Abstract
Background & Aims:
Levels of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and CA-125 in blood are used as markers to determine response of patients with cancer to therapy, but are not used to identify patients with pancreatic cancer.
Methods:
We obtained blood samples from 504 patients undergoing pancreatic surveillance from 2002 through 2018 who did not develop pancreatic cancer and measured levels of the tumor markers CA19-9, CEA, CA125, and thrombospondin-2. Single nucleotide polymorphisms (SNPs) in FUT3, FUT2, ABO, GAL3ST2 that have been associated with levels of tumor markers were used to establish SNP-defined ranges for each tumor marker. We also tested the association between additional SNPs (in FUT6 MUC16, B3GNT3, FAM3B and THBS2) with levels of tumor markers. To calculate the diagnostic specificity of each SNP-defined range, we assigned the patients under surveillance into training and validation sets. After determining the SNP-defined ranges, we determined the sensitivity of SNP-adjusted tests for the tumor markers, measuring levels in blood samples from 245 patients who underwent resection for pancreatic ductal adenocarcinoma (PDAC) from 2010 through 2017.
Results:
A level of CA19-9 that identified patients with PDAC with 99% specificity had 52.7% sensitivity. When we set the cutoff levels of CA19-9 based on each SNP, the test for CA19-9 identified patients with PDAC with 60.8% sensitivity and 98.8% specificity. Among patients with FUT3 alleles that encode a functional protein, levels of CA19-9 above the SNP-determined cutoff values identified 66.4% of patients with PDAC, with 99.3% specificity. In the validation set, levels of CEA varied among patients with vs without SNP in FUT2, by blood group, and among smokers vs non-smokers; levels of CA125 varied among patients with vs without the SNP in GAL3ST2. Using the SNPs to define the ranges of CEA and CA125 did not significantly increase the diagnostic accuracy of the assays for these proteins. Combining data on levels of CA19-9 and CEA, CA19-9 and CA125, or CA19-9 and thrombospondin-2 increased the sensitivity of detection of PDAC but slightly reduced specificity.
Conclusions:
Including information on SNPs associated with levels of CA19-9, CEA, and CA125 can improve the diagnostic accuracy of assays for these tumor markers in identification of patients with PDAC.
Keywords: early detection, diagnosis, genotype, screening
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the 3rd most common cause of cancer death in the United States with a 5-year survival of only ~8%1. Most patients with pancreatic cancer are diagnosed with advanced stage disease. Improving early detection is likely to be the most effective way of reducing pancreatic cancer mortality. Existing pancreatic surveillance protocols for individuals with an inherited/familial susceptibility to pancreatic cancer can increase the proportion of patients diagnosed with Stage I disease2, 3. However, patients that develop pancreatic cancer on a pancreatic screening protocol are often diagnosed with more advanced stage disease2-8. One reason is that current pancreatic imaging tests can miss small cancers9, another is that the window of time to detect pancreatic cancer clinically while at its most curative stage is short10 so some patients present with interval cancers even with annual surveillance. Some patients also find compliance with annual pancreatic imaging difficult11. Better non-invasive diagnostic tests are needed for patients under pancreatic surveillance.
Circulating biomarkers continue to be evaluated for their potential diagnostic utility to detect early pancreatic cancer12-14, but most biomarkers lack the needed performance characteristics. For example, CA19-9 is not expressed in ~10% of individuals who lack functional FUT3 (fucosyltransferase-3-null (FUT3−/−)15 and FUT2 status influences CA199 and CEA levels16-19. Despite the evidence that genetic factors influence tumor marker levels20, 21, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention. Recent studies have identified three genetic subgroups with respect to CA19-9 levels; FUT3-null, those without functional FUT2 (FUT2-null or FUT2−/−), and a third group with intact FUT2 and FUT3 17, 22, 23. Luo et al reported this approach improved CA19-9’s diagnostic performance for pancreatic cancer, though the authors did not describe marker performance using high-specificity cut-offs17.
In this study, we evaluated genetic variants for their association with tumor marker levels in a cohort of high-risk individuals (HRIs) undergoing pancreatic cancer surveillance. We then determined if diagnostic characteristics of a tumor marker panel for pancreatic cancer (diagnostic sensitivity at high specificity) could be improved by using a tumor marker SNP genotype test to create SNP-defined diagnostic cut-offs for each tumor marker.
MATERIALS AND METHODS
Subjects
This study included 504 HRIs prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies between 2002 and 2018 ( NCT00438906, NCT00714701, NCT02000089)2, 24-28 without evidence of pancreatic cancer after >1 year since their blood-draw, as well as 245 patients with blood samples collected prior to pancreatic resection for pancreatic ductal adenocarcinoma at the Johns Hopkins Hospital (2010-2017). Patients who underwent neoadjuvant chemotherapy were excluded. A detailed description of CAPS enrollment criteria has been published2. Twenty-four HRIs that had previous surgery for pancreatic cancer or high-grade dysplasia or had worrisome imaging findings were excluded. The final diagnoses were made by surgical pathology using World Health Organization criteria. All pathological diagnoses were made by an expert pathologist (R.H.H) 29. Tumor stage was defined by American Joint Committee on Cancer (AJCC) 8th edition. All authors had access to study data and reviewed and approved the final manuscript. This study was approved by the Johns Hopkins Institutional Review Board and written informed consent was provided from all enrolled patients.
Genotyping and ELISA
Sixteen SNPs (in FUT3, FUT2, FUT6, ABO, GAL3ST2, MUC16, B3GNT3, FAM3B and THBS2) associated with tumor marker levels (CA19-9, CEA, CA125, thrombospondin-2) from prior studies16-18, 20 were genotyped (Table S1).
All serum samples were assayed in duplicate and analyzed randomly. New serum aliquots were used for all measurements. Tumor marker levels were measured by ELISA; CA19-9 (EIA-1474R, DRG International, NJ, USA), CEA (EIA-1868R, DRG International), CA125 (CA125, Quantikine; R&D Systems, Minneapolis, MN, USA), thrombospondin-2 (DTSP20, Quantikine; R&D Systems). Since CA125 levels are higher prior to menopause30, CA125 levels in women were measured only in those age >55 (n=174). An internal reference serum was measured in duplicate for each ELISA plate. Coefficients of variation calculated using a serum reference sample were 9.0%, 6.9% and 7.9%, for CA19-9, CA125 and thrombospondin-2, respectively. Further description of methods are provided in Supplemental Materials.
Statistical analysis
Patient characteristics were compared and differences between groups were evaluated using Fisher’s exact test for categorical variables and Student t-tests for continuous measures. Control subjects were split randomly into discovery and validation sets. The training and validation study design was aimed at setting strict 99% specificity cut-offs that could be applied to the PDAC cases. Diagnostic cut-offs for each marker and corresponding SNP groups were set using data from the controls in the discovery set. The estimated 99th percentile cut-off for each subgroup in the discovery set controls was set as the mean+3SD. Diagnostic sensitivity was then estimated in the PDAC patients and specificity at the threshold established in the discovery set was estimated from the validation set controls. The area under the receiver operator characteristic curve (AUC) calculations were included for descriptive purposes (validation set controls versus PDAC cases).
Statistical analysis were conducted using R v3.4.2 and JMP-13 software (SAS Institute, Cary, NC, USA). P values <0.05 were considered statistically significant.
RESULTS
The characteristics of the pancreatic cancer cases and the discovery and validation set CAPS controls are described in Table 1.
Table 1.
Characteristics of patients with pancreatic cancer and controls
| Discovery set controls (n=245) |
Validation set controls (n=259) |
P Value | All Controls (n=504) |
PDAC (n=245) |
P Value | |
|---|---|---|---|---|---|---|
| Age - mean (SD) | 61.5 (9.5) | 56.4 (10.6) | <0.0001 | 58.9 (10.4) | 67.9 (10.6) | < 0.0001 |
| Race - no. (%) | 1 | 0.0002 | ||||
| White | 232 (94.7) | 244 (94.2) | 476 (94.4) | 211 (86.1) | ||
| Other | 13 (5.3) | 15 (5.8) | 28 (5.6) | 34 (13.9) | ||
| Sex - no. (%) | 0.0157 | 0.0231 | ||||
| Female | 121 (49.4) | 156 (60.2) | 277 (55.0) | 113 (46.1) | ||
| Male | 124 (50.6) | 103 (39.9) | 227 (45.0) | 132 (53.9) | ||
| BMI - mean (SD) | 27.4 (4.7) | 27.5 (5.0) | 0.5964 | 27.4 (4.8) | 25.6 (4.1) | < 0.0001 |
| Smoker - no. (%) | 0.8216 | 0.0507 | ||||
| Not current smoker | 236 (96.3) | 248 (95.8) | 484 (96.0) | 224 (91.4) | ||
| Current smoker | 9 (3.7) | 11 (4.2) | 20 (4.0) | 18 (7.3) | ||
| Diabetic~ - no. (%) | 1 | < 0.0001 | ||||
| No | 228 (93.1) | 240 (92.7) | 36 (7.1) | 90 (54.5) | ||
| Yes | 17 (6.9) | 19 (7.3) | 468 (92.9) | 75 (45.5) | ||
| Stage (8th AJCC) | ||||||
| I A | 18 (7.3) | |||||
| I B | 31 (12.6) | |||||
| IIA | 4 (1.6) | |||||
| IIB | 106 (43.3) | |||||
| III | 86 (3.5) |
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; SD, standard deviation; BMI, body mass index; ~status not known for some cases
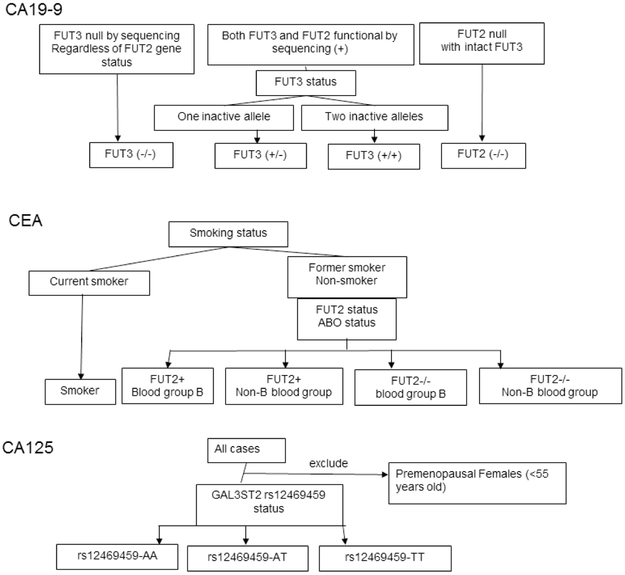
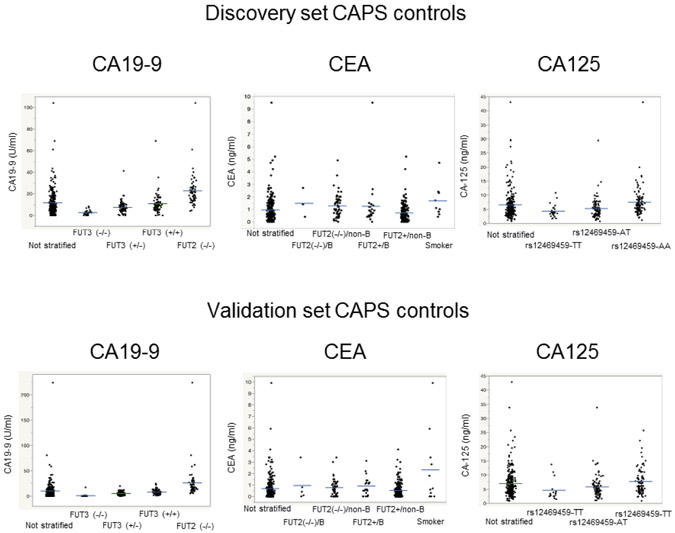
We compared mean CA19-9 levels by SNP group (Figure 1) within the control discovery set. The SNP(s) used to classify each tumor marker SNP group is summarized in Supplemental Table S1. As expected, FUT3-null subjects had minimal or absent CA19-9 levels and FUT2-null subjects had significantly higher CA19-9 levels than FUT2-intact subjects (Figure 2, Table 2). Among controls with intact FUT3, those with only one functional FUT3 allele (FUT3+/−) had lower CA19-9 levels than controls with two functional FUT3 alleles (FUT3+/+) in both the training (p=0.0038) and validation set (p<0.01)(Figure 2). Other FUT2/FUT3 SNP subgroups were not significantly different.
Figure 1.
Schematic outline of SNP subgroups for CA19-9, CEA and CA125.
Figure 2.
SNP-stratified tumor marker levels in (A) discovery set, (B) validation set controls. For CA19-9, discovery set controls; FUT3+/+ versus FUT3+/−, p=0.0038; all other comparisons, p<0.0001; for validation set controls all comparisons were p<0.001. CEA levels in discovery set controls were lower in FUT2+/non-blood group B (FUT2+/non-B) than all other groups; in the validation set, levels in the FUT2+/non-B group were lower than FUT2−/non-B and FUT2+/B groups controls (p=0.04 for both). For CA125, GAL3ST2 rs12469459-AA subjects had higher levels than rs12469459-TT and rs12469459-AT carriers (P=0.0007 and <0.0001, respectively, discovery set; both p=0.001, validation set). Rs12469459-TT carriers had lower levels than rs12469459-AT carriers (p=0.062 validation set, p=0.028 combined set). FUT2+ subjects include FUT2+/+ and FUT2+/−.
Table 2.
SNP stratified tumor marker levels in discovery set controls
| CA19-9 | number | Mean (U/mL) | SD | Mean +/− 3 SD* |
|---|---|---|---|---|
| FUT3 (−/−) | 26 | 2.7 | 2.3 | 9.5 |
| FUT3 (+/−) | 85 | 7.4 | 5.4 | 23.5 |
| FUT3 (+/+) | 75 | 10.9 | 9.1 | 38.1 |
| FUT2 (−/−) | 59 | 22.8 | 14.6 | 66.6 |
| Uniform diagnostic cut-off (Not SNP-stratified) | 245 | 11.7 | 11.5 | 46.1 |
| CEA | number | Mean (ng/mL) | SD | Mean +/− 3 SD* |
| FUT2+/blood B | 26 | 1.2 | 1.8 | 6.6 |
| FUT2+/non-blood B | 151 | 0.7 | 0.7 | 2.8 |
| FUT2 −/− /blood B | 4 | 1.5 | 0.9 | 4.3 |
| FUT2 −/− /non blood B | 55 | 1.3 | 0.9 | 4.0 |
| smoker | 9 | 1.7 | 0.8 | 4.2 |
| Uniform diagnostic cut-off (Not SNP-stratified) | 245 | 0.9 | 1.0 | 3.9 |
| CA125 | number | Mean (U/mL) | SD | Mean +/− 3 SD* |
| GAL3ST2 rs12469459-AA | 101 | 7.4 | 5.0 | 22.3 |
| GAL3ST2 rs12469459-TT | 90 | 5.2 | 3.7 | 16.2 |
| GAL3ST2 rs12469459-TT | 26 | 4.3 | 2.1 | 10.7 |
| Uniform diagnostic cut-off (Not SNP-stratified) | 217 | 6.1 | 4.4 | 19.2 |
Thus, in both training and validation sets results indicated FUT2 and FUT3 SNP status yielded 4 functional SNP subgroups each with a significantly different mean CA19-9 levels (Figure 2, Table 2). (Other SNP subgroups did not have evidence of effect on CA19-9). The four groups (FUT3-null, FUT3+/−, FUT3+/+ and FUT2-null) represented ~10%, 35%, 33% and 22% of the control population, respectively. SNP-stratified mean/SD CA19-9 levels, varied by ~3-fold between the lowest and highest SNP subgroups with intact FUT3 (Table 2). There was no significant difference in CA19-9 levels by age, diabetes status, presence or absence of cysts or worrisome features in controls (data not shown).
Having defined the SNP-defined subgroups, we set the diagnostic cut-off for each tumor maker using the observed 99th percentile of tumor marker in the discovery control set (Table 2). We then applied these cut-offs (i) to patients undergoing pancreatic resection for PDAC, (ii) to the control validation set, (iii) to another blood sample collected at a later time from controls.
The diagnostic sensitivity of CA19-9 applied to 245 (49 Stage 1, 196 Stage 2) localized PDAC cases using a uniform (not SNP-stratified) 99% specificity cut-off was 52.7% (95% CI: 46.2%, 59.0%), AUC 0.8827 (Table 3)(Figure 3). The diagnostic sensitivity of SNP-stratified CA19-9 at the 99% specificity cut-off was 60.8% (95% CI: 54.4, 67.0%). the AUC using SNP-stratified CA19-9 cut-offs was 0.9182. Applying SNP-stratified CA19-9 cut-offs to the validation set controls yielded 98.8% specificity (95% CI: 96.6%, 99.8%)(Table S2). The combined CA19-9-SNP test reclassified 28 of the 245 PDAC cases (11.4%) compared to the CA19-9 test alone (24 from negative-to-positive, 4 positive-to-negative)(Table S2).
Table 3.
Sensitivity for each tumor marker for localized PDAC
| CA19-9 | u/ml | All PDAC (n=245) | Stage I (n=49) |
|---|---|---|---|
| 99th percentile by SNP | 60.8% (95% CI: 54.40% to 66.97%) | 36.7% (95% CI: 23.42% to 51.71%) | |
| Uniform diagnostic cut-off | 46.1 | 52.7% (46.20% to 59.04%) | 28.6% (16.58% to 43.26%) |
| FUT3 intact only (n=217) | 66.4% (59.65% to 72.61%) | 40% (25.70% to 55.67%) | |
| CEA | ng/ml | All PDAC (n=245) | Stage I (n=49) |
| 99th percentile by SNP | 15.9% (11.57% to 21.11%) | 8.2% (2.27% to 19.60%) | |
| Uniform diagnostic cut-off | 3.9 | 13.1% (9.11% to 17.94%) | 8.2% (2.27% to 19.60%) |
| CA125 | u/ml | All PDAC (n=238) | Stage I (n=45) |
| 99th percentile by SNP | 17.6% (11.88% to 23.30%) | 11.1% (3.71% to 24.05%) | |
| Uniform diagnostic cut-off | 19.2 | 15.5% (11.19% to 20.79%) | 11.1% (3.71% to 24.05%) |
| thrombospondin-2 | u/ml | n=206 | Stage I (n=13) |
| Uniform diagnostic cut-off | 42.2 | 18% (12.97% to 23.90%) | 15.4% (1.92% to 45.45%) |
Figure 3.
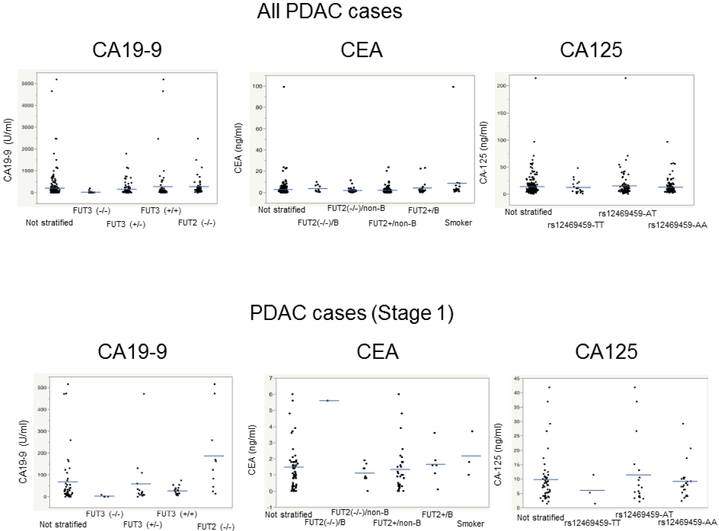
SNP-stratified tumor marker levels in patients with localized pancreatic ductal adenocarcinoma: All PDAC cases (top panel), Stage I cases only (bottom panel). CA19-9 levels differed within SNP subgroups (FUT2−/− versus other groups and FUT3−/− versus other groups, p<0.00001, Mann-Whitney).
Diagnostic specificity of CA19-9 was further evaluated using a 2nd longitudinal blood collected at a later time point from 217 of the 504 controls. Twenty of these 217 controls were FUT3-null; 2 of these 20 had elevated CA19-9 levels for this group. None of the 197 subjects with intact FUT3 had an elevated SNP-stratified CA19-9 level on repeat testing.
For CA19-9, genotyping is most helpful for the ~90% of subjects with functionally intact FUT3 genes. For patients with localized PDAC and intact FUT3, diagnostic sensitivity of SNP-stratified CA19-9 was 66.4% (95% CI: 59.6%, 72.6%). The specificity among intact FUT3 validation set controls was 99.2% (95% CI: 97.0%, 99.9%). The AUC for SNP-stratified CA19-9 in FUT3-intact subjects was 0.9315. The sensitivity of SNP-stratified CA19-9 in the 49 patients with Stage I PDAC was 36.7% (95% CI: 23.4%, 51.7%); among Stage I PDAC cases with intact FUT3, it was 40% (95% CI: 25.7%, 55.7%)(Table 3)(Figure 3). Among PDAC patients with similar-sized tumors (e.g. tumors of 2-4 cm diameter) and intact FUT3, the proportion with an elevated CA19-9 level was significantly higher if they had lymph node metastases and/or local invasion (Stage II) than if they did not (Stage IB disease)(79 of 107 versus 13 of 30 patients) (mean/SD tumor size, 3.07/0.6 versus 3.0/0.6 cm, p=0.016).
For CEA, levels were associated with FUT2 status and ABO blood group. FUT2-null controls had higher levels than those with intact FUT2 (p<0.001) and within the same FUT2 subgroup, CEA levels differed by blood-group B status (p=0.001), yielding 4 genetic subgroups (Table 2). CEA levels were higher in active smokers, as previously described18, so active smokers were grouped separately (Figure 2). For CEA, the overall specificity using SNP-stratified cut-offs in the validation set was >98% (Table S2).
This diagnostic sensitivity of CEA for PDAC using a uniform cut-off was 13.8% (9.1%, 17.9%); SNP-stratified CEA sensitivity was 15.9% (95% CI: 11.6%, 21.1%). The specificity of SNP-stratified cut-off for CEA levels in controls in the validation set was 98.1% (95% CI: 95.55% to 99.37%), a non-SNP cut-off had a similar specificity of 98.8% (95% CI: 96.65% to 99.76%). CEA’s AUC was 0.7849, uniform diagnostic cut-off; 0.7724 using SNP-stratified cut-offs. With genotyping, 13 PDAC cases (5.3%) had their CEA levels reclassified compared to the positive/negative results set with the uniform cut-off (3.9 ng/ml)(Table S3).
CA125 levels in controls were associated with one variant (rs12469459) in GAL3ST221. In both discovery and validation control sets, there were significant differences in CA125 levels between GAL3ST2 rs12469459-AA subjects and rs12469459-AT and between rs12469459-AA compared to rs12469459-TT subjects (p=0.0007, p<0.0001, respectively for the discovery set). CA125 levels in discovery set controls trended higher in rs12469459-AT subjects compared to rs12469459-TT subjects (p=0.062); in the combined discovery/validation sets, rs12469459-AT subjects had higher CA125 levels than rs12469459-TT subjects (p=0.028). The specificity of SNP-stratified and non-SNP-stratified CA125 in the validation set were similar (97.8%, 97.4%)(Table S2).
The diagnostic sensitivity of CA125 using a uniform cut-off was 15.5% (95% CI: 11.2%, 20.8%); for SNP-stratified CA125 it was 17.6% (95% CI: 11.9%, 23.3%). The specificity of SNP-stratified CA125 in the validation set was 97.8% (95% CI: 94.47% to 99.40%), whereas a non-SNP cut-off had a specificity of 97.4% (95% CI: 93.71% to 99.10%). CA125’s AUC was 0.7943 using the uniform cut-off; 0.7752 with SNP-stratified cut-offs. Genotyping reclassified the CA125 results of 6.3% of PDAC cases compared with a uniform cut-off (19.2 U/ml)(Table S3).
Combining tumor markers modestly improved diagnostic sensitivity but reduced specificity to a similar degree. Thus, overall sensitivity of combined CA19-9/CEA, CA19-9/CA125, and CA19-9/CEA/CA125 was 64.5%, 64.1%, and 66.1%, respectively, which yielded specificities in the validation set controls of 96.9%, 97.3%, and 95.4%, respectively (Table S4). Combining CA19-9 and CEA in patients with Stage I disease with intact FUT3 cases yielded a sensitivity of 44.4% (95% CI: 29.6%, 60.0%)(Table S4).
The diagnostic sensitivity of thrombospondin-2 was 18.0% (95% CI: 13.0%, 23.9%), and overall specificity (discovery and validation set controls) was 97.4% (95% CI: 95.4%, 98.6%). Within validation set controls, thrombospondin-2 had a specificity of 96.7% (95% CI: 93.2%, 98.6%). Adding thrombospondin-2 to SNP-stratified CA19-9 increased sensitivity to a similar degree as CEA or CA125 but reduced specificity more than combinations of SNP-stratified CA19-9 with CEA and/or CA-125 (Table S4). Additional results in Supplemental Materials.
DISCUSSION
We find that a CA19-9 SNP test improved CA19-9’s performance as a tumor marker, converting it into a test with improved diagnostic performance, especially in the ~90% of subjects with intact FUT3, achieving a 66.4% sensitivity for patients with localized PDAC at a specificity 99.3%. Most reclassified PDAC cases were FUT3 heterozygotes (who have a low CA19-9 reference range) classified as having elevated CA19-9 by SNP-stratified cut-offs but normal CA19-9 using the uniform diagnostic cut-off. Setting uniformly high diagnostic cut-offs for biomarkers can improve diagnostic specificity, but at the expense of reduced diagnostic sensitivity especially for small cancers14, 31; (in our study a uniform 99% specificity cut-off for CA19-9 yielded a sensitivity of 52.7%). We identify four genetic subgroups with respect to CA19-9; this four-group classification improves CA19-9’s performance as a diagnostic test compared to a three-group classification suggested in recent studies17,22, 32. The diagnostic value of SNP stratification for CA19-9 is particularly evident when diagnostic sensitivity is evaluated at 99% specificity.
Setting high specificity cut-offs to limit the number of false-positive tests and understanding causes of false-positive tests are required elements of a diagnostic test offered to an asymptomatic high-risk population such as those in the CAPS program. Although the average lifetime risk of pancreatic cancer in this cohort is relatively high (~5-20%, depending on the extent of familial and inherited susceptibility), the probability of having pancreatic cancer at any one point in time is low, perhaps 0.2 to 1% so diagnostic specificity is especially important. One strength of this study is that we selected individuals in the CAPS program as a control group. This group is one of the main targets of a pancreatic cancer detection blood test.
Our results show that tumor SNP-defined normal reference ranges can reduce the number of false positive results. Once the normal reference ranges of these tumor markers have been determined, one still has to account for non-neoplastic causes of elevated CA19-9 or tumor other markers. Most diseases that cause elevated CA19-9 cause symptoms (e.g. cholangitis, acute pancreatitis)12, are uncommon, and require diagnostic evaluation anyway, so these clinical scenarios are very different to the scenario of an asymptomatic high-risk individual undergoing tumor marker surveillance who presents with a positive tumor marker test. Studies have shown some FUT3-null subjects have detectable CA19-9 levels, perhaps from cross reactivity with other antigens33. Identifying other rare causes of elevated CA19-9, (e.g. polycystic or large liver/renal cysts)34, can usually be achieved with abdominal imaging undertaken during pancreatic surveillance.
A prior GWAS identified the GAL3ST2 rs12469459 variant as influencing CA125 levels 21. Our results provide evidence that this variant influences CA125 levels enough to improve its diagnostic performance. A CA125 SNP test could have diagnostic value for patients undergoing surveillance for ovarian cancer but this possibility requires further investigation.
Although our test performed well at detecting cases with localized pancreatic cancers, the toughest hurdle for a pancreatic cancer detection blood test is the detection of Stage I disease. Cancers generally shed biomarkers in proportion to their size and small Stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation making diagnosis more difficult. Evaluating biomarkers of Stage I pancreatic cancers is challenging because very few patients are diagnosed with Stage I disease. Such patients are usually asymptomatic and found through surveillance. We did enrich our study population with Stage I cases (20% of the localized PDAC cases) and found SNP-stratified CA19-9 had 40% sensitivity for detecting Stage I disease PDAC cases with intact FUT3.
Our results demonstrate that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and to a lesser extent CEA and CA125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer.
The precision of the specificity cut-offs was set using a large number of controls. Our sample size was adequate for defining diagnostic cut-offs for common genetic subgroups, but larger sample sizes are needed to define better tumor marker reference ranges in rarer genetic subgroups.
Overall, the diagnostic sensitivity and specificity achieved using SNP-stratified reference ranges for CA19-9 justifies further evaluation of a CA19-9 SNP test in a prospective study for subjects undergoing pancreatic surveillance.
In conclusion, a tumor marker SNP test can be used to define SNP-stratified reference ranges for CA19-9, CEA and CA125. Evaluation of the use of SNP-stratified CA19-9 measurements as an early pancreatic cancer detection test for high-risk individuals with intact FUT3 undergoing pancreatic surveillance appears warranted.
Supplementary Material
Need to Know.
Background: Single-nucleotide polymorphisms (SNPs) have been associated with blood levels of tumor proteins. These SNPs can affect accuracy of assays for these proteins in detection of pancreatic cancer.
Findings: Ranges of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), CA-125 in blood of patients without cancer vary based on SNPs in 4 genes. When we adjusted the cutoff values for these proteins based on the SNP information, these assays identified patients with pancreatic cancer with higher levels of specificity.
Implications for patient care: This blood test might be used to aid in detection of patients with early-stage pancreatic cancer.
Acknowledgments
Grant Support: This work was supported by NIH grants (U01210170, CA62924 and R01CA176828), Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, the Rolfe Pancreatic Cancer Foundation and by a Stand Up To Cancer-Lustgarten Foundation Pancreatic Cancer Interception Translational Cancer Research Grant (Grant Number: SU2C-AACR-DT25-17). Stand Up To Cancer is a program of the Entertainment Industry Foundation. SU2C research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. MG is the Sol Goldman Professor of Pancreatic Cancer Research.
Abbreviations used in this paper:
- PDAC
pancreatic ductal adenocarcinoma
- IPMN
intraductal papillary mucinous neoplasm
- PanIN
pancreatic intraepithelial neoplasia
- GWAS
Genome-wide association studies
- HRIs
high-risk individuals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any personal or financial conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Canto MI, Almario JA, Schulick RD, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology. 2018; 155(3):740–51.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34:2010–9. [DOI] [PubMed] [Google Scholar]
- 4.Konings IC, Harinck F, Poley JW, et al. Prevalence and Progression of Pancreatic Cystic Precursor Lesions Differ Between Groups at High Risk of Developing Pancreatic Cancer. Pancreas. 2017;46:28–34. [DOI] [PubMed] [Google Scholar]
- 5.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clinical Cancer Res. 2010;16(20):5028–37. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointestinal Surg. 2012;16:771–83.. [DOI] [PubMed] [Google Scholar]
- 8.Brentnall T, Bronner M, Byrd D, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. [DOI] [PubMed] [Google Scholar]
- 9.Krishna SG, Rao BB, Ugbarugba E, et al. Diagnostic performance of endoscopic ultrasound for detection of pancreatic malignancy following an indeterminate multidetector CT scan: a systemic review and meta-analysis. Surgical endoscopy. 2017;31:4558–67. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Blackford A, Dal Molin M, et al. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut. 2015;64:1783–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke FS, Matthai E, Slater EP, et al. German National Case Collection for familial pancreatic Cancer (FaPaCa) - acceptance and psychological aspects of a pancreatic cancer screening program. Hered Can Clin Prac. 2018;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clinical Cancer Res. 2011;17:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellby LD, Nyberg AP, Johansen JS, et al. Serum Biomarker Signature-Based Liquid Biopsy for Diagnosis of Early-Stage Pancreatic Cancer. J Clin Oncol. 2018;36:2887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Science translational medicine. 2017;9(398). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 1987;47:5501–3. [PubMed] [Google Scholar]
- 16.Narimatsu H, Iwasaki H, Nakayama F, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res 1998;58:512–8. [PubMed] [Google Scholar]
- 17.Luo G, Guo M, Jin K, et al. Optimize CA19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology 2016;16:1057–62. [DOI] [PubMed] [Google Scholar]
- 18.Kawai S, Suzuki K, Nishio K, et al. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int J Cancer 2008;123(12):2880–4. [DOI] [PubMed] [Google Scholar]
- 19.Guo M, Luo G, Lu R, et al. Distribution of Lewis and Secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS open bio. 2017;7:1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, Wu C, Xu J, et al. A genome wide association study of genetic loci that influence tumour biomarkers cancer antigen 19-9, carcinoembryonic antigen and alpha fetoprotein and their associations with cancer risk. Gut. 2014;63:143–51. [DOI] [PubMed] [Google Scholar]
- 21.Folkersen L, Fauman E, Sabater-Lleal M, et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS genetics. 2017;13:e1006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wannhoff A, Folseraas T, Brune M, et al. A common genetic variant of fucosyltransferase 2 correlates with serum carcinoembryonic antigen levels and affects cancer screening in patients with primary sclerosing cholangitis. UEGW. 2016;4:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wannhoff A, Hov JR, Folseraas T, et al. FUT2 and FUT3 genotype determines CA19-9 cut-off values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 2013;59:1278–84. [DOI] [PubMed] [Google Scholar]
- 24.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. [DOI] [PubMed] [Google Scholar]
- 25.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. [DOI] [PubMed] [Google Scholar]
- 26.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804; quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe T, Blackford AL, Tamura K, et al. Deleterious Germline Mutations Are a Risk Factor for Neoplastic Progression Among High-Risk Individuals Undergoing Pancreatic Surveillance. J Clin Oncol 2019;37:1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canto MI, Kerdsirichairat T, Yeo CJ, et al. Surgical Outcomes After Pancreatic Resection of Screening-Detected Lesions in Individuals at High Risk for Developing Pancreatic Cancer. Journal Gastrointestinal Surg 2019;doi: 10.1007/s11605-019-04230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosman F, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive System. 4th ed. 2010. [Google Scholar]
- 30.van Altena AM, Holtsema H, Hendriks JC, et al. Cancer antigen 125 level after a bilateral salpingo-oophorectomy: what is the contribution of the ovary to the cancer antigen 125 level? Menopause (New York, NY). 2011;18:133–7. [DOI] [PubMed] [Google Scholar]
- 31.Luo G, Fan Z, Cheng H, et al. New observations on the utility of CA19-9 as a biomarker in Lewis negative patients with pancreatic cancer. Pancreatology 2018;18:971–6. [DOI] [PubMed] [Google Scholar]
- 32.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. PNAS. 2017;114:10202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wannhoff A, Weiss KH, Hackert T, et al. Comment re: "Optimize CA19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping". Pancreatology 2017;17:354–5. [DOI] [PubMed] [Google Scholar]
- 34.Waanders E, van Keimpema L, Brouwer JT, et al. Carbohydrate antigen 19-9 is extremely elevated in polycystic liver disease. Liver international 2009;29:1389–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.