Abstract
Virtual resection of liver structures guided by three-dimensional visualization technology (3DVT) has been extensively used in China. This technique provides a safe and effective method for accurate diagnosis of liver tumors and has important applications in preoperative evaluation, surgical planning and intraoperative guidance of liver cancer surgeries. The technical advantages and clinical significance of 3DVT in the diagnosis and treatment of complex liver tumors have been recognized. In order to standardize the application of 3DVT in the precision diagnosis and treatment of complex liver tumors, this guideline provides explanations and recommendations in the following aspects: (1) the establishment of homogenization processing and quality control system of 3D reconstruction; (2) the establishment of 3D reconstructed models of abdominal organs and lesions; (3) the individualized classification and quantitative analysis of blood vessels based on 3DVT; (4) 3DVT-based classification and grading of hepatic vessels in complex hepatic tumors; (5) evaluation system for surgery evaluation after reconstruction of the 3D visualization model; (6) application of 3D printing in complex hepatectomy; (7) virtual reality technology; (8) ICG fluorescence imaging; (9) multi-modal images for real-time navigation; (10) three-dimensional visualization to guide the preoperative surgical planning of precision hepatectomy; (11) application of 3DVT guidance in other therapeutic methods of hepatocellular carcinoma; (12) application of 3DVT in follow-up evaluation of the patients after liver cancer surgeries. 3DVT for visualization of the liver structures has important clinical values for accurate preoperative evaluation, preoperative planning and surgical navigation of complex liver tumors, and it facilitates precision surgeries to improve the outcomes and promote postoperative recovery of the patients.
Keywords: three-dimensional visualization, three-dimensional printing, virtual reality, fluorescence imaging, complex liver tumors, hepatectomy
Abstract
基于肝脏三维可视化的虚拟肝切除在中国得到越来越广泛的应用, 为肝脏肿瘤精确诊断、术前评估、手术规划和术中精准指导提供了安全而有效的方法, 该技术在复杂性肝脏肿瘤诊治中的技术优势及重要意义也已逐渐得到认可。为规范三维可视化技术在复杂性肝脏肿瘤的精准诊疗中的应用, 本指南对以下几个方面进行了研究:(1)三维重建同质化处理与质控体系的建立; (2)个体化腹腔器官和病灶的三维可视化模型的建立; (3)三维可视化个体化血管分型和量化分析; (4)复杂性肝脏肿瘤肝脏血管三维可视化分型、分级; (5)三维可视化模型建立后的手术模拟评估体系; (6)三维可视化肝脏3D打印在复杂性肝切除术的应用; (7)虚拟现实技术; (8)ICG分子荧光成像; (9)多模图像融合实时导航技术; (10)三维可视化指导精准复杂性肝切除术的术前规划; (11)三维可视化技术对肝癌其他治疗手段的指导作用; (12)三维可视化技术对肝癌手术后复查的指导作用。肝脏三维可视化技术为复杂性肝脏肿瘤的术前精准评估病情、术前规划、手术导航等实施提供了新的方法, 为病人获得最佳的康复效果发挥强而有力的支持作用, 具有重要的临床应用价值。
Keywords: 三维可视化/3D打印, 虚拟现实, 荧光影像, 复杂性肝脏肿瘤, 解剖性/功能性/根治术/肝切除术
INTRODUCTION
Currently, there are different definitions for complex liver tumors: (1) centrally located hepatocellular carcinoma spreading to the porta hepatis; (2) tumors with vascular variations inside the liver; (3) intrahepatic vascular malformations caused by liver compression due to its large size; (4) hepatic malignancy with tumor thrombus in the inferior vena cava and/or the right atrium; (5) giant tumor regardless of its characters for which extended hepatectomy is planned; or (6) liver tumors encroaching on segments Ⅰ and Ⅷ to require complex hepatectomy[1-3].
Three-dimensional (3D) visualization technology (3DVT) for liver tumors encompasses the techniques involved in displaying, describing, and interpreting the 3D anatomy and morphological features of liver tumors. With the aid of computed tomography/magnetic resonance imaging (CT/MRI), 3DVT allows accurate description and interpretation of the morphology and spatial distribution of the targets including the liver, biliary tract system, blood vessels, and pathological tissues through such computer image processing techniques as data analysis and calculation, imaging fusion, segmentation, and visualization. This technique makes possible intuitive, accurate, and fast visual identification of the targets and thus offers decision-making support to preoperative diagnosis, individualized surgical planning, and selection of the surgical approach.
When using more conventional diagnostic imaging methods for liver tumors such as ultrasonography, CT, and MRI, clinicians have to rely on 2D information to deduce 3D structures. The results rely heavily on their personal experience. Nonetheless, the limitation and uncertainty of their experience may lead to uncertainty and inconsistency in reconstructed structures, which may hinder accurate preoperative assessment, resulting in a relatively high incidence of postoperative complications.
With the development of CT scanning techniques, more distinct and substantial image datasets of liver tumors are available to provide large amounts of diagnostic information that facilitates the research of 3DVT for liver tumors. Current advances in modern imaging technologies, including 3DVT, 3D printing, multimodal image fusion, virtual reality, and ICG fluorescence imaging[4]have promised a further increase in the diagnostic efficacy of the liver diseases. In 2017, "the Expert Consensus on the Application of 3DVT for the Accurate Diagnosis and Treatment of Complex Liver Tumors" was published in the Chinese Journal of Practical Surgery. It has received widespread acceptance nationwide after 3 years of clinical practice. A meta-analysis reported that the application of 3DVT in the diagnosis and management of primary hepatocellular carcinoma showed significant advantages over the control group in terms of intraoperative blood loss, postoperative complications, recovery of postoperative liver function, operation time, hospitalization time, and short-term postoperative tumor recurrence. According to the 2017 version of the Expert Consensus, this guideline, based on the efforts of the Chinese Society of Digital Medicine, Chinese Research Hospital Association of Digital Surgery Committee and Chinese experts in relevant fields, aims to standardize the application of 3DVT, 3D printing technology, virtual reality and fluorescence imaging technology in precision diagnosis and treatment for complex liver tumors.
This Guideline follows the Grading of Recommendations Assessment, Development and Evaluation (GRADE), which divides the quality of evidence into high, moderate, and low or very low levels, reported herein by tagging with the letters A, B and C, respectively (Tab. 1). The strength of the recommendations given herein, based on the GRADE grid method[5-7] (Tab. 1), is described as strong (1) and weak (2). The experts and patient representatives who participated in the formation of recommendations signed a declaration of no conflict of interest in advance, which was checked by the secretariats of guideline committees.
1.
Quality of evidence and strength of recommendations
| Grade | Classification | Content |
| Quality of evidence | ||
| High | A | We are very confidence that the true effect lies close to that of the estimated effect. |
| Moderate | B | We are moderately confident in the effect estimate: the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. |
| Low or very low | C | Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimated effect; we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimated effect. |
| Strength of recommendation | ||
| Strong recommendation | 1 | The desirable effects outweigh the undesirable effects. |
| Weak recommendation | 2 | The desirable effects possibly outweigh the undesirable effects. |
1. Homogenization processing and quality control system of 3D reconstruction
In the process of generating 3D models, manual segmentation is usually required besides automatic segmentation. Therefore, only by following the homogenization processing and quality control system can a high-standard 3D reconstruction and comprehensive evaluation of diseases be achieved.
1.1. Quality control of CT image data of complex liver tumors
The setting of scanning parameters and storage of the CT images (typically with a 64-slice helical CT scanner): the conventional supine position is chosen for a plain scan from head to foot. The range is from the top of the diaphragm to the inferior margin of the liver. The scanning parameter setting is 120 kV and 250 mAs, with the detector combinations of 0.625×64, slice thickness of 1.0 mm, an interval of 1.0 mm, and a screw pitch of 0.984. The time for one revolution of the bulb tube is 0.5 s. The delayed scan of the arterial phase is 20-25 s, and that of the portal phase is 50-55 s. The image data acquired are put into CT postprocessing workstation after the scan, and a disk is burned to store the three-phase data (the plain, arterial, and portal venous phases)[8].
Recommendation: Surgeons should work together with radiologists and radiographers to optimize the scanning parameter based on the tumor location, vessels adjacent to or invaded by the liver tumors, and the specific circumstances where CT scan is performed. The high-quality triple-phase helical CT data are the basis for constructing a 3D visualization evaluation model (strong recommendation).
1.2. Study of homogenization processing and quality control system of 3D reconstruction for complex liver tumors[8]
The establishment of the quality control system for 3D visualization evaluation is vital for scientific understanding of the diseases. Three-dimensional reconstruction should follow the quality control and homogenization research criteria: (1) the patient should be instructed to hold his/her breath during scanning to avoid the difficulties in image segmentation and registration; (2) the quality of the original CT images should meet the minimum requirement of the 3D reconstruction software; (3) 3D reconstruction should be carried out by qualified professionals; (4) 3D models should be manually checked and modified by a senior surgeon and a radiologist. Only after standardization and strict quality control can the 3D visualization models be used to guide clinical practice.
A standardized process of 3D reconstruction for complex liver tumors is currently unavailable in China. Failure to maintain standard procedures during the scan will lead to erroneous results, which has a direct impact on the understanding of the patient's performance status, the selection of surgical options and surgical navigation. Meanwhile, inconsistent operating standards also affect the accuracy of evaluating the clinical efficacy in different centers, thus causing great inconvenience to clinical practice and scientific research. To better apply 3DVT in the field of liver diseases, standardized evaluation and homogeneous operation flow are urgently needed. We previously proposed the quality control system and 3DVQS based on the 3 criteria concerning preoperative 3D surgical simulation, intraoperative 3D surgical navigation, and postoperative 3D reconstruction; they are further modified into 16 criteria, as elaborated in Tab. 2[15, 16].
2.
Process Measures
| Criteria | Points | |
| 1 | Diagnosis of liver diseases by preoperative imaging (ultrasound, CT or MRI) | +1 |
| 2 | Patients fast for at least 4 hours prior to the CT scan, orally take 0.5 L-1.0 L of clear liquid 20 to 30 mins before the exam and take another 500 ml before the exam. | +1 |
| 3 | Train the patient to hold their breath in full inspiration before scanning and instruct them to do so during each scan phase to achieve maximum management of artifacts due to respiratory motion. | +1 |
| 4 | Select 64-slice or above spiral CT scanning with a slice thickness of 0.625 to 1.0 mm. | +1 |
| 5 | CT scanning ranges from the top of the diaphragm to the lower level of both kidneys, and perform dynamic abdominal scan after intravenous contrast medium administration; perform CT celiac arteriography. The arterial phase, portal venous phase and delayed phase scans start at a delay of 20-25 s, 50-55 s, and 2 minutes, respectively. | +1 |
| 6 | 3D reconstruction should be performed by attending physicians or a level above who are engaged in the diagnosis and treatment of liver diseases. | +1 |
| 7 | Evaluate the integrity of the course, shape and continuity of hepatic artery reconstructed by 3D visualization to determine whether a manual revision is required (manual revision is unnecessary when the tertiary branches of artery can be reconstructed). | +1 (no manual revision); -1 (manual revision required) |
| 8 | Evaluate the integrity of the course, morphology and continuity of hepatic vein reconstructed by 3D visualization to determine whether a manual revision is required (Manual revision is unnecessary if the tertiary branches of hepatic vein can be reconstructed). | +1 (no manual revision); -1(manual revision required) |
| 9 | Evaluate the integrity of the course, morphology and continuity of portal vein reconstructed by 3D visualization to determine whether a manual revision is required. The branches of the portal vein system with the diameter ≥5 mm should be reconstructed (it is unnecessary if the tertiary branches of portal vein can be reconstructed) | +1 (no manual revision); -1(manual revision required) |
| 10 | Evaluate its course, morphology, continuity and integrity of the 3D reconstructed biliary tract (manual revision is unnecessary if the tertiary branches of the biliary tree can be reconstructed). | +1 (biliary system reconstructed); -1 (no biliary system reconstructed) |
| 11 | Evaluate the morphology, size and distribution of lesions in the 3D reconstructed model and whether they are consistent with CT images. | +3 (basically consistent, no manual revision required); +2 (mostly consistent, manual revision required); -1 (inconsistent, manual revision required) |
| 12 | The overall 3D model should be validated by at least 2 abdominal imaging attendings and at least 2 attending hepatologists in comparison with the original CT images, and finally confirmed by a senior physician. | +1 |
| 13 | Perform simulation surgery based on the 3D model. The simulation of various schemes should be carried out, and the optimal surgical approach and surgical resection plane should be selected by two attending physicians, and finally confirmed by a senior physician. | +2 |
| 14 | A multi-disciplinary team (MDT) should be formed based on the individualized 3D model and the results of clinical examinations; liver surgeons undertake the main tasks, assisted by the departments of hepatology, oncology, endoscopy, interventional therapy and radiotherapy. | +2 |
| 15 | The consistency between preoperative 3D models and intraoperative conditions (lesions, vascular variance and range of hepatectomy) should be assessed. | +3 (completely consistent); +2 (basically consistent); -1(inconsistent) |
| 16 | The volume of the virtual resected liver should be compared with that of the actual resected liver (reference standard is intraoperative dewatering method). The volume error (< 5%) is completely consistent, the volume error (< 10%) is basically consistent, and the volume error (> 10%) is inconsistent. | +3 (completely consistent); +2 (basically consistent); -1 (inconsistent) |
| Total score (24, 100%) | ||
Recommendation: An adequate quality control system must be established for 3D reconstruction of complex liver tumors. The reconstruction process should be carried out by qualified surgeons and radiologists and validated by senior physicians (strong recommendation).
2. Construction of individualized 3D models for abdominal organs and lesions
The acquired DICOM data should be imported into the independently developed MI-3DVS for data segmentation. The original 2D images of the abdominal organs, lesions and vascular system can be reconstructed automatically utilizing volume rendering for interactive 3D segmentation, regional self-growth method, and surface rendering with the intrahepatic vascular branches reaching the level of 3-4[9]. Based on the automatically generated liver contour and vascular model, clinicians and radiologists should work together to determine, manually check and modify the lesion scope to ensure the accuracy of reconstruction.
Recommendation: For patients with complex liver tumors scheduled for hepatectomy, clinicians and radiologists should cooperate to establish a standardized and individualized 3D visualization model (strong recommendation).
3. Individualized classification and quantitative analysis of blood vessels based on 3D visualization
Anatomical variations of the hepatic artery[10], hepatic vein[11]and portal vein[12]are common. A 3D visualization analyses of these blood vessels identify (1) the 3D distribution of blood vessels; (2) the course and variation of blood vessels, and (3) their spatial relationship with the tumors. Priority should be given to the assessment of vascular variations, which is helpful to the selection of a hepatectomy plane. The distance between the tumor and adjacent important vessels can be accurately estimated by classification according to the degree of compression or invasion of various blood vessels and vessels involved in the liver [13]. Accurate individualized vascular classification and quantitative analysis have important significance for guiding clinical diagnosis, surgical planning, and implementation of precision surgery.
3.1. Individualized arterial classification with 3D visualization
The incidence of hepatic artery variation is approximately 45%. Michels et al[10]divided them into 10 types. In type Ⅰ, the common hepatic artery is from coeliac trunk artery; in type Ⅱ, the left hepatic artery is from left gastric artery; in type Ⅲ, right hepatic artery is from the superior mesenteric artery; in type Ⅳ, the left hepatic artery is from the left gastric artery, and the right hepatic artery is from the superior mesenteric artery; in type Ⅴ, the accessory left hepatic artery is from the left gastric artery; in type Ⅵ, the accessory right hepatic artery is from the superior mesenteric artery; in type Ⅶ, the accessory left hepatic artery is from the left gastric artery, and the accessory right hepatic artery is from the superior mesenteric artery; in type Ⅷ, the accessory left hepatic artery is from the left gastric artery, the right hepatic artery is from the superior mesenteric artery (or the left hepatic artery is from the left gastric artery), and the accessory right hepatic artery is from the superior mesenteric artery; in type Ⅸ, the common hepatic artery is from the superior mesenteric artery; in type Ⅹ, the common hepatic artery is from the left gastric artery;
Recommendation: For patients with complex liver tumors scheduled for hepatectomy, these variations identified through 3D visualization analysis of the hepatic artery is crucial to clinical diagnosis, interventional therapy, and guiding precision surgery (weak recommendation).
3.2. Individualized portal vein classification with 3DVT[12]
The variation of the portal vein is also common, which can be classified into 5 types according to the analysis by 3DVT. These variations include: (1) the normal type, where the main portal vein is divided into left and right branches in the porta hepatis; (2) Type Ⅰ variation, where the main portal vein is divided into left, right anterior and right posterior branches, resembling a trident; (3) Type Ⅱ variation, where the main portal vein sends out the right posterior branch and then goes upward to divide into left and right anterior branches; (4) Type Ⅲ variation, where the right portal vein is divided horizontally into anterior and posterior branches; and (5) Type Ⅳ variation, where the horizontal part of the left portal vein is missing, in rare cases, the left portal vein is from the right anterior branch.
Recommendation: For patients undergoing hepatectomy for complex liver tumors, portal vein classification based on 3DVT is recommended to understand the vascular course, variations and its spatial relationship with tumors (strong recommendation).
3.3. Individualized three-dimensional visualization hepatic vein classification[11]
In hepatic surgery, the vascular control of hepatic vein is crucial to the entire procedure. It is important to summarize the character of hepatic vein and identify the variations to reserve normal hepatic tissues to the greatest extent. Based on the research on the classification of hepatic vein using 3D visualization technology, the variation of inferior right hepatic vein and hepatic veins for segments Ⅳ and Ⅷ are more valuable for hepatic surgery.
Recommendation: In the formulation of surgical plans, it is necessary to classify hepatic veins based on 3D visualization technology, especially the variation of right inferior hepatic vein, and the veins for segments Ⅳ and Ⅷ.
4. 3DVT-based classification and grading of hepatic vessels for complex hepatic tumors[14-15]
Hepatectomy mainly involves the portal vein, hepatic vein, inferior vena cava and hepatic artery system. Therefore, this guideline proposes a 3DVT-based classification system for hepatic vessels for complex liver tumors to guide the operation (Tab. 3).
3.
Three-dimensional visualization classification of complicated hepatocellular carcinoma
| Type | Grading |
| Type Ⅰ: Tumor involving portal vein | Grade 0: Vessels are not compressed by tumors. Grade 1: Vessels are compressed but not invaded by tumors. Grade 2: Vessels are invaded but not interrupted by tumors. Grade 3: Tumor invasion with continuity interruption of blood vessels |
| Type Ⅰ a: Tumor involving the right branch of portal vein | |
| Type Ⅰ b: Tumor involving the left branch of portal vein | |
| Type Ⅱ: Tumor involving hepatic vein | |
| Type Ⅱ a: Tumor involving right hepatic vein | |
| Type Ⅱb: Tumor involving middle hepatic vein | |
| Type Ⅱ c: Tumor involving left hepatic vein | |
| Type Ⅲ: Tumor involving hepatic artery | |
| Type Ⅲ a: Tumor involving right hepatic artery | |
| Type Ⅲ b: Tumor involving left hepatic artery | |
| Type Ⅳ: Tumor involving inferior vena cava | |
| Type Ⅴ: Tumor involving abdominal aorta |
Recommendation: Hepatic vessels of complex liver tumors should be classified by 3DVT to provide anatomical guidance for hepatectomy (weak recommendation).
5. Evaluation system for surgery simulation after 3D visualization model reconstruction
5.1. 3DVT-based individualized liver segmentation
The Couinaud classification in clinical use benefits from the results of in vitro liver cast study, and its coincidence rate is only 20%-30% among the general population. If digital medical technology can be used in liver segmentation, the individualized segmental partition can be performed based on blood topological relation for each patient and displayed in 3D visualization images. Each functional segment is supplied by the portal vein and drained by the hepatic vein[16]. There could be segments 7, 9, or 10 when intrahepatic vascular variation occurs. Regular and irregular liver segments can be precisely divided using 3DVT. Therefore, individualization can be achieved to increase the accuracy practically.
Recommendation: For patients undergoing hepatectomy for complex liver tumors, individualized liver segmentation should be performed before surgery (strong recommendation)
5.2. Individualized liver volume calculation based on 3DVT
Currently, there are 3 approaches to calculate the liver volume: (1) the computational formula of the liver volume[17]; (2) manual volumetry based on sectional data such as CT images[18]; (3) 3D reconstruction of CT images, through which the liver volume was calculated by 3D reconstruction based on the principle of voxel[19].
When the software is developed, the sum of dots (voxel) of the liver volume is calculated. Drainage and standard block methods are used to measure and proofread the volume. Total volume divided by the total dots makes the volume, and then the standard for the volume represented by each dot is obtained. Reports have shown that 3DVT-based calculation achieves accurate estimation of the liver volume[20-21].
Recommendation: For patients undergoing hepatectomy for complex liver tumors, individualized liver volumetry should be performed before surgery (strong recommendation).
5.3. Preoperative simulation surgery
The 3D model is imported into the simulation surgical system when individualized liver segmentation and volumetry are completed. By using the virtual environment, simulation surgical instruments and force feedback devices created by this system, simulation surgery is performed on the reconstructed 3D model to better understand the tumor location and the spatial relationship between the tumor and the intrahepatic vascular systems.
Recommendation: Preoperative simulation surgery could be performed when the hospital has adequate equipment. In recent years, for patients who may potentially have insufficient remnant liver volume or who can not receive a major hepatectomy, partial associating liver partition and portal vein ligation for staged hepatectomy or portal vein embolization can be an option (weak recommendation).
6. Application of 3D printing in complex hepatectomy[22]
The 3D printed model based on 3DVT can faithfully represent the characteristics of the intracorporeal organs (Fig. 1). With this technique, the location, size and shape of the tumor are faithfully represented, and the spatial relationship between the tumor and the vascular systems can be observed from different angles; this technique also provides real-time indirect navigation during the surgery and allows rapid recognition and localization of the vital positions.
1.
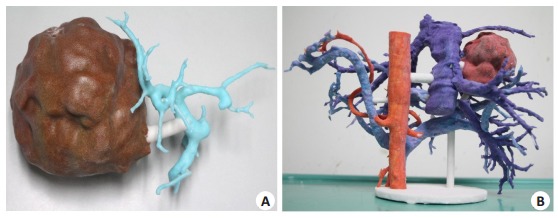
Complex hepatectomy guided by 3D printing technology. A: A 3D printing model of rare vascular aberration (segment Ⅳ portal vein stems from the right anterior branch); B: Type Ⅲ variation of the portal vein and the inferior right hepatic vein draining into the inferior vena cava (posterior view).
Recommendation: For patients undergoing hepatectomy for complex liver tumors, preoperative 3D printing can be used to guide surgery if the essential equipment is available (weak recommendation).
7. Virtual reality (VR) technology[4, 14-15]
VR technology provides surgeons with a variety of sensory simulations for preoperative planning and enriches the understanding of pathological changes. The STL format file of the 3D visualization model of complex liver tumors is imported into Unreal Engine 4 (UE4), and modeled in the VR environment. The individualized 3D model can be displayed in the VR environment. The operators can be integrated into the VR environment by wearing VR glasses and manipulating handles. Through the immersive human-computer interaction mode, the patients' individualized 3D model can be observed and manipulated stereoscopically in a manner closer to the real surgical experience.
Recommendation: It is suggested that a preoperative VR study be performed for patients scheduled for hepatectomy for complex liver tumors when adequate equipment is available (weak recommendation).
8. Indocyanine green (ICG)-fluorescence imaging
ICG fluorescence imaging can help to define the demarcation of tumors at the molecular and cellular levels, determine the resection line of lobe or segmental hepatectomy and detect small lesions or metastases. During the operation, the liver should be scanned using fluorescence detection equipment. The characteristics of the fluorescence signal of liver tumors and intraoperative rapid frozen pathology reports can be combined to preliminarily determine the differentiation degree of the space-occupying lesions in the liver (such as primary liver cancer). The residual tumor lesions and bile leakage can also be detected on the liver section after hepatectomy [23] to guide anatomical, functional, and radical hepatectomy for liver cancer[24].
Recommendation: ICG fluorescence detection should be performed preoperatively and intraoperatively in patients undergoing liver resection for complex hepatic tumors. Positive staining, negative staining or ultrasound-guided portal vein puncture and segmental staining can be selected during surgery according to the requirements of the surgical planning (strong recommendation).
9. Multi-modal images for real-time navigation[25]
Preoperatively, the portal venous-phase CT images and Gd-EOB-DTPA-enhanced MRI images are automatically registered and fused by the Mitworkbetch software. The fusion of CT-MRI images can provide more pathological information and improve the accuracy of diagnosis. At the same time, the CT-MRI-based 3D model and the virtual surgical images reconstructed are brought into the operating room to guide the critical procedures of the operation. The use of ICG fluorescence imaging makes possible the fusion of multi-modal images, which helps to define tumor demarcation, detect small lesions, and improve surgical precision[9]. Besides, during 3D surgical planning before the actual operation, the issues such as spatial and temporal separation and the lack of accuracy can be addressed by enhanced reality technology combined with multi-modal images to achieve a satisfactory real-time navigation effect.
Recommendation: Real-time navigation with multi-modal image fusion can be used in hepatectomy for hepatic tumors when adequate equipment is available (weak recommendation).
10. Surgical planning for 3DVT-guided precision hepatectomy
10.1. Surgical planning for complex hepatectomy in cases of hepatic vein variations
While it is challenging to detect hepatic vein variation with CT or MRI, 3DVT can display these variations in individual patients. The anatomy and variations of the segment Ⅳ and the inferior right hepatic vein are crucial in right hemihepatectomy. The segment Ⅳ vein drains mainly into the left and middle hepatic veins. Fig. 2 shows that in a specific case where the segment Ⅳ hepatic vein drains into the left hepatic vein, removal of the middle hepatic vein is safe in right hemihepatectomy[26].
2.
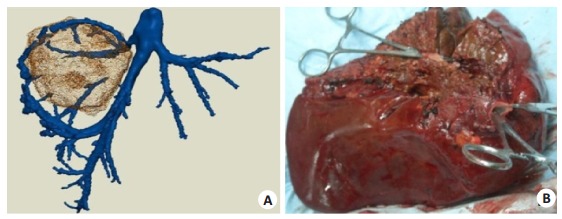
Three-dimensional visualization technology displays the middle hepatic vein to be removed in right hemihepatectomy. (A) the removed tissues in the actual operation (B). The tumor is located in the right liver, and the segment Ⅳ hepatic vein drains into the left hepatic vein, so segment Ⅳ is not affected after removal of the middle hepatic vein.
Recommendation: For patients undergoing right hemihepatectomy for liver tumors, 3DVT helps to identify the segment Ⅳ and the inferior right hepatic veins (strong recommendation).
10.2. Surgical planning for complex hepatectomy in cases of portal vein variations
With 3DVT, the course and variation of the portal vein can be clearly displayed preoperatively. Adequate surgical planning based on the variation types of the portal vein can significantly reduce the risk of vascular injuries and preserve more hepatic tissues, as exemplified by narrow-margin right hemihepatectomy in cases of type Ⅲ variation of the portal vein (Fig. 3) or in cases of specific variations of the portal vein. The segment Ⅳ of the portal vein stems from the right anterior branch. In a right hemihepatectomy, the trunk of the right portal vein needs to be severed, which causes insufficiency in blood supply of the segment Ⅳ. In such cases, narrow-margin right hemihepatectomy is a better option. Segment 4 of the portal vein from the right anterior branch is separated and protected, and the narrow-margin right hemihepatectomy is then performed (Fig. 4).
3.
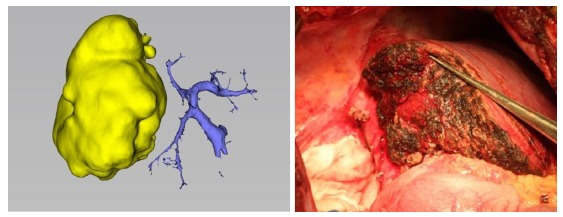
Narrow margin right hemihepatectomy is chosen for patients with type Ⅲ variation of portal vein.
4.

Preoperative surgical planning and actual operation of complex hepatectomy in a patient with anatomical variation of the portal vein. A: Blood supply of segment Ⅳ from the right portal vein is shown in three-dimensional visualization image (black arrow); B: Temporary blocking the trunk of the right portal vein shows that the ischemia area is around the falciform ligament (black arrow indicates the boundary of the right three sections, and the yellow arrow indicates the actual boundary of the left and right liver). The narrow margin right hemihepatectomy is performed and some part of segment Ⅴ and Ⅷ is preserved.
Recommendation: For patients undergoing hepatectomy for complex liver tumors, preoperative analysis using 3DVT is essential, which helps to identify and classify potential variations of the portal vein, determine liver segmentation and calculate the volume, thus facilitating the decision-making on the surgical plan (strong recommendation).
10.3. Narrow-margin hepatectomy is a choice in patients with insufficient liver volume following hepatectomy[27]
Narrow-margin right hemihepatectomy is an innovative procedure for the treatment of right hepatic tumor(s) in cases where (1) post hepatectomy liver failure may occur if standard right hemihepatectomy is performed; (2) parts of the sections Ⅴ and Ⅷ are preserved after partial right hepatectomy; (3) a normal type of the right portal veins is found, and the residual hepatic cross-section meets the requirements of the guidelines; and (4) the stub of the portal vein in sections Ⅵ and Ⅶ or the right posterior portal vein can be seen after hepatectomy. In such a complex hepatectomy, 3DVT can display the vessels that need to be severed or preserved, thus helping to preserve sufficient residual liver and maximally ensure the safety of the patient during tumor removal (Fig. 5).
5.
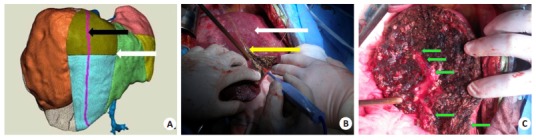
Narrow-margin right hemihepatectomy guided by 3DVT. A: Preoperative surgical planning based on 3DVT (the white arrow indicates the boundary of left and right liver, and the black arrow points the boundary of narrow-margin right hemihepatectomy); B: In the actual procedure, the yellow arrow shows the boundary of narrow-margin right hemihepatectomy and the white arrow shows the boundary of the left and right liver; C: The course of segments Ⅴ and Ⅷ portal vein and the nub of segments Ⅵ and Ⅶ are observed in the dissected plane after removal of the tumor.
Recommendation: 3DVT-guided narrow-margin right hemihepatectomy is a choice for patients with insufficient liver volume after right hemihepatectomy. Precision removal of the lesion can be performed to ensure the patient 's residual liver function and surgical safety (strong recommendation).
10.4. 3DVT-based classification and surgical planning for centrally located liver tumors
A centrally located liver tumor refers to one located mainly in segments Ⅰ, Ⅳ, Ⅴ, and Ⅷ. Due to its particular location and involvement of the important intrahepatic vascular system, the resection of such tumors can be extremely difficult and risky. The application of 3DVT better ensures the surgical safety by allowing individualized preoperative surgical planning[13-14](Tab. 4).
4.
3D visualization classification and surgical planning for centrally located liver tumor
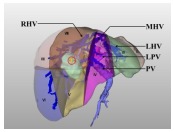
| Classification | Description | Surgical methods |
|
Type Ⅰ: The tumor is in the liver parenchyma of segments Ⅴ, Ⅷ, or both, characterized by their close proximity to or even direct invasion of the adjacent portal vein. They do not adhere to or compress the right hepatic vein trunk. | Resection of segments Ⅴ, Ⅷ ± partial resection of segment Ⅳ |
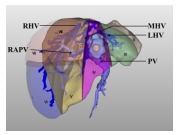
|
Type Ⅱ: The tumor is in the liver parenchyma of segments Ⅳ a, Ⅳ b, or both, characterized by its proximity to or even direct invasion of the left hepatic vein trunk. In addition, it does not adhere to or compress the left hepatic vein trunk. | Resection of segments Ⅳ a and Ⅳ b or left hepatectomy |
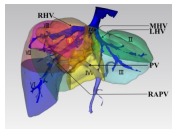
|
Type Ⅲ: The tumor occupies most liver parenchyma of segments Ⅳ, Ⅴ, and Ⅷ, characterized by a wide and deep invasion of the parenchyma, or their proximity to the middle hepatic vein. | Central bisectionectomy (resection of segments Ⅳ, Ⅴ, and Ⅷ ± Ⅰ) |
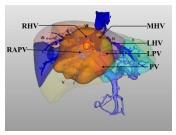
|
Type Ⅳ: This type of liver tumor occupies most liver parenchyma of segments Ⅳ, Ⅴ, and Ⅷ, characterized by their close proximity to, or direct invasion of, the left/ right portal vein trunk or the left/right hepatic vein. | Resection of segment Ⅳ, Ⅴ, Ⅵ, Ⅶ, Ⅷ. Resection of segment Ⅱ, Ⅲ, Ⅳ, Ⅴ, Ⅷ. Reduced right trisectionectomy or reduced left trisectionectomy. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). |
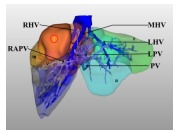
|
Type Ⅴ: This type of liver tumor occupies the superficial liver parenchyma of segments Ⅳ, Ⅴ and Ⅷ. The lesions are not close to either the portal branch or the hepatic vein. | Hepatectomy with a negative margin |
Recommendation: For centrally located liver tumors, 3DVT-based classification and surgical planning can be essential to preserve more liver parenchyma and achieve the goal of precision hepatectomy (strong recommendation).
11. 3DVT guidance for other therapeutic methods of hepatocellular carcinoma
Transarterial chemoembolization (TACE) is a useful treatment modality for hepatocellular carcinoma[28]. With 3DVT, the main blood artery supply and its small branches supplying the tumor can be displayed, and the hepatic artery variations can be assessed in an accurate 3D "vessel-tumor" model. 3DVT also provides a preoperative 3D approach to radiofrequency ablation and argon-helium knife technology for percutaneous liver tumors and allows accurate measurement of the volume of intraoperative electrode probe failure range.
Recommendation: For patients undergoing TACE, tumor ablation, and argon-helium knife, 3DVT can be used to evaluate the condition of the hepatic artery (weak recommendation).
12. Application of 3DVT in postoperative follow-up of patients with liver cancer
No matter what treatment procedures are chosen for patients with hepatocellular carcinoma, regular follow-up of the patients is essential. Ultrasonography[29], hepatic tri-phase contrast-enhanced helical CT, and Gd-EOB-DTPA-enhanced MR imaging[30] are used to track the outcomes of the patients dynamically. In cases experiencing neoplasm recurrence, reevaluation with 3DVT can provide 3D anatomical evidence for guiding the treatment.
Recommendation: Ultrasonography, hepatic tri-phase contrast-enhanced helical CT and Gd-EOB-DTPA-enhanced MR imaging should be performed regularly in postoperative patients. Comparing the 3D reconstruction results with the preoperative 3D images may help in the dynamic monitoring of tumor recurrence.
Conclusion
In the clinical setting, hepatectomy for complex liver tumors poses a challenge. 3DVT shows obvious advantages over conventional 2D imaging modalities in the treatment of complex liver tumors. Real-time guidance by ultrasonography is also vital to enhance the accuracy of surgical operation. For patients undergoing hepatectomy, adequate protection of the liver function during the perioperative period is of equal importance to the surgical techniques[31-32].
In conclusion, for patients undergoing hepatectomy for complex liver tumors and those who are tentatively diagnosed by ultrasonography and CT, 3DVT-based analysis of the target lesion is recommended due to high risk and difficulty in the surgical operation. Hepatic 3D printing evaluation, VR evaluation, and intraoperative ICG fluorescence detection can be used when equipment is available. These technologies are expected to play significant roles in enhancing the precisions of preoperative diagnosis and surgical operations and in improving the outcomes of the patients with complex liver tumors.
Validated by: LAU Wanyee
Directors of the Committee: FANG Chihua, JIANG Hongchi, LIANG Lijian
Participants: BAO Susu (School of Computer Science of South China Normal University), BIE Ping (First Hospital affiliated to AMU), CHEN Yajin (Sun Yat-Sen Memorial Hospital, Sun Yat-set University), CHEN Guihua (Third Affiliated Hospital, Sun Yat-set University), CHEN Rufu (Sun Yat-sen Memorial Hospital, Sun Yat-set University), CAI Xiujun (Sir Run-Run Shaw Hospital, Zhejiang University), CAI Xiangjun (General Hospital of Norrn War Zone of Chinese People's Liberation Army), CHENG Shuqun (Eastern Hepatobiliary Surgery Hospital, Naval Medical University), DONG Ming (First Hospital of China Medical University), DAI Chaoliu (Sheng Jing Hospital of China Medical University), FANG Chihua (Zhujiang Hospital, Sourn Medical University), FAN Jia (Zhongshan Hospital, Fudan University), FAN Haining (Qinghai University Affiliated Hospital), FAN Yingfang (Zhujiang Hospital, Sourn Medical University), GUO Wei (Beijing Friendship Hospital, Capital Medical University), GENG Xiaoping (Second Hospital of Anhui Medical University), HE Yu (First Hospital Affiliated to AMU), LI Yumin (Lanzhou University), LI Zongfang (Second Affiliated Hospital of Xi'an Jiaotong University), JIA Weidong (First Affiliated Hospital of University of Science and Technology of China), JIANG Xiaoqing (Eastern Hepatobiliary Surgery Hospital, Naval Medical University), JIANG Kewei (Peking University People 's Hospital), JIANG Hongchi (First Affiliated Hospital of Harbin Medical College), JIAN Zhixiang (Guangdong Provincial People's Hospital), JIANG Yi (Hospital of Joint Logistics Team), KONG Dexing (School of Mamatical Scinence, Zhejiang University), LAU Wanyee (Faculty of Medicine, Chinese University of Hong Kong), LIU Jun (First Affiliated Hospital of Xi'an Jiaotong University), LIU Bin (First Affiliated Hospital of Kunming Medical University), LIU Lianxing (Department of Hepatobiliary Surgery, First Affiliated Hospital of USTC, ), LIU Jingfeng (Mengchao Hepatobiliary Hospital of Fujian Medical University), LIU Jingang (4th Affiliated Hospital of China Medical University), LIU Chao (Sun Yat-san Memorial Hospital of Sun Yat-sun University), LIU Yingbin (Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine), LIANG Lijian (First Affiliated Hospital, Sun Yat-Sen University, ), LIANG Xiao (Sir Run-Run Shaw Hospital, Zhejiang University), LU Qiping (Department of General Surgery, General Hospital of Central ater Command), OU Jinrui (Guangdong Provincial People's Hospital), PENG Baogang (First Affiliated Hospital, Sun Yat-Sen University), QUAN Zhiwei, QI Xiaolong (First Hospital of Lanzhou University), QIN Lunxiu (Huashan Hospital, Fudan University), QIN Renyi (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology), QUAN Zhiwei (Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine), SUN Bei (First Affiliated Hospital of Harbin Medical University), SUN Chengyi (Affiliated Hospital of Guizhou Medical University), SUN Shijie (Yantai Yuhuangding Hospital), SHEN Feng (Eastern Hepatobiliary Surgery Hospital, Naval Medical University), TIAN Jie (Institute of Automation, Chinese Academy of Sciences), TIAN Liguo (Editorial Department of Chinese Journal of Practical Surgery), TANG Zhaohui (Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine), WANG Jian (Renji Hospital, Shanghai Jiaotong University School of Medicine), WANG Jianming (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology), WANG Xiaoyin (Zhongshan Hospital, Fudan University), WANG Huaizhi (First Hospital Affiliated to AMU), WANG Wei (Huadong Hospital, Fudan University), WANG Hongguang (General Hospital of PLA), WEN Hao (First Affiliated Hospital of Xinjiang Medical University), XIANG Nan (Zhujiang Hospital, Sourn Medical University), YANG Jian(Zhujiang Hospital, Sourn Medical University), YANG Yinmo (Peking University First Hospital), YANG Yang(Third Affiliated Hospital, Sun Yat-Set University), YIN Xiaoyu (First Affiliated Hospital of Sun Yat-Sen University), YIN Xinmin (Hunan Provincial People's Hospital), YUAN Yufeng (Zhongnan Hospital Affiliated to Wuhan University), ZHANG Shaoxiang (Army Medical University), ZENG Ning (Zhujiang Hospital, Sourn Medical University), ZHANG Bixiang (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology), ZHANG Yongjie (Eastern Hepatobiliary Surgery Hospital, Naval Medical University), ZHANG Xuewen (China-Japan Union Hospital, Jilin University), ZHANG Taiping (Peking Union Medical College Hospital), ZHOU Weiping (Eastern Hepatobiliary Surgery Hospital, Naval Medical University), ZHOU Jie (Nanfang Hospital, Southern Medical University), ZHONG Lin (First People's Hospital Affiliated to Shanghai Jiao Tong University), ZHI Xuting (Qilu Hospital of Shandong University)
Byliners: FANG Chihua, LU Qiping, LAU Wanyee
Funding Statement
This work was supported by grants from the National Key R & D Program (2016YFC0106500); Major Instrument Project of National Natural Science Foundation of China (81627805); National High Technology Research and Development Program of China (863 Program; 2012AA021105, 2006AA02Z346); the NSFC-GD Union Foundation (U1401254); Natural Science Foundation of Guangdong Province (6200171); Integration Project of Production, Teaching and Research of Department of Education of Guangdong Province (2009B080701077); Science and Technology Program of Guangdong Province (2012A080203013); Science and Technology Plan of Guangzhou (201604020144)
"十三五"国家重点研发计划数字诊疗装备研发重点专项(2016YFC0106500800);国家自然科学基金重大仪器项目(81627805);"十二五"国家高技术研究发展(863计划)(2012AA021105);"十一五"国家高技术研究发展(863计划)(2006AA02Z346);国家自然科学基金-广东联合基金重点支持项目(U1401254);广东省自然科学基金团队项目(6200171);广东省部产学研结合项目(2009B080701077);广东省重大科技专项计划项目(2012A080203013);广州市科技计划项目(201604020144)
Contributor Information
Chinese Society of Digital Medicine (中华医学会数字医学分会 ), Email: fangch_dr@126.com.
Clinical Precision Medicine Committee of Chinese Medical Doctor Association (中国医师协会临床精准医学专业委员会 ), Email: fangch_dr@126.com.
References
- 1.Chen XP, Zhang ZW. Continuously improve the therapeutic level of complicated hepatectomy. J Hepat Surg. 2005;6:401–3. [Chen XP, Zhang ZW. Continuously improve the therapeutic level of complicated hepatectomy[J]. J Hepat Surg, 2005, 6: 401-3.] [Google Scholar]
- 2.Liang LJ. Preoperative evaluation and decision of complicated hepatectomy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsywkzz201008008. Chin J Pract Surg. 2010;8:645–7. [Liang LJ. Preoperative evaluation and decision of complicated hepatectomy[J]. Chin J Pract Surg, 2010, 8: 645-7.] [Google Scholar]
- 3.Yan J, Bie P. Prevention and treatment for common complications of complex liver resection. http://cn.bing.com/academic/profile?id=08c4c1a374871ee60207284676cb05b6&encoded=0&v=paper_preview&mkt=zh-cn. Chin J Pract Surg. 2010;8:647–49. [Yan J, Bie P. Prevention and treatment for common complications of complex liver resection[J]. Chin J Pract Surg, 2010, 8: 647-49.] [Google Scholar]
- 4.Fang CH, Zhang P, Qi XL. Digital and intelligent liver surgery in the new era: Prospects and dilemmas. EBioMedicine. 2019;41:693–701. doi: 10.1016/j.ebiom.2019.02.017. [Fang CH, Zhang P, Qi XL. Digital and intelligent liver surgery in the new era: Prospects and dilemmas[J]. EBioMedicine, 2019, 41: 693-701.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations[J]. BMJ, 2008, 336(7650): 924-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology[J]. J Clin Epidemiol, 2011, 64(4): 380-2.] [DOI] [PubMed] [Google Scholar]
- 7.Meerpohl J J, Langer G, Perleth M, et al. GRADE guidelines: 3. Rating the quality of evidence (confidence in the estimates of effect) Z Evid Fortbild Qual Gesundhwes. 2012;106(6):449–56. doi: 10.1016/j.zefq.2012.06.013. [Meerpohl J J, Langer G, Perleth M, et al. GRADE guidelines: 3. Rating the quality of evidence (confidence in the estimates of effect)[J]. Z Evid Fortbild Qual Gesundhwes, 2012, 106(6): 449-56.] [DOI] [PubMed] [Google Scholar]
- 8.Fang CH, Lu CM, Huang YP, et al. Study on the application of value of digital medical technology in the operation on primary liver cancer. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhwk200907013. Chin J Surg. 2009;47(7):523–26. [Fang CH, Lu CM, Huang YP, et al. Study on the application of value of digital medical technology in the operation on primary liver cancer[J]. Chin J Surg, 2009, 47(7): 523-26.] [PubMed] [Google Scholar]
- 9.Fang CH, Zhang P, LAU WY, et al. Construction and application of the core technology system of digital intelligent diagnostic and treatment for hepato-biliary-pancreatic diseases. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhwk201904003. Chin J Surg. 2019;57(4):253–7. doi: 10.3760/cma.j.issn.0529-5815.2019.04.003. [Fang CH, Zhang P, LAU WY, et al. Construction and application of the core technology system of digital intelligent diagnostic and treatment for hepato-biliary-pancreatic diseases[J]. Chin J Surg, 2019, 57(4): 253-7.] [DOI] [PubMed] [Google Scholar]
- 10.Fang CH. Digital Liver Surgery. Beijing: People's Military Medical Press, Beijing; 2014. pp. 139–62. [Fang CH. Digital Liver Surgery[M]. Beijing: People's Military Medical Press, Beijing, 2014, 139-62.] [Google Scholar]
- 11.Fang CH, You JH, Lau WY, et al. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36(1):120–4. doi: 10.1007/s00268-011-1297-y. [Fang CH, You JH, Lau WY, et al. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects[J]. World J Surg, 2012, 36(1): 120-4.] [DOI] [PubMed] [Google Scholar]
- 12.Zhang YX. Three-dimensional reconstruction of individual hepatic and portal veins system in liver surgery[D]. J South Med Univ, 2013.
- 13.Fang CH, Tao HS, Yang J, et al. Impact of three -dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. http://cn.bing.com/academic/profile?id=dfb3d4aec3d6840b99e157818288499e&encoded=0&v=paper_preview&mkt=zh-cn. J Am CollSurg. 2015;220(1):28–37. doi: 10.1016/j.jamcollsurg.2014.09.023. [Fang CH, Tao HS, Yang J, et al. Impact of three -dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma[J]. J Am CollSurg, 2015, 220 (1): 28-37.] [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, He SH, Zeng SL, et al. Three-dimensional visualization evaluation and VR study of centrally located hepatocellular carcinoma with blood vessel as axis. http://d.old.wanfangdata.com.cn/Periodical/zhwk201905008. Chin J Surg. 2019;57(5):358–65. doi: 10.3760/cma.j.issn.0529-5815.2019.05.008. [Zhu W, He SH, Zeng SL, et al. Three-dimensional visualization evaluation and VR study of centrally located hepatocellular carcinoma with blood vessel as axis[J]. Chin J Surg, 2019, 57(5): 358-65.] [DOI] [PubMed] [Google Scholar]
- 15.Zeng SL, Zhu W, Fang CH, et al. Three-dimensional visualization evaluation and VR study of giant hepatocellular carcinoma with blood vessel as axis. Chin J Gen Surg. 2019;24(4):324–7. [Zeng SL, Zhu W, Fang CH, et al. Three-dimensional visualization evaluation and VR study of giant hepatocellular carcinoma with blood vessel as axis[J]. Chin J Gen Surg, 2019, 24(4): 324-7.] [Google Scholar]
- 16.Fan YF, Cai W, Fang CH. Segmental anatomy of liver and its research progress. Chin J Pract Surg. 2014;11:1105–8. [Fan YF, Cai W, Fang CH. Segmental anatomy of liver and its research progress[J]. Chin J Pract Surg, 2014, 11: 1105-8.] [Google Scholar]
- 17.Um EH, Hwang S, Song GW, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg. 2015;19(4):133–8. doi: 10.14701/kjhbps.2015.19.4.133. [Um EH, Hwang S, Song GW, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables[J]. Korean J Hepatobiliary Pancreat Surg, 2015, 19(4): 133-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitativeradiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. Am J Roentgenol. 2011;197(4):706–12. doi: 10.2214/AJR.10.5958. [Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitativeradiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry[J]. Am J Roentgenol, 2011, 197(4):706-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP) Surg Endosc. 2014;28(12):3408–12. doi: 10.1007/s00464-014-3611-x. [Begin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP)[J]. Surg Endosc, 2014, 28(12): 3408-12.] [DOI] [PubMed] [Google Scholar]
- 20.Chen XP. Development process and outlook of liver surgery. Chin J Dig Surg. 2015;14:cover9–10. [Chen XP. Development process and outlook of liver surgery[J]. Chin J Dig Surg, 2015, 14: cover9-10.] [Google Scholar]
- 21.Yang J, Tao HS, Cai W, et al. Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. J Surg Oncol. 2018;118(7):1081–7. doi: 10.1002/jso.25258. [Yang J, Tao HS, Cai W, et al. Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging[J]. J Surg Oncol, 2018, 118(7): 1081-7.] [DOI] [PubMed] [Google Scholar]
- 22.Xiang N, Fang CH, Fan YF, et al. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. http://cn.bing.com/academic/profile?id=3ce41b2a3b95275340a41dbdb674ba12&encoded=0&v=paper_preview&mkt=zh-cn. Int J Clin Exp Med. 2015;8(10):18873–8. [Xiang N, Fang CH, Fan YF, et al. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience[J]. Int J Clin Exp Med, 2015, 8(10): 18873-8.] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinese Society of Digital Medicine, Chinese Research Hospital Association of Digital Intelligent Surgery Committee Expert consensus on the application of computer-assisted combined indocyanine green fluorescence imaging for the diagnosis and surgical navigation of liver tumor. Chin J Pract Surg. 2017;37(5):531–8. [Chinese Society of Digital Medicine, Chinese Research Hospital Association of Digital Intelligent Surgery Committee. Expert consensus on the application of computer-assisted combined indocyanine green fluorescence imaging for the diagnosis and surgical navigation of liver tumor[J]. Chin J Pract Surg, 2017, 37(5): 531-8.] [Google Scholar]
- 24.Fang CH, Liang HB, CHI CW, et al. Application of indocyanine green-fluorescent imaging technique in planning resection line and real-time surgical navigation in small hepatocellular carcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhwk201606012. Chin J Surg. 2016;54(6):444–50. doi: 10.3760/cma.j.issn.0529-5815.2016.06.011. [Fang CH, Liang HB, CHI CW, et al. Application of indocyanine green-fluorescent imaging technique in planning resection line and real-time surgical navigation in small hepatocellular carcinoma[J]. Chin J Surg, 2016, 54(6): 444-50.] [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Zhu W, Fang CH, et al. Application of multimode imaging technology in real time navigation in anatomical liver resections. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsywkzz201905019. Chin J Pract Surg. 2019;39(05):480–6. [Zhang P, Zhu W, Fang CH, et al. Application of multimode imaging technology in real time navigation in anatomical liver resections[J]. Chin J Pract Surg, 2019, 39(05): 480-6] [Google Scholar]
- 26.Lau WY. Applied anatomy in liver resection and liver transplantation. Beijing: People's Medical Publishing House; 2010. pp. 51–6. [Lau WY. Applied anatomy in liver resection and liver transplantation. Beijing: People's Medical Publishing House, 2010: 51-6.] [Google Scholar]
- 27.Hu M, Wang K, Shang W, et al. The safety and feasibility of three -dimensional visualization technology assisted right posterior lobe allied with part of Ⅴ and Ⅷ sectionectomy for right hepatic malignancy therapy. J Laparoendosc Adv Surg Tech A. 2018;28:586–94. doi: 10.1089/lap.2017.0479. [Hu M, Wang K, Shang W, et al. The safety and feasibility of three -dimensional visualization technology assisted right posterior lobe allied with part of Ⅴ and Ⅷ sectionectomy for right hepatic malignancy therapy[J]. J Laparoendosc Adv Surg Tech A, 2018, 28: 586-94.] [DOI] [PubMed] [Google Scholar]
- 28.Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–62. doi: 10.1159/000367743. [Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma[J]. Liver Cancer, 2015, 4(4):253-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–2. doi: 10.1002/hep.24199. [Bruix J, Sherman M. Management of hepatocellular carcinoma: An update[J]. Hepatology, 2011, 53(3): 1020-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Matsui O, Kitao A, et al. Usefulness of Gd -EOB-DTPA-enhanced MR imaging in the evaluation of simple steatosis and nonalcoholic steatohepatitis. J Magn Reson Imaging. 2013;37(5):1137–43. doi: 10.1002/jmri.23921. [Wu Z, Matsui O, Kitao A, et al. Usefulness of Gd -EOB-DTPA-enhanced MR imaging in the evaluation of simple steatosis and nonalcoholic steatohepatitis[J]. J Magn Reson Imaging, 2013, 37(5): 1137-43.] [DOI] [PubMed] [Google Scholar]
- 31.Liao WJ, Mao YL. Standardized management of liver tumor during perioperative period. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1357124/ Chin J Pract Surg. 2014;8:783–5. [Liao WJ, Mao YL. Standardized management of liver tumor during perioperative period[J]. Chin J Pract Surg, 2014, 8: 783-5.] [Google Scholar]
- 32.Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. http://cn.bing.com/academic/profile?id=093d4283e1459c440f1232a68c5aad36&encoded=0&v=paper_preview&mkt=zh-cn. Gut. 2018:gutjnl-2018–315983. doi: 10.1136/gutjnl-2018-315983. [Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial[J]. Gut, 2018: gutjnl-2018-315983.] [DOI] [PubMed] [Google Scholar]


