Abstract
OBJECTIVE
Debate continues regarding the influence of dietary fats and sugars on the risk of developing metabolic diseases, including insulin resistance and nonalcoholic fatty liver disease (NAFLD). We investigated the effect of two eucaloric diets, one enriched with saturated fat (SFA) and the other enriched with free sugars (SUGAR), on intrahepatic triacylglycerol (IHTAG) content, hepatic de novo lipogenesis (DNL), and whole-body postprandial metabolism in overweight males.
RESEARCH DESIGN AND METHODS
Sixteen overweight males were randomized to consume the SFA or SUGAR diet for 4 weeks before consuming the alternate diet after a 7-week washout period. The metabolic effects of the respective diets on IHTAG content, hepatic DNL, and whole-body metabolism were investigated using imaging techniques and metabolic substrates labeled with stable-isotope tracers.
RESULTS
Consumption of the SFA diet significantly increased IHTAG by mean ± SEM 39.0 ± 10.0%, while after the SUGAR diet IHTAG was virtually unchanged. Consumption of the SFA diet induced an exaggerated postprandial glucose and insulin response to a standardized test meal compared with SUGAR. Although whole-body fat oxidation, lipolysis, and DNL were similar following the two diets, consumption of the SUGAR diet resulted in significant (P < 0.05) decreases in plasma total, HDL, and non-HDL cholesterol and fasting β-hydroxybutyrate plasma concentrations.
CONCLUSIONS
Consumption of an SFA diet had a potent effect, increasing IHTAG together with exaggerating postprandial glycemia. The SUGAR diet did not influence IHTAG and induced minor metabolic changes. Our findings indicate that a diet enriched in SFA is more harmful to metabolic health than a diet enriched in free sugars.
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of liver-related conditions, ranging from steatosis (characterized by an accumulation of intrahepatic triacylglycerol [IHTAG]) to nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma, and is the most prevalent liver disease worldwide (1). There appears to exist a bidirectional relationship between NAFLD and metabolic disease; the presence of NAFLD predicts the development of the metabolic syndrome/type 2 diabetes (T2D) and vice versa (2). Furthermore, the presence of NAFLD may exacerbate the metabolic abnormalities that occur with T2D (3). Obesity is a principal risk factor for NAFLD (1), and it is suggested that increased hepatic de novo lipogenesis (DNL) is an underlying cause in the development of NAFLD and/or insulin resistance (4,5). As excess nonlipid precursors (e.g., sugars and protein) can exacerbate hepatic DNL, dietary composition may be an important mediator of NAFLD development.
Observational studies report that diets high in fat and/or free sugars are associated with NAFLD, and a consistent finding from interventional studies is that hypercaloric diets enriched in fat or sugars increased IHTAG (6). Recently, Luukkonen et al. (7), reported that consuming 1,000 excess kcal/day as saturated fat (SFA) increased IHTAG content to a greater extent (55% relative increase) than consuming excess calories as unsaturated fatty acids (FA) (15% increase) or free sugars (33% increase); this effect was independent of changes in body weight. Others have reported that IHTAG increased to a greater extent with overfeeding of SFA compared with diets overfeeding either fructose (8) or n-6 polyunsaturated fat (9).
Of the limited number of studies that have investigated the influence of macronutrient composition in eucaloric diets, findings for the effects on IHTAG are inconsistent, with some demonstrating that diets enriched in fat/SFA (10) or sugars (11) increase IHTAG content, whereas others show no effect (12,13). To date, no study has directly compared eucaloric diets enriched in SFA or free sugars on IHTAG,and few studies assess the effect specific diets have on postprandial metabolism and intrahepatic FA synthesis and partitioning. Therefore, the aim of this study was to compare the effects of two eucaloric diets—one enriched in carbohydrate, specifically, free sugars, and the other enriched in fat, specifically, SFA—on IHTAG content, hepatic DNL, and hepatic and whole-body postprandial metabolism in overweight males. Based on the available evidence, we hypothesized that diets enriched in SFA or free sugars would differentially influence whole-body and hepatic FA metabolism, with an SFA-enriched diet increasing IHTAG to a greater extent than a sugar-enriched diet, and this would be driven by an increase in adipose tissue lipolysis, while a sugar-enriched diet would increase hepatic DNL.
Research Design and Methods
Participants
Participants were recruited from the Oxford BioBank (www.oxfordbiobank.org.uk) (14) (Supplementary Fig. 1). All volunteers were free from metabolic disease, had a BMI between 25 and 30 kg/m2, were not taking medication known to affect lipid or glucose metabolism, were nonsmokers, and consumed alcohol within recommended limits (1). The study was approved by the North West - Lancaster Research Ethics Committee (16/NW/0751), and all participants gave written informed consent.
Experimental Design
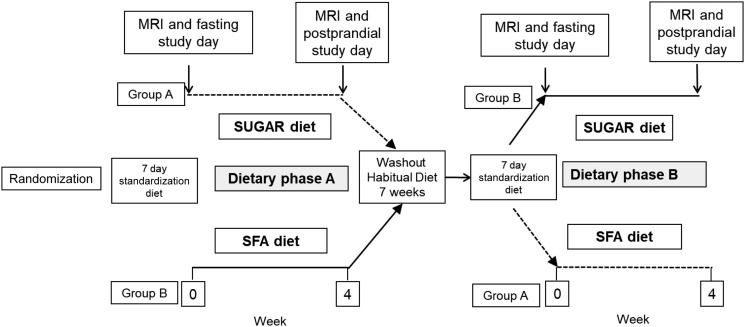
In a randomized crossover design, participants completed two 4-week dietary interventions separated by a 7-week washout period where they returned to their habitual diet. Participants also followed a 1-week standardization diet, based on the U.K. Eatwell plate, prior to starting the respective dietary interventions (i.e., before fasting study days). The two dietary interventions were 1) a relatively high-fat diet enriched in SFA (referred to as SFA), and 2) a relatively high-carbohydrate diet enriched with free sugars (referred to as SUGAR). Participants were randomized to the order in which they undertook each intervention diet (e.g., SFA then SUGAR or SUGAR then SFA) prior to the first study day through use of a random number generator by a statistician not involved in the running of the trial in order to avoid any effects of dietary sequence. Participants completed 3-day diet diaries during all standardized and experimental diet periods. Before beginning each dietary intervention, participants underwent a fasting study day, and upon completion of the intervention diet, participants underwent a postprandial study day that used stable-isotope tracers to investigate postprandial metabolism (Fig. 1).
Figure 1.
Overview of study design.
Anthropometric measures, IHTAG content, and fasting plasma biochemistry and lipidomics were assessed across each of the respective interventions (prediet vs. postdiet), while others, including postprandial plasma biochemistry, isotopic analysis, bile acid species, and indirect calorimetry, were compared between diets at the end of the respective dietary phase (postdiet vs. postdiet).
Fasting Study Day
Immediately before each dietary intervention, IHTAG was measured after an overnight fast by proton MRS (1H-MRS) using a 3 Tesla MRI scanner (Siemens Healthineers, Erlangen, Germany). A single voxel (20 × 20 × 20 mm3) was positioned in the posterior part of the left liver lobe, and both water-suppressed and non–water-suppressed stimulated acquisition mode (STEAM) measurements were performed (15). Sequence parameters were as follows: echo time 10 ms, mixing time 7 ms, and repetition time at least 2,000 ms for water-suppressed scans and at least 4,000 ms for non–water-suppressed scans with acquisitions synchronized to electrocardiogram. At the analysis stage, these two acquisitions were combined and the proportion of triacylglycerol (TAG) in the liver tissue was determined using the OXSA toolbox (16). Following the MRI scan, blood samples were collected from an antecubital vein, body weight and waist circumference measurements were made, and a DEXA scan was performed to assess body composition. Participants then had a consultation with the study dietitian, who provided diet sheets containing written and pictorial information about how to follow the respective experimental diets, including suggestions for suitable foods to be consumed. Participants were also provided with key foods to be consumed during experimental diets.
Experimental Diets
The SUGAR diet was composed of 20% total energy (TE) fat, 65% TE carbohydrate, and 15% TE protein and was enriched in free sugars (20% TE). Participants were advised to adopt a low-fat, high–glycemic index diet and were supplied with candy and sugar-sweetened beverages providing ∼100 g free sugars daily. The SFA diet was composed of 45% TE fat, 40% TE carbohydrate, and 15% TE protein and was enriched in SFA (20% TE). On this diet, participants were advised to include red meat and meat products, full-fat dairy products, and typical fast food items (e.g., hamburgers, pizza etc.) and were provided with foods (such as cheese/all-butter biscuits and milk chocolate) that provided ∼15 g SFA daily. Participants were instructed to maintain their usual body weight, physical activity levels, and alcohol intakes and were contacted weekly by a member of the research team to support adherence.
Postprandial Study Day
The evening before the postprandial study day participants consumed deuterated water (2H2O) (3 g/kg body water) (17). On the morning of the study day, participants arrived at the Clinical Research Unit after an overnight fast where a Teflon catheter was inserted into an antecubital vein for repeated blood sampling. A second catheter was inserted into the contralateral arm to allow for infusion of an isotopically labeled FA. Prior to the start of the FA infusion, blood samples were collected to determine fasting metabolite concentrations and background isotopic enrichment, and then the infusion of [2H2]palmitate (0.04 μmol/kg/min) bound to human albumin started and continued for the duration of the study period. The infusion was continued for 30 min to enable isotopic equilibrium, after which blood and breath samples (t0) were taken before participants were fed a standardized test meal containing 40 g carbohydrate, 40 g fat, and 200 mg [U13C]palmitic acid to trace the fate of dietary FA. Repeated blood and breath samples were taken 30, 60, 90, 120, 180, 240, 300, and 360 min after meal consumption. Breath samples were collected in EXETAINER tubes (Labco, High Wycombe, U.K.) to determine 13CO2 production. Indirect calorimetry was performed in the fasting state and 120 min after meal consumption using a GEM calorimeter (GEMNutrition Ltd., Cheshire, U.K.) to determine whole-body CO2 production, whole-body respiratory exchange ratio, and energy expenditure.
Analytical Procedures
Whole blood was collected into heparinized tubes (Sarstedt, Leicester, U.K.) and plasma immediately separated for analysis by centrifugation. Plasma glucose, NEFA, total and HDL cholesterol, TAG, β-hydroxybutyrate, adiponectin, and alanine aminotransferase were analyzed on a semiautomatic analyzer (ILab 600/650 clinical chemistry; Werfen, Warrington, U.K.). Plasma insulin levels were determined by radioimmunoassay as previously described (18). Analysis of plasma FGF21 and fetuin-A was performed via commercially available ELISAs (R&D Systems, Oxford, U.K.). Separation of chylomicrons (Svedberg flotation rate [Sf] >400) and VLDL-rich fractions (Sf 20–400) was made by sequential flotation using density gradient ultracentrifugation (17) and the Sf20–400 fraction separated by immunoaffinity chromatography (18).
FA Composition and Isotopic Enrichment
Total lipids were extracted from plasma and lipoprotein fractions and FA methyl esters prepared, and FA compositions (µmol/100 µmol total FA) were determined by gas chromatography (GC) from which palmitate concentrations were calculated (18).
Tracer enrichment in plasma NEFA, TAG, and lipoprotein-TAG fractions was determined by GC-mass spectrometry (19). Tracer-to-tracee ratios for [U13C]palmitate (M+16/M+0) and [2H2]palmitate (M+2/M+0) were calculated and multiplied by the corresponding palmitate concentration of the fraction to give tracer concentrations. The tracer-to-tracee ratio of a fasting sample obtained prior to tracer administration was subtracted from each sample to account for natural isotopic abundance. Analysis of 13C enrichment in breath CO2 samples and the relative rate of whole-body meal-derived FA oxidation was calculated (19) and corrected for lean mass.
Fasting and postprandial hepatic DNL was assessed by determining the incorporation of deuterium from 2H2O in plasma water (Finnigan GasBench II; Thermo Fisher Scientific, Paisley, U.K.) into VLDL-TAG palmitate using GC–mass spectrometry with monitoring ions with mass-to-charge ratios of 270 (M + 0) and 271 (M + 1) (20).
Plasma Lipidomics
Plasma lipidomics was performed as previously described (21). Quality criteria for the identified lipid metabolites were linearity R2 > 0.9 and coefficient of variation <20%.
Calculations and Statistics
The Ra of NEFA (Ra-NEFA) (22), the relative contribution of FA sources to VLDL-TAG (calculated at 360 min) (18), and HOMA-IR (23) were calculated as previously described. All data are presented as means ± SEM. Statistical analysis was performed using SPSS (version 21.0) for windows (SPSS). Paired t tests were used to make prediet to postdiet comparisons where appropriate (i.e., IHTAG, anthropometric measures, and fasting plasma biochemistry). Postprandial data were compared using a two-way repeated-measures ANOVA with time and experimental diet as within-subject effects, and Bonferroni post hoc analysis was performed where appropriate. Statistical significance was set at P < 0.05.
Results
Anthropometric and Fasting Biochemical Measures
Sixteen males (mean ± SEM age 47.9 ± 1.1 years and BMI 27.7 ± 0.4 kg/m2) completed the study. Body weight, BMI, and waist circumference significantly (P < 0.05) increased after consumption of the SFA but not the SUGAR diet (Table 1). Neither fasting plasma glucose nor insulin concentrations were altered in response to either dietary intervention (Table 1). Plasma total, HDL, and non-HDL cholesterol, adiponectin, and β-hydroxybutyrate concentrations all significantly (P < 0.05) decreased following the SUGAR diet but remained unchanged in response to the SFA diet (Table 1). Plasma FGF21 significantly (P < 0.05) increased in response to both diets, while fetuin-A was not affected by either diet (Table 1).
Table 1.
Characteristics of study participants and fasting biochemistry
| SFA | SUGAR | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Weight (kg) | 89.3 ± 2.6 | 90.8 ± 2.8* | 89.8 ± 2.5 | 90.1 ± 2.6 |
| BMI (kg/m2) | 27.7 ± 0.6 | 28.1 ± 0.6* | 27.9 ± 0.5 | 28.0 ± 0.5 |
| Waist (cm) | 98 ± 2 | 99 ± 2* | 99 ± 2 | 99 ± 2 |
| Fasting plasma biochemical parameters | ||||
| Glucose (mmol/L) | 5.3 ± 0.1 | 5.5 ± 0.1 | 5.3 ± 0.1 | 5.2 ± 0.1 |
| Insulin (mU/L) | 10.7 ± 0.9 | 9.2 ± 1.2 | 10.3 ± 1.4 | 9.3 ± 1.0 |
| HOMA-IR | 2.5 ± 0.3 | 2.2 ± 0.3 | 2.5 ± 0.3 | 2.2 ± 0.2 |
| NEFA (µmol/L) | 429 ± 56 | 378 ± 27 | 396 ± 42 | 410 ± 42 |
| Total cholesterol (mmol/L) | 4.8 ± 0.2 | 4.7 ± 0.2 | 5.0 ± 0.2 | 4.4 ± 0.2* |
| HDL cholesterol (mmol/L) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1* |
| Non-HDL cholesterol (mmol/L) | 3.6 ± 0.2 | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.4 ± 0.1* |
| TAG (mmol/L) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| 3-OHB (µmol/L) | 85.7 ± 33.1 | 45.1 ± 6.5 | 73.1 ± 16.5 | 39.6 ± 5.0* |
| Adiponectin (µg/mL) | 8.1 ± 0.9 | 8.8 ± 1.0 | 9.5 ± 0.9 | 7.2 ± 0.9* |
| ALT (IU/L) | 11 ± 1 | 12 ± 2 | 10 ± 1 | 9 ± 1 |
| FGF21 (pg/mL) | 138.7 ± 13.6 | 197.3 ± 24.3* | 193 ± 25.6 | 248.1 ± 37.7* |
| Fetuin-A (µg/mL) | 1,160.3 ± 39.1 | 1,119.4 ± 50.8 | 1,199.4 ± 61.9 | 1,154.1 ± 67.5 |
| Indirect calorimetry measures | ||||
| Fasting RQ | 0.73 ± 0.02 | 0.76 ± 0.01 | ||
| Postprandial RQ | 0.81 ± 0.03 | 0.82 ± 0.02 | ||
| Fasting REE (kcal) | 1,817.5 ± 81.2 | 1,665.0 ± 56.6 | ||
| Postprandial REE (kcal) | 1,829.6 ± 54.7 | 1,741.8 ± 84.8 | ||
Data are means ± SEM. ALT, alanine aminotransferase; FGF21, fibroblast growth factor 21; HOMA-IR, HOMA of insulin resistance; 3-OHB, β-hydroxybutyrate; Pre, before consumption of SFA or SUGAR diet for 4 weeks; Post, after consumption of SFA or SUGAR diet for 4 weeks; TAG, triacylglycerol. n = 16.
P < 0.05 prediet vs. postdiet.
Dietary Intakes
There was no difference in self-reported dietary intake between the two standardized run-in periods prior to the experimental interventions (Supplementary Table 1). Self-reported energy intake was greater during the SFA compared with the SUGAR diet (mean ± SEM 2,697 ± 126 kcal vs. 2,405 ± 88 kcal, respectively; P < 0.05). During the SFA diet, participants reported consuming 46 ± 1% TE from fat and 21 ± 1% TE from SFA, which was significantly (P < 0.05) greater than the fat and SFA consumed during the SUGAR diet (20 ± 2% total fat and 6 ± 17% SFA). In contrast, the relative contributions of carbohydrates (62 ± 2% TE) and free sugars (23 ± 23% TE) were significantly (P < 0.05) greater during the SUGAR compared with the SFA diet (35 ± 13% and 6 ± 1% for carbohydrate and free sugars, respectively) (Supplementary Table 1). There were no differences in the contribution of protein or alcohol between the two diets (Supplementary Table 1).
FA Composition and Lipid Profile
The FA composition of VLDL-TAG was analyzed as a biomarker of dietary FA intake. The FA composition of VLDL-TAG was similar after the SUGAR and SFA diets (Supplementary Table 2), except for pentadecanoic acid (15:0), a marker of dairy fat intake, which was greater after the SFA compared with the SUGAR diet (0.5 ± 0.1% SFA vs. 0.3 ± 0.1% SUGAR; P < 0.05). We also assessed the overall lipid profile of plasma after the diets, and although lipidomics analysis indicated lower acylcarnitines after consumption of the SUGAR and SFA diets, there were no overt differences in the profile (Supplementary Figs. 2 and 3).
IHTAG Content
IHTAG significantly (P < 0.05) increased by 39.0 ± 10.0% following the SFA diet, while it remained unchanged in response to the SUGAR diet (Fig. 2A). Linear regression indicated the increase in body weight observed after the SFA diet explained only 17.2% (P = NS) of the variance in IHTAG, suggesting the increase in IHTAG following SFA occurred independently of changes in body weight (Supplementary Fig. 4).
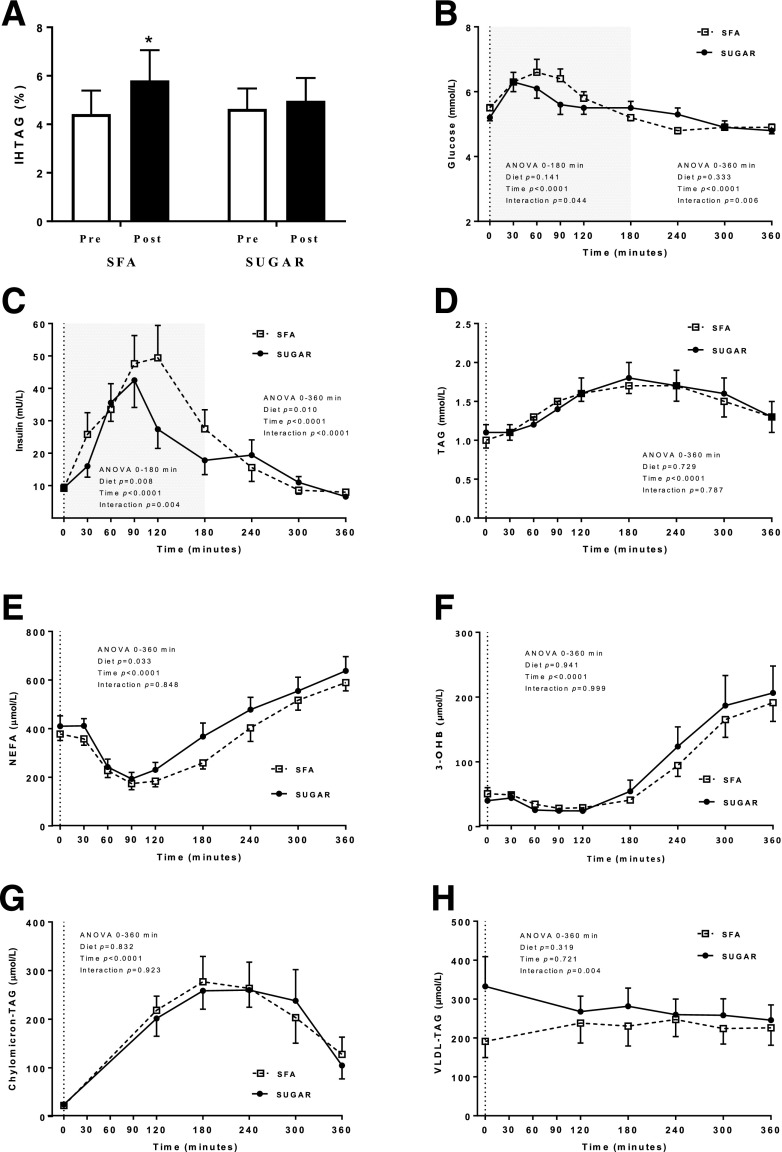
Figure 2.
A: IHTAG percentage before (pre) and after (post) consumption of SFA or SUGAR diet for 4 weeks. Systemic plasma glucose (B), insulin (C), TAG (D), NEFA (E), β-hydroxybutyrate (3-OHB) (F), chylomicron-TAG (G), and VLDL-TAG (H) following a standardized test meal conducted after consumption of SFA or SUGAR diet for 4 weeks. Data are presented are means ± SEM. n = 16 (A–G); n = 13 (H). *P < 0.05 prediet to postdiet. Dotted lines indicate consumption of test meal. Shading on B and C refers to additional statistical analysis performed due to the dynamic glucose and insulin response known to occur during the first 180 min of the postprandial period.
Postprandial Biochemical Measures
As humans spend a large proportion of the day in the postprandial state (24), we assessed the metabolic response to a standardized test meal at the end of each dietary intervention phase. Although there were no differences in fasting plasma glucose or insulin concentrations in response to the two diets, the postprandial excursions were greater and more prolonged for plasma glucose (diet × time interaction; P < 0.05) (Fig. 2B) and plasma insulin (main effect of diet, P < 0.05, and diet × time interaction, P < 0.05) (Fig. 2C) after consumption of the SFA compared with SUGAR diet. These differences remained significant (P < 0.05) whether comparisons were made between the early (0–180 min) postprandial responses or for the entire postprandial period (0–360 min). Postprandial plasma TAG concentrations were similar following the two diets (Fig. 2D). However, there was a significant main effect (P < 0.05) of diet for plasma NEFA concentrations across the postprandial period, where concentrations were greater after SUGAR compared with SFA (Fig. 2E). There was no difference in postprandial β-hydroxybutyrate concentrations following the diets (Fig. 2F), and there were not differences in the postprandial plasma chylomicron-TAG response (Fig. 2G). Although not significantly different, fasting plasma VLDL-TAG concentrations were higher after the SUGAR diet, which may in part explain the significant (P < 0.05) diet × time interaction for postprandial plasma VLDL-TAG (Fig. 2H).
Ra-NEFA
Increased lipolysis of adipose tissue has previously been observed in response to an SFA-enriched diet (7). We therefore investigated postprandial plasma Ra-NEFA after SFA and SUGAR and found no significant difference between the diets (Fig. 3A).
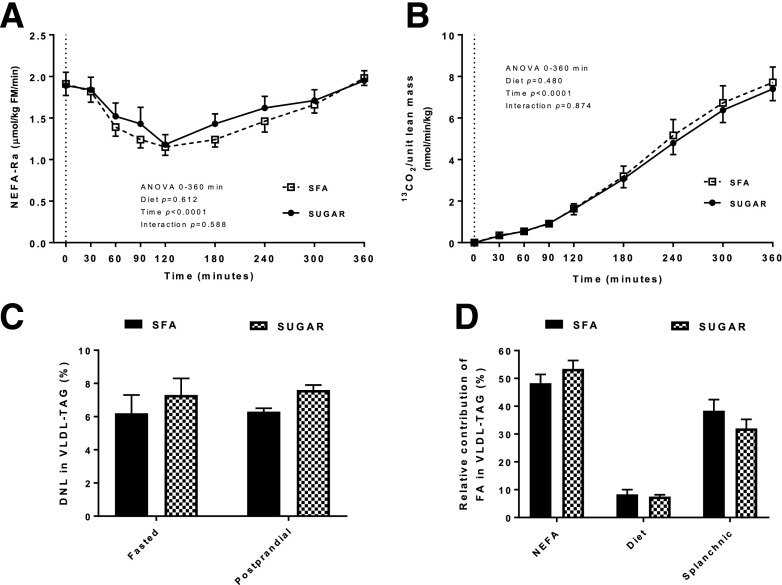
Figure 3.
Plasma NEFA Ra (A), expired 13CO2 (B), hepatic DNL (C), and the relative contribution of FA derived from systemic NEFA, diet, and splanchnic sources (i.e., from visceral adipose tissue and the intrahepatic pool) to VLDL-TAG (calculated at 360 min) (D) following a standardized test meal conducted after consumption of an SFA SUGAR diet for 4 weeks. Data are presented are means ± SEM. n = 16. Dotted lines indicate consumption of test meal. FM, fat mass.
FA Oxidation
The appearance of 13C (from meal [U13C]palmitate) in expired CO2 was similar after consumption of both diets (Fig. 3B), as was the recovery of tracer given (6.3 ± 0.8% SFA vs. 6.0 ± 0.6% SUGAR), indicating no difference in whole-body meal-derived FA oxidation. Similarly, there were no differences in fasting or postprandial respiratory quotient (RQ) or resting energy expenditure (REE) between the two diets (Table 1). We also calculated net substrate oxidation rates and found no significant difference in fasting or postprandial net carbohydrate or FA oxidation rate between the two diets (data not shown).
Intrahepatic DNL and FA Partitioning
We assessed the contribution of different FA sources in VLDL-TAG, as it has previously been suggested to reflect the contribution of different FA sources to IHTAG (25). Although previous studies have reported that diets enriched in carbohydrate increase hepatic DNL (26), we found no difference in fasting or postprandial hepatic DNL between SFA and SUGAR (Fig. 3C). There was also no difference in the relative contribution of systemic NEFA (from adipose tissue), meal-derived FA, and splanchnic FA (i.e., FA derived from visceral adipose tissue and stored hepatic TAG) to VLDL-TAG between SFA and SUGAR (Fig. 3D).
Systemic Bile Acids
There was no difference in total systemic bile acids, the concentration of specific bile acids, or their relative contribution to total concentration between the SFA and SUGAR diets (Supplementary Fig. 5).
Conclusions
Macronutrient composition may play a role in NAFLD development with increased consumption of SFA and/or free sugars being associated with NAFLD (6). A large proportion of experimental evidence is derived from overfeeding studies, and although they suggest that increased SFA intakes exaggerate IHTAG accumulation compared with unsaturated fat and dietary sugars (7–9), it is challenging to disentangle the effects of excess energy from those of the macronutrients per se. By using a combination of methodologies, we investigated the effect of two diets—one enriched in carbohydrate, specifically, free sugars, and the other enriched in fat, specifically, SFA—on IHTAG content, hepatic DNL, and hepatic and whole-body postprandial metabolism in overweight males. We found consumption of an SFA diet increased IHTAG, whereas consumption of the SUGAR diet did not. Despite no changes in fasting plasma glucose and insulin concentrations, we found consumption of the SFA diet resulted in exaggerated postprandial plasma glucose and insulin excursions compared with consumption of the SUGAR diet.
Effect of SFA and Free Sugars on Glycemic Control
Dietary composition has previously been reported to influence markers of glycemic control/whole-body insulin sensitivity, with SFA-induced impairments being reported by some (7,27) but not all (9,28). Although we found that consumption of SFA or SUGAR for 4 weeks had a negligible effect on fasting plasma glucose and insulin concentrations, by feeding of a standardized test meal at the end of the respective interventions we are able to demonstrate that consumption of the SFA compared with SUGAR diet led to exaggerated postprandial glucose and insulin excursions. The increased postprandial insulin concentrations following SFA may in part be explained by a reduced hepatic or peripheral insulin sensitivity resulting in increased endogenous glucose production/reduced peripheral glucose uptake and a compensatory increase in insulin secretion (29). Alternatively, the elevated insulin concentrations may be due to impaired hepatic insulin extraction, which has previously been associated with increased IHTAG and peripheral insulin resistance (30,31). Proposed mechanisms underpinning SFA-induced reductions in insulin sensitivity include increased ceramide production (32) and/or induction of metabolic endotoxemia and associated inflammation (7).
IHTAG and Dietary SFA and Free Sugars
We found IHTAG content increased by ∼37% after consumption of the SFA diet, while IHTAG was not significantly altered in response to the SUGAR diet. The negligible change in IHTAG in response to the SUGAR diet is in line with results of others who have fed sugar-enriched eucaloric diets for 4–10 weeks (13,33). In contrast, hypercaloric sugar-enriched diets, which result in weight gain, are associated with increased IHTAG (6). Although participants were instructed to maintain body weight during the dietary interventions, this was not achieved during the SFA diet, where on average participants gained ∼1.5 kg; linear regression indicated that the change in body weight in response to the SFA diet was not associated with IHTAG accumulation. This is in agreement with observations from hypercaloric studies, which found a notably greater increase in IHTAG after overfeeding of SFA compared with overfeeding free sugars and unsaturated fats after matching for increases in body weight (7,9,10). Taken together, these data indicate that diets enriched in SFA increase IHTAG independent of weight gain. Why SFA has a profound effect on IHTAG accumulation remains to be elucidated, but it has been hypothesized that the change is due to an increased endogenous NEFA flux to the liver and/or increased ceramide synthesis, which has been suggested to induce hepatic insulin resistance (7,9).
The negligible change in IHTAG in response to SUGAR is notable, as it has previously been suggested that a diet enriched in sugars would increase IHTAG content as a result of increased hepatic DNL (5). Hypercaloric feeding of carbohydrate/sugar enriched diets for 4 days up to 3 weeks upregulates DNL (7,34,35). In contrast, findings from isocaloric interventions are inconsistent, with one study suggesting a fourfold increase in fasting DNL after a high-sugar compared with low-sugar diet (36), while others have observed no significant difference between individuals with and without NAFLD in response to a 12-week eucaloric diet enriched in free sugars (26% TE) (11). We observed a nonsignificant increase in both fasting and postprandial hepatic DNL after SUGAR compared with SFA, despite increasing the intake of free sugars to a level equivalent to the 90th percentile of intake in the U.K. adult population (37). It is possible the lack of difference in hepatic DNL between the two diets is attributable to an adaptive response whereby differences may have been apparent earlier in the intervention period. Moreover, it is plausible that under conditions of energy balance other disposal pathways (e.g., storage as glycogen, oxidative glucose disposal, etc.) are sufficient.
Hepatic FA Input and Disposal
By using stable-isotope methodologies in combination with a standardized test meal, we were able to investigate intrahepatic FA partitioning across the postprandial period. As dietary composition has been suggested to influence adipose tissue TAG hydrolysis, we assessed Ra-NEFA and found no difference between the dietary interventions, suggesting a similar level of exposure of the liver to endogenous systemic NEFA. Within the liver, FA can be broadly partitioned into either oxidation or esterification pathways. We assessed FA oxidation in two ways: 1) via plasma β-hydroxybutyrate concentrations as a marker of hepatic FA oxidation and 2) the appearance of 13C (from the standardized test meal) in expired CO2 as a marker of whole-body FA oxidation, and found no difference for either between diets. Although there was no difference in the incorporation of either adipose tissue–derived or meal-derived FA into VLDL-TAG, there was a significant diet × time interaction for plasma VLDL-TAG concentrations, which may in part be explained by fasting VLDL-TAG concentrations being nonsignificantly higher at the end of the SUGAR compared with SFA diet.
While there were no significant differences in hepatic DNL, evidence from animal studies has suggested that newly synthesized FA are preferentially partitioned toward secretory pathways, which would lead to an increase in VLDL-TAG production and secretion. Others have found that a diet enriched in sugars increases IHTAG and upregulates VLDL-TAG secretion in overweight men and in those with NAFLD, and this occurs alongside a concomitant reduction in the fractional catabolic rate of plasma VLDL-TAG (11). We did not measure hepatic VLDL-TAG production or clearance, and differences between diets could be due to differences in either of these processes.
Adherence and Biomarkers
Food diaries completed during the intervention periods indicate that participants closely adhered to the experimental dietary interventions. We investigated a number of biomarkers that have been suggested to reflect changes in dietary intake and/or metabolism. We found a greater relative abundance of pentadecanoic acid (15:0), a marker of dairy fat intake, in VLDL-TAG after the SFA compared with SUGAR diet. There is currently no universally accepted biomarker for dietary sugar, although the hepatokine FGF21 has been shown to increase in response to sucrose consumption (38). We observed an increase in fasting plasma FGF21 concentrations in response to SUGAR; however, we also found an increase in fasting plasma FGF21 in response to SFA, with the latter corresponding with reports that IHTAG is the strongest predictor of FGF21 production (39). We found no difference in fetuin-A, which has previously been associated with hepatic steatosis (40) after either dietary intervention.
Limitations
Our study has a number of limitations. We did not provide all food to participants as others have done (28). Rather, we educated participants on how to meet the targeted dietary intakes for the interventions, allowing us to investigate participants in a real-world setting. However, this resulted in participants gaining weight during the SFA diet, likely explained by participants being encouraged to increase their consumption of energy-dense (i.e., high-fat) foods. For logistical reasons, we did not undertake postprandial study days at the start of the respective dietary interventions, and it would be of interest to compare postprandial responses across the interventions (prediet vs. postdiet). We only studied overweight males, who were representative of the U.K. adult population and considered to be at increased risk of NAFLD relative to females (41). As sexual dimorphism exists in the development of NAFLD, T2D, and intrahepatic FA metabolism (41,42), it is plausible that findings in females may differ from what we report here.
Conclusions
There has been much controversy about the role of SFA and free sugars in metabolic disease, and recently, low-carbohydrate, high-fat diets have been promoted for weight loss and for the management of T2D (43). The evidence suggests that these diets are safe and effective over the short-term but are not superior to other dietary strategies (43). However, in all studies conducted to date, hypocaloric diets specifically designed to induce weight loss were studied, and there is little evidence for eucaloric diets that are high in SFA. Our findings suggest that consumption of an SFA-enriched diet, in the absence of weight loss, had adverse metabolic effects (including increased IHTAG and exaggerated postprandial plasma glucose and insulin responses) compared with a diet enriched in free sugars; this may have implications for those who are not aiming for weight loss but choose to adopt a relatively high-fat diet. Moreover, despite careful monitoring and support, small weight gain was noted with the SFA as opposed to the SUGAR diet, suggesting that weight maintenance is challenging with diets high in SFA. The lack of substantial metabolic changes after consumption of the SUGAR diet for 4 weeks may be, in part, explained by participants being metabolically healthy, remaining weight stable, and maintaining energy balance over the course of the SUGAR diet. Others have reported an increase in IHTAG when a hypercaloric diet, high in sugar, is consumed (7), suggesting that the proposed unfavorable metabolic effects of a high-sugar diet are mediated through excess energy intake. Taken together, our findings indicate that a diet enriched in SFA is more harmful to metabolic health than a diet enriched in free sugars.
Supplementary Material
Article Information
Acknowledgments. The authors thank Louise Dennis and Rachel Craven-Todd (Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford) and all Clinical Research Unit staff for excellent nursing provision, Ruth Coleman (Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford) for helpful statistical advice and technical assistance, and Niall Dempster and Lia Anguelova (Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford) for providing medical support during study days. The authors thank Naira Beraza (Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford) of the Quadram Institute for assistance with the analysis of bile acids. The authors thank the volunteers from the Oxford BioBank (www.oxfordbiobank.org.uk) for their participation in this recall study.
Funding. L.H. is a British Heart Foundation (BHF) Senior Fellow in Basic Science. This study was funded by the World Sugar Research Organisation and Biotechnology and Biological Sciences Research Council (BB/N005600/1) and the BHF FS/15/56/31645 (L.H.). F.R. is supported by Henning and Johan Throne-Holsts Foundation, Swedish Society for Medical Research, Swedish Society of Medicine, and The Foundation Blanceflor. M.-E.P. was the recipient of a fellowship grant from Fonds de Recherche du Québec-Santé and the Heart and Lung Institute Foundation. The Oxford BioBank and Oxford BioResource are funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. P.D. is a member (unpaid) of the joint Scientific Advisory Committee on Nutrition/NHS England/Diabetes UK working group to review the evidence on lower carbohydrate diets compared with current government advice for adults with T2D.
The conduct of the trial, data analyses, and writing of the manuscript were all undertaken by the authors and were completely independent from the World Sugar Research Organisation. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.H. designed research and gained research funding. L.H., S.A.P., T.C., M.H., F.R., M.-E.P., and P.D. conducted the research. S.A.P., P.D., F.E.M., and L.H. analyzed data. S.A.P., F.R., P.D., and L.H. wrote the manuscript. L.H. helped with data interpretation and revisions of the manuscript. All authors read and approved the final manuscript. L.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT03145350, clinicaltrials.gov
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-2331/-/DC1.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al.; American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association . The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012;107:811–826 [DOI] [PubMed] [Google Scholar]
- 2.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol 2018;68:335–352 [DOI] [PubMed] [Google Scholar]
- 3.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammit VA. Hepatic triacylglycerol synthesis and secretion: DGAT2 as the link between glycaemia and triglyceridaemia. Biochem J 2013;451:1–12 [DOI] [PubMed] [Google Scholar]
- 5.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 2016;53:52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry SA, Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Investig Med 2017;65:1102–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luukkonen PK, Sädevirta S, Zhou Y, et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 2018;41:1732–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobrecases H, Lê KA, Bortolotti M, et al. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 2010;36:244–246 [DOI] [PubMed] [Google Scholar]
- 9.Rosqvist F, Kullberg J, Ståhlman M, et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab 2019;104:6207–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjermo H, Iggman D, Kullberg J, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr 2012;95:1003–1012 [DOI] [PubMed] [Google Scholar]
- 11.Umpleby AM, Shojaee-Moradie F, Fielding B, et al. Impact of liver fat on the differential partitioning of hepatic triacylglycerol into VLDL subclasses on high and low sugar diets. Clin Sci (Lond) 2017;131:2561–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marina A, von Frankenberg AD, Suvag S, et al. Effects of dietary fat and saturated fat content on liver fat and markers of oxidative stress in overweight/obese men and women under weight-stable conditions. Nutrients 2014;6:4678–4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–688 [DOI] [PubMed] [Google Scholar]
- 14.Karpe F, Vasan SK, Humphreys SM, et al. Cohort profile: the Oxford Biobank. Int J Epidemiol 2018;47:21–21g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frahm J, Merboldt KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson 1987;72:502–508 [DOI] [PubMed] [Google Scholar]
- 16.Purvis LAB, Clarke WT, Biasiolli L, Valkovič L, Robson MD, Rodgers CT. OXSA: an open-source magnetic resonance spectroscopy analysis toolbox in MATLAB. PLoS One 2017;12:e0185356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinnick KE, Gunn PJ, Hodson L. Measuring human lipid metabolism using deuterium labeling: in vivo and in vitro protocols. In Metabolic Signaling: Methods and Protocols. Fendt S-M, Lunt SY, Eds. New York, NY, Springer, 2019, p. 83–96 [DOI] [PubMed] [Google Scholar]
- 18.Hodson L, Bickerton AS, McQuaid SE, et al. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes 2007;56:2433–2441 [DOI] [PubMed] [Google Scholar]
- 19.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85:1511–1520 [DOI] [PubMed] [Google Scholar]
- 20.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 2009;119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narváez-Rivas M, Zhang Q. Comprehensive untargeted lipidomic analysis using core-shell C30 particle column and high field orbitrap mass spectrometer. J Chromatogr A 2016;1440:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruge T, Hodson L, Cheeseman J, et al. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 2009;94:1781–1788 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 24.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudgins LC, Baday A, Hellerstein MK, et al. The effect of dietary carbohydrate on genes for fatty acid synthase and inflammatory cytokines in adipose tissues from lean and obese subjects. J Nutr Biochem 2008;19:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vessby B, Uusitupa M, Hermansen K, et al.; KANWU Study . Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 2001;44:312–319 [DOI] [PubMed] [Google Scholar]
- 28.Lundsgaard AM, Holm JB, Sjoberg KA, et al. Mechanisms preserving insulin action during high dietary fat intake [published correction appears in Cell Metab 2019;29:229]. Cell Metab 2019;29:50–63.e4 [DOI] [PubMed] [Google Scholar]
- 29.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018;98:2133–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bril F, Barb D, Portillo-Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017;65:1132–1144 [DOI] [PubMed] [Google Scholar]
- 31.Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab 2019;104:1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaribeygi H, Bo S, Ruscica M, Sahebkar A. Ceramides and diabetes mellitus: an update on the potential molecular relationships. Diabet Med 2020;37:11–19 [DOI] [PubMed] [Google Scholar]
- 33.Johnston RD, Stephenson MC, Crossland H, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013;145:1016–1025.e2 [DOI] [PubMed] [Google Scholar]
- 34.McDevitt RM, Bott SJ, Harding M, Coward WA, Bluck LJ, Prentice AM. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am J Clin Nutr 2001;74:737–746 [DOI] [PubMed] [Google Scholar]
- 35.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43–50 [DOI] [PubMed] [Google Scholar]
- 36.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res 2000;41:595–604 [PubMed] [Google Scholar]
- 37. National Diet and Nutrition Survey. Results From Years 1, 2, 2 and 4 (Combined) of the Rolling Programme (2008/2009 – 2011/2012). London, U.K., Public Health England, 2014.
- 38.Søberg S, Sandholt CH, Jespersen NZ, et al. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab 2017;25:1045–1053.e6 [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Xia M, Chang X, et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One 2011;6:e24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Loeffelholz C, Horn P, Birkenfeld AL, et al. Fetuin A is a predictor of liver fat in preoperative patients with nonalcoholic fatty liver disease. J Invest Surg 2016;29:266–274 [DOI] [PubMed] [Google Scholar]
- 41.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr 2005;135:681–686 [DOI] [PubMed] [Google Scholar]
- 43.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol 2019;13:689–711.e1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.