Mycoplasma gallisepticum is the primary etiological agent of chronic respiratory disease in chickens. Live attenuated vaccines are most commonly used in the field to control the disease, but current vaccines have some limitations. Vaxsafe MG (strain ts-304) is a new vaccine candidate that is efficacious at a lower dose than the current commercial vaccine strain ts-11, from which it is derived. In this study, the transcriptional profiles of the trachea of unvaccinated chickens and chickens vaccinated with strain ts-304 were compared 2 weeks after challenge with M. gallisepticum strain Ap3AS during the chronic stage of infection.
KEYWORDS: chicken, host response, Mycoplasma gallisepticum, RNA-seq
ABSTRACT
Mycoplasma gallisepticum is the primary etiological agent of chronic respiratory disease in chickens. Live attenuated vaccines are most commonly used in the field to control the disease, but current vaccines have some limitations. Vaxsafe MG (strain ts-304) is a new vaccine candidate that is efficacious at a lower dose than the current commercial vaccine strain ts-11, from which it is derived. In this study, the transcriptional profiles of the trachea of unvaccinated chickens and chickens vaccinated with strain ts-304 were compared 2 weeks after challenge with M. gallisepticum strain Ap3AS during the chronic stage of infection. After challenge, genes, gene ontologies, pathways, and protein classes involved in inflammation, cytokine production and signaling, and cell proliferation were upregulated, while those involved in formation and motor movement of cilia, formation of intercellular junctional complexes, and formation of the cytoskeleton were downregulated in the unvaccinated birds compared to the vaccinated birds, reflecting immune dysregulation and the pathological changes induced in the trachea by infection with M. gallisepticum. Vaccination appears to protect the structural and functional integrity of the tracheal mucosa 2 weeks after infection with M. gallisepticum.
INTRODUCTION
Mycoplasma gallisepticum is a major respiratory pathogen of poultry, causing chronic respiratory disease (CRD) in chickens and infectious sinusitis in turkeys. After initial attachment to the respiratory epithelium and colonization (1–4), it induces a moderate to severe inflammatory response in the trachea of its host (3, 5, 6). Affected birds can develop clinical signs of disease, including coughing, sneezing, râles, and ocular and nasal discharge, and there is a significant subclinical effect on productivity (7, 8). M. gallisepticum remains predominantly on the mucosal surface, but can occur, rarely, inside phagosomes within the epithelial cells. Infection results in epithelial cell degeneration and inflammatory cell infiltration into the mucosa (5, 6). The tracheal mucosal thickness increases due to inflammatory cell infiltration and edema (9, 10). Some respiratory epithelial cells tend to lose their intercellular adhesions and exfoliate into the tracheal lumen, while other cells lyse (6, 9, 11). Colonization also results in rapid ciliostasis and loss of cilia from epithelial cells (6).
After initial colonization of the upper respiratory tract, M. gallisepticum spreads to the lower respiratory tract, resulting in bronchitis, pneumonia, and airsacculitis (12). Severe airsacculitis is often accompanied by infection with other respiratory pathogens, such as Newcastle disease virus, infectious bronchitis virus, and Escherichia coli (13).
Although the inflammatory pathological changes in the trachea after infection with M. gallisepticum are widely recognized, the underlying mechanisms or pathways responsible for these changes have not been well described. A few studies have explored the role of selected cytokines and chemokines (14), and lipid-associated membrane proteins of M. gallisepticum (15), in the host response. The transcriptional profile of the tracheal response to infection with M. gallisepticum strain Rlow in unvaccinated chickens has been examined up to 7 days after infection (16, 17), and these studies have suggested that the inflammation seen in the trachea is mediated by a panel of proinflammatory cytokines and chemokines via Toll-like receptor (TLR) 2 signaling through an NF-κB pathway. However, the regulatory pathways and mechanisms involved in the inflammation seen during the chronic stages of infection have not been explored previously.
M. gallisepticum Ap3AS is a pathogenic strain isolated from an infected chicken in Australia, and studies of its pathogenicity have revealed that, like other strains of M. gallisepticum, it causes severe airsacculitis, increases tracheal mucosal thickness due to infiltration of mononuclear cells, and causes degeneration and metaplasia of the respiratory epithelium (18). The cellular immune response to M. gallisepticum strain Ap3AS in the tracheal mucosa has been shown to be primarily mediated by T cells during the first 2 weeks after infection, with B cells appearing later, nearly 3 weeks after infection (19, 20). Although there is a considerable humoral response to infection, no correlation has been found between serum antibody concentrations and protective immunity (21), and birds remain chronically infected despite the presence of detectable concentrations of serum antibodies.
Prevention and control of infection with M. gallisepticum is achieved by maintaining disease-free breeding flocks (22) and by vaccination when maintaining freedom from infection is not feasible, such as in multiage commercial layer flocks (23). Both inactivated and live attenuated vaccines have been used (22), but live attenuated vaccines have been found to have greater efficacy (24). The commercially available live attenuated M. gallisepticum vaccines used for immunization of chickens include F strain (22), strain 6/85 (25), and strain ts-11 (26). A new vaccine candidate, MG ts-304 (27), has been shown to have a safety profile similar to that of ts-11 but to be efficacious at a lower dose (97). The transcriptional response in the tracheal mucosa has been investigated (17) after exposure to M. gallisepticum vaccine strains GT5 and Mg7 (16) over the first 2 days after exposure, but there have been no previous studies investigating the transcriptional profile in the trachea after challenge with wild-type M. gallisepticum in vaccinated birds.
This study aimed to explore the genes and pathways underlying the pathological and immune events in the tracheal mucosa in response to chronic infection with the virulent M. gallisepticum strain Ap3AS in unvaccinated chickens and in chickens vaccinated with Vaxsafe MG (strain ts-304) 2 weeks after infection. As M. gallisepticum is known to cause chronic infection, a comprehensive study of pathological and immune pathways involved at a later stage of infection will assist in understanding both the pathogenesis of mycoplasmosis in chickens and the effect of vaccination on progression of disease.
RESULTS
Increased thickness and inflammatory cell infiltrations in the tracheal mucosae of challenged-only birds.
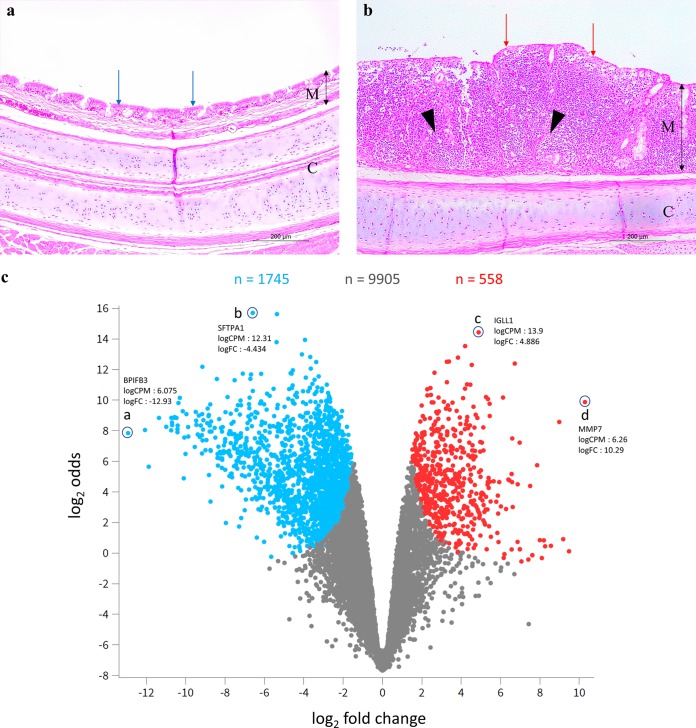
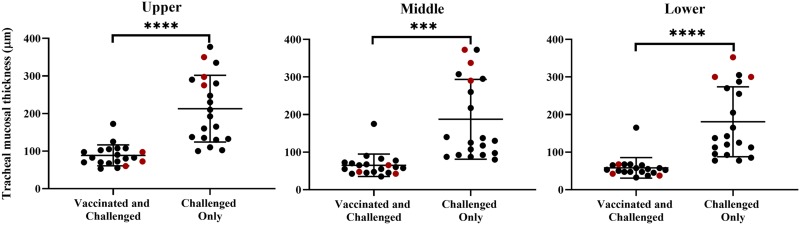
The mean mucosal thicknesses of the upper, middle, and lower trachea of the birds in the challenged-only group were significantly greater than those of the birds in the vaccinated-and-challenged group (P < 0.05) (Table 1). The tracheal mucosae of the birds in the challenged-only group had diffuse intraepithelial inflammatory cell infiltration with a disintegrated pseudostratified columnar epithelium, loss of cilia, and reduction or absence of the intraepithelial mucous glands (Fig. 1b). Most of the cells in the inflammatory cell infiltrates were lymphocytes and plasma cells, with a few heterophils and macrophages. The vaccinated-and-challenged birds had an intact pseudostratified ciliated columnar epithelium with abundant simple intraepithelial mucous glands containing several goblet cells (Fig. 1a).
TABLE 1.
Mean tracheal mucosal thicknesses of the upper, middle, and lower trachea of vaccinated and unvaccinated birds after challenge
| Group | No. of birds | Treatment (CCU/0.03 ml)a | Challenge (106.5 CCU/ml)a | Mean tracheal mucosal thickness ± SD (μm)b |
||
|---|---|---|---|---|---|---|
| Upper | Middle | Lower | ||||
| Vaccinated and challenged | 20 | ts-304 (106.0) | Ap3AS | 88.5 ± 27.8a | 64.9 ± 29.8a | 58.0 ± 27.3a |
| Challenged only | 20 | Merial diluent only | Ap3AS | 212.9 ± 88.8b | 177.6 ± 99.6b | 181.0 ± 93.0b |
CCU, color changing units.
Values marked with the same lowercase superscript letter were not significantly different (P > 0.05).
FIG 1.
(a) H&E-stained tracheal cross section from a typical bird in the vaccinated-and-challenged group. (b) H&E-stained tracheal cross section from a typical bird in the challenged-only group. M, mucosa; C, cartilage; double headed black arrows indicate the mucosal thickness; red arrows indicate damaged cilia; blue arrows indicate intact cilia; black arrowheads indicate inflammatory cell infiltrations. Magnification = 100×. (c) Volcano plot showing the levels of gene expression in the challenged-only group. Blue dots, downregulated genes (upregulated in the vaccinated-and-challenged group); red dots, upregulated genes (downregulated in the vaccinated-and-challenged group); gray dots, genes with no significant difference in expression between the two groups; n, number of genes; a, downregulated gene with the greatest log2 FC; b, downregulated gene with the greatest log2 odds; c, upregulated gene with the greatest log2 odds; d, upregulated gene with the greatest log2 FC.
Clear distinctions in gene expression between vaccinated and unvaccinated chickens after challenge.
RNA-seq reads were mapped to 18,346 Gallus gallus protein-coding genes, and 12,208 genes with a count per million (CPM) of >1 in three or more samples were retained for differential gene expression analysis between the two groups. At a false discovery rate (FDR) of <0.01, 2,303/12,208 (18.86%) genes were differentially expressed in the challenged-only group compared to the vaccinated-and-challenged group (Fig. 1c). More genes were downregulated (1,745/2,303, 75.77%) than upregulated (558/2,303, 24.23%). The multidimensional scaling (MDS) plot (see Fig. S1 in the supplemental material) generated based on the log2 CPM values to visualize the unsupervised relationships between samples demonstrated minimal variability within the groups and a distinct difference between the groups.
Genes with greatest differential expression.
The log2 fold change (FC) values for upregulated genes ranged from 1.52 to 10.29, and those for downregulated genes varied from –12.93 to –1.58. The top 25 up- and downregulated genes are listed separately in Tables 2 and 3, respectively. The FC of the genes showing the greatest differential expression ranged from 2.64 to 10.29 for the top 25 upregulated genes and from –10.57 to –3.67 for the top 25 downregulated genes. Genes encoding proteins involved in immune response signaling, immunoglobulin (Ig) production and secretion, antigen processing, and presentation via major histocompatibility complex (MHC) class II, MHC class I activity, the B cell response to antigen, inflammation, chemotaxis, phagocytosis, expression of cytokine receptors, degradation of the extracellular matrix, cell division and protein folding, and phosphorylation of deoxyguanosine and deoxyadenosine in the mitochondrial matrix were among the top 25 upregulated genes (Table 2). Proteins encoded by the top 25 downregulated genes were involved in microtubule assembly and stability, axonemal dynein complex assembly, formation and motor movement of cilia, cell cycle arrest, and expression of CD24 on cell surfaces (Table 3). Among the upregulated genes, SFTPA1 had the greatest average log2 CPM of 12.31 (and an FC of 4.886), while among the downregulated genes IGLL1 had an average log2 CPM of 13.9 (and an FC of –4.434) (Fig. 1c).
TABLE 2.
Top 25 upregulated genes based on FDR in the challenged-only group compared to the vaccinated-and-challenged groupa
| Gene symbol | Gene name | FC | FDR |
|---|---|---|---|
| MHC proteins | |||
| DMB2 | Major histocompatibility complex, class II, DM beta 2 | 4.096815 | 1.29e-05 |
| BF1 | MHC BF1 class I | 2.643041 | 1.48e-05 |
| Cytokine receptors and immune signaling | |||
| IL4I1 | Interleukin 4 induced 1 | 8.984358 | 9.92e-06 |
| IL13RA2 | Interleukin 13 receptor subunit alpha 2 | 5.445273 | 1.02e-05 |
| IL2RG | Interleukin 2 receptor subunit gamma | 5.263067 | 9.92e-06 |
| TNFRSF13B | TNF receptor superfamily member 13B | 5.124457 | 2.21e-05 |
| IFITM1 | Interferon-induced transmembrane protein 1 | 4.537843 | 6.94e-06 |
| EXFABP | Extracellular fatty acid-binding protein | 4.464134 | 8.39e-06 |
| POU2AF1 | POU class 2 associating factor 1 | 4.298379 | 8.46e-06 |
| Immunoglobulin-related proteins | |||
| MZB1 | Marginal zone B and B1 cell-specific protein | 6.723834 | 4.32e-06 |
| IGLL1 | Immunoglobulin lambda-like polypeptide 1 | 4.885844 | 3.29e-06 |
| JCHAIN | Joining chain of multimeric IgA and IgM | 3.837216 | 6.94e-06 |
| Chemotaxis, phagocytosis, cell killing | |||
| SRGN | Serglycin | 6.127048 | 8.79e-06 |
| BIN2 | Bridging integrator 2 | 5.362242 | 9.78e-06 |
| Degradation of extracellular matrix | |||
| MMP7 | Matrix metallopeptidase 7 | 10.28887 | 7.24e-06 |
| MMP9 | Matrix metallopeptidase 9 | 4.309264 | 2.45e-05 |
| Other | |||
| KCNMB1 | Potassium calcium-activated channel subfamily M regulatory beta subunit 1 | 5.451376 | 1.62e-05 |
| BLVRA | Biliverdin reductase A | 4.738442 | 1.94e-05 |
| TXNDC5 | Thioredoxin domain containing 5 | 4.19952 | 4.54e-06 |
| DGUOK | Deoxyguanosine kinase | 3.768688 | 2.45e-05 |
| GBP | Guanylate-binding protein 4-like | 3.558826 | 2.03e-05 |
| KPNA2 | Karyopherin subunit alpha 2 | 3.378235 | 7.24e-06 |
| CKS1B | CDC28 protein kinase regulatory subunit 1B | 3.273998 | 1.13e-05 |
| LMAN1 | Lectin, mannose binding 1 | 3.271608 | 7.24e-06 |
| MANF | Cerebral dopamine neurotrophic factor | 3.120726 | 2.58e-05 |
FC, log2 fold change. A false discovery rate (FDR) of < 0.01 was considered significant.
TABLE 3.
Top 25 downregulated genes based on FDR in the challenged-only group compared to the vaccinated-and-challenged groupa
| Gene symbol | Gene name | FC | FDR |
|---|---|---|---|
| Formation and motor movement of cilia | |||
| CFAP161 | Cilia and flagella-associated protein 161 | –10.37 | 4.32e-06 |
| DYDC1 | DPY30 domain containing 1 | –10.15 | 6.94e-06 |
| DEUP1 | Deuterosome assembly protein 1 | –9.164 | 7.24e-06 |
| PACRG | Parkin coregulated gene protein | –8.417 | 3.46e-06 |
| LRRC6 | Leucine rich repeat containing 6 | –7.098 | 4.10e-06 |
| ENKUR | Enkurin, TRPC channel interacting protein | –6.714 | 6.94e-06 |
| DAW1 | Dynein assembly factor with WD repeats 1 | –6.68 | 6.28e-06 |
| FOXJ1 | Forkhead box J1 | –6.594 | 1.04e-06 |
| DNAH3 | Dynein axonemal heavy chain 3 | –6.59 | 7.24e-06 |
| PIH1D3 | PIH1 domain containing 3 | –6.166 | 6.28e-06 |
| IFT81 | Intraflagellar transport 81 | –4.668 | 7.24e-06 |
| Microtubule assembly and stability | |||
| AQP5 | Aquaporin 5 | –5.383 | 3.29e-06 |
| EZR | Ezrin | –4.095 | 6.28e-06 |
| Other | |||
| DTHD1 | Death domain containing 1 | –10.57 | 7.24e-06 |
| Novel gene | Zinc finger B-box domain containing | –10.3 | 3.46e-06 |
| ANKEF1 | Ankyrin repeat and EF-hand domain containing 1 | –9.965 | 7.24e-06 |
| Novel gene | Kinase noncatalytic C-lobe domain containing 1 | –9.489 | 6.94e-06 |
| Novel gene | IQ motif containing M | –9.334 | 7.24e-06 |
| Novel gene | Uncharacterized | –9.153 | 3.29e-06 |
| FAM183BP | Family with sequence similarity 183 member B, pseudogene | –8.885 | 6.94e-06 |
| Novel gene | Glutamate-rich protein 3 | –7.515 | 6.28e-06 |
| MLF2 | Myeloid leukemia factor 2 | –5.362 | 1.19e-06 |
| CD24 | CD24 molecule | –4.555 | 7.24e-06 |
| CYP2C18 | Cytochrome P450 2C18 | –3.938 | 4.32e-06 |
| RBM38 | RNA binding motif protein 38 | –3.672 | 7.23e-06 |
FC, log2 fold change. A false discovery rate (FDR) of <0.01 was considered significant.
Challenged-only birds had altered expression of the genes that drive immune responses.
Because immune response genes were among the top 25 upregulated genes, further assessment of immune response-related genes was conducted. Genes for proinflammatory cytokines, including interleukin-22 (IL-22), IL-6, IL-17A, and IL-1β, were upregulated, along with that for IL-8-like 1 (chCXCLi1). The gene for interferon-γ (IFN-γ), a potent activator of macrophages that mediates T-helper cell (TH1) and inflammatory responses, was also upregulated and had the greatest FC (6.6) of the upregulated cytokine genes (Table 4). The gene for tumor necrosis factor (TNF) superfamily member 15 was the only cytokine gene that was downregulated. The genes for the chemokine C-C motif chemokine ligand 19 (CCL-19), CCL-4 (macrophage inflammatory protein-1β [MIP-1β]), CCL-1 (chCCLi5), CCL-26, C-X-C motif chemokine ligand 13 (CXCL-13), CXCL-13-like 2, X-C motif chemokine ligand 1 (XCL-1)/lymphotactin, and chemokine ah221 (chCCLi9) were upregulated, with FCs ranging from 3.2 to 7.9, while the gene for CCL-20 (MIP-3α) was the only chemokine gene that was downregulated, with an FC of –3.3 (Table 4).
TABLE 4.
Log2 fold change (FC) and false discovery rate (FDR) of differentially expressed immune-related genesa
| Gene | FC | FDR |
|---|---|---|
| Cytokines | ||
| Interferon gamma | 6.620001 | 0.0006 |
| Interleukin 8-like 1 (chCXCLi1) | 5.391872 | 0.0002 |
| Interleukin 17A | 4.321906 | 0.0039 |
| Interleukin 22 | 4.285866 | 0.0016 |
| Interleukin 6 | 3.927618 | 0.0017 |
| Interleukin 1, beta | 3.695163 | 0.0006 |
| TNF superfamily member 15 | –3.054895 | 0.0014 |
| Chemokines | ||
| C-X-C motif chemokine ligand 13-like 2 | 7.858709 | 6e-05 |
| C-C motif chemokine ligand 26 | 6.564404 | 0.0001 |
| Gallus gallus C-X-C motif chemokine ligand 13 | 6.448406 | 0.0002 |
| Chemokine (C-C motif) ligand 4 (MIP-1β) | 5.139185 | 0.0008 |
| X-C motif chemokine ligand 1 (lymphotactin) | 4.978717 | 0.0036 |
| C-C motif chemokine ligand 19 (MIP-3β) | 4.245883 | 3e-05 |
| Chemokine ah221 (chCCLi9) | 3.765825 | 0.0087 |
| C-C motif chemokine ligand 20 (MIP-3α) | –3.298172 | 0.0063 |
| C-C motif chemokine ligand 1 (chCCLi5) | 3.265933 | 0.0033 |
| Cytokine receptors | ||
| Interleukin-13 receptor alpha 2 | 5.445273 | 1e-05 |
| Interleukin-2 receptor subunit gamma | 5.263067 | 1e-05 |
| Tumor necrosis factor receptor superfamily member 13B | 5.124457 | 2e-05 |
| Granulocyte colony stimulating factor 3 receptor | 4.648147 | 0.0002 |
| Interleukin-21 receptor | 4.558267 | 0.0008 |
| Interleukin-1 receptor type 1 | –4.154731 | 0.0001 |
| Tumor necrosis factor receptor superfamily member 19 | –3.451626 | 0.0021 |
| Transforming growth factor beta receptor 3 | –3.298024 | 0.0005 |
| Interleukin-1 receptor-like 2 | –3.280008 | 0.0005 |
| Interleukin-20 receptor subunit alpha | 3.216855 | 0.0002 |
| Granulocyte-macrophage colony-stimulating factor receptor alpha like | 3.054296 | 0.0037 |
| Interleukin-1 receptor-associated kinase 1 binding protein 1 | –2.839971 | 0.0004 |
| Tumor necrosis factor receptor superfamily member 6B | 2.696494 | 0.0069 |
| Death domain containing TNF receptor superfamily member 23 | –2.5137 | 0.0088 |
| Tumor necrosis factor receptor superfamily member 1B | 2.435101 | 0.0016 |
| Chemokine receptors | ||
| C-C motif chemokine receptor 10 | 6.595925 | 3e-05 |
| C-C motif chemokine receptor 5 | 5.054953 | 0.0005 |
| C-X-C motif chemokine receptor 1 | 4.604372 | 0.003 |
| C-X3-C motif chemokine receptor 1 | 3.690204 | 0.0003 |
| C-X-C motif chemokine receptor 4 | 2.782461 | 0.0005 |
| Toll-like receptors | ||
| Gallus gallus Toll-like receptor 15 | 3.935744 | 0.0017 |
| Toll-like receptor 5 | –3.279345 | 0.0007 |
| Toll-like receptor 7 | 3.001301 | 0.0028 |
| MHC proteins | ||
| Major histocompatibility complex, class II, DM beta 1 | 5.013108 | 0.0004 |
| Major histocompatibility complex, class II, DM beta 2 | 4.096815 | 1e-05 |
| Gallus gallus major histocompatibility complex class II beta chain BLB2 | 3.429747 | 0.0003 |
| Major histocompatibility complex class II beta chain BLB1 | 3.391866 | 0.0004 |
| Major histocompatibility complex, class II, DM alpha | 3.289581 | 7e-05 |
| Class I histocompatibility antigen, F10 alpha chain-like 3 | 2.932091 | 0.0061 |
| Major histocompatibility complex class I antigen BF2 | 2.464203 | 4e-05 |
| Major histocompatibility complex class I antigen BF2 | 2.464203 | 4e-05 |
| Immunoglobulin and receptor proteins | ||
| Immunoglobulin superfamily member 1-like 7 | 5.210969 | 0.0019 |
| Immunoglobulin lambda-like polypeptide 1 | 4.885844 | 3e-06 |
| Immunoglobulin superfamily member 1-like 2 | 4.413245 | 0.0015 |
| Immunoglobulin superfamily member 1 | 4.030709 | 0.0008 |
| Immunoglobulin like domain containing receptor 1 | –2.810537 | 0.0042 |
| Polymeric immunoglobulin receptor | –2.056498 | 0.0002 |
| Immunoglobulin superfamily member 9 | –1.978692 | 0.0034 |
| V-set and immunoglobulin domain containing 10 | –1.725352 | 0.0093 |
An FDR of <0.01 was considered significant.
Genes for several Toll-like receptors (TLRs), including those for TLR7 and TLR15, were upregulated, and the expression of the chicken TLR5 gene, a highly conserved receptor for bacterial flagellin, was downregulated. In addition, there were several proinflammatory cytokine and chemokine receptors that were differentially expressed between the two groups (Table 4). Even though the gene for the proinflammatory cytokine IL-1β was upregulated, the genes for two of its receptors and the IL-1 receptor-associated kinase 1 binding protein 1 were downregulated (Table 4). There were six MHC protein coding genes, including a chicken-specific gene encoding MHC class II β chain, among the differentially expressed genes. In addition, a few Ig and Ig receptor protein genes were differentially expressed between the two groups (Table 4).
Gene ontologies enriched with upregulated genes were mainly those for immune response, regulation of cytokine production, and cell activation and proliferation.
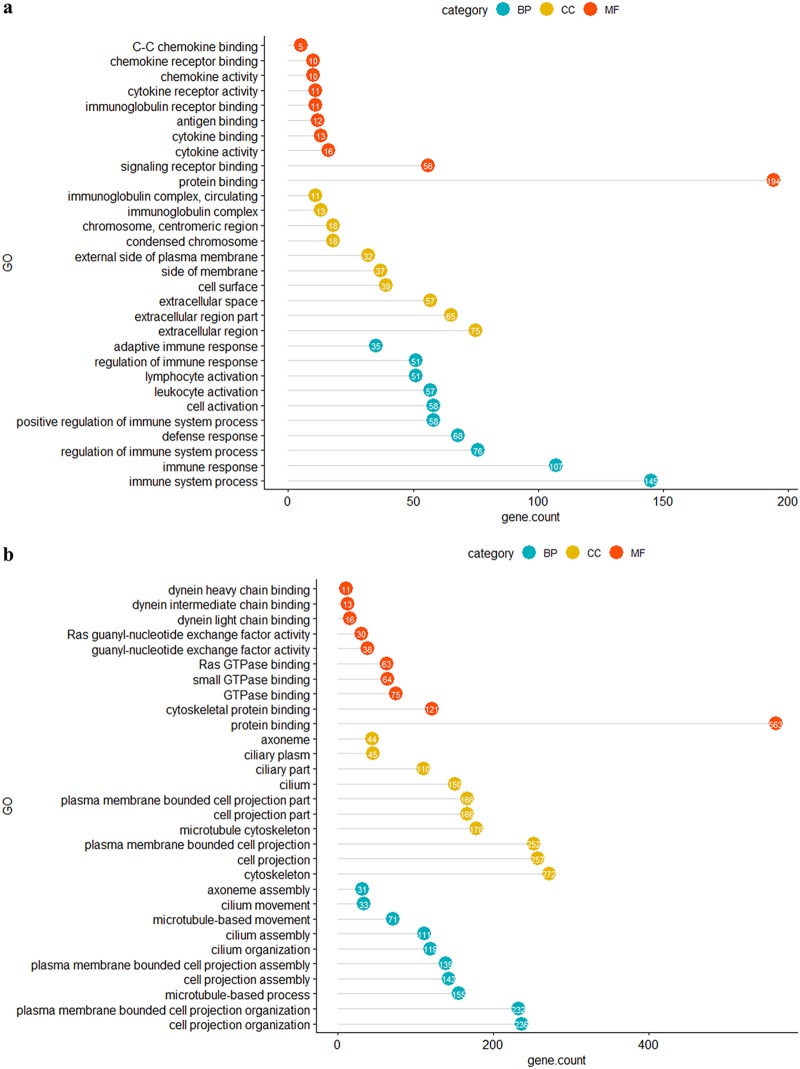
Functional gene ontology (GO) analysis of up- and downregulated genes enabled the genes to be categorized based on enriched cellular components (CCs), molecular functions (MFs), and biological processes (BPs). At an FDR of <0.01, upregulated genes were assigned to 25, 9, and 180 functional gene ontologies for CCs, MFs, and BPs, respectively. The 10 most significant gene ontologies for upregulated genes under each category are shown in Fig. 2a. The top 5 MFs enriched with upregulated genes were mainly associated with chemokine-receptor and Ig-receptor binding, the top 5 CCs enriched with upregulated genes were associated with the Ig complex and the condensed chromosome, and the top 5 BPs enriched with upregulated genes were linked mainly with leukocyte activation and regulation of immune responses. The REVIGO nonredundant functional GO summary highlighted the CCs in the tracheal mucosa that were enriched with upregulated genes. In brief, intracellular components that are involved in mitosis and extracellular components engaged in ligand-receptor binding, including T-cell receptors, Ig receptors, and cytokine/chemokine-receptor interactions, were highlighted (Fig. S2). The MFs that were enriched with upregulated genes were associated with protein binding, particularly antigen, Ig, cytokine and chemokine binding, and their subsequent functional roles, namely, Ig, cytokine, and chemokine activities (Fig. S3). Moreover, the BPs enriched with upregulated genes were associated with ongoing inflammation mediated by cytokines, chemokines, and activated lymphocytes, which undergo mitosis. These functions are associated with facilitation of phagocytosis and leukocyte-mediated cytotoxicity in response to infection (Fig. S4). The CC, MF, and BP with the highest proportions of upregulated genes per GO term were the T cell receptor complex (50%, 6/12), chemokine activity (37%, 10/27), and antigen processing and presentation of peptide antigen (64%, 9/14), respectively.
FIG 2.
The 10 most significant enriched functional gene ontologies based on FDR under each GO category. (a) GOs enriched with upregulated genes in the challenged-only birds; (b) GOs enriched with downregulated genes in the challenged-only birds. BP, biological process; CC, cellular component; MF, molecular function. The number of upregulated genes within each GO is indicated in the solid colored circle at the end of each horizontal line. The length of the horizontal lines corresponds to the number of genes included in each gene ontology.
Gene ontologies enriched with downregulated genes are associated with impaired ciliary movement and intercellular junctions.
Enrichment of gene ontologies for downregulated genes identified 50, 20, and 130 terms for CCs, MFs, and BPs, respectively, at an FDR of <0.01. The 10 most significant gene ontologies enriched with downregulated genes under each category are shown in Fig. 2b. The top 5 MFs were mainly associated with dynein chain binding and Ras guanyl-nucleotide exchange factor activity, the top 5 CCs were mainly linked with cilium assembly, and the top 5 BPs were associated with both assembly and movement of cilia. The summarized functional GOs included the apical cell membrane and axonemes, which constitute the cilia on the cell surface, and the cytoskeleton, which supports the ciliary projections, as well as the intercellular junctions (Fig. S5). The MF terms identified included the activity of the small GTPases, the Ras guanyl-nucleotide exchange factor, phosphotransferase, and dynein light chain-microtubule binding, suggesting downregulation of the ATP-dependent microtubule motor activity that drives the beating of the cilia (Fig. S6). BPs enriched with downregulated genes were those for cell-to-cell adhesion and cell-to-cell junction organization, cell organelle assembly, particularly the cilia and cytoskeleton, epithelial development, specifically the apical part of the cell, and the small GTPase activity in signal transduction, the Ras guanyl-nucleotide exchange factor, phosphotransferase, and dynein light chain-microtubule binding (Fig. S7). The CC, MF, and BP with the highest proportions of downregulated genes per GO term were those for intraciliary transport particle B (82%, 14/17), dynein light chain binding (80%, 16/20), and inner dynein arm assembly (100%, 10/10), respectively.
Immune responses and cell proliferation pathways were activated, while pathways involved in cilia formation were downregulated in infected chickens.
There were 26 Reactome pathways enriched with upregulated genes with a log2 fold enrichment of ≥2.21 at an FDR of <0.01. These pathways were associated with the immune system (10/26 pathways), the cell cycle (10/26 pathways), signal transduction (5/26 pathways), and hemostasis (1/26 pathways). Specifically, immune pathways involved in the complement cascade, neutrophil degranulation, MHC class I-mediated antigen processing, and MHC class II antigen presentation were activated by infection (Table 5). The log2 fold enrichment of downregulated Reactome pathways ranged from 0.01 to 6.36. At an FDR of <0.01, 10 pathways were downregulated in the challenged-only group. Downregulated pathways included those for cilium assembly (5/10 pathways), gene expression (1/10 pathways), metabolism of RNA (2/10 pathways), translation (1/10 pathways), and the cell cycle (1/10 pathways) (Table 6).
TABLE 5.
Reactome pathways enriched with upregulated genes in the challenged-only group compared with the vaccinated-and-challenged groupa
| Pathway | Log2 fold enrichment | FDR |
|---|---|---|
| Immune | ||
| ER-phagosome pathway | 27.97 | 4.72e-03 |
| Initial triggering of complement | 12.34 | 2.47e-03 |
| Antigen presentation: folding, assembly, and peptide loading of class I MHC | 12.34 | 2.60e-03 |
| Regulation of complement cascade | 9.64 | 7.00e-04 |
| MHC class II antigen presentation | 8.16 | 4.57e-03 |
| Complement cascade | 7.77 | 2.26e-03 |
| Neutrophil degranulation | 3.22 | 3.84e-07 |
| Innate immune system | 2.68 | 1.06e-07 |
| Immune system | 2.62 | 1.52e-12 |
| Adaptive immune system | 2.21 | 7.69e-03 |
| Cell cycle | ||
| Activation of ATR in response to replication stress | 6.99 | 8.66e-03 |
| Amplification of signal from the kinetochores | 5.32 | 1.92e-04 |
| Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal | 5.32 | 2.10e-04 |
| Mitotic spindle checkpoint | 4.77 | 2.59e-04 |
| Resolution of sister chromatid cohesion | 4.56 | 3.95e-04 |
| Cell cycle checkpoints | 3.99 | 4.54e-06 |
| Mitotic prometaphase | 3.88 | 1.52e-04 |
| Cell cycle, mitotic | 3.16 | 4.58e-07 |
| Cell cycle | 3 | 4.64e-07 |
| M phase | 2.61 | 3.07e-03 |
| Signal transduction | ||
| RHO GTPases activate NADPH oxidases | 13.45 | 6.75e-03 |
| Chemokine receptors bind chemokines | 11.12 | 1.16e-03 |
| RHO GTPase effectors | 4.79 | 1.33e-07 |
| RHO GTPases activate formins | 4.62 | 2.23e-04 |
| Signaling by Rho GTPases | 3.81 | 1.07e-07 |
| Hemostasis | ||
| Kinesins | 6.36 | 5.91e-03 |
A false discovery rate (FDR) of <0.01 was considered significant.
TABLE 6.
Reactome pathways enriched with downregulated genes in the challenged-only group compared with the vaccinated-and-challenged groupa
| Pathway | Log2 fold enrichment | FDR |
|---|---|---|
| Cell organelle formation | ||
| Intraflagellar transport | 6.36 | 1.26e-09 |
| Cilium assembly | 4.31 | 1.49e-16 |
| Cargo trafficking to the periciliary membrane | 4.22 | 3.91e-03 |
| Organelle biogenesis and maintenance | 3.73 | 3.47e-14 |
| Anchoring of the basal body to the plasma membrane | 2.95 | 2.16e-03 |
| Metabolism of RNA | ||
| Metabolism of RNA | 0.12 | 1.04e-09 |
| Processing of capped intron-containing pre-mRNA | 0.1 | 6.06e-04 |
| Gene expression | ||
| Gene expression (transcription) | 0.47 | 1.32e-03 |
| Metabolism of proteins | ||
| Translation | 0.06 | 5.96e-04 |
| Cell cycle | ||
| Cell cycle checkpoints | 0.1 | 6.05e-04 |
A false discovery rate (FDR) of <0.01 was considered significant.
Protein class analysis.
Panther protein class analysis of up- and downregulated genes determined the protein classes enriched at an FDR of <0.01 (Table 7). Seven protein classes were found to be enriched with upregulated genes, with a log2 fold enrichment of ≥2.10, and 8 protein classes were found to be enriched with downregulated genes, with a log2 fold enrichment of ≥0.29. Protein classes that play a role in immune responses were more highly expressed in response to infection, mainly cytokines, chemokines, Igs, Ig receptors, MHC antigens, and signaling molecules. Protein classes downregulated in response to infection included the microtubule family cytoskeletal proteins, cytoskeletal proteins, G-protein modulators, guanyl-nucleotide exchange factors, and microtubule binding motor proteins, reflecting impaired cytoskeletal formation and ciliary assembly (Table 7).
TABLE 7.
Panther protein classes enriched with up- and downregulated genes in the challenged-only group compared with the vaccinated-and-challenged groupa
| Protein class | Log2 fold enrichment | FDR |
|---|---|---|
| Upregulated | ||
| Defense/immunity protein | 6.17 | 1.05e-11 |
| Cytokine | 6.83 | 4.38e-07 |
| Chemokine | 12.95 | 3.24e-06 |
| Immunoglobulin receptor superfamily | 10.49 | 5.04e-05 |
| Immunoglobulin | 24.97 | 4.73e-04 |
| Major histocompatibility complex antigen | 12.34 | 1.09e-03 |
| Signaling molecule | 2.1 | 2.55e-03 |
| Downregulated | ||
| Nucleic acid binding | 0.46 | 2.65e-10 |
| Microtubule family cytoskeletal protein | 2.5 | 1.74e-06 |
| Cytoskeletal protein | 1.92 | 2.12e-06 |
| DNA binding protein | 0.29 | 4.55e-06 |
| RNA binding protein | 0.37 | 2.78e-05 |
| G-protein modulator | 2.03 | 6.29e-04 |
| Guanyl-nucleotide exchange factor | 2.81 | 4.57e-03 |
| Microtubule binding motor protein | 3.03 | 8.99e-03 |
A false discovery rate (FDR) of <0.01 was considered significant.
DISCUSSION
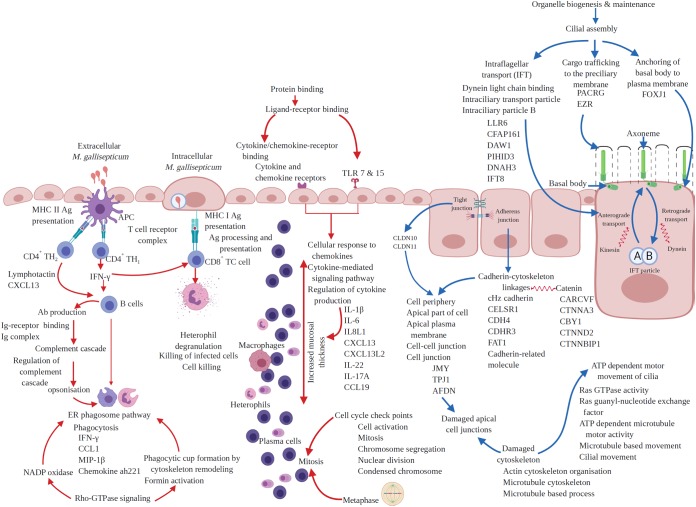
This study explored the transcriptional profiles of the trachea in unvaccinated chickens and chickens vaccinated with strain ts-304 2 weeks after challenge with M. gallisepticum strain Ap3AS. The RNA-seq data from three biological replicates from each group identified differences in gene expression between the two groups. The results identified the genes and pathways affected by infection and the protection against these affects provided by the vaccine. This is the first study to investigate the differences in the transcriptional profiles of the trachea of vaccinated and unvaccinated birds after infection with virulent M. gallisepticum. Previous studies have focused on the acute response to infection with M. gallisepticum (16, 17), while this study focused on the tracheal response at a more chronic stage. The similarities and differences between the findings of this study and previous transcriptome studies of acutely infected birds are summarized in Table S2. In addition, previous studies (16, 17, 28) have identified pathways and protein classes affected by infection using human orthologs of chicken genes for analyses, while this study used GO terms, protein classes, and biological pathways inferred directly from the chicken protein sequences, as summarized in Fig. 3.
FIG 3.
Chicken tracheal responses to chronic infection with M. gallisepticum. Red arrows, upregulated responses; blue arrows, downregulated responses. The text indicates genes, gene ontologies, protein classes, and pathways enriched with up- or downregulated genes. Created with BioRender.com.
The differential gene expression analysis, functional GO analysis, pathway analysis, and protein class analysis of the challenged-only birds in the study highlighted the adverse effect of infection on the formation and motor movement of cilia (Fig. 3). The effect of infection with M. gallisepticum on the genes and molecular pathways involved in ciliostasis and the loss of cilia have not been explored in detail previously, except in one recent study that detected downregulation of genes and pathways involved in formation and function of cilia in chickens coinfected with M. gallisepticum strain Rlow and low pathogenicity H3N8 avian influenza virus (28). As summarized in Fig. 3, infection with M. gallisepticum resulted in downregulation of cilial assembly, affecting the 3 main subpathways involved in ciliogenesis—intraflagellar transport (IFT), anchoring of the basal body to the plasma membrane, and cargo trafficking to the periciliary membrane (3, 29–34). The genes in the pathways involved in ciliogenesis were among the top 25 downregulated genes, and these downregulated genes are involved in formation and motility of cilia (35–45). Interestingly, the effect of infection with M. gallisepticum is more likely to be on retrograde IFT, as both downregulated MFs (Fig. S6) and the majority of the downregulated genes are involved in assembly and function of the dynein arms (Fig. 3). Dynein is a motor protein that mediates the retrograde movement of the IFT particles along the axoneme of cilia (32–34, 46). Apart from cilial assembly, downregulation of the ATP-dependent motor movement of cilia was reflected in the downregulation of functional GOs, genes (Fig. 3), and protein classes (Table 7). The hematoxylin and eosin (H&E)-stained tracheal sections revealed that the cilia were not obviously affected in the vaccinated-and-challenged group, suggesting the ability of the vaccine to protect the structural and functional integrity of the cilia.
The effect on impaired cellular cytoskeleton formation was also observed in the expression of genes involved in the formation of intercellular junctional complexes. Detailed analysis of functional GOs and downregulated genes (Fig. 3) revealed that M. gallisepticum had mainly affected the formation of apical junctional complexes, including adherens junctions and tight junctions. The downregulated genes JMY, TPJ1, and AFDN have been implicated in cell adhesion (47), maintenance of the intestinal epithelial barrier (48), and formation of both adherens and tight junctions (49). Furthermore, downregulation of cadherin, cadherin-related genes, and catenin-related genes (Fig. 3) also suggested impaired formation of adherens junctions, as formation of linkages between the protein cadherin and the cytoskeleton, regulated by the protein catenin (50, 51), is one of the major steps in formation of adherens junctions. Similarly, the members of the claudin multigene family make up the backbone of the tight junction strands in epithelial cells and are directly involved in their barrier function (52). Two claudin members were downregulated by infection with M. gallisepticum (Table S1). The functional role of adherens junctions in cell adhesion and cell signaling is well known (50, 51), and tight junctions have a role in cell signaling in addition to their role in maintenance of epithelial cell barriers (53). Therefore, the destruction of intercellular junctions by M. gallisepticum may result in impaired cell signaling and affect epithelial cell proliferation and development, resulting in disruption of the epithelial barrier, as suggested in previous studies (50, 51) and as seen in the histopathological assessment of the tracheal tissue sections in this study. Damage to the tracheal epithelial barrier may facilitate increased uptake of antigens via transepithelial migration of leukocytes, as is seen in the intestine (54). This is suggested by the presence of heterophils and macrophages in the tracheal sections of the challenged-only birds and the upregulation of the “junction adhesion molecule-like” gene, which encodes a protein that is expressed on granulocytes and monocytes and mediates the transepithelial migration of monocytes and granulocytes into the site of infection, a process stimulated by MIP-1 (55), the gene for which was also upregulated in this study. Vaccination appears to have prevented any destruction of intercellular junctions after challenge, as supported by the histopathological assessment of the tracheal tissues.
An increase in the tracheal mucosal thickness, caused by severe diffuse inflammatory cell infiltration, was seen in the histopathological assessment of the tracheal tissue sections from the challenged-only group. Inflammatory cell infiltration into the tracheal mucosa has been seen in chickens challenged with M. gallisepticum as soon as 1.5 hours after infection (14, 15, 17) and persists until at least 3 weeks after infection (19, 20). The enriched pathways and GOs (Fig. 3) indicating increased mitosis seen in this study probably reflect the activation and proliferation of inflammatory cells, suggesting the ability of infection to stimulate local proliferation of inflammatory cells that localize in the mucosa, increasing the mucosal thickness. Accumulations of T lymphocytes within the first 2 weeks after infection have been suggested to play a role in cytotoxic attack and promotion of antibody production, phagocytosis, and differentiation of other cells through cytokine production (19). These predicted effects are supported by the observations of upregulation of protein classes, GOs, genes, and pathways involved in Ig production, antigen processing and presentation by MHC I and II antigens, heterophil degranulation, phagocytosis, and chemokine and cytokine production in this study (Fig. 3). Recovery from infection is correlated with the onset of formation of follicles in the tracheal mucosa, accompanied by B cell infiltration from 3 weeks after infection (20). The absence of lymphocytic aggregations in the tracheal sections in this study suggests that the majority of the infiltrating cells are likely to be T cells and that the severe inflammatory response seen in the chronic phase of the disease is a result of immune dysregulation caused by M. gallisepticum (14–17, 20). There were some indications of B cell migration in the upregulated pathways and GOs (Fig. 3) and in the upregulation of the gene for the B cell chemoattractant CXCL13 and the lymphotactin gene (56, 57), as well as the immunoglobulin lambda-like polypeptide 1 gene, which is expressed on pre-B cells in humans (58). These findings suggest the onset of a humoral immune response at the end of the second week after infection. As in previous studies (20, 27), no inflammatory cells were seen in the tracheas of chickens challenged with M. gallisepticum after vaccination with strain ts-304. The polymeric Ig receptor, which mediates transcytosis of polymeric Ig from the basolateral to the apical cytoplasmic membrane of the cell (59), was downregulated in the challenged-only group, suggesting that vaccinated birds have increased capacity to secrete polymeric Igs such as IgA and mount a mucosal humoral immune response.
Even though the severe inflammatory response mounted in the trachea after infection with M. gallisepticum is believed to be mediated by TLR2 signaling (15) and a panel of cytokines and chemokines, no increased expression of the TLR2 gene was detected. However, upregulation of the TLR7 and TLR15 genes, and downregulation of the TLR5 gene, were detected. Beaudet et al. (16, 17) detected increased expression of the TLR2, TLR4, and TLR15 genes, but the increase in TLR2 gene expression was only seen up to 5 days after infection and was not as great as the upregulation of expression of the TLR4 and TLR15 genes. Increased expression of TLR7 is associated with cytokine production (60), and increased expression of TLR15 is associated with heterophil infiltration (61), while TLR5 is activated by bacterial flagellin (62), a protein not found in mycoplasmas. This may suggest that infection with M. gallisepticum could suppress TLR5-mediated responses against flagellated bacteria, such as E. coli, a common contributor to severe airsacculitis. The upregulated proinflammatory cytokine and chemokine genes in the challenged-only group included those for IL-1β, IL-6, IL-8L1, CXCL13, CXCL13L2, IL-22, IL-17A, CCL-19, lymphotactin, and MIP-1β, while the gene for CCL20 was downregulated. Previous studies at 24 hours after infection detected upregulation of the genes for IL-1β, IL-6, IL-8L1, CXCL13, CXCL13L2, lymphotactin, and MIP-1β, but not those for IL-22, IL-17A, and CCL-19, while the genes for CCL-20 and IL-12 were also upregulated at this early stage of infection (14, 15). The transcriptional profile of the trachea at 1, 3, 5, and 7 days after infection with M. gallisepticum strain Rlow was also found to be similar, but the gene for IL-22 was also upregulated, and the genes for IL-12p40 and CCL20 were downregulated by day 5 after infection (16, 17). At 8 days after infection with M. gallisepticum strain Rlow, the gene for IL-8 was also downregulated (14). IL-17A, the gene for which was found to be upregulated in the study described here, induces secretion of other proinflammatory cytokines, including IL-1β, IL-6, and IL-8 (63–65). Upregulation of IL-17A may indicate exacerbation of inflammation by M. gallisepticum, as immunopathological changes in response to infection with Eimeria tenella are enhanced by IL-17A (64). The detection of transcription of 2 of the 3 copies of the chicken CXCL13 gene (57) indicated that they play a role in inflammation and may contribute as B cell chemoattractants in concert with lymphotactin (56), which is a known chicken B-cell attractant. CCL19 may also contribute to the inflammatory response as a T-cell chemoattractant, as has been suggested in infections with infectious bursal disease virus (66). Although CCL20 has a functional role in inflammation (67), its gene appears to be upregulated only during the acute stage of infection (15), suggesting that CCL20 aids in establishment of the inflammatory response. Given all these findings, the severe inflammatory cell infiltration seen in the tracheal mucosa seemed to be primarily mediated by increased expression of proinflammatory cytokines, chemokines, and TLRs.
While expression of the IL-1β gene was upregulated, the genes for two receptors of IL-1, the IL-1 receptor 1 (IL-1R1) and IL-1R2, and the gene for the IL-1 receptor-associated protein (IL-1R-AcP) were downregulated. Both IL-1R1 and IL-1R2 bind IL-1α, IL-1β, and the IL-1 receptor antagonist (IL-1Ra) (68). There was no difference in the expression of IL-1Ra between the two groups in our study. It has been shown that binding of IL-1 to IL-1R1 results in negative feedback, leading to a decrease in the level of IL-1β (69), so the decreased expression of IL-1R1 would maintain a higher level of IL-1β by reducing this negative feedback. Moreover, the downregulation of IL-1R2 would also increase the level of active IL-1β, because IL-1R2 is a “decoy” receptor that reduces the availability of IL-1β (70).
In contrast to previous studies, we also observed upregulation of the genes for IFN-γ, chemokine ah221, and CCL1. This could be due to the migration of macrophages into the mucosa at 2 weeks after infection, as chemokine ah221 and CCL1 are members of the monocyte and macrophage chemotactic families, respectively (57, 71), and IFN-γ is a TH1-type cytokine and is a potent activator of macrophages (72). The role of macrophages in infections with M. gallisepticum is not yet clear; however, they are known to play a role in elimination of other mycoplasmas by phagocytosis (73–76). It has been suggested that incomplete activation of macrophages during infection with M. gallisepticum could be a result of immune dysregulation, as macrophage migration into the site of infection is not accompanied by increased levels of IFN-γ and IL-12. (16, 77). Our study detected evidence for enhanced phagocytosis in both pathway and GO analyses, associated with the upregulation of the IFN-γ gene (Fig. 3). The Rho GTPase pathway is likely to be the primary mediator of phagocytosis (78) (Fig. 3), and our findings suggest that there is macrophage-driven phagocytosis at 2 weeks after infection, coincident with the onset of protective immunity, after a phase of immune dysregulation. The gene for TNF superfamily member 15 was the only downregulated cytokine gene. This cytokine is known to mediate the innate immune response by inducing apoptosis of infected cells to provide protective immunity against Eimeria (79). The downregulation of this gene during infection with M. gallisepticum further suggests that the immune response is not protective but, rather, is dysregulated (16, 17). Previous studies detected increased expression of the CXCL14 gene up to 8 days after infection and the IL-12 gene in the first 24 hours after infection (14, 15), but neither the CXCL14 genes nor the IL-12 gene were differentially expressed in the challenged-only birds in our study. This suggests that CXCL14 only plays a role in the very early stages of the inflammatory response and that the TH1 response at 2 weeks after infection may be weak, as IL-12 is a TH1 type cytokine that regulates the function of cytotoxic T cells (80). These differences in TLR, cytokine, and chemokine expression profiles between our study and earlier studies are probably due to our focus on a later stage of infection. Differences could also be due to sampling methods, as Beaudet et al. (16, 17) and Mohammed et al. (14) used tracheal washings, while we used tracheal mucosal tissue. Thus, our findings will reflect gene expression in cells in the mucosal epithelial layer as well as those that have been lost from the mucosal surface. In addition, our comparison of challenged-only birds to vaccinated-and-challenged birds might have resulted in the differences, because Beaudet et al. (16, 17), Mohammed et al. (14), and Majumder and Silbart (77) compared infected birds to uninfected birds.
This study has enhanced our understanding of the tracheal response to chronic infection with virulent M. gallisepticum, highlighting the role of pathways and genes involved in immune dysregulation and the role of damage to ciliary function and intercellular junctions in the pathogenesis of this disease, as well as the protection afforded by the new vaccine strain against the adverse effects induced by M. gallisepticum.
MATERIALS AND METHODS
Experimental vaccination and infection of chickens.
Two groups of 20 birds each were experimentally vaccinated against M. gallisepticum and/or infected with M. gallisepticum (Table 1). Briefly, White Leghorn chicks were hatched from specific-pathogen-free (SPF) eggs (Australian SPF services Pty Ltd., Woodend, Victoria, Australia) and raised to 3 weeks of age. They were screened using serology and PCR 7 days prior to vaccination to confirm the absence of prior infection with M. gallisepticum and Mycoplasma synoviae. At 3 weeks of age, birds in the vaccinated-and-challenged group were inoculated by eye drop with 30 μl of the Vaxsafe MG (strain ts-304) vaccine containing 106.0 color-changing units (CCU) of the ts-304 strain, while those in the challenged-only group were inoculated by eye drop with 30 μl of sterile Merial diluent (Marek’s disease vaccine sterile diluent; Merial Select Inc., Gainesville, GA, USA). Four weeks after vaccination, all birds were exposed to an aerosol challenge with the M. gallisepticum wild-type strain Ap3AS by nebulization of a culture containing 106.5 CCU/ml into a purpose-built infection chamber for 40 minutes using an established protocol (81; Anna Kanci Condello et al., submitted for publication). Two weeks after challenge, all birds were humanely euthanized and necropsied. All procedures involving animals were reviewed and approved by the University of Melbourne Animal Ethics Committee under approval number 1714181.1.
Sample collection.
Approximately 2– to 3-mm-thick tracheal cross sections from the upper, middle, and lower trachea were collected from each bird and placed in 10% formalin for histopathology. The rest of the trachea was collected into RNAlater stabilization solution (Invitrogen, Carlsbad, CA, USA) and stored at –20°C until RNA extraction.
Histopathological examination of the tracheal mucosal thickness.
The formalin-fixed tracheal cross sections were embedded in paraffin, and 3-μm-thick sections were stained with hematoxylin and eosin (H&E). The mucosal thickness of the upper, middle, and lower trachea of each bird was calculated by averaging measurements taken at four points transected by vertical and horizontal lines under the ×10 power objective lens of a light microscope (10, 20). The mean mucosal thicknesses of the upper, middle, and lower trachea of the challenged-only group were compared to those of the vaccinated-and-challenged group using unpaired t tests in GraphPad Prism version 8.3.0 for Windows (GraphPad Software, La Jolla, CA, USA).
Total RNA extraction.
Total RNA was extracted separately from the trachea of three birds from each group as outlined in Table 1. Tracheal tissue samples from the three birds in the vaccinated-and-challenged group that had the lowest average mucosal thickness and three birds in the challenged-only group that had the greatest average mucosal thickness were selected for total RNA extraction (Fig. 4). Briefly, tracheas were halved along their longitudinal axis, and the mucosa was separated using sterile forceps. The mucosae were disrupted and homogenized in 600 μl of RLT buffer (RNeasy minikit; Qiagen, Hilden, Germany) using Discofix 3-way stopcocks (B. Braun, Melsungen, Germany). Total RNA was extracted from 20-mg samples of each of the tracheal mucosal homogenates using the RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. All the samples were treated with DNase using the Turbo DNA-free kit (Invitrogen, Carlsbad, CA, USA), and the RNA was then subjected to the cleanup steps in the RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturers’ instructions. RNA quality was confirmed using the Agilent 4200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA), and samples with RNA integrity numbers (RIN) of >7 were retained for further processing.
FIG 4.
Mucosal thicknesses of the upper, middle, and lower trachea of individual birds. Red dots indicate the mucosal thicknesses of the birds chosen for RNA-seq analysis. A P value of <0.05 was considered significant; ****, P < 0.0001; ***, P < 0.001.
Separation of chicken (eukaryotic) mRNA.
Total RNA was subjected to a polyadenosine [poly(A)] capture step to isolate the eukaryotic mRNA using a NEBNext poly(A) mRNA Magnetic Isolation Module (New England BioLabs, Ipswich, MA, USA) according to the manufacturer’s instructions (82). Briefly, poly(A) selection was performed using oligo(dT) magnetic beads on 5 μg of total RNA per sample to obtain mRNA. Poly(A)-selected RNA was cleaned and concentrated using a Zymo RNA Clean & Concentrator-25 kit (Zymo Research Corporation, Irvine, CA, USA) as recommended by the manufacturer, and the quality of the RNA was confirmed using an RNA high-sensitivity tape assay in a 4200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA).
Illumina sequencing.
The cDNA libraries were constructed using the TruSeq stranded mRNA library preparation kit (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s instructions, starting with the fragmentation of mRNA. Briefly, 10 to 400 ng of purified mRNA was fragmented using 20 μl of the Fragment, Prime, Finish mix, and fragmented RNA was used to synthesize first-strand cDNA using reverse transcriptase and random hexamer primers. Second-strand cDNA synthesis was performed using dUTP, DNA polymerase, and RNase. The products were amplified by PCR and purified after end repair and adaptor ligation. The correct fragment size (∼260 bp) and the purity of the libraries were confirmed using the Agilent 2200 TapeStation System. Libraries were then normalized to 1 nM, pooled, denatured, and sequenced on the NextSeq 500 sequencing platform (Illumina Inc., San Diego, CA, USA) to obtain 150-bp paired-end reads.
Quality control and preprocessing of RNA-seq reads.
The quality of the raw sequence data was assessed using FastQC version 0.11.8 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trim Galore version 0.4.5 (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) was used to remove the Illumina sequencing adapter sequences, and bases with a PHRED quality score of ≤20 and nonpaired reads were removed using BBMap version 38.22 (https://sourceforge.net/projects/bbmap/). The annotated chicken genome (general feature format, gene transfer format, and FASTA format) was downloaded from the Ensembl database release 94 (83), and the mRNAs for protein-coding genes were extracted, indexed, and used for high-quality paired-read RNAseq mapping (84) using Bowtie2 version 2.3.4.3 (85). The expected read count per gene was inferred using RSEM version 1.3.1 (84, 86).
Differential gene expression analysis.
Data exploration and differential gene transcription analysis were conducted using the expected counts per gene and Limma version 3.40.6 (87), Glimma version 1.12.0 (88), and edgeR version 3.26.8 (89) R/Bioconductor packages in the RStudio environment version 1.2.1335 following recommended guidelines (90). In brief, counts-per-million (CPM) were first calculated for each gene to standardize for differences in library size. Genes with low expression (equal to or below a threshold CPM value of 1; equivalent to a log2 CPM value of 0) in ≥3 samples were then excluded from further analysis. Filtered data were normalized for differences in distributions of expressed transcripts between samples using the trimmed mean of M-values (TMM) method (91). Heteroscedasticity was removed from count data, and raw CPMs were converted to log2 CPMs using voom (92). A linear model was fit to the log2 CPM values, which were assumed to be normally distributed, and the mean-variance relationship was accommodated using precision weights calculated using the voom function (92) to compare the two groups. The genes that had a log2 fold change of 1 (i.e., 2-fold) at a false discovery rate (FDR) of <0.01 were considered significant. Up- and downregulated genes reported in this study are the genes of the challenged-only group with abundances significantly different from those in the vaccinated-and-challenged group.
Enrichment of genes within gene ontology categories.
Differentially expressed genes in the challenged-only group compared with the vaccinated-and-challenged group were further analyzed with gene ontology (GO) enrichment using the Bioconductor R package topGO version 2.36.0 (93). Briefly GO-IDs linked to each protein coding gene of Gallus gallus (chicken) were extracted using the biomaRt annotation tool version 2.40.0 (94) to construct a G. gallus GO universe. Significantly up- and downregulated genes were then analyzed separately within the G. gallus GO universe to identify molecular functions (MFs), biological processes (BPs), and cellular components (CCs) enriched with up- or downregulated genes based on GO at an FDR of <0.01. GO terms significantly enriched with up- and downregulated genes were further summarized by removing redundant GO terms using the REVIGO Web server (95).
Pathway and protein class analysis.
Identification of biological pathways and protein classes enriched with up- and downregulated genes was performed using the Reactome and PANTHER databases available in the PANTHER classification tool version 14.1 (96). Enriched pathways and protein classes with an FDR of <0.01 were reported as significant in this study.
Data availability.
RNA-seq data from this study are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number PRJNA578757.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Australian Research Council Linkage Project grant LP160101105.
Sathya N. Kulappu Arachchige was supported by Melbourne International Fee Remission and Melbourne Research scholarships.
We gratefully acknowledge the assistance of June Daly and Angela Chircop for care of the birds, Faye Docherty and Paul Benham for their assistance in tissue processing and staining, and Stephen Wilcox for his assistance in cDNA library preparation and Illumina sequencing at the Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC, Australia.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Razin S, Yogev D, Naot Y. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62:1094–1156. doi: 10.1128/MMBR.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razin S. 1999. Adherence of pathogenic mycoplasmas to host cells. Biosci Rep 19:367–372. doi: 10.1023/a:1020204020545. [DOI] [PubMed] [Google Scholar]
- 3.Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK. 2003. Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp Cell Res 284:249–261. doi: 10.1016/S0014-4827(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 4.Papazisi L, Frasca S Jr, Gladd M, Liao X, Yogev D, Geary SJ. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect Immun 70:6839–6845. doi: 10.1128/iai.70.12.6839-6845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajima M, Nunoya T, Yagihashi T. 1979. An ultrastructural study on the interaction of Mycoplasma gallisepticum with the chicken tracheal epithelium. Am J Vet Res 40:1009–1014. [PubMed] [Google Scholar]
- 6.Abu-Zahr M, Butler M. 1976. Growth, cytopathogenicity and morphology of Mycoplasma gallisepticum and M. gallinarum in tracheal explants. J Comp Pathol 86:455–463. doi: 10.1016/0021-9975(76)90014-1. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento ER, Pereira V, Nascimento M, Barreto M. 2005. Avian mycoplasmosis update. Rev Bras Cienc Avic 7:1–9. doi: 10.1590/S1516-635X2005000100001. [DOI] [Google Scholar]
- 8.Ley D, Yoder H. 1997. Mycoplasma gallisepticum infection, p 194–207. In Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (ed), Diseases of poultry, 9th ed Iowa State University Press, Ames, IA. [Google Scholar]
- 9.Hod I, Yegana Y, Herz A, Levinsohn S. 1982. Early detection of tracheal damage in chickens by scanning electron microscopy. Avian Dis 26:450–457. doi: 10.2307/1590121. [DOI] [PubMed] [Google Scholar]
- 10.Nunoya T, Tajima M, Yagihashi T, Sannai S. 1987. Evaluation of respiratory lesions in chickens induced by Mycoplasma gallisepticum. Nihon Juigaku Zasshi 49:621–630. doi: 10.1292/jvms1939.49.621. [DOI] [PubMed] [Google Scholar]
- 11.Dykstra MJ, Levisohn S, Fletcher OJ, Kleven SH. 1985. Evaluation of cytopathologic changes induced in chicken tracheal epithelium by Mycoplasma gallisepticum in vivo and in vitro. Am J Vet Res 46:116–122. [PubMed] [Google Scholar]
- 12.Nunoya T, Kanai K, Yagihashi T, Hoshi S, Shibuya K, Tajima M. 1997. Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathol 26:391–398. doi: 10.1080/03079459708419221. [DOI] [PubMed] [Google Scholar]
- 13.Nunoya T, Yagihashi T, Tajima M, Nagasawa Y. 1995. Occurrence of keratoconjunctivitis apparently caused by Mycoplasma gallisepticum in layer chickens. Vet Pathol 32:11–18. doi: 10.1177/030098589503200102. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed J, Frasca S Jr, Cecchini K, Rood D, Nyaoke AC, Geary SJ, Silbart LK. 2007. Chemokine and cytokine gene expression profiles in chickens inoculated with Mycoplasma gallisepticum strains Rlow or GT5. Vaccine 25:8611–8621. doi: 10.1016/j.vaccine.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 15.Majumder S, Zappulla F, Silbart LK. 2014. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-κB dependent pathway. PLoS One 9:e112796. doi: 10.1371/journal.pone.0112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaudet J, Tulman ER, Pflaum K, Canter JA, Silbart LK, Geary SJ. 2019. Immunologic pathways in protective versus maladaptive host responses to attenuated and pathogenic strains of Mycoplasma gallisepticum. Infect Immun 87:e00613-18. doi: 10.1128/IAI.00613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaudet J, Tulman E, Pflaum K, Liao X, Kutish G, Szczepanek S, Silbart L, Geary S. 2017. Transcriptional profiling of the chicken tracheal response to virulent Mycoplasma gallisepticum strain Rlow. Infect Immun 85:e00343-17. doi: 10.1128/IAI.00343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soeripto, Whithear KG, Cottew GS, Harrigan KE. 1989. Virulence and transmissibility of Mycoplasma gallisepticum. Aust Vet J 66:65–72. doi: 10.1111/j.1751-0813.1989.tb09746.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaunson J, Philip C, Whithear K, Browning G. 2000. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 146:1223–1229. doi: 10.1099/00221287-146-5-1223. [DOI] [PubMed] [Google Scholar]
- 20.Gaunson J, Philip C, Whithear K, Browning G. 2006. The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine 24:2627–2633. doi: 10.1016/j.vaccine.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Soeripto, Whithear K, Cottew G, Harrigan K. 1989. Immunogenicity of Mycoplasma gallisepticum. Aust Vet J 66:73–77. doi: 10.1111/j.1751-0813.1989.tb09747.x. [DOI] [PubMed] [Google Scholar]
- 22.Levisohn S, Kleven S. 2000. Avian mycoplasmosis. Rev Sci Tech Off Int Epiz 19:425–442. doi: 10.20506/rst.19.2.1232. [DOI] [PubMed] [Google Scholar]
- 23.Kleven S. 2008. Control of avian mycoplasma infections in commercial poultry. Avian Dis 52:367–374. doi: 10.1637/8323-041808-Review.1. [DOI] [PubMed] [Google Scholar]
- 24.Whithear K. 1996. Control of avian mycoplasmoses by vaccination. Rev Sci Tech 15:1527–1554. doi: 10.20506/rst.15.4.985. [DOI] [PubMed] [Google Scholar]
- 25.Evans R, Hafez Y. 1992. Evaluation of a Mycoplasma gallisepticum strain exhibiting reduced virulence for prevention and control of poultry mycoplasmosis. Avian Dis 36:197–201. doi: 10.2307/1591490. [DOI] [PubMed] [Google Scholar]
- 26.Whithear K, Soeripto G, Ghiocas E. 1990. Efficacy of the ts11 attenuated Mycoplasma gallisepticum vaccine strain. IOM Lett 1:361–362. [Google Scholar]
- 27.Shil PK, Kanci A, Browning GF, Marenda MS, Noormohammadi AH, Markham PF. 2011. GapA+ Mycoplasma gallisepticum ts-11 has improved vaccine characteristics. Microbiology 157:1740–1749. doi: 10.1099/mic.0.046789-0. [DOI] [PubMed] [Google Scholar]
- 28.Canter JA, Tulman ER, Beaudet J, Lee D, May M, Szczepanek SM, Geary SJ. 2019. Transcriptional and pathological host responses to co-infection with virulent or attenuated Mycoplasma gallisepticum and low pathogenic avian influenza A virus in chickens. Infect Immun 88:e00607-19. doi: 10.1128/IAI.00607-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholey JM. 2003. Intraflagellar transport. Annu Rev Cell Dev Biol 19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum JL, Witman GB. 2002. Intraflagellar transport. Nat Rev Mol Cell Biol 3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 31.Porter ME. 1996. Axonemal dyneins: assembly, organization, and regulation. Curr Opin Cell Biol 8:10–17. doi: 10.1016/s0955-0674(96)80042-1. [DOI] [PubMed] [Google Scholar]
- 32.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. 1998. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piperno G, Mead K. 1997. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A 94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. 1993. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A 90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Ng CP, Habacher H, Roy S. 2008. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet 40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 36.Dawe HR, Farr H, Portman N, Shaw MK, Gull K. 2005. The Parkin co-regulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J Cell Sci 118:5421–5430. doi: 10.1242/jcs.02659. [DOI] [PubMed] [Google Scholar]
- 37.Inaba Y, Shinohara K, Botilde Y, Nabeshima R, Takaoka K, Ajima R, Lamri L, Takeda H, Saga Y, Nakamura T, Hamada H. 2016. Transport of the outer dynein arm complex to cilia requires a cytoplasmic protein Lrrc6. Genes Cells 21:728–739. doi: 10.1111/gtc.12380. [DOI] [PubMed] [Google Scholar]
- 38.Coutton C, Vargas AS, Amiri-Yekta A, Kherraf Z-E, Ben Mustapha SF, Le Tanno P, Wambergue-Legrand C, Karaouzène T, Martinez G, Crouzy S, Daneshipour A, Hosseini SH, Mitchell V, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Deleuze J-F, Boland A, Hennebicq S, Satre V, Jouk P-S, Thierry-Mieg N, Conne B, Dacheux D, Landrein N, Schmitt A, Stouvenel L, Lorès P, El Khouri E, Bottari SP, Fauré J, Wolf J-P, Pernet-Gallay K, Escoffier J, Gourabi H, Robinson DR, Nef S, Dulioust E, Zouari R, Bonhivers M, Touré A, Arnoult C, Ray PF. 2018. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat Commun 9:686. doi: 10.1038/s41467-017-02792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkerson CG, King SM, Koutoulis A, Pazour GJ, Witman GB. 1995. The 78,000 M (r) intermediate chain of Chlamydomonas outer arm dynein isa WD-repeat protein required for arm assembly. J Cell Biol 129:169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paff T, Loges NT, Aprea I, Wu K, Bakey Z, Haarman EG, Daniels JMA, Sistermans EA, Bogunovic N, Dougherty GW, Höben IM, Große-Onnebrink J, Matter A, Olbrich H, Werner C, Pals G, Schmidts M, Omran H, Micha D. 2017. Mutations in PIH1D3 cause X-linked primary ciliary dyskinesia with outer and inner dynein arm defects. Am J Hum Genet 100:160–168. doi: 10.1016/j.ajhg.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, Brody SL. 2003. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J Cell Sci 116:4935–4945. doi: 10.1242/jcs.00830. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Qiao Y, Di Q, Le X, Zhang L, Zhang X, Zhang C, Cheng J, Zong S, Koide SS, Miao S, Wang L. 2009. Interaction of SH3P13 and DYDC1 protein: a germ cell component that regulates acrosome biogenesis during spermiogenesis. Eur J Cell Biol 88:509–520. doi: 10.1016/j.ejcb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Zhu L, Zhu Y, Cao J, Li S, Huang Q, Xu T, Huang X, Yan X, Zhu X. 2013. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat Cell Biol 15:1434–1444. doi: 10.1038/ncb2880. [DOI] [PubMed] [Google Scholar]
- 44.Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. 2005. Characterization of the intraflagellar transport complex B core direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem 280:27688–27696. doi: 10.1074/jbc.M505062200. [DOI] [PubMed] [Google Scholar]
- 45.Blouin J, Gehrig C, Armengot M, Rutishauser M, Jorissen M, Jeganathan D, Bartoloni L, Rossier C, Duriaux-Sail G, Scamuffa N. 2002. DNAH3: characterization of the sequence and mutation search in patients with primary ciliary dyskinesia. Eur J Hum Genet 10:138. [Google Scholar]
- 46.Signor D, Wedaman KP, Rose LS, Scholey JM. 1999. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell 10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coutts AS, Weston L, La Thangue NB. 2009. A transcription co-factor integrates cell adhesion and motility with the p53 response. Proc Natl Acad Sci U S A 106:19872–19877. doi: 10.1073/pnas.0906785106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Wu J, Han X, Tan Z, Jiao J. 2019. Effects of rumen-protected glucose on ileal microbiota and genes involved in ileal epithelial metabolism and immune homeostasis in transition dairy cows. Anim Feed Sci Technol 254:114199. doi: 10.1016/j.anifeedsci.2019.06.003. [DOI] [Google Scholar]
- 49.Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y. 2007. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci 120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- 50.Knust E, Bossinger O. 2002. Composition and formation of intercellular junctions in epithelial cells. Science 298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 51.Jamora C, Fuchs E. 2002. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 52.Tsukita S, Furuse M, Itoh M. 2001. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 53.Matter K, Balda MS. 2003. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 54.Berkes J, Viswanathan V, Savkovic S, Hecht G. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Y-L, Bai R, Chen CX, Liu D-Q, Liu Y, Zhang C-Y, Zen K. 2009. Role of junctional adhesion molecule-like protein in mediating monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 29:75–83. doi: 10.1161/ATVBAHA.108.177717. [DOI] [PubMed] [Google Scholar]
- 56.Rossi D, Sanchez-García J, McCormack W, Bazan J, Zlotnik A. 1999. Identification of a chicken “C” chemokine related to lymphotactin. J Leukoc Biol 65:87–93. doi: 10.1002/jlb.65.1.87. [DOI] [PubMed] [Google Scholar]
- 57.Kaiser P, Poh TY, Rothwell L, Avery S, Balu S, Pathania US, Hughes S, Goodchild M, Morrell S, Watson M, Bumstead N, Kaufman J, Young JR. 2005. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res 25:467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- 58.Schiff C, Bensmana M, Guglielmi P, Milili M, Lefranc M, Fougereau M. 1990. The immunoglobulin λ-like gene cluster (14.1, 16.1, and Fλ1) contains gene(s) selectively expressed in pre-B cells and is the human counterpart of the mouse λ5 gene. Int Immunol 2:201–207. doi: 10.1093/intimm/2.3.201. [DOI] [PubMed] [Google Scholar]
- 59.Wieland WH, Orzaez D, Lammers A, Parmentier HK, Verstegen MW, Schots A. 2004. A functional polymeric immunoglobulin receptor in chicken (Gallus gallus) indicates ancient role of secretory IgA in mucosal immunity. Biochem J 380:669–676. doi: 10.1042/BJ20040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Philbin VJ, Iqbal M, Boyd Y, Goodchild MJ, Beal RK, Bumstead N, Young J, Smith AL. 2005. Identification and characterization of a functional, alternatively spliced Toll‐like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 114:507–521. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nerren JR, He H, Genovese K, Kogut MH. 2010. Expression of the avian-specific toll-like receptor 15 in chicken heterophils is mediated by Gram-negative and Gram-positive bacteria, but not TLR agonists. Vet Immunol Immunopathol 136:151–156. doi: 10.1016/j.vetimm.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 62.Iqbal M, Philbin VJ, Withanage GSK, Wigley P, Beal RK, Goodchild MJ, Barrow P, McConnell I, Maskell DJ, Young J, Bumstead N, Boyd Y, Smith AL. 2005. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect Immun 73:2344–2350. doi: 10.1128/IAI.73.4.2344-2350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S, Faris L, Cox CM, Sumners LH, Jenkins MC, Fetterer RH, Miska KB, Dalloul RA. 2012. Molecular characterization and immunological roles of avian IL-22 and its soluble receptor IL-22 binding protein. Cytokine 60:815–827. doi: 10.1016/j.cyto.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Liu R, Song M, Hu Y, Pan B, Cai J, Wang M. 2013. Eimeria tenella: interleukin 17 contributes to host immunopathology in the gut during experimental infection. Exp Parasitol 133:121–130. doi: 10.1016/j.exppara.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Kim WH, Jeong J, Park AR, Yim D, Kim Y-H, Kim KD, Chang HH, Lillehoj HS, Lee B-H, Min W. 2012. Chicken IL-17F: identification and comparative expression analysis in Eimeria-infected chickens. Dev Comp Immunol 38:401–409. doi: 10.1016/j.dci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Wang Q, Ou C, Wei X, Yu Y, Jiang J, Zhang Y, Ma J, Liu X, Zhang G. 2019. CC chemokine ligand 19 might act as the main bursal T cell chemoattractant factor during IBDV infection. Poult Sci 98:688–694. doi: 10.3382/ps/pey435. [DOI] [PubMed] [Google Scholar]
- 67.Munoz I, Berges M, Bonsergent C, Cormier-Aline F, Quéré P, Sibille P. 2009. Cloning, expression and functional characterization of chicken CCR6 and its ligand CCL20. Mol Immunol 47:551–559. doi: 10.1016/j.molimm.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Dinarello CA. 1998. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol 16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 69.Norman JG, Fink GW, Sexton C, Carter G. 1996. Transgenic animals demonstrate a role for the IL-1 receptor in regulating IL-1β gene expression at steady-state and during the systemic stress induced by acute pancreatitis. J Surg Res 63:231–236. doi: 10.1006/jsre.1996.0253. [DOI] [PubMed] [Google Scholar]
- 70.Colotta F, Dower SK, Sims JE, Mantovani A. 1994. The type II ‘decoy’ receptor: a novel regulatory pathway for interleukin 1. Immunol Today 15:562–566. doi: 10.1016/0167-5699(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 71.Hughes S, Haynes A, O’Regan M, Bumstead N. 2001. Identification, mapping, and phylogenetic analysis of three novel chicken CC chemokines. Immunogenetics 53:674–683. doi: 10.1007/s002510100368. [DOI] [PubMed] [Google Scholar]
- 72.Weining KC, Schultz U, Münster U, Kaspers B, Staeheli P. 1996. Biological properties of recombinant chicken interferon‐γ. Eur J Immunol 26:2440–2447. doi: 10.1002/eji.1830261026. [DOI] [PubMed] [Google Scholar]
- 73.Jones T, Minick R, Yang L. 1977. Attachment and ingestion of mycoplasmas by mouse macrophages. II. Scanning electron microscopic observations. Am J Pathol 87:347–358. [PMC free article] [PubMed] [Google Scholar]
- 74.Erb P, Bredt W. 1979. Interaction of Mycoplasma pneumoniae with alveolar macrophages: viability of adherent and ingested mycoplasmas. Infect Immun 25:11–15. doi: 10.1128/IAI.25.1.11-15.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis JK, Delozier KM, Asa DK, Minion FC, Cassell G. 1980. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun 29:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bredt W. 1975. Phagocytosis by macrophages of Mycoplasma pneumoniae after opsonization by complement. Infect Immun 12:694–695. doi: 10.1128/IAI.12.3.694-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majumder S, Silbart LK. 2016. Interaction of Mycoplasma gallisepticum with chicken tracheal epithelial cells contributes to macrophage chemotaxis and activation. Infect Immun 84:266–274. doi: 10.1128/IAI.01113-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall A. 2012. Rho family GTPases. Biochem Soc Trans 40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 79.Park SS, Lillehoj HS, Hong YH, Lee SH. 2007. Functional characterization of tumor necrosis factor superfamily 15 (TNFSF15) induced by lipopolysaccharides and Eimeria infection. Dev Comp Immunol 31:934–944. doi: 10.1016/j.dci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Degen WG, van Daal N, van Zuilekom HI, Burnside J, Schijns VE. 2004. Identification and molecular cloning of functional chicken IL-12. J Immunol 172:4371–4380. doi: 10.4049/jimmunol.172.7.4371. [DOI] [PubMed] [Google Scholar]
- 81.Kanci A, Wawegama NK, Marenda MS, Mansell PD, Browning GF, Markham PF. 2017. Reproduction of respiratory mycoplasmosis in calves by exposure to an aerosolised culture of Mycoplasma bovis. Vet Microbiol 210:167–173. doi: 10.1016/j.vetmic.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 82.Pflaum K, Tulman ER, Beaudet J, Liao X, Geary SJ. 2016. Global changes in Mycoplasma gallisepticum phase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Infect Immun 84:351–355. doi: 10.1128/IAI.01092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, et al. 2018. Ensembl 2018. Nucleic Acids Res 46:D754–D761. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lev Lafayette GS, Vu L, Meade B. 2016. Spartan performance and flexibility: an HPC-cloud chimera. OpenStack Summit, Barcelona, Spain. doi: 10.4225/49/58ead90dceaaa. [DOI] [Google Scholar]
- 87.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su S, Law CW, Ah-Cann C, Asselin-Labat M-L, Blewitt ME, Ritchie ME. 2017. Glimma: interactive graphics for gene expression analysis. Bioinformatics 33:2050–2052. doi: 10.1093/bioinformatics/btx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Law CW, Alhamdoosh M, Su S, Smyth GK, Ritchie ME. 2016. RNA-seq analysis is easy as 1-2-3 with limma, Glimma and edgeR. F1000 Res 5:1408. doi: 10.12688/f1000research.9005.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson MD, Oshlack A. 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Law CW, Chen Y, Shi W, Smyth GK. 2014. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexa A, Rahnenfuhrer J. 2010. topGO: enrichment analysis for gene ontology. R Package Version 2:2010. [Google Scholar]
- 94.Durinck S, Spellman PT, Birney E, Huber W. 2009. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Supek F, Bošnjak M, Škunca N, Šmuc T. 2011. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. 2019. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanci Condello A, Underwood GJ, Shil PK, Noormohammadi AH, Markham PF, Wawegama NK, Browning GF. Vet Microbiol, in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data from this study are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number PRJNA578757.