Summary
Background
Intravenous magnesium sulfate, a rescue therapy added to bronchodilator and systemic steroid therapy for moderate and severe asthma, is uncommonly administered. We hypothesized that nebulized magnesium would confer benefit without undue risk.
Design and Methods
Patients aged 2 to 14 y with moderate and severe asthma (PRAM severity score ≥4) admitted to infirmary/observation unit care were randomized double‐blind on admission to receive 800 mg nebulized magnesium or normal saline placebo after all received intensive therapy with combined nebulized albuterol–ipratropium and intravenous methylprednisolone. Time to medical readiness for discharge was the primary outcome; sample size was chosen to detect a 15% absolute improvement. Improvement over time in PRAM severity score and other secondary outcomes were compared for the overall group and severe asthma subset.
Results
One hundred and ninety‐one magnesium sulfates and 174 placebo patients met criteria for analysis. The groups were similar with mean baseline PRAM scores >7. Blinded active therapy significantly increased blood magnesium level 2 hr post‐treatment completion compared to placebo, 0.85 vs 0.82 mmol/L, P = 0.001. There were no important adverse effects. Accelerated failure time analysis showed a non‐significantly shortened time to medical readiness for discharge of 14% favoring the magnesium sulfate group, OR = 1.14, 95% CI 0.93 to 1.40, P = 0.20. Mean times until readiness for discharge were 14.7 hr [SD 9.7] versuss 15.6 hr [SD 11.3] for the investigational and placebo groups, respectively, P = 0.41.
Conclusions
Adding nebulized magnesium to combined nebulized bronchodilator and systemic steroid therapy failed to significantly shorten time to discharge of pediatric patients with moderate or severe asthma. Pediatr Pulmonol. 2015; 50:1191–1199. © 2015 Wiley Periodicals, Inc.
Keywords: pediatric emergency, nebulization, bronchodilator
ABBREVIATIONS
- ICU
Intensive care unit
- PRAM
Pediatric respiratory assessment measurement
- PEC
Pediatric emergency centres
- PCR
Polymerase chain reaction
INTRODUCTION
Adding magnesium sulfate treatment to inhaled combined bronchodilator therapy and systemic corticosteroid for moderate or severe pediatric asthma is controversial despite repeated and intensive study over more than a decade.1, 2 British and Scottish guidelines recommend magnesium by infusion for adults3 but in children, safety concerns,4 reticence toward providing iv access solely for magnesium therapy,4 and lack of evidence for benefit5 leave practitioners facing pediatric patients with a GINA (Global Initiative for Asthma) recommendation that magnesium treatment “should be considered”. In children, intravenous magnesium is typically reserved as rescue treatment for severely ill patients. Nebulized magnesium therapy seems ineffective in adults with severe asthma.6 In a recent placebo‐controlled multicenter trial7 of nebulized magnesium therapy in children with severe asthma, the investigators considered a reduced asthma severity score one hour after magnesium treatment statistically significant but clinically insignificant, with no difference in time to discharge or rate of hospital admission. There continues to be speculation about asthmatic patients that nebulized magnesium might benefit.2, 5, 7
Qatar's busiest Pediatric Emergency Centre (PEC) experienced 24,703 acute asthma visits during 2013, 9% of our total visits for the year. We escalate to infirmary (i.e., short‐stay unit) admission for children failing to improve substantially with inhaled bronchodilator and oral steroid therapy (17% of PEC asthma visits in 2013) and transfer patients failing in the infirmary to the pediatric ICU (0.06% of PEC asthma visits in 2013), where they often require intubation. Nebulizing magnesium sulfate is inexpensive and were it proven therapeutic, could be readily generalizable to rich and poor communities alike worldwide for preventing a potential downhill clinical course during worrisome pediatric asthma patient presentations. Since moderate and severe asthma patients already have a prolonged length of stay, we tested nebulized magnesium added when therapy was initiated, rather than randomized as rescue therapy some time after bronchodilator and steroid therapy had been deemed to have failed. We sought to enroll children presenting with moderate or severe asthma in a blinded, placebo‐controlled trial that would ensure standard therapy to all, optimize aerosol technology,8 confirm systemic magnesium sulfate delivery, and test for a significant difference in time to medical readiness for discharge.
MATERIALS AND METHODS
Population
We chose the largest and busiest of the PECs, Al‐Sadd, which serves approximately 274,000 visits annually and has 42 infirmary beds for prolonged stays. Eligible subjects were 2 to 14 y, previously known to have asthma, with a moderate or severe exacerbation, defined as a PRAM asthma severity score ≥4.9 Asthma was defined for children between 2 and 5 y as having a previous physician diagnosis of asthma with previous history of eczema in the child or asthma in a first degree family relative to increase the likelihood of true asthma; for children 5 to14 y, a previous physician diagnosis of asthma was sufficient. Patients were excluded for prematurity (<34 wk gestation); critical illness on presentation requiring ICU admission for intravenous bronchodilator therapy, noninvasive or invasive ventilation, etc; if they were transferred from another institution; a history of hypersensitivity to magnesium sulfate; a history of neuromuscular, cardiac, or renal disease; underlying structural lung disease; receiving systemic steroid, theophylline, or ipratropium within the prior 72 hr; pneumonic consolidation on chest radiograph at presentation; receipt of intravenous magnesium sulfate prior to randomization; prior participation in this study; or hemodynamic instability.
Randomization and Study Interventions
A computer‐generated randomization list without blocks provided to the pharmacy resulted in preparation of identical‐appearing sealed numbered vials containing either 15 ml 0.9% sodium chloride or 800 mg magnesium sulfate in 15 ml. There is no agreed nebulized magnesium sulfate dose for use in children; 1500 mg was given in a study of 13–60 year‐olds10 and 450 mg was given in a recent landmark study of 2–16 year‐olds.7 Vials were kept in the clinical area and identified only by random number. There was no blocking or stratification. Other than the study pharmacists, who had no patient interaction and were unaware of treatment assignment to patients, all study personnel were blinded to treatment. After obtaining verbal and written informed consent from accompanying parents, patients admitted to infirmary care had baseline asthma score recorded by trained staff, a chest radiograph, an intravenous line inserted, methylprednisone 1 mg/kg iv every 12 hr begun, and 3 doses begun of nebulized 1 ml albuterol (5 mg/ml) with 250 mcg ipratropium bromide and 2 ml normal saline. The three combination bronchodilator doses were administered over 1 hr. Magnesium sulfate or placebo was not added to this first hour nebulization due to osmolarity considerations and volume limitations of the Idehaler. Therefore, this was followed in the next hour by nebulization of three 5‐mL doses of study medication (active or placebo), to which 1 ml of albuterol (5 mg/ml) had been added (6 ml/nebulization, 433 mOsm/L, three nebulizations during the second hour). Afterward, each patient received albuterol nebulizations hourly for 3 hr, then q 2 hr until ready for discharge from infirmary care. Additional albuterol nebulizations, three back‐to‐back, could be administered by decision of the treating physician. Nebulized medication was given by tight face mask with pressurized O2 at 10 L/min, except for study medication which was given by Aeroneb Pro with an Idehaler holding chamber. All study patients had a nasal swab for viral and mycoplasma PCR studies. Oxygen was given by face mask if needed to keep the O2 saturation ≥94%. Vital signs were recorded at baseline, 2, and 4 hr and every 4 hr thereafter. Asthma severity score was recorded at baseline, 4, 8, 12, 24, 36, 48, 60, and 72 hr unless the patient was discharged from the infirmary earlier. The time when a patient was medically ready for discharge, which required feeding well, comfortable breathing, an O2 saturation ≥94% on room air, and an asthma severity score ≤3, was recorded. At discharge, all patients were given prednisolone pills or syrup to complete 5 d of steroid treatment. Telephone call follow‐up by a study nurse was done for each study patient. Revisits to PEC and need for repeat infirmary care or hospitalization were recorded.
Outcome Measures
Time to medical readiness for discharge was the primary outcome measure. We chose this, rather than, for example, patients requiring admission, to be generalizable across hospitals with a variety of criteria for requiring admission. Secondary outcomes included asthma severity scores at pre‐specified times after randomization, need for revisit, readmission to infirmary care, and admission to pediatric ICU within 2 wk after discharge. To ascertain lower airway magnesium delivery in the treated group, blood for baseline magnesium level was to be obtained when the iv was inserted and 2 hr after magnesium sulfate nebulizations were completed (3 hr after they were begun).
Sample Size and Analysis
We observed that approximately 50% of similar patients were medically ready for discharge by 24 hr. To enable detection of a 15% absolute improvement for the investigational group in discharge readiness with 80% power and two‐sided alpha = 0.05, we estimated 180 patients per group required, and aimed to enroll 200 per group to account for dropouts and exclusions. While 90% power would provide more certainty, other groups also estimated sample size with 80% power.7
We planned to use an accelerated failure time model to compare efficacy in the treatment groups. A statistically significant (P < 0.05) acceleration (HR > 1.0) in time to clinical readiness for discharge would favor nebulized magnesium sulfate over placebo therapy. In order to study efficacy, we analyzed a per‐protocol population and excluded patients with protocol (e.g., inclusion or procedure) violations. Outcomes in the overall patient groups were compared, and the patient subset meeting PRAM criteria for a severe asthma attack were also collected for exploratory comparison. Categorical and continuous variables were expressed as percentage and mean with standard deviation (SD). Quantitative variable means between the two treatment groups were analyzed by unpaired t and Wilcoxon rank sum tests. Associations between qualitative and categorical variables were assessed by chi‐square testing, with the continuity correction for smaller cell frequencies. Statistical analyses were performed using SPSS (version 19.0r, IBM SPSS Statistics, IBM Corporation). Data was transferred from SPSS package to STATA SE 11.0, Chicago, IL to for the accelerated time failure model analysis.
The Hamad Medical Corporation Human Studies Committee approved the study and its informed consent form on 21 May 2012.
Results
Between August 2012 and February 2014, 817 pediatric moderate and severe asthma patients were assessed for study eligibility, 400 were randomized after informed consent, and 1 was lost to follow‐up. This was a convenience sample, since although the PECs see a large number of asthmatic patients, recruitment was limited for practical reasons at nights, during very busy times, when key personnel were otherwise occupied or away, and by prior receipt of outpatient steroid in a large number of patients. One hundred and ninety‐one nebulized magnesium sulfate and 174 placebo nebulization patients were available for analysis; patient flow including reasons for exclusion from analysis are presented in Figure 1. Table 1 presents baseline demographic characteristics of the randomized patients. There were no clinically important differences between the two study groups. The mean asthma severity scores were over 7, mean baseline O2 saturation was 95%, over a third of patients had fever, and over 50% had a nasopharyngeal swab positive by PCR testing for one or more viral pathogens. Mean duration of symptoms ranged from 0.6 d (fever) to 2.4 d (cough). Baseline serum magnesium level was in the normal range and similar for both groups.
Figure 1.
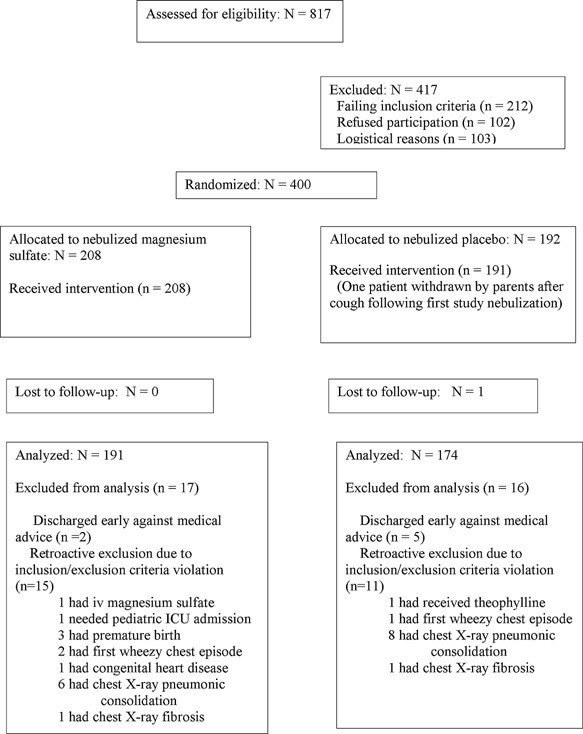
Study patient flow chart.
Table 1.
Study Patient Baseline Characteristics
| MgSo4 n = 208 | Placebo n = 192 | P Value | |
|---|---|---|---|
| Age, y , mean (SD) | 5.6 (3.1) | 5.8 (3.1) | 0.52 |
| Sex, male/female | 133/75 | 115/77 | |
| Moderate/severe asthma exacerbation | 168/40 | 163/29 | |
| Baseline asthma severity score, mean (SD) | 7.6 (1.3) | 7.5 (1.3) | 0.39 |
| Baseline oxygen saturation %, mean (SD) | 95 (2.6) | 95 (2.7) | 0.19 |
| Duration of symptoms d, mean (SD) | |||
| Duration of cough | 2.4 (2.6) | 2.2 (1.6) | 0.36 |
| Duration of difficulty of breathing | 1.6 (1.1) | 1.7 (1.5) | 0.46 |
| Duration of fever | 0.6 (1.2) | 0.7 (1.1) | 0.32 |
| Number of patients with fever on admission, n (%) | 72 (35) | 76 (40) | 0.3 |
| Mean age at first asthma diagnosis (SD) | 3.9 (3.0) | 4.0 (3.0) | 0.98 |
| Number of PEC visits for asthma, preceding year, mean (SD) | 3.7 (2.9) | 3.5 (3.1) | 0.36 |
| Number of admissions to infirmary for asthma, preceding year, mean (SD) | 2.0 (2.2) | 2.1 (2.2) | 0.76 |
| Number of patients requiring PICU admission for asthma since diagnosis, n (%) | 6 (2.9) | 9 (4.8) | 0.3 |
| Family history of asthma in father, mother or full sibling, n (%) | 158 (79) | 152 (79) | |
| History of eczema in patient, n (%) | 74 (36) | 74 (39) | 0.48 |
| Daily exposure to smoking, n (%) | 80 (39) | 71 (37) | 0.79 |
| Daily exposure to pets, n (%) | 36 (17) | 24 (13) | 0.18 |
| Use of prophylactic medication, n (%) | |||
| Montelukast | 13 (6.3) | 10 (5.2) | |
| Budesonide | 4(1.9) | 4 (2.1) | |
| Fluticasone | 12 (5.8) | 18 (9.4) | |
| Fluticasone –salmeterol combination | 3 (1.4) | 3 (1.6) | |
| Anti‐IgE antibody (omalizumab) | 2 (1.0) | 1 (0.5) | 0.29 |
| Chest radiograph, n (%) | |||
| Normal | 95 (45.7) | 88 (45.9) | 0.80 |
| Atalectasis | 16 (7.7) | 20 (10.4) | |
| Increased bronchovascular markings | 91 (43.8) | 76 (39.6) | |
| Pneumonia with consolidation | 6 (2.4) | 8 (3.6) | |
| Fibrotic changes | 1 (0.5) | 1 (0.5) | |
| Positive PCR in nasopharyngeal swab, n (%) | 105 (50.5) | 120 (62.5) | |
| Rhinovirus positivity in PCR nasopharyngeal swab, n (%) | 49 (23.7) | 73 (38.4) | |
| Enterovirus positivity in PCR nasopharyngeal swab, n (%) | 16 (7.7) | 17 (8.9) | |
| RSV positivity in PCR nasopharyngeal swab, n (%) | 14 (6.8) | 6 (3.2) | |
| Influenza/parainfluenza viruses positivity in PCR nasopharyngeal swab, n (%) | 12 (7) | 6 (3.14) | |
| Bocavirus positivity in PCR nasopharyngeal swab, n (%) | 9 (4.3) | 17 (8.9) | |
| Adenovirus positivity in PCR nasopharyngeal swab, n (%) | 8 (3.9) | 10 (5.3) | |
| Coronavirus positivity in PCR nasopharyngeal swab, n (%) | 10(4.8) | 5 (2.6) | |
| Human metapneumovirus positivity in PCR nasopharyngeal swab, n (%) | 5 (2.4) | 7 (3.7) | |
| Mycoplasma positivity in PCR nasopharyngeal swab, n (%) | 1 (0.5) | 2 (1.0) | |
| Co‐infection with more than 1 organism in PCR nasopharyngeal swab, n (%) | 20 (9.6) | 26 (13.5) | 0.157 |
| Basal magnesium level (mmol/l), mean ± SD | 0.83 (0.07) | 0.82 (0.06) | 0.46 |
Blood magnesium levels 2 hr after completion of 3 doses of nebulized magnesium sulfate (3 hr after initiation of nebulized magnesium or placebo) were obtained in over half the patients in each group and were 0.85 (SD 0.07) versus 0.82 (SD 0.06) mmol/L (P = 0.001, Student's t test) for the magnesium sulfate (n = 115 with levels) and placebo (n = 91 with levels) groups, respectively. Figure 2, a frequency histogram of asthma severity scores at baseline, 4 hr, and 8 hr, shows similar frequencies of severity score improvement for both the magnesium and placebo groups. Figure 3 shows the Kaplan–Meier plot of medical readiness for discharge from infirmary care. Accelerated failure time analysis showed a non‐significantly relatively shortened time to medical readiness for discharge of 14% favoring the magnesium sulfate group, OR = 1.14, 95% CI 0.93 to 1.40, P = 0.20. Mean times until readiness for discharge were 14.7 hr [SD 9.7] versus 15.6 hr [SD 11.3] for the investigational and placebo groups, respectively, a 5.8% absolute improvement, P = 0.41.
Figure 2.
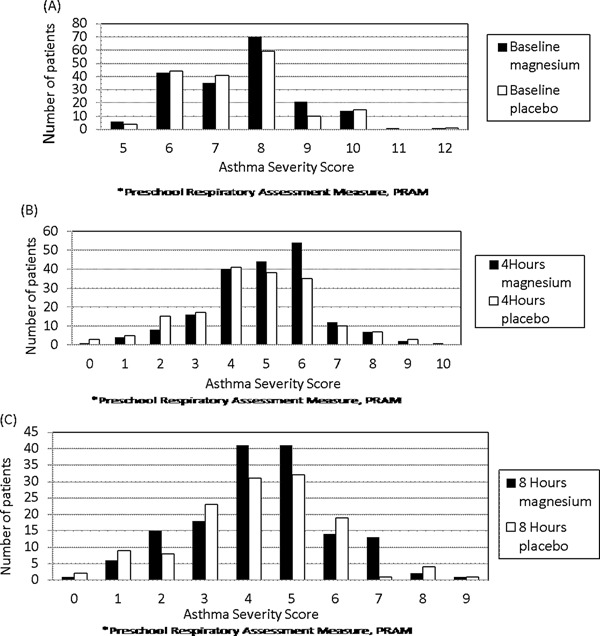
Asthma severity scores at baseline, 4 hr and 8 hr.
Figure 3.
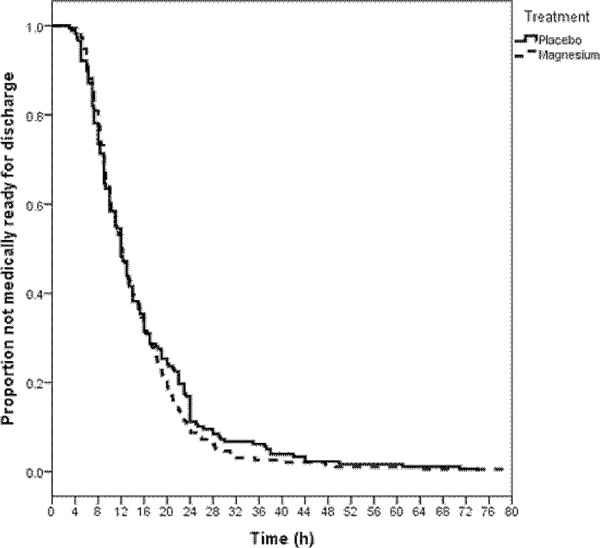
Asthma patient medical readiness for discharge.
Table 2 shows primary and secondary outcomes for the overall patient group and Table 3 for the subset with severe asthma. The preponderance of data and analyses presented failing to show a significant difference suggest that if a difference between treatment with inhaled magnesium sulfate and placebo in a relevant outcome truly does exist, it must be quite small. For the severe asthma subgroup (baseline PRAM score means 9.5 (SD 0.7) and 9.7 (SD 0.7) for magnesium sulfate and placebo groups, respectively), there were no clinically or statistically significant differences in mean (or median, data not shown) length of stay, need for oxygen, or time to readiness for discharge (Table 3).
Table 2.
Outcomes – Overall Set
| 95% Confidence interval | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | MgSO4 Overall: n = 191 | Placebo Overall: n = 174 | Mean difference | Lower | Upper | P value | ||
| Mean time to discharge readiness h (SD) | 14.7 (9.7) | 15.6 (11.3) | −0.9 | −3.1 | 1.3 | 0.41 | ||
| Readiness by 8 hr % (SE, %) | 21.5 (3.0) | 25.3 (3.3) | −3.8 | −12.5 | 4.9 | 0.39 | ||
| Readiness by 12 h | 49.7 (3.6) | 51.1 (3.8) | −1.4 | −11.7 | 9.9 | 0.79 | ||
| Readiness by 24 hr | 90.6 (2.1) | 88.5 (2.4) | 2.1 | −4.7 | 9.0 | 0.52 | ||
| Readiness by 36 hr | 97.4 (1.1) | 93.7 (1.8) | 3.7 | −1.1 | 1.1 | 0.08 | ||
| Readiness by 48 hr | 99.0 (0.7) | 97.7 (1.1) | 1.3 | −1.4 | 3.9 | 0.35 | ||
| Secondary outcomes | ||||||||
| Dischargeable by 24 hr, n (%) | 173 (90.6) | 154 (88.5) | (2.1) | (−5.7) | (9.0) | 0.52 | ||
| Antibiotic therapy, n (%) | 56 (29.3) | 56 (32.1) | (−2.9) | (−12.7) | (6.9) | 0.53 | ||
| Oxygen during infirmary care, n (%) | 14 (7.3) | 22 (12.6) | (−5.3) | (−12.2) | (1.3) | 0.09 | ||
| Mean asthma severity score (SD) | ||||||||
| Score on admission | 7.6 (1.3) | 7.4 (1.3) | −0.1 | −0.4 | −0.2 | 0.37 | ||
| Score at 4 hr | 5.0 (1.6) | 4.6 (1.8) | −0.4 | −0.7 | −0.01 | 0.05 | ||
| Score at 8 hr | 4.2 (1.5) | 4.2 (1.7) | 0.0 | −0.4 | 0.4 | 0.98 | ||
| Score at 12 hr | 4.0 (1.6) | 3.9 (1.5) | −0.1 | −0.5 | 0.5 | 0.94 | ||
| Score at 24 hr | 3.9 (1.6) | 3.3 (1.5) | −0.6 | −1.4 | 0.3 | 0.19 | ||
| Score at 36 hr | 4.4 (2.2) | 3.1 (1.3) | −1.3 | −2.8 | 0.3 | 0.12 | ||
| Score at 48 hr | 5.0 (1.7) | 4.4 (1.7) | −0.6 | −3.6 | 2.4 | 0.65 | ||
| Score when dischargeable | 2.1 (0.9) | 2.2 (0.9) | −0.1 | −0.2 | 0.1 | 0.57 | ||
| PEC revisit in 2 wk after discharge n(%) | 30 (15.7) | 30 (17.2) | (−1.5) | (−9.7) | (6.5) | 0.69 | ||
| Need for infirmary in 2 wk after discharge, n (%) | 9 (4.7) | 14 (8.0) | (−3.3) | (−9.2) | (2.1) | 0.19 | ||
| Hospital admission in 2 wk after discharge (%) | 1 (0.5) | 1 (0.6) | (−0.1) | (−3.1) | (2.8) | 0.95 | ||
Table 3.
Outcomes – Severe Asthma Subset
| 95% Confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | MgSO4 Overall: n = 37 | Placebo Overall: n = 26 | Mean difference | Lower | Upper | P value | |
| Mean time to discharge readiness h (SD) | 18.8 (11.8) | 17.9 (10.2) | 0.9 | −6.7 | 4.7 | 0.73 | |
| Readiness by 8 hr % (SE, %) | 8.1 (4.5) | 15.4 (7.1) | −7.3 | −23.7 | 9.1 | 0.07 | |
| Readiness by 12 hr | 32.4 (7.7) | 30.8 (9.1) | 1.7 | −23.6 | 25.1 | 0.89 | |
| Readiness by 24 hr | 81.1 (6.4) | 88.5 (6.3) | −7.4 | −24.4 | 12.5 | 0.46 | |
| Readiness by 36 hr | 94.6 (3.7) | 92.3 (5.2) | 2.3 | −13.2 | 21.7 | 0.74 | |
| Readiness by 48 hr | 97.3 (2.7) | 96.2 (3.8) | 1.1 | −12.4 | 19.1 | 0.83 | |
| Secondary outcomes | |||||||
| Dischargeable by 24 h, n (%) | 6 (16.2) | 2 (7.7) | (8.5) | (−12.6) | 26.2 | 0.38 | |
| Antibiotic therapy, n (%) | 18 (48.6) | 9 (34.6) | (14.0) | (−12.6) | (37.6) | 0.27 | |
| Oxygen during infirmary care, n (%) | 7 (18.9) | 8 (30.8) | (−11.9) | (−35.4) | (11.1) | 0.28 | |
| Mean asthma severity score (SD) | |||||||
| Score on admission | 9.5 (0.7) | 9.7 (0.7) | 0.2 | −0.2 | 0.5 | 0.31 | |
| Score at 4 hr | 5.8 (1.5) | 5.4 (2.2) | −0.4 | −1.4 | 0.5 | 0.39 | |
| Score at 8 hr | 4.9 (1.5) | 4.8 (1.7) | −0.1 | −1.0 | 0.8 | 0.80 | |
| Score at 12 hr | 4.6 (1.3) | 4.2 (1.6) | −0.5 | −1.4 | 0.4 | 0.29 | |
| Score at 24 hr | 3.7 (1.7) | 3.4 (1.5) | −0.2 | −2.0 | 1.5 | 0.78 | |
| Score at 36 hr | 4.7* | 3.5 (0.7) | −1.2 | −7.3 | 4.9 | 0.59 | |
| Score at 48 hr | 4.5 (2.1) | 4.0* | −0.5 | −33.5 | 32.5 | 0.88 | |
| Score when dischargeable | 2.3 (0.8) | 2.3 (0.8) | 0.0 | −0.37 | 0.5 | 0.86 | |
| PEC revisit in 2 wk after discharge n(%) | 5 (13.5) | 3 (11.5) | (2.0) | (‐19.5) | (20.5) | 1.00 | |
| Need for infirmary in 2 wk after discharge, n (%) | 3 (8.1) | 2 (7.7) | (0.4) | (‐16.6) | (19.4) | 0.95 | |
| Hospital admission in 2 wk after discharge (%) | 0 | 0 | 0 | (‐11.7) | (16.0) | 1.00 | |
Adverse Events
No hypotension was observed and vital signs at observation times were similar between the treatment groups. One patient had excessive cough after the first nebulization and was withdrawn from the study by his parents; he was in the placebo (normal saline nebulization) group. One patient in the magnesium sulfate group had chest tightness after the third nebulization and facial rash without oropharyngeal involvement; he received intravenous diphenhydramine and both resolved within 30 min. One placebo patient required pediatric ICU admission for refractory status asthmaticus.
DISCUSSION
Three nebulized back‐to‐back doses of magnesium sulfate totaling 800 mg after three nebulized doses of albuterol and ipratropium and intravenous methylprednisolone did not appear to favorably affect time to medical readiness for discharge nor other relevant clinical parameters compared to placebo recipients. The subset of patients with severe asthma on presentation behaved similarly.
Our magnesium sulfate dosing used a preferred nebulization system leading to a significantly elevated mean serum magnesium level 2 hr after nebulizations were completed, which we interpret as confirming dose delivery. If our point estimates for mean duration prior to readiness for discharge were hypothesized to reflect a significant benefit of an hour shortened stay (6.4% improvement) from the placebo mean of 15.6 hr, approximately 1400 patients per group would have been required to have 80% power to show that. If the non‐significant 14% improvement determined from accelerated failure time analysis were true, somewhat fewer patients would have been required. In either case, a large number of patients would require an hour of nebulized magnesium sulfate treatment for modest clinical gain. Despite its theoretical attractions, lack of adverse effects, and though we cannot exclude the possibility of a beneficial effect in rare patients, our results militate against making nebulized magnesium sulfate available as part of the standard armamentarium for pediatric patients presenting to community emergency departments with moderate or severe asthma.
Although a review of several much smaller studies of adults and children recommended focusing nebulized magnesium sulfate evaluation on severe rather than moderate asthmatics,2 that conclusion was drawn from accumulating small patient numbers, including adults, subjected to dosing through suboptimal delivery systems without biochemical confirmation that magnesium sulfate had indeed been delivered to study patients. Two recent randomized studies seeking to study solely severe asthmatics, children7 and adults,6 found their effort frustrated and included moderate asthmatics, as we set out to do. Many severe asthmatics have already received steroid therapy as outpatients and greatest severity often becomes apparent during treatment rather than clearly perceived at presentation. We reasoned that the therapeutic population for an optimized clinical trial of nebulized magnesium sulfate should include children presenting with moderate asthma as well. Nebulized magnesium could be further studied as a rescue agent solely in severe asthmatics. our study was not intended to assess efficacy solely in severe asthmatics and was underpowered to do so.
Interpreting our results requires noting some study limitations. After randomization, all our patients were administered systemic steroid as well as 3 combined bronchodilator treatments (intensive therapy consistent with existing guidelines)3 prior to nebulized investigational therapy, possibly diluting the proportion of patients remaining with moderate or severe asthma. An alternative design, studying nebulized magnesium solely as a rescue therapy after failure of bronchodilator and steroid therapy was unappealing because there is no uniform generalized definition of failure but it certainly would consume considerable time after presentation to have “failed”, and our goal was to test for efficacy, safety, and proof of delivery a therapy that could be added early to moderate and severe asthmatics. Counterbalancing this possible concern is the clinical course of our study patients, whose mean infirmary stay duration before medical readiness for discharge was nonetheless prolonged, about 15 hr despite receipt of systemic steroid therapy, a treatment for moderate to severe asthma. It is also possible a substantially higher nebulized magnesium sulfate dose than we gave, or more than three doses of it, may have provided demonstrable benefit. Although the treated group had persistently elevated magnesium levels 3 hr after initiation of magnesium nebulization, we did not obtain pharmacokinetic measurements to allow a comparison with intravenous magnesium dosing. However, our nebulized dose of 800 mg was 77% higher than the 453 mg nebulized in a recent trial in children7; it was 53% of the 1500 mg tested in adults.6 Moreover, all but one of our patients was able to be discharged from infirmary care without ICU admission.
We conclude that adding nebulized magnesium sulfate to combined nebulized bronchodilator and systemic steroid therapy is either very weakly or very rarely effective, or futile for benefitting pediatric patients with moderate or severe asthma.
ACKNOWLEDGMENTS
We thank Dr Prem Chandra for biostatics guidance and calculations and the patients, families, and study and PEC staff.
Conflict of interest: None.
REFERENCES
- 1. Rowe BH, Bretzlaff JA, Bourdon C, Bota GW. Camargo CA Jr. Systematic review of magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev 2000; 2:CD001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powell C, Dwan K, Milan SJ, Beasley R, Hughes R, Knopp‐Sihota JA, Rowe BH. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev 2012; 12:CD003898. [DOI] [PubMed] [Google Scholar]
- 3. British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma: A national clinical guideline. Thorax 2008;63 (Suppl IV):iv58. [Google Scholar]
- 4. Schuh S, Macias C, Freedman S, et al. North American practice patterns of IV magnesium therapy in severe acute asthma in children. Acad Emerg Med 2010; 17:1189–1196. [DOI] [PubMed] [Google Scholar]
- 5. Rowe BH. Intravenous and inhaled MgSO4 for acute asthma. Lancet Respir Med 2013; 1:276–277. [DOI] [PubMed] [Google Scholar]
- 6. Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T. On behalf of the 3Mg Research Team. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): A double‐blind, randomised controlled trial. Lancet Respir Med 2013; 1:293–300. [DOI] [PubMed] [Google Scholar]
- 7. Powell C, Kolamunnage‐Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. Magnesium sulphate in acute severe asthma in children (MAGNETIC): A randomised, placebo‐controlled trial. Lancet Respir Med 2013; 1:301–308. [DOI] [PubMed] [Google Scholar]
- 8. Coates AL, Leung K, Vecellio L, Schuh S. Testing of nebulizers for delivering magnesium sulfate to pediatric asthma patients in the emergency department. Respir Care 2011; 56:314–318. [DOI] [PubMed] [Google Scholar]
- 9. Ducharme FM, Chalut D, Plotnick L, et al. The pediatric respiratory assessment measure: A valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pedatr 2008; 152:476–480. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal P, Sharad S, Handa R. Et al Comparison of nebulised magnesium sulphate and salbutamol combined with salbutamol alone in the treatment of acute bronchial asthma: A randomised study. Emerg Med J 2006; 23:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]


