Abstract
Current vaccines that provide protection against infectious diseases have primarily relied on attenuated or inactivated pathogens. Virus‐like particles (VLPs), comprised of capsid proteins that can initiate an immune response but do not include the genetic material required for replication, promote immunogenicity and have been developed and approved as vaccines in some cases. In addition, many of these VLPs can be used as molecular platforms for genetic fusion or chemical attachment of heterologous antigenic epitopes. This approach has been shown to provide protective immunity against the foreign epitopes in many cases. A variety of VLPs and virus‐based nanoparticles are being developed for use as vaccines and epitope platforms. These particles have the potential to increase efficacy of current vaccines as well as treat diseases for which no effective vaccines are available. WIREs Nanomed Nanobiotechnol 2011 3 174–196 DOI: 10.1002/wnan.119
This article is categorized under:
-
1
Therapeutic Approaches and Drug Discovery > Nanomedicine for Infectious Disease
INTRODUCTION
The goal of vaccination is to initiate a strong immune response that leads to the development of lasting and protective immunity. Vaccines against pathogens are the most common, but approaches to develop vaccines against cancer cells, host proteins, or small molecule drugs have been developed as well.1,2 For the purposes of this article, we focus primarily on the use of virus‐based platforms in the development of vaccines for infectious disease.
The immune system is composed of the innate (nonspecific) and adaptive (specific) branches. After a pathogen breaches the host's physical barriers such as mucosal surfaces or skin, cells of the innate immune system can recognize general characteristics of the pathogen and initiate a response.3 Pathogen‐associated molecular patterns (PAMPs) are molecules that are common to many pathogens, such as lipopolysaccharide (LPS), which can be found on the cell wall of many bacterial species, double‐stranded RNA or unmethylated CpG motifs, which are normally associated with virus infection. PAMPs can be recognized by Toll‐like receptors (TLRs) and other pattern‐recognition receptors (PRRs) which are present on the surface of host cells.4 The intrinsic properties of multivalent display and highly ordered structure present in many pathogens also facilitate recognition by PAMPs, resulting in increased immunogenicity. PAMPs stimulate antigen uptake by antigen presenting cells and the subsequent presentation of antigens to cells of the adaptive immune response. Furthermore the initial response by polymorphonuclear (PMNs) leukocytes, granulocytes, and natural killer (NK) cells induces the release of pro‐inflammatory cytokines that promote elimination of pathogens.
The adaptive immune response takes longer to develop, on the order of days. Adaptive responses require the recognition of the pathogen by host lymphocytes through interaction of cell‐surface receptors that are unique to the lymphocyte. An enormous repertoire of possible receptor combinations allows for the recognition of almost any antigen presented.5 Once the antigen is delivered to the adaptive immune system and stimulates the proliferation of antigen‐specific effector cells, these cells begin eliminating the pathogen. The adaptive response is also the basis for immunological memory, which is important for ensuring a fast, strong response when an infectious pathogen is encountered again.6 Memory lymphocytes can form subsequent initiation for a rapid and immediate immune response upon reintroduction of the pathogen. Vaccines capitalize on immune memory by stimulating the formation of memory B‐ and T‐ cells and specific antibodies to a pathogen, which are then available to rapidly recognize the natural pathogen during future exposures. The ideal vaccine utilizes this process by introducing the immune system to a specific pathogen and allowing the formation of memory cells for future recognition, yet avoiding symptoms, illness, or transmission of the pathogen to other individuals.
VACCINE STRATEGIES FOR INITIATING IMMUNE RESPONSES
Viruses are continually evolving attributes that allow them to survive in a hostile environment, and hosts evolve strategies of recognizing and relieving themselves of the pathogen. We can take advantage of these natural strategies of recognition as we develop vaccines. Classical approaches to vaccine development involve chemical inactivation of virus particles or infected tissues (termed ‘killed’ vaccines), attenuation of virulence during passage in tissue culture or animal hosts (termed ‘live‐attenuated’ vaccines), or immunization with portions of the pathogen that can induce specific immune responses (termed ‘subunit’ vaccines). To generate vaccines against viruses, the delicate balance of protection and safety was first reached by using whole virus that was inactivated or killed. More recently, as understanding of the immune system activation has improved, it has become apparent that even small immunogenic peptides derived from a pathogen have the potential for inducing protective immunity.7
Currently, a large number of successful vaccines are based on whole viruses, whether live‐attenuated or inactivated. The first vaccine developed against smallpox by Edward Jenner in 1796, also involved the administration of less pathogenic cowpox (vaccinia) virus.8 The method for immunization with vaccinia was crude and imprecise, the mechanism of immune protection was not well understood, and the potential for vaccine‐induced illness was high. Over the next century, various vaccines for a range of infectious (primarily bacterial) agents, such as cholera, tetanus, and the bubonic plague were developed and used in humans.
A major advance toward the development of modern vaccines was the ability to grow and produce viruses in laboratory cell cultures, allowing better characterization and standardization. The first licensed vaccine produced from cell culture was an injected dose of killed poliovirus developed by Jonas Salk and announced to the American public in 1955.9,10 Other whole‐virus vaccines quickly followed suit and have been tremendously successful at reducing morbidity and mortality worldwide, including measles, mumps, rubella, influenza, and the inactivated hepatitis A vaccine.11, 12, 13
In spite of the success of the poliovirus and other vaccines, there are intrinsic disadvantages involved with the use of whole virus particles for immunization.7 Live‐attenuated viral vaccines are relatively unstable and difficult to deliver. The potential for genetic reversion of the attenuated strain to a more virulent form is always a concern. While administration of killed whole‐virus vaccines negates this particular problem, the immune response to these is weaker and a regimen of doses is generally required, increasing the expense and reducing vaccine coverage in a population. In addition, complete inactivation of the vaccine virus must be assured to prevent infection.
Subunit vaccines prime immune responses by delivery of a subset of immunogenic viral proteins, often portions of the virus capsid (Figure 1). Because there is no potential for replication, subunit vaccines are potentially much safer, but are often less immunogenic when expressed and purified in the absence of other viral components. An advance in this area involves the natural ability of many types of viral capsid subunits to self‐assemble into virus‐like particles (VLPs), which allows for a better mimic of the whole virus particle, and a resulting improvement in effectiveness as vaccines (Figure 2).18, 19, 20, 21, 22 Further advances utilize the VLP as a platform for the attachment or display of foreign epitopes such as nonstructural viral proteins or nonviral proteins. This strategy couples delivery of the chosen antigen with the ability of the VLP to induce strong immune responses. The ease with which VLPs are often made and various antigens are attached to them makes every potential platform promising from onset. However, application of the platform in a complex system involves unique challenges and problems that may limit their progression in clinical use. Each of these approaches is discussed below, as well as summarized in Table 1.
Figure 1.
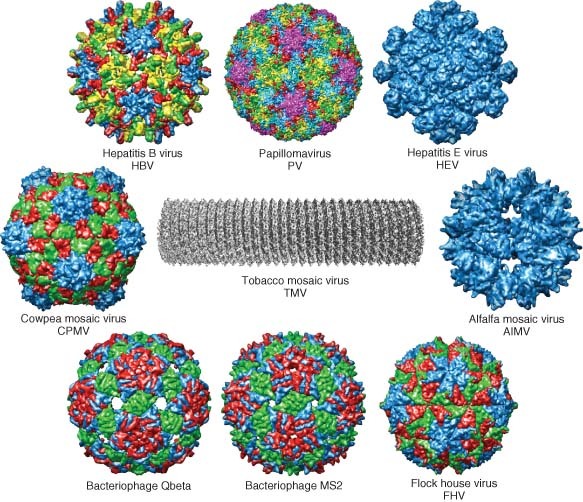
Examples of various virus capsids that have been developed as virus‐like particle (VLP) vaccines and platforms for heterologous antigen. PDB IDS: hepatitis B capsid, 2QIJ; Papillomavirus (PV), 3IYJ;14,15 Hepatitis E virus (HEV), 2ZTN; Cowpea mosaic virus (CPMV), 1NY7; Alfalfa mosaic virus (AlMV), AMV; bacteriophage Qβ capsid, 1QBE; bacteriophage MS2–2MS2; Flock house virus (FHV) VLP, 2Q26. (Tobacco mosaic virus (TMV) image has been reprinted with permission from Ref 16. Copyright 2007 Academic Press). (Other images have been reprinted with permission from Ref 17. Copyright 2009 Oxford University Press).
Figure 2.
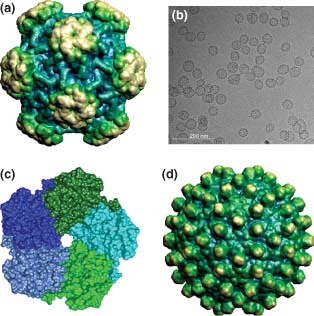
(a) Icosahedral capsid of human papilloma virus (HPV)16 L1 capsid, PDB ID: 1DZL. (b) Electron micrograph of HPV virus‐like particles (VLPs) (Image courtesy of Merck&Co and The Scripps Research Institute). (c) L1 pentamer of HPV rendered in chimera, PDB ID: 2R5H. (d) Human hepatitis B viral capsid (HBcAg), PDB ID: 1QGT. ((a) and (d) have been reprinted with permission from Ref 17. Copyright 2009 Oxford University Press).
Table 1.
VLP Vaccines and VLPs Developed for Display of Heterologous Epitopes
| Platform | Composition | Vaccine Target | Stage of Development | References |
|---|---|---|---|---|
| Poliovirus | VP0, VP3, VP1 | Poliovirus | Particles in development | 120 |
| HBV | Energix‐B | HBV | Testing in humans | 25,26,30 |
| HBV | HBsAg, envelope (major S, minor pre‐S2, pre‐S1) | HBV | Testing in humans | 27, 28, 29,121 |
| HPV | L1 and L2 | HPV | Approved | 31, 32, 33, 34,122,123 |
| Rotavirus | Variety | Rotavirus | Animal studies | 124, 125, 126 |
| HEV | Structural protein with truncated N terminus | HEV | Testing in humans | 39, 40, 41, 42 |
| Flu | HA, NA, and matrix proteins | Flu | Animal studies | 47, 48, 49 |
| HCV | Core, E1, E2 | HCV | Testing in nonhuman primates | 54, 55, 56, 57 |
| HIV/SIV | HIV or SIV capsid, and HIV immune epitopes (gag, pol) | HIV | Animal studies | 20,50,127,128 |
| Ebola virus and Marburg virus | Glycoprotein and matrix protein VP40 | EBOV and MARV | Testing in nonhuman primates | 58, 59, 60 |
| Norwalk virus | Major capsid protein | Norwalk virus | Testing in humans; potential as oral | 43, 44, 45 |
| SARS | S, E, M, and N structural proteins | SARS | Particles in development | 61 |
| FHV | FHV capsid with E1 of HCV | HCV | Particles in development | 112,113 |
| FHV | Core and E1 protein epitopes and HBsAg epitope on FHV capsid | HBV/HCV | Animal studies | 114,115 |
| FHV | FHV capsid with neutralizing domain from V3 loop of HIV‐1 | HIV | Animal studies | 116 |
| FHV | FHV capsid with ANTXR 2 PA‐binding domain | Anthrax—both protective and as a treatment | Animal studies | 65 |
| FHV | FHV capsid with CCR5 (HIV receptor) | HIV | Testing in humans | 129 |
| HBV | HBV core antigen and HIV env protein, conserved region of gp41, or p34 peptide | HIV | Animal studies | 69, 70, 71 |
| HBV | HBc with CS protein of malaria | Plasmodium falciparum | Testing in humans | 76,77 |
| HBV | HBsAg with env protein of DENV | Dengue virus | Animal studies | 118,119 |
| HBV | Epitope of FMDV on HBcAg | Foot and mouth disease virus | Animal studies | 73 |
| HBV | HBcAg with immunogenic HCMV epitope | Human cytomegalo virus | Animal studies | 130 |
| HBV | PUU nucleocapsid protein on HBc | Puumala hantavirus | Animal studies | 75 |
| HBV | HBc with M2 protein of influenza A | Influenza | Animal studies | 72 |
| HBV | HBcAg with CTL epitopes of HPV | HPV | Animal studies | 131 |
| HEV | HEV VLPs with B cell epitope tag | Stimulate mucosal immunity | Animal studies | 132 |
| HPV | L1 of HPV with SIV gag, HIV tat, and HIV rev | HIV | Testing in nonhuman primates | 79 |
| M13 | M13 version of S3Pvac | Taenia solium | Animal studies | 106 |
| M13 | Parasitic worm protein on M13 | Schistosoma japonicum | Animal studies | 107 |
| NDV | NP and M proteins of NDV with RSV G protein | RSV | Animal studies | 133 |
| Qβ | Qβ with allergen Der p1 | Allergen | Testing in humans | 134 |
| Qβ | Qβ with nicotine | Nicotine | Testing in humans | 2 |
| Qβ | CCR5 on Qβ | HIV | Animal studies | 135 |
| AP205 | Various heterologous epitopes on AP205 | Salmonella typhi, HIV, Influenza A | Animal studies | 108 |
| CPMV | CPMV with VP2 capsid protein of CPV | Canine parvovirus | Animal studies | 84,85 |
| CPMV | CPMV with outer membrane protein F of Pseudomonas aeruginosa | Pseudomonas aeruginosa | Animal studies | 89,90,101 |
| CPMV | Mink enteritis virus protein on CPMV | Mink enteritis virus | Animal studies | 86 |
| CPMV | Staphylococcus aureus protein on CPMV | Staphylococcus aureus | Animal studies | 91 |
| CPMV | Gp41 peptide inserted onto CPMV | HIV | Animal studies | 87,88 |
| CPMV | Outer membrane protein F of Pseudomonas aeruginosa on CPMV | Pseudomonas aeruginosa | Animal studies | 90 |
| Tobacco mosaic virus | Peptides from VP1 of FMDV on TMV | Foot and mouth disease virus | Animal studies | 95,96 |
| Tobacco mosaic virus | Peptide from spike protein of murine hepatitis virus on TMV | Murine hepatitis virus | Animal studies | 94 |
| Tobacco mosaic virus | Outer membrane protein F from Pseudomonas aeruginosa on TMV | Pseudomonas aeruginosa | Animal studies | 97 |
| Cucumber mosaic virus | R10 epitope of HCV on CMV | Hepatits C virus | Animal studies | 98 |
| Alfalfa mosaic virus | G protein peptide from RSV on AlMV coat protein | RSV | Testing in nonhuman primates | 100 |
| PapMV | LCMV peptide p33 on PapMV | LCMV | Animal studies | 64 |
| MS2 | Peptide from V3 of HIV gp20 or CCR5 on MS2 coat protein | HIV | Animal studies | 105 |
| HBV | Peptide from Bordatella pertussis on HBV core protein | Bordatella pertussis | Animal studies | 136 |
| HBV | Peptide from SPAG‐1 of Theileria annulata on HBcAg | Theileria annulata | Animal studies | 137,138 |
| ISCOMs | H1N1 peptide | H1N1 | Animal studies | 139,140 |
| ISCOMs | Core protein IMX | Hepatitis C virus | Testing in nonhuman primates | 141,142 |
| ISCOMs | Gp120, env, or gag peptides | HIV | Testing in nonhuman primates | 143, 144, 145, 146 |
| ISCOMs | Peptide F and G from RSV | RSV | Animal studies | 147,148 |
| ISCOMs | HSV‐2 antigen | Herpes simplex virus | Animal studies | 149 |
| ISCOMs | Rotavirus antigen | Rotavirus | Animal studies | 150,151 |
| Liposomes | Hepatitis A peptide | Hepatitis A virus | Testing in humans | 152, 153, 154 |
| Liposomes | Peptide from circumsporosoite protein of Plasmodium falciparum | Plasmodium falciparum | Testing in humans | 155 |
| Liposomes | Ha and Na from influenza | Influenza | Testing in humans | 156 |
| Liposomes | Gp120 from HIV | HIV | Animal studies | 157,158 |
| Liposomes | Toxins | Diptheria or tetanus | Animal studies | 159,160 |
| Liposomes | HA from influenza | Influenza | Animal studies | 161 |
| Virosomes | HA and NA from influenza | Influenza | Approved | 162 |
| Virosomes | Inactivated Hepatitis A virus | Hepatitis A virus | Animal studies | 153,163 |
| PLG microspheres | Whole cell lysate of Helicobacter pylori | Helicobacter pylori | Animal studies | 164 |
| PLG microspheres | Tetanus toxoid | Tetanus | Animal studies | 165 |
| PLG microspheres | HBsAg | Hepatitis B virus | Animal studies | 166,167 |
| PLG microspheres | Plasmodium falciparum antigen | Plasmodium falciparum | Animal studies | 168 |
| PLG microspheres | Yersinia pestis F1 antigen | Yersinia pestis | Animal studies | 169 |
| PLG microspheres | Phosphorylcholine antigen of Salmonella typhimuriun | Salmonella typhimurium | Animal studies | 170 |
| Nanoemulsion | Influenza A | Influenza | Animal studies | 171 |
| Nanoemulsion | Protective antigen from Bacillus anthracis | Anthrax | Animal studies | 172 |
| Nanoemusion | Gp120 from HIV | HIV | Animal studies | 173 |
| Nanoemulsion | Vaccinia virus | Smallpox | Animal studies | 174 |
| Nanoemulsion | HBsAg | Hepatitis B virus | Animal studies | 175 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
VLP AS VACCINES
VLPs are considered a subunit vaccine because they consist of a noninfectious subset of viral components, but mimic killed vaccines since they typically present a whole, but inactive virus particle to the host. Tailoring the viral proteins expressed can enable production of particles that are structurally and morphologically similar to infectious viruses, and retain the ability to bind and penetrate host cells. Noninfectious VLPs are inherently safer than killed or attenuated virus vaccines. In addition, due to their particulate nature, VLPs can be much more stable than soluble antigens, which have failed in many vaccine approaches. The multivalent and highly ordered structure of the VLP is not typical of host proteins, and thus constitutes a PAMP, which triggers innate immune sensing mechanisms. In addition, most VLPs encapsidate nucleic acids during their production, and these nucleic acids may stimulate particular TLRs as well. These qualities contribute to the effectiveness of VLPs as vaccines because they facilitate uptake by antigen presenting cells and long lasting CTL responses and antibody responses are possible.23,24
Nonenveloped VLPs
The two most successful VLP‐based vaccines have been licensed and approved for use in humans. The first to be commercialized was the hepatitis B virus (HBV) vaccine. HBV infection is transmitted by blood or sexual contact, and is a major cause of hepatitis and hepatocellular carcinoma, affecting approximately 300 million people worldwide. In the initial vaccine formulation, empty particles of small HBV‐derived surface antigen (HBsAg) that are naturally made during infection with hepatitis B and are present in the blood, were obtained from infected individuals and purified. The limited quantity of antigen produced by this method led to investigation of the potential for making the particles by recombinant protein expression. Expression systems in yeast, baculovirus, and mammalian cells are often utilized because these cultures can produce large amounts of proteins in vitro. In the case of HBV, vectors were prepared carrying the cDNA sequence for HBsAg, and the surface antigen proteins were expressed in recombinant yeast cell culture and VLPs formed by self‐assembly.25 Further studies confirmed the immunogenicity of these particles in human vaccination.26 Inclusion of surface proteins (preS1 and preS2 proteins), which are present in the native HBV envelope at lower levels than in HBsAg, allows for immune responses in the small percentage of patients who do not respond to VLPs of HBsAg alone.27, 28, 29 In this case, the better mimic of the authentic virion surface results in broadened effectiveness. The commercially successful vaccine, licensed in 1986, is a safe and effective example to which other vaccines are compared. The effectiveness of this vaccine has facilitated exploration into more complex issues such as administration in poorer countries where HBV is an epidemic and application of the current vaccine is limited due to cost. The most recent advances have tested the possibility of oral delivery of HBV particles that are produced in transgenic plants.30
The second commercially available VLP‐based vaccine is to human papillomavirus (HPV). An effective HPV vaccine is of particular interest because of the link of HPV infection, particularly genotypes HPV‐16 and HPV‐18, to cervical intraepithelial neoplasia (cervical cancer).31, 32, 33 The HPV capsid consists of two proteins, L1 and L2. Expression of the L1 major capsid protein of HPV16, either alone or in combination with L2 resulted in the production of 40 nm VLPs that induced high‐titer neutralizing antisera in rabbits.34,35 Immunizations in human females demonstrated that the HPV vaccine protected them from infection with HPV, and also reduced or prevented entirely the development of intraepithelial neoplasia. Of the control patients who received placebo, a subset was infected with HPV and some developed cervical intraepithelial neoplasia. The current HPV vaccine shows great promise, as it is almost completely protective against HPV types from which it is derived even in the absence of an adjuvant, and with adjuvant achieves partial protection against other phylogenetically related types.36 This VLP vaccine is considered a breakthrough for preventing cervical cancer, and it will be interesting to observe the impact of the vaccine on cervical cancer rates in the future. Since HPV‐16 has also recently been associated with oropharyngeal cancer,37 vaccination may impact the future rates of this disease as well. As with the HBV vaccine, production and characterization of VLPs is expensive and results in higher vaccine costs. Approaches to reduce costs by expressing the L1 protein as pentameric capsomeres, or in combinations with other viral proteins, are being explored.36
Other VLP‐based vaccines are in various stages of development.38 VLPs of hepatitis E virus (HEV), a virus thought to cause an acute severe liver disease in some individuals, were shown to be immunogenic and protective in macaques, and have now passed Phase I of clinical trials.39,40 Experimentation with the protein requirements for HEV VLP formation has facilitated further understanding of the formation of VLPs.41,42 However, circumstances specific to the disease hamper the testing of protective efficacy. The clinical attack rate of HEV is low and sporadic, requiring a large number of vaccinated and control individuals to achieve any interpretable results. VLPs of Norwalk virus, a highly infectious virus that is a major cause of gastrointestinal illness, have been tested in adults and are immunogenic.43 Strides toward an oral Norwalk virus vaccine produced in tobacco leaves and potato tubers have been successful, and oral immunization of mice resulted in production of serum and mucosal antibodies.44,45 In addition, a betanodavirus‐based VLP vaccine has found much success in preventing viral nervous necrosis disease in fish.46
Enveloped VLPs
Enveloped VLPs of viruses such as influenza, HIV, and HCV are similarly replication‐incompetent, and the immunogen consists of assembled particles containing some or all of the surface components of the virus embedded in plasma membrane. VLPs expressed in baculovirus and composed of influenza hemagglutinin (HA), neuraminidase (NA), and matrix structural proteins can induce production of specific antibodies in mice.47,48 Route of entry and presence of the cytokine interleukin‐12 (IL‐12) as an adjuvant alters the immune response and affords different levels of protection.49 In addition, although individual influenza proteins were capable of inducing protective immune responses, the use of baculovirus‐expressed recombinant revealed a problem that may be an underlying problem in many VLP‐based vaccines using recombinant proteins. The recombinant HA proteins were poorly immunogenic in people, perhaps due to differences in post‐translational modification such as glycosylation.47,48
The ability of HIV to consistently and effectively evade the immune system over time makes the development of an effective vaccine to HIV particularly difficult. On the surface, a VLP vaccine may be at a disadvantage compared to current replication‐based vaccine strategies because HIV‐1 VLPs are similar to the wild‐type viral structure, which does not elicit a protective response in infected individuals. However, modifications in the HIV VLP could advance this strategy. In addition, mucosal administration of the particles elicits strong neutralizing antibody and CTL responses.50 Efficient induction of broadly neutralizing antibodies is thought to be an essential component for inducing protection. Nevertheless, while such antibodies have been identified in a small subset of HIV‐infected patients, it is not yet possible to induce such antibodies experimentally. One significant challenge is the difficulty of producing antigens that are structurally similar to those found on the intact HIV particles. The production of an HIV VLP that confronts this problem has been attempted by mimicking the intact HIV particle containing the envelope proteins that associate with the viral gag protein core.50, 51, 52 The focus of recent research has been on the more conserved epitopes of the HIV‐1 gp‐41 envelope protein.53 Nevertheless the challenge of an efficient HIV vaccine will require further manipulation of the different VLPs and expression systems available.
Other VLP‐based vaccines for enveloped viruses have the potential to ultimately be used in humans. Progress has been made in developing VLPs for hepatitis C virus (HCV),54, 55, 56, 57 filoviruses Ebola virus (EBOV), Marburg virus (MARV),58, 59, 60 SARS coronavirus,61 and Chikungunya virus.62
USING VLPS AS PLATFORMS FOR DISPLAYING AND INDUCING IMMUNE RESPONSES AGAINST HETEROLOGOUS ANTIGENS
VLPs can also serve as a platform for multivalent heterologous epitope display, for the purpose of mounting an immune response to the protein or peptide that is attached through fusion or through genetic insertion into the capsid (Figure 3). VLPs thus serve as a multivalent particle platform; the capsid serves both as a presentation scaffold for epitopes from another viral, bacterial, or parasitic pathogen, and as an adjuvant to boost the immune response.2,63, 64, 65, 66 Because of the particulate nature of the VLPs, they are readily taken up into antigen‐presenting cells (APCs) and stimulate MHC Class I and Class II responses and therefore prime lasting T‐cell and antibody responses.
Figure 3.
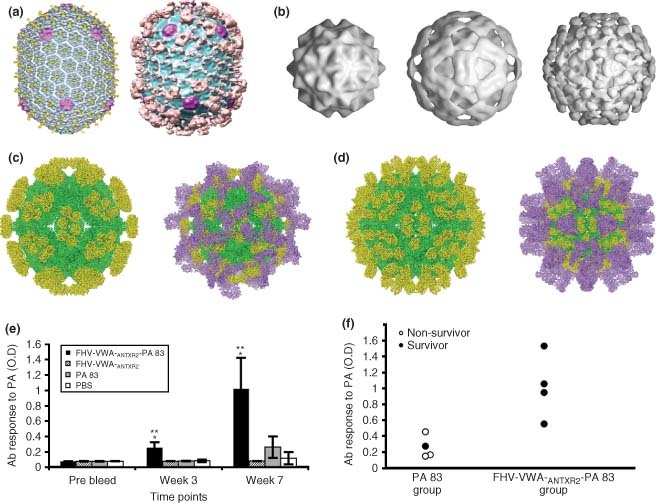
(a) Cryo‐EM reconstructions for wild‐type T4 capsid (left) and capsid with attached protective antigen fragment and N‐terminal domain of lethal factor of anthrax (right). (b) Three dimensional (*) surface shaded reconstructions of wild‐type flock house virus (FHV) (left), FHV–ANTXR2– protective antigen (PA) chimera 206 (center), and FHV–ANTXR2–PA chimera 264 (right). (c) Pseudoatomic models of FHV chimera 206 capsid protein (green) and anthrax VWA domain (yellow, left) and model including the protective antigen fragment bound to the surface (purple, right). (d) Pseudoatomic models of FHV chimera 264 capsid protein (green) and anthrax VWA domain (yellow, left) and model including the protective antigen fragment bound to the surface (purple, right). (e) Rats were immunized with FHV–ANTXR2–PA complex or controls and serum samples were collected at indicated time‐points and tested for IgG‐specific antibody responses to protective antigen. (f) Relationship between anti‐PA antibody level and survival of individual rats following challenge. ((a) has been reprinted with permission from Ref 67. Copyright 2007 Academic Press). ((b)–(f) have been reproduced with permission from Annette Schneemann Ref 65. Copyright 2007 Public Library of Science).
VLP‐based systems for epitope presentation are limited by certain constraints, including the difficulty of adding larger epitopes and proteins that might hinder VLP assembly. The viral capsid surface is a varied landscape, with local regions differing in charge, immunogenicity, and accessibility. Placement of antigen on this varied surface is the key to the immunogenicity and success of the vaccine. The first and most easily adaptable strategy is to introduce antigens into VLPs such as HBV and VLP that already have FDA approval as vaccines, such as HBV and HPV, and these are described below. In addition, novel VLP platforms displaying heterologous antigens are discussed.
Antigen Display on HBV VLPs
In many cases, the assembly and stability of VLPs is limited by the size of potential insertions, and small peptides (<50 aa) are often sufficient for the initiation of pathogen‐specific antibody production. For example, the addition of small peptides to the immunogenic c/e1 region of the VLP results in relatively strong responses. However, although the HBV core antigen (HBcAg) was shown to be strongly immunogenic after green fluorescent protein (GFP) was fused to the core protein and introduction resulted in robust humoral immune responses against native GFP in rabbits, the maintenance of the favorable properties of HBcAg with the addition of such a large protein remains to be determined.68
For HIV‐1, antigenic regions of the HIV envelope protein were linked to HBsAg, producing particles morphologically similar to HBcAg and which exhibited antigenic and immunogenic characteristics of both HBV and HIV.69 The short (6 aa) yet highly conserved region of HIV‐1 gp41 displayed on these particles elicited high titers of gp41‐reactive antibodies in mice, however they failed to neutralize HIV in vitro. 70 This same epitope was more successful in neutralizing antibody production when presented on the influenza A vaccine platform, thus the molecular context seems to be important when considering the ability to elicit protective responses. However, correct processing of particles or T‐cell recognition of HIV epitopes on particles is implied on HBV because both peptides and particles with gp41 epitope elicited similar responses.71
Other epitopes have found varying levels of success when displayed on the HBV platform. The invariant extracellular domain M2 of influenza A fused to HBc and administered intraperitoneally or intranasally gave >90% antibody‐mediated protection in mice.72 The attachment of an epitope of foot and mouth disease virus (FMDV) to HBcAg VLPs provides immunogenicity stronger than the peptide alone, approaching that of the FMDV particles themselves.73,74 The nucleocapsid of Puumala hantavirus on HBc provided protective immunity in voles.75
Parasitic infection can also be stemmed using HBV‐based vaccines. The circumsporozoite (CS) protein from malaria was expressed on HBc proteins, and proved to be highly immunogenic in both mice and monkeys and the first Phase I of trial of these particles was successful.76,77 Thus, HBV VLPs have proven to be a productive platform for attachment of epitopes for potential use as vaccines.
The HBV infection elicits a strong humoral response directed against the single immunodominant c/e1 epitope in humans. As expected, insertions at this epitope elicited much better responses than those at the C terminus.68 A thorough investigation into the effect of posititon on immunogenicity studied inserted epitopes of HBV envelope proteins at the amino terminus, the amino terminus with linker, truncated carboxy terminus, and an internal site of HBcAg. The most effective was the internal site, which resulted in very high antibody response. The weakest immune response was to the amino terminus because the epitope was not surface exposed.78
Antigen Display on HPV VLPs
Since the HPV vaccine has been approved for use in humans only recently, the study of these VLPs as platforms for vaccines is more limited. Chimeric HPV VLPs have made some progress as an HIV vaccine. Although administration of chimeras with epitopes from HIV showed significant protection in those macaques that showed protection, only partial protection was afforded, so there is plenty of opportunity for further improvement.79 To test how the location of the insertion affected immunogenicity of foreign peptides, an HBc epitope was introduced in various positions in the HPV VLP in order to assess and compare different positions. Although insertion sometimes reduced immunogenicity, each chimera formed particles and induced neutralizing antibodies against HBc.80 The development of HPV VLPs as a platform is still in the early stages, and the future success of these particles is uncertain but highly promising. Exploring the limits of increased sizes of inserted epitopes and tethering of proteins to the external portions of L1 or L2, will likely allow for more efficient formation and immunogenicity of chimeric VLPs.79
DISPLAY OF HETEROLOGOUS EPITOPES ON PLANT VIRUSES
Plant viruses in particular have shown promise as a platform for vaccine development. Many plant viruses are unable to replicate in mammals, and this characteristic allows the use of whole virus particles (often termed viral nanoparticles or VNPs) instead of VLPs. The viruses are modified to multivalently display antigens in a multivalent pattern through genetic introduction of foreign epitopes and proteins. These features augment the favorable safety profile and ease and low cost of large‐scale production, creating attractive alternatives to the currently available platforms.
Cowpea Mosaic Virus (CPMV)
CPMV has been extensively studied for vaccine applications.81,82 CPMV is a comovirus in the Picornavirus superfamily, and forms a 31 nm icosahedral particle composed of two capsid proteins termed large (L) and small (S). CPMV is a promising plant virus for epitope display for multiple reasons. Large amounts of icosahedral CPMV VLPs can be produced in the natural host Vigna unguiculata (cowpea), underscoring the potential for cost‐effective manufacture. CPMV particles are also taken up by a number of different antigen‐presenting cell types both in vitro, and in vivo following parenteral or oral administration.83 Particles are also found in the Peyer's patch‐associated lymphoid tissue after oral administration, underscoring their potential as a possible oral plant‐based vaccine.83 Heterologous peptides displayed on CPMV produce strong antibody responses when administered by parenteral and mucosal routes. As a result of mucosal administration, production of IgA antibodies indicates potential to protect against systemic and mucosal infections.84,85 Several exposed loops on the capsid surface, particularly one composed of amino acids 20–27 of the S capsid subunit, are of particular interest regarding development of CPMV as a vaccine.
CPMV particles have been genetically modified to include epitopes from Mink enteritis virus (MEV) VP2 and Canine parvovirus (CPV) VP2. Mink immunized with MEV CPMV particles were protected from viral challenge.86 Dogs immunized with CPV CPMV particles were protected from lethal challenge with CPV.84,85 Epitopes from the gp41 protein of HIV‐1 have also been inserted into the CPMV capsid and the resulting production of neutralizing antibodies suggests there is potential for application as an HIV vaccine.87,88
CPMV nanoparticles have been extensively studied for applications in bacterial vaccines as well. Administration of CPMV particles with peptides from Pseudomonas aeruginosa outer membrane (OM) protein F provided pathogen specific antibodies and protection from challenge.89 Expressing the same OM epitope in tandem with protein F peptide 18 on CPMV particles, it was shown that the site of peptide expression influences immune recognition.90 Administration of CPMV particles with a truncated D2 domain of Staphylococcus aureus induced neutrophil activity and macrophage phagocytosis in vitro, and provided protection from endocarditis in vivo. 91
The CPMV platform provides another example where the positioning of the inserted epitope influences the efficiency of protection. The relationship between a three‐dimensional structure of an epitope in the inserted site on CPMV and its immunogenicity were investigated with the display of NIm‐1A epitope from human rhinovirus 14 in different positions on the CPMV capsid. In this case, it was demonstrated that when the epitope was presented as a closed loop, the resulting polyclonal antisera had much greater capacity to bind intact human rhinovirus particles.92 One drawback toward using CPMV VLPs is frequent proteolytic cleavage of the inserted epitope; this can result in loss of the epitope and/or destabilization and heterogeneity of the particles.82 A recent advance toward generating noninfectious VLPs using Agrobacterium tumefaciens‐based heterologous expression in plants may solve these problems while retaining the possibility for large‐scale production.93
Tobacco Mosaic Virus (TMV)
Tobacco mosaic virus was the first plant virus casid to be genetically modified to contain a foreign epitope. TMV is a rod‐shaped RNA virus that infects tobacco and other plants in the Solanaceae family. The 18 by 300 nm capsid is made up of 2130 monomers surrounding genomic RNA. It provides an ideal platform for vaccines because it cannot infect animals and large quantities can be easily obtained. Particles with epitopes from murine hepatitis virus and FMDV were successful in providing protection against infectious particles in mice and guinea pigs, respectively.94, 95, 96
TMV particles have also been used for platforms for bacterial epitopes, including the outer membrane protein F of Pseudomonas aeruginosa. This formulation elicited protection from challenge when administered in mice.97 TMV may be a more efficient platform for epitope display when a highly ordered antigen array is required, because the number of potential epitopes per particle and the density of spacing is much higher than in an icosahedral arrangement.
Other Plant Viruses: CMV, AlMV, PVX, and PapMV
Cucumber mosaic virus (CMV) has a wide host range. Its genome is a linear positive‐sense single‐stranded RNA and its 31 nm icosahedral capsid is made up of 180 copies of a single capsid protein. CMV particles have been used for presentation of epitopes from HCV.98 Particles with the R9 epitope were successful in eliciting a pathogen specific response in rabbits, and HCV patients had a significant release of interferon‐gamma and interleukin‐12 and interleukin‐15.99 Alfalfa mosaic virus (AlMV) has four particle types (3 bacilliform and 1 spheroidal), all made up of the same coat protein. The virus infects over 600 plant species in 70 families. An antigenic eptide from Respiratory syncytial virus G protein was expressed on the capsid of AlMV, and specific antibodies were produced in vitro in human dendritic cells, and in vivo in nonhuman primates.100 Potato virus X (PVX) is a flexuous rod shape and the type member of the Potexvirus group. The capsid is made up of 1270 identical coat protein subunits. PVX particles have been utilized for attachment of an epitope of HIV‐1, and even yielded neutralizing antibodies in mice.101
Papaya mosaic virus (PapMV) forms a rod‐shaped 14 by 500 nm particle, and its immunogenicity is dependent on multimerization of the PapMV coat protein. Interestingly, PapMV has demonstrated efficacy as a platform when used to induce antigen‐specific CD8+ T‐cell responses, a process that is typically very inefficient when using a nonreplicating vaccine platform. Nevertheless, immunization with PapMV displaying the well‐characterized MHC Class I epitope peptide p33 (derived from lymphocytic choriomeningitis virus (LCMV)) that was expressed on the PapMV surface was able to protect vaccinated mice from subsequent challenge with LCMV.64 A similar result was observed with parvovirus VLPs displaying the same epitope,102 however most VLP platforms are not efficient at inducing Class I‐restricted CTL responses. It is not clear why some VLPs result in more efficient cross‐presentation of epitopes to Class I, but it may be due to differing routes of endocytosis of VLPs into antigen‐presenting cells governing their access to the antigen presentation pathway.
Bacteriophage Platforms for Heterologous Epitope Display: Qβ, MS2, AP205, M13, and T4
Bacteriophages present yet another platform for delivery of vaccine to animals. Bacteriophage Qβ is an RNA virus that infects Escherichia coli. It belongs to the positive‐stranded RNA phages family Leviviridae. The 24 nm icosahedral capsid is composed of 180 copies of a single coat protein. Displaying antigens on the Qβ particles has expanded the technology in other areas of disease besides infections. Immunization with Qβ particles coupled to nicotine succeeded in blocking entry of nicotine into the brain because the antibodies produced can sequester the molecules in the blood of immunized smokers. This is a very exciting advance for the treatment of smoking cessation, and these particles are currently in Phase II clinical trials.2,66
MS2 is another well‐characterized bacteriophage which has been utilized for attachment and display of viral epitopes.103 Structurally, MS2 is very similar to Qβ; sequence determination for the entire Qβ cDNA copy and replicase beta‐subunit of MS2 demonstrated regions of homology, and the coat proteins show 23% sequence identity.104 Peptides from the V3 loop of gp20 of HIV have been inserted into the coat proteins of MS2 and these particles were shown to be immunogenic in mice.105
Another bacteriophage, M13, has been used primarily for developing vaccines against parasitic infections. M13 is a filamentous bacteriophage made from 2700 copies of the major coat protein P8 and capped with five copies of each minor coat protein P9, P7, P6, and P3. A recent regimen of anticysticercosis tripeptide vaccine in pigs reduced incidence of infection by Taenia solium. This is a parasitic infection that primarily infects humans and pigs in Mexico and other Latin American countries and is characterized by headaches and seizures when infection is localized to the nervous system.106 Potential vaccine candidates have also been identified for Schistosoma japonicum infection, which infects humans and other wild mammals with a chronic illness that can damage internal organs.107 An effective vaccine is important in this case because there is little natural immunity to reinfection.
The RNA bacteriophage AP205 was investigated as a new platform for heterologous display of many antigens. The VLP is formed from 180 copies of the coat protein and allows both N‐terminal and C‐terminal fusion of epitopes. A fusion of a gonadotropin releasing hormone (GnRH) epitope successfully induced antibodies and a fusion of an extracellular domain of the Influenza A M2 protein elicited protective immunity in mice. Peptides from Salmonella typhi, HIV1 Nef, and others were also attached to this platform and resulted in immune responses.108
Bacteriophage T4 offers an ideal binding platform for antigen on the Hoc and Soc proteins because these accessory structural proteins are on the outer surface of the particle, provide little stabilization, and Hoc in particular is highly immunogenic.109, 110, 111 The four domains of the Hoc molecule have sites where exposure of epitopes to the immune system is ideal, in particular the linker regions between Domains 2 and 3, or between Domains 1 and 4. The T4 system is further discussed below.
INSECT VIRUS PLATFORMS FOR ANTIGEN DISPLAY
Flock House Virus (FHV)
Although the ability to act as an adjuvant may be reduced, nonanimal viruses also have potential for use as platform in animal vaccines. The insect virus flock house virus (FHV) is widely used for antigen display attachment and delivery in animals (Figure 3). FHV is a member of the insect virus family nodaviridae, but crosses the kingdom barrier and also replicates in plants and in neonatal mice. The icosahedral capsid is composed of 180 subunits of coat protein, and several surface exposed loops are promising sites for insertion of foreign epitopes. Insertions of HBV and HCV epitopes on various areas of the FHV capsid have been studied and compared. Many highly specific and reactive areas were identified for attachment.112, 113, 114 Testing in guinea pigs and in hepatitis B and C patients confirmed the potential for VLP‐based vaccines against HBV and HCV.115 FHV particles displaying HIV epitopes have also been tested, whereby a neutralizing domain of the V3 loop of HIV‐1 was inserted in the FHV capsid, and these particles initiated a strong immune response in guinea pigs.116
DISPLAY OF LARGE ANTIGENS ON VLPS
In some cases, it is extremely difficult to elicit effective antibody responses using epitopes displayed in a loop or on a carrier protein. This environment is very different from native and seldom mimicks the normal conformation of the epitopes. These constraints may require that whole or large protein domains be conjugated to a VLP surface. The large regions generally fold independently, allowing for native comformation of the important immunogenic epitopes. The potential for this has been investigated in the FHV, T4, and HBV systems.
A recent study showed that, in addition to small epitopes, the FHV surface loops at amino acids 206 and 264 could accommodate larger insertions of several hundred amino acids as confirmed by electron cryomicroscopy65,117 [Figure 3(B)–(D)]. To capitalize on this feature, FHV particles displaying large protein insertions were developed to potentially function as both, an antitoxin and vaccine for Bacillus anthracis, the causative agent of anthrax. Two enzymatic A subunits of the AB type toxin of B. anthracis are responsible for the toxic effects. These are delivered to the cell by binding to the B subunit, termed protective antigen (PA) that binds to cell‐surface receptors ANTXR1 and ANTXR2. The adenylate cyclase edema factor (EF) raises intracellular cyclic adenosine monophosphate levels, and the zinc protease lethal factor (LF) cleaves mitogen‐activated protein kinase kinases.65 Once PA binds to ANTXR1 or 2, it is cleaved to form heptamers that bind EF and LF to form edema toxin and lethal toxin, respectively. The FHV particles were genetically engineered to display the PA‐binding domain of anthrax receptor ANTXR2 at position 206 or 264, and the binding domain was deemed functional by its ability to bind PA and inhibit PA‐cellular receptor binding and intoxication in vitro and in vivo. Since they could bind PA, the FHV–ANTXR2 particles were then tested as a multivalent scaffold for displaying the antigenic PA protein. Following immunization with the FHV–ANTXR2–PA complex, strong toxin‐neutralizing antibody response along with protection against lethal toxin challenge in rats confirmed the dual‐action ability for treatment and protection.65 Interestingly, increased antibody responses and protection were observed following immunization multivalent FHV–ANTXR2–PA compared to monomeric PA alone (Figure 3(E) and (F)). In addition, protective responses were observed after a single immunization without additional adjuvants. These results further confirm the idea that multivalency of antigen presentation facilitates effective antibody responses. The use of the FHV platform to display other large antigens is currently under investigation.
HBV and T4 have also been used as platforms for display of large antigens. HBV was also used to display larger portions of Dengue virus antigens. A 395 amino acid portion of Dengue virus envelope protein type 2 (E2) is effective for priming a protective immune response. This large portion of E2 was expressed as a fusion protein with HBsAg in yeast and the resulting VLPs were purified and characterized.118 These well‐structured VLPs were shown to function as bivalent immunogens in mice.119 The Hoc and Soc molecules on bacteriophage T4 are ideal candidates to allow the attachment of large antigens because these proteins are not integral to capsid assembly. T4 can bind to up to 155 Hoc proteins, and fusions between Hoc and either PA, LF, or EF were shown to bind to T4 and induce immune responses against all of the individual antigens, proving the concept that this scaffold could be used for display of multiple antigens simultaneously. Consistent with the FHV studies, responses against multimeric PA were higher than monomeric PA.109 After assembly of the Soc‐PA proteins on T4 phage, cleaved anthrax protective antigen heptamers were attached and served as further scaffold for binding LF [Figure 3(A)]67. Together these results indicate that VLP scaffolds have excellent potential to display antigens with complex structure, and combinations of antigens. Since larger and more complex immunogens are in many cases more similar to natural antigens, it may be that these systems for immunizing with larger antigenic proteins will be particularly fruitful for VLP‐based vaccine design.
USING VLPS TO DISPLAY SELF ANTIGENS
Because of the success of attachment of antigen from foreign particles, exploration into attachment of self‐peptides for the purposes of treating diseases has been attempted. The antibodies formed following administration of self‐antigen often serve as competitive inhibitors to fight diseases. There are often intrinsic problems involved when administering the peptides alone, because in general it is difficult to break immunologic tolerance to self proteins and peptides are typically not very immunogenic. Attachment of self‐antigen to a VLP often produces a heightened immune response because of the addition of foreign protein and multivalent display that has the potential to break tolerance. A variety of VLPs have been used to display self‐antigens including HBV, FHV, MS2, and HPV.
One potential therapeutic avenue to prevent HIV infection is the production of autoantibodies against the HIV cellular receptor CCR5 as an ‘indirect’ route to vaccination, avoiding the problem of constant mutation of the viral antigens. A peptide from CCR5 was attached to HPV VLPs and when administered in mice, these particles initiated production of autoantibodies, which inhibited binding of the ligand and blocked infection of an indicator cell line expressing CCR5.176 Qβ has been used in a similar strategy for induction of anti‐CCR5 antibodies. Production of IgA antibodies at mucosal sites following administration of an aerosolized pulmonary formulation of the Qβ‐based vaccine confirmed the potential for focusing the attack on HIV at the first line of contact at the mucosa of the human body.135 The indirect route targeting CCR5 has been addressed using the FHV platform as well. Insertion of a CCR5 peptide on FHV, followed by administration results in reduced CCR5 on CD4+ cells in humans and mice.129 Furthermore, peptides from the ECL2 loop of the CCR5 coreceptor of HIV have been inserted into the coat proteins of MS2. The MS2 particles were shown to be potently immunogenic.105 While it is potentially risky to induce self‐responses, HIV may be one example where further risk is warranted since other strategies vaccine development against HIV infection have not been successful.
Alzheimer's disease is a neurodegenerative disease that results in accumulation of Aβ peptide, neurofibillary tangles, and loss of neurons leading to dementia. One therapeutic strategy has been the use of vaccination against Aβ to eliminate the accumulation of plaques in the brain in the hope of preserving neurons. The attachment of an Aβ peptide to papillomavirus VLP capsids induced high levels of specific antibodies and inhibited effective assembly of peptides into neurotoxic peptides in vitro. Aβ deposits were also reduced after immunization of a mouse model of Alzheimer's. However, clinical trials of another vaccine formulation against Aβ peptide resulted in development of encephalitis; therefore, it remains to be determined if vaccination will be effective in this and similar diseases.177
OTHER NANOPARTICLE PLATFORMS FOR VIRAL VACCINES
Since researchers have come to better understand the requirements to generate an effective vaccine, the goal of a synthetic mimic of VNPs has become more attainable. In principle, synthetic particles might be even safer and easier to produce than VLPs, but their properties of immunogenicity and elimination in vivo are not as favorable. Nevertheless, some progress has been made toward synthetic nanoparticle vaccines. A few examples follow.
Biodegradable and biocompatible microparticles have been utilized in oral immunizations for induction of local and systemic immune responses. Poly (D, L‐lactide‐coglycolide) (PLG) is a biodegradable material, and manipulation of biodegradation is possible through changes to polymer composition and molecular weight. Oral and intragastric administration of these microparticles has resulted in immune responses to antigens trapped inside the particles, including peptide, bacterial toxoids, and bacterial cells.178, 179, 180, 181 Although PLG microspheres have been studied more extensively as carriers of bacterial vaccines, a few examples of viral vaccines exist.166, 167, 168 This platform shows particular promise because it has been certified by the US Food and Drug Administration for its preparation as a delivery system.
Liposomes are made up of a phospholipid bilayer, often with cholesterol included to stabilize the artificial membrane.152, 153, 154, 155, 156,161 The preparation of empty liposomes has not been challenging; however, successful incorporation of antigen presents some difficulties.182 In addition, liposomes often do not elicit a strong immune response necessitating the use of adjuvants. Although there has continued to be a slow and steady increase, only formulations which perform very well in experimental animals ever enter expensive clinical trials. These products continue to perform below expectancy in man.182
ISCOMs, which are roughly 40 nm spherical micells composed of the saponin mixture Quil A (strong adjuvant), cholesterol, and phospholipids,182 have been licensed for use in horses and have made progress as an HSV‐2 and a rotavirus vaccine.149, 150, 151,182 In spite of these advances, ISCOMs perform similarly to liposomes, and are therefore limited in progression.
Nanoemulsion (NE)‐based vaccines are a promising noninflammatory mucosal adjuvant for use as nasal vaccine platform. The requirement of many current vaccines to be administered by injection has limited broad use of these vaccines worldwide. The use of a nasal‐based delivery platform would facilitate vaccination in the developing world.171, 172, 173,175 These synthetic platforms are an exciting area of development, and as more is learned about how biologically based particles efficiently prime protective immune responses these principles will likely be translated into synthetic systems as well.
CONCLUSION
Virus‐based vaccines share some of the same problems encountered with any vaccine. Even with efficient cellular and humoral immune responses, they rely on the longevity of the host response. In addition, use of VLPs as vaccines in immunocompromised patients will encounter the same problems with efficient immune response as other vaccine approaches. VLPs have shown more promise than many other subunit vaccines because they are conformationally authentic and safer due to lack of genetic material.183 The sucess of HBV and HPV‐based particles in humans has spurred intense interest in the development of VLPs and VNPs of all kinds, particularly the engineering of more complex structural antigens and multiple antigen delivery systems.
There are several obstacles unique to virus‐based vaccines when considering bringing these particles from research tool to use in patient populations. One of these is the concern over potential toxicity of the nanoparticles in the human body. The slow speed of biodegradability of some materials, the ability to span biological membranes, and high surface area and reactivity may lead to unacceptable toxic affects in humans. Another consideration is the complexity of clinical trials. Because vaccines are usually administered in healthy humans from infant to adult, trials in nonhuman primates are often needed to demonstrate safety and tolerability of the vaccine. In addition, preclinical studies involve immunization immediately followed by challenge to test for protection. Human trials require waiting for an outbreak to occur before protection can be analyzed. Nevertheless, the benefits to human health have historically outweighed these drawbacks, as vaccines are considered one of the most effective means of improving global health worldwide and thus warrant sustained efforts to continually improve safety and efficacy.
Acknowledgements
We thank Bridget Carragher for providing the image in Figure 2B and for helpful discussions. We would like to acknowledge Anette Schneemann, Leah Shriver, and Kris Koudelka for their helpful discussion and critical reading of the manuscript.
RELATED WIREs ARTICLES
REFERENCES
- 1. Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol 2007, 37(suppl 1):S148–S155. doi:10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- 2. Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, et al. A vaccine againstnicotine for smoking cessation: a randomized controlled trial. PLoS One 2008, 3:e2547. doi:10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies forovercoming host innate and adaptive immune responses. Nat Immunol 2002, 3:1033–1040. doi:10.1038/ni1102‐1033ni1102‐1033 [pii]. [DOI] [PubMed] [Google Scholar]
- 4. Barton GM, Medzhitov R. Toll‐like receptors and their ligands. CurrTop Microbiol Immunol 2002, 270:81–92. [DOI] [PubMed] [Google Scholar]
- 5. Starr TK, Jameson SC, Hogquist KA. Positive and negative selection ofT cells. Annu Rev Immunol 2003, 21:139–176. doi:10.1146/annurev.immunol.21.120601.141107120601.141107[pii]. [DOI] [PubMed] [Google Scholar]
- 6. Murali‐Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J,Ahmed R. Persistence of memory CD8 T cells in MHC class I‐deficientmice. Science 1999, 286:1377–1381. doi:7955 [pii]. [DOI] [PubMed] [Google Scholar]
- 7. Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis 2000, 30(suppl 3):S266–S270. doi:CID998806 [pii] 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 8. Baxby D. The Jenner bicentenary: the introduction and early distribution of smallpox vaccine. FEMS Immunol Med Microbiol 1996, 16:1–10. doi:S0928824496000685 [pii]. [DOI] [PubMed] [Google Scholar]
- 9. Bodian D. Emerging concept of poliomyelitis infection. Science 1955, 122:105–108. [DOI] [PubMed] [Google Scholar]
- 10. Bodian D. Viremia, invasiveness, and the influence of injections. Ann N Y Acad Sci 1955, 61:877–882. [DOI] [PubMed] [Google Scholar]
- 11. Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB, et al. Protection against hepatitis A by an inactivated vaccine. JAMA 1994, 271:1328–1334. [PubMed] [Google Scholar]
- 12. Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology 2006, 43:S164–S172. doi:10.1002/hep.21052. [DOI] [PubMed] [Google Scholar]
- 13. Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G, et al. A controlled trial of a formalin‐inactivated hepatitis A vaccine in healthy children. N Engl J Med 1992, 327:453–457. [DOI] [PubMed] [Google Scholar]
- 14. Wolf M, Garcea RL, Grigorieff N, Harrison SC. Subunit interactions in bovine papillomavirus. Proc Natl Acad Sci U S A 107:6298–6303. doi:0914604107 [pii] 10.1073/pnas.0914604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Modis Y, Trus BL, Harrison SC. Atomic model of the papillomavirus capsid. EMBO J 2002, 21:4754–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sachse C, Chen JZ, Coureux PD, Stroupe ME, Fandrich M, Grigorieff N. High‐resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J Mol Biol 2007, 371:812–835. doi:S0022‐2836(07)00759‐0 [pii] 10.1016/j.jmb.2007.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrillo‐Tripp M, Shepherd CM, Borelli IA, Venkataraman S, Lander G, Natarajan P, Johnson JE, Brooks CL. 3rd, Reddy VS. VIPERdb2: an enhanced and web API enabled relational database for structural virology. Nucleic Acids Res 2009, 37:D436–442. doi:gkn840 [pii] 10.1093/nar/gkn840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyanohara A, Imamura T, Araki M, Sugawara K, Ohtomo N, Matsubara K. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: synthesis of two polypeptides translated from different initiation codons. J Virol 1986, 59:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delchambre M, Gheysen D, Thines D, Thiriart C, Jacobs E, Verdin E, Horth M, Burny A, Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus‐like particles. EMBO J 1989, 8:2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV‐1 precursor Pr55gag virus‐like particles from recombinant baculovirus‐infected insect cells. Cell 1989, 59:103–112. doi:0092‐8674(89)90873‐8 [pii]. [DOI] [PubMed] [Google Scholar]
- 21. French TJ, Marshall JJ, Roy P. Assembly of double‐shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol 1990, 64:5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noad R, Roy P. Virus‐like particles as immunogens. Trends Microbiol 2003, 11:438–444. doi:S0966842X03002087 [pii]. [DOI] [PubMed] [Google Scholar]
- 23. Ludwig C, Wagner R. Virus‐like particles‐universal molecular toolboxes. Curr Opin Biotechnol 2007, 18:537–545. doi:S0958‐1669(07)00146‐2 [pii] 10.1016/j.copbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 2008, 60:915–928. doi:S0169‐409X(08)00045‐8 [pii] 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature 1984, 307:178–180. [DOI] [PubMed] [Google Scholar]
- 26. Andre FE, Safary A. Summary of clinical findings on Engerix‐B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad Med J 1987, 63(suppl 2):169–177. [PubMed] [Google Scholar]
- 27. Madalinski K, Sylvan SP, Hellstrom U, Mikolajewicz J, Zembrzuska‐Sadkowska E, Piontek E. Antibody responses to preS components after immunization of children with low doses of BioHepB. Vaccine 2001, 20:92–97. doi:S0264410X01003127 [pii]. [DOI] [PubMed] [Google Scholar]
- 28. Shouval D, Ilan Y, Adler R, Deepen R, Panet A, Even‐Chen Z, Gorecki M, Gerlich WH. Improved immunogenicity in mice of a mammalian cell‐derived recombinant hepatitis B vaccine containing pre‐S1 and pre‐S2 antigens as compared with conventional yeast‐derived vaccines. Vaccine 1994, 12:1453–1459. [DOI] [PubMed] [Google Scholar]
- 29. Yap I, Chan SH. A new pre‐S containing recombinant hepatitis B vaccine and its effect on non‐responders: a preliminary observation. Ann Acad Med Singapore 1996, 25:120–122. [PubMed] [Google Scholar]
- 30. Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci U S A 2001, 98:11539–11544. doi:10.1073/pnas.191617598 191617598 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, et al. Risks for incident human papillomavirus infection and low‐grade squamous intraepithelial lesion development in young females. JAMA 2001, 285:2995–3002. doi:joc02174 [pii]. [DOI] [PubMed] [Google Scholar]
- 32. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995, 87:796–802. [DOI] [PubMed] [Google Scholar]
- 33. Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, Koutsky LA. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002, 288:1749–1757. doi:joc11676 [pii]. [DOI] [PubMed] [Google Scholar]
- 34. Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion‐like particles. Virology 1991, 185:251–257. doi:0042‐6822(91)90772‐4 [pii]. [DOI] [PubMed] [Google Scholar]
- 35. Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self‐assembles into virus‐like particles that are highly immunogenic. Proc Natl Acad Sci U S A 1992, 89:12180–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jagu S, Kwak K, Garcea RL, Roden RB. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine 2010, 28:4478–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case‐control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007, 356:1944–1956. doi:356/19/1944 [pii] 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 38. Scheerlinck JP, Greenwood DL. Virus‐sized vaccine delivery systems. Drug Discov Today 2008, 13:882–887. doi:S1359‐6446(08)00242‐0 [pii] 10.1016/j.drudis.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 39. Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, Wong DC, Prieels JP, Emerson SU. Pre‐clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine 2003, 21:2607–2615. doi:S0264410X03001002 [pii]. [DOI] [PubMed] [Google Scholar]
- 40. Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med 2001, 7:462–466. doi:S1471‐4914(01)02106‐2 [pii]. [DOI] [PubMed] [Google Scholar]
- 41. Li TC, Takeda N, Miyamura T, Matsuura Y, Wang JC, Engvall H, Hammar L, Xing L, Cheng RH. Essential elements of the capsid protein for self‐assembly into empty virus‐like particles of hepatitis E virus. J Virol 2005, 79:12999–13006. doi:79/20/12999 [pii] 10.1128/JVI.79.20.12999‐13006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, Takeda N, Miyamura T. Expression and self‐assembly of empty virus‐like particles of hepatitis E virus. J Virol 1997, 71:7207–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus‐like particles in volunteers. Clin Immunol 2003, 108:241–247. doi:S1521661603001207 [pii]. [DOI] [PubMed] [Google Scholar]
- 44. Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A 1996, 93:5335–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santi L, Huang Z, Mason H. Virus‐like particles production in green plants. Methods 2006, 40:66–76. doi:S1046‐2023(06)00163‐0 [pii] 10.1016/j.ymeth.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thiery R, Cozien J, Cabon J, Lamour F, Baud M, Schneemann A. Induction of a protective immune response against viral nervous necrosis in the European sea bass Dicentrarchus labrax by using betanodavirus virus‐like particles. J Virol 2006, 80:10201–10207. doi:80/20/10201 [pii] 10.1128/JVI.01098‐06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus‐like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005, 23:5751–5759. doi:S0264‐410X(05)00754‐1 [pii] 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 48. Latham T, Galarza JM. Formation of wild‐type and chimeric influenza virus‐like particles following simultaneous expression of only four structural proteins. J Virol 2001, 75:6154–6165. doi:10.1128/JVI.75.13.6154‐6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galarza JM, Latham T, Cupo A. Virus‐like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol 2005, 18:244–251. doi:10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 50. Doan LX, Li M, Chen C, Yao Q. Virus‐like particles as HIV‐1 vaccines. Rev Med Virol 2005, 15:75–88. doi:10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- 51. Pantophlet R, Burton DR. GP120: target for neutralizing HIV‐1 antibodies. Annu Rev Immunol 2006, 24:739–769. doi:10.1146/annurev. immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 52. Grgacic EV, Anderson DA. Virus‐like particles: passport to immune recognition. Methods 2006, 40:60–65. doi:S1046‐2023(06)00162‐9 [pii]10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McGaughey GB, Barbato G, Bianchi E, Freidinger RM, Garsky VM, Hurni WM, Joyce JG, Liang X, Miller MD, Pessi A, et al. Progress towards the development of a HIV‐1 gp41‐directed vaccine. Curr HIV Res 2004, 2:193–204. [DOI] [PubMed] [Google Scholar]
- 54. Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol 1998, 72:3827–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jeong SH, Qiao M, Nascimbeni M, Hu Z, Rehermann B, Murthy K, Liang TJ. Immunization with hepatitis C virus‐like particles induces humoral and cellular immune responses in nonhuman primates. J Virol 2004, 78: 6995–7003. doi:10.1128/JVI.78.13.6995‐7003.200478/13/6995 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus‐like particles induce virus‐specific humoral and cellular immune responses in mice. Hepatology 2001, 34:417–423. doi:S0270‐9139(01)35142‐X [pii] 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 57. Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Immunization with hepatitis C virus‐like particles protects mice from recombinant hepatitis C virus‐vaccinia infection. Proc Natl Acad Sci U S A 2003, 100:6753–6758. doi:10.1073/pnas.11319291001131929100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus‐like particles exhibit potential as a pan‐filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 2005, 23:3033–3042. doi:S0264‐410X(04)00974‐0 [pii] 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 59. Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus‐like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A 2003, 100: 15889–15894. doi:10.1073/pnas.22370381002237038100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Warfield KL, Swenson DL, Demmin G, Bavari S. Filovirus‐like particles as vaccines and discovery tools. Expert Rev Vaccines 2005, 4:429–440. doi:10.1586/14760584.4.3.429. [DOI] [PubMed] [Google Scholar]
- 61. Mortola E, Roy P. Efficient assembly and release of SARS coronavirus‐like particles by a heterologous expression system. FEBS Lett 2004, 576:174–178. doi:S0014579304011238 [pii] 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, et al. A virus‐like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med 16:334–338. doi:nm.2105 [pii] 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Acosta‐Ramirez E, Perez‐Flores R, Majeau N, Pastelin‐Palacios R, Gil‐Cruz C, Ramirez‐Saldana M, Manjarrez‐Orduno N, Cervantes‐Barragan L, Santos‐Argumedo L, Flores‐Romo L, et al. Translating innate response into long‐lasting antibody response by the intrinsic antigen‐adjuvant properties of papaya mosaic virus. Immunology 2008, 124:186–197. doi:IMM2753 [pii] 10.1111/j.1365‐2567.2007.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lacasse P, Denis J, Lapointe R, Leclerc D, Lamarre A. Novel plant virus‐based vaccine induces protective cytotoxic T‐lymphocyte‐mediated antiviral immunity through dendritic cell maturation. J Virol 2008, 82:785–794. doi:JVI.01811‐07 [pii] 10.1128/JVI.01811‐07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manayani DJ, Thomas D, Dryden KA, Reddy V, Siladi ME, Marlett JM, Rainey GJ, Pique ME, Scobie HM, Yeager M, et al. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog 2007, 3:1422–1431. doi:07‐PLPA‐RA‐0044 [pii] 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol 2005, 35:2031–2040. doi:10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 67. Fokine A, Bowman VD, Battisti AJ, Li Q, Chipman PR, Rao VB, Rossmann MG. Cryo‐electron microscopy study of bacteriophage T4 displaying anthrax toxin proteins. Virology 2007, 367:422–427. doi:S0042‐6822(07)00383‐2 [pii] 10.1016/j.virol.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kratz PA, Bottcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci U S A 1999, 96:1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stahl SJ, Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A 1989, 86:6283–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eckhart L, Raffelsberger W, Ferko B, Klima A, Purtscher M, Katinger H, Ruker F. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J Gen Virol 1996, 77(Pt 9):2001–2008. [DOI] [PubMed] [Google Scholar]
- 71. Isaguliants MG, Nordlund S, Sallberg M, Smirnov VD, Ruden U, Wahren B. HIV‐1 epitopes exposed by hybrid hepatitis B core particles affect proliferation of peripheral blood mononuclear cells from HIV‐1 positive donors. Immunol Lett 1996, 52:37–44. doi:0165247896025795 [pii]. [DOI] [PubMed] [Google Scholar]
- 72. Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 1999, 5:1157–1163. doi:10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 73. Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, Highfield PE, Rowlands DJ, Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 1987, 330:381–384. doi:10.1038/330381a0. [DOI] [PubMed] [Google Scholar]
- 74. Clarke BE, Brown AL, Grace KG, Hastings GZ, Brown F, Rowlands DJ, Francis MJ. Presentation and immunogenicity of viral epitopes on the surface of hybrid hepatitis B virus core particles produced in bacteria. J Gen Virol 1990, 71(Pt 5):1109–1117. [DOI] [PubMed] [Google Scholar]
- 75. Koletzki D, Lundkvist A, Sjolander KB, Gelderblom HR, Niedrig M, Meisel H, Kruger DH, Ulrich R. Puumala (PUU) hantavirus strain differences and insertion positions in the hepatitis B virus core antigen influence B‐cell immunogenicity and protective potential of core‐derived particles. Virology 2000, 276:364–375. doi:10.1006/viro.2000.0540S0042‐6822(00)90540‐3 [pii]. [DOI] [PubMed] [Google Scholar]
- 76. Birkett A, Lyons K, Schmidt A, Boyd D, Oliveira GA, Siddique A, Nussenzweig R, Calvo‐Calle JM, Nardin E. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect Immun 2002, 70:6860–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nardin EH, Oliveira GA, Calvo‐Calle JM, Wetzel K, Maier C, Birkett AJ, Sarpotdar P, Corado ML, Thornton GB, Schmidt A. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect Immun 2004, 72:6519–6527. doi:72/11/6519 [pii] 10.1128/IAI.72.11.6519‐6527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schodel F, Moriarty AM, Peterson DL, Zheng JA, Hughes JL, Will H, Leturcq DJ, McGee JS, Milich DR. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol 1992, 66:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dale CJ, Liu XS, De Rose R, Purcell DF, Anderson J, Xu Y, Leggatt GR, Frazer IH, Kent SJ. Chimeric human papilloma virus‐simian/human immunodeficiency virus virus‐like‐particle vaccines: immunogenicity and protective efficacy in macaques. Virology 2002, 301:176–187. doi:S0042682202915898 [pii]. [DOI] [PubMed] [Google Scholar]
- 80. Sadeyen JR, Tourne S, Shkreli M, Sizaret PY, Coursaget P. Insertion of a foreign sequence on capsid surface loops of human papillomavirus type 16 virus‐like particles reduces their capacity to induce neutralizing antibodies and delineates a conformational neutralizing epitope. Virology 2003, 309:32–40. doi:S0042682202001344 [pii]. [DOI] [PubMed] [Google Scholar]
- 81. Liu L, Canizares MC, Monger W, Perrin Y, Tsakiris E, Porta C, Shariat N, Nicholson L, Lomonossoff GP. Cowpea mosaic virus‐based systems for the production of antigens and antibodies in plants. Vaccine 2005, 23:1788–1792. doi:S0264‐410X(04)00823‐0 [pii] 10.1016/j.vaccine.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 82. Lin T, Porta C, Lomonossoff G, Johnson JE. Structure‐based design of peptide presentation on a viral surface: the crystal structure of a plant/animal virus chimera at 2.8 A resolution. Fold Des 1996, 1:179–187. [DOI] [PubMed] [Google Scholar]
- 83. Gonzalez MJ, Plummer EM, Rae CS, Manchester M. Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo. PLoS One 2009, 4:e7981. doi:10.1371/journal.pone.0007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Langeveld JP, Brennan FR, Martinez‐Torrecuadrada JL, Jones TD, Boshuizen RS, Vela C, Casal JI, Kamstrup S, Dalsgaard K, Meloen RH, et al. Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parvovirus. Vaccine 2001, 19:3661–3670. doi:S0264‐410X(01)00083‐4 [pii]. [DOI] [PubMed] [Google Scholar]
- 85. Nicholas BL, Brennan FR, Martinez‐Torrecuadrada JL, Casal JI, Hamilton WD, Wakelin D. Characterization of the immune response to canine parvovirus induced by vaccination with chimaeric plant viruses. Vaccine 2002, 20:2727–2734. doi:S0264410X02002001 [pii]. [DOI] [PubMed] [Google Scholar]
- 86. Dalsgaard K, Uttenthal A, Jones TD, Xu F, Merryweather A, Hamilton WD, Langeveld JP, Boshuizen RS, Kamstrup S, Lomonossoff GP, et al. Plant‐derived vaccine protects target animals against a viral disease. Nat Biotechnol 1997, 15:248–252. doi:10.1038/nbt0397‐248. [DOI] [PubMed] [Google Scholar]
- 87. McLain L, Porta C, Lomonossoff GP, Durrani Z, Dimmock NJ. Human immunodeficiency virus type 1‐neutralizing antibodies raised to a glycoprotein 41 peptide expressed on the surface of a plant virus. AIDS Res Hum Retroviruses 1995, 11:327–334. [DOI] [PubMed] [Google Scholar]
- 88. McLain L, Durrani Z, Wisniewski LA, Porta C, Lomonossoff GP, Dimmock NJ. Stimulation of neutralizing antibodies to human immunodeficiency virus type 1 in three strains of mice immunized with a 22 amino acid peptide of gp41 expressed on the surface of a plant virus. Vaccine 1996, 14:799–810. doi:0264410X9500229T [pii]. [DOI] [PubMed] [Google Scholar]
- 89. Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WD, Gilleland HE Jr. A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology 1999, 145(Pt 8):2061–2067. [DOI] [PubMed] [Google Scholar]
- 90. Brennan FR, Jones TD, Gilleland LB, Bellaby T, Xu F, North PC, Thompson A, Staczek J, Lin T, Johnson JE, et al. Pseudomonas aeruginosa outer‐membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology 1999, 145(Pt 1):211–220. [DOI] [PubMed] [Google Scholar]
- 91. Rennermalm A, Li YH, Bohaufs L, Jarstrand C, Brauner A, Brennan FR, Flock JI. Antibodies against a truncated Staphylococcus aureus fibronectin‐binding protein protect against dissemination of infection in the rat. Vaccine 2001, 19:3376–3383. doi:S0264410X01000809 [pii]. [DOI] [PubMed] [Google Scholar]
- 92. Taylor KM, Lin T, Porta C, Mosser AG, Giesing HA, Lomonossoff GP, Johnson JE. Influence of three‐dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J Mol Recognit 2000, 13:71–82. doi:10.1002/(SICI)1099‐1352(200003/04)13:2<71::AID‐JMR489>3.0.CO;2‐V [pii] 10.1002/(SICI)1099‐1352(200003/04)13:2 <71::AID‐JMR489>3.0.CO;2‐V. [DOI] [PubMed] [Google Scholar]
- 93. Saunders K, Sainsbury F, Lomonossoff GP. Efficient generation of cowpea mosaic virus empty virus‐like particles by the proteolytic processing of precursors in insect cells and plants. Virology 2009, 393:329–337. doi:S0042‐6822(09)00515‐7 [pii] 10.1016/j.virol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 94. Koo M, Bendahmane M, Lettieri GA, Paoletti AD, Lane TE, Fitchen JH, Buchmeier MJ, Beachy RN. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci U S A 1999, 96:7774–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu L, Jiang L, Zhou Z, Fan J, Zhang Q, Zhu H, Han Q, Xu Z. Expression of foot‐and‐mouth disease virus epitopes in tobacco by a tobacco mosaic virus‐based vector. Vaccine 2003, 21:4390–4398. doi:S0264410X03004286 [pii]. [DOI] [PubMed] [Google Scholar]
- 96. Jiang L, Li Q, Li M, Zhou Z, Wu L, Fan J, Zhang Q, Zhu H, Xu Z. A modified TMV‐based vector facilitates the expression of longer foreign epitopes in tobacco. Vaccine 2006, 24:109–115. doi:S0264‐410X(05)01101‐1 [pii] 10.1016/j.vaccine.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 97. Staczek J, Bendahmane M, Gilleland LB, Beachy RN, Gilleland HE Jr. Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine 2000, 18:2266–2274. doi:S0264‐410X(99)00571‐X [pii]. [DOI] [PubMed] [Google Scholar]
- 98. Nuzzaci M, Piazzolla G, Vitti A, Lapelosa M, Tortorella C, Stella I, Natilla A, Antonaci S, Piazzolla P. Cucumber mosaic virus as a presentation system for a double hepatitis C virus‐derived epitope. Arch Virol 2007, 152:915–928. doi:10.1007/s00705‐006‐ 0916‐7. [DOI] [PubMed] [Google Scholar]
- 99. Piazzolla G, Nuzzaci M, Tortorella C, Panella E, Natilla A, Boscia D, De Stradis A, Piazzolla P, Antonaci S. Immunogenic properties of a chimeric plant virus expressing a hepatitis C virus (HCV)‐derived epitope: new prospects for an HCV vaccine. J Clin Immunol 2005, 25:142–152. doi:10.1007/s10875‐005‐2820‐4. [DOI] [PubMed] [Google Scholar]
- 100. Yusibov V, Mett V, Davidson C, Musiychuk K, Gilliam S, Farese A, Macvittie T, Mann D. Peptide‐based candidate vaccine against respiratory syncytial virus. Vaccine 2005, 23:2261–2265. doi:S0264‐410X(05)00042‐3 [pii] 10.1016/j.vaccine. 2005.01.039. [DOI] [PubMed] [Google Scholar]
- 101. Brennan FR, Jones TD, Longstaff M, Chapman S, Bellaby T, Smith H, Xu F, Hamilton WD, Flock JI. Immunogenicity of peptides derived from a fibronectin‐binding protein of S. aureus expressed on two different plant viruses. Vaccine 1999, 17:1846–1857. [DOI] [PubMed] [Google Scholar]
- 102. Sedlik C, Saron M, Sarraseca J, Casal I, Leclerc C. Recombinant parvovirus‐like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc Natl Acad Sci U S A 1997, 94:7503–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brown WL, Mastico RA, Wu M, Heal KG, Adams CJ, Murray JB, Simpson JC, Lord JM, Taylor‐Robinson AW, Stockley PG. RNA bacteriophage capsid‐mediated drug delivery and epitope presentation. Intervirology 2002, 45:371–380. [DOI] [PubMed] [Google Scholar]
- 104. Spingola M, Peabody DS. MS2 coat protein mutants which bind Qbeta RNA. Nucleic Acids Res 1997, 25:2808–2815. doi:gka445 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Peabody DS, Manifold‐Wheeler B, Medford A, Jordan SK. do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus‐like particles of RNA phage MS2. J Mol Biol 2008, 380:252–263. doi:S0022‐2836(08)00496‐8 [pii] 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Morales J, Martinez JJ, Manoutcharian K, Hernandez M, Fleury A, Gevorkian G, Acero G, Blancas A, Toledo A, Cervantes J, et al. Inexpensive anti‐cysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine 2008, 26:2899–2905. doi:S0264‐410X(08)00382‐4 [pii] 10.1016/j.vaccine.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 107. Wu HW, Hu XM, Wang Y, Kurtis JD, Zeng FJ, McGarvey ST, Wu GL, Zhang ZS, Hua ZC. Protective immunity induced by phage displayed mitochondrial related peptides of Schistosoma japonicum. Acta Trop 2006, 99:200–207. doi:S0001‐706X(06)00137‐9 [pii] 10.1016/j.actatropica.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 108. Tissot AC, Renhofa R, Schmitz N, Cielens I, Meijerink E, Ose V, Jennings GT, Saudan P, Pumpens P, Bachmann MF. Versatile virus‐like particle carrier for epitope based vaccines. PLoS One 5:e9809. doi:10.1371/journal.pone.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shivachandra SB, Li Q, Peachman KK, Matyas GR, Leppla SH, Alving CR, Rao M, Rao VB. Multicomponent anthrax toxin display and delivery using bacteriophage T4. Vaccine 2007, 25:1225–1235. doi:S0264‐410X(06)01115‐7 [pii] 10.1016/j.vaccine.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shivachandra SB, Rao M, Janosi L, Sathaliyawala T, Matyas GR, Alving CR, Leppla SH, Rao VB. In vitro binding of anthrax protective antigen on bacteriophage T4 capsid surface through Hoc‐capsid interactions: a strategy for efficient display of large full‐length proteins. Virology 2006, 345:190–198. doi:S0042‐6822(05)00671‐9 [pii] 10.1016/j.virol.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 111. Li Q, Shivachandra SB, Leppla SH, Rao VB. Bacteriophage T4 capsid: a unique platform for efficient surface assembly of macromolecular complexes. J Mol Biol 2006, 363:577–588. doi:S0022‐2836(06)01075‐8 [pii] 10.1016/j.jmb.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 112. Buratti E, Di Michele M, Song P, Monti‐Bragadin C, Scodeller EA, Baralle FE, Tisminetzky SG. Improved reactivity of hepatitis C virus core protein epitopes in a conformational antigen‐presenting system. Clin Diagn Lab Immunol 1997, 4:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Peng M, Dai CB, Chen YD. Expression and immunoreactivity of an epitope of HCV in a foreign epitope presenting system. World J Gastroenterol 2005, 11:3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xiong XY, Liu X, Chen YD. Expression and immunoreactivity of HCV/HBV epitopes. World J Gastroenterol 2005, 11:6440–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chen Y, Xiong X, Liu X, Li J, Wen Y, Dai Q, Cao Z, Yu W. Immunoreactivity of HCV/HBV epitopes displayed in an epitope‐presenting system. Mol Immunol 2006, 43:436–442. doi:S0161‐5890(05)00062‐3 [pii] 10.1016/j.molimm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 116. Scodeller EA, Tisminetzky SG, Porro F, Schiappacassi M, De Rossi A, Chiecco‐Bianchi L, Baralle FE. A new epitope presenting system displays a HIV‐1 V3 loop sequence and induces neutralizing antibodies. Vaccine 1995, 13:1233–1239. doi:0264‐410X(95)00058‐9 [pii]. [DOI] [PubMed] [Google Scholar]
- 117. Venter PA, Schneemann A. Recent insights into the biology and biomedical applications of Flock House virus. Cell Mol Life Sci 2008, 65:2675–2687. doi:10.1007/s00018‐008‐8037‐y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bisht H, Chugh DA, Swaminathan S, Khanna N. Expression and purification of Dengue virus type 2 envelope protein as a fusion with hepatitis B surface antigen in Pichia pastoris. Protein Expr Purif 2001, 23:84–96. doi:10.1006/prep.2001.1474 S1046‐5928(01)91474‐3 [pii]. [DOI] [PubMed] [Google Scholar]
- 119. Bisht H, Chugh DA, Raje M, Swaminathan SS, Khanna N. Recombinant dengue virus type 2 envelope/hepatitis B surface antigen hybrid protein expressed in Pichia pastoris can function as a bivalent immunogen. J Biotechnol 2002, 99:97–110. doi:S0168165602001815 [pii]. [DOI] [PubMed] [Google Scholar]
- 120. Brautigam S, Snezhkov E, Bishop DH. Formation of poliovirus‐like particles by recombinant baculoviruses expressing the individual VP0, VP3, and VP1 proteins by comparison to particles derived from the expressed poliovirus polyprotein. Virology 1993, 192:512–524. doi:S0042‐6822(83)71067‐6 [pii] 10.1006/viro.1993.1067. [DOI] [PubMed] [Google Scholar]
- 121. Yap I, Guan R, Chan SH. Recombinant DNA hepatitis B vaccine containing Pre‐S components of the HBV coat protein–a preliminary study on immunogenicity. Vaccine 1992, 10:439–442. [DOI] [PubMed] [Google Scholar]
- 122. Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002, 347:1645–1651. doi:10.1056/NEJMoa020586347/21/1645 [pii]. [DOI] [PubMed] [Google Scholar]
- 123. Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus‐like particle vaccine in young women: a randomised double‐blind placebo‐controlled multicentre phase II efficacy trial. Lancet Oncol 2005, 6:271–278. doi:S1470‐2045(05)70101‐7[pii] 10.1016/S1470‐2045(05)70101‐7. [DOI] [PubMed] [Google Scholar]
- 124. Bertolotti‐Ciarlet A, Ciarlet M, Crawford SE, Conner ME, Estes MK. Immunogenicity and protective efficacy of rotavirus 2/6‐virus‐like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine 2003, 21:3885–3900. doi:S0264410X03003086 [pii]. [DOI] [PubMed] [Google Scholar]
- 125. Crawford SE, Labbe M, Cohen J, Burroughs MH, Zhou YJ, Estes MK. Characterization of virus‐like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol 1994, 68:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vieira HL, Estevao C, Roldao A, Peixoto CC, Sousa MF, Cruz PE, Carrondo MJ, Alves PM. Triple layered rotavirus VLP production: kinetics of vector replication, mRNA stability and recombinant protein production. J Biotechnol 2005, 120:72–82. doi:S0168‐1656(05)00244‐0[pii] 10.1016/j.jbiotec.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 127. Deml L, Speth C, Dierich MP, Wolf H, Wagner R. Recombinant HIV‐1 Pr55gag virus‐like particles: potent stimulators of innate and acquired immune responses. Mol Immunol 2005, 42:259–277. doi:S0161‐5890(04)00250‐0 [pii] 10.1016/j.molimm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 128. Sakuragi S, Goto T, Sano K, Morikawa Y. HIV type 1 Gag virus‐like particle budding from spheroplasts of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2002, 99:7956–7961. doi:10.1073/pnas.082281199 99/12/7956 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Barassi C, Soprana E, Pastori C, Longhi R, Buratti E, Lillo F, Marenzi C, Lazzarin A, Siccardi AG, Lopalco L. Induction of murine mucosal CCR5‐reactive antibodies as an anti‐human immunodeficiency virus strategy. J Virol 2005, 79:6848–6858. doi:79/11/6848 [pii] 10.1128/JVI.79.11.6848‐6858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tarar MR, Emery VC, Harrison TJ. Expression of a human cytomegalovirus gp58 antigenic domain fused to the hepatitis B virus nucleocapsid protein. FEMS Immunol Med Microbiol 1996, 16:183–192. doi:S0928‐8244(96)00082‐X [pii]. [DOI] [PubMed] [Google Scholar]
- 131. Street M, Herd K, Londono P, Doan T, Dougan G, Kast WM, Tindle RW. Differences in the effectiveness of delivery of B‐ and CTL‐epitopes incorporated into the hepatitis B core antigen (HBcAg) c/e1‐region. Arch Virol 1999, 144:1323–1343. [DOI] [PubMed] [Google Scholar]
- 132. Niikura M, Takamura S, Kim G, Kawai S, Saijo M, Morikawa S, Kurane I, Li TC, Takeda N, Yasutomi Y. Chimeric recombinant hepatitis E virus‐like particles as an oral vaccine vehicle presenting foreign epitopes. Virology 2002, 293:273–280. doi:10.1006/viro.2001.1240 S004268 2201912401 [pii]. [DOI] [PubMed] [Google Scholar]
- 133. Murawski MR, McGinnes LW, Finberg RW, Kurt‐Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. Newcastle Disease Virus‐Like Particles Containing Respiratory Syncytial Virus (RSV) G protein Induced Protection in BALB/c Mice With No Evidence of Immunopathology. J Virol 2009, 1110–1123. doi:JVI.01709‐09 [pii] 10.1128/JVI.01709‐09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kundig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, Hennecke F, Sladko K, Jennings GT, Bachmann MF. Der p 1 peptide on virus‐like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol 2006, 117:1470–1476. doi:S0091‐6749(06)00298‐3 [pii] 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 135. Hunter Z, Smyth HD, Durfee P, Chackerian B. Induction of mucosal and systemic antibody responses against the HIV coreceptor CCR5 upon intramuscular immunization and aerosol delivery of a virus‐like particle based vaccine. Vaccine 2009.. doi:S0264‐410X(09)01563–1 [pii] 10.1016/j.vaccine.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Charles IG, Li JL, Roberts M, Beesley K, Romanos M, Pickard DJ, Francis M, Campbell D, Dougan G, Brennan MJ, et al. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur J Immunol 1991, 21:1147–1153. [DOI] [PubMed] [Google Scholar]
- 137. Boulter N, Brown D, Wilkie G, Williamson S, Kirvar E, Knight P, Glass E, Campbell J, Morzaria S, Nene V, et al. d'Oliveira C, Gubbels MJ, Jongejan F, Hall R. Evaluation of recombinant sporozoite antigen SPAG‐1 as a vaccine candidate against Theileria annulata by the use of different delivery systems. Trop Med Int Health 1999, 4:A71–77. doi:tmi453 [pii]. [DOI] [PubMed] [Google Scholar]
- 138. Boulter NR, Glass EJ, Knight PA, Bell‐Sakyi L, Brown CG, Hall R. Theileria annulata sporozoite antigen fused to hepatitis B core antigen used in a vaccination trial. Vaccine 1995, 13:1152–1160. doi:0264410X9500026W [pii]. [DOI] [PubMed] [Google Scholar]
- 139. Sambhara S, Woods S, Arpino R, Kurichh A, Tamane A, Underdown B, Klein M, Lovgren Bengtsson K, Morein B, Burt D. Heterotypic protection against influenza by immunostimulating complexes is associated with the induction of cross‐reactive cytotoxic T lymphocytes. J Infect Dis 1998, 177:1266–1274. [DOI] [PubMed] [Google Scholar]
- 140. Sjolander S, Drane D, Davis R, Beezum L, Pearse M, Cox J. Intranasal immunisation with influenza‐ISCOM induces strong mucosal as well as systemic antibody and cytotoxic T‐lymphocyte responses. Vaccine 2001, 19:4072–4080. doi:S0264‐410X(01)00110‐4 [pii]. [DOI] [PubMed] [Google Scholar]
- 141. Lin Y, Kwon T, Polo J, Zhu YF, Coates S, Crawford K, Dong C, Wininger M, Hall J, Selby M, et al. Induction of broad CD4+ and CD8+ T‐cell responses and cross‐neutralizing antibodies against hepatitis C virus by vaccination with Th1‐adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J Virol 2008, 82:7492–7503. doi:JVI.02743‐07 [pii] 10.1128/JVI.02743‐07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Polakos NK, Drane D, Cox J, Ng P, Selby MJ, Chien D, O'Hagan DT, Houghton M, Paliard X. Characterization of hepatitis C virus core‐specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J Immunol 2001, 166:3589–3598. [DOI] [PubMed] [Google Scholar]
- 143. Agrawal L, Haq W, Hanson CV, Rao DN. Generating neutralizing antibodies, Th1 response and MHC non restricted immunogenicity of HIV‐I env and gag peptides in liposomes and ISCOMs with in‐built adjuvanticity. J Immune Based Ther Vaccines 2003, 1:5. doi:10.1186/1476‐8518‐1‐5 1476‐8518‐1‐5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Boyle J, Eastman D, Millar C, Camuglia S, Cox J, Pearse M, Good J, Drane D. The utility of ISCOMATRIX adjuvant for dose reduction of antigen for vaccines requiring antibody responses. Vaccine 2007, 25:2541–2544. doi:S0264‐410X(06)01348‐X [pii]10.1016/j.vaccine.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 145. Pahar B, Cantu MA, Zhao W, Kuroda MJ, Veazey RS, Montefiori DC, Clements JD, Aye PP, Lackner AA, Lovgren‐Bengtsson K, et al. Single epitope mucosal vaccine delivered via immuno‐stimulating complexes induces low level of immunity against simian‐HIV. Vaccine 2006, 24:6839–6849. doi:S0264‐410X(06)00765‐1 [pii] 10.1016/j.vaccine.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 146. Verschoor EJ, Mooij P, Oostermeijer H, van der Kolk M, ten Haaft P, Verstrepen B, Sun Y, Morein B, Akerblom L, Fuller DH, et al. Comparison of immunity generated by nucleic acid‐, MF59‐, and ISCOM‐formulated human immunodeficiency virus type 1 vaccines in Rhesus macaques: evidence for viral clearance. J Virol 1999, 73:3292–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen XW, Liu XG, Zheng KQ, Liang NC. [Cloning, sequencing, expression, and primary identification of recombinant mouse protein kinase CK2 alpha subunit]. Ai Zheng 2002, 21:751–756. [PubMed] [Google Scholar]
- 148. Hu KF, Elvander M, Merza M, Akerblom L, Brandenburg A, Morein B. The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin Exp Immunol 1998, 113:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mohamedi SA, Heath AW, Jennings R. A comparison of oral and parenteral routes for therapeutic vaccination with HSV‐2 ISCOMs in mice; cytokine profiles, antibody responses and protection. Antiviral Res 2001, 49:83–99. doi:S0166‐3542(00)00142‐X [pii]. [DOI] [PubMed] [Google Scholar]
- 150. Iosef C, Van Nguyen T, Jeong K, Bengtsson K, Morein B, Kim Y, Chang KO, Azevedo MS, Yuan L, Nielsen P, et al. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6‐rotavirus‐like‐particles associated with immunostimulating complexes. Vaccine 2002, 20:1741–1753. doi:S0264410X02000312 [pii]. [DOI] [PubMed] [Google Scholar]
- 151. van Pinxteren LA, Bruce MG, Campbell I, Wood A, Clarke CJ, Bellman A, Morein B, Snodgrass DR. Effect of oral rotavirus/iscom vaccines on immune responses in gnotobiotic lambs. Vet Immunol Immunopathol 1999, 71:53–67. doi:S0165‐2427(99)00087‐2 [pii]. [DOI] [PubMed] [Google Scholar]
- 152. Gluck R. Immunopotentiating reconstituted influenza virosomes (IRIVs) and other adjuvants for improved presentation of small antigens. Vaccine 1992, 10:915–919. [DOI] [PubMed] [Google Scholar]
- 153. Gluck R, Mischler R, Brantschen S, Just M, Althaus B, Cryz SJ, Jr. Immunopotentiating reconstituted influenza virus virosome vaccine delivery system for immunization against hepatitis A. J Clin Invest 1992, 90:2491–2495. doi:10.1172/JCI116141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Just M, Berger R, Dreschsler H, Brantschen S, Gluck R. A single vaccination with an inactivated hepatitis A liposome vaccine induces protective antibodies after only two weeks. Vaccine 1992, 10:737–739. [DOI] [PubMed] [Google Scholar]
- 155. Fries LF, Gordon DM, Richards RL, Egan JE, Hollingdale MR, Gross M, Silverman C, Alving CR. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A 1992, 89:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kaji M, Kaji Y, Ohkuma K, Honda T, Oka T, Sakoh M, Nakamura S, Kurachi K, Sentoku M. Phase 1 clinical tests of influenza MDP‐virosome vaccine (KD‐5382). Vaccine 1992, 10:663–667. [DOI] [PubMed] [Google Scholar]
- 157. Alving CR, Detrick B, Richards RL, Lewis MG, Shafferman A, Eddy GA. Novel adjuvant strategies for experimental malaria and AIDS vaccines. Ann N Y Acad Sci 1993, 690:265–275. [DOI] [PubMed] [Google Scholar]
- 158. Locher CP, Witt SA, Ashlock BM, Levy JA. Evaluation of genetic immunization adjuvants to improve the effectiveness of a human immunodeficiency virus type 2 (HIV‐2) envelope DNA vaccine. DNA Cell Biol 2004, 23:107–110. doi:10.1089/104454904322759911. [DOI] [PubMed] [Google Scholar]
- 159. Chen H, Langer R. Oral particulate delivery: status and future trends. Adv Drug Deliv Rev 1998, 34:339–350. doi:S0169‐409X(98)00047‐7 [pii]. [DOI] [PubMed] [Google Scholar]
- 160. Mirchamsy H, Manhouri H, Hamedi M, Ahourai P, Fateh G, Hamzeloo Z. Stimulating role of toxoids‐laden liposomes in oral immunization against diphtheria and tetanus infections. Biologicals 1996, 24:343–350. doi:S1045‐1056(96)90049‐4 [pii] 10.1006/biol.1996.0049. [DOI] [PubMed] [Google Scholar]
- 161. Powers DC, Kilbourne ED, Johansson BE. Neuraminidase‐specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin Diagn Lab Immunol 1996, 3:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine 2002, 20(suppl 5):B17–B23. doi:S0264410X02005121 [pii]. [DOI] [PubMed] [Google Scholar]
- 163. Zurbriggen R, Novak‐Hofer I, Seelig A, Gluck R. IRIV‐adjuvanted hepatitis A vaccine: in vivo absorption and biophysical characterization. Prog Lipid Res 2000, 39:3–18. doi:S0163‐7827(99)00017‐X [pii]. [DOI] [PubMed] [Google Scholar]
- 164. Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori‐loaded poly(D, L‐lactide‐co‐glycolide) nanoparticles. Helicobacter 1999, 4:33–39. doi:hel9046 [pii]. [DOI] [PubMed] [Google Scholar]
- 165. Esparza I, Kissel T. Parameters affecting the immunogenicity of microencapsulated tetanus toxoid. Vaccine 1992, 10:714–720. [DOI] [PubMed] [Google Scholar]
- 166. Nellore RV, Pande PG, Young D, Bhagat HR. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis B surface antigen. J Parenter Sci Technol 1992, 46:176–180. [PubMed] [Google Scholar]
- 167. Singh M, Li XM, Wang H, McGee JP, Zamb T, Koff W, Wang CY, O'Hagan DT. Immunogenicity and protection in small‐animal models with controlled‐release tetanus toxoid microparticles as a single‐dose vaccine. Infect Immun 1997, 65:1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Audran R, Peter K, Dannull J, Men Y, Scandella E, Groettrup M, Gander B, Corradin G. Encapsulation of peptides in biodegradable microspheres prolongs their MHC class‐I presentation by dendritic cells and macrophages in vitro. Vaccine 2003, 21:1250–1255. doi:S0264410X02005212 [pii]. [DOI] [PubMed] [Google Scholar]
- 169. Reddin KM, Easterbrook TJ, Eley SM, Russell P, Mobsby VA, Jones DH, Farrar GH, Williamson ED, Robinson A. Comparison of the immunological and protective responses elicited by microencapsulated formulations of the F1 antigen from Yersinia pestis. Vaccine 1998, 16:761–767. doi:S0264410X97003058 [pii]. [DOI] [PubMed] [Google Scholar]
- 170. Allaoui‐Attarki K, Pecquet S, Fattal E, Trolle S, Chachaty E, Couvreur P, Andremont A. Protective immunity against Salmonella typhimurium elicited in mice by oral vaccination with phosphorylcholine encapsulated in poly(DL‐lactide‐co‐glycolide) microspheres. Infect Immun 1997, 65:853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Myc A, Kukowska‐Latallo JF, Bielinska AU, Cao P, Myc PP, Janczak K, Sturm TR, Grabinski MS, Landers JJ, Young KS, et al. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine 2003, 21:3801–3814. doi:S0264410X03003815 [pii]. [DOI] [PubMed] [Google Scholar]
- 172. Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR Jr. Mucosal immunization with a novel nanoemulsion‐based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun 2007, 75:4020–4029. doi:IAI.00070‐07 [pii] 10.1128/IAI.00070‐07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR Jr. Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses 2008, 24:271–281. doi:10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 174. Bielinska AU, Chepurnov AA, Landers JJ, Janczak KW, Chepurnova TS, Luker GD, Baker JR Jr. A novel, killed‐virus nasal vaccinia virus vaccine. Clin Vaccine Immunol 2008, 15:348–358. doi:CVI.00440‐07 [pii] 10.1128/CVI.00440‐07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR, et al. Pre‐clinical evaluation of a novel nanoemulsion‐based hepatitis B mucosal vaccine. PLoS One 2008, 3:e2954. doi:10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci U S A 1999, 96:2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid‐beta peptide: a case report. Nat Med 2003, 9:448–452. doi:10.1038/nm840 nm840 [pii]. [DOI] [PubMed] [Google Scholar]
- 178. Jepson MA, Simmons NL, O'Hagan DT, Hirst BH. Comparison of poly(DL‐lactide‐co‐glycolide) and polystyrene microsphere targeting to intestinal M cells. J Drug Target 1993, 1:245–249. doi:10.3109/10611869308996082. [DOI] [PubMed] [Google Scholar]
- 179. Jepson MA, Simmons NL, Savidge TC, James PS, Hirst BH. Selective binding and transcytosis of latex microspheres by rabbit intestinal M cells. Cell Tissue Res 1993, 271:399–405. [DOI] [PubMed] [Google Scholar]
- 180. Moser C, Metcalfe IC, Viret JF. Virosomal adjuvanted antigen delivery systems. Expert Rev Vaccines 2003, 2:189–196. doi:ERV020203 [pii] 10.1586/14760584.2.2.189. [DOI] [PubMed] [Google Scholar]
- 181. Shreedhar VK, Kelsall BL, Neutra MR. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T‐ and B‐cell areas of Peyer's patches. Infect Immun 2003, 71:504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kersten GF, Crommelin DJ. Liposomes and ISCOMs. Vaccine 2003, 21:915–920. doi:S0264410X020054 06 [pii]. [DOI] [PubMed] [Google Scholar]
- 183. Roy P, Noad R. Virus‐like particles as a vaccine delivery system: myths and facts. Hum Vaccin 2008, 4:5–12. doi:5559 [pii]. [DOI] [PubMed] [Google Scholar]


