Abstract
A 4‐month‐old male British Blue cat with catarrhal to haemorrhagic enteritis showed massive colonization of the stomach, small intestine and caecum with spiral‐shaped bacilli. In the stomach, organisms were located in foveolae and gland lumina and within unaltered and degenerate epithelial cells. Inflammatory infiltration was moderate and T cell dominated. In the intestine, bacilli were found in the gut lumen, between villi, in crypt lumina and within epithelial cells. Degeneration of crypt epithelial cells as well as crypt dilation and moderate to massive macrophage‐dominated infiltration of the mucosa and submucosa were observed. Immunohistochemically, bacilli were positive with an antibody against Helicobacter. Ultrastructurally, the organisms strongly resembled ‘Flexispira rappini’, a spiral‐shaped Helicobacter species known as a normal intestinal colonizer in dogs and mice.
Introduction
Helicobacter species and Helicobacter‐like organisms are frequently demonstrated in the feline gastric mucosa. Correlation with a variably intense inflammatory reaction is stated in some cases (Otto et al., 1994; Handt et al., 1995; Hermanns et al., 1995; Fox et al., 1996; Happonen et al., 1996; Neiger et al., 1998; Norris et al., 1999; Simpson et al., 2000). Intestinal Helicobacter infections in cats have been studied very little, presumably due to difficulties in identifying these organisms. There are only two reports on intestinal Helicobacter infections with clinical symptoms in cats (Foley et al., 1998, 1999). In dogs, mice and hamsters, however, these organisms are regarded as normal intestinal colonizers (Lockard and Boler, 1970; Gebhart et al., 1989; Schauer et al., 1993; Stanley et al., 1993; Fox et al., 1995).
This report describes a case of fatal enteritis in a cat, associated with gastrointestinal infection with spiral‐shaped bacilli. Immunohistochemically, the organisms stained positive with a polyclonal antiserum against H. pylori. Ultrastructurally, they closely resembled ‘Flexispira rappini’ (Lockard and Boler, 1970; Schauer et al., 1993). The findings indicate that spiral‐shaped organisms like ‘F. rappini’ have to be regarded as potential gastrointestinal pathogens in the cat.
Materials and Methods
Animal and tissue processing
A 4‐month‐old male British Blue cat had been imported from the Czech Republic to Germany 2 weeks before death. According to the owner’s knowledge, the animal had been vaccinated once against feline parvovirus, herpesvirus and calicivirus.
The cat was routinely necropsied. Samples from all parenchymatous organs, stomach, duodenum, jejunum, ileum and caecum, as well as mesenteric and caecal lymph nodes and thymus were fixed in 10% non‐buffered formalin for 18 h and subsequently embedded in paraffin wax. Sections (5 μm) were stained with haematoxylin and eosin, Warthin–Starry stain, Gram stain (modified by Weigert), and the periodic acid Schiff (PAS) reaction.
Immunohistochemical examination
Immunohistochemistry was performed for the demonstration of feline leukaemia virus (FeLV) antigens, parvovirus antigens, and coronavirus antigens as described (Kovacevic et al., 1997; Kipar et al., 1998a) and for Helicobacter species (rabbit anti‐H. pylori; Andersen et al., 1988). Inflammatory cells were characterized by cross‐reacting antibodies against the CD3 antigen of T cells, the CD45R antigen of B cells (B220; clone Lu5), and the myeloid/histiocyte antigen of monocytes/macrophages and neutrophils (clone MAC387) as described previously (Kipar et al., 1998b).
Electron microscopy
For transmission electron microscopy, formalin‐fixed samples of the small intestine were embedded in epoxy wax, and ultrathin sections were prepared and stained with uranyl acetate and lead citrate.
Preparation and amplification of bacterial ribosomal RNA genes from tissue samples
After removal of paraplast® embedding medium with xylene, the preparation of bacterial DNA was performed in duplicate using the QIAamp Tissue Kit according to the manufacturer’s recommendations (Qiagen, Hilden, Germany). A 5‐μl aliquot of each preparation was used for the polymerase chain reaction (PCR). Amplifications were performed in a total volume of 50 μl, containing 200 μM deoxynucleoside triphosphates (dATP, dCTP, dGRP, and dTTP), 10 pmol of each primer, 5 μl of 10‐fold concentrated polymerase synthesis buffer, and 1 U of AmpliTaq Gold® DNA polymerase (PE Biosystems, Weiterstadt, Germany). Partial‐length small subunit ribosomal RNA genes (16S) were amplified using the PCR technique with the primers fD1 and 342r (Lane, 1991; Weisburg et al., 1991). These primers can be used to amplify 16S rDNA of a phylogenetically and taxonomically wide range of bacteria (Lane, 1991; Weisburg et al., 1991). After 40 cycles, the amplification products were subjected to gel electrophoresis on 1% agarose gels, and visualized by staining with ethidium bromide. The presence of polymerase inhibitory substances was determined by spiking the material with positive controls. Negative controls for DNA extraction and for the PCR reagents were run in parallel.
Results
Clinical findings, serology and gross morphology
According to the owner where the cat lived for 2 weeks, the cat had repeatedly shown soft faeces and blood in the stools. It was then found dead without having developed any specific symptoms.
Post‐mortem, the animal serologically tested negative for FeLV p27 antigen and antibodies against feline immunodeficiency virus (FIV).
Macroscopically, haemorrhagic to catarrhal enteritis and massive enlargement of mesenteric and caecal lymph nodes were stated.
Histopathological, immunohistochemical and electron microscopic findings
Lesions were restricted to the gastrointestinal tract and lymphoid tissues. In the glandular region of the stomach, moderate T cell‐dominated mononuclear infiltration was observed. It was represented by disseminated lymphocytes mainly located in the upper third of the mucosa, and by focal T cell accumulations between or directly below gastric glands. Within gastric pits and gland lumina as well as within unaltered and degenerating epithelial cells, numerous bacilli were found, either solitary or in bundles (Fig. 1a,b). Organisms exhibited an undulatory or – when moved slightly out of focus – spiral shape (Fig. 1a,b).
Figure 1.

Stomach. (a) Numerous bacilli with undulating/spiral shape are found in gastric glands. (b) Bacilli are found within the cytoplasm of intact and degenerate (arrow) epithelial cells. Peroxidase anti‐peroxidase (PAP) method, Papanicolaou’s counterstain. × 330.
The small intestine and caecum showed degenerated crypt epithelial cells, crypt dilation and loss of crypts (Figs 2a, 3a). Crypt lumina often contained detrital material (Fig. 2a). Within crypt lumina, in the mucosa and – to a lesser extent – the submucosa, moderate to massive mononuclear infiltration, dominated by myeloid/histiocyte antigen‐positive macrophages, was observed (Fig. 2a,b). Masses of spiral‐shaped bacilli were found in the gut lumen close to the villous surface, between villi, within crypt lumina and within crypt epithelial cells (Figs 3a,b, 4). Release of bacilli from single degenerating epithelial cells was observed (Fig. 3a).
Figure 2.

Ileum. (a) Loss of crypts, degeneration of crypt epithelial cells and detrital material within crypts (arrow), massive mononuclear infiltration of the mucosa. Haematoxylin and eosin. × 50. (b) Infiltration of the mucosa by myeloid/histiocyte antigen‐positive macrophages. Peroxidase anti‐peroxidase (PAP) method, Papanicolaou’s counterstain. × 12 0.
Figure 3.

Ileum. (a) Spiral‐shaped bacilli are released from a degenerate crypt epithelial cell (arrow). Haematoxylin and eosin. × 3 50. (b) Spiral‐shaped bacilli are found in the crypt lumen and within crypt epithelial cells. Warthin–Starry stain. × 280.
Figure 4.

Caecum. Spiral‐shaped bacteria are found both in the lumen of crypts with intact epithelial cells and with detrital material (arrow). Peroxidase anti‐peroxidase (PAP) method, Papanicolaou’s counterstain. × 130.
Spiral‐shaped bacilli stained strongly with the Warthin–Starry stain (Fig. 3b). and showed a positive immunohistochemical reaction with the polyclonal antibody against H. pylori (Figs 1a,b, 3c). Staining for parvovirus, coronavirus and FeLV antigens was not observed. Special stains (Gram, Warthin–Starry, PAS) did not reveal other bacteria in significant amounts, nor fungal elements or parasites.
Ultrastructurally, the organisms had the morphology of a straight cylinder and ranged between 4 and 5 μm in length and 0.5–0.7 μm in width (Figs 5, 6). The spiral shape was due to a single periplasmic filament which coiled around the body (Figs 5, 6). It was located between the periplasmic envelope and the cell wall (Fig. 6). Additionally, polar flagella were observed (Fig. 5). Organisms were also demonstrated within the cytoplasm of epithelial cells in the small intestine (Fig. 7).
Figure 5.

The organism has the form of a straight cylinder. The spiral is formed by one periplasmic filament coiling around the bacterial body. Transmission electron microscopy. × 14 100.
Figure 6.

The periplasmic filament is located between the periplasmic envelope and the cell wall. Note polar flagellae. Transmission electron microscopy. × 28 200.
Figure 7.
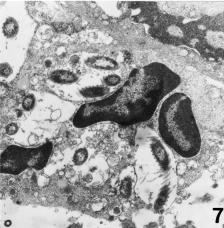
Jejunum. A spiral‐shaped bacterium within the cytoplasm of an intestinal crypt epithelial cell. Transmission electron microscopy. × 6000.
The spleen, mesenteric and caecal lymph nodes, and gut‐associated lymphoid tissue showed moderate follicular and T cell depletion with numerous apoptotic cells and tingible body macrophages. Within the sinuses of the caecal lymph nodes, single macrophages with a spot‐like positive immunohistochemical reaction for Helicobacter antigens were found. The thymus was massively involuted. Bone marrow activity was moderate.
PCR for bacterial ribosomal RNA genes
PCR did not yield any amplification product for bacterial ribosomal RNA genes (subunit of 16S gene). No inhibition of amplification was detectable in positive controls.
Discussion
This report describes a case of fatal enteritis in a 4‐month‐old cat, associated with massive gastrointestinal infection with spiral‐shaped bacilli. Organisms were demonstrated in the stomach, small intestine and caecum. They were located within the intestinal lumen, between intestinal villi and in gastric glands and pits, as well as within intestinal crypt lumina and gastric and intestinal crypt epithelial cells. Often, cellular colonization was associated with epithelial cell degeneration.
The stomach showed moderate T cell‐dominated infiltration. Regarding this feature, a difference can be stated to clinically healthy cats with gastric Helicobacter colonization who exhibit small focal B cell accumulations (Handt et al., 1995; Hermanns et al., 1995; Fox et al., 1996; Happonen et al., 1996; Norris et al., 1999).
The small intestine and caecum were intensely infiltrated by macrophages which were present in the lumen of affected crypts as well as in the mucosa and submucosa. This indicates macrophage recruitment due to epithelial cell degeneration and/or bacilli. In this respect, the present case is similar to a case of histiocytic colitis in a cat (van Kruiningen and Dobbins, 1979). However, in the latter case, infiltrating macrophages contained numerous bacilli, a feature which was lacking in the present case. Furthermore, the bacilli were morphologically different from those described here (van Kruiningen and Dobbins, 1979). The immunohistochemical demonstration of Helicobacter antigens in single sinus macrophages in the caecal lymph node indicate phagocytosis and lymphatic transport of single bacilli.
Light microscopy including immunohistochemistry and electron microscopy did not give any evidence of other enteric pathogens. The morphology of the gastrointestinal alterations together with the negative immunohistochemical results meant that FeLV‐associated enteritis, parvovirus enteritis and coronavirus enteritis could be excluded (Reinacher, 1987; Kipar et al., 1998a, 2000). Electron microscopy did not reveal virus particles in intestinal epithelial cells, rendering other viral infections, such as astrovirus, rotavirus, or torovirus infections, which are generally of minor pathological significance (Harbour, 1998), unlikely. Fungal elements or parasites were not demonstrated, and other bacteria were not observed in significant amounts. Furthermore, it has been shown that the antibody against H. pylori, applied for immunohistochemistry, does not cross‐react with Salmonella, Shigella, Yersinia, and Campylobacter jejuni/coli (Andersen et al., 1988). Unfortunately, a microbiological examination was not performed which would have served to definitely exclude other potential bacterial pathogens. The case history does not indicate drugs (cytostatica) or radiation as potential reasons for the alterations observed. Therefore, it seems likely that the described spiral‐shaped organisms are responsible for the lesions found.
Ultrastructurally, the spiral‐shaped bacilli have the morphology of a straight cylinder which is encoiled by a periplasmic filament. They are distinct from all Helicobacter species which have so far been demonstrated in cats, such as H. pylori, H. felis, ‘H. heilmannii’, H. canis and ‘H. colifelis’ (Lee et al., 1988; Happonen et al., 1996; Foley et al., 1998, 1999; Norris et al., 1999). The organisms bear more of a resemblance to ‘F. rappini’, H. bilis and H. canis, some closely related bacilli isolated from the intestine of various species (Lockard and Boler, 1970; Schauer et al., 1993; Stanley et al., 1993; Fox et al., 1995; Foley et al., 1999). The presence of a single periplasmic filament together with the lack of a basal plate at the point of flagella insertion strongly support that the organism is actually ‘F. rappini’ (Lockard and Boler, 1970; Stanley et al., 1993; Fox et al., 1995). However, a definite identification of the organism was not possible, as the PCR for bacterial ribosomal RNA genes (16S rDNA) did not yield any amplification product, thereby excluding determination of specific 16S rDNA sequences. It seems likely that the reason for the failure to detect 16S rDNA was DNA degradation due to prolonged formalin fixation and paraffin embedding.
Healthy cats are frequently inhabited by Helicobacter (‐like organisms) (Otto et al., 1994; Hermanns et al., 1995; Happonen et al., 1996; Neiger et al., 1998; Norris et al., 1999; Simpson et al., 2000). Species differentiation has most often identified ‘H. heilmannii’ and H. felis, whereas H. pylori infection has only been occasionally observed in commercial catteries, but not in pet or stray cats (Handt et al., 1994, 1995; El‐Zaatari et al., 1997; Neiger et al., 1998). Intestinal Helicobacter infection, however, has only been reported twice in cats (Foley et al., 1998, 1999). Helicobacter colifelis was isolated from the caecum and colon of a cat suffering from severe diarrhoea. The bacilli formed dense layers on the mucosal surface and within crypts which showed dilation and flattened epithelium (Foley et al., 1998). Helicobacter canis was isolated in a cat colony. The bacilli were found to be adhered to the colonic mucosal surface, and mixed cellular infiltrates and occasional crypt abscesses were observed. However, as both healthy cats and cats suffering from diarrhoea harboured the agent, the pathogenic role of H. canis could not be determined (Foley et al., 1999).
Intracellular spiral‐shaped organisms, as seen in the present case, have only occasionally been observed in gastric glandular epithelial cells in Helicobacter spp. and ‘F. rappini’ infection in cats and dogs (Lockard and Boler, 1970; Lee et al., 1992; Otto et al., 1994; Simpson et al., 2000), although both dogs and cats bear several Helicobacter species (Lee et al., 1992; Hermanns et al., 1995; Happonen et al., 1996; Foley et al., 1998, 1999; Jalava et al., 1998; Neiger et al., 1998; Norris et al., 1999; Simpson et al., 2000). There, however, infected gastric parietal cells did not show signs of degeneration (Lockard and Boler, 1970; Lee et al., 1992; Otto et al., 1994; Simpson et al., 2000) as observed in this case in gastric and intestinal epithelial cells harbouring intracellular organisms. The direct association of intracellular organisms and cellular degeneration can be regarded as additional evidence of the pathogenic significance of the ‘F. rappini’‐like organisms found in this case.
Helicobacter species like ‘F. rappini’ and H. bilis are regarded as normal colonizers of the lower gastrointestinal tract in the mouse (Schauer et al., 1993; Fox et al., 1995). The same is true for H. cinaedi in hamsters (Gebhart et al., 1989) and H. canis in the dog (Stanley et al., 1993). However, H. cinaedi and H. fennelliae have been shown to be potential enteric pathogens in humans (Totten et al., 1985). There is evidence for H. canis to be an enteric pathogen in cats (Foley et al., 1999), and a new Helicobacter species has been isolated from cotton‐top tamarins with chronic colitis (Saunders et al., 1999). Taking these observations and the present case into account, there is strong evidence that a group of phylogenetically closely related Helicobacter species are potentially enteropathogenic (Stanley et al., 1993; Fox et al., 1995; Saunders et al., 1999).
In the present case, substantial lymphoid depletion was observed. This finding indicates either primary or secondary immunosuppression, the reason for which, however, cannot be determined. Parvovirus, FeLV, and FIV infection, systemic viral infections frequently inducing lymphoid depletion, were not demonstrated. Furthermore, the case history did not give evidence of a specific treatment, intoxication, or exposure to radiation.
The source of the cat’s infection with ‘F. rappini’‐like organisms remains unidentified. However, the fact that ‘F. rappini’ belongs to the normal intestinal flora of mice (Schauer et al., 1993) may point to mice as the potential source of infection.
Acknowledgements
The authors wish to thank Professor Dr N. Lehn, Institut für Medizinische Mikrobiologie und Hygiene, Universität Regensburg, Germany, for helpful discussions. We are grateful to Mrs M. Wipplinger and Mrs I. Langhof for technical assistance, and Mrs U. Zeller for photographic support.
References
- 1. Andersen, L. P. , Holck S., Povlsen C. O., 1988: Campylobacter pylori detected by indirect immunohistochemical technique. Acta Pathol. Microbiol. Immunol. Scand. 96 , 559–564. [PubMed] [Google Scholar]
- 2. El‐Zaatari, F. A. , Woo J. S., Badr A., Osato M. S., Serna H., Lichtenberger L. M., Genta R. M., Graham D. Y., 1997: Failure to isolate Helicobacter pylori from stray cats indicates that H. pylori in cats may be an anthroponosis – an animal infection with a human pathogen. J. Med. Microbiol. 46 , 372–376. [DOI] [PubMed] [Google Scholar]
- 3. Foley, J. E. , Solnick J. V., Lapointe J.‐M., Jang S., Pedersen N. C., 1998: Identification of a novel enteric Helicobacter species in a kitten with severe diarrhea. J. Clin. Microbiol. 36 , 908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foley, J. F. , Marks S. L., Munson L., Melli A., Dewhirst F. E., Yu S., Shen Z., Fox J. G., 1999: Isolation of Helicobacter canis from a colony of Bengal cats with endemic diarrhea. J. Clin. Microbiol. 37 , 3271–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox, J. G. , Perkins S., Yan L., Shen Z., Attardo L., Pappo J., 1996: Local immune response in Helicobacter pylori‐infected cats and identification of H. pylori in saliva, gastric fluid and faeces. Immunology 88 , 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox, J. G. , Yan L. L., Dewhirst F. E., Paster B. J., Shames B., Murphy J. C., Hayward A., Belcher J. C., Mendes E. N., 1995: Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J. Clin. Microbiol. 33 , 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebhart, C. J. , Fennell C. L., Murtaugh M. P., Stamm W. E., 1989: Campylobacter cinaedi is normal intestinal flora in hamsters. J. Clin. Microbiol. 27 , 1692–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handt, L. K. , Fox J. G., Dewhirst F. E., Fraser G. J., Paster B. J., Yan L. L., Rozmiarek H., Rufo R., Stalis I. H., 1994: Helicobacter pylori isolated from the domestic cat: public health implications. Inf. Immun. 62 , 2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Handt, L. K. , Fox J. G., Stalis I. H., Rufo R., Lee G., Linn J., Li X., Kleanthous H., 1995: Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J. Clin. Microbiol. 33 , 2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Happonen, I. , Saari S., Castren L., Tyni O., Hänninen M.‐L., Westermarck E., 1996: Occurrence and topographical mapping of gastric Helicobacter‐like organisms and their association with histological changes in apparently healthy dogs and cats. J. Vet. Med. A 43 , 305–315. [DOI] [PubMed] [Google Scholar]
- 11. Harbour, D. A. , 1998: Feline enteric viral infections. In: Greene, C. E. (ed.), Infectious Diseases of the Dog and Cat, 2nd edn, pp. 69–71. W.B. Saunders, Philadelphia.
- 12. Hermanns, W. , Kregel K., Breuer W., Lechner J., 1995: Helicobacter‐like organisms: histopathological examination of gastric biopsies from dogs and cats. J. Comp. Path. 112 , 307–318. [DOI] [PubMed] [Google Scholar]
- 13. Jalava, K. , On S. L. W., Vandamme P. A., Happonene I., Sukura A., Hanninen M. L., 1998: Isolation and identification of Helicobacter spp. from canine and feline gastric mucosa. Appl. Environ. Microbiol. 64 , 3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kipar, A. , Kremendahl J., Addie D. D., Leukert W., Grant C. K., Reinacher M., 1998a: Fatal enteritis associated with coronavirus infection in cats. J. Comp. Path. 119 , 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kipar, A. , Bellmann S., Kremendahl J., Köhler K., Reinacher M., 1998b: Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Vet. Immunol. Immunopathol. 65 , 243–257.DOI: 10.1016/s0165-2427(98)00158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kipar, A. , Kremendahl J., Grant C. K., Von Bothmer I., Reinacher M., 2000: Expression of viral proteins in feline leukemia virus‐associated enteritis. Vet. Pathol. 37 , 129–136. [DOI] [PubMed] [Google Scholar]
- 17. Kovacevic, S. , Kipar A., Kremendahl J., Teebken‐Schuler D., Grant C. K., Reinacher M., 1997: Immunohistochemical diagnosis of feline leukemia virus infection in formalin‐fixed tissue. Eur. J. Vet. Pathol. 3 , 13–19. [Google Scholar]
- 18. Van Kruiningen, H. J. & Dobbins W. O., III, 1979: Feline histiocytic colitis. A case report with electron microscopy. Vet. Pathol. 16 , 215–222. [DOI] [PubMed] [Google Scholar]
- 19. Lane, D. J. , 1991: 16S/23S rRNA sequencing. In: Stackebrandt, E., and M. Goodfellow (eds), Nucleic Acid Techniques in Bacterial Systematics, pp. 155–175. John Wiley and Sons, Chichester.
- 20. Lee, A. , Hazell S. L., O'Rourke J., Kouprach S., 1988: Isolation of a spiral‐shaped bacterium from the cat stomach. Inf. Immun. 56 , 2843–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee, A. , Krakowka S., Fox J. G., Otto G., Eaton K. A., Murphy J. C., 1992: Role of Helicobacter felis in chronic canine gastritis. Vet. Pathol. 29 , 487–494. [DOI] [PubMed] [Google Scholar]
- 22. Lockard, V. G. & Boler R. K., 1970: Ultrastructure of a spiraled microorganism in the gastric mucosa of dogs. Am. J. Vet. Res. 31 , 1453–1462. [PubMed] [Google Scholar]
- 23. Neiger, R. , Dieterich C., Burnens A., Waldvogel A., Corthesy‐Theulaz I., Halter F., Lautenburg B., Schmassmann A., 1998: Detection and prevalence of Helicobacter infection in pet cats. J. Clin. Microbiol. 36 , 634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norris, C. R. , Marks S. L., Eaton K. A., Rorabian S. Z., Munn R. J., Solnick J. V., 1999: Healthy cats are commonly colonized with ‘Helicobacter heilmannii’ that is associated with minimal gastritis. J. Clin. Microbiol. 32 , 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otto, G. , Hazell H., Fox J. G., Howlett C. R., Murphy J. C., O'Rourke J. L., Lee A., 1994: Animal and public health implications of gastric colonization of cats by Helicobacter‐like organisms. J. Clin. Microbiol. 32 , 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinacher, M. , 1987: Feline leukemia virus‐associated enteritis – a condition with features of feline panleukopenia. Vet. Pathol. 24 , 1–4. [DOI] [PubMed] [Google Scholar]
- 27. Saunders, K. E. , Shen Z., Dewhirst F. E., Paster B. J., Dangler C. A., Fox J. G., 1999: Novel intestinal Helicobacter species isolated from cotton‐top tamarins (Saguinus oedipus) with chronic colitis. J. Clin. Microbiol. 37 , 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schauer, D. B. , Ghori N., Falkow S., 1993: Isolation and characterization of ‘Flexispira rappini’ from laboratory mice. J. Clin. Microbiol. 31 , 2709–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simpson, K. W. , Strauss‐Ayali D., Scanziani E., Straubinger R. K., McDonough P. L., Straubinger A. F., Chang Y.‐F., Domeneghini C., Arebi N., Calam J., 2000: Helicobacter felis infection is associated with lymphoid follicular hyperplasia and mild gastritis but normal gastric secretory function in cats. Inf. Immun. 68 , 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stanley, J. , Linton D., Burnens A. P., Dewhirst F. E., Owen R. J., Porter A., On S. L. W., Costas M., 1993: Helicobacter canis sp. nov., a new species from dogs: an integrated study of phenotype and genotype. J. Gen. Microbiol. 139 , 2495–2504. [DOI] [PubMed] [Google Scholar]
- 31. Totten, P. A. , Fennell C. L., Tenover F. C., Wezenberg J. M., Perine P. L., Stamm W. E., Holmes K. K., 1985: Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J. Inf. Dis. 151 , 131–139. [DOI] [PubMed] [Google Scholar]
- 32. Weisburg, W. G. , Barns S. M., Pelletier D. A., Lane D. J., 1991: 16S: ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173 , 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]


