Abstract
Blastocyst complementation is an emerging methodology in which human stem cells are transferred into genetically engineered preimplantation animal embryos eventually giving rise to fully developed human tissues and organs within the animal host for use in regenerative medicine. The ethical issues surrounding this method have caused the National Institutes of Health to issue a moratorium on funding for blastocyst complementation citing the potential for human cells to substantially contribute to the brain of the chimeric animal. To address this concern, we performed an in-depth review of the neural transplantation literature to determine how the integration of human cells into the nonhuman neural circuitry has altered the behavior of the host. Despite reports of widespread integration of human cell transplants, our review of 150 transplantation studies found no evidence suggestive of humanization of the animal host, and we thus conclude that, at present, concerns over humanization should not prevent research on blastocyst complementation to continue. We suggest proceeding in a controlled and transparent manner, however, and include recommendations for future research with careful consideration for how human cells may contribute to the animal host nervous system.
Keywords: Blastocyst complementation, Chimera, Stem cells, Cell transplantation, Cognition, Behavior
Introduction
In the broadest of definitions, a chimera is “a single biological entity that is composed of a mixing of materials from 2 or more different organisms” [1]. In Greek mythology, the chimera was a composite organism of different body parts from wildly divergent species. More recent variations of the chimera can be found in North American folklore (jackalope, hodag, Jersey Devil, etc.) as well as in modern literature such as Margaret Atwood’s speculative fiction Maddaddam trilogy. Far from the mythical and bizarre, however, chimerism—using the above definition—can commonly be found within the human brain. Microchimerism, the natural transfer of cells from a fetus which can cross the placenta and integrate within the maternal host, has been observed within the brain of over half of sampled women [2]. Similarly, female recipients of bone marrow transplantation contain neural and non-neural cells derived from the male donor marrow [3]. Human–human neurological chimeras have also existed as part of clinical trials investigating the efficacy of cell-mediated therapies for devastating neurological disorders such as Parkinson’s disease (PD), Huntington’s disease (HD), and spinal cord injury (SCI).
Blastocyst Complementation
Advances in mammalian gene editing, pluripotent stem cell culture, and embryo micromanipulation technology have culminated in attempts to grow authentic interspecies organs through blastocyst complementation (for a comprehensive review, see [4]). This emerging methodology has the potential to generate whole organs and tissues comprised entirely of cells from a single human donor (Fig. 1). To accomplish this, embryos from one organism are genetically engineered so that they lack functional gene(s) necessary for the development of the tissue of interest. The organogenesis-disabled embryos are then microinjected with healthy pluripotent stem cells (PSCs) from a second organism and are then transferred into a maternal surrogate. Through normal mammalian development, the microinjected PSCs occupy the niche left by the gene knockout and develop into a functional organ. This technique has successfully generated functioning allogeneic or xenogeneic pancreata in mice, rats, and pigs [5–8]. Microinjection of human cells into the wild-type porcine embryo has also led to human–animal chimerism across multiple organ systems, including neural cells [7].
Figure 1.
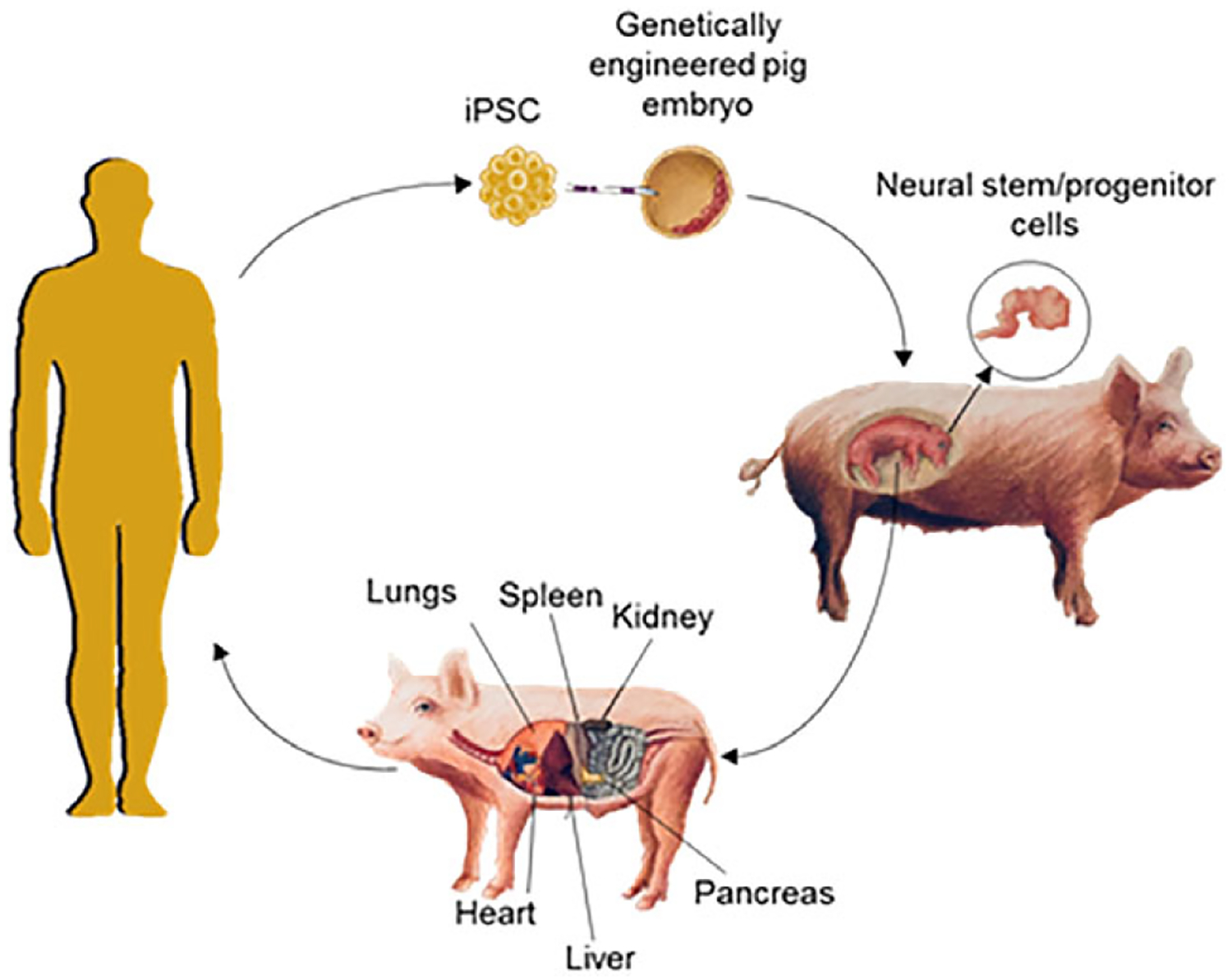
Cartoon schematic of blastocyst complementation. Human pluripotent stem cells grown in vitro are microinjected into genetically engineered porcine blastocysts which are then transferred to surrogate sows. The chimeric blastocysts develop to a fetal stage in which neural stem/progenitor cells can be harvested from the brain or to live-born animals where adult organs are processed for transplantation into patients.
A primary goal of blastocyst complementation is to meet the high-demand for human organs by producing fully functional human tissues and organs to be well-matched and ready for transplantation. Aside from the clinical potential of blastocyst complementation, the procurement of healthy human tissue also has the potential to impact the basic- and translational-sciences through disease modeling, drug discovery, and studies of transplantation biology.
Objections to Human–Animal Chimerism
A major concern echoed throughout the public response period to the National Institutes of Health (NIH) proposed changes in the guidelines regarding human–animal chimera research (NOTOD-16–128) is the “creation of human–animal beings with partly or substantially human brains” and whether such chimeras possess “humanized” characteristics. Given the current NIH moratorium on funding research proposals involving human–animal chimeras at the preimplantation embryo stage, it is difficult to secure funding to answer the question, “Will generation of human neural tissue within animals through blastocyst complementation produce ‘humanized’ animals?” However, we can ask the following surrogate question: “Has biomedical research involving transplantation of human tissue into the central nervous system (CNS) of animals altered the cytoarchitecture of the host brain resulting in an altered cognitive and behavioral state of the animal which could be considered human-like?”
In this review, we examine the outcomes of 150 transplantation studies in 112 peer-reviewed publications in which human cells have been targeted to the mammalian CNS (Fig. 2). These studies, not under moratorium by NIH, range from basic- to translational-science, and our focus is on the types of cells being transplanted within the nonhuman mammal and the degree to which the transplanted human cells are integrated. Although behavioral tests to identify human-specific attributes have not been performed in any transplant study, to date, we will also examine whether the transplanted human cells have enhanced the cognitive/behavioral abilities of the host to levels above wild-type animals. Because the ethical, legal, and social implications (ELSI) of human–animal chimerism and the potential for humanization of the animal host have been explored elsewhere [9–11], do not discuss the ELSI issues at length. The review aims to provide a necessary empirical foundation for those important ELSI debates.
Figure 2.
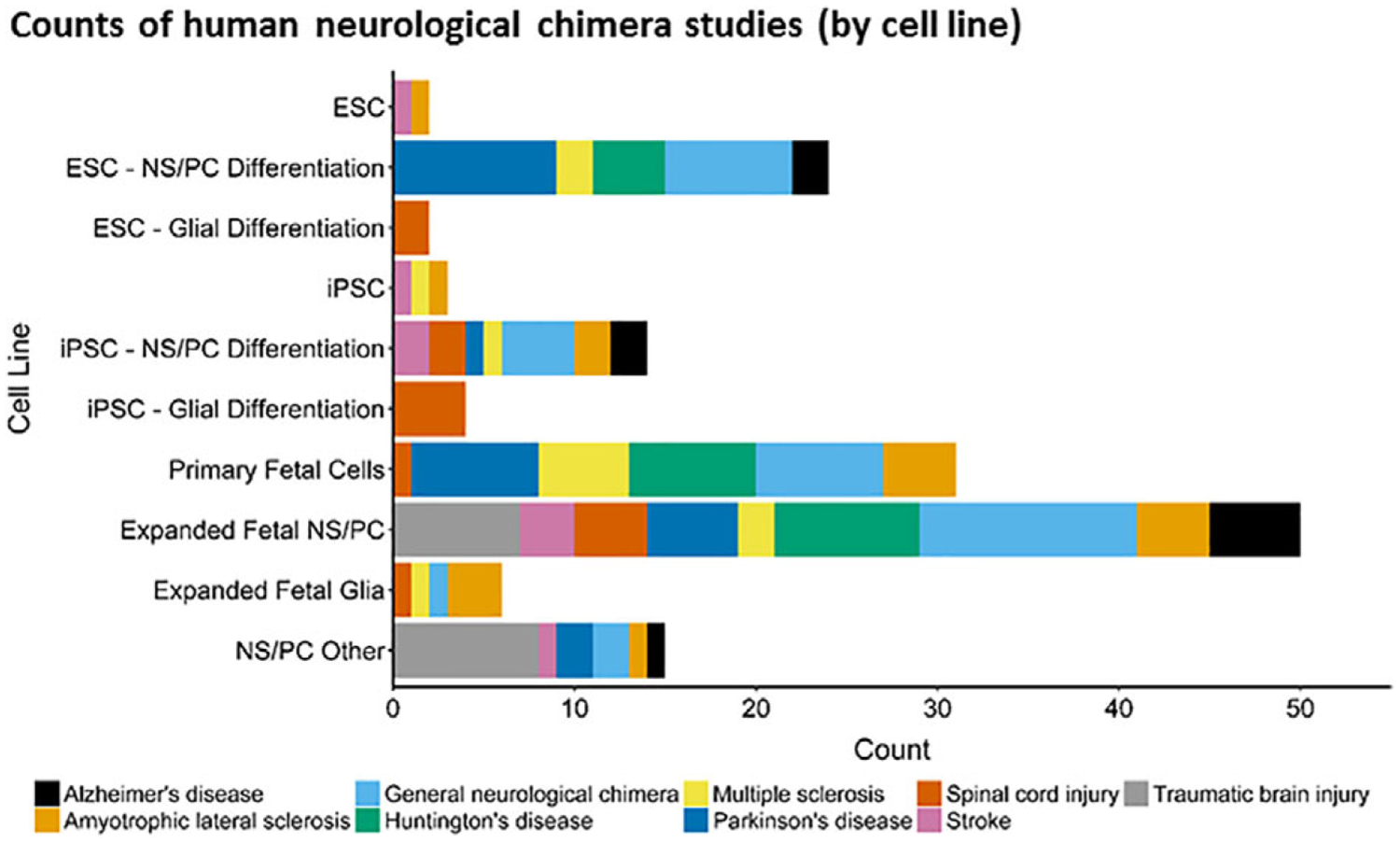
Human cells used for preclinical or biomedical neurological research. For scientific review, we sampled a small fraction of the available peer-reviewed primary research articles in which human cells are transplanted into the CNS of mice, rats, and nonhuman primates. From these studies, a variety of cell lines were used which primarily fall into 3 categories: Embryonic stem cell (ESC) derived, induced pluripotent stem cell (iPSC) derived and fetal-derived. These cells lines can either be expanded then differentiated into neural stem/progenitor cells (NS/PC) or glial progenitors, or transplanted directly with minimal in vitro manipulation (fluorescent- or magnetic- activated cell sorting) and no in vitro expansion (primary fetal cells). Within our review, an additional category of NS/PC cell lines is identified, which includes direct conversion of somatic tissue to NS/PC and the teratocarcinoma-derived Ntera2/D1 cell line.
Human–Animal Chimerism
General Neurological Chimerism
In an attempt to provide insight into the early stages of human neural development, several labs have transplanted clonally expanded human neural stem cells into the brains of perinatal mice and rats, a point at which neural development is still occurring, translating to mid-gestation in human prenatal development [12]. In these studies, up to 1 million cells from dissociated neurospheres were transplanted into the ventricles or subcortical regions. In some of these animals, human cells were observed over 1 year following transplantation [13, 14] with integration throughout the brain and migration of human cells along the rostral migratory stream to the olfactory bulbs [13–16] and into the proliferative subventricular zone (SVZ) [14]. The phenotype of the transplanted cells ranged from immature neuronal cells weeks following transplantation [15–18] and region-specific mature neuronal phenotypes months following transplantation [13, 15–17]. Ourednik and colleagues were interested in identifying the migration and differentiation potential of fetal-derived human neural stem cells following intraventricular transplantation into the brains of fetal Bonnet macaque at 12–13 gestational weeks [19]. This group observed terminal differentiation of human cells into neurons with appropriate cortical laminae that appeared to match the development of the host. These studies have provided evidence that a single cell type is likely to give rise to most cell types within the developing brain.
Experiments in transplantation of neural stem/progenitor cell (NS/PC) derived from fetal tissue [16, 20–25], ESCs [24, 26–30], or other sources [31–33] have been published that describe survival, neural maturation, and integration of unique cell lines. Survival of transplanted cells was highly variable with instances of complete cell rejection [27, 33], fewer than one observed transplanted cell per cubic mm [20, 28], or a complete doubling of transplanted cells [21]. The transplanted cells differentiated toward an immature neuronal phenotype early following transplantation that appeared more developed starting around 10 weeks post-transplantation [20, 28, 31]. Primary fetal tissue isolated from the developing cortex, thalamus, and striatum were transplanted into adult rats and were observed up to 40 weeks following transplantation and differentiated into immature or mature neurons as well as astrocytes that were spread throughout the SVZ, rostral migratory stream, and in the white matter of the corpus callosum [17, 24, 34, 35].
More recently, the group from the Gage lab developed a system to observe the growth and maturation of human brain like structures, termed organoids [36]. In this study, human ESC derived organoids were transplanted into a cavity in the retrosplenial cortex of immunocompromised mice. The transplanted organoids were observed to maturate and extend axons that integrated with the host neuronal circuitry, as measured by optogenetics and electrophysiology. Furthermore, the organoids were vascularized by the host and infiltrated with microglia. In a test of spatial learning and memory, none of the transplanted mice displayed significant alterations in behavior.
Transplantation of healthy neural cells into a nondiseased brain has improved our knowledge of neural development, cell migration and terminal differentiation. Although most of these studies did not directly measure behavior of the transplanted animals, none of the authors suggested the animals displayed altered characteristics that could be construed as human-like. Furthermore, none of these studies suggested an alteration of the cytoarchitecture of the host brain. To the contrary, multiple studies noted that the host organism dictated migration and transplanted cells followed the differentiation cues from the host [13, 14, 16, 19, 21].
Neurological Chimerism and Behavior
Neurological diseases and injuries can cause profound deficits in behavior and cognition. Current estimates for the prevalence of Alzheimer’s disease (AD), provided by the Alzheimer’s Association, suggest 5.5 million Americans are living with AD, a number expected to rise dramatically [37]. Transplantation of human NS/PCs into either transgenic mice or hippocampal lesioned rats ameliorated learning and memory deficits in the Morris water maze (MWM) task [38–41]. As AD is a disorder of global brain degeneration, the targeted site for transplantation is a debated issue. Direct transplantation into the affected hippocampal formation demonstrated a limited degree of migration [38, 39, 42], whereas transplantation into the lateral ventricles migrated out through the SVZ to the hippocampus and other subcortical regions through white matter tracts [40, 43]. Transplantation of human cells has also been used to model AD. In one study, human ESC-derived NS/PCs functionally integrated into the neonatal AD transgenic mouse brain and were present up to 8 months post-transplantation, during which time the human cells were showing signs of degeneration similar to AD patients [44]. Transplanted cells from all of these studies were observed to survive and differentiate into immature neurons as well as astrocytes and glia, but mature neurons expressing choline acetyltransferase were rarely observed [38, 39, 44].
Neurological injuries occurring as a result of ischemic stroke or traumatic brain injury (TBI) can also have a profound impact on cognitive and behavioral function. Reported statistics within the United States place the yearly incidence of stroke at nearly 800,000 individuals [45] and roughly 2,800,000 emergency department visits related to TBI [46]. Long-term disability is common in individuals surviving these injuries, often requiring years of physical-, speech-, and occupational-therapy. Although these disabilities are difficult to measure experimentally in animals, the MWM task can assess spatial learning and memory that are significantly impaired in stroke and TBI animals. Transplantation of NS/PCs derived from fetal brain as well as the NT2N cell line were able to attenuate MWM deficits back toward baseline levels as early as 2 weeks post-transplantation and lasting up to 12 weeks post-transplantation [47–51]. Hippocampal transplantation of fetal-derived NS/PCs 48-hours following middle cerebral artery occlusion reduced early sensorimotor deficits in the sticky-dot task in mice [49]. Many studies observed that transplanted cells remained in an immature neuronal phenotype up to 13 weeks post-transplantation [52–58]. Transplanted cells were also observed to differentiate toward a glial phenotype [47, 59, 60].
Cell-based regenerative therapies for AD, stroke and TBI are at a nascent stage in development, with a variety of hurdles to overcome before it is seen as a viable therapeutic option. Preclinical transplantation of human cells into rodent models were able to ameliorate some of the cognitive and behavioral deficits albeit rarely returning to, or rising above, healthy levels. This benefit may be due, in part, to trophic effects of the transplants, rather than integration of the graft as few studies noted alterations in synapse formation of host cells [38, 44, 49] and no study noted an alteration in the cytoarchitecture of the host brain.
Neurological Chimerism and Motor Function
Transplantation of neural progenitor cells as a therapy for PD has arguably advanced the furthest of any neurological cellular therapy, to date. Although PD affects multiple systems throughout the body, the clinical diagnosis of PD occurs as a result of the loss of dopaminergic neurons within the substantia nigra (SN) projecting to the striatum (STR). It is estimated that the prevalence of PD is near 2 individuals per every 1,000 in the population, with increased risk associated with age [61]. Preclinical success of cellular transplantation for PD is due to the identification of a population of neural progenitors that can innervate the STR and release dopamine to near physiological levels, which can be achieved using a variety of cell sources [62, 63]. Fetal ventral mesencephalic tissue is a dopaminerich source of cells that are capable of integrating with the denervated STR and has resulted in the rescuing functional deficits in the rat and nonhuman primate following transplantation [34, 64–69]. Within these grafted animals, a strong outgrowth of tyrosine hydroxylase (TH) fibers from the graft core into the host STR has been observed, suggesting that the grafts are integrating with the host neurons.
Due to the limited availability of human fetal tissue and the variability of cell populations in transplants derived from fetuses of different gestational ages, several labs have focused on identifying alternative sources of cells for transplantation into PD. Differentiation of PSCs, in vitro, toward a dopaminergic progenitor has advanced largely due to the dual SMAD inhibition and floor-plate specification protocols [70, 71]. Using variations of this protocol, multiple groups have focused on differentiation of ESCs, which have observed strong integration of graft-derived TH+ fibers in the host STR of mice, rats, and nonhuman primates [72–79]. Transplantation of iPSC derived dopaminergic progenitors have similarly displayed robust engraftment and functional benefits in unilaterally lesioned rats [80].
From analysis of post-mortem tissue correlated with behavioral outcomes in human fetal tissue clinical trials, Hagell and Brundin suggest, at a minimum, 100,000 surviving TH+ neurons need to be present for a sustained therapeutic benefit [81]. Within the rat, numerous studies report amelioration of amphetamine-induced rotational deficits with as little as 1,000 human TH+ neurons. In some cases, up to 20,000 human TH+ neurons were observed in the rodent striatum [71, 76] more than doubling the estimated number of dopaminergic neurons in the healthy rat SN pars compacta [82].
Similar to PD, HD is a movement related disorder that is a result of an autosomal dominant mutation of the Huntingtin gene leading to the death of medium spiny neurons within the STR and glutamatergic neurons of the cortex [83]. Transplantation of fetal tissue into HD patients has been seen as a relative success in clinical trials [84–86]. Preclinical data suggested that tissue derived from the human fetal ganglionic eminences could engraft into the STR in the quinolinic acid lesion model of HD, survive up to 9 months post-transplantation, and ameliorate apomorphine-induced rotational deficits [34, 87–90]. Primary fetal grafts from 6 to 11 postgestational week ganglionic eminences, still containing dividing cells, resulted in differentiation toward immature neurons and few region-specific mature neurons [91, 92].
Similar to the issues associated with fetal transplants in PD, the research field in HD moved preclinical transplantation toward finding new sources of tissue, such as in vitro expanded or Myc immortalized fetal-derived NS/PCs. Studies in which fetal NS/PCs were transplanted into the rodent striatum demonstrated poor outcomes with little or no improvement in behavioral measures, and poor survival or integration into the host circuitry [92–98]. However, differentiation of PSCs toward a neural stem cell fate or toward a medium spiny neuronal fate show promise in reducing deficits associated with the quinolinic acid model [99–102]. Transplants of PSC-derived NS/PCs survived up to 4 months post-transplantation, differentiated toward medium spiny neurons and also expressed synaptic marker PSD95, suggestive of integration with the host [102–104].
Transplantation of human tissue into animal models of PD or HD has proven invaluable as new regenerative therapies are advancing toward the clinic, providing benefit for the individuals diagnosed with these devastating neurological disorders. Early preclinical studies transplanting fetal-derived tissue into the animal host has laid the groundwork for PSC derived progenitor cells. In all of these studies, no group has reported findings in which the grafted cells altered the behavioral state of the animals above baseline levels or altered the cytoarchitecture of the host brain.
Neurological Chimerism in the Spinal Cord
SCI affects more than 250,000 people in the United States, with vehicular and fall-related injuries being the most common causes [105]. Injury of the spinal cord results in partial or complete loss of limb sensation and function depending on the severity of the injury. Rodent models of SCI attempt to reproduce injury from either a temporary compression or contusion of the spinal cord or a complete transection. In these rodent models, human ESCs or iPSCs differentiated toward NS/PCs or glial cells are most commonly transplanted into the lesion epicenter or surrounding vertebrae. Two- to six-months following transplantation, counts of surviving cells ranged from less than 1% to as high as 23.9% [106–109]. Another study observed a plateau of approximately 400,000 surviving human cells within the spinal cord, regardless of initial dose [110]. Although the vast majority of transplanted cells remain within the transplantation site, there have been reports of migration more than 7 mm distal to the site of transplantation, corresponding to approximately 3 vertebrae [108, 110, 111]. Multiple studies have observed that transplanted cells differentiate into oligodendrocytes, suggesting that the human cells may be supporting endogenous surviving neurons [106, 109–111]. Similarly, terminal differentiation of transplanted human NS/PCs into astrocytes also provides a therapeutic benefit, likely due to increased trophic support [107, 112]. Transplantation human cells into SCI rats demonstrate marginal behavioral improvements in open-field, flat beam, and rotarod tests, relative to SCI control animals [108–110, 113, 114].
The most common motor neuron disorder in adults is amyotrophic lateral sclerosis (ALS), which is characterized by the loss of both upper and lower motor neurons resulting in muscle atrophy and ultimately death most commonly due to the inability to contract the diaphragm. Over 90% of ALS cases are considered idiopathic, and death usually occurs 3–5 years after the onset of symptoms [115]. Variants of the superoxide dismutase 1 (SOD1) gene have been linked to familial ALS and is present in 5%–10% of all human ALS cases. Both ESCs and iPSCs differentiated toward NS/PCs or glial cells have been transplanted directly into the spinal cord of rodent models of ALS. Human cells have been observed within transplanted animals up to 9 months post-transplantation and have been shown to form neurites, axons, and even functional neuromuscular junctions [116–118]. However, the terminal differentiation of these cells is variable. One study demonstrated that human cells are still in an immature state at 9 months post-transplant, suggesting the human cells may not be at a phenotypic state to completely integrate into the rodent CNS [119]. Two separate groups observed that up to 1,600,000 human cells integrated into the spinal cord of mutant SOD1 transgenic rodents up to 9 months post-transplant, with the majority of transplanted human cells differentiating toward astrocytes [117, 120]. Although some studies have shown grafted human neural stem cells to extend axons and innervate muscle, it is likely that the benefits seen are at least in part due to the neuroprotective mechanisms exerted by the engrafted human cells [116, 119, 121–125].
Transplantation studies of human cells into the diseased and injured spinal cord of rodents has demonstrated a therapeutic benefit with encouraging cell survival and integration of the human graft with animal host tissue. Preclinical transplantation of human NS/PC or glial progenitors into the spinal cord of rodent models of SCI and ALS have provided new therapeutic options for future clinical trials. In these 2 conditions, transplantation of human cells has not altered the behavioral state of the host above baseline levels, nor altered the cytoarchitecture of the host CNS.
Neurological Chimerism Using Human Glial Progenitors
The demyelinating disease multiple sclerosis (MS) is characterized by the progressive loss of myelinating oligodendrocytes in the CNS, resulting in numbness, tingling, tremors, and loss of mobility. Human cell transplantation studies in adult rodent models of MS have shown variable results. Transplants of fetal glia progenitors into the adult brain as well as ESC- or iPSC-derived NS/PCs into the adult spinal cord have shown limited benefit, beyond an altered immunomodulatory effect [126–128]. Transplants of human fetal-derived NS/PCs were found to survive up to 9 weeks in the demyelinated primate brain while remaining in a progenitor state [129] and 25 weeks in the brain of the shiverer transgenic mouse focally myelinating cells around the site of transplantation [130].
The greatest degree of neurological chimerism in any of the studies and disease states mentioned thus far has been observed in multiple studies by Goldman and colleagues at the University of Rochester. In these studies, human glial precursors isolated from fetal NS/PCs are transplanted into the corpus callosum of neonatal shiverer transgenic mice, resulting in the ultimate replacement of the endogenous mouse glia with human glia [131]. This replacement has developed an entire human glial network within the mouse brain, where 300,000 initial human glial precursor cells proliferated into an estimated 12 million human glial cells throughout the entire shiverer mouse CNS [131–134]. Adult shiverer glial chimeric mice showed reduced seizure activity and extended overall survival as well as myelination patterns and glial networks similar to what is observed in wild-type mice. Human glial progenitors derived from human iPSCs have demonstrated similar effectiveness in myelinating the shiverer mouse CNS [135].
One study that deserves special attention transplanted fetal-derived glial precursors into neonatal immunodeficient mice and found human glia throughout the entire brain within 12–20 months and was organized in a laminar structure, a phenotype previously thought to be present only in humans and nonhuman primates [136]. The human astrocytes maintained a human astrocyte morphology (i.e., larger nuclei, long projections) within the mouse brain. Functionally, human astrocytes propagated calcium waves significantly faster than mouse astrocytes resulting in enhanced rates of field excitatory postsynaptic potentials and long-term potentiation. Behaviorally, chimeric mice displayed an improvement in the speed of acquisition of an auditory fear conditioning response, reduced latency to escape the Barnes maze, and an increase in the ability of mice to remember the locations of objects in the object-location memory task, relative to wild-type mice. It is likely that the enhanced ability to learn in the human/mouse astrocyte chimeras is a result of subtle differences between the functions of human and mouse astrocytes rather than human astrocytes forming novel neuronal pathways. However, the enhanced ability of these human/mouse chimeras to learn, regardless of whether the neuronal architecture is altered, is an important observation that necessitates further research.
Discussion
The purpose of this review was to summarize the literature in which human tissue has been transplanted into the CNS of mice, rats, and nonhuman primates, in order to answer the question: “Will generation of human neural tissue within animals through blastocyst complementation produce humanized animals?” Although the ultimate answer to this question requires additional research, our review of the scientific literature finds that human/animal chimerism has not yet generated animals that possess an altered cognitive or behavioral state which trends toward “human-like.” To the contrary, few studies transplanting human cells into a diseased or injured animal restored cognitive or motor function to levels of healthy animals.
Several key variables need to be considered when interpreting these findings. First, most studies implanted human cells into mice (30%) or rats (64%). The mouse brain contains an estimated 70 million neurons and 23 million glia, the rat brain contains an estimated 200 million neurons, and the human brain contains an estimated 86,000 million neurons and 85,000 million glia [137–140]. It would be unlikely that even a majority of human neurons within the rodent brain would substantially alter the cognitive abilities of the chimeric animals, due to the small size, limited cytoarchitecture and connectome of the rodent brain. Relevant to blastocyst complementation, the domestic pig has a gyrencephalic brain with roughly 2.5% of the total number of neurons relative to humans, similar to the total number of neurons in the rhesus macaque [141, 142]. Of the 6% of studies in this review in which human cells were transplanted into the nonhuman primate brain, no team identified an observational alteration in the behavior of the nonhuman primate.
The second variable that needs to be considered is the age of the host at the time of transplantation. The majority of studies transplanted human neural tissue into adult animals (84%) compared with prenatal or neonatal animals (16%). It is likely that the age of the transplant recipient will impact the degree of chimerism as the developing brain is more plastic to the integration of transplanted cells. One study observed that the neonatal mouse brain promoted the survival and migration of transplanted human cells when compared with adult mice [143]. Multiple studies transplanting human fetal glial progenitor cells into demyelinated neonatal mice show the human cells integrate and out-compete endogenous mouse glia, effectively creating a largely human glial network within the demyelinated mouse brain [131–136], whereas transplantation of other cell types into adult demyelinated animals results in limited engraftment [130, 144]. Although it is likely the case that transplant recipient age affects the outcome of transplantation, more work needs to be done to determine the extent of these effects.
Ethical concerns have been raised about the possibility that the introduction of human stem cells into a nonhuman blastocyst could potentially alter the brain connectome of the host to the degree to which we would observe evidence of neural connections of the type and magnitude that would be required to produce human-like thought and behavior [9]. Although more research is required to address the concern more completely, work by the Nakauchi group demonstrated that through generating a rat pancreas within the mouse, using blastocyst complementation, the size of the pancreas was similar to that of a normal mouse and not the rat. This suggests that in the context of interspecies complementation, the development of the host species likely dictates the ultimate size of the complemented organ [5]. Likewise, we speculate that generating human neural cells in the nonhuman host would produce a connectome that would be dictated by the host species. Thus the elaboration of human behavior through a human connectome would be unlikely.
A final consideration is that preclinical and clinical studies using human fetal brain tissue for transplantation demonstrate that neural precursors derived from the fetal brain are the most suitable for transplantation. Therefore the need to allow chimeras to come to term for the harvesting of neural cells for transplantation is not required and addresses the concerns of chimeras being born that express human behaviors.
Recommendations for Future Complementation Research
The 150 transplantation studies reviewed here suggest that complementation of nonhuman mammalian embryos with human stem cells is not likely to substantially alter the behavior of the chimera in a manner which can be construed as humanized. However, research on the potential humanization of chimera animals remains limited, and further investigation is required to more fully explore the plausible risks. Research in blastocyst complementation should be allowed to continue, carefully and with transparent milestones, in order to better evaluate these unknown risks. As the scientific community begins to discuss these milestones, we recommend the following be included:
Complementation of human cells in the livestock embryo should not surpass mid-gestation without determining the extent of chimerism in all tissues, including neurogenic regions, thus providing an early time point to alter or abandon the experimental protocol.
Blastocyst complementation for the purpose of nonneurological organs should not be allowed to come to term until a thorough and reproducible analysis of neurological structures can determine the extent of chimerism in preterm fetuses, with the goal of limiting neurologic chimerism.
Blastocyst complementation for the purpose of treating neurological disorders should harvest tissue at a progenitor stage, and therefore chimeric animals will not be allowed to come to term. However, neurological chimeras should be analyzed for the purity of human cells within the target structure as well as outside of target structure.
Blastocyst complementation for the purpose of neurological disease modeling should be allowed to come to term after a thorough analysis of the preterm brain has been established and if on-target chimerism is limited to motor regions of the brain, allaying concerns over human chimerism to prefrontal cortex and hippocampal regions.
Chimeric animals should be separated at weaning and not allowed to breed, in case human gametes are found in chimeric livestock.
Significance Statement.
Due to a severe shortage of human organs and tissues, thousands of patients die each year due to an inability to procure organs for transplantation. Blastocyst complementation is a methodology that has the potential to generate large quantities of functioning human organs and tissues but is hindered by a National Institutes of Health moratorium on funding, citing concern over substantial human cell contribution to the brain of the animal. This review summarizes published, peer-reviewed studies on human–animal neural transplantation and suggests that this concern over neurological chimerism should not prevent research to continue in a controlled and transparent manner.
Acknowledgments
We acknowledge Vibha Savanur, Antony Crane, and Georgette Danczyk for compiling and organizing background literature for this review and to Marra Evans who contributed illustrations for the figure and graphical abstract.
Footnotes
Disclosure of Potential Conflicts of Interest
W.L. declared leadership position with Regenevida. The other authors indicated no potential conflicts of interest.
References
- 1.Robert JS, Baylis F. Crossing species boundaries. Am J Bioeth 2003;3:1–13. [DOI] [PubMed] [Google Scholar]
- 2.Chan WFN, Gurnot C, Montine TJ et al. Male microchimerism in the human female brain. PLoS One 2012;7:e45592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mezey É, Key S, Vogelsang G et al. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci USA 2003;100:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suchy F, Nakauchi H. Lessons from interspecies mammalian chimeras. Annu Rev Cell Dev Biol 2017;33:203–217. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Yamaguchi T, Hamanaka S et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 2010;142:787–799. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Sato H, Kato-Itoh M et al. Interspecies organogenesis generates autologous functional islets. Nature 2017;542:191–196. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Platero-Luengo A, Sakurai M et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 2017;168:473–486.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunari H, Nagashima H, Watanabe M et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA 2013;110:4557–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farahany NA, Greely HT, Hyman S et al. The ethics of experimenting with human brain tissue. Nature 2018;556:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Greely HT, Jaenisch R et al. Stem cells and interspecies chimaeras. Nature 2016; 540:51–59. [DOI] [PubMed] [Google Scholar]
- 11.Bourret R, Martinez E, Vialla F et al. Human–animal chimeras: Ethical issues about farming chimeric animals bearing human organs. Stem Cell Res Ther 2016;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 2001;105:7–17. [DOI] [PubMed] [Google Scholar]
- 13.Englund U, Fricker-Gates RA, Lundberg C et al. Transplantation of human neural progenitor cells into the neonatal rat brain: Extensive migration and differentiation with long-distance axonal projections. Exp Neurol 2002;173:1–21. [DOI] [PubMed] [Google Scholar]
- 14.Uchida N, Buck DW, He D et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 2000;97: 14720–14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki S, Eckert K, He D et al. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J Neurosci Res 2002;69:976–986. [DOI] [PubMed] [Google Scholar]
- 16.Flax JD, Aurora S, Yang C et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol 1998;16:1033–1039. [DOI] [PubMed] [Google Scholar]
- 17.Brüstle O, Choudhary K, Karram K et al. Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nat Biotechnol 1998;16: 1040–1044. [DOI] [PubMed] [Google Scholar]
- 18.Tailor J, Kittappa R, Leto K et al. Stem cells expanded from the human embryonic hindbrain stably retain regional specification and high neurogenic potency. J Neurosci 2013; 33:12407–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ourednik V, Ourednik J, Flax JD et al. Segregation of human neural stem cells in the developing primate forebrain. Science 2001; 293:1820–1824. [DOI] [PubMed] [Google Scholar]
- 20.Maciaczyk J, Singec I, Maciaczyk D et al. Restricted spontaneous in vitro differentiation and region-specific migration of long-term expanded fetal human neural precursor cells after transplantation into the adult rat brain. Stem Cells Dev 2009;18:1043–1058. [DOI] [PubMed] [Google Scholar]
- 21.Englund U, Björklund A,Wictorin K. Migration patterns and phenotypic differentiation of long-term expanded human neural progenitor cells after transplantation into the adult rat brain. Brain Res Dev Brain Res 2002;134:123–141. [DOI] [PubMed] [Google Scholar]
- 22.Fricker RA, Carpenter MK, Winkler C et al. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci 1999;19:5990–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabaté O, Horellou P, Vigne E et al. Transplantation to the rat brain of human neural progenitors that were genetically modified using adenoviruses. Nat Genet 1995;9: 256–260. [DOI] [PubMed] [Google Scholar]
- 24.Kelly CM, Precious SV, Scherf C et al. Neonatal desensitization allows long-term survival of neural xenotransplants without immunosuppression. Nat Methods 2009;6:271–273. [DOI] [PubMed] [Google Scholar]
- 25.Aleksandrova MA, Saburina IN, Poltavtseva RA et al. Behavior of human neural progenitor cells transplanted to rat brain. Brain Res Dev Brain Res 2002;134:143–148. [DOI] [PubMed] [Google Scholar]
- 26.Zhang SC, Wernig M, Duncan ID et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 27.Nagashima F, Suzuki IK, Shitamukai A et al. Novel and robust transplantation reveals the acquisition of polarized processes by cortical cells derived from mouse and human pluripotent stem cells. Stem Cells Dev 2014;23: 2129–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim T-G, Yao R, Monnell T et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. STEM CELLS 2014;32:1789–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tennstaedt A, Aswendt M, Adamczak J et al. Human neural stem cell intracerebral grafts show spontaneous early neuronal differentiation after several weeks. Biomaterials 2015;44:143–154. [DOI] [PubMed] [Google Scholar]
- 30.Tennstaedt A, Mastropietro A, Nelles M et al. In vivo fate imaging of intracerebral stem cell grafts in mouse brain. PLoS One 2015;10:e0144262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hargus G, Ehrlich M, Araúzo-Bravo MJ et al. Origin-dependent neural cell identities in differentiated human iPSCs in vitro and after transplantation into the mouse brain. Cell Rep 2014;8:1697–1703. [DOI] [PubMed] [Google Scholar]
- 32.Torper O, Pfisterer U, Wolf DA et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA 2013; 110:7038–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trojanowski JQ, Mantione JR, Lee JH et al. Neurons derived from a human teratocarcinoma cell line establish molecular and structural polarity following transplantation into the rodent brain. Exp Neurol 1993;122: 283–294. [DOI] [PubMed] [Google Scholar]
- 34.Pundt LL, Kondoh T, Low WC. The fate of human glial cells following transplantation in normal rodents and rodent models of neurodegenerative disease. Brain Res 1995;695:25–36. [DOI] [PubMed] [Google Scholar]
- 35.Messina DJ, Alder L, Tresco PA. Comparison of pure and mixed populations of human fetal-derived neural progenitors transplanted into intact adult rat brain. Exp Neurol 2003;184:816–829. [DOI] [PubMed] [Google Scholar]
- 36.Mansour AA, Gonçalves JT, Bloyd CW et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 2018;36:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc 2017;13:325–373. [Google Scholar]
- 38.Ager RR, Davis JL, Agazaryan A et al. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015;25: 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marei HES, Farag A, Althani A et al. Human olfactory bulb neural stem cells expressing hngf restore cognitive deficit in Alzheimer’s disease rat model. J Cell Physiol 2015; 230:116–130. [DOI] [PubMed] [Google Scholar]
- 40.Park D, Joo SS, Kim TK et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant 2012;21:365–371. [DOI] [PubMed] [Google Scholar]
- 41.Park D, Yang Y-H, Bae DK et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol Aging 2013;34:2639–2646. [DOI] [PubMed] [Google Scholar]
- 42.McGinley LM, Sims E, Lunn JS et al. Human cortical neural stem cells expressing insulin-like growth factor-I: A novel cellular therapy for Alzheimer’s disease. STEM CELLS TRANSLATIONAL MEDICINE 2016;5:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee I-S, Jung K, Kim I-S et al. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener 2015; 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espuny-Camacho I, Arranz AM, Fiers M et al. Hallmarks of Alzheimer’s disease in stemcell-derived human neurons transplanted into mouse brain. Neuron 2017;93:1066–1081. [DOI] [PubMed] [Google Scholar]
- 45.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart disease and stroke statistics—2017 update. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor CA, Bell JM, Breiding MJ et al. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. Morb Mortal Wkly Rep 2017;66:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haus DL, López-Velázquez L, Gold EM et al. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol 2016;281:1–16. [DOI] [PubMed] [Google Scholar]
- 48.Gao J, Prough DS, McAdoo DJ et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol 2006; 201:281–292. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Saatman KE, Royo NC et al. Delayed transplantation of human neurons following brain injury in rats: A long-term graft survival and behavior study. J Neurotrauma 2005;22:1456–1474. [DOI] [PubMed] [Google Scholar]
- 50.Watson DJ, Longhi L, Lee EB et al. Genetically modified NT2N human neuronal cells mediate long-term gene expression as CNS grafts in vivo and improve functional cognitive outcome following experimental traumatic brain injury. J Neuropathol Exp Neurol 2003;62: 368–380. [DOI] [PubMed] [Google Scholar]
- 51.Yuan T, Liao W, Feng N-H et al. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther 2013;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abeysinghe HCS, Bokhari L, Quigley A et al. Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke. Stem Cell Res Ther 2015;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L, Wong S, Snyder EY et al. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res Ther 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jablonska A, Drela K, Wojcik-Stanaszek L et al. Short-lived human umbilical cord-bloodderived neural stem cells influence the endogenous secretome and increase the number of endogenous neural progenitors in a rat model of lacunar stroke. Mol Neurobiol 2016;53: 6413–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicks AU, Lappalainen RS, Narkilahti S et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur J Neurosci 2009;29:562–574. [DOI] [PubMed] [Google Scholar]
- 56.Tornero D,Wattananit S, Grønning Madsen M et al. Human induced pluripotent stem cell-derived cortical neurons integrate in strokeinjured cortex and improve functional recovery. Brain J Neurol 2013;136:3561–3577. [DOI] [PubMed] [Google Scholar]
- 57.Chang D-J, Lee N, Park I-H et al. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant 2013;22:1427–1440. [DOI] [PubMed] [Google Scholar]
- 58.Skardelly M, Gaber K, Burdack S et al. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma 2011;28:401–414. [DOI] [PubMed] [Google Scholar]
- 59.Jeong S-W, Chu K, Jung K-H et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 2003;34:2258–2263. [DOI] [PubMed] [Google Scholar]
- 60.Wennersten A, Meier X, Holmin S et al. Proliferation, migration, and differentiation of human neural stem/progenitor cells after Transplantation into a rat model of traumatic brain injury. J Neurosurg 2004;100:88–96. [DOI] [PubMed] [Google Scholar]
- 61.Pringsheim T, Jette N, Frolkis A et al. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 2014;29:1583–1590. [DOI] [PubMed] [Google Scholar]
- 62.Lindvall O. Treatment of Parkinson’s disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci 2015;370:20140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease—Past insights and future potential. Nat Rev Neurol 2015;11:492–503. [DOI] [PubMed] [Google Scholar]
- 64.Brundin P, Nilsson OG, Strecker RE et al. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp Brain Res 1986;65:235–240. [DOI] [PubMed] [Google Scholar]
- 65.Kondoh T, Pundt LL, Low WC. Development of human fetal ventral mesencephalic grafts in rats with 6-OHDA lesions of the nigrostriatal pathway. Neurosci Res 1995;21: 223–233. [DOI] [PubMed] [Google Scholar]
- 66.Kondoh T, Pundt LL, Blount JP et al. Transplantation of human fetal tissue from spontaneous abortions to a rodent model of Parkinson’s disease. Cell Transplant 1996;5: 69–75. [DOI] [PubMed] [Google Scholar]
- 67.Svendsen CN, Clarke DJ, Rosser AE et al. Survival and differentiation of rat and human epidermal growth factor-responsive precursor cells following grafting into the lesioned adult central nervous system. Exp Neurol 1996;137: 376–388. [DOI] [PubMed] [Google Scholar]
- 68.Clarkson ED, Zawada WM, Bell KP et al. IGF-I and bFGF improve dopamine neuron survival and behavioral outcome in parkinsonian rats receiving cultured human fetal tissue strands. Exp Neurol 2001;168:183–191. [DOI] [PubMed] [Google Scholar]
- 69.Redmond DE, Naftolin F, Collier TJ et al. Cryopreservation, culture, and transplantation of human fetal mesencephalic tissue into monkeys. Science 1988;242:768–771. [DOI] [PubMed] [Google Scholar]
- 70.Chambers SM, Fasano CA, Papapetrou EP et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kriks S, Shim J-W, Piao J et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011;480:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heuer A, Kirkeby A, Pfisterer U et al. hESC-derived neural progenitors prevent xenograft rejection through neonatal desensitisation. Exp Neurol 2016;282:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai J, Yang M, Poremsky E et al. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev 2010;19: 1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai J, Donaldson A, Yang M et al. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. STEM CELLS 2009;27:220–229. [DOI] [PubMed] [Google Scholar]
- 75.Grealish S, Diguet E, Kirkeby A et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014;15:653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirkeby A, Nolbrant S, Tiklova K et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell 2017;20:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wakeman DR, Redmond DE, Dodiya HB et al. Human neural stem cells survive long term in the midbrain of dopamine-depleted monkeys after GDNF overexpression and project neurites toward an appropriate target. STEMCELLS TRANSLATIONAL MEDICINE 2014;3:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakeman DR, Weiss S, Sladek JR et al. Survival and integration of neurons derived from human embryonic stem cells in MPTP-lesioned primates. Cell Transplant 2014;23: 981–994. [DOI] [PubMed] [Google Scholar]
- 79.Kang X, Xu H, Teng S et al. Dopamine release from transplanted neural stem cells in Parkinsonian rat striatum in vivo. Proc Natl Acad Sci USA 2014;111:15804–15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hargus G, Cooper O, Deleidi M et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA 2010;107: 15921–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal Transplantation in Parkinson disease. J Neuropathol Exp Neurol 2001;60:741–752. [DOI] [PubMed] [Google Scholar]
- 82.Nair-Roberts RG, Chatelain-Badie SD, Benson E et al. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 2008;152:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bates G, Tabrizi S, Jones L. Huntington’s Disease. USA: Oxford University Press, 2014. [Google Scholar]
- 84.Bachoud-Lévi AC, Rémy P, Nguyen JP et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet 2000;356:1975–1979. [DOI] [PubMed] [Google Scholar]
- 85.Bachoud-Lévi AC, Gaura V, Brugières P et al. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: A long-term follow-up study. Lancet Neurol 2006;5:303–309. [DOI] [PubMed] [Google Scholar]
- 86.Bachoud-Lévi AC. Neural grafts in Huntington’s disease: Viability after 10 years. Lancet Neurol 2009;8:979–981. [DOI] [PubMed] [Google Scholar]
- 87.Pundt LL, Kondoh T, Conrad JA et al. Transplantation of human striatal tissue into a rodent model of Huntington’s disease: Phenotypic expression of transplanted neurons and host-to-graft innervation. Brain Res Bull 1996;39:23–32. [DOI] [PubMed] [Google Scholar]
- 88.Pundt LL, Kondoh T, Conrad JA et al. Transplantation of human fetal striatum into a rodent model of Huntington’s disease ameliorates locomotor deficits. Neurosci Res 1996; 24:415–420. [DOI] [PubMed] [Google Scholar]
- 89.Pundt LL, Narang N, Kondoh T et al. Localization of dopamine receptors and associated mRNA in transplants of human fetal striatal tissue in rodents with experimental Huntington’s disease. Neurosci Res 1997;27:305–315. [DOI] [PubMed] [Google Scholar]
- 90.Sanberg PR, Borlongan CV, Koutouzis TK et al. Human fetal striatal transplantation in an excitotoxic lesioned model of Huntington’s disease. Ann N Y Acad Sci 1997;831:452–460. [DOI] [PubMed] [Google Scholar]
- 91.Hurelbrink CB, Armstrong RJE, Dunnett SB et al. Neural cells from primary human striatal xenografts migrate extensively in the adult rat CNS. Eur J Neurosci 2002;15:1255–1266. [DOI] [PubMed] [Google Scholar]
- 92.Kelly CM, Precious SV, Penketh R et al. Striatal graft projections are influenced by donor cell type and not the immunogenic background. Brain J Neurol 2007;130:1317–1329. [DOI] [PubMed] [Google Scholar]
- 93.El-Akabawy G, Medina LM, Jeffries A et al. Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the Hedgehog pathway. Stem Cells Dev 2011;20:1873–1887. [DOI] [PubMed] [Google Scholar]
- 94.Armstrong RJ, Watts C, Svendsen CN et al. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington’s disease. Cell Transplant 2000;9:55–64. [DOI] [PubMed] [Google Scholar]
- 95.McLeod MC, Kobayashi NR, Sen A et al. Transplantation of GABAergic cells derived from bioreactor-expanded human neural precursor cells restores motor and cognitive behavioral deficits in a rodent model of Huntington’s disease. Cell Transplant 2013;22:2237–2256. [DOI] [PubMed] [Google Scholar]
- 96.Ryu JK, Kim J, Cho SJ et al. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol Dis 2004;16:68–77. [DOI] [PubMed] [Google Scholar]
- 97.McBride JL, Behrstock SP, Chen E-Y et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol 2004;475:211–219. [DOI] [PubMed] [Google Scholar]
- 98.Lee S-T, Park J-E, Lee K et al. Noninvasive method of immortalized neural stem-like cell transplantation in an experimental model of Huntington’s disease. J Neurosci Methods 2006;152:250–254. [DOI] [PubMed] [Google Scholar]
- 99.Song J, Lee S-T, Kang W et al. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett 2007;423: 58–61. [DOI] [PubMed] [Google Scholar]
- 100.Delli Carri A, Onorati M, Lelos MJ et al. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ mediumsized spiny neurons. Dev Camb Engl 2013; 140:301–312. [DOI] [PubMed] [Google Scholar]
- 101.Jeon I, Lee N, Li J-Y et al. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. STEM CELLS 2012;30: 2054–2062. [DOI] [PubMed] [Google Scholar]
- 102.Ma L, Hu B, Liu Y et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 2012;10:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vazey EM, Dottori M, Jamshidi P et al. Comparison of transplant efficiency between spontaneously derived and noggin-primed human embryonic stem cell neural precursors in the quinolinic acid rat model of Huntington’s disease. Cell Transplant 2010;19:1055–1062. [DOI] [PubMed] [Google Scholar]
- 104.Aubry L, Bugi A, Lefort N et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA 2008; 105:16707–16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2017. [Google Scholar]
- 106.Bastidas J, Athauda G, De La Cruz G et al. Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia 2017;65:1278–1301. [DOI] [PubMed] [Google Scholar]
- 107.Li K, Javed E, Scura D et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp Neurol 2015;271:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim D-S, Jung SJ, Lee JS et al. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Exp Mol Med 2017;49:e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Erceg S, Ronaghi M, Oria M et al. transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. STEM CELLS 2010;28:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piltti KM, Funes GM, Avakian SN et al. Increasing human neural stem cell transplantation dose alters oligodendroglial and neuronal differentiation after spinal cord injury. Stem Cell Rep 2017;8:1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawabata S, Takano M, Numasawa-Kuroiwa Y et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep 2016;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lukovic D, Valdés-Sanchez L, Sanchez-Vera I et al. Brief report: Astrogliosis promotes functional recovery of completely transected spinal cord following transplantation of hESC-derived oligodendrocyte and motoneuron progenitors. STEM CELLS 2014;32:594–599. [DOI] [PubMed] [Google Scholar]
- 113.Ruzicka J, Machova-Urdzikova L, Gillick J et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant 2017;26:585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Führmann T, Tam RY, Ballarin B et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 2016;83:23–36. [DOI] [PubMed] [Google Scholar]
- 115.Mitsumoto HCD, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia, PA: F.A. Davis Company; 1998. [Google Scholar]
- 116.Pepper J-P, Wang TV, Hennes V et al. Human induced pluripotent stem cell-derived motor neuron transplant for neuromuscular atrophy in a mouse model of sciatic nerve injury. JAMA Facial Plast Surg 2017;19:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen H, Qian K, Chen W et al. Humanderived neural progenitors functionally replace astrocytes in adult mice. J Clin Invest 2015; 125:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao J, Coggeshall RE, Tarasenko YI et al. Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience 2005;131:257–262. [DOI] [PubMed] [Google Scholar]
- 119.Hefferan MP, Galik J, Kakinohana O et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal Transplantation. PLoS One 2012;7:e42614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lepore AC, O’Donnell J, Kim AS et al. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One 2011;6:e25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu L, Shen P, Hazel T et al. Dual Transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett 2011;494:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knippenberg S, Rath KJ, Böselt S et al. Intraspinal administration of human spinal cordderived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. J Tissue Eng Regen Med 2017;11:751–764. [DOI] [PubMed] [Google Scholar]
- 123.Klein SM, Behrstock S, McHugh J et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther 2005;16:509–521. [DOI] [PubMed] [Google Scholar]
- 124.Xu L, Yan J, Chen D et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 2006;82:865–875. [DOI] [PubMed] [Google Scholar]
- 125.Nizzardo M, Simone C, Rizzo F et al. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA41 neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 2014;23: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Plaisted WC, Zavala A, Hingco E et al. Remyelination is correlated with regulatory T cell induction following human embryoid bodyderived neural precursor cell transplantation in a viral model of multiple sclerosis. PLoS One 2016;11:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim H, Walczak P, Muja N et al. ICVtransplanted human glial precursor cells are short-lived yet exert immunomodulatory effects in mice with EAE. Glia 2012;60:1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen L, Coleman R, Leang R et al. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Rep 2014;2:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pluchino S, Gritti A, Blezer E et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol 2009;66:343–354. [DOI] [PubMed] [Google Scholar]
- 130.Uchida N, Chen K, Dohse M et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med 2012;4:155ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Windrem MS, Schanz SJ, Morrow C et al. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci 2014;34: 16153–16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Windrem MS, Roy NS, Wang J et al. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res 2002;69:966–975. [DOI] [PubMed] [Google Scholar]
- 133.Windrem MS, Nunes MC, Rashbaum WK et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 2004;10: 93–97. [DOI] [PubMed] [Google Scholar]
- 134.Windrem MS, Schanz SJ, Guo M et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2008;2:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S, Bates J, Li X et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 2013; 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han X, Chen M, Wang F et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013;12:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Azevedo FAC, Carvalho LRB, Grinberg LT et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009;513:532–541. [DOI] [PubMed] [Google Scholar]
- 138.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA 2006;103:12138–12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herculano-Houzel S. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci 2005;25:2518–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goffinet AM, Rakic P. Mouse Brain Development. Berlin, Germany: Springer Science & Business Media, 2012. [Google Scholar]
- 141.Kazu RS, Maldonado J, Mota B et al. Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Front Neuroanat 2014;8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kazu RS, Maldonado J, Mota B et al. Corrigendum: Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Front Neuroanat 2015;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tom CM, Younesi S, Meer E et al. Survival of iPSC-derived grafts within the striatum of immunodeficient mice: Importance of developmental stage of both transplant and host recipient. Exp Neurol 2017;297:118–128. [DOI] [PubMed] [Google Scholar]
- 144.Aharonowiz M, Einstein O, Fainstein N et al. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One 2008;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


