Abstract
Rice bran, a by-product after milling, is a rich source of phytonutrients like oryzanols, tocopherols, tocotrienols, phytosterols, and dietary fibers. Moreover, exceptional properties of the rice bran oil make it unparalleled to other vegetable oils. However, a lipolytic enzyme Phospholipase D alpha1 (OsPLDα1) causes rancidity and ‘stale flavor’ in the oil, and thus limits the rice bran usage for human consumption. To improve the rice bran quality, sequence based allele mining at OsPLDα1 locus (3.6 Kb) was performed across 48 accessions representing 11 wild Oryza species, 8 accessions of African cultivated rice, and 7 Oryza sativa cultivars. From comparative sequence analysis, 216 SNPs and 30 InDels were detected at the OsPLDα1 locus. Phylogenetic analysis revealed 20 OsPLDα1 cDNA variants which further translated into 12 protein variants. The O. officinalis protein variant, when compared to Nipponbare, showed maximum variability comprising 22 amino acid substitutions and absence of two peptides and two β-sheets. Further, expression profiling indicated significant differences in transcript abundance within as well as between the OsPLDα1 variants. Also, a new OsPLDα1 transcript variant having third exon missing in it, Os01t0172400-06, has been revealed. An O. officinalis accession (IRGC101152) had lowest gene expression which suggests the presence of novel allele, named as OsPLDα1-1a (GenBank accession no. MF966931). The identified novel allele could be further deployed in the breeding programs to overcome rice bran rancidity in elite cultivars.
Subject terms: Gene expression profiling, Agricultural genetics
Introduction
Rice (Oryza sativa L.) bran, a by-product after milling, is composed of pericarp, aleurone, seed coat, nucellus along with the germ and a small portion of endosperm1,2. It constitutes about 10% of the weight of rough rice, and is comprised of 12–23% oil, 14–16% protein, and 8–10% crude fibre. The rice bran oil is an oleic–linoleic-type fatty acid and is rich source of vitamin E, thiamin, niacin, and minerals like aluminium, calcium, chlorine, iron, magnesium, manganese, phosphorus, potassium, sodium, and zinc3. Further, the presence of ω-3 and ω-6 fatty acids, high level of unsaponifiables, and high levels of antioxidants (tocopherols, tocotrienols, and γ-Oryzanol) makes it superior to other vegetable oils as well as brightens the prospects of its utilization for humans as functional ingredient to mitigate the life-threatening disorders4–6. In addition, physico–chemical properties make it a good quality edible oil7. However, removel of husk from the paddy leads to direct contact of air with rice bran layer, which activates endogenous lipase, and results in development of off-flavor in brown rice. Further, decomposition of triacylglycerols (TAGs) in rice bran, immidiately after the process of milling, raise the levels of free fatty acids (FFAs) which makes the rice bran unsuitable for human consumption or for production of edible oil with acceptable quality8,9. In addition, rapid degradation and hydrolytic rancidity of rice bran oil limits its use for human consumption.
Rice bran is mainly comprised of TAGs, which act as the primary reserve lipids and occur in the phospholipid membrane bounded oil bodies. The aleuronic layer at maturation is comprised of living cells in which phospholipids are decomposed into fatty acids and some other chemicals by various phospholipid-degrading enzymes during storage. The phospholipid-degrading enzymes viz. phospholipases, acyl hydrolases, and lipid-oxidizing enzymes have been known as important contributors to membrane degradation10,11. The treatment of oil bodies, from rice bran fraction, with Phospholipase D (PLD) causes oil bodies disintegration followed by reduction of phosphatidylcholine levels and TAGs decomposition into FFAs12,13. Further, the FFAs interact with endosperm starches to reduces the edibility of the rice. In addition, lipoxygenases act on the FFAs which contain a 1, 4-pentadiene structure, such as linoleic and linolenic acids, and lead to their conversion into low molecular-weight volatile products which cause a stale flavor in the product14,15. Hence, it has been revealed by the the earlier studies that PLD acts as a trigger for the initiation of lipid decomposition which further leads to deterioration of the rice grain and rice bran fractions.
A total of 17 PLD genes including eight isoforms of PLDα, two of PLDβ, three of PLDγ, two of PLDξ, and one isoform each of PLDκ and PLDφ has been indicated in rice genome database16. In these isoforms, protein domain analysis has revealed several conserved domains, including the HKD (HxKxxxxD) domains (also known as PLD-C1 and PLD-C2 domains), having hydrolytic activity; the calcium/lipid-binding domain (C2 domain), resonsible for regulation of Ca2+-dependent enzyme activity through binding to Ca2+; and (3) the PX (phox consensus sequence) and PH (pleckstrin homology) domains, located at the N-terminus of Ca2+ independent PLDs in place of the C2 domain of Ca2+ dependent PLDs17. From the rice bran fraction, a PLD protein (designated RPLD1, synonymous with OsPLDα1) has been purified and is found to be responsible for rice bran oil rancidity18. Suzuki et al. (2011) cloned the sequence of OsPLDα1 from O. sativa japonica cv. Nipponbare. This gene is 6.28-kb in size including promoter region and is located on the chromosome 1 of rice19. The expression profiling reveals that most PLD-encoding genes are differentially expressed in many plant tissues, and during various developmental stages, suggesting their involvement in multiple developmental processes20. However, studies using transgenics have clarified that the suppressed OsPLDα1 expression results in the improvement of grain and bran stability. In addition, this gene has been reported to be unnecessary for seed maturation or germination21.
Although various stabilization methods are available to inhibit the OsPLDα1 lipolytic process22,23, such methods only lead to partial inactivation; reduce the nutritional value of rice bran; and increase the time stringency for treatment and cost of oil production24. Thus, a profitable substitute is required to reduce the rice bran rancidity. The use of breeding techniques could increase the rice bran stability against lipolytic process if genetic differences exist for this trait. However, the hassle of diminished gene pool in cultivated germplasm is specifically relevant in self pollinated crops where the degree of genetic variation in cultivars can be less than 5% of the total variation in natural populations. As a result of the selection deployed by humans during domestication in favour of desired traits, the acquired early varieties carry only a small portion of the genetic diversity available in wild species25. Hence, for the current study, we chose a representative subset of the wild rice germplasm as it constitutes a major gene pool for rice improvement26,27. Further, the allele mining technique have been successfully employed in wild species to find important variations at various loci including Badh2, OsC1, Pi ta, NBS-LRR class R-genes, Adh2, wx locus, and Rc locus28–34. However, thus far, wild germplasm of rice has not been assessed for the variability at OsPLDα1 locus.
Therefore, in the current study, a detailed analysis of DNA sequence variation at the OsPLDα1 locus (Os01g0172400) was performed in a panel of wild and cultivated rice (Oryza spp.) to identify the novel sources of alleles with lower or null activity of the enzyme. Further, validation of the identified OsPLDα1 allelic variants was conducted using quantitative reverse-transcription expression analysis.
Results
SNPs within the coding region of OsPLDα1
The complete coding region of OsPLDα1, in all the wild Oryza accessions and cultivars (Table 1), was found to be ~2248 bp long and was comprised of three exons. A total of 105 SNPs and 2 insertions were identified in the coding region of OsPLDα1 gene across wild species accessions and cultivars (see Supplementary Table S1), using multiple sequence alignments. Within the first exon of OsPLDα1 gene (located on the gene from nucleotide position 353 to 460), only one nucleotide change (T373C) was observed across the accessions of O. officinalis, O. australiensis, O. punctata, O. minuta, and O. latifolia spp.
Table 1.
Selected Oryza spp. accessions for allele mining at OsPLDα1 locus.
| S. No. | Species | Accession # | Genome | Country of origin |
|---|---|---|---|---|
| 1 | O. glaberrima | IRGC100854 | AA | Congo |
| 2 | O. glaberrima | IRGC101800 | AA | Senegal |
| 3 | O. glaberrima | IRGC102196 | AA | Liberia |
| 4 | O. glaberrima | IRGC102489 | AA | Liberia |
| 5 | O. glaberrima | IRGC102512 | AA | Liberia |
| 6 | O. glaberrima | IRGC102600b | AA | Liberia |
| 7 | O. glaberrima | IRGC102925 | AA | Burkina Faso |
| 8 | O. glaberrima | IRGC103750 | AA | Nigeria |
| 9 | O. barthii | IRGC100117 | AA | Mali |
| 10 | O. barthii | IRGC101317 | AA | Guinea |
| 11 | O. barthii | IRGC104102 | AA | Chad |
| 12 | O. barthii | IRGC 105990 | AA | Cameroon |
| 13 | O. barthii | IRGC106239 | AA | Nigeria |
| 14 | O. barthii | IRGC106294 | AA | Chad |
| 15 | O. nivara | CR100008 | AA | India |
| 16 | O. nivara | CR100400 | AA | India |
| 17 | O. nivara | CR100126 | AA | India |
| 18 | O. nivara | CR100429 | AA | India |
| 19 | O. nivara | IRGC80547 | AA | India |
| 20 | O. nivara | IRGC81847 | AA | India |
| 21 | O. nivara | IRGC92713 | AA | Cambodia |
| 22 | O. nivara | IRGC92930 | AA | Combodia |
| 23 | O. nivara | IRGC100189 | AA | Malaysia |
| 24 | O. nivara | IRGC106397 | AA | India |
| 25 | O. rufipogon | CR100013 | AA | India |
| 26 | O. rufipogon | IRGC80610 | AA | India |
| 27 | O. rufipogon | IRGC81976 | AA | Indonesia |
| 28 | O. rufipogon | IRGC83823 | AA | Vietnam |
| 29 | O. rufipogon | IRGC89224 | AA | Combodia |
| 30 | O. rufipogon | IRGC99551 | AA | Vietnam |
| 31 | O. rufipogon | IRGC103308 | AA | Taiwan |
| 32 | O. rufipogon | IRGC104308 | AA | Myanmar |
| 33 | O. rufipogon | IRGC104867 | AA | Thailand |
| 34 | O. rufipogon | IRGC105491 | AA | Malaysia |
| 35 | O. rufipogon | IRGC105569 | AA | Cambodia |
| 36 | O. rufipogon | IRGC105902 | AA | Bangladesh |
| 37 | O. rufipogon | IRGC106162 | AA | Laos |
| 38 | O. rufipogon | IRGC106336 | AA | Cambodia |
| 39 | O. rufipogon | IRGC106433 | AA | Vietnam |
| 40 | O. rufipogon | IRGC113652 | AA | Vietnam |
| 41 | O. longistaminata | IRGC101200 | AA | Nigeria |
| 42 | O. longistaminata | IRGC104301 | AA | Gambia |
| 43 | O. longistaminata | IRGC105206 | AA | Ethiopia |
| 44 | O. meridionalis | IRGC101146 | AA | Australia |
| 45 | O. glumaepatula | IRGC100184 | AA | Cuba |
| 46 | O. glumaepatula | IRGC104387 | AA | Brazil |
| 47 | O. officinalis | IRGC101152 | CC | Brunei |
| 48 | O. officinalis | IRGC105674 | CC | Indonesia |
| 49 | O. officinalis | IRGC106501 | CC | Indonesia |
| 50 | O. australiensis | IRGC105275 | EE | Australia |
| 51 | O. punctata | IRGC101434 | BBCC | Tanzania |
| 52 | O. punctata | IRGC105158 | BBCC | Kenya |
| 53 | O. minuta | IRGC101100 | BBCC | Philippines |
| 54 | O. minuta | IRGC101128 | BBCC | Philippines |
| 55 | O. latifolia | IRGC100165 | CCDD | Guatemala |
| 56 | O. latifolia | IRGC105139 | CCDD | Guatemala |
Codes: IRGC represents the wild species accessions from the International Rice Genetic Consortium, IRRI, Philippines; CR represents accessions from National Rice Research Institute, Cuttack, India.
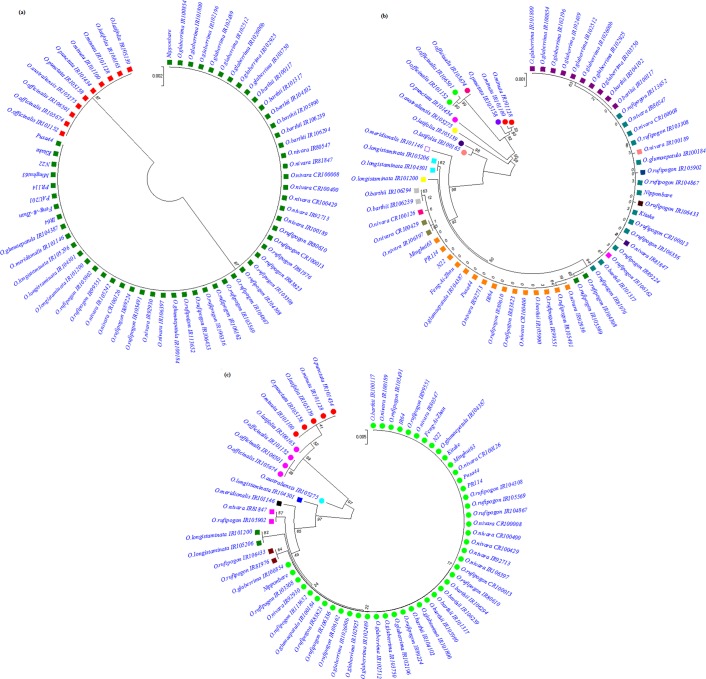
In addition, these species also had an insertion of nucleotide A at position 459. On the contrary, all the accessions belonging to ‘AA’ genome species and selected cultivars showed no polymorhism at the first exon and fall in the same cluster along with reference sequence of Nipponbare (Fig. 1a). The second exon of OsPLDα1, located on the gene from nucleotide position 1001 to 2897, was found to harbor the maximum variability (87 SNPs and an insertion of T1927) in the coding region. The detected SNPs were comprised of 65 transition changes and 22 transversions. G1141A was observed as the most frequent SNP followed by G1607A. Across all the selected wild species accessions and cultivars, maximum number of SNPs (73) were present in the species belonging to the O. officinalis complex (O. officinalis, O. australiensis, O. punctata, O. minuta, and O. latifolia). Moreover, O. officinalis spp. having 52 SNPs and an insertion of A at position 1927 was found most polymorphic among all (Fig. 1b). Of the total SNPs identified in O. officinalis complex, a few SNPs were also observed in the two AA genome species viz. O. meridionalis (T1135C, T1153C, T1207C, C1156T, A1747, A2099T, A2855G, and C1810T) and O. longistaminata (A1639G, A1747G, A2099T, and A2855G). AA genome species were found to carry only 27% of the total variations detected at second exon. Cultivars including Pusa44, Feng-Ai-Zhan, Minghui63, PR114, IR64, and N22 were observed to have two nucleotide changes, G1141A and G1607A, in the second exon, however, cultivar Kitake showed no polymorhism and, thus had more relatedness to the Nipponbare when compared to rest of the cultivars.
Figure 1.
Evolutionary relationship across different wild species accessions and cultivars based on the nucleotide sequence of OsPLDα1 exons (a) first exon, (b) second exon, (c) third exon using a neighbor –joining algorithm calculated by boot-strap value of 1000 replicate.
A total of 17 polymorphic sites were reported within the third exon (located on the gene from nucleotide position 3376 to 3618) of the gene. Accessions of O. punctata, O. latifolia, O. minuta and O. officinalis spp. were observed to harbor most of the variability present on third exon, and consequently were found least related to the Nipponbare sequence (Fig. 1c). However, all the cultivars were found monomorphic for the third exon of OsPLDα1.
SNP analysis of UTRs of OsPLDα1 gene
Untranslated regions (UTRs) play an importance role in stabilizing RNA and regulating the transcript expression. Moreover, variations within the 5′ UTR are also known to alter the transcription rate. Similar to the Nipponbare, UTR in all the wild species accessions and cultivars was found separated by an intronic region, however, variations in length and nucleotide sequences of two separeted UTRs (UTR1 and UTR2) were observed in different accessions due to the presence of SNPs and InDels. In comparison to the 142 nucleotide long UTR1 in Nipponbare, length of the UTR1 measured 139 nucleotides in O. officinalis and O. minuta spp. (see Supplementary Fig. S1) due to the presence of 5 InDels of 1 (Insertion of T), 3 (Deletion of TCT), 5 (Insertion of GCCTC), 1 (Deletion of T), and 5 nucleotides (Deletion of CCTCC) at positions 4, 58, 80, 102, and 124, respectively. Additionaly, SNPs A40G, C50A, C101G, C102T, and C141T were also observed in the UTR1 region of these species. Across all the sequenced rice cultivars and AA genomic wild species, an InDel of 3 nucleotides (Inserion of CTC) at position 99 was observed in the UTR1. Moreover, cultivars Feng-Ai-Zhan, Pusa44, IR64, and Minghui 63 also had a deletion of C at nucleotide position 69. In UTR2, a deletion of 10 nucleotides (AATCCAAATC) at nucleotide position 16, was detected in O. officinalis, O. minuta, O. punctata, and O. australiensis spp., when compared to the Nipponbare (see Supplementary Fig. S2). In addition, 3 SNPs, G5A, A21T, A22C and T23A were observed in these species as well. However, all the accessions of AA genomic species and all the cultivars were found monomorphic for UTR2 nucleotide sequence.
SNPs within intronic regions
Across the wild species and cultivars, we detected 101 SNPs and 22 InDels in the intronic region of OsPLDα1 gene, in comparison to the Nipponbare (see Supplementary Table S2). Within the first intron (located on the gene from nucleotide position 143 to 315), 50 nucleotide changes were identified and most of them (45) were detected in the accessions of O. officinalis (IR101152, IR105674, IR106501), O. minuta (IR101100, IR101128), O. punctata (IR105158), O. australiensis (IR105275), and O. latifolia (IR105139) spp. The remaining 5 SNPs (C187T, C257G, A286T, T289C, and G309T) were detected in the O. meridionalis accession (IR101146). In addition to the nucleotide changes, 4 InDels of 9 (+TCGCTGTAC222–230), 11 (+ATTTCTTATCC147–157), 13 (+ATCCTCGCTTACC147–159), and 6 (−AGGTAG176–181) nucleotides, were also observed in the species belonging to the O. officinalis complex. Across the cultivars, only a single InDel of 1 nucleotide long (+G315) was detected in Pusa44, Minghui63, and Feng-Ai-Zhan. No SNP or InDel was detected across the accessions of O. glaberrima, O. barthii, O. nivara, O. glumaepatula, and O. longistaminata. Phylogenetic tree generated from the nucleotide sequence of first intron showed that the accessions of selected species and cultivars fall into two clades (Fig. 2a).
Figure 2.
Evolutionary relationship across different wild species accessions and cultivars based on the nucleotide sequence of OsPLDα1 introns (a) first intron, (b) second intron, (c) third intron, using a neighbor –joining algorithm calculated by boot-strap value of 1000 replicate.
Second intron (located on the gene from nucleotide position 461 to 1000) sequence was only available for AA genome species and cultivars, and could not be obtained for the rest of species even after repeated efforts. In total, 11 SNPs and an Indel of 4 nucleotides were detected on this intron. A467G SNP was found as the most frequent as it was detected across all the AA genome species and rice cultivars. O. longistaminata accessions (IR104301 and IR101200) harbour maximum variability on the second exon, and thus were found most distant to the Nipponbare (Fig. 2b). The third intron (cover 2898 to 3375 nucleotide position on the gene) carried 40 nucleotide changes and 6 InDels. Among AA genome species, O. meridionalis had maximum number of SNPs; however O. officinalis was observed to have maximum variability (SNPs and InDels) across all the wild species. Within the third intron, two large InDels of 18bp (+ATGCATCAGAGATCATTT) and 30bp (CTAATGATCAAGCTAGTAACTTCATCTCCT) were detected from the nucleotide positions 2988 to 3006 and from 3295 to 3324, respectively. Accessions of O. officinalis, O. minuta, and O. punctata were falling in the same cluster, and were found least related to the Nipponbare (Fig. 2c).
OsPLDα1 cDNA and protein variants
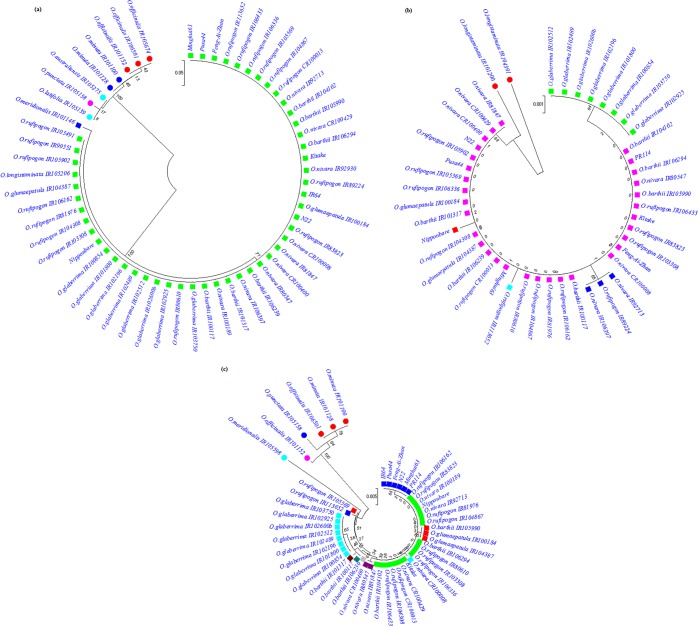
A panel of 63 OsPLDα1 cDNA sequence assemblies from wild Oryza species accessions and cultivars, each containing ~2248 bp were analyzed. Phylogenetic analysis revealed the presence of OsPLDα1 variants in 48 accessions from 11 wild Oryza spp., 8 accessions of O. glaberrima, and 7 Oryza sativa cultivars (Fig. 3). These OsPLDα1 variants were further classified into two major clusters that distinguish AA genomic spp. (O. glaberrima, O. barthii, O. nivara, O. rufipogon, O. longistaminata, O. meridionalis, and O. glumaepatula) from other genomic spp. (O. officinalis, O. australiensis, O. punctata, and O. minuta). Accessions of O. latifolia were falling in between the two clusters. The reference sequence of Nipponbare showed more closeness to japonica cultivar Kitake than to indica cvs. Minghui 63, IR 64, PR 114, Pusa 44, Feng-Ai-Zhan, and N22. The analysis revealed that polymorhic sites were frequently observed in the wild spp. having genome other than AA genome. A total of 20 OsPLDα1 variants were identified based on the nucleotide variations in the cDNA sequences of selected wild species accessions and cultivars.
Figure 3.
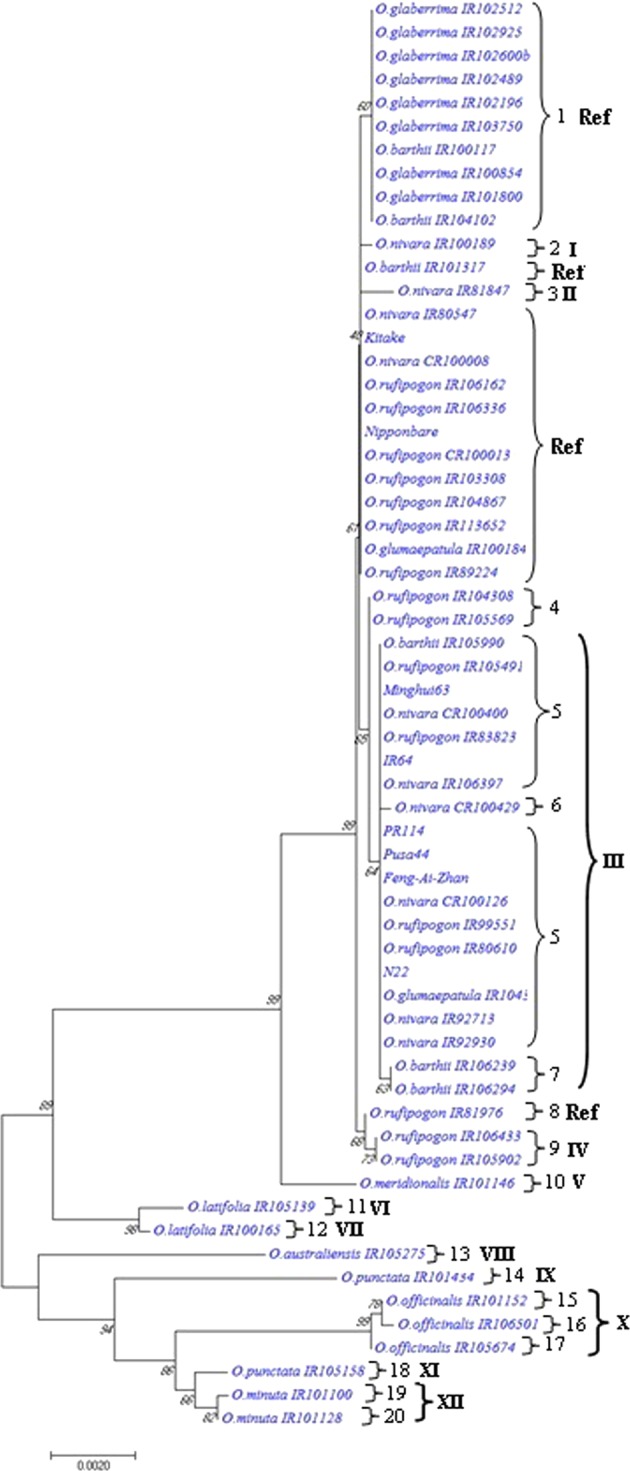
Phylogenetic relationship of OsPLDα1 across Nipponbare, wild species accessions, and cultivars of rice based on the nucleotide sequence data of cDNA. Phylogenetic tree was generated using a neighbor - joining algorithm calculated by boot-strap value of 1000 replicate. The number 1–20 indicates 20 OsPLDα1 variants based on the nucleotide sequences of cDNA while the numbers I to XII indicate protein variants. ‘Ref’ denotes the nucleotide sequences variants which translates into the amino acid sequence same as that of the reference OsPLDα1 protein sequence of Nipponbare.
To determine if the detected nucleotide variations in the coding region of the gene further lead to any alterations in the gene, the cDNA structures of the representative Oryza accessions were aligned with the Nipponbare (see Supplementary Fig. S3). The results revealed that all AA genomic spp. were having the similar number of exons as of the Nipponbare. Exon1 and Exon 3 were found to be of same length in all the studied species whereas Exon2 showed alterations in the species belonging to O. officinalis complex. At the end of first exon, a gap of 193bp (from nucleotide position 109 to 301) was detected in O. australiensis, O. punctata, and O. latifolia accessions while a smaller gap of 77 bp (from nucleotide position 109 to 184) was detected in the O. minuta and O. officinalis accessions. Interestingly, O. officinalis accession had an additional gap within the exon2 from the nucleotide position 1081 to 1137.
Further, from comparative sequence analysis, 107 nucleotide changes (105 SNPs and 2 insertions) were observed across the exons (see Supplementary Table S1). The identified SNPs included 81 transitions and 24 transversions, while G/A transition was the most common (23.80%). Of the identified nucleotide changes, 16 SNPs and 2 insertions were found to be non-synonymous SNPs/indels that really have the potential to become a novel functional alleles (Table 2). The identified 20 OsPLDα1 cDNA variants translated into 12 OsPLDα1 proteins variants (designated as I to XII) and the amino acids substitutions in these variants, in comparison to the reference protein sequence of Nipponbare, have been shown in the Table 3. The proteins predicted from O. officinalis complex clade had more polymorphic amino acids in comparison to the clade containing AA genome species. In addition to the amino acid substitutions across different regions of the the protein variants, 15 amino acids long peptide (KFVEGIEDTVGKGAT) was found missing at 36th position of the variants VI-XII (see Supplementary Fig. S4). Another 18 amino acids long peptide (RIVSFVGGLDLCDGRYDT) at position 336 was found missing only in the variant X (see Supplementary Fig. S5). As a result, X protein variant which comprised of three accessions of O. officinalis (IR101152, IR106501, and IR105674) was having maximum number, twenty two, of amino acid substitutions, and had both the peptides missing.
Table 2.
Translational modification sites observed at OsPLDα1 locus across the wild Oryza species accessions and Oryza cultivars as compared to the Nipponbare reference sequence.
| S.No. | Position# | Alleles* | Codon change | S.No. | Position# | Alleles* | Codon change |
|---|---|---|---|---|---|---|---|
| 1 | 373 | T/C† | — | 54 | 1964 | T/C† | — |
| 2 | 1027 | G/A† | — | 55 | 1967 | C/T† | — |
| 3 | 1043 | G/A† | — | 56 | 1997 | G/A† | — |
| 4 | 1060 | T/C† | — | 57 | 2048 | T/C† | — |
| 5 | 1084 | T/C† | — | 58 | 2077 | C/A†† | Thr395Asn |
| 6 | 1087 | A/T†† | — | 59 | 2081 | A/G† | — |
| 7 | 1087 | A/C†† | — | 60 | 2084 | A/G† | — |
| 8 | 1090 | G/C†† | — | 61 | 2087 | C/A†† | — |
| 9 | 1118 | A/G† | Ile76Val | 62 | 2087 | C/T† | — |
| 10 | 1120 | C/T† | — | 63 | 2089 | A/C†† | Lys399Thr |
| 11 | 1121 | A/G† | Asn77Asp | 64 | 2099 | A/T†† | — |
| 12 | 1123 | C/T† | — | 65 | 2102 | T/A†† | — |
| 13 | 1135 | T/C† | — | 66 | 2108 | G/A† | — |
| 14 | 1141 | G/A† | — | 67 | 2174 | G/A† | — |
| 15 | 1153 | T/C† | — | 68 | 2210 | T/A†† | — |
| 16 | 1156 | C/T† | — | 69 | 2232 | G/A† | — |
| 17 | 1162 | T/C† | — | 70 | 2258 | T/C† | — |
| 18 | 1168 | T/A†† | — | 71 | 2297 | A/C†† | — |
| 19 | 1207 | T/C† | — | 72 | 2335 | C/T† | Pro481Leu |
| 20 | 1210 | G/A† | — | 73 | 2363 | T/C† | — |
| 21 | 1243 | C/T† | — | 74 | 2414 | A/T†† | — |
| 22 | 1258 | T/C† | — | 75 | 2456 | A/T†† | — |
| 23 | 1273 | C/T† | — | 76 | 2498 | C/T† | — |
| 24 | 1275 | G/C†† | Arg128Thr | 77 | 2558 | G/T†† | — |
| 25 | 1282 | C/T† | — | 78 | 2582 | C/T† | — |
| 26 | 1303 | C/T† | — | 79 | 2627 | G/A† | — |
| 27 | 1308 | C/T† | Pro139Leu | 80 | 2636 | A/C†† | — |
| 28 | 1348 | C/T† | — | 81 | 2657 | A/T†† | — |
| 29 | 1369 | T/A†† | Asn159Lys | 82 | 2675 | G/A† | — |
| 30 | 1386 | G/A† | — | 83 | 2715 | C/T† | — |
| 31 | 1387 | C/T† | Arg165His | 84 | 2727 | G/A† | — |
| 32 | 1450 | C/G†† | — | 85 | 2738 | G/A† | — |
| 33 | 1516 | C/T† | — | 86 | 2794 | C/G†† | Ala634Gly |
| 34 | 1570 | A/G† | — | 87 | 2795 | T/C† | — |
| 35 | 1607 | G/A† | Glu239Lys | 88 | 2855 | A/G† | — |
| 36 | 1619 | G/A† | Val729Ile | 89 | 3407 | A/G† | — |
| 37 | 1639 | A/G† | — | 90 | 3425 | G/A† | — |
| 38 | 1723 | T/C† | — | 91 | 3446 | T/C† | — |
| 39 | 1747 | A/G† | — | 92 | 3452 | C/T† | — |
| 40 | 1753 | G/A† | — | 93 | 3461 | C/T† | — |
| 41 | 1760 | T/C† | — | 94 | 3481 | C/T† | — |
| 42 | 1762 | G/A† | — | 95 | 3485 | G/A† | — |
| 43 | 1788 | A/C†† | Glu299Ala | 96 | 3488 | C/T† | — |
| 44 | 1789 | A/T†† | — | 97 | 3494 | A/G† | — |
| 45 | 1808 | G/A† | Asp306Asn | 98 | 3497 | A/G† | — |
| 46 | 1810 | C/T† | — | 99 | 3503 | T/G†† | — |
| 47 | 1825 | A/G† | — | 100 | 3549 | G/A† | — |
| 48 | 1850 | G/A† | Gly320Ser | 101 | 3551 | A/C†† | — |
| 49 | 1858 | T/C† | — | 102 | 3553 | T/C† | Met728Thr |
| 50 | 1870 | G/A† | — | 103 | 3554 | G/A† | — |
| 51 | 1873 | G/A† | — | 104 | 3560 | T/C† | — |
| 52 | 1879 | A/G† | — | 105 | 3563 | T/C† | — |
| 53 | 1962 | A/G† | — |
*In addition to the identified SNPs, 2 insertions including A at nucleotide position 459 and T at nucleotide position 1927 were also detected. Insertion of A459 led to deletion of KFVEGIEDTVGVGKGAT peptide at amino acid position 36. Insertion of T1927 led to amino acid substitutions viz. Leu345Phe, Pro346Ala, Agn347Lys, Gln348Pro, Ser350Leu, Gln351Pro, Gln352Thr, Arg353Lys, Gln372Glu, Tyr373Asp, His374Ser, Ser375Gln, Phe377Arg, and Arg378Trp and a deletion of RIVSFVGGLDLCDGR peptide at amino acid position 354.
#The SNP position was calculated relative to the reference sequence of OsPLDα1 in Nipponbare (RAP locus ID Os01g0172400).
Transitions† and transversions ††observed as nucleotide substitutions.
Table 3.
Amino acid variations among 20 OsPLDα1 variants.
| Proteina | Ref.c | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variantb | 1, 4, 8 | 2 | 3 | 5, 6, 7 | 9 | 10 | 11 | 12 | 13 | 14 | 15, 16, 17 | 18 | 19, 20 |
| 76 | I | I | I | I | I | I | I | I | I | I | V | V | V |
| 77 | N | N | N | N | N | N | N | N | N | N | D | N | N |
| 128 | R | R | R | R | R | R | R | R | T | T | T | T | T |
| 139 | P | P | P | P | P | P | P | P | P | P | P | L | P |
| 159 | N | N | N | N | N | N | N | N | K | N | N | N | N |
| 165 | R | R | H | R | R | R | R | R | R | R | R | R | R |
| 239 | E | E | E | K | E | E | E | E | E | E | E | E | E |
| 243 | V | V | V | V | V | V | V | V | I | V | V | V | V |
| 299 | E | E | E | E | E | E | E | E | A | A | A | A | A |
| 306 | D | D | D | D | N | D | D | D | D | D | D | D | D |
| 320 | G | G | G | G | G | G | G | G | S | G | G | G | G |
| 345 | L | L | L | L | L | L | L | L | L | L | F | L | L |
| 346 | P | P | P | P | P | P | P | P | P | P | A | P | P |
| 347 | N | N | N | N | N | N | N | N | N | N | K | N | N |
| 348 | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | P | Q | Q |
| 350 | S | S | S | S | S | S | S | S | S | S | L | S | S |
| 351 | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | P | Q | Q |
| 352 | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | T | Q | Q |
| 353 | R | R | R | R | R | R | R | R | R | R | K | R | R |
| 372 | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | E | Q | Q |
| 373 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | D | Y | Y |
| 374 | H | H | H | H | H | H | H | H | H | H | S | H | H |
| 375 | S | S | S | S | S | S | S | S | S | S | Q | S | S |
| 377 | F | F | F | F | F | F | F | F | F | F | R | F | F |
| 378 | R | R | R | R | R | R | R | R | R | R | W | R | R |
| 395 | T | T | T | T | T | N | N | N | N | N | N | N | N |
| 399 | K | N | K | K | K | K | K | K | T | K | T | T | T |
| 481 | P | P | P | P | P | P | P | P | L | P | P | P | P |
| 634 | A | A | A | A | A | A | G | A | G | G | G | A | A |
| 728 | M | M | M | M | M | M | M | T | M | T | T | T | T |
Amino acids (indicated with the letter code) and their positions (indicated in numbers) in the OsPLDα1 variants. Amino acids with highlighted background show substitutions in OsPLDα1 protein variants in comparison to the OsPLDα1 protein (AB571657.1).
aGroups of OsPLDα1 variants based on amino acid sequences.
bGroups of OsPLDα1 variants based on nucleotide sequences.
cRef indicate the nucleotide sequences variants which translates into the amino acid sequence same as that of the reference OsPLDα1 protein sequence of Nipponbare.
Domains and motifs in OsPLDα1 variants
Domains/motifs were determined and compared in OsPLDα1 protein and its 12 variants. Three important domains including one copy of C2 domain and two copies of Phospholipase D Active site (PLDc) motif were detected in the reference OsPLDα1 protein. C2 domain was found to be present in all the OsPLDα1 variants, however, length of this domain was found 17 amino acids shorter than in VI-XII protein variants. The alignment of C2 domain from reference protein to the variants showed the absence of KFVEGIEDTVGVGKGAT peptide at 36 amino acid position (see Supplementary Fig. S4). In addition to the missing peptide, two amino acid substitutions have also been reported in the C2 domain which included Isoleucine to valine substitution (at position 76) in variant X, XI and XII and asparagine to aspartate substitution (at position 77) in variant X. Further analysis revealed the presence of two copies of PLDc motif in the PLDalpha1 protein (PLDc-I covering 330 to 368 amino acid position and PLDc-II covering 658 to 685 position of amino acids in the PLDalpha1 protein) in all the variants except variant X in which PLDc-I motif was found missing. Alignment of 330 to 368 amino acid sequences from PLDα1 protein to other variants revealed the absence of RIVSFVGGLDLCDGR peptide at amino acid position 354 and eight amino acid substitutions in variant X (see Supplementary Fig. S5).
Tertiary structure prediction of OsPLDα1 protein in Nipponbare and variant X
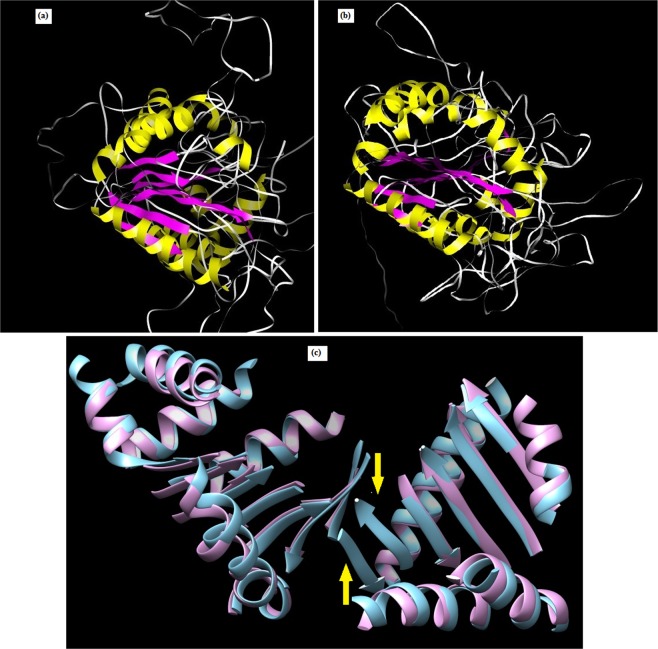
Homology modeling approach was employed to determine the three-dimensional structures of OsPLDα1 protein from Nipponbare and a representative accession (IR101152 accession O. officinalis) of variant X. The MPI Bioinformatics Toolkit (http://toolkit.tuebingen.mpg.de) selected 1v0w as the template for OsPLDα1 protein structure prediction. 1v0w represents the first crystal structure of Phospholipase-D from bacterial source Streptomyces sp. strain PMF35. By using this template, structures were predicted for OsPLDα1 protein in Nipponbare (Fig. 4a) and IR101152 accession of O. officinalis (Fig. 4b). RMSD (Root Mean Square Deviation) values were calculated using chimera and were found to be less than 2 Å (0.745 Å for Nipponbare and 0.825 Å for IR101152) indicating the accuracy of generated structures. Further, the predicted structures were superimposed and results showed the absence of two β-sheets in the IR101152 accession of O. officinalis species (Fig. 4c).
Figure 4.
Three-dimensional structures of OsPLDα1 protein in (a) Nipponbare and (b) IR101152 accession of O. officinalis. (c) Superimposition of OsPLDα1 protein from Nipponbare and IR101152 accession of O. officinalis. Two β-strands (shown with arrows) were found missing in the IR101152 (depicted in pink color) upon superimposition with Nipponbare (depicted in blue color).
Differential expression of OsPLDα1 variants
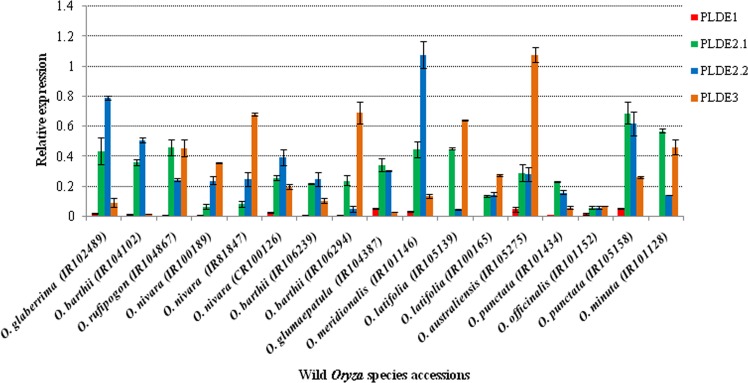
From each of the identified OsPLDα1 variants, at least one accession was selected for expression profiling. Significant differences were observed for the OsPLDα1 transcript levels in immature seeds from wild Oryza species accessions (Fig. 5). Expression differences for the transcripts acquired with primers designed from 5′ and 3′ ends of the second exon, signified the presence of truncated splice forms in most of the accessions. In addition, expression study also revealed significant expression variations between the genotypes for the same transcript variant and within the same genotype for different transcript variants. Further, for the confirmation of these results, amplification of full length transcript (Os01t0172400-1) and two alternate splice forms viz. Os01t0172400-4 and Os01t0172400-5 was performed in Oryza species accessions. Single sharp bands of expected amplicon size were obtained for all the three transcript forms (see Supplementary Figure S6), which validates that the full length as well as other transcripts with shorter lengths were present in the accessions. Moreover, it varified that the truncations obtained in the accessions are real and not due to failure of cDNA synthesis at the ends of mRNA. Of all the OsPLDα1 variants, lowest transcript expression (for all the four qRT-primers) was observed in the O. officinalis accession (IR101152) followed by O. punctata (IR101434). Further, the hierarchical clustering dendrogram represents the OsPLDα1 transcripts differences between as well as within the wild species accessions (Fig. 6).
Figure 5.
OsPLDα1 transcript levels in immature seeds from wild Oryza species accessions. Mean values for OsPLDα1 transcripts and standard deviation (S.D.) measured relative to Actin expression. Relative transcript levels of OsPLDα1, in accessions of wild Oryza species, for four qRT-PCR primers namely PLDE1 (designed from first exon of the gene), PLDE2.1 (designed from 5′end of second exon), PLDE2.2 (designed from 3′end of second exon), and PLDE3 (designed from third exon of the gene) are shown. Among all the wild species accessions, IR101152 accession of O. officinalis was found to have lowest transcript levels using all the four qRT-PCR primers.
Figure 6.
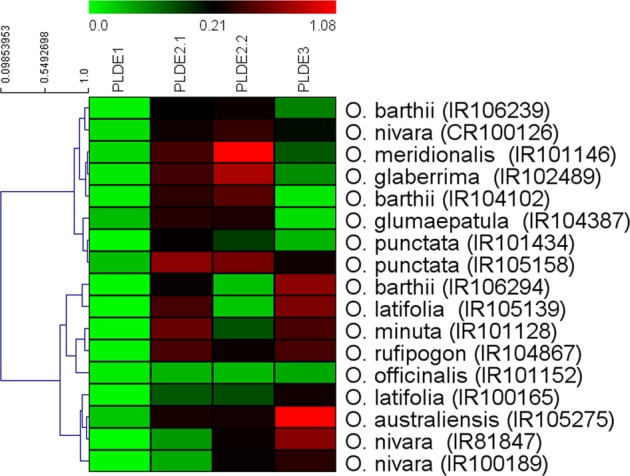
Heatmap showing differential expression of OsPLDα1 transcripts between as well as within the accessions of wild Oryza species. PLDE1, PLDE2.1, PLDE2.2 and PLDE3 denotes the qRT-primers designed from exons of OsPLDα1 gene. Wild species accessions (horizontal) were hierarchially clustered (Pearson sorrelation, average linkage). Color patterns from green to red indicate low to high transcript levels, thus IRGC101152 have the lowest expression for all the four exon specific qRT primers.
Discussion
The major bottleneck in improving the rice bran quality is narrow genetic base of germplasm on which breeders are working. Hence, the utilization of wild species germplasm to identify the ‘novel alleles’ through sequence based allele mining, and their further transfer to the elite lines has emerged as a good breeding strategy36. The progenitor Oryza species, in comparison to cultivated rice, are known to carry a number of functionally characterized genes with important coding variations37. It leads to the inference that useful coding variations for OsPLDα1 could be mined from primary and secondary Oryza gene pools. The present study depicts an in-depth survey of the genetic variability at OsPLDα1 in a large panel of genetically and geographically diverse wild rice germplasm. Despite repeated efforts to sequence the second intron in the accessions belonging to genomes other than AA genome, we could not obtain high quality sequence and only multiplets were obtained in that region. The reason for this could be significant sequence differences between the reference japonica variety Nipponbare and the species with genomes other than AA genome species. This resulted in fragmented assembly of OsPLDα1 gene sequence in wild species having CC, EE, BBCC, and CCDD genomes and hence polymorphic and phylogenetic analysis were conducted for individual exons, introns and UTRs to include all the wild species accessions in the study.
Phylogenetic relationships of OsPLDα1 gene among diverese wild species germplasm of rice
At exon 1, only two distinguished clusters were observed comprising all the AA genome species in one cluster and other diploid and tetraploid genome species in other cluster (Fig. 1a). The probable explanation for the lower variability is the smaller size of the first exon as compared to other two exons and might have fewer roles in controlling the trait. Among the AA genome species, O. glaberrima accessions were found closely related to O. barthii accessions while O. rufipogon accessions clustered close to O. sativa and O. nivara. It has been already established by earlier studies that African rice O. glaberrima was domesticated from the wild progenitor O. barthii approximately 3,000 years ago38 explaining the relative closeness between the clads. Also, the close genetic relationship between African rice O. glaberrima and O. barthii has been inferred way back using isozymes as markers39 and later by using SSRs and SNPs markers40,41. A comparison at major domesticated genes, for instance, Gn1a (Grain productivity), qSH1 (Shattering), Sd1 (Semi-dwarfing), Gw2 (Grain width), GIF1 (Grain incomplete filling), badh2 (Flavor or fragrance), Phr1 (Grain discoloration), OsLG1 (Closed panicle), Sh4 (Shattering), Moc1 (Tillering), Rc (Red pericarp), Sdr4 (Seed dormancy), Ep2 (Erect panicle), Ipa1 (Ideal plant architecture), Dep1 (Panicle architecture), and Sh4 (Shattering) by Wang et al.42 revealed reduced nucleotide diversity in O. glaberrima than O. barthii which correlates well with the hypothesis of O. barthii being the progenitor harbors greater diversity and during the process of domestication/selection it got reduced in O. glaberrima. At two other exons of OsPLDα1, O. rufipogon and O. nivara accessions showed admixture among them as well as with O. sativa (indica as well as japonica) with not much divergence (Fig. 1b,c). This observation could be ascribed to the fact that these two wild species are more closely related and collectively regarded as the progenitors of O. sativa43,44. Extensive allele sharing between O. rufipogon and O. nivara has also been documented by Banaticla‐Hilario et al.45. A taxonomic debate is still continued over whether O. rufipogon (the perennial species), O. nivara (the annual species), can be considered as two species or ecotypes of the same species46,47. Moreover, both O. rufipogon and O. nivara share a common geographical distribution in South and Southeast Asia therefore the probability of gene flow is higher between them. These species are cross compatible and exhibit little genetic differentiation and is supported by molecular phylogenetic analysis and population studies48–54.
At exon 2 and 3, the O. longistaminata accessions (distributed in Africa) consistently showed significant differentiation from other AA genome Oryza species. This divergence of O. longistaminata could be attributed to the unique morphological features such as self-incompatibility, distinctive characteristics of ligules and the presence of rhizomes. These features are not present in any other Oryza species which support to the data we obtained55–57. Further, haplotype diversity and feature-specific variation has been reported in O. longistaminata which the authors attributed to out-crossing nature of this species58. The other explanation could be the long distance dispersal of the seeds by animals, birds or any other way followed by ecological differentiation making this species different. The comparative study of genetic relationship using SSR and RAPD markers also revealed clear differentiation of O. longistaminata from other AA genome species59. Similar observations were also found by other scientists60–62. Interestingly, O. meridinolis did not group with any of the AA genome cluster. These results are consistent with the findings of evolutionary divergence study at PSTOL1 locus in wild, domesticated and weedy rice63 but contradictory to the findings based on plastome analysis which shows O. longistaminata to be most diverged from AA-species64.
The present study revealed that the species belonging to the O. officinalis complex (O. officinalis, O. australiensis, O. punctata, O. minuta, and O. latifolia) harbor maximum variability at the OsPLDα1 locus in comparison to AA genome spp. and Oryza cultivars. This complex is the largest one with 10 species and five genome types (BB, CC, EE, BBCC, CCDD) that are distributed widely in Asia, Africa, Australia and Latin America65, and hence might be capturing wider variability due to ecological speciation and polyploidization events. It is noteworthy that the O. officinalis (CC genome) accessions formed a distinct cluster at second and third exons then the counter diploid species O. punctata (BB genome) and two of the tetraploid species O. minuta (BBCC) and O. latifolia (CCDD) (Fig. 1b,c). These results signify that the O. officinalis species carry maximum variability at the OsPLDα1 locus in comparison to rest of the Oryza species. A study on polyploidy evolution in O. officinalis complex by Wang et al. (2009) states that the CC genome diverges with BB genome at ca.4.8 Mya followed by a series of speciation of C genome diploids and later successive events of polyploidization leads to the formation of tetraploid species i.e CCDD at 0.9 Mya and BBCC between ca. 0.3–0.6 Mya66. Further, O. latifolia (CCDD genome) clustered closer to O. australiensis (EE), this can be explained by the fact that EE genome is considered to be progenitor of DD genome67–69. Allele mining at Pi54 locus by Kumari et al. (2013) also observed that O. officinalis, O. punctata and O. latifolia forms a divergent cluster from other AA genome species70. A comparison of the sequences of Xa3/Xa26 orthologous family also revealed very low similarity between cultivated rice and wild Oryza species comprising of O. officinalis and O. minuta71.
In addition to the variations in coding region of the OsPLDα1 gene, the nucleotide changes including large InDels were also detected in the non-coding regions including first and third introns. Contrary to the other genome species, all the AA genome species were found in the same clade as of the Nipponbare, and O. meridionalis was found most distantly related of all the AA genome species (Fig. 2a,c). The Oryza officinalis complex, in a similar fashion of carrying maximum variability in the coding region, was found to carry maximum variability in the non-coding region as well. These variations included SNPs as well as large InDels (see Supplementary Table S2). The roles of intronic mutations have earlier been found evident in the expressions of tubulin, polyubiquitin and waxy (Wx) genes of rice72–74. The variations detected, in the current study, at the non-coding region of OsPLDα1 could play significant role in the transcript synthesis and accumulation which might lead to change in trait expression.
Protein domain analysis in OsPLDα1 variants
In this study, domains and motifs of the Nipponbare OsPLDα1 protein were aligned with the protein sequence of 12 OsPLDα1 variants (see Supplementary Fig. S7). Rice OsPLDα1 contains a single putative C2 domain that has been predicted to be involved in signal transduction and membrane trafficking, and is important in Ca2+-regulated binding to phospholipids75,76. In plants, Ca2+ is an important regulator of PLD activity, C2 domain has been known to play an important role in this regulation77,78. In the present study, seven of the identified protein variants viz. VI, VII, VIII, IX, X, XI, and XII were found to have a deletion of 17 amino acid long peptide that also included one of the four conserved amino acids (Glutamic acid at position 42) that are instrumental in Ca2+ binding79. The variants VIII, IX, X, XI and XII were having a common amino acid substitution at the position 111. In addition, variant X was detected with two unique amino acid substitutions in the C2 domain, at positions 59 and 60. The missing peptide, absence of conserved amino acid, and amino acid substitutions, may further lead to change in the protein function as this domain is important in translocating proteins to memberanes80,81. C2 domain deletion mutants in PI3K lead to loss of important inter-residue contacts and thereby lead to reduction in binding energy82. Downstream of the C2 domain, B-domain was found conserved among all the 12 protein varaints except for a single amino acid substitution in the varaint III. This region is similar to the B-domain of insect and cereal α-amylases that frequently regulate enzyme activity83–85.
Each rice PLD consists of two fully conserved HxKxxxxD motifs, which form the active catalytic site for phosphoester bond hydrolysis86. Any mutation in the HKD motifs abolishes the enzymatic activity of the PLD enzyme. Our inspection of predicted protein sequences revealed the presence of both of the HKD motifs in all the protein variants (see Supplementary Fig. S7) which is supported by the fact that most eukaryotic PLDs require two functional HKD sites to remain catalytically active87. Also, the three amino acid residues involved in PIP2 activation were found conserved in all the variants88. However, within the first PLD catalytic (PLDc-I) motif of the variant X, 15 amino acid long peptide was missing (see Supplementary Fig. S5) which might also had an altered effect on the enzyme activity.
OsPLDα1 gene expression profiling in Wild Oryza species and detection of a new OsPLDα1 transcript
To carry out expression analysis at OsPLDα1 locus, the plant development stage for RNA extraction was chosen on the basis of expression profiling of 17 PLD isoforms using the expression data from RiceXProv3.0 database (see Supplementary Fig. S8). The expression analysis using various plant tissues at different developmental stages indicated that the activity of OsPLDα1 enzyme was very high during early stages of grain development. Moreover, Suzuki (2011) reported the hike of PLD content in the seeds till 3 wk after flowering, becoming stagnant afterwards. Also, no PLD protein band was observed one week after flowering in the seeds of PLD-null rice mutant (03-s108), having <0.01% PLD activity in rice bran when compared to Nipponbare21. These results correlate the functional expression of OsPLDα1 in rice bran and immature seeds. Consequently, for the experiments conducted in the current study, RNA was isolated from immature seeds (one week after flowering). The quantitative gene expression studies have been successfully utilized to study the alterations in the transcript abundance during cell differentiation or development89; variation in expression for cells vulnerable to a chemical substance, for instance, drug, toxin, hormone or cytokine)90; and as a diagnostic tool91.
For expression analysis, in the present study, four exon-specific qRT-PCR primer pairs were designed from the exonic region of OsPLDα1 (Table S3). The designed primers aimed to assess the wild genotypes for variability in the gene expression as well as to unveil if the gene is alternatively spliced (see Supplementary Fig. S9). Alternate splicing has been known to control the gene expression and functional diversification of proteins in higher eukaryotes. Alternative splicing of the Ca2+-independent phospholipase A2 (iPLA2) pre-mRNA in humans can result in the production of regulatory subunits that can modify iPLA2 in vivo activity92. Alternative splicing is ubiquitous in rice with 36,650 known splicing events effecting 8772 genes including OsWRKY62 and OsWRKY7693,94. Further, differential expression levels of various genes involved in spikelet development in different rice species have been shown to manifest different phenotypes95. Our expression profiling results revealed significant differences in the OsPLDα1 transcript abundance, between the wild Oryza species, being lowest in O. officinalis spp. followed by O. punctata and O. latifolia (Fig. 5). In the O. officinalis accessions, two insertions viz. A at nucleotide position 459 and T at nucleotide position 1927 led to maximum alterations in the OsPLDα1 protein that included 14 amino acid substitutions and absence of two peptides (see Supplementary Fig. S4 and S5). The alterations observed in the protein could be the reason for lowest enzymatic activity in O. officinalis spp. The novel allele leading to low OsPLDα1 expression in O. officinalis accessions has been named as OsPLDα1-1a and is available in NCBI database (http://www.ncbi.nlm.nih.gov) with GenBank accession numbers MF966931, MF966932, and MF966933. In addition, significant differences were observed in the transcript abundance within the accessions for the primers designed from 5′ and 3′ ends of second exon, demonstrating the presence of 5′ and 3′ truncated mRNA (Fig. 5). Interestingly, IR102489 accession of O. glaberrima and accessions of wild Oryza species including O. barthii (IR104102 and IR106239), O. nivara (CR100126), O. glaumaepatula (IR104387), O. meridionalis (IR101146), and O. punctata (IR101434 and IR105158) had low abundance of transcripts having third exon when compared to the transcript levels of the first and second exons (Fig. 5). However, the five earlier reported OsPLDα1 transcript forms confirm the presence of third exon in all the splice forms (see Supplementary Fig. S9). Therefore, the current study revealed the presence of a new OsPLDα1 transcript variant, named as Os01t0172400-06, having truncations before the third exon (Fig. 7).
Figure 7.
Graphical representation of newly identified OsPLDα1 transcript variant, Os01t0172400-06. A new transcript form having only two exons was detected in the wild Oryza species accessions viz. O. barthii (IR104102 and IR106239), O. nivara (CR100126), O. glaumaepatula (IR104387), O. meridionalis (IR101146), and O. punctata (IR101434 and IR105158); and an accession of O. glaberrima (IR102489). Expression profiling in these accessions, using exon specific qRT-PCR primers, showed the low abundance of transcripts having third exon.
Conclusion
The species belonging to O. officinalis complex possess maximum variability at the OsPLDα1 locus. Of the O. officinalis complex species, OsPLDα1 allele of O. officinalis accessions has been reported to carry maximum number of non-synonymous SNPs/InDels which further led to alterations in the protein domains, that are responsible for regulating the enzyme activity. The lowest levels of OsPLDα1 transcript abundance in the O. officinalis accessions suggests that the reported polymorphism in the nucleotide and amino acid sequences, varied gene structure, and altered domains play an important role in regulating the enzyme activity in rice bran. Also, a new OsPLDα1 transcript variant named as Os01t0172400-06, having third exon missing in it, was discovered in the present study. We are in the process of transferring the superior OsPLDα1 allele, identified in O. officinalis accession (IRGC101152) i.e., OsPLDα1-1a (GenBank accession no. MF966931), into the elite rice cultivars.
Methods
Plant materials
A set of 56 accessions including 48 accessions representing 11 wild species of Oryza viz. O. barthii (n = 6), O. nivara (n = 10), O. rufipogon (n = 16), O. longistaminata (n = 3), O. meridionalis (n = 1), O. glumaepatula (n = 2), O. officinalis (n = 3), O. australiensis (n = 1), O. punctata (n = 2), O. minuta (n = 2), and O. latifolia (n = 2)]; and 8 accessions of African cultivated rice O. glaberrima were undertaken for the current study (Table 1). These germplasm accessions have been actively maintained at Punjab Agricultural University (PAU), Ludhiana, and were originally procured from the International Rice Research Institute (IRRI), Philippines and from National Rice Research Institute (NRRI), Cuttack. The sequence analysis in Oryza cultivars namely Punjab Rice 114 (PR 114), Nagina 22 (N22), IR64, Pusa 44, Minghui 63, Feng-Ai-Zhan, and Kitake, revealed the presence of OsPLDα1 allele. Therefore, these cultivars were used as positive checks to carry out the comparative OsPLDα1 sequence analysis with wild Oryza species accessions. Standard agronomic practices were followed to raise the crop. These practices included sowing of seeds in seedbeds and transplanting one-month-old seedlings in the field with a row-to-row distance of 70 cm and plant-to-plant distance of 45 cm; weed control using a Paddy Weeder, 15 days after transplanting and again after a fortnight; application of organic manures (15 tonnes of farmyard manure per hectare prior to transplanting of rice), bio-fertilizer (treat the nursery seedlings for 45 minutes in the solution made by dissolving 0.5 kg of Azorhizobium biofertilizer in requisite amount of water so as to soak seedlings needed to transplant one hectare, and then transplanting was done immediately) along with chemical fertilizers comprising 222 kg/ha Neem coated urea (provide 104 kg/ha Nitrogen), 67 kg/ha Diammonium Phosphate (provide 30 kg/ha phosphorus), and 49 kg/ha Muriate of potash (provide 30 kg/ha potassium) for higher crop yield and maintenance of soil health. 1/3 nitrogen was applied within 2 weeks of transplanting while the whole phosphorus and potassium was applied before the last puddling. Broadcasting of the remaining nitrogen was done in two equal splits, one three weeks after transplanting and the other three weeks afterwards; water was kept standing in the crop continuously for two weeks after transplanting, and thereafter irrigation was done two days after the ponded water has infiltrated into the soil; to facilitate harvesting, irrigation was stopped about a fortnight before maturity; panicles of the plants were covered with the mash bags to avoid shattering of the seeds; harvesting and threshing of different genotypes was done separately to avoid seed admixture.
DNA extraction, primer designing and PCR amplification
The current study followed a modified CTAB method to isolate genomic DNA from the selected genotypes96. 0.8% agarose gel was used to access the quantity and quality of DNA from each sample. For further use, DNA samples were diluted with 1X TE buffer and stored at −20 °C. To PCR amplify the coding and non-coding regions of the OsPLDα1 variants, Oryza sativa japonica cv. Nipponbare sequence (GenBank accession no. AB571657.1) was utilized to design five overlapping primer pairs (Table 4). Supplementary Fig. S10 shows the OsPLDα1 gene structure (RAPdb ID Os01g0172400) and positions of the designed primer pairs along the length of gene. First and last primers were designed from the upstream and downstream flanking regions of the gene to sequence the whole gene. PCR was performed in a 30 μl reaction mix containing 0.3 μl Phusion® high fidelity DNA polymerase, 3 μl of genomic DNA (20ng/μl), 6 μl of 5X HF buffer, 6 μl of dNTPs (1 mM), 3 μl of primer (5 μM), and 11.7 μl Nuclease Free Water. The thermal cycling conditions were as follows: an initial denaturation at 94 °C for 5 min; 35 cycles of 1 min denaturation at 94 °C followed by 1 min annealing at 55 °C and 1 min extension at 72 °C; and a final 5 min extension at 72 °C.
Table 4.
Overlapping PCR-primer pairs used for amplification of different segments of the OsPLDα1.
| Primer ID | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Amplicon sizea |
|---|---|---|---|
| PLD-1 | TTTAACCTCGCCTCCTCC | TCTCCAATTCTTGTCTACTACC | 783 bases (−154–629) |
| PLD-2 | GCCCGAATTTGATCTGCT | TTTGGAATGAAGTTGTCTGG | 946 bases (545–1472) |
| PLD-3 | GGAGAGGAGATTGACAGATGG | AGGAGAAGGTGGAATAATAGTG | 995 bases (1259–2233) |
| PLD-4 | CATGATATTCACTCACGGCT | TGTAACTCATCTGACATGCT | 862 bases (2114–2957) |
| PLD-5 | CTACCTCACTTTCTTCTGCT | ATGTCCCAGTACTTCTCC | 885 bases (2749–+16) |
aNumbers in parenthesis shows the position of bases covered by the primer pairs on OsPLDα1 locus.
Sequencing of OsPLDα1 gene in selected accessions and cultivars
Ethidium bromide stained 1.0% agarose gel electrophoresis was performed to analyze PCR products. 1 kb plus ladder (Thermo Scientific Generular) was used to estimate the DNA fragment size. We obtained single sharp bands of expected amplicon size for all the five overlapping primers (see Supplementary Fig. S11). The Wizard® SV PCR Clean-Up System (Promega, USA) as per the manufacturer’s protocol was followed to excise and purify the DNA fragments. The details of targeted DNA nucleotide sequence were created using separate sequencing reactions for forward and reverse primers. The ABI Big-dye Terminator v3.1 chemistry performed the sequencing reaction and ABI Sequencer 3730XL sequenced the DNA fragments, at the School of Agricultural Biotechnology, Punjab Agricultural University, Ludhiana. Experiment was carried out in two replications to confirm the presence of single nucleotide polymorphism (SNPs).
Analysis of the generated nucleotide sequences and protein prediction
For comparative sequence analysis, DNA Baser v4.23.0 (http://www.dnabaser.com/) software joined the contigs produced by overlapping primers, and generated the consensus sequence of OsPLDα1 gene. This software also helps in automatic identification and clipping of poor quality regions at both ends of the sequences. ClustalX 2.1.1 software was undertaken to generate the multiple sequence alignment97. OsPLDα1 sequence from ‘Nipponbare’ rice variety, which contains normal levels of PLD activity i.e., 133.2 units/mg21, was used as reference (wild type) in this study. The identified SNPs and InDels were then manually curated by comparing chromatogram files to the ClustalX alignment files.
HMM-based FGENESH online program (http://www.softberry.com/berry.phtml?topic=fgenesh) was used to predict the gene structure and amino acid sequences in different genotypes which were further compared with the Nipponbare protein to detect amino acid substitutions and InDels. Pfam (http://pfam.xfam.org/) online program predicted the domains and motifs in the protein variants. Bioinformatics toolkit (http://toolkit.tuebingen.mpg.de/) was used to predict the tertiary structures of protein. The MODELLER Homology modeling approach was followed98 to determine the structure of proteins based on the known strucure of template protein. Ramachandran plots were developed using Procheck through PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum) to check the quality of protein models. UCSF Chimera helped to visualize and compare the modeled protein structures99. All the developed tertiary structures were superimposed to detect the structural variations. Uniprot (http://www.uniprot.org/uniprot/P84147) online program determined the catalytic sites in the protein.
Phylogenetic analysis
The MEGA7 software100 was used to generate the phylogenetic tree using multiple sequence alignment file. The evolutionary distances were computed using the Maximum Composite Likelihood method with 1,000 bootstrap and are in the units of the number of base substitutions per site.
RNA isolation, cDNA synthesis, and expression analysis using qRT-PCR
To collect the RNA sample at the same stage (one week after flowering, at milking stage of grain development) from different wild species and cultivars, flowering data was collected on the daily basis. For each genotype, tissue for RNA isolation was collected in a way that each experimental replicate represents RNA from three biological replic ates101.
The TRIzol® reagent (Thermo Fisher Scientific) was used for RNA isolation according to the manufacturer’s protocol. The expression analysis part of the study wa s done at the School of Biology and Ecology, University of Maine, Orono, USA. NanoDrop® ND-1000 estimated the RNA quantity for the different samples. We employed an iScript cDNA kit (Bio-Rad laboratories, CA, USA) which produces first strand cDNAs by reverse transcribing RNA. Sequences of OsPLDα1 loci and its transcript forms (Locus ID Os01g0172400), for qRT primers designing, were retrieved from RAP data base (http://rapdb.dna.affrc.go.jp/viewer/gbrowse/).
Using the Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), four exon-specific qRT-primer pairs (PLDE1, PLDE2.1, PLDE2.2, and PLDE3) (see Supplementary Table S3) were designed from the exonic regions of OsPLDα1. qRT-primers were generated to assess the wild genotypes for variations in abundance of OsPLDα1 transcript variants (see Supplementary Fig. S9). Each primer was dissolved in 1X TE buffer (stock solution) to have a master stock of 100 µM. Real-time PCR was performed in MyiQ™ thermal cycler (Bio-Rad Laboratories, CA, USA) using the iQ™ SYBR® Green Supermix (Bio-Rad) according to the manufacturers protocol. The cycling conditions were as follows: 95 °C for 30s, 40 cycles of 95 °C for 5s and 60 °C for 30s. Each sample was amplified in triplicate to confirm the results. 2−∆CT method was used to calculate the relative expression levels102, and the actin (Locus ID Os10g0510000) gene was used as an internal control to normalize the data. For validation of expression results, primers were designed for the full length amplification of three alternate splice forms viz., Os01t0172400-1, Os01t0172400-4 and Os01t0172400-5 (see Supplementary Table S4).
Supplementary information
Acknowledgements
The authors are thankful to the International Rice Research Institute (IRRI), Philippines, Manila and Central Rice Research Institute (CRRI), Cuttack, India for providing wild species germplasm of rice. This work was supported by the Monsanto’s Beachell Borlaug International Scholarship Program, United States of America.
Author contributions
K.S. and B.G.D.R. designed the research and supervised experiments; A.K., K.K. and Ai. K. performed experiments; A.K. and K.N. analyzed data and wrote manuscript; all authors reviewed and approved the final manuscript.
Data availability
The DNA sequences of wild species accessions and cultivars generated and analyzed during the current study are available in the GenBank repository with accession numbers: O. glaberrima [IRGC100854 (MF774061), IRGC101800 (MF919319), IRGC102196 (MF919320), IRGC102489 (MF919321), IRGC102512 (MF919322), IRGC102600b (MF919323), IRGC102925 (MF919324), IRGC103750 (MF919325)]; O. bartii [IRGC100117 (MF919326), IRGC101317 (MF919327), IRGC104102 (MF919328), IRGC105990 (MF919329), IRGC106239 (MF919330), IRGC106294 (MF919331)]; O. nivara [CR100008 (MF919334), CR100400 (MF919335), CR100429 (MF91336), IRGC80547 (MF919332), IRGC81847 (MG725633), IRGC92713 (MF919337), IRGC92930 (MG725634), IRGC100189 (MF919338), IRGC106397 (MF919333)]; O. rufipogon [CR100013 (MG725637), IRGC80610 (MG725635), IRGC81976 (MG725638), IRGC83823 (MG725640), IRGC89224 (MG725641), IRGC99551 (MG725642), IRGC103308 (MF919339), IRGC104308 (MG725643) IRGC104867 (MF966922), IRGC105491 (MG7256360), IRGC105569 (MF966923), IRGC105902 (MF966924), IRGC106162 (MF966925), IRGC106336 (MF966926), IRGC106433 (MF966927), IRGC113652 (MF966928)]; O. longistaminata [IRGC101200 (MG725645), IRGC104301 (MG725646), IRGC105206 (MG725647)]; O. meridionalis [IRGC101146 (MG725648)]; O. glumaepatula [IRGC100184 (MF966930), IRGC104387 (MF966929)]; O. officinalis [IRGC101152 (MF966931), IRGC105674 (MF966932), IRGC106501 (MF966933)]; O. australiensis [IRGC105275 (MG725650)]; O. punctata [IRGC105158 (MG725651)]; O. minuta [IRGC101100 (MG725652), IRGC101128 (MG725653)]; O. latifolia [IRGC105139 (MG725654)]; Feng-Ai-Zhan (MF975521); Kitake (MF975522); Minghui63 (MF975523); Nagina22 (MF975524); PR114 (MF975525); Pusa44 (MF975526).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62649-w.
References
- 1.Hargrove, K. L. Processing and utilization of rice bran in the United States. Rice Science and Technology. Marshall, W. E. and Wadsworth, J. I. (Eds.). New York: Marcel Dekker Inc. (1994).
- 2.Hu W, Wells JH, Shin TS, Godber JS. Comparison of isopropanol and hexane for extraction of vitamin E and oryzanols from stabilized rice bran. J. Am. Oil Chem. Soci. 1996;73:1653–1656. doi: 10.1007/BF02517967. [DOI] [Google Scholar]
- 3.Saunders RM. The properties of rice bran as a foodstuff. Cereal Foods World. 1990;35:632–636. [Google Scholar]
- 4.Yoshino G. Effects of γ-oryzanol on hyperlipidemic subjects. Current Therapy Res. 1989;45:543–552. [Google Scholar]
- 5.Krishna AGG, Khatoon S, Babylatha R. Frying performance of processed rice bran oils. J. Food Lipids. 2005;12:1–11. doi: 10.1111/j.1745-4522.2005.00001.x. [DOI] [Google Scholar]
- 6.Nicolosi RJ, Ausman LM, Hegsted DM. Rice bran oil lowers serum total and low density lipoprotein cholesterol and apo B levels in nonhuman primates. Atherosclerosis. 1991;88:133–142. doi: 10.1016/0021-9150(91)90075-E. [DOI] [PubMed] [Google Scholar]
- 7.Salunkhe, D. K., Chavan, J. K., Adsule, R. N. & Kadam, S. S. Rice in World oilseeds: Chemistry, technology, and utilization. New York: Van Nostrand Reinhold. pp. 424–428 (1992).
- 8.Takano K, Kamoi I, Obara T. Changes in lipid components and lipolytic enzyme activities of rice bran during storage (Studies on the mechanisms of lipids-hydrolysing in rice bran part I) J. Jpn. Soc. Food Sci. Technol. 1986;33:310–315. doi: 10.3136/nskkk1962.33.5_310. [DOI] [Google Scholar]
- 9.Barnes P. and Galliard, T. Rancidity in cereal products. Lipid Technol. 1991;3:23–28. [Google Scholar]
- 10.List GR, Mounts TL, Lanser AC. Factors promoting the formation of nonhydratable soybean phosphatides. J. Am. Oil Chem. Soc. 1992;69:403–410. doi: 10.1007/BF02540945. [DOI] [Google Scholar]
- 11.Nakayama Y, Saio K, Kito M. Decomposition of phospholipids in soybean during storage. Cereal Chem. 1981;58:260–264. [Google Scholar]
- 12.Takano K, Kamoi I, Obara T. Purification and properties of rice bran phospholipase D. J. Jpn. Soc. Food Sci. Technol. 1987;34:8–13. doi: 10.3136/nskkk1962.34.8. [DOI] [Google Scholar]
- 13.Takano K, Kamoi I, Obara T. Properties and degradation of rice bran spherosome. J. Jpn. Soc. Food Sci. Technol. 1989;36:468–474. doi: 10.3136/nskkk1962.36.6_468. [DOI] [Google Scholar]
- 14.Suzuki Y, et al. Volatile components in stored rice [Oryza sativa (L.)] of varieties with and without lipoxygenase-3 in seeds. J. Agric. Food Chem. 1999;47:1119–1124. doi: 10.1021/jf980967a. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Robards K, Helliwell S, Blanchard C. Ageing of stored rice: Changes in chemical and physical attributes. J. Cereal Sci. 2002;35:65–78. doi: 10.1006/jcrs.2001.0418. [DOI] [Google Scholar]
- 16.Gang L, Fang L, Hong-Wei X. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLDβ1 in seed germination. Cell Res. 2007;17:881–894. doi: 10.1038/cr.2007.77. [DOI] [PubMed] [Google Scholar]
- 17.Qin C, Wang X. The Arabidopsis phospholipase D family: characterization of a Ca2+-independent and phosphatidylcholine-selective PLDζ1 with distinct regulatory domains. Plant Physiol. 2002;128:1057–1068. doi: 10.1104/pp.010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki J, Morioka S, Komari T, Kumashiro T. Purification and characterization of phospholipase D (PLD) from rice (Oryza sativa L.) and cloning of cDNA for PLD from rice and maize (Zea mays L.) Plant Cell Physiol. 1995;36:903–914. doi: 10.1093/oxfordjournals.pcp.a078837. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Takeuchi Y, Shirasawa K. Identification of a seed phospholipase D null allele in rice (Oryza sativa L.) and development of SNP markers for phospholipase D deficiency. Crop Sci. 2011;51:2113–2118. doi: 10.2135/cropsci2010.12.0716. [DOI] [Google Scholar]
- 20.Sato Y, et al. RiceXPro: a platform for monitoring gene expression in japonica rice grown under natural field conditions. Nucleic Acids Res. 2011;39:1141–1148. doi: 10.1093/nar/gkq1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki Y. Isolation and characterization of a rice (Oryza sativa L.) mutant deficient in seed phospholipase D, an enzyme involved in the degradation of oil-body membranes. Crop Sci. 2011;51:567–573. doi: 10.2135/cropsci2010.08.0460. [DOI] [Google Scholar]
- 22.Ramezanzadeh FM, Rao RM, Windhauser M, Prinyawiwatkul W, Marshall WE. Prevention of hydrolytic rancidity in rice bran during storage. J. Agric. Food Chem. 1999;47:2997–3000. doi: 10.1021/jf981168v. [DOI] [PubMed] [Google Scholar]
- 23.Malekian, F. et al. In: Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage: Bull 870. Louisiana Agric Exp Stn: LSU Agric Cent, Baton Rouge, LA. pp 1–56 (2000).
- 24.McCaskill DR, Zhang F. Use of rice bran oil in foods. Food Technol. 1999;53:50–53. [Google Scholar]
- 25.Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Khush GS, Ling KC. Inheritance of resistance to grassy stunt virus and its vector in rice. J. Hered. 1974;65:135–136. doi: 10.1093/oxfordjournals.jhered.a108483. [DOI] [Google Scholar]
- 27.Amante-Bordeos A, et al. Transfer of bacterial blight and blast resistance from the tetraploid wild rice Oryza minuta to cultivated rice (Oryza sativa) Theor. Appl. Genet. 2002;84:345–354. doi: 10.1007/BF00229493. [DOI] [PubMed] [Google Scholar]
- 28.Amarawathi Y, et al. Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L.) Mol. Breed. 2008;21:49–65. doi: 10.1007/s11032-007-9108-8. [DOI] [Google Scholar]
- 29.Saitoh K, Onishi K, Mikami I, Thidar K, Sano Y. Allelic diversification at the C (OsC1) locus of wild and cultivated rice: nucleotide changes associated with phenotypes. Genetics. 2004;168:997–1007. doi: 10.1534/genetics.103.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Jia Y, Shu QY, Wu D. Haplotype diversity at the Pi-ta locus in cultivated rice and its wild relatives. Phytopath. 2008;98:1305–1311. doi: 10.1094/PHYTO-98-12-1305. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, et al. Genetic variation of NBS-LRR class resistance genes in rice lines. Theor. Appl. Genet. 2008;116:165–177. doi: 10.1007/s00122-007-0656-4. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida K, Miyashita NT. Nucleotide polymorphism in the Adh2 region of the wild rice Oryza rufipogon. Theor. Appl. Genet. 2005;111:1215–1228. doi: 10.1007/s00122-005-0054-8. [DOI] [PubMed] [Google Scholar]
- 33.Mikami I, et al. Allelic diversification at the wx locus in landraces of Asian rice. Theor. Appl. Genet. 2008;116:979–989. doi: 10.1007/s00122-008-0729-z. [DOI] [PubMed] [Google Scholar]
- 34.Brooks SA, Yan W, Jackson AK, Deren CW. A natural mutation in rc reverts white rice pericarp to red and results in a new, dominant, wild-type allele: Rc-g. Theor. Appl. Genet. 2008;117:575–580. doi: 10.1007/s00122-008-0801-8. [DOI] [PubMed] [Google Scholar]
- 35.Leiros I, Secundo F, Zambonelli C, Servi S, Edward H. The first crystal structure of a phospholipase D. Structure. 2000;8:655–667. doi: 10.1016/S0969-2126(00)00150-7. [DOI] [PubMed] [Google Scholar]
- 36.Kumar GR, et al. Allele mining in Crops: prospects and potentials. Biotechnol. Adv. 2010;28:451–461. doi: 10.1016/j.biotechadv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Q, et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nature genetics. 2018;50:278–284. doi: 10.1038/s41588-018-0041-z. [DOI] [PubMed] [Google Scholar]
- 38.Sweeny M, McCouch S. The complex history of the domestication of rice. Ann. Bot. 2007;100:951–957. doi: 10.1093/aob/mcm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Second G. Origin of the genic diversity of cultivated rice growing environment in West Africa. (Oryza spp.): Study of the polymorphism scored at 40 isozyme loci. Japanese. Journal of Genetics. 1982;57:25–57. [Google Scholar]
- 40.Semon M, Nielsen R, Jones MP, McCouch SR. The population structure of African cultivated rice Oryza glaberrima (Steud.): evidence for elevated levels of linkage disequilibrium caused by admixture with O. sativa and ecological adaptation. Genetics. 2005;169:1639–1647. doi: 10.1534/genetics.104.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer, R. S. et al. Domestication history and geographical adaptation inferred from a SNP map of African rice. Nature Genetics; 10.1038/ng.3633 (2016). [DOI] [PubMed]
- 42.Wang M, et al. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nature genetics. 2014;46:982–988. doi: 10.1038/ng.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sang T, Ge S. Understanding rice domestication and implications for cultivar improvement. Curr. Opin. Plant. Biol. 2013;16:139–146. doi: 10.1016/j.pbi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Vaughan DA, Lu BR, Tomooka N. The evolving story of rice evolution. Plant Sci. 2008;174:394–408. doi: 10.1016/j.plantsci.2008.01.016. [DOI] [Google Scholar]
- 45.Banaticla‐Hilario MCN, van den Berg RG, Hamilton NRS, McNally KL. Local differentiation amidst extensive allele sharing in Oryza nivara and O. rufipogon. Ecology and Evolution. 2013;3:3047–3062. doi: 10.1002/ece3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morishima, H. Evolution and domestication of rice. Rice Genetics IV, IRRI. pp 63–78 (2001).
- 47.Zheng XM, Ge S. Ecological divergence in the presence of gene flow in two closely related Oryza species (Oryza rufipogon and O. nivara) Mol. Ecol. 2010;19:2439–2454. doi: 10.1111/j.1365-294X.2010.04674.x. [DOI] [PubMed] [Google Scholar]
- 48.Barbier P, Morishima H, Ishiharna A. Phylogenetic relationship of annual and perennial wild rice: probing by direct DNA sequencing. Theor. Appl. Genet. 1991;81:693–702. doi: 10.1007/BF00226739. [DOI] [PubMed] [Google Scholar]
- 49.Lu BR, Zheng KL, Qian HR. Genetic differentiation of wild relatives of rice as assessed by RFLP analysis. Theor. Appl. Genet. 2002;106:101–106. doi: 10.1007/s00122-002-1013-2. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Q, Ge S. Phylogenetic relationships among A-genome species of the genus Oryza revealed by intron sequences of four nuclear genes. New Phytol. 2005;167:249–265. doi: 10.1111/j.1469-8137.2005.01406.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Q, Zheng X, Luo J, Gaut BS, Ge S. Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol. Biol. Evol. 2007;24:875–888. doi: 10.1093/molbev/msm005. [DOI] [PubMed] [Google Scholar]
- 52.Huang X, et al. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490:497–501. doi: 10.1038/nature11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu R, Zheng XM, Zhou L, Zhou H. F. nd Ge, S. Population genetic structure of Oryza rufipogon and Oryza nivara: implications for the origin of O. nivara. Mol. Ecol. 2015;24:5211–5228. doi: 10.1111/mec.13375. [DOI] [PubMed] [Google Scholar]
- 54.Samal R, et al. Morphological and molecular dissection of wild rices from eastern India suggests distinct speciation between O. rufipogon and O. nivara populations. Scientific reports. 2018;8:1–13. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melaku, G. et al. Genetic diversity and differentiation of the African wild rice (Oryza longistaminata chev. et roehr) in Ethiopia. Scientific African; 10.1016/j.sciaf.2019.e00138 (2019).
- 56.Zhang Y, et al. Genome and comparative transcriptomics of African wild rice Oryza longistaminata provide insights into molecular mechanism of rhizomatousness and self-incompatibility. Molecular plant. 2015;8:1683–1686. doi: 10.1016/j.molp.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 57.He R, et al. A systems-wide comparison of red rice (Oryza longistaminata) tissues identifies rhizome specific genes and proteins that are targets for cultivated rice improvement. BMC Plant Biology. 2014;14:46. doi: 10.1186/1471-2229-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reuscher, S. et al. Assembling the genome of the African wild rice Oryza longistaminata by exploiting synteny in closely related Oryza species. Commun, Biol., 10.1038/s42003-018-0171-y (2018). [DOI] [PMC free article] [PubMed]
- 59.Ren F, Lu BR, Li S, Huang J, Zhu Y. A. comparative study of genetic relationships among the AA-genome Oryza species using RAPD and SSR markers. Theor. Appl. Genet. 2003;108:113–120. doi: 10.1007/s00122-003-1414-x. [DOI] [PubMed] [Google Scholar]
- 60.Iwamoto M, Nagashima H, Nagamine T, Higo H, Higo K. p-SINE1-like intron of the CatA catalase homologs and phylogenetic relationships among AA-genome Oryza and related species. Theor. Appl. Genet. 1999;98:853–861. doi: 10.1007/s001220051144. [DOI] [Google Scholar]
- 61.Cheng C, Tsuchimoto S, Ohtsubo H, Ohtsubo E. Evolutionary relationships among rice species with AA genome based on SINE insertion analysis. Genes Genet. Syst. 2002;77:323–334. doi: 10.1266/ggs.77.323. [DOI] [PubMed] [Google Scholar]
- 62.Wambugu PW, Brozynska M, Furtado A, Waters DL, Henry RJ. Relationships of wild and domesticated rices (Oryza AA genome species) based upon whole chloroplast genome sequences. Scientific Reports. 2015;5:13957. doi: 10.1038/srep13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cynthia CV, Linda LS, Kenneth MO. Long-term balancing selection at the Phosphorus Starvation Tolerance 1 (PSTOL1) locus in wild, domesticated and weedy rice (Oryza) BMC Plant Biol. 2016;16:101. doi: 10.1186/s12870-016-0783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao LZ, et al. Evolution of Oryza chloroplast genomes promoted adaptation to diverse ecological habitats. Commun. Boil. 2019;2:1–13. doi: 10.1038/s42003-018-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bao Y, Ge S. Origin and phylogeny of Oryza species with the CD genome based on multiple‐gene sequence data. Plant Syst. Evol. 2004;249:55–66. doi: 10.1007/s00606-004-0173-8. [DOI] [Google Scholar]
- 66.Wang B, et al. Polyploid evolution in Oryza officinalis complex of the genus Oryza. BMC Evol. Biol. 2009;9:250. doi: 10.1186/1471-2148-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, Zhang D, Ge S, Lu B, Hong D. Differentiation and inter‐genomic relationships among C, E and D genomes in the Oryza officinalis complex (Poaceae) as revealed by multicolor genomic in situ hybridization. Theor. Appl. Genet. 2001;103:197–203. doi: 10.1007/s001220100562. [DOI] [Google Scholar]
- 68.Guo YL, Ge S. Molecular phylogeny of Oryzeae (Poaceae) based on DNA sequences from chloroplast, mitochondrial, and nuclear genomes. Am. J. Bot. 2005;92:1548–1558. doi: 10.3732/ajb.92.9.1548. [DOI] [PubMed] [Google Scholar]
- 69.Hirsch CD, Wu Y, Yan H, Jiang J. Lineage‐specific adaptive evolution of the centromeric protein CENH3 in diploid and allotetraploid Oryza species. Mol. Biol. Evol. 2009;26:2877–2885. doi: 10.1093/molbev/msp208. [DOI] [PubMed] [Google Scholar]
- 70.Kumari A, et al. Mining of rice blast resistance gene Pi54 shows effect of single nucleotide polymorphisms on phenotypic expression of the alleles. Eur. J. Pl. Pathol. 2013;137:55–65. doi: 10.1007/s10658-013-0216-5. [DOI] [Google Scholar]
- 71.Li HJ, Li XH, Xiao JH, Wing RA, Wang SP. Ortholog alleles at Xa3/Xa26 locus confer conserved race-specific resistance against Xanthomonas oryzae in rice. Molecular Plant. 2012;5:281–290. doi: 10.1093/mp/ssr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammond SM, et al. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J. Biol. Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 73.Sung T, et al. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isshiki M, et al. A naturally occuring funcional allele of the rice waxy locus has a GT to TT mutation at the 5′splice site of the first intron. Plant J. 1998;15:133–138. doi: 10.1046/j.1365-313X.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 75.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structures of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J. Biol. Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 77.Pappan K, Wang X. Plant phospholipase Dα is an acidic phospholipase active at near-physiological Ca2+ concentrations. Arch. Biochem. Biophys. 1999;368:347–353. doi: 10.1006/abbi.1999.1325. [DOI] [PubMed] [Google Scholar]
- 78.Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J. 2000;345:401–415. doi: 10.1042/bj3450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ponting CP, Perker PJ. Extending the C2 domain family: C2s in PKCs delta, epsilon, eta, theta, phospholipases, GAPs, and perforin. Protein Sci. 1996;5:162–166. doi: 10.1002/pro.5560050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ryu, S. B. & Wang, X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochimica et. Biophysica Acta. 1303, 243–250. [DOI] [PubMed]
- 81.Zheng L, Krishnamoorthi R, Zolkiewski M, Wang X. Distinct Ca2+ binding properties of novel C2 domains of plant phospholipase D α and β. J. Biol. Chem. 2000;275:19700–19706. doi: 10.1074/jbc.M001945200. [DOI] [PubMed] [Google Scholar]
- 82.Croessmann S, et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (PI3K), generate oncogene dependence, and are exquisitely sensitive to PI3Kα inhibitors. Clinical Cancer Research. 2018;24:1426–1435. doi: 10.1158/1078-0432.CCR-17-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGee JD, et al. Rice phospholipase D isoforms show differential cellular location and gene induction. Plant Cell Physiol. 2003;44:1013–1026. doi: 10.1093/pcp/pcg125. [DOI] [PubMed] [Google Scholar]
- 84.Janecek S, Svensson B, Henrissat B. Domain evolution in the α-amylase family. J. Mol. Evol. 1997;45:322–331. doi: 10.1007/PL00006236. [DOI] [PubMed] [Google Scholar]
- 85.MacGregor EA. α-Amylase structure and activity. J. Protein Chem. 1988;7:399–415. doi: 10.1007/BF01024888. [DOI] [PubMed] [Google Scholar]
- 86.Ponting CP, Kerr ID. A novel family of phospholipase D homologues that include phospholipid synthases and putative endonucleases: identification of duplicated repeats and potential active site residues. Protein Sci. 1996;5:914–922. doi: 10.1002/pro.5560050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arhab Y, Abousalham A, Noiriel A. Plant phospholipase D mining unravels new conserved residues important for catalytic activity. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2019;1864:688–703. doi: 10.1016/j.bbalip.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Zheng L, Shan J, Krishnamoorthi R, Wang X. Activation of plant phospholipase Dα by phosphatidylinositol 4, 5-bisphosphate: characterization of binding site and mode of action. Biochemistry. 2002;41:4546–4553. doi: 10.1021/bi0158775. [DOI] [PubMed] [Google Scholar]
- 89.Higashibata A, et al. Decreased expression of myogenic transcription factors and myosin heavy chains in Caenorhabditis elegans muscles developed during space flight. J. Exp. Biol. 2006;209:3209–3218. doi: 10.1242/jeb.02365. [DOI] [PubMed] [Google Scholar]
- 90.Woods DC, Alvarez C, Johnson AL. Cisplatin-mediated sensitivity to TRAIL-induced cell death in human granulosa tumor cells. Gynecol. Oncol. 2008;108:632–640. doi: 10.1016/j.ygyno.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 91.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 92.Larsson PKA, Claesson HE, Kennedy BP. Multiple Splice Variants of the Human Calcium-independent Phospholipase A2 and Their Effect on Enzyme Activity. J. Biol. Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 93.Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC genomics. 2006;7:327. doi: 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J, et al. Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiology. 2016;171:1427–1442. doi: 10.1104/pp.15.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ta, K. N. et al. Differences in meristem size and expression of branching genes are associated with variation in panicle phenotype in wild and domesticated African rice. EvoDevo; 10.1186/s13227-017-0065-y (2017). [DOI] [PMC free article] [PubMed]
- 96.Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer length polymorphism in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc. Nat. Acad. Sci., USA. 1984;81:8014–8019. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 98.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 99.Pettersen EF, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 100.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaur A, et al. Novel cis-acting regulatory elements in wild Oryza species impart improved rice bran quality by lowering the expression of Phospholipase D alpha1 enzyme (OsPLDα1) Mol. Biol. Rep. 2019;47:401–422. doi: 10.1007/s11033-019-05144-4. [DOI] [PubMed] [Google Scholar]
- 102.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DNA sequences of wild species accessions and cultivars generated and analyzed during the current study are available in the GenBank repository with accession numbers: O. glaberrima [IRGC100854 (MF774061), IRGC101800 (MF919319), IRGC102196 (MF919320), IRGC102489 (MF919321), IRGC102512 (MF919322), IRGC102600b (MF919323), IRGC102925 (MF919324), IRGC103750 (MF919325)]; O. bartii [IRGC100117 (MF919326), IRGC101317 (MF919327), IRGC104102 (MF919328), IRGC105990 (MF919329), IRGC106239 (MF919330), IRGC106294 (MF919331)]; O. nivara [CR100008 (MF919334), CR100400 (MF919335), CR100429 (MF91336), IRGC80547 (MF919332), IRGC81847 (MG725633), IRGC92713 (MF919337), IRGC92930 (MG725634), IRGC100189 (MF919338), IRGC106397 (MF919333)]; O. rufipogon [CR100013 (MG725637), IRGC80610 (MG725635), IRGC81976 (MG725638), IRGC83823 (MG725640), IRGC89224 (MG725641), IRGC99551 (MG725642), IRGC103308 (MF919339), IRGC104308 (MG725643) IRGC104867 (MF966922), IRGC105491 (MG7256360), IRGC105569 (MF966923), IRGC105902 (MF966924), IRGC106162 (MF966925), IRGC106336 (MF966926), IRGC106433 (MF966927), IRGC113652 (MF966928)]; O. longistaminata [IRGC101200 (MG725645), IRGC104301 (MG725646), IRGC105206 (MG725647)]; O. meridionalis [IRGC101146 (MG725648)]; O. glumaepatula [IRGC100184 (MF966930), IRGC104387 (MF966929)]; O. officinalis [IRGC101152 (MF966931), IRGC105674 (MF966932), IRGC106501 (MF966933)]; O. australiensis [IRGC105275 (MG725650)]; O. punctata [IRGC105158 (MG725651)]; O. minuta [IRGC101100 (MG725652), IRGC101128 (MG725653)]; O. latifolia [IRGC105139 (MG725654)]; Feng-Ai-Zhan (MF975521); Kitake (MF975522); Minghui63 (MF975523); Nagina22 (MF975524); PR114 (MF975525); Pusa44 (MF975526).