Abstract
Purpose
Medications are commonly associated with acute kidney injury (AKI). However, in both clinical practice and research, consideration of specific medications as nephrotoxic varies widely. The Nephrotoxic Injury Negated by Just-in-time Action quality improvement collaborative was formed to focus on prevention or reduction of nephrotoxic medication-associated AKI in noncritically ill hospitalized children. However, there were discrepancies among institutions as to which medications should be considered nephrotoxic. The collaborative convened a Nephrotoxic Medication (NTMx) Subcommittee to develop a consensus for the classification of nephrotoxic medications.
Summary
The NTMx Subcommittee initially included pediatric nephrologists, a pharmacist, and a pediatric intensivist. The committee reviewed NTMx lists from the collaborative and identified changes from the initial NTMx list. The NTMx Subcommittee conducted a literature review of the disputed medications and assigned an evidence grade based on the reported association with nephrotoxicity and the quality of the data. The association between medication exposure and AKI was also determined using administrative data from the Pediatric Health Information Systems database. The NTMx Subcommittee then came to a majority consensus regarding which medications should be included on the list. The subcommittee’s recommendations were presented to the larger collaborative for approval, and consensus was achieved. The list continues to be reviewed and updated annually.
Conclusion
Formation of a multicenter quality-improvement initiative exposed current limitations as to which medications are considered nephrotoxic in clinical and research settings and presented an opportunity to approach this problem using an evidence-based process. A consensus definition of nephrotoxic-medication exposure was achieved.
Keywords: acute kidney injury, drug toxicity, nephrology, nephrotoxicity, pediatrics
KEY POINTS
Although nephrotoxic medications are associated with acute kidney injury, classification of specific medications as nephrotoxic varies greatly in clinical practice and research.
Using a systematic evidence-based process, a multicenter quality-improvement collaborative was able to reach a consensus as to which medications should be considered nephrotoxic.
Researchers and clinicians should continue to work to develop greater consistency among definitions of nephrotoxic-medication exposure.
Medications commonly contribute to acute kidney injury (AKI). In adult patients, nephrotoxic medications have been associated with 16%–25% of AKI cases.1 In critically ill children, exposure to nephrotoxic medications increases the odds of AKI by more than 3-fold, and AKI increases the risk of mortality by 3.5-fold.2 In noncritically ill children, nephrotoxic medications have been associated with 16%–42% of AKI cases, and exposure to an increasing number of nephrotoxic medications has been associated with an increasing incidence of AKI.3–5
A prospective quality-improvement project to assess nephrotoxic-medication exposure and nephrotoxic medication–associated AKI (NTMx-associated AKI) rates was conducted at a quaternary pediatric inpatient hospital through implementation of systematic electronic health record screening and decision support.6 Noncritically ill, hospitalized children receiving 3 or more nephrotoxic medications on 1 day or intravenous aminoglycosides for 3 or more consecutive days were considered “exposed” to nephrotoxic medications. The threshold of 3 or more medications was derived from work by Moffet and Goldstein which showed a near-doubling of the risk of severe AKI when concomitant nephrotoxic-medication exposure increased from 2 to 3 medications.5,6 Certain highly nephrotoxic medications were counted toward exposure for 7 days (day of administration plus 6 more days). All systemically administered forms of the nephrotoxic medications were included, but poorly bioavailable forms were not (e.g., inhaled or topical formulations). Nephrotoxic medications were counted irrespective of dose or frequency. Daily serum creatinine monitoring was recommended in exposed patients, and incidence of NTMx-associated AKI was tracked according to the Kidney Disease: Improving Global Outcomes (KDIGO) AKI criteria.7 Overall, 3.3% of patients met the nephrotoxic-medication exposure definition, and 25% of unique exposed patients developed AKI.6 In a 42-month period after initiating the quality-improvement measures, exposure to nephrotoxic medications decreased by 38% from 11.63 to 7.24 NTMx exposures/1,000 patient days, and the AKI rate decreased by 64% from 2.96 to 1.06 NTMx-associated AKI episodes/1,000 patient days.8 Projecting that initial baseline exposure rates would have persisted without the intervention, an estimated 633 exposures and 398 AKI episodes were avoided in the population over a 4-year period.
In 2014, a multicenter collaborative group of pediatric institutions was created to expand the initial single-center quality-improvement pilot. We aim to describe a critical component of our collaborative’s initiative in the implementation and refinement of the nephrotoxic medication list that defines exposure to nephrotoxic medications.
Problem
The variety of mechanisms by which medications can cause AKI makes defining a list of nephrotoxic medications difficult.9 Medications may lead to AKI via many mechanisms, including alterations in hemodynamics, acute tubular necrosis, crystalluria, acute interstitial nephritis, thrombotic microangiopathy, vasculitis, or thrombosis.1,10 There may be disagreement as to whether or not to consider a medication nephrotoxic, especially if it leads to AKI via indirect mechanisms versus directly nephrotoxic effects. Discrepancies in which medications are considered nephrotoxic have implications for clinical practice. The KDIGO Practice Guideline for AKI recommends discontinuing all nephrotoxic agents when possible for patients who are at risk for or have AKI.7 However, there is no standardized guidance as to which medications to avoid. Similarly, a lack of standardization regarding the type and number of medications included as nephrotoxic complicates NTMx-associated AKI research.
Inconsistencies in definitions of nephrotoxic medications are readily apparent when comparing published studies of pediatric AKI. In some cases, it is unclear which nephrotoxic medications were included because broad categories such as “antibiotics” and “chemotherapy” are used to describe the medications.3 In other cases, drug classes such as “nonsteroidal anti-inflammatory drugs” or “aminoglycosides” are used to define nephrotoxic medications without specifically stating which medications within the class were considered, presupposing that all medications within the class have a uniform effect on the occurrence of AKI.11,12 Using a sampling of 6 studies as an example of this variability, 88 individual medications were considered nephrotoxic.2,4-6,11,12 Some studies also included classes of nephrotoxic medications without explicitly stating which medications were included. Individual studies considered 5–62 individual medications and 0–6 medication classes as nephrotoxic. Inferring which individual medications could have been included within the classes, studies likely included 29–62 individual medications. Only methotrexate and vancomycin were explicitly included in all 6 studies. Inferring the medications included in each class, it is likely that 9 additional medications were included in all 6 studies: acyclovir, amikacin, cidofovir, cyclosporine, foscarnet, ganciclovir, gentamicin, tacrolimus, and tobramycin. Table 1 illustrates the differences in definitions of nephrotoxic medications among selected studies.
Table 1.
Nephrotoxic Medications and Medication Classes Included in Selected Studies
Similarly, inconsistencies were found within the definitions of nephrotoxic medications in the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) collaborative. When the NINJA program began at a single institution, 45 medications were included in the nephrotoxic medication list.6 This original list used for NINJA was developed by Moffett and Goldstein, who reviewed a standard drug reference and included all medications with AKI listed among the adverse reactions.5,13 The pharmacy team at the original institution then reviewed the list and added medications they thought were important based on their clinical knowledge.
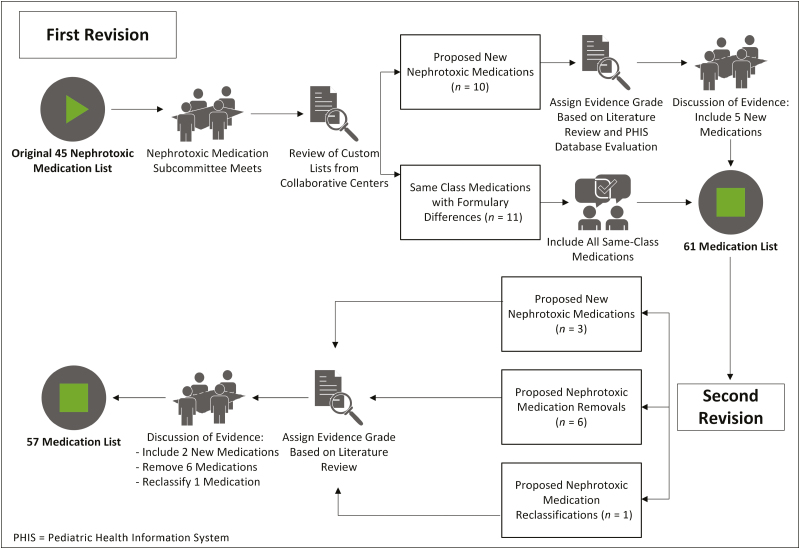
However, as the collaborative expanded to multiple centers, various other medications were added at specific institutions because of differences in institutional formularies and differences in beliefs regarding which medications posed a significant risk of AKI. Having custom lists at different institutions posed a problem, as outcome metrics were based on nephrotoxic-medication exposure. Therefore, it was essential for the collaborative to develop and use a uniform nephrotoxic medication list (Figure 1).
Figure 1.
Process map.
Analysis and resolution
After discussion at an in-person NINJA learning session in Cincinnati, Ohio, the NINJA Nephrotoxic Medication Subcommittee (NTMx Subcommittee) convened to address the problem of custom nephrotoxic medication lists among the participating NINJA sites. The committee was organized on a volunteer basis and originally included 3 pediatric nephrologists, a pediatric pharmacist, and a pediatric intensivist.
As noted above, the original nephrotoxic medication list contained 45 medications.6 The NTMx Subcommittee examined the custom lists for variation from the original nephrotoxic medication list, noting any additions or subtractions. There were 21 additional medications on one or more of the custom nephrotoxic medication lists that were not present on the original nephrotoxic medication list, and there were no suggested removals from the original list.
The additions to the list corresponded to 2 main categories: (1) medications in the same class that were on formulary at a collaborating center but not on the original list (n = 11) and (2) medications used across the collaborative that 1 or more centers considered to be important nephrotoxic medications (n = 10). The NTMx Subcommittee members agreed that all same-class medications should be included, because they were considered similarly nephrotoxic to their in-class counterparts. This left 10 medications that were present on 1 or more of the custom lists but not on the original nephrotoxic medication list.
To reconcile these differences, our approach was to gather evidence of nephrotoxicity and then discuss the strength of the evidence as a group. Our primary method for gathering evidence for nephrotoxicity was a literature search. Subcommittee members received instructions for collating and grading the evidence. To determine the strength of the evidence, we assigned an evidence grade to each potential addition. The strength of the evidence was graded from A to C and strength of the recommendation as strong (1) or weak (2), based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.14 For example, a 1A grade equated to a strong recommendation with high-quality evidence, while a 2C grade equated to a weak recommendation with low-quality evidence. Each team member assigned a recommendation and evidence grade based on the evidence review.
The secondary measure of nephrotoxicity used was the rate of AKI associated with each medication in approximately 4.8 million hospitalized children at 44 tertiary care children’s hospitals, as documented in the Pediatric Health Information System (PHIS) database, an administrative database that contains inpatient, emergency department, ambulatory surgery, and observation encounter-level data from more than 49 not-for-profit, tertiary care pediatric hospitals in the United States.15 These hospitals are affiliated with the Children’s Hospital Association (Lenexa, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. Portions of the data submission and data quality processes for the PHIS database are managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Nearly all of these hospitals also submit resource use data (e.g., pharmaceuticals, imaging, and laboratory) into PHIS. Data are de-identified at the time of data submission, and data are subjected to a number of reliability and validity checks before being included in the database. For this study, data from 44 hospitals were included.
We accessed all inpatient medications used from 2003 to 2013, and then obtained International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes for AKI (584.5, 584.6, 584.7, 584.8, and 584.9) associated with each encounter where that medication was received. Although we could not determine the timing of medication administration, we were able to perform a simple analysis of frequency of AKI according to each medication given. We compared the rate of AKI for each of the proposed additions to the rate of AKI for medications already on the NTMx list and for acetaminophen, which was considered a neutral comparator. This analysis was a useful secondary measure of nephrotoxicity where there was insufficient or conflicting literature. The subcommittee took into account that this analysis represented a simple association without correction for other factors.
The NTMx Subcommittee held a conference call to discuss evidence the next month. Subcommittee members presented a summary of the evidence, grade of evidence, and recommendation for their assigned medications. Following this, the subcommittee came to a consensus about each medication using the expert panel approach. Based on the evidence review, the subcommittee recommended 5 medications not be included and 5 new medications for inclusion (mitomycin, pentamidine isethionate, tenofovir disoproxil fumarate, zolendronic acid, and pamidronate disodium). Including the 11 same-class medications noted above, 16 new medications were added to the medication list based on the recommendation of the subcommittee.
During the next monthly collaborative-wide NINJA conference call, the NTMx Subcommittee presented its recommendations along with a grade of evidence for each recommended change. All the centers discussed the recommendations and agreed to the new nephrotoxic medication list, which contained 61 medications. A date was chosen for implementation of the new list, and centers planned and made the change as they were able. Because of differences in medical record systems and resources, there was institutional variability in how the list was used to identify exposed patients. All the participating NINJA institutions made the recommended changes to this list, resulting in a fully harmonized nephrotoxic medication list at all NINJA centers.
The collaborative decided that the list would be updated annually, because more frequent changes could lead to instability of the rates of nephrotoxic medication exposure and NTMx-associated AKI. Once a year, the NTMx Subcommittee accepts proposals for additions and subtractions from the list and reviews the proposals using the same methodology noted above. Between updates, each center agreed not to change its individual list until a new unified list was available for all.
In the most recent update to the nephrotoxic medication list, the subcommittee reviewed 10 proposed changes to the list, ultimately making the following changes: adding 2 medications because strong evidence of nephrotoxicity was available (deferasirox, polymixin b); removing 6 medications (dapsone, cefotaxime, ceftazidime, cefuroxime, gadodexate disodium, gadopentetate dimeglumine) as no strong evidence of nephrotoxicity was available in the literature; and changing the classification of 1 medication (vancomycin) to be considered an exposure after 3 or more days regardless of other nephrotoxic-medication use, given evidence of its nephrotoxicity independent of other NTMx use. This most recent list of 57 nephrotoxic medications was implemented in most NINJA centers on August 1, 2017, and has since been fully adopted by all NINJA centers (Appendix).
To explore the effect of the most recent changes made to the nephrotoxic medication list, the 2 exposure definitions (per the older, 61-mediation list versus the new 57-medication list) were compared using single-center data from October 1, 2016, to July 22, 2017. A comparison of nephrotoxic-medication exposure and NTMx-associated AKI was made using the same patient data with the 2 different exposure definitions. The computerized system to detect NTMx exposure and NTMx-associated AKI allowed comparison of the 2 lists within the exact same cohort of patients over the exact same period. The change in exposure definition led to an increase in the nephrotoxic-medication exposure rate (13.4% to 16.7%) and an increase in the percentage of exposed patients who developed AKI (15.7% to 17.3%). Therefore, the most recent medication list update seemed to reflect an increase in sensitivity and specificity of the exposure definition.
Discussion
Efforts within a multicenter collaborative to develop a standardized definition for nephrotoxic-medication exposure highlighted several limitations in the field of NTMx-associated AKI.
First, medications are inconsistently classified as nephrotoxic in the literature. This is largely the result of a lack of high-quality data describing the incidence of AKI with exposure to various medications. Thus, clinicians often rely on experience to make decisions as to which medications should be considered nephrotoxic, a method that is prone to bias. The NTMx Subcommittee addressed these limitations by conducting a thorough literature review for medications and by using a large database to assess the incidence of AKI in patients receiving candidate medications.
Second, the inclusion of every medication in the same class as nephrotoxic may lead to inclusion of medications with varying degrees of nephrotoxicity. A lack of data comparing nephrotoxicity within classes of medications makes it difficult to differentiate their nephrotoxicity, and our collaborative elected to allow member institutions to include medications from narrow classes (e.g., angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, nonsteroidal anti-inflammatory agents) according to institutional formularies. More research is needed to compare the nephrotoxicity of medications within classes to determine if same-class medications truly have equivalent nephrotoxicity.
Third, as new medications and formulations come into clinical use, an ongoing process to classify and monitor exposure to nephrotoxic medications is imperative to keep nephrotoxic-medication exposure definitions up to date. Based on our experience with a multicenter quality-improvement initiative, it is important that any change in the nephrotoxic-medication exposure definition be made simultaneously to ensure uniform data across the collaborative. The date of the change should be annotated in the data presentation to account for changes in outcomes measures related to changes in the exposure definition.
Future opportunities for research in this domain are abundant. In general, epidemiological study of NTMx-associated AKI is lacking, especially in pediatrics. Specifically, evidence regarding the nephrotoxic effects of various nephrotoxic-medication combinations is limited in children, and this should be further explored. Additionally, further analysis of nephrotoxic-medication dosing and frequency could be beneficial in refining the exposure definition, because certain medications may have varying nephrotoxicity at different doses. Next, future work to create tiers for these medications could help identify agents or combinations of agents that have differing effects on nephrotoxicity. With a few notable exceptions, all the medications on the collaborative’s nephrotoxic medication list carried the same weight in meeting the exposure definition.
In summary, definitions of nephrotoxic-medication exposure are inconsistent in the published literature, and expert opinion varies as to which medications should be considered nephrotoxic. Formation of our multicenter quality-improvement initiative exposed these limitations and presented an opportunity to approach this problem with an evidence-based process, through which we achieved consensus on a list of nephrotoxic medications that was used to define nephrotoxic-medication exposure. We encourage investigators in the field of NTMx-associated AKI to develop greater consistency among definitions of nephrotoxic-medication exposure. Future directions include sophisticated data analyses to identify and refine nephrotoxic-medication exposures that place patients at risk for NTMx-associated AKI, with the vision that children only get the nephrotoxic medications they need for the duration they need them. This will lead to our ultimate goal: to eliminate all preventable cases of NTMx-associated AKI in hospitalized children.
Conclusion
Formation of a multicenter quality-improvement initiative exposed current limitations as to which medications are considered nephrotoxic in clinical and research settings and presented an opportunity to approach this problem using an evidence-based process. A consensus definition of nephrotoxic- medication exposure was achieved.
Disclosures
This project was supported by grants from the Casey Lee Ball Foundation and the Agency for Healthcare Research and Quality (1R18HS023763-01). The authors have declared no potential conflicts of interest.
Appendix—Current Nephrotoxic Medication List for NINJA Collaborativea
| Acyclovir | Enalaprild | Ioxilanc | Polymixin B |
|---|---|---|---|
| Amikacinb | Enalaprilatd | Ketorolac | Sirolimus |
| Amphotericin liposomalc | Foscarnet | Lisinoprild | Sulfasalazine |
| Amphotericin B | Ganciclovir | Lithium | Tacrolimus |
| Aspirin | Gentamicinb | Losartand | Tenofovir |
| Captoprild | Ibuprofen | Mesalamine | Ticarcillin–clavulanate |
| Carboplatin | Ifosfamide | Methotrexate | Tobramycinb |
| Celecoxibd | Indomethacin | Mitomycin | Topiramate |
| Cidofovirc | Iodixanolc | Nafcillin | Valacyclovir |
| Cisplatin | Iohexolc | Naproxen | Valganciclovir |
| Cyclosporine | Iopamidolc | Pamidronate | Valsartand |
| Colistiethate | Iopromidec | Pentamidine | Vancomycinb |
| Deferasirox | Ioversolc | Piperacillin | Zoledronic acid |
| Diatrizoate megluminec | Ioxaglate meglumine–ioxaglate sodiumc | Piperacillin–tazobactam | Zonisamide |
| Diatrizoate sodiumc |
aNINJA = Nephrotoxic Injury Negated by Just-in-time Action.
bMedications that count as an exposure on day 3 of exposure, even if no other nephrotoxic medication is administered.
cMedications that count as an exposure for 7 days after a single administration.
dOther medications from the same classes may be added per institutional formulary.
Membership of the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) collaborative:
Eric S. Kirkendall, M.D.1, Devesh Dahale, M.S.1, Theresa Mottes, RN1, Kathleen Walsh, M.D.1, Stephen Muething, M.D.1, David J. Askenazi, M.D.2, Traci Henderson, RPh2, Lynn Dill, RN2, Jessica Kerr, M.P.H.3, Jennifer Gilarde, Pharm.D.3, Joshua Zaritsky, M.D.4, Valerie Bica, RN4, Patrick D. Brophy, M.D.5, Julia Steinke, M.D.6, Joann Mooney, RN6, Sara Ogrin, Pharm.D.6, Vimal Chadha, M.D.7, Bradley Warady, M.D.7, Wendy Hoebing, RPh7, Jordan Symons, M.D.8, Shina Menon, M.D.8, Lisa Abrams, RN8, Scott Sutherland, M.D.9, Patricia Weng, M.D.10, Michael G. Semanik, M.D.11, Monica C. Bogenschutz, Pharm.D.11, Jill Strayer, Pharm.D.11, Jeffrey J. Fadrowski, M.D, M.H.S.12, Elizabeth S. Goswami, Pharm.D.12, Alicia Neu, M.D.12, Emma Sexton, RN12, Amy Curran, RN12, Elys Bhatia12
1Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; 2Children’s Hospital of Alabama, Birmingham, AL; 3Children’s Hospital, Boston, MA; 4A.I. Dupont Children’s Hospital, Wilmington, DE; 5University of Iowa Stead Family Children’s Hospital, Iowa City, IA; 6Helen DeVos Children’s Hospital, Grand Rapids, MI; 7Children’s Mercy Hospital and Clinics, Kansas City, MO; 8Seattle Children’s Hospital, Seattle, WA; 9Lucille Packard Stanford Children’s Hospital, Palo Alto, CA; 10Mattel Children’s Hospital, Los Angeles, CA; 11American Family Children’s Hospital, Madison, WI; 12Johns Hopkins Children’s Center, Baltimore, MD
References
- 1. Joyce EL, Kane-Gill SL, Fuhrman DY, Kellum JA. Drug-associated acute kidney injury: who’s at risk? Pediatr Nephrol. 2017; 32:59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slater MB, Gruneir A, Rochon PA et al. Risk factors of acute kidney injury in critically ill children. Pediatr Crit Care Med. 2016; 17:e391-8. [DOI] [PubMed] [Google Scholar]
- 3. Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005; 45:96-101. [DOI] [PubMed] [Google Scholar]
- 4. McGregor TL, Jones DP, Wang L et al. Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis. 2016; 67:384-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011; 6:856-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldstein SL, Kirkendall E, Nguyen H et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013; 132:e756-67. [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2:1-138. [Google Scholar]
- 8. Goldstein SL, Mottes T, Simpson K et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016; 90:212-21. [DOI] [PubMed] [Google Scholar]
- 9. Mehta RL, Awdishu L, Davenport A et al. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015; 88:226-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patzer L. Nephrotoxicity as a cause of acute kidney injury in children. Pediatr Nephrol. 2008; 23:2159-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaddourah A, Basu RK, Bagshaw SM et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017; 376:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinclair EA, Yenokyan G, McMunn A et al. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014; 48:1555-62. [DOI] [PubMed] [Google Scholar]
- 13. Lacy C, Armstrong L, Goldman M et al. Drug information handbook. Hudson, OH: Lexi-Comp; 2009. [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Children’s Hospital Association. PHIS. www.childrenshospitals.org/phis (accessed 2019 Apr 5). [Google Scholar]