Abstract
Several reports have shown the presence of P2 receptors in hematopoietic stem cells (HSCs). These receptors are activated by extracellular nucleotides released from different sources. In the hematopoietic niche, the release of purines and pyrimidines in the milieu by lytic and nonlytic mechanisms has been described. The expression of P2 receptors from HSCs until maturity is still intriguing scientists. Several reports have shown the participation of P2 receptors in events associated with modulation of the immune system, but their participation in other physiological processes is under investigation. The presence of P2 receptors in HSCs and their ability to modulate this population have awakened interest in exploring the involvement of P2 receptors in hematopoiesis and their participation in hematopoietic disorders. Among the P2 receptors, the receptor P2X7 is of particular interest, because of its different roles in hematopoietic cells (e.g., infection, inflammation, cell death and survival, leukemias and lymphomas), making the P2X7 receptor a promising pharmacological target. Additionally, the role of P2Y12 receptor in platelet activation has been well-documented and is the main example of the importance of the pharmacological modulation of P2 receptor activity. In this review, we focus on the role of P2 receptors in the hematopoietic system, addressing these receptors as potential pharmacological targets.
Keywords: P2 receptor, Hematopoiesis, Hematopoietic stem cell, Leukemia, Platelets, Pharmacological target
Introduction
The regulation of quiescence, self-renewal, proliferation, differentiation, and cell death of the hematopoietic stem cell (HSC) is the core of the hematopoietic system. This process is known as hematopoiesis and is regulated by several signaling molecules, such as cytokines, which are the main regulators of hematopoiesis. However, other signaling molecules, such as ATP, are also relevant in this process. ATP is an important molecule linked to fundamental metabolic processes that also regulate the hematopoietic system by being the main agonist of ATP receptors (known as P2 receptors or purinergic receptors). These receptors are expressed in the most primitive hematopoietic cells until mature progeny. Although P2 receptors are mainly known to regulate the immune system, new physiological activities are being investigated.
ATP under normal conditions is at the millimolar range in the cytoplasm (3–10 mM) and at the nanomolar range in the extracellular side, providing a high chemical gradient [1]. ATP, in its ionized form (ATP4−) [2], can be released through the plasma membrane by lytic and nonlytic mechanisms in favor of a chemical gradient and due to the electrical potential difference across the plasma membrane, which is negative on the inside. A loss of plasma membrane integrity by stress mechanisms, or by cell death, leads to the release of intracellular ATP. Over the years, several nonlytic mechanisms by vesicles and conductive ATP release have been described [3]. For example, a cornerstone study published almost half a century ago demonstrated the release of ATP contained in vesicles with other neurotransmitters [4], which initiated the boom of investigations of purinergic receptors, while more recent ones showed the release of ATP under hypoxia by endothelial cells [5] and osteoblasts [6]. The activation of mechanoreceptors also promotes the release of ATP by osteoblasts [7, 8]. Another mechanism of ATP release was described by the autocrine activation of P2 receptors by ATP itself, with the participation of membrane channels formed by the pannexin-1 protein, which is highly expressed in various tissues [9–11]. Hemichannels formed by connexins are also another form of ATP release [12, 13]. Paracrine/autocrine ATP signaling on neutrophils has also been investigated either through the pannexin channels or by the vesicular nucleotide transporter [14, 15]. In addition to the cells described above, other cell types, such as erythrocytes [16], epithelial cells [17], and hepatocytes [18] can also release ATP. These reports have demonstrated the range of physiological situations in which P2 receptors can be activated.
Over the last decades, it has been shown that all hematopoietic cell types express P2X (ionotropic) and/or P2Y (metabotropic) P2 receptors that are activated by ATP and its analogs. The effects of ATP vary according to cell type, the degree of differentiation, agonist concentration, and the time of stimulus due to the large number of receptors and differences in the pharmacological characteristics of these receptors. In this review, we focus on the participation of P2 receptors in the hematopoietic system and how the knowledge of purinergic regulation can be used as potential pharmacological targets in blood disorders.
P2 receptors
Purinergic receptors are responsible for the transduction of signal triggers by extracellular nucleotides and nucleosides. P2X receptors are ligand-gated ion channels and assembled as homotrimers or heterotrimers [19]. To date, seven isoforms have been described (P2X1-7) (Fig. 1). These receptors have intracellular N- and C-subunits that bind to protein kinases and also have two transmembrane regions, the TM1, which is involved in the channel activation and the TM2, which coats the ionic pore [20, 21]. The homology among the P2X receptor genes is around 40–60% in mammals and the subtypes differ in their rates of desensitization; ion conductivity; and sensitivity to agonists, antagonists, and allosteric modulators [22, 23]. P2X1-7 receptors have three subunits that form a stretched trimer or a hexamer of conjugated trimers [24, 25], while transition metals such as Zn2+ and Cu2+ ions act as allosteric modulators [26]. Other metals, such as lanthanides, may also modulate P2X receptors, and ethanol has been shown to reduce the potency of ATP in several P2X receptors [27]. A comparative study shows 180 putative P2X genes in 32 animal species and four members in three species of lower plants exploring the evolutional relationship among different subtypes of P2X genes [28].
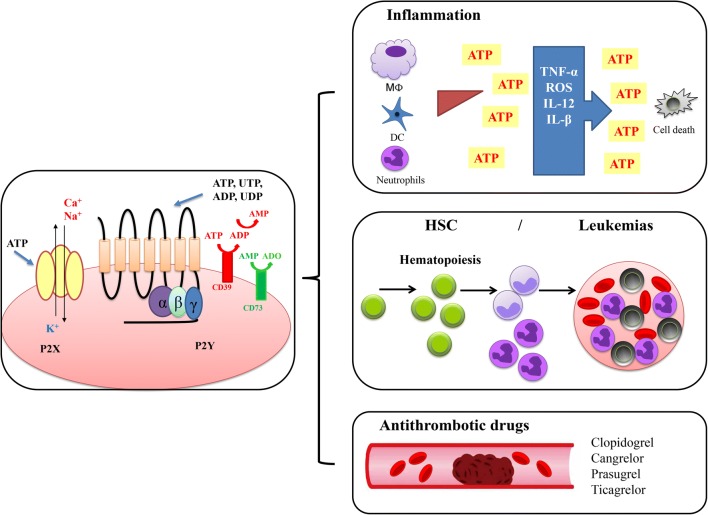
Fig. 1.
Roles of purinergic regulation in the hematopoiesis. ATP is an important molecule that also regulates the hematopoietic system by activation of receptors, known as P2 or purinergic receptors. Purinergic receptors are responsible for the signal transduction triggered by extracellular nucleotides and nucleosides. The release of ATP directly promotes the activation of P2X (ionotropic) and P2Y (metabotropic) receptors triggering signaling associated to Ca2+ mobilization, or by activation of enzymes linked to G proteins. The activation of these intracellular pathways affects the hematopoiesis, for instance modulating inflammation, HSC differentiation, and coagulation. The complexity of purinergic regulation involves not only the primary agonists and their receptors but also enzymes such as CD39 that hydrolyzes ATP to ADP and ADP to AMP, which can subsequently be hydrolyzed by CD73 to adenosine, which activates P1 receptors. The most emblematic clinical application of knowledge about the regulation of P2 receptors is the use of inhibitors of P2Y12 receptor such as clopidogrel, used as an antithrombotic drug
P2X1 receptor is widely expressed in smooth muscle and mediates the neurotransmitter actions of ATP released by the sympathetic and parasympathetic nerves [29, 30]. P2X1 receptor is also responsible for mediation of Ca2+ influx in platelet aggregation [31]. P2X2 receptors are widely expressed in central and peripheral neurons and have been implicated in neurotransmission [32, 33]. P2X3 receptors are expressed in sensory neurons, including nociceptive nerve endings [34, 35]. The agonist α,β-methylene-ATP distinguish the receptors (P2X1, P2X3) from the other homomeric forms (P2X2, P2X4, P2X6 and P2X7) [30]. However, the antagonist NF023, an analog of suramin, is about 20 times less effective in P2X3 compared to P2X1 receptors [36]. There are no selective agonists known for P2X2 receptors [19].
The receptors P2X4 and P2X6 are expressed in the central nervous system, endothelial cells, placenta, and thymus [37, 38]. Carbamazepine derivatives are potent agonists of the P2X4 receptor [39], and the allosteric modulator ivermectin increases ATP concentration and potentiates its effects [39]. The receptor P2X5 is highly expressed in skeletal muscle, skin, and epithelium [35]. ATP and 2-methylthio-ATP are full agonists for the P2X5 receptor, which in turn is inhibited by TNP-ATP, PPADS, and suramin [40, 41]. The P2X6 receptor was discovered in rat brain and by homology [42], being only functionally expressed as a heteromultimer [38, 43]. P2X7 receptor is expressed in immune cells, pancreas, skin, and microglia [35, 44]. Prolonged exposure of P2X7 receptor to high concentrations of ATP may lead to cell death [45–47]. BzATP is currently the most potent agonist at P2X7 receptors [42, 48], while oxidized ATP and KN-62 are selective antagonists [49, 50]. However, anti-inflammatory effects of oxidized ATP, independent of the expression or activation of P2 receptors, were described [51]. In addition, new antagonists for P2X7 receptor such as brilliant blue G, A740003, calmidazolium, AZ11645373, and ZINC58368839 have improved the pharmacological characterization of this receptor [52, 53]. Alternatively, a nanobody that specifically inhibits the P2X7 receptor was development [54]. This nanobody was 1000 times more potent in preventing IL-1b release than the P2X7 antagonists JNJ47065567 and AZ10606120 [54].
P2Y receptors are a family of G protein-coupled receptors, stimulated by nucleotides such as ATP, ADP, UTP, UDP, and UDP-glucose (Fig. 1). So far, there are eight P2Y receptors found in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 [55]. Members of the P2Y1 receptor subgroup (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) are conjugated with Gαq/11 proteins that activate phospholipase C. P2Y11 also binds to Gαs via protein/adenylate cyclase [56]. Members of the P2Y12 receptor subgroup (P2Y12, P2Y13, P2Y14) are coupled to Gαi/o protein. P2Y1, P2Y12, and P2Y13 are activated by ATP and ADP [57–59], P2Y2 and P2Y4 are activated also by UTP [60, 61], and P2Y6 is activated by UTP [61], but P2Y14 receptor is only activated by UDP-glucose [62].
Presence and participation of P2 receptors in the immune system
Among the initial descriptions of the participation of purinergic receptors in cells of the hematopoietic system, we can highlight the work of Cockcroft & Gomperts in mast cells [63]. In this study, it was observed that ATP produced the release of histamine and the secretion of metabolites [63]. One particular receptor was described in macrophages in the murine J774 lineage, which was initially called the P2Z receptor [64] and is currently known as the P2X7 receptor [42]. Activation of the P2X7 receptor has been implicated in the formation of a cytolytic pore that allows the passage of molecules up to 900 Da [42, 64]. Subsequently, the P2X7 receptor was described in monocytes and macrophages of various origins [47, 65–67]. However, the P2X7 receptor is not the only receptor expressed in macrophages; in contrast, it is common that primary cells express more than one P2 receptor.
In addition to monocytes/macrophages, other myeloid cells also express P2 receptors. ATP and UTP promote an increase in the concentration of cytoplasmic Ca2+ ([Ca2+]cyt) in eosinophils, which was inhibited by the pertussis toxin, an inhibitor of Gαi/o protein, and also by U73122, an inhibitor of phospholipase C [68]. Different types of P2X and P2Y receptor agonists promote several effects in eosinophils, such as an increase in [Ca2+]cyt, actin polymerization, reactive oxygen (ROS), and nitrogen (RNS) production increased CD11b expression and chemotaxis [69]. The P2Y2 receptor was shown to be involved in asthmatic airway inflammation in a mouse model caused by mediating ATP-triggered dendrocytes and eosinophils [70].
Macrophages stimulated with ATP were able to kill both bacteria Escherichia coli e Staphylococcus aureus, but macrophages isolated from P2X4−/− animals were not able to induce Escherichia coli death, whereas no differences were observed in macrophages isolated from P2X7−/− animals [71]. In addition, infected animals P2X4−/− have a lower survival rate than their normal counterpart [71]. P2X7−/− deficient mice were more susceptible to sepsis and the floxed P2X7 conditional knockout mouse model (P2X7fl/fl-LysM-Cre), without expression of P2X7 receptor in myeloid cells, showed higher levels of cytokines and infection [72].
Studies performed in granulocytes have shown that P2X7 receptor activation generated superoxide anion and secretory granule exocytosis by increasing [Ca2+]cyt and protein kinase C activation [73, 74]. It has also been reported that activation of the P2X7 receptor promotes apoptosis in murine granulocytic cells and that the ability to promote apoptosis is reduced by aging, with a concomitant decrease in P2X7 expression [46]. P2X1 receptor is involved in endotoxemia by its participation in the chemotaxis of neutrophils [75], P2X1−/− mouse shows reduced inflammatory response with resistance to LPS-induced death [76]. Additionally, the evaluation of P2 receptor expression in murine granulocytes showed the presence of the P2Y1, P2Y2, and P2Y4 receptors, with heteromeric association of the P2Y1/P2Y2 and P2Y1/P2Y4 receptors [77].
Although there are several works on the release of ATP and osmotic regulation in erythrocytes and those related to the presence of P2 receptors and ectoATPases on the plasma membrane of erythrocytes [78–80], there are few works addressing the physiological mode of action regarding the signaling of P2 receptors in this cell type and its progenitor cells. The expression of P2X1, P2X4, P2X7, and P2Y1 receptors but not the P2Y2, P2Y4, or P2Y6 receptors was observed in erythroid precursor cells derived from human CD34+ cells [81]. Human erythrocytes were also responsive to ATP and BzATP by activation of the P2X7 receptor [82]. Murine erythroblasts express P2Y1, P2Y4, and P2Y12 receptors, and activation of the P2Y1 receptor in these cells promoted a biphasic increase in [Ca2+]cyt. The initial transient phase was dependent on inositol 1,4,5-trisphosphate, and the sustained phase was dependent on the activation of protein kinase C and Ca2+ calmodulin kinase II [83].
Although the presence of P2 receptors in hematopoietic cells has been addressed in previous reviews [44, 84, 85], a complete molecular and pharmacological characterization of P2 receptors in different types of hematopoietic cells is still required.
One of the main functions of differentiated leukocytes (granulocytes, monocytes/macrophages, dendritic cells, and B and T lymphocytes) is their involvement in inflammation and the immune response (Fig. 1). Extracellular nucleotides can act as immunomodulators in the cellular response of these hematopoietic lineages [86–88].
Extracellular ATP may act as an endogenous adjuvant to initiate the inflammatory process by stimulating P2 receptors. The participation of the P2X7 receptor is the most studied in the immune system, since it participates in innate and adaptive immune responses. The activation of the P2X7 receptor by ATP is a signal for the activation of the inflammasome, inducing both maturation and release of proinflammatory cytokines such as IL-1β and IL-18 and the production of ROS and RNS [89, 90]. Moreover, during the adaptive immune response, the P2X7 receptor modulates the balance between the generation of T helper lymphocytes type 17 (Th17) cells and T regulatory (Treg) cells [89, 91].
The main function of ROS production during the inflammatory response is the destruction of microorganisms, and neutrophils as well as other leukocytes (lymphocytes and monocytes/macrophages), play a central role in this process [92]. After recruitment, neutrophils migrate to the inflammatory and/or infectious site, and together with macrophages, are able to eliminate the aggressor agent. There are primary and secondary granules inside neutrophils. The primary granule content enzymes, such as myeloperoxidase, are associated with the phagocytic process mainly in neutrophils (rather than macrophages). In the phagocytic phase, activation of the enzyme NADPH oxidase occurs, which together with the influx of ions, induces the myeloperoxidase activity. The activation of myeloperoxidase leads to the production of hypochlorous acid (HClO) that possesses microbicidal activity, which is produced from H2O2 and Cl− ions, and may also cause oxidative damage [93].
There is evidence that activation of the neuronal P2X7 receptor leads to the production of ROS and subsequent nociceptive pain in mice [94]. ROS produced by activation of the P2X7 receptor play a critical role in the P2X7 signaling pathway of macrophages and microglia in the central nervous system [94]. It has been shown that pretreatment with a P2X7 receptor antagonist or an antioxidant efficiently protected cells from caspase-dependent cell death triggered by ultraviolet irradiation [95]. These results showed that autocrine signaling by the release of ATP and activation of the P2X7 receptor are required for the stimulation of oxidative stress in monocytes.
It should be noted that numerous intracellular pathways related to inflammation and activation by the P2X7 receptor have been described, including the inflammasome NLRP3, NF-kB, NFAT, GSK3β, and VEGF [96–98].
Inflammasome NLRP3 is an intracellular sensor that detects a broad range of viral infection, bacterial DNA, bacterial toxins, and protozoaries, and by P2X7 activation, resulting in the formation and activation of the NLRP3 inflammasome [99–101]. Activation of Toll-like receptor, tumor necrosis factor receptor, or IL-1 receptor leading to the transcriptional upregulation inflammasome components by NF-kB [99]. Initially, it was described that secreted ATP through pannexin-1 in bone marrow cells triggers activation of the complement cascade activation, initiating the mobilization of HSC from bone marrow to peripheral blood, whereas P2X7−/− mice were poor mobilizers [102]. Then, the same group showed that HSC mobilization to peripheral blood by the ATP-driven NLRP3 inflammasome leads to the release of IL-1β and IL-18, as well as several danger-associated molecular pattern molecules [103]. In addition, adenosine exhibited an inhibitory function in this process, working as a negative feedback molecule [104]. These reports showed the participation of NLRP3 inflammasome/P2X7 in HSC mobilization.
P2X7 activation was usually associated with cell death. An unexpected role of P2X7 receptor in the immune system is its participation in the maintenance of immune cells. For instance, the P2X7 receptor is required for the establishment, maintenance, and functionality of long-lived central and tissue-resident memory CD8+ T cell populations [105]. This capability of P2X7 receptor provides a common currency that both alerts the nervous and immune systems to tissue damage, promoting a durable adaptive immunological memory related to the immune response against reinfection, which is the basis of vaccines [105].
The P2X7 receptor may be involved in the pathophysiology of chronic inflammatory diseases, and this receptor could be a therapeutic target by the use of antagonists [96, 106].
The development of a tumor generated by injection of murine B16 melanoma was compared in a P2X7−/− deficiency mouse model versus the use of P2X7 antagonist, A740003. P2X7 pharmacological blockade does not replicate the immunosuppressive effects due to genetic ablation, rather it enhances tumor infiltration by CD4+ T effector cells and decreases CD39 and CD73 expression, thus reducing immunosuppression in the tumor microenvironment [53].
The P2X7 receptor expressed in macrophages may be implicated in several pathophysiological mechanisms in infections, such as in the spread of human immunodeficiency virus infection since the stimulation of ATP promoted the release of microvesicles loaded with the human immunodeficiency virus [107]. In addition, the P2X7 receptor may be involved in inflammation and coagulation. The stimulation of P2X7 receptors in macrophages increased the expression and release of microvesicles containing tissue factor, producing a strong prothrombotic response [108]. It is important to emphasize that the activation of the P2X7 receptor induces the release of proinflammatory factors, such as IL-6 (fibroblasts, microglia, mast cells, and dendritic cells), and may also release anti-inflammatory factors, such as IL-10 and TGF-β, in macrophages [109–112].
P2 receptors and their involvement in hematopoiesis
Although hematopoietic cells are known to express P2 receptors, their involvement in hematopoiesis has not yet been fully elucidated. To date, the presence of these receptors, even in the most undifferentiated cells, and the variation in their expression with differentiation, indicate their probable participation in hematopoiesis. Endothelial cells and osteoblasts, which can release ATP [5, 6, 8], are cells present in the different bone marrow niches where HSCs are present; ATP and other signaling molecules released by these cells or by another mechanism can regulate the activity of HSCs [113, 114] (Fig. 2).
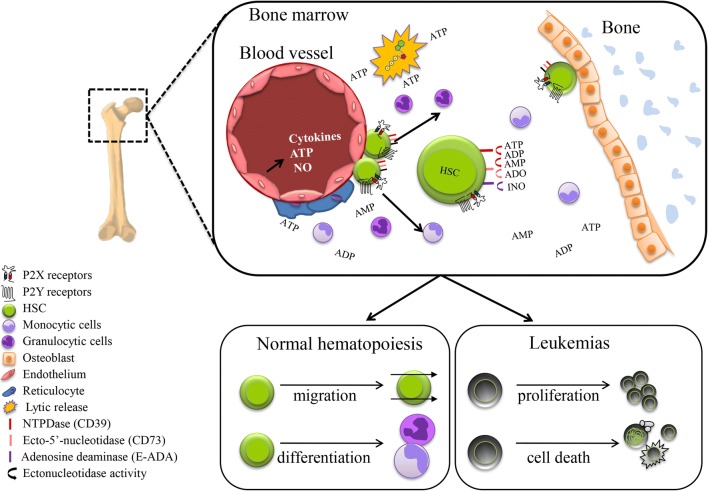
Fig. 2.
Regulation of HSC by purinergic regulation. The bone marrow is the hematopoietic environment where HSC resides. HSC is mainly regulated by cytokines, but other signaling molecules such as ATP also can modulate hematopoiesis. ATP can be released by lytic and non-lytic mechanisms by cells of the hematopoietic environment, such as endothelial cells and osteoblasts. Recent reports have shown that ATP and analogs promote differentiation and migration of HSC. But the real participation of P2 receptors and whether the purinergic signaling foments or avoids the growth of leukemias, is not clear. The presence of several P2 and P1 receptors and ectonucleotidases, which hydrolysis of ATP/UTP, evince the complexity of purinergic regulation in the hematopoietic microenvironment
Synergism between ATP and cytokines has been shown by increasing the primitive human hematopoietic CD34+ population in methylcellulose [115]. This primitive human hematopoietic CD34+ subset expresses several P2 receptors, including P2Y1, P2Y2, P2Y12, and P2X1-7 [115, 116]. Furthermore, UTP stimulation increased the migration of HSCs, inhibited the downregulation of the CXC type 4 chemokine receptor and increased the fibronectin adhesion of human CD34+, which were observed in vitro [117]. Using an in vivo assay, immunodeficient animals showed that the stimulation of human CD34+ cells with UTP increased the homing efficiency [117]. Differentiation by the direct modulation of primitive hematopoietic cells by ATP has also been reported. ATP and its analogs produce a large increase in [Ca2+]cyt, leading to the myeloid differentiation of hematopoietic primitive cells in bone marrow cultures, unlike cytokines, which produce a small [Ca2+]cyt increase [118]. Subsequently, it was shown that the differentiation promoted by ATP mainly affected HSCs and granulocyte-macrophage progenitor populations [119]. Moreover, it has been reported that ATP reduced the number of hematopoietic primitive populations, increased the myeloid population Gr-1+Mac-1+, decreased Notch-1 receptor expression, and reduced both quiescence and the engraftment ability of HSCs [119]. Another interesting aspect observed was the increase in [Ca2+]cyt by ATP, which was reduced in the presence of cytokines such as interleukin-3 and stem cell factor in HSCs, reducing the differentiation effect of ATP [119].
The P2Y14 receptor was described as a hematopoietic modulator. Under normal conditions, the absence of the receptor in knockout animals (P2Y14−/−) did not affect the biology of the cells of the hematopoietic system, but in situations such as radiation stress, aging, chemotherapy, and transplantation, the senescence pattern and cell death of HSCs were modified by increasing the levels of ROS production, the expression of p16INK4a, and the hypophosphorylation of retinoblastoma protein [120].
The participation of the P2X7 receptor in hematopoiesis has also been demonstrated. The P2X7 receptor is expressed weakly in murine HSCs, but overexpression of the P2X7 receptor in this population by viral transfection reduced both the ability to form colonies in vitro (mainly affecting the most primitive colony-forming unit of granulocytes/erythrocytes/macrophages/megakaryocytes), as well as its engraftment potential in vivo [121]. The mobilization of HSCs is important in processes of the immune response when damage to cells or tissues occurs or in response to drugs that mobilize HSCs, such as granulocytic colony-stimulating factor, a physiological cytokine. The mobilization of HSCs was affected by the release of ATP via pannexin-1 and the formation of its metabolite adenosine [102], whereas P2X7−/− knockout animals showed a low mobilization ability [102].
P2X7 polymorphism induces a function gain of P2X7, as in the presence of SNP Gln460Arg which is co-inherited with Ala348Thr to form the gain-of-function haplotype 4, then the mobilization ability increases [122].
The role of ectonucleotidases such as NTPDase (CD39) and ecto-5′-nucleotidase (CD73) may also be associated with regulation of the undifferentiated state of HSCs. In addition to the ATP-dependent differentiation of murine HSCs, HSCs exhibit higher expression of CD39 and CD73 than do mature hematopoietic cells [119]. Moreover, primitive c-Kit+ cells exhibited higher ectonucleotidase activity than did mature hematopoietic cells or CD45− Ter119− populations [119]. A previous report described the presence of a Treg CD150high population located in the niche of bone marrow HSCs [123]. A reduction in the Treg CD150high population from bone marrow, obtained by the conditional deletion of C-X-C chemokine receptor type 4, increased the number of HSCs [123]. Experiments that used animals with the conditional expression of CD39 on Treg CD150high cells showed that extracellular adenosine generated by the activity of the CD39 enzyme protects against oxidative stress and maintains HSCs in a quiescent state [123]. Moreover, Treg cells modulated the HSC population via activation of the CD73 enzyme [80]. Deletion of the CD73 enzyme increased the number, cell cycle frequency, and ROS production of HSCs [123, 124]. The use of antioxidants inhibited the increase in HSCs in CD73−/− knockout mice and maintained HSCs in a quiescent state [80]. Thus, the activity of CD39 and CD73 enzymes could prevent HSC differentiation promoted by ATP through its degradation by the production of adenosine, which maintains the quiescent state of HSCs (Fig. 2).
Purinergic receptors as likely therapeutic targets in leukemia
The importance of P2 receptors in cells from the hematopoietic system has been demonstrated by several studies; therefore, the investigation of the role of P2 receptors in related disorders has great relevance. Leukemias as well the other main hematological cancers arise from problems in the maturation and proliferation of primitive hematological cells. The alteration in purinergic regulation can currently be well-evaluated, and the investigation of basic mechanisms and alterations can provide novel diagnoses, prognoses, and treatments.
Initial studies have shown the presence of P2 receptors in leukemic lineages, such as HL-60 (acute promyelocytic leukemia) and CB1 (acute lymphoblastic leukemia) [125, 126]. Variation in P2 receptor expression and their functional responses has been reported during myeloid differentiation in leukemia lineages, particularly in the HL-60 lineage [125, 127, 128].
The expression and function of the P2X7 receptor are particularly evaluated for its unique pharmacological characteristics. Activation of the P2X7 receptor was initially correlated with cell death by its ability to form pores in the membrane [42, 45, 64, 129], but its activation was also related to cellular proliferation [130]. In leukemias, the participation of the P2X7 receptor in proliferation and cell death by apoptosis and necrosis was described. Initially, it was found that the P2X7 receptor was highly expressed in U-937 (histiocytic lymphoma) and KG-1 (acute myeloid leukemia-AML) lineages. In HL-60 and J6-1 (myeloid leukemia) lineages, the P2X7 receptor had moderate expression, while in K562 (chronic myeloid leukemia) and Burkitt lymphoma (Raji and Ramos) lineages, the expression of this receptor was not detected [131]. Another study reported functional expression of the P2X7 receptor in KG-1 cells [132]. In the murine erythroleukemia cell line, the P2X7 receptor was responsible for inducing cell death and the release of microparticles [133].
Some studies have identified alterations in the gene expression of P2 receptors in myeloid leukemias. Cells extracted from patients with AML exhibited higher expression of P2X1, P2X4, P2X5, and P2X7 receptors than those extracted from normal cells, whereas P2X2, P2X3, and P2X6 were not detected [131].
Among the positively regulated receptors in AML, the P2X7 receptor stands out. Studies have shown that the remission rate of AML is higher in patients with lower P2X7 receptor expression after chemotherapy and that patients with relapse have the highest levels of P2X7 expression [131, 134]. The morphological and immunophenotypic variations that differentiate myeloid leukemias are also reflected in the expression of the P2X7 receptor. High levels of the P2X7 receptor were observed in some AML subtypes, such as M4, M5, and M6, and low expression of this receptor was observed in the M2 and M3 subtypes, suggesting that its expression is higher due to the greater number of immature monocytes [131]. Antiproliferative effects were observed in blast cells and in leukemic stem cells of AML patients by stimulation with ATP, but the same was not observed in normal cells, generating expectations for innovative therapies [135, 136].
The effect of ATP release from chemotherapy-treated dying leukemia cells was investigated. The immunosuppressor effect of ATP release by the activation of P2X7 receptors induces the increase of Treg and dendritic cells [137].
A relationship between P2X7 receptor expression and apoptosis induction has been related in myeloid leukemias. The apoptosis elicited by aminopyridine 4, a proapoptotic agent, was Ca2+-dependent and blocked by KN-62, an antagonist of the P2X7 receptor [138]. The same results occur when cells of myeloid leukemia lineages are treated with ATP (enhanced Ca2+ influx, alteration of the cell cycle, the activation of intracellular caspases and consequent cellular apoptosis), suggesting a possible therapeutic ability of ATP [139]. However, some authors suggest that this cytotoxic effect mediated by P2X7 receptors may occur independently of the influx of Ca2+ [140]. Activation of the P2X7 receptor induced ROS formation in murine erythroleukemia, which may be involved with apoptosis [141].
A comparative study observed that the lineage of acute leukemia T cells (Jurkat) possess a larger number and more active mitochondria than their healthy counterparts promoting autocrine purinergic signaling and uncontrolled proliferation of leukemia cells [142]. The inhibition of P2X1 and P2X7 receptors reduced the decrease in mitochondrial activity and proliferation of leukemia cells [142].
B-cell chronic lymphocytic leukemia (B-CLL) is the most frequent leukemia among adults in the Western World. It is caused by the clonal expansion of B lymphocytes and is characterized by a loss of apoptotic capacity in CD5+ leukemic lymphocytes that accumulate in the blood [143, 144]. P2X7 receptor expression was correlated with disease severity in patients with B-CLL [145]. The expression of the P2X7 receptor was higher in patients with the most aggressive form of B-CLL [145].
Single nucleotide polymorphisms were observed in the gene encoding the P2X7 receptor in the KG1 lineage; polymorphisms such as H155Y and A348T were related to gain of function, and T357S and Q460R were related to loss of function [132]. Among the single nucleotide polymorphisms in the P2X7 receptor, 1513A/C is characterized by the substitution of adenine for cytosine at position 1513; this alteration converts glutamic acid to alanine at residue 496 and causes an almost complete loss of function of the P2X7 receptor [132]. Some studies investigated a possible relationship between this polymorphism and B-LLC. It was proposed that the loss of receptor function caused by this mutation could favor antiapoptotic effects and, consequently increase the pathogenesis [144]. The correlation between this polymorphism and the improvement of clinical conditions in patients with B-LLC was already described [146]. However, further studies did not support the role of the P2X7 genotype as a prognostic marker in B-cell CLL [147–150].
B cells from CLL patients have P2X7 receptor and ASC (apoptosis-associated speck-like protein containing a CARD) overexpressed while NLRP3 is down-modulated and promotes growth. On the other hand, NLRP3 overexpression stimulates cell death by apoptosis [151].
Although previous reports use the term apoptosis or necrosis-like to describe the P2X7 receptor-dependent cell death the revision of this term is necessary. The cell death elicited by P2X7 receptor activation is usually associated with membrane permeabilization, loss of cell and mitochondrial potential membrane, caspase activation, and release of interleukins [44–46, 67, 135, 140]. Pyroptosis is a programmed cell death initially described in monocytes/macrophages by caspase 1 activation [152]. Recent findings indicate that pyroptosis can be also driven by several other caspases including caspase 3 and murine caspase 11, human caspase 4 and 5 and can also occur in cell types other than cells from the immune system [153]. Sometimes it is associated with IL-1β and IL18 secretion, promoting the inflammation process, culminating with chromatin condensation as well as cellular swelling of plasma membrane permeabilization [152, 153]. Current studies are associating the cell death elicited by pannexin-1, caspases, and P2X7 with pyroptosis [154–157].
Taken together, these studies related to normal and altered hematopoiesis, illustrate an important role of P2 receptors, and among them, the P2X7 receptor arises as an important target to investigate against leukemias. The involvement of P2X7 receptor for or against tumor progression is still unclear. However, participation and function of other P2 receptors in leukemias and other disorders deserve more attention (Fig. 2).
Therapeutic application of a purinergic antagonist as an antithrombotic
Other hematopoietic populations such as platelets also respond to the ATP- and αβMeATP-induced increase in [Ca2+]cyt by activating the P2X1 receptor [158]. Among the P2Y receptors, the P2Y1 receptor initiates platelet aggregation by increasing [Ca2+]cyt, and the P2Y12 receptor modulates platelet aggregation by inhibiting adenylate cyclase [59, 159, 160]. Both receptors, P2Y1 and P2Y12, are mainly activated by ADP and analogs such as 2-methylthio-ADP, whereas ATP acts as a partial agonist or can even act as a competitive antagonist of P2Y1 and P2Y2 receptors [161–163]; the P2Y1 receptor is mainly coupled to the Gαq protein, while the P2Y12 receptor is coupled to protein Gαi [164]. The knowledge of purinergic signaling has been very promising in the development of antithrombotic drugs in platelet aggregation (Fig. 1).
There are already some commercial drugs that act as inhibitors of purinergic receptors blocking platelet aggregation. The first commercial drug that inhibited a P2 receptor as medicine was ticlopidine, which was discovered in 1972 and commercialized in 1978; used as a selective inhibitor of ADP-dependent platelet aggregation [165]. This investigation contributed to elucidating the role of ADP in arterial thrombosis. Clopidogrel was developed in 1986 and marketed in 1997 [166]. Ticlopinide and clopidogrel reduce the risk of stroke and heart attacks, especially when combined with aspirin [167, 168]. Ticlopinide and clopidogrel are inhibitors of the P2Y12 receptor and act as prodrugs [169]. Owing to the increased safety attributed to treatment with clopidogrel, it has become the most widely used inhibitor of the P2Y12 receptor, both clinically and experimentally [170].
Two new P2Y12 receptor inhibitors were approved for clinical use by the FDA in 2009 and 2011: prasugrel and ticagrelor [171]. They are more potent and have a faster action and a more predictable effect than clopidogrel, representing an advance in the treatment of acute coronary syndrome but with an increased risk of hemorrhage [171]. Prasugrel, similar to clopidogrel, is also a prodrug. Their active metabolites have the same affinity for the P2Y12 receptor, but the metabolism of prasugrel results in the most availability of the active component in vivo [172], in contrast to ticagrelor, which does not require metabolic activation [173].
The choice of drug in patients should be based on individual characteristics. Clopidogrel can be used when prasugrel and ticagrelor were not indicated [174]. However, there may be cases in which none of these oral inhibitors of the P2Y12 receptor can be indicated, since platelets may remain activated by other routes and are not inhibited by these agents [171]. Owing to the limitations of oral application, intravenous P2Y12 receptor antagonist drugs have been developed.
Cangrelor, an inhibitor of the P2Y12 receptor that does not require metabolic activation, was recently approved for use by the FDA [175]. This inhibitor has a similar structure to ATP, and ticagrelor binds directly and reversibly to the P2Y12 receptor [176, 177].
Elinogrel is also an intravenous drug that acts on the P2Y12 receptor but is still under clinical testing [171, 175]. It is also available in oral form and binds reversibly and directly to the P2Y12 receptor [171]. Elinogrel presents better levels of platelet aggregation than does clopidogrel but is associated with increased levels of liver enzymes and dyspnea [178].
Studies using P2Y1-knockout mice, thrombosis models, and P2Y1 receptor antagonists have shown that this receptor can also be an important potential target for novel antithrombotic drugs [159]. Some antagonists of the P2Y1 receptor are under investigation, such as MRS2179 and MRS2500 [171, 179]. In addition, the synergistic effect of the use of P2Y1 receptor antagonists in the presence of clopidogrel has been demonstrated in the prevention of thromboembolism [180].
Antagonists of P2 receptors are being tested in pathologies not related to platelet aggregation. Suramin, the oldest P2 antagonists, was synthesized in 1916 to treat protozoan infections. Its mechanism of action is related to the nonspecific and reversible inhibition of P2 receptors [181, 182]. Diquafosol acts on the P2Y2 receptors as an agonist, increasing the transport of liquid via chloride channels [183] and facilitating the secretion of mucin by conjunctival epithelial cells [184].
As ATP is degraded to adenosine by ectonucleotidases, it was observed that adenosine exerts regulatory effects by activating P1 receptors; therefore, ectonucleotidases have also become a target of research. Preclinical studies have shown that it is possible to reduce tumor growth by inhibiting CD73 [185]. Phase I clinical studies have been conducted to inhibit this ectoenzyme through monoclonal antibodies (nivolumab and durvalumab) and small molecules as substituted derivatives of benzothiadiazine, osimertinib, and CPI-444 [186].
Conclusions
The presence of these P2 receptors, even in the most undifferentiated cells of the hematopoietic system, and the variation in their expression in the differentiation process, suggests the participation of P2 receptors in several aspects of hematopoiesis. Important reports in this area have shown the participation of P2 receptors in the modulation of normal and leukemic HSCs. This complex system is controlled by P2 receptors with the participation of ectonucleotidases and the activation of P1 receptors by adenosine. These studies demonstrate complex purinergic regulation that can be used for clinical purposes. The perspective of developing new drugs for hematological and inflammatory diseases by the modulation of purinergic receptors is very promising. Many drugs that are under preclinical testing are also very promising and expected to revolutionize the treatment of these diseases, thereby improving the lives of patients.
AcknowledgmentsThe authors would like to thank Professor Danilo Wilhelm Filho and Professor Ed Yates for constructive criticism of the manuscript.
Funding information
This publication and the previous related results were supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP. Proc. 2018/23870-4) and “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq 425965/2018-0).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alice Teixeira Ferreira, Email: atferreira@unifesp.br.
Edgar J. Paredes-Gamero, Email: edgar.gamero@ufms.br
References
- 1.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615(1-2):7–32. doi: 10.1016/S0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 2.Cockcroft S, Gomperts BD (1979) Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol:296229–296243. 10.1113/jphysiol.1979.sp013002 [DOI] [PMC free article] [PubMed]
- 3.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8(3):359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G, Dumsday B, Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim To WK, Kumar P, Marshall JM. Hypoxia is an effective stimulus for vesicular release of ATP from human umbilical vein endothelial cells. Placenta. 2015;36(7):759–766. doi: 10.1016/j.placenta.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220(1):155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 7.Hayton MJ, Dillon JP, Glynn D, Curran JM, Gallagher JA, Buckley KA. Involvement of adenosine 5′-triphosphate in ultrasound-induced fracture repair. Ultrasound Med Biol. 2005;31(8):1131–1138. doi: 10.1016/j.ultrasmedbio.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Alvarenga EC, Rodrigues R, Caricati-Neto A, Silva-Filho FC, Paredes-Gamero EJ, Ferreira AT. Low-intensity pulsed ultrasound-dependent osteoblast proliferation occurs by via activation of the P2Y receptor: role of the P2Y1 receptor. Bone. 2010;46(2):355–362. doi: 10.1016/j.bone.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580(1):239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828(1):15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Miteva AS, Gaydukov AE, Shestopalov VI, Balezina OP. Mechanism of P2X7 receptor-dependent enhancement of neuromuscular transmission in pannexin 1 knockout mice. Purinergic Signal. 2018;14(4):459–469. doi: 10.1007/s11302-018-9630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95(26):15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28(18):4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledderose C, Bao Y, Zhang J, Junger WG. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signalling during immune cell activation. Acta Physiol (Oxf) 2015;213(2):334–345. doi: 10.1111/apha.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada Y, Kato Y, Miyaji T, Omote H, Moriyama Y, Hiasa M. Vesicular nucleotide transporter mediates ATP release and migration in neutrophils. J Biol Chem. 2018;293(10):3770–3779. doi: 10.1074/jbc.M117.810168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41(5):525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors--recent progress and persisting challenges. Purinergic Signal. 2012;8(3):375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves LA, da Silva JH, Ferreira DN, Fidalgo-Neto AA, Teixeira PC, de Souza CA, Caffarena ER, de Freitas MS. Structural and molecular modeling features of P2X receptors. Int J Mol Sci. 2014;15(3):4531–4549. doi: 10.3390/ijms15034531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokic MB, Stojilkovic SS (2013) Two open states of P2X receptor channels. Front Cell Neurosci 7215. 10.3389/fncel.2013.00215 [DOI] [PMC free article] [PubMed]
- 22.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18(10):601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 23.North RA (2016) P2X receptors. Philos Trans R Soc Lond B Biol Sci 371(1700). 10.1098/rstb.2015.0427 [DOI] [PMC free article] [PubMed]
- 24.Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17(11):3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stelmashenko O, Lalo U, Yang Y, Bragg L, North RA, Compan V. Activation of trimeric P2X2 receptors by fewer than three ATP molecules. Mol Pharmacol. 2012;82(4):760–766. doi: 10.1124/mol.112.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans RJ. Orthosteric and allosteric binding sites of P2X receptors. Eur Biophys J. 2009;38(3):319–327. doi: 10.1007/s00249-008-0275-2. [DOI] [PubMed] [Google Scholar]
- 27.Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J Biol Chem. 2007;282(51):36879–36886. doi: 10.1074/jbc.M706925200. [DOI] [PubMed] [Google Scholar]
- 28.Hou Z, Cao J. Comparative study of the P2X gene family in animals and plants. Purinergic Signal. 2016;12(2):269–281. doi: 10.1007/s11302-016-9501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 30.Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48(2):178–183. [PubMed] [Google Scholar]
- 31.Savi P, Bornia J, Salel V, Delfaud M, Herbert JM. Characterization of P2x1 purinoreceptors on rat platelets: effect of clopidogrel. Br J Haematol. 1997;98(4):880–886. doi: 10.1046/j.1365-2141.1997.3133126.x. [DOI] [PubMed] [Google Scholar]
- 32.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 33.Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995;18(5):224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- 34.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377(6548):428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 35.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 36.Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38(1):141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- 37.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175(2):105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 38.Padilla K, Gonzalez-Mendoza D, Berumen LC, Escobar JE, Miledi R, Garcia-Alcocer G. Differential gene expression patterns and colocalization of ATP-gated P2X6/P2X4 ion channels during rat small intestine ontogeny. Gene Expr Patterns. 2016;21(2):81–88. doi: 10.1016/j.gep.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Tian M, Abdelrahman A, Weinhausen S, Hinz S, Weyer S, Dosa S, El-Tayeb A, Muller CE. Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorg Med Chem. 2014;22(3):1077–1088. doi: 10.1016/j.bmc.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Torres GE, Haines WR, Egan TM, Voigt MM. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol. 1998;54(6):989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- 41.Haines WR, Torres GE, Voigt MM, Egan TM. Properties of the novel ATP-gated ionotropic receptor composed of the P2X(1) and P2X(5) isoforms. Mol Pharmacol. 1999;56(4):720–727. [PubMed] [Google Scholar]
- 42.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16(8):2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem. 2005;280(11):10759–10765. doi: 10.1074/jbc.M412265200. [DOI] [PubMed] [Google Scholar]
- 44.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 45.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275(6):F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- 46.Paredes-Gamero EJ, Dreyfuss JL, Nader HB, Miyamoto Oshiro ME, Ferreira AT. P2X7-induced apoptosis decreases by aging in mice myeloblasts. Exp Gerontol. 2007;42(4):320–326. doi: 10.1016/j.exger.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Paredes-Gamero EJ, Franca JP, Moraes AA, Aguilar MO, Oshiro ME, Ferreira AT. Problems caused by high concentration of ATP on activation of the P2X7 receptor in bone marrow cells loaded with the Ca2+ fluorophore fura-2. J Fluoresc. 2004;14(6):711–722. doi: 10.1023/b:jofl.0000047221.51493.e3. [DOI] [PubMed] [Google Scholar]
- 48.Fischer W, Urban N, Immig K, Franke H, Schaefer M. Natural compounds with P2X7 receptor-modulating properties. Purinergic Signal. 2014;10(2):313–326. doi: 10.1007/s11302-013-9392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268(11):8199–8203. [PubMed] [Google Scholar]
- 50.Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120(8):1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol. 2003;140(3):507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bin Dayel A, Evans RJ, Schmid R. Mapping the site of action of human P2X7 receptor antagonists AZ11645373, Brilliant Blue G, KN-62, Calmidazolium, and ZINC58368839 to the intersubunit allosteric pocket. Mol Pharmacol. 2019;96(3):355–363. doi: 10.1124/mol.119.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, Colombo MP, Di Virgilio F, Adinolfi E. The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene. 2019;38(19):3636–3650. doi: 10.1038/s41388-019-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danquah W, Meyer-Schwesinger C, Rissiek B, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop JH, Hammel A, Bergmann P, Schwarz N, Assuncao J, Rotthier W, Haag F, Tolosa E, Bannas P, Boue-Grabot E, Magnus T, Laeremans T, Stortelers C, Koch-Nolte F. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med. 2016;8(366):366ra162. doi: 10.1126/scitranslmed.aaf8463. [DOI] [PubMed] [Google Scholar]
- 55.von Kugelgen I (2019) Pharmacology of P2Y receptors. Brain Res Bull:15112–15124. 10.1016/j.brainresbull.2019.03.010 [DOI] [PubMed]
- 56.Niss Arfelt K, Fares S, Sparre-Ulrich AH, Hjorto GM, Gasbjerg LS, Molleskov-Jensen AS, Benned-Jensen T, Rosenkilde MM (2017) Signaling via G proteins mediates tumorigenic effects of GPR87. Cell Signal:309–318. 10.1016/j.cellsig.2016.11.009 [DOI] [PubMed]
- 57.Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 58.Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64(1):104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 59.Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98(12):3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- 60.Erb L, Lustig KD, Sullivan DM, Turner JT, Weisman GA. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci U S A. 1993;90(22):10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Communi D, Motte S, Boeynaems JM, Pirotton S. Pharmacological characterization of the human P2Y4 receptor. Eur J Pharmacol. 1996;317(2-3):383–389. doi: 10.1016/s0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- 62.Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, Sarau HM, Ames RS, Wilson S, Livi GP, Chambers JK. Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics. 2001;78(3):124–128. doi: 10.1006/geno.2001.6662. [DOI] [PubMed] [Google Scholar]
- 63.Cockcroft S, Gomperts BD. The ATP4- receptor of rat mast cells. Biochem J. 1980;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinberg TH, Silverstein SC. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262(7):3118–3122. [PubMed] [Google Scholar]
- 65.Naumov AP, Kuryshev YA, Kaznacheyeva EV, Mozhayeva GN. ATP-activated Ca(2+)-permeable channels in rat peritoneal macrophages. FEBS Lett. 1992;313(3):285–287. doi: 10.1016/0014-5793(92)81210-d. [DOI] [PubMed] [Google Scholar]
- 66.Nuttle LC, Dubyak GR. Differential activation of cation channels and non-selective pores by macrophage P2z purinergic receptors expressed in Xenopus oocytes. J Biol Chem. 1994;269(19):13988–13996. [PubMed] [Google Scholar]
- 67.Hickman SE, el Khoury J, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84(8):2452–2456. [PubMed] [Google Scholar]
- 68.Mohanty JG, Raible DG, McDermott LJ, Pelleg A, Schulman ES. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: activation of purinergic P2Y receptors. J Allergy Clin Immunol. 2001;107(5):849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- 69.Idzko M, Dichmann S, Panther E, Ferrari D, Herouy Y, Virchow C, Jr, Luttmann W, Di Virgilio F, Norgauer J. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188(3):329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 70.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 71.Csoka B, Nemeth ZH, Szabo I, Davies DL, Varga ZV, Paloczi J, Falzoni S, Di Virgilio F, Muramatsu R, Yamashita T, Pacher P, Hasko G (2018) Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight 3(11). 10.1172/jci.insight.9943199431 [DOI] [PMC free article] [PubMed]
- 72.Csoka B, Nemeth ZH, Toro G, Idzko M, Zech A, Koscso B, Spolarics Z, Antonioli L, Cseri K, Erdelyi K, Pacher P, Hasko G. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015;29(9):3626–3637. doi: 10.1096/fj.15-272450fj.15-272450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balazovich KJ, Boxer LA. Extracellular adenosine nucleotides stimulate protein kinase C activity and human neutrophil activation. J Immunol. 1990;144(2):631–637. [PubMed] [Google Scholar]
- 74.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166(11):6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 75.Lecut C, Faccinetto C, Delierneux C, van Oerle R, Spronk HM, Evans RJ, El Benna J, Bours V, Oury C. ATP-gated P2X1 ion channels protect against endotoxemia by dampening neutrophil activation. J Thromb Haemost. 2012;10(3):453–465. doi: 10.1111/j.1538-7836.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 76.Maitre B, Magnenat S, Heim V, Ravanat C, Evans RJ, de la Salle H, Gachet C, Hechler B. The P2X1 receptor is required for neutrophil extravasation during lipopolysaccharide-induced lethal endotoxemia in mice. J Immunol. 2015;194(2):739–749. doi: 10.4049/jimmunol.1401786. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro-Filho AC, Buri MV, Barros CC, Dreyfuss JL, Nader HB, Justo GZ, Craveiro RB, Pesquero JB, Miranda A, Ferreira AT, Paredes-Gamero EJ. Functional and molecular evidence for heteromeric association of P2Y1 receptor with P2Y2 and P2Y4 receptors in mouse granulocytes. BMC Pharmacol Toxicol. 2016;17(1):29. doi: 10.1186/s40360-016-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leal Denis MF, Alvarez HA, Lauri N, Alvarez CL, Chara O, Schwarzbaum PJ. Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes. PLoS One. 2016;11(6):e0158305. doi: 10.1371/journal.pone.0158305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaziri C, Downes CP. G-protein-mediated activation of turkey erythrocyte phospholipase C by beta-adrenergic and P2y-purinergic receptors. Biochem J. 1992;284(Pt 3):917–922. doi: 10.1042/bj2840917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Light DB, Attwood AJ, Siegel C, Baumann NL. Cell swelling increases intracellular calcium in Necturus erythrocytes. J Cell Sci. 2003;116(Pt 1):101–109. doi: 10.1242/jcs.00202. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman JF, Dodson A, Wickrema A, Dib-Hajj SD. Tetrodotoxin-sensitive Na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts. Proc Natl Acad Sci U S A. 2004;101(33):12370–12374. doi: 10.1073/pnas.0404228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem. 2004;279(43):44749–44755. doi: 10.1074/jbc.M405631200. [DOI] [PubMed] [Google Scholar]
- 83.Paredes-Gamero EJ, Craveiro RB, Pesquero JB, Franca JP, Oshiro ME, Ferreira AT. Activation of P2Y1 receptor triggers two calcium signaling pathways in bone marrow erythroblasts. Eur J Pharmacol. 2006;534(1-3):30–38. doi: 10.1016/j.ejphar.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Burnstock G. Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signal. 2015;11(4):411–434. doi: 10.1007/s11302-015-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Virgilio F, Vuerich M (2015) Purinergic signaling in the immune system. Auton Neurosci:191117–191123. 10.1016/j.autneu.2015.04.011 [DOI] [PubMed]
- 86.Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012;120(12):2365–2375. doi: 10.1182/blood-2012-04-422378. [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Vieira JM, Gutierres JM, Carvalho FB, Stefanello N, Oliveira L, Cardoso AM, Morsch VM, Pillat MM, Ulrich H, Duarte MMF, Schetinger MRC, Spanevello RM (2018) Caffeine and high intensity exercise: Impact on purinergic and cholinergic signalling in lymphocytes and on cytokine levels. Biomed Pharmacother:1081731–1081738. 10.1016/j.biopha.2018.10.006 [DOI] [PubMed]
- 89.Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R (2018) The P2X7 Receptor in Inflammatory Diseases: angel or Demon? Front Pharmacol 952. 10.3389/fphar.2018.00052 [DOI] [PMC free article] [PubMed]
- 90.Granata S, Masola V, Zoratti E, Scupoli MT, Baruzzi A, Messa M, Sallustio F, Gesualdo L, Lupo A, Zaza G. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS One. 2015;10(3):e0122272. doi: 10.1371/journal.pone.0122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4(162):ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 92.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 93.Gutteridge JMC, Halliwell B. Mini-review: oxidative stress, redox stress or redox success? Biochem Biophys Res Commun. 2018;502(2):183–186. doi: 10.1016/j.bbrc.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 94.Munoz FM, Gao R, Tian Y, Henstenburg BA, Barrett JE, Hu H. Neuronal P2X7 receptor-induced reactive oxygen species production contributes to nociceptive behavior in mice. Sci Rep. 2017;7(1):3539. doi: 10.1038/s41598-017-03813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawano A, Hayakawa A, Kojima S, Tsukimoto M, Sakamoto H (2015) Purinergic signaling mediates oxidative stress in UVA-exposed THP-1 cells. Toxicol Rep:2391–2400. 10.1016/j.toxrep.2015.01.015 [DOI] [PMC free article] [PubMed]
- 96.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F (2018) The P2X7 receptor: a main player in inflammation. Biochem Pharmacol:151234–151244. 10.1016/j.bcp.2017.12.021 [DOI] [PubMed]
- 97.Hill LM, Gavala ML, Lenertz LY, Bertics PJ. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol. 2010;185(5):3028–3034. doi: 10.4049/jimmunol.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y, Xiao Y, Li Z. P2X7 receptor positively regulates MyD88-dependent NF-kappaB activation. Cytokine. 2011;55(2):229–236. doi: 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amores-Iniesta J, Barbera-Cremades M, Martinez CM, Pons JA, Revilla-Nuin B, Martinez-Alarcon L, Di Virgilio F, Parrilla P, Baroja-Mazo A, Pelegrin P. Extracellular ATP activates the NLRP3 Inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 2017;21(12):3414–3426. doi: 10.1016/j.celrep.2017.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratajczak MZ, Mack A, Bujko K, Domingues A, Pedziwiatr D, Kucia M, Ratajczak J, Ulrich H, Kucharska-Mazur J, Samochowiec J. ATP-Nlrp3 inflammasome-complement cascade axis in sterile brain inflammation in psychiatric patients and its impact on stem cell trafficking. Stem Cell Rev Rep. 2019;15(4):497–505. doi: 10.1007/s12015-019-09888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adamiak M, Bujko K, Cymer M, Plonka M, Glaser T, Kucia M, Ratajczak J, Ulrich H, Abdel-Latif A, Ratajczak MZ. Novel evidence that extracellular nucleotides and purinergic signaling induce innate immunity-mediated mobilization of hematopoietic stem/progenitor cells. Leukemia. 2018;32(9):1920–1931. doi: 10.1038/s41375-018-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lenkiewicz AM, Adamiak M, Thapa A, Bujko K, Pedziwiatr D, Abdel-Latif AK, Kucia M, Ratajczak J, Ratajczak MZ. The Nlrp3 inflammasome orchestrates mobilization of bone marrow-residing stem cells into peripheral blood. Stem Cell Rev Rep. 2019;15(3):391–403. doi: 10.1007/s12015-019-09890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adamiak M, Bujko K, Brzezniakiewicz-Janus K, Kucia M, Ratajczak J, Ratajczak MZ (2019) The inhibition of CD39 and CD73 cell surface ectonucleotidases by small molecular inhibitors enhances the mobilization of bone marrow residing stem cells by decreasing the extracellular level of adenosine. Stem Cell Rev Rep. 10.1007/s12015-019-09918-y [DOI] [PMC free article] [PubMed]
- 105.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, Hippen KL, Blazar BR, Masopust D, Kelekar A, Vulchanova L, Hogquist KA, Jameson SC. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature. 2018;559(7713):264–268. doi: 10.1038/s41586-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14(1):1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graziano F, Desdouits M, Garzetti L, Podini P, Alfano M, Rubartelli A, Furlan R, Benaroch P, Poli G. Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc Natl Acad Sci U S A. 2015;112(25):E3265–E3273. doi: 10.1073/pnas.1500656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee R, Williams JC, Mackman N. P2X7 regulation of macrophage tissue factor activity and microparticle generation. J Thromb Haemost. 2012;10(9):1965–1967. doi: 10.1111/j.1538-7836.2012.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47(1):15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 110.Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J Cell Sci. 1999;112(Pt 3):297–305. doi: 10.1242/jcs.112.3.297. [DOI] [PubMed] [Google Scholar]
- 111.Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H (2012) Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun 31034. 10.1038/ncomms2023 [DOI] [PMC free article] [PubMed]
- 112.Bianchi G, Vuerich M, Pellegatti P, Marimpietri D, Emionite L, Marigo I, Bronte V, Di Virgilio F, Pistoia V, Raffaghello L (2014) ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis:5e1135. 10.1038/cddis.2014.109 [DOI] [PMC free article] [PubMed]
- 113.Nogueira-Pedro A, Dias CC, Regina H, Segreto C, Addios PC, Lungato L, D’Almeida V, Barros CC, Higa EM, Buri MV, Ferreira AT, Paredes-Gamero EJ. Nitric oxide-induced murine hematopoietic stem cell fate involves multiple signaling proteins, gene expression, and redox modulation. Stem Cells. 2014;32(11):2949–2960. doi: 10.1002/stem.1773. [DOI] [PubMed] [Google Scholar]
- 114.Paredes-Gamero EJ, Barbosa CM, Ferreira AT (2012) Calcium signaling as a regulator of hematopoiesis. Front Biosci (Elite Ed), 41375-84. [DOI] [PubMed]
- 115.Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Di Virgilio F. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104(6):1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Jacobsen SE, Bengtsson A, Erlinge D (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 516. 10.1186/1471-2172-5-16 [DOI] [PMC free article] [PubMed]
- 117.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Di Virgilio F, Baccarani M, Lemoli RM. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109(2):533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 118.Paredes-Gamero EJ, Leon CM, Borojevic R, Oshiro ME, Ferreira AT. Changes in intracellular Ca2+ levels induced by cytokines and P2 agonists differentially modulate proliferation or commitment with macrophage differentiation in murine hematopoietic cells. J Biol Chem. 2008;283(46):31909–31919. doi: 10.1074/jbc.M801990200. [DOI] [PubMed] [Google Scholar]
- 119.Barbosa CM, Leon CM, Nogueira-Pedro A, Wasinsk F, Araujo RC, Miranda A, Ferreira AT, Paredes-Gamero EJ (2011) Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines. Cell Death Dis:2e165. 10.1038/cddis.2011.49 [DOI] [PMC free article] [PubMed]
- 120.Cho J, Yusuf R, Kook S, Attar E, Lee D, Park B, Cheng T, Scadden DT, Lee BC. Purinergic P2Y(1)(4) receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest. 2014;124(7):3159–3171. doi: 10.1172/JCI61636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng W, Yang F, Wang R, Yang X, Wang L, Chen C, Liao J, Lin Y, Ren Q, Zheng G. High level P2X7-mediated signaling impairs function of hematopoietic stem/progenitor cells. Stem Cell Rev. 2016;12(3):305–314. doi: 10.1007/s12015-016-9651-y. [DOI] [PubMed] [Google Scholar]
- 122.Koldej R, Collins J, Ritchie D. P2X7 polymorphisms and stem cell mobilisation. Leukemia. 2018;32(12):2724–2726. doi: 10.1038/s41375-018-0232-8. [DOI] [PubMed] [Google Scholar]
- 123.Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, Robson SC, Frenette PS, Fujisaki J. CD150(high) Bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22(3):445–453. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hirata Y, Kakiuchi M, Robson SC, Fujisaki J (2018) CD150high CD4 T cells and CD150high Tregs regulate hematopoietic stem cell quiescence via CD73. Haematologica. 10.3324/haematol.2018.198283 [DOI] [PMC free article] [PubMed]
- 125.Montero M, Garcia-Sancho J, Alvarez J. Biphasic and differential modulation of Ca2+ entry by ATP and UTP in promyelocytic leukaemia HL60 cells. Biochemical Journal. 1995;305(3):879–887. doi: 10.1042/bj3050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Biffen M, Alexander DR. Mobilization of intracellular Ca2+ by adenine nucleotides in human T-leukaemia cells: evidence for ADP-specific and P2y-purinergic receptors. Biochemical Journal. 1994;304(3):769–774. doi: 10.1042/bj3040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Communi D, Janssens R, Robaye B, Zeelis N, Boeynaems J-M. Rapid up-regulation of P2Y messengers during granulocytic differentiation of HL-60 cells. FEBS Lett. 2000;475(1):39–42. doi: 10.1016/s0014-5793(00)01618-5. [DOI] [PubMed] [Google Scholar]
- 128.Adrian K, Bernhard MK, Breitinger HG, Ogilvie A. Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim Biophys Acta. 2000;1492(1):127–138. doi: 10.1016/s0167-4781(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 129.Zoetewij JP, van de Water B, de Bont HJ, Nagelkerke JF. The role of a purinergic P2z receptor in calcium-dependent cell killing of isolated rat hepatocytes by extracellular adenosine triphosphate. Hepatology. 1996;23(4):858–865. doi: 10.1002/hep.510230429. [DOI] [PubMed] [Google Scholar]
- 130.Yu T, Junger WG, Yuan C, Jin A, Zhao Y, Zheng X, Zeng Y, Liu J. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am J Physiol Cell Physiol. 2010;298(3):C457–C464. doi: 10.1152/ajpcell.00342.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X-J, Zheng G-G, Ma X-T, Yang Y-H, Li G, Rao Q, Nie K, Wu K-F. Expression of P2X7 in human hematopoietic cell lines and leukemia patients. Leuk Res. 2004;28(12):1313–1322. doi: 10.1016/j.leukres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 132.Gadeock S, Pupovac A, Sluyter V, Spildrejorde M, Sluyter R. P2X7 receptor activation mediates organic cation uptake into human myeloid leukaemic KG-1 cells. Purinergic Signal. 2012;8(4):669–676. doi: 10.1007/s11302-012-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJ, Wiley JS, Sluyter R. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2010;1798(9):1797–1804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 134.Chong JH, Zheng GG, Zhu XF, Guo Y, Wang L, Ma CH, Liu SY, Xu LL, Lin YM, Wu KF. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem Biophys Res Commun. 2010;391(1):498–504. doi: 10.1016/j.bbrc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 135.Salvestrini V, Orecchioni S, Talarico G, Reggiani F, Mazzetti C, Bertolini F, Orioli E, Adinolfi E, Di Virgilio F, Pezzi A, Cavo M, Lemoli RM, Curti A. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget. 2017;8(4):5895–5908. doi: 10.18632/oncotarget.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Salvestrini V, Zini R, Rossi L, Gulinelli S, Manfredini R, Bianchi E, Piacibello W, Caione L, Migliardi G, Ricciardi MR. Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood. 2012;119(1):217–226. doi: 10.1182/blood-2011-07-370775. [DOI] [PubMed] [Google Scholar]
- 137.Lecciso M, Ocadlikova D, Sangaletti S, Trabanelli S, De Marchi E, Orioli E, Pegoraro A, Portararo P, Jandus C, Bontadini A, Redavid A, Salvestrini V, Romero P, Colombo MP, Di Virgilio F, Cavo M, Adinolfi E, Curti A (2017) ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front Immunol:81918. 10.3389/fimmu.2017.01918 [DOI] [PMC free article] [PubMed]
- 138.Wang W, Xiao J, Adachi M, Liu Z, Zhou J. 4-aminopyridine induces apoptosis of human acute myeloid leukemia cells via increasing [Ca2+]i through P2X7 receptor pathway. Cell Physiol Biochem. 2011;28(2):199–208. doi: 10.1159/000331731. [DOI] [PubMed] [Google Scholar]
- 139.Yoon MJ, Lee HJ, Kim JH, Kim DK. Extracellular ATP induces apoptotic signaling in human monocyte leukemic cells, HL-60 and F-36P. Arch Pharm Res. 2006;29(11):1032–1041. doi: 10.1007/BF02969288. [DOI] [PubMed] [Google Scholar]
- 140.Zhang X, Meng L, He B, Chen J, Liu P, Zhao J, Zhang Y, Li M, An D. The role of P2X7 receptor in ATP-mediated human leukemia cell death: calcium influx-independent. Acta Biochim Biophys Sin (Shanghai) 2009;41(5):362–369. doi: 10.1093/abbs/gmp016. [DOI] [PubMed] [Google Scholar]
- 141.Wang B, Sluyter R. P2X7 receptor activation induces reactive oxygen species formation in erythroid cells. Purinergic Signal. 2013;9(1):101–112. doi: 10.1007/s11302-012-9335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ledderose C, Woehrle T, Ledderose S, Strasser K, Seist R, Bao Y, Zhang J, Junger WG. Cutting off the power: inhibition of leukemia cell growth by pausing basal ATP release and P2X receptor signaling? Purinergic Signal. 2016;12(3):439–451. doi: 10.1007/s11302-016-9510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig VH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 144.Di Virgilio F, Wiley JS. The P2X7 receptor of CLL lymphocytes-a molecule with a split personality. The Lancet. 2002;360(9349):1898–1899. doi: 10.1016/S0140-6736(02)11933-7. [DOI] [PubMed] [Google Scholar]
- 145.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Di Virgilio F, Baricordi OR. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99(2):706–708. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
- 146.Thunberg U, Tobin G, Johnson A, Söderberg O, Padyukov L, Hultdin M, Klareskog L, Enblad G, Sundström C, Roos G. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. The Lancet. 2002;360(9349):1935–1939. doi: 10.1016/S0140-6736(02)11917-9. [DOI] [PubMed] [Google Scholar]
- 147.Nückel H, Frey UH, Dürig J, Dührsen U, Siffert W. 1513A/C polymorphism in the P2X7 receptor gene in chronic lymphocytic leukemia: absence of correlation with clinical outcome. Eur J Haematol. 2004;72(4):259–263. doi: 10.1111/j.0902-4441.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 148.Sellick GS, Rudd M, Eve P, Allinson R, Matutes E, Catovsky D, Houlston RS. The P2X7 receptor gene A1513C polymorphism does not contribute to risk of familial or sporadic chronic lymphocytic leukemia. Cancer Epidemiol Prev Biomarkers. 2004;13(6):1065–1067. [PubMed] [Google Scholar]
- 149.Starczynski J, Pepper C, Pratt G, Hooper L, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The P2X7 receptor gene polymorphism 1513 A → C has no effect on clinical prognostic markers, in vitro sensitivity to fludarabine, Bcl-2 family protein expression or survival in B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;123(1):66–71. doi: 10.1046/j.1365-2141.2003.04563.x. [DOI] [PubMed] [Google Scholar]
- 150.Zhang L, Ibbotson R, Orchard J, Gardiner A, Seear R, Chase A, Oscier D, Cross N. P2X7 polymorphism and chronic lymphocytic leukaemia: lack of correlation with incidence, survival and abnormalities of chromosome 12. Leukemia. 2003;17(11):2097. doi: 10.1038/sj.leu.2403125. [DOI] [PubMed] [Google Scholar]
- 151.Salaro E, Rambaldi A, Falzoni S, Amoroso FS, Franceschini A, Sarti AC, Bonora M, Cavazzini F, Rigolin GM, Ciccone M, Audrito V, Deaglio S, Pelegrin P, Pinton P, Cuneo A, Di Virgilio F (2016) Involvement of the P2X7-NLRP3 axis in leukemic cell proliferation and death. Sci Rep 626280. 10.1038/srep26280 [DOI] [PMC free article] [PubMed]
- 152.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 153.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang D, Zheng J, Hu Q, Zhao C, Chen Q, Shi P, Chen Q, Zou Y, Zou D, Liu Q, Pei J, Wu X, Gao X, Ren J, Lin Z (2019) Magnesium protects against sepsis by blocking gasdermin D N-terminal-induced pyroptosis. Cell Death Differ. 10.1038/s41418-019-0366-x [DOI] [PMC free article] [PubMed]
- 155.Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43(5):923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Faliti CE, Gualtierotti R, Rottoli E, Gerosa M, Perruzza L, Romagnani A, Pellegrini G, De Ponte CB, Rossi RL, Idzko M, Mazza EMC, Bicciato S, Traggiai E, Meroni PL, Grassi F. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J Exp Med. 2019;216(2):317–336. doi: 10.1084/jem.20171976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mitra S, Sarkar A. Microparticulate P2X7 and GSDM-D mediated regulation of functional IL-1beta release. Purinergic Signal. 2019;15(1):119–123. doi: 10.1007/s11302-018-9640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91(9):3172–3181. [PubMed] [Google Scholar]
- 159.Gachet C. Identification, characterization, and inhibition of the platelet ADP receptors. Int J Hematol. 2001;74(4):375–381. doi: 10.1007/BF02982079. [DOI] [PubMed] [Google Scholar]
- 160.Hall DA, Hourani SM. Effects of suramin on increases in cytosolic calcium and on inhibition of adenylate cyclase induced by adenosine 5′-diphosphate in human platelets. Biochem Pharmacol. 1994;47(6):1013–1018. doi: 10.1016/0006-2952(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 161.Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53(4):727–733. [PubMed] [Google Scholar]
- 162.Fagura MS, Dainty IA, McKay GD, Kirk IP, Humphries RG, Robertson MJ, Dougall IG, Leff P. P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y(ADP)-receptors. Br J Pharmacol. 1998;124(1):157–164. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y(1) receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62(5):1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol:24031–24304. 10.1016/S0074-7696(04)40002-3 [DOI] [PubMed]
- 165.Maffrand JP, Bernat A, Delebassée D, Defreyn G, Cazenave JP, Gordon JL. ADP plays a key role in thrombogenesis in rats. Thromb Haemost. 1988;59(2):225–230. [PubMed] [Google Scholar]
- 166.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84(5):891–896. [PubMed] [Google Scholar]
- 167.Boeynaems JM, van Giezen H, Savi P, Herbert JM. P2Y receptor antagonists in thrombosis. Curr Opin Investig Drugs. 2005;6(3):275–282. [PubMed] [Google Scholar]
- 168.Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM. ADP receptors--targets for developing antithrombotic agents. Curr Pharm Des. 2003;9(28):2303–2316. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- 169.Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86(1):222–232. [PubMed] [Google Scholar]
- 170.Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, Investigators C. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting : the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102(6):624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 171.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12(1):30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 172.Sugidachi A, Ogawa T, Kurihara A, Hagihara K, Jakubowski JA, Hashimoto M, Niitsu Y, Asai F. The greater in vivo antiplatelet effects of prasugrel as compared to clopidogrel reflect more efficient generation of its active metabolite with similar antiplatelet activity to that of clopidogrel’s active metabolite. J Thromb Haemost. 2007;5(7):1545–1551. doi: 10.1111/j.1538-7836.2007.02598.x. [DOI] [PubMed] [Google Scholar]
- 173.Anderson SD, Shah NK, Yim J, Epstein BJ. Efficacy and safety of ticagrelor: a reversible P2Y12 receptor antagonist. Ann Pharmacother. 2010;44(3):524–537. doi: 10.1345/aph.1M548. [DOI] [PubMed] [Google Scholar]
- 174.Husted S, Boersma E. Case study: ticagrelor in PLATO and Prasugrel in TRITON-TIMI 38 and TRILOGY-ACS trials in patients with acute coronary syndromes. Am J Ther. 2016;23(6):e1876–e1889. doi: 10.1097/MJT.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Patelis N, Kakavia K, Maltezos K, Damascos C, Spartalis E, Matheiken S, Georgopoulos S (2018) An update on novel antiplatelets in vascular patients. Curr Pharm Des. 10.2174/1381612825666181226144129 [DOI] [PubMed]
- 176.Xiang B, Zhang G, Ren H, Sunkara M, Morris AJ, Gartner TK, Smyth SS, Li Z. The P2Y(12) antagonists, 2MeSAMP and cangrelor, inhibit platelet activation through P2Y(12)/G(i)-dependent mechanism. PLoS One. 2012;7(12):e51037. doi: 10.1371/journal.pone.0051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Franchi F, Rollini F, Muñiz-Lozano A, Cho JR, Angiolillo DJ. Cangrelor: a review on pharmacology and clinical trial development. Expert Rev Cardiovasc Ther. 2013;11(10):1279–1291. doi: 10.1586/14779072.2013.837701. [DOI] [PubMed] [Google Scholar]
- 178.Welsh RC, Rao SV, Zeymer U, Thompson VP, Huber K, Kochman J, McClure MW, Gretler DD, Bhatt DL, Gibson CM, Angiolillo DJ, Gurbel PA, Berdan LG, Paynter G, Leonardi S, Madan M, French WJ, Harrington RA, Investigators I-P. A randomized, double-blind, active-controlled phase 2 trial to evaluate a novel selective and reversible intravenous and oral P2Y12 inhibitor elinogrel versus clopidogrel in patients undergoing nonurgent percutaneous coronary intervention: the INNOVATE-PCI trial. Circ Cardiovasc Interv. 2012;5(3):336–346. doi: 10.1161/CIRCINTERVENTIONS.111.964197. [DOI] [PubMed] [Google Scholar]
- 179.Pfefferkorn JA, Choi C, Winters T, Kennedy R, Chi L, Perrin LA, Lu G, Ping YW, McClanahan T, Schroeder R, Leininger MT, Geyer A, Schefzick S, Atherton J. P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg Med Chem Lett. 2008;18(11):3338–3343. doi: 10.1016/j.bmcl.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 180.Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316(2):556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dunn PM, Blakeley AG. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure-activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8(26):2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- 183.Murakami T, Fujihara T, Horibe Y, Nakamura M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004;36(2):89–93. doi: 10.1159/000076887. [DOI] [PubMed] [Google Scholar]
- 184.Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002;18(4):363–370. doi: 10.1089/10807680260218524. [DOI] [PubMed] [Google Scholar]
- 185.Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, Di Leo A, Loi S, Piccart-Gebhart M, Willard-Gallo K, Sotiriou C, Stagg J. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol. 2018;29(4):1056–1062. doi: 10.1093/annonc/mdx730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J (2018) Targeting the CD73-adenosine axis in immuno-oncology. Immunol Lett. 10.1016/j.imlet.2018.05.001 [DOI] [PubMed]