Abstract
Objective
This study aimed to analyse the phenotype of systemic lupus erythematosus (SLE) at first presentation and during follow-up in a newly established SLE cohort based at ‘Attikon’ University Hospital. The hospital combines primary, secondary and tertiary care for the region of Western Attica, Greece.
Methods
This study comprised a mixed prevalent and incident cohort of 555 Caucasian patients diagnosed with SLE according to American College of Rheumatology 1997 criteria and/or the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) 2012 criteria. Demographic and clinical characteristics, patterns of severity, treatments and SLICC damage index were recorded for each patient at the time of diagnosis and at last evaluation.
Results
The mean age at lupus diagnosis was 38.3 years (standard deviation = 15.6 years), with a median disease duration at last follow-up of two years (interquartile range 1-11). At initial presentation, the most common ‘classification’ manifestations were arthritis (73.3%), acute cutaneous lupus (65%) and unexplained fever (25%), while among symptoms not included in any criteria set, Raynaud’s phenomenon (33%) was the most common. Kidney and neuropsychiatric involvement as presenting manifestations were present in 10.3% and 11.5% cases, respectively. Irreversible damage accrual was present in 17.8% within six months of disease diagnosis, attributed mainly to thrombotic and neuropsychiatric disease. At last evaluation, 202 (36.4%) patients had developed severe disease, of whom more than half were treated with pulse cyclophosphamide.
Conclusion
In this cohort of Caucasian patients, lupus nephritis is not as common as in older cohorts, while neuropsychiatric disease is emerging as a major frontier in lupus prevention and care. These data may help to document changes in the natural history and treatment of SLE over time and may have implications for its early recognition and management.
Keywords: Prevalent cohort, incident cohort, lupus criteria, non-lupus criteria, damage
Introduction
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease with a strong female predominance estimated to affect more than 8000 individuals in Greece (total population approximately 10 million).1 Its clinical presentation encompasses a widely heterogeneous spectrum of phenotypes, ranging from mild or ‘organ limited’ to full-blown life-threatening disease. Mild manifestations, such as skin rashes, inflammatory arthritis, leucopaenia/lymphopaenia, non-scarring alopecia and oral ulcers, are common among patients, while involvement of major organs is less frequent.2 Common manifestations are useful for an early diagnosis, and their presence should raise the suspicion of underlying SLE, but they typically lack specificity, as they may also occur in other diseases.3
Approximately half of lupus patients are diagnosed with mild disease initially, with less than 20% having severe disease at onset.4 For those presenting with mild disease in the absence of specific autoantibodies (e.g. anti-dsDNA) or characteristic lupus manifestations (e.g. malar rash), definite diagnosis represents a challenge.5 Lupus diagnosis remains clinical because existing classification criteria for the disease6,7 fail to classify up to 25% of patients, especially in the early stages.8,9 In this regard, ‘non-criteria’ manifestations may aid in an earlier diagnosis of lupus.
The phenotype, clinical course and outcome of lupus differ around the world, depending on the population under study. Caucasians are more likely to have less severe disease, and a mild phenotype is maintained throughout the disease course in 50% of patients.1 In contrast, Afro-Americans and Hispanics exhibit a more aggressive course, with a high incidence of lupus nephritis (LN)10 and neuropsychiatric manifestations.11 Childhood-onset SLE (cSLE) usually displays worse outcomes and more severe disease compared to adult-onset patients,12 while patients with late-onset lupus typically have lower disease activity and a milder disease course.13,14
In this study, we sought to assess and describe the phenotype of lupus systematically at the time of presentation and throughout the disease course in a newly established cohort, the ‘Attikon’ lupus cohort, consisting exclusively of Caucasians. To this end, we recorded clinical manifestations, treatment, damage accrual and co-morbidities.
Methods
The ‘Attikon’ cohort
The ‘Attikon’ University Hospital is the largest tertiary medical centre of Western Attica, responsible for the care of approximately two million local citizens. In 2014, a rheumatology unit was established, serving as a referral centre for patients with lupus. Starting in September 2014, a cohort of patients with SLE was established in the rheumatology unit. The cohort (still ongoing) includes 555 Caucasian patients. It consists of a ‘prevalent cohort’ and an ‘inception’ cohort. The ‘prevalent cohort’ includes 237 patients with a SLE diagnosis prior to the establishment of the ‘Attikon’ cohort, who continue their regular follow-up in ‘Attikon’ University Hospital. The ‘inception cohort’ includes 318 SLE patients who have been diagnosed in ‘Attikon’ University Hospital and who have been followed ever since. For each patient, the first visit to the unit and registration in the cohort is defined as the ‘enrolment’ visit. Patient registration for the purpose of the study was completed in June 2019.
Patients and clinical assessment
Diagnosis of SLE was established by the American College of Rheumatology (ACR) 19976 and/or the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) 2012 criteria,7 either at diagnosis or during the disease course.
We used a standardized form containing the ACR and SLICC classification criteria, as well as an additional list of clinical items not captured in these criteria sets. Patient files were also scrutinized for the following variables: (a) main demographic characteristics, (b) co-morbidities based on the Charlson Comorbidity Index,15 (c) immunological tests and (d) past and present medications. The timing of the appearance of each clinical item and of serological tests was documented as present either (a) at diagnosis or (b) during the course of the disease. At every patient visit, any new manifestation was added to the database, thus ensuring a continuous recording.
Definitions
Kidney involvement was defined as (a) a kidney biopsy with a diagnosis of LN according to the 2003 International Society of Nephrology/Renal Pathology Society classification16 or previous histological criteria for LN, and/or (b) by fulfilment of classification criteria for SLE (ACR and SLICC criteria) after exclusion of other causes.6,7 The latter was mainly the case for patients diagnosed with SLE in the past (1995 or earlier), when kidney biopsy was not performed routinely. Chronic kidney disease (CKD) was defined as a glomerular filtration rate of <60 mL/min/1.73 m2 for three months or more, and end-stage renal disease (ESRD) as initiation of kidney-replacement therapy.17 Neuropsychiatric manifestations were classified as either primary neuropsychiatric SLE (NPSLE, attributed to SLE;18 using a combination of multidisciplinary physician judgment with attribution models,19 as previously described) or secondary NPSLE (neuropsychiatric manifestations not attributed to SLE) or manifestations of uncertain attribution. Neuropsychiatric manifestations were classified as ‘minor’ and ‘major’, according to the definition by Ainiala et al.20 Minor manifestations include headache, anxiety disorders, mild mood disorders, mild cognitive impairment and polyneuropathy without electrophysiological confirmation. The SLICC damage index (SDI) was used for the assessment of irreversible organ damage.21 The revised Sydney classification criteria were used for definite diagnosis of antiphospholipid syndrome (APS).22 For the definition of cSLE and late-onset SLE, cut-offs of 18 and 50 years, respectively, were used. All patients were categorized as having ‘mild’, ‘moderate’ or ‘severe’ lupus based on physician assessment and the presence of BILAG group A (for severe), group B (for moderate) or groups C/D/E (for mild) manifestations cumulatively during the course of their disease.23
Statistical analysis
Descriptive statistics were undertaken for continuous variables, and mean values/standard deviation (SD) or median/interquartile range (IQR) were calculated for normally and non-normally distributed variables, respectively. The chi-square test and Student’s t-test were used to compare categorical and continuous variables, respectively. For all comparisons, a p-value of <0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics for Windows v25.0 (IBM Corp., Armonk, NY). Captured data are stored electronically at ‘Attikon’ University Hospital and are accessible only by rheumatologists in the unit.
Results
Demographics and co-morbidities
The ‘Attikon’ cohort consists of 555 SLE patients, all of whom are Caucasian. The mean age at lupus diagnosis was 38.3 (SD = 15.6) years, and the median disease duration at last follow-up was two years (IQR 10 years). The female-to-male ratio was approximately 9:1, with a less pronounced ratio in patients diagnosed after 50 years of age (late-onset; approximately 4:1). A total of 135 (24.3%) patients were diagnosed with late-onset SLE, while 57 (10.3%) patients were identified as having cSLE. At the time of diagnosis, 294 (53%) subjects had mild disease, while 143 (25.8%) and 118 (21.2%) were diagnosed as having a moderate or severe phenotype, respectively. Irreversible damage accrual was prevalent in 99 (17.8%) patients already within six months from disease diagnosis. The respective items of the SDI are shown in Supplemental Table S1.
The most frequent associated diseases and co-morbidities were thyroid disease (29.1%; mainly Hashimoto’s thyroiditis), obesity (22.2%), hypertension (20.2%), dyslipidaemia (15.7%), major depression (11.2%), osteoporosis (8.2%), diabetes mellitus (5.4%) and valvular heart disease (4.5%). The percentage of active smokers at enrolment was as high as 32.8%, which is consistent with the rate of the general population in Greece.24
Clinical manifestations and immunological profile
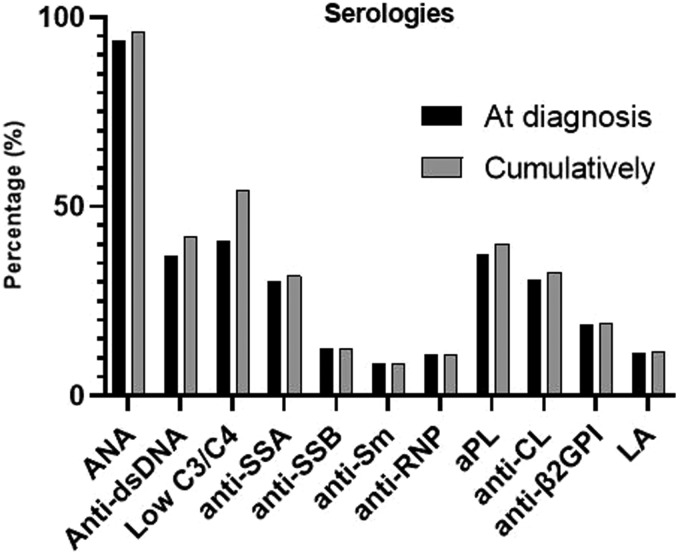
The most common clinical manifestations at disease onset are summarized in Table 1. Of manifestations included in previous sets of classification criteria, inflammatory arthritis (73.3%), acute cutaneous lupus (65%; mainly photosensitive rash (50.8%) and malar rash (39.8%)) and leucopaenia (23.8%) were the most common. A quarter of patients (25%) presented with unexplained fever, an item recently included in the new European League Against Rheumatism (EULAR)/ACR classification criteria. Among ‘non-criteria’ symptoms, the most frequent at diagnosis were Raynaud’s phenomenon (33.0%), while livedo reticularis and lymphadenopathy were observed in 6.8% and 6.7%, respectively. At the time of diagnosis, 6.3% of patients were negative for antinuclear antibodies (ANA), while only 8.5% were positive for anti-Smith, 36.9% for anti-dsDNA and 37.3% for antiphospholipid antibodies (aPL). Serological items at diagnosis and cumulatively are summarized in Figure 1.
Table 1.
Clinical manifestations at diagnosis and at last follow-up (N = 555)
| Clinical items | At diagnosis | Cumulatively |
|---|---|---|
| Arthritis, n (%) | 407 (73.3) | 473 (85.2) |
| Acute cutaneous lupus, n (%) | 361 (65.0) | 393 (70.8) |
| Malar rash, n (%) | 221 (39.8) | 250 (45.0) |
| Photosensitivity, n (%) | 282 (50.8) | 297 (53.5) |
| Chronic cutaneous lupus, n (%) | 55 (9.9) | 62 (11.2) |
| Oral/nasal ulcers, n (%) | 98 (17.7) | 143 (25.8) |
| Non-scarring alopecia, n (%) | 124 (22.3) | 175 (31.5) |
| Lupus nephritis, n (%) | 57 (10.3) | 118 (21.3) |
| Primary NPSLE, n (%) | 64 (11.5) | 98 (17.6) |
| Serositis, n (%) | 64 (11.5) | 104 (18.7) |
| Leucopaenia, n (%) | 132 (23.8) | 196 (35.3) |
| AIHA, n (%) | 15 (2.7) | 19 (3.4) |
| Thrombocytopaenia, n (%) | 68 (12.3) | 88 (15.9) |
| Raynaud’s, n (%) | 183 (33.0) | 205 (37.0) |
| Fever, n (%) | 138 (25.0) | 171 (31.0) |
| Livedo reticularis, n (%) | 38 (6.8) | 57 (10.2) |
| Lymphadenopathy, n (%) | 37 (6.7) | 51 (9.2) |
NPSLE: neuropsychiatric systemic lupus erythematosus; AIHA: autoimmune haemolytic anaemia.
Figure 1.
Immunological profile of subjects with SLE in the ‘Attikon’ cohort at diagnosis and cumulatively. SLE: systemic lupus erythematosus; LA: lupus anticoagulant; aPL: antiphospholipid antibodies.
Lupus nephritis and neuropsychiatric disease
At the time of disease diagnosis, kidney involvement was present in only 57 (10.3%) patients, while 61 (11%) more patients exhibited LN during follow-up, reaching an overall prevalence of 21.3%. Among patients with biopsy-proven LN, the most common histological patterns were class III/IV (45.3%), class V (23.8%) and a combination of class III/IV and class V (19%). Eight (6.8%) patients reached ESRD, with four already at the time of diagnosis and four over the course. Fifteen (12.7% of those with kidney involvement) patients developed CKD.
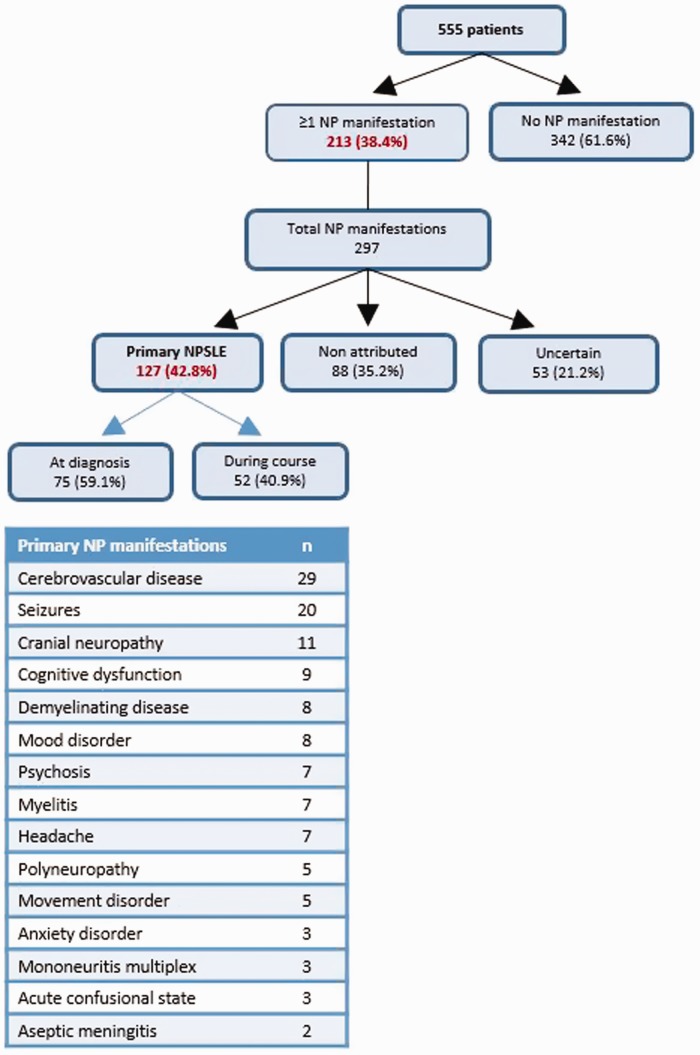
In our cohort, 213 (38.4%) patients developed at least one neuropsychiatric manifestation, while the total number of neuropsychiatric manifestations captured was 297. A total of 129 primary neuropsychiatric manifestations were observed in 98 (17.6% of total cohort population) patients. Approximately two-thirds (64/98) of NPSLE patients had at least one SLE-related neuropsychiatric manifestation at the time of diagnosis, while 34 (34.7%) patients manifested NPSLE during follow-up. The most common primary neuropsychiatric manifestations were stroke, seizure disorder and cranial neuropathy (Figure 2).
Figure 2.
Flow chart of all neuropsychiatric manifestations and types of events of the ‘Attikon’ cohort. Among 297 manifestations recorded, 127 were attributed to SLE, corresponding to 98 patients (17.6% of the whole cohort).
Rare and severe ‘non-criteria manifestations’
The use of classification criteria for diagnosis has raised concerns about the possibility of missing a diagnosis, especially in patients with early and incomplete disease. A high cumulative prevalence of moderate to severe ‘non-criteria manifestations’ was captured in our cohort. Non-criteria manifestations attributed to SLE were (number of patients at diagnosis/cumulatively): vasculitis (12/22), pulmonary embolism (11/22), pneumonitis (7/15) interstitial lung disease (6/15), autoimmune hepatitis (8/11), ocular involvement including uveitis, episcleritis and retinal vasculitis (4/8), pulmonary arterial hypertension (3/8), myocarditis (3/7), diffuse alveolar haemorrhage (3/6), peritonitis (2/6), thrombotic thrombocytopenic purpura-like syndrome (3/5), myositis (2/4) and macrophage activation syndrome (2/4). Although these manifestations were individually rare, in sum 67 (12.1%) patients presented with such a manifestation at onset. Also, 108 (19.5%) patients developed one or more ‘non-criteria’ major organ involvement during the course of their disease, suggesting a high cumulative prevalence of non-typical SLE manifestations.
Secondary APS
Fifty-seven (10.3%) SLE patients (female:male approximately 3:1) were diagnosed with secondary APS. Among them, 51 (89.5%) patients exhibited thrombotic APS, 12 (21%) obstetric APS and six (10.5%) both thrombotic and obstetric APS. Nine (15.8%) of these patients had been diagnosed with APS prior to the diagnosis of SLE (mean years to SLE diagnosis = 7.9 (SD = 6 years)), while in 30 (52.6%) patients, the diagnoses of lupus and APS were made simultaneously. Eighteen (31.6%) patients developed APS over the course of the disease (mean disease duration until APS diagnosis = 6.9 years (SD = 8.5 years)). The most common thrombotic events were deep-venous thrombosis (47.4%; n = 27) and stroke (29.8%; n = 17). Among APS patients, lupus anticoagulant positivity was detected in 28 (49.1%), while triple positivity was observed in 14 (24.6%) subjects.
cSLE versus late-onset SLE
The frequency of individual manifestations at the time of diagnosis was not different between cSLE and late-onset SLE (Table 2), with the exception of fever (more prevalent in cSLE: 40.4% vs. 12.6% in late-onset; p < 0.001). Over the course of the disease, the cSLE population developed LN, acute cutaneous lupus, oral ulcers and non-scarring alopecia more frequently. Of 57 cSLE patients, 22 (38.6%) and 10 (17.6%) developed LN and NPSLE, respectively. Accordingly, LN and NPSLE were observed in 24 (17.8%) and 23 (17.1%) patients, respectively, among the late-onset group (n = 135). Contrary to LN, NPSLE appears to have a steady prevalence, irrespective of age group.
Table 2.
Clinical characteristics of SLE patients with cSLE versus late-onset SLE
| Clinical items | At diagnosis |
p-Value | Ever |
p-Value | ||
|---|---|---|---|---|---|---|
| cSLE (N = 57) | Late-onset SLE (N = 135) | cSLE (N = 57) | Late-onset SLE (N = 135) | |||
| Arthritis, n (%) | 35 (61.4) | 95 (70.4) | 0.22 | 45 (78.9) | 109 (80.8) | 0.77 |
| Acute cutaneous lupus, n (%) | 41 (71.9) | 84 (62.2) | 0.19 | 47 (82.4) | 87 (64.4) | 0.013 |
| Chronic cutaneous lupus, n (%) | 6 (10.5) | 15 (11.1) | 0.90 | 6 (10.5) | 15 (11.1) | 0.90 |
| Oral/nasal ulcers, n (%) | 12 (21.1) | 13 (9.6) | 0.055 | 20 (35.1) | 18 (13.3) | <0.001 |
| Non-scarring alopecia, n (%) | 12 (21.2) | 24 (17.8) | 0.59 | 22 (38.6) | 31 (23.0) | 0.026 |
| Lupus nephritis, n (%) | 8 (14.0) | 19(14.1) | 0.46 | 22 (38.6) | 24 (17.8) | 0.002 |
| Primary NPSLE, n (%) | 7 (12.3) | 19 (14.1) | 0.74 | 10 (17.6) | 23 (17.1) | 0.93 |
| Serositis, n (%) | 9 (15.8) | 23 (17.0) | 0.83 | 10 (17.6) | 29 (21.4) | 0.53 |
| Leucopaenia, n (%) | 19 (33.3) | 31 (23.0) | 0.13 | 26 (45.6) | 43 (31.9) | 0.07 |
| Thrombocytopaenia, n (%) | 7 (12.3) | 21 (15.6) | 0.55 | 13 (22.8) | 24 (17.8) | 0.42 |
| Raynaud’s, n (%) | 17 (29.8) | 50 (36.3) | 0.33 | 21 (36.8) | 51 (37.0) | 0.90 |
| Fever, n (%) | 23 (40.4) | 17 (12.6) | <0.0001 | 25 (43.9) | 20 (14.8) | <0.001 |
Statistically significant values are shown in bold.
SLE: systemic lupus erythematosus; cSLE: childhood-onset SLE.
Therapies
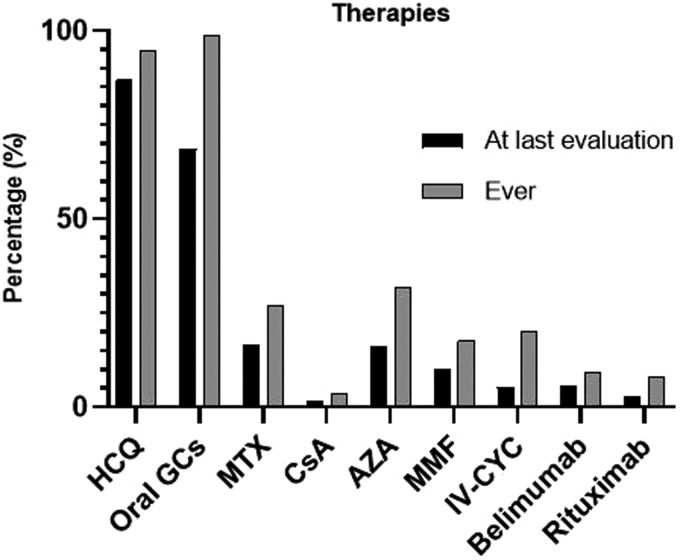
All administered immunosuppressive drugs (both current and past medications) are summarized in Figure 3. Azathioprine (AZA) and methotrexate (MTX) were the most commonly used immunosuppressive agents for mild/moderate disease (31.7% and 26.8%, respectively), while calcineurin inhibitors were rarely used. Belimumab was used in 51 (9.2%) patients. Mycophenolate mofetil was administered at a lower rate (17.7%) in moderate/severe cases. Specifically, it was used in 20 patients with moderate disease (11.5%; 21/182) and in 70 with severe lupus (36.8%; 77/209). For life-threatening, refractory or severe SLE (n = 209), intravenous cyclophosphamide (CYC) was the main therapeutic option (56.5%; 118/209). Rituximab (RTX) was administered ‘off-label’ for severe/refractory disease in 39 (20.6% of those with severe disease) patients. A significant percentage of patients (31.5%; 175/555) did not receive glucocorticoids at enrolment. Hydroxychloroquine was discontinued in 44 (7.9%) patients due to side effects, mainly due to allergic reactions and ocular toxicity.
Figure 3.
Types of treatment of subjects with SLE at both last evaluation and ever received in the ‘Attikon’ cohort. GCs: glucocorticoids; IV-MP: intravenous methylprednisolone; HCQ: hydroxychloroquine; IV-CYC: intravenous cyclophosphamide; MMF: mycophenolate; AZA: azathioprine; CsA: cyclosporine; MTX: methotrexate.
Discussion
There are several well-established lupus cohorts around the globe. As the phenotype of the disease and the available treatments evolve over time, it is also essential to assess relatively ‘fresh’ cohorts, which may provide a more accurate picture for modern lupus. We present such a SLE cohort, consisting exclusively of Caucasian patients, with approximately two-thirds of patients having an early diagnosis (e.g. disease duration of less than five years). This communication includes a thorough description with regards to clinical manifestations, particularly at the time of diagnosis, autoantibodies, demographics, co-morbidities, severity pattern, damage accrual over time and administered treatments.
A comparison of clinical manifestations at first presentation among our cohort and other cohorts around the world is summarized in Table 3.3,25–27 In Caucasian populations, musculoskeletal and skin involvement is common at disease onset, while the incidence of LN and positivity for anti-dsDNA or other lupus-specific autoantibodies are lower compared to non-Caucasian races. Neuropsychiatric disease represents an emerging phenotype among Caucasians.1 In cSLE, kidney and haematological involvement is common, accounting for more than 40% of lupus patients. Importantly, among ‘non-criteria manifestations’, Raynaud’s phenomenon is often present at initial presentation and could alert physicians towards a diagnosis of lupus. Recently, unexplained fever was added in the new EULAR/ACR classification criteria9,28 for SLE, which probably increases the sensitivity for early classification, since fever is prevalent in more than 25% of patients at the time of diagnosis.
Table 3.
Comparison of clinical features of SLE patients at the time of diagnosis from large SLE cohorts around the world
| Items | ‘Attikon’ cohort | Mosca et al.3 | Pons-Estel et al.25 | Joo et al.26 | Fiorot et al.27 | Total |
|---|---|---|---|---|---|---|
| Centre based | Europe | Multi-centre | Latin America | Asia | Latin America (childhood onset) | |
| No. patients | N = 555 | N = 389 | N = 1214 | N = 996 | N = 1312 | N = 4466 |
| Malar rash | 39.8% | 49.5% | 23.6% | 44% | 52.9% | 41.1% |
| Photosensitivity | 50.8% | 31.6% | 24.5% | 35% | 45.0% | 36.8% |
| Discoid | 7.4% | 9.3% | 5.3% | 8% | 5.3% | 6.5% |
| Oral ulcers | 17.7% | 21.6% | 10.5% | 36% | 32.8% | 24.6% |
| Alopecia | 22.3% | 30.6% | 20.3% | – | 21.7% | 22.3% |
| Arthritis | 73.3% | 57.6% | 67.3% | 65% | 68.4% | 67.0% |
| Pericarditis | 7.0% | 18.8% | 2.7% | 15% | 19.1% | 12.2% |
| Pleuritis | 7.6% | 22.4% | 3.6% | 19% | 17.6% | 13.3% |
| Renal involvement | 10.3% | 13.1% | 5.3% | 42% | 40.8% | 25.1% |
| Neuropsychiatric | 11.5% | 9.2% | 4.1% | 6% | 11.0% | 7.9% |
| Leucopaenia | 23.8% | 16.2% | 5.1% | 61% | 41.8% | 31.6% |
| Thrombocytopaenia | 12.3% | 6.6% | 5.2% | 24% | 18.9% | 15.5% |
| AIHA | 2.7% | 4.6% | 2.4% | 14% | 21.4% | 10.8% |
| Fever | 25.0% | 34.5% | 28.6% | – | – | 28.7% |
| Raynaud’s | 33.0% | 22.1% | 10.2% | – | – | 18.2% |
| ANA | 93.7% | 99.5% | – | 100% | 93.4% | 96.1% |
| Anti-dsDNA | 36.6% | 71.7% | – | 79% | 59.4% | 62.1% |
ANA: antinuclear antibodies; AIHA; Autoimmune hemolytic anemia; Anti-dsDNA; antidouble-strand DNA.
The kidney represents the most common major organ involved in SLE and is associated with the worst outcomes.29 Hispanics and African Americans are more likely to develop LN compared to Caucasians.10 In a large Asian cohort, LN was also found to be present in 42% of patients at the time of diagnosis.26 More recently, in the SLICC multi-ethnic cohort, consisting of approximately 2000 lupus individuals, the true incidence of LN was 38%, of whom 80% developed kidney involvement close to lupus diagnosis.29 In contrast, in our cohort, half of LN cases occurred after diagnosis and during the disease course. Notably, in the SLICC cohort,29 LN prevalence among Caucasians was approximately 20% (40–50% in Hispanics and African Americans), which is compatible with our results. Thus, LN may represent an overestimated feature in Caucasian lupus patients.
Compared to other large cohort studies, neuropsychiatric disease in our cohort does not seem to have significant differences in terms of risk factors, attribution rates, incidence, epidemiology and timing of NPSLE appearance.11,19,30–34 In our cohort, 15.1% of lupus subjects developed at least one primary neuropsychiatric manifestation, consistent with findings in other cohorts.11,30 Only 14 (2.5%) patients developed ‘minor’ neuropsychiatric manifestations that were considered as attributed to SLE based on the presence of risk factors (e.g. generalized disease activity, aPL positivity and history of primary NPSLE) and multidisciplinary expert physician judgement.18,19 The incidence of distinct and relatively common neuropsychiatric manifestations, such as seizures, cerebrovascular events, neuropathies and psychosis, are also consistent with currently published large studies,31–34 with more than half of primary neuropsychiatric manifestations occurring at the time of diagnosis. Notably, our data indicate that neuropsychiatric involvement is more frequent at the time of diagnosis in Caucasians compared to Asians and Hispanics,25,26 while cSLE seems to have comparable prevalence of primary NPSLE at disease onset compared to our results27 (Table 3). Thus, neuropsychiatric disease represents an increasingly recognized phenotype in lupus patients.
Late-onset lupus represents a distinct phenotype, accounting in most series for up to 10% of lupus patients, generally characterized by a milder disease pattern and lower disease activity over time.13,14 A quarter of our patients were diagnosed as late-onset SLE, a relatively high percentage not previously reported in the literature. Our results confirm the lower incidence of LN in late-onset disease, while NPSLE seems to have a steady frequency among different age groups. Late-onset patients exhibited a stable clinical course without significant accumulation of additional manifestations over the course of their disease. Moreover, the initial presentation of lupus in terms of clinical manifestations and disease severity did not differ between cSLE and late-onset SLE (Table 2). Only fever at the time of diagnosis was significantly more prevalent in cSLE and adult-onset SLE compared to late-onset SLE. Concerning cSLE, in our experience, initial disease phenotype was not as severe as indicated in the current literature.2 However, this group ultimately developed more severe disease over the course, with a high incidence of LN.
Our data indicate a more restricted immunological profile due to low rates of positivity of multiple autoantibodies, as was recently reported in a Caucasian cohort.1 In contrast, the prevalence of multiple autoantibodies is almost double in large cohort consisting of Hispanics, Afro-Americans and Asians.2,25–27 Approximately 6% of our patients were ANA negative at the time of diagnosis, a finding identical to the multi-centre SLICC lupus cohort.35
AZA and MTX remain the main medications for mild/moderate lupus as first-line agents. Despite the progress in the management of severe SLE over the last three decades, cytotoxic therapies such as CYC still represent commonly used drugs for the treatment of severe disease. During the last decades, in addition to cytotoxic therapies, new immunosuppressive and biological agents have been introduced to the armamentarium of SLE treatment.36 Our data indicate that belimumab and RTX are increasingly used in clinical practice for the management of moderate and severe lupus, respectively.
Our study is limited by the retrospective data collection in approximately one-third of patients in the ‘prevalent cohort’, which includes approximately one-third of patients diagnosed prior to the establishment of our registry. In this group, we included only lupus subjects with comprehensive medical records and adequate information from their medical history, reflecting the true course of the disease of each patient. The low incidence of some severe manifestations captured in our cohort, such as LN in 21.3%, may be attributed to the skewed distribution of disease duration, since the median disease duration in our cohort is only two years. Yet, lower rates of LN have also been reported in the ‘Leto’ lupus cohort in Crete.1
In summary, our data confirm the relatively low incidence of LN in Caucasians compared to other racial backgrounds, and describe a more contemporary phenotype of NPSLE with a higher rate of late-onset SLE than previously reported. These data may help to document changes in the natural history and treatment of SLE over time.
Supplemental Material
Supplemental material, LUP908932 Supplemental Material for Evolving phenotype of systemic lupus erythematosus in Caucasians: low incidence of lupus nephritis, high burden of neuropsychiatric disease and increased rates of late-onset lupus in the ‘Attikon’ cohort by D Nikolopoulos, M Kostopoulou, A Pieta, T Karageorgas, D Tseronis, K Chavatza, S Flouda, P Rapsomaniki, A Banos, E Kremasmenou, V Tzavara, P Katsimbri, A Fanouriakis and D T Boumpas in Lupus
Acknowledgements
We thank the staff physicians (Drs M. Aggelakos, K. Thomas, E. Atsali, S. Boiu and L. Fotis) and nurses (A. Ntourou, K. Togia and T. Gerogianni) of the Rheumatology Unit of the ‘Attikon’ University Hospital of Athens for their referral and care of the patients with SLE.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was funded in part by: The Hellenic Society of Rheumatology; the Foundation for Research in Rheumatology (FOREUM); the Greek General Secretariat of Research and Technology ‘Aristeia’ action of the Operational Program ‘Education and Lifelong Learning’ (co-funded by the European Social Fund and National Resources, Aristeia I 2344 to DB); the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 742390); and the SYSCID (A Systems Medicine Approach to Chronic Inflammatory Diseases) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 733100).
ORCID iD
D Nikolopoulos https://orcid.org/0000-0002-9894-6966
Supplemental material
Supplemental material for this article is available online.
References
- 1.Gergianaki I, Fanouriakis A, Repa A, et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: data from the community-based lupus registry of Crete, Greece. Ann Rheum Dis 2017; 76: 1992–2000. [DOI] [PubMed] [Google Scholar]
- 2.El Hadidi KT, Medhat BM, Abdel Baki NM, et al. Characteristics of systemic lupus erythematosus in a sample of the Egyptian population: a retrospective cohort of 1109 patients from a single center. Lupus 2018; 27: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 3.Mosca M, Costenbader KH, Johnson SR, et al. Brief report: how do patients with newly diagnosed systemic lupus erythematosus present? A multicenter cohort of early systemic lupus erythematosus to inform the development of new classification criteria. Arthritis Rheumatol 2019; 71: 91–98. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Yoshida K, Feldman CH, et al. Initial disease severity, cardiovascular events and all-cause mortality among patients with systemic lupus erythematosus. Rheumatology (Oxford). Epub ahead of print 3 September 2019. DOI: 10.1093/rheumatology/kez433. [Google Scholar]
- 5.Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol 2013; 9: 687–694. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725–1725. [DOI] [PubMed] [Google Scholar]
- 7.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inês L, Silva C, Galindo M, et al. Classification of systemic lupus erythematosus: Systemic Lupus International Collaborating Clinics versus American College of Rheumatology Criteria. A comparative study of 2,055 patients from a real-life, international systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2015; 67: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 9.Adamichou C, Nikolopoulos D, Genitsaridi I, et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann Rheum Dis 2020; 79: 232–241. [DOI] [PubMed] [Google Scholar]
- 10.Alarcón GS, McGwin G, Brooks K, et al. Systemic lupus erythematosus in three ethnic groups. XI. Sources of discrepancy in perception of disease activity: a comparison of physician and patient visual analog scale scores. Arthritis Care Res (Hoboken) 2002; 47: 408–413. [DOI] [PubMed] [Google Scholar]
- 11.Flower C, Hambleton I, Corbin D, Marquez S, Edghill R. The spectrum of neuropsychiatric lupus in a Black Caribbean population: a report of the Barbados National Lupus Registry. Lupus 2017; 26: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez Gómez LA, Uribe Uribe O, Osio Uribe O, et al. Childhood systemic lupus erythematosus in Latin America. The GLADEL experience in 230 children. Lupus 2008; 17: 596–604. [DOI] [PubMed] [Google Scholar]
- 13.Bertoli AM, Alarcón GS, Calvo-Alén J, Fernández M, Vilá LM, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort: clinical features, course, and outcome in patients with late-onset disease. Arthritis Rheum 2006; 54: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 14.Catoggio LJ, Soriano ER, Imamura PM, et al. Late-onset systemic lupus erythematosus in Latin Americans: a distinct subgroup? Lupus 2015; 24: 788–795. [DOI] [PubMed] [Google Scholar]
- 15.Bello GA, Brown MA, Kelly JA, Thanou A, James JA, Montgomery CG. Development and validation of a simple lupus severity index using ACR criteria for classification of SLE. Lupus Sci Med 2016; 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004; 65: 521–530. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 18.Liang MH, Corzillius M, Bae SC, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed] [Google Scholar]
- 19.Bortoluzzi A, Scirè CA, Bombardieri S, et al. Development and validation of a new algorithm for attribution of neuropsychiatric events in systemic lupus erythematosus. Rheumatol (Oxford) 2014; 54: 891–898. [DOI] [PubMed] [Google Scholar]
- 20.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001; 45: 419–423. [DOI] [PubMed] [Google Scholar]
- 21.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum 1996; 39: 363–369. [DOI] [PubMed] [Google Scholar]
- 22.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 23.Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005; 44: 902–906. [DOI] [PubMed] [Google Scholar]
- 24.Vardavas CI, Kafatos AG. Smoking policy and prevalence in Greece: an overview. Eur J Public Health 2007; 17: 211–213. [DOI] [PubMed] [Google Scholar]
- 25.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus. Medicine (Baltimore) 2004; 83: 1–17. [DOI] [PubMed] [Google Scholar]
- 26.Joo YB, Bae SC. Assessment of clinical manifestations, disease activity and organ damage in 996 Korean patients with systemic lupus erythematosus: comparison with other Asian populations. Int J Rheum Dis 2015; 18: 117–128. [DOI] [PubMed] [Google Scholar]
- 27.Fiorot FJ, Islabão AG, Pereira RM, et al. Disease presentation of 1312 childhood-onset systemic lupus erythematosus: influence of ethnicity. Clin Rheumatol 2019; 38: 2857–2863. [DOI] [PubMed] [Google Scholar]
- 28.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 29.Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatol (Oxford) 2015; 55: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn GY, Kim D, Won S, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus 2018; 27: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 31.Hanly JG, Urowitz MB, Su L, et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Ann Rheum Dis 2015; 71: 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanly JG, Li Q, Su L, et al. Cerebrovascular events in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Care Res 2018; 70: 1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanly JG, Li Q, Su L, et al. Peripheral nervous system disease in systemic lupus erythematosus: results from an international, inception cohort study. Arthritis Rheumatol 2020; 72: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanly JG, Li Q, Su L, et al. Psychosis in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol 2019; 71: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi MY, Clarke AE, St Pierre Y, et al. Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res (Hoboken) 2019; 71: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, LUP908932 Supplemental Material for Evolving phenotype of systemic lupus erythematosus in Caucasians: low incidence of lupus nephritis, high burden of neuropsychiatric disease and increased rates of late-onset lupus in the ‘Attikon’ cohort by D Nikolopoulos, M Kostopoulou, A Pieta, T Karageorgas, D Tseronis, K Chavatza, S Flouda, P Rapsomaniki, A Banos, E Kremasmenou, V Tzavara, P Katsimbri, A Fanouriakis and D T Boumpas in Lupus