and 15 nonforaging individuals total that were evenly distributed across conspecific abundance levels. If present, conspecifics were generally observed in variable locations with respect to the focal squid, and all animals were in constant motion. Thus, it was difficult to determine the extent to which chromatic behaviors may have been directed at particular conspecifics, but, based on the bilateral symmetry of most behaviors (SI Appendix, Fig. S1A), it is likely that they were visible at many conspecific locations. Depth, temperature, oxygen, and salinity for each observed D. gigas were recorded at the middle of the 14-s observation (i.e., the 7.5-s mark) from the VARS database. Squid were observed from normal depth, temperature, oxygen, and salinity distributions, which, respectively, ranged from 265.97 to 848.39 m, 4.39 to 10.04 °C, 0.14 to 3.59 mlO2 per liter, and 33.99 to 34.69 practical salinity units (Shapiro–Wilk normality tests, alpha = 0.05). Focal squid and surrounding squid appeared to be of similar size. However, the size, sex, and maturity of observed squid could not be directly quantified. Collections of D. gigas made at the surface at night after ROV dives from 2013 to 2014, and also in a study examining D. gigas in the California Current from 2007 to 2010 (60), suggest that observed squid comprised both sexes, were generally in the medium to large size range (50- to 70-cm dorsal mantle length), and were maturing or mature.
Behavioral Quantification and Analysis.
Each D. gigas was observed for the first 14 contiguous s that its entire body was in frame. This duration was feasibly short enough for effective data collection, yet long enough to see repetition of behavioral components. As described by ref. 61, behavioral components are defined as the individual postural, chromatic, and locomotor behaviors that compose the overall physical appearance of a squid (or body configuration) at any moment in time. During all playback observations, we paused the footage once every second and recorded all postural, chromatic, and locomotor components as present or absent (62). Depending on a squid’s orientation relative to the camera, all body surfaces may not have been visible, and, thus, some chromatic behaviors could have been missed. Where appropriate, we used the same names and descriptions for components as refs. 16 and 26 (Fig. 1, Table 1, and SI Appendix, Fig. S1 and Table S1). The footage was also replayed to look for variable chromatic displays, flickering and flashing (20), and we noted at which times (out of 14-s total observation) these dynamic chromatic displays were present. All squid were observed in detail by the same viewer on at least three separate occasions, where all 14 s of behavioral footage were examined numerous times at multiple playback speeds.
All data analyses were made in R (Version 3.5.2) (63). Unless otherwise noted, all tests were two-tailed with alpha set to 0.05. In order to relate specific behaviors to foraging and conspecific abundance, we calculated the total duration of all postural, chromatic, and locomotor components for each of the 30, 14-s focal observations. Total duration was defined as the total time (in seconds) a component or pattern was displayed during the 14 s of observation. The two postural components used to define foraging squid (arm strike and tentacle strike) were excluded from analyses. Components that were displayed by fewer than four squid were also excluded from analyses. We pooled data by component type and analyzed postural, chromatic, and locomotor components separately. Component datasets were log-transformed in order to place all behavioral data on a similar scale, thus enhancing the signal of nondominant behaviors. Each component dataset was converted into a resemblance matrix (based on Euclidean distance), and then we performed PCAs on the resemblance matrices (63). PCA plots represent each squid as a single point, with proximity indicating behavioral similarity (Figs. 1 A and C, 2 C and D, and SI Appendix, Fig. S2C). For simplicity, behavioral comparisons of conspecific abundance categories in these analyses were made between squid in the presence of few conspecifics (n = 10) and squid in the presence of some or many conspecifics (n = 20) (Fig. 1B and SI Appendix, Fig. S2C). PCA plots were overlaid with behavioral-component vectors, and foraging status or conspecific abundance clusters were encircled by 50% probability ellipses. This facilitated the identification of covariation between squid behavior and ecological categories, illuminated the specific components driving behavioral trends, and linked covariation to the percent variance explained by the relevant principal component (SI Appendix, Table S1). Foraging status aligned with principal component one (PC1) for all component types (Figs. 1A and 2 C and D), and conspecific abundance aligned with principal component two (PC2) only for chromatic components (Fig. 1B and SI Appendix, Fig. S2C). Behaviors represented by PC1 eigenvalues greater than 0.11 or less than −0.11 were determined to contribute most to behavioral trends associated with foraging status. The same eigenvalue threshold for PC2 determined which chromatic components contributed most to behavioral trends associated with conspecific abundance (SI Appendix, Table S1). To determine how variable chromatic displays were associated with foraging or group behaviors, we calculated the mean and SD of the log-transformed total duration of flashing or flickering within foraging status and conspecific abundance categories; ANOVA models with Tukey’s post hoc HSD tests were used to compare averages between factors within ecological categories (SI Appendix, Fig. S2B and Table S2).
To determine how squid combined components to create body configurations, we calculated pairwise component associations using a probabilistic co-occurrence model (64) applied to a presence/absence data matrix of all behavioral components displayed by the 30 squid (SI Appendix, Fig. S2A). The model calculated the expected co-occurrence frequency of components, assuming independent distribution across squid, and then determined the probability that the observed co-occurrence frequency was greater than expected (positive association; 7.6% of component pairs) or less than expected (negative association; 1.6% of component pairs). Randomly associated components (91% of pairs) were determined as those that did not deviate from expected co-occurrences by more than 10% of the total number of squid. Of the 780 component-pair combinations, 31 pairs (4%) were removed from the analysis because the expected co-occurrence was <1.
The postures and locomotion displayed by foraging squid were consistent (Fig. 2 C and D), owing to the stereotyped manner with which they pursued, captured, and processed prey items (Fig. 2E). To elucidate how chromatic components were arranged with respect to a fixed event, we examined patterns in the timing of chromatic component display surrounding foraging attempts. We calculated the frequency of all chromatic components every second from 4 s before to 4 s after foraging attempts by dividing the number of foraging squid which displayed a component at a given time point by the total number of foraging attempts among all of the squid (n = 15) (Fig. 2E). To investigate how behavioral components interact with one another in an ecological context, we explored their arrangement during foraging by constructing adjacency network heatmaps* for each component type. The prolonged display of some components masked nondominant behavioral adjacencies that were potentially informative (SI Appendix, Fig. S2D). To visualize these interactions, component adjacencies were portrayed with loops (i.e., prolonged component display) removed (Fig. 2 F–H) and retained (SI Appendix, Fig. S2D).
Photophore Quantification and Analysis.
Two male and two female small (20 to 40 cm dorsal mantle length), mature D. gigas were arbitrarily (i.e., no particular criteria) selected from a collection made in the Gulf of California, Mexico. Squid were frozen after capture and then thawed prior to photophore quantification. This enhanced the visibility of s.c. photophores in D. gigas by making them more opaque (48). For each animal, the mantle was cut open along the ventral surface, from the anterior margin to the posterior tip, and the head was removed and cut in half along the lateral axis, from the mantle margin through the arm crown pillar. All organs and skin were removed, as well as the eyes, beak, and gladius. We divided the remaining muscular body tissue into four pieces: the port fin; mantle and starboard fin; dorsal head with arm pairs I to III; and ventral head with arm pair IV and the tentacles. These divisions were chosen in order to get the thinnest, and thus most translucent, body sections possible. Each piece was laid flat on a 115-V Portable Light Table (Gagne Associates Inc.), illuminated from below, and photographed alongside a scale bar by using an Olympus E-300 DC 9-V digital camera outfitted with a 45-mm lens (Olympus America Inc.) suspended overhead. Ommastrephids can have parasitic cysts in their muscle tissue, and if they were present in the analyzed specimens, it was impossible to distinguish them from photophores by using this method. From high-resolution images, s.c. photophores embedded in the muscle tissue were categorized as being large (1 to 2 mm diameter) or small (≤1 mm diameter) and quantified within 13 different body regions pertaining to specific chromatic components (e.g., fin edge, mantle base, dorsal head, etc.) (Fig. 3A). The average density of small and large photophores (±1 SD) was 4.17 (±0.81) and 1.44 (±0.30) photophores per cm2, respectively. To examine how s.c. photophore density was related to different pigmentation-changing regions of the four D. gigas, which were both nonindependent measures, we performed two linear mixed-effects analyses (65) (one for each photophore type) with photophore density as the dependent variable and the different body regions pertaining to specific chromatic components as independent variables. In each analysis, pigmentation-changing regions were the fixed effects, and to account for variation among the four squid, by-squid intercepts for the influence of pigmentation-changing region on photophore density were random effects. We plotted photophore density at each pigmentation-changing region and used the slope as determined by linear mixed-effects analyses to compare the photophore density of each region to the overall body density of the specified photophore type (Fig. 3A and SI Appendix, Table S3). All methods were carried out in accordance with relevant guidelines and regulations. All data are contained in the main text and SI Appendix.
Supplementary Material
Acknowledgments
This research would not be possible without the support and collaboration of the pilots of the MBARI ROVs Ventana, Tiburon, and Doc Ricketts, as well as the captains and ship’s crews of the research vessels Rachel Carson, Point Lobos, and Western Flyer. We thank R. Sherlock and K. Reisenbichler for assistance on land and at sea; MBARI’s Video Lab for support with data collection; M. Denny, G. Somero, and W. Gilly for providing comments that improved the manuscript; W. Gilly, C. Lowe, and J. Goldbogen for conversations that benefited this work; P. Daniel and E. Portner for assistance with specimen collection; O. Drake for assistance with specimen preparation; S. Haddock for providing images in Fig. 3 B and C; and G. Sanchez for sharing the Decabranchia phylogeny in SI Appendix, Fig. S3. B.P.B. acknowledges support from National Science Foundation Grant IOS-1557754 (to W. Gilly). The David and Lucile Packard Foundation and the Department of Biology of Stanford University supported this research.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
*K. Ognyanova, “Network visualization with R” in POLNET Workshop (2016).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920875117/-/DCSupplemental.
References
- 1.Guilford T., Dawkins M. S., Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14 (1991). [Google Scholar]
- 2.Bradbury J. W., Vehrencamp S. L., Principles of Animal Communication (Sinauer Associates Inc., Sunderland, MA, 1998). [Google Scholar]
- 3.Herring P., The Biology of the Deep Ocean (Biology of Habitats Series, Oxford University Press, Oxford, UK, 2002). [Google Scholar]
- 4.Boyle P., Rodhouse P., Cephalopods: Ecology and Fisheries (Blackwell, Oxford, UK, 2005). [Google Scholar]
- 5.Hoving H. J. T., et al. , The study of deep-sea cephalopods. Adv. Mar. Biol. 67, 235–359 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Hanlon R. T., Messenger J. B., Cephalopod Behavior (Cambridge University Press, Cambridge, UK, 2018). [Google Scholar]
- 7.Jereb P., Roper C. F. E., Vecchione M., “Cephalopods of the World: An Annotated and Illustrated Catalogue of Species Known to Date, Volume 2, Myopsid and Oegopsid Squids” (FAO Species Catalogue for Fishery Purposes, Rome, Italy, 2010). [Google Scholar]
- 8.Benoit-Bird K. J., Gilly W. F., Coordinated nocturnal behavior of foraging jumbo squid Dosidicus gigas. Mar. Ecol. Prog. Ser. 455, 211–228 (2012). [Google Scholar]
- 9.Ibánez C. M., Keyl F., Cannibalism in cephalopods. Rev. Fish Biol. Fish. 20, 123–136 (2010). [Google Scholar]
- 10.Hoving H. J. T., Robison B. H., Deep-sea in situ observations of gonatid squid and their prey reveal high occurrence of cannibalism. Deep Sea Res. Part I Oceanogr. Res. Pap. 116, 94–98 (2016). [Google Scholar]
- 11.Moynihan M., Rodaniche A. F., The behavior and natural history of the Caribbean Reef Squid Sepioteuthis sepioidea. Adv. Ethol. 25, 1–152 (1982). [Google Scholar]
- 12.Lin C. Y., Tsai Y. C., Chiao C. C., Quantitative analysis of dynamic body patterning reveals the grammar of visual signals during the reproductive behavior of the oval squid Sepioteuthis lessoniana. Front. Ecol. Evol. 5, 30 (2017). [Google Scholar]
- 13.Laidre M. E., Johnstone R. A., Animal signals. Curr. Biol. 23, R829–R833 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Bush S. L., Robison B. H., Caldwell R. L., Behaving in the dark: Locomotor, chromatic, postural, and bioluminescent behaviors of the deep-sea squid Octopoteuthis deletron Young 1972. Biol. Bull. 216, 7–22 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Burford B. P., Robison B. H., Sherlock R. E., Behaviour and mimicry in the juvenile and subadult life stages of the mesopelagic squid Chiroteuthis calyx. J. Mar. Biol. Assoc. U. K. 95, 1221–1235 (2015). [Google Scholar]
- 16.Trueblood L. A., Zylinski S., Robison B. H., Seibel B. A., An ethogram of the Humboldt squid Dosidicus gigas Orbigny (1835) as observed from remotely operated vehicles. Behaviour 152, 1911–1932 (2015). [Google Scholar]
- 17.Zeidberg L. D., Robison B. H., Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl. Acad. Sci. U.S.A. 104, 12948–12950 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart J. S., et al. , Combined climate- and prey-mediated range expansion of Humboldt squid (Dosidicus gigas), a large marine predator in the California Current System. Glob. Change Biol. 20, 1832–1843 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Pitcher T. J., Heuristic definitions of fish shoaling behavior. Anim. Behav. 31, 611–612 (1983). [Google Scholar]
- 20.Rosen H., Gilly W., Bell L., Abernathy K., Marshall G., Chromogenic behaviors of the Humboldt squid (Dosidicus gigas) studied in situ with an animal-borne video package. J. Exp. Biol. 218, 265–275 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Gilly W. F., et al. , Vertical and horizontal migrations by the jumbo squid Dosidicus gigas revealed by electronic tagging. Mar. Ecol. Prog. Ser. 324, 1–17 (2006). [Google Scholar]
- 22.Bazzino G., Gilly W. F., Markaida U., Salinas-Zavala C. A., Ramos-Castillejos J., Horizontal movements, vertical-habitat utilization and diet of the jumbo squid (Dosidicus gigas) in the Pacific Ocean off Baja California Sur, Mexico. Prog. Oceanogr. 86, 59–71 (2010). [Google Scholar]
- 23.Gilly W. F., et al. , Locomotion and behavior of Humboldt squid, Dosidicus gigas, in relation to natural hypoxia in the Gulf of California, Mexico. J. Exp. Biol. 215, 3175–3190 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Stewart J. S., Hazen E. L., Foley D. G., Bograd S. J., Gilly W. F., Marine predator migration during range expansion: Humboldt squid Dosidicus gigas in the northern California Current System. Mar. Ecol. Prog. Ser. 471, 135–150 (2012). [Google Scholar]
- 25.Pinhassi J., DeLong E. F., Béjà O., González J. M., Pedrós-Alió C., Marine bacterial and archaeal ion-pumping rhodopsins: Genetic diversity, physiology, and ecology. Microbiol. Mol. Biol. Rev. 80, 929–954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell L. E., “Behavioral ecology of the Humboldt squid, Dosidicus gigas: Insights from an animal-borne video and data logging system,” Undergraduate thesis, Stanford University, Stanford, CA (2011).
- 27.Vail A. L., Manica A., Bshary R., Referential gestures in fish collaborative hunting. Nat. Commun. 4, 1765 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Cheroske A. G., Cronin T. W., Durham M. F., Caldwell R. L., Adaptive signaling behavior in stomatopods under varying light conditions. Mar. Freshwat. Behav. Physiol. 42, 219–232 (2009). [Google Scholar]
- 29.Brown C., Garwood M. P., Williamson J. E., It pays to cheat: Tactical deception in a cephalopod social signalling system. Biol. Lett. 8, 729–732 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domenici P., et al. , How sailfish use their bills to capture schooling prey. Proc. Biol. Sci. 281, 20140444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams E. S., Caldwell R. L., Deceptive communication in asymmetric fights of the stomatopod crustacean Gonodactylus bredini. Anim. Behav. 39, 706–716 (1990). [Google Scholar]
- 32.Herberholz J., McCurdy C., Edwards D. H., Direct benefits of social dominance in juvenile crayfish. Biol. Bull. 213, 21–27 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Bickerton D., Language and Species (Chicago University Press, Chicago, IL, 1990). [Google Scholar]
- 34.Pearce J. M., Animal Learning and Cognition (Psychology Press, Hove, UK, 2008). [Google Scholar]
- 35.Barbato M., Bernard M., Borrelli L., Fiorito G., Body patterns in cephalopods: “Polyphenism” as a way of information exchange. Pattern Recognit. Lett. 28, 1854–1864 (2007). [Google Scholar]
- 36.Candolin U., The use of multiple cues in mate choice. Biol. Rev. Camb. Philos. Soc. 78, 575–595 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Mauris E., Colour patterns and body postures related to prey capture in Sepiola affinis (Mollusca: Cephalopoda). Mar. Freshwat. Behav. Physiol. 14, 189–200 (1989). [Google Scholar]
- 38.Adamo S. A., Ehgoetz K., Sangster C., Whitehorne I., Signaling to the enemy? Body pattern expression and its response to external cues during hunting in the cuttlefish Sepia officinalis (Cephalopoda). Biol. Bull. 210, 192–200 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Martini S., Haddock S. H. D., Quantification of bioluminescence from the surface to the deep sea demonstrates its predominance as an ecological trait. Sci. Rep. 7, 45750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haddock S. H. D., Moline M. A., Case J. F., Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2, 443–493 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Pietsch T. W., Oceanic Anglerfishes: Extraordinary Diversity in the Deep Sea (University of California Press, Berkeley, CA, 2009). [Google Scholar]
- 42.Morin J. G., et al. , Light for all reasons: Versatility in the behavioral repertoire of the flashlight fish. Science 190, 74–76 (1975). [Google Scholar]
- 43.Woodland D. J., Cabanban A. S., Taylor V. M., Taylor R. J., A synchronized rhythmic flashing light display by schooling Leiognathus splendens (Leiognathidae: Perciformes). Mar. Freshw. Res. 53, 159–162 (2002). [Google Scholar]
- 44.Kubodera T., Koyama Y., Mori K., Observations of wild hunting behaviour and bioluminescence of a large deep-sea, eight-armed squid, Taningia danae. Proc. Biol. Sci. 274, 1029–1034 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García‐Tello P., Nota preliminar sobre una observación de bioluminiscencia en Dosidicus gigas (D’Orb) Cephalopoda. Bol. Univ. Chile 46, 27–28 (1964). [Google Scholar]
- 46.Wormuth J. H., The Biogeography and Numerical Taxonomy of the Oegopsid Squid Family Ommastrephidae in the Pacific Ocean (University of California Press, Berkeley, CA, 1976). [Google Scholar]
- 47.Nesis K. N., The biology of the giant squid of Peru and Chile, Dosidicus gigas. Oceanology (Mosc.) 10, 108–118 (1970). [Google Scholar]
- 48.Lohrmann K. B., Subcutaneous photophores in the jumbo squid Dosidicus gigas (d’Orbigny, 1835) (Cephalopoda: Ommastrephidae). Rev. Biol. Mar. Oceanogr. 43, 275–284 (2008). [Google Scholar]
- 49.Galeazzo G. A., et al. , Characterizing the bioluminescence of the Humboldt squid, Dosidicus gigas (d’Orbigny, 1835): One of the largest luminescent animals in the world. Photochem. Photobiol. 95, 1179–1185 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Ruby E. G., McFall-Ngai M. J., A squid that glows in the night: Development of an animal-bacterial mutualism. J. Bacteriol. 174, 4865–4870 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herring P. J., Sex with the lights on? A review of bioluminescent sexual dimorphism in the sea. J. Mar. Biol. Assoc. U. K. 87, 829–842 (2007). [Google Scholar]
- 52.Schmitz L., et al. , Allometry indicates giant eyes of giant squid are not exceptional. BMC Evol. Biol. 13, 45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeney A. M., Haddock S. H. D., Johnsen S., Comparative visual acuity of coleoid cephalopods. Integr. Comp. Biol. 47, 808–814 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Maxmen A., The hidden lives of deep-sea creatures caught on camera. Nature 561, 296–297 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Hebets E. A., Papaj D. R., Complex signal function: Developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214 (2005). [Google Scholar]
- 56.Messié M., Shulman I., Martini S., Haddock S. H. D., Using fluorescence and bioluminescence sensors to characterize auto- and heterotrophic plankton communities. Prog. Oceanogr. 171, 76–92 (2019). [Google Scholar]
- 57.Sanchez G., et al. , Genus-level phylogeny of cephalopods using molecular markers: Current status and problematic areas. PeerJ 6, e4331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrop J., Vecchione M., Felley J. D., In situ observations on behaviour of the ommastrephid squid genus Illex (Cephalopoda: Ommastrephidae) in the northwestern Atlantic. J. Nat. Hist. 48, 2501–2516 (2014). [Google Scholar]
- 59.Schlinning B. M., Stout N. J., MBARI’s video annotation and reference system. Oceans 2006, 1–5 (2006). [Google Scholar]
- 60.Field J. C., et al. , Foraging ecology and movement patterns of jumbo squid (Dosidicus gigas) in the California Current System. Deep Sea Res. Part II Top. Stud. Oceanogr. 95, 37–51 (2013). [Google Scholar]
- 61.Hanlon R. T., Smale M. J., Sauer W. H. H., An ethogram of body patterning behavior in the squid Loligo vulgaris reynaudii on spawning grounds in South Africa. Biol. Bull. 187, 363–372 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Jantzen T. M., Havenhand J. N., Reproductive behavior in the squid Sepioteuthis australis from South Australia: Interactions on the spawning grounds. Biol. Bull. 204, 305–317 (2003). [DOI] [PubMed] [Google Scholar]
- 63.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria 2018). https://www.R-project.org. Accessed 20 December 2018.
- 64.Griffith D. M., Veech J. A., Marsh C. J., Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 69, 1–17 (2016). [Google Scholar]
- 65.Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team, nlme: Linear and Nonlinear Mixed Effects Models (R package version 3.1–137, 2018). https://cran.r-project.org/web/packages/nlme/index.html. Accessed 7 April 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.