Treponema denticola is one of numerous host-associated spirochetes, a group including commensals, pathobionts, and at least one frank pathogen. While most T. denticola research concerns its role in periodontitis, its relative tractability for growth and genetic manipulation make it a useful model for studying Treponema physiology, metabolism, and host-microbe interactions. Metal micronutrient acquisition and homeostasis are highly regulated both in microbial cells and by host innate defense mechanisms that severely limit metal cation bioavailability. Here, we characterized the T. denticola troABCDR operon, the role of TroA-mediated iron and manganese uptake in growth, and the effects of TroR on global gene expression. This study contributes to our understanding of the mechanisms involved in cellular metal homeostasis required for survival in the host environment.
KEYWORDS: metalloregulation, spirochetes, transcription
ABSTRACT
The availability of divalent metal cations required as cofactors for microbial metabolism is severely limited in the host environment. Bacteria have evolved highly regulated uptake systems to maintain essential metal homeostasis to meet cellular demands while preventing toxicity. The Tro operon (troABCDR), present in all sequenced Treponema spp., is a member of a highly conserved family of ATP-binding cassette transporters involved in metal cation uptake whose expression is controlled by TroR, a DtxR-like cation-responsive regulatory protein. Transcription of troA responds to divalent manganese and iron (T. denticola) or manganese and zinc (T. pallidum), and metal-dependent TroR binding to the troA promoter represses troA transcription. We report here the construction and complementation of defined T. denticola ΔtroR and ΔtroA strains to characterize (i) the role of TroA in metal-dependent T. denticola growth and (ii) the role of TroR in T. denticola gene expression. We show that TroA expression is required for T. denticola growth under iron- and manganese-limited conditions. Furthermore, TroR is required for the transcriptional regulation of troA in response to iron or manganese, and deletion of troR results in significant differential expression of more than 800 T. denticola genes in addition to troA. These results suggest that (i) TroA-mediated cation uptake is important in metal homeostasis in vitro and may be important for Treponema survival in the host environment and (ii) the absence of TroR results in significant dysregulation of nearly one-third of the T. denticola genome. These effects may be direct (as with troA) or indirect due to dysregulation of metal homeostasis.
IMPORTANCE Treponema denticola is one of numerous host-associated spirochetes, a group including commensals, pathobionts, and at least one frank pathogen. While most T. denticola research concerns its role in periodontitis, its relative tractability for growth and genetic manipulation make it a useful model for studying Treponema physiology, metabolism, and host-microbe interactions. Metal micronutrient acquisition and homeostasis are highly regulated both in microbial cells and by host innate defense mechanisms that severely limit metal cation bioavailability. Here, we characterized the T. denticola troABCDR operon, the role of TroA-mediated iron and manganese uptake in growth, and the effects of TroR on global gene expression. This study contributes to our understanding of the mechanisms involved in cellular metal homeostasis required for survival in the host environment.
INTRODUCTION
Iron and other divalent cations are essential cofactors for numerous aspects of metabolism for both microbes and their eukaryotic hosts. Host mechanisms for sequestering iron and other transition metals are recognized as a key feature of innate defenses controlling or preventing microbial colonization and proliferation and have been described as a system of “nutritional immunity” (1). Because divalent cation availability is severely limited in the host environment through sequestration mechanisms (reviewed in reference 2), bacteria have evolved highly regulated specific uptake systems to obtain these crucial cofactors. Metal-sensing transcription factors respond to fluctuating metal ion concentrations by controlling the expression of metal ion transporter genes, thus helping to maintain intracellular concentrations of essential metal cations at levels sufficient to meet cellular demands but low enough to prevent toxicity.
The DtxR/MntR family of transcriptional regulators was first characterized in Gram-positive bacteria (3, 4), but reasonably homologous systems have been identified throughout both Bacteria and Archaea (5, 6). Binding of divalent metal ions by these regulatory proteins allows them to dimerize and bind to palindromic sequences within the promoter region of the target genes, thus repressing the transcription of the gene(s) they regulate (7). The decreased intracellular metal ion concentration as a consequence of host sequestration results in reduced binding of the regulatory protein to the promoter of the uptake operon, resulting in increased expression and target cation uptake.
Regulation and uptake of divalent cations are linked to virulence in numerous microbes (8–10). Interestingly, this includes microbes such as Yersinia pestis (6) and Streptococcus mutans (11) that have very different levels of virulence under the different environmental conditions that they encounter. Of particular interest, manganese uptake systems regulated by DtxR homologues in Enterococcus faecalis and oral streptococci are required for endocarditis virulence in animal models (8, 11, 12). In oral streptococci, this manganese uptake system has been found to potentiate the superoxide dismutase activity that is required for growth in serum but is not required for colonization of the teeth (13).
Treponema denticola is an obligately host-associated anaerobic spirochete, and as such it is exquisitely adapted to survival in the human oral cavity. T. denticola is highly associated with severe periodontitis (14, 15). Using the most sensitive PCR conditions, it can generally be detected at very low levels in the oral microbiomes of periodontally healthy individuals (16), including young children (17, 18). The proportions of oral Treponema increase dramatically during the development of periodontal disease, reflecting major changes in both the disease-associated microbiome and the tissue environment (14, 19, 20).
As do all bacteria, T. denticola requires metal ions as micronutrients for growth. The T. denticola genome includes eight annotated ATP-binding cassette (ABC) transporters with predicted iron compound uptake function, as well as several other predicted metal ion transporters (21). Most of these are uncharacterized as to either cation specificity or actual function. While at least 20 genes encoding metalloproteins are annotated in the genome (22), specific metal dependence of very few T. denticola enzymes has been demonstrated. These include an iron-dependent superoxide reductase encoded by TDE1754 (23) and a manganese-dependent 1,2-diacylglycerol choline phosphotransferase encoded by TDE0021 that is required for phosphatidylcholine synthesis (24).
Based on genome analysis, a T. denticola genetic locus (TDE1222 to TDE1226) has significant homology to the T. pallidum troABCDR operon. This operon encodes TroA, a cation-specific binding protein, TroB, an ATPase, TroC and TroD, cytoplasmic membrane permeases, and TroR, a predicted manganese- and iron-dependent transcription repressor. TroR in both T. denticola and T. pallidum shares high homology with a large family of iron-dependent regulatory proteins typified by DtxR from Corynebacterium diphtheriae and the manganese-dependent MntR from Bacillus subtilis (25). Function and regulation of the tro operon in Treponema have been studied primarily in vitro or in an Escherichia coli background. Overexpression of T. pallidum troABCD in E. coli increased its sensitivity to Zn2+, Mn2+ and Fe2+, and expression of TroA was repressed by Mn2+ or Zn2+ in E. coli harboring a plasmid carrying the entire troABCDR operon, suggesting that TroR suppresses tro expression in the presence of metal cations (26). In both T. pallidum and T. denticola, expression of TroR could repress activity of a tro promoter-lacZ reporter in E. coli (27, 28). When T. denticola was grown in chelated medium, supplementation with either Mn2+ or Fe2+, but not Zn2+, resulted in decreased troA transcription (27).
The present work, conducted in T. denticola, was undertaken to expand understanding of the tro operon in its native genetic context, including its regulation and its role in Treponema growth in metal cation-limited conditions. Here, we report the construction and complementation of isogenic T. denticola ΔtroA and ΔtroR mutant strains and characterization of their behavior under various nutrient conditions.
RESULTS
Transcription of the tro operon.
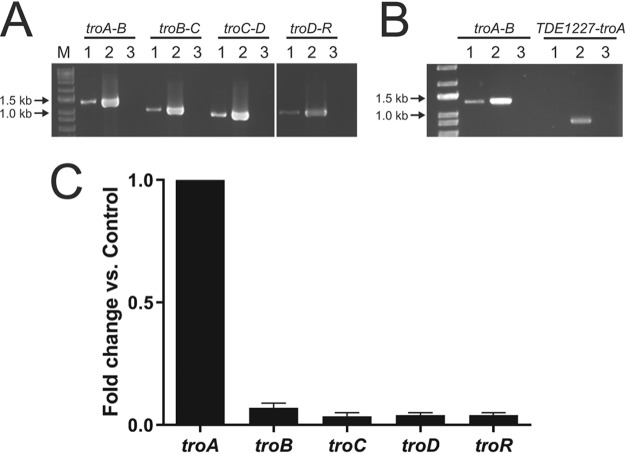
The T. denticola genes troA, troB, troC, troD, and troR (troA-R) are predicted to be transcribed as a single mRNA (27), as has been reported in T. pallidum (29). To confirm this and provide the basis for further studies, we assayed transcription in the tro operon by reverse transcription-PCR (RT-PCR) and quantitative RT-PCR (RT-qPCR). To determine whether troABCDR comprise a single mRNA transcript, primer pairs were designed to amplify across junctions between each set of neighboring genes. As shown in Fig. 1A, RT-PCR products of the expected size were seen for each pair of genes, indicating that troA-R comprise a single mRNA. To confirm this, RT-PCR was conducted on a cDNA generated with an oligonucleotide primer complementary to the 3′ region of troR. As shown in Fig. 1B, while RT-PCR using this template resulted in the expected troA-troB amplicon, no RT-PCR amplicon was detected using a primer set designed to amplify between troA and TDE1227, the gene immediately 5′ to troA. In contrast to T. pallidum, in which the gene directly 3′ to the tro operon is in the same transcriptional orientation, TDE1221 is encoded on the opposite strand relative to troA-R in T. denticola. To determine the relative transcript levels of each region of the operon, we performed RT-qPCR using primer sets specific for each gene. After 2 days growth in NOS medium (early log phase), the transcription levels of troB, troC, troD, and troR were only about 10% of that of troA (Fig. 1C).
FIG 1.
RT-PCR analysis of the tro locus. (A) RT-PCR using oligonucleotide primer sets to amplify junctions of troA-troB, troB-troC, troC-troD, and troD-troR . Lanes showing troD-R PCR products were spliced from a different region of the same agarose gel due to the number of lanes required. (B) RT-PCR using oligonucleotide primer sets specific for TDE1227-troA. T. denticola PCR templates: cDNA (lanes 1), genomic DNA (lanes 2), and RNA (lanes 3). Template cDNAs were made with random hexamer primers (A) or a troR “reverse” primer (B). (C) Quantitative RT-PCR (RT-qPCR) following 2 days of growth in NOS medium. The transcript levels of individual genes in the tro operon are shown relative to troA. The oligonucleotide primer sets are listed in Table S2.
Transcription of troA responds to cation availability.
A previous study reported that the transcription of troA was repressed by addition of Fe2+ or Mn2+ to T. denticola grown under conditions of metal cation chelation (27). We assayed the transcription of troA and troR under conditions of varying cation availability. As shown in Fig. 2, compared to expression after growth in normal medium, troA transcription was significantly increased after 2 days of growth in chelated medium, and troR transcription was also increased to a lesser degree. Compared to transcription after growth in chelated medium, transcription of both troA and troR was reduced ∼3-fold in chelated medium supplemented with Fe2+ or Mn2+ at 20 μM. Supplementation of chelated media with Fe2+ or Mn2+ at 5 or 10 μM resulted in smaller decreases in the expression of troA and troR (data not shown). These results are consistent with those reported by Brett et al. (27), though they did not include data on transcription in “normal” medium.
FIG 2.
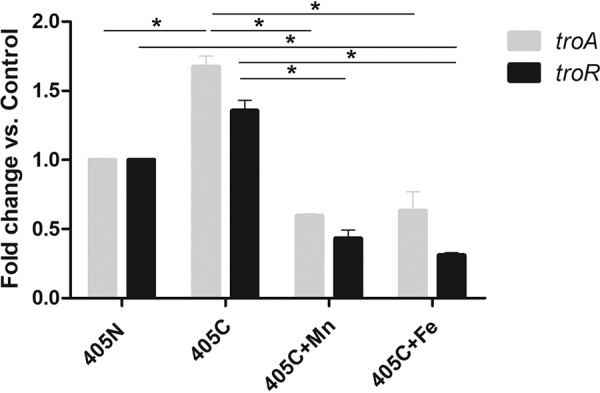
RT-qPCR analysis of troA and troR transcription in response to cation starvation and supplementation with Mn2+ or Fe2+. T. denticola 35405 was grown for 2 days in NOS medium under standard conditions (405N), chelated conditions (405C), chelated conditions plus Mn2+ at 20 μM (405C+Mn), or chelated conditions plus Fe2+ at 20 μM (405C+Fe). Statistically significant (P < 0.05) differences between gene expressions under different growth conditions are indicated by asterisks.
Expression of TroA increases during growth in culture.
The availability of nutrients, including metal cation micronutrients, decreases during growth in culture. To investigate how closely expression of TroA responded to changes in metal cation availability, we performed Western immunoblotting of T. denticola 35405 over 4 days of culture in NOS medium. As shown in Fig. 3, expression of TroA protein increased over time, consistent with lessening of TroR repression of troA transcription as levels of available Fe2+ or Mn2+ per cell decreased as cell numbers increased.
FIG 3.
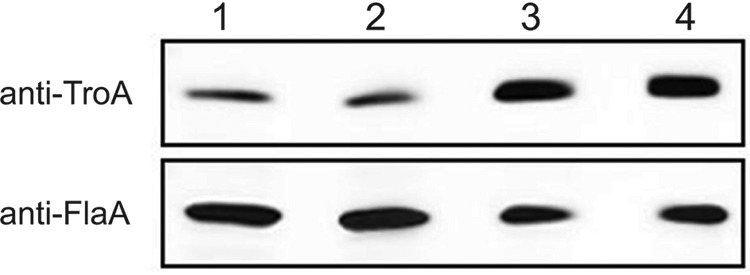
Expression of TroA during growth. Samples of T. denticola 35405 were taken on days 1 to 4 during growth in NOS medium and subjected to Western immunoblotting. The upper panel was probed with anti-TroA antibodies. The lower panel was probed with anti-FlaA antibodies. Lanes 1 to 4 represent samples taken on the indicated day.
Mutagenesis and complementation of troA and troR.
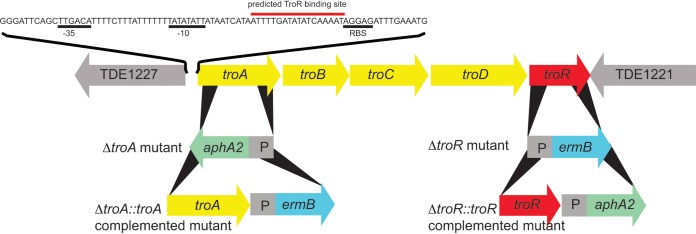
To determine the roles of TroA and TroR in T. denticola growth, we constructed isogenic mutants in which either troA or troR was disrupted by insertion of an antibiotic cassette encoding resistance to kanamycin (Km) or erythromycin (Em), respectively. The strategy for allelic replacement mutagenesis and complementation in the tro operon is illustrated in Fig. 4. Details of mutant construction are provided in the supplemental material. To determine the role of TroA-mediated uptake function in T. denticola, we constructed a T. denticola troA deletion strain (ΔtroA strain). A 358-bp internal fragment of troA was replaced by the aphA2 cassette in opposite transcriptional orientation to troA. The ΔtroA strain was selected by growth in TYGVS medium containing kanamycin. The ΔtroA strain was then complemented by replacing the aphA2 cassette in the ΔtroA strain with the wild-type troA linked to the ermB cassette encoding Emr, yielding a ΔtroA::troA strain (C-ΔtroA). To avoid disrupting the troA promoter or the adjacent uncharacterized gene (TDE1227), the complementation construct was designed so that the ermB cassette was localized between troA and troB and in the same transcriptional orientation.
FIG 4.
Mutagenesis and complementation of the T. denticola tro operon. The construction of isogenic T. denticola strains mutated in troA or troR (designated ΔtroA and ΔtroR strains, respectively) and the ΔtroA::troA and ΔtroR::troR complemented mutant strains (designated C-ΔtroA and C-ΔtroR, respectively) are illustrated below the map. Each of the four mutant strains was constructed independently. Each antibiotic cassette (aphA2 encoding Kmr and ermB encoding Emr) is under transcriptional control of a ermB promoter (P). The sequence of the tro promoter/operator (P/O) region containing the promoter and putative TroR binding site in T. denticola 35405 is shown above the map.
To demonstrate the regulatory function of TroR on transcription of the tro operon, we constructed a T. denticola troR deletion strain (ΔtroR strain). All but 66 nucleotides comprising the extreme 5′ and 3′ ends of troR were replaced with the ermB gene under transcriptional control of its own promoter (30). The ΔtroR mutant was then complemented by replacing the ermB cassette in the ΔtroR strain with the wild-type troR linked to the aphA2 cassette encoding Kmr, yielding a ΔtroR::troR strain (C-ΔtroR).
TroA is required for growth in medium lacking added supplemental cations.
The two most commonly used media for culturing T. denticola are NOS and TYGVS (31). The ΔtroA mutant was isolated and maintained in TYGVS medium. While there are minor differences between them in amounts of specific components, TYGVS contains four times the amount of yeast extract as NOS, as well as a mixture of salts including neither iron nor manganese. To determine the contribution of TroA to T. denticola growth, the wild-type parent strain 35405 and the ΔtroA and ΔtroR mutants were grown in NOS medium with or without supplementation with Fe2+, Mn2+, or both. The ΔtroA mutant grew extremely slowly in NOS medium (Fig. 5) and did not grow in chelated NOS medium (data not shown). Supplementation of NOS medium with Fe2+ or Mn2+ partially rescued the growth defect in the ΔtroA strain. Increasing the concentration of rabbit serum in NOS medium from 2.5 to 10% did not result in improved growth of the ΔtroA strain in NOS medium (data not shown). Complementation of the ΔtroA strain resulted in partial rescue of its growth defect in NOS medium (Fig. 5). The ΔtroR strain showed no growth defect in NOS medium. To confirm that the growth phenotype of the ΔtroA strain was specifically due to the mutagenesis of troA, we independently constructed a second ΔtroA mutant strain and determined that both mutants had the same growth phenotype (data not shown). We then constructed a complemented strain T. denticola ΔtroA::troA (designated C-ΔtroA) (Fig. 4). The C-ΔtroA strain was able to grow in NOS medium without Fe2+ or Mn2+ supplementation but reached a lower cell density than did the parent strain (Fig. 5).
FIG 5.

T. denticola ΔtroA mutant requires cation supplementation. T. denticola parent strain 35405 (“405”) and isogenic ΔtroR and ΔtroA were grown in NOS medium or NOS medium supplemented with Fe2+, Mn2+, or both Fe2+ and Mn2+ .
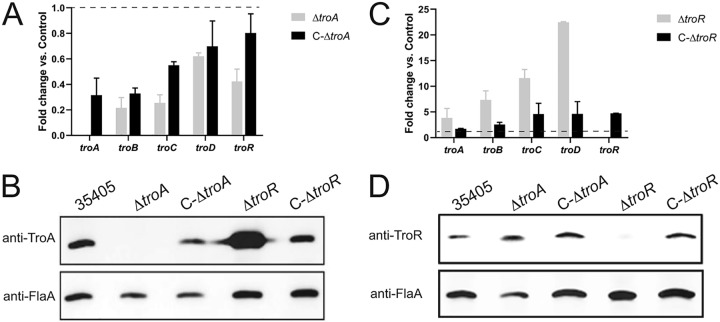
In order to understand why complementation of the ΔtroA mutant only partially restored the wild-type phenotype, we compared transcription and protein expression levels in parent, mutant, and complemented strains. Transcription of troA in the C-ΔtroA strain was only about 30% of that in the parent strain (Fig. 6A), and C-ΔtroA produced lower levels of TroA protein than did the parent strain (Fig. 6B). The transcription levels of troB, troC, troD, and troR were reduced 2- to 4-fold in the ΔtroA strain compared to the relatively low levels in the parent strain and were only partially restored in the C-ΔtroA strain (Fig. 6A).
FIG 6.
Expression of the T. denticola tro locus in wild-type, mutant, and complemented mutant strains. (A) RT-qPCR of each of the genes in the tro operon in T. denticola ΔtroA and C-ΔtroA strains relative to its expression level in T. denticola 35405, which is set at 1.0 (dashed line). (B) TroA protein expression after 2 days of growth. Immunoblots were probed with antibodies raised against TroA (upper) or FlaA (lower). (C) RT-qPCR of each of the genes in the tro operon in T. denticola ΔtroR and C-ΔtroR strains relative to its expression level in T. denticola 35405, which is set at 1.0 (dashed line). (D) TroR protein expression after 2 days growth. Immunoblots were probed with antibodies raised against TroR (upper) or FlaA (lower).
Mutagenesis of troR results in loss of repression of troA expression.
TroR binds to the troA promoter in the presence of Fe2+ or Mn2+ and suppresses transcription (27). As expected, mutagenesis of troR resulted in increased levels of troA transcript (Fig. 6C) and TroA protein (Fig. 6B) compared to the parent strain. Derepression of tro transcription in the ΔtroR mutant also resulted in 5- to 15-fold increases in troB, troC, and troD transcription compared to the relatively low levels in the parent strain (Fig. 6C and Fig. 1B). Unlike the ΔtroA mutant, the ΔtroR mutant showed no growth defect compared to the wild-type strain (Fig. 5).
Complementation of the ΔtroR mutant rescues repression of troA expression.
To confirm that the phenotype of the ΔtroR mutant was not an artifact of mutagenesis, we complemented the mutation in the ΔtroR mutant by replacing the ermB cassette with DNA consisting of troR plus the aphA2 cassette (Fig. 4 and the text in the supplemental material). The complemented ΔtroR::troR (C-ΔtroR) strain was selected for Kmr and assayed for transcription and protein expression. Complementation of the ΔtroR mutant resulted in reversion of tro operon transcription to levels closer to that of the wild-type strain (Fig. 6C). Similarly, TroA and TroR proteins were expressed at approximately wild-type levels in the complemented C-ΔtroR strain (Fig. 6B and D).
Transcriptomic changes resulting from troR mutagenesis.
To determine whether mutagenesis of troR resulted in effects beyond loss of repression of transcription of the tro operon, we performed transcriptome sequencing (RNA-seq) analysis on T. denticola parent and ΔtroR strains after 2 days growth in broth culture, which represents early to mid-logarithmic growth phase (Fig. 5). RNA-seq data are included in Table S4. Compared to the parent strain, 902 genes were differentially expressed in the ΔtroR strain, including 214 whose expression was significantly higher and more than 688 whose expression was significantly lower (cutoff, ±1.5-fold). Consistent with the results of a separate RT-qPCR experiment (Fig. 6C), RNA-seq showed that the transcriptions of troA and troD were increased approximately 2- and 3-fold, respectively, in the ΔtroR strain. Interestingly, 146 genes showed greater transcription increases in the ΔtroR strain than did troA. These include TDE0439 encoding a hypothetical protein (28.2-fold increase) and TDE0610 encoding a predicted 3-hydroxyacyl coenzyme A dehydrogenase (27.4-fold increase). In E. coli, this enzyme (Pfam PF00725, PF02737) is part of a multienzyme complex involved in fatty acid metabolism (32). Eleven of the twenty genes with the greatest transcription increases and forty-four of the fifty genes with the greatest transcription decreases in the ΔtroR strain are annotated as encoding hypothetical proteins (data not shown). Annotated genes with significantly increased transcription levels in the ΔtroR strain included those encoding a wide variety of enzymatic functions as well as ABC transporters for peptide uptake and iron uptake. More than 20 significantly upregulated genes encode ribosomal components or functions. Annotated genes with significantly reduced transcription levels in the ΔtroR strain included those encoding a wide variety of functions, including ABC transporters for peptide uptake and iron uptake as well as group putative bacteriophage genes. To validate the RNA-seq results, we conducted RT-qPCR analysis on a subset of eleven genes with the greatest expression changes in the ΔtroR strain (nine with higher expression and two with lower expression levels) plus troA. As shown in Fig. 7, RT-qPCR results closely paralleled RNA-seq results.
FIG 7.
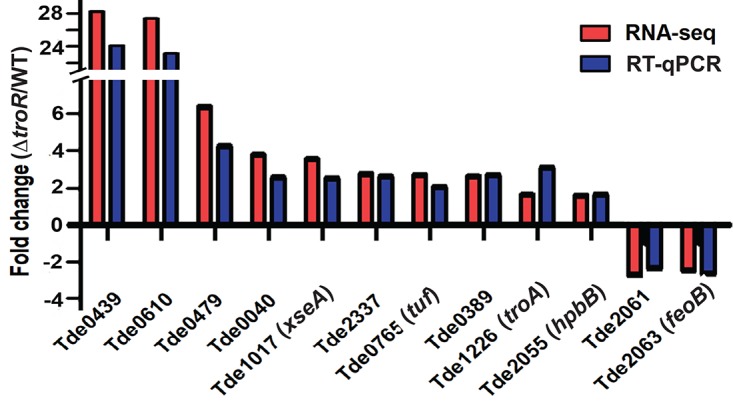
Comparison of RNA-seq and RT-qPCR data. Comparison of transcription levels (fold change, base 10) between T. denticola parent strain (WT) and ΔtroR mutant in 12 differentially expressed genes as determined by the two methods. The Pearson correlation coefficient between RNA-seq and RT-qPCR was 0.997. TDE0439, TDE0610, TDE0479, TDE0040, TDE1017, TDE2337, TDE0765, and TDE0389 were among the top 20 upregulated genes in RNA-seq in T. denticola ΔtroR, while TDE2061 and TDE2063 were downregulated. As expected, TDE1226 (troA) was also upregulated in both RNA-seq and RT-qPCR.
DISCUSSION
Understanding of Treponema biology and physiology remains relatively limited, despite decades of research on both frank pathogens (T. pallidum) and pathobionts (T. denticola). While these two microbes have radically different pathologies, they share many important features, including physiology and coevolutionary interactions with the human mucosal immune system. Recent methodological advances in culturing T. pallidum (33) and in genetic manipulation of T. denticola (34, 35) have the potential to drive significant advances in this field. The purpose of the present study was to extend knowledge of regulation of metal homeostasis in T. denticola, which shares a conserved troABCDR divalent cation uptake operon with T. pallidum. As in other members of the DtxR family, TroR dimerizes in the presence of specific cations and inhibits troA transcription by binding to a palindromic sequence in the promoter region. While the specificity of both systems for manganese has been reported, it is of interest that T. pallidum TroR responds to zinc but not to iron, whereas the T. denticola TroR responds to iron but not to zinc (26–28). The Tro system in both organisms has been studied in some depth, but the present work is the first to focus on characterization of its function in a native Treponema background.
As noted by Brett et al., the organization of the tro operon is conserved between T. denticola and T. pallidum, though the genomic context and flanking genes are not conserved (27). We confirmed the predicted operon structure by RT-PCR and quantified transcription throughout the operon. While RT-PCR results shown in Fig. 1A and B indicate that a single mRNA encompasses troABCDR, the RT-qPCR results were not as straightforward (Fig. 1C). The level of troA transcription detected after 2 days growth was much higher than that of the other genes. Since we found no evidence of a transcription termination signal 3′ to troA (data not shown), lower levels of transcription 3′ to troA may reflect intrinsic mRNA instability. Alternatively, it is possible that troB, troC, troD, and troR are constitutively transcribed at low levels. In an independently constructed ΔtroA strain carrying the transcription terminator of the T. denticola msp gene (36), we were still able to detect low levels of troB, troC, troD, and troR transcription (data not shown). In either scenario, regulation at the level of troA transcription may be sufficient for regulation of uptake function. Consistent with this interpretation, SloR (the TroR orthologue in Streptococcus mutans), is expressed constitutively, independent of manganese concentration (37). Further studies are in progress to address these issues in T. denticola.
TroA expression is responsive to availability of Fe2+ and Mn2+. This was evident when assaying transcription of both troA and troR in the presence or absence of these cations in parent and mutant strains. Similarly, nutrients, including cation micronutrients, are consumed during growth in culture. We were able to confirm the predicted response of increased production of TroA protein over the course of 4 days of growth in culture, during which the levels of available nutrients drop (Fig. 3). We did not quantitate the levels of bioavailable Fe2+ and Mn2+ over the course of this growth study, but this interpretation is consistent with results of the growth studies in which Fe2+ and Mn2+ levels were controlled (Fig. 2 and 5).
Construction of isogenic T. denticola strains mutated in troA and troR, coupled with complementation of the mutations in each of these strains facilitated analysis of transcription, protein expression and growth behavior dependent on TroA and TroR. As expected, expression of TroA was greatly increased in the ΔtroR strain and reverted to near wild-type levels in the complemented C-ΔtroR strain. Similarly, complementation of the ΔtroA strain resulted in partial restoration of TroA expression. It is helpful to note here that the large observed fold changes in expression of troB, troC, and troD between wild-type, mutant, and complemented strains are in part reflective of the relatively low expression levels of troB, troC, and troD in the wild-type strain (Fig. 1C; Fig. 6A and B). An initial metal analysis showed that total iron and manganese levels in TYGVS medium were approximately 50% higher than in NOS medium though both were at less than 1 μM (data not shown). It should be noted that this assay identifies neither bioavailability nor oxidation state of metals. Presumably, overloading NOS with Fe2+ or Mn2+ resulted in sufficient nonspecific uptake sufficient to support limited growth of the ΔtroA strain. The fact that neither metal supplementation nor genetic complementation completely restored growth of the ΔtroA strain to wild-type levels was of great concern. In the case of the ΔtroA strain, the only specific Mn2+ uptake mechanism was absent. Given that T. denticola has as many as eight annotated iron uptake systems (21), the inability of the ΔtroA strain to grow in NOS medium without Fe2+ supplementation is more difficult to understand. This issue is further addressed below. As for the complemented ΔtroA mutant, Fig. 6A and B show that transcription and translation of troA were still markedly lower in C-ΔtroA than in the wild type. We interpret the incomplete restoration of wild-type growth in the complemented mutant as consistent with both the mRNA and protein expression data. The troA complementation strategy was adopted because preliminary mutagenesis studies suggested that TDE1227, whose predicted promoter appears to overlap that of troA, may be an essential gene (data not shown). As described in the supplemental material, the C-ΔtroA strain contains the entire wild-type troA ORF and its promoter region exactly as in the wild-type strain. The troA-troB intergenic region is 26 nucleotides, with a predicted ribosome binding site starting at residue 15. Compared to the wild-type sequence, the C-ΔtroA construct is missing the first seven of these intergenic nucleotides, which have been replaced with the ermB ORF in the same orientation as the tro operon. While the ermB cassette does not include transcription signals, insertion of the ermB cassette may modulate expression downstream due either to the loss of seven nucleotides or to attenuation of transcription due to increased transcript length. Further analysis of the tro mRNA is required to address this issue.
Mutagenesis of troR resulted in significant changes in T. denticola global gene expression patterns (Table S4). Although most of these changes are likely secondary effects on multiple metabolic pathways due to derepression of Mn2+ and Fe2+ uptake, we conducted a preliminary survey of the promoter regions of some of the genes whose expression was most increased in the ΔtroR mutant. As illustrated in Fig. S1 in the supplemental material, promoter regions of several of these genes contained palindromic sequences similar to the TroR binding sites of T. denticola and T. pallidum. Interestingly, SloR, the TroR ortholog in Streptococcus mutans, has been shown to directly regulate expression of at least four target genes in addition to SloABC by metal-dependent binding to their promoters (38). While our observations here are intriguing, further studies are required to determine whether TroR directly regulates expression of genes other than the tro operon.
Our studies confirm the basic model of substrate-regulated gene expression in that manganese availability contributes to regulation of its own transport. While the role of manganese in Treponema metabolism has not yet been studied in great depth (22), it is likely, as in other microbes, to have a key role in oxidative stress responses (39, 40). Consistent with this, McHardy et al. reported that transcription of troA, troB, and troR was upregulated in T. denticola subjected to oxidative stress (41). Planned future studies will address global gene expression in T. denticola strains carrying tro mutations when grown under various conditions, including oxidative stress, that could be encountered in the host environment.
Manganese has been suggested as a regulator of bacterial iron homeostasis in that manganese may modulate iron-dependent gene expression (42). A recent report that either manganese depletion in wild-type strain or mutagenesis of a Mn2+ transporter in Bradyrhizobium japonicum resulted in cellular iron deficiency is entirely consistent with the results presented here (42). This could provide an explanation for our observation that, even though T. denticola has several annotated iron uptake systems, mutagenesis of troA resulted in inability of the mutant to grow in NOS medium in the absence of iron supplementation. Consistent with the data shown in Fig. 5, this interpretation is supported by the fact that the ΔtroA mutant was isolated in TYGVS medium, which contains considerably more iron and manganese than does standard NOS medium (31; data not shown). Interestingly, our initial RNA-seq studies revealed a mixed pattern of up- and downregulation of various iron transport-related proteins. Further studies are required to address the potential role of TroR in directly or indirectly regulating other uptake systems.
The studies reported here demonstrate that T. denticola TroA-mediated Fe2+ and Mn2+ uptake regulated by TroR is important for growth in culture and suggest a key role of manganese-dependent and TroR-regulated gene expression in T. denticola survival in the host environment. Future studies will address global effects of dysregulation of the tro operon, including the potential roles of TroR and TroA in directly or indirectly affecting metabolic pathways, including those involved in oxidative stress responses and iron uptake and utilization.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
T. denticola strains were grown as previously described (31) under anaerobic conditions in NOS or TYGVS medium supplemented with erythromycin (Em; 40 μg ml−1) and kanamycin (Km; 25 μg ml−1) as appropriate. Unless otherwise noted, growth medium contained 2.5% rabbit serum or horse serum. For some studies, broth medium was pretreated with Chelex (Bio-Rad Laboratories, Hercules, CA) with or without subsequent supplementation with ferrous sulfate or manganese sulfate at the indicated concentrations, as described previously (27) and in the supplemental material. All T. denticola growth media were incubated under anaerobic conditions for at least 18 h prior to use (43). Purity of spirochete cultures was monitored by dark-field microscopy. For growth studies, T. denticola was grown to an optical density at 600 nm (OD600) of 0.2 in NOS or TYGVS medium and then diluted 1:20 in the appropriate medium. Growth was then monitored spectrophotometrically for 7 days. Each of at least two biological replicates was performed with three independent technical measurements. Growth data were plotted as means ± the standard deviations. E. coli JM109 (44), used for plasmid construction, was grown in Luria-Bertani medium supplemented as appropriate with kanamycin (50 μg ml−1), carbenicillin (Cb; 50 μg ml−1), or erythromycin (200 μg ml−1).
Construction of plasmids for mutagenesis studies.
All plasmids were constructed using either restriction fragments of previously verified plasmids or PCR products generated with high-fidelity DNA polymerases (Phusion [New England Biolabs, Beverly, MA] or Taq-HF [Invitrogen, Carlsbad, CA]) using oligonucleotide primers listed in Table S1 in the supplemental material. Details of individual plasmid constructs are presented in the supplemental material.
Allelic replacement mutagenesis of T. denticola.
Defined isogenic mutants in troA and troR were constructed as described previously (45, 46) by electroporation of T. denticola with linear DNA fragments consisting of the selectable ermB or aphA2 cassette cloned between DNA fragments flanking the mutagenesis target (see the supplemental material). Mutants were selected for growth in NOS or TYGVS semisolid medium containing 0.8% Noble agar, with Em (40 μg ml−1) or Km (25 μg ml−1) added as appropriate. Isolated colonies were passaged twice in broth medium containing methylcellulose (0.95% [wt/vol]) and appropriate antibiotics before transfer to standard broth medium for further analysis (24). Mutations were verified by PCR analysis and DNA sequencing of the target region in genomic DNA of the mutants.
Protein gel electrophoresis and immunoblotting.
SDS-PAGE and Western immunoblotting were performed as described previously (36). Total cell lysates of T. denticola strains expressing TroR constructs were heated at 95°C for 5 min and then separated by SDS-PAGE and transferred to nitrocellulose membranes, which were then probed with rabbit polyclonal antibodies raised against TroR (27), FlaA (47), or rat polyclonal antibodies raised against TroA (48), followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or HRP-conjugated goat anti-rat IgG (Thermo Scientific, Rockford, IL) as appropriate. Protein bands of interest were visualized using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) and a G:Box imaging system (Syngene, Frederick, MD).
DNA sequence analysis.
Recombinant constructs in plasmids and in T. denticola mutants were confirmed by DNA sequencing at the University of Michigan DNA Sequencing Core Facility and analyzed using DNASTAR sequence analysis software (DNASTAR, Inc., Madison, WI).
RNA extraction.
Total RNA was extracted from cell pellets of T. denticola cultures using TRIzol reagent (Sigma, St. Louis, MO), followed by extraction with chloroform. The upper aqueous phase was precipitated with isopropanol, washed with 70% ethanol, and suspended in RNase-free water. After heating (10 min, 60°C), RNA was treated with DNase (Turbo DNA-free kit; Thermo-Fisher, Inc.) and then processed in an RNA Mini-Spin column (Qiagen) to remove residual DNase. RNA concentration was determined on a NanoDrop spectrophotometer (Thermo Fisher, Inc.). RNA quality was assayed by agarose gel electrophoresis, and DNA contamination was assayed by a standard PCR using a 16S rRNA primer set.
RT-PCR.
RNA samples were reversed transcribed using either random hexamer primers supplied with the SuperScript first-strand synthesis system for RT-PCR (Invitrogen) or with an oligonucleotide primer complementary to the 3′ region of troR. A portion (1 μl) of the resulting first-strand cDNA was amplified according to the manufacturer’s instructions. The gene-specific primer pairs used to generate and amplify cDNAs (Table S2) were designed using NCBI-PrimerBlast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). RT-PCR products, including positive (genomic DNA template) and negative controls (RNA not treated with RT enzyme), were analyzed by agarose gel electrophoresis.
For RT-qPCR, total RNA was extracted from T. denticola cultures harvested at specific growth stages and growth conditions as noted in Results, treated with DNase, and reverse transcribed to cDNA using random hexamer primers. Gene-specific primers were designed using the NCBI-PrimerBlast tool so that they all had similar annealing temperatures and amplicon sizes of 80 to 100 bp (Table S2). Then, 1 μl of the resulting cDNA was amplified using a QuantiTect SYBR green PCR kit (Qiagen) in 25 μl of reaction buffer, with T. denticola flaA serving as an internal reference control for normalization between samples. Unlike some flagellar genes in enterobacteria, flaA is constitutively expressed in spirochetes. It has been used as a reference gene for RT-qPCR in a number of studies in T. denticola (49–51), as well as in other spirochetes, including Borrelia burgdorferi (52, 53) and Leptospira interrogans (54, 55). Thermal cycling was performed in a MyiQ single-color real-time PCR detection system (Bio-Rad) at 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s, and 68°C for 30 s. Each individual assay was performed in triplicate. Threshold values were calculated using baseline cycles 2 to 10. Data were analyzed using by the ΔΔCT method (56) using software supplied by the manufacturer. Experiments were performed on three biologically independent replicates, each containing triplicate samples. Transcription data are presented as means ± the standard deviations. The statistical significance (P < 0.05) of differences in gene expression under different growth conditions was determined using two-way analysis of variance and is indicated by asterisks in the figures.
Transcriptome analysis by RNA-seq.
T. denticola strains were grown in NOS medium and harvested at early log phase (OD600 of ∼0.2). Total RNA was isolated, treated with DNase, and assayed for quality and concentration as described above for RT-PCR. Subsequent steps in RNA-seq analysis were conducted at the University of Michigan DNA Sequencing Core Facility. rRNA was depleted from purified RNA samples by using a RiboZero rRNA removal kit for Gram-negative bacteria (Illumina, San Diego, CA). RNA integrity was determined using an Agilent 2100 Bioanalyzer (San Diego, CA). Only samples with RNA integrity number greater than 8 were used for the construction of cDNA libraries. Sequencing was performed on a HiSeq 4000 system instrument using the clustering and sequencing reagents provided by Illumina. Paired-end raw reads in FASTQ format were trimmed using the Trimmomatic. Trimmed reads were assessed for quality, for each sample and end, using FastQC. Reads were aligned to the reference T. denticola ATCC 35405 genome. Gene expression levels were calculated using EDGE-pro with Bowtie2 for mapping and alignment. Normalized values are given in fragments per kilobase million (FPKM). Differential expression was calculated using log ratios of the FPKM values.
Metals analysis.
Inductively coupled plasma mass spectrometry (ICP-MS) analysis was conducted by the University of Michigan Elemental Analysis Laboratory. Prior to trace metal analysis, a 0.5-ml aliquot of each sample was transferred to perfluoroalkoxy vials containing 0.5 ml of concentrated HNO3 (Optima Grade) plus 0.5 ml of 30% H2O2 (Optima Grade). The vials were tightly closed and placed on a hot plate overnight (∼100°C, ∼12 h). The samples were then evaporated to dryness and redissolved in 2% HNO3 for ICP-MS analysis, which was performed on a Thermo Scientific high-resolution ICP-MS apparatus (Element 2). Mn, Fe, Co, Ni, Cu, and Zn were analyzed in medium resolution, and Se was analyzed in high resolution. Mass drift was corrected for using bracketing standards.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH grant DE025225 to J.C.F., the University of Michigan School of Dentistry Research Immersion Program, and the Department of Biologic and Materials Sciences and Prosthodontics, University of Michigan School of Dentistry.
We thank Justin Radolf for kindly providing anti-TroA antibodies.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson N. 1999. Metal ion transporters and homeostasis. EMBO J 18:4361–4371. doi: 10.1093/emboj/18.16.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd J, Oza MN, Murphy JR. 1990. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A 87:5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 5.Leyn SA, Rodionov DA. 2015. Comparative genomics of DtxR family regulons for metal homeostasis in Archaea. J Bacteriol 197:451–458. doi: 10.1128/JB.02386-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L, Ying W, Han Y, Chen M, Yan Y, Li L, Zhu Z, Zheng Z, Jia W, Yang R, Qian X. 2012. A proteome reference map and virulence factors analysis of Yersinia pestis 91001. J Proteomics 75:894–907. doi: 10.1016/j.jprot.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Tao X, Murphy JR. 1992. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem 267:21761–21764. [PubMed] [Google Scholar]
- 8.Colomer-Winter C, Flores-Mireles AL, Baker SP, Frank KL, Lynch AJL, Hultgren SJ, Kitten T, Lemos JA. 2018. Manganese acquisition is essential for virulence of Enterococcus faecalis. PLoS Pathog 14:e1007102. doi: 10.1371/journal.ppat.1007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaharik ML, Finlay BB. 2004. Mn2+ and bacterial pathogenesis. Front Biosci 9:1035–1042. doi: 10.2741/1317. [DOI] [PubMed] [Google Scholar]
- 10.Eijkelkamp BA, McDevitt CA, Kitten T. 2015. Manganese uptake and streptococcal virulence. Biometals 28:491–508. doi: 10.1007/s10534-015-9826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol 185:5967–5975. doi: 10.1128/jb.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnette-Curley D, Wells V, Viscount H, Munro CL, Fenno JC, Fives-Taylor P, Macrina FL. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect Immun 63:4669–4674. doi: 10.1128/IAI.63.12.4669-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump KE, Bainbridge B, Brusko S, Turner LS, Ge X, Stone V, Xu P, Kitten T. 2014. The relationship of the lipoprotein SsaB, manganese and superoxide dismutase in Streptococcus sanguinis virulence for endocarditis. Mol Microbiol 92:1243–1259. doi: 10.1111/mmi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loesche WJ. 1988. The role of spirochetes in periodontal disease. Adv Dent Res 2:275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- 15.Ellen RP, Galimanas VB. 2005. Spirochetes at the forefront of periodontal infections. Periodontol 2000 38:13–32. doi: 10.1111/j.1600-0757.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 16.Asai Y, Jinno T, Igarashi H, Ohyama Y, Ogawa T. 2002. Detection and quantification of oral treponemes in subgingival plaque by real-time PCR. J Clin Microbiol 40:3334–3340. doi: 10.1128/jcm.40.9.3334-3340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortelli JR, Fernandes CB, Costa FO, Cortelli SC, Kajiya M, Howell SC, Kawai T. 2012. Detection of periodontal pathogens in newborns and children with mixed dentition. Eur J Clin Microbiol Infect Dis 31:1041–1050. doi: 10.1007/s10096-011-1405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papaioannou W, Gizani S, Haffajee AD, Quirynen M, Mamai-Homata E, Papagiannoulis L. 2009. The microbiota on different oral surfaces in healthy children. Oral Microbiol Immunol 24:183–189. doi: 10.1111/j.1399-302X.2008.00493.x. [DOI] [PubMed] [Google Scholar]
- 19.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 20.Hajishengallis G. 2014. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, Davidsen TM, DeBoy RT, Fouts DE, Haft DH, Selengut J, Ren Q, Brinkac LM, Madupu R, Kolonay J, Durkin SA, Daugherty SC, Shetty J, Shvartsbeyn A, Gebregeorgis E, Geer K, Tsegaye G, Malek J, Ayodeji B, Shatsman S, McLeod MP, Smajs D, Howell JK, Pal S, Amin A, Vashisth P, McNeill TZ, Xiang Q, Sodergren E, Baca E, Weinstock GM, Norris SJ, Fraser CM, Paulsen IT. 2004. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A 101:5646–5651. doi: 10.1073/pnas.0307639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gherardini FC, Boylan JA, Brett PJ. 2006. Metal utilization and oxidative stress, p 101–126. In Radolf JD, Lukehart SA (ed), Pathogenic Treponema: molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 23.Caranto JD, Gebhardt LL, MacGowan CE, Limberger RJ, Kurtz DM Jr. 2012. Treponema denticola superoxide reductase: in vivo role, in vitro reactivities, and a novel [Fe(Cys)(4)] site. Biochemistry 51:5601–5610. doi: 10.1021/bi300667s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vences-Guzmán MÁ, Paula Goetting-Minesky M, Guan Z, Castillo-Ramirez S, Córdoba-Castro LA, López-Lara IM, Geiger O, Sohlenkamp C, Christopher Fenno J. 2017. 1,2-Diacylglycerol choline phosphotransferase catalyzes the final step in the unique Treponema denticola phosphatidylcholine biosynthesis pathway. Mol Microbiol 103:896–912. doi: 10.1111/mmi.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchant AT, Spatafora GA. 2014. A role for the DtxR family of metalloregulators in Gram-positive pathogenesis. Mol Oral Microbiol 29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazlett KR, Rusnak F, Kehres DG, Bearden SW, La Vake CJ, La Vake ME, Maguire ME, Perry RD, Radolf JD. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J Biol Chem 278:20687–20694. doi: 10.1074/jbc.M300781200. [DOI] [PubMed] [Google Scholar]
- 27.Brett PJ, Burtnick MN, Fenno JC, Gherardini FC. 2008. Treponema denticola TroR is a manganese- and iron-dependent transcriptional repressor. Mol Microbiol 70:396–409. doi: 10.1111/j.1365-2958.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posey JE, Hardham JM, Norris SJ, Gherardini FC. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci U S A 96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardham JM, Stamm LV, Porcella SF, Frye JG, Barnes NY, Howell JK, Mueller SL, Radolf JD, Weinstock GM, Norris SJ. 1997. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene 197:47–64. doi: 10.1016/S0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 30.Goetting-Minesky MP, Fenno JC. 2010. A simplified erythromycin resistance cassette for Treponema denticola mutagenesis. J Microbiol Methods 83:66–68. doi: 10.1016/j.mimet.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenno JC. 2005. Laboratory maintenance of Treponema denticola. Curr Protoc Microbiol Chapter 12:Unit 12B.1. [DOI] [PubMed] [Google Scholar]
- 32.Yang SY, Li JM, He XY, Cosloy SD, Schulz H. 1988. Evidence that the fadB gene of the fadAB operon of Escherichia coli encodes 3-hydroxyacyl-coenzyme A (CoA) epimerase, delta 3-cis-delta 2-trans-enoyl-CoA isomerase, and enoyl-CoA hydratase in addition to 3-hydroxyacyl-CoA dehydrogenase. J Bacteriol 170:2543–2548. doi: 10.1128/jb.170.6.2543-2548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edmondson DG, Hu B, Norris SJ, Edmondson DG, Hu B, Norris SJ. 2018. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. mBio 9:e01153-18. doi: 10.1128/mBio.01153-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuramitsu HK, Chi B, Ikegami A. 2005. Genetic manipulation of Treponema denticola. Curr Protoc Microbiol Chapter 12:Unit 12B 2. [DOI] [PubMed] [Google Scholar]
- 35.Godovikova V, Goetting-Minesky MP, Shin JM, Kapila YL, Rickard AH, Fenno JC. 2015. A modified shuttle plasmid facilitates expression of a flavin mononucleotide-based fluorescent protein in Treponema denticola ATCC 35405. Appl Environ Microbiol 81:6496–6504. doi: 10.1128/AEM.01541-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenno JC, Müller K-H, McBride BC. 1996. Sequence analysis, expression and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J Bacteriol 178:2489–2497. doi: 10.1128/jb.178.9.2489-2497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monette P, Brach R, Cowan A, Winters R, Weisman J, Seybert F, Goguen K, Chen J, Glasfeld A, Spatafora G. 2018. Autoregulation of the S. mutans SloR metalloregulator is constitutive and driven by an independent promoter. J Bacteriol 200:e00214-18. doi: 10.1128/JB.00214-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, Spatafora G. 2006. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J Bacteriol 188:5033–5044. doi: 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisher JP, Giedroc DP. 2013. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol 3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crepps SC, Fields EE, Galan D, Corbett JP, Von Hasseln ER, Spatafora GA. 2016. The SloR metalloregulator is involved in the Streptococcus mutans oxidative stress response. Mol Oral Microbiol 31:526–539. doi: 10.1111/omi.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McHardy I, Keegan C, Sim JH, Shi W, Lux R. 2010. Transcriptional profiles of Treponema denticola in response to environmental conditions. PLoS One 5:e13655. doi: 10.1371/journal.pone.0013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puri S, Hohle TH, O’Brian MR. 2010. Control of bacterial iron homeostasis by manganese. Proc Natl Acad Sci U S A 107:10691–10695. doi: 10.1073/pnas.1002342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMinn MT, Crawford JJ. 1970. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol 19:207–213. doi: 10.1128/AEM.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Ruby J, Charon N, Kuramitsu H. 1996. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol 178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenno JC, Wong GWK, Hannam PM, McBride BC. 1998. Mutagenesis of outer membrane virulence determinants of the oral spirochete Treponema denticola. FEMS Microbiol Lett 163:209–215. doi: 10.1111/j.1574-6968.1998.tb13047.x. [DOI] [PubMed] [Google Scholar]
- 47.Bian X-L, Wang H-T, Ning Y, Lee SY, Fenno JC. 2005. Mutagenesis of a novel gene in the prcA-prtP protease locus affects expression of Treponema denticola membrane complexes. Infect Immun 73:1252–1255. doi: 10.1128/IAI.73.2.1252-1255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anand A, Luthra A, Edmond ME, Ledoyt M, Caimano MJ, Radolf JD. 2013. The major outer sheath protein (Msp) of Treponema denticola has a bipartite domain architecture and exists as periplasmic and outer membrane-spanning conformers. J Bacteriol 195:2060–2071. doi: 10.1128/JB.00078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frederick JR, Rogers EA, Marconi RT. 2008. Analysis of a growth-phase-regulated two-component regulatory system in the periodontal pathogen Treponema denticola. J Bacteriol 190:6162–6169. doi: 10.1128/JB.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDowell JV, Huang B, Fenno JC, Marconi RT. 2009. Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect Immun 77:1417–1425. doi: 10.1128/IAI.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar J, Frederick J, Marconi RT. 2010. The Hpk2-Rrp2 two-component regulatory system of Treponema denticola: a potential regulator of environmental and adaptive responses. Mol Oral Microbiol 25:241–251. doi: 10.1111/j.2041-1014.2010.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motaleb MA, Sal MS, Charon NW. 2004. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J Bacteriol 186:3703–3711. doi: 10.1128/JB.186.12.3703-3711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sal MS, Li C, Motalab MA, Shibata S, Aizawa S, Charon NW. 2008. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. J Bacteriol 190:1912–1921. doi: 10.1128/JB.01421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. 2007. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun 75:2864–2874. doi: 10.1128/IAI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adhikarla H, Wunder EA Jr, Mechaly AE, Mehta S, Wang Z, Santos L, Bisht V, Diggle P, Murray G, Adler B, Lopez F, Townsend JP, Groisman E, Picardeau M, Buschiazzo A, Ko AI. 2018. Lvr, a signaling system that controls global gene regulation and virulence in pathogenic Leptospira. Front Cell Infect Microbiol 8:45. doi: 10.3389/fcimb.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔC(T) Method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.