Abstract
Background: In sarcoidosis patients, pulmonary hypertension (PH) is associated with significant morbidity and mortality. Early identification of sarcoidosis-associated pulmonary hypertension (SAPH) has substantial clinical implications. While a number of pulmonary function testing (PFT) variables have been associated with SAPH, the optimal use of PFT’s in screening for SAPH is unknown. Objectives: To examine the predictive value of PFT’s for echocardiographic PH in a cohort of sarcoidosis patients. Methods: We conducted a retrospective cohort study of patients with sarcoidosis from a single center over a period of five years. All consecutive adult patients with a diagnosis of biopsy-proven sarcoidosis (determined by review of the medical chart) who underwent PFT and echocardiographic testing were included. Echocardiographic risk of PH (either intermediate or high) was determined by the presence of echocardiographic PH signs and tricuspid regurgitant jet velocity. Data analysis was performed using multivariate logistic regression analysis with least absolute shrinkage and selection operator. Results: Of the 156 patients included in the study, 42 (27%) met the criteria for echocardiographic PH. Roughly equal proportions met the criteria for intermediate risk (45%) as did for high risk of PH (55%). The percent predicted of diffusion capacity for carbon monoxide (%DLCO) and forced vital capacity (%FVC) were predictive of echocardiographic PH. No other PFT variables outperformed these two markers, and the incorporation of additional PFT variables failed to significantly enhance the model. Conclusions: The %FVC and %DLCO emerged as being predictive of echocardiographic PH in this cohort of biopsy-proven sarcoidosis patients. Potentially reflecting the multifactorial pathogenesis of PH in sarcoidosis, incorporation of other PFT variables failed to enhance screening for PH in this population. (Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 308-316)
Keywords: sarcoidosis, pulmonary hypertension, pulmonary function testing
Introduction
Sarcoidosis is a granulomatous disorder that affects multiple organs, with pulmonary manifestations occurring in the vast majority of cases (1). Pulmonary hypertension (PH), part of group 5 of the current WHO classification system, is a complication of sarcoidosis that is associated with significant morbidity and mortality (2,3). The etiology of sarcoidosis-associated PH (SAPH) is thought to be multifactorial, with contributions from cardiac, vascular, pulmonary parenchymal, and comorbid disease processes. While there is currently no approved treatment for SAPH, a number of targeted pulmonary vascular therapies have shown promise, and early identification of this condition has both prognostic and therapeutic implications (3-5).
While right heart catheterization (RHC) demonstrating a mean pulmonary artery pressure at rest (mPAP) of ≥25mmHg is required for the diagnosis of PH, it is an invasive and costly procedure. As a result, echocardiography has emerged as the screening tool of choice when evaluating patients with suspected SAPH in whom further confirmatory testing is warranted. Both morphological abnormalities such as right ventricular chamber dilation, as well as echocardiographic measurements such as tricuspid regurgitant jet velocity, are incorporated into assessing the risk of PH (6). However, due to the significant clinical overlap between pulmonary parenchymal and pulmonary vascular disease in sarcoidosis, signs and symptoms of PH are often mistaken for sarcoidosis lung disease and delays in echocardiographic screening can result (3,7).
Given the propensity for pulmonary disease in sarcoidosis, patients often regularly undergo serial pulmonary function tests (PFT) over the course of their management. A number of PFT variables including diffusion capacity for carbon monoxide (DLCO) and forced vital capacity (FVC) have emerged as potentially predictive of SAPH. Due to the multiple pulmonary manifestations of sarcoidosis (such as interstitial lung disease, bronchiectasis, significant emphysema and airflow obstruction) these variables alone may have trouble differentiating pulmonary vascular disease from pulmonary parenchymal disease (7-9). We hypothesized that a combination of PFT variables may be effective in predicting the risk of Echocardiographic PH in a group of sarcoidosis patients.
Methods
All consecutive adult (≥18 years old) patients with a diagnosis of sarcoidosis who underwent echocardiographic and pulmonary function testing between August 30th, 2002 and September 30th, 2016 at The George Washington University’s Medical Faculty Associates Pulmonary Disease Clinic were included in the study. Echocardiographic testing was performed at the discretion of the primary clinician, either for an abnormality on pulmonary function testing (such as a decreased DLCO) or clinical signs or symptoms of cardiovascular disease (including dyspnea on exertion, chest discomfort at rest or with exertion, the presence of a murmur on cardiac auscultation, or the presence of lower extremity edema). This strategy was consistent with current recommendations regarding when to initiate echocardiographic screening for pulmonary hypertension in patients, including those with sarcoidosis (6, 10). A diagnosis of sarcoidosis was obtained from review of the referral information for the pulmonary function test and the medical chart. Only patients with a biopsy-proven diagnosis of sarcoidosis and a compatible clinical presentation were included. Patients without a biopsy-confirmed diagnosis and missing clinical or demographic data were excluded from the final analysis (11, 12). Data was retrospectively collected, and for patients with multiple diagnostic studies only the first study was used. Age, gender, ethnicity, height, and weight were collected at the time of echocardiographic testing. PFT’s and chest roentgenograms were obtained within six months of echocardiographic testing. At the time of diagnostic testing, a board-certified cardiologist reviewed all echocardiographic testing and a board-certified radiologist reviewed all chest roentgenograms. A Scadding Stage was retrospectively assigned to each patient based on the chest roentgenogram reports by an author who was board-certified in Pulmonary Diseases (13).
The presence or absence of echocardiographic PH was based on the 2015 ESC/ERS guidelines (6). Echocardiographic PH signs include a ratio of the right to left ventricular basal diameter of >1, flattening of the interventricular septum in either systole or diastole, a pulmonary artery diameter of >25 mm, an inferior vena cava diameter of >21 mm with <50% collapse with inspiration, a right atrial end-systolic area of >18 cm2, a right ventricular outflow Doppler acceleration time <105 msec, or midsystolic notching on the right ventricular outflow signal. An intermediate risk of echocardiographic PH required the presence of echocardiographic signs of PH for those patients with an absent tricuspid regurgitant jet velocity or those with a velocity ≤2.8 m/sec, and an absence of echocardiographic signs of PH for those with a tricuspid regurgitant jet velocity between 2.9 m/sec and 3.4 m/sec. A high risk of echocardiographic PH required a tricuspid regurgitant jet velocity >3.4 m/sec, or the presence of echocardiographic PH signs for those patients with a regurgitant jet velocity between 2.9 m/sec and 3.4 m/sec. This study was reviewed and approved by The George Washington University Institutional Review Board (IRB 031708), and written consent was not required due to the retrospective nature of the study.
Continuous data are expressed as median with interquartile range, categorical data as frequencies and percentages. Continuous variables were compared using the Wilcoxon-Mann-Whitney Test, and categorical variables using the Chi-square Test or Fisher’s Exact Test where appropriate. The presence or absence of echocardiographic PH was treated as a binary variable, and a multivariate logistic regression model was constructed.
The initial model-building methodology used least absolute shrinkage and selection operator (LASSO) to screen PFT candidate variables. The LASSO is a process by which the optimal number of prognostic factors is selected through coefficient shrinkage, and is particularly useful when selecting from a large number of correlated variables (such as in PFTs) (14). Given that patients with pulmonary sarcoidosis can exhibit a wide variety of functional pulmonary defects (such as obstruction or restriction), and the development of PH for a given defect (such as obstruction) is associated with a different PFT abnormality (such as hyperinflation), we utilized the LASSO to avoid selection bias and find the smallest number of prognostic factors for PH from among candidate PFT variables (15-16). To avoid missing clinically relevant predictors, other candidate PFT variables (based on a p-value of <0.05 in the comparison between groups) were then sequentially added to the model, and comparisons between models were made using the Likelihood Ratio Test. Only variables that generated a statistically significant improvement to the model fit were retained. Discrimination power was quantified using the C statistic [area under the receiver-operating curve (AUC)] and goodness of fit was assessed using the Hosmer-Lemeshow and Likelihood Ratio Tests. Odds ratios and confidence intervals for the independent variables in the final model were also calculated. The final multivariate model was also adjusted for baseline demographics (age, gender, ethnicity, BMI) to assess the effect of these variables on model prediction. A receiver-operating curve (ROC) analysis was performed on the final model PFT variables to determine the optimal combination with the highest sensitivity and specificity for predicting echocardiographic PH. All statistical tests were two-tailed, and p-values of <0.05 were considered statistically significant.
All statistical analyses were conducted using R version 3.3.2 (R Foundation for Statistical Computing, Vienna Austria).
Results
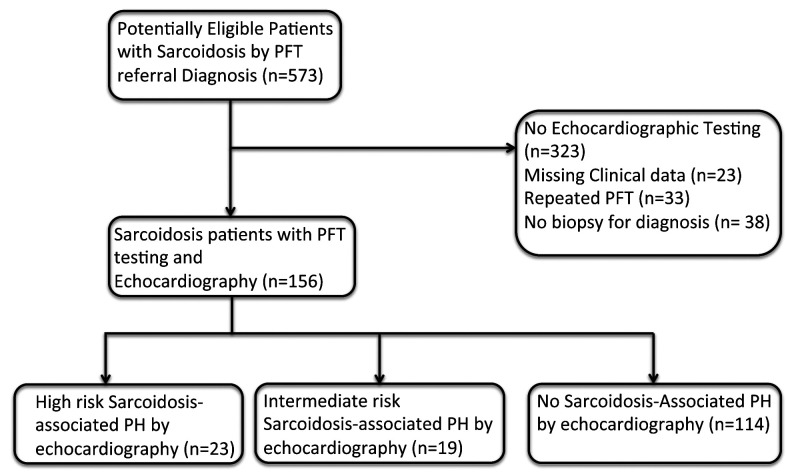
Of the 250 patients with echocardiographic and PFT testing, a total of 156 patients (62.4%) had complete data and biopsy-proven sarcoidosis and were included in the final analysis (Figure 1). A total of 42 (27%) met the criteria for echocardiographic PH and the remaining 114 (73%) did not.
Fig. 1.
Flow of Participants Through Study.
Abbreviations: PFT = Pulmonary function test, PH = pulmonary hypertension
The baseline characteristics of the patients included in the study stratified by diagnosis of echocardiographic PH are shown in Table 1. Those with echocardiographic PH were significantly more likely to have lower expiratory flows [percent-predicted of FEV1, FVC, and forced expiratory flow at 25-75% of the pulmonary volume (FEF25-75)], lower lung volumes [percent-predicted of total lung capacity (TLC), vital capacity (VC), functional residual capacity (FRC), inspiratory capacity (IC), and expiratory reserve volume (ERV)], and more diffusion capacity impairment [percent-predicted of DLCO and DLCO corrected for alveolar volume (DLCO/VA)]. There was no statistically significant difference in the degree of spirometric obstruction between the two groups, as measured by the FEV1/FVC ratio. There were no statistically significant differences in the radiographic severity of sarcoidosis as measured by the individual Scadding Stage between the two groups or the composite presence of parenchymal lung disease (Scadding Stage 2, 3, or 4). There were no statistically significant differences in baseline demographics between the two groups.
Table 1.
Baseline Characteristics of Patients
| Variable | No PH (n=114) | PH (n=42) | P-value | ||
| Med/F | IQR/% | Med/F | IQR/% | ||
| Race (%AA) | 95 | 83% | 41 | 98% | 0.063 |
| Gender (%F) | 78 | 68% | 28 | 67% | 0.848 |
| Age (years) | 52 | 45-57 | 56 | 45-63 | 0.173 |
| Height (cm) | 168 | 163-175 | 169 | 160-180 | 0.992 |
| Weight (kg) | 88.0 | 75.0-107.3 | 86.0 | 74.3-99.1 | 0.282 |
| %FVC | 73 | 60-81 | 53 | 45-69 | <0.001* |
| %FEV1 | 69 | 57-83 | 51 | 42-64 | <0.001* |
| FEV1/FVC | 79 | 73-83 | 79 | 73-85 | 0.965 |
| %FEF25-75 | 60 | 44-89 | 48 | 33-68 | 0.019* |
| %TLC | 78 | 65-86 | 63 | 53-76 | <0.001* |
| %RV | 75 | 65-97 | 68 | 62-93 | 0.242 |
| %FRC | 70 | 60-84 | 61 | 51-82 | 0.026* |
| %VC | 75 | 63-85 | 53 | 46-71 | <0.001* |
| %IC | 83 | 70-92 | 58 | 46-79 | <0.001* |
| %ERV | 61 | 36-77 | 49 | 29-60 | 0.014* |
| %DLCO | 57 | 42-66 | 35 | 26-49 | <0.001* |
| %DLCO/%VA | 81 | 68-93 | 73 | 57-87 | 0.038* |
| BMI (kg/m2) | 31.2 | 26.7-37.5 | 30.1 | 25.0-34.9 | 0.225 |
| Echo RVS (mmHg) | 27 | 23-28 | 41 | 38-56 | <0.001* |
| Echo RA (mmHg) | 3 | 3-5 | 3 | 3-11 | 0.186 |
| Echo TR Jet (m/s) | 2.34 | 2.00-2.48 | 3.13 | 2.92-3.66 | <0.001* |
| DD (%) | 17 | 18% | 11 | 34% | 0.083 |
| EF | 67 | 60-72 | 61 | 55-65 | 0.002* |
| Scadding Stage 0 | 44 | 40% | 16 | 40% | 1 |
| Scadding Stage 1 | 9 | 8% | 2 | 5% | 0.728 |
| Scadding Stage 2 | 30 | 27% | 8 | 20% | 0.403 |
| Scadding Stage 3 | 11 | 10% | 5 | 13% | 0.766 |
| Scadding Stage 4 | 16 | 15% | 9 | 22% | 0.322 |
| †Lung Disease | 57 | 52% | 22 | 55% | 0.854 |
Abbreviations: AA = African-American, F=female, %FVC = percent-predicted of forced vital capacity, %FEV1 = percent-predicted of forced expiratory volume in one second, %FEF25-75 = percent-predicted of forced expiratory flow at 25-75% of the pulmonary volume, %TLC = percent-predicted of total lung capacity, %RV = percent-predicted of residual volume, %FRC = percent-predicted of functional residual capacity, %VC = percent-predicted of vital capacity, %IC = percent-predicted of inspiratory capacity, %ERV = percent-predicted of expiratory reserve volume, %DLCO = percent-predicted of diffusion capacity for carbon monoxide, %DLCO/VA = percent-predicted of DLCO when corrected for alveolar volume, BMI = body-mass index, RVS = Right ventricular systolic pressure estimate, RA = right atrial pressure estimate, DD = presence of echocardiographic diastolic dysfunction, EF = ejection fraction, Scadding Stage 0 = No evidence of parenchymal disease or adenopathy on chest imaging PH = echocardiographic pulmonary hypertension
Med/F = Median or Frequency (for continuous or categorical variables respectively)
IQR/% = Interquartile range or percentage (for continuous or categorical variables respectively)
*P-value is statistically significant (P<0.05)
†Lung Disease = Presence of Scadding Stages 2, 3, or 4 on chest imaging
Table 2 shows the characteristics of patients with echocardiographic PH separated out into intermediate-risk or high-risk groups. As expected, patients with a high risk of PH by echocardiography had significantly higher tricuspid regurgitant jet velocity and higher estimated right ventricular systolic pressure. There were no differences between the groups in other echocardiographic parameters, and no difference between groups in either the %FVC or %DLCO. There was no difference in the proportion of patients from either group who had confirmatory RHC testing available for analysis. Of the patients who underwent confirmatory RHC testing there was no difference in either the severity of pulmonary vascular disease (as measured by RHC hemodynamics) or pulmonary parenchymal disease (as measured by the individual or composite Scadding Stage) between the two groups. When separating out patients with echocardiographic PH by individual Scadding stage, there was no significant difference between groups across demographic or pulmonary function test variables (Supplementary Table S2). While there was no difference in the proportion of patients with pre-capillary PH (mPAP ≥25 mmHg, PCWP ≤15 mmHg) between the intermediate-risk and high-risk groups, a substantial proportion of patients who underwent confirmatory RHC testing (40%) exhibited a post-capillary PH phenotype (mPAP ≥25 mmHg, PCWP >15 mmHg).
Table 2.
Characteristics of Patients with Echocardiographic PH
| Variable | Intermediate Risk PH (n=19) | High Risk PH (n=23) | P-value | ||
| Med/F | IQR/% | Med/F | IQR/% | ||
| Echo RVS (mmHg) | 38 | 34-42 | 55 | 42-76 | 0.003* |
| Echo RA (mmHg) | 3 | 3-10 | 8 | 3-15 | 0.228 |
| Echo TR Jet (m/s) | 2.93 | 2.82-3.13 | 3.50 | 3.05-3.85 | 0.005* |
| DD (%) | 3 | 16% | 8 | 35% | 0.291 |
| EF | 62 | 55-69 | 60 | 53-65 | 0.742 |
| %FVC | 54 | 49-70 | 52 | 44-66 | 0.250 |
| %DLCO | 38 | 34-57 | 32 | 24-48 | 0.114 |
| Confirmatory RHC | 7 | 37% | 8 | 35% | 1 |
| TR Velocity Only | 8 | 42% | 2 | 9% | |
| Echo Signs Only | 11 | 58% | --- | --- | |
| Both TR Velocity and Echo Signs | 21 | 91% | |||
| Variable | Intermediate Risk PH confirmed by RHC (n=7) | High Risk PH confirmed by RHC (n=8) | P-value | ||
| Med/F | IQR/% | Med/F | IQR/% | ||
| RHC mPAP (mmHg) | 33 | 30-46 | 37 | 34-40 | 0.862 |
| RHC PCWP (mmHg) | 18 | 8-17 | 17 | 7-21 | 0.728 |
| RHC CO (L/min) | 5.08 | 3.81-6.40 | 3.82 | 3.48-5.25 | 0.452 |
| RHC PVR (Wu) | 6.27 | 3.17-7.29 | 5.27 | 4.15-6.41 | 0.908 |
| Pre-Capillary PH | 5 | 71% | 4 | 50% | 0.608 |
| Scadding Stage 0 | 5 | 83% | 3 | 38% | --- |
| Scadding Stage 1 | 0 | --- | 1 | 13% | --- |
| Scadding Stage 2 | 1 | --- | 3 | 38% | --- |
| Scadding Stage 3 | 0 | --- | 1 | 13% | --- |
| Scadding Stage 4 | 1 | 17% | 0 | --- | --- |
| †Lung Disease | 2 | 29% | 4 | 50% | 0.608 |
Abbreviations: PH = Echocardiographic pulmonary hypertension, RVS = right ventricular systolic pressure estimate, RA = right atrial pressure estimate, DD = presence of diastolic dysfunction, EF = ejection fraction, RHC = right heart catheterization, mPAP = mean pulmonary artery pressure, PCWP = pulmonary capillary wedge pressure, CO = cardiac output, PVR = pulmonary vascular resistance, Wu = woods units, %FVC = percent predicted of forced vital capacity, %DLCO = percent predicted of diffusion capacity for carbon monoxide, Scadding Stage 0 = No evidence of parenchymal disease or adenopathy on chest imaging
Pre-Capillary PH = PH (mPAP ≥ 25mmHg at rest) with PCWP ≤ 15mmHg. Patients with PH and PCWP > 15mmHg were deemed to have post-capillary PH.
Med/F = Median or Frequency (for continuous or categorical variables respectively)
IQR/% = Interquartile range or percentage (for continuous or categorical variables respectively)
*P-value is statistically significant (P<0.05)
†Lung Disease = Presence of Scadding Stages 2, 3, or 4 on chest imaging
Table 3 shows the final regression model variables selected by the LASSO. In the initial selection by the LASSO, the percent-predicted FVC (%FVC) and percent-predicted DLCO (%DLCO) were selected as predictive variables. In the unadjusted model, echocardiographic PH patients were significantly more likely to have both a lower %FVC (OR 0.97, 95% Confidence Interval 0.93-0.99, p=0.031) and a lower %DLCO (OR 0.97, 95% Confidence Interval 0.94-0.99, p=0.031). After adjusting for baseline demographics (age, gender, body mass index (BMI), ethnicity) and the presence or absence of lung parenchymal disease, only the %FVC remained significantly predictive (OR 0.95, 95% Confidence Interval 0.92-0.99, p=0.01). While not statistically significant, after adjustment the %DLCO did retain a trend towards significance (OR 0.97, 95% Confidence Interval 0.94-1.00, p=0.094). The area under the receiver-operating curve for the final adjusted model was 0.7956 (Figure 2), indicating good model discrimination.
Table 3.
Regression Model Statistics
| Variable | Unadjusted Regression Model | P-value | |
| OR | 95% CI | ||
| %DLCO | 0.97 | 0.93-0.99 | 0.031* |
| %FVC | 0.97 | 0.94-0.99 | 0.033* |
| AUC = 0.7663 | |||
| HL = 0.4177 | |||
| Variable | † Adjusted Regression Model | P-value | |
| OR | 95% CI | ||
| %DLCO | 0.97 | 0.94-1.00 | 0.094 |
| %FVC | 0.95 | 0.92-0.99 | 0.010* |
| AUC=0.7956 | |||
Abbreviations: %DLCO = percent predicted of diffusion capacity for carbon monoxide, %FVC = percent predicted of forced vital capacity, AUC = Area under the receiver-operating curve, OR=odds ratio, 95% CI = 95% Confidence Interval, HL = Hosmer-Leme- show Goodness of Fit p-value
*P-value is statistically significant (P≤0.05)
†Model is adjusted for age, gender, ethnicity, body mass index, and lung disease (presence of Scadding Stages 2-4 on chest imaging)
Fig. 2.
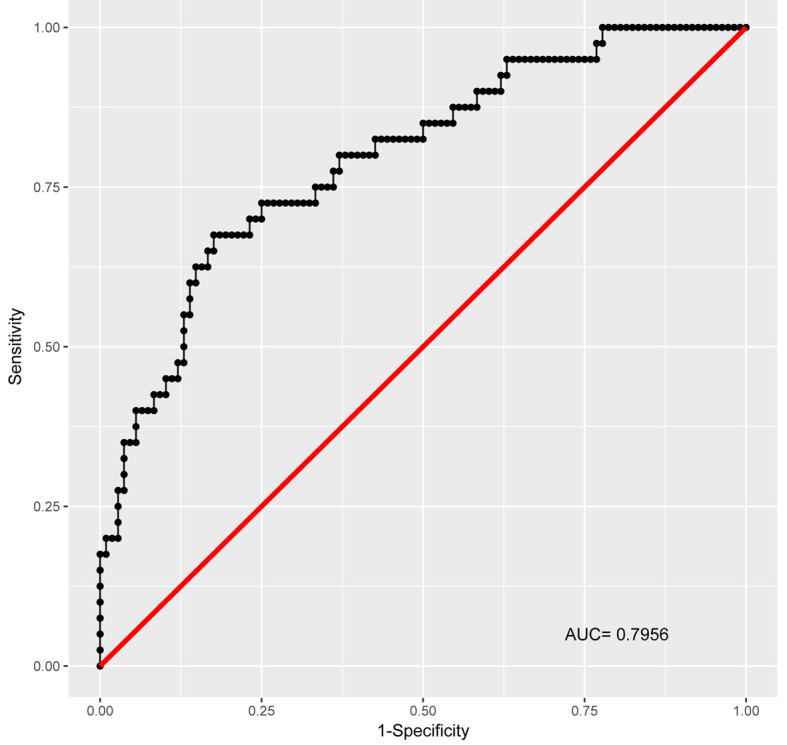
Full Adjusted Model Receiver-Operating Curve
Final model of percent-predicted diffusion capacity for carbon monoxide (%DLCO) and forced vital capacity (%FVC), adjusted for age, gender, ethnicity, body mass index, and lung disease (presence of Scadding Stages 2-4 on chest imaging)
An ROC analysis was then performed to determine the optimal cutoff of %FVC and %DLCO with the highest combined sensitivity and specificity to predict echocardiographic PH (Supplementary Table S1). The highest combined sensitivity and specificity occurred with a %DLCO <50% and a %FVC <60%. This cutoff had a sensitivity of 62%, a specificity of 82%, and a negative predictive value of 85%.
Discussion
In this cohort of patients with sarcoidosis who underwent echocardiographic and pulmonary function testing, a combination of the %FVC and %DLCO emerged as predictive of echocardiographic PH. The predictive power of the %FVC held even after controlling for baseline demographics and disease severity (age, gender, BMI, ethnicity, presence of lung parenchymal disease on chest imaging). Incorporation of additional PFT variables failed to significantly enhance the model predictive power.
While rare, the development of SAPH is associated with increased morbidity and mortality. As seen in our results, pulmonary vascular disease occurred in patients with any degree of pulmonary parenchymal disease and the presence or absence was not predictive of echocardiographic PH in the final model. There was also no difference in the degree of pulmonary parenchymal disease between those patients who had an intermediate or high risk of echocardiographic PH, even among the sub-set who had confirmatory RHC testing. A substantial proportion of patients with echocardiographic PH (45%) had radiographically “normal” lung parenchyma (Scadding Stage 0-1), and a significant proportion of patients undergoing confirmatory RHC testing (40%) exhibited a post-capillary PH phenotype. These results agree with the prevailing view that, although more likely with increasing severity of lung disease (as measured by %FVC), SAPH can occur in the absence of pulmonary parenchymal disease (3,15). While this study was not designed to investigate the precise pathological mechanisms underlying PH in a cohort of patients with sarcoidosis, we suspect this finding may be reflective of the multifactorial pathogenesis of SAPH with both pulmonary vascular and pulmonary parenchymal abnormalities contributing. Patients with lung fibrosis (Scadding Stage 2, 3, 4) may have PH secondary to pulmonary parenchymal capillary bed destruction, whereas those with an absence of pulmonary parenchymal involvement (Scadding Stage 0-1) may have PH as a result of granulomatous inflammation of the pulmonary arterial system, mechanical compression due to enlarged hilar and mediastinal lymph nodes, or a post-capillary phenotype secondary to left heart disease (3, 9, 10, 17). Consequently, while the burden of pulmonary parenchymal and pulmonary vascular disease may be reflected by the predictive power of %FVC and %DLCO respectively, no clear combination of clinical and diagnostic variables has emerged as predictive of SAPH and an accurate screening tool is lacking (3,14).
As seen in our results, a cutoff of %FVC < 60 and %DLCO < 50 was only able to obtain a sensitivity of 62% and a negative predictive value of 85%. The highest sensitivity and negative predictive value was obtained with a cutoff of %FVC < 80% and %DLCO < 60%, with a sensitivity of 83% and a negative predictive value of 89% (Supplementary Table S1). Of note all the patients who underwent confirmatory RHC testing in this cohort had a %DLCO <60%, and 14/15 (93%) had a %FVC <80%. These PFT thresholds likely reflect pulmonary parenchymal (%FVC) and pulmonary arterial (%DLCO) disease, and may have value when screening sarcoidosis patients for occult pulmonary vascular disease. These findings agree with previous studies evaluating PFT parameters to identify PH in sarcoidosis patients, the in which abnormalities in %FVC and %DLCO have consistently emerged as the most predictive (3, 7-9, 17-19). Our findings also suggest that other PFT variables are of limited value when evaluating a sarcoidosis patient for PH. For example, in contrast to patients with scleroderma where the FVC/DLCO ratio has been shown to be predictive of pulmonary hypertension, in our study that or any other ratio of PFT variables was not retained in the final model (20). Incorporation of additional predictive PFT variables, such as the %FEV1 or FVC/DLCO, failed to significantly improve the predictive or discriminative power of our model. This may be due to the more protean manifestations of pulmonary disease in sarcoidosis as compared to the predominance for nonspecific interstitial pneumonitis in other chronic diseases like scleroderma (21). As a result SAPH can occur in patients with varying levels of parenchymal lung involvement, and our results suggest applying PH screening methods from other chronic lung diseases to the sarcoidosis population may lead to significant misclassification. However, there may be value to the bedside clinician of utilizing the %FVC and %DLCO to quantify the risk of SAPH when confronted with a symptomatic sarcoidosis patient, and further investigation into the optimal cutoff of %FVC and %DLCO is warranted.
While the strengths of our study include a large cohort of patients with biopsy-proven sarcoidosis with complete echocardiographic and pulmonary function testing data, there are also a number of limitations. The study population is drawn from a single center and is overwhelmingly African-American in ethnicity (as expected based on the local epidemiology of the disease), however this limits the generalizability of our results to other ethnicities. Only those patients referred for PFT testing with complete data were included in the final analysis, and this may have introduced some selection bias.
Some patients with sarcoidosis and PFT’s did not undergo echocardiographic testing, as the decision to undergo screening was at the discretion of the treating clinician based on signs or symptoms of cardiovascular disease. While some of those patients may have had occult pulmonary vascular disease, it was not severe enough to manifest clinically with signs or symptoms that would prompt their clinician to screen for PH with echocardiographic testing. While this strategy may have introduced a selection bias to our data, it also enriched the data for clinically significant pulmonary vascular disease, and may have increased the applicability of our results to the bedside clinician confronted with a symptomatic sarcoidosis patient.
The diagnosis of pulmonary hypertension was made by echocardiography based on the 2015 ESC/ERS guidelines for pulmonary hypertension, and not all patients with echocardiographic PH got confirmatory RHC testing for the diagnosis of PH. However, even although PAH in sarcoidosis is most strongly associated with poor prognosis, echocardiographic pulmonary vascular abnormalities have also been associated with adverse clinical outcomes in sarcoidosis patients (22). While it would have been preferable to obtain RHC testing in all patients, the retrospective nature of our study prevented this. Of the 42 patients with echocardiographic PH in our study, only 15 (36%) underwent a RHC within 3 months of their echocardiography that confirmed the presence of PH. We cannot exclude that a number of patients with an intermediate or high risk of PH on echocardiography did not have true pulmonary vascular disease on RHC testing, and while this is a limitation common to echocardiographic screening for PH it may also affect the significance of our result (6).
Based on the frequency of PFT testing performed for PH screening and monitoring of parenchymal lung disease, we allowed a maximum of six months to elapse between the screening PFT and confirmatory echocardiographic testing (6, 23-24).While it would have been ideal to obtain PFT and echocardiographic data on the same day, the retrospective nature of our study prevented this. While we think it unlikely, it is still possible that the echocardiographic testing did not accurately reflect the PFT abnormalities in some patients, and this may have affected our results.
A substantial proportion of patients with echocardiographic PH (45%) did not have pulmonary parenchymal disease on chest roentgenogram (Scadding stage 0 and 1), despite some having evidence of impairment in measures of pulmonary function such as percent predicted of TLC. While the reason for this is unclear, this may be due in part to the high prevalence of obesity in our patient cohort leading to a restrictive defect not involving the lung parenchyma. Using a cutoff of 30 kg/m2, a total of 44% of patients with Scadding stage 0 and 1 and a total of 52% of all echocardiographic PH patients were classified as obese. Of these obese Scadding stage 0 and 1 patients, the vast majority (88%) had pulmonary restriction as evidenced by a TLC <80% of predicted, despite not having pulmonary parenchymal disease on their chest roentgenogram. This discrepancy between pulmonary parenchymal and pulmonary vascular disease may also be due to the Scadding stage classification underestimating the presence of pulmonary parenchymal disease, particularly in those patients with normal chest roentgenograms. As a result, a number of patients with subtle pulmonary parenchymal disease not evident on plain chest radiography may have been misclassified as Scadding stage 0 and 1 (25-26). These factors may have contributed to the attenuated relationship between pulmonary parenchymal and pulmonary vascular disease seen in this study, and our results will need to be confirmed in future larger studies.
Additional clinical variables known to be associated with SAPH, such as six-minute walk distance and serum brain natriuretic peptide, were unavailable and therefore could not be included in the final prediction model (19). While these variables may have predictive value in screening for SAPH, they are not as frequently collected in patients with sarcoidosis as compared to PFT’s, and may not be readily available to the clinician at the bedside. It is unknown what the best clinical prediction model of SAPH is, but it likely incorporates multiple clinical variables including PFTs, serum biomarkers, and functional testing like six-minute walk distance. Further studies incorporating these variables are needed to answer this question.
Conclusion
The %FVC and %DLCO emerged as being predictive to rule out echocardiographic PH in this cohort of sarcoidosis patients. In particular, an %FVC cutoff of 80% and %DLCO cutoff of 60% may be of value to the clinician at the bedside in quantifying the risk of PH when confronted with a symptomatic sarcoidosis patient. Likely reflecting the multifactorial pathogenesis of SAPH, incorporation of other PFT variables or the degree of parenchymal lung disease failed to significantly enhance the model predictive power. Our results confirm the importance of these PFT measures in predicting SAPH and, due to the lack of correlation between parenchymal lung involvement and pulmonary vascular disease, PH screening methods in other chronic lung diseases may not be applicable to sarcoidosis patients.
Acknowledgements
The authors wish to acknowledge Alexander Cho, M.D. for his assistance in coordinating the data acquisition for the project.
Contributions:
Conception, hypothesis generation, design of the study: J.E.A. and A.J. Data analysis and interpretation: J.D., J.G., H.G., and A.J. Writing the article or substantial involvement in its revision before submission: J.E.A., J.D., J.G., H.G., A.J.
References
- 1.Baughman RP, Tierstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Shlobin O, Baughman R. Sarcoidosis-associated pulmonary hypertension. Semin Respir Crit Care Med. 2017;38:450–62. doi: 10.1055/s-0037-1603767. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R, Culver D, Cordova F, et al. Bosentan for sarcoidosis-associated pulmonary hypertension. CHEST. 2014;145:810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 5.Ford HJ, Baughman R, Aris R, Engel P, Donohue JF. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ. 2016;6:557–562. doi: 10.1086/688775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 7.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. CHEST. 2005;128:1483–9. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 8.Rapti A, Kouranos V, Gialafos E, et al. Elevated pulmonary arterial systolic pressure in patients with sarcoidosis: prevalence and risk factors. LUNG. 2013;191:61–67. doi: 10.1007/s00408-012-9442-4. [DOI] [PubMed] [Google Scholar]
- 9.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J. 2008;32:296–302. doi: 10.1183/09031936.00175907. [DOI] [PubMed] [Google Scholar]
- 10.Huitema MP, Grutters JC, Rensing BJ, Reesink JH, Post MC. Pulmonary hypertension complicating pulmonary sarcoidosis. Neth Heart J. 2016;24(6):390–399. doi: 10.1007/s12471-016-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183(5):573–81. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J. 1961;2:1165–72. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B. 1996;59:267–288. [Google Scholar]
- 15.Minai OA, Fessler H, Stoller JK, Criner GJ, Scharf SM, Meli Y, et al. Clinical characteristics and prediction of pulmonary hypertension in severe emphysema. Respiratory Medicine. 2014;108(3):482–90. doi: 10.1016/j.rmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Meyer FJ, Ewert R, Hoeper MM, Olschewski H, Behr J, Winkler J, et al. Peripheral airway obstruction in primary pulmonary hypertension. Thorax. 2002;57(6):473–6. doi: 10.1136/thorax.57.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baughman R, Engel P, Nathan S. Pulmonary hypertension in sarcoidosis. Clin Chest Med. 2015;36:703–14. doi: 10.1016/j.ccm.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Mirsaeidi M, Omar H, Baughman R, Machado R, Sweiss N. The association between BNP, 6MWD test, DLCO% and pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:317–320. [PubMed] [Google Scholar]
- 20.Launay D, Humbert M, Berezne A, et al. Clinical characteristics and survival in systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease. CHEST. 2011;140:1016–1024. doi: 10.1378/chest.10-2473. [DOI] [PubMed] [Google Scholar]
- 21.Doyle T, Dellaripa P. Lung manifestations in the rheumatic diseases. CHEST. 2017 doi: 10.1016/j.chest.2017.05.015. pii:S0012-3692:30963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce E, Kamperidis V, Ninaber MK, et al. Prevalence and correlates of early right ventricular dysfunction in sarcoidosis and its association with outcome. J Am Soc Echocardiogr. 2016;29:871–8. doi: 10.1016/j.echo.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Glaude H, Altorok N, Townsend W, Mclaughlin V, Khanna D. Screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension: a systematic review. Semin Arthritis Rheum. 2014;43(4):536–41. doi: 10.1016/j.semarthrit.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):315–321. doi: 10.1513/pats.200602-022TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma R, Guleria R, Mohan A, Das C. Scadding criteria for diagnosis of sarcoidosis: Is there a need for change. CHEST. 2004;126(4) [Google Scholar]
- 26.Greco FG, Spagnolo P, Muri M, Paladini I, Chizzolini F, Piciucci S, et al. The value of chest radiograph and computed tomography in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(2):108–16. [PubMed] [Google Scholar]