Abstract
The successful use of leptin for the treatment of individuals with lipodystrophy and leptin deficiency is well established. However, pharmacological approaches of leptin therapy for the treatment of diet-induced obesity have been ineffective. There is ample room for a better understanding of the much famed “leptin resistance” phenomenon. Our recent data in this area prompt us to call for a conceptual shift. This shift entails a model in which a reduction of bioactive leptin levels in the context of obesity triggers a high degree of leptin sensitization and improved leptin action, both centrally and peripherally. Put another way, hyperleptinemia per se causes leptin resistance and associated metabolic disorders. In this perspective, we briefly discuss the underlying conceptual steps that led us to explore partial leptin reduction as a viable therapeutic avenue. We hope this discussion will contribute to potential future applications of partial leptin reduction therapy for the treatment of obesity and type 2 diabetes.
Introduction
The landmark discovery of the Lep gene and the demonstration of the physiological role of leptin generated great excitement in the metabolism field (1,2). Along with leptin, the identification of the second important adipocyte-derived hormone, adiponectin (3), fundamentally changed our view of adipose tissue and its communication with other organs in the mid-1990s. Adipose tissue was no longer merely considered an energy sink; rather, we began to appreciate its role as a highly active endocrine organ. The early studies also highlighted the importance of adipose tissue per se as a key regulator of systemic energy metabolism.
Leptin, the product of the Lep gene, is a 167-residue peptide hormone. It is primarily secreted by adipose tissue. Functional inactivation of the Lep gene leads to undetectable levels of leptin in circulation (4). Once released by adipose tissue into the bloodstream, leptin reaches its targets, including the hypothalamus, through different mechanisms (5). Though the mechanisms are still somewhat unclear, leptin has been reported to reach the brain via direct transport through circumventricular organs, saturable transport through the blood-brain barrier (6), and uptake into the brain parenchyma and choroid plexus (7). Leptin binding to the long form of its receptor (LepRb) activates a well-characterized downstream signaling pathway, which regulates food intake and energy expenditure (8). The central melanocortin system is a target of leptin to regulate energy and glucose homeostasis (9). This system consists of intermingled neurons expressing pro-opiomelanocortin (POMC) and neurons producing agouti-related protein (AgRP), which are respectively activated and inhibited by leptin. In particular, LepR-expressing POMC neurons regulate hepatic glucose homeostasis (9), while LepR-expressing AgRP neurons regulate food intake and energy expenditure (10).
Unlike insulin, which is stored in granules for immediate release in response to a proper stimulus (11), the rate of leptin release is primarily dependent on the rate of Lep gene transcription and translation. The adipocyte lacks a classical “triggered” exocytic pathway and, at best, responds in an inducible fashion by releasing factors that are withheld at the level of the endoplasmic reticulum within a 15- to 30-min delay. In other words, leptin levels in circulation are rather stable on a short-term basis (i.e., meal to meal) and require (under normal physiological conditions) hours rather than minutes/seconds to respond to various metabolic stimuli. Similarly, it remains quite puzzling how leptin regulates food intake on a day-to-day basis. Elevated insulin and glucocorticoid levels are robust stimulators of leptin secretion. In contrast, the sympathetic nervous system, through activation of adrenergic receptors, significantly represses leptin release (9).
Acute and chronic administration of recombinant leptin into lean individuals or mice (either wild-type or leptin-deficient ob/ob mice) yields a number of responses, such as reducing food intake and body weight (12). This and other observations formed the basis for leptin therapy for the U.S. Food and Drug Administration–approved treatment of individuals with leptin deficiency and generalized lipodystrophy (13). Originally, there was the expectation that leptin therapy would have the same successful potential for the treatment of diet-induced obesity as insulin has for diabetes (14). However, it was rapidly established that the vast majority of obese individuals do not have a shortage of leptin. Rather, most common forms of obesity are associated with excessive circulating levels of leptin (coined “hyperleptinemia”), which results in a still ill-defined state of “leptin resistance” (15). The most accepted definition of leptin resistance is the inability of pharmacological doses of leptin to suppress food intake and body weight. However, “selective leptin resistance” is also proposed to explain the preservation of leptin action in hypertension and in the reproductive axis in the context of diet-induced obesity (16). Unlike insulin resistance, which can be at least partially overcome by further increasing the levels of insulin, enhancing the circulating levels of leptin is completely ineffective. As such, numerous attempts over the years to restore the physiological role of leptin have failed. As a consequence, the initial idea that leptin therapy could be utilized to effectively overcome diet-induced obesity in preclinical and clinical settings had to be abandoned.
Hyperleptinemia: A Cause or Consequence of Obesity?
As obesity gradually progresses with increased adipose tissue mass (as a result of an imbalance between energy intake and energy expenditure), a concomitant prominent increase in circulating levels of leptin is typically observed. Under normal physiological conditions, circulating levels of leptin are proportional to adipose tissue mass for a given individual, despite a substantial variation at any given BMI. As such, it seems plausible that obesity-associated hyperleptinemia may be a consequence of obesity. Therefore, hyperleptinemia was not thought to play a direct role in the onset of obesity-associated disorders. However, given the robust and positive association of hyperleptinemia with many metabolic disorders, such as insulin resistance, kidney disease, and cardiovascular disease, is it possible the role of hyperleptinemia has been overlooked? Put another way: is hyperleptinemia per se contributing to the metabolic dysfunction commonly seen in obese subjects (17)? For example, hyperleptinemia, independent of adiposity, has been shown to be predictive in the outcome of cardiovascular disease progression (18). We propose that the contribution of chronic hyperleptinemia (within a physiological range) has not received the attention it deserves.
It should be noted that high (pharmacological) levels of leptin are necessary, but not sufficient, to induce leptin resistance. In the absence of other stimulating factors, such as high-fat diet (HFD), hyperleptinemia improves glucose metabolism and insulin sensitivity, as observed in transgenic lean mice overexpressing leptin and in mice receiving chronic intracerebroventricular (i.c.v.) leptin perfusions (12,19). Upon exposure to HFD, hyperleptinemia promotes leptin resistance (20). Chronically elevated levels of leptin in mice (through means of constitutive very high-level leptin overexpression from a transgenic cassette in adipose tissue) results in increased susceptibility to diet-induced obesity (21) as well as enhanced adipose tissue mass with aging. Consistent with these observations, we recently reported that further increasing the levels of leptin even well within a physiological range in the context of preexisting obesity (by utilizing a titratable doxycycline [Dox]-inducible adipose tissue-specific leptin overexpression cassette) accelerates diet-induced obesity and dramatically worsens obesity-associated insulin resistance (22). Surprisingly, less than a doubling of the concentration of leptin in circulation was sufficient to trigger these very profound metabolic alterations. This is in contrast to previous approaches that delivered leptin at pharmacological levels (12,19). Based on these observations, we speculated that, in the context of obesity, any intervention that promotes leptin secretion, directly or indirectly, could be considered in the long run as an “obesogenic” driver with negative metabolic consequences. This model is supported by a number of observations. For instance, insulin and glucocorticoids, both hormones that stimulate leptin release, also promote diet-induced obesity (9,15). Moreover, certain inflammatory factors, such as tumor necrosis factor-α and lipopolysaccharide, enhance leptin secretion, providing a crucial link between obesity and its associated metabolic disorders.
However, one persistent problem is that none of these models can functionally dissociate the changes in leptin levels from other changes occurring simultaneously at the level of the adipocyte.
Hyperleptinemia in Evolution
The long-term evolutionary challenge has been food scarcity rather than food oversupply. The selection pressure was clearly aimed to develop a number of strategies to effectively cope with the starvation response to survive through a period of caloric shortage. In response to fasting, a drop in leptin levels initiates a series of characteristic neuroendocrine responses, including heightening of appetite, reduction of fertility, slowing down metabolic rates and suppression of growth, to increase food intake and suppress noncritical physiological functions for survival (2). Put another way, leptin levels within a physiological range inform the brain that it is not a time of energy deficit. This well-preserved starvation response may be the major functional purpose of the fall in leptin. However, in the context of a food oversupply, the obesity-associated hyperleptinemia, rather than a starvation-induced drop in leptin levels, becomes the major effector of leptin in circulation. However, neurons still respond to changes of elevated leptin levels based on multiple observations. First, in obese mice, leptin receptor levels in the hypothalamic region are increased rather than decreased (23). Second, basal phospho-STAT3 levels in diet-induced obese mice are increased rather than decreased (24). Third, a complete block of leptin receptor action through leptin receptor antagonists or specific inhibitors further increases food intake and weight gain (25). Fourth, i.c.v.injection of leptin into obese mice is still efficient in inducing profound weight loss and insulin sensitivity (26).
All of these observations support a model wherein subsets of leptin-responsive circuits can be engaged even in the context of obesity. Moreover, we predict that the leptin resistance in obesity is differentially triggered in distinct neuronal groups. For example, leptin-responsive neurons regulating feeding become resistant earlier than leptin-responsive neurons that regulate autonomic outflow including blood pressure. This prediction is an extension of the concept of “selective leptin resistance” (27). This model is analogous to the concept of selective insulin resistance in the liver described by Brown and Goldstein (28) in which insulin fails to suppress hepatic glucose production, but high insulin continues to stimulate lipogenesis. These responses subsequent to rising leptin levels may contribute to increased food intake and obesity-associated metabolic disorders, such as diabetes, hypertension, and cardiovascular disease. All of these eventually lead to reduced health span and life span (29). In that sense, constraining leptin action within a low “physiological” range may actually enhance fitness.
Partial Leptin Reduction: Beneficial or a Source of Further Metabolic Deterioration?
Given the possibility that hyperleptinemia contributes to obesity and its associated metabolic disorders, we probed for any potentially beneficial effects associated with a reduction in circulating levels of leptin. Notably, we assessed the effects of partial leptin reduction in two disparate settings: 1) in the context of high leptin sensitivity and 2) in the context of baseline leptin resistance.
In the context of increased leptin sensitivity (as observed in an extremely lean state and at a young age), the circulating leptin levels are low. Under these circumstances, the response to a further reduction in leptin levels coincides with the classical model, which emphasizes that low circulating levels of an adipocyte-derived factor (i.e., leptin) reflect an energy deficit and prompt increased food intake and decreased energy expenditure (30). Predictably, we observed a rapid weight gain upon leptin reduction in the lean and young state, demonstrated by utilizing young, lean inducible leptin knockout mice (22). This is also apparent in mice and humans that are heterozygous for one of the functional copies of the Lep gene (31,32). In the heterozygous state, the gene dosage is only 50%, and hence the degree of hyperleptinemia achieved in the obese state is also reduced by 50%. In a state of elevated leptin sensitivity, a further reduction in the circulating levels of leptin is not desired. In this context, treatment with recombinant leptin is an effective approach to improve glucose metabolism, as demonstrated with the dramatic results obtained with leptin treatment of ob/ob mice and lipodystrophic patients. A subset of obese individuals display lower levels of leptin, suggesting a state of higher leptin sensitivity, and, as such, these obese individuals respond adequately to leptin therapy (33).
However, the biological dose-response curve for leptin’s action on inhibiting food intake and promoting energy expenditure is within a relatively narrow range, i.e., transitioning between a state of energy deficit with low leptin levels and “energy sufficiency” evoked by even modestly higher leptin levels. The leptin response rapidly plateaus off at higher levels, beyond which leptin remains absolutely ineffective. Therefore, even under conditions of leptin sensitivity, leptin levels need to be maintained narrowly within a physiological level. For example, hyperleptinemia also induces leptin resistance, even in highly leptin-sensitive ob/ob mice (34).
In stark contrast to the leptin-sensitive state, in the context of leptin resistance, an approach toward leptin reduction displays beneficial antidiabetic and antiobesogenic effects. A number of independent lines of observations support this model. A reduction in leptin levels (induced either by genetic strategies or through neutralizing monoclonal antileptin antibodies) restores leptin sensitivity in the context of obesity, concomitant with reduced food intake and significant weight loss (22). Moreover, β3-adrenergic receptor agonist treatment inhibits insulin-stimulated leptin secretion (9) to trigger reduced weight gain upon HFD feeding (35). In addition, inverse agonist treatment for the peripheral cannabinoid-1 receptor (CB1R) decreases the expression and secretion of leptin from adipocytes and increases leptin clearance via the kidney; this collectively counteracts the progression of obesity (36). These antiobesogenic effects are exerted in a leptin-dependent manner, as judged by the observation that the CB1R inverse agonists completely lack any effects in ob/ob or db/db mice. Furthermore, phenolic compounds, such as resveratrol, which inhibit leptin expression in adipocytes, protect mice from obesity (37). Similarly, interleukin-6 has been shown to attenuate obesity in a leptin-dependent manner (38). Metformin, a first-line antidiabetic drug with pleiotropic beneficial effects on energy homeostasis, also inhibits leptin secretion from adipocytes (39). All these observations support a model that “partial leptin reduction” restores leptin sensitivity and manifests antiobesogenic effects in the context of leptin-resistant states. If additional studies support this model, it leads to the provocative prediction that any compound or therapeutic intervention that directly or indirectly inhibits leptin levels (via reduction of transcription or secretion) has the potential to be beneficial for weight control and metabolic homeostasis under obese conditions.
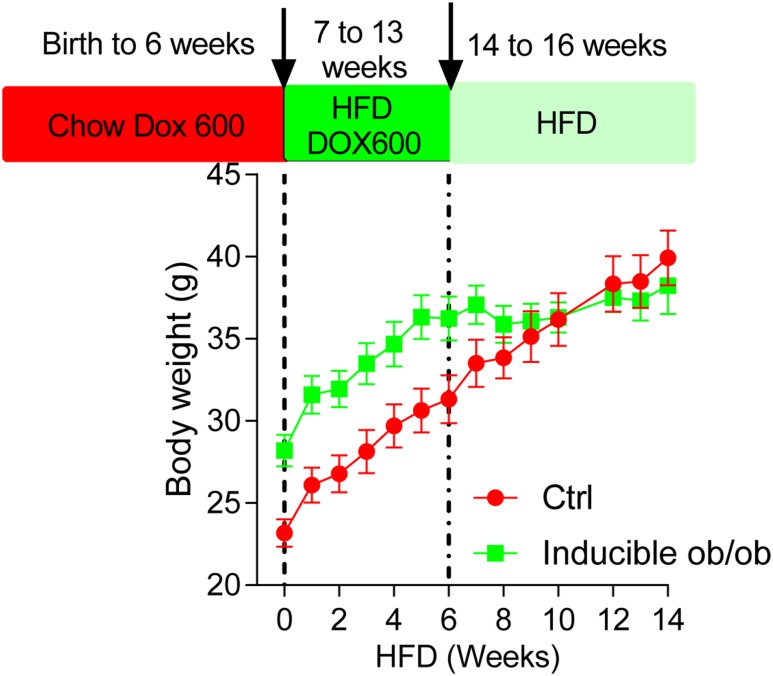
Our inducible leptin knockout mice (generated through inducible Lep gene deletion during embryonic development) are a means to directly test components of this model (Fig. 1). These mice display more than 90% reduction in circulating levels of leptin. From birth to 6 weeks of chow diet feeding, inducible leptin-deficient mice display rapid weight gain when compared with littermate control mice. However, upon switching to an HFD, the rate of weight gain in leptin-deficient mice is much slower than in control littermates. This suggests that the inability to increase leptin levels, in the context of an obesogenic condition, preserves some degree of leptin sensitivity, thus rendering the mice resistant to diet-induced obesity.
Figure 1.
Body weight of inducible ob/ob mice and littermate controls. Inducible ob/ob and littermate control (Ctrl) mice were placed on a chow diet with Dox 600 from birth to 6 weeks of age, followed by a switch to HFD plus Dox 600 and HFD only diets at the indicated weeks. Body weights were monitored on a weekly basis.
Weight loss via caloric restriction is the first-line recommendation for obesity and its associated metabolic disorders (40). Numerous studies have documented the beneficial effects of caloric restriction in inducing weight loss and slowing down the progression of multiple metabolic disorders. Leptin reduction via caloric restriction increased leptin sensitivity and is predicted to further aid in weight loss or weight maintenance (41). However, this prediction is not supported by the observation of rapid recovery of lost weight following caloric restriction (42). A series of clinical studies from the Leibel laboratory has demonstrated that repletion of leptin to individuals after weight loss reverses skeletal muscle and autonomic and neuroendocrine adaptations to achieve the maintenance of reduced weight (43,44). Thus, dropping leptin levels has been proposed as a cause of body weight rebound (45). An alternative explanation is that once obesity is established, alterations in key central circuits are induced, such that the new, elevated level of body weight and fat mass are biologically defended. However, some other clinical reports suggest that obese individuals with higher basal leptin levels are more prone to regain weight (46). A greater reduction of leptin levels offers a better correlate to weight-loss maintenance (47). More recently, another clinical study concluded that leptin reduction does not counteract weight loss and it is indeed correlated with further weight loss in the long run (48). Future studies will be required to determine whether a partial leptin reduction in the obese setting favors a further weight-loss or at least a weight-maintenance phenotype.
The Underlying Mechanisms Involved in the Beneficial Effects of Partial Leptin Reduction
An examination of the existing literature reveals many more examples of either genetic or pharmacological interventions that result in metabolic improvements associated with pronounced reductions in leptin levels. We ourselves observed critical effects directly linking the lowering of leptin levels to metabolic improvements. Combining these observations with the existing literature, we argue that there is a compelling case for lowered leptin levels as the underlying driving force for the metabolically beneficial effects of many interventions.
Mechanistically, how is leptin sensitization achieved? Several mechanisms have been proposed (15,16), including 1) leptin being more efficiently transported into the brain, 2) the fact that this leads to a removal of a feedback inhibition mechanism to enhance leptin signaling, and 3) a restoration of leptin sensing in key “metabolic” neurons, such as POMC and AgRP cells.
The central leptin-sensing mechanisms critically rely on changes in peripheral circulating leptin concentrations (14). Leptin levels are rather stable in the short term; however, there is a significant degree of variation over the course of a day, as part of diurnal oscillations (49). While the physiological significance of these diurnal variations is still unclear, the changes in circulating leptin could be “resetting” the central leptin signaling pathway. In the context of obesity-associated hyperleptinemia, we propose that a “flattened” peak and trough in the diurnal variation results in a diminished response of hypothalamic neurons in leptin actions. Thus, an exogenously imposed leptin reduction may restore leptin oscillations and restore proper signaling in hypothalamic neurons. We therefore propose that the fasting-induced fall in leptin levels acutely restores leptin signaling, which leads to enhanced leptin signaling during a period of reduced leptin concentrations in the periphery. This fall in leptin levels, which is acutely out of proportion to fat mass loss, is a critical signal for neurons to coordinate a fasting response (2). The acute response to leptin-neutralizing antibody treatment (that very effectively lowers leptin levels) may be associated counterintuitively with enhanced leptin signaling. This model will require direct assessment utilizing reporter mice that can acutely provide central readouts for both leptin and insulin signaling.
It is also well established that leptin exerts many of its effects with actions on key groups of neurons. Notably, recent evidence suggests that key neurons, including POMC neurons, regulate the dynamics of leptin transcription and secretion (50). Thus, leptin sensitivity in key neurons may be required to maintain leptin sensitivity globally. Leptin signaling in the hypothalamus undergoes feedback regulation to prevent overactivation. The key feedback mechanisms are executed by the suppressor of cytokine signaling 3 (SOCS3) and phosphatases, including protein tyrosine phosphatase 1B (PTP1B) and T-cell protein tyrosine phosphatase (TCPTP) (51,52). Leptin binding to LepRb results in activation, phosphorylation, and translocation of STAT3 into the nucleus to modulate the expression of important genes, including SOCS3 (53). SOCS3 then binds to janus kinase 2 (JAK2) to inhibit its activity (54). As a classical feedback inhibition scheme, this effectively dampens leptin signaling (55). Indeed, SOCS3 expression in the hypothalamic region is leptin dependent; higher leptin levels promote increased SOCS3 expression (53). In addition, similar to SOCS3, PTP1B also functions as part of the feedback mechanism to reduce leptin signaling (56). Obesity-associated hyperleptinemia induces PTP1B expression and contributes to leptin resistance (56).
As such, in the context of obesity, the major cause of impaired leptin action may not be a defect in leptin signaling per se but rather the potent feedback mechanisms induced by constitutive activation of leptin signaling. Importantly, this also feeds back on insulin signaling (57). Partial reduction of leptin action in the hypothalamus ameliorates these feedback mechanisms of leptin signaling and restores both leptin and insulin sensitivity. This may well be true in other areas of the brain and in the periphery, thus leading to potent system-wide insulin sensitizing effects, even prior to weight loss.
Applications of Partial Leptin Reduction Therapy
In the context of obesity, a partial leptin deletion (as achieved by monoclonal leptin-neutralizing antibodies) triggers significant weight loss concomitant with antidiabetic outcomes. Monotherapy with leptin-neutralizing antibodies could be complemented with combination therapies with other antidiabetic and antiobesity drugs. For instance, GLP-1 receptor agonists, a unique family of compounds with potent effects on weight loss, have been shown to inhibit food intake and promote energy expenditure through actions in the central nervous system. It has been further demonstrated that GLP-1 enhances central leptin action and exerts its anorexic effects in synergy with leptin. Previous reports have examined the potential combination therapy of GLP-1 agonists with leptin. However, this combination requires a diet consisting of low lipids (i.e., a switch from HFD to chow diet) accompanied by a dramatic weight loss (more than ∼28%) to observe a restoration of leptin responsiveness (58). An attractive approach in this context may be combination therapy of partial leptin reduction with GLP-1 receptor agonists.
Similar to GLP-1, FGF21 induces significant weight loss in obese mice, primarily by regulating glucose metabolism and energy expenditure (59). FGF21 and its derivatives serve as potential drug targets and, as such, are currently under development. Recent data suggest that FGF21-induced weight loss is centrally mediated, as FGF21 receptors have been identified in the mediobasal hypothalamus, a region of the brain that is also highly enriched in leptin receptor. This renders the attractive possibility for synergistic effects for FGF21 and leptin. However, combination therapies of leptin with FGF21 encounter similar problems as GLP-1/leptin combination therapies (58). This suggests that a partial leptin reduction therapy may be a better choice for the combination. In addition to this, undesirable side effects on bone loss associated with FGF21 greatly hinder its clinical potential. Leptin is a powerful inhibitor of bone formation in vivo, and, as such, reducing leptin levels enhances bone mass (60). Taken together, combination therapy of FGF21 and a leptin-neutralizing antibody may be a rational strategy to reverse FGF21-induced bone loss.
Rosiglitazone, a potent PPARγ agonist, enhances insulin sensitivity and serves as an effective therapy for type 2 diabetes. However, treatment with rosiglitazone is frequently associated with weight gain, a potential reduction in bone density, and presumed cardiovascular side effects (that are at this stage questioned). This collectively dampened the enthusiasm for the wide usage of rosiglitazone in the treatment of individuals with type 2 diabetes (61). The effects of PPARγ agonists on leptin are still not settled. The leptin promoter contains a 17-base-pair noncanonical PPARγ/RXRα-binding site, suggesting that leptin gene expression is directly regulated by PPARγ (62). Uncertainty emerges as to whether the reported side effects of rosiglitazone are leptin dependent or leptin independent. Direct studies will be needed to assess whether partial leptin reduction therapy could alleviate some of the side effects of PPARγ agonists.
Finally, insulin replacement is the most widely utilized therapy in the treatment of both type 1 and type 2 diabetes. One of the most documented side effects of insulin therapy, however, is significant weight gain (63). As mentioned above, insulin is also a potent physiological stimulator of leptin secretion. It remains unclear as to whether insulin-induced weight gain is leptin dependent or leptin independent. Our studies show that partial leptin reduction in obese mice elicits beneficial effects for weight loss. Importantly, leptin physiology is preserved extremely well from mice to humans. As such, combination approaches of insulin and leptin-neutralizing antibodies may produce highly positive effects for the future of diabetes and obesity management.
Conclusions
In the context of a physiological state of enhanced leptin sensitivity (observed in leptin-deficient ob/ob mice, in lipodystrophy, and in a small subset of obese individuals), leptin therapy works effectively for glucose and body weight control and improving metabolic health. In the context of leptin resistance (as widely observed in diet-induced obesity, following genetic or pharmacologically induced leptin reduction, either by itself or in combination with other pharmacological interventions), a restoration of leptin sensitivity in hypothalamic neurons can be observed, which leads to reduced food intake, a significant reduction in body weight, and improved insulin sensitivity. Improvements in insulin sensitivity can occur as a result of interventions targeted at directly reducing circulating levels of leptin. The metabolic improvements in this context critically rely on the reduction of leptin levels in circulation. This offers a novel basis for the explanation of the mechanism underlying the metabolic improvements observed for numerous interventions.
Article Information
Acknowledgments. The authors thank Alexandre Caron for critical review of the manuscript, in addition to the rest of the Scherer and Elmquist laboratories for helpful discussions.
Funding. This work was supported by U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01-DK55758, R01-DK099110, RC2-DK118620, and P01-DK088761 (to P.E.S.) and was also supported by NIDDK grants R01-DK118725 and R01-DK088423 (to J.K.E). S.Z. was supported by a postdoctoral fellowship from Fonds de recherche du Québec – Santé (FRQS).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Prabakaran D, Mantzoros C, et al. . Role of leptin in the neuroendocrine response to fasting. Nature 1996;382:250–252 [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–26749 [DOI] [PubMed] [Google Scholar]
- 4.Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology 2014;155:4316–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000;62:413–437 [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996;17:305–311 [DOI] [PubMed] [Google Scholar]
- 7.Yuan X, Caron A, Wu H, Gautron L. Leptin receptor expression in mouse intracranial perivascular cells. Front Neuroanat 2018;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan WW, Myers MG Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 2018;19:95–105 [DOI] [PubMed] [Google Scholar]
- 9.Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci 2018;19:153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Bartolome CL, Low CS, et al. . Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 2018;556:505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Mugabo Y, Iglesias J, et al. . α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab 2014;19:993–1007 [DOI] [PubMed] [Google Scholar]
- 12.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A 1997;94:8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oral EA, Simha V, Ruiz E, et al. . Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–578 [DOI] [PubMed] [Google Scholar]
- 14.Flier JS. Starvation in the midst of plenty: reflections on the history and biology of insulin and leptin. Endocr Rev 2019;40:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers MG Jr, Heymsfield SB, Haft C, et al. . Challenges and opportunities of defining clinical leptin resistance. Cell Metab 2012;15:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol 2008;52:1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace AM, McMahon AD, Packard CJ, et al. . Plasma leptin and the risk of cardiovascular disease in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 2001;104:3052–3056 [DOI] [PubMed] [Google Scholar]
- 19.Ogawa Y, Masuzaki H, Hosoda K, et al. . Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 1999;48:1822–1829 [DOI] [PubMed] [Google Scholar]
- 20.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One 2010;5:e11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogus S, Ke Y, Qiu J, Wang B, Chehab FF. Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology 2003;144:2865–2869 [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Zhu Y, Schultz RD, et al. . Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab 2019;30:706–719.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Münzberg H, Björnholm M, Bates SH, Myers MG Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 2005;62:642–652 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav 2006;88:249–256 [DOI] [PubMed] [Google Scholar]
- 25.Ottaway N, Mahbod P, Rivero B, et al. . Diet-induced obese mice retain endogenous leptin action. Cell Metab 2015;21:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Heek M, Compton DS, France CF, et al. . Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997;99:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol 2013;305:R566–R581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 2008;7:95–96 [DOI] [PubMed] [Google Scholar]
- 29.Galletti F, D’Elia L, De Palma D, et al. . Hyperleptinemia is associated with hypertension, systemic inflammation and insulin resistance in overweight but not in normal weight men. Nutr Metab Cardiovasc Dis 2012;22:300–306 [DOI] [PubMed] [Google Scholar]
- 30.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 2000;21:263–307 [DOI] [PubMed] [Google Scholar]
- 31.Begriche K, Lettéron P, Abbey-Toby A, et al. . Partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice. Am J Physiol Endocrinol Metab 2008;294:E939–E951 [DOI] [PubMed] [Google Scholar]
- 32.Farooqi IS, Keogh JM, Kamath S, et al. . Partial leptin deficiency and human adiposity. Nature 2001;414:34–35 [DOI] [PubMed] [Google Scholar]
- 33.Depaoli A, Long A, Fine GM, Stewart M, O’ Rahilly S. Efficacy of metreleptin for weight loss in overweight and obese adults with low leptin levels. Diabetes 2018;67(Suppl. 1):296 [Google Scholar]
- 34.Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A. High-fat diet induces leptin resistance in leptin-deficient mice. J Neuroendocrinol 2014;26:58–67 [DOI] [PubMed] [Google Scholar]
- 35.Shao M, Ishibashi J, Kusminski CM, et al. . Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab 2016;23:1167–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tam J, Cinar R, Liu J, et al. . Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 2012;16:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco JG, Dias-Rocha CP, Fernandes TP, et al. . Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur J Nutr 2016;55:601–610 [DOI] [PubMed] [Google Scholar]
- 38.Wallenius V, Wallenius K, Ahrén B, et al. . Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002;8:75–79 [DOI] [PubMed] [Google Scholar]
- 39.Mueller WM, Stanhope KL, Gregoire F, Evans JL, Havel PJ. Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res 2000;8:530–539 [DOI] [PubMed] [Google Scholar]
- 40.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994;17:30–36 [DOI] [PubMed] [Google Scholar]
- 41.Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol 2004;181:297–306 [DOI] [PubMed] [Google Scholar]
- 42.Crujeiras AB, Díaz-Lagares A, Abete I, et al. . Pre-treatment circulating leptin/ghrelin ratio as a non-invasive marker to identify patients likely to regain the lost weight after an energy restriction treatment. J Endocrinol Invest 2014;37:119–126 [DOI] [PubMed] [Google Scholar]
- 43.Rosenbaum M, Goldsmith R, Bloomfield D, et al. . Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118:2583–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumithran P, Prendergast LA, Delbridge E, et al. . Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604 [DOI] [PubMed] [Google Scholar]
- 46.Crujeiras AB, Goyenechea E, Abete I, et al. . Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab 2010;95:5037–5044 [DOI] [PubMed] [Google Scholar]
- 47.Murer SB, Knöpfli BH, Aeberli I, et al. . Baseline leptin and leptin reduction predict improvements in metabolic variables and long-term fat loss in obese children and adolescents: a prospective study of an inpatient weight-loss program. Am J Clin Nutr 2011;93:695–702 [DOI] [PubMed] [Google Scholar]
- 48.Neseliler S., et al. . Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metab 2019;29:39–49.e4 [DOI] [PubMed] [Google Scholar]
- 49.Sinha MK, Ohannesian JP, Heiman ML, et al. . Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 1996;97:1344–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caron A, Dungan Lemko HM, Castorena CM, et al. . POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. eLife 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loh K, Fukushima A, Zhang X, et al. . Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab 2011;14:684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 2004;59:287–304 [DOI] [PubMed] [Google Scholar]
- 53.Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1998;1:619–625 [DOI] [PubMed] [Google Scholar]
- 54.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999;274:30059–30065 [DOI] [PubMed] [Google Scholar]
- 55.Bjorbak C, Lavery HJ, Bates SH, et al. . SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem 2000;275:40649–40657 [DOI] [PubMed] [Google Scholar]
- 56.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. . PTP1B regulates leptin signal transduction in vivo. Dev Cell 2002;2:489–495 [DOI] [PubMed] [Google Scholar]
- 57.Williams KW, Liu T, Kong X, et al. . Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab 2014;20:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller TD, Sullivan LM, Habegger K, et al. . Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J Pept Sci 2012;18:383–393 [DOI] [PubMed] [Google Scholar]
- 59.Owen BM, Ding X, Morgan DA, et al. . FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 2014;20:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elefteriou F, Takeda S, Ebihara K, et al. . Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A 2004;101:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Dallner OS, Nakadai T, et al. . A noncanonical PPARγ/RXRα-binding sequence regulates leptin expression in response to changes in adipose tissue mass. Proc Natl Acad Sci U S A 2018;115:E6039–E6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes Metab 2007;9:799–812 [DOI] [PubMed] [Google Scholar]