M. tuberculosis retains the unique ability to establish an asymptomatic latent infection. To understand the mechanisms involved in hypoxic stress which play a critical role in persistence, we show that the virulence regulator PhoP is linked to hypoxia, the dormancy signal. In keeping with this, phoP was shown to play a major role in M. tuberculosis growth under hypoxia even in the presence of surplus nitrogen, the alternate electron acceptor. Our results showing regulation of hypoxia-responsive genes provide new biological insights into role of the virulence regulator in metabolic switching by sensing hypoxia and integrating nitrogen metabolism with hypoxia by the assistance of the hypoxia regulator DosR.
KEYWORDS: hypoxia regulator, M. tuberculosis PhoP, metabolic switching, protein-protein interactions, virulence regulator
ABSTRACT
Mycobacterium tuberculosis retains the ability to establish an asymptomatic latent infection. A fundamental question in mycobacterial physiology is to understand the mechanisms involved in hypoxic stress, a critical player in persistence. Here, we show that the virulence regulator PhoP responds to hypoxia, the dormancy signal, and effectively integrates hypoxia with nitrogen metabolism. We also provide evidence to demonstrate that both under nitrogen limiting conditions and during hypoxia, phoP locus controls key genes involved in nitrogen metabolism. Consistently, under hypoxia a ΔphoP strain shows growth attenuation even with surplus nitrogen, the alternate electron acceptor, and complementation of the mutant restores bacterial growth. Together, our observations provide new biological insights into the role of PhoP in integrating nitrogen metabolism with hypoxia by the assistance of the hypoxia regulator DosR. The results have significant implications on the mechanism of intracellular survival and growth of the tubercle bacilli under a hypoxic environment within the phagosome.
IMPORTANCE M. tuberculosis retains the unique ability to establish an asymptomatic latent infection. To understand the mechanisms involved in hypoxic stress which play a critical role in persistence, we show that the virulence regulator PhoP is linked to hypoxia, the dormancy signal. In keeping with this, phoP was shown to play a major role in M. tuberculosis growth under hypoxia even in the presence of surplus nitrogen, the alternate electron acceptor. Our results showing regulation of hypoxia-responsive genes provide new biological insights into role of the virulence regulator in metabolic switching by sensing hypoxia and integrating nitrogen metabolism with hypoxia by the assistance of the hypoxia regulator DosR.
INTRODUCTION
A hallmark of tuberculosis (TB) is the unique ability of Mycobacterium tuberculosis to establish an asymptomatic latent infection and persist within granulomas in a dormant form, sometimes for a very long time, before reactivation to cause the active disease. Since survival and persistence in this environment depend on the sensing of signals and ability to induce a robust adaptive response, one of the major aspects to understand latent TB relates to the mechanism of adaptation of the tubercle bacilli in response to environmental stress.
Despite its requirement of oxygen for growth, M. tuberculosis can survive during latency without oxygen for a surprisingly long time. Thus, two in vivo conditions are often linked to latent TB. These are hypoxia and exposure to immune effectors such as nitric oxide (NO) (1–3). The ability to produce reactive nitrogen species by host-inducible nitric oxide synthase (iNOS) contributes to TB infections by its effect on both the host and the pathogen (4). Therefore, functional iNOS expression could be detected in the lung macrophages of human TB patients (5, 6). Consistently, hypoxia and nitric oxide-dependent bacterial adaptation to a dormant state induce latent TB in mice (1, 3). During limiting oxygen concentrations, M. tuberculosis induces reduction of nitrate (NO3–) to nitrite (NO2–) to control redox homeostasis and energy production (7). When nitrate is taken up by mycobacteria via passive diffusion (8, 9), the mycobacterial nitrate reductase (encoded by narGHIJ) is expressed at a low level under aerobic conditions (7). Also, hypoxia promotes induction of NarK2, a nitrate transporter enabling rapid accumulation of nitrite by M. tuberculosis cultures growing under oxygen-limiting conditions but in the presence of nitrate (8). Consistent with these results, transcripts from narG, encoding a nitrate reductase subunit, and narX, encoding a nonfunctional nitrate reductase, were identified within granulomas of human TB samples (10, 11). These results suggest that intracellular mycobacteria within the human host encounter a very low oxygen tension and most likely adapt to the microenvironment by respiring nitrate. However, the key regulators that connect hypoxia and nitrogen metabolism of mycobacteria and that define the mechanisms that promote and maintain TB latency in humans remain poorly understood.
Previous studies have shown that upon exposure to hypoxia, carbon monoxide or nitric oxide, the dosR-dosS system activates expression of ∼48 genes that are part of the dormancy survival (Dos) regulon (12–14). Although DosR is essential for mycobacterial survival under dormancy (12, 15), phosphorylated DosR induces the expression of hypoxia-responsive genes (13). DosR cooperatively binds to target promoters containing a minimum of two tandem binding sites, and the proximal DosR binding site often juxtaposes with the –35 sequence element of the promoters (16, 17). In keeping with this, DosR-SigA interaction remains essential in the dormancy survival program of M. tuberculosis (18). Although previous studies suggest that dosR is regulated by PhoP (19–21) via recruitment of the regulator within the dosR promoter region (22–24), the role of PhoP during mycobacterial hypoxia remains unknown.
Recently, Voskuil and coworkers have shown that acidic conditions of growth significantly inhibited anaerobic survival of a ΔdosR mutant (25). However, an alkaline growth environment improved the mutant’s survival. Because PhoP controls pH-driven adaptations of M. tuberculosis (26–29), in this study we investigated whether the virulence-associated phoP locus is linked to the hypoxic response of the tubercle bacilli. Transcript profiling indicates that induction of dosR regulon was subdued in ΔphoP mutant, suggesting that PhoP functions as an activator of hypoxia-inducible genes. We provide evidence showing a striking increase in nitrate and nitrite reduction under hypoxia (7), a state related to nonreplicating persistence of M. tuberculosis either for redox balance maintenance or to provide energy during shift-down (8), is controlled by the phoP locus. In keeping with these results, phoP strongly impacts the expression of genes related to nitrogen metabolism and under oxygen austerity even in the presence of surplus nitrogen conditions the M. tuberculosis ΔphoP strain was significantly growth defective relative to the wild-type (WT) bacilli. Together, these results establish that (i) metabolic switching of M. tuberculosis under hypoxia is achieved by integration of hypoxia with nitrogen metabolism and (ii) the convergence of PhoP and DosR as coactivators of hypoxia-inducible genes, controlled by protein-protein contacts, coordinates nitrogen metabolism in response to hypoxia.
RESULTS
phoP impacts hypoxia-inducible gene expression of M. tuberculosis.
To investigate whether phoP impacts hypoxia, we compared the expression levels of hypoxia-inducible genes in WT and ΔphoP mutant M. tuberculosis strains using an in vitro model of hypoxia (3) (Fig. 1). The results suggest that under normal conditions the major hypoxia-inducible genes, which belong to the dosR regulon (12, 13, 15), are significantly downregulated in the ΔphoP mutant relative to the WT bacilli. However, during hypoxia their expressions remain comparable in the WT and the mutant strain (compare Fig. 1A and B). In contrast, dosR regulon genes which are involved in N2 metabolism (30) show a phoP-dependent activation both under normal conditions and during hypoxia (Fig. 1). Because narG and nirB control the reduction of nitrate and nitrite, respectively (30) and hypoxia is accompanied by an increase in nitrate reduction (7), these results together suggest that during hypoxia PhoP appears to activate expression of genes involved in N2 metabolism. In keeping with these results, glnR, a major regulator of nitrate assimilation (30), is strongly repressed by PhoP during hypoxia but not under normal conditions (compare Fig. 1A and B). Although GlnR is known for its role in the nitrate assimilation of M. tuberculosis (30), repression of glnR by PhoP during hypoxia rules out the possibility of activation of narG and nirB via GlnR. Table S1 in the supplemental material lists the PAGE-purified oligonucleotides used in reverse transcription-PCR (RT-PCR) experiments. Although a previous study by Smith and coworkers had identified PhoP-regulated transcriptome, DNA arrays using exponentially growing cells under normal conditions did not reveal an influence of PhoP on the expression of dosR or other hypoxia-inducible genes (31). In contrast, using a different phoP mutant strain, Gonzalo-Asensio et al. showed that PhoP functions as an activator of dosR (20). Although different experimental conditions and various quantitative approaches appear to account for this discrepancy, clearly our results are consistent with those of Gonzalo-Asensio et al. showing a significant effect of PhoP on the expression of dosR. More recently, Vashist et al. have shown that PhoP functions as a repressor of dosR (24). Although both our study and this report have investigated the regulation of dosR by PhoP, the use of laboratory-attenuated M. tuberculosis H37Ra, a different genetic background comprising a genomic copy of a mutant PhoP (S219L) by Vashist et al., versus the clean mutant used in our study most likely accounts for this discrepancy. Also, it should be noted that except for PhoP binding to nirB promoter in a genome-wide SELEX experiment (32), there has been no new report linking PhoP with hypoxia-inducible genes other than dosR itself.
FIG 1.
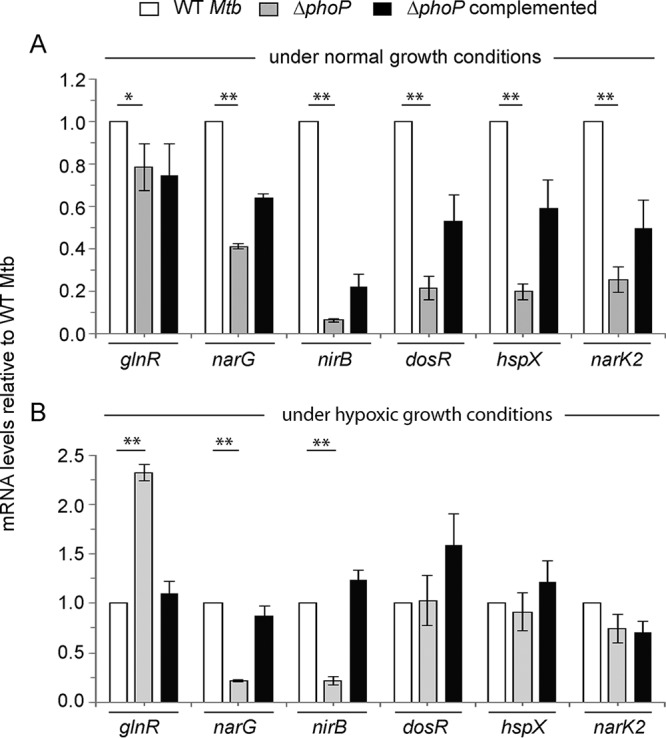
M. tuberculosis phoP regulates expression of hypoxia-responsive genes in vivo. Real-time RT-qPCR was carried out to determine relative expression levels of indicated hypoxia-inducible genes in WT (wild-type), ΔphoP, and complemented mutant strains both under normal conditions (A) and hypoxia (B), as described in Materials and Methods. The fold differences in mRNA levels with standard deviations from replicate experiments were determined from at least three independent RNA preparations (**, P < 0.01; *, P < 0.05). Mtb, M. tuberculosis (in this and subsequent figures).
Next, to investigate the expression levels of glnR, narG, and nirB, we grew WT M. tuberculosis in Dubos medium with either limiting (1 mM) or surplus nitrogen (30 mM) conditions (see Fig. S1 in the supplemental material), as described earlier (33). Although mycobacterial genes involved in N2 metabolism showed a relatively lower level of expression under hypoxia coupled with limiting ammonium chloride concentration (Fig. S1A), these genes displayed a significant induction during hypoxia coupled with surplus nitrogen (Fig. S1B). In sharp contrast, regardless of the ammonium chloride concentration, the mycobacterial genes dosR, hspX, and narK2 showed a significantly higher expression level under normoxia (compare Fig. S1A and B). It should be noted that hypoxic growth conditions in WT M. tuberculosis alone were unable to induce the expression of narG and nirB. However, the dosR regulon is strongly induced under hypoxia, showing activation of dosR expression (Fig. S1A). In sharp contrast, narG and nirB show a striking activation only when hypoxia is coupled with an excess of N2 source (Fig. S1B), clearly suggesting that activation of narG and nirB in M. tuberculosis is not attributable to hypoxia-dependent DosR activation alone.
To examine whether PhoP controls expression of mycobacterial N2 metabolism, we grew WT and ΔphoP M. tuberculosis strains in Dubos medium under either limiting nitrogen (1 mM) or a nitrogen surplus (30 mM) conditions (Fig. 2). In agreement with the results presented above (Fig. 1), during hypoxia coupled with surplus nitrogen concentration, the expression of glnR was significantly higher in the ΔphoP mutant, whereas narG and nirB were much less expressed in the mutant relative to the WT bacilli (Fig. 2A). This is consistent with reduced nitrogen scavenging by GlnR (34) and enhanced nitrite and nitrate reduction by the respective reductases (nirB and narG, respectively) (30) (see below). In contrast, (i) under identical conditions the phoP locus had no effect on the expression of dosR, hspX, and narK2 (Fig. S2A) and (ii) under normoxia coupled with surplus ammonium chloride concentration, these genes were comparably expressed in WT and ΔphoP mutant strains (Fig. 2B). On the other hand, regardless of the ammonium concentration, under normoxia the phoP locus had a striking effect on the expression of the hypoxia-responsive genes dosR, hspX, and narK2 (Fig. S2B). Based on these results, we conclude that phoP plays a major regulatory role in mycobacterial nitrogen metabolism under oxygen-limiting (hypoxia) conditions. A regulatory scheme suggesting activation of narG and nirB and repression of glnR to lower nitrogen scavenging is shown in Fig. 2C.
FIG 2.
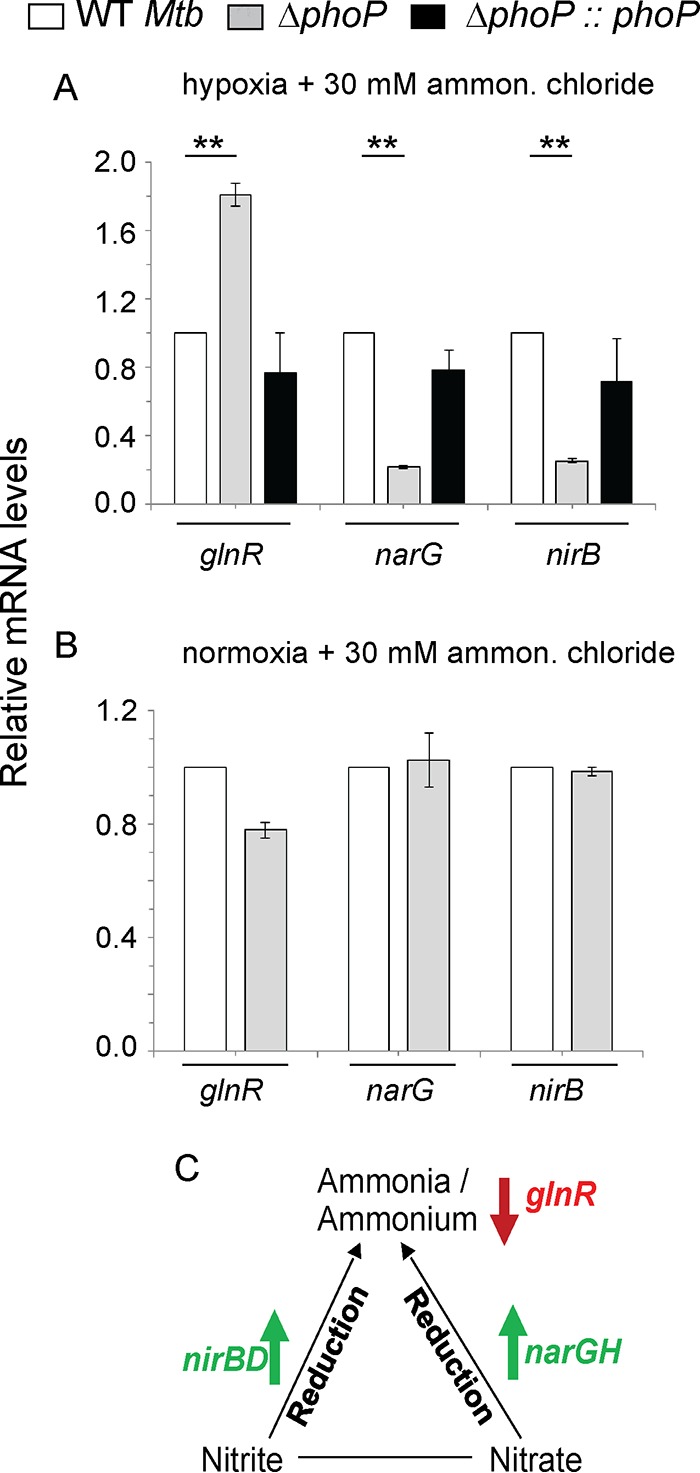
Regulation of genes related to nitrogen metabolism by the phoP locus. (A and B) RT-qPCR was carried out to compare expression levels of hypoxia-inducible genes in the WT, ΔphoP, and complemented mutant strains under specific and indicated conditions of growth. The differences in mRNA levels with the standard deviations were determined from at least three independent RNA preparations (**, P < 0.01). (C) Scheme summarizing phoP-dependent regulation of nitrogen metabolism during hypoxia. While PhoP acts as an activator of nitrite and nitrate reductases (nirB and narG, respectively) during hypoxia (as shown by upward arrows), repression of glnR (as shown by a downward arrow) signifies lower scavenging of nitrogen source under oxygen austerity.
Regulation of hypoxia-responsive promoters by PhoP and DosR.
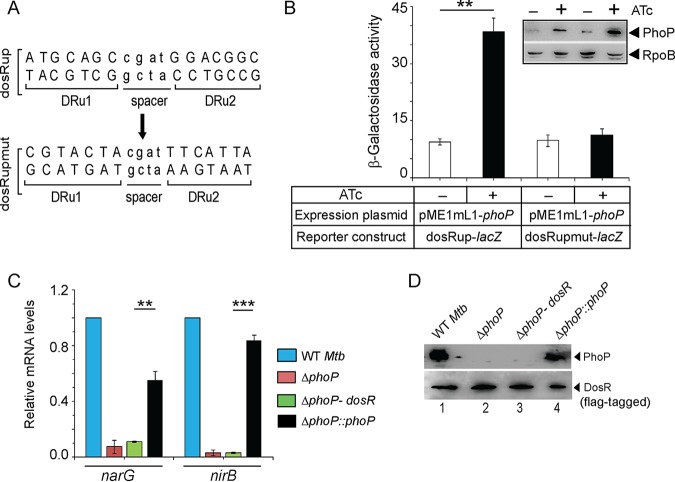
To examine PhoP-dependent dosR expression, we probed the PhoP binding site within the dosR promoter (dosRup, spanning positions –1438 to –774 relative to the start of the open reading frame [ORF]). Based on the knowledge of consensus PhoP binding site (32, 33) and more recent results on PhoP binding (24), we could locate a region spanning positions –1117 to –1100 within dosRup as the likely PhoP box. Next, mutant dosRup (dosRupmut) was generated by introducing mutations at the PhoP box, as shown in Fig. 3A, and transcriptional fusions of the WT (dosRup) and the mutant promoter (dsoRupmut) were cloned at the ScaI site of pSM128 (35), a promoter-less integrative lacZ reporter vector with a streptomycin resistance gene. M. tuberculosis PhoP was expressed in M. smegmatis by using pME1mL1-phoP as described previously (36) (see Materials and Methods for details). Consistent with phoP-dependent activation of dosR expression (Fig. 1), the dosRup-lacZ fusion showed a significant activation [(4 ± 0.9)-fold] of promoter activity with induction of phoP expression relative to the uninduced culture (Fig. 3B). However, dosRupmut-lacZ showed comparable β-galactosidase activity with or without induction of PhoP [a (1.2 ± 0.05)-fold difference]. The inset image in Fig. 3B compares M. tuberculosis PhoP expression in M. smegmatis harboring WT or the mutant promoter. These results establish that PhoP-dependent activation of dosR expression involves the above-noted direct repeat motif as the primary target site of PhoP.
FIG 3.
Regulatory mechanism of hypoxia-inducible promoter activity by PhoP. (A) PhoP binding direct repeat motif showing upstream (DRu1) and downstream (DRu2) repeat units. The mutant promoter of dosR carrying changes only at the PhoP binding site, dosRupmut, was generated by changing A’s to C’s and G’s to T’s (of both repeat units) and vice versa. Next, the orientation of the DRu2 sequence was reversed relative to DRu1. (B) M. smegmatis strains harboring either WT (dosRup-lacZ) or the mutant promoter (dosRupmut-lacZ) construct, along with PhoP expression plasmid (pME1mL1-phoP), were grown for 24 h in absence (–) or presence (+) of ATc, as an inducer of PhoP expression. The β-galactosidase activities were determined as described in Materials and Methods. The activities represent averages of multiple experiments with standard deviations from at least three independent cultures. The inset compares the expression of PhoP in ∼10 μg crude extracts as detected by anti-PhoP antibody; RpoB was used as the loading control. (C) FLAG-tagged DosR was cloned and expressed in mycobacterial expression vector p19Kpro (54) as described in Materials and Methods. To examine the effect of dosR expression in the ΔphoP mutant, the mRNA levels of hypoxia-responsive narG and nirB were determined in the indicated strains by RT-qPCR analyses, as described in the legend to Fig. 1. (D) To verify the expression of PhoP and DosR in the indicated strains, mycobacterial cell extracts containing comparable amount of total proteins were resolved by SDS-PAGE, and the presence of the regulators was confirmed by Western blotting with anti-PhoP and anti-FLAG antibodies, respectively.
To examine whether phoP-dependent regulation of hypoxic response is achieved via DosR expression, we next expressed dosR in the M. tuberculosis ΔphoP mutant and compared the expression levels of narG and nirB (Fig. 3C). The data unambiguously suggest that narG and nirB are significantly downregulated in a ΔphoP mutant under normoxic growth conditions. Clearly, ectopic expression of DosR (ΔphoP-dosR) was unable to restore narG and nirB expression, whereas the complemented ΔphoP mutant (ΔphoP::phoP) restored gene expression. These results suggest that activation of narG and nirB expression by PhoP is not attributable to PhoP dependent dosR activation under normoxia (Fig. 1A). Figure 3D confirms the expression of regulators in the indicated strains. From these results, we conclude that PhoP-dependent dosR expression cannot account for its regulation of hypoxic response. This observation fits well with the above finding that PhoP-dependent regulation of hypoxia-inducible genes (dosR, hspX, and narK2) is limited to normoxia only (Fig. 1) and thus possibly remain unlinked to the hypoxic response of M. tuberculosis.
Since both PhoP and DosR were shown to regulate hypoxia-responsive genes, we next compared the in vivo recruitment of DosR within hypoxia-responsive promoters of WT and ΔphoP strains (Fig. 4A). This is because the hypoxia-responsive narG, nirB, and glnR genes showed a phoP-dependent expression during hypoxia (Fig. 1). Note that despite a comparable level of DosR in the WT and ΔphoP strains under hypoxia (inset in Fig. 4A), we observed a significantly lower DosR recruitment, approximately 2- to 6-fold, within the target promoters in the ΔphoP bacilli relative to the WT bacilli. Thus, the presence of PhoP is necessary for effective recruitment of DosR within the hypoxia-inducible promoters involved in mycobacterial N2 metabolism. Notably, this finding is consistent with the previously reported dosR-dependent regulation of narG expression (13).
FIG 4.
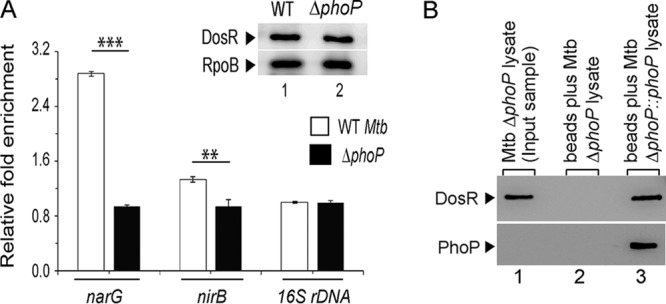
Recruitment of PhoP and DosR within hypoxia-inducible promoters. (A) In vivo experiments compared the recruitment of DosR in WT and ΔphoP strains grown under hypoxia. ChIP-qPCR experiments utilized anti-FLAG antibodies (Thermo Scientific) to determine the fold enrichments with respect to mock IP (without adding antibody) sample, as described in Materials and Methods, and the inset compares DosR expression in 10-μg crude cell lysates; RpoB was used as the loading control. **, P < 0.01; *, P < 0.05. (B) Crude cell lysates of ΔphoP strains expressing His6-tagged PhoP (p19kpro-phoP) (Table S3) were incubated with Ni-NTA and eluted with 200 mM imidazole. Lane 1, input sample; lane 2, elution from the crude lysate of cells lacking phoP expression; lane 3, detection of DosR coelution with PhoP. The upper and lower panels identify DosR and PhoP by using anti-DosR and anti-His antibodies, respectively, and Western blotting as described in Materials and Methods.
Considering these results, the recently reported PhoP-DosR interaction (24) emerged as a possible explanation for the regulation of hypoxia-inducible genes. To examine this, a whole-cell lysate of the ΔphoP mutant expressing His-tagged PhoP, as described previously (37), was incubated with Ni-nitrilotriacetic acid (Ni-NTA), and proteins were eluted from an Ni-NTA column using imidazole (Fig. 4B). While the eluent showed clear presence of DosR (lane 3), we were unable to detect DosR from the cell lysate of the ΔphoP mutant carrying the empty vector (p19Kpro) (lane 2; see Table S3). In agreement with the PhoP-DosR interaction recently reported by Vashist et al. (24), these results suggest a specific in vivo interaction between PhoP and DosR.
Next, we utilized a mycobacterial protein fragment complementation (M-PFC) assay to examine PhoP-DosR interaction using M. smegmatis as the surrogate host (Fig. S3) (see Table S3 for the plasmids used in this study). In agreement with results reported previously (24), our results also showed specific interaction between PhoP and DosR (Fig. S3A). However, under identical conditions, we were unable to detect interaction between PhoP and GlnR (Fig. S3B), suggesting that the regulation of narG and nirB expression during hypoxia is most likely GlnR independent. Using phosphorylation-defective mutants of PhoP and DosR (PhoPD71N and DosRD54N, respectively), we further showed that phosphorylation of the response regulators do not appear to influence PhoP-DosR protein-protein contacts (Fig. S3C and D).
Probing PhoP-DosR protein-protein interactions.
To investigate interacting domains of PhoP and DosR, we next performed in vitro protein-protein interaction analysis using glutathione S-transferase (GST)–DosR and the domains of His6-tagged PhoP (Fig. 5A), previously shown to be functioning on their own (38). In pulldown assays, GST-DosR was immobilized on glutathione-Sepharose, followed by incubation with His6-tagged PhoP domains (Fig. 5A). Upon elution, PhoPN (comprising PhoP residues 1 to 141) (Fig. 5A, lane 1) is coeluted with GST-DosR from the bound glutathione-Sepharose by 10 mM glutathione (similar to PhoP [lane 4]). However, under identical conditions, none of the PhoP C domain constructs (PhoPC1 [comprising residues 141 to 247] or PhoPC2 [comprising residues 150 to 247]) is coeluted with GST-DosR (lanes 2 and 3).
FIG 5.
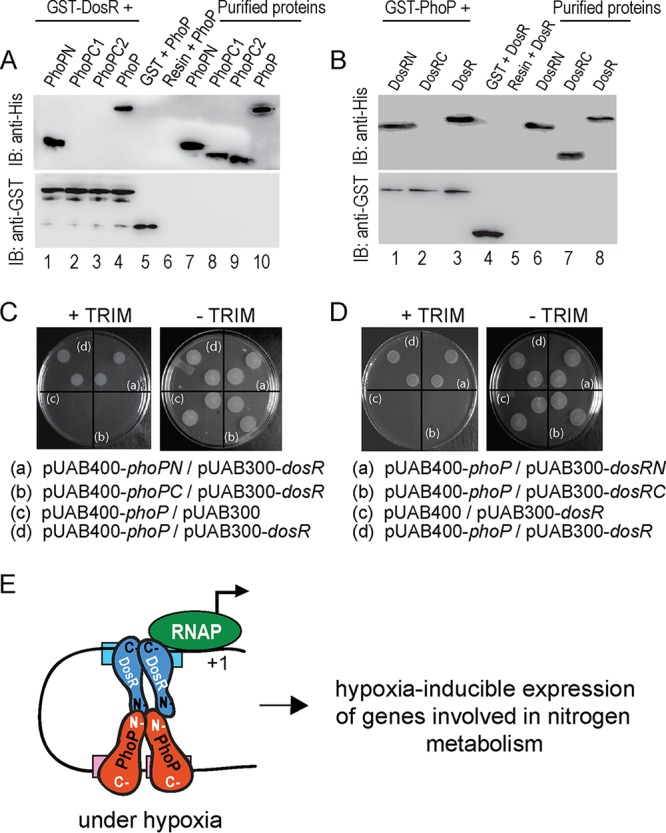
Probing interacting domains of PhoP and DosR. (A) The indicated His6-tagged PhoP domains were incubated with glutathione-Sepharose, previously immobilized with GST-DosR. Fractions of bound proteins (lanes 1 to 4) were analyzed by anti-His (upper panel) or anti-GST antibody (lower panel). Control sets include GST alone (lane 5) or the resin alone (lane 6); lanes 7 to 10 resolve purified PhoP constructs. (B) Likewise, indicated His6-tagged DosR domains were incubated with glutathione-Sepharose previously immobilized with GST-PhoP. Fractions of bound proteins (lanes 1 to 3) were probed with anti-His or anti-GST antibody (as described above). Control sets include GST alone (lane 4) or the resin alone (lane 5); lanes 6 to 8 resolved purified DosR constructs. The results suggest that DosRN and not DosRC retains the ability to interact with PhoP. (C and D) M-PFC experiments examined interactions between the indicated PhoP domains and DosR (C) or between PhoP and indicated DosR domains (D), respectively, using the full-length PhoP/DosR pair as the positive control. (E) Model depicting how PhoP and DosR function as coactivators of hypoxia-inducible gene expression (right). While PhoP-DosR interaction via their received domains contributed to the additional stability of the transcription initiation complex, mycobacterial RNA polymerase bound to the target site of the promoter to initiate transcription.
To identify the corresponding interacting domain of DosR, we used GST-PhoP and His-tagged DosR domain constructs in pulldown assays similar to those described above (Fig. 5B; see Table S3). Interestingly, DosRN (comprising residues 1 to 193) coeluted with GST-PhoP (lane 1); however, DosRC (comprising residues 143 to 217) under identical conditions, did not coelute with GST-PhoP (lane 2), suggesting a specific interaction between DosRN and PhoP. Figure S4 shows the purified form of the recombinant regulators (and their domains) used in these experiments. Consistent with these results, M. smegmatis cells coexpressing PhoPN and DosR (Fig. 5C), as well as PhoP and DosRN (Fig. 5D), pairs grew well on trimethoprim (TRIM) plates. However, cells coexpressing PhoPC/DosR or PhoP/DosRC pairs failed to grow on TRIM plates (compare Fig. 5C and D). Note that all of these cells grew well on plates lacking TRIM. Together, these results suggest that the N domain of PhoP interacts with the N domain of DosR. Based on the promoter occupancy of PhoP (23, 32) and DosR (16, 39) and the orientation of the two interacting regulators, Fig. 5E summarizes a regulatory scheme showing coactivation of hypoxia-inducible genes (involved in N2 metabolism) by PhoP and DosR.
phoP-dependent growth restoration of M. tuberculosis under hypoxia.
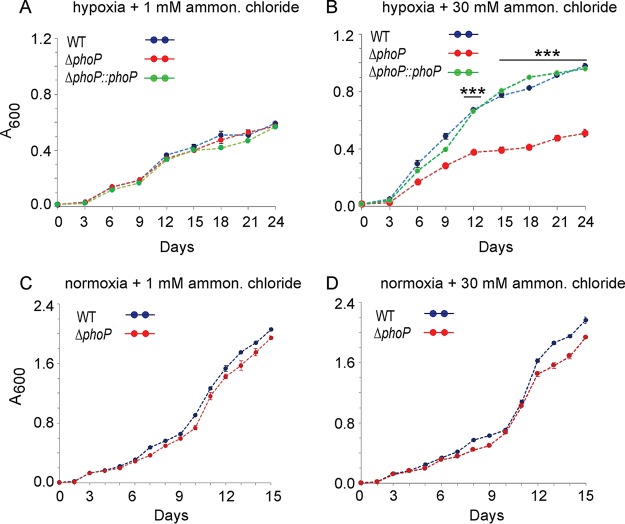
With the results showing a striking impact of PhoP on expression of hypoxia-inducible genes involved in N2 metabolism, we sought to examine growth of WT and ΔphoP strains under hypoxia (Fig. 6). When compared under hypoxia coupled with N2-limiting conditions, both strains showed comparably limited growth (Fig. 6A). However, under hypoxia coupled with N2 surplus conditions, WT bacilli showed significant growth restoration (Fig. 6B). In striking contrast, the M. tuberculosis ΔphoP mutant strain under identical conditions failed to resume growth, as had the WT bacilli, and consistently showed a lower growth [(2 ± 0.1)-fold] relative to the WT bacilli. Importantly, growth defect of the ΔphoP mutant could be restored to the WT level with the complementation of the mutant (see Fig. S5), underscoring the importance of phoP in utilizing an available nitrogen resource during hypoxia. As controls, under normoxia, the WT and ΔphoP strains grew comparably well regardless of the available ammonium chloride concentration (Fig. 6C and D). We conclude that PhoP, under hypoxic conditions, integrates nitrogen metabolism and hypoxia.
FIG 6.
Mycobacterial growth under hypoxia coupled with surplus or limited nitrogen availability. Growth curves of indicated strains were compared under hypoxia (A and B) or normoxia (C and D), coupled with either limiting nitrogen (1 mM NH4Cl) or surplus nitrogen (30 mM NH4Cl) conditions. The values represent averages from replicate experiments with standard deviations from at least three independent cultures (***, P < 0.001).
DISCUSSION
M. tuberculosis, during infection, survives in environments with oxygen-limiting conditions, and yet the mechanisms that promote survival of the metabolically inactive bacilli remain largely unknown. The fact that oxygen pressure within granulomas remains low (40, 41), yet reactivation from latency takes place within oxygen-rich sites of the lung, suggests that oxygen availability remains the key determinant. In keeping with this, mycobacterial growth both in vitro and in vivo is strongly influenced by the available oxygen pressure (2, 42), suggesting hypoxia as one of the major trigger factors of latency and reactivation.
Although hypoxia is known to increase nitrate reduction in M. tuberculosis (7), mechanism of regulation of nitrogen metabolism during oxygen austerity remained obscure. While DosR remains essential for M. tuberculosis adaptation and survival under hypoxic environment, PhoP coordinates pH homeostasis via regulation of pH-inducible gene expression. Interestingly, DosR was recently implicated in M. tuberculosis growth at low pH under anaerobic conditions (25). Thus, we sought to investigate role of PhoP on hypoxic response of M. tuberculosis. Results reported in this study suggest that PhoP activates expression of mycobacterial genes related to N2 metabolism under oxygen-limiting conditions with effective assistance from the hypoxia regulator DosR. Together, our results provide a new biological insight showing metabolic switching of M. tuberculosis in response to hypoxia by the convergence of two major response regulators.
It is noteworthy that M. tuberculosis, which evolved as an obligate human pathogen, has lost the majority of the regulatory pathways that are part of the metabolism of easily available nutrients. Thus, the nitrogen metabolism regulon is largely restricted to nitrite and nitrate reductases, making available the most likely nitrogen sources within the host (43). Notably, nitrate remains the final electron acceptor in lieu of oxygen to support growth of many bacteria during oxygen austerity. Therefore, hypoxia-induced nonreplicating persistence in M. tuberculosis is accompanied by nitrate reduction as a way to maintain redox balance and save the energy reservoir during downshift (8). In keeping with these results, under hypoxia, (i) activation of nirBD and narGHIJ loci, which function in nitrite and nitrate reductions, respectively, and (ii) repression of glnR, which controls nitrogen scavenging (43) by the phoP locus, unravel a strikingly novel role of PhoP in nitrogen metabolism during hypoxia.
Considering the involvement of both the regulators, it was of interest to examine whether these two are functionally connected. Clearly, the recruitment of PhoP and DosR (Fig. 4), most likely controlled by protein-protein interactions (Fig. 4), regulates the activation of hypoxia-inducible genes and provides an integrated view of our results. Under such a situation, for a more effective functioning DosR recruitment is possibly ensured within target promoters already bound by PhoP. Based on the involvement of both regulators, coupled with our findings that the respective N domains interact with each other (Fig. 5A to D), we propose a model (Fig. 5E) suggesting how PhoP-DosR interaction controls precise regulation of hypoxic response, a key step in the intracellular survival of M. tuberculosis. These considerations take on more significance in the light of previously identified PhoP and DosR binding sites within the aforementioned promoters. The arrangement and spacing between the binding sites are strongly indicative of DNA looping, possibly to assist transcription initiation. Together, these results (i) suggest a critical role of PhoP in binding and transcriptional control of narG and nirB and (ii) account for why DosR could not be recruited at these promoters in the ΔphoP M. tuberculosis strain (Fig. 4B), a finding that fits well with the previously reported PhoP-DosR interaction data (24) (Fig. 4). Although the model (Fig. 5E) shows an equimolar PhoP and DosR in the ternary complex, there is no evidence to suggest 1:1 binding stoichiometry. However, as independent regulators, in both cases a dimer of PhoP or DosR has been shown to bind DNA (16, 23, 39). Based on the chromatin immunoprecipitation (ChIP) results (Fig. 4), DosR remains ineffective in the ΔphoP mutant and therefore the ΔphoP mutant is expected to display a growth defect under hypoxia. However, we found comparably limited growth by both the WT and the mutant bacteria under hypoxia (Fig. 6A). We argue that PhoP-DosR interaction-dependent regulation possibly controls a few critical genes related to nitrogen metabolism, and therefore a significant growth defect of the ΔphoP mutant relative to the WT bacilli is apparent only when surplus nitrogen is available during oxygen austerity (Fig. 6B).
The integration of two signaling systems, either similar systems or systems of different types, has been shown in numerous other biological systems. An earlier study demonstrates the role of PknH in the phosphorylation of DosR, which is necessary for complete induction of the M. tuberculosis dosR regulon (44). Although these results suggest the convergence of two different types of signaling modules for a common function, along a similar line, DosR interacts with the housekeeping sigma factor SigA for bacterial survival under dormancy (18). Recently, we showed that PhoP interacts with the nucleoid-associated protein EspR to control ESAT-6 secretion (37). In the present study, we show that N domains that are phosphorylated for activation of respective regulators interact with each other (Fig. 5). This is in sharp contrast to recently reported PhoP-HspR and PhoP-HrcA interactions, where PhoPN was shown to interact with the C-terminal ends of mycobacterial heat shock repressors (45). Although the phosphorylation of either of the regulators does not seem to influence PhoP-DosR protein-protein contacts (see Fig. S3C and D in the supplemental material), the involvement of additional regulatory control by the N domains other than phosphorylation (Fig. 5), which enables appropriate in vivo functioning of the regulators via protein-protein contacts (Fig. 4), offers a new mechanistic insight. However, based on these results we cannot rule out the involvement of PhoR and DosS/DosT during hypoxia. In fact, a previous study suggests cross talk between the two signaling systems (46), suggesting that phosphorylation by a noncognate kinase(s) may even have in vivo significance. Given the facts that complex regulation of the level of phosphorylated DosR is determined by the balancing act of kinase/phosphatase functions of DosS (47) and that multiple signals are sensed by DosS and DosT (12, 14, 48, 49) to activate DosR, based on the results presented above we cannot completely exclude the possibility of the phosphorylation of DosR playing a role in PhoP-DosR interaction. Clearly, more experiments are needed to assess how specific stress conditions phosphorylate PhoP and DosR and coordinate their functioning to support mycobacterial survival in a phagosomal environment.
Since several examples establish that either two different or similar regulatory systems are often integrated toward a common regulatory function, integration via a single interaction (or lack thereof) possibly could have a large or a small impact on the transcriptional control of different target promoters. It is conceivable that a diversity of interactions at numerous promoters (belonging to various regulons) may significantly enhance the available combinations of potential regulatory interactions to fine-tune context-dependent gene expression. Since a hypoxic response is believed to play a major role in dormancy adaptation, DosR activation is associated with metabolic changes where the tubercle bacilli move into a nonreplicating persistent state. Therefore, it is not too difficult to imagine that control mechanisms (for example, PhoP-DosR interactions) exist that interfere with bacterial persistence unless an appropriate signal is recognized. In fact, this is expected, since activation of the regulon consisting of ∼48 genes would otherwise be energy demanding, and it therefore appears that a second molecular control system (in addition to DosS/DosT) is in place to regulate the induction of DosR only under the most appropriate stress conditions.
In conclusion, we have identified a signaling mechanism in M. tuberculosis that regulates hypoxia-inducible genes related to nitrogen metabolism. To our knowledge, this is the first report of a regulatory pathway that links the virulence regulator PhoP to the expression of nitrite and nitrate reductases under hypoxia. This study explains, at least in part, a fundamental mechanism of metabolic switching underlying how nitrogen metabolism genes are activated for survival of M. tuberculosis under oxygen austerity for a long period of time.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli DH5α and E. coli BL21(DE3) strains were grown at 37°C in Luria-Bertani medium containing appropriate antibiotics and used for cloning and for the overexpression of mycobacterial proteins, respectively. Although the ΔphoP mutant and the complemented mutant have been described previously (31), the ΔdosR mutant and the complemented mutant are also described below. M. tuberculosis strains were grown aerobically at 37°C in Middlebrook 7H9 liquid broth (containing 0.2% glycerol, 0.05% Tween 80, and 10% albumin-dextrose-catalase) or on 7H10 agar medium (containing 0.5% glycerol and 10% oleic acid-albumin-dextrose-catalase [OADC]). For growth under hypoxic conditions, stock cultures were aerobically subcultured twice to mid-log phase (A600 of 0.3 to 0.4) in Dubos (Difco) medium supplemented with 0.045% Tween 80, 10% albumin, and dextrose and subsequently inoculated into fresh medium at an A600 of 0.01. Oxyrase was used to remove excess oxygen, and 1.5 μg/ml methylene blue was added as an indicator of oxygen depletion (7). M. tuberculosis growth under nitrogen excess or nitrogen-limiting conditions are as described previously (43), and the expression of nirB was measured by quantitative RT-PCR (RT-qPCR) using RNA from three independent cultures with gapdh as an internal control. Briefly, M. tuberculosis strains were grown as described above, washed twice in nitrogen-free Dubos medium, and inoculated in the same medium supplemented with either 1 mM (nitrogen limiting) or 30 mM (nitrogen surplus) ammonium chloride (Sigma). Transformation of WT and mutant M. tuberculosis strains and selection of transformants on appropriate antibiotics were carried out as described previously.
RNA isolation and quantitative real-time RT-PCR.
Total RNA from M. tuberculosis was extracted as described previously (29) using exponentially growing bacterial cultures grown with or without stress. After extraction with chloroform-isoamyl alcohol, RNA was precipitated with chilled ethanol. Finally, RNA was treated with RNase-free DNase I (Invitrogen) for 20 min at room temperature to remove genomic DNA contamination. The integrity of the RNA samples was checked by determining the intactness of 23S and 16S rRNA using formaldehyde-agarose gel electrophoresis. RNA concentrations were determined by measuring the A260 and stored at –80°C.
cDNA synthesis and PCRs using appropriate PAGE-purified primer pairs (200 nM) (see Table S1 in the supplemental material) were performed in an Applied Biosystems real-time PCR detection system using a Superscript III platinum-SYBR green one-step qRT-PCR kit (Invitrogen) and cycling conditions as described previously (29). For each pair of primers, a standard curve was generated using serially diluted RNA samples, and the PCR efficiency was evaluated. The average fold change in the expression of each sample relative to endogenously expressed M. tuberculosis gapdh (Rv1436) was calculated by the ΔΔCT method (50). Note that the CT values for gapdh remained mostly unchanged under variable conditions of M. tuberculosis growth and that standard deviations were derived from at least three independent RNA preparations. Platinum Taq DNA polymerase (Invitrogen) was used to confirm absence of genomic DNA in our RNA preparations.
Cloning.
Isolation and purification of nucleic acids, digestion with restriction enzymes, and analyses of nucleic acids or its fragments by agarose gel electrophoresis followed standard procedures. M. tuberculosis PhoP or its domain-specific overexpressing constructs have been described earlier (38). Likewise, the full-length DosR (encoding 654 bp) ORF and truncated DosR proteins (encoding the N-terminal 579 bp and C-terminal 222 bp of the dosR ORF) were cloned in T7-lac-based expression system pET28b (Novagen) as recombinant fusion proteins containing an N-terminal His6 tag. The cloning strategy resulted in pET-dosR, pET-dosRN, and pET-dosRC comprising 217-amino-acid full-length DosR, 193-amino-acid DosRN (lacking the C-terminal 24 residues), and 74-residue DosRC (lacking the N-terminal 142 residues), respectively (51). Plasmid pGEX-dosR expressing DosR with an N-terminal glutathione S-transferase (GST) tag was generated by cloning PCR-derived dosR ORF fragment between BamHI and XhoI sites of pGEX 4T-1 (GE Healthcare) as described previously for pGEX-phoP (52). To complement dosR expression in the respective mycobacterial mutant, the ORF was cloned and expressed in the mycobacterial expression vector pSTKi (53). To express FLAG-tagged dosR in M. tuberculosis, the ORFs were cloned and expressed in the mycobacterial expression vector p19Kpro (54). Point mutations in phoP and dosR genes were introduced by the two-stage overlap extension method and verified by DNA sequencing. The oligonucleotide primers used in cloning and construction of the plasmids are provided in Tables S2 and S3, respectively.
Promoter regulation by M. tuberculosis PhoP in M. smegmatis.
M. smegmatis strains carrying indicated lacZ fusions and pME1mL1-phoP, expressing M. tuberculosis PhoP under the Pmyc1tetO promoter and the TetR repressor (or no expression plasmid as control), were grown either in absence or in the presence of 50 ng/ml anhydrotetracycline (ATc) as described previously (55). To determine promoter activity, cells from both induced and uninduced cultures were grown for 24 h, cell suspensions were sonicated, and the β-galactosidase activity of the extracts was determined. To assess TetR-dependent PhoP expression, crude cell lysates of M. smegmatis (∼10 μg of protein) were resolved by 12% SDS-PAGE and probed with anti-PhoP antibody (AlphaOmega Sciences, India).
Proteins.
M. tuberculosis PhoP and its domains were expressed and purified as described previously (38). Full-length and truncated DosR proteins were expressed in E. coli BL21(DE3) as fusion proteins containing an N-terminal His6 tag and purified by immobilized metal affinity chromatography (Ni-NTA; Qiagen). Full-length DosR was also expressed with N-terminal GST tag, as described for GST-PhoP (56). Finally, the proteins were stored in buffer containing 50 mM Tris-HCl (pH 7.9), 300 mM NaCl, and 10% glycerol. In all cases, the purity was verified by SDS-PAGE, and protein concentrations were determined by Bradford reagent with BSA as the standard, and expressed in equivalent of protein monomers.
Immunoblotting.
Crude cell lysates of M. tuberculosis were resolved by 12% SDS-PAGE and visualized by Western blotting. For immunoblotting, resolved samples were electroblotted onto polyvinyl difluoride (polyvinylidene difluoride) membranes (Millipore) and were detected by affinity-purified antibodies elicited in rabbit (AlphaOmega Sciences). RNA polymerase, used as a loading control, was detected by anti-RpoB (Abcam). Anti-His and anti-GST antibodies were from GE Healthcare. Goat anti-rabbit and goat anti-mouse secondary antibodies conjugated to horseradish peroxidase were procured from AlphaOmega Sciences, and blots were developed with Luminata Forte chemiluminescence reagent (Millipore).
ChIP-qPCR.
M. tuberculosis was grown as described above and processed for ChIP experiments using purified anti-PhoP (Alpha Omega Sciences) or anti-FLAG (Thermo Scientific) antibody and protein A/G-agarose beads (Pierce) as described previously (57). qPCR was performed using PAGE purified primer pairs (Sigma) (Table S1) that spanned appropriate promoter regions of interest. PCR mix contained suitable dilutions of immunoprecipitation (IP) DNA in a reaction buffer containing SYBR green mix and specific primers (200 nM). An IP experiment without adding antibody (mock) served as a negative control, and data were normalized against PCR signal from a mock-treated sample. Typically, 40 cycles of amplification were carried out using an Applied Biosystems real-time PCR detection system with serially diluted DNA samples (mock, IP treated, and total input). Melting curve analysis was carried out to confirm amplification of a single product in all cases. Enrichment of PCR signal from anti-PhoP or anti-FLAG IP relative to the signal from an IP experiment without adding any antibody (mock) was measured to determine efficiency of recruitment. Specific PCR enrichment was ensured by performing ChIP-qPCR of the identical IP samples using gapdh/16S rRNA gene-specific primers. Each data point represents the mean of duplicate qPCR measurements using at least three independent M. tuberculosis cultures.
Mycobacterial protein fragment complementation assays.
To express M. tuberculosis PhoP or its domains in M. smegmatis, phoP and/or its appropriate domain constructs were cloned in the integrative vector pUAB400 (Kanr) (Table S3) between MfeI and HindIII sites, as described previously (56). Similarly, dosR, glnR, and truncated dosR ORFs were cloned in episomal plasmid pUAB300 (Hygr) (Table S3) between BamHI/HindIII sites to generate pUAB300-dosR, pUAB-glnR, pUAB300-dosRN, and pUAB300-dosRC, respectively. Next, cotransformed cells were selected on 7H10/Kan/Hyg plates in the absence or presence of 15 μg/ml trimethoprim (TRIM; Sigma), as described previously (58). In this assay, two interacting proteins as separate fusion constructs of two domains of murine dihydrofolate reductase (mDHFR) when coexpressed in M. smegmatis reconstitute functional mDHFR and enable the bacteria to grow on medium containing TRIM. As a positive control, ESAT-6/CFP-10-expressing constructs were used in the M-PFC experiments. Note that all of the strains used in this study grew well in the absence of TRIM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Adrie Steyn for pUAB300 and pUAB400 plasmids, G. Marcela Rodriguez and Issar Smith for the ΔphoP and complemented mutant strains, Harsh Goar for purification of recombinant DosR, and Ashwani Kumar for very helpful discussions.
This study was supported by a research grant (to D.S.) from the SERB-Department of Science and Technology (DST; EMR/2016/004904), Government of India, and by intramural funding from CSIR-IMTECH. P.R.S. and V.A.K. received financial support from DST and CSIR, respectively.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rustad TR, Sherrid AM, Minch KJ, Sherman DR. 2009. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol 11:1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 3.Wayne LG, Sohaskey CD. 2001. Nonreplicating persistence of mycobacterium tuberculosis. Annu Rev Microbiol 55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat Immunol 14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson S, Bonecini-Almeida MG, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JL. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med 183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. 2002. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med 166:178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 7.Sohaskey CD, Wayne LG. 2003. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol 185:7247–7256. doi: 10.1128/jb.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne LG, Hayes LG. 1998. Nitrate reduction as a marker for hypoxic shift-down of Mycobacterium tuberculosis. Tuberc Lung Dis 79:127–132. doi: 10.1054/tuld.1998.0015. [DOI] [PubMed] [Google Scholar]
- 9.Wayne LG, Doubek JR. 1965. Classification and identification of mycobacteria. II. Tests employing nitrate and nitrite as substrate. Am Rev Respir Dis 91:738–745. [DOI] [PubMed] [Google Scholar]
- 10.Rachman H, Strong M, Ulrichs T, Grode L, Schuchhardt J, Mollenkopf H, Kosmiadi GA, Eisenberg D, Kaufmann SH. 2006. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect Immun 74:1233–1242. doi: 10.1128/IAI.74.2.1233-1242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenhalls G, Stevens L, Moses L, Bezuidenhout J, Betts JC, Helden Pv P, Lukey PT, Duncan K. 2002. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect Immun 70:6330–6338. doi: 10.1128/iai.70.11.6330-6338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioanoviciu A, Meharenna YT, Poulos TL, Ortiz de Montellano PR. 2009. DevS oxy complex stability identifies this heme protein as a gas sensor in Mycobacterium tuberculosis dormancy. Biochemistry 48:5839–5848. doi: 10.1021/bi802309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini DK, Malhotra V, Dey D, Pant N, Das TK, Tyagi JS. 2004. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150:865–875. doi: 10.1099/mic.0.26218-0. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan S, Tyagi JS. 2008. Cooperative binding of phosphorylated DevR to upstream sites is necessary and sufficient for activation of the Rv3134c-devRS operon in Mycobacterium tuberculosis: implication in the induction of DevR target genes. J Bacteriol 190:4301–4312. doi: 10.1128/JB.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauhan S, Tyagi JS. 2009. Powerful induction of divergent tgs1-Rv3131 genes in Mycobacterium tuberculosis is mediated by DevR interaction with a high-affinity site and an adjacent cryptic low-affinity site. J Bacteriol 191:6075–6081. doi: 10.1128/JB.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautam US, Sikri K, Vashist A, Singh V, Tyagi JS. 2014. Essentiality of DevR/DosR interaction with SigA for the dormancy survival program in Mycobacterium tuberculosis. J Bacteriol 196:790–799. doi: 10.1128/JB.01270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalo-Asensio J, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, Winter N, Daffe M, Gicquel B, Martin C, Jackson M. 2006. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem 281:1313–1316. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalo-Asensio J, Mostowy S, Harders-Westerveen J, Huygen K, Hernández-Pando R, Thole J, Behr M, Gicquel B, Martín C. 2008. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One 3:e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryndak M, Wang S, Smith I. 2008. PhoP, a key player in Mycobacterium tuberculosis virulence. Trends Microbiol 16:528–534. doi: 10.1016/j.tim.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. 2013. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X, Wang S. 2014. DNA consensus sequence motif for binding response regulator PhoP, a virulence regulator of Mycobacterium tuberculosis. Biochemistry 53:8008–8020. doi: 10.1021/bi501019u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vashist A, Malhotra V, Sharma G, Tyagi JS, Clark-Curtiss JE. 2018. Interplay of PhoP and DevR response regulators defines expression of the dormancy regulon in virulent Mycobacterium tuberculosis. J Biol Chem 293:16413–16425. doi: 10.1074/jbc.RA118.004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichlen MJ, Leistikow RL, Scobey MS, Born SEM, Voskuil MI. 2017. Anaerobic Mycobacterium tuberculosis cell death stems from intracellular acidification mitigated by the DosR regulon. J Bacteriol 199:e00320-17. doi: 10.1128/JB.00320-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramovitch RB, Rohde KH, Hsu FF, Russell DG. 2011. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol Microbiol 80:678–694. doi: 10.1111/j.1365-2958.2011.07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JJ, Johnson BK, Abramovitch RB. 2014. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol Microbiol 94:56–69. doi: 10.1111/mmi.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S, Sukumar N, Abramovitch RB, Parish T, Russell DG. 2013. Mycobacterium tuberculosis responds to chloride and pH as synergistic cues to the immune status of its host cell. PLoS Pathog 9:e1003282. doi: 10.1371/journal.ppat.1003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal R, Anil Kumar V, Sevalkar RR, Singh PR, Sarkar D. 2017. Mycobacterium tuberculosis virulence-regulator PhoP interacts with alternative sigma factor SigE during acid-stress response. Mol Microbiol 104:400–411. doi: 10.1111/mmi.13635. [DOI] [PubMed] [Google Scholar]
- 30.Malm S, Tiffert Y, Micklinghoff J, Schultze S, Joost I, Weber I, Horst S, Ackermann B, Schmidt M, Wohlleben W, Ehlers S, Geffers R, Reuther J, Bange FC. 2009. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology 155:1332–1339. doi: 10.1099/mic.0.023275-0. [DOI] [PubMed] [Google Scholar]
- 31.Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol 60:312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 32.Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martín C, Cole ST. 2014. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog 10:e1004183. doi: 10.1371/journal.ppat.1004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X, Wang L, Wang S. 2016. Structural basis of DNA sequence recognition by the response regulator PhoP in Mycobacterium tuberculosis. Sci Rep 6:24442. doi: 10.1038/srep24442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins VA, Barton GR, Robertson BD, Williams KJ. 2013. Genome-wide analysis of the complete GlnR nitrogen-response regulon in Mycobacterium smegmatis. BMC Genomics 14:301. doi: 10.1186/1471-2164-14-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dussurget O, Timm J, Gomez M, Gold B, Yu S, Sabol SZ, Holmes RK, Jacobs WR Jr, Smith I. 1999. Transcriptional control of the iron-responsive fxbA gene by the mycobacterial regulator IdeR. J Bacteriol 181:3402–3408. doi: 10.1128/JB.181.11.3402-3408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrt S, Guo XV, Hickey CM, Ryou M, Monteleone M, Riley LW, Schnappinger D. 2005. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anil Kumar V, Goyal R, Bansal R, Singh N, Sevalkar RR, Kumar A, Sarkar D. 2016. EspR-dependent ESAT-6 protein secretion of Mycobacterium tuberculosis requires the presence of virulence regulator PhoP. J Biol Chem 291:19018–19030. doi: 10.1074/jbc.M116.746289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathak A, Goyal R, Sinha A, Sarkar D. 2010. Domain structure of virulence-associated response regulator PhoP of Mycobacterium tuberculosis: role of the linker region in regulator-promoter interaction(s). J Biol Chem 285:34309–34318. doi: 10.1074/jbc.M110.135822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauhan S, Tyagi JS. 2008. Interaction of DevR with multiple binding sites synergistically activates divergent transcription of narK2-Rv1738 genes in Mycobacterium tuberculosis. J Bacteriol 190:5394–5403. doi: 10.1128/JB.00488-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol 8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 41.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE III. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun 76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wayne LG. 1977. Synchronized replication of Mycobacterium tuberculosis. Infect Immun 17:528–530. doi: 10.1128/IAI.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams KJ, Jenkins VA, Barton GR, Bryant WA, Krishnan N, Robertson BD. 2015. Deciphering the metabolic response of Mycobacterium tuberculosis to nitrogen stress. Mol Microbiol 97:1142–1157. doi: 10.1111/mmi.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chao JD, Papavinasasundaram KG, Zheng X, Chavez-Steenbock A, Wang X, Lee GQ, Av-Gay Y. 2010. Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J Biol Chem 285:29239–29246. doi: 10.1074/jbc.M110.132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevalkar RR, Arora D, Singh PR, Singh R, Nandicoori VK, Karthikeyan S, Sarkar D, Sevalkar RR, Arora D, Singh PR, Singh R, Nandicoori VK, Karthikeyan S, Sarkar D. 2019. Functioning of mycobacterial heat shock repressors requires the master virulence regulator PhoP. J Bacteriol 201:e00013-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal R, Pandey A, Rajankar MP, Dixit NM, Saini DK. 2015. The two-component signalling networks of Mycobacterium tuberculosis display extensive cross-talk in vitro. Biochem J 469:121–134. doi: 10.1042/BJ20150268. [DOI] [PubMed] [Google Scholar]
- 47.Kaur K, Kumari P, Sharma S, Sehgal S, Tyagi JS. 2016. DevS/DosS sensor is bifunctional and its phosphatase activity precludes aerobic DevR/DosR regulon expression in Mycobacterium tuberculosis. FEBS J 283:2949–2962. doi: 10.1111/febs.13787. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Toledo JC, Patel RP, Lancaster JR Jr, Steyn AJ. 2007. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc Natl Acad Sci U S A 104:11568–11573. doi: 10.1073/pnas.0705054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vos MH. 2008. Ultrafast dynamics of ligands within heme proteins. Biochim Biophys Acta 1777:15–31. doi: 10.1016/j.bbabio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Gautam US, Chauhan S, Tyagi JS. 2011. Determinants outside the DevR C-terminal domain are essential for cooperativity and robust activation of dormancy genes in Mycobacterium tuberculosis. PLoS One 6:e16500. doi: 10.1371/journal.pone.0016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta S, Pathak A, Sinha A, Sarkar D. 2009. Mycobacterium tuberculosis PhoP recognizes two adjacent direct-repeat sequences to form head-to-head dimers. J Bacteriol 191:7466–7476. doi: 10.1128/JB.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parikh A, Kumar D, Chawla Y, Kurthkoti K, Khan S, Varshney U, Nandicoori VK. 2013. Development of a new generation of vectors for gene expression, gene replacement, and protein-protein interaction studies in mycobacteria. Appl Environ Microbiol 79:1718–1729. doi: 10.1128/AEM.03695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Smet KA, Kempsell KE, Gallagher A, Duncan K, Young DB. 1999. Alteration of a single amino acid residue reverses fosfomycin resistance of recombinant MurA from Mycobacterium tuberculosis. Microbiology 145:3177–3184. doi: 10.1099/00221287-145-11-3177. [DOI] [PubMed] [Google Scholar]
- 55.Goyal R, Das AK, Singh R, Singh PK, Korpole S, Sarkar D. 2011. Phosphorylation of PhoP protein plays direct regulatory role in lipid biosynthesis of Mycobacterium tuberculosis. J Biol Chem 286:45197–45208. doi: 10.1074/jbc.M111.307447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh R, Anil Kumar V, Das AK, Bansal R, Sarkar D. 2014. A transcriptional co-repressor regulatory circuit controlling the heat-shock response of Mycobacterium tuberculosis. Mol Microbiol 94:450–465. doi: 10.1111/mmi.12778. [DOI] [PubMed] [Google Scholar]
- 57.Fol M, Chauhan A, Nair NK, Maloney E, Moomey M, Jagannath C, Madiraju MV, Rajagopalan M. 2006. Modulation of Mycobacterium tuberculosis proliferation by MtrA, an essential two-component response regulator. Mol Microbiol 60:643–657. doi: 10.1111/j.1365-2958.2006.05137.x. [DOI] [PubMed] [Google Scholar]
- 58.Singh A, Mai D, Kumar A, Steyn AJ. 2006. Dissecting virulence pathways of Mycobacterium tuberculosis through protein-protein association. Proc Natl Acad Sci U S A 103:11346–11351. doi: 10.1073/pnas.0602817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.