Abstract
Colletotrichum graminicola is a hemibiotrophic fungus that causes anthracnose leaf blight (ALB) and anthracnose stalk rot (ASR) in maize. Despite substantial economic losses caused by these diseases, the defence mechanisms against this pathogen remain poorly understood. Several hormones are suggested to aid in defence against C. graminicola, such as jasmonic acid (JA) and salicylic acid (SA), but supporting genetic evidence was not reported. Green leaf volatiles (GLVs) are a group of well‐characterized volatiles that induce JA biosynthesis in maize and are known to function in defence against necrotrophic pathogens. Information regarding the role of GLVs and JA in interactions with (hemi)biotrophic pathogens remains limited. To functionally elucidate GLVs and JA in defence against a hemibiotrophic pathogen, we tested GLV‐ and JA‐deficient mutants, lox10 and opr7 opr8, respectively, for resistance to ASR and ALB and profiled jasmonates and SA in their stalks and leaves throughout infection. Both mutants were resistant and generally displayed elevated levels of SA and low amounts of jasmonates, especially at early stages of infection. Pretreatment with GLVs restored susceptibility of lox10 mutants, but not opr7 opr8 mutants, which coincided with complete rescue of JA levels. Exogenous methyl jasmonate restored susceptibility in both mutants when applied before inoculation, whereas methyl salicylate did not induce further resistance in either of the mutants, but did induce mutant‐like resistance in the wild type. Collectively, this study reveals that GLVs and JA contribute to maize susceptibility to C. graminicola due to suppression of SA‐related defences.
Keywords: Colletotrichum graminicola, green leaf volatile (GLV), hormone cross‐talk, jasmonic acid (JA), lipoxygenase (LOX), salicylic acid (SA), Zea mays (maize)
Jasmonic acid and green leaf volatiles act as key susceptibility factors of maize to Colletotrichum graminicola by suppressing salicylic acid‐mediated defence during the biotrophic phase of growth.

1. INTRODUCTION
Colletotrichum is one of the most widespread and prolific genera of plant pathogenic fungi in the world (Dean et al., 2012). Co lletotrichum graminicola is an economically relevant representative of this genus, and is one of the greatest sources of yield loss among maize pathogens (Mueller et al., 2016). Though C. graminicola infects several tissues of maize, its most devastating forms of disease arise from infection of stalks and leaves, which result in anthracnose stalk rot (ASR) and anthracnose leaf blight (ALB). Infection begins with germination of conidia 12 hr after contact with the plant surface and is followed by formation of melanized appressoria within 24 hr (Vargas et al., 2012). A penetration peg is then formed after 24–36 hr, which leads to a phase of biotrophic colonization by primary hyphae (Mims and Vaillancourt, 2002; Vargas et al., 2012). After approximately 48–72 hr the fungus switches to a phase of necrotrophic growth. This is characterized by the formation of thinner secondary hyphae that kill cells prior to infection, which results in the formation of necrotic lesions on infected tissues (O’Connell et al., 1985; Bergstrom and Nicholson, 1999; Wharton et al., 2001; Mims and Vaillancourt, 2002; Vargas et al., 2012). Though disease progression of C. graminicola is well documented, less is known regarding defences employed by maize against it. Existing literature implicates salicylic acid (SA), jasmonic acid (JA), and various other metabolites as potential regulators of defence against C. graminicola; however, genetic evidence has not yet been provided to validate these hypotheses (Vargas et al., 2012; Balmer et al., 2013; Miranda et al., 2017).
JA and green leaf volatiles (GLVs) are well‐known oxylipins produced in the lipoxygenase (LOX) pathway (Feussner and Wasternack, 2002; Andreou and Fuessner, 2009; Borrego and Kolomiets, 2016). JA biosynthesis in the allene oxide synthase (AOS) pathway begins in the chloroplast with the oxygenation of C18:3 by a 13‐LOX to form 13S‐hydroperoxy octadecatrienoic acid (13S‐HPOTE) (Blée, 2002; Howe and Shilmiller, 2002). 13S‐HPOTE is subsequently acted upon by a 13‐AOS and then an allene oxide cyclase (AOC) to form (+)‐cis‐12‐oxo‐phytodienoic acid (12‐OPDA), which possesses signalling activity distinct from JA (Vick and Zimmerman, 1983; Kramell et al., 2000; Stintzi et al., 2001; Taki et al., 2005; Ribot et al., 2008; Dave et al., 2011; Dave and Graham, 2012). 12‐OPDA is then transported to the peroxisome, where it is reduced to 8‐[3‐oxo‐2‐cis‐[(Z)‐2‐pentenyl]cyclopentyl]octanoic acid (OPC‐8:0) by an oxo‐phytodienoate reductase (OPR). Maize possesses two functionally redundant JA‐producing OPRs, ZmOPR7 and ZmOPR8 (Yan et al., 2012). OPC‐8:0 undergoes three rounds of β‐oxidation to form (+)‐7‐iso‐JA, which itself is biologically inactive (Staswick and Tiryaki, 2004). Once synthesized, JA may be converted into a variety of derivatives, including methyl jasmonate (MeJA) and the main biologically active conjugate (+)‐7‐iso‐JA‐Ile (JA‐Ile) (Vick and Zimmerman, 1983; Staswick and Tiryaki, 2004; Fonseca et al., 2009; Yan et al., 2016; Caarls et al., 2017; Wasternack and Strnad, 2018). JA signalling regulates many diverse physiological processes and is a key regulator of the defence response to wounding, herbivory, and pathogen infection via induction of defensive metabolites and proteolytic enzymes (Farmer and Ryan, 1990; Rodriguez‐Saona et al., 2001; Howe and Jander, 2008) or emission of volatile organic compounds (VOCs), such as GLVs (Kessler and Baldwin, 2001), which recruit insect parasitoids (Turlings et al., 1995; Allman and Baldwin, 2010).
GLVs are an important class of VOC and are produced in the hydroperoxide lyase (HPL) pathway, functioning in inter‐ and intraplant signalling, plant–insect communication, and defence against a variety of environmental stresses. GLVs include C6 aldehydes, alcohols, and their corresponding esters, and are almost ubiquitously emitted in response to abiotic and biotic stresses. As with JA, GLV biosynthesis begins in chloroplasts with oxygenation of C18:3 by a 13‐LOX to produce 13S‐HPOTE (Blée, 2002; Howe and Schilmiller, 2002; Borrego and Kolomiets, 2016). Maize possesses a single LOX isoform, LOX10, that supplies substrate to the HPL pathway for GLV biosynthesis (Christensen et al., 2013). A 13‐HPL acts upon LOX10‐derived 13S‐HPOTE to form a short‐lived hemiacetal, which quickly splits into C6 GLVs and traumatin, a C12 compound (Mukhtarova et al., 2018). Exposure to GLVs induces broad‐spectrum defence to various stresses through up‐regulation of a variety of defence‐related genes, including genes involved in the biosynthesis and signalling of JA (Bate and Rothstein, 1998; Engelberth et al., 2004; 2007; 2013; Farag et al., 2005; Frost et al., 2008; Hirao et al., 2012; Christensen et al., 2013; Yamauchi et al., 2015). In addition to altering plant responses, GLVs can also directly inhibit growth of fungal and bacterial pathogens (Major et al., 1960; Zeringue and McCormick, 1989; Hamilton‐Kemp et al., 1992; Nakamura and Hatanaka, 2002; Prost et al., 2005; Kishimoto et al., 2006; Shiojiri et al., 2006).
Thus far, the effects of GLVs on plant–pathogen interactions have almost exclusively been studied in the Arabidopsis–Botrytis cinerea pathosystem, where GLVs induce resistance (Archbold et al., 1997, Kishimoto et al., 2006; 2008; Shijori et al., 2006). In this pathosystem, defence conferred by GLVs is probably mediated through increased JA‐dependent signalling, which typically induces resistance to necrotrophic pathogens (Thomma et al., 1998). JA shares strong antagonism with another important defence phytohormone, SA, which typically induces resistance to (hemi)biotrophic pathogens (Glazebrook, 2005). Using the maize GLV‐deficient mutant lox10 and the JA‐deficient double mutant opr7-5 opr8-2, we show that GLVs facilitate susceptibility to C. graminicola through JA‐dependent suppression of SA shortly after inoculation.
2. RESULTS
2.1. GLV and JA deficiency results in increased resistance to ASR
To investigate the roles of GLVs and JA in ASR, we inoculated stalks of lox10 mutants in two different genetic backgrounds, W438 and B73, with C. graminicola. Mutants displayed significantly smaller lesions relative to their respective near‐isogenic line wild types (NIL‐WTs), indicating increased resistance to ASR (Figure 1a–c). Furthermore, mutants in both genetic backgrounds were equally resistant, indicating LOX10 function in mediating susceptibility is well‐conserved across diverse genetic backgrounds (Figure 1a–c). Because GLVs are one of the major LOX10‐derived products and are well‐known inducers of JA synthesis (Engelberth et al., 2004), we hypothesized that the increased resistance in lox10 mutants is due to reduced JA. To test whether JA is a susceptibility factor for ASR, we inoculated stalks of single opr7‐5 and opr8‐2 mutants, opr7‐5 opr8‐2 double mutants, and their respective NIL‐WTs. Of these genotypes, only the opr7‐5 opr8‐2 double mutant is devoid of JA, while opr7‐5 and opr8‐2 single mutants maintain WT levels of JA due to functional redundancy of the OPR7 and OPR8 genes (Yan et al., 2012). Accordingly, only opr7‐5 opr8‐2 double mutants displayed increased resistance against ASR, whereas opr7‐5 and opr8‐2 single mutants and their respective NIL‐WTs all exhibited equally large lesions (Figure 1d). Taken together, these results imply that lox10 and opr7‐5 opr8‐2 mutant resistance is due to reduced GLV and JA biosynthesis.
Figure 1.
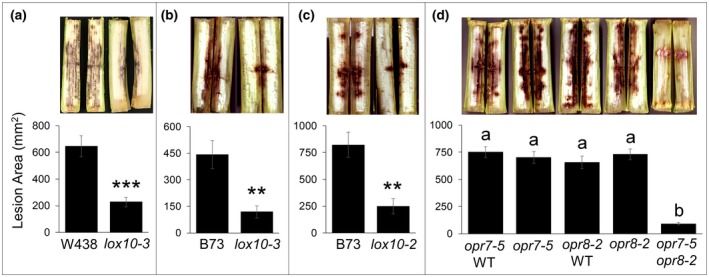
lox10 and opr7‐5 opr8‐2 mutant stalks are more resistant to Colletotrichum graminicola. (a) Representative stalks of wild‐type (WT) and lox10‐3 mutants in the W438 genetic background 11 days post‐inoculation (dpi). (b) lox10‐2 and (c) lox10‐3 mutants and their near‐isogenic WTs in the B73 genetic background at 10 dpi. (d) Stalks of opr7‐5, opr8‐2, and opr7‐5 opr8‐2 mutants and their respective WTs in the B73 background at 10 dpi. Stalks were split and imaged and lesions were quantified from the digital images. Mean ± SE, mm2. For (a), (b), and (c) Student's t test was used to determine statistical significance (**p < .005, ***p < .0005). Tukey's HSD test was used to determine the statistical significance for (d), where different letters denote statistical difference (p < .05)
2.2. Increased resistance to ASR correlates with reduced JA and increased SA
To investigate the biochemical mechanisms behind increased resistance of both GLV‐ and JA‐deficient mutants, we used liquid chromatography tandem mass spectrometry (LC‐MS/MS) to quantify accumulation of diverse hormones and metabolites, including SA, and the jasmonates 12‐OPDA, JA, and JA‐Ile during ASR progression in lox10‐3 and opr7‐5 opr8‐2 mutant stalks. lox10‐3 mutants were significantly impaired in their ability to accumulate 12‐OPDA throughout the course of infection, as well as in uninoculated controls (Figure 2a). Similarly, opr7‐5 opr8‐2 mutants accumulated low levels of 12‐OPDA at 1 day post‐inoculation (dpi) (Figure 2e). Unlike in lox10‐3 mutants, however, levels of 12‐OPDA in opr7‐5 opr8‐2 mutants recovered to WT levels by 3 dpi, presumably due to increased synthesis of 12‐OPDA by LOX10. This suggests that LOX10 is a major producer of 12‐OPDA in stalks.
Figure 2.

Hormone analysis shows 12‐OPDA, jasmonic acid (JA), and JA‐Ile are largely low or absent and salicylic acid (SA) is high in lox10‐3 and opr7‐5 opr8‐2 mutant stalks after inoculation. The left column shows 12‐OPDA (a), JA (b), JA‐Ile (c), and SA (d) levels in wild‐type (WT) and lox10‐3 mutant stalks in the W438 background 1, 4, and 6 days post‐inoculation (dpi). The right column shows 12‐OPDA (e), JA (f), JA‐Ile (g), and SA (h) levels in WT and opr7‐5 opr8‐2 mutants stalks in the B73 background 1, 3, and 5 dpi. n.d., not detected. Control = mock‐treated 1 dpi. Mean ± SE, pmol per g of fresh weight. Student's t test was used to determine the statistical difference between genotypes of each timepoint/treatment (*p < .05, **p < .005, ***p < .0005)
JA and JA‐Ile accumulation were also impaired in lox10‐3 mutants but, as in WT stalks, steadily increased throughout the duration of the experiment (Figure 2b,c). As expected, opr7‐5 opr8‐2 mutants are largely devoid of JA and JA‐Ile, with the notable exception of 5 dpi, where JA, but not JA‐Ile, exceeded levels seen in WT stalks (Figure 2f,g). This induction of JA was unexpected, as opr7‐5 opr8‐2 mutants were thought to be JA‐deficient (Yan et al., 2012). However, many fungi are able to directly synthesize JA themselves (Tsukada et al., 2010; Oliw and Hamberg, 2019), so we hypothesized that JA detected in opr7‐5 opr8‐2 mutants was synthesized and secreted by C. graminicola in order to reduce host defences. To test this hypothesis, we measured jasmonates in C. graminicola mycelial mass filtered from liquid culture, as well as the liquid medium itself. Much to our surprise, no jasmonates of any kind were detected in either C. graminicola biomass or the liquid medium in which it was grown (data not shown).
Metabolite analysis revealed that SA was elevated in lox10‐3 and opr7‐5 opr8‐2 mutant stalks relative to their respective WTs (Figure 2d,h). Both mutants displayed elevated levels of SA at 1 dpi relative to their respective WTs. opr7‐5 opr8‐2 mutants were particularly high, which is probably a result of them possessing higher basal SA, a result not observed in lox10‐3 mutants (Figures 2d,h and S2). Importantly, the most significant differences of jasmonates and SA between both mutants and their respective WTs occurred at the earliest timepoints of infection, which coincide with a phase of biotrophic growth by C. graminicola (Figure 2). Overall, lox10‐3 and opr7‐5 opr8‐2 mutant resistance seems to correlate with increased SA and decreased JA in stalks at early stages of infection.
2.3. GLV and JA deficiency results in increased resistance to ALB
To test whether GLVs and JA mediate susceptibility to C. graminicola in leaves, we drop‐inoculated C. graminicola spore suspension onto leaves of lox10‐2 and lox10‐3 mutants in the W438 and B73 backgrounds, as well as opr7‐5 opr8‐2 mutants in the B73 background. Similar to ASR, ALB assays consistently showed that lox10‐2 and lox10‐3 mutant leaves in both genetic backgrounds displayed significantly smaller lesions compared with their WT counterparts (Figure 3). Additionally, opr7‐5 opr8‐2 mutant leaves also had significantly smaller lesions compared with WT (Figure 3c), and in direct side‐by‐side comparisons were similar to lesions on lox10‐3 mutant leaves. Overall, this confirms that, as with ASR, GLVs and/or other LOX10‐derived products and JA promote virulence of C. graminicola.
Figure 3.

lox10 and opr7‐5 opr8‐2 mutant leaves are resistant to Colletotrichum graminicola. Mean areas of lesion after inoculation of lox10‐2 (b) and (d), lox10‐3 (a) and (b), and opr7‐5 opr8‐2 (b) mutants. (a) and (b) lox10‐2 and lox10‐3 mutants in the W438 background. (c) and (d) lox10‐2, lox10‐3, and opr7‐5 opr8‐2 mutants in the B73 background. Leaves were harvested at 5 days post‐inoculation (dpi) (a) and (c) or 6 dpi (b) and (d), and scanned to produce digital images from which lesion areas were measured. Mean ± SE, lesion area (mm2). For (a), (b), and (d), Student's t test was performed to determine the statistical significance of lesion areas (**p < .005, ***p < .0001). Tukey's HSD test was performed for (c), where different letters denote statistical significance (p < .05)
2.4. Increased resistance to ALB correlates with reduced JA and increased SA
To determine if the same mechanisms behind ASR resistance of lox10‐3 and opr7‐5 opr8‐2 mutant stalks also underlie ALB resistance in leaves, we quantified 12‐OPDA, JA, JA‐Ile, and SA throughout progression of ALB in leaves of lox10‐3 and opr7‐5 opr8‐2 mutants. As in stalks, 12‐OPDA and JA levels were low in lox10‐3 mutants throughout the duration of disease progression (Figure 4a). JA‐Ile largely mimicked this same pattern, but by 5 dpi, lox10‐3 mutants accumulated more JA‐Ile than WT (Figure 4c). opr7‐5 opr8‐2 mutant leaves, with the exception of 1 dpi, displayed lower amounts of 12‐OPDA compared with WT, similar to results obtained for stalks (Figure 4e). Unsurprisingly, opr7‐5 opr8‐2 mutant leaves were devoid of JA or JA‐Ile throughout 3 dpi (Figure 4f,g), but at 5 dpi both accumulated to levels greater than seen in WT (Figure 4f,g). This late and robust accumulation of JA in opr7‐5 opr8‐2 mutant leaves at 5 dpi mirrors results in stalks, but unlike in stalks of opr7‐5 opr8‐2 mutants, also includes a proportionate increase of JA‐Ile. Interestingly, this late increase in JA and/or JA‐Ile was seen in leaves of both lox10‐3 and opr7‐5 opr8‐2 mutants, which characteristically produce low amounts of JA. These results again suggest potential differing roles of JA during biotrophic and necrotrophic phases of growth.
Figure 4.
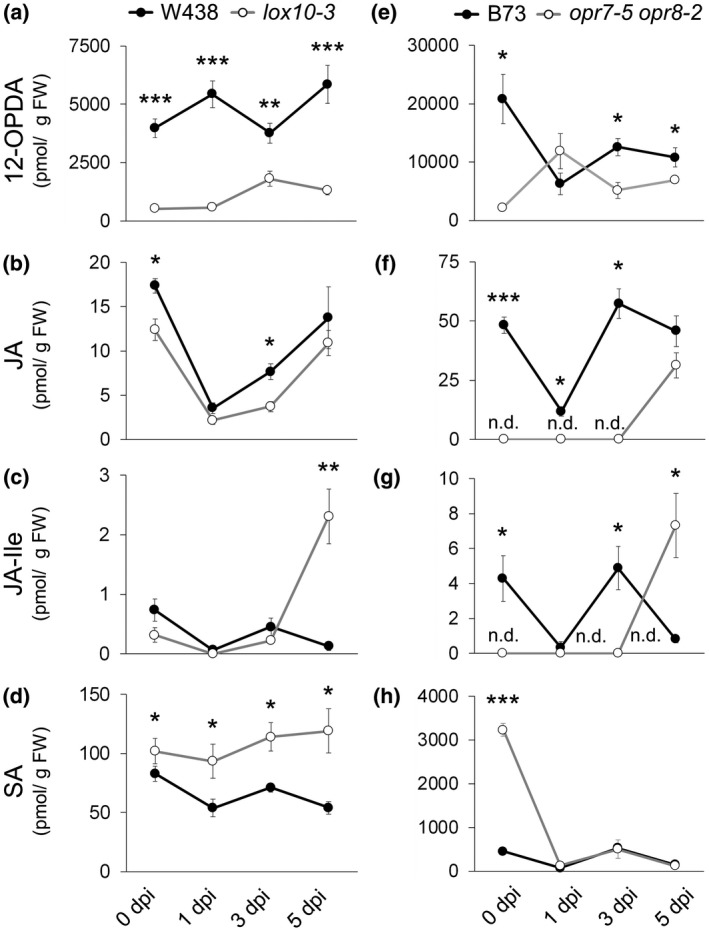
Hormone analysis shows 12‐OPDA, jasmonic acid (JA), and JA‐Ile are largely low or absent, and that salicylic acid (SA) is high in lox10‐3 and opr7‐5 opr8‐2 mutant leaves before or throughout infection. The left column shows 12‐OPDA (a), JA (b), JA‐Ile (c), and SA (d) levels in wild‐type (WT) and lox10‐3 mutants leaves in the W438 background 0, 1, 3, and 5 days post‐inoculation (dpi). The right column shows 12‐OPDA (e), JA (f), JA‐Ile (g), and SA (h) levels in WT and opr7‐5 opr8‐2 mutant leaves in the B73 background at 0, 1, 3, and 5 dpi. 0 dpi plants were untreated. Mean ± SE, pmol per g of fresh weight. n.d., not detected. Student's t test was used to determine the statistical difference between genotypes of each timepoint/treatment (*p < .05, **p < .005, ***p < .0005)
As in stalks, SA is elevated in lox10‐3 mutant leaves throughout the course of infection (Figure 4d). In opr7‐5 opr8‐2 mutant leaves, SA levels were equal to WT throughout infection (Figure 4h). This was surprising given the high amounts of SA seen in opr7‐5 opr8‐2 mutant stalk tissues. However, it is important to note that the levels of SA prior to infection (0 dpi) were strikingly higher relative to WT. Furthermore, the levels of SA in leaves of opr7‐5 opr8‐2 mutant were much higher compared to the amounts detected in their stalks. This suggests that high basal amounts of SA present in opr7‐5 opr8‐2 mutant leaves may prevent initial colonization by C. graminicola, resulting in limited infection and thus limited defence induction. Conversely, lox10‐3 mutants possessed high amounts of SA after infection, but, as in stalks, had normal basal levels of SA. This suggests that although the mechanisms behind SA‐related defence in lox10‐3 and opr7‐5 opr8‐2 mutant leaves and stalks are different, both mutants are resistant due to enhanced amounts of SA. Taken in concert with the relatively low amount of jasmonates present in both mutants, especially at the earliest points of infection, this suggests that early LOX10‐, OPR7‐, and OPR8‐dependent accumulation of JA suppresses SA‐mediated defence against C. graminicola. However, in addition to producing potent JA‐inducing GLVs, LOX10 may also provide substrate for synthesis of a variety of other metabolites, including JA (Borrego and Kolomiets, 2016). As such, it was unclear whether LOX10 increases JA through GLV‐dependent induction, direct synthesis of JA, both, or other LOX10‐derived metabolites.
2.5. LOX10 localizes to chloroplasts, the site of JA and GLV biosynthesis
We first sought to establish whether LOX10 localizes to chloroplasts, the known site of both GLV and JA biosynthesis, as with other GLV‐producing LOXs (Bell et al., 1995; Chehab et al., 2006; Shen et al., 2014; Mochizuki et al., 2016). Using maize containing yellow fluorescent protein (YFP)‐tagged LOX10 (LOX10‐YFP), Christensen et al. (2013) previously reported that LOX10 localized to unknown microbodies but its localization to plastids was uncertain. To further understand LOX10 localization, we performed additional confocal microscopy on LOX10‐YFP lines and found LOX10 also localized to chloroplasts (Figure 5). LOX10 seemed to accumulate most prominently in chloroplasts of bundle sheath cells and guard cells, as well as in chloroplasts of mesophyll cells, albeit to a lesser degree. These results suggest that 13S‐HPOTE produced by LOX10 may potentially feed into the AOS pathway for JA synthesis as well as into the HPL pathway for GLV synthesis.
Figure 5.

LOX10‐yellow fluorescent protein (YFP) tagged maize lines reveal that LOX10 localizes to chloroplasts of bundle sheath cells. (a)–(d) Images of untransformed leaves of B73 inbred line, (e)–(l) images of transgenic lines in the B73 background expressing YFP‐tagged LOX10 (LOX10‐YFP) under its native promoter, where (e)–(h) show images comparable to (a)–(d) and (i)–(l) show zoomed‐in views of (e)–(h) detailing LOX10 localization to bundle sheath chloroplast (i)–(l). Columns in order of left to right show brightfield views, chlorophyll autofluorescence, YFP fluorescence, and a merged view. Scale bars = 50 µm
2.6. GLVs mediate susceptibility to C. graminicola through induction of JA
To uncover the mechanism behind LOX10‐mediated JA induction, we exposed lox10‐3 and opr7‐5 opr8‐2 mutants to exogenous GLV treatment for 1 hr prior to inoculation of their leaves. Treatment consisted of a mixture containing 10 nmol of each major GLV molecular species emitted by maize, (Z)‐3‐hexenal, (Z)‐3‐hexenol, and (Z)‐3‐hexenyl acetate. Exposure of lox10‐3 mutants to GLVs resulted in lesion sizes equal to those observed on WT controls, and were significantly larger than those on lox10‐3 mutant controls (Figure 6a). In contrast, opr7‐5 opr8‐2 mutants exposed to GLVs did not display increased susceptibility compared to opr7‐5 opr8‐2 mutant controls (Figure 6b). These results show that small amounts of GLVs can completely rescue lox10‐3 mutant resistance, but are unable to rescue JA‐deficient opr7‐5 opr8‐2 mutants (Figure 6a,b). This confirms that GLVs are a major susceptibility factor and suggests that GLVs act through JA to induce susceptibility.
Figure 6.

Green leaf volatiles (GLVs) exposure rescues lox10‐3 mutant, but not opr7‐5 opr8‐2 mutant, susceptibility by increasing jasmonic acid (JA) and JA‐Ile. lox10‐3 and opr7‐5 opr8‐2 mutants in the W438 (a) or B73 (b) backgrounds were exposed to either an exogenous GLV mixture or triacetin (control) for 1 hr, followed by inoculation with Colletotrichum graminicola. Infected leaves were harvested and scanned at 4 days post‐inoculation to produce digital images from which lesions were measured. Mean ± SE, mm2. 12‐OPDA (c), JA (d), and JA‐Ile (e) were measured in lox10‐3 mutants after GLV exposure. Mean ± SE, pmol per g of fresh weight. Tukey's HSD test was used to determine the statistical significance, where different letters denote statistical significance (p < .05)
To confirm that rescue of lox10‐3 mutants by GLV exposure is mediated by increased JA, we exposed lox10‐3 mutants to GLVs and quantified 12‐OPDA, JA, and JA‐Ile. Accumulation of these jasmonates was significantly diminished in lox10‐3 mutant controls compared to WT controls; however, JA and JA‐Ile levels in lox10‐3 mutants were completely restored after GLV exposure (Figure 6c,d,e). Interestingly, 12‐OPDA was not increased by GLV exposure (Figure 6c). This could be due to GLV induction of genes in the AOS pathway downstream of 12‐OPDA synthesis, such as OPR7 and OPR8 (Christensen et al., 2013), and/or LOX10 is the major producer of 12‐OPDA. These results confirm that LOX10‐derived GLVs induce JA in a LOX10‐independent manner and are able to fully rescue both JA levels and susceptibility in lox10-3 mutants. Collectively, these data support the hypothesis that GLVs increase susceptibility to C. graminicola through induction of JA, and thus suppression of SA.
2.7. SA induces defence and JA promotes susceptibility to C. graminicola
SA is commonly required for resistance to biotrophs or hemibiotrophs and is implicated in systemic acquired resistance (SAR) to C. graminicola in maize (Balmer et al., 2013), but the roles of JA and SA in local infections remain untested. To confirm these respective roles, we exposed WT, lox10‐3, and opr7‐5 opr8‐2 mutants to methyl salicylate (MeSA) or methyl jasmonate (MeJA) prior to infecting their leaves with C. graminicola. WT plants in both the W438 and B73 backgrounds exposed to MeSA exhibited significantly smaller lesions compared with their respective controls, and were comparable to lox10‐3 and opr7‐5 opr8‐2 mutant controls (Figure 7a,b). In contrast, WT plants in the W438 background exposed to MeJA exhibited larger lesions than WT controls (Figure 7c). This increased susceptibility due to exogenous MeJA was not seen in the B73 background (Figure 7d). MeSA was unable to further decrease lesion sizes in lox10‐3 or opr7‐5 opr8‐2 mutants compared to their respective controls, presumably due to their innate saturation of SA‐dependent defences (Figure 7a,b). These results conclusively showed that SA induces resistance and JA promotes susceptibility to C. graminicola during its biotrophic phase of growth, and confirmed that high SA and low JA early in the infection process are responsible for lox10‐3 and opr7‐5 opr8‐2 mutant resistance.
Figure 7.

Treatment with methyl jasmonate (MeJA) rescues susceptibility in lox10‐3 and opr7‐5 opr8‐2 mutants while treatment with methyl salicylate (MeSA) has no effect. Wild type (WT) and lox10‐3 mutant in the W438 genetic background (a) and (b), and WT and opr7‐5 opr8‐2 mutants in the B73 genetic background were exposed to MeSA (a) and (b), MeJA (c) and (d), or ethanol (control) before inoculation with Colletotrichum graminicola. Treatments consisted of exposing plants to 10 µmol MeSA or MeJA dissolved in ethanol, or ethanol (control) for 2 days post‐inoculation (dpi) (a) and (b) or 6 dpi (c), (d), and (h). Leaves were harvested and scanned at 4 dpi to produce digital images from which lesions were measured. Mean ± SE, mm2. Tukey's HSD test was used to determine statistical significance, where different letters denote statistical significance (p < .05)
2.8. GLVs, but not JA, induce susceptibility after the switch to necrotrophy by C. graminicola
As GLVs and JA are known to aid in defence against necrotrophic pathogens, we sought to better clarify the roles of these metabolites after the switch to necrotrophy. To determine this, leaves of lox10‐3 and opr7‐5 opr8‐2 mutants were inoculated and infection was allowed to proceed for 3 days. At 3 dpi and the formation of visible lesions, we exposed the infected plants to either GLVs or MeJA and leaves were harvested at 6 dpi. As with the treatment before inoculation, GLV treatment during necrotrophy was able to fully rescue WT levels of susceptibility in lox10‐3 mutants (Figure 8a). However, MeJA treatment only slightly, but not significantly, enhanced susceptibility of lox10‐3 and opr7‐5 opr8‐2 mutants (Figure 8b,c). This indicates that JA only significantly contributes to disease progression during the earliest points of infection, when C. graminicola grows biotrophically, but not during the necrotrophic phase of growth. Furthermore, this indicates that while GLVs rely on JA induction for susceptibility during biotrophic growth, these molecules contribute to susceptibility through unknown, JA‐independent mechanisms during necrotrophic growth.
Figure 8.

Green leaf volatiles (GLVs) treatment during necrotrophy rescued susceptibility in lox10‐3 mutants, but methyl jasmonate (MeJA) treatment did not. lox10‐3 and opr7‐5 opr8‐2 mutants were inoculated with Colletotrichum graminicola and infection was left to proceed for 3 days before they were exposed to GLVs (a), MeJA (b) and (c), and their respective control treatments (triacetin and ethanol, respectively). Leaves were harvested and scanned 6 days post‐inoculation to produce digital images from which lesions were measured. Mean ± SE, mm2. Tukey's HSD test was used to determine statistical significance, where different letters denote statistical significance
3. DISCUSSION
Inoculation of stalks and leaves of GLV‐deficient lox10 mutants and JA‐deficient opr7‐5 opr8‐2 mutants clearly revealed that LOX10 and JA are susceptibility factors during C. graminicola infection (Figures 1 and 4). Importantly, resistance was observed in lox10‐2 and lox10‐3 mutants in both the B73 and W438 genetic backgrounds, indicating that LOX10‐mediated susceptibility is conserved across diverse genetic backgrounds in leaves and stalks. As LOX10, OPR7, and OPR8 are involved in the synthesis and/or induction of JA, it was not surprising that lox10‐3 and opr7‐5 opr8‐2 mutant stalks and leaves displayed impaired accumulation of several jasmonates during the early stages of infection. However, we were surprised to find that lox10‐3 mutant leaves and opr7‐5 opr8‐2 mutant leaves and stalks experienced increased amounts of JA at later stages of infection, 5–6 dpi, after the switch to necrotrophy (Figures 3 and 5). Interestingly, opr7‐5 opr8‐2 mutant leaves, but not stalks, also accumulated JA‐Ile at this time, which could be due to differences in JAR1 expression between the two tissue types. We hypothesized this JA was directly synthesized and secreted by C. graminicola, but jasmonates were not detected in analysis of either C. graminicola or its growth medium (data not shown). Despite this result, we cannot rule out that C. graminicola is directly producing this JA, as it may require physical or chemical cues and/or access to plant substrate from the plant surface to initiate JA biosynthesis. Alternatively, it is possible this JA was synthesized by the plant through a non‐OPR7/OPR8 dependent pathway, as has been reported for opr3 mutants in Arabidopsis (Chini et al., 2018). Despite its unknown origins, this late JA increase reveals a possible shift in the role of JA upon the switch from biotrophy to necrotrophy. Ultimately, however, lox10‐3 and opr7‐5 opr8‐2 mutant leaves and stalks possess low amounts of jasmonates during the early stages of infection, which are probably the most critical for establishing effective SA‐mediated defence.
In contrast to JA, lox10‐3 and opr7‐5 opr8‐2 mutants had higher amounts of SA in both stalks (Figure 3d,h) and leaves (Figure 4d,h). lox10‐3 mutant stalks and leaves had similar levels of SA compared with WT prior to infection, but had significantly higher amounts as soon as 1 dpi (Figure 3d). Unlike lox10‐3 mutants, stalks and leaves of opr7‐5 opr8‐2 mutants had high basal amounts of SA compared to WT. As early as 1 dpi, stalks of opr7‐5 opr8‐2 experienced even greater accumulation of SA relative to WT; however, the amount of SA in leaves of opr7‐5 opr8‐2 mutants returned to WT levels after 1 dpi. This is probably because of the very high amounts of SA present in opr7‐5 opr8‐2 mutant leaves, which were significantly higher than in stalks. This high basal amount may effectively prevent infection, thus resulting in diminished defence hormone induction. It should be noted that all leaves, but not stalks, of all genotypes experienced a decrease in most metabolites and hormones at 1 dpi. This is attributed to the inoculation method for leaves, which involved a 24 hr incubation in a humidity chamber with comparatively limited air flow and available light for photosynthesis. The relatively high amounts of SA present in both lox10‐3 and opr7‐5 opr8‐2 mutants before or soon after infection (1 dpi) directly coincide with appressoria formation and initiation of biotrophic growth by C. graminicola (Vargas et al., 2012). Reactive oxygen species are rapidly produced by plants on infection via SA signalling and are involved in fungal defence and growth inhibition, including C. graminicola (Apostol et al., 1989; Mellersh et al., 2002; Albarouki and Deising, 2013). It is possible that the quick induction of SA‐related defences inhibits catalase‐mediated degradation of H2O2, allowing build‐up of H2O2 around points of appressorial penetration, which stops penetration and/or growth of C. graminicola.
The mechanism behind low concentrations of JA was clear in opr7‐5 opr8‐2 mutants, but less so in lox10‐3 mutants as GLV‐producing LOXs are also usually the major JA‐producing LOXs. In maize, there are several LOXs that may directly contribute to JA synthesis, such as LOX8, a known producer of JA in response to wounding (Acosta et al., 2009; Christensen et al., 2013). As such, there was uncertainty as to whether LOX10 increases JA upon inoculation through direct synthesis, through GLV‐mediated signalling, both, or perhaps through other LOX10‐derived metabolites. Previous microscopy of LOX10‐YFP in maize did not show LOX10 localization to plastids, despite it containing a predicted plastid localization signal and LOX10 protein being previously isolated from chloroplasts (Majeran et al., 2005; Christensen, et al., 2013). As such, we first wanted to confirm if LOX10 localizes to chloroplasts, the initial site of both GLV and JA synthesis. These new results clearly detail LOX10 localization to chloroplasts of bundle sheath, mesophyll, and guard cells, indicating potential for JA biosynthesis (Figure 5 and S3). Importantly, LOX10‐YFP signal was distinctly more intense in bundle sheath chloroplasts compared to those of mesophyll cells, highlighting a potentially pivotal role in systemic vascular communication (Figure S3). These findings agree with analysis of LOX10 by ChloroP software, which predicts the presence of a 58 amino acid chloroplast transit peptide (Nemchenko et al., 2006), and with prior proteome analysis revealing LOX10 is abundant in both mesophyll and bundle sheath plastids (Majeran et al., 2005).
To uncover the mechanism behind LOX10‐mediated increase of JA on infection, we exposed lox10‐3 and opr7‐5 opr8‐2 mutants to GLVs prior to infection. GLV exposure of lox10‐3 and opr7‐5 opr8‐2 mutants resulted in a full rescue of susceptibility in lox10‐3 mutants, but not opr7‐5 opr8‐2 mutants (Figure 6a,b). Furthermore, GLV exposure fully restored JA and JA‐Ile in lox10‐3 mutants (Figure 6c). This result agrees with previous analysis of JA in lox10‐2 mutants after GLV exposure (Christensen et al., 2013). This demonstrates that GLVs alone can induce susceptibility to C. graminicola and that GLV‐mediated susceptibility, at least during biotrophy, requires intact JA signalling. Furthermore, this shows that biologically relevant amounts of GLVs increase JA and susceptibility independent of direct LOX10 JA synthesis. Remarkably, 12‐OPDA levels were not rescued in lox10‐3 mutants exposed to GLVs, suggesting that LOX10 is the major producer of 12‐OPDA. This also indicates that GLV‐mediated JA induction is achieved by activation of steps in the AOS pathway following AOC‐mediated synthesis of 12‐OPDA, implicating OPR7 and OPR8 as potential specific targets of GLVs for JA induction (Christensen et al., 2013).
Exposure of lox10‐3 and opr7‐5 opr8‐2 mutants to MeJA fully restored WT levels of susceptibility in both mutants (Figure 7c,d), agreeing with the low levels of jasmonates detected in both mutants at early stages of infection. Conversely, MeSA exposure was unable to induce further resistance in either mutant, which is probably due to their already saturated SA‐dependent defence signalling before or on infection. Furthermore, MeSA was also able to induce resistance in WT on a par with either mutant and MeJA was able to induce further susceptibility of WT only in the W438 background, which could be due to underlying differences in the two backgrounds (Figure 7). These data provide strong genetic and chemical evidence that JA is a susceptibility factor, and that SA provides resistance to C. graminicola.
However, as both lox10‐3 and opr7‐5 opr8‐2 mutant leaves displayed low initial amounts of JA, followed by high amounts by 5 dpi, we sought to determine if the role of JA and GLVs changed after the shift from biotrophic growth to necrotrophic growth of C. graminicola. Exposure of lox10‐3 and opr7‐5 opr8‐2 mutants to MeJA after 3 days of infection, after the switch to necrotrophy, was not able to rescue susceptibility as with the treatment before inoculation (Figure 8b,c). This indicates that while JA initially acts as a susceptibility factor, its contribution to susceptibility becomes negligible after the fungus switches to a necrotrophic phase of growth. In contrast to JA, GLVs were still able to fully restore susceptibility in lox10‐3 mutants after the switch to necrotrophy (Figure 8a). This shows that during necrotrophy, GLVs rely on other mechanisms than JA after the switch to necrotrophy. These mechanisms remain unknown and should be the subject of future studies. Furthermore, this shows that GLVs contribute to susceptibility in both phases of growth. This result contrasts with the previous study by Ameye et al. (2015), in which GLV treatment after the switch to necrotrophy by another hemibiotroph, Fusarium graminearum, in wheat induced resistance. The role GLVs play in plant–pathogen interactions may largely depend on the lifestyle of the pathogen; however, we should not discount the possibility that the effect of GLVs on plant–pathogen defence is pathosystem‐specific.
Little is known regarding maize defence mechanisms against C. graminicola. JA, SA, and other metabolites were suggested to be important for defence, but these hypotheses were not investigated with the use of knockout mutants or exogenous chemical treatment. Using JA‐ and GLV‐deficient mutants, we have definitively shown that JA, and GLVs by virtue of JA induction, cause maize susceptibility to C. graminicola in the biotrophic phase of growth. Much of this susceptibility can be attributed to JA‐mediated antagonism of SA‐related defences at this time, although there could be additional SA‐independent effects of JA or GLVs responsible as well. This is particularly true of GLV‐mediated susceptibility during necrotrophy. This suggests that LOX10, OPR7, OPR8, and other genes involved in synthesis or induction of GLVs and JA are potential targets for induction by C. graminicola shortly after colonization. It is important to note that herbivory and mechanical damage are strong inducers of GLVs and JA in maize (Christensen et al., 2013) and that ASR frequency in field settings shows a direct correlation with herbivory by the European corn borer (ECB) (Ostrinia nubilalis) (Keller et al., 1986; Bergstrom and Nicholson, 1999; Venard and Vaillancourt, 2007). This is in part because ECB can vector C. graminicola and allow it to bypass the tough rind of the stalk. However, this work reveals that a critical biochemical mechanism behind this correlation may also be the suppression of SA‐mediated defences by GLV and JA induction on herbivory (Bergstrom and Nicholson, 1999; Venard and Vaillancourt, 2007). This study also expands on the known functions of GLVs in plant–pathogen interactions, adding to a small number of publications that show GLVs can induce susceptibility to (hemi)biotrophs (Tong et al., 2012; Scala et al., 2013; Ameye et al., 2015).
4. EXPERIMENTAL PROCEDURES
4.1. Plant and fungal material
As previously described, mutant alleles of ZmLOX10, ZmOPR7, and ZmOPR8 were obtained by PCR screening of the Mutator‐transposon insertional genetics resource at DuPont‐Pioneer, Inc. (http://www.pioneer.com) for insertions in these genes (Yan et al., 2012; Christensen et al., 2013). The lox10‐2, lox10‐3, opr7‐5, and opr8‐2 alleles are all confirmed exon‐insertional knockout mutants. Original mutants were backcrossed into the B73 (lox10‐2, lox10‐3, opr‐5, and opr8‐2) and W438 (lox10‐2 and lox10‐3) backgrounds and genetically advanced to the backcross (BC) BC5 to BC7 stages. All mutants were genotyped by PCR analysis for conformation of homozygous mutant status. Transgenic C‐terminal YFP‐tagged LOX10 used in this study were generated as described by Mohanty et al. (2009) and advanced to the BC3 stage in the B73 genetic background. All C. graminicola plates used in infection assays were grown from culture stock (C. graminicola 1.001 strain) kept in a −80 °C freezer. Cultures were grown on potato dextrose agar (PDA) plates for at least 2 weeks before conidia were collected for use in plant inoculations. Spore extractions were performed as previously described by Gao et al. (2007) and were used within 2 hr of extraction.
4.2. Microscopy
LOX10‐YFP plants were grown to the V3 stage in TX‐360 Metro Mix soil (Sun Gro Horticulture) in a growth chamber under 16 hr of light (c.600 µmol⋅m−2⋅s−1) at 28 °C and 8 hr of dark at 24 °C with 50% humidity. Leaves were harvested and then imaged using a Digital Eclipse C1 confocal microscope (Nikon) (Tolley et al., 2018).
4.3. Anthracnose leaf blight assays and volatile treatments
Plants used in ALB assays were grown as previously described to the V4 stage, and were inoculated as previously described by Gao et al. (2007) except six plants per genotype/treatment were inoculated at six different points. opr7 opr8 mutants and their WT were grown in sterile soil, as they cannot survive in normal soil (Yan et al., 2012). For lesion size determination, plants were left for 4–6 days after inoculation before the infected leaves were excised and scanned to produce digital images. Lesion sizes were determined from digital images using ImageJ software (Schneider et al., 2012). For volatile treatments, plants were exposed to GLVs for 1 hr, MeSA for 2 hr, or MeJA for 6 hr before being inoculated 1 hr after volatile exposure ended. Six plants of each genotype/treatment were placed into a 6‐L glass container along with a cotton ball containing 100 µl of the chosen volatile(s). GLV treatment consisted of a mix containing 10 nmol (Z)‐3‐hexenal (50% in triacetin), 10 nmol (Z)‐3‐hexenol (>98%), and 10 nmol (Z)‐3‐hexenyl acetate (>98%,) dissolved in triacetin. MeSA (>98%) and MeJA (>98%) treatment consisted of 10 µmol of either chemical dissolved in ethanol. All chemical standards were purchased from Sigma‐Aldrich. For GLV and JA treatments during necrotrophy, plants were exposed to the volatile treatments at 3 dpi, and leaves were harvested for lesion size determination after 6 dpi.
4.4. Anthracnose stalk rot assays
lox10‐2 and lox10‐3 mutants and their NIL or inbred WTs used in ASR assays were grown to the VT stage outdoors under natural conditions throughout late spring and summer (College Station, TX, USA) in 14‐L pots filled with TX‐360 Metro Mix soil. Stalks were inoculated as previously described by Gao et al. (2007). For determination of lesion areas in lox10 mutants, three internodes of 10 plants per genotype were infected and harvested at 10–11 dpi. Postharvest, infected internodes were split and photographed to produce digital images that were analysed by ImageJ software. opr7‐5 and orp8‐2 mutants were grown in 14‐L pots filled with sterile TX‐360 Metro Mix soil in greenhouses during the summer. Four internodes of four plants per genotype were inoculated, harvested, and analysed at 10 dpi as described above.
4.5. Hormone analysis
For analysis of hormones in response to GLV exposure, plants were grown in a growth chamber to the V4 stage and exposed to GLVs as previously described. Leaves of the plants were harvested, frozen in liquid N2, and stored in a −80 °C freezer. For hormone analysis ALB, shoots were inoculated using an atomizer to spray a mist of 5 ml of 106 spores/ml onto each plant (0 hr timepoint plants were not treated) before plants were sealed in a humidity chamber as previously described by Gao et al. (2007). Five plants per genotype/timepoint were used. After 1, 3, or 5 dpi, their leaves were harvested, immediately frozen in liquid N2, and stored in a −80 °C freezer until further use. For hormone analysis of ASR, plant stalks were inoculated with either C. graminicola or sterile distilled water (control) as previously described. For lox10‐3 mutants, three internodes of five plants per genotype/timepoint were inoculated and harvested 1, 4, and 6 dpi as described above. For opr7‐5 opr8‐2 mutants, four internodes of four plants per genotype/timepoint were inoculated and harvested after 1, 3, or 5 dpi. A mortar and pestle were used to grind frozen plant material into a fine powder under liquid N2. Hormones were extracted from tissue and quantified by LC‐MS/MS. Ground tissue (100 mg) was mixed with 10 μl of 5 μM internal standards of d‐JA (2,4,4‐d3; acetyl‐2,2‐d2 JA [CDN Isotopes]), d6‐SA (Sigma‐Aldrich), and 500 μl phytohormone extraction buffer (1‐propanol/water/HCl [2:1:0.002 vol/vol/vol]). The samples were agitated for 30 min at 4 °C under darkness and then 500 μl dichloromethane was added to each sample. The samples were again agitated for 30 min at 4 °C in darkness and then centrifuged at 17,000 × g for 5 min. The lower organic layer of each sample was transferred to a glass vial for evaporation under nitrogen gas. Samples were resuspended in 150 μl methanol, transferred to a 1.5 ml microcentrifuge tube, and centrifuged at 17,000 × g for 2 min to separate any debris. Approximately 90 µl of supernatant of each sample was transferred into autosampler vials for LC‐MS/MS. The simultaneous detection of several phytohormones used methods of Müller and Munné‐Bosch (2011) with modifications. An Ascentis Express C‐18 Column (3 cm × 2.1 mm, 2.7 µm) (Sigma‐Aldrich) connected to an API 3200 LC‐MS/MS (Sciex) using electrospray ionization with multiple reaction mentoring was used. The injection volume was 10 μl and had a 600 μl/min mobile phase consisting of solution A (0.2% acetic acid in water) and solution B (0.2% acetic acid in acetonitrile) with a gradient consisting of 0.5 min–10% B, 1 min–20% B, 21 min–70% B, 24.6 min–100% B, 24.8 min–10% B, 29 min–stop. All hormones were quantified by comparing against isotopically labelled internal standards from Sigma‐Aldrich and Cayman Chemical.
Supporting information
ACKNOWLEDGEMENTS
The work performed in this study was supported by USDA‐NIFA (2017‐67013‐26524) grant awarded to M.V.K. There is no conflict of interest among authors of this study.
Gorman Z, Christensen SA, Yan Y, He Y, Borrego E, Kolomiets MV. Green leaf volatiles and jasmonic acid enhance susceptibility to anthracnose diseases caused by Colletotrichum graminicola in maize. Molecular Plant Pathology. 2020;21:702–715. 10.1111/mpp.12924
Funding information
The work performed in this study was supported by USDA‐NIFA (2017‐67013‐26524) grant awarded to M.V.K.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- Acosta, I.F. , Laparra, H. , Romero, S.P. , Schmelz, E. , Hamberg, M. , Mottinger, J.P. et al (2009) tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science, 323, 262–265. [DOI] [PubMed] [Google Scholar]
- Albarouki, E. and Deising, H.B. (2013) Infection structure‐specific reductive iron assimilation is required for cell wall integrity and full virulence of the maize pathogen Colletotrichum graminicola . Molecular Plant‐Microbe Interactions, 26, 695–708. [DOI] [PubMed] [Google Scholar]
- Allmann, S. and Baldwin, I.T. (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science, 329, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Ameye, M. , Audenaert, K. , De Zutter, N. , Steppe, K. , Van Meulebroek, L. , Vanhaecke, L. et al (2015) Priming of wheat with the green leaf volatile Z‐3‐hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production. Plant Physiology, 167, 1671–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou, A. and Feussner, I. (2009) Lipoxygenases – structure and reaction mechanism. Phytochemistry, 70, 1504–1510. [DOI] [PubMed] [Google Scholar]
- Apostol, I. , Heinstein, P.F. and Low, P.S. (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiology, 90, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold, D.D. , Hamilton‐Kemp, T.R. , Barth, M.M. and Langlois, B.E. (1997) Identifying natural volatile compounds that control gray mold (Botrytis cinerea) during postharvest storage of strawberry, blackberry, and grape. Journal of Agricultural and Food Chemistry, 45, 4032–4037. [Google Scholar]
- Balmer, D. , de Papajewski, D.V. , Planchamp, C. , Glauser, G. and Mauch‐Mani, B. (2013) Induced resistance in maize is based on organ‐specific defence responses. The Plant Journal, 74, 213–225. [DOI] [PubMed] [Google Scholar]
- Bate, N.J. and Rothstein, S.J. (1998) C6‐volatiles derived from the lipoxygenase pathway induce a subset of defense‐related genes. The Plant Journal, 16, 561–569. [DOI] [PubMed] [Google Scholar]
- Bell, E. , Creelman, R.A. and Mullet, J.E. (1995) A chloroplast lipoxygenase is required for wound‐induced jasmonic acid accumulation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 92, 8675–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom, G.C. and Nicholson, R.L. (1999) The biology of corn anthracnose: knowledge to exploit for improved management. Plant Disease, 83, 596–608. [DOI] [PubMed] [Google Scholar]
- Blée, E. (2002) Impact of phyto‐oxylipins in plant defense. Trends in Plant Science, 7, 315–322. [DOI] [PubMed] [Google Scholar]
- Borrego, E.J. and Kolomiets, M.V. (2016) Synthesis and functions of jasmonates in maize. Plants, 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caarls, L. , Elberse, J. , Awwanah, M. , Ludwig, N.R. , de Vries, M. , Zeilmaker, T. et al (2017) Arabidopsis JASMONATE‐INDUCED OXYGENASES down‐regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proceedings of the National Academy of Sciences of the United States of America, 114, 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab, E.W. , Raman, G. , Walley, J.W. , Perea, J.V. , Banu, G. , Theg, S. et al (2006) Rice HYDROPEROXIDE LYASES with unique expression patterns generate distinct aldehyde signatures in Arabidopsis . Plant Physiology, 141, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini, A. , Monte, I. , Zamarreño, A.M. , Hamberg, M. , Lassueur, S. , Reymond, P. et al (2018) An OPR3‐independent pathway uses 4, 5‐didehydrojasmonate for jasmonate synthesis. Nature Chemical Biology, 14, 171. [DOI] [PubMed] [Google Scholar]
- Christensen, S.A. , Nemchenko, A. , Borrego, E. , Murray, I. , Sobhy, I.S. , Bosak, L. et al (2013) The maize lipoxygenase, ZmLOX 10, mediates green leaf volatile, jasmonate and herbivore‐induced plant volatile production for defense against insect attack. The Plant Journal, 74, 59–73. [DOI] [PubMed] [Google Scholar]
- Dave, A. and Graham, I.A. (2012) Oxylipin signaling: a distinct role for the jasmonic acid precursor cis‐(+)‐12‐oxo‐phytodienoic acid (cis‐OPDA). Frontiers in Plant Science, 3, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave, A. , Hernández, M.L. , He, Z. , Andriotis, V.M. , Vaistij, F.E. , Larson, T.R. et al (2011) 12‐Oxo‐phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis . The Plant Cell, 23, 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. et al (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth, J. , Alborn, H.T. , Schmelz, E.A. and Tumlinson, J.H. (2004) Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth, J. , Contreras, C.F. , Dalvi, C. , Li, T. and Engelberth, M. (2013) Early transcriptome analyses of Z‐3‐hexenol‐treated Zea mays revealed distinct transcriptional networks and anti‐herbivore defense potential of green leaf volatiles. PLoS ONE, 8, e77465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth, J. , Seidl‐Adams, I. , Schultz, J.C. and Tumlinson, J.H. (2007) Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12‐oxo phytodienoic acid reductases in Zea mays . Molecular Plant‐Microbe Interactions, 20, 707–716. [DOI] [PubMed] [Google Scholar]
- Farag, M.A. , Fokar, M. , Abd, H. , Zhang, H. , Allen, R.D. and Pare, P.W. (2005) (Z)‐3‐Hexenol induces defense genes and downstream metabolites in maize. Planta, 220, 900–909. [DOI] [PubMed] [Google Scholar]
- Farmer, E.E. and Ryan, C.A. (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences of the United States of America, 87, 7713–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feussner, I. and Wasternack, C. (2002) The lipoxygenase pathway. Annual Review of Plant Biology, 53, 275–297. [DOI] [PubMed] [Google Scholar]
- Fonseca, S. , Chico, J.M. and Solano, R. (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Current Opinion in Plant Biology, 12, 539–547. [DOI] [PubMed] [Google Scholar]
- Frost, C.J. , Mescher, M.C. , Dervinis, C. , Davis, J.M. , Carlson, J.E. and De Moraes, C.M. (2008) Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis‐3‐hexenyl acetate. New Phytologist, 180, 722–734. [DOI] [PubMed] [Google Scholar]
- Gao, X. , Shim, W.B. , Göbel, C. , Kunze, S. , Feussner, I. , Meeley, R. et al (2007) Disruption of a maize 9‐lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Molecular Plant‐Microbe Interactions, 20, 922–933. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology, 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Hamilton‐Kemp, T.R. , McCracken, C.T. , Loughrin, J.H. , Andersen, R.A. and Hildebrand, D.F. (1992) Effects of some natural volatile compounds on the pathogenic fungi Alternaria alternata and Botrytis cinerea . Journal of Chemical Ecology, 18, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Hirao, T. , Okazawa, A. , Harada, K. , Kobayashi, A. , Muranaka, T. and Hirata, K. (2012) Green leaf volatiles enhance methyl jasmonate response in Arabidopsis . Journal of Bioscience and Bioengineering, 114, 540–545. [DOI] [PubMed] [Google Scholar]
- Howe, G.A. and Jander, G. (2008) Plant immunity to insect herbivores. Annual Review of Plant Biology, 59, 41–66. [DOI] [PubMed] [Google Scholar]
- Howe, G.A. and Schilmiller, A.L. (2002) Oxylipin metabolism in response to stress. Current Opinion in Plant Biology, 5, 230–236. [DOI] [PubMed] [Google Scholar]
- Keller, N.P. , Bergstrom, G.C. and Carruthers, R.I. (1986) Potential yield reductions in maize associated with an anthracnose/European corn borer pest complex in New York. Phytopathology, 76, 586–589. [Google Scholar]
- Kessler, A. and Baldwin, I.T. (2001) Defensive function of herbivore‐induced plant volatile emissions in nature. Science, 291, 2141–2144. [DOI] [PubMed] [Google Scholar]
- Kishimoto, K. , Matsui, K. , Ozawa, R. and Takabayashi, J. (2006) Components of C6‐aldehyde‐induced resistance in Arabidopsis thaliana against a necrotrophic fungal pathogen, Botrytis cinerea . Plant Science, 170, 715–723. [Google Scholar]
- Kishimoto, K. , Matsui, K. , Ozawa, R. and Takabayashi, J. (2008) Direct fungicidal activities of C6‐aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea . Phytochemistry, 69, 2127–2132. [DOI] [PubMed] [Google Scholar]
- Kramell, R. , Miersch, O. , Atzorn, R. , Parthier, B. and Wasternack, C. (2000) Octadecanoid‐derived alteration of gene expression and the “oxylipin signature” in stressed barley leaves. Implications for different signaling pathways. Plant Physiology, 123, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran, W. , Cai, Y. , Sun, Q. and van Wijk, K.J. (2005) Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics. The Plant Cell, 17, 3111–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major, R.T. , Marchini, P. and Sproston, T. (1960) Isolation from Ginkgo biloba L. of an inhibitor of fungus growth. Journal of Biological Chemistry, 235, 3298–3299. [PubMed] [Google Scholar]
- Matsui, K. , Sugimoto, K. , Mano, J.I. , Ozawa, R. and Takabayashi, J. (2012) Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE, 7, e36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellersh, D.G. , Foulds, I.V. , Higgins, V.J. and Heath, M.C. (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. The Plant Journal, 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Mims, C.W. and Vaillancourt, L.J. (2002) Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology, 92, 803–812. [DOI] [PubMed] [Google Scholar]
- Miranda, V.J. , Porto, W.F. , da Rocha Fernandes, G. , Pogue, R. , Nolasco, D.O. , Araujo, A.C.G. , et al, 2017. Comparative transcriptomic analysis indicates genes associated with local and systemic resistance to Colletotrichum graminicola in maize. Scientific reports, 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, A. , Luo, A. , DeBlasio, S. , Ling, X. , Yang, Y. , Tuthill, D.E. et al (2009) Advancing cell biology and functional genomics in maize using fluorescent protein‐tagged lines. Plant Physiology, 149, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, S. , Sugimoto, K. , Koeduka, T. and Matsui, K. (2016) Arabidopsis lipoxygenase 2 is essential for formation of green leaf volatiles and five‐carbon volatiles. FEBS Letters, 590, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Mueller, D.S. , Wise, K.A. , Sisson, A.J. , Allen, T.W. , Bergstrom, G.C. , Bosley, D.B. , et al. (2016) Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Progress, 17, 211–222. [Google Scholar]
- Mukhtarova, L.S. , Brühlmann, F. , Hamberg, M. , Khairutdinov, B.I. and Grechkin, A.N. (2018) Plant hydroperoxide‐cleaving enzymes (CYP74 family) function as hemiacetal synthases: Structural proof of hemiacetals by NMR spectroscopy. Biochimica et Biophysica Acta, 1863, 1316–1322. [DOI] [PubMed] [Google Scholar]
- Müller, M. and Munné‐Bosch, S. (2011) Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods, 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S. and Hatanaka, A. (2002) Green‐leaf‐derived C6‐aroma compounds with potent antibacterial action that act on both gram‐negative and gram‐positive bacteria. Journal of Agricultural and Food Chemistry, 50, 7639–7644. [DOI] [PubMed] [Google Scholar]
- Nemchenko, A. , Kunze, S. , Feussner, I. and Kolomiets, M. (2006) Duplicate maize 13‐lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. Journal of Experimental Botany, 57, 3767–3779. [DOI] [PubMed] [Google Scholar]
- O'Connell, R.J. , Bailey, J.A. and Richmond, D.V. (1985) Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum . Physiological Plant Pathology, 27, 75–98. [Google Scholar]
- Oliw, E.H. and Hamberg, M. (2019) Biosynthesis of jasmonates from linoleic acid by the fungus Fusarium oxysporum. Evidence for a novel allene oxide cyclase. Lipids, 54, 543–556. [DOI] [PubMed] [Google Scholar]
- Prost, I. , Dhondt, S. , Rothe, G. , Vicente, J. , Rodriguez, M.J. , Kift, N. et al (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiology, 139, 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot, C. , Zimmerli, C. , Farmer, E.E. , Reymond, P. and Poirier, Y. (2008) Induction of the Arabidopsis PHO1; H10 gene by 12‐oxo‐phytodienoic acid but not jasmonic acid via a CORONATINE INSENSITIVE1‐dependent pathway. Plant Physiology, 147, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Saona, C. , Crafts‐Brandner, S.J. , ParÉ, P.W. and Henneberry, T.J. (2001) Exogenous methyl jasmonate induces volatile emissions in cotton plants. Journal of Chemical Ecology, 27, 679–695. [DOI] [PubMed] [Google Scholar]
- Scala, A. , Mirabella, R. , Mugo, C. , Matsui, K. , Haring, M.A. and Schuurink, R.C. (2013) (E)‐2‐hexenal promotes susceptibility to Pseudomonas syringae by activating jasmonic acid pathways in Arabidopsis . Frontiers in Plant Science, 4, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. and Eliceiri, K.W. (2012) NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Tieman, D. , Jones, J.B. , Taylor, M.G. , Schmelz, E. , Huffaker, A. et al (2014) A 13‐lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. Journal of Experimental Botany, 65, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri, K. , Kishimoto, K. , Ozawa, R. , Kugimiya, S. , Urashimo, S. , Arimura, G. et al (2006) Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences of the United States of America, 103, 16672–16676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick, P.E. and Tiryaki, I. (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis . The Plant Cell, 16, 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi, A. , Weber, H. , Reymond, P. and Farmer, E.E. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proceedings of the National Academy of Sciences of the United States of America, 98, 12837–12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki, N. , Sasaki‐Sekimoto, Y. , Obayashi, T. , Kikuta, A. , Kobayashi, K. , Ainai, T. et al (2005) 12‐oxo‐phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound‐induced gene expression in Arabidopsis . Plant Physiology, 139, 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma, B.P. , Eggermont, K. , Penninckx, I.A. , Mauch‐Mani, B. , Vogelsang, R. , Cammue, B.P. et al (1998) Separate jasmonate‐dependent and salicylate‐dependent defense‐response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America, 95, 15107–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley, J.P. , Nagashima, Y. , Gorman, Z. , Kolomiets, M.V. and Koiwa, H. (2018) Isoform‐specific subcellular localization of Zea mays lipoxygenases and oxo‐phytodienoate reductase 2. Plant Gene, 13, 36–41. [Google Scholar]
- Tong, X. , Qi, J. , Zhu, X. , Mao, B. , Zeng, L. , Wang, B. et al (2012) The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. The Plant Journal, 71, 763–775. [DOI] [PubMed] [Google Scholar]
- Tsukada, K. , Takahashi, K. and Nabeta, K. (2010) Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae . Phytochemistry, 71, 2019–2023. [DOI] [PubMed] [Google Scholar]
- Turlings, T.C. , Loughrin, J.H. , Mccall, P.J. , Röse, U.S. , Lewis, W.J. and Tumlinson, J.H. (1995) How caterpillar‐damaged plants protect themselves by attracting parasitic wasps. Proceedings of the National Academy of Sciences of the United States of America, 92, 4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, W.A. , Martín, J.M.S. , Rech, G.E. , Rivera, L.P. , Benito, E.P. , Díaz‐Mínguez, J.M. et al (2012) Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiology, 158, 1342–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venard, C. and Vaillancourt, L. (2007) Penetration and colonization of unwounded maize tissues by the maize anthracnose pathogen Colletotrichum graminicola and the related nonpathogen C. sublineolum . Mycologia, 99, 368–377. [DOI] [PubMed] [Google Scholar]
- Vick, B.A. and Zimmerman, D.C. (1983) The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochemical and Biophysical Research Communications, 111, 470–477. [DOI] [PubMed] [Google Scholar]
- Wasternack, C. and Strnad, M. (2018) Jasmonates: news on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. International Journal of Molecular Sciences, 19, e2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton, P.S. , Julian, A.M. and O'Connell, R.J. (2001) Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum . Phytopathology, 91, 149–158. [DOI] [PubMed] [Google Scholar]
- Yamauchi, Y. , Kunishima, M. , Mizutani, M. and Sugimoto, Y. (2015) Reactive short‐chain leaf volatiles act as powerful inducers of abiotic stress‐related gene expression. Scientific Reports, 5, 8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. , Li, S. , Gu, M. , Yao, R. , Li, Y. , Chen, J. et al (2016) Endogenous bioactive jasmonate is composed of a set of (+)‐7‐iso‐JA‐amino acid conjugates. Plant Physiology, 172, 2154–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y. , Christensen, S. , Isakeit, T. , Engelberth, J. , Meeley, R. , Hayward, A. et al (2012) Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. The Plant Cell, 24, 1420–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeringue, H.J. and McCormick, S.P. (1989) Relationships between cotton leaf‐derived volatiles and growth of Aspergillus flavus . Journal of the American Oil Chemists’ Society, 66, 581–585. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are not shared.


