Summary
The genus Quercus is among the most widespread and species-rich tree genera in the northern hemisphere. The extraordinary species diversity in America and Asia together with the continuous continental distribution of a limited number of European species raise questions about how macro- and microevolutionary processes made the genus Quercus an evolutionary success. Synthesizing conclusions reached during the past three decades by complementary approaches in phylogenetics, phylogeography, genomics, ecology, paleobotany, population biology and quantitative genetics, this review aims to illuminate evolutionary processes leading to the radiation and expansion of oaks. From opposing scales of time and geography, we converge on four overarching explanations of evolutionary success in oaks: (1) accumulation of large reservoirs of diversity within populations and species; (2) ability for rapid migration contributing to ecological priority effects on lineage diversification; (3) high rates of evolutionary divergence within clades combined with convergent solutions to ecological problems across clades; and (4) propensity for hybridization, contributing to adaptive introgression and facilitating migration. Finally, we explore potential future research avenues, emphasizing the integration of microevolutionary and macroevolutionary perspectives.
Keywords: Quercus, macroevolution, microevolution, phylogeny, adaptation, migration, hybridization
Introduction: oak evolution in context
Oaks arose an estimated 56 million years ago and have radiated and expanded subsequently across the Northern Hemisphere (Manos & Stanford, 2001; Hipp et al., 2019). Today they extend from the equator (Colombia and Indonesia) to the boreal regions up to 60° latitude N in Europe, and from sea level to 4000 meters in the Yunnan province in China (Camus, 1936; Camus, 1938; Camus, 1952; Menitsky, 2005; De Beaulieu & Lamant, 2010). Oaks range in habitat from dense bottomland forests to open grasslands and savannas, and from alkaline to acidic soils (Abrams, 1992; Nixon, 1997). The genus has diversified into numerous species in America and Asia, with highest diversity at 15–30° N in Mexico and east Asia (Valencia, 2004; Menitsky, 2005; Nixon, 2006). Europe exhibits lower species richness (up to 30 species), but the genus is nearly as widespread there as it is in North America and east Asia, as a limited number of European species have expanded across the continent (Camus, 1936,). Most recent estimates place diversity of oaks at approximately 435 species (Denk et al., 2017), though ongoing work in Mexico and Central America suggests that this number may be an underestimate. The genus is in any case among the most species-rich tree genera in the Northern Hemisphere together with Salix, and the most important in terms of species and biomass in the Americas (Cavender-Bares et al., 2016b, 2019).
Oaks are, by at least these measures of diversity and distribution, an evolutionary success. Although there might be some arbitrariness and anthropomorphic overtone in defining “success,” we sidestep for the moment the semantic embarrassment and we investigate the evolutionary underpinnings of the increase in species abundance and geographic range of the genus Quercus from its origin to the current day. Our review complements the integrative review of the American oaks in Cavender-Bares (2019), which emphasizes the reciprocal effects of ecological and evolutionary processes on oak diversity. In our review, we focus on the question of how global patterns of oak diversity and abundance are shaped by the joint effects of microevolutionary processes—processes at or below the species level—and macroevolutionary processes—processes at the clade level. The extraordinary species diversity in America and Asia as well as the continuous continental distribution of a limited number of European species together plead for a joint consideration of macro- and microevolutionary processes that triggered the radiation and expansion of oaks. Oaks have been scrutinized at both scales of inquiry over the last three decades, taking the North American, European, and East Asian settings as study cases (Manos et al., 1999; Manos & Stanford, 2001; Petit et al., 2002; Pearse & Hipp, 2009; Kremer et al., 2010; Torres-Miranda et al., 2013; Rodríguez-Correa et al., 2017; Deng et al., 2018; Jiang et al., 2019 Ohsawa et al., 2007; Okaura et al., 2007). In North America, two oak clades arose simultaneously in the late Eocene to early Oligocene and radiated in sympatry, maintaining similar levels of diversity and range (Cavender-Bares et al., 2018; Hipp et al., 2018; Cavender-Bares, 2019). Europe affords a contrasting evolutionary stage, where two species (Q. petraea and Q. robur) underwent repeated continental retraction and expansion during the late Pleistocene, generated by peculiar demographic and genetic processes (Brewer et al., 2002; Petit et al., 2003). We review major findings regarding radiation and expansion in oaks and extend our review beyond these emblematic cases by addressing more recent research in Asian species (Deng et al., 2018).
Our review is not exhaustive, and many important papers will have been missed in it. This fact notwithstanding, our separate examinations of microevolution and macroevolution and consideration of their reciprocal effects on oak evolutionary success offer general insights into the success of the genus Quercus across the northern hemisphere. We believe our review demonstrates the power of integrating perspectives across diverse evolutionary scales for understanding the past and future of oak diversity.
I. Microevolution in oaks
Most studies of oak microevolution have either quantified the amount of evolutionary change within a given spatiotemporal interval or have investigated the pace and mechanism of evolutionary processes driving evolutionary change. These studies have made use of complementary approaches and disciplines, including paleobotany, ecology, phylogeography, population biology and quantitative genetics, as partially reviewed previously (Manos et al., 1999; Kremer et al., 2010; Barron et al., 2017; Cavender-Bares, 2019). In general, microevolutionary processes have been studied from synchronic monitoring of data for traits and gene markers in extant in situ natural populations or ex situ common gardens (provenance tests). Allochronic approaches based on ancient DNA have only recently been attempted to explore the persistence and/or replacement of temperate European oak populations during the Holocene (Wagner et al., 2018). The overall time frame encompassed by allochronic and synchronic microevolutionary investigations spans the last 15,000 years. Most microevolutionary studies have focused on oak species widely distributed in the northern temperate zone, the populations of which were established during the most recent postglacial warming (i.e., during the Holocene). The major evolutionary changes during this period were triggered by macroecological changes, comprising a general trend towards warming since the last glacial maximum, but also shorter alternating periods of cooling and warming as the “Little Ice Ages” (Jones & Mann, 2004). Impacts of climate change on forest ecosystems lasting only a few centuries are also well documented in northern latitudes (Kullman & Kjallgren, 2006; MacDonald et al., 2008). In other parts of the world—in Mexico, for example, where oak species diversity is at its highest—climate change has been complemented by local orogeny (mountain formation) during the Holocene (Ferrari et al., 2012). Climatic and orogenic changes have served as strong selective drivers in plant and animal species, triggering migration, the colonization of new habitats, adaptation, and introgression (Svennig et al., 2015; Meng et al., 2017; Muellner-Riehl, 2019).
1. Rapid migration limiting the erosion of diversity
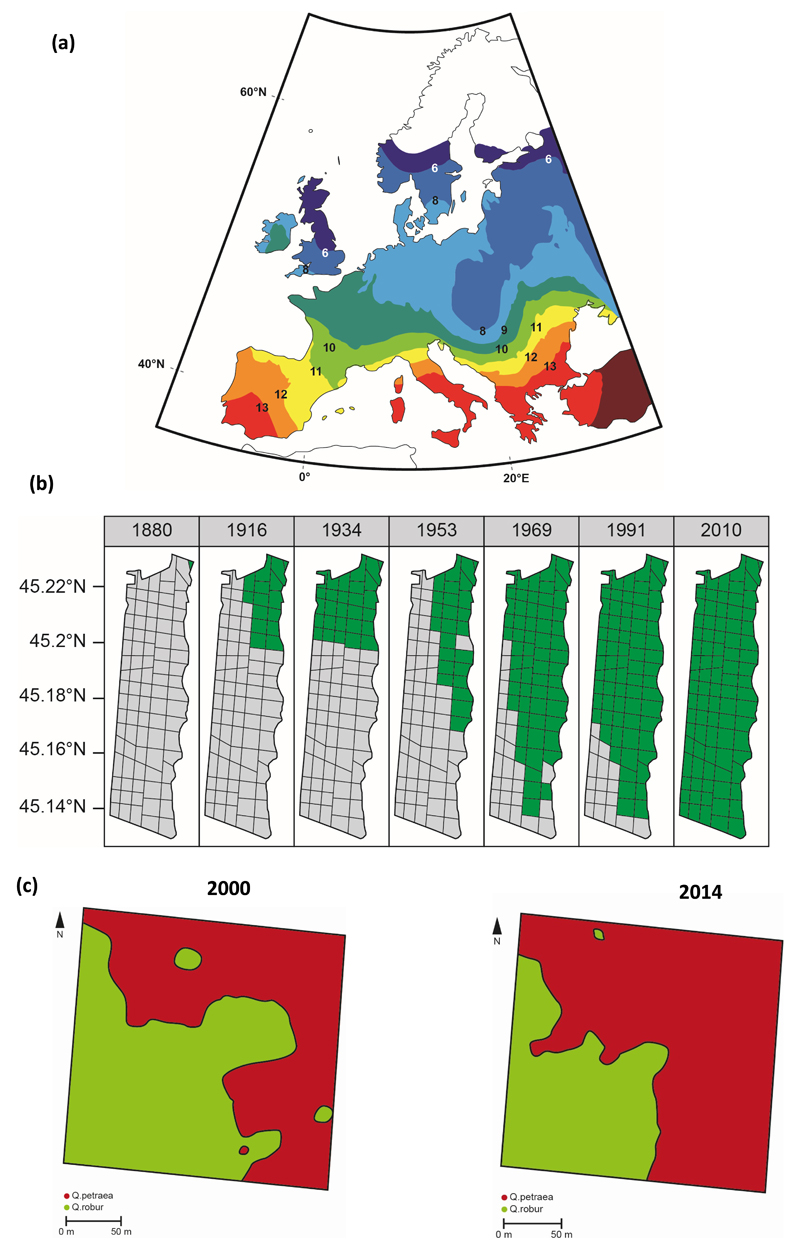
Empirical reconstructions of post-glacial oak colonization in Europe based on fossilized pollen have indicated a mean migration velocity of 400 to 500 m per year (Brewer et al., 2002; Giesecke & Brewer, 2018), with maximum values as high as 1000 m/year (Fig. 1) (Brewer et al., 2005). These estimates outpace migration rates inferred from data on dispersal by jays or rodents (Bossema, 1979). This apparent discrepancy suggests that postglacial migration dynamics involved a composite mechanism combining diffusion at the migration front with rare long distance dispersal events (Le Corre et al., 1997). Computer simulations have shown that even very rare long-distance dispersal events can increase overall migration velocity, as new focal populations generated stochastically by these events ultimately merge through population growth (Le Corre et al., 1997; Bialozyt et al., 2006). A somewhat different picture has emerged from postglacial migration reconstructions in North America. East of the Rocky Mountains and in the Pacific Northwest, oak stands persisted closer to the Laurentide ice sheet during the glacial period and extended northward to lower latitudes than in Europe (Schlarbaum et al., 1982; Magni et al., 2005; Soltis et al., 2006; Marsico et al., 2009). West of the Rocky Mountains, in California, populations were maintained in situ during glacial periods, albeit at reduced sizes and these populations subsequently expanded during postglacial warming, but over limited total distances (Grivet et al., 2006). In China, postglacial colonisation occurred as in Europe over very large distances as oaks were restricted to tropical and subtropical areas at the Last Glacial Maximum and expanded up to the north-eastern rim of the Tibetan Plateau (Cao et al., 2015). Rapid migration seems to have been triggered by quaternary climatic oscillations (Svennig et al., 2015), but at spatial scales that differed between continents. While in central Europe (Brewer et al., 2002) and Asia (Cao et al., 2015), postglacial colonization dynamics of temperate oaks were dominated by large scale expansion, in Mediterranean regions of Europe (de Heredia et al., 2007) and California (Grivet et al., 2006), oaks species expanded at a limited scale from multiple refugial foci.
Figure 1. Velocity of oak migration at different time and spatial scales.
-
a)Migration velocity of deciduous oaks retrieved from palynological records retracing postglacial colonization in Europe.This map shows isochrones pollen maps at 1000 year time slices from 13000 BP to 6000 BP, representing arrival times of oaks as derived from the European Pollen Data (EPD) base (Brewer et al., 2002). Velocity varied over time and space, with average values amounting to 500 m./year and maximum values up to 1000 m./year (Brewer et al., 2005). Although palynological records may overestimate migration effects due to the persistence of small refugial populations, these figures were recently confirmed by updating EPD data (Giesecke et al., 2016; Giesecke and Brewer, 2018).
-
b)Migration velocity of Holm oak (Quercus ilex) during the last century at the northern margin of its distribution (Delzon et al., 2013). Holm oak is a Mediterranean evergreen species and its northern margin stretches along the Atlantic coast in France. These maps illustrate its distribution (in green colors) in a national forest located on the Atlantic coast at different times during the last century. Forest maps show compartment subdivisions (black lines). Maps were derived from floristic inventories conducted at the compartment level by the forest service every 10 to 20 years when management plans of national forest are renewed. Migration velocity amounted on average to 20 m./year with maximum values up to 57 m./year. These figures are likely underestimated as Holm oak is locally considered as a weed by foresters and screened during thinning operations.
-
c)Expansion of Quercus petraea over two successive generations in La Petite Charnie Forest, located in the North Western part of France (Truffaut et al., 2017). The map shows the distribution of individual trees of each species (red area: Quercus petraea; green area: Q. robur) in 2000 and 2014, before and after natural regeneration occurred. Q. petraea has expanded its distribution by up 50 %, corresponding to maximum linear shifts of 20 meters.
What were the evolutionary consequences of rapid migration? Long-distance dispersal imprinted strong phylogeographic structures, because such dispersal events were rare and gave rise to small populations, with founding events for chloroplast genomes (Le Corre & Kremer, 1998). While haplotype fixation within population is nearly complete in most oak species in Europe (Vitelli et al., 2017 and Petit et al., 2002), North America (Whittemore and Schaal, 1991 and Cavender et al., 2015) and Asia (Yang et al., 2017 and Harada et al., 2018), geographical haplotype distributions have also been shaped by postglacial movements: oak species that expanded over large distances usually exhibit widely distributed haplotypes, while species with regional expansion show locally distributed haplotypes (Pham et al., 2017; Sork et al., 2016). Studies of ancient DNA retrieved from wood remains (Wagner et al., 2018) have shown that chloroplast genomes have been maintained since the establishment of these populations. Thus, the extant phylogeographic structures were imprinted during postglacial migration (Wagner et al., 2018). A somewhat counterintuitive consequence of long-distance dispersal is the maintenance of diversity despite founding events during colonization. Computer simulations have shown that the multiple local populations created by long-distance dispersal maintain higher levels of diversity than populations displaying mainstream diffusion during colonization (Bialozyt et al., 2006).
However, founding events and the strong phylogeographic structures they generated have tended to persist only in organellar genomes, as subsequent gene flow mediated by pollen travelling among widely separated populations within species progressively erased nuclear differentiation among geographic regions (Kremer et al., 2010). Contrasts between organellar and nuclear differentiation are widespread in most oak species studied to date, as a result of strong differences of seed vs pollen movement that is common in oak species (Petit et al., 2005; Petit & Excoffier, 2009). Rapid migration associated with fast establishment may lead ultimately to priority effects, resulting from novel evolutionary and ecological opportunities favoring species that arrive first (Cavender-Bares et al., 2016a; Cavender-Bares, 2019). Impacts of evolutionary priority effects may include demographic growth, expansion, ecological divergence and diversification. The most recent comparative data regarding postglacial expansion velocities in the three continents (Giesecke et al., 2017 in Europe; Cao et al., 2015 in Asia and Harnik et al., 2017 in North America) suggest that oaks benefited from priority effects in many circumstances.
2. Regional adaptation despite extensive gene flow
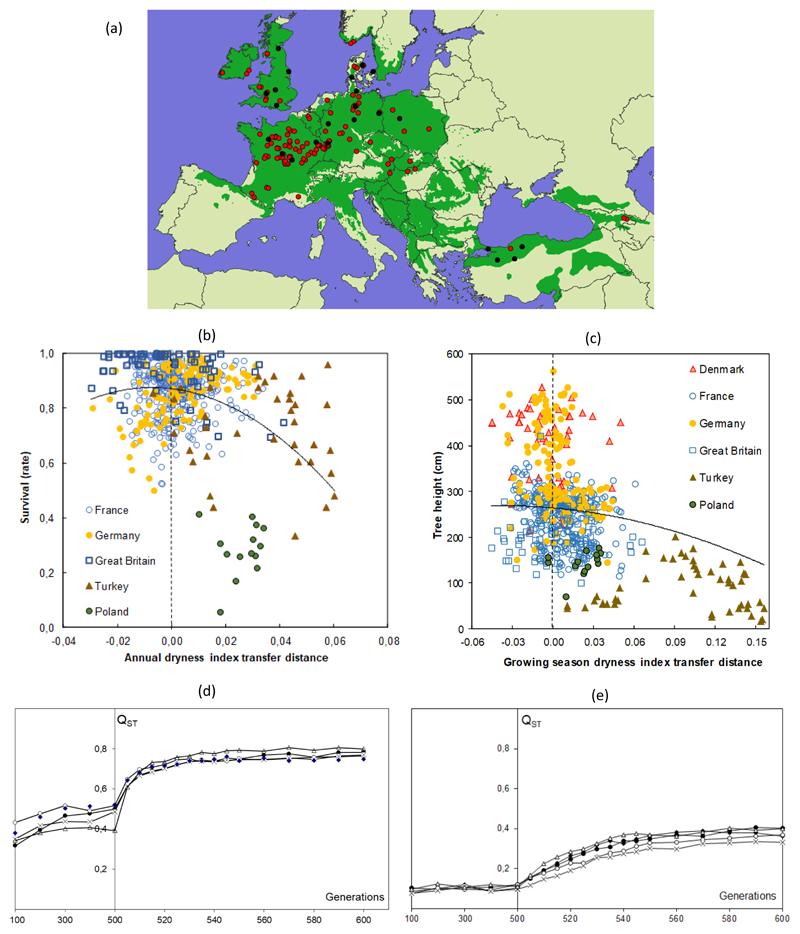
Evidence for adaptation during the Holocene has been provided by many common-garden experiments, demonstrating the occurrence of adaptive divergence between modern populations established after postglacial colonization. Range-wide population-level collections from species with continental distributions have revealed high levels of population differentiation for adult growth-related traits in species representing three major clades of the genus: Quercus rubra (Kriebel et al., 1976; Kriebel et al., 1988), representing section Lobatae ; Quercus macrocarpa (Dicke & Bagley, 1980) and Quercus petraea (Kremer et al., 2010), representing section Quercus; and Quercus suber (Gandour et al., 2007; Ramirez-Valiente et al., 2014), representing section Cerris. Reported Qst values (a metric of genetic population differentiation for traits) reach up to 0.8, greatly exceeding typical differentiation values for neutral genetic markers (Kremer et al., 2010; Ramirez-Valiente et al., 2018), suggesting that natural selection is the predominant driver of differentiation in phenotypic traits (Figure 3d). Similar results have been obtained for juvenile traits and in limited population samples from other species (e.g., Quercus acutissima (Zhang et al., 2017), Quercus oleoides (Ramirez-Valiente et al., 2017, 2019; Koehler et al., 2012)).
Figure 3. Adaptive divergence, plasticity and local adaptation in oaks.
-
a)Acorns were collected in 116 forest stands (populations, red dots) distributed across Europe, saplings were redistributed in 23 field tests (black dots) in 6 different countries following standard experimental designs. Monitoring of adaptive phenotypic traits were conducted since installation.
-
b)And c) The response of two fitness related traits (survival and height growth at age 10) are plotted for each population as a function of the climatic transfer distance between the climate of the origin of the populations and the climate of the test site. Populations are labelled according to the country where the test site is established. Various climatic data were tested to account for the transfer distance. For survival and height, dryness related indexes explained the largest variation of population responses. Both traits show an overall quadratic curve, with a maximum near the null transfer distance, suggesting that populations are nearly locally adapted. On average populations grow on slighter dryer conditions than where their optimum is.d) And e) Local adaptation due to diversifying selection resulted therefore in adaptive divergence. How fast adaptive divergence builds up is largely unknown, but simulations suggest that adaptive differentiation can be quite rapid. Two evolutionary scenario calibrated to the oak case study (55 populations undergoing diversifying selection and connected by gene flow) with two extreme within population selection intensities (strong, d, and moderate e) were simulated to give hand on the pace of adaptive divergence measured here as Qst (a metric of genetic population differentiation, varying between 0 and 1). At generation 500, environmental changes were introduced to assess the response of adaptive divergence following environmental changes. In a few tens of generation following environmental changes, genetic differentiation reached again a plateau (adapted from (Kremer & Le Corre, 2012)).
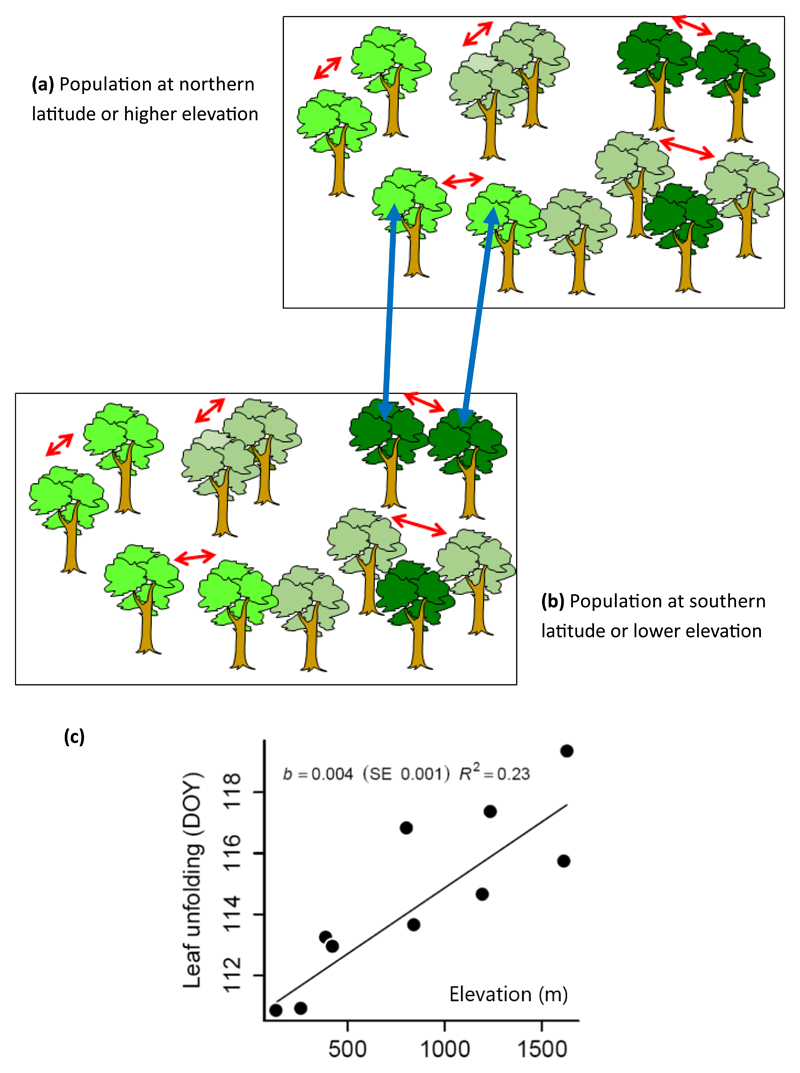
These studies also suggest that divergent selection has shaped genetic variation across species ranges. Clinal variation tracking geographic temperature-driven trends is often observed for growth and phenological traits (Fig 2) (Soularue & Kremer, 2014). However, the direction of the cline may differ between species or continents. Bud burst, for example, tends to occur later in spring for European oak populations from higher elevation or latitude than for more southerly populations (Ducousso et al., 1996; Vitasse et al., 2009), whereas the opposite pattern is observed for northern red oak (McGee, 1970,1974; Kriebel et al., 1976; Daubree & Kremer, 1993). This raises the question of whether the abiotic or biotic factors driving divergent selection for flushing date differ between the two continents or between different species. More recent studies have focused on physiological traits thought to contribute to adaptation to climate in Quercus petraea (Torres-Ruiz et al., 2019) and Quercus suber (Ramirez-Valiente et al., 2014). Interestingly, in these two cases, population differentiation was less pronounced for leaf traits and traits relating to water metabolism, suggesting that divergent selection has disproportionately targeted more integrated traits, such as overall growth or bud phenology. The opposite trend was observed in Quercus oleoides, in which leaf functional traits and water use efficiency varied according to populations’ climate of origin (Cavender-Bares & Ramirez-Valiente, 2017; Ramirez-Valiente & Cavender-Bares, 2017. At a more local scale, local adaptation was demonstrated in reciprocal transplantations for leaf herbivore resistance in Q. rubra (Sork et al., 1993).
Figure 2. Assortative mating, gene flow and diversifying selection drive clinal phenological responses in oaks.
-
a)and b) Within natural populations early flushing trees tend to mate with early flushing trees, resulting in positive assortative mating. Trees with similar phenotypes regarding date of bud burst preferentially mate within populations. Positive assortative mating is shown by red arrows between trees sharing similar colours on graph a) (colours indicate here the timing of bud burst). However matings resulting from immigrant pollen flow are likely to result in negative assortative mating especially if source populations are farther away in latitude or elevation. Populations at northern latitudes or higher altitude flush on average later than populations from more southern latitudes or from lower elevations because of temperature differences. Hence successful matings resulting from pollen flow can only associate late flushing trees from the south (or low elevation) to early flushing trees in the north (or at higher elevation) (negative assortative mating shown by blue arrows on b). As a result, assortative mating and gene flow contribute to a directional filtering of late flushing genes to the northern (or higher elevation) populations (Soularue & Kremer, 2012; Soularue & Kremer, 2014).
-
c)Within population positive assortative mating generates higher within population genetic variance, whereas negative assortative mating and gene flow creates genetic clines along temperature gradients in the landscape, as shown here by the genetic divergence of the time of bud burst along an elevational gradient in sessile oak in the Pyrénées (Firmat et al., 2017)
High levels of adaptive divergence for phenotypic traits have led to studies aiming to identify genomic footprints of diversifying selection based on two different approaches. In one approach, genome scans have been used to search for genome-environment associations (Rellstab et al., 2016; Sork et al., 2016b; Martins et al., 2018). In the other, associations between genomic and trait variation have been explored in situations for which common garden results were available (Alberto et al., 2013). Significant statistical associations were detected in each case study, but there has been a lack of consistency across studies at the SNP, gene or function level (Alberto et al., 2013). More recently, whole-genome sequencing in different populations has revealed the existence of thousands of SNPs significantly correlated with bud burst in Q. petraea and displaying clinal variation with temperature for allelic frequencies (Leroy et al., 2019a). The genetic architecture of the traits contributing to climate adaptation is therefore highly polygenic. Under such circumstances, diversifying selection proceeds by first combining allelic effects at different genes rather than targeting single genes, an approach that can lead to rapid adaptive responses, occurring over a few tens of generations (Fig 3) (Kremer & Le Corre, 2012). Indeed, the existence of extensive genetic differentiation for integrated traits raises questions about the timing of divergence and adaptation (recently, during the Holocene) and whether divergence has reached optimal levels. In European temperate oaks, it is likely that the existing divergence developed gradually and exclusively during the Holocene, as evidenced by the lack of association between populations’ trait values and their glacial refugial origins (Kremer et al., 2002). Furthermore, based on theory mentioned above, polygenic architecture of traits enhances “rapid divergence”. However, just as divergence can be limited by the extensive pollen flow reported in oaks, adaptation also can be limited by pollen swamping. This fact notwithstanding, we argue that adaptation can develop from standing genetic variation despite gene swamping, especially for polygenic traits, for which the contributions of individual alleles are small. As predicted by simulations (Kremer & Le Corre, 2012) and by theory (Yeaman, 2015), greater genetic variation due to gene flow provides more opportunities for allelic combinations with favorable effects, facilitating adaptation.
3. Hybridization facilitating migration and reinforcing adaptation
Hybridization is widespread within sections of the Quercus genus and has received considerable attention during the last decade, as illustrated by examples in white oaks, sect. Quercus (Ishida et al., 2003; Curtu et al., 2007; Lepais & Gerber, 2011; Castillo-Mendoza et al., 2019), southern live oaks, sect. Virentes (Cavender-Bares & Pahlich, 2009; Eaton et al., 2015), red oaks, sect. Lobatae (Dodd & Afzal-Rafii, 2004; Penaloza-Ramirez et al., 2010; Moran et al., 2012; Sullivan et al., 2016), golden cup oaks, sect. Protobalanus (Ortego et al., 2018), holly oaks, sect. Ilex (Neophytou et al., 2007), and ring-cupped oaks, sect. Cyclobalanopsis (Song et al., 2015; An et al., 2017). Most of these studies focused on the ecological, demographic and genetic factors shaping the rates and variation of hybridization in extant sympatric stands. Much less attention has been paid to the evolutionary implications of hybridization and introgression, which we explore here.
Recent studies based on whole-genome sequencing or reduced-representation genomic sequencing have shown that hybridization can occur without jeopardizing species integrity, even if only a limited part of the genome, comprising numerous widely distributed small regions, is responsible for maintaining species barriers (Eaton et al., 2015; Leroy et al., 2017, 2019b). Genes under selection for drought-tolerance (Oney-Birol et al., 2018) or involved in photoperiodic control of growth and development (Lind-Riehl et al., 2014) may be disproportionately responsible for genetic divergence between species: selection in ecologically distinct species may suffice to explain divergence in the face of gene flow. Equally importantly, these observations are inconsistent with the notion that hybridization triggers speciation by creating new hybrid species (Abbott et al., 2013), or favoring the assimilation of one species by another, although one cannot exclude the possibility that relictual hybrid populations might in rare cases behave as new species (Hauser et al., 2017).
Instead, hybridization should be seen as a microevolutionary process reinforcing adaptation (Cavender-Bares, 2019; Leroy et al., 2019a), and, more surprisingly, enhancing migration (Petit et al., 2003), as shown by ecological and population genomics studies of various oak complexes. Evidence for a role of hybridization in facilitating colonization comes from the continent-wide sharing of chloroplast genomes by sympatric European or North American white oaks (Whittemore & Schaal, 1991; Petit et al., 1997, 2002). In each case study, two species cohabiting the same forest have the same chloroplast haplotype, whereas the same species co-occurring in a nearby mixed stand may share a different chloroplast haplotype. These findings suggest that hybridization and subsequent backcrossing between two species can result in the chloroplast genome of one species being captured by the other. Furthermore according to this scenario, the hybridization/backcrossing mechanism necessarily occurred recently (during the Holocene), at some time since post-glaciation establishment of the two species. Interestingly in the European example, ABC simulations have supported these conclusions by showing that secondary contacts between white oak species began at the onset of the last glacial period, before recolonization (Leroy et al., 2017; Leroy et al., 2019b). In the particular context of the Quercus petraea - Q. robur complex, in which backcrossing is preferentially unidirectional, from Q. petraea to hybrids, Q. petraea (the pollen “invader” species) should be progressively “regenerated” within Q. robur (the “resident” species) stands as a result of recurrent backcrossing after the initial hybridization. A recent demographic and genetic study of the spatial distribution of pure species and admixed forms conducted over two successive generations confirmed the progressive invasion of Q. robur by Q. petraea as a result of hybridization (Truffaut et al., 2017). To sum up, the pioneer species Q. robur is more readily dispersed long distance by jays (Bossema, 1979) while Q. petraea migrates more readily by long distance pollen movement. Ultimately Q. petraea invades Q. robur as a consequence of the recurrent backcrossing after initial hybridizition (Petit et al., 2003). We conclude that this mechanism—introgression-driven migration—was active during post-glacial colonization in Europe and was probably selected to increase vagility during the repeated climatic oscillations that prevailed during the quaternary period (Dynesius & Jansson, 2000). Artificial directional backcrossing in chestnut breeding programs has shown that the pollen invader species can largely be regenerated in a relatively small number of generations, compatible with the post-glacial time frame (Diskin et al., 2006; Steiner et al., 2017). This directional introgression is still evident at the northern margins of the temperate European oak range, at which the frequency of introgressed forms is much higher than in the center of the distribution (Beatty et al., 2016). According to this scenario, introgression occurs mostly from the resident to the invading species, as predicted by theory (Currat et al., 2008), and observed in sympatric Q. petraea - Q. robur stands (Guichoux et al., 2013).
While the direction of introgression has been elucidated, the genes actually transmitted, even the classes of genes, remain a matter of speculation. An early study based on a rangewide genome scan of four co-occurring California red oaks (Dodd & Afzal-Rafii, 2004) demonstrated that the amount and source of introgression in a landscape context is predictable by climatic conditions and that introgressed amplified fragment length polymorphisms (AFLPs) could be found well outside the natural range of their source species. This suggested that selective introgression could facilitate species migration. A more recent study (Leroy et al., 2019a) builds on this finding, confirming expectations that introgression may facilitate adaptation of the invading population. In a broad collection of Q. petraea populations sampled along elevational and latitudinal gradients, whole genome sequencing detected signatures of introgression of temperature tolerance related genes from Q. robur, facilitating adaptation at higher latitudes and elevation of the invading species (Leroy et al., 2019a). This appears to be the first reported case of adaptive introgression in oaks supported by genomic and phenotypic evidence.
4. Pace and targets of microevolutionary change
Synchronic approaches, in common garden experiments or in extant natural populations, have been valuable for estimating the magnitude and direction of evolutionary change. However, because such approaches integrate divergence or differentiation estimates accumulating over time during the Holocene, including changes in opposing directions that effectively cancel each other out, they tend to underestimate the instantaneous rate of evolution, or evolution occurring over a very small number of generations (Gingerich, 2001). It is unclear, for example, whether observed divergence in bud-break phenology between red oak (Quercus rubra) populations at two ends of the species range includes only the changes triggered by the minor environmental changes of the few centuries since the stabilization of overall climate in the northern hemisphere (6000 years BP). Alternative explanations are either longer-term divergence between populations throughout the Holocene or conversely early equilibrium reached within a few generations after post-glacial establishment.
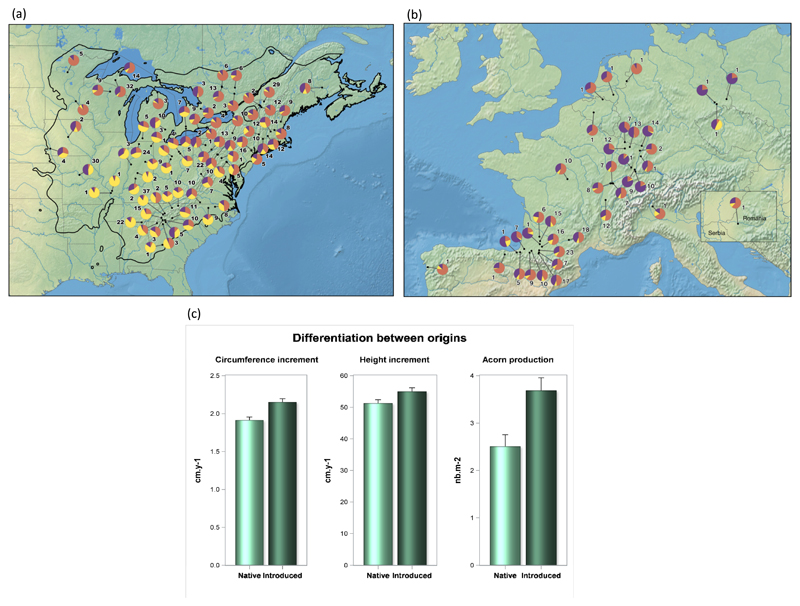
There are a few documented examples in oaks of evolutionary change occurring over a small number of generations, mostly in conditions of strong environmental change. Many North American oak species were introduced into Europe during the last three centuries. These species were initially established in botanical gardens, then in planted forests (Timbal et al., 1994). Northern red oak, Q. rubra, was introduced into Europe in the 18th century and is now widely used in operational forestry throughout the continent. A genetic survey conducted in Europe and throughout the natural range of this species showed that a diverse germplasm had been introduced, probably from the northern part of the natural distribution (Merceron et al., 2017). At the same time, a range-wide common garden of plants from introduced and native populations was established in Europe, and studies of this common garden showed that the introduced populations had diverged from the native gene pool in phenological and growth traits (Daubree & Kremer, 1993; Merceron, 2017). As there is evidence to suggest that founder effects were limited (Merceron et al., 2017), the observed divergence between the two gene pools probably results from selection, due to the difference in environment between the two continents and the particular colonization dynamics of an exotic species (Fig. 4).
Figure 4. Evolutionary change assessed in transferred populations.
-
a)Distribution of the native populations included in the genetic survey. Colours in the pie chart correspond to the genetic clusters assigned by the genetic clustering analysis (orange: northeastern populations, yellow: southeastern; purple: northcentral/northwestern)
-
b)Distribution of the introduced populations included in the genetic survey.
-
c)Comparison of native and introduced populations for height yearly increment (cm/year), circumference increment (cm/year), and seed production (seed/m2)
Another example is provided by the monitoring of genetic, phenotypic and demographic changes in two successive generations of Quercus petraea and Q. robur (Truffaut et al., 2017). Predicted and observed changes were detected for highly integrated traits, such as height and diameter growth, whereas physiological traits tended to be more static (Alexandre, unpublished material). These rare examples suggest that evolutionary change may occur over short periods of time, even a single generation. We would argue that extensive and rapid change is possible because oaks harbor large reservoirs of diversity. Such rapid evolutionary changes may shape species coexistence in oaks through selection for divergent competitive abilities (Hart et al., 2019) and thus shape oak diversity at scales ranging from single forests to entire continents.
5. Accumulation of fuel for evolutionary change
Expected evolutionary change increases with the level of diversity residing within populations, regardless of whether drift or natural selection dominates. Studies of genetic diversity in natural oak populations date to the advent of tools for monitoring diversity. Initially, such studies focused on isozymes or morphology (Baranski, 1975), but they now consider whole-genome sequences. The diversity statistics compiled in an earlier review based on isozymes (Kremer & Petit, 1993) were subsequently confirmed by a myriad of studies encompassing species from three continents, which highlighted the very high degree of genetic diversity residing within populations. If we restrict the analysis to diversity inventories based on microsatellites, then a general picture of high heterozygosity (> 0.7) with numerous rare alleles emerges. This is the case not only for species with a broad distribution (Q. macrocarpa (Craft & Ashley, 2007); Q. rubra (Lind-Riehl & Gailing, 2015; Alexander & Woeste, 2017); Q. candicans, Q. castanea and Q. crassifolia (Oyama et al., 2018); Q. petraea and Q. robur (Mariette et al., 2002; Neophytou et al., 2010); Q. acutissima (Zhang et al., 2013); and Q. mongolica (Zeng et al., 2015)), but also for species with much narrower ranges (Q. hinckelyi (Backs et al., 2015); Q. engelmannii (Ortego et al., 2012); Q. tomentella (Ashley et al., 2018); Q. alnifolia (Neophytou et al., 2011); and Q. austrocochinchinensis (An et al., 2017)). Whole-genome sequencing results have recently confirmed marker estimates, highlighting a strikingly high level of nucleotide diversity (π values of 0.0114 (Plomion et al., 2018), and one SNP every 23 bp (Leroy et al., 2019b). Despite potential constraints due to either natural vegetative reproduction (Ainsworth et al., 2003; Valbuena-Carabana et al., 2008; Backs et al., 2015; Ashley et al., 2018) or scattered distributions at the edges of the main range (Ohsawa et al., 2008; Marsico et al., 2009; Ortego et al., 2015), the erosion of diversity has been limited.
The high level of within-population polymorphism that appears to characterize almost all oaks raises the question of whether properties intrinsic to Quercus might enhance the accumulation of genetic diversity. Diversity is mechanistically driven by population size, which is itself determined by population history and life history traits (Ellegren & Galtier, 2016). According to neutral theory, new mutations are more likely to remain within a population, and for longer periods of time before fixation, in large populations. Thus species with large population sizes tend to accumulate diversity simply because they do not lose new mutations. It could be argued that oak species, despite their different evolutionary histories, have several life history traits in common that collectively contribute to the establishment and maintenance of large populations and high levels of diversity. Longevity increases the opportunity for somatic mutations, which may ultimately enter the germline, increasing overall diversity (Plomion et al., 2018). Longevity, and the long juvenile period in particular, facilitate the replenishment of population density through colonization, allowing migrants to enter the recipient population before reproduction takes place (Austerlitz et al., 2000; Austerlitz & Garnier-Gere, 2003). Polyandry similarly increases with longevity, contributing to greater diversity (Nakanishi et al., 2005; Craft et al., 2009). Long-distance pollination has now been repeatedly demonstrated by paternity analysis in various oak species (in Q. macrocarpa (Dow & Ashley, 1996; Dow & Ashley, 1998; Craft & Ashley, 2010); and Quercus robur (Buschbom et al., 2011)), suggesting that oaks maintain large effective population sizes even if their populations are spatially isolated (Oyama et al., 2017). Perhaps equally important in oaks is the role of interspecific gene exchange as a mechanism for enriching within-population diversity. Multispecies oak communities are common, and genetic diversity of the widespread Quercus castanea has been shown to increase with the number of co-occurring oak species in the species-rich Transmexican Volcanic Belt (Valencia-Cuevas et al., 2014).
In addition to neutral diversity, oaks also retain variation that is potentially adaptive (Ramirez-Valiente et al., 2014). Empirical estimates of within-population heritability and evolvability show that oaks possess large within-population phenotypic variance encompassing a number of functional traits. Historically, estimates of genetic variance were limited to species and traits of economic relevance, mostly growth and wood-related traits. Previous reviews of North American oaks (mainly Q. alba, Q. rubra, Q. falcata, Q. pagoda) (Kriebel, 1993) and European oaks (mainly Q. petraea and Q. robur) (Kleinschmit, 1993) highlighted the existence of already extremely variable phenotypes within and between progenies raised in common gardens in terms of height and diameter growth, crown form and phenology. Similar outcomes were also observed more recently in Asian species for growth (in Q. acutissima (Na et al., 2015) and Q. serrata (Kang et al., 2007)). Within the context of environmental change, attention has shifted towards fitness-related traits, such as phenology, resistance to biotic and abiotic stresses, and reproduction (Ramirez-Valiente et al., 2019; Brendel et al., 2008). Strikingly, the heritability of spring phenology is very high (>0.8) in various oak species (Q. robur (Baliuckas & Pliura, 2003); Q. petraea (Alberto et al., 2011) and (Firmat et al., 2017); Q. suber (Ramirez-Valiente et al., 2014)), probably due to assortative mating, with preferential mating between extreme flushing phenotypes (Soularue & Kremer, 2012; Soularue & Kremer, 2014). Artificial cloning by cuttings has made it possible to assess the wide genotypic variation in water use efficiency (Brendel et al., 2008; Roussel et al., 2009), resistance to waterlogging (Parelle et al., 2007) and fecundity (Caignard et al., 2019). Estimates of genetic variation are highly variable among species where traits have been studied, but there is on average inflated genetic variance within natural populations for a number of traits, including fitness-related traits such as growth and fecundity (Alexandre et al., 2019).
Convergent assessments of within-population diversity regardless of the species and traits considered raise questions about the inherent mechanisms responsible for the maintenance of this variation. In addition to generic processes, such as extensive gene flow, spatially and temporally fluctuating selection may act over long time periods, during which environmental or climatic changes may alter natural selection in contrasting or opposite directions. Stabilizing selection, favoring intermediate phenotypes, and the polygenic architecture of most of the traits studied in oaks to date probably also contribute to the maintenance of genetic variation in natural populations.
II. Macroevolution in oaks
Macroevolutionary studies in oaks trace the history of lineage diversification from the birth of the genus ca. 56 Mya to the present day. Phylogenetic research in oaks initially proceeded from synthetic inferences based on morphology and biogeography (Trelease, 1924; Camus, 1936; Camus, 1938; Camus, 1952; Axelrod, 1983) to morphological cladistics (Nixon, 1985; Nixon., 1993) (reviewed in Manos et al., 1999; Manos, 2016). These first studies make evident the importance of molecular data for distinguishing between hypotheses that emphasize morphology vs. biogeography. The first molecular phylogenetic studies analyzed relatively small numbers of genes (Whittemore & Schaal, 1991; Manos et al., 1999; Manos et al., 2008; Oh & Manos, 2008; Hubert et al., 2014; Deng et al., 2013) and were successful at recovering many broad relationships, but not robust species-level relationships near the tips of the phylogeny. DNA fingerprinting techniques (Pearse & Hipp, 2009) and phylogenomic approaches of the past 5 years based primarily on RAD-seq reduced-representation genomic sequencing (Hipp et al., 2014; Cavender-Bares et al., 2015; Fitz-Gibbon et al., 2017; Deng et al., 2018) succeeded in recovering fine-scale phylogenetic relationships and lay the groundwork for this review.
1. Biogeographic transitions and trait evolution shape oak-dominated ecosystems
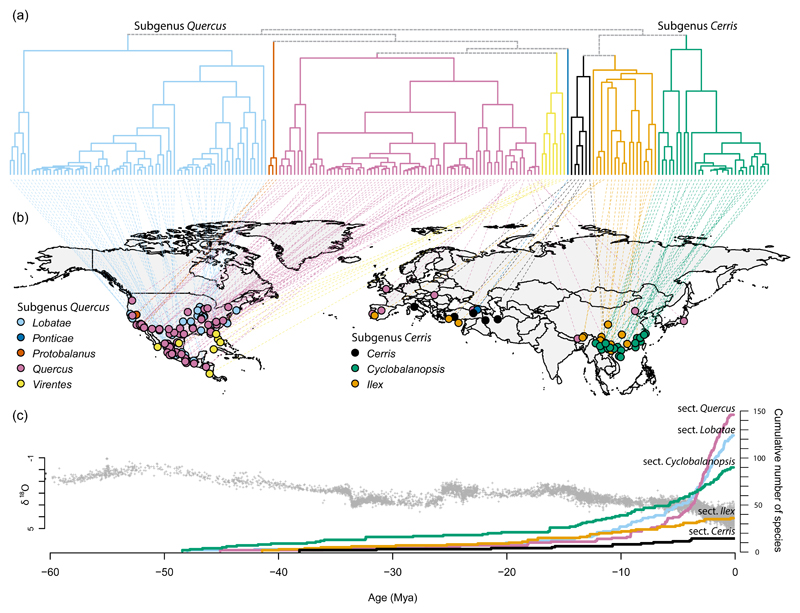
The stem age of Quercus dates to at least the Paleocene / Eocene boundary, 56 Ma, based on fossilized pollen records (Hofmann, 2010; Hofmann et al., 2011). At that time, global temperatures were approximately 10°C warmer than current conditions (Zachos et al., 2001). Oaks underwent a basal vicariance between Eurasia and North America (Manos and Stanford, 2001; Hipp et al., 2019). Fossil data suggest that several of the major lineages that we now recognize as sections (Denk et al. (2017)) - viz. sections Cyclobalanopsis (Manchester, 1994; Manchester, 2011), Lobatae (Greenwood et al., 2016; Grimsson et al., 2016), Quercus (McIntyre, 1991; Eberle & Greenwood, 2012), and possibly section Ilex (Hofmann, 2010) - originated within about 10 million years following the early Eocene climatic optimum. The early diversification of Quercus thus rapidly divided the genus into geographically structured clades (Fig. 5). As temperatures cooled over the past 40 million years, they diversified largely in situ (Axelrod, 1983; Manos & Stanford, 2001), diversifying ecologically within each region.
Figure 5. Phylogeny shapes global oak biodiversity.
(a) Oak diversification has been a repeated story of region-specific diversification of multiple lineages, each of which diversifies ecologically within the region. Within each of the eight sections recognized today, species have diversified ecologically, converging on similar habitats between sections, so that most geographic regions have representatives of at least two sections. The diversity we observe today is due in part to the fact that divergent lineages have evolved convergent ecologies. (b) In the Americas, the red and white oaks dominate (sections Quercus and Lobatae), followed by sections Virentes and Protobalanus. Red and white oaks radiated in sympatry in western and eastern North America, subsequently in Mexico and Central America. In Europe, sections Ilex, Cerris, and Quercus overlap in distribution, whereas in southeast Asia, the highly diverse section Cyclobalanopsis is a dominant of broad-leaved evergreen forests, accompanied by sections Ilex and Quercus in part. (c) Oaks arose at northern latitudes ca. 56 Ma, at around the time of the early Eocene climatic optimum. Temperatures at this time were ca. 10°C warmer than they are today, as evidenced by global deep-sea oxygen isotope (δ18O) records (here represented by gray ‘+’ signs), which serve as ocean temperature proxies (Zachos et al., 2001). The eight major lineages now named as sections all appear to have originated within the first ca. 10 million years of oak evolution and have subsequently diversified largely on the continents where they arose. Standing diversity, however, has mostly arisen in the past 10 million years. Figures are all adapted from Hipp et al. (2019a), with infrageneric taxonomy following Denk et al. (2017). Sources: (a) Phylogeny pruned to one tip per species, including only tips with a georeferenced collection. (b) Georeferenced specimens sequenced to make the phylogeny, colored by section. (c) Lineage-through-time (LTT) plot with unsampled species added at random positions within the clades where they occur to recover the contemporary standing diversity of the genus.
Each of the eight sections of Quercus overlaps with one or more others in ecological and geographic space (Cavender-Bares et al., 2015; Eaton et al., 2015; Fitz-Gibbon et al., 2017; Hauser et al., 2017; Leroy et al., 2017; McVay et al., 2017a; Ortego et al., 2017). This has been explored in depth in the Americas, where the white and red oaks (sections Quercus and Lobatae respectively) arose from a northern ancestor, then diversified southward as temperatures warmed from the early Eocene climatic optimum until the mid-Oligocene (Zachos et al., 2001), radiating in parallel in the western U.S., eastern North America, and Mexico and the southwestern U.S. (Hipp et al., 2018; Cavender-Bares, 2019). In both the red and white oaks, the move into Mexico resulted in an abrupt uptick in net diversification rate in the topographically diverse Mexican mountains (Hipp et al., 2018). A similar history played out in Europe, where the Cerris and Ilex clades radiated to cover a broadly overlapping geographic range, ecologically diversifying within clades (Hipp et al., 2019). As in North America, increases in diversification rate in the Eurasian oaks were driven by ecological opportunity in two clades, but not in sympatry as we find in the Americas. One of the Eurasian oak clades, the Roburoid white oaks, arrived in Eurasia from an eastern North American ancestor somewhere between 10 and 20 Mya (McVay et al. 2017b). Arriving to a continent lacking in closely related oaks, the Roburoid white oaks diversified at a higher rate than the Ilex and Cerris clades present in Europe when they arrived. By way of comparison, the Roburoid net diversification rate, at 0.174 (0.07–0.29, 95% CI) spp My-1, is 2.8× the net diversification rate of sections Cerris and Ilex—both 0.063 (0.05–0.08) spp My-1—the only other oak clades present in Europe when the Roburoids arrived, and 2.0× the net diversification rate of Quercus alba and allies, the sister clade that it left behind in eastern North America, at 0.085 (0.05–0.20) spp My-1 (Hipp et al., 2019a).
While the Roburoids were diversifying in Europe and east Asia, a portion of section Cyclobalanopsis, comprising the Glauca, Acuta, and Semiserrata clades (Deng et al., 2018), was radiating in southeast Asia, reaching a net diversification rate of 0.31 (0.14–0.47) spp My-1 (Hipp et al., 2019a). Section Cyclobalanopsis—by far the largest of the Old World clades, with ca. 90 species—originated with uplift of the Himalayas at the Oligocene/Miocene boundary (Deng et al., 2018). As in the oaks of Mexico, increased topographic heterogeneity is implicated in the initial diversification of the section as well as an uptick in diversification ca. 8 Ma, coinciding with the uplift of the eastern Tibetan plateau (Deng et al., 2018). Initiation of the Asian summer monsoons in the late Oligocene may have contributed to spread of the section, which is a dominant of evergreen broadleaf forests. Global oak diversification is thus a story of repeated diversification into the same areas of geographic and ecological space by different lineages, with occupation of new territory facilitating diversification.
2. Adaptation has shaped macroevolution of oak traits
Oaks adapted to new climatic conditions throughout their radiation. The Ornstein-Uhlenbeck (O-U) adaptation model adaptive half-time (t½)—the expected time for a trait to evolve halfway from its ancestral state to its optimum (Hansen, 1997; Hansen et al., 2008)—has been estimated at 7.53 Ma for adaptation of oak clades to transitions in climate (Hipp et al., 2018). The model-averaged t½ of an index of herbivore resistance evolving toward an optimum predicted by the multivariate climate has been estimated at 12.8 Ma (Pearse & Hipp, 2012). These significant but relatively slow estimated rates of adaptation seem to stand at odds with the high variation of phenological traits among populations within oak species (Firmat et al., 2017; Cavender-Bares, 2019). This mirrors the contrast between high rates of adaptation for individual populations and low rates of macroevolutionary change observed for trees as a whole (Petit & Hampe, 2006). While the phylogenetic O-U model may be thought of as a framework for translating microevolutionary dynamics into phylogenetic expectations (Martins, 1995), aggregating traits up to species means masks variation within species that underlies local and regional adaptation; thus, the particular value of t½ should not be taken as an estimate of the rate at which species will adapt to future climate change.
Convergence between the red and white oak clades and divergence within those clades, combined most likely with niche differentiation between clades along resource-acquisition axes that we are only beginning to understand (González-Rodríguez et al., 2019), allowed oaks to pack niche space more densely (Cavender-Bares et al., 2004; Cavender-Bares et al., 2006; Pearse & Hipp, 2012; Cavender-Bares et al., 2016; Kaproth & Cavender-Bares, 2016; Cavender-Bares et al., 2018; Hipp et al., 2018; Cavender-Bares 2019). The high diversity of Mexican oaks is thus a consequence of both increased diversification rates within Mexico, likely due to increased topographic heterogeneity as oaks entered the mountains on their way south (Nixon, 1993; Valencia, 2004; Torres-Miranda et al., 2011; Rodriguez-Correa et al., 2017; Hipp et al., 2018), and the fact that white oaks and red oaks were able to radiate simultaneously across the region.
As discussed in the previous section, diversification of oaks in Eurasia parallels the American oak diversification. Eurasian oaks comprise four major clades, three constituting subgenus Cerris, which diversified exclusively in the Old World—sections Ilex and Cerris, together sister to section Cyclobalanopsis—and a clade of ca. 25 white oaks of section Quercus (Manos & Stanford, 2001; Hipp et al., 2018), the ancestor of which arrived from the Americas presumably via the North Atlantic Land Bridge between 15 and 18 Ma (Axelrod, 1983; Manos & Stanford, 2001; but see Denk et al., 2010 who argue for a late Miocene origin of the Eurasian white oaks, also from an eastern North American progenitor). The uplift of the Himalayas is implicated in diversification of the holly oaks, section Ilex, and in the diversification of the ring-cupped oaks, section Cyclobalanopsis. Phylogenomic data, combined with morphometric analysis of fossil and living taxa (Jiang et al., 2019), suggest that the European holly oaks arose from an East Asian ancestor via migration along the southern foothills of the Himalayas. This connection had been suggested previously (Denk & Grimm, 2010), but without phylogenetic evidence in support of the direction of migration. The authors also demonstrate a morphological transition in the two fossil section Ilex species of Europe, suggesting rapid ecological and morphological differentiation of European section Ilex species during the early diversification of the clade (Jiang et al., 2019).
In aggregate, these studies suggest that (1) ecological opportunity drives oak diversification; (2) convergent patterns of diversification within co-occurring clades results in high regional oak diversity in the Americas and Europe; and (3) plant traits and climatic niche exhibit both adaptation and phylogenetic niche conservatism in their macroevolutionary responses to novel climates (as also argued by Cavender-Bares, 2019). Oaks’ ability to take advantage of novel ecological conditions has shaped the global distribution of oak biodiversity.
3. Deep genomic incongruence shapes oak macroevolution
Early efforts at molecular phylogenetic reconstruction in oaks demonstrated that the chloroplast tracks phylogeographic history rather than population and species level divergences (Whittemore & Schaal, 1991; Dumolin-Lapègue et al., 1997; Manos et al., 1999). As has been shown in microevolutionary studies in the European white oaks reviewed above, chloroplast DNA markers are more informative about local and historical gene flow at regional scales than about species relationships. Within the North American white oaks alone, for example, phylogenetic history as inferred from multilocus nuclear (RAD-seq) DNA explains very little chloroplast variation, while geography explains 11%–21% of chloroplast phylogenetic variance (Pham et al., 2017). In practical terms, chloroplast data has little use in solving fine-scale phylogenetic questions, and they fail to recover monophyly of even some very deep divergences in the tree. A particularly remarkable example of nuclear / chloroplast discordance is found in section Protobalanus, which segregates into two chloroplast types (Manos et al., 1999; Pham et al., 2017) (Fig. 6b), in spite of the strong nuclear coherence of the section (Ortego et al., 2018). In this case, microevolutionary dynamics of introgression and / or inheritance of ancestral allele polymorphisms near the base of the oak phylogeny appear to be frozen in the chloroplast phylogeny.
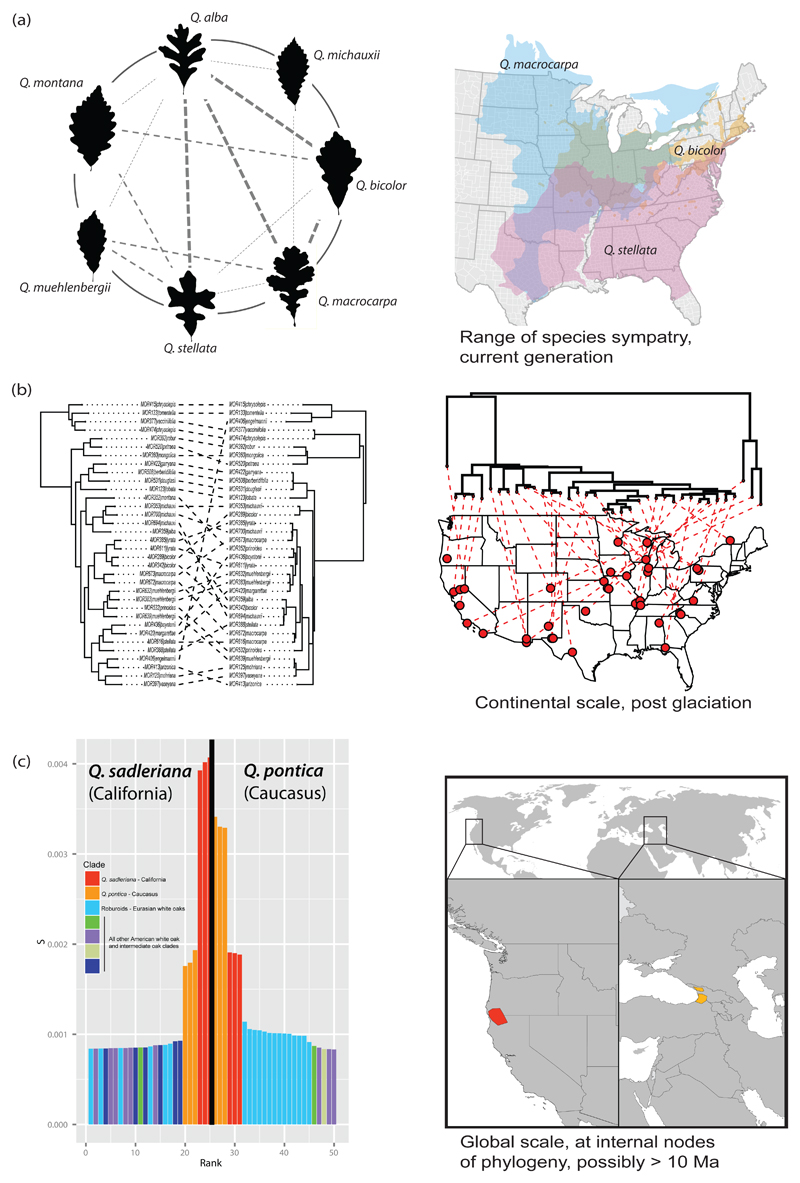
Figure 6. Contemporary introgression becomes ancient introgression.
(a) Contemporary introgression among the eastern North American white oaks has been a case study in the oak syngameon since the mid-1970s (Burger, 1975; Hardin, 1975; Van Valen, 1976) and was the focus of the first chloroplast phylogenetic study in oaks (Whittemore & Schaal, 1991). Introgression at this scale occurs primarily within the range of species sympatry and reflects recent generations of gene flow. The figure illustrates observed gene flow between seven eastern North American white oaks based on analysis of 80 SNPs that map back to all 12 Quercus linkage groups, with distances between uniquely mapped loci an average of 7.47 million bp (± 8.74 million bp, s.d.). The figure replicates the 16-species figure of Hardin 1975 (his Fig. 1), including only the subset of species investigated in Hipp et al. (2019b), with lines indicating hybridizations that Hardin inferred from morphological study. Thin dashed lines indicate hybridizations identified by Hardin but not by the SNP genotyping; medium dashed lines were identified by both Hardin and SNP genotyping, at an admixture level of 0.10 to 0.19 for at least one specimen; and thick dashed lines indicate SNP admixture levels of 0.20 or higher for at least one specimen. (Hipp et al. 2019b). (b) As species ranges change in response to climatic transitions, species that were once sympatric may become allopatric. Gene flow between them may still be detected by, for example, incongruence between chloroplast and nuclear topologies (Whittemore & Schaal, 1991; Manos et al., 1999). Here, a RAD-seq (nuclear) phylogeny for predominantly North American white oaks (left side) shows strong species cohesion, whereas the phylogeny of whole chloroplasts for the same samples sorts better by geography than by species (Pham et al., 2017). Such events may also be detected by phylogenomic incongruence in nuclear loci (McVay et al., 2017a; e.g., Kim et al., 2018). (c) With enough generations, ancient introgression events that were sufficiently pervasive may remain detectable. A fascinating case involves the sister species Q. pontica of the Caucasus, Q. sadleriana of California, and the Eurasian white oaks, or Roburoids. Because Q. pontica and Q. sadleriana are sister species, they are perforce equally closely related to all other oaks. With careful study, John McVay (2017b) and colleagues found that there are loci shared among the Roburoid white oaks that ally them much more closely to the Eurasian Q. pontica than to the American Q. sadleriana. Panel (c) shows genomic evidence for introgressive hybridization between wild Q. pontica samples and the Roburoid oaks: scaled locus similarity on the y-axis between Q. pontica and the Roburoids is significantly stronger than expected when data are simulated under incomplete lineage sorting alone (p < 0.002). Observed rankings of Roburoids to Q. sadleriana sample did not differ significantly from simulated distribution. The fact that these loci are shared among the Roburoids in spite of the fact that most are not sympatric with Q. pontica suggests a history of ancient introgression between the lineage that contains Q. pontica but not Q. sadleriana (allowing for extinction, there may have been other species) and an ancestral Roburoid. This finding is borne out with analysis of a separate phylogenomic dataset using species tree methods that account for hybridization. Thus a microevolutionary process, inferred to have preceded the crown divergence of the Roburoids an estimated 7.6-12.1 Ma (Hipp et al. 2019a), became frozen in the genome, like a fly in amber.
The first study to investigate broad phylogenetic patterns in oaks using a combination of cpDNA and the nuclear ribosomal internal transcribed spacer (ITS) + 5.8S region recovered the monophyly of the American oak clade (subgenus Quercus) and the among-section topology within that clade (Manos et al., 1999). Addition of seven low-copy nuclear genes + ITS provided some support for clades and species monophyly, but with extensive incongruence and low statistical support (Hubert et al., 2014). High among-locus incongruence in oaks demands large numbers of loci to reconstruct population divergence history: even AFLP markers, which are relatively difficult to model probabilistically (Luo et al., 2007), yield more strongly supported phylogenies (Pearse & Hipp, 2009) than the longest multigene alignments to date (Hubert et al., 2014). Extending the genome-wide sampling of AFLPs to nucleotide-level data from more readily characterized regions of the genome (Leroy et al., 2017; Hipp et al., 2014; Crowl et al., 2019) provides highly resolved trees that track biogeography, morphology, and species boundaries. Study of the genomic architecture of phylogenetic signal in RAD-seq markers demonstrates why such large numbers of loci are needed to reconstruct the history of population divergence in oaks: different regions of the genome resolve different portions of the oak tree of life, and the high variation in among-population relationships encoded along the length of the mosaic oak genome demands a large sample of loci to clearly pick out the dominant phylogenetic history (Hipp et al., 2019).
As has been argued previously (Hipp, 2015) and shown in numerous studies (cited above), a consensus phylogenetic signal arises from the oak genome in spite of genomic mosaicism. This consensus divergence history has been the target of most phylogenetic work in the genus to date (though the phylogenetic network has been investigated for some groups; e.g., McVay et al., 2017a, b; Crowl et al., 2019; Ortego et al., 2018). It remains to be seen, however, how gene trees that track alternatives to the primary population divergence history may record the history of trait transfers among lineages (Bastide et al., 2017). Integrating our understanding of phylogenetic network inference, genomic mosaicism, and phylogenetic comparative methods that account for both will be a major challenge to the study of forest tree macroevolution in the genomic era.
4. Ancient introgression events are encoded in the genome
Because introgression is common across the tree of life (Arnold, 2016; Vargas et al., 2017), we consequently expect different genes to track different histories of adaptation to shifting climates, population divergence, and gene flow in sympatry. Microevolutionary studies, reviewed in the first half of this review, have focused on contemporary and often localized hybridization in oaks (Fig. 6a). Phylogenetic studies allow us to distinguish ongoing recent hybridization from ancient introgression in a multispecies system. Four dominant European white oaks, for example, show evidence of early divergence with gene flow only following secondary contact (Leroy et al., 2017). In the California white oaks, a species of Mexican origin (Q. engelmanii) exhibits a signature of introgression with the ancestor of a distantly-related clade comprising Q. durata and Q. berberidifolia, suggesting that weaker reproductive isolation between this species and the white oaks of Californian origin may be a consequence of more secondary contact (Kim et al., 2018). Even more dramatically, populations of Q. engelmanii (section Quercus) harbor a section Protobalanus chloroplast (Manos et al., 1999; Pham et al., 2017). There is no evidence of ongoing gene flow between these two, as attempts to make crosses between the sections have been unsuccessful (Cottam et al., 1982), and we know of no other cases of interbreeding between white oaks and intermediate oaks. Thus this is the residue of hybridizations or lineage sorting long past.
Macroevolutionary studies also allow us to identify how hybridization shapes the tree of life. The European white oaks, for example, carry alleles received by ancient introgression from Quercus pontica into the ancestor of the European white oak clade (McVay et al., 2017b, Crowl et al., 2019) (Fig. 6c). The western North American Quercus gambelii similarly appears to be a genetic conduit between the white oaks of California and the eastern North American Quercus macrocarpa and allies (McVay et al., 2017a, Crowl et al., 2019). While many species-level studies using nuclear markers recover the history of species divergence (Muir et al., 2000), they are usually conducted at the level of species pairs. Because oaks occur in multispecies communities and have undergone in situ radiation, introgression may be complex, involving numerous species and long time periods. Phylogenetic studies like the ones discussed here allow us to reconstruct ancient events that, at the time, had we been able to sequence, would have been microevolutionary processes. It remains to be seen whether the genes that fail to introgress at microevolutionary scales are also those that preserve phylogenetic history across the tree.
At the same time, phylogenetic studies based on relatively small numbers of samples for large numbers of species allow us to test for species coherence across large numbers of loci and the origins of alleles with respect to species boundaries. The overwhelming evidence is that there are at least enough genes not shared locally among species to permit species coherence across wide geographic ranges. In the largest species sample to date, 97 out of 147 species for which multiple samples were sequenced were monophyletic (Hipp et al., 2019a). A study of 221 individuals representing 13 species of section Cerris (Simeone et al., 2018) found that the 5S-IGS region is effective at both delineating clades and identifying species boundaries, while providing some evidence against the putative hybrid-species status of Quercus afares (Welter et al., 2012). The same general finding results from analysis of larger numbers of individuals in closely related species groups, e.g. the oaks of section Virentes (Cavender-Bares et al., 2015; Eaton et al., 2015), section Protobalanus (Ortego et al., 2017), the eastern North American white oaks (Hipp et al., 2019b), and the California scrub white oaks (Fitz-Gibbon et al., 2017) and red oaks (Hauser et al., 2017). Macroevolutionary studies demonstrate with broad species sampling that regional assemblages of related oak species true syngameons, “good species” in spite of a long history of introgression (Hardin 1975; Cannon and Petit, 2019).
III. Microevolution and macroevolution interact to shape oak success
In this review, we have somewhat artificially separated evolutionary processes within species from evolution above the species level. In fact, microevolutionary processes intergrade with and shape macroevolution (Fig. 7). Hybridization among co-occurring species within populations, for example, results in introgression of adaptive alleles among species (Leroy et al., 2019a), facilitating recolonization of habitats following displacement by climate change (Petit et al., 2003) or range extensions (Dodd & Afzal-Rafii, 2004). These migrations in turn afford oaks access to novel areas of niche space that may enable lineage diversification (Abbott et al., 2013). At the population level, high genetic and phenotypic variation within species allows for niche complementarity and high species diversity within stands even when species are not highly divergent on average (Clark, 2010; Clark et al., 2011; Hausch et al., 2018). The capacity for rapid adaptation within populations (Petit & Hampe, 2006) may in addition allow for rapid divergence between species in competitive abilities, promoting differentiation and coexistence of species at both ecological and evolutionary scales (Hart et al., 2019), as has been demonstrated previously in the American oaks (Cavender-Bares, 2019) and as we have argued here for the Eurasian oak clades (Hipp et al., 2019).
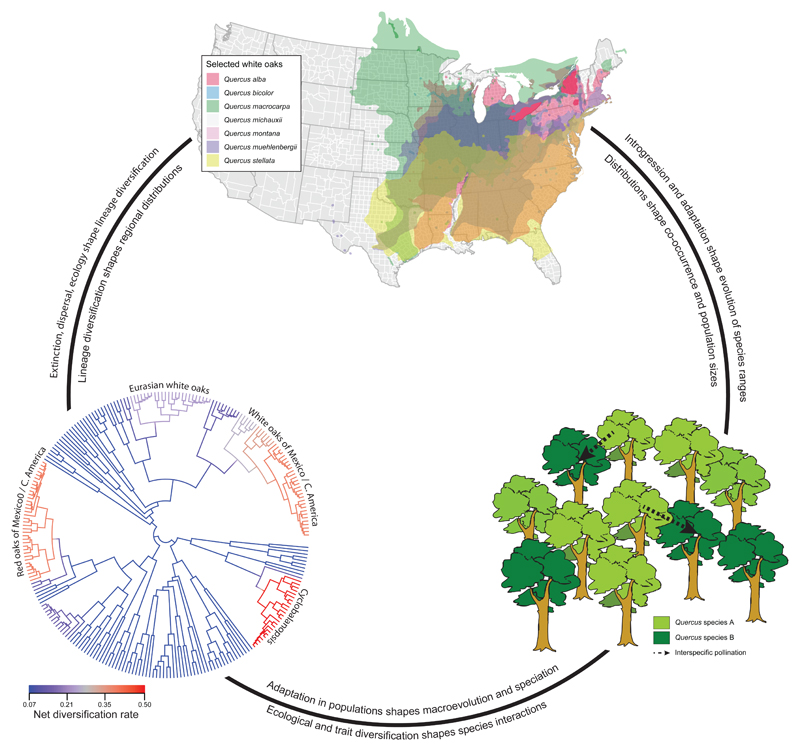
Figure 7. Microevolution and macroevolution shape one another and the success of the oaks.
The story of oak diversification and success rests on the interplay between microevolution and macroevolution. In this review, we have identified four attributes that combine in oaks to shape their diversity and abundance. Here we summarize some of the ways in which these attributes—set off in bold-face in this caption—shape interactions between microevolutionary and macroevolutionary processes in oaks, and how these in turn affect oak diversity, distributions, and abundance. High rates of evolutionary divergence within clades (bottom left panel) shape the global and regional distributions of oak diversity. Radiations within the red oaks and white oaks of America, for example, have shaped regional diversity and distributions (top panel). These distributions in turn affect genetic diversity within populations and species, and coexistence at the site level that influence rates of hybridization and introgression (bottom right panel). Species distributions are further modified by migration combined with the ability to adapt to new conditions. Introgression and natural selection at these site scales then feedback to shape species migration and adaptation to climate, which drive extinction and ecological opportunity for establishment of new populations. These forces shape diversification rates. In this way, microevolutionary dynamics shape the tree of life, and the tree of life creates the opportunity of microevolution. Figure details: Phylogeny of the world’s oaks (Hipp et al., 2019a), based on RAD-seq data, with net diversification rates by branch estimated using reversible-jump Markov chain Monte Carlo; four major increases in diversification rate are labeled (bottom left panel). A hypothetical forest stand of two species, with arrows indicating the potential for hybridization between species (bottom right panel). Range map for co-occuring eastern North American white oaks from Little (1971, 1979), adapted from Hipp et al. (2019b) (top panel). Text along the inside of the circle connecting panels indicates processes operating clockwise around circle; text along the outside indicates processes operating counterclockwise.
By the same token, macroevolution shapes microevolution, providing the backdrop of lineage diversity against which introgression, maintenance of genetic variation within species, adaptation, genetic drift and other population-level processes play out. The high lineage diversity of oaks allows them to benefit from potentially adaptive multispecies introgression (Baranski, 1975; Hardin, 1975; Curtu et al., 2007; Peñaloza-Ramírez et al., 2010; Lepais & Gerber, 2011; Eaton et al., 2015; Cannon and Petit, 2019). At the same time, trait divergence among species within clades enables close relatives to diverge ecologically and occupy a wide range of habitats, enabling high regional diversity among habitats (Cavender-Bares et al., 2004; Cavender-Bares et al., 2006), while among-clade convergence in habitat and climatic niche (Cavender-Bares et al., 2018; Hipp et al., 2018) and differentiation in ecosystem functions among clades (González-Rodríguez et al., 2019) allow distantly related oaks to co-occur, enabling high local diversity. These processes have played out in parallel in the Americas among red and white oaks, in particular, and in Eurasia among sections Ilex, Cerris, and Cyclobalanopsis, and the Eurasian white oaks of section Quercus. The diversity we observe in local oak communities is thus a product of regional diversity resulting from diversification history, filtered by ecological interactions (Ricklefs, 2004; Cavender-Bares et al., 2004).
In both the Americas and Eurasia, lineage extinctions and migrations have laid the groundwork for subsequent cladogenesis and microevolution in oaks, while cladogenesis has placed populations into novel habitats that exerted strong natural selection. The rapid crown diversification of oaks, with origin of the major Quercus clades in the first 10 million years following the origin of the genus followed by ca. 20 million years for which we have limited evidence of lineage diversification (either due to extinction or low net diversification rate) (Hipp et al., 2019), tracks the southward movement of oaks following the early Eocene climactic optimum, where their diversification was facilitated by reduced competition in the lee of receding tropical lineages (Graham, 2011; Cavender-Bares et al., 2018). Following what appears to have been a relatively even pace of diversification until the late Miocene, oaks underwent a second pulse of diversification in four young clades ca. 10 Mya: the white oaks of Mexico, the red oaks of Mexico, the white oaks of Europe, and a clade of ring-cupped oaks (sect. Cyclobalanopsis) (Fig. 7). The first three of these three diversification events reflect migrations into novel territory, either topographically heterogeneous (in the case of Mexico) or altogether lacking closely related oaks (in the case of Europe, where only sections Ilex and Cerris, with possibly some red oaks (Barrón et al., 2017), were to be found when the white oaks arrived). In the fourth, section Cyclobalanopsis, diversification appears to have been driven by climatic changes associated with the Himalayan orogeny (Deng et al., 2018). Thus cladogenesis was driven by population extinction and migrations, then drove ecological diversification among populations.
On more recent time-frames, tree species extinction at the Pliocene-Pleistocene transition had profound impacts on microevolution of Pleistocene tree populations. Extinction of tree species was severe up to the Middle Pleistocene, then dramatically reduced during more recent periods (Svenning, 2003; Magri et al., 2017). As is the case for other woody genera (Latham & Ricklefs, 1993), the fossil record indicates widespread European oak extinction beginning at the Pliocene (Barron et al., 2017). While there are no solid figures on oak species numbers at the beginning of the Quaternary, there are today about eight times as many species in Asia and in the Americas as in Europe. Late Cenozoic extinction most likely contributed to this disparity in species richness (Hubert et al., 2014). Across trees, more cold-tolerant genera, as judged by the temperature requirements of their living relatives in Europe, persisted into Pleistocene in Europe (Svenning, 2003). While the early Pleistocene extinctions may have severely constrained expansion of Quercus and other tree genera in Europe, oaks rapidly became the dominant genus across the continent in the late Pleistocene (Magri et al., 2017; Goni et al., 2018), during both glacial and interglacial periods (Tzedakis, 1994; Tzedakis et al., 2006). This appears not to have been a macroevolutionary (speciation) process, as the Eurasian species mostly predate the Pleistocene. Rather, it was a consequence of population dynamics and microevolution within the few remaining species both during the period of rapid extinctions and afterward.
Today’s European white oak species exhibit a unique integration over macroevolutionary and microevolutionary history. Their crown diversification began an estimated 10 to 15 million years ago from an Eastern North American ancestor presumably well adapted to cold, as it wended its way across the North Atlantic Landbridge. A subset of its European descendents escaped extinction during the early glacial-interglacial cycles. As severe cold constricted populations, maintenance of fecundity, genetic variation, and connectivity among populations by pollen flow would have been essential to the success of surviving oak species (Gomulkiewicz & Holt, 1995). Glacial extinctions were followed by interglacial range extensions, selecting for high migration rates and optimal exploitation of local resources. Priority effects would have been equally important. Large climatic oscillations over short evolutionary times scales, inducing alternating expansion and contraction of species ranges, would have had strong microevolutionary consequences (Dynesius & Jansson, 2000), selecting for clinal variation in dispersal ability and plasticity. Both processes have the potential to reduce speciation rates while mitigating extinction risk by increasing the rate of intraspecific gene flow and facilitating wide distributions. Whether the widely reported gene flow and plasticity in oaks was selected for by climate oscillation is unknown, but this might be tested by comparing these properties between European and North American species, where Pleistocene extinctions were reduced, and species underwent more limited expansion-contraction cycles. In either case, the Eurasian white oaks are a case history in the joint effects of macroevolution and microevolution on oak success that complements the history of American oak diversification (Hipp et al., 2018; Cavender-Bares 2019).
At the finest scales, much has been made of the role of hybridization in oak evolution, but with possible very rare exceptions—e.g., Quercus gambelii, which may be a species of hybrid origin (McVay et al., 2017b)—hybrid speciation per se (Abbott et al., 2013) has not been a source of new Quercus species and lineages. Rather, introgression of alleles from one species into another has been the predominant outcome of hybridization in oaks. The current review has pointed to evidence that interspecific gene flow in oaks, which was once viewed to be a potentially rampant force, does not seem to be homogenizing oak species (though see Ortego et al., 2018), but may facilitate adaptive gene flow at selected loci. What is less clear is how introgression affects trait evolution among species—shuttling ecologically relevant traits among close relatives, for example. Understanding how and to what degree gene flow impacts oak adaptation and diversity is a fruitful area for future research.
Our review illustrates how macroevolution and microevolution feed one another and jointly affect diversity and abundance of oaks. In some cases, constraints on macroevolution (for example extinctions) have been compensated for by microevolutionary dynamics (adaptation and migration). In others, microevolution is driven by lineage diversity and macroevolutionary legacy effects. We highlight also the peculiar role of hybridization, typically considered in the context of microevolution, but that renders the macroevolutionary history of oaks a mosaic of individual gene histories bearing different traits along different evolutionary routes. While we have based our inferences on the most strongly studied systems, we believe our conclusions generalize well to oaks as a whole (Box 1). In the end, understanding oak diversity, abundance, and distributions demands a synthetic approach in which macroevolutionary and microevolutionary forces are considered in close relation to one another.
Box 1. Generality of oak study systems, and research gaps.
Our review of microevolutionary processes is dominated by results and conclusions from the two European white oaks (Q. petraea and Q. robur), as they have been investigated longer than any other species, and insights into these two species have done much to inform our understanding of temperate tree speciation and population dynamics. Conversely, macroevolution in oaks has until quite recently received more attention in North America than in Asia or Europe, though recent work (e.g., Deng et al., 2018; Jiang et al, 2019), including a paper in the current volume (Hipp et al., 2019a), does much to amend this. What can we learn from these case studies regarding other congeneric species? What research gaps should be addressed in the future, and what new research avenues have become available?
The European story has demonstrated the impact of postglacial colonization on the distribution and pace of adaptive diversity and divergence. However, with recent phylogeographical and palynological investigations in an increasing number of oak species (Vitelli et al, 2017; Sork et al., 2016b; Muellner-Riehl, 2019), it is becoming clear that the clean relationship between postglacial migration and genetic diversity seen in the European white oaks is not universal. In the widespread temperate European and Asian species, postglacial migration occurred over very large distances starting from a reduced number of refugial areas, resulting in a relatively tight correlation between geography and chloroplast diversity, as reviewed in part one of this review. In the North Eastern American and Mexican species case, however, refugial populations were more diffuse and some persisted closer to the glacial margin (McLachlan et al., 2005). As a consequence, chloroplast variation in North American white oaks is structured geographically, but apparently only at very large scales, entailing separation between the western and eastern species but not obviously among regions within eastern North America (Pham et al., 2017). This suggests that the scale of migration was smaller and mixing among refugial populations larger in eastern North American than in Europe. Migration range was also limited in Californian (Sork et al., 2016b) and Mediterranean species (Vitelli et al., 2017). It remains to be seen what evolutionary legacies persist from the European and American migration histories regarding ecological divergence and adaptation. It is also not yet clear whether the history of recent secondary contact between European species (Leroy et al., 2017, 2019a) played out the same way in the Americas. More multispecies, geographically widespread samples will be needed in America, paralleling the work done in Europe, to test whether these alternative histories led to different timings in species contacts. Recent and ongoing work in the eastern North American sections Virentes (Cavender-Bares et al., 2015) and Quercus (Hipp et al., 2019b; Garner et al., 2019), the western North American sections Quercus and Protobalanus (Sork et al., 2016b), and widespread Mexican oaks of sections Quercus and Lobatae (Rodriguez-Correa et al., 2017) are particularly promising in this regard.
The effects of introgression on both migration (Petit et al., 2003) and adaptation (Leroy et al., 2019a) in the European white oaks are probably echoed in other clades and areas. Indirect evidence of large scale introgression comes from the chloroplast genome sharing that has been shown in many sympatric oaks (Whittemore & Schaal, 1991; Pham et al., 2017), which, in combination with coherence at nuclear loci, demonstrates that introgression is strong and likely a long-term phenomenon in oaks, but that selection is strong enough to counterbalance introgression and maintain species genetically. A strong argument for introgression-driven migration requires a demonstration also of asymmetric gene flow (as has been shown, for example, in section Virentes; Eaton et al., 2015) and different colonization dynamics between the species crossing, with one species dispersing and establishing better by seed than the other, i.e. being more of a pioneer species. Regarding adaptive introgression, the availability of reference genomes in the white oaks (Sork et al., 2016a; Plomion et al., 2018) and the existence of common garden experiments for the same species should facilitate the detection of adaptive introgression, complementing landscape genomic approaches that have already demonstrated differential levels of introgression across loci associated with ecological variation (Dodd and Afzal-Rafii, 2004) and gene function (Oney-Birol et al., 2018). A challenging and stimulating avenue of research would be a global comparative study of which portions of the genome of sympatric species introgress, in a climatic and ecological context (cf. Hipp et al., 2019a). Such a synthetic study is within grasp with new genomic and analytical tools.
Finally, a striking feature in European oaks is the large genetic variation in phenological responses to latitudinal or altitudinal temperature gradients shared among species. Equally striking is the opposing genetic cline found in common garden studies between European white oaks, in which populations originating from higher latitudes or elevation flush later than populations from lower latitude or elevation (Firmat et al., 2017), and North American red oaks, in which northern populations flush earlier in the common garden (McGee, 1974; Kriebel et al., 1976). The timing of bud burst is a highly integrated trait closely correlated to reproduction and growth and consequently to fitness and responding to temperature variation. These contrasting patterns observed in a limited number of species should stimulate extended investigations of a larger number of species and populations encompassing contrasting temperature gradients. Such comparative research would ultimately help clarify the role of genetic variation in phenological traits for adaptation to ongoing climate change.
Conclusion
And so we return to the question with which we started: what makes the genus Quercus an evolutionary success? In this review, at both microevolutionary and macroevolutionary scales, we find at least four overarching explanations for the evolutionary success of oaks:
1. High diversity within populations and species. Genetic and phenotypic diversity within species increase the adaptive potential of species and facilitate coexistence of species.
2. Rapid migration combined with the ability to adapt to new conditions. As documented by palynological reconstruction during the late Pleistocene, rapid migration was essential for oak expansion during interglacial periods. It was likely as important during periods of oak diversification, during the late Miocene.
3. High rates of ecological divergence within clades, combined with convergent solutions to ecological problems across clades. Exceptionally well documented in the Americas, the combination of divergence within and convergence among clades appears to be characteristic of the European oak radiations as well and may be key to the high diversity of oaks across the three continents.
4. Hybridization and introgression. Gene flow in oaks is a largely creative force, increasing diversity, moving adaptive alleles between species, and serving as a migratory mechanism. While phylogenetic reconstruction has shown how introgression contributed to the diversification of oak genomes across species, this review also demonstrates how introgression has enhanced adaptation and stimulated migration.
While these attributes are individually shared with other tree genera, their combined effects together in oaks uniquely explain the evolutionary success of Quercus (Box 2). These explanations are presented here as drivers of evolution, but they may also be understood as evolutionary outcomes. Whether genetic diversity, migration rate, evolvability, and capacity for introgressive hybridization have actually been selected for remains to be investigated. There may be other emergent or aggregate species attributes that contribute equally to lineage diversification rates and form linkages between microevolutionary and macroevolutionary dynamics: population size, geographic range, plasticity, and ecological range all have the potential to influence species coexistence and the degree to which species can exchange genes, and thus shape patterns of lineage diversification. Demonstrating that such attributes may be driven by macroevolution would require that they respond to selection at a species level, suggesting investigations aimed at estimating phylogenetic heritability and effects on diversification rates, or fitness expressed at the species level (Rabosky & McCune, 2010). Range size, for example, appears to be phylogenetically heritable in the American oaks (Hipp, 2017), suggesting that the diversification of the clade shapes a key demographic attribute that governs patterns of sympatry and consequently may shape rates of hybridization and speciation. More nuanced investigations of species selection in oaks have the potential to demonstrate how macroevolution and microevolution operate jointly to shape diversification.
Box 2. Oaks: unique among tree genera?
We point to four non-mutually exclusive potential causes of oak evolutionary success: high genetic diversity, rapid migration rates, high rates of ecological divergence, and propensity for hybridization and introgression. Are these attributes peculiar to oaks or are they shared with other woody genera? High genetic diversity is frequent in trees and has been argued to be a major cause of their rapid microevolution (Petit & Hampe, 2006), but recent results based on whole genome sequences suggest that Quercus has accumulated even larger levels than is typical of forest trees generally (Plomion et al., 2018). Maintenance of high genetic diversity is likely an outcome of life history traits that convergently manifest in large population sizes in unrelated woody plant lineages, as discussed in the main text. Regarding rapid migration, comparative analysis of postglacial spread of woody species in Europe suggests that oak migration rates were similar to and sometimes exceeded rates of such pioneer genera as Alnus and Corylus (alders and hazelnuts; Brewer et al., 2005; Giesecke et al., 2017). Similar comparative analysis in North American species is less convincing (Harnik et al., 2018) although Davis (1983) in her earlier work on the late Pleistocene spread of trees underpins the rapid migration of oaks: “Oak range expansion was rapid, more rapid than any of the deciduous trees that today grow in major forests” (Davis, 1983 p. 558). The Asian picture also emphasizes “the remarkably fast spread of Quercus from Southern China to the North East rim of the Tibetan Plateau” in comparison to other species (Cao et al. 2015, p. 89).
Across a number of forest tree genera, ecological shifts may contribute to biodiversity and biomass. Bursera for example exhibits convergent transitions from seasonal dry tropical forest to xerophytic scrub and oak forest (De-Nova et al., 2012) that may explain some degree of the diversity of the clade across biomes. Inga exhibits high species diversity within sites that has been shown to be associated with exceptionally high diversification in antiherbivore defenses (Kursar et al., 2009). The temperate shrub genus Viburnum exhibits increases in diversification rate in association with increased rates of transition among forest types and colonization of mountainous regions (Spriggs et al., 2015). The effect of ecological diversification on diversity of oaks, however, is exceptional among tree genera, as are the effects of biogeographic transitions and convergence on rates of lineage diversification (Hipp et al., 2018, 2019a) and species coexistence over long periods (Cavender-Bares et al., 2004, 2018). The closest analogue to Quercus in the temperate Americas, Pinus—the tree genus with the second highest biodiversity in the U.S. and the second highest biomass in both the U.S. and Mexico, after Quercus (Cavender-Bares 2016, 2019)—exhibits differentiation of clades by fire tolerance, but not the simultaneous parallel diversification that shaped the diversity of Quercus (He et al., 2012; Keeley, 2012).
Similarly, the contribution of introgression to adaptation (Suarez-Gonzalez et al., 2018) has been demonstrated in Populus (Chhatre et al., 2018) and Cupressus (Ma et al., 2019), and the role of hybridization in species expansion was earlier shown in Eucalyptus (Potts & Reid, 1990). Introgression has also been demonstrated to lead to transgressive segregation in Picea (spruce), allowing for adaptive gene flow that is expressed in the second generation and potentially later (Hamilton & Miller, 2016). Increasingly, introgression appears to be widespread among forest trees, especially in the tropics (Cannon & Lerdau, 2015; Caron et al., 2019). It is however the widespread hybridization within each of the different oak clades recognized as sections (Denk et al., 2017), supported by widespread choroplast capture and nuclear population-level studies reported in the main text, that suggests that introgression may have significantly contributed to migration and coexistence of oak species.
While some of the micro- and macroevolutionary explanations we have offered for the global success of oaks might individually be shared with other genera, it is likely that oaks possess a unique suite of attributes. Additionally, and not mentioned previously in this review, one cannot dismiss the minor but locally and temporally significant role of early human populations on the maintenance and spread of oak forests (Kremer, 2015; Chassé, 2016). Anthropogenic fire alone has greatly shaped the forest composition of North America, favoring oaks throughout the vast prairie province and bordering regions (Kline, 1997; Abrams, 1992). More generally, the exceptional diversity of services oaks provide for food, shelter, medicine, and culture make it highly likely that human activities have in many contexts favored oaks (Logan 2006) and shaped their evolutionary trajectories (Toumi & Lumaret, 1998).
Our review also points to the potential insights to be gained from comparative analysis of intraspecific selection patterns across clades, investigating parallel microevolutionary dynamics in different species, habitats, and continents. Candidate traits for comparative analysis include phenology, growth rates, and leaf morphology, traits that have already been investigated in separate studies in different species. An immediate application would be to test whether gradients of selection are of similar magnitude and direction in species distributed along similar environmental clines. At the same time, these comparative studies, paired with macroevolutionary studies of selection at the clade level, may cast light on the biological underpinnings of variation in evolutionary rates at different scales of inquiry, on different continents, and among clades.
Finally, this review provides insights into contemporary evolutionary challenges that oaks face in the context of environmental changes. What lessons did we gain from the past that may enlighten ongoing processes sustaining the evolutionary success of oaks? Migration triggered by climatic variation has already been reported for Mediterranean species, and it is likely that this trend will continue despite constraints generated by land management (Delzon et al., 2013). Species shifts are expected to increase sympatry between Mediterranean and temperate oaks, potentially triggering hybridization and most likely adaptive introgression as well. But what will happen at the extreme southern margin? This and related questions will require long term monitoring and research. Ongoing climate change thus offers a unique opportunity to disentangle evolutionary processes in action at contemporary time scales.
Acknowledgements
This review assembles results accumulated over the last decades within the oaks biology scientific community that has steadily increased. It benefited from discussions and cooperation with colleagues from Europe, North America and Asia, whom we acknowledge for their insights and inputs: Hermine Alexandre, Catherine Bodénès, Chuck Cannon, Jeannine Cavender-Bares, Richard Cronn, Andrew Crowl, Lucian Curtu, Sylvain Delzon, Min Deng, Thomas Denk, Alexis Ducousso, Jean Luc Dupouey, Deren Eaton, Elisabeth Fitzek, Alain Franc, Mira Garner, Sorel Fitz-Gibbon, Oliver Gailing, Pablo Goicoechea, M. Socorro González-Elizondo, Santiago Gonzalez-Martinez, Antonio González-Rodríguez, Guido Grimm, Felix Gugerli, Marlene Hahn, Thomas Hansen, Sean Hoban, Xiaolong Jiang, Matthew Kaproth, Martin Lascoux, Myriam Legay, Thibault Leroy, Paul Manos, John McVay, Brigitte Musch, Ian Pearse, Rémy Petit, Csaba Matyas, Kasey Pham, Christophe Plomion, Annabel Porte, Hernando Rodríguez-Correa, Jeanne Romero-Severson, Cuauhtemoc Saenz-Romero, Dounia Saleh, Victoria Sork, Susana Valencia Avalos, Stéfanie Wagner, Jaime Weber, Alan Whittemore, and Lindsey Worcester. We thank Lucie Kremer and Annabel Porte for their assistance during the preparation of the manuscript. We are grateful to J. Cavender-Bares and two anonymous referees for their very insightful reviews and helpful suggestions, which we consider to have significantly improved the manuscript.
This review was supported by grants from the U.S. National Science Foundation (Award # 1146488), The Morton Arboretum Center for Tree Science, a Fulbright Fellowship to AH funded by the Franco-American Commission, and grants from the European Union (ERC Advanced grant Treepeace #339728) and the French National Research Agency (ANR) within the Labex COTE (ANR-10-LABX-45).
References
- Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N, Boughman JW, Brelsford A, Buerkle CA, Buggs R, et al. Hybridization and speciation. Journal of Evolutionary Biology. 2013;26(2):229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- Abrams MD. Fire and the devlopment of oak forest. Bioscience. 1992;42:346–353. [Google Scholar]
- Ainsworth EA, Tranel PJ, Drake BG, Long SP. The clonal structure of Quercus geminata revealed by conserved microsatellite loci. Molecular Ecology. 2003;12(2):527–532. doi: 10.1046/j.1365-294x.2003.01749.x. [DOI] [PubMed] [Google Scholar]
- Alberto F, Bouffier L, Louvet JM, Lamy JB, Delzon S, Kremer A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. Journal of Evolutionary Biology. 2011;24(7):1442–1454. doi: 10.1111/j.1420-9101.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- Alberto FJ, Derory J, Boury C, Frigerio JM, Zimmermann NE, Kremer A. Imprints of natural selection along environmental gradients in phenology related genes of Quercus petraea. Genetics. 2013;195(2):495–512. doi: 10.1534/genetics.113.153783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Woeste K. Pollen gene flow, male reproductive success, and genetic correlations among offspring in a northern red oak (Quercus rubra L.) seed orchard. Plos One. 2017;12(2) doi: 10.1371/journal.pone.0171598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre H, Truffaut L, Ducousso A, Louvet JM, Nepveu G, Torres-Ruiz JM, Lagane F, Firmat C, Musch B, Delzon S, et al. In situ estimation of genetic variation of functional and ecological traits in Q. petraea and Q.robur. Tree Genetics & Genomes. 2019 doi: 10.1007/s11295-019-1407-9. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M, Deng M, Zheng SS, Jiang XL, Song YG. Introgression threatens the genetic diversity of Quercus austrocochinchinensis (Fagaceae), an Endangered Oak: A Case Inferred by Molecular Markers. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. Introgressive hybridization. New York: John Wiley & Sons, Inc; 1949. [Google Scholar]
- Arnold ML. Divergence with genetic exchange. Oxford: Oxford University Press; 2016. [Google Scholar]
- Ashley MV, Backs JR, Kindsvater L, Abraham ST. Genetic variation and structure in an endemic island oak, Quercus tomentella and mainland canyon oak, Quercus chrysolepis. International Journal of Plant Sciences. 2018;179(2):151–161. [Google Scholar]
- Austerlitz F, Garnier-Gere PH. Modelling the impact of colonisation on genetic diversity and differentiation of forest trees: interaction of life cycle, pollen flow and seed long-distance dispersal. Heredity. 2003;90(4):282–290. doi: 10.1038/sj.hdy.6800243. [DOI] [PubMed] [Google Scholar]
- Austerlitz F, Mariette S, Machon N, Gouyon PH, Godelle BL. Effects of colonization processes on genetic diversity: Differences between annual plants and tree species. Genetics. 2000;154(3):1309–1321. doi: 10.1093/genetics/154.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod DI. Biogeography of oaks in the Arcto-tertiary province. Annals of the Missouri Botanical Garden. 1983;70(4):629–657. [Google Scholar]
- Backs JR, Terry M, Klein M, Ashley MV. Genetic analysis of a rare isolated species: A tough little West Texas oak, Quercus hinckleyi CH Mull. Journal of the Torrey Botanical Society. 2015;142(4):302–313. [Google Scholar]
- Baliuckas V, Pliura A. Genetic variation and phenotypic plasticity of Quercus robur populations and open-pollinated families in Lithuania. Scandinavian Journal of Forest Research. 2003;18(4):305–319. [Google Scholar]
- Baranski MJ. An analysis of variation within white oak (Quercus alba L.) Raleigh: North Carolina Agricultural Experimental Station; 1975. [Google Scholar]
- Barrón E, Averyanova A, Kvacek Z, Momohara A, Pigg KB, Popova S, Maria Postigo-Mijarra J, Tiffney BH, Utescher T, Zhou ZK. The Fossil History of Quercus. In: Gil-Pelegrin E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological Ecology. Exploring the functional diversity of the genus Quercus L. Springer; 2017. pp. 39–105. [Google Scholar]
- Bastide P, Solís-Lemus C, Kriebel R, William Sparks K, Ané C. Phylogenetic Comparative Methods on Phylogenetic Networks with Reticulations. Systematic Biology. 2018;67:800–820. doi: 10.1093/sysbio/syy033. [DOI] [PubMed] [Google Scholar]
- Beatty GE, Montgomery WI, Spaans F, Tosh DG, Provan J. Pure species in a continuum of genetic and morphological variation: sympatric oaks at the edge of their range. Annals of Botany. 2016;117(4):541–549. doi: 10.1093/aob/mcw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialozyt R, Ziegenhagen B, Petit RJ. Contrasting effects of long distance seed dispersal on genetic diversity during range expansion. Journal of Evolutionary Biology. 2006;19(1):12–20. doi: 10.1111/j.1420-9101.2005.00995.x. [DOI] [PubMed] [Google Scholar]
- Bossema I. Jays and oaks. Eco-ethological study of a symbiosis. Behaviour. 1979;70:1–117. [Google Scholar]
- Brendel O, Le Thiec D, Scotti-Saintagne C, Bodenes C, Kremer A, Guehl JM. Quantitative trait loci controlling water use efficiency and related traits in Quercus robur L. Tree Genetics & Genomes. 2008;4(2):263–278. [Google Scholar]
- Brewer S, Cheddadi R, de Beaulieu JL, Reille M, Data Contributors The spread of deciduous Quercus throughout Europe since the last glacial period. Forest Ecology and Management. 2002;156(1-3):27–48. [Google Scholar]
- Brewer S, Hely-Alleaume C, Cheddadi R, De Beaulieu JL, Laurent JM, Cuziat J. Postglacial history of Atlantic oakwoods: context, dynamics and controlling factors. Botanical Journal of Scotland. 2005;57:41–57. [Google Scholar]
- Burger WC. The species concept in Quercus. Taxon. 1975;24:45–50. [Google Scholar]
- Buschbom J, Yanbaev Y, Degen B. Efficient long-distance gene flow into an isolated relict oak stand. Journal of Heredity. 2011;102(4):464–472. doi: 10.1093/jhered/esr023. [DOI] [PubMed] [Google Scholar]
- Caignard T, Delzon S, Bodenes C, Dencausse B, Kremer A. Heritability and genetic architecture of reproduction-related traits in a temperate oak species. Tree Genetics & Genomes. 2019;15(1) doi: 10.1007/s11295-018-1309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A. Les chênes. Monographie du genre Quercus. Tome I. Genre Quercus. Sous-genre Cyclobalanopsis et sous-genre Euquercus (Section Cerris et Mesobalanus) Paris: Editions Paul Lechevalier; 1936. [Google Scholar]
- Camus A. Les chênes. Monographie du genre Quercus. Tome II. Genre Quercus. Sous-genre Euquercus (Section Lepidobalanus et Macrobalanus) Paris: Editions Paul Lechevalier; 1938. [Google Scholar]
- Camus A. Monographie du genre Lithocarpus. Paris: Editions Paul Lechevalier; 1952. Les chênes. Monographie du genre Quercus. Tome III. Genre Quercus. Sous-genre Euquercus (Section Protobalanus et Erythrobalanus) [Google Scholar]
- Cannon CH, Lerdau M. Variable mating behaviors and the maintenance of tropical biodiversity. Frontiers in Genetics. 2015;6:183. doi: 10.3389/fgene.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CH, Petit RJ. The oak syngameon : more than the sum of its parts. New Phytologist. 2019 doi: 10.1111/nph.16091. (this issue) [DOI] [PubMed] [Google Scholar]
- Cao X, Herzschuh U, Ni J, Zhao Y, Böhmer T. Spatial and temporal distribution of major tree taxa in eastern continental Asia during the last 22000 Years. The Holocene. 2015;25:79–91. [Google Scholar]
- Caron H, Molino J-F, Sabatier D, Léger P, Chaumeil P, Scotti-Saintagne C, Frigério J-M, Scotti I, Franc A, Petit RJ. Chloroplast DNA variation in a hyperdiverse tropical tree community. Ecology and Evolution. 2019;9:4897–4905. doi: 10.1002/ece3.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Mendoza E, Salinas-Sanchez D, Valencia-Cuevas L, Zamilpa A, Tovar-Sanchez E. Natural hybridisation among Quercus glabrescens, Q. rugosa and Q. obtusata (Fagaceae): Microsatellites and secondary metabolites markers. Plant Biology. 2019;21(1):110–121. doi: 10.1111/plb.12899. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytologist. 2019;221(2):669–692. doi: 10.1111/nph.15450. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. American Naturalist. 2004;163(6):823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Ackerly D, Baum D, Bazzaz F. Diversity, distribution, and ecosystem services of the North American oaks. International Oaks: The Journal of the International Oak Society. 2016b;27:37–48. [Google Scholar]
- Cavender-Bares J, Ackerly DD, Hobbie SE, Townsend PA. Evolutionary legacy effects on ecosystems: biogeographic origins, plant traits and implications for management in the Era of global change. Annu Rev Ecol Evol Syst. 2016c;47:433–462. [Google Scholar]
- Cavender-Bares J, Gonzalez-Rodriguez A, Eaton DAR, Hipp AAL, Beulke A, Manos PS. Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): a genomic and population genetics approach. Molecular Ecology. 2015;24(14):3668–3687. doi: 10.1111/mec.13269. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Keen A, Miles B. Phylogenetic structure of floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87(7):S109–S122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Kothari S, Meireles JE, Kaproth MA, Manos PS, Hipp AL. The role of diversification in community assembly of the oaks (Quercus L.) across the continental U.S. American Journal of Botany. 2018;105(3):565–586. doi: 10.1002/ajb2.1049. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Meireles JE, Couture JJ, Kaproth MA, Kingdon CC, Singh A, Serbin SP, Center A, Zuniga E, Pilz G, et al. Associations of leaf spectra with genetic and phylogenetic variation in oaks: prospects for remote detection of biodiversity. Remote Sensing. 2016a;8(3) [Google Scholar]
- Cavender-Bares J, Pahlich A. Molecular, morphological and ecological niche differentiation of sympatric sister oak species, Quercus virginiana and Q. geminata (Fagaceae) American Journal of Botany. 2009;96(9):1690–1702. doi: 10.3732/ajb.0800315. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Ramirez-Valiente JA. Physiological evidence from common garden experiments for local adaptation and adaptive plasticity to climate in American Live Oaks (Quercus Section Virentes): implications for conservation under global change. In: Gil-Pelegrin E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological Ecology. Exploring the functional diversity of the genus Quercus L. Springer; 2017. pp. 107–135. [Google Scholar]
- Chassé B. Eating acorns: what story do the distant, far, and near past tell us, and why? International Oaks: The Journal of the International Oak Society. 2016;27:107–135. [Google Scholar]
- Chhatre VE, Evans LM, DiFazio SP, Keller SR. Adaptive introgression and maintenance of a trispecies hybrid complex in range-edge populations of Populus. Molecular Ecology. 2018;27(23):4820–4838. doi: 10.1111/mec.14820. [DOI] [PubMed] [Google Scholar]
- Clark JS. Individuals and the Variation Needed for High Species Diversity in Forest Trees. Science. 2010;327(5969):1129–1132. doi: 10.1126/science.1183506. [DOI] [PubMed] [Google Scholar]
- Clark JS, Bell DM, Hersh MH, Kwit MC, Moran E, Salk C, Stine A, Valle D, Zhu K. Individual-scale variation, species-scale differences: inference needed to understand diversity. Ecology Letters. 2011;14(12):1273–1287. doi: 10.1111/j.1461-0248.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- Cottam WP, Tucker JM, Santamour FS. Oak hybridization at the University of Utah. Salt Lake City: State Arboretum of Utah; 1982. [Google Scholar]
- Craft KJ, Ashley MV. Landscape genetic structure of bur oak (Quercus macrocarpa) savannas in Illinois. Forest Ecology and Management. 2007;239(1–3):13–20. [Google Scholar]
- Craft KJ, Ashley MV. Pollen mediated gene flow in isolated and continuous stands of bur oak (Quercus macrocarpa (Fagaceae) American Journal of Botany. 2010;97(12):1999–2006. doi: 10.3732/ajb.0900390. [DOI] [PubMed] [Google Scholar]
- Craft KJ, Brown JS, Golubski AJ, Ashley MV. A model for polyandry in oaks via female choice: a rigged lottery. Evolutionary Ecology Research. 2009;11(3):471–481. [Google Scholar]
- Crowl AA, McVay JD, Manos PS, Hipp AL, Lemmon A, Lemmon E. Uncovering the genomic signature of ancient introgression between white oak lineages (Quercus) using anchored enrichment. New Phytologist (this issue) 2019 doi: 10.1111/nph.15842. [DOI] [PubMed] [Google Scholar]
- Currat M, Ruedi M, Petit RJ, Excoffier L. The hidden side of invasions: Massive introgression by local genes. Evolution. 2008;62(8):1908–1920. doi: 10.1111/j.1558-5646.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- Curtu AL, Gailing O, Finkeldey R. Evidence for hybridization and introgression within a species-rich oak (Quercus spp.) community. BMC Evolutionary Biology. 2007;7 doi: 10.1186/1471-2148-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubree JB, Kremer A. Genetic and phenological differentiation between introduced and natural populations of Quercus rubra L. Annales Des Sciences Forestières. 1993;50(Suppl 1):271s–280s. [Google Scholar]
- Davis MB. Quaternary history of deciduous forests of Eastern North America and Europe. Annals of Missouri Botanical Garden. 1983;70(3):550–563. [Google Scholar]
- De Beaulieu A, Lamant T. Guide illustré des chênes. Geers, Belgium: Edilens; 2010. [Google Scholar]
- De Heredia UL, Carrion JS, Jimenez P, Collada C, Gil L. Molecular and palaeoecological evidence for multiple glacial refugia for evergreen oaks on the Iberian Peninsula. Journal of Biogeography. 2007;34(9):1505–1517. [Google Scholar]
- Delzon S, Urli M, Samalens JC, Lamy JB, Lischke H, Sin F, Zimmermann NE, Porte AJ. Field evidence of colonisation by Holm Oak, at the Northern Margin of Its Distribution Range, during the Anthropocene Period. Plos One. 2013;8(11) doi: 10.1371/journal.pone.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Zhou Z, Li Q. Taxonomy and systematics of Quercus subgenus Cyclobalanopsis. International Oaks: The Journal of the International Oak Society. 2013;24:48–60. [Google Scholar]
- Deng M, Jiang XL, Hipp AL, Manos PS, Hahn M. Phylogeny and biogeography of East Asian evergreen oaks (Quercus section Cyclobalanopsis; Fagaceae): Insights into the Cenozoic history of evergreen broad-leaved forests in subtropical Asia. Molecular Phylogenetics and Evolution. 2018;119:170–181. doi: 10.1016/j.ympev.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Denk T, Grimm GW. The oaks of western Eurasia: Traditional classifications and evidence from two nuclear markers. Taxon. 2010;59(2):351–366. [Google Scholar]
- Denk T, Grimm GW, Manos PS, Deng M, Hipp AL. An updated infrageneric classification of the oaks: review of previous taxonomic schemes and synthesis of evolutionary patterns. In: Gil-Pelegrin E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological Ecology. Exploring the functional diversity of the genus Quercus L. Springer; 2017. pp. 13–38. [Google Scholar]
- Denk T, Grimsson F, Zetter R. Episodic migration of oaks to Iceland: Evidence for a North Atlantic ‘land bridge’ in the latest Miocene. American Journal of Botany. 2010;97:276–287. doi: 10.3732/ajb.0900195. [DOI] [PubMed] [Google Scholar]
- De-Nova JA, Medina R, Montero JC, Weeks A, Rosell JA, Olson ME, Eguiarte LE, Magallón S. Insights into the historical construction of species-rich Mesoamerican seasonally dry tropical forests: the diversification of Bursera (Burseraceae, Sapindales) New Phytologist. 2012;193:276–287. doi: 10.1111/j.1469-8137.2011.03909.x. [DOI] [PubMed] [Google Scholar]
- Dicke SG, Bagley WT. Variability of Quercus macrocarpa Michx in an eastern Nebraska provenance study. Silvae Genetica. 1980;29(5–6):171–176. [Google Scholar]
- Diskin M, Steiner KC, Hebard FV. Recovery of American chestnut characteristics following hybridization and backcross breeding to restore blight-ravaged Castanea dentata. Forest Ecology and Management. 2006;223(1–3):439–447. [Google Scholar]
- Dodd RS, Afzal-Rafii Z. Selection and dispersal in a multispecies oak hybrid zone. Evolution. 2004;58(2):261–269. [PubMed] [Google Scholar]
- Dow BD, Ashley MV. Microsatellite analysis of seed dispersal and parentage of saplings in bur oak, Quercus macrocarpa. Molecular Ecology. 1996;5(5):615–627. [Google Scholar]
- Dow BD, Ashley MV. High levels of gene flow in bur oak revealed by paternity analysis using microsatellites. Journal of Heredity. 1998;89(1):62–70. [Google Scholar]
- Ducousso A, Guyon JP, Kremer A. Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt) Liebl) Annales Des Sciences Forestières. 1996;53(2–3):775–782. [Google Scholar]
- Dumolin-Lapègue S, Demesure B, Fineschi S, Le Corre V, Petit RJ. Phylogeographic structure of white oaks throughout the European continent. Genetics. 1997;146(4):1475–1487. doi: 10.1093/genetics/146.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynesius M, Jansson R. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(16):9115–9120. doi: 10.1073/pnas.97.16.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DAR, Hipp AL, Gonzalez-Rodriguez A, Cavender-Bares J. Historical introgression among the American live oaks and the comparative nature of tests for introgression. Evolution. 2015;69(10):2587–2601. doi: 10.1111/evo.12758. [DOI] [PubMed] [Google Scholar]
- Eberle JJ, Greenwood DR. Life at the top of the greenhouse Eocene world-A review of the Eocene flora and vertebrate fauna from Canada's High Arctic. Geological Society of America Bulletin. 2012;124(1–2):3–23. [Google Scholar]
- Ellegren H, Galtier N. Determinants of genetic diversity. Nature Reviews Genetics. 2016;17(7):422–433. doi: 10.1038/nrg.2016.58. [DOI] [PubMed] [Google Scholar]
- Ferrari L, Orozco-Esquivel T, Manea V, Manea M. The dynamic history of the Trans-Mexican Volcanic Belt and the Mexico subduction zone. Tectonophysics. 2012;522:122–149. [Google Scholar]
- Firmat C, Delzon S, Louvet JM, Parmentier J, Kremer A. Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. Journal of Evolutionary Biology. 2017;30(12):2116–2131. doi: 10.1111/jeb.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Hipp AL, Pham KK, Manos PS, Sork VL. Phylogenomic inferences from reference-mapped and de novo assembled short-read sequence data using RADseq sequencing of California white oaks (Quercus subgenus Quercus) Genome. 2017;30:125–130. doi: 10.1139/gen-2016-0202. [DOI] [PubMed] [Google Scholar]
- Gandour M, Khouja ML, Toumi L, Triki S. Morphological evaluation of cork oak (Quercus suber): Mediterranean provenance variability in Tunisia. Annals of Forest Science. 2007;64(5):549–555. [Google Scholar]
- Garner M, Pham KK, Whittemore AT, Cavender-Bares J, Gugger PF, Manos PS, Pearse IS, Hipp AL. From Manitoba to Texas: A study of the population genetic structure of bur oak (Quercus macrocarpa) International Oaks: The Journal of the International Oak Society. 2019;30:131–138. [Google Scholar]
- Giesecke T. Did thermophilous trees spread into central Europe during the Late Glacial? New Phytologist. 2016;212(1):15–18. doi: 10.1111/nph.14149. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Brewer S. Notes on the postglacial spread of abundant European tree taxa. Vegetation History and Archaeobotany. 2018;27(2):337–349. [Google Scholar]
- Giesecke T, Brewer S, Finsinger W, Leydet M, Bradshaw RHW. Patterns and dynamics of European vegetation change over the last 15,000years. Journal of Biogeography. 2017;44(7):1441–1456. [Google Scholar]
- Gingerich PD. Rates of evolution on the time scale of the evolutionary process. Genetica. 2001;112:127–144. [PubMed] [Google Scholar]
- Gomulkiewicz R, Holt RD. When does evolution by natural selection prevent extinction. Evolution. 1995;49(1):201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Goni MFS, Desprat S, Fletcher WJ, Morales-Molino C, Naughton F, Oliveira D, Urrego DH, Zorzi C. Pollen from the deep-sea: A breakthrough in the mystery of the Ice Ages. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rodríguez A, Garcia-Oliva F, Tapie-Torres Y, Moron-Cruz A, Chavez-Vergara B, Baca-Patino B, Cuevas-Reyes P. Oak community diversity affects nitrogen in litter and soil. International Oaks: The Journal of the International Oak Society. 2019;30:125–130. [Google Scholar]
- Graham A. The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. American Journal of Botany. 2011;98:336–351. doi: 10.3732/ajb.1000353. [DOI] [PubMed] [Google Scholar]
- Greenwood DR, Pigg KB, Basinger JF, DeVore ML. A review of paleobotanical studies of the Early Eocene Okanagan (Okanogan) Highlands floras of British Columbia, Canada, and Washington, USA. Canadian Journal of Earth Sciences. 2016;53(6):548–564. [Google Scholar]
- Grimsson F, Grimm GW, Zetter R, Denk T. Cretaceous and Paleogene Fagaceae from North America and Greenland: evidence for a late Cretaceous split between Fagus and the remaining Fagaceae. Acta Palaeobotanica. 2016;56:247–305. [Google Scholar]
- Grivet D, Deguilloux MF, Petit RJ, Sork VL. Contrasting patterns of historical colonization in white oaks (Quercus spp.) in California and Europe. Molecular Ecology. 2006;15(13):4085–4093. doi: 10.1111/j.1365-294X.2006.03083.x. [DOI] [PubMed] [Google Scholar]
- Guichoux E, Garnier-Gere P, Lagache L, Lang T, Boury C, Petit RJ. Outlier loci highlight the direction of introgression in oaks. Molecular Ecology. 2013;22(2):450–462. doi: 10.1111/mec.12125. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Miller JM. Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conservation Biology. 2016;30:33–41. doi: 10.1111/cobi.12574. [DOI] [PubMed] [Google Scholar]
- Hansen TF. Stabilizing selection and the comparative analysis of adaptation. Evolution. 1997;51(5):1341–1351. doi: 10.1111/j.1558-5646.1997.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Hansen TF, Pienaar J, Orzack SH. A comparative method for studying adaptation to a randomly evolving environment. Evolution. 2008;62(8):1965–1977. doi: 10.1111/j.1558-5646.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- Hardin JW. Hybridization and introgression in Quercus alba. Journal of the Arnold Arboretum. 1975;56(3):336–363. [Google Scholar]
- Harada K, Dwiyabti FG, Liu HZ, Takeichi Y, Nakatami N, Kamiya K. Genetic variation and strcuture of Ubame oak (Quercus phillyraeoides, in Japan revealed by chloroplast DNA and nuclear microsatellite markers. Genes Genet Syst. 2018;93:37–50. doi: 10.1266/ggs.17-00026. [DOI] [PubMed] [Google Scholar]
- Harnik PG, Maherali H, Miller JH, Manos PS. Geographic range velocities and its association with phylogeny and life history traits in North America woody plants. Ecology and Evolution. 2018;8:2632–2644. doi: 10.1002/ece3.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SP, Turcotte MM, Levine JM. Effects of rapid evolution on species coexistence. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(6):2112–2117. doi: 10.1073/pnas.1816298116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausch S, Vamosi SM, Fox JW. Effects of intraspecific phenotypic variation on species coexistence. Ecology. 2018;99(6):1453–1462. doi: 10.1002/ecy.2346. [DOI] [PubMed] [Google Scholar]
- Hauser DA, Keuter A, McVay JD, Hipp AL, Manos PS. The evolution and diversification of the red oaks of the California Floristic Province (Quercus section Lobatae, series Agrifoliae) American Journal of Botany. 2017;104(10):1581–1595. doi: 10.3732/ajb.1700291. [DOI] [PubMed] [Google Scholar]
- He T, Pausas JG, Belcher CM, Schwilk DW, Lamont BB. Fire-adapted traits of Pinus arose in the fiery Cretaceous. New Phytologist. 2012;194:751–759. doi: 10.1111/j.1469-8137.2012.04079.x. [DOI] [PubMed] [Google Scholar]
- Hipp AL. Should hybridization make us skeptical of the oak phylogeny? International Oaks: The Journal of the International Oak Society. 2015;26:9–18. [Google Scholar]
- Hipp AL. American oaks in an evolutionary context. In: Jerome D, Beckman E, Kenny L, Wenzell K, Kua CS, Westwood M, editors. The red list of US oaks. Lisle: US Forest Service and Morton Arboretum; 2017. pp. 10–11. [Google Scholar]
- Hipp AL, Eaton DAR, Cavender-Bares J, Fitzek E, Nipper R, Manos PS. A Framework Phylogeny of the American Oak Clade Based on Sequenced RAD Data. Plos One. 2014;9(4) doi: 10.1371/journal.pone.0093975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp AL, Manos PS, Gonzalez-Rodriguez A, Hahn M, Kaproth M, McVay JD, Avalos SV, Cavender-Bares J. Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytologist. 2018;217(1):439–452. doi: 10.1111/nph.14773. [DOI] [PubMed] [Google Scholar]
- Hipp AL, Manos PS, Hahn M, Avishai M, Bodénès C, Cavender-Bares J, Crowl A, Deng M, Denk T, Gailing O, et al. The genomic landscape of the global oak phylogeny. New Phytologist. 2019a doi: 10.1111/nph.16162. This issue. [DOI] [PubMed] [Google Scholar]
- Hipp AL, Whittemore AT, Garner M, Hahn M, Fitzek E, Guichoux E, Cavender-Bares J, Gugger PF, Manos PS, Pearse IS, Cannon CH. Genomic identity of white oak species in an eastern North American syngameon. Annals of the Missouri Botanical Garden. 2019b;104:455–477. [Google Scholar]
- Hofmann CC. Microstructure of Fagaceae pollen from Austria (Paleocene/Eocene boundary) and Hainan island (middle Eocene) 8th European Palaeobotany-Palynology Conference; Budapest: Hungarian National Museum, 119: 2010. [Google Scholar]
- Hofmann CC, Mohamed O, Egger H. A new terrestrial palynoflora from the Palaeocene/Eocene boundary in the northwestern Tethyan realm (St. Pankraz, Austria) Review of Palaeobotany and Palynology. 2011;166(3-4):295–310. [Google Scholar]
- Hubert F, Grimm GW, Jousselin E, Berry V, Franc A, Kremer A. Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity. 2014;12(4):405–423. [Google Scholar]
- Ishida TA, Hattori K, Sato H, Kimura MT. Differentiation and hybridization between Quercus crispula and Q. dentata (Fagaceae): Insights from morphological traits, amplified fragment length polymorphism markers, and leaf miner composition. American Journal of Botany. 2003;90(5):769–776. doi: 10.3732/ajb.90.5.769. [DOI] [PubMed] [Google Scholar]
- Jiang X-L, Hipp AL, Deng M, Su T, Zho Z-K, Yan M-X. East Asian origins of European holly oaks via the Tibet-Himalayas. Journal of Biogeography. 2019 doi: 10.1111/jbi.13654. [DOI] [Google Scholar]
- Jones PD, Mann ME. Climate over past millennia. Reviews of Geophysics. 2004;42(2) [Google Scholar]
- Kang KS, Cheon BH, Kim CS, Han SU, Choi WY. Genetic gain and diversity under different selection methods in a breeding seed orchard of Quercus serrata. Silvae Genetica. 2007;56(6):277–281. [Google Scholar]
- Kaproth MA, Cavender-Bares J. Drought Tolerance and climatic distributions of the American oaks. International Oaks: The Journal of the International Oak Society. 2016;27:49–60. [Google Scholar]
- Keeley JE. Ecology and evolution of pine life histories. Annals of Forest Science. 2012;69:445–453. [Google Scholar]
- Kim BY, Wei XZ, Fitz-Gibbon S, Lohmueller KE, Ortego J, Gugger PF, Sork VL. RADseq data reveal ancient, but not pervasive, introgression between Californian tree and scrub oak species (Quercus sect. Quercus: Fagaceae) Molecular Ecology. 2018;27(22):4556–4571. doi: 10.1111/mec.14869. [DOI] [PubMed] [Google Scholar]
- Kleinschmit J. Intraspecific variation of growth and adaptive traits in European oak species. Annales Des Sciences Forestières. 1993;50(Suppl 1):166s–185s. [Google Scholar]
- Kline VM. Orchards of oak and a sea of grass. In: Packard S, Mutel CF, editors. The Tallgrass restoration handbook: for prairies, savannas, and woodlands. Washington, D.C.: Island Press; 1997. pp. 3–21. [Google Scholar]
- Koehler K, Center A, Cavender-Bares J. Evidence for a freezing tolerance-growth rate trade-off in the live oaks (Quercus series Virentes) across the tropical-temperate divide. New Phytologist. 2012;193:730–744. doi: 10.1111/j.1469-8137.2011.03992.x. [DOI] [PubMed] [Google Scholar]
- Kremer A. Did early human populations in Europe facilitate the dispersion of oaks? International Oaks: The Journal of the International Oak Society. 2015;26:19–28. [Google Scholar]
- Kremer A, Kleinschmit J, Cottrell J, Cundall EP, Deans JD, Ducousso A, Konig AO, Lowe AJ, Munro RC, Petit RJ, et al. Is there a correlation between chloroplastic and nuclear divergence, or what are the roles of history and selection on genetic diversity in European oaks? Forest Ecology and Management. 2002;156(1-3):75–87. [Google Scholar]
- Kremer A, Le Corre V. Decoupling of differentiation between traits and their underlying genes in response to divergent selection. Heredity. 2012;108(4):375–385. doi: 10.1038/hdy.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, Le Corre V, Petit RJ, Ducousso A. Historical and contemporary dynamics of adaptive differentiation in European oaks. In: DeWoody JA, Bickham JW, Michler CH, Nichols KM, Rhodes OE, Woeste KE, editors. Molecular Approaches in Natural Resource Conservation and Management. Academic Press; 2010. pp. 101–122. [Google Scholar]
- Kremer A, Petit RJ. Gene diversity in natural populations of oak species. Annales Des Sciences Forestières. 1993;50(Suppl 1):186s–202s. [Google Scholar]
- Kriebel HB. Intraspecific variation of growth and adaptive traits in North American species. Annales Des Sciences Forestières. 1993;50(Suppl 1):153s–165s. [Google Scholar]
- Kriebel HB, Bagley WT, Deneke FJ, Funsch RW, Roth P, Jokela JJ, Merritt C, Wright JW, Williams RD. Geographic variation in Quercus rubra in North Central United States plantations. Silvae Genetica. 1976;25(3-4):118–122. [Google Scholar]
- Kriebel HB, Merritt C, Stadt T. Genetics of growth-rate in Quercus rubra provenance and family effects by the early third decade in the North central USA. Silvae Genetica. 1988;37(5-6):193–198. [Google Scholar]
- Kullman L, Kjallgren L. Holocene pine tree-line evolution in the Swedish Scandes: Recent tree-line rise and climate change in a long-term perspective. Boreas. 2006;35(1):159–168. [Google Scholar]
- Kursar TA, Dexter KG, Lokvam J, Pennington RT, Richardson JE, Weber MG, Murakami ET, Drake C, McGregor R, Coley PD. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proceedings of the National Academy of Sciences. 2009;106:18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham RE, Ricklefs RE. Global patterns of tree species richness in moist forests. Energy-diversity theory does not account for variation in species richness. Oikos. 1993;67(2):325–333. [Google Scholar]
- Le Corre V, Kremer A. Cumulative effects of founding events during colonisation on genetic diversity and differentiation in an island and stepping-stone model. Journal of Evolutionary Biology. 1998;11(4):495–512. [Google Scholar]
- Le Corre V, Machon N, Petit RJ, Kremer A. Colonization with long-distance seed dispersal and genetic structure of maternally inherited genes in forest trees: a simulation study. Genetical Research. 1997;69(2):117–125. [Google Scholar]
- Lepais O, Gerber S. Reproductive patterns shape introgression dynamics and species succession within the European white oak species complex. Evolution. 2011;65(1):156–170. doi: 10.1111/j.1558-5646.2010.01101.x. [DOI] [PubMed] [Google Scholar]
- Leroy T, Louvet JM, Lalanne C, Le Provost G, Labadie K, Aury JM, Delzon S, Plomion C, Kremer A. Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytologist. 2019a doi: 10.1111/nph.16095. (This issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy T, Rougemont Q, Dupouey JL, Bodénès C, Lalanne C, Belser C, Labadie K, Le Provost G, Aury JM, Kremer A, et al. Massive postglacial gene flow between European white oaks uncovered genes underlying species barriers. New Phytologist. 2019b doi: 10.1111/nph.16039. (This issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy T, Roux C, Villate L, Bodénès C, Romiguier J, Paiva JAP, Dossat C, Aury JM, Plomion C, Kremer A. Extensive recent secondary contacts between four European white oak species. New Phytologist. 2017;214(2):865–878. doi: 10.1111/nph.14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind-Riehl JF, Gailing O. Fine-scale spatial genetic structure of two red oak species, Quercus rubra and Quercus ellipsoidalis. Plant Systematics and Evolution. 2015;301(6):1601–1612. [Google Scholar]
- Lind-Riehl JF, Sullivan AR, Gailing O. Evidence for selection on a CONSTANS-like gene between two red oak species. Annals of Botany. 2014;113(6):967–975. doi: 10.1093/aob/mcu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan WB. Oak. The frame of civilization. New York, NY, USA: W.W. Norton& Company; 2006. [Google Scholar]
- Luo R, Hipp AL, Larget B. A Bayesian Model of AFLP Marker Evolution and Phylogenetic Inference. Statistical Applications in Genetics and Molecular Biology. 2007;6:1–30. doi: 10.2202/1544-6115.1152. Article 11. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang J, Hu Q, Li J, Sun Y, Zhang L, Abbott RJ, Liu J, Mao K. Ancient introgression drives adaptation to cooler and drier mountain habitats in a cypress species complex. Communications Biology. 2019;2:213. doi: 10.1038/s42003-019-0445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald GM, Kremenetski KV, Beilman DW. Climate change and the northern Russian treeline zone. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363(1501):2285–2299. doi: 10.1098/rstb.2007.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni CR, Ducousso A, Caron H, Petit RJ, Kremer A. Chloroplast DNA variation of Quercus rubra L. in North America and comparison with other Fagaceae. Molecular Ecology. 2005;14(2):513–524. doi: 10.1111/j.1365-294X.2005.02400.x. [DOI] [PubMed] [Google Scholar]
- Magri D, Di Rita F, Aranbarri J, Fletcher W, Gonzalez-Samperiz P. Quaternary disappearance of tree taxa from Southern Europe: Timing and trends. Quaternary Science Reviews. 2017;163:23–55. [Google Scholar]
- Manchester SR. Fruits and seed of the middle Eocene nut beds flora, Clarno Formation, Oregon. Palaeontographica Americana. 1994;58:1–205. [Google Scholar]
- Manchester SR. Fruits of Ticodendraceae (Fagales) from the Eocene of Europe and North America. International Journal of Plant Sciences. 2011;172:1179–1187. [Google Scholar]
- Manos PS. Systematics and biogeography of the American oaks. International Oaks : The Journal of the International Oak Society. 2016;27:23–36. [Google Scholar]
- Manos PS, Cannon CH, Oh SH. Phylogenetic relationships and taxonomic status of the paleoendemic Fagaceae of western North America: recognition of a new genus, Notholithocarpus. Madrono. 2008;55:181–190. [Google Scholar]
- Manos PS, Doyle JJ, Nixon KC. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae) Molecular Phylogenetics and Evolution. 1999;12(3):333–349. doi: 10.1006/mpev.1999.0614. [DOI] [PubMed] [Google Scholar]
- Manos PS, Stanford AM. The historical biogeography of Fagaceae: Tracking the tertiary history of temperate and subtropical forests of the Northern Hemisphere. International Journal of Plant Sciences. 2001;162:S77–S93. [Google Scholar]
- Mariette S, Cottrell J, Csaikl UM, Goikoechea P, Konig A, Lowe AJ, Van Dam BC, Barreneche T, Bodenes C, Streiff R, et al. Comparison of levels of genetic diversity detected with AFLP and microsatellite markers within and among mixed Q. petraea (Matt.) Liebl. and Q. robur L. stands. Silvae Genetica. 2002;51(2–3):72–79. [Google Scholar]
- Marsico TD, Hellmann JJ, Romero-Severson J. Patterns of seed dispersal and pollen flow in Quercus garryana (Fagaceae) following post-glacial climatic changes. Journal of Biogeography. 2009;36(5):929–941. [Google Scholar]
- Martins EP. Phylogenies and comparative data, a microevolutionary perspective. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1995;349(1327):85–91. doi: 10.1098/rstb.1995.0094. [DOI] [PubMed] [Google Scholar]
- Martins K, Gugger PF, Llanderal-Mendoza J, Gonzalez-Rodriguez A, Fitz-Gibbon ST, Zhao JL, Rodriguez-Correa H, Oyama K, Sork VL. Landscape genomics provides evidence of climate-associated genetic variation in Mexican populations of Quercus rugosa. Evolutionary Applications. 2018;11(10):1842–1858. doi: 10.1111/eva.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CE. Bud-break on red oak seedlings varies with elevation of seed source. Tree Planters Notes. 1970;21(2):18–19. [Google Scholar]
- McGee CE. Elevation of seed sources and planting sites affects phenology and development of red oak seedlings. Forest Science. 1974;20:160–164. [Google Scholar]
- McIntyre DJ. Pollen and spore flora of an Eocene forest, eastern Axel Heiberg island. N.W.T. Geological Survey of Canada Bulletin. 1991;403:83–97. [Google Scholar]
- McLachlan JS, Clark JS, Manos PS. Molecular indicators of tree migration capacity under rapid climate change. Ecology. 2005;86:2088–2098. [Google Scholar]
- McVay JD, Hauser D, Hipp AL, Manos PS. Phylogenomics reveals a complex evolutionary history of lobed-leaf white oaks in western North America. Genome. 2017a;60(9):733–742. doi: 10.1139/gen-2016-0206. [DOI] [PubMed] [Google Scholar]
- McVay JD, Hipp AL, Manos PS. A genetic legacy of introgression confounds phylogeny and biogeography in oaks. Proceedings of the Royal Society B-Biological Sciences. 2017b;284(1854) doi: 10.1098/rspb.2017.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng HH, Su T, Gao XY, Li J, Jiang XL, Sun H, Zhou ZK. Warm-cold colonization: response of oaks to uplift of the Himalaya-Hengduan mountains. Molecular Ecology. 2017;26:3276–3294. doi: 10.1111/mec.14092. [DOI] [PubMed] [Google Scholar]
- Menitsky YL. Oaks of Asia. New York: CRC Press; 2005. [Google Scholar]
- Merceron NR. Processus écologiques et évolutifs impliqués dans le succès de l'introduction de Quercus rubra L. en Europe. PhD thesis; Université de Bordeaux: 2017. [Google Scholar]
- Merceron NR, Leroy T, Chancerel E, Romero-Severson J, Borkowski DS, Ducousso A, Monty A, Porte AJ, Kremer A. Back to America: tracking the origin of European introduced populations of Quercus rubra L. Genome. 2017;60(9):778–790. doi: 10.1139/gen-2016-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EV, Willis J, Clark JS. Genetic evidence for hybridization in red oaks (Quercus sect. Lobatae, Fagaceae) American Journal of Botany. 2012;99(1):92–100. doi: 10.3732/ajb.1100023. [DOI] [PubMed] [Google Scholar]
- Muir G, Fleming CC, Schlotterer C. Taxonomy - Species status of hybridizing oaks. Nature. 2000;405(6790):1016–1016. doi: 10.1038/35016640. [DOI] [PubMed] [Google Scholar]
- Muellner-Riehl AN. Mountains as evolutionary arenas: patterns, emerging approaches, paradigm shifts, and their implications for plant phylogeographic research in the Tibeto-Himalayan region. Front. Plant Science. 2019 doi: 10.3389/fpls.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na SJ, Lee HS, Han SU, Park JM, Kang KS. Estimation of genetic gain and diversity under various genetic thinning scenarios in a breeding seed orchard of Quercus acutissima. Scandinavian Journal of Forest Research. 2015;30(5):377–381. [Google Scholar]
- Nakanishi A, Tomaru N, Yoshimaru H, Manabe T, Yamamoto S. Interannual genetic heterogeneity of pollen pools accepted by Quercus salicina individuals. Molecular Ecology. 2005;14(14):4469–4478. doi: 10.1111/j.1365-294X.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- Neophytou C, Aravanopoulos FA, Fink S, Dounavi A. Detecting interspecific and geographic differentiation patterns in two interfertile oak species (Quercus petraea (Matt.) Liebl. and Q. robur L.) using small sets of microsatellite markers. Forest Ecology and Management. 2010;259(10):2026–2035. [Google Scholar]
- Neophytou C, Aravanopoulos FA, Fink S, Dounavi A. Interfertile oaks in an island environment. II. Limited hybridization between Quercus alnifolia Poech and Q. coccifera L. in a mixed stand. European Journal of Forest Research. 2011;130(4):623–635. [Google Scholar]
- Neophytou C, Palli G, Dounavi A, Aravanopoulos FA. Morphological differentiation and hybridization between Quercus alnifolia Poech and Quercus coccifera l. (Fagaceae) in Cyprus. Silvae Genetica. 2007;56(6):271–277. [Google Scholar]
- Nixon KC. A biosytematic study of Quercus series Virentes (the Live Oaks) with phylogenetic analyses of Fagales, Fagaceae and Quercus. PhD Thesis; University of Texas, Austin: 1985. [Google Scholar]
- Nixon KC. The genus Quercus in Mexico. In: Ramamoorthy TP, Dye R, Lot A, Fa J, editors. Biological diversity of Mexico. Oxford University Press; 1993a. pp. 448–458. [Google Scholar]
- Nixon KC. Infrageneric classification of Quercus (Fagaceae) and typification of sectional names. Annales Des Sciences Forestières. 1993b;50(Suppl 1):25s–34s. [Google Scholar]
- Nixon KC. Quercus. In: Committee FoNAE, editor. Flora of North America North of Mexico. New York: Oxford University Press; 1997. pp. 445–447. [Google Scholar]
- Nixon KC. Global and neotropical distribution and diversity of oaks (genus Quercus) and oak forest. In: Kappelle M, editor. Ecology and conservation of Neotropical Montane oak forests. Springer; 2006. pp. 3–13. [Google Scholar]
- Oh SH, Manos PS. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 2008;57(2):434–451. [Google Scholar]
- Ohsawa T, Saito Y, Sawada H, Ide Y. Impact of altitude and topography on the genetic diversity of Quercus serrata populations in the Chichibu Mountains, central Japan. Flora. 2008;203(3):187–196. [Google Scholar]
- Ohsawa T, Tsuda Y, Saito Y, Sawada H, Ide Y. Altitudinal genetic diversity and differentiation of Quercus crispula in the Chichibu Mountains, central Japan. International Journal of Plant Sciences. 2007;168(3):333–340. [Google Scholar]
- Okaura T, Quang ND, Ubukata M, Harada K. Phylogeographic structure and late Quaternary population history of the Japanese oak Quercus mongolica var. crispula and related species revealed by chloroplast DNA variation. Genes & Genetic Systems. 2007;82(6):465–477. doi: 10.1266/ggs.82.465. [DOI] [PubMed] [Google Scholar]
- Oney-Birol S, Fitz-Gibbon S, Chen J-M, Gugger PF, Sork VL. Assessment of shared alleles in drought-associated candidate genes among southern California white oak species (Quercus sect. Quercus) BMC Genetics. 2018;19 doi: 10.1186/s12863-018-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Bonal R, Munoz A, Espelta JM. Living on the edge: the role of geography and environment in structuring genetic variation in the southernmost populations of a tropical oak. Plant Biology. 2015;17(3):676–683. doi: 10.1111/plb.12272. [DOI] [PubMed] [Google Scholar]
- Ortego J, Gugger PF, Riordan EC, Sork VL. Influence of climatic niche suitability and geographical overlap on hybridization patterns among southern Californian oaks. Journal of Biogeography. 2012;41(10):1895–1908. [Google Scholar]
- Ortego J, Gugger PF, Sork VL. Genomic data reveal cryptic lineage diversification and introgression in Californian golden cup oaks (section Protobalanus) New Phytologist. 2018;218(2):804–818. doi: 10.1111/nph.14951. [DOI] [PubMed] [Google Scholar]
- Oyama K, Herrera-Arroyo ML, Rocha-Ramirez V, Benitez-Malvido J, Ruiz-Sanchez E, Gonzalez-Rodriguez A. Gene flow interruption in a recently human-modified landscape: the value of isolated trees for the maintenance of genetic diversity in a Mexican endemic red oak. Forest Ecology and Management. 2017;390:27–35. [Google Scholar]
- Oyama K, Ramirez-Toro W, Penaloza-Ramirez JM, Pedraza AEP, Torres-Miranda CA, Ruiz-Sanchez E, Gonzalez-Rodriguez A. High genetic diversity and connectivity among populations of Quercus candicans, Quercus crassifolia, and Quercus castanea in a heterogeneous landscape in Mexico. Tropical Conservation Science. 2018;11 [Google Scholar]
- Parelle J, Brendel O, Jolivet Y, Dreyer E. Intra- and interspecific diversity in the response to waterlogging of two co-occurring white oak species (Quercus robur and Q. petraea) Tree Physiology. 2007;27(7):1027–1034. doi: 10.1093/treephys/27.7.1027. [DOI] [PubMed] [Google Scholar]
- Pearse IS, Hipp AL. Phylogenetic and trait similarity to a native species predict herbivory on non-native oaks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18097–18102. doi: 10.1073/pnas.0904867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse IS, Hipp AL. Global patterns of leaf defenses in oak species. Evolution. 2012;66(7):2272–2286. doi: 10.1111/j.1558-5646.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- Peñaloza-Ramírez JM, Gonzalez-Rodriguez A, Mendoza-Cuenca L, Caron H, Kremer A, Oyama K. Interspecific gene flow in a multispecies oak hybrid zone in the Sierra Tarahumara of Mexico. Annals of Botany. 2010;105(3):389–399. doi: 10.1093/aob/mcp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A. Hybridization as a mechanism of invasion in oaks. New Phytologist. 2003;161(1):151–164. [Google Scholar]
- Petit RJ, Csaikl UM, Bordacs S, Burg K, Coart E, Cottrell J, van Dam B, Deans JD, Dumolin-Lapegue S, Fineschi S, et al. Chloroplast DNA variation in European white oaks - Phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management. 2002;156(1–3):5–26. [Google Scholar]
- Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG. Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Molecular Ecology. 2005;14(3):689–701. doi: 10.1111/j.1365-294X.2004.02410.x. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Excoffier L. Gene flow and species delimitation. Trends in Ecology & Evolution. 2009;24:386–393. doi: 10.1016/j.tree.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Hampe A. Some evolutionary consequences of being a tree. Annual Review of Ecology Evolution and Systematics. 2006;37:187–214. [Google Scholar]
- Petit RJ, Pineau E, Demesure B, Bacilieri R, Ducousso A, Kremer A. Chloroplast DNA footprints of postglacial recolonization by oaks. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(18):9996–10001. doi: 10.1073/pnas.94.18.9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham KK, Hipp AL, Manos PS, Cronn RC. A time and a place for everything: phylogenetic history and geography as joint predictors of oak plastome phylogeny. Genome. 2017;60(9):720–732. doi: 10.1139/gen-2016-0191. [DOI] [PubMed] [Google Scholar]
- Plomion C, Aury JM, Amselem J, Leroy T, Murat F, Duplessis S, Faye S, Francillonne N, Labadie K, Le Provost G, et al. Oak genome reveals facets of long lifespan. Nature Plants. 2018;4(7):440–452. doi: 10.1038/s41477-018-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts BM, Reid JB. The evolutionary significance of hybridization in Eucalyptus . Evolution. 1990;44(8):2151–2152. doi: 10.1111/j.1558-5646.1990.tb04319.x. [DOI] [PubMed] [Google Scholar]
- Rabosky DL, McCune AR. Reinventing species selection with molecular phylogenies. Trends in Ecology & Evolution. 2010;25(2):68–74. doi: 10.1016/j.tree.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Alia R, Aranda I. Geographical variation in growth form traits in Quercus suber and its relation to population evolutionary history. Evolutionary Ecology. 2014;28(1):55–68. [Google Scholar]
- Ramirez-Valiente JA, Cavender-Bares J. Evolutionary trade-offs between drought resistance mechanisms across a precipitation gradient in a seasonally dry tropical oak (Quercus oleoides) Tree Physiology. 2017;37(7):889–901. doi: 10.1093/treephys/tpx040. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Center A, Sparks JP, Sparks KL, Etterson JR, Longwell T, Pilz G, Cavender-Bares J. Population-level differentiation in growth rates and leaf traits in seedlings of the Neotropical Live Oak Quercus oleoides Grown under Natural and Manipulated Precipitation Regimes. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Deacon NJ, Etterson JR, Center A, Sparks JP, Sparks KL, Longwell T, Pilz G, Cavender-Bares J. Natural selection and neutral evolutionary processes contribute to genetic divergence in leaf traits across a precipitation gradient in the tropical oak Quercus oleoides. Molecular Ecology. 2018;27:2176–2192. doi: 10.1111/mec.14566. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Etterson JR, Deacon NJ, Cavender-Bares J. Evolutionary potential varies across populations and traits in the neotropical oak Quercus oleoides. Tree Physiology. 2019;39(3):427–439. doi: 10.1093/treephys/tpy108. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Valladares F, Aranda I. Exploring the impact of neutral evolution on intrapopulation genetic differentiation in functional traits in a long-lived plant. Tree Genetics & Genomes. 2014;10(5):1181–1190. [Google Scholar]
- Rellstab C, Zoller S, Walthert L, Lesur I, Pluess AR, Graf RE, Bodenes C, Sperisen C, Kremer A, Gugerli F. Signatures of local adaptation in candidate genes of oaks (Quercus spp.) with respect to present and future climatic conditions. Molecular Ecology. 2016;25(23):5907–5924. doi: 10.1111/mec.13889. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. A comprehensive framework for global patterns in biodiversity. Ecology Letters. 2004;7:1–15. [Google Scholar]
- Rodriguez-Correa H, Oyama K, Quesada M, Fuchs EJ, Quezada M, Ferrufino L, Valencia-Avalos S, Cascante-Marin A, Gonzalez-Rodriguez A. Complex phylogeographic patterns indicate Central American origin of two widespread Mesoamerican Quercus (Fagaceae) species. Tree Genetics & Genomes. 2017;13(3) [Google Scholar]
- Roussel M, Dreyer E, Montpied P, Le-Provost G, Guehl JM, Brendel O. The diversity of C-13 isotope discrimination in a Quercus robur full-sib family is associated with differences in intrinsic water use efficiency, transpiration efficiency, and stomatal conductance. Journal of Experimental Botany. 2009;60(8):2419–2431. doi: 10.1093/jxb/erp100. [DOI] [PubMed] [Google Scholar]
- Sáenz-Romero C, Kremer A, Nagy L, Újvári-Jármay É, Ducousso A, Kóczán-Horváth A, Hansen JK, Mátyás C. Common garden comparisons confirm inherited differences in sensitivity to climate change between forest tree species. PeerJ. 2019;7:e6213. doi: 10.7717/peerj.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz-Romero C, Lamy JB, Ducousso A, Musch B, Ehrenmann F, Delzon S, Cavers S, Chalupka W, Dagdas S, Hansen JK, et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Global Change Biology. 2017;23(7):2831–2847. doi: 10.1111/gcb.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlarbaum SE, Adams RP, Bagley WT, Wayne WJ. Post-glacial migration pathways of Quercus rubra L, northern red oak, as indicated by regional genetic variation patterns. Silvae Genetica. 1982;31(5–6):150–158. [Google Scholar]
- Simeone MC, Cardoni S, Piredda R, Imperatori F, Avishai M, Grimm GW, Denk T. Comparative systematics and phylogeography of Quercus Section Cerris in western Eurasia: inferences from plastid and nuclear DNA variation. Peerj. 2018;6:e5793. doi: 10.7717/peerj.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Morris A, McLachlan J, Manos PS, Soltis PS. Comparative phylogeography of unglaciated eastern North America. Molecular Ecology. 2006;15:4261–4293. doi: 10.1111/j.1365-294X.2006.03061.x. [DOI] [PubMed] [Google Scholar]
- Song Y, Deng M, Hipp AL, Li Q. Leaf morphological evidence of natural hybridization between two oak species (Quercus austrocochinchinensis and Q. kerrii) and its implications for conservation management. European Journal of Forest Research. 2015;134(1):139–151. [Google Scholar]
- Sork VL, Fitz-Gibbon ST, Pui D, Crepeau M, Gugger PF, Sherman R, Stevens K, Langley CH, Pellegrini M, Salzberg SL. First draft assembly and annotation of the genome of a California endemic oak Quercus lobata Née (Fagaceae) Genes Genomes Genetics. 2016a;6(11):3485–3495. doi: 10.1534/g3.116.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sork VL, Gugger PF, Chen JM, Werth S. Evolutionary lessons from California plant phylogeography. PNAS. 2016b;113:8064–8071. doi: 10.1073/pnas.1602675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sork VL, Squire K, Gugger PF, Steele SE, Levy ED, Eckert AJ. Landscape genomic analysis of candidate genes for climate adaptation in a California endemic oak, Quercus lobata. American Journal of Botany. 2016;103(1):33–46. doi: 10.3732/ajb.1500162. [DOI] [PubMed] [Google Scholar]
- Sork VL, Stowe KA, Hochwender C. Evidence for local adaptation in closely adjacent subpopulations of Northern red oak (Quercus rubra L.) expressed as resistance to leaf herbivores. American Naturalist. 1993;142(6):928–936. doi: 10.1086/285581. [DOI] [PubMed] [Google Scholar]
- Soularue JP, Kremer A. Assortative mating and gene flow generate clinal phenological variation in trees. BMC Evolutionary Biology. 2012;12 doi: 10.1186/1471-2148-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soularue JP, Kremer A. Evolutionary responses of tree phenology to the combined effects of assortative mating, gene flow and divergent selection. Heredity. 2014;113(6):485–494. doi: 10.1038/hdy.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner KC, Westbrook JW, Hebard FV, Georgi LL, Powell WA, Fitzsimmons SF. Rescue of American chestnut with extraspecific genes following its destruction by a naturalized pathogen. New Forests. 2017;48(2):317–336. [Google Scholar]
- Suarez-Gonzalez A, Lexer C, Cronk Q. Adaptive introgression: a plant perspective. Biology Letters. 2018;14 doi: 10.1098/rsbl.2017.0688. 20170688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AR, Owusu SA, Weber JA, Hipp AL, Gailing O. Hybridization and divergence in multi-species oak (Quercus) communities. Botanical Journal of the Linnean Society. 2016;181(1):99–114. [Google Scholar]
- Svenning JC. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecology Letters. 2003;6(7):646–653. [Google Scholar]
- Svenning JC, Eiserhardt WL, Normand S, Ordonez A, Sandel B. The influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Ann Rev Ecol Evol Syst. 2015;46:551–572. [Google Scholar]
- Timbal J, Kremer A, Le Goff N, Nepveu G. Le Chêne rouge d'Amérique. Paris: Editions de INRA; 1994. [Google Scholar]
- Torres-Miranda A, Luna-Vega I, Oyama K. Conservation biogeography of red oaks (Quercus, section Lobatae) in Mexico and central America. American Journal of Botany. 2011;98(2):290–305. doi: 10.3732/ajb.1000218. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Kremer A, Carins-Murphy MR, Brodribb TJ, Lamarque LJ, Truffaut L, Bonne F, Ducousso A, Delzon S. Genetic differentiation in functional traits among European sessile oak populations growing. Tree Physiology. 2019 doi: 10.1093/treephys/tpz090. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumi L, Lumaret R. Allozyme variation in cork oak (Quercus suber L.): the role of phylogeography and genetic introgression by other Mediterranean oak species and human activities. Theoretical and Applied Genetics. 1998;97:647–656. [Google Scholar]
- Trelease W. The American oaks. Memoirs of the National Academy of Sciences. 1924;20:1–255. [Google Scholar]
- Truffaut L, Chancerel E, Ducousso A, Dupouey JL, Badeau V, Ehrenmann F, Kremer A. Fine-scale species distribution changes in a mixed oak stand over two successive generations. New Phytologist. 2017;215(1):126–139. doi: 10.1111/nph.14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzedakis PC. Vegetation change through glacial-interglacial cycles. A long pollen sequence perspective. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1994;345(1314):403–432. [Google Scholar]
- Tzedakis PC, Hooghiemstra H, Palike H. The last 1.35 million years at Tenaghi Philippon: revised chronostratigraphy and long-term vegetation trends. Quaternary Science Reviews. 2006;25(23–24):3416–3430. [Google Scholar]
- Valbuena-Carabana M, Gonzalez-Martinez SC, Gil L. Coppice forests and genetic diversity: A case study in Quercus pyrenaica Willd. from Central Spain. Forest Ecology and Management. 2008;254(2):225–232. [Google Scholar]
- Valencia S. Diversidad del género Quercus (Fagaceae) en México. Bol Soc Bot Mex. 2004;75:33–53. [Google Scholar]
- Valencia-Cuevas L, Pinero D, Mussali-Galante P, Valencia-Avalos S, Tovar-Sanchez E. Effect of a red oak species gradient on genetic structure and diversity of Quercus castanea (Fagaceae) in Mexico. Tree Genetics & Genomes. 2014;10(3):641–652. [Google Scholar]
- Van Valen L. Ecological species, multispecies, and oaks. Taxon. 1976;25:233–239. [Google Scholar]
- Vargas OM, Ortiz EM, Simpson BB. Conflicting phylogenomic signals reveal a pattern of reticulate evolution in a recent high-Andean diversification (Asteraceae: Astereae: Diplostephium) New Phytologist. 2017;214(4):1736–1750. doi: 10.1111/nph.14530. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research. 2009;39(7):1259–1269. [Google Scholar]
- Vitelli M, Vessella F, Cardoni S, Pollegioni P, Denkk T, Grimm GW, Simeone MC. Phylogeographic structuring of plastome diversity in Mediterranean oaks (Quercus Group Ilex, Fagaceaea) Tree Genetics & Genomes. 2017;13:3. [Google Scholar]
- Wagner S, Lagane F, Seguin-Orlando A, Schubert M, Leroy T, Guichoux E, Chancerel E, Bech-Hebelstrup I, Bernard V, Billard C, et al. High-Throughput DNA sequencing of ancient wood. Molecular Ecology. 2018;27(5):1138–1154. doi: 10.1111/mec.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter S, Bracho-Nuñez A, Mir C, Zimmer I, Kesselmeier J, Lumaret R, Schnitzler J-P, Staudt M. The diversification of terpene emissions in Mediterranean oaks: lessons from a study of Quercus suber, Quercus canariensis and its hybrid Quercus afares. Tree Physiology. 2012;32:1082–1091. doi: 10.1093/treephys/tps069. [DOI] [PubMed] [Google Scholar]
- Whittemore AT, Schaal BA. Interspecific gene flow in sympatric oaks. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2540–2544. doi: 10.1073/pnas.88.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Di X, Meng X, Feng L, Liu Z, Zhao G. Phylogeography and evolution of two closely related oak species (Quercus) from north and northeast China. Tree Genetics & Genomes. 2017;12:89. [Google Scholar]
- Yeaman S. Local Adaptation by Alleles of Small Effect. American Naturalist. 2015;186:S74–S89. doi: 10.1086/682405. [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- Zeng YF, Wang WT, Liao WJ, Wang HF, Zhang DY. Multiple glacial refugia for cool-temperate deciduous trees in northern East Asia: the Mongolian oak as a case study. Molecular Ecology. 2015;24(22):5676–5691. doi: 10.1111/mec.13408. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang XQ, Yu MK, Han YZ, Wu TG. Variations in seed size and seed mass related to tree growth over 5 years for 23 provenances of Quercus acutissima from across China. Journal of Forestry Research. 2017;28(5):917–923. [Google Scholar]
- Zhang XW, Li Y, Zhang Q, Fang YM. Ancient east-west divergence, recent admixture, and multiple marginal refugia shape genetic structure of a widespread oak species (Quercus acutissima) in China. Tree Genetics & Genomes. 2018;14(6) [Google Scholar]
- Zhang YY, Fang YM, Yu MK, Li XX, Xia T. Molecular characterization and genetic structure of Quercus acutissima germplasm in China using microsatellites. Molecular Biology Reports. 2013;40(6):4083–4090. doi: 10.1007/s11033-013-2486-6. [DOI] [PubMed] [Google Scholar]