Abstract
Psilocybin is the psychoactive compound of mushrooms in the psilocybe species. Psilocybin directly affects a number of serotonin receptors, with highest affinity for the serotonin 2A receptor (5HT-2Ar). Generally, the effects of psilocybin, and its active metabolite psilocin, are well established and include a range of cognitive, emotional, and perceptual perturbations. Despite the generality of these effects, there is a high degree of inter-individual variability in subjective psilocybin experiences that are not well understood. Others have shown brain morphology metrics derived from magnetic resonance imaging (MRI) can predict individual drug response. Due to high expression of serotonin 2A receptors (5HT-2Ar) in the cingulate cortex, and its prior associations with psilocybin, we investigate if cortical thickness of this structure predicts the psilocybin experience in healthy adults. We hypothesized that greater cingulate thickness would predict higher subjective ratings in sub-scales of the Five-Dimensional Altered State of Consciousness (5D-ASC) with high emotionality in healthy participants (n = 55) who received oral psilocybin (either low dose: 0.160 mg/kg or high dose: 0.215 mg/kg). After controlling for sex, age, and using false discovery rate (FDR) correction, we found the rostral anterior cingulate predicted all four emotional sub-scales, whereas the caudal and posterior cingulate did not. How classic psychedelic compounds induce such large inter-individual variability in subjective states has been a long-standing question in serotonergic research. These results extend the traditional set and setting hypothesis of the psychedelic experience to include brain structure metrics.
Keywords: psilocybin, 5HT2Ar, cingulate, emotion
1. Introduction
In 1953, relatively high concentrations of serotonin (5-hydroxytryptamine or 5-HT) were found in the brain [1]. Five years later, the Swiss chemist Albert Hofmann isolated psilocybin (4-phosphor yloxy-N,N-dimethyltryptamine), an indole alkaloid of the tryptamine family, from mushrooms of the genus Psilocybe used for ceremonial purposes in Mexico. Psilocybin is rapidly dephosphorylated into the psychoactive metabolite psilocin (4-hydroxy-N,N-dimethyltryptamine) in vitro [2] and in vivo [3]. Pharmacological studies in rats have shown that psilocin primarily binds to 5-HT2A receptors (5-HT2Ar), with a lower affinity for the 5-HT1Ar and several other 5-HT receptor subtypes [4,5,6]. Generally, the effects of psilocybin, and its active metabolite psilocin, on human behavior are well established and include a range of cognitive, emotional, and perceptual perturbations [7,8,9,10,11,12,13,14,15,16,17]. Studies using a preferential 5-HT2Ar antagonist have demonstrated psilocybin’s psychoactive effects are primarily mediated through this receptor [18].
Despite the generality of the psilocybin experience, there is also a high amount of inter and intra-individual variability in psilocybin subjective responses. Psychedelic research of the 1960s attributed variability in drug response with the concept of set and setting (for review see [19]). The set and setting principle posits that the psychedelic experience is highly determined by the extra-pharmacological parameters of set (personality, preparation, expectation, and intention) and setting (the physical, social, and cultural environment). Our group and others have postulated and demonstrated the relative importance of set and setting variables for the subjective response to psychedelics for over half a century [20,21,22,23]. For example, we previously used a pooled analyses of 261 individuals to test 24 non-pharmacological variables as predictors of the subjective effects of psilocybin and found, indeed, a variety of set and setting variables are associated with many aspects of the psilocybin response in healthy volunteers [22]. However, there is a lack of research specifically assessing biological drivers of the psilocybin experience.
Individual brain morphology measures can be used to predict various pharmacological challenges and behavior. For example, thicker frontal cortices predict less D-amphetamine induced striatal dopamine release measured by positron emission tomography (PET) [24,25,26]. Brain structure metrics are associated with several aspects of depression such as risk factors, neurobiological correlates, and pharmacological treatment response [27,28,29,30]. Furthermore, individual differences in the personality dimensions of novelty seeking, harm avoidance, reward dependence, and persistence also reflect structural variance in specific brain regions [31,32,33]. Therefore, it is reasonable to presume individual differences in brain morphologic measures may predict the individual subjective experience of psilocybin.
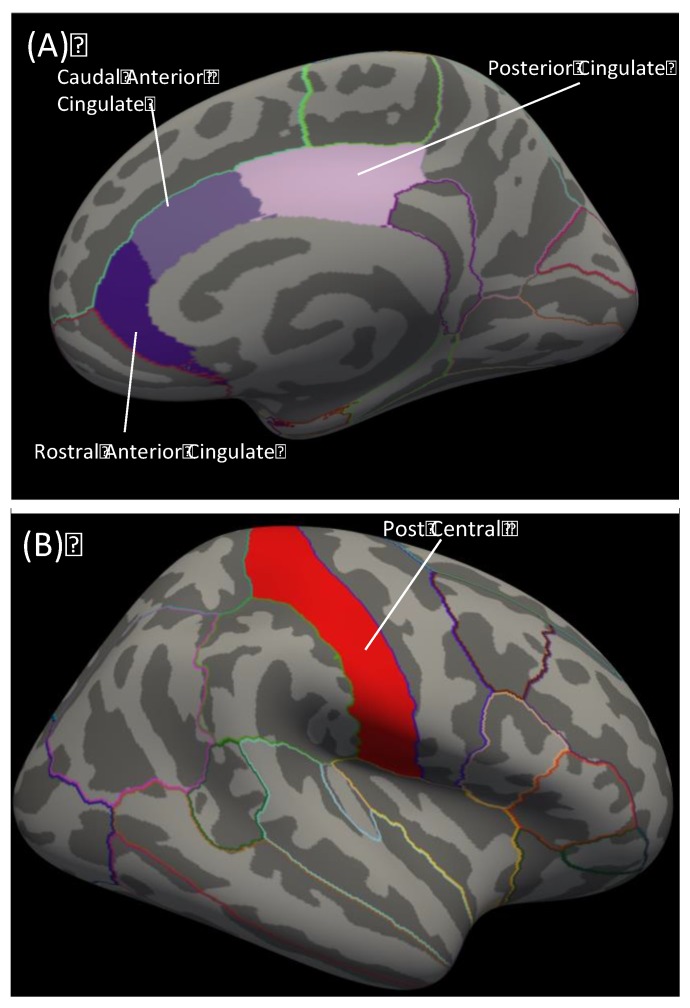
In addition to cognitive and perceptual distortions, the effects of psilocybin on emotionality have been a growing point of interest due to the psycho-therapeutic potential of these compounds. Therefore, we aimed to identify morphological features that predict the subjective emotional experience of psilocybin. The Five-Dimensional Altered States of Consciousness Scale (5D-ASC) is a reliable and validated tool often used to measure the subjective psilocybin experience [34,35]. The 5D-ASC comprises 11 subscales derived from questions assessing the subjective experiences of an altered state of consciousness in retrospect [34]. Of the 11 5D-ASC constructs, the four constructs with high emotionality are: Feeling of Unity, Bliss, Spiritual Experience, and Insightfulness. Psilocybin modulates activity and functional connectivity throughout the limbic system, which is considered the central emotional network [13,16,17,36,37]. Because the subjective effects of psilocybin are primarily mediated by the 5-HT2Ar [18], we chose regions of the limbic system with the highest 5-HT2Ar protein expression, and identified the cingulate cortex based on prior research (Figure 1A) [38]. We hypothesized that greater cingulate thickness would predict higher subjective ratings in healthy participants (n = 55) who received psilocybin (either low dose: 0.160 mg/kg or high dose: 0.215 mg/kg).
Figure 1.
Schematic of FreeSurfer brain regions used in analyses. (A) Right hemisphere cingulate cortex parcellations used in primary analyses. Dark purple = rostral anterior cingulate; medium purple = caudal anterior cingulate; light purple = posterior cingulate. (B) Red = right hemisphere post central parcellation used as the control region analysis.
2. Materials and Methods
2.1. Participants
Participants were recruited through advertisements in local universities for two separate groups, either low dose (0.16 mg/kg: Low) or a high dose (0.215 mg/kg: High). All participants were healthy based on medical history, physical examination, blood analysis, and electrocardiography, had normal or corrected-to-normal vision, and were urine tested for drug abuse and pregnancy. The combined data used for this analysis included 55 participants (33 males, 22 females; mean age 25, SD 3.96 years, range 20–37 years). Participants received written and oral descriptions of the study procedures as well as the effects and possible risks of psilocybin administration. All participants provided written informed-consent statements in accordance with the declaration of Helsinki before participation in the study. The Swiss Federal Office of Public Health, Bern, Switzerland authorized the use of psilocybin in humans. Furthermore, the Cantonal Ethics Committee of Zurich approved the study (KEK 2014-0496 12-12-2014; KEK 2012-0303 13-09-12).
2.2. Experimental Design and Psilocybin Administration
In a randomized, double blind, and placebo-controlled study, subjects received either placebo (maltose) or oral psilocybin (0.16 mg/kg or 0.215mg/kg) in two separate sessions at least 10 days apart. These doses reflect the low and high end of the medium dose range commonly used in the literature [12,39]. Participants had to abstain from smoking for at least 60 min before MRI assessments and from drinking caffeine during the test day. An anatomical scan was conducted 60 min after drug administration. The 5D-ASC [34], a self-report questionnaire retrospectively assessing the subjective experience after drug intake, was completed 360 min after intake. The 5D-ASC comprises 94 items to be answered on visual analogue scale. Scores have been validated for 11 sub-scales [35], of which we used four in this study: experience of unity (example item: “I experienced past, present, and future as a oneness”), spiritual experience (example item: “I experienced a kind of awe”), blissful state (example item: “I enjoyed boundless pleasure”), and insightfulness (example item: “I gained clarity into connections that puzzled me before”). All items that comprise these subscales can be found here [35]. Participant report of psilocybin minus placebo was used to calculate the delta (Δ) variables used in analyses.
2.3. Neuro-Imaging Acquisition
All MR data were acquired on a Philips Achieva 3.0T whole-body scanner (Best, The Netherlands). Inflatable pillows (Multipad, Pearltec AG, Zurich, Switzerland) were used to increase participant comfort in the scanner and to reduce motion induced artifacts. High-resolution anatomical images (voxel size, 1 × 1 × 1 mm) were acquired using a standard T1-weighted three-dimensional (3D) magnetization prepared rapid gradient echo sequence (MP-RAGE). Each session consisted of a resting state arterial spin labeling perfusion-weighted scan and several task-related blood-oxygen-level-dependent scans [13,16,17].
2.4. Image Data Processing
T1-weighted images from the placebo session were processed to obtain cortical thickness using FreeSurfer version 6.0 image analysis suite (surfer.nmr.mgh.harvard.edu/). These processing steps have been described in detail elsewhere [40,41,42]. Cortical thickness was defined as the distance between the white/gray matter boundary to the pial surface. We chose cortical thickness, rather than surface area or volume, because of previous literature associating this metric with drug response [24,25,26]. Segmentations were visually inspected to ensure accuracy of automated processes. Regions of interest were based on high 5HT-2Ar expression in limbic regions (rostral anterior cingulate, caudal anterior cingulate, and posterior cingulate; Figure 1) [38]. A control region was chosen on lowest 5HT-2Ar expression in a cortical region (post central; Figure 1B) [38]. Cortical thickness values for the three areas of the cingulate and the post central gyrus were extracted from the Desikan atlas.
2.5. Statistical Analyses
To assess a difference in the sub-scales between dose groups we ran an analysis of covariance (ANCOVA), controlling for sex and age. Because there was no difference between groups, for further analyses we collapsed across dose and used it as a covariate. We ran multivariate linear regressions controlling for sex, age, control region, and dose with rostral anterior cingulate, caudal anterior cingulate, and posterior cingulate thickness as predictor variables for all models for the four emotional sub-scales. The 5D-ASC sub-scale variables were calculated as psilocybinScale – placeboScale = ΔScale. The Benjamini-Hochberg (false-discovery rate; FDR) procedure was conducted to control for multiple comparisons and Type 1 error within each hemisphere. Lastly, as a follow up to the significant associations, we compared the correlated correlation coefficients between the three cingulate regions in the right hemisphere for each sub-scale to determine if they were significantly different using methods outlined here [43].
3. Results
3.1. Dose Comparison
There were no significant differences between the two doses on the behavioral sub-scales (Table 1). Therefore, for further analyses we collapsed across dose and included dose as a covariate.
Table 1.
Comparison between doses on sub-scales.
| Low (0.16 mg/kg) | High (0.215mg/kg) | p-Value | |||
|---|---|---|---|---|---|
| Construct | Mean | SE | Mean | SE | |
| Unity | 26 | 5 | 33 | 5 | 0.495 |
| Spiritual | 12 | 4 | 16 | 4 | 0.975 |
| Bliss | 38 | 6 | 43 | 6 | 0.75 |
| Insight | 25 | 5 | 36 | 6 | 0.516 |
Max value = 100. Values reported as psilocybin - placebo.
3.2. Cingulate Thickness Predicting Sub-Scales
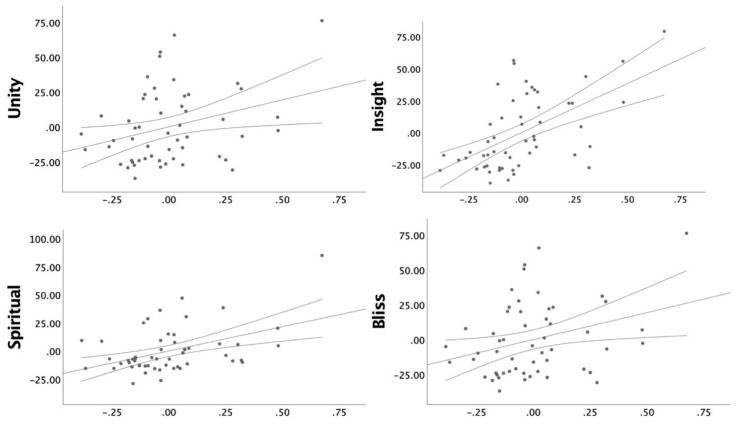
We found a significant positive association between the right hemisphere rostral anterior cingulate thickness and all ΔScales; Feeling of Unity, Bliss, Spiritual Experience, and Insightfulness (Figure 2). Standardized beta values, SE, p, and FDR corrected p values can be found for all the rostral anterior cingulate models in Table 2. We found no significant associations with the caudal anterior cingulate or posterior cingulate cortex thickness and ΔScales (Table 3).
Figure 2.
Partial correlation plots controlling for sex, age, and dose between responses to psilocybin (Unity, Bliss, Spiritual, and Insight) and estimates of right hemisphere rostral anterior cingulate cortex (R rACC) thickness with 95% confidence intervals. Scatter plots for non-significant results can be found in Supplementary Materials. Axes represent normalized values. Response to psilocybin is represented as the delta value; psilocybin – placebo.
Table 2.
Right Hemisphere Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience.
| Sub-Scale | Rostral Anterior Cingulate Cortex (rACC) | |||
|---|---|---|---|---|
| β | SE | p | FDR p | |
| Unity | ||||
| LH | 0.295 | 17.93 | 0.037 | 0.114 |
| RH | 0.324 | 17.18 | 0.027 | 0.027 |
| Bliss | ||||
| LH | 0.106 | 25.14 | 0.465 | 0.465 |
| RH | 0.386 | 18.95 | 0.008 | 0.011 |
| Spiritual | ||||
| LH | 0.266 | 13.84 | 0.057 | 0.114 |
| RH | 0.465 * | 12.35 | 0.001 | 0.002 |
| Insight | ||||
| LH | 0.179 | 20.26 | 0.202 | 0.269 |
| RH | 0.572 *# | 16.46 | 0.00002 | 0.00008 |
FDR: false discovery rate; LH: left hemisphere; RH right hemisphere; β: standardized regression coefficient; SE: standard error; * Age < 0.05; # Dose < 0.05.
Table 3.
Caudal and posterior cingulate thickness do not predict psilocybin emotional experience.
| Sub-Scale | Caudal Cingulate | Posterior Cingulate | ||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Unity | ||||||
| LH | −0.021 | 22.383 | 0.884 | 0.108 | 22.289 | 0.454 |
| RH | 0.275 | 16.976 | 0.065 | 0.062 | 30.219 | 0.676 |
| Bliss | ||||||
| LH | −0.106 | 25.141 | 0.465 | −0.046 | 25.287 | 0.749 |
| RH | 0.006 | 19.852 | 0.969 | 0.01 | 34.181 | 0.947 |
| Spiritual | ||||||
| LH | 0.213 | 16.749 | 0.132 | 0.052 | 17.144 | 0.711 |
| RH | 0.109 | 13.39 | 0.463 | −0.028 | 23.175 | 0.852 |
| Insight | ||||||
| LH | 0.186 | 24.158 | 0.186 | 0.125 | 24.428 | 0.371 |
| RH | 0.241 | 18.781 | 0.099 | −0.033 | 33.242 | 0.82 |
FDR: false discovery rate; LH: left hemisphere; RH right hemisphere; β: standardized regression coefficient; SE: standard error; * Age < 0.05; # Dose < 0.05.
3.3. Comparing Correlated Correlation Coefficients
We found the correlation coefficients between right hemisphere rostral anterior, caudal, and posterior cingulate for Unity (p = 0.05), Spiritual (p < 0.001), Insight (p < 0.001), and Insight (p < 0.001) were significantly different using methods outlined here [43].
4. Discussion
This is the first study to evaluate brain morphology as a predictor of the emotional subjective experience of psilocybin in healthy controls. We focused our analyses on the cingulate cortex based on high 5-HT2Ar expression levels compared to other limbic regions. We found rostral anterior cingulate thickness specifically predicted all four sub-scales of Unity, Spiritual Experience, Blissful State, and Insightfulness, whereas the caudal anterior and posterior cingulate did not. These results point towards the possibility that morphology metrics such as cortical thickness may reflect differences in brain substrates that mediate drug effects.
The anterior portion of the cingulate is a unique region of the brain for its connections to both the limbic system and the more cognitive prefrontal cortex [44]. Our results of rostral, but not other sub-divisions of the cingulate, predicting emotional experiences aligns well with current anatomical knowledge of the cingulate. Briefly, the anterior cingulate has extensive connections with areas known to be important for emotion, memory, and reward related functions whereas the caudal cingulate has extensive connections with cognitive and motor-related areas of cortex [41]. Lastly, the posterior cingulate has connections throughout the neocortex, is crucial component of the default mode network, and is thought to play a pivotal role in the recall of autobiographical memories [45]. While it was surprising to us that posterior cingulate thickness did not predict the psilocybin emotional experience due to the structure’s previous associations with psilocybin [46], our results further highlight the importance of anterior cingulate in emotional processing. For example, others have found the anterior cingulate to be involved in emotional and self-processing in healthy controls [47,48], and anterior cingulate activity changes have been associated with Mindfulness-Based Stress Reduction interventions in back pain patients with depression [49]. While the amygdala is typically considered the key limbic structure with a central role in emotion [50], emotional control is considered a “top-down” regulation process from several areas of frontal cortex [51]. For example, the anterior cingulate cortex projects to both the amygdala and the prefrontal cortex, which is thought to provide the capacity for emotional regulation [52]. Taken together, one might speculate that cingulate 5-HT2A activation by psilocybin/psilocin plays a major role in inducing the profound emotional occurrences often associated with psilocybin.
There is some evidence suggesting the experience of emotion (i.e., mood and affect) is predominantly regulated by the right hemisphere [53,54]. Our results indicate right hemisphere cingulate morphology is a better predictor of psilocybin-induced emotional experiences compared to the left hemisphere. These results align well with the previous findings from our group and others that classic psychedelic compounds induce hyperfrontal effects particularly in the right hemisphere [9,13,55,56,57]. Right-hemisphere processing is historically described to possess holistic, gestalt, or integrative characteristics compared to the left hemisphere [58,59]. Specifically, the right hemisphere is superior in processing emotional affect and affective linguistic characteristics such as metaphor [53,60]. Because the right hemisphere appears to be involved in emotional processing, perhaps the right lateralized effects of psilocybin play a role in its reported psychotherapeutic effectiveness across a wide range of psychiatric disorders [61].
A recent open-label trial using psilocybin for treatment resistant depression found the composite variable of Unity, Spiritual Experience, and Blissful State along with Insightfulness predicted therapeutic outcomes [62]. The spiritual experience of psilocybin, along with the persistent increased sense of well-being and life satisfaction, has been reported to account for sustained reductions in smoking and drinking behavior and decreased depression and anxiety [39,63,64,65,66,67]. While the underlying mechanism(s) linking these subjective states with long-term reductions in maladaptive behavior are unknown, these emotional states reflect common therapeutic goals such as decreasing social disconnection, negative rumination, and hopelessness [68,69,70,71,72]. Furthermore, it is hypothesized that the direct 5-HT2Ar agonist properties of psilocybin enhance sensitivity and facilitates emotional release, which, when combined with psychological support, leads to symptom reduction [73]. If psilocybin clinical trials continue to demonstrate effective therapeutic properties, discovering individual biomarkers to help predict which patients are most likely to benefit from these therapies will be an important line of research [74]. Biomarkers prospectively predicting effective treatment options could assist in individualized treatment planning, reducing time spent on ineffective treatments, shortening patient suffering, and reducing the overall cost burden of depression [75]. Our results suggest neuroimaging may benefit precision medicine approaches of future psychedelic assisted treatment options.
One limitation of this study is that our design does not allow for a causal inference between brain morphology and psilocybin subjective states. However, our self-report data does take into account the participant’s baseline emotional states by using self-report from both psilocybin and placebo sessions. This is important as it allows us to correlate cingulate thickness with psilocybin-induced states while functionally controlling for variability in participant baseline mood, excitement, or study stress. While including two separate doses of psilocybin is a strength, it is also a limitation that doses were both in the medium range; thus, we did not detect dose effects. Another strength of this study is the use of modern neuroimaging methods to extend the set and setting hypothesis to include in vivo individual brain structure information. However, future research should also include genomic and epigenetic predictors of individual psilocybin response since allelic variations in monoaminergic genes have been linked to brain structure and therapeutic response to pharmacological treatments [76,77,78,79,80,81]. Lastly, because we assessed these relationships with a healthy sample, future research with patient populations will need to determine if our results prove to be of clinical value.
How classic psychedelic compounds induce such large inter- and intra-variability in subjective states has been a long-standing question in serotonergic research. Set and setting has been the prevailing theory for over half a century. Several studies validated this theory and found significant relationships between non-pharmacological predictors and the psilocybin experience; however, there are still relatively large proportions of unexplained variance in individual emotional experiences [19,22]. Investigating the relationship between psilocybin modulation and cingulate structure could provide insight into mechanisms underlying psilocybin’s profound emotional effects and provides a theoretical framework for brain-based predictors of 5HT2A psychedelic modulation. These results provide the basis for a potential new unified theory of set, setting, and structure as predictors of the individual psychedelic experience.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2227-9059/8/2/34/s1.
Author Contributions
F.X.V., K.H.P., and C.R.L. conceived and designed the experiments; K.H.P. performed the experiments; C.R.L., B.B.B., and C.R. analyzed the data; C.R.L., B.B.B., K.H.P., and F.X.V. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by grants from the Heffter Research Institute and the Swiss Neuromatrix Foundation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Twarog B.M., Page I.H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 1953:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Eivindvik K., Rasmussen K.E., Sund R.B. Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta Pharm. Nord. 1989;1:295–302. [PubMed] [Google Scholar]
- 3.Hasler F., Studerus E., Lindner K., Ludewig S., Vollenweider F.X. Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J. Psychopharmacol. 2009;23:923–935. doi: 10.1177/0269881108094650. [DOI] [PubMed] [Google Scholar]
- 4.Passie T., Seifert J., Schneider U., Emrich H.M. The pharmacology of psilocybin. Addict. Biol. 2002;7:357–364. doi: 10.1080/1355621021000005937. [DOI] [PubMed] [Google Scholar]
- 5.Mckenna D.J., Repke D.B., Lo L., Peroutka S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 6.Vollenweider F.X., Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Publ. Gr. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 7.Carter O.L., Burr D.C., Pettigrew J.D., Wallis G.M., Hasler F., Vollenweider F.X. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cogn. Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 8.Gouzoulis-Mayfrank E., Schreckenberger M., Sabri O., Hermle L., Büll U., Sass H. Neurometabolic Effects of Psilocybin, (MDE) and d-Methamphetamine in Healthy Volunteers. Neuropsychopharmacology. 1999;20:565–581. doi: 10.1016/S0893-133X(98)00089-X. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths R.R., Richards W. a., McCann U., Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl). 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 10.Carbonaro T.M., Johnson M.W., Hurwitz E., Griffiths R.R. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berl). 2018;235:521–534. doi: 10.1007/s00213-017-4769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths R.R., Johnson M.W., Richards W.A., Richards B.D., McCann U., Jesse R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl). 2011;218:649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis C.R., Preller K.H., Kraehenmann R., Michels L., Stämpfli P., Vollenweider F.X. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage. 2017;159:70–78. doi: 10.1016/j.neuroimage.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Pokorny T., Preller K.H., Kometer M., Dziobek I., Vollenweider F.X. Effect of psilocybin on empathy and moral decision-making. Int. J. Neuropsychopharmacol. 2017;20:747–757. doi: 10.1093/ijnp/pyx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollenweider F.X., Csomor P., Knappe B., Geyer M., Quednow B.B. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- 15.Kraehenmann R., Schmidt A., Friston K., Preller K.H., Seifritz E., Vollenweider F.X. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. NeuroImage Clin. 2015;11:53–60. doi: 10.1016/j.nicl.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preller K.H., Pokorny T., Hock A., Kraehenmann R., Stãmpfli P., Seifritz E., Scheidegger M., Vollenweider F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kometer M., Schmidt A., Bachmann R., Studerus E., Seifritz E., Vollenweider F.X. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol. Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Hartogsohn I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law. 2017;3:2050324516683325. doi: 10.1177/2050324516683325. [DOI] [Google Scholar]
- 19.Metzner R., Litwin G.H., Weil G.M. The Relation of Expectation and Mood to Psilocybin Reactions: A Questionnaire Study. Psychedelic Rev. 1965;1:3–39. [Google Scholar]
- 20.Becker H.S. History, culture and subjective experience: an exploration of the social bases of drug-induced experiences. J. Health Soc. Behav. 1967:163–176. doi: 10.2307/2948371. [DOI] [PubMed] [Google Scholar]
- 21.Studerus E., Gamma A., Kometer M., Vollenweider F.X. Prediction of psilocybin response in healthy volunteers. PLoS ONE. 2012;7:e30800. doi: 10.1371/journal.pone.0030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panton Y., Fischer R. Hallucinogenic Drug-Induced Behavior Under Sensory Attenuation: Prediction of Response to Psilocybin. Arch. Gen. Psychiatry. 1973;28:434–438. doi: 10.1001/archpsyc.1973.01750330106017. [DOI] [PubMed] [Google Scholar]
- 23.Casey K.F., Cherkasova M.V., Larcher K., Evans A.C., Baker G.B., Dagher A., Benkelfat C., Leyton M. Individual Differences in Frontal Cortical Thickness Correlate with the d-Amphetamine-Induced Striatal Dopamine Response in Humans. J. Neurosci. 2013;33:15285–15294. doi: 10.1523/JNEUROSCI.5029-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherkasova M.V., Faridi N., Casey K.F., Larcher K., O’Driscoll G.A., Hechtman L., Joober R., Baker G.B., Palmer J., Evans A.C., et al. Differential Associations between Cortical Thickness and Striatal Dopamine in Treatment-Naïve Adults with ADHD vs. Healthy Controls. Front. Hum. Neurosci. 2017;11:1–14. doi: 10.3389/fnhum.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaworska N., Cox S.M., Casey K.F., Boileau I., Cherkasova M., Larcher K., Dagher A., Benkelfat C., Leyton M. Is there a relation between novelty seeking, striatal dopamine release and frontal cortical thickness? PLoS ONE. 2017;12:1–19. doi: 10.1371/journal.pone.0174219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaney A., Carballedo A., Amico F., Fagan A., Skokauskas N., Meaney J., Frodl T. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J. Psychiatry Neurosci. 2014;39:50. doi: 10.1503/jpn.120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redlich R., Almeida J.R., Grotegerd D., Opel N., Kugel H., Heindel W., Arolt V., Phillips M.L., Dannlowski U. Brain morphometric biomarkers distinguishing unipolar and bipolar depression: A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71:1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung J., Kang J., Won E., Nam K., Lee M.S., Tae W.S., Ham B.J. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: A voxel-based morphometry study. J. Affect. Disord. 2014;169:179–187. doi: 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Young R.C., Kalayam B., Nambudiri D.E., Kakuma T., Alexopoulos G.S. Brain morphology and response to nortriptyline in geriatric depression. Am. J. Geriatr. Psychiatry. 1999;7:147–150. doi: 10.1097/00019442-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Schilling C., Kühn S., Paus T., Romanowski A., Banaschewski T., Barbot A., Barker G.J., Brühl R., Büchel C., Conrod P.J., et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol. Psychiatry. 2013;36:4038–4049. doi: 10.1038/mp.2012.56. [DOI] [PubMed] [Google Scholar]
- 31.Holmes A.J., Hollinshead M.O., Roffman J.L., Smoller J.W., Buckner R.L. Individual Differences in Cognitive Control Circuit Anatomy Link Sensation Seeking, Impulsivity, and Substance Use. J. Neurosci. 2016;36:4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardini S., Cloninger C.R., Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res. Bull. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31:80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- 34.Studerus E., Gamma A., Vollenweider F.X. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraehenmann R., Preller K.H., Scheidegger M., Pokorny T., Bosch O.G., Seifritz E., Vollenweider F.X. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol. Psychiatry. 2014;78:1–9. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Mayberg S. The Neuropsychiatry of Limbic and Subcortical Disorders. American Psychiatric Press; Washington, DC, USA: 1997. Limbic-Cortical Dysregulation: Depression. [Google Scholar]
- 37.Beliveau V., Ganz M., Feng L., Ozenne B., Højgaard L., Fisher P.M., Svarer C., Greve D.N., Knudsen G.M. A high-resolution in vivo atlas of the human brain’s serotonin system. J. Neurosci. 2017;37:120–128. doi: 10.1523/JNEUROSCI.2830-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths R.R., Johnson M.W., Carducci M.A., Umbricht A., Richards W.A., Richards B.D., Cosimano M.P., Klinedinst M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosman E., Jr., Fischl B., Wells III W., Dale A. Topology Correction for Cortical Surface Models. Massachusetts Institute of Technology; Cambridge, MA, USA: 1999. pp. 169–170. [Google Scholar]
- 40.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han X., Han X., Jovicich J., Jovicich J., Salat D., Salat D., van der Kouwe A., van der Kouwe A., Quinn B., Quinn B., et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 42.Meng X.L., Rosenthal R., Rubin D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992;111:172. doi: 10.1037/0033-2909.111.1.172. [DOI] [Google Scholar]
- 43.Stevens F.L., Hurley R.A., Taber K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011;23:121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- 44.Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebedev A.V., Lövdén M., Rosenthal G., Feilding A., Nutt D.J., Carhart-Harris R.L. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum. Brain Mapp. 2015;36:3137–3153. doi: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith R., Fadok R.A., Purcell M., Liu S., Stonnington C., Spetzler R.F., Baxter L.C. Localizing sadness activation within the subgenual cingulate in individuals: A novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging Behav. 2011;5:229–239. doi: 10.1007/s11682-011-9127-2. [DOI] [PubMed] [Google Scholar]
- 47.Liotti M., Mayberg H.S., Brannan S.K., McGinnis S., Jerabek P., Fox P.T. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol. Psychiatry. 2000;48:30–42. doi: 10.1016/S0006-3223(00)00874-X. [DOI] [PubMed] [Google Scholar]
- 48.Braden B.B., Pipe T.B., Smith R., Glaspy T.K., Deatherage B.R., Baxter L.C. Brain and behavior changes associated with an abbreviated 4-week mindfulness-based stress reduction course in back pain patients. Brain Behav. 2016;6:e00443. doi: 10.1002/brb3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeDoux J. The amygdala. Curr. Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Blair K.S., Smith B.W., Mitchell D.G.V., Morton J., Vythilingam M., Pessoa L., Fridberg D., Zametkin A., Sturman D., Nelson E.E., et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 52.Demaree H.A., Everhart D.E., Youngstrom E.A., Harrison D.W. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance. ” Behav. Cogn. Neurosci. Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- 53.Simon-Thomas E.R., Role K.O., Knight R.T. Behavioral and electrophysiological evidence of a right hemisphere bias for the influence of negative emotion on higher cognition. J. Cogn. Neurosci. 2005;17:518–529. doi: 10.1162/0898929053279504. [DOI] [PubMed] [Google Scholar]
- 54.Hermle L., Fünfgeld M., Oepen G., Botsch H., Borchardt D., Gouzoulis E., Fehrenbach R.A., Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol. Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 55.Riba J., Romero S., Grasa E., Mena E., Carrió I., Barbanoj M.J. Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology (Berl). 2006;186:93–98. doi: 10.1007/s00213-006-0358-7. [DOI] [PubMed] [Google Scholar]
- 56.Vollenweider F.X., Leenders K.L., Scharfetter C., Maguire P., Stadelmann O., Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 57.Ley R.G., Bryden M.P. Hemispheric differences in processing emotions and faces. Brain Lang. 1979;7:127–138. doi: 10.1016/0093-934X(79)90010-5. [DOI] [PubMed] [Google Scholar]
- 58.Bradshaw J.L., Nettleton N.C. The nature of hemispheric specialization in man. Behav. Brain Sci. 1981;4:51–63. doi: 10.1017/S0140525X00007548. [DOI] [Google Scholar]
- 59.Bottini G., Corcoran R., Sterzi R., Paulesu E., Schenone P., Scarpa P., Frackowiak R.S.J., Frith D. The role of the right hemisphere in the interpretation of figurative aspects of language a positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- 60.Thomas K., Malcolm B., Lastra D. Psilocybin-Assisted Therapy: A Review of a Novel Treatment for Psychiatric Disorders. J. Psychoactive Drugs. 2017;49:446–455. doi: 10.1080/02791072.2017.1320734. [DOI] [PubMed] [Google Scholar]
- 61.Carhart-Harris R.L., Bolstridge M., Day C.M.J., Rucker J., Watts R., Erritzoe D.E., Kaelen M., Giribaldi B., Bloomfield M., Pilling S., et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235:399–408. doi: 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson M.W., Garcia-Romeu A., Cosimano M.P., Griffiths R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014;28:983–992. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogenschutz M.P., Forcehimes A.A., Pommy J.A., Wilcox C.E., Barbosa P., Strassman R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015;29:289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 64.Ross S., Bossis A., Guss J., Agin-Liebes G., Malone T., Cohen B., Mennenga S.E., Belser A., Kalliontzi K., Babb J., et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 2016;30:1165–1180. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carhart-Harris R.L., Bolstridge M., Rucker J., Day C.M.J., Erritzoe D., Kaelen M., Bloomfield M., Rickard J.A., Forbes B., Feilding A., et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. The Lancet Psychiatry. 2016;0366:11–13. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 66.Roseman L., Nutt D.J., Carhart-Harris R.L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 2018;8:974. doi: 10.3389/fphar.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abramson L.Y., Metalsky G.I., Alloy L.B. Hopelessness Depression: A Theory-Based Subtype of Depression. Psychol. Rev. 1989;96:358. doi: 10.1037/0033-295X.96.2.358. [DOI] [Google Scholar]
- 68.Watkins E.R. Depressive rumination: Investigating mechanisms to improve cognitive behavioural treatments. Cogn. Behav. Ther. 2009;38:8–14. doi: 10.1080/16506070902980695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherry S.B., Law A., Hewitt P.L., Flett G.L., Besser A. Social support as a mediator of the relationship between perfectionism and depression: A preliminary test of the social disconnection model. Pers. Individ. Dif. 2008;45:339–344. doi: 10.1016/j.paid.2008.05.001. [DOI] [Google Scholar]
- 70.Remmers C., Michalak J. Losing your gut feelings. Intuition in depression. Front. Psychol. 2016;7:1291. doi: 10.3389/fpsyg.2016.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruwys T., Alexander Haslam S., Dingle G.A., Jetten J., Hornsey M.J., Desdemona Chong E.M., Oei T.P.S. Feeling connected again: Interventions that increase social identification reduce depression symptoms in community and clinical settings. J. Affect. Disord. 2014;159:139–146. doi: 10.1016/j.jad.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Carhart-Harris R.L., Goodwin G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology. 2017;42:2105–2113. doi: 10.1038/npp.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strawbridge R., Young A.H., Cleare A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017;16:194–209. doi: 10.2147/NDT.S114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop B.W., Craighead W.E., Franco A.R., Craddock R.C., Mayberg H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durston S., Fossella J.A., Casey B.J., Hulshoff Pol H.E., Galvan A., Schnack H.G., Steenhuis M.P., Minderaa R.B., Buitelaar J.K., Kahn R.S., et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol. Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 76.Shaw P., Gornick M., Lerch J., Addington A., Seal J., Greenstein D., Sharp W., Evans A., Giedd J.N., Castellanos F.X., et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- 77.Fernández-Jaén A., López-Martín S., Albert J., Fernández-Mayoralas D.M., Fernández-Perrone A.L., de La Peña M.J., Calleja-Pérez B., Rodríguez M.R., López-Arribas S., Muñoz-Jareño N. Cortical thickness differences in the prefrontal cortex in children and adolescents with ADHD in relation to dopamine transporter (DAT1) genotype. Psychiatry Res. -Neuroimaging. 2015;233:409–417. doi: 10.1016/j.pscychresns.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Lesch K.P. Molecular foundation of anxiety disorders. J. Neural Transm. 2001;108:717–746. doi: 10.1007/s007020170048. [DOI] [PubMed] [Google Scholar]
- 79.Müller J., Dreisbach G., Brocke B., Lesch K.P., Strobel A., Goschke T. Dopamine and cognitive control: The influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Res. 2007;1131:155–162. doi: 10.1016/j.brainres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Lesch K.P., Gutknecht L. Focus on the 5-HT1A receptor: Emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int. J. Neuropsychopharmacol. 2004;7:381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- 81.Lin J.Y., Jiang M.Y., Kan Z.M., Chu Y. Influence of 5-HTR2A genetic polymorphisms on the efficacy of antidepressants in the treatment of major depressive disorder: a meta-analysis. J. Affect Disord. 2014;168:430–438. doi: 10.1016/j.jad.2014.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.